Abstract
The anatomy and morphology of gonadotropin‐releasing hormone (GnRH) neurons makes them both a joy and a challenge to investigate. They are a highly unique population of neurons given their developmental migration into the brain from the olfactory placode, their relatively small number, their largely scattered distribution within the rostral forebrain, and, in some species, their highly varied individual anatomical characteristics. These unique features have posed technological hurdles to overcome and promoted fertile ground for the establishment and use of creative approaches. Historical and more contemporary discoveries defining GnRH neuron anatomy remain critical in shaping and challenging our views of GnRH neuron function in the regulation of reproductive function. We begin this review with a historical overview of anatomical discoveries and developing methodologies that have shaped our understanding of the reproductive axis. We then highlight significant discoveries across specific groups of mammalian species to address some of the important comparative aspects of GnRH neuroanatomy. Lastly, we touch on unresolved questions and opportunities for future neuroanatomical research on this fascinating and important population of neurons.
Keywords: human, luteinizing hormone‐releasing hormone, mouse, reproduction, sheep
1. A BRIEF HISTORICAL OVERVIEW
A major force in the understanding of systems neuroscience and the system regulating reproduction arose from the concept that “function follows form.” Studies of neuron morphology reveal changes during development, maturation, senescence, experience and plasticity following injury. Information about these important components leads to understanding of normal function and how dynamic changes alter the way the system works. For neuroendocrinology, anatomy has had a seminal role. First and foremost, discovery of neurosecretion and its unique role in endocrine processing changed the perspective of the roles that neurons play in homeostasis and brought to light how neurons can work as secretory units directed into the peripheral circulation. The acceptance of the concept of neurosecretion arose from the results of anatomical studies that began in the late 1930s and extended through the 1960s. Those studies established that neurosecretory material originated from neurons that extended their axons from the brain to the posterior lobe of the pituitary. The neurohormones released from the axon terminals near fenestrated blood vessels were transported by the blood circulation to distant sites. As the cited reviews indicate, 1 , 2 these seminal studies initiated the field of neurosecretion and provided key insights into the unique roles of neuroendocrine neurons in the control of both posterior and anterior pituitary functions. However, the study of the reproductive neuroendocrine system would still be a black box had it not been for the discovery of GnRH. There is little doubt that the award of the Nobel Prize to Andrew Schally and Roger Guillemin in 1977 set the stage for unravelling the mysteries of reproductive physiology.
For the control of anterior pituitary‐related functions (regulation of growth hormone, thyroid hormone, gonadotropin, prolactin and corticosteroids secretion), anatomical studies led to the primary role of the median eminence and pituitary stalk as the sites of factor release. The unique blood vessels forming loops within the median eminence 3 were found to collect into “private” vessels that led exclusively to the anterior pituitary to control adenohypophyseal functions. As evidence mounted suggesting that the brain made the factors regulating the anterior pituitary, anatomical studies describing the reproductive function upon transplantation of the anterior pituitary into the medial basal hypothalamus (MBH) 4 , 5 compared to the kidney capsule led to the initial evidence of where the neural control systems resided. They emphasized the need for the pituitary gland to have a close proximity to the hypothalamus and its portal blood system and that placement of median eminence extracts into the pituitary enabled ovulation. 4 , 5 , 6 , 7 , 8 While these early studies indicated that the anterior pituitary needed close proximity to the median eminence, data did not reveal where the brain cells providing the stimulating factors for gonadotropins resided.
Use of reproductive function assays (ovulation or eventually measurement of LH or FSH) after brain stimulation 9 or knife cuts placed stereotaxically into the brain 10 began to suggest that reproductive control in a number of species required an intact preoptic area (POA) connection to MBH. While it is beyond the scope of this article to review each of those studies in all the species that have been used with these approaches, one must be cognizant of the fact that interpretation of studies using knife cuts, lesions or electrical stimulation were impaired or easily misinterpreted when the locations of the system and its pathways were not yet defined. An understanding of where the GnRH neurons were located and where their axons projected were required for a deeper understanding of how the neurons controlled LH and FSH secretion. In the 1970s, as antibodies against GnRH were produced and techniques for visualizing GnRH became available, scientists began to search for the elusive GnRH neurons. Few of those early studies were successful due to suboptimal tissue processing conditions and antibody qualities. Investigators tried to apply the same preparations for hypothalamic tissue that were used for routine histology both in terms of selection of fixatives and preparation of tissue blocks in paraffin for cutting. GnRH was easily extracted during tissue dehydration in EtOH and embedment in paraffin which resulted in a striking loss of the GnRH antigen in paraffin‐embedded material. If embedding in paraffin was avoided by processing free‐floating sections, staining greatly improved. 11 Fixation known to be optimal for routine histology (Bouin's) included formaldehyde, picric acid and acetic acid and had a pH close to 1.0. It turned out that staining of tissue fixed in a neutral formaldehyde ‐ picric acid fixative (Zamboni's) preserved GnRH better and provided clear locations of the cells and their axons compared to staining of tissue fixed with the more acidic fixative. Moreover, initial recommendations for fixation of peptides suggested short fixation times and cold solutions. Missed was the fact that formaldehyde‐based fixatives are not able to complex proteins quickly. If the fixative was cool, the process became even slower. It was later learned that fixatives that penetrated better and reacted more readily than buffered formaldehyde/formalin fixatives markedly improved the detection. 12 Once GnRH antisera with high titres were produced or purified after production, antigen detection improved further. Scientists could then distinguish immunoreaction products and details of cell morphology. It is essential, however, that one considers the method selected for detection. Methods that generate few molecules of product not only require more antibody but may disable detection of structures with lower expression. Comparisons of methods 13 reveal the striking differences in antigen detection, especially using immunofluorescence approaches.
Early studies often assumed that the cells of the GnRH system would be found in the MBH. Some may not have probed carefully other brain areas or based their conclusions on assays of extracts that showed high activity in the MBH, in virtually all species. Some attempts to stain those cells had used an antibody later found contaminated by proopiomelanocortin antibodies. Arcuate nucleus (ARC) stained cells ended up being opiate cells since the antibodies had been repurposed after first immunizing the rabbits with proopiomelanocortin peptides and not GnRH. 14 As localization improved it was realized that the high GnRH concentrations in the ARC were due to the axons converging into the median eminence rather than cells (if present in the area, the cells were often positioned lateral to the ARC). Once descriptions of cell locations accumulated, locations widely scattered in the forebrain seemed to contradict the notion that neurons developed from the ventricular surfaces and migrated to their final destination in a programmed fashion like most other CNS neurons. GnRH neurons were different. Diverse species were found to have GnRH neurons in different sites, and initially this made no sense. It was then the seminal studies of Schwanzel‐Fukuda and Pfaff 15 and Wray et al. 15 both in 1989, that lit the light bulb indicating those neurons developed from the olfactory placode unlike most neurons of the central nervous system. That discovery also paved our way for understanding how genetic disorders of olfactory placode cell migration linked anosmia to reproductive dysfunction (Kallmann's Disease). Once the manner in which the olfactory system generated GnRH neurons was uncovered 15 we could better recognize that GnRH cells all fell into a part of what we now know as the rostral migratory stream. Table 1 shows site variation in a variety of species. 16 Note there are vast differences in the location of cells, ranging from small ganglia at the interface of the olfactory nerve whose cells extended into the rostral septum in the opossum, 17 to the midbrain in primates and dogs. Cells were positioned in the lateral anterior tuberal hypothalamus (along the lateral borders of the ARC) in species where the forebrain is vertically shifted (primates, sheep, ferrets and guinea pigs), whereas a dominance of medial POA and septal cells were found in rodents, cats, hamsters, voles, and pigs. Nonetheless, there are a small number of GnRH neurons in the latter group that migrate into the arcuate nucleus and sometimes the pituitary stalk along with the axons from more rostrally positioned neurons. While not yet known why the cells move further along the migratory stream in some but not all species, the association of the neuroendocrine members of the GnRH system do seem to follow the known roads from the nose into the brain. 16 A feature of the GnRH cells that travel into more caudal brain areas is that they often reside between rather than within the resident nuclei. This phenomenon might be explained by the fact that in those animals, GnRH neurons arrive in those areas at the time when the nuclei have already formed. For example, in the rat, migration of GnRH neurons is completed by embryonic day 16.5, 18 whereas at that time, neurons of the medial POA have already left the mitotic cycle and occupy their adult locations. 19
TABLE 1.
Diversity of distribution of GnRH neurons in mammals. Note these data are derived from use of antibodies that appeared not to stain truncated or modified forms of GnRH. Modified from Hoffman and Berghorn 16
| Septum | Preoptic area | Anterior hypothalamus | Tuberal hypothalamus | Premammalian | |
|---|---|---|---|---|---|
| Rodents | |||||
| Mouse | ++ | ++++ | ++ | +/− | − |
| Hamster | +++ | ++++ | + | − | − |
| Rat | ++ | ++++ | ++ | +/− | − |
| Guinea pig | + | +++ | ++ | + | − |
| Lagomorphs | |||||
| Rabbit | ++ | +++ | + | + | + |
| Chiroptera | |||||
| Bat | −/+ | −/+ | + | ++++ | − |
| Carnivores | |||||
| Ferret | −/+ | + | + | ++++ a | − |
| Dog | + | ++ | ++ | ++ | + |
| Cat | + | +++ | ++ | − | − |
| Ungulates | |||||
| Sheep | ++ | ++++ | + | + | − |
| Pig | ++ | ++++ | + | − | − |
| Marsupials | |||||
| Opposum | +/− | − | − | − | − |
| Primates | |||||
| Rhesus monkey | ++ | ++++ | +++ | +++ a | + |
| Baboon a | ++ | ++++ | +++ | +++ a | + |
Lateral to the Arcuate nucleus.
The fact that the axons of GnRH neurons reached the pituitary portal blood supply provided an opportunity for anatomy to reveal which of the GnRH cells have the ability to influence the pituitary. In the late 1980s and 1990, application of retrogradely transported molecules administered into the median eminence, and taken up by the axons of the portal blood supply or injected into the peripheral circulation, gave insight into which GnRH cells were potentially able to release GnRH into the peripheral circulation (horseradish peroxidase, 20 wheat germ agglutinin, 21 and fluorogold 22 , 23 ). In all those studies, only a portion of the GnRH neurons possessed the tracer. The fact that some GnRH cells lacked connection to the median eminence is noteworthy in that it stresses the fact that subsets of GnRH neurons have a neuroendocrine role not shared by all GnRH cells in the area. Comparisons across species speak to a similarity of subsets of neuroendocrine GnRH cells in mice, guinea pigs, sheep, and primates.
Scientists were then faced with the question of whether examining the GnRH neuron architecture could provide insight into the transitions across reproductive status. Do the cells visibly change during the transition to puberty or senescence? Overall, the data are inconsistent and with high variation in the quality of staining. When changes are observed, such as development of spines in rats during adolescence as an indicator of the pubertal period, some of those changes do not accurately reflect the functional status of the GnRH cells 24 , 25 but may instead reflect the capacity to cycle rather than the actual presence of altered cyclic hormone function. In contrast, studies in mice elegantly revealed that at the time of activation of GnRH neurons spines were increased by 60% over quiescent times. 26 Studies of this type address the plasticity of spines on neurons and emphasize the importance of knowing the details of the animal's physiological status when probing for morphological changes.
