Abstract
Background
Family members of critically ill patients face considerable uncertainty and distress during their close others’ intensive care unit (ICU) stay. About 20–60% of family members experience adverse mental health outcomes post-ICU, such as symptoms of anxiety, depression, and posttraumatic stress. Guidelines recommend structured family inclusion, communication, and support, but the existing evidence base around protocolized family support interventions is modest and requires substantiation.
Methods
To test the clinical effectiveness and explore the implementation of a multicomponent, nurse-led family support intervention in ICUs, we will undertake a parallel, cluster-randomized, controlled, multicenter superiority hybrid-type 1 trial. It will include eight clusters (ICUs) per study arm, with a projected total sample size of 896 family members of adult, critically ill patients treated in the German-speaking part of Switzerland. The trial targets family members of critically ill patients with an expected ICU stay of 48 h or longer. Families in the intervention arm will receive a family support intervention in addition to usual care. The intervention consists of specialist nurse support that is mapped to the patient pathway with follow-up care and includes psycho-educational and relationship-focused family interventions, and structured, interprofessional communication, and shared decision-making with families. Families in the control arm will receive usual care. The primary study endpoint is quality of family care, operationalized as family members’ satisfaction with ICU care at discharge. Secondary endpoints include quality of communication and nurse support, family management of critical illness (functioning, resilience), and family members’ mental health (well-being, psychological distress) measured at admission, discharge, and after 3, 6, and 12 months. Data of all participants, regardless of protocol adherence, will be analyzed using linear mixed-effects models, with the individual participant as the unit of inference.
Discussion
This trial will examine the effectiveness of the family support intervention and generate knowledge of its implementability. Both types of evidence are necessary to determine whether the intervention works as intended in clinical practice and could be scaled up to other ICUs. The study findings will make a significant contribution to the current body of knowledge on effective ICU care that promotes family participation and well-being.
Trial registration
ClinicalTrials.gov NCT05280691. Prospectively registered on 20 February 2022.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-022-06454-y.
Keywords: Intensive care (MeSH), Family (MeSH), Family nursing (MeSH), Anxiety (MeSH), Depression (MeSH), Post-traumatic stress disorder (MeSH), Postintensive care syndrome – family (non-MeSH), Satisfaction with care (non-MeSH), Cluster-randomized controlled trial (non-MeSH)
Administrative information
| Title (1) | A multicomponent family support intervention in intensive care units: study protocol for a multicenter cluster-randomized trial (FICUS Trial) |
| Trial registration (2a and 2b). | ClinicalTrials.gov, NCT05280691, and the Swiss National Clinical Trials Portal (SNCTP), SNCTP000004842 |
| Protocol version (3) | Version 1.0, 25. October 2021 |
| Funding (4) | The study is funded by the Swiss National Science Fund (SNSF, grant no. 33IC30_198778/1). |
| Author details (5a) |
Rahel Naef: Institute for Implementation Science in Health Care, Faculty of Medicine, University of Zurich and Center of Clinical Nursing Science, University Hospital Zurich, Universitätsstrasse 84, CH-8006 Zurich, Switzerland. Miodrag Filipovic: Surgical Intensive Care Unit, Rescue and Pain Medicine, Intensive Care, Division of Anesthesiology, Cantonal Hospital of St. Gallen, Rorschacher Strasse 95, CH-9007 St. Gallen, Switzerland. Marie-Madlen Jeitziner: Department of Intensive Care Medicine, University Hospital Bern, Inselspital, University of Bern, Freiburgstrasse 10, 3010 Bern, Switzerland. Stefanie von Felten: Department of Biostatistics, Epidemiology, Biostatistics, and Prevention Institute, Faculty of Medicine, University of Zurich, Hirschengraben 84, 8001 Zurich, Switzerland. Judith Safford: Patient representative, no affiliation Marco Riguzzi: Institute for Implementation Science in Health Care, Faculty of Medicine, University of Zurich and Center of Clinical Nursing Science, University Hospital Zurich, Universitätsstrasse 84, 8006 Zurich, Switzerland. Michael Rufer: Department of Psychiatry, Psychotherapy, and Psychosomatics, Psychiatric University Hospital Zurich, University of Zurich and Center for Psychiatry and Psychotherapy, Psychiatric Hospital Zugersee, Triaplus AG, Widenstrasse 55, 6317 Oberwil-Zug, Switzerland. |
| Name and contact information of the trial sponsor (5b) |
Rahel Naef, PhD, RN Institute for Implementation Science in Health Care, Faculty of Medicine, University of Zurich and Center of Clinical Nursing Science, University Hospital Zurich, Universitätsstrasse 84, CH-8006 Zurich, Switzerland. E-Mail: rahel.naef@uzh.ch Phone: +41 44 634 37 49 |
| Role of sponsor (5c) |
The sponsor-investigator acts as the coordinating investigator of the trial and has a primary role regarding study design; collection, management, analysis, and interpretation of the data; and writing of the report. She has ultimate authority over these activities. The funder (Swiss National Science Fund) has no role in the development of the study design; collection, analysis, or interpretation of the data; writing of the manuscript; or decision to submit the manuscript for publication. |
Introduction
Family members are important to the well-being and recovery of critically ill persons, yet they are themselves profoundly affected by the critical illness [1, 2]. During a close other’s treatment in an intensive care unit (ICU), families experience high levels of stress and uncertainty [3], which often negatively affects their coping ability and mental health [4–6]. Research reports that 20–60% of family members experience subsequent adverse mental health outcomes, such as symptoms of anxiety, depression, posttraumatic stress, and complicated grief [7, 8], also known as post-intensive care syndrome – family (PICS-F) [9–11]. With the COVID-19 pandemic, incidences of PICS-F are likely to be even higher [12, 13].
The impact of critical illness on family members’ health and well-being and the need to increase family access, inclusion, and support are gaining recognition [10, 14–16], even more so since the COVID-19 pandemic [13, 17–19]. A move towards structured inclusion, communication, and support of family is recommended [20]. While an abundance of research exists about how to better address families’ needs during critical illness, empirical knowledge about the effectiveness and successful implementation of family interventions and models of care is only modest.
State of evidence on family interventions in ICU
Prior research has identified a promising effect of interventions seeking to increase family inclusion, communication, and support in ICU, such as participation in patient care or rounds, information/education, structured communication, or ICU diary, on quality of family care and family health outcomes [21–26]. While there is a general trend towards improved communication, decision-making, and family satisfaction and reports of reduced symptoms of anxiety, depression, and post-traumatic stress following such family interventions, effects were mostly found to be statistically non-significant with unclear clinical significance. The effects on length of ICU stay remain controversial [21, 22, 27], and most studies have used non-controlled designs [24, 25].
Studies that investigated specific, multicomponent, bundled family interventions, which included specific family support roles combined with facilitated communication, have demonstrated an increase in satisfaction with care and quality of communication [28–32], and have established feasibility and acceptability of family liaison and navigator roles [28, 31, 33, 34], most recently in the context of the COVID-19 pandemic [35–37]. However, there is little evidence of the effectiveness of such interventions to reduce symptoms of anxiety, depression, or posttraumatic stress [30, 32, 34, 38, 39]. Only three randomized trials on family support interventions in ICU have been conducted to date. Curtis and colleagues [38], testing a communication facilitator intervention for family members of incapacitated patients involved in surrogate decision-making, found a decrease in depressive symptoms but not in anxiety 6 months post-ICU, and there was no statistically significant difference after 3 months. White and colleagues [30], who investigated a multicomponent, nurse-delivered family support intervention (FOUR SUPPORT) for the same target group, did not identify a clear trend. Just recently, Kentish-Barnes and colleagues [39], who implemented a physician-driven, nurse-aided support strategy for family members of patients dying in ICU following a decision to withdraw or withhold life support, found a significant reduction in the proportion of family members with prolonged grief symptoms and significantly lower grief scores in the intervention group.
Trial rationale
There is a clear clinical need for increased family communication and support during critical illness to improve evidence-based ICU care delivery [40, 41]. However, there is a lack of evidence-based family support programs. Our group has developed and pilot-feasibility tested a nurse-delivered family systems intervention program in a general ICU population using a mixed-method design. We were unable to demonstrate a favorable impact on post-ICU psychological distress, possibly due to the uncontrolled before-and-after comparison [32], but found statistically significant improved satisfaction levels. The qualitative evaluation showed self-perceived benefits for family management of critical illness and its aftermath when the intervention is initiated shortly after ICU admission, responsive to families’ unique needs, and perceived to be delivered with high proficiency [32]. The pre-existing evidence base around multicomponent family support interventions is modest and requires further substantiation, particularly using randomized controlled designs [24–26]. Hence, the here proposed trial seeks to generate high-quality evidence on the intervention effectiveness of such multicomponent, bundled family support interventions in ICU while also generating knowledge around implementation processes and outcomes.
Objectives
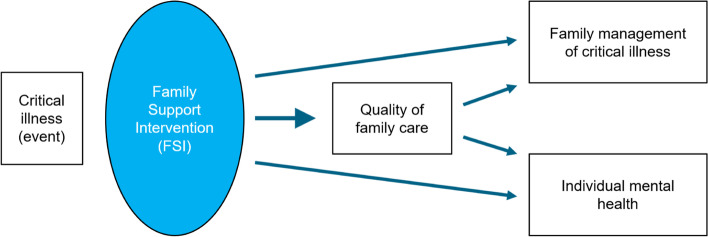
The primary aim of the Family in Intensive Care UnitS (FICUS) trial is to test the effectiveness of a multicomponent, nurse-led family support intervention (FSI), on the quality of family care, family management of critical illness, and mental health of individual index family members of critically ill patients compared with usual ICU care (Fig. 1). We hypothesize that the FSI will (1) increase the quality of family care, i.e., satisfaction with ICU care (primary endpoint); (2) increase the quality of communication and nurse support as reported by family members after patient discharge/death; (3) improve family management of critical illness and individual well-being; and (4) decrease symptoms of psychological distress post-ICU. The secondary aim is to examine the implementation processes, that is, identify implementation barriers/enablers in the real-world context in which the study intervention is implemented to discern determinants and strategies of implementation success.
Fig. 1.
Intervention mechanism
Design and methods
The FICUS trial is designed as a parallel, cluster-randomized, controlled, multicenter superiority hybrid type 1 trial with an equal number of clusters per study arm and a primary endpoint of quality of family care in ICU assessed after patient ICU discharge or death. We employ an effectiveness-implementation hybrid design type 1 [42], in which, alongside clinical effectiveness, contextual determinants (barriers/facilitators), together with implementation processes and outcomes are explored. This protocol publication follows the SPIRIT guidance [43] (see the SPIRIT Checklist as an additional file to this manuscript).
