Abstract
Introduction:
Although insufficient or prolonged sleep duration is associated with cardiovascular disease (CVD), sleep duration is not included in most lifestyle scores. This study evaluates the relationship between a lifestyle score including sleep duration and CVD risk.
Methods:
A prospective analysis among 67,250 women in the Nurses’ Health Study and 29,114 men in Health Professionals Follow-up Study (1986–2016) was conducted in 2021. Lifestyle factors were updated every 2–4 years using self-reported questionnaires. The traditional lifestyle score was defined as not smoking, normal BMI, being physically active (≥30 minutes/day of moderate physical activity), a healthy diet, and drinking alcohol in moderation. Low-risk sleep duration, defined as sleeping ≥6 to <8 hours/day, was included as an additional component in the updated lifestyle score. Cox proportional hazard regression models were used to estimate CVD risk. The likelihood ratio test and C-statistics were used to compare both scores.
Results:
A total of 11,710 incident CVD cases during follow-up were documented. The multivariable-adjusted hazard ratios comparing 6 with 0 low-risk factors in the healthy lifestyle score including sleep duration were 0.17 (95% CI=0.12, 0.23) for CVD, 0.14 (95% CI=0.10, 0.21) for coronary heart disease, and 0.20 (95% CI=0.12, 0.33) for stroke. Approximately 66% (95% CI=56%, 75%) of CVD, 67% (95% CI=54%, 77%) of coronary heart disease, and 62% (95% CI=42%, 76%) of stroke cases were attributable to poor adherence to a healthy lifestyle including sleep. Adding sleep duration to the score slightly increased the C-statistics from 0.64 (95% CI=0.63, 0.64) to 0.65 (95% CI=0.64, 0.65) (p<0.001).
Conclusions:
Adopting a healthy lifestyle including sleep recommendations could substantially reduce the risk of CVD in U.S. adults.
INTRODUCTION
One of every 4 deaths in the U.S. is attributable to cardiovascular disease (CVD), with 1.5 million cases occurring every year.1,2 Accruing evidence has demonstrated that the combination of several unhealthy lifestyle factors, including a Western-style diet, physical inactivity, smoking, high alcohol intake, and obesity, can explain a large number of chronic disease cases.3 Conversely, epidemiological studies have observed that a healthy lifestyle combining several low-risk factors is inversely associated with the risk of CVD and chronic diseases.4–6
Although sleep generally has not been considered a traditional cardiovascular risk factor, growing evidence supports the independent association of sleep duration with cardiovascular risk factors and CVD incidence.7 The National Sleep Foundation recommends 7–9 hours/day of sleep for people aged 26–64 years and 7–8 hours/day for people aged ≥64 years, and the American Heart Association has similar recommendations.8 Several meta-analyses have suggested significant associations of short (<6 hours/day) and long (>9 hours/day) sleep duration with higher CVD incidence, including stroke and coronary heart disease (CHD), and mortality.9–14 A recent dose–response meta-analysis, including 74 studies, illustrated a J-shaped relationship, with the lowest cardiovascular and all-cause mortality risk at 7–8 hours/day.7 Despite sleep duration being clearly associated with CVD risk, it has not been included in most lifestyle indices and there is no evidence on the added value of considering adequate sleep duration as part of healthy lifestyle scores in relation to CVD risk.
Therefore, the relationship between 2 lifestyle scores, a traditional lifestyle score and an updated score including sleep duration, and subsequent CVD risk in 2 large prospective cohort studies of predominantly White U.S. men and women was evaluated. The proportion of CVD cases attributable to poor adherence to both healthy lifestyle scores was estimated. The joint associations between sleep duration and other lifestyle factors including smoking, BMI, physical activity, alcohol intake, and diet on the risk of CVD was evaluated.
METHODS
Study Population
The Nurses’ Health Study (NHS) is an ongoing prospective cohort study of 121,701 U.S. female registered nurses aged 30–55 years at study baseline in 1976. The Health Professionals Follow-up Study (HPFS) is a prospective cohort study of 51,529 male health professionals aged 40–75 years at study inception in 1986.15,16
For the current analysis, baseline for NHS was set as 1986, and 1987 for HPFS. Participants who reported CVD at baseline; those with implausible energy intakes; BMI <18.5 kg/m2 at baseline; or with missing information on BMI, physical activity, alcohol intake, sleep duration, or smoking were excluded. After these exclusions, 67,250 women and 29,114 men remained in the analysis.
Measures
The primary outcome measure was major CVD defined as a combined endpoint of nonfatal myocardial infarction, nonfatal stroke, or fatal CVD. Secondary outcomes were: total CHD, which was defined as fatal CHD and nonfatal myocardial infarction; total stroke, which included all fatal and nonfatal cases (ischemic, hemorrhagic, and undetermined subtypes); and fatal CVD, which included all fatal CHD, fatal stroke, and other cardiovascular death. Nonfatal events were confirmed through review of medical records. Myocardial infarction was confirmed if the WHO criteria were met on the basis of symptoms plus diagnostic electrocardiogram changes or elevated cardiac enzymes. Strokes were confirmed if data in the medical records fulfilled the National Survey of Stroke criteria.17
Deaths were identified by reports of families, the U.S. postal system, or death certificates from state vital statistics departments and the National Death Index, and confirmed through review of medical records or autopsy reports.
Every 2 years, participants returned a mailed validated questionnaire that obtained updated information on age, body weight, smoking status, physical activity, medication use, and physician diagnosis of chronic diseases. Diet and alcohol information was assessed every 4 years using a validated food frequency questionnaire asking the frequency, on average, a participant had consumed a particular amount of a specific type of food during the previous year.18 Quality of diet was assessed using the Alternate Healthy Eating Index (AHEI) score. Detailed information on the assessment of lifestyle factors is provided in Appendix 1.
