Abstract
Background
Children with Down syndrome (DS) have a high risk of cardiac disease that may prompt consideration for heart transplantation (HTx). However, transplantation in patients with DS is rarely reported. This project aimed to collect and describe waitlist and post– HTx outcomes in children with DS.
Methods and Results
This is a retrospective case series of children with DS listed for HTx. Pediatric HTx centers were identified by their participation in 2 international registries with centers reporting HTx in a patient with DS providing detailed demographic, medical, surgical, and posttransplant outcome data for analysis. A total of 26 patients with DS were listed for HTx from 1992 to 2020 (median age, 8.5 years; 46% male). High‐risk or failed repair of congenital heart disease was the most common indication for transplant (N=18, 69%). A total of 23 (88%) patients survived to transplant. All transplanted patients survived to hospital discharge with a median posttransplant length of stay of 22 days. At a median posttransplant follow‐up of 2.8 years, 20 (87%) patients were alive, 2 (9%) developed posttransplant lymphoproliferative disorder, and 8 (35%) were hospitalized for infection within the first year. Waitlist and posttransplant outcomes were similar in patients with and without DS (P=non‐significant for all).
Conclusions
Waitlist and post‐HTx outcomes in children with DS selected for transplant listing are comparable to pediatric HTx recipients overall. Given acceptable outcomes, the presence of DS alone should not be considered an absolute contraindication to HTx.
Keywords: Down syndrome, health disparities, heart transplantation, outcomes
Subject Categories: Mortality/Survival, Quality and Outcomes, Disparities, Transplantation, Heart Failure
Nonstandard Abbreviations and Acronyms
- DS
Down syndrome
- HTx
heart transplantation
- PTLD
posttransplant lymphoproliferative disorder; PVR, indexed pulmonary vascular resistance
Clinical Perspective
What Is New?
This multicenter cases series demonstrates that children with Down syndrome can have acceptable outcomes following heart transplantation.
What Are the Clinical Implications?
Given these findings, patients with Down syndrome should be considered for heart transplantation if otherwise acceptable.
Down syndrome (DS) is the most common chromosomal abnormality with an incidence of 10 to 16 per 10 000 live births. 1 , 2 , 3 , 4 DS is associated with a number of comorbidities that can impact multiple organ systems, including the heart. 5 , 6 Congenital heart disease (CHD) in this population is common and occurs in nearly 50% of patients. 5 , 7 , 8 , 9 However, acquired heart disease can also occur either spontaneously or secondary to cardiotoxic therapies given the elevated risk of hematologic malignancies in this population. 10 , 11 Although overall survival for patients with DS has improved over time, cardiac disease remains a leading cause of early death in this group. 12
Despite the high burden of cardiac disease in patients with DS, reports of heart transplantation (HTx) in this group are exceedingly rare. 8 , 13 , 14 A number of studies have reported lower than expected rates of referral for transplantation in children with DS. 15 , 16 This suggests that there may be an overarching perception that children with DS are not acceptable candidates for transplantation. DS is associated with several extracardiac comorbidities that may impact posttransplant outcomes, including pulmonary hypertension, immunologic dysfunction with an increased risk for infections and autoimmune disorders, obesity, and an increased risk of acute leukemia. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Whether these potential risk factors impact waitlist or posttransplant outcomes, however, remains unclear. There are currently no large‐scale studies to suggest that HTx is contraindicated in patients with DS, and the limited available studies suggest that acceptable outcomes can be acheieved. 8 , 13 A better understanding of how patients with DS fare with HTx is critical to assess the feasibility of this procedure in this population and to allow for equitable access to this life‐saving therapy.
The aim of this study is to describe waitlist and post‐HTx outcomes of children with DS across a multicenter cohort. We hypothesized that children with DS would have acceptable waitlist and post‐HTx outcomes without increased risks of posttransplant infection, posttransplant lymphoproliferative disorder (PTLD), or posttransplant mortality.
Methods
Study Cohort and Data Source
This is a retrospective case series of children with DS who were listed for HTx. Pediatric HTx centers belonging to the International Society of Heart and Lung Transplantation or Pediatric Heart Transplant Society listservs were contacted to identify patients with DS who were listed for HTx at their center at any timepoint. Centers reporting a patient with DS listed for HTx were then asked to participate in detailed retrospective data collection.
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Vanderbilt University Medical Center. 26 , 27 REDCap is a secure, web‐based software platform designed to support data capture for research studies. Data were collected pertaining to cardiac diagnosis, comorbidities, pretransplant support, the transplant procedure, and patient outcomes. Outcomes assessed included waitlist and posttransplant survival, rejection, posttransplant malignancy, and hospitalization for infection in the first year after transplant. Rejection was defined by each center as a clinical event, with or without biopsy confirmation, that prompted augmentation of the immunosuppression regimen. Detailed data collection forms are provided in Data S1.
Data from the Organ Procurement and Transplantation Network (OPTN) were used to generate matched control groups. Controls were randomly selected (4:1) after matching for age (±2 years), sex, diagnosis (CHD with prior surgery, CHD without prior surgery, anthracycline cardiomyopathy, dilated cardiomyopathy, or myocarditis), year of transplant, and the need for ventricular assist device, extracorporeal membrane oxygenation, or mechanical ventilatory support. Separate control groups were generated for waitlist and posttransplant outcomes. Because of limitations of OPTN data, an analysis of hospitalization for infection in the first year after transplant was not possible for the matched control group.
Statistical Analysis
Data are presented using summary statistics and reported as frequency (percentage) for categorical and median (interquartile range [IQR]) for continuous data. The Kaplan–Meier method was used to assess posttransplant graft survival, freedom from rejection, freedom from posttransplant malignancy, and freedom from hospitalization for infection in the first year after transplant. The log‐rank test was used to compare outcomes between patients with DS and the matched control groups.