Patient and public involvement
A patient and family advisory group (PFAG) with five members (one patient, three family members, one patient representative) ensures that the trial is relevant and meaningful to critically ill patients and their family members, contributes to the design of the study processes, and guarantees that participation is feasible and acceptable to family members [44–46]. An ongoing partnership has been formed between the research team and the PFAG, with one member acting as a Patient and Public Involvement (PPI) liaison. This person co-designed the involvement strategy, co-leads consultation and communication, and acts as a liaison between the research team and the PFAG. Furthermore, PFAG members give advice, contribute their expertise to the trial design, and are actively involved in trial implementation.
To date, users have advised on the trial conception, namely the recruitment strategy, study outcomes, data collection processes, and the study intervention. They have provided feedback on the study protocol before submission to the funding body, and co-written user involvement strategy and lay abstracts. Future involvement during the preparatory phase includes the preparation of study information sheets, training of data assessors and interventionists, and communication recommendations for both the general public and for the recruitment of trial participants. The PFAG will continue to be engaged in the recruitment, data collection (i.e., retention procedures, ongoing reflection on study course), data analysis (i.e., interpretation of results, critical review), and dissemination (i.e., planning, dissemination activities, lay summaries) phases.
Participants and setting
Study setting
The FICUS trial will take place in ICUs in the German-speaking part of Switzerland.
Cluster-level eligibility criteria
Study ICUs need to be able to offer the highest level of patient care and treat patients who are hemodynamically unstable, require ventilation with multiple-organ failure, and need multidisciplinary intervention [47]. They may offer different or combined specialty care, including surgical, trauma, medical, cardiac, or neurological care. ICUs certified by the Swiss Society for Intensive Medicine (SGI) to run at least eight beds are eligible. ICUs with less than 300 admissions per year of patients with an ICU stay of 48 h or longer are excluded, as are ICUs with a protocolized, interprofessional family support program.
Participant-level eligibility criteria
Participants are adult family members (≥ 18 years of age) of critically ill persons who receive treatment in an eligible ICU for at least 48 h. Critically ill persons are defined as those with an expected length of stay in ICU of ≥ 48 h, combined with a life-threatening condition with a high risk of death or long-lasting functional impairment, or a high risk of prolonged mechanical ventilation (> 24 h) as appraised by the intaking clinician. Family members are defined as close others from the patient’s perspective, as noted in clinical records or advanced directives, or as legally defined surrogate decision-makers. Legal or blood kinship is not a requirement. They have to be a primary support person of the critically ill person, able to complete the baseline data collection and family-reported questionnaires in German within the required time frame and sign a written informed consent form. Family members of patients with refused general consent will not be eligible to take part. Family members with prior inclusion in the FICUS trial in another ICU, with cognitive inability to understand the study or inability to complete the questionnaire as appraised by clinicians or study recruitment staff, will be excluded. Inability to complete the baseline data collection within the required time frame after admission/study enrollment will lead to exclusion [48].
Informed consent processes
The investigator or his/her delegates will be responsible for obtaining written informed consent. They will explain to each potential family member participant the nature of the study, its purpose, the procedures involved, the expected duration, the potential risks and benefits, and any discomfort it may entail. Each participant will be informed that the participation in the study is voluntary, that s/he may withdraw from the study at any time, and that withdrawal of consent will not affect his/her or their close other’s care. Family members will also be asked to state in an additional consent form whether their data obtained in the context of this trial may be used for secondary data analyses.
To extract clinical patient data, consent will be obtained from patients. If the patient has pre-signed a general consent form for the use of routine clinical data for research purposes, no additional consent form is required. If not, and in case the patient lacks cognitive ability to do so upon ICU admission, the participating family member will provide his/her surrogate written informed consent based on the presumed will of the critically ill patient. As soon as the critically ill patient regains his/her ability of judgment, his/her written informed consent will be obtained.
Study intervention
The family support intervention (FSI), which has been developed and pilot-tested by the coordinating investigator [32, 33], has been slightly adapted in consultation with five users, seven ICU nurses, and three physicians to increase the implementability across the different study ICUs while maintaining its core components [49]. The aims of the FSI are (1) to increase the quality of interaction and communication between families and the ICU team and (2) to improve the ICU team’s capacity to provide the necessary care and support to families. The intervention also seeks (1) to strengthen family illness management capacity and well-being and (2) to alleviate the impact of the critical illness and/or loss on family members’ mental health.
Explanation of choice of comparator
The FSI will be introduced to the study ICUs of the intervention arm in addition to usual care, which is the control condition.
Usual care
Usual care is defined as a non-protocolized approach to family care and services offered by nurses, physicians, teams, or other health professionals that are an established part of the ICU’s routine care delivery before the trial start. These include granting access (visitation), interacting with family members and informing about the patient’s condition and treatment (written and oral information), communicating with family members as surrogate decision-makers (communication structure), supporting families (support structures), and making referrals (auxiliary services). ICU staff in control units will be allowed to act upon patient/family needs. However, the introduction of a new protocolized family support intervention, a family nursing or other family support role, or a structured family support pathway is not permitted.
Intervention description
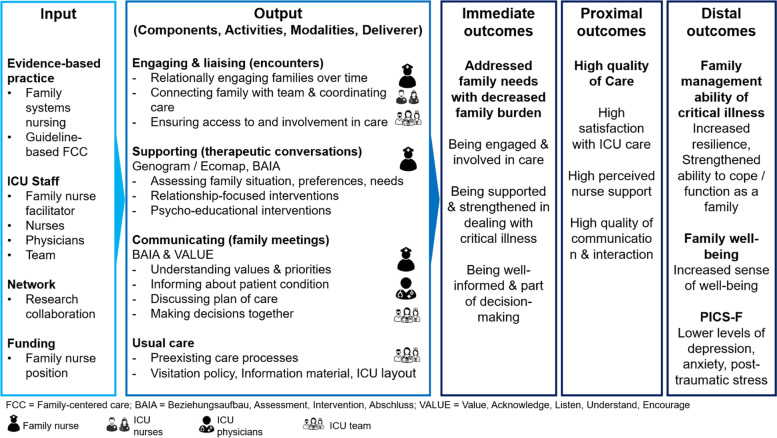
The FSI is grounded in a family-systems nursing approach [50–53]. It proposes that critical illness affects families’ affective, cognitive, and behavioral functioning, requiring therapeutic relational nurse-family engagement including relationship-focused and psycho-educational interventions to support family management of illness and to alleviate their suffering. The development was based on evidence around systemic family interventions for chronic illness [54–57], which suggests that the use of a combination of psycho-educational and relationship-focused interventions is most effective in strengthening family and individual health and well-being. The FSI also builds on guideline-based recommendations around ICU care to families that include the use of a specific family consultation role and of structured, interprofessional communication with families to ensure high quality of family care [15] (Fig. 2).
Fig. 2.
FSI program logic model
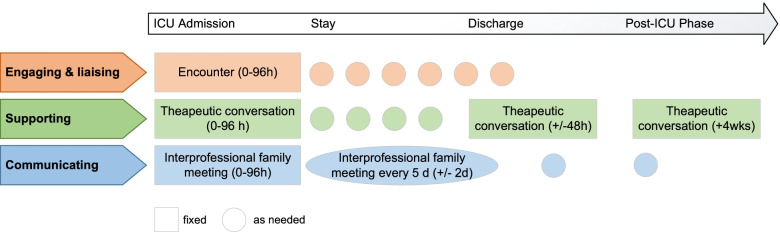
The FSI consists of a new family nursing role that delivers three interacting, closely intertwined intervention components that are mapped to a family care pathway (Fig. 3):
Engaging and liaising (family encounters): This intervention component involves relationship-building with families, connecting and coordinating family care activities as well as transition and follow-up care for family members and surviving patients.
Supporting (therapeutic family conversations): This component is based on a relational family systems nursing approach and includes assessing family structure, processes, and resources and supporting families through relationship-focused and psycho-educational interventions at the family systems level.
Communicating (interprofessional family meetings): This component focuses on structured, interprofessional communication, and shared decision-making with families.
Fig. 3.
Family care pathway
The FSI will be delivered by two to three designated family nurses per ICU in close collaboration with the ICU team. Interventionists will be registered nurses with a certification in ICU nursing or equivalent and training in family systems nursing. They hold a Master of Science in Nursing degree or work under the supervision of an advanced practice nurse. Advanced competencies in family nursing [58] are necessary for the following three reasons: First, families are in a vulnerable situation and/or crisis, which requires specific knowledge and expertise around family processes and illness management. Second, the relational, systemic family interventions require specific knowledge and skills beyond the general competencies of ICU nurses. Third, interventionists will assume a combined role encompassing clinical practice with families, consultation, collaboration, and leadership.
Criteria for discontinuing/modifying interventions
As this is a cluster-randomized trial (RCT), there will be no individual-level assignment that could be discontinued. However, the intervention will be discontinued if a family member withdraws the consent.
Strategies to improve adherence to interventions
The intervention is standardized in terms of components, intervention content, and a minimum dose along the clinical patient pathway (see Fig. 3) [59]. The frequency of intervention contacts and the dose of each intervention component can be increased and tailored according to the patients’ and/or families’ preferences and needs. Individual patient or family adherence to a particular health behavior is not required. Fidelity to intervention protocol is ensured at the individual and cluster levels. Adherence will be promoted by a 5-day interventionist training, monthly supervision and case conferences with all study interventionists, and site visits for quality assurance purposes.
Relevant concomitant care permitted/prohibited
The intervention components will be delivered within predefined time windows along the patient pathway, that is, within 96 h after admission, 48 h before or after discharge, and within the first 4 weeks after ICU discharge. A higher intervention dose and frequency of each intervention component are permitted to tailor to patient illness course, length of ICU stay, and family needs and preferences. Any interaction between ICU shift or primary nurses, ICU, or other treating physicians and other health or social care professionals is explicitly permitted. There are no medical interventions that are prohibited during the study.
Implementation strategy
The FSI will be implemented using a combination of strategies that showed promise in the pilot study [33]. These implementation strategies include leadership endorsement, nurse and physician champions, team education, and external/internal implementation support practitioners [60]. They will be adapted and tailored to the local context to address potential barriers, which will be identified prior to the enrollment start [61, 62].
Outcomes
Primary outcome
The primary outcome is the quality of family care in the ICU, operationalized as family satisfaction with ICU care, which is an established core indicator of the quality of family care [63–65]. It will be assessed at discharge from ICU by the Family Satisfaction in ICU Questionnaire (FS-ICU-24R) [66, 67].
Secondary outcomes
Quality of family care is further operationalized as the quality of communication and nurse support (see Table 1 for measures). As the intervention also targets families’ ability to cope with critical illness, family management of critical illness was chosen as a further secondary outcome. Individual family members’ mental health will be operationalized to cover the spectrum from well-being to psychological distress. Family management and mental health indicators are more distal than the quality of family care but highly relevant outcomes of interventions that address families’ needs for support and promote family capacity and health [68, 69] (Fig. 1). They will be obtained at baseline, discharge, and 3, 6, and 12 months thereafter.
