Abstract
Background
Corticosteroids are first‐line therapy for induction of remission in ulcerative colitis. Although corticosteroids may improve symptoms, they have significant adverse effects. Steroids which act topically, with less systemic side‐effects may be more desirable. Budesonide is a topically acting corticosteroid with extensive first pass hepatic metabolism. There are currently three formulations of budesonide: two standard formulations including a controlled‐ileal release capsule and a pH‐dependent capsule both designed to release the drug in the distal small intestine and right colon; and the newer Budesonide‐MMX® capsule designed to release the drug throughout the entire colon.
Objectives
The primary objective was to evaluate the efficacy and safety of oral budesonide for the induction of remission in ulcerative colitis.
Search methods
We searched MEDLINE, EMBASE, CENTRAL, and the Cochrane IBD Group Specialised Register from inception to April 2015. We also searched reference lists of articles, conference proceedings and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials comparing oral budesonide to placebo or another active therapy for induction of remission in ulcerative colitis were considered eligible. There were no exclusions based on patient age or the type, dose, duration or formulation of budesonide therapy.
Data collection and analysis
Two independent investigators reviewed studies for eligibility, extracted data and assessed study quality. Methodological quality was assessed using the Cochrane risk of bias tool. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria. The primary outcome was induction of remission (as defined by the primary studies) at week eight. Secondary outcomes included clinical, endoscopic and histologic improvement, adverse events and early withdrawal. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for each dichotomous outcome and the mean difference (MD) and corresponding 95% CI for each continuous outcome. Data were analysed on an intention‐to‐treat basis.
Main results
Six studies (1808 participants) were included. Four studies compared budesonide‐MMX® with placebo, one small pilot study looked at clinical remission at week four, and was subsequently followed by three large, studies that assessed combined clinical and endoscopic remission at week eight. Although two placebo‐controlled studies had mesalamine and Entocort (standard budesonide) treatment arms, these studies were not sufficiently powered to compare Budesonide‐MMX® with these active comparators. One small study compared standard budesonide with prednisolone and one study compared standard budesonide to mesalamine. Four studies were rated as low risk of bias and two studies had an unclear risk of bias. A pooled analysis of three studies (900 participants) showed that budesonide‐MMX® 9 mg was significantly superior to placebo for inducing remission (combined clinical and endoscopic remission) at 8 weeks. Fifteen per cent (71/462) of budesonide‐MMX® 9 mg patients achieved remission compared to 7% (30/438) of placebo patients (RR 2.25, 95% CI 1.50 to 3.39). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (101 events). A subgroup analysis by concurrent mesalamine use suggests higher efficacy in the 442 patients who were not considered to be mesalamine‐refractory (RR 2.89, 95% CI 1.59 to 5.25). A subgroup analysis by disease location suggests budesonide is most effective in patients with left‐sided disease (RR 2.98, 95% CI 1.56 to 5.67; 289 patients). A small pilot study reported no statistically significant difference in endoscopic remission between budesonide and prednisolone (RR 0.75, 95% CI 0.23 to 2.42; 72 patients). GRADE indicated that the overall quality of the evidence supporting this outcome was very low due to unclear risk of bias and very sparse data (10 events). Standard oral budesonide was significantly less likely to induce clinical remission than oral mesalamine after 8 weeks of therapy (RR 0.72, 95% CI 0.57 to 0.91; 1 study, 343 patients). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (161 events). Another study found no difference in remission rates between budesonide‐MMX® 9 mg and mesalamine (RR 1.48, 95% CI 0.81 to 2.71; 247 patients). GRADE indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (37 events). One study found no difference in remission rates between budesonide‐MMX® 9 mg and standard budesonide 9 mg (RR 1.38, 95% CI 0.72 to 2.65; 212 patients). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (32 events). Suppression of plasma cortisol was more common in prednisolone‐treated patients (RR 0.02, 95% CI 0.0 to 0.33). While budesonide does appear to suppress morning cortisol to some extent, mean morning cortisol values remained within the normal range in 2 large studies (n = 899) and there was no difference in glucocorticoid‐related side‐effects across different treatment groups. Further, study withdrawal due to adverse events was not more common in budesonide compared with placebo treated patients (RR 0.85, 95% CI 0.53 to 1.38). Common adverse events included worsening ulcerative colitis, headache, pyrexia, insomnia, back pain, nausea, abdominal pain, diarrhoea, flatulence and nasopharyngitis.
Authors' conclusions
Moderate quality evidence to supports the use of oral budesonide‐MMX® at a 9 mg daily dose for induction of remission in active ulcerative colitis, particularly in patients with left‐sided colitis. Budesonide‐MMX® 9 mg daily is effective for induction of remission in the presence or absence of concurrent 5‐ASA therapy. Further, budesonide‐MMX® appears to be safe, and does not lead to significant impairment of adrenocorticoid function compared to placebo. Moderate quality evidence from a single study suggests that mesalamine may be superior to standard budesonide for the treatment of active ulcerative colitis. Low quality evidence from one study found no difference in remission rates between budesonide MMX® and mesalamine. Very low quality evidence from one small study showed no difference in endoscopic remission rates between standard budesonide and prednisolone. Low quality evidence from one study showed no difference in remission rates between budesonide‐MMX® and standard budesonide. Adequately powered studies are needed to allow conclusions regarding the comparative efficacy and safety of budesonide versus prednisolone, budesonide‐MMX® versus standard budesonide and budesonide versus mesalamine.
Plain language summary
Oral budesonide for treatment of people with active ulcerative colitis
What is ulcerative colitis?
Ulcerative colitis is a debilitating long‐term (chronic), inflammatory bowel disease that affects the large bowel. When people with ulcerative colitis are experiencing symptoms which may include bleeding, diarrhoea and abdominal pain, the disease is said to be 'active'; periods when the symptoms stop are called 'remission'. A common initial treatment of ulcerative colitis is oral steroid therapy. Unfortunately, conventional steroids are usually absorbed into the body and cause significant unwanted side‐effects. These may include but are not limited to weight gain, diabetes, growth retardation, acne, mood instability, and high blood pressure.
What is budesonide?
Budesonide is a steroid that is quickly metabolised by the liver thereby reducing corticosteroid‐related side‐effects. There are currently three formulations of budesonide: two standard capsules both designed to release the drug in the outer part of the small intestine and right colon; and the newer Budesonide‐MMX® capsule designed to release the drug throughout the entire colon.
What did the researchers investigate?
The researchers investigated whether budesonide (both standard budesonide and a new specialised formulation called budesonide‐MMX®) produces remission in people with active ulcerative colitis; and whether these medications cause any harm (side‐effects). The researchers searched the medical literature up to April 28, 2015.
What did the researchers find?
We found six studies that included a total of 1808 participants. One study (343 participants) compared standard budesonide to mesalamine (an anti‐inflammatory drug composed of 5‐aminosalicylic acid), one study (72 participants) compared standard budesonide to conventional corticosteroids, four studies (1393 participants) compared budesonide‐MMX® to placebo (a fake medicine with no active ingredients such as a sugar pill) or active comparators including Entocort (standard budesonide), prednisolone (a conventional steroid drug) or mesalamine. Four studies were judged to be of high quality and two studies were judged to be of low quality.
Evidence from three studies including 900 participants indicates that the newer formulation, budesonide‐MMX® at a dose of 9 mg/day was superior to placebo for induction of remission irrespective of mesalamine use. There is evidence to suggest that budesonide‐MMX® at a dose of 9 mg/day is particularly effective in patients with left‐sided disease as opposed to patients with more extensive disease. One small study (32 participants) comparing standard budesonide to placebo found no difference in remission rates. Evidence from one study (343 participants) comparing standard budesonide to mesalamine suggests that standard budesonide was significantly less effective than mesalamine for induction of remission. However, another study (247 participants) found no difference in remission rates between patients treated with budesonide‐MMX® and mesalamine. One study (212 participants) found no difference in remission rates between patients treated with budesonide‐MMX® 9 mg/day and standard budesonide 9 mg/day. One small study (72 participants) found no difference in endoscopic remission rates between patients treated with standard budesonide and prednisolone, however budesonide patients were less likely than prednisolone patients to experience adrenal suppression, a condition in which the adrenal glands do not produce adequate amounts of steroid hormones. Commonly reported side‐effects in the studies include worsening ulcerative colitis, headache, pyrexia (raised body temperature), insomnia (difficulty sleeping), back pain, nausea, abdominal pain, diarrhoea, flatulence and nasopharyngitis (common cold). More studies with larger numbers of participants are needed to allow conclusions regarding the comparative effectiveness of budesonide versus conventional steroid drugs, budesonide‐MMX® versus standard budesonide and budesonide versus mesalamine.
Summary of findings
Background
There are two major forms of inflammatory bowel disease (IBD): ulcerative colitis (UC) and Crohn's disease (CD). Both conditions have been distinguished from each other for decades (Lockhart‐Mummery 1960). The first pathological description of UC occurred in the 1800's (Kirsner 2001). The cause of the disease is unknown but most likely results from of a combination of genetic factors, host environment and abnormal host immune responses (Hanauer 2006; Kucharzik 2006; Xavier 2007).
Description of the condition
UC is a chronic, relapsing and remitting inflammatory disorder affecting the colonic mucosa. It can present at any age. Symptoms include bloody diarrhoea, abdominal pain or discomfort, tenesmus and urgency. Patients typically experience relapses and remissions throughout the course of their disease. The inflammation in UC extends proximally from the rectum and involves the mucosa in a continuous fashion. It can be sub‐categorised based on disease extent. In children, the disease extends proximal to the splenic flexure in approximately 80% of cases, whereas in adult patients, the disease is more commonly limited to the left side of the colon (Griffiths 2004; Van Limbergen 2008). To date, there is no cure for the condition, and treatments are aimed at inducing and maintaining remission and suppressing inflammation. Severe and prolonged inflammation of the colonic mucosa is a risk factor for the development of colorectal carcinoma (Ekbom 1990; Eaden 2001; Itzkowitz 2004; Rutter 2004; Lakatos 2006).
Therapeutic options are varied and choice of medication depends on the severity of inflammation as well as the extent of the disease. Current treatments include 5‐aminosalicylic acid (5‐ASA) compounds (Travis 2006), glucocorticoids (Truelove 1955), and immunomodulators (Timmer 2012). TNF‐α antagonist therapy has now been established for the management of patients with UC who are refractory to conventional medical therapy (Rutgeerts 2005; Lawson 2006). More recently, vedolizumab, a selective antibody against α4ß7‐integrin, which targets leukocyte trafficking in the gastrointestinal tract has been demonstrated to be an effective agent in the induction and maintenance of remission in ulcerative colitis (Feagan 2013; Bickston 2014). Patients with ongoing, severe inflammation, unresponsive to medical therapy, or patients who are steroid‐dependent may require a colectomy.
Description of the intervention
Glucocorticoids have been used in the management of ulcerative colitis for decades. Current guidelines recommend corticosteroids when treatment with 5‐ASA medication has been unsuccessful (Kornbluth 2010; Dignass 2012). A meta‐analysis has demonstrated that corticosteroids are more likely to induce remission than placebo in patients with active UC (Ford 2011). Corticosteroids act by inhibiting protein synthesis and transcription. This ultimately results in down‐regulation of cytokines known to have a role in inflammation. These include NF‐kappa B, TNF‐α, interleukin‐1 and interleukin‐6 (Barnes 2005;Silverman 2011). Budesonide is a non‐halogenated glucocorticosteroid which binds with the glucocorticoid receptor with 195‐fold greater affinity than hydrocortisone (Seow 2009). It exhibits low systemic bioavailability as a result of its first pass hepatic metabolism, reducing the likelihood of adverse effects (Gionchetti 2014). Budesonide is metabolized in the liver via a cytochrome P450 (CYP) enzyme into two main metabolites (6b‐hydroxybudesonide and 16a‐hydroxyprednisolone), which have negligible glucocorticoid activity (Jönsson 1995). In addition, P‐glycoprotein mediates the GI efflux of budesonide (Dilger 2004). Overall, only 10‐15% of budesonide circulates systemically (Seow 2009).
Several formulations of budesonide exist. The plain formulation of oral budesonide is completely absorbed in the proximal GI tract, making it unsuitable for the treatment of colonic disease. For this reason, a controlled ileal release (CIR) formulation was developed. Oral budesonide is administered as a 3 mg enteric‐coated tablet. It is usually prescribed at a dose of 6 to 9 mg daily for 2 to 3 months. Entocort and Budenofalk (both enteric‐coated formulations, which deposit active budesonide in the terminal ileum and right colon), have been found to be efficacious at inducing remission in patients with ileocolonic Crohn’s disease (Seow 2009; Kuenzig 2014; Rezaie 2015). Entocort (AstraZeneca) releases active drug in a pH and time‐dependent manner. It consists of a gelatin capsule that contains budesonide in an ethylcellulose matrix. Eudragit, the enteric coating, prevents release in the stomach, permitting delivery to the small intestine where the pH is above 5.5. Budenofalk (Dr Falk Pharma) is a pH‐dependent release formulation. Budesonide micro‐granules, coated with Eudragit, are contained within a capsule. The micro‐granules are designed to dissolve at pH values above 6.4 (Fedorak 2005; Seow 2009). Budesonide MMX® (Cosmo Pharmaceuticals, Santarus, Salix Pharmaceuticals) utilizes a multi matrix system technology platform designed to produce controlled release of budesonide throughout the colon, while limiting systemic absorption. Tablets are coated in acrylic polymers which are resistant to degradation while passing through the upper gastrointestinal tract. It is available as a 9 mg tablet.
Budesonide has been shown to be more effective than placebo and non inferior to conventional oral steroids for inducing remission in patients with mild to moderate CD involving the distal ileum and/or right colon (Rezaie 2015). It has also been demonstrated to be superior to mesalamine therapy for the treatment of active CD and has proved to be an effective therapeutic option as enema therapy in patients with distal UC (Danielsson 1992; Thomsen 1998; Bar‐Meir 2003) Furthermore, budesonide appears to be safe. A pooled safety analysis revealed that serious or clinically important adverse effects such as sepsis, cataracts, adrenal insufficiency were very infrequent and similar between patients treated with maintenance budesonide and those receiving placebo (Lichtenstein 2009).
How the intervention might work
Corticosteroids inhibit protein synthesis and transcription, ultimately down‐regulating inflammatory cytokines such as NF‐kappa B, TNF‐α, interleukin‐1 and interleukin‐6 (Barnes 2005; Silverman 2011). Ulcerative colitis is a disease which is limited to the mucosa and submucosa of the colon. Medication such as budesonide, which predominantly acts topically, is desirable for reducing this inflammation and potentially inducing remission in patients with active disease.
Why it is important to do this review
Current UC treatment strategies vary. For acute disease flares, a 5‐ASA product or oral corticosteroid, such as prednisone or prednisolone, may be prescribed. Medications such as TNF‐α antagonists and selective leukocyte trafficking inhibitors are very expensive and are often not readily available; hence corticosteroids are frequently the drug of choice for an acute flare of UC. However, conventional corticosteroids are associated with a large range of adverse events including hypertension, diabetes, osteopenia and osteoporosis, cataracts and glaucoma, as well as the risk of opportunistic infection (Lichtenstein 2006). Given that oral corticosteroids (usually prednisone or prednisolone) are frequently the drug of choice during acute flares of UC, corticosteroids which predominantly act topically with a lower adverse effect profile, such as budesonide, are desirable. It is important that this medication and its role in the induction of remission of ulcerative colitis be formally reviewed. This systematic review is an update of a previously published Cochrane review (Sherlock 2010).
Objectives
The primary objective was to evaluate the efficacy and safety of oral budesonide for the induction of remission in ulcerative colitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials evaluating the use of oral budesonide (standard formulation or the MMX‐® formulation) for induction of remission in ulcerative colitis were considered for inclusion in this review. Eligible trial designs included parallel arm, placebo‐controlled trials or trials comparing two active agents. Cross‐over designs were also eligible for inclusion. Studies of human subjects, published in all languages were considered. Studies published in abstract format within the past 3 years were considered only if sufficient outcome data could be retrieved from the abstract or following contact with the authors.
Types of participants
Participants of all ages with a confirmed diagnosis of active UC, using a combination of clinical symptoms and signs, radiologic, endoscopic and histologic criteria, were eligible for inclusion in the review. Heterogeneity in defined disease activity was anticipated, therefore the definitions used by the original authors were accepted. Acceptable activity indices included the following: the Ulcerative Colitis Disease Activity Index (UCDAI) (Sutherland 1987), the Clinical Activity Index (CAI) (Rachmilewitz 1989), the Powell‐Tuck Index (Powell‐Tuck 1978), the Simple Clinical Colitis Activity Index (SCCAI) (Walmsley 1998), Beattie's Colitis Symptom Score (Beattie 1996), Lichtiger Symptom Score for acute Ulcerative Colitis (Lichtiger 1990), the Mayo Index (Schroeder 1987), the Seo Index (Seo 1992), the Truelove and Witt's Severity Index (Truelove 1955), and the Paediatric Ulcerative Colitis Activity Index (Turner 2007) and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) (Travis 2012a).
Types of interventions
Trials were eligible for inclusion if the intervention included oral budesonide versus a control, which could be either a placebo or an active agent such as a traditional corticosteroid or 5‐ASA product. All doses and formulations of budesonide as well as different durations of therapy were eligible for inclusion.
Types of outcome measures
Primary outcomes
The primary outcome was induction of remission of active ulcerative colitis. Clinical remission was defined by the primary studies and was expressed as the percentage of patients randomised (intention‐to‐treat analysis).
Secondary outcomes
Secondary outcomes included:
clinical, endoscopic and histologic improvement as defined by the authors;
endoscopic mucosal healing;
change in disease activity index score
quality of life;
hospital admissions;
the need for intravenous corticosteroids;
surgery;
adverse events; and
study withdrawal.
Search methods for identification of studies
See: Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Group Methods used in reviews.
Electronic searches
We searched the following databases from inception to 28 April 2015:
MEDLINE;
EMBASE;
Cochrane Central Register of Controlled Trials;
Cochrane Inflammatory Bowel Disease (IBD) Group Specialised Register; and
Ongoing trials were identified using the registry link http://ClinicalTrials.gov
The search strategies with MeSH headings and text word items used to search the MEDLINE and EMBASE databases are outlined in Appendix 1 and Appendix 2.
Searching other resources
We searched the reference lists of reported studies and review articles identified by the literature search to identify further eligible studies. The abstracts and proceedings of major gastrointestinal meetings (Digestive Diseases Week ‐ USA, Canadian Digestive Diseases Week, American Gastroenterology Association, British Society of Gastroenterology, United European Gastroenterology Week), were manually searched (Appendix 3). We contacted experts in the field as well as pharmaceutical companies involved in the manufacturing of budesonide in an attempt to identify any additional trials or unpublished studies with negative or positive findings (Appendix 4).
Data collection and analysis
Selection of studies
All articles identified by the literature search were independently reviewed for eligibility by two authors (MES and CHS). Disagreements were recorded and resolved by consensus under the guidance of the third and fourth authors (AHS and AMG). The full text articles of potentially eligible abstracts were retrieved and reviewed by two authors (MES and CHS). Trials published in abstract format were included only if the authors were able to provide protocol details or if there were sufficient data provided in the abstract. Disagreements were recorded and resolved by consensus under the guidance of a third author (JKM).
Data extraction and management
Eligible full text articles were reviewed and data were extracted independently by two authors (MES and CHS). Any disagreements were resolved by consensus under the guidance of a third author (JKM).
A customised data extraction form was developed and included the following information:
General article information: title, authors, publication year;
Study design: randomisation process, allocation concealment, blinding;
Study participants: country where the study was performed, inclusion/exclusion criteria, years patients were enrolled, numbers randomised, baseline patient characteristics (age, gender, disease extent, disease severity);
Intervention: dose and duration of treatment with budesonide;
Control: placebo or active medication; and
Primary and secondary outcomes.
Assessment of risk of bias in included studies
We assessed the quality of included studies using the Cochrane risk of bias tool (Higgins 2011). Factors assessed included: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias. Two authors (MES and CHS) independently assessed the risk of bias and any disagreements were recorded and resolved by consensus under the guidance of another author (AHS, or AMG or JKM).
Studies were considered to have a 'low risk of bias' if there was a low risk of bias for all key domains, an 'unclear risk of bias' if there was an unclear risk of bias for one or more of the key domains or a 'high risk of bias' if there was a high risk of bias for one or more key domains. When insufficient data were provided to allow adequate assessment of risk of bias, the study was classified as having an 'unclear risk of bias'.
We used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) criteria to assess the overall quality of evidence supporting the primary outcome and selected secondary outcomes. Evidence from randomised controlled trials begin as high quality evidence. The quality of evidence can be downgraded due to: (1) high risk of bias, (2) indirect evidence, (3) inconsistency (unexplained heterogeneity), (4) imprecision in data, and (5) publication bias. The overall quality of evidence for each outcome was determined and classified as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); and very low quality (i.e. we are very uncertain about the estimate) (Guyatt 2008; Schünemann 2011).
Measures of treatment effect
For dichotomous outcomes, we calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI). For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI An intention‐to‐treat analysis was used.
Unit of analysis issues
Each study was reviewed to ensure that the number of observations in the final analysis equalled the number of subjects initially randomised.
Dealing with missing data
Authors of studies were contacted for further details and to provide original data if the published paper or abstract contained insufficient information.
