Highlights
-
•
Women with early menopause are at greater risk for heart disease over the long-term.
-
•
Over half of middle-aged women with early menopause were free of CAC, similar to women without early menopause.
-
•
Coronary calcium scores were similar between women with and without early menopause.
-
•
Women without calcium had low risk for heart disease regardless of menopause history.
Keywords: ASCVD, CAC, Early menopause, Women
Abbreviations: ARIC, Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CHD, coronary heart disease; CVD, cardiovascular disease; EM, early menopause; HT, hormone therapy; MESA, Multi-Ethnic Study of Atherosclerosis; PCE, Pooled Cohort Equations; SWAN, Study of Women's Health Across the Nation
Abstract
Background and Aims
We aimed to determine the utility of coronary artery calcium (CAC) for atherosclerotic cardiovascular disease (ASCVD) risk stratification in women with and without early menopause (EM).
Methods
To examine the association between CAC and incident ASCVD, we performed Kaplan-Meier survival analysis and multivariable Cox proportional hazards modeling using data from 2,456 postmenopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA) with or without EM, defined as occurring at <45 years of age.
Results
The cohort was 64.1 ± 9.1 years old and 28.0% experienced EM. There were 291 ASCVD events over 12.5 ± 3.6 year follow-up with a higher event rate among those with EM compared to those without EM of 13.6 vs. 9.0 per 1,000 year follow-up (p < 0.01). Women with EM had a slightly lower prevalence of CAC = 0 (55.1%) than women without EM (59.7%) (p = 0.04) despite no difference in mean age. Among women with CAC = 0, the cumulative incidence of ASCVD at 10 years was low-to-borderline for women with (5.4%) and without EM (3.2%) (p = 0.06). However, women with EM had a significantly higher 15-year risk with an adjusted HR of 1.96 (95% CI: 1.26–3.04). In multivariable Cox models, women with CAC ≥ 1 had progressively increased ASCVD risk that did not significantly differ by EM status.
Conclusion
In MESA, >50% of middle-aged postmenopausal women with EM had CAC = 0, similar to those without EM. Among women with CAC = 0, those with EM had a low to borderline 10-year risk of ASCVD, but the 15-year risk was significantly higher for women with EM versus those without EM. When CAC ≥ 1, the incidence of ASCVD was similar for women with and without EM. These findings support the use of CAC to help improve ASCVD risk stratification in women with EM.
Condensed abstract
This study investigated the association between coronary artery calcium (CAC) and incident atherosclerotic cardiovascular disease (ASCVD) in postmenopausal women with and without early menopause (EM). We found that >50% of women had CAC = 0 and an associated low-to-borderline 10-year cumulative incidence of ASCVD. However, the risk for ASCVD was significantly higher for women with EM after 15-years follow-up. Additional research is needed to better understand the differences in long-term ASCVD risk between women with and without EM who have CAC = 0.
Graphical abstract
1. Introduction
Cardiovascular disease (CVD) is the leading cause of mortality among women in the United States and was responsible for more than 400,000 deaths among women in 2015 [1]. The incidence of CVD for women lags by approximately ten years compared to men and women experience a sharp increase in CVD incidence following menopause [2], [3], [4], [5], [6], [7], [8], [9]. This phenomenon has been predominantly attributed to the decrease in estrogen levels that is associated with menopause, although the Women's Health Initiative (WHI) randomized trial did not find a decrease in risk with the use of exogenous hormone therapy [10,11]. Contemporary risk prediction tools, such as the American Heart Association (AHA)/American College of Cardiology (ACC) Pooled Cohort Equations (PCEs), utilize traditional CVD risk factors to estimate an individual's 10-year atherosclerotic cardiovascular disease (ASCVD) risk to guide treatment. However, these tools underestimate ASCVD risk in populations with atherogenic inflammatory disease and may also underpredict risk in women who experience early menopause (EM) [12], [13], [14], [15], [16], [17], [18]. The AHA/ACC 2018 Cholesterol Treatment Guidelines further identifies premature menopause, defined as occurring at <40 years of age, as an ASCVD risk-enhancing factor in favor of lipid-lowering therapy [19].
Coronary artery calcium (CAC) is a non-invasive measurement of an individual's subclinical atherosclerotic burden and a robust predictor of ASCVD beyond traditional risk factors that is equally prognostic in both men and women [20,21]. Its use has been recommended by the 2018 AHA/ACC Cholesterol Treatment Guidelines to refine ASCVD risk prediction in individuals with uncertain ASCVD risk [19,[22], [23], [24], [25], [26]]. Although women who experience EM seem to be at increased ASCVD risk, their burden of CAC compared to women who do not experience EM is unknown. In addition, it is unknown whether there is a difference in the long-term association of the absence of CAC (CAC = 0) and ASCVD risk between women with and without EM. Therefore, we aimed to describe the prevalence of CAC and the association of CAC with incident ASCVD among women with and without EM in the Multi-Ethnic Study of Atherosclerosis (MESA).
2. Methods
2.1. Design and overview
MESA is a multi-center, longitudinal cohort study of participants who were enrolled between July 2000 and August 2002. Eligible study participants were between 45 to 84 years of age and free of CVD. Recruitment and follow-up occurred in 6 US communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota. The study was approved by the Institutional Review Board of each Field Center and informed consent was obtained from each participant at the time of enrollment.
2.2. Inclusion/exclusion criteria
Of the 3,601 women enrolled in the study, we included those who were postmenopausal at entry as determined by self-report, history of prior oophorectomy, and/or age >55 years using a previously published algorithm for MESA (Supplementary Fig. 1) [27]. Self-reported menopausal status is a validated and reliable method of determining menopausal age as demonstrated in the Nurses’ Health Study where 99% of women accurately reporting their menopausal age within one year [5]. We excluded premenopausal women and those with indeterminate status (n = 516) to avoid selection bias that would result from classifying premenopausal women as women without EM due to the study's minimum age criteria. At the baseline examination, trained interviewers obtained information on women's menopause history, including their menopause status, age of menopause, type of menopause (natural vs. surgical), and use of hormone therapy (HT). Using this information, we additionally excluded women with inconsistencies between their reported ages of menopause and histories of surgical menopause (n = 496) or natural menopause (n = 58) as well as women with missing data on menopausal age (n = 75). For this analysis, we defined EM as occurring at age <45 years, which is older than the AHA/ACC definition of premature menopause of age <40 years-old. We used age 45 years-old for our definition of premature menopause, because this was the minimum age eligible for entry to the MESA cohort and this definition is consistent with prior studies [2,8,28]. In addition, a large multi-country meta-analysis demonstrated a prevalence of nonsurgical menopause of 9% among postmenopausal women who experienced menopause at <45 years-old [28]. Our final cohort included 2,456 women, of whom 688 were classified as experiencing EM (Supplementary Fig. 1 and Supplementary Fig. 2).