Five lifestyle-related factors—smoking, physical activity, diet quality (AHEI), BMI, and alcohol intake—were included in the score traditional score. Sleep duration was added as an additional factor in the updated score (Appendix Table 1). These factors were selected based on the evidence for their associations with CVD, and the same low-risk categories were used as in previous publications with these cohorts.6,7
For smoking, the low-risk group was defined as not currently smoking. Low-risk physical activity was defined as engaging in at least 3.5 hours/week of moderate to vigorous–intensity exercise (≥30 minutes/day), consistent with WHO guidelines.19 Diet quality was assessed using the AHEI, which is strongly associated with the onset of cardiometabolic disease in the general population.20 A healthy diet was defined as a diet score in the top 40% of each cohort distribution, consistent with previous publications in the cohorts.6,21 Low-risk body weight was defined as BMI in the range of 18.5–24.9 kg/m2.22 Low-risk alcohol consumption was defined as moderate, 5–14.9 grams/day for women and 5–30 grams/day for men, as defined in the U.S. dietary guidelines.23 Low-risk sleep duration was defined ≥6 to <8 hours/day as per the distribution at lower risk in the study population, and consistent with guidelines for older adults.8
For each low-risk factor, the participant received a score of 1 if they met the criterion for low risk and 0 otherwise (high-risk factor). The sum of these 6 scores provided a final low-risk factor of 0–6, with higher scores indicating a healthier lifestyle.
Statistical Analysis
Participants contributed person-time from the return of the baseline questionnaire to the date of CVD diagnosis, death, or the end of follow-up (June 30, 2016 for NHS and January 31, 2016 for HPFS), whichever came first. Cox proportional hazard models were used to calculate the adjusted hazard ratios (HRs) with their 95% CIs across categories of each individual factor and combinations of low-risk factors with the risk of total CVD, CHD, and stroke.
For the primary analysis, for each lifestyle factor and covariates, simple updated levels of each variable in which outcomes were predicted from the most recent questionnaire were used. For example, events that occurred between 1990 and 1992 were examined in relation to exposures reported on the 1990 questionnaire, events occurring between 1992 and 1994 were examined in relation to exposures reported on the 1992 questionnaire, and so forth. In a sensitivity analysis, cumulative averages instead of simple updated levels were used for AHEI, alcohol intake, physical activity, and sleep duration by taking the average of all prior values from previous questionnaires. The models were stratified by age and time period, and adjusted for ethnicity, multivitamin and aspirin use, menopausal status and hormone use (women), and family history of diabetes, myocardial infarction, and cancer. In pooled analysis, models were further stratified by cohort.
Likelihood ratio tests were used to compare 2 nested models: Model 1 was defined as the traditional lifestyle score (not smoking, normal BMI, being physically active, healthy diet, and drinking alcohol in moderation). Model 2, the updated score, included the traditional lifestyle score plus sleep duration. To explore the potential predictive ability, receiver operating characteristic analysis was conducted and C-statistics with 95% CIs were calculated comparing the 2 models including age. Integrated discrimination improvement and the net reclassification improvement were calculated as well.24
To estimate the proportion of cases attributable to poor adherence to a healthy lifestyle, the population-attributable risk percentage was calculated, an estimate of the percentage cases in this population that would not have occurred if all participants had been in the optimal group, assuming a causal relationship.25 Population-attributable risk percentage for adhering to all low-risk lifestyle factors of the score (5 for the Traditional Lifestyle score and 6 for the updated score) versus all other categories (not adhering to all low-risk factors) adjusted for age and time period is presented. Pooled logistic regression models were used with age and time period included in the model.
The risk of CVD was calculated using joint variables between sleep duration in 3 categories (<6 hours/day, ≥6 to <8 hours/day (low risk), and ≥8 hours/day) and the other risk factors (being in the low-risk versus not being in the low-risk group for all other risk factors), using as the reference those who slept <6 hours/day and were not in the low-risk group for other factors (not low-risk diet, current smoking, no low-risk BMI, no low-risk alcohol intake, or no low-risk physical activity).
Stratified and sensitivity analyses were conducted to test the robustness of the results. First, models were further adjusted by history of hypertension, hypercholesterolemia, and diabetes. Second, because many wish to avoid alcohol for various reasons, a healthy lifestyle score based on all the other risk factors except alcohol was created. In men, 1 drink/day was used as the criterion for low risk, following the recent new guidelines.23 Third, the primary analysis was repeated using ≥7 to <9 hours/day as an alternative definition of optimal sleep duration and in a different model ≥2.5 hours/week of optimal physical activity levels. Fourth, additional analyses were conducted excluding 1 lifestyle factor at a time while adjusting for the excluded factor. Fifth, to account for reverse causation, lifestyle variables were no longer updated following a report of cancer, diabetes, coronary artery bypass, or angina. Finally, in NHS, stratified analysis by never versus ever rotating night shifts was conducted.
Analyses were performed with SAS, version 9.4. Statistical tests were 2-sided, and p-values of <0.05 indicated statistical significance.
RESULTS
During an average of 30 years of follow-up, a total of 11,710 CVD cases were documented, 6,984 in the NHS and 4,726 in the HPFS. Baseline characteristics of participants are presented in Table 1. Participants who slept ≥6 to <8 hours/day had the lowest BMI, the highest AHEI and physical activity levels, and were less likely to smoke (Appendix Table 2).
Table 1.
