Abstract
Background:
Our current understanding of right heart failure (RHF) post-LVAD is lacking. Recently, a new INTERMACS definition of RHF was introduced. Based on this definition, we investigated natural history, risk factors and outcomes of post-LVAD RHF.
Methods:
Patients implanted with continuous flow LVAD between 6/2/2014–6/30/2016 and registered in the INTERMACS/STS Database were included. RHF incidence and predictors, and survival after RHF were assessed. The manifestations of RHF which were separately analyzed were elevated central venous pressure, peripheral edema, ascites and use of inotropes.
Results
Among 5,537 LVAD recipients (mean 57±13 years, 49% destination therapy, support 18.9 months) prevalence of 1-month RHF was 24%. Of these, RHF persisted at 12 months in 5.3%. In contrast, de novo RHF, first identified at 3 months, occurred in 5.1% and persisted at 12 months in 17% of these, and at 6 months occurred in 4.8% and persisted at 12 months in 25%. Higher pre-implant blood urea nitrogen (ORs:1.03–1.09 per 5 mg/dl increase; P<0.0001), previous tricuspid valve repair/replacement (ORs:2.01–10.09; P<0.001), severely depressed right ventricular systolic function (ORs:1.17–2.20, P=0.004) and centrifugal vs. axial LVAD (ORs:1.15–1.78; P=0.001) represented risk factors for RHC incidence at 3 months. Patients with persistent RHF at 3 months had the lowest 2-year survival (57%) while patients with de novo RHF or RHF which resolved by 3 months had more favorable survival outcomes (75% and 78% at 2-years, respectively; P<0.001).
Conclusions:
RHF at 1 or 3 months post-LVAD was a common and frequently transient condition, which, if resolved, was associated with relatively favorable prognosis. Conversely, de novo, late RHF post-LVAD (>6 months) was more frequently a persistent disorder and associated with increased mortality. The 1-, 3- and 6-month time points may be used for RHF assessment and risk stratification in LVAD recipients.
Subject terms: heart failure
Keywords: LVAD, right ventricular failure, mechanical circulatory support
Introduction
Right heart failure (RHF) often complicates the post-operative course of patients undergoing a left ventricular assist device (LVAD) implantation and has a dramatic impact on outcomes [1,2]. Although it more commonly occurs in the early post-operative period (early RHF [ERHF]) [3], the potential for late presentation, months to years after LVAD implantation (late RHF [LRHF]), has been recently described [4–6]. The incidence of post-LVAD RHF ranges between 5% and 46% [7,8]. Small cohorts, variable definitions of RHF and differences between ERHF and LRHF partly account for this heterogeneity. Therefore, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) introduced a universal definition of RHF post-LVAD in 2014 [8,9]; RHF was defined as the presence of findings or symptoms of persistent right ventricular failure characterized by both documented signs of increased central venous pressure (CVP) and presence of relevant manifestations (peripheral edema, ascites or palpable hepatomegaly, worsening hepatic or renal dysfunction) [9]. Furthermore, RHF was graded as mild, moderate and severe based on (a) the duration of post-LVAD inotropic support for the LVAD implantation admission and (b) the presence and number of RHF-related hospitalizations, the need for inotropes or a right ventricular assist device (RVAD) and/or RHF-related death for the post-implant surveillance period [9].
Several risk scores predicting the ERHF post-LVAD have been developed but have significant limitations [10–13], being derived from small, single-center populations with heterogeneous definitions of RHF and from populations predominantly bridged to transplantation (BTT) with first-generation LVADs [13]. Similarly, the few studies to date on LRHF have similar limitations. The characteristics previously associated with development of LRHF are pre-implant serum creatinine, systematic vascular resistance [4]; blood type O, hemoglobin [5]; body surface area (BSA), blood urea nitrogen (BUN) and central venous to pulmonary capillary wedge pressure (CVP/PCWP) ratio [6]. Thus, in the era of continuous flow LVADs we investigated the epidemiology, natural history, and prognostic significance of RHF following the device implantation.
Methods
Access & Publications Acknowledgment (A&P)
STS INTERMACS
The data for this research were provided by The Society of Thoracic Surgeons’ National Database Access and Publications Research Program. The Society of Thoracic Surgeons (STS) Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) is a prospective registry of all FDA approved durable mechanical circulatory support (MCS) devices in the United States [14]. It is funded by the NHLBI and participation is mandatory for all Centers for Medicare and Medicaid Services approved implantation centers. All patient level data are securely processed and analyzed by the Kirklin Institute for Research in Surgical Outcomes (KIRSO) at the University of Alabama at Birmingham (UAB) with the UAB IRB oversight for all such research activities. The database is maintained by the University of Alabama and is approved by the institutional review of board of each participating center.
Study population
The study patients were 5,537 patients, implanted with a continuous flow LVAD between 6/2/2014 and 6/30/2016 and registered in the STS/INTERMACS Database.
Study design and definitions
We used the new definition of RHF according to INTERMACS [9].
RHF definition
Right Heart Failure (RHF) was defined as “symptoms or findings of persistent right ventricular failure characterized by both:
- Documentation of elevated central venous pressure (CVP) by:
-
A1.Direct measurement (e.g., right heart catheterization) with evidence of a central venous pressure (CVP) or right atrial pressure (RAP)>16 mmHg, OR
-
A2.Findings of significantly dilated inferior vena cava with absence of inspiratory variation by echocardiography, OR
-
A3.Clinical findings of elevated jugular venous distension at least half way up the neck in an upright patient.
- AND
-
A1.
- Manifestations of elevated central venous pressure characterized by:
-
B1.Clinical findings of peripheral edema (≥2+ either new or unresolved), OR
-
B2.Presence of ascites or palpable hepatomegaly on physical examination (unmistakable abdominal contour) or by diagnostic imaging, OR
-
B3.Laboratory evidence of worsening hepatic (total bilirubin>2.0 mg/dl) or renal dysfunction (creatinine>2.0 mg/dl).”
-
B1.
Importantly, presence of RHF was assessed by each INTERMACS investigator, who also provided the RHF elements of elevated CVP manifestation and documentation that were present.
RHF manifestations
The manifestations of RHF which were separately assessed and analyzed were a) elevated right atrial/central venous pressure (>16mmHg), b) presence of peripheral edema, c) presence of ascites and d) use of inotropes.
Definitions of RHF subtypes based on RHF persistence
When studying changes in the incidence of RHF between two sequential assessment time points (1–3 months and 3–6 months) RHF was further divided into:
de novo RHF: present at latter but absent at former time point,
persistent RHF: present at both time points,
resolved RHF: absent at latter but present at former time point
no RHF: absent at both time points.
Variable selection
Medical history, clinical, laboratory, echocardiography and hemodynamic data at LVAD implantation and intra-operative factors from all patients were recorded. Data on medical history and clinical status are listed in Table 1.
Table 1.
