Abstract
Background
The prevalence of hypertension subtypes changes with age. However, little is known regarding the age‐dependent association of hypertension subtypes with incident heart failure (HF).
Methods and Results
We conducted an observational cohort study including 2 612 570 people (mean age, 44.0 years; 55.0% men). No participants were taking blood pressure–lowering medications or had a known history of cardiovascular disease. Participants were categorized as aged 20 to 49 years (n=1 825 756), 50 to 59 years (n=571 574), or 60 to 75 years (n=215 240). We defined stage 1 hypertension as systolic blood pressure (SBP) 130 to 139 mm Hg or diastolic blood pressure (DBP) 80 to 89 mm Hg and stage 2 hypertension as SBP ≥140 mm Hg or DBP ≥90 mm Hg. Among participants with stage 2 hypertension, isolated diastolic hypertension was defined as SBP <140 mm Hg and DBP ≥90 mm Hg, isolated systolic hypertension as SBP ≥140 mm Hg and DBP <90 mm Hg, and systolic diastolic hypertension as SBP ≥140 mm Hg and DBP ≥90 mm Hg. During a mean follow‐up of 1205±934 days, 43 415 HF, 4807 myocardial infarction, 45 365 angina pectoris, 22 179 stroke, and 10 420 atrial fibrillation events occurred. Although the incidence of HF and other cardiovascular disease events increased with age, hazard ratios and relative risk reductions of each hypertension subtype for HF decreased with age. An age‐dependent relationship between hypertension subtypes and incident HF was similarly observed in both men and women.
Conclusions
The contribution of isolated diastolic hypertension, isolated systolic hypertension, and systolic diastolic hypertension to the development of HF and other cardiovascular disease events was attenuated with age, suggesting that preventive efforts for blood pressure control could provide a greater benefit in younger individuals.
Keywords: age, epidemiology, heart failure, hypertension subtype
Subject Categories: Hypertension, Heart Failure, Epidemiology
Nonstandard Abbreviations and Acronyms
- AP
angina pectoris
- DBP
diastolic blood pressure
- IDH
isolated diastolic hypertension
- ISH
isolated systolic hypertension
- PAF
population attributable fraction
- RRR
relative risk reduction
- SBP
systolic blood pressure
- SDH
systolic diastolic hypertension
Clinical Perspective
What Is New?
Our analysis of a nationwide administrative claims database including 2 612 570 people showed that isolated diastolic hypertension, isolated systolic hypertension, and systolic diastolic hypertension were associated with an elevated risk for heart failure in all age categories (20–49, 50–59, and 60–75 years).
The hazard ratio and relative risk reduction of each hypertension subtype for heart failure decreased with age.
This age‐dependent relationship of hypertension subtypes was similarly seen in other cardiovascular disease events.
What Are the Clinical Implications?
The present analysis of a large‐scale administrative claims database showed that all hypertension subtypes increased the risk of heart failure and other cardiovascular disease events in all age categories.
Although the incidence of heart failure and other cardiovascular disease events increased with age, the contribution of each hypertension subtype to the development of heart failure and other cardiovascular disease events was attenuated with age.
Hypertension has several subtypes, including isolated diastolic hypertension (IDH), isolated systolic hypertension (ISH), and systolic diastolic hypertension (SDH), all of which may be associated with an elevated risk of cardiovascular disease (CVD). However, previous analyses of the relationship between IDH and ISH with incident CVD have yielded conflicting results, 1 , 2 , 3 and several issues remain to be clarified. Systolic blood pressure (SBP) increases with age, whereas diastolic blood pressure (DBP) decreases with age, and the prevalence of ISH and IDH changes with age. Therefore, the prognostic effect of each hypertension subtype varies with age. Furthermore, little is known about the age‐dependent association between hypertension and its subtypes and incident CVD. Most preceding studies on the relationship between hypertension subtypes and CVD outcomes have analyzed the influence of hypertension subtypes on the subsequent risk of overall CVD, ischemic heart disease, and stroke. 4 , 5 , 6 , 7 On the other hand, there have been scarce clinical data on the association of hypertension subtypes with incident heart failure (HF). The prevalence of HF is increasing worldwide, and the public health importance of HF is becoming more increasingly recognized. Furthermore, a previous study showed that the prognostic impact of each hypertension subtype on the future risk of developing HF might differ between men and women. 1 Using a nationwide administrative claims database, we here examined (1) whether the association of each hypertension subtype with the incidence of HF varied with age, (2) whether the proportion of HF that are potentially preventable when blood pressure (BP) is lowered to the normal range was different between age categories, and (3) whether the influence of each hypertension subtype on the incidence of HF differed between men and women.
Methods
The JMDC Claims Database is available for purchase from JMDC Inc. (https://www.jmdc.co.jp/en/index).
Study Population
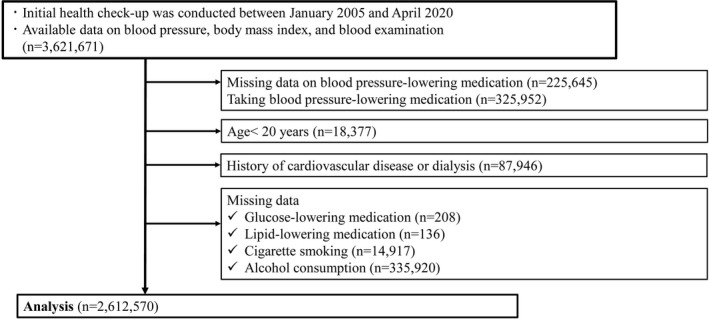
We conducted this retrospective cohort study using the JMDC Claims Database (JMDC Inc., Tokyo, Japan), a health check‐up and administrative claims database, between January 2005 and April 2020. 2 , 8 The JMDC Claims Database includes the records of participants’ annual health check‐up data, including data on BP, body mass index, medical history, current medications, and administrative claims records, including the diagnosis of CVDs based on the International Classification of Diseases, Tenth Revision (ICD‐10) coding. Among the 3 621 671 individuals with available health data, including physical examination and blood test data, we excluded individuals who met the following criteria: (1) missing data on BP‐lowering medications taken (n=225 645) or taking BP‐lowering medications (n=325 952); (2) age <20 years (n=18 377); (3) history of CVD, including HF, myocardial infarction (MI), angina pectoris (AP), stroke, atrial fibrillation (AF), or dialysis (n=87 946); and (4) missing data on medications for the treatment of diabetes (n=208), dyslipidemia (n=136), cigarette smoking (n=14 917), and alcohol consumption (n=335 920). The final study population included 2 612 570 participants (Figure 1).
