Abstract
Trauma-focused psychotherapies show general efficacy in posttraumatic stress disorder (PTSD), but outcomes vary substantially among individuals with PTSD and many patients do not achieve clinically meaningful symptom improvement. Several factors may contribute to poor treatment response, including genetic or environmental (e.g., stress) effects on neurobiological factors involved in learning and memory processes critical to PTSD recovery. In this review, we discuss the relationship between deficient GABAergic neurosteroid metabolites of progesterone, allopregnanolone (Allo) and pregnanolone (PA) and PTSD symptoms in men and women or PTSD-like behavioral abnormalities observed in male rodent models of PTSD. We also review the role and molecular underpinnings of learning and memory processes relevant to PTSD recovery, including extinction, extinction retention, reconsolidation of reactivated aversive memories, and episodic non-aversive memory. We then discuss preclinical and clinical research that supports a role in these learning and memory processes for GABAergic neurosteroids and sulfated metabolites of Allo and PA that allosterically antagonize N-methyl-D-aspartate (NMDA) receptor function. Studies supporting the possible therapeutic impact of appropriately timed, acutely administered Allo or Allo analogues to facilitate extinction retention and/or block reconsolidation of aversive memories are also reviewed. Finally, we discuss important future directions for research in this area. Examining the varied and composite effects in PTSD of the several metabolites of progesterone, as well as neuroactive derivatives of other parent steroids produced in the brain and the periphery will likely enable a broadening of targets for treatment development. Defining the contributions of these neuroactive steroids to common PTSD-comorbid psychiatric and medical conditions, as well as subpopulation-specific underlying dysfunctional physiological processes such as hypothalamic-pituitary-adrenal axis and immune system dysregulation, may also enable development of more effective multi-system precision medicines to prevent and treat the broader, polymorbid sequelae of extreme and chronic stress.
Keywords: Neurosteroids, Extinction Retention, Reconsolidation Blockade, Allopregnanolone, Memory, Post-Traumatic Stress Disorder
Introduction
Posttraumatic stress disorder (PTSD) is a major public health concern, affecting 8.3% of the general U.S. population overall (11.0% of women and 5.4% of men)1 and higher percentages of individuals exposed to particularly high-risk types of trauma, such as combat, sexual assault and compound community trauma. Trauma-focused psychotherapies, such as Cognitive Processing Therapy (CPT) and Prolonged Exposure (PE) show general efficacy in the treatment of PTSD, but outcomes vary substantially among individuals and across PTSD subpopulations and many clients do not achieve clinically meaningful reductions in symptoms by the end of treatment2–4. For example, among published studies of CPT for PTSD, initial PTSD symptom severity is on average, severe and similar across studies, but the percent reduction in symptoms from pre- to post-treatment ranges from 15% to 86%, with a general pattern reflecting substantially less improvement in male and female veterans and active duty military personnel compared to civilian non-veterans5–17.
Several factors may contribute to poor responses to these often highly effective PTSD psychotherapies, including genetic, environmental, and stress effects on neurobiological factors involved in learning and memory processes18 critical to: a) reprocessing of trauma memories and modification of maladaptive thoughts about the trauma19–21, b) extinction of threat-conditioned behavioral and neurophysiological defense responses22–27, and c) consolidation of resulting reconfigured brain circuits28,29. Recent work by Etkin et al.21 demonstrated that a subgroup of PTSD patients who responded poorly to PE therapy exhibited both poor delayed recall on a verbal memory word-list learning task and aberrant functional connectivity within the ventral attention network (VAN) during functional magnetic resonance imaging (fMRI). Further, reduced VAN connectivity was associated with prolonged below-baseline alpha-range desynchronization in response to single pulse transcranial magnetic stimulation (spTMS) — a phenomenon thought to be related to aberrant gamma-amino-butyric acid (GABA) system function21. This work thus suggests that resistance to the beneficial effects of trauma-focused psychotherapy may be associated with dysfunction in more than one relevant neural domain (i.e., coordinated neuronal connectivity patterns as well as learning and memory)—and/or in a neurobiological factor or factors, such as GABAergic neuroactive steroids, that have impact across multiple neural domains critical to PTSD recovery.
Association Between Deficits in GABAergic Neurosteroids and PTSD
Allopregnanolone (Allo) and pregnanolone (PA) are GABAergic neurosteroids produced from progesterone (Fig. 1) in the brain, adrenal gland, ovaries, and testes that equipotently facilitate inhibitory effects of GABA acting at GABAA receptors—increasing chloride influx in response to GABA binding by seven to ten times30,31. As recently reviewed32, preclinical and clinical research from this investigative team’s laboratories implicate deficits in the synthesis of these GABAergic stereoisomers in the pathophysiology and recovery from PTSD. For example, our group initially demonstrated markedly reduced cerebrospinal fluid (CSF) levels of Allo+PA (measured together by gas chromatography-mass spectrometry or GC-MS) in follicular phase women with PTSD33. The women with PTSD also exhibited a decrease in the ratio of Allo+PA to the Allo precursor 5α-dihydroprogesterone (5α-DHP), suggesting the presence of a block in Allo synthesis at the enzyme 3α-hydroxysteroid dehydrogenase (3α-HSD) (Fig. 1). Pineles et al.34 confirmed a PTSD-related reduction in the ratio of Allo+PA (measured separately by GC-MS) to 5α-DHP in the plasma of women with PTSD tested both in the early follicular (eFol) and mid-luteal (mLut) phases of the menstrual cycle. Furthermore, the women with PTSD failed to significantly increase the ratio of Allo+PA to 5α-DHP in response to a moderately stressful laboratory fear-conditioning task, whereas healthy trauma-exposed control subjects responded to the stress of the task with a robust and significant increase in this ratio. A PTSD-related block in the synthesis of Allo from its steroid precursors was also confirmed by our group in the CSF of men with PTSD, although the block in men appeared to be at the enzyme 5α-reductase instead of 3α-HSD35 (Fig. 1)—a finding consistent with previous work by Gillespie et al.36, demonstrating a risk polymorphism in the 5α-reductase II gene in men with PTSD, but not women. Moreover, in both men and women with PTSD, CSF Allo+PA levels correlate negatively and strongly with PTSD reexperiencing and depressive symptoms, accounting for about 50% of the variance in severity—while the ratio of Allo+PA to dehydroepiandrosterone [DHEA: an adrenally-produced neuroactive steroid that allosterically antagonizes GABAA receptors and facilitates N-Methyl-D-Aspartate (NMDA) receptor function] correlated even more strongly, accounting for over 60% of the variance in the severity of these symptoms. This suggests that an imbalance in inhibitory to excitatory neurotransmission in the central nervous system (CNS) contributes to PTSD severity. Consistent with these studies in CSF and plasma, Cruz et al37. recently reported reduced Allo levels in post-mortem medial orbitofrontal cortical brain samples from males with PTSD; androsterone (another potent GABAergic neurosteroid produced from androstenedione by the serial activity of 5α-reductase and 3α-HSD; see Fig. 1) was reduced when controlling for age, post-mortem interval, and smoking.
