Abstract
Palliative care remains suboptimal in advanced cirrhosis, in part relating to a lack of evidence-based interventions. Ascites remains the most common cirrhosis complication resulting in hospitalisation. Many patients with refractory ascites are not candidates for liver transplantation or transjugular intrahepatic portosystemic shunt, and therefore, require recurrent palliative large volume paracentesis in hospital. We review the available evidence on use of palliative long-term abdominal drains in cirrhosis. Pending results of a national trial (REDUCe 2) and consistent with recently published national and American guidance, long-term abdominal drains cannot be regarded as standard of care in advanced cirrhosis. They should instead be considered only on a case-by-case basis, pending definitive evidence. This manuscript provides consensus to help standardise use of long-term abdominal drains in cirrhosis including patient selection and community management. Our ultimate aim remains to improve palliative care for this under researched and vulnerable cohort.
Keywords: LIVER CIRRHOSIS, PERITONITIS, CLINICAL TRIALS, ASCITES
Key points.
Nationally, cirrhosis-related mortality has increased significantly over the last four decades.
Ascites is the most common cirrhosis complication. Despite many patients with refractory ascites due to cirrhosis not being liver transplant candidates, palliative interventions remains a clear unmet need.
Palliative long-term abdominal drains (LTADs) are routinely used in refractory malignant ascites but are not standard of care in cirrhosis, pending results of a national definitive trial.
Currently, outside of a research setting, LTADs should only be considered in cirrhosis on a case by case basis after careful patient selection.
Patients being considered for LTADs should be referred to palliative care services.
The key to successful implementation of LTAD in cirrhosis will be integrated working between the hospital and community teams.
Introduction
Liver disease-related deaths in England have increased by >250% since 1971.1 Nationally, the COVID-19 pandemic has resulted in a 20% increase in all cause alcohol-related deaths in 2020 compared with 2019.2
The current manuscript focuses on palliative management of refractory ascites due to decompensated cirrhosis (henceforth referred as advanced cirrhosis), with emphasis on long-term abdominal drains (LTADs). After the recent feasibility study,3 4funding has just been obtained for a definitive randomised controlled trial (REDUCe 2) comparing large volume paracentesis (LVP) vs palliative LTADs in refractory ascites due to cirrhosis (Health Technology Assessment (HTA) project: National Institute for Health and Care Research (NIHR 133889). This intervention is also undergoing National Institute for Health and Care Excellence (NICE) assessment (GID-IPG10194). Therefore, at present, LTAD cannot be regarded as standard of care in advanced cirrhosis. However, following on from the feasibility study and recently published case series/systematic review,4–6 use of LTADs has increased nationally, but without oversight. To help standardise LTAD usage and improve practice, we provide guidance on patient selection and community management, based on the current best current available evidence.4–6
The guidance was developed through the consensus of an expert panel, who were invited on behalf of the British Association for the Study of the Liver/British Society of Gastroenterology (BASL/BSG) End of Life Special Interest Group. This included specialists in hepatology and liver transplantation, palliative medicine, community and liver nursing, interventional radiology (IR) and patient groups. The quality (level) of the evidence and the strength of each guidance statement are not formally rated, owing to a current paucity of high-quality data in this area.
Refractory ascites due to advanced cirrhosis
Ascites remains the most common cirrhosis complication requiring hospitalisation,7 8 up to a third of patients progressing to refractory ascites.9 10 The International Ascites Club Criteria defines refractory ascites as either (1) diuretic-resistant ascites or (2) diuretic-intractable ascites.11 12 Once refractory ascites develops, transplant-free survival is 6–12 months.9 10 13 14 However, patients with refractory ascites are a heterogenous group, older age (>60 years), presence of hepatocellular cancer and diabetes mellitus predicting poorer survival, while alcohol abstinence is independently associated with improved survival.13
Many patients with refractory ascites are not candidates for transplantation (9–10, 13–14), transjugular intrahepatic portosystemic shunt (TIPS) or the Automated Low Flow Ascites pump.15 16 Data from one UK secondary liver centre showed that from 2013 to 2015, only 14% of patients with refractory ascites were listed/underwent liver transplantation and/or TIPS,10 consistent with studies from Europe and America.9 13 14 LVP remains the most common palliative intervention for refractory ascites. An English mortality study noted that of the 44 923 patients who died from liver disease in England between 2013 and 2015, 13 181 (29%) required LVP in their last year of life, mean annual cost/person being >£21 000.17
In a recent systematic review pain and breathlessness, commonly observed with ascites, were reported by up to 88% of patients with cirrhosis.18 Unsurprisingly, health-related quality of life (HRQoL) is more significantly impaired in patients with cirrhosis than in both healthy controls and those with non-cirrhotic chronic liver disease, the impairment increasing with worsening cirrhosis severity.18–22 Ascites is one of the main drivers of impaired HRQoL in advanced cirrhosis, both in patients and caregivers.21 23–27
Refractory ascites and palliative care
Despite refractory ascites being a reliable prognostic guide, only a minority of patients with advanced cirrhosis are referred to palliative care, often in the last few days before death.10 28–31 Timely palliative care in cirrhosis can improve symptom control,32 33 address goals of care/advance care planning34 35 and reduce hospitalisations.4 29 36 Approximately 75% of patients with advanced cirrhosis die in hospital,17 37 compared with 40% with advanced cancer.38 Lack of evidence-based guidelines remains an obstacle to optimal palliative care in advanced cirrhosis.
