Abstract
Background
Adverse pregnancy outcomes (APOs) (hypertensive disorders of pregnancy [HDP], preterm delivery [PTD], or low birth weight [LBW]) are associated adverse maternal and offspring cardiovascular outcomes. Therefore, we sought to describe nationwide temporal trends in the burden of each APO (HDP, PTD, LBW) from 2007 to 2019 to inform strategies to optimize maternal and offspring health outcomes.
Methods and Results
We performed a serial cross‐sectional analysis of APO subtypes (HDP, PTD, LBW) from 2007 to 2019. We included maternal data from all live births that occurred in the United States using the National Center for Health Statistics Natality Files. We quantified age‐standardized and age‐specific rates of APOs per 1000 live births and their respective mean annual percentage change. All analyses were stratified by self‐report of maternal race and ethnicity. Among 51 685 525 live births included, 15% were to non‐Hispanic Black individuals, 24% Hispanic individuals, and 6% Asian individuals. Between 2007 and 2019, age standardized HDP rates approximately doubled, from 38.4 (38.2–38.6) to 77.8 (77.5–78.1) per 1000 live births. A significant inflection point was observed in 2014, with an acceleration in the rate of increase of HDP from 2007 to 2014 (+4.1% per year [3.6–4.7]) to 2014 to 2019 (+9.1% per year [8.1–10.1]). Rates of PTD and LBW increased significantly when co‐occurring in the same pregnancy with HDP. Absolute rates of APOs were higher in non‐Hispanic Black individuals and in older age groups. However, similar relative increases were seen across all age,racial and ethnic groups.
Conclusions
In aggregate, APOs now complicate nearly 1 in 5 live births. Incidence of HDP has increased significantly between 2007 and 2019 and contributed to the reversal of favorable trends in PTD and LBW. Similar patterns were observed in all age groups, suggesting that increasing maternal age at pregnancy does not account for these trends. Black–White disparities persisted throughout the study period.
Keywords: adverse pregnancy outcomes, maternal morbidity, racial disparities
Subject Categories: Pregnancy, Preeclampsia, Disparities, Epidemiology
Nonstandard Abbreviations and Acronyms
- APO
adverse pregnancy outcome
- HDP
hypertensive disorders of pregnancy
- PTD
preterm delivery
Clinical Perspective
What Is New?
Maternal age at delivery increased by only 2 years, whereas rates of hypertensive disorders of pregnancy more than doubled from 2007 to 2019 in the United States, with similar increases in all maternal age groups between 15 and 44 years.
The increasing rate of hypertensive disorders of pregnancy appears to be a driver in the unfavorable trends observed for other adverse pregnancy outcomes: delivery of a low birth weight infant and preterm delivery.
Rates of adverse pregnancy outcomes were higher in non‐Hispanic Black birthing individuals compared with non‐Hispanic White, Hispanic, and Asian birthing individuals, without narrowing of disparities across the study period.
What Are the Clinical Implications?
Clinical and public health efforts focusing on prevention, awareness, and long‐term risk stratification among individuals who experience an adverse pregnancy outcome are urgently needed to optimize maternal and offspring health.
Specifically, focused interventions targeting hypertensive disorders of pregnancy are needed.