Where neuroanatomy has been valuable in reflecting functional changes in the GnRH system arose from the observations that many neurons, when stimulated, express immediate early gene proteins. FOS is one of these proteins and has the advantage that it is expressed within 30–45 min after the cells are stimulated. Fortunately, the FOS protein is also highly conserved across species. The induction of FOS is the consequence of sharp changes in second messenger signals arising from synaptic activity. Baseline cell firing alone often cannot reach the threshold for FOS induction. FOS has enabled the identification of those GnRH neurons that give rise to the preovulatory LH surge in female rats, mice, sheep, and ferrets. In rats, taking into account the time it takes for FOS translation (30–60 min), the number of cells displaying FOS is tightly coupled to the rise in LH that triggers ovulation. 27 In these species FOS is not triggered by hormonal signalling in males. This reflects the absence of the oestrogen‐sensitive cells driving GnRH secretion. 28 Surprisingly, a study attempting to show that FOS identified activated GnRH neurons in primates during the preovulatory LH surge did not show evidence of activation of the GnRH system. 29 However, the primate LH surge persists for days due to pituitary drive of LH. Also, since FOS will only remain in stimulated cells for 2–3 h after onset of stimulation the fact that the time of examination was not linked to the onset of LH secretion could explain why the cells did not show signs of recent stimulation. Indeed, examination of GnRH release from the median eminence of cycling female rhesus monkeys showed a marked surge in GnRH at the time of the preovulatory LH surge. 30 What this reinforces is that unless accompanied by careful monitoring of LH secretion at the onset of LH surges, FOS can appear unreliable as an activity marker. Another consideration is the fact that FOS induction does not persist, but rather initial induction of second messenger signals turns on the c‐fos gene. FOS then appears to turn off its own mRNA production and protein turnover stops the signal within a couple of hours even when stimulation persists for days. Only after the system normalizes can FOS be induced again. Repeated surges of LH if separated by 24 h permit the re‐expression of FOS in the GnRH neurons. 31 An important problem unresolved for a long time was how GnRH neuronal function enables GnRH pulsatile release at times other than the preovulatory surge. Both males and females in multiple species show pulses of GnRH and LH that are spaced between 30 and 60 min apart. It has been suggested that pulses might simply represent the random firing of two GnRH neurons, but the timing of pulsatile release seems too regular for events to be random. Over the years it has also been hypothesized that GnRH–GnRH synapses (axodendritic, dendrodendritic) could link cell activity or that gap junctional dendrodendritic connections suggested by Witkin et al. 32 from intercellular bridges could provide synchronous activity between GnRH neurons. An additional explanation for the function of the dendritic interactions could be ephaptic transmission but the diffuse organization of the cells and challenge of knowing which cells should be assessed have made it difficult to determine the nature of connections that underlie the pulses. The interaction of cellular processes in GnRH neurons is seen in both primates and rat and could be the result of dendron or dendritic interactions in mice. Examples of dendritic associations in a primate and a rat is presented in Figure 1. 33 The advent of genetic animal models expressing reporter proteins under the control of the GnRH promoter has since richly contributed to our understanding of the connectedness of GnRH neurons. As discussed in detail below, the absence of electrical coupling between GnRH neurons was initially reported in mice expressing GFP in GnRH neurons, 34 and later confirmed in other reports. 35 Evidence accumulated in recent years now indicates that kisspeptin (KP) release from MBH KP neurons which cosynthesize KP, neurokinin B (NKB) and dynorphin (Dyn; aka “KNDy neurons”), plays a pivotal role in shaping the patterned secretion of GnRH into the hypophysial portal circulation. 36 , 37
FIGURE 1.
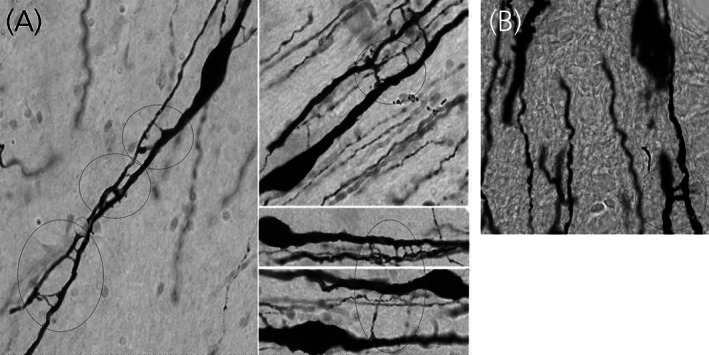
Dendrodendritic interactions of GnRH neurons. Intercellular bridges are seen between GnRH dendrites. (A) Examples of dendritic processes from a rhesus monkey whose bridges between immunoreactive GnRH neurons appear as fine ladders. (B) Example of two intercellular bridges across GnRH neurons in a rat brain (red circle). Figure reproduced from Hoffman 33
Another challenge in the studies of the GnRH system is with little doubt that while the male control of gonadotropin section is vastly different from that of the female, scientists have found little evidence of GnRH neuron sexual dimorphisms. In that domain, a major clue arose from the fact that the alpha oestrogen receptor isoform (ER‐α) was essential for cyclic release of GnRH and gonadotrophins, but GnRH neurons had no ER‐α. 38 Further investigation of oestrogen's site of action led to the KP neurons which not only possess ER‐α but also display striking sexually dimorphic distributions. 39
2. SPECIES SPECIFIC GNRH NEUROANATOMY: RODENTS
The first visualisation of GnRH neurons with immunohistochemistry was reported in the guinea pig 40 (reviewed in 41 ) and described as a diffuse distribution of bipolar neurons and their fibre processes through the basal forebrain. Since that time, the distribution and morphology of GnRH neurons has been described and studied most frequently in the rat 42 , 43 and mouse, 44 but also in several other rodent and small mammal species, for example the hamster, 45 , 46 , 47 bat, 48 and rabbit, 49 and in larger mammal species (see further below). In general, rodent GnRH neurons are found dispersed throughout the septal, preoptic and anterior hypothalamic areas. 50 They are evident along a rostral to caudal continuum, from the olfactory bulbs to the medial basal hypothalamus, following their developmental migratory path into the brain. 15 , 18 In the rat and mouse, the diffuse somata of GnRH neurons are frequently described by three anatomical subpopulations, including a rostral most population that is found to run in dorsoventral bilateral streams from the medial septum to the rostral POA, a central population in an inverted Y distribution around the organum vasculosum of the lamina terminalis (OVLT), and a smaller lateral population that sits ventrally on both sides of the third ventricle in the anterior hypothalamus (Figure 2A). While it is not clear whether GnRH neurons residing in different locations possess specialised functions, a large proportion of those neurons surrounding the OVLT colabel with the immediate early gene marker FOS at the time of the LH surge in rodents, 52 suggesting that this subpopulation of neurons is particularly important in surge generation. GnRH neuron axon processes in rodents can be visualised along the rostral to caudal continuum of the GnRH neuron soma, extending into the OVLT and densely infiltrating the external zone of the median eminence. GnRH projections have also been described in a number of hypothalamic and extra hypothalamic regions outside of this zone, including the olfactory system, amygdala, limbic cortex and midbrain 45 , 53 , 54 supporting the notion for extra‐hypophysiotropic roles for GnRH neurons as a central nervous system neurotransmitter.
FIGURE 2.
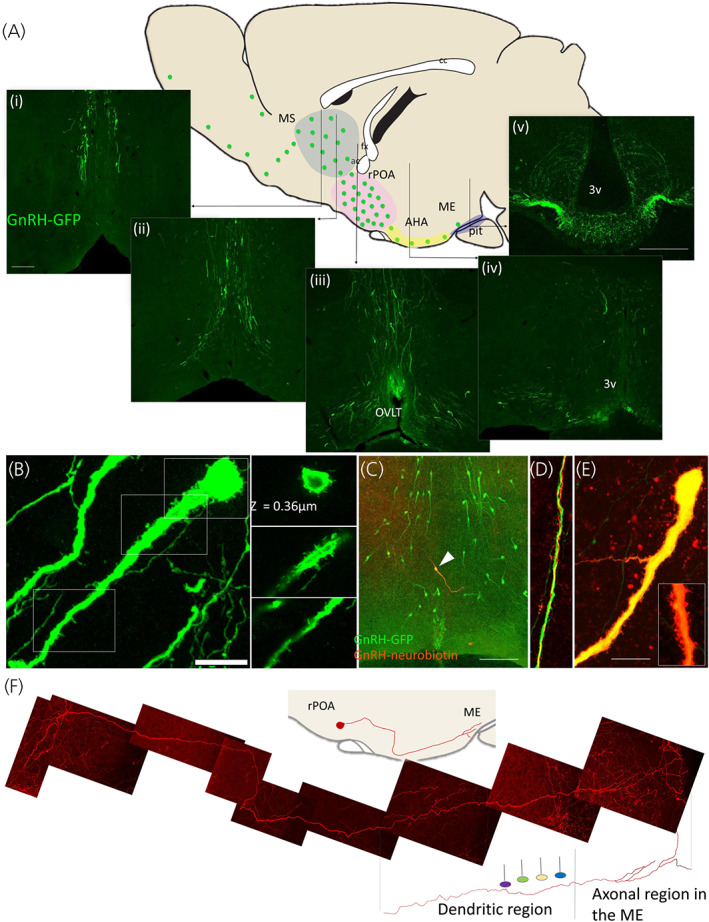
Transgenic approaches to reveal GnRH neuron morphology in the rodent brain. (A) A sagittal brain schematic depicts the scattered distribution of GnRH neurons in a mouse brain (green dots). Coloured regions correspond to commonly referenced anatomical zones, that is, the medial septal region (MS, blue), the rostral preoptic area (rPOA, pink) and the anterior hypothalamic area (AHA, yellow). Vertical lines indicate the location of representative confocal images in the coronal plane of a GnRH‐green fluorescent protein transgenic mouse (i–v). (B) GnRH neuron spines can be visualised in confocal images of the GnRH‐GFP mouse brain. Fixed labelled brain sections and following neurobiotin cell‐filling in ex vivo brain slices from GnRH‐GFP mice. Bar = 10 μm. (C) Neurobiotin filling of GnRH neurons in GnRH‐GFP muse brain slices reveals long dendritic projections, found to bundle with other GnRH dendrites (D) and exhibit proximal and distal spiny protrusion (E). (F) An individual GnRH neuron originating in the rPOA and terminating in the median eminence can be visualised through sparse viral mediated transfection of membrane linked fluorophores in GnRH‐Cre transgenic mice coupled with optical clearing techniques. Together with functional evidence, the long dendritic processes of GnRH neurons that transition into axonal processes supports the presence of a blended dendron. Bar = 100 μm in (A) and (C) and 10 μm in (B) and (E); fx: fornix, ac: anterior commissure, cc: corpus callosum. OVLT: organum vasculosum of the lamina terminalis, 3v: third ventricle, ME: median eminence, pit: anterior pituitary gland. Parts of figure adapted from Campbell 51
Studies of GnRH neuron morphology in rodents, through both classical and transgenic approaches, have advanced and challenged our understanding of GnRH neuron function. Collectively, these anatomical discoveries have formed a foundation for understanding how GnRH neurons are regulated by upstream networks, behave across different physiological states, and operate in a synchronized fashion for pulsatile release.
2.1. Distinct regulation zones
As the final downstream effector neurons driving the central regulation of fertility, a large focus has been on understanding how GnRH neurons are regulated by higher order inputs. Early ultrastructural studies described GnRH neuron somata and proximal dendrites in several species as largely ensheathed by glial membrane and very sparsely innervated relative to surrounding neurons. 42 , 46 , 55 , 56 These early studies promoted the idea that the integration of synaptic input required for successful pituitary‐gonadal axis control probably occurs upstream from the GnRH neurons themselves. This idea has been both challenged and reframed with the development of new ways to visualise GnRH neuron morphology and the discovery of the KP system.
Filling murine GnRH neurons in brain slices with low molecular weight dyes 57 , 58 , 59 or expressing membrane bound fluorescent markers in small numbers of GnRH neurons in vivo 60 has expanded our view of GnRH neurons beyond regions limited by GnRH peptide detection (Figures 2C–E). GnRH neurons have been found to extend exceptionally long processes that possess dendritic morphology, suggesting large anatomical zones important in receiving and processing afferent information. Most GnRH neurons extend two of these long dendrites, and in many cases, both project ventrally and caudally toward the median eminence, with dorsally oriented dendrites frequently taking a hairpin turn to follow other projection toward the median eminence. 61 , 62 Labelling for ankyrin G has identified the axon initiation zone in one of these dendrites, typically 50–150 μm away from the soma. 61 Histological evidence for synaptic inputs places the majority of inputs within this dendritic zone between the soma and the axon initiation zone, 58 , 61 , 63 and quantification of apposed vesicular transporters suggests that glutamatergic inputs are twice as likely as GABAergic inputs within this zone. 63 In addition, the number of putative synapses to the GnRH neuron proximal dendrite drops precipitously beyond about 50 μm. 58 , 63 These data suggest that although the GnRH neuron dendrite can extend for millimetres through the mouse brain, there is still a concentrated area in the proximal dendrite where the majority of synaptic input is received, at least at this “end” of the GnRH neuron.
Cell‐filling and reporter expression in GnRH neurons also discovered that as dendrites approach the ME, they branch into multiple processes with axonal morphology that extend to and terminate on blood vessels 60 , 62 (Figure 2F). This was not entirely new, as early morphological studies also described dendritic projections that appeared to transition into axonal projections. 42 More recently, the blended dendritic and axonal features of this process, have been confirmed with electrophysiology and electron microscopy, 62 and led to the term “dendron” (reviewed in Herbison 64 ). Morphological evidence suggests that this distal region of the GnRH neuron is much more densely innervated than the proximal dendrite, 60 , 65 and that synaptic inputs are restricted to the dendritic portion of the dendron and absent from axons. 60 Expansion microscopy suggests that dendrons are also responsive to short‐diffusion volume transmission, as local KP‐expressing axons do not appear to make synaptic contacts with the dendron. 66 Evidence for a dendron‐like structure has also been described in the rat, 67 although ultrastructural studies will be required to confirm this morphology.
