ABSTRACT
Sexual reproduction of Plasmodium falciparum parasites is critical to the spread of malaria in the human population. The factors that regulate gene expression underlying formation of fertilization-competent gametes, however, remain unknown. Here, we report that P. falciparum expresses a protein with an AT-rich interaction domain (ARID) which, in other organisms, is part of chromatin remodeling complexes. P. falciparum ARID (PfARID) localized to the parasite nucleus and is critical for the formation of male gametes and fertility of female gametes. PfARID gene deletion (Pfarid–) gametocytes showed downregulation of gene expression important for gametogenesis, antigenic variation, and cell signaling and for parasite development in the mosquito. Our study identifies PfARID as a critical nuclear protein involved in regulating the gene expression landscape of mature gametocytes. This establishes fertility and also prepares the parasite for postfertilization events that are essential for infection of the mosquito vector.
KEYWORDS: ARID, chromatin, differentiation, gametocyte, transmission
INTRODUCTION
Malaria remains a major cause of mortality and morbidity in developing countries across the world. The disease is caused by Plasmodium parasites, with most deaths attributed to infection with Plasmodium falciparum. Malaria parasites are alveolates that reproduce asexually within two hosts: a vertebrate such as humans and a mosquito vector. The Plasmodium life cycle also has an obligate sexual phase, which initiates in the vertebrate host and is completed in mosquitoes (1). P. falciparum male and female gametocytes are formed by a subset of asexually replicating parasites and develop over 12 to 14 days as morphologically distinct stages I, II, III, IV, and V within infected red blood cells (RBCs). When male and female stage V gametocytes are taken up by the mosquito during a blood meal, they are activated to form male microgametes and female macrogametes. This process is completed within 10 to 15 minutes, and mature gametes then egress from the infected RBCs. The factors controlling gametocyte activation include a decrease in temperature (2), increase in pH (3), and/or exposure to xanthurenic acid (XA), a metabolite of tryptophan (4). Gametogenesis is also linked to mobilization of intracellular calcium (Ca2+) stores, which can regulate Ca2+-dependent protein function via protein kinase G (PKG), important for calcium mobilization-1 (ICM1) (4, 5) and Ca2+-dependent protein kinase 4 (CDPK4) (6). In P. falciparum, Gametogenesis is further regulated by the activity of a perforin-like protein, PPLP2 (7), which in turn is regulated by a patatin-like phospholipase (PATPL1) (8). Microgametes fertilize macrogametes to form a zygote, which transforms into a motile ookinete within 24 h. Ookinetes penetrate the mosquito midgut epithelium and each form an oocyst, which produces sporozoites for transmission to the next host.
In all organisms, cellular differentiation is accompanied by activation and/or repression of specific genes via genetic and epigenetic mechanisms (9–12). Sexual differentiation, including germ cell formation and gametophyte formation is controlled by diverse master regulatory factors across the animal and plant kingdoms, respectively (13–15). Over the past decade, the molecular basis of sexual stage differentiation (gametocytogenesis) in Plasmodium has begun to be better understood. It involves hierarchical transcriptional control, where a subset of genes is specifically expressed or predominantly expressed in sexual forms of the parasite (16, 17). Members of the plant-like ApiAP2 family of transcription factors, AP2-G, AP2-G2, and AP2-G5, have been shown to play critical roles in regulating sexual stage commitment and gametocytogenesis in human malaria parasite species (18–20). Mature gametocytes also exhibit large-scale translational repression, which represents a major mechanism that prepares the parasite progeny for postfertilization development (21).
For transcription factors to access DNA information, histone proteins must first be repositioned or evicted from the chromosomes, a function that is performed by ATP-dependent chromatin remodeling complexes such as BAF (BRG1/BRM-associated factor) (mammalian SWI/SNF) complexes, which are composed of 14 to 16 individual protein subunits in human cells (22). These complexes control multiple cellular processes such as cell proliferation, transcriptional activation, differentiation, and chromatin remodeling (23–25). However, chromatin remodeling complexes regulating gametogenesis have not been identified in P. falciparum although, Plasmodium gametocytogenesis and gametogenesis likely requires chromatin remodeling to regulate expression of specific genes. In searching for putative epigenetic regulators of gametogenesis, we have identified one AT-rich interaction domain (ARID) domain-containing protein in Plasmodium, including the human malaria parasite P. falciparum. ARID is an ancient ~100-amino acid DNA-binding module present in various eukaryotic transcriptional regulators, which can be part of chromatin remodeling complexes (26). Although, ARID proteins were initially named due to their preference for AT-rich target DNA, it is now known that most of the ARID family proteins do not prefer an AT-rich target sequence (27).
Here, we explored the role of P. falciparum ARID (PfARID) in the parasite life cycle. We show that PfARID is a nuclear protein and is expressed in asexual and sexual erythrocytic stages. PfARID gene deletion parasites (Pfarid–) showed normal asexual blood-stage replication and differentiated into mature stage V gametocytes. In contrast, Pfarid– parasites exhibited a complete block in transmission to mosquitoes. Further analysis revealed that Pfarid– parasites suffered a severe defect in microgametogenesis, while macrogametes appeared to form normally. Strikingly, however, genetic crosses with male-only sterile parasite lines and female-only sterile lines demonstrated that ARID is also critical for female gamete fertility. We further show that PfARID regulates gene expression that is important for gametogenesis but also appears to be a regulator for expression of genes that prepare the parasite for the postfertilization steps that are necessary for mosquito infection.
RESULTS
A Plasmodium ARID domain-containing protein is expressed in asexual and sexual erythrocytic stages.
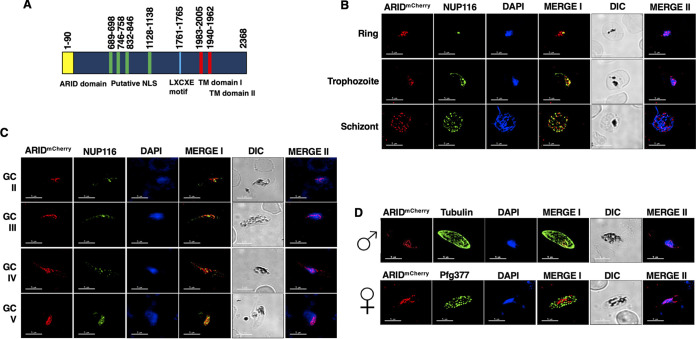
To identify ARID orthologs in the Plasmodium falciparum genome, we searched for PFAM domain PF01388 using PlasmoDB v.50, revealing a single gene with an encoded ARID domain, which we have termed PfARID (PF3D7_0603600). Predicted amino acid sequence analysis revealed that the ARID domain is located at the N terminus of the protein (Fig. 1A). PfARID has multiple predicted internal nuclear localization signals (NLS) downstream of the ARID domain and has two predicted transmembrane (TM) domains toward the C-terminal part of the protein (Fig. 1A) The presence of TMs appears unique compared to other ARID proteins, which have no TM domains (28). In addition, PfARID also shows an LXCXE motif (Fig. 1A) which, in other proteins, has a role in facilitating interaction between the retinoblastoma (RB) tumor suppressor and many cellular proteins (29). To analyze the conservation of ARID among different Plasmodium species, we performed an amino acid sequence alignment for P. falciparum, P. vivax (Pv), P. berghei (Pb), and P. yoelii (Py) ARID, which revealed high sequence similarities in their respective ARID domains (Fig. S1A). 3-D structure models generated using SWISS Model (https://swissmodel.expasy.org/) revealed that the P. falciparum ARID domain has closest structural similarity with the human ARID3a protein (Fig. S1B), which is involved in the differentiation of hematopoietic progenitors (30).
FIG 1.
Expression and localization of PfARID in intraerythrocytic parasite stages. (A) Domain architecture of the P. falciparum ARID protein showing the ARID domain (1 to 90 amino acids [aa]) in yellow, the putative NLS in green, an LXCXE motif in blue, and two transmembrane domains (TM) in red. (B and C) IFAs were performed on asexual (ring, trophozoite, and schizonts) and sexual stages (stage II to V gametocytes) using anti-mCherry antibody (in red) in combination with anti-PfNUP116 antibody (in green). (D) IFAs were performed on sexual stages using an α-mCherry antibody in combination with α-tubulin II (male gametocytes) or α-Pfg377 antibodies (female gametocytes). The parasite DNA was stained with DAPI (blue). Scale bar = 5 μm. Images are shown from representative experiments. Merge I, merged image for red and green channels; merge II, merged image for red and DAPI (blue) channels; GC, gametocytes.
PfARID conservation and generation of PfARIDmCherry parasites. (A) ARID domain of ARID proteins from P. falciparum (PfARID), Plasmodium vivax (Pv), Plasmodium berghei (Pb), and Plasmodium yoelii (Py). Conserved residues are in white font on a red background. (B) Homology-based predicted three-dimensional structure of PfARID. PDB template used 4LJX (HsARID3A). (C) The schematic for endogenous tagging of the PfARID locus with mCherry. The pFCL3_ARID_mCherry plasmid has homology regions from 5′ (5′HR) and 3′ (3′HR) of the PfARID locus, single guide RNA-seq (sgRNA), and the human dihydrofolate reductase (hDHFR) locus cloned. (D) Confirmation of PfARIDmCherry parasite generation by diagnostic PCR. The oligonucleotides were designed from outside 5′HR and 3′HR and the PfARID locus, and their positions are indicated by arrows in panel C. (E) The expected sizes for different sets of PCRs. (F and G) IFAs were performed on sexual stages using α-mCherry antibody in combination with H3K9ac. Parasite nucleus was stained with DAPI (blue). Scale bar = 5 μm. Images are shown from representative experiments. Merge I, merged image for red and green channel; merge II, merged image for red and DAPI (blue) channel; GC, gametocytes. Download FIG S1, TIF file, 2.5 MB (2.6MB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To analyze expression of PfARID, a transgenic line with an mCherry tag at the C terminus (PfARIDmCherry) was generated by double crossover recombination (Fig. S1C to E). Indirect immunofluorescence assays (IFAs) were performed on fixed and permeabilized thin blood-stage culture smears using mCherry antibodies and counterlabeling with an anti-NUP116 antibody, which marks nuclear pores (31). Expression of PfARID was detected in ring, trophozoite, and schizont stages (Fig. 1B). In each stage ARID partially colocalized with DNA and also appeared to be in close proximity to NUP116. PfARID expression was also detected in gametocytes, from stage II through stage V (Fig. 1C). In these stages, ARID also partially colocalized with DNA and also appeared to be in close proximity to NUP116. Counterstaining with male (anti-tubulin) or female (anti-Pfg377) gametocyte-specific antibodies revealed that PfARID is expressed in both male and female gametocytes (Fig. 1D). Further analysis performed using an acetylated histone-specific antibody (H3K9Ac) revealed that PfARID is mainly expressed in euchromatic regions in stage II to V gametocytes (Fig. S1F and G).
