Abstract
Background
Although the impact of dietary fats on cardiovascular disease (CVD) risk is widely researched, longitudinal associations between dietary patterns (DPs) based on fat type and early markers of CVD risk remain unclear.
Methods and Results
UK Biobank participants (46.9% men, mean age 55 years) with data on early markers of CVD risk (n=12 706) were followed longitudinally (2014–2020; mean 8.4 years). Two DPs (DP1, DP2) were derived using reduced rank regression (response variables: monounsaturated fat, polyunsaturated fat, and saturated fat based on two 24‐hour dietary assessments. Multivariable logistic and linear regression were used to investigate associations between DPs and odds of elevated CVD risk (using the nonlaboratory Framingham Risk Score) and changes in early CVD markers, respectively. DP1 (characterized by higher nuts and seeds and lower fruit and legumes intake) was positively correlated with saturated fat, monounsaturated fat, and polyunsaturated fat; DP2 (characterized by higher butter and high‐fat cheese, lower nuts and seeds intake) was positively correlated with saturated fat and negatively with polyunsaturated fat and monounsaturated fat. DP2 was associated with slightly higher odds of elevated CVD risk (odds ratio, 1.04 [95% CI, 1.00–1.07]). DP1 was associated with higher diastolic blood pressure (β, 0.20 [95% CI, 0.01–0.37]) and lower cardiac index (β, −0.02 [95% CI, −0.04 to −0.01]); DP2 was associated with higher carotid intima medial thickness (β, 1.80 [95% CI, 0.01–3.59]) and lower left ventricular ejection fraction (β, −0.15 [95% CI, −0.24 to −0.07]) and cardiac index (β, −0.01 [95% CI, −0.02 to −0.01]).
Conclusions
This study suggests small but statistically significant associations between DPs based on fat type and some early markers of CVD risk. Further research is needed to confirm these associations.
Keywords: cardiovascular disease, dietary fat, dietary patterns, Framingham Risk Score, reduced rank regression
Subject Categories: Diet and Nutrition, Epidemiology, Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- AI
augmentation index
- CIMT
carotid intima medial thickness
- DP
dietary pattern
- FRS
Framingham Risk Score
- MUFA
monounsaturated fat
- PUFA
polyunsaturated fat
- SFA
saturated fat
Clinical Perspective
What Is New?
This is the first study to derive dietary patterns based on fat type and investigate their association with early markers of cardiovascular disease (CVD) risk.
The dietary pattern characterized by foods rich in saturated fat, such as butter and high‐fat cheese, and low in foods rich in polyunsaturated fat, such as nuts and seeds, was associated with worsened cardiac function and vascular health. Similarly, the other dietary pattern identified was characterized by high saturated fat, monounsaturated fat, and polyunsaturated fat foods, such as nuts, seeds, and butter, and was also associated with worsened cardiac function and vascular health.
This suggests that dietary patterns with foods high in fat and specifically high in saturated fat worsened the CVD risk in the UK Biobank cohort in the follow‐up period of 8.4 years.
What Are the Clinical Implications?
Because dietary guidelines internationally are shifting from a single nutrient focus to a dietary pattern focus, this study provides important insight for dietary pattern recommendations specific to dietary fat and CVD risk.
Although the changes in markers of CVD were small, these are signs of early changes in CVD risk that could be indicative of an important window of opportunity for interventions to prevent CVD progression.
Cardiovascular disease (CVD) is the leading cause of death worldwide, with 31% of all deaths attributed to it. 1 CVD risk can be predicted using the Framingham Risk Score (FRS) according to an individual’s age, sex, body mass index (BMI), systolic blood pressure, antihypertensive medication use, smoking, and diabetes status. 2 Because CVD can take years to develop, detecting early changes to the cardiovascular system can also provide important insight into CVD prevention. 3 , 4 Changes in cardiac function, such as left ventricular ejection fraction (LVEF) and cardiac index, measure the ability of the heart to relax and contract and have been used to predict heart disease, CVD mortality, and nonfatal events. 5 , 6 , 7 Similarly, early signs of vasculature changes, such as changes in arterial stiffness, augmentation index (AI), and carotid intima medial thickness (CIMT), have also been strong predictors of CVD risk. 8 , 9 , 10 , 11 Most CVDs can be prevented by lifestyle changes, such as a healthy diet; therefore, understanding how to reduce CVD risk and detect early changes in the cardiovascular system are crucial. 1
Dietary fat has long been a focus in CVD prevention, 12 , 13 , 14 with recent dietary guidelines suggesting that the type of fat may play a more important role rather than total dietary fat. 12 , 15 , 16 Diets high in saturated fat (SFA) intake have been associated with higher CVD risk, 12 , 17 , 18 , 19 , 20 and replacing SFA with polyunsaturated fat (PUFA) 18 or monounsaturated fat (MUFA) shows benefits for reducing CVD risk. 17 However, results from observational studies remain inconsistent, 21 , 22 and a recent meta‐analysis identified that the diverse dietary sources of SFA are likely to play a role in these inconsistencies. 23 Thus, a dietary pattern (DP) approach that considers the combined effect of foods and nutrients on CVD risk may be a more physiologically relevant method for understanding the impact of dietary fat on early markers of CVD risk. 24
DP methodologies have been increasingly used to investigate the relationship between diet and CVD risk 25 , 26 , 27 , 28 , 29 ; however, few have considered the type of fat in creating the DPs. 30 , 31 , 32 Reduced rank regression, a method that generates DPs based on nutrient intakes of interest, has been used to investigate associations between a DP derived based on SFA intake and markers of CVD 30 and CVD incidence 31 ; however, none have considered SFA together with MUFA and PUFA. Moreover, to the best of our knowledge, no studies have investigated DPs based on fat type and associations with the FRS and subclinical markers of CVD risk. Understanding the associations between dietary fat as part of an overall DP, with early markers of CVD risk, has the potential to inform future dietary strategies to reduce early CVD risk.
Thus, the primary aim of this study was to derive DPs associated with intake of SFA, PUFA, and MUFA, and to examine their longitudinal associations with the FRS in the population‐based UK Biobank cohort study. The secondary aim was to examine the longitudinal association between these DPs and early markers of cardiac function (LVEF, cardiac index) and vascular health (blood pressure, AI, CIMT).
METHODS
This research has been conducted using the UK Biobank resource under applications number 14990 and 34894.
This study was approved by the North West Multi‐Centre Research Ethics Committee. All participants signed an informed consent to participate in the UK Biobank study. Further information about the study can be found at http://www.ukbiobank.ac.uk.
Data from UK Biobank were used, which is a prospective cohort study that included over 500 000 individuals, aged 40 to 69 years, living in the United Kingdom. Full details about the UK Biobank protocol have been published elsewhere. 33 Briefly, the volunteers attended the assessment center located across England, Scotland, and Wales for the baseline measurements from 2006 to 2010. In this first visit, participants who signed a consent form completed self‐reported questionnaires related to their lifestyle and undertook various physical measurements. Data described in the article, codebook, and analytic code will be made available upon request to UK Biobank and the pending return of data to the UK Biobank.
Diet
Dietary Intake
Dietary intake information was collected using a 24‐hour dietary assessment tool, the Oxford WebQ. 34 The Oxford WebQ was developed and compared against an interviewer‐administered 24‐hour recall for energy and nutrient intake. 34 It was also validated against recovery biomarkers. 35 The online questionnaire consisted of questions about the quantity of consumption of each of the 206 food and 32 beverages from the previous 24 hours. Information on energy and macronutrient intake was calculated using the UK Nutrient Databank food composition tables for years 2012 to 2013 and 2013 to 2014. 36 The quantity of each food and beverage consumed was calculated by multiplying the portion size by the amount consumed. 36 Dietary data were collected at 5 different time points: April 2009 to September 2009, February 2011 to April 2011, June 2011 to September 2011, October 2011 to December 2011, and April 2012 to June 2012. Consistent with our previous use of these data, 37 and the stable dietary intake reported over this period, 38 these were averaged (2009–2012) and considered baseline dietary data, and only participants with 2 or more valid dietary questionnaires were included.
