Abstract
Prenatal maternal diet is a critical factor in offspring neurodevelopment. Emerging evidence suggests that prenatal diet may also play a role in the etiology autism spectrum disorder (ASD). This review summarizes studies published in English that examined prenatal nutrients or maternal diet in association with ASD from PubMed as of July 2020. 36 studies from nine countries were included in this systematic review; these focused on multivitamin (n=5), prenatal vitamin (n=3), folic acid (n=14), vitamin D (n=11), polyunsaturated fatty acid (PUFA) or fish/supplement intake (n=7), iron (n=3), vitamin B12 (n=1), calcium (n=1), magnesium (n=1), and broad maternal dietary habits (n=3). Overall, higher or moderate intake of prenatal/multivitamin, folic acid, and vitamin D was associated with reductions in odds of ASD, though results have not been uniform and there is a need to clarify differences in findings based on biomarkers versus reported intake. Evidence was inconclusive or insufficient for other nutrients. Differences in the timing and measurement of these dietary factors, as well as potential residual confounding, may contribute to existing discrepancies. Key areas for future research to better understand the role of maternal diet in ASD include the need to address potential critical windows, examine the combined effect of multiple nutrients, and consider interactions with genetic or environmental factors.
Keywords: ASD autism spectrum disorders, FA folic acid, multivitamin, prenatal vitamin, vitamin D, PUFAs polyunsaturated fatty acids, maternal diet
Lay summary
Maternal diet during pregnancy is important for child neurodevelopment. We reviewed 36 studies examining maternal diet and autism spectrum disorder (ASD) and found that prenatal vitamin/multivitamin use and adequate intake of folic acid and vitamin D were each associated with lower likelihood of having a child with ASD. Future studies on these and other dietary factors are needed to better understand the role of maternal diet in the development of ASD.
1. Introduction
Autism spectrum disorder (ASD) is a developmental condition characterized by social, communication, and behavioral challenges. The etiology is not well understood, and multiple complex etiologies may likely coexist (Newschaffer et al., 2007). The current consensus suggests both genetic and environmental factors are involved (Lyall et al., 2017). Furthermore, evidence of differences in brain anatomy and connectivity in children with ASD compared to those who are typically developing (Ha, Sohn, Kim, Sim, & Cheon, 2015) point to prenatal origins (Kaushik & Zarbalis, 2016). Thus, factors influencing neurodevelopment during the prenatal period are of potential etiologic relevance to ASD.
It is a well-accepted paradigm that prenatal maternal diet can influence neurodevelopment. Perhaps the most established findings are the relationships between folate deficiency and neural tube defects (Blencowe, Cousens, Modell, & Lawn, 2010) and between prenatal nutrient deprivation and schizophrenia (Susser et al., 1996). Prenatal fish intake, a source of both long-chain polyunsaturated fatty acids (PUFAs) but also of heavy metals, has been associated with certain child cognitive outcomes (E. Oken, Osterdal, et al., 2008; Strain et al., 2012), as have iron/maternal anemia, and vitamin D (Boksa, 2010; Garcia-Serna & Morales, 2019; Kočovská, Fernell, Billstedt, Minnis, & Gillberg, 2012; Rice & Barone, 2000; Sachdev, Gera, & Nestel, 2005). Studies examining these factors and ASD have emerged over the past decade, though the majority to date have focused on folate, prenatal vitamin supplements, and vitamin D.
Several reviews have summarized associations between specific nutrients (DeVilbiss, Gardner, Newschaffer, & Lee, 2015; Kawicka & Regulska-Ilow, 2013) and ASD, and one has reviewed dietary factors in association with neurodevelopmental outcomes including ASD, though did not include vitamin D (M. Li, Francis, Hinkle, Ajjarapu, & Zhang, 2019). The objective of this review is to summarize the evidence regarding the role of prenatal diet in ASD/ASD-related traits and to highlight future directions. Given below-recommended intake of many critical nutrients in pregnant women (Kominiarek & Rajan, 2016), diet may represent a source of modifiable risk factors with the potential for large public health impact.
2. Materials and Methods
2.1. Study Criteria
In order to be included in this review, studies had to assess the independent effect of a maternal dietary factor or nutrient deficiency in association with offspring ASD diagnosis or ASD-related traits. Alcohol or other substance use and toxicants like mercury were not included. Dietary exposures were required to have been measured using self-reported intake and/or biomarkers during or representing the periconceptional (i.e., the 3 months before pregnancy through the 1st month of pregnancy) or prenatal period (anytime during pregnancy), though studies using nutrients measured in neonatal and/or early postnatal samples were also considered for inclusion when supported by evidence that biomarkers in this time period reflect prenatal exposures. ASD diagnosis was defined according to clinical assessment, register, or parent report; ASD phenotype was defined as measurement by a validated quantitative scale (such as the Social Responsiveness Scale (SRS); see Appendix 1 for a full list of specific measures). Additionally, studies had to be published in English, conducted in human subjects, and were required to have a comparison group; as such, case reports, ecological studies, and other review papers were excluded. No limitations were placed on the age of mothers or children, or timing of offspring ASD-related outcome assessment, or geographic location of the study population. Only studies published or accepted for publication through July 2020 were included.
2.2. Search Strategy
Published articles on dietary factors and nutrients and ASD were identified using the PubMed database and adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Search terms included maternal or time-period keywords (e.g., prenatal, periconceptional, etc.), individual nutrients or diet, and ASD outcome terms. Further details of the search, including keywords used, are provided in Appendix 2.
2.3. Data Extraction And Assessment
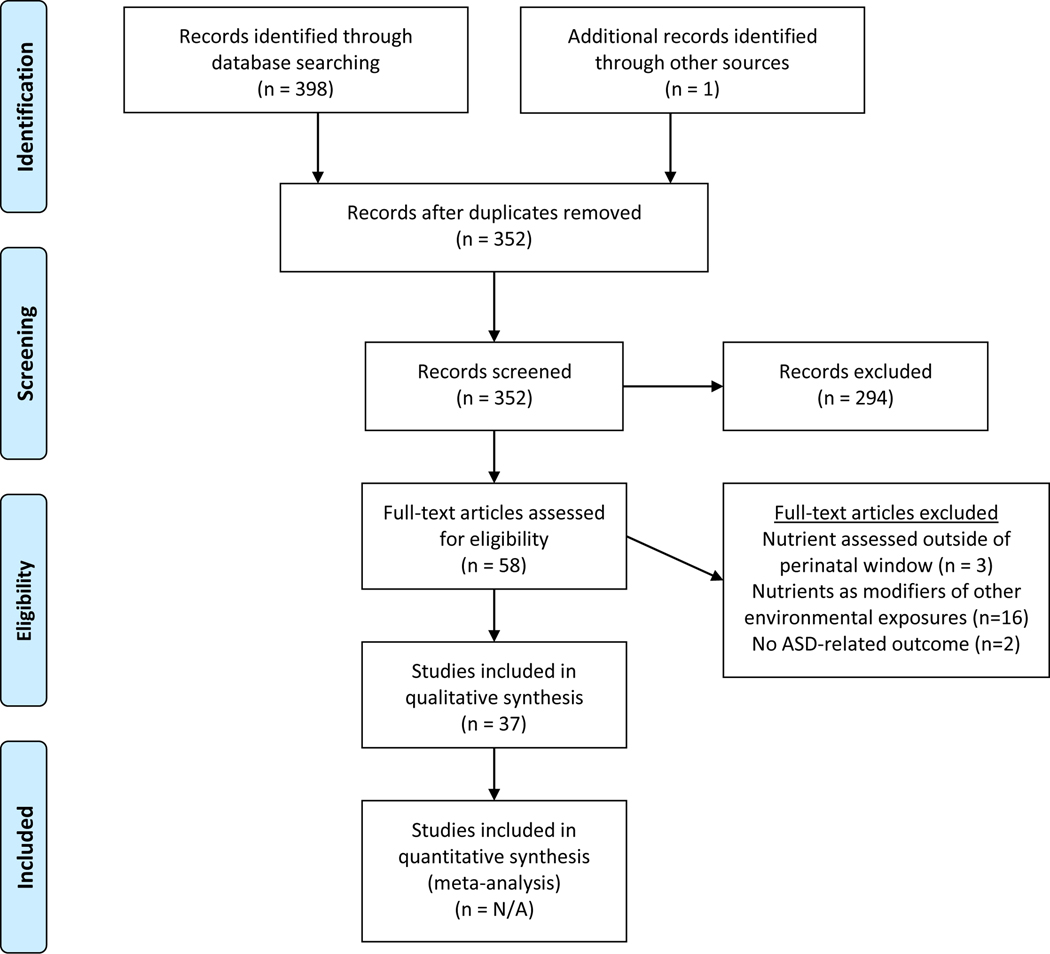
As of July 2020, our search yielded a total of 398 publications in English. After removing duplicate citations (n=46) and adding 1 additional article meeting criteria and known to be in press, the abstracts for 287 papers were screened by J.T. and C.Z., in which 57 full-text articles were reviewed to determine eligibility. In total, 37 articles were included in this systematic review. The study selection process is further summarized in Figure 1.
Fig 1.
PRISMA 2009 flow diagram
Given substantial heterogeneity across studies, results were summarized qualitatively to determine overall patterns in the existing literature and to identify any inconsistencies, biases, and gaps where future work may be needed. Study characteristics, including study population, location, design, and methodology, were summarized across studies, as was definition and timing of maternal diet/nutrient measurements, ASD-related outcome, and covariates considered as potential confounders. Key findings and final effect estimates were extracted (J.T. and C.Z.) from each publication and summarized in forest plots (for analyses of ASD diagnosis) and/or tables (for quantitatively-assessed ASD outcomes). For ease of comparison across studies, we summarized findings according to the association between higher intake of the nutrient and ASD (with lower/lowest levels as the referent group). Therefore, for any studies utilizing higher intake as the referent group, we calculated the inverse of estimates and their confidence intervals.
3. Results
The 37 studies (including 10 studies addressing more than one nutrient) included in this review examined multivitamin (n=5), prenatal vitamin (n=3), folic acid (n=15), vitamin D (n=11), PUFA intake or a source of PUFAs (seafood or fish oil supplementation) (n=8), other nutrients (n=8; including iron, vitamin B12, calcium, magnesium, and micronutrient supplement use), and broad maternal dietary habits or patterns (n=3). These studies utilized data from nine different countries, including the US (n=13), China (n=5), Sweden (n=5), Israel (n=2), Spain (n=2), Denmark (n=2), Netherland (n=4), Norway (n=2), and Australia (n=1). Some studies represented the same study population: 4 were from the CHARGE (Childhood Autism Risks from Genetics and Environment) case-control study in California (USA), 4 from the Generation R prospective cohort study in Rotterdam (Netherlands), 2 from the MARBLES (Markers of Autism Risk in Babies: Learning Early Signs) ASD high-familial risk prospective cohort study in California (USA), 2 from the MoBa (Norwegian Mother, Father, and Child Cohort) Study in Norway, 2 from the DNBC (Danish National Birth Cohort) in Denmark, and 3 from the SYC (Stockholm Youth Cohort) in Sweden.
Exposure assessment methods across these studies varied: 14 relied on self-reported dietary data alone, 13 used prenatal biomarkers alone, 6 used a combination of reported diet and biomarkers, and 3 extracted data from medical/pharmacy dispensation records or registry data. Of studies relying on self-reported data, 8 used standardized Food Frequency Questionnaires (FFQs), while the remaining utilized brief dietary screeners or questionnaires including questions on intake. Likewise, sources and definitions of the primary outcome (i.e. ASD diagnosis or autism-related traits) also varied across studies: for ASD diagnosis, 21 relied on registry-based data according to ICD/DSM codes or receiving services for ASD, 7 relied on clinical diagnosis including the Autism Diagnostic Observation Schedule (ADOS) and/or Autism Diagnostic Interview-Revised (ADI-R), and 1 relied on maternal report; 10 assessed autism-related traits using quantitative measures (4 of these used the SRS or a subset of its items). Further details (and study references) are summarized in Table 1 and in below subsections.
Table 1.
Key characteristics of studies published through April 2020 examining maternal dietary factors in association with ASD or ASD-traits.
