Abstract
Background
Ulcerative colitis (UC) is a chronic inflammatory disorder of the colon that has a relapsing‐remitting course. Health related quality of life (HRQL) is significantly lower in patients with UC than the general population due to the negative effects of the disease on physical, psychological and social well‐being. Randomized controlled trials (RCTs) evaluating medical interventions for UC have traditionally used clinical disease activity indices that focus on symptoms to define primary outcomes such as clinical remission or improvement. However, this approach does not evaluate benefits that are highly relevant to patients such as HRQL
Objectives
The primary objective was to assess the impact of biologic therapy on the HRQL of UC patients.
Search methods
We searched PubMed, MEDLINE, EMBASE and CENTRAL from inception to September, 2015. Conference abstracts and reference lists were also searched.
Selection criteria
RCTs that compared biologics to placebo in UC patients and reported on HRQL using the Inflammatory Bowel Disease Questionnaire (IBDQ), or the SF‐36 or EQ‐5D to measure HRQL were included.
Data collection and analysis
Two authors independently screened studies for inclusion, extracted data and assessed study quality using the Cochrane risk of bias tool. The primary outcome was improvement in HRQL. For dichotomous outcomes we calculated the risk ratio (RR) and 95% confidence interval (CI). For continuous outcomes we calculated the mean difference (MD) and 95% CI. The overall quality of the evidence supporting the primary outcome was assessed using GRADE.
Main results
Nine RCTs (n = 4143) were included. Biologics included rituximab (one small study), interferon‐ß‐1a (one study), vedolizumab (one study), and the tumor necrosis factor‐alpha (TNF‐α) antagonists infliximab (two studies), adalimumab (three studies), and golimumab (one study). Risk of bias was low in eight studies. The rituximab study was judged to be at high risk of bias due to attrition bias. The studies comparing interferon‐ß‐1a and rituximab to placebo found no clear evidence of a difference in the proportion of patients who experienced an improvement in HRQL at 8 or 12 weeks respectively. The proportion of patients with a clinically meaningful improvement in HRQL at 6 or 52 weeks was significantly higher in vedolizumab patients compared to placebo. At 6 weeks 37% (83/225) of vedolizumab patients had an improvement in IBDQ score of at least 16 points from baseline compared to 23% (34/149) of placebo patients (RR 1.62, 95% CI 1.15 to 2.27; 1 study). At 52 weeks, 64% (157/247) of vedolizumab patients had an improvement in IBDQ score of at least 16 points from baseline compared to 38% (48/126) of placebo patients (RR 1.62, 95% CI 1.15 to 2.27; 1 study). A GRADE analysis indicated that the overall quality of the evidence supporting these outcomes was moderate due to sparse data (< 400 events). Patients who received maintenance vedolizumab every eight weeks had significantly higher mean SF‐36 scores than placebo patients at 52 weeks (MD 3.40, 95% CI 1.56 to 5.24, 1 study 248 patients). This difference appears to be clinically meaningful as the lower boundary for a clinically meaningful change in SF‐36 is three points. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (< 400 events). Adalimumab patients had significantly higher mean IBDQ scores than placebo patients at weeks 8 (MD 9.00, 95% CI 2.65 to 15.35; 1 study, 494 patients) and 52 (MD 8.00, 95% CI 0.68 to 15.32; 1 study, 494 patients). However, these differences may not be clinically meaningful as the lower boundary for a clinically meaningful change in IBDQ is 16 points. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (< 400 events). Golimumab patients who received a dose of 200/100 mg (MD 12.20, 95% CI 6.52 to 17.88; 504 patients) or 400/200 mg (MD 12.10, 95% CI 6.40 to 17.80; 508 patients) had significantly higher mean IBDQ scores than placebo patients at week 6. Although a GRADE analysis indicated that the overall quality of the evidence supporting these outcomes was high, the difference in IBDQ scores may not be clinically meaningful. Infliximab patients had significantly higher mean IBDQ scores at week 6 or 8 than placebo patients (MD 18,58, 95% CI 13.19 to 23.97; 2 studies, 529 patients). This difference in HRQL is clinically meaningful. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was high. The proportion of patients with a clinically meaningful improvement in HRQL at eight weeks was significantly higher in infliximab patients compared to placebo. Sixty‐nine per cent (333/484) of infliximab patients had an improvement in IBDQ score of > 16 points from baseline compared to 50% of placebo patients (RR 1.39, 95% CI 1.21 to 1.60; 1 study). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was high. Similar results were found between infliximab and placebo when HRQL was measured using the SF‐36 instrument. One small study (n = 43) found no difference in HRQL between infliximab and placebo when measured by the EQ‐5D. Pooled analyses of TNF‐α antagonists showed a benefit in HRQL favouring TNF‐α over placebo.
Authors' conclusions
These results suggest that biologics have the potential to improve HRQL in UC patients. High quality evidence suggests that infliximab provides a clinically meaningful improvement in HRQL in UC patients receiving induction therapy. Moderate quality evidence suggests that vedolizumab provides a clinically meaningful improvement in HRQL in UC patients receiving maintenance therapy. These findings are important since there is a paucity of effective drugs for the treatment of UC that have the potential to both decrease disease activity and improve HRQL. More research is needed to assess the long‐term effect of biologic therapy on HRQL in patients with UC. More research is needed to assess the impact of golimumab and adalimumab on HRQL in UC patients. Trials involving direct head to head comparisons of biologics would help determine which biologics provide optimum benefit for HRQL.
Plain language summary
The impact of biological interventions for ulcerative colitis on health‐related quality of life
What is ulcerative colitis?
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by abdominal pain, urgent bowel movements and bloody diarrhea. Treatment of UC focuses on induction of remission (treatment of symptoms of active disease) and prevention of clinical relapse (resumption of symptoms of active disease) in patients in remission (known as maintenance therapy). UC has a major impact on patients' health related quality of life (HRQL). HRQL refers to a person's physical functioning, social and emotional well‐being, ability to work and freedom from disease symptoms. HRQL is significantly lower in patients with UC compared to the general population. Randomized controlled trials (RCTs) evaluating medical interventions for UC have traditionally used clinical disease activity indices which focus on subjective symptoms to define primary outcomes such as clinical remission or improvement. This focus on disease symptoms results in a failure to assess other important indicators of successful treatment such as HRQL.
What are biological interventions for ulcerative colitis?
Biologics are genetically engineered medications made from living organisms. They work by targeting specific cells in the gut that are involved in the inflammation process.
What did the researchers investigate?
The researchers assessed the impact of biologic medications (e.g. interferon‐ß‐1a, rituximab, infliximab, adalimumab, golimumab and vedolizumab) on HRQL in people with ulcerative colitis. The researchers extensively searched the medical literature up to September 9, 2015.
What did the researchers find?
The researchers identified nine RCTs that included a total of 4143 people with ulcerative colitis. One small study investigated rituximab, one study investigated interferon‐ß‐1a, one study investigated vedolizumab, and the remaining studies investigated tumor necrosis factor‐alpha (TNF‐α) antagonists including infliximab (two studies), adalimumab (three studies), and golimumab (one study). All of the studies compared the biologic medication to a placebo (a fake medicine) administered by intravenous infusion (an IV bag) or subcutaneous injection needle injection (a shot given into the fat layer between the skin and muscle). Eight of the studies were judged to of high quality and the study on rituximab was judged to be of poor quality due to a high drop out rate. The study that compared interferon‐ß‐1a to placebo found no clear evidence of a difference in the proportion of patients who experienced an improvement in HRQL at eight weeks. The study that compared rituximab to placebo found no clear evidence of a difference in HRQL at 12 weeks. Moderate quality evidence from the study comparing vedolizumab to placebo suggests that vedolizumab provides a clinically meaningful improvement in HRQL in UC patients receiving maintenance therapy. A clinically meaningful improvement would be a difference in HRQL that can be detected by the person with ulcerative colitis. Moderate quality evidence from the studies comparing adalimumab to placebo suggest that adalimumab may provide a benefit in terms of improved HRQL in people with UC receiving induction or maintenance therapy. However, the differences between adalimumab and placebo may not be clinically meaningful. High quality evidence from a study comparing golimumab to placebo suggests that golimumab patients had a better HRQL at six weeks than placebo patients. However, this difference in HRQL may not be clinically meaningful. High quality evidence suggests that infliximab provides a clinically meaningful improvement in HRQL in UC patients receiving induction therapy. High quality evidence shows that TNF‐α antagonists (as a class of biologics) provide a clinically meaningful improvement in HRQL in UC patients receiving induction therapy. More research is needed to assess the long‐term effect of biologic therapy on HRQL in people with UC. Future research should also focus on determining whether golimumab and adalimumab can provide UC patients with a clinically meaningful improvement in HRQL. Future research should involve direct head to head comparisons of biologics to determine which biologics provide the most benefit in terms of HRQL.
Summary of findings
Background
Ulcerative colitis (UC) is an idiopathic chronic intestinal inflammation of the colon characterized by periods of abdominal pain and bloody diarrhea. UC has a major impact on patients' health related quality of life (HRQL). HRQL and general life satisfaction are significantly lower in patients with UC compared to the general population (Petrak 2001; Janke 2004; Bernklev 2005; Janke 2005; Irvine 2008). Variables that influence the HRQL of patients with UC include disease course (extent, severity, and relapse pattern), medical therapy (efficacy, adverse events and adherence issues), and demographic, psychosocial and socioeconomic characteristics (Irvine 2008). Disease activity is the most important predictor of HRQL (Janke 2005; Irvine 2008).
Patients with UC experience difficulty with regular daily activities resulting in workplace and school absenteeism (Boonen 2002; Marri 2005; Bernklev 2006). Randomized controlled trials (RCTs) evaluating medical interventions for UC have traditionally used clinical disease activity indices which focus on subjective symptoms to define primary outcomes such as clinical remission or improvement. This focus on symptomatology results in a failure to assess other important indicators of successful treatment such as HRQL, work productivity and mucosal healing. Mucosal healing is associated with a reduced likelihood of future relapses, need for surgery and hospitalizations (Ha 2010).
The introduction of effective but costly treatments (e.g. biologics) for UC has forced physicians and health care authorities to make decisions regarding the allocation of scarce resources (Feagan 1999). Such decisions are often based on pharmacoeconomic analyses that evaluate the cost of a drug vis‐a‐vis clinically meaningful outcomes such as disease symptoms and complications, surgery, hospitalization and HRQL (Feagan 1999). Assessing the HRQL of patients receiving biologic interventions allows for the performance of cost‐utility analyses, which can be used to guide future clinical decision‐making and health care policy (Feagan 1999; Irvine 2008).
HRQL includes four main components: physical function, social and emotional well‐being, ability to work and freedom from disease symptoms (Feagan 1999). General HRQL assessment tools include the Short Form‐36 Health Survey (SF‐36) (Ware 1992) and the European HRQL index (EQ‐5D) (Konig 2002). These are mainly self‐reported outcome measures used in health economics and cost‐effectiveness studies (Achleitner 2012). There are also IBD‐specific tools for measuring HRQL, such as the Inflammatory Bowel Disease Questionnaire (IBDQ) (Guyatt 1989; Irvine 1994; Irvine 1996a), the Short Form‐36 Health Survey (SF‐36) (Ware 1992), and the Cleveland Global Quality of Life Questionnaire (CGQL) (Fazio 1999). Each of these instruments has been extensively validated in patients with IBD.
The impact of biologic interventions on HRQL in UC has not been studied comprehensively, although a review published in 2009 found that one out of eight studies failed to observe an improvement in HRQL among patients with Crohn's disease (CD) and UC treated with biologic agents (Vogelaar 2009). Seven of the eight studies included in this review only enrolled CD patients. The intent of the current systematic review was to assess the impact of biologic therapy on HRQL in patients with active or quiescent UC.
Objectives
The primary objective was to systematically assess the impact of biologic therapy on the HRQL of UC patients.
Methods
Criteria for considering studies for this review
Types of studies
RCTs comparing biologics to placebo were considered for inclusion. Examples of potential biologics include but are not limited to infliximab, adalimumab, certolizumab pegol, golimumab, vedolizumab, natalizumab, interferon alpha and rituximab.
Types of participants
Adult patients with UC (active or quiescent) defined by a combination of clinical, radiographic, endoscopic and histological criteria were considered for inclusion.
Types of interventions
Studies that incorporated the use of biologics for active or quiescent UC were considered for inclusion. Studies that did not measure HRQL as an outcome were excluded.
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of patients achieving improvement in HRQL as defined by the studies (e.g. validated HRQL instruments such as the IBDQ,SF‐36 or EQ‐5D) expressed as a percentage of patients randomized or absolute counts.
Secondary outcomes
Changes in mean difference in quality of life scores (e.g. IBDQ, EQ‐5D and SF‐36) were considered as secondary outcomes.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases from inception to 9 September 2015:
1. MEDLINE;
2. EMBASE;
3. Cochrane Register of Controlled Trials (CENTRAL); and
4. DDW abstracts of randomized controlled and controlled clinical trials.
The databases were searched for randomized controlled and controlled clinical trials using the search strategies described in Appendix 1. There were no language or date restrictions.
Searching other resources
We searched the reference lists of studies and review articles identified by the literature search to identify other potential studies. We also searched ClinicalTrials.gov to identify ongoing studies.
Data collection and analysis
Selection of studies
All studies identified by the literature search were independently assessed for eligibility by two authors (KL and MM or CEP and JKM) based on the inclusion criteria described above. The full text of potentially relevant citations were reviewed for inclusion and the study investigators were contacted to clarify any unclear or missing data. Disagreements were resolved by discussion and consensus.