2.3. Data collection
At the baseline examination, participants’ medical histories, anthropometric measurements, blood pressures, and laboratory data were obtained using standardized protocols [29]. Ever smoking status was defined as having smoked ≥100 cigarettes in a lifetime. For this analysis, hypertension was defined according to 2017 ACC/AHA Blood Pressure Treatment guidelines as a systolic blood pressure (SBP) ≥130 mm Hg, a diastolic blood pressure (DBP) of ≥80 mm Hg, or current use of an antihypertensive medication [30]. A serum panel that included total cholesterol, HDL cholesterol (HDL-C), and glucose was drawn after a 12 h fast. Type 2 diabetes mellitus was defined according to the 2003 American Diabetes Association guidelines as an impaired fasting glucose of ≥126 mg/dL or current use of a hypoglycemic medication. Ten-year ASCVD risk was calculated using the 2013 ACC/AHA PCEs [31]. The data underlying this article were provided by MESA by permission and will be shared on reasonable request to the corresponding author with permission of MESA.
2.4. ASCVD event ascertainment
Follow-up telephone interviews were conducted at intervals of 9 to 12 months. In addition, the study cohort also completed five in-person follow-up clinic examinations between September 2002 and June 2018. A participant's follow-up duration was defined as the period from the baseline examination until the first ASCVD event, loss to follow-up, death, or December 31, 2015 – whichever came first. ASCVD events were adjudicated by members of the MESA morbidity and mortality committee. A detailed description of follow-up methodology is available at http://www.mesa-nhlbi.org.
In our analysis, an ASCVD event was the primary outcome and defined as a composite of fatal or non-fatal myocardial infarction (MI), fatal or non-fatal stroke, and other fatal or non-fatal ASCVD events (e.g., resuscitated cardiac arrest and angina with revascularization). A coronary heart disease (CHD) event was defined as a composite of fatal or non-fatal MI, resuscitated cardiac arrest, definite angina, probable angina with subsequent revascularization, and fatal CHD. Calculated ASCVD risk was categorized as low (<5.0%), borderline (≥5.0% and <7.5%), intermediate (≥7.5% and <20%), and high risk (≥20%) based on the 2013 ACC/AHA PCEs [31].
2.5. CAC measurement
At baseline, CAC was measured with either electron-beam computed tomography (EBT) (Chicago, Los Angeles, and New York Field Centers) or multidetector computed tomography (MDCT) (Baltimore, Forsyth County, and St. Paul Field Centers). In previous studies, EBT and MDCT were shown to produce similar results [32]. Furthermore, intra-observer and inter-observer agreements were excellent (κ = 0.93 and 0.90, respectively) [32,33]. CT images were independently read at a centralized reading center (Los Angeles Biomedical Research Institute at UCLA-Harbor) where the phantom-adjusted mean value was reported. The calcium score was calculated using the Agatston method, which uses a sum of the area of calcium at each plaque multiplied by a density factor [34]. For this analysis, we categorized CAC scores into clinically relevant cut-points of 0, 1 to 99, and ≥100.
2.6. Statistical analyses
We compared demographics and baseline data of women with and without EM. Univariable comparisons of groups were performed using two-sample Student t-tests for continuous variables and Pearson's Chi-Square for categorical variables. We calculated age-adjusted event rates per 1,000 person-years follow-up and further stratified this calculation by CAC. In women with EM, we calculated the difference in CAC scores for those with and without HT use. We also performed Kaplan-Meier survival analysis and log-rank testing to determine 10-year and 15-year cumulative ASCVD-free survival rates in women with and without EM to examine differences over time. We examined the 10-year cumulative ASCVD rate in addition to the maximal follow-up time of 15 years, because 10 years is the timeframe for which ASCVD risk prediction is most commonly performed [31]. We also performed multivariable Cox proportional hazards modeling using women without EM as the reference group in order to examine whether there were differences in the associations between CAC and incident ASCVD by EM status. Three progressively adjusted models were constructed: Model 1 was adjusted for demographics, including age and race/ethnicity. Model 2 additionally adjusted for traditional CVD risk factors including SBP, DBP, total cholesterol, HDL-C, diabetes, smoking status, lipid-lowering medication use, anti-hypertensive medication use, family history of MI, income, and education level. Model 3 additionally adjusted for baseline HT use. We also performed Cox proportional hazards regression with these adjusted models to determine the risk of CHD and ASCVD with graded increases in CAC scores in both women with and without EM using CAC = 0 as the reference group. We also calculated an interaction term between EM and CAC. Nine (3 EM, 6 non-EM) patients were excluded from ASCVD survival analysis due to an absence of follow-up after the initial baseline visit. To evaluate the association between traditional risk factors and CAC burden, we compared the prevalence of CAC ≥ 1 between women with and without EM within each PCEs 10-year risk category.
Complementary log-log plots and Schoenfeld residual statistics were used to validate the assumption of proportionality of hazards in each model. Multicollinearity was checked using variance inflation factors. The statistical significance of association between early menopause and ASCVD or CHD was evaluated using the Wald test of the beta coefficients. A two-tailed p-value of <0.05 was considered statistically significant for all analyses. Statistical analyses were performed using Stata Version 15.1 (StataCorp, College Station, TX).