Table 1.
Individual-level primary and secondary outcomes
| Domain/construct | Measurea | Range | Cronbach’s αb | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|---|---|
| Quality of family care | ||||||||
| Satisfaction with care (primary outcome) | Family satisfaction with ICU care (FS-ICU-24R) | 0–100 | > .85 | X | ||||
| Quality of communication | Questionnaire on Quality of Physician-Patient Interaction (QQPPI-14) | 1–5 | .95 | X | ||||
| Support from nurses | Family Perceived Support Questionnaire (ICE-FPSQ-14) | 14–70 | > 0.87 | X | ||||
| Family management | ||||||||
| Family functioning | Family Assessment Device - General Functioning Scale (FAD-GF-12) | 1–4 | .87 | X | X | X | X | X |
| Family resilience | Brief Resilience Scale (BRS-6)c | 1–5 | .85 | X | X | X | X | X |
| Mental health | ||||||||
| Subjective well-being | Satisfaction with Life Scale (SWLS-5) | 5–35 | .89–.92 | X | X | X | X | X |
| WHO-5 Well-Being Index (WHO-5) | 0–100 | .92 | X | X | X | X | X | |
| Adapted VAS on Quality of Life (QoL-VAS) | 0–100 | n/a | X | X | X | X | X | |
| Psychological distress | Distress Thermometer (DT) | 0–10 | n/a | X | X | X | X | X |
| Impact of Events Scale-6 (IES-6) | 0–4 | .80 | X | X | X | X | X | |
| Hospital Anxiety and Depression Scale (HADS-14) | 0–21 | > .80 | X | X | X | X | X | |
aUse of German versions of measures
bReferences for Cronbach’s alpha are listed in the text
cAdapted for family
Cluster-level data
Data will also be obtained at the cluster level, that is, from participating ICUs, and include data on ICU characteristics and family care processes and policies.
Process data
Process data will be obtained in the intervention arm, namely information on implementation activities and costs (cluster level) and on intervention delivery (individual level).
Accompanying studies
To address the second study aim, a three-phased implementation study will be undertaken among the clusters of the intervention arm. First, a context assessment will be conducted to identify barriers to the implementation of FSI in the specific ICU. Next, a tailored implementation plan will be developed, and an adaptive process put in place. Then, the implementation process and outcomes will be evaluated using a mixed-method approach with a case-study format.
An economic evaluation will be undertaken to assess the cost associated with intervention delivery and implementation of the FSI. Working hours per intervention (FSI) will be analyzed in relation to family members’ mental health and their absenteeism/presentism at work.
Cluster and participant timelines
Eligible clusters, that is, ICUs that have signed a study agreement, will be enrolled and randomly assigned to the intervention or control arm after cluster-level baseline data collection has been completed. Cluster data will be obtained at baseline, then yearly, and finally after the conclusion of the discharge data collection (first follow-up) of the last participant.
Participant eligibility screening and enrollment will be conducted at the individual level using a consecutive sampling strategy. The participant timeline is displayed in Table 2.
Table 2.
Participant timeline
| Task/study period | Admission to ICU | Screening | Informaiton | ICF | Baseline assessment (T0) | FU 1 to discharge from ICU (T1) | EOT and FU 2–3 months (T2) | FU 3–6 months (T3) | FU 4–12 months (T4) = EOS |
|---|---|---|---|---|---|---|---|---|---|
| Time point | 0–24 h | 0–48 h | 0–72 h | 0–96 h | 0–96 h | Y (− 24 h/+ 14 days) | Y + 90 days (± 14 days) | Y + 180 days (± 14 days) | Y + 365 days (± 14 days) |
| Enrollment | |||||||||
| Eligibility screen of patient | X | ||||||||
| Identification of eligible family member | X | ||||||||
| Information of family member | X | ||||||||
| Informed consent | X | ||||||||
| Interventions | |||||||||
| Intervention arm: family support intervention including intervention log | X | X | X | ||||||
| Usual care | X | X | X | X | X | X | |||
| Assessments | |||||||||
| Patient demographics, health and functional status, care utilizationa | X | X | X | X | X | X | |||
| Family member demographics, self-perceived health, care utilization | X | X | X | X | X | ||||
| Satisfaction with care (FS-ICU-24R) (primary endpoint) | X | ||||||||
| Quality of communication (QQPPI-14) | X | ||||||||
| Nurse support (ICE-FPSQ-14) | X | ||||||||
| Family functioning (FAD-GF-12) | X | X | X | X | X | ||||
| Family resilience (BRS-6) | X | X | X | X | X | ||||
| Distress Thermometer (DT) | X | X | X | X | X | ||||
| Depression and anxiety (HADS-14) | X | X | X | X | X | ||||
| Posttraumatic stress (IES-6) | X | X | X | X | X | ||||
| Life satisfaction (SWLS-5) | X | X | X | X | X | ||||
| Well-being (WHO-5, QoL-VAS) | X | X | X | X | X | ||||
ICF informed consent form, FU follow-up, EOT end of treatment, EOS end of study, Tx assessment time point
aT0–T1 from clinical record, T2–T4 family member reported
Sample size
Assuming a difference in FS-ICU-24 total score between the intervention and control groups of 5.5 and a within-group standard deviation of the FS-ICU-24 total score of 16.3, as observed in Naef and colleagues [32], we would need a total of 278 subjects in a standard RCT (with nRCT = 139 per arm) with 1:1 randomization to have a power of 0.8 at a significance level of 0.05. However, due to the design effect [70], the number of participants required for our cluster-randomized trial is bigger than the sample size of a standard RCT. We assumed an intra-cluster correlation coefficient (ICC) of 0.03 and a coefficient of variation in cluster size of 0.2.
Since the number of clusters is more limiting for this study than cluster size, we used the approach described by Hemming and colleagues [71] to derive the minimum number of clusters required for each condition in our trial. Assuming an unlimited cluster size, the minimum number is simply nRCT × ICC = 5 clusters. Increasing the number of clusters to eight (three more than the minimum), the cluster size could be reduced to =47.
We then conducted a series of precise power calculations, using the R packages clusterPower [72] and sse [73], considering a range of clusters per condition (3,…,20), mean cluster sizes (10–300), and ICCs (0.01–0.1). With an average cluster size of 50 evaluable patients and an ICC of 0.03, 8 clusters need to be randomized to each condition to achieve a power of 0.8. To account for a drop-out rate of 10% of patients within clusters (but no drop-out of whole clusters), an average number of 56 patients should be recruited per cluster.
Recruitment
Eligible ICUs in German-speaking Switzerland were identified based on the list of Swiss Society for Intensive Medicine-certified ICUs. The coordinating investigator contacted eligible ICUs to invite participation and enroll them in the study.
Eligible family members will be recruited within 96 h after patient admission to ICU. Designated ICU nurses or physicians will perform daily eligibility screening of newly admitted patients and potential family participants. They will obtain contact details of at least one family member and provide initial information to family members and hand out the study flyer and information. Study flyers will also be displayed in the waiting area and included in ICU information packages routinely handed to family members. Written study information will also be available on websites.
Next, the clinician or study coordinator will contact eligible family members in person when they visit their critically ill close other or via phone, explain the study, invite participation, and enroll participants. Family members will receive time to decide for or against participation in accordance with ethical guidelines. Participants will not receive payment or any other form of compensation. A thank you letter will be written to them upon study completion.
Assignment of interventions
Clusters will be assigned 1:1 to the intervention and the control arms. To reduce a potential imbalance of study groups at baseline, restricted randomization will be used [74, 75].
Assignment
The assignment of the participating ICUs will be generated after their recruitment and baseline cluster data collection using minimization at a central location. Variables used in the minimization procedure are the degree of specialization (specialized vs. general ICU) and the hospital. The first cluster will be assigned completely at random. To avoid subsequent assignments being deterministic, a random component of 10% will be introduced.
Concealment mechanism
The assignment will be generated by the trial statistician and only be revealed to the investigators after cluster-level baseline data collection has been completed.
Implementation
Clusters will be identified and enrolled by the coordinating investigator. The cluster assignment will be generated by the trial statistician, using the R package Minirand [76]. The coordinating investigator will then communicate the assignment to the local investigators.
Blinding
This is a non-blinded study. Blinding of study participants and clinical and research staff will not be possible due to the nature of the study intervention and cluster design.
Data collection and management
Assessment and collection of cluster-level data
Data at the cluster level will be collected by a paper case report form (pCRF) at baseline before cluster assignment to a study arm, every 12 months, and after the last participant in each cluster has completed the first follow-up (discharge).
ICU characteristics
ICU characteristics, such as the number of beds, admissions per year, percentage of high risk and mechanically ventilated patients, length of stay, mortality rate, will be obtained from the most recent Minimal Data Set (MDSi), which is routinely collected using a standardized data capture system provided by the Swiss Society of Intensive Medicine.
Quality of family care
To describe usual care processes offered to family members in study ICUs prior to and during the study, a brief form and two self-assessment instruments will be completed by a team of ICU clinicians. These instruments capture usual care processes and family care policies pre-intervention and allow to distinguish between usual care and the FSI post-intervention at the cluster level.
Self-Assessment Tool for Family-Centered Care in ICU [77, 78], which is based on the Society of Critical Care Medicine’s guideline for family-centered care [15]. The tool assesses on a 4-point Likert scale the degree to which recommended family engagement practices are currently delivered, such as family presence, support, communication, consultation, and family-friendly layout.
Patient- and Family-Centered Care Organizational Self-Assessment Tool [79] assesses the degree of family engagement at the hospital level, using eleven domains, such as leadership, quality improvement, personnel, mission & visions, and charting and documentation.
Implementation log
Implementation strategies used to introduce and maintain the fidelity of the intervention will be recorded by an internal implementation facilitator or local investigator in a structured format. Cost indicators include (but are not necessarily limited to) implementation of structures and processes, acquisition of staff to deliver the intervention, and intervention-specific training.
Assessment and collection of individual-level data
All participants who were assessed for study eligibility, irrespective of whether they were enrolled, considered non-eligible, or were eligible but not enrolled in the study, have to be recorded in a screening log. Investigators will document each study participant in an enrollment log and generate an individual participant schedule. Individual participant-level data will be collected by local study coordinators with an electronic case report form (eCRF), after participant enrollment at admission (baseline), discharge, and 3, 6, and 12 months thereafter via online or paper/pencil survey from family members and via direct data entry from clinical records. To access the online survey, a personalized link will be generated for each participant and assessment time point, which will be sent to participants via email, a text message, or other predetermined means. Participants can opt to answer the survey on a printed paper and pencil version of the eCRF, which will be sent to them with a stamped return envelope. Data collection at ICU admission and discharge will involve either phone or face-to-face interactions to facilitate the timely completion of the survey during this particularly vulnerable phase of a critical illness. During the post-ICU phase, emails or letters will be sent to family members to inform them about the upcoming follow-up data collection. If the survey is not completed within 2 weeks, study coordinators will be followed up by phone every week for 2 weeks. Follow-up calls for data collection reasons will be recorded in the participant list.
