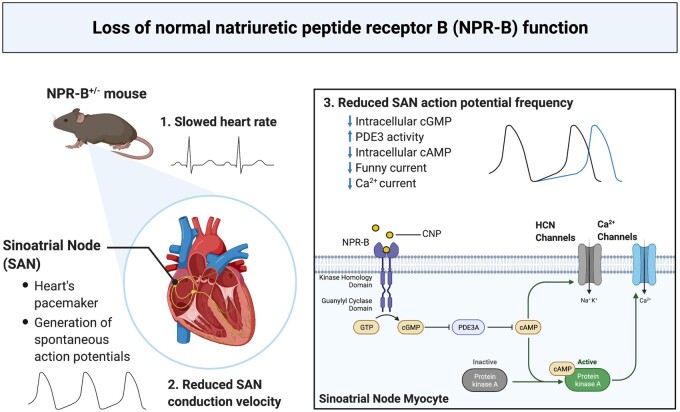
Abstract
Aims
Heart rate (HR) is a critical indicator of cardiac performance that is determined by sinoatrial node (SAN) function and regulation. Natriuretic peptides, including C-type NP (CNP), have been shown to modulate ion channel function in the SAN when applied exogenously. CNP is the only NP that acts as a ligand for natriuretic peptide receptor-B (NPR-B). Despite these properties, the ability of CNP and NPR-B to regulate HR and intrinsic SAN automaticity in vivo, and the mechanisms by which it does so, are incompletely understood. Thus, the objective of this study was to determine the role of NPR-B signalling in regulating HR and SAN function.
Methods and results
We have used NPR-B deficient mice (NPR-B+/−) to study HR regulation and SAN function using telemetry in conscious mice, intracardiac electrophysiology in anaesthetized mice, high-resolution optical mapping in isolated SAN preparations, patch-clamping in isolated SAN myocytes, and molecular biology in isolated SAN tissue. These studies demonstrate that NPR-B+/− mice exhibit slow HR, increased corrected SAN recovery time, and slowed SAN conduction. Spontaneous AP firing frequency in isolated SAN myocytes was impaired in NPR-B+/− mice due to reductions in the hyperpolarization activated current (If) and L-type Ca2+ current (ICa,L). If and ICa,L were reduced due to lower cGMP levels and increased hydrolysis of cAMP by phosphodiesterase 3 (PDE3) in the SAN. Inhibiting PDE3 or restoring cGMP signalling via application of 8-Br-cGMP abolished the reductions in cAMP, AP firing, If, and ICa,L, and normalized SAN conduction, in the SAN in NPR-B+/− mice. NPR-B+/− mice did not exhibit changes in SAN fibrosis and showed no evidence of cardiac hypertrophy or changes in ventricular function.
Conclusions
NPR-B plays an essential physiological role in maintaining normal HR and SAN function by modulating ion channel function in SAN myocytes via a cGMP/PDE3/cAMP signalling mechanism.
Keywords: Natriuretic peptides, Natriuretic peptide receptors, Sinoatrial node, Heart rate, Phosphodiesterase
Graphical Abstract
Graphical Abstract.
1. Introduction
Heart rate (HR) is determined by a specialized group of cells located in the sinoatrial node (SAN) within the right atrium of the heart. The SAN generates spontaneous action potentials (APs), the frequency of which determines intrinsic heart rate (HR) in vivo.1 Spontaneous APs in SAN myocytes occur due to the presence of a diastolic depolarization (DD) between successive APs. The hyperpolarization-activated current (If, carried by HCN channels) and the L-type Ca2+ current (ICa,L, carried by CaV1.2 and CaV1.3 channels) play critical roles in determining the slope of the DD and the frequency of spontaneous AP firing in SAN myocytes.1,2 Conduction within the SAN and from the SAN to the atria is also affected by Na+ current (INa) and gap junctions formed by connexins (Cx).3–5 HR and SAN function can be modified by both neural and humoral factors in order to regulate cardiac performance.6 For example, AP firing and ion channel function in the SAN are regulated by cyclic nucleotides, such as cyclic GMP (cGMP) and cyclic AMP (cAMP), enabling regulation of HR to meet physiological demands.1,6 Cyclic nucleotide levels in cardiomyocytes are critically regulated by phosphodiesterases (PDEs), which are the primary enzymes responsible for the degradation of cGMP and cAMP.7
Natriuretic peptides (NPs) are a family of cardioprotective hormones composed of atrial (ANP), B-type (BNP), and C-type (CNP) NPs that play important roles in maintaining cardiovascular homeostasis.8–10 NPs bind to three distinct membrane receptors (NPRs) called NPR-A, NPR-B, and NPR-C, all of which are expressed in the SAN.8,11 CNP is the lone agonist for NPR-B, which is a particulate guanylyl cyclase (GC) linked receptor that increases intracellular cGMP concentration upon receptor activation.8
NPs are produced in the atrial myocardium where they can be released into the circulation or exert local effects within the heart.8,10 CNP is present at very low levels in the circulation suggesting it mainly acts through local paracrine mechanisms.10 Previous studies demonstrate that acute activation of NPR-B by exogenous application of CNP can potently regulate HR and SAN function.11–14 Furthermore, alterations in the expression of NPR-B have been noted in a number of disease conditions15–18 that are associated with reduced HR or impaired SAN function19,20 suggesting that NPR-B may play an important role in maintaining normal SAN physiology. Nevertheless, how NPR-B contributes to the maintenance of normal intrinsic SAN automaticity in vivo remains incompletely understood. Accordingly, the goal of the present study was to determine how NPR-B deficiency impacts HR and SAN function using mice with a heterozygous deletion of NPR-B (NPR-B+/−). Our findings demonstrate that NPR-B plays an essential physiological role in maintaining SAN automaticity via cGMP-mediated effects on ion channel function in SAN myocytes.
2. Methods
2.1 Mice
This study was conducted using male and female NPR-B+/− mice and wildtype littermate controls. All experimental procedures were approved by the University of Calgary Animal Care and Use Committee or the Dalhousie University Committee for Laboratory Animals and were in accordance with the guidelines of the Canadian Council on Animal Care and the NIH guide for the care and use of laboratory animals.
2.2 Experimental techniques
HR, heart rate variability (HRV), and SAN function were assessed in anaesthetized mice in vivo using intracardiac electrophysiology21,22 or in conscious mice using telemetry.23,24 SAN function and conduction patterns were assessed using high-resolution optical mapping in isolated SAN preparations.12,21 Spontaneous AP firing patterns and ionic currents (If, ICa,L, INa) were investigated using patch-clamping in isolated SAN myocytes.21,22 Cyclic nucleotide (cAMP and cGMP) levels and PDE activity were assessed using commercially available kits. SAN mRNA expression and protein levels were investigated using qPCR and Western blotting.22,25 SAN fibrosis was assessed using histology and immunohistochemistry.22 Cardiac structure was measured in anaesthetized mice by echocardiography.22,26 Blood pressure was measured in conscious, restrained mice by tail-cuff plethysmography.26,27 For surgeries and tissue isolations, mice were anaesthetized using isoflurane (2%, inhalation). Mice were euthanized by cervical dislocation under isoflurane anaesthesia. Expanded details for these experimental techniques are available in the Supplementary material online.
2.3 Statistics
All data are presented as means ± SEM. Statistical analysis was conducted using Prism version 8.3.1 (GraphPad Software). Data were analysed using Student’s t-test, two-way analysis of variance (ANOVA) with a Holm–Sidak posthoc test, two-way repeated-measures ANOVA with a Holm–Sidak posthoc test, or a Fisher’s exact test as indicated in each figure legend. Non-parametric data were analysed by Mann–Whitney U test where indicated. P < 0.05 was considered significant.
3. Results
3.1 Effects of NPR-B deficiency on HR and SAN function in vivo
Initially, the mRNA and protein expression of NPR-A, NPR-B, and NPR-C were measured in the SAN of wildtype and NPR-B+/− mice. NPR-B mRNA and protein levels were reduced by ∼50% in the SAN in NPR-B+/− mice (Supplementary material online, Figure S1). NPR-A and NPR-C mRNA expression and protein levels were unchanged in the SAN of NPR-B+/− mice (Supplementary material online, Figure S1). NPR-B mRNA and protein levels were also reduced in the atria and left ventricle in NPR-B+/− mice (Supplementary material online, Figure S1).
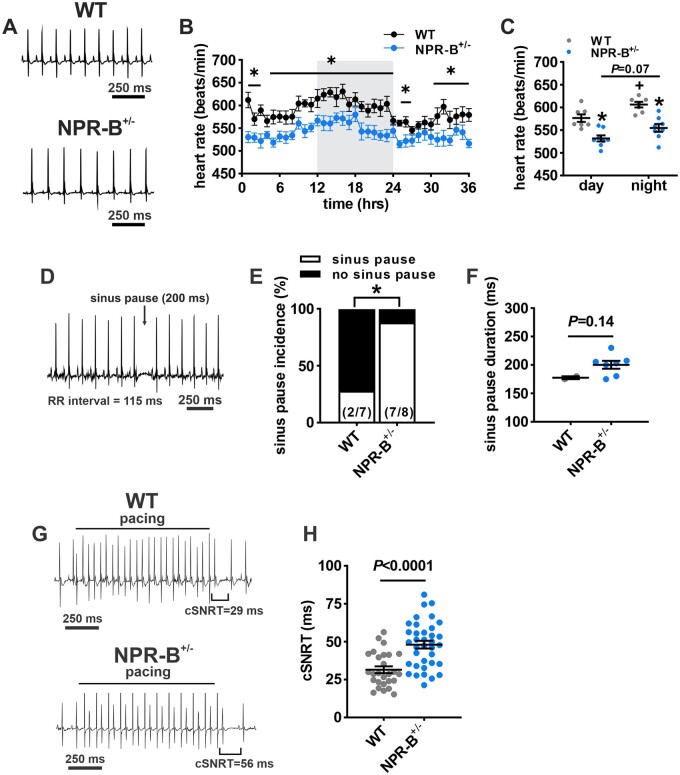
Next, HR was assessed in awake, freely moving wildtype and NPR-B+/− mice using telemetric ECG recordings. HR was reduced in NPR-B+/− mice (Figure 1A,B) as indicated by lower average HRs during the day and night phases of the diurnal cycle (Figure 1C). SAN dysfunction was evident in NPR-B+/− mice as shown by an increase in spontaneous sinus pause incidence compared to wildtype controls (Figure 1D,E). There was no difference in the duration of sinus pauses between genotypes (Figure 1F).