3. Results
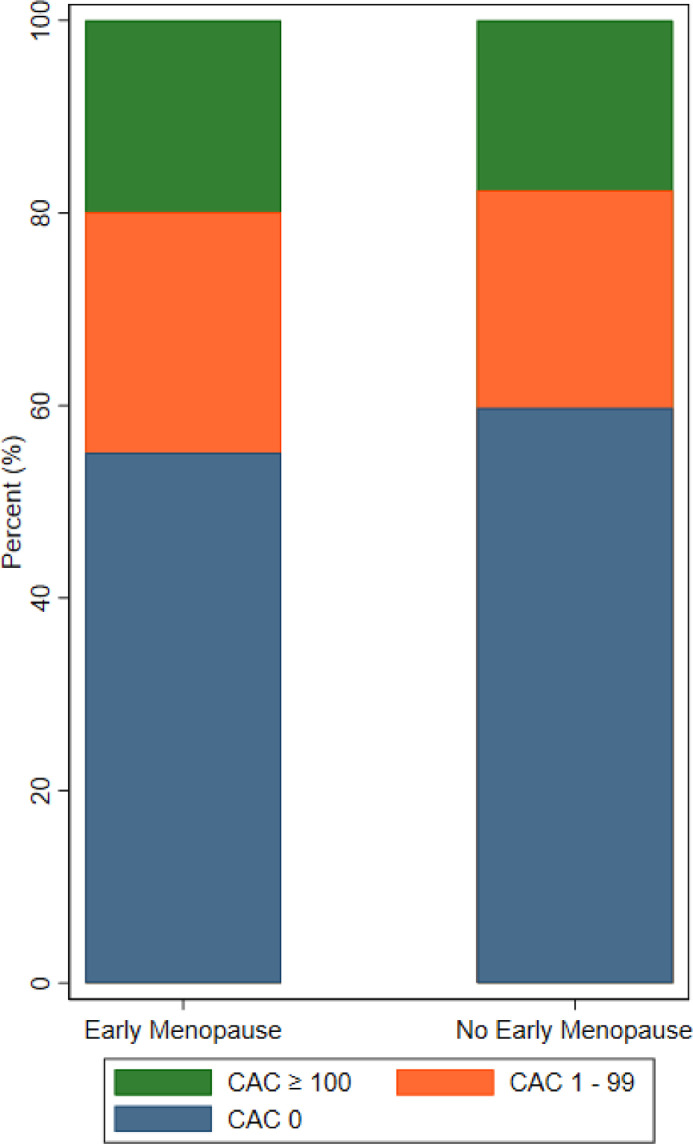
Our study included 2,456 postmenopausal women with reliable menopausal data (mean age 64.1 ± 9.1 years, 28.0% with history of EM, 39.4% White). There were 291 ASCVD events over a median follow-up of 13.9 years (IQR: 11.1, 14.6). At baseline, there were no differences in age, blood pressure, cholesterol levels, number of live births, or number of pregnancies between groups (Table 1). However, women with EM had a higher prevalence of diabetes and current smoking along with a higher body mass index, although the absolute differences were small. Menopause occurred 10.5 years earlier for women with EM compared to those without EM. Women with EM were more likely to have undergone surgical rather than natural menopause (41.8% versus 14.3%, p < 0.01) and to have used HT (53.3% versus 47.2%, p < 0.01). Women with EM also had a slightly lower prevalence of CAC =0 at 55.1% compared to women without EM at 59.7% (p = 0.04). Among women with EM, 38.9% of those who reported HT use had CAC ≥ 1 with a median CAC score of 0.0 (IQR: 0.0, 27.0) compared with 50.0% of women without HT use with a median CAC score of 0.4 (IQR: 0.0, 72.0). The relative distribution of CAC was similar between groups (Pearson's χ2 = 0.11) (Fig. 1).
Table 1.
Baseline characteristics of postmenopausal women in MESA with vs. without EM (N = 2,456).
| Early Menopause (n = 688) | No Early Menopause (n = 1,768) | p-value | |
|---|---|---|---|
| Age | 64.0 ± 10.4 | 64.1 ± 9.0 | 0.87 |
| Age at menopause | 40.7 ± 5.0 | 51.2 ± 3.3 | <0.01 |
| Race/Ethnicity | |||
| White | 34.6% | 41.3% | <0.01 |
| Black | 30.8% | 23.0% | <0.01 |
| Hispanic | 26.5% | 20.5% | <0.01 |
| Chinese | 8.1% | 15.2% | <0.01 |
| Systolic Blood Pressure (mmHg) | 129.1 ± 24.9 | 128.2 ± 22.9 | 0.39 |
| Diastolic Blood Pressure (mmHg) | 69.2 ± 10.5 | 69.0 ± 10.0 | 0.67 |
| Total Cholesterol (mg/dL) | 202.8 ± 36.6 | 201.5 ± 35.8 | 0.43 |
| HDL Cholesterol (mg/dL) | 56.3 ± 15.4 | 56.8 ± 15.2 | 0.44 |
| Diabetes | 14.2% | 10.9% | 0.02 |
| Hypertension | 60.9% | 59.6% | 0.54 |
| Smoking Status | |||
| Never | 54.4% | 61.0% | <0.01 |
| Former | 30.2% | 29.2% | 0.63 |
| Current | 14.8% | 9.4% | <0.01 |
| Body Mass Index (kg/m2) | 28.9 ± 6.4 | 28.2 ± 6.0 | 0.02 |
| Reproductive history | |||
| Median # Live births a. | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0.24 |
| Median # of Pregnancies a. | 3.0 (2.0, 5.0) | 3.0 (2.0, 5.0) | 0.35 |
| Natural Menopause | 58.2% | 85.7% | <0.01 |
| Ever Use Hormone Therapy | 53.3% | 47.2% | <0.01 |
| 10-year ASCVD risk | |||
| Low (<5.0%) | 35.0% | 38.9% | 0.08 |
| Borderline (≥5.0 and <7.5%) | 9.9% | 13.0% | 0.04 |
| Intermediate (≥7.5 and <20%) | 29.9% | 30.3% | 0.86 |
| High (≥20%) | 25.1% | 17.9% | <0.01 |
| CAC =0 | 55.1% | 59.7% | 0.04 |
| Median CACa. | 0 (0, 56.1) | 0 (0, 38.3) | 0.03 |
| Income < $40,000 | 37.5% | 46.0% | <0.01 |
| High School Education | 77.9% | 79.4% | 0.44 |
Values are denoted as mean ± SD, unless otherwise stated.
ASCVD = atherosclerotic cardiovascular disease, CAC = coronary artery calcium, EM = early menopause, MESA = Multi-Ethnic Study of Atherosclerosis.
median and interquartile range.
Fig. 1.
Distribution of CAC scores by EM status. CAC scores when classified into cut-points of 0, 1 to 99, and ≥ 100 were distributed similarly between women with and without EM (Pearson χ2 = 0.11). CAC = coronary artery calcium, EM = early menopause.