Evidence for palliative interventions for refractory ascites due to advanced cirrhosis remains a clear unmet need
In ascites due to advanced abdominal malignancy there is evidence to support the use of palliative LTADs.39–42 These tunnelled drains are inserted in hospital under local anaesthetic into the peritoneal cavity. Community nurses or informal caregivers (if willing), then drain small amounts (1–2 L) of ascitic fluid in the community, up to three times a week. LTADs could reduce hospitalisation, improve symptom control and HRQoL and be cost-effective to the National Health Service.39–42 Currently LTADs are not standard of care in advanced cirrhosis, ongoing concerns being community management and the increased peritonitis16 risk in cirrhosis. These concerns were evident in our national survey of BSG and BASL members.43
LTAD use in advanced cirrhosis
An earlier systematic review assessed LTADs in refractory ascites due to advanced cirrhosis,6 though most studies were rated as ‘poor’ (Newcastle-Ottawa Scale).44 Nonetheless, LTAD insertion success was 100%, no further ascites-related hospitalisations needed in 14/18 studies where data were provided. Peritonitis rates (12.7%) were however more than two fold higher than reported in malignant ascites (median 5.9%, range 2.5%–34%).6 39
Recent data come from the feasibility REpeated Drainage in Untreatable Cirrhosis (REDUCe) trial,3 4 comparing palliative LTADs vs LVP in refractory ascites due to advanced cirrhosis. Thirty-six patients were randomised with 21 (58%) completing the 3-month study, both groups receiving prophylactic antibiotics for the study duration. LTAD insertion was successful in all participants, only 2/15 (13%) requiring further hospitalisations specifically for ascites. Peritonitis incidence (LTAD vs LVP) was 6% vs 11%, self-limiting cellulitis (treated if needed with antibiotics, none requiring hospitalisation), being 41% vs 11%, respectively. Median fortnightly total costs were about 15% lower in the LTAD group. Symptom and HRQoL scores were highly variable in both groups, likely reflecting the small sample size.4 An embedded qualitative study indicated LTAD acceptability by patients and nurses.45 The REDUCe study demonstrated feasibility with preliminary evidence of LTAD effectiveness, safety, acceptability and reduced health resource utilisation supporting a future definitive trial.
Guidance for the management of patients requiring LTADs
1. Selection of patients for LTAD insertion
1.1 LTADs can be considered on a case-by-case basis in patients with refractory ascites who are not under consideration for/listed for liver transplantation or TIPS.
1.2 The decision for LTAD insertion should be made by a multidisciplinary team.
1.3 LTADs may be less appropriate in patients if there is a reasonable prospect of recompensation (eg, alcohol-related liver disease with subsequent abstinence).
1.4 LTAD insertion is not appropriate for patients with chylous or loculated ascites.
1.5 LTAD may not be appropriate for patients who are likely to be in their last days/weeks of life.
1.6 Hepatic encephalopathy and paucity of caregiver support should not be considered absolute contraindications to LTAD insertion.
1.7 Appropriate community nursing support should be available in the specific region.
Consistent with recently published national and American guidance,16 46 LTADs cannot be regarded as standard of care in advanced cirrhosis, pending results of the definitive trial. The decision for LTAD insertion should therefore be made on a case-by-case basis at a multidisciplinary meeting where suitability for transplantation or TIPS should also be discussed. While some non-UK centres are inserting LTADs in potential transplant candidates,47 pending definitive evidence, our current recommendation is that LTADs not to be inserted in patients who are under consideration, or listed for liver transplantation and or TIPS. This is because of risk of potential infection and/or sclerosing peritonitis increasing surgical risk. Rarely, patients initially deemed unsuitable for transplantation may become eligible (eg, with improved nutritional status). In such instances, however, the presence of an LTAD should not be an absolute contraindication for transplantation.
Once deemed to have true refractory ascites11 12 and TIPS/transplant ineligibility, an LTAD could be a considered a potential option. Table 1 shows the indications and contraindications for LTADs. LTADs may be more suitable than repeated LVPs when recompensation is less likely (eg, non-alcoholic fatty liver disease). In particular, the propensity to recompensate in alcohol-related liver disease (on alcohol cessation) and chronic viral hepatitis (after antiviral treatment) should be considered prior to LTAD insertion (although LTAD insertion can still be considered in these aetiologies).
Table 1.
Indications and contraindications for long-term abdominal drains (LTADs) in refractory ascites due to cirrhosis
| Indications for LTAD | Contraindications for LTAD | |
| Refractory ascites defined as per International Ascites Club criteria with need for repeated large volume paracentesis | Absolute | Relative |
| Not eligible for TIPS±liver transplant | Loculated/chylous ascites | Stage 4 CKD (eGFR <30 mL/min) |
| Candidate for liver transplant/TIPS | Prior life-threatening SBP | |
| Actively dying, that is, expected die within days | Active infection | |
| Reasonable possibility of recompensation | ||
CKD, chronic kidney disease; SBP, spontaneous bacterial peritonitis; TIPS, transjugular intrahepatic portosystemic shunt.
Not all patients with refractory ascites due to cirrhosis would find an LTAD acceptable. Those who are socially isolated, hospital-based LVP maybe their only opportunity for social interaction. LTAD insertion may also not be appropriate in most patients likely to be in the last few days/weeks of life, as the benefits of short-term LTAD insertion are unlikely to be greater than an isolated LVP procedure. Presence of hepatic encephalopathy and absence of caregivers should not be considered absolute contraindications to LTAD insertion. However, practicalities of use and care in these patients groups needs careful consideration and planning. As patients with advanced cirrhosis can deteriorate suddenly, pragmatic, individualised decision making is often the best way forward.
