Abstract
Background
Persistent sensorimotor impairments after stroke can negatively impact quality of life. The hippocampus is vulnerable to poststroke secondary degeneration and is involved in sensorimotor behavior but has not been widely studied within the context of poststroke upper‐limb sensorimotor impairment. We investigated associations between non‐lesioned hippocampal volume and upper limb sensorimotor impairment in people with chronic stroke, hypothesizing that smaller ipsilesional hippocampal volumes would be associated with greater sensorimotor impairment.
Methods and Results
Cross‐sectional T1‐weighted magnetic resonance images of the brain were pooled from 357 participants with chronic stroke from 18 research cohorts of the ENIGMA (Enhancing NeuoImaging Genetics through Meta‐Analysis) Stroke Recovery Working Group. Sensorimotor impairment was estimated from the FMA‐UE (Fugl‐Meyer Assessment of Upper Extremity). Robust mixed‐effects linear models were used to test associations between poststroke sensorimotor impairment and hippocampal volumes (ipsilesional and contralesional separately; Bonferroni‐corrected, P<0.025), controlling for age, sex, lesion volume, and lesioned hemisphere. In exploratory analyses, we tested for a sensorimotor impairment and sex interaction and relationships between lesion volume, sensorimotor damage, and hippocampal volume. Greater sensorimotor impairment was significantly associated with ipsilesional (P=0.005; β=0.16) but not contralesional (P=0.96; β=0.003) hippocampal volume, independent of lesion volume and other covariates (P=0.001; β=0.26). Women showed progressively worsening sensorimotor impairment with smaller ipsilesional (P=0.008; β=−0.26) and contralesional (P=0.006; β=−0.27) hippocampal volumes compared with men. Hippocampal volume was associated with lesion size (P<0.001; β=−0.21) and extent of sensorimotor damage (P=0.003; β=−0.15).
Conclusions
The present study identifies novel associations between chronic poststroke sensorimotor impairment and ipsilesional hippocampal volume that are not caused by lesion size and may be stronger in women.
Keywords: hippocampus, MRI, sensorimotor impairment, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Big Data and Data Standards, Magnetic Resonance Imaging (MRI), Rehabilitation
Nonstandard Abbreviations and Acronyms
- ENIGMA
Enhancing NeuroImaging Genetics through Meta‐Analysis
- FMA‐UE
Fugl‐Meyer Assessment of Upper Extremity
- SD
spreading depression
Clinical Perspective
What Is New?
In this study, we report a novel association between more severe sensorimotor impairment and smaller ipsilesional hippocampal volume in patients with chronic stroke, which is not caused by lesion size or lesion damage to sensorimotor‐specific regions, and which may be stronger in women than in men.
What Are the Clinical Implications?
These findings are of clinical interest because they provide initial evidence linking the integrity of the non‐lesioned hippocampus (a key region of the limbic system associated with cognition and mood) to poststroke sensorimotor outcomes. This result provides support for holistic therapeutic approaches that are applicable to multiple domains of well‐being.
Sensorimotor impairments are a major burden of disease for stroke survivors. 1 To help clinicians, caregivers, and patients make informed and effective rehabilitation treatment decisions, there is a critical need to identify biomarkers that accurately predict a patient’s potential for sensorimotor recovery. 2 , 3 Magnetic resonance imaging (MRI) studies of regional brain volumes suggest that secondary degeneration of adjacent or remote regions may contribute to sensorimotor impairment and could influence poststroke sensorimotor outcomes. 4 , 5
The hippocampus is a brain region that is particularly vulnerable to poststroke secondary degeneration. Both rodent 6 and human 7 , 8 , 9 stroke studies have shown evidence of damage within the hippocampus in the same hemisphere as an infarct (ipsilesional), but outside of the lesion itself. Using structural MRI, smaller ipsilesional hippocampal volumes in patients with stroke have been reported in comparison to healthy controls, 7 , 8 , 9 as well as smaller bilateral hippocampal volumes at the time of stroke 10 and accelerated hippocampal atrophy observed most prominently 3 months after stroke. 6 , 9 Studies have also reported magnetic resonance spectroscopy evidence of contralesional hippocampal neuronal loss 8 and contralesional hippocampal atrophy measured with longitudinal MRI. 9 Given that stroke‐related infarctions of the hippocampus are uncommon, 11 poststroke hippocampal atrophy may be attributed to secondary degenerative mechanisms such as spreading depression (SD) 6 or reduced connectivity to lesioned structures, 11 among others. However, the extent to which lesion volume relates to hippocampal damage remains unclear.
The hippocampus is widely known for its key role in memory, and this has led the field of stroke recovery research to primarily focus on the role of hippocampal damage in cognitive impairment. 6 , 7 , 8 Although not typically considered a primary sensorimotor region, there is evidence that the hippocampus may also be involved in sensorimotor behavior. The hippocampus is densely connected to brain areas that play an important role in sensorimotor processing such as the thalamus and basal ganglia through the spinal‐limbic pathway. 12 Reports of hippocampal activity during sensorimotor behavior such as sensorimotor integration, 13 sensorimotor learning, 14 and motor control 14 suggest that the hippocampus plays a role in sensorimotor circuits. Sensorimotor task‐related functional connectivity with the hippocampus has also been reported with the thalamus, 15 sensorimotor cortex, 14 and the supplementary motor area. 16 However, the relationship between hippocampal structural integrity and poststroke upper‐limb sensorimotor impairment remains unclear. Given the potential involvement of the hippocampus in sensorimotor circuits, hippocampal damage caused by secondary degeneration after stroke could further weaken sensorimotor circuits, leading to greater chronic sensorimotor impairment. Alternatively, damage to the thalamus, basal ganglia, sensorimotor cortex, or supplementary motor area, which are typically associated with greater sensorimotor impairment, may lead to downstream degeneration of the hippocampus through functional or structural connections.
Dementia studies 17 and healthy aging 18 research also suggest that hippocampal atrophy may differ by sex, because hippocampal atrophy has been found to accelerate in women after menopause. Estrogen levels may play a mediating role in these trends 19 and have been associated with stroke severity and mortality. 20 Stroke‐related outcomes including disability and quality of life are generally poorer in women than men, 1 , 21 although conclusive sex differences have not been reported in terms of poststroke sensorimotor impairment. 22 As such, sex could moderate the relationship between sensorimotor impairment and hippocampal volume following a stroke. In particular, women may have smaller hippocampal volumes and worse sensorimotor impairment, potentially leading to stronger effect sizes compared with men.
In addition, associations between lesion size and hippocampal volume remain unclear. One study reports that larger lesion size is directly associated with smaller hippocampal volumes, 7 while other studies report no clear relationship. 6 , 8 Given this lack of consensus, we also investigated whether lesion size was independently associated with hippocampal volume, using a large sample of brain MRI scans with manually segmented stroke lesions.
