Abstract
Pseudomonas aeruginosa (P. aeruginosa) is a Gram-negative opportunistic pathogen that infects patients with cystic fibrosis, burn wounds, immunodeficiency, chronic obstructive pulmonary disorder (COPD), cancer, and severe infection requiring ventilation, such as COVID-19. P. aeruginosa is also a widely-used model bacterium for all biological areas. In addition to continued, intense efforts in understanding bacterial pathogenesis of P. aeruginosa including virulence factors (LPS, quorum sensing, two-component systems, 6 type secretion systems, outer membrane vesicles (OMVs), CRISPR-Cas and their regulation), rapid progress has been made in further studying host-pathogen interaction, particularly host immune networks involving autophagy, inflammasome, non-coding RNAs, cGAS, etc. Furthermore, numerous technologic advances, such as bioinformatics, metabolomics, scRNA-seq, nanoparticles, drug screening, and phage therapy, have been used to improve our understanding of P. aeruginosa pathogenesis and host defense. Nevertheless, much remains to be uncovered about interactions between P. aeruginosa and host immune responses, including mechanisms of drug resistance by known or unannotated bacterial virulence factors as well as mammalian cell signaling pathways. The widespread use of antibiotics and the slow development of effective antimicrobials present daunting challenges and necessitate new theoretical and practical platforms to screen and develop mechanism-tested novel drugs to treat intractable infections, especially those caused by multi-drug resistance strains. Benefited from has advancing in research tools and technology, dissecting this pathogen’s feature has entered into molecular and mechanistic details as well as dynamic and holistic views. Herein, we comprehensively review the progress and discuss the current status of P. aeruginosa biophysical traits, behaviors, virulence factors, invasive regulators, and host defense patterns against its infection, which point out new directions for future investigation and add to the design of novel and/or alternative therapeutics to combat this clinically significant pathogen.
Subject terms: Microbiology, Infection
Introduction to Pseudomonas aeruginosa, a critical clinical pathogen and model microorganism
Pseudomonas aeruginosa is a multi-drug resistance (MDR) opportunistic pathogens, causing acute or chronic infection in immunocompromised individuals with chronic obstructive pulmonary disease (COPD), cystic fibrosis, cancer, traumas, burns, sepsis, and ventilator-associated pneumonia (VAP) including those caused by COVID-19.1–3 P. aeruginosa in biofilm states may survive in a hypoxic atmosphere or other extremely harsh environments.4,5 In addition, treatments of P. aeruginosa infection are extremely difficult due to its rapid mutations and adaptation to gain resistance to antibiotics.6 Furthermore, P. aeruginosa is also one of the top-listed pathogens causing hospital-acquired infections, which are widely found in medical devices (ventilation) because they tend to thrive on wet surfaces.7 Importantly, P. aeruginosa is one of the MDR ESKAPE pathogens, which stand for pathogens Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter. P. aeruginosa with arbapenem-resistance is listed among the “critical” group of pathogens by WHO, which urgently need novel antibiotics in the clinics.8
Epidemiological studies have shown that nearly 700,000 people died of the antibiotic resistance bacterial infections each year. P. aeruginosa that was isolated from European populations with a combined resistance was 12.9%.9 Hospital-acquired infection caused by P. aeruginosa continues to produce resistance to conventionally effective antibiotics becoming a main healthcare problem.10 The 2016 EPINE survey found that Escherichia coli and P. aeruginosa are the most common cause of hospital-acquired infections in Spain, P. aeruginosa accounting for 10.5% of clinically isolated bacteria infections.11 The 2011–2012 HCAIs report that P. aeruginosa caused almost nosocomial 9% of infections, which is the fourth commonest pathogen of the European hospitals.12 Similarly, 7.1% of HCAI are caused by P. aeruginosa in the United States.13 In addition, the 2016 European Center for Disease Prevention and Control (ECDC) epidemiological reported that P. aeruginosa causes a variety of ICU-hospital-acquired infections, including pneumonia flares, urinary tract infections, and bloodstream infections.9,10,14,15 Furthermore, data from China Antimicrobial Surveillance Network (CHINET) (http://www.chinets.com/) identified 301,917 clinically isolated pathogenic strains and found P. aeruginosa was the fourth nosocomial infections after Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus, accounting for 7.96%. Altogether, P. aeruginosa is not a local, but a global major threat to human health.
The aforementioned statistics necessitate the identification drug targets and development of new treatments and effective vaccines for P. aeruginosa to improve human health. However, both efforts have met huge difficulty due to the surging cases with MDR strains. This article broadly reviews the recent progress in P. aeruginosa research towards the regulatory and functional mechanisms of virulence factors, gene expression regulators, secretion systems, quorum sensing, and antibiotic resistance, as well as host-pathogen interaction, new technologic advances, and therapeutic development (Fig. 1).
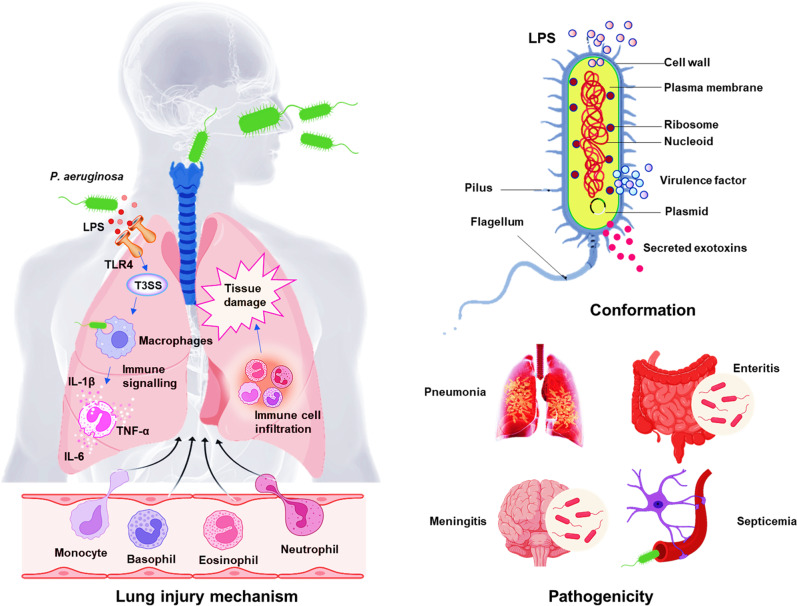
Fig. 1.
Schema of P. aeruginosa pathogenesis. P. aeruginosa can be traced everywhere including hospital environments and cause serious infection of almost any organ. LPS induces TLR-4-dependent and -independent inflammatory responses in the lung after bacterial infection, epithelial cells secrete cytokines and chemokines, thereby recruiting and activating innate immune cells and adaptive immune cells. The recruitment of neutrophils is a sign of inflammatory response activation. Although the activation of neutrophils is critical for host defense, excessively activated immune cell infiltration will cause severe tissue damage and aggravate bacterial infections.478 Therefore, studying the balance between the virulence factors secreted by bacteria and corresponding host immunity is important for the treatment of infections
Virulence factors
P. aeruginosa is able to adapt to the adverse environment in hosts by secreting a variety of virulence factors, which contribute to successful infection and causing disease.16 First, lipopolysaccharide (LPS) is an important surface structural component to protect the external leaflet and posion host cells and the endotoxicity of the lipid A in LPS enable tissue damage, attachment, and recognition by host receptors.17 LPS may be related to antibiotic tolerance and biofilm formation.18 Second, out membrane proteins (OMPs) contributes to nutrient exchange, adhesion, and antibiotic resistance.19 In addition, drug resistance caused by the formation of biofilms is associated with the flagellum, pili, and other adhesins.20 Fourth, there are six types of secretion systems, including flagella (T6SS-associated), pili (T4SS), and multi-toxin components type 3 secretion system (T3SS), which function at colonisation of the host, adhesion, swimming, and swarming responding chemotactic signaling. Finally, exopolysaccharides, such as alginate, Psl and Pel, may help facilitate the formation of biofilms, while impairing bacterial clearance.20
In terms of toxins, T3SS is a complex system and may severely impede host defense via injection of cytotoxins including ExoU, ExoT, ExoS and ExoY, which affect the intracellular environment, especially blocking phagocytosis and bacterial clearance. Exotoxin A (ETA) can inhibit host protein synthesis through ADP ribosylation activity.21,22 Pyocyanin is also toxic to hosts to cause disease severity, damage host tissue, and impair organs’ function.23 In addition, LasA and LasB elastases, alkaline protease (AprA) LipC lipases, phospholipase C, and esterase A enzymes comprise a large group of lytic enzymes that modulate other virulence factors.24 Moreover, rhamnolipids-mediated lung surfactant degrading and tight junction destroying can directly injure trachea or lung epithelial cells.25 Furthermore, siderophores (pyoverdine and pyochelin) as iron uptake systems help in bacterial survival in iron-depleted environment to augment virulence strength.26 Finally, antioxidant enzymes, such as catalases (KatA, KatB, and KatE), alkyl hydroperoxide reductases, and superoxide dismutases, neutralize activity of reactive oxygen species (ROS) in phagocyte environments to avoid clearance.27
Virulence factors related to membranes
Lipopolysaccharide as a virulence factor widely interacting with hosts and also target for vaccines
LPS, an important classical structural component of the outer membrane (OM) of most Gram-negative bacteria, is a known potent agonist that elicits robust innate inflammatory immunity, its distal end may be capped with O antigen, a long polysaccharide that can range from a few to hundreds of sugars in length, which is critical for bacterial physiology and pathogenesis.28 At the early stage, scientists were interested in developing vaccines to prevent infection by focusing on LPS, which were later proven highly difficult due to the various serotypes and inefficacious outcomes.28–30
Pathogen-associated molecular patterns (PAMPs), as small molecular motifs conserved in a class of microorganisms, can be sensed by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) to activate innate immune responses, which effectively protect the host from infection.31 LPS, as the prototypical PAMP, can be recognized by multiple host receptors, including TLRs, PRRs, and nucleotide-binding oligomerization domain-like (NOD-like) receptors.32 The LPS-PRRs/NOD-like molecules activate inflammasome to produce proinflammatory cytokines,33,34 activating TNF-α and IL-1β, two of the most eminent inflammatory cytokines.35 Furthermore, LPS amongst five major Gram-negative bacteria have the ability to induce the production of NF-κB and proinflammatory IL-8 in a TLR4-dependent manner, suggesting that the pathogenesis of bacterial enhancement of chronic inflammatory diseases may be related to its serotype-specific LPS response.36
Apparently, LPS exhibits a crucial role in regulating the interaction of bacteria with their host, is the main cause of tissue degeneration and chronic damage. LPS induces respiratory tract infections by regulating epithelial-mesenchymal transition (EMT)-mediated airway remodeling.37 The mutations of LPS can result in attenuated virulence.38,39 Caffeine alleviates the excessive inflammatory response caused by P. aeruginosa infection by inhibiting the activation of LPS-mediated TLR4/MyD88/NF-κB/miR-301b signaling pathway, and improves lung tissue injury.40 Notably, LPS mutations confer bacteria gain tolerance to phage infection.41 Taken together, in addition to the direct interaction with the host PRRs receptors, LPS may use its unique molecular features to adjust bacterial pathogenesis and damage host immune defense, ultimately benefiting the fitness and invasive strength.
OMVs are important part of virulence platforms
OMVs are bacterial components that can be released spontaneously to the environment during growth by many Gram-negative bacteria. Bacterial-derived OMVs have been characterized as a novel secretion mechanism that can deliver a variety of bacterial proteins and lipids into host cells without direct contact with host cells.42–44 OMVs can package and enrich a wide variety of proteins and nucleic acids, including lipoproteins, periplasmic proteins (E. coli cytolysin A, enterotoxigenic E. coli heat-labile enterotoxin, and Actinobacillus actinomycetemcomitans leukotoxin), plasmid containing chromosomal DNA fragments, phage DNA, virulence factors (LPS, alkaline phosphatase, phospholipase C, β-lactamase, and Cif et al.45,46 P. aeruginosa secretion of OMVs have been implicated in many virulence-associated behaviors, including the acquisition of drug resistance, the regulation of bacterial density and host immune escape.47–51 Mechanistically, P. aeruginosa secretes OMVs to deliver virulence factors and sRNAs into lung epithelial cells through the diffusion the mucus layer.44,47,52–55 Some studies also illustrate that OMVs could lead to an increased hydrophobicity of cell surface, resulting in enhanced ability to form biofilms.56 OMVs is controlled by quorum-sensing systems, which enable bacteria to colonize and immune escape.54,57 Interestingly, OMVs are naturally immunogenic and self-adjuvation, making them have potential to be developed as antibacterial vaccine, such as OMV vaccine for Neisseria meningitidis.58–60 Therefore, OMVs are not only an important functional constitute, but also a potential biotechnological engineering carrier for vaccination or drug delivery. More details about virulence factors and their associated treatment strategies in P. aeruginosa are listed in Table 1 (45, 82–100).
Table 1.
The major pathogenesis factors of P. aeruginosa and therapeutics
| Pathogenic factor | Features and biological role | Therapeutic intervention | Vaccine availability |
|---|---|---|---|
| Proteases | P. aeruginosa secreted proteases include elastase A, elastase B, large protease, protease IV, alkaline protease, Pseudomonas small protease, MucD, and P. aeruginosa aminopeptidase. They exhibit high proteolytic enzyme activity that damages host tissues by degrading proteins. | Protease inhibitors | Preclinical369,392,408 |
| Toxins | P. aeruginosa produces a variety of extracellular toxins, including pigments, phytotoxic factors, hydrocyanic acid, phospholipase, protein convertase, enterotoxin, exotoxin, and mucus. These exotoxins can cause leukopenia, acidosis, liver necrosis, pulmonary edema, circulatory failure, renal tubular necrosis and bleeding, and many other serious damages. | Bacteriophages | Preclinical410,415,436 |
| LPS | LPS is an integral component of cell envelope. It is the major virulence factor of P. aeruginosa and can be recognized by host pattern-recognition receptors to initiate inflammation and immunity response. | Antibody | Phase III380,389,425 |
| Pili and fimbriae | Pili and fimbriae are the major adherence factors. They contribute to the adherence and motility of P. aeruginosa in host. | Phages380,422,424 | None |
| Flagella | The main protein component of flagella is flagellin. Flagella provide motility and chemotaxis toward specific substrates and provide the ligand for clearance by phagocytic cells. | Bacteriophages | Phase III366,393,410 |
| Leukocidin (cytotoxin) | They are secreted by the typical secretion system (e.g., ExoU secreted by Type III secretion system) and are the main cytotoxin targeting lymphocytes and neutrophils. | Natriuretic peptides376,380 | None |
| Siderophores | There are two siderophores produced by P. aeruginosa: pyoverdine (formerly called fluorescein) and pyochelin. In addition to the iron needs, siderophores can support other virulence factors production by transferring iron, such as biofilms and toxic themselves. | Antibiotic-siderophore387 | None |
| Urease | Urease enzyme is a virulence factor (limited extent) of P. aeruginosa. It can hydrolyze urea to produce ammonia and carbon dioxide (CO2). It is associated with urinary tract infection.378,427 | None | None |
| Outer membrane proteins | The outer membrane contains a large number of outer membrane proteins. These protein members are involved in the transportation of amino acids and peptides, the absorption of antibiotics, and the transportation of carbon sources. They are essential for bacterial adherence, virulence secretion, and host recognition. | Potential receptor for the internalization of host | Phase I372,456,457 |
Secretion systems, an integral part of virulence platforms and mechanisms
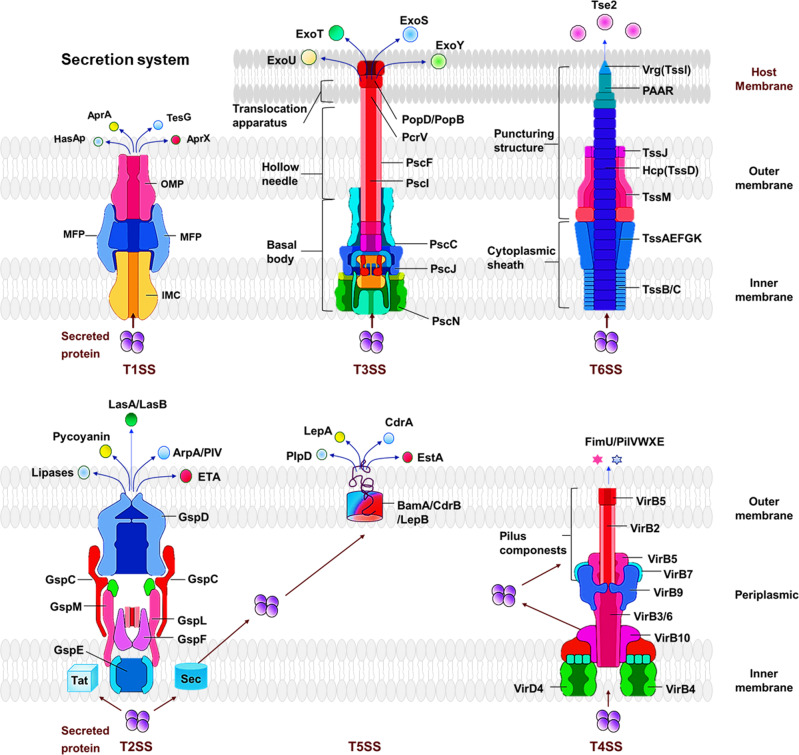
Gram-negative pathogens cause various types of nosocomial infections, and secreted virulence factors often mediate their interactions with the host.61 Bacteria can modulate the host immune response through the secretion system for secreting virulence factors into host cells, which facilitates immune escape and enable bacterial colonization.62 Currently, 6 types of secretion systems (T1SS to T6SS) have been identified in P. aeruginosa. Based on the secretion routes of transport proteins, the secretion systems are divided into two major classes, one-step secretion system (T1SS, T3SS, T4SS, and T6SS) and two-step secretion system (T2SS and T5SS). The one-step secretion system directly secretes proteins from bacterial cytosol to the surface, while the two-step secretion system requires a brief periplasmic stay of the secreted proteins on the export way and then releases the proteins to outside environments of the bacterium (Fig. 2).
Fig. 2.
Protein secretion systems in P. aeruginosa. The secretion systems are divided into two major classes, one-step secretion system (T1SS, T3SS, T6SS) and two-step secretion system (T2SS, T5SS). One-step secretion system exoproteins are directly absorbed into the cytoplasm through their cognate secretion mechanism. In contrast, the exoproteins of two-step secretion system are first exported to the periplasm through the Sec or Tat system, and then crossing outer membranes through specific secretion mechanisms
One-step secretion systems
T1SS
Two different T1SS types in P. aeruginosa elucidated, Apr secretion system and Has secretion system.63 The Apr secretion system consists of three major components: AprD (ATP-binding cassette transporter, ABC transporter), AprE (adaptor), AprF (outer membrane factor, OMF), and secretes two proteins: AprA (alkaline protease), and AprX (protein with unknown function).64 T1SS is found in a variety of bacteria (P. aeruginosa Salmonella enterica, Neisseria meningitidis, and E. coli).65 T1SS major transport proteins (such as proteases and lipases). The substrate protein containing a C-terminal uncleaved secretion signal were recognized by the ABC transporter, were directly transferred across bacterial inner and outer membranes in a one-step process.66 The Has secretion system is composed of HasD (ABC transporter), HasE (adaptor), HasF, and OMF.67 Has secretion system participates in iron regulation by secreting an extracellular haem-binding protein (hasAp).68 Thus far, data relating to T1SS is very limited and its function in pathogenesis and significance for bacterium physiology and fitness are largely unknown, requiring further elucidation in order to know whether it has potential important functions.
T3SS
The T3SS of P. aeruginosa, playing a key role in virulence like quorum sensing, was first discovered in 199669 and is one of the most extensively-studied secreted toxins with increasing evidence for its important virulent effects.70 The T3SS regulon comprises five distinct operons, including the pscN to pscU, the exsD-pscB to pscL operons, the popN-pcr1-pcr2-pcr3-pcr4-pcrD-pcrR operon, the pcrGVH-popBD operon and finally the exsCEBA operon.71,72 The five distinct operons play important roles in the biogenesis and translocation mechanisms of type III secretions. Structurally, the T3SS, similar to a molecular syringe, comprises five components: the needle complex, the translocation apparatus, the regulatory proteins, the effector proteins, and the chaperones.73 T3SS secretes virulence effectors (ExoS, ExoT, ExoY, and ExoU) into the eukaryotic host cells to disrupt intracellular signaling and ultimately causing cell death.74–76 Many bacterial factors regulate T3SS genes of P. aeruginosa. The MgtC and OprF of PAO1 regulate T3SS and ExoS-induced host macrophage damage.77 The T3SS is positively regulated by PsrA,78 HigB,79 Vfr,80 and DeaD,81 but negatively regulated by MexT,82 AlgZR, GacAS/LadS/RetS,83–88 and MgtE.89 No only for P. aeruginosa, the T3SS is a highly important secretion mechanism for Gram-negative bacterial invasion factors, which may also facilitate the bacterial evasion from the host immune responses to establish invasion, colonization, replication, and spread.
T6SS is a novel, important virulence machinery
In P. aeruginosa, T6SS is a newly identified powerful system with diverse and vital functions in virulence, bacterial interaction, and competition with the environmental microorganisms.90 Initially, the genome of P. aeruginosa was thought to constitute three gene clusters called Hcp Secretion Island (HSI) encoding T6SS components, which are later renamed H1-T6SS to H3-T6SS,91,92 with ~15–20 genes for each of them.93 In addition, the apparatus of T6SS, consisting of 13 core components, is divided into a baseplate-like structure, a sheathed inner tube assembled from the baseplate-like structure and a trans-membrane complex.94 The assembled T6SS appears to be an inverted phage tail, with the Hcp (hemolysin coregulated protein)/Vgr (valine-glycine repeat protein) complex forming the distal end of the cell-puncturing device.95 The sheath transits the effectors into targeted cells by a contraction-based mechanism.96 Furthermore, ClpV, an AAA+ family ATPase of T6SS, also provides the energy necessary to drive the secretory apparatus.97
Mechanically, the secretion process by T6SS needs other elements; for example, the H2-T6SS machinery to deliver the novel antibacterial toxin Tle3 requiring a cytoplasmic adaptor Tla3.98 The GacAS/Rsm regulates T6SS (H1-T6SS and H3-T6SS) by activating RsmY/Z to inhibit the binding activity of RsmA/RsmN to fha1/tssA1.99 H3-T6SS secrete TseF to facilitate the import of the PQS-Fe3+ complex into cells by incorporating it into OMVs with Pseudomonas quinolone signal (PQS).100 Interestingly, it was found that the quorum sensing (QS) system plays an important role in the expression of this secretion system. In P. aeruginosa, QS differentially regulates three loci of encoding T6SS (HSI-I, HSI-II, and HSI-III) by LasR and MvfR.101 The QS systems regulator of Las and Rhl controlls the expression of H2-T6SS in PAO1 strains102 and the QS regulator of MvfR directly modulates the expression of multiple proteins, including virulence factors and other regulators in PA14,103 respectively.