The identification of these two distinct input zones, at opposite poles of the GnRH neuron, has evolved alongside the discovery of two distinct anatomical and functional populations of KP neurons in the mouse. Selective viral‐mediated tracing has identified that the most rostral population of KP neurons, associated with surge generation, project most heavily to the proximal zone of GnRH neurons, while the KNDy neurons of the ARC, the putative pulse generators, 68 project exclusively to the distal zone. 69 This morphology, supported by functional evidence, 65 suggests that the proximal zone is critical for surge generation and the distal dendron is a separately regulated domain critical for pulsatile GnRH secretion.
To date, evidence for a GnRH dendron‐like process has only been described in the mouse, 62 and the rat 67 and it remains unclear whether the same feature is exists in other mammals. Of interest, the GnRH neurons of the small brown bat, which have a distribution that is more similar to the primate pattern than the rodent pattern, is described to have one thin and one thicker process leaving the soma, both of which give rise to fine varicose fibres. 48 Presumably the thicker process is dendritic in nature and the varicose fibres are axonal, describing a similar morphology to that seen in the mouse. Another clue that this feature may be more universal comes from the ewe, where median eminence explants are found to respond to KP, 70 suggesting that distal GnRH neuron elements are responsive to neurotransmission.
2.2. Function‐related morphological plasticity
Visualisation of rodent GnRH neurons has repeatedly demonstrated morphological plasticity associated with changes in circuitry, function and physiological state. Morphological plasticity of the GnRH neuron soma and proximal dendrite was suggested by early immunodetection studies reporting somatic profiles of GnRH neurons as “smooth” or “thorny.” These shape‐types were found to vary with age and hormonal status 71 , 72 , 73 and with the number of ultrastructurally identified synaptic inputs, 42 suggesting that these ‘spines’ were presumptive sites of excitatory input, as in other neuronal populations. 74 , 75 Later studies, using transgenic mice and rats expressing green fluorescent protein (GFP) in GnRH neurons, 34 , 76 , 77 , 78 afforded an enhanced view of spine morphology (Figure 2B) and found the density of these putative spines to increase in several physiological states, including over pubertal development, 79 in FOS activated neurons at the time of the preovulatory LH surge, 26 and in association with hyperactivity in prenatally androgenised mice. 80 Although the presence of these spines correlates with physiological states in which greater synaptic inputs may be anticipated, and in some case has been shown, 81 there is currently limited evidence to support that the spines identified on GnRH neuron soma or dendrites are indeed sites of synaptic input or reflect morphological rearrangements in synaptic inputs, in particular over the female oestrous cycle. 63 , 82 In contrast, seasonal breeders, including the ewe and hamster, have been found to exhibit dramatic plasticity synaptic input density to GnRH neurons. 83 It remains unknown whether GnRH neurons in these species possess spines similar to the rat and mouse and whether they exhibit morphological plasticity. To date, all evidence for morphological plasticity comes from the proximal input zone of the GnRH and it remains to be determined whether morphological plasticity occurs in the distal region of the dendron.
The nerve terminal zone of rat GnRH neuron axons has also been well described to undergo morphological plasticity to facilitate hypophysiotropic peptide release into the perivascular space. 84 Ultrastructural studies show GnRH nerve terminals in the external zone of the median exhibit neuro‐glial remodelling over the estrous cycle, dependent upon the actions of semaphorin 7A expressed in local tanycytes. 85 Similar structural rearrangements have been shown in the ewe between the breeding and anoestrus seasons 86 and with altered metabolic status. 87
2.3. Dendritic bundling: A morphology to support synchronisation or simply a product of migration?
Ultrastructural evidence for closely associated GnRH neuron dendrites and shared synaptic input (a single axon synapsing with two GnRH neuron dendrites) was first described in rats in the mid 80s. 88 More recently, colabelling of biocytin‐filled GnRH neurons and GFP in brain tissue from GnRH‐GFP transgenic mice also identified several examples of these ‘bundled’ GnRH neuron processes, where the dendritic processes of 2–4 distinct GnRH neurons were found intertwining and coming in close contact with one another 89 (Figure 2D). At the ultrastructural level, these bundles are found frequently connected via zonula adherens and contacted by shared synaptic input from single afferents. 89 While tempting to speculate that GnRH neurons are electrically coupled via these close associations to support synchronised activity, GnRH neurons do not express gap junctions. 35 This suggests that the main function of these bundles may serve as sites for efficient innervation by upstream regulators, aimed at simultaneously targeting several GnRH neurons. To date, these elements have only been described for more proximal regions of the GnRH neuron dendrite and it remains to be determined whether similar features exist in more distal regions where pulse generation appears to be predominantly regulated. 65 While postulated as a potential morphological substrate for synchronisation, an alternative hypothesis is that the bundling morphology of GnRH neurons is a byproduct of early migration and dendritic pathfinding. While dendritic bundling suggests a mechanism by which the largely scattered rodent GnRH neurons can be in physical contact with one another, it remains to be determined if or how this morphology is important for GnRH neuron function.
3. SPECIES SPECIFIC GNRH NEUROANATOMY: SHEEP
Early work in the sheep provided some of the seminal observations characterizing the morphology of GnRH neurons and their neuroendocrine functions. These include initial observations of the close, intimate anatomical relationship of GnRH neurons with surrounding glia, as well as evidence suggesting regional differences among GnRH subpopulations in their activation during pulsatile versus surge secretion of LH. In addition, because sheep are seasonal breeders, they have provided a model demonstrating seasonal plasticity of inputs onto GnRH neurons, work that has more recently been extended into plasticity of specific sets of afferents. Finally, it is worth noting that because of the unique ability in sheep to directly monitor GnRH in portal blood in awake animals, 90 it has been possible to directly correlate patterns of GnRH neuronal activity using FOS and other markers with their neurosecretory output. In the following sections, we will briefly review some of these key observations, and how they formed a foundation and, in some cases, presaged more recent findings.
3.1. GnRH neuron morphology and projections in sheep
Early immunocytochemical work using free‐floating, unembedded sections, and including the use of detergent (Triton‐X100) in processing, revealed a complex morphology of GnRH neurons in the sheep brain, with significant numbers of multipolar neurons 91 in contrast to the predominantly bipolar morphology of GnRH neurons in rodents (see above). While the majority of GnRH neurons in the sheep were seen in the POA, smaller but significant numbers were seen more caudally in the anterior hypothalamus and MBH. 91 , 92 Subsequent tract tracing studies in sheep, 93 similar to such studies in rodents, showed that despite the regional variation in GnRH cell number, the percentage of GnRH cells in each area projecting to the median eminence remained consistent across regions, suggesting that GnRH neurons in all areas were capable of controlling the release of pituitary LH. Perhaps the most remarkable observation of these early studies in sheep was the degree to which GnRH cells, unlike adjacent non‐GnRH neurons, were found to be almost entirely ensheathed by the processes of adjacent astrocytes. 94 Afferent axon terminals were seen to penetrate this glial sheath before synapsing upon preoptic GnRH somata and dendrites. 94 This observation set the stage for subsequent studies of plasticity of inputs to ovine GnRH neurons, related to neuroendocrine control by season 86 as well as alterations in the adult GnRH system due to prenatal androgen treatment. 95 Interestingly, it also complemented work in rodents documenting the loose functional relationship between glial and GnRH cells in sexual maturation, including recent demonstration of mechanisms by which GnRH neurons “recruit” astrocytes in postnatal development to ensure appropriate wiring and firing of GnRH neurons. 96
3.2. Seasonal plasticity of the GnRH system in sheep
Sheep and other seasonally breeding mammals display a reversible annual cycle of fertility in which GnRH neurons are the critical, final common output controlling reproduction. In sheep and most other mammals, the key factor responsible for seasonal reproduction is a profound change in the ability of estradiol to inhibit pulsatile secretion of GnRH. 97 The early recognition that GnRH neurons in sheep and other species lacked nuclear ER‐alpha, 98 the isoform required for oestradiol's negative feedback influence, implied that the influence of estradiol must be conveyed to GnRH via afferents. Given the striking degree of glial ensheathment of GnRH cells in the sheep, it was logical to compare the ultrastructure and synaptic inputs of GnRH neurons between the breeding and nonbreeding (anoestrous) animals as a possible structural basis for seasonality. The results of an incredibly labour‐intensive, electron microscopic analysis revealed that GnRH neurons in the breeding season received more than twice the density of synaptic inputs onto their dendrites and somas. 86 These changes in synaptic density were complemented by a reciprocal change in the amount of glial ensheathment of GnRH cells, with cells in anestrous animals being more completely surrounded by glial processes. Importantly, both the changes in synaptic input and glial ensheathment were only in GnRH cells and not in adjacent nonidentified neurons in the POA. Later studies provided details of the neurochemical phenotype of the seasonally changing inputs, 99 including work showing increased number of KP, and decreased GnIH, contacts into GnRH neurons during the breeding season. 100 This demonstration of seasonal plasticity in the GnRH system also provided a conceptual foundation for other studies documenting changes in the number and phenotype of afferents onto adult GnRH neurons, including studies of changes associated with the preovulatory GnRH/LH surge, 101 and long‐term changes seen as a result of prenatal androgen exposure in female sheep. 95 , 102 Finally, seasonal synaptic changes in the reproductive neuroendocrine system of sheep are not limited to GnRH neurons, but also seen in inputs onto upstream afferent neurons, notably KNDy cells of the ARC. 103 Upstream changes in the number and density of synaptic inputs onto KNDy cells are also seen in prenatally androgenized ewe 95 , 102 and prenatal androgen mouse 104 models of polycystic ovary syndrome (PCOS).
3.3. Functional subpopulations of GnRH neurons in sheep
As discussed above, when used in conjunction with monitoring of secretory LH patterns, FOS has been a useful marker for GnRH neuron activation in a number of species. This has been particularly true in the sheep, where discrete and repeated measurements of portal GnRH are possible over long periods of time in unanaesthetized animals. 90 Indeed, the ability to precisely monitor the dynamics of GnRH pulses and surge has allowed for examination of the activation of GnRH and other upstream neurons (e.g., KNDy neurons) during both pulsatile 105 , 106 and surge secretion. 92 , 101 , 107 Whereas about half of all GnRH neurons across the entire range of their distribution, including the POA and MBH, expressed FOS during the preovulatory LH surge, 107 using two models that acutely induced an LH pulse, FOS expression was seen to be limited to GnRH cells located in MBH. 105 Interestingly, in the latter experiment, the occurrence of endogenous (not induced) LH pulses was also accompanied by FOS activation in MBH but not POA GnRH neurons. 105 More recent work in the sheep has reinforced the view that MBH GnRH neurons are functionally involved in pulse generation. First, unlike POA GnRH cells, they are heavily innervated by Dyn terminals 108 presumably arising from KNDy neurons which constitute a core component of the GnRH pulse generator. 36 In addition, recent work using internalization of kappa opioid receptor as a marker has shown that Dyn is released onto MBH, but not POA, GnRH neurons at the time of the termination of each GnRH pulse. 103 Thus, at least in sheep, there is strong evidence for the most caudal subpopulation of GnRH neurons in the MBH being selectively involved in pulsatile secretion, perhaps in parallel to actions at the GnRH “dendron”, also located in the MBH, as a locus of pulse regulation in rodents.
4. SPECIES SPECIFIC GNRH NEUROANATOMY: PRIMATES
While the bulk of the neuroanatomical literature is available from rodents and ruminants, immunohistochemical and in situ hybridization experiments on monkeys and humans have revealed some interesting species‐specific anatomical characteristics of the GnRH neuronal system in primates. Results of these studies may become particularly useful for the understanding of the central regulation of human fertility and its disorders. This chapter aims to highlight some unique features of primate GnRH cells.
4.1. Presence of two GNRH genes in the primate genome
Reproduction in nearly all mammals is controlled by the mammalian form of GnRH decapeptide (mGnRH) encoded by the sole GNRH1 gene. Unlike laboratory rodents, primates contain a second GNRH gene (GNRH2) in their genome. 109 This gene is inactivated in the orangutan and the chimpanzee, whereas it remains fully functional in rhesus monkeys, humans and several other primates. 109 In situ hybridization studies of the macaque brain localized GNRH2 mRNA expressing neurons in the midbrain, hippocampus and the hypothalamic supraoptic, paraventricular and suprachiasmatic nuclei and the ARC. 110 The oestrogenic induction of GNRH2 expression in this species raised the intriguing possibility that, in some primates, GnRH‐II neurons may contribute to oestradiol‐positive feedback and the midcycle preovulatory LH surge. 110 We note that the biosynthesis of the functional GnRH‐II peptide in mesencephalic and hypothalamic neurons of the rhesus monkey brain still awaits confirmation. 110 Mapping the distribution of GNRH2 expressing neurons in the human brain remains an interesting challenge for future research. The following sections will focus on the neuroanatomy of the primate GnRH‐I system.