The current study is a first step towards examining whether there is an association between the volume of the non‐lesioned poststroke hippocampus and sensorimotor impairment using a large cross‐sectional data set. In this study, we aimed to investigate the relationship between sensorimotor impairment and ipsilesional and contralesional hippocampal volumes (separately) in 357 participants with chronic stroke across 18 cohorts from the ENIGMA (Enhancing NeuoImaging Genetics through Meta‐Analysis) Stroke Recovery Working Group. 23 Because of the heterogeneity of poststroke brain reorganization across individuals, large consortium‐based multisite studies such as the ENIGMA Stroke Recovery Working Group are important for achieving large and diverse samples that can identify associations that may have otherwise been undetectable in a smaller single‐site sample. 24 In addition, the diversity of data allows us to verify whether associations are maintained beyond a single cohort, improving the robustness and generalizability of research findings. First, we investigated associations between sensorimotor impairment and hippocampal volume, controlling for lesion size and additional covariates of age, sex, and lesioned hemisphere. The Fugl‐Meyer Assessment of Upper Extremity (FMA‐UE) was used as a measure of sensorimotor impairment of the paretic upper limb. 25 We hypothesized that greater poststroke sensorimotor impairment would be correlated with smaller ipsilesional but not contralesional hippocampal volume. We hypothesized, based on the involvement of the hippocampus in sensorimotor circuits, that the association between ipsilesional hippocampal volume and sensorimotor impairment would be independent of lesion size. Second, in an exploratory analysis, we tested to see whether sex had a moderating effect on the relationship between sensorimotor impairment and hippocampal volume. Because of more severe hippocampal vulnerability and poorer stroke outcome trends in women, we hypothesized that women would have a stronger relationship between more severe sensorimotor impairment and smaller hippocampal volume than men. Given the lack of consensus in the literature regarding lesion size and hippocampal volume, we independently tested associations between lesion size and hippocampal volume, without the FMA‐UE included in the model. We hypothesized that larger lesion size would be significantly associated with smaller ipsilesional but not contralesional hippocampal volumes. Finally, we also tested to see whether damage to sensorimotor regions specifically was associated with ipsilesional hippocampal volume. We hypothesized that greater damage to sensorimotor regions would be correlated with smaller ipsilesional hippocampal volumes, independent of lesion size.
METHODS
Data Availability
To protect the privacy of research participants, individual subject data used in this study are not available in a public repository. Participating research cohorts vary in public data‐sharing restrictions as determined by the following: (1) ethical review board and consent documents; (2) national and transnational sharing laws; and (3) institutional processes that may require signed data transfer agreements for limited, predefined data use. However, data sharing is possible for new and participating ENIGMA Stroke Recovery Working Group members who agree to the consortium’s ethical standards for data use and upon the submission of a secondary analysis plan for group review. Upon the approval of the proposed analysis plan, access to relevant data is provided contingent on local principal investigator approval, data availability, and compliance with supervening regulatory boards. Deidentified summary data as well as code used for this study can be made available upon reasonable request to the corresponding author.
ENIGMA Stroke Recovery Data Set
A subset of cross‐sectional data from the ENIGMA Stroke Recovery Working Group database (available as of December 15, 2020) was used. Details of the ENIGMA Stroke Recovery procedures and methods are available in Liew and colleagues. 23 The data were derived from 18 research studies (cohorts) conducted at 10 different research institutes in 6 countries (Table 1). Informed consent was obtained from all subjects, and data were collected in compliance with each institution’s local ethical review boards and in accordance with the Declaration of Helsinki.
Table 1.
Demographics for ENIGMA Stroke Recovery Working Group Participants Included in the Study by Cohort
| Cohort | Total No. (women/men) |
Median age (y) (IQR, min–max) |
Median FMA‐UE (IQR, min–max) |
Median lesion size (cm3) (IQR, min–max) |
|---|---|---|---|---|
| Cohort 1 | 39 (10/29) | 61 (17, 31–80) | 43 (16, 0–58) | 6.1 (20.3, 0.04–120.8) |
| Cohort 2 | 12 (6/6) | 69.5 (12, 39–85) | 33 (27, 13–48) | 28.3 (28.5, 4.2–137.4) |
| Cohort 3 | 15 (6/9) | 61 (17, 33–85) | 16 (13, 5–40) | 21.1 (68.7, 0.6–182.2) |
| Cohort 4 | 19 (6/13) | 44 (15, 30–68) | 10 (11, 1–34) | 35.8 (54.4, 4.5–313.5) |
| Cohort 5 | 28 (12/16) | 64 (18, 44–81) | 52 (33, 8–65) | 1.9 (25.7, 0.1–237.7) |
| Cohort 6 | 10 (3/7) | 61 (12.5, 49–72) | 65 (3, 45–65) | 1.4 (1.1, 0.5–9.1) |
| Cohort 7 | 14 (5/9) | 58 (12, 45–69) | 63 (14, 6–65) | 2.0 (2.9, 0.04–6.9) |
| Cohort 8 | 11 (4/7) | 56 (12, 45–74) | 48 (15, 25–55) | 35.8 (50.2, 0.7–103.9) |
| Cohort 9 | 11 (3/8) | 59 (3, 45–68) | 38 (18, 15–49) | 2.6 (21.7, 0.7–53.7) |
| Cohort 10 | 8 (4/4) | 58 (8, 46–73) | 48 (16, 35–59) | 28.4 (43.2, 0.4–59) |
| Cohort 11 | 22 (6/16) | 61.5 (11, 23–75) | 49 (22, 23–64) | 5.6 (41.5, 0.4–201.4) |
| Cohort 12 | 13 (4/9) | 57 (13, 32–80) | 54 (15, 38–63) | 4.8 (18.2, 0.3–98) |
| Cohort 13 | 12 (4/8) | 66 (16, 31–83) | 51 (26, 19–62) | 4.4 (37.6, 0.2–107.5) |
| Cohort 14 | 29 (18/11) | 50 (15, 25–79) | 41 (13, 24–53) | 12.1 (28.6, 0.1–143.6) |
| Cohort 15 | 10 (3/7) | 61.5 (11, 42–76) | 29 (16, 11–60) | 9.1 (23.4, 3–186.1) |
| Cohort 16 | 40 (14/26) | 66.5 (11, 43–93) | 47 (30, 4–65) | 9.2 (26.1, 0.5–111.8) |
| Cohort 17 | 36 (15/21) | 70 (14, 37–80) | 53 (27, 8–65) | 7.6 (29.3, 0.3–188.4) |
| Cohort 18 | 28 (12/16) | 64 (14, 34–85) | 27 (5, 14–34) | 5 (29.4, 0.7–136.9) |
| Total | 357 (135/222) | 61 (18, 23–93) | 41 (28, 0–65) | 7.6 (33.4, 0.04–313.5) |
Total sample size (N), number of women and men, and information about age (years), Fugl‐Meyer Assessment of Upper Extremity (FMA‐UE), and raw lesion size in cubic centimeters (cm3) are listed. For more information regarding cohort demographics by sex, see Tables S1 and S2. ENIGMA indicates Enhancing NeuoImaging Genetics through Meta‐Analysis; and IQR, interquartile range.
ENIGMA Stroke Recovery participants with the following data were included: (1) high‐resolution (1‐mm isotropic) T1‐weighted brain MRI (T1w) acquired with a 3T MRI scanner; (2) Fugl‐Meyer Assessment of Upper Extremity score (FMA‐UE; acquired on a scale from 0 to 66: 0=severe sensorimotor impairment, 66=no sensorimotor impairment); (3) age; and (4) sex. Because we were interested in studying effects of secondary degeneration of the hippocampus, we only included participants with chronic stroke (defined as data acquired at least 180 days poststroke 26 ). Behavioral data were collected within ≈72 hours of the MRI. Exclusion criteria included site‐reported bilateral, brainstem, or cerebellar lesions; participants with no identifiable lesions; and participants with no sensorimotor impairment (FMA‐UE=66). In addition, each hippocampus was visually inspected with lesion masks overlaid, and any brains with hippocampal lesions were excluded. The total initial sample size was N=357 (age: median=61 years, interquartile range=18, range=23–93; FMA‐UE: median=41, interquartile range=28, range=0–65; 135 women and 222 men) (Table 1).