The H1-T6SS-dependent substrates have a broad research foundation, while only little is known about functional roles of H2-T6SSs and H3-T6SSs due to the limited substrates available for research.104 With collaboration efforts, we characterize H2-T6SS-dependent secretomes that are related to copper (Cu2+)-binding effector azurin (Azu).104 Azu and H2-T6SS are both inhibited by CueR while induced by low levels of Cu2+. Furthermore, our studies reveal an Azu-interacting partner OprC, which is a Cu2+-specific TonB-dependent outer membrane transporter and is also modulated by CueR. The Azu-OprC-mediated Cu2+ transport network may contribute to P. aeruginosa virulence. Our follow-up studies indeed illuminate that oprC dampens host response in cells and mice to Pseudomonas infection by potently enhancing Quorum-Sensing-associated virulence.105
In addition, we recently describe a function for H2-T6SS of P. aeruginosa for specific delivery of AmpDh3 (a paralogous zinc protease) to the periplasm of a prey bacterium upon contact.106 AmpDh3 can hydrolyze peptidoglycan located on the cell wall of the prey bacterium to induce prey cell death, which serves as evolutionary advantage for P. aeruginosa in a competitive environment.
In spite of the relative short time since discovery of T6SS, the progress in understanding the potent virulence pathway is fascinating and fast-moving, which may define many unknown functions that can be attributed to T6SS’ virulence and indicate new ways for treating patients who suffer the most severe disease and difficult to treat.107
T3SS and T6SS in cooperation for regulating host and bacterial responses
T3SS and T6SS are indicated to regulate host and bacterial responses, including host cell apoptosis, inflammatory response, colonization, motility, biofilm formation, and bacterial competition/interaction108,109 (Fig. 3). Interaction regulation and inter-conversion of T6SS and T3SS may be especially helpful for coping with complex environmental pressures.110 The switch between T6SS and T3SS is directly regulated by the RNA-binding protein RtcB controlling colonization, establishment, and pathogenicity in P. aeruginosa.111 YbeY regulates T3SS and T6SS secretion systems and biofilm formation by controlling RetS.112 The function of T3SS is regulated by various regulators, including four main regulators genes (exsA, exsC, exsD, and exsE), which is involved in the transcription activation of the aforementioned classical effectors (exoS, exoT, exoU, and exoY).72,113 ExoS is a 48.3 kDa protein containing 453 amino acid length. It has been reported early that ExoS participates in host cell apoptosis via its GAP region or ADP-ribosyltransferase (ADPr) activity.114,115 Furthermore, the ExoS possesses ADPRT activity, which induces P. aeruginosa-afflicted host cell apoptosis by targeting a variety of Ras proteins.116 ExoU is the longest P. aeruginosa effector containing 687 amino acids (73.9 kDa).117 ExoU is the most acutely cytotoxic among the four effector proteins because it can induce rapid cell death and is considered to be the main driver of the cytotoxic phenotype.118 ExoU dysregulates the host’s innate inflammatory response by poisoning and killing immune cells, including macrophages, neutrophils, epithelial cells, and endothelial cells, allowing bacteria to persist, proliferate, and spread, and ultimately leading to sepsis, Alzheimer’s disease, acute respiratory distress syndrome, etc.114 Mechanistically, ExoU transiently represses Capase 1 and NLRC4 inflammasome activation, inhibiting the release of IL-1β, IL-18, and proinflammatory DAMPs, and thereby suppressing the host immune response.119,120 In addition, ExoU can activate AP-1 transcription factors to increase IL-8 production and induce tissue-damaging inflammatory by JNK/MAPK) pathway.121 ExoT containing 457 amino acids (48.5 kDa) has GAP and ADPRT activities, and can induce host cell apoptosis by targeting Crk proteins phosphorylation of p38β and JNK induces apoptosis, which subsequently interferes with integrin-mediated survival signaling via destroying the stability of focal adhesion sites.122 Notably, recent studies have shown that P. aeruginosa ExoT induces G1 cell cycle arrest in melanoma cells, suggesting its potential for regulating the cell cycle.123 ExoY is a 378 amino acid protein with a molecular size of 41.7 kDa, detected in 80–100% of P. aeruginosa.124 ExoY plays a direct role in immune escape by inhibiting TAK1 activation, which is a key factor in the TGF-inducible pathway that directly modulates immune responses, contributing to P. aeruginosa survival and infection severity.125 In addition, ExoY regulates host inflammatory responses by delaying activation of NF-κB and caspase-1.126
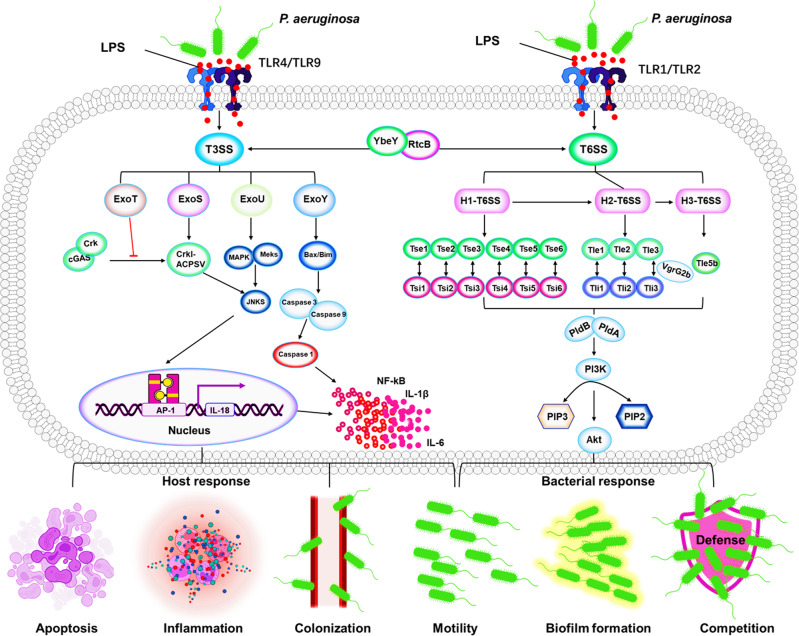
Fig. 3.
Mechanisms of T3SS and T6SS in regulating bacterial pathogenesis and host responses in P. aeruginosa. LPS is recognized by TLRs (TLR1/2 or TLR4/9) and then activates T3SS and T6SS.479 T3SS and and T6SS represent a critical network in regulating bacterial behaviors (growth, biofilm formation, and competition) and host defense (host cell apoptosis, inflammatory response, colonization, and motility). T6SS and T3SS interaction and inter-conversion are regulated by RtcB and YbeY. ExoS/ExoU induce P. aeruginosa-afflicted host cell apoptosis and colonization by targeting JNKS signal pathway. ExoY/ExoT reduces inflammasome activity through inhibition of bacterial motility to dampen NF-κB and caspase-1 activation. T6SS is a powerful antibacterial weapon that can be injected through multiple effectors to compete with other bacteria and allows P. aeruginosa colonization and biofilm formation108,109
The T6SS components and their effectors are diverse and complex beyond bacterial-cell targeting. T6SS systems have been detected in ~200 Gram-negative bacteria, including P. aeruginosa.109 To compete for survival in the living environment, H1-T6SS kills other bacteria by injecting Tse2 effector molecules into other target bacteria possessing antibacterial activity and providing advantages for P. aeruginosa growth. In addition, to protect itself from Tse2 toxins, P. aeruginosa also produces the antitoxin Tsi2.127 Similarly, H1-Tse1 and Tse3 are injected into the periplasm of other bacteria to hydrolyze peptidoglycan, which can be counteracted by periplasmic immune proteins Tse1 and Tse3.128 Tse4, Tse5, Tse6, and Tse7 that also show antibacterial activity are associated with homologous immunity.129 The phospholipase D enzymes PldA and PldB of Tle5 were injected to other bacteria by H2-T6SS and H3-T6SS to exert antibacterial activity.130 Further, VgrG2b is injected into epithelial cells by H2-T6SS, in which it targets the γ-tubulin ring complex component (γ-TuRC) and promotes the recruitment of PI3K at the apical membrane.131,132 Moreover, PldA/B targets the host PI3K (phosphoinositide 3-kinase)/Akt pathway to remodel the PIP3 (phosphatidylinositol-3,4,5-triphosphate) and actin in the apical membrane, which is essential for successful colonization of the host by P. aeruginosa.133 T6SS-related genes hcp1/hcp3 and lasR were significantly higher in the strong biofilm formation (SBF) compared to nonbiofilm formation (NBF) groups, which contributes to the biofilm production by P. aeruginosa.90 Collectively, T6SS is a powerful antibacterial weapon that can be injected with many different effectors to compete with other bacteria and allow P. aeruginosa colonization and biofilm formation.
Two-step secretion systems
Different from the One-Step secretion, Two-step secretion requires a brief periplasmic phase of the secreted proteins on the export route before being exported to the outside of the cell through general export pathways, which plays an important role in the transport of periplasmic and outer membrane proteins.
T2SS
The function of T2SS is one of the less characterized secretion systems and is thought to secrete folded proteins from the periplasm.134 Two different pathways exist in T2SS: the general secretory (Sec) and twin-arginine translocation (Tat).135 The secreted proteins are first transited through the inner membrane, stays briefly in the periplasm and then secreted into the extracellular environment.136,137 The Sec pathway consists of a protein targeting component, a motor protein, and a membrane integrated conducting channel called SecYEG translocase, the secreted proteins with a SecB-specific signal sequence might be guided to the periplasm or the extracellular environment.138 The twin-arginine translocation (Tat) pathway of Gram-negative consists of TatA and TatB, which can decide whether the secreted is retained in the periplasm or translocated to the extracellular space with a twin-arginine motif.139 Functionally, T2SS participates in the secretion of guanylate cyclase ExoA, proteases lasA/B and multiple other factors and many of which have emerged as potential therapeutic targets.140,141
T5SS
The T5SS of P. aeruginosa is composed of five subtypes (type Va to Ve) and exports the proteins across the inner membrane via the Sec pathway.142 The proteins of the V-type secretion system are often referred to as autotransporters (ATs). Typically, the T5SS consists of only one polypeptide chain with a β-barrel translocator domain in the membrane, and an extracellular passenger or effector region.143 Under the regulation of the Bam complex (β-barrel assembly mechanism) and TAM complex (translocation and assembly module), outer membrane proteins fold to form a β-barrel conformation and insert into the outer membrane.144 T5SS secretes a variety of proteins, including EstA, LepB, and LepA. EstA has esterase activity and is involved in rhamnolipid production and biofilm formation.143
The type Vb secretion system comprises two distinct polypeptide chains encoded in one operon, therefore, it is also known as the dual-partner secretion system (TPSS) containing the passenger domain (TpsA) and the β domain (TpsB).145 TpsA has a TPS secretion motif and a functional/catalytic domain and the TpsB is a 16-chain OM-integrating β-barrel protein with two periplasmic POTRA (polypeptide transport-associated) domains.146 The Vc-type secretion system forms a highly intertwined trimeric structure and is therefore also known as a trimeric autotransporter adhesin (TAA).147 The C-terminal β-barrel domain of the Type Vd consists of 16 β-strands, similar to the β-barrel of TpsB proteins.145 The Ve-type ATs share obvious similarities with Va-type ATs, the main difference is that Ve-type ATs have an inverted domain order, with the β-barrel at the N-terminus and the passenger at the C-terminus.148 Despite these preliminary studies, there is much more to be learnt regarding T5SS for the physiology and virulence in P. aeruginosa.
T4SS
T4SS is a multisubunit cell-envelope-spanning structure that can transfer protein and nucleoprotein complexes across membranes, which is related to horizontal gene transfer-mediated antibiotic resistance, adaptation, evolution, and virulence.149 T4SS in bacteria is divided into the IVA-type secretion system represented by Agrobacterium tumefaciens VirB/VirD4 and the IVB-type secretion existed as Legionella pneumophila Dot/Icm system. Those distinct from the above are classified as “Other T4SS”, which contain genomic islands pKLC102 and PAPI in P. aeruginosa and are less characterized than other two types.149 P. aeruginosa T4SS comprises abundant major pilin subunits, PilA, and low abundance minor pilins FimU and PilVWXE. Both major pilin and minor pilins are processed by the pre-prepilin peptidase, PilD to exert function. T4SS assembly system is evolutionarily associated with T2SS contributing to the process of assembly and disassembly of pili. Minor pilins can impact assembly, retraction, extension, and adhesion.150
Again, the chief function of T4SS is horizontal genetic transfer (HGT) between different microorganisms and potentially relating to pathogenesis.151 For the genetic island containing T4SS, there is a discrepancy: whether a conjugation mechanism exists but this is likely related to differences between strains.152 Nevertheless, there are relatively limited studies of this system compared to other secretion systems such as T3SS. We will further discuss the potential role of T4SS in host response context, initiating/activating inflammasome independent on nucleotide oligomerization domain (NOD)-like receptor (NLR) family CARD (C-terminal caspase recruitment domain) domain-containing protein 4 (NLRC4),153 in addition, the virulence factors and their associated secretion systems in P. aeruginosa were summarized in Table 2.
Table 2.
The virulence factors and their associated secretion systems in P. aeruginosa
| Secretion systems | Virulence factors | Features | Pathogenicity | Inhibition | Interaction\regulation |
|---|---|---|---|---|---|
| T1 | Alkaline protease | Adherence and Colonization342 | Corneal infections | APRin363 | Ca2+372 |
| HasAp | Proliferation375 | Scavenges heme | Metal complexes382 | hasR | |
| T2 | LasB (elastase) | Biofilm formation353 | Antibiotic resistance | Ag-phendione, Cu(phendione)395 | IL-1β438, LasA384,395,397,404,438,451,458 |
| LasA | Biofilm formation and Colonization | Antibiotic resistance459 and Corneal infections370 | Satureja khuzistanica essential oil339,343,369 | LasD | |
| Phospholipase C (PlcH, PlcN) | Host inflammation regulation | Cell hemolysis and Neutrophil activity344,354 | Global regulator Anr | Ca2+, Pi, Zn+, PlcR1, PlcR2, IL-8375,385,420,421,440 | |
| PrpL protease | Biofilm formation | Wound healing and Corneal virulence403 | – | Iron, Pvds, AprA, LasB434,447 | |
| Lipases (LipA, LipC) | Motility, Biofilm formation and Rhamnolipid production | Enhanced PLC-induced 12-HETE and LTB4 generation | DsbC protein381 | PmrA/PmrB401,411 | |
| ToxA | Colonization and Proliferation | Impairment of host defense, Inhibition of protein synthesis and Interference with cellular immune functions | Norepinephrine340 | Iron and PvdS404 | |
| LapA (via Hxc T2SS) | Biofilm formation and Localization adhesin | Antibiotic resistance346 | – | LapF, c-di-GMP374,377,405,419 | |
| Mep72 | – | – | – | PopD, PcrV, ExoS, FliD | |
| T3 | ExoS | Avoid phagocytosis and Kill the host cell | Inhibition of autophagy and mTOR, ROS Production and Induces apoptosis | Cinnamaldehyde and Carvacrol and honey413,417 | RpoS, Rac116,418,429,444,449 |
| ExoY | Enhance acute pathogenicity and infection | Impairs endothelial cell proliferation and vascular repair116,418,429,444,449 | Aspirin | GSH, NF-κB, AP-1, TAK1 | |
| ExoT | Inhibition of internalization by epithelial cells and macrophages | Inhibits lung epithelial wound repair386,398,399,407,437 | – | RhoA, Rac1, Cdc42 | |
| ExoU | Overexpression efflux pumps and AmpC β-lactamase genes | Antibiotic resistance and Augments neutrophil transepithelial migration | Arylsulfonamides388,409,426 | NF-κB, IL-8/KC | |
| ExsE | Negative regulator of type III secretion gene430,455 | – | – | T3SS | |
| T5 | Esterase (EstA) | Enhanced rhamnolipid production, Cell motility and Biofilm formation | – | – | ExsC, ExsD443,448 |
| CupB5 | Activates alginate overproduction | – | – | AlgU, MucA, TpsB4\LepB381,390 | |
| Exoprotease (Lep) | Producing pyocyanin | – | – | Iron396 | |
| T6 | Tse1 (amidase) | Defense against other organisms | Lysis of cells | Tsi1 | Tsi1360 |
| Tse2 | Defense against other organisms | Lysis of cells | Tsi2 | Tsi2431 | |
| Tse3 (muramidase) | Defense against other organisms | Lysis of cells | Tsi3 | Ca2+, Tsi3416,445 | |
| Other secretion systems | HCN | – | – | – | Glycine, AlgR, LasR and RhlR382,383 |
| Pyocyanin | – | Interferes with multiple cellular functions | Baicalin, 4-aminopyridine | BrlR, PhzM383,387,397 | |
| Pyoverdine | High-affinity iron uptake from transferrin and lactoferrin | Keratitis | Phenylthiourea | PvdP tyrosinase439,446 | |
| Rhamnolipids | Biofilm formation | Potentiate aminoglycoside antibiotics | H2S | Oxygen, Polyhydroxyalkanoate371,418,421,422 | |
| Alkyl quinolones | Bacterial communication and quorum sensing | – | – | PqsR, LysR433 | |
| N-acyl homoserine | Biofilm formation | – | – | Propolis379 | |
| Lactones | Biofilm formation | Accelerate host immunomodulation | Th1 and Th2 cytokines401 | – |
Virulence regulatory systems
The regulation of all these virulence factors is cell density-dependent through release of autoinducers of critical quorum sensing (QS) (e.g., Las, Rhl, Pqs, and Iqs), a mass communication system.154 QS may help large population fitness by a hierarchical signal pattern to survive in fierce host environments and thrive, leading to persistent infection in individuals with cystic fibrosis, which cannot be completely cured even with tremendous progress in drug development, drastically improved medcare systems and living conditions.155 Hence, QS systems along with some other critical virulence factors, such as six types of secretion systems (of toxic molecules), two-component systems (TCSs), have become an intense interest in mechanistic understanding of this bacterium.
Quorum sensing is the most-studied systems for regulating gene expression and virulence
Roles and acting mechanisms of QS
QS describes a method that is widely utilized by bacteria for cell–cell mass communication. Both Gram-negative and -positive bacteria detect the local population density by sensing chemical signals and coordinate gene expression and group-beneficial behaviors.156,157 Bacteria produce autoinducer or quoromone as diffusion signaling molecules and release into the environment for communication. Once the population reaching a threshold, the autoinducers activate their cognate receptors to directly or indirectly induce gene expression.158 Over the past two decades, QS has been extensively studied as a potential target for ‘antivirulence agents’, which may be harnessed to counteract bacterial virulence via a noncytotoxic mechanism as alternatives of traditional antibiotics.159,160
Another essential function for QS in P. aeruginosa is to regulate the production of multiple virulence factors, such as extracellular proteases,161 iron chelators,162 efflux pump expression,163 biofilm development,164 swarming motility,165 and the response to host immunity.166 As a model organism, P. aeruginosa serves as one of the most suitable bacteria to study the fundamental mechanisms of QS signaling regulating virulence and search for chemical agents to block the QS system.167,168
Interaction between quorum-sensing systems and environments
Bacteria communicate with each other through the QS, which acts as a global regulatory system by directly or indirectly controlling the expression of genes, has been the central point of research.157 For example, scientists have made significant efforts in understanding the interactions between all the four QS systems and also how environmental cues may affect gene expression and function of the QS. Two canonical N-acyl L-homoserine lactone (AHL) based (Las and Rhl) and two 2-alkyl-4 quinolones (AQ) based (Pqs and its precursor Hhq) signaling systems.70 These systems connect and coregulate each other. Rhl and Pqs were positively regulated by the Las system, while Rhl represses Pqs and Pqs augments Rhl.169 For example, in response to various nutritional and environmental stimuli, the regulatory relationship between Rhl and Pqs systems can change independently of Las.170
The activation of QS genes generally requires a large number of regulatory factors to control receptor expression or function, and/or to coregulate some QS-controlled target genes since the QS systems are functional diverse, organizational complex, and consisting of a spectrum of key regulators (including rpoS, vqsR, mvfR, rhlR, rsaL et al.).171 RpoS indirectly plays a subtle role in activating lasR and rhlR expression and modulating ~40% QS-controlled gene expression during the stationary phase.172 VqsR controls the production of AHL signaling molecules and virulence factors by inhibiting the LuxR-type regulator QscR, which represses lasI expression to regulate the timing of QS activation.173 VqsM positively regulates the QS systems by controlling several relevant QS regulators ranging from QS to antibiotic resistance, and P. aeruginosa pathogenicity.174 MvaT and its homolog MvaU control the magnitude and timing of QS-dependent gene expression,175 which have a massive impact on all three QS systems by directly regulating lasR, lasI, rsaL, pprB, mvfR, algT/U, mexR, and rpoS.176 RsaL binds to the lasI promoter and prevents LasR-mediated activation, regulates las signaling,177,178 and modulates the activity of PqsH and a recently identified regulator, CdpR, which are required for PQS synthesis.179 AmpR activates QS-regulated genes to positively influence acute virulence, while negatively regulating biofilm formation.180 CdpR negatively modulates bacterial virulence by impacting the expression of pqsH, which is positively regulated by LasI and VqsM along with QscR and RsaL.181 We also recently found that BfmR/S and/or its variants modulate the rhl QS system in P. aeruginosa.182 Crc regulates rhl QS by promoting Hfq-mediated suppression of lon gene expression.183 More recently, our laboratory delineated that AnvM is a critical regulator of virulence in P. aeruginosa by directly interacting with the QS regulator MvfR and anaerobic regulator Anr.184 The aforementioned discoveries have highlighted the rapid progress in understanding diverse, heterogenous regulatory mechanisms of QS coordinated by seemingly a large number of unprecedented factors, which may finally characterize powerful, versatile regulatory proteins or systems to be applied to better control the notorious pathogen.
Several factors (such as QscR and QteE) have been identified to regulate the activation threshold of quorum-regulated genes, which control QS activation timing through additional homeostatic mechanisms.174 In addition to the QscR mentioned above, QteE also blocks QS-regulated genes’ expression by preventing LasR and RhlR accumulation and blocking Rhl-mediated signaling.185 Moreover, QslA is found to interact with LasR.186
Based on extensive studies to date, we may presume that QS may be one of the most important regulatory systems in Pseudomonas contributing substantially to bacterial physiology, adaptation, and pathogenesis. Although, various studies have tested the ideas of targeting QS for potential therapy of bacterial infection, the effect of using QS-associated approach for treatment is unsatisfactory.187 In particular, there were only limited reports of in vivo treatments targeting QS.188 Hence, it is necessary to further study the fundamental role of QS in bacterial pathogenesis and identify new anti-virulence targets and approaches that would help develop urgently needed medicines for treating refractory infection in clinics for QS bearing bacteria.
The two-component regulatory systems are critical gene regulators and virulence factors
The two-component systems (TCSs) are ubiquitous, complex signaling regulators that play vital roles in bacterial survival, metabolism, and virulence.189 As a versatile opportunistic pathogen, P. aeruginosa virulence network is tightly controlled by a growing number of TCSs.190 In general, a TCS pair of genes consists of a membrane-bound sensory histidine kinase (HK) and a cytoplasmic response regulator (RR). In P. aeruginosa, 64 HKs and 72 RRs have been identified.191 More than 50% of the TCSs and their corresponding HKs are linked to virulence and/or antibiotic resistance of P. aeruginosa.192 For instance, CzcR/CzcS is implicated in carbapenem-resistance;193 KinB/AlgB is involved in the alginate synthesis and virulence;194 and GacS/GacA is essential for pathogenicity.195 Remarkably, an attenuation in virulence behavior can be achieved by blocking TCS signaling. Goswami et al. reported that inhibition of HKs, especially Rilu-4 and 12, significantly reduced the production of virulence factors and toxins, and severely impacted the motility behavior of PA14.195
Our recent studies discovered a new copper-responsive TCS called DsbRS in P. aeruginosa, in which DsbS (sensor of histidine kinase) and DsbR (cognate response regulator) modulate gene transcription for disulfide bond formation (Dsb). DsbS (phosphatase) targets DsbR to interfere with the transcription of Dsb genes and help the bacterium cope with copper stress.196 Intriguingly, transcription factors can also regulate the behaviors of bacteria to adapt host environments; for instance, imidazole-4-acetic acid (ImAA) and its receptor HinK are recently implicated in the response of P. aeruginosa to histamine.197 These findings help understand the communication between P. aeruginosa and hosts to adjust bacterial health. We have summarized TCSs and their roles in controlling the key virulence factors in P. aeruginosa (Table 3).