2. Provision of palliative care and advance care planning
2.1 Patients with refractory ascites should be counselled around their prognosis.
2.2 Patients undergoing LTAD insertion and their caregivers should be aware that it is a procedure carried out with palliative intent.
2.3 All patients in whom LTAD insertion is considered should be afforded an opportunity to engage in advance care planning, and should have access to specialist palliative care services if and when required.
We recommend that palliative care and advance care planning discussions are initiated in parallel with consideration of LTAD insertion. These discussions should focus on the goals and priorities of the individual to help guide treatment decisions (eg, is there a desire to be managed at home if possible, attitudes to LTAD). It should encompass discussions around prognosis (and prognostic uncertainty), and advance care planning. Some patients and caregivers may find it difficult to accept that refractory ascites, like advanced cancer, is a life-limiting condition. This is consistent with REDUCe study qualitative data where in some instances LTADs were misinterpreted as active treatment rather than a palliative intervention.45
3. Periprocedural management of LTAD insertion
3.1 Patients undergoing LTAD insertion should be counselled regarding risks and alternatives of the procedure, and ideally provided with written material prior to the procedure.
3.2 Clotting parameters (INR and platelets) should be checked within 7 days of LTAD insertion, and corrected as per IR protocols.
3.3 Patients should have a diagnostic ascitic tap within 7 days of LTAD insertion to exclude spontaneous bacterial peritonitis (ascitic neutrophil count <250 cells/mm3/white cell count <500 cells/mm3 and negative ascitic fluid culture). Patients in whom spontaneous bacterial peritonitis is diagnosed should be fully treated prior to LTAD insertion.
3.4 Patients undergoing LTAD insertion should be offered ongoing prophylactic antibiotics to reduce peritonitis risk (as per local trust protocol).
There are currently two LTADs available in the UK: PleurX, recently rebranded as PeriX (UK Medical, Basingstoke, UK) and Rocket (Rocket Medical plc, Watford, UK). These devices have a CE mark for intermittent, long-term drainage of symptomatic, recurrent, malignant and non-malignant ascites. In absence of head-to-head trials comparing the devices, the choice of LTAD remains at clinician’s discretion.
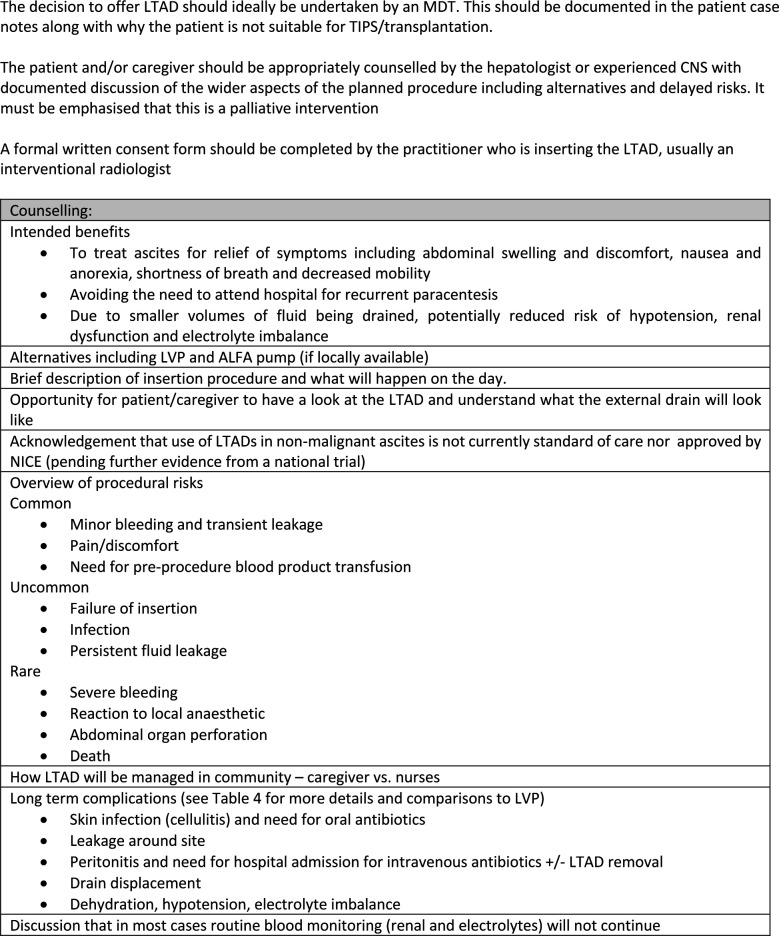
Box 1 shows the recommended checklist prior to LTAD insertion and figure 1 shows important facets of informed consent. It must be emphasised to patients and caregivers that this is a palliative intervention with a limited evidence base in cirrhosis. Unlike LVP where routine testing of INR and platelet is not recommended,16 48 insertion of LTAD is more invasive as it involves tunnelling. Therefore haemostatic function should be checked within 7 days of LTAD insertion and necessary products administered if INR >1.5 and or platelet count ≤50×109/L (box 1). This would be standard practice for most interventional radiologists.49
Box 1. Checklist prior to long-term abdominal drain insertion.
