Abstract
Purpose:
i) To examine the time window during which intercellular signaling though gap junctions mediates non-targeted (bystander) effects induced by moderate doses of ionizing radiation. ii) To investigate the impact of gap junction communication on genomic instability in distant progeny of bystander cells.
Materials and Methods:
A layered cell culture system was developed to investigate the propagation of harmful effects from irradiated normal or tumor cells that express specific connexins to contiguous bystander normal human fibroblasts. Irradiated cells were exposed to moderate mean absorbed doses from 3.7 MeV α particle, 1000 MeV/u iron ions, 600 MeV/u silicon ions, or 137Cs γ rays. Following 5 h of co-culture, pure populations of bystander cells, unexposed to secondary radiation, were isolated and DNA damage and oxidative stress was assessed in them and in their distant progeny (20–25 population doublings).
Results:
Increased frequency of micronucleus formation and enhanced oxidative changes were observed in bystander cells co-cultured with confluent cells exposed to either sparsely ionizing (137Cs γ rays) or densely ionizing (α particles, energetic iron or silicon ions) radiations. The irradiated cells propagated signals leading to biological changes in bystander cells within one hour of irradiation, and the effect required cellular coupling by gap junctions. Notably, the distant progeny of isolated bystander cells also exhibited increased levels of spontaneous micronuclei. This effect was dependent on the type of junctional channels that coupled the irradiated donor cells with the bystander cells. Previous work showed that gap junctions composed of connexin26 (Cx26) or connexin43 (Cx43) mediate toxic bystander effects within 5 h of co-culture, whereas gap junctions composed of connexin32 (Cx32) mediate protective effects. In contrast, the long-term progeny of bystander cells expressing Cx26 or Cx43 did not display elevated DNA damage, whereas those coupled by Cx32 had enhanced DNA damage.
Conclusions:
In response to moderate doses from either sparsely or densely ionizing radiations, toxic and protective effects are rapidly communicated to bystander cells through gap junctions. We infer that bystander cells damaged by the initial co-culture (expressing Cx26 or Cx43) die or undergo proliferative arrest, but that the bystander cells that were initially protected (expressing Cx32) express DNA damage upon sequential passaging. Together, the results inform the roles that intercellular communication play under stress conditions, and aid assessment of the health risks of exposure to ionizing radiation. Identification of the communicated molecules may enhance the efficacy of radiotherapy and help attenuate its debilitating side-effects.
Keywords: Bystander effects, Genomic instability, Non-targeted effects, Gap junctions, Channel permeability, Radiation quality
INTRODUCTION
The expression of non-targeted biological responses in living systems exposed to ionizing radiation is thought to contribute to cancer and non-cancer effects (Morgan and Sowa 2015). The study of these responses, specifically biological changes induced in non-irradiated cells that respond to signal(s) communicated by irradiated cells (i.e. bystander effects), or effects occurring in the progeny of an irradiated cell generations after the parental cell has been irradiated (i.e. genomic instability), was a central theme in the research of William Francis Morgan and his reflections on the risk of detrimental health effects linked to exposure to radiation (Morgan and Bair 2013). William Morgan, or Bill as he is known by his friends and colleagues, championed and promoted research in this topic at the onset (Marder and Morgan 1993, Morgan 2003a, Morgan 2003b, Morgan 2003c) due to its immense impact on issues related to occupational and environmental radiation protection when a small fraction of cells in tissues receive any direct exposure, or to high dose radiotherapy conditions in which a significant portion of the healthy tissue is not directly targeted by radiation (Coates et al. 2004, Hamada et al. 2007, Hei et al. 2011, Held 2009, Little 2003, Morgan 2003a, Mothersill and Seymour 2004, Prise and O’Sullivan 2009). A discussion of these effects between one of the authors of this manuscript (EIA), while still a student, and Bill occurred for the first time in May 1993, during a road trip from Whistler to Vancouver in British Columbia, Canada, following a symposium on the molecular, cellular and genetic basis of radio-sensitivity at low doses. Shortly before that time, the late Sheldon Wolf, a mentor of Bill, was presented with the ‘Failla Award’ of the Radiation Research Society for his pioneering work on radiation-induced adaptive protection (Wolff 1992), a low dose phenomenon. Furthermore, two seminal papers were also published describing non-targeted effects of cellular exposure to low fluences of particulate radiation (Kadhim et al. 1992, Nagasawa and Little 1992). Inspired by these influential discoveries and the discussions that took place at the symposium in Whistler, our dialogue continued and was enriched over time with collaborations (Sowa et al. 2011) that lasted until the second week in November 2015 when suddenly Bill left us physically (Brooks 2015, Hamada et al. 2016, Schwartz 2016). This article is merely a continuation of these discussions and a joyful memory of having known an altruistic stalwart of the radiation sciences, one who will continue to inspire future generations of researchers.
Whereas our previous work examined bystander responses induced following in vitro and in vivo exposures to low mean absorbed doses of α particles or high atomic number (Z) and high energy (E) (HZE) particles (Azzam et al. 1998, Azzam et al. 2001, Azzam et al. 2002, Buonanno, De Toledo, Pain, et al. 2011, Gaillard et al. 2009, Gonon et al. 2013, Jain et al. 2011), this study examines in parallel the role of gap junction intercellular communication in mediating bystander effects induced by moderate doses of sparsely and densely ionizing radiations, which differ in their biophysical characteristics (i.e. quality).
Firm evidence for the participation of junctional communication in radiation-induced bystander effects (Azzam, de Toledo, Gooding and Little 1998, Azzam, de Toledo and Little 2001, Desai et al. 2014, Gaillard, Pusset, de Toledo, Fromm and Azzam 2009, Mancuso et al. 2008, Shao, Furusawa, et al. 2003, Zhou et al. 2001) was generated soon after the landmark report of Nagasawa and Little indicating that genetic changes occurred in a greater number of cells than were exposed to low fluences of α particles (Nagasawa and Little 1992). Further, we have described, in previous studies, the effects of different connexins that form junctional channels with specific permeability properties in responses of human cells to ionizing radiation when all the cells in an exposed culture were directly traversed by the impacting radiation (Autsavapromporn, de Toledo, et al. 2013, Autsavapromporn, de Toledo, Little, et al. 2011). Here, we extend these studies and report novel findings on the permeability aspects of junctional channels in modulating the spread of stressful effects from irradiated cells to the bystander cells with which they are coupled. We also characterize the impact of bystander effects propagated in the first few hours after irradiation on ensuing biological alterations in distant progeny of the bystanders.
Gap junctions are critical for diverse physiological functions (Mehta et al. 1986). They are dynamic structures that allow direct intercellular transfer of cytoplasmic molecules, thereby providing a powerful and rapid pathway for molecular signaling between cells, which is essential for the coordination of normal tissue functions (Bruzzone and Meda 1988). Gap junctions are formed by two apposed subunits called hemichannels or connexons, one contributed by each cell. Each hemichannel is a hexamer of connexin proteins, and can be composed of more than one connexin isoform (Saez and Leybaert 2014). In general, connexin pores are considered to allow permeation by molecules up to ~1000 Da, well above the size of most second messengers (Harris 2001). However, recent evidence has indicated that molecules as large as microRNA can also permeate using gap junctions (Hong et al. 2015, Lim et al. 2011). In mammals, there are ~ 20 known connexins encoded by a multi-gene family, and each connexin isoform has a particular set of unique properties that are essential for specific functions and developmental processes (Willecke et al. 2002). Notably, junctional channels formed of different connexins (Cx) are highly selective among molecular permeants (Harris 2001, Harris 2007, Harris and Contreras 2014). For example, ATP, ADP, AMP, glutamate and glutathione are significantly more permeable through junctional Cx43 channels than Cx32 channels. Conversely, adenosine and IP3 are more permeable through Cx32 channels than Cx43 channels. Depending on their composition, connexin channels can discriminate between highly similar second messengers (e.g. cAMP and cGMP, and among inositol triphosphates (Ayad et al. 2006, Bevans et al. 1998, Elsayed and Harris 2004, Elsayed et al. 2004, Locke et al. 2004). Therefore, it is not surprising that functional deletion of a particular connexin isoform produces a specific and distinct pathology (Brisset et al. 2009).
Evidence for the involvement of gap junctions in propagation of radiation-induced bystander effects has been derived by several approaches (reviewed in (Azzam et al. 2013)). Up- or down-regulation of connexin expression/gap-junction gating by chemical agents, forced connexin expression by transfection, and connexin gene knockout studies have uniformly generated strong evidence for the participation of gap junctions in radiation-induced bystander effects (Azzam et al. 2003b). This is supported by stabilization and up-regulation of connexin mRNA and protein by ionizing radiation (Azzam et al. 2003a). Disruption of cholesterol rich areas of the plasma membrane where gap-junction channels partition (Schubert et al. 2002) attenuated the propagation to bystander cells of harmful effects resulting from direct traversal of other cells by radiation (Nagasawa et al. 2002, Shao et al. 2004). However, the role of junctional permeability in the short- and long-term consequences of the propagation of bystander effects remains unclear (Buonanno, de Toledo and Azzam 2011, Buonanno, De Toledo, Pain and Azzam 2011, Gonon, Groetz., detoledo, Howell, Fromm and Azzam 2013, Zhao et al. 2014).
