Abstract
Background
Preeclampsia is a major cause of maternal and fetal morbidity and mortality. Given its large public health burden, there is a need to identify modifiable factors that can be targeted for preeclampsia prevention. In this study, we examined whether a Mediterranean‐style diet is protective for preeclampsia in a large cohort of racially and ethnically diverse, urban, low‐income women.
Methods and Results
We used data from the Boston Birth Cohort. Maternal sociodemographic and dietary data were obtained via interview and food frequency questionnaire within 24 to 72 hours postpartum, respectively. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records. We derived a Mediterranean‐style diet score from the food frequency questionnaire and performed logistic regression to examine the association of the Mediterranean‐style diet score with preeclampsia. Of 8507 women in the sample, 848 developed preeclampsia. 47% were Black, 28% were Hispanic, and the remaining were White/Other. After multivariable adjustment, greatest adherence with MSD was associated with lower preeclampsia odds (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% CI, 0.64–0.96). A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76–0.96).
Conclusions
Self‐report of higher adherence to a Mediterranean‐style diet is associated with lower preeclampsia odds, and benefit of this diet is present among Black women as well.
Keywords: diet, preeclampsia, pregnancy
Subject Categories: Cardiovascular Disease, Epidemiology, Pregnancy, Women
Nonstandard Abbreviations and Acronyms
- DASH
Dietary Approaches to Stop Hypertension
- ESTEEM
Effect of Simple, Targeted Diet in Pregnant Women With Metabolic Risk Factors on Pregnancy Outcomes
- MSDS
Mediterranean‐style diet score
- PREDIMED
Primary Prevention of Cardiovascular Disease With a Mediterranean Diet
Clinical Perspective
What Is New?
Women with preexisting cardiometabolic disorders (chronic hypertension, obesity, and diabetes) are at higher risk for preeclampsia.
In a racially and ethnically diverse high‐risk population, adherence to a Mediterranean‐style diet is associated with lower odds of preeclampsia.
The benefit of a Mediterranean‐style diet is also present when the analysis is restricted to Black women only.
What Are the Clinical Implications?
Our findings suggest that pregnant women may benefit from a Mediterranean‐style diet to reduce the risk of preeclampsia.
Preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end‐organ dysfunction, is a disorder that occurs in up to 5% to 10% of all pregnant women worldwide. 1 It is a major cause of maternal and fetal morbidity and raises the risk for long‐term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure. 1 , 2 , 3 It is possible that the observed increased risk of CVD may be related to shared cardiometabolic risk factors, such as hypertension, diabetes, and obesity, between preeclampsia and CVD, although studies have suggested that the increased CVD risk conferred by preeclampsia exceeds that expected by cardiometabolic factors alone. 1 , 4 , 5 , 6 , 7 Children born to mothers with preeclampsia have also shown to be at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters including flow‐mediated dilation, which are markers of elevated CVD risk. 8 , 9 Given these health hazards to both mothers and children, it is important to prevent development of preeclampsia.
Multiple studies have demonstrated a benefit of the Mediterranean diet, characterized primarily by high intake of vegetables, fruits, and healthy fats, in reducing CVD risk in the nonpregnant population. 10 , 11 , 12 , 13 In a prior study by our group, we found that in pregnant women, greater adherence to a Mediterranean‐style diet was associated with reduced risks of preterm birth and low birth weight. 14 Given the shared risk factors between CVD, preterm birth, and preeclampsia, we hypothesized that adherence to a dietary pattern such as the Mediterranean diet can reduce the risk of preeclampsia.
Additionally, hypertensive disorders of pregnancy, such as preeclampsia, occur more frequently in Black women. 15 , 16 , 17 Studies evaluating potential therapies in high‐risk women, including Black women, are limited. Furthermore, studies investigating the effect of diet on development of preeclampsia have been discrepant, 18 , 19 , 20 , 21 and evidence of the effect in women at high risk of preeclampsia is lacking, especially, among understudied, underrepresented, and underreported minority populations. In this study, we examined the independent association of a Mediterranean‐style diet in a large cohort of racially and ethnically diverse, urban, low‐income, minority women at high risk of preeclampsia.