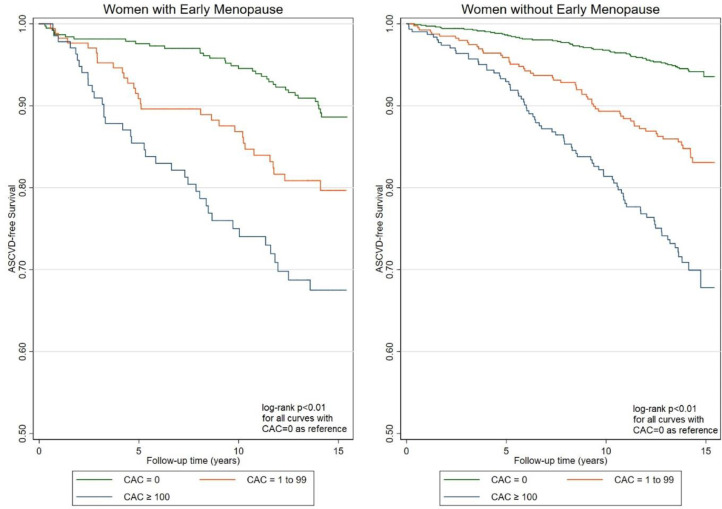
The overall age-adjusted ASCVD event rate was greater for women with EM at 13.6 per 1,000 person-years compared to women without EM at 9.0 per 1,000 person-years (p < 0.01) (Table 2). In both women with and without EM, the ASCVD event rates increased with CAC and the Kaplan-Meier trends were similar across both groups (log-rank p < 0.01 for all comparisons with CAC = 0 as reference group) (Fig. 2).
Table 2.
Crude and age-adjusted ASCVD and CHD event rates per 1,000 person-years follow-up, stratified by baseline CAC score.
|
Early Menopause |
No Early Menopause |
||||||
|---|---|---|---|---|---|---|---|
| CAC Score | Events/Participants | Crude Event Rate | Age Adjusted Event Rate | Events/ Participants | Crude Event Rate | Age Adjusted Event Rate | |
| ASCVD | Overall | 103/688 | 12.9 | 13.6 | 188/1,768 | 8.7 | 9.0 |
| 0 | 32/376 | 7.5 | 7.8 | 55/1,052 | 4.0 | 4.2 | |
| 1-99 | 54/255 | 15.5 | 15.7 | 94/603 | 11.8 | 11.9 | |
| ≥100 | 17/57 | 28.0 | 28.7 | 39/113 | 23.1 | 23.4 | |
| CHD | Overall | 62/688 | 7.7 | 8.0 | 117/1,768 | 5.3 | 5.5 |
| 0 | 16/376 | 3.4 | 3.5 | 32/1,052 | 2.4 | 2.4 | |
| 1-99 | 36/255 | 10.6 | 11.0 | 57/603 | 6.0 | 6.0 | |
| ≥100 | 10/57 | 17.5 | 17.8 | 28/113 | 16.3 | 16.5 | |
ASCVD = atherosclerotic cardiovascular disease, CAC = coronary artery calcium, CHD = coronary heart disease.
Fig. 2.
Kaplan-Meier ASCVD-free survival curves for women with and without early menopause stratified by baseline CAC score. The difference in survival was statistically significant (log-rank p < 0.01 for all comparisons). A calculated interaction term between EM and CAC was not statistically significant with p = 0.09.
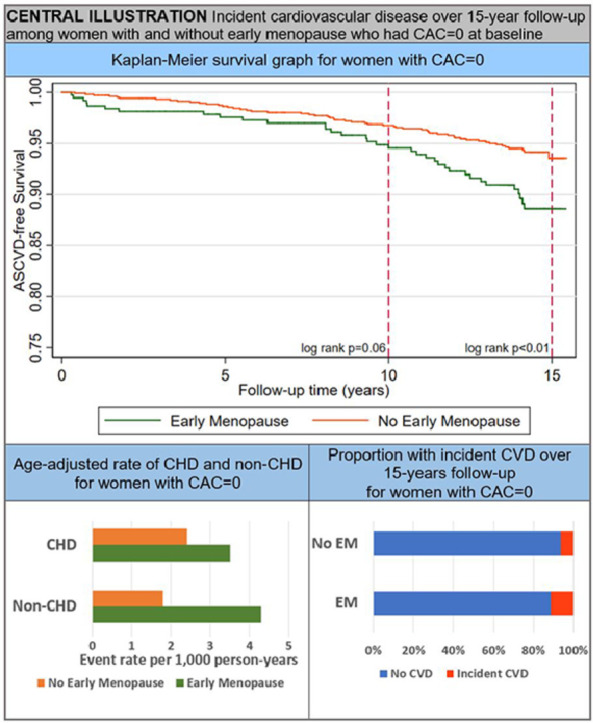
Among women with CAC = 0 at baseline, the age-adjusted ASCVD event rate was nearly double for women with EM at 7.8 per 1,000 person-years compared to women without EM at 4.2 per 1,000 person-years. Additionally, among women with CAC = 0 at baseline, the age-adjusted non-CHD (ASCVD minus CHD) event rate was higher for women with EM compared to women without EM (4.3 vs. 1.8 per 1,000 person-years). In Kaplan-Meier survival analysis of women with CAC = 0, the cumulative incidence of ASCVD was low-to-borderline over 10-year follow-up at 5.4% for women with EM and 3.2% for women without EM (p = 0.06). However, the curves began to noticeably separate at approximately 7-8 years follow-up. Over maximal 15-year follow-up, women with EM had a significantly greater cumulative incidence of ASCVD compared to women without EM (11.4% vs. 6.4%, p < 0.01) (Central Illustration).
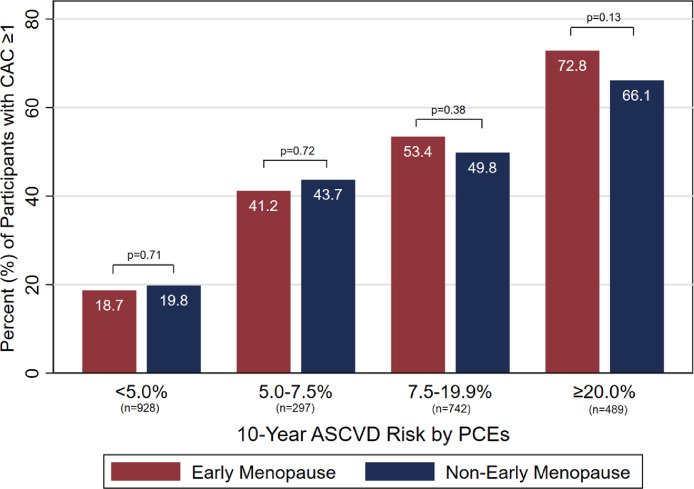
The prevalence of CAC ≥ 1 increased with PCEs risk score. Approximately 20 percent of low-risk women as defined by PCEs < 5.0% had CAC ≥ 1 (Fig. 3). Conversely, nearly 70% of high-risk women as defined by PCEs ≥ 20% had CAC ≥ 1. Within each 10-year ASCVD risk category, there were no differences in the prevalence of CAC ≥ 1 between women with and without EM (all p > 0.05).