| Reference | Nutrient/Category | Sample size (n) | Study type, population, location† (PC=prospective cohort, RC= retrospective cohort, CC= case control) | Exposure assessment‡ (P= prospective, R= retrospective; SR=self-report, B= biomarker; M= medical/dispensation record/ registry, FFQ=food frequency questionnaire) | Outcome assessment information§ (Dx= ASD diagnosis; AT=autism-related traits) | Key finding(s) |
|---|---|---|---|---|---|---|
| Schmidt et al 2011 PMID: 21610500 | Prenatal Vitamins (PV) | 288 ASD, 141 DD controls, 278 TD controls | CC; CHARGE; Northern California, USA | PV use from 3 months preconception to breastfeeding according to R SR | Dx at age 2–5 yrs based on SCQ, ADOS-G, ADI-R (2–3 yrs)¶ | Periconceptional PV use ↓ ASD odds; Interaction with susceptible genes involved in one-carbon metabolism |
| Braun et al 2014 PMID: 24710813 | 209 mother-child pairs | PC; HOME; Ohio, USA | PV use at 14–39 wks according to P SR | AT at age 4–5 yrs using SRS Total T scores | PV use ↓ odds of clinically elevated SRS scores. | |
| Schmidt et al., 2019 PMID: 30810722 | 305 mothers 332 children (55 ASD) | PC; MARBLES; California, USA | PV use from preconception to after birth according to P SR | Dx at age 3 yrs based on DSM-IV, ADOS and MSEL†† | 1st month pregnancy PV intake ↓ ASD risk. | |
| Schmidt et al 2011 (See above in PV) | Multivitamin (MVS) | 288 ASD, 141 DD controls, 278 TD controls | CC; CHARGE; Northern California, USA | MVS use from 3 months preconception to breastfeeding according to R SR | Dx at age 2–5 yrs based on SCQ, ADOS-G, ADI-R (2–3 yrs) ¶ | No significant association overall |
| DeVilbiss et al., 2017 PMID: 28978695 | 27, 3107 mother-child pairs | PC; SYC; Sweden | MVS use at 1st trimester (interquartile range 9.0–12.7 weeks) according to P SR | Dx at age 4–15 yrs based on ICD-10 or DSM-IV from registry | MVS use during early pregnancy↓ odds of ASD with intellectual disability | |
| Raghavan et al, 2018 PMID: 28984369 | 1,257 mother-child pairs (86 ASD) | PC; Boston Birth Cohort, USA | MVS use at 1st trimester according to P SR & plasma folate concentration at 24–72 hrs post-delivery according to P B | Dx at mean age 6 yrs based on ICD-9 from EMR | U-shaped relationship between MVS frequency and ASD risk | |
| Levine et al., 2018 PMID: 29299606 | 45,300 children (572 ASD) | CC (nested); Israel | MVS use before and/or during pregnancy according to P M | Dx up until 12 yrs based on ICD-9 from registry | MVS use before and/or during pregnancy ↓ ASD risk | |
| Virk et al 2015 PMID: 26408631 | 35,059 mothers (552 ASD) | PC; DNBC; Denmark | MVS use during periconception period according to P SR | Dx at age 8.1–11.4 yrs based on ICD-10 from National Hospital Registry | No significant association overall | |
| Tan et al 2019 PMID: 31759952 | 416 ASD, 201 TD | CC; China | Micronutrient supplement use from last menstrual period to birth according to R SR | Dx at mean age 4.68 yrs based on DSM-5; AT using ABC, CARS, SRS§§§ | Micronutrient supplement during pregnancy ↓ ASD odds and severe AT symptoms | |
| Schmidt et al., 2019 (See above in PV) | Folic Acid (FA) | 305 mothers 332 children (55 ASD) | PC; MARBLES; California, USA | Calculated FA intake based on PV use from preconception to after birth according to P SR | Dx at age 3 yrs based on DSM-IV, ADOS and MSEL†† | Highest tertile of 1st month pregnancy FA intake and FA intake at or above 600 μg ↓ ASD risk. |
| Schmidt et al 2012 PMID: 22648721 | 429 ASD, 130 DD, 278 TD controls | CC; CHARGE; Northern California, USA | Calculated quantified FA intake from supplement and food from preconception to pregnancy according to R SR | Dx at age 2–5 yrs (see above- CHARGE criteria) | High periconceptional FA consumption of >=600 μg FA/day (relative to <600 μg) ↓ ASD risk | |
| Suren et al 2013 PMID: 23403681 | 85,176 children (270 ASD) | PC; MoBa; Norway | FA use at 18, 22 wks gestation according to P SR (FFQ) | Dx screen at age 3,5,7 yrs, referral, or registry; based on ADI-R, ADOS | Periconceptional FAS use ↓ ASD risk | |
| Bjork et al., 2018 PMID: 29279889 | 104,946 children | PC; MoBa; Norway | FA use and dose from preconception, periconception (−4 to 12 wks), to pregnancy according to P SR & plasma folate concentration at 17–19 wks gestation according to P B | AT based on screener scores (M-CHAT‡‡ at age 18 months, SCQ at age 3 yrs) | Periconceptional FAS use and dose and prenatal folate status ↓ ASD risk | |
| Levine et al., 2018 (See above in MVS) | 45,300 children (572 ASD) | CC (nested); Israel | FA use before and/or during pregnancy according to P M | Dx up until 12 yrs based on ICD-9 from registry | Maternal FAS use before and/or during pregnancy ↓ ASD risk | |
| Steenweg-de Graaff et al 2014 PMID: 25085472 | 3,893 children | PC; Generation R; Netherlands | FA use according to P SR & plasma folate concentration at 13 wks gestation according to P B | AT at age 6 yrs using SRS subset | Prenatal FAS use ↓ child autism-related traits; No association with plasma folate concentration. | |
| Virk et al 2015 (See above in MVS) | 35059 women (552 ASD) | PC; DNBC; Denmark | FA use during periconceptional and prenatal according to P SR (FFQ) | Dx at age 8.1–11.4 yrs (see above- DNBC criteria) | No significant association overall | |
| Strom et al., 2018 PMID:28946926 | 87210 mother-child pairs (1234 ASD) | PC; DNBC; Denmark | FA use during periconceptional and prenatal according to P SR (FFQ) | Dx at age 8.1–11.4 yrs (see above- DNBC criteria) | No significant association overall | |
| DeVilbiss et al., 2017 (See above in MVS) | 27,3107 mother-child pairs | PC; SYC; Sweden | FA use at first antenatal visit according to P SR | Dx at age 4–15 yrs (see above-SYC criteria) | No significant association overall | |
| Li et al., 2018 PMID: 30593205 | 374 ASD, 354 controls | CC; ACED; China | FA use during preconception, pregnancy, and breastfeeding according to R SR | Dx at age 3–6 based on DSM-IV-TR and total raw SRS T scores ≥ 56.5 | No significant association overall | |
| Sharman et al, 2019 PMID: 31376478 | 2009 ASD, 19,886 controls | CC (nested); Israel | FA use during pregnancy according to P M | Dx up until 6 yrs based on DSM§§ from registry | No significant association overall | |
| Braun et al 2014 (See above in PV) | 209 mother-child pairs | PC; HOME; Ohio, USA | WBF concentration at 11–21 wks gestation according to P B | AT at age 4–5 yrs (see above- HOME criteria) | No significant association with measured levels; | |
| Raghavan et al, 2018 (see above in MVS) | 1,257 mother-child pairs | PC; Boston Birth Cohort, USA | Plasma folate concentration at 24–72 hrs post-delivery according to P B | Dx at mean age 6 yrs based on ICD-9 from EMR | Low and extreme high plasma folate concentration levels ↑ ASD risk | |
| Egorova et al, 2020 PMID: 32131900 | 100 ASD, 100 TD controls | CC (matched); Sweden | Total folate concentration from stored pre-diagnostic blood samples at 14 wks gestation according to P B | Dx based on ICD-10, DSM-IV (born between 1996 and 2009) | Higher early plasma folate concentration ↑ offspring ASD odds | |
| Tan et al 2019 (See above in MVS) | 416 ASD, 201 TD | CC; China | FA use from 12 wks before (LMP) to 12 wks after LMP according to R SR | Dx at mean age 4.68 yrs; AT using ABC, CARS, SRS | FAS during pregnancy ↓ ASD odds and severe AT symptoms | |
| Whitehouse et al 2013 PMID: 23070790 | Vitamin D | 406 children | PC; Raine; Australia | Maternal serum 25(OH)D at 18 wks gestation according to P B | Dx at age 5,8,10,14,17 yrs based on DSM-IV; AT using total ASQ scores7 | Low 25(OH)D ↑ risk of higher scores on the Attention Switching subscale; No association with Dx |
| Chen et al., 2016 PMID: 27663117 | 68 ASD, 68 controls | CC (matched); China | Maternal 25(OH)D3 at first trimester pregnancy according to P B | Dx at age 3–7 yrs based on DSM-V, clinically confirmed | Lower 1st trimester 25(OH) D levels ↑ ASD risk | |
| Vinkhuyzen et al 2017 PMID: 28446959 | 4,334 children (68 ASD) | PC; Generation R; Netherlands | Maternal serum 25(OH)D at 18.1–24.9 wks gestation, and neonatal from cord blood at birth according to P B | Dx at age 6–9 yrs based on SRS, CBC, SCQ total score; | Mid-gestational, but not neonatal, vitamin D deficiency ↑ ASD risk | |
| Vinkhuyzen et al 2018 PMID: 27895322 | 4,229 children | PC; Generation R; Netherlands | Maternal 25(OH)D at 18.1–24.9 wks gestation, and neonatal from cord blood at birth according to P B | AT at mean age 6 yrs using 18-item SRS subset | Mid-gestational and neonatal 25OHD deficient ↑ SRS scores | |
| Fernell et al 2015 PMID: 25874075 | 58 sibling pairs | CC; Sweden | Neonatal 25(OH)D levels from stored dried blood spots at birth according to P B | Dx at preschool age based on clinical assessments | ASD cases ↓ 25OHD level at birth than siblings | |
| Lee et al., 2019 PMID: 31695167 | Maternal sample (449 ASD, 574 controls); neonatal sample (1399 ASD, 1607 controls)¶¶ | CC; SYC; Sweden | Maternal 25OHD level at 10.9 wks gestation and neonatal 25OHD from stored DBS at 3–5 days of age according to P B | Dx screened at age 0–5 yrs and diagnosed based on ICD-9, ICD-10, DSM-IV from registry | Neonatal, but not maternal, vitamin D deficiency ↑ ASD odds | |
| Windham et al., 2019 PMID: 30883046 | 563 ASD, 190 ID, 436 control | CC; EMA; Southern California | Neonatal 25(OH)D from stored DBS at birth according to P B | Dx at age 4.5–7 yrs based on DSM-IV-TR | No significant association overall | |
| Schmidt et al., 2019 PMID: 31094097 | 357 ASD, 134 DD, 234 TD controls | CC; CHARGE; Northern California, USA | Neonatal 25(OH)D from stored DBS at birth according to P B | Dx at age 2–5 yrs (see above- CHARGE criteria) | Higher neonatal 25(OH)D ↓ ASD odds only in females. | |
| Lopez-Vicente et al., 2019 PMID: 31616023 | 2,107 mother-child pairs | PC; INMA; Spain | Maternal 25(OH)D3 at 10–13 wks gestation according to P B | AT at age 5 yrs using CAST | No significant association overall | |
| Wu et al., 2017 31094097 | 310 ASD, 1240 controls | CC (matched); NBSIB; China | Neonatal 25(OH)D3 from stored DBS at birth according to P B | Dx at age 3 yrs based on DSM-5 | Higher neonatal vitamin D ↓ ASD risk | |
| Egorova et al, 2020 (See above in FA) | 100 ASD, 100 TD controls | CC (matched); Sweden | Maternal 25(OH)D3 from stored pre-diagnostic blood samples at 14 wks gestation according to P B | Dx based on ICD-10, DSM-IV (born between 1996 and 2009) | No significant association overall | |
| Lyall et al 2013 PMID: 23813699 | Fish and polyunsaturated fatty acid (PUFAs) ††† | 317 ASD, 17728 controls | PC; NHS II; USA | PUFA intake and fish oil supplement use during prenatal, pregnancy, postnatal according to P SR (FFQ) | Dx based on maternal report‡‡‡ | Higher PUFA intake ↓ ASD risk |
| Steenweg-de Graaff et al, 2016 PMID: 27052119 | 4,624 children | PC; Generation R; Netherlands | Fish intake at 10.8–21.4 wks in early pregnancy according to P SR (FFQ) & maternal plasma PUFA concentration and ω−3: ω−6 ratio at mid-pregnancy according to P B | AT at mean age 6.2yrs using SRS subset and the pervasive developmental problems subscale scores | Lower maternal ω−3: ω−6 ratio during pregnancy ↑ autism-related traits | |
| Julvez et al., 2016 PMID: 26740026 | 1,892 and 1589 mother-child pairs | PC; SCEP; Spain | Fish intake at 10–13wks, 28–32 of pregnancy, at child age14 months and 5 years according to P SR (FFQ) | AT at age 5 yrs using CAST scores | Maternal large fatty fish consumption ↓ CAST scores | |
| Gao et al., 2016 PMID: 28081749 | 108 ASD, 79 ID, 108 controls | CC (matched); China | Parental dietary habits from preconception to childbirth according to P SR | Dx at age 4–17 yrs based on DSM-IV, CARS ≥ 30 | Parental fish consumption ↓ odds of ASD with intellectual disability | |
| Suren et al 2013 (See above in FA) | 85,176 children (270 ASD) | PC; MoBa; Norway | Prenatal fish oil supplement according to P SR | Dx at age 3,5,7 yrs (see above – MoBa criteria) | No significant association overall | |
| Huang et al 2020 PMID:31958995 | 258 mother–child pairs (57 ASD, 62 non-TD, 139 TD) | PC; MARBLES; California, USA | Maternal PUFAs intake during pregnancy according to P SR (FFQ) & plasma PUFAs concentration at 3rd trimester according to P B | Dx at age 3 yrs (see above-MARBLES criteria) | Higher total omega-3 reported intake in 2nd half of pregnancy ↓ ASD risk; No significant associations with 3rd trimester plasma PUFA concentration | |
| Lyall et al, 2020, In press | 499 ASD cases, 501 controls | CC; California, USA | Maternal mid-pregnancy PUFA levels according to P B | Dx at (minimum age 5 yrs) from registry | No significant association overall; lower levels of PUFA ↑ ASD with ID | |
| Vecchione et al, 2020 PMID: 32519188 | 426 children | PC; EARLI & HOME; USA | Maternal fish intake across pregnancy according to P SR | AT at age 3 yrs using SRS | Higher overall fish intake ↑ autism-related traits; Higher salmon intake ↓ autism-related traits | |
| Raghavan et al, 2018 (See above in FA) | Other Nutrients: Vitamin B12 | 1,257 mother-child pairs (86 ASD) | PC; Boston Birth Cohort, USA | Vitamin B12 supplements use during prenatal according to P SR | Dx at mean age 6 yrs based on ICD-9 from EMR | Very high B12 levels ↑ASD risk; No association between B12 deficiency and ASD risk |
| Li et al., 2018 (See above in FA) | Other Nutrients: Calcium | 374 ASD, 354 controls | CC (matched); ACED; China | Maternal preference of calcium during preconception, pregnancy, and breastfeeding according to R SR | Dx at age 3–6 yrs (see above-ACED criteria) | Preconception calcium supplementation ↓ ASD risk |
| DeVilbiss et al., 2017 (See above in MVS, FA) | Other Nutrients: Iron | 27, 3107 mother-child pairs | PC; SYC; Sweden | Iron supplement at first antenatal visit according to P SR | Dx at age 4–15 yrs (see above-SYC criteria) | No significant association overall |
| Schmidt et al., 2014 PMID: 25249546 | 510 ASD, 341 TD controls | CC; CHARGE; Northern California, USA | Quantified mean maternal daily iron intake from supplements and cereals during 3 months preconception, pregnancy, and breastfeeding according to R SR | Dx at age 2–5 yrs (see above- CHARGE criteria) | Highest quintile of iron intake relative to lowest ↓ ASD risk, especially during breastfeeding. | |
| Wiegersma et al., 2019 PMID:31532497 | 532 232 children | PC; SYC; Sweden | Maternal anemia diagnosis during pregnancy (≤30 or >30 weeks) according to P M | Dx at age 4–15 yrs (see above-SYC criteria) | Maternal anemia diagnosed in early pregnancy ↑ ASD risk | |
| Schmidt et al., 2019 (See above in PV and FA) | 305 mothers 332 children (55 ASD) | PC; MARBLES; California, USA | Calculated iron intake from PV use in the 1st months of pregnancy according to P SR | Dx at age 3 yrs based on DSM-IV, ADOS and MSEL†† | The top two tertiles of 1st month pregnancy iron intake ↓ ASD risk. | |
| Bakian et al., 2018 PMID: 30246047 | Other Nutrients: Magnesium | 4855 children (112 ASD) | PC; Utah, USA | Neonatal magnesium level from serum at 24–48 hours after birth according to P B | Dx at age 4–12 yrs based on ICD-9 from registry | No significant association overall |
| Li et al., 2018 (See above in FA) | Dietary habit: balanced diet | 374 ASD, 354 controls | CC (matched); ACED; China | Maternal dietary habits during preconception, pregnancy, and breastfeeding according to R SR | Dx at age 3–6 (see above- ACED criteria) | Mostly meat or mostly vegetables (relative to balanced diet) ↑ ASD odds |
| House et al., 2018 PMID: 30246009 | Dietary habit: Mediterranean Diet | 325 mother-child pairs | PC; NEST; North Carolina, USA | Mediterranean diet adherence (MDA) scores during periconceptional/ early pregnancy according to P SR (FFQ) | AT at age 2 yrs using ITSEA (problem and competency portions) | Middle and high tertile of Mediterranean diet (relative to lowest tertile) ↓ ASD behaviors |
| Gao et al., 2016 (See above in PUFAs/fish) | Dietary habit: Fruit | 108 ASD, 79 ID, 108 controls. | CC (matched); China | Maternal dietary habits from preconception to birth according to R SR (FFQ) | Dx at age 4–17 yrs based on DSM-IV, CARS ≥ 30 | Low fruit intake during preconception and pregnancy relative to high intake ↑ ASD odds. |
Study names/acronyms are provided as relevant. SYC=Stockholm Youth Cohort; MoBa=Norwegian Mother and Child Cohort Study; NES=Newborn Epigenetics Study; CHARGE=Childhood Autism Risks from Genetics and the Environment; SCEP=Spanish Childhood and Environment Project; NHS II= Nurses’ Health Study II; Raine=The Western Australian Pregnancy Cohort Study; HOME=the Health Outcomes and Measures of the Environment Study; ACED=Autism Clinical and Environmental Database; DNBC=Danish National Birth Cohort; MARBLES=Markers of Autism Risk in Babies Learning Early Signs; NBSIB= the Expanded newborn screening in Beijing Study; EMA= the Early Markers for Autism Study; INMA= the INfancia y Medio Ambiente (Environment and Childhood) Project.