Outcome measures obtained via survey
The following family-reported outcome measures will be used in their German versions as part of the family member survey (see Table 1):
Family satisfaction in ICU (FS-ICU-24R): The FS-ICU-24-R yields a transformed mean score for overall satisfaction, satisfaction with care (16 items), and satisfaction with involvement in decision-making (ten items). The test-retest reliability of this assessment was high in the original pilot r = .85 [65], and the instrument is sensitive to change in several intervention studies. The German version of the FS-ICU-24R has demonstrated no floor or ceiling effects [66, 67].
Questionnaire on the Quality of Physician-Patient Interaction (QQPPI): The QQPPI assesses relationship-building, information exchange, and shared decision-making (14 items) and has been appraised as one of the most psychometrically sound measures of health professional-patient interaction [80] with a test-retest reliability of .59 [81].
Family Perceived Support Questionnaire (ICE-FPSQ): The ICE-FPSQ-14 measures families’ perception of support provided by nurses on two subscales: emotional support (nine items) and cognitive support (five items) [82, 83]. It has been translated into German employing a systematic procedure [84] and is currently under psychometric validation by the research group.
Family Assessment Device - General Functioning Scale (FAD-GF): The FAD-GF-12 is used to assess the overall functioning of the family system [85–87] and has demonstrated good test-retest reliability (r = .60 after 12 weeks) among patients with PTSD as well as responsiveness in intervention studies [88].
Brief Resilience Scale (BRS): The BRS-6 is conceived to measure the essence of resilience as the ability to bounce back from stress [89], and evidence from intervention studies suggests that the scale is sensitive to change [90, 91]. Its items will be reformulated from “I” to “we” statements to assess family rather than individual ability.
Satisfaction with Life Scale (SWLS-5): Developed to measure the global dimension of subjective well-being [92], the SWLS-5 can detect changes over time as well as responses to treatment [93]. The sum of the scores (range 5–35) can be transformed to an ordinal scale with seven levels ranging from extremely dissatisfied to extremely satisfied (with 20 indicating neutrality).
WHO-5 Well-being Index (WHO-5): The WHO-5 was developed to measure subjective psychological well-being [94]. It is widely used in European surveys [95, 96] and can detect clinically relevant changes while a score ≤ 50 (on a 0–100 scale) is indicative of depression.
Adapted VAS on Quality of Life (QoL-VAS): An adapted version of a visual analog scale (VAS) similar to that used in the EuroQol EQ-5D Questionnaire [97] will measure the self-perceived general quality of life (QoL) rather than health-related QoL.
Distress Thermometer (DT): The Distress Thermometer is originally and still primarily used among cancer patients [98–102], yet it is a generic visual analog scale (VAS) [103]. A common cutoff of 4 indicates potential distress [98, 104], and the thermometer can detect changes over time [105–107].
Impact of Events Scale-6 (IES-6): The IES-R is a widely used instrument to assess psychological distress in ICU family members [3, 108], which measures the presence and severity of symptoms associated with a traumatic event during the past week. The brief version (IES-6) includes two items from each of the three subscales of the IES-R-22 version (intrusion, avoidance, hyperarousal) [109, 110], is highly correlated to the IES-R [111], and detects changes over time [110].
Hospital Anxiety and Depression Scale (HADS): HADS-14 [9, 108, 112] is scored on two subscales (anxiety and depression) and has thresholds for mild/caseness for depression and anxiety, respectively [9, 108, 112]. The HADS has been shown to be an effective measure of psychological distress [113] and can detect changes over time [114, 115] as well as varying degrees of severity [115].
Further family member- and patient-related data
At baseline and first follow-up, information on the patients’ health condition and ICU treatment will be extracted from the clinical record. After that, family members will be asked to provide proxy information on patients’ care utilization and functional status. Family members will complete a demographic form together with information on their work situation, health status, and care utilization as part of the survey at each assessment time point (Table 3).
Table 3.
Patient- and family member-related data
| Time point of assessment | Patient | Family member | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T0 | T1 | T2 | T3 | T4 | |
| Demographics | ||||||||||
| Age, gender, civil status (T2–T4 change), Education | X | X | X | X | X | |||||
| Type of family member (T0), primary carer (yes/no) | X | X | X | X | ||||||
| Co-habiting with patient (yes/no), frequency of contact | X | |||||||||
| Travel time to hospital | X | |||||||||
| Health status | ||||||||||
| Medical diagnosis (MDSi) | X | X | ||||||||
| Trauma (yes/no); if yes, AISa | X | |||||||||
| Mechanical ventilation and circulary support (T0: yes/no, T1: # of days) | X | X | ||||||||
| SAPS-2b | X | |||||||||
| NEMSc, SOFAd | X | X | ||||||||
| Death (yes/no); if yes, date, cause, placee | X | X | X | X | X | |||||
| Organ donation (yes/no); if yes, organ, surrogate dm) | X | |||||||||
| Functional status (Katz ADL/Lawton IADL)e | X | X | X | |||||||
| Prior/present psychiatric diagnosis (yes/no) | X | |||||||||
| Self-perceived health (VAS) | X | X | X | X | X | |||||
| Care utilization | ||||||||||
| Cause of admission | X | |||||||||
| Type of admission (expected, unexpected), admitted from | X | |||||||||
| Surgery (emergency, planned, none) | X | |||||||||
| Previous ICU stays (yes/no) | X | |||||||||
| Prior need for informal/ formal care (yes/no)f | X | |||||||||
| Length of ICU stay | X | |||||||||
| Discharge/transfer destination, planned vs. unexpected | X | |||||||||
| Need for informal/formal care (yes / no); if yes, change, hours/frequency, more/less than before ICU treatment)e | X | X | X | |||||||
| Prior/current/new psychological/psychiatric treatment (yes / no) | X | X | X | X | X | |||||
| Use of prescription drugs | X | X | X | X | X | |||||
| Current treatment for a chronic condition (yes/no) | X | |||||||||
| Number of family physician visits/hospitalizations (if yes: related to critical illness yes/no/length of stay) | X | X | X | X | ||||||
| Previous ICU experience (as patient, as family of the patient) | X | |||||||||
| Family satisfaction with prior care experience (VAS) | X | |||||||||
| Family satisfaction with care on hospital wards (VAS) | X | |||||||||
| Work situation | ||||||||||
| Level of employment, income | X | |||||||||
| Change in level of employment (yes / no); if yes: related to critical illness yes/no), new income | X | X | X | X | ||||||
| Sick leave/absence from work (if yes: # of (half-)days) | X | X | X | X | X | |||||
| Presenteeism | X | X | X | X | ||||||
aAbbreviated Injury Scale
bSequential Organ Failure Assessment Score
cNine equivalents of nursing manpower use score
dSimplified Acute Physiology Score
ePatient-related information at T2, T3, T4 obtained from family member
fPatient-related information at T0 obtained from family member
Intervention log
For each intervention contact, the following data will be recorded by interventionists in the eCRF: date, ICU or post-ICU day, duration in minutes, delivery mode (face-to-face, phone, online), number/type of family members and ICU staff present, type of intervention component (encounter, therapeutic conversation, interprofessional family meeting), nurse intervention activity (relational engaging, family assessment, psycho-educational intervention, relationship-focused interventions, liaison and coordination, interprofessional communication, shared decision-making, making referrals), and referrals to auxiliary services. Hence, all concomitant interventions or interventions exceeding usual care have to be recorded. Usual care itself will not be documented.
Retention and complete follow-up
To promote retention at the cluster level, the trial steering committee will hold quarterly study group meetings to discuss study progress, and provide quarterly communication via newsletters to study sites and staff. The trial manager and further members of the research team will interact regularly with the local study team and site.
To ensure retention of individual study participants, local study teams will make the utmost effort to obtain completed questionnaires at admission, discharge, and at the three post-ICU follow-ups at months 3, 6, and 12 [48]. As the study endpoints rely mainly on the completion of questionnaires by the participants, system-supported automatic notifications on the eCRF completion status will be implemented to ensure data completeness. Study coordinators will check the completeness of the paper-reported outcome measures and follow-up with participants if they identify missing values.
If participants withdraw consent, the reason for withdrawal will be assessed at the time point of withdrawal, and—if the participant agrees—data on the clinical status of the patient will be collected (change in health status/or death, time point of death). In this case, the intervention and the data collection will be discontinued, and no follow-up is planned. Participants who withdraw or are discontinued before the first follow-up (discharge) will be replaced to achieve the required sample size. The data of the last completed assessment time point will be included in the analysis (as per intention-to-treat analysis).
Data management
For data processing and management, the electronic data capture (EDC) system REDCap [116] will be used. A data monitoring plan specific to the study assessment schedule has been prepared by the coordinating investigator site’s clinical trial unit. Data monitoring will be initiated after the inclusion of ten study participants at each study center. Regular monitoring will be undertaken by the trial manager and/or external monitor. Observations and findings will be documented and made available to the coordinating investigator and local study site.
Confidentiality
Role- and user-based access control with personal login regulate permission to access the EDC system, which includes individual user rights for data entry, review, export, and reports. Appropriate coded identification is used to enter participant data into the database; no patient identifying information will be entered into the EDC system.
Data storage
The servers hosting the EDC system and study database are kept in an off-site restricted access locked server room. A copy of the study database will be stored securely by the Clinical Trial Unit at the coordinating investigator’s site for at least 10 years. Investigators maintain the essential documents and source data in the Trial Master File and Investigator Site Files and archive interim and final reports in electronic and hard copy format for at least 10 years.
Statistical methods
A more detailed statistical analysis plan will be finalized before database closure. All analyses will be performed in R [117].
Statistical methods for primary and secondary outcomes
The FS-ICU-24-R total score at patient discharge from ICU will be analyzed by a linear mixed-effects model (LMM) with a random intercept per cluster to account for the non-independence of family members from the same cluster. Due to the small number of clusters, the main model will include the treatment (intervention vs. control) as the only explanatory variable in the main analysis, and the Satterthwaite approximation for the denominator degrees of freedom will be used, as recommended by Leyrat and colleagues [118]. The ICC will be estimated from this model based on the residual variance and between-cluster variance.
The following covariate-adjusted sensitivity analyses will be conducted to adjust the treatment effect estimate for potential confounding: At the cluster level, the specialization of the ICU (as used in the cluster randomization), overall ICU staffing, and a quality-of-care indicator will each be added separately to the main model described above. At the individual participant’s level, patient age, cause of admission, the SAPS-2 score of the patient (mortality risk), and the family member’s previous ICU experience will be added to the main model together (and potentially to the cluster-level adjusted models).
