Abstract
Cultures of a purple nonsulfur bacterium, Rhodobacter sphaeroides, amended with ∼1 or ∼100 ppm selenate or selenite, were grown phototrophically to stationary phase. Analyses of culture headspace, separated cells, and filtered culture supernatant were carried out using gas chromatography, X-ray absorption spectroscopy, and inductively coupled plasma spectroscopy-mass spectrometry, respectively. While selenium-amended cultures showed much higher amounts of SeO32− bioconversion than did analogous selenate experiments (94% uptake for SeO32− as compared to 9.6% for SeO42−-amended cultures from 100-ppm solutions), the chemical forms of selenium in the microbial cells were not very different except at exposure to high concentrations of selenite. Volatilization accounted for only a very small portion of the accumulated selenium; most was present in organic forms and the red elemental form.
The roles of selenium in the biosphere, both beneficial and deleterious, are gradually being determined (2, 5, 7, 15), and it is becoming apparent that bacteria play a major role in the global selenium cycle. Selenate (SeO42−) and selenite (SeO32−) seem to be the most abundant forms of bioavailable selenium in the environment, and both can serve as electron acceptors for many microorganisms, including some phototrophic bacteria. These organisms are widespread and exhibit enormous metabolic and ecological diversity, and some are known to be able to use selenium oxyanions in their anaerobic metabolism (12, 13, 14, 24). Rhodobacter sphaeroides is a purple nonsulfur bacterium that often serves as a model species for the group. It can tolerate high concentrations of selenite and selenate (13, 14) and can reduce and/or methylate these compounds (24).
Recent work with phototrophic bacteria has shown that some can grow in the presence of 0.1, 1.0, even 10 mM selenate or selenite. Though growth rates were somewhat decreased, five phototrophic species grown on Sistrom minimal medium were shown to exhibit overall stationary-phase biomass production similar to that of controls. Methylated organosulfur, organoselenium, and dimethyl selenenyl sulfide have been detected in anaerobic culture headspace, and elemental Se was produced in some cultures (13, 21, 23). This reduction to the elemental form may be an important process in the biosphere, since soils from an evaporation pond in the Kesterson Reservoir show that, nearest the surface, Se0 is the most prominent selenium species (16).
Although it is relatively clear that phototrophic bacteria can grow in the presence of selenium oxyanions and that small amounts are reduced and/or methylated, little is known of the extent and path of this process. Here we report the results of quantifying and speciating selenium in the culture medium, the bacteria, and the gaseous headspace above the culture after R. sphaeroides was grown phototrophically in the presence of different concentrations of selenite and selenate to stationary phase. Cell supernatants were analyzed for total selenium using inductively coupled plasma spectroscopy-mass spectrometry, volatile selenium species in the headspace were analyzed by gas chromatography (GC) (23, 24), and cells were analyzed for inorganic and organoselenium species via selenium X-ray absorption spectroscopy (16, 17).
MATERIALS AND METHODS
Dimethyl selenone was synthesized as reported elsewhere (25, 26). Dimethyl selenide and dimethyl diselenide, used as chromatographic standards, were purchased from Strem Chemical and used as received. Other model compounds were purchased from Aldrich or Sigma or synthesized as discussed by Pickering et al. (17). All culture medium components were purchased and used as received.
R. sphaeroides 2.4.1. (DSM 158) was cultured in a manner reported elsewhere (24). Briefly, test tube cultures grown on minimal medium at 30°C with succinate as the carbon source (20) were amended with either sodium selenite or sodium selenate. Samples for headspace analysis were grown in 16-ml tubes with Teflon septa. These tubes had 10 ml of liquid medium added, leaving 6 ml of headspace gas. Duplicates of each Se-amended concentration (<1 or ∼100 ppm Se) were grown under incandescent light (10 W/m2) to stationary phase, and the cells were washed and harvested via microfiltration or centrifugation. After being concentrated into pellets, cell samples were stored at 4°C or below for less than a week until analysis by X-ray absorption spectroscopy. Supernatants were treated identically until analyzed by inductively coupled plasma spectroscopy-mass spectrometry (ICP/MS). All cultures were grown for 14 days before being sampled.