Baseline characteristics of study cohort*
| Patient characteristics | Data available on N patients | Value |
|---|---|---|
| Age (years) | 5537 | 57.0 ± 12.8 |
| Male | 5522 | 4340 (78.6) |
| Caucasian | 5537 | 3661 (66.1) |
| Married | 5405 | 3406 (63.0) |
| Body mass index (kg/m2) | 5514 | 28.6 ± 7.4 |
| Body surface area (m2) | 5514 | 2.1 ± 0.3 |
| Blood type O | 5468 | 2540 (46.5) |
| Severely depressed RV systolic function pre-operatively | 4442 | 584 (13.2) |
| Unspecified | 1 (0.0) | |
| Destination Therapy | 2688 (48.6) | |
| New York Heart Association class 4 | 5349 | 4399 (82.2) |
| Stroke | 5537 | 200 (3.6) |
| Cancer | 5537 | 260 (4.7) |
| Severe diabetes | 5537 | 553 (10.0) |
| Peripheral vascular disease | 5537 | 251 (4.5) |
| Current smoker | 5537 | 265 (4.8) |
| Current drug abuse | 5537 | 427 (7.7) |
| Alcohol abuse | 5537 | 412 (7.4) |
| History of hepatitis | 5537 | 73 (1.3) |
| Dialysis | 5537 | 71 (1.3) |
| History of CABG | 5537 | 1026 (18.5) |
| History of valve surgery | 5537 | 380 (6.9) |
| Intra-cardiac defibrillator | 5501 | 4411 (80.2) |
| Ventilator | 5537 | 284 (5.1) |
| Intra-aortic balloon pump | 5537 | 1031 (18.6) |
| B-Blockers | 5348 | 4230 (79.1) |
| ACE Inhibitors | 5131 | 2522 (49.2) |
| Systolic blood pressure (mmHg) | 5450 | 106.3 ± 16.1 |
| Diastolic blood pressure (mmHg) | 5435 | 65.3 ± 11.3 |
| Heart rate (beats per minute) | 5515 | 89.0 ± 17.3 |
| Hemoglobin (mg/dL) | 5518 | 11.4 ± 2.10 |
| White blood cells (K/uL) | 5521 | 8.5 ± 3.7 |
| Platelet (K/uL) | 5518 | 198.4 ± 77.9 |
| INR | 5349 | 1.3 ± 0.4 |
| Albumin (g/dL) | 5231 | 3.4 ± 0.6 |
| Total bilirubin (mg/dL) | 5300 | 1.3 ± 1.8 |
| Creatinine (mg/dL) | 5531 | 1.4 ± 0.7 |
| Blood urea nitrogen (mg/dL) | 5528 | 28.4 ± 16.7 |
| Cholesterol (mg/dL) | 3281 | 130.4 ± 42.3 |
| AST (u/L) | 5301 | 49.7 ± 149.4 |
| ALT (u/L) | 5290 | 60.3 ± 190.7 |
| Sodium (mmol/L) | 5530 | 135.1 ± 4.7 |
| BNP (pg/ml) | 2602 | 1102.6 ± 1045.1 |
| CRP (mg/L) | 1781 | 19.2 ± 38.7 |
| LVEDD (cm) | 4370 | 6.8 ± 1.1 |
| Moderate/Severe mitral regurgitation | 5184 | 2956 (57.0) |
| Moderate/Severe tricuspid regurgitation | 5152 | 2114 (41.0) |
| Moderate/Severe aortic regurgitation | 4841 | 212 (4.4) |
| LVEF < 20% | 5304 | 3679 (69.4) |
| Severely depressed pre-operative RV function | 4442 | 584 (13.15) |
| Right atrial pressure (mmHg) | 3761 | 12.4 ±8.0 |
| Pulmonary systolic pressure (mmHg) | 4927 | 49.9± 14.7 |
| Pulmonary diastolic pressure (mmHg) | 4886 | 24.8 ± 8.9 |
| Pulmonary wedge pressure (mmHg) | 4096 | 24.9 ± 9.3 |
| Cardiac index (L/min/m2) | 4695 | 2.2± 0.9 |
| Pulmonary vascular resistance (wood units) | 3885 | 2.3 ± 2.4 |
| Pulmonary artery pulsatility index (PAPi) ‡ | 4764 | 3.3 ± 3.4 |
| Pulmonary circulation elastance (PCE, mmHg/ml) ‡ | 4602 | 1.2 ± 0.7 |
| Concomitant surgery | 5535 | 2163 (39.1) |
| Failure to wean | 5537 | 31 (0.6) |
| ECMO | 5537 | 162 (2.9) |
| Patient profile modifier TCS | 4742 | 1336 (28.2) |
Values are shown as means ± SD or N (%), as appropriate.
ACE: Angiotensin-converting enzyme; ALT: alanine aminotransferase; AST: aspartate transaminase; BNP: B-type natriuretic peptide; CABG: coronary artery bypass grafting; CRP: c-reactive protein; ECMO: extra-corporeal membrane oxygenation; INR: international normalized ratio; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; RV: right ventricular; TCS: temporary circulatory support
The remaining 27 patients were either Rescue Therapy or ‘Other’ device strategy.
PAPi was calculated based on the formula (systolic PAP-diastolic PAP)/RAP and if RAP was missing (systolic PAP-diastolic PAP)/CVP. PCE was calculated based on the formula systolic PAP/Stroke volume and stroke volume from Cardiac output*1000/heart rate.
Outcomes
The STS/INTERMACS registry routinely collects follow-up data post-LVAD implantation at 1 week, 1, 3, 6 months and every 6 months thereafter. Major outcomes (i.e. death, readmission, explant, and adverse events) are entered as part of the defined follow-up and within 30 days of occurrence. The outcome of interest for the current analysis were a) RHF and (with censoring at death) b) all-cause mortality. For post-LVAD RHF, conditional analyses including only the patients who survived at the time-point of interest (1, 3, and 6 months) were used.
Statistical analysis
Continuous variables are expressed as mean ± SD and were compared using unpaired t-test. Categorical variables are expressed as counts and percentages and were compared using chi-square test. Kaplan-Meier survival curves were created to evaluate survival, which was defined as continued LVAD support at the time of the end of study time-window, LVAD exchange, or explant due to recovery, or cardiac transplant. Survival between groups was analyzed by log-rank for linear trend. Risk factors associated for the dichotomous outcome of RHF at 3 months were identified using logistic regression. The prognostic significance of the different RHF subtypes was assessed with Cox proportional hazard models. All baseline covariates shown in Supplemental Table 1 were tested in the univariate model. If they were significant at a level <0.05 and had at least 80% of available data, they were entered in a stepwise selection. Covariates remaining at the last step were included in the multivariable model to identify independent associations. In order to address the potential bias due to early death, a competing outcomes analysis of death after 3months (looking at patterns of RHF before 3 months) was also performed. All significance tests were 2-tailed, and P<0.05 was considered statistically significant. Statistical analyses were done using SAS.PC 9.4.
Results
Baseline characteristics
Among 5,537 patients with primary LVAD implant, mean age was 57.0 ±12.8 years, 79% were male and 49% of patients received LVAD as DT. Mean duration of support was 18.9 months which represented 8,734 patient-years of follow-up. Baseline characteristics are shown in Table 1.
Epidemiology and natural history of right heart failure
The prevalence of RHF at 1 month was 24%. Thereafter, the prevalence of RHF among LVAD recipients fell and remained relatively constant at a rate of 8–10% over the entire 3-year follow-up period (Figure 1). The stable prevalence of RHF, at least during the first 12 months of follow-up, was not a result of persistence of RHF in a fixed percentage, but a combination of de novo, resolved and persistent RHF. Namely, de novo RHF at the 3-, 6- and 12-month time points developed in 5.1%, 4.8% and 5.0% of patients, respectively (Figure 1). Among patients with RHF at 1 month, RHF persisted at 3, 6 and 12 months in 27%, 9.2% and 3.5%, respectively. De novo RHF first identified at 3 months persisted at 6 months in 32% and at 12 months in 11% of cases, while de novo RHF first identified at 6 months persisted at 12 months in 25% of cases, thus indicating that the further away from implantation RHF appears the higher the risk of persisting. The diagram of persistence of RHF according to timing of onset post-LVAD is depicted in Figure 2.
Figure 1. Incidence of de novo right heart failure and prevalence of right heart failure following LVAD implantation.