The secondary outcomes regarding the quality of care, which are only measured once at discharge from ICU, will be analyzed with an LMM as described above for the primary outcome. All other secondary outcomes, which are measured at baseline, at discharge from ICU, and at three other follow-up points in time, will be analyzed by an LMM with a random intercept per cluster and a random intercept per family member (nested within the cluster) to additionally account for the non-independence of repeated measurements from the same study participant. The serial autocorrelation of residuals will be modeled using a first-order autoregressive correlation structure. The models will include the treatment (intervention vs. control), the corresponding baseline measurement, the visit, and the visit-treatment interaction.
Methods for additional analyses
Subgroup analyses are planned regarding the primary outcome for the following baseline characteristics: specialization (specialized vs. general ICU), overall ICU staffing, and quality of care indicator (sum score) at the cluster level and patient age, gender, cause of admission (expected vs. unexpected), mortality risk (SPAS-2 score upon admission), type of relationship between patient and family member, family member prior ICU experience, family resilience (BRS-6), family functioning (FAD-FG-12), and anxiety and depression (HADS) at the participant level. A separate LMM will be fitted for each subgroup variable, adding the subgroup variable and the interaction between the subgroup variable and the treatment as explanatory variables to the main model. A significant interaction between a subgroup variable and the treatment would indicate a different treatment effect in the corresponding subgroups (or along a gradient for the continuous subgroup variables). Furthermore, patient survival status and ICU length of stay are two participant-level covariates that are of interest but are measured during or after the intervention and therefore do not qualify as baseline characteristics to adjust for in these subgroup models. However, for exploratory purposes, a model including these two variables together with the treatment and the three two-way interaction terms will be fitted, which will require cautious interpretation. Similarly, a model that includes the intervention dose will be fitted (with a dose of zero for the control group). No interim analyses are planned.
Methods in analysis to handle protocol non-adherence and missing data
The analysis will follow the intention-to-treat principle. Clusters and their participants will be analyzed given the condition to which they were assigned. Should a patient be transferred to another cluster, the family will be analyzed in the original cluster. This will be possible due to individual informed consent. The main analysis will be complete cases, but multilevel multiple imputation of missing data at the participant level [119] will be performed (potentially also to the covariate-adjusted models) to assess the sensitivity of the results with regard to missing outcomes. We do not expect any clusters to be missing as a whole but would exclude them from the analysis if present.
Plans to give access to the full protocol, participant-level data, and statistical code
The full protocol has been made available at ClinicalTrials.gov, NCT05280691. Participant-level data (after anonymization) and statistical code for data analysis will be made available upon reasonable request.
Oversight and monitoring
A trial steering committee; that is, coordinating investigator, co-investigators, trial manager, data manager, statistician, implementation support practitioner, and patient representative, will oversee the trial implementation and conduct at the study sites. Data management and monitoring are provided by the coordinating investigator site’s clinical trial unit. The trial will follow national and international standards for good clinical practice and comply with regulatory and ethical requirements. Related or unrelated serious adverse events (SAEs) affecting the participants (family members) are collected and documented in source documents.
Dissemination plans
The scientific output will be published and made available as widely as possible to support knowledge transfer, meta-analyses, and general reproduction efforts. All study centers and study participants will be provided with a lay summary of the study outcome (upon interest), and preliminary findings will be shared through poster presentations, scientific talks, and scientific publications on a national and international level at appropriate platform events typically attended by ICU staff, health care professionals, or the public.
Discussion
The FICUS trial has the dual aim to establish the clinical effectiveness of the FSI, a nurse-delivered, interprofessional multicomponent intervention that is introduced into routine care delivery and to explore its implementation. In line with the MRC framework for developing and evaluating complex interventions [120], the current testing of intervention effectiveness and exploration of implementation builds on previous phases of the FSI development and feasibility-pilot testing [32, 33].
Given the state of research in the field of family intervention in the context of critical illness [22, 24–26, 121, 122], rigorous real-world evidence generated by a randomized controlled design is now necessary. There is a clear need to clarify the clinical effectiveness of specific, yet multicomponent or bundled, nurse-led family interventions [24] that build on guideline-based recommendations for family care in the ICU [15]. Two groups have already tested similar multicomponent interventions combining communication and support with a family navigator role [30, 38], which have shown promising effects on the quality of family care but less clear effects on post-ICU family member health.
The FSI adds, in addition to engagement/liaison and communication, a family systems intervention component. This component, delivered through therapeutic family conversations, is based on a relational family systems nursing approach and includes assessing family structure, processes, and resources, and supporting families through relationship-focused and psycho-educational interventions at the family systems level [52, 53, 55]. Such a systemic family intervention has been found to be effective in chronic illness [54, 123, 124]. In the context of critical illness, one group has pilot-tested a single component family health conversation intervention, delivered in the early post-ICU phase [125]. They found that families who received the intervention were able to improve their family and social functioning from baseline to month three, and demonstrated better functioning and mental health as well as mental health after twelve months. The FSI, while building on existing knowledge, denotes a novel, feasible and acceptable nurse-led family intervention in ICU, adding a relationship-focused, systemic component to the engagement/liaison and communication/shared-decision-making, spanning from ICU admission into the early post-ICU phase [33].
In addition to the well-established service and clinical outcomes that such family interventions seek to improve, we added family management as a third relevant clinical outcome [125]. We also chose to target a more general critically ill patient group to account for those family members whose close others survive critical illness. Critical illness survivors exhibit high levels of post-ICU physical, cognitive, and mental impairments, requiring considerable and often new-onset family caregiving [8, 68, 126–128]. Given the increased prevalence of critically ill patients due to the COVID-19 pandemic, who have a high prevalence of post-intensive care syndrome [129, 130], more families are affected by the health impact of critical illness. In addition, pandemic-related access restrictions increase family suffering and risk for negative health outcomes [12, 13, 19].
Patient and family member representatives are involved in the trial design and implementation [46, 131]. They actively collaborate with the research team in ensuring that participation in the FICUS trial is meaningful and feasible. In addition, they participate in activities of the FICUS study group and meet regularly to advise the lead researchers on study processes. Patient and public involvement (PPI) has been increasingly called for to ensure the relevance, feasibility, and impact of clinical and critical care research [45, 46, 132]. It has been reported that many challenges often inhibit meaningful engagement and effective collaboration [133, 134]. Hence, the early installment and ongoing partnership with the patient and family representatives, together with the integration of the patient liaison in the research team, build a solid foundation for the implementation of effective PPI in the FICUS trial.
In conclusion, the FICUS trial will evaluate a nurse-led, multicomponent, bundled family support intervention that is embedded in interprofessional care delivery in the ICU and aims to generate high-quality evidence on intervention effectiveness and knowledge of its implementability. Both types of evidence are necessary to determine whether the intervention works as intended in clinical practice and could be scaled up to other ICUs. The study findings will make a significant contribution to the current body of knowledge on effective ICU care that promotes family participation and well-being.
Trial status
At the time of manuscript submission, the FICUS trial has been approved by the responsible Swiss cantonal ethics committees (Nr. 2021-2300). Fifteen of the sixteen required clusters have been recruited. Enrollment of the first participant is expected in spring 2022. Recruitment is expected to be completed by early 2024.
Protocol version: 1.0, 25 October 2021.
Supplementary Information
Acknowledgements
We are very grateful to the ICU nurse and physician leadership teams who decided to participate in the trial. We would also like to acknowledge the support of our research staff Andrea Thesenvitz, Lotte Verweij, and Saskia Oesch, as well as the four persons acting as patient and family member representatives.
Collaborators: FICUS Study Group
Ursula Betschart, RN, Clinic of Intensive Medicine, Cantonal Hospital St. Gallen
Philipp Buehler, MD, Center of Intensive Care Medicine, Cantonal Hospital Winterthur
Hanna Burkhalter, PhD, RN, Graubünden Cantonal Hospital Chur
Alexander Dullenkopf, MD, Clinic of Anesthesiology and Intensive Care, Cantonal Hospital Frauenfeld, Spital Thurgau AG
Antje Heise, MD, Interdisciplinary Intensive Care Unit, Hospital Thun
Benjamin Hertler, MD, Institute of Intensive Medicine, University Hospital Zurich
Yvonne Liebert, MScN, RN, Department of Nursing, Cantonal Hospital Baden
Fabienne Lussmann, BScN, RN, Center of Intensive Care Medicine, Lucerne Cantonal Hospital
Paola Massarotto, MScN, RN, Institute of Intensive Medicine, University Hospital Zurich
Urs Pietsch, MD, Surgical Intensive Care Unit, Division of Anesthesiology, Intensice Care, Rescue and Pain Medicine, Cantonal Hospital of St. Gallen
Esther Siegrist, MScN, RN, Institute of Intensive Medicine, University Hospital Zurich
Peter Steiger, MD, Institute of Intensive Medicine, University Hospital Zurich
Christoph von Dach, DNP, RN, Cantonal Hospital Olten, Solothurn Hospitals
Monique Wenzler, RN, Institute for Anesthesiology and Intensive Medicine, Hirslanden Clinic Zurich, Hirslanden AG
Jan Wiegand, MD, Interdisciplinary Intensive Care, Lindenhof Hospital Bern
Björn Zante, MD, University Department of Intensive Care Medicine, Inselspital, University Hospital Bern
Abbreviations
- BRS
Brief Resilience Scale
- DT
Distress Thermometer
- EDC
Electronic Data Capture
- FAD-GF
Family Assessment Device - General Functioning Scale
- FICUS
Family in Intensive Care UnitS
- FSI
Family support intervention
- FS-ICU-24R
Family Satisfaction in ICU Questionnaire Revised
- eCRF
Electronic case report form
- HADS
Hospital Anxiety and Depression Scale
- ICC
Intra-cluster correlation coefficient
- ICE-FPSQ
Icelandic Family Perceived Support Questionnaire
- ICU
Intensive care unit
- IES-6
Impact of Events Scale-6
- MDSi
Minimal Data Set
- MRC
Medical Research Council
- QoL-VAS
Adapted VAS on Quality of Life
- QQPPI
Questionnaire on the Quality of Physician-Patient Interaction
- pCRF
Paper case report form
- PFAG
Patient and Family Advisory Group
- PICS-F
Post-intensive care syndrome-family
- PPI
Patient and Public Involvement
- RCT
Randomized controlled trial
- SAPS-2
Simplified Acute Physiology Score
- SGI
Swiss Society for Intensive Medicine
- SWLS-5
Satisfaction with Life Scale
- WHO-5
WHO-5 Well-being Index
Authors’ contributions
RN initiated the study conception and design of the FICUS trial, was responsible for the funding application, and acts as the sponsor and coordinating investigator. MF, MMJ, and MRu made a substantial contribution to the conception and trial design and co-wrote the funding application. MF and MMJ will be responsible for the implementation of the trial. SvF designed the statistical analysis plan. MRi and RN co-wrote the manuscript. MF, MMJ, SvF, JS, and MRu critically reviewed the manuscript for important intellectual content. All authors have reviewed the drafts and approved the final version.