Supernatants from bacterial cultures and controls were filtered through 0.2-μm-pore-size sterile filters and were analyzed by inductively coupled plasma using U.S. Environmental Protection Agency standard method 3120 B on a Perkin-Elmer Elan 6000 ICP/MS.
Samples from bacterial culture headspace were analyzed as described elsewhere (23). Briefly, after cells had reached stationary phase, 1 ml of headspace gas was removed by gas syringe and analyzed immediately by capillary GC with fluorine-induced chemiluminescence detection. Organosulfur and organoselenium headspace components were identified by retention time and by GC-MS. Gas phase headspace concentrations (reported in parts per billion by volume [ppbv]) were determined from calibration curves prepared by injecting known amounts of chromatographic standards into acetonitrile solvent. Since a commercial standard is not available, dimethyl selenenyl sulfide concentrations were extrapolated based on dimethyl disulfide and dimethyl diselenide instrumental responses as reported elsewhere (23).
Selenium K X-ray absorption near-edge spectra were measured on beamline 7-3 of the Stanford Synchrotron Radiation Laboratory with a Si(220) double crystal monochromator, a 1-mm vertical aperture, and no focusing optics. Incident intensity was measured using a nitrogen-filled ion chamber, and absorption spectra were recorded in fluorescence using a Canberra 13-element germanium detector (4). Spectra were calibrated with respect to the first energy inflection of a simultaneously collected spectrum of hexagonal selenium, the energy of which was assumed to be 12,658 eV.
Edge fitting was carried out as previously described (8, 16, 22). Briefly, a spectrum of a mixture of selenium species can be considered to be the sum of the spectra of the individual selenium components, weighted by their mole fraction of selenium. Curve fitting of the edge spectrum of an unknown to a sum of model compounds can therefore be used to deduce the fractions of species present. In this work a large number of spectra were tested (all in aqueous solution unless noted): red elemental selenium (solid), dimethyl diselenide (solution in acetonitrile), the mixed selenium-sulfur analog of cystine, selenocystine, dimethyl selenide, selenomethionine, selenocysteine, dimethyl selenone, selenite, and selenate. Each spectrum was fit to convergence, and then the estimated standard deviation for the fraction of each component was examined. If the estimated standard deviation was greater than 33% of the value, then that component was excluded from subsequent fits. The final fits therefore involved a much smaller number of parameters.
RESULTS
R. sphaeroides grows to stationary phase under our conditions in about 7 to 10 days on Sistrom medium. Cultures were maintained for 14 days in the light and at 30°C before they were sampled for headspace or the cells were harvested and supernatant was collected. Selenium oxyanion concentrations of slightly less than 1 ppm and slightly above 100 ppm were chosen as “representing” realistic low and high environmental concentrations. Kesterson soil content of selenium oxyanions can be as high as 5 to 50 ppm (11, 16). The total Se content in supernatants from selenite- or selenate-amended sterile media and bacterial cultures, determined by ICP/MS, are listed in Table 1. Values reported are an average of triplicates reported along with standard errors. The percent uptake by bacterial cells was computed as the percent difference between what was found in sterile controls and in bacterial cultures. Immediately obvious are the high conversion of Se in the high-selenite-concentration case and the very low conversion in that of the low-selenate-concentration samples.
TABLE 1.
Total selenium in supernatants from sterile controls and cultures of R. sphaeroides amended with selenite and selenatea
| Treatment | Selenium content (ppm) in or after:
|
% Uptake (SE) | |
|---|---|---|---|
| sterile control | bacterial growth | ||
| Low SeO32− | 0.86 | 0.32 | 62.9 (1.2) |
| High SeO32− | 104 | 5.88 | 94.3 (0.8) |
| Low SeO42− | 0.80 | 0.72 | 10.6 (1.9) |
| High SeO42− | 114 | 103 | 9.6 (0.5) |
Data are the average of triplicates.