Exposure acronyms are provided as relevant. MVS=Multivitamin supplement; PV=Prenatal Vitamins; FAS=Folic Acid supplement; WBF=maternal whole blood folate concentration; LMP=last menstrual period; DBS= Dried blood spots.
Outcome assessment/sources are provided as relevant. ICD=International Classification of Diseases; Diagnostic and Statistical Manual of Mental Disorders=DSM; EMR= Electronic medical records; SCQ= Social communication Questionnaire; ADOS= Autism Diagnostic Observation Schedule; ADOS-G= Autism Diagnostic Observation Schedule-Generic; ADI-R= Autism Diagnostic Interview-Revised; MSEL= Mullen Scales of Early Learning; M-CHAT= Modified Checklist for Autism in Toddlers; CARS= Childhood Autism Rating Scale; AQ= Autism Spectrum Quotient; SRS= Social Responsiveness Scale; ITSEA= Infant Toddler Social and Emotional Assessment; CAST=Childhood Asperger Syndrome Test; VABS= Vineland Adaptive Behavior Scale; ABC= the Autism Behaviour Checklist; Bayley=Bayley Scales of Infant Development Second Edition;
To be defined as a case, children must have scored ≥ 15 (with >7 or more in the social domain) on SCQ; a score of 4 or more in the communication domain and a combined social-communication total of at least 12 (with scored 10 for the Social Interaction Domain, 8 for the Communication Domain for verbal children and 7 for nonverbal children, and 3 for the Restricted/repetitive Domain) on ADOS-G.
To be defined as a case, children must have scored below 70 for composite scores. (2 or more MSEL subscale scores more than 1.5 SD below the normative mean or at least 1 MSEL subscale score more than 2 SD below the normative mean).
On M-CHAT, to be defined as case, children aged 16 to 30 months need to miss any 3 of 23 items or 2 of 6 critical items.
DSM version was not specified
The study included a total of four groups: maternal sample (449 ASD, 574 controls); neonatal sample (1399 ASD, 1607 controls), matched neonatal-sibling sample (357 ASD, 364 unaffected siblings), and paired maternal-neonatal sample (340 ASD, 426 controls)
A few studies have also examined methylmercury in fish in association with ASD traits and diagnosis; these have generally not seen associations and are briefly summarized in the text.
Maternal report of diagnosis was validated in a subset of participants using the ADI-R
Autism-related traits are measured as >=53 on the Autism Behaviour Checklist (ABC), >=30 on Childhood Autism Rating Scale (CARS), and > 65 on Social Responsiveness Scale (SRS).
Supplemental Table 1 summarizes covariates adjusted for across these studies. Most studies included adjustment for basic demographic factors, maternal age, child age and sex, and many adjusted for maternal BMI, but there was wide variability in adjustment strategies. While several studies adjusted for certain other dietary factors, as well as smoking and alcohol use, many did not include adjustment for other nutrients or dietary factors.
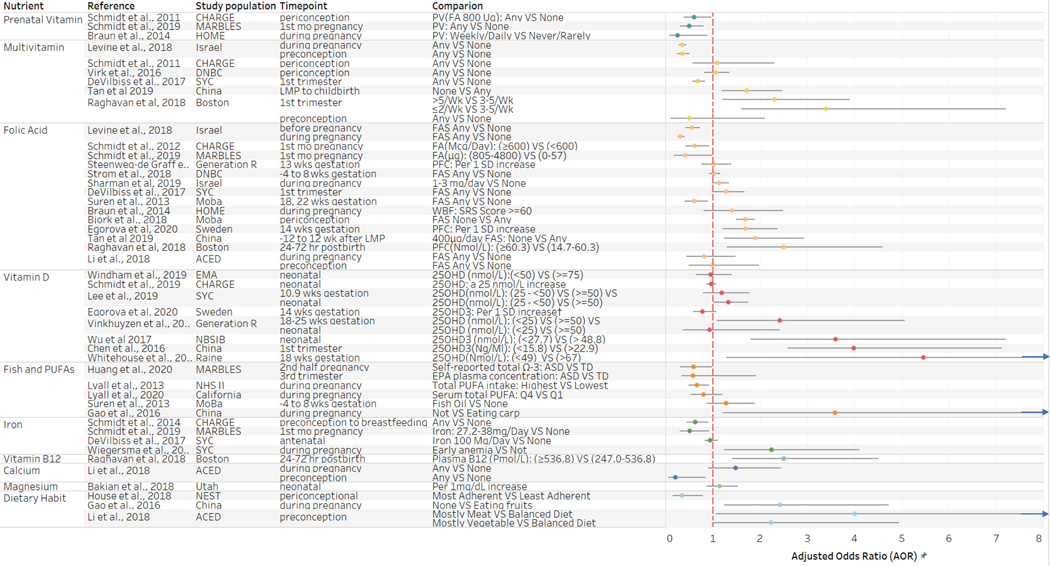
Figure 2 provides primary estimates by nutrient from those studies examining adjusted associations between the dietary factor and dichotomously defined ASD outcome. Most studies reported adjusted odds ratio (AORs), though a few studies reported incidence rate ratios or hazard ratios (HR), and these were plotted on the same axis based on the assumption of comparability of these estimates of relatively modest associations (Davies, Crombie, & Tavakoli, 1998). Plotted estimates demonstrate relationships according to higher vs lower intake of the nutrients (for those studies in which the inverse of estimates was calculated in order to facilitate comparison in this manner, original estimates can be found in Supplemental Figure 1). Overall, a higher or moderate intake of most nutrients examined was associated with reduced odds of ASD, though these were not uniformly statistically significant.
Fig 2.
Adjusted odds ratios and confidence intervals for primary findings of included studies by dietary factor
Table 2 presents primary results from the studies estimating adjusted associations with quantitative measures of ASD-related traits. Overall, strong associations consistent with clinically-impairing symptoms were not observed across these studies, though several studies reported modest inverse associations between the dietary factor and ASD-related traits. An additional study (the Tan et al. Chinese case-control study further described below within the folic acid (FA) and other nutrients sections) suggested increases in ASD-related traits according to the SRS in those without FA and micronutrient supplement use but did not conduct adjusted analyses(Tan et al., 2020).
Table 2.
Association Between Dietary Factors and ASD-related traits (continuous scores) according to reported adjusted betas (95% CI).
| Study | Exposure assessment | Outcome measure¶ | Beta (Confidence Interval) | |
|---|---|---|---|---|
|
| ||||
| Folic Acid | Steenweg-de Graaff et al., 2014 | Maternal preconception FAS†: use vs. not | SRS (18-item subset) | −0.042 (−0.068, −0.017) |
|
| ||||
| Braun et al., 2014 | PVS‡: weekly/daily vs. rarely/never | SRS total T scores | −2.7 (−6.5, 1.1) | |
| WBF§ per standard deviation increase | 0.6 (0.3,1.5) | |||
|
| ||||
| Vitamin D | Vinkhuyzen et al., 2016 | Maternal 25OHD (nmol/L): deficient (<25) vs. sufficient (≥ 50) | SRS (18-item subset) | 0.06 (0.04, 0.08) |
| Neonatal 25OHD (nmol/L): deficient (<25) vs. sufficient (≥ 50) | 0.03 (0.01, 0.05) | |||
|
| ||||
| Lopez-Vicente et al., 2019 | 25OHD3 (ng/mL) per 10-unit increase | CAST | −0.02 (−0.17, 0.12) | |
| ≥30 ng/mL vs. <20ng/mL 25OHD3 | −0.26 (−0.71, 0.19) | |||
|
| ||||
| Fish and PUFAs | Steenweg-de Graaff et al.,2016 | ω−3:ω−6 ratio quintile: highest vs. lowest | SRS (18-item subset) | −0.024 (−0.047, −0.000) |
| 1-unit increase in linoleic acid | 0.012 (0.001–0.023) | |||
|
| ||||
| Julvez et al., 2016 | Fish intake quintile: highest vs. lowest | CAST | −0.55 (−1.06, −0.04) | |
|
| ||||
| Vecchione et al, 2020 | Overall fish intake: High (Daily) VS Low (Monthly/None) in second half of pregnancy | SRS raw total score | 5.60 (1.76, 12.97) | |
| Salmon: Any VS None | −4.66 (−10.29, 0.97) | |||
FAS=Folic acid supplementation.
PVS=prenatal vitamin supplementation.
WBF= whole blood folate; 25OHD= 25 hydroxycholecalciferol.
SRS= Social Responsiveness Scale; CAST=Childhood Autism Spectrum Test. Higher scores on both indicate greater ASD traits or severity.
Suspected “critical windows” (that may represent particularly influential time periods for potential associations with ASD given neurodevelopmental timelines) for folic acid, PUFA, and vitamin D (as the three nutrients with largest number of prior studies examining associations with ASD), are summarized in Supplemental Figure 2. In general, evidence supports the periconceptional period as a potential critical window for folic acid, while PUFAs and vitamin D may have greater influence later in pregnancy(M. Li et al., 2019; Wagner, Taylor, Johnson, & Hollis, 2012), though critical windows for these have not been definitively defined.
3.1. Prenatal vitamins
Prenatal vitamins typically contain higher amounts of folic acid, iron, and vitamin B6 and B12 as compared to standard adult multivitamins, in order to meet the extra nutrient requirements of pregnancy(Schmidt et al., 2011). However, there is also wide variability across formulations(Saldanha et al., 2017), and certain prenatal vitamins (such as many gummy vitamins) do not contain key nutrients like iron(WIC., 2018). Studies examining prenatal vitamins in association with ASD have for the most part attributed observed protective associations to folic acid within the supplements, although the specificity of effects is difficult to determine.
We identified 3 studies examining prenatal vitamin use in association with ASD; all suggested decreased odds with prenatal vitamin use during pregnancy and/or preconception(Braun et al., 2014; Schmidt et al., 2011; Schmidt, Iosif, Guerrero Angel, & Ozonoff, 2019). In the first of these, conducted within the CHARGE study, the odds of ASD was approximately 40% lower in mothers who retrospectively reported any prenatal vitamin use during the periconceptional period as compared to mothers reporting no use (Schmidt et al., 2011). When examining frequency of use, a similar relationship was found with moderate frequency of periconceptional prenatal vitamin use (4 days/week) and the odds of ASD. The study also reported interaction effects with polymorphisms in two genes, specifically that children who had the COMT 472G>A polymorphism, or whose mothers had the MTHFR 677 C>T polymorphism, and whose mothers reported no prenatal vitamin use, were at a greater risk for autism. In the second study, conducted within the HOME (Health Outcomes and Measures of the Environment) cohort in Cincinnati (USA), prospectively reported prenatal vitamin use was associated with reduced odds of higher autistic traits, measured by SRS scores (Braun et al., 2014). The third study, conducted within the MARBLES prospective cohort (a high-familial risk study including families who already had at least one child with ASD and follows subsequent children), reported a 50% lower risk of ASD in children of mothers reporting prenatal vitamin use during the first month of pregnancy as compared to those of mothers reported no use during this period of time (Schmidt, Iosif, et al., 2019). Although we could not confirm brands of the prenatal vitamins used in each study, results across prenatal vitamin studies have yielded consistent findings.
Key areas for future studies of prenatal vitamin use in association with ASD and ASD-related traits to clarify include more detailed consideration of timing and frequency, and assessment of whether individual nutrients vs the combination of them may be driving these associations.
3.2. Multivitamins
Studies on multivitamins serve as a complement to the prenatal vitamin studies by adding larger sample sizes, often with prospectively collected data. To date, there have been 5 published studies (DeVilbiss et al., 2017; Levine et al., 2018; Raghavan et al., 2018; Schmidt et al., 2011; Virk et al., 2016) examining maternal multivitamin supplement (MVS) use during preconception or pregnancy and ASD. Three of these reported results consistent with a significant inverse association between MVS use and ASD, with estimates ranging from 0.3 to 0.69 (DeVilbiss et al., 2017; Levine et al., 2018; Raghavan et al., 2018). Two prospective cohort studies, one within the SYC cohort(DeVilbiss et al., 2017) and the other within a Boston Birth Cohort(Raghavan et al., 2018), reported a decrease in the risk of ASD in children of mothers who prospectively self-reported any MVS use during the antenatal/preconception period as compared to non-users. In addition, the Boston Birth Cohort study also reported a U-shaped relationship, with increased risks of ASD of 150% for low (2 or fewer vitamins/week) as well as high-frequency MVS (5 or more vitamins/week) relative to moderate preconception (3–5 times/week) MVS use. In the largest study to date investigating this association, an Israeli nested case-cohort study using data from the Maccabi Healthcare Services (MHS), MVS use both before and during pregnancy according to dispensation records was associated with an approximately 65% lower likelihood of ASD in the offspring (Levine et al., 2018). In contrast, in a large subsample of the DNBC study, no association between prospectively self-reported periconceptional (−4 to 8 weeks) MVS use and ASD or autistic disorder was found (Virk et al., 2016). The previously mentioned CHARGE case-control study reported no statistically significant association between retrospectively self-reported multivitamin use around conception and risk of autism (Schmidt et al., 2011).
In addition, one investigation examined micronutrient supplementation in unadjusted analyses. This Chinese case control study (which also examined FA use) reported increased odds of ASD, as well as greater autism-related traits according to the SRS, in children of mothers retrospectively reporting no micronutrient supplementation use compared to those reporting use (Tan et al., 2020). A prospective cohort study, previously mentioned in the folic acid section, also examined self-reported maternal use of other vitamins/minerals supplements from 4 weeks before to 8 weeks after pregnancy in the exploratory analyses and reported no statistically significant association with risk of ASD(Suren et al., 2013).