Between 1987 and 2018, annual maternal mortality rates in the United States increased by >2‐fold, from 7.2 to 17.4 deaths per 100 000 live births. Although this perhaps reflects improved death certificate reporting of pregnancy‐related mortality, US maternal mortality rates still exceed every other high‐income country in the world. 1 Adverse pregnancy outcomes (APOs), such as hypertensive disorders of pregnancy (HDP), preterm delivery (PTD), and low birth weight (LBW) are key risk factors for maternal morbidity and mortality as well as long‐term cardiovascular outcomes for the birthing person and their offspring. 2 , 3
Cardiovascular disease (CVD) is the leading cause of maternal mortality in pregnancy and in the postpartum period and accounts for 26.5% of maternal deaths in the United States. 4 Recently, data have emerged that individuals who experience APOs are at greater risk for long‐term CVD, including heart failure, coronary artery disease, peripheral arterial disease, and ischemic stroke, compared with individuals with uncomplicated term deliveries. 5 , 6 , 7 Specifically, HDPs are associated with earlier development of CVD 8 and a higher risk of all‐cause cardiovascular mortality. 9 As a result, the American College of Obstetrics and Gynecology, American Academy of Family Physicians, American College of Cardiology, and American Heart Association all now emphasize the importance of targeted surveillance and earlier interventions to reduce cardiovascular risk among those who experienced an APO. In addition, APOs are associated with adverse cardiovascular profiles in the offspring, which make APOs a significantly important public health problem that impacts multiple generations.
Before pregnancy, suboptimal cardiovascular health may contribute to increased risk of APOs. 10 On a population level, the cardiovascular health of young adults of reproductive age has been worsening in the United States, and rates of obesity, 11 prediabetes, and diabetes 12 have all increased since 1999. Additionally, the mean age of pregnant individuals 13 has increased. Given these changing risk factors 14 and demographic 15 profiles in pregnant individuals, a better understanding of changes in nationwide rates of APOs is needed to direct targeted prevention strategies. We therefore analyzed trends in rates of APOs in the United States between 2007 and 2019 using age‐standardization as well as age‐specific rates. We focused on HDP, PTD, and LBW given that these 3 APOs account for a large majority of maternal and neonatal morbidity. We also stratified based on maternal self‐report of race and ethnicity, which are social constructs, to examine racial and ethnic disparities that reflect upstream social determinants of health, such as structural and systemic barriers (eg, racism).
METHODS
We performed a serial, cross‐sectional analysis using maternal data from the National Center for Health Statistics Natality Files, which include all live births in the United States. We included maternal and neonatal data from all 51 685 525 live births to individuals in the United States aged 15 to 44 years between January 1, 2007 and December 31, 2019. Consistent with prior publications, we selected births to individuals aged 15 to 44 years, given 99% of all births occur in this reproductive aged group, with a small minority of US births falling outside of this window (0.05% of births occur in those aged <15 years and 0.26% in those ages >44 years). All data and materials are publicly available at https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm.
Demographic and health data for registration of live births are completed by the professional attendant present at delivery according to National Center for Health Statistics protocols using the standard certificate of birth. 16 Specifically, new‐onset HDP is coded as yes or no and includes both gestational hypertension and preeclampsia together. Birth weight is entered in grams, and estimated gestational age is quantified in weeks. Based on American College of Obstetrics and Gynecology definitions, we defined LBW as <2500 g 17 and PTD as <37 weeks of gestation. 18 Maternal race and ethnicity was based on self‐identified data and was included to describe disparities in APOs with the acknowledgement that race and ethnicity are social constructs. We also performed a sensitivity analysis restricting the sample to only nulliparous mothers with singleton live births (N=19 789 132).
We calculated age‐standardized rates of each APO subtype in the overall sample based on the age distribution of the sample midpoint in 2012. We also calculated rates of each APO subtype in subgroup analyses by race and ethnicity (non‐Hispanic White, non‐Hispanic Black, Hispanic, and Asian) and in 5‐year standardized age categories (15–19, 20–24, 25–29, 30–34, 35–39, and 40–44 years). We calculated and examined trends in rates (per 1000 live births) for each APO subtype overall and stratified by racial and ethnic and age subgroups.
Statistical Analysis
We used the Joinpoint Regression Program (National Cancer Institute) to fit log‐linear regression models to identify statistically significant inflection points (or joinpoints) in the trend of HDP across the study period from 2007 to 2019. 19 , 20 Once inflection points (or joinpoints) were identified in the rate of HDP, this was applied to the other APO subtypes to have a standard reference point, and we calculated the slopes (average annual percent change) of the segments connecting the joinpoints in the overall population. In secondary analyses, we also examined trends in rates of LBW and PTD stratified by presence or absence of HDP. Two‐sided statistical significance was defined as P<0.05. Analyses were performed using SAS (SAS Institute, Cary, NC).