Funding
The study is funded by the Swiss National Science Fund (SNSF, No. 33IC30_198778/1). The funder has no role in the development of the study design; collection, analysis, or interpretation of the data; writing of the manuscript; or decision to submit the manuscript for publication.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study (study protocol).
Declarations
Ethics approval and consent to participate
Cantonal Ethics Committee of Zurich BASEC ID 2021-02300 approval on 28 January 2022. Written, informed consent will be obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rahel Naef, Email: rahel.naef@uzh.ch.
Miodrag Filipovic, Email: miodrag.filipovic@kssg.ch.
Marie-Madlen Jeitziner, Email: marie-madlen.jeitziner@insel.ch.
Stefanie von Felten, Email: stefanie.vonfelten@uzh.ch.
Judith Safford, Email: judith@saffordconsulting.ch.
Marco Riguzzi, Email: marco.riguzzi@uzh.ch.
Michael Rufer, Email: michael.rufer@triaplus.ch.
References
- 1.Eggenberger SK, Nelms TP. Being family: the family experience when an adult member is hospitalized with a critical illness. J Clin Nurs. 2007;16(9):1618–1628. doi: 10.1111/j.1365-2702.2007.01659.x. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins RO. Emotional processing/psychological morbidity in the ICU. In: Netzer G, editor. Families in the Intensive care unit: a guide to understanding, engaging, and supporting at the bedside. Cham: Springer Nature; 2018. pp. 31–48. [Google Scholar]
- 3.Alfheim HB, Hofso K, Smastuen MC, Toien K, Rosseland LA, Rustoen T. Post-traumatic stress symptoms in family caregivers of intensive care unit patients: a longitudinal study. Intensive Crit Care Nurs. 2019;50:5–10. doi: 10.1016/j.iccn.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Minton C, Batten L, Huntington A. A multicase study of prolonged critical illness in the intensive care unit: families’ experiences. Intensive Crit Care Nurs. 2019;50:21–27. doi: 10.1016/j.iccn.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Turner-Cobb JM, Smith PC, Ramchandani P, Begen FM, Padkin A. The acute psychobiological impact of the intensive care experience on relatives. Psychol Health Med. 2016;21(1):20–26. doi: 10.1080/13548506.2014.997763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfheim HB, Rosseland LA, Hofsø K, Småstuen MC, Rustøen T. Multiple symptoms in family caregivers of intensive care unit patients. J Pain Symptom Manage. 2018;55(2):387–394. doi: 10.1016/j.jpainsymman.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Kentish-Barnes N, Chaize M, Seegers V, Legriel S, Cariou A, Jaber S, et al. Complicated grief after death of a relative in the intensive care unit. Eur Respir J. 2015;45(5):1341–1352. doi: 10.1183/09031936.00160014. [DOI] [PubMed] [Google Scholar]
- 8.Inoue S, Hatakeyama J, Kondo Y, Hifumi T, Sakuramoto H, Kawasaki T, et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg. 2019;6(3):233–246. doi: 10.1002/ams2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson JE, Jones C, Bienvenu OJ. Family responses to critical illness: postintensive care syndrome - family. Crit Care Med. 2012;40(2):618–624. doi: 10.1097/CCM.0b013e318236ebf9. [DOI] [PubMed] [Google Scholar]
- 10.Harvey MA, Davidson J. Long-term consequences of critical illness: a new opportunity for high-impact critical care nurses. Crit Care Nurse. 2011;31(5):12–15. doi: 10.4037/ccn2011597. [DOI] [PubMed] [Google Scholar]
- 11.van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care. 2016;20:16. doi: 10.1186/s13054-016-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattelan J, Castellano S, Merdji H, Audusseau J, Claude B, Feuillassier L, et al. Psychological effects of remote-only communication among reference persons of ICU patients during COVID-19 pandemic. J Intensive Care. 2021;9(1):5. doi: 10.1186/s40560-020-00520-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kentish-Barnes N, Cohen-Solal Z, Morin L, Souppart V, Pochard F, Azoulay E. Lived experiences of family members of patients with severe COVID-19 who died in intensive care units in France. JAMA Netw Open. 2021;4(6):e2113355. doi: 10.1001/jamanetworkopen.2021.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Netzer G, editor. Families in the intensive care unit: a guide to understanding, engaging, and supporting at the bedside. Springer Nature: Cham; 2018. [Google Scholar]
- 15.Davidson JE, Aslakson RA, Long AC, Puntillo KA, Kross EK, Hart J, et al. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017;45(1):103–128. doi: 10.1097/CCM.0000000000002169. [DOI] [PubMed] [Google Scholar]
- 16.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Translat Internal Med. 2017;5(2):90–92. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azoulay É, Curtis JR, Kentish-Barnes N. Ten reasons for focusing on the care we provide for family members of critically ill patients with COVID-19. Intensive Care Med. 2021;47(2):230–233. doi: 10.1007/s00134-020-06319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montauk TR, Kuhl EA. COVID-related family separation and trauma in the intensive care unit. Psychol Trauma. 2020;12(S1):S96–SS7. doi: 10.1037/tra0000839. [DOI] [PubMed] [Google Scholar]
- 19.Zante B, Erne K, Grossenbacher J, Camenisch SA, Schefold JC, Jeitziner MM. Symptoms of post-traumatic stress disorder (PTSD) in next of kin during suspension of ICU visits during the COVID-19 pandemic: a prospective observational study. BMC Psychiatry. 2021;21(1):477. doi: 10.1186/s12888-021-03468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerritsen RT, Hartog CS, Curtis JR. New developments in the provision of family-centered care in the intensive care unit. Intensive Care Med. 2017;43(4):550–553. doi: 10.1007/s00134-017-4684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HW, Park Y, Jang EJ, Lee YJ. Intensive care unit length of stay is reduced by protocolized family support intervention: a systematic review and meta-analysis. Intensive Care Med. 2019;45(8):1072–1081. doi: 10.1007/s00134-019-05681-3. [DOI] [PubMed] [Google Scholar]
- 22.Goldfarb MJ, Bibas L, Bartlett V, Jones H, Khan N. Outcomes of patient- and family-centered care interventions in the ICU: a systematic review and meta-analysis. Crit Care Med. 2017;45(10):1751–1761. doi: 10.1097/CCM.0000000000002624. [DOI] [PubMed] [Google Scholar]
- 23.Scheunemann LP, McDevitt M, Carson SS, Hanson LC. Randomized, controlled trials of interventions to improve communication in intensive care: a systematic review. Chest. 2011;139(3):543–554. doi: 10.1378/chest.10-0595. [DOI] [PubMed] [Google Scholar]
- 24.Kiwanuka F, Sak-Dankosky N, Alemayehu YH, Nanyonga RC, Kvist T. The evidence base of nurse-led family interventions for improving family outcomes in adult critical care settings: a mixed method systematic review. Int J Nurs Stud. 2022;125:104100. doi: 10.1016/j.ijnurstu.2021.104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xyrichis A, Fletcher S, Philippou J, Brearley S, Terblanche M, Rafferty AM. Interventions to promote family member involvement in adult critical care settings: a systematic review. BMJ Open. 2021;11(4):e042556. doi: 10.1136/bmjopen-2020-042556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zante B, Camenisch SA, Schefold JC. Interventions in post-intensive care syndrome-family: a systematic literature review. Crit Care Med. 2020;48(9):e835–ee40. doi: 10.1097/CCM.0000000000004450. [DOI] [PubMed] [Google Scholar]
- 27.Iftikhar IH, Goldfarb M. Effect of patient- and family-centered care interventions on ICU length of stay. Crit Care Med. 2018;46:e186–7. [DOI] [PubMed]
- 28.Shelton W, Moore CD, Socaris S, Gao J, Dowling J. The effect of a family support intervention on family satisfaction, length-of-stay, and cost of care in the intensive care unit. Crit Care Med. 2010;38(5):1315–1320. doi: 10.1097/CCM.0b013e3181d9d9fe. [DOI] [PubMed] [Google Scholar]
- 29.White DB, Cua SM, Walk R, Pollice L, Weissfeld L, Hong S, et al. Nurse-led intervention to improve surrogate decision making for patients with advanced critical illness. Am J Crit Care. 2012;21(6):396–409. doi: 10.4037/ajcc2012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White DB, Angus DC, Shields AM, Buddadhumaruk P, Pidro C, Paner C, et al. A randomized trial of a family-support intervention in intensive care units. N Engl J Med. 2018;378(25):2365–2375. doi: 10.1056/NEJMoa1802637. [DOI] [PubMed] [Google Scholar]
- 31.Moore CD, Bernardini GL, Hinerman R, Sigond K, Dowling J, Wang DB, et al. The effect of a family support intervention on physician, nurse, and family perceptions of care in the surgical, neurological, and medical intensive care units. Crit Care Nurs Q. 2012;35(4):378–387. doi: 10.1097/CNQ.0b013e318268fde3. [DOI] [PubMed] [Google Scholar]
- 32.Naef R, von Felten S, Petry H, Ernst J, Massarotto P. Impact of a nurse-led family support intervention on family members’ satisfaction with intensive care and psychological wellbeing: a mixed-methods evaluation. Aust Crit Care. 2021;34(6):594–603. [DOI] [PubMed]
- 33.Naef R, Massarotto P, Petry H. Families’ and health professionals’ experience with a nurse-led family support intervention in ICU: a qualitative evaluation study. Intensive Crit Care Nurs. 2020;61:102916. doi: 10.1016/j.iccn.2020.102916. [DOI] [PubMed] [Google Scholar]
- 34.Torke AM, Wocial LD, Johns SA, Sachs GA, Callahan CM, Bosslet GT, et al. The family navigator: a pilot intervention to support intensive care unit family surrogates. Am J Crit Care. 2016;25(6):498–507. doi: 10.4037/ajcc2016730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor SP, Short RT, Asher AM, Muthukkumar R, Sanka P. Family engagement navigators: a novel program to facilitate family-centered care in the intensive care unit during COVID-19. NEJM Catalyst. 2020. 10.1056/CAT.20.0396.
