Abstract
Introduction:
Rotavirus is the leading cause of acute diarrhea among children <5 years worldwide. As all children are equally susceptible to infection and disease development, rotavirus vaccination programs are the best upstream approach to preventing rotavirus disease, and the subsequent risk of hospitalization or death.
Areas covered:
We provide an overview of global rotavirus vaccine policy, summarize the burden of rotavirus disease in developing countries, review data on the effectiveness, impact, safety, and the cost-effectiveness of rotavirus vaccination programs, and identify areas for further research and improvement.
Expert opinion:
Rotavirus vaccines continue to be an effective, safe, and cost-effective solution to preventing rotavirus disease. As two new rotavirus vaccines enter the market (Rotasiil and Rotavac) and Asian countries continue to introduce rotavirus vaccines into their national immunization programs, documenting vaccine safety, effectiveness, and impact in these settings will be paramount.
Keywords: Rotavirus, rotavirus vaccine, vaccines, vaccine implementation, low and middle-income countries, developing countries, diarrhea
1. Introduction
In 2013, an estimated 215,000 deaths were attributed to rotavirus infection globally, with 99.8% occurring in developing countries [1]. In contrast to the prevention of all-cause diarrhea mortality, rotavirus is a unique cause of infectious diarrhea as all children are equally susceptible to infection and disease development, independent of location, socioeconomic status, or hygiene and sanitation level [2]. As such, rotavirus vaccination programs are the best upstream approach to preventing rotavirus disease, and the subsequent risk of hospitalization or death. To help encourage the continued use and adoption of rotavirus vaccines, the goal of this review is to provide an up-to-date overview of the global rotavirus vaccine policy, the burden of rotavirus disease in developing countries, the implementation, effectiveness, impact, safety, and cost-effectiveness of rotavirus vaccination programs, and to identify areas for further research and improvement.
2. Global rotavirus vaccine policy
In 2006, the World Health Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) first recommended the inclusion of rotavirus vaccine into national immunization programs in regions with data showing that the vaccine was effective. The recommendation followed two pre-licensure trials conducted in the Americas and Europe that enrolled >60,000 infants each; these trials found no increased risk of intussusception following rotavirus vaccination and exhibited high vaccine efficacy against severe rotavirus disease [3-5]. At the time, implementation of vaccination in developing countries in Africa and Asia was not recommended by WHO due to the lack of available data on rotavirus vaccine efficacy in these regions [3]. Because of a number of factors, including the greater prevalence of concurrent enteric infections, malnutrition, and gut enteropathy, concerns existed that live oral rotavirus vaccines may not perform well in these regions. Indeed, later trials performed in Africa and Asia showed a moderate vaccine efficacy, lower than the initial trials in developed countries [6-8]. However, given that even moderately effective rotavirus vaccines would have a substantial health impact in developing countries given the especially high burden of rotavirus disease, including mortality, SAGE recommended the integration of rotavirus vaccine into all immunization programs worldwide in 2009 [9,10].
Currently, four rotavirus vaccines have been pre-qualified by WHO for use internationally (Table 1). In developing countries, WHO recommends the use of Rotarix vaccine in a 2-dose schedule given at 6 and 10 weeks of age, and RotaTeq, Rotavac, and Rotasiil in a 3-dose schedule at 6, 10, and 14 weeks of age, with vaccine doses given concurrently with other childhood vaccines given at these ages. Because of safety concerns about the risk of intussusception particularly with the first dose of rotavirus vaccine, strict age restrictions were initially placed on the vaccination schedule to help minimize the risk of intussusception [11]. Baseline rates of naturally occurring intussusception are very low in the first two to 3 months of life, rapidly increase and peak at four to 7 months, and then decline, with few cases occurring after 12 months of age [12]. Thus, WHO recommended that the rotavirus vaccine series be started before the child was 15 weeks old [13]. They also recommended that the full vaccine series be completed by 32 weeks of age, as the vaccine had not been tested in children older than this age in the clinical trials. While these recommendations were reasonable from a safety standpoint, they hampered rotavirus vaccine coverage in developing countries where children frequently present at later than recommended ages to receive immunizations, and could be disqualified based on the recommended age windows [11]. Indeed, a mathematical modeling analysis found that, by removing the age restrictions on rotavirus vaccination in developing countries and the consequent increase in vaccine coverage, an additional 47,200 rotavirus deaths could be prevented each year while potentially causing an additional 294 intussusception deaths [14,15]. These findings led WHO to revise its recommendation for rotavirus vaccine use in 2013; WHO currently recommends that the rotavirus vaccine series can be started at 6 weeks of age and subsequent vaccine doses can be given until 24 months of age [13,15]. However, on-time vaccination at 6 and 10 weeks, or 6, 10, and 14 weeks of age is strongly encouraged to achieve protection against rotavirus disease early in life [13].
Table 1.
Rotavirus vaccines currently pre-qualified by the World Health Organization.