In this report, we describe the effectiveness of a layered cell culture system (Domoguaer et al. 2016) in shedding light on the permeability properties of junctional channels in the nature of long-term outcomes of the radiation-induced bystander effect. By using this system, confounding factors in interpreting results attributed to bystander responses, due to scattering or fragmentation of the incident radiation as it crosses the culture vessel and the biological material, is eliminated. This is particularly important in the case of experiments involving HZE particles (Gonon, Groetz., detoledo, Howell, Fromm and Azzam 2013, Nikjoo et al. 2001, Ponomarev and Cucinotta 2006). Secondary radiations arising from interactions of the target material with an incident HZE particle may include energetic electrons (δ rays), photons, protons, neutrons, α particles and other heavier ions with different Linear Energy Transfer (LET). The range of 𝛿 rays produced following HZE particle-traversals can extend up to several cell diameters (Cucinotta et al. 1998), thereby potentially irradiating and contributing to biochemical changes in cells that are near those targeted by the primary particle track. In particular, protective mechanisms induced by low LET secondary radiations may mitigate stressful effects propagated from cells traversed by the primary particle (Elmore et al. 2009). Therefore, cells that were thought to be bystanders may be significantly irradiated by secondaries. Using our cell culture system, we show that isolated bystander cells that have been in coculture with cells exposed to moderate or high mean absorbed doses from either high LET α or HZE particles, or low LET 137Cs γ rays, experience significant stress as measured by increased micronucleus formation, protein carbonylation and an altered pattern of proteins conjugated with 4-hydroxynonenal (4-HNE), a product of lipid peroxidation. Furthermore, we show that the expressed connexins determine the nature of biological changes in the distant progeny of bystander cells.
MATERIAL AND METHODS
Cell culture
Apparently normal AG1522 human diploid skin fibroblasts were obtained from the Genetic Cell Repository at the Coriell Institute for Medical Research. These cells express Cx26, Cx32 and Cx43, and functionally communicate with other normal or tumor human cells by gap junctions and secreted factors (Autsavapromporn, de Toledo, Jay-Gerin, Harris and Azzam 2013, Azzam, de Toledo and Little 2003a, Zhao, De Toledo, Hu, Hei and Azzam 2014). Stock cultures were routinely maintained in 37 °C humidified incubators in an atmosphere of 5 % CO2 (vol/vol) in air. The cells were grown in Eagles’ Minimum Essential Medium (MEM) (CellGro) containing 12.5 % (vol/vol) heat inactivated (30 min at 56 °C) fetal bovine serum (FBS) (Sigma), supplemented with 4 mM L-alanyl-L-glutamine (CellGro), 100 U/mL penicillin and 100 μg/mL streptomycin (CellGro).
In experiments, cells at passage 10 were used. The cells destined for irradiation with HZE-particles or 137Cs γ rays were grown in 12.5 cm2 polystyrene flasks (Greiner). Cells destined for α-particle-irradiation were seeded in stainless steel dishes with a circular 36-mm-diameter growing surface that consists of 1.5 μm-thick replaceable polyethylene terephthalate (PET). To facilitate cell attachment, the PET surface was pre-coated with FNC solution comprising fibronectin and collagen (AthenaES™), overlaid with 2 mL of MEM and incubated at 37 °C. After 30 min, the medium was aspirated and the cells were seeded at a density of ~ 1.5×105 cells/culture vessel, and fed twice every other day after reaching the confluent, density-inhibited state (usually within 4–5 days). They were irradiated 48 h after the last feeding. Under these conditions, 95–98 % of the cells were in G0/G1 phase of the cell cycle. The synchronization of cells in G0/G1 phase, by density-inhibition of growth, eliminates complications in interpretation of results that arise from changes in responses to ionizing radiation at different phases of the cell cycle (Terasima and Tolmach 1961).
Cells destined to be bystanders were cultured onto inverted Transwell inserts containing 10 μm-thick polyester permeable microporous membranes with 1 μm pores (Costar #3452) as we have recently described in detail (Domoguaer, De Toledo and Azzam 2016). Briefly, the cells destined to be bystanders were seeded at ~ 1.5×105 cells in 50 μL of MEM supplemented with 25% FCS on one side of the insert. After 40–50 min, the inserts containing adherent cells were inverted (i.e. with the cells now growing on the bottom side) and placed into 6-well plates containing 1 mL of growth medium, and were fed daily with MEM supplemented with 12.5% FCS. Experiments were carried 2–4 days after a confluent monolayer of cells was formed across the bottom side of the inserts. For co-culture, irradiated (donor) cells were gently trypsinized, and 2–3×105 cells were seeded in 1 mL of growth medium on the top side of the insert (see scheme in Figure 1A). An additional 1 mL of growth medium was added to the bottom of the wells. The irradiated and bystander cells established coupling via gap junctions within 1–2 h of co-culture as described below. In all experiments, control cells were sham-treated and handled in parallel with the test cells.
Figure 1. AG1522 normal human fibroblasts establish gap-junction intercellular communication in a layered cell co-culture system.
(A) Schematic representation of the layered cell co-culture system. (B) AG1522 cells labeled with CellTracker Orange and Calcein were seeded on the top side of permeable microporous membrane inserts with 1 m-pores. By 2 h, Calcein permeated to bystander cells grown on the bottom side of the insert.
Irradiation
Cells were exposed at 37°C to γ rays (LET ~0.9 keV/μm in liquid water) from a 137Cs source (J.L. Shepherd Mark I, San Fernando, CA) at a dose rate of 1.3 Gy/min. The culture flasks were placed on a rotating platform to ensure uniform exposure, and control cultures were handled in parallel but were sham treated.
For α-particle-irradiation, the cell cultures were exposed at 37°C to a 7.4 MBq 241Am-collimated source housed in a helium-filled plexiglass box at a mean absorbed dose rate of 2 cGy/min. Irradiation was carried out from below, through the PET growing surface. At the growing surface, measurements using a Canberra (Meridien, CT) CAM300 passive implanted planar silicon (PIPS) detector showed that the α particles had a mean energy of 3.7 MeV (0.92 MeV/u) with Full Width at Half Maximum (FWHM) of 0.5 MeV (Neti et al. 2004). The LET corresponding to a mean energy of 3.7 MeV is ~109 keV/μm in liquid water (ICRU 1993). The 241Am source was fitted with a photographic shutter to allow accurate delivery of the specific radiation dose. Microscopic examination of pits etched in CR-39 plastic after a 1 min-exposure showed no source hot-or cold spots (Neti, de Toledo, Perumal, Azzam and Howell 2004). The α particle irradiator system was housed in a chamber (BioSpherix) maintained at 37°C and 5 % CO2 (vol/vol), and the cell incubators housing the cell cultures destined for irradiation are an integral part of the chamber.
Experiments with 1000 MeV/u 56Fe26+ or 600 MeV/u 28Si14+ ions were performed at the NASA Space Radiation Laboratory (NSRL) located at Brookhaven National Laboratory, Upton NY. Description of the facility and radiation beam characteristics can be found at http://www.bnl.gov/medical/nasa/LTSF.asp. The tissue culture flasks, with adherent cells, were placed in the plateau region of the Bragg curve and irradiated at a dose-rate of 0.5 Gy/min. They were positioned orthogonal to the beam such that the irradiating particles impacted first the plastic of the culture vessel, followed by the adherent cells and then the growth medium. At the place where they were positioned, the LET in liquid water of 1000 MeV/u iron ions (56Fe26+) is ~151 keV/μm, and of 600 MeV/u silicon ions (28Si14+) is ~51 keV/μm. Three to six hours before the radiation exposure, the flasks were filled to capacity with 37 ˚C growth medium supplemented with 0.5% FBS that was equilibrated at pH 7.4. This ensured that during the irradiation, temperature fluctuations were attenuated and the cells were immersed in medium when in the vertical position, which alleviates changes in osmolarity and partial oxygen tension that affect their responses to ionizing radiation (Gray et al. 1953, Raaphorst et al. 1984, Rueckert and Mueller 1960). In all cases, control cells were handled in parallel but were sham-irradiated. A detailed description of the fraction of cells whose nuclei were traversed by an average of one or more particle tracks according to the method given by Charlton and Sephton (Charlton and Sephton 1991) is found in our earlier publication (Gonon, Groetz., detoledo, Howell, Fromm and Azzam 2013). At mean absorbed doses of 0.8 or 1.0 Gy from 3.7 MeV α particles or 1000 MeV/u iron ions or 600 MeV/u silicon ions, respectively, 95–100% of the cells in the exposed populations would be traversed through the nucleus by an average of one or more particle tracks (Autsavapromporn, de Toledo, Buonanno, et al. 2011, Autsavapromporn, de Toledo, Little, Jay-Gerin, Harris and Azzam 2011, Gonon, Groetz., detoledo, Howell, Fromm and Azzam 2013). In case of confluent cultures exposed to 4 Gy of 137Cs γ rays, every cell in the population is uniformly traversed by thousands of electron tracks resulting from γ-irradiation.
Calcein dye transfer
AG1522 donor cells were loaded with Calcein AM (gap junction permeable) and CellTracker Orange (gap junction impermeable) dyes. They were irradiated or sham-treated and immediately trypsinized and seeded on the top side of the insert with bystander cells growing on the bottom side of the insert. Within 1.5 to 2 h after co-culture, transfer of Calcein to bystander cells can be observed (Figure 1B) indicating that donor AG1522 cells (green and red labeled) are able to establish gap junction communication through the insert pores with the lower layer of bystander cells (green labeled).