Participant Characteristics at Baseline According to the Number of Low-Risk Lifestyle Factors
| Variable | Healthy Lifestyle Score including sleep duration | ||||||
|---|---|---|---|---|---|---|---|
| None | 1 | 2 | 3 | 4 | 5 | 6 | |
| Nurses’ Health Study (1986) | |||||||
| n (%) | 1,282 (1.9) | 8,808 (13.1) | 20,133 (29.9) | 20,763 (30.8) | 11,717 (17.4) | 3,861 (5.7) | 686 (1.02) |
| Age, years | 45.4 (6.6) | 46.3 (7) | 46.3 (7.1) | 46.2 (7.3) | 46.6 (7.2) | 46.7 (7) | 46.7 (7) |
| BMI, kg/m2 | 29.5 (4.3) | 28.6 (5.3) | 26.8 (5.1) | 24.5 (4.1) | 23.2 (2.9) | 22.4 (2) | 22 (1.6) |
| AHEI | 40.8 (6.9) | 41.4 (7.8) | 43.5 (9.1) | 47.3 (10.5) | 52.5 (10.4) | 57.4 (8.7) | 60.5 (6.7) |
| Physical activity, hours/week | 0.4 (0.7) | 0.6 (1.2) | 1 (2.1) | 1.8 (3.1) | 3.4 (4.3) | 5.6 (4.7) | 7.6 (4.9) |
| Alcohol intake, g/day | 6.3 (14.5) | 5.7 (12.7) | 5.6 (11.5) | 5.9 (10) | 7.2 (9.2) | 8.2 (8) | 9.6 (2.9) |
| Sleep, hours/day | 5.4 (1.1) | 5.6 (1.1) | 6 (1) | 6.1 (0.8) | 6.2 (0.7) | 6.3 (0.6) | 6.3 (0.5) |
| Smoking, current% | 100.0 | 45.9 | 25.5 | 12.6 | 6.0 | 2.1 | 0.0 |
| White, % | 97.2 | 97.2 | 97.8 | 97.9 | 98.3 | 98.3 | 99.4 |
| Multivitamin use, % | 33.1 | 36.9 | 39.8 | 43.6 | 47.7 | 50.5 | 52.8 |
| Aspirin use, % | 61.2 | 67.6 | 68.3 | 67.8 | 67.8 | 66.4 | 68.1 |
| Baseline high blood pressure, % | 22.4 | 21.9 | 18.6 | 14.9 | 11.8 | 10.8 | 7.9 |
| Baseline high blood cholesterol, % | 15.4 | 13.9 | 13.7 | 12.4 | 12.3 | 11.8 | 12.6 |
| Baseline diabetes, % | 7.7 | 6.5 | 5.0 | 3.2 | 2.1 | 1.1 | 0.9 |
| Family history of diabetes, % | 36.5 | 35.1 | 31.9 | 28.5 | 27.3 | 24.5 | 22.3 |
| Family history of cancer, % | 14.0 | 14.0 | 14.4 | 15.1 | 15.2 | 15.6 | 17.7 |
| Family history of myocardial infarction, % | 29.5 | 27.8 | 26.6 | 24.5 | 25.0 | 24.2 | 23.9 |
| Health Professionals Follow-up Study (1987) | |||||||
| n (%) | 237 (0.8) | 2,461 (8.5) | 7,163 (24.6) | 9,124 (31.3) | 6,591 (22.6) | 2,893 (9.9) | 645 (2.2) |
| Age, years | 56.2 (8.7) | 56.7 (9.9) | 55.2 (9.8) | 54.7 (9.8) | 54.7 (9.7) | 55.1 (9.6) | 54.8 (9.1) |
| BMI, kg/m2 | 27.7 (2.8) | 27.4 (3) | 26.5 (3.2) | 25.2 (2.9) | 24.2 (2.4) | 23.5 (1.8) | 23 (1.3) |
| AHEI | 38.5 (7.9) | 40.6 (7.6) | 42.8 (8.8) | 46.2 (10.4) | 50.6 (10.9) | 56 (10) | 61.1 (6.3) |
| Physical activity, hours/week | 0.5 (0.8) | 0.8 (1.2) | 1.3 (2.2) | 2.4 (3.7) | 4.1 (4.9) | 6 (5.3) | 8.1 (5.9) |
| Alcohol intake, g/day | 24.6 (26.9) | 14.8 (22.3) | 11.3 (18.5) | 10.9 (14.4) | 11.1 (11.9) | 11.5 (9.3) | 12.7 (5.7) |
| Sleep, hours/day | 8 (1) | 7.8 (1) | 7.3 (0.9) | 7.1 (0.8) | 6.9 (0.7) | 6.8 (0.6) | 6.7 (0.5) |
| Smoking, current% | 100.0 | 30.6 | 12.4 | 5.4 | 1.7 | 0.7 | 0.0 |
| White, % | 92.9 | 95.4 | 95.0 | 94.2 | 94.5 | 95.6 | 95.1 |
| Multivitamin use, % | 58.2 | 56.5 | 59.3 | 60.9 | 64.5 | 69.9 | 71.6 |
| Aspirin use, % | 34.7 | 32.8 | 30.0 | 29.9 | 30.5 | 29.1 | 31.3 |
| Baseline high blood pressure, % | 33.4 | 31.0 | 27.4 | 24.9 | 22.3 | 21.5 | 21.6 |
| Baseline high blood cholesterol, % | 10.1 | 12.6 | 12.3 | 12.3 | 12.7 | 15.3 | 14.5 |
| Baseline diabetes, % | 3.3 | 4.0 | 3.3 | 3.0 | 2.4 | 2.1 | 1.1 |
| Family history of myocardial infarction, % | 29.9 | 32.7 | 33.1 | 33.6 | 34.8 | 34.0 | 33.7 |
| Family history of cancer, % | 37.6 | 36.1 | 36.4 | 36.8 | 37.7 | 37.5 | 39.9 |
| Family history of diabetes, % | 28.1 | 27.1 | 26.3 | 23.9 | 22.9 | 22.8 | 23.5 |
Notes: Values are means (SD) or percentages for categorical variables, and are standardized to the age distribution of the study population except age. Low-risk factors in the score included cigarette smoking (not smoking), physically active (≥3.5 hours/week of moderate to vigorous intensity activity), high diet quality (top 40% of Alternate Healthy Eating Index), moderate alcohol intake of 5 to 14.9 g/day (women) or 5 to 30 g/day (men), normal weight (BMI, 18.5–24.9 kg/m2), and sleep duration (≥6 to <8 hours/day).