Figure 1.
Heart rate and SAN function in NPR-B+/− mice. (A) Representative telemetric ECG recordings from awake and freely moving wildtype (WT) and NPR-B+/− mice during daytime low activity phase. (B) Heart rate (HR) fluctuations in WT (n = 7 mice) and NPR-B+/− (n = 8 mice) mice over a 36 h period. Grey shading indicates night-time hours (7:00 pm–7:00 am). *P < 0.05 vs. WT at the indicated timepoint by two-way ANOVA with Holm–Sidak post hoc test. (C) Summary of mean HR in WT and NPR-B+/− mice during day and night cycles. *P < 0.05 vs. WT within diurnal phase; +P < 0.05 vs. day within genotype by two-way ANOVA with Holm–Sidak post hoc test. (D) Representative telemetric ECG recording from an NPR-B+/− mouse illustrating the occurrence and duration of sinus pauses. (E) Incidence of sinus pauses in WT and NPR-B+/− mice. Numbers in parentheses indicate the number of mice in each group that developed sinus pauses. *P < 0.05 vs. WT by Fisher’s exact test. (F) Summary of sinus pause duration in the subset of WT (n = 2) and NPR-B+/− (n = 7) mice that exhibited sinus pauses. (G) Representative surface ECG recordings during intracardiac programmed stimulation in anaesthetized WT and NPR-B+/− mice. (H) Summary of cSNRT in WT (n = 27 mice) and NPR-B+/− (n = 36 mice) mice. P < 0.001 vs. WT by Student’s t-test.
SAN function was assessed directly by intracardiac programmed stimulation to measure corrected SAN recovery time (cSNRT) in anaesthetized mice (Figure 1G). These data demonstrate that cSNRT was prolonged in NPR-B+/− mice compared to wildtype controls (Figure 1H). SAN dysfunction can affect male and female patients;28 therefore, HR and cSNRT were also stratified by sex in wildtype and NPR-B+/− mice. HR was reduced and cSNRT recovery time was prolonged similarly in NPR-B+/− mice of both sexes (Supplementary material online, Figure S2); thus, male and female data were combined for subsequent analysis.
HR was also assessed as a function of activity from telemetric ECG recordings (Supplementary material online, Figure S3). As expected, HR was higher during high activity phases; however, HR remained reduced in NPR-B+/− mice during both low and high activity. There were no differences in activity levels between WT and NPR-B+/− mice (Supplementary material online, Figure S3). Maximum HR elicited by the injection of isoproterenol (ISO; 10 mg/kg) was also assessed (Supplementary material online, Figure S3). ISO increased HR similarly in both genotypes such that maximum HR was reduced in NPR-B+/− mice.
Additional measures of cardiac conduction were assessed from the surface and intracardiac ECG recordings that included His bundle signals (Supplementary material online, Figure S4). AV node effective refractory period was prolonged in NPR-B+/− mice; however, there were no differences in PR interval, AH interval, or HV interval between genotypes (Supplementary material online, Figure S4).
3.2 Autonomic nervous system activity in NPR-B deficient mice
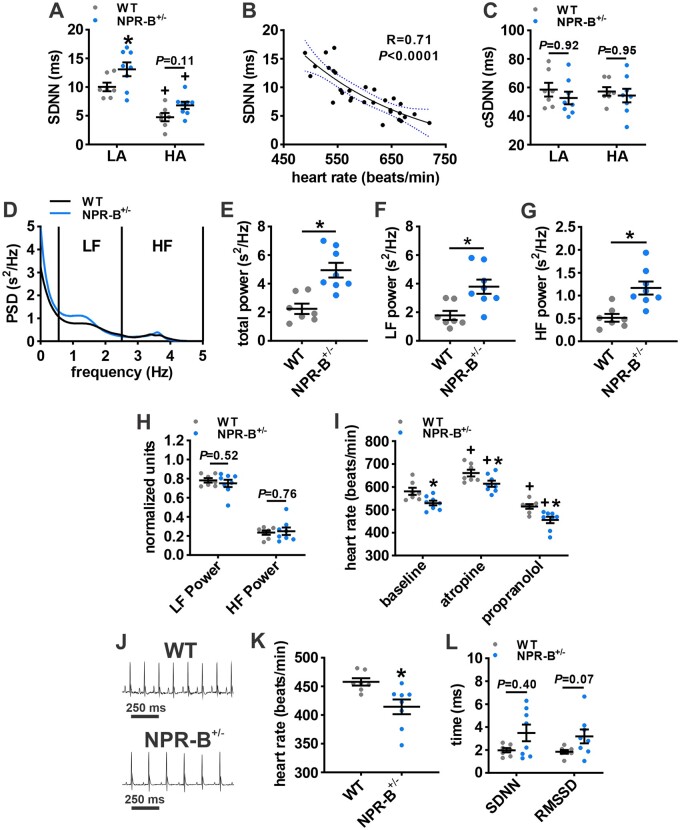
NPs can regulate autonomic nervous system (ANS) function,29 which could have an impact on resting or intrinsic HR. This was assessed by determining if NPR-B deficiency affects heart rate variability (HRV; a surrogate marker of ANS activity30) or the HR response to ANS blockers in conscious mice (Figure 2). Total HRV, as assessed by the standard deviation of R-R intervals (SDNN), was increased in NPR-B+/− mice compared to wildtypes during the low activity phase. High activity reduced SDNN in both groups with NPR-B+/− mice trending (P = 0.11) towards higher SDNN during the high activity phase (Figure 2A). It is important to recognize that HR itself, which is reduced in NPR-B+/− mice, has an important effect on overall HRV.31 Accordingly, SDNN was corrected for HR by plotting SDNN from high and low activity phases in wildtype and NPR-B+/− mice as a function of the corresponding HR and fitting the data with an exponential equation (Figure 2B). Linear regression analysis of the relationship between SDNN and HR shows an R2 value of 0.71 indicating that HR accounts for 71% of the variation in SDNN in these mice. When corrected for HR, there were no differences in SDNN (cSDNN) between wildtype and NPR-B+/− mice during high and low activity phases (Figure 2C).
Figure 2.
Autonomic regulation of HR in NPR-B+/− mice. (A) Summary of variability in RR-interval (SDNN) in WT and NPR-B+/− mice during low activity (LA) and high activity (HA) phases. (B) Plot of SDNN in WT and NPR-B+/− mice as a function of HR. Data were fit with an exponential function to correct for HR as detailed in the methods. Correlation coefficient obtained using Pearson’s correlation. (C) Summary of corrected SDNN (cSDNN) in WT and NPR-B+/− mice. For (A,C), *P < 0.05 vs. WT within activity phase; +P < 0.05 vs. low activity within genotype by two-way ANOVA with Holm–Sidak post hoc test. (D) Representative power spectral density curve for WT and NPR-B+/− mice showing increased power in NPR-B+/− mice and cut-off ranges for low-frequency (LF) and high-frequency (HF) powers. (E–G) Summary of total power (E) LF power (F) and HF power (G) in WT and NPR-B+/− mice. (H) Summary of normalized LF and HF power. For (E–H), *P < 0.01 vs. WT by Student’s t-test. (I) Effects of intraperitoneal injection of atropine (10 mg/kg) or propranolol (10 mg/kg) on HR in WT and NPR-B+/− mice. *P < 0.05 vs. WT within injection condition; +P < 0.05 vs. baseline within genotype by two-way repeated-measures ANOVA with Holm–Sidak post hoc test. (J) Telemetric ECG recordings from WT and NPR-B+/− mice following combined intraperitoneal injection of propranolol and atropine. (K,L), Summary data illustrating HR (K) and time domain measures of intrinsic HRV (SDNN, RMSSD; L) in NPR-B+/− mice following ANS blockade. *P < 0.05 vs. WT by Mann–Whitney Rank Sum test (SDNN) or Student’s t-test (RMSSD). N = 7 WT and 8 NPR-B+/− mice.
Consistent with the increased SDNN in NPR-B+/− mice, power spectral density plots (baseline conditions) demonstrate that total power, low-frequency (LF) power, and high-frequency (HF) power were all increased in NPR-B+/− mice (Figure 2D–G). Similar to cSDNN, when LF power and HF power were normalized to total power, no differences in frequency domain metrics of HRV between wildtype and NPR-B+/− mice were observed (Figure 2H).
HRV was also assessed using Poincaré plots measured at baseline and after autonomic nervous system (ANS) blockade following combined injection of atropine (10 mg/kg) and propranolol (10 mg/kg) (Supplementary material online, Figure S5). Standard deviations from these Poincaré plots (i.e. SD1 and SD2) were larger in NPR-B+/− mice at baseline and after ANS blockade (Supplementary material online, Figure S5).
To further investigate ANS regulation of HR, wildtype and NPR-B+/− mice were injected with atropine (10 mg/kg) and propranolol (10 mg/kg) separately to assess parasympathetic and sympathetic nervous system regulation of HR (Figure 2I). Atropine increased HR while propranolol decreased HR in wildtype and NPR-B+/− mice compared to baseline. These effects were similar in both genotypes and HR remained lower in NPR-B+/− mice in all conditions. Following complete ANS blockade (Supplementary material online, Figure S6), intrinsic HR was lower in NPR-B+/− mice compared to wildtypes (Figure 2J,K). After ANS blockade NPR-B+/− mice showed trends for increases in SDNN and RMSSD (Figure 2L). Collectively, these data indicate that the increase in HRV observed in NPR-B+/− mice is due to changes in intrinsic SAN activity rather than due to changes in sympatho-vagal balance or ANS activity.