Institutional review board approval or exemption was obtained at each participating institution with a waiver of informed consent. The corresponding author had full access to all the data in the study and takes responsibility for its integrity and the data analysis. A subset of deidentified data that support the findings of this study may be made available upon reasonable request from the corresponding author.
Results
A total of 17 centers across 4 different countries (Canada, Sweden, the United Kingdom, and the United States) reported 28 patients with DS who were listed for HTx. Of this group, 15 (88%) centers agreed to participate in detailed retrospective data collection, accounting for 26 (93%) of the 28 identified patients. The earliest reported HTx listing was in 1992. The timeline of HTx listings in patients with DS is shown in Figure S1. Most patients were listed for HTx only, with 1 patient listed for combined heart–kidney transplantation.
Baseline Demographics and Clinical Characteristics
Demographic data for the 26 included patients are presented in Table 1. The median age at listing was 8.5 years (IQR, 2.6–12.5 years), 62% were White, and 46% were male. Of this group, 6 (23%) were aged <1 year at the time of listing (Figure S2). A total of 21 (81%) patients had a history of CHD, with atrioventricular septal defects being the most common lesion. Failed repair of CHD was the most frequent indication for HTx (N=16, 62%) followed by cardiomyopathy secondary to anthracycline administration (N=5, 19%). Most patients were inpatient at the time of listing, with 18/20 (90%) requiring intensive care. A total of 9 (35%) patients were supported with a ventricular assist device, and 3 required biventricular support. Of the listed patients, 19 (73%) underwent a diagnostic catherization in the pretransplant period. The median pretransplant indexed pulmonary vascular resistance (PVR) was 3.3 Woods units (WU)×m2 (IQR, 2.5–5.7 WU×m2). The maximum pretransplant PVR in this cohort was 11.3 WU×m2. Detailed data regarding pretransplant PVR is presented in Figure S3. Data pertaining to the reactivity of PVR were not collected.
Table 1.
Patient Demographics at the Time of Listing (N=26)
| Age, y | 8.5 (2.6–12.5) |
| Male sex | 12 (46.2) |
| History of CHD | 21 (80.8) |
| CHD lesions (N=21) | |
| Complete atrioventricular septal defect | 9 (42.9) |
| Unbalanced atrioventricular septal defect | 5 (23.8) |
| Hypoplastic left heart syndrome | 2 (9.5) |
| Tetralogy of fallot | 2 (9.5) |
| Atrial septal defect/patent ductus arteriosus* | 1 (4.8) |
| Patent ductus arteriosus* | 1 (4.8) |
| Tetralogy of fallot/atrioventricular septal defect | 1 (4.8) |
| Prior CHD surgery | 19 (73.1) |
| Indication for listing | |
| Failed repair of CHD | 16 (61.5) |
| Anthracycline induced cardiomyopathy | 5 (19.2) |
| CHD deemed too high risk for repair | 2 (7.7) |
| Dilated cardiomyopathy | 2 (7.7) |
| Lymphocytic myocarditis | 1 (3.8) |
| Blood type | |
| O | 7 (29.2) |
| A | 12 (50) |
| B | 4 (16.7) |
| AB | 1 (4.2) |
| Race or ethnicity | |
| White race | 16 (61.5) |
| Black race | 5 (19.2) |
| Hispanic ethnicity | 5 (19.2) |
| Location | |
| ICU | 18 (69.2) |
| Inpatient, not in ICU | 2 (7.7) |
| Outpatient | 6 (23.1) |
| Support at listing | |
| Ventilator | 4 (15.4) |
| ECMO | 1 (3.8) |
| Inotropes | 15 (57.7) |
| Inhaled nitric oxide | 1 (3.8) |
| Prostacyclin | 1 (3.8) |
| Ventricular assist device | 9 (34.6) |
| Ventricular assist device type (N=9) | |
| Berlin EXCOR | 5 (55.6) |
| HeartWare HVAD | 3 (33.3) |
| Thoratec PVAD | 1 (11.1) |
| Biventricular support | 3 (33.3) |
| Functional status | |
| Performs most age‐appropriate activities | 3 (12) |
| Performs age‐appropriate activities with assistance | 10 (40) |
| Requires assistance for all activities | 6 (24) |
| Not applicable (patient aged <1 y) | 6 (24) |
| Prior malignancy | 5 (19.2) |
| Malignancy type (N=5) | |
| AML | 4 (80) |
| ALL | 1 (20) |
| Underwent pretransplant catheterization | 19 (73.1) |
| Listing status (United States only; N=21) | |
| 1A | 14 (66.7) |
| 1B | 3 (14.3) |
| 2 | 4 (19) |
Data are reported as frequency (percentage) for categorical and median (interquartile range) for continuous variables. ALL indicates acute lymphocytic leukemia; AML, acute myelocytic leukemia; CHD, congenital heart disease; ECMO, extracorporeal membrane oxygenation; and ICU, intensive care unit.
Indication for transplantation in these patients was dilated cardiomyopathy.