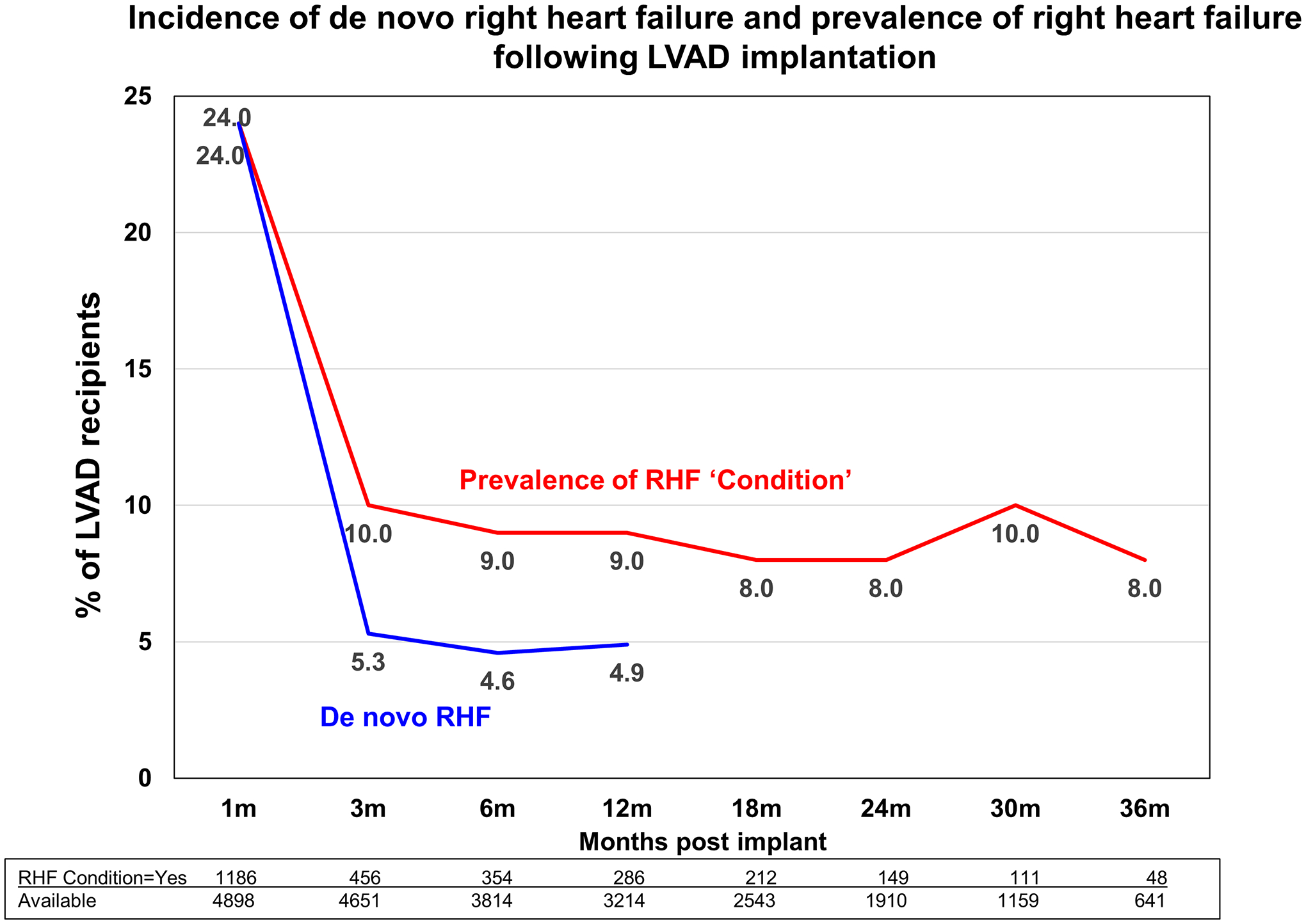
Follow-up was performed at 1, 3 and 6 months, and every 6 months thereafter.
Figure 2. Bar chart depicting persistence of post-LVAD right heart failure (RHF) according to timing of onset.
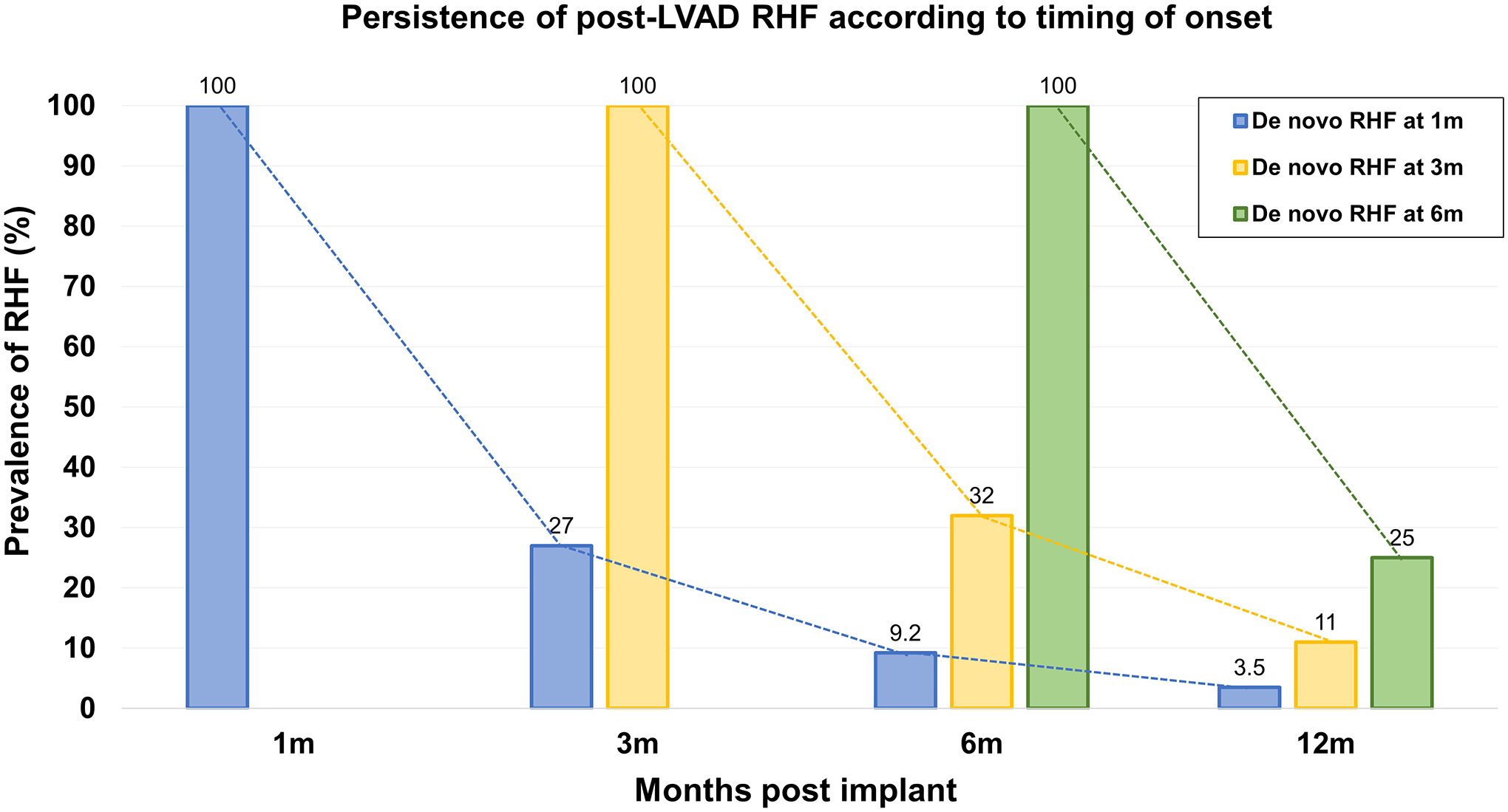
RHF presenting for the first time at 1 month resolves during follow-up in most patients, persisting up to 12 months in only 3.5%. RHF presenting at 3 months persists up to 12 months in 11% of patients. Contrary, RHF presenting for the first time at 6 months is more often a persistent condition, continuing to affect 25% of patients at 12 months post-implant.
Risk factors for RHF at 3 months post implantation
RHF at 3 months was present in 456/4651 patients (9.8%), of who 263 (58%) had persistent RHF present from 1 month, 174 (38%) presented with de novo RHF at month 3 and 19 (4%) patients were missing data on RHF status at 1 month. The pre-implant variables that were independently associated with presence of RHF at 3 months are depicted in Table 2. Significant associations were demonstrated between implantation of a centrifugal LVAD (HR: 1.43; 95% CI: 1.15–1.78, P=0.001), pre-implant BUN (HR: 1.06 per 5-mg/dl increase; 95% CI: 1.03–1.09, P<0.001), severely depressed pre-operative RV function (HR: 1.60; 95% CI:1.17–2.20, P=0.004) and previous repair/replacement of the tricuspid valve (HR: 4.50; 95% CI: 2.01–10.09, P<0.001) and a higher risk of RHF at 3 months.
Table 2.
Multi-variable logistic regression for RHF at 3 months post-implant*
| Effect | Odds ratio (95%CI) | p-value |
|---|---|---|
| Pre-Implant Characteristics | ||
| Previous TV Repair/Replacement | 4.50 (2.01 −10.09) | <0.001 |
| Severely depressed pre-operative RV function | 1.60 (1.17 −2.20) | 0.004 |
| Elevated Systolic Blood Pressure (>120 mmHg) | 1.40 (1.07 −1.82) | 0.014 |
| Blood urea nitrogen (mg/dL)† | 1.06 (1.03 −1.09) | <0.001 |
| Pulmonary Diastolic Pressure (mmHg) | 1.02 (1.01 −1.03) | 0.004 |
| LVEF < 20% | 0.75 (0.59 −0.94) | 0.013 |
| Implant Details | ||
| Left ventricular centrifugal pump vs axial | 1.43 (1.15 −1.78) | 0.001 |
| Post Implant Events | ||
| RHF at 1 Month Post Implant | 6.35 (5.13 −7.86) | <0.001 |
| Sepsis Post Implant | 2.74 (1.16 −6.50) | 0.022 |
LVEF: left ventricular ejection fraction diameter; RHF: right heart failure; RV: right ventricular; TV: tricuspid valve. All variables shown in Supplemental Table 1 were potential covariates in the regression models.