Results are therefore less consistent for associations with prenatal multivitamin use than for use of prenatal vitamins, perhaps supporting the role of the specific nutrients with increased content in most prenatal vitamins. As the studies represent 4 different countries, differences in formulizations in MVS may have influenced results. While all studies utilizing prospectively collected information on MVS use identified inverse associations, the studies reporting no association relied on either retrospectively (n=1) or prospectively (n=1) collected information. Exposure timing and definition varied somewhat across studies, though generally patterns were not evident based on this. Only 1 study classified MVS use as a categorical variable according to levels of frequency of use (Raghavan et al., 2018). The other 4 MVS studies classified MVS use as a binary variable (any vs none) during pregnancy. Thus, the examination of dose is lacking, and future work may consider increased resolution of timing and dose for MVS studies.
3.3. Folic acid (FA)/ folate
Folate is a B-vitamin obtained from dietary sources, such as leafy greens, beans, and legumes, or supplementation. Evidence of prenatal deficiencies leading to neural tube defects led to widespread fortification in the US in 1998 and 53 other countries (Crider, Bailey, & Berry, 2011), though insufficiency and deficiency are still common (DeVilbiss et al., 2015; Rogers et al., 2018). Folate acts as a cofactor in one-carbon metabolism, particularly in the conversion of homocysteine to S-adenosyl-methionine, a key methyl-donor, and thus plays a critical role in methylation(Mahmoud & Ali, 2019), a process with suspected importance for DNA synthesis and DNA methylation directly related to neurodevelopment(Lintas, 2019; Zeisel, 2011). Therefore deficiency may result in DNA damage and neuronal death (Mattson & Shea, 2003). Sufficient levels of folate are necessary for favorable neurodevelopment in the fetus during the critical windows of pre-conception and early pregnancy (DeVilbiss et al., 2015; Geiman & Muegge, 2010). Further details of folate mechanisms have been previously summarized in several systematic reviews and meta-analyses (Castro et al., 2016; DeVilbiss et al., 2015; Y. Gao et al., 2016; Lintas, 2019; Moretti et al., 2004; Naninck, Stijger, & Brouwer-Brolsma, 2019; Wang, Li, Zhao, & Li, 2017).
To date, a total of 15 studies evaluated the association between maternal folic acid and ASD: 12 of these utilized self-reported FA supplementation use (9 prospective cohort studies, 2 of which relied on medical dispensation records, and 3 case-control studies with retrospective reporting), 4 used maternal folate biomarkers from samples collected during pregnancy (3 prospective cohort studies and one case-control study), and 1 (a prospective cohort) used both reported supplementation and biomarkers (Bjork et al., 2018; Levine et al., 2018; Raghavan et al., 2018; Schmidt, Iosif, et al., 2019; Schmidt et al., 2012; Steenweg-de Graaff, Ghassabian, Jaddoe, Tiemeier, & Roza, 2015; Suren et al., 2013; Tan et al., 2020). Several studies are drawn from the same study population; 2 came from the DNBC cohort (Strom, Granstrom, Lyall, Ascherio, & Olsen, 2018; Virk et al., 2016), and 2 from the MoBa cohort (Bjork et al., 2018; Suren et al., 2013). Eleven of these 15 studies were discussed in detail in a recent systematic review(M. Li et al., 2019). Thus, we provide somewhat greater information below on the 4 more recent studies(Bjork et al., 2018; Egorova et al., 2020; Sharman Moser et al., 2019; Tan et al., 2020).
Eight studies(3mentioned above in the multivitamin section), including 3 case-control studies, and 5 prospective cohort studies, found inverse relationships between reported FA and ASD risk. Effect estimates across these studies ranged from 0.3–0.6. Of these studies, 5 relied on prospectively self-reported FA supplement use, 2 on retrospectively self-reported FA supplement use, and 1 on dispensation records; 6 examined ASD diagnosis and 2 examined ASD-related traits (Bjork et al., 2018; Levine et al., 2018; Raghavan et al., 2018; Schmidt, Iosif, et al., 2019; Schmidt et al., 2012; Steenweg-de Graaff et al., 2015; Suren et al., 2013; Tan et al., 2020). Of the more recent studies, this includes a Chinese case-control study and an analysis in the MoBa cohort. The Chinese study examined periconceptional FA supplement use (400 μg/day) and ASD as well as autism-related traits, with decreases in risk according to both outcome measures (Tan et al., 2020). The MoBa study found a significant inverse relationship between prospectively self-reported FA supplements and risk of autistic traits as measured by M-CHAT and SCQ (Bjork et al., 2018).
In contrast to expected protective effects, 2 studies reported a positive relationship between FA and ASD (Egorova et al., 2020; Raghavan et al., 2018). In addition to associations with overall multivitamin use, the previously described Boston Birth Cohort study also reported a 150% increased risk of ASD in children of a mother with high plasma folate levels (>60nmol/L) compared to mothers with plasma folate level between 14.7 nmol/L and 60.3 nmol/L, again suggesting a U-shape relationship for folate specifically(Raghavan et al., 2018). A recent Swedish matched case-control study reported a weak positive association between maternal total folate concentration (total detected 5-methyltetrahydropholate (mTHF) and 4-alfahydroxy-5-methyl-tetrahydrofolate (hmTHF)), which was not statistically significant after adjustment for multiple testing (Egorova et al., 2020).
Seven studies reported no association between periconceptional and/or prenatal FA and ASD, including 2 case-control studies(Y. M. Li et al., 2018; Sharman Moser et al., 2019) and 5 prospective cohort studies(Braun et al., 2014; DeVilbiss et al., 2017; Steenweg-de Graaff et al., 2015; Strom et al., 2018; Virk et al., 2016)). Of the cohort studies, 3 relied on prospectively self-reported FA supplement use, including 2 analyses in the DNBC(Strom et al., 2018; Virk et al., 2016), and 1 in the SYC cohort(DeVilbiss et al., 2017)). Of the case control studies, one relied on retrospectively self-reported information (Y. M. Li et al., 2018), and one on national registry data (Sharman Moser et al., 2019). Lastly, two studies assessed offspring autistic traits and maternal folate concentration using biomarkers from blood samples collected during pregnancy from the HOME cohort(Braun et al., 2014) and the Generation R cohort(Steenweg-de Graaff et al., 2015).
Results across the rapidly growing number of folate/folic acid supplementation studies therefore suggest that, while protective associations have been replicated, there is also a fair degree of conflicting information. Such discrepancies may be due to differences in folate measurement. While the majority of studies relying on self-reported folic acid supplementation (at 800μg, 600μg, or 400μg) support an inverse association between folic acid and ASD, all studies relying on folate biomarkers have reported null associations. However, these biomarkers were measured outside of the suspected critical window of the periconceptional period, suggesting the need to clarify temporal variability in folate biomarkers and to examine associations with folate levels earlier in pregnancy. Another potential driver of discrepancies may be study location, suggesting a potential influence of fortification practices and regional diet; though not fully consistent, most of the null studies were conducted outside the US. In addition, several studies reporting null associations had larger sample sizes than most of the positive association studies, though a number indicating protective associations have also had large sample sizes and prospective data, suggesting discrepancies are not driven solely by statistical power or reporting biases. Comparing results across studies by outcome definition, both positive and null studies were identified for ASD diagnosis and ASD-related traits, though a greater proportion studies examining association with the latter reported null findings. Differences in results by timing of outcome assessment are not evident across the studies.
Despite the many studies examining maternal folate/folic acid supplementation, work addressing mechanisms is still generally lacking, and there is a need for pathway analyses assessing measured methylation levels. In 1 recent study taking perhaps the most comprehensive approach thus far in terms of investigating folate as well as other nutrients and serum biomarkers, an increased risk of ASD with higher folate initially suggested was attenuated to a null association after adjustment for other factors in the potential pathway(Egorova et al., 2020). Future studies in this area may benefit from use of similar comprehensive approaches, use of biospecimens in the suspected periconceptional susceptibility time frame, and the ability to examine associations across self-reported supplement use, dietary sources, and biomarker data.
3.4. Vitamin D
Vitamin D, a family of steroid compounds, is mostly obtained through the transformation of 7-dehydrocholesterol in the body upon exposure to ultraviolet light from the sun. The active form of vitamin D, 25-hydroxyvitamin D (25(OH)D), which is the sum of 25(OH)D2 and 25(OH)D3, is a critical hormone expressed in placenta (J. S. Shin, Choi, Longtine, & Nelson, 2010) and the brain for physiological, neurological, and immune development and function (Cannell, 2008) (Eyles, Burne, & McGrath, 2013). 25(OH)D also serves as an indicator of levels of vitamin D sufficiency. During the early weeks of pregnancy, vitamin D may act as an immune modulator to prevent maternal-fetal rejection, and serum vitamin D concentration levels are elevated in early pregnancy and throughout the pregnancy, relative to non-pregnancy (Hollis & Wagner, 2017). Neonatal levels of vitamin D level are dependent on maternal vitamin D during pregnancy, reflecting 60–80% maternal vitamin D level(Hollis, Johnson, Hulsey, Ebeling, & Wagner, 2011; Kiely, Hemmingway, & O’Callaghan, 2017; Marshall, Mehta, Ayers, Dhumal, & Petrova, 2016); hence, studies examining neonatal levels were considered to meet our inclusion criteria of representing gestational exposures.
We identified 11 studies evaluating maternal and/or neonatal biomarker-measured vitamin D status in relation to ASD, including 4 prospective cohort studies (two of these from the Generation R cohort)(Lopez-Vicente et al., 2019; Vinkhuyzen et al., 2018; Vinkhuyzen et al., 2017; Whitehouse et al., 2013) and 7 case-control studies(Chen, Xin, Wei, Zhang, & Xiao, 2016; Fernell et al., 2015; Lee et al., 2019; Schmidt, Niu, Eyles, Hansen, & Iosif, 2019; Windham et al., 2019; Wu et al., 2018).
Of these, 7 studies reported an inverse association between ASD-related outcomes in offspring and maternal (n=4) and/or neonatal (n=4) vitamin D status according to continuous 25(OH)D or 25(OH)D3 levels (ranging from a 16% to 82% reduced risk (Chen et al., 2016; Lee et al., 2019; Schmidt, Niu, et al., 2019; Vinkhuyzen et al., 2018; Vinkhuyzen et al., 2017; Whitehouse et al., 2013; Wu et al., 2018)). Of these studies reporting significant inverse associations, 3 defined vitamin D deficiency as total 25(OH)D concentration <25 nmol/L, vitamin D insufficiency as total 25(OH)D concentration 25 - <50 nmol/L, and vitamin D sufficiency as total 25(OH)D concentration >=50 nmol/L(Lee et al., 2019; Vinkhuyzen et al., 2018; Vinkhuyzen et al., 2017). Two analyses in the Dutch Generation R cohort suggested associations between vitamin D and ASD diagnosis as well as quantitatively-assessed ASD-related traits. One found a more than twofold increased odds of ASD in children of mothers with mid-gestational vitamin D deficiency, but no association with neonatal vitamin D deficiency (Vinkhuyzen et al., 2017). The other reported an association between higher SRS scores (indicating a greater degree of ASD-related traits) and both mid-gestation and neonatal vitamin D deficiency according to 25(OH)D levels (Vinkhuyzen et al., 2018). A study within the SYC cohort reported a higher odds of ASD in children with neonatal vitamin D deficiency and in children of Nordic-born mothers with first-trimester vitamin D insufficiency. Increased odds were also observed in children with low maternal and neonatal 25(OH)D levels (below 55.1 nmol/L and 27.2 nmol/L, respectively)(Lee et al., 2019). In the remaining studies reporting inverse associations between 25(OH)D level and ASD, exposure categorization varied. The Australian Raine cohort study, the first to examine the prospective association, reported an increased odds of having a high score on the Attention Switching subscale of Autism Spectrum Quotient (AQ) in children of mothers with mid-gestation total 25(OH)D concentrations <49 nmol/L (vs >67 nmol/L), though the effect estimate was imprecise (Whitehouse et al., 2013). In a small Swedish study including 58 sibling pairs, siblings with ASD were found to have lower levels of 25(OH)D measured in newborn bloodspot samples as compared to their siblings without ASD(Fernell et al., 2015). Two Chinese matched case-control studies measured 25(OH)D3 levels, 1 in children of mothers with lower first-trimester 25(OH)D level (<15.8 vs > 22.9 ng/ml)(Chen et al., 2016), and the other in children with low neonatal 25(OH)D level (< 27.7 vs > 48.8 nmol/L)(Wu et al., 2018). Finally, an analysis in the CHARGE study (in California) found reduced odds of ASD associated with an increased neonatal total 25(OH)D (per 25nmol/L), though in female children only (Schmidt, Niu, et al., 2019).
In comparison, 3 studies (1 prospective cohort and 2 case-control) all found no statistically significant relationship between 25(OH)D levels and ASD (Egorova et al., 2020; Lopez-Vicente et al., 2019; Windham et al., 2019). The INfancia y Medio Ambiente (INMA; Environment and Childhood) Project in Spain reported no association between maternal first trimester 25(OH)D3 and offspring autism-related traits(Lopez-Vicente et al., 2019). A Swedish matched case-control study from the Northern Sweden Maternity Cohort (NSMC) (described in the folic acid section), reported no association between maternal 25(OH)D3 level during early pregnancy and ASD(Egorova et al., 2020). The Early Markers for Autism (EMA) case-control study conducted in Southern California (USA) reported no association between neonatal total 25(OH)D deficiency (<50 nmol/L) compared to sufficiency (>=75 nmol/L) (Windham et al., 2019). However, potential differences by sex (a protective association in boys but not in girls) and race/ethnicity (a protective association in non-Hispanic Whites but not other groups) were noted, though these did not reach statistical significance.
The weight of evidence across vitamin D studies therefore supports a protective association between higher 25(OH)D levels and ASD or ASD-related traits. However, there are discrepancies across work in the levels corresponding to such relationships and in the strength of the association. While several studies reporting protective associations were from lower-sunlight regions, and 2/3 reporting null associations were from higher sunlight areas, results were not fully concordant with an explanation of geographic region influencing the ability to detect associations. In addition to the differences regarding geographic region and sunlight, as the primary source of vitamin D, comparison across studies is also challenged by the different parameterizations of 25(OH)D levels to define sufficiency, insufficiency and deficiency status. Most studies reported an inverse association between low 25(OH)D levels less than or equal to 25 nmol/L and ASD, while no associations were found when comparing slightly higher 25(OH)D level (ranging from 20 to 50 nmol/L) to higher 25(OH)D levels. All studies relied on prospectively collected biomarkers to reflect vitamin D levels. Three studies examined both maternal and neonatal (measured at birth from newborn blood spots) 25(OH)D level(Lee et al., 2019; Vinkhuyzen et al., 2018; Vinkhuyzen et al., 2017); 2 of these reported consistent findings according to levels measured across these timepoints. Thus, the use of neonatal vs. maternal 25(OH)D level do not appear to drive discrepancies in study findings. Future work may seek to clarify dose-response relationships, address the role of dietary sources of vitamin D, and further consider potential differences by geographic region and within certain subgroups of the population.