Inhibitors
The cells were incubated 30 min before co-culture with 50 μM of 18-α-glycyrrhetinic acid (AGA), a reversible inhibitor of connexin channels and hemi-channels, or 100 μM LaCl3 (La 3+), an inhibitor of hemi-channels (Sigma, St. Louis, MO). The inhibitors were also present during the coculture period of 5 h. Under these conditions, the inhibitors resulted in minimal toxicity to AG1522 cells as verified by clonogenic survival.
Metabolic labeling
To generate additional evidence for junctional communication between irradiated and contiguous bystander cells cultured on the inserts (Figure 1) we performed metabolic labeling experiments. To this end, AG1522 donor cells were incubated for 20 h in growth medium containing MEM deficient in glucose but containing 37 MBq/mL D-[14C(U)]-glucose (NEN, Boston, MA, 100MBq/nmol). The cells were then chased in non-radioactive medium for 4 h. and were exposed to 4 Gy from 137Cs γ rays, trypsinized shortly thereafter and seeded on the top side of the insert described in Figure 1, with bystander cells cultured on the bottom side of the insert. Following 3 h of co-culture, the bystander cells were harvested, and the incorporated radioactivity was measured as cpm/ug protein. Co-culture in the presence of the gap-junction inhibitor AGA attenuated the transfer of labeled metabolites (p < 0.003). In contrast, inhibition of hemi-channels with lanthanum ions (La3+) had no effect on transfer of radioactivity (Figure 2), showing that junctional communication was involved in the transfer.
Figure 2. Transfer of 14C-labeled metabolites from irradiated to bystander cells through gap-junctions.
AG1522 cells labeled with D[14C-U]-glucose for 24 h were chased in non-radioactive medium for 4 h. They were then exposed to 4 Gy from 137Cs γ rays, harvested by trypsinization, and seeded on the top side of permeable microporous membrane inserts containing a monolayer of bystander cells growing on the bottom side of the insert. Following 4 h of co-culture in the absence or presence of 50 μM AGA or 100 μM La3+, bystander cells were harvested and incorporated radioactivity was measured as cpm/ug of protein.
Studies in progeny cells
For studies in progeny cells, bystander cells that were in co-culture with control or irradiated cells were isolated with 99.8% purity and maintained in cell culture for additional twenty to twenty five population doublings. The cells were then submitted for analyses of long-term effects on chromosomal damage using micronucleus formation as an endpoint.
Micronucleus formation
The frequency of micronuclei (a surrogate form of DNA damage that results mainly from DNA double strand breaks) was measured by the cytokinesis-block technique (Fenech and Morley 1985). After trypsinization, cells were seeded in chamber flaskettes (Nunc) in the presence of 2 μg/ml cytochalasin B (Sigma Aldrich) and incubated at 37°C. After 72 hours, the cells were rinsed in phosphate buffered solution (PBS), fixed in ethanol, stained with Hoechst 33342 solution (1 μg/ml) and viewed under a fluorescent microscope (Azzam, de Toledo and Little 2001). One thousand to 2000 binucleated cells were examined, and micronuclei in the binucleate cells only were considered for analysis. At the concentration used, cytochalasin B was non-toxic to AG1522 cells. Chi-Square analysis was used to determine whether the frequency of micronucleus formation in test cells was different from that in control sham-treated cells.
Western Blot Analysis of Proteins with 4-Hydroxynonenal (4-HNE) Adducts
The cells were lysed in chilled radio-immuno-precipitation assay (RIPA) buffer supplemented with inhibitor cocktails (P8340, P2850 and P5726, Sigma Aldrich) (Azzam, de Toledo and Little 2001) and equal amount of protein were analyzed by SDS-PAGE gels followed by immunoblotting as per standard procedures. The primary antibody to 4-HNE (#AB5605, Millipore) was used to detect lipid peroxidation. Secondary antibodies conjugated with horseradish peroxidase (Bio-Rad and Santa Cruz Biotechnology, Inc) and the enhanced chemiluminescence system from GE Healthcare was used for protein detection. Luminescence was determined by exposure to X-ray film, and densitometry was performed with an EPSON scanner and National Institutes of Health Image J software (NIH Research Services Branch, Bethesda, MD). Staining of the nitrocellulose membranes with Ponceau S Red was used to verify equal loading of samples. Hydroxyalkenals, such as 4-HNE, are among the major products of lipid peroxidation (Voulgaridou et al. 2011).
Protein oxidation
When proteins are oxidized by reactive oxygen species (ROS), some amino acids are modified generating carbonyl groups. These carbonyl groups, specifically of aldehydes or ketones, can react with 2,4-dinitrophenyl hydrazine (DNPH), which may be recognized by anti-2,4 dinitrophenol (DNP) antibodies on immunoblots (Stadtman 1993). For experiments, the OxyBlot Protein Oxidation Detection Kit (Millipore) was used. Protein samples were denaturated with 6 % (wt/vol) SDS and derivatized with DNPH. Negative controls were derivatized with a Derivatization-Control solution. After 15 min incubation at room temperature, neutralization solution (2 M Tris/30 % glycerol; vol/vol) was added and samples were immunoblotted. The DNPH-bound proteins were detected with rabbit anti-2,4-dinitrophenyl IgG (Millipore).
The role of different connexins
To investigate the role of specific connexins in propagation of the radiation induced bystander effects, HeLa adenocarcinoma cells that lack endogenous connexins were stably transfected with either empty vector (henceforth called HeLa parental) or inducible vectors containing rat Cx26, Cx32 or Cx43 cDNA (henceforth called HeLa Cx26, HeLa Cx32 or HeLa Cx43). In the transfected cells, the expressed connexins localize in the plasma membrane, and form functional gap junctions that discriminate among communicated signaling molecules (Koreen et al. 2004). To induce the connexins, the cells were incubated with 1 μg/mL doxycycline (BD Biosciences, San Jose, CA) 16 h before irradiation. We have shown that HeLa cells expressing Cx26 or Cx32 communicate via junctional channels with cocultured AG1522 cells grown on Transwell inserts (Autsavapromporn, de Toledo, Jay-Gerin, Harris and Azzam 2013). We have also shown that junctional communication occurs between HeLa Cx43 cells and AG1522 cells (Zhao, De Toledo, Hu, Hei and Azzam 2014). Hela cells transfected with different connexins were selected as an experimental system as they have been well characterized in basic studies of intercellular communication and its involvement in propagation of non-targeted effects of ionizing radiation (Autsavapromporn, de Toledo, Jay-Gerin, Harris and Azzam 2013, Elfgang et al. 1995, Koreen, Elsayed, Liu and Harris 2004, Manthey et al. 1999, Zhao, De Toledo, Hu, Hei and Azzam 2014). Whereas transfection of these cells with rat cDNA was conducive to the study of the role of specific connexins in propagation of radiation induced bystander effects (Zhao, De Toledo, Hu, Hei and Azzam 2014), this does not preclude that transfection with human connexin cDNA may alter the extent of propagation of bystander effects. Nonetheless, junctional channels consisting of mouse, rat or human connexins were shown to mediate radiation bystander effects (Autsavapromporn, Suzuki, et al. 2013, Azzam, de Toledo and Little 2001, Azzam, de Toledo and Little 2003a).
RESULTS
Propagation of the harmful effects of ionizing radiation: The time window and the role of intercellular communication by connexin channels and hemi-channels in bystander effects
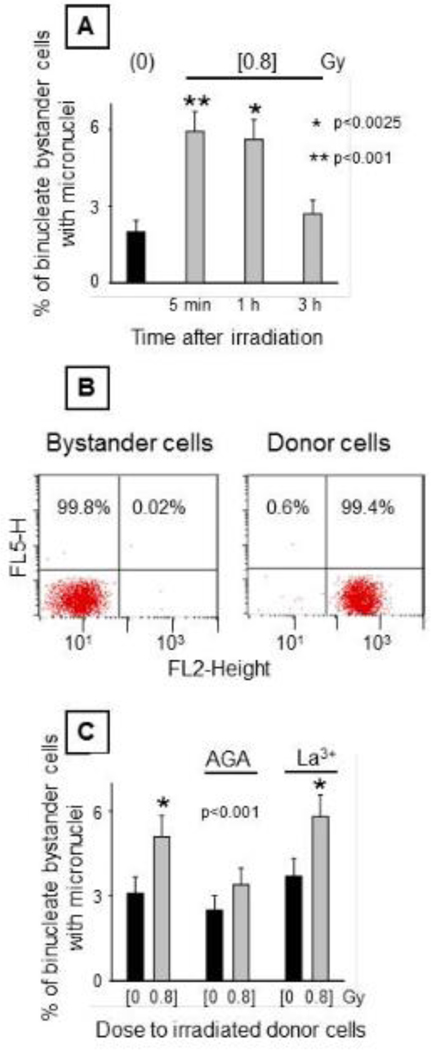
Different strategies utilizing high precision microbeams (Braby 1992, Hei et al. 1997), low fluence broad beam irradiators (Azzam, de Toledo, Gooding and Little 1998, Lehnert and Goodwin 1997, Nagasawa and Little 1992), transfer of growth medium from irradiated to non-irradiated cell cultures (Lorimore et al. 2008, Mothersill and Seymour 1997), and co-culture of irradiated and non-irradiated cell populations (Bishayee et al. 1999, Gerashchenko and Howell 2003) have been used to study radiation-induced bystander effects. Whereas medium transfer strategies allow simple and non-stressful isolation of pure bystander cells for the study of short- and long-term biological changes, this is not the case when the bystander cells are growing alongside irradiated cells in the same culture flask/plate. As described in Methods and elsewhere (Domoguaer, De Toledo and Azzam 2016), the layered cell culture system is highly effective at generating pure populations of either irradiated or bystander cells following prolonged co-cultures when the choice of the insert’s pore size and the length of the co-culture period is well characterized (Domoguaer, De Toledo and Azzam 2016). Notably, the layered cell culture strategy is conducive to the study of mechanisms underlying non-targeted effects mediated by direct communication via junctional channels or secreted factors. However, by using this co-culture system, it is important to determine the window of time during which the irradiated cells continue to transmit factors/signals capable of inducing biological changes in the bystander cells. The results shown in Figure 3 (panel A) indicate that confluent AG1522 normal human fibroblasts exposed to a mean absorbed dose of 80 cGy from 3.7 MeV α particles and harvested up 1 h after irradiation, and then seeded on the top side of the insert, induce significant increase in the level of micronuclei in bystander AG1522 cells growing on the bottom side of the insert. A 3-fold increase (n=2, p<0.002) in micronuclei, a surrogate form of chromosomal damage, was detected in bystander cells that were co-cultured with the irradiated cells. If the irradiated cells were harvested by trypsinization from their vessel 3 h after irradiation and then seeded for co-culture, the bystander effect measured by the endpoint of micronucleus formation was insignificant.
