Abstract
Background
EAST‐AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) demonstrated clinical benefit of early rhythm‐control therapy (ERC) in patients with new‐onset atrial fibrillation (AF) and concomitant cardiovascular conditions compared with current guideline‐based practice. This study aimed to evaluate the generalizability of EAST‐AFNET 4 in routine practice.
Methods and Results
Using a US administrative database, we identified 109 739 patients with newly diagnosed AF during the enrollment period of EAST‐AFNET 4. Patients were classified as either receiving ERC, using AF ablation or antiarrhythmic drug therapy, within the first year after AF diagnosis (n=27 106) or not receiving ERC (control group, n=82 633). After propensity score overlap weighting, Cox proportional hazards regression was used to compare groups for the primary composite outcome of all‐cause mortality, stroke, or hospitalization with the diagnoses heart failure or myocardial infarction. Most patients (79 948 of 109 739; 72.9%) met the inclusion criteria for EAST‐AFNET 4. ERC was associated with a reduced risk for the primary composite outcome (hazard ratio [HR], 0.85; 95% CI, 0.75–0.97 [P=0.02]) with largely consistent results between eligible (HR, 0.89; 95% CI, 0.76–1.04 [P=0.14]) or ineligible (HR, 0.77; 95% CI, 0.60–0.98 [P=0.04]) patients for EAST‐AFNET 4 trial inclusion. ERC was associated with lower risk of stroke in the overall cohort and in trial‐eligible patients.
Conclusions
This analysis replicates the clinical benefit of ERC seen in EAST‐AFNET 4. The results support adoption of ERC as part of the management of recently diagnosed AF in the United States.
Keywords: antiarrhythmic drugs, atrial fibrillation, cather ablation, rhythm‐control therapy, trial generalizability
Subject Categories: Arrhythmias, Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator, Epidemiology, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- AAD
antiarrhythmic drug
- CABANA
Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation
- EARLY‐AF
Early Aggressive Invasive Intervention for Atrial Fibrillation
- EAST‐AFNET 4
Early Treatment of Atrial Fibrillation for Stroke Prevention Trial
- ERC
early rhythm control therapy
- STOP AF First
Cryoballoon Catheter Ablation in an Antiarrhythmic Drug Naive Paroxysmal Atrial Fibrillation
Clinical Perspective
What Is New?
The majority of patients with newly diagnosed atrial fibrillation treated in routine US practice would be eligible for early rhythm control as tested in EAST‐AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial).
What Are the Clinical Implications?
Our data support the routine initiation of early rhythm‐control therapy as part of the management of recently diagnosed atrial fibrillation in patients.
Atrial fibrillation (AF) is associated with an increased risk for cardiovascular complications such as death, stroke and myocardial infarction (MI), particularly in the first year after diagnosis. 1 , 2 Restoring and maintaining sinus rhythm has been associated with reduced mortality in large observational data sets 3 ; however, previous randomized trials have failed to demonstrate superiority over rate control. 4 , 5 , 6 Recently, EAST‐AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) randomized patients with recently diagnosed AF and increased cardiovascular risk (CHA2DS2‐VASc score ≥2) to early rhythm‐control therapy (ERC) or current guideline‐based usual care, consisting of rate‐control therapy initially with rhythm‐control therapy added to improve AF–related symptoms. 7 In EAST‐AFNET 4, which was stopped for efficacy, early rhythm control was associated with reduced risk in the composite end point of death from cardiovascular causes, stroke, or hospitalization with worsening of heart failure (HF) or acute coronary syndrome (hazard ratio [HR], 0.79; 96% CI, 0.66 to 0.94]). 7 ERC included AF ablation in 25% of patients, added to continued anticoagulation and therapy for concomitant cardiovascular conditions. These characteristics distinguish EAST‐AFNET 4 from prior “rhythm versus rate” strategy trials. Furthermore, rhythm control was initiated early, which may increase the effectiveness and safety of rhythm‐control therapy. 8 , 9 Especially the early initiation of therapy raised questions with regards to the generalizability of the trial results in routine care.
To assess the generalizability of the EAST‐AFNET 4 findings to routine practice in a large cohort of US patients with AF, we assessed the proportion of patients who would have met trial eligibility criteria and examined the association between early rhythm control and clinical outcomes, stratified by trial eligibility.
Methods
The Mayo Clinic’s institutional review board exempted this study from review because it used preexisting, deidentified data. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to OptumLabs.
Study Population
This study was a retrospective cohort analysis using deidentified administrative claims data from the OptumLabs Data Warehouse, which contains medical and pharmacy claims and enrollment records for private insurance and Medicare Advantage enrollees of all ages and races throughout the United States. 10 , 11
The study population included adult patients (aged ≥18 years) who had newly diagnosed AF between July 28, 2011, and December 30, 2016, the enrollment period of EAST‐AFNET 4. Patients were divided into 2 treatment groups. The ERC group included patients who underwent ERC, ie, AF ablation or antiarrhythmic drug (AADs; Table S1) therapy, within the first year after AF diagnosis. Some patients were treated with both AF ablation and AADs. Cardioversion was not considered a chronic prophylactic treatment to prevent recurrence of AF and therefore not considered a criteria for ERC. AF ablation was identified using procedure codes (Table S2). 12 , 13 The control group included patients who did not receive rhythm‐control therapy within the first year after AF diagnosis. These treatment groups approximated the randomized groups in EAST‐AFNET 4. For analysis, the date 12 months after the first AF diagnosis was defined as the index date and the start of the follow‐up period. The patient selection flow diagram is shown in Figure 1.