PfARID is essential for male gametogenesis.
To assess the importance of ARID in the P. falciparum life cycle, the endogenous PfARID gene was disrupted using CRISPR/Cas9 methodology (Fig. S2). Gene deletion parasites were confirmed by a set of diagnostic PCRs with oligonucleotides specific for the PfARID locus and genomic regions 5′ (upstream) and 3′ (downstream) of the open reading frame (Fig. S2A to C). To analyze a potential function of PfARID in asexual blood stages, a comparative growth rate assay was set up using two clones of Pfarid– (clones 4E and 6A) along with wild-type (WT) PfNF54 parasites, starting with synchronized ring stages. Growth was monitored over two replication cycles using Giemsa-stained thin blood culture smears, which revealed that the growth rate of Pfarid– was similar to that of WT PfNF54 parasites (Fig. 2A). This indicated that PfARID does not have a critical role in asexual blood-stage replication.
FIG 2.
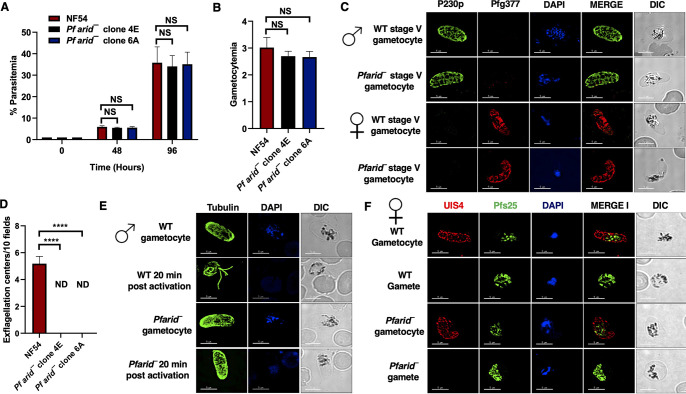
Pfarid– parasites grow normally as asexual parasites and undergo gametocytogenesis but fail to form microgametes. (A) Parasite growth rate for WT PfNF54 and Pfarid– (clone 4E and 6A) was measured over two erythrocytic cycles using Giemsa-stained smears. Data were averaged from three biological replicates and are presented as the mean ± standard deviation (SD). (B) Gametocytemias for WT PfNF54 and Pfarid– (clones 4E and 6A) parasites were measured on day 15 using Giemsa-stained smears. Data were averaged from three biological replicates and are presented as the mean ± SD. (C) IFAs performed for day 15 WT or Pfarid– stage V gametocytes (clone 4E) using α-P230p (green), a stage V male-specific marker, and Pfg377 (red), a marker for female gametocytes. (D) The number of exflagellation centers (vigorous flagellar beating of microgametes in clusters of RBCs) per field at 15 min postactivation. Data were averaged from three biological replicates and are presented as the mean ± SD. (E) IFAs performed on WT or Pfarid– (clone 4E) gametocytes activated for 20 min in vitro using α-tubulin II (green), a male-specific marker. α-Tubulin II staining showed male gametes emerging from an exflagellating male gametocyte in WT PfNF54. The Pfarid– gametocytes were defective for male gametocyte exflagellation. ND, not detected. (F) IFAs performed on WT PfNF54 and Pfarid– (clone 4E) gametocytes activated for 20 min in vitro using Pfs25 (green), a marker for female gametes, and PfUIS4, marker for the parasitophorous vacuole membrane. Female gametes did not show any defect in egress from the infected RBC. NS, not significant; ND, not detected.
Disruption of the PfARID locus via CRISPR/Cas9. (A) The schematic for disrupting the PfARID gene. The pFC_ARID_KO plasmid has homology regions from 5′ (5′HR) and 3′ (3′HR) of the PfARID locus, single guide RNAseq (sgRNA), and the human dihydrofolate reductase (hDHFR) locus cloned. (B) Confirmation of PfARID deletion by diagnostic PCR. The oligonucleotides were designed from outside 5′HR and 3′HR and the PfARID locus, and positions are indicated by arrows in panel A. (C) The expected sizes for different sets of PCRs are indicated. Download FIG S2, TIF file, 0.5 MB (558.4KB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To gain insight into the role of PfARID in sexual-stage development, we next analyzed the ability of Pfarid– to undergo gametocytogenesis. WT PfNF54 and Pfarid– (clones 4E and 6A) gametocytes were induced as described previously (32). Gametocytemia was scored for all the cultures on day 15 using Giemsa-stained thin blood culture smears. This revealed that Pfarid– parasites were able to develop into mature stage V gametocytes and showed a similar gametocytemia as WT PfNF54 parasites (Fig. 2B). Next, IFAs were performed to analyze the formation of mature male and female stage V gametocytes using anti-P230p and anti-Pfg377 antibodies, respectively. This revealed the apparently normal formation of both male and female Pfarid– stage V gametocytes (Fig. 2C).
We next analyzed Pfarid– gametogenesis. Day 15 gametocyte cultures for WT PfNF54 and Pfarid– (clones 4E and 6A) were activated by addition of O+ human serum and a decrease in the temperature from 37°C to room temperature (RT). Activated gametocyte cultures were used to prepare a wet mount, and the emergence of microgametes was assessed using the formation of exflagellation centers in 10 random fields of view under bright-field microscopic illumination at ×400 magnification. Strikingly, and in contrast to WT PfNF54 parasites, we did not observe any exflagellation centers for Pfarid– (Fig. 2D), indicating a severe defect in male gametogenesis. To investigate this defect further, IFAs were performed on thin culture smears for WT PfNF54 and Pfarid– activated gametocytes 20 min postactivation, and parasites were stained with anti-tubulin antibody, which labels the axoneme of microgametes. The complete absence of microgametes emerging from the male gametocyte body confirmed a severe defect of microgamete formation in Pfarid– (Fig. 2E). To analyze formation and egress of female gametes, IFAs were performed using anti-Pfs25 antibody (33) and anti-PfUIS4 antibody, which marks the parasitophorous vacuolar membrane (34). These IFAs revealed that Pfarid– female gametes formed and egressed from RBCs normally and did not show any apparent morphological defect (Fig. 2F). Taken together, these results show that PfARID is critical for male gametogenesis.
PfARID is also essential for female gamete fertility and for transmission to the mosquito.
After establishing the critical role of PfARID in male gametogenesis, we next investigated the transmissibility of Pfarid– gametocytes to mosquitoes. Infectious blood meals were prepared for WT PfNF54 and Pfarid– stage V gametocytes, and gametocytes were fed to mosquitoes using standard membrane feeders. Oocyst stages were analyzed in the mosquito midguts on day 7 postinfection, which revealed a complete absence of oocysts for both Pfarid– clones. This was in contrast to WT PfNF54 parasites, which yielded an average oocyst number of ~24/mosquito (Fig. 3A). These results indicated that PfARID is essential for transmission to the mosquito vector, presumably due to its critical role in male gametogenesis.
FIG 3.
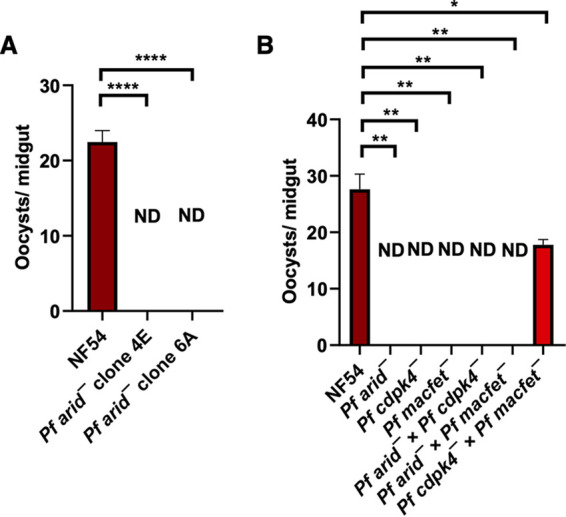
Genetic crosses reveal that PfARID also regulates fertility of female gametes. (A) WT PfNF54 and Pfarid– gametocytes were fed to A. stephensi mosquitoes, and the number of oocysts per mosquito midgut were enumerated on day 7 postfeed. Data were averaged from three biological replicates with a minimum of 50 mosquito guts and are presented as the mean ± standard deviation (SD). Pfarid– did not infect the mosquito vector. ND, not detected. (B) Oocyst formation of WT PfNF54, Pfarid–, Pfcdpk4–, and Pfmacfet– single parasite line feeds and genetic crosses of Pfarid– × Pfcdpk4–, Pfarid– × Pfmacfet–, and Pfcdpk4– × Pfmacfet–. Genetic crosses revealed that the Pfarid– did not show productive cross-fertilization with the Pfmacfet– parasites (which produce functional males only), and also not with Pfcdpk4– parasites (which produce functional females only). This demonstrates that Pfarid– parasites suffer a functional defect in both genders. Error bar indicates mean ± SD; n = 2. ND, not detected.