Figure 1. Neurosteroid Synthesis Pathways.
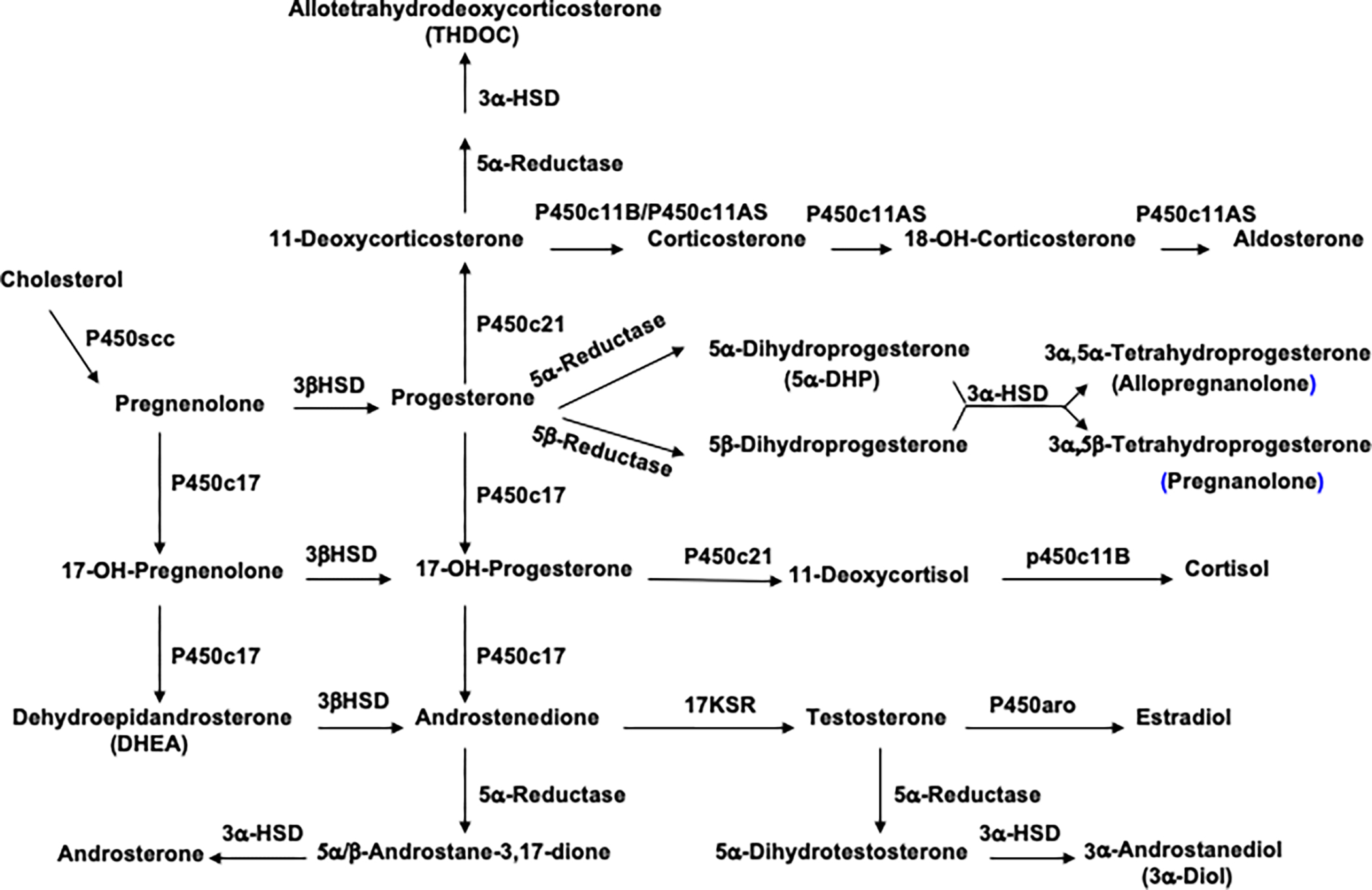
The enzyme pathways involved in neurosteroidogenesis are detailed above. Note that not all pathways are active in all neurosteroidogenic cells107. Abbreviations include P450 scc: P450 side chain cleavage enzyme; 3a-HSD: 3a-hydroxysteroid dehydrogenase; 3b-HSD: 3b-hydroxysteroid dehydrogenase; 17KSR: 17 ketosteroid reductase.
Given the reproducible cross-species PTSD phenotype associated with deficits in GABAergic neurosteroid synthesis deficits and the reciprocal relationship between GABAergic neurosteroid levels and GABA levels observed in occipital cortex38 and across the menstrual cycle in healthy women, Arditte Hall et al.39 also measured plasma GABA levels in samples from the study of women with PTSD by Pineles et al.29 using a slight modification of the high pressure liquid chromatography/mass spectrometry (LC-MS/MS) method of Schür et al.40 to ensure adequate assay sensitivity and specificity. The results indicated that plasma GABA levels were not modulated by experimentally induced stress or menstrual cycle phase. In addition, although plasma GABA levels did not differ significantly by PTSD diagnosis in this relatively small study (ps > 0.18), GABA levels were positively associated with total PTSD symptom severity (p=.06), and with PTSD dysphoria (p=0.03) and avoidance (p=0.06) cluster symptom severity as defined by Simms et al.41 These data suggest that GABA levels may increase but compensate inadequately for reduced Allo+PA levels among more symptomatic individuals with PTSD. These findings also align with the work of Schür et al.40 in male military personnel showing a positive association between post-deployment plasma GABA levels and symptoms of PTSD and depression at 6 months, but not 1 month after deployment. In contrast to these studies measuring plasma GABA at time points remote from trauma exposure, Vaiva et al. 42,43 found that low GABA levels (measured by comparable GC-MS methodology) immediately after a motor vehicle accident were associated with having a PTSD diagnosis 6 weeks and 1 year later.
Relationship Between GABAergic Neurosteroid Levels and Extinction Retention Deficits in PTSD
Laboratory-based fear conditioning and extinction models have been used in preclinical and clinical research to model PTSD development and maintenance. In recent years, extinction retention (i.e., the ability to recall extinction learning when tested at a later date) has gained increasing attention as a construct potentially contributing to PTSD chronicity and the suboptimal impact of trauma-focused treatments for a significant subpopulation of PTSD patients. Studies have often (although not always) shown comparable acquisition and extinction of conditioned fear in male PTSD and control subjects, but consistent extinction retention deficits in those with PTSD28. Studies in women show more complicated menstrual cycle and PTSD effects on extinction retention. For example, healthy women show optimum extinction retention when 17β-estradiol levels are highest (menstrual phase not assessed)44 or during the mLut phase of the menstrual cycle when 17β-estradiol as well as progesterone and Allo+PA levels are high29. However, trauma-exposed, medically healthy women with and without PTSD show comparable extinction retention during the eFol phase when plasma progesterone and Allo+PA levels are relatively low, while women with PTSD show marked extinction retention deficits during the mLut phase compared to the eFol phase and trauma-exposed women without PTSD in the mLut phase29.