Figure 1. Patient selection flow chart.
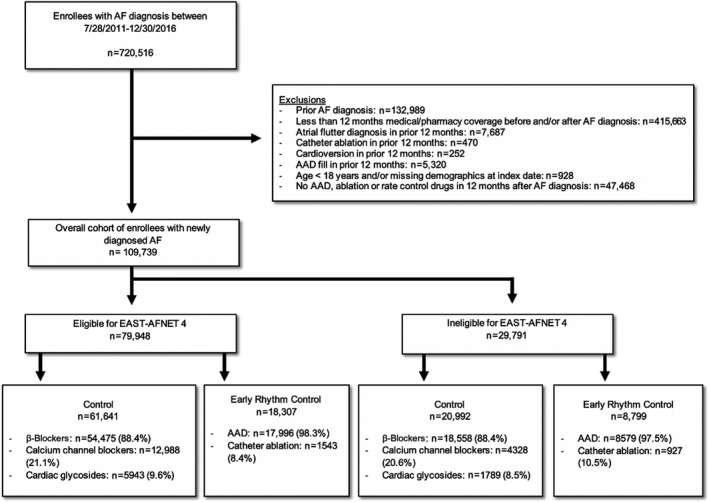
From July 28, 2011, to December 30, 2016, the enrollment period of EAST‐AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial), we identified 109 739 patients with newly diagnosed atrial fibrillation (AF) (overall cohort). The majority of patients (72.9%; 79 948 of 109 739) would have been eligible for EAST‐AFNET 4. AAD indicates antiarrhythmic drug.
Enrolled patients in EAST‐AFNET 4 (and the “trial‐eligible” subgroup of the current study) who were either aged >75 years or had a previous transient ischemic attack or stroke, or met 2 of the following criteria: age >65 years, female sex, HF, hypertension, diabetes, severe coronary artery disease, chronic kidney disease (Modification of Diet in Renal Disease stage 3 or 4 [glomerular filtration rate, 15 to 59 mL/min per 1.73 m2 of body surface area]), and left ventricular hypertrophy (diastolic septal wall width >15 mm).
Patients were required to have at least 12 months of continuous enrollment in health insurance plans before the first AF diagnosis date (baseline period) in order to capture an adequate medical history and to exclude those with AF diagnoses before the enrollment period. Also, patients were required to have AF diagnoses on at least 2 different days to exclude coding errors. Patients whose demographic or residence data were missing or invalid were excluded.
Outcomes
The primary outcome was a composite of all‐cause mortality, stroke, or hospitalization with the diagnoses of HF or MI, ie, comparable to the primary outcome assessed in EAST‐AFNET 4. The secondary outcomes included each of these outcomes considered separately. Mortality was identified based on the Social Security Death Master File and discharge status. Secondary analyses of administrative databases typically cannot ascertain the cause of death, therefore all‐cause mortality was used rather than cardiovascular death. Patients were followed until December 31, 2019, the end of enrollment in health insurance plans, or death, whichever occurred first.
Statistical Analysis
The proportion of patients who were not eligible for the trial was calculated and patients were divided into 3 subgroups based on the operational definition in Table S3: (1) patients who would be eligible for EAST‐AFNET 4; (2) patients who failed to meet the inclusion criterion, ie, those aged <75 years without 2 stroke risk factors; and (3) patients who met at least one of the exclusion criteria. Some patients may have both failed to meet the inclusion criterion and met the exclusion criteria. Such patients were classified as those who met the exclusion criteria. In the stratified analyses for clinical outcomes, patients of subgroups 2 and 3 were summarized as patients ineligible for the trial.
Propensity score overlap weighting was used to balance differences in 83 baseline characteristics between patients who underwent ERC and controls in the overall cohort and in each subgroup stratified by trial egilibilty. The standardized mean difference was used to assess the balance of covariates after weighting and a difference <0.1 was considered acceptable. 14
Cox proportional hazards regression was used to compare patients treated with ERC and controls in the propensity score–weighted cohort, with a robust sandwich estimator for variance estimation. The regression was performed in the overall cohort as well as in the groups stratified by trial eligibility. The Fine and Gray method was used to consider death as a competing risk when assessing nonfatal outcomes (ie, stroke, or hospitalization with the diagnoses of HF or MI when considered separately). 15 The proportional hazards assumption was tested on the basis of Schoenfeld residuals. 16
A 2‐sided P value <0.05 was considered statistically significant for all tests. All analyses except those related to the primary outcome were considered to be exploratory and conducted using SAS Enterprise Guide 7.1 (SAS Institute Inc.) and Stata 16.0 (StataCorp).
Sensitivity Analyses
We conducted several sensitivity analyses to assess the robustness of the findings. First, we performed subgroup analyses for the primary outcome stratified by age, sex, race, CHA2DS2‐VASc score, hypertension with left ventricular hypertrophy, HF, cardiomyopathy, sleep apnea, and prior thromboembolism. Second, we conducted a stratified analysis based on whether patients with early rhythm control were treated with AF ablation or AADs only. Third, a similar stratified analysis was conducted based on the adherence to AADs in the early rhythm‐control group. Adherence to AAD therapy was defined as the proportion of days covered ≥80%. Patients treated without early rhythm control were compared separately with those adherent and nonadherent AAD‐treated patients. Last, we assessed residual confounding by testing 2 falsification end points that are unlikely to be a result of ERC but might be related to unmeasured confounders such as frailty: pneumonia and fracture. The prespecified analysis plan, including more details of the methods, is available in Data S1.