AHEI, Alternative Healthy Eating Index-2010.
In multivariable-adjusted analysis, inverse associations between both the traditional and the updated score and CVD, CHD, and stroke risk were observed (Table 2). A combination of the 6 low-risk lifestyle factors was associated with an HR of 0.17 (95% CI=0.12, 0.23) for total CVD, 0.14 (95% CI=0.10, 0.21) for CHD, and 0.20 (95% CI=0.12, 0.33) for stroke, as compared with participants with 0 low-risk factors (Table 2). Each individual component of the score showed a significant association with total CVD, CHD, and stroke (Appendix Table 3).
Table 2.
HR (95% CI) of Cardiovascular Disease, Coronary Heart Disesase, and Stroke According to Lifestyle Scores
| Person years | NHS | Person years | HPFS | Pooled | |||
|---|---|---|---|---|---|---|---|
| Lifestyle score | Cases | HR (95% CI) | Cases | HR (95% CI) | HR (95% CI) | ||
| Total cardiovascular disease | |||||||
| Healthy Lifestyle Score including sleep durationa | |||||||
| None | 22,335 | 89 | 1.00 (ref) | 3,500 | 62 | 1.00 (ref) | 1.00 (ref) |
| 1 | 257,211 | 1,131 | 0.75 (0.60, 0.93) | 49,172 | 646 | 0.59 (0.45, 0.76) | 0.68 (0.58, 0.80) |
| 2 | 611,210 | 2,390 | 0.64 (0.51, 0.79) | 144,160 | 1,361 | 0.46 (0.35, 0.59) | 0.56 (0.47, 0.66) |
| 3 | 638,254 | 2,144 | 0.54 (0.43, 0.66) | 185,345 | 1,444 | 0.40 (0.31, 0.52) | 0.48 (0.41, 0.56) |
| 4 | 369,399 | 981 | 0.44 (0.36, 0.55) | 146,464 | 842 | 0.32 (0.25, 0.42) | 0.39 (0.33, 0.46) |
| 5 | 129,995 | 238 | 0.35 (0.27, 0.45) | 73,136 | 326 | 0.27 (0.21, 0.36) | 0.32 (0.27, 0.39) |
| 6 | 20,949 | 11 | 0.11 (0.06, 0.21) | 18,063 | 45 | 0.16 (0.11, 0.23) | 0.17 (0.12, 0.23) |
| Traditional lifestyle score | |||||||
| None | 51,175 | 209 | 1.00 (ref.) | 8,234 | 134 | 1.00 (ref.) | 1.00 (ref.) |
| 1 | 514,563 | 2,001 | 0.64 (0.56, 0.74) | 110,635 | 1,155 | 0.52 (0.44, 0.63) | 0.60 (0.53, 0.67) |
| 2 | 751,328 | 2,837 | 0.55 (0.48, 0.64) | 193,863 | 1,653 | 0.42 (0.35, 0.51) | 0.50 (0.45, 0.56) |
| 3 | 502,821 | 1,517 | 0.44 (0.38, 0.51) | 176,816 | 1,180 | 0.34 (0.28, 0.41) | 0.40 (0.35, 0.44) |
| 4 | 196,033 | 391 | 0.33 (0.28, 0.40) | 101,262 | 510 | 0.28 (0.23, 0.33) | 0.31 (0.28, 0.36) |
| 5 | 33,433 | 29 | 0.17 (0.11, 0.25) | 29,031 | 94 | 0.19 (0.14, 0.25) | 0.20 (0.17, 0.25) |
| Coronary heart disease | |||||||
| Healthy Lifestyle Score including sleep duration | |||||||
| None | 30,166 | 80 | 1.00 (ref) | 3,518 | 45 | 1.00 (ref) | 1.00 (ref) |
| 1 | 287,230 | 724 | 0.65 (0.50, 0.84) | 49,321 | 492 | 0.62 (0.46, 0.84) | 0.64 (0.52, 0.77) |
| 2 | 620,358 | 1,347 | 0.56 (0.44, 0.72) | 144,360 | 1,101 | 0.51 (0.38, 0.69) | 0.54 (0.44, 0.65) |
| 3 | 618,104 | 1,179 | 0.45 (0.35, 0.58) | 185,678 | 1,105 | 0.43 (0.32, 0.58) | 0.44 (0.36, 0.54) |
| 4 | 354,487 | 499 | 0.39 (0.30, 0.51) | 146,629 | 648 | 0.35 (0.26, 0.47) | 0.37 (0.30, 0.45) |
| 5 | 122,805 | 133 | 0.29 (0.21, 0.39) | 73,231 | 216 | 0.26 (0.19, 0.36) | 0.27 (0.22, 0.34) |
| 6 | 18,874 | 9 | 0.09 (0.04, 0.21) | 18,076 | 31 | 0.16 (0.10, 0.25) | 0.14 (0.10, 0.21) |
| Traditional lifestyle score | |||||||
| None | 51,207 | 139 | 1.00 (ref) | 8,263 | 101 | 1.00 (ref) | 1.00 (ref) |
| 1 | 515,143 | 1,179 | 0.61 (0.51, 0.73) | 110,841 | 931 | 0.56 (0.46, 0.69) | 0.59 (0.52, 0.68) |
| 2 | 752,458 | 1,588 | 0.52 (0.44, 0.62) | 194,171 | 1,304 | 0.45 (0.37, 0.55) | 0.49 (0.43, 0.56) |
| 3 | 503,547 | 825 | 0.40 (0.34, 0.49) | 177,084 | 888 | 0.34 (0.28, 0.42) | 0.37 (0.33, 0.43) |
| 4 | 196,210 | 226 | 0.32 (0.26, 0.40) | 101,390 | 357 | 0.26 (0.21, 0.33) | 0.29 (0.25, 0.34) |
| 5 | 33,459 | 14 | 0.13 (0.08, 0.23) | 29,064 | 57 | 0.16 (0.11, 0.22) | 0.16 (0.12, 0.21) |
| Stroke | |||||||
| Healthy Lifestyle Score including sleep duration | |||||||