3.5. Fish and PUFAs
Polyunsaturated fatty acids (PUFAs), including omega-3 (ω−3) and omega-6 (ω−6) fatty acids, play a critical role in neurodevelopment as components of neuronal membranes and as factors involved in neurogenesis, neuronal migration, synapse formation, as well as metabolism of neurotransmitters (Wurtman, 2008). PUFAs are also involved in both pro- and anti-inflammatory processes(Calder, 2010). Key fatty acids within this class include the essential fats alpha-linolenic acid (ALA) and linoleic (LA) acid, which must be obtained from the diet, as well as the ω−3 PUFA docosahexaenoic acid (DHA) acid, given its high abundance in the brain (Hadders-Algra, 2010). While PUFAs are required for neurodevelopmental processes throughout pregnancy, uptake of PUFAs (and DHA in particular) in the fetal brain is most rapid during the third trimester (Gil-Sanchez, Demmelmair, Parrilla, Koletzko, & Larque, 2011). PUFAs are also critical for postnatal brain growth in the first year of life (Lauritzen et al., 2016). Fatty fish is a key source of the critical long-chain PUFAs including DHA as well as eicosapentaenoic acid (EPA), though certain ω−3, as well as ω−6 PUFAs, can also be obtained from vegetable oils, flaxseed, and nuts (Saini & Keum, 2018). Along with PUFAs, fish may contain heavy metals and other contaminants with potentially adverse and counteractive effects on neurodevelopment and could serve as potential confounders. However, this depends on the fish type, and some work has suggested an overall beneficial effect of fish even when accounting for mercury levels(E. Oken, Radesky, et al., 2008). A number of studies have examined methylmercury (MeHg), a known neurotoxin that accumulates in fatty fish, in association with ASD, and reported no statistically significant associations with ASD(McKean et al., 2015; van Wijngaarden et al., 2013).
A total of 8 studies examining PUFAs or primary PUFA sources (fish intake) were identified in this review. Four studies examined PUFA levels according to biospecimens or reported diet in or around pregnancy(Huang, Iosif, Hansen, & Schmidt, 2020; Lyall, Munger, O’Reilly, Santangelo, & Ascherio, 2013; Lyall, Windham, Whitman, Snyder, & Newschaffer, In press; Steenweg-de Graaff et al., 2016; Suren et al., 2013), with three reporting associations but in somewhat differing directions (Huang et al., 2020; Lyall et al., 2013; Steenweg-de Graaff et al., 2016). In the US-based Nurses’ Health Study II (NHS II), decreased risk of ASD was found with higher maternal total PUFAs (as well as total n-6 and linoleic acid) according to prospectively reported intake. Increased risk with very low intake of ω−3 PUFAs was also observed (Lyall et al., 2013). The Generation R cohort of the Netherlands reported a significantly increased risk between a low ω−3 to ω−6 ratio according to measured levels during mid-pregnancy; however, the finding was primarily attributed to higher ω−6 fatty acids, suggesting a conflicting result to the prior NHSII work (Steenweg-de Graaff et al., 2016). Two other studies have reported no overall association between measured PUFA levels and ASD-related traits or diagnosis, though both reported other potentially suggestive findings (Huang et al., 2020; Lyall et al., In press). Specifically, in the MARBLES cohort, decreased risk of ASD was found with higher total ω−3 according to reported diet in the second half of pregnancy(Huang et al., 2020), but not according to measured 3rd-trimester levels. However, the sample size was small and there was a suggestive decrease in risk for one ω−3 fatty acid (EPA). In a relatively large population-based case control study from California, levels of several ω−3 and ω−6 PUFAs, as well as total PUFA, measured in mid-pregnancy samples were not significantly associated with ASD, though lower levels of total and ω−3 PUFAs were associated with increased risk of ASD with comorbid intellectual disability(Lyall et al., In press).
Two studies have also reported no associations with maternal fish oil supplementation (usually containing DHA and/or DHA and DHA+EPA) and ASD(Lyall et al., 2013; Suren et al., 2013), though 1 had limited power (Lyall et al., 2013) and the other may have examined use outside the critical window(Suren et al., 2013).
Three studies have examined associations with fish or seafood consumption during pregnancy and ASD(L. Gao et al., 2016; Julvez et al., 2016; Vecchione et al., 2020). In a population-based Spanish birth cohort, retrospectively-reported higher seafood consumption (increases of 10g/week) was associated with decreased ASD symptom scores as measured by the Childhood Asperger Syndrome Test(Julvez et al., 2016). A small Chinese matched case-control study also found an inverse association between retrospectively reported maternal carp intake during pregnancy and offspring ASD(L. Gao et al., 2016). Most recently, prospectively reported higher maternal fish intake, particularly in the second half of pregnancy, was associated with increases in autistic traits as measured by the SRS in 2 pregnancy cohorts. However, differences by fish type were suggested; shellfish and large fish species accounted for observed increases in SRS scores, while salmon was associated with decreases in child SRS scores. Since salmon does not tend to bioaccumulate toxins as do the large fatty fish, this work reiterates the need to consider fish type(Vecchione et al., 2020).
Thus, while there are several suggestive findings, results across PUFA studies do not paint a clear picture. No consistent pattern was observed regarding study design and exposure measurement in the context of the findings. For the few studies utilizing biomarker-based levels, use of absolute concentrations (as in Huang et al., 2020 and Lyall et al., In press) versus those expressed as a percent of total fatty acids (as in Steenweg-de Graaff et al., 2016) may be a driver of differences. There is some evidence that the latter approach could lead to reversals of associations with outcomes(Schwertner & Mosser, 1994; Sergeant et al., 2016), though there is debate about the best approach, and consideration of PUFAs within the context of other lipids and their metabolism is needed. It could be PUFAs do not play a strong role in social communication traits characteristic of ASD diagnosis. Alternatively, it may also be that existing work has not captured the critical window for PUFAs, which, in addition to during late pregnancy, could also extend into infancy. Existing work has also not sufficiently addressed potential associations with ASD phenotypes, which may deserve further attention given suggestive findings with ASD-related traits(Steenweg-de Graaff et al., 2016) and associations with ASD with ID(Lyall et al., In press). These areas represent opportunities for future work addressing the association between PUFAs and ASD.
3.6. Other nutrients
Several other nutrients have been examined in association with ASD, though in only a few studies. Iron status has been linked with neurotransmitter production, myelination, and immune function (Kim & Wessling-Resnick, 2014). Sufficient maternal iron level is crucial both during pregnancy and post-delivery as the developing infant requires external sources of iron until the age of 4–6 months (Burke, Leon, & Suchdev, 2014). Meat, poultry and fish, are key sources of iron, and particularly of the more bioavailable form, heme iron; iron is also available from plants, though in the primarily non-heme form (West & Oates, 2008). To date, only 4 studies have evaluated the association between maternal iron and ASD (DeVilbiss et al., 2017; Schmidt, Tancredi, Krakowiak, Hansen, & Ozonoff, 2014; Suren et al., 2013; Wiegersma, Dalman, Lee, Karlsson, & Gardner, 2019). One of these studies used data from the CHARGE case-control study, and reported a 51% lower odds of ASD among mothers in the highest quintile of iron intake (≥ 86mg/day) from 3 months before pregnancy through breastfeeding according to retrospective report on FFQ, as compared to mothers in the lowest quintile. This association was strongest during breastfeeding after adjustment for folic acid intake and other covariates (Schmidt et al., 2014). In a study conducted within the SYC (also discussed above with regard to FA and multivitamins), no consistent evidence was found for a relationship between prospectively self-reported antenatal iron use, obtained from the medical birth register, and the risk of ASD (DeVilbiss et al., 2017). However, another study conducted within the SYC reported an association with maternal anemia diagnosed within the first 30 weeks of pregnancy and increased risk of offspring ASD (Wiegersma et al., 2019), while no association was found for anemia later in pregnancy. Lastly, a previously mentioned study from the MARBLES prospective cohort reported an inverse association between the top two tertiles of the daily iron intake during the first month of pregnancy and the risk of ASD compared to the lowest tertile, though no statistically significant association was found between iron intake at or above 27mg and ASD risk(Schmidt, Iosif, et al., 2019).
Only one study has specifically focused on vitamin B12 and ASD, though several others have considered this and other B vitamins. Vitamin B12 is involved in the one-carbon metabolism pathway supporting cell division and differentiation(Chandyo et al., 2017). The previously discussed prospective Boston Birth Cohort Study found that elevated maternal plasma vitamin B12 in the days after birth (>600 pmol/L), but not deficiency (<200 pmol/L), was associated with increased ASD risk in children (Raghavan et al., 2018). A handful of other studies, focused on folate’s association with ASD, have examined adjustment for vitamin B12(Chen et al., 2016; Schmidt et al., 2017; Schmidt et al., 2012), and one explored interactions between vitamin B12 (as well as vitamin B6) and pesticide exposure (Schmidt et al., 2017). Though adjusted associations were not examined for B12 and B6 in this study, crude data provided suggested somewhat lower maternal levels of both in cases, though differences were overall not statistically significant. Two studies also examined the independent association between B12 and ASD in their secondary or sensitivity analysis, but no statistically significant association was found(Chen et al., 2016; Strom et al., 2018).
For other nutrients, independent associations with ASD have been examined in only a single study. Calcium mainly serves as a building block supporting bone and teeth development (Kawicka & Regulska-Ilow, 2013), but also plays a role in key signaling processes, particular in the depolarizing signal transmission and synaptic activity (Brini, Cali, Ottolini, & Carafoli, 2014). A Chinese matched case-control study (previously described in the folic acid section) reported a decreased odds of ASD in mothers who reported calcium supplementation during preconception, but not during pregnancy or lactation, compared to those who did not (Y. M. Li et al., 2018). Magnesium is a cofactor in over 300 enzymatic reactions, is needed for DNA synthesis, and is also required for conversion of vitamin D into its active form(Gröber, Schmidt, & Kisters, 2015). Antenatal magnesium administration is recommended to prevent children born preterm against cerebral palsy(ACOG, 2012). One prospective cohort study conducted in the US found that neonatal serum magnesium level (reflecting maternal magnesium level at the end of pregnancy) had no association with ASD risk in preterm children(Bakian, Bilder, Korgenski, & Bonkowsky, 2018).
Finally, a Swedish matched case-control study, discussed in the folic acid and vitamin D sections, examined a large number of other nutrient biomarkers, including several involved in the one-carbon metabolism pathway (such as homocysteine and vitamin B2), as well as tryptophan, and the lipid-soluble vitamins A, E, and K from maternal serum collected at 14 weeks gestation, and found no associations with these other nutrients and offspring ASD, though the sample size was small (Egorova et al., 2020).
3.7. Dietary patterns/habits
Dietary patterns capture usual intake of foods and can be a useful summary of combined effects of foods and nutrients on health outcomes (Hu et al., 1999). Most established dietary patterns are created based on reported intake using techniques to identify groupings of foods (with Prudent (A. D. Wood et al., 2014), Western (Lopez-Garcia et al., 2004), and Mediterranean patterns (Bach et al., 2006) as some examples), or according to adherence to prescribed guidelines (such as the Healthy Eating Index (HEI) or Alternative Healthy Eating Index (AHEI) (McCullough et al., 2002)). While most of these patterns have been associated with outcomes like cardiovascular disease, mortality, and stroke, with inflammation a key suspected mechanism, limited work has examined dietary patterns during pregnancy in association with offspring outcomes (Rifas-Shiman, Rich-Edwards, Kleinman, Oken, & Gillman, 2009).
We identified 3 studies evaluating associations between combined dietary factors or dietary patterns and ASD (L. Gao et al., 2016; House et al., 2018; Y. M. Li et al., 2018). Two Chinese matched case-control studies (previously described above in the folic acid and fish/PUFAs sections) reported associations between self-reported maternal unbalanced dietary “habits” and ASD (L. Gao et al., 2016; Y. M. Li et al., 2018), including increases in risk of ASD in children whose mothers retrospectively reported diets consisting of mostly meat or mostly vegetables during the preconception period(Y. M. Li et al., 2018) and lack of fruits during preconception and pregnancy period(L. Gao et al., 2016). The Newborn Epigenetics STudy (NEST), a prospective cohort study conducted in U.S., reported lower odds of having autism-related behavior outcomes (measured according to the problem and competency domains from the Infant Toddler Social and Emotional Assessment (ITSEA) (Kruizinga et al., 2014)), in children of mothers who had higher adherence to a Mediterranean diet around the periconceptional period (House et al., 2018). Future studies considering these and other established dietary patterns are therefore needed.
4. Discussion
Although research examining maternal diet in association with ASD has begun relatively recently, the field is rapidly growing and has provided suggestive evidence for the importance of a number of nutrients. Most notably, the greatest attention has been paid to the potential role of folate/folic acid, with several replicated findings supportive of a protective association. The results of this review also suggested relative support for a protective association with vitamin D and ASD. Evidence was less consistent for PUFAs. Insufficient evidence exists for other prenatal nutrients, including iron, vitamin B12, calcium, and magnesium. Across all identified studies, most reported reductions in risk of ASD with higher or sufficient nutrient levels, though a number also found evidence for increases in risk with lower levels.
As noted, the bulk of literature examining any single prenatal nutrient in relationship to ASD has focused on folate. To large extent, this work was spawned by initial reports of protective associations with prenatal vitamin use and ASD, for which studies were fairly uniform. These studies were followed by several larger studies on multivitamin use and ASD, obtained via prospective register data, and specific folic acid supplementation and folate biomarker studies. However, findings across these more recent studies were not uniform, with several null studies and even an increased risk with higher folate reported.
Vitamin D also emerged as a nutrient with a growing literature base supporting a protective association with ASD. Studies relying on neonatal vs. maternal levels, both expected to represent prenatal exposure at least to some degree, yielded similar findings. As only three studies assessed autism-related traits, including one reporting a null association, there is a need to further examine the potential association between vitamin D and ASD-related outcome and severity, in addition to addressing potential differences by region.
As noted, the available evidence is conflicting for an association with PUFAs/fish intake. While PUFAs have an established role in neurodevelopment, this has not been borne out in associations with ASD to date. However, there is insufficient evidence to conclusively determine the role of PUFAs in ASD. Additional work is needed examining specific PUFAs individually, in addition to total PUFAs and the omega-3 and omega-6 classes, including in other time windows of neurodevelopment. In addition, given some potential suggestive findings for subgroups and the known importance of PUFAs in neurodevelopment more broadly, further work examining associations with ASD sub-phenotypes is needed in large studies sufficiently powered to address these questions. Studies of fish may also benefit from consideration of potential confounding by contaminants.
