Abstract
Objectives: To determine incidence trends of Staphylococcus aureus bacteremia (SAB) from population-based studies from multiple countries.
Methods: A contemporary systematic review was conducted using Ovid Cochrane Central Register of Controlled Trials (1991+), Ovid Embase (1974+), Ovid Medical Literature Analysis and Retrieval System Online (MEDLINE) (1946+ including epub ahead of print, in-process & other non-indexed citations), and Web of Science Core Collection (Science Citation Index Expanded 1975+ and Emerging Sources Citation Index 2015+). Two authors (J.R.H. and J.A.Q.M.) independently reviewed all studies and included those that reported population-based incidence of SAB in patients aged 18 years and older.
Results: Twenty-six studies met inclusion criteria with the highest number (n=6) of studies conducted in Canada. The incidence of SAB ranged from 9.3 to 65 cases/100,000/year. The median age of patients with SAB ranged from 62 to 72 years and SAB cases were more commonly observed in men than in women. The most common infection sources were intravascular catheters and skin and soft tissue infections. SAB incidence trends demonstrated high variability for geographic regions and calendar years. Overall, there was no change in the incidence trend across all studies during the past two decades.
Conclusion: Multiple factors, both pros, and cons are likely responsible for the overall stable SAB incidence in countries included in this systematic review. Some of these factors vary in geographic location and prompt additional investigations from countries not included in the current review so that a more global characterization is defined.
Keywords: staphylococcus aureus, bacteremia, trends, population-based study, incidence
Introduction and background
Staphylococcus aureus is a predominate pathogen in cases of bacteremia in both the community and healthcare settings [1,2], with a mortality rate as high as 30% [3]. The incidence of S. aureus bacteremia (SAB) varies depending on risk factors of the studied population and infection control practices in healthcare facilities. Surveillance of SAB trend incidence is crucial to evaluate its impact on public health agencies and to enhance the development of infection prevention and control strategies [4]. Unlike hospital-based studies, population-based studies prevent selection biases and allow standardization of incidence rates for a reference population, making them one of the best tools to assess infectious diseases epidemiology [5]. The most recent systematic review of population-based studies assessing the incidence of bacteremias due to a variety of organisms was conducted in 2013, with incidence rates reported through 2008 [6]. Therefore, we conducted a systematic review of contemporary population-based studies investigating the incidence of SAB from numerous geographic regions during the past two decades as presented herein.
Review
Methods
Data Sources and Searches
A literature search was done by a medical librarian (D.J.G.) for the concepts of SAB and incidence rates. Search strategies were created using a combination of keywords and standardized index terms. Searches were run on September 2, 2021, in Ovid Cochrane Central Register of Controlled Trials (1991+), Ovid Embase (1974+), Ovid Medical Literature Analysis and Retrieval System Online (MEDLINE) (1946+ including epub ahead of print, in-process, and other non-indexed citations), and Web of Science Core Collection (Science Citation Index Expanded 1975+ and Emerging Sources Citation Index 2015+). After limiting the results based on exclusion criteria (listed below), a total of 26,552 citations were retrieved. Deduplication was performed in Covidence leaving 16,499 citations. Full search strategies are provided in the appendices. Two reviewers (J.R.H. and J.A.Q.M.) performed the literature review and any disagreements were solved by a discussion with an additional reviewer (L.M.B.). This review was registered in Open Science Framework (OSF).
Inclusion and Exclusion Criteria
All population-based investigations published in the English language from 2001 through 2020 on SAB incidence trends in the adult population were included in this review. Studies that did not report population-based estimates were not included, such as case reports, single-center, and multi-center studies, clinical trials, conference abstracts, systematic reviews, and studies on animals [7]. Studies conducted that focused on specific sub-groups (e.g., patients with HIV infection or end-stage renal disease [ESRD]) or published in a language other than English were also excluded.
Data Extraction
The following information was extracted from the included studies: first author's last name, year of publication, country of origin (+/- specific region studied), population size, and incidence rate (per 100,000 person-year). Two reviewers (J.R.H. and J.A.Q.M.) extracted data from the studies independently; study investigators were contacted if additional information was needed. The incidence rate of SAB was the primary outcome. Secondary outcomes were age, sex, comorbidities, methicillin resistance of the blood culture isolate, site of onset, and mortality rates.
Statistical Analysis
When available, incidence rate estimates from separate periods within each study were extracted. If estimates from separate periods were not available, overall incidence rate estimates were used. When necessary, these incidence rate estimates were transformed to represent incidence rates per 100,000 population. To visualize the data, incidence rate estimates were plotted by time point and study. For periods where reported incidence rates spanned multiple years, the median of the period was chosen for plotting. To look at trends across the entire period of interest, a simple linear regression model was fit to the individual estimates to help visually interpret the overall trend over time. The SAS, version 9.4 (SAS Institute, Cary, NC, USA) was used for plotting and regression.