Blood urea nitrogen represents a 5-unit increase
Prognostic significance of type and number of right heart failure manifestations
The detrimental impact of individual RHF manifestations at 1 month on patient outcomes is cumulative and shown in Figure 3a. LVAD patients with no RHF had survival of 92% at 1-year of follow-up. The respective survival with 1, 2 and 3 or more RHF manifestations were 86%, 68% and 58% (P<0.0001). The 2-year survival rates for patients with no RHF and with 1, 2, and 3 or more RHF manifestations at 1-month post-LVAD were 82%, 76%, 56% and 50% (P<0.0001), respectively. For example, presenting with isolated elevation of CVP was accompanied by a much more favorable prognosis compared to simultaneous presentation with elevated CVP, ascites and peripheral edema, as the presence of each additional RHF manifestation increased the risk of death by >60% (HR: 1.62 per manifestation; 95%CI:1.52–1.73, P<0.001). Patients with no or one RHF manifestation had comparable survival at 1- (86% vs 92%, P<0.0001) and 2-year follow-up (76% vs 82%, P<0.0001, Figure 3a), which was significantly better than the respective of patients with >1 RHF manifestation (65% at 1 year and 55% at 2 years, P<0.0001 for both comparisons, Figure 3b).The type of RHF manifestation carried prognostic significance (Supplemental Figure 1), but the dominant factor was the number of manifestations, with low survival among patients presenting with multiple manifestations of RHF at 1 month.
Figure 3. Survival by RHF groups for patients who are alive at 1 month post-implant according to number of RHF manifestations.
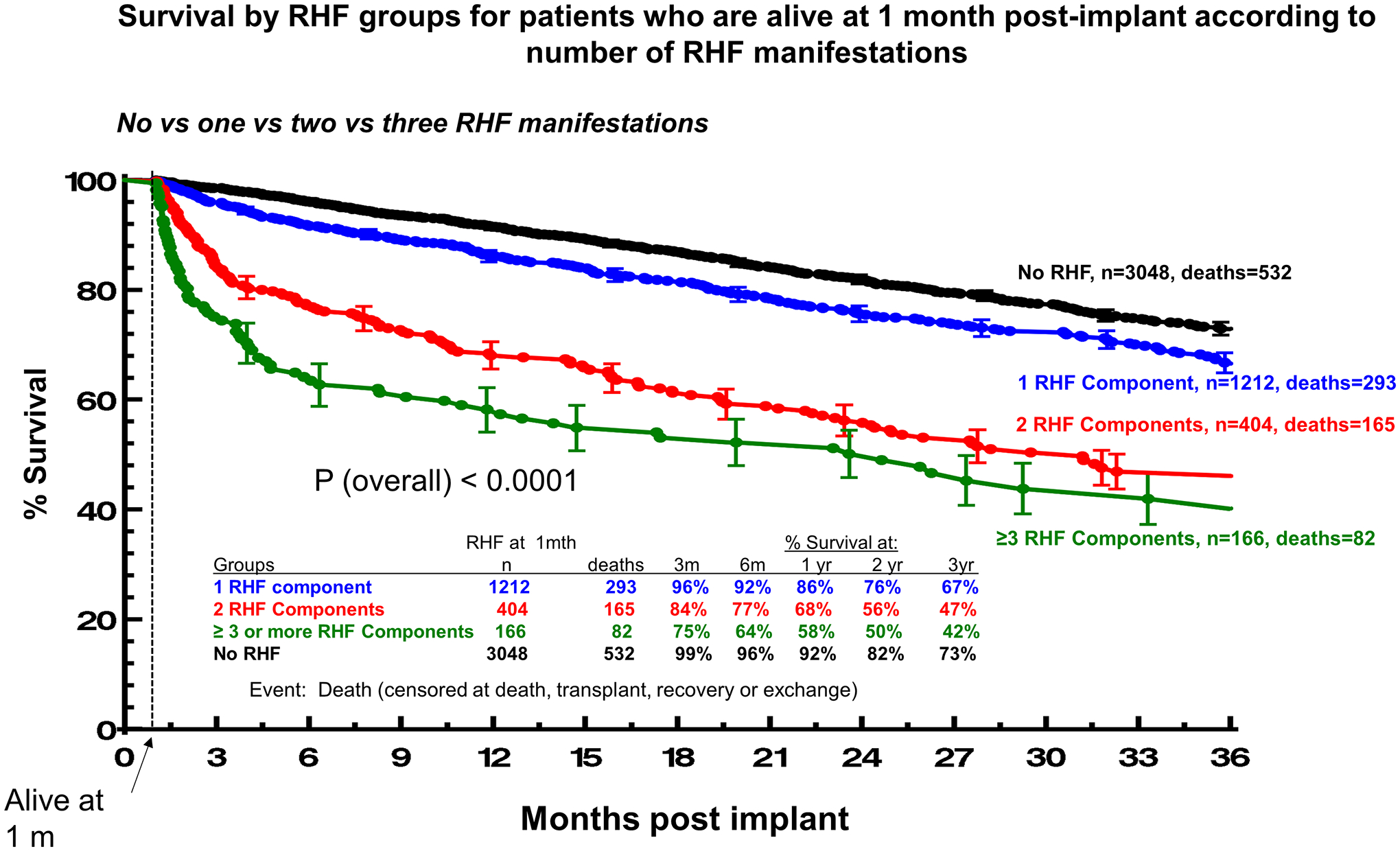
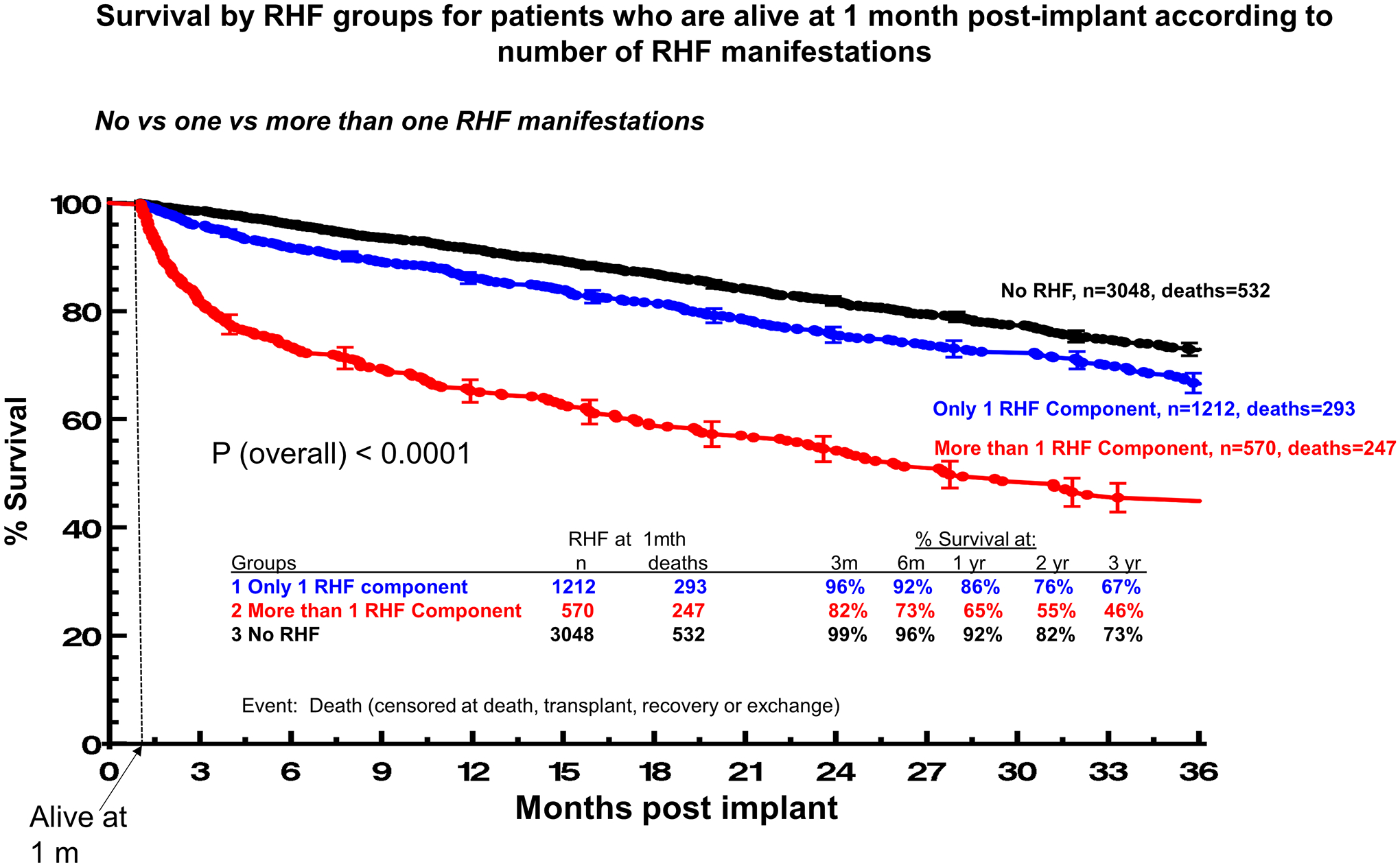
Panel A. No vs one vs two vs three RHF manifestations. Panel B. No vs one vs more than one RHF manifestations. Patients alive at 1 month were N=5176. Event was death censored at transplant, recovery or exchange. Patients with vena cava distension (n=17, deaths=8) and patients with missing RHF information (n=329, deaths=75) were excluded from the analysis.