Table 2 reports the average gas phase concentrations of the organosulfur and organoselenium components detected in culture headspace. This method's detection limit is about 15 ppbv for the volatile compounds reported. The average percentage of the added Se volatilized is also included in Table 2. A660 is shown as a simple measure of culture growth, and it can be noted that emission of volatile metabolites or percent Se conversion does not necessarily correlate with high A660 values.
TABLE 2.
Headspace components of R. sphaeroides cultures amended with selenite or selenatea
| Amt of selenite or selenate added (ppm) | A660 | Concns (ppbv) for:
|
% Se conversion | n | |||
|---|---|---|---|---|---|---|---|
| dimethyl sulfide (SE) | dimethyl selenide (SE) | dimethyl selenenyl sulfide (SE) | dimethyl diselenide (SE) | ||||
| No added Se | 2.33 | 2,076 (85) | 0 | 0 | 0 | 3 | |
| SeO32− (0.86) | 2.21 | 3,057 (151) | 0 | 0 | 0 | 3 | |
| SeO32− (104) | 1.45 | 2,279 (260) | 2,057 (110) | 49 (8) | 594 (155) | <0.06 | 3 |
| SeO42− (0.80) | 2.22 | 3,249 (158) | 0 | 0 | 0 | 3 | |
| SeO42− (114) | 1.62 | 1,787 (243) | 21,530 (3,618) | 0 | 0 | <0.5 | 2 |
A660 provides an estimate of the relative culture density and shows the slight inhibition of growth at the highest concentrations of selenite and selenate. n is the number of observations. Gas concentrations are reported in ppbv.
Sterile controls yielded no organosulfur or organoselenium, and Se-free bacterial cultures produced only volatile organosulfur (Table 2), mirroring previous work with this microbial strain (21, 23). The relative amounts of added Se that were converted to the volatile forms reported in Table 2 take into account the amount of the organoselenium compounds detected in the gas phase and an adjustment based on Henry's law (10, 24) to estimate the amount of the volatile species that remains in solution at a specific temperature. For dimethyl selenide and dimethyl diselenide, the ratio of organoselenide dissolved in the aqueous phase to that found in the gas phase (at 30°C in this culture medium) is about 11/1. A similar distribution was assumed for dimethyl selenenyl sulfide. While the low-concentration-amendment samples probably reduced and methylated some Se, the amounts produced were below the detection limit for our headspace method. The experimental protocol of 14 days' incubation before sampling was chosen in light of previous work with this microbe, which showed that the volatilization of metabolized selenium remained high even after 10 days (24). Again of significance is the low volatilization rate even after the 14-day incubation period.
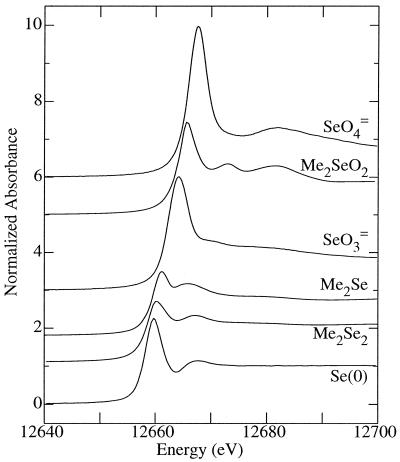
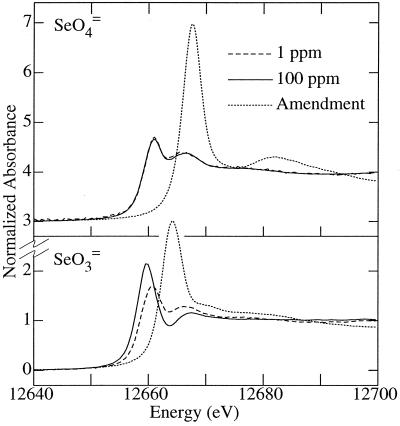