4.2. GnRH neuron development in primates
As reported for different mammals, GnRH neurons in primates develop in the olfactory placodes and migrate to the brain prenatally. 18 The detailed spatiotemporal profile of this migration in the human embryo has been characterized recently by the Giacobini laboratory. 111 These authors confirmed the existence of a ventral migratory pathway also known in other species which carries ~2,000 neurons to the developing human hypothalamus to regulate reproduction after puberty. In addition, they discovered and reported a second dorsal route previously known only in monkeys 112 whereby ~8,000 neurons migrated toward extrahypothalamic structures of the human brain. 111 It is currently unclear where these neurons finally settle and whether they survive into adulthood. Interestingly, it has only been established recently that the number of extrahypothalamic GnRH neurons in the adult human brain is much higher than 8,000 (104,000–229,000 in different brain samples), 113 suggesting that the majority of extrahypothalamic GnRH neurons in humans might be brain‐born, instead of originating from the olfactory placodes. 113
4.3. Topography and morphology of GnRH neurons in the primate hypothalamus
The majority of the anatomical studies in the primate brain used immunohistochemistry to localize GnRH neurons. 113 , 114 , 115 , 116 , 117 , 118 , 119 Perikaryon distribution in monkeys and humans was found to be even looser than in rodents. The large hypothalamic volume contains cell bodies from the septal‐preoptic to the retromammillary region. GnRH neurons were reported within the preopticoseptal region, diagonal band of Broca, lamina terminalis, periventricular and infundibular nuclei and the mammillary region. 115 , 116 , 119 , 120 Some investigators observed a few cell bodies in the ventromedial nucleus. 118 Unlike in laboratory rodents, the OVLT contained relatively few GnRH cells. 119 Morphologically, the majority of GnRH neurons in primates are fusiform and bipolar, with two processes emanating from the opposite poles of a thin soma 116 , 119 , 121 (Figure 3A). A smaller subpopulation are multipolar, with triangular or rounded cell body. 118 , 119 , 120 GnRH neurons were found to be considerably larger in adult human males (37× 14 μm) and in rhesus monkeys (28× 12 μm) than in bats (16 × 9 μm) or rats (16 × 6 μm). 50 Information about the fine structure of primate GnRH neurons is limited to the subcellular compartments visualized with immunohistochemistry, in the absence of efficient cell filling and 3D neuronal reconstruction techniques to study dendritic arborization, filopodia, spines and network connectivity.
FIGURE 3.
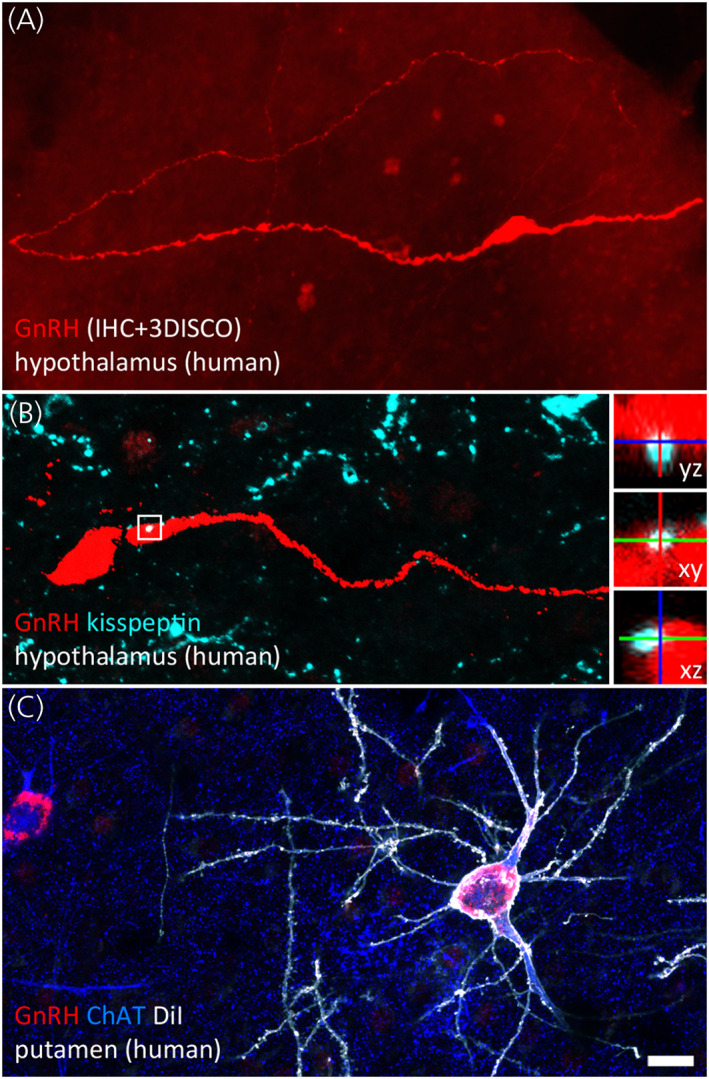
Hypothalamic and extrahypothalamic GnRH neurons of the adult human brain. (A) Hypothalamic GnRH neurons regulating reproduction are typically fusiform. In 1 mm‐thick slices made transparent with the 3DISCO clearing technology, lengthy GnRH dendrites can be followed occasionally for several millimetres. Dendrites may represent the main cellular compartment receiving afferent inputs. (B) Kisspeptin‐immunoreactive inputs to GnRH neurons (turquoise) from the infundibular (arcuate) nucleus convey information about circulating sex steroid levels. High‐power insets show orthogonal views of a neuronal apposition. (C) Unlike rodents, humans contain hundreds of thousands of GnRH‐immunoreactive (red) neurons in extrahypothalamic brain regions. The dendritic tree of GnRH cells in the putamen can be visualized post mortem using the lipophilic dye DiI (shown in white) delivered to the sections with the aid of a Gene Gun. Labelled GnRH neurons exhibit smooth surfaced dendrites and correspond to a subpopulation of cholinergic interneurons immunoreactive to choline acetyltransferase (ChAT; blue). Scale bar: 20 μm in (A) and (C), 16 μm in (B) (insets: 5 μm). Photograph courtesy of Dr Katalin Skrapits, Institute of Experimental Medicine, Budapest
Rance and coworkers analysed the distribution of human GnRH neurons with in situ hybridization. Based on perikaryon size and labelling density, they classified GNRH1 mRNA expressing neurons into three main categories. 122 The highest levels of GNRH1 mRNA signal were observed in small oval or fusiform (“type 1”) GnRH neurons in the medial basal hypothalamus, ventral POA and periventricular zone. These cells probably correspond to the bulk of hypothalamic GnRH neurons detectable with immunohistochemistry. 119 Interestingly, in situ hybridization also revealed thousands of additional “type 2” and “type 3” GNRH1 mRNA expressing neurons at various extrahypothalamic sites of the human brain. 122
4.4. GnRH fibre projections
As described in different species, 50 , 123 several circumventricular organs, including the ME, the OVLT and the subfornical area, receive input from GnRH neurons in primates. 50 , 116 , 120 As shown in monkeys, POA as well as MBH GnRH neurons contribute to the neuroendocrine regulation of the pituitary because both populations can be labelled by microinjection of retrograde tracers into the median eminence. 117 The anatomical route of neurosecretory GnRH fibres in primates shows some important species‐specific features. Accordingly, the median eminence containing the neurohemal junction is positioned rostral to the infundibular stalk in laboratory rodents, whereas it is postinfundibular in primates. 124 In humans, some neuroendocrine GnRH cell bodies can be more than a centimetre away from this site. Further, neuroendocrine GnRH axons all terminate within the external zone of the median eminence in rodents, whereas a considerable subset enter the infundibular stalk and some travel all the way down to the neurohypophysis in primates. 50 , 121 , 125 In humans, these descending GnRH fibres are accompanied and occasionally contacted by other peptidergic fibres containing KP, NKB and Substance P (SP). 126 GnRH secreted from these processes may have access to adenohypophysial gonadotrophs via the short portal veins. 125 Neuroendocrine GnRH projections outside the blood–brain barrier are exposed to blood‐born substances which also explains the mechanism whereby systemic KP injection causes prompt LH secretory responses also in humans. 127
Presence of nonhypophysiotropic GnRH fibres in the habenula, amygdala, hippocampus and around the mamillary bodies 50 , 115 , 119 , 120 , 128 indicates that GnRH also serves as a neurotransmitter at hypothalamic and extrahypothalamic sites. This non‐neuroendocrine role is supported by the electron microscopic demonstration of symmetrical synapses between GnRH‐immunoreactive axons and GnRH‐immunoreactive perikarya and dendrites in monkeys 129 and by light microscopic evidence for direct contacts between GnRH containing axons and SP neurons in humans. 130
4.5. Afferent regulation
Various neurotransmitter systems contributing to the neuronal regulation of human GnRH neurons have been reviewed recently. 131 Neuropeptides detected previously with light‐ or confocal microscopy in afferent contacts to GnRH perikarya and dendrites include neuropeptide Y, SP, galanin, corticotropin releasing hormone, KP (Figure 3B), NKB, endorphins, enkephalins, Dyn, RF‐amide related peptides, GnRH, orexins and melanin concentrating hormone. 131 , 132 , 133 The incidence of such contacts is region‐dependent and, as a general tendency, higher to GnRH neurons in the infundibular versus the POA. 132 Similarly, GnRH neurons in the ARC of monkeys receive higher numbers of synapses than those in the POA. 134 The number of appositions is also sex‐ and age dependent. GnRH neurons receive more KP‐immunoreactive and NKB‐immunoreactive appositions in aged versus young men 135 and in postmenopausal women versus age‐matched middle aged/aged men. 136 It would be difficult to determine if these differences reflect the anatomical plasticity of afferent connections or simply a better detectability of higher antigen levels in aged individuals due to lower sex steroid and negative feedback levels.
The cell bodies and proximal dendrites of GnRH neurons in different species receive relatively few classical synapses, as also demonstrated with electron microscopy in monkeys. 134 The number of synapses decreases following ovariectomy and can be restored by steroid replacement. 134 These observations somewhat contrast with the opposite changes in the number of KP and NKB contacts detected on human GnRH neurons with light microscopy 136 and also imply that synaptic specializations may often be absent from peptidergic afferent contacts.
In addition to various neuropeptides, fibres innervating human GnRH neurons contain the classic neurotransmitters histamine, catecholamines, γ‐aminobutyric acid and glutamate. 131 , 132 , 137 , 138
A substantial proportion of neuronal inputs may target the dendritic compartment of primate GnRH neurons, as it was proposed in mice. While the putative existence of “dendrons” in primates needs to be established, GnRH neurons possess lengthy dendrites which are likely to receive and integrate a substantial number of synaptic signals. The primary importance of dendritic inputs is supported by the observation that 75%–80% of the orexin‐ and melanin concentrating hormone‐containing afferents target the dendritic rather than the somatic compartment of human GnRH cells. 133 Similarly, each 100 μm dendritic segment receives similar numbers of light microscopic GABAergic and glutamatergic appositions as does the human GnRH perikaryon. 139
4.6. Paracrine regulation of GnRH fibres
Many thin GnRH‐immunoreactive processes in the primate hypothalamus show the typical appearance of varicose axons and this fibre phenotype becomes dominant around the human portal capillaries of the median eminence and in the neurohypophysis. 126 Nonsynaptic communication between KP and GnRH fibres within the median eminence was proposed to represent the primary mechanism whereby endogenous KP regulates GnRH neurons in monkeys 140 and similar axoaxonal contacts were detected later in the human median eminence and hypophysial stalk. 126
4.7. GnRH neuron cotransmitters in primates
Although little is currently known about the classical and peptide neurotransmitters of primate GnRH neurons, existing species differences deserve some discussion. Galanin, which is cosynthesized in a sexually dimorphic and oestrogen‐dependent manner in GnRH neurons of rats, 141 , 142 , 143 seems to be absent from human GnRH neurons. 144 Similarly, while GnRH neurons of rats exhibit immunoreactivity to γ‐aminobutyric acid prenatally 145 and the glutamatergic marker type‐2 vesicular glutamate transporter (VGLUT2) in adulthood, 146 earlier studies using confocal microscopy failed to reveal amino acid neurotransmitters or the more readily detectable vesicular amino acid transporter markers 139 in human GnRH neurons.