Figure 1. Participant flowchart.
Ethics
This study was approved by the Ethical Committee of the University of Tokyo (number 2018‐10862) and was conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived because all of the data contained in the JMDC Claims Database were deidentified after combining individual’s health check‐up and administrative claims records.
Measurements and Definitions
Data on the following items were collected using standardized protocols at the health check‐up: BP, body mass index, medication status, history of CVD or dialysis, and fasting blood test data. In the Japanese health check‐up system, trained health care professionals measure the BP at least twice after the participant has been in a resting condition using a mercury or aneroid sphygmomanometer or a validated automated device. The average BP values were recorded, based on the recommendations of the Ministry of Health, Labour and Welfare, and the Japanese Society of Cardiovascular Disease Prevention. 8 Detailed BP measurement methods are summarized in Data S1. Consistent with guidelines, we defined stage 1 hypertension as SBP of 130 to 139 mm Hg or DBP of 80 to 89 mm Hg and stage 2 hypertension as SBP ≥140 mm Hg or DBP ≥90 mm Hg. Among participants with stage 2 hypertension, IDH was defined as SBP <140 mm Hg and DBP ≥90 mm Hg, ISH as SBP ≥140 mm Hg and DBP <90 mm Hg, and SDH as SBP ≥140 mm Hg and DBP ≥90 mm Hg. We defined nonhypertension (normotension) as SBP <130 mm Hg and DBP <80 mm Hg. We defined obesity as a body mass index ≥25 kg/m2. We defined diabetes as a fasting glucose level ≥126 mg/dL or use of glucose‐lowering medications. We defined dyslipidemia as low‐density lipoprotein cholesterol level ≥140 mg/dL, high‐density lipoprotein cholesterol level <40 mg/dL, triglyceride level ≥150 mg/dL, or use of lipid‐lowering medications. 9 Information regarding cigarette smoking (current or noncurrent) and alcohol consumption (every day or not every day) was self‐reported.
Outcomes
Outcomes were collected between January 2005 and April 2020. The primary outcome was HF. Secondary outcomes included MI, AP, stroke, and AF. ICD‐10 codes used in this study are summarized in Data S2.
Statistical Analysis
Continuous variables are presented as mean (SD), and categorical variables are presented as number (percentage). We categorized study participants by age category (20–49, 50–59, and 60–75 years) based on a previous study using the Framingham Heart Study, which examined the age‐dependent association between BP and incident CVD. 4 We compared continuous variables between the 3 age categories using analysis of variance, and categorical variables using χ2 tests with the Cramer V statistic to assess effect size. We conducted Cox regression analysis to identify the association of each hypertension subtype with the incidence of HF or other CVDs. Model 1 included hypertension subtypes alone (unadjusted model). Model 2 included hypertension subtypes, age, and sex, and we conducted the multivariable Cox regression analyses (forced entry model). Furthermore, in model 3, we added conventional CVD risk factors, including obesity, diabetes, dyslipidemia, cigarette smoking, and alcohol consumption, to model 2, and we performed the multivariable Cox regression analyses (forced entry model). The relative risk reduction (RRR) indicates the proportion of people with a disease that can be attributed to each hypertension subtype, that is, the proportional reduction expected to occur if the exposure to that particular risk factor (eg, IDH, ISH, and SDH) is reduced to the nonhypertension level. We estimated the RRR with its corresponding 95% CI, adjusted for the covariates included in the multivariable Cox regression model.
We analyzed the association between hypertension subtypes and incident HF stratified by sex. We performed 7 sensitivity analyses. First, we defined IDH as DBP ≥80 mm Hg and SBP <130 mm Hg, ISH as SBP ≥130 mm Hg and DBP <80 mm Hg, and SDH as SBP ≥130 mm Hg and DBP ≥80 mm Hg. Second, we defined stage 1 IDH as SBP <130 mm Hg and DBP 80 to 89 mm Hg, stage 1 ISH as SBP 130 to 139 mm Hg and DBP <80 mm Hg, stage 1 SDH as SBP 130 to 139 mm Hg and DBP 80 to 89 mm Hg, stage 2 IDH as SBP <140 mm Hg and DBP ≥90 mm Hg, stage 2 ISH as SBP ≥140 mm Hg and DBP <90 mm Hg, and stage 2 SDH as SBP ≥140 mm Hg and DBP ≥90 mm Hg. Third, we changed the cutoff value of the age categories to 20 to 44, 45 to 54, and 55 to 75 years. Fourth, we categorized the study participants using the tertiles of age (20–40, 40–48, 48–75 years). Fifth, as death could be regarded as a competing risk with HF events, we performed cause‐specific Cox proportional hazard modeling as a competing risks analysis. 8 Sixth, to consider the influence of renal function, we added estimated glomerular filtration rate in model 3. Seventh, we analyzed the relationship of each hypertension subtype with all‐cause mortality.
We calculated population attributable fraction (PAF), interpreted as the reduction that would be possible if each risk factor was normalized in the whole population, using the following formula: RRR×(no. of events in people having a risk factor [eg, IDH, ISH, or SDH]/no. of total events).
The null hypothesis was rejected for (2‐tailed) values of P<0.05. Statistical analyses were performed using SPSS version 25 (IBM Inc., Armonk, NY) and Stata version 17 (StataCorp LLC, College Station, TX).
Results
Baseline Characteristics
The baseline characteristics of the study participants are summarized in Table 1. Participants were categorized into the following age groups according to their age at the baseline health check‐up: 20 to 49 years (n=1 825 756), 50 to 59 years (n=571 574), and 60 to 75 years (n=215 240). The prevalence of stage 1 hypertension, ISH, and SDH increased with age. Among participants with stage 2 hypertension, the prevalence of IDH decreased from 30.9% in those aged 20 to 49 years to 13.6% in those aged 60 to 75 years. In contrast, the prevalence of ISH among participants with stage 2 hypertension increased from 23.7% in those aged 20 to 49 years to 43.8% in those aged 60 to 75 years.