Not a transjugular intrahepatic portosystemic shunt/liver transplant candidate
Absence of loculated/chylous ascites.
Clear discussions with patients and caregivers that LTAD is a palliative intervention, current evidence being from a small trial and case series.
Community nursing team able to support such patients.
Referral made to hospital and community palliative care team.
Schedule appointment for LTAD insertion with interventional radiology/clinician.
Check INR and platelet count up to 7 days days prior to LTAD insertion. If INR is >1. 5 and platelet count ≤50×109/L consider blood products.
Perform a diagnostic ascitic tap for cell count and culture up to 7 days days prior to LTAD insertion.
Antibiotic prophylaxis (as per local trust guidelines) for duration that LTAD remains in situ.
Discuss with caregivers if they are willing to help with home drainage.
Inform community nursing team and ensure that they are provided with a contact number for the parent medical team.
Inform LTAD manufacturer so that additional bespoke training can be organised for patients and caregivers if needed.
General practitioner notification letter including details of required prescription for ongoing drainage bag supply.
If the LTAD is being inserted outside of a research setting, ensure clinical outcomes audited and reviewed.
Figure 1.
Considerations when counselling a patient/caregiver for insertion of a long-term abdominal drain. ALFA, automated low flow ascites; CNS, clinical nurse specialist; INR, international normalised ratio; LTAD long-term abdominal drain; LVP, large volume paracentesis; MDT, multidisciplinary team; NICE, National Institute for Health and Care Excellence; TIPS, transjugular intrahepatic portosystemic shunt.
There are no evidence-based guidelines on use of prophylactic antibiotics in setting of LTADs. NICE, European and BSG guidelines16 48 50 recommend prophylactic antibiotics if total ascitic fluid protein is <15 g/L. However, recent studies suggest that ascitic fluid protein may not predict peritonitis risk.51 52 As already stated, peritonitis risk is more than twofold higher when LTADs are inserted in patients with cirrhosis compared with those with malignant ascites.6 39 We would therefore recommend that all patients be offered prophylactic antibiotics (as per local protocols), as long as the LTAD remains in situ, especially if planned duration is for 3 months or longer.53 Since this is a palliative cohort, the duration of antibiotic usage will in most patients be short-term in-keeping with overall life expectancy. Risk/benefits of prophylactic antibiotics should however be discussed with patients and their caregivers.
4. Practicalities of LTAD insertion (box 1 and table 2)
Table 2.
Do’s and don’ts when inserting long-term abdominal drains (LTADs) for refractory ascites
| Do’s | Don’ts |
| Emphasise that this is a palliative intervention, the evidence being limited to a small trial and case series | Do not do routine blood tests and ascitic fluid analysis in asymptomatic patients following LTAD insertion |
| Ensure that patients have been referred to palliative care. | Do not routinely administer human albumin solution as an outpatient |
| Check haemostatic function and screen for peritonitis prior to LTAD insertion. | Do not assume that LTAD will be suitable for every patient with refractory ascites. |
| Provide a contact number for the hospital parent medical team. | |
| Work closely with community nursing teams. | |
| Ensure good nutritional intake. | |
| Encourage caregivers to participate in home drainage. |
4. 1 LTADs can be inserted by any appropriately trained clinician
4.2 LTADs should be inserted under ultrasound guidance
4.3 Ascites should be drained to dryness (with human albumin solution as required) at the time of LTAD insertion
LTAD insertion is done as a day case with ultrasound guidance, the technique having been previously described.3 While at most sites, LTAD insertion will be performed by IR, this is not essential. Individuals inserting drains outside of IR should undergo a period of supervised practice in IR, and be assessed as competent to perform the procedure independently. Once an LTAD has been inserted it is recommended that the ascites is drained to dryness with HAS (20%) administered as per LVP protocol.16 48 This makes subsequent community management of ascites easier. On discharge, incontinence sheets should be provided as some leakage is to be expected along with approximately 2 weeks supply of drainage bags with discharge notification being sent to the general practitioner (GP) to organise ongoing supply. Patients are advised to keep the wound sites dressed until the community nurses remove the stitches.
5. Community management of LTADs
5. 1 Community teams should be informed of the decision to proceed with LTAD insertion in advance, and have access to support and advice in secondary care when required (see online supplemental file 1 for community standard operating procedure).
flgastro-2022-102128supp001.pdf (118.2KB, pdf)
5.2 Patients should have approximately 2–3 drainage procedures/week with up to 2 L of ascites being removed on each occasion, with a maximum 5 L of ascites drained/week. This will be sufficient for most patients.
5.3 Caregivers can be trained in LTAD drainage when appropriate/willing.
5.4 Patients undergoing community drainage of ascites do not require human albumin solution replacement.
Multidisciplinary working between hepatology, community, primary and specialist palliative care, and family caregivers is essential to the successful management of a patient with an LTAD. This is a complex patient group with multiple distressing symptoms increasing as end of life approaches. The management of the LTAD is a component of community nursing care that should be incorporated into the provision of end of life care for this patient group. Following LTAD insertion, the patient’s GP and the community nursing team should be informed to ensure continuity of care between hospital and community. Most community nursing teams are familiar with LTAD as they are used in malignant ascites, however, experience in advanced cirrhosis is very limited. Based on REDUCe study data,4 we would recommend two to three nursing visits per week with 1–2 L being drained at each visit with initially a maximum of 5 L being drained each week (see online supplemental file 1) for community standard operating procedure). This will be sufficient for most patients.