Fig. 3.
Prevalence of CAC ≥ 1 by estimated 10-year ASCVD risk. Within each 10-year ASCVD risk category, the prevalence of CAC ≥ 1 at baseline exam was similar between women with vs. without EM (all p > 0.05). ASCVD = atherosclerotic cardiovascular disease, CAC = coronary artery calcium, PCEs = Pooled Cohort Equations.
There was a graded increase in hazard of ASCVD with higher CAC values for women with and without early menopause (Table 3). For women with EM, the fully adjusted hazard ratio (HR) was 1.19 (95% CI: 0.69-2.05) for CAC 1-99 and 1.85 (95% CI: 1.08-3.20) for CAC ≥ 100 compared to women with CAC = 0. For women without EM, the fully adjusted HRs were 2.09 (95% CI: 1.14-3.09) for CAC 1-99 and 3.08 (95% CI: 2.05-4.65) for CAC ≥ 100 compared to women with CAC = 0. The risk of CHD increased in a similar manner with a fully adjusted HR of 2.01 (95% CI 0.99-4.11) for CAC 1-99 and 2.85 (95% CI 1.37-5.96) for CAC ≥ 100 in women with EM. In women without EM, the fully adjusted HRs were 1.81 (95% CI: 1.08-3.05) for CAC 1-99 and 3.98 (95% CI: 2.40-6.59) for CAC ≥ 100. There was attenuation of the hazard ratios with adjustment for cardiovascular risk factors, but additional adjustment for HT use did not significantly change the observed hazard ratios. The interaction term between EM status and CAC was not statistically significant with a p-value of 0.09. In multivariable Cox proportional hazards models comparing the risk of ASCVD between women with and without EM, women with early EM and CAC = 0 had a long-term risk of ASCVD that was nearly twice as high compared to women without EM with a fully adjusted HR of 1.96 (95% CI: 1.26–3.04) (Table 4). Among women with CAC = 0, there was no statistically significant difference in risk for CHD for women with EM compared to women without EM with a HR of 1.48 (95% CI: 0.08-2.75). The hazard of ASCVD and CHD for women with EM compared to women without EM were not significantly different when CAC ≥ 1. In these Cox models, the hazard ratios showed minimal change with progressive adjustment for traditional cardiovascular risk factors including the model with HT use.
Table 3.
Progressively adjusted hazard ratios of ASCVD and CHD by CAC scores in women with and without EM.
|
Coronary Artery Calcium Score |
||||
|---|---|---|---|---|
| 0 | 1-99 | ≥100 | ||
| ASCVD | Early menopause | |||
| Model 1a | Ref | 1.37 (0.81–2.31) | 2.20 (1.32–3.66) | |
| Model 2b | Ref | 1.19 (0.69–2.34) | 1.91 (1.12–3.25) | |
| Model 3c | Ref | 1.19 (0.69–2.05) | 1.85 (1.08–3.20) | |
| No early menopause | ||||
| Model 1 | Ref | 2.49 (1.70–3.64) | 4.22 (2.87–6.19) | |
| Model 2 | Ref | 2.10 (1.42–3.10) | 3.13 (2.08–4.70) | |
| Model 3 | Ref | 2.09 (1.41–3.09) | 3.08 (2.05–4.65) | |
| CHD | Early menopause | |||
| Model 1 | Ref | 2.36 (1.18–4.74) | 3.56 (1.77–7.16) | |
| Model 2 | Ref | 2.02 (0.99–4.12) | 2.99 (1.46–6.11) | |
| Model 3 | Ref | 2.01 (0.99–4.11) | 2.85 (1.37–5.96) | |
| No early menopause | ||||
| Model 1 | Ref | 2.23 (1.34–3.70) | 5.61 (3.48–9.01) | |
| Model 2 | Ref | 1.82 (1.08–3.07) | 4.08 (2.47–6.71) | |
| Model 3 | Ref | 1.81 (1.08–3.05) | 3.98 (2.40–6.59) | |
ASCVD = atherosclerotic cardiovascular disease, CHD = coronary heart disease.
Model 1: Adjusted for age, race/ethnicity.
Model 2: Model 1 plus smoking, diabetes, family history of heart disease, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL-C, lipid-lowering medication, anti-hypertensive medication, income, and education level.
Model 3: Model 2 plus use of hormone therapy.
Table 4.
Adjusted hazard ratios of ASCVD and CHD for women with vs. without EM by CAC score.
|
Coronary Artery Calcium Score |
||||
|---|---|---|---|---|
| 0 | 1-99 | ≥100 | ||
| ASCVD | No Early Menopause | Ref | Ref | Ref |
| Early Menopause | ||||
| Model 1a. | 2.03 (1.33–3.12) | 1.18 (0.75–1.85) | 1.20 (0.81–1.79) | |
| Model 2b. | 1.94 (1.25–3.01) | 1.12 (0.71–1.77) | 1.22 (0.80–1.85) | |
| Model 3c. | 1.96 (1.26–3.04) | 1.10 (0.69–1.76) | 1.17 (0.76–1.81) | |
| CHD | No Early Menopause | Ref | Ref | Ref |
| Early Menopause | ||||
| Model 1 | 1.54 (0.84–2.81) | 1.67 (0.95–2.96) | 1.07 (0.66–1.75) | |
| Model 2 | 1.45 (0.78–2.68) | 1.71 (0.95–3.09) | 1.15 (0.70–1.90) | |
| Model 3 | 1.48 (0.80–2.75) | 1.69 (0.93–03.10) | 1.10 (0.65–1.88) | |
ASCVD = atherosclerotic cardiovascular disease, CAC = coronary artery calcium, CHD = coronary heart disease.
Model 1: Adjusted for age, race/ethnicity.
Model 2: Model 1 plus smoking, diabetes, family history of heart disease, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL-C, lipid-lowering medication, anti-hypertensive medication, income, and education level.
Model 3: Model 2 plus use of hormone therapy.
4. Discussion
Consistent with prior studies, we found that women who experienced EM had an increased risk for ASCVD [2], [3], [4], [5], [6], [7], [8]. However, our study demonstrates that the overall distribution of CAC and the association of CAC ≥ 1 with ASCVD were similar between middle-aged women with and without EM. We found the prevalence of CAC = 0 was similar between women with and without EM and that both groups had a very low 10-year rate of ASCVD, although over the maximal 15-year follow-up, women with EM had a significantly higher risk for ASCVD compared to women without EM.