Data extraction and management
Data extraction forms were used to collect information from the included studies. Two authors (KL and MM or CEP and JKM) independently extracted data. Disagreements were resolved by consensus. The following data were retrieved from eligible studies:
1. General information: title, journal, year, published/unpublished;
2. Study information: design, methods of randomization, concealment of allocation and blinding, power calculation, a priori and post hoc analyses;
3. Intervention and control: type and dose of a medication, placebo or active comparator;
4. Eligibility: inclusion/exclusion criteria, total number screened and randomized;
5. Baseline characteristics (in each group) age, sex, race, disease severity (and how evaluated) concurrent medications used;
6. Follow‐up: length of follow‐up, assessment of compliance of treatment, withdrawals and loss to follow‐up; and
7. Outcomes: primary and secondary outcomes, HRQL outcomes, adverse events.
Assessment of risk of bias in included studies
All studies were independently reviewed to assess methodological quality using the Cochrane risk of bias tool (Higgins 2011). Factors assessed included: 1) sequence generation (i.e. was the allocation sequence adequately generated?); 2) allocation sequence concealment (i.e. was allocation adequately concealed?); 3) blinding (i.e. was knowledge of the allocated intervention adequately prevented during the study?); 4) incomplete outcome data (i.e. were incomplete outcome data adequately addressed?); 5) selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and 6) other potential sources of bias (i.e. was the study apparently free of other problems that could put it at a high risk of bias?). A judgement of 'Yes' indicates low risk of bias, 'No' indicates high risk of bias, and 'Unclear' indicates unclear or unknown risk of bias.
The GRADE criteria were used to evaluate the overall quality of evidence for the primary outcomes and selected secondary outcomes (Guyatt 2008; Schünemann 2011). RCTs started out as high quality evidence, but were downgraded due to: (1) risk of bias, (2) indirectness of evidence, (3) unexplained heterogeneity, (4) sparse data, and (5) publication bias. The overall quality of evidence for each outcome was determined after considering each of these elements, and categorized as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); or very low quality (i.e. we are very uncertain about the estimate).
Measures of treatment effect
Data were analyzed using Review Manager (RevMan 5.3.5). The relative risk (RR) with 95% confidence intervals (95% CI) was calculated for each dichotomous outcome. The number needed to treat (NNT) and risk difference (RD) was calculated where appropriate. For continuous variables, the mean difference (MD) or standardized mean difference (SMD) with 95% CI was calculated using inverse variance (IV). In cross‐over studies only data from the first arm was included. All data were analyzed on an intention‐to‐treat (ITT) basis. The presence of heterogeneity among studies was assessed using the Chi2 test (a P value of 0.10 was regarded as statistically significant). The l2 statistic was used to estimate the degree of heterogeneity (Higgins 2003). This measure describes the percentage of total variation across studies that results from heterogeneity rather than chance. A value of 25% is considered to indicate low heterogeneity, 50% moderate heterogeneity and 75% high heterogeneity (Higgins 2003). Data were not pooled for analysis if interventions, patient populations, and outcome measures were not similar enough to justify pooling (determined by consensus). Data were not pooled for meta‐analysis if a high degree of heterogeneity was detected (i.e. l2 > 75%). A fixed‐effect model was used to pool data in the absence of heterogeneity. A random‐effects model was used if significant heterogeneity was detected. The pooled RR and 95% CI were calculated for dichotomous outcomes. For continuous outcomes the pooled MD or SMD and 95% CI was calculated as appropriate.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were performed by type of biological intervention (e.g. monoclonial antibodies, leukocyte trafficking inhibitors and other). When significant heterogeneity was detected, potential causes for heterogeneity were explored, including differences in patient populations, outcomes and interventions.
Sensitivity analysis
Planned sensitivity analyses included the exclusion of poor quality studies and studies published in abstract form.
Results
Description of studies
The literature search was conducted on 9 September 2015. There were 381 studies identified through database searching. After 111 duplicates were removed, the titles and abstracts of 270 reports were screened by two independent reviewers (KL and MM or CEP and JKM). Two hundred and thirty‐six reports were flagged as non‐applicable and 34 full‐text reports were assessed for eligibility (See Figure 1). Four reports were excluded (See: Characteristics of excluded studies), and 30 reports of 9 studies were included in the review as they met the pre‐defined inclusion criteria (Feagan 2013; Leiper 2011; Pena‐Rossi 2008; Probert 2003; Reinisch 2011; Rutgeerts 2005; Sandborn 2012; Sandborn 2014; Suzuki 2014). Four ongoing studies were identified (NCT00488631; NCT01551290; NCT01863771; NCT02039505).
1.
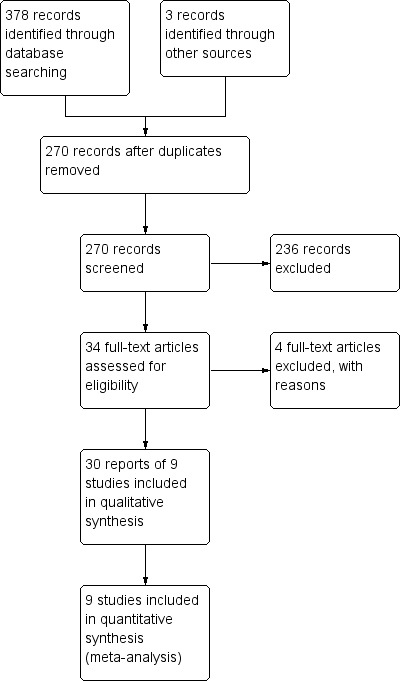
Study flow diagram.
Three studies were excluded as they were not placebo controlled (Armuzzi 2005; Madsen 2001; Parikh 2013), and one study was excluded because it utilized the Work Productivity and Activity Impairment Questionnaire for ulcerative colitis (WPAI‐UC), which is not a validated HRQL instrument (Miner 2011).
Of the included studies, one trial investigated the efficacy of interferon‐ß‐1a (Pena‐Rossi 2008), one trial tested rituximab (an anti‐CD20 antibody) (Leiper 2011), and the remaining trials investigated tumor necrosis factor‐alpha (TNF‐α) antagonists (i.e. infliximab, adalimumab, and golimumab) and vedolizumab (a leukocyte trafficking inhibitor). Two studies investigated infliximab (Probert 2003; Rutgeerts 2005), three trials investigated adalimumab (Reinisch 2011; Sandborn 2012; Suzuki 2014), one trial studied golimumab (Sandborn 2014), and one study investigated vedolizumab (Feagan 2013).
Studies in Crohn's disease have shown that an increase in the IBDQ score of 16 to 32 points from baseline constitutes the lower and upper bounds of clinically meaningful improvement in HRQL (Feagan 2007). Based on these cut‐offs Rutgeerts 2005 defined an improvement in IBDQ as an increase of either > 16 or > 32 points from baseline. Sandborn 2012, Feagan 2013 and Suzuki 2014 defined an improvement in IBDQ as an increase of > 16 points from baseline. Pena‐Rossi 2008 defined an improvement in IBDQ as a > 15 point increase from baseline. Samsa 1999 determined that an increase of 3 to 5 points from baseline for the SF‐36 physical and mental component summary scores (PCS and MCS respectively) reflected a clinically meaningful improvement in HRQL. Rutgeerts 2005 defined an improvement in SF‐36 as an increase of either > 3 or > 5 points from baseline.
Risk of bias in included studies
The risk of bias assessment is summarized in Figure 2. The risk of bias was judged to be low in eight studies. Sequence generation was rated low risk for all studies. Eight of the nine trials utilized a centralized randomization technique and were rated as low risk of bias for allocation concealment. One study did not describe the method of allocation concealment and was rated as unclear risk of bias for this item (Probert 2003). Three studies were rated as having unclear risk of bias for blinding of participants and study personnel (Sandborn 2012; Sandborn 2014; Suzuki 2014). Eight of the nine included studies did not describe how outcome assessment was blinded and were therefore rated as having an unclear risk of bias. All of the included studies used adequate methods to deal with missing data except for Leiper 2011 which was rated as high risk for incomplete outcome data. Six out of sixteen patients in the rituximab group and two out of eight patients in the placebo group completed this twelve week study. All of the included studies were rated as low risk of bias for the selective reporting and other sources of bias domains.
2.
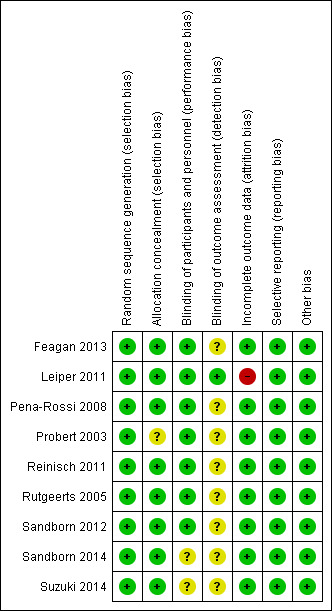
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. Interferon‐B‐1a versus placebo.
| Interferon‐B‐1a versus placebo | ||||||
| Patient or population: patients with active ulcerative colitis Settings: Outpatient Intervention: Interferon‐B‐1a versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Interferon‐B‐1a versus placebo | |||||
| Improved IBDQ at week 8 | 406 per 10001 | 463 per 1000 (325 to 654) | RR 1.14 (0.80 to 1.61) | 194 (1 study) | ⊕⊕⊕⊝ moderate2 | Improvement > 15 points from baseline |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (86 events)
Summary of findings 2. Rituximab versus placebo.
| Rituximab versus placebo | ||||||
| Patient or population: patients with active ulcerative colitis Settings: Outpatient Intervention: Rituximab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rituximabversus placebo | |||||
| IBDQ at week 12 | The mean IBDQ change score in the placebo group was 2 | The mean IBDQ score in the intervention group was 15 points higher | 22 (1 study) | ⊕⊝⊝⊝ very low1, 2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded two levels due to small sample size (24 participants)
2 Downgraded one level due to high risk of bias
Summary of findings 3. Infliximab versus placebo.
| Infliximab versus placebo | ||||||
| Patient or population: patients with active ulcerative colitis Settings: Outpatient Intervention: Infliximab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Infliximab versus placebo | |||||
| Improved IBDQ at week 8 | 496 per 10001 | 689 per 1000 (600 to 794) | RR 1.39 (1.21 to 1.60) | 728 (1 study) | ⊕⊕⊕⊕ high | Improvement > 16 points from baseline |
| Improved IBDQ at week 8 | 328 per 10001 | 538 per 1000 (449 to 666) | RR 1.67 (1.37 to 2.03) | 728 (1 study) | ⊕⊕⊕⊝ moderate2 | Improvement > 32 points from baseline |
| IBDQ at week 6 or 8 | The mean IBDQ score ranged across placebo groups from 21 to 25 | The mean IBDQ scores in the intervention groups was on average 18.6 points higher (95% CI 13.2 to 24.0) | 529 (2 studies) | ⊕⊕⊕⊕ high | Participants in the active drug group received infliximab 5 mg/kg | |
| IBDQ at week 6 or 8 | The mean IBDQ score in the placebo group was 21 | The mean IBDQ score in the intervention group was on average 15 points higher (95% CI 9.46 to 20.54) | 486 (1 study) | ⊕⊕⊕⊕ high | Participants in the active drug group received infliximab 10 mg/kg | |
| EQ‐5D at week 6 | The mean EQ‐5D score in the placebo group was 4 | The mean EQ‐5D score in the intervention group was on average 3 points higher (95% CI ‐6.87 to 12.87) | 43 (1 study) | ⊕⊕⊝⊝ low3 | ||
| Improved SF‐36 PCS at week 8 | 324 per 10001 | 489 per 1000 ( 399 to 599) | RR 1.51 (1.23 to 1.85) | 728 (1 study) | ⊕⊕⊕⊝ moderate4 | Improvement > 5 points from baseline |
| Improved SF‐36 MCS at week 8 | 299 per 10001 | 431 per 1000 (347 to 535) | RR 1.44 (1.16 to 1.79) | 728 (1 study) | ⊕⊕⊕⊝ moderate5 | Improvement > 5 points from baseline |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials
2 Downgraded one level due to sparse data (345 events)
3 Downgraded two levels due to a wide confidence interval crossing the line of no effect and small sample size (43 participants)
4 Downgraded one level due to sparse data (316 events)
5 Downgraded one level due to sparse data (281 events)
Summary of findings 4. Adalimumab versus placebo.
| Adalimumab versus placebo | ||||||
| Patient or population: patients with active ulcerative colitis Settings: Outpatient Intervention: Adalimumab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Adalimumab versus placebo | |||||
| Improved IBDQ at week 8 | 439 per 10001 | 540 per 1000 (465 to 628) | RR 1.23 (1.06 to 1.43) | 767 (2 studies) | ⊕⊕⊕⊝ moderate2 | Improvement > 16 points from baseline |
| Improved IBDQ at week 52 | 152 per 10001 | 263 per 1000 (195 to 356) | RR 1.73 (1.28 to 2.34) | 767 (2 studies) | ⊕⊕⊕⊝ moderate3 | Improvement > 16 points from baseline |
| IBDQ at week 8 | The mean IBDQ score in the placebo group was 20 | The mean IBDQ score in the intervention group was on average 9 points higher (95% CI 2.65 to 15.35) | 494 (1 study) | ⊕⊕⊕⊝ moderate4 | ||
| IBDQ at week 52 | The mean IBDQ score in the placebo group was 19 | The mean IBDQ score in the intervention group was on average 8 points higher (95% CI 0.68 to 15.32) | 494 (1 study) | ⊕⊕⊕⊝ moderate4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (374 events)
3 Downgraded one level due to sparse data (162 events)
4 Downgraded one level due to imprecision of results (wide confidence interval)
Summary of findings 5. Golimumab versus placebo.