| Characteristic | Rotavirus Vaccine | |||
|---|---|---|---|---|
| Trade name | Rotarix | RotaTeq | Rotavac | Rotasiil |
| Manufacturer | GlaxoSmithKline | Merck and Co., Inc. | Bharat Biotech International Limited | Serum Institute of India Pvt. Ltd. |
| Country of manufacture | Belgium | USA | India | India |
| Composition | Live-attenuated G1P1A [8] human rotavirus strain | Live human-bovine reassortant rotavirus strains: G1P7 [5], G2P7 [5], G3P7 [5], G4P7 [5], G6P1A [8] | Live-attenuated G9P [11] human rotavirus strain | Human-bovine reassortant rotavirus strains: G1, G2, G3, G4, G9 |
| Pharmaceutical form | Liquid | Liquid | Liquid | Lyophilized + diluent |
| Presentation | a) 1 dose plastic tube b) 1 dose applicator c) 5 dose plastic tube |
1 dose plastic tube | a) 5 dose vial b) 10 dose vial |
a) 1 dose two vial set b) 2 dose two vial set |
| Route of Administration | Oral | Oral | Oral | Oral |
| Recommended schedule of administration | 1st dose: 6 weeks 2nd dose: 10 weeks |
1st dose: 6 weeks 2nd dose: 10 weeks 3rd dose: 14 weeks |
1st dose: 6 weeks 2nd dose: 10 weeks 3rd dose: 14 weeks |
1st dose: 6 weeks 2nd dose: 10 weeks 3rd dose: 14 weeks |
| Volume per dose | 1.5 ml | 2 ml | 0.5 ml | 2.5 ml |
| Storage temperature | 2–8°C | 2–8°C | −20°C | 2–8°C |
| Shelf life | a) 24 months b) 36 months c) 24 months |
24 months | 60 months | 30 months |
| Cold chain volume (cm3/dose) | a) 17.1–115.3 b) 85.3–134.0 c) 11.8 |
46.3–75.3 | a) 4.2 b) 3.2 |
a) 17.6 b) 10.5 |
| Date of WHO prequalification | 2009, 2019 | 2008 | 2018 | 2018 |
| GAVI 2019–2021 price per dose (USD) | a) 2.29 USD b) 2.29 USD c) - |
_1 | a) 0.85 USD b) 0.85 USD |
a) 1.55 USD b) 0.95 USD |
| GAVI 2020 price per fully immunized person (USD) | a) 4.58 USD b) 4.58 USD c) - |
_1 | a) 2.55 USD b) 2.55 USD |
a) 4.65 USD b) 2.85 USD |
| Indicative Wastage Rate | a) 4% b) 4% |
_1 | a) 23% b) 41% |
a) 4% b) 10% |
| 2020 waste-adjusted price per fully immunized person (USD) | a) 4.77 USD b) 4.77 USD c) - |
_1 | a) 3.31 USD b) 4.32 USD |
a) 4.65 USD b) 3.17 USD |
Underling data source: https://www.gavi.org/library/gavi-documents/supply-procurement/rotavirus-vaccine-profiles/.
RotaTeq was withdrawn from the Gavi market at the end of 2019, therefore Gavi prices and wastage rates are no longer available.
In 2019, various rotavirus vaccine schedules were reevaluated to assess the risk-to-benefit ratio of allowing later vaccination in low- and middle-income countries. While a higher risk-to-benefit ratio was obtained utilizing the original WHO recommendation compared with the new recommendation to relax age restrictions (512:1 vs 148:1 rotavirus deaths averted per excess intussusception deaths), removing the upper age limit resulted in 12,000 fewer rotavirus deaths and 79 additional intussusception deaths per year, supporting the current WHO recommendation [16]. Due to a lack of data on the safety, effectiveness, and cost effectiveness of these relaxed age restrictions, however, some countries still utilize the original vaccine schedule.
3. High burden in developing countries
In 2013, an estimated 214,664 deaths were attributed to rotavirus infection in developing countries. By region, most deaths attributed to rotavirus occurred in Sub-Saharan Africa (121,000), Southern Asia (70,109), and Southeast Asia (10,765) with a lower burden among other regions [1]. Four countries accounted for nearly half of all rotavirus deaths globally, with 47,100 deaths in India, 30,800 in Nigeria, 14,700 in Pakistan, and 13,526 in the Democratic Republic of Congo [1].
In the absence of rotavirus vaccine introduction, 38% of all hospitalized diarrhea cases among <5 children globally are due to rotavirus infection [1,17]. This proportion remains fairly consistent by region, with 38.2%, 37.5%, 35.7%, 36.3%, 37.2%, and 42.3% of diarrhea hospitalizations attributed to rotavirus infection in the African, American, Eastern Mediterranean, European, Southeast Asian, and Western Pacific regions, respectively [17].
4. Vaccine implementation, efficacy, effectiveness, impact, and safety
4.1. Vaccine implementation
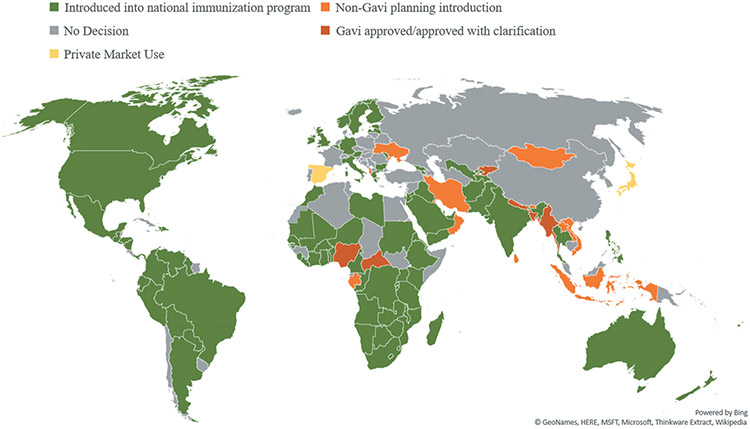
As of December 2019, 100 countries have introduced a rotavirus vaccine into their national immunization programs, with 48 countries having introduced rotavirus vaccine with support from Gavi, the Vaccine Alliance. An additional 6 low-income countries with a gross national income of <$1,580 USD per capita have been approved for funding support for vaccine introduction from Gavi and are awaiting a national introduction, and another 13 countries are preparing to introduce independent of Gavi support (Figure 1). Given that only 47 countries had introduced rotavirus vaccine into their national immunization program by 2012, including only one country in Africa and Asia, remarkable progress has been made in vaccine implementation over the last few years, particularly in low-income countries eligible for Gavi support [18,19].
Figure 1.
National rotavirus vaccine introduction, by geographic region – as of December 2019. Underlying data source: http://view-hub.org/viz/.
4.2. Vaccine efficacy and effectiveness
In the preliminary clinical trials in developed countries (US, Latin America, and Europe) RotaTeq and Rotarix had an efficacy of 85–98% at preventing severe rotavirus disease [2,4,5,20]. However, mirroring the findings from other oral vaccines (e.g. cholera, polio), initial efficacy studies in Africa and Asia found that rotavirus vaccines were less effective at preventing severe rotavirus disease (39–77%) when compared to developed countries, with wide variation by region [8,11,20-29]. Later systematic reviews using clinical trial data have found efficacies of 90.6% in developed countries, compared with 88.4% in East and Southeast Asia, 79.6% in Latin America and the Caribbean, 50.0% in Southern Asia, and 46.1% in Sub-Saharan Africa [30].
