Abstract
Background
Heart failure (HF) is the leading cause of mortality and associated with significant morbidity in adults with congenital heart disease. We sought to assess the association between HF and patient‐report outcomes in adults with congenital heart disease.
Methods and Results
As part of the APPROACH‐IS (Assessment of Patterns of Patient‐Reported Outcomes in Adults with Congenital Heart disease—International Study), we collected data on HF status and patient‐reported outcomes in 3959 patients from 15 countries across 5 continents. Patient‐report outcomes were: perceived health status (12‐item Short Form Health Survey), quality of life (Linear Analogue Scale and Satisfaction with Life Scale), sense of coherence‐13, psychological distress (Hospital Anxiety and Depression Scale), and illness perception (Brief Illness Perception Questionnaire). In this sample, 137 (3.5%) had HF at the time of investigation, 298 (7.5%) had a history of HF, and 3524 (89.0%) had no current or past episode of HF. Patients with current or past HF were older and had a higher prevalence of complex congenital heart disease, arrhythmias, implantable cardioverter‐defibrillators, other clinical comorbidities, and mood disorders than those who never had HF. Patients with HF had worse physical functioning, mental functioning, quality of life, satisfaction with life, sense of coherence, depressive symptoms, and illness perception scores. Magnitudes of differences were large for physical functioning and illness perception and moderate for mental functioning, quality of life, and depressive symptoms.
Conclusions
HF in adults with congenital heart disease is associated with poorer patient‐reported outcomes, with large effect sizes for physical functioning and illness perception.
Registration
URL: https://clinicaltrials.gov; Unique identifier: NCT02150603.
Keywords: adult congenital heart disease, heart failure, patient‐reported outcomes, quality of life
Subject Categories: Congenital Heart Disease, Heart Failure
Nonstandard Abbreviations and Acronyms
- APPROACH‐IS
Assessment of Patterns of Patient Reported Outcomes in Adults with Congenital Heart disease ‐ International Study
- PROs
patient‐reported outcomes
- QoL
quality of life
Clinical Perspective
What Is New?
Heart failure is associated with patient‐reported health status, quality of life, sense of coherence, anxiety, depression, and illness perceptions, illustrating the importance of patient‐reported outcomes in understanding the impact of heart failure from a patient perspective.
What Are the Clinical Implications?
Heart failure is an important factor that explains patient‐reported outcomes, and therefore, it is an indispensable covariate in patient‐reported outcomes research in congenital heart disease.
Heart failure (HF) is the leading cause of mortality in adults with congenital heart disease (CHD), 1 with population trends indicating a sharp rise in the proportion of deaths attributable to HF over time. 2 Despite the success in management of CHD which has greatly improved survival, many long‐term survivors possess variable degrees of sequelae and hemodynamic abnormalities. 3 Unlike patients with HF because of acquired heart diseases, adults with CHD often have abnormal baseline cardiovascular function and are subject to lifelong adaptation to their cardiac physiological derangement. Therefore, the clinical presentation and outcomes of HF in adults with CHD are often quite different from those with HF because of acquired heart disease. For example, compared with patients with non‐CHD admitted because of HF, adults with CHD have longer lengths of stay and a higher incidence of arrhythmia and in‐hospital mortality. 4 The substantial symptom burden and high incidence of mortality and morbidity associated with HF may impact their psychosocial status, functional status, and general health perception.
Patient‐reported outcomes (PROs) refer to any report obtained directly from patients about how they feel or function in relation to their health condition and therapy. 5 Through the various domains of PROs, such as patient‐reported health status, health‐related quality of life (QoL), sense of coherence, psychological distress, and illness perceptions, healthcare providers can better understand patient perspectives and deliver more patient‐centered care. Although HF has been associated with poor QoL and psychosocial functioning in adults with acquired heart disease, 6 the psychosocial impact of HF on adults with CHD has not previously been reported. We, therefore, assessed the interaction between HF and PROs and explored correlates of HF in adults with CHD.
Methods
The data underlying this article can be shared on reasonable request to the corresponding author.
Study Population
This study is part of the APPROACH‐IS (Assessment of Patterns of Patient‐Reported Outcomes in Adults with Congenital Heart disease—International Study). 7 APPROACH‐IS is a cross‐sectional study in which data were collected from 24 medical centers across 15 countries: Argentina, Australia, Belgium, Canada, France, India, Italy, Japan, Malta, Norway, Sweden, Switzerland, Taiwan, the Netherlands, and the United States. A total of 4028 patients were enrolled between 2013 and 2015. Patients were eligible if they met all of the following criteria: (1) 18 years of age or older; (2) established diagnosis of CHD before adolescence; and (3) possessing physical, cognitive, and language capabilities required to complete self‐report questionnaires. Excluding criteria consisted of: (1) prior heart transplantation or (2) idiopathic pulmonary hypertension. The study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board of all participating centers. All participants provided informed consent, in line with the national requirements. Detailed information on the rationale, design, and methods of APPROACH‐IS has been previously described 7 and the study protocol was registered at ClinicalTrials.gov: NCT02150603.
Variables and Measurement
Background characteristics pertained to sociodemographic, clinical, and behavioral variables. Age, sex, and patient‐reported New York Heart Association functional class assessment were based on self‐report. Complexity of heart defect (ie, simple, moderate, complex), 8 history of arrhythmias, cardiac device implantation, physical and mental comorbidities, and cardiac admissions were obtained by medical chart review. Behavioral variables, more specifically smoking status, substance use, and sports participation, were evaluated using the Health Behavior Scale‐CHD. 9
The diagnosis of HF was determined by a review of medical records. It was based on the evaluation from available clinical information including history (dyspnea, orthopnea, paroxysmal nocturnal dyspnea, fatigue and/or history of weight gain), current and past treatments (diuretics, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers), physical examination (neck vein distention, pulmonary rales, ascites or pedal edema), hemodynamic assessment (imaging studies or cardiac catheterization), and other diagnostic tests (radiographic pulmonary congestion, signs of impaired end‐organ perfusion, and natriuretic peptide levels greater that the upper limit of normal). Patients were categorized into 3 groups: never HF; past but not current HF; or current HF.