Figure 3. Biological changes in AG1522 bystander cells when co-cultured with irradiated cells.
Donor control or irradiated AG1522 cells exposed to mean absorbed dose of 80 cGy from 3.7 MeV α particles were seeded on the top side of permeable microporous membrane inserts with bystander AG1522 cells growing on their underside. After 5 h of co-culture, the bystander cells were collected. [A] Effect of elapsed time between irradiation of donor cells and co-culture with bystander cells on formation of micronuclei in bystander cells. [B] Flow cytometric analyses of non-dyed bystander cells co-cultured with dyed donor cells. AG1522 donor cells labeled with CellTracker Orange and Calcein were seeded on the top side of the inserts containing non-dyed bystander cells on the bottom side. After 5 h of co-culture, the cells were collected and submitted to Flow Cytometry to determine purity. [C] Effect of inhibitors of connexin channels and hemi channels (50 μM AGA) and hemi channels (100 μM La3+) during 5 h co-culture of bystander cells with irradiated donor cells on the induction of micronuclei in bystander cells.
To eliminate the possibility that the increase in the fraction of micronucleated cells in the bystander population may be due to irradiated cells that have crawled through the 1 μm pore of the insert, we labeled the irradiated AG1522 donor cells with CellTracker green, harvested them and seeded them on the top side of the insert with bystander AG1522 cells growing on the bottom side of the insert. At different times after initiation of the culture (6 and 24 h), bystander cells were collected and analyzed to determine if fluorescent green labeled donor cells were present in the population. Microscopic examination indicated no contamination occurred. Furthermore, flow cytometry analyses (n=4) performed following 5 h of co-culture of CellTracker Orange labeled irradiated cells and bystander cells confirmed the microscopic results and indicated a 99.8% purity of the bystander population (Figure 3, panel B), hence ruling out cross-contamination with irradiated cells.
To verify that gap junction intercellular communication was involved in the induction of micronuclei in the bystander cells, donor AG1522 cell monolayers destined for irradiation (80 cGy of 3.7 MeV α particles that result in 10% clonogenic survival) were treated either with 18-α-glycyrrhetinic acid (AGA), a reversible inhibitor of connexin channels and hemi-channels, or 100 μM LaCl3 (La 3+), an inhibitor of hemi-channels 30 min before irradiation. Within 2–5 min after irradiation, they were trypsinized and co-cultured with the bystander AG1522 cells for 5 h in the presence of either of the inhibitors. The results in Figure 3 C indicate that the increase of micronuclei frequency in bystander cells (n=4, p<0.001) was inhibited by AGA but not La3+ ions indicating that the signals leading to the DNA damaging effects were transmitted primarily through gap junctions. Therefore, these results further support the role of junctional communication in the propagation of radiation induced bystander effects. They also indicate that in spite of the experimental manipulations of rinsing irradiated cells with PBS followed by trypsinization and resuspension in growth medium, a significant bystander effect is still expressed: donor irradiated cells continue to propagate signals leading to bystander effects for at least 1 h after irradiation.
Radiation quality and the propagation of bystander effects
In previous studies, we have shown that bystander AG1522 normal human fibroblasts or RKO36 human colon carcinoma incubated in medium harvested from their respective counterparts following exposure to low LET X rays (250 kVp), 137Cs γ rays or 50 keV electrons, or high LET 1000 MeV/u Fe ions failed to show a significant increase in micronucleus formation or decrease in clonogenic survival (Sowa et al. 2010). In contrast, bystander AG1522 cells recipient of growth medium from α particle irradiated counterparts experienced a significant decrease in clonogenic survival (Sowa, Goetz, Baulch, Pyles, Dziegielewski, Yovino, Snyder, de Toledo, Azzam and Morgan 2010). Here, we extend these studies using the layered cell culture strategy where the irradiated and bystander cells are growing in the same culture vessel and are coupled by gap junctions, rather than using a protocol where growth medium is transferred from one culture vessel to another culture vessel (Lorimore, Chrystal, Robinson, Coates and Wright 2008, Mothersill and Seymour 1998).
Exposure of AG1522 confluent density-inhibited fibroblasts to mean absorbed doses of 80 cGy of 3.7 MeV α particles (LET ~ 109 keV/μm), 200 cGy of 1000 MeV/u 56Fe26+ ions (LET ~ 151 keV/μm), or 400 cGy of 137Cs γ rays (LET ~ 0.9 keV/μm) that result in clonogenic survival levels of ~ 10% caused, in all cases, the propagation of events leading to a significant increase in micronuclei formation in the contiguous AG1522 bystander cells (n=5, p<0.03 in case of 3.7 MeV α particles; n=4, p<0.003 in case of 1 GeV/u Fe ions; n=2, p<0.003 in case of 137Cs γ rays) (Figure 4A). Therefore, following exposures to such toxic doses of radiation (10% survival dose), significant increases in micronucleus formation are induced in the coupled bystander cells. As was the case for α particles, the spread of harmful effects propagated from cells exposed to 1000 MeV/u 56Fe26+ ions to bystander cells is gap junction dependent (n=4, p<0.05) and not hemi-channel dependent (Figure 4B). Therefore, both low and high LET radiations propagate harmful effects following cellular exposures to moderate/high mean absorbed doses.
Figure 4. Micronucleus formation in AG1522 bystander cells co-cultured with AG1522 cells exposed to different types of radiation that differ in their LET.
[A] Donor control or irradiated cells exposed to 3.7 MeV α particles (80 cGy), 1000 MeV/u iron ions (2 Gy) or 137Cs γ rays (4 Gy) were seeded, immediately after irradiation, on the top side of permeable microporous membrane inserts with bystander AG1522 cells growing on their underside. Following 5 h of co-culture, the bystander cells were harvested and assayed for micronuclei formation. [B] Effect of inhibitors of connexin channels and hemi channels (50 μM AGA) and hemi channels (100 μM La3+) during 5 h co-culture of bystander cells with irradiated (2 Gy of 1 GeV/u 56Fe ions) donor cells on induction of micronuclei in bystander cells.
The layered cell culture strategy reveals that cultures exposed to low doses of α particles also propagate signals leading to oxidative effects in bystander cells
At a mean absorbed dose of 80 cGy of α particles, most of the cells in the exposed culture are traversed by multiple tracks of radiation (Gonon, Groetz., detoledo, Howell, Fromm and Azzam 2013). To test if cellular exposure to lower mean absorbed doses would still result in propagation of harmful bystander effects by using the layered cell culture strategy, donor AG1522 cells were irradiated with mean doses of 5 or 10 cGy of 3.7 MeV α particles and were co-cultured with AG1522 bystander cells within 5 min after the exposure. In contrast to earlier studies where DNA damaging events were detected in situ in bystander AG1522 cells in cultures exposed to doses as low as 0.2 cGy of α particles but no trypsinization or co-culture was involved (Azzam, de Toledo and Little 2001, Gonon, Groetz., detoledo, Howell, Fromm and Azzam 2013), we were not able to detect significant increases in the frequency of micronuclei formation in the isolated bystander cells following 5 h of co-culture. However, an altered pattern of expression of carbonylated proteins and proteins conjugated to 4-hydroxynonenal (4-HNE) was observed. The representative results in Figure 5A show that the overall level of protein carbonylation was increased (1.4–1.9 fold) reinforcing the observation that the bystander cells have experienced oxidative stress, which can lead to DNA damage (Cadet et al. 2012). Interestingly, lipid peroxidation (Figure 5 B) showed a mixed pattern of 4-HNE conjugation in the bystander cells. Some proteins were more conjugated (A) and others were less conjugated (B). These results show that the layered cell culture system is a sensitive strategy for detecting bystander effects resulting in oxidative stress even under conditions when only 30 or 50 % of donor cells have experienced a nuclear traversal. Notably, the results are consistent with earlier in vitro and in vivo studies showing extensive protein carbonylation and lipid peroxidation long after exposure to very low fluences of or HZE particles (Buonanno, De Toledo, Pain and Azzam 2011, Gonon, Groetz., detoledo, Howell, Fromm and Azzam 2013, Li et al. 2014)
Figure 5. Oxidative stress in AG1522 bystander cells.