| Golimumab versus placebo | ||||||
| Patient or population: patients with active ulcerative colitis Settings: Outpatient Intervention: Golimumab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Golimumab versus placebo | |||||
| IBDQ at week 6 | The mean IBDQ score in the placebo group was 14.8 | The mean IBDQ score in the intervention group was on average 12.2 points higher (95% CI 6.52 to 17.88) | 504 (1 study) | ⊕⊕⊕⊕ high | Participants in the active drug group received golimumab 200/100 mg | |
| IBDQ at week 6 | The mean IBDQ score in the placebo group was 14.8 | The mean IBDQ score in the intervention group was on average 12.1 points higher (95% CI 6.40 to 17.80) | 508 (1 study) | ⊕⊕⊕⊕ high | Participants in the active drug group received golimumab 400/200 mg | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (86 events)
Summary of findings 6. Vedolizumab versus placebo.
| Vedolizumab versus placebo | ||||||
| Patient or population: patients with active ulcerative colitis Settings: Outpatient Intervention: Vedolizumab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vedolizumab versus placebo | |||||
| Improved IBDQ at week 6 | 228 per 10001 | 370 per 1000 (262 to 518) | RR 1.62 (1.15 to 2.27) | 374 (1 study) | ⊕⊕⊕⊝ moderate2 | Improvement > 16 points from baseline |
| Improved IBDQ at week 52 | 381 per 10001 | 636 per 1000 (499 to 808) | RR 1.67 (1.31 to 2.12) | 373 (1 study) | ⊕⊕⊕⊝ moderate3 | Improvement > 16 points from baseline |
| SF‐36 PCS at week 6 | The mean SF‐36 PCS score in the placebo group was 1.4 | The mean SF‐36 PCS score in the intervention group was on average 2.6 points higher (95% CI 1.22 to 3.98) | 374 (1 study) | ⊕⊕⊕⊝ moderate4 | ||
| SF‐36 MCS at week 6 | The mean SF‐36 MCS score in the placebo group was ‐0.2 | The mean SF‐36 MCS score in the intervention group was on average 4.6 points higher (95% CI 2.69 to 6.51) | 374 (1 study) | ⊕⊕⊕⊝ moderate4 | ||
| SF‐36 PCS at week 52 | The mean SF‐36 PCS score in the placebo group was 7.46 | The mean SF‐36 PCS score in the intervention group was on average 3.4 points higher (95% CI 1.56 to 5.24) | 248 (1 study) | ⊕⊕⊕⊝ moderate4 | Participants in the active drug group received vedolizumab every 8 weeks | |
| SF‐36 MCS at week 52 | The mean SF‐36 MCS score in the placebo group was 3.9 | The mean SF‐36 MCS score in the intervention group was on average 4.80 points higher (95% CI 2.29 to 7.31) | 248 (1 study) | ⊕⊕⊕⊝ moderate5 | Participants in the active drug group received vedolizumab every 8 weeks | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials.
2 Downgraded one level due to sparse data (117 events)
3 Downgraded one level due to sparse data (205 events)
4 Downgraded one level because sample size was < 400 (374 participants)
5 Downgraded one level because sample size was < 400 (248 participants)
Summary of findings 7. TNF‐alpha antagonists versus placebo.
| TNF‐alpha antagonists versus placebo | ||||||
| Patient or population: patients with active ulcerative colitis Settings: Outpatient Intervention: TNF‐alpha antagonists versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | TNF‐alpha antagonistsversus placebo | |||||
| Improved IBDQ at weeks 6 or 8 | 462 per 10001 | 610 per 1000 (550 to 675) | RR 1.32 (1.19 to 1.46) | 1495 (3 study) | ⊕⊕⊕⊕ high | Improvement > 16 points from baseline |
| IBDQ at weeks 6 or 8 | The mean IBDQ score ranged across placebo groups from 14.8 to 25 | The mean IBDQ scores in the intervention groups was on average 13.71 points higher (95% CI 13.2 to 24.0) | 1565 (4 studies) | ⊕⊕⊕⊝ moderate2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials.
2 Downgraded one level due to unexplained heterogeneity (I2 = 50%)
Interferon‐ß‐1a versus placebo
Improved IBDQ at week 8
One study (N = 194) compared interferon‐ß‐1a to placebo (Pena‐Rossi 2008). There was no statistically significant difference in the proportion of patients who had improved IBDQ scores at week eight. Forty‐six percent (60/130) of interferon‐ß‐1a patients had improved IBDQ scores compared to 41% (26/64) of placebo patients (RR 1.14, 95% CI 0.80 to 1.61). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 1).
Rituximab versus placebo
IBDQ at week 12
One study (N = 24) compared rituximab to placebo in patients with active, steroid‐refractory UC (Leiper 2011). There was no statistically significant difference in mean IBDQ change scores at week 12. The mean (SD) improvement in IBDQ score was 17 (45) points for rituximab patients compared to 2 (29) points for placebo patients (MD 15.00, 95% CI ‐14.83 to 44.83). A GRADE analysis indicated that the quality of evidence supporting this outcome was very low due to very sparse data (24 participants) and high risk of bias (See Table 2).
Infliximab versus placebo
IBDQ at week 6 or 8
Two studies (Probert 2003; Rutgeerts 2005), reported mean IBDQ scores at week 6 or 8 among patients who received a 5 mg/kg infusion of infliximab (n = 265) or placebo (n = 264). There was a statistically significant improvement in the mean IBDQ score among infliximab patients compared to placebo (MD 18.58, 95% CI 13.19 to 23.97). A GRADE analysis indicated that the quality of evidence supporting this outcome was high (See Table 3). One study (Rutgeerts 2005) reported mean IBDQ scores at week 6 among patients who received 10 mg/kg infliximab (n = 242) or placebo (n = 244). There was a statistically significant improvement in the mean IBDQ score among infiximab patients compared to placebo (MD 15.00, 95% CI 9.46 to 20.54). A GRADE analysis indicated that the quality of evidence supporting this outcome was high (See Table 3).
Improved IBDQ (> 16 points or > 32 points from baseline) at week 8
One study reported the proportion of patients who had improved IBDQ scores at week eight (Rutgeerts 2005). There was a statistically significant difference in the proportion of patients who had improved IBDQ scores at week eight. At week 8, 69% (333/484) of infliximab patients had an improvement in IBDQ score of at least 16 points from baseline compared to 50% (121/244) of placebo patients (RR 1.39, 95% CI 1.21 to 1.60). A GRADE analysis indicated that the quality of evidence supporting this outcome was high (See Table 3). Fifty‐five per cent (265/484) of infliximab patients had an improvement in IBDQ score of at least 32 points from baseline compared to 33% (80/244) of placebo patients (RR 1.67, 95% CI 1.37 to 2.03). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 3).
EQ‐5D at week 6
One study (N = 43) reported mean EQ‐5D scores at 6 weeks (Probert 2003). There was no statistically significant difference in mean EQ‐5D scores at week 6. The mean (SD) improvement in EQ‐5D score was 7 (17) points for infliximab patients compared to 4 (16) points for placebo patients (MD 3.00, 95% CI ‐6.87 to 12.87). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to sparse data and wide confidence interval (See Table 3).
Improved SF‐36 PCS (> 3 or > 5 points from baseline)
One study (N = 728) reported the proportion of patients who had an improved SF‐36 PCS at week eight (Rutgeerts 2005). There was a statistically significant difference in the proportion of patients who had an improved SF‐36 PCS at week eight. Fifty‐nine per cent (286/484) of infliximab patients had an improvement in SF‐36 PCS of at least 3 points from baseline compared to 41% (99/244) of placebo patients (RR 1.46, 95% CI 1.23 to 1.72). Forty‐nine per cent (237/484) of infliximab patients had an improvement in SF‐36 PCS of at least 5 points from baseline compared to 33% (80/244) of placebo patients (RR 1.51, 95% CI 1.23 to 1.85). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 3).
Improved SF‐36 MCS (> 3 or > 5 points from baseline)
One study (n = 728) reported the proportion of patients who had an improved SF‐36 MCS at week eight (Rutgeerts 2005). There was a statistically significant difference in the proportion of patients who had an improved SF‐36 MCS at week eight. Fifty per cent (242/484) of infliximab patients had an improvement in SF‐36 MCS of at least 3 points from baseline compared to 34% (83/244) of placebo patients (RR 1.47, 95% CI 1.21 to 1.79). Forty‐three per cent (208/484) of infliximab patients had an improvement in SF‐36 MCS of at least 5 points from baseline compared to 30% (73/244) of placebo patients (RR 1.44, 95% CI 1.16 to 1.79). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 3).
Adalimumab versus placebo
Three trials evaluated the change in HRQL with adalimumab administration. Reinisch 2011 investigated adalimumab over an eight week period as an induction agent for UC. Patients (N = 576) were randomized (1:1:1) to adalimumab 160/80 mg (160 mg at week 0, 80 mg at week 2 and 40 mg at weeks 5 and 6), adalimumab 80/40 mg (80 mg at week 0 and 40 mg at weeks 2, 4 and 6) or placebo. The authors found that patients in the 160/80 mg group had significantly improved IBDQ and SF‐36 PCS scores at week 8. Patients in the 80/40 mg group only showed an improvement in SF‐36 score at week 4. There was no improvement in the MCS dimension of the SF‐36 score across any of the groups. While the mean HRQOL scores were reported at baseline, week 4 and week 8 for all treatment groups this data could not be included in analyses because standard deviations were not reported.
IBDQ at week 8 or 52
Sandborn 2012 (n = 494) studied the effect of adalimumab for induction and maintenance treatment of UC. Patients received adalimumab 160 mg at week 0, 80 mg at week 2 and 40 mg every other week or placebo for 52 weeks. At week 8, there was a statistically significant improvement in the mean IBDQ score among adalimumab patients compared to placebo (MD 9.00, 95% CI 2.65 to 15.35). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to a wide confidence interval (See Table 4). At week 52, there was a statistically significant improvement in the mean IBDQ score among adalimumab patients compared to placebo (MD 8.00, 95% CI 0.68 to 15.32). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to a wide confidence interval (See Table 4).
Improved IBDQ (> 16 points from baseline) at week 8 or 52
Two studies (n = 768 patients) reported the proportion of patients who had improved IBDQ scores at week eight or 52 (Sandborn 2012; Suzuki 2014). There was a statistically significant difference in the proportion of patients who had improved IBDQ scores at week eight. At week 8, 53% (224/425) of adalimumab patients had an improvement in IBDQ score of at least 16 points from baseline compared to 44% (150/342) of placebo patients (RR 1.23, 95% CI 1.06 to 1.43). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 4). Although IBDQ scores tended to decrease over time there was a statistically significant difference in the proportion of patients who had improved IBDQ scores at week 52. Twenty‐six per cent (110/425) of adalimumab patients had an IBDQ score of at least 16 points greater than baseline compared to 15% (52/342) of placebo patients (RR 1.73, 95% CI 1.28 to 2.34). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 4).
Golimumab versus placebo
IBDQ at week 6
Sandborn 2014 reported on the effect of golimumab administration on IBDQ scores in patients receiving induction therapy. There was a statistically significant difference in the proportion of patients who had improved IBDQ scores at week 6 in patients who received 200mg/100 mg (MD 12.20, 95% CI 6.52, 17.88; 504 patients) and 400 mg/200 mg (MD 12.10, 95% CI 6.40 to 17.80; 508 patients) dosing regimens compared to placebo. GRADE analyses indicated that the quality of evidence supporting this outcome for both dose groups was high (See Table 5).
Vedolizumab versus placebo
Vedolizumab was investigated as an induction (week 6) and maintenance agent (week 52) in a large multi‐center, randomized, double‐blind, placebo‐controlled trial (GEMINI1) that integrated two study cohorts and involved 1406 patients with moderate to severe UC (Feagan 2013). In the induction phase , 374 patients (cohort 1) were assigned to two intravenous doses of 300 mg of vedolizumab, at weeks 0 and 2 with an additional 521 patients (cohort 2) receiving open‐label vedolizumab at weeks 0 and 2. In the maintenance phase of the trial, patients from either cohort who responded to vedolizumab at week 6 (defined as a change in Mayo Clinic score of > 3 points and a decrease of at least 30% from baseline, with an decrease in the rectal bleeding subscore of >1 point or an absolute rectal bleeding subscore of 0 or 1) were randomly assigned to either continued vedolizumab therapy every 4 or 8 weeks or placebo for up to 52 weeks of treatment.
Improved IBDQ (> 16 points from baseline) at week 6 or 52
There was a statistically significant difference in the proportion of patients who had improved IBDQ scores at week six. Thirty‐seven per cent (83/225) of vedolizumab patients had an improvement in IBDQ score of at least 16 points from baseline compared to 23% (34/149) of placebo patients (RR 1.62, 95% CI 1.15 to 2.27). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 6). There was a statistically significant difference in the proportion of patients who had improved IBDQ scores at 52 weeks. Sixty‐four percent of patients receiving maintenance vedolizumab had an improvement in IBDQ score of at least 16 points from baseline compared to 38% (48/126) of placebo patients (RR 1.67, 95% CI 1.31 to 2.12). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 6).
SF‐36 PCS at week 6 or 52
Feagan 2013 reported mean SF‐36 PCS at week six and 52. At week 6, there was a statistically significant improvement in the mean SF‐36 PCS among vedolizumab patients compared to placebo (MD 2.60, 95% CI 1.22 to 3.98). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 6). At week 52 there was a statistically significant difference in mean SF‐36 PCS among patients receiving maintenance vedolizumab every four weeks (MD 2.80, 95% CI 0.96 to 4.64) and every eight weeks compared to placebo (MD 3.40, 95% CI 1.56 to 5.24). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 6).