Results
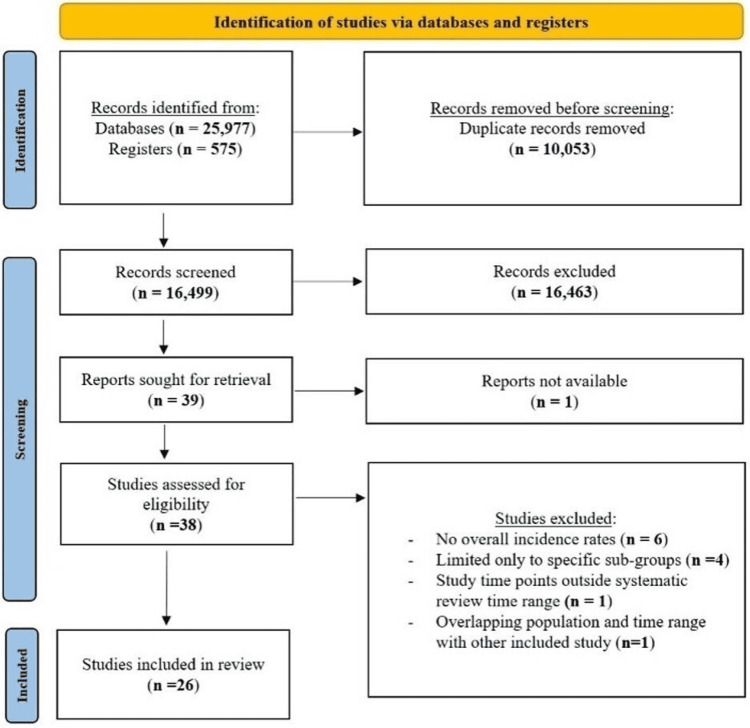
After removing all duplicates, a total of 16,499 citations were identified from the search engines (supplemental data) and their abstracts were screened. Thirty-eight of them were chosen for full-text review and 26 studies met inclusion criteria (Table 1).
Table 1. Incidence rates of SAB reported in the 26 included population-based studies.
SAB: Staphylococcus aureus bacteremia
*This study included only community-onset SAB
| First author, publication year | Country (+/- region) | Years | Population | SAB/105/year |
| Mejer et al., 2012 [8] | Denmark | 1995–2008 | 5,350,000 | 22.7 |
| Nielsen et al., 2014 [9] | Denmark, Funen County | 2000–2008 | 390,000 | 27.3 |
| Thorlacius-Ussing et al., 2019 [10] | Denmark | 2008–2015 | 5,475,791 | 24.9 |
| Wilson et al., 2011 [11] | England | 2004–2008 | 61,500,000 | 21.8 |
| Lyytikäinen et al., 2005 [12] | Finland | 1995–2001 | 5,000,000 | 14 |
| Skogberg et al., 2012 [13] | Finland | 2004–2007 | 5,300,000 | 20 |
| Jokinen et al., 2018 [14] | Finland, Pirkanmaa County | 2005–2015 | 543,700 | 31.5 |
| Asgeirsson et al., 2011 [15] | Iceland | 1995–2008 | 300,000 | 24.5 |
| Asgeirsson et al., 2011 [16] | Iceland | 2003–2008 | 300,000 | 29 |
| Mehl et al., 2017 [17] | Norway, Nord-Trøndelag County | 2002–2013 | 72,000 | 25 |
| Blomfeldt et al., 2016 [18] | Norway, Oslo | 2011–2014 | 500,000 | 27.6 |
| Ruiz-Azcona et al., 2020 [19] | Spain, Valencia | 2013–2017 | 5,000,000 | 9.3 |
| Allard et al., 2008 [20] | Canada, Quebec | 1991–2008 | 445,000 | 27.6 |
| Laupland et al., 2013 [21] | Canada, Victoria | 1998–2005 | 750,000 | 15.5 |
| *Laupland et al., 2007 [22] | Canada, Calgary | 2000–2004 | 1,000,000 | 13.5 |
| Laupland et al., 2008 [23] | Canada, Calgary | 2000–2006 | 1,000,000 | 19.7 |
| Lam et al., 2019 [24] | Canada, Calgary | 2012–2014 | 1,288,000 | 27.8 |
| Laupland et al., 2021 [25] | Canada, British Columbia | 2010–2020 | 191,000 | 31.9 |
| Morin et al., 2001 [26] | USA, Connecticut | 1998 | 1,124,337 | 20.9 |
| El Atrouni et al., 2009 [27] | USA, Minnesota, Olmsted County | 1998–2005 | 124,277 | 38.2 |
| Hindy et al., 2022 [28] | USA, Minnesota, Olmsted County | 2006–2020 | 164,365 | 31.1 |
| Tong et al., 2009 [29] | Australia, Northern territory | 2006–2007 | 176,000 | 65 |
| Tong et al., 2012 [30] | Australia | 2007–2010 | 21,750,000 | 11.2 |
| Mitchell et al., 2012 [31] | Australia, Tasmania | 2009–2010 | 503,292 | 21.3 |
| Huggan et al., 2010 [32] | New Zealand, Canterbury | 1998–2006 | 478,000 | 21.5 |
| Laupland et al., 2013 [33] | Australia, Canberra, Queanbeyan, and New South Wales; Canada, Sherbrooke (Quebec), Victoria (British Columbia), and Calgary (Alberta); Denmark, North Denmark; Finland; Sweden, Skaraborg County | 2000–2008 | 83,000,000 | 26.1 |
Figure 1 is a flow diagram showing the included studies as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [34].