Transplant Hospitalization Outcomes
A total of 23 (88%) patients survived to transplantation (including 1 concomitant heart–kidney multiorgan transplant), with a median waitlist time of 105 days (IQR, 27–189 days). Waitlist survival was similar when comparing patients with DS to controls (P=0.433; Figure 1). Causes of waitlist death were not collected as part of the study protocol. Demographics of patients who survived to HTx are shown in Table 2. The median donor‐to‐recipient weight ratio was 1.3 (IQR, 1.1–1.6), and the median donor ischemic time was 3.6 hours (IQR, 2.9–4.2 hours). Of those who survived to transplant, 1 (4%) required extracorporeal membrane oxygenation in the posttransplant period, and 1 (4%) required dialysis (heart‐only transplant). No centers reported alterations in their standard immunosuppression protocols. Induction therapy was used in 13 (57%) patients with 10 (43%) receiving antithymocyte globulin and 3 (13%) receiving interleukin‐2 receptor antagonists. All patients who underwent transplantation survived to hospital discharge with a median posttransplant length of stay of 22 days (IQR, 13–32 days). A total of 17 (74%) patients received maintenance steroids at the time of hospital discharge. The most common maintenance immunosuppression at hospital discharge was tacrolimus and mycophenolate mofetil (N=12) followed by cyclosporine and azathioprine (N=6).
Figure 1. Kaplan–Meier survival curve demonstrating overall waitlist survival.
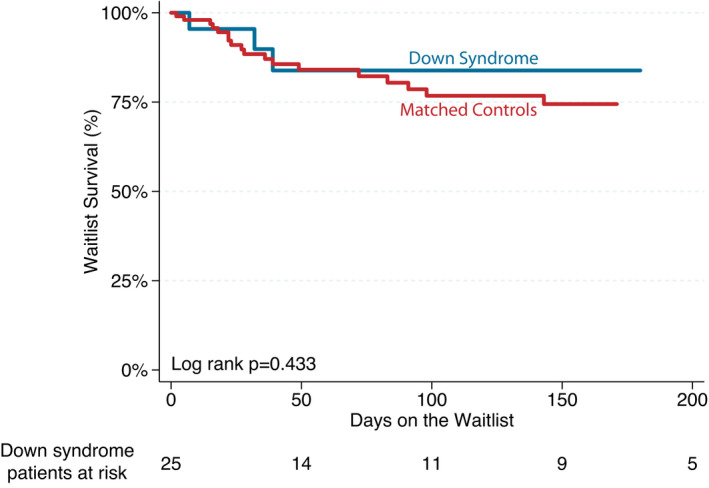
Table 2.
Patient Demographics at the Time of Transplant (N=23)
| Age, y | 10.4 (3–13.8) |
| Male sex | 10 (43.5) |
| Prior CHD surgery | 16 (69.6) |
| Indication for listing | |
| Failed repair of CHD | 13 (56.5) |
| Anthracycline induced cardiomyopathy | 5 (21.7) |
| CHD deemed too high risk for repair | 2 (8.7) |
| Dilated cardiomyopathy | 2 (8.7) |
| Lymphocytic myocarditis | 1 (4.3) |
| Support at transplant | |
| Ventilator | 3 (13) |
| ECMO | 0 (0) |
| Inotropes | 12 (52.2) |
| Inhaled nitric oxide | 2 (8.7) |
| Prostacyclin | 1 (4.3) |
| Ventricular assist device | 9 (39.1) |
| Donor ischemic time, h | 3.6 (2.9–4.2) |
| Donor‐to‐recipient weight ratio | 1.3 (1.1–1.6) |
| Required ECMO posttransplant | 1 (4.3) |
| Required dialysis posttransplant | 1 (4.3) |
| Survived to hospital discharge | 23 (100) |
| Posttransplant length of stay, d | 22 (13–32) |
| Maintenance steroids | 17 (73.9) |
| Maintenance immunosuppression at discharge | |
| Tacrolimus/mycophenolate | 12 (54.5) |
| Cyclosporine/azathioprine | 6 (27.3) |
| Cyclosporine/mycophenolate | 2 (9.1) |
| Tacrolimus monotherapy | 1 (4.5) |
| Sirolimus/mycophenolate | 1 (4.5) |
Data are reported as frequency (percentage) for categorical and median (interquartile range) for continuous variables. CHD indicates congenital heart disease, and ECMO, extracorporeal membrane oxygenation.
Posttransplant Outcomes
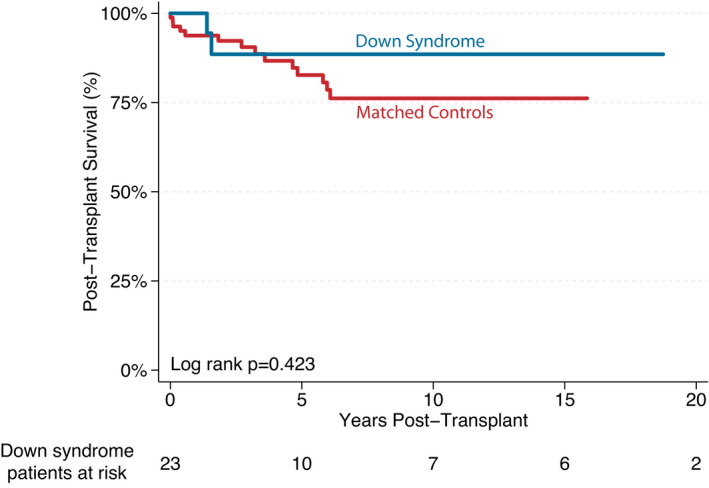
The median posttransplant follow‐up time was 2.8 years (IQR, 1.4–15.5 years). A total of 3 (13%) patients have died as of the last data collection in August 2021. The causes of death were (1) coronary allograft vasculopathy at 22.6 years posttransplant, (2) multiple episodes of rejection and PTLD at 1.6 years posttransplant, and (3) respiratory arrest at 1.4 years posttransplant. Posttransplant survival was similar between patients with DS and controls (P=0.423; Figure 2).
