Abstract
Shiga toxin-producing Escherichia coli (STEC) is an important cause of food-borne illness in humans. Ruminants appear to be more frequently colonized by STEC than are other animals, but the reason(s) for this is unknown. We compared the frequency, magnitude, duration, and transmissibility of colonization of sheep by E. coli O157:H7 to that by other pathotypes of E. coli. Young adult sheep were simultaneously inoculated with a cocktail consisting of two strains of E. coli O157:H7, two strains of enterotoxigenic E. coli (ETEC), and one strain of enteropathogenic E. coli. Both STEC strains and ETEC 2041 were given at either 107 or 1010 CFU/strain/animal. The other strains were given only at 1010 CFU/strain. We found no consistent differences among pathotypes in the frequency, magnitude, and transmissibility of colonization. However, the STEC strains tended to persist to 2 weeks and 2 months postinoculation more frequently than did the other pathotypes. The tendency for persistence of the STEC strains was apparent following an inoculation dose of either 107 or 1010 CFU. One of the ETEC strains also persisted when inoculated at 1010 CFU. However, in contrast to the STEC strains, it did not persist when inoculated at 107 CFU. These results support the hypothesis that STEC is better adapted to persist in the alimentary tracts of sheep than are other pathotypes of E. coli.
Escherichia coli O157:H7 and other serotypes of Shiga toxin-producing E. coli (STEC) are important causes of food-borne illnesses in people. Most clusters of disease are traced to contaminated food or water, but person-to-person transmissions also occurs (24, 32, 56). Contaminated beef has been the source of several large outbreaks of disease, and cattle are considered to be a reservoir for E. coli O157:H7 and other STEC serotypes (24, 32, 48, 58). Healthy cattle shed E. coli O157:H7 intermittently. Among U.S. cattle the overall individual animal prevalence of E. coli O157:H7 is approximately 2 to 3%, while the herd prevalence is much higher (16, 20, 25, 46, 58, 59). Fecal shedding of E. coli O157:H7 is often seasonal, with increased shedding during the summer (7, 25, 27, 41), which corresponds to an increased incidence of human disease (27, 37). Frequently individual cattle are transiently colonized (<1 month) by one particular strain, but occasional animals excrete multiple strains of E. coli O157:H7 (1, 16, 53). Individual strains of E. coli O157:H7 can be isolated from some herds for as long as 2 years, whereas other herds remain culture negative for several years (53). E. coli O157:H7 has also been isolated from healthy sheep (7, 29, 36), sheep's milk (50), and wild deer (47, 51). Food products from other ruminants, such as venison jerky (33) and goat's milk (3), have also been implicated as sources of human STEC infection. Both cattle and sheep also harbor other serotypes of STEC, usually at a much higher prevalence than serotype O157:H7 (2, 18, 35, 46, 58).
In contrast to ruminants, E. coli O157:H7 is isolated much less frequently from other domestic and wild animals (7, 26, 53, 57). This may be partially due to a sampling bias toward cattle. However, in a survey of 4,229 market swine in the United States, the incidence of E. coli O157:H7 was less than 0.07% (5). E. coli O157:H7 was not isolated from 1,000 fecal samples from either swine or poultry in England (7). There are recent reports of E. coli O157:H7 being isolated from swine in Chile (49) and Japan (45). Although E. coli O157:H7 occurs in nonruminant animals such as dogs, horses, birds, and flies, there is no evidence that the agent is as prevalent or as persistent in these animals as it is in ruminants (6, 26, 53, 57). Furthermore, the prevalence of all types of STEC appears to be greater in ruminants than in other types of domestic animals (2).
Both calves and adult cattle have been experimentally inoculated with E. coli O157:H7 (4, 11, 28, 30). The magnitude of shedding is greatest during the first 2 weeks postinoculation (p.i.) and decreases thereafter. In general, calves shed higher numbers of E. coli O157:H7 organisms for a longer duration than do mature animals (11). This parallels the results of on-farm studies that show a greater percentage of young animals colonized with E. coli O157:H7 than adults (25, 27, 41, 58). However, there is considerable animal-to-animal variability in both the numbers of bacteria shed and the duration of shedding (4, 11). The infection persists for several months in some cattle and calves (11). Sheep have also been experimentally inoculated with E. coli O157:H7 (34, 37). The quantity and duration of fecal shedding are similar to those for cattle. Diet appears to influence the colonization of E. coli O157:H7 in both cattle and sheep (10, 30, 34, 37). Serum antibody to O157 lipopolysaccharide or Shiga toxin 1 (Stx1) acquired from a prior infection does not protect calves from reinfection (31). Sheep and cattle that have cleared a previous colonization with E. coli O157:H7 can also be reinfected (11, 37).