To further substantiate the finding that the lack of ARID causes a male-specific defect, we assessed the fertility of male and female Pfarid– gametes using genetic crosses with gene deletion parasite lines which either formed only fertile female gametes (Pfcdpk4–) (6) or only fertile male gametes (Pfmacfet–) (35). WT PfNF54, Pfarid–, Pfcdpk4–, and Pfmacfet– gametocytes were generated in vitro followed by pairwise mixing and fed to female Anopheles stephensi mosquitoes on day 15 of culture. Genetic crosses were set up as follows: Pfarid– × Pfcdpk4–, Pfarid– × Pfmacfet–, Pfcdpk4– × Pfmacfet–. Mosquitoes were dissected 7 days postfeed to enumerate oocysts in the midgut. As expected, WT PfNF54 gametocytes showed robust mosquito midgut infection, but Pfarid–, Pfcdpk4–, and Pfmacfet– single line-fed mosquitoes showed no oocysts in the midgut (Fig. 3B). As anticipated, the Pfarid– × Pfcdpk4– cross showed no mosquito midgut infection, further establishing a complete defect in Pfarid– microgametogenesis. Unexpectedly however, the Pfarid– × Pfmacfet– cross (Fig. 3B) also showed no mosquito midgut oocysts. This indicated that Pfarid– parasites suffer an additional severe female fertility defect, which we did not predict based on the apparently normal formation of female gametes (Fig. 2F).
Widespread transcriptome perturbances in mature Pfarid– gametocytes.
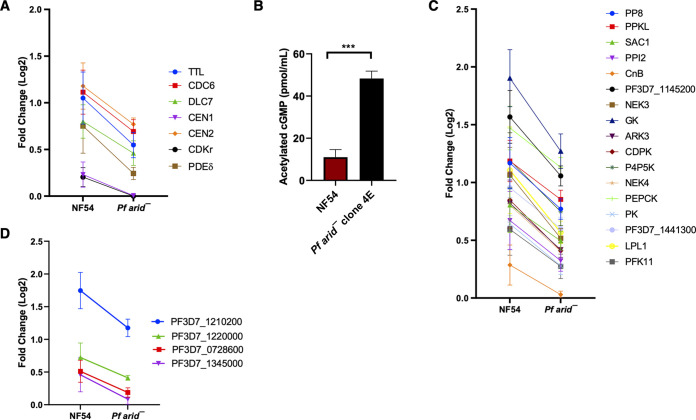
Based on the nuclear localization of PfARID and its association with euchromatic regions, as well as a potential function in chromatin remodeling, we predicted that the lack of PfARID would cause changes in gene expression. To determine these changes, we performed comparative RNA-seq analysis on WT PfNF54 and Pfarid– stage V gametocytes. We focused on this stage, as we anticipated that potential changes in the transcriptome might precede the observed phenotypic defects. RNA-seq was carried out on three biological replicates each for WT PfNF54 and Pfarid–. This revealed 489 differentially expressed genes (DEGs), of which 78 were upregulated and 411 were downregulated in Pfarid– stage V gametocytes (Data set S1A to C). Initially, we curated the DEGs manually to identify genes related to gametogenesis in the malaria parasite and other organisms. We observed that several transcripts encoding proteins such as cGMP-specific 3′,5′-cyclic phosphodiesterase delta (PDE δ), tubulin-tyrosine ligase (TTL), cell division control protein 6 (CDC6), cyclin-dependent kinase regulatory subunit (CDKr), centrin-1 and centrin-2, and flagellar outer arm dynein-associated protein (DLC7) were downregulated in Pfarid– (Fig. 4A and Data set S1D). PfPDEδ has been shown to play a role during gametogenesis by regulating cyclic GMP (cGMP) levels (36). We thus measured the levels of cGMP in Pfarid– and WT PfNF54 gametocytes, which revealed that cGMP levels were significantly increased in the absence of PfARID (Figure. 4B). This showed that Pfarid– parasites suffer high levels of cGMP during gametocyte development, caused by downregulation of PfPDEδ, and this is deleterious to microgametogenesis.
FIG 4.
Target-by-target comparison of differential gene expression in WT PfNF54 and Pfarid– stage V game oocytes. (A) Graph showing differentially expressed genes (DEGs) from genes predicted to have a role in gametogenesis. Log2 fold changes are indicated. (B) Acetylated cGMP levels in day 15 gametocyte extracts prepared for WT PfNF54 and Pfarid–. Bar graph represents means standard errors of the means. Unpaired t test with Welch’s correction was used for statistical analysis. (C) Graph showing DEGs belonging to signaling (kinases, phosphatases, and phospholipases). (D) Graph showing DEGs belonging zinc finger proteins (ZFPs). Log2 fold changes are indicated.
Summary of differentially expressed genes (DEGs). (S1A) Summary of all DEGs. (S1B) Summary of all upregulated DEGs. (S1C) Summary of all downregulated DEGs. (S1D) Summary of gametogenesis-related DEGs. (S1E) Summary of signaling-related DEGs. (S1F) Summary of DEGs, zinc finger proteins. (S1G) Summary of DEGs, AP2-O targets. (S1H) Summary of DEGs, crystalloid. Download Data Set S1, XLSX file, 0.8 MB (862.1KB, xlsx) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
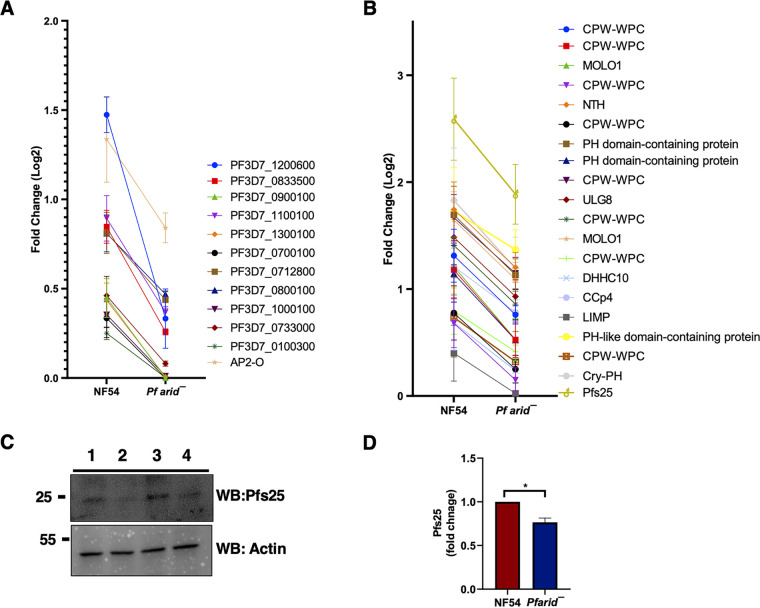
Interestingly, several signaling molecules, such as kinases and phosphatases and a lysophospholipase (LPL1), were also downregulated in Pfarid– (Figure. 4C and Data set S1E). This suggested PfARID-mediated regulation of expression of genes involved in cellular signaling events, which may be relevant to gametogenesis. The phospholipase PfLPL1 has been shown to regulate neutral lipid synthesis (37). Since a proportion of neutral lipids increase during gametocyte maturation (38), LPL1-mediated generation of neutral lipids may have a role in formation of fertile gametes. Another group of downregulated genes encode zinc finger proteins (ZFPs) (Figure. 4D and Data set S1F). ZFPs are involved in transcriptional regulation, chromatin remodeling, proteostasis, and signal transduction, as well as cell proliferation and differentiation (39, 40). The Plasmodium genome encodes 170 putative ZFPs (41). Downregulation of the ZFPs suggested that PfARID functions upstream of ZFPs and that it might have a regulatory role for expression of these genes during gametogenesis and beyond. This hypothesis is also supported by the recent functional studies in the rodent malaria parasite P. berghei, where female development 4 (FD4) (an ortholog of PF3D7_1220000) is important for completion of female-specific development (42). An additional two ZFPs, PBANKA_1357900 (PF3D7_1345000 ortholog) and PBANKA_0608600 (PF3D7_1210200 ortholog) are critical for the blood to midgut oocyst transition (43), suggesting roles in sexual-stage development.
Pfarid– gametocytes show dysregulation of heterochromatinized gene expression and expression of genes encoding ookinete/crystalloid proteins.
After manual curation, we performed Gene Ontology term enrichment analysis for the DEGs. This analysis revealed that parasite transcripts encoding gene products related to host interactions, host cell, extraorganismal space, and Maurer’s clefts were upregulated in Pfarid– stage V gametocytes (Fig. S3A and B). Maurer’s clefts are parasite-derived membranous structures in the infected red blood cell cytosol (44). This group majorly represented multigene family genes such as members of VAR, RIFIN, and PHIST (Data set S1B and 2A and B), which mediate host/parasite interactions. Among the downregulated transcripts, we also found gene terms related to the crystalloid, pellicle, infected host cell surface knobs, inner membrane complex, nucleosome, basal part of the cell, apical part of the cell, signal peptidase complex, and host cell plasma membrane (Fig. S3C D). The majority of the genes belonging to “host cell plasma membrane” included VAR/RIFIN/PHIST genes (Fig. S4 and Data set S1C and S2C and D). Since these VAR/RIFIN/PHIST multigene families are associated with heterochromatin and heterochromatin protein 1 (HP1) (45–47), their dysregulation may be a result of perturbations of chromatin structure in the Pfarid– stage V gametocytes.