A small proportion of patients (13% in the REDUCe study),4 who remain symptomatic from ascites despite drainage of 5 L/week in the community should undergo supplementary LVP in hospital (via the LTAD using drain specific adaptors), with HAS replacement as per LVP protocol.16 48 In this small subset of patients who require LVPs in hospital despite 5 L/week community drainage, higher volume community LTAD drainage can be considered on a case-by-case basis, in discussion with the consultant/community teams. Community nurses should be provided with a named contact from the hospital hepatology team to address queries for care provision in the community. This allows management of increasing symptom distress as disease progresses, facilitates individualised care and supports the community teams thus reducing unplanned hospital visits. Family caregivers if available and able to be involved with drainage can be supported to do so by the community nurses and hospital team.
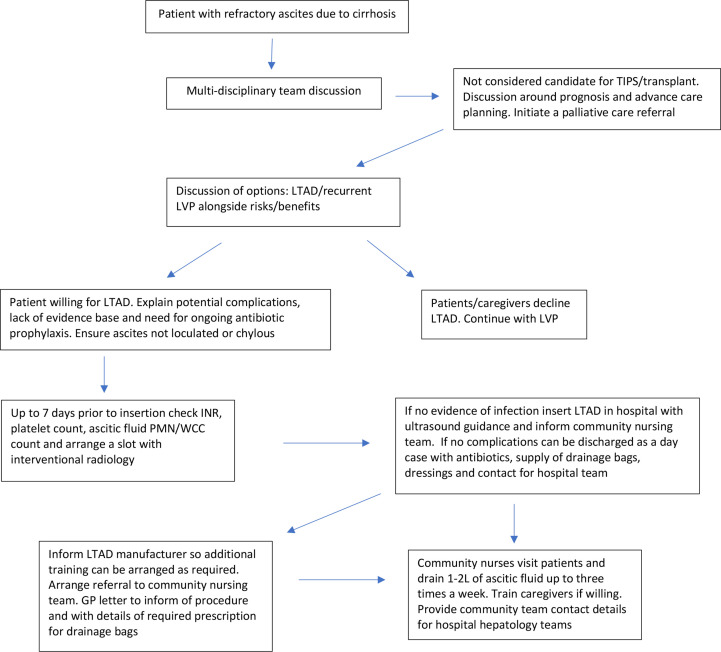
Use of long-term outpatient HAS remains contentious. Two recent studies gave conflicting results, those with advanced ascites less likely to benefit.54 55 LTAD is a palliative intervention, focus being on symptom control, improving HRQoL and moving care to the community. Currently, therefore, outpatient HAS cannot be routinely recommended in this cohort. In the REDUCe study, there was a decrease in week 2 serum albumin (g/L) (median, IQR) compared with baseline in the LTAD group as regular HAS was not administered: 29.5 (27.5–31.5) vs 33.33–36 However, serum albumin levels then remained stable until end of study.4 Week 12 serum albumin and serum creatinine were similar in both LTAD and LVP groups.4 Figure 2 summarises the process for LTAD selection and management and table 2 lists the do’s and don’ts.
Figure 2.
Flow chart showing process for long-term abdominal drain insertion and community management (also see online supplemental file 1, for community standard operating procedure). GP, general practitioner; INR, international normalised ratio; LTAD, long-term abdominal drain; LVP, large volume paracentesis; PMN, polymorhonuclear; TIPS, transjugular intrahepatic portosystemic shunt; WCC, white cell count.
6. Potential complications following LTAD insertion
6.1 Patients should not undergo routine post LTAD insertion ascitic fluid sampling and/or clinical blood tests unless there is clinical suspicion of peritonitis.
6.2 LTAD removal is not necessarily required in patients who develop peritonitis.
6.3 Episodes of leakage and cellulitis are typically self-limiting and do not usually require LTAD removal.
6.4 Patients should be provided with written information describing LTAD management in case of an out-of-hours hospitalisation.
Peritonitis remains the main concern following LTAD insertion. In malignant ascites, tunnelled catheters reduce the risk of peritonitis (tunnelled vs non-tunnelled catheters 4.4% vs 21 %).39 In a recent systematic review assessing LTAD in cirrhosis, peritonitis rates were 12.7%, the LTADs being removed in about half.6 In the REDUCe study, peritonitis incidence in LTAD vs LVP group were 6% (1/17) vs 11% (2/194 (table 1)). We would not recommend routine sampling of ascitic fluid in asymptomatic patients as colonisation is almost universal after LTAD insertion,56 the clinical significance of which remains unknown. Therefore, after LTAD insertion, only symptomatic patients (fever, abdominal pain, worsening hepatic decompensation or renal function) should be screened and treated for suspected infection/peritonitis as clinically appropriate. In those with suspected peritonitis, a sample should be taken from both the LTAD and via a separate ascitic tap. Removal of LTAD may not be necessary in all cases of peritonitis. Leakage and cellulitis post LTAD insertion are usually self-limiting with antibiotic treatment, rates being 8% and 6%, respectively, in the systematic review,6 consistent with a recent case series (3%).5 In the REDUCe study, a higher incidence of cellulitis/leakage was observed (41%), though all were self-limiting.4 Strategies to reduce leakage include: draining ascites to dryness following insertion, ensuring incisions are of appropriate size (may require a suture if too large) and ensuring that the tunnelled portion of the LTAD is not under undue tension.