Figure 1. Flow diagram of study selection per 2020 PRISMA guidelines.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
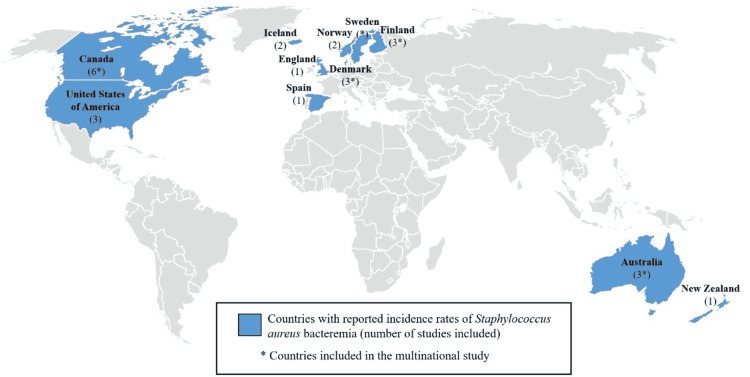
The highest number (n=6) of studies were from Canada; other countries that were included are shown in Figure 2. Of note, 20 (76.9%) of the 26 investigations were regional studies.
Figure 2. Geographical representation of the 11 countries included in the systematic review.
Overall Incidence
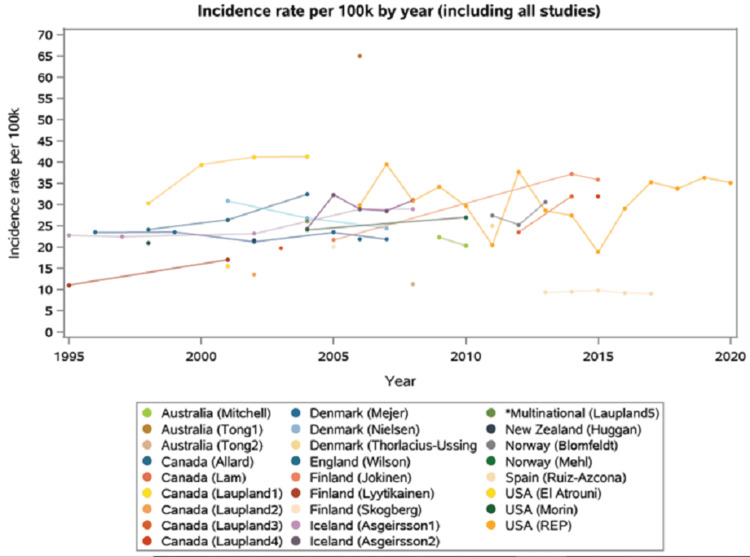
Fourteen studies reported temporal trends in SAB incidences and 12 represented only one time point (Figure 3).
Figure 3. Incidence rate per 100,000/year in included studies.
*The multinational study included five countries: Australia, Canada, Denmark, Finland, and Sweden
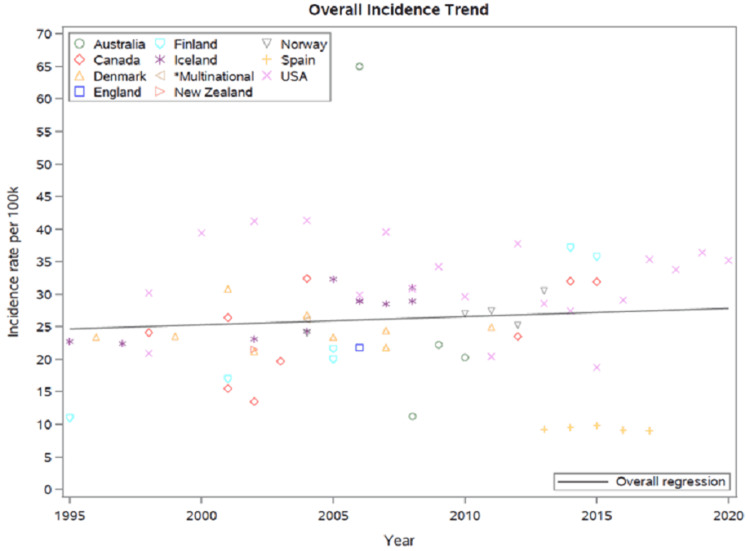
Trends across all included studies demonstrated high variability for geographic regions and calendar years with a SAB incidence ranging from 9.3 to 65 cases/100,000/year. While some showed an increasing incidence of SAB, others revealed decreasing or even unchanging rates over time. Overall, there was no change in the incidence trend across all studies during the past two decades (Figure 4).
Figure 4. Overall incidence trend of SAB in included studies.
SAB: Staphylococcus aureus bacteremia
*The multinational study included five countries: Australia, Canada, Denmark, Finland, and Sweden.