Assessment of heterogeneity
At least two authors (MES, CHS or JKM) independently evaluated the eligible studies for clinical and methodological heterogeneity. We used the Chi2 test to assess heterogeneity, with a P‐value of < 0.10 considered statistically significant. To estimate the degree of heterogeneity across studies, we used the I2 statistic. A value of 25% is considered to indicate low heterogeneity, 50% moderate heterogeneity and 75% high heterogeneity (Higgins 2003). However, we were unable to pool the results of all studies in a combined analysis due to significant clinical heterogeneity. Löfberg 1996 compared budesonide with prednisolone, Gross 2011 compared budesonide with mesalamine and D'Haens 2010 compared budesonide‐MMX® with placebo for four weeks, followed by budesonide‐MMX® in both groups for a further four weeks. Sandborn 2012, Travis 2014 and Rubin 2014 compared budesonide‐MMX® with placebo and the results of these studies are pooled for analysis. Since all participants in the Rubin 2014 study were on concomitant 5‐ASA therapy while concurrent 5‐ASA therapy was not permitted in the Sandborn 2012 and Travis 2014 trials, a sensitivity analysis was performed to determine the effect of mesalamine refractoriness on outcomes.
Assessment of reporting biases
We planned to assess publication bias by means of a funnel plot. However, given that we identified only six eligible studies, three of which were not suitable for combined analysis, a funnel plot was not constructed.
Data synthesis
We planned to perform a meta‐analysis on the included study results using either a fixed‐effect or random‐effects model, with a Mantel‐Haenszel method of weighting (as it is more robust with small studies) depending on the presence or absence of heterogeneity. Meta‐analysis was performed using a fixed‐effect model combining data from three studies which had comparable methodology, interventions and outcome measurements. The remaining three studies were not pooled for meta‐analysis due to significant heterogeneity; two compared budesonide with a different study medication (mesalamine or prednisolone) and one study used a different outcome measure. Therefore, the results of each of these three studies are presented separately.
Subgroup analysis and investigation of heterogeneity
A priori subgroup analyses were planned for different budesonide doses, different durations of treatment, disease severity and disease location (proctitis, left‐sided colitis, pan‐colitis). We also planned to perform a subgroup analysis on paediatric and adult UC patients separately. However, all patients in the included studies were greater than 18 years old, therefore subgroup analysis of paediatric versus adult patients was not possible. We were able to perform a subgroup analysis looking at the efficacy of oral budesonide for inducing remission in patients with different disease extent (combined proctosigmoiditis and left‐sided disease versus extensive disease). We also explored the effect of concomitant 5‐ASA therapy.
Sensitivity analysis
We planned to perform sensitivity analyses including and excluding poor quality studies, and including or excluding those published only in abstract format. Only one identified study was published in abstract form (Rubin 2014), and a sensitivity analysis was performed on the pooled analysis comparing budesonide‐MMX® compared with placebo. Incidentally, the same study was also subject to a sensitivity analysis based on the presence or absence of concurrent mesalamine therapy.
Results
Description of studies
Results of the search
A literature search conducted on 28 April 2015 identified a total of 2014 records. After duplicates were removed, a total of 1941 studies remained for review of titles and abstracts. Two authors (MES and CHS) independently reviewed the titles and abstracts of these trials (See Figure 1). Thirty‐one potentially relevant full text articles were identified and considered for inclusion in this review. Following review of the complete manuscripts, six studies met inclusion criteria (Löfberg 1996; D'Haens 2010; Gross 2011; Sandborn 2012; Rubin 2014; Travis 2014). There was 100% agreement amongst authors regarding eligibility of included studies.
1.
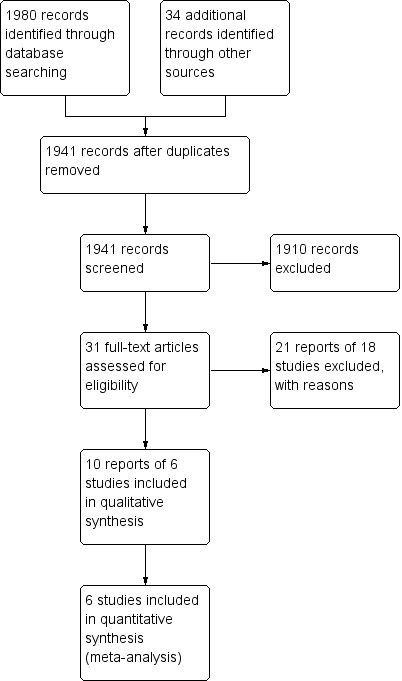
Study flow diagram.
We searched the reference lists of main review articles to identify any additional studies not identified by the primary search strategy (Baumgart 2007; Sands 2007; Biancone 2008; Kozuch 2008; Ford 2011; Silverman 2011; De Cassan 2012; Danese 2014; Gionchetti 2014; Hoy 2015). Two potentially relevant articles were in Spanish (Gomollón 1999; Díaz Blasco 1995) and one was in French (Sabate 1998). These reviews were translated to English and their reference lists manually examined, but no additional studies were identified. We identified a meta‐analysis of budesonide therapy for inflammatory bowel disease (Nos 2001). The article, published in Spanish, was translated to English. It reviewed the use of oral budesonide in CD. For UC, only studies of rectal formulations of budesonide were discussed. The reference list was searched and no new studies were identified. A meta‐analysis of treatments for left‐sided UC and proctitis did not include the use of oral budesonide (Cohen 2000). Pharmaceutical companies (Salix Pharmaceuticals, Dr. Falk Phama and AstraZeneaca) manufacturing budesonide were contacted. No additional or unpublished studies were identified.
Included studies
Six eligible studies (1808 participants) were identified. Löfberg 1996 was a pilot study evaluating the efficacy of budesonide versus prednisolone. Gross 2011 compared budesonide with mesalamine. D'Haens 2010 was a pilot study which compared budesonide‐MMX® with placebo. Sandborn 2012, Travis 2014 and Rubin 2014 were large randomised double‐blind placebo‐controlled trials that compared budesonide‐MMX® to placebo. Patients in the Rubin 2014 study were all on concomitant 5‐ASA therapy whereas patients in the Sandborn 2012 and Travis 2014 studies were excluded if they were taking concomitant 5‐ASA. Detailed characteristics of each study are included in the 'Characteristics of included studies' tables.
INCLUDED STUDY COMPARING ORAL BUDESONIDE (STANDARD FORMULATION) TO PREDNISOLONE:
This was a multicenter, double‐blind, double‐dummy, randomised controlled trial that compared the efficacy and safety of budesonide to prednisolone for the treatment of active UC. Participants were either inpatients or outpatients with active UC. Active disease activity was defined as an endoscopic inflammation score of ≥ 2 (Appendix 5) in at least one colonic segment along with the clinical symptoms of bloody stools and increased stool frequency of ≥ 3 stools per day. All participants were over 18 years (range 18 to 71) and had confirmed disease that extended proximal to the sigmoid colon. The use of concomitant oral 5‐ASA products was allowed. However, the use of topical therapy, systemic corticosteroids or antibiotics were exclusion criteria. The sample size calculation was based on detecting a difference of 0.8 in the change in endoscopic score, using a power of 80% and an alpha of 0.05.
The interventional medication was budesonide capsules (10 mg total daily dose), manufactured by Astra Draco (Lund, Sweden). Patients received 6 mg in the morning and 4 mg in the evening for the first four weeks. The dose was reduced to 4 mg twice daily from weeks five to seven and patients received 4 mg once daily during weeks eight and nine. The control medication was prednisolone 40 mg daily for two weeks; thereafter the dose was reduced by 5 mg each week until the eighth week. Study medication was administered for a total of nine weeks. Thirty‐four patients were randomised to budesonide and 38 were randomised to prednisolone. The primary outcome was improvement in the endoscopic inflammation score. Secondary outcomes included improvement in histologic score, achievement of endoscopic remission, improvement in gastrointestinal symptoms, change in laboratory parameters and adverse events.
INCLUDED STUDY COMPARING ORAL BUDESONIDE (STANDARD FORMULATION) TO MESALAMINE:
This was a multicenter, double‐blind, double‐dummy, randomised controlled trial that compared the efficacy and safety of budesonide (9 mg/day) to mesalamine (3 g/day) for the treatment of active UC. Patients were treated for eight weeks.
Adult, non‐pregnant patients (aged 18 to 75 years) were eligible for inclusion. Active ulcerative colitis was defined as a clinical activity index (CAI) of ≥ 6 and an endoscopic index (EI) of ≥ 4 (Rachmilewitz 1989). Patients with disease limited to the rectum were excluded. Patients with newly diagnosed UC or those with established disease were eligible for inclusion. Patients treated with immunosuppressant medications or corticosteroids (oral or intravenous) within four weeks of study enrolment were excluded. Almost 80% of patients had proctosigmoiditis or left‐sided disease. Three hundred and forty‐three patients were enrolled, with 177 patients randomised to budesonide and 166 to Mesalazine. The primary outcome was clinical remission (defined as a CAI ≤ 4 with rectal bleeding and stool frequency sub‐score of '0') at eight weeks. Secondary outcomes included mucosal healing (as defined by Sutherland's Disease Activity Index Score ≤ 1) (Sutherland 1987), histologic healing (Histologic Index as described by Riley 1991), changes in disease activity and symptoms from baseline and therapeutic success or benefit (as defined by Hanauer's Physician Global Assessment) (Hanauer 1993).
INCLUDED STUDIES COMPARING ORAL BUDESONIDE (Budesonide‐MMX®) TO PLACEBO:
This pilot study was a multi‐centre, randomised, double‐blind, trial that evaluated the safety and efficacy of a new formulation of oral budesonide (Budesonide‐MMX®) for inducing clinical remission in active left‐sided ulcerative colitis at four weeks. During the first four weeks, patients were randomly assigned to receive either budesonide(n = 18) or placebo (n = 18). For the last four weeks of the trial, all patients received budesonide 9 mg daily. Concomitant oral 5‐ASAs or immunomodulators were allowed. Patients were excluded if they were being treated with topical agents, antibiotics, systemic corticosteroids or a biologic agent.
The primary outcome was clinical remission (CAI ≤ 4) (Rachmilewitz 1989) or clinical improvement (defined as a reduction in CAI score by at least 50%) at four weeks. Secondary outcomes included a reduction in clinical symptoms at eight weeks, a reduction in CAI score by 70%, changes in the Rachmilewitz Endoscopic Index Score and histological changes (Saverymuttu 1986) at four and eight weeks. To assess the influence of budesonide on the adrenocortical axis, patients had a morning cortisol level drawn following four and eight weeks of therapy and a short ACTH test performed at week eight. Only the first arm of the study (duration four weeks), was utilized for outcome assessment.
The CORE 1 study was a randomised double‐blind placebo‐controlled trial, conducted at 108 centres in North America and India. The study aim was to examine the efficacy of oral budesonide‐MMX® for inducing remission in adult patients with mild to moderate ulcerative colitis (UCDAI score of 4 ‐ 10). Patients were randomly assigned to four treatment groups: budesonide‐ MMX® 9 mg or 6 mg, Asacol or placebo. Patients were treated for eight weeks. Patients were excluded for oral or rectal steroid use within four weeks of study enrolment. The use of immunosuppressants within eight weeks of enrolment and treatment with TNF‐alpha antagonists within 12 weeks of enrolment were exclusion criteria.
The primary outcome was remission (defined as combined clinical and endoscopic remission) at eight weeks. Secondary outcomes included clinical improvement (≥3 point reduction in UCDAI), endoscopic improvement, symptom resolution, histologic healing, and adverse events. Although 509 patients were initially randomised; 20 patients were excluded due to major protocol violations or because they did not meet study inclusion criteria. The modified intention‐to‐treat population included: budesonide‐MMX® 9 mg/day (n = 123), budesonide‐ MMX® 6mg/day (n = 121), Asacol 2.4g/day (n = 124) or placebo (n = 121). The study was funded by Santarus Inc and Cosmo Pharmaceuticals (manufacturers of budesonide‐ MMX®).
The CORE II study was a randomised double‐blind placebo‐controlled trial, conducted at 69 centres in 15 countries (Europe, Russia, Israel and Australia). The study aim was to examine the efficacy of oral budesonide‐MMX® for inducing remission in adult patients with mild to moderate ulcerative colitis (UCDAI score of 4 ‐ 10). Patients were randomly assigned to four treatment groups: budesonide‐MMX® 9mg or 6mg, Entocort or placebo and were treated for eight weeks. Patients were excluded if they were treated with oral or rectal steroids within four weeks of entry. The use of immunosuppressants within eight weeks of enrolment and treatment with TNF‐alpha antagonists within 12 weeks of enrolment were exclusion criteria.
The primary outcome was remission (defined as combined clinical and endoscopic remission) at eight weeks. Secondary outcomes included clinical improvement (≥ 3 point reduction in UCDAI), endoscopic improvement, symptom resolution, histologic healing and adverse events. Although 511 patients were initially randomised; 101 patients were excluded due to good clinical practice violations or because they did not meet study inclusion criteria. The modified intention‐to‐treat population included: budesonide‐MMX® 9 mg/day (n = 109), budesonide‐ MMX® 6 mg/day (n = 109), Entocort 9 mg/day (n = 103) or placebo (n = 89). The study was funded by Cosmo Pharmaceuticals (manufacturer of budesonide‐ MMX®).
This is a prospective randomised double‐blind placebo controlled trial. The study was conducted in the United States and Europe with the aim to evaluate the efficacy and safety of budesonide‐MMX® for the induction of remission of active, mild to moderate UC (UCDAI score of 4 to 10) not adequately controlled by stable, oral mesalamine therapy > 2.4 g/day (or equivalent) for > 6 weeks prior to entry. Patients were randomly assigned to budesonide‐MMX® or placebo for eight weeks of treatment . The same preparation and dosage of oral mesalamine (or equivalent) at study entry was continued through the trial. Minimum required doses were 2.4 g/day for mesalamine, 4.0 g/day for sulfasalazine, 2.0 g/day for olsalazine and 6.75 g/day for balsalazide.
The primary outcome was combined clinical and endoscopic remission at week eight. Secondary outcomes included clinical remission, endoscopic remission, histological healing and adverse events. Although 510 patients were randomised, 52 patients were excluded as they demonstrated normal baseline mucosal histology or infectious colitis. The modified intention‐to‐treat population included 230 patients receiving budesonide‐MMX® and 228 who received placebo.
Excluded studies
Three studies were excluded as they failed to meet the inclusion criteria (Chopra 2006; Keller 1997; Kolkman 2004). Keller 1997 was excluded as it was a pilot study, with no control arm, reporting the use of budesonide in 14 patients with steroid‐dependent UC. Budesonide was not used as an induction medication, but rather it was introduced as a 'maintenance' medication during the weaning phase of traditional corticosteroids. Kolkman 2004 was a multicenter, randomised, open phase II clinical trial. This study was designed to evaluate the pharmacokinetics, pharmacodynamics and efficacy of two budesonide dosage regimes and was excluded because the control arm was not a placebo or another active medication. The comparison arms were budesonide 9 mg once daily and budesonide 3 mg three times daily. Chopra 2006 was excluded as it was a retrospective chart review of budesonide therapy in patients with CD and UC.
Seven studies were excluded because they were review articles (Díaz Blasco 1995; Feagan 1996; Lamers 1996; Gomollón 1999; Fedorak 2005; Marín‐Jiménez 2006; Silverman 2011). The reference lists of these papers were manually searched and no new studies were identified. Travis 2011 and Lichtenstein 2012 were excluded for being open label extension studies. Six studies were excluded for being pooled analyses of the CORE I and CORE II studies (Travis 2012b; Danese 2013; Lichtenstein 2013; Sandborn 2013a; Sandborn 2013b; Sandborn 2015). Further details regarding excluded studies are described in the 'characteristics of excluded studies' tables.
Risk of bias in included studies
The risk of bias assessment summarised in Figure 2.
2.
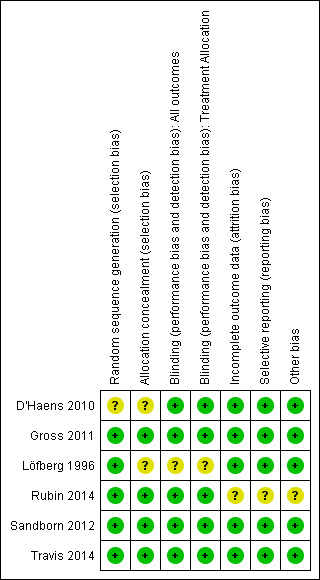
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Löfberg 1996 allocated patients by block randomisation at each of the nine participating sites. Random sequence generation was rated as low risk. Allocation concealment was not described and this item was rated as unclear. We confirmed allocation concealment in Gross 2011 following personal communication with the authors. The randomisation process was performed by a contracted company. A computer‐generated randomisation list was created using randomly permuted blocks. Allocation concealment was maintained for all study investigators and was only known by the contracted company, who, otherwise, had no involvement in the study. The methods used for random sequence generation or allocation concealment were not described in D'Haens 2010 and these items were rated as unclear. Allocation concealment was satisfactory in Sandborn 2012 and Travis 2014. The randomisation process was performed by an external contracted company. Patients were assigned to treatment groups in blocks of four, using random numbers. Rubin 2014 utilised a computer‐generated randomisation scheme and stratified by study centre. Allocation concealment was ensured by centralized randomisation via an interactive voice response system.
Blinding
Löfberg 1996 used a double‐blind, double‐dummy design. Patients were blinded to their treatment group. Pathologists were blinded to patient treatment groups during assessment of histological inflammation. However, from the published paper, we were unable to confirm that the treating physicians and study analysts were blinded to patient treatment groups. Gross 2011 used a double‐blind, double‐dummy design. All patients, treating physicians and outcome assessors were blinded to the treatments received. Patients and treating physicians were blinded in D'Haens 2010. Sandborn 2012 and Travis 2014 were both double‐blind, double‐dummy studies. Treating physicians and outcome assessors were blinded to the treatment groups. Rubin 2014 was a double‐blind study and patients, physicians and outcomes assessors were blinded to treatment allocation.
Incomplete outcome data
Löfberg 1996 randomised 75 patients, with 72 receiving a study drug. Thirty‐four patients received budesonide and 38 received prednisolone. No further information is provided on the 3 patients who were randomised but did not receive either study drug. The analysis in the published paper was a 'per‐protocol' analysis. All patients treated were accounted for by the authors. D'Haens 2010 report efficacy data for 32 of 36 patients initially randomised. Four patients were excluded from the efficacy analysis as they failed to meet study inclusion criteria (one patient had pan‐colitis and three patients were in clinical remission at entry. Outcome data were complete for the 32 included patients. Eighty‐four per cent (288/342) of patients completed the Gross 2011 study. All patients (including those who did not complete the study) were accounted for in the final analysis. Seventy‐one per cent (349/489) of patients completed the Sandborn 2012 study. All patients (including those who did not complete the study) were accounted for in the final analysis. The proportions of patients who did not complete the study as well as reasons for study discontinuation were similar across the treatment groups. Sixty‐six per cent (272/410) of patients completed the Travis 2014 study. The proportions of patients who did not complete the study as well as reasons for study discontinuation were similar across different treatment groups. All patients (including those who did not complete the study) were accounted for in the final analysis. Rubin 2014 reported on study discontinuation due to adverse events in the abstract. As per 'personal communication' with the author, Rubin accounted for all patients who did not complete the study as well as reasons for study discontinuation. The proportion of patients who completed the study was 89.1% (408/458).
Selective reporting
We found no evidence to indicate selective reporting in any of the six included studies.