Table 1.
Characteristics of Study Participants
|
Ages 20 to 49 y (n=1 825 756) |
Ages 50 to 59 y (n=571 574) |
Ages 60 to 75 y (n=215 240) |
P value | |
|---|---|---|---|---|
| Systolic BP, mm Hg, mean (SD) | 115 (15) | 121 (17) | 126 (18) | <0.001 |
| Diastolic BP, mm Hg, mean (SD) | 71 (11) | 76 (12) | 77 (11) | <0.001 |
| BP classification, n (%) | ||||
| Nonhypertension | 1 370 035 (75.0) | 328 157 (57.4) | 107 472 (49.9) | |
| Stage 1 hypertension | 322 516 (17.7) | 148 267 (25.9) | 59 294 (27.5) | |
| Stage 2 hypertension | ||||
| Isolated diastolic hypertension | 41 147 (2.3) | 24 558 (4.3) | 6579 (3.1) | |
| Isolated systolic hypertension | 31 511 (1.7) | 24 260 (4.2) | 21 255 (9.9) | |
| Systolic diastolic hypertension | 60 547 (3.3) | 46 332 (8.1) | 20 640 (9.6) | |
| Age, y, mean (SD) | 38.7 (7.4) | 53.9 (2.8) | 63.5 (3.3) | … |
| Men, n (%) | 1 003 021 (54.9) | 313 155 (54.8) | 121 082 (56.3) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 22.6 (3.8) | 22.8 (3.4) | 22.5 (3.0) | <0.001 |
| Obesity, n (%) | 395 231 (21.6) | 130 877 (22.9) | 41 695 (19.4) | <0.001 |
| Diabetes, n (%) | 30 822 (1.7) | 28 990 (5.1) | 18 141 (8.4) | <0.001 |
| Dyslipidemia, n (%) | 560 441 (30.7) | 282 598 (49.4) | 119 028 (55.3) | <0.001 |
| Fasting glucose, mg/dL, mean (SD) | 91 (14) | 97 (19) | 100 (20) | <0.001 |
| LDL‐C, mg/dL, mean (SD) | 116 (31) | 130 (31) | 132 (31) | <0.001 |
| HDL‐C, mg/dL, mean (SD) | 63 (16) | 66 (18) | 66 (17) | <0.001 |
| Triglyceride, mg/dL, mean (SD) | 96 (79) | 109 (84) | 109 (72) | <0.001 |
| Cigarette smoking, n (%) | 474 635 (26.0) | 144 742 (25.3) | 44 787 (20.8) | <0.001 |
| Alcohol consumption, n (%) | 342 884 (18.8) | 159 773 (28.0) | 64 017 (29.7) | <0.001 |
P values were calculated using χ2 tests for categorical variables and analysis of variance for continuous variables. Based on age at health check‐up, study participants were categorized into the following age groups: 20 to 49 years (n=1 825 756), 50 to 59 years (n=571 574), and 60 to 75 years (n=215 240). Cramer V values were as follows: BP classification, 0.16; men (sex), 0.01; obesity, 0.02; diabetes, 0.13; dyslipidemia, 0.20; cigarette smoking, 0.03; and alcohol consumption, 0.11. BP indicates blood pressure; HDL‐C, high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol.
Incidence of HF and Hazard Ratios of Each Hypertension Subtype Stratified by Age
The incidence of HF and the hazard ratios (HRs) of the hypertension subtypes for HF stratified by age group are summarized in Figure 2. During a mean±SD follow‐up of 1205±934 days and a median (quartile 1–quartile 3) of 954 (501–1701) days, 43 415 HF events were recorded. The incidence of HF increased with age. Compared with normal BP, stage 1 hypertension, IDH, ISH, and SDH were all associated with a greater incidence of HF in the 3 age categories. The HRs of stage 1 hypertension, IDH, ISH, and SDH for HF decreased with age. Compared with nonhypertension, HRs (95% CIs) of stage 1 hypertension, IDH, ISH, and SDH for HF were 1.35 (1.31–1.40), 2.03 (1.90–2.16), 1.96 (1.81–2.11), and 3.10 (2.95–3.25), respectively, in participants aged 20 to 49 years. HRs (95% CIs) of stage 1 hypertension, IDH, ISH, and SDH for HF in participants aged 50 to 59 years were 1.28 (1.23–1.33), 1.63 (1.52–1.75), 1.72 (1.60–1.84), and 2.12 (2.02–2.23), respectively. HRs (95% CIs) of stage 1 hypertension, IDH, ISH, and SDH for HF in participants aged 60 to 75 years were 1.14 (1.08–1.21), 1.36 (1.19–1.54), 1.44 (1.34–1.56), and 1.72 (1.60–1.85), respectively.
Figure 2. Hypertension subtype and heart failure event.
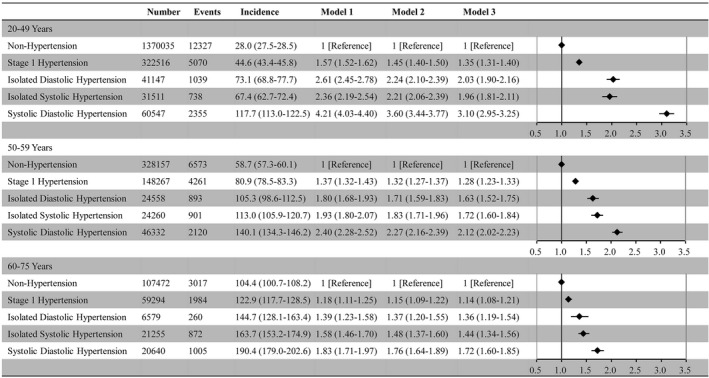
Number of heart failure events, incidence (per 10 000 person‐years), and hazard ratio (95% CI) of each hypertension subtype for heart failure are shown.