X-ray absorption spectroscopy provides a novel way of determining the chemical nature of almost any element without the need for any chemical pretreatment (18). Selenium is a particularly suitable element for this approach, since different chemical forms exhibit significantly different spectra (Fig. 1) (16, 17). Figure 2 shows the X-ray absorption edge spectra of four Se-amended samples (low- or high-concentration selenite or selenate amendments), and Table 3 and Figure 3 show the results of fits to the model compound spectra (16). For possible seleno-amino acid components in our fits (17), we used selenomethionine, selenocysteine, selenocystine, and the mixed selenium-sulfur analog of cystine. However, the only one of these components found to be present in significant amounts in any of the amendments was selenomethionine. The spectrum of aqueous dimethyl selenide (Figure 1) is very similar to that of aqueous selenomethionine, and we found that using the two spectra in our fits invariably yielded highly correlated results where it was difficult to assign respective fractions reliably. The local environment of the selenium in the two compounds is very similar (both have two —CH2— substituents), so this result is not entirely unexpected. Since we do not believe that the spectral-fitting approach used here can reliably distinguish between aqueous solutions of selenomethionine and dimethyl selenide when they are present in a mixture, we have used an average spectrum of the two components in the fits reported here. We refer to this average aliphatic selenoether spectrum as RSeR in Fig. 3 and Table 3.
FIG. 1.
Selenium K X-ray absorption near-edge spectra of some model compounds. Compounds from top to bottom (in aqueous solution unless otherwise stated): selenate (SeO42−), dimethyl selenone (Me2SeO2), selenite (SeO32−), dimethyl selenide (Me2Se), dimethyl diselenide solution in acetonitrile (Me2Se2), and solid red elemental selenium (Se0).
FIG. 2.
Selenium K X-ray absorption near-edge spectra of R. sphaeroides cultures amended with selenite or selenate. For each oxyanion, spectra of two cultures are shown, amended with approximately 1 and 100 ppm, respectively. Also shown is the solution spectrum of the oxyanion itself (Amendment).
TABLE 3.
Selenium speciation (as percentage) in R. sphaeroides cells grown with selenium oxyanions, based on least-squares fitting of selenium K X-ray absorption near-edge spectra
| Amt of selenite or selenate added (ppm) | % Selenium speciation
|
R (104) | |||
|---|---|---|---|---|---|
| Se0 | RSeR | SeO32− | Me2SeO2 | ||
| SeO32− (0.86) | 36 (2) | 60 (2) | 4 (1) | 1.5 | |
| SeO32− (104) | 97 (1) | 3 (1) | 3.1 | ||
| SeO42− (0.80) | 13 (2) | 84 (3) | 4 (2) | 3.4 | |
| SeO42− (114) | 15 (1) | 81 (2) | 2 (1) | 2 (1) | 1.0 |
Abbreviations are same as for Fig. 1, except that the spectrum of RSeR represents the mean of the spectra of aqueous dimethyl selenide and selenomethionine (see text for details). A value equivalent to three times the estimated standard deviation, obtained from the diagonal element of the covariance matrix, is shown in parentheses after each value. R is a goodness-of-fit parameter, defined as R = Σ(Io − Ic)/n, where Io and Ic are the observed and calculated intensities and n is the number of observations.
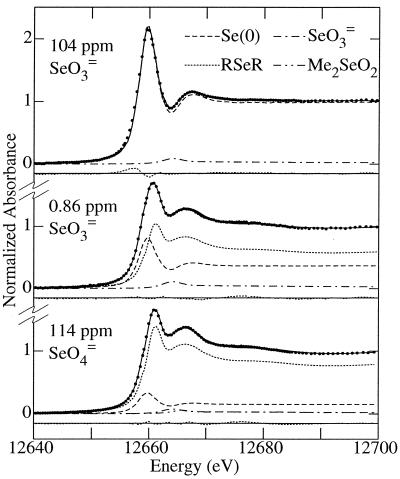
FIG. 3.