MRI Data Analysis
Hippodeep, an automated convolutional neural network‐based hippocampal segmentation algorithm, was used to segment ipsilesional and contralesional hippocampal volumes as well as estimated total head size from the T1‐weighted MRI scan. 27 Hippodeep was previously found to be the most robust out of the freely available methods for segmenting the hippocampus in people with stroke pathology. 28 Hippocampal segmentations were visually inspected according to previously described protocols. 23 , 28 Any segmentations that were not properly segmented were marked as failed and excluded from the analysis. This resulted in different sample sizes for the ipsilesional and contralesional analyses. More information on demographics of samples after quality control can be found in Tables S1 and S2. We performed a supplemental analysis using only participants with hippocampal segmentations that passed quality control for both ipsilesional and contralesional hippocampi and confirmed that differences in sample sizes did not significantly influence the results (Tables S3 through S6). To account for differences in head size, hippocampal volume was normalized for head size by taking the ratio of hippocampal volume to head size for each participant and multiplying it by the average head size across the sample, as done in previous studies of poststroke hippocampal volume. 7 , 8
Manually Segmented Lesions
Lesions were manually segmented on the T1w MRI by B.L., M.D., J.S., A.Z.P., and S.‐L.L. according to an updated version of the Anatomical Tracings of Lesions After Stroke (ATLAS) protocol. 29 Briefly, brain lesions were identified, and masks were manually drawn on each individual brain in native space using ITK‐Snap. 30 Each lesion was checked for quality by at least 2 different tracers. An expert neuroradiologist (G.B.) was also consulted to ensure lesion segmentation accuracy. The ATLAS protocol for manually tracing stroke lesions has been technically validated, with high inter‐ and intrarater reliability. More details on the criteria used to manually trace lesions, including links to the lesion‐tracing protocol, can be found in Liew and colleagues. 29 The sample includes 81 participants with cortical lesions, 100 participants with subcortical lesions, and 176 participants with mixed cortical and subcortical lesions. Although all participants were listed by the providing research sites as having unilateral lesions, additional secondary lesions were discovered in 100 participants during manual tracing, which were likely silent, subclinical, and/or prior strokes. Secondary lesions were found in both hemispheres, the brainstem, and the cerebellum, and ranged in size. For this article, we refer to the primary lesioned hemisphere as the lesioned hemisphere noted by the research site. We also performed follow‐up analyses excluding participants with any identified secondary lesions (Figure S1), which did not significantly impact results (Tables S7 and S8). Lesion probability maps were generated by nonlinearly normalizing lesion masks and registering them to the MNI‐152 template (Figure 1).
Figure 1. Lesion density maps for primary lesions from participants with cohort‐reported left and right hemisphere lesions are overlaid on a coronal (left) and axial (right) slice of the Montreal Neurological Institute (MNI) MNI‐152 template.

Lesioned hemisphere refers to the primary lesion, as reported by the research cohort. The color bar refers to the percentage of overlapping lesions across participants.
Finally, lesion volume was calculated by summing the voxels within each lesion mask. Lesion size was also normalized for head size as previously described for hippocampal volume in MRI Data Analysis Section. Lesion size was then log transformed to normalize the distribution of the data.
Sensorimotor Damage
Sensorimotor damage was estimated by calculating the percentage of lesion voxels that overlapped with the sensorimotor cortex, supplementary motor cortex, thalamus, or the basal ganglia, as calculated using the open‐source software Pipeline for Analyzing Lesions after Stroke (PALS 31 ). Briefly, T1w images and their accompanying lesion masks were nonlinearly registered to the MNI‐152 template. Regions of interest reflecting the sensorimotor cortex, supplementary motor cortex, thalamus, and basal ganglia were identified using the Desikan‐Killiany Atlas. 32 Sensorimotor cortex and supplementary motor cortex were estimated using the pre‐ and postcentral gyrus. The basal ganglia were estimated using the caudate, pallidum, putamen, and nucleus accumbens. The number of lesioned voxels in the sensorimotor cortex, supplementary motor cortex, thalamus, and basal ganglia was summed and divided by the sum of the size of each sensorimotor region. This value was then multiplied by 100 to create a percent of lesion overlap with sensorimotor regions, reflecting sensorimotor damage.
Statistical Analysis
Hippocampal Volume and Sensorimotor Impairment
We first tested our primary hypothesis that more severe poststroke sensorimotor impairment is correlated with smaller ipsilesional but not contralesional hippocampal volumes by performing robust mixed‐effects linear regressions with hippocampal volume as the dependent variable (see Model 1). Sensorimotor impairment was measured using the FMA‐UE. 33 Sex (coded as a binary variable: women=0, men=1), age, and lesioned hemisphere (coded as binary variable: left hemisphere lesion=0.5, right hemisphere lesion=1.5) were included in the model as fixed effects and cohort was included in the model as a random effect (Model 1):
| (1) |
Next, we tested our exploratory hypothesis that sex may have a moderating effect on the relationship between sensorimotor impairment and hippocampal volume by including an FMA‐UE×Sex interaction covariate as a fixed effect (Model 2):
| (2) |
Sex differences in sensorimotor impairment, lesion size, and age were tested using an independent t test.
Finally, we tested our hypothesis that sensorimotor impairment is independently associated with hippocampal volume regardless of lesion size by including lesion size as a fixed covariate (Model 3):
| (3) |
Associations Between Lesion Size and Hippocampal Volume
To investigate associations between lesion size and hippocampal volume, regardless of sensorimotor impairment, we performed a robust mixed‐effects regression with ipsilesional and contralesional hippocampal volume as dependent variables with lesion size, age, sex, and lesioned hemisphere as fixed effects, and cohort as a random effect (Model 4):
| (4) |
Associations Between Sensorimotor Damage and Hippocampal Volume
We investigated our hypothesis that greater sensorimotor‐specific lesion damage would be associated with smaller ipsilesional hippocampal volumes with a robust mixed‐effects regression with ipsilesional hippocampal volume as the dependent variable and percent of sensorimotor damage, age, sex, and lesioned hemisphere as fixed effects, and cohort as a random effect (Model 5):
| (5) |
We also tested the hypothesis that sensorimotor damage is independently associated with hippocampal volume regardless of lesion size by including lesion size as a fixed covariate (Model 6):
| (6) |
Statistical Tools
All statistical analyses were performed in R (version 4.0.2 33 ). The Mahalanobis distance was used to detect multivariate outliers, 34 which were then removed from the analyses (Data S1). All continuous measures were normalized using the scale function in R and analyzed as z‐scores to calculate standardized β coefficients. All mixed‐effects regressions were initially run as linear mixed‐effects regressions (lmer function from nlme package). To ensure that there was no redundancy in the included independent variables, collinearity for variables in every model tested was ruled out (variance inflation factor ≤2.5; Tables S9 and S10). Regression assumptions of linearity, normality of the residuals, and homogeneity of the residual variance were tested by visually inspecting residuals versus fits plots as well as qq‐plots. After detecting influential observations using Cook’s distance in each analysis, 35 the analyses were repeated using robust mixed‐effects regression. Robust mixed‐effects regression (rlmer from the robustlmm package) avoids excluding data by reducing the weight of influential observations. 36 We therefore report the results of the robust mixed‐effects regression. For all analyses, β coefficients for the factor of interest and CIs (β [CI]), SE), and uncorrected P values are reported. For each analysis, a Bonferroni correction was applied for 2 comparisons (ipsilesional, contralesional; corrected P<0.025). Effect sizes were mapped onto a template of the hippocampus to visualize the results using ggseg. 37
RESULTS
Hippocampal Volume and Sensorimotor Impairment
Greater sensorimotor impairment was significantly associated with smaller ipsilesional (β=0.16, P=0.005, R 2=0.27) but not contralesional (β=0.003, P=0.96, R 2=0.29) hippocampal volume after adjusting for age, sex, lesioned hemisphere, and cohort (Table S11). When including FMA‐UE×Sex interaction as a covariate, we observed a better model fit and an increase in effect size for the association between sensorimotor impairment and ipsilesional hippocampal volume (β=0.31, P<0.001, R 2=0.30; Table 2). Furthermore, FMA‐UE remained independently associated with ipsilesional hippocampal volume after including lesion size in the model (β=0.26, P=0.001, R 2=0.33; Table 3, Figure 2). This association remained significant when excluding participants with secondary lesions (β=0.30, P=0.001, R 2=0.35; Table S7).