Each participant completed a set of questionnaires to assess PROs. The following PROs were measured: (1) perceived health status using the 12‐item Short Form Health Survey version 2, and further divided into physical and mental component scores; (2) QoL using a Linear Analog Scale and the Satisfaction With Life Scale; (3) sense of coherence assessed by Orientation to Life Questionnaire; (4) psychological distress using the Hospital Anxiety and Depression Scale; (5) illness perceptions assessed by Brief Illness Perception Questionnaire. The measurements, description, scale, interpretation, and references of PROs in APPROACH‐IS are detailed in Table S1.
Diversity Information
The present study is conducted and authored by researchers from 15 countries in 5 continents. Furthermore, 13 of the authors of the present study (43%) are women. In addition, both established and junior researchers are involved and represented as co‐authors. This study also included patients from low‐ and middle‐income countries (376/3959, 9.5%, defined according to the report from the World Bank Group in 2014). Patients from such countries are seldomly included in studies on PROs. Altogether, diversity received substantial attention in the set‐up of the present study.
Statistical Analysis
Categorical variables are summarized by frequency and percentage. Continuous variables are expressed as the mean and 95% CI or median and quartiles. The distribution of baseline characteristics and associated factors among patients with past or current HF versus without history of HF were compared by Chi‐Square test, Cochran‐Mantel‐Haenszel trend test, or 1‐way ANOVA. A logistic regression model was developed to identify potential factors associated with current or past HF in the adult CHD population. Variables with a P value <0.2 in univariable analyses were included in the multivariable logistic regression model. One‐way ANOVA tests were used to compare the results of PROs among patients with past or current HF versus without history of HF. Effect sizes were calculated by Cohen’s d. Values between 0.2 to 0.5 were considered indicative of a small effect; >0.5 to 0.8 of a moderate effect; and >0.8 of a large effect. 10 To define the relationship between HF and PROs, multivariable regression models were created that included age, sex, CHD complexity, comorbidity, smoking, substance abuse, arrhythmia, cardiac devices, physical exercise, psychiatric disorders, and participating countries as potential confounding factors. Variables with 2‐tailed P values <0.05 in the multivariable regression model were considered statistically significant. The statistics software SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analysis.
Results
Demographic, Clinical, and Behavioral Characteristics of Patients With and Without HF
Of the 4028 patients enrolled in APPROACH‐IS, 69 were excluded because of insufficient information to determine HF status. In the remaining 3959 patients, 137 (3.5%) were experiencing HF at the time of investigation, 298 (7.5%) had HF in the past but not currently, and 3524 (89.0%) had no history of HF. Background characteristics are summarized in Table 1. Significant differences in most background characteristics were observed in the 3 groups of patients with the exception of sex, cognitive impairment, and smoking status. Patients with HF were significantly more likely to have a history of arrhythmia, implanted cardiac devices, other medical conditions, psychiatric disorders, and a higher number of adult cardiac admissions and cardiac admissions within the past year (Table 1). Heart defects associated with the highest prevalence of HF were cyanotic CHD or Eisenmenger syndrome, univentricular anatomy, congenitally corrected transposition of great arteries, and tricuspid atresia. (Figure 1) In patients with CHD of moderate complexity, those with Ebstein anomaly or ventricular septal defects with other complications were associated with a higher occurrence of HF (Figure 1).
Table 1.
Background Characteristics of 3959 Adult Patients With Congenital Heart Disease With (Past, Current)/Without Heart Failure
| Current HF | Past, not current HF | Never HF | P value | |
|---|---|---|---|---|
| Median age, y | 42 (Q1=30;Q3=55)* | 35 (Q1=26;Q3=48)* | 31 (Q1=24;Q3=41)* | <0.0001* |