Other potential sources of bias
Rubin 2014 was rated as unclear for other sources of bias because it was an abstract publication. The other studies appeared to be free of other sources of bias and were rated as low risk for this item.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Budesonide MMX® 9 mg versus placebo for induction of remission in ulcerative colitis.
| Budesonide MMX® 9 mg versus placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: Adult patients with active ulcerative colitis Settings: Outpatient Intervention: Budesonide MMX® 9 mg versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Budesonide MMX® 9 mg versus placebo | |||||
| Remission (combined clinical and endoscopic remission) | 68 per 10001 | 154 per 1000 (103 to 232) | RR 2.25 (1.5 to 3.39) | 900 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| Clinical improvement | 286 per 1000 | 371 per 1000 (283 to 486) | RR 1.3 (0.99 to 1.7) | 442 (2 studies) | ⊕⊕⊕⊝ moderate3 | |
| Endoscopic improvement | 324 per 1000 | 418 per 1000 (327 to 538) | RR 1.29 (1.01 to 1.66) | 442 (2 studies) | ⊕⊕⊕⊝ moderate4 | |
| Histologic remission | 123 per 1000 | 186 per 1000 (137 to 254) | RR 1.51 (1.11 to 2.06) | 900 (3 studies) | ⊕⊕⊝⊝ low5,6 | |
| Endoscopic remission | 143 per 1000 | 223 per 1000 (161 to 309) | RR 1.56 (1.13 to 2.16) | 695 (2 studies) | ⊕⊕⊕⊝ moderate7 | |
| Serious adverse events | 31 per 1000 | 27 per 1000 (10 to 74) | RR 0.88 (0.33 to 2.4) | 513 (2 studies) | ⊕⊕⊝⊝ low8 | |
| Adverse events | 412 per 1000 | 449 per 1000 (391 to 519) | RR 1.09 (0.95 to 1.26) | 971 (3 studies) | ⊕⊕⊕⊝ moderate9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials 2 Downgraded one level due to sparse data (101 events) 3 Downgraded one level due to sparse data (147 events) 4 Downgraded one level due to sparse data (165 events) 5 Downgraded one level due to moderate heterogeneity (I2 = 47%) 6 Downgraded one level due to sparse data (139 events) 7 Downgraded one level due to sparse data (128 events) 8 Downgraded two levels due to very sparse data (15 events) 9 Downgraded one level due to moderate heterogeneity (I2 = 54%)
Summary of findings 2. Budesonide MMX® 6 mg versus placebo for induction of remission in ulcerative colitis.
| Budesonide MMX® 6 mg versus placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: Adult patients with active ulcerative colitis Settings: Outpatient Intervention: Budesonide MMX® 6 mg versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Budesonide MMX® 6 mg versus placebo | |||||
| Remission (combined clinical and endoscopic remission | 62 per 10001 | 111 per 1000 (58 to 212) | RR 1.8 (0.94 to 3.42) | 440 (2 studies) | ⊕⊕⊝⊝ low2 | |
| Clinical improvement | 286 per 10001 | 283 per 1000 (209 to 380) | RR 0.99 (0.73 to 1.33) | 440 (2 studies) | ⊕⊕⊝⊝ low3,4 | |
| Endoscopic improvement | 324 per 10001 | 311 per 1000 (236 to 411) | RR 0.96 (0.73 to 1.27) | 440 (2 studies) | ⊕⊕⊕⊝ moderate5 | |
| Histologic remission | 67 per 10001 | 82 per 1000 (42 to 160) | RR 1.23 (0.63 to 2.4) | 440 (2 studies) | ⊕⊕⊝⊝ low6 | |
| Serious adverse events | 31 per 10001 | 20 per 1000 (7 to 59) | RR 0.63 (0.21 to 1.91) | 512 (2 studies) | ⊕⊕⊝⊝ low7 | |
| Adverse events | 535 per 10001 | 604 per 1000 (519 to 706) | RR 1.13 (0.97 to 1.32) | 512 (2 studies) | ⊕⊝⊝⊝ very low8,9 | |
| Withdrawal due to adverse events | 163 per 10001 | 179 per 1000 (90 to 362) | RR 1.10 (0.55 to 2.22) | 512 (2 studies) | ⊕⊕⊝⊝ low10,11 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials 2 Downgraded two levels due to very sparse data (38 events) 3 Downgraded one level due to moderate heterogeneity (I2 = 60%) 4 Downgraded one level due to sparse data (125 events) 5 Downgraded one level due to sparse data (139 events) 6 Downgraded two levels due to very sparse data (33 events) 7 Downgraded two levels due to very sparse data (13 events) 8 Downgraded two levels due to high heterogeneity (I2 = 86%) 9 Downgraded one level due to sparse data (292 events) 10 Downgraded one level due to moderate heterogeneity (I2 = 69%) 11 Downgraded one level due to sparse data (88 events)
Summary of findings 3. Budesonide 10 mg versus prednisolone 40 mg for induction of remission in ulcerative colitis.
| Budesonide 10 mg versus prednisolone 40 mg for induction of remission in ulcerative colitis | ||||||
| Patient or population: Adult patients with active ulcerative colitis Settings: Multicentre study in Sweden (outpatients) Intervention: Budesonide 10 mg/day versus prednisolone 40 mg/day | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Budesonide versus prednisolone | |||||
| Endoscopic improvement | 658 per 10001 | 618 per 1000 (434 to 875) | RR 0.94 (0.66 to 1.33) | 72 (1 study) | ⊕⊕⊝⊝ low2,3 | |
| Endoscopic remission | 158 per 10001 | 118 per 1000 (36 to 382) | RR 0.75 (0.23 to 2.42) | 72 (1 study) | ⊕⊝⊝⊝ very low2,4 | |
| Histologic remission | 158 per 10001 | 88 per 1000 (24 to 325) | RR 0.56 (0.15 to 2.06) | 72 (1 study) | ⊕⊝⊝⊝ very low2,5 | |
| Adverse event ‐ Reduction in plasma cortisol below lower reference limit | 758 per 10001 | 15 per 1000 (0 to 227) | RR 0.02 (0 to 0.3) | 67 (1 study) | ⊕⊝⊝⊝ very low2,6 | Per‐protocol analysis7 |
| Study withdrawals | 211 per 10001 | 236 per 1000 (99 to 558) | RR 1.12 (0.47 to 2.65) | 72 (1 study) | ⊕⊝⊝⊝ very low2,8 | |
| Withdrawal due to adverse event | 211 per 10001 | 207 per 1000 (84 to 508) | RR 0.98 (0.40 to 2.41) | 72 (1 study) | ⊕⊝⊝⊝ very low2,9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials 2 Downgraded one level because sequence generation and allocation concealment were unclear 3 Downgraded one level due to sparse data (46 events) 4 Downgraded two levels due to very sparse data (10 events) 5 Downgraded two levels due to very sparse data (9 events) 6 Downgraded two levels due to very sparse data (25 events) 7 Suppression of plasma cortisol below baseline at 2 weeks. Cortisol levels improved over time in the prednisolone‐treated group, with no significant difference in levels compared to budesonide at 9 weeks 8 Downgraded two levels due to very sparse data (16 events) 9 Downgraded two levels due to very sparse data (15 events)
Summary of findings 4. Budesonide versus mesalamine for induction of remission in ulcerative colitis.
| Budesonide versus mesalazine for induction of remission in ulcerative colitis | ||||||
| Patient or population: Adult patients with active ulcerative colitis Settings: Outpatient Intervention: Budesonide versus mesalazine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Budesonide versus mesalazine | |||||
| Clinical remission (ITT analysis) | 548 per 10001 | 395 per 1000 (312 to 499) | RR 0.72 (0.57 to 0.91) | 343 (1 study) | ⊕⊕⊕⊝ moderate2 | |
|
Endoscopic improvement Gross 2011 |
819 per 10001 | 688 per 1000 (606 to 778) | RR 0.84 (0.74 to 0.95) | 343 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Endoscopic remission (EI ≤ 1) | 392 per 10001 | 305 per 1000 (227 to 407) | RR 0.78 (0.58 to 1.04) | 343 (1 study) | ⊕⊕⊕⊝ moderate4 | |
|
Histologic remission Gross 2011 |
584 per 10001 | 473 per 1000 (385 to 578) | RR 0.81 (0.66 to 0.99) | 343 (1 study) | ⊕⊕⊕⊝ moderate5 | |
|
Adverse events Gross 2011 |
253 per 10001 | 266 per 1000 (185 to 380) | RR 1.05 (0.73 to 1.50) | 343 (1 study) | ⊕⊕⊕⊝ moderate6 | |
| Remission (combined clinical and endoscopic remission) | 121 per 10001 | 179 per 1000 (98 to 328) | RR 1.48 (0.81 to 2.71) | 247 (1 study) | ⊕⊕⊝⊝ low7 | |
| Clinical improvement | 339 per 10001 | 332 per 1000 (234 to 474) | RR 0.98 (0.69 to 1.4) | 247 (1 study) | ⊕⊕⊕⊝ moderate8 | |
| Serious adverse events | 31 per 10001 | 24 per 1000 (5 to 103) | RR 0.75 (0.17 to 3.28) | 254 (1 study) | ⊕⊕⊝⊝ low9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials 2 Downgraded one level due to sparse data (161 events) 3 Downgraded one level due to sparse data (258 events) 4 Downgraded one level due to sparse data (119 events) 5 Downgraded one level due to sparse data (181 events) 6 Downgraded one level due to sparse data (89 events) 7 Downgraded two levels due to very sparse data (37 events) 8 Downgraded one level due to sparse data (83 events) 9 Downgraded two levels due to very sparse data (7 events)
Summary of findings 5. Budesonide MMX® 9 mg versus Entocort EC 9mg for induction of remission in ulcerative colitis.
| Budesonide MMX® 9mg versus Entocort EC 9mg for induction of remission in ulcerative colitis | ||||||
| Patient or population: Adult patients with active ulcerative colitis Settings: Outpatients Intervention: Budesonide MMX® 9mg versus Entocort EC 9mg | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Budesonide MMX® 9mg versus Entocort EC 9mg | |||||
| Remission (combined clinical and endoscopic remission) | 126 per 1000 | 174 per 1000 (91 to 334) | RR 1.38 (0.72 to 2.65) | 212 (1 study) | ⊕⊕⊝⊝ low2 | |
| Clinical improvement | 330 per 1000 | 423 per 1000 (297 to 601) | RR 1.28 (0.9 to 1.82) | 212 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Endoscopic improvement | 369 per 1000 | 421 per 1000 (302 to 590) | RR 1.14 (0.82 to 1.60) | 212 (1 study) | ⊕⊕⊕⊝ moderate4 | |
| Histologic remission | 136 per 1000 | 164 per 1000 (87 to 314) | RR 1.21 (0.64 to 2.31) | 212 (1 study) | ⊕⊕⊝⊝ low2 | |
| Serious adverse events | 8 per 1000 | 31 per 1000 (4 to 276) | RR 3.94 (0.45 to 34.74) | 254 (1 study) | ⊕⊕⊝⊝ low5 | |
| Adverse events | 548 per 1000 | 553 per 1000 (444 to 690) | RR 1.01 (0.81 to 1.26) | 254 (1 study) | ⊕⊕⊕⊝ moderate6 | |
| Withdrawal due to adverse events | 190 per 1000 | 179 per 1000 (106 to 300) | RR 0.94 (0.56 to 1.58) | 254 (1 study) | ⊕⊕⊕⊝ moderate7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials 2 Downgraded two levels due to very sparse data (32 events) 3 Downgraded one level due to sparse data (80 events) 4 Downgraded one level due to sparse data (84 events) 5 Downgraded two levels due to very sparse data (5 events) 6 Dowgraded one level due to sparse data (140 events) 7 Dowgraded one level due to sparse data (47 events)
PRIMARY OUTCOME:
Induction of clinical remission in ulcerative colitis:
We assessed the primary outcome of interest in five studies (D'Haens 2010; Gross 2011; Sandborn 2012; Rubin 2014; Travis 2014).
Budesonide versus placebo
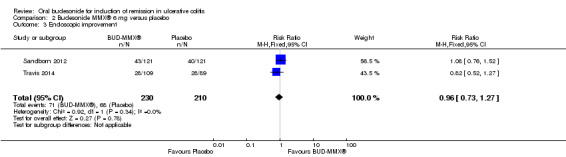
Data from three studies (900 participants) were combined in a meta‐analysis (Sandborn 2012; Rubin 2014; Travis 2014). The primary outcome was a combined clinical and endoscopic remission. Budesonide‐MMX® 9 mg daily was superior to placebo for inducing remission at eight weeks. Fifteen per cent (71/462) of patients in the budesonide‐MMX® 9 mg group achieved remission compared to 7% (30/438) placebo patients (RR 2.25, 95% CI 1.50 to 3.39; Figure 3). A GRADE analysis indicated that the quality of evidence supporting the primary outcome was moderate due to sparse data (101 events, See Table 1). A pooled analysis of two studies (440 participants) suggests that a lower dose of budesonide‐MMX® 6 mg was not superior to placebo for induction of remission (Sandborn 2012; Travis 2014). Eleven per cent (25/230) of patients in the budesonide‐MMX® 6 mg group achieved remission compared to 6% (13/210) of placebo patients (RR 1.80, 0.94 to 3.42) (Analysis 2.1). A GRADE analysis indicated that the quality of evidence supporting the primary outcome was low due to very sparse data (38 events, See Table 2).
3.
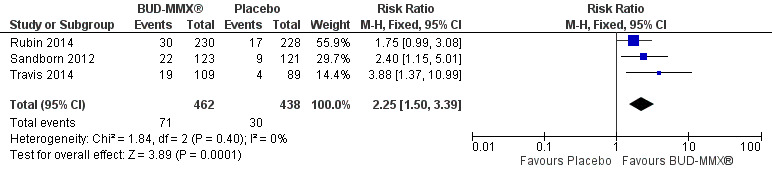
Forest plot of comparison: 1 Budesonide MMX® 9 mg versus placebo, outcome: 1.1 Remission (combined clinical and endoscopic remission).
2.1. Analysis.
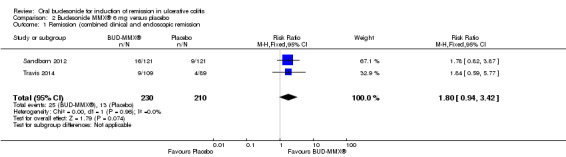
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 1 Remission (combined clinical and endoscopic remission.
D'Haens 2010 found no difference in the proportion of patients who achieved either clinical remission or a 50% reduction in CAI score at four weeks. Forty‐seven per cent (8/17) of budesonide patients were in clinical remission or had a reduction in CAI score of at least 50% at 4 weeks compared to 33% (5/15) of placebo patients (RR 1.41, 95% CI 0.59 to 3.39)..
Subgroup analysis: remission rates according to concurrent mesalamine use:
While Rubin 2014 evaluated the efficacy of budesonide‐MMX® in patients with active disease despite treatment with mesalamine, Sandborn 2012 and Travis 2014 excluded patients who used mesalamine. In this latter population, 18% (41/232) of budesonide‐MMX® 9 mg patients achieved remission compared to 6% (13/210) of placebo patients. The relative risk was 2.89 (95% CI 1.59 to 5.25; Figure 4), suggesting that budesonide‐MMX® may be more effective in patients who are not mesalamine‐refractory. As pre‐specified, we performed a sensitivity analysis excluding studies published as abstracts. This did not change the conclusion that budesonide‐MMX® 9 mg was more efficacious than placebo for inducing remission in patients with active ulcerative colitis. The Rubin 2014 study was the only study published in abstract form, therefore the sensitivity analysis mirrors the above subgroup analysis on the use of concurrent mesalamine.
4.
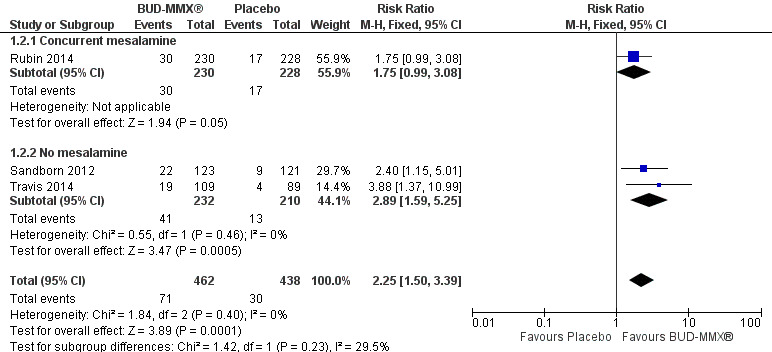
Forest plot of comparison: 1 Budesonide MMX® 9 mg versus placebo, outcome: 1.2 Remission (combined clinical and endoscopic remission): subgroup by mesalamine use.
Subgroup analysis: Remission rates according to disease location:
A pooled analysis of two studies (Sandborn 2012; Travis 2014), shows that budesonide‐MMX® 9 mg daily was significantly more efficacious than placebo for treatment of patients with left‐sided disease (289 patients) but not for patients with extensive disease (145 patients). Among those with left‐sided disease 22% (32/145) of budesonide‐MMX® 9 mg patients entered remission compared to 8% (11/144) of placebo patients (RR 2.98, 95% CI 1.56 to 5.67). Among those with extensive disease 9% (8/85) of budesonide‐MMX® 9 mg patients entered remission compared to 3% (2/60) of placebo patients (RR 2.41, 95% CI 0.61 to 9.56) (Analysis 1.3, Figure 5).
1.3. Analysis.
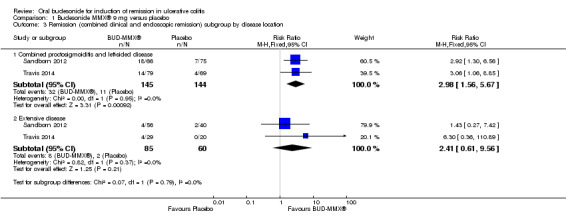
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 3 Remission (combined clinical and endoscopic remission) subgroup by disease location.
5.
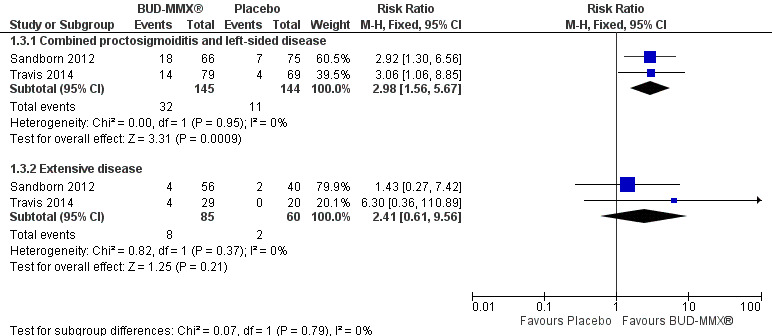
Forest plot of comparison: 4 Budesonide MMX® 9mg versus Placebo, outcome: 4.2 Remission (combined clinical and endoscopic remission) according to disease location.
Budesonide versus prednisolone
Clinical remission was not assessed as an outcome in the Löfberg 1996 study.
Budesonide versus mesalamine
Data were not pooled for meta‐analysis for this comparison because of differences in drug regimens and outcomes. Gross 2011 utilized clinical remission as an outcome and compared 9 mg/day budesonide to 3 g/day mesalamine (Salofalk®). The definition of remission in the Sandborn 2012 study included clinical and endoscopic remission. This study compared budesonide MMX® 9 mg/day to mesalamine 2.4 g/day (Asacol®).
For the Gross 2011 study 40% (70/177) of patients in the budesonide group were in clinical remission at 8 weeks compared to 55% (91/166) of patients in the mesalamine group (RR 0.72, 95% CI 0.57 to 0.91) (Analysis 4.1 & Figure 3). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (161 events, See Table 4).
4.1. Analysis.
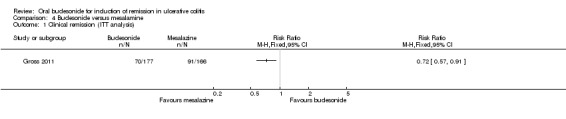
Comparison 4 Budesonide versus mesalamine, Outcome 1 Clinical remission (ITT analysis).
Sandborn 2012 did not find a significant difference in remission rates at eight weeks between Budesonide‐MMX® 9 mg daily and Asacol (mesalamine) 2.4 g daily. Eighteen per cent (22/123) of budesonide patients achieved remission compared to 12% (15/124) placebo patients (RR 1.48, 95% CI 0.81 to 2.71); however the study was not sufficiently powered to make this comparison. A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to very sparse data (37 events, See Table 4).
Budesonide MMX® versus standard budesonide
Travis 2014 did not find a significant difference between in remission rates at eight weeks between budesonide‐MMX® 9 mg dally and Entocort (budesonide controlled ileal release) 9 mg daily. Seventeen per cent (19/109) of budesonide‐MMX® 9 mg patients achieved remission compared to 13% of Entocort patients (RR 1.38, 95% CI 0.72 to 2.65; Analysis 5.1); however the study was not sufficiently powered to make this comparison.
5.1. Analysis.
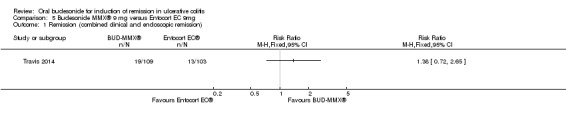
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 1 Remission (combined clinical and endoscopic remission).
SECONDARY OUTCOMES:
1. Clinical, Endoscopic and Histologic Improvement:
(i) Clinical Improvement:
Budesonide versus placebo
A pooled analysis of two studies (442 participants) showed no statistically significant difference in clinical improvement rates at week eight between budesonide‐MMX® 9 mg daily and placebo treated patients (Sandborn 2012; Travis 2014). Thirty‐eight per cent (87/332) of budesonide‐MMX® 9 mg patients experienced clinical improvement compared to 29% (60/210) placebo (RR 1.30, 95% CI 0.99 to 1.70) (Analysis 1.10). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (147 events, See Table 1). A pooled analysis of two studies (440 participants showed no statistically significant difference in clinical improvement rates between budesonide‐MMX® 6 mg daily and placebo treated patients. Twenty‐eight per cent (65/230) of budesonide‐MMX® 6 mg patients improved clinically compared to 29% (60/210) of placebo patients (RR 0.99, 95% CI 0.73 to 1.33) (Analysis 2.2). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to moderate heterogeneity (I2 = 60%) and sparse data (125 events, See Table 2).
1.10. Analysis.
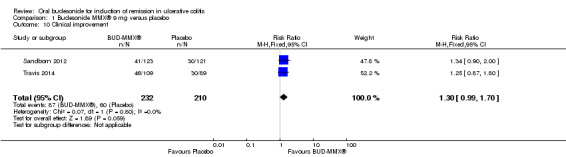
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 10 Clinical improvement.
2.2. Analysis.
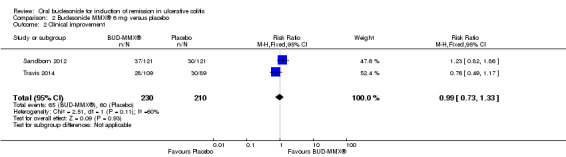
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 2 Clinical improvement.
A pooled analysis of two studies (442 participants) shows that treatment with budesonide‐MMX® 9 mg was significantly more likely to result in resolution of symptoms than treatment with placebo (Sandborn 2012; Travis 2014). Twenty‐six per cent (61/232) of budesonide‐MMX® 9 mg patients experienced resolution of symptoms compared to 14% (30/210) of placebo patients (RR 1.86; 95% CI 1.25 to 2.77) (Analysis 1.11, Figure 5).
1.11. Analysis.
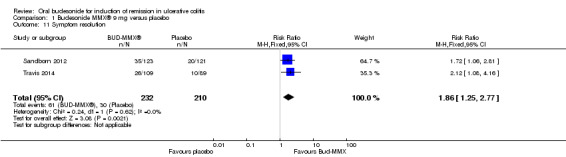
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 11 Symptom resolution.