This study was deemed exempt from review by the institutional review board at Northwestern University and no informed consent was required because of the deidentified nature of this publicly available data set.
RESULTS
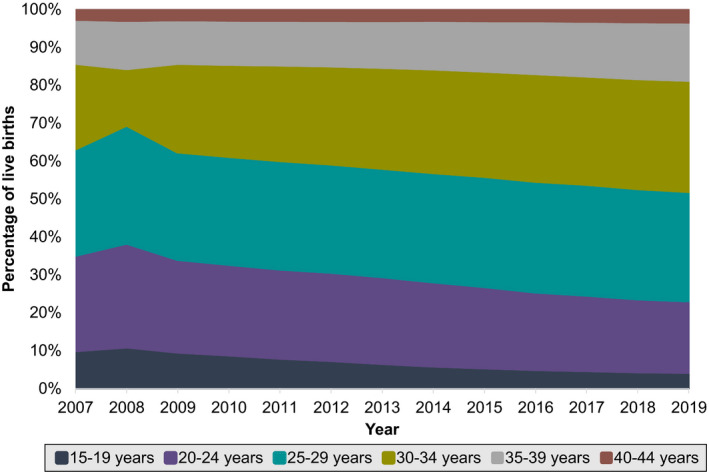
Between 2007 to 2019, a total of 51 685 525 live births occurred to individuals aged 15 to 44 years in the United States; 54% of live births occurred in individuals who were non‐Hispanic White, 15% non‐Hispanic Black, 24% Hispanic, and 6% Asian (Table 1). Mean (SD) maternal age at delivery increased from 27.4 (6.1) to 29.1 (5.7) years. The percentage of individuals in the middle age groups (30–39 years) increased, whereas the percentage of individuals in the youngest age groups (15–24 years) decreased. There was no major change in the percentage of individuals in the oldest age group (40–44 years) (Figure 1). Less than 0.5% of individuals had missing data for each APO subtype.
Table 1.
Demographic Characteristics in Women Aged 15 to 44 Years With Live Births in the United States, Overall and in 2007 and 2019
| Overall, N=51 685 525 | 2007, N=4 302 685 | 2019, N=3 736 144 | |
|---|---|---|---|
| Age, y, mean (SD) | 28.2 (5.9) | 27.4 (6.1) | 29.1 (5.7) |
| Age categories, % | |||
| 15–19 y | 7.4 | 10.3 | 4.6 |
| 20–24 y | 22.4 | 25.2 | 18.9 |
| 25–29 y | 28.7 | 28.1 | 28.9 |
| 30–34 y | 26.0 | 22.4 | 29.2 |
| 35–39 y | 12.8 | 11.6 | 15.3 |
| 40–44 y | 2.8 | 2.4 | 3.2 |
| Race and ethnicity, % | |||
| Non‐Hispanic White | 53.5 | 53.6 | 52.2 |
| Non‐Hispanic Black | 14.8 | 14.5 | 15.4 |
| Asian | 6.4 | 5.6 | 7.0 |
| Hispanic | 23.5 | 24.6 | 23.7 |
Figure 1. Age of women who had live births in the United States between 2007 and 2019, categorized by 5‐year age intervals.
Between 2007 to 2019, age‐standardized rates of HDP increased from 38.4 (38.2–38.6) in 2007 to 77.8 (77.5–78.1) in 2019 (Table S1, Figure 2). A significant inflection point was observed in 2014. The average annual percent change for rates of HDP accelerated from 4.1% per year (3.6–4.7) between 2007 and 2014 to 9.1% per year (8.1–10.1) between 2014 and 2019. (Table 2).