Dietary Patterns
DPs were generated through reduced rank regression, derived from food groups and nutrient intake data collected from the Oxford WebQ. Reduced rank regression is a method for generating DPs, which combines the strengths of both a priori methods by using prior knowledge of relationships between nutrient intakes and health outcomes, and the exploratory method with data‐driven approaches. The number of response variables chosen for reduced rank regression determined the number of DPs generated. 39 DPs were generated based on food group as predictor variables and nutrient intake as response variables. The predictor variables were 48 food groups (Table S1), divided from previously defined food groups in the UK Biobank, 40 and the response variables were percentage energy from SFA, PUFA, and MUFA, selected based on established relationships between fat type and CVD. 12 , 14 , 17 Food items were grouped based on the food groupings used in the UK National Diet and Nutrition Survey and were adapted according to differences in SFA, PUFA, and MUFA content. Higher DP scores reflect a higher adherence to the DP, and lower scores indicate a lower adherence to the DPs.
Cardiovascular Outcomes
Nonlaboratory FRS
CVD risk was calculated at baseline (2006–2010) and first follow‐up (2014–2020) using the nonlaboratory FRS. 2 This method estimates risk based on the following variables: age (categorical), sex (binary), BMI (categorical), systolic blood pressure (categorical), antihypertensive medication use (binary), smoking (binary), and diabetes status (binary) (Data S1). It differs from the original FRS method by using BMI instead of total cholesterol concentrations, which were not available in the UK Biobank for the time points used in this analysis. 2 The change in nonlaboratory FRS from baseline (2006–2010) to first follow‐up (2014–2020) was used, because the change followed a normal distribution. A binary variable was created for FRS indicating low (<10%) and high (>10%) CVD risk. 41 , 42 Weight (in kilograms) was measured by digital scales (Tanita BC‐418MA body analyzer; Tanita Corporation of America, Arlington Heights, IL), and standing height (in centimeters) was measured using a Seca 202 device (Seca, Hamburg, Germany). 43 BMI (in kilograms per square meter) was calculated from information on weight (in kilograms) divided by height (in meters) squared. These were grouped into underweight (≤18.5 kg/m2), normal weight (>18.5 to ≤24.9 kg/m2), overweight (>24.9 to ≤29.9 kg/m2), or obese (>29.9 kg/m2) according to World Health Organization classification. 44
Blood Pressure
Brachial systolic and diastolic blood pressure (in millimeters of mercury) was collected at baseline (2006–2010), first follow‐up (2014–2020), and second follow‐up (2019+). The Omron 705 IT electronic blood pressure monitor (Omron, Kyoto, Japan) connected to an appropriately sized cuff (determined by measuring the participant's arm circumference) was used. 45 This was measured by registered nurses who were trained and certified to conduct the assessment. The measurement was done twice in each instance, with 1 minute between each measurement. To maximize the sample size for this analysis, data on blood pressure available at the first follow‐up (n=11 116) and second follow‐up (n=1340) were used.
Augmentation Index
AI (in percentage) was assessed at the first (2014–2020) and second (2019+) follow‐ups. 46 The measurements were made 4 times using a VICORDER (Skidmore Medical, Bristol, UK) blood pressure device by trained staff members. The measurement was performed 4 times in each instance, and values were averaged. To maximize the sample size for this analysis, data on AI available at the first follow‐up (n=11 209) and second follow‐up (n=1247) were used.
Carotid Intima Medial Thickness
Information on CIMT (in micrometers) was assessed via ultrasound at the first (2014–2020) and second (2019+) follow‐ups. 47 The CardioHealth Station (Panasonic Biomedical Sales Europe BV, Leicestershire, UK) ultrasound machine was used, with a linear array transducer with a frequency of 5 to 13 MHz. The scans were performed according to standard operating procedures, and every operator went through training. These were measured at 2 different angles at each side (right: 120°, 150°; and left: 210°, 240°) in each instance, and values were averaged. To maximize the sample size for this analysis, data on CIMT available at the first follow‐up (n=11 114) and second follow‐up (n=1342) were used.
LVEF and Cardiac Index
LVEF (in percentage) and cardiac index (in liters per minute per square meter) measurements were obtained through cardiovascular magnetic resonance at the first (2014–2020) and second (2019+) follow‐ups. The clinical wide bore 1.5T scanner (MAGNETOM Area, Syngo Platform VD13A; Siemens Healthcare, Erlangen, Germany) was used. Measurements were performed once in each instance by radiographers who underwent standardized central training and followed the standard operating procedures. The body surface area for cardiac index was calculated at the testing site based on the following formula: body surface area=0.20247×(weight0.425)×(height0.725), as previously reported. 48 To maximize the sample size for this analysis, data on LVEF and cardiac index available at the first follow‐up (n=12 030) and second follow‐up (n=426) were used.
Confounders
Confounders considered included age, sex, Townsend deprivation index, ethnicity, BMI, physical activity, smoking, and antihypertensive medication use. 33 The Townsend deprivation index was used based on national census output areas. Participants received a score corresponding to the output area of their postcode. This was assigned before their commencement in the study. Data on ethnicity were based on the following question: What is your ethnic group? Categories were collapsed into White, Mixed (Asian or Asian British, Black or Black British, Chinese), or other based on previous use in the UK Biobank. 49
Data on physical activity were assessed through an adapted version of the short International Physical Activity Questionnaire, covering the duration, intensity, and frequency of walking and moderate and vigorous activity. The metabolic equivalent, minutes per week, was derived from the time spent in each category of physical activity. Physical activity was divided into light, moderate, and vigorous. 43 Further details can be found elsewhere. 50
A continuous and binary (plausible/energy misreporters) indicator of energy misreporting was created. 51 , 52 For the continuous indicator, a ratio of reported energy intake to estimated energy requirement was calculated. The estimated energy requirement was based on the predictive formulas from the US Dietary Reference Intake, 53 where physical activity level was calculated based on total metabolic equivalent hours per day divided by 24 hours and included in the formula as such. In addition, a binary variable was created (plausible/energy misreporters), where individuals who reported energy intake in the 95% CI range for the ratio were considered adequate reporters. 52 Participants with values outside of this range were categorized as energy misreporters (over‐ and underreporters of energy intake), and this information was used for sensitivity analyses.
Inclusion and Exclusion Criteria
For the present analysis, participants were included if they had complete data for exposure and outcomes (n=28 130). Of these, participants were excluded if (1) they did not complete at least 2 valid dietary assessments (valid dietary assessments are defined as energy intake within the range of 500 to 3500 kcal for women and 800 to 4200 kcal for men) (n=14 923); (2) were pregnant during the exposure period (2009–2012) (n=11); (3) had a CVD diagnosis: hospital admission or death based on International Classification of Diseases, Tenth Revision (ICD‐10) codes, including coronary heart disease (I20‐I25, K49, K50, K75, K40–K46), congestive heart failure or cardiomyopathy (I50.0, I50.1, I50.9, I11.0, I13.0, I13.2, I42–43), and stroke (I60–I64) before or during baseline exposure period (2009–2012) (n=284); (4) had missing confounder data (n=399). For the secondary outcomes only, individuals were further excluded if they had LVEF <40%, indicating heart failure (n=241) (Figure S1). 54
Statistical Analysis
Descriptive statistics were used for participant characteristics and were presented as mean and standard deviation or frequency counts, and unadjusted linear regression analyses were used to investigate sex differences in participants’ characteristics. Each DP was treated as a continuous variable (DP scores), except for descriptive purposes where they were treated as tertiles. The primary outcome (FRS) and the secondary outcomes (markers of CVD risk) were treated as continuous variables. Multivariable adjusted linear regression analyses were used to examine associations between DPs at baseline and change in FRS from baseline to follow‐up. Restricted cubic splines, using 4 knots at the default percentile by Harrell, 55 were used to investigate other nonlinear associations between the DPs and changes in FRS. A likelihood ratio test was used to compare the linear and spline models, where the null hypothesis is that the linear model is a better fit. To align with clinical cut points for the FRS, 56 logistic regression analyses were also used to investigate the associations between the DPs and FRS at follow‐up as a binary variable (<10% low risk and ≥10% high risk). For the secondary outcomes, diastolic and systolic blood pressure, cardiac index, LVEF, CIMT, and AI outcomes were modeled using just the follow‐up data (Figure S2).