Table 3.
The composition and function of the two-component regulatory systems
| Composition | Virulence factor | Function |
|---|---|---|
| PilSR | Type IV pili460 | Twitching and Swimming Motilities |
| RocS1-RocR-RocA1 | CupC fimbriae461 | Biofilm formation/Antibiotic tolerance |
| FimS-AlgR, KinB-AlgB | Alginate462 | Exopolysaccharide alginate synthesis |
| BfiSR | Rnase G463 | Regulation sRNA levels |
| GacSA, RetS | Exopolysaccharides (Pel, Psl)464 | Biofilm formation |
| RcsCB, PvrSR | CupD fimbriae204 | Fimbriae assembly/Biofilm formation |
| BfmSR | Edna465 | Bacteriophage-mediated lysis and DNA release/Biofilm formation |
| PprAB | Cup E fimbriae466 | Fimbriae assembly/Biofilm formation |
| BqsSR | Rhamnolipid467 | Quorum sensing-dependent biofilm |
| FleSR | Flagella468 | Flagellar biogenesis |
| FimS-AlgR | Type IV pili469 | Twitching motility/Biofilm maturation, Surface adhesion/Virulence |
| PA2573-PA2572 | ExoS469 | Virulence and antibiotic tolerance |
| TtsSR | CbpE470 | Specific secretion of the CbpE chitin-binding protein |
| GtrS-GltR | ToxA471 | The expression of exotoxin A and glucose catabolic enzymes |
| GacS-LadS-RetS, CsrA/RsmA | Type III secretion472,473 | Virulence/Secretion systems/Biofilm formation, Quorum sensing/Motility |
| RsmA | Elastase474 | Pyocyanin, rhamnolipids and elastase production |
| LadS | ExoU475 | Virulence and the switch between acute and chronic infections |
| RocS1-RocR-RocA1 | ExoY, ExoT476 | Biofilm formation |
| CbrAB | ExoT, ExoS477 | Metabolism, virulence, and antibiotic resistance |
| FimS-AlgR | Regulated by PilY1469 | Virulence |
The number of TCSs related to the virulence of P. aeruginosa has recently grown considerably, which may be highlighted by the multi-kinase networks containing multiple sensor kinases through crosstalk (network) to impact virulence.192 The networks include: (1) GacS network governs the switch between acute and chronic infections;198–201 (2) Roc network and Rcs/Pvr network control cup fimbriae production;202–204 (3) A complex network of five TCSs (PmrB, PhoQ, ColS, ParS, and CprS) regulates the aminoarabinose modification of lipopolysaccharide;205–207 (4) Wsp chemosensory pathway modulates biofilm formation and chronic infection;208,209 and (5) Chp/FimS/AlgR network involves biofilm formation and virulence.210,211 Although TCSs may control many functions, some of them may have unknown roles that require further studying. TCSs play a significant role in controlling either P. aeruginosa virulence or virulence-related behaviors (such as biofilm formation and antibiotic resistance). The functions of TCSs may contribute to the clinical significance exemplified by a more recently identified BfmRS TCS.182 Indeed, the plasticity of TCSs mediated by spontaneous mutations of BfmRS in patients has been assessed, and the data suggest that mutation-induced activation of BfmRS may be related to host adaptation by P. aeruginosa in chronic infections.212 Despite increasing identification of novel TCSs, several critical questions remain to be answered in future investigation: how multi-kinase networks process and integrate signals, which of the kinases plays a dominant role in multi-kinase networks, and how these TCSs interact with other systems, quorum sensing and secondary messenger signals to confer pathogenicity (e.g., cAMP and c-di-GMP).
Small non-coding regulatory RNAs play roles in regulating gene expression and virulence
Small non-coding regulatory RNAs (sRNAs) range from 50 to 400 nucleotides in length.213 Since the discovery as part of a large group of transcriptional regulators in Escherichia coli in the 1960s, sRNAs are gradually recognized as key modulators to mount rapid responses when necessary and are encoded widespread in the bacterial genomes213–215 despite to different extent (the number of sRNAs in PAO1 is approximately twice higher than that in PA14). sRNAs play an essential role in bacterial pathogenicity and virulence mechanisms, such that participation in quorum-sensing regulation, ion metabolism, biofilm formation, stress responses, host cell invasion, and adaption to growth conditions.216–218 Hfq-dependent sRNAs are instrumental for modulating P. aeruginosa virulence.219 rsmY and rsmZ act as early responders to finely modulate bacterial cooperation under environmental stimuli to optimize population density.220
In P. aeruginosa, sRNAs can regulate bacterial metabolism instantly and precisely to establish successful infection in the hosts.218 A total of 573 sRNAs were detected in PAO1 and 233 sRNAs in PA14, indicating their quantity variation in different strains.201 Although 126 sRNAs are found in common to both strains, sRNAs can evolve rapidly, and many sRNAs exhibit strain‐specific or environmental-dependent expression.221 Interestingly, we recently revealed that sRNA PhrS may help generate CRISPR RNA (crRNA) for initiating bacterial immunity against bacteriophages, which is achieved through inhibiting Rho function on transcription-termination.222 Collectively, it would be intriguing to further understand the functions and underpinning mechanisms involving sRNA in this bacterium, which may identify novel pathway regulators and drug targets for controlling bacterial invasion.
Acquisition of antibiotic resistance contributes to bacterial survival and thriving
The acquisition of drug resistance by P. aeruginosa depends primarily on multiple intrinsic and acquired antibiotic resistance mechanisms, including the biofilm-mediated formation of resistant and multi-drug-resistant persistent cells.223 Therefore, P. aeruginosa can quickly develop resistance to various antibiotics, including aminoglycosides, quinolones, and β-lactams.224
In the course of long-term evolution, bacteria have developed a variety of ancient genetic resistance mechanisms that have significant genetic plasticity against harmful antibiotic molecules, enabling them to respond to various environmental threats, including possible harm (e.g., antibiotics, chemical compounds, and antimicrobial peptides) to their survival. The acquisition of antibiotic resistance with P. aeruginosa is quite diverse, and some primary mechanisms including outer membrane permeability, efflux systems, and antibiotic-inactivating enzymes will be addressed below225 (Fig. 4).
Fig. 4.
Mechanisms of antimicrobial resistance in P. aeruginosa. Mechanisms of antimicrobial resistance in P. aeruginosa can be divided into intrinsic antibiotic resistance (① outer membrane permeability, ② efflux systems, and ④antibiotic-modifying enzymes or ⑤ antibiotic-inactivating enzymes), acquired antibiotic resistance (⑥ resistance by mutations and acquisition of resistance genes), and adaptive antibiotic resistance (③ biofilm-mediated resistance). Alteration of outer membrane protein porins decreases the penetration of drugs into cells by reducing membrane permeability. The efflux system directly pumps out drugs. Drug-hydrolyzing and modification enzymes render them inactive. Similarly, some enzymes cause target alterations so that the drug cannot bind its target, resulting in drug inactivity. Antibiotic resistance genes carried on plasmids can be acquired via horizontal gene transfer from the same or different bacterial species,225 quorum-sensing signaling molecules activate the formation of biofilms, which act as physical barriers and prevent antibiotics penetrating the cell
Outer membrane permeability
To treat P. aeruginosa infections, most antibiotics need to penetrate the cell membrane to reach the intracellular compartment to function.226 The outer membrane of P. aeruginosa can act as a specific hurdle inhibiting antibiotic penetration. The outer membrane of P. aeruginosa is chiefly composed of bilayer phospholipid molecules, LPS and porins embedded in phospholipids. The outer membrane is responsible for the specific and non-specific uptake of extracellular substances relying on different porin functions, including non-specific porins (OprF), specific porins (OprB, OprD, OprE, OprO, and OprP), gated porins (OprC and OprH), and efflux porins (OprM, OprN, and OprJ).227–230 P. aeruginosa manipulates different porins to limit antibiotic penetration and increase antibiotic resistance. OprF promotes the formation and attachment function of P. aeruginosa biofilm to protect the bacterium against antibiotics.231–233 Mutations of specific porins OprD involving conformational features cause carbapenem resistance, a serious challenge for medical treatment practices.234 Outer membrane protein H (OprH) of P. aeruginosa enhances the stability of the outer membrane through direct interaction with LPS to regulate antibiotic resistance.235,236 Efflux porins (OprM, OprN, and OprJ) contribute to the active efflux of several antibiotics, including tetracycline, norfloxacin, and β-lactam antibiotics.237 These findings demonstrate that the elegant effects and diverse mechanisms by which porins determine antibiotic resistance.
In a separate account, in recent years, OMVs secreted by P. aeruginosa are shown to be able to transport multiple virulence factors, like hemolytic phospholipase C, mRNA, DNA, β-lactamase, alkaline phosphatase, to the host cytoplasm, which may be a part of pathogenic mechanisms of antibiotic resistance. OMVs may be fused with the host plasma membrane through receptor-mediated endocytosis. While OMVs are detrimental to the host by delivering antibiotic resistance molecules or enzymes (β-lactamase), they have been harnessed as alternative delivery vehicles for transporting treatments or vaccines for various diseases including infection and cancer.47,238
Efflux systems for pumping off drug resistance factors
The toxic compounds derived from metabolism or antimicrobials inside bacterial cells are harmful to bacterial survival, and require mechanisms to expel. The efflux pump is a main, conserved mechanism to remove antibiotics. The efflux pump may upregulate virulence genes (QS) for enhancing antibiotic resistance and maintaining bacterial homeostasis. Currently, five components are described in the efflux pump family: ATP-binding cassette (ABC) superfamily, major facilitator superfamily (MFS), and multidrug, toxic compound extrusion (MATE) family, resistance-nodulation-division (RND) family, and small multidrug resistance (SMR) family.239 Among all efflux pumps, the RND efflux pump family has the strongest correlation with antibiotic resistance. Twelve RND family efflux pumps are identified in P. aeruginosa: multidrug efflux AB-outer membrane porin M (MexAB-OprM), multidrug efflux CD-outer membrane porin J (MexCD-OprJ), multidrug efflux EF-outer membrane porin N (MexEF-OprN) and multidrug efflux XY-outer membrane porin M (Mex XY-OprM) can increase resistance to a variety of antibiotics through efflux.240 MexAB-OprM is crucial for developing carbapenem-resistant P. aeruginosa, which is a lingering clinical issue.237 Upregulation of MexCD-OprJ is closely associated with increased resistance of most clinical strains to ciprofloxacin, cefepime, and chloramphenicol.241 Quinolones MexEF-OprN are overproduced by the QS deficiency due to kynurenine extrusion.242 Furthermore, the resistance of P. aeruginosa to aminoglycosides, fluoroquinolones, and zwitterionic cephalosporins depends on the efflux function of MexXY-OprM.243 These findings suggest that despite some similarity in substrates, their affinity of efflux pumps may vary greatly, displaying different extent of anti-antimicrobial activities.
Multiple lines of evidence suggest that the chief mechanism for P. aeruginosa success in infection is highly related to its stubborn resistance to antibiotics or other therapeutics, which is regulated by the efflux pump. Hence, targeting this mechanism such as inhibiting the critical efflux pump—mexAB-oprM or enhancing the repressor—mexR, will likely reveal new strategies to overcome antibiotic resistance mechanisms in the bacterium and achieve the improved treatment efficacy.244
Antibiotic-inactivating enzymes that cleave enzymatic drugs
Antibiotics often contain chemical bonds (e.g., amides and esters), and bacteria can produce antibiotic-inactivating enzymes (hydrolase) to degrade or alter antibiotics, leading to antibiotic resistance.245 P. aeruginosa is highly resistant to various antibiotics including penicillin, cephalosporin, and aztreonam by producing extended-spectrum β-lactamases (ESBLs).246,247 In addition, the bacterium is also resistant to the cefazolin-tazobactam combination therapy via ESBL GES-6.248 Again, the ESBL of P. aeruginosa is thought to be the most significant mechanism in terms of countering antibiotics, which would be a major target for designing and developing the most potent antimicrobial drugs.
From enzymatic angles, P. aeruginosa can modify the amino groups and glycosidic groups of aminoglycoside antibiotics to produce antibiotic resistance. The currently known aminoglycoside modifying enzymes utilize three common mechanisms: aminoglycoside phosphotransferase (APH), aminoglycoside acetyltransferase (AAC), and aminoglycoside nucleotide transferase (ANT).249,250 These enzymes trigger the resistance of different antibiotics through various mechanisms, providing powerful resistant activities towards different types of antibiotics. APH can inactivate streptomycin by transferring the phosphate group to the 3′-hydroxyl group of aminoglycosides.251,252 AAC may cause gentamicin resistance by transferring the acetyl group to the amino group at the 3′ and 6′ positions of the aminoglycoside.253 ANT confers P. aeruginosa resistance to amikacin by transferring adenosine groups to the amino or hydroxyl groups of these aminoglycosides.254 The diverse enzyme list is growing (currently more than 50 enzymes), which helps in bacterial success in the anti-drug battle with humans.
Acquiring the antibiotic resistance throughout bacterial life cycle
Bacteria can acquire antibiotic resistance through mutations or horizontal gene transfer.255,256 Mutations of OprD in P. aeruginosa confers resistance to carbapenems234 and mutations of DNA gyrase (GyrA) causes resistance to quinolone antibiotics.257 Importantly, mutations in the β-lactamase gene ampC causes a significant increase in resistance to cephalosporins.258 There are already a host of enzymes in this bacterium may counter antibiotics while it continues to gain new resistance factors, which is debatably the biggest challenge for drug industry and scientific research. As bacteria can conveniently obtain antibiotic resistance genes from the same or different bacterial species through horizontal gene transfer, despite challenging targeting this mechanism may be a niche to search for better treatments.259 A typical example is that P. aeruginosa may obtain aminoglycoside and β-lactam resistance genes through horizontal gene transfer from the environment or other microbes at an unpredictable, fast pace,223,260 it may be highly difficult for scientists and clinicians to design new tools in impeding this natural robust mechanism in this bacterium.
Adaptive antibiotic resistance
When facing the diverse environmental stimuli, bacteria obtain adaptive resistance to increase the resistance to antibiotics through transient changes in gene and/or protein expression by a spectrum of approaches.261 In P. aeruginosa, the formation of biofilms is the most typical strategy to acquire adaptive antibiotic resistance.262,263 P. aeruginosa can produce biofilms to enhance pathogenicity. Meanwhile, P. aeruginosa infection can also cope with antibiotic treatment by forming persistent cells or persisters, thereby preventing the synthesis of antibiotic targets.223,262,263 Persistent molecules from the persisters can maintain vitality and refill biofilms; once antibiotics are not present, bacteria will resume growth and cause chronic infections.264
Altogether, P. aeruginosa can become exceedingly resistant to antibiotics through myriads of mechanisms, and currently we probably only know a tip of iceberg regarding these bacterial behaviors. It necessitates accelerated research efforts to fully understand the detailed mechanisms by which bacteria constantly grow antibiotic resistance, providing insight into the design and development of innovative and efficious drugs to overcome drug resistance.
Highly complex and multiple mechanisms in host immune responses to the bacterium
The opportunistic pathogen P. aeruginosa exists almost everywhere and in any environmental conditions. In immunosuppressed people, there is extreme susceptibility to P. aeruginosa infection, developing either acute or chronic phenotypes. As the first line of host defense systems, the innate immune system plays a vital role in battling with P. aeruginosa via multiple mechanisms, such as phagocytosis and inflammatory responses. Several types of host systems, such as pattern recognition receptors (PRRs), plasma membrane signals, intracellular enzymes, and secreted cytokines/chemokines participate in inflammatory response against the bacterial infections. Although a well-balanced inflammatory response is required for restraining P. aeruginosa invasion, overzealous inflammation is associated with rapid disease progression, tissue injury, and even death. Some host molecules including cytosolic protein annexin A2 (AnxA2), autophagy-related protein 7 (ATG7), NLRC4, as well as non-coding RNAs (lncRNAs and microRNAs), are also implicated in P. aeruginosa-induced inflammation and/or other aspects of host defense mechanisms,265–267 and understanding of the mechanisms of inflammatory response is just beginning to be unfolded.
Toll-like receptors (TLRs) instantly recognize invasive pathogens
TLRs are highly conserved transmembrane PRRs, containing leucine-rich repeats and Toll/interleukin-1 receptor (TIR) homolog domains, which rapidly recognize invading microorganisms and elicit innate immunity and inflammatory response upon bacterial invasion.268 TLRs play vital roles in initiating innate immunity for eradicating invading pathogens.269 Upon the sensing of pathogen-associated molecular patterns (PAMPs), TLRs activate NF-κB and AP-1 to mediate inflammatory response signal pathways.270 Correspondingly, TLRs are capable of recognizing different pathogen-associated molecular patterns found in P. aeruginosa. LPS is a major component of the outer membrane of P. aeruginosa, responsible for maintaining bacterial membrane structure and initiating immune response as a major antigenic factor. Research shows that a large amount of hex-acylated lipid A from LPS is isolated from infected patients, which is a strong TLR4 agonist, capable of activating TLR4-dependent inflammatory response.271,272 In addition, several TLR4 co-receptors MD-2 and CD14 are indicated necessary for TLR4 recognition of LPS and TLR4 activation.273 Airway epithelial cells and macrophages, both expressed TLR4 in the cell membrane, serve as the first line of host defense for the initial contact to P. aeruginosa. As TLR4 is the essential trigger of host immunity against P. aeruginosa infection, its deficient mice show higher bacterial burdens and severe lung injury under infection.274 Nevertheless, the TLR4 pathway does not function alone and is very complex, which may be impacted or coregulated by a number of signaling systems including AnxA2,275 autophagy,276,277 inflammasome,278 cGAS,279,280 ion channel proteins,281 DNA repair proteins,282 miRNAs,40,266,283 and lncRNA,229 to name a few.
Apart from TLR4, TLR2 has also been reported as an LPS recognizer, which is capable of recognizing P. aeruginosa‐derived lipoproteins and T3SS effector ExoS.269 In addition, TLR5 is responsible for detecting P. aeruginosa flagellin, which can induce specific inflammasome.284 TLR9 is responsible for detecting P. aeruginosa unmethylated bacterial CpG DNA intracellularly.285 Interestingly, TLR9 is also activated by P. aeruginosa infection-induced mtDNA release (Wang Biao et al., under revision, Immunology).
Although paradoxical at times, TLRs are arguably the single most important PPRs for initiating the initial recognition to mount a robust immune defense against both bacteria and viruses. It is imperative that scientists be focused on verifying the roles of played out by TLRs using a comprehensive approach or systems biology in an unbiased manner in animals as well as human subjects,277 which may provide insight into the disease pathogenesis and suggesting new therapeutic development.
Inflammasomes drive inflammation and pyroptosis
The inflammasome is a multiprotein complex, which is attributed to the production and release of inflammatory cytokines, IL-1β and IL-18.286,287 Recent work reveals that inflammasome is involved in pyroptosis dependent on the cleavage of Gasdermin D, which contributed to the formation of plasma membrane pores, and in turn promoting the release of inflammatory cytokines and pyroptosis.288,289 Typically, inflammasome consists of cytosolic PRRs, ASC (an apoptosis-associated speck-like protein containing a CARD), and caspase 1. Inflammasome PRRs are responsible for detecting exogenous PAMPs like TLRs, which are also essential for monitoring P. aeruginosa invasion and activate host inflammatory response for promoting the clearance of P. aeruginosa.269,290 Remarkably, TLRs are involved in the priming of inflammasome activation by promoting the transcription of inflammasome-related genes.290 NLRC4 and AIM2 (absent in melanoma 2) are well characterized among numerous inflammasome PRRs linked to infection of P. aeruginosa.
NLRC4 inflammasome is shown capable of recognizing needle and inner rod (PrgJ) T3SS proteins, independently of T3SS exotoxins, or intracellular flagellin utilizing different murine NAIP as adaptors.291–294 Unexpectively, only one factor, human NAIP (hNAIP), has been found, homologous to murine NAIP5, responsible for sensing P. aeruginosa T3SS inner-rod protein PscI and needle protein PscF.295 Apart from T3SS, the T4SS pilin is also capable of activating inflammasome independent of NLRC4 and ASC.153 P. aeruginosa-induced mitochondrial dysfunction also promotes the assembly and activation of NLRC4 via T3SS.296 Mitochondrial ROS and release of mitochondrial DNA are key to NLRC4 activation under P. aeruginosa infection, which is also dependent on autophagy.296 Removal of damaged mitochondria blocks the activation of NLRC4.296 However, AIM2 appears to be dispensable for recognizing and promoting P. aeruginosa infection-induced inflammation in most cases.297
Although several P. aeruginosa components are implicated in activating inflammasome-related immune defense, little is known about how the bacterium evades immune response after inflammasome activation.298 Recent research broadens our knowledge that P. aeruginosa takes advantage of bacterial QS-dependent secretant, including proteases, pyocyanin, and 3-oxo-C12-HSL, to inhibit the activation of NLRC4 or NLR family, pyrin domain containing 3 (NLRP3) inflammasomes.298 A further study supports that pyocyanin specifically inhibits activation of the NLRP3 inflammasome (but not NLRC4 and AIM2) through induced excessive oxidation,299 contrary to the positive role of oxidation in NLRP3 activation.300 Hence, the potential role of oxidation in P. aeruginosa infection and inflammasome activation requires further study. In addition, K+ efflux is necessary for the activation of inflammasome against P. aeruginosa infection.301
To date, the role and underlying mechanisms of the inflammasome in host defense against P. aeruginosa remain largely unclear and sometimes paradoxical: some researches show that inflammasome activation enhances host defense to clear P. aeruginosa,302,303 whereas others present opposite results that inflammasome activation triggered severe host tissue damage, which may impact disease progression and mortality.304,305 One explanation for such a mixed response is that infection may involve different pathways dependent on the bacterial strains, model systems, environments, and experimental conditions. Another caveat is that most experiments have not been performed at holistic and/or spatiotemporal levels to evaluate the dynamics, rather a single cell type, specific location, and one or two timepoints. Therefore, enhanced or advanced approaches are needed to further clarify the role and underlying regulatory mechanisms of inflammasome activation in P. aeruginosa infection.277
Nucleic acid sensor cGAS is pivotal for eliciting immune reaction
cGAS is a recently discovered nucleic acid sensor that is initially identified to recognize viruses.306 Typically, activation of cGAS contributes to the induction of inflammasome as a means against viral or intracellular bacterial infection.307 However, the latest research shows that cGAS downstream effector STING may also play an anti-inflammatory role under extracellular bacterial P. aeruginosa infection by inhibiting NF-κB activity.308 More recently, we have found that cGAS may be involved in an uncoupled protein response (UPR) during P. aeruginosa infection. Mechanistically, our studies uncover cGAS as a novel nucleic acids’ sensor to initiate immune responses against P. aeruginosa infection through a canonical pathway involving STING and IRF-1,280,309 suggesting that cGAS pathways may be a critical target for control of this bacterium. It is a new and important function for cGAS since previous reports were primarily focused on viral sensing and intracellular bacterial sensing.