| Women | 76/137 (55.5%) | 164/296 (55.4%) | 1836/3510 (52.3%) | 0.471 |
| Participating countries | 0.440 | |||
| High‐income countries (n=3583) | 127/3583 (3.5%) | 265/3583 (7.4%) | 3191/3583 (89.1%) | |
| Low‐ and middle‐income countries (n=376) | 10/376 (2.7%) | 33/376 (8.8%) | 333/376 (88.6%) | |
| Patient‐reported NYHA functional class | <0.0001* | |||
| Class I | 20/133 (15.0%)* | 89/286 (31.1%)* | 1966/3441 (57.1%)* | |
| Class II | 53/133 (39.9%)* | 149/286 (52.1%)* | 1150/3441 (33.4%)* | |
| Class III | 37/133 (27.8%)* | 28/286 (9.8%)* | 217/3441 (6.3%)* | |
| Class IV | 23/133 (17.3%)* | 20/286 (7%)* | 108/3441 (3.1%)* | |
| Complexity of heart defect | <0.0001* | |||
| Simple (n=1029) | 19/137 (13.9%)* | 38/298 (12.8%)* | 972/3524 (27.6%)* | |
| Moderate (n=1916) | 56/137 (40.9%)* | 150/298 (50.3%)* | 1718/3524 (48.7%)* | |
| Great (n=1014) | 62/137 (45.3%)* | 110/298 (36.9%)* | 834/3524 (23.7%)* | |
| History of arrhythmia | 95/136 (69.9%)* | 151/297 (50.8%)* | 817/3514 (23.2%)* | <0.0001* |
| Cardiac device | <0.0001* | |||
| None | 79/128 (61.7%)* | 213/282 (75.5%)* | 2802/3085 (90.8%)* | |
| Pacemaker | 23/128 (18.0%)* | 43/282 (15.2%)* | 190/3085 (6.2%)* | |
| ICD | 26/128 (20.3%)* | 26/282 (9.2%)* | 93/3085 (3.0%)* | |
| Other medical condition | 105/137 (76.6%)* | 152/298 (51.0%)* | 1447/3506 (41.3%)* | <0.0001* |
| Cognitive impairment | 2/137 (1.5%) | 6/298 (2.0%) | 40/3504 (1.1%) | 0.406 |
| Chart documented psychiatric disorder | ||||
| Mood disorder | 27/136 (19.9%)* | 27/296 (9.1%)* | 196/3512 (5.6%)* | <0.0001* |
| Anxiety disorder | 12/137 (8.8%)* | 22/297 (7.4%)* | 156/3517 (4.4%)* | 0.006* |
| Other psychiatric diagnosis | 7/137 (5.1%)* | 1/296 (0.3%)* | 63/3516 (1.8%)* | 0.002* |
| Smoking | 10/136 (7.4%) | 33/294 (11.2%) | 426/3484 (12.2%) | 0.210 |
| Substance abuse score (0–100) | 16.1 (95% CI, 12.0–20.1)* | 21.1 (95% CI, 18.2–24.0)* | 22.2 (95% CI, 21.4–23.1)* | 0.020* |
| Total sport score | 1.97 (95% CI, 0.91–3.03)* | 2.45 (95% CI, 1.88–3.02)* | 3.72 (95% CI, 3.46–3.98)* | 0.001* |
| Physical exercise score | 2.11 (95% CI, 1.04–3.19)* | 2.61 (95% CI, 2.04–3.19)* | 3.93 (95% CI, 3.67–4.19)* | 0.001* |
| Total number of adult cardiac admissions | 3.2 (95% CI, 2.6–3.8)* | 2.1 (95% CI, 1.8–2.4)* | 0.7 (95% CI, 0.7–0.8)* | <0.0001* |
| Cardiac admission within past 1 year | 56/137 (40.8%)* | 78/298 (26.2%)* | 518/3472 (14.9%)* | <0.0001* |
Q1: 25th percentile; Q3: 75th percentile. HF indicates heart failure; ICD, implantable cardioverter‐defibrillator; and NYHA, New York Heart Association.
Represents statistical significance at P<0.05.
Figure 1. The occurrence of heart failure in different congenital heart disease severities. Patients are assigned to the types of heart defect in a mutually exclusive way.
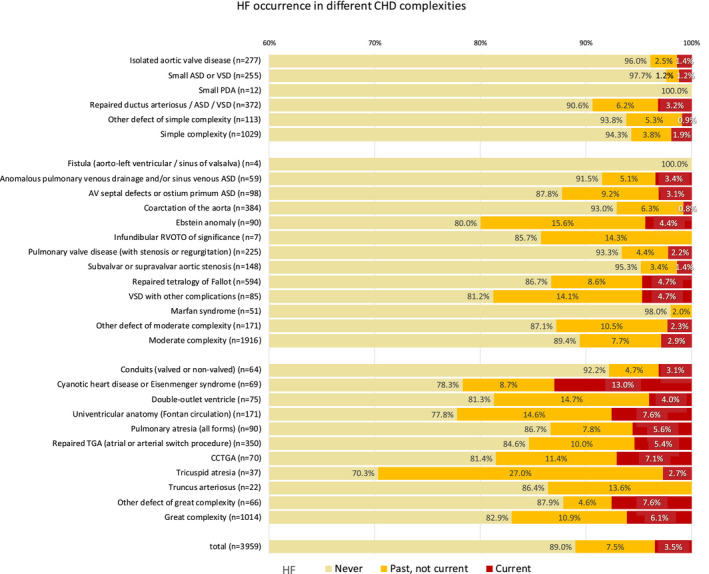
When >1 defect was present in patients, the patient was assigned to the defect of highest complexity or with the biggest functional impact. ASD indicates atrial septal defect; CHD, congenital heart disease; CCTGA, congenitally corrected transposition of great arteries; HF, heart failure; PDA, patent ductus arteriosus; RVOTO, right ventricle outflow track obstruction; and VSD, ventricular septal defect.
Multivariable logistic regression analysis showed that patients with current HF, when compared with patients who never had HF, were older (odds ratio [OR], 1.04 per year; 95% CI, 1.02–1.06; P<0.0001) and more likely to have complex CHD versus a simple defect (OR, 2.75; 95% CI, 1.49–5.07; P=0.0012), history of arrhythmia (OR, 2.94; 95% CI, 1.85–4.66; P<0.0001), an implantable cardioverter‐defibrillator (OR, 4.09; 95% CI, 1.02–1.06; P<0.0001), other medical conditions (OR, 3.34; 95% CI, 2.12–5.27; P<0.0001), and mood disorders (OR, 2.67; 95% CI, 1.52–4.68; P<0.0001) (Figure 2A). The factors associated with past but not current HF versus never HF included older age (OR, 1.02 per year; 95% CI, 1.01–1.03; P=0.0017), complex CHD versus simple defect (OR, 2.78; 95% CI, 1.81–4.26; P<0.0001), CHD of moderate complexity versus simple defect (OR, 1.99; 95% CI, 1.34–2.95; P=0.0007), history of arrhythmia (OR, 2.05; 95% CI, 1.53–2.77; P<0.0001) and presence of an implantable cardioverter‐defibrillator (OR, 1.84; 95% CI, 1.02–3.02; P=0.016) (Figure 2B).
Figure 2. Patient characteristics associated with past/current history of heart failure.