A systematic review of observational studies stratified by region aligned with these findings, with rotavirus vaccines 88.9% effective at preventing rotavirus hospitalizations in developed regions, compared to 67.6% in Latin America and the Caribbean and 57.0% in Sub-Saharan Africa [30]. When stratified by vaccine similar findings were observed. In a systematic review that analyzed post-licensure vaccine effectiveness data stratified by the countries’ childhood mortality rates, Rotarix had a vaccine effectiveness of 84% (13 studies), 75% (8 studies), and 57% (9 studies) in countries with low, medium, and high childhood mortality rates, while RotaTeq had a vaccine effectiveness of 90% (20 studies) and 45% (7 studies) in countries with low and high child mortality rates, respectively, [31].
Currently, no data have been published on the effectiveness of Rotavac or Rotasiil in any developed or developing countries; however, efficacy data from three clinical trials are available. Rotavac’s clinical trial conducted in India showed the vaccine to be 53.6% effective at preventing severe rotavirus gastroenteritis [32]. Rotasiil’s trials, conducted in Niger and India, found the vaccine efficacy against severe rotavirus gastroenteritis to be 66.7% and 39.5%, respectively, [33,34].
Of note, in addition to lower vaccine efficacy and effectiveness, vaccine efficacy wanes more rapidly over time in developing countries [8,22,35-37]. A recent meta-analysis combined follow-up data from 50 studies in low (15 studies), medium (11 studies), and high (24 studies) childhood mortality strata to examine the efficacy of rotavirus vaccines by follow-up duration [38]. In low mortality settings, efficacy was 98% at 2 weeks, 94% at 12 months, and 91% at 60 months, in medium mortality settings efficacy was 82% at 2 weeks, 77% at 12 months, and 75% at 60 months, and in high mortality settings efficacy was 66% at 2 weeks, 44% at 12 months, and 30% at 60 months [38].
There are a number of possible explanations for why rotavirus vaccine effectiveness is lower and wanes more rapidly in developing countries, including: higher transmission rates of rotavirus, differences in gut microbiota, host mucosal factors, inhibitory effect of higher maternal antibodies in serum or breast milk, higher rates of malnutrition, micronutrient deficiencies (specifically zinc, vitamin A, or vitamin D), interference from other concurrent enteric viruses or helminth infections, microbial overload of mucosal surfaces, presence of other comorbidities, and co-administration with oral polio vaccine [2,18,29,39-42].
While the exact effect that many of these factors have on vaccine effectiveness is still inconclusive, or have been shown to have a minimal effect (breastfeeding/maternal antibodies in breast milk [43], zinc or probiotic supplementation at the time of vaccination) [44], some interventions (alternative immunization schedules [11,45], staggered administration of oral rotavirus and poliovirus vaccines) [46-48] have been shown to improve seroconversion rates by 15–20%, but they are hard to translate into practical recommendations for programmatic use [49].
4.3. Vaccine impact
4.3.1. Global
Utilizing data from WHO’s Global Rotavirus Surveillance Network (GRSN), including rotavirus test results from 305,789 children enrolled at 198 sites in 69 countries, rotavirus vaccine introduction has reduced the global proportion of hospitalized diarrhea cases attributed to rotavirus among children <5 years from 38.0% in the pre-vaccine period, to 23.0% in the post-vaccine period, a 39.6% relative decline [17]. While the magnitude of the decline varies by WHO region, due to varying vaccine effectiveness and vaccine coverage, overall reductions remain relatively consistent with a 34.5% relative decline in Africa, 39.6% in the Americas, 26.4% in Eastern Mediterranean, and 55.2% in the European Region [17].
A systematic review utilizing data from 57 studies and 27 countries globally found even greater declines after vaccine introduction, with a median 38% reduction in all-cause AGE hospitalizations and/or ED visits among children <5 years overall, and a 41%, 30%, and 46% reduction in countries with low, medium, and high child mortality strata, respectively, [50]. AGE mortality among children <5 years also declined 42% overall (medium and high mortality strata only), with a 50% decline in medium and 36% decline in high child mortality strata [50]. When looking at rotavirus-specific hospitalizations and/or ED visits among children <5 years, the impact was even greater with a 67% overall decline, and 71%, 59%, and 60% decline in countries with low, medium, and high child mortality strata, respectively, [50].
Of note, in these impact evaluations, and those discussed later in this article, the role of rotavirus vaccine is supported by the sharp declines observed after vaccine introduction, the greater declines that occurred during the months of the year corresponding to the rotavirus season, and the larger impact first observed among younger vaccine-eligible children before expanding to older age groups [40].
4.3.2. Latin America
When looking at specific regions, a recent systematic review utilizing data from 31 impact studies in Latin America and the Caribbean found that among countries that had implemented rotavirus vaccine, rotavirus hospitalizations declined 64%, AGE hospitalizations declined 32%, and AGE related mortality declined 54% among children <5 years [51]. As a result of this, in 2015, Chavers et al., estimated that 123,000 rotavirus hospitalizations and 660 rotavirus-related deaths were prevented among the 15 countries that had implemented rotavirus vaccine in Latin America. They additionally estimated that if all Latin American countries had introduced by 2015, an additional 18,000 rotavirus hospitalizations and 50 rotavirus-related deaths could have been prevented that year [51]. Another study using data from WHO’s GRSN found similar results. Pooling rotavirus testing data from 60,433 individuals across 14 Latin American countries, rotavirus positivity among hospitalized AGE cases declined from 37.5% to 22.7% after vaccine introduction, and 39.6% reduction [17].