Somewhat unexpectedly, a large subset (~35%) of hypothalamic GnRH neurons in the adult human brain was found to be immunoreactive to the cholinergic phenotype marker choline acetyltransferase. The cholinergic phenotype, which has not been reported in other species yet, develops prenatally after human GnRH neurons enter the brain and is typical both in hypothalamic and extrahypothalamic GnRH neurons. 113
4.8. Ovarian oestrogen signalling to primate GnRH neurons
Oestrogen feedback in primates appears to involve a stronger pituitary component than in laboratory rodents. For example, a study using graded oestrogen infusion to postmenopausal volunteers, followed by 18FDG positron emission tomography, came to the conclusion that positive estrogen feedback takes place dominantly in the pituitary, whereas negative feedback on LH is primary hypothalamic. 147 Another study using diffusion MRI and MR spectroscopy techniques identified transient microstructural and metabolic changes in the human female hypothalamus (but not in the thalamus) following the withdrawal of a combined low‐dose oestrogen‐progesterone treatment. This treatment paradigm is thought to serve as a model for hypothalamic plasticity during negative feedback in the early follicular phase of the menstrual cycle. 148 Reduced oestrogen levels in postmenopausal women cause robust neuronal hypertrophy of estrogen‐sensitive neurons in the human infundibular region; hypertrophied neurons are devoid of GnRH mRNA. 149 Although a subpopulation of human GnRH neurons are immunoreactive to the beta oestrogen receptor isoform, 150 oestrogen feedback to GnRH neurons is likely to be primarily indirect, in accordance with the conclusion drawn from results in other species.
The hypothalamic ARC (also called infundibular nucleus in the human) has long been known as the primary site of sex steroid feedback in primates. The loss of estrogens in postmenopausal women causes the hypertrophy of neurons expressing ER‐α, 149 SP, 151 NKB, 151 KP 152 and proDyn 153 in this area. The homologous “KNDy” neurons (expressing KP, NKB and Dyn) are thought to be important players of negative oestrogen feedback and GnRH/LH pulsatility in other species, but some primate‐specific characteristics deserve to be mentioned. First, human KP neurons exhibit a unique pattern of neuropeptide colocalization. Differences from their rodent KNDy neuron counterparts include presence of SP and cocaine‐ and amphetamine regulated transcript and absence of Dyn and galanin in human KP neurons. 154 Second, the human KP system exhibits an unusually robust sexual dimorphism and aging‐dependent plasticity, as reviewed recently. 155 Third, while the somatodendritic compartment of GnRH neurons in mice receives only sparse innervation from KNDy neurons of the MBH, 156 at least ~10%–30% of the KP input to human GnRH neurons originates from this site. 135 , 157
Positive oestrogen feedback to GnRH neurons in rodents if thought to be accounted by KP neurons located in the rostral periventricular area of the third ventricle mediate These neurons contain estrogen receptors, innervate GnRH neurons, express FOS during the LH surge and are substantially more numerous in females than in males. 158 Surgical isolation of the MBH in rhesus monkeys did not prevent spontaneous ovulation and the oestrogen‐induced LH surge, giving support to the prevailing view that this region accounts for positive oestrogen feedback in primates. 159 , 160 The POA/rostral hypothalamus of humans and monkeys contains KP cell groups which anatomically resemble the homologous rodent neurons. 155 , 161 , 162 These cell groups may also contribute to positive feedback because they exhibit increased FOS immunoreactivity 163 and KISS1 mRNA expression 164 in response to a surge‐inducing oestradiol regimen in monkeys. Further, KISS1 also seems to be regulated positively by oestrogens in the human rostral hypothalamus, which was concluded from the reduced number of KP‐immunoreactive neurons after menopause. 161
4.9. Functional networking of GnRH neurons
The rhythmic LH secretory bursts from the anterior pituitary requires a coordinated secretory activity of GnRH neurons. Although the mechanisms underlying this coordination are still not entirely understood, regulation of neurosecretory GnRH preterminals/terminals by volume transmission may play an essential role in this process also in primates.
Classical synapses interconnecting GnRH neurons in nonhuman primates 129 may also contribute to the synchronized secretion of a larger GnRH neuron population. Frequent axosomatic and axodendritic contacts were also reported between human GnRH neurons. 119 Witkin et al. described an unusual syncytium‐like connection of neighbouring GnRH neurons in which GnRH‐GnRH contact sites appeared to exhibit cytoplasmic confluence at the ultrastructural level. 32 Equivalent to this could be the end‐to‐end dendritic continuity observed between close pairs of GnRH neurons at the light microscopic level. 119 Somewhat against the idea of cytoplasmic continuity is the lack of evidence for dye coupling between murine GnRH neurons during their studies with slice electrophysiology. We note that the resolution of light‐ or confocal microscopy in most anatomical studies on the primate GnRH system was unable to visualize intertwined dendrites and dendritic bundles similar to those described in mice. 165
4.10. The extrahypothalamic GnRH neuron population of primates
While GnRH neurons in adult laboratory rodents are located nearly exclusively in septal, anterior hypothalamic and preoptic areas and subserve functions related to reproduction, a handful of anatomical studies on primates identified large numbers of additional GNRH1 mRNA expressing and/or GnRH immunoreactive neurons at extrahypothalamic sites not related closely to reproductive regulation. These sites included several basal ganglia, the basal forebrain and the amygdala. 112 , 118 , 122 , 166 , 167 Initial interest toward these cells somewhat decreased because extrahypothalamic GnRH neurons in the developing monkey brain could not be immunostained with all GnRH antibodies and they also showed immunoreactivity to metalloendopeptidase which can cleave GnRH at the Tyr5‐Gly6 position. 166 These observations raised the possibility that extrahypothalamic GnRH neurons of primates contain the GnRH degradation product GnRH1‐5, instead of the bona fide GnRH decapeptide.
Recently the Hrabovszky laboratory has provided a more comprehensive characterization of extrahypothalamic GnRH neurons located in the human basal ganglia and basal forebrain 113 which correspond to the large and round “type 3” neurons expressing intermediate levels of GNRH1 mRNA between the heavily labelled “type 1” neurons of the hypothalamus and the lightly labelled small, oval “type 2” neurons reported previously in the septum, dorsal POA, bed nucleus of the stria terminalis and amygdala. 122 Immunohistochemical studies combined with stereology revealed that these regions contain 150,000–200,000 GnRH‐immunoreactive neurons. 82% of labelled cells were observed in the putamen, 5.5% in the accumbens nucleus, 4.9% in the caudate nucleus, 3.5% in the basal nucleus of Meynert, 1.8% in the globus pallidus, 1.3% in the ventral pallidum and 0.8% in the bed nucleus of the stria terminalis. 113 The neurons were detectable with a series of different GnRH and GnRH‐associated peptide antibodies and high performance liquid chromatography/tandem mass spectrometry (HPLC‐MS/MS) analysis of putamen extracts revealed presence of the GnRH decapeptide and its dominance over its degradation product GnRH1‐5. These studies established that extrahypothalamic GnRH neurons express the cholinergic marker choline acetyltransferase and correspond to subsets of previously known cholinergic interneuron populations in the putamen and cholinergic projection neurons in the basal nucleus of Meynert 113 (Figure 3C).
The functional significance of GnRH cosynthesis in cholinergic systems is difficult to study in the absence of appropriate rodent models. Deep transcriptome profiling of cholinergic interneurons and spiny projection neurons, which represent main target cells of the former, established that the GNRHR1 transcript is only expressed in cholinergic interneurons. Therefore, at least in the human putamen, GnRH appears to act on GnRHR1 autoreceptors to regulate higher order nonreproductive functions. 113
5. UNRESOLVED QUESTIONS AND FUTURE RESEARCH OPPORTUNITIES
Comparative and complementary neuroanatomical approaches across mammalian species have led to a greater understanding of how GnRH neurons orchestrate reproductive function. While rodent models provide a wealth of readily available transgenic neuroscience tools that can be harnessed, sheep models have the advantage of being able to couple neuroanatomical findings with GnRH secretion dynamics and seasonal reproductive function, and primates may be our best models of the human condition. Ongoing neuroanatomical research across all of these species is required if we are to fully understand GnRH neuron biology.
Over the past two decades, we have seen technical advances in transgenics, viral mediated transduction tools, tissue clearing and expanding methods and image analysis tools that have revolutionised our ability to assess information about GnRH neuron anatomy and network connectivity. Transgenic expression of GFP, 34 , 76 , 77 , 78 , 168 or Cre recombinase 38 , 67 , 169 in rodent GnRH neurons has been instrumental in visualising regions of the GnRH neuron that are difficult to detect with antibodies detecting GnRH peptide alone and for driving the expression of circuit tracing and functional measurement tools in a cell‐specific way. In addition to enabling visualisation, GnRH neurons expressing fluorescent markers have provided the opportunity to sort and isolate the hypothalamic GnRH neuron populations in the postnatal brains of both mice 170 and rats. 96 The expression of calcium indicators has enabled researchers to visualise the activity of GnRH neuron elements in real time and in situ. 67 , 171 , 172 , 173 , 174 Tissue clearing and advanced microscopy methods in rodents, sheep and primates have made it possible to visualise whole GnRH neurons in detail and whole GnRH neuron populations in situ throughout the brain. 60 , 111 , 175 While we have learned a great deal through these advances over the past several decades, many important questions remain to be answered that will probably require additional technological advances. Below are just some of the remaining questions that will be interesting to target going forward.
Is the dendron described in rodents a universal feature of mammalian GnRH neurons? The ability to visualise and functionally assess the rodent dendron is currently dependent upon transgenic approaches that enable visualising or targeting individual GnRH neurons. Will transgenic approaches or other methodologies become available that will allow us to visualise the full extent of individuals GnRH neurons in other species?
What is the role of extrahypothalamic GnRH signalling? While it is known that GnRH neurons across species possess subpopulations of nonhypophysiotropic cells and that GnRH neurons project to several brain regions outside of the median eminence, the role of central GnRH signalling is largely unknown. Can functional neuroscience tools such as opto‐ or chemogenetics help us to identify the roles of these extrahypothalamic pathways?
Do GNRH2 neurons play an essential role in the regulation of fertility in primates? To date, hypothalamic GNRH2 neurons were only studied with in situ hybridization in Rhesus monkeys. The estrogenic regulation of their GNRH2 mRNA expression raises the intriguing possibility that these cells contribute to the regulation of mammalian fertility in humans and some other primates.
How does transcriptome plasticity of GnRH neurons regulate reproductive physiology and pathology? Newly available single‐cell RNA‐seq technologies open the way for deep transcriptome profiling of GnRH neurons from laboratory animals and even from post mortem human brains.
Can in vivo monitoring of cellular activity be engineered across species? The advances in nanotechnology and genetic modification enable following neurons, dendrites, and spines in living organisms. Extension of these approaches to GnRH systems in multiple species could fill the gaps in our knowledge of species‐specific differences in GnRH regulation.
AUTHOR CONTRIBUTIONS
Rebecca E Campbell: Conceptualization; funding acquisition; writing – original draft; writing – review and editing. Lique M. Coolen: Conceptualization; funding acquisition; writing – original draft; writing – review and editing. Gloria E. Hoffman: Conceptualization; funding acquisition; writing – original draft; writing – review and editing. Erik Hrabovszky: Conceptualization; funding acquisition; writing – original draft; writing – review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jne.13115.
ACKNOWLEDGMENTS
Studies discussed in this neuroanatomy review were funded by multiple awards form the National Institute of Neurologic Disease and Stroke to GEH, Eunice Kennedy Shriver National Institute of Child Health and Human Development to GEH and to LMC, MN Lehman and RG Goodman, the Royal Society Marsden Fund (17‐064; 15‐097) and the Health Research Council of New Zealand (18‐671; 14‐077) to RC and the National Science Foundation of Hungary (K128317; K138137; PD134837) and the Hungarian Brain Research Program (2017‐1.2.1‐NKP‐2017‐00002) to EH. Open access publishing facilitated by University of Otago, as part of the Wiley ‐ University of Otago agreement via the Council of Australian University Librarians. [Correction added on 26 May 2022, after first online publication: CAUL funding statement has been added.]