Incidence of Other CVDs and HRs of Each Hypertension Subtype Stratified by Age
The incidence of MI, AP, stroke, and AF, and the HRs of each hypertension subtype for these CVD events stratified by age group are summarized in Table 2. During the follow‐up period, 4807 MI events, 45 365 APs, 22 179 strokes, and 10 420 AF events were recorded. The incidence of all CVD events increased with age. Compared with nonhypertension, stage 1 hypertension, IDH, ISH, and SDH were all associated with a greater incidence of MI, AP, stroke, and AF in the 3 age categories.
Table 2.
Frequency of Events, Corresponding Incidence Rates, and Hazard Ratios for Cardiovascular Events
| Ages 20 to 49 y | Ages 50 to 59 y | Ages 60 to 75 y | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonhypertension (n=1 370 035) | Stage 1 hypertension (n=322 516) | IDH (n=41 147) | ISH (n=31 511) | SDH (n=60 547) | Nonhypertension (n=328 157) | Stage 1 hypertension (n=148 267) | IDH (n=24 558) | ISH (n=24 260) | SDH (n=46 332) | Nonhypertension (n=107 472) | Stage 1 hypertension (n=59 294) | IDH (n=6579) | ISH (n=21 255) | SDH (n=20 640) | |
| Myocardial infarction | |||||||||||||||
| No. of events | 1166 | 605 | 122 | 101 | 249 | 696 | 614 | 111 | 113 | 235 | 321 | 234 | 45 | 85 | 110 |
| Incidence | 2.6 (2.5–2.8) | 5.3 (4.9–5.7) | 8.4 (7.1–10.1) | 9.1 (7.5–11.0) | 12.1 (10.7–13.7) | 6.1 (5.7–6.6) | 11.5 (10.6–12.4) | 12.8 (10.7–15.5) | 13.9 (11.5–16.7) | 15.1 (13.3–17.1) | 10.9 (9.8–12.2) | 14.2 (12.5–16.1) | 24.5 (18.3–32.7) | 15.5 (12.6–19.2) | 20.2 (16.8–24.4) |
| Model 1 | 1 (ref) | 1.97 (1.79–2.18) | 3.19 (2.65–3.85) | 3.37 (2.75–4.14) | 4.60 (4.01–5.27) | 1 (ref) | 1.86 (1.67–2.08) | 2.09 (1.71–2.56) | 2.26 (1.85–2.75) | 2.47 (2.13–2.86) | 1 (ref) | 1.30 (1.10–1.54) | 2.25 (1.65–3.08) | 1.43 (1.12–1.81) | 1.86 (1.50–2.31) |
| Model 2 | 1 (ref) | 1.52 (1.38–1.68) | 2.13 (1.76–2.57) | 2.79 (2.27–3.42) | 3.16 (2.75–3.64) | 1 (ref) | 1.58 (1.42–1.76) | 1.63 (1.34–2.00) | 2.04 (1.67–2.49) | 2.01 (1.73–2.34) | 1 (ref) | 1.17 (0.99–1.39) | 1.87 (1.37–2.56) | 1.31 (1.03–1.67) | 1.58 (1.27–1.97) |
| Model 3 | 1 (ref) | 1.36 (1.22–1.50) | 1.83 (1.51–2.21) | 2.22 (1.80–2.73) | 2.44 (2.11–2.82) | 1 (ref) | 1.56 (1.40–1.74) | 1.62 (1.32–1.99) | 1.90 (1.56–2.33) | 1.91 (1.64–2.22) | 1 (ref) | 1.21 (1.02–1.44) | 2.04 (1.49–2.79) | 1.33 (1.04–1.69) | 1.67 (1.34–2.08) |
| Angina pectoris | |||||||||||||||
| No. of events | 14146 | 5458 | 948 | 732 | 1763 | 7630 | 4508 | 872 | 861 | 1825 | 2993 | 1931 | 221 | 715 | 762 |
| Incidence | 32.2 (31.7–32.8) | 48.1 (46.8–49.4) | 66.7 (62.6–71.1) | 67.0 (62.3–72.0) | 87.6 (83.6–91.8) | 68.4 (66.8–69.9) | 85.8 (83.4–88.4) | 103.1 (96.5–110.2) | 108.2 (101.2–115.7) | 120.3 (114.9–125.9) | 103.7 (100.1–107.5) | 119.9 (114.7–125.4) | 122.8 (107.6–140.0) | 133.8 (124.4–144.0) | 143.4 (133.6–154.0) |
| Model 1 | 1 (ref) | 1.48 (1.43–1.53) | 2.07 (1.94–2.21) | 2.05 (1.90–2.21) | 2.72 (2.59–2.86) | 1 (ref) | 1.25 (1.21–1.30) | 1.51 (1.41–1.62) | 1.58 (1.48–1.70) | 1.76 (1.67–1.85) | 1 (ref) | 1.16 (1.09–1.22) | 1.19 (1.03–1.36) | 1.29 (1.19–1.40) | 1.38 (1.28–1.50) |
| Model 2 | 1 (ref) | 1.30 (1.26–1.35) | 1.66 (1.56–1.78) | 1.86 (1.73–2.00) | 2.20 (2.09–2.31) | 1 (ref) | 1.22 (1.18–1.27) | 1.46 (1.36–1.56) | 1.53 (1.43–1.64) | 1.70 (1.61–1.79) | 1 (ref) | 1.14 (1.08–1.21) | 1.19 (1.04–1.36) | 1.23 (1.13–1.33) | 1.36 (1.25–1.47) |
| Model 3 | 1 (ref) | 1.22 (1.18–1.26) | 1.51 (1.41–1.61) | 1.65 (1.53–1.78) | 1.90 (1.81–2.01) | 1 (ref) | 1.19 (1.14–1.23) | 1.40 (1.30–1.50) | 1.44 (1.34–1.55) | 1.60 (1.52–1.69) | 1 (ref) | 1.12 (1.05–1.18) | 1.16 (1.01–1.33) | 1.18 (1.08–1.28) | 1.30 (1.20–1.41) |