3.3 Cardiac structure and blood pressure in NPR-B deficient mice
Echocardiography was used to assess cardiac structure and function in wildtype and NPR-B+/− mice. M-mode imaging of the left ventricle demonstrates there were no differences in left ventricular structure or function between wildtype and NPR-B+/− mice (Supplementary material online, Figure S7). Left ventricular internal diameter, ventricular wall thickness (anterior and posterior), ejection fraction, and fractional shortening were not different between wildtype and NPR-B+/− mice (Supplementary material online, Figure S7). Furthermore, there were no differences in systolic or diastolic blood pressure between genotypes (Supplementary material online, Figure S7). These data demonstrate that reductions in HR and impaired SAN function in NPR-B+/− mice are not related to pathological changes in cardiac structure or alterations in systemic blood pressure.
To assess if the changes in HR and SAN function in NPR-B+/− mice were associated with structural remodelling of the pacemaker complex, SAN fibrosis was measured. Histological sections cut perpendicularly to the crista terminalis through the SAN (identified as the HCN4 positive region) demonstrate that there were no differences in SAN fibrosis between wildtype and NPR-B+/− mice (Supplementary material online, Figure S8). Similarly, there were no differences in interstitial fibrosis in the right atrium or the left ventricle in NPR-B+/− mice (Supplementary material online, Figure S8).
3.4 Effects of NPR-B deficiency on SAN electrical conduction
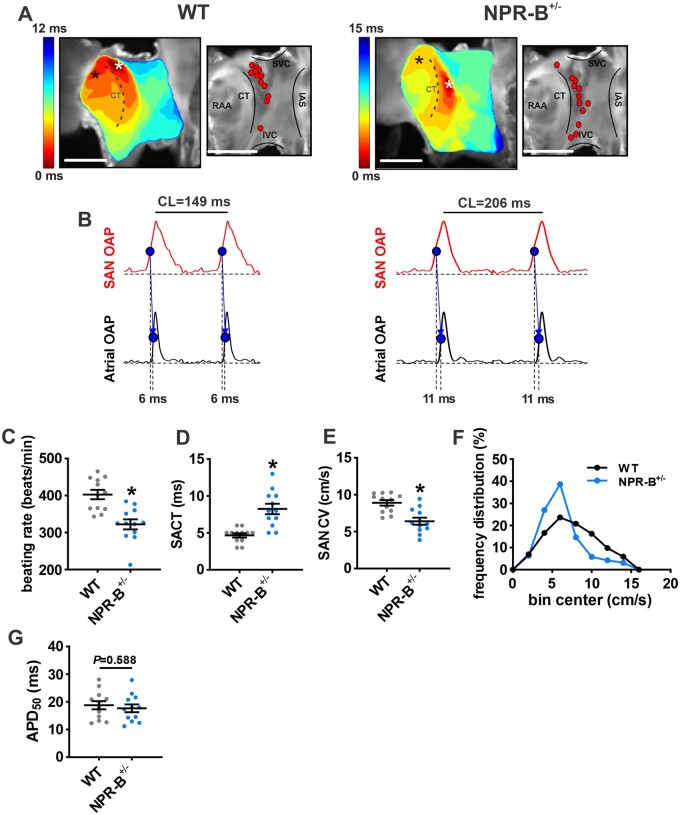
To directly assess electrical conduction within the SAN of wildtype and NPR-B+/− mice we used high-resolution optical mapping of the right atrial posterior wall adjacent to the crista terminalis, which corresponds to the anatomical position of the SAN (Figure 3). Activation maps in this region were used to identify the leading activation site within the SAN (Figure 3A). The majority of wildtype hearts had leading activation sites in the superior part of the SAN in the right atrial posterior wall as expected.12,22,32 In contrast, NPR-B+/− hearts showed leading activation sites that spread across the full right atrial posterior wall from superior to inferior vena cava with more hearts demonstrating inferior leading activation sites compared to wildtype controls (Figure 3A).
Figure 3.
Assessment of electrical conduction in the SAN in NPR-B+/− mice. (A) Representative activation maps (left) and maps showing location of leading activation sites (right) for WT and NPR-B+/− mice. Red dots represent leading activation sites for different WT and NPR-B+/− hearts. Scale bars = 2 mm. (B) Optical action potentials (OAPs) from the initial pacemaker site in the SAN region (white asterisk on activation map) and earliest right atrial activation site (black asterisk on activation map) from WT (left) and NPR-B+/− (right) mice. These optical APs were used to quantify SAN to atrial conduction time (SACT). (C–F) Summary of atrial preparation beating rate (C), SACT (D), SAN conduction velocity (CV; E), frequency distribution of CV in the SAN (F) and optical APD50 (G) for WT (n = 12) and NPR-B+/− (n = 12) hearts. For (C,D,G), *P < 0.001 vs. WT by Student’s t-test. For (E), *P < 0.001 vs. WT by Mann–Whitney U test.
Optical APs were recorded from the SAN in the region of the leading activation site and in the adjacent atrial myocardium (first atrial activation adjacent to the CT on the side of the right atrial free wall) (Figure 3B). These optical APs show that the diastolic period between successive APs is longer in NPR-B+/− hearts. Consistent with this, and the reduction in HR in vivo, atrial preparations from NPR-B+/− mice demonstrated lower intrinsic beating rates compared to wildtype controls (Figure 3C). These optical APs were also used to measure SAN to atrial conduction time (SACT) for each genotype (Figure 3B). SACT was longer in NPR-B+/− mice compared to wildtype controls (Figure 3D). Finally, local conduction velocity (CV) in the right atrial posterior wall (i.e. SAN region) was slower in NPR-B+/− mice (Figure 3E) which is also evident by a leftward shift in the right atrial posterior wall CV histogram from NPR-B+/− mice (Figure 3F). There were no differences in optical AP duration at 50% repolarization (APD50) in the SAN region in NPR-B+/− mice (Figure 3G).
3.5 NPR-B deficiency impairs SAN myocyte electrophysiology
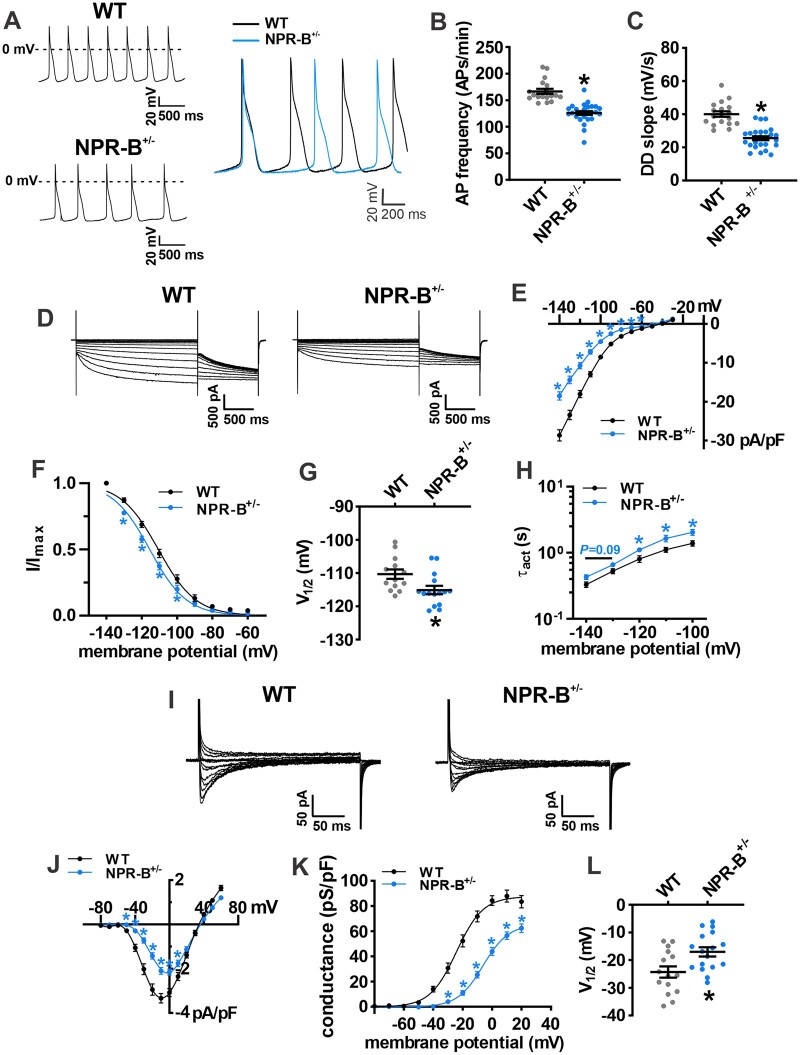
To further investigate the basis for SAN dysfunction in NPR-B deficient mice spontaneous APs were recorded in isolated SAN myocytes from wildtype and NPR-B+/− mice (Figure 4; Supplementary material online, Table S1). Consistent with the reductions in in vivo and in situ beating rates, isolated SAN myocytes from NPR-B+/− mice demonstrated reduced AP firing frequency (Figure 4A,B) and diastolic depolarization (DD) slope (Figure 4C) compared to wildtype controls. These differences were similar for SAN myocytes isolated from male and female wildtype and NPR-B+/− mice, further supporting that these findings are independent of sex (Supplementary material online, Figure S9). Other measurements of SAN AP morphology were not different between genotypes (Supplementary material online, Table S1).
Figure 4.