D'Haens 2010 found no statistically significant difference in clinical improvement at four weeks. Forty‐seven per cent (8/17) of budesonide patients improved clinically by 4 weeks compared to 33% (5/15) of placebo patients (RR 1.41, 95% CI 0.59 to 3.39). Six per cent (1/17) of budesonide‐treated patients compared to 33% (5/15) of placebo‐treated patients experienced either no change or worsening clinical status at 4 weeks (RR 0.18, 95% CI 0.02 to 1.35).
Budesonide versus prednisolone
Löfberg 1996 report that there was a similar improvement in bowel symptoms (i.e. number of bowel movements, mucus discharge, with and without blood) in both the budesonide and prednisolone treatment groups. The exact number of patients with improved clinical symptoms in each treatment group was not reported.
Budesonide versus mesalamine
Gross 2011 used the Physician's Global assessment to define 'therapeutic success' (marked clinical improvement) and 'therapeutic benefit' (at least slight improvement seen with treatment). There was a statistically significant difference in therapeutic success favouring mesalamine over budesonide. Fifty‐one per cent (91/177) of budesonide patients experienced 'therapeutic success compared to 69% (114/166) of mesalamine patients (RR 0.75, 95% CI 0.63 to 0.89) (Analysis 4.3). There was a statistically significant difference in therapeutic benefit favouring mesalamine over budesonide. Seventy‐seven per cent (136/177) of budesonide patients experienced 'benefit' compared to 86% (142/166) of mesalamine patients (RR 0.90, 95% CI 0.81 to 0.99) (Analysis 4.4).
4.3. Analysis.
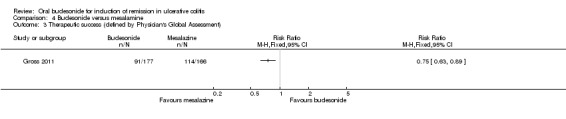
Comparison 4 Budesonide versus mesalamine, Outcome 3 Therapeutic success (defined by Physician's Global Assessment).
4.4. Analysis.
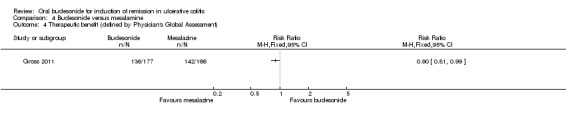
Comparison 4 Budesonide versus mesalamine, Outcome 4 Therapeutic benefit (defined by Physician's Global Assessment).
Sandborn 2012 found no significant difference in clinical improvement rates at eight weeks. Thirty‐three per cent (41/123) of budesonide‐MMX® 9 mg patients improved clinically at eight weeks compared to 34% (42/124) of Asacol (mesalamine) patients (RR 0.98, 95% CI 0.69‐1.40). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (83 events, See Table 4).
Budesonide MMX® versus standard budesonide
Travis 2014 found no statistically significant difference in clinical improvement rates at eight weeks. Forty‐two per cent (46/109) of budesonide‐MMX® 9 mg patients improved clinically at eight weeks compared to 33% (34/103) Entocort (budesonide controlled ileal release) patients (RR 1.28, 95% CI 0.90 to 1.82) (Analysis 5.2). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (80 events, See Table 5).
5.2. Analysis.
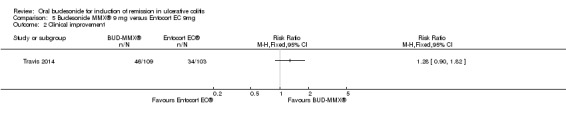
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 2 Clinical improvement.
(ii) Endoscopic Improvement:
Budesonide versus placebo
A pooled analysis of two studies (442 participants) showed a statistically significant difference in endoscopic improvement rates at week eight between budesonide‐MMX® 9 mg daily and placebo treated patients (Sandborn 2012; Travis 2014). Forty‐one per cent (97/232) of budesonide‐MMX® 9 mg patients had endoscopic improvement at 8 weeks compared to 32% (68/210) of placebo patients (RR 1.29, 95% CI 1.01 to 1.66) (Analysis 1.12). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (165 events, See Table 1). A pooled analysis of two studies (440 participants) showed no statistically significant difference in endoscopic improvement rates at eight weeks in patients randomised to budesonide‐MMX® 6 mg daily compared to placebo (Sandborn 2012; Travis 2014). Thirty‐one per cent (71/230) of budesonide‐MMX® 6 mg patients had endoscopic improvement at week 8 compared to 32% (68/210) of placebo patients (RR 0.96, 95% CI 0.73 to 1.27).(Analysis 2.3). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (139 events, See Table 2).
1.12. Analysis.
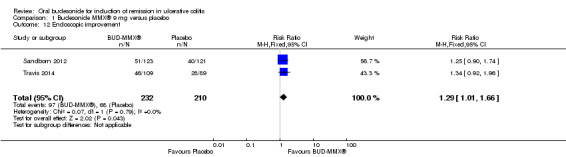
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 12 Endoscopic improvement.
2.3. Analysis.
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 3 Endoscopic improvement.
In D'Haens 2010, 32 patients underwent endoscopy at baseline and 31 of 32 underwent a repeat endoscopy at 4 weeks. There was no significant difference in baseline endoscopic scores between the treatment groups (MD 0.53, 95% CI ‐0.83 to 1.89). There was an improvement in endoscopic index scores in both treatment groups over 4 weeks. In the budesonide‐treated group, the mean difference in endoscopic index score at baseline and 4 weeks was 2.62 (95% CI 0.81 to 4.43). In the placebo‐treated group, the mean difference in endoscopic index score at baseline and at 4 weeks was 2.20 (95% CI 0.49 to 3.91). There was no statistically significant difference in the mean endoscopic index scores at 4 weeks in the budesonide‐ and placebo‐treated groups. The mean difference in endoscopic index score was 0.11 (95% CI ‐1.98 to 2.20).
Budesonide versus prednisolone
In Löfberg 1996, an improvement in endoscopic score was a primary outcome of the study. There was no statistically significant difference in endoscopic improvement at four weeks. Sixty‐two per cent (21/34) of budesonide patients had endoscopic improvement at 4 weeks compared to 66% (25/38) of prednisolone patients (RR 0.94, 95% CI 0.66 to 1.33) (Analysis 3.1). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to unclear risk of bias and sparse data (46 events, See Table 3). The authors analysed endoscopic scores separately for each colonic segment and found that in the sigmoid segment, the reduction in endoscopic score was greatest in the prednisolone‐treated group (P = 0.04) at 4 weeks, based upon a per‐protocol analysis. Original data were not provided; therefore we were unable to perform an intention‐to‐treat analysis.
3.1. Analysis.
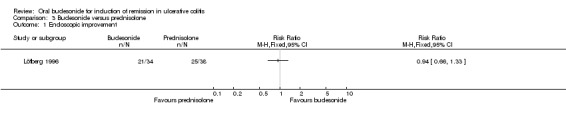
Comparison 3 Budesonide versus prednisolone, Outcome 1 Endoscopic improvement.
Budesonide versus mesalamine
Gross 2011 found a statistically significant difference in the proportion of patients who had endoscopic improvement (drop in EI (endoscopic index) ≥ 1) at eight weeks. Sixty‐nine per cent (122/177) of budesonide patients had endoscopic improvement at 8 weeks compared to 82% (136/166) of mesalamine patients (RR 0.84, 95% CI 0.74 to 0.95) (Analysis 4.5). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (258 events, See Table 4). Sandborn 2012 found no statistically significant difference in rates of endoscopic improvement amongst those treated with budesonide‐MMX® 9mg daily and Asacol 2.4 mg daily. Forth‐one per cent (51/123) had endoscopic improvement at eight weeks compared to 33% of Asacol patients (RR 1.25, 95% CI 0.90 to 1.74).
4.5. Analysis.
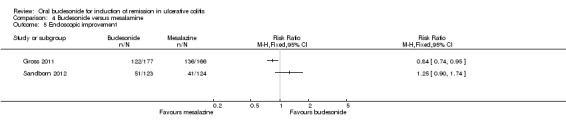
Comparison 4 Budesonide versus mesalamine, Outcome 5 Endoscopic improvement.
Budesonide MMX® versus standard budesonide
Travis 2014 found no statistically significant difference in endoscopic improvement rates amongst budesonide‐MMX® treated patients and those receiving Entocort. Forty‐two per cent (46/109) of budesonide‐MMX® patients had endoscopic improvement at eight weeks compared to 37% (38/103) of Entocort patients (RR 1.14, 95% CI 0.82 to 1.60) (Analysis 5.3). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (84 events, See Table 5).
5.3. Analysis.
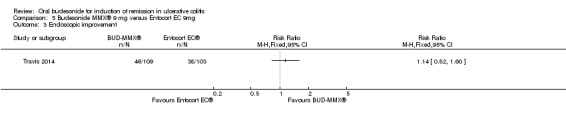
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 3 Endoscopic improvement.
(iii) Histologic Improvement or remission:
Budesonide versus placebo
A pooled analysis of three studies (900 participants) showed a statistically significant difference in histologic remission rates at eight weeks (Sandborn 2012; Rubin 2014; Travis 2014). Eighteen per cent (85/462) of budesonide‐MMX® 9 mg patients achieved histologic remission at week 8 compared to 12% (54/438) of placebo patients (RR 1.51, 95% CI 1.11 to 2.06). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to moderate heterogeneity (I2 = 47%) and sparse data (139 events, See Table 1). A sensitivity analysis (two studies, 442 participants) excluding the study where patients received concurrent mesalamine showed no statistically significant difference in histologic remission rates (Sandborn 2012; Travis 2014). Ten per cent (23/232) of budesonide‐MMX® 9 mg patients achieved histologic remission compared to 7% (14/210) of placebo patients (RR 1.44, 95% CI 0.75 to 2.75). This suggests that mesalamine may improve histologic outcomes. A pooled analysis of two studies (440 patients) showed no statistically significant difference in histologic remission rates among patients receiving 6 mg budesonide‐MMX® or placebo (Sandborn 2012; Travis 2014). Eight per cent (19/230) of budesonide‐MMX® 6 mg patients were in histologic remission at week eight compared to 7% (14/210) of placebo patients (RR 1.23, 95% CI 0.63 to 2.40) (Analysis 2.4). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due very sparse data (33 events, See Table 2).
2.4. Analysis.
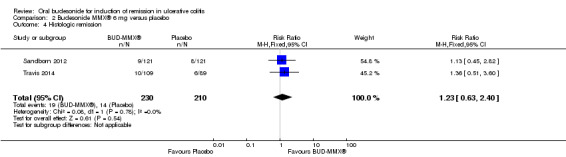
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 4 Histologic remission.
In D'Haens 2010, there was no statistically significant difference in histologic scores at baseline and at four weeks in either the budesonide‐ or placebo‐treated groups. In the budesonide‐treated group, the mean difference in the histologic index score at baseline and at 4 weeks was 0.13 (95% CI ‐0.30 to 0.56). In the placebo‐treated group, the mean difference in histologic index score at baseline and at 4 weeks was ‐0.13 (95% CI ‐0.79 to 0.53). At 4 weeks, there was no statistically significant difference in histologic scores in the budesonide‐treated group in comparison with the placebo‐treated group. The mean difference was ‐0.11 (95% CI ‐0.63 to 0.41).
Budesonide versus prednisolone
In Löfberg 1996, the authors report that histological inflammation scores were significantly reduced from baseline in both treatment groups and that the reduction was significantly greater in patients treated with prednisolone (P = 0.02). However, original data were not reported and the authors used a per‐protocol analysis in the published paper. The improvement seen in the prednisolone‐treated group was limited to the sigmoid and descending colonic segments. There was no statistically significant difference in histologic remission rates at eight weeks. Nine per cent of (3/34) budesonide patients achieved histologic remission at eight weeks compared to 16% (6/38) of prednisolone patients (RR 0.56, 95% CI 0.15 to 2.06) (Analysis 3.3). A GRADE analysis indicated that the quality of evidence supporting this outcome was very low due to unclear risk of bias and very sparse data (9 events, See Table 3).
3.3. Analysis.
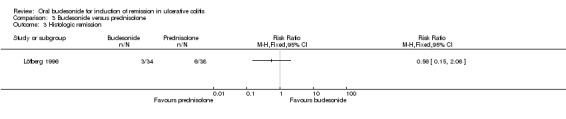
Comparison 3 Budesonide versus prednisolone, Outcome 3 Histologic remission.
Budesonide versus mesalamine
In Gross 2011, there was a statistically significant difference in histologic remission rates at eight weeks. Histological remission was achieved in 48% (84/177) of budesonide patients compared to 58% (97/166) of mesalamine patients (RR 0.81, 95% CI 0.66 to 0.99) (Analysis 4.7). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (181 events, See Table 4). Sandborn 2012 also found a statistically significant difference in histologic remission rates at eight weeks. Four per cent (5/123) of budesonide‐MMX® 9 mg patients achieved histologic remission at 8 weeks compared to 11% (14/124) of Asacol patients (RR 0.36, 95% CI 0.13 to 0.97).
4.7. Analysis.
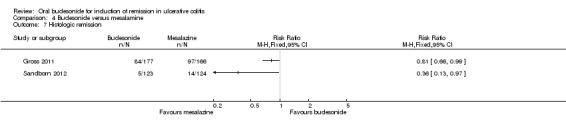
Comparison 4 Budesonide versus mesalamine, Outcome 7 Histologic remission.
Budesonide MMX® versus standard budesonide
Travis 2014 found no statistically significant difference in histologic remission rates in patients treated with budesonide‐MMX® 9 mg daily or Entocort (budesonide controlled ileal release) (RR 1.21, 95% CI 0.64 to 2.31) (Analysis 5.4). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to very sparse data (32 events, See Table 5).
5.4. Analysis.
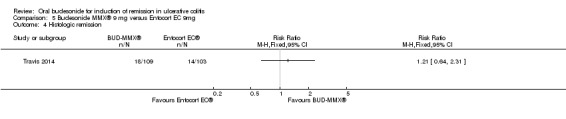
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 4 Histologic remission.
2. Endoscopic Mucosal Healing (also known as Endoscopic Remission):
Budesonide versus placebo
A pooled analysis of two studies (695 participants) showed a statistically significant difference in endoscopic remission rates at eight weeks (Sandborn 2012; Rubin 2014). Twenty‐two per cent (79/352) of budesonide‐MMX® 9 mg patients had endoscopic remission at week eight compared to 14% (49/343) of placebo patients (RR 1.56, 95% CI 1.13 to 2.16; Figure 6). .A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (128 events, See Table 1). A sensitivity analysis removing the study where patients were co‐treated with mesalamine found no statistically significant difference endoscopic remission rates at eight weeks. Twenty‐seven per cent (33/122) of budesonide‐MMX® 9 mg patients entered endoscopic remission at week 8 compared to 18% (21/115) of placebo patients (RR 1.48, 95% CI 0.91 to 2.40). This suggests that mesalamine may independently improve endoscopic outcomes.
D'Haens 2010 did not assess endoscopic mucosal healing as an outcome.
Budesonide versus prednisolone
Löfberg 1996 found no statistically significant difference in endoscopic remission rates (defined as a normal or non‐inflamed mucosa) at four weeks. Twelve per cent (4/34) of budesonide patients were in endoscopic remission at 4 weeks compared to 16% (6/38) of prednisolone patients (RR 0.75, 95% CI 0.23 to 2.42) (Analysis 3.2). A GRADE analysis indicated that the quality of evidence supporting this outcome was very low due to unclear risk of bias and very sparse data (10 events, See Table 3).
3.2. Analysis.
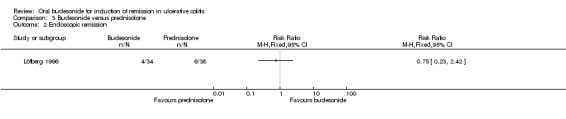
Comparison 3 Budesonide versus prednisolone, Outcome 2 Endoscopic remission.
Budesonide versus mesalamine
There was no statistically significant difference in the proportion of patients achieving mucosal healing (EI ≤ 1) in the Gross 2011 study. Thirty per cent (54/177) of budesonide patients entered endoscopic remission at week eight compared to 39% (65/166) of mesalamine patients (RR 0.78, 95% CI 0.58 to 1.04) (Analysis 4.6). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (119 events, See Table 4).
4.6. Analysis.
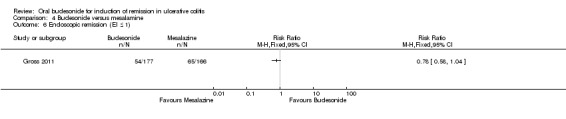
Comparison 4 Budesonide versus mesalamine, Outcome 6 Endoscopic remission (EI ≤ 1).
Budesonide MMX® versus standard budesonide
Data on mucosal healing were not reported in isolation from clinical remission data in the Travis 2014 study.
3. Change in Disease Activity Index Score.
Budesonide versus placebo
In D'Haens 2010, a reduction in CAI of at least 50% was seen 8 of 17 (47.1%) patients in the budesonide group and in 5 of 15 (33.3%) patients in the placebo group. The relative risk was 1.41 (95% CI 0.59 to 3.39). Sandborn 2012 and Travis 2014 defined clinical improvement as a ≥ 3 point reduction in UCDAI score. Clinical improvement rates are described above.
Budesonide versus prednisolone
Change in disease activity index score was not a study outcome in Löfberg 1996.
Budesonide versus mesalamine
Gross 2011 found that there was a reduction in CAI score in both treatments group, with a greater reduction in the mesalamine group. The mean difference in CAI score at the end of treatment was 1.0 (95% CI 0.21 to 1.79).
Budesonide MMX® versus standard budesonide
The Travis 2014 study did not report on change in mean UCDAI scores.
4‐7. Quality of Life, Hospital Admissions, the Need for Intravenous Corticosteroids and Surgery
These outcomes were not measured in any of the six included studies.
8. Adverse Events:
Budesonide versus placebo
A pooled analysis of three studies (971 participants) showed no statistically significant difference in the proportion of patients who experienced at least one adverse event (Sandborn 2012; Rubin 2014; Travis 2014). Forty‐five per cent (217/485) of budesonide MMX® 9 mg patients experienced at least one adverse event compared to 41% (200/486) of placebo patients (RR 1.09, 95% CI 0.95 to 1.26). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to moderate heterogeneity (I2 = 54%, See Table 1). Similar results were found for the comparison budesonide MMX® 6 mg versus placebo (2 studies, 512 participants; Sandborn 2012; Travis 2014). Sixty‐one per cent (154/254) of budesonide MMX® 6 mg patients experienced at least one adverse event compared to 53% of placebo patients (RR 1.13, 95% CI 0.97 to 1.32). A GRADE analysis indicated that the quality of evidence supporting this outcome was very low due to a high level of heterogeneity (I2 = 86%) and sparse data (292 events, See Table 2). Commonly reported adverse events include worsening ulcerative colitis, headache, pyrexia, insomnia, back pain, nausea, abdominal pain, diarrhoea, flatulence and nasopharyngitis (Sandborn 2012; Travis 2014).
Pooled data from Sandborn 2012 and Travis 2014 showed no statistically significant difference in the proportion of patients who experienced a serious adverse event. Serious adverse events occurred in 3% (7/255) of patients randomised to 9 mg budesonide‐MMX® daily compared to 3% (8/258) of patients randomised to placebo (RR 0.88, 95% CI 0.33 to 2.40) (Analysis 1.19). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to very sparse data (15 events, See Table 1). Two events in the budesonide‐treated group were felt to be treatment‐related compared to no events in the placebo‐treated group (RR 3.04, 95% CI 0.32 to 28.99). When budesonide MMX® 6 mg was compared to placebo there was no statistically significant difference in serious adverse event rates (2 studies, 512 participants; Sandborn 2012; Travis 2014). Two per cent (5/254) of patients in the budesonide MMX® 6 mg group experienced a serious adverse event compared to 3% (8/258) of placebo patients. A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to very sparse data (13 events, See Table 2). Three events in the budesonide‐treated group were felt to be treatment‐related compared to no events in the placebo group (RR 4.06, 95% CI 0.46 to 36.10).
1.19. Analysis.
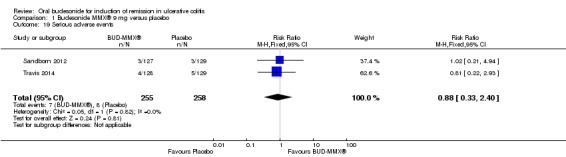
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 19 Serious adverse events.
Changes in plasma cortisol levels occurred in both budesonide‐MMX® groups in Sandborn 2012 and Travis 2014. Sandborn 2012 reported a reduction in baseline cortisol of 17.9% and 9.4% in patients randomised to the 9 mg and 6 mg doses, respectively. In contrast there was a +0.9% and a +5.3% increase in baseline cortisol level in the Asacol (mesalamine) and placebo groups. Travis 2014 reported a reduction in baseline cortisol of 28.8% and 13.2% in the budesonide‐MMX® 9 mg and 6 mg groups, respectively. The Entocort (budesonide controlled ileal release) group had a reduction in baseline morning cortisol of 12.7% and the placebo group had an increase in baseline morning cortisol of 8.3%. Throughout the study period, in all treatment groups (both studies), the mean baseline cortisol remained within normal limits (5 to 25 μg/dL). Despite the reduction in morning cortisol levels in the budesonide‐MMX® groups, this did not appear to be related to the presence of glucocorticoid side‐effects which occurred with similar frequency in budesonide and placebo groups with a risk ratio of 0.90 (95% CI 0.53 to 1.53).