CRISPR-mediated adaptive immunity, a double-edged sword for bacterial self-survival and invasion
CRISPR-Cas (clustered regularly interspaced short palindromic repeats-CRISPR-associated) is widely existing in bacteria and archaea providing protection against genetic intruders (plasmids), phages (phage) and other parasites.310 To date, two classes, six types of CRISPR-CRISPR-associated (Cas) systems, based on the characteristic of Cas proteins, are present in bacteria.311–313 Class I CRISPR-Cas systems (types II, V, and VI systems) rely on multiple CRISPR-Cas protein effector complexes, while Class II CRISPR-Cas systems (types I, III, and IV systems) are dependent on a single CRISPR-Cas effector protein.314–316 CRISPR-Cas I-C, type I-E and type I-F systems have been found in many clinically isolated P. aeruginosa.317,318 It is known that CRISPR-Cas systems provide adaptive immunity against the invasion of bacteriophages or plasmids.319 However, the role of CRISPR-Cas systems is far beyond the adaptive immunity of bacteria based on the current reports.320 Our research showed that type I CRISPR-Cas targeted the QS regulator LasR to inhibit toll-like receptor 4 recognition, thereby evading mammalian host immunity, suggesting that the CRISPR system is linked to host immunity modulation by targeting their own genes, potentially evading host defense mechanisms.321 Type I CRISPR-Cas systems may elicit inflammasome activation in mammalian hosts by regulating autophagy in P. aeruginosa.278 To ensure maximum CRISPR-Cas function, P. aeruginosa PA14 utilized quorum sensing to activate the expression of cas genes to promote CRISPR adaptation when bacteria are at high risk of phage invasion.322 The adaptability and virulence of P. aeruginosa can be regulated by CRISPR-Cas adaptive immunity based on the biological complexity of microbial communities in natural environments.323 The CRISPR-Cas system actively maintains the virulence of P. aeruginosa by limiting virus-like accessory genomic elements.324 Increased expression of phage-related genes initiates CRISPR-mediated biofilm-specific death of P. aeruginosa.325 CRISPR-Cas systems may help mediate antibiotic resistance to multiple membrane irritants, including enhancing the integrity of the envelope in pathogen Francisella novicida.326 Consequently, various corresponding anti-CRISPR (Acr) proteins have evolved by phages to inhibit bacterial CRISPR systems.315,327–333 Our previous research identified a series of type VI-A anti-CRISPRs (acrVIA1-7) genes that block the activities of Type VI-A CRISPR-Cas13a system,333 and designed Type III CRISPR endonuclease antivirals for coronaviruses (TEAR-CoV) as an experimental therapeutic to combat SARS-CoV-2 infection,334 suggesting that exploiting the mechanism of CRISPR-mediated adaptive immunity has great potential for treating bacterial and viral infections. However, it is not clear whether CRISPR-Cas systems can regulate antibiotic resistance in P. aeruginosa, which may be studied in future (Fig. 5).
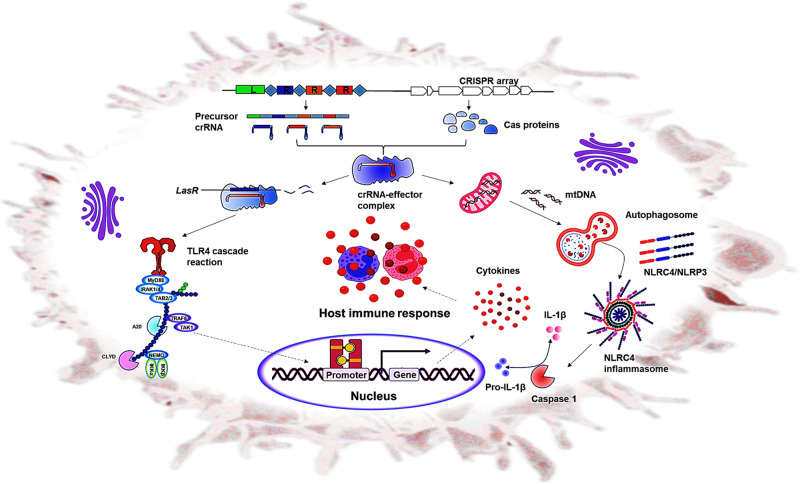
Fig. 5.
CRISPR-mediated adaptive immunity. Type I-C, type I-E, and type I-F CRISPR-Cas systems have been identified in P. aeruginosa. Type I CRISPR-Cas targeted endogenous LasR gene to decrease TLR4 expression and TLR4-mediated host inflammatory responses. Similarly, type I CRISPR-Cas systems elicited inflammasome activation by promoting mitochondrial-mediated autophagy. Ultimately, CRISPR-mediated adaptive immunity helps P. aeruginosa evade mammalian host immunity
Development of novel therapeutics for P. aeruginosa is not up to the speed
P. aeruginosa have strong ability to develop natural and acquired drug resistance through various mechanisms, including the production of antibiotic inactivating enzymes or antibiotic modifying enzymes, inhibiting the penetration of the drug through the cell wall, changing the target of antibacterial action, and limiting the drug to reach its target and adaptation to the adverse environment.223 Due to the increasing difficulty in treating antibiotic resistance strain infection, the development of the anti-P. aeruginosa therapy depends on targeting the resistance mechanism. Research on the resistance mechanism of P. aeruginosa has been an urgent topic for decades since antibiotic resistance has escalated exceedingly. Even with the intense interest, development of new antibiotics and other therapeutic strategies for P. aeruginosa infections is at a painstakingly slow pace due to the variability and complexity of drug resistance, as well as the lack of a deep understanding of the pathogenic mechanisms for P. aeruginosa. Designing effective therapeutic approaches (including phage therapy, immunotherapy, gene editing therapy, antimicrobial peptides, and vaccine therapies) to counteracting P. aeruginosa invasion has been an increasing urgency, requiring consorted efforts (Fig. 6).
Fig. 6.
Novel therapeutics for P. aeruginosa. Multidrug-resistant P. aeruginosa poses a major challenge to traditional antibiotics therapeutics, which have limited efficacy and cause serious side effects. Phage therapy, immunotherapy, gene editing therapy, antimicrobial peptides, and vaccine therapies have become the most promising strategy and garnered great expectations to overcome multidrug-resistant bacterial infections. A full-scale network of regulatory understanding of P. aeruginosa virulence is expected to be unveiled, thus, we will be in a much better position for rationale drug design to control Pseudomonas aeruginosa infections
New antibiotic formulations and compounds
With an alarming rise in pathogens with resistance to existing drugs, a number of new antibiotics have recently entered the antibiotic development pipeline; however, the hope for patients and clinicians is rapidly dwindling once a new antibiotic resistance strain emerges. Hence, we should invest unconventionally research efforts in searching for new treatments of MDR bacteria. Recently developed antimicrobials are discussed below.
The biological activity of substituted guanidines was known in the mid-1930s when a series of guanidines and metformin compounds were found to possess bactericidal and disinfectant properties. Subsequently, many guanidine derivatives were studied for therapeutic purposes. Chin et al., recently reported a polyguanidine polycarbonates with strong antimicrobial activities through a distinctive mechanism that does not intensify drug resistance in multi-drug resistance (MDR) superbugs including methicillin-resistant S. aureus, P. aeruginosa, A. baumannii, K. pneumoniae,335 suggesting that the polyguanidine compounds may be potential antibacterial candidates.
In addition, novel antimicrobial peptides are promising to become the next generation of antimicrobials. The sequences of amino acids with antibiotic nature can be found in insects, soil bacteria, amphibians, plants, and even mammals. With the diversity of species and antimicrobial mechanisms, peptides render a niche as an alternative treatment for the MDR bacteria.336 A variety of new antibiotic formulations for treating P. aeruginosa infection have been tested in the clinic despite mostly at preliminary stages. Plazomicin is a novel semi-synthetic parenteral aminoglycoside that inhibits bacterial protein synthesis, and is shown to inhibit P. aeruginosa growth.337 Plazomicin is assessed in two Phase III randomized controlled trials, with an EPIC trial compared with meropenem in Complicated Urinary Tract Infection (cUTI). Plazomicin demonstrated a composite cure 81.7% (P) vs. 70.1% (M), a difference of 11.6%.337 Similarly, another trial CARE with plazomicin-based or colistin-based combinations to treat infection by carbapenem-resistant Enterobacteriaceae (CRE) shows that therapy with plazomicin-based combinations reduced mortality and complications vs. colistin-based combinations (23.5% vs 50%). This latter (CARE) trial seems more effective than the former (Plazomicin) and is achieved by enhanced sustained microbiological eradication.
Eravacycline is a novel fluorocycline that is evaluated for antimicrobial activity against anaerobic Gram-negative and Gram-positive bacteria. Eravacycline demonstrates potent broad-spectrum activity against 90% of bacterial isolates having a concentration range of ≤0.008 to 2 μg/ml.338 Eravacycline is more effective when an expression of tetracycline-specific efflux and ribosomal protection mechanisms is present. Eravacycline is effective against multi-drug-resistant bacteria towards other antibiotic classes. Eravacycline has shown broad-spectrum antibacterial activity when put unique chemical modifications at C-9 and C-7 of the tetracycline core.338
Baxdela, also known as delafloxacin meglumine, is a broad-spectrum anionic fluoroquinolone and has distinct chemical structures that increase potency in acidic environments. Delafloxacin is known for inhibiting DNA replication and repairs targeting DNA gyrase and topoisomerase IV. Currently, Baxdela is under a Phase III clinical trial for therapy of community-acquired pneumonia.308 Zidebactam (WCK 5222) is a dual mechanism antibiotic in a development phase, which is involved in binding Gram-negative PBP2 to exert β-lactamase inhibition. As an active antibiotic against Enterobacteriacease producing CTX-M-15, Zidebactam has some effects against Enterobacteriaceae and P. aeruginosa.339 Although we only reviewed a small portion of ongoing development of new therapeutics for P. aeruginosa with some hope, development of effective therapeutics to counter the growing drug resistance is still challenging.
Nanoparticles may be useful for delivering drugs or vaccines
Nanoparticles have being tested in a number of therapeutic applications, such as drug delivery, gene delivery, and vaccine delivery in addition to direct killing of bacteria by its membrane piercing ability and induction of ROS. Since the CRISPR/Cas system can target to self-genes within a bacterium, delivery of CRISPR-Cas targeting a vital gene (for survival) by nanoparticles may directly kill the bacterium. Use of nanoparticles for delivering vaccines can also be achieved but preparation of optimal particles that can readily control protein loading and folding remains challenging.340 We will focus on the studies of vaccines and nanoparticles. There are several types of nanoparticles: nanoantibiotics liposomes, polymers, hydrogels, metallic particles, magnetic structures, carbon-based materials, and mesoporous with silica and each has its advantages and disadvantages. Hence, a multivalent nanoparticle strategy is considered a superior means and is used in vaccine delivery with P. aeruginosa, specifically to target T6SS. T6SS can divide into two distinct forms: phage tail-like structure and transmembrane complex, which can be exploited to create multivalent nanoparticle delivery of vaccine antigens. For the delivery of T6SS structural device, many proteins in a single nanoparticle may be delivered simultaneously.
The drawback of using nanoparticle structures is the likelihood that host immune responses may be activated against the delivery system, which can prohibit the repeated delivery.340 Future experiments need to be conducted to control antigen exposure to generate a uniform particle. The advantages of T6SS delivery system can be adopted for other gene targets with different nanoparticles. The advancement of nanotechnology has allowed a variety of nanostructures as a strategy to minimize the undesirable characteristic and natural and synthetic AMPs. Reports have indicated that in nanostructures, peptides present lower cytotoxicity and better efficiency of desired targets. Hence, nanostructures may help produce biomolecules and implementation of vaccines and drugs against extracellular degradation and target treatment. Using two approaches for encapsulating AMPs, nanotechnology can use an indirect approach where a passive delivery occurs involving a conventional nano-delivery system. Direct delivery is involved in an active target for modifying the surface chemistry of the nano-carrier with ligands. When comparing the two approaches, both have their rpos and cons, using the passive system involving the encapsulated peptide results in the best fitting solution.336
As biofilms are a major cause of drug resistance, nanoparticles targeting biofilms are also a promising strategy. Zhang et al. found that magnetite hybrid nanocomplexes can penetrate and disrupt bacterial biofilms by breaking through the biofilm matrix barrier.341 Others also demonstrate that silver nanoparticles (metallic particles) combined with polymyxin B can target P. aeruginosa biofilms with significant effects.342 Biofilms are also an important factor to aggravate cystic fibrosis disease. Hence, exotoxin A (ETA) is encapsulated into poly-lactic-co-glycolic acid (PLGA) nanoparticles, forming the ETA-PLGA nanoparticles, which may function as a continuous immunogen to trigger cellular immunity.343 Therefore, these nanoparticles may be one of the potential vaccine candidates to penetrate biofilms. To date, many nanoparticles-mediated compounds are in clinical development and several of them have been approved for human therapy, especially cancer therapy, hepatic fibrosis, virus infection, fungal infections, hypercholesterolemia, and pneumonia.344–346 In particular, the recently approved lipid nanoparticle (LNP) mRNA vaccines for COVID-19 was an acclaimed success in nanoparticle research for contribution to novel vaccines to combat diseases.347
Phage therapy is a reviving alternative therapy for severe and incurable cases
Due to the lack of effective therapy for drug-resistant superbugs, phage therapy has been a viable alternative for bacterial treatment, especially for patients who have been non-responsive to conventional antibiotics. There are two types of bacteriophages, lytic and temperate, only lytic phages are utilized for clinical therapy. Lytic phages adhere to the host cell surface and inject DNA into host cells. Progeny phages release and migrate towards infection sites through targeting specific bacteria. For P. aeruginosa, flagella vaccines have been used together with phage or other therapies and showed the remarkable therapeutic results. However, P. aeruginosa displays heterogeneity and varying clinical outcomes with acute infections in later phases, where bacteria start losing their flagella and pili. A vaccine based on flagella will direct toward motile bacteria instead of established/colonized biofilms-forming strains.348 Advanced techniques have been developed for formulating phage cocktails to improve efficacy in vitro. For example, Forti et al. made a cocktail with six phages: E215, E217, PAK_P1, PYO2, DEV, and PAK_P4 and showed some effects against clinical P. aeruginosa strains.349 A number of studies showed that phage cocktails possess enhanced efficiency in killing P. aeruginosa compared to single phage therapy.350,351 As strains from patients are distinct, personalized phages combined with antibiotics have also been applied for effective and safe therapy in clinics. An excellent such application was reported by Forti et al., who constructed phage cocktails based on the genomic information and host range.349 Clinical trials of phage therapy have shown viable and sustained efficacy in therapy. Marked reduction in bacterial colonies with a single dose of phage therapy has been reported in small studies.352 Previous studies have primarily been on patients with burns. Phages were applied on the wounds, but the minimal effect was observed.353 With P. aeruginosa being a ubiquitous pathogen that infects lungs and many other organs, further research will investigate how phage therapy would help control chronic CF or COPD diseases. Phage neutralizing antibodies that are induced by the body’s immune system and the safety concern about exotoxins during phage preparations are among the biggest challenge of phage therapy.
Another limitation is that bacteria can become resistant rapidly to phage therapy due in part to CRISPR-Cas systems, which are a bacterial adaptive immunity system that can degrade invading DNA in a high specificity. Research has been conducted with a CRISPR-Cas system that targets endogenous genes to alter host defense.324 To conquer the bacterial immune response for promoting phage replication, phages should gain access to do the job by evolving an anti-CRISPR system (Acrs). With this mechanism, future goals are to use natural anti-CRISPR or synthetic proteins that can counteract the CRISPR systems.319 A protein called AcrIF was shown to be a potent inhibitor of the CRISPR system in P. aeruginosa. This protein is locked with the Cys complex of CRISPR and diminished the phages’ ability to bind to DNA.354 Further research may be conducted using the same mechanism for bacteria to become resistant to CRISPR. Creating a synthetic phage with desired characteristics and genomic content would be a creative approach.355 Recently, mutations in phage host-range-determining regions (HRDRs) to cope with resistance mutation of bacteria have been implemented as an effective method to prevent Escherichia coli infection.41 Engineered bacteriophages BPsΔ33HTH_HRM10 and D29_HRMGD40 cocktail cured a cystic fibrosis patient with a disseminated drug-resistant Mycobacterium abscessus, which shows that the therapeutics of engineered phages to break the evolutionary constraints, holding great potential to create the next-generation of antibacterial antimicrobials.356,357 In spite of recent progress, the strategy for phage therapy is highly difficult. Through creative design, basic research and clinical testing, phage therapy can be rejuvenated against bacterial infections. Hence, it is promising to combine the phage therapy and anti-CRISPR approach for development of clinically feasible antibacterials.333
Gene delivery has made notable progress
Genome editing and nucleic acid-based antibiotics, such as single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), plasmid DNA, and ssRNA358 have emerged as the new types of antimicrobials. For gene editing, there is no doubt that editing with CRISPR systems is of high promise. Since scientists first used Cas9 to edit genomes in mice and human cells in 2013,359 the technology has been thought to have the greatest potential to cure some of the deadly diseases that humans cannot control today, including against bacteria-plagued diseases (i.e., CF), cancer and more importantly hereditary disorders. Although there is no therapy in clinics yet, the advance in off-target control and improved editing efficiency will translate gene therapy from bench to bedside in the foreseeable future. Both the gene editing and nucleic acid-based antibiotics need to be delivered into the host and cross spontaneously bacterial cell walls and membranes.
The major strategies for gene delivery are viral and non-viral vector systems. Non-viral strategy includes electroporation (direct injection, in vitro), lipids, and polymers. Research has also shown that LPN (Liposome polymer nucleic acid) is a versatile platform for efficiently delivering diverse nucleic acids to Gram-negative bacteria.358 Intracellular delivery of LPN can be directed against essential genes, resulting in bacterial growth inhibition or death.360 For viral delivery strategy, lentivirus, adenovirus-associated virus (AAV), and adenovirus vectors are often used.361 The lentiviral vector can efficiently integrate a foreign gene into the host chromosome, thereby achieving the effect of persistently expressing the sequence of interest. For some cells that are difficult to transfect, such as primary cells, stem cells, undifferentiated cells, etc., the use of lentiviral vectors has shown versatility to greatly improve the transduction efficiency of the target gene or the target shRNA. Therefore, in vitro and in vivo experiments, lentivirus has become one of the commonly used forms of expression for expressing foreign genes or exogenous shRNAs, and is gaining increasing attention.362 AAV’s function mechanism is very similar to lentiviruses. Compared to the lentiviruses, AAV shows better safety, a wide range of host cells, and low immunogenicity, so it has become the most promising gene transfer vector, especially for the CRISPR system.363,364 The AAV-based gene delivery may bear high transduction efficiency for delivering nucleic acid-based antibiotics, leading to better therapeutic effects.
Development of vaccines is painfully slow
Nip in the bud, vaccines have been a viable approach for preventing millions of people from suffering devastating pandemic infections. Vaccine formulations can be efficiently targeted by antigen-presenting cells to generate both humoral and cellular immune responses.
Only can a few of vaccines that have being tested pass the hurdles of clinical trials to inoculate human populations. A major component of the bacterium targeted by vaccines are LPS and OMP with high rates of variations and mutations, which present challenges in vaccine designs. Vaccines based on these components have shown effects against homologous strains, but no effect against different serotypes. Two strategies have been adopted with LPS-vaccines: the first was to use lipid droplets for carrying LPS components into the host; and the second was stripping the lipid portion off LPS to create serotype-specific protection, which is considered the best option.365 Proteins or genetic vaccines appear to be the best candidates to vaccinate for P. aeruginosa. Emerging strains of P. aeruginosa are a key model system for investigating T4P structure and function, which may provide insight into vaccine design. Research indicates a serious nosocomial pathogen with facile growth requirements or contribution to motility.366 Flagellin and flagella have high immunogenicity, therefore, they are potential targets for vaccination against P. aeruginosa.367 The use of flagella is not only for motility purpose, but also for assisting attachment to the respiratory epithelium and activating TLR5. P. aeruginosa flagellin is a major unit of flagellar filament that is required to target protective antibodies and virulence. Flagella vaccines seem to help target acute infections and provide substantial relief of disease by preventing bacterial invasions and spreads. Klebsiella surface O polysaccharides (OPS) are protective antigens against infection in animals. The development of combined K. pneumoniae and P. aeruginosa glycoconjugate vaccine of four common Klebsiella OPS types may improve the control of human infections. OPS is chemically associated with two flagellin types, FlaA and FlaB.368 Monoclonal antibodies as passive immunity have shown protection of susceptible individuals to delay or prevent initial infections, especially those with cystic fibrosis.369 Increasing vaccines targeting iron chelation, lectin inhibitors, QS inhibition, have been developed. Finally, OMVs are being tested in clinical settings either vaccines or therapeutic carriers.58 Taken together, despite the strong interest in research and urgent need in public health, vaccines against P. aeruginosa are still unavailable for clinical application.
Future directions
In the past several decades, although human beings have witnessed great scientific advances in basic research of pathogenesis and host defense as well as preventative and therapeutic development with P. aeruginosa, our understanding of this bacterium is still limited, impeding the hunt for novel effective therapeutic in the clinical settings. There are a large number of questions requiring clarification. (1) How do drug-resistant invasive strains emerge in the context with abuse of antibiotics or normal evolution? (2) How many other virulence factors are not discovered and characterized but critical for bacterial pathogenesis and evolution, especially those with remarkable virulence? (3) How the elements of the bacterium trigger and how the bacterium evade the activation, phagocytosis, and clearance by the human immune system? (4) What are new mechanisms responsible for increasing resistance in antimicrobial therapy? (5) How to better identify new host factors for improved immunity against this bacterium? (6) Can the scientific community establish a systematic and all-inclusive guideline to facilitate the discovery and revelation of novel antimicrobials, immune modulators, and other disease-modifying therapeutics?
Summary
As an opportunistic pathogen, P. aeruginosa has a complex regulatory system that is closely connected and mutually regulated to cope with the harsh external and internal environment, which causes substantial morbidity, debilitating diseases, shortened life span, and high mortality in humans (Fig. 7). In this comprehensive review and other articles, scientists have discussed the virulence factors of P. aeruginosa, such as LPS, adherence factors, elastase, secretion systems, and OMVs.116,153,212,366,369–455 We also introduced the recent progress in new antibiotic formulations and compounds, phage therapy strategy, vaccine approaches, nanoparticle fabrication as well as gene editing and nucleic acid-based antibiotics. Furthermore, we have included a large set of immune responses from hosts, including cell types, innate and adaptive immunity, and emerging advances in immunological research.
Fig. 7.
Interlinked and mechanistic regulatory network in P. aeruginosa. Interactions between various regulatory systems of P. aeruginosa are linked to regulate adaptation, survival, and resistance to multiple antibiotics, enabling P. aeruginosa to survive environmental stresses. QS systems primarily comprise Las, Rhl, and Pqs and are driven by autoinducer signaling molecules N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL) N-butanoyl-L-homoserine lactone (C4-HSL), and quinolone signal (PQS) activated through the interaction of transcription factors LasR/RhlR/Pqs and 3-oxo-C12-HSL/C4-HSL/PQS. When signaling molecules reach a putative threshold concentration in the cell environment, QS regulates toxicity expression by regulating two-component systems (TCSs). TCSs regulatory systems, consisting of sensor kinase and response regulator pairs, play roles in bacterial adaptation by regulating the expression of a variety of extracellular enzymes, virulence factors, and QS molecules. GacS/GacA TCS is regulated by sensor kinases RetS (positive regulation) and LadS (negative regulation). Transcription of non-coding regulatory RNAs of the RsmY/Z depends on the activation of GacS/GacA to activate RsmA and regulate the T3SS-mediated virulence secretion and biofilm formation. Biofilms are encapsulated in a self-generating extracellular polymer (EPS) matrix for species survival in surprising alterations of living conditions, like temperature fluctuations and nutrient availability, especially the antibiotic threat. Likewise, the secretory system activates the host immune response through virulence factors. TLRs play a key role in innate immunity. TLR1, 2, 4, 5, 6, and 9 are reported for recognizing P. aeruginosa infection and mediating inflammatory response signal pathways. T3SS and bacterial QS-dependent secretants have roles in modulating NLRP3 and NLRC4 inflammasome activation under P. aeruginosa challenge
Our paper is a rarely extensive review that covers both bacterial pathogenesis and host defense in a great depth, serving as an irreplaceable reference for both students, doctors, and scientists who want to better understand P. aeruginosa. This comprehensive and analytic summary of current literature may enrich our knowledge in the balance between P. aeruginosa invasion and host responses. Despite the vast progress made over the years, a number of questions ranging from basic to clinical and applied aspects remain to be answered and further increased research efforts are still needed to study P. aeruginosa, which will improve our design to more effectively combat the infection caused by emerging drug-resistance strains.