| Stroke | |||||||||||||||
| No. of events | 5220 | 2220 | 494 | 270 | 1098 | 3786 | 2373 | 506 | 423 | 1250 | 1926 | 1263 | 173 | 513 | 664 |
| Incidence | 11.8 (11.5–12.1) | 19.4 (18.6–20.2) | 34.4 (31.5–37.6) | 24.4 (21.7–27.5) | 54.0 (50.9–57.3) | 33.6 (32.6–34.7) | 44.7 (42.9–46.5) | 59.2 (54.2–64.5) | 52.4 (47.6–57.6) | 81.4 (77.0–86.1) | 66.3 (63.4–69.3) | 77.7 (73.5–82.1) | 95.5 (82.3–110.9) | 95.1 (87.3–103.7) | 124.4 (115.3–134.3) |
| Model 1 | 1 (ref) | 1.62 (1.54–1.70) | 2.90 (2.64–3.18) | 2.03 (1.79–2.29) | 4.56 (4.28–4.87) | 1 (ref) | 1.33 (1.26–1.40) | 1.76 (1.61–1.93) | 1.56 (1.41–1.72) | 2.43 (2.28–2.59) | 1 (ref) | 1.17 (1.09–1.26) | 1.44 (1.24–1.69) | 1.44 (1.30–1.59) | 1.88 (1.72–2.05) |
| Model 2 | 1 (ref) | 1.43 (1.36–1.51) | 2.32 (2.11–2.55) | 1.83 (1.62–2.07) | 3.61 (3.38–3.86) | 1 (ref) | 1.29 (1.22–1.36) | 1.71 (1.56–1.88) | 1.46 (1.32–1.62) | 2.33 (2.18–2.48) | 1 (ref) | 1.17 (1.09–1.25) | 1.50 (1.28–1.75) | 1.33 (1.21–1.47) | 1.86 (1.70–2.04) |
| Model 3 | 1 (ref) | 1.40 (1.33–1.48) | 2.24 (2.04–2.47) | 1.74 (1.54–1.97) | 3.41 (3.18–3.65) | 1 (ref) | 1.28 (1.22–1.35) | 1.71 (1.55–1.88) | 1.42 (1.28–1.57) | 2.28 (2.13–2.44) | 1 (ref) | 1.16 (1.08–1.25) | 1.50 (1.28–1.75) | 1.30 (1.18–1.44) | 1.84 (1.68–2.01) |
| Atrial fibrillation | |||||||||||||||
| No. of events | 2475 | 1114 | 189 | 130 | 381 | 1722 | 1196 | 254 | 220 | 485 | 981 | 631 | 115 | 239 | 288 |
| Incidence | 5.6 (5.4–5.8) | 9.7 (9.2–10.3) | 13.1 (11.4–15.1) | 11.7 (9.9–13.9) | 18.6 (16.8–20.5) | 15.2 (14.5–16.0) | 22.4 (21.2–23.7) | 29.5 (26.1–33.4) | 27.1 (23.7–30.9) | 31.2 (28.6–34.1) | 33.5 (31.5–35.6) | 38.5 (35.6–41.6) | 63.1 (52.5–75.7) | 43.9 (38.7–49.8) | 53.2 (47.4–59.7) |
| Model 1 | 1 (ref) | 1.71 (1.59–1.84) | 2.33 (2.01–2.70) | 2.05 (1.72–2.45) | 3.31 (2.97–3.69) | 1 (ref) | 1.47 (1.36–1.58) | 1.94 (1.70–2.22) | 1.78 (1.54–2.04) | 2.06 (1.86–2.28) | 1 (ref) | 1.15 (1.04–1.27) | 1.89 (1.56–2.29) | 1.32 (1.14–1.52) | 1.59 (1.40–1.82) |
| Model 2 | 1 (ref) | 1.31 (1.22–1.41) | 1.54 (1.33–1.79) | 1.69 (1.41–2.01) | 2.27 (2.03–2.53) | 1 (ref) | 1.25 (1.16–1.35) | 1.52 (1.33–1.74) | 1.62 (1.41–1.87) | 1.69 (1.53–1.87) | 1 (ref) | 1.05 (0.95–1.16) | 1.63 (1.34–1.98) | 1.20 (1.04–1.38) | 1.39 (1.22–1.58) |
| Model 3 | 1 (ref) | 1.25 (1.16–1.34) | 1.42 (1.22–1.66) | 1.54 (1.29–1.84) | 2.02 (1.80–2.26) | 1 (ref) | 1.21 (1.12–1.31) | 1.45 (1.27–1.66) | 1.55 (1.34–1.78) | 1.60 (1.44–1.77) | 1 (ref) | 1.04 (0.94–1.15) | 1.59 (1.31–1.93) | 1.18 (1.02–1.36) | 1.35 (1.18–1.54) |
The incidence rate was per 10 000 person‐years. Unadjusted and adjusted hazard ratios (95% CIs) associated with hypertension subtypes are shown. Model 1 is unadjusted. Model 2 includes adjustment for age and sex. Model 3 includes adjustment for age, sex, obesity, diabetes, dyslipidemia, cigarette smoking, and alcohol consumption. IDH indicates isolated diastolic hypertension; ISH, isolated systolic hypertension; ref, reference; and SDH, systolic diastolic hypertension.
Relative Risk Reduction
The RRR of stage 1 hypertension, IDH, ISH, and SDH for HF and other CVDs, including MI, AP, stroke, and AF events, are summarized in Table S1 and Figure 3. The RRR of stage 1 hypertension, IDH, ISH, and SDH for HF events decreased with age. The RRR of stage 1 hypertension, IDH, ISH, and SDH for MI, AP, stroke, and AF events also decreased with age, except for the RRR of stage 1 hypertension for MI and the RRR of IDH for MI and AF.
Figure 3. Relative risk reduction.