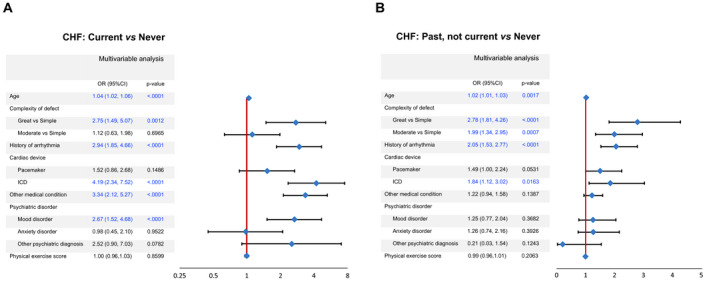
A, Comparison of current heart failure versus never heart failure (reference). B, Comparison of past heart failure versus never heart failure (reference). CHF indicates chronic heart failure; ICD, implantable cardioverter‐defibrillator; and OR, odds ratio.
PROs in Patients With and Without HF
Significant differences between patients with and without HF were observed for all PROs (Figure 3). Patients with current or past HF had lower scores for physical and mental functioning, QoL, satisfaction with life, and sense of coherence. They also had more anxiety and depressive symptoms, and more negative illness perceptions (Figure 3). The PROs were worst in patients with current HF. The magnitude of differences between patients with current HF compared with those who never had HF were large for physical functioning and illness perceptions; moderate for mental functioning, QoL, and depressive symptoms; and small for satisfaction with life, sense of coherence, and anxiety. When comparing patients with a past but not current history of HF to those who never had HF, the differences for physical functioning, mental functioning, QoL, satisfaction with life, depressive symptoms, and illness perceptions were small; and the differences for sense of coherence and anxiety were negligible (Figure 3).
Figure 3. Standardized effect size (Cohen’s d) for patients with congenital heart disease with current or past heart failure versus no heart failure.
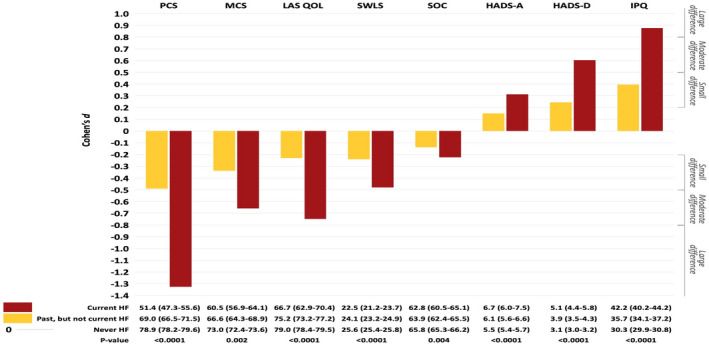
Scores: mean (95% CI). HADS‐A indicates Hospital Anxiety and Depression Scale—Anxiety; HADS‐D, Hospital Anxiety and Depression Scale—Depression; HF, heart failure; IPQ, Illness Perception Questionnaire; LAS QOL, Linear Analog Scale Quality of Life; MCS, Mental Component Summary; PCS, Physical Component Summary; SOC, Sense of Coherence; and SWLS, Satisfaction with Life Scale.
Adjusting for demographic, clinical, and behavioral factors, multivariable linear regression analysis showed that all PROs except for anxiety were significantly worse in patients with current HF compared with patients who never had HF (Table 2). Patients with a past but not current history of HF had poorer physical functioning and satisfaction with life than patients who never had HF (Table 2).
Table 2.
Patient‐Reported Outcomes in Adult Patients With Congenital Heart Disease With/Without History of Heart Failure
| Current vs Never* | P value | Past, not current vs Never* | P value | |
|---|---|---|---|---|
| Coefficient (95%CI) | Coefficient (95%CI) | |||
| Physical health status (0−100) | −14.57 (−18.03 to −11.11) † | <.0001 † | −3.38 (−5.76 to −1.00) † | 0.006 † |
| Mental health status (0–100) | −4.81 (−8.09 to −1.53) † | 0.004 † | −1.77 (−4.03 to 0.49) | 0.12 |
| Linear analog quality of life (0–100) | −6.59 (−9.54 to −3.65) † | <.0001 † | −1.27 (−3.29 to 0.75) | 0.22 |
| Satisfaction with life scale (5–35) | −1.44 (−2.62 to −0.26) † | 0.017 † | −0.87 (−1.69 to −0.06) † | 0.036 † |
| Sense of coherence scale (13–91) | −2.27 (−4.47 to −0.06) † | 0.044 † | −1.51 (−3.05 to 0.03) | 0.055 |
| Hospital anxiety scale (0–21) | 0.25 (−0.43 to 0.93) | 0.47 | 0.08 (−0.39 to 0.55) | 0.75 |
| Hospital depression scale (0–21) | 0.78 (0.20 to 1.37) † | 0.009 † | 0.38 (−0.02 to 0.79) | 0.063 |
| Illness perception (0–80) | 5.04 (2.72 to 7.35) † | <.0001 † | 1.06 (−0.56 to 2.68) | 0.20 |
CHD complexity, comorbidity, smoking, substance abuse, arrhythmia, cardiac devices, physical exercise, psychiatric disorders, and participating country.
CHD indicates congenital heart disease.
Adjusted for age, sex.
Represents statistical significance at P<0.05.
Discussion
HF poses great challenges to adults with CHD from many perspectives, given its impact on increased mortality, morbidity, symptom burden, and hospitalizations. 1 , 2 , 4 To our knowledge, this multicenter study is the first to extensively assess the impact of HF on PROs in adults with CHD. HF independently associated with older age, more complex defects, arrhythmias, implantable cardioverter‐defibrillators, clinical comorbidities, and mood disorders. Importantly, we found that current HF was associated with an adverse impact on a broad of range of PROs including patient‐reported health status, quality of life, sense of coherence, depression, and illness perceptions. In patients with a history of, but no current, HF, the effect on PROs was less marked, although significantly lower PRO scores were observed for physical functioning and satisfaction with life.