Figure 2. Trends in age‐standardized rates of adverse pregnancy outcome subtypes per 1000 live births in women aged 15 to 44 years in the United States, 2007 to 2019.
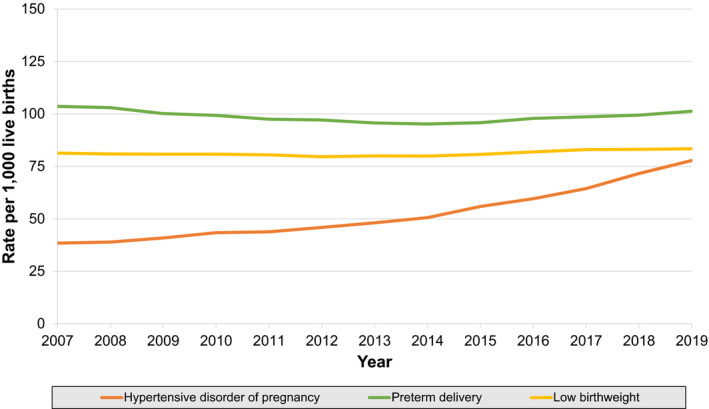
Adverse pregnancy outcomes defined as hypertensive disorder of pregnancy, preterm delivery (<37 weeks gestation), or low birth weight (<2500 g).
Table 2.
Average Annual Percent Change in Age‐Standardized Rates of Adverse Pregnancy Outcomes Per 1000 Live Births in Women Aged 15 to 44 Years in the United States, 2007 to 2019
| APO subtypes | Average annual percent change (95% CI) | ||
|---|---|---|---|
| 2007–2019 | 2007–2014 | 2014–2019 | |
| Hypertensive disorders of pregnancy | 6.2 (5.7 to 6.6)* | 4.1 (3.6 to 4.7)* | 9.1 (8.1 to10.1)* |
| Preterm delivery | −0.2 (−0.4 to 0.0)* | −1.2 (−1.5 to −1.0)* | 1.3 (1.0 to 1.7)* |
| Low birth weight | 0.2 (−0.1 to 0.5) | −0.3 (−0.4 to −0.1)* | 0.9 (0.2 to 1.6)* |
APO indicates adverse pregnancy outcome.
Indicates that the average annual percent change is significantly different from 0 at the α=0.05 level.
Patterns in rates of APOs were heterogeneous. Rates of LBW per 1000 live births decreased slightly from 81.3 (81.0–81.5) in 2007 to 79.9 (79.7–80.2) in 2014, and then increased modestly to 83.4 (83.1–83.7) in 2019. Similarly, PTD rates per 1000 live births decreased from 103.6 (103.3–103.9) in 2007 to 95.2 (94.9–95.5) in 2014, and then increased to 101.3 (101.0–101.7) in 2019 (Figure 2). For both LBW and PTD, the declines between 2007 and 2014 (−1.2% per year [−1.5 to −1.0] and −0.3% per year [−0.4 to −0.1], respectively) reversed in 2014, with subsequent increases from 2014 to 2019 (+1.3% per year [1.0–1.7] and +0.9% per year [0.2–1.6], respectively). Secondary analyses of LBW and PTD rates stratified by presence or absence of concomitant HDP identified heterogeneous patterns. Age‐standardized rates of LBW or PTD without concomitant HDP did not change or decreased over the study period, across all racial and ethnic groups (Figure S1A and S1C). However, in contrast, age‐standardized rates of LBW or PTD with concomitant HDP increased across the study period in all racial and ethnic groups (Figure S1B and S1D).