SAN myocyte electrophysiology in NPR-B+/− mice. (A) Representative spontaneous AP recordings in isolated SAN myocytes from WT and NPR-B+/− mice. (B,C) Summary of AP firing rate (B) and DD slope (C) in isolated SAN myocytes from WT (n = 18 cells from six mice) and NPR-B+/− (n = 26 cells from 10 mice) mice. *P < 0.0001 vs. WT by Student’s t-test. See Supplementary material online, Table S1 for additional AP parameters. (D) Representative If recordings in SAN myocytes isolated from WT and NPR-B+/− mice. (E–G), Summary If IV curves (E), activation curves (F), V1/2 activation (G), and time constant of activation (τact; H) for WT (n = 13 cells from seven mice) and NPR-B+/− (n = 15 cells from six mice) mice. (I) Representative ICa,L recordings in SAN myocytes isolated from WT and NPR-B+/− mice. (J–L) Summary ICa,L IV curves (J), activation curves (K), and V1/2 activation (L) for WT (n = 15 cells from six mice) and NPR-B+/− (n = 17 cells from six mice) mice. For (E,F,H,J,K), *P < 0.01 vs. WT at each membrane potential by two-way repeated-measures ANOVA with Holm–Sidak post hoc test. For panels G and K *P < 0.05 vs. WT by Student’s t-test.
The ionic basis for these alterations in SAN AP morphology was investigated next. If and ICa,L are important contributors to DD slope and can be activated acutely by exogenous applications of NPs.11 IV curve analysis demonstrates that If density was reduced in NPR-B+/− mice (Figure 4D,E). Analysis of If activation kinetics demonstrates that the reduction in If density in NPR-B+/− mice was associated with a leftward shift (Figure 4F,G) in the voltage for 50% channel activation (V1/2act). The slope factor (k) for the If activation curves was not different between genotypes (Supplementary material online, Table S2). The time constants for If activation were prolonged in NPR-B+/− SAN myocytes (Figure 4H). There were no differences in expression of mRNAs for Hcn1, Hcn2, or Hcn4 in the SAN in NPR-B+/− mice (Supplementary material online, Figure S10). Similarly, the protein levels for HCN1, HCN2, and HCN4 were not different in NPR-B+/− mice (Supplementary material online, Figure S10).
ICa,L density was also reduced in SAN myocytes isolated from NPR-B+/− mice (Figure 4I,J). The reduction in ICa,L occurred in association with a rightward shift in V1/2(act) (Figure 4K,L) and a reduction in maximum ICa,L conductance (Gmax; Supplementary material online, Table S2). There were no differences in expression of Ca2+ channel mRNAs (Cacna1a or Cacna1d) or CaV1.2 and CaV1.3 proteins in the SAN in NPR-B+/− mice (Supplementary material online, Figure S11).
Sodium channels have been shown to affect SAN conduction;4 however, there were no differences in INa amplitude (Supplementary material online, Figure S12) or INa steady-state activation (Supplementary material online, Figure S12) in SAN myocytes in NPR-B+/− mice. Expression of Scn5a mRNA (encodes NaV1.5 channels) was also not different in the SAN in NPR-B+/− mice (Supplementary material online, Figure S12). Finally, there were no differences in expression of Gja5 (encodes Cx40), Gja1 (encodes Cx43), or Gjc1 (encodes Cx45) in the SAN in NPR-B+/− mice (Supplementary material online, Figure S13).
3.6 Cyclic nucleotide signalling in NPR-B deficient mice
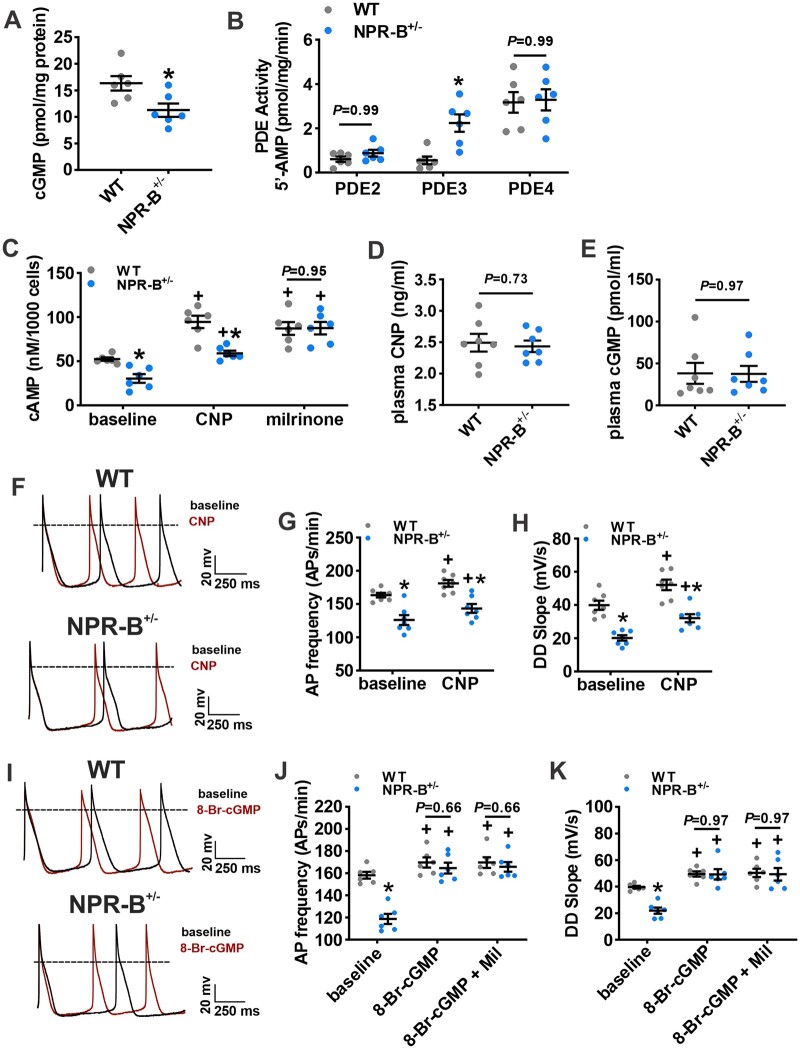
Reductions in If and ICa,L with corresponding changes in V1/2(act) suggests that these currents are reduced due to alterations in the biophysical modulation of these channels by cyclic nucleotides. Accordingly, we next assessed whether the reductions in AP firing in NPR-B+/− mice were due to alterations in SAN cyclic nucleotide handling. NPR-B is a GC-linked receptor which increases intracellular cGMP upon CNP binding.8 Whole atrial tissue lysates from NPR-B+/− mice had lower levels of cGMP compared to wildtype mice (Figure 5A). cGMP potently inhibits PDE3 activity7 and PDE3 plays a critical role in regulating basal SAN function by hydrolyzing cAMP;33,34 therefore, the PDE3 inhibitor milrinone (10 µM) was used to determine the fraction of total SAN PDE activity that was due to PDE3 in wildtype and NPR-B+/− mice. PDE3 activity in the SAN was increased in NPR-B+/− mice compared to wildtype controls (Figure 5B) while PDE2 and PDE4 activity were not different (Figure 5B). Consistent with increased PDE3 activity, intracellular cAMP was lower in SAN myocytes isolated from NPR-B+/− mice (Figure 5C). Exogenous application of CNP (100 nM) increased cAMP concentrations in SAN myocytes from wildtype and NPR-B+/−; however, overall cAMP concentrations remained lower in NPR-B+/− mice (Figure 5C). Conversely, inhibition of PDE3 with milrinone (10 µM) increased cAMP concentration in both genotypes and abolished the differences in SAN cAMP concentrations between wildtype and NPR-B+/− mice (Figure 5C). There were no differences in plasma CNP levels (Figure 5D) or in plasma cGMP levels (Figure 5E) in NPR-B+/− mice. The mRNA expression of PDE3A and other PDEs in the SAN, as well as in the right atrium and left ventricle, where not different in NPR-B+/− mice (Supplementary material online, Figure S14). There were also no differences in intracellular cAMP in the right atrium or left ventricle (Supplementary material online, Figure S14). Collectively, these data demonstrate that NPR-B deficiency lowers intracellular cGMP levels leading to increased PDE3 activity and diminished cAMP signalling locally in the SAN.
Figure 5.
Altered cyclic nucleotide handling in the SAN of NPR-B+/− mice. (A) Whole atrial tissue cGMP levels in WT (n = 6 hearts) and NPR-B+/− (n = 6 hearts) mice. (B) Summary of phosphodiesterase 2, 3, and 4 (PDE2, PDE3, PDE4) activity in the SAN of WT and NPR-B+/− mice (n = 6 pooled SANs for each genotype). For (A and B), *P < 0.05 vs. WT by Student’s t-test. (C) SAN myocyte intracellular cAMP production at baseline and in response to CNP (100 nM) or the PDE3 inhibitor milrinone (10 µM). n = 6 pooled SANs for each genotype; *P < 0.05 vs. WT within condition; +P < 0.05 vs. baseline within genotype by two-way repeated-measures ANOVA with Holm–Sidak post hoc test. (D) Summary of plasma CNP in WT (n = 7) and NPR-B+/− (n = 7) mice. (E) Summary of plasma cGMP in WT (n = 7) and NPR-B+/− (n = 7) mice. (D, E) analysed by Student’s t-test. (F) Representative spontaneous AP recordings in isolated SAN myocytes from WT and NPR-B+/− mice at baseline (black trace) and in response to CNP (100 nM; red trace). (G,H) Summary of the effects of CNP on spontaneous AP firing frequency (G) and DD slope (H) for WT (n = 7 cells from two mice) and NPR-B+/− (n = 7 cells from three mice) SAN myocytes. (I) Representative spontaneous AP recordings in isolated SAN myocytes from WT and NPR-B+/− mice at baseline (black trace) and in response intracellular dialysis of 8-Br-cGMP (10 µM; red trace). (J, K) Summary of the effects of 8-Br-cGMP and 8-Br-cGMP + Milrinone (Mil) on spontaneous AP firing frequency (J) and DD slope (K) in WT (n = 6 cells from six mice) and NPR-B+/− (n = 6 cells from five mice) SAN myocytes. For (G, H, J, and K) *P < 0.05 vs. WT within condition; +P < 0.05 vs. baseline within genotype by two-way repeated-measures ANOVA with Holm–Sidak post hoc test.