An interaction term was added to the models to test for moderation effects by sex. According to recommendations for reporting sex differences in CVD associations, analyses were still presented stratified by sex regardless of whether interactions were significant. 57 Among other reasons: (1) Women and men have biological differences, and stratifying results by sex could uncover mechanisms that partially explain these differences. (2) Because each sex makes up roughly 50% of the population, potential differences found could have general relevance. (3) There are sex differences in CVD. 57 Linear regression analyses were presented in 3 models. Model 1 analyses were adjusted for age (continuous) and sex (binary). Model 2, for the primary outcome, included confounders identified using a directed acyclic graph (Figure S3): Townsend deprivation index (continuous), physical activity (categorical), ethnicity (categorical), follow‐up time (continuous), and energy misreporting (continuous). Model 3, for the secondary outcomes, included adjusted analyses included the following confounders (Figure S4): BMI (continuous), energy misreporting, physical activity, ethnicity, sex, age, smoking (binary), follow‐up time (continuous), and medication use (categorical). For CIMT and AI, robust linear regressions were used, because these variables were skewed. All results in the text will refer to the fully adjusted models unless otherwise specified. DPs were generated in SAS software (SAS Institute, Cary, NC), whereas all other analyses were performed in Stata SE 15 (64 bit; StataCorp, College Station, TX).
Exploratory and Sensitivity Analysis
To further investigate any effect of energy misreporting, under‐ and overreporters of energy intake were excluded before generating DPs and associations between these revised DPs, and outcomes were investigated. Tertiles of DPs were also derived to present descriptive statistics of total energy (kilojoules per day) and nutrient intakes by DP tertiles. Nutrient intakes investigated included carbohydrate (percent energy [E] per day), protein (percent E per day), total fat (percent E per day), animal fat (percent E per day), vegetable fat (percent E per day), transfat (percent E per day), omega‐3 (grams per day), omega‐6 (grams per day), energy density (kilojoules per gram per day), and fiber (grams per day) and were presented as mean and SD and by sex. Linear regression analyses were used to investigate sex differences in the above‐mentioned nutrients, and analyses were adjusted for age, smoking status, and BMI.
RESULTS
Participant Characteristics
The final sample included for the primary analysis was 12 706 participants who were followed up for an average of 8.4 (±1.7) years (minimum of 4.3 and maximum of 12.3 years). For secondary outcomes, the final sample was 12 485 (Figure S1).
Individuals had similar characteristics across tertiles of DPs (Table S2). The characteristics of the participants who were excluded were comparable to those who were included (Table S3). Participants included in this study had a mean age of 55 (±7.4) years, and 53.1% were women. Most individuals were White (97.8%), and more than half of the participants reported moderate physical activity levels (54%). Men were on average 1.5 years older (55.8±7.5 versus 54.3±7.2 years) and had a higher proportion of smokers (6.2% versus 4.8%), and more men were overweight compared with women (50.8% versus 33.7%) (Table 1).
Table 1.
Overall Characteristics of the Participants at Baseline and According to Sex
| Characteristics | All, n=12 706 | Men, n=5965 | Women, n=6741 | P value* |
|---|---|---|---|---|
| Age, y, mean (±SD) | 55.0 (7.4) | 55.8 (7.5) | 54.3 (7.2) | <0.001 |
| Townsend deprivation index, n (%) | 0.12 | |||
| Low | 5160 (40.6) | 2478 (41.5) | 2682 (39.8) | |
| Medium | 4392 (34.6) | 2025 (34.0) | 2367 (35.1) | |
| High | 3154 (24.8) | 1462 (24.5) | 1692 (25.1) | |
| Race or ethnicity, n (%) | 0.45 | |||
| White | 12 287 (97.8) | 5812 (97.7) | 6584 (97.9) | |
| Mixed § | 238 (1.9) | 120 (2.0) | 117 (1.7) | |
| Other | 45 (0.3) | 19 (0.3) | 26 (0.4) | |
| Smoking, n (%) | 0.001 | |||
| Yes | 698 (5.5) | 373 (6.2) | 325 (4.8) | |
| No | 12 008 (94.5) | 5592 (93.8) | 6416 (95.2) | |
| Physical activity, n (%) † | 0.10 | |||
| Light | 2659 (20.9) | 1299 (20.6) | 1431 (21.2) | |
| Moderate | 6873 (54.1) | 3198 (53.5) | 3678 (54.6) | |
| Vigorous | 3174 (25.0) | 1546 (25.9) | 1632 (24.2) | |
| BMI category, n (%) ‡ | <0.001 | |||
| Underweight/normal weight | 5484 (43.1) | 2002 (33.5) | 3482 (51.7) | |
| Overweight | 5303 (41.7) | 3022 (50.7) | 2281 (33.8) | |
| Obesity | 1919 (15.2) | 941 (15.8) | 978 (14.5) |
BMI indicates body mass index.
P value for unadjusted linear regression analysis for sex differences in baseline characteristics where variables were continuous. For categorical variables, P value represents unadjusted χ2 analysis for sex differences in baseline characteristics.
Physical activity: light (total metabolic equivalent–hours a week <10), moderate (total metabolic equivalent–hours a week ≥10 and <50), and vigorous (total metabolic equivalent–hours a week >50).
Underweight/normal weight (BMI <25 kg/m2), overweight (BMI ≥25 and <30 kg/m2), obese (BMI ≥30 kg/m2).
At baseline, the mean value for FRS was 13.1±8.72, whereas for systolic and diastolic blood pressure the mean values were 135.0±17.9 and 81.3±10.0 mm Hg, respectively. At follow‐up, the mean value for FRS was 14.9±10.4, whereas for systolic and diastolic blood pressure the mean values were 137.5±18.6 and 78.2±10.1 mm Hg, respectively. The mean values for cardiac index and LVEF were 2.54±0.93 L/min per m2 and 55.7±6.62%, respectively, whereas for CIMT and AI the mean values were 681.1±124.5 µm and 20.0±8.87%, respectively.
Characteristics of DPs
Explained variation in food intake and response variables for each of the DPs generated through reduced rank regression are presented in Table 2. The results generated 3 DPs designated DP1, DP2, and DP3, explaining 41.9%, 23.8%, and 2.22% amount of variability in the response variables intakes, respectively. DP3 was not further investigated, because the explained variation in the response variables was lower than 10%. DP1 scores ranged from −3.41 to 6.68, and DP2 scores ranged from −5.89 to 5.03, with a higher score indicating the participant’s diet was better characterized by that pattern. DP1 was positively associated with intake of food groups such as nuts and seeds, vegetable dishes, and butter and negatively associated with fruits, legumes, and beer and cider (Tables S4 and S5). The full list of food groups factor loadings can be found in Figure S5. DP1was positively correlated with MUFA (r=0.67), PUFA (r=0.55), and SFA (r=0.50) intake, suggesting participants with higher DP1 scores were consuming similar amounts of all 3 types of fats. Individuals in the third tertile of DP1 had higher intakes of both animal and vegetable fat. No major differences in protein and energy intake were observed between tertiles of DP1 (Tables S6 and S7).
Table 2.