Patient Demographics
The median age of patients with SAB ranged from 62 to 72 years [13,15,20-22,25,27,28,31,32], with the oldest populations from Olmsted County in Minnesota (MN), USA and Oslo, Norway [18,27]. In most of the countries, the rates of SAB were rising with increasing age [8,10,12,14,15,20,26-28,31,32]. However, Ruiz-Azcona et al. reported higher SAB rates in the age range between 45 and 64 years and lower rates in patients aged 65 years and older in Spain [19]. When comparing the two periods of time from 1995 to 1997 and 2004 to 2006, Mejer et al. demonstrated an increase in the proportion of cases in patients older than 75 years and a decrease in the proportion of cases aged one to 55 years [8]. During the past two decades, more than half of SAB cases reported occurred in men [11,13,15,17,18,20-22,25,27,31,32]. Moreover, higher rates of SAB were reported in men than in women in Norway, Denmark, Finland, and Spain [8,10,12,14,19].
In the Danish study by Mejer et al., the proportion of SAB patients with a Charlson comorbidity index (CCI) >0 increased from 1995 to 2008 and the majority of this population had a CCI of 1-2 (39.1%) [8]. In addition, another Danish study restricted to Funen County showed that most of the SAB patients had no comorbidities [9]. However, the majority of the Danish population according to Thorlacius-Ussing et al. had a CCI >3 [10], similar to the SAB populations in Quebec, Canada, and Oslo, Norway [18,20]. Three-fourths of the Canadian population in Calgary from 2000 to 2006 had significant chronic comorbid conditions and/or alcohol use disorder [23]. Compared to SAB patients with no comorbidities, there was increased mortality with increased CCI scores [10].
The prevalence of diabetes mellitus in patients with SAB ranged from 13% to 38% with the highest numbers observed in the USA [16,24,26,27,29]. Moreover, two Canadian studies demonstrated the relative risk of developing SAB in patients with diabetes mellitus was seven to 10.6 [23,24]. The percentage of SAB patients undergoing hemodialysis in the Icelandic and Australian populations ranged between 7.5% and 10.5% [16,30]. However, El Atrouni et al. reported a slightly higher (18.6%) percentage of patients with end-stage renal disease in Olmsted County, MN [27]. A Canadian investigation demonstrated a relative risk of developing SAB in patients undergoing hemodialysis of 360 [23]. According to Tong et al., 33.9% of SAB cases in Australia from 2007 to 2010 were device-related [30]. Ten percent of SAB patients from Calgary, Canada had a prosthetic joint and 5.6% had a permanent pacemaker or implantable cardioverter-defibrillator (ICD) [24].
Only six studies from Australia, Iceland, the USA, and Canada reported percentages of injection drug use (IDU) in their populations [24,26,27,29,30]. The highest number (28%) was observed by Morin et al. in Connecticut, USA in 1998 [26]. However, El Atrouni et al. described a lower percentage (1.6%) in Olmsted County, MN from 1998-to 2005 [27], which was comparable to that reported in Australian and Icelandic studies (0.74% to 5.7%) [16,29,30]. In addition, Lam et al. noted that 18.7% of the population was abusing substances and was not limited to IDU [24].
Based on the New Zealand deprivation index, the least deprived patients had a significantly lower incidence rate of SAB compared to the most deprived [32].
Staphylococcus aureus Bacteremia Characteristics
The highest percentages of methicillin-resistant Staphylococcus aureus (MRSA) causing SAB were reported in Canada and the USA (9% to 32%) [20,24-27]; whereas the lowest percentages were described in Norway, Iceland, Denmark, and New Zealand (0.4% to 1.7%) [10,15,18,32]. Two studies from Australia reported different percentages of MRSA in SAB depending on the region (24% in the Northern Territory and 10% in Tasmania) [29,31]. Jokinen et al. reported decreased incidence rates of SAB caused by MRSA in Pirkanmaa County, Finland during the period 2005 to 2015 [14].
The majority of SAB cases from Iceland and Canada were nosocomial (39% to 46%) [15,23], from Norway, USA, and Denmark healthcare-associated (42% to 59%) [8,18,27], and from New Zealand and Australia community-acquired (58% to 64%) [31,32]. In Pirkanmaa County, Finland, the number of healthcare-acquired and community-acquired SAB increased from 2005 to 2015 [14]. Ruiz-Azcona et al. demonstrated an incidence rate of 18.87 per 100,000 inhabitants/year for nosocomial SAB [19].
The most common infection sources of SAB were intravascular catheters and skin and soft tissue infections [20,23,29,30], although four studies from Norway, Iceland, USA, and Canada reported that the majority of their observed SAB did not have an identified source of infection [16,18,24,27]. Other sources were also identified, including infective endocarditis, bone or joint, and respiratory tract infections [16,20,23,24,27,30].
Mortality
The majority of included studies reporting annual mortality rates of SAB in their populations during the past two decades were from Europe [10,12,14-18], with numbers ranging from two to 7.6 per 100,000 inhabitants per year [10,12,14-18,23,32]. The two studies from Iceland demonstrated a gradual decrease in their annual SAB death rates during the periods of 1995 to 2008 and 2003 to 2008 [15,16].