3.7 Augmented cGMP levels restore regular SAN function in NPR-B deficient mice
Our results suggest that the reductions in HR observed in NPR-B deficient mice are due to lower levels of myocardial cGMP and increased PDE3 hydrolysis of cAMP in the SAN. To confirm this mechanism, we tested if enhancing intracellular cGMP signalling in SAN myocytes from NPR-B deficient mice would restore spontaneous SAN AP firing. Acute CNP (100 nM) application caused an increase in AP firing rate and DD slope in isolated SAN myocytes from both genotypes; however, AP firing frequency and DD slope remained reduced in NPR-B+/− mice (Figure 5F–H).
To bypass NPR-B and directly increase cGMP signalling SAN myocytes were dialysed with 8-Bromo-cGMP (8-Br-cGMP; 10 µM), a slowly hydrolyzed analogue of cGMP, via the patch pipette. 8-Br-cGMP increased spontaneous AP frequency and DD slope in SAN myocytes from both wildtype and NPR-B+/− mice to levels that no longer differed between genotypes (Figure 5I– K). Application of milrinone (10 µM) after 8-Br-cGMP dialysis did not cause any further increases in AP firing rate or DD slope in both groups suggesting that 8-Br-cGMP fully inhibited PDE3 in SAN myocytes (Figure 5J,K).
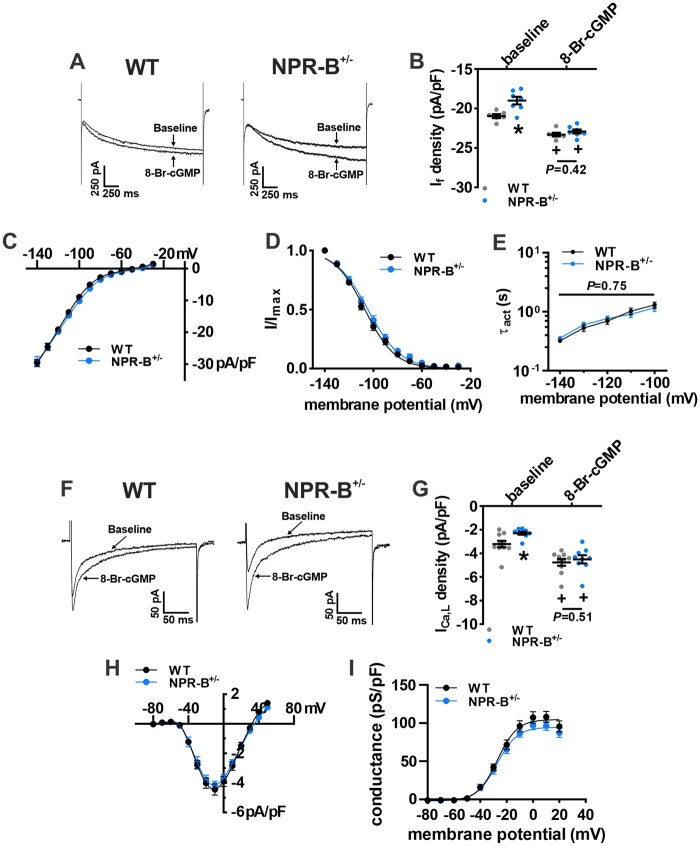
The effects of enhancing cGMP signalling on If and ICa,L in NPR-B+/− SAN myocytes were also investigated (Figure 6; Supplementary material online, Table S3). If density was measured at -130 mV immediately after rupture during whole-cell recordings and after dialysis of 8-Br-cGMP (10 µM; Figure 6A). 8-Br-cGMP increased If density in SAN myocytes from both wildtype and NPR-B+/− mice and abolished the difference in If current density seen at baseline (Figure 6B). Summary IV curves demonstrate that 8-Br-cGMP normalized If density (Figure 6C) as well as If steady-state activation properties (Figure 6D; Supplementary material online, Table S3). 8-Br-cGMP also normalized the If time constants of activation (Figure 6E) in NPR-B+/− SAN myocytes.
Figure 6.
Effect of 8-Br-cGMP on If and ICa,L in SAN myocytes from NPR-B+/− mice. (A) Representative If recordings (measured at –130 mV) at baseline and following intracellular dialysis of 8-Br-cGMP (10 µM) in isolated SAN myocytes from WT and NPR-B+/− mice. (B) Summary of the effects of 8-Br-cGMP on If density at –130 mV in WT (n = 6 cells from three mice) and NPR-B+/− (n = 7 cells from four mice) SAN myocytes. (C–E) Summary If IV curves (C), activation curves (D), and τact (E) for WT (n = 10 cells from six mice) and NPR-B+/− (n = 9 cells from five mice) SAN myocytes in the presence of 8-Br-cGMP. (E) Representative ICa,L recordings (measured at –10 mV) at baseline and following intracellular dialysis of 8-Br-cGMP (10 µM) in isolated SAN myocytes from WT and NPR-B+/− mice. (F) Summary of the effects of 8-Br-cGMP on ICa,L density at –10 mV in WT (n = 10 cells from three mice) and NPR-B+/− (n = 9 cells from two mice) SAN myocytes. (G,H) Summary ICa,L IV curves (G) and activation curves (H) for WT (n = 10 cells from three mice) and NPR-B+/− (n = 9 cells from two mice) SAN myocytes in the presence of 8-Br-cGMP. For (B, F), *P < 0.05 vs. WT within condition; +P < 0.05 vs. baseline within genotype by two-way repeated-measures ANOVA with the Holm–Sidak post hoc test. For (C, D, E, H, and I) data analysed by two-way repeated-measures ANOVA with Holm–Sadak post hoc test.
Similarly, 8-Br-cGMP dialysis increased ICa,L density in SAN myocytes from both wildtype and NPR-B+/− mice and abolished the differences in ICa,L seen at baseline between genotypes (Figure 6F,G). Following 8-Br-cGMP application, summary ICa,L IV curves did not differ between wildtype and NPR-B+/− mice (Figure 6H). ICa,L activation properties from NPR-B+/− mice were also not different from wildtype controls (Figure 6I; Supplementary material online, Table S3).
ICa,L has also been shown to be regulated by cGMP activated protein kinase G (PKG).35 Accordingly, we next performed patch-clamp experiments to assess if the 8-Br-cGMP-stimulated increase in ICa,L was due to enhanced activation of PKG in SAN myocytes. First, we examined the effects of the PKG inhibitor KT-5823 (0.5 µM) on basal ICa,L in wildtype and NPR-B+/− mice (Supplementary material online, Figure S15A,B). As demonstrated previously, basal ICa,L was reduced in NPR-B+/− mice compared to wildtype controls. Application of KT-5823 had no effect on ICa,L current density in either wildtype or NPR-B+/− mice (Supplementary material online, Figure S15B). Additionally, we observed no effect of KT-5823 on 8-Br-cGMP stimulated increases in ICa,L (Supplementary material online, Figure S15C,D). 8-Br-cGMP increased ICa,L in both groups and abolished the differences in ICa,L between wildtype and NPR-B+/− mice seen at baseline. Further application of KT-5823 had no effect on ICa,L in either group suggesting that the effects of 8-Br-cGMP are not mediated through PKG.
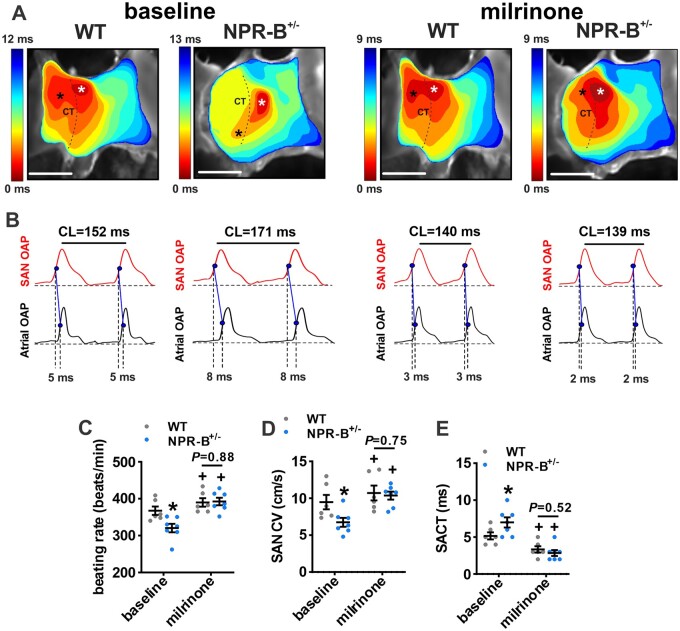
Finally, the effects of milrinone (10 µM) on SAN conduction patterns were assessed using optical mapping (Figure 7). Activation maps (Figure 7A) and optical AP measurements (Figure 7B) demonstrate that milrinone increased beating rate (Figure 7C), increased conduction velocity in the SAN region in the right atrial posterior wall (Figure 7D) and decreased SACT (Figure 7E) to values that did not differ between wildtype and NPR-B+/− mice. These data demonstrate that the increase in PDE3 activity it the SAN accounts for impaired SAN function in NPR-B+/− mice.
Figure 7.
Effects of PDE3 inhibition on electrical conduction in the SAN in NPR-B+/− mice. (A) Representative activation maps for WT and NPR-B+/− hearts at baseline and after PDE3 inhibition with milrinone (10 µM). Scale bars = 2 mm. (B) Optical action potentials (OAPs) from the initial pacemaker site in the SAN region (white asterisk on activation maps) and earliest right atrial activation site (black asterisk on activation maps) from WT and NPR-B+/− hearts at baseline and in the presence of milrinone. Dashed lines illustrate SACT for these examples. (C–E) Summary of atrial preparation beating rate (C), SAN conduction velocity (CV; D), and SACT (E) for WT (n = 6) and NPR-B+/− (n = 7) hearts at baseline and after application of milrinone. For (C–E), *P < 0.05 vs. WT; +P < 0.05 vs. baseline by two-way repeated measures ANOVA with Holm–Sidak post hoc test.