Methods
Study Population
The data that support the findings of this study are available from Dr Xiaobin Wang (the principal investigator of the Boston Birth Cohort; xwang82@ jhu.edu) upon reasonable request and after review and approval of the institutional review board. Data were obtained from the Boston Birth Cohort, a prospective cohort study based in Boston, Massachusetts. Full enrollment details and protocol have been described previously. 22 Briefly, participants were enrolled from 1998 to 2016. All the study participants were recruited from the Boston Medical Center. Thus, the composition of race and ethnicity of the Boston Birth Cohort reflects the patient population of the Boston Medical Center, which is the largest safety net hospital in Boston, serving a predominantly urban, low‐income, minority population. This population is traditionally understudied and underrepresented. The Boston Birth Cohort oversampled preterm birth, where mothers with singleton pregnancies delivering infants who were preterm (<37 weeks) or low birth weight (<2500 grams) were selected as cases and matched in an ≈1:2 case control ratio. 22 Race and ethnicity information was per self‐report from the mother. In this study, women who identified as self‐reported "Asian, Pacific Islander, Cape Verdean, mixed race or other" were included together in the category called "Other." Consistent with other studies demonstrating higher rates of preterm birth among Black mothers, a large number of Black women were therefore enrolled into the Boston Birth Cohort. 22 , 23 Newborns with birth defects or chromosomal abnormalities were excluded. Informed consent was obtained from the mothers. Institutional Review Boards of the Boston Medical Center, and the Johns Hopkins Bloomberg School of Public Health approved the study. The study is also registered on ClinicalTrials.gov (NCT03228875).
Mediterranean‐Style Diet Score
Among 8623 participants, dietary data were available for 8507. Food frequency questionnaires were administered by interviewers 24 to 72 hours after delivery. Questionnaires included information regarding maternal intake of various foods by weekly frequency for the duration of pregnancy. The categories for days/week for reporting each food group were as follows: 0=none; 1=<1 day; 2=1 to 2 days; 3=3 to 5 days; 4=6 to 7 days; 5=do not know.
We used the food frequency questionnaire data to create a Mediterranean‐style diet score (MSDS), as previously published. 14 Specifically, we used food groups associated with Mediterranean diet (vegetables, fruits, legumes, grains, dairy, and seafood). Rice and pasta were included under whole grains. Dairy was considered low‐fat dairy, and meat was considered red/processed meat. The missing responses in the food frequency questionnaire were imputed with the mode within each race and age group, as previously reported. 14
For the final diet score, each food group was added on the basis of the reported intake with a score assigned (0–4 as above in the categories). The final MSDS was calculated by summing the individual score for each food group (only meat was inversely scored). MSDS was further divided into tertiles for analysis, with the third tertile representing the highest adherence to Mediterranean‐style diet and the first tertile corresponding to the lowest. Given that there were ties, the number of women in each tertile was not always equal. The first tertile corresponds to an MSDS of 4 to 23, second tertile to an MSDS of 24 to 26 and the third tertile to an MSDS of 27 to 38.
Demographic Classifications
Demographic variables were self‐reported in the postpartum questionnaire. Race and ethnicity groups included White, Black (African American and Haitian), Hispanic, and Other (Cape Verdian, Asian, Pacific Islander, mixed race, and other). Maternal age was categorized into <21, 21 to 30, and ≥30 years groups. Education (at the time of birth) was categorized into “no school/elementary school,” “high school,” and “some college or above” groups. Parity before the index pregnancy was categorized as a binary variable (nulliparous versus parous). Marital status (at the time of delivery) was categorized into “married,” “unmarried,” and “unknown.” Smoking status during pregnancy, collected after pregnancy, was treated as a binary variable (ever or never smoking during pregnancy). Annual income was categorized as <$30 000 or ≥30 000. Because of a high number of women missing data on income (46%), we did not include income in our final adjusted models.
Clinical Outcomes
Trained research staff extracted the physician‐classified clinical diagnoses from the electronic medical records. The primary outcome for this study was preeclampsia. We included any form of preeclampsia, including mild or severe preeclampsia, eclampsia, or HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome in our preeclampsia definition. For covariates, we considered prepregnancy obesity as body mass index ≥30 kg/m2. Prepregnancy height and weight were obtained at the postpartum interview and used to calculate prepregnancy body mass index.
Statistical Analysis
Descriptive statistics were performed for comparison of demographics and clinical variables across tertiles of MSDS. The normality assumption was tested for continuous variables with use of histograms (evaluation for Gaussian distribution) and the Skewness Kurtosis normality test (null hypothesis of normality rejected if P<0.05). Normally distributed continuous variables were reported as means and SDs, while nonnormally distributed continuous variables were reported as medians and interquartile ranges. The Kruskal‐Wallis 1‐way test was performed for comparisons of continuous variables. Categorical variables were reported as number (frequency as percentage) and the chi‐squared test was used to compare categorical variables.
Next, we used logistic regression to calculate the odds ratios of preeclampsia with demographic, clinical, and socioeconomic factors in univariate models. Then to minimize the potential for reverse causation, we excluded women with preexisting hypertension or diabetes from subsequent analyses on the association of MSDS and preeclampsia.
We evaluated the potential nonlinear associations between MSDS and preeclampsia using restricted cubic splines with knots at the 10th (MSDS=20), 50th (MSDS=25) and 90th (MSDS=30) percentiles of their sample distribution. We used an adjusted multivariable restricted cubic spline model (same variables as the logistic regression model above).