The limited number of studies on iron suggests only a very low level of iron may increase ASD risk(DeVilbiss et al., 2017; Wiegersma et al., 2019). However, there is insufficient research on iron, as well as vitamin B12, calcium, magnesium, and broader dietary habits in association with ASD; therefore, these factors require further study to confirm initial findings.
A final consideration in comparing results across existing studies of dietary factors is that differences in study populations may also play a large role in discrepancies across existing work. Specifically, differences in formulation across supplements, fortification policies, demographic and health characteristics relating to dietary preferences, as well as differences in the distribution of other ASD risk factors, including other environmental exposures that may interact with nutrients, may lead to true differences across study populations and may pose challenges to generalizing and replicating findings. These factors should therefore be considered when placing individual study findings in context.
4.1. Common mechanisms
Several key mechanisms have been cited as potential pathways linking individual prenatal nutrients to ASD-related traits, as described above. For folate and vitamin B12, a primary suspected mechanism is through the one-carbon metabolism pathway and impacts on DNA methylation. Altered one-carbon metabolism has shown to be associated with aberrant DNA replication, repair process, and redox homeostasis dysregulation in children with ASD(Schaevitz & Berger-Sweeney, 2012). In addition, differences in DNA methylation have been noted in affected children and their parents, including global hypomethylation of DNA and hypermethylation of specific genes, such as CpG binding protein 2 (MeCP2), leading to downregulation of gene expression(Schaevitz & Berger-Sweeney, 2012). Some animal studies have also linked maternal and postnatal methyl-donor nutrients supplements, which are essential for one-carbon metabolism, with favorable offspring neurodevelopment outcomes, including autism-related traits(McKee & Reyes, 2018). In addition to potential influences on DNA methylation, nutrient roles in immune functioning and inflammation represent another key pathway (particularly for vitamin D, but also relevant for PUFAs and several other nutrients) (Hollis & Wagner, 2017; McDougle et al., 2015; Zerbo et al., 2015). Growing evidence supports immune dysregulation in ASD, including evidence of inflammation, altered cytokine production activity, and anti-brain autoantibody production, which can lead to inhibited neurogenesis and changes in neuronal development and function (Meltzer & Van de Water, 2017; Siniscalco, Schultz, Brigida, & Antonucci, 2018). Oxidative stress is another common mechanism that is linked with prenatal inflammation (Banik et al., 2017; Lyall, Ashwood, Van de Water, & Hertz-Picciotto, 2014; Zerbo et al., 2015), which may directly induce brain damage via oxidative species (Bjørklund et al., 2020) and could, therefore, increase risk of ASD(Frustaci et al., 2012; Gardener, Spiegelman, & Buka, 2011; James et al., 2004; Weisskopf, Kioumourtzoglou, & Roberts, 2015). Lastly, as described in the fish and PUFAs section, certain nutrients may also act through direct impacts on neurodevelopment.
Considering the co-involvement of multiple nutrients in each of these biological processes, the potential convergence of multiple pathways should also be considered. Further details on suspected underlying mechanisms are reviewed elsewhere(DeVilbiss et al., 2015; M. Li et al., 2019; Marques, O’Connor, Roth, Susser, & Bjorke-Monsen, 2013).
4.2. Limitations of existing work
While validated approaches have been developed(W. C. Willett et al., 1985), assessment of diet is challenging and known to be measured with error(Spiegelman, McDermott, & Rosner, 1997; W. Willett, 2013). Studies based on reported intake, including self-reported dietary screeners, food frequency questionnaires, and 24-hour recalls, have not considered strategies to combat this, such as adjustment for measurement error. Several studies utilized retrospective reporting of dietary factors, which may have led to recall bias and exposure misclassification. Compared to studies relied on self-reported information, studies utilizing dispensation records may not accurately represent what was actually taken, though no pattern of discrepancy was found in this review according to this exposure source. A common limitation across many studies here was a lack of measured levels of nutrients; approximately 50% of all studies reviewed had biomarker-based assessment of nutrients. While potential error due to reporting is removed with these studies, variability from factors such as metabolism, processing, and fasting status may play a role. In addition, timing of measurement may influence results; several studies utilizing biomarker-based levels may have been collected outside the nutrient’s suspected relevant time point for influence on ASD (such as early pregnancy or the periconceptional period for folic acid). No study to date has measured levels of nutrients across multiple time points over pregnancy, an important consideration given potential dietary variability over the course of pregnancy particularly for nutrients whose biomarkers may represent more transient exposures and for which prior evidence does not clearly indicate which time period may be most influential on risk. Studies utilizing neonatal or early postnatal samples may be influenced by recent intake of nutrients and therefore could also lead to differences across studies. In addition, few studies had both biomarker and FFQ/reported intake data, as may help to better enable more comprehensive assessment of diet and resolve potential discrepancies by exposure assessment.
Furthermore, a number of studies included in this review (about 30%) had small sample sizes (<500) and/or had a small number of exposed cases (<60), suggesting limited statistical power to detect associations (Braun et al., 2014; Chen et al., 2016; Egorova et al., 2020; Fernell et al., 2015; L. Gao et al., 2016; House et al., 2018; Huang et al., 2020; Schmidt, Iosif, et al., 2019; Vinkhuyzen et al., 2017; Whitehouse et al., 2013). Only a handful of studies considered ASD outcomes according to both diagnosis and quantitative traits, as may help to inform strength of associations across the range of the autism spectrum(L. Gao et al., 2016; Y. M. Li et al., 2018; Schmidt et al., 2011; Schmidt, Niu, et al., 2019; Schmidt et al., 2014; Schmidt et al., 2012; Tan et al., 2020; Whitehouse et al., 2013). Finally, while a number of studies considered certain other dietary factors that may influence associations in their analyses(Braun et al., 2014; Chen et al., 2016; House et al., 2018; Huang et al., 2020; Julvez et al., 2016; Y. M. Li et al., 2018; Lyall et al., 2013; Schmidt et al., 2011; Schmidt et al., 2014; Schmidt et al., 2012; Steenweg-de Graaff et al., 2016; Vecchione et al., 2020), the majority of studies included in this review did not(discussed below). Moreover, as those with higher nutrient intake may be healthier in other ways that may relate to offspring neurodevelopment, studies may have been subject to potential residual confounding by health-related factors.
4.3. Future research
Even for nutrients with replicated findings, limitations in existing work demonstrate the need for replication and continued study. Future work should strive to use biomarkers for exposure assessment with consideration of timing, and ideally with information on reported diet to enable complementary comparisons. Analyses of a broader range of nutrients will need to keep in mind optimal assessment measures, and that these may differ for different nutrients (for example, serum 25(OH)D level may be the best indicator of vitamin D status as compared to dietary recall given sunlight represents the main source of exposure) (Jones, 2008). For folate, a key goal remains to resolve discrepancies between biomarker and reported supplement studies as well as clarification of dose-response effects. Work investigating vitamin D should further consider differences by geographic region, and comparisons of associations with quantitatively measured ASD-related traits and categorical ASD diagnosis. Priorities for studies of PUFAs include clarifying existing discrepancies and examining of associations with sub-phenotypes of ASD. For iron, vitamin B12, calcium, and magnesium, replication of existing findings is needed. Common gaps across studies include a need to understand how and whether intake influences the level of severity, comorbidities, or functioning within those with ASD and whether differences in risk exist by sex or other subgroups. Future work should also consider potential interactions with genetic factors. In addition, pathway analyses are also needed to better understand mechanisms and advance our understanding of how these factors may influence the complex etiology of ASD.
4.3.1. Additional nutrients and combined effects
While some studies included other dietary factors in adjusted analyses, such as fruit and vegetable intake (Braun et al., 2014; Lyall et al., 2013), certain vitamins (including vitamins A, B-6, C, E, and B-12) (Schmidt et al., 2012), cereal (Schmidt et al., 2011), whole grains and total fat (Lyall et al., 2013), estimates for independent associations between dietary factors outside those reviewed here and ASD are largely lacking. Nutrients that should be targeted for future investigation in association with ASD include those in common pathways implicated in ASD etiology as described above. Specifically, these would include co-factors in methionine synthesis with folate, other B vitamins, and nutrients involved in one-carbon metabolism (such as vitamins B12, B6, and choline) and those that impact folate bioavailability (such as methyl-donors and vitamin C, niacin, or riboflavin). Antioxidants such as vitamins A, C, and E also hold potential relevance, given the suspected role of oxidative stress in ASD etiologic pathways as well as evidence of improved cognitive, motor, language, and socio-emotional development with prenatal or perinatal supplementation with certain antioxidants (Frustaci et al., 2012; McGinnis, 2004; Narciso et al., 2016). Other nutrients of potential interest include tryptophan (investigated in one prior review of tryptophan depletion clinical and preclinical studies on treating mood and cognition disorders, though no association found), which is involved in serotonin synthesis in brain regions highly involved in social reasoning (Jenkins, Nguyen, Polglaze, & Bertrand, 2016; Steenbergen, Jongkees, Sellaro, & Colzato, 2016); and flavonoids, which have been shown to have anti-neuroinflammatory effects (Spencer, 2009; Vafeiadou et al., 2009). Given these associations with outcomes relevant to ASD and aforementioned mechanistic relationships, consideration of these factors in association with ASD seems warranted.
In addition to an expanded line of research examining a wider range of nutrients individually in association with ASD, future work should also consider maternal diet more comprehensively to address collective effects. Further consideration of dietary patterns is therefore warranted, in order to consider combined effects of foods and nutrients across common food intake. For consideration of combined effects of specific nutrients, additional studies using statistical methods capable of handling a large number of correlated nutrients, such as the Bayesian techniques used by Egorova (Egorova et al., 2020), may be useful in advancing ASD research. Several studies have utilized Bayesian techniques to examine multiple nutrients (some in combination with other environmental exposures) in association with other outcomes, supporting a role of combined effects over and above individual nutrients and/or identifying novel interactions(Liu et al., 2018; Sordillo et al., 2018; Zhao, Singh, & Naumova, 2019). Thus, ASD research may benefit from similar considerations of combined effects of dietary factors.
Finally, future work considering the potential role of dietary factors as modifiers of other ASD risk factors, and/or in interaction with genetic factors, is needed. Several studies provide support for interactive effects between nutrients and environmental risk factors on other neurodevelopmental outcomes, including PUFAs and pesticides(Ogaz-Gonzalez et al., 2018; Schmidt et al., 2017), folic acid and antiepileptic drugs(Bjork et al., 2018; Veiby et al., 2013; A. G. Wood et al., 2015) folic acid and smoking(Jiang et al., 2016), and prenatal methyl-donor nutrient intake (particularly methionine) and air pollution exposure(Stingone et al., 2017), suggesting that sufficient or higher intake of nutrients can mitigate risks and/or that low levels may increase risks from these exposures. For ASD specifically, a few studies have emerged examining joint effects of folic acid or prenatal vitamin use with environmental exposures, including phthalates(Oulhote et al., 2020; H. M. Shin et al., 2018), air pollution(Goodrich et al., 2018), and pesticides (Schmidt et al, 2017), with several findings supportive of modification. Even in the face of weak or null independent associations with nutrients, interaction effects may exist, suggesting the need, again, to consider a wider range of nutrients in such work. Because diet is a factor that can be intervened on at the individual level, it may represent a more accessible way to modulate risks than factors regulated at the population level like air pollution and environmental chemicals.
5. Conclusions
Research on maternal diet and ASD has rapidly expanded in recent years, with promising findings for maternal folate and vitamin D as potential protective factors, and emerging evidence for associations with other nutrients. Modifications to maternal dietary factors during pregnancy may provide the potential to influence disability associated with ASD at the individual and population level. Continued research in this area is needed, not only to clarify existing discrepancies and consider timing and dose in greater depth, but also to expand the list of nutrients considered for associations with ASD, employ more comprehensive approaches to address combined effects, examine underlying mechanisms, and explore associations with ASD severity, comorbidity, and phenotypic subgroups. Given evidence of nutrient deficiencies and/or suboptimal intake of a number of dietary factors in a large proportion of women of childbearing age (Bird, Murphy, Ciappio, & McBurney, 2017; Drewery, Gaitán, Thaxton, Xu, & Lammi-Keefe, 2016; Mei et al., 2011; Oken et al., 2003), the examination of maternal diet as a potential modifiable risk factor for ASD holds great promise.