D'Haens 2010 report that 6 of 12 subjects tested had morning cortisol levels below the normal range in the budesonide group at 4 weeks in comparison to 0 of 14 patients tested in the placebo group. The risk ratio was 15.0 (95% CI 0.93 to 241.52). Headaches and abdominal upset were experienced in 11.9% and 8.5% of budesonide‐treated patients respectively. Adverse events occurring in the placebo‐treated group are not described.
Budesonide versus prednisolone
In Löfberg 1996 adverse events are described as being 'mild' in both groups. No changes in blood pressure were noted in either group. Budesonide had no impact on mean plasma cortisol levels; however, there was a significant depression of plasma cortisol in the prednisolone group. Morning cortisol levels were tested in 31 of 34 (91.2%) patients in the budesonide group and 33 of 38 (86.8%) patients in the prednisolone group. No patient in the budesonide group had a plasma cortisol level below the lower reference limit, while 25 of 33 (75.8%) patients in the prednisolone group had plasma cortisol levels below the lower reference limit at some point during the study. The risk ratio was 0.02 (95% CI 0.00 to 0.33) (Analysis 3.4). A GRADE analysis indicated that the quality of evidence supporting this outcome was very low due to unclear risk of bias and very sparse data (25 events, See Table 3). One patient in the prednisolone group developed a Cushing's‐like syndrome. Gastrointestinal adverse events were more common in the budesonide group (35%) compared to the prednisolone group (10%). The authors state that weight gain was greater in the prednisolone treated group; however no original data were provided in the published paper and the authors reported a per‐protocol analysis.
3.4. Analysis.
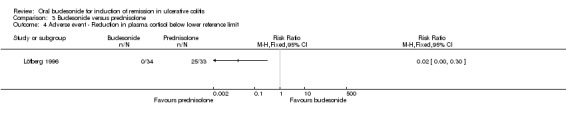
Comparison 3 Budesonide versus prednisolone, Outcome 4 Adverse event ‐ Reduction in plasma cortisol below lower reference limit.
Budesonide versus mesalamine
In both the Gross 2011 and Sandborn 2012 studies there was no statistically significant difference in the proportion of patients who experienced at least one adverse event. In the Gross 2011 study 27% (47/177) of budesonide patients had at least one adverse event compared to 25% (42/166) of mesalamine patients (RR 1.05, 95% CI 0.73 to 1.50). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (89 events, See Table 4). In the Sandborn 2012 study 57% (73/127) of budesonide patients experienced at least one adverse event compared to 63% (80/127) of mesalamine patients (RR 0.91, 95% CI 0.75 to 1.11). Gross 2011 reported that potential treatment‐related adverse events occurred in 2 of 177 (1.1%) budesonide‐treated patients and 7 of 166 (4.2%) mesalamine‐treated patients (RR 0.27, 95% CI 0.06 to 1.27) (Analysis 4.9). Sandborn 2012 reported that potential treatment‐related adverse events occurred in 1 of 127 (0.8%) budesonide‐treated patients and 0 of 127 (0%) mesalamine‐treated patients (RR 3.00, 95% CI 0.12 to 72.95). Adverse effects reported by Gross 2011 included flatulence, constipation and insomnia in the budesonide group and nausea, dyspepsia, elevated lipase and worsening cholestasis in the mesalamine group. Adverse events reported by Sandborn 2012 include worsening ulcerative colitis, headache, pyrexia, insomnia, back pain, nausea, abdominal pain, diarrhoea, and flatulence. Gross 2011 reported three serious adverse events in the budesonide group (all were worsening of UC disease activity) and two serious adverse events (both acute appendicitis) in the mesalamine group (RR 1.41, 95% CI 0.24 to 8.31). None of these serious adverse events were felt to be treatment‐related. Sandborn 2012 reported three serious adverse events in the budesonide group compared to four in the mesalamine group (RR 0.75, 95% CI 0.17 to 3.28). One of these serious adverse events was thought to be related to budesonide treatment. Measurement of plasma cortisol levels was not an a priori outcome in Gross 2011 and therefore not all patients had plasma cortisol levels drawn. Twenty‐one per cent (19/91) of budesonide‐treated patients and 1% (1/83) of mesalamine‐treated patients, for whom cortisol levels were tested, had a cortisol level that was below the normal range (defined as 6.2 µg/dL) at the end of the study (RR 17.33, 95% CI 2.37 to 126.62) (Analysis 4.11).
4.9. Analysis.
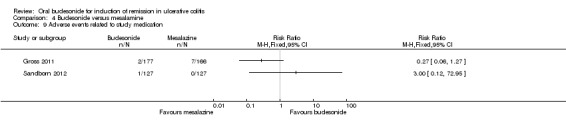
Comparison 4 Budesonide versus mesalamine, Outcome 9 Adverse events related to study medication.
4.11. Analysis.
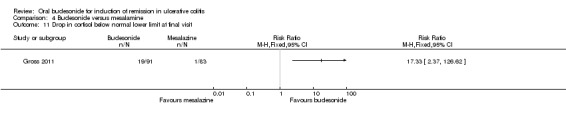
Comparison 4 Budesonide versus mesalamine, Outcome 11 Drop in cortisol below normal lower limit at final visit.
Budesonide MMX® versus standard budesonide
Travis 2014 found no statistically significant difference in the proportion of patients who experienced at least one adverse event. Fifty‐five per cent (71/128) of patients in the budesonide MMX® 9 mg group experienced at least one adverse event compared to 55% (69/126) of patients in the Entocort group (RR 1.01, 95% CI 0.81 to 1.26). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (140 events, See Table 5).
9. Study Withdrawals
Budesonide versus placebo
Thirty‐seven per cent (45/121) of patients randomised to placebo in Sandborn 2012 did not complete the study. Reasons for withdrawal included treatment failure in 14 patients, adverse events in 10 patients, withdrawal of consent in 10 patients, protocol violation in 2 patients, and investigator decision to remove the patient in 2 cases. Four patients were lost to follow‐up. Three patients did not complete the study for unspecified reasons. Of those randomised to budesonide‐MMX® 9 mg, 28% (34/123) patients did not complete the study. Reasons for withdrawal included treatment failure in 9 patients, adverse events in 6 patients, withdrawal of consent in 11 patients, protocol violation in 1 patient, and investigator decision to remove the patient in 2 cases. Five patients were lost to follow‐up. In Travis 2014 30% (33/109) of patients randomised to budesonide‐MMX® 9 mg did not complete the study. Reasons for withdrawal included treatment failure in 21 patients, adverse events in 2 patients, withdrawal of consent in 6 patients, and investigator decision to remove the patient in 2 cases. One patient was lost to follow‐up. Of patients randomised to the placebo group, 32% (28/89) patients did not complete the study. Reasons for withdrawal included treatment failure in 17 patients, adverse events in 1 patient, withdrawal of consent in 7 patients, and investigator decision to remove the patient in 1 case. 1 patient was lost to follow‐up. Rubin 2014 reported that study withdrawal related to adverse events occurred in 4.7% and 3.5% of the budesonide‐MMX® and placebo groups accordingly. A pooled analysis of three studies (971 participants) showed no statistically significant difference in withdrawal due to adverse events (Sandborn 2012; Rubin 2014; Travis 2014). Ten per cent (49/485) of budesonide MMX® 9 mg patients withdrew due to an adverse event compared to 10% (50/486) of placebo patients (RR 0.99, 95% CI 0.68 to 1.43). A pooled analysis of two studies (512 participants) showed no statistically significant difference in withdrawal due to adverse events (Sandborn 2012; Travis 2014). Eighteen per cent (46/254) of budesonide MMX® 6 mg patients withdrew due to an adverse event compared to 16% (42/258) of placebo patients (RR 1.10, 95% CI 0.55 to 2.22). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to moderate heterogeneity (I2 = 69%) and sparse data (88 events, See Table 2).
In D'Haens 2010, during the first arm of the study (duration four weeks) one patient was withdrawn at two weeks because of treatment failure (treatment group not reported). Five patients who were initially randomised to the placebo arm were switched to open label treatment with budesonide at two weeks because of failure to improve or disease worsening with placebo.
Budesonide versus prednisolone
In Löfberg 1996, there were eight withdrawals from the budesonide group: 5 of 34 (14.7%) patients withdrew due to a deterioration in their disease. One patient withdrew consent, two withdrew because of side‐effects of the budesonide (one patient had vomiting and one developed a rash). Eight patients withdrew from the prednisolone group: 7 of 38 (18.4%) patients had a deterioration in their disease and 1 patient withdrew because of insomnia. There were no statistically significant differences in study withdrawals or withdrawals due to adverse events. Twenty‐four per cent (8/34) of budesonide patients withdrew before the end of the study compared to 21% (8/38) of prednisolone patients (RR 1.12, 95% CI 0.47 to 2.65). A GRADE analysis indicated that the quality of evidence supporting this outcome was very low due unclear risk of bias and very sparse data (16 events; See Table 3), Twenty‐one per cent (7/34) of budesonide patients withdrew due to adverse events compared to 21% (8/38) of prednisolone patients (RR 0.98, 95% CI 0.40 to 2.41). A GRADE analysis indicated that the quality of evidence supporting this outcome was very low due unclear risk of bias and very sparse data (15 events; See Table 3),
Budesonide versus mesalamine
In Gross 2011, 20% (35/177) of patients randomised to budesonide did not complete the study. Reasons for discontinuation included lack of efficacy in 25 patients, adverse events in 2 patients, lack of cooperation in 3 patients and non‐specified reasons in 5 patients. Twelve per cent (20/166) of patients randomised to mesalamine did not complete the study. Reasons for study discontinuation included lack of efficacy in nine patients, adverse events in three patients, lack of cooperation in seven patients and non‐specified reasons in one patient. There was a statistically significant difference in withdrawals due to adverse events in the Gross 2011 study but not in the Sandborn 2012 study. In the Gross 2011 study, 15% (27/177) of budesonide patients withdrew due to an adverse event compared to 7% (12/166) of mesalamine patients (RR 2.11, 95% CI 1.11 to 4.03). In the Sandborn 2012 study 12% (15/127) of budesonide patients withdrew due to an adverse event compared to 11% (14/127) of mesalamine patients (RR 1.07, 95% CI 0.54 to 2.13).
Budesonide MMX® versus standard budesonide
Travis 2014 found no statistically significant difference in the proportion of patients who withdrew due to an adverse event. Eighteen per cent (23/128) of patients in the budesonide MMX® 9 mg group withdrew due to an adverse event compared to 19% (24/126) of patients in the Entocort group (RR 0.94, 95% CI 0.56 to 1.58). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (47 events, See Table 5).
Discussion
Summary of main results
Oral budesonide is a corticosteroid with high first pass hepatic metabolism, limiting the systemic adverse effects caused by conventional corticosteroids. This systematic review and meta‐analysis summarises the available evidence from randomised controlled clinical trials evaluating the efficacy and safety of oral budesonide, compared to placebo or other active agents, for the induction of remission in UC. Several formulations of budesonide exist. While more readily available enterically coated formulations of budesonide overcame the problem of gastric dissolution, these formulations (Entocort CIR® and Budenofalk®) were designed to deposit active budesonide in the terminal ileum or the ascending colon and accordingly were demonstrated to be effective for induction of remission in Crohn's disease (Rezaie 2015). However, neither Entocort CIR® or Budenofalk® have been approved for the treatment of UC. However, a newer formation known as budesonide MMX® utilises a Multi Matrix MMX® technology platform which is designed to provide homogenous release of budesonide throughout the entire colon and was subsequently investigated for its ability to induce remission in UC.
Six trials (1808 participants) met the inclusion criteria. Löfberg 1996 compared standard (non‐MMX®) budesonide with prednisolone and found that both medications were similar with respect to endoscopic score improvement; however, induction of clinical remission was not a primary study outcome. In addition, the study was small and unlikely to be adequately powered to demonstrate a difference amongst the groups if this outcome had been examined. A pilot study by D'Haens 2010 compared budesonide MMX® with placebo and found no significant difference in clinical remission rates between the two treatment groups at four weeks. The fact that this study was small (N = 32), underpowered and evaluated outcomes early at four weeks, may account for the negative study findings. In comparison, pooled data from three recent large trials with a total of 900 randomised participants demonstrated that budesonide‐MMX® 9 mg daily was more than twice as likely to induce remission (defined stringently as combined endoscopic and clinical remission) than placebo (RR 2.25, 95% CI 1.50 to 3.39) at 8 weeks (Sandborn 2012; Rubin 2014; Travis 2014). Several exploratory analyses were performed. The Rubin 2014 data are currently available in abstract form only, and this study included participants who were on concurrent 5‐ASA therapy whereas the Sandborn 2012 and Travis 2014 studies excluded patients on 5‐ASA. A sensitivity analysis excluding the Rubin 2014 study demonstrated that budesonide‐MMX® 9 mg daily was almost three times more effective than placebo for induction of remission in UC (RR 2.89; 95% CI 1.59 to 5.25). This may reflect that the Rubin 2014 cohort was a 'harder to treat' patient population, as all participants had active disease despite 5‐ASA therapy prior to entering the budesonide‐MMX® clinical trial. A subgroup analysis based on disease location suggests that budesonide‐MMX® 9 mg daily provides the most benefit in patients with left‐sided colitis (RR 2.98, 95% CI 1.56 to 5.67) compared to patients with pan‐colitis (RR 2.42, 95%CI 0.61 to 9.56). Symptom resolution was also more likely to occur in budesonide‐treated patients. Budesonide MMX® 9 mg daily was more likely to induce endoscopic improvement in comparison to placebo (Sandborn 2012; Travis 2014). It appeared that budesonide‐MMX® at a 9 mg daily dose was more likely to induce endoscopic remission and histologic remission than placebo, although this effect may be confounded by the use of concurrent 5‐ASA therapy which was permitted in the Rubin 2014 study. The 6 mg daily dose of budesonide‐MMX® was not more effective than placebo for induction of remission, clinical improvement, symptom resolution, endoscopic improvement, or histologic remission (Sandborn 2012; Travis 2014).
Gross 2011 compared controlled ileal release budesonide (Budenofalk®) with mesalamine and found that mesalamine performed significantly better at inducing clinical remission than budesonide. The Sandborn 2012 study included a mesalamine (Asacol) reference arm found no difference in terms of clinical remission and improvement between budesonide‐MMX® and mesalamine. However, the Sandborn 2012 study was not adequately powered to detect differences in efficacy between these agents. Adequately powered trials comparing budesonide‐MMX® with a 5‐ASA medication, including 5‐ASA MMX® formulations are required to determine the relative efficacy and safety of these agents.
One small study (N = 72) compared budesonide (starting dose 10 mg/day) to prednisolone (starting dose 40 mg/day) and found no differences in terms of endoscopic improvement, endoscopic remission and histologic remission (Löfberg 1996). Clinical remission was not assessed. Although there were no differences in study withdrawals or withdrawals due to adverse events prednisolone patients had significantly reduced plasma cortisol levels. This study was not adequately powered to detect differences in efficacy and safety. Adequately powered trials comparing budesonide to prednisolone are required to determine the relative efficacy and safety of these agents.
The Travis 2014 study included an Entocort 9 mg arm and no differences were found between budesonide MMX® 9 mg and Entocort in terms of remission (combined clinical and endoscopic remission), clinical improvement, endoscopic improvement, histologic remission, adverse events, serious adverse events and withdrawal due to adverse events. However. it is likely that this study was not adequately powered to detect differences in efficacy and safety between these budesonide formulations. Adequately powered trials comparing standard budesonide to budesonide‐MMX® formulations are required to determine the relative efficacy and safety of these budesonide formulations.
The included trials provide data regarding tolerability of budesonide and effects on adrenocortical function. Budesonide‐MMX® was not more likely than placebo to induce potential glucocorticoid effects including moon face, striae rubrae, flushing, fluid retention, mood changes, sleep changes, insomnia, acne and hirsutism. Although it was not possible to meta‐analyse the data from Sandborn 2012 and Travis 2014 with respect to changes in mean morning cortisol, both studies reported that while there was a decrement in the mean morning cortisol values from the beginning to the end of the study, the absolute mean concentrations of cortisol remained within the normal reference range for all treatment groups at all time points. When compared to mesalamine budesonide was significantly more likely to be associated with a decrease in plasma cortisol below the normal lower limit (Gross 2011). Budesonide did not appear to impact upon the adrenocortical axis when compared to prednisolone (Löfberg 1996).
Serious adverse events and treatment‐related serious adverse effects were equally likely to occur in the budesonide‐MMX® groups and the placebo groups (Sandborn 2012; Travis 2014). Furthermore, adverse effects were not more likely in budesonide‐MMX® treated patients compared with placebo in a pooled analysis of almost 1000 patients (Sandborn 2012; Rubin 2014; Travis 2014). The majority of adverse effects were considered mild or moderate in severity and were not considered to be related to the study drug.
Overall completeness and applicability of evidence
In general the results of this review are applicable to patients with mild‐to‐moderate ulcerative colitis. Most of the included studies were multicenter trials conducted in countries where the burden of ulcerative colitis is greatest. This review makes use of six published randomised trials. Five studies assessed the primary outcome of this review (D'Haens 2010; Gross 2011; Sandborn 2012; Rubin 2014; Travis 2014). Two studies were pilot studies were relatively small (Löfberg 1996; D'Haens 2010). The remaining four studies were large multi‐centre studies (Gross 2011; Sandborn 2012; Rubin 2014; Travis 2014). The studies were conducted amongst adult patients with mild‐to‐moderate ulcerative colitis, with the majority of the patients having left‐sided disease. The overall findings of this review support the use of oral budesonide‐MMX® for inducing remission in active ulcerative colitis.
Quality of the evidence
Three of the studies were high quality (Gross 2011, Sandborn 2012, Travis 2014), with a low risk of bias across all domains. The quality of the evidence in D'Haens 2010 appears to be high. However, we were unable to assess the risk of bias due to allocation concealment or sequence generation as this information was not available. Therefore we reported the study to be of moderate quality. Löfberg 1996 was rated as unclear risk of bias for allocation concealment and blinding. Rubin 2014 was published in abstract form and was rated as unclear risk of bias for incomplete outcome data, selective reporting and other sources of bias.
A GRADE analysis indicated that the overall quality of the evidence supporting the primary outcome (clinical remission) for the budesonide‐MMX® 9 mg versus placebo comparison was moderate due top sparse data (101 events). The overall quality of the evidence supporting the primary outcome for the budesonide‐MMX® 6 mg versus placebo comparison was low due to very sparse data (38 events). The small study comparing budesonide to prednisolone did not assess the primary outcome clinical remission (Löfberg 1996). GRADE analyses indicated that the overall quality of the evidence supporting the outcomes assessed in this study was very low due to unclear risk of bias (allocation concealment and sequence generation) and very sparse data. The overall quality of the evidence supporting the primary outcome for the study comparing budesonide 9 mg/day to 3 g/day mesalamine (Salofalk®) was moderate due to sparse data (161 events). The overall quality of the evidence supporting the primary outcome for the study that compared budesonide MMX® to mesalamine (Asacol®) was low due to very sparse data (37 events). The overall quality of the evidence supporting the primary outcome for the study that compared budesonide MMX® 9 mg to Entocort 9 mg was low due to very sparse data (32 events).
Potential biases in the review process
To reduce potential bias we performed a comprehensive literature search to identify all eligible studies. We also searched Clinicaltrials.gov to identify ongoing studies. Two review authors independently assessed studies for inclusion, extracted data and assessed study quality. Given the relative paucity of published literature on the use of oral budesonide in ulcerative colitis in comparison to the many trials of oral budesonide in CD, it is possible that studies with negative results have been performed but have never been published. We contacted representatives of the main budesonide pharmaceutical manufacturers as well as experts in the field, but to date we have not identified any unpublished studies.
Agreements and disagreements with other studies or reviews
The findings of this systematic review are in keeping with recent review articles (Danese 2014; Gionchetti 2014). We previously performed a systematic review on the use of oral budesonide for induction of remission in ulcerative colitis (Sherlock 2010). At that time there was insufficient evidence to draw any conclusions on the efficacy of budesonide for inducing remission in ulcerative colitis. However, with the addition of three large, high quality, adequately powered studies, all utilising the newer formulation of budesonide with Multi Matrix technology which allows for homogenous release of the active ingredient budesonide throughout the colon, there is now moderate quality evidence supporting the use of budesonide‐MMX®, for the induction of remission in patients with UC.
Authors' conclusions
Implications for practice.
There is moderate quality evidence to support the clinical use of oral budesonide‐MMX® at a 9 mg daily dose for induction of remission in active ulcerative colitis, particularly in patients with proctosigmoiditis or left‐sided colitis. Budesonide‐MMX® 9 mg daily is effective for induction of remission in the presence or absence of concurrent 5‐ASA therapy. Further, budesonide‐MMX® appears to be safe, and does not lead to significant impairment of adrenocorticoid function compared to placebo. Moderate quality evidence from a single study suggests that mesalamine may be superior to standard (non‐MMX®) budesonide for the treatment of active ulcerative colitis. Low quality evidence from one study found no difference in remission rates between budesonide MMX® and mesalamine. Very low quality evidence from one small study showed no difference in endoscopic remission rates between standard budesonide and prednisolone. Low quality evidence from one study showed no difference in remission rates between budesonide‐MMX® and standard budesonide.
Implications for research.