[A] Protein carbonylation, and [B] lipid peroxidation (i.e. 4-HNE protein adduct accumulation) as revealed by immunoblotting in confluent bystander cells that had been in co-culture for 5 h with cell populations exposed to mean doses of 0, 5 or 10 cGy of 3.7 MeV α particles using the layered cell culture strategy. Relative to controls, bystander cells exhibited different levels of protein cabonylation and lipid peroxidation.
Increased levels of spontaneous DNA damage in distant progeny of bystander cells is dependent on early signaling events propagated by irradiated parental cells through gap junctions
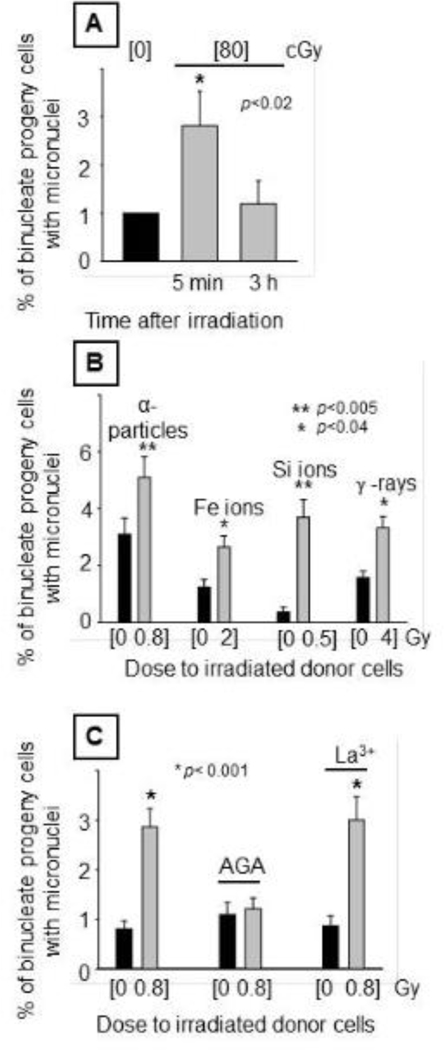
In previous studies, we reported that the progeny of bystander AG1522 cells that were co-cultured with AG1522 cells exposed to 1000 MeV/u, 56Fe26+ or 600 MeV/u 28Si14+ exhibited after twenty population doublings, reduced cloning efficiency, increased frequency of micronuclei, higher levels of protein oxidation and lipid peroxidation, decreased activity of antioxidant enzymes, inactivation of aconitase enzyme and altered translation of proteins encoded by mitochondrial DNA (Buonanno, De Toledo, Pain and Azzam 2011). In those studies, the irradiated cells were co-cultured with bystander cells within 5–10 min after irradiation. Here, we test whether such long-term stressful effects also occur after exposure to α particles and whether gap junction intercellular communication between the parental cells impacts changes in distant progeny of the bystander cells. To this end, bystander AG1522 cells were co-cultured with donor AG1522 cells that were derived from cultures exposed to 80 cGy of 3.7 MeV α particles and harvested for co-culture with bystander cells within 5 minutes of exposure or 3 h later. Consistent with the results in Figure 3A, initial experiments revealed an increase (p<0.02) in the frequency of spontaneous micronucleus formation in progeny cells (25 population doublings later) when the parental cells were co-cultured with cells harvested within 5 min after irradiation and not 3 h later, indicating that the signaling events leading to persistent DNA damage in progeny of bystander cells decay with time after irradiation (Figure 6A). Subsequent experiments (Figure 6B), confirmed (n=9) the results described in Figure 6A, and also showed similar persistent harmful effects when bystander cells were initially co-cultured within minutes after irradiation with donor cells derived from density-inhibited AG1522 cells exposed to a mean dose of 200 cGy of 1000 MeV/u Fe ions (n=4, p<0.005), 50 cGy of 600 MeV/u Si ions (n=3, p<0.005), or 400 cGy of 137Cs γ rays (n=2, p<0.04) (Figure 6B).
Figure 6. Micronucleus formation in distant progeny of bystander cells.
Control or irradiated donor AG1522 cells were seeded on the top side of permeable microporous membrane inserts with bystander AG1522 cells growing on the bottom side of the inserts. Following 5 h of co-culture, bystander cells were harvested, sub-cultured for 25 population doublings and assayed for micronuclei formation. [A] Effect of elapsed time between irradiation of donor cells (80 cGy from 3.7 MeV α particles) and co-culture with bystander cells. [B] Genomic instability induced in distant progeny of bystander cells whose parental cells were co-cultured with cells from cultures exposed to 3.7 MeV α particles (80 cGy), 1000 MeV/u Fe ions (200 cGy), 600 MeV/u Si ions (50 cGy), or 137Cs γ rays (400 cGy). [C] Effect of inhibitors of intercellular communication: 50 μ M AGA or 100 μ M La3+.
To test the role of gap-junction communication in the persistence of stressful effects in progeny of bystander cells, irradiated AG1522 cells were harvested within 5 min after exposure to a mean dose of 80 cGy from α particles, and the 5 h co-culture between these parental irradiated cells and bystander AG1522 cells occurred in the presence or absence of AGA or La3+ ions. Twenty five populations later, an enhanced level of micronucleus formation (n=3, p<0.001) was observed in progeny of the bystander cells (Figure 6C), which is consistent with the oxidative stress observed by Buonanno et al. (Buonanno, De Toledo, Pain and Azzam 2011). This increase in chromosomal damage (n=3, p<0.001) was also observed in progeny cells whose parental cells were derived from La3+-treated co-cultures, but not AGA-treated co-cultures (Figure 6C), indicating that this persistent harmful effect was gap junction dependent. Together, these results indicate that gap junction intercellular communication is not only a major pathway for the propagation of signaling events leading to induction of early harmful effects in bystander cells, but also for their persistence in their distant progeny.
Connexins forming channels with different permeability properties induce differential long-term biological changes in distant progeny of bystander cells
In previous studies, we have shown that intercellular communication between irradiated and bystander normal human fibroblasts through Cx43 junctional channels plays a major role in the spread of radiation-induced bystander effects (Azzam, De Toledo, Harris, Ivanov, Zhou, Amundson, lieberman and Hei 2013). Furthermore, gap junctions consisting of Cx26, but not Cx32, promoted the expression of stressful radiation induced bystander effects assessed 6–26 h after co-culture (Zhao, De Toledo, Hu, Hei and Azzam 2014). To investigate the role of intercellular communication through connexin channels with specific permeability properties in events that may modulate the induction of DNA damage in distant progeny of AG1522 fibroblasts, we used irradiated HeLa cells as a model system (Koreen, Elsayed, Liu and Harris 2004). HeLa cells, which lack endogenous functional connexins (Mesnil et al. 1995), were stably transfected with either empty vector (henceforth called HeLa parental) or inducible vectors containing Cx26, Cx32 or Cx43 cDNA (henceforth called HeLa Cx26, HeLa Cx32 or HeLa Cx43). In the transfected cells, the expressed connexins localize in the plasma membrane, and form functional gap junctions (Koreen, Elsayed, Liu and Harris 2004). The HeLa cells were exposed to a mean absorbed dose of 50 cGy from 3.7 MeV α particles that results in ~50% clonogenic survival (Autsavapromporn, de Toledo, Jay-Gerin, Harris and Azzam 2013), and within 5 min after exposure, the irradiated cells were intimately co-cultured with bystander AG1522 cells using the layered cell culture system. Bystander AG1522 cells co-cultured with α-particle-irradiated AG1522 cells were maintained in parallel. After 6 h, the bystander cells were isolated with high purity and propagated for 24 population doublings.
Similar to the results in Figure 6, a significant increase in the fraction of progeny cells harboring micronuclei occurred when parental bystander AG1522 cells were co-cultured with AG1522 cells originating from cultures exposed to 50 cGy of α particles (n=5, p<0.05). In contrast, co-culture of bystander AG1522 cells with irradiated HeLa parental cells (lacking endogenous connexins) resulted in no significant change in the fraction of bystander AG1522 progeny with micronuclei (n=5, p=0.6), which is consistent with the absence of an effect when AG1522 bystanders were assessed for micronucleus formation at 6 h following co-culture with irradiated HeLa cells (Zhao, De Toledo, Hu, Hei and Azzam 2014).