Campbell RE, Coolen LM, Hoffman GE, Hrabovszky E. Highlights of neuroanatomical discoveries of the mammalian gonadotropin‐releasing hormone system. J Neuroendocrinol. 2022;34:e13115. doi: 10.1111/jne.13115
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development; Hungarian Brain Research Program, Grant/Award Number: 2017‐1.2.1‐NKP‐2017‐00002; National Institute of Neurologic Disease and Stroke; National Science Foundation of Hungary, Grant/Award Numbers: K128317, K138137, PD134837; the Health Research Council of New Zealand, Grant/Award Numbers: 18‐671, 14‐077; the Royal Society Marsden Fund, Grant/Award Numbers: 17‐064, 15‐097
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Scharrer B. Neurosecretion—comparative and evolutionary aspects. Prog Brain Res. 1976;451:25‐37. [DOI] [PubMed] [Google Scholar]
- 2. Scharrer B. Neurosecretion: beginnings and new directions in neuropeptide research. Annu Rev Neurosci. 1987;101:17‐18. [DOI] [PubMed] [Google Scholar]
- 3. Page RB, Bergland RM. The neurohypophyseal capillary bed. I. Anatomy and arterial supply. Am J Anat. 1977;148(3):345‐357. [DOI] [PubMed] [Google Scholar]
- 4. Nikitovitch‐Winer M, Everett JW. Functional restitution of pituitary grafts re‐transplanted from kidney to median eminence. Endocrinology. 1958;63(6):916‐930. [DOI] [PubMed] [Google Scholar]
- 5. McLean BK, Nikitovitch‐Winer MB. Effects of pituitary transplantation and hypothalamic lesions on luteal function in the Mongolian gerbil. Neuroendocrinology. 1974;15(2):120‐125. [DOI] [PubMed] [Google Scholar]
- 6. Nikitovitch‐Winer M, Everett JW. Comparative study of luteotropin secretion by hypophysial autotransplants in the rat; effects of site and stages of the estrus cycle. Endocrinology. 1958;62(4):522‐532. [DOI] [PubMed] [Google Scholar]
- 7. Nikitovitch‐Winer MB. Induction of ovulation in rats by direct intrapituitary infusion of median eminence extracts. Endocrinology. 1962;70:350‐358. [DOI] [PubMed] [Google Scholar]
- 8. Nikitovitch‐Winer MB. Effect of hypophysial stalk transection on luteotropic hormone secretion in the rat. Endocrinology. 1965;77(4):658‐666. [DOI] [PubMed] [Google Scholar]
- 9. Clemens JA, Shaar CJ, Kleber JW, Tandy WA. Areas of the brain stimulatory to LH and FSH secretion. Endocrinology. 1971;88(1):180‐184. [DOI] [PubMed] [Google Scholar]
- 10. Koves K, Halasz B. Location of the neural structures triggering ovulation in the rat. Neuroendocrinology. 1970;6(3):180‐193. [DOI] [PubMed] [Google Scholar]
- 11. Burchanowski BJ, Knigge KM, Sternberger LA. Rich ependymal investment of luliberin (LHRH) fibers revealed immunocytochemically in an image like that from Golgi stain. Proc Natl Acad Sci U S A. 1979;76(12):6671‐6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. King JC, Lechan RM, Kugel G, Anthony EL. Acrolein: a fixative for immunocytochemical localization of peptides in the central nervous system. J Histochem Cytochem. 1983;31(1):62‐68. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman GE, Murphy KJ, Sita LV. The importance of titrating antibodies for Immunocytochemical methods. Curr Protoc Neurosci. 2016;76:2.12.1–2.12.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clayton CJ, Hoffman GE. Immunocytochemical evidence for anti‐LHRH and anti‐ACTH activity in the "F" antiserum. Am J Anat. 1979;155(1):139‐145. [DOI] [PubMed] [Google Scholar]
- 15. Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone‐releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86(20):8132‐8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoffman GE, Berghorn KA. Gonadotropin‐releasing hormone neurons: their structure and function. Semin Reprod Endocrinol. 1997;15(1):5‐17. [DOI] [PubMed] [Google Scholar]
- 17. Schwanzel‐Fukuda M, Fadem BH, Garcia MS, Pfaff DW. Immunocytochemical localization of luteinizing hormone‐releasing hormone (LHRH) in the brain and nervus terminalis of the adult and early neonatal gray short‐tailed opossum (Monodelphis domestica). J Comp Neurol. 1988;276(1):44‐60. [DOI] [PubMed] [Google Scholar]
- 18. Schwanzel‐Fukuda M, Pfaff DW. Origin of luteinizing hormone‐releasing hormone neurons. Nature. 1989;338(6211):161‐164. [DOI] [PubMed] [Google Scholar]
- 19. Altman J, Bayer SA. Development of the diencephalon in the rat. I. Autoradiographic study of the time of origin and settling patterns of neurons of the hypothalamus. J Comp Neurol. 1978;182(4 Pt 2):945‐971. [DOI] [PubMed] [Google Scholar]
- 20. Jennes L, Stumpf WE. Gonadotropin‐releasing hormone immunoreactive neurons with access to fenestrated capillaries in mouse brain. Neuroscience. 1986;18(2):403‐416. [DOI] [PubMed] [Google Scholar]
- 21. Merchenthaler I, Setalo G, Csontos C, Petrusz P, Flerko B, Negro‐Vilar A. Combined retrograde tracing and immunocytochemical identification of luteinizing hormone‐releasing hormone‐ and somatostatin‐containing neurons projecting to the median eminence of the rat. Endocrinology. 1989;125(6):2812‐2821. [DOI] [PubMed] [Google Scholar]
- 22. Witkin JW. Access of luteinizing hormone‐releasing hormone neurons to the vasculature in the rat. Neuroscience. 1990;37(2):501‐506. [DOI] [PubMed] [Google Scholar]
- 23. Silverman AJ, Witkin JW, Silverman RC, Gibson MJ. Modulation of gonadotropin‐releasing hormone neuronal activity as evidenced by uptake of fluorogold from the vasculature. Synapse. 1990;6(2):154‐160. [DOI] [PubMed] [Google Scholar]
- 24. Wray S, Gainer H. Effect of neonatal gonadectomy on the postnatal development of LHRH cell subtypes in male and female rats. Neuroendocrinology. 1987;45(5):413‐419. [DOI] [PubMed] [Google Scholar]
- 25. Junier MP, Wolff A, Hoffman GE, Ma YJ, Ojeda SR. Effect of hypothalamic lesions that induce precocious puberty on the morphological and functional maturation of the luteinizing hormone‐releasing hormone neuronal system. Endocrinology. 1992;131(2):787‐798. [DOI] [PubMed] [Google Scholar]
- 26. Chan H, Prescott M, Ong Z, Herde MK, Herbison AE, Campbell RE. Dendritic spine plasticity in gonadatropin‐releasing hormone (GnRH) neurons activated at the time of the preovulatory surge. Endocrinology. 2011;152(12):4906‐4914. [DOI] [PubMed] [Google Scholar]
- 27. Lee WS, Smith MS, Hoffman GE. cFos activity identifies recruitment of LHRH neurons during the ascending phase of the proestrous luteinizing hormone surge. J Neuroendocrinol. 1992;4:161‐166. [DOI] [PubMed] [Google Scholar]
- 28. Hoffman GE, Le WW, Schulterbrandt T, Legan SJ. Estrogen and progesterone do not activate Fos in AVPV or LHRH neurons in male rats. Brain Res. 2005;1054(2):116‐124. [DOI] [PubMed] [Google Scholar]
- 29. Witkin JW, Xiao E, Popilskis S, Ferin M, Silverman AJ. FOS expression in the gonadotropin‐releasing hormone (GnRH) neuron does not increase during the ovarian steroid‐induced GnRH surge in the rhesus monkey. Endocrinology. 1994;135(3):956‐961. [DOI] [PubMed] [Google Scholar]
- 30. Pau KY, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin‐releasing hormone surge in ovarian‐intact rhesus macaques. Endocrinology. 1993;133(4):1650‐1656. [DOI] [PubMed] [Google Scholar]
- 31. Hoffman GE, Smith MS, Verbalis JG. C‐Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14(3):173‐213. [DOI] [PubMed] [Google Scholar]
- 32. Witkin JW, O'Sullivan H, Silverman AJ. Novel associations among gonadotropin‐releasing hormone neurons. Endocrinology. 1995;136(10):4323‐4330. [DOI] [PubMed] [Google Scholar]
- 33. Hoffman GE. Anatomical markers of activity in hypothalamic neurons. Compr Physiol. 2020;10(2):549‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin‐releasing hormone neurons: characterization of whole‐cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412‐419. [DOI] [PubMed] [Google Scholar]
- 35. Campbell RE, Ducret E, Porteous R, et al. Gap junctions between neuronal inputs but not gonadotropin‐releasing hormone neurons control estrous cycles in the mouse. Endocrinology. 2011;152(6):2290‐2301. [DOI] [PubMed] [Google Scholar]
- 36. Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219‐3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore AM, Coolen LM, Lehman MN. In vivo imaging of the GnRH pulse generator reveals a temporal order of neuronal activation and synchronization during each pulse. Proc Natl Acad Sci U S A. 2022;119(6):e2117767119. doi: 10.1073/pnas.2117767119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin‐releasing hormone neurons and fertility. Neuron. 2006;52(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steiner RA. Kisspeptin: past, present, and prologue. Adv Exp Med Biol. 2013;784:3‐7. [DOI] [PubMed] [Google Scholar]
- 40. Barry J, Dubois MP, Poulain P. LRF producing cells of the mammalian hypothalamus. A fluorescent antibody study. Z Zellforsch Mikrosk Anat. 1973;146(3):351‐366. [DOI] [PubMed] [Google Scholar]
- 41. Barry J. Immunohistochemistry of luteinizing hormone‐releasing hormone‐producing neurons of the vertebrates. Int Rev Cytol. 1979;60:179‐221. [DOI] [PubMed] [Google Scholar]
- 42. Jennes L, Stumpf WE, Sheedy ME. Ultrastructural characterization of gonadotropin‐releasing hormone (GnRH)‐producing neurons. J Comp Neurol. 1985;232(4):534‐547. [DOI] [PubMed] [Google Scholar]
- 43. Witkin JW, Paden CM, Silverman AJ. The luteinizing hormone‐releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology. 1982;35(6):429‐438. [DOI] [PubMed] [Google Scholar]
- 44. Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986;7(1):45‐48. [DOI] [PubMed] [Google Scholar]
- 45. Jennes L, Stumpf WE. LHRH‐systems in the brain of the golden hamster. Cell Tissue Res. 1980;209(2):239‐256. [DOI] [PubMed] [Google Scholar]
- 46. Lehman MN, Silverman AJ. Ultrastructure of luteinizing hormone‐releasing hormone (LHRH) neurons and their projections in the golden hamster. Brain Res Bull. 1988;20(2):211‐221. [DOI] [PubMed] [Google Scholar]
- 47. Silverman AJ, Silverman R, Lehman MN, Witkin JW, Millar RP. Localization of a peptide sequence contained in the precursor to gonadotropin releasing hormone (GnRH). Brain Res. 1987;402(2):346‐350. [DOI] [PubMed] [Google Scholar]
- 48. King JC, Anthony EL, Gustafson AW, Damassa DA. Luteinizing hormone‐releasing hormone (LH‐RH) cells and their projections in the forebrain of the bat Myotis lucifugus lucifugus. Brain Res. 1984;298(2):289‐301. [DOI] [PubMed] [Google Scholar]
- 49. Foster WG, Younglai EV. An immunohistochemical study of the GnRH neuron morphology and topography in the adult female rabbit hypothalamus. Am J Anat. 1991;191(3):293‐300. [DOI] [PubMed] [Google Scholar]
- 50. King JC, Anthony EL. LHRH neurons and their projections in humans and other mammals: species comparisons. Peptides. 1984;5(Suppl 1):195‐207. [DOI] [PubMed] [Google Scholar]
- 51. Campbell RE. Morphology of the adult GnRH neuron. In: Herbison AE, Plant TM, eds. The GnRH neuron and its Control. International Neuroendocrine Federation (INF) Master Class Series Wiley; 2017:121‐148. [Google Scholar]
- 52. Wang HJ, Hoffman GE, Smith MS. Increased GnRH mRNA in the GnRH neurons expressing cFos during the proestrous LH surge. Endocrinology. 1995;136(8):3673‐3676. [DOI] [PubMed] [Google Scholar]
- 53. Jennes L. Sites of origin of gonadotropin releasing hormone containing projections to the amygdala and the interpeduncular nucleus. Brain Res. 1987;404(1–2):339‐344. [DOI] [PubMed] [Google Scholar]
- 54. Jennes L. Dual projections of gonadotropin releasing hormone containing neurons to the interpeduncular nucleus and to the vasculature in the female rat. Brain Res. 1991;545(1–2):329‐333. [DOI] [PubMed] [Google Scholar]
- 55. Witkin JW, O'Sullivan H, Ferin M. Glial ensheathment of GnRH neurons in pubertal female rhesus macaques. J Neuroendocrinol. 1995;7(9):665‐671. [DOI] [PubMed] [Google Scholar]
- 56. Kozlowski GP, Chu L, Hostetter G, Kerdelhue B. Cellular characteristics of immunolabeled luteinizing hormone releasing hormone (LHRH) neurons. Peptides. 1980;1(1):37‐46. [DOI] [PubMed] [Google Scholar]
- 57. Roberts CB, Best JA, Suter KJ. Dendritic processing of excitatory synaptic input in hypothalamic gonadotropin releasing‐hormone neurons. Endocrinology. 2006;147(3):1545‐1555. [DOI] [PubMed] [Google Scholar]
- 58. Campbell RE, Han SK, Herbison AE. Biocytin filling of adult gonadotropin‐releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology. 2005;146(3):1163‐1169. [DOI] [PubMed] [Google Scholar]