The 30-day mortality rates of SAB ranged from 18% to 29% [10,18,20,25], with the highest number observed in Quebec, Canada from 1997 to 1999 [20]. There was rising 30-day mortality with age [18,25,32], with increasing underlying comorbidities including immunosuppression [18,20,25], MRSA compared to methicillin-susceptible Staphylococcus aureus (MSSA) [24], and with healthcare-associated (HCA)-SAB compared to care-associated (CA-SAB) [18,25]. In two studies, women with SAB had higher 30-day mortality compared to men [18,20], whereas one study reported higher mortality rates in men [17]. Also, when grouped by potential sources of SAB, mortality was the highest in patients with pneumonia or infective endocarditis followed by an unknown source of infection [18,20,25]. Interestingly, there was a lower 30-day fatality rate when infectious diseases specialists were consulted [25].
Discussion
The current systematic review represents a contemporary review of SAB incidence in 26 geographical population-based investigations over two decades and demonstrates the striking burden of disease due to SAB. The incidence of SAB ranged from 9.3 to 65 cases/100,000/year. The highest SAB incidence appeared to be an outlier; it was seen in an aboriginal population of the Northern Territory of Australia and was likely due to unique risk factors associated with SAB [29]. This considerable variability in SAB incidence rates between geographic regions and calendar year is likely due to changes in population demographics, socioeconomic status, cultural influences, clinical practices, including those related to infection prevention and control, and surveillance processes. While some studies showed an increased incidence of SAB, others revealed decreasing rates over time. Overall, SAB incidence trends observed during the study period were stable.
The most recent systematic review of population-based studies was conducted in 2013 and assessed both overall bacteremia incidences and SAB incidence more specifically [6]. It showed high variability in rates according to the geographic location with an overall SAB incidence rate of 25 per 100,000 annually. Moreover, this review did not provide overall incidence trends of SAB or demographic data regarding the SAB population.
North America had higher percentages of MRSA causing SAB [20,24,26,27] as compared to Iceland and Denmark [10,15,32]. In the early 21st century, patients in Northern European countries were undergoing aggressive screening for MRSA with eradication in colonized patients [35]. The implementation of this new policy to search and destroy MRSA could account for the low percentages of MRSA in the region [10,15].
During the past two decades, the highest rates of SAB were observed in the Northern Territory of Australia’s population [29]. When stratified by ethnicity, Aboriginal patients had a six-times higher rate of SAB than non-Aboriginal patients. This significantly correlates with socioeconomic disadvantages related to access to healthcare and education, as well as housing and employment in this population as decreasing socioeconomic status was linked to an increased risk of SAB [32,36].
A Danish study reported high incidence rates of SAB in an HIV-infected population that had IDU from 1995 to 2007 [37]. The percentage of IDU in a SAB population was the highest (28%) in Connecticut, USA in 1998 [26]. In contrast, a much lower (1.6%) percentage was reported more recently in MN, USA [27]; of course, this could be an underestimation since IDU can be underreported, or related to geographic heterogeneity related to opioid use in more rural areas of the USA [38]. The highest percentages (9% to 32%) of MRSA causing SAB were also observed in the USA [26,27], which could be explained by the rising IDU prevalence since one-fourth of SAB caused by MRSA were IDU-related in the USA [39].
One-third of SAB cases were device-related [30]; the increasing utilization of indwelling foreign devices including vascular catheters, and orthopedic prostheses in clinical practice is a well-established risk factor associated with SAB [40]. Also, patients undergoing hemodialysis have an increased SAB incidence rate, particularly when chronic indwelling vascular catheters are used for hemodialysis access [23,41]. Of note, patients are most at risk during the first three months of hemodialysis [41].
There were several limitations to this systematic review despite its rigorousness. First, while multinational, studies included in our review do not represent a global aspect of SAB incidence since it does not include coverage of many geographic regions, namely Africa, Asia, and South and Central America, highlighting the need for population-based investigations from regions worldwide. Second, while our systematic review included all studies, regardless of geographic location, the inclusion of non-English language articles could have enhanced the likelihood of finding data from other countries, particularly developing countries. Third, not all included studies provided consistent data regarding their population demographics, comorbidities, and mortality rates. Fourth, some studies reported incidence trends of SAB, whereas others reported only one time point; this may have contributed to the absence of clear change in SAB incidence trends overall.
Conclusions
The current systematic review represents the most contemporary review of SAB incidence in 26 geographical population-based investigations during the past two decades. It shows an overall stable SAB incidence in the 11 countries included ranging from 9.3 to 65 cases/100,000/year. Multiple factors, both pros, and cons are likely responsible for the overall stable SAB incidence. Some of these factors vary in geographic location and prompt additional investigations from countries not included in the current review are required so that a more global characterization of SAB is defined.
Acknowledgments
The authors are extremely grateful for the philanthropic support provided by a gift from Eva and Gene Lane (L.M.B.), which was paramount in our work to advance the science of cardiovascular infections, an ongoing focus of investigation at Mayo Clinic for over 60 years.
Appendices
Table 2. The number of citations per database and registers .