Following this, we used unadjusted and adjusted logistic regression to calculate the odds ratios of preeclampsia with tertiles of MSDS. Multivariable‐adjusted models included age (categorical: <21, 21–30, ≥30 years), race and ethnicity (White, Black, Hispanic, and other), education (categorical: 1=no school/elementary school, 2=high school, 3=some college or above), marital status (categorical: 1=married, 2=unmarried, 3=unknown), smoking status (binary: 0=never smoker during pregnancy, 1=smoking during pregnancy), parity (binary: 0=nulliparous, 1=parous), and prepregnancy obesity (binary: 0=body mass index <30 kg/m2, 1=body mass index ≥30 kg/m2).
We then investigated the relationship of MSDS with preeclampsia stratified by Black versus non‐Black race, as prior literature indicates that Black women are at the highest risk for preeclampsia. Evaluation of the MSDS‐preeclampsia association was not performed in other racial and ethnic subgroups because we lacked sufficient sample size to estimate robust parameter estimates. In addition to conducting Black race–specific associations, we examined the joint association of MSDS and Black race with preeclampsia. Finally, given the possibility that the effect of a Mediterranean diet may be different among nulliparous versus parous women, we then performed a sensitivity analysis limited to nulliparous women only.
Results
A total of 8507 women were included in our study. There were some significant differences in demographic and clinical variables across tertiles of MSDS. Women in the highest tertile were older, were more likely to be parous, had lower prepregnancy obesity and diabetes, were more likely to be married, and were less likely to have a college education (Table 1). Overall, a total of 848 (10%) of women developed preeclampsia (Table 1). A greater percentage (11%) of women in the lowest MSDS tertile had preeclampsia, compared with the middle and highest tertiles (9% and 10%, respectively).
Table 1.
Demographics and Clinical Variables by Tertile of MSDS
|
Overall (n=8507) |
MSDS Tertile 1 (n=2842) |
MSDS Tertile 2 (n=2644) |
MSDS Tertile 3 (n=3021) |
P value | |
|---|---|---|---|---|---|
| MSDS, median (range) | 25 (22–28) | 21 (19–22) | 25 (24–26) | 28 (27–30) | N/A |
| Maternal age, y | 28 (23–33) | 26 (22–32) | 28 (23–33) | 29 (24–34) | <0.001 |
| Race and ethnicity, n (%) | <0.001 | ||||
| White | 1005 (12) | 495 (17) | 270 (10) | 240 (8) | |
| Black | 4030 (47) | 1328 (48) | 1260 (48) | 1442 (48) | |
| Hispanic | 2423 (28) | 727 (25) | 806 (30) | 890 (29) | |
| Other* | 1049 (12) | 292 (10) | 308 (12) | 449 (15) | |
| Parity before index pregnancy, n (%) | 0.024 | ||||
| Nulliparous | 3675 (43) | 1279 (45) | 1143 (43) | 1253 (42) | |
| Parous | 4832 (57) | 1563 (55) | 1501 (57) | 1786 (58) | |
| Sex of infant, male, n (%) | 4235 (50) | 1403 (49) | 1321 (50) | 1511 (50) | 0.86 |
| Gestational age at delivery, wk | 39 (37–40) | 39 (36–40) | 39 (37–40) | 39 (37–40) | <0.001 |
| Low birth weight, n (%) | 2221 (26) | 859 (30) | 656 (27) | 706 (23) | <0.001 |
| Preeclampsia, n (%) | 848 (10) | 312 (11) | 235 (9) | 301 (10) | 0.038 |
| Prepregnancy obesity, n (%) | 1606 (19) | 607 (21) | 490 (19) | 509 (17) | <0.001 |
| Gestational diabetes, n (%) | 576 (7) | 213 (7) | 161 (6) | 202 (7) | 0.11 |
| Preexisting diabetes, n (%) | 307 (4) | 125 (4) | 96 (4) | 86 (3) | 0.006 |
| Chronic hypertension, n (%) | 442 (5) | 160 (6) | 128 (5) | 154 (5) | 0.41 |
| Ever smoking, n (%) | 1635 (19) | 794 (28) | 460 (17) | 381 (13) | <0.001 |
| Annual income, $, n (%) | <0.001 | ||||
| <30 000 | 3485 (41) | 1054 (37) | 1118 (42) | 1313 (44) | |
| ≥30 000 | 1094 (13) | 296 (10) | 336 (13) | 462 (15) | |
| Missing | 3928 (46) | 1492 (53) | 1190 (45) | 1246 (41) | |
| Education, n (%) | <0.001 | ||||
| College or higher | 2629 (31) | 963 (34) | 840 (32) | 559 (26) | |
| High school | 2957 (35) | 999 (35) | 904 (34) | 732 (34) | |
| Less than high school | 2926 (34) | 880 (31) | 900 (34) | 1145 (38) | |
| Marital status, n (%) | <0.001 | ||||
| Married | 2871 (34) | 720 (25) | 916 (34) | 1235 (41) | |
| Unmarried | 5467 (64) | 2065 (73) | 1683 (64) | 1719 (57) | |
| Unknown | 174 (2) | 57 (2) | 45 (2) | 67 (2) |
For continuous variables, normally distributed continuous variables are reported as means and standard deviations, while nonnormally distributed continuous variables are reported as medians and interquartile ranges. MSDS indicates Mediterranean‐style diet score.