Feagan 2013 reported mean SF‐36 MCS at week six and 52. At week 6, there was a statistically significant improvement in the mean SF‐36 MCS among vedolizumab patients compared to placebo (MD 4.60, 95% CI 2.69 to 6.51). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 6). At week 52 there was a statistically significant difference in mean SF‐36 MCS among patients receiving maintenance vedolizumab every four weeks (MD 4.80, 95% CI 2.33 to 7.27) and every eight weeks compared to placebo (MD 4.80, 95% CI 2.29 to 7.31). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 6).
TNF‐alpha antagonists versus placebo
IBDQ at week 6 or 8
The results of theTNF‐alpha antagonist trials were pooled to estimate the overall effect of this kind of therapy on HRQL in UC. The 10 mg/kg infliximab group and the 400 mg/200 mg golimumab group were omitted from this analysis since 5mg/kg of infliximab and 200 mg/100 mg of golimumab are more commonly used doses in clinical practice. Four studies (Probert 2003; Rutgeerts 2005; Sandborn 2012; Sandborn 2014), reported mean IBDQ scores at week 6 or 8 among patients who received TNF‐alpha antagonists (n = 784) or placebo (n = 781). The pooled analysis revealed a statistically significant improvement in the mean IBDQ scores favouring TNF‐alpha antagonist treatment (MD 13.71, 95% CI 10.40 to 17.01). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to unexplained heterogeneity (See Table 7).
Improved IBDQ (> 16 points from baseline) at week 6 or 8
Three studies (n = 1495) reported the proportion of patients who had improved IBDQ scores at week six or eight (Rutgeerts 2005; Sandborn 2012; Suzuki 2014). There was a statistically significant difference in the proportion of patients who had improved IBDQ scores. Sixty‐two per cent (557/909) of patients who received TNF‐alpha antagonists had an improvement in IBDQ score of at least 16 points from baseline compared to 46% (271/586) of placebo patients (RR 1.32, 95% CI 1.19 to 1.46). A GRADE analysis indicated that the quality of evidence supporting this outcome was high (See Table 7).
Discussion
Summary of main results
Improvements in clinical disease activity indices and evidence of mucosal healing are often used to determine the efficacy of treatments for UC. Due to the chronicity of the disease, and the variable responsiveness and toxicity of certain therapies, HRQL measures are increasingly employed in clinical trials to determine overall improvement in patient health status (Irvine 1996a; Irvine 1996b). Over the past 15 years biologics have become an effective, albeit expensive, therapeutic option for the treatment of moderate to severely active UC. The goal of this review was to assess whether biologics are effective for improving a patient's HRQL. Several instruments have been developed to assess HRQL in UC patients, including the EQ‐5D, SF‐36 and IBDQ questionnaires. Our search identified nine studies including a total of 4143 participants. One study investigated the use of interferon‐ß‐1a (Pena‐Rossi 2008), and another investigated rituximab (Leiper 2011). There was no evidence to suggest that these therapies were effective for treating UC, nor were they associated with an improvement in IBDQ score relative to placebo.
The remaining studies investigated TNF‐α antagonists and vedolizumab. In a pooled analysis of two high‐quality studies examining the TNF‐α antagonist infliximab, a significantly greater change in mean IBDQ score was observed with infliximab treatment compared to placebo (Probert 2003; Rutgeerts 2005). Furthermore, Rutgeerts 2005 reported that a statistically significant proportion of patients randomized to infliximab achieved an increase in IBDQ score of both > 16 and > 32 points, which represents the lower and upper bounds for a clinically meaningful improvement in HRQL (Feagan 2007). Rutgeerts 2005 also found that patients treated with infliximab were significantly more likely to achieve both a > 3 point increase and a > 5 point increase in the mental and physical component scales that comprise the SF‐36. The minimal clinically important difference for the MCS and PCS ranges from three to five points (Samsa 1999). The Cochrane risk of bias tool was used to assess the methodological quality of the infliximab trials and the possibility of bias was judged to be low for these studies. Furthermore, the overall quality of evidence supporting the HRQL outcomes (e.g. proportions of patients with improved IBDQ or components of SF‐36, mean IBDQ) was rated as either 'high' or 'moderate' using the GRADE criteria. In the former case this indicates that future research is unlikely to change our confidence in the point estimate of effect. In the latter case this indicates that further research may change our confidence in the point estimate of effect. The outcomes rated as moderate were downgraded one level due to sparse data (i.e. fewer than 400 events). These results suggest that infliximab provides a substantial benefit for UC patients in terms of improved HRQL.
Two trials compared adalimumab to placebo in patients with moderate to severely active UC (Sandborn 2012; Suzuki 2014). A pooled analysis of these studies revealed a statistically significant trend toward improved IBDQ scores (defined as > 16 points from baseline) at weeks 8 and 52 in the adalimumab groups. The risk of bias for these studies was judged to be low. A GRADE analysis indicated that the overall quality of the evidence supporting the HRQL outcomes was rated as moderate due to sparse data (i.e. fewer than 400 events). Sandborn 2012 also found that the mean difference in IBDQ score between the adalimumab and placebo groups was statistically significant at weeks 8 and 52, however this improvement may not be a clinically meaningful improvement in HRQL (i.e. > 16 points) as the mean difference between adalimumab and placebo was only eight points. We rated the evidence supporting this outcome as moderate quality due to imprecision (wide confidence intervals). This evidence suggests that adalimumab may be effective in improving HRQL in patients with UC.
One trial investigated the effect of golimumab on HRQL. In Sandborn 2014 there was a statistically significant trend toward improvement in mean IBDQ score at week 6 among patients receiving 200/100 mg and 400/200 mg doses of golimumab compared to placebo. Although the evidence supporting this outcome was rated as high quality for the GRADE analysis, this improvement may not be clinically meaningful (i.e. > 16 points) as the mean difference between golimumab and placebo was only 12 points for both dosage comparisons.
Feagan 2013 compared vedolizumab to placebo in patients with moderately to severely active ulcerative colitis. Individuals randomized to vedolizumab had significantly improved IBDQ scores (defined as > 16 points from baseline) at weeks 6 and 52. Improvement in HRQL was further reflected in the SF‐36 scores of patients receiving vedolizumab. At weeks 6 and 52 there was a statistically significant mean difference in the mental and physical component scales between the vedolizumab and placebo groups. These differences reflect a clinically meaningful improvement in HRQL as most of the mean differences were greater than three points on the SF‐36 for most comparisons (Samsa 1999).
The results of the infliximab, adalimumab and golimumab studies were pooled to assess whether anti‐TNF‐α therapy is associated with an overall improvement in HRQL. The pooled analysis indicated that patients assigned to TNF‐α antagonists were significantly more likely than placebo patients to have improved IBDQ scores at weeks 6 or 8. The GRADE analysis indicated that the evidence supporting this outcome was high in quality. The pooled results also demonstrated a statistically significant difference in mean IBDQ scores at weeks 6 or 8. While these results are statistically significant, the change in mean IBDQ score may not be clinically meaningful (i.e. > 16 points) as the mean difference between TNF‐α and placebo was approximately 14 points.The evidence supporting this outcome was rated as moderate for the GRADE analysis due to unexplained heterogeneity (I2 = 50%). Overall, the results of this pooled analysis suggest that TNF‐α antibodies are effective for improving both disease activity and HRQL in patients with UC. The results of the infliximab studies provided the strongest evidence in favour of this conclusion. Additional research is needed to determine whether golimumab and adalimumab can provide a clinically meaningful change in mean IBDQ score among patients with UC.
It is worth noting that the maintenance trials included in this review fall into one of two methodological categories. In Reinisch 2011, Rutgeerts 2005, Sandborn 2012 and Suzuki 2014 patients in the maintenance phase continued to receive the treatment to which they were randomized during the induction phase (either placebo or active drug). Alternatively, only the induction‐phase responders were re‐randomized to placebo or active drug during the maintenance arm in Feagan 2013 and Sandborn 2014. Patients who entered the maintenance phase as responders may have had a higher HRQL than those who entered as non‐responders. Unfortunately, there were not enough data to explore this potential relationship.
Overall completeness and applicability of evidence
Biologics particularly infliximab and vedolizumab have the potential to improve HRQL in patients with UC. In general the results of this review are applicable to patients with moderate to severe ulcerative colitis despite treatment with corticosteroids and immunosuppressives. Most of the included studies were multicenter trials conducted in countries where the burden of ulcerative colitis is greatest including USA, Canada, Argentina, Iceland, Ireland, UK, Spain, Germany, Austria, Belgium, Denmark, The Netherlands, France, Switzerland, Sweden, Italy, Greece, Turkey, Czech Republic, Bulgaria, Hungary, Lithuania, Estonia, Poland, Romania, Serbia, Slovakia, Ukraine, Russian Federation, Israel, South Africa, India, Republic of Korea, Malaysia, Hong Kong, Singapore, Taiwan, Japan, Australia, and New Zealand. More research is needed to assess the impact of adalimumab and golimumab on the HRQL in UC patients with moderate to severe disease. Although the results of the Pena‐Rossi 2008 study are applicable to patients with moderate to severe UC, we are uncertain whether interferon‐ß‐1a provides any benefit in terms of HRQL. The Leiper 2011 study was the only trial that was not conducted at multiple centres. This study conducted at a single centre in the UK, and we are uncertain whether rituximab provides any benefit in terms of HRQL
Quality of the evidence
Eight of the nine included studies were judged to be at low risk of bias. The rituximab study was judged to be at high risk of bias due to high drop out rates. A GRADE analysis indicated that the overall quality of the evidence supporting the primary outcome from the interferon‐ß‐1a study was moderate due to sparse data (86 events). A GRADE analysis indicated that the overall quality of the evidence supporting the primary outcome from the rituximab study was very low due to very sparse data (24 events) and high risk of bias (high drop‐out rate). GRADE analyses indicated that the overall quality of the evidence supporting the primary outcome from the infliximab studies was high. A GRADE analysis indicated that the overall quality of the evidence supporting the primary outcome from the golimumab study was high. GRADE analyses indicated that the overall quality of the evidence supporting the primary outcome from the adalimumab studies was moderate due to imprecision. GRADE analyses indicated that the overall quality of the evidence supporting the primary outcome from the pooled TNF‐α antagonist studies ranged from moderate to high quality. The pooled analysis that was rated as moderate was downgraded one level due to unexplained heterogeneity (I2 = 50%).
Potential biases in the review process
To reduce potential bias in the review process we performed a comprehensive literature search to identify all eligible studies. We also searched Clinicaltrials.gov to identify ongoing studies. Two review authors independently assessed studies for inclusion, extracted data and assessed study quality.
Agreements and disagreements with other studies or reviews
The results of our review agree with another published review on biologics and HRQL. The review article by Vogelaar 2009 assessed the impact of biologics on HRQL in patients with inflammatory bowel disease. This review included eight RCTs. Seven of these studies assessed the impact of biologics on HRQL in patients Crohn's disease and one study assessed the impact of infliximab on HRQL in patients with UC. Vogelaar 2009 reported that HRQL was significantly greater in ulcerative colitis patients treated with infliximab compared to placebo. Our systematic review provides high quality evidence that infliximab provides a clinically meaningful improvement in HRQL in patients with moderate to severe UC.
Authors' conclusions
Implications for practice.
The results of this review suggest that biologic agents have the potential to improve HRQL in patients with UC. High quality evidence suggests that infliximab provides a clinically meaningful improvement in HRQL in UC patients receiving induction therapy. Moderate quality evidence suggests that vedolizumab provides a clinically meaningful improvement in HRQL in UC patients receiving maintenance therapy. These findings are important since there is a paucity of effective drugs for the treatment of UC that have the potential to both decrease disease activity and improve HRQL.
Implications for research.
More research is needed to assess the long‐term effect of biologic therapy on HRQL in patients with UC. More research is needed to assess the impact of golimumab and adalimumab on HRQL in UC patients. These trials should ensure adequate sample size and reduce the likelihood of sparse data by performing a priori power and sample size calculations. Trials involving direct head to head comparisons of biologics would be helpful in determining which biologics provide optimum benefit in terms of HRQL.
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. Search strategies
Pubmed (1946 – Present)
Search Query
#5 Search (#1 AND #2 AND #3 AND #4)
#4 Search (HRQoL OR HRQL OR “quality of life” OR SF‐36 OR “short form‐36” OR SF‐36V2 OR IBDQ OR OR “inflammatory bowel disease questionnaire” OR EQ‐5D OR WPAI* OR “work productivity” OR “activity impairment” OR questionnaire OR questionnaire* OR “activities of daily living” OR ADL OR questionnaire [MH])
#3 Search ((colitis AND ulcerat*) OR proctosigmoiditis OR rectocolitis OR rectosigmoiditis OR (ulcerative AND rectocolitis) OR (ulcerative AND proctocolitis) OR (haemorrhagic AND ulcerative) OR (hemorrhagic AND ulcerative) OR (haemorrhagic AND proctocolitis) OR (hemorrhagic AND proctocolitis) OR (proctitis)
#2 Search ("anti tnf" OR anti‐tnf OR anti‐TNF* OR "anti TNF*" OR anti‐tum* OR antitum* OR "anti IL*" OR anti‐IL* OR etanercept OR infliximab OR "mab CA2" OR ustekinumab OR "CNTO 1275" OR certolizumab* OR CDP870 OR natalizumab OR anti‐alpha* OR "anti alpha*" OR onercept OR r‐hTBP‐1 OR vedolizumab OR MLN0002 OR basiliximab "CHI 621" OR certolizumab OR "rhuMAb*" OR visilizumab OR "HuM291" OR daclizumab OR "DAC HYP" OR briakinumab OR ABT‐874 OR adalimumab OR D2E7 OR anti‐CD* OR "anti CD*" OR anti‐integr* OR antiintegr* OR "anti madcam" OR anti‐madcam OR CDP571 OR PF00547 OR PF‐00547 OR IFN* OR interferon* OR RDP58 OR antibodies, monoclonal [MH])
#1 Search (single* OR double* OR triple* OR treble* OR blind* OR mask* OR placebo* OR single‐blind* OR double‐blind* OR triple‐blind* OR random* OR controlled)
EMBASE (1974 – Present)
# Searches
1 random$.tw.