Table 2.
Summary Statistics From Robust Mixed‐Effects Linear Regression to Test Associations Between Ipsilesional Hippocampal Volume and Sensorimotor Impairment and Contralesional Hippocampal Volume and Sensorimotor Impairment When Including a Sensorimotor Impairment and Sex Interaction
| Hippocampus~FMA‐UE×Sex+FMA‐UE+sex+lesioned hemisphere+age+random (cohort) | |||
|---|---|---|---|
| Covariates | β (CI) | SE | P value |
| Ipsilesional hippocampal volume (N=336; R 2=0.30) | |||
| FMA‐UE* | 0.31 (0.15 to 0.46)* | 0.08* | <0.001* |
| FMA‐UE×sex* | −0.26 (−0.46 to −0.07)* | 0.10* | 0.009* |
| Sex* | −0.53 (−0.73 to −0.33)* | 0.10* | <0.001* |
| Lesioned hemisphere | 0.17 (−0.03 to 0.37) | 0.10 | 0.09 |
| Age* | −0.32 (−0.42 to −0.22)* | 0.05* | <0.001* |
| Contralesional hippocampal volume (N=349; R 2=0.32) | |||
| FMA‐UE | 0.16 (0.01 to 0.31) | 0.08 | 0.04 |
| FMA‐UE×sex* | −0.27 (−0.46 to −0.08)* | 0.10* | 0.006* |
| Sex* | −0.51 (−0.70 to −0.32)* | 0.10* | <0.001* |
| Lesioned hemisphere* | −0.35 (−0.54 to −0.16)* | 0.10* | <0.001* |
| Age* | −0.41 (−0.51 to −0.32)* | 0.05* | <0.001* |
The full model as well as the sample size (N), conditional R 2, β coefficient (β) with 95% CI, SE, and uncorrected P value for all fixed‐effect covariates are reported. FMA‐UE indicates Fugl‐Meyer Assessment of Upper Extremity.
Significant covariates.
Table 3.
Summary Statistics From Robust Mixed‐Effects Linear Regression to Test Associations Between Ipsilesional Hippocampal Volume and Sensorimotor Impairment and Contralesional Hippocampal Volume and Sensorimotor Impairment When Including Lesion Size as a Covariate
| Hippocampus~lesion size+FMA‐UE×Sex+FMA‐UE+sex+lesioned hemisphere+age+random (cohort) | |||
|---|---|---|---|
| Covariates | β (CI) | SE | P value |
| Ipsilesional hippocampal volume (N=336; R 2=0.33) | |||
| FMA‐UE* | 0.26 (0.10 to 0.41)* | 0.08* | 0.001* |
| FMA‐UE×sex* | −0.26 (−0.45 to −0.07)* | 0.10* | 0.008* |
| Lesion size* | −0.19 (−0.29 to −0.09)* | 0.05* | <0.001* |
| Sex* | −0.58 (−0.78 to −0.39)* | 0.10* | <0.001* |
| Lesioned hemisphere | 0.17 (−0.03 to 0.36) | 0.10 | 0.09 |
| Age* | −0.36 (−0.46 to −0.26)* | 0.05* | <0.001* |
| Contralesional hippocampal volume (N=349; R 2=0.32) | |||
| FMA‐UE | 0.15 (0.00 to 0.30) | 0.08 | 0.05 |
| FMA‐UE×sex* | −0.27 (−0.46 to −0.08)* | 0.10* | 0.006* |
| Lesion size | −0.03 (−0.13 to 0.07) | 0.05 | 0.58 |
| Sex* | −0.52 (−0.71 to −0.33)* | 0.10* | <0.001* |
| Lesioned hemisphere* | −0.35 (−0.54 to −0.16)* | 0.10* | <0.001* |
| Age* | −0.42 (−0.52 to −0.32)* | 0.05* | <0.001* |
The full model as well as the sample size (N), conditional R 2, β coefficient (β) with 95% CI, SE, and uncorrected P value for all fixed‐effect covariates are reported. FMA‐UE indicates Fugl‐Meyer Assessment of Upper Extremity.
Significant covariates.
Figure 2. Effect sizes (standardized β values) for ipsilesional and contralesional hippocampi are mapped onto a template for associations between hippocampal volumes and sensorimotor impairment (top left) and lesion size (bottom left), with warmer colors representing stronger positive associations.
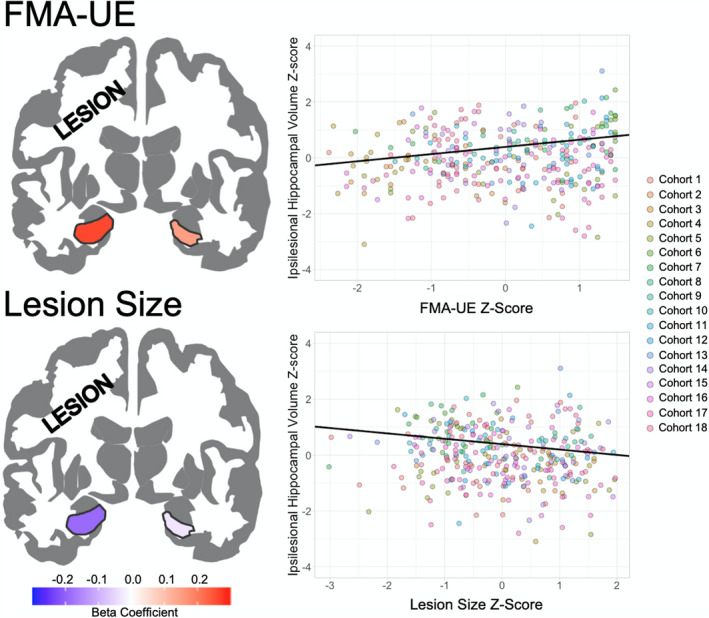
Trend lines (black line) are plotted for the association between ipsilesional hippocampal volume z‐scores with Fugl‐Meyer Assessment of Upper Extremity (FMA‐UE) z‐scores (top right) and lesion size z‐scores (bottom right). Scatterplot points are colored by research cohort.