While data currently supports the use of budesonide‐MMX® in patients with left‐sided colitis, the effect of concurrent oral or rectal 5‐ASA therapy in this subset of patients is unknown. Adequately powered studies are needed to allow conclusions regarding the comparative efficacy and safety of budesonide versus prednisolone, budesonide‐MMX® versus standard budesonide and budesonide versus mesalamine. Trials comparing budesonide‐MMX® with a 5‐ASA medication, including a comparison with the more novel 5‐ASA MMX® formulation will likely provide more information to help guide therapy. Further, there are no existing data comparing budesonide‐MMX® with systemic steroids, and data on efficacy, tolerability and safety and required. Lastly, studies exploring the use of budesonide‐MMX® for maintenance of remission in UC are currently ongoing and should be reported separately.
What's new
| Date | Event | Description |
|---|---|---|
| 28 April 2015 | New search has been performed | New literature searches conducted on April 28, 2015. Three new studies added. |
| 28 April 2015 | New citation required and conclusions have changed | Substantively updated review with new conclusions and author |
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE (1950 ‐ present) ‐ Most recent search performed on 28 April 2015
pregnenediones/ or budesonide/
BUDESONIDE (nm) or (horacort or budesonide or Budecol or Budecort or Budefat or Budes or Budeson or Budon or Entocort or Preferid or "S 1320" or S1320 or "S‐1320").mp.
1 or 2
colitis/ or colitis, ulcerative/ or proctocolitis/ or inflammatory bowel diseases/
3 and 4
limit 5 to (clinical trial, all or clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or clinical trial or controlled clinical trial or meta analysis or multicenter study or randomized controlled trial)
(RCT or RCTS or random: or (clin: adj10 trial:) or ((singl: doubl: or tripl: or trebl:) adj5 (blind: or mask:))).mp.
5 and 7
6 or 8
exp Administration, Oral/ or oral:.mp.
5 and 10
exp case‐control studies/ or exp cohort studies/ or exp clinical trials as topic/
5 and 12
9 or 13
14 not 11
from 15 keep 1‐41
from 9 keep 1‐71
16 or 17
Appendix 2. EMBASE search strategy
EMBASE (1980 ‐ present) ‐ Most recent search performed on 28 April 2015
Pregnane Derivative/ or pregnenediones.mp. or budesonide/ or (horacort or budesonide or Budecol or Budecort or Budefat or Budes or Budeson or Budon or Entocort or Preferid or "S 1320" or S1320 or "S‐1320").mp. or 51333‐22‐3.rn.
oral drug administration/
1 and 2
1 and po.fs.
3 or 4
Enteritis/
1 and 6
colitis/ or proctitis/ or proctocolitis/ or ulcerative colitis/ or (inflammatory adj2 bowel).mp.
1 and 8
5 and 8
cohort analysis/ or crossover procedure/ or double blind procedure/ or parallel design/ or single blind procedure/ or triple blind procedure/ or intervention study/ or longitudinal study/ or prospective study/ or retrospective study/ or exp case control study/ or exp clinical trial/ or (RCT or RCTS or random: or (clin: adj10 trial:) or ((singl: doubl: or tripl: or trebl:) adj5 (blind: or mask:))).mp. or ct.fs.
10 and 11
Appendix 3. Conference Proceedings Searched
Digestive Disease Week (DDW) ‐ 2011 to 2014
Canadian Digestive Diseases Week ‐ 2011 to 2015
British Society of Gastroenterology (BSG) ‐ 2011 to 2014
American Gastroenterology Association (AGA) ‐ 2011 to 2014
United European Gastroenterology Week (UEGW) 2011 to 2014
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) ‐ 2011 to 2014
Appendix 4. Contact with Experts and Pharmaceutical Companies
The following experts were contacted:
Dr. William Sandborn, University of California, San Diego, United States.
Dr. Simon Travis, John Radcliffe Hospital, Oxford, United Kingdom.
Dr. Ralph Mueller, affiliated with Dr. Falk Pharma (manufacturers of budesonide capsules)
Dr. Andy Barrett, Associate Director, Medical and Scientific Communications at Salix
No additional studies were identified. The experts are unaware of any other unpublished studies (with negative or positive findings).
Appendix 5. Endoscopic Index Score used by Löfberg et al.
Score of Endoscopic Inflammation
0 Normal/non‐inflamed mucosa
1 Granularity, edema and absence of normal vascular pattern
2 Hyperemia, friable mucosa, petechiae (plus all the characteristics of score '1')
3 Ulcerations (plus all the characteristics of scores '1' and '2')
Appendix 6. UCDAI ‐ Disease activity index used by Sandborn et al and Travis et al
Stool Frequency:
Normal 0
1 to 2 stools above usual number 1
3 to 4 stools above normal 2
> 4 stools above normal 3
Rectal Bleeding:
None 0
Streaks of blood 1
Obvious blood 2
Mostly blood 3
Mucosal Appearance:
Normal 0
Mild friability 1
Moderate friability 2
Exudation 3
Physician Global Assessment if
Disease Activity:
Normal 0
Mild 1
Moderate 2
Severe 3
Data and analyses
Comparison 1. Budesonide MMX® 9 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Remission (combined clinical and endoscopic remission) | 3 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.50, 3.39] |
| 2 Remission (combined clinical and endoscopic remission): subgroup by mesalamine use | 3 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.50, 3.39] |
| 2.1 Concurrent mesalamine | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.99, 3.08] |
| 2.2 No mesalamine | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [1.59, 5.25] |
| 3 Remission (combined clinical and endoscopic remission) subgroup by disease location | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Combined proctosigmoiditis and left‐sided disease | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.56, 5.67] |
| 3.2 Extensive disease | 2 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.61, 9.56] |
| 4 Clinical Improvement (without remission) at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Clinical remission or reduction in CAI of at least 50% at 4 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 No change or worsening of disease at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Endoscopic Improvement at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Histologic Improvement at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Morning Cortisol Suppression at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Clinical improvement | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.99, 1.70] |
| 11 Symptom resolution | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.25, 2.77] |
| 12 Endoscopic improvement | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.01, 1.66] |
| 13 Histologic remission | 3 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.11, 2.06] |
| 14 Histologic remission: sensitivity analysis | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.75, 2.75] |
| 15 Endoscopic remission | 2 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.13, 2.16] |
| 16 Endoscopic remission: sensitivity analysis | 1 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.91, 2.40] |
| 17 Endoscopic remission according to disease location | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Proctosigmoiditis | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.75, 3.65] |
| 17.2 Left‐sided disease | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.76, 3.09] |
| 17.3 Extensive disease | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.65, 6.00] |
| 18 Adverse events | 3 | 971 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 19 Serious adverse events | 2 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.40] |
| 20 Treatment‐related serious adverse events | 2 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.32, 28.99] |
| 21 Potential glucocorticoid effects | 2 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.53] |
| 22 Withdrawal due to adverse events | 3 | 971 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.68, 1.43] |
1.1. Analysis.
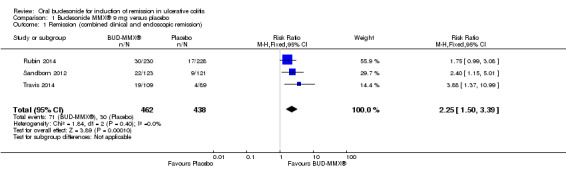
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 1 Remission (combined clinical and endoscopic remission).
1.2. Analysis.
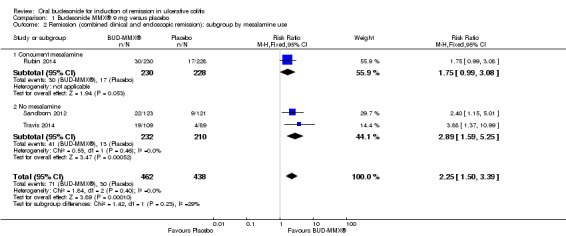
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 2 Remission (combined clinical and endoscopic remission): subgroup by mesalamine use.
1.4. Analysis.
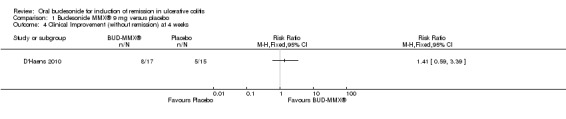
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 4 Clinical Improvement (without remission) at 4 weeks.
1.5. Analysis.
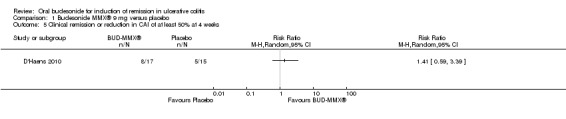
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 5 Clinical remission or reduction in CAI of at least 50% at 4 weeks.
1.6. Analysis.
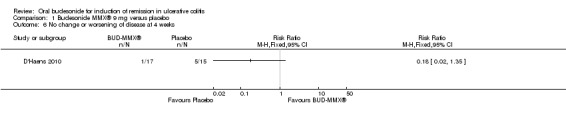
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 6 No change or worsening of disease at 4 weeks.
1.7. Analysis.
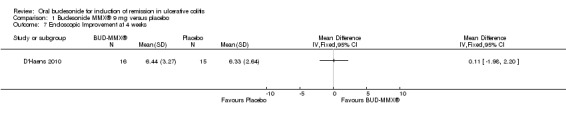
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 7 Endoscopic Improvement at 4 weeks.
1.8. Analysis.
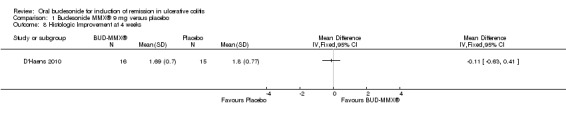
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 8 Histologic Improvement at 4 weeks.
1.9. Analysis.
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 9 Morning Cortisol Suppression at 4 weeks.
1.13. Analysis.
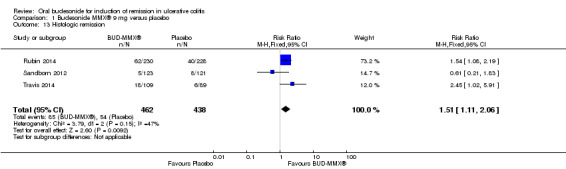
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 13 Histologic remission.
1.14. Analysis.
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 14 Histologic remission: sensitivity analysis.
1.15. Analysis.
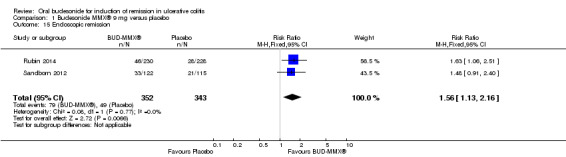
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 15 Endoscopic remission.
1.16. Analysis.
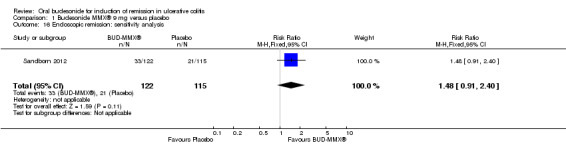
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 16 Endoscopic remission: sensitivity analysis.
1.17. Analysis.
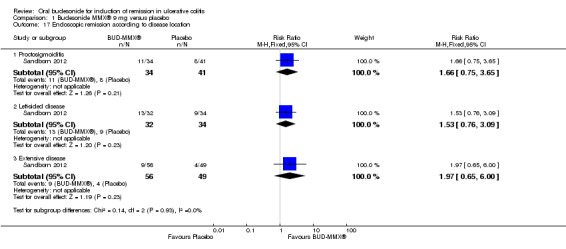
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 17 Endoscopic remission according to disease location.
1.18. Analysis.
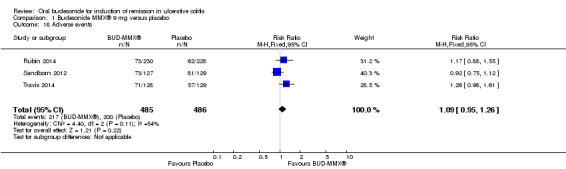
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 18 Adverse events.
1.20. Analysis.
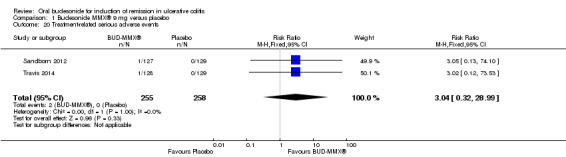
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 20 Treatment‐related serious adverse events.
1.21. Analysis.
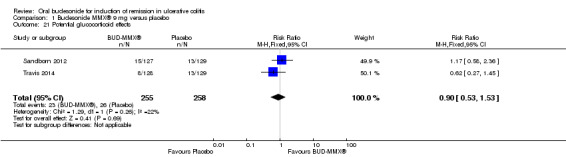
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 21 Potential glucocorticoid effects.
1.22. Analysis.
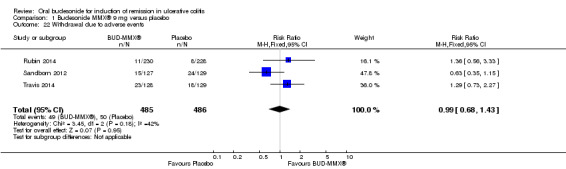
Comparison 1 Budesonide MMX® 9 mg versus placebo, Outcome 22 Withdrawal due to adverse events.
Comparison 2. Budesonide MMX® 6 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Remission (combined clinical and endoscopic remission | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.94, 3.42] |
| 2 Clinical improvement | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.33] |
| 3 Endoscopic improvement | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.27] |
| 4 Histologic remission | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.63, 2.40] |
| 5 Symptom resolution | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.04, 2.35] |
| 6 Serious adverse events | 2 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.91] |
| 7 Treatment‐related serious adverse events | 2 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.06 [0.46, 36.10] |
| 8 Potential glucocorticoid effects | 2 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.97] |
| 9 Adverse events | 2 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.97, 1.32] |
| 10 Withdrawal due to adverse events | 2 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.55, 2.22] |
2.5. Analysis.
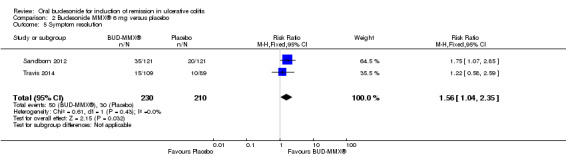
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 5 Symptom resolution.
2.6. Analysis.
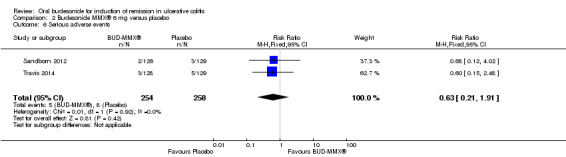
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 6 Serious adverse events.
2.7. Analysis.
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 7 Treatment‐related serious adverse events.
2.8. Analysis.
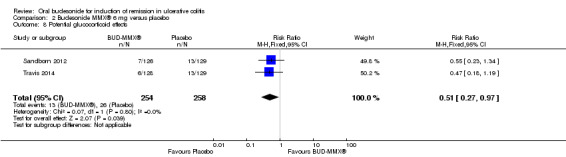
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 8 Potential glucocorticoid effects.
2.9. Analysis.
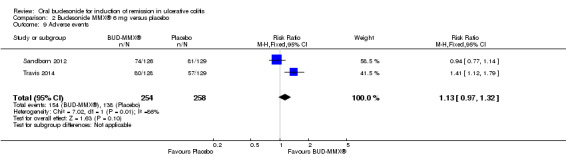
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 9 Adverse events.
2.10. Analysis.
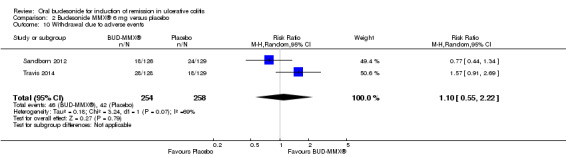
Comparison 2 Budesonide MMX® 6 mg versus placebo, Outcome 10 Withdrawal due to adverse events.
Comparison 3. Budesonide versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endoscopic improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Endoscopic remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Histologic remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse event ‐ Reduction in plasma cortisol below lower reference limit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Study withdrawals | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Withdrawal due to adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.5. Analysis.
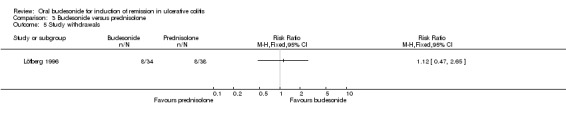
Comparison 3 Budesonide versus prednisolone, Outcome 5 Study withdrawals.
3.6. Analysis.
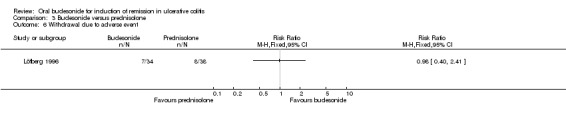
Comparison 3 Budesonide versus prednisolone, Outcome 6 Withdrawal due to adverse event.
Comparison 4. Budesonide versus mesalamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical remission (ITT analysis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical remission according to disease location | 1 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.91] |
| 2.1 Distal Disease (combined proctosigmoiditis and left‐sided disease) | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.96] |
| 2.2 Extensive disease (extending proximal to the splenic flexure) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.39, 1.05] |
| 3 Therapeutic success (defined by Physician's Global Assessment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Therapeutic benefit (defined by Physician's Global Assessment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Endoscopic improvement | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Endoscopic remission (EI ≤ 1) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Histologic remission | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Adverse events related to study medication | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Withdrawal due to adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Drop in cortisol below normal lower limit at final visit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Remission (combined clinical and endoscopic remission) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Clinical improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Symptom resolution | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Serious adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16 Potential glucocorticoid effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.2. Analysis.
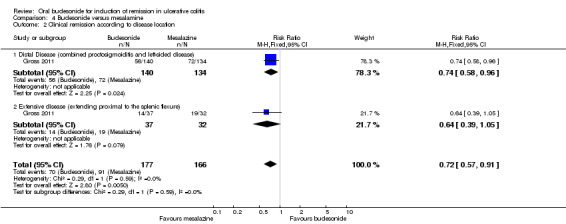
Comparison 4 Budesonide versus mesalamine, Outcome 2 Clinical remission according to disease location.
4.8. Analysis.
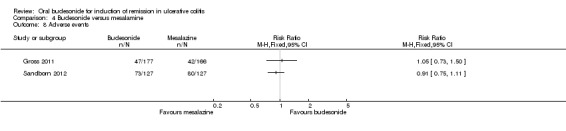
Comparison 4 Budesonide versus mesalamine, Outcome 8 Adverse events.
4.10. Analysis.
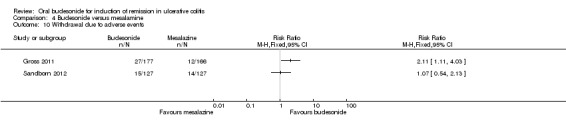
Comparison 4 Budesonide versus mesalamine, Outcome 10 Withdrawal due to adverse events.
4.12. Analysis.
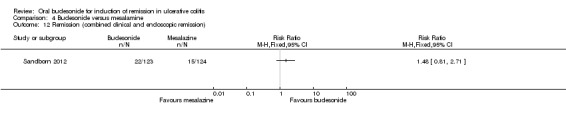
Comparison 4 Budesonide versus mesalamine, Outcome 12 Remission (combined clinical and endoscopic remission).
4.13. Analysis.
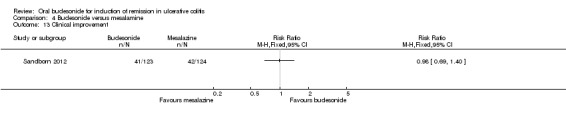
Comparison 4 Budesonide versus mesalamine, Outcome 13 Clinical improvement.
4.14. Analysis.
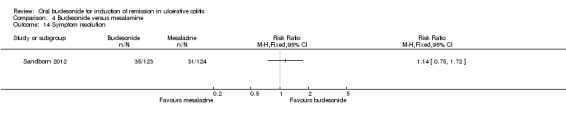
Comparison 4 Budesonide versus mesalamine, Outcome 14 Symptom resolution.
4.15. Analysis.
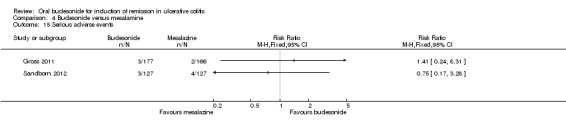
Comparison 4 Budesonide versus mesalamine, Outcome 15 Serious adverse events.
4.16. Analysis.
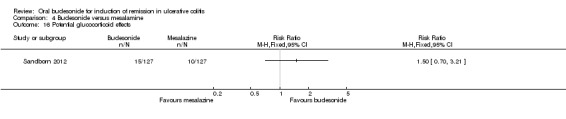
Comparison 4 Budesonide versus mesalamine, Outcome 16 Potential glucocorticoid effects.
Comparison 5. Budesonide MMX® 9 mg versus Entocort EC 9mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Remission (combined clinical and endoscopic remission) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Endoscopic improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Histologic remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Symptom resolution | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Treatment‐related adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Potential glucocorticoid effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Withdrawal due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.5. Analysis.
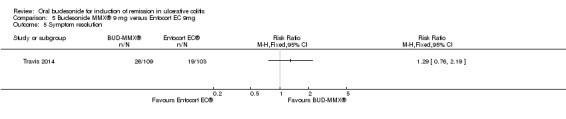
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 5 Symptom resolution.
5.6. Analysis.
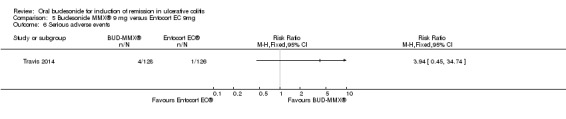
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 6 Serious adverse events.
5.7. Analysis.
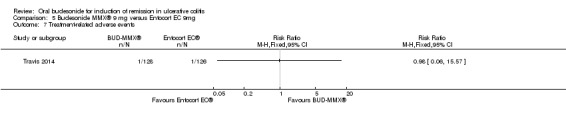
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 7 Treatment‐related adverse events.