| None | 30,168 | 53 | 1.00 (ref) | 3,520 | 17 | 1.00 (ref) | 1.00 (ref) |
| 1 | 287,219 | 508 | 0.80 (0.57, 1.12) | 49,378 | 154 | 0.52 (0.31, 0.85) | 0.71 (0.54, 0.94) |
| 2 | 620,332 | 1,086 | 0.65 (0.47, 0.90) | 144,610 | 260 | 0.32 (0.19, 0.52) | 0.54 (0.41, 0.71) |
| 3 | 618,031 | 993 | 0.58 (0.42, 0.81) | 185,817 | 339 | 0.34 (0.21, 0.55) | 0.50 (0.38, 0.66) |
| 4 | 354,453 | 430 | 0.44 (0.31, 0.62) | 146,781 | 194 | 0.26 (0.16, 0.42) | 0.38 (0.29, 0.50) |
| 5 | 122,828 | 99 | 0.40 (0.28, 0.58) | 73,252 | 110 | 0.31 (0.18, 0.51) | 0.40 (0.29, 0.53) |
| 6 | 18,872 | 6 | 0.15 (0.06, 0.35) | 18,077 | 14 | 0.16 (0.08, 0.33) | 0.20 (0.12, 0.33) |
| Traditional lifestyle score | |||||||
| None | 51,218 | 85 | 1.00 (ref) | 8,274 | 33 | 1.00 (ref) | 1.00 (ref) |
| 1 | 515,132 | 892 | 0.68 (0.55, 0.85) | 111,025 | 224 | 0.41 (0.28, 0.59) | 0.60 (0.50, 0.73) |
| 2 | 752,379 | 1,298 | 0.59 (0.47, 0.74) | 194,412 | 349 | 0.35 (0.25, 0.51) | 0.52 (0.43, 0.63) |
| 3 | 503,504 | 706 | 0.47 (0.37, 0.59) | 177,205 | 292 | 0.33 (0.23, 0.47) | 0.43 (0.35, 0.52) |
| 4 | 196,226 | 178 | 0.35 (0.27, 0.46) | 101,461 | 153 | 0.31 (0.21, 0.45) | 0.36 (0.29, 0.45) |
| 5 | 33,444 | 16 | 0.22 (0.13, 0.38) | 29,058 | 37 | 0.27 (0.16, 0.43) | 0.30 (0.22, 0.42) |
Notes: Multivariable-adjusted HR was adjusted for age; ethnicity; current multivitamin use; current aspirin use; family history of diabetes mellitus, myocardial infarction, or cancer; and menopausal status and hormone use (women only). P-value for likelihood ratio test comparing traditional lifestyle score vs traditional lifestyle score + sleep duration in categories was <0.001. P for trend was <0.01 in all the analyses.
Low-risk factors in the score included cigarette smoking (not smoking), physically active (≥3.5 hours/week of moderate to vigorous intensity activity), high diet quality (top 40% of Alternate Healthy Eating Index), moderate alcohol intake of 5 to 14.9 g/day (women) or 5 to 30 g/day (men), normal weight (BMI, 18.5–24.9 kg/m2), and sleep duration (≥6 to <8 hours/day). Traditional lifestyle score included all factors except sleep duration.
CVD, cardiovascular disease; HPFS, Health Professionals Follow-up Study; HR, hazard ratio. NHS, Nurses’ Health Study.
Figure 1 shows the association between 3 categories of adherence to the lifestyle score including sleep duration (low, moderate, and ideal). Compared with those in the low category, ideal adherence to the score was associated with 52% lower CVD, 56% lower CHD, and 46% lower stroke risk. The p-values for likelihood ratio tests comparing the traditional lifestyle score versus the updated score were significant (p<0.001) for total CVD, CHD, and stroke. The C-statistic was slightly greater for the lifestyle score including sleep duration than the traditional score (0.65, 95% CI=0.64, 0.65 vs 0.64, 95% CI=0.63, 0.64, p<0.001). Compared with the traditional lifestyle score, significantly (p<0.0001) higher net reclassification improvement was observed for the score adding sleep duration (15%, 95% CI=13.3%, 16.8%). A statistically significant integrated discrimination improvement (p<0.0001) was observed (Appendix Table 4). When the healthy lifestyle scores were collapsed into binary categories, adhering to 6 low-risk lifestyle factors was associated with substantially lower risk of CVD, CHD, and stroke, as compared with those in all other categories (score points=0–5) (Appendix Table 8). The population-attributable risk percentage of no adherence to 6 low-risk lifestyle factors was 66% (95% CI=56%, 75%) for the lifestyle score including sleep and 54% (95% CI=45%, 62%) for the traditional score (5 low-risk factors) (Appendix Table 8).
Figure 1.