Results
Patient Characteristics
We identified 109 739 patients with newly diagnosed AF from July 28, 2011, to December 30, 2016 (Table 1). The majority of patients (72.9%; 79 948 of 109 739) would have been eligible for EAST‐AFNET 4 (Figure 1). Only 6926 patients (6.3%) failed to meet the trial inclusion criterion and 22 865 patients (20.8%) met the trial exclusion criteria. In the overall cohort, 27 106 patients (24.7%) received ERC, ie, AF ablation or AAD therapy, within the first year after AF diagnosis; 82 633 patients (75.3%) did not receive ERC. The mean age was 71.0±11.6 years, 52 417 patients (47.8%) were women, 21 582 patients (19.7%) had a history of stroke, and 76 921 patients (70.1%) had a CHA2DS2‐VASc score of ≥4. Only 35 898 patients (32.7%) were using oral anticoagulation (before propensity score weighting: 29.2% in the control group and 43.4% in the early rhythm‐control group; after propensity score weighting: 28.0% in the control group and 28.0% in the early rhythm‐control group). The rates of catheter ablation among patients receiving ERC were similar in both trial‐eligible (8.4%; 1543 of 18 307) and trial‐ineligible (10.5%; 927 of 8799, Figure 1) patients. After propensity score weighting, patients receiving ERC and patients not receiving ERC were balanced on 83 dimensions (Table S4 through S6).
Table 1.
Selected Baseline Characteristics Before and After PS Weighting in the Overall Cohort
| Before PS weighting | After PS weighting | |||||
|---|---|---|---|---|---|---|
| Controls (n=82 633) | Early rhythm control (n=27 106) | Standardized difference | Controls (n=82 633) | Early rhythm control (n=27 106) | Standardized difference | |
| Age, mean±SD, y | 71.7±11.6 | 68.9±11.4 | 0.245 | 70.1±12.3 | 70.1±11.9 | 0.000 |
| 18–64 | 24.5% | 33.6% | 0.202 | 29.9% | 29.9% | 0.000 |
| 65–74 | 27.4% | 30.9% | 0.077 | 26.5% | 26.5% | 0.000 |
| 75+ | 48.1% | 35.5% | 0.258 | 43.6% | 43.6% | 0.000 |
| Women | 50.1% | 40.8% | 0.188 | 40.3% | 40.3% | 0.000 |
| Race or ethnicity | ||||||
| Asian | 2.5% | 2.0% | 0.031 | 2.7% | 2.7% | 0.000 |
| Black | 11.7% | 8.8% | 0.094 | 10.2% | 10.2% | 0.000 |
| Hispanic | 6.6% | 5.6% | 0.042 | 7.0% | 7.0% | 0.000 |
| Unknown | 2.4% | 2.4% | 0.001 | 2.2% | 2.2% | 0.000 |
| White | 76.8% | 81.1% | 0.105 | 77.9% | 77.9% | 0.000 |
| Comorbidities | ||||||
| Systolic HF | 16.9% | 22.5% | 0.142 | 25.7% | 25.7% | 0.000 |
| Cardiomyopathy | ||||||
| None | 80.4% | 74.9% | 0.133 | 68.9% | 68.9% | 0.000 |
| Hypertrophic | 1.3% | 1.7% | 0.032 | 2.7% | 2.7% | 0.000 |
| Ischemic | 4.6% | 6.0% | 0.060 | 8.1% | 8.1% | 0.000 |
| Dilated | 13.6% | 17.4% | 0.105 | 20.3% | 20.3% | 0.000 |
| Implanted device | ||||||
| None | 87.1% | 85.3% | 0.053 | 75.1% | 75.1% | 0.000 |
| CRT defibrillator | 0.6% | 0.9% | 0.038 | 1.9% | 1.9% | 0.000 |
| ICD | 5.2% | 5.6% | 0.019 | 12.3% | 12.3% | 0.000 |
| CRT pacemaker | 0.1% | 0.1% | 0.002 | 0.3% | 0.3% | 0.000 |
| Dual‐chamber pacemaker | 5.3% | 5.9% | 0.025 | 7.5% | 7.5% | 0.000 |
| Single‐chamber pacemaker | 1.8% | 2.3% | 0.033 | 3.0% | 3.0% | 0.000 |
| Hypertension | 94.0% | 90.7% | 0.123 | 92.2% | 92.2% | 0.000 |
| Diabetes | 42.7% | 36.7% | 0.123 | 44.3% | 44.3% | 0.000 |
| Thromboembolism | 26.2% | 20.7% | 0.130 | 25.4% | 25.4% | 0.000 |
| Stroke | 21.0% | 15.6% | 0.139 | 20.1% | 20.1% | 0.000 |
| CAD | 62.0% | 65.5% | 0.071 | 74.9% | 74.9% | 0.000 |
| Myocardial infarction | 24.8% | 26.1% | 0.032 | 34.0% | 34.0% | 0.000 |