Despite the widespread epidemiological evidence that most ruminants are colonized by STEC, the reasons for this observation are not known. We hypothesized that STEC bacteria are better adapted to colonize and persist in the alimentary tracts of ruminants than are other pathotypes of E. coli. We tested this hypothesis by analyzing the frequency, magnitude, duration, and transmissibility of E. coli O157:H7 colonization compared to those for other pathotypes of E. coli when sheep were inoculated with a cocktail consisting of multiple strains representing three pathotypes of E. coli. We found that the inoculated STEC strains tended to persist longer than the enterotoxigenic (ETEC) and enteropathogenic (EPEC) strains included in the same inoculum. We also found that all of the strains were transmitted to naïve animals from infected donors and again that the STEC strains tended to persist longer in the recipient animals than did two of the other strains.
(A preliminary report of this work was made at the 99th General Meeting of the American Society for Microbiology, 1999, abstr. D/B 309); at the annual meeting of the Food Research Institute, University of Wisconsin—Madison, 1999; and at the Verocytotoxigenic E. coli in Europe: Pathogenicity and Virulence meeting, Liège, Belgium, 1999.)
MATERIALS AND METHODS
Inoculum cocktail.
Strains of E. coli used in the inoculum cocktail are listed in Table 1. All of the strains used in the cocktail are animal or human pathogens. STEC strain 86-24 was isolated from an outbreak of human disease and causes attaching and effacing lesions in experimentally infected animals (15, 23). STEC strain 3081 was isolated from a healthy bovine, can persist asymptomatically as long as 6 months in the alimentary tracts of experimentally infected cattle, and causes attaching and effacing lesions in the intestines of neonatal pigs and calves (11, 13). ETEC strains 2041 and 637 cause diarrhea in experimentally inoculated weaned and neonatal pigs, respectively (43, 52, 54). F4 fimbriae (K88) host adapt ETEC 2041 for swine, and F5 fimbriae (K99) host adapt ETEC 637 for neonatal calves, pigs, and lambs. EPEC strain E2348/69 causes diarrhea in experimentally inoculated humans and attaching and effacing lesions in animal models (39, 44). Generally, EPEC and ETEC strains colonize the lower small intestine (ileum). It is not known if STEC strains colonize a specific site in mature ruminants. In short-term experimental studies (2 to 30 days), STEC bacteria have been recovered from the rumen, ileum, and sites throughout the lower intestinal tract (4, 11, 13).
TABLE 1.
Strains of E. coli used in the inoculum cocktail
| Strain | Pathotype | Serogroup | Virulence determinant(s)a | Source | Reference(s) | Selective mediumb/ carbohydrate reaction |
|---|---|---|---|---|---|---|
| 86-24 | STEC | O157:H7 | eae, Stx2 | Human | 15, 23 | SN dulcitol/positive |
| 3081 | STEC | O157:H7 | eae, Stx1, Stx2 | Bovine | 11, 13 | KA sorbitol/negative |
| 2041 | ETEC | O157:H43 | F4, F41, LT, STb | Porcine | 52 | TN sorbose/positive |
| 637 | ETEC | O64:NM | F5, STa | Porcine | 43, 54 | TN sorbose/negative |
| E2348/69 | EPEC | O127:H6 | eae | Human | 39, 44 | SN dulcitol/negative |
LT, heat labile enterotoxin; STb, heat-stable enterotoxin b; STa, heat-stable enterotoxin a; eae, gene encoding intimin; F4, F4 (K88) fimbriae; F5, F5 (K99) fimbriae; F41, F41 fimbriae.
MacConkey agar base; S, streptomycin (100 μg/ml); N, nalidixic acid (10 or 20 μg/ml); T, tetracycline (10 μg/ml); K, kanamycin (30 μg/ml); A, ampicillin (100 μg/ml).