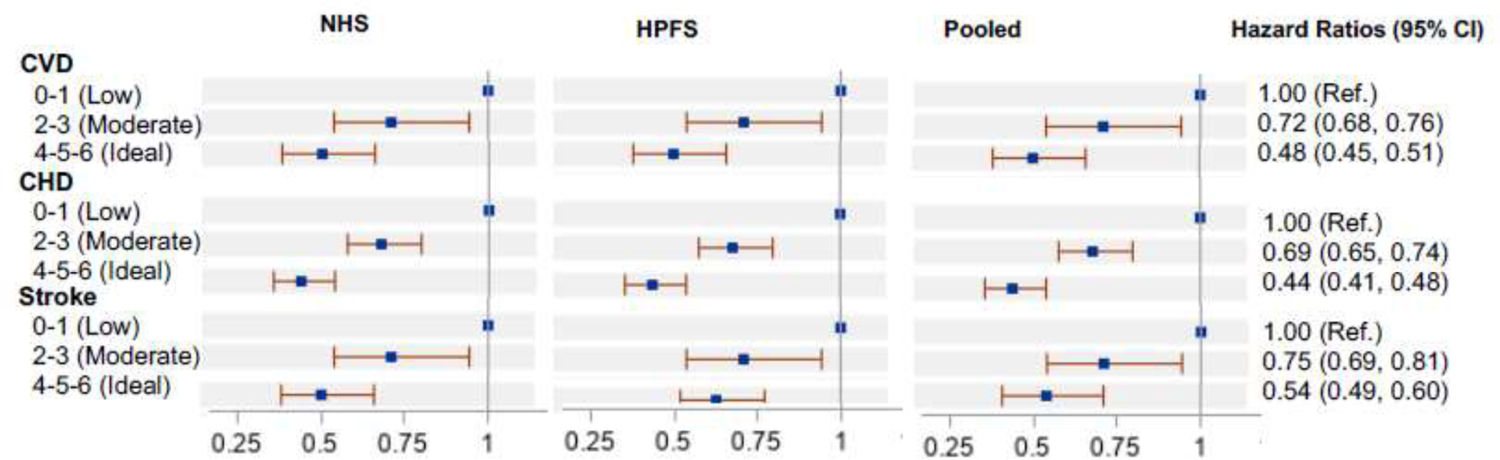
Hazard ratios for cardiovascular disease, coronary heart disease, and stroke according to low-risk lifestyle factors.
Notes: Multivariable-adjusted hazard ratios adjusted for age; ethnicity; current multivitamin use; current aspirin use; family history of diabetes, myocardial infarction, or cancer; and menopausal status and hormone use (women only). Low-risk factors in the score included cigarette smoking (not smoking), physically active (≥3.5 hour/week of moderate to vigorous intensity activity), high diet quality (top 40% of AHEI), moderate alcohol intake of 5 to 14.9 g/day (women) or 5 to 30 g/day (men), normal weight (BMI, 18.5–24.9 kg/m2), and sleep duration (≥6 to <8 hours/day) (Score ranging from 0 to 6).
CVD, cardiovascular disease; CHD, coronary heart disease; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study.
Low versus high risk were cross-classified for each of the lifestyle factors according to 3 categories of sleep duration: sleeping <6 hours/day, ≥6 to <8 hours/day (low risk), and ≥8 hours/day. Sleeping ≥6 to <8 hours/day was associated with lower risk of CVD within each lifestyle category, after adjusting for the other risk factors (Figure 2). Compared with those not adhering to the low risk level, the HRs ranged from 0.36 (95% CI=0.33, 0.40) for low-risk physical activity combined with optimal sleep to 0.59 (95% CI=0.54, 0.64) for low-risk diet combined with optimal sleep.
Figure 2.

Joint analysis of sleep duration and other lifestyle factors and risk of CVD.
Notes: Multivariable models adjusted for age, ethnicity, family history of diabetes, family history of myocardial infarction, family history of cancer, current aspirin use, current multivitamin use. Joint variables were used to estimate the HR and CIs of CVD. Sleep duration in 3 categories (<6 hours/day, ≥6 hours to <8 hours (low-risk), and ≥8 hours/day) and the other risk factors (being in the low-risk vs not being in the low-risk group for all other risk factors), using as the reference those who sleep <6 hours/day and are not in the low-risk group for other factors (not low-risk diet, current smoking, no low-risk BMI, no low-risk alcohol intake, or no low-risk physical activity). Low-risk factors: cigarette smoking(not smoking), physically active(≥3.5 hours/week of moderate to vigorous intensity activity), high diet quality(top 40% of AHEI), moderate alcohol intake of 5 to 14.9 g/day (women) or 5 to 30 g/day (men), normal weight (BMI, 18.5–24.9 kg/m2), and sleep duration (≥6 to <8 hours/day).
The primary results remained unchanged in a sensitivity analysis adjusting for history of diabetes, hypertension, and hypercholesterolemia (comparing extremes of the score and CVD: HR=0.17, 95% CI=0.12, 0.25). In stratified analysis by age, history of diabetes, hypertension, or hypercholesterolemia, a higher number of low-risk factors was associated with lower risk of CVD; this was observed in all subgroups (Appendix Table 5).
Appendix Table 6 shows the associations between the lifestyle risk scores and total CVD incidence after excluding 1 lifestyle factor at a time. When using 1 drink/day for men as the low-risk criterion, the results were consistent with the primary analysis (comparing score extremes: HR=0.15, 95% CI=0.10, 0.24 for CVD; HR=0.15, 95% CI=0.09, 0.26 for CHD; and HR=0.14, 95% CI=0.06, 0.36 for stroke).