5.8. Analysis.
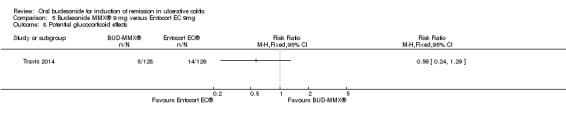
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 8 Potential glucocorticoid effects.
5.9. Analysis.
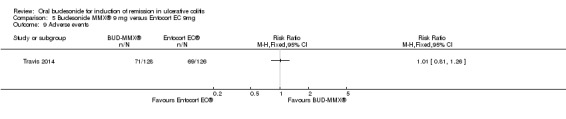
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 9 Adverse events.
5.10. Analysis.
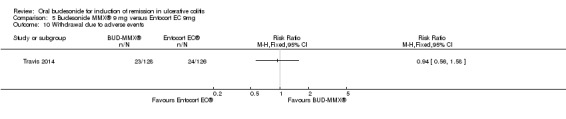
Comparison 5 Budesonide MMX® 9 mg versus Entocort EC 9mg, Outcome 10 Withdrawal due to adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
D'Haens 2010.
| Methods | Randomised double‐blind study comparing Budesonide‐MMX® with placebo at 4 weeks Patients who had worsening of their disease or who were not improving after 2 weeks were switched to open‐label budesonide therapy In an open‐label extension, from 4 to 8 weeks, all patients received oral Budesonide‐MMX® Only outcomes following the first arm of the trial were considered in this review |
|
| Participants | Adult patients with mild to moderate, active left‐sided ulcerative colitis (N = 36) Active disease was defined as a clinical activity index (CAI) of < 14 (Rachmilewitz 1989) Stable doses of immunomodulators (methotrexate or azathioprine) or 5‐ASA products were allowed Participants were excluded if they had severe disease (CAI > 14), extensive disease (inflammation extending proximal to the splenic flexure) or distal proctitis Additional exclusion criteria included the use of systemic or topical steroids within the preceding 4 weeks, or previous use of TNF‐α antagonists. |
|
| Interventions | Budesonide‐MMX® 9 mg once daily for 8 weeks (n = 18), or placebo (n = 18) for 4 weeks followed by Budesonide‐MMX® for a further 4 weeks | |
| Outcomes | Primary outcome was the proportion of patients achieving clinical remission (CAI ≤ 4) or a clinical improvement (50% reduction in their CAI score) at 4 weeks Secondary outcomes included a reduction in clinical symptoms at 8 weeks, a reduction in CAI by 70% and changes in the Rachmilewitz Endoscopic Index Score at 4 and 8 weeks In both groups, morning cortisol levels were tested and ACTH stimulation tests were performed at 4 and 8 weeks |
|
| Notes | This trial was supported by Crinos S.p.A., Italy and Cosmo Technologies, Ireland, manufacturers of budesonide‐MMX® | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to the treatment allocation |
| Blinding (performance bias and detection bias) Treatment Allocation | Low risk | Physicians, patients and outcome assessors were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient was withdrawn at 2 weeks because of treatment failure (treatment group not stated) 5 patients who were initially randomised to the placebo arm were switched to open label treatment with budesonide at 2 weeks because of failure to improve or disease worsening with placebo |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gross 2011.
| Methods | Randomised, double‐blind, double‐dummy, multicenter trial comparing budesonide with mesalamine The study was conducted across 48 centres in Europe |
|
| Participants | Adult patients (age 18‐75 years), with mild to moderate active ulcerative colitis (N = 343) Patients were required to have a Clinical Activity Index (CAI) of ≥ 6 and an Endoscopic Index (EI) ≥ 4 (Rachmilewitz 1989) Participants with disease limited to the rectum were excluded Participants were also excluded if they had toxic megacolon, a diagnosis of Crohn's disease, indeterminate colitis, ischemic colitis, radiation colitis or microscopic colitis Other exclusion criteria included: gastrointestinal infection, diarrhoea due to other GI conditions, bleeding diathesis, active peptic ulcer disease, prior or concurrent history of colorectal malignancy, the use of immunosuppressant or corticosteroid medication within the preceding 3 months and 4 weeks, respectively Patients who were experiencing a relapse while being treated with a maintenance dose of mesalamine of > 2.4 g/day were also excluded |
|
| Interventions | Patients received budesonide 9 mg once daily (3 x 3 mg capsules) for 8 weeks (n = 177) or mesalamine 3 g once daily (3 x 1000 mg tablets) for 8 weeks (n = 166) | |
| Outcomes | The primary outcome was clinical remission (CAI ≤ 4, with rectal bleeding and stool frequency sub‐scores of '0') at week 8 Subgroup analysis included clinical remission rates according to disease location and disease severity at the outset Secondary outcomes included CAI score changes, mucosal healing (EI ≤ 1), endoscopic remission (EI ≤ 3), histological remission and therapeutic success and benefit (defined by Physician Global Assessment (Hanauer 1993)) |
|
| Notes | This trial was supported by Dr. Falk Pharma GmbH, manufacturers of the budesonide evaluated in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators used a computer‐generated randomisation list using randomly permuted blocks |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from all study investigators |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to the treatment allocation |
| Blinding (performance bias and detection bias) Treatment Allocation | Low risk | Physicians, patients and outcome assessors were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data appear to be complete |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Löfberg 1996.
| Methods | Double‐blind, double dummy, randomised controlled trial, comparing oral budesonide with prednisolone | |
| Participants | Adult patients with mild to moderate, extensive and left‐sided UC, participated in the study (N = 72)
Both hospitalised and outpatient UC patients were eligible for study inclusion Eligible patients had an endoscopic index score ≥ 2 in one or more colonic segments (Appendix 5) and had at least 4 bloody stools per day Oral sulphasalazine or 5‐ASA products were the only concomitant medications permitted, the doses of which were kept constant for the study duration Patients were excluded if they were treated with corticosteroids within 2 weeks of the study start date or if they were receiving acid‐blocking medications (H2‐antagonists and proton‐pump inhibitors) Pregnant and breast‐feeding patients were excluded in addition to patients with liver disease, diabetes or untreated hypertension Participants were withdrawn from the trial if their medical condition deteriorated significantly or if there was no improvement after 2 weeks of treatment |
|
| Interventions | Intervention: Budesonide Capsules, starting dose 6 mg in the morning and 4 mg in the evening, for the first 4 weeks (n = 34) During weeks 5 to 7 the dose was reduced to 4 mg in the morning and evening. For the final 2 weeks, the dose administered was 4 mg in the morning The active drug was contained within a capsule and consisted of acid‐resistant pellets, designed to have a sustained release throughout the colon Active Control: Prednisolone, starting dose was 40 mg once daily (n =38) Tapering began after 2 weeks, with a reduction in the dose of 5 mg weekly until week 8, during which patients received 7.5 mg once daily A dose of 5 mg daily was administered during week 9 |
|
| Outcomes | The primary outcome was a change in endoscopic and histological scores of inflammation and an improvement in laboratory parameters. Clinical symptoms (daily bowel motions, presence or absence of blood or mucus) were recorded in a daily diary. Patients had a colonoscopy at study entry and following 4 weeks of therapy. A sigmoidoscopy was performed at the 2 and 9 week visits. Biopsies were taken from each colonic segment and from the most severely inflamed sites. The authors also aimed to evaluate to effect of budesonide on the adrenocortical system. At each follow‐up visit an early morning cortisol level was measured. Outcomes were assessed at 2, 4 and 9 weeks. | |
| Notes | This trial was supported by Astra Draco AB, Lund Sweden, the manufacturers of the budesonide evaluated in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated to treatment with either oral budesonide or oral prednisolone from blocks of four at each of the participating nine centres |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | All biopsy specimens were examined for the presence of inflammation by a pathologist who was blinded to the patient treatment group It is unclear if endoscopists were blinded to the patient treatment group. |
| Blinding (performance bias and detection bias) Treatment Allocation | Unclear risk | Patients were blinded to their treatment ‐ dummy pills of the alternative medication were used throughout the study The pathologist was blinded to the treatment group for their assessment of endoscopic inflammation We are not told whether the treating physicians were also blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Analyses and results are in accordance with the predefined study protocol |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rubin 2014.
| Methods | Randomised double‐blind, placebo controlled trial | |
| Participants | Non‐pregnant patients, age 18‐75 years, with active mild to moderate UC (UCDAI score ≥ 4 and ≤ 10) with a mucosal appearance score of ≥ 1, despite the use of oral 5‐ASA (≥ 2.4g daily for at least 6 weeks) were included (N = 510) Patients with disease limited to the rectum were excluded Other exclusion criteria included infectious colitis, malignancy within the past 5 years (with the exception of non‐melanoma skin cancers), active peptic ulcer disease, a history of toxic megacolon, Crohn's disease or indeterminate colitis. Patient with tuberculosis, Hepatitis B, Hepatitis C or HIV were excluded. Patients with severe disease in other organs or systems were excluded, including those with type 1 diabetes The use of oral corticosteroids, other than budesonide, the use of immunosuppressant medications and the use of biologic therapy, within 4 weeks, 8 weeks and 3 months of randomisation, respectively, were also exclusion criteria Patients who had used rectal 5‐ASA or corticosteroid products within 2 weeks of randomisation were also excluded |
|
| Interventions | Budesonide multi‐matrix system (MMX®) 9 mg (n =230) Placebo (n = 228) |
|
| Outcomes | Primary outcome: induction of remission (combination of clinical and endoscopic remission) following 8 weeks of therapy Secondary outcomes: clinical remission, clinical response, histologic remission and histologic healing, evaluation of treatment failures, quality of life, C‐reactive protein and fecal calprotectin levels at 8 weeks |
|
| Notes | Currently only published in abstract form Modified intention‐to‐treat population was 458 This trial was supported by Santarus, Inc. a wholly owned subsidiary of Salix Pharmaceuticals, Inc. which manufactures budesonide‐MMX®. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation via an interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to the treatment allocation |
| Blinding (performance bias and detection bias) Treatment Allocation | Low risk | Physicians, patients and outcome assessors were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract publication |
| Selective reporting (reporting bias) | Unclear risk | Abstract publication |
| Other bias | Unclear risk | Abstract publication |
Sandborn 2012.
| Methods | Prospective, multicenter, double‐blind, double‐dummy, randomised, placebo‐controlled trial | |
| Participants | Participants (N = 509) were recruited from 108 centres from North America (United States, Canada, Mexico) and India Non‐pregnant adult patients (age 18 ‐ 75 years) with mild to moderate UC as defined by an Ulcerative Colitis Disease Activity Index score of ≥ 4 and ≤ 10 (Sutherland 1987) were eligible A ≥ 2‐day wash out period for oral mesalamine or other 5‐ASA product was required Exclusion criteria: history of oral or rectal corticosteroid, immunosuppressant or biologic use within the preceding 4 weeks, 8 weeks and 3 months, respectively, patients with severe UC (UCDAI > 10 points) or those with disease limited to the rectum, severe anaemia, leukopenia, granulocytopenia, pregnancy or lactation, cirrhosis, liver or renal insufficiency, severe disease in other organs or systems, underlying conditions (other than UC) requiring corticosteroid therapy as well patients with type 1 diabetes or glaucoma The median age of the patients included in the final analysis was 42 years with a median disease duration of 3.3 years Approximately half of the enrolled patients were Caucasian and one third were Asian. 28.6% of enrolled patients had proctosigmoiditis, 29% had left‐sided colitis and 40.5% had extensive or pan‐colitis The median UCDAI score at study entry was 7.0 |
|
| Interventions | Budesonide‐MMX® 9 mg (n = 123) Budesonide‐MMX® 6 mg (n = 121) Placebo (n = 121) Asacol® 2.4g/day (mesalamine 800mg 3 times daily) daily(n = 124) Placebo formulations were available for the Asacol and the Budesonide‐MMX® tablets |
|
| Outcomes | The primary outcome was combined clinical and endoscopic remission at 8 weeks. (UCDAI score ≤ 1, with sub‐scores of zero for rectal bleeding and stool frequency, no mucosal friability at colonoscopy and a reduction of ≥ 1 point in the endoscopic index score (Rachmilewitz 1989)). Secondary outcomes included clinical improvement (≥3 point reduction in UCDAI), endoscopic improvement, symptom resolution, histologic healing as well as an assessment of adverse effects and potential glucocorticoid side‐effects. |
|
| Notes | Modified intention‐to‐treat population was 489 The presence of confirmed active UC based on histologic findings was an eligibility criterion; however, there was a lag time from study enrolment to the availability of histology results and therefore participants with normal histology were excluded from the analysis The authors also excluded participants who had a diagnosis of infectious colitis which had not been recognised at the time of enrolment We contacted Dr Andy Barrett, Associate Director, Medical and Scientific Communications at Salix in order to clarify the authors decision to use a modified intention‐to‐treat analysis and we are in agreement with the authors decision to proceed with a modified intention‐to‐treat analysis Further details on the reasons for use of the modified intention‐to‐treat analysis are available in the FDA Review document produced by Dr Marjorie Dennis, available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203634_uceris_toc.cfm This trial was supported by Cosmo pharmaceuticals SpA, Italy, and Santarus manufacturers of budesonide‐MMX® |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An interactive voice response system was responsible for randomising patients in blocks of 4 to each of the treatment arms |
| Allocation concealment (selection bias) | Low risk | An external contractor, located centrally, was responsible for the randomisation process. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to the treatment allocation |
| Blinding (performance bias and detection bias) Treatment Allocation | Low risk | Physicians, patients and outcome assessors were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for in the final analysis which was a modified intention‐to‐treat analysis. Outcome data appear to be complete. 349 of 489 (71.4%) patients completed the study The proportions of patients who did not complete the study as well as reasons for study discontinuation were similar across different treatment groups |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes |
| Other bias | Low risk | No other sources of bias identified |
Travis 2014.
| Methods | This is a prospective, multicenter, double‐blind, double‐dummy, randomised, placebo‐controlled trial | |
| Participants | Participants (N = 509) were recruited from 69 centres in 15 countries (Europe, Israel, Russia and Australia) All non‐pregnant adult patients (age 18 ‐ 75 years) with mild to moderate UC as defined by an Ulcerative Colitis Disease Activity Index score of ≥ 4 and ≤ 10 (Sutherland 1987) were eligible for inclusion A ≥ 2‐day wash out period for oral mesalamine or other 5‐ASA product was required A 4 week washout period was required for rectal 5‐ASA use Exclusion criteria: history of oral or rectal corticosteroid, immunosuppressant or biologic use within the preceding 4 weeks, 8 weeks and 3 months, respectively, severe UC (UCDAI > 10 points) or those with disease limited to the rectum, severe anaemia, leukopenia, granulocytopenia, pregnancy or lactation, cirrhosis, liver or renal insufficiency, severe disease in other organs or systems, underlying conditions (other than UC) requiring corticosteroid therapy as well patients with type 1 diabetes or glaucoma, concomitant use of antibiotics or any rectal medication The median age of the patients included in the final analysis was 36 years with a median disease duration of 3.9 years. Patients were predominantly Caucasian. 43.2% of enrolled patients had proctosigmoiditis, 32% had left‐sided colitis and 24.4% had extensive or pan‐colitis. The median UCDAI score at study entry was 7.0 |
|
| Interventions | Budesonide‐MMX® 9 mg (n = 126) Budesonide‐MMX® 6 mg (n = 128) Placebo (n = 129) Entocort® (budesonide controlled ileal release) 9 mg daily (n = 126) Placebo formulations were available for the Entocort® capsules and the Budesonide‐MMX® tablets |
|
| Outcomes | Primary outcome: combined clinical and endoscopic remission at 8 weeks defined as UCDAI score ≤ 1, with sub‐scores of zero for rectal bleeding and stool frequency, no mucosal friability at colonoscopy and a reduction of ≥ 1 point in the endoscopic index score (Rachmilewitz 1989) Secondary outcomes: clinical improvement defined as ≥ 3 point reduction in UCDAI, endoscopic improvement, symptom resolution, histologic healing, adverse effects and potential glucocorticoid side‐effects |
|
| Notes | Modified intention‐to‐treat population was 410 The presence of confirmed active UC based on histologic findings was an eligibility criterion; however, there was a lag time from study enrolment to the availability of histology results and therefore participants with normal histology were excluded from the analysis The authors also excluded participants who had a diagnosis of infectious colitis which had not been recognised at the time of enrolment All patients from centres where significant Good Clinical Practice (GCP) violations had occurred were excluded from the final analysis We contacted Dr Andy Barrett, Associate Director, Medical and Scientific Communications at Salix in order to clarify the authors decision to use a modified intention‐to‐treat analysis and we are in agreement with the authors decision to proceed with a modified intention‐to‐treat analysis Further details on the reasons for use of the modified intention‐to‐treat analysis are available in the FDA Review document produced by Dr Marjorie Dennis, available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203634_uceris_toc.cfm This trial was supported by Cosmo pharmaceuticals SpA, Italy, and Santarus manufacturers of budesonide‐MMX® |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An interactive voice response system was responsible for randomising patients in blocks of 4 to each of the treatment arms |
| Allocation concealment (selection bias) | Low risk | An external contractor, located centrally, was responsible for the randomisation process. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to the treatment allocation |
| Blinding (performance bias and detection bias) Treatment Allocation | Low risk | Physicians, patients and outcome assessors were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for in the final analysis which was a modified intention‐to‐treat analysis Outcome data appear to be complete. 272 of 410 (66.3%) patients completed the study The proportions of patients who did not complete the study as well as reasons for study discontinuation were similar across different treatment groups |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes |
| Other bias | Low risk | No other sources of bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chopra 2006 | Retrospective chart review of patients with IBD (CD and UC) who received oral budesonide |
| Danese 2013 | This study analysed pooled data from Sandborn 2012 and Travis 2014 and found that remission rates (combined clinical and endoscopic remission) as well as symptom resolution in budesonide‐MMX® treated patients was independent of previous mesalamine exposure |
| Díaz Blasco 1995 | Review paper, Spanish article was translated to English The reference list was manually searched and no new trials were identified |
| Feagan 1996 | Overview paper describing the use of oral budesonide for UC The reference list was manually searched and no new trials were identified |
| Fedorak 2005 | Review paper The reference list was manually searched and no new trials were identified |
| Gomollón 1999 | Review paper, Spanish article was translated to English The reference list was manually searched and no new trials were identified |
| Keller 1997 | Not a randomised controlled trial Pilot study describing the use of oral budesonide in 14 patients with steroid‐dependent ulcerative colitis Budesonide was not commenced to induce remission, but rather it was commenced during the phase of weaning of conventional corticosteroids once remission had been induced with conventional corticosteroids |
| Kolkman 2004 | Budesonide was not compared to either a placebo or another active agent Two different budesonide dosing regimens were compared ‐ budesonide 9 mg once daily with 3 mg three times daily (TID) The aim of the study was to assess the pharmacokinetics, pharmacodynamics and efficacy of 2 dosing regimens for patients with active UC Although, it was a multicenter trial, only a small number of patients were actually randomised ‐ 7 patients were randomised to receive 9 mg once daily and 8 patients were randomised to receive 3 mg TID |
| Lamers 1996 | Review paper The reference list was manually searched and no new trials were identified |
| Lichtenstein 2012 | Open label extension study |
| Lichtenstein 2013 | This study analysed pooled data from Sandborn 2012 and Travis 2014 and found that remission rates (combined clinical and endoscopic remission) as well as symptom resolution in budesonide‐MMX® treated patients was independent of previous mesalamine exposure |
| Marín‐Jiménez 2006 | Review paper The reference list was manually searched and no new trials were identified |
| Sandborn 2013a | This study analysed pooled data from Sandborn 2012 and Travis 2014 and looked at quality of life in these patients |
| Sandborn 2013b | This study analysed pooled data from Sandborn 2012 and Travis 2014 and looked at improvement in symptoms |
| Sandborn 2015 | This study analysed pooled data from Sandborn 2012 and Travis 2014 and looked at clinical and endoscopic remission |
| Silverman 2011 | Review paper The reference list was manually searched and no new trials were identified |
| Travis 2011 | Open label extension study |
| Travis 2012b | This study analysed pooled data from Sandborn 2012 and Travis 2014 and looked at clinical and endoscopic remission |
Differences between protocol and review
We stated in the protocol that we would perform a meta‐analysis of relevant studies. However, we were only able to meta‐analyse 3 of 6 studies due to significant heterogeneity amongst the remaining studies.
Contributions of authors
MES, CHS or JKM performed independent assessment of article abstracts and full text papers to assess eligibility for inclusion in this review. MES, CHS or JKM independently performed data extraction and analyses. Preparation of the final manuscript was the responsibility of MES, CHS and JKM. AMG and AHS provided supervisory support and content expert advice. All authors approved the final manuscript.
Declarations of interest
MES: Dr. Sherlock has received fees for consultancy from Abbvie Canada for attending an advisory board meeting. All of the fees received are outside the scope of the submitted work.
AMG: Anne Marie Griffiths has received fee(s) from Johnson and Johnson for Board membership; fee(s) from Janssen, Abbvie and Ferring for consultancy; grants or grants pending from Johnson and Johnson and Abbive; lecture fee(s) from: Abbvie and Merck and payment for development of educational presentations from Ferring. All of these activities are outside the submitted work.
AHS: Hillary Steinhart has received fee(s) from Janssen, Abbvie, Shire, Pendopharm, Pfizer, and Takeda for consultancy; and lecture fee(s) from: Janssen, Abbvie, Shire, Warner Chilcott, Aptalis, and Takeda. His institution has received grants or grants pending from Janssen, Abbvie, Pfizer, Amgen, Takeda and Actavis. All of these activities are outside the submitted work.