Sex Effects on the Association Between Hippocampal Volume and Sensorimotor Impairment
A t test revealed no significant differences in FMA‐UE (t[260])=1.13, P=0.26) or age (t[249]=1.12, P=0.26) between women and men. Women did have significantly larger lesions than men (t[277]=2.9, P=0.004) (Figure 3). The FMA‐UE×Sex interaction was a significant covariate for both ipsilesional (β=−0.26, P=0.009, R 2=0.30) and contralesional (β=−0.27, P=0.006, R 2=0.32) hippocampal volumes (Figure 3, Table 2), even after accounting for lesion size (ipsilesional: β=−0.26, P=0.008, R 2=0.33; contralesional: β=−0.27, P=0.006, R 2=0.32; Table 3). In the ipsilesional hippocampus, women had a positive slope (β=0.26) and men had a negative slope close to 0 (β=−0.002). In the contralesional hippocampus, women had a positive slope (β=0.15) while men had a negative slope (β=−0.12) (Figure 3). The FMA‐UE×Sex interaction remained significantly associated with both ipsilesional (β=−0.31, P=0.008, R 2=0.35) and contralesional (β=−0.28, P=0.017, R 2=0.32) hippocampal volumes, even when excluding participants with secondary lesions (Table S7).
Figure 3. Trend lines are plotted for the association between Fugl‐Meyer Assessment of Upper Extremity (FMA‐UE) z‐score (x‐axis) and hippocampal volumes z‐score (y‐axis) for women (red) and men (blue) calculated from the FMA‐UE×Sex interactions.

Histograms for FMA‐UE scores (bottom left), age (bottom middle), and lesion size (bottom right) are plotted by sex (women in red and men in blue).
Hippocampal Volume and Lesion Size
Larger lesion size was significantly associated with smaller ipsilesional (β=−0.21, P<0.001, R 2=0.33) but not contralesional hippocampal volume (β=−0.03, P=0.60, R 2=0.30), after adjusting for age, sex, lesioned hemisphere, and cohort (Table S12; Figure 2). Lesion size remained significantly associated with smaller ipsilesional, but not contralesional, hippocampal volume, even when excluding participants with secondary lesions (β=−0.18, P=0.003, R 2=0.33; Table S8).
Hippocampal Volume and Sensorimotor Damage
More sensorimotor damage was significantly associated with a smaller ipsilesional hippocampal volume (β=−0.15, P=0.003, R 2=0.30; Table S13). However, this association did not remain significant after including lesion size in the model (β=−0.03, P=0.66, R 2=0.32; Table S14).
DISCUSSION
In this study, associations between sensorimotor impairment, lesion size, sex, and hippocampal volume were investigated in participants with chronic stroke from 18 research cohorts in the ENIGMA Stroke Recovery Working Group. 23 Greater sensorimotor impairment and larger lesion sizes were both significantly associated with smaller ipsilesional hippocampal volumes, and the association between sensorimotor impairment and hippocampal volume was stronger in women than in men.
To our knowledge, this is the first study to report associations between hippocampal volume and sensorimotor impairment in patients with chronic stroke. Greater sensorimotor impairment was independently associated with smaller ipsilesional hippocampal volume, even after adjusting for lesion size. This suggests that poststroke ipsilesional hippocampal integrity may be related to sensorimotor impairment. SD might explain the sensitivity of stroke‐related ipsilesional hippocampal atrophy, where damaging neurotoxic signals from the core of the lesion propagate to adjacent gray matter regions. 6 , 7 , 8 The blockage of blood supply during the acute phase of a stroke leads to an ionic imbalance that causes a buildup of extracellular glutamate, triggering a self‐propagating wave of cell depolarization throughout neighboring gray matter. 38 The hippocampus is filled with tightly packed, easily excitable glutamatergic neurons and a high density of N‐methyl‐d‐aspartate receptors, 39 making it more susceptible to damage from SD. Overexcitation of the hippocampal glutamatergic network leads to hippocampal excitotoxicity, resulting in hippocampal neuron apoptosis, which is thought to be reflected on a macroscale as reduced hippocampal volume. 8 The damaging effects of SD are likely more prominent in the lesioned hemisphere because SD waves do not propagate easily through white matter 38 ; therefore, the waves cannot easily traverse to the contralesional hippocampus. While a magnetic resonance spectroscopy study reported evidence of hippocampal neuronal loss in the contralesional hippocampus, it was less severe and not detectable using volumetric MRI. 8 SD still is not well understood; therefore, contralesional hippocampal damage may be caused by mild SD or might be attributed to other forms of secondary degeneration such as diaschisis. 5 The available evidence is insufficient to support SD as the key cause of reduced ipsilesional hippocampal volumes observed in this study, and there are several other mechanisms that may explain stroke‐related hippocampal damage such as chronic inflammation. 40 However, these findings could provide future directions for research investigating the mechanisms of stroke‐related hippocampal damage.
One question is whether the smaller hippocampal volumes observed is reflective of general atrophy of regions across the entire ipsilesional hemisphere, or if it is specific to the hippocampus. Although the current work cannot directly address this, because Hippodeep only provides measures of hippocampal volume and total brain volume, recent work examined relationships between the volumes of 5 subcortical regions associated with sensorimotor processing (ie, thalamus, caudate, putamen, pallidum, and nucleus accumbens) in relation to sensorimotor impairment after stroke. 4 In chronic stroke, only the ipsilesional putamen was significantly associated with sensorimotor impairment specifically, suggesting that volumes of distinct regions are related with sensorimotor deficits. Thus, it is likely that the hippocampal association is a true relationship and not reflective of general ipsilesional atrophy.
We initially hypothesized that in addition to hippocampal damage incurred by SD, ipsilesional disruption to sensorimotor circuits may cause secondary degeneration of the ipsilesional hippocampus, possibly as a result of anatomical connectivity to damaged areas (eg, through the thalamus, 15 basal ganglia, 12 sensorimotor cortex, 14 or supplementary motor area 16 ) via anterograde degeneration. While we found a significant relationship between extent of sensorimotor damage and ipsilesional hippocampal volume, this relationship became insignificant once lesion size was added into the model. This suggests that smaller hippocampal volumes are likely not attributed to secondary degeneration derived from ipsilesional disruption to sensorimotor circuits specifically. Further structural and functional connectivity research as well as longitudinal investigations may provide a more complete picture of the role of hippocampal damage in sensorimotor circuitry.
Furthermore, because the hippocampus is an important limbic system structure, it is heavily involved in learning, memory, and emotion. 39 Poststroke cognitive impairment, depression, and anxiety are all common pervasive symptoms in stroke survivors that interfere with rehabilitation and are associated with poor stroke outcomes. 41 Limbic system disruption caused by secondary poststroke hippocampal damage may cause cognitive impairment or aggravate symptoms of depression and anxiety, which in turn, may interfere with stroke sensorimotor rehabilitation efforts. Further functional and longitudinal research is necessary to understand the relationship between hippocampal damage and sensorimotor circuits and how hippocampal volume loss may impact sensorimotor rehabilitation.