- 36.Lopez-Soto C, Bates E, Anderson C, Saha S, Adams L, Aulakh A, et al. The role of a liaison team in ICU family communication during the COVID 19 pandemic. J Pain Symptom Manage. 2021;62(3):e112–9. [DOI] [PMC free article] [PubMed]
- 37.Keen A, George A, Stuck BT, Snyder C, Fleck K, Azar J, et al. Nurse perceptions of a nurse family liaison implemented during the COVID-19 pandemic: a qualitative thematic analysis. Intensive Crit Care Nurs. 2021;70:103185. [DOI] [PMC free article] [PubMed]
- 38.Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med. 2016;193(2):154–162. doi: 10.1164/rccm.201505-0900OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kentish-Barnes N, Chevret S, Valade S, Jaber S, Kerhuel L, Guisset O, et al. A three-step support strategy for relatives of patients dying in the intensive care unit: a cluster randomised trial. Lancet. 2022;399:656–664. doi: 10.1016/S0140-6736(21)02176-0. [DOI] [PubMed] [Google Scholar]
- 40.Liu K, Nakamura K, Katsukawa H, Nydahl P, Ely EW, Kudchadkar SR, et al. Implementation of the ABCDEF bundle for critically Ill ICU patients during the COVID-19 pandemic: a multi-national 1-day point prevalence study. Front Med. 2021;8:735860. doi: 10.3389/fmed.2021.735860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinpell R, Heyland DK, Lipman J, Sprung CL, Levy M, Mer M, et al. Patient and family engagement in the ICU: report from the task force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care. 2018;48:251–256. doi: 10.1016/j.jcrc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Landes SJ, McBain SA, Curran GM. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res. 2019;280:112513. doi: 10.1016/j.psychres.2019.112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan A-W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandya-Wood R, Barron DS, Elliott J. A framework for public involvement at the design stage of NHS health and social care research: time to develop ethically conscious standards. Res Involve Engage. 2017;3(1):6. doi: 10.1186/s40900-017-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burns KEA, Devlin JW, Hill NS. Patient and family engagement in designing and implementing a weaning trial: a novel research paradigm in critical care. Chest. 2017;152(4):707–711. doi: 10.1016/j.chest.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al. Frameworks for supporting patient and public involvement in research: systematic review and co-design pilot. Health Expect. 2019;22(4):785–801. doi: 10.1111/hex.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall JC, Bosco L, Adhikari NK, Connolly B, Diaz JV, Dorman T, et al. What is an intensive care unit? A report of the task force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care. 2017;37:270–276. doi: 10.1016/j.jcrc.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO Extension. Jama. 2018;319(5):483–494. doi: 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 49.Movsisyan A, Arnold L, Evans R, Hallingberg B, Moore G, O’Cathain A, et al. Adapting evidence-informed complex population health interventions for new contexts: a systematic review of guidance. Implement Sci. 2019;14(1):105. doi: 10.1186/s13012-019-0956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doane GH, Varcoe C. Family nursing as relational inquiry: developing health-promoting practice. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 51.Gottlieb LN. Strengths-based nursing care: health and healing for person and family: Springer Publishing Company; 2012.
- 52.Rolland JS, Emanuel LL, Torke AM. Applying a family systems lens to proxy decision making in clinical practice and research. Fam Syst Health. 2017;35(1):7–17. doi: 10.1037/fsh0000250. [DOI] [PubMed] [Google Scholar]
- 53.Shajani Z, Snell D. Wright & Leahey’s nurses and families: a guide to family assessment and intervention. 7. Davis: F. A; 2019. [Google Scholar]
- 54.Hartmann M, Bazner E, Wild B, Eisler I, Herzog W. Effects of interventions involving the family in treatment of adult patients with chronic diseases: a meta-analysis. Psychother Psychosom. 2010;79(3):136–148. doi: 10.1159/000286958. [DOI] [PubMed] [Google Scholar]
- 55.Östlund U, Persson C. Examining family responses to family systems nursing interventions: an integrative review. J Fam Nurs. 2014;20(3):259–286. doi: 10.1177/1074840714542962. [DOI] [PubMed] [Google Scholar]
- 56.Mahrer-Imhof R, Bruylands M. Is it beneficial to involve family members? A literature review to psychosocial interventions in family-centred nursing [German] Pflege. 2014;27(5):285–296. doi: 10.1024/1012-5302/a000376. [DOI] [PubMed] [Google Scholar]
- 57.Chesla CA. Do family interventions improve health? J Fam Nurs. 2010;16(4):355–377. doi: 10.1177/1074840710383145. [DOI] [PubMed] [Google Scholar]
- 58.International Family Nursing Association . Position statement on advanced competencies for family nursing. 2017. [Google Scholar]
- 59.Sidani S, Braden CJ. Design, evaluation, and translation of nursing interventions. Chichester: Wiley-Blackwell; 2011. [Google Scholar]
- 60.Albers B, Metz A, Burke K. Implementation support practitioners – a proposal for consolidating a diverse evidence base. BMC Health Serv Res. 2020;20(1):368. doi: 10.1186/s12913-020-05145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp SA, et al. Tailored interventions to address determinants of practice. Cochrane Database Syst Rev. 2015;4:CD005470. doi: 10.1002/14651858.CD005470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powell BJ, Beidas RS, Lewis CC, Aarons GA, McMillen JC, Proctor EK, et al. Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res. 2017;44(2):177–194. doi: 10.1007/s11414-015-9475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padilla Fortunatti C, Munro CL. Factors associated with family satisfaction in the adult intensive care unit: a literature review. Aust Crit Care. 2021;31(5):318–24. [DOI] [PubMed]
- 64.Scott P, Thomson P, Shepherd A. Families of patients in ICU: a scoping review of their needs and satisfaction with care. Nursing Open. 2019;6(3):698–712. [DOI] [PMC free article] [PubMed]
- 65.van den Broek JM, Brunsveld-Reinders AH, Zedlitz AM, Girbes AR, de Jonge E, Arbous MS. Questionnaires on family satisfaction in the adult ICU: a systematic review including psychometric properties. Crit Care Med. 2015;43(8):1731–1744. doi: 10.1097/CCM.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 66.Harrison DA, Ferrando-Vivas P, Wright SE, McColl E, Rowan KM, Investigators FS. Psychometric assessment of the family satisfaction in the intensive care unit (FS-ICU-24) questionnaire among family members of patients admitted to adult general ICUs in the United Kingdom. Intensive Care Med Exp. 2015;3(Suppl 1):A152. [Google Scholar]
- 67.Stricker KH, Niemann S, Bugnon S, Wurz J, Rohrer O, Rothen HU. Family satisfaction in the intensive care unit: cross-cultural adaptation of a questionnaire. J Crit Care. 2007;22(3):204–211. doi: 10.1016/j.jcrc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 68.Cameron JI, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. One-year outcomes in caregivers of critically ill patients. N Engl J Med. 2016;374(19):1831–1841. doi: 10.1056/NEJMoa1511160. [DOI] [PubMed] [Google Scholar]
- 69.Stepanovic K, Van J, Jackson JJ. Family psychological morbidity after the intensive care unit. In: Netzer G, editor. Families in the intensive care unit: a guide to understanding, engaging, and supporting at the bedside. Cham: Springer Nature; 2018. pp. 49–60. [Google Scholar]
- 70.Kerry SM, Bland JM. Sample size in cluster randomisation. Bmj. 1998;316(7130):549. doi: 10.1136/bmj.316.7130.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hemming K, Eldridge S, Forbes G, Weijer C, Taljaard M. How to design efficient cluster randomised trials. BMJ. 2017;358:j3064. doi: 10.1136/bmj.j3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kleinman K, Moyer J, Reich N, Obeng D. clusterPower: power calculations for cluster-randomized and cluster-randomized crossover trials. R package version 0.6.111. 2017. [Google Scholar]
- 73.Fabbro T. sse: sample size estimation. R package version 0.7-13. 2019. [Google Scholar]
- 74.Esserman D, Allore HG, Travison TG. The method of randomization for cluster-randomized trials: challenges of including patients with multiple chronic conditions. Int J Stat Med Res. 2016;5(1):2–7. doi: 10.6000/1929-6029.2016.05.01.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ivers NM, Halperin IJ, Barnsley J, Grimshaw JM, Shah BR, Tu K, et al. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials. 2012;13:120. doi: 10.1186/1745-6215-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin M, Polis A, Hartzel J. R package ‘Minirand’. 0.1.3 ed: CRAN; 2020. https://cran.r-project.org/web/packages/Minirand/Minirand.pdf
- 77.Hwang DY, El-Kareh R, Davidson JE. Implementing intensive care unit family-centered care: resources to identify and address gaps. AACN Adv Crit Care. 2017;28(2):148–154. doi: 10.4037/aacnacc2017636. [DOI] [PubMed] [Google Scholar]
- 78.Hwang DY, Yagoda D, Perrey HM, Tehan TM, Guanci M, Ananian L, et al. Assessment of satisfaction with care among family members of survivors in a neuroscience intensive care unit. J Neurosci Nurs. 2014;46(2):106–116. doi: 10.1097/JNN.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Institute for Healthcare Improvement . Patient- and family-centered care organizational self-assessment tool. 2013. [Google Scholar]
- 80.Zill J, Christalle E, Müller E, Härter M, Dirmaier J, Scholl I. Measurement of physician-patient communication—a systematic review. PLoS One. 2014;9(12):e112637. [DOI] [PMC free article] [PubMed]
- 81.Bieber C, Müller KG, Nicolai J, Hartmann M, Eich W. How does your doctor talk with you? Preliminary validation of a brief patient self-report questionnaire on the quality of physician-patient interaction. J Clin Psychol Med Settings. 2010;17(2):125–136. doi: 10.1007/s10880-010-9189-0. [DOI] [PubMed] [Google Scholar]
- 82.Bruce E, Dorell A, Lindh V, Erlingsson C, Lindkvist M, Sundin K. Translation and testing of the Swedish version of Iceland-Family Perceived Support Questionnaire with parents of children with congenital heart defects. J Fam Nurs. 2016;22(3):298–320. doi: 10.1177/1074840716656343. [DOI] [PubMed] [Google Scholar]
- 83.Sveinbjarnardottir EK, Svavarsdottir EK, Hrafnkelsson B. Psychometric development of the Iceland-Family Perceived Support Questionnaire (ICE-FPSQ) J Fam Nurs. 2012;18(3):328–352. doi: 10.1177/1074840712449203. [DOI] [PubMed] [Google Scholar]
- 84.Sousa VD, Rojjanasrirat W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J Eval Clin Pract. 2011;17(2):268–274. doi: 10.1111/j.1365-2753.2010.01434.x. [DOI] [PubMed] [Google Scholar]