2 factorial$.tw.
3 (crossover$ or cross over$ or cross‐over$).tw.
4 placebo$.tw.
5 single blind.mp.
6 double blind.mp.
7 triple blind.mp.
8 (singl$ adj blind$).tw.
9 (double$ adj blind$).tw.
10 (tripl$ adj blind$).tw.
11 assign$.tw.
12 allocat$.tw.
13 crossover procedure/
14 double blind procedure/
15 single blind procedure/
16 triple blind procedure/
17 randomized controlled trial/
18 or/1‐17
19 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human
or humans).ti.)
20 18 not 19
21 exp monoclonal antibody/
22 anti‐tum*.mp. or exp anti tumor necrosis factor/
23 exp tumor necrosis factor antibody/ or exp tumor necrosis factor alpha antibody/ or
anti‐TNF*.mp.
24 exp interleukin 2 receptor antibody/ or anti‐IL*.mp.
25 etanercept.mp. or exp etanercept/
26 infliximab.mp. or exp infliximab/
27 ustekinumab.mp. or exp ustekinumab/
28 exp certolizumab pegol/ or certolizumab*.mp.
29 natalizumab.mp. or exp natalizumab/
30 anti‐alpha.mp.
31 onercept.mp. or exp onercept/
32 vedolizumab.mp. or exp vedolizumab/
33 basiliximab.mp. or exp basiliximab/
34 visilizumab.mp. or exp visilizumab/
35 daclizumab.mp. or exp daclizumab/
36 briakinumab.mp. or exp briakinumab/
37 adalimumab.mp. or exp adalimumab/
38 anti‐CD*.mp.
39 exp mucosal addressin cell adhesion molecule 1/ or anti‐madcam.mp.
40 IFN.mp. or exp interferon/
41 interferon*.mp. [mp=title, abstract, subject headings, heading word, drug trade name,
original title, device manufacturer, drug manufacturer, device trade name, keyword] 42 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or
36 or 37 or 38 or 39 or 40 or 41
43 20 and 42
44 exp ulcerative colitis/ or exp colitis/
45 (rectocolitis or proctitis or proctocolitis or rectocolitis or rectosigmoiditis or
proctosigmoiditis).mp. [mp=title, abstract, subject headings, heading word, drug trade
name, original title, device manufacturer, drug manufacturer, device trade name,
keyword]
46 44 or 45
47 43 and 46
48 exp "quality of life"/
49 quality of life.mp. [mp=title, abstract, subject headings, heading word, drug trade name,
original title, device manufacturer, drug manufacturer, device trade name, keyword]
50 (HRQL or HRQoL).mp. [mp=title, abstract, subject headings, heading word, drug trade
name, original title, device manufacturer, drug manufacturer, device trade name,
keyword]
51 SF‐36.mp. or exp Short Form 36/
52 short form 36.mp.
53 inflammatory bowel disease questionnaire.mp.
54 IBDQ.mp.
55 EQ‐5D.mp.
56 exp productivity/ or WPAI*.mp.
57 activity impairment.mp. or exp absenteeism/
58 exp questionnaire/ or questionnair*.mp.
59 activities of daily living.mp. or exp daily life activity/
60 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59
61 47 and 60
MEDLINE (In‐process and other non‐indexed citations) (1946 – present)
# Searches
1 random$.tw.
2 factorial$.tw.
3 (crossover$ or cross over$ or cross‐over$).tw.
4 placebo$.tw.
5 single blind.mp.
6 double blind.mp.
7 triple blind.mp.
8 (singl$ adj blind$).tw.
9 (double$ adj blind$).tw.
10 (tripl$ adj blind$).tw.
11 assign$.tw.
12 allocat$.tw.
13 crossover procedure/
14 double blind procedure/
15 single blind procedure/
16 triple blind procedure/
17 randomized controlled trial/
18 or/1‐17
19 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or
humans).ti.)
20 18 not 19
21 anti‐tum*.mp. or exp anti tumor necrosis factor/
22 exp tumor necrosis factor antibody/ or exp tumor necrosis factor alpha antibody/ or anti‐
TNF*.mp.
23 exp interleukin 2 receptor antibody/ or anti‐IL*.mp.
24 etanercept.mp. or exp etanercept/
25 infliximab.mp. or exp infliximab/
26 ustekinumab.mp. or exp ustekinumab/
27 exp certolizumab pegol/ or certolizumab*.mp.
28 natalizumab.mp. or exp natalizumab/
29 anti‐alpha.mp.
30 onercept.mp. or exp onercept/
31 vedolizumab.mp. or exp vedolizumab/
32 basiliximab.mp. or exp basiliximab/
33 visilizumab.mp. or exp visilizumab/
34 daclizumab.mp. or exp daclizumab/
35 briakinumab.mp. or exp briakinumab/
36 adalimumab.mp. or exp adalimumab/
37 exp mucosal addressin cell adhesion molecule 1/ or anti‐madcam.mp.
38 IFN.mp. or exp interferon/
39 interferon*.mp. [mp=title, abstract, original title, name of substance word, subject heading
word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
40 exp Antibodies, Monoclonal/ or monoclonal antibod*.mp.
41 21 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
or 38 or 39 or 40
42 20 and 41
43 ulcerative colitis.mp. or exp Colitis, Ulcerative/
44 (rectocolitis or proctitis or proctocolitis or rectocolitis or rectosigmoiditis or
proctosigmoiditis).mp. [mp=title, abstract, original title, name of substance word, subject
heading word, keyword heading word, protocol supplementary concept, rare disease
supplementary concept, unique identifier]
45 43 or 44
46 42 and 45
47 quality of life.mp. or exp "Quality of Life"/
48 (HRQL or HRQoL).mp. [mp=title, abstract, original title, name of substance word, subject
heading word, keyword heading word, protocol supplementary concept, rare disease
supplementary concept, unique identifier]
49 short form 36.mp.
50 SF‐36.mp.
51 inflammatory bowel disease questionnaire.mp.
52 IBDQ.mp.
53 EQ‐5D.mp.
54 exp Absenteeism/ or WPAI*.mp.
55 exp Efficiency/ or activity impairment.mp.
56 questionnair*.mp.
57 questionnaire.mp. or Questionnaires/
58 activities of daily living.mp. or exp "Activities of Daily Living"/
59 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58
60 46 and 59
Cochrane Central Library
ID Search
#1 ulcerative colitis or rectocolitis or proctitis or proctocolitis or rectocolitis or rectosigmoiditis or proctosigmoiditis
#2 "anti tnf" or anti‐tnf or anti‐TNF* or "anti TNF*" or anti‐tum* or antitum* or "anti IL*" or anti‐IL* or etanercept or infliximab or "mab CA2" or ustekinumab or "CNTO 1275" or certolizumab* or CDP870 or natalizumab or anti‐alpha* or "anti alpha*" or onercept or r‐hTBP‐1 or vedolizumab or MLN0002 or basiliximab "CHI 621" or certolizumab or
"rhuMAb*" or visilizumab or "HuM291" or daclizumab or "DAC HYP" or briakinumab
or ABT‐874 or adalimumab or D2E7 or anti‐CD* or "anti CD*" or anti‐integr* or
antiintegr* or "anti madcam" or anti‐madcam or CDP571 or PF00547 or PF‐00547 or
IFN* or interferon* or RDP58
#3 HRQoL or HRQL or "quality of life" or SF‐36 or "short form‐36" or SF‐36V2 or IBDQ
or “inflammatory bowel disease questionnaire" or EQ‐5D or WPAI* or "work productivity" or "activity impairment" or questionnaire or questionnaire* or "activities
of daily living" or ADL
#4 #1 and #2 and #3
DDW Abstracts – (1981 – 2010)
The terms“ulcerative colitis or rectocolitis or proctitis or proctocolitis or rectocolitis or rectosigmoiditis or proctosigmoiditis” will be cross‐referenced with “HRQoL or HRQL or "quality of life" or SF‐36 or "short form‐36" or SF‐36V2 or IBDQ or “inflammatory bowel disease questionnaire" or EQ‐5D or WPAI* or "work productivity" or "activity impairment" or questionnaire or questionnaire* or "activities of daily living" or ADL” and the abstracts reviewed to determine their relevance to this review.
Data and analyses
Comparison 1. Interferon‐B‐1a versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improved IBDQ (≥15 points from baseline) at week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1 Interferon‐B‐1a versus placebo, Outcome 1 Improved IBDQ (≥15 points from baseline) at week 8.
Comparison 2. Rituximab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IBDQ at week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
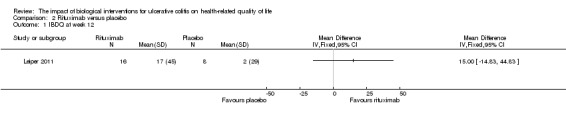
Comparison 2 Rituximab versus placebo, Outcome 1 IBDQ at week 12.
Comparison 3. Infliximab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IBDQ at week 6 or 8 | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 5 mg/kg | 2 | 529 | Mean Difference (IV, Fixed, 95% CI) | 18.58 [13.19, 23.97] |
| 1.2 10 mg/kg | 1 | 486 | Mean Difference (IV, Fixed, 95% CI) | 15.0 [9.46, 20.54] |
| 2 Improved IBDQ (≥16 points from baseline) at week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Improved IBDQ (≥32 points from baseline) at week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 EQ‐5D at week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Improved SF‐36 PCS (≥3 points from baseline) at week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Improved SF‐36 PCS (≥5 points from baseline) at week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Improved SF‐36 MCS (≥3 points from baseline) at week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Improved SF‐36 MCS (≥5 points from baseline) at week 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
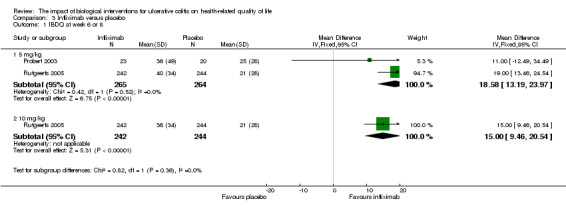
Comparison 3 Infliximab versus placebo, Outcome 1 IBDQ at week 6 or 8.
3.2. Analysis.

Comparison 3 Infliximab versus placebo, Outcome 2 Improved IBDQ (≥16 points from baseline) at week 8.
3.3. Analysis.
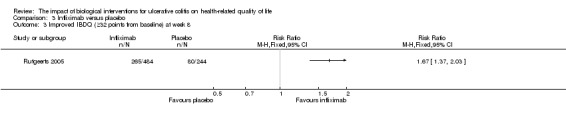
Comparison 3 Infliximab versus placebo, Outcome 3 Improved IBDQ (≥32 points from baseline) at week 8.
3.4. Analysis.
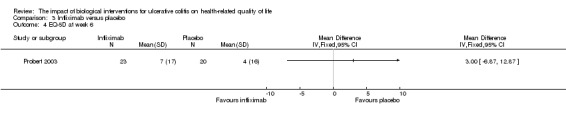
Comparison 3 Infliximab versus placebo, Outcome 4 EQ‐5D at week 6.
3.5. Analysis.
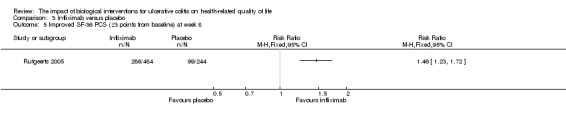
Comparison 3 Infliximab versus placebo, Outcome 5 Improved SF‐36 PCS (≥3 points from baseline) at week 8.
3.6. Analysis.
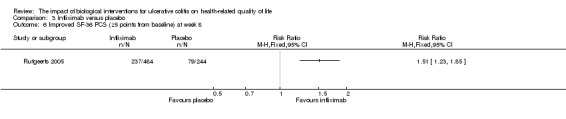
Comparison 3 Infliximab versus placebo, Outcome 6 Improved SF‐36 PCS (≥5 points from baseline) at week 8.
3.7. Analysis.
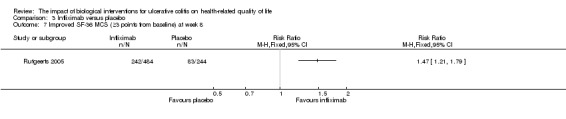
Comparison 3 Infliximab versus placebo, Outcome 7 Improved SF‐36 MCS (≥3 points from baseline) at week 8.
3.8. Analysis.
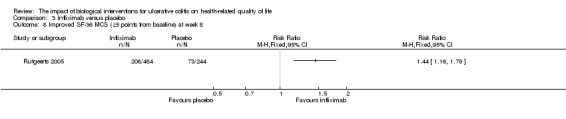
Comparison 3 Infliximab versus placebo, Outcome 8 Improved SF‐36 MCS (≥5 points from baseline) at week 8.
Comparison 4. Adalimumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IBDQ at week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 IBDQ at week 52 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Improved IBDQ (≥16 points from baseline) at week 8 | 2 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.06, 1.43] |
| 4 Improved IBDQ (≥16 points from baseline) at week 52 | 2 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.28, 2.34] |
4.1. Analysis.
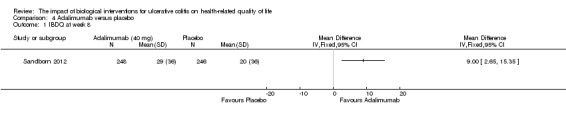
Comparison 4 Adalimumab versus placebo, Outcome 1 IBDQ at week 8.