Results of curve-fitting the Se K X-ray absorption near-edge spectra of the cultures described in Fig. 2. Dots, data; solid line, the calculated curve; lower dotted line, the residual. The constituent model compounds have been scaled according to their contributions to the fit (Table 3). The fit to the spectrum of the 0.80-ppm-SeO42−-amended experiment (not shown) was essentially identical to that of the 114-ppm SeO42− spectrum, except that the low-concentration-amendment spectrum had a slightly poorer signal-to-noise ratio (Fig. 2), which accounts for the higher value of R (Table 3).
The major components found to be present in all four amendments were elemental selenium and aliphatic selenoether. For the selenate-amended cultures, the fractions of these three components present were the same within the precisions of the fit for the two different selenate levels. In contrast, for the selenite amendments, the fractions were strikingly different for different levels, the high-level amendment producing almost exclusively elemental selenium.
DISCUSSION
It is not clear whether selenium is an essential element for R. sphaeroides, although this seems likely (19). Selenium is not routinely added to culture media, but trace levels are probably always present. We were unable to detect selenium in cells grown without added selenium by X-ray absorption spectroscopy, but this technique is not sensitive to extremely low concentrations. Selenium is definitely an essential element for many eukaryotes, bacteria, and archaea (9), and its major role is in selenium-substituted amino acids (1), although it is also found at the active sites of some metalloenzymes.
Although R. sphaeroides can clearly metabolize selenite and selenate, it exhibits a profound preference for the former under the conditions described here. Furthermore, selenium uptake was greater on both an absolute and a percent basis at 100 ppm selenite than at 1 ppm, suggesting that selenite uptake becomes limiting when the concentration of selenite is below about 500 ppb. Perhaps this limitation can explain the very high uptake seen when selenite was initially present at 100 ppm, if the selenite-reduction pathway has a very high affinity for selenite to compensate for relatively poor selenite binding in the initial step. The biochemical basis for the greater uptake of selenite than of selenate remains to be clarified, as indeed does the question of whether there is a specific selenite and/or selenate transport system or whether these oxyanions are transported by a broad-specificity system.
Our data indicate that volatilization is a statistically insignificant fate for selenite or selenate taken up by R. sphaeroides under the conditions used here. Instead, the vast majority of the selenium is retained in or on the cell. Almost all of it is reduced from the oxyanion to organic or elemental forms. Even at the lowest levels of selenium tested here, some was reduced to the red elemental form, suggesting that the selenium was present at levels far beyond the requirements of the cell for selenoamino acids. This suggests, as discussed above, that the cells have a very efficient metabolism for using any selenium that they acquire. Most of the selenium in cells grown on low levels of selenite and on either level of selenate is present in a form that is very similar or identical to selenomethionine or perhaps to dimethyl selenide. In view of the low levels of dimethyl selenide detected in the headspace of these cultures (Table 2), we attribute the selenium to selenomethionine-like species rather than to dimethyl selenide, but it is possible that it is actually some precursor to dimethyl selenide that might be converted to the volatile form if there was exchange of the headspace gas.
There are also significant amounts of selenium that can be modeled as elemental selenium in all the selenium-amended cells. While dimethyl selenone could be included at 2% for the high-selenate-concentration-amended sample, such a component is probably at the limit of accuracy of the fitting process and its significance is questionable. Finally, though selenocystine, the mixed selenium-sulfur analog of cystine, and selenocysteine were included in the fit algorithm, they were invariably rejected as significant contributors.