Finally, several approaches were used to test robustness and the results remained consistent when: (1) cumulative average of alcohol intake, physical activity, AHEI, and sleep was used for calculating the score (comparing score extremes: HR=0.27, 95% CI=0.20, 0.36 for CVD; HR=0.26, 95% CI=0.18, 0.37 for CHD; and HR=0.30, 95% CI=0.18, 0.49 for stroke); (2) lifestyle factors were no longer updated following a diagnosis of an intermediate outcome (comparing extremes: HR=0.28, 95% CI=0.19, 0.39 for CVD; HR=0.19, 95% CI=0.12, 0.30 for CHD; and HR=0.49, 95% CI=0.29, 0.83 for stroke); (3) both of the above were used (comparing score extremes: HR=0.25, 95% CI=0.18, 0.34 for CVD; HR=0.21, 95% CI=0.14, 0.31 for CHD; and HR=0.35, 95% CI=0.21, 0.58 for stroke); (4) using ≥7 to <9 hours/day as the optimal sleep duration in the score (Appendix Table 7); (5) using ≥2.5 hours/week as optimal physical activity (comparing extremes for CVD: HR=0.19, 95% CI=0.15, 0.25); and (6) stratified analysis by never versus ever rotating night shifts (in NHS) showed inverse associations with CVD in both subgroups.
DISCUSSION
In 2 large, prospective studies of U.S. men and women, individuals with a low-risk lifestyle (not smoking, exercising daily, consuming a healthy diet, having a healthy BMI, and sleeping ≥6 to <8 hours/day) had a significantly lower risk of total CVD, CHD, and stroke. Individuals who engaged in all 6 healthy lifestyle choices had 83% lower CVD, 86% lower CHD, and 80% lower stroke risk. These findings underpin the importance of promoting healthy sleep habits in combination with other traditional low-risk factors in lifestyle and health guidelines. Within these study populations, it was estimated that approximately 66% of total CVD, 67% of CHD, and 62% of stroke cases might be avoidable by adherence to these 6 lifestyle factors.
Findings from several meta-analyses have shown associations of short (<6 hours/day) and long (>9 hours/day) sleep duration with higher CVD incidence, including stroke and CHD, and mortality.9–13,26 In a recent meta-analysis including 3,340,684 participants, it was shown that divergence from the recommended 7–8 hours/day of sleep was associated with a higher risk of mortality and cardiovascular events.7 Epidemiological evidence has also shown that decreases in sleep duration were adversely associated with changes in diet quality and physical activity, while increases in sleep duration were associated with greater weight gain.27 Although sleep duration has been clearly associated with type 2 diabetes and CVD,7,28 few previous studies addressed the impact of adding sleep duration into traditional lifestyle scores and its association with CVD risk. The findings from this study indicate that adhering to the recommendations of sleep duration jointly with low risk for each of the other lifestyle factors including a healthy diet, not smoking, BMI in the normal range, moderate-to-light alcohol intake, and higher physical activity, was associated with the lowest risk of total CVD.
Previous studies in the NHS and HPFS compared 5 versus 0 lifestyle risk factors (cigarette smoking, lack of physical activity, low diet quality, alcohol intake ≥15 grams/day, and being overweight or obese) and reported significant relative risks of 3.26 for cancer, 8.17 for cardiovascular, and 4.31 for total mortality.29 More recently, findings from these cohorts demonstrated that adopting a healthy lifestyle with the same 5 low-risk factors was associated with substantially lower premature mortality and prolonged life expectancy in U.S. adults.6,21 In younger women, a healthy lifestyle was associated with reductions in the incidence of CHD and clinical CVD risk factors.4 Furthermore, a recent meta-analysis including 22 prospective cohort studies showed that adherence to several healthy lifestyle behaviors simultaneously was associated with a 66% lower CVD risk compared with adopting none or only 1 behavior.30 Most lifestyle indices included physical activity, smoking, diet, alcohol consumption, and body weight, although the definitions and components varied substantially across the studies. In the present population, it was estimated that when sleep duration was included in the traditional lifestyle score, 66% of total CVD, 67% CHD, and 62% stroke cases were attributed to not following these 6 low-risk factors. When sleep was not included in the lifestyle score, the corresponding percentages were 54%, 60%, and 40% for the traditional score. This work goes beyond previous papers by adding sleep duration in a healthy lifestyle score for the prevention of CVD. Including sleep duration into the traditional lifestyle score slightly improved C-statistics. Although the improvement in C-statistics was low, when sleep was included in the lifestyle score there was a substantial improvement in net reclassification improvement, risk ratios, and population-attributable risk, suggesting the importance of sleep duration in clinical practice to assess CVD risk.
Strengths of this study include the prospective design, detailed information on a large number of lifestyle factors collected multiple times during follow-up, and the large number of confirmed CVD cases.
Limitations
Limitations of this study warrant discussion. As in any observational study, measurement error in self-reported variables is inevitable. However, the use of repeated measures of these variables reduces measurement error and represents long-term diet and lifestyle. The study population, consisting of predominantly White health professionals, is not representative of the general population. Because population-attributable risks depend on both relative risks and the prevalence of risk factors (the higher the prevalence, the higher the population-attributable risk) the prevalence of risk factors has to be considered when generalizing these findings to other populations. However, the risk estimates between lifestyle factors and CVD are most likely generalizable to other populations because the underlying biology should be similar. As the focus was on modifiable lifestyle factors, this study did not attempt to estimate the additional gain in risk reduction through optimal control of hypertension, lipids, and diabetes through medication, which may further reduce CVD risk. Sleep duration was based on self-reports rather than objective measurements, which may be susceptible to measurement error. Finally, sleep duration is only one dimension of sleep health, and future studies are needed to evaluate the performance of other sleep health dimensions that have been linked to CVD risk, such as sleep quality, sleep apnea, and sleep regularity.31–33
CONCLUSIONS
A healthy lifestyle score including sleep duration was associated with substantially lower risk of total CVD, CHD, and stroke in U.S. adults. Adding sleep duration as a prevention target into traditional lifestyle scores and inclusion in behavioral recommendations could further reduce the risk of CVD associated with unhealthy lifestyle factors.