4. Discussion
In the present study, we have identified a critical functional role for NPR-B in maintaining normal intrinsic SAN automaticity and in the physiological regulation of HR in vivo. Our findings demonstrate that NPR-B deficiency in mice results in reduced HR, impaired SAN conduction, and altered electrophysiological properties of SAN myocytes due to impaired cGMP-dependent regulation of If and ICa,L by PDE3. These findings are highly consistent with clinical data in humans showing that heterozygous loss of function mutations in the NPR-B gene result in lower HRs compared to non-carrier family members.18 Similarly, a recent study demonstrated that cardiac-specific deletion of CNP in mice results in reduced HR,36 supporting the conclusion that CNP-mediated NPR-B signalling contributes to the maintenance of normal HR and SAN function under physiological conditions. NPs are best known for their ability to regulate cardiovascular homeostasis via their effects on blood pressure and blood volume.10 Here we show that NPR-B-mediated cGMP signalling regulates HR via direct effects in the SAN and that this is a physiological determinant of HR regulation in vivo. Thus, our study provides new insight into the mechanisms by which NP signalling can regulate cardiac performance via NPR-B in the SAN.
Our study shows that awake and freely moving NPR-B+/− mice exhibit reduced HR in vivo during both phases of the diurnal cycle, following administration of ANS blockers, and during β-adrenergic receptor stimulation. There were no differences in activity level in NPR-B+/− mice. This indicates that the reductions in HR observed in NPR-B+/− mice were associated with impaired intrinsic SAN function and were independent of the ANS suggesting a key role for NPR-B in the SAN itself. This is consistent with recent studies demonstrating that intrinsic SAN beating rate is a critical determinant of HRV in vivo.23,37,38 Nevertheless, previous studies have also identified a role for NP signalling and NPR-B in regulating sympathetic and parasympathetic nervous system activity leading to changes in HR.13,29,39 For example, transgenic rats with neuronal-specific dominant-negative overexpression of NPR-B develop higher HRs and increased blood pressure due to elevated post-ganglionic sympathetic nerve activity.39 CNP has also been shown to modulate vagal neurotransmission via presynaptic effects.13 Conversely, in our model of global NPR-B deficiency, HR was reduced in association with increased HRV and increases in intrinsic SAN beating rate variability. Some of these differences could be related to the different models used in each study. The ability of CNP/NPR-B to affect HR via direct effects in the SAN as well as via effects on cardiac neurotransmission indicates site-specific NPR-B signalling is likely important and that, overall, NPR-B is a key regulator of HR.
SAN dysfunction in NPR-B+/− mice was evident as shown by increased cSNRT in vivo. Consistent with this, high-resolution optical mapping studies demonstrate that NPR-B+/− mice exhibited increased SAN conduction time and more inferior leading activation sites compared to WT mice. Inferior shifts in the leading activation site in the SAN, which can occur in disease states or following parasympathetic nervous system activation, are well known to be associated with lower HR.22,32,40–42 In agreement with these findings, we have previously shown that acute exogenous application of CNP (the ligand for NPR-B) increases HR in association with superior shifts in leading activation site in the SAN.12
Impaired SAN function in NPR-B+/− mice occurred due to reductions in spontaneous AP firing and DD slope in SAN myocytes. These impairments were the result of reductions in If and ICa,L, which are critical regulators of SAN automaticity. Mechanistically, If and ICa,L were reduced in association with shifts in the voltage dependence of activation (V1/2(act)) for each current. These biophysical alterations were due to a reduction in intracellular cGMP and increased PDE3 activity, leading to a reduction in cAMP in the SAN in NPR-B+/− mice. Consistent with this, direct application of cGMP or milrinone (PDE3 inhibitor) normalized cAMP levels, AP firing, If and ICa,L in SAN myocytes from NPR-B+/− mice while exogenous application of CNP did not. These findings indicate that impaired SAN function occurs due to reduced NPR-B levels and attenuated cGMP-mediated signalling downstream of this receptor in SAN myocytes. This is in agreement with previous studies showing that NPR-B deficiency in rats results in lower cardiomyocyte cGMP generation43 while HEK293A cells with a gain of function mutation in NPR-B exhibit elevated intracellular cGMP.44 Thus, our study demonstrates that NPR-B regulates SAN AP firing via the cross-talk between cGMP and cAMP via PDE3. PDE3-dependent cAMP signalling is a critical determinant of SAN AP firing.33,34,45 Reduced HR and impaired intrinsic SAN function in NPR-B+/− mice is consistent with previous studies demonstrating that acute application of exogenous CNP can increase HR, SAN conduction and SAN AP firing via PDE3.11–14,46 We did not observe alterations in INa or Cx expression in the SAN of NPR-B+/− mice suggesting that conduction abnormalities and reduced HR are associated with the slower rate of diastolic depolarization due to reductions in If and ICa,L identified in this study.
In addition to inhibiting PDE3, cGMP can also activate PDE2; however, it requires higher concentrations of cGMP to activate PDE2 than to inhibit PDE3.7 PDE2 activity was not altered in the SAN in NPR-B+/− mice indicating that the reduction in cGMP in the SAN of NPR-B+/− mice was not able to alter PDE2 activity. PDE4 is not directly modulated by cGMP, but is importantly involved in cAMP hydrolysis.7 Nevertheless, PDE4 activity was also not altered in NPR-B+/− mice. cGMP can also activate PKG,47,48 which has been shown to modulate ICa,L density in atrial and ventricular myocytes.49–51 We found that there was no effect of PKG inhibition on basal ICa,L or 8-Br-cGMP stimulated increases in ICa,L in SAN myocytes. This is consistent with previous literature showing that the HR response to GC stimulation by nitric oxide donors is significantly reduced following PKA inhibition, but not PKG inhibition.52 It is also conceivable that cGMP could directly affect HCN activation kinetics; however, while both cAMP and cGMP can enhance the activity of HCN channels,53 the apparent affinities of HCN channels for cAMP are about 10-fold higher than for cGMP.54 This, in conjunction with the low levels of cGMP (∼10 nM) in adult cardiomyocytes compared to cAMP (∼1 µM), would suggest that the direct action of cGMP on HCN channel activation in the SAN is relatively minimal. Thus, while cGMP can activate a number of signalling pathways, our data show that cAMP levels in the SAN were normalized by milrinone and that SAN AP firing, DD slope, and SAN conduction properties were normalized by 8-Br-cGMP and milrinone in NPR-B+/− mice. From these data, we conclude that that the primary effect of NPR-B-mediated cGMP signalling in the SAN is via the inhibition of PDE3 and a subsequent increase in cAMP, which modulates the biophysical properties of key ionic currents (If and ICa,L) in SAN myocytes.
There was no evidence of hypertension or left ventricular dysfunction in NPR-B+/− mice in our study. We also found that SAN fibrosis was unchanged in NPR-B+/− mice. These observations are consistent with previous studies showing young NPR-B-/- mice are normotensive, with normal ventricular structure and function and normal ventricular fibrosis.36 Previous studies have also shown that ventricular fibrosis is unchanged in NPR-B dominant-negative rats.43 On the other hand, aged NPR-B deficient mice have been shown to develop ventricular hypertrophy and fibrosis17 indicating loss of NPR-B may lead to further alterations with aging. We also observed no changes in expression of mRNAs or proteins for If and ICa,L in the SAN of NPR-B+/− mice. Thus, our findings demonstrate that reduced HR and impaired SAN function in NPR-B+/− mice are not associated with pathological cardiac remodelling or changes in ion channel expression. Rather, these data, in conjunction with our electrophysiological and molecular studies, indicate that the alterations in SAN function in NPR-B+/− occur due to the loss of normal physiological signalling via CNP due to reduced NPR-B levels and NPR-B that acts to maintain normal SAN function. These findings demonstrate an essential physiological role for the regulation of HR and intrinsic SAN function by CNP via NPR-B signalling and the modulation of ion channel function in SAN myocytes. We observed no changes in plasma CNP or cGMP concentrations despite reduced cGMP in the SAN. These findings agree with the hypothesis that CNP can elicit localized effects within the heart, including in a paracrine fashion and support the conclusion that impaired SAN function in NPR-B+/− mice occur due to impaired cGMP-mediated signalling secondary to reduced NPR-B expression in SAN myocytes rather than a change in CNP availability. Despite reduced expression of NPR-B in the atria and ventricles, there were no differences in atrial and ventricular cAMP levels and there was no evidence of changes in conduction from the atria to the ventricles in NPR-B+/− mice, based on analysis of AH and HV intervals. The relative expression of NPR-B is approximately two-fold higher in the SAN compared to the left ventricle.21 Similarly, adenylyl cyclase activity and PDE3 activity are high in the SAN compared to the working myocardium.11,33,45,55 These factors may partially account for the impact of NPR-B deficiency on HR and SAN function with an absence of alterations in ventricular function.
In addition to activating NPR-B, CNP can also activate the NPR-C receptor.9 We previously showed that NPR-C knockout mice also exhibit SAN dysfunction; however, this occurred via distinct mechanisms from those observed in the present study in NPR-B+/− mice. Specifically, SAN dysfunction in NPR-C knockout mice occurred due to enhanced SAN fibrosis without alterations in SAN AP firing and AP morphology.21,22 These findings suggest that CNP can maintain SAN function via NPR-B-mediated effects on ion channel function as well as via NPR-C-mediated effects on SAN fibrosis. This highlights the importance of the NP system in maintaining HR and SAN function and suggests that multiple NPRs are involved in these effects. There were no changes in expression of other NPRs (i.e. NPR-A and NPR-C) in the SAN in NPR-B+/− mice indicating that the alterations in SAN function identified in the present study are the direct result of loss of NPR-B function.