Acknowledgements
This work was supported by National Institutes of Health Grants R01 AI109317-06 and AI138203-3 to M.W. Some icons or graphic element in the Figures (Figs. 1–7) are adapted from BioRender.com (2022). Retrieved from https://app.biorender.com/. Final schematic illustrations were created and integrated by our original design.
Author contributions
M.W. conceived, supervised, and revised the paper; S.Q. organized figures and formatted the paper; S.Q., X.S., W.X., C.Z., Q.P., X.D., L.L., H.L., and M.W. participated in different parts of writing.
Competing interests
The authors have no financial conflict of interest. Xiangrong Song and Min Wu are editorial board members of Signal Transduction and Targeted Therapy, but they have not been involved in the process of the manuscript handling.
Footnotes
These authors contributed equally: Shugang Qin, Wen Xiao, Chuanmin Zhou.
Contributor Information
Xiangrong Song, Email: songxr@scu.edu.cn.
Min Wu, Email: min.wu@und.edu.
References
- 1.Rossi E, et al. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 2021;19:331–342. doi: 10.1038/s41579-020-00477-5. [DOI] [PubMed] [Google Scholar]
- 2.Jurado-Martin, I., Sainz-Mejias, M. & McClean, S. Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int. J. Mol. Sci. 22, 1–35 (2021). [DOI] [PMC free article] [PubMed]
- 3.Cendra MDM, Torrents E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021;49:107734. doi: 10.1016/j.biotechadv.2021.107734. [DOI] [PubMed] [Google Scholar]
- 4.Sinha, M. et al. Pseudomonas aeruginosa theft biofilm require host lipids of cutaneous wound. Ann. Surg. 5252, 1–23 (2021). [DOI] [PMC free article] [PubMed]
- 5.Tang P, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat. Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 6.Blomquist KC, Nix DE. A critical evaluation of newer beta-lactam antibiotics for treatment of Pseudomonas aeruginosa infections. Ann. Pharmacother. 2021;55:1010–1024. doi: 10.1177/1060028020974003. [DOI] [PubMed] [Google Scholar]
- 7.Jangra, V., Sharma, N. & Chhillar, A. K. Therapeutic approaches for combating Pseudomonas aeruginosa Infections. Microbes Infect. 24, 104950 (2022). [DOI] [PubMed]
- 8.Daikos, G. L. et al. Review of ceftazidime-avibactam for the treatment of infections caused by Pseudomonas aeruginosa. Antibiotics.10, 1–24 (2021). [DOI] [PMC free article] [PubMed]
- 9.Botelho, J., Grosso, F. & Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa—mechanisms, epidemiology and evolution. Drug Resist. Updat.44, 100640 (2019). [DOI] [PubMed]
- 10.Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections—an overview. Infect. Drug Resist. 2018;11:2321–2333. doi: 10.2147/IDR.S177247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Calleja AI, et al. Antimicrobial activity of ceftolozane-tazobactam against multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa clinical isolates from a Spanish hospital. Rev. Esp. Quimioter. 2019;32:68–72. [PMC free article] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control publishes Annual epidemiological report 2011. Euro. Surveill. 16, 20012 (2011). [PubMed]
- 13.McCarthy KL, Paterson DL. Increased risk of death with recurrent Pseudomonas aeruginosa bacteremia. Diagn. Microbiol. Infect. Dis. 2017;88:152–157. doi: 10.1016/j.diagmicrobio.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Feng W, et al. Epidemiology and resistance characteristics of Pseudomonas aeruginosa isolates from the respiratory department of a hospital in China. J. Glob. Antimicrob. Resist. 2017;8:142–147. doi: 10.1016/j.jgar.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Xin XF, Kvitko B, He SY. Pseudomonas syringae: what it takes to be a pathogen. Nat. Rev. Microbiol. 2018;16:316–328. doi: 10.1038/nrmicro.2018.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidaillac C, Chotirmall SH. Pseudomonas aeruginosa in bronchiectasis: infection, inflammation, and therapies. Expert Rev. Respir. Med. 2021;15:649–662. doi: 10.1080/17476348.2021.1906225. [DOI] [PubMed] [Google Scholar]
- 17.Park, W. S. et al. Benzyl isothiocyanate attenuates inflammasome activation in Pseudomonas aeruginosa LPS-stimulated THP-1 cells and exerts regulation through the MAPKs/NF-kappaB pathway. Int. J. Mol. Sci. 23, 1–10 (2022). [DOI] [PMC free article] [PubMed]
- 18.Chambers, J. R., Cherny, K. E. & Sauer, K. Susceptibility of Pseudomonas aeruginosa dispersed cells to antimicrobial agents is dependent on the dispersion cue and class of the antimicrobial agent used. Antimicrob. Agents Chemother. 61, 1–18 (2017). [DOI] [PMC free article] [PubMed]
- 19.Sabnis, A. et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. Elife. 10, 1–26 (2021). [DOI] [PMC free article] [PubMed]
- 20.Ozer, E. et al. An inside look at a biofilm: Pseudomonas aeruginosa flagella biotracking. Sci. Adv. 7, 1–15 (2021). [DOI] [PMC free article] [PubMed]
- 21.Yang, J. J., Tsuei, K. C. & Shen, E. P. The role of Type III secretion system in the pathogenesis of Pseudomonas aeruginosa microbial keratitis. Tzu. Chi. Med. J.34, 8–14 (2022). [DOI] [PMC free article] [PubMed]
- 22.Karash S, Nordell R, Ozer EA, Yahr TL. Genome sequences of two Pseudomonas aeruginosa isolates with defects in type III secretion system gene expression from a chronic ankle wound infection. Microbiol. Spectr. 2021;9:e0034021. doi: 10.1128/Spectrum.00340-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alatraktchi, F. A., Svendsen, W. E. & Molin, S. Electrochemical detection of pyocyanin as a biomarker for Pseudomonas aeruginosa: a focused review. Sensors.20, 1–15 (2020). [DOI] [PMC free article] [PubMed]
- 24.Chadha, J., Harjai, K. & Chhibber, S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: a chronicle through the perspective of quorum sensing. Environ. Microbiol.00, 1–27 (2021). [DOI] [PubMed]
- 25.Zhao F, Wang Q, Zhang Y, Lei L. Anaerobic biosynthesis of rhamnolipids by Pseudomonas aeruginosa: performance, mechanism and its application potential for enhanced oil recovery. Micro. Cell Fact. 2021;20:103. doi: 10.1186/s12934-021-01593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perraud Q, et al. Opportunistic use of catecholamine neurotransmitters as siderophores to access iron by Pseudomonas aeruginosa. Environ. Microbiol. 2022;24:878–893. doi: 10.1111/1462-2920.15372. [DOI] [PubMed] [Google Scholar]
- 27.Dar, H. H. et al. A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(*) sabotage of theft-ferroptosis. Redox. Biol. 45, 102045 (2021). [DOI] [PMC free article] [PubMed]
- 28.Maldonado RF, Sa-Correia I, Valvano MA. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016;40:480–493. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross, A. S. Anti-endotoxin vaccines: back to the future. Virulence.5, 219–225 (2014). [DOI] [PMC free article] [PubMed]
- 30.Goldberg JB, Pler GB. Pseudomonas aeruginosa lipopolysaccharides and pathogenesis. Trends Microbiol. 1996;4:490–494. doi: 10.1016/S0966-842X(97)82911-3. [DOI] [PubMed] [Google Scholar]
- 31.Amarante-Mendes GP, et al. Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 2018;9:2379. doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct. Target Ther. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadatani Y, et al. NOD-like receptor protein 3 inflammasome priming and activation in Barrett’s epithelial cells. Cell Mol. Gastroenterol. Hepatol. 2016;2:439–453. doi: 10.1016/j.jcmgh.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sebastian-Valverde, M. & Pasinetti, G. M. The NLRP3 inflammasome as a critical actor in the inflammaging process. Cells. 9, 1–28 (2020). [DOI] [PMC free article] [PubMed]
- 36.Stephens, M. & von der Weid, P. Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes.11, 421–432 (2020). [DOI] [PMC free article] [PubMed]
- 37.Liu W, Sun T, Wang Y. Integrin alphavbeta6 mediates epithelial-mesenchymal transition in human bronchial epithelial cells induced by lipopolysaccharides of Pseudomonas aeruginosa via TGF-beta1-Smad2/3 signaling pathway. Folia Microbiol. 2020;65:329–338. doi: 10.1007/s12223-019-00728-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramjeet M, et al. Truncation of the lipopolysaccharide outer core affects susceptibility to antimicrobial peptides and virulence of Actinobacillus pleuropneumoniae serotype 1. J. Biol. Chem. 2005;280:39104–39114. doi: 10.1074/jbc.M502852200. [DOI] [PubMed] [Google Scholar]
- 39.Murray GL, et al. Mutations affecting Leptospira interrogans lipopolysaccharide attenuate virulence. Mol. Microbiol. 2010;78:701–709. doi: 10.1111/j.1365-2958.2010.07360.x. [DOI] [PubMed] [Google Scholar]
- 40.Li X, et al. Pseudomonas aeruginosa infection augments inflammation through miR-301b repression of c-Myb-mediated immune activation and infiltration. Nat. Microbiol. 2016;1:16132. doi: 10.1038/nmicrobiol.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yehl, K. et al. Engineering phage host-range and suppressing bacterial resistance through phage tail fiber mutagenesis. Cell.179, 459–469. e459 (2019). [DOI] [PMC free article] [PubMed]
- 42.Sartorio MG, Pardue EJ, Feldman MF, Haurat MF. Bacterial outer membrane vesicles: from discovery to applications. Annu Rev. Microbiol. 2021;75:609–630. doi: 10.1146/annurev-micro-052821-031444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnington, K. E. & Kuehn, M. J. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta.1843, 1612–1619 (2014). [DOI] [PMC free article] [PubMed]
- 45.Kim JY, et al. Engineered bacterial outer membrane vesicles with enhanced functionality. J. Mol. Biol. 2008;380:51–66. doi: 10.1016/j.jmb.2008.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jan AT. Outer membrane vesicles (OMVs) of Gram-negative bacteria: a perspective update. Front. Microbiol. 2017;8:1053. doi: 10.3389/fmicb.2017.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bomberger JM, et al. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5:e1000382. doi: 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Micro. Pathog. 2002;32:1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- 50.Wai, S. N. et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell.115, 25–35 (2003). [DOI] [PubMed]
- 51.Kesty NC, Kuehn MJ. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 2004;279:2069–2076. doi: 10.1074/jbc.M307628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bomberger JM, et al. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 2011;7:e1001325. doi: 10.1371/journal.ppat.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006;8:2400–2408. doi: 10.1016/j.micinf.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koeppen K, et al. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog. 2016;12:e1005672. doi: 10.1371/journal.ppat.1005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballok AE, et al. Epoxide-mediated differential packaging of Cif and other virulence factors into outer membrane vesicles. J. Bacteriol. 2014;196:3633–3642. doi: 10.1128/JB.01760-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanton BA. Effects of Pseudomonas aeruginosa on CFTR chloride secretion and the host immune response. Am. J. Physiol. Cell Physiol. 2017;312:C357–C366. doi: 10.1152/ajpcell.00373.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furuyama N, Sircili MP. Outer membrane vesicles (OMVs) produced by Gram-negative bacteria: structure, functions, biogenesis, and vaccine application. Biomed. Res. Int. 2021;2021:1490732. doi: 10.1155/2021/1490732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015;10:1689–1706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balhuizen MD, Veldhuizen EJA, Haagsman HP. Outer membrane vesicle induction and isolation for vaccine development. Front. Microbiol. 2021;12:629090. doi: 10.3389/fmicb.2021.629090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martins P, et al. Outer membrane vesicles from Neisseria Meningitidis (Proteossome) used for nanostructured Zika virus vaccine production. Sci. Rep. 2018;8:8290. doi: 10.1038/s41598-018-26508-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Disco. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 62.Sharma AK, et al. Bacterial virulence factors: secreted for survival. Indian J. Microbiol. 2017;57:1–10. doi: 10.1007/s12088-016-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filloux A. Protein secretion systems in Pseudomonas aeruginosa: an essay on diversity, evolution, and function. Front. Microbiol. 2011;2:155. doi: 10.3389/fmicb.2011.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Sousa, T. et al. Genomic and metabolic characteristics of the pathogenicity in Pseudomonas aeruginosa. Int. J. Mol. Sci. 22, 1–28 (2021). [DOI] [PMC free article] [PubMed]
- 65.Thomas, S., Holland, I. B. & Schmitt, L. The Type 1 secretion pathway—the hemolysin system and beyond. Biochim. Biophys. Acta.1843, 1629–1641 (2014). [DOI] [PubMed]
- 66.Smith, T. J., Sondermann, H. & O’Toole, G. A. Type 1 does the two-step: type 1 secretion substrates with a functional periplasmic intermediate. J. Bacteriol. 200, e00168–18 (2018). [DOI] [PMC free article] [PubMed]
- 67.Alav I, et al. Structure, assembly, and function of tripartite efflux and type 1 secretion systems in Gram-negative bacteria. Chem. Rev. 2021;121:5479–5596. doi: 10.1021/acs.chemrev.1c00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cassat, J. E. & Skaar, E. P. Iron in infection and immunity. Cell Host Microbe.13, 509–519 (2013). [DOI] [PMC free article] [PubMed]
- 69.Yahr TL, Goranson J, Frank DW. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- 70.Jimenez PN, et al. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soscia C, et al. Cross talk between type III secretion and flagellar assembly systems in Pseudomonas aeruginosa. J. Bacteriol. 2007;189:3124–3132. doi: 10.1128/JB.01677-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coburn B, Sekirov I, Finlay BB. Type III secretion systems and disease. Clin. Microbiol. Rev. 2007;20:535–549. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Francis MS, Wolf-Watz H, Forsberg A. Regulation of type III secretion systems. Curr. Opin. Microbiol. 2002;5:166–172. doi: 10.1016/S1369-5274(02)00301-6. [DOI] [PubMed] [Google Scholar]
- 75.Zhu M, et al. Modulation of type III secretion system in Pseudomonas aeruginosa: involvement of the PA4857 gene product. Front. Microbiol. 2016;7:7. doi: 10.3389/fmicb.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galle M, Carpentier I, Beyaert R. Structure and function of the Type III secretion system of Pseudomonas aeruginosa. Curr. Protein Pept. Sci. 2012;13:831–842. doi: 10.2174/138920312804871210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garai P, et al. Killing from the inside: intracellular role of T3SS in the fate of Pseudomonas aeruginosa within macrophages revealed by mgtC and oprF mutants. PLoS Pathog. 2019;15:e1007812. doi: 10.1371/journal.ppat.1007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen DK, et al. PsrA is a positive transcriptional regulator of the type III secretion system in Pseudomonas aeruginosa. Infect. Immun. 2006;74:1121–1129. doi: 10.1128/IAI.74.2.1121-1129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li M, et al. HigB of Pseudomonas aeruginosa enhances killing of phagocytes by up-regulating the type III secretion system in ciprofloxacin induced persister cells. Front. Cell Infect. Microbiol. 2016;6:125. doi: 10.3389/fcimb.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marsden AE, et al. Vfr directly activates exsA transcription to regulate expression of the Pseudomonas aeruginosa Type III secretion system. J. Bacteriol. 2016;198:1442–1450. doi: 10.1128/JB.00049-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Intile PJ, Balzer GJ, Wolfgang MC, Yahr TL. The RNA helicase DeaD stimulates ExsA translation to promote expression of the Pseudomonas aeruginosa Type III secretion system. J. Bacteriol. 2015;197:2664–2674. doi: 10.1128/JB.00231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin Y, Yang H, Qiao M, Jin S. MexT regulates the type III secretion system through MexS and PtrC in Pseudomonas aeruginosa. J. Bacteriol. 2011;193:399–410. doi: 10.1128/JB.01079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goodman, A. L. et al. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell.7, 745–754 (2004). [DOI] [PubMed]
- 84.Intile PJ, et al. The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. J. Bacteriol. 2014;196:357–366. doi: 10.1128/JB.01199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brencic A, et al. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 2009;73:434–445. doi: 10.1111/j.1365-2958.2009.06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laskowski MA, Osborn E, Kazmierczak BI. A novel sensor kinase-response regulator hybrid regulates type III secretion and is required for virulence in Pseudomonas aeruginosa. Mol. Microbiol. 2004;54:1090–1103. doi: 10.1111/j.1365-2958.2004.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ventre, I. et al. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl Acad. Sci. USA.103, 171–176 (2006). [DOI] [PMC free article] [PubMed]
- 88.Goodman AL, et al. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 2009;23:249–259. doi: 10.1101/gad.1739009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chakravarty, S. et al. Pseudomonas aeruginosa magnesium transporter MgtE inhibits type III secretion system gene expression by stimulating rsmYZ transcription. J. Bacteriol. 199, e00268–17 (2017). [DOI] [PMC free article] [PubMed]
- 90.Chen, L., Zou, Y., Kronfl, A. A. & Wu, Y. Type VI secretion system of Pseudomonas aeruginosa is associated with biofilm formation but not environmental adaptation. Microbiologyopen.9, e991 (2020). [DOI] [PMC free article] [PubMed]
- 91.Vettiger, A. & Basler, M. Type VI secretion system substrates are transferred and reused among sister cells. Cell.167, 99–110. e112 (2016). [DOI] [PubMed]
- 92.Chen L, Zou Y, She P, Wu Y. Composition, function, and regulation of T6SS in Pseudomonas aeruginosa. Microbiol. Res. 2015;172:19–25. doi: 10.1016/j.micres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 93.Bleves S, et al. Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int. J. Med. Microbiol. 2010;300:534–543. doi: 10.1016/j.ijmm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Zoued, A. et al. Architecture and assembly of the Type VI secretion system. Biochim Biophys. Acta.1843, 1664–1673 (2014). [DOI] [PubMed]
- 95.Hood, R. D. et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe.7, 25–37 (2010). [DOI] [PMC free article] [PubMed]
- 96.Wang J, Brodmann M, Basler M. Assembly and subcellular localization of bacterial type VI secretion systems. Annu. Rev. Microbiol. 2019;73:621–638. doi: 10.1146/annurev-micro-020518-115420. [DOI] [PubMed] [Google Scholar]
- 97.Basler, M. Type VI secretion system: secretion by a contractile nanomachine. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 1–11 (2015). [DOI] [PMC free article] [PubMed]
- 98.Berni B, et al. A type VI secretion system trans-kingdom effector is required for the delivery of a novel antibacterial toxin in Pseudomonas aeruginosa. Front. Microbiol. 2019;10:1218. doi: 10.3389/fmicb.2019.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang H, et al. An integrated genomic regulatory network of virulence-related transcriptional factors in Pseudomonas aeruginosa. Nat. Commun. 2019;10:2931. doi: 10.1038/s41467-019-10778-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li C, et al. T6SS secretes an LPS-binding effector to recruit OMVs for exploitative competition and horizontal gene transfer. ISME J. 2022;16:500–510. doi: 10.1038/s41396-021-01093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lesic, B. et al. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology.155, 2845–2855 (2009). [DOI] [PMC free article] [PubMed]
- 102.Sana TG, et al. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J. Biol. Chem. 2012;287:27095–27105. doi: 10.1074/jbc.M112.376368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maura D, et al. Evidence for direct control of virulence and defense gene circuits by the Pseudomonas aeruginosa quorum sensing regulator, MvfR. Sci. Rep. 2016;6:34083. doi: 10.1038/srep34083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han Y, et al. A Pseudomonas aeruginosa type VI secretion system regulated by CueR facilitates copper acquisition. PLoS Pathog. 2019;15:e1008198. doi: 10.1371/journal.ppat.1008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao P, et al. oprC impairs host defense by increasing the quorum-sensing-mediated virulence of Pseudomonas aeruginosa. Front. Immunol. 2020;11:1696. doi: 10.3389/fimmu.2020.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang T, et al. A type VI secretion system delivers a cell wall amidase to target bacterial competitors. Mol. Microbiol. 2020;114:308–321. doi: 10.1111/mmi.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shao X, et al. Novel therapeutic strategies for treating Pseudomonas aeruginosa infection. Expert Opin. Drug Disco. 2020;15:1403–1423. doi: 10.1080/17460441.2020.1803274. [DOI] [PubMed] [Google Scholar]
- 108.Horna G, Ruiz J. Type 3 secretion system of Pseudomonas aeruginosa. Microbiol. Res. 2021;246:126719. doi: 10.1016/j.micres.2021.126719. [DOI] [PubMed] [Google Scholar]
- 109.Sana TG, Berni B, Bleves S. The T6SSs of Pseudomonas aeruginosa strain PAO1 and their effectors: beyond bacterial-cell targeting. Front. Cell Infect. Microbiol. 2016;6:61. doi: 10.3389/fcimb.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Records AR, Gross DC. Sensor kinases RetS and LadS regulate Pseudomonas syringae type VI secretion and virulence factors. J. Bacteriol. 2010;192:3584–3596. doi: 10.1128/JB.00114-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dadashi, M. et al. Putative RNA ligase RtcB affects the Switch between T6SS and T3SS in Pseudomonas aeruginosa. Int. J. Mol. Sci. 22, 1–21 (2021). [DOI] [PMC free article] [PubMed]
- 112.Xia, Y. et al. YbeY controls the type III and type VI secretion systems and biofilm formation through RetS in Pseudomonas aeruginosa. Appl. Environ. Microbiol.87, e02171–20 (2020). [DOI] [PMC free article] [PubMed]
- 113.Diaz MR, King JM, Yahr TL. Intrinsic and extrinsic regulation of type III secretion gene expression in Pseudomonas Aeruginosa. Front. Microbiol. 2011;2:89. doi: 10.3389/fmicb.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaminski A, et al. Pseudomonas aeruginosa ExoS induces intrinsic apoptosis in target host cells in a manner that is dependent on its GAP domain activity. Sci. Rep. 2018;8:14047. doi: 10.1038/s41598-018-32491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kroken AR, et al. Exotoxin S secreted by internalized Pseudomonas aeruginosa delays lytic host cell death. PLoS Pathog. 2022;18:e1010306. doi: 10.1371/journal.ppat.1010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jia J, Wang Y, Zhou L, Jin S. Expression of Pseudomonas aeruginosa toxin ExoS effectively induces apoptosis in host cells. Infect. Immun. 2006;74:6557–6570. doi: 10.1128/IAI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Finck-Barbancon V, Frank DW. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 2001;183:4330–4344. doi: 10.1128/JB.183.14.4330-4344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hardy, K. S. et al. ExoU induces lung endothelial cell damage and activates pro-inflammatory caspase-1 during Pseudomonas aeruginosa Infection. Toxins.14, 152 (2022). [DOI] [PMC free article] [PubMed]