The average age of onset of menopause among women without EM in MESA is consistent with findings from the Study of Women's Health Across the Nation (SWAN) which estimated onset of natural menopause at a median age of 51.4 years [35]. In our study, women with EM reported onset about 10 years earlier than their counterparts. Although the relative distributions of CAC scores were similar between women with and without EM, women without EM had a slightly higher prevalence of CAC = 0. However, the absolute difference was small (5.0%) and may reflect the higher baseline prevalence of diabetes and smoking among women with EM. Our estimate of CAC = 0 prevalence among women in MESA is consistent with results from the Framingham cohort, which found CAC = 0 in 56% of asymptomatic women between the ages 55 to 64 [36]. Although this study examined a contemporary cohort of women, this group was homogenous and predominantly White. A secondary analysis of SWAN reported similar results with 48% of postmenopausal or late peri-menopausal women with CAC = 0 [37]. Overall, the estimates from these three different cohorts suggest that roughly half of postmenopausal women in the US from ages 55 to 64 may have CAC = 0. Our study adds to this data by demonstrating a similar prevalence of CAC = 0 for women with and without EM in MESA.
The similarity in the distribution of CAC scores between women with and without EM in MESA is noteworthy as women with EM had accrued greater time since menopause and had a worse cardiovascular profile than their peers at the time of the CAC scan. However, our finding is concordant with the CARDIA study, which found that early menopause was not associated with higher odds of CAC ≥ 1 in women of a similar age group [38]. One possible explanation is that CAC = 0 under-captures the extent of coronary atherosclerosis in women with EM because it does not quantify noncalcified plaque burden, which itself serves as a precursor for CAD and an independent predictor of cardiovascular events. Furthermore, CAC may not capture microvascular coronary disease or atherosclerotic disease in other vascular beds, which are more prevalent in women than men and similarly increases the risk of ASCVD [39,40]. In addition, we found that compared to women without EM, women with EM had a higher prevalence of diabetes and ASCVD risk ≥20%, both of which are associated with a shorter time for conversion from CAC = 0 to CAC ≥ 1 [41].
Among women in MESA with CAC = 0, the ASCVD event rate was low-to-borderline at 10-year follow-up and the difference between those with and without EM had marginal statistical significance. Although 10-year ASCVD risk was not statistically significant between the two groups of women, the survival curves began to diverge at approximately 7-8 years of follow-up with a significantly higher ASCVD event rate for women with EM at 15-year follow-up. This divergence may be partially explained by the higher baseline prevalence of some traditional CVD risk factors among women with EM. In addition, non-CHD outcomes such as stroke and heart failure (which are less strongly associated with CAC) likely contribute to differences in long-term ASCVD risk among women with CAC = 0 [24,[42], [43], [44]]. In our study, the non-CHD event rate among women with CAC = 0 was significantly higher for women with EM, while the CHD event rates were similar. Despite the difference in ASCVD-free survival, the total incidence of ASCVD remained low in both groups of women with CAC = 0 at 15-years follow-up, at which time the mean age in this analysis was 79 years.
The precise mechanisms through which EM increases ASCVD risk remains unclear but has been largely attributed to the association between the loss of estrogen and atherosclerosis [45]. Estrogen is thought to protect against atherosclerosis by preventing endothelial deposition of LDL-C, proliferation of smooth muscle cells, aggregation of platelets, and oxidative damage [46], [47], [48]. In addition, estrogen has vasodilatory properties that may oppose thrombotic ischemia [46,49,50]. The loss of these protective mechanisms during menopause may contribute to the increased risk for ASCVD in EM. However, the WHI randomized trial conversely showed that the use of HT actually increased the incidence of ASCVD, which suggests a potential difference in the vascular impact between natural and exogenous hormones [11]. Additionally, recent studies have also demonstrated that the age of initiation, timing in relation to menopause, and duration of HT can affect ASCVD outcomes with results favoring the use of HT in younger postmenopausal women and initiation closer to the onset of menopause [51,52]. Our results did not show any substantial difference after adjustment for the use of HT, although MESA did not collect data on age of initiation, timing in relation to menopause, or duration of hormone therapy. Therefore, while earlier initiation of exogenous HT appears likely to be safe based on prior evidence, further research is needed to investigate these nuanced questions with regards to EM, CAC, and ASCVD.
The 2018 AHA/ACC Cholesterol Treatment Guidelines identify premature menopause (defined as occurring <40 years of age) as a risk-enhancing factor that favors statin therapy for women with uncertain risk based on their calculated 10-year PCEs score [19]. In our study, we have defined EM as occurring before the age of 45 in accordance with several contemporary studies [2,8,28]. The significance of this 5-year difference in classification of menopause on ASCVD outcomes is uncertain due to mixed findings from prior studies. However, a recent large pooled meta-analysis demonstrated increasing hazard of ASCVD with earlier onset of menopause [28]. Accordingly, while there is likely a continuous relationship between younger age of menopause and increase in risk for ASCVD, the age cutoff of <45 years that we use in this study still appears to define a group of women at increased risk for ASCVD. As in MESA, this heterogenous study population included women with natural and surgical EM. Although our present study did not stratify women with EM by type of menopause, it is noteworthy that there were nearly three times as many cases of surgical menopause among women with EM. Several studies have shown that both surgical and natural premature menopause are associated with an increased risk for ASCVD, but that women with premature surgical menopause have a higher ASCVD risk than women with premature natural menopause [53], [54], [55]. This observation is incompletely understood but may be related to the acuity and degree of estrogen loss in surgical EM compared to natural EM. Collectively, this data underscores the importance of preventive care – including lipid-lowering therapy and HT – especially in women who experience surgical menopause at an early age.
In this study, we also found that the prevalence of CAC ≥ 1 was similar between middle-aged women with and without EM in various strata of PCEs scores. Fewer than 20 percent of the women in our study with PCE < 5.0% had CAC ≥ 1. In a meta-analysis examining five large population-based cohorts including MESA, Kavousi et al. estimated CAC ≥ 1 in only 36.1% of low-risk women [56]. Therefore, EM alone may not necessarily warrant CAC screening among middle-aged women with low predicted ASCVD risk as the net population benefit is indeterminate and between 50-60% of women with borderline or intermediate ASCVD risk had baseline CAC = 0 in our study. Silverman et al. previously demonstrated that CAC = 0 was associated with low 10-year ASCVD event rates, even among individuals with unfavorable cardiometabolic profiles [57]. Therefore, a substantial number of women with EM who are classified as borderline- or intermediate-risk by PCEs may benefit from additional CAC screening as CAC = 0 can help to identify those in whom it may be reasonable to defer statin therapy based on a shared patient-clinician decision making process.