In an exploratory analysis, we found significant sex differences in the association between FMA‐UE and bilateral hippocampal volume, where women showed progressively greater sensorimotor impairment with smaller hippocampal volumes compared with men. This observation suggests that women with greater sensorimotor impairment may also have more hippocampal damage or more pre‐existing hippocampal atrophy compared with men. In addition, sex differences observed in the association between sensorimotor impairment and hippocampal volume did not appear to be driven by age or severity of sensorimotor impairment. Although lesion size was significantly larger in women, the FMA‐UE×Sex interaction covariate was independently associated with hippocampal volume, even when accounting for lesion size. Overall, these findings should be considered exploratory given the unequal number of men and women in the sample. Further research is needed to confirm these findings, because our sample was unable to account for additional variables thought to influence the hippocampus in a sex‐dependent way such as estrogen levels, 19 dementia, 17 and depression. 42 Additionally, the current sample does not have information about race, which is known to affect stroke risk factors, possibly in a sex‐dependent way. 43 Furthermore, the extent to which sex differences observed in stroke research are a result of physiological differences between sexes versus different contextual factors such as treatment received by women poststroke remains unclear. 21 For example, a recent meta‐analysis showed that women are less likely to receive thrombolytic treatment thought to improve stroke outcomes, compared with men. 44 Further research on sex differences in stroke is crucial to improve our understanding of the relationship between hippocampal damage and sensorimotor impairment.
Lastly, we found that larger lesion sizes were significantly associated with smaller hippocampal volumes, but only within the lesioned hemisphere, independent of sensorimotor impairment. This finding is in line with a previous study 7 and may indicate that smaller hippocampal volumes observed in patients with stroke may be specific to the amount of stroke‐related damage within the lesioned hemisphere beyond that which is attributed to age‐related atrophy 39 or other stroke risk factors such as hypertension 45 or changes in estrogen 19 that are typically observed bilaterally.
Limitations and Future Directions
This study only considered gross hippocampal volume. However, the hippocampus is composed of structurally and functionally distinct subfields, each differentially vulnerable to disease. 46 Structurally, reduced neuron density has been observed in the cells of the CA1 subfield of postmortem patients with stroke when compared with controls, 47 and larger white matter hyperintensity volume has been associated with a smaller hippocampal‐amygdala transition area. 48 Functionally, while the posterior extents of the hippocampus along the long axis are thought to be more involved with memory and cognitive processing, 49 the anterior extents have been implicated in sensorimotor integration. 39 Further research investigating sensorimotor impairment and the hippocampus at a finer resolution, such as at the level of hippocampal subfields 49 or vertex‐wise associations, 50 may reveal more specific and robust relationships that can better inform the understanding of the impact of hippocampal damage on recovery and rehabilitation.
One limitation of the Hippodeep software used in this study is that it only generates measurements for hippocampal volume and total head size and does not provide information about other brain regions such as cortical and subcortical volumes. Further research is necessary to compare the strength of associations between sensorimotor impairment and volumes of other cortical and subcortical regions in relation to associations with hippocampal volume.
In addition, although secondary lesions were discovered while manually tracing lesion masks, our findings did not change when participants with secondary lesions were excluded. Additionally, our preliminary results on lesion location only investigated sensorimotor region damage. As mentioned previously, the hippocampus is a densely connected region and further research is necessary to investigate the impact of lesion location on the association between hippocampal volume and sensorimotor impairment.
Given the focus on hippocampal volumes, another limitation of this study is the lack of cognitive and depression data. While cognitive and depressive scores are available for a small number of cohorts in the ENIGMA Stroke Recovery database, the participants with available data have very limited information. In addition, many of the participating ENIGMA Stroke Recovery research cohorts used cognitive impairment as an exclusion criterion, which is a fairly common practice in sensorimotor rehabilitation research, 51 resulting in participants with no or mild cognitive deficits. However, given the high prevalence of poststroke dementia, 52 future prospective studies collecting both sensorimotor and cognitive behavioral information across a range of impairment levels are necessary to improve our understanding of how poststroke cognitive impairment may interfere with sensorimotor rehabilitation. 53
Finally, the current sample is cross‐sectional and cannot account for the extent of longitudinal hippocampal atrophy that may have occurred as a result of stroke, mild cognitive impairment, pre‐existing dementia, or normal aging. Additionally, without longitudinal data, we cannot investigate the directionality of the association between sensorimotor impairment and hippocampal volume. Our findings reflect a cross‐sectional association, which does not imply causality. Future well‐powered longitudinal research should examine causal relationships between these 2 factors.
This sample also does not contain data on type or dose of rehabilitation treatment received, which could also influence sensorimotor outcomes. However, the current cross‐sectional analysis serves as a first step to examining the relationship between hippocampal volumes, sensorimotor impairment, lesion volume, and sex and can be used to guide future questions using a longitudinal data set.
CONCLUSIONS
Our findings demonstrate a novel association between chronic poststroke sensorimotor impairment and hippocampal volume that may be modulated by sex. We provide supporting evidence to existing literature that reduced hippocampal volume is likely a consequence of stroke‐related damage within the lesioned hemisphere. Overall, these findings provide unique insight into the role that the hippocampus may play in poststroke sensorimotor impairment.
Sources of Funding
S.‐L.L. is supported by NIH K01 HD091283; NIH R01 NS115845. A.B. and M.S.K. are supported by National Health and Medical Research Council (NHMRC) GNT1020526, GNT1045617 (A.B.), GNT1094974, and Heart Foundation Future Leader Fellowship 100784 (A.B.). P.M.T. is supported by NIH U54 EB020403. L.A.B. is supported by the Canadian Institutes of Health Research (CIHR). C.M.B. is supported by NIH R21 HD067906. W.D.B. is supported by the Heath Research Council of New Zealand. J.M.C. is supported by NIH R00HD091375. A.B.C. is supported by NIH R01NS076348‐01, Hospital Israelita Albert Einstein 2250‐14, CNPq/305568/2016‐7. A.N.D. is supported by funding provided by the Texas Legislature to the Lone Star Stroke Clinical Trial Network. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Government of the United States or the State of Texas. N.E.‐B. is supported by Australian Research Council NIH DE180100893. W.F. is supported by NIH P20 GM109040. F.G. is supported by Wellcome Trust (093957). B.H. is funded by and NHMRC fellowship (1125054). S.A.K is supported by NIH P20 HD109040. F.B. is supported by Italian Ministry of Health, RC 20, 21. N.S. is supported by NIH R21NS120274. N.J.S. is supported by NIH/National Institute of General Medical Sciences (NIGMS) 2P20GM109040‐06, U54‐GM104941. S.R.S. is supported by European Research Council (ERC) (NGBMI, 759370). G.S. is supported by Italian Ministry of Health RC 18‐19‐20‐21A. M.T. is supported by National Institute of Neurological Disorders and Stroke (NINDS) R01 NS110696. G.T.T. is supported by Temple University sub‐award of NIH R24 –NHLBI (Dr Mickey Selzer) Center for Experimental Neurorehabilitation Training. N.J.S. is funded by NIH/National Institute of Child Health and Human Development (NICHD) 1R01HD094731‐01A1.