Gene Ontology enrichment analysis of DEGs. (S2A) GO term enrichment analysis (molecular function) of upregulated DEGs. (S2B) GO term enrichment analysis (cellular component) of upregulated DEGs. (S2C) GO term enrichment analysis (molecular function) of downregulated DEGs. (S2D) GO term enrichment analysis (cellular component) of downregulated DEGs. Download Data Set S2, XLSX file, 0.02 MB (20.3KB, xlsx) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Gene Ontology term enrichment analysis. (A) Molecular function and (B) cellular component terms of upregulated genes. (C) Molecular function and (D) cellular component terms of downregulated genes. Download FIG S3, TIF file, 0.7 MB (689.3KB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PfARID regulates transcription of the heterochromatin-related genes. Heterochromatin-associated gene families with significant dysregulation in Pfarid–. Geometric mean fold changes and P values (two-sided, one sample t test) are indicated. Download FIG S4, TIF file, 0.4 MB (382.4KB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Interestingly, three members of the ApiAP2 transcription factor family, including AP2-O, were also downregulated in Pfarid– gametocytes, while AP2-L was upregulated (Data set S1A). PfAP2-O has been shown to bind upstream regions of var genes (48) and regulate their transcription (49). It also regulates sexual stage development and parasite transmission to the mosquito vector (49). A comparative analysis of DEGs from RNA-seq data with known PfAP2-O target genes (48, 49) revealed downregulation of numerous PfAP2-O target genes in Pfarid– (Fig. 5A and Data set S1G), indicating PfARID-mediated regulation of PfAP2-O transcription factor function.
FIG 5.
PfARID regulates expression of the PfAP2-O and PfHMGB2 target genes. (A) Graph showing DEGs related to AP2-O and its VAR targets. Log2 fold changes are indicated. (B) Graph showing DEGs related to PfHMGB2 regulated genes. Log2 fold changes are indicated. (C) Western blot analysis of Pfs25 expression in WT PfNF54 and Pfarid– gametocytes (upper panel). β-Actin was used as the loading control (lower panel). 1,3-WT PfNF54; 2,4-Pfarid– gametocytes.
The downregulation of transcripts encoding P. falciparum orthologs of the P. berghei crystalloid proteins in Pfarid– gametocytes was interesting. (Fig. S3D and Data set S1H). To identify a possible link between PfARID and crystalloid component-encoding genes, we searched published studies for chromatin regulators and other proteins which might regulate the expression of these genes. A high-mobility group box (HMGB) protein, HMGB2 has been demonstrated to control expression of several ookinete/oocyst-specific gene products in P. yoelii (50). Microarray analyses on Pyhmgb2– parasites (asexual and gametocyte mix) revealed 30 genes to be downregulated, out of which 12 are expressed in ookinete/oocyst stages (50). Another study in P. berghei reported that these 12 ookinete/oocyst-specific proteins are present in a complex with LCCL lectin adhesive-like protein 3 (LAP3) along with crystalloid proteins (51). Interestingly, PfHMGB2 was downregulated in Pfarid– gametocytes (Data set S1C). While PfHMGB2 shows 100% identity with P. yoelii HMGB2 (PyHMGB2), attempts at disrupting the gene have failed (52). To establish a possible link between PfARID and PfHMGB2, we compared PyHMGB2 crystalloid gene targets (50) with those downregulated in Pfarid–. This analysis revealed common gene expression perturbations between these two data sets (Fig. 5B). Pfs25, which is an activated gametocyte/gamete and ookinete protein, was also among downregulated DEGs in Pfarid– gametocytes (Data set S1C). Its ortholog Pys25 was also shown to be downregulated in Pyhmgb2– gametocytes (50). We performed protein expression analysis for Pfs25 in WT PfNF54 and Pfarid– gametocytes via Western blotting, which revealed that Pfs25 levels were indeed reduced in Pfarid– gametocytes (Fig. 5C and Fig. 5D).
DISCUSSION
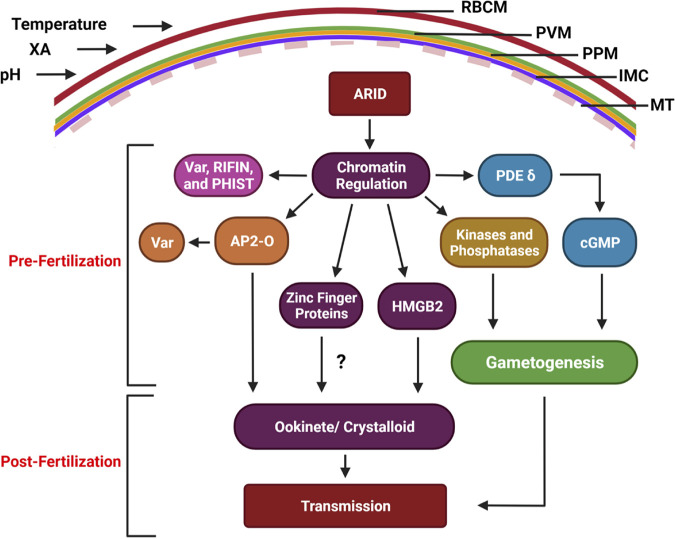
Differentiation of a small subset of asexually replicating Plasmodium parasites into fertilization-competent gametes is a critical step for continuation of the parasite life cycle. The gametocytes taken up by the mosquito vector during a blood meal are rapidly activated to form gametes. For this, male gametocytes undergo three rounds of rapid DNA replication and each form eight flagellated male gametes (exflagellae). Female gametocytes undergo a marked reduction in cytoplasmic density and nuclear changes to each form a single female gamete. Gametocytogenesis and gametogenesis are critical steps and bottlenecks in the parasites’ life cycle. The well-studied ApiAP2 family protein AP2-G functions in the initiation of the transcriptional program that regulates the onset of gametocytogenesis (18, 53), but factors that regulate gene expression to drive gametogenesis and fertilization competence remain largely unknown. Our study demonstrates an essential regulatory role of the ARID domain-containing protein PfARID in microgametogenesis and macrogamete fertility, as well as postfertilization events that are transcriptionally established in mature gametocytes (Fig. 6).
FIG 6.
A model for PfARID function. PfARID regulates the stage V gametocyte chromatin landscape, likely as part of a chromatin remodeling complex. This in part controls expression of AP2-O, HMGB2, and zinc finger proteins (ZFPs), which in turn regulate heterochromatinized genes (VAR, RIFIN, PHIST), and ookinete/crystalloid-specific genes. ARID also controls expression of signaling molecules (kinases and phosphatase) which are known to regulate parasite functions. In addition, PfARID regulates expression of PDEδ, which in turn regulates cGMP levels and, thereby, gametogenesis. Collectively, these molecules regulate male gamete formation, female gamete fertility, and parasite infection of the mosquito vector.
The ARID family proteins ARID1A/ARID1B (also known as BAF250a/BAF250b) are part ATP-dependent chromatin remodeling complexes such as BAF (BRG1/BRM-associated factor or mammalian SWI/SNF) complexes in human cells (22). ARID proteins also form a complex with histone deacetylases in plants and regulate sperm cell formation (54). ARID complexes regulate maintenance of open chromatin at enhancer elements to drive gene expression programs in a developmentally regulated and cell context-specific manner (22). Plasmodium contains components of chromatin-modifying proteins (55), and genome-wide nucleosome mapping has indicated that chromatin remodeling might be an important mechanism of gene regulation in these parasites (56). However, no chromatin remodeling complexes or BAF complex components have been characterized in P. falciparum. Using CRIPSR/Cas9-based gene editing, we generated Pfarid– parasites and demonstrated that PfARID is critical for microgametogenesis but also regulates fertility of macrogametes. Although PfARID is expressed in the asexual erythrocytic stages, the deletion of the gene did not cause an overt phenotypic defect in these parasite stages. We thus analyzed the perturbation of the transcriptome in the sexual stages and found that PfARID is regulating expression of genes that drive gametogenesis, of gene products that form signaling cascades, and of gene products critical for the ookinete crystalloid organelle and expression of multigene families.
PfARID possesses multiple NLS signals, a LXCXE motif and two TM domains. In agreement with the NLS prediction, we showed that PfARID displayed a nuclear localization in both asexual and sexual stages. An Internal bipartite NLS has been shown to regulate mammalian ARID1A nuclear localization (57). The presence of TM domains in PfARID is unique and intriguing. Nuclear envelope transmembrane proteins (NETs) have been described to control the cell cycle (58) and organize spatial control of the genome (59), but no ARID family protein is known to possess TM domains (28). We have also shown that PfARID localized in close proximity to nuclear pore complexes, further suggesting an association with the nuclear envelope. It is possible that its two predicted TM domains anchor PfARID to the nuclear envelope. PfARID also contained an LXCXE motif, which in other proteins has a role in facilitating interaction with the retinoblastoma (RB) tumor suppressor (29). ARID4A is an RB-binding protein and regulates cell cycle progression in a variety of organisms (60–62). ARID4A and ARID4B are involved in the control of male fertility by acting in the RB pathway (63). An RB pathway and its components have, however, not been identified in Plasmodium. It is thus possible that other parasite proteins might bind to PfARID via the LXCXE motif and regulate its function. The ARID domain of PfARID displayed high structural similarity to the ARID domain of human ARID3a, which implicates it in transcriptional regulation, as ARID3a has been shown to play a role in regulation of transcription factors associated with hematopoietic lineage decisions and regulation of myeloid and B lineage pathways (30).
Our gene deletion analysis showed that PfARID is not required for asexual blood-stage replication or gametocyte development but uncovered its critical role in male gamete formation, specifically the formation of flagellated microgametes. Male Pfarid– gametocytes also did not show the typical morphological changes that lead to the formation of a spheroid infected RBCs upon activation. In contrast, we observed no discernible defect in Pfarid– macrogamete formation. The Pfarid– genetic crosses we performed with transgenic lines producing either fertile microgametes (6) or macrogametes (35) confirmed a completely penetrant male defect but, surprisingly, also showed that PfARID is required for fertility of female gametes. A recent study describing screening for fertility-related genes in the rodent malaria parasite P. berghei, showed that the P. berghei ARID (PbARID) ortholog named MD4 (PBANKA_0102400) is involved in fertility of male gametes only (42). Thus, ARID might differ in its sex-specific functions among different malaria parasite species.