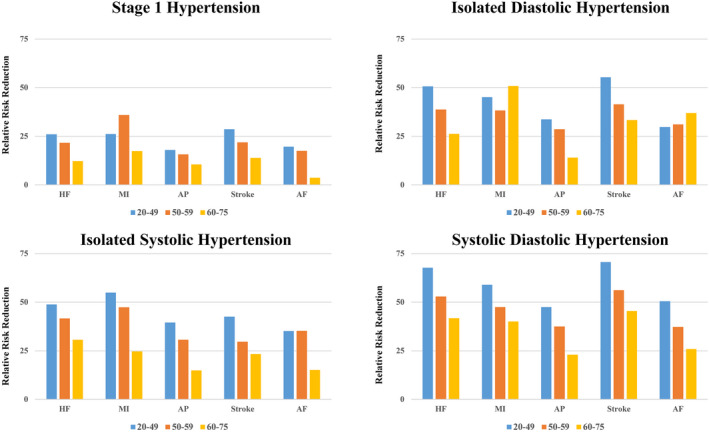
Relative risk reductions of stage1 hypertension, isolated diastolic hypertension, isolated systolic hypertension, and systolic diastolic hypertension for heart failure (HF), myocardial infarction (MI), angina pectoris (AP), stroke, and atrial fibrillation (AF) according to age category are shown.
Stratified Analysis by Sex
The number of HF events, incidence of HF events, and HRs of hypertension subtypes for HF events stratified by sex are presented in Figure S1. Multivariable Cox regression analyses showed that, compared with nonhypertension, stage 1 hypertension, IDH, ISH, and SDH were associated with an increased incidence of HF in all age categories in both men (Figure S1A) and women (Figure S1B). The RRR of stage 1 hypertension, IDH, ISH, and SDH for HF decreased with age in both men and women (Table S2 and Figure 4).
Figure 4. Relative risk reduction stratified by sex.
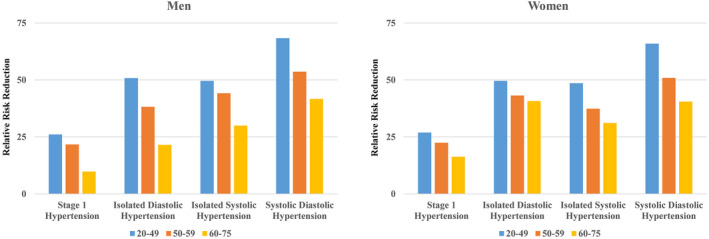
Relative risk reductions of stage1 hypertension, isolated diastolic hypertension, isolated systolic hypertension, and systolic diastolic hypertension for heart failure according to age category among men and women are shown.
Sensitivity Analyses
First, even when we categorized stage 1 and stage 2 hypertension into IDH, ISH, and SDH, IDH, ISH, and SDH were associated with a higher risk of HF events in all age categories (Figure S2). Second, we categorized stage 1 hypertension into stage 1 IDH, ISH, and SDH and stage 2 hypertension into stage 2 IDH, ISH, and SDH. The relationship between each hypertension subtype and incident HF was attenuated with age (Figure S3). Third, we categorized study participants into people aged 20 to 44 years (n=1 378 067), 45 to 54 years (n=786 922), and 55 to 75 years (n=447 581). In this model, the HRs of stage 1 hypertension, IDH, ISH, and SDH for HF decreased with age as well (Figure S4). Fourth, when we categorized the study participants by the tertile of age, our main results did not change (Figure S5). Fifth, the main results were unchanged in the cause‐specific Cox proportional hazard modeling as a competing risks analysis. In participants aged 20 to 49 years, compared with participants without hypertension, the HRs (95% CIs) of stage 1 hypertension, IDH, ISH, and SDH for HF were 1.35 (1.31–1.40), 2.03 (1.90–2.16), 1.96 (1.81–2.11), and 3.10 (2.95–3.25), respectively. In participants aged 50 to 59 years, compared with participants without hypertension, the HRs (95% CIs) of stage 1 hypertension, IDH, ISH, and SDH for HF were 1.28 (1.23–1.33), 1.63 (1.52–1.75), 1.72 (1.60–1.84), and 2.12 (2.02–2.23), respectively. In participants aged 60 to 75 years, compared with participants without hypertension, the HRs (95% CIs) of stage 1 hypertension, IDH, ISH, and SDH for HF were 1.14 (1.08–1.21), 1.36 (1.19–1.54), 1.44 (1.34–1.56), and 1.72 (1.60–1.85), respectively. Sixth, we analyzed 1 029 517 participants with available data on estimated glomerular filtration rate. Even after adding estimated glomerular filtration rate to model 3, our main results did not change (Figure S6). Seventh, although the all‐cause mortality increased with age, HRs of each hypertension subtype for all‐cause mortality decreased with age (Figure S7).
Population Attributable Fraction
PAFs of each hypertension subtype are summarized in Figure S8. Compared with the results of RRR, the age‐dependent tendency of PAF was not clear.
Discussion
The current study used data from a nationwide administrative claims database, including a general population of >2.5 million people, with no history of CVD and not taking BP‐lowering medications. The results demonstrated that although all hypertension subtypes were associated with increased risk of incident HF in all age categories, all hypertension subtypes conferred a higher proportion of HF events and a greater RRR in younger than in older people. To the best of our knowledge, this is the largest study to present age‐associated differences in the strength of associations between hypertension subtypes and the incidence of HF.
Although hypertension is known to increase the risk of HF, data on the relationship between hypertension subtypes and the incidence of HF are limited. The pathological mechanisms and hemodynamic patterns differ between IDH and ISH. Increased vascular resistance is a major feature of IDH (higher DBP), whereas higher stroke volume and/or increased aortic stiffness are major contributors to ISH (higher SBP). 10 Considering these differences in the pathological basis of IDH and ISH, the prognostic value of IDH and ISH for HF and other CVD events may vary.
Consistent with previous studies, we confirmed that the proportion of IDH was lower, and that of ISH was higher, in older compared with younger people. Despite the differences in the proportion of IDH and ISH among age categories, the contribution of IDH, ISH, and SDH to HF declined with age. An age‐dependent relationship with hypertension subtypes was also observed in other forms of CVD, including MI, AP, stroke, and AF, except for stage 1 hypertension for MI and IDH for MI and AF. Although hypertension is known to be associated with an elevated risk of CVD events in young adults, 5 it is clinically important that this relationship is stronger in young people than in older people.
These results are consistent with findings of previous studies suggesting that the association of hypertension with CVD outcomes is attenuated with advancing age. 6 , 11 However, the current study is the first to demonstrate the age‐dependent relationship of each hypertension subtype with incident HF and with various CVD events using a large‐scale epidemiological data set. In addition, by calculating the RRR of each hypertension subtype, we showed the clinical impact of normalizing the BP of each hypertension subtype. Moreover, we confirmed the robustness of the results through sensitivity analyses using different definitions of hypertension subtypes and cutoff values for age categories.