One limitation of our study is that HT confounds the association between menopause and ASCVD depending on the time of its initiation relative to menopause. Prescriptions for HT declined after 2002 following the enrollment period due to findings of increased ASCVD risk from the WHI trial [11]. As a result, changes in clinical practice would modify ASCVD outcomes among women who had initiated HT prior to enrollment. Additionally, survival bias may be present, because MESA excluded participants with prior CVD and women who experienced early CVD events would not be included in this cohort. However, a large meta-analysis of women with EM found that only 4% had a CVD event prior to the age of 60 years (mean age of MESA participants in this study was 64 years-old) and we found that among women <65 years of age, the distribution of CAC was very similar (Supplementary Fig. 3) [28]. Our study uses self-reported menopausal status, but we note that this method was validated and shown to be reliable in the Nurses’ Health Study where 99% of women accurately reported their menopausal age within one year [5]. We also excluded a small proportion of participants, because they either reported uncertainty about their menopause status or discrepant information regarding menopausal status and history. Finally, to establish a cohort of postmenopausal women for our study, we applied an algorithm to infer postmenopausal status, although this algorithm has been used in prior publications [27].
Strengths of this study include a robust baseline exam and consistent longitudinal follow-up for ASCVD events over 15 years. In addition, the data was collected from a large, well-studied cohort of women with diverse ethnic representation.
5. Conclusions
In this multi-ethnic cohort of middle-aged women, there was a slightly lower prevalence of CAC = 0 in women with EM, but >50% had CAC = 0. The absence of CAC was associated with a low-to-borderline 10-year cumulative incidence of ASCVD regardless of EM status, although there was a divergence in cumulative ASCVD incidence between women with and without EM before 10-year follow-up. At 15-year follow-up, women with EM had a significantly higher incidence of ASCVD compared to women without EM. When CAC ≥ 1, the incidence of ASCVD was similar for women with and without EM. These findings support the use of CAC to help improve ASCVD risk stratification in women with EM. Further research is needed to better understand the differences in long-term ASCVD risk between women with and without EM who have CAC = 0.
CRediT authorship contribution statement
Jian H. Chu: Conceptualization, Methodology, Formal analysis, Writing – original draft. Erin D. Michos: Writing – review & editing. Pamela Ouyang: Writing – review & editing. Dhananjay Vaidya: Formal analysis, Writing – review & editing. Roger S. Blumenthal: Writing – review & editing. Matthew J. Budoff: Writing – review & editing. Michael J. Blaha: Writing – review & editing. Seamus P. Whelton: Conceptualization, Methodology, Supervision, Formal analysis, Writing – original draft.
Declaration of Competing Interest
The authors have no relevant conflicts of interests and relationships with industry to disclose.
Acknowledgements
Dr. Whelton has been supported by the PJ Schafer Memorial Foundation. This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2022.100362.
Appendix. Supplementary materials
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19(10):1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 4.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40(4):1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen B, Nilssen S, Heuch I, Kvåle G. Does age at natural menopause affect mortality from ischemic heart disease? J Clin Epidemiol. 1997;50(4):475–479. doi: 10.1016/s0895-4356(96)00425-8. [DOI] [PubMed] [Google Scholar]
- 7.Peters S, Woodward M. Women's reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104(13):1069–1075. doi: 10.1136/heartjnl-2017-312289. [DOI] [PubMed] [Google Scholar]
- 8.Muka T, Oliver-Williams C, Kunutsor S, Laven J, Fauser B, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systemic review and meta-analysis. JAMA Cardiol. 2016;1(7):767–776. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease advantage. Arch Intern Med. 1995;155(1):57–61. [PubMed] [Google Scholar]
- 10.Chae C, Derby C. The menopausal transition and cardiovascular risk. Obstet Gynecol Clin North Am. 2011;38(3):477–488. doi: 10.1016/j.ogc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Rossouw J, Anderson G, Prentice R, LaCroix A, Kooperberg C, Stefanick M, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Arts E, Popa C, Den Broeder A, Donders R, Sandoo A, Toms T, et al. Prediction of cardiovascular risk in rheumatoid arthritis: performance of original and adapted SCORE algorithms. Ann Rheum Dis. 2016;75(4):674–680. doi: 10.1136/annrheumdis-2014-206879. [DOI] [PubMed] [Google Scholar]
- 13.Arts E, Popa C, Den Broeder A, Semb A, Toms T, Kitas G, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74(4):668–674. doi: 10.1136/annrheumdis-2013-204024. [DOI] [PubMed] [Google Scholar]
- 14.Solomon D, Greenberg J, Curtis J, Liu M, Farkouh M, Tsao P, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: a Consortium of Rheumatology Researchers of North America Registry Study. Arthritis Rheumatol. 2015;67(8):1995–2003. doi: 10.1002/art.39195. [DOI] [PubMed] [Google Scholar]
- 15.Castelli W, Anderson K, Wilson P, Levy D. Lipids and risk of coronary heart disease. The Framingham study. Ann Epidemiol. 1992;2(1-2):23–28. doi: 10.1016/1047-2797(92)90033-m. [DOI] [PubMed] [Google Scholar]
- 16.Thompson-Paul A, Lichtenstein K, Armon C, Palella F, Skarbinski J, Chmiel J, et al. Cardiovascular disease risk prediction in the HIV outpatient study. Clin Infect Dis. 2016;63(11):1508–1516. doi: 10.1093/cid/ciw615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monroe AK, Haberlen SA, Post WS, Palella FJ, Kinsgley LA, Witt MD, et al. Cardiovascular disease risk scores' relationship to subclinical cardiovascular disease among HIV-infected and HIV-uninfected men. AIDS. 2016;30(13):2075–2084. doi: 10.1097/QAD.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone N, Robinson J, Lichtenstein A, Bairey Merz C, Blum C, Eckel R, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol. 2018;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Kelkar A, Schultz W, Khosa F, Schulman-Marcus J, O'Hartaigh B, Gransar H, et al. Long-term prognosis after coronary artery calcium scoring among low-intermediate risk women and men. Circ Cardiovasc Imaging. 2016;9(4) doi: 10.1161/CIRCIMAGING.115.003742. [DOI] [PubMed] [Google Scholar]