| Left ventricular hypertrophy | 33.6% | 40.7% | 0.149 | 41.3% | 41.3% | 0.000 |
| Prior valve procedure | 2.9% | 9.5% | 0.274 | 6.4% | 6.4% | 0.000 |
| Mitral stenosis | 2.6% | 3.7% | 0.063 | 4.4% | 4.4% | 0.000 |
| Mitral regurgitation | 40.1% | 50.5% | 0.210 | 49.1% | 49.1% | 0.000 |
| Major bleeding | 31.5% | 30.4% | 0.023 | 32.0% | 32.0% | 0.000 |
| Intracranial bleeding | 3.6% | 2.9% | 0.041 | 3.2% | 3.2% | 0.000 |
| Stage 3–5 CKD | 20.0% | 17.3% | 0.069 | 20.4% | 20.4% | 0.000 |
| COPD | 24.6% | 23.0% | 0.037 | 25.5% | 25.5% | 0.000 |
| Obstructive sleep apnea | 21.7% | 28.7% | 0.164 | 27.4% | 27.4% | 0.000 |
| Previous drug treatment | ||||||
| No. of previous AADs | ||||||
| 0 | 99.2% | 1.9% | 8.365 | 31.2% | 31.2% | 0.000 |
| 1 | 0.8% | 88.6% | 3.757 | 67.0% | 67.0% | 0.000 |
| 2+ | 0.0% | 9.5% | 0.457 | 1.7% | 1.7% | 0.000 |
| Amiodarone use | 0.6% | 58.7% | 1.651 | 47.5% | 47.5% | 0.000 |
| No. of previous rate control drugs | ||||||
| 0 | * | * | 0.466 | 0.2% | 0.2% | 0.000 |
| 1 | 61.1% | 48.4% | 0.258 | 48.3% | 48.3% | 0.000 |
| 2 | 28.4% | 29.0% | 0.012 | 33.1% | 33.1% | 0.000 |
| 3+ | * | * | 0.075 | 18.3% | 18.3% | 0.000 |
| Concurrent Medication | ||||||
| Oral anticoagulants | ||||||
| None | 70.8% | 56.6% | 0.298 | 72.0% | 72.0% | 0.000 |
| Warfarin | 14.8% | 15.8% | 0.027 | 12.6% | 12.6% | 0.000 |
| NOAC | 14.4% | 27.6% | 0.329 | 15.4% | 15.4% | 0.000 |
| ACEIs | 28.2% | 26.7% | 0.034 | 28.3% | 28.3% | 0.000 |
| ARBs | 17.4% | 17.1% | 0.008 | 17.9% | 17.9% | 0.000 |
| β‐Blockers (rate control) | 70.0% | 53.2% | 0.350 | 67.2% | 67.2% | 0.000 |
| Calcium channel blockers (rate control) | 14.3% | 10.5% | 0.118 | 10.8% | 10.8% | 0.000 |
| Digitalis | 6.4% | 4.3% | 0.093 | 6.9% | 6.9% | 0.000 |
| Statin | 48.7% | 48.3% | 0.009 | 52.1% | 52.1% | 0.000 |
| Insulin | 8.8% | 6.2% | 0.100 | 9.8% | 9.8% | 0.000 |
| CHA2DS2‐VASc, mean±SD | 4.7±2.0 | 4.3±2.1 | 0.224 | 4.7±2.1 | 4.7±2.1 | 0.000 |
AAD indicates antiarrhythmic drug; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD; coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter–defibrillator; HF, heart failure; NOAC, non–vitamin K antagonist oral anticoagulant; and PS, propensity score.
To maintain deidentification, OptumLabs does not allow researchers to disclose the number of events when the number is ≤10.
Outcomes
Patients were followed for a mean of 2.6±1.8 years. In the overall cohort, ERC was associated with a reduction in the primary composite outcome of all‐cause mortality, stroke, or hospitalization with the diagnoses HF or MI compared with the control group (9.45 versus 11.13 events per 100 person‐years; HR, 0.85 [95% CI, 0.75–0.97]; P=0.02), and reduced risk for stroke (1.10 versus 1.70 events per 100 person‐years; HR, 0.66 [95% CI, 0.47–0.93]; P=0.02) (Table 2 and Figure 2). There was no significant risk reduction for all‐cause mortality (5.49 versus 6.24 events per 100 person‐years; HR, 0.88 [95% CI, 0.75–1.04]; P=0.14) or hospitalization with the diagnoses HF (3.69 versus 3.94 events per 100 person‐years; HR, 0.95 [95% CI, 0.76–1.18]; P=0.61) or MI (1.16 versus 1.52 events per 100 person‐years; HR, 0.76 [95% CI, 0.54–1.08]; P=0.13). The observed results were largely consistent between patients eligible or ineligible for the trial; however, the reduction of stroke risk associated with ERC was only significant in patients eligible for the trial (1.27 versus 1.94 events per 100 person‐years; HR, 0.67 [95% CI, 0.45–0.98]; P=0.04).
Table 2.