- 119.Sutterwala FS, et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindsey AS, et al. Analysis of pulmonary vascular injury and repair during Pseudomonas aeruginosa infection-induced pneumonia and acute respiratory distress syndrome. Pulm. Circ. 2019;9:2045894019826941. doi: 10.1177/2045894019826941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Foulkes, D. M. et al. Pseudomonas aeruginosa toxin ExoU as a therapeutic target in the treatment of bacterial infections. Microorganisms. 7, 707 (2019). [DOI] [PMC free article] [PubMed]
- 122.Wood S, Goldufsky J, Shafikhani SH. Pseudomonas aeruginosa ExoT induces atypical anoikis apoptosis in target host cells by transforming Crk adaptor protein into a cytotoxin. PLoS Pathog. 2015;11:e1004934. doi: 10.1371/journal.ppat.1004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mohamed MF, et al. Pseudomonas aeruginosa ExoT induces G1 cell cycle arrest in melanoma cells. Cell Microbiol. 2021;23:e13339. doi: 10.1111/cmi.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Winsor GL, et al. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016;44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He, C. et al. Bacterial nucleotidyl cyclase inhibits the host innate immune response by suppressing TAK1 activation. Infect. Immun. 85, e00239–17 (2017). [DOI] [PMC free article] [PubMed]
- 126.Jeon J, Kim YJ, Shin H, Ha UH. T3SS effector ExoY reduces inflammasome-related responses by suppressing bacterial motility and delaying activation of NF-kappaB and caspase-1. FEBS J. 2017;284:3392–3403. doi: 10.1111/febs.14199. [DOI] [PubMed] [Google Scholar]
- 127.Yang, X., Long, M. & Shen, X. Effector(-)immunity pairs provide the T6SS nanomachine its offensive and defensive capabilities. Molecules. 23, 1009 (2018). [DOI] [PMC free article] [PubMed]
- 128.Russell, A. B. et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature.475, 343–347 (2011). [DOI] [PMC free article] [PubMed]
- 129.Pérez-Lorente, A. I., Molina-Santiago, C., de Vicente, A. & Romero, D. Sporulation activated via σW protects Bacillus from a Tse1 peptidoglycan hydrolase T6SS effector. Preprint at bioRxiv10.1101/2022.02.23.481616 (2022). [DOI] [PMC free article] [PubMed]
- 130.Coulthurst, S. The Type VI secretion system: a versatile bacterial weapon. Microbiology.165, 503–515 (2019). [DOI] [PubMed]
- 131.Sana, T. G. et al. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. mBio.6, e00712 (2015). [DOI] [PMC free article] [PubMed]
- 132.Kollman JM, Merdes A, Mourey L, Agard DA. Microtubule nucleation by gamma-tubulin complexes. Nat. Rev. Mol. Cell Biol. 2011;12:709–721. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kierbel, A., Gassama-Diagne, A., Mostov, K. & Engel, J. N. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell.16, 2577–2585 (2005). [DOI] [PMC free article] [PubMed]
- 134.Naskar S, Hohl M, Tassinari M, Low HH. The structure and mechanism of the bacterial type II secretion system. Mol. Microbiol. 2021;115:412–424. doi: 10.1111/mmi.14664. [DOI] [PubMed] [Google Scholar]
- 135.Lee PA, Tullman-Ercek D, Georgiou G. The bacterial twin-arginine translocation pathway. Annu. Rev. Microbiol. 2006;60:373–395. doi: 10.1146/annurev.micro.60.080805.142212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nivaskumar, M. & Francetic, O. Type II secretion system: a magic beanstalk or a protein escalator. Biochim. Biophys. Acta.1843, 1568–1577 (2014). [DOI] [PubMed]
- 137.Green, E. R. & Mecsas, J. Bacterial secretion systems: an overview. Microbiol. Spectr. 4, VMBF-0012-2015 (2016). [DOI] [PMC free article] [PubMed]
- 138.Veenendaal, A. K., van der Does, C. & Driessen, A. J. The protein-conducting channel SecYEG. Biochim. Biophys. Acta.1694, 81–95 (2004). [DOI] [PubMed]
- 139.Goosens, V. J., Monteferrante, C. G. & van Dijl, J. M. The Tat system of Gram-positive bacteria. Biochim. Biophys. Acta.1843, 1698–1706 (2014). [DOI] [PubMed]
- 140.Filloux A, Michel G, Bally M. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol. Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 141.Swietnicki W, et al. Identification of a potent inhibitor of type II secretion system from Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2019;513:688–693. doi: 10.1016/j.bbrc.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 142.Costa TR, et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat. Rev. Microbiol. 2015;13:343–359. doi: 10.1038/nrmicro3456. [DOI] [PubMed] [Google Scholar]
- 143.Meuskens I, Saragliadis A, Leo JC, Linke D. Type V secretion systems: an overview of passenger domain functions. Front. Microbiol. 2019;10:1163. doi: 10.3389/fmicb.2019.01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stubenrauch, C. J. & Lithgow, T. The TAM: a translocation and assembly module of the beta-barrel assembly machinery in bacterial outer membranes. EcoSal Plus. 8, ESP-0036-2018 (2019). [DOI] [PubMed]
- 145.Leo JC, Grin I, Linke D. Type V secretion: mechanism(s) of autotransport through the bacterial outer membrane. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:1088–1101. doi: 10.1098/rstb.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guerin J, et al. Two-partner secretion: combining efficiency and simplicity in the secretion of large proteins for bacteria-host and bacteria-bacteria interactions. Front Cell Infect. Microbiol. 2017;7:148. doi: 10.3389/fcimb.2017.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lyskowski A, Leo JC, Goldman A. Structure and biology of trimeric autotransporter adhesins. Adv. Exp. Med. Biol. 2011;715:143–158. doi: 10.1007/978-94-007-0940-9_9. [DOI] [PubMed] [Google Scholar]
- 148.Leo JC, Oberhettinger P, Schutz M, Linke D. The inverse autotransporter family: intimin, invasin and related proteins. Int. J. Med. Microbiol. 2015;305:276–282. doi: 10.1016/j.ijmm.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 149.Juhas M, Crook DW, Hood DW. Type IV secretion systems: tools of bacterial horizontal gene transfer and virulence. Cell Microbiol. 2008;10:2377–2386. doi: 10.1111/j.1462-5822.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tammam S, et al. PilMNOPQ from the Pseudomonas aeruginosa type IV pilus system form a transenvelope protein interaction network that interacts with PilA. J. Bacteriol. 2013;195:2126–2135. doi: 10.1128/JB.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu Y, et al. Transposon insertion sequencing reveals T4SS as the major genetic trait for conjugation transfer of multi-drug resistance pEIB202 from Edwardsiella. BMC Microbiol. 2017;17:112. doi: 10.1186/s12866-017-1013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Juhas M, et al. Genomic islands: tools of bacterial horizontal gene transfer and evolution. FEMS Microbiol. Rev. 2009;33:376–393. doi: 10.1111/j.1574-6976.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Arlehamn CS, Evans TJ. Pseudomonas aeruginosa pilin activates the inflammasome. Cell Microbiol. 2011;13:388–401. doi: 10.1111/j.1462-5822.2010.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang D, et al. Paeonol attenuates quorum-sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2021;12:692474. doi: 10.3389/fmicb.2021.692474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Elshaer, S. L. & Shaaban, M. I. Inhibition of quorum sensing and virulence factors of Pseudomonas aeruginosa by biologically synthesized gold and selenium nanoparticles. Antibiotics.10, 1461 (2021). [DOI] [PMC free article] [PubMed]
- 156.Fuqua C, Greenberg EP. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 157.Rutherford, S. T. & Bassler, B. L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2, a012427 (2012). [DOI] [PMC free article] [PubMed]
- 158.Hense BA, Schuster M. Core principles of bacterial autoinducer systems. Microbiol. Mol. Biol. Rev. 2015;79:153–169. doi: 10.1128/MMBR.00024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gerdt JP, Blackwell HE. Competition studies confirm two major barriers that can preclude the spread of resistance to quorum-sensing inhibitors in bacteria. ACS Chem. Biol. 2014;9:2291–2299. doi: 10.1021/cb5004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: can we make evolution-proof drugs? Nat. Rev. Microbiol. 2014;12:300–308. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 161.Oh J, Li XH, Kim SK, Lee JH. Post-secretional activation of Protease IV by quorum sensing in Pseudomonas aeruginosa. Sci. Rep. 2017;7:4416. doi: 10.1038/s41598-017-03733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaufmann, G. F. et al. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl Acad. Sci. USA.102, 309–314 (2005). [DOI] [PMC free article] [PubMed]
- 163.Dietrich LE, et al. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 164.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blus-Kadosh, I., Zilka, A., Yerushalmi, G. & Banin, E. The effect of pstS and phoB on quorum sensing and swarming motility in Pseudomonas aeruginosa. PLoS One.8, e74444 (2013). [DOI] [PMC free article] [PubMed]
- 166.Cooley M, Chhabra SR, Williams P. N-Acylhomoserine lactone-mediated quorum sensing: a twist in the tail and a blow for host immunity. Chem. Biol. 2008;15:1141–1147. doi: 10.1016/j.chembiol.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 167.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu. Rev. Microbiol. 2013;67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 168.Venturi V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 2006;30:274–291. doi: 10.1111/j.1574-6976.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- 169.Welsh MA, Blackwell HE. Chemical probes of quorum sensing: from compound development to biological discovery. FEMS Microbiol. Rev. 2016;40:774–794. doi: 10.1093/femsre/fuw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee, J. & Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell.6, 26–41 (2015). [DOI] [PMC free article] [PubMed]
- 171.LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013;77:73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schuster, M. & Greenberg, E. P. Early activation of quorum sensing in Pseudomonas aeruginosa reveals the architecture of a complex regulon. BMC Genomics.8, 287 (2007). [DOI] [PMC free article] [PubMed]
- 173.Fuqua C. The QscR quorum-sensing regulon of Pseudomonas aeruginosa: an orphan claims its identity. J. Bacteriol. 2006;188:3169–3171. doi: 10.1128/JB.188.9.3169-3171.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liang H, et al. Molecular mechanisms of master regulator VqsM mediating quorum-sensing and antibiotic resistance in Pseudomonas aeruginosa. Nucleic Acids Res. 2014;42:10307–10320. doi: 10.1093/nar/gku586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Diggle SP, et al. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 2002;184:2576–2586. doi: 10.1128/JB.184.10.2576-2586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Castang, S., McManus, H. R., Turner, K. H. & Dove, S. L. H-NS family members function coordinately in an opportunistic pathogen. Proc. Natl Acad. Sci. USA.105, 18947–18952 (2008). [DOI] [PMC free article] [PubMed]
- 177.Rampioni G, et al. Contribution of the RsaL global regulator to Pseudomonas aeruginosa virulence and biofilm formation. FEMS Microbiol. Lett. 2009;301:210–217. doi: 10.1111/j.1574-6968.2009.01817.x. [DOI] [PubMed] [Google Scholar]
- 178.Rampioni G, et al. RsaL provides quorum sensing homeostasis and functions as a global regulator of gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 2007;66:1557–1565. doi: 10.1111/j.1365-2958.2007.06029.x. [DOI] [PubMed] [Google Scholar]
- 179.Kang H, et al. Crystal structure of Pseudomonas aeruginosa RsaL bound to promoter DNA reaffirms its role as a global regulator involved in quorum-sensing. Nucleic Acids Res. 2017;45:699–710. doi: 10.1093/nar/gkw954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Balasubramanian, D. et al. The regulatory repertoire of Pseudomonas aeruginosa AmpC ss-lactamase regulator AmpR includes virulence genes. PLoS One.7, e34067 (2012). [DOI] [PMC free article] [PubMed]
- 181.Zhao J, et al. Structural and molecular mechanism of CdpR involved in quorum-sensing and bacterial virulence in Pseudomonas aeruginosa. PLoS Biol. 2016;14:e1002449. doi: 10.1371/journal.pbio.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cao Q, et al. A novel signal transduction pathway that modulates rhl quorum sensing and bacterial virulence in Pseudomonas aeruginosa. PLoS Pathog. 2014;10:e1004340. doi: 10.1371/journal.ppat.1004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang N, et al. The Crc protein participates in down-regulation of the Lon gene to promote rhamnolipid production and rhl quorum sensing in Pseudomonas aeruginosa. Mol. Microbiol. 2015;96:526–547. doi: 10.1111/mmi.12954. [DOI] [PubMed] [Google Scholar]
- 184.Zhang, Y. et al. Pseudomonas aeruginosa regulatory protein AnvM controls pathogenicity in anaerobic environments and impacts host defense. MBio.10, e01362–01319 (2019). [DOI] [PMC free article] [PubMed]
- 185.Siehnel, R. et al. A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA.107, 7916–7921 (2010). [DOI] [PMC free article] [PubMed]
- 186.Seet Q, Zhang LH. Anti-activator QslA defines the quorum sensing threshold and response in Pseudomonas aeruginosa. Mol. Microbiol. 2011;80:951–965. doi: 10.1111/j.1365-2958.2011.07622.x. [DOI] [PubMed] [Google Scholar]
- 187.Ghosh, S. et al. Phytocompound mediated blockage of quorum sensing cascade in ESKAPE pathogens. Antibiotics.11, 61 (2022). [DOI] [PMC free article] [PubMed]
- 188.Piewngam P, Chiou J, Chatterjee P, Otto M. Alternative approaches to treat bacterial infections: targeting quorum-sensing. Expert Rev. Anti. Infect. Ther. 2020;18:499–510. doi: 10.1080/14787210.2020.1750951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 2006;9:143–152. doi: 10.1016/j.mib.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 190.Balasubramanian D, Schneper L, Kumari H, Mathee K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013;41:1–20. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rodrigue A, et al. Two-component systems in Pseudomonas aeruginosa: why so many? Trends Microbiol. 2000;8:498–504. doi: 10.1016/S0966-842X(00)01833-3. [DOI] [PubMed] [Google Scholar]
- 192.Francis, V. I., Stevenson, E. C. & Porter, S. L. Two-component systems required for virulence in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 364, 1–22 (2017). [DOI] [PMC free article] [PubMed]
- 193.Bem AE, et al. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem. Biol. 2015;10:213–224. doi: 10.1021/cb5007135. [DOI] [PubMed] [Google Scholar]
- 194.Ma S, Wozniak DJ, Ohman DE. Identification of the histidine protein kinase KinB in Pseudomonas aeruginosa and its phosphorylation of the alginate regulator algB. J. Biol. Chem. 1997;272:17952–17960. doi: 10.1074/jbc.272.29.17952. [DOI] [PubMed] [Google Scholar]
- 195.Goswami M, Espinasse A, Carlson EE. Disarming the virulence arsenal of Pseudomonas aeruginosa by blocking two-component system signaling. Chem. Sci. 2018;9:7332–7337. doi: 10.1039/C8SC02496K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yu, L. et al. A novel copper-sensing two-component system for inducing Dsb gene expression in bacteria. Science Bulletin. 67, 198–212 (2022). [DOI] [PubMed]
- 197.Wang, Y. et al. Histamine activates HinK to promote the virulence of Pseudomonas aeruginosa. Science Bulletin. 66, 1101–1118 (2021). [DOI] [PubMed]
- 198.Broder UN, Jaeger T, Jenal U. LadS is a calcium-responsive kinase that induces acute-to-chronic virulence switch in Pseudomonas aeruginosa. Nat. Microbiol. 2016;2:16184. doi: 10.1038/nmicrobiol.2016.184. [DOI] [PubMed] [Google Scholar]
- 199.Sall, K. M. et al. A gacS deletion in Pseudomonas aeruginosa cystic fibrosis isolate CHA shapes its virulence. PLoS One.9, e95936 (2014). [DOI] [PMC free article] [PubMed]
- 200.Morris, E. R. et al. Structural rearrangement in an RsmA/CsrA ortholog of Pseudomonas aeruginosa creates a dimeric RNA-binding protein, RsmN. Structure.21, 1659–1671 (2013). [DOI] [PMC free article] [PubMed]
- 201.Mikkelsen, H., McMullan, R. & Filloux, A. The Pseudomonas aeruginosa reference strain PA14 displays increased virulence due to a mutation in ladS. PLoS One.6, e29113 (2011). [DOI] [PMC free article] [PubMed]
- 202.Kazmierczak BI, Schniederberend M, Jain R. Cross-regulation of Pseudomonas motility systems: the intimate relationship between flagella, pili and virulence. Curr. Opin. Microbiol. 2015;28:78–82. doi: 10.1016/j.mib.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rao F, Yang Y, Qi Y, Liang ZX. Catalytic mechanism of cyclic di-GMP-specific phosphodiesterase: a study of the EAL domain-containing RocR from Pseudomonas aeruginosa. J. Bacteriol. 2008;190:3622–3631. doi: 10.1128/JB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mikkelsen H, Hui K, Barraud N, Filloux A. The pathogenicity island encoded PvrSR/RcsCB regulatory network controls biofilm formation and dispersal in Pseudomonas aeruginosa PA14. Mol. Microbiol. 2013;89:450–463. doi: 10.1111/mmi.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gellatly SL, et al. The Pseudomonas aeruginosa PhoP-PhoQ two-component regulatory system is induced upon interaction with epithelial cells and controls cytotoxicity and inflammation. Infect. Immun. 2012;80:3122–3131. doi: 10.1128/IAI.00382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lewenza S. Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Front. Microbiol. 2013;4:21. doi: 10.3389/fmicb.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Turner KH, et al. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet. 2014;10:e1004518. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 2008;69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.O’Toole GA, Wong GC. Sensational biofilms: surface sensing in bacteria. Curr. Opin. Microbiol. 2016;30:139–146. doi: 10.1016/j.mib.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Luo, Y. et al. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. MBio. 6, e02456–14 (2015). [DOI] [PMC free article] [PubMed]
- 212.Cao, Q. et al. Mutation-induced remodeling of the BfmRS two-component system in Pseudomonas aeruginosa clinical isolates. Sci. Signal. 13, eaaz1529 (2020). [DOI] [PubMed]
- 213.Nitzan M, Rehani R, Margalit H. Integration of Bacterial Small RNAs in Regulatory Networks. Annu Rev. Biophys. 2017;46:131–148. doi: 10.1146/annurev-biophys-070816-034058. [DOI] [PubMed] [Google Scholar]
- 214.Mikulik K. Structure and functional properties of prokaryotic small noncoding RNAs. Folia Microbiol. 2003;48:443–468. doi: 10.1007/BF02931326. [DOI] [PubMed] [Google Scholar]
- 215.Papenfort K, et al. SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol. Microbiol. 2006;62:1674–1688. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Klein G, Raina S. Small regulatory bacterial RNAs regulating the envelope stress response. Biochem. Soc. Trans. 2017;45:417–425. doi: 10.1042/BST20160367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Michaux, C., Verneuil, N., Hartke, A. & Giard, J. C. Physiological roles of small RNA molecules. Microbiology.160, 1007–1019 (2014). [DOI] [PubMed]
- 218.Pita, T., Feliciano, J. R. & Leitao, J. H. Small noncoding regulatory RNAs from Pseudomonas aeruginosa and Burkholderia cepacia complex. Int. J. Mol. Sci. 19, 3759 (2018). [DOI] [PMC free article] [PubMed]
- 219.Sonnleitner E, et al. Reduced virulence of a hfq mutant of Pseudomonas aeruginosa O1. Micro. Pathog. 2003;35:217–228. doi: 10.1016/S0882-4010(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 220.Zhao, K., Li, Y., Yue, B. & Wu, M. Genes as early responders regulate quorum-sensing and control bacterial cooperation in Pseudomonas aeruginosa. PLoS One.9, e101887 (2014). [DOI] [PMC free article] [PubMed]
- 221.Gomez-Lozano M, et al. Diversity of small RNAs expressed in Pseudomonas species. Environ. Microbiol. Rep. 2015;7:227–236. doi: 10.1111/1758-2229.12233. [DOI] [PubMed] [Google Scholar]
- 222.Lin P, et al. High-throughput screen reveals sRNAs regulating crRNA biogenesis by targeting CRISPR leader to repress Rho termination. Nat. Commun. 2019;10:3728. doi: 10.1038/s41467-019-11695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pang Z, et al. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 224.Hancock, R. E. & Speert, D. P. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist. Updat.3, 247–255 (2000). [DOI] [PubMed]
- 225.Breidenstein EB, de la Fuente-Nunez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 226.Lambert PA. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. R. Soc. Med. 2002;95:22–26. [PMC free article] [PubMed] [Google Scholar]
- 227.Welte W, Nestel U, Wacker T, Diederichs K. Structure and function of the porin channel. Kidney Int. 1995;48:930–940. doi: 10.1038/ki.1995.374. [DOI] [PubMed] [Google Scholar]
- 228.Hancock RE, Brinkman FS. Function of pseudomonas porins in uptake and efflux. Annu. Rev. Microbiol. 2002;56:17–38. doi: 10.1146/annurev.micro.56.012302.160310. [DOI] [PubMed] [Google Scholar]
- 229.Angeletti S, et al. Multi-drug resistant Pseudomonas aeruginosa nosocomial strains: molecular epidemiology and evolution. Micro. Pathog. 2018;123:233–241. doi: 10.1016/j.micpath.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 230.Samanta S, et al. Getting drugs through small pores: exploiting the porins pathway in Pseudomonas aeruginosa. ACS Infect. Dis. 2018;4:1519–1528. doi: 10.1021/acsinfecdis.8b00149. [DOI] [PubMed] [Google Scholar]
- 231.Song F, Wang H, Sauer K, Ren D. Cyclic-di-GMP and oprF are involved in the response of Pseudomonas aeruginosa to substrate material stiffness during attachment on polydimethylsiloxane (PDMS) Front. Microbiol. 2018;9:110. doi: 10.3389/fmicb.2018.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Simm R, et al. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004;53:1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- 233.Valentini M, Filloux A. Biofilms and Cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2016;291:12547–12555. doi: 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li H, et al. Structure and function of OprD protein in Pseudomonas aeruginosa: from antibiotic resistance to novel therapies. Int. J. Med. Microbiol. 2012;302:63–68. doi: 10.1016/j.ijmm.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee J, et al. Refinement of OprH-LPS interactions by molecular simulations. Biophys. J. 2017;112:346–355. doi: 10.1016/j.bpj.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Young ML, Bains M, Bell A, Hancock RE. Role of Pseudomonas aeruginosa outer membrane protein OprH in polymyxin and gentamicin resistance: isolation of an OprH-deficient mutant by gene replacement techniques. Antimicrob. Agents Chemother. 1992;36:2566–2568. doi: 10.1128/AAC.36.11.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li XZ, Nikaido H, Poole K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1995;39:1948–1953. doi: 10.1128/AAC.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Farjah, A. et al. Immunological evaluation of an alginate-based conjugate as a vaccine candidate against Pseudomonas aeruginosa. APMIS.123, 175–183 (2015). [DOI] [PubMed]