Given the nuclear localization of PfARID, its colocalization with the euchromatic acetylated histone marker H3K9Ac and the severe gamete defects of Pfarid– parasites, we hypothesized that PfARID might be controlling gametogenesis and fertility by regulating stage V gametocyte gene expression. Indeed, comparative transcriptome analysis of PfNF54 WT and Pfarid– parasites using RNA-seq identified 411 differentially expressed genes (DEGs) which were downregulated in parasites lacking PfARID as well as 78 upregulated DEGs. Likely, these DEGs might be directly regulated by PfARID but also indirectly by the perturbation of transcription factor expression. We observed downregulation of key gametogenesis-regulating gene products, including PfPDEδ. Previous work has demonstrated that PfPDEδ activity, optimal cGMP levels, and cGMP-dependent kinase PKG are required for microgametogenesis and liberation of the male gametes from the infected RBC during exflagellation (36, 64). We observed an increase of cGMP levels in Pfarid– parasites, which would severely impact microgametogenesis. Also, DEGs observed in Pfarid– parasites may represent targets of cGMP-PfPDEδ-mediated homeostasis and signaling and other parasite proteins which may have a role in microgametogenesis. Indeed, expression of GTP cyclohydrolase 1 (GCH1) was elevated in Pfarid– gametocytes (Data set S1B). GCH1 catalyzes conversion of GTP into DHNTP (7,8-dihydroneopterin triphosphate) and is critical for parasite transmission in P. berghei (43). Other DEGs that were downregulated in the absence of PfARID, such as NIMA-related kinase 3 (NEK3), cell division control protein 6 (CDC6), DLC7, centrin 1, and centrin 2, as well as CDKr show very high expression in late-stage gametocytes (PlasmoDB). These proteins are currently uncharacterized in P. falciparum, but P. berghei orthologs of some of these proteins have been shown to play a role in sexual development (65). It would thus be important to study their function during P. falciparum microgametogenesis. Interestingly, transcripts encoding several signaling molecules, such as kinases and phosphatases, were also downregulated in Pfarid–, indicating a potential perturbation of phosphorylation of parasite proteins in stage V gametocytes. Kinases and phosphatases have been shown to play a role in gametogenesis and transmission to the mosquito vector in both P. falciparum (6, 64, 66) and P. berghei (67, 68).
Surprisingly, another group of DEGs downregulated in Pfarid– parasites encode ookinete/crystalloid-related genes. Crystalloids are unique organelles of the ookinete stages, which develop after fertilization from the zygote and are built to invade the mosquito midgut to form oocysts. They appear as clusters of tightly packed electron-dense spherical units in the ookinete cytoplasm (69) and are involved in sporogony, the formation of sporozoites within the oocyst (70). Recent studies in P. berghei have identified components of crystalloids, including LCCL lectin adhesive proteins (LAPs), CPW-WPC family proteins, secreted ookinete proteins (SOPs), a palmitoyl-S-acyl transferase (PAT) protein named DHHC10, NAD(P) transhydrogenase (NTH), a multipass transmembrane protein that generates NADPH, and several PH domain-containing proteins (51). We found that a number of transcripts, including those for DHHC10, NTH, CPW-WPC family proteins, and LAP6 were downregulated in Pfarid– parasites. This might be indirectly driven by the observed downregulation of PfHMGB2 in Pfarid– parasites, a member of the high mobility group box (HMGB) family, which in other organisms actively participates in chromatin remodeling by increasing nucleosome sliding and accessibility of the chromatin (71). Previous studies have implicated PyHMGB2 in regulating expression of the orthologous genes in P. yoelii (50). Interestingly, many of the HMGB2-regulated gene transcripts are then translationally repressed by the development of zygote inhibited (DOZI) mRNA storage complex (72). This complex represses premature translation of mRNAs in gametocytes that encode proteins which function postfertilization. Thus, PfARID in part regulates the expression of mRNAs that are stored and translationally repressed in gametocytes and are translated only after fertilization to drive infection of the mosquito vector.
Transcriptional regulation in Plasmodium is thought to mainly involve members of the ApiAP2 transcription factor family. We found that some ApiAP2 family members such as AP2-O were downregulated in Pfarid– parasites. AP2-O has been previously implicated in regulating expression of genes involved in parasite transmission to the mosquito vector (49). This suggests an additional level of complexity to PfARID function, as it might not only directly regulate accessibility of transcription factors to DNA but might also directly regulate expression levels of transcription factors.
Lastly, gametocytes lacking PfARID also showed dysregulation (both upregulated and downregulated) of a large number of heterochromatin-associated multigene families such as VAR (73), RIFIN (74), and PHISTa/b/c (75), which are known to regulate cytoadherence of infected RBCs, immune escape, and other parasite/host interactions, mainly in the parasites’ asexual blood stages. These gene families are, however, also expressed in gametocytes (76–79). VAR gene expression encoding PfEMP1 proteins might continue to provide variant antigen expression and immune escape in gametocytes during maturation (76), and PHIST family proteins are exported during gametocytogenesis (77) and control infected RBC rigidity (80). Host cell deformability and rigidity change late in gametocytogenesis and are possibly critical factors in gametocyte transmission to the mosquito vector (81). Since we have shown that PfARID is also expressed in asexual blood stages, but its deletion did not result in a parasite growth defect, it will be of interest to analyze the perturbation of multigene family expression in these stages in the future.
Our demonstration that PfARID serves an essential role in driving microgametogenesis and macrogamete fertility via regulation of gene expression constitutes a critical entry point for understanding the regulation of P. falciparum gamete formation and fertilization competence on the molecular level. Since ARID proteins in other organisms are part of BAF chromatin-remodeling complexes (22), the identification PfARID will enable the isolation of equivalent complexes in Plasmodium. A fuller understanding of PfARID-mediated chromatin regulation might also inform novel transmission-blocking interventions against malaria parasites.
MATERIALS AND METHODS
Reagents and primary antibodies.
All molecular biology reagents and oligonucleotides were purchased from MilliporeSigma, USA, unless otherwise stated. All oligonucleotides were purchased from Integrated DNA Technologies, Inc. (IDT), USA. The following primary antibodies and antisera and dilutions were used: mouse α-P230p (1:100, kindly gifted by Kim Williamson, Uniformed Services University of the Health Sciences, USA) α-Pfg377 (1:500, kindly gifted by Pietro Alano at Istituto Superiore di Sanità, Italy), mouse α-tubulin antibody (1:200, Sigma-Aldrich, catalog [cat.] no. T5168), rat α-mCherry antibody (1:200, Thermo Scientific cat. no. M11217, clone 16D7), α-NUP116 (1:100, rabbit, kindly gifted by Artur Scherf at Institut Pasteur, France). The following reagents were obtained through BEI Resources, NIAID, NIH: hybridoma 4B7 anti-Pfs25-kilodalton gamete surface protein (Pfs25), MRA-315, contributed by Louis H. Miller and Allan Saul and α-Pfs25 (1:1, mouse).
P. falciparum culture and transfection.
P. falciparum parasites (WT PfNF54 and Pfarid–) were cultured as asexual blood stages according to standard procedures and received complete RPMI medium supplemented either with 0.5% AlbuMAX II (Thermo Scientific) medium or 10% (vol/vol) human serum every 24 h. In vitro gametocytes were generated using O+ human RBCs (Valley Biomedical, Virginia, USA) and O+ human serum (Interstate Blood Bank, Tennessee, USA) using methods published elsewhere (32).
Oligonucleotide primers used for the creation and analysis of P. falciparum Pfarid– and PfARIDmCherry parasites are detailed in Table S1. Deletion of PfARID (PlasmoDB identifier gene PF3D7_0603600) was achieved based on the previously reported CRISPR/Cas9 strategy. Gene deletion was confirmed by a set of genotyping PCRs (Fig. S2B). Two individual clones for Pfarid– (clones 4E and 6A) were used for phenotypic characterization.
Oligonucleotides used in the study. Download Table S1, DOCX file, 0.02 MB (23.1KB, docx) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Measurement of asexual blood-stage growth and gametocyte development.
To compare asexual blood stage replication and growth between the WT PfNF54 and Pfarid– parasites, synchronized parasites were set up at an initial ring stage parasitemia of 1% and cultured in 6-well plates. Thin smears were prepared at 48 and 96 h. For preparation of Giemsa-staining, parasitemia was scored per 1,000 erythrocytes.
To compare gametocyte formation between WT PfNF54 and Pfarid–, gametocytes were cultured as described above. Parasites were removed on day 15 of in vitro culture for preparation of Giemsa-stained thin blood smears, and gametocytemia was scored per 1,000 erythrocytes.
Indirect immunofluorescence.
For IFAs on gametocytes and exflagellating gametes, thin smears were prepared on Teflon-coated slides and fixed with 4% paraformaldehyde/0.0025% glutaraldehyde solution for 30 min. Slides were kept in a humidity chamber for each step. Fixed parasites were washed twice with phosphate-buffered saline (PBS) and permeabilized using 0.1% Triton X-100/PBS solution for 10 min. Parasites were washed twice with PBS for 5 min each and blocked with 3% bovine serum albumin (BSA)/PBS for 45 min. Primary antisera in 3% BSA/PBS were added to the parasites, and the slides were incubated at 4°C. Antigens were visualized using anti-species antibodies. Images were obtained using a ×100 1.4-numerical aperture (NA) objective 90 (Olympus) on a Delta Vision Elite high-resolution microscope (GE Healthcare Life Sciences).
Comparative RNA-Seq and data analysis.