Prognostic significance of RHF subtypes based on RHF persistence
Survival during follow-up also differed according to the subtype of RHF and the time points at which it was present (Figures 4a and 4b, Supplemental Figures 2a and 2b). For patients who were alive at 3 months post implant and had completed both 1-month and 3-month follow-up, survival was highest among the ones without RHF and lowest among those with persistent RHF (P<0.0001, Figure 4a). Patients with RHF which either resolved or presented de novo at 3 months had comparable and relatively favorable survival (P for comparison=0.22, Figure 4a). In multivariable Cox regression analysis, all subtypes of RHF were significantly associated with an increased risk of death compared with no RHF but the magnitude of the association was considerably higher with persistent vs de novo and resolved RHF (Supplemental Table 2). This was also the case when a multivariable competing risk model was used (Supplemental Table 3).
Figure 4.
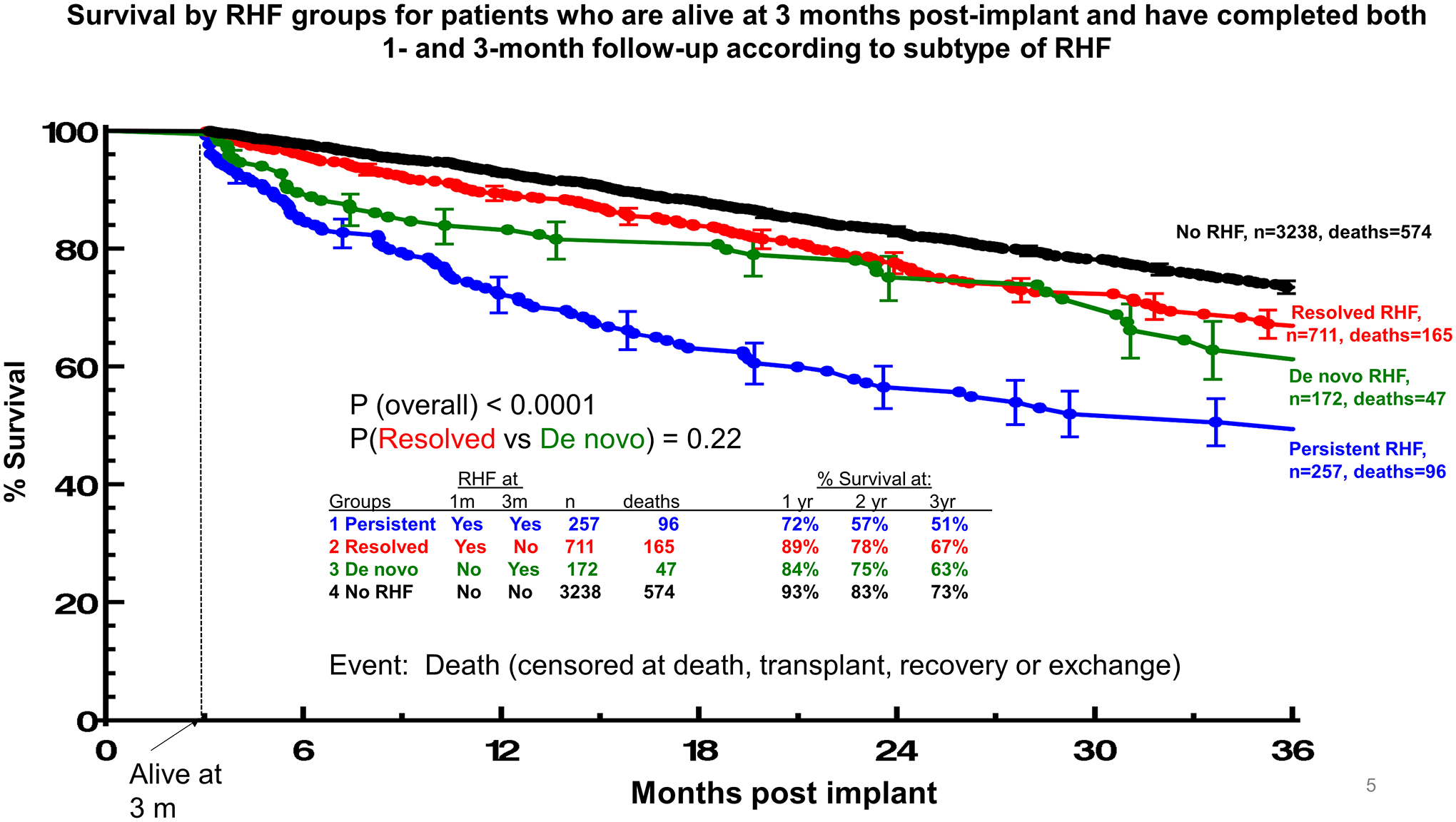
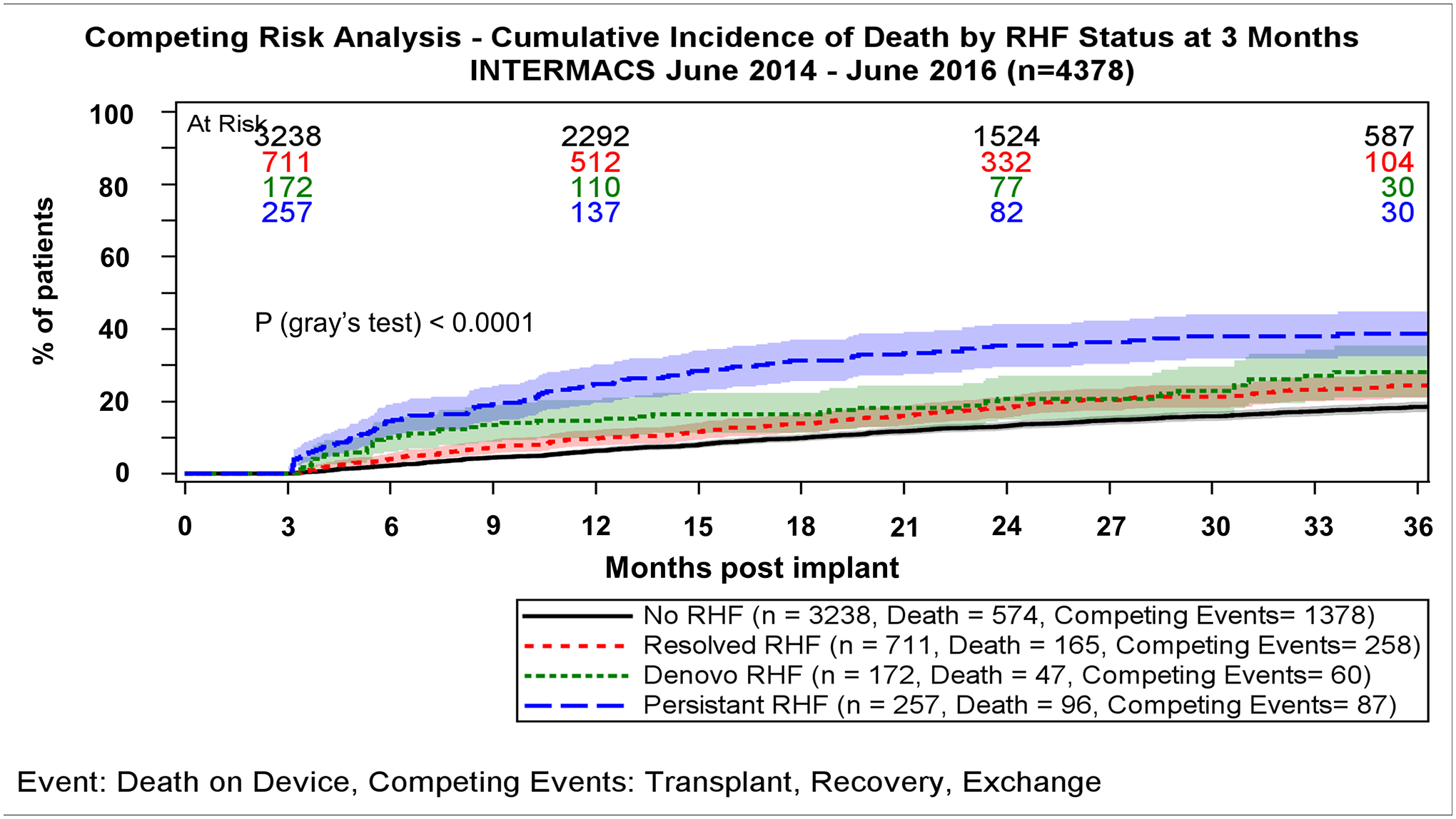
Panel A. Survival by RHF groups for patients who are alive at 3 months post-implant and have completed both 1- and 3-month follow-up according to subtype of RHF. Curves estimated using Kaplan-Meier survival analysis with transplant, and recovery or exchange as censoring events. Panel B. Mortality by RHF groups for patients who are alive at 3 months post-implant and have completed both 1- and 3-month follow-up according to subtype of RHF.