Explained Variation in Food Intake and Nutrient Response Variables for Each DP and Correlation Coefficient Between DPs and Response Variables (n=12 706)
| DP | Explained variation, % | Correlation coefficient | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Nutrient response variables | |||||||
| Food intakes | Nutrient response variables | SFA, %E | PUFA, %E | MUFA, %E | SFA, %E | PUFA, %E | MUFA, %E | |
| DP1 | 2.25 | 41.9 | 31.3 | 38.2 | 56.3 | 0.50 | 0.55 | 0.67 |
| DP2 | 3.03 | 23.8 | 71.5 | 69.5 | 56.3 | 0.75 | −0.66 | −0.01 |
| DP3 | 2.65 | 2.22 | 72.2 | 71.2 | 60.0 | 0.43 | 0.51 | −0.74 |
%E indicates percentage of total energy; DP, dietary pattern; MUFA, monounsaturated fat; PUFA, polyunsaturated fat; and SFA, saturated fat.
DP2 was associated with higher butter, high‐fat cheese, and ice cream intake, and lower consumption of nuts and seeds, vegetables, and vegetable dishes (Table S4). DP2 was negatively associated with MUFA (r=−0.01) and PUFA (r=−0.66) intake, whereas being positively correlated with SFA (r=0.75) intake suggesting participants with higher DP2 scores were consuming higher amounts of SFA and lower amounts of PUFA. Individuals in the third tertile of DP2 had higher intakes of animal fat, but lower intake of vegetable fat. No major differences in carbohydrate, protein, and energy intake were observed between tertiles of DP2 (Tables S6 and S7).
Association of DPs With FRS
As shown in Table 3, there were no significant associations between DP1 and DP2 and change in FRS from baseline to follow‐up in the overall sample (β coefficient per DP unit increase: DP1: 0.01 [95% CI, −0.14 to 0.16]; DP2: 0.09 [95% CI, −0.03 to 0.21]) or when stratified by sex. There was also no evidence for a nonlinear relationship between the DPs and FRS (DP1 splines and FRS: P=0.71; DP2 splines and FRS: P=0.81). When FRS was treated as a binary variable (<10% low risk and ≥10% high risk), DP1 was not associated with CVD risk. DP2 was associated with slightly higher odds of elevated CVD risk in the overall sample (odds ratio [OR] per DP unit increase, 1.04 [95% CI, 1.00–1.07]), and this association was only observed in men (OR per DP unit increase, 1.07 [95% CI, 1.07–1.12]) (Figure). No sex interaction was observed with DPs on FRS (β coefficient for sex by DP interaction term: DP1: 0.06 [95% CI, −0.22 to 0.35]; DP2: 0.01 [95% CI, −0.23 to 0.26]).
Table 3.
Changes in Markers of CVD Risk Overall and Stratified by Sex Per 1‐Unit Increase in DP Score
| Overall, n=12 706 | Men, n=5965 | Women, n=6741 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DP1 | DP2 | DP1 | DP2 | DP1 | DP2 | |||||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Framingham Risk Score* | ||||||||||||
| Model 1 ‖ | 0.06 | −0.09 to 0.22 | 0.08 | −0.04 to 0.21 | 0.15 | −0.09 to 0.41 | 0.05 | −0.16 to 0.25 | −0.03 | −0.20 to 0.14 | 0.09 | −0.06 to 0.23 |
| Model 2 ‖ | 0.01 | −0.14 to 0.16 | 0.09 | −0.03 to 0.21 | 0.03 | −0.21 to 0.27 | 0.13 | −0.07 to 0.33 | −0.03 | −0.20 to 0.14 | 0.07 | −0.07 to 0.21 |
| Systolic blood pressure*,§ | ||||||||||||
| Model 1 ‖ | 0.21 | −0.10 to 0.52 | 0.21 | −0.06 to 0.46 | 0.31 | −0.11 to 0.73 | 0.36 † | 0.02–0.71 † | 0.11 | −0.35 to 0.56 | 0.01 | −0.38 to 0.40 |
| Model 3 ‖ | −0.27 | −0.55 to 0.01 | −0.16 | −0.39 to 0.07 | −0.30 | −0.68 to 0.08 | −0.04 | −0.34 to 0.26 | −0.20 | −0.61 to 0.20 | −0.26 | −0.61 to 0.09 |
| Diastolic blood pressure*,§ | ||||||||||||
| Model 1 ‖ | 0.23 † | 0.05–0.40 † | 0.09 | −0.06 to 0.24 | 0.32 † | 0.06–0.57 † | 0.16 | −0.04 to 0.37 | 0.13 | −0.12 to 0.38 | 0.02 | −0.19 to 0.23 |
| Model 3 ‖ | −0.04 | −0.21 to 0.11 | 0.02 | −0.11 to 0.16 | −0.05 | −0.27 to 0.18 | 0.08 | −0.11 to 0.26 | −0.06 | −0.28 to 0.17 | −0.02 | −0.22 to 0.18 |
| Cardiac index ‡ , § | ||||||||||||
| Model 1 ‖ | −0.02 † | −0.04 to −0.01 † | −0.01 | −0.03 to 0.01 | −0.02 † | −0.04 to −0.01 † | −0.02 † | −0.03 to −0.01 † | −0.02 | −0.05 to 0.01 | −0.01 | −0.03 to 0.02 |
| Model 3 ‖ | −0.02 † | −0.04 to −0.01 † | −0.01 † | −0.02 to −0.01 † | −0.02 † | −0.04 to −0.01 † | −0.02 † | −0.03 to −0.01 † | −0.02 | −0.05 to 0.01 | −0.01 | −0.02 to 0.01 |
| LVEF ‡ , § | ||||||||||||
| Model 1 ‖ | 0.01 | −0.12 to 0.13 | −0.16 † | −0.27 to −0.06 † | −0.04 | −0.21 to 0.13 | −0.16 † | −0.31 to −0.02 † | 0.05 | −0.11 to 0.23 | −0.16 † | −0.31 to −0.01 † |
| Model 3 ‖ | −0.05 | −0.15 to 0.06 | −0.15 † | −0.24 to −0.07 † | −0.09 | −0.23 to 0.06 | −0.16 † | −0.28 to −0.04 † | 0.02 | −0.12 to 0.17 | −0.15 † | −0.27 to −0.02 † |
| Carotid IMT ‡ , § | ||||||||||||
| Model 1 ‖ | −0.94 | −3.11 to 1.22 | 1.85 † | 0.07–3.63 † | −1.43 | −4.71 to 1.85 | 2.43 | −0.23 to 5.09 | −0.43 | −3.21 to 2.34 | 1.27 | −1.06 to 3.59 |
| Model 3 ‖ | −1.27 | −3.46 to 0.92 | 1.10 | −0.67 to 2.87 | −1.72 | −5.07 to 1.62 | 1.41 | −1.24 to 4.06 | −0.69 | −3.47 to 2.09 | 0.70 | −1.59 to 3.00 |
| Carotid IMT ‡ , § | ||||||||||||
| Model 1 ‖ | 0.05 | −0.11 to 0.21 | −0.02 | −0.15 to 0.12 | 0.04 | −0.16 to 0.25 | 0.01 | −0.17 to 0.18 | 0.06 | −0.18 to 0.30 | −0.06 | −0.27 to 0.15 |
| Model 3 ‖ | 0.06 | −0.10 to 0.22 | −0.09 | −0.23 to 0.04 | 0.07 | −0.14 to 0.28 | −0.07 | −0.24 to 0.11 | 0.04 | −0.21 to 0.28 | −0.14 | −0.35 to 0.07 |
CVD indicates cardiovascular disease; DP, dietary pattern; IMT, intima medial thickness; and LVEF, left ventricular ejection fraction.
Regression coefficients from linear regression analyses represent change in outcome from baseline (2006–2010) to follow‐up (2014–2020) per 1‐unit increase in DP scores. DP1 scores ranged from −3.41 to 6.68, and DP2 scores ranged from −5.89 to 5.03.
Statistically significant associations.
Regression coefficients from linear regression analyses represent values for the outcome at follow‐up (2014–2020) per 1‐unit increase in DP scores. DP1 scores ranged from −3.41 to 6.68, and DP2 scores ranged from −5.89 to 5.03.
Secondary outcome analyses included 12 486 individuals.