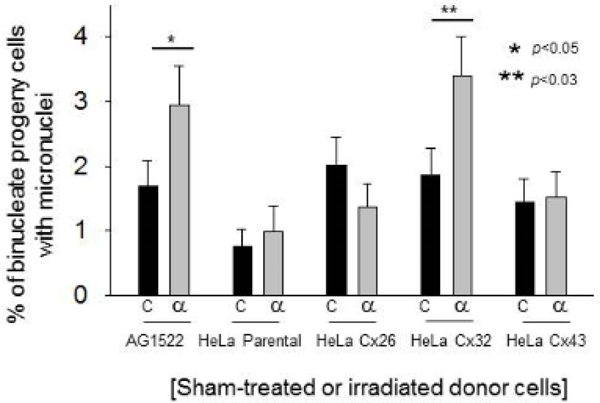
In initial studies examining early bystander effects, inducible expression of gap junctions composed of Cx26 in irradiated HeLa cells enhanced the induction of micronuclei in bystander cells (p<0.01) and reduced the co-culture time necessary for manifestation of the effect. In contrast, expression of Cx32 conferred protective effects (Zhao, De Toledo, Hu, Hei and Azzam 2014). Further, the role of Cx43 channels in mediating bystander effects within hours of traversal of a small fraction of cells in a culture by α particles delivered by broad beam or microbeam irradiators was described in reports from several laboratories (Azzam, De Toledo, Harris, Ivanov, Zhou, Amundson, lieberman and Hei 2013). To investigate the long-term outcome of the bystander effect mediated by different connexin channels, we evaluated spontaneous micronucleus formation in progeny of bystander cells AG1522 cells that were co-cultured with irradiated or sham-treated HeLa Cx26, HeLa Cx32 or HeLa Cx43. The bystanders were isolated following 6 h of co-culture, they were propagated for 24 population doublings and then examined for micronucleus formation. There was no difference in micronucleus formation in progeny of AG1522 bystanders that were co-cultured with irradiated or sham-treated HeLa Cx26 cells (n=5, p=0.2) or HeLa Cx43 cells (n=5, p=0.8) (Figure 7). In contrast, progeny of bystander cells that were co-cultured with irradiated HeLa Cx32 cells, which did not show an increase in micronucleus formation within 6 h following coculture (Zhao, De Toledo, Hu, Hei and Azzam 2014), exhibited an enhanced genomic instability reflected in a greater fraction of cells harboring micronuclei (n=5, p<0.03) (Figure 7). Together, these results support a critical role for gap junction permeability in determining the nature and magnitude of induced early bystander effects and their persistence in progeny cells.
Figure 7. The effect of the connexin expressed in irradiated cells on genomic instability in distant progeny of bystander cells.
Micronucleus formation in the progeny of bystander AG1522 cells that were co-cultured with either AG1522 cells, HeLa cells devoid of functional connexins, or HeLa cells expressing either Cx26, Cx32 or Cx43 within minutes following exposure to mean absorbed doses of 0 or 50 cGy of 3.7 MeV α particles. Micronuclei formation was assayed in progeny cells that underwent 24 population doublings.
DISCUSSION
In vitro and in vivo observations made over the last 25 years have provided strong evidence indicating that molecular events leading to various biological effects, including genetic damage, can be transmitted from irradiated to non-irradiated cells. The phenomenon occurs in a variety of cell types of human and rodent origin and involves gap junction intercellular communication, oxidative metabolism, secreted factors and DNA repair (Azzam, De Toledo, Harris, Ivanov, Zhou, Amundson, lieberman and Hei 2013, Hamada, Matsumoto, Hara and Kobayashi 2007, Hei, Zhou, Chai, Ponnaiya and Ivanov 2011, Held 2009, Little 2003, Lorimore et al. 2003, Morgan 2003a, Mothersill and Seymour 2004, Prise and O’Sullivan 2009). Here the focus has been on further characterizing the impact of junctional communication between irradiated cells and contiguous bystander cells in the first few hours after irradiation on the long term consequences of this direct type of communication. Specifically, we have examined the impact of intercellular communication by connexin channels formed by Cx26, Cx32 or Cx43 on DNA damage in distant progeny of the bystander cells (20–25 population doublings). In addition, we have investigated the effect of radiation quality on bystander effects induced following exposures to low or high LET radiations at doses that result in significant cell killing. To this end, we used a layered cell culture strategy that facilitates the understanding of intercellular communication, while permitting a simplified approach that permits metabolic coupling of cells and offering the advantage of separation of two heterotypic cell populations (adenocarcinoma HeLa cells and normal human AG1522 fibroblasts) grown on either side of a permeable microporous membrane insert in the same environmental conditions. The protocol offers the added advantage of allowing various modes of intercellular communication to operate through the membrane pores. Though physically separated, the two cell populations used in the studies were metabolically coupled through gap-junctional channels (Figures 1 and 2), while communication via secreted elements was not excluded. Notably, using our strategy, the two cell populations (irradiated or bystander) can be easily isolated with high purity (99.8%), without fluorescent tagging and/or the stress of cell sorting (Figure 3B).
Our use of the layered cell culture strategy has shown that the effects of signals capable of causing chromosomal damage in recipient bystander cells can be detected for at least one hour after irradiation of the donor cells (Figure 3A). Although by 3 h, we were not able to observe increased micronuclei frequency in the bystander cells, it is possible that for other endpoints, biological changes can still be detected. The increased micronuclei frequency that we observed in the bystander cells when irradiated and bystander normal AG1522 cells were co-cultured together for 5 hours was dependent on the presence of functional gap junctional communication (Figure 3C). Incubation of the co-culture with the gap junction inhibitor AGA (Davidson et al. 1986) inhibited the effect, whereas incubation with an inhibitor of hemi-channels (La3+) (John et al. 1999) was ineffective in its prevention (Figure 3C). Hemi-channels contribute to intercellular communication by allowing interchange of metabolites between cells via their surrounding medium. Additional experiments where the donor irradiated cells and bystander cells are incubated with the same concentration of La3+ used here but for longer periods of time, or with higher concentrations of the drug (Wang et al. 2013) may yield different outcome; however, interpretations of the results may be complicated due to toxic effects.
The results reported here strongly indicate that bystander effects are not solely a low dose phenomenon as was originally thought (Azzam, de Toledo and Little 2001, Mothersill et al. 2000, Nagasawa and Little 1992, Seymour and Mothersill 2000, Shao, Stewart, et al. 2003, Zhou et al. 2000). Our study shows that harmful bystander effects are induced following exposures to both low (Figure 5) as well as moderate/high doses of radiations that differ in their quality (Figure 4). Cellular exposure to either low LET (137Cs γ rays) or high LET radiations (3.7 MeV α particles, 600 MeV/u 28Si ions, and 1000 MeV/u 56Fe ions) resulted in generation of signals that induce DNA damage in bystander cells (Figure 4). Clearly, these results have relevance to both radiotherapy with external beam radiation or radionuclide therapies and to issues related to space exploration. Although, in the latter case, astronauts are likely to be exposed during space travel to very low fluences of HZE particles received at very low dose rate rather than to high mean absorbed doses (Norbury et al. 2016). Notably, the bystander effects observed following exposure to low LET γ rays are consistent with in vivo effects observed by others (Chai et al. 2013, Desai et al. 2016, Mancuso, Pasquali, Leonardi, Tanori, Rebessi, Di Majo, Pazzaglia, Toni, Pimpinella, Covelli and Saran 2008).
Since genomic instability is considered a predisposition factor for carcinogenesis (Sigurdson and Jones 2003), the persistence of DNA damage in progeny of bystander cells following exposure to radio-therapeutic doses (Figures 6–7) is likely to have a role in the emergence of second primary cancers or to the proliferation of dormant tumor cells in radiotherapy patients. Importantly, expression of this genomic instability is strongly dependent on the type of junctional communication between the irradiated and bystander cells in the first few hours after exposure to radiation (Figure 6).
In earlier studies, we have shown that expression of Cx26 or Cx43 channels propagated radiation effects that resulted in excess DNA damage in bystander cells when assayed within 24 h after irradiation (Azzam, de Toledo and Little 2001, Zhao, De Toledo, Hu, Hei and Azzam 2014). In contrast, intercellular communication through Cx32 channel helped prevent the expression of such effect in the bystander cells (Zhao, De Toledo, Hu, Hei and Azzam 2014). Here, we show that in the distant progeny of the bystanders (24 population doublings later), opposing results to the early effects were observed (Figure 7). Likely, the harmful effects propagated by Cx26 or Cx43 channels resulted through passaging of the bystander cells in elimination of damaged cells, perhaps though cell death or permanent cell cycle arrest. In contrast, bystander cells coupled with irradiated cells through Cx32 channels, which appeared to be protected during early time, must have harbored a perturbation (e.g., in oxidative metabolism) that resulted in enhanced damaging effects with serial passaging. Analyses of DNA damage as a function of passaging of bystander cells whose parental cells were coupled with cells expressing either Cx26, Cx32 or Cx43 would be informative. Together with analyses of the epigenome, oxidative metabolism and different modes of cell death, these studies will enhance understanding of the mechanisms involved. Furthermore, experiments wherein HeLa Cx26 cells, HeLa Cx32 or HeLa Cx43 cells are co-cultured with AG1522 cells in which Cx26, Cx32, or Cx43 are knocked down would shed light on the specificity of these connexins in short- and long-term outcomes of bystander effects and the ways by which they mediate their effects.
Gap junctions exist between different types of normal cells and in tumor cells, particularly at the metastatic stage (Cronier et al. 2009), and specific connexins exist in certain cells in tissues and not in others (Bruzzone and Meda 1988). Therefore, understanding the role of junctional permeability in propagation of bystander effects has profound implications to both radioprotection and radiotherapy. Identifying the molecules communicated through the various connexin channels under radiation stress conditions would be informative for counteracting adverse health effects caused by exposure to ionizing radiation. Primarily, such knowledge is basic to the understanding of intercellular communication under various stress conditions.
ACKNOWLEDGMENT
We are ever grateful to Bill Morgan’s guidance through the years.