Similarly, for patients who were alive at 6 months and had completed both 3-and 6-month follow-up, survival was also highest among those without RHF and lowest among those with persistent RHF (P<0.0001, Supplemental Figure 2a). In contrast to de novo RHF at 3 months, new onset RHF at 6 months was accompanied by a worse survival, which approximated that of the persistent RHF group (P=0.36, Supplemental Figure 2a). If RHF had resolved between 3 and 6 months patients presented with favorable prognosis, comparable to the one of no RHF patients (P=0.16), and significantly higher survival compared with persistent/de novo RHF (HR: 2.14; 95%CI: 1.51–3.03, P<0.001). Finally, for patients who were alive at 12 months and had completed both 3-, 6 and 12-month follow-up, survival was high only among those without RHF (Supplemental Figure 2b). Conversely, presence of RHF at this time point was accompanied by unfavorable survival at 2 and 3 years of follow-up, irrespectively of whether RHF was de novo, persistent or even resolved (P>0.05, Supplemental Figure 2b).
Discussion
In this large contemporary and generalizable cohort of LVAD patients included in the STS/INTERMACS Registry after the introduction of the new RHF definition, we demonstrated that the incidence of RHF was as high as 24% at 1-month post-LVAD but declines and remains stable with a prevalence of 8–10% thereafter. Several pre-implant factors predicted RHF at 3 months. The increase in the number of RHF manifestations present was paralleled by an increase in mortality. The prognostic impact of RHF differed significantly according to timing and subtype of RHF.
Prevalence and natural history of right heart failure
The prevalence of RHF was 24% at 1 month and ranged between 8–10% at all time points following the first month, whereas de novo RHF at the 3-, 6- and 12-month time points developed in approximately 5%. RHF persisted less the earlier it occurred. Most studies investigating the incidence of RHF have focused on the immediate post-operative period. A meta-analysis of 36 studies including a total of 4,428 LVAD patients (fewer than in our single but generalizable cohort) reported a 25% pooled incidence of RHF, which differed substantially according to study design and proportion of continuous-flow LVADs implanted [7]. This entity seems to differ significantly in terms of incidence, natural history and risk factors compared with RHF that manifests later on, weeks to years after LVAD implantation [5,6,15]. However, sparse data on the latter are available and have limited value due to variable definitions of RHF. Imamura et al. reported in a small cohort that the prevalence of LRHF, defined as right ventricular stroke work index<4.0 g/m2 was 36.8% at 5 weeks post-implantation [15]. Another single-center reported LRHF, defined as HF requiring medical intervention >4 weeks after implantation, in 15% of BTT patients and in 11% of all patients at a median 141 days post-implant [4,16]. In another small cohort of DT patients RHF, defined as symptoms and signs necessitating up-titration of diuretics or use of inotropes, presented in 45% of patients after 2.3±1.5 years (median 2.1 years) of support. However, only 4/20 (20%) patients required inotropes and were the only ones that subsequently died due to RHF [5]. Finally, in a large cohort of 537 DT LVAD recipients LRHF, defined as the development of clinical RHF accompanied by need for inotropes and re-hospitalization occurring >30 days from LVAD implant hospitalization, was reported in 8% a median 480 days post-implant [6].
Heterogeneity in RHF definitions, leading to the inconsistency of reported results incited INTERMACS to universally define RHF [8]. Nonetheless, few studies have investigated the epidemiology of RHF based on this new definition. In a recently published study examining 526 LVAD patients, 16.5% developed ERHF and 14.4% LRHF, defined as RHF occurring more than 30 days after LVAD implantation [17]. Importantly, 16 (3%) patients presented with both ERHF and LRHF, a condition that would fulfill our definition of persistent RHF. Even fewer data are available on dynamic changes of RHF prevalence over time. Teuteberg et al. demonstrated in a population of 3,065 patients from the STS INTERMACS Registry that the prevalence of RHF at 1 week, 1, 3, 6 and 12 months post-implantation was 34%, 16%, 12%, 11%, and 14%, respectively [18], whereas a second STS INTERMACS analysis raised the prevalence of RHF at 3 months post-implant to 9%, with mild RHF representing 4% and moderate 5% [19]. Finally, in another analysis from the same database the incidence of mild RHF at 1, 3, 6 and 12 months was reported to be 6%, 5%, 6%, and 6%, respectively, whereas that of moderate RHF 15%, 3%, 3% and 3% respectively [20], findings which are in accordance with ours. Our study is among the largest to date studies on post-LVAD RHF. It provides for the first-time insight into the fact that the relatively stable prevalence of RHF overtime is not the result of persistence of RHF in a fixed proportion of patients, but rather a mix of de novo, resolved and persistent RHF. Furthermore, we are the first to demonstrate that prevalence of RHF remains stable between 8 and 10% of patients up to at least 3 years post-implant.
Risk factors for development of RHF
We have identified multiple pre-implant factors, which are independently associated with appearance of RHF at 3 months after LVAD implantation. Several of the predictors we recognized, such as BUN, LVEF etc. have been previously reported to differ between patients with and without RHF [4–6,14–16]. Nonetheless, our analysis did not confirm other findings, such as the presumed prognostic significance of body mass index, hemoglobin, platelets, LV end-diastolic diameter and concomitant aortic valve repair. However, these early reports were limited by their selected study populations as well as the different definitions of LRHF, thus rendering their results of limited clinical significance. Therefore, contrary to the multitude of risk factors and scores available for ERHF [7,8,10–13,21], this is not the case with LRHF. Apart from the Columbia group which recently recognized tricuspid annulus diameter as an independent predictor of LRHF [22], the only systematic attempt to find risk factors for LRHF was based on an STS/INTERMACS registry analysis [23]. Although this study attributed prognostic value to several parameters which were also identified as risk factors in our analysis (pulmonary arterial pressures, RV systolic function), its limitations lie in that it defined RHF based on the old INTERMACS definition and that it considered LRHF as every RHF occurring >14 days from implantation, thus encompassing a large proportion of ERHF events. Interestingly, previous tricuspid valve repair/replacement was associated with an increased risk for RHF incidence at 3 months of follow-up, possibly indicating the presence of subtle, unidentified RV dysfunction in these patients. Furthermore, this is the first time that implantation of a centrifugal (vs an axial-flow) pump has been independently associated with the appearance of RHF post-LVAD, though a higher incidence of RHF following centrifugal (vs an axial-flow) pump implantation has been previously reported [24]. Hints that incidence of RHF may be higher with centrifugal pumps were also evident in the seminal MOMENTUM-3 trial [25]. Although the study was not powered to detect differences in RHF events and statistical significance was not reached (P=0.18), a considerable higher incidence of RHF was reported with centrifugal (34.2%) vs an axial-flow (28.3%) pump [25]. This finding, which may be inherent to the different way of unloading of the two pump types [26], warrants further investigation in properly designed studies. Importantly, our analysis covered a period prior to the initiation of Heartmate III data collection in INTERMACS. In regard to BSA, several studies have demonstrated an association between BSA and post-implant RHF. Given that this association was inverse in the early days of LVADs [27,28] but direct in more recent studies possibly indicates LVAD-specific parameters as the underlying mechanism of this relationship [5,29]. Focused studies on the topic are necessary to clarify mediators and prevent RHF, possibly through adjusting device parameters.