Virulence genes from all of the strains were confirmed using multiplex PCR (19; B. T. Bosworth and T. A. Casey, Abstr. 97th Gen. Meet. Am. Soc. Microbiol. 1997, abstr. B-509, p. 116, 1997). Resistance to antibiotics (ampicillin, chloramphenicol, kanamycin, nalidixic acid, streptomycin, and tetracycline) and carbohydrate fermentation (dulcitol, sorbose, sorbitol, and sucrose) (12, 17) were determined by standard methods. Spontaneous mutants resistant to nalidixic acid were isolated from strains 86-24, 2041, 637, and E2348/69. Strains were selected so that only two of the five bacteria in the cocktail were resistant to the same antibiotics. These two strains were differentiated from each other by carbohydrate fermentation. This approach allowed for five strains to be differentiated by using three different selective media (Table 1). The recovery of individual strains was compared on selective and nonselective media to ensure that the selective medium fostered the growth of each strain equally (data not shown). The bacterial inoculum was made as previously described (52). Briefly, strains were grown individually in Trypticase soy broth (TSB) overnight at 37°C with agitation (200 rpm), concentrated 10-fold in TSB, and frozen individually at −80°C in glycerol. The inoculum was thawed and diluted in TSB just prior to inoculation.
Experimental design.
Young adult sheep (5 to 12 months old) were obtained from the Iowa State University herd and acclimated for ≥2 weeks. They were housed two to a room with shared water and feed on concrete floors in BL-2 containment and were fed a low-energy diet of concentrate (40% corn, 22% alfalfa, 15% wheat millings, 10% oat hulls, 7.5% soybean meal; 1 lb/sheep/day) and grass and alfalfa hay (ad libitum). Water buckets were emptied daily and filled with chlorinated city water. During the acclimation period, feces from each sheep were screened to ensure that the background fecal flora did not contain enteric bacteria with the same antibiotic resistance patterns as the cocktail strains. Sheep were inoculated by mixing each of the five strains into a small amount of the feed concentrate. The inoculum was consumed within 2 h. In vitro experiments to determine the survival of the cocktail strains in the feed did not reveal any significant differences between strains (data not shown). Controls (n = 4) were not inoculated. Paired serum samples (prior to and 2 months after inoculation) were collected and analyzed for the development of antibodies to Stx1, Stx2 (n = 18), and O157 lipopolysaccharide (n = 16).
On days when fecal samples were to be collected, the floor was cleaned, the sheep were individually penned, and fecal samples were collected from the floor within 2 h of passage. Individual fecal samples were collected on days 2, 3, 4, 14, 15, 16, 58, 59, and 60 p.i. Previous work with cattle demonstrated that the decrease in the magnitude of STEC shedding is comparatively rapid between 2 days and 2 weeks and is gradual between 2 weeks and 2 months; therefore, samples were not collected between the latter time points (11). Two months p.i. was selected as the final period because shedding terminates in some, but not all, cattle by this time (11).
Samples (5 g) were processed immediately by fivefold dilution with phosphate-buffered saline (PBS) and mixing in a Stomacher blender for 1 min; then serial 10-fold dilutions were made in PBS. Aliquots were plated directly onto selective media in triplicate and incubated for 24 h at 37°C. The sensitivity of the direct plating was ≥50 CFU/g. Samples (10 g in 100 ml of medium) were also cultured at 37°C in enrichment broth (TSB with 0.15% bile salts) with agitation for 24 h and then plated onto selective media (11). Samples from water buckets (10 ml) were cultured in enrichment broth. The identities of the isolates recovered were confirmed by serology (slide agglutination in antisera or a commercial kit for O157) for O antigens. Sheep were necropsied at the end of the experiment (2 months p.i.). The contents of the rumen, ileum, cecum, colon, and rectum as well as tissues from the tonsil and lymph nodes were cultured in enrichment broth. When the enrichment cultures were positive, the original contents (which were held at 4°C overnight) were plated directly the next day. Preliminary experiments comparing the bacterial counts of tissue contents cultured immediately versus after holding for 24 h at 4°C did not show quantitative differences between the treatments (data not shown).
Transmission experiments.
Sheep were acclimated for ≥2 weeks prior to the experiment as described above. Donor sheep (6 sheep total in 3 replicates) were orally inoculated with the cocktail shown in Table 1. The STEC strains, 86-24 and 3081, were given at 107 CFU/strain, and the other three strains were given at 1010 CFU/strain. Three days p.i., each donor was moved into a clean room with a naïve animal of the same age and remained there for the duration of the experiment. Fecal samples were collected from each donor prior to moving. Fecal samples were collected on days 2, 3, 4, 14, 15, 16, 58, 59, and 60 postexposure from both recipient and donor sheep. Samples were cultured as described above. Sheep were necropsied 2 months postexposure.