We thank Drs. Adam Rusek, Michael Sivertz, Peter Guida, and the late I-Hung Chang, and their colleagues, for their support during the experiments at the NASA Space Radiation Laboratory. We also thank Drs. Roger W. Howell, Géraldine Gonon, Jie Zhang, Narongchai Autsavapromporn, Nicholas W. Colangelo and Jason D. Domogauer in the RUTGERS Division of Radiation Research for their support during experiments and for scientific exchanges. Grant CA049062 from the National Institutes of Health, and grants NNJ06HD91G and NNX15AD62G from the National Aeronautics and Space Administration supported this research.
Footnotes
DISCLOSURES
The authors declare that they have no competing or conflicting interests.
REFERENCES
- Autsavapromporn N, de Toledo SM, Buonanno M, Jay-Gerin JP, Harris AL, Azzam EI. 2011. Intercellular communication amplifies stressful effects in high-charge, high-energy (HZE) particle-irradiated human cells. J Radiat Res (Tokyo).52:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autsavapromporn N, de Toledo SM, Jay-Gerin JP, Harris AL, Azzam EI. 2013. Human Cell Responses to Ionizing Radiation are Differentially Affected by the Expressed Connexins. J Radiat Res (Tokyo).54:251–259. Epub 2012 Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autsavapromporn N, de Toledo SM, Little JB, Jay-Gerin J-P, Harris AL, Azzam EI. 2011. The role of gap junction communication and oxidative stress in the propagation of toxic effects among high-dose-particle-irradiated human cells. Radiat Res. Mar;175:347–357. Epub 2011/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autsavapromporn N, Suzuki M, Funayama T, Usami N, Plante I, Yokota Y, Mutou Y, Ikeda H, Kobayashi K, Kobayashi Y, et al. 2013. Gap junction communication and the propagation of bystander effects induced by microbeam irradiation in human fibroblast cultures: the impact of radiation quality. Radiat Res. Oct;180:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad WA, Locke D, Koreen IV, Harris AL. 2006. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J Biol Chem.281:16727–16739. [DOI] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Gooding T, Little JB. 1998. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res.150:497–504. [PubMed] [Google Scholar]
- Azzam EI, De Toledo SM, Harris AL, Ivanov V, Zhou H, Amundson SA, lieberman HB, Hei TK. 2013. The Ionizing Radiation-Induced Bystander Effect: Evidence, Mechanism and Significance. In: Pathobiology of Cancer Regimen-Related Toxicities. New York, NY: Springer. p. 42–68. [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. 2001. Direct evidence for the participation of gapjunction mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to non-irradiated cells. Proc Natl Acad Sci USA.98:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. 2003a. Expression of connexin43 is highly sensitive to ionizing radiation and environmental stresses. Cancer Res.63:7128–7135. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. 2003b. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. Oct 13;22:7050–7057. [DOI] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Spitz DR, Little JB. 2002. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particleirradiated normal human fibroblast cultures. Cancer Res. Oct 1;62:5436–5442. [PubMed] [Google Scholar]
- Bevans CG, Kordel M, Rhee SK, Harris AL. 1998. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem.273:2808–2816. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Rao DV, Howell RW. 1999. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model. Radiat Res.152:88–97. [PMC free article] [PubMed] [Google Scholar]
- Braby LA. 1992. Microbeam studies of the sensitivity of structures within living cells. Scanning Microsc. Mar;6:167–174; discussion 174–165. [PubMed] [Google Scholar]
- Brisset AC, Isakson BE, Kwak BR. 2009. Connexins in vascular physiology and pathology. Antioxid Redox Signal. Feb;11:267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AL. 2015. Dr. William F. Morgan 1952–2015. Int J Radiat Biol.91:913. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Meda P. 1988. The gap junction: a channel for multiple functions? Eur J Clin Invest.18:444–453. [DOI] [PubMed] [Google Scholar]
- Buonanno M, de Toledo SM, Azzam EI. 2011. Increased frequency of spontaneous neoplastic transformation in progeny of bystander cells from cultures exposed to denselyionizing radiation. PloS one.6: art. no. e21540. Epub 2011. June 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BuonannM, De ToledM, PaiD, AzzaI. 2011. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat Res.175:405–415. Epub 2011 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J, Ravanat JL, TavernaPorro M, Menoni H, Angelov D. 2012. Oxidatively generated complex DNA damage: tandem and clustered lesions. Cancer Lett. Dec 31;327:5–15. [DOI] [PubMed] [Google Scholar]
- Chai Y, Calaf GM, Zhou H, Ghandhi SA, Elliston CD, Wen G, Nohmi T, Amundson SA, Hei TK. 2013. Radiation induced COX-2 expression and mutagenesis at non-targeted lung tissues of gpt delta transgenic mice. Br J Cancer. Jan 15;108:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton DE, Sephton R. 1991. A relationship between microdosimetric spectra and cell survival for high-LET irradiation. Int J Radiat Biol.59:447–457. [DOI] [PubMed] [Google Scholar]
- Coates PJ, Lorimore SA, Wright EG. 2004. Damaging and protective cell signalling in the untargeted effects of ionizing radiation. Mutat Res. Dec 2;568:5–20. [DOI] [PubMed] [Google Scholar]
- Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. 2009. Gap junctions and cancer: new functions for an old story. Antioxid Redox Signal. Feb;11:323–338. [DOI] [PubMed] [Google Scholar]
- Cucinotta F, Nikjoo H, Goodhead DT. 1998. The effect of delta rays on the number of particle traversals per cell in laboratory and space exposures. Radiat Res.150:115–119. [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM, Harley EH. 1986. Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun. Jan 14;134:29–36. [DOI] [PubMed] [Google Scholar]
- Desai S, Kobayashi A, Konishi T, Oikawa M, Pandey BN. 2014. Damaging and protective bystander cross-talk between human lung cancer and normal cells after proton microbeam irradiation. Mutat Res. May-Jun;763–764:39–44. [DOI] [PubMed] [Google Scholar]
- Desai S, Srambikkal N, Yadav HD, Shetake N, Balla MM, Kumar A, Ray P, Ghosh A, Pandey BN. 2016. Molecular Understanding of Growth Inhibitory Effect from Irradiated to Bystander Tumor Cells in Mouse Fibrosarcoma Tumor Model. PloS one.11:e0161662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domoguaer J, De Toledo SM, Azzam EI. 2016. A Mimic of the Tumor Microenvironment: a Simple Method for Generating Enriched Cell Populations and Investigating Intercellular Communication. Journal of Visualized Experiments. Epub 9/20/2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. 1995. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol.129:805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore E, Lao XY, Kapadia R, Redpath JL. 2009. Threshold-type dose response for induction of neoplastic transformation by 1 GeV/nucleon iron ions. Radiat Res. Jun;171:764–770. Epub 2009/07/08. [DOI] [PubMed] [Google Scholar]
- Elsayed W, Harris AL. 2004. Selective permeability of connexin channels among inositol phosphates. Biophys J.86:583a. [Google Scholar]
- Elsayed WA, Koreen IV, Liu YL, Harris AL. 2004. Inositol phosphates are selectively permeable through heteromeric connexin channels. Mol Biol Cell.15:183a. [Google Scholar]
- Fenech M, Morley AA. 1985. Measurement of micronuclei in lymphocytes. Mutat Res.147:29–36. [DOI] [PubMed] [Google Scholar]
- Gaillard S, Pusset D, de Toledo SM, Fromm M, Azzam EI. 2009. Propagation distance of the alpha-particle-induced bystander effect: the role of nuclear traversal and gap junction communication. Radiat Res. May;171:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko BI, Howell RW. 2003. Flow cytometry as a strategy to study radiationinduced bystander effects in co-culture systems. Cytometry.54A:1–7. [DOI] [PubMed] [Google Scholar]
- Gonon G, Groetz JE, detoledo SM, Howell RW, Fromm M, Azzam EI. 2013. Non-Targeted Stressful Effects in Normal Human Fibroblast Cultures Exposed to Low Fluences of High Charge, High Energy (HZE) Particles: Kinetics of Biologic Responses and Significance of Secondary Radiations. Radiat Res. 2013 Mar 6;179:444–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. 1953. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. Dec;26:638–648. [DOI] [PubMed] [Google Scholar]
- Hamada N, Hei TK, Bouffler S, Woloschak GE. 2016. Morgan William F. (1952–2015). Mutat Res.770, Part B:387–388. [Google Scholar]
- Hamada N, Matsumoto H, Hara T, Kobayashi Y. 2007. Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects. J Radiat Res (Tokyo). Mar;48:87–95. [DOI] [PubMed] [Google Scholar]
- Harris AL. 2001. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. Aug;34:325–472. [DOI] [PubMed] [Google Scholar]
- Harris AL. 2007. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. May-Jun;94:120–143. Epub 2007/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL, Contreras JE. 2014. Motifs in the permeation pathway of connexin channels mediate voltage and Ca (2+) sensing. Frontiers in physiology.5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei TK, Wu LJ, Liu SX, Vannais D, Waldren CA, Randers-Pehrson G. 1997. Mutagenic effects of a single and an exact number of alpha particles in mammalian cells. Proc Natl Acad Sci U S A.94:3765–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. 2011. Radiation induced non-targeted response: mechanism and potential clinical implications. Current molecular pharmacology.4 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held KD. 2009. Effects of low fluences of radiations found in space on cellular systems. Int J Radiat Biol. May;85:379–390. [DOI] [PubMed] [Google Scholar]