Model 1: analysis adjusted for age and sex (except when used to stratify). Model 2: analysis adjusted for Model 1 plus Townsend deprivation index, physical activity, follow‐up time, and energy misreporting. Model 3: analysis adjusted for Model 2 plus body mass index, smoking status, and blood pressure medication use.
Figure 1. OR (95% CI) of cardiovascular disease risk after an average 8.4 years of follow‐up, as assessed using the nonlaboratory Framingham Risk Score, for DP1 and DP2 per 1‐unit increase.
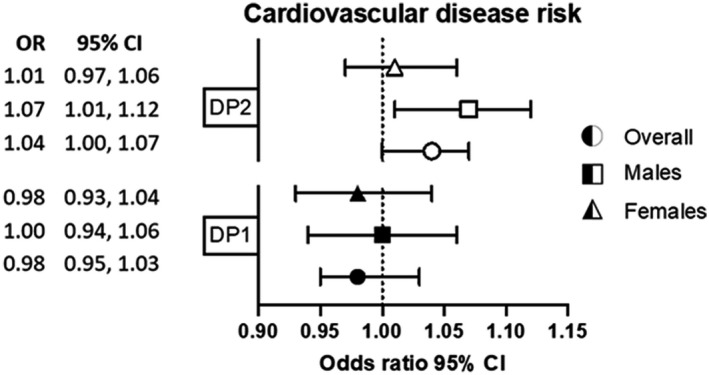
DP1 scores ranged from −3.41 to 6.68, and DP2 scores ranged from −5.89 to 5.03. Analysis adjusted for age and sex (except when used to stratify), Townsend deprivation index, physical activity, and energy misreporting. Logistic regression analyses represent OR and 95% CI for high Framingham Risk Score (≥10% risk) compared with low‐risk score (<10% risk), at follow‐up (2014–2020) in DP scores at baseline (2009–2012). DP indicates dietary pattern; and OR, odds ratio.
Association of DPs With Markers of CVD Risk
DP1 was associated with slightly higher diastolic blood pressure (β coefficient per DP unit increase: 0.23 [95% CI, 0.05–0.40]) in the minimally adjusted model and lower cardiac index (β coefficient per DP unit increase: −0.02 [95% CI, −0.04 to −0.01]) in the overall sample and men only (diastolic blood pressure, β coefficient per DP unit increase: 0.32 [95% CI, 0.06–0.57]; cardiac index, β coefficient per DP unit increase: −0.02 [95% CI, −0.04 to −0.01]) (Table 3). There was no evidence of DP1 being associated with systolic blood pressure, LVEF, CIMT, or AI. There was also no evidence of sex interactions with DP1 on markers of CVD risk (β coefficient for sex by DP interaction term: −0.16 [95% CI, −0.70 to 0.38]; diastolic blood pressure: 0.01 [95% CI, −0.30 to 0.33]; cardiac index: −0.01 [95% CI, −0.03 to 0.03]; LVEF: −0.06 [95% CI, −0.26 to 0.14]; CIMT: −0.76 [95% CI, −5.01 to 3.49]; AI: 0.04 [95% CI, −0.28 to 0.35]).
DP2 was associated with lower cardiac index (β coefficient per DP unit increase: −0.01 [95% CI, −0.02 to −0.01]), LVEF (β coefficient per DP unit increase: −0.15 [95% CI, −0.24 to −0.07]), and slightly higher CIMT in the minimally adjusted model only (β coefficient per DP unit increase: 1.85 [95% CI, 0.07–3.63]) in the overall sample. In men, DP2 was associated with lower cardiac index (β coefficient per DP unit increase: −0.02 [95% CI, −0.03 to −0.01]) and higher systolic blood pressure (β coefficient per DP unit increase: 0.36 [95% CI, 0.02–0.71]) in the minimally adjusted model only. DP2 was also associated with lower LVEF in both men (β coefficient per DP unit increase: −0.16 [95% CI, −0.28 to −0.04]) and women (β coefficient per DP unit increase: −0.15 [95% CI, −0.27 to −0.02]). There was no evidence of DP2 being associated with diastolic blood pressure or AI. There was also no evidence of sex interactions with DP2 on markers of CVD risk (β coefficient for sex by DP interaction term, systolic blood pressure: 0.15 [95% CI, −0.31 to 0.61]; diastolic blood pressure: 0.06 [95% CI, −0.21 to 0.32]; cardiac index: −0.02 [95% CI, −0.04 to 0.01]; LVEF: −0.02 [95% CI, −0.19 to 0.15]; CIMT: 1.06 [95% CI, −2.42 to 4.55]; AI: 0.08 [95% CI, −0.19 to 0.35]).
Exploratory and Sensitivity Analyses
After excluding energy misreporters, results remained consistent for DP1, except for systolic blood pressure and cardiac index, which were negatively associated with DP1 in the overall sample (β coefficient per DP unit increase: −0.34 [95% CI, −0.68 to −0.01] and −0.03 [95% CI, −0.03 to −0.01], respectively) and diastolic blood pressure, which was no longer associated with DP1 in the overall sample (β coefficient per DP unit increase: 0.19 [95% CI, −0.02 to 0.41]). Results remained consistent for DP2, except for being associated with higher systolic blood pressure (β coefficient per DP unit increase: 0.33 [95% CI, 0.01–0.65]) in the overall sample in the minimally adjusted model only and with higher CIMT in men only (β coefficient per DP unit increase: 3.95 [95% CI, 0.74–7.16]) in the minimally adjusted model only. DP2 was also no longer associated with LVEF in the women only sample (β coefficient per DP unit increase: −0.12 [95% CI, −0.27 to 0.03]) (Table S8).
When comparing sex differences in intakes of the highest loading food groups, men had a higher intake of the following food groups with high factor loadings for DP1: buns, cakes, and pastries (58.0±46.9 versus 49.2±39.9), and beer (277±438 versus 39.8±129); and of the following food group with highest loading for both DP1 and DP2: butter (6.80±9.60 versus 5.33±7.48) compared with women (Table S5). In addition, men had a lower intake of the following food group with high negative factor loading for DP1: fruits (192±145 versus 210±145) and for the food group with high negative factor loading for DP2: vegetables (153±117 versus 196±128) compared with women.
DISCUSSION
The present study investigated the longitudinal associations between DPs characterized by fat type and the FRS and early markers of CVD risk in a population‐based sample of older UK adults. None of the DPs were associated with FRS in the main models. Only when FRS was treated as a binary variable, a DP positively correlated with SFA and negatively with PUFA (DP2) was associated with slightly higher odds of having a high FRS. DP2 was also associated with lower LVEF and cardiac index, meaning worsened cardiac function and higher CIMT. In men only, DP2 was associated with higher systolic blood pressure and lower cardiac index. A DP positively correlated with SFA, MUFA, and PUFA (DP1) was associated with higher diastolic blood pressure and lower cardiac index. These early changes in vascular health and cardiac function after 8.4 years of follow‐up, though small, provide evidence for the importance of early interventions that could prevent a possible progression to CVD. Furthermore, these results indicate that the type of dietary fat within the context of a DP may be important for addressing some, but not all, early markers of CVD risk.
In this study, neither DP generated based on fat type was associated with the FRS (when treated as a continuous variable). This was an unexpected result, because other studies indicate associations between dietary fat type and CVD risk. Although there is limited research on the role of dietary fat type as part of a DP, a recent meta‐analysis of 15 randomized controlled trials reported that reducing SFA intake was associated with a 21% decrease in risk of CVD events, 18 whereas another meta‐analysis has reported higher CVD mortality with higher SFA intake. 19 No studies to date have used reduced rank regression DPs to investigate associations with FRS; however, one study has investigated the association between data‐driven DPs using factor analysis, and FRS using a binary cutoff (<10% low risk and >10% high risk of CVD). This study of 1196 adults followed up over 7 years identified 3 DPs, including a refined foods pattern, high in corn tortillas, refined grains, and soft drinks that was associated with high FRS; a prudent pattern, high in fresh fruits and vegetables that was associated with lower FRS 41 ; and a meat and fish pattern, high in red and processed meats, fish, and poultry that was not associated with CVD risk. Because the high fat meat and fish pattern did not differentiate between different meat sources, this hinders the comparability with the present study, where we separated food groups based on fat type. However, it could indicate that other components, other than dietary fat, such as high refined grains and soft drinks or low fruit and vegetable intake, may be a stronger predictor of CVD risk. Our study only found evidence of associations between a DP positively correlated with SFA and negatively with PUFA (DP2) and a slightly higher FRS when FRS was treated as a binary variable. However, we cannot rule out that this result is spurious because of sample variability or unknown confounding factors. Further research is needed to determine whether these discrepancies were caused by study design limitations, such as sample size and generalizability, or the distribution of the outcome itself.