Factors Related to HF in Adults With CHD
The diagnosis of HF, especially in adults with CHD, can be challenging. Since, by definition, CHD is present at birth, adults with CHD have faced lifelong adaptation to their chronic physical limitations. As a result, adults with CHD may not be aware of HF‐related symptoms and notoriously underreport their objective degree of exercise impairment. 11 Therefore, identifying those patients with HF related risk factors may help the caregivers to detect the presence of HF through more comprehensive diagnostic work‐up and provide timely treatments to prevent adverse outcomes.
A recent study by Arnaert et al. identified several risk factors associated with the presence of HF in adults with CHD including: older age, infective endocarditis, atrial arrhythmia, pacemaker, end‐organ dysfunction, advanced New York Heart Association class, faster heart rate, ventricular dysfunction, and pulmonary hypertension severity. 12 Consistent with our findings, they reported a higher prevalence of HF in patients with cyanotic CHD (41%), Fontan circulation (30%) and systemic right ventricle (25%). 12 Another study by Cohen et al. identified factors associated with hospitalization for HF during the following year: age ≥50 years, male sex, CHD lesion severity, HF admission over the previous 12 months, pulmonary arterial hypertension, chronic kidney disease, coronary artery disease, systemic arterial hypertension, and diabetes. 13 The factors identified with HF in our study, including older age, complex CHD, arrhythmias, implantable cardioverter‐defibrillators, and comorbidities are coherent with the prior literature.
In addition, we found that mood disorders were independently associated with current HF in adults with CHD. In adults with HF because of acquired heart disease, mood disorders have been associated with the development and progression of HF. 14 The interaction between mood disorders and HF appears to be bidirectional. Mediating mechanisms likely involve both physiologic (e.g., enhanced inflammation, autonomic derangement, platelet aggregation, vascular endothelial dysfunction) and behavioral (e.g., poorer adherence to physical activity, healthy diet, or medical therapy) factors. 14 However, there is a paucity of research examining the association between mood disorders and HF in adults with CHD. The prevalence of mood disorder in adults with CHD is similar to rates identified for those with acquired heart disease. 15 In a recent population‐based study, adults with CHD had a significantly higher risk of depression than matched controls (adjusted hazard ratio, 1.43), with coronary artery disease and chronic obstructive pulmonary disease identified as significant comorbidities mediating the relationship between adult CHD and depression. 16 Depressive symptomatology was also independently impacted by the presence of HF in the current study. Therefore, the interaction between mood disorders and HF in adults with CHD may be multifactorial and involve similar mechanisms invoked in those with HF attributable to acquired heart disease.
HF and PROs in Adult Congenital Heart Disease
The importance of PROs in understanding the impact of HF from a patient perspective is increasingly recognized. 17 PROs can provide information about the impact of HF on the patient’s symptoms, functional limitations, daily activities, emotional well‐being, psychological health and social functioning. 17 At the same time, PROs can help clinicians and researchers to assess the patient’s baseline status, treatment effectiveness, and clinical trial end points. PROs can also serve as important prognostic predictors for subsequent clinical events and even mortality according to HF‐related research in acquired heart disease. 18 Although PROs are widely used in studies involving HF attributable to acquired heart disease, studies on the impact of HF on PROs in adults with CHD are scarce. A few previous studies revealed that poorer New York Heart Association functional class was associated with impaired physical and emotional QoL, and higher perception of distress among adolescents and young adults with CHD. 19 , 20 However, New York Heart Association class is based on patient‐reported symptoms in relation to physical activity. Therefore, it is not an accurate surrogate for the severity of HF associated with CHD. Our study demonstrated that after adjusting for confounding covariates, HF remains significantly associated with impaired psychosocial functioning and QoL in adults with CHD. The presence of co‐existing factors that may also strongly impact PROs in patients with CHD with HF are common, such as complex CHD (45.3%), arrhythmia (69.9%), cardiac device (38.3%), medical comorbidities (76.6%), and mood disorder (19.9%). 21 , 22 , 23 , 24 , 25 Therefore, we speculate that, in addition to these co‐existing factors associated with HF, the stress and illness perceptions associated with HF‐related symptoms, repeated hospitalizations, and uncertainty or side effects of treatment, together with impaired executive functions and coping mechanisms for HF‐related stress can contribute to the extensive and profound impact of HF on PROs in adults with CHD. 20 , 26 , 27 , 28 , 29 , 30
Strengths and Limitations
The APPROACH‐IS study is a prospective collaboration involving 24 medical centers from 15 countries, which enrolled >4000 adult patients with CHD worldwide. 7 The PRO instruments used are comprehensive and validated. The clinical information was obtained and interpreted by professional investigators and the data collection of structural questionnaires had a high degree of completeness. Therefore, the relationship between HF and PROs could be delineated more clearly across a broad spectrum of patients. However, this study also has several limitations. First, the APPROACH‐IS is a cross‐sectional study, therefore causality cannot be inferred and the influence of PROs on long‐term outcomes cannot be determined. Such would require longitudinal follow‐up studies. Secondly, since the APPROACH‐IS study enrolled patients from collaborative medical centers all over the world, these patients tended to have more complex CHD (25.6% with great complexity, 48.3% with moderate complexity) than the general population of adults with CHD. The patient selection process may, therefore, impact generalizability of results to the larger target population of adults with CHD. Third, the presence of HF in participating patients was determined by on‐site investigators on the basis of available medical records. The criteria for diagnosis of HF may vary among different medical centers.
Conclusions
The presence of current HF in adults with CHD is independently associated with an adverse effect on a broad range of PROs across multiple domains, including patient‐reported health status, QoL, sense of coherence, anxiety, depression, and illness perceptions. Large effect sizes are observed for physical functioning and illness perceptions.