Furthermore, during the mLut phase, women with PTSD showed a strong positive association between resting plasma Allo+PA levels and extinction retention45. During the eFol phase, the ratio of resting plasma Allo+PA to DHEA, rather than Allo+PA levels alone, correlated strongly and positively with extinction retention, but only when extinction retention was compared to late extinction45. During the eFol phase, DHEA released by the adrenal gland in response to maximally activating adrenocorticotropin hormone (ACTH) doses46 reaches levels that are comparable to eFol phase Allo+PA levels—and thus may compete effectively with Allo+PA at brain GABAA receptors to influence extinction retention. During the mLut phase, plasma DHEA levels are much lower than plasma Allo+PA levels. It is therefore notable that 17β-estradiol, which is higher during the mLut than eFol phase of the menstrual cycle, upregulates expression of 3α-HSD in the hippocampus of healthy female rodents47—an effect possibly mediated by estrogen receptor (ER)β, given that activation of ERβ but not ERα is associated with improvements in extinction and extinction retention48. In women with PTSD, 17β-estradiol signaling thus may be inadequate or at least unable to overcome the impact of other possible causes of deficits in 3α-HSD enzyme function during the mLut phase.
These studies in humans demonstrating a role for GABAergic neurosteroids in the pathophysiology of PTSD align with research in male rodent models of PTSD49–53. In male rodents, experimentally induced Allo deficits in brain and blood are associated with behavioral manifestations of anxiety, depression, and aggression, as well as enhanced contextual fear conditioning, slow extinction, and poor extinction retention—a phenotype akin to PTSD in humans. In turn, administration of ganaxolone, a synthetic 3β-analogue of Allo with similar effects at GABAA receptors54 or drugs such as selective serotonin reuptake inhibitors (SSRIs) that enhance Allo synthesis50,55 reverse these PTSD-like behaviors. For example, Pinna and Rasmusson51 demonstrated the capacity of a single dose of ganaxolone to block the reconsolidation of contextual fear and/or enhance extinction and extinction retention when administered immediately after the first re-exposure to a fear-conditioned context of male mice with social isolation (SI)-induced reductions in brain Allo; ganaxolone had no apparent effect in group-housed male mice with normal brain Allo levels. In contrast, vehicle-treated mice with SI-induced brain Allo deficiency extinguished very slowly compared to group-housed mice and showed spontaneous recovery of fear a week after extinction training ended. In a subsequent study using the same conditioning and single injection treatment paradigm53, the endocannabinoid congener N-palmitoylethanolamine (PEA), which activates the peroxisome proliferator-activated receptor alpha (PPARα) to induce biosynthesis of Allo, had effects like those of ganaxolone. Furthermore, the beneficial effects of PEA in socially isolated mice were blocked by prior administration of finasteride, which competitively antagonizes 5α-reductase type I at sub-micromolar concentrations (Ki=300 nM) and inactivates 5α-reductase II at sub-nanomolar concentrations56, thus blocking Allo synthesis.
Mechanisms by which GABAergic Neurosteroids and their Metabolites May Influence Extinction Retention and Aversive Memory Reconsolidation in PTSD
During trauma-focused therapies for PTSD such as PE and CPT, activation of a threat-related memory renders the memory “labile” and engages two competing processes: extinction and reconsolidation57,58. Extinction involves both: a) prefrontal cortical inhibition of fear-conditioned, amygdala-mediated sympathetic nervous system, cardiovascular, hypothalamic-pituitary-adrenal (HPA) axis, monoaminergic and behavioral defense responses59, and b) acquisition and consolidation of new learning (e.g., the conditioned threat stimulus or CS+ no longer signals threat, at least in the new time-space context)60,61. At the molecular level, extinction involves both synaptic long-term potentiation (LTP) and long-term depression (LTD)62. Extinction thus improves function, but is not permanent, as amygdala-mediated defense responses may be ‘renewed’ in a new context, ‘reinstated’ upon re-exposure to the unconditioned threat stimulus (US), or ‘spontaneously recovered’ with the passage of time58. Reconsolidation blockade also may contribute to PTSD recovery (reviewed in Elsey et al.63). β-blockers (e.g., propranolol64,65), protein kinase A (PKA) inhibitors66, and protein synthesis inhibitors (not feasible in humans)67 block reconsolidation (if given within an hour of brief threat memory reactivation) by blocking phosphorylation of serine 845 residues on glutamate receptor 1 (Glu-R1) α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, thus limiting their synaptic incorporation—a prerequisite for memory consolidation and reconsolidation58,68. Some but not all human studies have demonstrated propranolol-induced blockade of episodic and aversive memory reconsolidation63, as well as PTSD symptom improvement in paradigms combining reconsolidation blockade and extinction69. Furthermore, Soeter and Kindt70–72 showed that fear responses could not be reinstated or renewed after reconsolidation blockade, and that subsequent reacquisition of fear was no faster than during the initial fear conditioning experience, consistent with a lack of “savings” of the CS+-US association.
As noted above, individuals with PTSD are at high risk for deficiencies in Allo+PA—and deficits in Allo or Allo+PA production have been associated with poor extinction retention in male rodents and women with PTSD. Synthesis of these GABAergic neurosteroids is initiated de novo by ACTH in the adrenal gland, luteinizing hormone (LH) in the ovaries and testes, and NMDA receptor activation (with facilitation by co-activation of G-protein coupled receptors) in neurons (reviewed in Rasmusson and Pineles32)--and appears to peak within an hour of synthesis initiation, as demonstrated in response to swim stress76, maximum load exercise77, and activation of hippocampal neurons78. Izumi et al.78 showed in turn that activation of neuronal synthesis of these GABAergic neurosteroids is essential to the production of LTD and/or LTP interference. While LTD contributes directly to extinction62, LTP interference is thought to promote extinction retention by protecting recently modulated neuronal synapses (as may occur during extinction training) from further modification during the subsequent one- to six-hour period of memory consolidation79. Slowly rising intraneuronal levels of Allo or PA may contribute to LTD or LTP interference via: 1) facilitation of GABAA receptor-mediated inhibition of p38 mitogen-activated protein kinase (MAPK)/extracellular-signal regulated kinase (ERK) pathway production of PKA80,81, resulting in disruption of Glu-R1 serine 845 phosphorylation and synaptic incorporation of AMPA receptors, and 2) slowly rising levels of the sulfated metabolites of Allo and PA (i.e., Allo-S and PA-S) that antagonize NMDA receptor activation82,83. Simultaneous enhancement of the function of extrasynaptic GABAA receptors upregulated or persisting in amygdala after aversive conditioning84 by increasing Allo and PA levels during re-exposure to conditioned aversive stimuli likely restrains downstream activation of brainstem monoamine inputs to prefrontal cortex (PFC) (that, if to high, can disrupt working memory and PFC inhibition of the amygdala60). PFC-amygdala connectivity thus may be promoted as a substrate for consolidation—as critical for the subsequent expression of extinction.