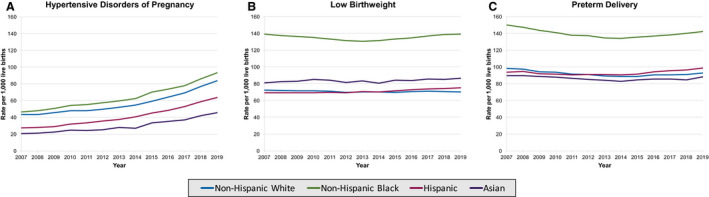
Non‐Hispanic Black individuals experienced higher rates of every APO subtype compared with non‐Hispanic White, Hispanic, and Asian individuals (Figure 3A through 3C). Patterns in the rates of APOs within each racial and ethnic group resembled the overall patterns. HDP rates increased across all racial and ethnic groups from 2007 to 2014, and then further accelerated in all racial and ethnic groups from 2014 to 2019. LBW rates changed variably in racial and ethnic groups from 2007 to 2014 (decreased in non‐Hispanic White and non‐Hispanic Black groups, increased in Hispanic and Asian groups) and from 2014 to 2019 (stable in non‐Hispanic White groups, reversed to rising in non‐Hispanic Black groups, continued rising in Hispanic and Asian groups). PTD rates declined in all racial and ethnic groups from 2007 to 2014, and then began rising in all groups from 2014 to 2019 (Table S2).
Figure 3. Trends in age‐standardized rates of adverse pregnancy outcome subtypes per 1000 live births in women aged 15 to 44 years in the United States, 2007 to 2019, stratified by race and ethnicity, for hypertensive disorders of pregnancy (A), low birth weight (B), and preterm delivery (C).
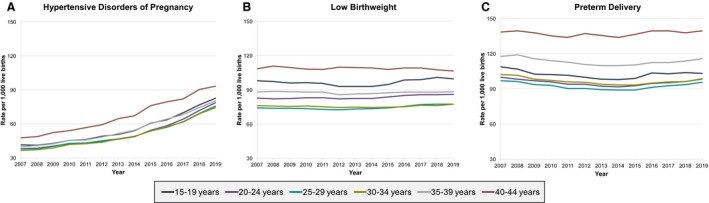
Age‐specific rates of each APO subtype were consistently higher in individuals aged 40 to 44 years compared with younger individuals aged 15 to 39 years throughout the study period (Figure 4A through 4C). For age‐specific subgroups, the average annual percent change of HDP from 2014 to 2019 was higher in the younger age groups (eg, 10.2% per year [9.2–11.3] in those aged 20–24 years compared with 6.1% per year [5.6–6.5] in those aged 40–44 years). Similarly, the average annual percent change between 2014 and 2019 was higher in younger age groups for both LBW (eg, 1.5% per year [0.8–2.1] in those aged 15–19 years compared with no change of −0.1% [−0.3 to 0.0] in those aged 40–44 years) and for PTD (eg, 1.5% per year [1.2–1.8] in those aged 20–24 years compared with 0.4% per year [0.0–0.9] in those aged 40–44 years) (Table S3).
Figure 4. Trends in rates of adverse pregnancy outcome subtypes per 1000 live births in women aged 15 to 44 years in the United States, 2007 to 2019, stratified by 5‐year age intervals, for hypertensive disorders of pregnancy (A), low birth weight (B), and preterm delivery (C).
Nulliparous singleton births had higher rates of HDP and lower rates of PTD and LBW when compared with all live births (Figure S2A through S2C).
DISCUSSION
Incident rates of each APO subtype (HDP, PTD, LBW) increased significantly between 2014 to 2019. Furthermore, rates of HDP more than doubled over the entire study period between 2007 to 2019. Significant racial and ethnic disparities persisted with up to 90% higher rates in certain APOs in non‐Hispanic Black individuals compared with non‐Hispanic White individuals. Although average maternal age at delivery increased modestly, relative increases in age‐specific rates of APOs were higher in younger age groups, suggesting that increasing age at pregnancy is not a driver for unfavorable trends.