Evidence for associations between DPs based on dietary fat and blood pressure is unclear. 58 Our findings indicated that a DP positively correlated with SFA, PUFA, and MUFA (DP1) was associated with higher diastolic blood pressure, whereas DP2 was associated with higher systolic blood pressure. This is consistent with literature where DPs with a lower fat content from dairy and meat sources, such as the Dietary Approaches to Stop Hypertension program, have been associated with lower blood pressure. 58 , 59 A recent meta‐analysis suggests that the Dietary Approaches to Stop Hypertension DP is associated with lower blood pressure. 59 However, it is important to note that the Dietary Approaches to Stop Hypertension DP is also higher in fruits and vegetables and lower in sodium, and that reductions seen in blood pressure could also be caused by these other dietary components. 59 Therefore, although DP1 and DP2 were associated with higher blood pressure, it may be that lower intake of other foods present in these DPs, such as fruits and vegetables, could also have played a role. Unexpectedly, blood pressure was only positively associated with DPs in men but not women. It is worth noting that men and women can have differences in physiology, body composition, hormones, and metabolism, which can differentially impact on their risk of CVD. 60 For example, fat deposition in men is more prominent in the abdominal region, whereas in women it is concentrated on hips and thighs. 61 , 62 These differences have been suggested to be positively linked to a healthier metabolic profile in women and inversely in men. 61 , 62 Although men had a higher intake of high‐fat cheese and butter and lower intake of fruits and vegetables, whether the effect of sex was because of differences in food intake or physiological mechanisms warrants further investigation.
Our findings for associations with markers of CVD risk are comparable with other studies. 63 , 64 A cross‐sectional study of 4601 US adults used reduced rank regression to generate DPs using metabolic syndrome components as response variables to investigate associations with left ventricle mass and function. 63 A DP high in high‐fat meats, cheese, and processed foods and low in fruits, vegetables, nuts, and fish was associated with a 0.21% decrease in LVEF. 63 Moreover, a Mediterranean DP, characterized by a higher intake of fruits, vegetables, nuts, olive oil, and fatty fish and a lower intake of processed and red meat is associated with a 0.20% higher LVEF. 64 Therefore, it seems that a DP higher in SFA and lower in PUFA could be associated with impaired cardiac function, but further randomized controlled trials are needed to investigate causality.
Consistent with previous research, 65 , 66 a high SFA DP identified in this study was negatively associated with markers of vascular health. Research suggests that a higher SFA intake is associated with higher CIMT. 65 , 66 For every 10‐g/day increase in SFA, an increase of 0.03 mm in CIMT has been reported. 66 Moreover, in a DP context, the Mediterranean DP has also been associated with lower CIMT. 65 However, in the present study, DP2 was only associated with CIMT in the overall sample, and results were not consistent when stratified by sex, suggesting no sex differences. Although limited, recent evidence suggests that a Mediterranean DP could be associated with lower AI. 67 A 1‐year randomized controlled trial of 1294 European individuals free from chronic disease, investigated the effects of a Mediterranean diet on AI in a small subset (n=225) and reported lower augmentation AI following the intervention (−12.4 [95% CI, −24.4 to −0.5]). 67 However, individuals in this intervention were older (aged 65–79 years) than in our study (aged 50–69 years), which could explain the lack of associations in our study. Future research is needed to confirm if the effect of diet on AI is dependent on age.
This study acknowledges several strengths and limitations. The Oxford WebQ has been validated for energy and nutrient intake, and this study used repeated assessments to estimate an individual’s usual intake. 35 However, it is a self‐reported measurement and therefore could be subjected to misreporting biases. By only including individuals with at least 2 dietary assessments, selection bias could have been introduced. Although our use of consecutive dietary intake assessments did not investigate change in dietary intakes between time points, previous research suggests a moderate‐to‐substantial agreement between time points. 38 Reduced rank regression generates DPs that reflect the dietary habits of the population of interest; however, these may not reflect other populations with varying dietary habits. Nevertheless, this method has the strength of generating DPs based on specific nutrients (SFA, MUFA, and PUFA), which are known to be associated with CVD risk. 12 , 17 , 19 Although this study presented results overall and stratified by sex, the creation of DPs was conducted in the overall population. This was done based on previous studies that have used a similar approach, 29 , 68 but other studies may consider generating DPs stratified by sex. 69 To retain a larger sample size and therefore increase our statistical power, this study investigated the association between diet and CVD risk from baseline to first follow‐up, and where first follow‐up data were not available, second follow‐up data were used. Because CVD risk increases with age, this variation in follow‐up time points should be considered when interpreting the results. Nonetheless, the proportion of participants for whom we used the second follow‐up time point was small, ranging from 3% to 11% depending on the outcome, so this is unlikely to have significantly impacted on our findings. Another limitation of this study was the lack of baseline data for some of the subclinical markers of CVD risk, which may have limited our ability to detect prospective associations. A strength of this study was its prospective design, with over 8 years of follow‐up, which appeared to be sufficient for capturing early changes in vascular health. Furthermore, this study investigated the association between DPs based on the fat type and clinical markers of early CVD risk in a large sample free from chronic disease at baseline.
In conclusion, this study identified 2 DPs based on fat type. No strong associations between these DPs and early markers of CVD were observed. The DP characterized by SFA‐rich foods such as butter and high‐fat cheese and low in PUFA‐rich foods such as nuts and seeds (DP2) was not associated with FRS in its continuous form, but we found a small association between DP and higher odds of high FRS in its binary form. This DP was also associated with lower LVEF and cardiac index, and higher CIMT. In men only, this DP was associated with higher systolic blood pressure and lower cardiac index, which suggests that sex may influence these associations. Conversely, the other DP identified in this study (DP1) was characterized by high SFA, MUFA, and PUFA foods, such as nuts, seeds, and butter, and was associated with higher diastolic blood pressure and lower cardiac index. Therefore, both DPs were associated with worsened early markers of CVD risk. Though the changes in markers of CVD were small, these are signs of early changes in CVD risk and could be indicative of an important window of opportunity for interventions that could prevent their progression. Because the early origins of disease suggest that biomarkers first manifest in early life, future studies are needed to investigate if these findings are consistent in a younger cohort. Moreover, the results described in this study could be specific to the sample in question, and further investigation in different cohorts is warranted to confirm these findings. This is the first study to derive DPs based on the fat type and investigate their associations with the FRS and early changes in vasculature and cardiac function. Our findings suggest that the type of fat, in the context of a DP, may be associated with small changes in some but not all early markers of CVD risk.
Sources of Funding
None.
Disclosures
Dr Livingstone is supported by a National Health and Medical Research Council Emerging Leadership Fellowship (APP1173803). Dr Piernas received a British Nutrition Foundation pump priming award that paid for access to the data. Dr Piernas is funded by the National Institute for Health and Care Research Oxford and Thames Valley Applied Research Collaboration. Dr Perez‐Cornago is supported by a Cancer Research UK Population Research Fellowship (C60192/A28516) and by the World Cancer Research Fund (WCRF UK), as part of the World Cancer Research Fund International grant program (2019/1953). The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S8
Figures S1–S5
Acknowledgments
The authors thank Dr Abbott (Deakin University) for his contributions in developing the statistical analysis for this study. This study has been conducted using data from the UK Biobank study, and as such, the authors thank all participants who accepted to be part of it.