In contrast to the potential benefit of administering Allo or an Allo analog after extinction training to maintain extinction training-induced changes in synaptic strength while protein-dependent synaptic consolidation occurs in the service of extinction retention, Allo given shortly after a single brief CS+ re-exposure, but before extinction training, may be expected to block CS+-US reconsolidation—and possibly more effectively than propranolol or PKA inhibitors. Like β-blockers, Allo facilitation of GABAA receptor function may disrupt Glu-R1 serine 845 phosphorylation by inhibiting the PKA/cyclic AMP response element binding protein (CREB) signaling pathway. In addition, Allo would be expected to inhibit amygdala activation of the locus coeruleus and reduce norepinephrine (NE) activation of noradrenergic β-receptors, while Allo-S may directly interfere with NMDA receptor activation. While propranolol also may block NE activation of the PKA/CREB signaling pathway and prevent re-incorporation of AMPA receptors into synapses previously strengthened by aversive conditioning, it may also reduce neurosteroidogenesis32,85 and interfere with the learning or consolidation of learned safety signals.
The Timing of GABAergic Neurosteroid Administration May be Critical to Treatment Efficacy
As can be inferred from the discussion above, co-activation of NMDA and G-protein coupled monoamine receptors and downstream PKA/CREB signaling upon re-exposure to a fear-conditioned memory32 may be prevented by the prior administration of Allo. Therefore, chronic Allo dosing or acute dosing of Allo before activation of trauma memories may be countertherapeutic. This possibility is supported by numerous studies in rodents. Johansson et al.86 demonstrated markedly negative effects on spatial learning and/or recall in adult male Wistar rats by a (relatively low) 2 mg/kg intravenous (IV) dose of Allo administered at 8 minutes, but not 20 minutes, before each of 9 training sessions in a Morris water maze. Notably, brain levels of Allo were 1.5 to 2.5 times higher in relevant brain regions at the 8- versus 20-minute training condition and paralleled those in plasma. Matthews et al.87 demonstrated a dose dependent, negative effect in adult male Long-Evans hooded rats of a single intraperitoneal (i.p.) injection of Allo (12.5 mg/kg, 17 mg/kg, or 20 mg/kg) 20 minutes before a test of spatial memory retrieval in a Morris water maze after 16 days of training to criterion in the absence of drug treatment. Similar results were obtained in adolescent male Sprague-Dawley rats88.
Increases in endogenous GABAergic neurosteroid levels before reactivation of fear memory circuits can potentially be induced by the intake of substances, activities, or experiences that activate the HPA axis, including some psychiatric medications such as clozapine and olanzepine89, smoking90, acute exercise77 and other physiological or psychological stressors such as pain. Preliminary work in our laboratory shows that even placement of an IV induces a rise in plasma Allo+PA levels that peaks at about 30 minutes and returns to the resting baseline by 60 minutes. We speculate that raising GABAergic neurosteroid levels under these circumstances may acutely minimize PTSD reexperiencing or hyperarousal symptoms, but at the expense of trauma memory circuit activation, which is critical to both extinction and reconsolidation blockade. In contrast, SSRIs have been shown to increase Allo levels in male rodents at doses < 1/10 of the effective dose 50 (ED50) for serotonin reuptake blockade in association with increases in the expression of 5α-reductase I (effects on 5α-reductase II not tested)55 or perhaps function of 3α-HSD91 (however, see Trauger et al.92). Similarly, PEA administration has been shown to upregulate several neurosteroidogenic enzymes85 downregulated in the hippocampus of socially isolated mice, including PPARα, steroidogenic acute regulatory protein (StAR), cholesterol side-chain cleavage enzyme (cytochrome P450 11A1 [CYP11A1]), and 5α-reductase I52. This would be expected to magnify the normally timed, de novo synthesis of Allo and PA when brain neurons and the adrenal cortex are activated by re-exposure to trauma reminders, and potentially facilitate extinction and extinction retention. Consistent with this formulation, Schneier et al.93 reported a relatively hastened and increased magnitude of PTSD improvement and remission among 37 World Trade Center attack trauma survivors treated with paroxetine compared to placebo in combination with PE for 10 weeks; differences between these treatment groups waned in a subsample of 26 participants who continued treatment to 22 weeks. In contrast, a study of 207 mostly male Veterans showed no benefit of sertraline compared to placebo combined with PE and a lower overall effect size, consistent with the generally smaller effect sizes seen in pharmacotherapy and trauma-focused treatment studies in Veterans 94. Future treatment studies thus should consider both the timing of GABAergic neurosteroid-based treatment administration and underlying fluctuations in endogenous GABAergic neurosteroid levels relative to learning and memory processes relevant to PTSD recovery—keeping in mind that diverse trauma-exposed populations may vary in their capacity for GABAergic neurosteroid synthesis due to genomic factors as well as environmental exposures.
GABAergic Neurosteroids and Non-Aversive Memory
The impact of neurosteroid metabolites of progesterone on non-aversive learning and memory should also be considered in relation to trauma-focused PTSD therapies. Ideally, non-aversive learning proceeds in tandem with extinction learning to allow future ‘informed contextualization’ and recalibration of the intensity of defensive responses to previously conditioned threat cues. Optimization of GABAergic neurosteroid levels during therapeutic encounters is likely necessary to modulate ‘arousal dependent’ activation and connectivity among the sensory cortices, frontal lobe, hippocampus, and amygdala – to enable collation of conditioned contextual threat cues and current sensory experiences for construction of the new context and revised cognitive evaluations of risk.
Unfortunately, studies directly evaluating a potential role for GABAergic neurosteroids in non-aversive learning and memory tasks have been few. Rabinowitz et al.95 used a relatively non-aversive, novel object recognition task to examine effects of Allo on non-spatial, hippocampus-dependent memory. In that study, Allo was administered at doses of 3.2 mg/kg, 10 mg/kg, or 17 mg/kg, i.p., fifteen minutes before exposing male mice to two sample objects. Twenty-four hours later, mice treated with Allo vs. vehicle demonstrated dose-dependent reductions in the normal preference to explore a novel object presented with one of the familiar sample objects—consistent with impaired encoding and/or consolidation of memory for the objects presented the day before. Allo administered systemically or by microinjection into the dorsal hippocampus immediately after exposure to the sample objects had similar effects on novel object recognition95.