Some limitations of our study should be noted. SAN function and regulation of ion channels by PDEs in mice may exhibit differences compared to humans. Investigating the mechanisms identified in our study in large animal models and ultimately humans will be necessary in future studies. In addition, our study focused on If and ICa,L, which are critical ionic currents involved in pacemaker function in the SAN. Nevertheless, other mechanisms such as sarcoplasmic reticulum (SR) Ca2+ release and the Na+-Ca2+ exchanger also contribute to DD slope generation and spontaneous AP firing.56,57 Furthermore, ion channel function and SR Ca2+ handling are linked such that effects on L-type Ca2+ channels may lead to downstream of effects on SR Ca2+ handling. Whether these other currents and pacemaker mechanisms are regulated by cAMP and PKA downstream of NPR-B is not known and requires further investigation. HR is a major determinant of cardiac output; however, cardiac output was not directly assessed in the present study. qPCR and Western blot studies were performed in whole tissues that will contain myocytes as well as other cell types, such as fibroblasts and neurons. Our study used mice with global NPR-B+/− deficiency; however, the degree of reduction of NPR-B in SAN myocytes compared to other tissues such as autonomic ganglia was not investigated. Finally, we did not use homozygous NPR-B knockout mice as these mice exhibit severe dwarfism58 making them unsuitable for many of our experimental approaches. Nevertheless, using NPR-B+/− mice as a model of NPR-B deficiency, rather than complete loss of NPR-B, more closely mimics known human mutations associated with loss of NPR-B function.
5. Conclusion
Our multi-level assessment of HR and SAN function in NPR-B+/− mice provides novel mechanistic insight into the role of NPR-B in regulating SAN automaticity. This work demonstrates that NPR-B deficiency is associated with impaired SAN function due to altered cGMP-dependent effects on ion channel function in SAN myocytes. From these studies, we conclude that NPR-B-mediated cGMP signalling plays a fundamental role in maintaining normal HR and SAN function, thereby providing new insight into the critical role of endocrine/paracrine regulation of cardiovascular homeostasis by NPs. These findings have important implications for identifying mechanisms of SAN dysfunction and alterations in cardiac performance in conditions associated with altered NP signalling. These findings also suggest that NPR-B stimulators could be a novel approach for improving or maintaining SAN function in conditions associated with SAN disease, which will be an important area of ongoing study.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
T.W.D. and R.A.R. contributed to conception and experimental design. T.W.D., H.J.J., and L.B.J. performed telemetry and intracardiac studies. T.W.D., M.M., and L.J.B. performed and analysed optical mapping experiments. T.W.D. performed and analysed all patch clamp experiments. M.M. and M.D.M. performed and analysed sinoatrial node histology. M.M., H.J.J., and L.A. performed qPCR. T.W.D. and Y.L. performed all western blots. D.D.B. performed and analysed echocardiography experiments. T.W.D. and R.A.R. prepared the figures and wrote the manuscript. All authors contributed to editing the final version of the manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research to R.A.R. (MOP 142486 and PJT 1666105). T.W.D. and M.M. each hold a Canadian Institutes of Health Research Doctoral Research Award. H.J.J. was supported by a Killam Postdoctoral Fellowship and a Libin Cardiovascular Institute Postdoctoral Fellowship. L.J.B. holds a Libin Cardiovascular Institute Doctoral Research Award. Y.L. holds a Canadian Institutes of Health Research Postdoctoral Fellowship.
Conflict of interest: none declared.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Contributor Information
Tristan W Dorey, Department of Cardiac Sciences, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAC66, Health Research Innovation Centre, 3280 Hospital Drive N.W., Calgary, AB T2N 4Z6, Canada.
Martin Mackasey, Department of Cardiac Sciences, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAC66, Health Research Innovation Centre, 3280 Hospital Drive N.W., Calgary, AB T2N 4Z6, Canada.
Hailey J Jansen, Department of Cardiac Sciences, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAC66, Health Research Innovation Centre, 3280 Hospital Drive N.W., Calgary, AB T2N 4Z6, Canada.
Megan D McRae, Department of Cardiac Sciences, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAC66, Health Research Innovation Centre, 3280 Hospital Drive N.W., Calgary, AB T2N 4Z6, Canada.
Loryn J Bohne, Department of Cardiac Sciences, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAC66, Health Research Innovation Centre, 3280 Hospital Drive N.W., Calgary, AB T2N 4Z6, Canada.
Yingjie Liu, Department of Cardiac Sciences, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAC66, Health Research Innovation Centre, 3280 Hospital Drive N.W., Calgary, AB T2N 4Z6, Canada.
Darrell D Belke, Department of Cardiac Sciences, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAC66, Health Research Innovation Centre, 3280 Hospital Drive N.W., Calgary, AB T2N 4Z6, Canada.
Logan Atkinson, Department of Physiology and Biophysics, Faculty of Medicine, Dalhousie University, Halifax, NS, Canada.
Robert A Rose, Department of Cardiac Sciences, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAC66, Health Research Innovation Centre, 3280 Hospital Drive N.W., Calgary, AB T2N 4Z6, Canada.
Translational perspective.
Natriuretic peptides are critical regulators of cardiac function. We report that natriuretic peptide receptor B (NPR-B) deficiency results in reduced heart rate due to impaired sinoatrial node (SAN) function. These effects are due in part to alterations in SAN ion channel function, including If and ICa,L, in association with reduced cGMP and alterations in cAMP regulation via phosphodiesterase 3 in the SAN. These data demonstrate that NPR-B plays an essential functional role in maintaining normal heart rate and SAN function suggesting that NPR-B may be a novel target for regulating HR clinically.
References
- 1. Mangoni ME, Nargeot J.. Genesis and regulation of the heart automaticity. Physiol Rev 2008;88:919–982. [DOI] [PubMed] [Google Scholar]
- 2. Bartos DC, Grandi E, Ripplinger CM.. Ion channels in the heart. Compr Physiol 2015;5:1423–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lei M, Jones SA, Liu J, Lancaster MK, Fung SS, Dobrzynski H, Camelliti P, Maier SK, Noble D, Boyett MR.. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol 2004;559:835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lei M, Zhang H, Grace AA, Huang CL.. SCN5A and sinoatrial node pacemaker function. Cardiovasc Res 2007;74:356–365. [DOI] [PubMed] [Google Scholar]
- 5. Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN.. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res 2001;52:40–50. [DOI] [PubMed] [Google Scholar]
- 6. MacDonald EA, Rose RA, Quinn TA.. Neurohumoral control of sinoatrial node activity and heart rate: insight from experimental models and findings from humans. Front Physiol 2020;11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bender AT, Beavo JA.. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 2006;58:488–520. [DOI] [PubMed] [Google Scholar]
- 8. Moghtadaei M, Polina I, Rose RA.. Electrophysiological effects of natriuretic peptides in the heart are mediated by multiple receptor subtypes. Prog Biophys Mol Biol 2016;120:37–49. [DOI] [PubMed] [Google Scholar]
- 9. Rose RA, Giles WR.. Natriuretic peptide C receptor signalling in the heart and vasculature. J Physiol 2008;586:353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potter LR, Abbey-Hosch S, Dickey DM.. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev 2006;27:47–72. [DOI] [PubMed] [Google Scholar]
- 11. Springer J, Azer J, Hua R, Robbins C, Adamczyk A, McBoyle S, Bissell MB, Rose RA.. The natriuretic peptides BNP and CNP increase heart rate and electrical conduction by stimulating ionic currents in the sinoatrial node and atrial myocardium following activation of guanylyl cyclase-linked natriuretic peptide receptors. J Mol Cell Cardiol 2012;52:1122–1134. [DOI] [PubMed] [Google Scholar]
- 12. Azer J, Hua R, Krishnaswamy PS, Rose RA.. Effects of natriuretic peptides on electrical conduction in the sinoatrial node and atrial myocardium of the heart. J Physiol 2014;592:1025–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herring N, Zaman JA, Paterson DJ.. Natriuretic peptides like NO facilitate cardiac vagal neurotransmission and bradycardia via a cGMP pathway. Am J Physiol Heart Circ Physiol 2001;281:H2318–2327. [DOI] [PubMed] [Google Scholar]
- 14. Beaulieu P, Cardinal R, De Lean A, Lambert C.. Direct chronotropic effects of atrial and C-type natriuretic peptides in anaesthetized dogs. Br J Pharmacol 1996;118:1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dickey DM, Flora DR, Bryan PM, Xu X, Chen Y, Potter LR.. Differential regulation of membrane guanylyl cyclases in congestive heart failure: natriuretic peptide receptor (NPR)-B, Not NPR-A, is the predominant natriuretic peptide receptor in the failing heart. Endocrinology 2007;148:3518–3522. [DOI] [PubMed] [Google Scholar]
- 16. Del Ry S, Cabiati M, Lionetti V, Emdin M, Recchia FA, Giannessi D.. Expression of C-type natriuretic peptide and of its receptor NPR-B in normal and failing heart. Peptides 2008;29:2208–2215. [DOI] [PubMed] [Google Scholar]
- 17. Blaser MC, Wei K, Adams RLE, Zhou YQ, Caruso LL, Mirzaei Z, Lam AY, Tam RKK, Zhang H, Heximer SP, Henkelman RM, Simmons CA.. Deficiency of natriuretic peptide receptor 2 promotes bicuspid aortic valves, aortic valve disease, left ventricular dysfunction, and ascending aortic dilatations in mice. Circ Res 2018;122:405–416. [DOI] [PubMed] [Google Scholar]