*Asian, Pacific Islander, Cape Verdean, Mixed or Other.
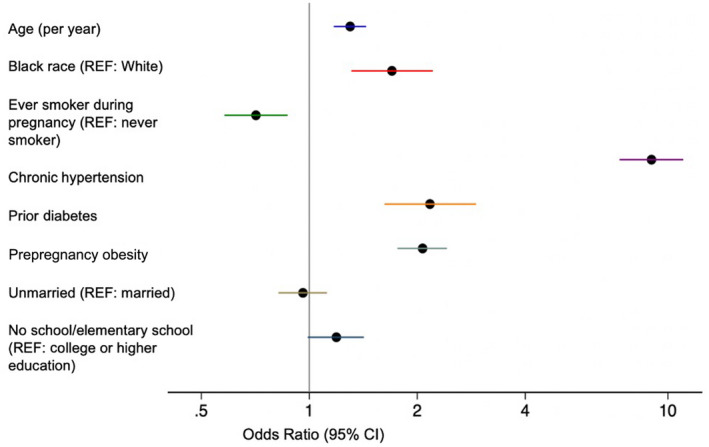
Multiple factors were associated with higher odds of preeclampsia (Figure 1). In particular, women entering pregnancy with chronic hypertension had 9 (95% CI, 7.33–11.04) times the odds of preeclampsia compared with women entering pregnancy without hypertension. Women with preexisting diabetes and prepregnancy obesity had 2.17 (95% CI, 1.62–2.92) and 2.07 (95% CI, 1.76–2.42) times the odds of preeclampsia compared with women without those conditions, respectively (Figure 1, Table S1).
Figure 1. Unadjusted associations of potential risk factors with preeclampsia.
Next, we focused on the association of MSDS and preeclampsia. Again, for these analyses we excluded women with preexisting diabetes or hypertension (n=667; 8% of total sample). We clarified with a restricted cubic spline model that the association of MSDS with preeclampsia was approximately inverse, with a possible threshold effect around MSDS of 25 (Figure 2). Compared with women who scored in the lowest tertile of the MSDS, women in the middle tertile and highest tertile had 28% (11%–41%) and 22% (95% CI, 4%–36%) lower odds of preeclampsia after adjustment for covariates (Table 2).
Figure 2. Relationship between adjusted* odds of preeclampsia and Mediterranean‐style diet score.
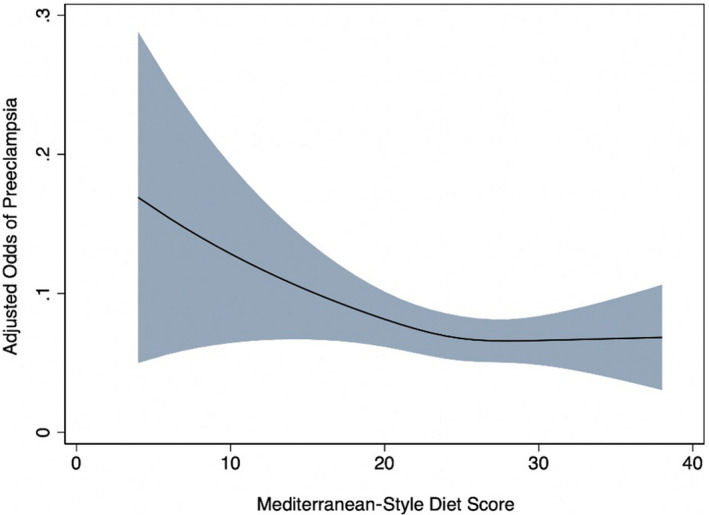
*Variables included in the model: maternal age, race, education, marital status, smoking in pregnancy, parity, and prepregnancy obesity.
Table 2.