Appendix
APPROACH‐IS consortium: Luis Alday, Héctor Maisuls, Betina Vega (Córdoba, Argentina, Hospital de Niños); Samuel Menahem, Sarah Eaton, Qi Feng Wang, Ruth Larion (Melbourne, Australia, Monash Medical Center); Werner Budts, Kristien Van Deyk (Leuven, Belgium, University Hospitals of Leuven); Silke Apers, Eva Goossens, Jessica Rassart, Koen Luyckx, Philip Moons (Leuven, Belgium, University of Leuven); Gwen Rempel, Andrew Mackie, Ross Ballantyne, Kathryn Rankin, Colleen Norris, Dylan Taylor, Isabelle Vondermuhll, Jonathan Windram, Pamela Heggie, Gerri Lasiuk (Edmonton, Canada, University of Alberta); Paul Khairy, Anna Proietti, Annie Dore, Lise‐Andrée Mercier, François‐Pierre Mongeon, François Marcotte, Reda Ibrahim, Blandine Mondésert, Marie‐Claude Côté (Montreal, Canada, Montreal Heart Institute); Adrienne Kovacs, Erwin Oechslin, Mimi Bandyopadhyay (Toronto, Canada, University Health Network); Alexandra Soufi, Sylvie Di Filippo, François Sassolas, André Bozio, Cécile Chareyras (Lyon, France, Louis Pradel Hospital); Shanthi Chidambarathanu, Farida Farzana, Nitya Lakshmi (Chennai, India, Frontier Lifeline Hospital, Dr. K. M. Cherian Heart Foundation); Edward Callus, Emilia Quadri, Massimo Chessa, Giovanna Campioni, Alessandro Giamberti (Milan, Italy, IRCCS Policlinco San Donato Hospital); Junko Enomoto, Yoshiko Mizuno (Chiba, Japan, Chiba Cardiovascular Center); Maryanne Caruana, Victor Grech, Sheena Vella, Anabel Mifsud, Neville Borg, Daniel Chircop, Matthew Mercieca Balbi, Rachel Vella Critien, James Farrugia, Yanika Gatt, Darlene Muscat (Msida, Malta, Mater Dei Hospital); Katrine Eriksen, Mette‐Elise Estensen (Oslo, Norway, Oslo University Hospital); Mikael Dellborg, Malin Berghammer (Gothenburg, Sweden, Sahlgrenska University Hospital); Eva Mattsson, Anita Strandberg, Pia Karlström‐Hallberg (Stockholm, Sweden, Karolinska University Hospital); Bengt Johansson, Anna‐Karin Kronhamn (Umeå, Sweden, Umeå University Hospital); Markus Schwerzman, Corina Thomet, Margrit Huber (Bern, Switzerland, University Hospital Bern); Jou‐Kou Wang, Chun‐Wei Lu, Hsiao‐Ling Yang, Yu Chuan Hua (Taipei, Taiwan, National Taiwan University Hospital); Barbara Mulder, Maayke Sluman (Amsterdam, the Netherlands, Amsterdam Medical Center); Marco Post (Nieuwegein, the Netherlands, St. Antonius Hospital); Els Pieper (Groningen, the Netherlands, University Medical Center Groningen); Kathinka Peels (Eindhoven, the Netherlands, Catharina Hospital); Marc Waskowsky (Zwolle, the Netherlands, Isala Clinic); Gruschen Veldtman, Michelle Faust, Colin Lozier, Christy Reed, Jamie Hilfer (Cincinnati, USA, Cincinnati Children’s Hospital Medical Center); Curt Daniels, Jamie Jackson (Columbus, USA, Nationwide Children’s Hospital); Shelby Kutty, Carolyn Chamberlain, Sara Warta (Omaha, USA, Children’s Hospital & Medical Center); Stephen Cook, Morgan Hindes (Pittsburgh, USA, Children's Hospital of Pittsburgh of UPMC); Ari Cedars, Kamila White (Saint Louis, USA, Washington University and Barnes Jewish Heart & Vascular Center, University of Missouri); Susan Fernandes, Anitra Romfh, Kirstie MacMillen (Palo Alto, USA, Stanford University).
Sources of Funding
This work was supported by the Research Fund—KU Leuven (Leuven, Belgium) through grant OT/11/033 to K.L. and P.M.; by the Swedish Heart‐Lung Foundation (Sweden) through grant number 20130607 to M.D.; by the University of Gothenburg Centre for Person‐centred Care (Gothenburg, Sweden) to M.D. and P.M.; and by the Cardiac Children's Foundation (Taiwan) through grant CCF2013_02 to H.L.Y. Furthermore, this work was endorsed by and conducted in collaboration with the International Society for Adult Congenital Heart Disease.
Disclosures
None.
Supporting information
Acknowledgments
The authors would like to express our thanks to the staff of National Taiwan University Hospital‐Statistical Consulting Unit for statistical consultation and analyses.