4.3.3. Africa
A review by Shah et al. estimated the impact of rotavirus vaccine introduction in Africa, and found that among the 29 African countries that had introduced rotavirus vaccine by 2015, there was a 47% reduction in the number of rotavirus hospitalizations and a 39% reduction in rotavirus-related deaths when compared to the burden estimates from 2013 [52]. As a result, among countries that had introduced the vaccine an estimated 130,000 rotavirus hospitalizations and 21,000 rotavirus-related deaths were prevented in 2016 among children <5 years [52]. If all countries in Africa had introduced rotavirus vaccine, an additional 139,000 hospitalizations and 27,000 deaths could have been prevented that year [52]. These findings align closely with those from WHO’s GRSN, which using data from 24 countries in Africa and 56,301 individuals found that rotavirus positive declined after vaccine introduction, from 38.2% among hospitalized AGE cases to 25.0%, a 34.5% decline [17]. Of note, the declines in rotavirus hospitalizations vary from country to country, ranging from 23% to 76% among children <5, and 28–82% among children <1 year of age [53].
4.3.4. European region
In developed countries in Europe, a review found that rotavirus hospitalizations declined 65–84% among countries that introduced rotavirus vaccine, however, no developing countries were included in this review [54]. Of the four developing countries in Europe who have introduced the rotavirus vaccine, no impact data are available for two countries (Bulgaria – introduced 2017, Georgia – introduced 2013). Data are available for the Republic of Moldova and the Republic of Armenia, both of which introduced rotavirus vaccine in 2012. In Moldova, the percent of diarrhea hospital admissions positive for rotavirus among children <5 years fell 67% 2 years after vaccine introduction [27]. In Armenia, near identical declines were observed with rotavirus hospital admissions reduced 69% among children <5 years of age in the 3 years after vaccine introduction [55]. Additionally, in Armenia over 30% of the reduction was observed among children who were not age eligible to receive the vaccine, suggesting herd immunity [55].
4.3.5. Asia
Since countries in Asia have been delayed in implementing rotavirus vaccines into their national immunization programs, no data on vaccine impact currently exists for any Asian country [17,40]. To help encourage the implementation of rotavirus vaccine in Asian countries, Burnett et al. estimated the potential impact of rotavirus vaccine in Asia if all countries introduced. They estimated that 1,452,257 rotavirus hospitalizations and 88,889 rotavirus-related deaths occur annually in Asia. However, with universal rotavirus vaccine introduction, an estimated 710,580 rotavirus hospitalizations and 35,865 rotavirus-related deaths would be prevented annually [56].
4.4. Vaccine safety
Because the first commercial rotavirus vaccine (RotaShield) was introduced and subsequently withdrawn from the market due to an association with intussusception, this adverse event has been closely monitored with current vaccines through large safety studies and post-marketing surveillance [57]. The initial clinical trials for Rotarix and RotaTeq both enrolled 60,000–70,000 infants in the United States, Europe, and Latin America and did not identify any increased risk of intussusception in the 30–42 day window post vaccination [4,5,57]. However, later post-marketing evaluations conducted in Australia and the Americas have identified a slight increase in risk of intussusception 1–7 days following vaccination for both Rotarix and RotaTeq at a rate of 1 excess case per 20,000–100,000 vaccinated infants [57-60]. Given that the benefits greatly exceed the risk, these findings have not resulted in any vaccine policy change in these countries (Table 2).
Table 2.
Annual benefits and risks associated with rotavirus vaccination in Mexico, Brazil, Australia, the United States, and England.
| Country | Diarrhea hospitalizations prevented by vaccination |
Diarrhea deaths prevented by vaccination |
Intussusception cases potentially caused by vaccination |
Intussusception deaths potentially caused by vaccination |
Reference |
|---|---|---|---|---|---|
| Mexico | 11,600 | 663 | 41 | 2 | [58] |
| Brazil | 69,600 | 640 | 55 | 3 | [58] |
| Australia | 7,000 | 0 | 14 | 0 | [61] |
| United States 1 | 53,444 | 14 | 45–213 | 0.1–0.5 | [62] |
| England | 13,000 | 3 | 35 | 0 | [63] |
data for one vaccinated birth cohort followed until age 5.
To evaluate the risk of intussusception after Rotarix vaccination among children residing in Sub-Saharan Africa, two active surveillance systems were set up to enroll intussusception cases that occurred in Ethiopia, Ghana, Kenya, Malawi, Tanzania, Zambia, Zimbabwe, and South Africa [64,65]. In contrast to what has been observed in developed countries, both studies found no increased risk of intussusception associated with either dose of Rotarix [64,65].
While the exact cause for the lack of association between intussusception risk and rotavirus vaccination in developing countries is unknown, there are a few possible explanations. First, intussusception risk is linked to the high viral replication of the vaccine virus after vaccination. As rotavirus vaccine has lower effectiveness in these settings (and is commonly coadministered with oral poliovirus vaccine which further reduces its effectiveness), there may be lower viral replication occurring in the guts of children in developing countries and thus a subsequently lower intussusception risk [46,48,64]. Second, the first dose of rotavirus vaccine is given at an earlier age in these developing compared to developed countries (6 weeks vs 2 months) when children have a naturally lower baseline risk of intussusception when they are vaccinated, resulting in lower overall risk [12,64,65]. Finally, it could be due to other mechanisms for which lower intussusception risk is not known (e.g. differences in microbiome, maternal antibodies, diet, or breastfeeding practices) [64]. Because of the lag in adoption of rotavirus vaccines by Asian countries, no post-marketing data currently exists on the intussusception risk following vaccination for infants residing in Asia [40].
To date, there has been no increased intussusception risk associated with the administration of Rotavac or Rotasiil; however, with only 3,500–7,500 children enrolled in the clinical trials, these studies were not powered to detect the level of risk observed with Rotarix and RotaTeq [32-34]. As such, post-marketing surveillance data is needed to monitor intussusception risk as countries begin to introduce these vaccines.
The WHO Global Advisory Committee on Vaccine Safety has reviewed all of this data and reaffirmed the recommendation for continued rotavirus vaccine use. While there is a slight risk associated with intussusception in some settings, the benefits of vaccination far outweigh the observed risks [11,15,57].