Association of MSDS With Preeclampsia
| MSDS |
Preeclampsia cases/total participants (%) (n=618/7770) |
Unadjusted model, odds ratio (95% CI) |
Multivariable model,* odds ratio (95% CI) |
|---|---|---|---|
| Tertile 1 |
234/2569 (9) |
Referent | Referent |
| Tertile 2 |
170/2420 (7) |
0.75 (0.61–0.93) | 0.72 (0.59–0.89) |
| Tertile 3 |
214/2781 (8) |
0.83 (0.68–1.01) | 0.78 (0.64–0.96) |
MSDS indicates Mediterranean‐style diet score.
Variables included in the model: maternal age, race, education, marital status, smoking in pregnancy, parity, and prepregnancy obesity.
In our subgroup analyses by race, we found that a 1‐SD increment in MSDS was associated with 15% (4%–24%) times the odds of preeclampsia in Black women (Table 3). Looking at the joint association of MSDS and Black race with preeclampsia, we found that Black women in the lowest tertile of MSDS had the highest adjusted odds (1.72; 95% CI, 1.16–2.55) for preeclampsia compared with non‐Black women in the highest tertile of MSDS (Figure 3, Table S2). A formal P value for interaction test was performed to compare the interaction of tertiles of MSDS with race and demonstrated a null association (P=0.82). Finally, after limiting our analyses to nulliparous women only in a sensitivity analysis, we again found that women with greater ‐adherences to a Mediterranean style diet (ie, tertiles 2 and 3 of MSDS) had lower odds of preeclampsia compared with women in the lowest tertile (Table S3).
Table 3.
Association of MSDS With Preeclampsia, Stratified by Race
| MSDS |
Preeclampsia cases/ total participants (%) (n=311/3592) |
Unadjusted model, odds ratio (95% CI) |
Multivariable model,* odds ratio (95% CI) |
|---|---|---|---|
| Black women | |||
| Tertile 1 | 123/1182 (10) | Referent | Referent |
| Tertile 2 | 87/1124 (8) | 0.72 (0.54–0.96) | 0.71 (0.53–0.95) |
| Tertile 3 | 101/1286 (8) | 0.73 (0.56–0.97) | 0.74 (0.55–0.98) |
| MSDS |
Preeclampsia cases/ total participants (%) (n=368/4384) |
Unadjusted model, odds ratio (95% CI) |
Multivariable model*, odds ratio (95% CI) |
|---|---|---|---|
| Non‐Black women† | |||
| Tertile 1 | 133/1463 (9) | Referent | Referent |
| Tertile 2 | 100/1359 (7) | 0.79 (0.61–1.04) | 0.72 (0.54–0.95) |
| Tertile 3 | 135/1562 (9) | 0.95 (0.74–1.22) | 0.81 (0.62–1.05) |
MSDS indicates Mediterranean‐style diet score.
Variables included in the model: Maternal age, education, marital status, smoking in pregnancy, parity, and prepregnancy obesity.
†Non‐Black includes White, Hispanic, Asian, Cape Verdian, Pacific Islander, Mixed Race, or Other.
Figure 3. Adjusted* odds ratios of preeclampsia according to joint categories of Mediterranean‐style diet score and race.
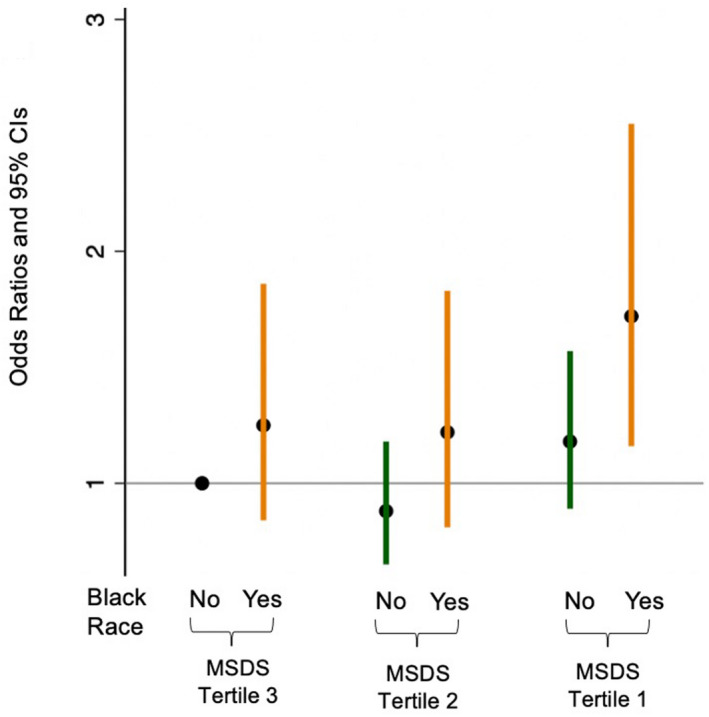
*Variables included in the model: maternal age, education, marital status, smoking in pregnancy, parity, and prepregnancy obesity. MSDS indicates Mediterranean‐style diet score.