4.2. Analysis.
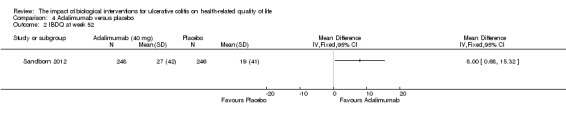
Comparison 4 Adalimumab versus placebo, Outcome 2 IBDQ at week 52.
4.3. Analysis.
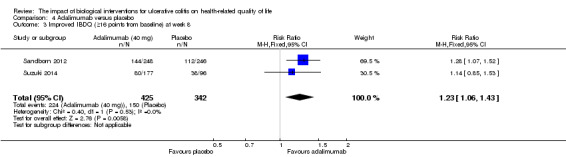
Comparison 4 Adalimumab versus placebo, Outcome 3 Improved IBDQ (≥16 points from baseline) at week 8.
4.4. Analysis.
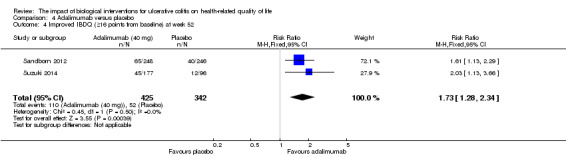
Comparison 4 Adalimumab versus placebo, Outcome 4 Improved IBDQ (≥16 points from baseline) at week 52.
Comparison 5. Golimumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IBDQ at week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 200/100 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 400/200 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
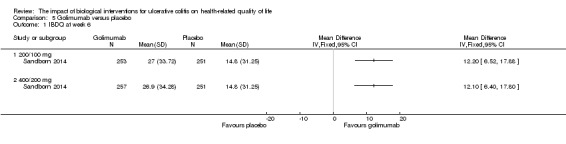
Comparison 5 Golimumab versus placebo, Outcome 1 IBDQ at week 6.
Comparison 6. Vedolizumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improved IBDQ (≥16 points from baseline) at week 6 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Improved IBDQ (≥16 points from baseline) at week 52 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 SF‐36 PCS at week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 SF‐36 MCS at week 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 SF‐36 PCS at week 52 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Vedolizumab every 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Vedolizumab every 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 SF‐36 MCS at week 52 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Vedolizumab every 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Vedolizumab every 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
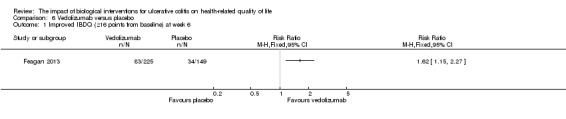
Comparison 6 Vedolizumab versus placebo, Outcome 1 Improved IBDQ (≥16 points from baseline) at week 6.
6.2. Analysis.

Comparison 6 Vedolizumab versus placebo, Outcome 2 Improved IBDQ (≥16 points from baseline) at week 52.
6.3. Analysis.
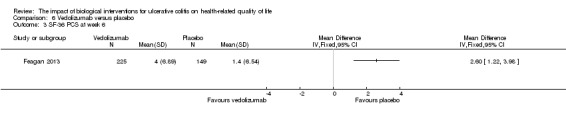
Comparison 6 Vedolizumab versus placebo, Outcome 3 SF‐36 PCS at week 6.
6.4. Analysis.
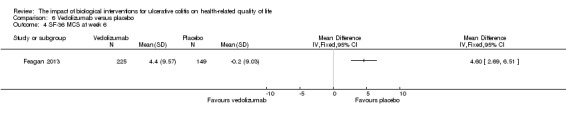
Comparison 6 Vedolizumab versus placebo, Outcome 4 SF‐36 MCS at week 6.
6.5. Analysis.
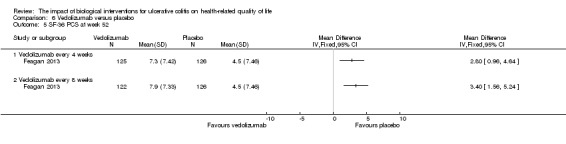
Comparison 6 Vedolizumab versus placebo, Outcome 5 SF‐36 PCS at week 52.
6.6. Analysis.
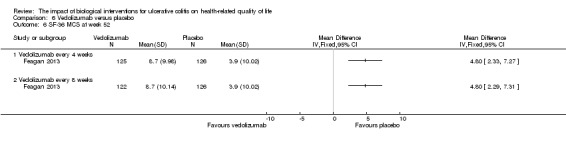
Comparison 6 Vedolizumab versus placebo, Outcome 6 SF‐36 MCS at week 52.
Comparison 7. TNF‐alpha antagonists (Adalimumab, Infliximab and Golimumab) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IBDQ at weeks 6 or 8 | 4 | 1565 | Mean Difference (IV, Fixed, 95% CI) | 13.71 [10.40, 17.01] |
| 1.1 Infliximab ‐ 5 mg/kg | 2 | 567 | Mean Difference (IV, Fixed, 95% CI) | 18.26 [12.98, 23.54] |
| 1.2 Adalimumab ‐ 160/80/40 mg | 1 | 494 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [2.65, 15.35] |
| 1.3 Golimumab ‐ 200/100 mg | 1 | 504 | Mean Difference (IV, Fixed, 95% CI) | 12.2 [6.52, 17.88] |
| 2 Improved IBDQ at weeks 6 or 8 | 3 | 1495 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [1.19, 1.46] |
| 2.1 Infliximab | 1 | 728 | Risk Ratio (IV, Fixed, 95% CI) | 1.39 [1.21, 1.60] |
| 2.2 Adalimumab | 2 | 767 | Risk Ratio (IV, Fixed, 95% CI) | 1.24 [1.07, 1.44] |
7.1. Analysis.
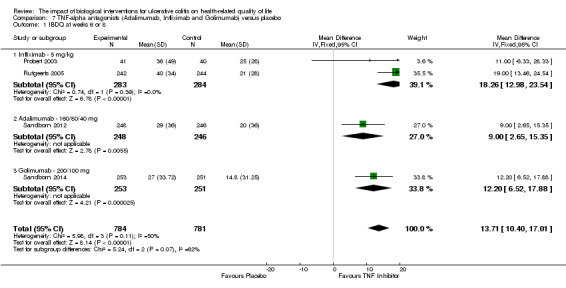
Comparison 7 TNF‐alpha antagonists (Adalimumab, Infliximab and Golimumab) versus placebo, Outcome 1 IBDQ at weeks 6 or 8.
7.2. Analysis.
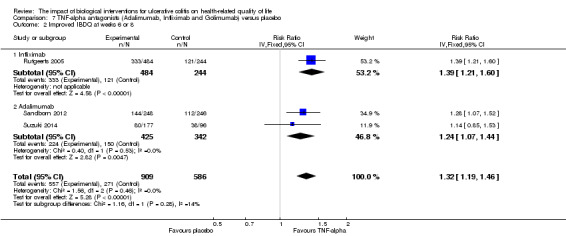
Comparison 7 TNF‐alpha antagonists (Adalimumab, Infliximab and Golimumab) versus placebo, Outcome 2 Improved IBDQ at weeks 6 or 8.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Feagan 2013.
| Methods | A phase 3, randomized, double‐blind, placebo‐controlled study consisting of separate induction and maintenance trials conducted at 211 medical centers (including 15 that discontinued enrolment) in 34 countries from 2008 to 2012 | |
| Participants | 1406 patients were evaluated for eligibility; 895 were enrolled and included in the analysis, of whom 58 (6.5%) did not meet one or more inclusion criteria or met one or more exclusion criteria In the induction trial 225 patients were randomly assigned to receive vedolizumab and 149 to receive placebo (cohort 1); 521 patients (cohort 2) received open‐label vedolizumab Patients who had a response to vedolizumab at week 6 were enrolled in the maintenance trial, with 122, 125, and 126 patients randomly assigned to receive vedolizumab every 8 weeks, vedolizumab every 4 weeks, and placebo, respectively | |
| Interventions | Patients were randomly assigned, in a 3:2 ratio, to receive intravenous vedolizumab (300 mg) or placebo at days 1 and 15 (cohort 1), with two stratification factors: concomitant use or nonuse of glucocorticoids, and concomitant use or nonuse of immunosuppressive agents or prior use or nonuse of TNF antagonists | |
| Outcomes | The primary outcome for induction therapy was a clinical response at week 6 (defined as a reduction in the Mayo Clinic score of at least 3 points and a decrease of at least 30% from the baseline score, with a decrease of at least 1 point on the rectal bleeding subscale or an absolute rectal bleeding score of 0 or 1) Secondary outcomes at week 6 were clinical remission (defined as a Mayo Clinic score of 2 or lower and no subscore higher than 1, and mucosal healing, defined as an endoscopic subscore of 0 or 1) The primary outcome for maintenance therapy was clinical remission at week 52 Secondary measures were durable clinical response (response at both weeks 6 and 52), durable clinical remission (remission at both weeks 6 and 52), mucosal healing at week 52, and glucocorticoid‐free remission at week 52 in patients receiving glucocorticoids at baseline HRQL was evaluated with the IBDQ |
|
| Notes | NCT00783718 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned in a 3:2 ratio using computer‐generated randomization schedules |
| Allocation concealment (selection bias) | Low risk | Central allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study; both the participant and physician were blinded to the treatment administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Analyses were performed according to the intention‐to‐treat principle" The number of subjects who withdrew during the induction phase were 14 and 7 in the placebo and VDZ groups respectively |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Leiper 2011.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | Patients (n = 24) over 18 years of age with active steroid‐resistant UC (Mayo score: 6‐12 points, failure to respond to at least 2 weeks of 40 mg/day of prednisolone treatment) | |
| Interventions | Patients received either an infusion of 1 g of rituximab or placebo on day 1 and at 2 weeks | |
| Outcomes | Primary outcome was remission at week 4 Secondary outcomes consisted of clinical response at weeks 4 and 8, remission at weeks 8 and 12, mucosal healing at weeks 4 and 12 and improvement in the IBDQ | |
| Notes | This drug was not shown to be an effective therapy for active steroid‐resistant UC NCT00261118 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized 2:1 (treatment:placebo) in blocks of 5 by the hospital pharmacy department. The pharmacists had no other involvement in the trial |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from patients and investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Allocation was not revealed until the last patient completed the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of response or remission was made before unblinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a high drop‐out rate in both groups Only 6 out of 16 patients in the rituximab group and 2 out of 8 patients in the placebo group completed the 12 week study Last value was carried forward for analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Pena‐Rossi 2008.
| Methods | Randomized, double‐blind, parallel‐group, placebo‐controlled multi‐center trial (43 centers in 17 countries) | |
| Participants | Patients (n = 194) over 18 years of age with moderately‐active UC, based on clinical (UCSS score: 6‐10 and a Physicians Global Assessment score < 3), radiological and endoscopic (proctosigmoidoscopy score of 2‐3) or histological findings | |
| Interventions | Patients received either placebo or 44 or 66 μg of IFN‐β‐1a subcutaneously 3 times a week for 8 weeks | |
| Outcomes | The primary outcome was endoscopically‐confirmed remission Secondary outcomes were clinical response, HRQL (IBDQ) and changes in biomarkers of inflammation |
|
| Notes | This drug was not shown to be an effective therapy for moderately active UC NCT00303381 ClinicalTrials.gov website listed 7 countries rather than 17 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization (1:1:1) using stochastic minimisation, considering overall balance, center, region and use of maintenance therapy (i.e. amino‐salicylic acid) as minimisation factors |
| Allocation concealment (selection bias) | Low risk | The existing balance of allocated treatments influenced allocation of patients to treatment (see above) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physician and patient were blind to the treatment administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs were adequately explained and balanced among treatment groups |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes and patients reported in the text |
| Other bias | Low risk | No other apparent sources of bias |
Probert 2003.
| Methods | Randomized, double blind, placebo‐controlled study at 4 centers in the United Kingdom and Germany | |
| Participants | 43 male and female patients (at least 18 years of age) with moderately severe glucocorticoid resistant UC Patients had to have received at least 30 mg prednisolone (or equivalent) for at least 1 week, for relapse but still had clinical activity Patients had to have an ulcerative colitis symptom score (UCSS) ≥6 and a sigmoidoscopy score of at least 2 on the Baron scale Patients had biopsies taken to verify the presence of active disease |
|
| Interventions | Patients were randomized to receive either placebo or 5 mg of infliximab/kg of body weight at 0 and 2 weeks Consectuive patients were randomized in blocks of 4 within each center |
|
| Outcomes | The primary outcome was the proportion of patients in remission at week 6 (UCSS ≤ 2 and a Baron score of 0) Secondary outcomes included change in the UCSS, Baron score, HRQL, C‐reactive protein levels and change in daily glucocorticoid dose HRQL was assessed with the IBDQ and EQ‐5D indices | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive patients randomized in blocks of 4 at each center |
| Allocation concealment (selection bias) | Unclear risk | Method of randomisation and allocation was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pharmacists, investigators and participants were blinded to the treatment administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in the methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the 6 week study and all results reported |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes and patients reported in the text |
| Other bias | Low risk | No other apparent sources of bias |
Reinisch 2011.
| Methods | Randomized, double blind, placebo‐controlled induction study in 94 centers across North America and Europe (ULTRA 1) | |
| Participants | Anti‐tumor necrosis factor (anti‐TNF)‐naïve patients with moderately to severely active UC (Mayo score > 6 points and endoscopic subscore > 2 points) despite treatment with corticosteroids and/or immunosuppressants 186 patients were randomized under the first protocol; after the protocol was amended there were 576 patients randomized |
|
| Interventions | 1:1 treatment with subcutaneous adalimumab (160 mg at Week 0, 80 mg at Week 2, and 40 mg at Weeks 4 and 6) or placebo At the request of European regulatory authorities the protocol was amended ("Amendment 3") and a second induction group was added (80 mg at Week 0, 40 mg at Weeks 2, 4 and 6) |
|
| Outcomes | HRQL was measured by the Inflammatory Bowel Disease Questionnaire (IBDQ) and Mental and Physical Component Summary (MCS and PCS) scores of the Short Form 36 Health Survey (SF‐36) | |
| Notes | NCT00385736 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization was performed using a scheme developed by the study sponsor Patients were randomized to adalimumab induction (ADA 160/80) or placebo (1:1 ratio, original protocol), or one of two adalimumab induction doses (ADA160/80 or ADA 80/40) or placebo (1:1:1 ratio, after study was amended) |
| Allocation concealment (selection bias) | Low risk | Centralized treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, study site personnel, study investigators, and the study sponsor were blinded to treatment assignment throughout the study" The study drug and placebo were administered subcutaneously using pre‐filled syringes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Since Amendment 3 added a second adalimumab dose group, patients enrolled before the amendment were not included in the primary analysis (intention‐to‐treat) Patients randomized under the original protocol and all of the amendments were included in a second population, intention‐to‐treat‐ The safety population included all patients who received at least one dose of study drug or placebo |
| Selective reporting (reporting bias) | Low risk | Results of all patients and outcomes are reported in the text |
| Other bias | Low risk | No other apparent sources of bias |
Rutgeerts 2005.