Rabinowitz et al.95 also tested hippocampus-specific effects of Allo on non-aversive memory. Allo (10 mg/kg, i.p.) versus vehicle was given 10 minutes before pre-exposure of male mice to a novel conditioning chamber for 5 minutes. Twenty-four hours later, vehicle was administered 10 minutes before fear conditioning, during which a 30 second, 90 decibel (db) tone co-terminating with a 1-second, 0.5 milliAmp (mA) footshock was presented 3 times over 5 minutes. The next day, mice treated with Allo versus vehicle before pre-exposure to the conditioning chamber showed a reduction in contextual fear but no difference in expression of fear to the tone. The authors claimed that Allo diminished context learning during the non-aversive, pre-exposure of the mice to the conditioning chamber95. However, the findings may be better explained by effects of Allo on ‘latent inhibition’. Allo may have exerted a mild anxiolytic effect in mice pre-exposed to the ‘novel’ conditioning chamber and perhaps facilitated memory consolidation as well. Thus, an association of the chamber with safety strengthened by Allo administration may have competed with formation of a fear association engendered by subsequent fear-conditioning in the same context. It will be important for future work to discriminate these possibilities.
Only one human study of Allo effects on non-aversive memory has been conducted. In healthy women tested during the follicular phase of the menstrual cycle, there was no effect of IV Allo compared to placebo on episodic memory tested via 10-minute recall of a 12-item word list96. Tests of IV Allo effects on semantic and working memory also were negative. Unfortunately, the timing of the IV-line placement relative to the 45-minute battery of memory tests conducted before and after drug administration was not reported, preventing consideration of potentially confounding effects of such a stressor on endogenous Allo levels and memory performance.
Individuals with PTSD have been shown to have deficits in non-aversive episodic memory97 and source memory for trauma-related stimuli98. Indeed, Golier et al.98 observed a positive correlation in combat veterans between PTSD symptom severity and memory for general and personally relevant combat trauma-related words, but not neutral words. This is consistent with the observation that chronic stress-related neurobiological changes reduce the signal-to-noise ratio of sensory inputs tightly associated with unconditioned threat and increase defensive responding to adventitious contextual stimuli99, thereby facilitating threat generalization and degrading source memory.
Lesion studies suggest that the frontal lobe100, and perhaps more specifically, the prefrontal cortex101 is important for source memory. The parietal cortex also appears to be involved in encoding and remembering the source of episodic memories, while posterior visual areas contribute to source memory accuracy97. As reviewed by Letzkus et al.102, ‘GABA-mediated disinhibition’ operationalized by the inhibitory effects of GABA on post-synaptic inhibitory interneurons is thought to be involved in the rapid activation and coordination of neuronal activity across these brain regions that contribute to state-dependent learning and coordination of defensive responses to threat or approach responses to the arousing prospects of reward. GABA-mediated disinhibition is also involved in the rapid activation and synchronization of more discrete neuronal subpopulations involved in non-aversive learning. It thus may be important to understand the potential capacity of GABAergic neurosteroids to modulate ‘GABA-mediated disinhibition’, as well as define the location and subtype of target GABA receptors within neuronal assemblies that mediate this phenomenon—if we are to most efficaciously use GABAergic neurosteroid based interventions to modulate aversive and non-aversive learning and memory in the service of PTSD recovery.
Future Directions
Recent work in very large human samples by Shalev and collaborators from the International Consortium to Predict PTSD103 demonstrated a remarkably high predictive relationship between PTSD symptom severity rated within a month of trauma exposure and chronic PTSD assessed 4–15 months later. Further research will be necessary to determine if this important observation is related to the presence of deficits in GABAergic neurosteroid synthesis, which now have been associated with PTSD and depressive symptom severity, as well as learning and memory processes relevant to recovery from PTSD, in multiple pre-clinical and clinical studies. Work on the role of neuroactive steroids in the brain and psychiatric disorders such as PTSD is likely to continue for some time given our growing awareness of the variety and varied effects of the isomers and metabolites of progesterone derivatives, as well as derivatives of other parent steroids produced in the brain and periphery104. As an exciting potential example, given the effects of ketamine on PTSD and depressive symptoms105, it might be helpful to learn the extent to which the endogenous NMDA receptor antagonists, Allo-S and PA-S, either alone or in addition to Allo and PA, influence neuropsychiatric symptom expression, reconsolidation blockade, or the production of LTP interference with effects on aversive and non-aversive memory formation and consolidation. There is also much yet to learn about the mechanisms by which these neuroactive steroids may contribute to PTSD risk, severity, and recovery. In addition, there appear to be contributions of these neuroactive steroids to the etiology of common PTSD-comorbid psychiatric conditions (e.g., major depression, post-partum depression, premenstrual dysphoric disorder, and possibly alcohol, nicotine, and cannabinoid dependence) as well as PTSD-comorbid medical conditions (e.g., chronic pain, psychogenic seizures, traumatic brain injury, neurodegenerative disorders, pre-term birth, sexual dysfunction, and autoimmune disorders)106,107. Research on the regulatory effects of neuroactive steroids on more fundamental physiological processes dysregulated in these conditions also will be important. For example, Allo provides local inhibition and long-loop negative feedback to the HPA axis108,109. As previously reviewed106,107, the hyperreactivity and persistent increases in ACTH and cortisol observed in multiple studies of women with PTSD and co-morbid depression may be secondary to a primary deficiency in GABAergic neurosteroid synthesis. On the other hand, childhood abuse-related methylation of an FK506 binding protein 5 (FKBP-5) risk polymorphism and related downregulation of glucocorticoid receptor sensitivity could contribute to deficient upregulation of 3α-HSD and a secondary deficiency in the production of Allo and PA. Understanding the sources of these converging PTSD-related glucocorticoid system endophenotypes, as well as others (e.g., that characterized by low cortisol and upregulated glucocorticoid receptor sensitivity110) could potentially guide development and targeting of subpopulation-specific precision therapies for PTSD. However, we must also point out the importance of measuring cortisol by GC/MS instead of immunoassay based on the replicated observations by Yehuda et al.111,112 showing that cortisol deficits related to PTSD risk and severity (when measured by immunoassay) were instead deficits in the 5α-reduced metabolite of cortisol synthesized by 5α-reductase (as determined by GC/MS). This work thus appears to align with the relationship between PTSD severity and possible 5α-reductase dysregulation as discussed earlier in this review. Finally, recent research has defined a role for Allo in the regulation of inflammation113,114, which may provide additional insight into possible PTSD subpopulation-specific pathophysiologic processes that impede recovery but that might yield to novel targeted treatments.