- 239.Li XZ, Plesiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015;28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dreier J, Ruggerone P. Interaction of antibacterial compounds with RND e ffl ux pumps in Pseudomonas aeruginosa. Front. Microbiol. 2015;6:660. doi: 10.3389/fmicb.2015.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jeannot K, et al. Resistance and virulence of Pseudomonas aeruginosa clinical strains overproducing the MexCD-OprJ efflux pump. Antimicrob. Agents Chemother. 2008;52:2455–2462. doi: 10.1128/AAC.01107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Llanes C, et al. Role of the MexEF-OprN efflux system in low-level resistance of Pseudomonas aeruginosa to ciprofloxacin. Antimicrob. Agents Chemother. 2011;55:5676–5684. doi: 10.1128/AAC.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guenard S, et al. Multiple mutations lead to MexXY-OprM-dependent aminoglycoside resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014;58:221–228. doi: 10.1128/AAC.01252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Biswas R, Panja AS, Bandopadhyay R. Molecular mechanism of antibiotic resistance: the untouched area of future hope. Indian J. Microbiol. 2019;59:254–259. doi: 10.1007/s12088-019-00781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rafiee R, Eftekhar F, Tabatabaei SA, Minaee Tehrani D. Prevalence of extended-spectrum and metallo beta-lactamase production in AmpC beta-lactamase producing Pseudomonas aeruginosa isolates from burns. Jundishapur J. Microbiol. 2014;7:e16436. doi: 10.5812/jjm.16436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rawat D, Nair D. Extended-spectrum beta-lactamases in Gram negative bacteria. J. Glob. Infect. Dis. 2010;2:263–274. doi: 10.4103/0974-777X.68531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Poirel, L. et al. Acquisition of extended-spectrum beta-lactamase GES-6 leading to resistance to ceftolozane-tazobactam combination in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 63, e01809-18 (2019). [DOI] [PMC free article] [PubMed]
- 249.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ratjen F, Brockhaus F, Angyalosi G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: a review. J. Cyst. Fibros. 2009;8:361–369. doi: 10.1016/j.jcf.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 251.Hachler H, Santanam P, Kayser FH. Sequence and characterization of a novel chromosomal aminoglycoside phosphotransferase gene, aph (3′)-IIb, in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1996;40:1254–1256. doi: 10.1128/AAC.40.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hainrichson M, et al. Overexpression and initial characterization of the chromosomal aminoglycoside 3′-O-phosphotransferase APH(3’)-IIb from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2007;51:774–776. doi: 10.1128/AAC.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Poole K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005;49:479–487. doi: 10.1128/AAC.49.2.479-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Subedi D, Vijay AK, Willcox M. Overview of mechanisms of antibiotic resistance in Pseudomonas aeruginosa: an ocular perspective. Clin. Exp. Optom. 2018;101:162–171. doi: 10.1111/cxo.12621. [DOI] [PubMed] [Google Scholar]
- 255.Woodford N, Ellington MJ. The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect. 2007;13:5–18. doi: 10.1111/j.1469-0691.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- 256.Lerminiaux NA, Cameron ADS. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019;65:34–44. doi: 10.1139/cjm-2018-0275. [DOI] [PubMed] [Google Scholar]
- 257.Aldred, K. J., Kerns, R. J. & Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry.53, 1565–1574 (2014). [DOI] [PMC free article] [PubMed]
- 258.Berrazeg M, et al. Mutations in beta-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob. Agents Chemother. 2015;59:6248–6255. doi: 10.1128/AAC.00825-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Husnik F, McCutcheon JP. Functional horizontal gene transfer from bacteria to eukaryotes. Nat. Rev. Microbiol. 2018;16:67–79. doi: 10.1038/nrmicro.2017.137. [DOI] [PubMed] [Google Scholar]
- 260.Bonomo RA, Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006;43:S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 261.Motta, S. S., Cluzel, P. & Aldana, M. Adaptive resistance in bacteria requires epigenetic inheritance, genetic noise, and cost of efflux pumps. PLoS One.10, e0118464 (2015). [DOI] [PMC free article] [PubMed]
- 262.Maurice NM, Bedi B, Sadikot RT. Pseudomonas aeruginosa biofilms: host response and clinical implications in lung infections. Am. J. Respir. Cell Mol. Biol. 2018;58:428–439. doi: 10.1165/rcmb.2017-0321TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cohen, N. R., Lobritz, M. A. & Collins, J. J. Microbial persistence and the road to drug resistance. Cell Host Microbe.13, 632–642 (2013). [DOI] [PMC free article] [PubMed]
- 264.Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017;15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 265.Li R, et al. Annexin A2 regulates autophagy in Pseudomonas aeruginosa infection through the Akt1-mTOR-ULK1/2 signaling pathway. J. Immunol. 2015;195:3901–3911. doi: 10.4049/jimmunol.1500967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhou X, et al. MicroRNA-302b augments host defense to bacteria by regulating inflammatory responses via feedback to TLR/IRAK4 circuits. Nat. Commun. 2014;5:3619. doi: 10.1038/ncomms4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ye Y, et al. Atg7 deficiency impairs host defense against Klebsiella pneumoniae by impacting bacterial clearance, survival and inflammatory responses in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L355–L363. doi: 10.1152/ajplung.00046.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Imbert PR, et al. A Pseudomonas aeruginosa TIR effector mediates immune evasion by targeting UBAP1 and TLR adaptors. EMBO J. 2017;36:1869–1887. doi: 10.15252/embj.201695343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.McIsaac SM, Stadnyk AW, Lin TJ. Toll-like receptors in the host defense against Pseudomonas aeruginosa respiratory infection and cystic fibrosis. J. Leukoc. Biol. 2012;92:977–985. doi: 10.1189/jlb.0811410. [DOI] [PubMed] [Google Scholar]
- 270.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 271.Ernst, R. K. et al. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science.286, 1561–1565 (1999). [DOI] [PubMed]
- 272.Hajjar AM, et al. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 273.da Silva Correia J, et al. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 274.Power MR, et al. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J. Biol. Chem. 2004;279:49315–49322. doi: 10.1074/jbc.M402111200. [DOI] [PubMed] [Google Scholar]
- 275.Zhou, C. M. et al. Annexin A2 regulates unfolded protein response via IRE1-XBP1 axis in macrophages during P. aeruginosa infection. J. Leukoc. Biol. 110, 375–384 (2020). [DOI] [PMC free article] [PubMed]
- 276.Pu Q, et al. Atg7 deficiency intensifies inflammasome activation and pyroptosis in Pseudomonas Sepsis. J. Immunol. 2017;198:3205–3213. doi: 10.4049/jimmunol.1601196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pu Q, et al. Interaction among inflammasome, autophagy and non-coding RNAs: new horizons for drug. Precis Clin. Med. 2019;2:166–182. doi: 10.1093/pcmedi/pbz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wu, Q. et al. Bacterial Type I CRISPR-Cas systems influence inflammasome activation in mammalian host by promoting autophagy. Immunology.158, 240–251 (2019). [DOI] [PMC free article] [PubMed]
- 279.Zhou, C. M. et al. Identification of cGAS as an innate immune sensor of extracellular bacterium Pseudomonas aeruginosa. iScience.24, 101928 (2021). [DOI] [PMC free article] [PubMed]
- 280.Qin S, et al. Small-molecule inhibitor of 8-oxoguanine DNA glycosylase 1 regulates inflammatory responses during Pseudomonas aeruginosa infection. J. Immunol. 2020;205:2231–2242. doi: 10.4049/jimmunol.1901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhou X, et al. Transient receptor potential channel 1 deficiency impairs host defense and proinflammatory responses to bacterial infection by regulating protein kinase calpha signaling. Mol. Cell Biol. 2015;35:2729–2739. doi: 10.1128/MCB.00256-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wu M, et al. Host DNA repair proteins in response to Pseudomonas aeruginosa in lung epithelial cells and in mice. Infect. Immun. 2011;79:75–87. doi: 10.1128/IAI.00815-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Huang T, et al. MicroRNA-302/367 cluster impacts host antimicrobial defense via regulation of mitophagic response against Pseudomonas aeruginosa infection. Front. Immunol. 2020;11:569173. doi: 10.3389/fimmu.2020.569173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature.410, 1099–1103 (2001). [DOI] [PubMed]
- 285.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 286.Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell.10, 417–426 (2002). [DOI] [PubMed]
- 287.Sharma D, Kanneganti TD. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature.526, 660–665 (2015). [DOI] [PubMed]
- 289.Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature.535, 153–158 (2016). [DOI] [PMC free article] [PubMed]
- 290.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol. Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lin CK, Kazmierczak BI. Inflammation: a double-edged sword in the response to Pseudomonas aeruginosa infection. J. Innate Immun. 2017;9:250–261. doi: 10.1159/000455857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature.477, 592–595 (2011). [DOI] [PMC free article] [PubMed]
- 293.Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature.477, 596–600 (2011). [DOI] [PubMed]
- 294.Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA.107, 3076–3080 (2010). [DOI] [PMC free article] [PubMed]
- 295.Grandjean T, et al. The human NAIP-NLRC4-inflammasome senses the Pseudomonas aeruginosa T3SS inner-rod protein. Int. Immunol. 2017;29:377–384. doi: 10.1093/intimm/dxx047. [DOI] [PubMed] [Google Scholar]
- 296.Jabir, M. S. et al. Mitochondrial damage contributes to Pseudomonas aeruginosa activation of the inflammasome and is downregulated by autophagy. Autophagy.11, 166–182 (2015). [DOI] [PMC free article] [PubMed]
- 297.Pang Z, Sun G, Junkins RD, Lin TJ. AIM2 inflammasome is dispensable for the host defense against Pseudomonas aeruginosa infection. Cell Mol. Biol. 2015;61:63–70. [PubMed] [Google Scholar]
- 298.Yang J, et al. Bacterial secretant from Pseudomonas aeruginosa dampens inflammasome activation in a quorum sensing-dependent manner. Front. Immunol. 2017;8:333. doi: 10.3389/fimmu.2017.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Virreira Winter S, Zychlinsky A. The bacterial pigment pyocyanin inhibits the NLRP3 inflammasome through intracellular reactive oxygen and nitrogen species. J. Biol. Chem. 2018;293:4893–4900. doi: 10.1074/jbc.RA117.001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Abais, J. M. et al. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal.22, 1111–1129 (2015). [DOI] [PMC free article] [PubMed]
- 301.Arlehamn CS, et al. The role of potassium in inflammasome activation by bacteria. J. Biol. Chem. 2010;285:10508–10518. doi: 10.1074/jbc.M109.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Miao, E. A. et al. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl Acad. Sci. USA.105, 2562–2567 (2008). [DOI] [PMC free article] [PubMed]
- 303.Kupz A, et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8(+) T cells. Nat. Immunol. 2012;13:162–169. doi: 10.1038/ni.2195. [DOI] [PubMed] [Google Scholar]
- 304.Faure E, et al. Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am. J. Respir. Crit. Care Med. 2014;189:799–811. doi: 10.1164/rccm.201307-1358OC. [DOI] [PubMed] [Google Scholar]
- 305.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J. Clin. Invest. 2013;123:1630–1637. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sun, L. et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science.339, 786–791 (2013). [DOI] [PMC free article] [PubMed]
- 307.Collins, A. C. et al. Cyclic GMP-AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe.17, 820–828 (2015). [DOI] [PMC free article] [PubMed]
- 308.Chen K, et al. Stimulator of interferon genes promotes host resistance against Pseudomonas aeruginosa keratitis. Front. Immunol. 2018;9:1225. doi: 10.3389/fimmu.2018.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhou, C. et al. Identification of cGAS as an innate immune sensor of extracellular bacterium Pseudomonas aeruginosa. iScience. 24, 101928 (2020). [DOI] [PMC free article] [PubMed]
- 310.Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 2014;23:R40–R46. doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- 311.Makarova KS, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015;13:722–736. doi: 10.1038/nrmicro3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Koonin EV, Makarova KS, Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017;37:67–78. doi: 10.1016/j.mib.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Stanley, S. Y. & Maxwell, K. L. Phage-encoded anti-CRISPR defenses. Annu. Rev. Genet.52, 445-464 (2018). [DOI] [PubMed]
- 314.Sternberg, S. H., Richter, H., Charpentier, E. & Qimron, U. Adaptation in CRISPR-Cas systems. Mol. Cell.61, 797–808 (2016). [DOI] [PubMed]
- 315.Marino, N. D. et al. Discovery of widespread type I and type V CRISPR-Cas inhibitors. Science.362, 240–242 (2018). [DOI] [PMC free article] [PubMed]
- 316.van Belkum, A. et al. Phylogenetic distribution of CRISPR-Cas systems in antibiotic-resistant Pseudomonas aeruginosa. mBio.6, e01796–01715 (2015). [DOI] [PMC free article] [PubMed]
- 317.Vorontsova D, et al. Foreign DNA acquisition by the I-F CRISPR-Cas system requires all components of the interference machinery. Nucleic Acids Res. 2015;43:10848–10860. doi: 10.1093/nar/gkv1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Marraffini, L. A. CRISPR-Cas immunity in prokaryotes. Nature.526, 55–61 (2015). [DOI] [PubMed]
- 319.Cady KC, et al. The CRISPR/Cas adaptive immune system of Pseudomonas aeruginosa mediates resistance to naturally occurring and engineered phages. J. Bacteriol. 2012;194:5728–5738. doi: 10.1128/JB.01184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Louwen R, et al. The role of CRISPR-Cas systems in virulence of pathogenic bacteria. Microbiol. Mol. Biol. Rev. 2014;78:74–88. doi: 10.1128/MMBR.00039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Li R, et al. Type I CRISPR-Cas targets endogenous genes and regulates virulence to evade mammalian host immunity. Cell Res. 2016;26:1273–1287. doi: 10.1038/cr.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hoyland-Kroghsbo, N. M. et al. Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system. Proc. Natl Acad. Sci. USA.114, 131–135 (2017). [DOI] [PMC free article] [PubMed]
- 323.Alseth, E. O. et al. Bacterial biodiversity drives the evolution of CRISPR-based phage resistance. Nature.574, 549–552 (2019). [DOI] [PMC free article] [PubMed]
- 324.Vasquez-Rifo A, et al. The Pseudomonas aeruginosa accessory genome elements influence virulence towards Caenorhabditis elegans. Genome Biol. 2019;20:270. doi: 10.1186/s13059-019-1890-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Heussler, G. E. et al. Clustered regularly interspaced short palindromic repeat-dependent, biofilm-specific death of Pseudomonas aeruginosa mediated by increased expression of phage-related genes. mBio.6, e00129–00115 (2015). [DOI] [PMC free article] [PubMed]
- 326.Sampson, T. R. et al. A CRISPR-Cas system enhances envelope integrity mediating antibiotic resistance and inflammasome evasion. Proc. Natl Acad. Sci. USA.111, 11163–11168 (2014). [DOI] [PMC free article] [PubMed]
- 327.Pawluk A, et al. Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat. Microbiol. 2016;1:16085. doi: 10.1038/nmicrobiol.2016.85. [DOI] [PubMed] [Google Scholar]
- 328.Bondy-Denomy, J., Pawluk, A., Maxwell, K. L. & Davidson, A. R. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature.493, 429–432 (2013). [DOI] [PMC free article] [PubMed]
- 329.Pawluk, A. et al. Naturally occurring off-switches for CRISPR-Cas9. Cell.167, 1829–1838. e1829 (2016). [DOI] [PMC free article] [PubMed]
- 330.Harrington, L. B. et al. A broad-spectrum inhibitor of CRISPR-Cas9. Cell.170, 1224–1233. e1215 (2017). [DOI] [PMC free article] [PubMed]
- 331.Rauch, B. J. et al. Inhibition of CRISPR-Cas9 with bacteriophage proteins. Cell.168, 150–158. e110 (2017). [DOI] [PMC free article] [PubMed]
- 332.Watters, K. E. et al. Systematic discovery of natural CRISPR-Cas12a inhibitors. Science.362, 236–239 (2018). [DOI] [PMC free article] [PubMed]
- 333.Lin, P. et al. CRISPR-Cas13 inhibitors block RNA editing in bacteria and mammalian cells. Mol. Cell.78, 850–861. e855 (2020). [DOI] [PMC free article] [PubMed]
- 334.Lin, P. et al. Type III CRISPR-based RNA editing for programmable control of SARS-CoV-2 and human coronaviruses. Nucleic. Acids Res.50, e47 (2022). [DOI] [PMC free article] [PubMed]
- 335.Chin W, et al. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nat. Commun. 2018;9:917. doi: 10.1038/s41467-018-03325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Biswaro LS, et al. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018;9:855. doi: 10.3389/fmicb.2018.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Eljaaly, K. et al. Plazomicin: a novel aminoglycoside for the treatment of resistant Gram-negative bacterial infections. Drugs.79, 243–269 (2019). [DOI] [PubMed]
- 338.Sutcliffe JA, O’Brien W, Fyfe C, Grossman TH. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob. Agents Chemother. 2013;57:5548–5558. doi: 10.1128/AAC.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Sader HS, et al. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant beta-lactamases. J. Antimicrob. Chemother. 2017;72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 340.Del Tordello, E., Danilchanka, O., McCluskey, A. J. & Mekalanos, J. J. Type VI secretion system sheaths as nanoparticles for antigen display. Proc. Natl Acad. Sci. USA.113, 3042–3047 (2016). [DOI] [PMC free article] [PubMed]
- 341.Zhang C, et al. Synthesis of magnetite hybrid nanocomplexes to eliminate bacteria and enhance biofilm disruption. Biomater. Sci. 2019;7:2833–2840. doi: 10.1039/C9BM00057G. [DOI] [PubMed] [Google Scholar]
- 342.Salman M, et al. Synergistic effect of silver nanoparticles and polymyxin B against biofilm produced by Pseudomonas aeruginosa isolates of pus samples in vitro. Artif. Cells Nanomed. Biotechnol. 2019;47:2465–2472. doi: 10.1080/21691401.2019.1626864. [DOI] [PubMed] [Google Scholar]
- 343.Safari Zanjani L, et al. Exotoxin A-PLGA nanoconjugate vaccine against Pseudomonas aeruginosa infection: protectivity in murine model. World J. Microbiol. Biotechnol. 2019;35:94. doi: 10.1007/s11274-019-2669-y. [DOI] [PubMed] [Google Scholar]
- 344.Xiao Y, et al. Engineering nanoparticles for targeted delivery of nucleic acid therapeutics in tumor. Mol. Ther. Methods Clin. Dev. 2019;12:1–18. doi: 10.1016/j.omtm.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Adler-Moore J, et al. Preclinical safety, tolerability, pharmacokinetics, pharmacodynamics, and antifungal activity of liposomal amphotericin B. Clin. Infect. Dis. 2019;68:S244–S259. doi: 10.1093/cid/ciz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Oliver SE, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Hoggarth A, et al. Mechanistic research holds promise for bacterial vaccines and phage therapies for Pseudomonas aeruginosa. Drug Des. Devel Ther. 2019;13:909–924. doi: 10.2147/DDDT.S189847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Forti, F. et al. Design of a broad-range bacteriophage cocktail that reduces Pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob. Agents Chemother. 62, e02573–17 (2018). [DOI] [PMC free article] [PubMed]
- 350.Hall AR, et al. Effects of sequential and simultaneous applications of bacteriophages on populations of Pseudomonas aeruginosa in vitro and in wax moth larvae. Appl. Environ. Microbiol. 2012;78:5646–5652. doi: 10.1128/AEM.00757-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Alves DR, et al. A novel bacteriophage cocktail reduces and disperses Pseudomonas aeruginosa biofilms under static and flow conditions. Micro. Biotechnol. 2016;9:61–74. doi: 10.1111/1751-7915.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wright A, Hawkins CH, Anggard EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009;34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 353.Merabishvili, M. et al. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One.4, e4944 (2009). [DOI] [PMC free article] [PubMed]
- 354.Niu, Y. et al. A type I-F anti-CRISPR protein inhibits the CRISPR-Cas surveillance complex by ADP-ribosylation. Mol. Cell.80, 512–524. e515 (2020). [DOI] [PubMed]
- 355.Bikard D, et al. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 2014;32:1146–1150. doi: 10.1038/nbt.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Nick, J. A. et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell.185, 1860–1874 (2022). [DOI] [PMC free article] [PubMed]
- 357.Dedrick RM, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019;25:730–733. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Perche, F. et al. Cardiolipin-based lipopolyplex platform for the delivery of diverse nucleic acids into Gram-negative bacteria. Pharmaceuticals.12, 81 (2019). [DOI] [PMC free article] [PubMed]
- 359.Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR-Cas9 system. Science.343, 80–84 (2014). [DOI] [PMC free article] [PubMed]
- 360.Tan XX, Actor JK, Chen Y. Peptide nucleic acid antisense oligomer as a therapeutic strategy against bacterial infection: proof of principle using mouse intraperitoneal infection. Antimicrob. Agents Chemother. 2005;49:3203–3207. doi: 10.1128/AAC.49.8.3203-3207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Shim G, et al. Therapeutic gene editing: delivery and regulatory perspectives. Acta Pharm. Sin. 2017;38:738–753. doi: 10.1038/aps.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol. Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 363.Fakhiri J, Nickl M, Grimm D. Rapid and simple screening of CRISPR guide RNAs (gRNAs) in cultured cells using adeno-associated viral (AAV) vectors. Methods Mol. Biol. 2019;1961:111–126. doi: 10.1007/978-1-4939-9170-9_8. [DOI] [PubMed] [Google Scholar]
- 364.Lau CH, Suh Y. In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease. F1000Res. 2017;6:2153. doi: 10.12688/f1000research.11243.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Delucia, A. M. et al. Lipopolysaccharide (LPS) inner-core phosphates are required for complete LPS synthesis and transport to the outer membrane in Pseudomonas aeruginosa PAO1. MBio. 2, e00142–11 (2011). [DOI] [PMC free article] [PubMed]
- 366.Burrows LL. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 2012;66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 367.Campodonico VL, et al. Evaluation of flagella and flagellin of Pseudomonas aeruginosa as vaccines. Infect. Immun. 2010;78:746–755. doi: 10.1128/IAI.00806-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Hegerle, N. et al. Development of a broad spectrum glycoconjugate vaccine to prevent wound and disseminated infections with Klebsiella pneumoniae and Pseudomonas aeruginosa. PLoS One.13, e0203143 (2018). [DOI] [PMC free article] [PubMed]
- 369.Sharma, A., Krause, A. & Worgall, S. Recent developments for Pseudomonas vaccines. Hum. Vaccin.7, 999–1011 (2011). [DOI] [PMC free article] [PubMed]
- 370.Alionte LG, et al. Pseudomonas aeruginosa LasA protease and corneal infections. Curr. Eye Res. 2001;22:266–271. doi: 10.1076/ceyr.22.4.266.5509. [DOI] [PubMed] [Google Scholar]
- 371.Allured, V. S., Collier, R. J., Carroll, S. F. & McKay, D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc. Natl Acad. Sci. USA.83, 1320–1324 (1986). [DOI] [PMC free article] [PubMed]
- 372.Arhin, A. & Boucher, C. The outer membrane protein OprQ and adherence of Pseudomonas aeruginosa to human fibronectin. Microbiol. (Read.).156, 1415–1423 (2010). [DOI] [PubMed]