Figure 2. Kaplan–Meier survival curve demonstrating overall posttransplant survival.
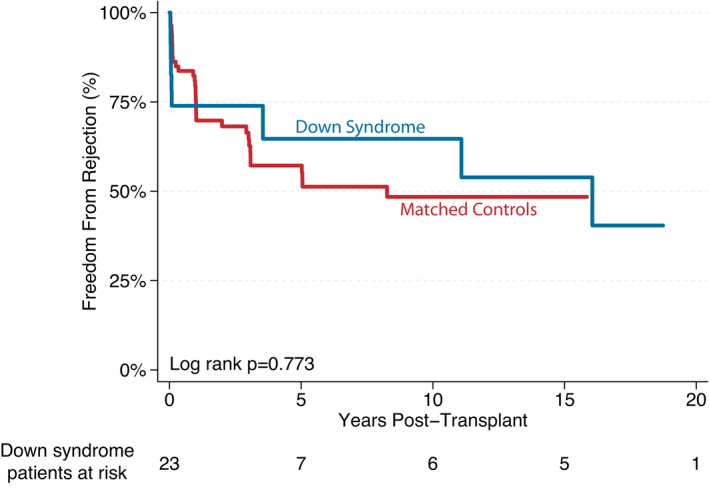
A total of 10 (43%) patients experienced at least 1 episode of acute rejection, with a median time to first rejection of 30 days posttransplant. Of this group, 7 patients had an isolated episode of rejection (80% cellular rejection), and 3 patients demonstrated recurrent rejection events with episodes of cellular, antibody‐mediated, and mixed forms of rejection. Hemodynamic compromise occurred in 2 patients. Overall freedom from rejection is presented in Figure 3. There was no difference in the incidence of rejection between patients with DS and controls (P=0.773).
Figure 3. Kaplan–Meier survival curve demonstrating freedom from rejection after transplant.
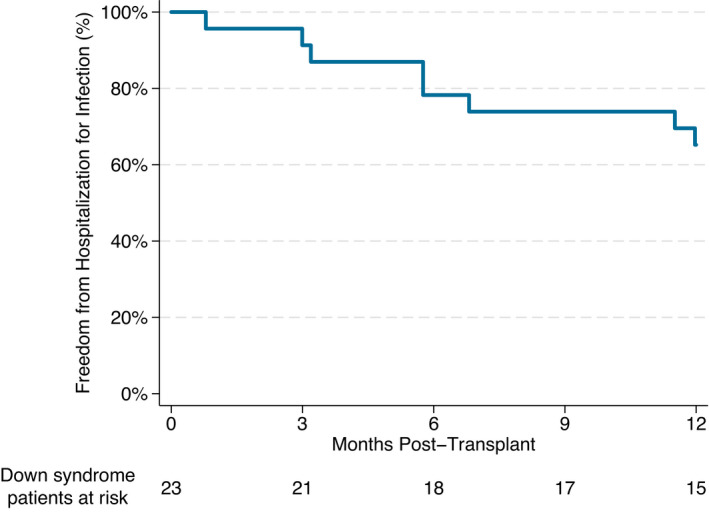
A total of 8 (35%) patients were rehospitalized for infection in the first year after transplant. Of this group, 5 patients had a single hospitalization, whereas the remaining 3 had multiple readmission encounters. Freedom from rehospitalization for infection is shown in Figure 4. The median time to first readmission was 175 days (IQR, 94–279 days) posttransplant. Documented infections were predominantly viral and included viral upper respiratory tract infection (N=5) and cytomegalovirus viremia (N=2) as well as norovirus, human herpesvirus 6, adenovirus, and BK viremia (N=1 each). Bacterial infections included bacterial sinusitis, methicillin‐resistant Staphylococcus aureus tracheitis, Clostridium difficile, and disseminated Pseudomonas. One patient was admitted with oral candidiasis and feeding intolerance.
Figure 4. Kaplan–Meier survival curve demonstrating freedom from hospitalization for infection in the first year after transplant.
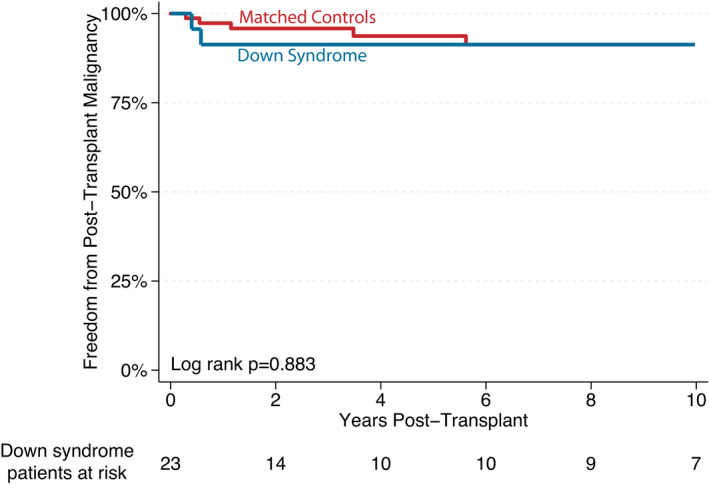
A total of 5 (19%) patients had histories of pretransplant malignancy. Each of these patients survived to HTx, and at a median follow‐up of 7.4 years, all remained cancer free. Across the entire cohort, 2 (9%) patients were diagnosed with PTLD in the posttransplant period, including 1 patient with monomorphic diffuse large B cell lymphoma and 1 with polymorphic Epstein‐Barr virus–positive PTLD presenting at 5 and 7 months after transplant, respectively. Freedom from posttransplant malignancy is shown in Figure 5, with no statistical difference between patients with DS and controls (P=0.883).