In summary, we attribute our findings to the presence of a selenium uptake system in R. sphaeroides that normally functions at very low levels of selenium oxyanions. Its poor initial affinity for selenite, as shown by only a 63% uptake from 0.86 ppm selenite (Table 1) and even lower affinity for selenate, seems to be compensated for by a very effective subsequent reduction to trap any selenium that enters the cell. This is supported by the fact that no selenate and only traces of selenite were detected in any cells. We expect that the primary requirement for selenium is incorporation into amino acids, but the necessary levels are so low that we are unable to detect them by X-ray absorption spectroscopy. Under the conditions described here, where even the lowest additions of selenite or selenate exceed the requirements for growth, the system initially seems to put the excess into a form very similar or perhaps identical to selenomethionine. Further excess seems to be detoxified as the red elemental form, which has a very low bioavailability (3, 5), and Moore and Kaplan have suggested that members of the family Rhodospirillaceae can use oxidized compounds to get rid of excess electrons produced in anaerobic photosynthesis (14). While volatilization is not a common fate under our conditions, work has shown that R. sphaeroides grown in the light produced more reduced volatile selenium than cultures kept in the dark (24).
Our results with selenite mirror those of Combs et al. (3) with Bacillus subtilis and Microbacterium arborescens, and we agree with them that the immobilization of selenite and selenate into the much less bioavailable red elemental form of selenium may provide a useful approach for remediating selenium contamination (see reference 6 for a recent review), both in soils and especially in aqueous streams where the Se-enriched bacteria can be trapped in “activated sludge.”
ACKNOWLEDGMENTS
Stanford Synchrotron Radiation Laboratory is funded by the Offices of Basic Energy Sciences and Biological and Environmental Research, U.S. Department of Energy; the National Institutes of Health; National Center for Research Resources; Biomedical Technology Program; and the National Institute of General Medical Sciences. V.V.F.-S. and T.G.C. were supported by a Cottrell College Science Award of Research Corporation, The Texas Research Institute for Environmental Studies, Sam Houston State University Research Enhancement Funds, and the Robert A. Welch Foundation.
We are grateful to F.C. McElroy and A.S. Mennito for the ICP/MS measurements of Table 1.
REFERENCES
- 1.Boeck A, Forchhammer K, Heider J, Leinfleder W, Sawers G, Veprek B, Zinoni F. Selenocysteine: the 21st amino acid. Mol Microbiol. 1991;5:515–520. doi: 10.1111/j.1365-2958.1991.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 2.Bowie G L, Sanders J G, Riedel G F, Gilmour C C, Breitburg D L, Cutter G A, Porcella D B. Assessing selenium cycling and accumulation in aquatic ecosystems. Water Air Soil Pollut. 1996;90:93–104. [Google Scholar]
- 3.Combs G F, Jr, Garbisu C, Lee B C, Yee A, Carlson D E, Smith N R, Magyarosy A C, Leighton T, Buchanan B B. Bioavailability of selenium accumulated by selenite-reducing bacteria. Biol Trace Elem Res. 1996;52:209–225. doi: 10.1007/BF02789163. [DOI] [PubMed] [Google Scholar]
- 4.Cramer S P, Trench O, Yocum M, George G N. A 13-element Ge detector for fluorescence EXAFS. Nucl Instrum Methods Phys Red. 1988;266:586–591. [Google Scholar]
- 5.Daniels L A. Selenium metabolism and bioavailability. Biol Trace Elem Res. 1996;54:185–199. doi: 10.1007/BF02784430. [DOI] [PubMed] [Google Scholar]
- 6.Dungan R S, Frankenberger W T. Microbial transformations of selenium and the bioremediation of seleniferous environments. Biorem J. 1999;3:171–188. [Google Scholar]
- 7.Frankenberger W T, Jr, Benson S. Selenium in the environment. New York, N.Y: Marcel Dekker, Inc.; 1994. [Google Scholar]