Non-infectious LTAD complications such as catheter blockage and displacement are rare (6% and 1%, respectively),6 bleeding is also very uncommon, only two cases being reported in the systematic review,6 none of these complications observed in the feasibility trial.4 In the afore-mentioned systematic review,6 increase in serum creatinine was observed in 8%. In the REDUCe trial,4 mean serum creatinine remained stable in both groups (table 3). All patients should be provided with written information regarding LTAD management to assist medical teams in the event of an out of hours hospitalisation (see online supplemental file 2).
Table 3.
Potential long-term abdominal drain (LTAD)-related complications when used in end-stage liver disease
| Complication | Recommended management | Incidence observed in the REDUCe trial (LTAD vs LVP)5 |
| Leakage | Usually self-limiting, if persists may need an extra suture. Continue ascites drainage via LTAD | Leakage/cellulitis 41% vs 11% |
| Cellulitis | Usually results due to leakage and is again self-limiting. If persist may need a short course of antibiotics. Very rarely LTAD needs to be removed and can be resited | |
| Suspected peritonitis | Do a diagnostic tap for cell count and culture from peritoneum as well as taking sample from LTAD. Treat as per usual peritonitis guidelines. Decision to remove LTAD must be made on a case by case basis after discussion with patient/caregiver Routine sampling of ascitic fluid from LTAD and or routine blood tests in asymptomatic patients is not recommended. |
6% vs 11% |
| Elevation in serum creatinine | Manage as clinically indicated | Baseline and week 12 serum creatinine (μmol/L) (median, IQR) LTAD vs LVP groups: 109 (79–141) vs 113.5 (89–134) and 104.5 (81–115.5) vs127(63–158), respectively. |
| LTAD blockage | Admit to hospital and discuss need for replacement | 0% |
| LTAD displacement | Admit to hospital if necessary and discuss need for replacement | 6% |
| Bleeding | Usually self-limiting | 0% vs 5% |
| Unable to manage ascites symptoms despite draining 1–2 L three times a week from LTAD | Will need LVP in hospital—drain ascitic fluid via LTAD using adaptor with human albumin solution as per standard LVP protocols | 13% |
LVP, large volume paracentesis.
flgastro-2022-102128supp002.pdf (87.3KB, pdf)
Conclusions
Development of refractory ascites in advanced cirrhosis is a difficult time in the lives of patients and their caregivers as most are coming to terms with entering a palliative phase of their illness. Palliative interventions for refractory ascites remain a clear unmet need. Data from a recent small trial provides preliminary evidence of LTAD safety, efficacy, acceptability and cost-effectiveness. These results, however, need to be confirmed by the future definitive trial. Not all patients will be suitable for palliative LTAD, some preferring hospital-based LVPs, this being their only opportunity for social interaction. The complexities of a palliative intervention that crosses healthcare boundaries cannot be underestimated. The key to successful implementation of LTAD will be collaborative working between the hospital, community (including palliative services), primary care, patients and their caregivers. The future national LTAD study, besides providing definitive evidence, will increase knowledge, skills and confidence in managing advanced cirrhosis out of hospital, through shared learning between primary and secondary care. Hopefully this will improve palliative care for this disenfranchised and under-researched cohort.
Footnotes
Twitter: @jomcDonagh2, @NeilRaj1, @MhairiDonnelly, @marktheliverdoc
Contributors: SV and LM wrote the first draft with input from BH. MC, WP, FF, JM, NR, CS, MD, CE, BG, MW provided critical revisions. JB and SS provided service user perspective. All coauthors reviewed and approved the final draft of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SV: Rocket Medical plc provided the LTAD free of cost for the REDUCe trial. They were not involved in data collection or preparation of manuscript and nor will they be claiming any intellectual property based on the trial.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Public Health England . The 2nd atlas of variation in risk factors and healthcare for liver disease in England, 2017. Available: https://fingertips.phe.org.uk/profile/atlas-of-variation
- 2. Quarterly alcohol-specific deaths in England and Wales: 2001-2009 registrations and quarter 1 (Jan to Mar) to quarter 4 (Oct to Dec) 2020 provisional registrations. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/quarterlyalcoholspecificdeathsinenglandandwales/2001to2019registrationsandquarter1jantomartoquarter4octtodec2020provisionalregistrations
- 3. Macken L, Mason L, Evans C. Palliative long-term abdominal drains versus repeated drainage in individuals with untreatable ascites due to advanced cirrhosis: study protocol for a feasibility randomised controlled trial. Trials 2018;19:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macken L, Bremner S, Gage H. Randomised clinical trial: palliative long-term abdominal drains versus large volume paracentesis in refractory ascites due to cirrhosis. Aliment Pharmacol Ther 2020;52:107–22. [DOI] [PubMed] [Google Scholar]
- 5. Corrigan M, Thomas R, McDonagh J. Tunnelled peritoneal drainage catheter placement for the palliative management of refractory ascites in patients with liver cirrhosis. Frontline Gastroenterol 2020;12:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macken L, Hashim A, Mason L. Permanent indwelling peritoneal catheters for palliation of refractory ascites in end stage liver disease: a systematic review. Liver Int 2019;39:1594–607. [DOI] [PubMed] [Google Scholar]
- 7. Ginés P, Quintero E, Arroyo V. Compensated cirrhosis: natural history and prognostic factors. Hepatol 1987;7:122–8. [DOI] [PubMed] [Google Scholar]
- 8. Lucena MI, Andrade RJ, Tognoni G. Spanish collaborative Study Group on therapeutic management in liver disease. multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol 2002;58:435–40. [DOI] [PubMed] [Google Scholar]
- 9. Salerno F, Borroni G, Moser P. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol 1993;88:514–9. [PubMed] [Google Scholar]
- 10. Macken L, Hashim A, Potts J. Care of patients with end stage liver disease and refractory ascites remains suboptimal: need for earlier input from palliative care. Gut 2017;66:A161. [Google Scholar]
- 11. Moore KP, Wong F, Gines P. The management of ascites in cirrhosis: report on the consensus conference of the International ascites Club. Hepatol 2003;38:258–66. [DOI] [PubMed] [Google Scholar]
- 12. Arroyo V, Gines P, Gerbes AL. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club.Hepatol 1996;23:164–76. [DOI] [PubMed] [Google Scholar]
- 13. Moreau R, Delègue P, Pessione F. Clinical characteristics and outcome of patients with cirrhosis and refractory ascites. Liver Int 2004;24:457–64. [DOI] [PubMed] [Google Scholar]
- 14. Medici V, Rossaro L, Wegelin JA. The utility of the Model for End-Stage Liver Disease Score - A reliable guide for liver transplant candidacy and, for select patients, simultaneous hospice referral. Liver Transpl 2008;14:1100–6. [DOI] [PubMed] [Google Scholar]
- 15. Subcutaneous automated low-flow pump implantation for refractory ascites caused by cirrhosis Interventional procedures guidance [IPG631].