- 373.Baumann U, Wu S, Flaherty KM, McKay DB. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993;12:3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Berka RM, Gray GL, Vasil ML. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect. Immun. 1981;34:1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bezzerri V, et al. Phospholipase C-beta3 is a key modulator of IL-8 expression in cystic fibrosis bronchial epithelial cells. J. Immunol. 2011;186:4946–4958. doi: 10.4049/jimmunol.1003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Blier, A. S. et al. C-type natriuretic peptide modulates quorum sensing molecule and toxin production in Pseudomonas aeruginosa. Microbiol.157, 1929–1944 (2011). [DOI] [PMC free article] [PubMed]
- 377.Boyd CD, et al. Structural features of the Pseudomonas fluorescens biofilm adhesin LapA required for LapG-dependent cleavage, biofilm formation, and cell surface localization. J. Bacteriol. 2014;196:2775–2788. doi: 10.1128/JB.01629-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Broomfield RJ, Morgan SD, Khan A, Stickler DJ. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J. Med. Microbiol. 2009;58:1367–1375. doi: 10.1099/jmm.0.012419-0. [DOI] [PubMed] [Google Scholar]
- 379.Bulman Z, Le P, Hudson AO, Savka MA. A novel property of propolis (bee glue): anti-pathogenic activity by inhibition of N-acyl-homoserine lactone mediated signaling in bacteria. J. Ethnopharmacol. 2011;138:788–797. doi: 10.1016/j.jep.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 380.Burrows LL. The therapeutic pipeline for Pseudomonas aeruginosa infections. ACS Infect. Dis. 2018;4:1041–1047. doi: 10.1021/acsinfecdis.8b00112. [DOI] [PubMed] [Google Scholar]
- 381.Cai, X. et al. Structural and functional characterization of Pseudomonas aeruginosa CupB chaperones. PLoS One.6, e16583 (2011). [DOI] [PMC free article] [PubMed]
- 382.Carterson AJ, et al. The transcriptional regulator AlgR controls cyanide production in Pseudomonas aeruginosa. J. Bacteriol. 2004;186:6837–6844. doi: 10.1128/JB.186.20.6837-6844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Castric PA. Glycine metabolism by Pseudomonas aeruginosa: hydrogen cyanide biosynthesis. J. Bacteriol. 1977;130:826–831. doi: 10.1128/jb.130.2.826-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Cathcart GR, et al. Novel inhibitors of the Pseudomonas aeruginosa virulence factor LasB: a potential therapeutic approach for the attenuation of virulence mechanisms in pseudomonal infection. Antimicrob. Agents Chemother. 2011;55:2670–2678. doi: 10.1128/AAC.00776-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Cota-Gomez A, et al. PlcR1 and PlcR2 are putative calcium-binding proteins required for secretion of the hemolytic phospholipase C of Pseudomonas aeruginosa. Infect. Immun. 1997;65:2904–2913. doi: 10.1128/iai.65.7.2904-2913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Cowell BA, Evans DJ, Fleiszig SM. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol. Lett. 2005;250:71–76. doi: 10.1016/j.femsle.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 387.Cox CD, Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect. Immun. 1985;48:130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.de Lima, C. D. et al. ExoU activates NF-kappaB and increases IL-8/KC secretion during Pseudomonas aeruginosa infection. PLoS One.7, e41772 (2012). [DOI] [PMC free article] [PubMed]
- 389.de Mattos, K. A., Sarno, E. N., Pessolani, M. C. & Bozza, P. T. Deciphering the contribution of lipid droplets in leprosy: multifunctional organelles with roles in Mycobacterium leprae pathogenesis. Mem. Inst. Oswaldo Cruz.107, 156–166 (2012). [DOI] [PubMed]
- 390.de Regt AK, et al. Overexpression of CupB5 activates alginate overproduction in Pseudomonas aeruginosa by a novel AlgW-dependent mechanism. Mol. Microbiol. 2014;93:415–425. doi: 10.1111/mmi.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Ding J, et al. Structural insights into the Pseudomonas aeruginosa type VI virulence effector Tse1 bacteriolysis and self-protection mechanisms. J. Biol. Chem. 2012;287:26911–26920. doi: 10.1074/jbc.M112.368043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Everett, M. J. & Davies, D. T. Pseudomonas aeruginosa elastase (LasB) as a therapeutic target. Drug Discov. Today. 26, 2108–2123 (2021). [DOI] [PubMed]
- 393.Feldman M, et al. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 1998;66:43–51. doi: 10.1128/IAI.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Feltzer RE, Gray RD, Dean WL, Pierce WM., Jr. Alkaline proteinase inhibitor of Pseudomonas aeruginosa. Interaction of native and N-terminally truncated inhibitor proteins with Pseudomonas metalloproteinases. J. Biol. Chem. 2000;275:21002–21009. doi: 10.1074/jbc.M002088200. [DOI] [PubMed] [Google Scholar]
- 395.Galdino ACM, et al. Disarming Pseudomonas aeruginosa virulence by the inhibitory action of 1,10-phenanthroline-5,6-dione-based compounds: elastase B (LasB) as a chemotherapeutic target. Front. Microbiol. 2019;10:1701. doi: 10.3389/fmicb.2019.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Garcia-Contreras, R. et al. Rhamnolipids stabilize quorum sensing mediated cooperation in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 367, fnaa080 (2020). [DOI] [PubMed]
- 397.Garner AL, et al. 3-Hydroxy-1-alkyl-2-methylpyridine-4(1H)-thiones: inhibition of the Pseudomonas aeruginosa virulence factor LasB. ACS Med. Chem. Lett. 2012;3:668–672. doi: 10.1021/ml300128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Garrity-Ryan L, et al. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 2000;68:7100–7113. doi: 10.1128/IAI.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Geiser TK, et al. Pseudomonas aeruginosa ExoT inhibits in vitro lung epithelial wound repair. Cell Microbiol. 2001;3:223–236. doi: 10.1046/j.1462-5822.2001.00107.x. [DOI] [PubMed] [Google Scholar]
- 400.Grande KK, Gustin JK, Kessler E, Ohman DE. Identification of critical residues in the propeptide of LasA protease of Pseudomonas aeruginosa involved in the formation of a stable mature protease. J. Bacteriol. 2007;189:3960–3968. doi: 10.1128/JB.01828-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Gupta, R. K., Chhibber, S. & Harjai, K. Acyl homoserine lactones from culture supernatants of Pseudomonas aeruginosa accelerate host immunomodulation. PLoS One.6, e20860 (2011). [DOI] [PMC free article] [PubMed]
- 402.Gutierrez-Gomez U, Soto-Aceves MP, Servin-Gonzalez L, Soberon-Chavez G. Overproduction of rhamnolipids in Pseudomonas aeruginosa PA14 by redirection of the carbon flux from polyhydroxyalkanoate synthesis and overexpression of the rhlAB-R operon. Biotechnol. Lett. 2018;40:1561–1566. doi: 10.1007/s10529-018-2610-8. [DOI] [PubMed] [Google Scholar]
- 403.Howe TR, Iglewski BH. Isolation and characterization of alkaline protease-deficient mutants of Pseudomonas aeruginosa in vitro and in a mouse eye model. Infect. Immun. 1984;43:1058–1063. doi: 10.1128/iai.43.3.1058-1063.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Islamieh DI, Afshar D, Esmaeili D. Effect of Satureja khuzistanica essential oil (SKEO) extract on expression of lasA and lasB genes in Pseudomonas aeruginosa. Iran. J. Microbiol. 2019;11:55–59. [PMC free article] [PubMed] [Google Scholar]
- 405.Ivanov IE, et al. Atomic force and super-resolution microscopy support a role for LapA as a cell-surface biofilm adhesin of Pseudomonas fluorescens. Res. Microbiol. 2012;163:685–691. doi: 10.1016/j.resmic.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Jepkorir G, et al. Structural, NMR spectroscopic, and computational investigation of hemin loading in the hemophore HasAp from Pseudomonas aeruginosa. J. Am. Chem. Soc. 2010;132:9857–9872. doi: 10.1021/ja103498z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Kazmierczak BI, Engel JN. Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42. Infect. Immun. 2002;70:2198–2205. doi: 10.1128/IAI.70.4.2198-2205.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Kida, Y. Roles of Pseudomonas aeruginosa-derived proteases as a virulence factor. Nihon Saikingaku Zasshi.68, 313–323 (2013). [DOI] [PubMed]
- 409.Kim D, et al. Identification of arylsulfonamides as ExoU inhibitors. Bioorg. Med. Chem. Lett. 2014;24:3823–3825. doi: 10.1016/j.bmcl.2014.06.064. [DOI] [PubMed] [Google Scholar]
- 410.Kim S, et al. Pseudomonas aeruginosa bacteriophage PA1O requires type IV pili for infection and shows broad bactericidal and biofilm removal activities. Appl. Environ. Microbiol. 2012;78:6380–6385. doi: 10.1128/AEM.00648-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Konig, B. et al. Role of Pseudomonas aeruginosa lipase in inflammatory mediator release from human inflammatory effector cells (platelets, granulocytes, and monocytes. Infect Immun. 64, 3252–3258 (1996). [DOI] [PMC free article] [PubMed]
- 412.Kumar, R. et al. Replacing the axial ligand tyrosine 75 or its hydrogen bond partner histidine 83 minimally affects hemin acquisition by the hemophore HasAp from Pseudomonas aeruginosa. Biochemistry.53, 2112–2125 (2014). [DOI] [PMC free article] [PubMed]
- 413.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 414.Li W, et al. Norepinephrine represses the expression of toxA and the siderophore genes in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2009;299:100–109. doi: 10.1111/j.1574-6968.2009.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Liu PV. Extracellular toxins of Pseudomonas aeruginosa. J. Infect. Dis. 1974;130:S94–S99. doi: 10.1093/infdis/130.Supplement.S94. [DOI] [PubMed] [Google Scholar]
- 416.Lu D, et al. Structural insights into the T6SS effector protein Tse3 and the Tse3-Tsi3 complex from Pseudomonas aeruginosa reveal a calcium-dependent membrane-binding mechanism. Mol. Microbiol. 2014;92:1092–1112. doi: 10.1111/mmi.12616. [DOI] [PubMed] [Google Scholar]
- 417.Miller LC, et al. Development of potent inhibitors of pyocyanin production in Pseudomonas aeruginosa. J. Med. Chem. 2015;58:1298–1306. doi: 10.1021/jm5015082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Mohammadzamani Z, et al. Inhibitory effects of Cinnamaldehyde, Carvacrol, and honey on the expression of exoS and ampC genes in multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infections. Micro. Pathog. 2020;140:103946. doi: 10.1016/j.micpath.2019.103946. [DOI] [PubMed] [Google Scholar]
- 419.Monds RD, et al. Di-adenosine tetraphosphate (Ap4A) metabolism impacts biofilm formation by Pseudomonas fluorescens via modulation of c-di-GMP-dependent pathways. J. Bacteriol. 2010;192:3011–3023. doi: 10.1128/JB.01571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Moroz OV, et al. The structure of a calcium-dependent phosphoinositide-specific phospholipase C from Pseudomonas sp. 62186, the first from a Gram-negative bacterium. Acta Crystallogr D. Struct. Biol. 2017;73:32–44. doi: 10.1107/S2059798316019616. [DOI] [PubMed] [Google Scholar]
- 421.Ostroff RM, Wretlind B, Vasil ML. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect. Immun. 1989;57:1369–1373. doi: 10.1128/iai.57.5.1369-1373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Paranchych W, et al. Fimbriae (pili): molecular basis of Pseudomonas aeruginosa adherence. Clin. Invest. Med. 1986;9:113–118. [PubMed] [Google Scholar]
- 423.Park S, Galloway DR. Pseudomonas aeruginosa LasD processes the inactive LasA precursor to the active protease form. Arch. Biochem. Biophys. 1998;357:8–12. doi: 10.1006/abbi.1998.0787. [DOI] [PubMed] [Google Scholar]
- 424.Piepenbrink KH, Sundberg EJ. Motility and adhesion through type IV pili in Gram-positive bacteria. Biochem Soc. Trans. 2016;44:1659–1666. doi: 10.1042/BST20160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int. J. Med. Microbiol. 2007;297:277–295. doi: 10.1016/j.ijmm.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Rabin SD, Hauser AR. Functional regions of the Pseudomonas aeruginosa cytotoxin ExoU. Infect. Immun. 2005;73:573–582. doi: 10.1128/IAI.73.1.573-582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Raissy HH, et al. A proof of concept study to detect urease producing bacteria in lungs using aerosolized (13)C-urea. Pediatr. Allergy Immunol. Pulmonol. 2016;29:68–73. doi: 10.1089/ped.2015.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Raju H, Sundararajan R, Sharma R. The structure of BrlR reveals a potential pyocyanin binding site. FEBS Lett. 2018;592:256–262. doi: 10.1002/1873-3468.12950. [DOI] [PubMed] [Google Scholar]
- 429.Rao L, et al. Pseudomonas aeruginosa survives in epithelia by ExoS-mediated inhibition of autophagy and mTOR. EMBO Rep. 2021;22:e50613. doi: 10.15252/embr.202050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Rietsch, A., Vallet-Gely, I., Dove, S. L. & Mekalanos, J. J. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA.102, 8006–8011 (2005). [DOI] [PMC free article] [PubMed]
- 431.Robb, C. S., Robb, M., Nano, F. E. & Boraston, A. B. The structure of the toxin and type six secretion system substrate Tse2 in Complex with its immunity protein. Structure.24, 277–284 (2016). [DOI] [PubMed]
- 432.Rosenau F, et al. Lipase LipC affects motility, biofilm formation and rhamnolipid production in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2010;309:25–34. doi: 10.1111/j.1574-6968.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- 433.Saalim, M., Villegas-Moreno, J. & Clark, B. R. Bacterial Alkyl-4-quinolones: discovery, structural diversity and biological properties. Molecules. 25, 5689 (2020). [DOI] [PMC free article] [PubMed]
- 434.Sarkisova S, et al. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J. Bacteriol. 2005;187:4327–4337. doi: 10.1128/JB.187.13.4327-4337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Schultz MJ, et al. Impairment of host defence by exotoxin A in Pseudomonas aeruginosa pneumonia in mice. J. Med. Microbiol. 2001;50:822–827. doi: 10.1099/0022-1317-50-9-822. [DOI] [PubMed] [Google Scholar]
- 436.Shiau, J. W. et al. Mice immunized with DNA encoding a modified Pseudomonas aeruginosa exotoxin A develop protective immunity against exotoxin intoxication. Vaccine.19, 1106–1112 (2000). [DOI] [PubMed]
- 437.Sun J, Barbieri JT. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J. Biol. Chem. 2003;278:32794–32800. doi: 10.1074/jbc.M304290200. [DOI] [PubMed] [Google Scholar]
- 438.Sun, J. et al. The Pseudomonas aeruginosa protease LasB directly activates IL-1beta. EBioMedicine.60, 102984 (2020). [DOI] [PMC free article] [PubMed]
- 439.Suzuki, T. et al. Role of pvdE pyoverdine synthesis in Pseudomonas aeruginosa keratitis. Cornea.37, S99–S105 (2018). [DOI] [PubMed]
- 440.Terada LS, et al. Pseudomonas aeruginosa hemolytic phospholipase C suppresses neutrophil respiratory burst activity. Infect. Immun. 1999;67:2371–2376. doi: 10.1128/IAI.67.5.2371-2376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Uehara H, et al. Structures of the heme acquisition protein HasA with iron(III)-5,15-diphenylporphyrin and derivatives thereof as an artificial prosthetic group. Angew. Chem. Int. Ed. Engl. 2017;56:15279–15283. doi: 10.1002/anie.201707212. [DOI] [PubMed] [Google Scholar]
- 442.Urban A, Leipelt M, Eggert T, Jaeger KE. DsbA and DsbC affect extracellular enzyme formation in Pseudomonas aeruginosa. J. Bacteriol. 2001;183:587–596. doi: 10.1128/JB.183.2.587-596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.van den Berg B. Crystal structure of a full-length autotransporter. J. Mol. Biol. 2010;396:627–633. doi: 10.1016/j.jmb.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 444.Vareechon, C. et al. Pseudomonas aeruginosa effector ExoS inhibits ROS production in human neutrophils. Cell Host Microbe.21, 611–618. e615 (2017). [DOI] [PMC free article] [PubMed]
- 445.Wang, T. et al. Complex structure of type VI peptidoglycan muramidase effector and a cognate immunity protein. Acta Crystallogr D. Biol. Crystallogr.69, 1889–1900 (2013). [DOI] [PMC free article] [PubMed]
- 446.Wibowo JP, et al. A novel mechanism of inhibition by phenylthiourea on PvdP, a tyrosinase synthesizing pyoverdine of Pseudomonas aeruginosa. Int. J. Biol. Macromol. 2020;146:212–221. doi: 10.1016/j.ijbiomac.2019.12.252. [DOI] [PubMed] [Google Scholar]
- 447.Wilderman PJ, et al. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect. Immun. 2001;69:5385–5394. doi: 10.1128/IAI.69.9.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wilhelm S, et al. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J. Bacteriol. 2007;189:6695–6703. doi: 10.1128/JB.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Wurtele M, et al. Structure of the ExoS GTPase activating domain. FEBS Lett. 2001;491:26–29. doi: 10.1016/S0014-5793(01)02105-6. [DOI] [PubMed] [Google Scholar]
- 450.Yarlagadda V, Wright GD. Membrane-active rhamnolipids overcome aminoglycoside resistance. Cell Chem. Biol. 2019;26:1333–1334. doi: 10.1016/j.chembiol.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 451.Yu H, et al. Elastase LasB of Pseudomonas aeruginosa promotes biofilm formation partly through rhamnolipid-mediated regulation. Can. J. Microbiol. 2014;60:227–235. doi: 10.1139/cjm-2013-0667. [DOI] [PubMed] [Google Scholar]
- 452.Zhang H, Gao ZQ, Su XD, Dong YH. Crystal structure of type VI effector Tse1 from Pseudomonas aeruginosa. FEBS Lett. 2012;586:3193–3199. doi: 10.1016/j.febslet.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 453.Zhao F, et al. Production of rhamnolipids by Pseudomonas aeruginosa is inhibited by H2S but resumes in a co-culture with P. stutzeri: applications for microbial enhanced oil recovery. Biotechnol. Lett. 2015;37:1803–1808. doi: 10.1007/s10529-015-1859-4. [DOI] [PubMed] [Google Scholar]
- 454.Zhao F, et al. Oxygen effects on rhamnolipids production by Pseudomonas aeruginosa. Micro. Cell Fact. 2018;17:39. doi: 10.1186/s12934-018-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Zheng Z, Ma D, Yahr TL, Chen L. The transiently ordered regions in intrinsically disordered ExsE are correlated with structural elements involved in chaperone binding. Biochem. Biophys. Res. Commun. 2012;417:129–134. doi: 10.1016/j.bbrc.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Wang Y, et al. Optimization of antitumor immunotherapy mediated by type III secretion system-based live attenuated bacterial vectors. J. Immunother. 2012;35:223–234. doi: 10.1097/CJI.0b013e31824747e5. [DOI] [PubMed] [Google Scholar]
- 457.Lin YM, et al. Outer membrane protein I of Pseudomonas aeruginosa is a target of cationic antimicrobial peptide/protein. J. Biol. Chem. 2010;285:8985–8994. doi: 10.1074/jbc.M109.078725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Cowell, B. A. et al. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology.149, 2291–2299 (2003). [DOI] [PubMed]
- 459.Barequet IS, et al. Pseudomonas aeruginosa LasA protease in treatment of experimental staphylococcal keratitis. Antimicrob. Agents Chemother. 2004;48:1681–1687. doi: 10.1128/AAC.48.5.1681-1687.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Kilmury, S. L. N. & Burrows, L. L. The Pseudomonas aeruginosa PilSR Two-Component System Regulates Both Twitching and Swimming Motilities. mBio.9, e01310-18 (2018). [DOI] [PMC free article] [PubMed]
- 461.Mikkelsen, H., Ball, G., Giraud, C. & Filloux, A. Expression of Pseudomonas aeruginosa CupD fimbrial genes is antagonistically controlled by RcsB and the EAL-containing PvrR response regulators. PLoS One.4, e6018 (2009). [DOI] [PMC free article] [PubMed]
- 462.Yu H, et al. Identification of the algZ gene upstream of the response regulator algR and its participation in control of alginate production in Pseudomonas aeruginosa. J. Bacteriol. 1997;179:187–193. doi: 10.1128/jb.179.1.187-193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Miller CL, et al. RsmW, Pseudomonas aeruginosa small non-coding RsmA-binding RNA upregulated in biofilm versus planktonic growth conditions. BMC Microbiol. 2016;16:155. doi: 10.1186/s12866-016-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Chambonnier G, et al. The hybrid histidine kinase LadS forms a multicomponent signal transduction system with the GacS/GacA two-component system in Pseudomonas aeruginosa. PLoS Genet. 2016;12:e1006032. doi: 10.1371/journal.pgen.1006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Petrova OE, Schurr JR, Schurr MJ, Sauer K. The novel Pseudomonas aeruginosa two-component regulator BfmR controls bacteriophage-mediated lysis and DNA release during biofilm development through PhdA. Mol. Microbiol. 2011;81:767–783. doi: 10.1111/j.1365-2958.2011.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Giraud C, et al. The PprA-PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone-usher pathway system assembling fimbriae. Environ. Microbiol. 2011;13:666–683. doi: 10.1111/j.1462-2920.2010.02372.x. [DOI] [PubMed] [Google Scholar]
- 467.Reis RS, Pereira AG, Neves BC, Freire DM. Gene regulation of rhamnolipid production in Pseudomonas aeruginosa—a review. Bioresour. Technol. 2011;102:6377–6384. doi: 10.1016/j.biortech.2011.03.074. [DOI] [PubMed] [Google Scholar]
- 468.Dasgupta N, et al. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 2003;50:809–824. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 469.Marko VA, Kilmury SLN, MacNeil LT, Burrows LL. Pseudomonas aeruginosa type IV minor pilins and PilY1 regulate virulence by modulating FimS-AlgR activity. PLoS Pathog. 2018;14:e1007074. doi: 10.1371/journal.ppat.1007074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Cadoret F, Ball G, Douzi B, Voulhoux R. Txc, a new type II secretion system of Pseudomonas aeruginosa strain PA7, is regulated by the TtsS/TtsR two-component system and directs specific secretion of the CbpE chitin-binding protein. J. Bacteriol. 2014;196:2376–2386. doi: 10.1128/JB.01563-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Daddaoua A, et al. GtrS and GltR form a two-component system: the central role of 2-ketogluconate in the expression of exotoxin A and glucose catabolic enzymes in Pseudomonas aeruginosa. Nucleic Acids Res. 2014;42:7654–7663. doi: 10.1093/nar/gku496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Marden, J. N. et al. An unusual CsrA family member operates in series with RsmA to amplify posttranscriptional responses in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA.110, 15055–15060 (2013). [DOI] [PMC free article] [PubMed]
- 473.Ryan Kaler KM, Nix JC, Schubot FD. RetS inhibits Pseudomonas aeruginosa biofilm formation by disrupting the canonical histidine kinase dimerization interface of GacS. J. Biol. Chem. 2021;297:101193. doi: 10.1016/j.jbc.2021.101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Heurlier K, et al. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2004;186:2936–2945. doi: 10.1128/JB.186.10.2936-2945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Chen G, et al. Oligoribonuclease is required for the type III secretion system and pathogenesis of Pseudomonas aeruginosa. Microbiol. Res. 2016;188-189:90–96. doi: 10.1016/j.micres.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 476.Sultan, M., Arya, R. & Kim, K. K. Roles of two-component systems in Pseudomonas aeruginosa virulence. Int. J. Mol. Sci. 22, 12152 (2021). [DOI] [PMC free article] [PubMed]
- 477.Yeung AT, Bains M, Hancock RE. The sensor kinase CbrA is a global regulator that modulates metabolism, virulence, and antibiotic resistance in Pseudomonas aeruginosa. J. Bacteriol. 2011;193:918–931. doi: 10.1128/JB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Lehman HK, Segal BH. The role of neutrophils in host defense and disease. J. Allergy Clin. Immunol. 2020;145:1535–1544. doi: 10.1016/j.jaci.2020.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Shin HS, et al. Pseudomonas aeruginosa-dependent upregulation of TLR2 influences host responses to a secondary Staphylococcus aureus infection. Pathog. Dis. 2013;69:149–156. doi: 10.1111/2049-632X.12074. [DOI] [PubMed] [Google Scholar]