Author Contributions: All authors designed the research. Dr Brayner analyzed the data and wrote the article. Dr Livingstone had primary responsibility for the final content. All authors interpreted the data and provided critical evaluation. All authors read and approved the final article.
For Sources of Funding and Disclosures, see page 11.
Footnotes
Mixed includes Asian or Asian British, Black or Black British, Chinese.
REFERENCES
- 1. World Health Organization . Cardiovascular diseases (CVDs). Available at: https://www.who.int/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds). Accessed November 5, 2020.
- 2. D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 3. Ghebre YT, Yakubov E, Wong WT, Krishnamurthy P, Sayed N, Sikora AG, Bonnen MD. Vascular aging: implications for cardiovascular disease and therapy. Transl Med (Sunnyvale). 2016;6:183. doi: 10.4172/2161-1025.1000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease: the Bogalusa Heart Study. Am J Cardiol. 2002;90:L3–L7. doi: 10.1016/S0002-9149(02)02953-3 [DOI] [PubMed] [Google Scholar]
- 5. Carlsson M, Andersson R, Markenroth Bloch K, Steding‐Ehrenborg K, Mosén H, Stahlberg F, Ekmehag B, Arheden H. Cardiac output and cardiac index measured with cardiovascular magnetic resonance in healthy subjects, elite athletes and patients with congestive heart failure. J Cardiovasc Magn Reson. 2012;14:51. doi: 10.1186/1532-429X-14-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423 [DOI] [PubMed] [Google Scholar]
- 7. Kosaraju A, Goyal A, Grigorova Y. Left Ventricular Ejection Fraction. StatPearls Publishing; 2021. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459131/. Accessed September 29, 2021. [PubMed] [Google Scholar]
- 8. Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb. 1991;11:1245–1249. doi: 10.1161/01.ATV.11.5.1245 [DOI] [PubMed] [Google Scholar]
- 9. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103 [DOI] [PubMed] [Google Scholar]
- 10. Tardif J‐C, Heinonen T, Orloff D, Libby P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation. 2006;113:2936–2942. doi: 10.1161/CIRCULATIONAHA.105.598987 [DOI] [PubMed] [Google Scholar]
- 11. Nürnberger J, Keflioglu‐Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schäfers RF. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20:2407–2414. doi: 10.1097/00004872-200212000-00020 [DOI] [PubMed] [Google Scholar]
- 12. Wang DD, Hu FB. Dietary fat and risk of cardiovascular disease: recent controversies and advances. Annu Rev Nutr. 2017;37:423–446. doi: 10.1146/annurev-nutr-071816-064614 [DOI] [PubMed] [Google Scholar]
- 13. Harcombe Z. US dietary guidelines: is saturated fat a nutrient of concern? Br J Sports Med. 2019;53:1393–1396. doi: 10.1136/bjsports-2018-099420 [DOI] [PubMed] [Google Scholar]
- 14. Kris‐Etherton PM, Krauss RM. Public health guidelines should recommend reducing saturated fat consumption as much as possible: YES. Am J Clin Nutr. 2020;112:13–18. doi: 10.1093/ajcn/nqaa110 [DOI] [PubMed] [Google Scholar]
- 15. Dietary Guidelines Advisory Committee . Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service; 2020. [Google Scholar]
- 16. Zhu Y, Bo Y, Liu Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: a dose‐response meta‐analysis of cohort studies. Lipids Health Dis. 2019;18:91. doi: 10.1186/s12944-019-1035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris‐Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–e23. doi: 10.1161/CIR.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 18. Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020;5:Cd011737. doi: 10.1002/14651858.CD011737.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim Y, Je Y, Giovannucci EL. Association between dietary fat intake and mortality from all‐causes, cardiovascular disease, and cancer: a systematic review and meta‐analysis of prospective cohort studies. Clin Nutr. 2021;40:1060–1070. doi: 10.1016/j.clnu.2020.07.007 [DOI] [PubMed] [Google Scholar]
- 20. Livingstone KM, Givens DI, Cockcroft JR, Pickering JE, Lovegrove JA. Is fatty acid intake a predictor of arterial stiffness and blood pressure in men? Evidence from the Caerphilly Prospective Study. Nutr Metab Cardiovasc Dis. 2013;23:1079–1085. doi: 10.1016/j.numecd.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 21. Siri‐Tarino PW, Sun Q, Hu FB, Krauss RM. Meta‐analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta‐analysis of observational studies. BMJ. 2015;351:h3978. doi: 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Sullivan TA, Hafekost K, Mitrou F, Lawrence D. Food sources of saturated fat and the association with mortality: a meta‐analysis. Am J Public Health. 2013;103:e31–e42. doi: 10.2105/AJPH.2013.301492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- 25. Kahleova H, Levin S, Barnard ND. Vegetarian dietary patterns and cardiovascular disease. Prog Cardiovasc Dis. 2018;61:54–61. doi: 10.1016/j.pcad.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 26. Shen J, Wilmot KA, Ghasemzadeh N, Molloy DL, Burkman G, Mekonnen G, Gongora MC, Quyyumi AA, Sperling LS. Mediterranean dietary patterns and cardiovascular health. Annu Rev Nutr. 2015;35:425–449. doi: 10.1146/annurev-nutr-011215-025104 [DOI] [PubMed] [Google Scholar]
- 27. Nanri A, Mizoue T, Shimazu T, Ishihara J, Takachi R, Noda M, Iso H, Sasazuki S, Sawada N, Tsugane S. Dietary patterns and all‐cause, cancer, and cardiovascular disease mortality in Japanese men and women: the Japan public health center‐based prospective study. PLoS One. 2017;12:e0174848. doi: 10.1371/journal.pone.0174848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagner S, Lioret S, Girerd N, Duarte K, Lamiral Z, Bozec E, Berghe LVD, Hoge A, Donneau AF, Boivin JM, et al. Association of dietary patterns derived using reduced‐rank regression with subclinical cardiovascular damage according to generation and sex in the STANISLAS cohort. J Am Heart Assoc. 2020;9:e013836. doi: 10.1161/JAHA.119.013836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livingstone KM, Abbott G, Bowe SJ, Ward J, Milte C, McNaughton SA. Diet quality indices, genetic risk and risk of cardiovascular disease and mortality: a longitudinal analysis of 77 004 UK Biobank participants. BMJ Open. 2021;11:e045362. doi: 10.1136/bmjopen-2020-045362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livingstone KM, McNaughton SA. Dietary patterns by reduced rank regression are associated with obesity and hypertension in Australian adults. Br J Nutr. 2017;117:248–259. doi: 10.1017/S0007114516004505 [DOI] [PubMed] [Google Scholar]
- 31. Gao M, Jebb SA, Aveyard P, Ambrosini GL, Perez‐Cornago A, Carter J, Sun X, Piernas C. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all‐cause mortality in 116,806 individuals from the UK Biobank: a prospective cohort study. BMC Med. 2021;19:83. doi: 10.1186/s12916-021-01958-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livingstone KM, McNaughton SA. Association between diet quality, dietary patterns and cardiometabolic health in Australian adults: a cross‐sectional study. Nutr J. 2018;17:19. doi: 10.1186/s12937-018-0326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. UK Biobank: protocol for a large‐scale prospective epidemiological resource. 2007. Available at: https://www.ukbiobank.ac.uk/media/gnkeyh2q/study‐rationale.pdf. Accessed November 24, 2019.