5. Cost-effectiveness
Current Gavi prices for Rotarix, Rotavac, and Rotasiil range from 0.85 USD-$2.29 USD per dose (Table 1; Merck supplied RotaTeq to Gavi from 2012 to 2019). While vaccine prices decline when multidose vials are purchased, this can lead to higher wastage rates as a vaccine container must be fully used the day it is opened, or the remaining doses are discarded at the end of the day. Accounting for the wastage rates and the number of doses needed for each vaccine, prices to fully immunize a child against rotavirus range from 3.17 USD-$4.77 USD with Gavi prices (Table 1).
When looking at all countries, previous work has found that at 5 USD per dose, the cost-effectiveness of rotavirus vaccine per disability-adjusted life year (DALY) averted in low, low-middle, and upper-middle-income countries was 88, USD 291, USD, and 329 USD USD, respectively, [66]. Debellut et al. evaluated the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi eligible or formerly eligible countries from 2018 to 2027 [67]. This work found that rotavirus vaccine would prevent 158.6 million cases of rotavirus gastroenteritis, 80.7 million outpatient visits, 7.9 million hospitalizations and 576,567 deaths over the 10-year period, and in turn, would avert 14.7 million DALYs [67]. For all countries included in the analysis the cost-effectiveness on average was 325 USD per DALY averted for the entire time period, ranging from a low of 195 USD for countries in the African region to a high of 1158 USD for countries in the Americas [67]. In this analysis, the cost per DALY averted was on average was 0.16 times a country’s GDP per capita (GDP used as the threshold for DALY cost-effectiveness), four countries, however, did have costs exceeding 1.0 times GDP per capita, including; Armenia, Honduras, Moldova, and Ukraine. This is likely due to the fact that these countries are entirely self-financing their rotavirus vaccine purchases [67]. Of note, other studies have occasionally found rotavirus vaccine not to be cost effective, but this generally occurs when only direct medical costs are considered, and not those incurred by the family or society [18].
6. Expert Opinion
Rotavirus vaccines continue to be the most effective, safe, and cost-effective solution to preventing rotavirus disease in developing countries. To ensure the continued introduction and sustained use of rotavirus vaccines; however, work must continue in a number of areas.
6.1. Generating effectiveness and safety data in Asia
Although efficacy data exist for rotavirus vaccine’s performance in low-income Asian countries, no data currently exists on rotavirus vaccine’s effectiveness, safety, or impact in routine use in Asian countries. Given regional and socioeconomic variability in rotavirus vaccine performance, as Asian countries continue to introduce rotavirus vaccines, gathering, analyzing, and distributing these data will be paramount [17,40]. This will allow for better informed risk-to-benefit assessments, which in turn will aid countries in their decision to introduce and/or continue to use the rotavirus vaccine.
6.2. Generating effectiveness and safety data for Rotavac and Rotasiil
As the demand for rotavirus vaccines among Gavi eligible countries is projected to increase to 66 million doses per year, the addition of Rotavac and Rotasiil to the marketplace should help increase vaccine availability, limit stockouts, and help maintain affordable vaccine prices [49]. However, minimal data currently exists on their effectiveness and safety in routine use. With the introduction of both vaccines in India and recent and impending introduction of these Indian-manufactured vaccines in several low and middle-income countries globally, the first reports of their real-world effectiveness and safety should be available soon. These data will allow countries to be better informed when selecting a vaccine for introduction or advocating for its continued use.
6.3. Ensuring vaccine affordability
One emerging threat to the continued use and implementation of rotavirus vaccines is their cost. While 45 countries are currently purchasing rotavirus vaccine through Gavi, after their gross national income per capita surpasses 1,580 USD, they begin to enter a transition phase and after 5 years assume the full financial responsibility of their vaccination program [68]. Unfortunately, prices over 1 USD per dose in the absence of Gavi likely mean that once countries are no longer eligible for subsidized vaccine prices, the vaccination program may become unsustainable [29]. As the cost-effectiveness of a vaccine is almost entirely dictated by its price, additional reductions in market prices are essential to ensure that these countries will be able to maintain their vaccination programs [68,69]. To help ease this transition, some rotavirus vaccine manufacturers (e.g. GSK) have agreed to maintain the Gavi level discounted prices after countries graduate from Gavi assistance, locking prices at Gavi levels for 10 years. In addition, the recent introduction of Rotavac and Rotasiil into the market should help keep vaccine prices low by adding to market pressure and help ensure an adequate vaccine supply globally [47]. Despite these improvements, however, further reductions in vaccine prices are essential to ensure that countries will be able to maintain their vaccination programs after co-financing has ended [11].
Among non-Gavi eligible countries, or those who are transitioning from Gavi assistance, another barrier to vaccine introduction and use is transparency in vaccine pricing. While prices for Gavi eligible countries are easily accessible, many countries that have not introduced have assumed that prices would be the same as those paid by developed countries, or prices that were reflected in the private market. Based on this feedback, organizing ways for countries to join a pooled purchase group (similar to PAHO’s revolving fund) or setting up a reliable source for vaccine pricing, procurement options, and suppliers (particularly in Asia and Africa) could aid additional countries in introducing rotavirus vaccine in the future [69].
6.4. Expanding cold-chain capacity
Even with favorable cost-effectiveness analyses and pricing, many countries who are planning to introduce a rotavirus vaccine may face challenges due to a lack of cold-chain storage capacity. Because of the large packaging volume of the vaccine, many countries do not have space to accommodate the vaccine once introduced [29]. Unfortunately, the highest burden of rotavirus disease is also in countries with the lowest ability to store and effectively give the vaccine. To ensure vaccines are not wasted, understanding the cold-chain storage capacity and resources required prior to procuring the vaccine is essential [70]. Continued efforts should be directed toward the expansion of the cold-chain capacity in developing countries, and adequately evaluating a country’s capacity prior to vaccine introduction. Of note, this issue could also be relieved by the further development and production of heat-stable vaccines, such as the one developed by Serum Institute of India (stable for 2 years at 37°C or 6 months at 40°C) [34].