- Hong X, Sin WC, Harris AL, Naus CC. 2015. Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget. May 4. ICRU. 1993. Stopping Powers and Ranges for Protons and Alpha Particles. (Report 49). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain MR, Li M, Chen W, Liu T, De Toledo SM, Pandey BN, Li H, Rabin BM, Azzam EI. 2011. In vivo space radiation-induced non-targeted responses: late effects on molecular signaling in mitochondria. Current molecular pharmacology .4:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. 1999. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. Jan 1;274:236–240. [DOI] [PubMed] [Google Scholar]
- Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG. 1992. Transmission of chromosomal instability after plutonium-particle irradiation [see comments]. Nature.355:738–740. [DOI] [PubMed] [Google Scholar]
- Koreen IV, Elsayed WA, Liu YJ, Harris AL. 2004. Tetracycline-regulated expression enables purification and functional analysis of recombinant connexin channels from mammalian cells. Biochem J. Oct 1;383:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert BE, Goodwin EH. 1997. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res.57:2164–2171. [PubMed] [Google Scholar]
- Li M, Gonon G, Buonanno M, Autsavapromporn N, De Toledo SM, Pain D, Azzam EI. 2014. Health Risks of Space Exploration: Targeted and Non-targeted Oxidative Injury by High Charge and High Energy Particles. Antioxidants and Redox Signaling. 2014 Mar 20;20:1501–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. 2011. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. Mar 1;71:1550–1560. [DOI] [PubMed] [Google Scholar]
- Little JB. 2003. Genomic instability and bystander effects: a historical perspective. Oncogene. Oct 13;22:6978–6987. [DOI] [PubMed] [Google Scholar]
- Locke D, Stein T, Davies C, Morris J, Harris AL, Evans WH, Monaghan P, Gusterson B. 2004. Altered permeability and modulatory character of connexin channels during mammary gland development. Exp Cell Res. Aug 15;298:643–660. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. 2008. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. Oct 1;68:8122–8126. Epub 2008/10/03. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Coates PJ, Wright EG. 2003. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. Oct 13;22:7058–7069. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, Pazzaglia S, Toni MP, Pimpinella M, Covelli V, et al. 2008. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc Natl Acad Sci U S A. Aug 26;105:12445–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey D, Bukauskas F, Lee CG, Kozak CA, Willecke K. 1999. Molecular cloning and functional expression of the mouse gap junction gene connexin-57 in human HeLa cells. J Biol Chem. May 21;274:14716–14723. [DOI] [PubMed] [Google Scholar]
- Marder BA, Morgan WF. 1993. Delayed chromosomal instability induced by DNA damage. Mol Cell Biol. Nov;13:6667–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PP, Bertram JS, Loewenstein WR. 1986. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. Jan 17;44:187–196. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, Yamasaki H. 1995. Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer Res. Feb 1;55:629–639. Epub 1995/02/01. [PubMed] [Google Scholar]
- Morgan WF. 2003a. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene. Oct 13;22:7094–7099. [DOI] [PubMed] [Google Scholar]
- Morgan WF. 2003b. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. May;159:567–580. [DOI] [PubMed] [Google Scholar]
- Morgan WF. 2003c. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. May;159:581–596. [DOI] [PubMed] [Google Scholar]
- Morgan WF, Bair WJ. 2013. Issues in low dose radiation biology: the controversy continues. A perspective. Radiat Res. May;179:501–510. [DOI] [PubMed] [Google Scholar]
- Morgan WF, Sowa MB. 2015. Non-targeted effects induced by ionizing radiation: mechanisms and potential impact on radiation induced health effects. Cancer Lett. Jan 01;356:17–21. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Kadhim MA, O’Reilly S, Papworth D, Marsden SJ, Seymour CB, Wright EG. 2000. Dose- and time-response relationships for lethal mutations and chromosomal instability induced by ionizing radiation in an immortalized human keratinocyte cell line. Int J Radiat Biol. Jun;76:799–806. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. 1997. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol.71:421–427. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. 1998. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: Evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res.149:252–262. [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. 2004. Radiation-induced bystander effects--implications for cancer. Nat Rev Cancer. Feb;4:158–164. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Cremesti A, Kolesnick R, Fuks Z, Little JB. 2002. Involvement of membrane signaling in the bystander effect in irradiated cells. Cancer Res. May 1;62:2531–2534. [PubMed] [Google Scholar]
- Nagasawa H, Little JB. 1992. Induction of sister chromatid exchanges by extremely low doses of-particles. Cancer Res.52:6394–6396. [PubMed] [Google Scholar]
- Neti PV, de Toledo SM, Perumal V, Azzam EI, Howell RW. 2004. A multi-port lowfluence alpha-particle irradiator: fabrication, testing and benchmark radiobiological studies. Radiat Res. Jun;161:732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikjoo H, Uehara S, Khvostunov IG, Cucinotta FA, Wilson WE, Goodhead DT. 2001. Monte Carlo track structure for radiation biology and space applications. Phys Med.17 Suppl 1:38–44. [PubMed] [Google Scholar]
- Norbury JW, Schimmerling W, Slaba TC, Azzam EI, Badavi FF, Baiocco G, Benton E, Bindi V, Blakely EA, Blattnig SR, et al. 2016. Galactic cosmic ray simulation at the NASA Space Radiation Laboratory. Life sciences in space research. Feb;8:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev AL, Cucinotta FA. 2006. Nuclear fragmentation and the number of particle tracks in tissue. Radiat Prot Dosimetry.122:354–361. Epub 2007/01/31. [DOI] [PubMed] [Google Scholar]
- Prise KM, O’Sullivan JM. 2009. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. May;9:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaphorst GP, Azzam EI, Borsa J, Sargent MD. 1984. Modification by anisotonic treatment of repair and fixation of radiation damage in cell killing and transformation. Br J Cancer Suppl.6:239–242. [PMC free article] [PubMed] [Google Scholar]
- Rueckert RR, Mueller GC. 1960. Effect of oxygen tension on HeLa cell growth. Cancer Res. Jul;20:944–949. [PubMed] [Google Scholar]
- Saez JC, Leybaert L. 2014. Hunting for connexin hemichannels. FEBS Lett. Apr 17;588:1205–1211. [DOI] [PubMed] [Google Scholar]
- Schubert AL, Schubert W, Spray DC, Lisanti MP. 2002. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. May 7;41:5754–5764. [DOI] [PubMed] [Google Scholar]
- Schwartz JL. 2016. Dr. William Francis (Bill) Morgan (1952–2015). Environ Mol Mutagen. Jun;57:416–417. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. 2000. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat Res. May;153:508–511. [DOI] [PubMed] [Google Scholar]
- Shao C, Folkard M, Michael BD, Prise KM. 2004. Targeted cytoplasmic irradiation induces bystander responses. Proc Natl Acad Sci U S A. Sep 14;101:13495–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Furusawa Y, Aoki M, Ando K. 2003. Role of gap junctional intercellular communication in radiation-induced bystander effects in human fibroblasts. Radiat Res. Sep;160:318–323. [DOI] [PubMed] [Google Scholar]
- Shao C, Stewart V, Folkard M, Michael BD, Prise KM. 2003. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. Dec 1;63:8437–8442. [PubMed] [Google Scholar]
- Sigurdson AJ, Jones IM. 2003. Second cancers after radiotherapy: any evidence for radiation-induced genomic instability? Oncogene. Oct 13;22:7018–7027. Epub 2003/10/15. [DOI] [PubMed] [Google Scholar]
- Sowa MB, Goetz W, Baulch JE, Lewis AJ, Morgan WF. 2011. No Evidence for a Low Linear Energy Transfer Adaptive Response in Irradiated Rko Cells. Radiat Prot Dosimetry. Jan 6. [DOI] [PubMed] [Google Scholar]
- Sowa MB, Goetz W, Baulch JE, Pyles DN, Dziegielewski J, Yovino S, Snyder AR, de Toledo SM, Azzam EI, Morgan WF. 2010. Lack of evidence for low-LET radiation induced bystander response in normal human fibroblasts and colon carcinoma cells. Int J Radiat Biol. Feb;86:102–113. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. 1993. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem.62:797–821. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- Terasima T, Tolmach LJ. 1961. Changes in x-ray sensitivity of HeLa cells during the division cycle. Nature. Jun 24;190:1210–1211. Epub 1961/06/24. [DOI] [PubMed] [Google Scholar]
- Voulgaridou GP, Anestopoulos I, Franco R, Panayiotidis MI, Pappa A. 2011. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res. Jun 3;711:13–27. Epub 2011/03/23. [DOI] [PubMed] [Google Scholar]
- Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. 2013. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta. Jan;1828:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. 2002. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. May;383:725–737. [DOI] [PubMed] [Google Scholar]
- Wolff S. 1992. Failla Memorial Lecture. Is radiation all bad? The search for adaptation. Radiat Res.131:117–123. [PubMed] [Google Scholar]
- Zhao Y, De Toledo SM, Hu G, Hei TK, Azzam EI. 2014. Connexins and Cyclooxygenase-2 Crosstalk in Expression of Radiation-Induced Bystander. Br J Cancer. 2014 July 8;111:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. 2000. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci USA.97:2099–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HN, Suzuki M, Randers-Pehrson R, Chen G, Trosko J, Vannais D, Waldren CA, Hall EJ, Hei TK. 2001. Radiation risk at low fluences of alpha particles may be greater than we thought. Proc Natl Acad Sci USA.98:14410–14415. [DOI] [PMC free article] [PubMed] [Google Scholar]