Discussion
In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean‐style diet during pregnancy had >20% lower odds of developing preeclampsia, after adjusting for potential confounders. In addition, the evidence for the protective effect of a Mediterranean‐style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women.
In the Spanish population, the PREDIMED (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet) trial demonstrated that the traditional Mediterranean diet lowers blood pressure. 24 Blood pressure improvement with a Mediterranean diet has also been seen in pregnancy, and a prior cohort study in Australian women suggested an inverse relationship between a Mediterranean diet and preeclampsia. 25 , 26 In contrast, results from a recent randomized clinical trial, the ESTEEM (Effect of Simple, Targeted Diet in Pregnant Women With Metabolic Risk Factors on Pregnancy Outcomes) trial, did not demonstrate any reduction in preeclampsia by adherence to a Mediterranean diet during early pregnancy, but did show a benefit for gestational diabetes and maternal weight gain. 18 However, compared with our study, only a small number of women were enrolled in this trial, and the study was not powered to look at preeclampsia as an outcome.
Similarly, studies looking at the benefit of a Dietary Approaches to Stop Hypertension (DASH) diet—which is compositionally similar to the Mediterranean diet—in pregnancy have also been mixed. A large Dutch study demonstrated a reduction in midpregnancy diastolic blood pressure by adhering to a DASH diet, but did not show benefit for hypertensive disorders of pregnancy. 19 A smaller case‐control study in China and a recent paper from the Nurses’ Health Study II further showed reduction in the odds of preeclampsia with a DASH diet. 20 , 21 Conversely, an observational study of pregnant women in the United States did not show any benefit in blood pressure reduction during pregnancy with a DASH diet. 27
These incongruent results of dietary patterns with preeclampsia may be related to the heterogeneity of the pregnant women included in prior studies, specifically by including fewer higher‐risk and Black women. In our current study, we find that Black women are at the highest risk for preeclampsia and also have the greatest reduction in odds of preeclampsia by increased adherence to a Mediterranean‐style diet. As shown in Figure 3, compared with non‐Black women (White, Hispanic, Asian, Cape Verdian, Pacific Islander, Mixed Race, or Other) in MSDS tertile 3, only Black women in MSDS tertile 1 had a significantly higher risk of preeclampsia; other joint categories of race and MSDS tertiles were not statistically significant as compared with the referent group (non‐Black women in MSDS tertile 3). A strength of this study includes a relatively large number of Black women and women with preeclampsia, which is in part attributable to the study design to oversample women who delivered preterm or Low‐birth‐weight infants, who are more likely to be Black and have preeclampsia. 23 , 28 This allowed us to examine the association of a Mediterranean‐style diet with preeclampsia in this high‐risk group.
The benefit of a Mediterranean‐style diet may be attributable to improvement in oxidative stress or endothelial cell function. Increases in fetoplacental vascular resistance have been associated with preeclampsia. 29 A prior study showed improvement in placental vascular function with the DASH diet in pregnancy. 19 Similarly, in other populations, a Mediterranean diet has been shown to result in improvement in microvascular function and endothelial function, as measured via flow‐mediated dilation. 30 , 31 It is possible that through improved endothelial function, a Mediterranean‐style diet may contribute to improved placental vascular function in the setting of pregnancy. Adherence to a Mediterranean diet has also been shown to result in a unique metabolic signature, which has been predictive of lower future CVD risk. 32 Given the arguably shared pathogenesis between preeclampsia and CVD, it is also possible that increased adherence to a Mediterranean‐style diet may reduce risk of preeclampsia through metabolomic changes. 1 , 2
Our findings should be interpreted in the context of certain limitations. The current study is cross sectional, which limits our ability to establish temporality. Prospective studies, in which diet is assessed or intervened upon before the diagnosis of preeclampsia, are needed to verify our results. Also, the food frequency questionnaire was conducted at a single time point 24 to 72 hours postpartum, so misclassification of the diet is possible, as is the potential for recall bias. Since at the time of the questionnaire interview (over a decade ago), neither the mothers nor the interviewers were aware of the relationship between diet and preeclampsia, the potential recall bias is likely nondifferential, thus bias the association towards Another limitation is that while we relied on physician diagnosis for preeclampsia, maternal blood pressure measurements during pregnancy were not available. Despite these limitations, this study uses data from a large, racially and ethnically diverse urban population, and therefore provides insight into identifying the women who may be most likely to benefit from a dietary intervention for reducing risk of preeclampsia.
Conclusions
In summary, a Mediterranean‐style diet is associated with lower odds of preeclampsia. This finding remains significant after adjusting for various sociodemographic and preexisting clinical risk factors. Black women may be the most likely to benefit from a Mediterranean‐style diet.