- 59. Herde MK, Geist K, Campbell RE, Herbison AE. Gonadotropin‐releasing hormone neurons extend complex highly branched dendritic trees outside the blood‐brain barrier. Endocrinology. 2011;152(10):3832‐3841. [DOI] [PubMed] [Google Scholar]
- 60. Moore AM, Prescott M, Czieselsky K, et al. Synaptic innervation of the GnRH neuron distal Dendron in female mice. Endocrinology. 2018;159(9):3200‐3208. [DOI] [PubMed] [Google Scholar]
- 61. Herde MK, Herbison AE. Morphological characterization of the action potential initiation segment in GnRH neuron dendrites and axons of male mice. Endocrinology. 2015;156(11):4174‐4186. [DOI] [PubMed] [Google Scholar]
- 62. Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long‐range projection with shared axonal and dendritic functions. J Neurosci. 2013;33(31):12689‐12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yeo S‐H, Herde MK, Herbison AE. Morphological assessment of GABA and glutamate inputs to GnRH neurons in intact female mice using expansion microscopy. J Neuroendocrinol. 2021; in revision;33:e13021. [DOI] [PubMed] [Google Scholar]
- 64. Herbison AE. The dendron and episodic neuropeptide release. J Neuroendocrinol. 2021;33(11):e13024. doi: 10.1111/jne.13024 [DOI] [PubMed] [Google Scholar]
- 65. Wang L, Guo W, Shen X, et al. Different dendritic domains of the GnRH neuron underlie the pulse and surge modes of GnRH secretion in female mice. Elife. 2020;9:e53945. doi: 10.7554/eLife.53945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu X, Yeo SH, McQuillan HJ, et al. Highly redundant neuropeptide volume co‐transmission underlying episodic activation of the GnRH neuron dendron. Elife. 2021;10:e62455. doi: 10.7554/eLife.62455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yip SH, Campos P, Liu X, Porteous R, Herbison AE. Innervation of GnRH neuron distal projections and activation by Kisspeptin in a new GnRH‐Cre rat model. Endocrinology. 2021;162(1):bqaa186. doi: 10.1210/endocr/bqaa186 [DOI] [PubMed] [Google Scholar]
- 68. Herbison AE. The gonadotropin‐releasing hormone pulse generator. Endocrinology. 2018;159(11):3723‐3736. [DOI] [PubMed] [Google Scholar]
- 69. Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct Kisspeptin neuron populations to gonadotropin‐releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156(7):2582‐2594. [DOI] [PubMed] [Google Scholar]
- 70. Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001‐1012. [DOI] [PubMed] [Google Scholar]
- 71. Witkin JW, Demasio K. Ultrastructural differences between smooth and thorny gonadotropin‐releasing hormone neurons. Neuroscience. 1990;34(3):777‐783. [DOI] [PubMed] [Google Scholar]
- 72. Witkin JW. Morphology of luteinizing hormone‐releasing hormone neurons as a function of age and hormonal condition in the male rat. Neuroendocrinology. 1989;49(4):344‐348. [DOI] [PubMed] [Google Scholar]
- 73. Wray S, Hoffman G. Postnatal morphological changes in rat LHRH neurons correlated with sexual maturation. Neuroendocrinology. 1986;43(2):93‐97. [DOI] [PubMed] [Google Scholar]
- 74. Andersen P, Blackstad TW, Lomo T. Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp Brain Res. 1966;1(3):236‐248. [DOI] [PubMed] [Google Scholar]
- 75. Km H, Sultan P. Variation in the number, location and size of synaptic vesicles provides an anatomical basis for the nonuniform probability of release at hippocampal CA1 synapses. Neuropharmacology. 1995;34(11):1387‐1395. [DOI] [PubMed] [Google Scholar]
- 76. Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA‐ and glutamate‐activated channels in green fluorescent protein‐tagged gonadotropin‐releasing hormone neurons in transgenic mice. J Neurosci. 1999;19(6):2037‐2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin‐releasing hormone neurons. Endocrinology. 2004;145(2):495‐499. [DOI] [PubMed] [Google Scholar]
- 78. Xue H, Gai X, Sun W, Li C, Liu Q. Morphological changes of gonadotropin‐releasing hormone neurons in the rat preoptic area across puberty. Neural Regen Res. 2014;9(13):1303‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cottrell EC, Campbell RE, Han SK, Herbison AE. Postnatal remodeling of dendritic structure and spine density in gonadotropin‐releasing hormone neurons. Endocrinology. 2006;147(8):3652‐3661. [DOI] [PubMed] [Google Scholar]
- 80. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen‐induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796‐806. [DOI] [PubMed] [Google Scholar]
- 81. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin‐releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci U S A. 2015;112(2):596‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moore AM, Abbott G, Mair J, Prescott M, Campbell RE. Mapping GABA and glutamate inputs to gonadotrophin‐releasing hormone neurones in male and female mice. J Neuroendocrinol. 2018;30(12):e12657. [DOI] [PubMed] [Google Scholar]
- 83. Lehman MN, Goodman RL, Karsch FJ, Jackson GL, Berriman SJ, Jansen HT. The GnRH system of seasonal breeders: anatomy and plasticity. Brain Res Bull. 1997;44(4):445‐457. [DOI] [PubMed] [Google Scholar]
- 84. Prevot V. Glial‐neuronal‐endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol. 2002;14(3):247‐255. [DOI] [PubMed] [Google Scholar]
- 85. Parkash J, Messina A, Langlet F, et al. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat Commun. 2015;6:63‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xiong JJ, Karsch FJ, Lehman MN. Evidence for seasonal plasticity in the gonadotropin‐releasing hormone (GnRH) system of the ewe: changes in synaptic inputs onto GnRH neurons. Endocrinology. 1997;138(3):1240‐1250. [DOI] [PubMed] [Google Scholar]
- 87. Pouchain Ribeiro Neto R, Clarke IJ, Conductier G. Alteration in the relationship between tanycytes and gonadotrophin‐releasing hormone neurosecretory terminals following long‐term metabolic manipulation in the sheep. J Neuroendocrinol. 2017;29(10). doi: 10.1111/jne.12509 [DOI] [PubMed] [Google Scholar]
- 88. Witkin JW, Silverman AJ. Synaptology of luteinizing hormone‐releasing hormone neurons in rat preoptic area. Peptides. 1985;6(2):263‐271. [DOI] [PubMed] [Google Scholar]
- 89. Campbell RE, Gaidamaka G, Han SK, Herbison AE. Dendro‐dendritic bundling and shared synapses between gonadotropin‐releasing hormone neurons. Proc Natl Acad Sci U S A. 2009;106(26):10835‐10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moenter SM, Evans NP. Gonadotropin‐releasing hormone (GnRH) measurements in pituitary portal blood: a history. J Neuroendocrinol. 2021;e13065. Online ahead of print. doi: 10.1111/jne.13065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone‐releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid‐luteal phase of the estrous cycle. J Comp Neurol. 1986;244(1):19‐35. [DOI] [PubMed] [Google Scholar]
- 92. Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology. 2011;152(1):214‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jansen HT, Hileman SM, Lubbers LS, Kuehl DE, Jackson GL, Lehman MN. Identification and distribution of neuroendocrine gonadotropin‐releasing hormone neurons in the ewe. Biol Reprod. 1997;56(3):655‐662. [DOI] [PubMed] [Google Scholar]
- 94. Lehman MN, Karsch FJ, Robinson JE, Silverman AJ. Ultrastructure and synaptic organization of luteinizing hormone‐releasing hormone (LHRH) neurons in the anestrous ewe. J Comp Neurol. 1988;273(4):447‐458. [DOI] [PubMed] [Google Scholar]
- 95. Cernea M, Padmanabhan V, Goodman RL, Coolen LM, Lehman MN. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015;156(9):3277‐3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pellegrino G, Martin M, Allet C, et al. GnRH neurons recruit astrocytes in infancy to facilitate network integration and sexual maturation. Nat Neurosci. 2021;24(12):1660‐1672. [DOI] [PubMed] [Google Scholar]
- 97. Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res. 1984;40:185‐232. [DOI] [PubMed] [Google Scholar]
- 98. Lehman MN, Karsch FJ. Do gonadotropin‐releasing hormone, tyrosine hydroxylase‐, and beta‐endorphin‐immunoreactive neurons contain estrogen receptors? A double‐label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133(2):887‐895. [DOI] [PubMed] [Google Scholar]
- 99. Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin‐releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology. 2003;144(8):3663‐3676. [DOI] [PubMed] [Google Scholar]
- 100. Smith JT, Coolen LM, Kriegsfeld LJ, et al. Variation in kisspeptin and RFamide‐related peptide (RFRP) expression and terminal connections to gonadotropin‐releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770‐5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH Neurones during the Preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Porter DT, Moore AM, Cobern JA, et al. Prenatal testosterone exposure alters GABAergic synaptic inputs to GnRH and KNDy neurons in a sheep model of polycystic ovarian syndrome. Endocrinology. 2019;160(11):2529‐2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Weems PW, Coolen LM, Hileman SM, et al. Evidence that Dynorphin acts upon KNDy and GnRH neurons during GnRH pulse termination in the ewe. Endocrinology. 2018;159(9):3187‐3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Moore AM, Lohr DB, Coolen LM, Lehman MN. Prenatal androgen exposure alters KNDy neurons and their afferent network in a model of polycystic ovarian syndrome. Endocrinology. 2021;162(11):bqab158. doi: 10.1210/endocr/bqab158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN. A subset of gonadotropin‐releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology. 1999;140(12):5929‐5936. [DOI] [PubMed] [Google Scholar]
- 106. Merkley CM, Porter KL, Coolen LM, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406‐5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol‐induced gonadotropin‐releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133(2):896‐903. [DOI] [PubMed] [Google Scholar]
- 108. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin‐releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959‐2967. [DOI] [PubMed] [Google Scholar]
- 109. Stewart AJ, Katz AA, Millar RP, Morgan K. Retention and silencing of prepro‐GnRH‐II and type II GnRH receptor genes in mammals. Neuroendocrinology. 2009;90(4):416‐432. [DOI] [PubMed] [Google Scholar]
- 110. Urbanski HF, White RB, Fernald RD, Kohama SG, Garyfallou VT, Densmore VS. Regional expression of mRNA encoding a second form of gonadotropin‐releasing hormone in the macaque brain. Endocrinology. 1999;140(4):1945‐1948. [DOI] [PubMed] [Google Scholar]
- 111. Casoni F, Malone SA, Belle M, et al. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development. 2016;143(21):3969‐3981. [DOI] [PubMed] [Google Scholar]
- 112. Quanbeck C, Sherwood NM, Millar RP, Terasawa E. Two populations of luteinizing hormone‐releasing hormone neurons in the forebrain of the rhesus macaque during embryonic development. J Comp Neurol. 1997;380(3):293‐309. [PubMed] [Google Scholar]
- 113. Skrapits K, Sarvari M, Farkas I, et al. The cryptic gonadotropin‐releasing hormone neuronal system of human basal ganglia. Elife. 2021;10:e67714. doi: 10.7554/eLife.67714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Barry J. Immunofluorescence study of LRF neurons in man. Cell Tissue Res. 1977;181(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 115. Barry J. Septo‐epithalamo‐habenular LRF‐reactive neurons in monkeys. Brain Res. 1978;151(1):183‐187. [DOI] [PubMed] [Google Scholar]
- 116. King JC, Anthony EL, Fitzgerald DM, Stopa EG. Luteinizing hormone‐releasing hormone neurons in human preoptic/hypothalamus: differential intraneuronal localization of immunoreactive forms. J Clin Endocrinol Metab. 1985;60(1):88‐97. [DOI] [PubMed] [Google Scholar]
- 117. Goldsmith PC, Thind KK, Song T, Kim EJ, Boggant JE. Location of the neuroendocrine gonadotropin‐releasing hormone neurons in the monkey hypothalamus by retrograde tracing and immunostaining*,**. J Neuroendocrinol. 1990;2(2):157‐168. [DOI] [PubMed] [Google Scholar]
- 118. Stopa EG, Koh ET, Svendsen CN, Rogers WT, Schwaber JS, King JC. Computer‐assisted mapping of immunoreactive mammalian gonadotropin‐releasing hormone in adult human basal forebrain and amygdala. Endocrinology. 1991;128(6):3199‐3207. [DOI] [PubMed] [Google Scholar]