There are several possible explanations for our results. Baseline CVD risk in young people is generally low, and therefore the influence of hypertension would be more pronounced in younger adults. In contrast, the aging process and its associated pathological changes would make large contributions to the development of HF or other CVD events (eg, cardiac fibrosis in HF with preserved ejection fraction), which would overwhelm the influence of hypertension in older people. Further investigations are needed to clarify the underlying mechanisms of our results.
Furthermore, given sex differences in hormonal influences, cardiovascular biology, and social environmental factors, 12 there may be a difference between sexes in the prognostic value of IDH and ISH for the development of CVD including HF. The increase in BP with age is steeper in women than in men and begins at a younger age. 13 Moreover, the influence of BP on CVD outcomes is more pronounced in women than in men. 14 An analysis of the Chicago Heart Association Detection Project in Industry Study of young and middle‐aged adults showed that the CVD risk of ISH in men was similar to the CVD risk in men with high‐normal BP and was lower than that of IDH, whereas the CVD risk of ISH in women was higher than that of women with high‐normal BP or IDH. 1 The present study showed a similar influence of IDH, ISH, and SDH on the risk of HF events in men and women. An attenuated relationship between each hypertension subtype and incident HF with age was seen in both men and women. On the other hand, the RRR of stage 1 hypertension and IDH seemingly decreased less in women than in men particularly for the age category of 60 to 75 years. These results need to be confirmed using other independent data sets.
The present study has several clinical implications. The importance of hypertension in young adults is frequently underestimated, and elevated BP is often left without adequate evaluation and treatment. For example, ISH in young adults is sometimes thought to be a “false” or “spurious” hypertension. Furthermore, IDH tends to be disregarded because the prognostic influence of DBP is thought to be lower than that of SBP. However, similar to our results, the importance of ISH and IDH in the development of CVD in young adults has been shown in other studies. 1 , 15 Furthermore, even people aged 20 to 49 years with stage 1 IDH and ISH were at a higher risk for HF than individual without hypertension, as shown in Figure S3. Therefore, we should not underestimate the clinical significance of IDH and ISH, even in young people. Because the prevalence of hypertension (particularly ISH) is increasing in young adults as a result of the obesity epidemic 16 , 17 and the incidence of CVD in young people is increasing or stagnating compared with the older population, the optimal management of each hypertension subtype may provide greater than expected benefit to prevent future CVD events in young adults. However, it should also be noted that our results did not deny the importance of hypertension in older people. As shown in Figure S7, the age‐dependent relationship of the PAF for each hypertension subtype was not clear compared with that of the RRR because the PAF is strongly influenced by the prevalence of each risk factor and the prevalence of each hypertension subtype is lower in younger people than in older people. For example, the RRR of ISH for HF decreased with age as shown in Figure 3, whereas the prevalence of ISH increased with age and the PAF of ISH for HF also increased with age. From this point of view, even if the RRR of each hypertension subtype decreased with age, the clinical importance of BP control should not be underestimated in older people. Rather, results of the present study simply indicate that the underlying strength of relationship of each hypertension subtype with the risk for developing HF is relatively greater in younger people, which should motivate efforts to manage each hypertension subtype early in life.
From a public health perspective, increasing awareness of hypertension is also important. Young adults are reported to be less aware of the importance of controlling risk factors such as hypertension. 18 , 19 Specific measures focusing on the younger generation (eg, information technology such as smartphone apps and Internet of Things) are needed to improve the quality of care for young patients with hypertension. Indeed, a novel interactive smartphone app with a web‐based patient management system has recently been reported to improve the control of hypertension. 20 Such cutting‐edge technology is highly anticipated for the treatment of hypertension in the population.
Strengths of the present study include the use of a large administrative claims database with a high retention of study participants. The JMDC Claims Database includes administrative insurance claims data from participants’ insurance programs in a deidentified format. In particular, this database can track an individual as long as he/she remains under the same insurance coverage, and therefore clinical event data (eg, the diagnosis of HF) can be obtained even if the individual visits multiple medical providers.
This study has several limitations that warrant discussion. First, the BP measurements taken on a single occasion (eg, health check‐up) may not fully represent the BP phenotype of the study participants. Second, although trained health care professionals (eg, nurses) measured the BP of the study participants based on the standard protocol recommended by the Japanese Ministry of Health, Labour and Welfare, 8 adherence to this BP measurement protocol could be limited on a nationwide scale. We have no data on the compliance rate for this protocol. We measured BP using a mercury or aneroid sphygmomanometer or a validated automated device. Using different measuring methods would lead to biased results. Third, the diagnoses recorded in administrative databases are generally considered to be less well validated than those recorded in prospective registries. However, our data on the incidence of CVD from the JMDC Claims Database are comparable with other epidemiological data in Japan. 21 Fourth, this data set primarily included an employed population. Therefore, a selection bias (ie, a healthy worker bias) may be present. Further investigations are needed to determine whether our results can be generalized to other populations of different ethnicities and races or socioeconomic levels. Fifth, data on cause of death and the cause of HF (eg, HF with reduced or preserved ejection fraction) were not available in the JMDC Claims Database. Sixth, regarding the categorization of smoking and alcohol consumption, we categorized study participants into current or noncurrent smoker and drinking alcohol every day or not every day based on self‐reported questionnaire at health check‐up. This categorization is somewhat simplistic and may have caused a bias. Finally, the JMDC Claims Database does not include people aged >75 years. Therefore, we could not assess the association of hypertension subtypes with incident HF and other CVD events in the very elderly population.
Conclusions
Our analysis of a large‐scale administrative claims database demonstrated that the incidence of HF increased with age and that all hypertension subtypes were associated with an increased incidence of HF in all age categories. Despite this, the contribution of each hypertension subtype to the development of HF was attenuated with age. Optimal BP control is indispensable across the life course to reduce the burden of HF. In particular, preventive efforts for BP control have the potential to provide a greater benefit in younger individuals.