- 34. Liu B, Young H, Crowe FL, Benson VS, Spencer EA, Key TJ, Appleby PN, Beral V. Development and evaluation of the Oxford WebQ, a low‐cost, web‐based method for assessment of previous 24 h dietary intakes in large‐scale prospective studies. Public Health Nutr. 2011;14:1998–2005. doi: 10.1017/S1368980011000942 [DOI] [PubMed] [Google Scholar]
- 35. Greenwood DC, Hardie LJ, Frost GS, Alwan NA, Bradbury KE, Carter M, Elliott P, Evans CEL, Ford HE, Hancock N, et al. Validation of the Oxford WebQ online 24‐hour dietary questionnaire using biomarkers. Am J Epidemiol. 2019;188:1858–1867. doi: 10.1093/aje/kwz165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perez‐Cornago A, Pollard Z, Young H, van Uden M, Andrews C, Piernas C, Key TJ, Mulligan A, Lentjes M. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur J Nutr. 2021;60:4019–4030. doi: 10.1007/s00394-021-02558-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brayner B, Kaur G, Keske MA, Perez‐Cornago A, Piernas C, Livingstone KM. Dietary patterns characterized by fat type in association with obesity and type 2 diabetes: a longitudinal study of UK Biobank participants. J Nutr. 2021;151:3570–3578. doi: 10.1093/jn/nxab275 [DOI] [PubMed] [Google Scholar]
- 38. Bradbury KE, Young HJ, Guo W, Key TJ. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6. doi: 10.1017/jns.2017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoffmann K. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol. 2004;159:935–944. doi: 10.1093/aje/kwh134 [DOI] [PubMed] [Google Scholar]
- 40. Piernas C, Perez‐Cornago A, Gao M, Young H, Pollard Z, Mulligan A, Lentjes M, Carter J, Bradbury K, Key TJ, et al. Describing a new food group classification system for UK Biobank: analysis of food groups and sources of macro‐ and micronutrients in 208,200 participants. Eur J Nutr. 2021;60:2879–2890. doi: 10.1007/s00394-021-02535-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Denova‐Gutiérrez E, Tucker KL, Flores M, Barquera S, Salmerón J. Dietary patterns are associated with predicted cardiovascular disease risk in an urban Mexican adult population. J Nutr. 2016;146:90–97. doi: 10.3945/jn.115.217539 [DOI] [PubMed] [Google Scholar]
- 42. American Heart Association . Heart attack risk assessment 2020. Available at: https://www.heart.org/en/news/2018/05/01/risk‐assessment‐calculator‐accurately‐predicts‐heart‐attacks‐strokes. Accessed November 24, 2020.
- 43. Anderson J, Celis‐Morales C, Mackay D, Iliodromiti S, Lyall D, Sattar N, Gill J, Pell J. Adiposity among 132 479 UK Biobank participants; contribution of sugar intake vs other macronutrients. Int J Epidemiol. 2016;46:492–501. doi: 10.1093/ije/dyw173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Organization WH . Obesity: Preventing and Managing the Global Epidemic. World Health Organization; 2000. [PubMed] [Google Scholar]
- 45. UK Biobank Blood pressure 2011. Available at: http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/Bloodpressure.pdf. Accessed September 17, 2019.
- 46. UK Biobank Arterial Pulse‐Wave Velocity 2011. Available at: http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/Pulsewave.pdf. Accessed September 17, 2019.
- 47. UK Biobank Imaging modality: carotid ultrasound 2015. Available at: http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/carult_explan_doc.pdf. Accessed September 17, 2019.
- 48. UK biobank body surface area. Available at: https://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=22427. Accessed January 22, 2022.
- 49. Cox SR, Ritchie SJ, Tucker‐Drob EM, Liewald DC, Hagenaars SP, Davies G, Wardlaw JM, Gale CR, Bastin ME, Deary IJ. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun. 2016;7:13629. doi: 10.1038/ncomms13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cassidy S, Chau JY, Catt M, Bauman A, Trenell MI. Cross‐sectional study of diet, physical activity, television viewing and sleep duration in 233 110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ. 2016;6:e010038. doi: 10.1136/bmjopen-2015-010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murakami K, Livingstone MBE. Eating frequency in relation to body mass index and waist circumference in British adults. Int J Obes. 2014;38:1200. doi: 10.1038/ijo.2014.1 [DOI] [PubMed] [Google Scholar]
- 52. Leech RM, Worsley A, Timperio A, McNaughton SA. The role of energy intake and energy misreporting in the associations between eating patterns and adiposity. Eur J Clin Nutr. 2018;72:142–147. doi: 10.1038/ejcn.2017.90 [DOI] [PubMed] [Google Scholar]
- 53. Institute of Medicine . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. National Academy Press; 2002. [DOI] [PubMed] [Google Scholar]
- 54. American Heart Association . Ejection fraction heart failure measurement 2017. Available at: https://www.heart.org/en/health‐topics/heart‐failure/diagnosing‐heart‐failure/ejection‐fraction‐heart‐failure‐measurement. Accessed July 27, 2021.
- 55. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; 2001. [Google Scholar]
- 56. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 57. Woodward M. Rationale and tutorial for analysing and reporting sex differences in cardiovascular associations. Heart. 2019;105:1701. doi: 10.1136/heartjnl-2019-315299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nestel PJ. Dietary fat and blood pressure. Curr Hypertens Rep. 2019;21:17. doi: 10.1007/s11906-019-0918-y [DOI] [PubMed] [Google Scholar]
- 59. Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LI, Chrysochoou CA, Nihoyannopoulos PI, Tousoulis DM. Dietary Approaches to Stop Hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta‐analysis of randomized controlled trials. Adv Nutr. 2020;11:1150–1160. doi: 10.1093/advances/nmaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vaccarezza M, Papa V, Milani D, Gonelli A, Secchiero P, Zauli G, Gemmati D, Tisato V. Sex/gender‐specific imbalance in CVD: could physical activity help to improve clinical outcome targeting CVD molecular mechanisms in women? Int J Mol Sci. 2020;21:1477. doi: 10.3390/ijms21041477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99:931–940. doi: 10.1017/S0007114507853347 [DOI] [PubMed] [Google Scholar]
- 62. Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu L, Nettleton JA, Bertoni AG, Bluemke DA, Lima JA, Szklo M. Dietary pattern, the metabolic syndrome, and left ventricular mass and systolic function: the Multi‐Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:362–368. doi: 10.3945/ajcn.2009.27538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Levitan EB, Ahmed A, Arnett DK, Polak JF, Hundley WG, Bluemke DA, Heckbert SR, Jacobs DR Jr, Nettleton JA. Mediterranean diet score and left ventricular structure and function: the Multi‐Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2016;104:595–602. doi: 10.3945/ajcn.115.128579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Petersen KS, Clifton PM, Keogh JB. The association between carotid intima media thickness and individual dietary components and patterns. Nutr Metab Cardiovasc Dis. 2014;24:495–502. doi: 10.1016/j.numecd.2013.10.024 [DOI] [PubMed] [Google Scholar]
- 66. Merchant AT, Kelemen LE, de Koning L, Lonn E, Vuksan V, Jacobs R, Davis B, Teo KK, Yusuf S, Anand SS, et al. Interrelation of saturated fat, trans fat, alcohol intake, and subclinical atherosclerosis. Am J Clin Nutr. 2008;87:168–174. doi: 10.1093/ajcn/87.1.168 [DOI] [PubMed] [Google Scholar]
- 67. Jennings A, Berendsen AM, de Groot LCPGM, Feskens EJM, Brzozowska A, Sicinska E, Pietruszka B, Meunier N, Caumon E, Malpuech‐Brugère C, et al. Mediterranean‐style diet improves systolic blood pressure and arterial stiffness in older adults. Hypertension. 2019;73:578–586. doi: 10.1161/HYPERTENSIONAHA.118.12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Livingstone KM, Abbott G, Ward J, Bowe SJ. Unhealthy lifestyle, genetics and risk of cardiovascular disease and mortality in 76,958 individuals from the UK Biobank cohort study. Nutrients. 2021;13:4283. doi: 10.3390/nu13124283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huybrechts I, Lioret S, Mouratidou T, Gunter MJ, Manios Y, Kersting M, Gottrand F, Kafatos A, De Henauw S, Cuenca‐García M, et al. Using reduced rank regression methods to identify dietary patterns associated with obesity: a cross‐country study among European and Australian adolescents. Br J Nutr. 2017;117:295–305. doi: 10.1017/S0007114516004669 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S8
Figures S1–S5