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Philip Moons, Email: philip.moons@kuleuven.be.
the APPROACH‐IS consortium, the International Society for Adult Congenital Heart Disease (ISACHD):
Luis Alday, Héctor Maisuls, Betina Vega, Samuel Menahem, Sarah Eaton, Qi Feng Wang, Ruth Larion, Werner Budts, Kristien Van Deyk, Silke Apers, Eva Goossens, Jessica Rassart, Koen Luyckx, Philip Moons, Gwen Rempel, Andrew Mackie, Ross Ballantyne, Kathryn Rankin, Colleen Norris, Dylan Taylor, Isabelle Vondermuhll, Jonathan Windram, Pamela Heggie, Gerri Lasiuk, Paul Khairy, Anna Proietti, Annie Dore, Lise‐Andrée Mercier, François‐Pierre Mongeon, François Marcotte, Reda Ibrahim, Blandine Mondésert, Marie‐Claude Côté, Adrienne Kovacs, Erwin Oechslin, Mimi Bandyopadhyay, Alexandra Soufi, Sylvie Di Filippo, François Sassolas, André Bozio, Cécile Chareyras, Shanthi Chidambarathanu, Farida Farzana, Nitya Lakshmi, Edward Callus, Emilia Quadri, Massimo Chessa, Giovanna Campioni, Alessandro Giamberti, Junko Enomoto, Yoshiko Mizuno, Maryanne Caruana, Victor Grech, Sheena Vella, Anabel Mifsud, Neville Borg, Daniel Chircop, Matthew Mercieca Balbi, Rachel Vella Critien, James Farrugia, Yanika Gatt, Darlene Muscat, Katrine Eriksen, Mette‐Elise Estensen, Mikael Dellborg, Malin Berghammer, Eva Mattsson, Anita Strandberg, Pia Karlström‐Hallberg, Bengt Johansson, Anna‐Karin Kronhamn, Markus Schwerzman, Corina Thomet, Margrit Huber, Jou‐Kou Wang, Chun‐Wei Lu, Hsiao‐Ling Yang, Yu Chuan Hua, Barbara Mulder, Maayke Sluman, Marco Post, Els Pieper, Kathinka Peels, Marc Waskowsky, Gruschen Veldtman, Michelle Faust, Colin Lozier, Christy Reed, Jamie Hilfer, Curt Daniels, Jamie Jackson, Shelby Kutty, Carolyn Chamberlain, Sara Warta, Stephen Cook, Morgan Hindes, Ari Cedars, Kamila White, Susan Fernandes, Anitra Romfh, and Kirstie MacMillen
References
- 1. Verheugt CL, Uiterwaal CSPM, van der Velde ET, Meijboom FJ, Pieper PG, van Dijk APJ, Vliegen HW, Grobbee DE, Mulder BJM. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220–1229. doi: 10.1093/eurheartj/ehq032 [DOI] [PubMed] [Google Scholar]
- 2. Engelings CC, Helm PC, Abdul‐Khaliq H, Asfour B, Bauer UM, Baumgartner H, Kececioglu D, Korten MA, Diller GP, Tutarel O. Cause of death in adults with congenital heart disease ‐ an analysis of the German national register for congenital heart defects. Int J Cardiol. 2016;211:31–36. [DOI] [PubMed] [Google Scholar]
- 3. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal A, Dudley CW, Nah G, Hayward R, Tseng ZH. Clinical outcomes during admissions for heart failure among adults with congenital heart disease. J Am Heart Assoc. 2019;8:e012595. doi: 10.1161/JAHA.119.012595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weldring T, Smith SM. Patient‐reported outcomes (pros) and patient‐reported outcome measures (proms). Health Serv Insights. 2013;6:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moradi M, Daneshi F, Behzadmehr R, Rafiemanesh H, Bouya S, Raeisi M. Quality of life of chronic heart failure patients: a systematic review and meta‐analysis. Heart Fail Rev. 2020;25:993–1006. [DOI] [PubMed] [Google Scholar]
- 7. Apers S, Kovacs AH, Luyckx K, Alday L, Berghammer M, Budts W, Callus E, Caruana M, Chidambarathanu S, Cook SC, et al. Assessment of patterns of patient‐reported outcomes in adults with congenital heart disease ‐ international study (APPROACH‐IS): rationale, design, and methods. Int J Cardiol. 2015;179:334–342. [DOI] [PubMed] [Google Scholar]
- 8. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, Del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008;118:e714–e833. [DOI] [PubMed] [Google Scholar]
- 9. Goossens E, Luyckx K, Mommen N, Gewillig M, Budts W, Zupancic N, Moons P; i‐Detach investigators . Health risk behaviors in adolescents and emerging adults with congenital heart disease: psychometric properties of the health behavior scale‐congenital heart disease. Eur J Cardiovasc Nurs. 2013;12:544–557. [DOI] [PubMed] [Google Scholar]
- 10. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 11. Diller G‐P, Dimopoulos K, Okonko D, Li W, Babu‐Narayan SV, Broberg CS, Johansson B, Bouzas B, Mullen MJ, Poole‐Wilson PA, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800 [DOI] [PubMed] [Google Scholar]
- 12. Arnaert S, De Meester P, Troost E, Droogne W, Van Aelst L, Van Cleemput J, Voros G, Gewillig M, Cools B, Moons P, et al. Heart failure related to adult congenital heart disease: prevalence, outcome and risk factors. ESC Heart Fail. 2021;8:2940–2950. doi: 10.1002/ehf2.13378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen S, Liu A, Wang F, Guo L, Brophy JM, Abrahamowicz M, Therrien J, Beauchesne LM, Bedard E, Grewal J, et al. Risk prediction models for heart failure admissions in adults with congenital heart disease. Int J Cardiol. 2021;322:149–157. [DOI] [PubMed] [Google Scholar]
- 14. Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and anxiety in heart failure: a review. Harv Rev Psychiatry. 2018;26:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson JL, Leslie CE, Hondorp SN. Depressive and anxiety symptoms in adult congenital heart disease: prevalence, health impact and treatment. Prog Cardiovasc Dis. 2018;61:294–299. [DOI] [PubMed] [Google Scholar]
- 16. Yang HL, Chang NT, Wang JK, Lu CW, Huang YC, Moons P. Comorbidity as a mediator of depression in adults with congenital heart disease: a population‐based cohort study. Eur J Cardiovasc Nurs. 2020;19:732–739. [DOI] [PubMed] [Google Scholar]