Outcomes in PS–Weighted Patients Stratified by Trial Eligibility
| Control | Early rhythm control | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of events | Person‐years | Event rate | No. of events | Person‐years | Event rate | HR (95% CI) | P value | |
| Overall cohort | n= 82 633 | n=27 106 | ||||||
| Composite | 228 | 2049 | 11.13 | 195 | 2065 | 9.45 | 0.85 (0.75–0.97) | 0.015 |
| Stroke | 37 | 2185 | 1.70 | 24 | 2191 | 1.10 | 0.66 (0.47–0.93) | 0.017 |
| HF | 84 | 2125 | 3.94 | 78 | 2124 | 3.69 | 0.95 (0.76–1.18) | 0.613 |
| MI | 34 | 2203 | 1.52 | 25 | 2188 | 1.16 | 0.76 (0.54–1.08) | 0.127 |
| Mortality | 140 | 2243 | 6.24 | 122 | 2223 | 5.49 | 0.88 (0.75–1.04) | 0.135 |
| Eligible for trial | n=61 641 | n=18 307 | ||||||
| Composite | 165 | 1507 | 10.98 | 143 | 1466 | 9.76 | 0.89 (0.76–1.04) | 0.138 |
| Stroke | 31 | 1598 | 1.94 | 20 | 1560 | 1.27 | 0.67 (0.45–0.98) | 0.041 |
| HF | 56 | 1566 | 3.60 | 56 | 1512 | 3.67 | 1.03 (0.79–1.34) | 0.843 |
| MI | 26 | 1613 | 1.59 | 19 | 1558 | 1.24 | 0.78 (0.53–1.17) | 0.236 |
| Mortality | 102 | 1644 | 6.23 | 86 | 1586 | 5.40 | 0.87 (0.72–1.06) | 0.168 |
| Ineligible for trial | n=20 992 | n=8799 | ||||||
| Composite | 63 | 543 | 11.55 | 52 | 600 | 8.69 | 0.77 (0.60–0.98) | 0.035 |
| Stroke | 6 | 587 | 1.04 | 4 | 631 | 0.69 | 0.67 (0.33–1.34) | 0.254 |
| HF | 27 | 560 | 4.89 | 23 | 611 | 3.73 | 0.79 (0.54–1.15) | 0.214 |
| MI | 8 | 589 | 1.35 | 6 | 630 | 0.94 | 0.71 (0.36–1.41) | 0.330 |
| Mortality | 37 | 599 | 6.25 | 36 | 637 | 5.69 | 0.92 (0.68–1.26) | 0.621 |
The event rate was calculated as the number of events per 100 person‐years. Propensity score (PS) weight was applied when calculating number of events, person‐years, event rates, absolute reduction, and hazard ratios (HRs). HF indicates hospitalization with the diagnosis of heart failure; and MI, hospitalization with the diagnosis of myocardial infarction.
Figure 2. Primary composite end point and cumulative incidence of stroke stratified by EAST‐AFNET 4 (Early treatment of atrial fibrillation for stroke prevention trial) eligibility criteria.
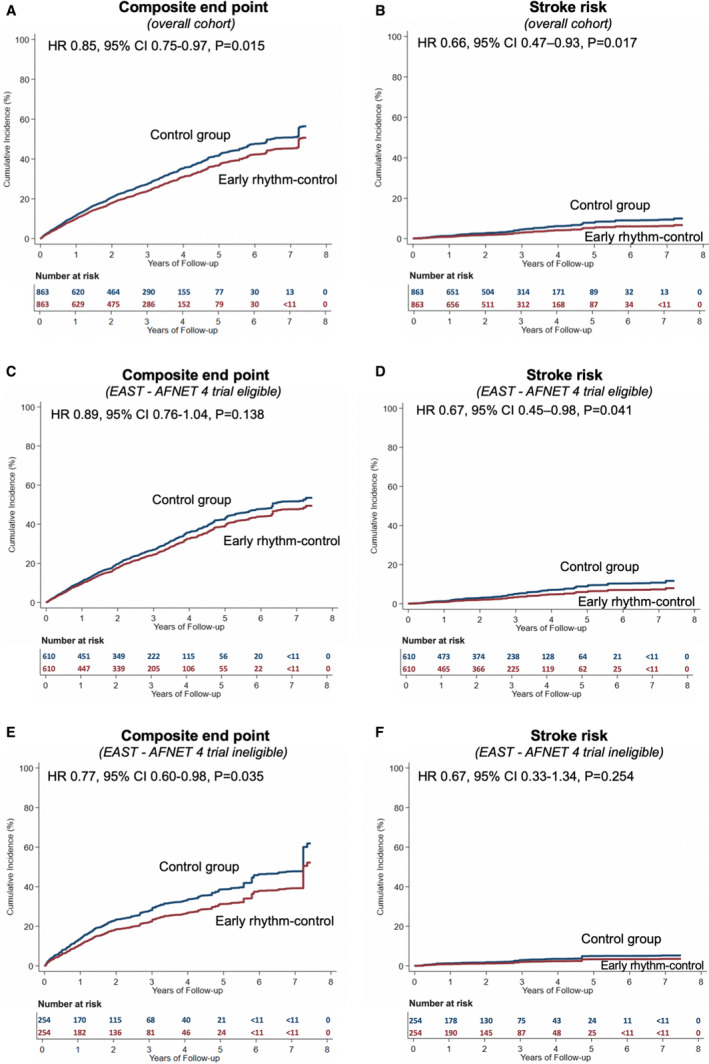
Cumulative incidence curves for the primary outcome, a composite of all‐cause mortality, stroke, or hospitalization with the diagnoses of heart failure or myocardial infarction in the early rhythm‐control group (red) or control group (blue), stratified by EAST‐AFNET 4 trial eligibility criteria. Overall cohort (A and B), patients who would be potentially eligible for EAST‐AFNET 4 (C and D), and patients who would be ineligible for EAST‐AFNET 4 (E and F). The control group was the reference group in the Cox proportional hazards regression analyses. All of the curves and numbers were calculated using propensity score weighting. *To maintain deidentification, OptumLabs does not allow researchers to disclose the number of events when the number is ≤10. HR indicates hazard ratio.