Supplementary Material
Acknowledgement:
This research was supported by Department of Defense grant W81XWH-16-1-0753, NIH grant 1R21HD096356-01, NINDS 1R01NS107607-01A1, and through research funding from the Eagles Autism Challenge.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- ACOG. (2012). Patient safety checklist No. 7: magnesium sulfate before anticipated preterm birth for neuroprotection. Obstetrics and gynecology, 120(2 Pt 1), 432–433. doi: 10.1097/AOG.0b013e318268054c [DOI] [PubMed] [Google Scholar]
- Bach A, Serra-Majem L, Carrasco JL, Roman B, Ngo J, Bertomeu I, & Obrador B. (2006). The use of indexes evaluating the adherence to the Mediterranean diet in epidemiological studies: a review. Public Health Nutr, 9(1A), 132–146. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16512961 [DOI] [PubMed] [Google Scholar]
- Bakian AV, Bilder DA, Korgenski EK, & Bonkowsky JL (2018). Autism Spectrum Disorder and Neonatal Serum Magnesium Levels in Preterm Infants. Child Neurol Open, 5, 2329048×18800566. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik A, Kandilya D, Ramya S, Stunkel W, Chong YS, & Dheen ST (2017). Maternal Factors that Induce Epigenetic Changes Contribute to Neurological Disorders in Offspring. Genes (Basel), 8(6). doi: 10.3390/genes8060150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JK, Murphy RA, Ciappio ED, & McBurney MI (2017). Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States. Nutrients, 9(7), 655. doi: 10.3390/nu9070655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork M, Riedel B, Spigset O, Veiby G, Kolstad E, Daltveit AK, & Gilhus NE (2018). Association of Folic Acid Supplementation During Pregnancy With the Risk of Autistic Traits in Children Exposed to Antiepileptic Drugs In Utero. JAMA Neurol, 75(2), 160–168. doi: 10.1001/jamaneurol.2017.3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørklund G, Meguid NA, El-Bana MA, Tinkov AA, Saad K, Dadar M, … Chirumbolo S. (2020). Oxidative Stress in Autism Spectrum Disorder. Molecular Neurobiology, 57(5), 2314–2332. doi: 10.1007/s12035-019-01742-2 [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Modell B, & Lawn J. (2010). Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol, 39 Suppl 1, i110–121. doi: 10.1093/ije/dyq028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. (2010). Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun, 24(6), 881–897. doi: 10.1016/j.bbi.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Braun JM, Froehlich T, Kalkbrenner A, Pfeiffer CM, Fazili Z, Yolton K, & Lanphear BP (2014). Brief report: are autistic-behaviors in children related to prenatal vitamin use and maternal whole blood folate concentrations? J Autism Dev Disord, 44(10), 2602–2607. doi: 10.1007/s10803-014-2114-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Cali T, Ottolini D, & Carafoli E. (2014). Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci, 71(15), 2787–2814. doi: 10.1007/s00018-013-1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RM, Leon JS, & Suchdev PS (2014). Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients, 6(10), 4093–4114. doi: 10.3390/nu6104093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC (2010). Omega-3 fatty acids and inflammatory processes. Nutrients, 2(3), 355–374. doi: 10.3390/nu2030355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell JJ (2008). Autism and vitamin D. Med Hypotheses, 70(4), 750–759. doi: 10.1016/j.mehy.2007.08.016 [DOI] [PubMed] [Google Scholar]
- Castro K, Klein Lda S, Baronio D, Gottfried C, Riesgo R, & Perry IS (2016). Folic acid and autism: What do we know? Nutr Neurosci, 19(7), 310–317. doi: 10.1179/1476830514y.0000000142 [DOI] [PubMed] [Google Scholar]
- Chandyo RK, Ulak M, Kvestad I, Shrestha M, Ranjitkar S, Basnet S, … Strand TA (2017). The effects of vitamin B12 supplementation in pregnancy and postpartum on growth and neurodevelopment in early childhood: Study Protocol for a Randomized Placebo Controlled Trial. BMJ Open, 7(8), e016434. doi: 10.1136/bmjopen-2017-016434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xin K, Wei J, Zhang K, & Xiao H. (2016). Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring. J Psychosom Res, 89, 98–101. doi: 10.1016/j.jpsychores.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Crider KS, Bailey LB, & Berry RJ (2011). Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients, 3(3), 370–384. doi: 10.3390/nu3030370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HT, Crombie IK, & Tavakoli M. (1998). When can odds ratios mislead? Bmj, 316(7136), 989–991. doi: 10.1136/bmj.316.7136.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVilbiss EA, Gardner RM, Newschaffer CJ, & Lee BK (2015). Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. Br J Nutr, 114(5), 663–672. doi: 10.1017/s0007114515002470 [DOI] [PubMed] [Google Scholar]
- DeVilbiss EA, Magnusson C, Gardner RM, Rai D, Newschaffer CJ, Lyall K, … Lee BK (2017). Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: population based cohort study. Bmj, 359, j4273. doi: 10.1136/bmj.j4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewery ML, Gaitán AV, Thaxton C, Xu W, & Lammi-Keefe CJ (2016). Pregnant Women in Louisiana Are Not Meeting Dietary Seafood Recommendations. J Pregnancy, 2016, 1853935. doi: 10.1155/2016/1853935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova O, Myte R, Schneede J, Hagglof B, Bolte S, Domellof E, … Silfverdal SA (2020). Maternal blood folate status during early pregnancy and occurrence of autism spectrum disorder in offspring: a study of 62 serum biomarkers. Mol Autism, 11, 7. doi: 10.1186/s13229-020-0315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles DW, Burne TH, & McGrath JJ (2013). Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol, 34(1), 47–64. doi: 10.1016/j.yfrne.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Fernell E, Bejerot S, Westerlund J, Miniscalco C, Simila H, Eyles D, … Humble MB (2015). Autism spectrum disorder and low vitamin D at birth: a sibling control study. Mol Autism, 6, 3. doi: 10.1186/2040-2392-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, & Bonassi S. (2012). Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med, 52(10), 2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Gao L, Cui SS, Han Y, Dai W, Su YY, & Zhang X. (2016). Does Periconceptional Fish Consumption by Parents Affect the Incidence of Autism Spectrum Disorder and Intelligence Deficiency? A Case-control Study in Tianjin, China. Biomed Environ Sci, 29(12), 885–892. doi: 10.3967/bes2016.118 [DOI] [PubMed] [Google Scholar]
- Gao Y, Sheng C, Xie RH, Sun W, Asztalos E, Moddemann D, … Wen SW (2016). New Perspective on Impact of Folic Acid Supplementation during Pregnancy on Neurodevelopment/Autism in the Offspring Children - A Systematic Review. PLOS ONE, 11(11), e0165626. doi: 10.1371/journal.pone.0165626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Serna AM, & Morales E. (2019). Neurodevelopmental effects of prenatal vitamin D in humans: systematic review and meta-analysis. Mol Psychiatry. doi: 10.1038/s41380-019-0357-9 [DOI] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, & Buka SL (2011). Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. PEDIATRICS, 128(2), 344–355. doi: 10.1542/peds.2010-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiman TM, & Muegge K. (2010). DNA methylation in early development. Mol Reprod Dev, 77(2), 105–113. doi: 10.1002/mrd.21118 [DOI] [PubMed] [Google Scholar]
- Gil-Sanchez A, Demmelmair H, Parrilla JJ, Koletzko B, & Larque E. (2011). Mechanisms involved in the selective transfer of long chain polyunsaturated Fatty acids to the fetus. Front Genet, 2, 57. doi: 10.3389/fgene.2011.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich AJ, Volk HE, Tancredi DJ, McConnell R, Lurmann FW, Hansen RL, & Schmidt RJ (2018). Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res, 11(1), 69–80. doi: 10.1002/aur.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröber U, Schmidt J, & Kisters K. (2015). Magnesium in Prevention and Therapy. Nutrients, 7(9), 8199–8226. doi: 10.3390/nu7095388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Sohn IJ, Kim N, Sim HJ, & Cheon KA (2015). Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan. Exp Neurobiol, 24(4), 273–284. doi: 10.5607/en.2015.24.4.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M. (2010). Effect of long-chain polyunsaturated fatty acid supplementation on neurodevelopmental outcome in full-term infants. Nutrients, 2(8), 790–804. doi: 10.3390/nu2080790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Johnson D, Hulsey TC, Ebeling M, & Wagner CL (2011). Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res, 26(10), 2341–2357. doi: 10.1002/jbmr.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, & Wagner CL (2017). New insights into the vitamin D requirements during pregnancy. Bone Res, 5, 17030. doi: 10.1038/boneres.2017.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Mendez M, Maguire RL, Gonzalez-Nahm S, Huang Z, Daniels J, … Hoyo C. (2018). Periconceptional Maternal Mediterranean Diet Is Associated With Favorable Offspring Behaviors and Altered CpG Methylation of Imprinted Genes. Front Cell Dev Biol, 6, 107. doi: 10.3389/fcell.2018.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, … Willett WC (1999). Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr, 69(2), 243–249. doi: 10.1093/ajcn/69.2.243 [DOI] [PubMed] [Google Scholar]
- Huang Y, Iosif AM, Hansen RL, & Schmidt RJ (2020). Maternal polyunsaturated fatty acids and risk for autism spectrum disorder in the MARBLES high-risk study. Autism, 1362361319877792. doi: 10.1177/1362361319877792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, & Neubrander JA (2004). Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr, 80(6), 1611–1617. doi: 10.1093/ajcn/80.6.1611 [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Nguyen JC, Polglaze KE, & Bertrand PP (2016). Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients, 8(1). doi: 10.3390/nu8010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Liu L, Sun DL, Yin XN, Chen ZD, Wu CA, & Chen WQ (2016). [Interaction between passive smoking and folic acid supplement during pregnancy on autism spectrum disorder behaviors in children aged 3 years]. Zhonghua Liu Xing Bing Xue Za Zhi, 37(7), 940–944. doi: 10.3760/cma.j.issn.0254-6450.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Jones G. (2008). Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr, 88(2), 582S–586S. doi: 10.1093/ajcn/88.2.582S [DOI] [PubMed] [Google Scholar]
- Julvez J, Mendez M, Fernandez-Barres S, Romaguera D, Vioque J, Llop S, … Sunyer J. (2016). Maternal Consumption of Seafood in Pregnancy and Child Neuropsychological Development: A Longitudinal Study Based on a Population With High Consumption Levels. Am J Epidemiol, 183(3), 169–182. doi: 10.1093/aje/kwv195 [DOI] [PubMed] [Google Scholar]
- Kaushik G, & Zarbalis KS (2016). Prenatal Neurogenesis in Autism Spectrum Disorders. Front Chem, 4, 12. doi: 10.3389/fchem.2016.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawicka A, & Regulska-Ilow B. (2013). How nutritional status, diet and dietary supplements can affect autism. A review. Rocz Panstw Zakl Hig, 64(1), 1–12. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23789306 [PubMed] [Google Scholar]
- Kiely M, Hemmingway A, & O’Callaghan KM (2017). Vitamin D in pregnancy: current perspectives and future directions. Ther Adv Musculoskelet Dis, 9(6), 145–154. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, & Wessling-Resnick M. (2014). Iron and mechanisms of emotional behavior. The Journal of nutritional biochemistry, 25(11), 1101–1107. doi: 10.1016/j.jnutbio.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kočovská E, Fernell E, Billstedt E, Minnis H, & Gillberg C. (2012). Vitamin D and autism: clinical review. Res Dev Disabil, 33(5), 1541–1550. doi: 10.1016/j.ridd.2012.02.015 [DOI] [PubMed] [Google Scholar]
- Kominiarek MA, & Rajan P. (2016). Nutrition Recommendations in Pregnancy and Lactation. Med Clin North Am, 100(6), 1199–1215. doi: 10.1016/j.mcna.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruizinga I, Visser JC, van Batenburg-Eddes T, Carter AS, Jansen W, & Raat H. (2014). Screening for autism spectrum disorders with the brief infant-toddler social and emotional assessment. PLOS ONE, 9(5), e97630. doi: 10.1371/journal.pone.0097630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen L, Brambilla P, Mazzocchi A, Harsløf LBS, Ciappolino V, & Agostoni C. (2016). DHA Effects in Brain Development and Function. Nutrients, 8(1), 6. doi: 10.3390/nu8010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Eyles DW, Magnusson C, Newschaffer CJ, McGrath JJ, Kvaskoff D, … Gardner RM (2019). Developmental vitamin D and autism spectrum disorders: findings from the Stockholm Youth Cohort. Mol Psychiatry. doi: 10.1038/s41380-019-0578-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SZ, Kodesh A, Viktorin A, Smith L, Uher R, Reichenberg A, & Sandin S. (2018). Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods Before and During Pregnancy With the Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry, 75(2), 176–184. doi: 10.1001/jamapsychiatry.2017.4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Francis E, Hinkle SN, Ajjarapu AS, & Zhang C. (2019). Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients, 11(7). doi: 10.3390/nu11071628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Shen YD, Li YJ, Xun GL, Liu H, Wu RR, … Ou JJ (2018). Maternal dietary patterns, supplements intake and autism spectrum disorders: A preliminary case-control study. Medicine (Baltimore), 97(52), e13902. doi: 10.1097/MD.0000000000013902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas C. (2019). Linking genetics to epigenetics: The role of folate and folate-related pathways in neurodevelopmental disorders. Clin Genet, 95(2), 241–252. doi: 10.1111/cge.13421 [DOI] [PubMed] [Google Scholar]
- Liu SH, Bobb JF, Claus Henn B, Gennings C, Schnaas L, Tellez-Rojo M, … Coull BA (2018). Bayesian varying coefficient kernel machine regression to assess neurodevelopmental trajectories associated with exposure to complex mixtures. Statistics in Medicine, 37(30), 4680–4694. doi: 10.1002/sim.7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, & Hu FB (2004). Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr, 80(4), 1029–1035. doi: 10.1093/ajcn/80.4.1029 [DOI] [PubMed] [Google Scholar]
- Lopez-Vicente M, Sunyer J, Lertxundi N, Gonzalez L, Rodriguez-Dehli C, Espada Saenz-Torre M, … Guxens M. (2019). Maternal circulating Vitamin D3 levels during pregnancy and behaviour across childhood. Sci Rep, 9(1), 14792. doi: 10.1038/s41598-019-51325-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Ashwood P, Van de Water J, & Hertz-Picciotto I. (2014). Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord, 44(7), 1546–1555. doi: 10.1007/s10803-013-2017-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, … Newschaffer C. (2017). The Changing Epidemiology of Autism Spectrum Disorders. Annu Rev Public Health, 38, 81–102. doi: 10.1146/annurev-publhealth-031816-044318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Munger KL, O’Reilly EJ, Santangelo SL, & Ascherio A. (2013). Maternal dietary fat intake in association with autism spectrum disorders. Am J Epidemiol, 178(2), 209–220. doi: 10.1093/aje/kws433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Windham GC, Whitman C, Snyder NW, & Newschaffer CJ (In press). Maternal serum polyunsaturated fatty acid levels in association with autism spectrum disorder: Results from a population-based case control study in California. American Journal of Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AM, & Ali MM (2019). Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients, 11(3), 608. doi: 10.3390/nu11030608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AH, O’Connor TG, Roth C, Susser E, & Bjorke-Monsen AL (2013). The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci, 7, 120. doi: 10.3389/fnins.2013.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I, Mehta R, Ayers C, Dhumal S, & Petrova A. (2016). Prevalence and risk factors for vitamin D insufficiency and deficiency at birth and associated outcome. BMC Pediatr, 16(1), 208. doi: 10.1186/s12887-016-0741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, & Shea TB (2003). Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci, 26(3), 137–146. doi: 10.1016/S0166-2236(03)00032-8 [DOI] [PubMed] [Google Scholar]
- McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, … Willett WC (2002). Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr, 76(6), 1261–1271. doi: 10.1093/ajcn/76.6.1261 [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Landino SM, Vahabzadeh A, O’Rourke J, Zurcher NR, Finger BC, … Carlezon WA Jr. (2015). Toward an immune-mediated subtype of autism spectrum disorder. Brain Res, 1617, 72–92. doi: 10.1016/j.brainres.2014.09.048 [DOI] [PubMed] [Google Scholar]