Figure 5. Kaplan–Meier survival curve demonstrating freedom from malignancy after transplant.
Functional Status
The majority of patients continue to be followed by pediatric providers. A total of 5 (22%) patients have been transitioned to adult practices. At last known follow‐up, 5/22 (23%) patients require assistance for all activities, 14/22 (64%) perform age‐appropriate activities with some assistance, and 3/22 (14%) perform most age‐appropriate activities without assistance.
Discussion
We present the largest report of HTx in patients with DS to date. We found that children with DS who are listed for HTx have waitlist and posttransplant survival similar to their non‐DS counterparts. 28 , 29 Moreover, patients with DS have comparable rates of posttransplant infection requiring hospitalization, rejection, and PTLD as other pediatric HTx recipients. 28 , 30 , 31 , 32 , 33 , 34 , 35 Based on these data, despite historical concerns about transplant candidacy in this population, a diagnosis of DS alone does not appear to lead to inferior posttransplant outcomes and therefore should not be considered an a priori contraindication to HTx.
There are limited prior reports of HTx in individuals with DS. Broda and colleagues used administrative data from the Pediatric Health Information System (PHIS) to analyze HTx outcomes in children with chromosomal anomalies. 13 There were 5 patients with DS in their cohort with a reported 20% mortality before hospital discharge. This is in contrast to our data where all patients survived to hospital discharge. This difference highlights potential limitations of administrative data in identification of in‐hospital mortality or identification of patients with DS using International Classification of Diseases, Ninth Revision (ICD‐9) codes as 22 of the patients from our cohort are from centers that participate in PHIS and likely overlap with the patients reported by Broda et al. Consistent with our data, 2 previous single‐center reports demonstrate acceptable posttransplant outcomes in patients with DS. 8 , 14
One important consequence of DS is dysfunction of the innate as well as adaptive immune system, which likely plays a critical role in the heightened risk of hematologic malignancies, autoimmune disorders, and infectious complications in this population. 21 , 22 , 23 , 24 , 25 , 36 , 37 , 38 Although it would have been reasonable to consider alterations in induction and/or posttransplant immunosuppression for patients with DS undergoing HTx, no centers in our study reported doing so. Although a number of patients were hospitalized for posttransplant infections, the frequency was not out of proportion to published reports in pediatric HTx, where >60% of patients are readmitted in the first year and infection accounts for up to 25% of these admissions. 30 , 31 Importantly, the risk of rejection is similar in patients with DS. This finding further supports that a standard immunosuppression strategy is likely warranted in patients with DS undergoing HTx.
Patients with DS have a 20‐fold increased risk of acute leukemia. 19 , 20 , 21 , 39 , 40 , 41 The risk is highest between the ages of 1 and 4 years but persists well into adulthood. 42 However, with the exception of testicular cancer, there is a low incidence of solid tumors in this population, and the overall risk of cancer is similar to the general population. 10 , 42 Importantly, no patients in our cohort with anthracycline‐induced cardiomyopathy experienced recurrence of their primary malignancy or any secondary malignancies, and only 2 patients in our cohort developed PTLD. Although the potential for malignancy remains a concern in patients with DS and expert oncology consultation may be warranted, our data suggest that posttransplant malignancy is not exaggerated in those with DS.
Intellectual disability is nearly universal in patients with DS, with a high degree of variability among individuals and a wide spectrum of cognitive capabilities. 43 , 44 , 45 Although the presence of cognitive delay may inappropriately influence consideration of HTx listing, a number of studies have demonstrated that the presence of cognitive delay does not negatively impact solid organ transplant outcomes in the pediatric population and should not discourage programs from offering HTx to a patient with sufficient social support. 46 , 47 , 48 , 49 Ensuring a strong social support structure likely represents a critical step to ensure long‐term success in patients with DS undergoing HTx. This is underscored by our data showing that the majority of patients in our cohort required at least some degree of assistance for activities of daily living at the time of last known follow‐up.
Children with DS are at higher risk for the development of pulmonary hypertension, which is more common with coexisting cardiac disease. 17 , 18 Fixed and significantly elevated PVR has traditionally been regarded as a contraindication to HTx given concerns for acute right ventricular failure in the graft. 50 , 51 Although this may have been the basis by which patients with DS were excluded from transplant consideration previously, a number of patients in our cohort were successfully transplanted in the face of elevated PVR. Evolution of mechanical circulatory support strategies, donor selection, and posttransplant management has helped to mitigate some of the risks associated with elevated PVR. 52 , 53 , 54 Therefore, although elevated PVR may identify higher risk patients, it may not be prohibitive to HTx in the current era.
Although the lower than expected rates of referral for transplantation in patients with DS likely suggests an underlying systematic bias, parental decision making and potential misconceptions about transplant candidacy may also play a role. 15 , 16 There are limited data addressing the reasons for the lack of transplant consideration in this population, representing an important area for future research to ensure equitable access to potentially life‐saving therapies. Importantly, our data highlight that acceptable outcomes can be achieved following HTx in patients with DS, providing critical insights for both providers and families when considering transplant candidacy.