MEDLINE: Medical Literature Analysis and Retrieval System Online
| Databases & Registers | # of initial hits |
| Central (clinical trial register) | 575 |
| Embase | 10,880 |
| MEDLINE | 6,134 |
| Web of Science | 8,963 |
| Duplicates | 10,053 |
| Totals | 16,499 |
Table 3. Cochrane Central Register of Controlled Trials (CCTR) via Ovid (1991+)).
| # | Query | Results from 2 Sep 2021 |
| 1 | (staph* or s-aureus).ab,hw,ti. | 6,268 |
| 2 | (epidemiology* or incidence or surveillance or biosurveillance or population* or cohort* or prevalence or cross-section* or seroepidemiology* or frequency* or occur* or rate or rates or morbidity or mortality or trend* or distribution or geography*).ab,hw,ti. | 830,218 |
| 3 | ((blood* adj1 (infection* or poisoning*)) or bacteremia* or bacteraemia* or septic* or sepsis or bacillemia* or bacillaemia* or bacteriemia* or pyemi* or pyaemi* or pyohemi*).ab,hw,ti. | 19,172 |
| 4 | 1 and 2 and 3 | 912 |
| 5 | (infant or infants or neonate* or newborn* or toddler* or preschool* or pre-school* or child* or pediatric* or paediatric* or girl* or boy* or adolesc* or preteen* or teen* or youth*).m_titl. | 143,148 |
| 6 | 4 not 5 | 768 |
| 7 | limit 6 to yr="2000 -Current" | 575 |
Table 4. Embase via Ovid (1974+).
| # | Query | Results from 2 Sep 2021 |
| 1 | exp Staphylococcus aureus/ or exp Staphylococcus infection/ | 207,435 |
| 2 | (staph* or s-aureus).ab,dj,kw,ti. | 203,208 |
| 3 | 1 or 2 | 273,335 |
| 4 | exp epidemiological data/ or exp epidemiology/ | 4,555,005 |
| 5 | exp trend study/ | 39,019 |
| 6 | (epidemiolog* or incidence or surveillance or biosurveillance or population* or cohort* or prevalence or cross-section* or seroepidemiolog* or frequenc* or occur* or rate or rates or morbidity or mortality or trend* or distribution or geograph*).ab,dj,kw,ti,fx,sh. | 12,961,416 |
| 7 | 4 or 5 or 6 | 13,631,494 |
| 8 | 3 and 7 | 111,463 |
| 9 | bacteremia/ or staphylococcal bacteremia/ or exp bloodstream infection/ or sepsis/ or ((blood* adj1 (infection* or poisoning*)) or bacteremia* or bacteraemia* or septic* or sepsis or bacillemia* or bacillaemia* or bacteriemia* or pyemi* or pyaemi* or pyohemi*).ab,kw,ti. | 353,305 |
| 10 | 8 and 9 | 23,047 |
| 11 | limit 10 to English language | 21,167 |
| 12 | limit 11 to yr="2000 -Current" | 18,670 |
| 13 | exp juvenile/ not adult/ | 2,363,604 |
| 14 | 12 not 13 | 15,696 |
| 15 | limit 14 to conference abstract | 4,299 |
| 16 | 14 not 15 | 11,397 |
| 17 | exp animal/ not exp human/ | 4,829,043 |
| 18 | 16 not 17 | 10,880 |
Table 5. MEDLINE via Ovid (1946+ and Epub ahead of print, in-process and other non-indexed citations and Ovid MEDLINE(R) Daily).
MEDLINE: Medical Literature Analysis and Retrieval System Online
| # | Query | Results from 2 Sep 2021 |
| 1 | exp Staphylococcus aureus/ or exp Staphylococcal Infections/ | 115,177 |
| 2 | (staph* or s-aureus).ab,kw,ti. | 151,134 |
| 3 | 1 or 2 | 179,717 |
| 4 | exp Morbidity/ or exp Mortality/ | 962,601 |
| 5 | (epidemiolog* or incidence or surveillance or biosurveillance or population* or cohort* or prevalence or cross-section* or seroepidemiolog* or frequenc* or occur* or rate or rates or morbidity or mortality or trend* or distribution or geograph*).ab,hw,kw,ti,fx. | 9,233,452 |
| 6 | 4 or 5 | 9,287,700 |
| 7 | 3 and 6 | 65,570 |
| 8 | exp Bacteremia/ or Sepsis/ or ((blood* adj1 (infection* or poisoning*)) or bacteremia* or bacteraemia* or septic* or sepsis or bacillemia* or bacillaemia* or bacteriemia* or pyemi* or pyaemi* or pyohemi*).ab,kf,ti. | 196,177 |
| 9 | 7 and 8 | 11,385 |
| 10 | limit 9 to English language | 9,883 |
| 11 | limit 10 to yr="2000 -Current" | 7,202 |
| 12 | (exp CHILD/ or Adolescent/) not exp ADULT/ | 1,500,200 |
| 13 | 11 not 12 | 6,528 |
| 14 | exp Animals/ not Humans/ | 4,879,221 |
| 15 | 13 not 14 | 6,134 |
Figure 5. Web of Science core collection via Clarivate Analytics (Science Citation Index Expanded 1975+ and Emerging Sources Citation Index 2015+).
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared financial relationships, which are detailed in the next section.
Larry M. Baddour declare(s) personal fees from Roivant Sciences, Botanix Pharmaceuticals, and Boston Scientific.