- McGinnis WR (2004). Oxidative stress in autism. Altern Ther Health Med, 10(6), 22–36; quiz 37, 92. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15624347 [PubMed] [Google Scholar]
- McKean SJ, Bartell SM, Hansen RL, Barfod GH, Green PG, & Hertz-Picciotto I. (2015). Prenatal mercury exposure, autism, and developmental delay, using pharmacokinetic combination of newborn blood concentrations and questionnaire data: a case control study. Environ Health, 14, 62. doi: 10.1186/s12940-015-0045-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SE, & Reyes TM (2018). Effect of supplementation with methyl-donor nutrients on neurodevelopment and cognition: considerations for future research. Nutr Rev, 76(7), 497–511. doi: 10.1093/nutrit/nuy007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, & Grummer-Strawn LM (2011). Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr, 93(6), 1312–1320. doi: 10.3945/ajcn.110.007195 [DOI] [PubMed] [Google Scholar]
- Meltzer A, & Van de Water J. (2017). The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology, 42(1), 284–298. doi: 10.1038/npp.2016.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, & Bava A. (2004). Vitamin B12 and folate depletion in cognition: a review. Neurol India, 52(3), 310–318. [PubMed] [Google Scholar]
- Naninck EFG, Stijger PC, & Brouwer-Brolsma EM (2019). The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv Nutr, 10(3), 502–519. doi: 10.1093/advances/nmy120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narciso L, Parlanti E, Racaniello M, Simonelli V, Cardinale A, Merlo D, & Dogliotti E. (2016). The Response to Oxidative DNA Damage in Neurons: Mechanisms and Disease. Neural Plast, 2016, 3619274. doi: 10.1155/2016/3619274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, … Windham GC (2007). The epidemiology of autism spectrum disorders. Annu Rev Public Health, 28, 235–258. doi: 10.1146/annurev.publhealth.28.021406.144007 [DOI] [PubMed] [Google Scholar]
- Ogaz-Gonzalez R, Merida-Ortega A, Torres-Sanchez L, Schnaas L, Hernandez-Alcaraz C, Cebrian ME, … Lopez-Carrillo L. (2018). Maternal dietary intake of polyunsaturated fatty acids modifies association between prenatal DDT exposure and child neurodevelopment: A cohort study. Environ Pollut, 238, 698–705. doi: 10.1016/j.envpol.2018.03.100 [DOI] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Berland WE, Simon SR, Rich-Edwards JW, & Gillman MW (2003). Decline in fish consumption among pregnant women after a national mercury advisory. Obstetrics and gynecology, 102(2), 346–351. doi: 10.1016/s0029-7844(03)00484-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Osterdal ML, Gillman MW, Knudsen VK, Halldorsson TI, Strom M, … Olsen SF (2008). Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: a study from the Danish National Birth Cohort. Am J Clin Nutr, 88(3), 789–796. doi: 10.1093/ajcn/88.3.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, … Gillman MW (2008). Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol, 167(10), 1171–1181. doi: 10.1093/aje/kwn034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Lanphear B, Braun JM, Webster GM, Arbuckle TE, Etzel T, … Muckle G. (2020). Gestational Exposures to Phthalates and Folic Acid, and Autistic Traits in Canadian Children. Environ Health Perspect, 128(2), 27004. doi: 10.1289/ehp5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan R, Riley AW, Volk H, Caruso D, Hironaka L, Sices L, … Wang, X. (2018). Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr Perinat Epidemiol, 32(1), 100–111. doi: 10.1111/ppe.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, & Barone S Jr. (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect, 108 Suppl 3, 511–533. doi: 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, & Gillman MW (2009). Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. Journal of the American Dietetic Association, 109(6), 1004–1011. doi: 10.1016/j.jada.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LM, Cordero AM, Pfeiffer CM, Hausman DB, Tsang BL, De-Regil LM, … Bailey LB (2018). Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann N Y Acad Sci, 1431(1), 35–57. doi: 10.1111/nyas.13963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev H, Gera T, & Nestel P. (2005). Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr, 8(2), 117–132. doi: 10.1079/phn2004677 [DOI] [PubMed] [Google Scholar]
- Saini RK, & Keum YS (2018). Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci, 203, 255–267. doi: 10.1016/j.lfs.2018.04.049 [DOI] [PubMed] [Google Scholar]
- Saldanha LG, Dwyer JT, Andrews KW, Brown LL, Costello RB, Ershow AG, … Pehrsson PR (2017). Is Nutrient Content and Other Label Information for Prescription Prenatal Supplements Different from Nonprescription Products? Journal of the Academy of Nutrition and Dietetics, 117(9), 1429–1436. doi: 10.1016/j.jand.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaevitz LR, & Berger-Sweeney JE (2012). Gene–Environment Interactions and Epigenetic Pathways in Autism: The Importance of One-Carbon Metabolism. ILAR Journal, 53(3–4), 322–340. doi: 10.1093/ilar.53.3-4.322 [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, … Hertz-Picciotto I. (2011). Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology, 22(4), 476–485. doi: 10.1097/EDE.0b013e31821d0e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Iosif AM, Guerrero Angel E, & Ozonoff S. (2019). Association of Maternal Prenatal Vitamin Use With Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry, 76(4), 391–398. doi: 10.1001/jamapsychiatry.2018.3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Kogan V, Shelton JF, Delwiche L, Hansen RL, Ozonoff S, … Volk HE (2017). Combined Prenatal Pesticide Exposure and Folic Acid Intake in Relation to Autism Spectrum Disorder. Environ Health Perspect, 125(9), 097007. doi: 10.1289/ehp604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Niu Q, Eyles DW, Hansen RL, & Iosif AM (2019). Neonatal vitamin D status in relation to autism spectrum disorder and developmental delay in the CHARGE case-control study. Autism Res, 12(6), 976–988. doi: 10.1002/aur.2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, & Ozonoff S. (2014). Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol, 180(9), 890–900. doi: 10.1093/aje/kwu208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, … Hertz-Picciotto I. (2012). Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr, 96(1), 80–89. doi: 10.3945/ajcn.110.004416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertner HA, & Mosser EL (1994). Lipid fatty acids calculated on a concentration vs percentage basis. The American journal of clinical nutrition, 59(5), 1093–1093. doi: 10.1093/ajcn/59.5.1093 [DOI] [PubMed] [Google Scholar]
- Sergeant S, Ruczinski I, Ivester P, Lee TC, Morgan TM, Nicklas BJ, … Chilton FH (2016). Impact of methods used to express levels of circulating fatty acids on the degree and direction of associations with blood lipids in humans. British Journal of Nutrition, 115(2), 251–261. doi: 10.1017/S0007114515004341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman Moser S, Davidovitch M, Rotem RS, Chodick G, Shalev V, & Koren G. (2019). High dose folic acid during pregnancy and the risk of autism; The birth order bias: A nested case-control study. Reprod Toxicol, 89, 173–177. doi: 10.1016/j.reprotox.2019.07.083 [DOI] [PubMed] [Google Scholar]
- Shin HM, Schmidt RJ, Tancredi D, Barkoski J, Ozonoff S, Bennett DH, & Hertz-Picciotto I. (2018). Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ Health, 17(1), 85. doi: 10.1186/s12940-018-0428-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JS, Choi MY, Longtine MS, & Nelson DM (2010). Vitamin D effects on pregnancy and the placenta. Placenta, 31(12), 1027–1034. doi: 10.1016/j.placenta.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalco D, Schultz S, Brigida AL, & Antonucci N. (2018). Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals (Basel, Switzerland), 11(2), 56. doi: 10.3390/ph11020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo JE, Switkowski KM, Coull BA, Schwartz J, Kloog I, Gibson H, … Gold DR (2018). Relation of Prenatal Air Pollutant and Nutritional Exposures with Biomarkers of Allergic Disease in Adolescence. Sci Rep, 8(1), 10578. doi: 10.1038/s41598-018-28216-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP (2009). Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr, 4(4), 243–250. doi: 10.1007/s12263-009-0136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman D, McDermott A, & Rosner B. (1997). Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr, 65(4 Suppl), 1179s–1186s. doi: 10.1093/ajcn/65.4.1179S [DOI] [PubMed] [Google Scholar]
- Steenbergen L, Jongkees BJ, Sellaro R, & Colzato LS (2016). Tryptophan supplementation modulates social behavior: A review. Neurosci Biobehav Rev, 64, 346–358. doi: 10.1016/j.neubiorev.2016.02.022 [DOI] [PubMed] [Google Scholar]
- Steenweg-de Graaff J, Ghassabian A, Jaddoe VW, Tiemeier H, & Roza SJ (2015). Folate concentrations during pregnancy and autistic traits in the offspring. The Generation R Study. Eur J Public Health, 25(3), 431–433. doi: 10.1093/eurpub/cku126 [DOI] [PubMed] [Google Scholar]
- Steenweg-de Graaff J, Tiemeier H, Ghassabian A, Rijlaarsdam J, Jaddoe VW, Verhulst FC, & Roza SJ (2016). Maternal Fatty Acid Status During Pregnancy and Child Autistic Traits: The Generation R Study. Am J Epidemiol, 183(9), 792–799. doi: 10.1093/aje/kwv263 [DOI] [PubMed] [Google Scholar]
- Stingone JA, Luben TJ, Carmichael SL, Aylsworth AS, Botto LD, Correa A, … Olshan AF (2017). Maternal Exposure to Nitrogen Dioxide, Intake of Methyl Nutrients, and Congenital Heart Defects in Offspring. Am J Epidemiol, 186(6), 719–729. doi: 10.1093/aje/kwx139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JJ, Davidson PW, Thurston SW, Harrington D, Mulhern MS, McAfee AJ, … Myers GJ (2012). Maternal PUFA status but not prenatal methylmercury exposure is associated with children’s language functions at age five years in the Seychelles. J Nutr, 142(11), 1943–1949. doi: 10.3945/jn.112.163493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M, Granstrom C, Lyall K, Ascherio A, & Olsen SF (2018). Research Letter: Folic acid supplementation and intake of folate in pregnancy in relation to offspring risk of autism spectrum disorder. Psychol Med, 48(6), 1048–1054. doi: 10.1017/s0033291717002410 [DOI] [PubMed] [Google Scholar]
- Suren P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, … Stoltenberg C. (2013). Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA, 309(6), 570–577. doi: 10.1001/jama.2012.155925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, & Gorman JM (1996). Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry, 53(1), 25–31. [DOI] [PubMed] [Google Scholar]
- Tan M, Yang T, Zhu J, Li Q, Lai X, Li Y, … Li T. (2020). Maternal folic acid and micronutrient supplementation is associated with vitamin levels and symptoms in children with autism spectrum disorders. Reprod Toxicol, 91, 109–115. doi: 10.1016/j.reprotox.2019.11.009 [DOI] [PubMed] [Google Scholar]
- Vafeiadou K, Vauzour D, Lee HY, Rodriguez-Mateos A, Williams RJ, & Spencer JP (2009). The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch Biochem Biophys, 484(1), 100–109. doi: 10.1016/j.abb.2009.01.016 [DOI] [PubMed] [Google Scholar]
- van Wijngaarden E, Davidson PW, Smith TH, Evans K, Yost K, Love T, … Myers GJ (2013). Autism spectrum disorder phenotypes and prenatal exposure to methylmercury. Epidemiology, 24(5), 651–659. doi: 10.1097/EDE.0b013e31829d2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchione R, Vigna C, Whitman C, Kauffman EM, Braun JM, Chen A, … Lyall K. (2020). The Association Between Maternal Prenatal Fish Intake and Child Autism-Related Traits in the EARLI and HOME Studies. J Autism Dev Disord. doi: 10.1007/s10803-020-04546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiby G, Daltveit AK, Schjolberg S, Stoltenberg C, Oyen AS, Vollset SE, … Gilhus NE (2013). Exposure to antiepileptic drugs in utero and child development: a prospective population-based study. Epilepsia, 54(8), 1462–1472. doi: 10.1111/epi.12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkhuyzen AAE, Eyles DW, Burne THJ, Blanken LME, Kruithof CJ, Verhulst F, … McGrath JJ (2018). Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol Psychiatry, 23(2), 240–246. doi: 10.1038/mp.2016.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkhuyzen AAE, Eyles DW, Burne THJ, Blanken LME, Kruithof CJ, Verhulst F, … McGrath JJ (2017). Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open, 3(2), 85–90. doi: 10.1192/bjpo.bp.116.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk J, Liew Z, Olsen J, Nohr EA, Catov JM, & Ritz B. (2016). Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism, 20(6), 710–718. doi: 10.1177/1362361315604076 [DOI] [PubMed] [Google Scholar]
- Wagner CL, Taylor SN, Johnson DD, & Hollis BW (2012). The role of vitamin D in pregnancy and lactation: emerging concepts. Women’s health (London, England), 8(3), 323–340. doi: 10.2217/whe.12.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li K, Zhao D, & Li L. (2017). The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: a meta-analysis. Mol Autism, 8, 51. doi: 10.1186/s13229-017-0170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Kioumourtzoglou MA, & Roberts AL (2015). Air Pollution and Autism Spectrum Disorders: Causal or Confounded? Curr Environ Health Rep, 2(4), 430–439. doi: 10.1007/s40572-015-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A-R, & Oates P-S (2008). Mechanisms of heme iron absorption: current questions and controversies. World journal of gastroenterology, 14(26), 4101–4110. doi: 10.3748/wjg.14.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Hart PH, & Kusel MM (2013). Maternal vitamin D levels and the autism phenotype among offspring. J Autism Dev Disord, 43(7), 1495–1504. doi: 10.1007/s10803-012-1676-8 [DOI] [PubMed] [Google Scholar]
- WIC. (2018). Prenatal Vitamins. Retrieved from https://www.health.state.mn.us/docs/people/wic/nutrition/english/pgprenatalvits.pdf
- Wiegersma AM, Dalman C, Lee BK, Karlsson H, & Gardner RM (2019). Association of Prenatal Maternal Anemia With Neurodevelopmental Disorders. JAMA Psychiatry, 1–12. doi: 10.1001/jamapsychiatry.2019.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W. (2013). Nutritional epidemiology (Third;3rd; ed. Vol. 40;40.;). New York;Oxford;: Oxford University Press. [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, … Speizer FE (1985). Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol, 122(1), 51–65. doi: 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- Windham GC, Pearl M, Anderson MC, Poon V, Eyles D, Jones KL, … Croen LA (2019). Newborn vitamin D levels in relation to autism spectrum disorders and intellectual disability: A case-control study in california. Autism Res, 12(6), 989–998. doi: 10.1002/aur.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AD, Strachan AA, Thies F, Aucott LS, Reid DM, Hardcastle AC, … Macdonald HM (2014). Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr, 112(8), 1341–1352. doi: 10.1017/S0007114514001962 [DOI] [PubMed] [Google Scholar]
- Wood AG, Nadebaum C, Anderson V, Reutens D, Barton S, O’Brien TJ, & Vajda F. (2015). Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia, 56(7), 1047–1055. doi: 10.1111/epi.13007 [DOI] [PubMed] [Google Scholar]
- Wu DM, Wen X, Han XR, Wang S, Wang YJ, Shen M, … Zheng YL (2018). Relationship Between Neonatal Vitamin D at Birth and Risk of Autism Spectrum Disorders: the NBSIB Study. J Bone Miner Res, 33(3), 458–466. doi: 10.1002/jbmr.3326 [DOI] [PubMed] [Google Scholar]
- Wurtman RJ (2008). Synapse formation and cognitive brain development: effect of docosahexaenoic acid and other dietary constituents. Metabolism: clinical and experimental, 57 Suppl 2(Suppl 2), S6–S10. doi: 10.1016/j.metabol.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH (2011). The supply of choline is important for fetal progenitor cells. Semin Cell Dev Biol, 22(6), 624–628. doi: 10.1016/j.semcdb.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B, & Croen LA (2015). Immune mediated conditions in autism spectrum disorders. Brain Behav Immun, 46, 232–236. doi: 10.1016/j.bbi.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Singh G, & Naumova E. (2019). Joint Association of Multiple Dietary Components on Cardiovascular Disease Risk: A Machine Learning Approach (OR06–02-19). Current Developments in Nutrition, 3(Supplement_1). doi: 10.1093/cdn/nzz039.OR06-02-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.