There are a number of limitations to our analysis. We only queried pediatric HTx centers and therefore may have missed adults with DS who have undergone HTx. However, given the lack of published reports of HTx in adults with DS and the fact that pediatric providers are less likely to view DS as a contraindication to transplantation, 55 we believe that we have captured a large extent of the worldwide experience in this population. Although this represents the largest report of HTx in DS to date, the numbers remain small. Therefore, the study is largely descriptive. There is almost certainly a selection bias encompassed in our data. Centers are less likely to pursue transplantation in a patient with DS who has multiple comorbidities, and therefore our data may contain only the most ideal candidates. Despite this, there were patients in our cohort with high‐risk features, including the need for biventricular mechanical support, multiorgan transplant, and elevated PVR. Our results should also be interpreted with caution because of the potential for survivorship bias. Centers may be less likely to report patients who did not survive to transplantation or who experienced posttransplant mortality, potentially biasing our results toward improved patient outcomes. Lastly, event definitions within the OPTN data used as our comparison group may differ from the definitions used in our study, representing a potential source of error.
Despite the noted limitations, patients with DS transplanted in this multicenter cohort appear to have acceptable posttransplant outcomes. Therefore, the presence of DS alone should not serve as an absolute contraindication to HTx.
Sources of Funding
This project was supported by Clinical and Translational Science Award UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosures
None.
Supporting information
Data S1
Figures S1–S3
For Sources of Funding and Disclosures, see page 8.
See Editorial by Schumacher et al.
REFERENCES
- 1. Weijerman ME, van Furth AM, Vonk Noordegraaf A, van Wouwe JP, Broers CJ, Gemke RJ. Prevalence, neonatal characteristics, and first‐year mortality of Down syndrome: a national study. J Pediatr. 2008;152:15–19. doi: 10.1016/j.jpeds.2007.09.045 [DOI] [PubMed] [Google Scholar]
- 2. Owens JR, Harris F, Walker S, McAllister E, West L. The incidence of Down's syndrome over a 19‐year period with special reference to maternal age. J Med Genet. 1983;20:90–93. doi: 10.1136/jmg.20.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes‐McCormack LA, McGowan R, Pell JP, Mackay D, Henderson A, O'Leary L, Cooper SA. Birth incidence, deaths and hospitalisations of children and young people with Down syndrome, 1990–2015: birth cohort study. BMJ Open. 2020;10:e033770. doi: 10.1136/bmjopen-2019-033770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stoll C, Alembik Y, Dott B, Roth MP. Epidemiology of Down syndrome in 118,265 consecutive births. Am J Med Genet Suppl. 1990;7:79–83. doi: 10.1002/ajmg.1320370715 [DOI] [PubMed] [Google Scholar]
- 5. Stoll C, Dott B, Alembik Y, Roth MP. Associated congenital anomalies among cases with Down syndrome. Eur J Med Genet. 2015;58:674–680. doi: 10.1016/j.ejmg.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 6. Cleves MA, Hobbs CA, Cleves PA, Tilford JM, Bird TM, Robbins JM. Congenital defects among liveborn infants with Down syndrome. Birth Defects Res A Clin Mol Teratol. 2007;79:657–663. doi: 10.1002/bdra.20393 [DOI] [PubMed] [Google Scholar]
- 7. Weijerman ME, van Furth AM, van der Mooren MD, van Weissenbruch MM, Rammeloo L, Broers CJ, Gemke RJ. Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur J Pediatr. 2010;169:1195–1199. doi: 10.1007/s00431-010-1200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irving CA, Chaudhari MP. Cardiovascular abnormalities in down's syndrome: spectrum, management and survival over 22 years. Arch Dis Child. 2012;97:326–330. doi: 10.1136/adc.2010.210534 [DOI] [PubMed] [Google Scholar]
- 9. Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, Khoury MJ, Saker DM. Population‐based study of congenital heart defects in down syndrome. Am J Med Genet. 1998;80:213–217. doi: [DOI] [PubMed] [Google Scholar]
- 10. Patja K, Pukkala E, Sund R, Iivanainen M, Kaski M. Cancer incidence of persons with down syndrome in Finland: a population‐based study. Int J Cancer. 2006;118:1769–1772. doi: 10.1002/ijc.21518 [DOI] [PubMed] [Google Scholar]
- 11. Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Cancers and immune related diseases associated with Down's syndrome: a record linkage study. Arch Dis Child. 2004;89:1014–1017. doi: 10.1136/adc.2003.046219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Leary L, Hughes‐McCormack L, Dunn K, Cooper SA. Early death and causes of death of people with Down syndrome: a systematic review. J Appl Res Intellect Disabil. 2018;31:687–708. doi: 10.1111/jar.12446 [DOI] [PubMed] [Google Scholar]
- 13. Broda CR, Cabrera AG, Rossano JW, Jefferies JL, Towbin JA, Chin C, Shamszad P. Cardiac transplantation in children with down syndrome, turner syndrome, and other chromosomal anomalies: a multi‐institutional outcomes analysis. J Heart Lung Transplant. 2018;37:749–754. doi: 10.1016/j.healun.2018.01.1296 [DOI] [PubMed] [Google Scholar]
- 14. Wilkens SJ, Priest J, Kaufman BD, Barkoff L, Rosenthal DN, Hollander SA. Pediatric waitlist and heart transplant outcomes in patients with syndromic anomalies. Pediatr Transplant. 2020;24:e13643. doi: 10.1111/petr.13643 [DOI] [PubMed] [Google Scholar]