- 8.George G N, Gorbaty M L, Keleman S R, Sansone M. Direct determination and quantification of sulfur forms in coals from the Argonne Premium Sample Program. Energy Fuels. 1991;5:93–97. [Google Scholar]
- 9.Guimaraes M J, Peterson D, Vicari A, Cocks B G, Copeland N G, Gilbert D J, Jenkins N A, Ferrick D A, Kastelein R A, Bazan J F, Zlotnik A. Identification of a novel selD homolog from eukaryotes, bacteria, and archaea: is there an autoregulatory mechanism in selenocysteine metabolism? Proc Natl Acad Sci USA. 1996;93:15086–15091. doi: 10.1073/pnas.93.26.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gürleyük H. Investigation of the biological reduction and methylation of antimony compounds. M.S. thesis. Huntsville, Tex: Sam Houston State University; 1996. [Google Scholar]
- 11.Martens D A, Suarez D L. Selenium speciation of marine shales, alluvial soils, and evaporation basin soils of California. J Environ Qual. 1997;26:424–432. [Google Scholar]
- 12.McCarty S L, Chasteen T G, Marshall M, Fall R, Bachofen R. Phototrophic bacteria produce volatile, methylated sulfur and selenium compounds. FEBS Lett. 1993;112:93–98. [Google Scholar]
- 13.Moore M D, Kaplan S. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J Bacteriol. 1992;174:1505–1514. doi: 10.1128/jb.174.5.1505-1514.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore M D, Kaplan S. Members of the family Rhodospirillaceae reduce heavy-metal oxyanions to maintain redox poise during photosynthetic growth. ASM News. 1994;60:17–23. [Google Scholar]
- 15.Paveglio F L, Kilbride K M, Bunck C M. Selenium in aquatic birds from central California. J Wildl Manag. 1997;61:832–839. [Google Scholar]
- 16.Pickering I J, Brown G E, Jr, Tokunaga T K. Quantitative speciation of selenium in soils using X-ray absorption spectroscopy. Environ Sci Tech. 1995;29:2456–2459. doi: 10.1021/es00009a043. [DOI] [PubMed] [Google Scholar]
- 17.Pickering I J, George G N, Van Fleet-Stalder V, Chasteen T G, Prince R C. X-ray absorption spectroscopy of selenium-containing amino acids. J Biol Inorg Chem. 1999;4:791–794. doi: 10.1007/s007750050352. [DOI] [PubMed] [Google Scholar]
- 18.Pickering I J, Prince R C. X-ray absorption spectroscopy as a probe of elemental speciation. In: Alleman B C, Leeson A, editors. In situ and on-site bioremediation. Vol. 2. Columbus, Ohio: Battelle Press; 1997. pp. 381–386. [Google Scholar]
- 19.Rosenfeld I, Beath O A. Selenium geobotany, biochemistry, toxicity and nutrition. New York, N.Y: Academic Press; 1964. [Google Scholar]
- 20.Sistrom W R. A requirement for sodium in the growth of Rhodopseudomonas sphaeroides. J Gen Microbiol. 1960;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- 21.Stalder V, Bernard N, Hanselmann K W, Bachofen R, Chasteen T G. A method of repeated sampling of static headspace above anaerobic bacterial cultures with fluorine-induced chemiluminescence detection. Anal Chim Acta. 1995;303:91–97. [Google Scholar]
- 22.Tokunaga T K, Pickering I J, Brown G E., Jr Selenium transformations in ponded sediments. Soil Sci Soc Am J. 1996;60:781–790. [Google Scholar]
- 23.Van Fleet-Stalder V, Chasteen T G. Using fluorine-induced chemiluminescence to detect organo-metalloids in the headspace of phototrophic bacterial cultures amended with selenium and tellurium. J Photochem Photobiol. 1998;43:193–203. [Google Scholar]
- 24.Van Fleet-Stalder V, Gürleyük H, Bachofen R, Chasteen T G. Effects of growth conditions on production of methyl selenides in cultures of Rhodobacter sphaeroides. Ind Microbiol Biotechnol. 1997;19:98–103. [Google Scholar]
- 25.Yu R, Coffman J P, Van Fleet-Stalder V, Chasteen T G. Toxicity of oxyanions of selenium and of a proposed bioremediation intermediate, dimethyl selenone. Environ Toxicol Chem. 1997;16:140–145. [Google Scholar]
- 26.Zhang L, Chasteen T G. Amending cultures of selenium resistant bacteria with dimethyl selenone. Appl Organomet Chem. 1994;8:501–508. [Google Scholar]