Prognostic significance of type and number of right heart failure manifestations
Although appearance of LRHF has been consistently associated with worse prognosis [2–7,13,15,16], our data indicate that RHF should not be considered, from a risk stratification standpoint, as a uniform clinical entity. Patients presenting only with elevated CVP or peripheral edema have a relatively favorable prognosis whereas need for inotropes has a significant association with worse survival. Signals that inotropes are the manifestation of LRHF which mainly determines outcome have been previously reported [5,18]. Apart from type of manifestation, number of manifestations also differentiates patients regarding their outcomes; patients with one RHF manifestation have impressively better prognosis compared with the ones with >1 RHF manifestations, a finding which has not been reported to date.
Prognostic significance of RHF subtypes based on RHF persistence
The differential association of timing and subtype of RHF with outcomes is also reported for the first time. RHF which persists between assessment time points (1–3 months or 3–6 months) was associated with the worse overall survival, finding which has been previously reported among patients with both ERHF and LRHF compared with patients with one of the two disorders alone [17]. Moreover, de novo RHF at 3 months or resolved RHF up to 6 months had relatively favorable prognosis, contrary to RHF being present from 6 months onward which was accompanied by dismal prognosis, regardless of its subtype. The significant differences in outcomes, based on the subtypes and timing of RHF, indicate that the 1-, 3- and 6-month time points may be routinely used to assess RHF, stratify patients’ risk and potentially guide therapeutic interventions, if not redefine RHF.
Limitations
As with any observational study, causality cannot be inferred from our study. Furthermore, as only a few intra-operative and post-operative data were collected and analyzed, we could not identify additional such parameters that may have been associated with RHF appearance. Thus, although variables from different domains were associated with the post-operative appearance of RHF, our study cannot clarify whether RHF is attributable to patient factors, device factors, treatment factors or combination of all the above, as well as the mechanisms underlying these associations. Further studies targeted on mechanistic aspects of RHF would be needed to clarify this issue and set the grounds for studies aiming the prevention or treatment of post-LVAD RHF. The diagnosis of RHF was based on treating physicians and not adjudicated by a central committee. However, the need for documentation of elevated CVP based on the new INTERMACS definition reduces the risk of RHF misdiagnosis. Nonetheless, a proportion of the de novo RHF cases could still represent RHF pre-existing from an earlier timepoint post-operatively which was not recognized promptly. The STS/INTERMACS registry that was used is of high external and internal validity, though our analysis was limited to the data collected which might have omitted confounders. Finally, recently a new definition of updated definitions of adverse events, among which RHF, for trials and registries of mechanical circulatory support was introduced by the mechanical circulatory support academic research consortium [30]. Unfortunately, our data did not suffice to capture epidemiology of RHF with this new definition. Finally, our predictive model for RHF at 3 months does not separately account for death prior to 3 months, possibly due to RHF. However, death after 3 months is analyzed with a competing outcomes model to examine the relationship between patterns of RHF and subsequent mortality.
Conclusion
The incidence of RHF, according to the new INTERMACS definition, was 24% at 1-month post-LVAD and the prevalence approximately 8–10% at all time points thereafter. Multiple pre-implant variables independently predicted the presence of RHF post-implantation. Presence of >1 RHF manifestation has a significant adverse effect on patients’ outcomes; among these manifestations elevated RAP/CVP is the most benign and inotropes the most malignant in terms of prognostication. RHF at 1-month post-LVAD or de novo RHF occurring relatively early post-LVAD (≤3 months) was a common and often transient condition, which if resolved was not accompanied by considerable long-term survival compromise. Conversely, RHF which presents late post-LVAD (>3–6 months) was more frequently a persistent disorder, which carries a significant risk of mortality. The 1-, 3- and 6-month time points should be used to assess RHF, stratify risk and potentially guide therapeutic interventions, if not redefine post-LVAD RHF.
Supplementary Material
Short commentary.
a. What is new?
Prevalence of post-LVAD RHF is 24% at 1-month post-implantation but declines and ranges between 8–10% up to 36 months thereafter. The later RHF presents, the higher the chance it represents a persistent, rather than a transient, abnormality. Several variables can predict the incidence of 3-month RHF, including pre-existing (pre-operative or post-operative) RV disease and implantation of a centrifugal LVAD. Increase in the number of manifestations with which RHF presents is paralleled by an increase in mortality. The prognostic impact of RHF differs significantly according to timing and subtype of RHF with persistent RHF representing the most unfavorable subtype.
b. What are the clinical implications?
Our study provides insight in the epidemiology of RHF post-LVAD. First, it clarifies that the stable prevalence of RHF over time is a result of the combination of de novo, resolved and persistent RHF. Second, it indicates that post-LVAD RHF should not be considered and dealt with as a uniform, in terms of prognosis, entity, as resolved and de novo RHF may have a quite favorable course, especially when presenting early post implantation. Third, the 1-, 3- and 6-month time points should be used to assess RHF, stratify risk and potentially guide therapeutic interventions in patient post-LVAD implantation.
Acknowledgments:
The data for this research were provided by The Society of Thoracic Surgeons’ National Database Access and Publications Research Program.
Funding:
AHA Heart Failure Strategically Focused Research Network 16SFRN29020000 and Nora Eccles Treadwell Foundation.
Disclosures:
Mrs. Myers and Drs. Adamopoulos, Alharethi, Bonios, Cantor, Chamogeorgakis, Gilbert, Kapelios, Kfoury, Koliopoulou, McKellar, Selzman and Wever-Pinzon have no relevant conflict of interest;
Dr. Drakos is a consultant to Abbott (steering committee of INTELLECT-2 multicenter trial); Research grants from Merck, Novartis, NIH, AHA, Department of Veterans Affairs and Nora Eccles Treadwell Foundation.
Dr. Fang has the following disclosures: Novartis (Steering Committee of EVALUATE Trial), Amgen (Steering Committee of GALACTIC-HF), Johnson & Johnson (was Actelion) (DSMB Chair of SOPRANO Trial), AstraZeneca (Steering Committee of DELIVER-HF trial and DSMB of PRESERVED-HF trial), CardioRenal (Advisory Board), Boerhinger-Ingelheim/Lilly (DSMB of EMBRACE-HF), Abbott (Adjudication Committee of abtMI study), Capricor (CEC member of ALLSTAR and HOPE Trials, CEC Chair of COVID trial), Windtree (DSMB of SEISMiC trial), AHA (Training Director of SFRN HF Center), NIH (5% co-investigator of HFpEF grant [PI: DiBella], Co-investigator in TRANSFORM-HF substudy [PI: Testani], Steering Committee and Site PI of LOFT-HF), OSMB member of PVOMICS, Medical Monitor of CTSN, DSMB Chair of U01 CABG with Ang1-7, DSMB of Jennifer Ho R01 metformin PH trial), outside the submitted work;
Dr. Kirklin is the Director of the Data Coordinating Center for the STS INTERMACS database and receives partial salary support through funds paid to his institution;
Dr. Lund reports personal fees from Merck, grants and personal fees from Boehringer Ingelheim, personal fees from Sanofi, grants and personal fees from Vifor-Fresenius, grants and personal fees from AstraZeneca, grants and personal fees from Relypsa, personal fees from Bayer, grants from Boston Scientific, grants and personal fees from Novartis, personal fees from Pharmacosmos, personal fees from Abbott, grants and personal fees from Mundipharma, personal fees from Myocardia, personal fees from Medscape, outside the submitted work;
Dr. Stehlik is a consultant to Abbott and Medtronic.