- 16. Aithal GP, Palaniyappan N, China L, et al. Guidelines on the management of ascites in cirrhosis. Gut 2021;70:9–29. 10.1136/gutjnl-2020-321790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hudson B, Round J, Georgeson B, et al. Cirrhosis with ascites in the last year of life: a nationwide analysis of factors shaping costs, health-care use, and place of death in England. Lancet Gastroenterol Hepatol 2018;3:95–103. 10.1016/S2468-1253(17)30362-X [DOI] [PubMed] [Google Scholar]
- 18. Peng J-K, Hepgul N, Higginson IJ, et al. Symptom prevalence and quality of life of patients with end-stage liver disease: a systematic review and meta-analysis. Palliat Med 2019;33:24–36. 10.1177/0269216318807051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther 2009;30:469–76. 10.1111/j.1365-2036.2009.04061.x [DOI] [PubMed] [Google Scholar]
- 20. Björnsson E, Verbaan H, Oksanen A, et al. Health-related quality of life in patients with different stages of liver disease induced by hepatitis C. Scand J Gastroenterol 2009;44:878–87. 10.1080/00365520902898135 [DOI] [PubMed] [Google Scholar]
- 21. Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology 2001;120:170–8. 10.1053/gast.2001.21193 [DOI] [PubMed] [Google Scholar]
- 22. Younossi ZM, Boparai N, Price LL, et al. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol 2001;96:2199–205. 10.1111/j.1572-0241.2001.03956.x [DOI] [PubMed] [Google Scholar]
- 23. Hansen L, Chang MF, Lee CS, et al. Physical and mental quality of life in patients with end-stage liver disease and their informal caregivers. Clin Gastroenterol Hepatol 2021;19:155–61. 10.1016/j.cgh.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Les I, Doval E, Flavià M, et al. Quality of life in cirrhosis is related to potentially treatable factors. Eur J Gastroenterol Hepatol 2010;22:221–7. 10.1097/MEG.0b013e3283319975 [DOI] [PubMed] [Google Scholar]
- 25. Jara M, Bednarsch J, Malinowski M, et al. Predictors of quality of life in patients evaluated for liver transplantation. Clin Transplant 2014;28:1331–8. 10.1111/ctr.12426 [DOI] [PubMed] [Google Scholar]
- 26. Kok B, Whitlock R, Ferguson T, et al. Health-Related quality of life: a rapid predictor of hospitalization in patients with cirrhosis. Am J Gastroenterol 2020;115:575–83. 10.14309/ajg.0000000000000545 [DOI] [PubMed] [Google Scholar]
- 27. Macdonald S, Jepsen P, Alrubaiy L, et al. Quality of life measures predict mortality in patients with cirrhosis and severe ascites. Aliment Pharmacol Ther 2019;49:321–30. 10.1111/apt.15084 [DOI] [PubMed] [Google Scholar]
- 28. Poonja Z, Brisebois A, van Zanten SV, et al. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol 2014;12:692–8. 10.1016/j.cgh.2013.08.027 [DOI] [PubMed] [Google Scholar]
- 29. Patel AA, Walling AM, Ricks-Oddie J, et al. Palliative care and health care utilization for patients with end-stage liver disease at the end of life. Clin Gastroenterol Hepatol 2017;15:1612–9. 10.1016/j.cgh.2017.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holden JH, Shamseddeen H, Johnson AW, et al. Palliative care and hospice referrals in patients with decompensated cirrhosis: what factors are important? J Palliat Med 2020;23:1066–75. 10.1089/jpm.2019.0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kathpalia P, Smith A, Lai JC. Underutilization of palliative care services in the liver transplant population. World J Transplant 2016;6:594–8. 10.5500/wjt.v6.i3.594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baumann AJ, Wheeler DS, James M, et al. Benefit of early palliative care intervention in end-stage liver disease patients awaiting liver transplantation. J Pain Symptom Manage 2015;50:882–6. 10.1016/j.jpainsymman.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 33. Kimbell B, Murray SA, Byrne H, et al. Palliative care for people with advanced liver disease: a feasibility trial of a supportive care liver nurse specialist. Palliat Med 2018;32:919–29. 10.1177/0269216318760441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ufere NN, O'Riordan DL, Bischoff KE, et al. Outcomes of palliative care consultations for hospitalized patients with liver disease. J Pain Symptom Manage 2019;58:766–73. 10.1016/j.jpainsymman.2019.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ufere NN, Robinson B, Donlan J, et al. Pilot randomized controlled trial of an advance care planning video decision tool for patients with advanced liver disease. Clin Gastroenterol Hepatol 2021. 10.1016/j.cgh.2021.10.027. [Epub ahead of print: 27 Oct 2021]. [DOI] [PubMed] [Google Scholar]