Our results defining recent nationwide trends in rates of APO subtypes extend recent studies with contemporary data that demonstrates acceleration of HDP rates since 2014. 21 In addition, we now demonstrate for the first time that favorable patterns in overall LBW and PTD have been reversed in pregnancies complicated by HDP and either PTD or LBW. 15 Because these APOs often co‐occur, complicating the same pregnancy, these data documenting trends by linking adverse outcomes in the same pregnancy are critical to design prevention strategies. These trends suggest that HDPs is the driving contributor for the increases seen in PTD and LBW.
Our analysis further expands upon well‐established Black–White disparities in APOs, which may inform strategies to mitigate substantial disparities in maternal morbidity and mortality. Given that APOs are associated with up to a 3‐fold increased maternal risk of incident hypertension and CVD, 22 , 23 prevention of APOs may have the greatest population‐level benefit across the life course. Furthermore, APOs are associated with increased risk of cardiometabolic disease in offspring and may contribute to intergenerational transmission of health inequities. 24 , 25 , 26 The higher rates of APOs noted in non‐Hispanic Black individuals compared with non‐Hispanic White individuals are likely multifactorial and parallel pervasive disparities in cardiometabolic health in the United States. 27 Approaches focusing on social determinants of health, including targeting structural and systemic racism, and improving access to quality health care following pregnancy, are needed to mitigate exacerbation of disparities among those who experienced an APO. 28
Although increasing maternal age may be a contributing factor to higher crude rates of APOs, the change in average maternal age at delivery was modest (<2 years), and in recent years, greater relative increases in HDP, LBW, and PTD occurred in younger groups. Given the greater proportion of live births in younger individuals aged 30 to 34 years (58%), the absolute number of live births complicated by each APO was greatest in this age range in 2019. Secular changes in risk factor burden among individuals of reproductive age with earlier age of onset of obesity as well as higher levels of systolic blood pressure and fasting glucose may all be contributing to these unfavorable trends. 29
Given the known higher rates of short‐ and long‐term CVD following APOs, the American College of Obstetrics and Gynecology and the American Heart Association now recommend that APOs should be taken into account when determining need for implementation and intensification of CVD preventive efforts in the postpartum period. The 2019 American College of Cardiology/American Heart Association Scientific Statement on the Primary Prevention of Cardiovascular Diseases 30 likewise identifies APOs as a risk‐enhancing factor in the algorithm for prescribing lipid‐lowering therapy. This is particularly important for those individuals who experienced HDP, given the robust data linking gestational hypertension and preeclampsia, postpartum hypertension, and future risk of CVD. Ideally, individuals with HDP would be seen in close follow‐up postpartum for surveillance and monitoring of blood pressure. However, use of primary care remains low, even among individuals with medically complicated pregnancies. 31 Furthermore, awareness of the association between APOs and future maternal CVD varies by specialty, 32 and dedicated physician and patient education is crucial.
Strengths of this study include the use of the National Center for Health Statistics Natality Files, which include all live births in the United States, and is the largest and most complete data set available to study rates of APOs. Furthermore, this data set is composed of high‐quality and reliable data because the forms are completed by the health care professional present at the time of delivery using standardized protocols, as compared with many other data sets that capture APO rates based on self‐report alone.