Supplementary Material
ACKNOWLEDGMENTS
The Nurses’ Health Study and Health Professionals Follow-up Study were approved by the IRBs of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health in Boston.
MGF and FBH conceived the study design. JEM, QS, ER, KMR, WCW, MJS, and FBH were involved in data collection. MGF, YL, SNB, TH, JPDC, QS, ER, WCW, MJS, and FBH provided statistical expertise. MGF wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
We thank the participants and staff of the Nurses’ Health Study and Health Professionals Follow-up Study for their valuable contributions.
The authors have no conflicts of interest to declare.
This work was supported by research grants UM1 CA186107, U01 CA176726, U01 CA167552, P01 CA87969, P01 CA055075, R01 HL034594, HL088521, HL35464, and HL60712, DK120870 from NIH. MG-F is supported by the American Diabetes Association grant #1-18-PMF-029 and 1R21AG070375-01A1. TH is supported by K01HL143034 from NIH.
None of the funding sources played a role in the design, collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement:
Marta Guasch-Ferré: Conceptualization, Methodology, Investigation, Data curation, Formal Analysis, Writing-Original draft preparation. Yanping Li: Conceptualization, Visualization, Data Curation, Validation, Investigation, Writing-Review and Edit. Shilpa N Bhupathiraju: Conceptualization, Visualization, Investigation, Writing-Review and Edit. Tianyi Huang: Conceptualization, Visualization, Investigation, Writing-Review and Edit. Jean-Philippe Drouin-Chartier: Conceptualization, Visualization, Data Curation, Investigation, Writing-Review and Edit. JoAnn E Manson: Conceptualization, Visualization, Investigation, Funding Acquisition, Writing-Review and Edit. Qi Sun: Conceptualization, Visualization, Investigation, Funding Acquisition, Writing-Review and Edit. Eric Rimm: Conceptualization, Visualization, Investigation, Funding Acquisition, Writing-Review and Edit. Kathryn M Rexrode: Conceptualization, Visualization, Investigation, Funding Acquisition, Writing-Review and Edit. Walter C Willett: Conceptualization, Visualization, Investigation, Funding Acquisition, Writing-Review and Edit. Meir J Stampfer: Conceptualization, Visualization, Investigation, Funding Acquisition, Supervision, Writing-Review and Edit. Frank B Hu: Conceptualization, Visualization, Investigation, Supervision, Funding Acquisition, Writing-Review and Edit.
REFERENCES
- 1.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gakidou E, Afshin A, Abajobir AA, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345–1422. 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJL, Mokdad AH, Ballestros K, et al. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–1472. 10.1001/jama.2018.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65(1):43–51. 10.1016/j.jacc.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114(2):160–167. 10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138(4):345–355. 10.1161/CIRCULATIONAHA.117.032047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwok CS, Kontopantelis E, Kuligowski G, et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: a dose-response meta-analysis. J Am Heart Assoc. 2018;7(15):e008552. 10.1161/JAHA.118.008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11(8):931–952. 10.5664/jcsm.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 10.He Q, Sun H, Wu X, et al. Sleep duration and risk of stroke: a dose-response meta-analysis of prospective cohort studies. Sleep Med. 2017;32:66–74. 10.1016/j.sleep.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 11.da Silva AA, de Mello RG, Schaan CW, Fuchs FD, Redline S, Fuchs SC. Sleep duration and mortality in the elderly: a systematic review with meta-analysis. BMJ Open. 2016;6(2):e008119. 10.1136/bmjopen-2015-008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Li W, Cui X, et al. Sleep duration and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;219:231–239. 10.1016/j.ijcard.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Krittanawong C, Tunhasiriwet A, Wang Z, et al. Association between short and long sleep durations and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2019;8(8):762–770. 10.1177/2048872617741733. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 16.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 17.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 2016;12(2 Pt 2 Suppl 1):I13–44. [PubMed] [Google Scholar]
- 18.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051–1063. 10.1093/aje/kwx328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Physical activity. WHO. https://www.who.int/news-room/fact-sheets/detail/physical-activity. Accessed November 18, 2021. [Google Scholar]
- 20.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Schoufour J, Wang DD, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368:I6669. 10.1136/bmj.l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Defining Adult Overweight & Obesity. Centers for Disease Control and Prevention. https://www.cdc.gov/obesity/adult/defining.html. Accessed November 18, 2021.
- 23.Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services and the Sectraty of Agriculture. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service, 2020. [Google Scholar]
- 24.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–579. 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 26.Leng Y, Cappuccio FP, Wainwright NW, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology. 2015;84(11):1072–1079. 10.1212/WNL.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cespedes EM, Bhupathiraju SN, Li Y, Rosner B, Redline S, Hu FB. Long-term changes in sleep duration, energy balance and risk of type 2 diabetes. Diabetologia. 2016;59(1):101–109. 10.1007/s00125-015-3775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337(7672):742–745. 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbaresko J, Rienks J, Nöthlings U. Lifestyle indices and cardiovascular disease risk: a meta-analysis. Am J Prev Med. 2018;55(4):555–564. 10.1016/j.amepre.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 31.Huang T, Zeleznik OA, Poole EM, et al. Habitual sleep quality, plasma metabolites and risk of coronary heart disease in post-menopausal women. Int J Epidemiol. 2019;48(4):1262–1274. 10.1093/ije/dyy234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. 10.1161/CIRCULATIONAHA.117.029400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2020;75(9):991–999. 10.1016/j.jacc.2019.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