RNA-seq methodology was adapted from previous articles with modifications (82, 83). On day 15 of gametocyte development, stage V gametocytes were harvested using saponin lysis. The RNA preparation, library preparation, and RNA-seq analysis were done at Azenta/Genewiz, USA. Total RNA from saponin-lysed parasites was extracted using TRIzol (Invitrogen) and a Qiagen RNA-extraction kit. Following RNA isolation, total RNA integrity was checked using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). RNA concentrations were measured using the NanoDrop system. rRNA was removed from total RNA using an Illumina Ribo Zero Gold for human/mouse/rat kit. The libraries were multiplexed and clustered on one lane of a flow cell and loaded on an Illumina HiSeq platform according to the manufacturer’s instructions. After the quality of the raw data was investigated, sequence reads were trimmed to remove possible adapter sequences and nucleotides with poor quality using Trimmomatic v.0.36. The trimmed reads were mapped to the Plasmodium falciparum reference genome using the STAR aligner v.2.5.2b. BAM files were generated because of this step. Unique gene hit counts were calculated by using the Counts feature from the Subread package v.1.5.2. R software v.3.4.1 was used when executing DESeq2 analysis for DEG identification and graphic tools. All the analyses were performed with default parameters; DEGs were defined as genes with an absolute log2 fold change (log2FC) of >1 and adjusted P value of <0.05.
Gene Ontology term enrichment analyses were carried out with Cytoscape v.3.9.0 (84) with the BiNGO plugin (85). Gene Ontology (GO) terms for P. falciparum genes were downloaded from the PlasmoDB database. GO terms from all three categories were fetched from this and used as input against all the known GO terms in the BiNGO plugin. The hypergeometric distribution test was performed at a P value of ≤0.05 with Bonferroni correction. The network of enriched GO terms thus obtained was reported as the result.
Measurement of cGMP levels.
The assay for determining cGMP levels in gametocytes was performed using the cGMP enzyme immunoassay (EIA) kit (cat. no. 581021; Cayman Chemical) following the manufacturer’s instructions. Gametocytes for the assay were purified on a Percoll gradient to get rid of uninfected RBCs, and gametocyte extracts were prepared by two rounds of freezing on dry ice-ethanol, thawing on ice, and passaging through a 21-gauge needle from the same number of gametocytes for each line. Equal volumes of extract from WT PfNF54 and Pfarid– gametocytes were used to assay for cGMP.
Statistical analysis.
All data are expressed as the mean ± standard deviation (SD). Statistical differences were determined using one-way analysis of variance (ANOVA) with the post hoc Bonferroni multiple-comparison test or unpaired two-tailed Student’s t test, as indicated. Values of P < 0.05 were considered statistically significant. Significances were calculated using GraphPad Prism 8 and are represented in the figures as follows: ns, not significant, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Data and material availability.
All the correspondence and request for materials used in these studies should be addressed to the corresponding authors.
ACKNOWLEDGMENTS
WE acknowledge and thank W. Betz and T. Seltzer for maintaining insectaries at the Center for Global Infectious Disease Research, Seattle Children’s Research Institute, and for timely provision of uninfected mosquitoes for this research.
This work was funded by seed funds from Seattle Children’s to S.H.I.K.
Conceptualization: S.K. and S.H.I.K.; methodology: S.K., V.K.B., and S.H.I.K.; investigation: S.K., K.M.Z.O., B.A.A., M.T.H., and S.Y.K.; visualization: S.K., V.K.B., and S.H.I.K.; supervision: A.M.V., and S.H.I.K.; writing-original draft: S.K., V.K.B., K.M.Z.O., and S.H.I.K.; writing-review and editing: S.K., V.K.B., K.M.Z.O., A.M.V., and S.H.I.K.
We declare no competing financial or nonfinancial interests.
Contributor Information
Sudhir Kumar, Email: sudhir.kumar@seattlechildrens.org.
Stefan H. I. Kappe, Email: stefan.kappe@seattlechildrens.org.
Louis M. Weiss, Albert Einstein College of Medicine
REFERENCES
- 1.Aly AS, Vaughan AM, Kappe SH. 2009. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol 63:195–221. doi: 10.1146/annurev.micro.091208.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinden RE, Croll NA. 1975. Cytology and kinetics of microgametogenesis and fertilization in Plasmodium yoelii nigeriensis. Parasitology 70:53–65. doi: 10.1017/s0031182000048861. [DOI] [PubMed] [Google Scholar]
- 3.Sinden RE. 1983. Sexual development of malarial parasites. Adv Parasitol 22:153–216. doi: 10.1016/s0065-308x(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 4.Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. 1998. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 5.Balestra AC, Koussis K, Klages N, Howell SA, Flynn HR, Bantscheff M, Pasquarello C, Perrin AJ, Brusini L, Arboit P, Sanz O, Castaño LP, Withers-Martinez C, Hainard A, Ghidelli-Disse S, Snijders AP, Baker DA, Blackman MJ, Brochet M. 2021. Ca(2+) signals critical for egress and gametogenesis in malaria parasites depend on a multipass membrane protein that interacts with PKG. Sci Adv 7:eabe5396. doi: 10.1126/sciadv.abe5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Haile MT, Hoopmann MR, Tran LT, Michaels SA, Morrone SR, Ojo KK, Reynolds LM, Kusebauch U, Vaughan AM, Moritz RL, Kappe SHI, Swearingen KE. 2021. Plasmodium falciparum calcium-dependent protein kinase 4 is critical for male gametogenesis and transmission to the mosquito vector. mBio 12:e0257521. doi: 10.1128/mBio.02575-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirth CC, Glushakova S, Scheuermayer M, Repnik U, Garg S, Schaack D, Kachman MM, Weißbach T, Zimmerberg J, Dandekar T, Griffiths G, Chitnis CE, Singh S, Fischer R, Pradel G. 2014. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol 16:709–733. doi: 10.1111/cmi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh P, Alaganan A, More KR, Lorthiois A, Thiberge S, Gorgette O, Guillotte Blisnick M, Guglielmini J, Aguilera SS, Touqui L, Singh S, Chitnis CE. 2019. Role of a patatin-like phospholipase in Plasmodium falciparum gametogenesis and malaria transmission. Proc Natl Acad Sci USA 116:17498–17508. doi: 10.1073/pnas.1900266116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierre-Jerome E, Drapek C, Benfey PN. 2018. Regulation of division and differentiation of plant stem cells. Annu Rev Cell Dev Biol 34:289–310. doi: 10.1146/annurev-cellbio-100617-062459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satory D, Gordon AJ, Halliday JA, Herman C. 2011. Epigenetic switches: can infidelity govern fate in microbes? Curr Opin Microbiol 14:212–217. doi: 10.1016/j.mib.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Norman TM, Lord ND, Paulsson J, Losick R. 2015. Stochastic switching of cell fate in microbes. Annu Rev Microbiol 69:381–403. doi: 10.1146/annurev-micro-091213-112852. [DOI] [PubMed] [Google Scholar]
- 12.Pepper JW, Sprouffske K, Maley CC. 2007. Animal cell differentiation patterns suppress somatic evolution. PLoS Comput Biol 3:e250. doi: 10.1371/journal.pcbi.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. 2009. A signaling principle for the specification of the germ cell lineage in mice. Cell 137:571–584. doi: 10.1016/j.cell.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls PK, Schorle H, Naqvi S, Hu YC, Fan Y, Carmell MA, Dobrinski I, Watson AL, Carlson DF, Fahrenkrug SC, Page DC. 2019. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc Natl Acad Sci USA 116:25677–25687. doi: 10.1073/pnas.1910733116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hisanaga T, Yamaoka S, Kawashima T, Higo A, Nakajima K, Araki T, Kohchi T, Berger F. 2019. Building new insights in plant gametogenesis from an evolutionary perspective. Nat Plants 5:663–669. doi: 10.1038/s41477-019-0466-0. [DOI] [PubMed] [Google Scholar]
- 16.López-Barragán MJ, Lemieux J, Quiñones M, Williamson KC, Molina-Cruz A, Cui K, Barillas-Mury C, Zhao K, Su XZ. 2011. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genomics 12:587. doi: 10.1186/1471-2164-12-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Biljon R, van Wyk R, Painter HJ, Orchard L, Reader J, Niemand J, Llinás M, Birkholtz LM. 2019. Hierarchical transcriptional control regulates Plasmodium falciparum sexual differentiation. BMC Genomics 20:920. doi: 10.1186/s12864-019-6322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kafsack BF, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE, Drought LG, Kwiatkowski DP, Baker DA, Cortés A, Llinás M. 2014. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang X, Shen S, Tang J, He X, Zhao Y, Wang C, He X, Guo G, Liu M, Wang L, Zhu Q, Yang G, Jiang C, Zhang M, Yu X, Han J, Culleton R, Jiang L, Cao J, Gu L, Zhang Q. 2021. A cascade of transcriptional repression determines sexual commitment and development in Plasmodium falciparum. Nucleic Acids Res 49:9264–9279. doi: 10.1093/nar/gkab683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh S, Santos JM, Orchard LM, Yamada N, van Biljon R, Painter HJ, Mahony S, Llinás M. 2021. The PfAP2-G2 transcription factor is a critical regulator of gametocyte maturation. Mol Microbiol 115:1005–1024. doi: 10.1111/mmi.14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasonder E, Rijpma SR, van Schaijk BC, Hoeijmakers WA, Kensche PR, Gresnigt MS, Italiaander A, Vos MW, Woestenenk R, Bousema T, Mair GR, Khan SM, Janse CJ, Bártfai R, Sauerwein RW. 2016. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res 44:6087–6101. doi: 10.1093/nar/gkw536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodges C, Kirkland JG, Crabtree GR. 2016. The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb Perspect Med 6:a026930. doi: 10.1101/cshperspect.a026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwahara J, Clubb RT. 1999. Solution structure of the DNA binding domain from Dead ringer, a sequence-specific AT-rich interaction domain (ARID). EMBO J 18:6084–6094. doi: 10.1093/emboj/18.21.