6.5. Understanding indirect protection
In developed settings, large reductions in rotavirus disease have been documented among children who were unvaccinated and among age groups too old to be vaccinated (herd protection), implicating infants as the primary transmitters of rotavirus infection [18,20]. These benefits are of lesser importance in areas with high vaccine effectiveness and uptake (developed countries), but could help swing the pendulum further in favor of rotavirus vaccination in areas where vaccine effectiveness and coverage may be lower [20,40]. Data on indirect protection in developing countries is less clear, and additional work is needed to better understand the extent of herd protection in these settings [40,71,72].
6.6. Improving vaccine performance in developing countries
As previously discussed, rotavirus vaccine’s effectiveness and the duration of immunity in developing countries are nearly half of that observed in developed countries. Fortunately, there are a number of potential interventions that can be explored (e.g. staggering poliovirus and rotavirus vaccine administration, providing catch-up doses, transitioning from oral to microneedle skin patch vaccinations, parenteral vaccination) that could improve vaccine performance [49,73,74]. Given that over 85% of the world’s children live in developing countries, even interventions that offer small increases in vaccine performance could have a dramatic impact on alleviating the burden of rotavirus disease globally. Improvements in vaccine performance could also help ensure that rotavirus vaccine remains the most cost-effective solution to preventing rotavirus disease.
6.7. Conclusion
As two new rotavirus vaccines enter the market (Rotasiil and Rotavac) and Asian countries continue to introduce rotavirus vaccines into their national immunization programs, documenting vaccine safety, effectiveness, and impact in these settings will be paramount. As issues around vaccine effectiveness, cost, and indirect protection are addressed in the coming years, it is likely that the effectiveness and cost-effectiveness of rotavirus vaccines in developing countries will continue to improve. As rotavirus vaccine introductions continue, efforts toward evaluating and expanding their cold-chain capacity should continue. Until universal rotavirus vaccine introduction is achieved, the full benefits of the rotavirus vaccine have yet to be realized.
Article highlights.
In 2006 the World Health Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) first recommended the inclusion of rotavirus vaccines into national immunization programs in Europe and the Americas, and in 2009 SAGE recommended the integration of rotavirus vaccine into all immunization programs worldwide.
Currently, four rotavirus vaccines have been pre-qualified by WHO for use globally. In developing countries, WHO recommends the use of Rotarix vaccine in a 2-dose schedule given at 6 and 10 weeks of age, and RotaTeq, Rotavac, and Rotasiil in a 3-dose schedule at 6, 10, and 14 weeks of age, with vaccine doses given concurrently with other childhood vaccines given at these ages.
WHO currently recommends that the rotavirus vaccine series can be started at 6 weeks of age and subsequent vaccine doses can be given until 24 months of age. However, on-time vaccination at as early of an age as possible is strongly encouraged to achieve protection against rotavirus disease early in life.
In 2013, an estimated 214,664 deaths were attributed to rotavirus infection in developing countries, and in the absence of rotavirus vaccine introduction, 38% of all hospitalized diarrhea cases among children <5 years globally are due to rotavirus infection.
As of December 2019, 100 countries have introduced a rotavirus vaccine into their national immunization programs; an additional 6 low-income countries with a gross national product of <US$1,580 per capita have been approved for funding support for vaccine introduction from Gavi, the Vaccine Alliance and are awaiting national introduction and another 13 countries are preparing to introduce independent of Gavi support.
A systematic review that analyzed post-licensure vaccine effectiveness data stratified by a country’s childhood mortality rates aligned with these findings, with Rotarix vaccine effectiveness at 84% (13 studies), 75% (8 studies), and 57% (9 studies) in countries with low, medium, and high childhood mortality rates, and RotaTeq vaccine effectiveness at 90% (20 studies) and 45% (7 studies) in countries with low and high child mortality rates, respectively.
Rotavirus vaccine introduction has reduced the global proportion of hospitalized diarrhea cases attributed to rotavirus among children <5 years from 38% in the pre-vaccine period, to 23.0% in the post-vaccine period, a 39.6% relative decline. While the magnitude of the decline varies by WHO region, due to varying vaccine effectiveness and vaccine coverage, overall reductions remain relatively consistent.
Post-marketing surveillance conducted in several high and middle-income countries has identified a slight increase in risk of intussusception 1-7 days following vaccination for both Rotarix and RotaTeq at a rate of 1 excess case per 20,000 – 100,000 vaccinated infants. The WHO Global Advisory Committee on Vaccine Safety has reviewed all of these data and reaffirmed the recommendation for continued rotavirus vaccine use.
Currently, minimal data have been published on the effectiveness or intussusception risk associated with Rotavac or Rotasiil in routine use.
When looking at all countries, previous work has found that at $5 per dose, the cost effectiveness of rotavirus vaccine per DALY averted in low, low-middle, and upper-middle-income countries was $88, $291, and $329, respectively. While occasionally some countries have found rotavirus vaccine not to be cost effective, this generally occurred when only direct medical costs were considered, and not those incurred by the family or society.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Tate JE, Burton AH, Boschi-Pinto C, et al. Global, regional, and national estimates of rotavirus mortality in children< 5 years of age, 2000–2013. Clinl Infect Dis. 2016;62(suppl_2):S96–S105. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood CD, Ma L, Carey ME, et al. The rotavirus vaccine development pipeline. Vaccine. 2017;37(50):7328–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Rotavirus vaccines. Weekly Epidemiological Rec. 2007;82:285–295. [PubMed] [Google Scholar]
- 4. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human–bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. •Presents results from a large Phase III clinical trial, demonstrates that pentavalent rotavirus vaccine is safe and efficacious against severe rotavirus disease in developed countries.
- 5. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. •Presents results from a large Phase III clinical trial, demonstrates that monovalent rotavirus vaccine is safe and efficacious against severe rotavirus disease in developed countries.
- 6.Phua KB, Lim FS, Lau YL, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine. 2009;27(43):5936–5941. [DOI] [PubMed] [Google Scholar]
- 7. Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. Malawi Med J. 2016;28(3):108–114. •Presents results from a large Phase III clinical trial, demonstrates that monovalent rotavirus vaccine is safe and efficacious against severe rotavirus disease in two African countries.