References
- 1.Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Uslan DZ, Crane SJ, Steckelberg JM, Cockerill FR, St Sauver JL, Wilson WR, Baddour LM. Arch Intern Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 2.Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Chang F-Y, MacDonald BB, Peacock JE, et al. Medicine (Baltimore. 2003;82:322–332. doi: 10.1097/01.md.0000091185.93122.40. [DOI] [PubMed] [Google Scholar]
- 4.Enhanced surveillance of Staphylococcus aureus bacteraemia to identify targets for infection prevention. Morris AK, Russell CD. J Hosp Infect. 2016;93:169–274. doi: 10.1016/j.jhin.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Importance of population-based studies in clinical practice. Ronnie G, Ve RS, Velumuri L, Asokan R, Vijaya L. Indian J Ophthalmol. 2011;59:0. doi: 10.4103/0301-4738.73681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Incidence of bloodstream infection: a review of population-based studies. Laupland KB. Clin Microbiol Infect. 2013;19:492–500. doi: 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- 7.How should systematic reviewers handle conference abstracts? A view from the trenches. Scherer RW, Saldanha IJ. Syst Rev. 2019;8:264. doi: 10.1186/s13643-019-1188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 9.Population-based burden of bloodstream infections in Finland. Skogberg K, Lyytikäinen O, Ollgren J, Nuorti JP, Ruutu P. Clin Microbiol Infect. 2012;18:170–176. doi: 10.1111/j.1469-0691.2012.03845.x. [DOI] [PubMed] [Google Scholar]
- 10.Temporal trends in the incidence of Staphylococcus aureus bacteremia in Olmsted County, Minnesota, 1998 to 2005: a population-based study. El Atrouni WI, Knoll BM, Lahr BD, Eckel-Passow JE, Sia IG, Baddour LM. Clin Infect Dis. 2009;49:0. doi: 10.1086/648442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991-2005. Allard C, Carignan A, Bergevin M, et al. Clin Microbiol Infect. 2008;14:421–428. doi: 10.1111/j.1469-0691.2008.01965.x. [DOI] [PubMed] [Google Scholar]
- 12.Burden of community-onset bloodstream infection: a population-based assessment. Laupland KB, Gregson DB, Flemons WW, Hawkins D, Ross T, Church DL. Epidemiol Infect. 2007;135:1037–1042. doi: 10.1017/S0950268806007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Population-based laboratory assessment of the burden of community-onset bloodstream infection in Victoria, Canada. Laupland KB, Kibsey PC, Gregson DB, Galbraith JC. Epidemiol Infect. 2013;141:174–180. doi: 10.1017/S0950268812000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The epidemiology of Staphylococcus aureus bacteraemia in Tasmania. Mitchell BG, Gardner A, Stewart L. Healthcare Infection. 2012;17:98–103. [Google Scholar]
- 15.Population-based epidemiology of Staphylococcus aureus bloodstream infection in Canterbury, New Zealand. Huggan PJ, Wells JE, Browne M, Richardson A, Murdoch DR, Chambers ST. Intern Med J. 2010;40:117–125. doi: 10.1111/j.1445-5994.2009.01910.x. [DOI] [PubMed] [Google Scholar]
- 16.Staphylococcus aureus bacteraemia in Iceland, 1995-2008: changing incidence and mortality. Asgeirsson H, Gudlaugsson O, Kristinsson KG, Heiddal S, Kristjansson M. Clin Microbiol Infect. 2011;17:513–518. doi: 10.1111/j.1469-0691.2010.03265.x. [DOI] [PubMed] [Google Scholar]
- 17.Staphylococcus aureus bloodstream infection: Secular changes associated with the implementation of a de novo clinical infectious diseases service in a Canadian population. Laupland KB, Steele L, Pasquill K, Parfitt EC. Int J Infect Dis. 2021;104:45–49. doi: 10.1016/j.ijid.2020.12.064. [DOI] [PubMed] [Google Scholar]
- 18.Incidence of monomicrobial Staphylococcus aureus bacteremia: a population-based study in Olmsted County, Minnesota - 2006 to 2020. Hindy J-R, Quintero-Martinez JA, Lahr BD, et al. Open Forum Infect Dis. 2022;190 doi: 10.1093/ofid/ofac190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Population-based epidemiology of Staphylococcus aureus bloodstream infection: clonal complex 30 genotype is associated with mortality. Blomfeldt A, Eskesen AN, Aamot HV, Leegaard TM, Bjørnholt JV. Eur J Clin Microbiol Infect Dis. 2016;35:803–813. doi: 10.1007/s10096-016-2601-4. [DOI] [PubMed] [Google Scholar]
- 20.Stable incidence and continued improvement in short term mortality of Staphylococcus aureus bacteraemia between 1995 and 2008. Mejer N, Westh H, Schønheyder HC, Jensen AG, Larsen AR, Skov R, Benfield T. BMC Infect Dis. 2012;12:260. doi: 10.1186/1471-2334-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Age-dependent increase in incidence of Staphylococcus aureus bacteremia, Denmark, 2008-2015. Thorlacius-Ussing L, Sandholdt H, Larsen AR, Petersen A, Benfield T. Emerg Infect Dis. 2019;25 doi: 10.3201/eid2505.181733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trends in incidence and resistance patterns of Staphylococcus aureus bacteremia. Jokinen E, Laine J, Huttunen R, Lyytikäinen O, Vuento R, Vuopio J, Syrjänen J. Infect Dis (Lond) 2018;50:52–58. doi: 10.1080/23744235.2017.1405276. [DOI] [PubMed] [Google Scholar]