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilsker D, Probst L, Wain HM, Maltais L, Tucker PW, Moran E. 2005. Nomenclature of the ARID family of DNA-binding proteins. Genomics 86:242–251. doi: 10.1016/j.ygeno.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Doyle DZ, Lam MM, Qalieh A, Qalieh Y, Sorel A, Funk OH, Kwan KY. 2021. Chromatin remodeler Arid1a regulates subplate neuron identity and wiring of cortical connectivity. Proc Natl Acad Sci USA 118:e2100686118. doi: 10.1073/pnas.2100686118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Nagl NG, Wilsker D, Van Scoy M, Pacchione S, Yaciuk P, Dallas PB, Moran E. 2004. Two related ARID family proteins are alternative subunits of human SWI/SNF complexes. Biochem J 383:319–325. doi: 10.1042/BJ20040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patsialou A, Wilsker D, Moran E. 2005. DNA-binding properties of ARID family proteins. Nucleic Acids Res 33:66–80. doi: 10.1093/nar/gki145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C, Song W, Bi X, Zhao J, Huang Z, Li Z, Zhou J, Cai J, Zhao H. 2014. Recent advances in the ARID family: focusing on roles in human cancer. Onco Targets Ther 7:315–324. doi: 10.2147/OTT.S57023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourgo RJ, Thangavel C, Ertel A, Bergseid J, McClendon AK, Wilkens L, Witkiewicz AK, Wang JY, Knudsen ES. 2011. RB restricts DNA damage-initiated tumorigenesis through an LXCXE-dependent mechanism of transcriptional control. Mol Cell 43:663–672. doi: 10.1016/j.molcel.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratliff ML, Mishra M, Frank MB, Guthridge JM, Webb CF. 2016. The transcription factor ARID3a is important for in vitro differentiation of human hematopoietic progenitors. J Immunol 196:614–623. doi: 10.4049/jimmunol.1500355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guizetti J, Martins RM, Guadagnini S, Claes A, Scherf A. 2013. Nuclear pores and perinuclear expression sites of var and ribosomal DNA genes correspond to physically distinct regions in Plasmodium falciparum. Eukaryot Cell 12:697–702. doi: 10.1128/EC.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathi AK, Mlambo G, Kanatani S, Sinnis P, Dimopoulos G. 2020. Plasmodium falciparum gametocyte culture and mosquito infection through artificial membrane feeding. J Vis Exp doi: 10.3791/61426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackellar DC, O'Neill MT, Aly AS, Sacci JB, Jr, Cowman AF, Kappe SH. 2010. Plasmodium falciparum PF10_0164 (ETRAMP10.3) is an essential parasitophorous vacuole and exported protein in blood stages. Eukaryot Cell 9:784–794. doi: 10.1128/EC.00336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Abatiyow BA, Haile MT, Oualim KMZ, Leeb AS, Vaughan AM, Kappe SHI. 2022. A putative plasmodium RNA-binding protein plays a critical role in female gamete fertility and parasite transmission to the mosquito vector. Front Cell Dev Biol 10:825247. doi: 10.3389/fcell.2022.825247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor CJ, McRobert L, Baker DA. 2008. Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis. Mol Microbiol 69:110–118. doi: 10.1111/j.1365-2958.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asad M, Yamaryo-Botté Y, Hossain ME, Thakur V, Jain S, Datta G, Botté CY, Mohmmed A. 2021. An essential vesicular-trafficking phospholipase mediates neutral lipid synthesis and contributes to hemozoin formation in Plasmodium falciparum. BMC Biol 19:159. doi: 10.1186/s12915-021-01042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran PN, Brown SH, Rug M, Ridgway MC, Mitchell TW, Maier AG. 2016. Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar J 15:73. doi: 10.1186/s12936-016-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassandri M, Smirnov A, Novelli F, Pitolli C, Agostini M, Malewicz M, Melino G, Raschellà G. 2017. Zinc-finger proteins in health and disease. Cell Death Discov 3:17071. doi: 10.1038/cddiscovery.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh JK, van Attikum H. 2021. DNA double-strand break repair: putting zinc fingers on the sore spot. Semin Cell Dev Biol 113:65–74. doi: 10.1016/j.semcdb.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Ngwa CJ, Farrukh A, Pradel G. 2021. Zinc finger proteins of Plasmodium falciparum. Cell Microbiol 23:e13387. doi: 10.1111/cmi.13387. [DOI] [PubMed] [Google Scholar]
- 42.Russell AJC, Sanderson T, Bushell E, Talman AM, Anar B, Girling G, Hunziker M, Kent RS, Metcalf T, Montandon R, Pandey V, Brett Roberts A, Sayers C, Schwach F, Rayner JC, Voet T, Modrzynska KK, Waters AP, Lawniczak MKN, Billker O. 2021. Regulators of male and female sexual development critical for transmission of a malaria parasite. bioRxiv doi: 10.1101/2021.08.04.455056. [DOI] [PMC free article] [PubMed]
- 43.Bushell E, Gomes AR, Sanderson T, Anar B, Girling G, Herd C, Metcalf T, Modrzynska K, Schwach F, Martin RE, Mather MW, McFadden GI, Parts L, Rutledge GG, Vaidya AB, Wengelnik K, Rayner JC, Billker O. 2017. Functional profiling of a Plasmodium genome reveals an abundance of essential genes. Cell 170:260–272.e8. doi: 10.1016/j.cell.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mundwiler-Pachlatko E, Beck HP. 2013. Maurer’s clefts, the enigma of Plasmodium falciparum. Proc Natl Acad Sci USA 110:19987–19994. doi: 10.1073/pnas.1309247110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Rubio JJ, Mancio-Silva L, Scherf A. 2009. Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe 5:179–190. doi: 10.1016/j.chom.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Bunnik EM, Cook KB, Varoquaux N, Batugedara G, Prudhomme J, Cort A, Shi L, Andolina C, Ross LS, Brady D, Fidock DA, Nosten F, Tewari R, Sinnis P, Ay F, Vert JP, Noble WS, Le Roch KG. 2018. Changes in genome organization of parasite-specific gene families during the Plasmodium transmission stages. Nat Commun 9:1910. doi: 10.1038/s41467-018-04295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraschka SA, Filarsky M, Hoo R, Niederwieser I, Yam XY, Brancucci NMB, Mohring F, Mushunje AT, Huang X, Christensen PR, Nosten F, Bozdech Z, Russell B, Moon RW, Marti M, Preiser PR, Bártfai R, Voss TS. 2018. Comparative heterochromatin profiling reveals conserved and unique epigenome signatures linked to adaptation and development of malaria parasites. Cell Host Microbe 23:407–420.e8. doi: 10.1016/j.chom.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell TL, De Silva EK, Olszewski KL, Elemento O, Llinás M. 2010. Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog 6:e1001165. doi: 10.1371/journal.ppat.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cubillos EFG, Prata IO, Fotoran WL, Ranford-Cartwright L, Wunderlich G. 2021. The transcription factor PfAP2-O influences virulence gene transcription and sexual development in Plasmodium falciparum. Front Cell Infect Microbiol 11:669088. doi: 10.3389/fcimb.2021.669088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gissot M, Ting LM, Daly TM, Bergman LW, Sinnis P, Kim K. 2008. High mobility group protein HMGB2 is a critical regulator of plasmodium oocyst development. J Biol Chem 283:17030–17038. doi: 10.1074/jbc.M801637200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tremp AZ, Saeed S, Sharma V, Lasonder E, Dessens JT. 2020. Plasmodium berghei LAPs form an extended protein complex that facilitates crystalloid targeting and biogenesis. J Proteomics 227:103925. doi: 10.1016/j.jprot.2020.103925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu B, Liu M, Gu L, Li Y, Shen S, Guo G, Wang F, He X, Zhao Y, Shang X, Wang L, Yang G, Zhu Q, Cao J, Jiang C, Culleton R, Wei G, Zhang Q. 2021. The architectural factor HMGB1 is involved in genome organization in the human malaria parasite Plasmodium falciparum. mBio 12:e00148-21. doi: 10.1128/mBio.00148-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bancells C, Llorà-Batlle O, Poran A, Nötzel C, Rovira-Graells N, Elemento O, Kafsack BFC, Cortés A. 2019. Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat Microbiol 4:144–154. doi: 10.1038/s41564-018-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng B, He H, Zheng Y, Wu W, McCormick S. 2014. An ARID domain-containing protein within nuclear bodies is required for sperm cell formation in Arabidopsis thaliana. PLoS Genet 10:e1004421. doi: 10.1371/journal.pgen.1004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horrocks P, Wong E, Russell K, Emes RD. 2009. Control of gene expression in Plasmodium falciparum: ten years on. Mol Biochem Parasitol 164:9–25. doi: 10.1016/j.molbiopara.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Westenberger SJ, Cui L, Dharia N, Winzeler E, Cui L. 2009. Genome-wide nucleosome mapping of Plasmodium falciparum reveals histone-rich coding and histone-poor intergenic regions and chromatin remodeling of core and subtelomeric genes. BMC Genomics 10:610. doi: 10.1186/1471-2164-10-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bateman NW, Shoji Y, Conrads KA, Stroop KD, Hamilton CA, Darcy KM, Maxwell GL, Risinger JI, Conrads TP. 2016. Identification and functional characterization of a novel bipartite nuclear localization sequence in ARID1A. Biochem Biophys Res Commun 469:114–119. doi: 10.1016/j.bbrc.2015.11.080. [DOI] [PubMed] [Google Scholar]