Sensitivity Analyses
The risk reduction in the primary composite outcome associated with ERC was observed with significant differences in patients aged <75 years, patients with CHA2DS2‐VASc scores ≥4, patients without systolic HF or cardiomyopathy, and patients with prior thromboembolism. Of note, the most significant interaction was observed by cardiomyopathy status (Figure 3). ERC was never associated with an increased risk in any of the outcomes analyzed or any of the subgroups (Table S7 through S10). Patients aged <75 years had significantly reduced stroke risk and reduced overall mortality. In patients eligible for the trial, event rates were highest and the risk reduction in the composite outcome was greatest in patients with prior thromboembolism. The subgroup analyses for the primary outcome stratified by trial eligibility can be found in Table S11 and Table S12.
Figure 3. Subgroup analysis for the primary outcome in propensity score–weighted patients.
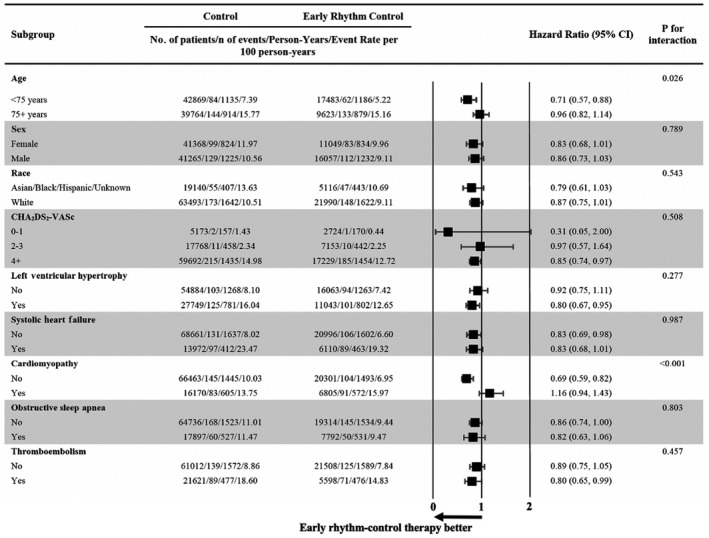
Hazard ratios and P values for interaction are based on Cox proportional hazards regression analyses on the composite end point of all‐cause mortality, stroke, or hospitalization with the diagnoses of heart failure or myocardial infarction. There were significant interactions between early rhythm control and age, as well as cardiomyopathy, which imply that the reduction in the composite end point associated with early rhythm control was greater in patients aged <75 years and patients without cardiomyopathy.
In the stratified analysis based on whether patients with early rhythm control were treated with AF ablation or without AF ablation, ie, with AADs only, early rhythm control was associated with a lower risk of the primary composite end point in patients treated without AF ablation (11.39 versus 13.28 events per 100 person‐years; HR, 0.86 [95% CI, 0.74–1.00]; P=0.05) but not in patients treated with AF ablation; however, event rates were much lower in these patients and this subsample was relatively small (4.36 versus 5.40 events per 100 person‐years; HR, 0.80 [95% CI, 0.55–1.18]; P=0.26) (Table S13).
For patients in the early rhythm‐control group who adhered to AAD therapy, ERC was associated with a lower stroke risk in both the overall cohort and in trial‐eligible patients, but the magnitude was greater in trial‐eligible patients (0.92 versus 2.15 events per 100 person‐years; HR, 0.43 [95% CI, 0.25–0.74]; P<0.01) (Table S14 through Table S16).
There was no difference in the rate of fracture or pneumonia, the chosen falsification end points between patients treated with early rhythm control and control patients (Table S17).
Discussion
In this large US data set of 109 739 patients with newly diagnosed AF, ERC was associated with a lower risk of death, stroke, or hospitalization with the diagnoses HF or MI, with the greatest reduction in stroke risk. The majority of patients (72.9%; 79 948 of 109 739) treated in routine US practice appear to meet enrollment criteria for EAST‐AFNET 4 and the observed results associated with ERC in routine practice are largely consistent between patients eligible or ineligible for the trial.
Patients in routine practice had higher rates of adverse outcomes than the trial, but the relative risk reduction with ERC was similar: EAST‐AFNET 4 reported a 21% reduction in the composite end point associated with early rhythm control, with low overall event rates of 3.9 events per 100 person‐years in the early rhythm‐control group and 5.0 events per 100 person‐years in the usual care group. 7 Event rates in this analysis were higher, but the relative stroke risk reduction associated with ERC observed in routine practice of 34% is consistent with EAST‐AFNET 4. Absolute stroke rates were higher in this analysis than in the trial, possibly because of the lower rate of anticoagulation (≈90% in EAST‐AFNET 4 compared with only 32.7% of patients in this data set). Furthermore, patients in this analysis had more cardiovascular comorbidities (the mean CHA2DS2‐VASc score was 4.6 in OptumLabs compared with 3.4 in EAST‐AFNET 4). Interestingly, the mean age was quasi‐identical to the EAST‐AFNET 4 population (70.2±8.4 years). In addition, differences in absolute event rates could be related to the different methods of event adjudication/ascertainment between retrospective claims–based analyses and prospective trial event classification.