- 23.Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995-2001. Lyytikäinen O, Ruotsalainen E, Järvinen A, Valtonen V, Ruutu P. Eur J Clin Microbiol Infect Dis. 2005;24:399–404. doi: 10.1007/s10096-005-1345-3. [DOI] [PubMed] [Google Scholar]
- 24.Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. Morin CA, Hadler JL. J Infect Dis. 2001;184:1029–1034. doi: 10.1086/323459. [DOI] [PubMed] [Google Scholar]
- 25.Etiology of bloodstream infections at a population level during 2013-2017 in the autonomous community of Valencia, Spain. Ruiz-Azcona L, Santibañez M, Gimeno A, et al. Rev Esp Quimioter. 2020;33:200–206. doi: 10.37201/req/024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trends among pathogens reported as causing bacteraemia in England, 2004-2008. Wilson J, Elgohari S, Livermore DM, et al. Clin Microbiol Infect. 2011;17:451–458. doi: 10.1111/j.1469-0691.2010.03262.x. [DOI] [PubMed] [Google Scholar]
- 27.Burden of bloodstream infection in an area of Mid-Norway 2002-2013: a prospective population-based observational study. Mehl A, Åsvold BO, Lydersen S, et al. BMC Infect Dis. 2017;17:205. doi: 10.1186/s12879-017-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decreasing incidence rates of bacteremia: a 9-year population-based study. Nielsen SL, Pedersen C, Jensen TG, Gradel KO, Kolmos HJ, Lassen AT. J Infect. 2014;69:51–59. doi: 10.1016/j.jinf.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. Laupland KB, Ross T, Gregson DB. J Infect Dis. 2008;198:336–343. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 30.Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous Northern Australia: epidemiology and outcomes. Tong SYC, Bishop EJ, Lilliebridge RA, et al. J Infect Dis. 2009;199:1461–1470. doi: 10.1086/598218. [DOI] [PubMed] [Google Scholar]
- 31.Epidemiology and outcome determinants of Staphylococcus aureus bacteremia revisited: a population-based study. Lam JC, Gregson DB, Robinson S, Somayaji R, Conly JM, Parkins MD. Infection. 2019;47:961–971. doi: 10.1007/s15010-019-01330-5. [DOI] [PubMed] [Google Scholar]
- 32.Staphylococcus aureus bacteraemia--nationwide assessment of treatment adequacy and outcome. Asgeirsson H, Kristjansson M, Kristinsson KG, Gudlaugsson O. J Infect. 2011;62:339–346. doi: 10.1016/j.jinf.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Impact of ethnicity and socio-economic status on Staphylococcus aureus bacteremia incidence and mortality: a heavy burden in Indigenous Australians. Tong SY, van Hal SJ, Einsiedel L, Currie BJ, Turnidge JD. BMC Infect Dis. 2012;12:249. doi: 10.1186/1471-2334-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adjustment of the MRSA search and destroy policy for outpatients in the Netherlands: a prospective cohort study with repeated prevalence measurements. van Rijen MM, Kluytmans JA. Antimicrob Resist Infect Control. 2014;3:3. doi: 10.1186/2047-2994-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The associations between socioeconomic status and risk of Staphylococcus aureus bacteremia and subsequent endocarditis - a Danish nationwide cohort study. Oestergaard LB, Schmiegelow MD, Bruun NE, Skov RL, Petersen A, Andersen PS, Torp-Pedersen C. BMC Infect Dis. 2017;17:589. doi: 10.1186/s12879-017-2691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Major but differential decline in the incidence of Staphylococcus aureus bacteraemia in HIV-infected individuals from 1995 to 2007: a nationwide cohort study. Larsen MV, Harboe ZB, Ladelund S, et al. HIV Med. 2012;13:45–53. doi: 10.1111/j.1468-1293.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- 37.Opioid-related mortality in rural America: geographic heterogeneity and intervention strategies. Rigg KK, Monnat SM, Chavez MN. Int J Drug Policy. 2018;57:119–129. doi: 10.1016/j.drugpo.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Methicillin-resistant Staphylococcus aureus bloodstream infections and injection drug use, Tennessee, USA, 2015-2017. Parikh MP, Octaria R, Kainer MA. Emerg Infect Dis. 2020;26:446–453. doi: 10.3201/eid2603.191408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Steinberg JP, Clark CC, Hackman BO. Clin Infect Dis. 1996;23:255–259. doi: 10.1093/clinids/23.2.255. [DOI] [PubMed] [Google Scholar]
- 40.Increased risk of Staphylococcus aureus bacteremia in hemodialysis - a nationwide study. Chaudry MS, Gislason GH, Kamper A-L, et al. Hemodial Int. 2019;23:230–238. doi: 10.1111/hdi.12728. [DOI] [PubMed] [Google Scholar]
- 41.The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Laupland KB, Lyytikäinen O, Søgaard M, et al. Clin Microbiol Infect. 2013;19:465–471. doi: 10.1111/j.1469-0691.2012.03903.x. [DOI] [PubMed] [Google Scholar]