- 58.Korfali N, Srsen V, Waterfall M, Batrakou DG, Pekovic V, Hutchison CJ, Schirmer EC. 2011. A flow cytometry-based screen of nuclear envelope transmembrane proteins identifies NET4/Tmem53 as involved in stress-dependent cell cycle withdrawal. PLoS One 6:e18762. doi: 10.1371/journal.pone.0018762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuleger N, Boyle S, Kelly DA, de las Heras JI, Lazou V, Korfali N, Batrakou DG, Randles KN, Morris GE, Harrison DJ, Bickmore WA, Schirmer EC. 2013. Specific nuclear envelope transmembrane proteins can promote the location of chromosomes to and from the nuclear periphery. Genome Biol 14:R14. doi: 10.1186/gb-2013-14-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harbour JW, Dean DC. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 14:2393–2409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- 61.Borghi L, Gutzat R, Fütterer J, Laizet Y, Hennig L, Gruissem W. 2010. Arabidopsis retinoblastoma-related is required for stem cell maintenance, cell differentiation, and lateral organ production. Plant Cell 22:1792–1811. doi: 10.1105/tpc.110.074591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebel C, Mariconti L, Gruissem W. 2004. Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature 429:776–780. doi: 10.1038/nature02637. [DOI] [PubMed] [Google Scholar]
- 63.Wu RC, Jiang M, Beaudet AL, Wu MY. 2013. ARID4A and ARID4B regulate male fertility, a functional link to the AR and RB pathways. Proc Natl Acad Sci USA 110:4616–4621. doi: 10.1073/pnas.1218318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA. 2008. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol 6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balestra AC, Zeeshan M, Rea E, Pasquarello C, Brusini L, Mourier T, Subudhi AK, Klages N, Arboit P, Pandey R, Brady D, Vaughan S, Holder AA, Pain A, Ferguson DJ, Hainard A, Tewari R, Brochet M. 2020. A divergent cyclin/cyclin-dependent kinase complex controls the atypical replication of a malaria parasite during gametogony and transmission. Elife 9:e56474. doi: 10.7554/eLife.56474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bansal A, Molina-Cruz A, Brzostowski J, Liu P, Luo Y, Gunalan K, Li Y, Ribeiro JMC, Miller LH. 2018. PfCDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proc Natl Acad Sci USA 115:774–779. doi: 10.1073/pnas.1715443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tewari R, Straschil U, Bateman A, Böhme U, Cherevach I, Gong P, Pain A, Billker O. 2010. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8:377–387. doi: 10.1016/j.chom.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guttery DS, Poulin B, Ramaprasad A, Wall RJ, Ferguson DJ, Brady D, Patzewitz EM, Whipple S, Straschil U, Wright MH, Mohamed AM, Radhakrishnan A, Arold ST, Tate EW, Holder AA, Wickstead B, Pain A, Tewari R. 2014. Genome-wide functional analysis of Plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell Host Microbe 16:128–140. doi: 10.1016/j.chom.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dessens JT, Tremp AZ, Saeed S. 2021. Crystalloids: fascinating parasite organelles essential for malaria transmission. Trends Parasitol 37:581–584. doi: 10.1016/j.pt.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Dessens JT, Saeed S, Tremp AZ, Carter V. 2011. Malaria crystalloids: specialized structures for parasite transmission? Trends Parasitol 27:106–110. doi: 10.1016/j.pt.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Briquet S, Marinach C, Silvie O, Vaquero C. 2020. Preparing for transmission: gene regulation in Plasmodium sporozoites. Front Cell Infect Microbiol 10:618430. doi: 10.3389/fcimb.2020.618430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, Khan SM, Dimopoulos G, Janse CJ, Waters AP. 2006. Regulation of sexual development of Plasmodium by translational repression. Science 313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kyes SA, Kraemer SM, Smith JD. 2007. Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. Eukaryot Cell 6:1511–1520. doi: 10.1128/EC.00173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng Q, Cloonan N, Fischer K, Thompson J, Waine G, Lanzer M, Saul A. 1998. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol Biochem Parasitol 97:161–176. doi: 10.1016/s0166-6851(98)00144-3. [DOI] [PubMed] [Google Scholar]
- 75.Frech C, Chen N. 2013. Variant surface antigens of malaria parasites: functional and evolutionary insights from comparative gene family classification and analysis. BMC Genomics 14:427. doi: 10.1186/1471-2164-14-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharp S, Lavstsen T, Fivelman QL, Saeed M, McRobert L, Templeton TJ, Jensen AT, Baker DA, Theander TG, Sutherland CJ. 2006. Programmed transcription of the var gene family, but not of stevor, in Plasmodium falciparum gametocytes. Eukaryot Cell 5:1206–1214. doi: 10.1128/EC.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warncke JD, Passecker A, Kipfer E, Brand F, Pérez-Martínez L, Proellochs NI, Kooij TWA, Butter F, Voss TS, Beck HP. 2020. The PHIST protein GEXP02 targets the host cytoskeleton during sexual development of Plasmodium falciparum. Cell Microbiol 22:e13123. doi: 10.1111/cmi.13123. [DOI] [PubMed] [Google Scholar]
- 78.Mwakalinga SB, Wang CW, Bengtsson DC, Turner L, Dinko B, Lusingu JP, Arnot DE, Sutherland CJ, Theander TG, Lavstsen T. 2012. Expression of a type B RIFIN in Plasmodium falciparum merozoites and gametes. Malar J 11:429. doi: 10.1186/1475-2875-11-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petter M, Bonow I, Klinkert MQ. 2008. Diverse expression patterns of subgroups of the rif multigene family during Plasmodium falciparum gametocytogenesis. PLoS One 3:e3779. doi: 10.1371/journal.pone.0003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maier AG, Rug M, O’Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF. 2008. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 134:48–61. doi: 10.1016/j.cell.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aingaran M, Zhang R, Law SK, Peng Z, Undisz A, Meyer E, Diez-Silva M, Burke TA, Spielmann T, Lim CT, Suresh S, Dao M, Marti M. 2012. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell Microbiol 14:983–993. doi: 10.1111/j.1462-5822.2012.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Z, Cui H, Guan J, Liu C, Yang Z, Yuan J. 2021. Plasmodium transcription repressor AP2-O3 regulates sex-specific identity of gene expression in female gametocytes. EMBO Rep 22:e51660. doi: 10.15252/embr.202051660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morillo RC, Tong X, Xie W, Abel S, Orchard LM, Daher W, Patel DJ, Llinás M, Le Roch KG, Kafsack BFC. 2021. The essential transcriptional regulator HDP1 drives expansion of the inner membrane complex during early sexual differentiation of malaria parasites. bioRxiv doi: 10.1101/2020.10.26.352583. [DOI]
- 84.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maere S, Heymans K, Kuiper M. 2005. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PfARID conservation and generation of PfARIDmCherry parasites. (A) ARID domain of ARID proteins from P. falciparum (PfARID), Plasmodium vivax (Pv), Plasmodium berghei (Pb), and Plasmodium yoelii (Py). Conserved residues are in white font on a red background. (B) Homology-based predicted three-dimensional structure of PfARID. PDB template used 4LJX (HsARID3A). (C) The schematic for endogenous tagging of the PfARID locus with mCherry. The pFCL3_ARID_mCherry plasmid has homology regions from 5′ (5′HR) and 3′ (3′HR) of the PfARID locus, single guide RNA-seq (sgRNA), and the human dihydrofolate reductase (hDHFR) locus cloned. (D) Confirmation of PfARIDmCherry parasite generation by diagnostic PCR. The oligonucleotides were designed from outside 5′HR and 3′HR and the PfARID locus, and their positions are indicated by arrows in panel C. (E) The expected sizes for different sets of PCRs. (F and G) IFAs were performed on sexual stages using α-mCherry antibody in combination with H3K9ac. Parasite nucleus was stained with DAPI (blue). Scale bar = 5 μm. Images are shown from representative experiments. Merge I, merged image for red and green channel; merge II, merged image for red and DAPI (blue) channel; GC, gametocytes. Download FIG S1, TIF file, 2.5 MB (2.6MB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Disruption of the PfARID locus via CRISPR/Cas9. (A) The schematic for disrupting the PfARID gene. The pFC_ARID_KO plasmid has homology regions from 5′ (5′HR) and 3′ (3′HR) of the PfARID locus, single guide RNAseq (sgRNA), and the human dihydrofolate reductase (hDHFR) locus cloned. (B) Confirmation of PfARID deletion by diagnostic PCR. The oligonucleotides were designed from outside 5′HR and 3′HR and the PfARID locus, and positions are indicated by arrows in panel A. (C) The expected sizes for different sets of PCRs are indicated. Download FIG S2, TIF file, 0.5 MB (558.4KB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Summary of differentially expressed genes (DEGs). (S1A) Summary of all DEGs. (S1B) Summary of all upregulated DEGs. (S1C) Summary of all downregulated DEGs. (S1D) Summary of gametogenesis-related DEGs. (S1E) Summary of signaling-related DEGs. (S1F) Summary of DEGs, zinc finger proteins. (S1G) Summary of DEGs, AP2-O targets. (S1H) Summary of DEGs, crystalloid. Download Data Set S1, XLSX file, 0.8 MB (862.1KB, xlsx) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Gene Ontology enrichment analysis of DEGs. (S2A) GO term enrichment analysis (molecular function) of upregulated DEGs. (S2B) GO term enrichment analysis (cellular component) of upregulated DEGs. (S2C) GO term enrichment analysis (molecular function) of downregulated DEGs. (S2D) GO term enrichment analysis (cellular component) of downregulated DEGs. Download Data Set S2, XLSX file, 0.02 MB (20.3KB, xlsx) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Gene Ontology term enrichment analysis. (A) Molecular function and (B) cellular component terms of upregulated genes. (C) Molecular function and (D) cellular component terms of downregulated genes. Download FIG S3, TIF file, 0.7 MB (689.3KB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PfARID regulates transcription of the heterochromatin-related genes. Heterochromatin-associated gene families with significant dysregulation in Pfarid–. Geometric mean fold changes and P values (two-sided, one sample t test) are indicated. Download FIG S4, TIF file, 0.4 MB (382.4KB, tif) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotides used in the study. Download Table S1, DOCX file, 0.02 MB (23.1KB, docx) .
Copyright © 2022 Kumar et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.