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled clinical studies (ACT 1 and ACT 2) | |
| Participants | 728 patients with moderately‐to‐severely active UC (defined as a Mayo score of 6‐12 points) | |
| Interventions | Patients were randomized in a 1:1:1 ratio to receive either placebo or 5 mg/kg or 10 mg/kg infusions of infliximab at 0, 2, 6 and every 8 weeks through week 22 (Act 22) or week 46 (Act 1) | |
| Outcomes | Mean difference in IBDQ score at week 8 | |
| Notes | See Rutgeerts 2005 for a description of the methods NCT00096655 NCT00036439 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned in a 1:1:1 ratio. Central randomization with a dynamic treatment allocation stratified according the the investigational site and whether the patients had UC refractory to corticosteroid treatment |
| Allocation concealment (selection bias) | Low risk | Centralized dynamic treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participant and physician were blinded to the treatment administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were included in the analysis Patients taking prohibited medication, discontinued study medication, had a colectomy or ostomy, were scored as non‐responders |
| Selective reporting (reporting bias) | Low risk | All patients and outcomes were included in the results |
| Other bias | Low risk | No other apparent sources of bias |
Sandborn 2012.
| Methods | Phase 3, multicenter, randomized, double‐blind, placebo‐controlled study (ULTRA 2) conducted at 103 centers in North America, Europe, Australia, New Zealand and Israel | |
| Participants | Patients with moderately‐to‐severely active ulcerative colitis (defined as a Mayo score of 6‐12 points) despite concurrent treatment with oral corticosteroids or immunosuppressants (N = 494) Concominant medication remained at stable doses except steroids which could be tapered at week 8 if the patient was deemed to have a satisfactory clinical response At week 12 patients with an inadequate response could switch to open‐label adalimumab (40 mg EOW) | |
| Interventions | Patients received subcutaneous injections of adalimumab 160 mg at week 0, 80 mg at week 2 and 40 mg EOW beginning at week 4 or matched placebo until week 52 | |
| Outcomes | Primary outcomes were remission at weeks 8 and 52 (defined as a total Mayo score < 2 points,with no subscore exceeding 1 point) Secondary outcomes included HRQL (measured by the IBDQ); clinical response (defined as a decrease from baseline in total Mayo score of at least 3 points and a decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1); and mucosal healing (defined as an endoscopy subscore of 0 or 1) | |
| Notes | See Reinisch 2011 for details on ULTRA 1 NCT00408629 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized randomization was performed. Patinets were stratified by prior exposure to infliximab or other anti‐TNF drugs |
| Allocation concealment (selection bias) | Low risk | Centralized treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind and matched placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were included in the analysis Patients who switched to open‐label adalimumab were considered treatment failures |
| Selective reporting (reporting bias) | Low risk | All outcomes and patients were included in the results |
| Other bias | Low risk | No other apparent sources of bias |
Sandborn 2014.
| Methods | An integrated double‐blind phase 2 dose‐finding and phase 3 dose‐confirmation trial | |
| Participants | 1064 adults with UC (Mayo score: 6‐12; endoscopic subscore > 2; n=774 patients in phase 3) were included | |
| Interventions | Patients were randomized (1:1:1:1) to receive subcutaneous injections of placebo or golimumab 100/50 mg, 200/100 mg or 400/200 mg in phase 2 Patients were randomized (1:1:1) to received subcutaneous injections of placebo or golimumab 200/100 mg or 400/200 mg at weeks 0 and 2 in phase 3 |
|
| Outcomes | The phase 3 primary end point was week‐6 clinical response Secondary end points included week‐6 clinical remission, mucosal healing, and Inflammatory Bowel Disease Questionnaire (IBDQ) score change | |
| Notes | NCT00487539 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization using an interactive voice response system In phase 2 patients were allocated using an adaptive randomization procedure with stratification by investigative site Following phase 2 allocation was performed using a permuted block randomization schema |
| Allocation concealment (selection bias) | Low risk | Central allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind and matched placebo but further detail not provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/774 (1.7%) patients were excluded from the efficacy analysis due to noncompliance with good clinical practice (e.g. source documentation, informed consent process) at the study site |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Suzuki 2014.
| Methods | 52‐week, phase 2/3, randomized, double‐blind study evaluated adalimumab for induction and maintenance treatment. | |
| Participants | 273 anti‐TNF‐naïve Japanese patients with UC who were refractory to corticosteroids, immunosuppressives , or both | |
| Interventions | Patients received placebo, adalimumab 80/40 (80 mg at week 0, then 40 mg every other week), or adalimumab 160/80 (160/80 mg at weeks 0/2, then 40 mg every other week) in addition to background UC therapy | |
| Outcomes | Outcomes included: week 8, 32 and 52 clinical response, clinical remission, and mucosal healing Other efficacy analyses at weeks 8, 32, and 52 included RBS, PGA, and stool frequency indicative of mild disease (score B1) and IBDQ response (C16‐point increase from baseline in IBDQ score) |
|
| Notes | NCT00853099 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized 1:1:1 (adalimumab 80/40 mg:adalimumab160/80 mg:placebo) using a centrally designed randomization table |
| Allocation concealment (selection bias) | Low risk | Central allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind but no further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses included all 273 patients enrolled in the study |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes and patients reported |
| Other bias | Low risk | No other apparent sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armuzzi 2005 | Study was not placebo‐controlled |
| Madsen 2001 | Open label observational study |
| Miner 2011 | This study used the Work Productivity Activity Impairment Index (WPAII) as an outcome measure, which is not a validated HRQL instrument |
| Parikh 2013 | Open label observational study |
Characteristics of ongoing studies [ordered by study ID]
NCT00488631.
| Trial name or title | A phase 3 multicenter, randomized, placebo‐controlled, double‐blind study to evaluate the safety and efficacy of golimumab maintenance therapy, administered subcutaneously, in subjects with moderately to severely active ulcerative colitis |
| Methods | Phase III, multicenter, randomized, double‐blind, placebo‐controlled trial |
| Participants | Adult patients (> 18 years) with ulcerative colitis in remission or response induced by golimumab |
| Interventions | Subcutaneous golimumab 100 mg administered every 4 weeks through week 52 Subcutaneous golimumab 50 mg administered every 4 weeks through week 52 Placebo |
| Outcomes | Primary outcome: number of participants in clinical response through week 54 Secondary outcomes: number of participants with clinical remission at both week 30 and week 54, number of participants with mucosal healing at both week 30 and week 54, number of participants with clinical remission at both week 30 and 54 among participants with clinical remission at week 0 of maintenance study, number of participants with clinical remission at week 54 and not receiving concomitant corticosteroids among participants on corticosteroids at week 0 of maintenance study |
| Starting date | September 2007 |
| Contact information | Janssen Research & Development, LLC |
| Notes |
NCT01551290.
| Trial name or title | A phase 3, multicenter, randomized, double‐blind, placebo‐controlled study evaluating the efficacy and safety of infliximab in Chinese subjects with active ulcerative colitis |
| Methods | Phase III, multicenter, randomized, double‐blind, placebo‐controlled trial |
| Participants | Adult patients (18 to 65 years) with active ulcerative colitis of at least 3 months duration at screening with score of > 2 on the endoscopy subscore of the Mayo score and baseline Mayo score of 6 to 12 |
| Interventions | Infliximab 5 mg/kg infusion at weeks 0, 2, 6, 14, and 22 Placebo |
| Outcomes | Primary outcome: clinical response at week 8 Secondary outcomes: clinical remission at week 8, mucosal healing at week 8, clinical response at week 26, clinical remission at week 26 |
| Starting date | April 2012 |
| Contact information | Xian‐Janssen Pharmaceutical Ltd |
| Notes |
NCT01863771.
| Trial name or title | A phase 3 multicenter, placebo‐controlled, double‐blind, randomized‐withdrawal study to evaluate the safety and efficacy of golimumab maintenance therapy, administered subcutaneously, in Japanese subjects with moderately to severely active ulcerative colitis |
| Methods | Phase III, multicenter, randomized, double‐blind, placebo‐controlled trial |
| Participants | Adult patients (18 to 70 years) with moderately to severely active ulcerative colitis, defined as a baseline Mayo score of 6 to 12, inclusive |
| Interventions | All patients received subcutaneous golimumab 200 mg at week 0 and 100 mg golimumab at week 2, patients with a clinical response were randomized to subcutaneous golimumab 100 mg every 4 weeks or placebo through week 52 |
| Outcomes | Primary outcome: clinical response Secondary outcomes: IBDQ, clinical remission, EQ‐5D, mucosal healing, adverse events |
| Starting date | April 2013 |
| Contact information | Janssen Pharmaceutical KK |
| Notes |
NCT02039505.
| Trial name or title | Phase III, multicenter, randomized, double‐blinded, placebo‐controlled, parallel‐group study to examine the efficacy, safety, and pharmacokinetics of intravenous MLN0002 (300 mg) Infusion in induction and maintenance therapy in Japanese patients with moderately or severely active ulcerative colitis |
| Methods | Phase III, multicenter, randomized, double‐blind, placebo‐controlled trial |
| Participants | Patients with moderately or severely active ulcerative colitis as determined by baseline complete Mayo score of 6 to 12 (inclusive) with an endoscopic subscore of > 2 |
| Interventions | Vedolizumab (300 mg) administered at weeks 0, 2, and 6 and every 8 weeks thereafter Placebo |
| Outcomes | Primary outcomes: clinical response at week 10, clinical remission at week 60, adverse events Secondary outcomes: clinical remission at week 10, mucosal healing at week 10, durable clinical response, mucosal healing at week 60, durable clinical remission, corticosteroid‐free clinical remission at week 60, serum vedolizumab concentration, human anti‐human antibody, neutralizing antibody |
| Starting date | March 2014 |
| Contact information | Takeda Study Registration Call Center +1‐800‐778‐2860 |
| Notes |
Differences between protocol and review
In the protocol we reported that workplace productivity and participation and will be assessed if enough data exists to complete such an analysis. We did not collect any data for these outcomes but will consider doing so for future updates of this review. We searched ClinicalTrials.gov to identify ongoing studies. This was not pre‐specified in the protocol.
Contributions of authors
KL and MM or CEP and JKM scanned the papers for inclusion and extracted data. KL, JKM, CEP and MM were involved in the writing of the manuscript.
Declarations of interest
None known.
New
References
References to studies included in this review
Feagan 2013 {published data only}
- Feagan B, Colombel JF, Rubin D, Mody R, Sankoh S, Lasch K. Improvements in health‐related quality of life in patients with ulcerative colitis treated with vedolizumab. Journal of Crohn's and Colitis 2014;8:S51‐2. [Google Scholar]
- Feagan BG, Colombel JF, Rubin DT, Mody R, Sankoh S, Lasch K. Health‐related quality of life in patients with ulcerative colitis after treatment with vedolizumab: Results from the GEMINI 1 study. Gastroenterology 2014;146(5 Suppl 1):S‐590. [Google Scholar]
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699‐710. [DOI] [PubMed] [Google Scholar]
- Sandborn W, Colombel JF, Panaccione R, Lasch K, Mody R, Green A, et al. Deep remission as a predictor of clinical outcomes in vedolizumab‐treated patients with ulcerative colitis. Gastroenterology 2015;148(4 Suppl 1):S256. [Google Scholar]
Leiper 2011 {published data only}
- Leiper K, Martin K, Ellis A, Subramanian S, Watson AJ, Christmas SE, et al. Randomised placebo‐controlled trial of rituximab (anti‐CD20) in active ulcerative colitis. Gut 2011;60(11):1520‐26. [DOI] [PubMed] [Google Scholar]
Pena‐Rossi 2008 {published data only}
- Pena‐Rossi C, Schreiber S, Golubovic G, Mertz‐Nielsen A, Panes J, Rachmilewitz D, et al. Clinical trial: a multicentre, randomized, double‐blind, placebo‐controlled, dose‐finding, phase II study of subcutaneous interferon‐ß‐1a in moderately active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2008;28(6):758‐67. [DOI] [PubMed] [Google Scholar]
Probert 2003 {published data only}
- Probert CSJ, Hearing SD, Schreiber S, Kuhbacher T, Ghosh S, Arnott IDR, Forbes A. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut 2003;52:998‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinisch 2011 {published data only}
- Reinisch W, Sandborn W, Thakkar R, Lazar A, Huang B, Mulani P, et al. Adalimumab induction therapy improves health‐related quality of life in patients with moderately to severely active ulcerative colitis. American Journal of Gastroenterology 2010;105:S441‐2. [Google Scholar]
- Reinisch W, Sandborn WJ, Hommes, DW, D’Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780‐7. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Assche G, Thakkar R, Lazar A, Huang B, Mulani P, et al. Adalimumab induction therapy improves health‐related quality of life in patients with moderately to severely active ulcerative colitis. Inflammatory Bowel Diseases 2011;17:S60‐1. [Google Scholar]
Rutgeerts 2005 {published data only}
- Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, Eisenberg D, et al. The effects of infliximab therapy on health‐related quality of life in ulcerative colitis patients. American Journal of Gastroenterology 2007;102:794‐802. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Feagan B, Yan S, Sandborn WJ, Rutgeerts P, Eisenberg D, et al. Improvement in health related quality of life in infliximab‐treated moderate‐to‐severe active ulcerative colitis patients: Improvement overall and by baseline disease activity. UEGW Abstract Database. 2006:Abstract MON‐G‐298.