Sources of Funding
The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20‐FY02‐56, #21‐FY07‐605); National Institutes of Health grants (R21ES011666, 2R01HD041702, R21HD066471, R01HD086013, R01HD098232, R01 ES031272, and R01ES031521); and the Health Resources and Services Administration of the US Department of Health and Human Services (UJ2MC31074). Dr Minhas was supported by the National Heart, Lung, and Blood Institute training grant T32HL007024; the Lou and Nancy Grasmick Research Fellowship; and the Marie‐Josée and Henry R. Kravis Endowed Fellowship in Honor of Dr James L. Weiss. M Zhang is supported by the American Heart Association (Award Number: 827990). Dr Michos is supported by the Amato Fund for Women’s Cardiovascular Health at Johns Hopkins. Dr Mueller is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (K01HL141589). This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, any funding agencies.
Disclosures
None.
Supporting information
Tables S1–S3
This material was presented as an oral presentation at the American Heart Association EPI|Lifestyle Scientific Sessions, May 20‐21, 2021.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022589
For Sources of Funding and Disclosures, see page 8.
References
- 1. Lane‐Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long‐term cardiovascular risks associated with adverse pregnancy outcomes. J Am Coll Cardiol. 2019;73:2106–2116. doi: 10.1016/j.jacc.2018.12.092 [DOI] [PubMed] [Google Scholar]
- 2. Minhas AS, Ying W, Ogunwole SM, Miller M, Zakaria S, Vaught AJ, Hays AG, Creanga AA, Cedars A, Michos ED, et al. The association of adverse pregnancy outcomes and cardiovascular disease: current knowledge and future directions. Curr Treat Options Cardio Med. 2020;22:61. doi: 10.1007/s11936-020-00862-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, et al. Preeclampsia and future cardiovascular health: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10: doi: 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 4. Timpka S, Fraser A, Schyman T, Stuart JJ, Åsvold BO, Mogren I, Franks PW, Rich‐Edwards JW. The value of pregnancy complication history for 10‐year cardiovascular disease risk prediction in middle‐aged women. Eur J Epidemiol. 2018;33:1003–1010. doi: 10.1007/s10654-018-0429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, Tanz LJ, Haug EB, Fraser A, Timpka S, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J. 2019;40:1113–1120. doi: 10.1093/eurheartj/ehy863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellamy L, Casas J‐P, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 8. Zhang M, Michos ED, Wang G, Wang X, Mueller NT. Associations of cord blood vitamin D and preeclampsia with offspring blood pressure in childhood and adolescence. JAMA Netw Open. 2020;3:e2019046. doi: 10.1001/jamanetworkopen.2020.19046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayet P‐Y, Rimoldi SF, Stuber T, Salmòn CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori‐Cucchia C, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–494. doi: 10.1161/CIRCULATIONAHA.110.941203 [DOI] [PubMed] [Google Scholar]
- 10. Estruch R, Ros E, Salas‐Salvadó J, Covas M‐I, Corella D, Arós F, Gómez‐Gracia E, Ruiz‐Gutiérrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- 11. Kesse‐Guyot E, Ahluwalia N, Lassale C, Hercberg S, Fezeu L, Lairon D. Adherence to Mediterranean diet reduces the risk of metabolic syndrome: a 6‐year prospective study. Nutr Metab Cardiovasc Dis. 2013;23:677–683. doi: 10.1016/j.numecd.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 12. Martínez‐González MA, Gea A, Ruiz‐Canela M. The Mediterranean diet and cardiovascular health: a critical review. Circ Res. 2019;124:779–798. doi: 10.1161/CIRCRESAHA.118.313348 [DOI] [PubMed] [Google Scholar]
- 13. Hernáez Á, Castañer O, Elosua R, Pintó X, Estruch R, Salas‐Salvadó J, Corella D, Arós F, Serra‐Majem L, Fiol M, et al. Mediterranean diet improves high‐density lipoprotein function in high‐cardiovascular‐risk individuals: a randomized controlled trial. Circulation. 2017;135:633–643. doi: 10.1161/CIRCULATIONAHA.116.023712 [DOI] [PubMed] [Google Scholar]
- 14. Rhee DK, Ji Y, Hong X, Pearson C, Wang X, Caulfield LE. Mediterranean‐style diet and birth outcomes in an urban, multiethnic, and low‐income US population. Nutrients. 2021;13:1188. doi: 10.3390/nu13041188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fingar KR, Hambrick MM, Heslin KC, Moore JE. Trends and Disparities in Delivery Hospitalizations Involving Severe Maternal Morbidity, 2006–2015: Statistical Brief #243. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Available at http://www.ncbi.nlm.nih.gov/books/NBK532465/. Accessed December 27, 2020. [PubMed]