Limitations associated with the use of the National Center for Health Statistics Natality Files include the potential for miscoding based on birth registration, as well as the potential for over‐ or underestimation of rates. HDP, in particular, may be underestimated, given that the data capture ends at the time of delivery, whereas HDP can occur up to 12 weeks postpartum. Although the change in the definition of mild and severe preeclampsia made by the American College of Obstetrics and Gynecology Task Force on Hypertension in Pregnancy in 2013 shifted classification of some women from gestational hypertension to preeclampsia, this change should not have impacted trends in HDP in our study, because our HDP definition included both gestational hypertension and preeclampsia. Second, our trends reflecting changes in rates of new‐onset HDP in aggregate are not able to differentiate between gestational hypertension and preeclampsia rates. Third, the definition of HDP did not include eclampsia or hemolysis, elevated liver enzymes, and low platelets syndrome in this data set, leading to possible underestimation. However, given that these conditions make up a minority of HDP cases, this likely did not significantly affect the trends observed. Fourth, given the deidentified nature of this publicly available data set, repeat pregnancies resulting in live births were included in the estimation of rates. However, fertility rates and prevalence of multiple births declined during the study period. 15 Furthermore, results were similar in our sensitivity analysis of nulliparous individuals with singleton births only, so these factors are unlikely to account for all of the observed trends. Fifth, HDP is a major contributor to neonatal morbidity and mortality, but pregnancies that resulted in fetal death were excluded from this analysis. However, these represent <0.6% of all births so were unlikely to affect trends in APOs. Sixth, although patterns in delivery, including induction of labor at 39 weeks gestational age, may have changed during the study period, this was unlikely to affect trends in PTD defined as <37 weeks gestation. Lastly, our results are ecological in nature and therefore cannot identify causes for these trends. Despite these limitations, these data reflect the most comprehensive assessment of cause‐specific pregnancy complications at a national level.
In summary, rates of each APO subtype have increased significantly in the United States since 2014, and substantial Black–White disparities persist. Increased rates of HDP may underpin the observed increases in PTD and LBW and require urgent studies to examine causal links and evaluate the best targeted interventions. Our findings highlight the importance of clinical and public health efforts focusing on the equitable prevention of APOs and cardiovascular sequelae in birthing individuals and their offspring.
Sources of Funding
Supported by grants from the National Institutes of Health (KL2TR001424, P30DK092939; P30AG059988, and HL161514 to Dr Khan and K23HL14510102 to Dr Perak), the American Heart Association (19TPA34890060) to Dr Khan, and the American College of Cardiology to Dr Freaney.
Disclosures
The authors report no conflicts of interest.
Supporting information
Tables S1–S3
Figures S1–S2
For Sources of Funding and Disclosures, see page 7.
REFERENCES
- 1. Hoyert DL, Miniño AM. Maternal mortality in the United States: changes in coding, publication, and data release, 2018. 2020. [PubMed]
- 2. von Dadelszen P, Magee LA. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2016;36:83–102. doi: 10.1016/j.bpobgyn.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang I‐K, Tsai I‐J, Chen P‐C, Liang C‐C, Chou C‐Y, Chang C‐T, Kuo H‐L, Ting I‐W, Lin C‐C, Chuang F‐R, et al. Hypertensive disorders in pregnancy and subsequent diabetes mellitus: a retrospective cohort study. Am J Med. 2012;125:251–257. doi: 10.1016/j.amjmed.2011.07.040 [DOI] [PubMed] [Google Scholar]
- 4. ACOG Practice Bulletin No. 212: pregnancy and heart disease. Obstet Gynecol. 2019;133:e320–e356. doi: 10.1097/AOG.0000000000003243 [DOI] [PubMed] [Google Scholar]
- 5. Lane‐Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long‐term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2106–2116. doi: 10.1016/j.jacc.2018.12.092 [DOI] [PubMed] [Google Scholar]