Support:
NIMH 1R01MH122867-01 (Rasmusson, Pineles, Brown, Pinna)
List of Abbreviations
- 3α-HSD
3α-Hydroxysteroid Dehydrogenase
- 5α-DHP
5α-Dihydroprogesterone
- ACTH
Adrenocorticotropin Hormone
- Allo
Allopregnanolone
- Allo-S
Allopregnanolone Sulfate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CNS
Central Nervous System
- CPT
Cognitive Processing Therapy
- CREB
Cyclic AMP Response Element Binding Protein
- CS+
Conditioned Threat Stimulus
- CSF
Cerebrospinal Fluid
- CYP11A1
Cytochrome P450 11A1
- dB
Decibel
- DHEA
Dehydroepiandrosterone
- ED50
Effective Dose 50
- eFol
Early Follicular
- ER
Estrogen Receptor
- ERK
Extracellular Signal-Regulated Kinase
- FKBP-5
FK506 Binding Protein 5
- fMRI
Functional Magnetic Resonance Imaging
- GABA
Gamma-Aminobutyric Acid
- GC-MS
Gas Chromatography-Mass Spectrometry
- Glu-R1
Glutamate Receptor 1
- HPA
Hypothalamic-Pituitary-Adrenal
- IV
Intravenous
- LC-MS/MS
Liquid Chromatography-Mass Spectrometry/ Mass Spectrometry
- LH
Luteinizing Hormone
- LTD
Long-Term Depression
- LTP
Long-Term Potentiation
- mA
Milliamp
- MAPK
Mitogen-Activated Protein Kinase
- mLut
Mid-Luteal
- NE
Norepinephrine
- NMDA
N-Methyl-D-Aspartate
- PA
Pregnanolone
- PA-S
Pregnanolone Sulfated
- PE
Prolonged Exposure
- PEA
Palmitoylethanolamine
- PFC
Prefrontal Cortex
- PKA
Protein Kinase A
- PPAR
Peroxisome Proliferator-Activated Receptor
- PTSD
Posttraumatic Stress Disorder
- SI
Social Isolation
- spTMS
Single Pulse Transcranial Magnetic Stimulation
- SSRIs
Selective Serotonin Reuptake Inhibitors
- StAR
Steroidogenic Acute Regulatory Protein
- US
Unconditioned Threat Stimulus
- VAN
Ventral Attention Network
Footnotes
Potential Conflicts of Interest:
AMR was a paid consultant to Praxis Precision Medicines, Inc., in 2020. SLP and KDB declare no potential conflicts of interest. GP is a paid consultant to PureTech Health and has two pending patent applications, one on N-palmitoylethanolamine (PEA) and peroxisome proliferator-activated receptor alpha (PPAR-α) agonists, and one on allopregnanolone analogs in the treatment of neuropsychiatric disorders.
Data Availability:
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Kilpatrick DG, Resnick HS, Milanak ME, et al. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schottenbauer MA, Glass CR, Arnkoff DB, et al. Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry Interpers Biol Process. 2008;71:134–168. [DOI] [PubMed] [Google Scholar]
- 3.Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128–141. [DOI] [PubMed] [Google Scholar]
- 4.Asmundson GJ, Thorisdottir AS, Roden-Foreman JW, et al. A meta-analytic review of cognitive processing therapy for adults with posttraumatic stress disorder. Cogn Behav Ther. 2019;48:1–14. [DOI] [PubMed] [Google Scholar]
- 5.Surís A, Link-Malcolm J, Chard K, et al. A randomized clinical trial of cognitive processing therapy for veterans with PTSD related to military sexual trauma. J Trauma Stress. 2013;26:28–37. [DOI] [PubMed] [Google Scholar]
- 6.Resick PA, Suvak MK, Johnides BD, et al. The impact of dissociation on PTSD treatment with cognitive processing therapy. Depress Anxiety. 2012;29:718–730. [DOI] [PubMed] [Google Scholar]
- 7.Galovski TE, Blain LM, Mott JM, et al. Manualized therapy for PTSD: Flexing the structure of cognitive processing therapy. J Consult Clin Psychol. 2012;80:968–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes D, Lloyd D, Nixon RD, et al. A multisite randomized controlled effectiveness trial of cognitive processing therapy for military-related posttraumatic stress disorder. J Anxiety Disord. 2012;26:442–452. [DOI] [PubMed] [Google Scholar]
- 9.Chard KM. An evaluation of cognitive processing therapy for the treatment of posttraumatic stress disorder related to childhood sexual abuse. Clin Psychol. 2005;73:965–971. [DOI] [PubMed] [Google Scholar]
- 10.Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83:1058–1068. [DOI] [PubMed] [Google Scholar]
- 11.Resick PA, Nishith P, Weaver TL, et al. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consult Clin Psychol. 2002;70:867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resick PA, Galovski TE, Uhlmansiek MO, et al. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. J Consult Clin Psychol. 2008;76:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morland LA, Mackintosh MA, Rosen CS, et al. Telemedicine versus in-person delivery of cognitive processing therapy for women with posttraumatic stress disorder: A randomized noninferiority trial. Depress Anxiety. 2015;32:811–820. [DOI] [PubMed] [Google Scholar]
- 14.Morland LA, Mackintosh MA, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75:470–476. [DOI] [PubMed] [Google Scholar]
- 15.Monson CM, Schnurr PP, Resick PA, et al. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006;74:898–907. [DOI] [PubMed] [Google Scholar]
- 16.Maieritsch KP, Smith TL, Hessinger JD, et al. Randomized controlled equivalence trial comparing videoconference and in person delivery of cognitive processing therapy for PTSD. J Telemed Telecare. 2016;22:238–243. [DOI] [PubMed] [Google Scholar]
- 17.Kaysen D, Lindgren K, Sabir Zangana GA, et al. Adaptation of cognitive processing therapy for treatment of torture victims: Experience in Kurdistan, Iraq. Psychol Trauma Theory, Res Pract Policy. 2013;5:184–92. [Google Scholar]
- 18.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann New York Acad Sci. 2003;1008:112–121. [DOI] [PubMed] [Google Scholar]
- 19.Nijdam MJ, Gersons BP, Olff M. Response to psychotherapy for posttraumatic stress disorder: the role of pretreatment verbal memory performance. J Clin Psychiatry. 2015;76:e1023–1028. [DOI] [PubMed] [Google Scholar]
- 20.Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141:105–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etkin A, Maron-Katz A, Wu W, et al. Using fMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Sci Transl Med. 2019;11:eaal3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orr SP, Milad MR, Metzger LJ, et al. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 23.Norrholm SD, Jovanovic T, Olin IW, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norrholm SD, Anderson KM, Olin IW, et al. Versatility of fear-potentiated startle paradigms for assessing human conditioned fear extinction and return of fear. Front Behav Neurosci. 2011;5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glover LE, Jovanovic T, Mercer KB, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jovanovic T, Kazama A, Bachevalier J, et al. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuj DV, Palmer MA, Hsu CM, et al. Impaired fear extinction associated with PTSD increases with hours-since-waking. Depress Anxiety. 2016;33:203–210. [DOI] [PubMed] [Google Scholar]
- 28.Milad MR, Orr SP, Lasko NB, et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pineles SL, Nillni YI, Patton SC, et al. Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. J Abnorm Psychol. 2016;125:349–355. [DOI] [PubMed] [Google Scholar]
- 30.Majewska MD, Harrison ND, Schwartz RD, et al. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;323:1004–1007. [DOI] [PubMed] [Google Scholar]