AF ablation was used in a minority of patients treated with early rhythm control (≈1 in 10 patients), similar to EAST‐AFNET 4. Patients treated with AF ablation in this data set had a lower event rate, most likely reflecting the clinical tendency to offer AF ablation to younger and healthier patients as reflected by lower age and CHA2DS2‐VASc scores. The lower event rate and the lower number of patients are likely reasons that the risk reduction associated with early rhythm control showed a comparable hazard rate but no statistical significance (HR, 0.80; 95% CI, 0.55–1.18) compared with control. Adding to earlier reports assessing AF ablation as first‐line rhythm‐control therapy, 17 the recently published EARLY‐AF (Early Aggressive Invasive Intervention for Atrial Fibrillation) and STOP AF First (Cryoballoon Catheter Ablation in an Antiarrhythmic Drug Naive Paroxysmal Atrial Fibrillation) trials both demonstrated the safety of AF ablation using cryoballoon devices compared with AAD therapy with lower AF recurrence rates. 18 , 19 Consistent with these findings and with the safety profile of AAD and AF ablation therapy in the CABANA (Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation) trial, 20 none of our analyses found a signal for harm associated with early rhythm control.
This is the largest comparison of patients treated with ERC and controls, including >100 000 patients. The strengths of the study are the close modeling of the EAST‐AFNET 4 inclusion criteria and the well‐documented information on events. The estimate for eligibility thus should be robust. Taken together with the main findings from the EAST‐AFNET 4 randomized clinical trial and with a recent analysis in the Korean Health Data showing lower event rates in Korean patients treated with ERC (early rhythm control 7.42 events per 100 patient‐years, controls 9.25 events per 100 patient‐years; HR, 0.81 [95% CI, 0.71–0.93]), 21 our data support the inclusion of ERC in the management of all patients with recently diagnosed AF and concomitant conditions to avoid missing positive effects, calling for an update of international guidelines. 22 , 23
Limitations
First, the comparison between treatment groups was not randomized and is therefore prone to residual confounding despite careful adjustment. 12 , 13 However, many of the measured variables are strongly correlated with unmeasured variables and the propensity matching procedure used here resulted in identical values on 83 baseline characteristics. Furthermore, the lack of effect of early rhythm control of other health outcomes (pneumonia, fracture) associated with frailty and multimorbidity support the robustness of our matching algorithms.
Second, administrative data can be subject to misclassification. However, the billing codes used in this study are robustly monitored by payors and hospitals during the reimbursement process and have been commonly used and have demonstrated good performance in validation studies with positive predictive values around 90%. 24 , 25 , 26 , 27 , 28 The information contained in health data sets such as OptumLabs is less precise than the more granular information collected in a clinical trial, hence excluding cause of death in this analysis, which could be be a potential source of bias. Also, in OptumLabs, there is no reliable way to adjudicate paroxysmal versus persistent AF given the reliance on diagnosis codes. Therefore, we have not analyzed the AF type.
Third, the findings are reflective of insured patients in the United States. The generalizability to uninsured patients and those not in Medicare Advantage are uncertain.
Last, within administrative claims databases such as OptumLabs it is challenging to accurately ascertain arrhythmia outcomes and quality of life because in routine practice not all patients are regularly monitored. Therefore, in contrast to EAST‐AFNET 4, we have not assessed the efficacy of rhythm‐control therapy, the severity of AF symptoms, or the quality of life.
Conclusions
In this large routine‐care data set, three quarters of patients with new‐onset AF would be eligible for early rhythm control as tested in EAST‐AFNET 4. ERC was associated with lower rates of a composite of stroke, death, and hospitalization for HF or MI. Our data support the routine initiation of ERC as part of the management of patients with recently diagnosed AF.
Sources of Funding
Dr Dickow was supported by the German Heart Foundation (S/06/19; Mit Fördermitteln der Deutsche Herzstiftung e.V.). Dr Kirchhof is partially supported by European Union BigData@Heart (grant agreement EU IMI 116074), British Heart Foundation (FS/13/43/30324; PG/17/30/32961 and PG/20/22/35093; AA/18/2/34218), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK), and Leducq Foundation. Dr Packer is funded in part by a Clinician Investigator award from the Mayo Foundation. Dr Noseworthy receives research funding from the National Institutes of Health, including the National Heart, Lung, and Blood Institute (R21AG 62580–1, R01HL 131535‐4, R01HL 143070‐2), the National Institute on Aging (R01AG 062436‐1), the Agency for Healthcare Research and Quality (R01HS 25402‐3), the Food and Drug Administration (FD 06292), and the American Heart Association (18SFRN34230146). Over the past 36 months, Dr Yao has received research support through Mayo Clinic from the National Institutes of Health (R21HL140205, R01AG062436), the Agency for Healthcare Research and Quality (R01HS025402), the Food and Drug Administration (U01FD005938), the University of Nebraska Medical Center, and the Medical Devices Innovation Consortium/National Evaluation System for health Technology.