- 8.Zaman K, Anh DD, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–623. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Rotavirus vaccines: an update. Wkly Epidemiol Rec. 2009;84(50):533–540. [PubMed] [Google Scholar]
- 10.World Health Organization. Meeting of the immunization Strategic Advisory Group of Experts, April 2009–conclusions and recommendations. Wkly Epidemiol Rec. 2009;84(23):220–236. [PubMed] [Google Scholar]
- 11.Tate JE, Patel MM, Cortese MM, et al. Remaining issues and challenges for rotavirus vaccine in preventing global childhood diarrheal morbidity and mortality. Expert Rev Vaccines. 2012;11(2):211–220. [DOI] [PubMed] [Google Scholar]
- 12.Rha B, Tate JE, Weintraub E, et al. Intussusception following rotavirus vaccination: an updated review of the available evidence. Expert Rev Vaccines. 2014;13(11):1339–1348. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Rotavirus vaccines: WHO position paper—January 2013. Wkly Epidemiol Rec. 2013;88(5):49–64. [PubMed] [Google Scholar]
- 14.Weintraub ES, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014;370(6):513–519. [DOI] [PubMed] [Google Scholar]
- 15.Yen C, Tate JE, Hyde TB, et al. Rotavirus vaccines: current status and future considerations. Hum Vaccine Immunother. 2014;10(6):1436–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark A, Tate J, Parashar U, et al. Mortality reduction benefits and intussusception risks of rotavirus vaccination in 135 low-income and middle-income countries: a modelling analysis of current and alternative schedules. Lancet Glob Health. 2019;7(11):e1541–e1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aliabadi N, Antoni S, Mwenda JM, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health. 2019;7(7):e893–e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glass RI, Parashar U, Patel M, et al. Rotavirus vaccines: successes and challenges. J Infect. 2014;68:S9–S18. [DOI] [PubMed] [Google Scholar]
- 19.Vesikari T. Rotavirus vaccination: a concise review. Clin Microbiol Infect. 2012;18:57–63. [DOI] [PubMed] [Google Scholar]
- 20.Patel MM, Steele D, Gentsch JR, et al. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011;30(1):S1–S5. [DOI] [PubMed] [Google Scholar]
- 21.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–298. [DOI] [PubMed] [Google Scholar]
- 22. Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–614. •Presents results from a large Phase III clinical trial, demonstrates that pentavalent rotavirus vaccine is safe and efficacious against severe rotavirus disease in three African countries.
- 23.Patel M, Pedreira C, De Oliveira LH, et al. Effectiveness of pentavalent rotavirus vaccine against a diverse range of circulating strains in Nicaragua. Clinl Infect Dis. 2016;62(suppl_2):S127–S132. [DOI] [PubMed] [Google Scholar]
- 24.Tate JE, Ngabo F, Donnen P, et al. Effectiveness of pentavalent rotavirus vaccine under conditions of routine use in Rwanda. Clinl Infect Dis. 2016;62(suppl_2):S208–S212. [DOI] [PubMed] [Google Scholar]
- 25.Pringle KD, Patzi M, Tate JE, et al. Sustained effectiveness of rotavirus vaccine against very severe rotavirus disease through the second year of life, Bolivia 2013–2014. Clinl Infect Dis. 2016;62(suppl_2):S115–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar-Zeev N, Jere KC, Bennett A, et al. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clinl Infect Dis. 2016;62(suppl_2):S213–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gheorghita S, Birca L, Donos A, et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clinl Infect Dis. 2016;62(suppl_2):S140–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gastañaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clinl Infect Dis. 2016;62(suppl_2):S161–S167. [DOI] [PubMed] [Google Scholar]
- 29.Babji S, Kang G. Rotavirus vaccination in developing countries. Curr Opin Virol. 2012;2(4):443–448. [DOI] [PubMed] [Google Scholar]
- 30.Lamberti LM, Ashraf S, Walker CLF, et al. A systematic review of the effect of rotavirus vaccination on diarrhea outcomes among children younger than 5 years. Pediatr Infect Dis J. 2016;35(9):992–998. [DOI] [PubMed] [Google Scholar]
- 31.Jonesteller CL, Burnett E, Yen C, et al. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global post-licensure data, 2006–2016. Clinl Infect Dis. 2017;65(5):840–850. [DOI] [PubMed] [Google Scholar]
- 32.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9935):2136–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni PS, Desai S, Tewari T, et al. A randomized Phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35(45):6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isanaka S, Guindo O, Langendorf C, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N Engl J Med. 2017;376(12):1121–1130. [DOI] [PubMed] [Google Scholar]
- 35.Correia JB, Patel M, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P [4] Strains in Brazil. J Infect Dis. 2010;201(3):363–369. [DOI] [PubMed] [Google Scholar]
- 36.de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ. 2010;340:c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justino MCA, Linhares AC, Lanzieri TM, et al. Effectiveness of the monovalent G1P [8] human rotavirus vaccine against hospitalization for severe G2P [4] rotavirus gastroenteritis in Belém, Brazil. Pediatr Infect Dis J. 2011;30(5):396–401. [DOI] [PubMed] [Google Scholar]
- 38.Clark A, van Zandvoort K, Flasche S, et al. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis. 2019;19(7):717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qadri F, Bhuiyan TR, Sack DA, et al. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine. 2013;31(3):452–460. [DOI] [PubMed] [Google Scholar]
- 40.Parashar UD, Johnson H, Steele AD, et al. Health impact of rotavirus vaccination in developing countries: progress and way forward. Clinl Infect Dis. 2016;62(suppl_2):S91–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhaskaram P, Nair KM, Hemalatha P, et al. Systemic and mucosal immune response to polio vaccination with additional dose in newborn period. J Trop Pediatr. 1997;43(4):232–234. [DOI] [PubMed] [Google Scholar]
- 42.Albert MJ, Qadri F, Wahed M, et al. Supplementation with zinc, but not vitamin A, improves seroconversion to vibriocidal antibody in children given an oral cholera vaccine. J Infect Dis. 2003;187(6):909–913. [DOI] [PubMed] [Google Scholar]