- Reinisch W, Sandborn WJ, Bala M, Yan S, Feagan BG, Rutgeerts P, et al. Response and remission are associated with improved quality of life, employment and disability status, hours worked, and productivity of patients with ulcerative colitis. Inflammatory Bowel Diseases 2007;13(9):1135‐40. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2005;353(23):2462‐76. [DOI] [PubMed] [Google Scholar]
- Sands B, Feagan B, Yan S, Sandborn W, Rutgeerts P, Eisenberg D, et al. Improvement in health related quality of life in infliximab‐treated moderate‐to‐severe active ulcerative colitis patients: Improvement overall and by baseline disease activity. Inflammatory Bowel Diseases 2007;13(Supp 5):648. [Google Scholar]
Sandborn 2012 {published data only}
- Sandborn W, Colombel JF, Yang M, Thakkar R, Mulani P, Chao J. Sustained clinical remission of ulcerative colitis is associated with greater improvements in quality of life, work productivity and activity. American Journal of Gastroenterology 2011;106:S456. [Google Scholar]
- Sandborn W, Thakkar R, Lazar A, Skup M, Yang M, Chao J, et al. Time‐in‐remission analysis of adalimumab vs. placebo for the treatment of ulcerative colitis. American Journal of Gastroenterology 2012;107:S641‐2. [Google Scholar]
- Sandborn WJ, Assche G, Thakkar R, Lazar B, Kron A, Yang M, et al. Adalimumab improves health‐related quality of life for 52 weeks in patients with ulcerative colitis. Journal of Crohn's and Colitis 2011;5(1):S87‐8. [Google Scholar]
- Sandborn WJ, Assche G, Thakkar RB, Lazar A, Kron M, Yang M, et al. Adalimumab improves health‐related quality of life for 52 weeks in patients with ulcerative colitis. Gut 2012;61:A400‐1. [Google Scholar]
- Sandborn WJ, Assche GA, Thakkar R, Lazar A, Kron M, Yang M, et al. Adalimumab improves health‐related quality of life for 52 weeks in patients with ulcerative colitis. Gastroenterology 2011;140(5 Suppl 1):S263. [Google Scholar]
- Sandborn WJ, Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology 2012;142(2):257‐65. [DOI] [PubMed] [Google Scholar]
Sandborn 2014 {published data only}
- Feagan B, Gibson P, Marano C, Strauss R, Han C, Johanns J, et al. Impact of golimumab sc on disease specific and generic hrqol in patients with moderately to severely active UC: Pursuit‐SC induction. Inflammatory Bowel Diseases 2012;18:S43. [Google Scholar]
- Feagan B, Gibson P, Marano C, Strauss R, Han C, Johanns J, et al. Impact of subcutaneously administered golimumab on disease specific and generic health‐related quality of life in patients with moderately to severely active ulcerative colitis: Results from PURSUIT‐SC induction. Journal of Crohn's and Colitis 2013;7:S99‐S100. [Google Scholar]
- Gibson P, Feagan B, Marano C, Strauss R, Han C, Johanns J, et al. Subcutaneously administered golimumab induction therapy improves health states measured by EQ‐5D in patients with moderately to severely active ulcerative colitis: Results from PURSUIT‐SC. Journal of Crohn's and Colitis 2013;7:S65. [Google Scholar]
- Rutgeerts P, Feagan B, Marano C, Strauss R, Johanns J, Zhang H, et al. Phase 2/3 randomized, placebo‐controlled, double‐blind study of SC golimumab induction in moderate to severe UC. Journal of Gastroenterology and Hepatology 2013;28:591. [Google Scholar]
- Sandborn W, Colombel JF, Feagan B, Marano C, Strauss R, Han C, et al. Early and sustained remission after treatment with subcutaneously administered golimumab is associated with normalized health‐related quality of life in patients with moderate to severe ulcerative colitis: Post‐HOC analysis from pursuit induction and maintenance trials. American Journal of Gastroenterology 2013;108:S519. [Google Scholar]
- Sandborn W, Feagan B, Marano C, Strauss R, Johanns J, Zhang H, et al. Clinical response is a meaningful endpoint in ulcerative colitis clinical studies. Inflammatory Bowel Diseases 2012;18:S26‐7. [Google Scholar]
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology 2014;146(1):85‐95. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Feagan BG, Marano CW, Strauss R, Johanns J, Zhang H, et al. A phase 2/3 randomized, placebo‐controlled, double‐blind study to evaluate the safety and efficacy of subcutaneous golimumab induction therapy in patients with moderately to severely active ulcerative colitis: Pursuit sc. Gastroenterology 2012;142(5 Suppl 1):S161. [Google Scholar]
Suzuki 2014 {published data only}
- Suzuki Y, Motoya S, Hanai H, Matsumoto T, Hibi T, Robinson A, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. Journal of Gastroenterology 2014;49(2):283‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Armuzzi 2005 {published data only}
- Armuzzi A, Lupascu A, Pascalis B, Fedeli P, Vincenti F, Gasbarrini G, et al. Infliximab in the treatment of glucocorticoid‐dependent ulcerative colitis: a 54‐week randmoized methylprednisolone‐controlled trial. Gastroenterology 2005;128(4 Suppl 2):A575. [Google Scholar]
Madsen 2001 {published data only}
- Madsen SM, Schlichting P, Davidsen B, Nielsen OH, Federspiel B, Riis P, et al. An open‐labeled, randomized study comparing systemic interferon‐α‐2A and prednisolone enemas in the treatment of left‐sided ulcerative colitis. American Journal of Gastroenterology 2001;96:1807‐15. [DOI] [PubMed] [Google Scholar]
Miner 2011 {published data only}
- Miner P, Mannon P, Rajagopalan K, McAllister A, Xi Y, Cataldi F. Improvement in work productivity: analysis from a proof of concept in ulcerative colitis with Interferon‐B‐1a. Inflammatory Bowel Diseases 2011;17:S55. [Google Scholar]
Parikh 2013 {published data only}
- Parikh A, Fox I, Leach T, Xu J, Scholz C, Patella M, et al. Long‐term clinical experience with vedolizumab in patients with inflammatory bowel disease. Inflammatory Bowel Disease 2013;19(8):1691‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00488631 {published data only}
- NCT00488631. A phase 3 multicenter, randomized, placebo‐controlled, double‐blind study to evaluate the safety and efficacy of golimumab maintenance therapy, administered subcutaneously, in subjects with moderately to severely active ulcerative colitis. clinicaltrials.gov/ct2/show/NCT00488631 (accessed 9 September 2015).
NCT01551290 {published data only}
- NCT01551290. A phase 3, multicenter, randomized, double‐blind, placebo‐controlled study evaluating the efficacy and safety of infliximab in Chinese subjects with active ulcerative colitis. clinicaltrials.gov/ct2/show/NCT01551290 (accessed 9 September 2015).
NCT01863771 {published data only}
- NCT01863771. A phase 3 multicenter, placebo‐controlled, double‐blind, randomized‐withdrawal study to evaluate the safety and efficacy of golimumab maintenance therapy, administered subcutaneously, in Japanese subjects with moderately to severely active ulcerative colitis. clinicaltrials.gov/ct2/show/NCT01863771 (accessed 9 September 2015).
NCT02039505 {published data only}
- NCT02039505. Phase III, multicenter, randomized, double‐blinded, placebo‐controlled, parallel‐group study to examine the efficacy, safety, and pharmacokinetics of intravenous MLN0002 (300 mg) Infusion in induction and maintenance therapy in Japanese patients with moderately or severely active ulcerative colitis. clinicaltrials.gov/ct2/show/study/NCT02039505 (accessed 9 September 2015).
Additional references
Achleitner 2012
- Achleitner U, Coenen M, Colombel JF, Peyrin‐Biroulet L, Sahakyan N, Cieza A. Identification of areas of functioning and disability addressed in inflammatory bowel disease‐specific patient reported outcome measures. Journal of Crohn's and Colitis 2012;6(5):507‐17. [DOI] [PubMed] [Google Scholar]
Bernklev 2005
- Bernklev T, Jahnsen J, Lygren I, Henriksen M, Vatn M, Moum B. Health‐related quality of life in patients with inflammatory bowel disease measured with the short form‐36: psychometric assessments and a comparison with general population norms. Inflammatory Bowel Diseases 2005;11(10):909‐18. [DOI] [PubMed] [Google Scholar]
Bernklev 2006
- Bernklev T, Jahnsen J, Henriksen M, Lygren I, Aadland E, Sauar J, et al. Relationship between sick leave, unemployment, disability, and health‐related quality of life in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2006;12(5):402‐12. [DOI] [PubMed] [Google Scholar]
Boonen 2002
- Boonen A, Dagnelie PC, Feleus A, Hesselink MA, Muris JW, Stockbrügger RW, et al. The impact of inflammatory bowel disease on labor force participation: results of a population sampled case‐control study. Inflammatory Bowel Diseases 2002;8(6):382‐9. [DOI] [PubMed] [Google Scholar]
Fazio 1999
- Fazio VW, O’Riordain MG, Lavery IC, Church JM, Lau P, Strong SA, et al. Long‐term functional outcome and quality of life after stapled restorative proctocolectomy. Annals of Surgery 1999;230(4):575‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feagan 1999
- Feagan BG. Review article: economic issues in Crohn's disease‐‐assessing the effects of new treatments on health‐related quality of life. Alimentary Pharmacology and Therapeutics 1999;13(Suppl 4):29‐37; discussion 38. [DOI] [PubMed] [Google Scholar]
Feagan 2007
- Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Songkai Y, Eisenberg D, et al. The effects of infliximab therapy on health‐related quality of life in ulcerative colitis patients. American Journal of Gastroenterology 2007;102:794–802. [DOI] [PubMed] [Google Scholar]
Guyatt 1989
- Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96(3):804‐10. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ha 2010
- Ha C, Kornbluth A. Mucosal healing in inflammatory bowel disease: where do we stand?. Current Gastroenterology Reports 2010;12(6):471‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Irvine 1994
- Irvine EJ, Feagan B, Rochon J, Archambault A, Fedorak RN, Groll A, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology 1994;106(2):287‐96. [DOI] [PubMed] [Google Scholar]
Irvine 1996a
- Irvine EJ, Zhou Q, Thompson AK. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn's Relapse Prevention Trial. American Journal of Gastroenterology 1996;91(8):1571‐8. [PubMed] [Google Scholar]
Irvine 1996b
- Irvine EJ, Feagan BG, Wong CJ. Does self‐administration of a quality of life index for inflammatory bowel disease change the results?. Journal of Clinical Epidemiology 1996;49(10):1177‐85. [DOI] [PubMed] [Google Scholar]
Irvine 2008
- Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflammatory Bowel Diseases 2008;14(4):554‐65. [DOI] [PubMed] [Google Scholar]
Janke 2004
- Janke KH, Raible A, Bauer M, Clemens P, Meisner C, Häuser W, et al. Questions on life satisfaction (FLZM) in inflammatory bowel disease. International Journal of Colorectal Disease 2004;19(4):343‐53. [DOI] [PubMed] [Google Scholar]
Janke 2005
- Janke KH, Klump B, Gregor M, Meisner C, Haeuser W. Determinants of life satisfaction in inflammatory bowel disease. Inflammatory Bowel Diseases 2005;11(3):272‐86. [DOI] [PubMed] [Google Scholar]
Konig 2002
- Konig HH, Ulsofer A, Gregor M, Tirpiz C, Reinshagen M, Adler G, et al. Validation of the EuroQoL questionnaire in patients with inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2002;14(11):1205‐15. [DOI] [PubMed] [Google Scholar]
Marri 2005
- Marri SR, Buchman AL. The education and employment status of patients with inflammatory bowel diseases. Inflammatory Bowel Diseases 2005;11(2):171‐7. [DOI] [PubMed] [Google Scholar]
Petrak 2001
- Petrak F, Hardt J, Clement T, Börner N, Egle UT, Hoffmann SO. Impaired health‐related quality of life in inflammatory bowel diseases: psychosocial impact and coping styles in a national German sample. Scandinavian Journal of Gastroenterology 2001;36(4):375‐82. [DOI] [PubMed] [Google Scholar]
Samsa 1999
- Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomic 1999;15(2):141‐55. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Vogelaar 2009
- Vogelaar L, Spijker AV, Woude CJ. The impact of biologics on health‐related quality of life in patients with inflammatory bowel disease. Clinical and Experimental Gastroenterology 2009;2:101‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]