Disclosures
Dr Kirchhof receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and the German Centre for Cardiovascular Research, from several drug and device companies active in AF, and has received honoraria from several such companies in the past but not in the past 3 years. Dr Kirchhof is listed as inventor on 2 patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). Dr Packer in the past 12 months has provided consulting services for Abbott; AtriFix; Biosense Webster, Inc.; Cardio Syntax; EBAmed; Johnson & Johnson $0; MediaSphere Medical; LLC<$5000; MedLumics; Medtronic; NeuCures; St. Jude Medical; Siemens; Spectrum Dynamics; Centrix; and Thermedical. Dr Packer received no personal compensation for these consulting activities, unless noted. Dr Packer receives research funding from Abbott; Biosense Webster; Boston Scientific/EPT; CardioInsight; EBAmed; German Heart Foundation; Medtronic; National Institutes of Health; Robertson Foundation; St. Jude Medical; Siemens; Thermedical; Inc.; Vital Project Funds, Inc.; and Mr. and Mrs. J. Michael Cook. Dr Packer and Mayo Clinic jointly have equity in a privately held company, External Beam Ablation Medical Devices. Royalties from Wiley & Sons, Oxford, and St Jude Medical. Dr Noseworthy is a study investigator in an ablation trial sponsored by Medtronic. Dr Noseworthy and Mayo Clinic are involved in potential equity/royalty relationship with AliveCor. Dr Noseworthy has served on an expert advisory panel for Optum. Dr Noseworthy and Mayo Clinic have filed patents related to the application of artificial intelligence to the ECG for diagnosis and risk stratification. All other authors have nothing to declare.
Supporting information
Data S1
Tables S1–S17
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024214
For Sources of Funding and Disclosures, see page 10.
References
- 1. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- 2. Friberg L, Hammar N, Pettersson H, Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort‐Study of Atrial Fibrillation (SCAF). Eur Heart J. 2007;28:2346–2353. doi: 10.1093/eurheartj/ehm308 [DOI] [PubMed] [Google Scholar]
- 3. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJM, Tijssen JGP, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375 [DOI] [PubMed] [Google Scholar]
- 4. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328 [DOI] [PubMed] [Google Scholar]
- 5. Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U. Randomized trial of rate‐control versus rhythm‐control in persistent atrial fibrillation: the strategies of treatment of atrial fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. doi: 10.1016/S0735-1097(03)00332-2 [DOI] [PubMed] [Google Scholar]
- 6. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JMO, Buxton AE, Camm AJ, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789 [DOI] [PubMed] [Google Scholar]
- 7. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Bax J, Blomstrom‐Lundquist C, Calkins H, Camm AJ, Cappato R, Cosio F, Crijns H, Diener H‐C, Goette A, et al. Early and comprehensive management of atrial fibrillation: executive summary of the proceedings from the 2nd AFNET‐EHRA consensus conference “research perspectives in AF”. Eur Heart J. 2009;30:2969–2980. doi: 10.1093/eurheartj/ehp235 [DOI] [PubMed] [Google Scholar]
- 9. Kirchhof P. Can we improve outcomes in AF patients by early therapy? BMC Med. 2009;7:72. doi: 10.1186/1741-7015-7-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum labs: building a novel node in the learning health care system. Health Aff. 2014;33:1187–1194. doi: 10.1377/hlthaff.2014.0038 [DOI] [PubMed] [Google Scholar]
- 11. Optum Research Data Assets . Available at https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf. Accessed October 17, 2020.
- 12. Noseworthy PA, Gersh BJ, Kent DM, Piccini JP, Packer DL, Shah ND, Yao X. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J. 2019;40:1257–1264. doi: 10.1093/eurheartj/ehz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noseworthy PA, Van Houten HK, Gersh BJ, Packer DL, Friedman PA, Shah ND, Dunlay SM, Siontis KC, Piccini JP, Yao X. Generalizability of the CASTLE‐AF trial: catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm. 2020;17:1057–1065. doi: 10.1016/j.hrthm.2020.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 16. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 17. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566 [DOI] [PubMed] [Google Scholar]
- 18. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, Roux J‐F, Yung D, Skanes A, Khaykin Y, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980 [DOI] [PubMed] [Google Scholar]
- 19. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, Niebauer M, Makati K, Halperin B, Gauri A, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–324. doi: 10.1056/NEJMoa2029554 [DOI] [PubMed] [Google Scholar]
- 20. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim D, Yang P‐S, You SC, Sung J‐H, Jang E, Yu HT, Kim T‐H, Pak H‐N, Lee M‐H, Lip GYH, et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ. 2021;373:n991. doi: 10.1136/bmj.n991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 23. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C, Healey JS, Bell A, Cairns J, Connolly S, et al. 2018 Focused update of the canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34:1371–1392. doi: 10.1016/j.cjca.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 24. Kumamaru H, Judd SE, Curtis JR, Ramachandran R, Hardy NC, Rhodes JD, Safford MM, Kissela BM, Howard G, Jalbert JJ, et al. Validity of claims‐based stroke algorithms in contemporary medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with medicare claims. Circ Cardiovasc Qual Outcomes. 2014;7:611–619. doi: 10.1161/CIRCOUTCOMES.113.000743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1 [DOI] [PubMed] [Google Scholar]
- 26. Fan J, Arruda‐Olson AM, Leibson CL, Smith C, Liu G, Bailey KR, Kullo IJ. Billing code algorithms to identify cases of peripheral artery disease from administrative data. J Am Med Informatics Assoc. 2013;20:e349–e354. doi: 10.1136/amiajnl-2013-001827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non‐vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69:2779–2790. doi: 10.1016/j.jacc.2017.03.600 [DOI] [PubMed] [Google Scholar]
- 28. Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, Noseworthy PA. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:2621–2632. doi: 10.1016/j.jacc.2017.09.1087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S17