JKM: None known.
CHS: Dr. Cynthia Seow has served as a consultant and on advisory boards for Janssen Pharmaceuticals, Abbvie, Takeda, Actavis, and Shire. She has a grant through Janssen Pharmaceuticals. Dr. Seow has also provided lectures for Janssen Pharmaceuticals and Warner Chilcott. All of these activities are outside the submitted work.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
D'Haens 2010 {published and unpublished data}
- D'Haens G, Kovács Á, Vergauwe P, Lonovics J, Bouhnik Y, Weiss W, et al. Safety and efficacy of a novel extended release budesonide formulation in patients with active left‐sided ulcerative colitis. Gastroenterology 2007;132(4 Suppl 2):A505‐6. [Google Scholar]
- D'Haens GR, Kovács Á, Vergauwe P, Nagy F, Molnár T, Bouhnik Y, et al. Clinical trial: Preliminary efficacy and safety study of a new budesonide‐MMX® 9 mg extended‐release tablets in patients with active left‐sided ulcerative colitis. Journal of Crohn's and Colitis 2010;4(2):153‐60. [DOI] [PubMed] [Google Scholar]
Gross 2011 {published and unpublished data}
- Gross V, Buganic I, Belousova EA, Mikhailova TL, Kupcinkas L, Kiudelis G, et al. 3g mesalazine granules are superior to 9mg budesonide for achieving remission in active ulcerative colitis: A double‐blind, double‐dummy, randomised trial. Journal of Crohn's and Colitis 2011;5(2):129‐138. [DOI] [PubMed] [Google Scholar]
- Gross V, Bunganic I, Mikhailova TL, Kupcinskas L, Kiudelis G, Tulassay Z, et al. Efficacy and tolerability of a once daily treatment with budesonide capsules versus mesalamine granules for the treatment of active ulcerative colitis: A randomized, double‐blind, double‐dummy, multicenter study. Gastroenterology 2009;136(5 Suppl 1):A15. [Google Scholar]
Löfberg 1996 {published data only}
- Löfberg R, Danielsson Å, Suhr O, Nilsson Å, Schiöler R, Nyberg A, et al. Oral budesonide versus prednisone in patients with active extensive and left‐sided ulcerative colitis. Gastroenterology 1996;110(6):1713‐8. [DOI] [PubMed] [Google Scholar]
Rubin 2014 {published data only}
- Rubin D, Cohen R, Sandborn W, Lichtenstein G, Axler J, Riddell R, et al. Budesonide MMX 9 mg for inducing remission in patients with mild‐to‐moderate ulcerative colitis not adequately controlled with oral 5‐asas. Inflammatory Bowel Diseases 2014;20(Suppl 1):S1. [Google Scholar]
Sandborn 2012 {published data only}
- Sandborn WJ, Travis S, Moro L, Ballard ED, Yeung P, Bleker W, et al. Budesonide Mxx 9mg for the induction of remission of mild‐to‐moderate ulcerative colitis (UC): Data from a multicenter, randomized, double‐blind placebo‐controlled study in North America and India. Gastroenterology 2011;140(5 Suppl 1):S124. [Google Scholar]
- Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, et al. Once‐daily Budesonide MMX® extended‐release tablets induce remission in patients with mild to moderate ulcerative colitis: Results from the CORE 1 study. Gastroenterology 2012;143(5):1218‐26. [DOI] [PubMed] [Google Scholar]
Travis 2014 {published data only}
- Sandborn WJ, Travis S, Danese S, Kupcinskas L, Alexeeva O, Moro L, et al. Budesonide‐MMx 9 mg for induction of remission of mild‐to‐moderate ulcerative colitis (UC): Data from a multicenter, randomized, double‐blind placebo‐controlled study in the Europe, Russia, Israel and Australia. Gastroenterology 2011;140(5 Suppl 1):S65. [Google Scholar]
- Travis S, Danese S, Kupcinskas L, Alexeeva O, D'Haens G, Gibson PR, et al. Once‐daily budesonide MMX in active mild‐to‐moderate ulcerative colitis: results from the randomised CORE II study. Gut 2014;63(3):433‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Chopra 2006 {published data only}
- Chopra A, Pardi DS, Loftus EV Jr, Tremaine WJ, Egan LJ, Faubion WA, et al. Budesonide in the treatment of inflammatory bowel disease: the first year of experience in clinical practice. Inflammatory Bowel Diseases 2006;12(1):29‐32. [DOI] [PubMed] [Google Scholar]
Danese 2013 {published data only}
- Danese S, Kupcinskas L, Ballard ED, Harris‐Collazo R, Haridman Y, Huang M, Travis SP. Budesonide MMX® efficacy is independent of previous mesalazine exposure in ulcerative colitis: pooled analysis of CORE I and CORE II. United European Gastroenterology Journal 2013;1(1 Suppl):OP262. [Google Scholar]
Díaz Blasco 1995 {published data only}
- Días Blasco J, Robledo Andrés P. Advances in the topical steroid treatment of inflammatory bowel disease [Avances en el tratamiento esteroideo tópico de la enfermedad inflamatoria intestinal]. Revista Española de Enfermedades Digestivas 1995;87(11):802‐7. [PubMed] [Google Scholar]
Feagan 1996 {published data only}
- Feagan B. Budesonide therapy for ulcerative colitis: a topical tale. Gastroenterology 1996;110(6):2000‐17. [DOI] [PubMed] [Google Scholar]
Fedorak 2005 {published data only}
- Fedorak RN, Bistritz L. Targeted delivery, safety, and efficacy of oral enteric‐coated formulations of budesonide. Advanced Drug Delivery Reviews 2005;57(2):303‐16. [DOI] [PubMed] [Google Scholar]
Gomollón 1999 {published data only}
- Gomollón F, Hinojosa J, Nos P. Budesonide and inflammatory bowel disease [Budesonida y enfermedad inflamatoria intestinal]. Gastroenterología y Hepatología 1999;22(10):525‐32. [PubMed] [Google Scholar]
Keller 1997 {published data only}
- Keller R, Stoll R, Foerster EC, Gutsche N, Domschke W. Oral budesonide therapy for steroid‐dependent ulcerative colitis. Alimentary Pharmacology and Therapeutics 1997;11(6):1047‐52. [DOI] [PubMed] [Google Scholar]
Kolkman 2004 {published data only}
- Kolkman JJ, Möllmann HW, Möllmann AC, Peńa AS, Greinwald R, Tauschel HD, et al. Evaluation of oral budesonide in the treatment of active distal ulcerative colitis. Drugs of Today 2004;40(7):589‐601. [PubMed] [Google Scholar]
- Kolkman JJ, Mölmann HW, Möllman AC, Nelis FG, Viergever P, Peña S. Beneficial effect of oral budesonide for distal ulcerative colitis: A comparative study of Budenfalk® 3 MG TID VS 9 MG OD. Gastroenterology 2000;118(4 Suppl 2):A779. [Google Scholar]
Lamers 1996 {published data only}
- Lamers CB, Wagtmans MJ, Sluys Veer A, Hogezand RA, Griffioen G. Budesonide in inflammatory bowel disease. Netherlands Journal of Medicine 1996;48(2):60‐3. [DOI] [PubMed] [Google Scholar]
Lichtenstein 2012 {published data only}
- Lichtenstein GR, Danese S, Ballard ED, Moro L, Jones RJ, Bagin R, et al. Effect of budesonide MMX 6 mg on the hypothalamic‐pituitary‐adrenal (HPA) axis in patients with ulcerative colitis: Results from a phase III, 12 month safety and extended use study. Gastroenterology 2012;142(5 Suppl 1):S785. [Google Scholar]
Lichtenstein 2013 {published data only}
- Lichtenstein G, Sandborn W, Huang M, Hardiman Y, Bagin R, Yeung P, et al. Budesonide MMX versus placebo in patients with active, mild‐to‐moderate ulcerative colitis who were 5‐ASA naive or previously treated with 5‐ASA. Inflammatory Bowel Diseases 2013;19:S83. [Google Scholar]
- Lichtenstein GR, Sandborn W, Huang M, Hardiman Y, Bagin R, Yeung P, et al. Budesonide MMX 9 mg induces remission in mild‐to‐moderately active UC patients regardless of prior history of 5‐ASA therapy. Gastroenterology 2013;144(5 Suppl 1):S234. [Google Scholar]
Marín‐Jiménez 2006 {published data only}
- Marín‐Jiménez I, Peña AS. Budesonide for ulcerative colitis. Revista Española de Enfermedades Digestivas 2006;98(5):362‐73. [DOI] [PubMed] [Google Scholar]
Sandborn 2013a {published data only}
- Sandborn W, Bagin R, Zhao J, Huang M, Hardiman Y, Schulteis C, et al. Symptom resolution and clinical and endoscopic remission both resulted in improved quality of life in patients with ulcerative colitis. Inflammatory Bowel Diseases 2013;19:S82. [Google Scholar]
Sandborn 2013b {published data only}
- Sandborn W, Hardiman Y, Huang M, Harris‐Collazo R, Ballard ED, Travis S. Efficacy of budesonide MMX in reduction of symptoms in patients with mild‐to‐moderately active ulcerative colitis: A pooled analysis of the core I and core II studies. Gastroenterology 2013;144(5 Suppl 1):S233‐4. [Google Scholar]
Sandborn 2015 {published data only}
- Sandborn W, Travis S, Bala N, Danese S, Moro L, Ballard ED, et al. Induction of clinical and endoscopic remission of mild to moderately active ulcerative colitis with budesonide MMX 9 MG: Analysis of pooled data from two phase 3 studies. American Journal of Gastroenterology 2011;106:S485. [Google Scholar]
- Sandborn WJ, Danese S, D'Haens G, Moro L, Jones R, Bagin R, et al. Induction of clinical and colonoscopic remission of mild‐to‐moderate ulcerative colitis with budesonide MMX 9 mg: pooled analysis of two phase 3 studies. Alimentary Pharmacology and Therapeutics 2015;41(5):409‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 2011 {published data only}
- Silverman J, Otley A. Budesonide in the treatment of inflammatory bowel disease. Expert Review of Clinical Immunology 2011;7(4):419‐28. [DOI] [PubMed] [Google Scholar]
Travis 2011 {published data only}
- Travis S, Ballard ED II, Bagin B, Gautille T, Huang M. Induction of remission with oral budesonide MMXVR (9 mg) tablets in patients with mild to moderate, active ulcerative colitis: A multicenter, open‐label efficacy and safety study. Inflammatory Bowel Diseases 2011;17(Suppl 2):S20. [Google Scholar]
Travis 2012b {published data only}
- Travis S, Danese S, Moro L, Ballard ED, Bagin R, Gautille T, et al. Induction of clinical and endoscopic remission with budesonide MMX in mild to moderately active ulcerative colitis: Pooled data from two phase 3 studies. Journal of Crohn's and Colitis 2012;6:S74. [Google Scholar]
Additional references
Bar‐Meir 2003
- Bar‐Meir S, Fidder HH, Faszczyk M, Bianchi Porro G, Sturniolo GC, Mickisch O, et al. Budesonide foam vs. hydrocortisone acetate foam in the treatment of active ulcerative proctosigmoiditis. Diseases of the Colon and Rectum 2003;46(7):929‐36. [DOI] [PubMed] [Google Scholar]
Barnes 2005
- Barnes PJ. Molecular mechanisms and cellular effects of glucocorticosteroids. Immunology and allergy clinics of North America 2005;25(3):451‐68. [DOI] [PubMed] [Google Scholar]
Baumgart 2007
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007;369(9573):1641‐57. [DOI] [PubMed] [Google Scholar]
Beattie 1996
- Beattie RM, Nicholls SW, Domizio P, Williams CB, Walker‐Smith JA. Endoscopic assessment of the colonic response to corticosteroids in children with ulcerative colitis. Journal of Pediatric Gastroenterology and Nutrition 1996;22(4):373‐9. [DOI] [PubMed] [Google Scholar]
Biancone 2008
- Biancone L, Michetti P, Travis S, Escher J, Moser G, Forbes A, et al. European evidence‐based consensus on the management of ulcerative colitis: special situations. Journal of Crohn's and Colitis 2008;2(1):63‐92. [DOI] [PubMed] [Google Scholar]
Bickston 2014
- Bickston SJ, Behm BW, Tsoulis DJ, Cheng J, MacDonald JK, Khanna R, Feagan BG. Vedolizumab for induction and maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD007571.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2000
- Cohen RD, Woseth DM, Thisted RA, Hanauer SB. A meta‐analysis and overview of the literature on treatment options for left‐sided ulcerative colitis and ulcerative proctitis. American Journal of Gastroenterology 2000;95(5):1263‐76. [DOI] [PubMed] [Google Scholar]
Danese 2014
- Danese S, Siegel CA, Peyrin‐Biroulet L. Review article: integrating budesonide‐MMX into treatment algorithms for mild‐to‐moderate ulcerative colitis. Alimentary Pharmacology and Therapeutics 2014;39(10):1095‐103. [DOI] [PubMed] [Google Scholar]
Danielsson 1992
- Danielsson A, Löfberg R, Persson T, Salde L, Schiöler R, Suhr O, Willén R. A steroid enema, budesonide, lacking systemic effects for the treatment of distal ulcerative colitis or proctitis. Scandinavian Journal of Gastroenterology 1992;27(1):9‐12. [DOI] [PubMed] [Google Scholar]
De Cassan 2012
- Cassan C, Fiorino G, Danese S. Second‐generation corticosteroids for the treatment of Crohn's disease and ulcerative colitis: more effective and less side effects?. Digestive Diseases 2012;30(4):368‐75. [DOI] [PubMed] [Google Scholar]
Dignass 2012
- Dignass A, Lindsay JO, Sturm A, Windsor A, Colombet LF, Allez M, et al. Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 2: current management. Journal of Crohn's and Colitis 2012;6(10):991‐1030. [DOI] [PubMed] [Google Scholar]
Dilger 2004
- Dilger K, Schwab M, Fromm MF. Identification of budesonide and prednisone as substrates of the intestinal drug efflux pump P‐glycoprotein. Inflammatory Bowel Diseases 2004;10(5):578‐83. [DOI] [PubMed] [Google Scholar]
Eaden 2001
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta‐analysis. Gut 2001;48(4):526‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekbom 1990
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population‐based study. New England Journal of Medicine 1990;323(18):1228‐33. [DOI] [PubMed] [Google Scholar]
Feagan 2013
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699‐710. [DOI] [PubMed] [Google Scholar]
Ford 2011
- Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Takkey NJ, Moayyedi P. Glucocorticosteroid therapy and inflammatory bowel disease: a systematic review and meta‐analysis. American Journal of Gastroenterology 2011;106(4):590‐9. [DOI] [PubMed] [Google Scholar]
Gionchetti 2014
- Gionchetti P, Praticò C, Rizzello F, Calafiore A, Capozzi N, Campieri M, Calabrese C. The role of budesonide‐MMX in active ulcerative colitis. Expert Review of Gastroenterology and Hepatology 2014;8(3):215‐22. [DOI] [PubMed] [Google Scholar]
Griffiths 2004
- Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Practice and Research. Clinical Gastroenterology 2004;18(3):509‐23. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 1993
- Hanauer S, Schwartz J, Robinson M, Roufail W, Arora S, Cello J, et al. Mesalamine capsules for the treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. American Journal of Gastroenterology 1993;88(8):1188‐97. [PubMed] [Google Scholar]
Hanauer 2006
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflammatory Bowel Diseases 2006;12 Suppl 1:S3‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hoy 2015
- Hoy SM. Budesonide MMX(®): a review of its use in patients with mild to moderate ulcerative colitis. Drugs 2015;75(8):879‐86. [DOI] [PubMed] [Google Scholar]
Itzkowitz 2004
- Itzkowitz SH, Yio X. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American Journal of Physiology. Gastrointestinal and Liver Physiology 2004;287(1):G7‐17. [DOI] [PubMed] [Google Scholar]
Jönsson 1995
- Jönsson G, Aström A, Andersson P. Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver. Drug Metabolism and Disposition: The Biological Fate of Chemicals 1995;23(1):137‐42. [PubMed] [Google Scholar]
Kirsner 2001
- Kirsner JB. Historical origins of current IBD concepts. World Journal of Gastroenterology 2001;7(2):175‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kornbluth 2010
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. American Journal of Gastroenterology 2010;105(3):501‐23. [DOI] [PubMed] [Google Scholar]
Kozuch 2008
- Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World Journal of Gastroenterology 2008;14(3):354‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kucharzik 2006
- Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflammatory Bowel Diseases 2006;12(11):1068‐83. [DOI] [PubMed] [Google Scholar]
Kuenzig 2014
- Kuenzig ME, Rezaie A, Seow CH, Otley AR, Steinhart AH, Griffiths AM, Kaplan GG, Benchimol EI. Budesonide for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD002913.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakatos 2006
- Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, et al. Risk factors for ulcerative colitis‐associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population‐based study. Inflammatory Bowel Diseases 2006;12(3):205‐11. [DOI] [PubMed] [Google Scholar]
Lawson 2006
- Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005112.pub2] [DOI] [PubMed] [Google Scholar]
Lichtenstein 2006
- Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological Association. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130(3):940‐87. [DOI] [PubMed] [Google Scholar]
Lichtenstein 2009
- Lichtenstein GR, Bengtsson B, Hapten‐White L, Rutgeerts P. Oral budesonide for maintenance of remission of Crohn's disease: a pooled safety analysis. Alimentary Pharmacology and Therapeutics 2009;29(6):643‐53. [DOI] [PubMed] [Google Scholar]
Lichtiger 1990
- Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active ulcerative colitis. Lancet 1990;336(8706):16‐9. [DOI] [PubMed] [Google Scholar]
Lockhart‐Mummery 1960
- Lockhart‐Mummery HE, Morson BC. Crohn's disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut 1960;1:87‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nos 2001
- Nos P, Hinojosa J. Gomollón F, Ponce J. Budesonide in inflammatory bowel disease: a meta‐analysis [Metaanálisis sobre la efectividad de la budesonida en la enfermedad inflamatoria intestinal]. Medicina Clínica 2001;116(2):47‐53. [DOI] [PubMed] [Google Scholar]
Powell‐Tuck 1978
- Powell‐Tuck J, Bown RL, Lennard‐Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scandinavian Journal of Gastroenterology 1978;13(7):833‐7. [DOI] [PubMed] [Google Scholar]
Rachmilewitz 1989
- Rachmilewitz D. Coated mesalamine (5‐aminosalicylic acid) versus sulphasalazine in active ulcerative colitis: a randomised trial. BMJ 1989;298(6666):82‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rezaie 2015
- Rezaie A, Kuenzig ME, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH, Kaplan GG, Seow CH. Budesonide for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD000296.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 1991
- Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean?. Gut 1991;32(2):174‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutgeerts 2005
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2005;353(23):2462‐76. [DOI] [PubMed] [Google Scholar]
Rutter 2004
- Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126(2):451‐9. [DOI] [PubMed] [Google Scholar]
Sabate 1998
- Sabate JM, Chaussade S. Modalities of corticosteroid therapy in inflammatory bowel disease [Modalités de la corticothérapie au cours des maladies inflammatoires du tube digestif]. Semaine des Hôpitaux de Paris 1998;74:1079‐89. [Google Scholar]
Sands 2007
- Sands BE. Inflammatory bowel disease: past, present, and future. Journal of Gastroenterolgy 2007;42(1):16‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saverymuttu 1986
- Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJ, Chadwick VS. Indium 111‐granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111‐graunlocyte excretion. Gastroenterology 1986;90(5 Pt 1):1121‐8. [DOI] [PubMed] [Google Scholar]
Schroeder 1987
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New England Journal of Medicine 1987;317(26):1625‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Seo 1992
- Seo M, Okada M, Yao T, Ueki M, Arima A, Okumura M. An index of disease activity in patients with ulcerative colitis. American Journal of Gastroenterology 1992;87(8):971‐6. [PubMed] [Google Scholar]
Seow 2009
- Seow CH, Benchimol EI, Steinhart AH, Griffiths AM, Otley AR. Budesonide for Crohn's disease. Expert Opinion on Drug Metabolism and Toxicology 2009;5(8):971‐9. [DOI] [PubMed] [Google Scholar]
Sutherland 1987
- Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, et al. 5‐Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis and proctitis. Gastroenterology 1987;92(6):1894‐8. [DOI] [PubMed] [Google Scholar]
Thomsen 1998
- Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, et al. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide‐Mesalamine Study Group. New England Journal of Medicine 1998;339(6):370‐4. [DOI] [PubMed] [Google Scholar]
Timmer 2012
- Timmer A, McDonald JW, Tsoulis DJ, MacDonald JK. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000478.pub3] [DOI] [PubMed] [Google Scholar]
Travis 2006
- Travis SP. Review article: induction therapy for patients with active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;24(s1):10‐6. [DOI] [PubMed] [Google Scholar]
Travis 2012a
- Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012;61(4):535‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Truelove 1955
- Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. British Medical Journal 1955;2(4947):1041‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2007
- Turner D, Otley AR, Mack D, Hyams J, Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133(2):423‐32. [DOI] [PubMed] [Google Scholar]
Van Limbergen 2008
- Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, et al. Definition of the phenotypic characteristics of childhood‐onset inflammatory bowel disease. Gastroenterology 2008;135(4):1114‐22. [DOI] [PubMed] [Google Scholar]
Walmsley 1998
- Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998;43(1):29‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xavier 2007
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448(7152):427‐34. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sherlock 2010
- Sherlock ME, Seow CH, Steinhart HS. Griffiths AM. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007698.pub2] [DOI] [PubMed] [Google Scholar]