Disclosures
Amy Brodtmann is on the editorial boards of Neurology and International Journal of Stroke and sits on a Biogen Australia Scientific Advisory Committee. Steven C. Cramer serves as a consultant for Abbvie, Constant Therapeutics, MicroTransponder, Neurolutions, SanBio, Panaxium, NeuExcell, Elevian, Medtronic, and TRCare. Brenton Hordacre has a clinical partnership with Fourier Intelligence. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S14
Figure S1
Acknowledgments
We thank Sophia Thomopoulos for her assistance.
Preprint posted on BioRxiv October 28, 2021. doi: https://doi.org/10.1101/2021.10.26.465924
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025109
For Sources of Funding and Disclosures, see page 12.
References
- 1. Gittler M, Davis AM. Guidelines for adult stroke rehabilitation and recovery. JAMA. 2018;319:820. doi: 10.1001/jama.2017.22036 [DOI] [PubMed] [Google Scholar]
- 2. Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, Carter AR, Leff AP, Copland DA, Carey LM, et al. Biomarkers of stroke recovery: consensus‐based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2017;12:480–493. doi: 10.1177/1747493017714176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stinear CM, Stinear C, Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol. 2017;16:826–836. doi: 10.1016/S1474-4422(17)30283-1 [DOI] [PubMed] [Google Scholar]
- 4. Liew S‐L, Zavaliangos‐Petropulu A, Schweighofer N, Jahanshad N, Lang CE, Lohse KR, Banaj N, Barisano G, Baugh LA, Bhattacharya AK, et al. Smaller spared subcortical nuclei are associated with worse post‐stroke sensorimotor outcomes in 28 cohorts worldwide. Brain Commun. 2021;3:fcab254. doi: 10.1093/BRAINCOMMS/FCAB254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Zhang Y, Xing S, Liang Z, Zeng J. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management? Stroke. 2012;43:1700–1705. doi: 10.1161/STROKEAHA.111.632448 [DOI] [PubMed] [Google Scholar]
- 6. Xie M, Yi C, Luo X, Xu S, Yu Z, Tang Y, Zhu W, Du Y, Jia L, Zhang Q, et al. Glial gap junctional communication involvement in hippocampal damage after middle cerebral artery occlusion. Ann Neurol. 2011;70:121–132. doi: 10.1002/ana.22386 [DOI] [PubMed] [Google Scholar]
- 7. Schaapsmeerders P, van Uden IWM, Tuladhar AM, Maaijwee NAM, van Dijk EJ, Rutten‐Jacobs LCA, Arntz RM, Schoonderwaldt HC, Dorresteijn LDA, de Leeuw F‐E, et al. Ipsilateral hippocampal atrophy is associated with long‐term memory dysfunction after ischemic stroke in young adults. Hum Brain Mapp. 2015;36:2432–2442. doi: 10.1002/hbm.22782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang X, Wang C, Xia L, Zhu W, Zhao L, Zhu W. Volumetric MRI and 1H MRS study of hippocampus in unilateral MCAO patients: relationship between hippocampal secondary damage and cognitive disorder following stroke. Eur J Radiol. 2012;81:2788–2793. doi: 10.1016/j.ejrad.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 9. Brodtmann A, Khlif MS, Egorova N, Veldsman M, Bird LJ, Werden E. Dynamic regional brain atrophy rates in the first year after ischemic stroke. Stroke. 2020;51:183–192. doi: 10.1161/STROKEAHA.120.030256 [DOI] [PubMed] [Google Scholar]
- 10. Werden E, Cumming T, Li Q, Bird L, Veldsman M, Pardoe HR, Jackson G, Donnan GA, Brodtmann A. Structural MRI markers of brain aging early after ischemic stroke. Neurology. 2017;89:116–124. doi: 10.1212/WNL.0000000000004086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Dang G, Dang C, Liu G, Xing S, Chen Y, Xu Q, Zeng J. An ischemic stroke model of nonhuman primates for remote lesion studies: a behavioral and neuroimaging investigation. Restor Neurol Neurosci. 2015;33:131–142. doi: 10.3233/RNN-140440 [DOI] [PubMed] [Google Scholar]
- 12. Maller JJ, Welton T, Middione M, Callaghan FM, Rosenfeld JV, Grieve SM. Revealing the hippocampal connectome through super‐resolution 1150‐direction diffusion MRI. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-018-37905-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki WA. Integrating associative learning signals across the brain. Hippocampus. 2007;17:842–850. doi: 10.1002/hipo.20321 [DOI] [PubMed] [Google Scholar]
- 14. Burman DD. Hippocampal connectivity with sensorimotor cortex during volitional finger movements: laterality and relationship to motor learning. PLoS One. 2019;14:e0222064. doi: 10.1371/journal.pone.0222064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baumgartner P, El Amki M, Bracko O, Luft AR, Wegener S. Sensorimotor stroke alters hippocampo‐thalamic network activity. Sci Rep. 2018;8:15770. doi: 10.1038/s41598-018-34002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single‐neuron responses in humans during execution and observation of actions. Curr Biol. 2010;20:750–756. doi: 10.1016/j.cub.2010.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, Mallampalli MP, Mormino EC, Scott L, Yu WH, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. 2018;14:1171–1183. doi: 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wierenga LM, Doucet GE, Dima D, Agartz I, Aghajani M, Akudjedu TN, Albajes‐Eizagirre A, Alnæs D, Alpert KI, Andreassen OA, et al. Greater male than female variability in regional brain structure across the lifespan. Hum Brain Mapp. 2020;7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albert K, Hiscox J, Boyd B, Dumas J, Taylor W, Newhouse P. Estrogen enhances hippocampal gray‐matter volume in young and older postmenopausal women: a prospective dose‐response study. Neurobiol Aging. 2017;56:1–6. doi: 10.1016/j.neurobiolaging.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pappa T, Vemmos K, Mantzou E, Savvari P, Stamatelopoulos K, Alevizaki M. Estradiol levels predict short‐term adverse health outcomes in postmenopausal acute stroke women. Eur J Neurol. 2012;19:1300–1304. doi: 10.1111/j.1468-1331.2012.03714.x [DOI] [PubMed] [Google Scholar]
- 21. Dehlendorff C, Andersen KK, Olsen TS. Sex disparities in stroke: women have more severe strokes but better survival than men. J Am Heart Assoc. 2015;4:e001967. doi: 10.1161/JAHA.115.001967 doi: 10.1161/JAHA.115.001967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawe RL, Cluff T, Dowlatshahi D, Hill MD, Dukelow SP. Assessment of sex differences in recovery of motor and sensory impairments poststroke. Neurorehabil Neural Repair. 2020;34:746–757. doi: 10.1177/1545968320935811 [DOI] [PubMed] [Google Scholar]
- 23. Liew S‐L, Zavaliangos‐Petropulu A, Jahanshad N, Lang CE, Hayward KS, Lohse KR, Juliano JM, Assogna F, Baugh LA, Bhattacharya AK, et al. The ENIGMA Stroke Recovery Working Group: big data neuroimaging to study brain–behavior relationships after stroke. Hum Brain Mapp. 2020;25:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thompson PM, Ching CRK, Dennis EL, Salminen LE, Turner JA, van Erp TGM, Jahanshad N. Big data initiatives in psychiatry: global neuroimaging studies. In: Kubicki M, Shenton ME, eds. Neuroimaging in Schizophrenia. Springer; 2020:411–426. [Google Scholar]
- 25. See J, Dodakian L, Chou C, Chan V, McKenzie A, Reinkensmeyer D, Cramer S. A standardized approach to the Fugl‐Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27:732–741. doi: 10.1177/1545968313491000 [DOI] [PubMed] [Google Scholar]