- 17. Kelkar AA, Spertus J, Pang P, Pierson RF, Cody RJ, Pina IL, Hernandez A, Butler J. Utility of patient‐reported outcome instruments in heart failure. JACC Heart Fail. 2016;4:165–175. doi: 10.1016/j.jchf.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 18. Johansson I, Joseph P, Balasubramanian K, McMurray JJV, Lund LH, Ezekowitz JA, Kamath D, Alhabib K, Bayes‐Genis A, Budaj A, et al. Health‐related quality of life and mortality in heart failure: the global congestive heart failure study of 23 000 patients from 40 countries. Circulation. 2021;143:2129–2142. doi: 10.1161/CIRCULATIONAHA.120.050850 [DOI] [PubMed] [Google Scholar]
- 19. Jackson JL, Hassen L, Gerardo GM, Vannatta K, Daniels CJ. Medical factors that predict quality of life for young adults with congenital heart disease: what matters most? Int J Cardiol. 2016;202:804–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson JL, Gerardo GM, Daniels CJ, Vannatta K. Perceptions of disease‐related stress a key to better understanding patient‐reported outcomes among survivors of congenital heart disease. J Cardiovasc Nurs. 2017;32:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Callus E, Pagliuca S, Boveri S, Ambrogi F, Luyckx K, Kovacs AH, Apers S, Budts W, Enomoto J, Sluman MA, et al. Phenotypes of adults with congenital heart disease around the globe: a cluster analysis. Health Qual Life Outcomes. 2021;19:53. doi: 10.1186/s12955-021-01696-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casteigt B, Samuel M, Laplante L, Shohoudi A, Apers S, Kovacs AH, Luyckx K, Thomet C, Budts W, Enomoto J, et al. Atrial arrhythmias and patient‐reported outcomes in adults with congenital heart disease: an international study. Heart Rhythm. 2021;18:793–800. doi: 10.1016/j.hrthm.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 23. Lévesque V, Laplante L, Shohoudi A, Apers S, Kovacs AH, Luyckx K, Thomet C, Budts W, Enomoto J, Sluman MA, et al. Implantable cardioverter‐defibrillators and patient‐reported outcomes in adults with congenital heart disease: an international study. Heart Rhythm. 2020;17:768–776. doi: 10.1016/j.hrthm.2019.11.026 [DOI] [PubMed] [Google Scholar]
- 24. Ko JM, White KS, Kovacs AH, Tecson KM, Apers S, Luyckx K, Thomet C, Budts W, Enomoto J, Sluman MA, et al. Differential impact of physical activity type on depression in adults with congenital heart disease: a multi‐center international study. J Psychosom Res. 2019;124:109762. [DOI] [PubMed] [Google Scholar]
- 25. Moons P, Luyckx K, Thomet C, Budts W, Enomoto J, Sluman MA, Lu CW, Jackson JL, Khairy P, Cook SC, et al. Physical functioning, mental health, and quality of life in different congenital heart defects: comparative analysis in 3538 patients from 15 countries. Can J Cardiol. 2021;37:215–223. [DOI] [PubMed] [Google Scholar]
- 26. Schoormans D, Mulder BJ, van Melle JP, Pieper PG, van Dijk AP, Sieswerda GT, Hulsbergen‐Zwarts MS, Plokker TH, Brunninkhuis LG, Vliegen HW, et al. Illness perceptions of adults with congenital heart disease and their predictive value for quality of life two years later. Eur J Cardiovasc Nurs. 2014;13:86–94. [DOI] [PubMed] [Google Scholar]
- 27. Rassart J, Apers S, Kovacs AH, Moons P, Thomet C, Budts W, Enomoto J, Sluman MA, Wang JK, Jackson JL, et al. Illness perceptions in adult congenital heart disease: a multi‐center international study. Int J Cardiol. 2017;244:130–138. [DOI] [PubMed] [Google Scholar]
- 28. Moons P, Luyckx K, Thomet C, Budts W, Enomoto J, Sluman MA, Wang J‐K, Jackson JL, Khairy P, Cook SC, et al. Patient‐reported outcomes in adults with congenital heart disease following hospitalization (from APPROACH‐IS). Am J Cardiol. 2021;145:135–142. doi: 10.1016/j.amjcard.2020.12.088 [DOI] [PubMed] [Google Scholar]
- 29. Jackson JL, Gerardo GM, Monti JD, Schofield KA, Vannatta K. Executive function and internalizing symptoms in adolescents and young adults with congenital heart disease: the role of coping. J Pediatr Psychol. 2018;43:906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andonian C, Beckmann J, Ewert P, Freilinger S, Kaemmerer H, Oberhoffer‐Fritz R, Sack M, Neidenbach R. Assessment of the psychological situation in adults with congenital heart disease. J Clin Med. 2020;9. doi: 10.3390/jcm9030779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ware JE, Kosinski M, Turner‐Bowker DM, Sundaram M, Gandek B, Maruish ME. User's Manual for the sf‐12v2 Health Survey. 2nd ed. Lincoln, RI: QualityMetric, Incorporated; 2009. [Google Scholar]
- 32. Moons P, Van Deyk K, De Bleser L, Marquet K, Raes E, De Geest S, Budts W. Quality of life and health status in adults with congenital heart disease: a direct comparison with healthy counterparts. Eur J Cardiovasc Prev Rehabil. 2006;13:407–413. [DOI] [PubMed] [Google Scholar]
- 33. Moons P, Marquet K, Budts W, De Geest S. Validity, reliability and responsiveness of the "schedule for the evaluation of individual quality of life‐direct weighting" (seiqol‐dw) in congenital heart disease. Health Qual Life Outcomes. 2004;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49:71–75. [DOI] [PubMed] [Google Scholar]
- 35. Antonovsky A. The structure and properties of the sense of coherence scale. Soc Sci Med. 1993;36:725–733. [DOI] [PubMed] [Google Scholar]
- 36. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 37. Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631–637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