Footnotes
References
- 1.Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, et al. ; HeartMate II Clinical Investigators. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–1324. [DOI] [PubMed] [Google Scholar]
- 2.LaRue SJ, Raymer DS, Pierce BR, Nassif ME, Sparrow CT, Vader JM. Clinical outcomes associated with INTERMACS-defined right heart failure after left ventricular assist device implantation. J Heart Lung Transplant. 2017;36:475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teuteberg JJ, Studdard G, Kiernan M, Oliveria G, Rame E, Alturi P, Gaffey A, Grandin EW, Kirklin J, Myers S, et al. The Incidence of Early and Late Clinical Right Heart Failure and the Impact on Survival After Continuous Flow Mechanical Support: Insights from the New INTERMACS Definition of Right Heart Failure. J Heart Lung Transplant. 2017;36:356. Abstract [Google Scholar]
- 4.Takeda K, Takayama H, Colombo PC, Jorde UP, Yuzefpolskaya M, Fukuhara S, Manicini DM, Naka Y. Late right heart failure during support with continuous-flow left ventricular assist devices adversely affects posttransplant outcome. J Heart Lung Transplant. 2015;34:667–674. [DOI] [PubMed] [Google Scholar]
- 5.Kapelios CJ, Charitos C, Kaldara E, Malliaras K, Nana E, Pantsios C, Repasos E, Tsamatsoulis M, Toumanidis S, Nanas JN. Late-onset right ventricular dysfunction after mechanical support by a continuous-flow left ventricular assist device. J Heart Lung Transplant. 2015;34:1604–1610. [DOI] [PubMed] [Google Scholar]
- 6.Rich JD, Gosev I, Patel CB, Joseph S, Katz JN, Eckman PM, Lee S, Sundareswaran K, Kilic A, Bethea B, et al. ; Evolving Mechanical Support Research Group (EMERG) Investigators. The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J Heart Lung Transplant. 2017;36:50–58. [DOI] [PubMed] [Google Scholar]
- 7.Bellavia D, Iacovoni A, Scardulla C, Moja L, Pilato M, Kushwaha SS, Senni M, Clemenza F, Agnese V, Falletta C, et al. Prediction of right ventricular failure after ventricular assist device implant: systematic review and meta-analysis of observational studies. Eur J Heart Fail. 2017;19:926–946. [DOI] [PubMed] [Google Scholar]
- 8.Turner KR. Right Ventricular Failure After Left Ventricular Assist Device Placement-The Beginning of the End or Just Another Challenge? J Cardiothorac Vasc Anesth. 2019;33:1105–1121. [DOI] [PubMed] [Google Scholar]
- 9.University of Alabama at Birmingham School of Medicine. Interagency Registry for Mechanical Circulatory Support. Adverse Event Definitions. Accessed September 22, 2020. Available online at: https://www.uab.edu/medicine/intermacs/intermacs-documents.
- 10.Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick JR 3rd, Frederick JR, Hsu VM, Kozin ED, O’Hara ML, Howell E, Dougherty D, McCormick RC, Laporte CA, Cohen JE, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008;27:1286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, Horton K, Haddad F, Li DY, Renlund DG, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010;105:1030–1035. [DOI] [PubMed] [Google Scholar]
- 13.Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015;34:1123–1130. [DOI] [PubMed] [Google Scholar]
- 14.Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, Miller MA, Ulisney K, Young JB. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27:1065–1072. [DOI] [PubMed] [Google Scholar]
- 15.Imamura T, Kinugawa K, Kato N, Muraoka H, Fujino T, Inaba T, Maki H, Kinoshita O, Hatano M, Kyo S, et al. Late-onset right ventricular failure in patients with preoperative small left ventricle after implantation of continuous flow left ventricular assist device. Circ J. 2014;78:625–633. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Takayama H, Colombo PC, Yuzefpolskaya M, Fukuhara S, Han J, Kurlansky P, Mancini DM, Naka Y. Incidence and clinical significance of late right heart failure during continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2015;34:1024–1032. [DOI] [PubMed] [Google Scholar]
- 17.Kurihara C, Critsinelis AC, Kawabori M, Sugiura T, Loor G, Civitello AB, Morgan JA. Frequency and Consequences of Right-Sided Heart Failure After Continuous-Flow Left Ventricular Assist Device Implantation. Am J Cardiol. 2018;121:336–342. [DOI] [PubMed] [Google Scholar]
- 18.Teuteberg J, Kormos RL, Pagani FD, Kiernan MS, Naftel DC, Myers SL, Pamboukian SV, Kirklin JK. New Definition, Same Old Problem: Characterizing the Condition of Right Heart Failure in INTERMACS. J Heart Lung Transplant.2016;35:S266. Abstract [Google Scholar]
- 19.Rame JE, Birati EY, Teuteberg J, Grandin EW, Atluri P, Kiernan M, Oliveira GH, Myers SL, Naftel DC, Pagani FD, et al. Outcomes in Late Right Heart Failure after LVAD: A Contemporary Analysis of the New Intermacs 4.0 Definition. J Heart Lung Transplant. 2018;37:S86. Abstract [Google Scholar]
- 20.Teuteberg J, Studdard G, Pagani F, Kiernan M, Oliveria G, Rame E, Alturi P, Gaffey A, Grandin E, Kirklin J, et al. The Ebb and Flow of Right Heart Failure in INTERMACS: Does Right Heart Failure Get Better or Worse Over Time? J Heart Lung Transplant. 2018;37:S377–8. Abstract [Google Scholar]
- 21.Argiriou M, Kolokotron SM, Sakellaridis T, Argiriou O, Charitos C, Zarogoulidis P, Katsikogiannis N, Kougioumtzi I, Machairiotis N, Tsiouda T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis. 2014;6:S52–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanishi K, Homma S, Han J, Takayama H, Colombo PC, Yuzefpolskaya M, Garan AR, Farr MA, Kurlansky P, Di Tullio MR, et al. Usefulness of Tricuspid Annular Diameter to Predict Late Right Sided Heart Failure in Patients With Left Ventricular Assist Device. Am J Cardiol. 2018;122:115–120. [DOI] [PubMed] [Google Scholar]
- 23.Loghmanpour NA, Kormos RL, Kanwar MK, Teuteberg JJ, Murali S, Antaki JF. A Bayesian Model to Predict Right Ventricular Failure Following Left Ventricular Assist Device. JACC Heart Fail. 2016;4:711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teuteberg JJ, Cleveland JC Jr, Cowger J, Higgins RS, Goldstein DJ, Keebler M, Kirklin JK, Myers SL, Salerno CT, Stehlik J, et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications.Ann Thorac Surg. 2020;109:649–660. [DOI] [PubMed] [Google Scholar]
- 25.Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, et al. ; MOMENTUM 3 Investigators. A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med. 2019;380:1618–27. [DOI] [PubMed] [Google Scholar]
- 26.Giridharan GA, Koenig SC, Soucy KG, Choi Y, Pirbodaghi T, Bartoli CR, Monreal G, Sobieski MA, Schumer E, Cheng A, et al. Left ventricular volume unloading with axial and centrifugal rotary blood pumps. ASAIO J. 2015;61:292–300. [DOI] [PubMed] [Google Scholar]
- 27.Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J, Hsu AP, Yeager ML, Buda T, Hoercher KJ, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002;106:I198–202. [PubMed] [Google Scholar]
- 28.Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, et al. ; HeartMate II Clinical Investigators. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes.J Thorac Cardiovasc Surg. 2010;139:1316–24. [DOI] [PubMed] [Google Scholar]
- 29.Grandin EW, Zamani P, Mazurek JA, Troutman GS, Birati EY, Vorovich E, Chirinos JA, Tedford RJ, Margulies KB, Atluri P, et al. Right ventricular response to pulsatile load is associated with early right heart failure and mortality after left ventricular assist device. J Heart Lung Transplant. 2017;36:97–105. [DOI] [PubMed] [Google Scholar]
- 30.Kormos RL, Antonides CFJ, Goldstein DJ, Cowger JA, Starling RC, Kirklin JK, Rame JE, Rosenthal D, Mooney ML, Caliskan K, et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: A consensus statement of the mechanical circulatory support academic research consortium. J Heart Lung Transplant. 2020;39:735–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