- 85.Mansfield AK, Keitner GI, Dealy J. The family assessment device: an update. Fam Process. 2015;54(1):82–93. doi: 10.1111/famp.12080. [DOI] [PubMed] [Google Scholar]
- 86.Epstein N, Baldwin L, Bishop D. The McMaster Family Assessment Device. J Marital Fam Ther. 1983;9(2):171–180. [Google Scholar]
- 87.Beierlein V, Bultmann JC, Möller B, von Klitzing K, Flechtner H-H, Resch F, et al. Measuring family functioning in families with parental cancer: reliability and validity of the German adaptation of the Family Assessment Device (FAD). J Psychosom Res. 2016;93:110–7 [DOI] [PubMed]
- 88.Staccini L, Tomba E, Grandi S, Keitner GI. The evaluation of family functioning by the family assessment device: a systematic review of studies in adult clinical populations. Fam Process. 2015;54(1):94–115. doi: 10.1111/famp.12098. [DOI] [PubMed] [Google Scholar]
- 89.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194–200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 90.Bluth K, Eisenlohr-Moul TA. Response to a mindful self-compassion intervention in teens: a within-person association of mindfulness, self-compassion, and emotional well-being outcomes. J Adolesc. 2017;57:108–118. doi: 10.1016/j.adolescence.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romcevich LE, Reed S, Flowers SR, Kemper KJ, Mahan JD. Mind-body skills training for resident wellness: a pilot study of a brief mindfulness intervention. J Med Educ Curric Dev. 2018;5:1–10. [DOI] [PMC free article] [PubMed]
- 92.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 93.Pavot W, Diener E. The Satisfaction With Life Scale and the emerging construct of life satisfaction. J Positive Psychol. 2008;3(2):137–152. [Google Scholar]
- 94.Bech P, Olsen LR, Kjoller M, Rasmussen NK. Measuring well-being rather than the absence of distress symptoms: a comparison of the SF-36 Mental Health subscale and the WHO-Five Well-Being Scale. Int J Methods Psychiatr Res. 2003;12(2):85–91. doi: 10.1002/mpr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eurofund. European Quality of Life Survey 2016. Luxembourg: Publications Office of the European Union; 2017. [Google Scholar]
- 96.Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84(3):167–176. doi: 10.1159/000376585. [DOI] [PubMed] [Google Scholar]
- 97.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 98.Donovan KA, Grassi L, McGinty HL, Jacobsen PB. Validation of the Distress Thermometer worldwide: state of the science. Psychooncology. 2014;23(3):241–250. doi: 10.1002/pon.3430. [DOI] [PubMed] [Google Scholar]
- 99.Garrubba M. Use of Distress Thermometers in settings outside of oncology Centre for Clinical Effectiveness: Monash Health. 2019. [Google Scholar]
- 100.Mehnert A, Müller D, Lehmann C, Koch U. Die deutsche Version des NCCN Distress-Thermometers. Zeitschrift für Psychiatrie, Psychol und Psychother. 2006;54(3):213–223. [Google Scholar]
- 101.Zwahlen D, Hagenbuch N, Carley MI, Recklitis CJ, Buchi S. Screening cancer patients’ families with the Distress Thermometer (DT): a validation study. Psychooncology. 2008;17:959–66. [DOI] [PubMed]
- 102.Zwahlen D, Hagenbuch N, Jenewein J, Carley MI, Buchi S. Adopting a family approach to theory and practice: measuring distress in cancer patient-partner dyads with the Distress Thermometer. Psychooncology. 2011;20(4):394–403. doi: 10.1002/pon.1744. [DOI] [PubMed] [Google Scholar]
- 103.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007–1019. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- 104.Ma X, Zhang J, Zhong W, Shu C, Wang F, Wen J, et al. The diagnostic role of a short screening tool--the Distress Thermometer: a meta-analysis. Support Care Cancer. 2014;22(7):1741–1755. doi: 10.1007/s00520-014-2143-1. [DOI] [PubMed] [Google Scholar]
- 105.Gessler S, Low J, Daniells E, Williams R, Brough V, Tookman A, et al. Screening for distress in cancer patients: is the Distress Thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psychooncology. 2008;17(6):538–547. doi: 10.1002/pon.1273. [DOI] [PubMed] [Google Scholar]
- 106.Ohnhäuser S, Wüller J, Foldenauer AC, Pastrana T. Changes in distress measured by the Distress Thermometer as reported by patients in home palliative care in Germany. J Palliat Care. 2018;33(1):39–46. doi: 10.1177/0825859717751932. [DOI] [PubMed] [Google Scholar]
- 107.Thalen-Lindstrom A, Larsson G, Hellbom M, Glimelius B, Johansson B. Validation of the Distress Thermometer in a Swedish population of oncology patients; accuracy of changes during six months. Eur J Oncol Nurs. 2013;17(5):625–663. doi: 10.1016/j.ejon.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 108.Kentish-Barnes N, Lemiale V, Chaize M, Pochard F, Azoulay E. Assessing burden in families of critical care patients. Crit Care Med. 2009;37(10 Suppl):S448–S456. doi: 10.1097/CCM.0b013e3181b6e145. [DOI] [PubMed] [Google Scholar]
- 109.Maercker A, Schützwohl M. Erfassung von psychischen Belastungssfolgen: Die Impact of Event Skala-revidierte Version. Diagnostica. 1998;44:130–141. [Google Scholar]
- 110.Weiss DS, Marmar CR. Assessing psychological trauma and PTSD. New York: Guilford; 1996. [Google Scholar]
- 111.Thoresen S, Tambs K, Hussain A, Heir T, Johansen VA, Bisson JI. Brief measure of posttraumatic stress reactions: impact of Event Scale-6. Soc Psychiatry Psychiatr Epidemiol. 2010;45(3):405–412. doi: 10.1007/s00127-009-0073-x. [DOI] [PubMed] [Google Scholar]
- 112.McAdam JL, Puntillo K. Symptoms experienced by family members of patients in intensive care units. Am J Crit Care. 2009;18(3):200–209. doi: 10.4037/ajcc2009252. [DOI] [PubMed] [Google Scholar]
- 113.Norton S, Cosco T, Doyle F, Done J, Sacker A. The Hospital Anxiety and Depression Scale: a meta confirmatory factor analysis. J Psychosom Res. 2013;74(1):74–81. doi: 10.1016/j.jpsychores.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Angst F, Verra ML, Lehmann S, Aeschlimann A. Responsiveness of five condition-specific and generic outcome assessment instruments for chronic pain. BMC Med Res Methodol. 2008;8:26. doi: 10.1186/1471-2288-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 116.Research Electronic Data Capture software: REDCap Consortium; 2022. https://www.project-redcap.org. Accessed 22 Feb 2022.
- 117.R Core Team . R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. 2021. [Google Scholar]
- 118.Leyrat C, Morgan KE, Leurent B, Kahan BC. Cluster randomized trials with a small number of clusters: which analyses should be used? Int J Epidemiol. 2018;47(3):1012. doi: 10.1093/ije/dyy057. [DOI] [PubMed] [Google Scholar]
- 119.van Buuren S. Flexible imputation of missing data. 2nd ed: Chapman and Hall/CRC; 2018.
- 120.Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. doi: 10.1136/bmj.n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kynoch K, Chang A, Coyer F, McArdle A. The effectiveness of interventions to meet family needs of critically ill patients in an adult intensive care unit: a systematic review update. JBI Database System Rev Implement Rep. 2016;14(3):181–234. doi: 10.11124/JBISRIR-2016-2477. [DOI] [PubMed] [Google Scholar]
- 122.Mackie BR, Mitchell M, Marshall PA. The impact of interventions that promote family involvement in care on adult acute-care wards: an integrative review. Collegian. 2018;25(1):131–140. [Google Scholar]
- 123.Deek H, Hamilton S, Brown N, Inglis SC, Digiacomo M, Newton PJ, et al. Family-centred approaches to healthcare interventions in chronic diseases in adults: a quantitative systematic review. J Adv Nurs. 2016;72(5):968–979. doi: 10.1111/jan.12885. [DOI] [PubMed] [Google Scholar]
- 124.Hopkinson JB, Brown JC, Okamoto I, Addington-Hall JM. The effectiveness of patient-family carer (couple) intervention for the management of symptoms and other health-related problems in people affected by cancer: a systematic literature search and narrative review. J Pain Symptom Manage. 2012;43(1):111–142. doi: 10.1016/j.jpainsymman.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 125.Ågren S, Eriksson A, Fredrikson M, Hollman-Frisman G, Orwelius L. The health promoting conversations intervention for families with a critically ill relative: a pilot study. Intensive Crit Care Nurs. 2019;50:103–110. doi: 10.1016/j.iccn.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 126.Steenbergen S, Rijkenberg S, Adonis T, Kroeze G, van Stijn I, Endeman H. Long-term treated intensive care patients outcomes: the one-year mortality rate, quality of life, health care use and long-term complications as reported by general practitioners. BMC Anesthesiol. 2015;15:142. doi: 10.1186/s12871-015-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wintermann GB, Petrowski K, Weidner K, Strauß B, Rosendahl J. Impact of post-traumatic stress symptoms on the health-related quality of life in a cohort study with chronically critically ill patients and their partners: age matters. Crit Care. 2019;23(1):39. doi: 10.1186/s13054-019-2321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Torres J, Veiga C, Pinto F, Ferreira A, Sousa F, Jacinto R, et al. Caregiving burden: the impact of post intensive care syndrome. Intensive Care Med Exp. 2015;3(1):A967. [Google Scholar]
- 129.Banno A, Hifumi T, Takahashi Y, Soh M, Sakaguchi A, Shimano S, et al. One-year outcomes of postintensive care syndrome in critically ill coronavirus disease 2019 patients: a single institutional study. Crit Care Explor. 2021;3(12):e0595. doi: 10.1097/CCE.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weidman K, LaFond E, Hoffman KL, Goyal P, Parkhurst CN, Derry-Vick H, et al. Post-ICU syndrome in a cohort of COVID-19 survivors in New York City. Ann Am Thorac Soc. 2021. 10.1513/AnnalsATS.202104-520OC. [DOI] [PMC free article] [PubMed]
- 131.Concannon TW, Grant S, Welch V, Petkovic J, Selby J, Crowe S, et al. Practical guidance for involving stakeholders in health research. J Gen Intern Med. 2019;34(3):458–463. doi: 10.1007/s11606-018-4738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Swiss Clinical Trial Organisation. Guide for researchers to address patient and public involvement (PPI) in clinical trials Bern: Swiss Clinical Trial Organisation & Swiss National Science Fund; 2021. https://www.scto.ch/de/publications/fact-sheets.html. Accessed 22 Feb 2022.
- 133.Mathie E, Smeeton N, Munday D, Rhodes G, Wythe H, Jones J. The role of patient and public involvement leads in facilitating feedback: “invisible work”. Res Involve Engage. 2020;6(1):40. doi: 10.1186/s40900-020-00209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Selman LE, Clement C, Douglas M, Douglas K, Taylor J, Metcalfe C, et al. Patient and public involvement in randomised clinical trials: a mixed-methods study of a clinical trials unit to identify good practice, barriers and facilitators. Trials. 2021;22(1):735. doi: 10.1186/s13063-021-05701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study (study protocol).