Sources of Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907, 21H03159, and 21K08123). The funding sources had no role in the design and conduct of the current study.
Disclosures
Drs Kaneko and Fujiu report research funding and scholarship funds from Medtronic Japan; Biotronik Japan; SIMPLEX QUANTUM; Boston Scientific Japan; and Fukuda Denshi, Central Tokyo. Drs Okada and Yamaguchi are members of the Department of Prevention of Diabetes and Lifestyle‐related Diseases, which is a cooperative program between The University of Tokyo and Asahi Mutual Life Insurance Company. The remaining authors have nothing to disclose.
Supporting information
Data S1–S2
Tables S1–S2
Figures S1–S8
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, Liu K, Greenland P, Lloyd‐Jones DM. Isolated systolic hypertension in young and middle‐aged adults and 31‐year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. J Am Coll Cardiol. 2015;65:327–335. doi: 10.1016/j.jacc.2014.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Takeda N, et al. Association of isolated diastolic hypertension based on the cutoff value in the 2017 American College of Cardiology/American Heart Association blood pressure guidelines with subsequent cardiovascular events in the general population. J Am Heart Assoc. 2020;9:e017963. doi: 10.1161/JAHA.120.017963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McEvoy JW, Daya N, Rahman F, Hoogeveen RC, Blumenthal RS, Shah AM, Ballantyne CM, Coresh J, Selvin E. Association of isolated diastolic hypertension as defined by the 2017 ACC/AHA blood pressure guideline with incident cardiovascular outcomes. JAMA. 2020;323:329–338. doi: 10.1001/jama.2019.21402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.CIR.103.9.1245 [DOI] [PubMed] [Google Scholar]
- 5. Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, Gidding SS, Bress AP, Greenland P, Muntner P, et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA. 2018;320:1774–1782. doi: 10.1001/jama.2018.13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee H, Yano Y, Cho SMJ, Park S, Lloyd‐Jones DM, Kim HC. Cardiovascular risk of isolated diastolic hypertension defined by the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline: a Nationwide Age‐Stratified Cohort Study. Hypertension. 2020;76:e44–e46. doi: 10.1161/HYPERTENSIONAHA.120.16018 [DOI] [PubMed] [Google Scholar]
- 7. McEvoy JW, Yang WY, Thijs L, Zhang ZY, Melgarejo JD, Boggia J, Hansen TW, Asayama K, Ohkubo T, Dolan E, et al. Isolated diastolic hypertension in the IDACO study: an age‐stratified analysis using 24‐hour ambulatory blood pressure measurements. Hypertension. 2021;78:1222–1231. doi: 10.1161/HYPERTENSIONAHA.121.17766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, Fujiu K, Michihata N, Jo T, Takeda N, et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with risk of heart failure and atrial fibrillation. Circulation. 2021;143:2244–2253. doi: 10.1161/CIRCULATIONAHA.120.052624 [DOI] [PubMed] [Google Scholar]
- 9. Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25:846–984. doi: 10.5551/jat.GL2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McEniery CM, Yasmin WS, Maki‐Petaja K, McDonnell B, Sharman JE, Retallick C, Franklin SS, Brown MJ, Lloyd RC, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–226. doi: 10.1161/01.HYP.0000165310.84801.e0 [DOI] [PubMed] [Google Scholar]
- 11. Tromp J, Paniagua SMA, Lau ES, Allen NB, Blaha MJ, Gansevoort RT, Hillege HL, Lee DE, Levy D, Vasan RS, et al. Age dependent associations of risk factors with heart failure: pooled population based cohort study. BMJ. 2021;372:n461. doi: 10.1136/bmj.n461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heise L, Greene ME, Opper N, Stavropoulou M, Harper C, Nascimento M, Zewdie D, Gender Equality N, Health SC. Gender inequality and restrictive gender norms: framing the challenges to health. Lancet. 2019;393:2440–2454. doi: 10.1016/S0140-6736(19)30652-X [DOI] [PubMed] [Google Scholar]
- 13. Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 2020;5:19–26. doi: 10.1001/jamacardio.2019.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, Claggett B, Merz CNB, Cheng S. Sex Differences in blood pressure associations with cardiovascular outcomes. Circulation. 2021;143:761–763. doi: 10.1161/CIRCULATIONAHA.120.049360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee H, Yano Y, Cho SMJ, Park JH, Park S, Lloyd‐Jones DM, Kim HC. Cardiovascular risk of isolated systolic or diastolic hypertension in young adults. Circulation. 2020;141:1778–1786. doi: 10.1161/CIRCULATIONAHA.119.044838 [DOI] [PubMed] [Google Scholar]
- 16. Grebla RC, Rodriguez CJ, Borrell LN, Pickering TG. Prevalence and determinants of isolated systolic hypertension among young adults: the 1999–2004 US National Health and Nutrition Examination Survey. J Hypertens. 2010;28:15–23. doi: 10.1097/HJH.0b013e328331b7ff [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Rodriguez CJ, Wang K. Prevalence and trends of isolated systolic hypertension among untreated adults in the United States. J Am Soc Hypertens. 2015;9:197–205. doi: 10.1016/j.jash.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension. 2017;70:736–742. doi: 10.1161/HYPERTENSIONAHA.117.09801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahajan S, Feng F, Hu S, Lu Y, Gupta A, Murugiah K, Gao Y, Lu J, Liu J, Zheng X, et al. Assessment of prevalence, awareness, and characteristics of isolated systolic hypertension among younger and middle‐aged adults in China. JAMA Netw Open. 2020;3:e209743. doi: 10.1001/jamanetworkopen.2020.9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kario K, Nomura A, Harada N, Okura A, Nakagawa K, Tanigawa T, Hida E. Efficacy of a digital therapeutics system in the management of essential hypertension: the HERB‐DH1 pivotal trial. Eur Heart J. 2021;42:4111–4122. doi: 10.1093/eurheartj/ehab559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M, Tsugane S. Association between mortality and incidence rates of coronary heart disease and stroke: the Japan Public Health Center‐based prospective (JPHC) study. Int J Cardiol. 2016;222:281–286. doi: 10.1016/j.ijcard.2016.07.222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1–S2
Tables S1–S2
Figures S1–S8