- 15. Leonard H, Eastham K, Dark J. Heart and heart‐lung transplantation in Down's syndrome. The lack of supportive evidence means each case must be carefully assessed. BMJ. 2000;320:816–817. doi: 10.1136/bmj.320.7238.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arenson EB Jr, Forde MD. Bone marrow transplantation for acute leukemia and Down syndrome: report of a successful case and results of a national survey. J Pediatr. 1989;114:69–72. doi: 10.1016/S0022-3476(89)80603-1 [DOI] [PubMed] [Google Scholar]
- 17. Bush D, Galambos C, Dunbar ID. Pulmonary hypertension in children with Down syndrome. Pediatr Pulmonol. 2021;56:621–629. doi: 10.1002/ppul.24687 [DOI] [PubMed] [Google Scholar]
- 18. Bush D, Galambos C, Ivy DD, Abman SH, Wolter‐Warmerdam K, Hickey F. Clinical characteristics and risk factors for developing pulmonary hypertension in children with down syndrome. J Pediatr. 2018;202:212–219.e2. doi: 10.1016/j.jpeds.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 19. Mili F, Khoury MJ, Flanders WD, Greenberg RS. Risk of childhood cancer for infants with birth defects. I. A record‐linkage study, Atlanta, Georgia, 1968‐1988. Am J Epidemiol. 1993;137:629–638. [DOI] [PubMed] [Google Scholar]
- 20. Mili F, Lynch CF, Khoury MJ, Flanders WD, Edmonds LD. Risk of childhood cancer for infants with birth defects. Ii. A record‐linkage study, Iowa, 1983‐1989. Am J Epidemiol. 1993;137:639–644. [DOI] [PubMed] [Google Scholar]
- 21. Satge D, Seidel MG. The pattern of malignancies in Down syndrome and its potential context with the immune system. Front Immunol. 2018;9:3058. doi: 10.3389/fimmu.2018.03058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bloemers BL, Bont L, de Weger RA, Otto SA, Borghans JA, Tesselaar K. Decreased thymic output accounts for decreased naive T cell numbers in children with down syndrome. J Immunol. 2011;186:4500–4507. doi: 10.4049/jimmunol.1001700 [DOI] [PubMed] [Google Scholar]
- 23. Bloemers BL, van Bleek GM, Kimpen JL, Bont L. Distinct abnormalities in the innate immune system of children with Down syndrome. J Pediatr. 2010;156:804–809, 809 e801–809 e805. doi: 10.1016/j.jpeds.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 24. Karlsson B, Gustafsson J, Hedov G, Ivarsson SA, Anneren G. Thyroid dysfunction in Down's syndrome: relation to age and thyroid autoimmunity. Arch Dis Child. 1998;79:242–245. doi: 10.1136/adc.79.3.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin Exp Immunol. 2011;164:9–16. doi: 10.1111/j.1365-2249.2011.04335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The redcap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rossano JW, Singh TP, Cherikh WS, Chambers DC, Harhay MO, Hayes D, Hsich E, Khush KK, Meiser B, Potena L, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: twenty‐second pediatric heart transplantation report ‐ 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1028–1041. doi: 10.1016/j.healun.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zafar F, Castleberry C, Khan MS, Mehta V, Bryant R III, Lorts A, Wilmot I, Jefferies JL, Chin C, Morales DLS. Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. J Heart Lung Transplant. 2015;34:82–88. doi: 10.1016/j.healun.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 30. Hollander SA, McElhinney DB, Almond CS, McDonald N, Chen S, Kaufman BD, Bernstein D, Rosenthal DN. Rehospitalization after pediatric heart transplantation: incidence, indications, and outcomes. Pediatr Transplant. 2017;21:e12857. doi: 10.1111/petr.12857 [DOI] [PubMed] [Google Scholar]
- 31. Lambert AN, Weiner JG, Hall M, Thurm C, Dodd DA, Bearl DW, Soslow JH, Feingold B, Smith AH, Godown J. Rehospitalization following pediatric heart transplantation: incidence, indications, and risk factors. Pediatr Cardiol. 2020;41:584–590. doi: 10.1007/s00246-020-02326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gossett JG, Canter CE, Zheng J, Schechtman K, Blume ED, Rodgers S, Naftel DC, Kirklin JK, Scheel J, Fricker FJ, et al. Decline in rejection in the first year after pediatric cardiac transplantation: a multi‐institutional study. J Heart Lung Transplant. 2010;29:625–632. doi: 10.1016/j.healun.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 33. Ameduri RK, Zheng J, Schechtman KB, Hoffman TM, Gajarski RJ, Chinnock R, Naftel DC, Kirklin JK, Dipchand AI, Canter CE. Has late rejection decreased in pediatric heart transplantation in the current era? A multi‐institutional study. J Heart Lung Transplant. 2012;31:980–986. doi: 10.1016/j.healun.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 34. Godown J, Cantor R, Koehl D, Cummings E, Vo JB, Dodd DA, Lytrivi I, Boyle GJ, Sutcliffe DL, Kleinmahon JA, et al. Practice variation in the diagnosis of acute rejection among pediatric heart transplant centers: an analysis of the pediatric heart transplant society (PHTS) registry. J Heart Lung Transplant. 2021;40:1550–1559. doi: 10.1016/j.healun.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 35. Lamour JM, Mason KL, Hsu DT, Feingold B, Blume ED, Canter CE, Dipchand AI, Shaddy RE, Mahle WT, Zuckerman WA, et al. Early outcomes for low‐risk pediatric heart transplant recipients and steroid avoidance: a multicenter cohort study (clinical trials in organ transplantation in children–CTOTC‐04). J Heart Lung Transplant. 2019;38:972–981. doi: 10.1016/j.healun.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bloemers BL, Broers CJ, Bont L, Weijerman ME, Gemke RJ, van Furth AM. Increased risk of respiratory tract infections in children with Down syndrome: the consequence of an altered immune system. Microbes Infect. 2010;12:799–808. doi: 10.1016/j.micinf.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 37. Bloemers BL, van Furth AM, Weijerman ME, Gemke RJ, Broers CJ, van den Ende K, Kimpen JL, Strengers JL, Bont LJ. Down syndrome: a novel risk factor for respiratory syncytial virus bronchiolitis–a prospective birth‐cohort study. Pediatrics. 2007;120:e1076–e1081. doi: 10.1542/peds.2007-0788 [DOI] [PubMed] [Google Scholar]