- 18. Olney RC, Bukulmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML.. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab 2006;91:1229–1232. [DOI] [PubMed] [Google Scholar]
- 19. Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM.. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation 2004;110:897–903. [DOI] [PubMed] [Google Scholar]
- 20. Urena M, Hayek S, Cheema AN, Serra V, Amat-Santos IJ, Nombela-Franco L, Ribeiro HB, Allende R, Paradis J-M, Dumont E, Thourani VH, Babaliaros V, Francisco Pascual J, Cortés C, Del Blanco BG, Philippon F, Lerakis S, Rodés-Cabau J.. Arrhythmia burden in elderly patients with severe aortic stenosis as determined by continuous electrocardiographic recording: toward a better understanding of arrhythmic events after transcatheter aortic valve replacement. Circulation 2015;131:469–477. [DOI] [PubMed] [Google Scholar]
- 21. Egom EE, Vella K, Hua R, Jansen HJ, Moghtadaei M, Polina I, Bogachev O, Hurnik R, Mackasey M, Rafferty S, Ray G, Rose RA.. Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor C. J Physiol 2015;593:1127–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mackasey M, Egom EE, Jansen HJ, Hua R, Moghtadaei M, Liu Y, Kaur J, McRae MD, Bogachev O, Rafferty SA, Ray G, Kirkby AW, Rose RA.. Natriuretic peptide receptor-C protects against angiotensin II-mediated sinoatrial node disease in mice. JACC Basic Transl Sci 2018;3:824–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorey TW, Moghtadaei M, Rose RA.. Altered heart rate variability in angiotensin II-mediated hypertension is associated with impaired autonomic nervous system signaling and intrinsic sinoatrial node dysfunction. Heart Rhythm 2020;17:1360–1370. [DOI] [PubMed] [Google Scholar]
- 24. Moghtadaei M, Langille E, Rafferty SA, Bogachev O, Rose RA.. Altered heart rate regulation by the autonomic nervous system in mice lacking natriuretic peptide receptor C (NPR-C). Sci Rep 2017;7:17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moghtadaei M, Jansen HJ, Mackasey M, Rafferty SA, Bogachev O, Sapp JL, Howlett SE, Rose RA.. The impacts of age and frailty on heart rate and sinoatrial node function. J Physiol 2016;594:7105–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jansen HJ, Mackasey M, Moghtadaei M, Liu Y, Kaur J, Egom EE, Tuomi JM, Rafferty SA, Kirkby AW, Rose RA.. NPR-C (natriuretic peptide receptor-C) modulates the progression of angiotensin ii-mediated atrial fibrillation and atrial remodeling in mice. Circ Arrhythm Electrophysiol 2019;12:e006863. [DOI] [PubMed] [Google Scholar]
- 27. Jansen HJ, Mackasey M, Moghtadaei M, Belke DD, Egom EE, Tuomi JM, Rafferty SA, Kirkby AW, Rose RA.. Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillation. J Mol Cell Cardiol 2018;124:12–25. [DOI] [PubMed] [Google Scholar]
- 28. Jensen PN, Gronroos NN, Chen LY, Folsom AR, deFilippi C, Heckbert SR, Alonso A.. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol 2014;64:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ajijola OA, Shivkumar K, Habecker BA.. Natriuretic peptides and peripheral autonomic neurotransmission: back to the A, B, and C's. Cardiovasc Res 2016;112:619–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yaniv Y, Lyashkov AE, Lakatta EG.. The fractal-like complexity of heart rate variability beyond neurotransmitters and autonomic receptors: signaling intrinsic to sinoatrial node pacemaker cells. Cardiovasc Pharm Open Access 2013;2:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monfredi O, Lyashkov AE, Johnsen AB, Inada S, Schneider H, Wang R, Nirmalan M, Wisloff U, Maltsev VA, Lakatta EG, Zhang H, Boyett MR.. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension 2014;64:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krishnaswamy PS, Egom EE, Moghtadaei M, Jansen HJ, Azer J, Bogachev O, Mackasey M, Robbins C, Rose RA.. Altered parasympathetic nervous system regulation of the sinoatrial node in Akita diabetic mice. J Mol Cell Cardiol 2015;82:125–135. [DOI] [PubMed] [Google Scholar]
- 33. Hua R, Adamczyk A, Robbins C, Ray G, Rose RA.. Distinct patterns of constitutive phosphodiesterase activity in mouse sinoatrial node and atrial myocardium. PLoS One 2012;7:e47652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vinogradova TM, Sirenko S, Lukyanenko YO, Yang D, Tarasov KV, Lyashkov AE, Varghese NJ, Li Y, Chakir K, Ziman B, Lakatta EG.. Basal spontaneous firing of rabbit sinoatrial node cells is regulated by dual activation of PDEs (phosphodiesterases) 3 and 4. Circ Arrhythm Electrophysiol 2018;11:e005896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahapatra S, Marcantoni A, Zuccotti A, Carabelli V, Carbone E.. Equal sensitivity of Cav1.2 and Cav1.3 channels to the opposing modulations of PKA and PKG in mouse chromaffin cells. J Physiol 2012;590:5053–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moyes AJ, Chu SM, Aubdool AA, Dukinfield MS, Margulies KB, Bedi KC, Hodivala-Dilke K, Baliga RS, Hobbs AJ.. C-type natriuretic peptide co-ordinates cardiac structure and function. Eur Heart J 2020;41:1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yaniv Y, Lyashkov AE, Lakatta EG.. Impaired signaling intrinsic to sinoatrial node pacemaker cells affects heart rate variability during cardiac disease. J Clin Trials 2014;4:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Papaioannou VE, Verkerk AO, Amin AS, de Bakker JM.. Intracardiac origin of heart rate variability, pacemaker funny current and their possible association with critical illness. Curr Cardiol Rev 2013;9:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buttgereit J, Shanks J, Li D, Hao G, Athwal A, Langenickel TH, Wright H, da Costa Goncalves AC, Monti J, Plehm R, Popova E, Qadri F, Lapidus I, Ryan B, Ozcelik C, Paterson DJ, Bader M, Herring N.. C-type natriuretic peptide and natriuretic peptide receptor B signalling inhibits cardiac sympathetic neurotransmission and autonomic function. Cardiovasc Res 2016;112:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fedorov VV, Hucker WJ, Dobrzynski H, Rosenshtraukh LV, Efimov IR.. Postganglionic nerve stimulation induces temporal inhibition of excitability in rabbit sinoatrial node. Am J Physiol Heart Circ Physiol 2006;291:H612–623. [DOI] [PubMed] [Google Scholar]
- 41. Glukhov AV, Fedorov VV, Anderson ME, Mohler PJ, Efimov IR.. Functional anatomy of the murine sinus node: high-resolution optical mapping of ankyrin-B heterozygous mice. Am J Physiol Heart Circ Physiol 2010;299:H482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bleeker WK, Mackaay AJ, Masson-Pevet M, Bouman LN, Becker AE.. Functional and morphological organization of the rabbit sinus node. Circ Res 1980;46:11–22. [DOI] [PubMed] [Google Scholar]
- 43. Langenickel TH, Buttgereit J, Pagel-Langenickel I, Lindner M, Monti J, Beuerlein K, Al-Saadi N, Plehm R, Popova E, Tank J, Dietz R, Willenbrock R, Bader M.. Cardiac hypertrophy in transgenic rats expressing a dominant-negative mutant of the natriuretic peptide receptor B. Proc Natl Acad Sci USA 2006;103:4735–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miura K, Namba N, Fujiwara M, Ohata Y, Ishida H, Kitaoka T, Kubota T, Hirai H, Higuchi C, Tsumaki N, Yoshikawa H, Sakai N, Michigami T, Ozono K.. An overgrowth disorder associated with excessive production of cGMP due to a gain-of-function mutation of the natriuretic peptide receptor 2 gene. PLoS One 2012;7:e42180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, Yang D, Ruknudin AM, Spurgeon H, Lakatta EG.. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res 2008;102:761–769. [DOI] [PubMed] [Google Scholar]
- 46. Beaulieu P, Cardinal R, Page P, Francoeur F, Tremblay J, Lambert C.. Positive chronotropic and inotropic effects of C-type natriuretic peptide in dogs. Am J Physiol 1997;273:H1933–1940. [DOI] [PubMed] [Google Scholar]
- 47. Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F.. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res 2003;93:907–916. [DOI] [PubMed] [Google Scholar]
- 48. Lohmann SM, Fischmeister R, Walter U.. Signal transduction by cGMP in heart. Basic Res Cardiol 1991;86:503–514. [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Wagner MB, Joyner RW, Kumar R.. cGMP-dependent protein kinase mediates stimulation of L-type calcium current by cGMP in rabbit atrial cells. Cardiovasc Res 2000;48:310–322. [DOI] [PubMed] [Google Scholar]
- 50. Vandecasteele G, Verde I, Rucker-Martin C, Donzeau-Gouge P, Fischmeister R.. Cyclic GMP regulation of the L-type Ca(2+) channel current in human atrial myocytes. J Physiol 2001;533:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kumar R, Namiki T, Joyner RW.. Effects of cGMP on L-type calcium current of adult and newborn rabbit ventricular cells. Cardiovasc Res 1997;33:573–582. [DOI] [PubMed] [Google Scholar]
- 52. Musialek P, Rigg L, Terrar DA, Paterson DJ, Casadei B.. Role of cGMP-inhibited phosphodiesterase and sarcoplasmic calcium in mediating the increase in basal heart rate with nitric oxide donors. J Mol Cell Cardiol 2000;32:1831–1840. [DOI] [PubMed] [Google Scholar]
- 53. Kaupp UB, Seifert R.. Molecular diversity of pacemaker ion channels. Annu Rev Physiol 2001;63:235–257. [DOI] [PubMed] [Google Scholar]
- 54. Craven KB, Zagotta WN.. CNG and HCN channels: two peas, one pod. Annu Rev Physiol 2006;68:375–401. [DOI] [PubMed] [Google Scholar]
- 55. Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG.. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 2006;98:505–514. [DOI] [PubMed] [Google Scholar]
- 56. Lakatta EG, Maltsev VA, Vinogradova TM.. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 2010;106:659–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Neco P, Rose B, Huynh N, Zhang R, Bridge JH, Philipson KD, Goldhaber JI.. Sodium-calcium exchange is essential for effective triggering of calcium release in mouse heart. Biophys J 2010;99:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL.. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA 2004;101:17300–17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.