- 31.Puia G, Santi M, Vicini S, et al. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. [DOI] [PubMed] [Google Scholar]
- 32.Rasmusson AM, Pineles SL. Neurotransmitter, Peptide, and Steroid Hormone Abnormalities in PTSD: Biological Endophenotypes Relevant to Treatment. Curr Psychiatry Rep. 2018;20:52. [DOI] [PubMed] [Google Scholar]
- 33.Rasmusson AM, Pinna G, Paliwal P, et al. Decreased Cerebrospinal Fluid Allopregnanolone Levels in Women with Posttraumatic Stress Disorder. Biol Psychiatry. 2006;60:704–713. [DOI] [PubMed] [Google Scholar]
- 34.Pineles SL, Nillni YI, Pinna G, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 2018;93:133–141. [DOI] [PubMed] [Google Scholar]
- 35.Rasmusson AM, King MW, Gregor K, et al. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. 2019;102:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillespie CF, Almli LM, Smith AK, et al. Sex dependent influence of a functional polymorphism in steroid 5-α-reductase type 2 (SRD5A2) on post-traumatic stress symptoms. Am J Med Genet Part B Neuropsychiatr Genet. 2013;162:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz DA, Glantz LA, McGaughey KD, et al. Neurosteroid levels in the orbital frontal cortex of subjects with PTSD and controls: A preliminary report. Chronic Stress. 2019;3:2470547019838570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epperson CN, Haga K, Mason GF, et al. Cortical γ-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. [DOI] [PubMed] [Google Scholar]
- 39.Arditte Hall KA, DeLane SE, Anderson GM, et al. Plasma gamma-aminobutyric acid (GABA) levels and posttraumatic stress disorder symptoms in trauma-exposed women: a preliminary report. Psychopharmacology (Berl). 2021;238:1541–1552. [DOI] [PubMed] [Google Scholar]
- 40.Schür RR, Boks MP, Geuze E, et al. Development of psychopathology in deployed armed forces in relation to plasma GABA levels. Psychoneuroendocrinology. 2016;73:263–270. [DOI] [PubMed] [Google Scholar]
- 41.Simms LJ, Watson D, Doebbeling BN. Confirmatory factor analyses of posttraumatic stress symptoms in deployed and nondeployed veterans of the Gulf War. J Abnorm Psychol. 2002;111:637–647. [DOI] [PubMed] [Google Scholar]
- 42.Vaiva G, Thomas P, Ducrocq F, et al. Low posttrauma GABA plasma levels as a predictive factor in the development of acute posttraumatic stress disorder. Biol Psychiatry. 2004;55:250–254. [DOI] [PubMed] [Google Scholar]
- 43.Vaiva G, Boss V, Ducrocq F, et al. Relationship between posttrauma GABA plasma levels and PTSD at 1-year follow-up. Am J Psychiatry. 2006;163:1446–1448. [DOI] [PubMed] [Google Scholar]
- 44.Milad MR, Zeidan MA, Contero A, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pineles SL, Nillni YI, Pinna G, et al. Associations between PTSD-Related extinction retention deficits in women and plasma steroids that modulate brain GABAA and NMDA receptor activity. Neurobiol Stress. 2020;13:100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmusson A, Vasek J, Lipschitz DS, et al. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29:1546–1557. [DOI] [PubMed] [Google Scholar]
- 47.Mitev YA, Darwish M, Wolf SS, et al. Gender differences in the regulation of 3α-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. 2003;120:541–549. [DOI] [PubMed] [Google Scholar]
- 48.Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: Implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pibiri F, Nelson M, Guidotti A, et al. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci. 2008;105:5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinna G, Rasmusson AM. Up-regulation of neurosteroid biosynthesis as a pharmacological strategy to improve behavioural deficits in a putative mouse model of post-traumatic stress disorder. J Neuroendocrinol. 2012;24:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinna G, Rasmusson AM. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Front Cell Neurosci. 2014;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang LM, Qiu ZK, Zhao N, et al. Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. J Neuropsychopharmacol. 2014;17:1659–1669. [DOI] [PubMed] [Google Scholar]
- 53.Locci A, Pinna G. No Stimulation of peroxisome proliferator activation of PPAR-α by N-palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biol Psychiatry. 2019;85:1036–1045. [DOI] [PubMed] [Google Scholar]
- 54.Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABAA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. [DOI] [PubMed] [Google Scholar]
- 55.Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drury JE, Costanzo L, Penning TM, et al. Inhibition of human steroid 5α-reductase (AKR1D1) by finasteride and structure of the enzyme-inhibitor complex. J Biol Chem. 2009;284:19786–19790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mamiya N, Fukushima H, Suzuki A, et al. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monfils MH, Cowansage KK, Klann E, et al. Extinction-Reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324:951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldstein LE, Rasmusson AM, Bunney BS, et al. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dunsmoor JE, Kroes MC, Li J, et al. Role of human ventromedial prefrontal cortex in learning and recall of enhanced extinction. J Neurosci. 2019;39:3264–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maren S Out with the old and in with the new: Synaptic mechanisms of extinction in the amygdala. Brain Res. 2015;1621:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elsey JW, Van Ast VA, Kindt M. Human memory reconsolidation: A guiding framework and critical review of the evidence. Psychol Bull. 2018;144:797–848. [DOI] [PubMed] [Google Scholar]
- 64.Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. [DOI] [PubMed] [Google Scholar]
- 65.Hu H, Real E, Takamiya K, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. [DOI] [PubMed] [Google Scholar]
- 66.Tronson NC, Wiseman SL, Olausson P, et al. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. [DOI] [PubMed] [Google Scholar]
- 67.Nader K, Shafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. [DOI] [PubMed] [Google Scholar]
- 68.Oh MC, Derkach VA, Guire ES, et al. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. [DOI] [PubMed] [Google Scholar]
- 69.Brunet A, Saumier D, Liu A, et al. Reduction of PTSD symptoms with pre-reactivation propranolol therapy: a randomized controlled trial. Am J Psychiatry. 2018;175:427–433. [DOI] [PubMed] [Google Scholar]
- 70.Soeter M, Kindt M. Erasing fear from memory. Neurobiol Learn Mem. 2010;94:30–41. [DOI] [PubMed] [Google Scholar]
- 71.Soeter M, Kindt M. Disrupting reconsolidation: Pharmacological and behavioral manipulations. Learn Mem. 2011;18:357–366. [DOI] [PubMed] [Google Scholar]
- 72.Soeter M, Kindt M. Erasing fear for an imagined threat event. Psychoneuroendocrinology. 2012;37:1769–1779. [DOI] [PubMed] [Google Scholar]
- 73.Wood NE, Rosasco ML, Suris AM, et al. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: Three negative psychophysiological studies. Psychiatry Res. 2015;225:31–39. [DOI] [PubMed] [Google Scholar]
- 74.Brunet A, Orr SP, Tremblay J, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. [DOI] [PubMed] [Google Scholar]