- 38. Schoch J, Rohrer TR, Kaestner M, Abdul‐Khaliq H, Gortner L, Sester U, Sester M, Schmidt T. Quantitative, phenotypical, and functional characterization of cellular immunity in children and adolescents with down syndrome. J Infect Dis. 2017;215:1619–1628. doi: 10.1093/infdis/jix168 [DOI] [PubMed] [Google Scholar]
- 39. Windham GC, Bjerkedal T, Langmark F. A population‐based study of cancer incidence in twins and in children with congenital malformations or low birth weight, Norway, 1967–1980. Am J Epidemiol. 1985;121:49–56. doi: 10.1093/oxfordjournals.aje.a113982 [DOI] [PubMed] [Google Scholar]
- 40. Satge D, Sommelet D, Geneix A, Nishi M, Malet P, Vekemans M. A tumor profile in Down syndrome. Am J Med Genet. 1998;78:207–216. doi: [DOI] [PubMed] [Google Scholar]
- 41. Ross JA, Spector LG, Robison LL, Olshan AF. Epidemiology of leukemia in children with Down syndrome. Pediatr Blood Cancer. 2005;44:8–12. doi: 10.1002/pbc.20165 [DOI] [PubMed] [Google Scholar]
- 42. Hasle H, Friedman JM, Olsen JH, Rasmussen SA. Low risk of solid tumors in persons with Down syndrome. Genet Med. 2016;18:1151–1157. doi: 10.1038/gim.2016.23 [DOI] [PubMed] [Google Scholar]
- 43. Startin CM, D'Souza H, Ball G, Hamburg S, Hithersay R, Hughes KMO, Massand E, Karmiloff‐Smith A, Thomas MSC, LonDown SC, et al. Health comorbidities and cognitive abilities across the lifespan in Down syndrome. J Neurodev Disord. 2020;12:4. doi: 10.1186/s11689-019-9306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maatta T, Tervo‐Maatta T, Taanila A, Kaski M, Iivanainen M. Mental health, behaviour and intellectual abilities of people with down syndrome. Downs Syndr Res Pract. 2006;11:37–43. doi: 10.3104/reports.313 [DOI] [PubMed] [Google Scholar]
- 45. Couzens D, Cuskelly M, Haynes M. Cognitive development and Down syndrome: age‐related change on the Stanford‐Binet test (fourth edition). Am J Intellect Dev Disabil. 2011;116:181–204. doi: 10.1352/1944-7558-116.3.181 [DOI] [PubMed] [Google Scholar]
- 46. Wightman A, Young B, Bradford M, Dick A, Healey P, McDonald R, Smith J. Prevalence and outcomes of renal transplantation in children with intellectual disability. Pediatr Transplant. 2014;18:714–719. doi: 10.1111/petr.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wightman A, Hsu E, Zhao Q, Smith J. Prevalence and outcomes of liver transplantation in children with intellectual disability. J Pediatr Gastroenterol Nutr. 2016;62:808–812. doi: 10.1097/MPG.0000000000001071 [DOI] [PubMed] [Google Scholar]
- 48. Wightman A, Bartlett HL, Zhao Q, Smith JM. Prevalence and outcomes of heart transplantation in children with intellectual disability. Pediatr Transplant. 2017;21:e12839. doi: 10.1111/petr.12839 [DOI] [PubMed] [Google Scholar]
- 49. Prendergast C, McKane M, Dodd DA, Godown J. The impact of cognitive delay on pediatric heart transplant outcomes. Pediatr Transplant. 2017;21. doi: 10.1111/petr.12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shaddy RE. Pulmonary hypertension in pediatric heart transplantation. Prog Pediatr Cardiol. 2000;11:131–136. doi: 10.1016/S1058-9813(00)00040-0 [DOI] [PubMed] [Google Scholar]
- 51. Addonizio LJ, Gersony WM, Robbins RC, Drusin RE, Smith CR, Reison DS, Reemtsma K, Rose EA. Elevated pulmonary vascular resistance and cardiac transplantation. Circulation. 1987;76:V52–V55. [PubMed] [Google Scholar]
- 52. Richmond ME, Law YM, Das BB, Everitt MD, Kukreja M, Naftel DC, Kemna MS, Henderson HT, Beddows K, Fricker FJ, et al. Elevated pre‐transplant pulmonary vascular resistance is not associated with mortality in children without congenital heart disease: a multicenter study. J Heart Lung Transplant. 2015;34:448–456. doi: 10.1016/j.healun.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 53. Chiu P, Russo MJ, Davies RR, Addonizio LJ, Richmond ME, Chen JM. What is high risk? Redefining elevated pulmonary vascular resistance index in pediatric heart transplantation. J Heart Lung Transplant. 2012;31:61–66. doi: 10.1016/j.healun.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 54. Thangappan K, Morales DLS, Vu Q, Lehenbauer D, Villa C, Wittekind S, Hirsch R, Lorts A, Zafar F. Impact of mechanical circulatory support on pediatric heart transplant candidates with elevated pulmonary vascular resistance. Artif Organs. 2021;45:29–37. doi: 10.1111/aor.13747 [DOI] [PubMed] [Google Scholar]
- 55. Wall A, Lee GH, Maldonado J, Magnus D. Genetic disease and intellectual disability as contraindications to transplant listing in the United States: a survey of heart, kidney, liver, and lung transplant programs. Pediatr Transplant. 2020;24:e13837. doi: 10.1111/petr.13837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Figures S1–S3