- 36. Shinall MC, Karlekar M, Martin S, et al. Compass: a pilot trial of an early palliative care intervention for patients with end-stage liver disease. J Pain Symptom Manage 2019;58:614–22. 10.1016/j.jpainsymman.2019.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng J-K, Higginson IJ, Gao W. Place of death and factors associated with hospital death in patients who have died from liver disease in England: a national population-based study. Lancet Gastroenterol Hepatol 2019;4:52–62. 10.1016/S2468-1253(18)30379-0 [DOI] [PubMed] [Google Scholar]
- 38. End of life care CCG profile . Cause of death and place of death, 2015. Available: http://www.endoflifecareintelligence.org.uk/profiles/CCGs/PlaceandCauseofDeath/atlas.html
- 39. Fleming ND, Alvarez-Secord A, Von Gruenigen V, et al. Indwelling catheters for the management of refractory malignant ascites: a systematic literature overview and retrospective chart review. J Pain Symptom Manage 2009;38:341–9. 10.1016/j.jpainsymman.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 40. White J, Carolan-Rees G. PleurX peritoneal catheter drainage system for vacuum-assisted drainage of treatment-resistant, recurrent malignant ascites: a NICE medical technology guidance. Appl Health Econ Health Policy 2012;10:299–308. 10.1007/BF03261864 [DOI] [PubMed] [Google Scholar]
- 41. Stukan M. Drainage of malignant ascites: patient selection and perspectives. Cancer Management Res 2017;9:115–30. 10.2147/CMAR.S100210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caldwell J, Edriss H, Nugent K. Chronic peritoneal indwelling catheters for the management of malignant and nonmalignant ascites. Proc 2018;31:297–302. 10.1080/08998280.2018.1461525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abbot J, Verma S, Saxsena A. Long term abdominal drain for palliation in advanced liver cirrhosis: a survey of risks & barriers. Gut 2020;69:A7. [Google Scholar]
- 44. Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses [Internet]. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 45. Cooper M, Pollard A, Pandey A, et al. Palliative long-term abdominal drains versus large volume paracentesis in refractory ascites due to cirrhosis (REDUCe study): qualitative outcomes. J Pain Symptom Manage 2021;62:312–25. 10.1016/j.jpainsymman.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 46. Rogal SS, Hansen L, Patel A, et al. AASLD practice guidance: palliative care and symptom-based management in decompensated cirrhosis. Hepatology 2022. 10.1002/hep.32378. [Epub ahead of print: 01 Feb 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Solbach P, Höner Zu Siederdissen C, Taubert R, et al. Home-based drainage of refractory ascites by a permanent-tunneled peritoneal catheter can safely replace large-volume paracentesis. Eur J Gastroenterol Hepatol 2017;29:539–46. 10.1097/MEG.0000000000000837 [DOI] [PubMed] [Google Scholar]
- 48., European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. 10.1016/j.jhep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 49. Patel IJ, Rahim S, Davidson JC, et al. Society of interventional radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided Interventions-Part II: recommendations: endorsed by the Canadian association for interventional radiology and the cardiovascular and interventional radiological Society of Europe. J Vasc Interv Radiol 2019;30:1168–84. 10.1016/j.jvir.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 50. Harrison P, Hogan BJ, Floros L, et al. Assessment and management of cirrhosis in people older than 16 years: summary of NICE guidance. BMJ 2016;354:2850. 10.1136/bmj.i2850 [DOI] [PubMed] [Google Scholar]
- 51. Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol 2015;62:1056–60. 10.1016/j.jhep.2014.11.036 [DOI] [PubMed] [Google Scholar]
- 52. Bruns T, Lutz P, Stallmach A, et al. Low ascitic fluid protein does not indicate an increased risk for spontaneous bacterial peritonitis in current cohorts. J Hepatol 2015;63:527–8. 10.1016/j.jhep.2015.03.040 [DOI] [PubMed] [Google Scholar]
- 53. Reinglas J, Amjadi K, Petrcich B, et al. The palliative management of refractory cirrhotic ascites using the PleurX (©) catheter. Can J Gastroenterol Hepatol 2016;2016:1–7. 10.1155/2016/4680543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391:2417–29. 10.1016/S0140-6736(18)30840-7 [DOI] [PubMed] [Google Scholar]
- 55. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol 2018;69:1250–9. 10.1016/j.jhep.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 56. Kimer N, Riedel AN, Hobolth L, et al. Tunneled peritoneal catheter for refractory ascites in cirrhosis: a randomized Case-Series. Medicina 2020;56:565. 10.3390/medicina56110565 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2022-102128supp001.pdf (118.2KB, pdf)
flgastro-2022-102128supp002.pdf (87.3KB, pdf)