- 75.Brunet A, Poundja J, Tremblay J, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2011;31:547–550. [DOI] [PubMed] [Google Scholar]
- 76.Purdy RH, Morrow AL, Moore PH, et al. Stress-induced elevations of gamma-aminobutyric acid type a receptor-active steroids in the rat brain. Proc Natl Acad Sci. 1991;88:4553–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scioli-Salter E, Forman DE, Otis JD, et al. Potential neurobiological benefits of exercise in chronic pain and posttraumatic stress disorder: Pilot study. J Rehabil Res Dev. 2016;53:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Izumi Y, O’dell KA, Zorumski CF. Metaplastic LTP inhibition after LTD induction in CA1 hippocampal slices involves NMDA receptor-mediated neurosteroidogenesis. Physiol Rep. 2013;1:e00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stefan K, Wycislo M, Gentner R, et al. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex. 2006;16:376–385. [DOI] [PubMed] [Google Scholar]
- 80.Tyagi N, Gillespie W, Vacek JC, et al. Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 activation by ERK pathway. J Cell Physiol. 2009;220:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim DH, Kim JM, Park SJ, et al. Hippocampal extracellular signal-regulated kinase signaling has a role in passive avoidance memory retrieval induced by GABA A receptor modulation in mice. Neuropsychopharmacology. 2012;37:1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park-Chung M, Wu FS, Purdy FH, et al. Distinct Sites for Inverse Modulation of N-Methyl-D-Aspartate Receptors by Sulfated Steroids. Mol Pharmacol. 1997;52:1113–1123. [DOI] [PubMed] [Google Scholar]
- 83.Gibbs TT, Russek SJ, Farb DH. Sulfated steroids as endogenous neuromodulators. Pharmacol Biochem Behav. 2006;84:555–567. [DOI] [PubMed] [Google Scholar]
- 84.Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang JL, Rasmusson AM. Overview of allopregnanolone biosynthesis and corresponding steroidogenic regulation. Chronic Stress. 2019;2:1–17. [Google Scholar]
- 86.Johansson IM, Birzniece V, Lindblad C, et al. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934:125–131. [DOI] [PubMed] [Google Scholar]
- 87.Matthews DB, Morrow AL, Tokunaga S, et al. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res. 2002;26:1747–1751. [DOI] [PubMed] [Google Scholar]
- 88.Chin VS, Van Skike CE, Berry RB, et al. Effect of acute ethanol and acute allopregnanolone on spatial memory in adolescent and adult rats. Alcohol. 2011;45:473–483. [DOI] [PubMed] [Google Scholar]
- 89.Marx CE, Van Doren MJ, Duncan GE, et al. Olanzapine and clozapine increase the GABAergic neuroactive steroid allopregnanolone in rodents. Neuropsychopharmacology. 2003;28:1–13. [DOI] [PubMed] [Google Scholar]
- 90.Porcu P, Sogliano C, Cinus M, et al. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74:683–690. [DOI] [PubMed] [Google Scholar]
- 91.Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci. 1999;96:13512–13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41:13451–13459. [DOI] [PubMed] [Google Scholar]
- 93.Schneier FR, Neria Y, Pavlicova M, et al. Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: a randomized controlled trial. Am J Psychiatry. 2012;169:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rauch SA, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry. 2019;76:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rabinowitz A, Cohen SJ, Finn DA, et al. The neurosteroid allopregnanolone impairs object memory and contextual fear memory in male C57BL/6J mice. Horm Behav. 2014;66:238–246. [DOI] [PubMed] [Google Scholar]
- 96.Kask K, Bäckström T, Nilsson LG, et al. Allopregnanolone impairs episodic memory in healthy women. Psychopharmacology (Berl). 2008;199:161–168. [DOI] [PubMed] [Google Scholar]
- 97.Mitchell KJ, Johnson MK. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135:638–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Golier J, Harvey P, Steiner A, et al. Source Monitoring in PTSD. Ann N Y Acad Sci. 1997;21:472–475. [DOI] [PubMed] [Google Scholar]
- 99.Rasmusson AM, Shalev A. Integrating the Neuroendocrinology, Neurochemistry, and Neuroimmunology of PTSD to Date and the Challenges Ahead. In: Friedman M, Keane T, Resick P (eds) Handbook of PTSD. New York, NY: Guilford Publications Inc., 2014, pp. 166–189. [Google Scholar]
- 100.Davidson PS, Cook SP, Glisky EL, et al. Source Memory in the Real World: A Neuropsychological Study of Flashbulb Memory. J Clin Exp Neuropsychol. 2005;27:915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swick D, Senkfor AJ, Van Petten C. Source memory retrieval is affected by aging and prefrontal lesions: Behavioral and ERP evidence. Brain Res. 2006;1107:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Letzkus JJ, Wolff SB, Lüthi A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron. 2015;88:264–276. [DOI] [PubMed] [Google Scholar]
- 103.Shalev AY, Gevonden M, Ratanatharathorn A, et al. Estimating the risk of PTSD in recent trauma survivors: results of the International Consortium to Predict PTSD (ICPP). World Psychiatry. 2019;18:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. [DOI] [PubMed] [Google Scholar]
- 105.Duman RS, Shinohara R, Fogaça MV, et al. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry. 2019;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rasmusson AM, Marx CE, Pineles SL, et al. Neuroactive steroids and PTSD treatment. Neurosci Lett. 2019;649:156–163. [DOI] [PubMed] [Google Scholar]
- 107.Rasmusson AM, Kim BK, Lago TR, et al. The Neuroendocrinology, Neurochemistry, and Neuroimmunology of PTSD. In: Friedman M, Keane T, Schnurr P (eds) Handbook of PTSD. New York, NY: Guilford Publications Inc., 2021, pp. 168–191. [Google Scholar]
- 108.Barbaccia ML, Roscetti G, Trabucchi M, et al. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. [DOI] [PubMed] [Google Scholar]
- 109.Barbaccia ML, Serra M, Purdy RH, et al. Stress and neuroactive steroids. Int Rev of Neurobiol. 2001;46:243–272. [DOI] [PubMed] [Google Scholar]
- 110.Yehuda R Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002:25;341–368. [DOI] [PubMed] [Google Scholar]
- 111.Yehuda R, Bierer LM, Andrew R, Schmeidler J, Seckl JR. Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. J Psychiatr Res. 2009:43;877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yehuda R, Bierer LM, Sarapas C, et al. Cortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the World Trade Center attacks on September 11, 2001. Psychoneuroendocrinology. 2009:34;1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balan I, Beattie MC, O’Buckley TK, Aurelian L, Morrow AL. Endogenous neurosteroid (3α,5α) 3-hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory Signaling in macrophages and brain. Sci Rep. 2019:9;1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balan I, Aurelian L, Schleicher R, Boero G, O’Buckley T, Morrow AL. Neurosteroid allopregnanolone (3α,5α-THP) inhibits inflammatory signals induced by activated MyD88-dependent toll-like receptors. Transl Psychiatry. 2021:11;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