- 43.Ali A, Kazi AM, Cortese MM, et al. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine—a randomized trial. PLoS One. 2015;10(6):e0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazarus RP, John J, Shanmugasundaram E, et al. The effect of probiotics and zinc supplementation on the immune response to oral rotavirus vaccine: a randomized, factorial design, placebo-controlled study among Indian infants. Vaccine. 2018;36(2):273–279. [DOI] [PubMed] [Google Scholar]
- 45.Steele A, De Vos B, Tumbo J, et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine. 2010;28(39):6542–6548. [DOI] [PubMed] [Google Scholar]
- 46.Ramani S, Mamani N, Villena R, et al. Rotavirus serum IgA immune response in children receiving rotarix coadministered with bOPV or IPV. Pediatr Infect Dis J. 2016;35(10):1137–1139. [DOI] [PubMed] [Google Scholar]
- 47.Cherian T, Wang S, Mantel C. Rotavirus vaccines in developing countries: the potential impact, implementation challenges, and remaining questions. Vaccine. 2012;30:A3–A6. [DOI] [PubMed] [Google Scholar]
- 48.Emperador DM, Velasquez DE, Estivariz CF, et al. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in rural Bangladesh. Clinl Infect Dis. 2015;62(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steele AD, Victor JC, Carey ME, et al. Experiences with rotavirus vaccines: can we improve rotavirus vaccine impact in developing countries? Hum Vaccin Immunother. 2019;15(6):1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnett E, Jonesteller CL, Tate JE, et al. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215(11):1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chavers T, De Oliveira LH, Parashar UD, et al. Post-licensure experience with rotavirus vaccination in Latin America and the Caribbean: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17(11):1037–1051. [DOI] [PubMed] [Google Scholar]
- 52.Shah MP, Tate JE, Mwenda JM, et al. Estimated reductions in hospitalizations and deaths from childhood diarrhea following implementation of rotavirus vaccination in Africa. Expert Rev Vaccines. 2017;16(10):987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mwenda JM, Parashar UD, Cohen AL, et al. Impact of rotavirus vaccines in Sub-Saharan African countries. 2018;36(47):7119–7123. [DOI] [PubMed] [Google Scholar]
- 54.Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006-2014. Vaccine. 2015;33(18):2097–2107. [DOI] [PubMed] [Google Scholar]
- 55.Sahakyan G, et al. Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clinl Infect Dis. 2016;62(suppl_2):S147–S154. [DOI] [PubMed] [Google Scholar]
- 56.Burnett E, Tate JE, Kirkwood CD, et al. Estimated impact of rotavirus vaccine on hospitalizations and deaths from rotavirus diarrhea among children <5 in Asia. Expert Rev Vaccines. 2018;17(5):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tate JE, Steele AD, Bines JE, et al. Research priorities regarding rotavirus vaccine and intussusception: a meeting summary. Vaccine. 2012;30:A179–A184. [DOI] [PubMed] [Google Scholar]
- 58.Patel MM, López-Collada VR, Bulhões MM, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283–2292. [DOI] [PubMed] [Google Scholar]
- 59.Buttery J, Danchin MH, Lee KJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29(16):3061–3066. [DOI] [PubMed] [Google Scholar]
- 60.Lopman BA, Payne DC, Tate JE, et al. Post-licensure experience with rotavirus vaccination in high and middle income countries; 2006 to 2011. Curr Opin Virol. 2012;2(4):434–442. [DOI] [PubMed] [Google Scholar]
- 61.Australian Government Therapeutic Goods Administration. Rotavirus vaccination and the risk of intussusception. 2013. Dec 16 [2019]; [cited 2019 Dec 15]. Available from: https://www.tga.gov.au/alert/rotavirus-vaccination-and-risk-intussusception-0
- 62.Cortese MM. Summary of intussusception risk and benefits of rotavirus vaccination in the United States. Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, 20 June 2013. 2014. [Google Scholar]
- 63.Clark A, Jit M, Andrews N, et al. Evaluating the potential risks and benefits of infant rotavirus vaccination in England. Vaccine. 2014;32(29):3604–3610. [DOI] [PubMed] [Google Scholar]
- 64.Tate JE, Mwenda JM, Armah G, et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med. 2018;378(16):1521–1528. [DOI] [PubMed] [Google Scholar]
- 65.Groome MJ, et al. Evaluation of intussusception after oral monovalent rotavirus vaccination in South Africa. Clinl Infect Dis. 2020;70(8):1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rheingans RD, Antil L, Dreibelbis R, et al. Economic costs of rotavirus gastroenteritis and cost-effectiveness of vaccination in developing countries. J Infect Dis. 2009;200(Supplement_1):S16–S27. [DOI] [PubMed] [Google Scholar]
- 67.Debellut F, Clark A, Pecenka C, et al. Re-evaluating the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi countries: a modelling study. Lancet Glob Health. 2019;7(12):e1664–e1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clinl Infect Dis. 2014;59(9):1291–1301. [DOI] [PubMed] [Google Scholar]
- 69.Makinen M, Kaddar M, Molldrem V, et al. New vaccine adoption in lower-middle-income countries. Health Policy. 2012;27(suppl_2):ii39–ii49. [DOI] [PubMed] [Google Scholar]
- 70.De Oliveira LH, Danovaro-Holliday MC, Matus CR, et al. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7(3):345–353. [DOI] [PubMed] [Google Scholar]
- 71.Groome MJ, Zell ER, Solomon F, et al. Temporal association of rotavirus vaccine introduction and reduction in all-cause childhood diarrheal hospitalizations in South Africa. Clinl Infect Dis. 2016;62(suppl_2):S188–S195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mpabalwani EM, Simwaka CJ, Mwenda JM, et al. Impact of rotavirus vaccination on diarrheal hospitalizations in children aged< 5 years in Lusaka, Zambia. Clinl Infect Dis. 2016;62(suppl_2):S183–S187. [DOI] [PubMed] [Google Scholar]
- 73.Moon S, Wang Y, Edens C, et al. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013;31(34):3396–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burke RM, et al. Current and new rotavirus vaccines. Current opinion in infectious diseases. 2019;32(5):435. [DOI] [PMC free article] [PubMed] [Google Scholar]