- 119. Dudas B, Mihaly A, Merchenthaler I. Topography and associations of luteinizing hormone‐releasing hormone and neuropeptide Y‐immunoreactive neuronal systems in the human diencephalon. J Comp Neurol. 2000;427(4):593‐603. [DOI] [PubMed] [Google Scholar]
- 120. Barry J. Characterization and topography of LH‐RH neurons in the human brain. Neurosci Lett. 1976;3(5–6):287‐291. [DOI] [PubMed] [Google Scholar]
- 121. Anthony EL, King JC, Stopa EG. Immunocytochemical localization of LHRH in the median eminence, infundibular stalk, and neurohypophysis. Evidence for multiple sites of releasing hormone secretion in humans and other mammals. Cell Tissue Res. 1984;236(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 122. Rance NE, Young WS 3rd, McMullen NT. Topography of neurons expressing luteinizing hormone‐releasing hormone gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1994;339(4):573‐586. [DOI] [PubMed] [Google Scholar]
- 123. Silverman AJ, Krey LC, Zimmerman EA. A comparative study of the luteinizing hormone releasing hormone (LHRH) neuronal networks in mammals. Biol Reprod. 1979;20(1):98‐110. [DOI] [PubMed] [Google Scholar]
- 124. Duvernoy H, Koritke JG, Monnier G. Vascularization of the posterior tuber in man and its relation to the tuber‐hypophyseal vasculature. J Neurovisc Relat. 1971;32(2):112‐142. [DOI] [PubMed] [Google Scholar]
- 125. Silverman AJ, Antunes JL, Ferin M, Zimmerman EA. The distribution of luteinizing hormone‐releasing hormone (LHRH) in the hypothalamus of the rhesus monkey. Light microscopic studies using immunoperoxidase technique. Endocrinology. 1977;101(1):134‐142. [DOI] [PubMed] [Google Scholar]
- 126. Borsay BA, Skrapits K, Herczeg L, et al. Hypophysiotropic gonadotropin‐releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin B and substance p immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology. 2014;100(2–3):141‐152. [DOI] [PubMed] [Google Scholar]
- 127. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin‐54 stimulates the hypothalamic‐pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609‐6615. [DOI] [PubMed] [Google Scholar]
- 128. Silverman AJ, Antunes JL, Abrams GM, et al. The luteinizing hormone‐releasing hormone pathways in rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) monkeys: new observations on thick, unembedded sections. J Comp Neurol. 1982;211(3):309‐317. [DOI] [PubMed] [Google Scholar]
- 129. Thind KK, Goldsmith PC. Infundibular gonadotropin‐releasing hormone neurons are inhibited by direct opioid and autoregulatory synapses in juvenile monkeys. Neuroendocrinology. 1988;47(3):203‐216. [DOI] [PubMed] [Google Scholar]
- 130. Dudas B, Merchenthaler I. Close juxtapositions between LHRH immunoreactive neurons and substance P immunoreactive axons in the human diencephalon. J Clin Endocrinol Metab. 2002;87(6):2946‐2953. [DOI] [PubMed] [Google Scholar]
- 131. Hrabovszky E, Liposits Z. Afferent neuronal control of type‐I gonadotropin releasing hormone neurons in the human. Front Endocrinol (Lausanne). 2013;4:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dudas B, Merchenthaler I. Three‐dimensional representation of the neurotransmitter systems of the human hypothalamus: inputs of the gonadotrophin hormone‐releasing hormone neuronal system. J Neuroendocrinol. 2006;18(2):79‐95. [DOI] [PubMed] [Google Scholar]
- 133. Skrapits K, Kanti V, Savanyu Z, et al. Lateral hypothalamic orexin and melanin‐concentrating hormone neurons provide direct input to gonadotropin‐releasing hormone neurons in the human. Front Cell Neurosci. 2015;9:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology. 1991;129(2):1083‐1092. [DOI] [PubMed] [Google Scholar]
- 135. Molnar CS, Vida B, Sipos MT, et al. Morphological evidence for enhanced kisspeptin and neurokinin B signaling in the infundibular nucleus of the aging man. Endocrinology. 2012;153(11):5428‐5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hrabovszky E. Neuroanatomy of the human hypothalamic kisspeptin system. Neuroendocrinology. 2014;99(1):33‐48. [DOI] [PubMed] [Google Scholar]
- 137. Fekete CS, Strutton PH, Cagampang FR, et al. Estrogen receptor immunoreactivity is present in the majority of central histaminergic neurons: evidence for a new neuroendocrine pathway associated with luteinizing hormone‐releasing hormone‐synthesizing neurons in rats and humans. Endocrinology. 1999;140(9):4335‐4341. [DOI] [PubMed] [Google Scholar]
- 138. Dudas B, Merchenthaler I. Catecholaminergic axons innervate LH‐releasing hormone immunoreactive neurons of the human diencephalon. J Clin Endocrinol Metab. 2001;86(11):5620‐5626. [DOI] [PubMed] [Google Scholar]
- 139. Hrabovszky E, Molnar CS, Nagy R, et al. Glutamatergic and GABAergic innervation of human gonadotropin‐releasing hormone‐I neurons. Endocrinology. 2012;153(6):2766‐2776. [DOI] [PubMed] [Google Scholar]
- 140. Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149(9):4387‐4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Merchenthaler I, Lopez FJ, Negro‐Vilar A. Colocalization of galanin and luteinizing hormone‐releasing hormone in a subset of preoptic hypothalamic neurons: anatomical and functional correlates. Proc Natl Acad Sci U S A. 1990;87(16):6326‐6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Coen CW, Montagnese C, Opacka‐Juffry J. Coexistence of gonadotrophin‐releasing hormone and Galanin: Immunohisto‐chemical and functional studies. J Neuroendocrinol. 1990;2(2):107‐111. [DOI] [PubMed] [Google Scholar]
- 143. Merchenthaler I, Lennard DE, Lopez FJ, Negro‐Vilar A. Neonatal imprinting predetermines the sexually dimorphic, estrogen‐dependent expression of galanin in luteinizing hormone‐releasing hormone neurons. Proc Natl Acad Sci U S A. 1993;90(22):10479‐10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Dudas B, Merchenthaler I. Bi‐directional associations between galanin and luteinizing hormone‐releasing hormone neuronal systems in the human diencephalon. Neuroscience. 2004;127(3):695‐707. [DOI] [PubMed] [Google Scholar]
- 145. Tobet SA, Chickering TW, King JC, et al. Expression of gamma‐aminobutyric acid and gonadotropin‐releasing hormone during neuronal migration through the olfactory system. Endocrinology. 1996;137(12):5415‐5420. [DOI] [PubMed] [Google Scholar]
- 146. Hrabovszky E, Turi GF, Kallo I, Liposits Z. Expression of vesicular glutamate transporter‐2 in gonadotropin‐releasing hormone neurons of the adult male rat. Endocrinology. 2004;145(9):4018‐4021. [DOI] [PubMed] [Google Scholar]
- 147. Ottowitz WE, Dougherty DD, Fischman AJ, Hall JE. [18F]2‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography demonstration of estrogen negative and positive feedback on luteinizing hormone secretion in women. J Clin Endocrinol Metab. 2008;93(8):3208‐3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Baroncini M, Jissendi P, Catteau‐Jonard S, et al. Sex steroid hormones‐related structural plasticity in the human hypothalamus. Neuroimage. 2010;50(2):428‐433. [DOI] [PubMed] [Google Scholar]
- 149. Rance NE, McMullen NT, Smialek JE, Price DL, Young WS 3rd. Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71(1):79‐85. [DOI] [PubMed] [Google Scholar]
- 150. Hrabovszky E, Kallo I, Szlavik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin‐releasing hormone neurons express estrogen receptor‐beta. J Clin Endocrinol Metab. 2007;92(7):2827‐2830. [DOI] [PubMed] [Google Scholar]
- 151. Rance NE, Young WS 3rd. Hypertrophy and increased gene expression of neurons containing neurokinin‐B and substance‐P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239‐2247. [DOI] [PubMed] [Google Scholar]
- 152. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744‐2750. [DOI] [PubMed] [Google Scholar]
- 153. Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Skrapits K, Borsay BA, Herczeg L, Ciofi P, Liposits Z, Hrabovszky E. Neuropeptide co‐expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Front Neurosci. 2015;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hrabovszky E, Takacs S, Rumpler E, Skrapits K. The human hypothalamic kisspeptin system: functional neuroanatomy and clinical perspectives. Handb Clin Neurol. 2021;180:275‐296. [DOI] [PubMed] [Google Scholar]
- 156. Kallo I, Vida B, Deli L, et al. Co‐localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol. 2012;24(3):464‐476. [DOI] [PubMed] [Google Scholar]
- 157. Hrabovszky E, Molnar CS, Sipos MT, et al. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front Endocrinol (Lausanne). 2011;2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Herbison AE. Estrogen positive feedback to gonadotropin‐releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Plant TM, Moossy J, Hess DL, Nakai Y, McCormack JT, Knobil E. Further studies on the effects of lesions in the rostral hypothalamus on gonadotropin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology. 1979;105(2):465‐473. [DOI] [PubMed] [Google Scholar]
- 160. Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53‐88. [DOI] [PubMed] [Google Scholar]
- 161. Rumpler E, Skrapits K, Takacs S, et al. Characterization of Kisspeptin neurons in the human rostral hypothalamus. Neuroendocrinology. 2021;111(3):249‐262. [DOI] [PubMed] [Google Scholar]
- 162. Vargas Trujillo M, Kalil B, Ramaswamy S, Plant TM. Estradiol upregulates Kisspeptin expression in the preoptic area of both the male and female rhesus monkey (Macaca mulatta): implications for the hypothalamic control of ovulation in highly evolved primates. Neuroendocrinology. 2017;105(1):77‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Watanabe Y, Uenoyama Y, Suzuki J, et al. Oestrogen‐induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol. 2014;26(12):909‐917. [DOI] [PubMed] [Google Scholar]
- 164. Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non‐human primate. Biol Reprod. 2010;83(4):568‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Campbell RE, Suter KJ. Redefining the gonadotrophin‐releasing hormone neurone dendrite. J Neuroendocrinol. 2010;22(7):650‐658. [DOI] [PubMed] [Google Scholar]
- 166. Terasawa E, Busser BW, Luchansky LL, et al. Presence of luteinizing hormone‐releasing hormone fragments in the rhesus monkey forebrain. J Comp Neurol. 2001;439(4):491‐504. [DOI] [PubMed] [Google Scholar]
- 167. Krajewski SJ, Abel TW, Voytko ML, Rance NE. Ovarian steroids differentially modulate the gene expression of gonadotropin‐releasing hormone neuronal subtypes in the ovariectomized cynomolgus monkey. J Clin Endocrinol Metab. 2003;88(2):655‐662. [DOI] [PubMed] [Google Scholar]
- 168. Kato M, Ui‐Tei K, Watanabe M, Sakuma Y. Characterization of voltage‐gated calcium currents in gonadotropin‐releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology. 2003;144(11):5118‐5125. [DOI] [PubMed] [Google Scholar]
- 169. Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123(4):669‐682. [DOI] [PubMed] [Google Scholar]
- 170. Messina A, Langlet F, Chachlaki K, et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat Neurosci. 2016;19(6):835‐844. [DOI] [PubMed] [Google Scholar]
- 171. Spergel DJ. Calcium and small‐conductance calcium‐activated potassium channels in gonadotropin‐releasing hormone neurons before, during, and after puberty. Endocrinology. 2007;148(5):2383‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Jasoni CL, Todman MG, Strumia MM, Herbison AE. Cell type‐specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin‐releasing hormone neurons. J Neurosci. 2007;27(4):860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yip SH, Liu X, Hessler S, Cheong I, Porteous R, Herbison AE. Indirect suppression of pulsatile LH secretion by CRH neurons in the female mouse. Endocrinology. 2021;162(3):bqaa237. doi: 10.1210/endocr/bqaa237 [DOI] [PubMed] [Google Scholar]
- 174. Iremonger KJ, Porteous R, Herbison AE. Spike and neuropeptide‐dependent mechanisms control GnRH neuron nerve terminal ca(2+) over diverse time scales. J Neurosci. 2017;37(12):3342‐3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Moore AM, Lucas KA, Goodman RL, Coolen LM, Lehman MN. Three‐dimensional imaging of KNDy neurons in the mammalian brain using optical tissue clearing and multiple‐label immunocytochemistry. Sci Rep. 2018;8(1):2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