- 21.Bellasi A, Lacey C, Taylor A, Raggi P, Wilson P, Budoff M, et al. Comparison of prognostic usefulness of coronary artery calcium in men versus women (results from a meta- and pooled analysis estimating all-cause mortality and coronary heart disease death or myocardial infarction) Am J Cardiol. 2007;100(3):409–414. doi: 10.1016/j.amjcard.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Michos ED, Blaha MJ, Blumenthal RS. Use of the coronary artery calcium score in discussion of initiation of statin therapy in primary prevention. Mayo Clin Proc. 2017;92(12):1831–1841. doi: 10.1016/j.mayocp.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 24.Gibson AO, Blaha MJ, Arnan MK, Sacco RL, Szklo M, Herrington DM, et al. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort. The MESA Study. JACC Cardiovasc Imaging. 2014;7(11):1108–1115. doi: 10.1016/j.jcmg.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 27.Subramanya V, Zhao D, Ouyang P, Ying W, Vaidya D, Ndumele C, et al. Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA) J Cardiovasc Comput Tomogr. 2019;13(1):41–47. doi: 10.1016/j.jcct.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu D, Chung H, Dobson A, Pandeya N, Giles G, Bruinsma F, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4(11):e553–ee64. doi: 10.1016/S2468-2667(19)30155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bild D, Bluemke D, Burke G, Detrano R, Diez Roux A, Folsom A, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 30.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 31.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 32.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 33.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113(1):30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 34.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 35.Gold E, Bromberger J, Crawford S, Samuels S, Greendale G, Harlow S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann U, Massaro JM, Fox CS, Manders E, O'donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102(Generic):1136–1141. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang N, Matthews K, Barinas-Mitchell E, Chang C, El Khoudary S. Inflammatory/hemostatic biomarkers and coronary artery calcification in midlife women of African-American and White race/ethnicity: the Study of Women's Health Across the Nation (SWAN) heart study. Menopause. 2016;23(6):653–661. doi: 10.1097/GME.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freaney P, Petito L, Colangelo L, Lewis C, Schreiner P, Terry J, et al. Association of premature menopause With coronary artery calcium: the CARDIA study. Circ Cardiovasc Imaging. 2021;14(11) doi: 10.1161/CIRCIMAGING.121.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coutinho T, Yam Y, Chow B, Dwivedi G, Inácio J. Sex Differences in associations of arterial compliance with coronary artery plaque and calcification burden. J Am Heart Assoc. 2017;6(8) doi: 10.1161/JAHA.117.006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plank F, Beyer C, Friedrich G, Wildauer M, Feuchtner G. Sex differences in coronary artery plaque composition detected by coronary computed tomography: quantitative and qualitative analysis. Neth Heart J. 2019;27(5):272–280. doi: 10.1007/s12471-019-1234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzaye O, Dardari Z, Cainzos-Achirica M, Blankstein R, Agatston A, Duebgen M, et al. Warranty Period of a Calcium Score of Zero: Comprehensive Analysis From MESA. JACC Cardiovasc Imaging. 2021;14(5):990–1002. doi: 10.1016/j.jcmg.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appiah D, Schreiner P, Demerath E, Loehr L, Chang P, Folsom A. Association of age at menopause with incident heart failure: a prospective cohort study and Meta-Analysis. J Am Heart Assoc. 2016;5(8) doi: 10.1161/JAHA.116.003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebong I, Watson K, Goff D, Bluemke D, Srikanthan P, Horwich T, et al. Age at menopause and incident heart failure: the multi-ethnic study of atherosclerosis. Menopause. 2014;21(6):585–591. doi: 10.1097/GME.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermann D, Gronewold J, Lehmann N, Moebus S, Jöckel K, Bauer M, et al. Coronary artery calcification is an independent stroke predictor in the general population. Stroke. 2013;44(4):1008–1013. doi: 10.1161/STROKEAHA.111.678078. [DOI] [PubMed] [Google Scholar]
- 45.Bleil M, Gregorich S, McConnell D, Rosen M, Cedars M. Does accelerated reproductive aging underlie premenopausal risk for cardiovascular disease? Menopause. 2013;20(11):1139–1146. doi: 10.1097/GME.0b013e31828950fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;89(12A):12E–17E. doi: 10.1016/s0002-9149(02)02405-0. [DOI] [PubMed] [Google Scholar]
- 47.Wild RA. Estrogen: effects on the cardiovascular tree. Obstet Gynecol. 1996;87(2 Suppl):27S–35S. doi: 10.1016/0029-7844(95)00434-3. [DOI] [PubMed] [Google Scholar]
- 48.Samaan SA, Crawford MH. Estrogen and cardiovascular function after menopause. J Am Coll Cardiol. 1995;26(6):1403–1410. doi: 10.1016/0735-1097(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 49.Glasser SP, Selwyn AP, Ganz P. Atherosclerosis: risk factors and the vascular endothelium. Am Heart J. 1996;131(2):379–384. doi: 10.1016/s0002-8703(96)90370-1. [DOI] [PubMed] [Google Scholar]
- 50.Collins P, Rosano GM, Jiang C, Lindsay D, Sarrel PM, Poole-Wilson PA. Cardiovascular protection by oestrogen–a calcium antagonist effect? Lancet. 1993;341(8855):1264–1265. doi: 10.1016/0140-6736(93)91158-i. [DOI] [PubMed] [Google Scholar]
- 51.Rossouw J, Prentice R, Manson J, Wu L, Barad D, Barnabei V, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 52.Manson J, Aragaki A, Rossouw J, Anderson G, Prentice R, LaCroix A, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The Women's Health Initiative Randomized Trials. JAMA. 2017;318(10):927–938. doi: 10.1001/jama.2017.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu D, Chung H, Dobson A, Pandeya N, Brunner E, Kuh D, et al. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: pooled analysis of individual data from 10 international studies. Hum Reprod. 2020;35(8):1933–1943. doi: 10.1093/humrep/deaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atsma F, Bartelink M, Grobbee D, van der Schouw Y. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 55.Honigberg M, Zekavat S, Aragam K, Finneran P, Klarin D, Bhatt D, et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA. 2019;322(24):2411–2421. doi: 10.1001/jama.2019.19191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kavousi M, Desai C, Ayers C, Blumenthal R, Budoff M, Mahabadi A, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA. 2016;316(20):2126–2134. doi: 10.1001/jama.2016.17020. [DOI] [PubMed] [Google Scholar]
- 57.Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35(33):2232–2241. doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.