- 6. Freaney PM, Khan SS, Lloyd‐Jones DM, Stone NJ. The role of sex‐specific risk factors in the risk assessment of atherosclerotic cardiovascular disease for primary prevention in women. Curr Atheroscler Rep. 2020;22:46. doi: 10.1007/s11883-020-00864-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bassily E, Bell C, Verma S, Patel N, Patel A. Significance of obstetrical history with future cardiovascular disease risk. Am J Med. 2019;132:567–571. doi: 10.1016/j.amjmed.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 8. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, et al. Preeclampsia and future cardiovascular health: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. doi: 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 9. Wang YX, Arvizu M, Rich‐Edwards JW, Wang L, Rosner B, Stuart JJ, Rexrode KM, Chavarro JE. Hypertensive disorders of pregnancy and subsequent risk of premature mortality. J Am Coll Cardiol. 2021;77:1302–1312. doi: 10.1016/j.jacc.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011;22:724–730. doi: 10.1097/EDE.0b013e318225c960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, Hales CM. Trends in obesity prevalence by race and hispanic origin—1999–2000 to 2017–2018. JAMA. 2020;324:1208–1210. doi: 10.1001/jama.2020.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. [Google Scholar]
- 13. Matthews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000–2014. CDC/NCHS Data Brief No 232. 2016. [PubMed]
- 14. Perak AM, Ning H, Khan SS, Van Horn LV, Grobman WA, Lloyd‐Jones DM. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. J Am Heart Assoc. 2020;9:e015123. doi: 10.1161/JAHA.119.015123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1–47. [PubMed] [Google Scholar]
- 16. Statistics NCfH . Guide to Completing the Facility Worksheets for the Certificate of Live Birth and Report of Fetal Death (2003 Revision). National Center for Health Statistics; 2006. [Google Scholar]
- 17. McDonald SD, Han Z, Mulla S, Beyene J; Knowledge Synthesis G . Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta‐analyses. BMJ. 2010;341:c3428. doi: 10.1136/bmj.c3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Committee on Practice Bulletins—Obstetrics, The American College of Obstetricians and Gynecologists Practice bulletin no. 130: prediction and prevention of preterm birth. Obstetrics and gynecology, 2012;120: 964–973. doi: 10.1097/AOG.0b013e3182723b1b [DOI] [PubMed] [Google Scholar]
- 19. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: [DOI] [PubMed] [Google Scholar]
- 20. Joinpoint Regression Program, Version 4.8.0.1—April 2020. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.
- 21. Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. 2018;7:e009382. doi: 10.1161/JAHA.118.009382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, et al. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications: systematic review and meta‐analysis. Circulation. 2019;139:1069–1079. doi: 10.1161/CIRCULATIONAHA.118.036748 [DOI] [PubMed] [Google Scholar]
- 23. Søndergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, Gemmill A, Van Horn L, Park KI, Salmoirago‐Blotcher E, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. 2020;5:1390. doi: 10.1001/jamacardio.2020.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andraweera PH, Lassi ZS. Cardiovascular risk factors in offspring of preeclamptic pregnancies—systematic review and meta‐analysis. J Pediatr. 2019;208:104–113.e6. doi: 10.1016/j.jpeds.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 25. Knop MR, Geng TT, Gorny AW, Ding R, Li C, Ley SH, Huang T. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta‐analysis of 7 646 267 participants from 135 studies. J Am Heart Assoc. 2018;7:e008870. doi: 10.1161/JAHA.118.008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, Jaddoe VW. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension. 2014;63:683–691. doi: 10.1161/HYPERTENSIONAHA.113.02671 [DOI] [PubMed] [Google Scholar]
- 27. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Willis M, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 28. Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 29. Azeez O, Kulkarni A, Kuklina EV, Kim SY, Cox S. Peer reviewed: hypertension and diabetes in non‐pregnant women of reproductive age in the United States. Prev Chronic Dis. 2019;16:1–9. doi: 10.5888/pcd16.190105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett WL, Chang HY, Levine DM, Wang L, Neale D, Werner EF, Clark JM. Utilization of primary and obstetric care after medically complicated pregnancies: an analysis of medical claims data. J Gen Intern Med. 2014;29:636–645. doi: 10.1007/s11606-013-2744-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gogineni VSM, Manfrini D, Aroda SH, Zhang Y, Nelson DS, Egerman R, Park K. Variations in awareness of association between adverse pregnancy outcomes and cardiovascular risk by specialty. Cardiol Ther. 2021;10:577–592. doi: 10.1007/s40119-021-00220-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2