- 16. Johnson JD, Louis JM. Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature. Am J Obstet Gynecol. 2022;226:S876–S885. doi: 10.1016/j.ajog.2020.07.038 [DOI] [PubMed] [Google Scholar]
- 17. Nelson DB, Moniz MH, Davis MM. Population‐level factors associated with maternal mortality in the United States, 1997–2012. BMC Public Health. 2018;18:1007. doi: 10.1186/s12889-018-5935-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. H. Al Wattar B, Dodds J, Placzek A, Beresford L, Spyreli E, Moore A, Gonzalez Carreras FJ, Austin F, Murugesu N, Roseboom TJ, et al; for the ESTEEM study group . Mediterranean‐style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLoS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wiertsema CJ, Mensink‐Bout SM, Duijts L, Mulders AGMGJ, Jaddoe VWV, Gaillard R. Associations of DASH diet in pregnancy with blood pressure patterns, placental hemodynamics, and gestational hypertensive disorders. JAHA. 2021;10: doi: 10.1161/JAHA.120.017503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao Y, Liu Y, Zhao X, Duan D, Dou W, Fu W, Chen H, Bo Y, Qiu Y, Chen G, et al. Adherence to a dietary approaches to stop hypertension (DASH)‐style diet in relation to preeclampsia: a case‐control study. Sci Rep. 2020;10:9078. doi: 10.1038/s41598-020-65912-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arvizu M, Stuart JJ, Rich‐Edwards JW, Gaskins AJ, Rosner B, Chavarro JE. Prepregnancy adherence to dietary recommendations for the prevention of cardiovascular disease in relation to risk of hypertensive disorders of pregnancy. Am J Clin Nutr. 2020;112:1429–1437. doi: 10.1093/ajcn/nqaa214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311:587. doi: 10.1001/jama.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin JA, Osterman MJK. Describing the increase in preterm births in the United States, 2014‐2016. NCHS Data Brief. 2018;1–8. [PubMed] [Google Scholar]
- 24. Toledo E, Hu FB, Estruch R, Buil‐Cosiales P, Corella D, Salas‐Salvadó J, Covas MI, Arós F, Gómez‐Gracia E, Fiol M, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med. 2013;11:207. doi: 10.1186/1741-7015-11-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Timmermans S, Steegers‐Theunissen RPM, Vujkovic M, Bakker R, den Breeijen H, Raat H, Russcher H, Lindemans J, Hofman A, Jaddoe VWV, et al. Major dietary patterns and blood pressure patterns during pregnancy: the Generation R Study. Am J Obstet Gynecol. 2011;205:337.e1–337.e12. doi: 10.1016/j.ajog.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 26. Schoenaker DA, Soedamah‐Muthu SS, Callaway LK, Mishra GD. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian Longitudinal Study on Women’s Health. Am J Clin Nutr. 2015;102:94–101. doi: 10.3945/ajcn.114.102475 [DOI] [PubMed] [Google Scholar]
- 27. Fulay AP, Rifas‐Shiman SL, Oken E, Perng W. Associations of the dietary approaches to stop hypertension (DASH) diet with pregnancy complications in Project Viva. Eur J Clin Nutr. 2018;72:1385–1395. doi: 10.1038/s41430-017-0068-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sibai BM. Preeclampsia as a cause of preterm and late preterm (near‐term) births. Semin Perinatol. 2006;30:16–19. doi: 10.1053/j.semperi.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 29. Gaillard R, Arends LR, Steegers EAP, Hofman A, Jaddoe VWV. Second‐ and third‐trimester placental hemodynamics and the risks of pregnancy complications. Am J Epidemiol. 2013;177:743–754. doi: 10.1093/aje/kws296 [DOI] [PubMed] [Google Scholar]
- 30. Klonizakis M, Alkhatib A, Middleton G, Smith MF. Mediterranean diet‐ and exercise‐induced improvement in age‐dependent vascular activity. Clin Sci. 2013;124:579–587. doi: 10.1042/CS20120412 [DOI] [PubMed] [Google Scholar]
- 31. Davis CR, Hodgson JM, Woodman R, Bryan J, Wilson C, Murphy KJ. A Mediterranean diet lowers blood pressure and improves endothelial function: results from the MedLey randomized intervention trial. Am J Clin Nutr. 2017;ajcn146803. doi: 10.3945/ajcn.116.146803 [DOI] [PubMed] [Google Scholar]
- 32. Li J, Guasch‐Ferré M, Chung W, Ruiz‐Canela M, Toledo E, Corella D, Bhupathiraju SN, Tobias DK, Tabung FK, Hu J, et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J. 2020;41:2645–2656. doi: 10.1093/eurheartj/ehaa209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