- 26. Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, Krakauer JW, Boyd LA, Carmichael ST, Corbett D, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12:444–450. doi: 10.1177/1747493017711816 [DOI] [PubMed] [Google Scholar]
- 27. Thyreau B, Sato K, Fukuda H, Taki Y. Segmentation of the hippocampus by transferring algorithmic knowledge for large cohort processing. Med Image Anal. 2018;43:214–228. doi: 10.1016/j.media.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 28. Zavaliangos‐Petropulu A, Tubi MA, Haddad E, Zhu A, Braskie MN, Jahanshad N, Thompson PM, Liew S‐L. Testing a convolutional neural network‐based hippocampal segmentation method in a stroke population. Hum Brain Mapp. 2022;43:234–243. doi: 10.1002/hbm.25210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liew S‐L, Anglin JM, Banks NW, Sondag M, Ito KL, Kim H, Chan J, Ito J, Jung C, Khoshab N, et al. A large, open source dataset of stroke anatomical brain images and manual lesion segmentations. Sci Data. 2018;5:180011. doi: 10.1038/sdata.2018.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User‐guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 31. Ito KL, Kumar A, Zavaliangos‐Petropulu A, Cramer SC, Liew S‐L. Pipeline for analyzing lesions after stroke (PALS). Front Neuroinform. 2018;12:63. doi: 10.3389/fninf.2018.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 33. Team RC . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018. Available at: https://wwwR‐project.org. Accessed June, 2021. [Google Scholar]
- 34. Huberty CJ. Mahalanobis distance. Wiley StatsRef Stat Ref Online. 2014. doi: 10.1002/9781118445112.STAT06485 [DOI]
- 35. Nieuwenhuis R, Te Grotenhuis M, Pelzer B. Influence.ME: tools for detecting influential data in mixed effects models. R J. 2012;4:38. doi: 10.32614/RJ-2012-011 [DOI] [Google Scholar]
- 36. Greco L, Luta G, Krzywinski M, Altman N. Analyzing outliers: robust methods to the rescue. Nat Methods. 2019;16:275–276. [DOI] [PubMed] [Google Scholar]
- 37. Mowinckel AM, Vidal‐Piñeiro D, Mowinckel AM, Vidal‐Piñeiro D. Visualization of brain statistics with R packages ggseg and ggseg3d. Adv Meth Pract Psychol Sci. 2020;3:466–483. doi: 10.1177/2515245920928009 [DOI] [Google Scholar]
- 38. Chung DY, Oka F, Ayata C. Spreading depolarizations: a therapeutic target against delayed cerebral ischemia after subarachnoid hemorrhage. J Clin Neurophysiol. 2016;33:196. doi: 10.1097/WNP.0000000000000275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nikonenko AG, Radenovic L, Andjus PR, Skibo GG. Structural features of ischemic damage in the hippocampus. Anat Rec. 2009;292:1914–1921. [DOI] [PubMed] [Google Scholar]
- 41. Quinn T, Elliott E, Langhorne P. Cognitive and mood assessment tools for use in stroke. Stroke. 2018;49:483–490. doi: 10.1161/STROKEAHA.117.016994 [DOI] [PubMed] [Google Scholar]
- 42. Shi Y, Zeng Y, Wu L, Liu W, Liu Z, Zhang S, Yang J, Wu W. A study of the brain abnormalities of post‐stroke depression in frontal lobe lesion. Sci Rep. 2017;7:13203. doi: 10.1038/s41598-017-13681-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA, et al. Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurol. 2019;76:179–186. doi: 10.1001/jamaneurol.2018.3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Strong B, Lisabeth LD, Reeves M. Sex differences in IV thrombolysis treatment for acute ischemic stroke: a systematic review and meta‐analysis. Neurology. 2020;95:11–22. doi: 10.1212/WNL.0000000000009733 [DOI] [PubMed] [Google Scholar]
- 45. Fiford CM, Nicholas JM, Biessels GJ, Lane CA, Cardoso MJ, Barnes J. High blood pressure predicts hippocampal atrophy rate in cognitively impaired elders. Alzheimers Dement. 2020:12:e12035. doi: 10.1002/dad2.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu T, Hu R, Qiu X, Taylor M, Tso Y, Yiannoutsos C, Navia B, Mori S, Ekholm S, Schifitto G, et al. Quantification of accuracy and precision of multi‐center DTI measurements: a diffusion phantom and human brain study. NeuroImage. 2011;56:1398–1411. doi: 10.1016/j.neuroimage.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gemmell E, Bosomworth H, Allan L, Hall R, Khundakar A, Oakley AE, Deramecourt V, Polvikoski TM, O’Brien JT, Kalaria RN. Hippocampal neuronal atrophy and cognitive function in delayed poststroke and aging‐related dementias. Stroke. 2012;43:808–814. doi: 10.1161/STROKEAHA.111.636498 [DOI] [PubMed] [Google Scholar]
- 48. Etherton MR, Fotiadis P, Giese AK, Iglesias JE, Wu O, Rost NS. White matter hyperintensity burden is associated with hippocampal subfield volume in stroke. Front Neurol. 2020;11:588883. doi: 10.3389/fneur.2020.588883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khlif MS, Bird LJ, Restrepo C, Khan W, Werden E, Egorova‐Brumley N, Brodtmann A. Hippocampal subfield volumes are associated with verbal memory after first‐ever ischemic stroke. Alzheimers Dement. 2021;13:e12195. doi: 10.1002/dad2.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hibar DP, Adams HHH, Jahanshad N, Chauhan G, Stein JL, Hofer E, Renteria ME, Bis JC, Arias‐Vasquez A, Ikram MK, et al. Novel genetic loci associated with hippocampal volume. Nat Commun. 2017;8:13624. doi: 10.1038/ncomms13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lingo VanGilder J, Hooyman A, Peterson DS, Schaefer SY. Post‐stroke cognitive impairments and responsiveness to motor rehabilitation: a review. Curr Phys Med Rehabil Rep. 2020;8:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leys D, Hénon H, Mackowiak‐Cordoliani M‐A, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0 [DOI] [PubMed] [Google Scholar]
- 53. McDonald MW, Black SE, Copland DA, Corbett D, Dijkhuizen RM, Farr TD, Jeffers MS, Kalaria RN, Karayanidis F, Leff AP, et al. Cognition in stroke rehabilitation and recovery research: consensus‐based core recommendations from the second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2019;14:774–782. doi: 10.1177/1747493019873600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S14
Figure S1
Data Availability Statement
To protect the privacy of research participants, individual subject data used in this study are not available in a public repository. Participating research cohorts vary in public data‐sharing restrictions as determined by the following: (1) ethical review board and consent documents; (2) national and transnational sharing laws; and (3) institutional processes that may require signed data transfer agreements for limited, predefined data use. However, data sharing is possible for new and participating ENIGMA Stroke Recovery Working Group members who agree to the consortium’s ethical standards for data use and upon the submission of a secondary analysis plan for group review. Upon the approval of the proposed analysis plan, access to relevant data is provided contingent on local principal investigator approval, data availability, and compliance with supervening regulatory boards. Deidentified summary data as well as code used for this study can be made available upon reasonable request to the corresponding author.