Antibody titers.
Serum was heat inactivated for 1 h at 56°C. Neutralizing antibody titers to Stx1 and Stx2 were determined using monolayers of Vero cells (22). Antibody to O157 lipopolysaccharide was detected using a blocking enzyme-linked immunosorbent assay (ELISA) as described previously except that 1% skim milk (rather than fetal calf serum) was used to block nonspecific binding (38).
Fecal toxin titers.
Assays for free fecal Shiga toxin were performed on Vero cells as previously described except that the sheep feces were diluted 1:5 with PBS and mixed in a Stomacher blender prior to centrifugation (9, 21).
Statistics.
Bacterial counts (CFU per gram of feces) from individual sheep were converted to log10 units and averaged over days 2, 3, and 4 (initial period), days 14, 15, and 16 (2 weeks), and days 58, 59, and 60 (2 months). Differences in the magnitude of shedding between strains were compared by a paired t test using Bonferroni's method to correct for type 1 error (55). Differences in the magnitude of shedding due to treatment were compared using repeated-measures analysis of variance (ANOVA). Differences in the duration of shedding between strains were compared using a sign test (55).
RESULTS
Treatment 1.
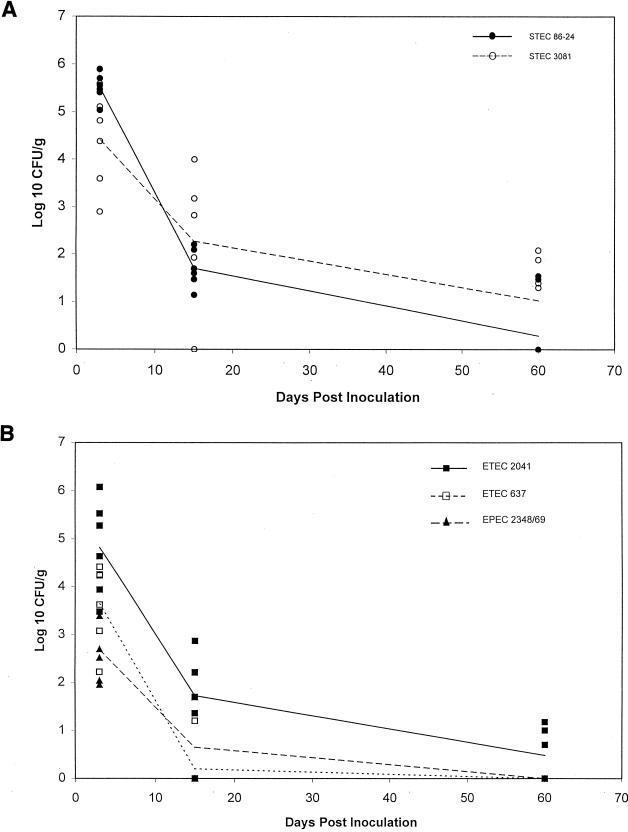
Sheep (n = 6) were simultaneously inoculated with all five strains in the cocktail at a dose of 1010 CFU/strain. During the initial period p.i., all of the strains were recovered from all of the sheep (Fig. 1). The magnitude of colonization by all strains varied considerably for individual animals. In general, both STEC strains and ETEC 2041 were shed in greater numbers than the other two strains. These differences were significant between STEC 86-24 and EPEC E2348/69 and between STEC 86-24 and ETEC 637 (P ≤ 0.002).
FIG. 1.
Fecal shedding of E. coli strains from six sheep inoculated with a cocktail containing five strains given at a dose of 1010 CFU/strain/animal (treatment 1). Lines represent the means for each strain. (A) Strains STEC 86-24 and STEC 3081; (B) strains ETEC 2041, ETEC 637, and EPEC E2348/69.
At 2 weeks p.i., STEC 86-24 was recovered from 6 of 6 sheep, while STEC 3081 and ETEC 2041 were recovered from 5 of 6 sheep. Again, the magnitude of shedding of both STEC strains and ETEC 2041 tended to be greater than those of the other two strains. Only STEC 86-24 and ETEC 637 were significantly different at this time (P ≤ 0.002).
At 2 months p.i., STEC 86-24 was recovered from 2 of 6 sheep, STEC 3081 from 4 of 6 sheep, and ETEC 2041 from 3 of 6 sheep. Neither EPEC E2348/69 nor ETEC 637 was recovered at 2 months p.i. These six sheep were not necropsied.
Treatment 2.
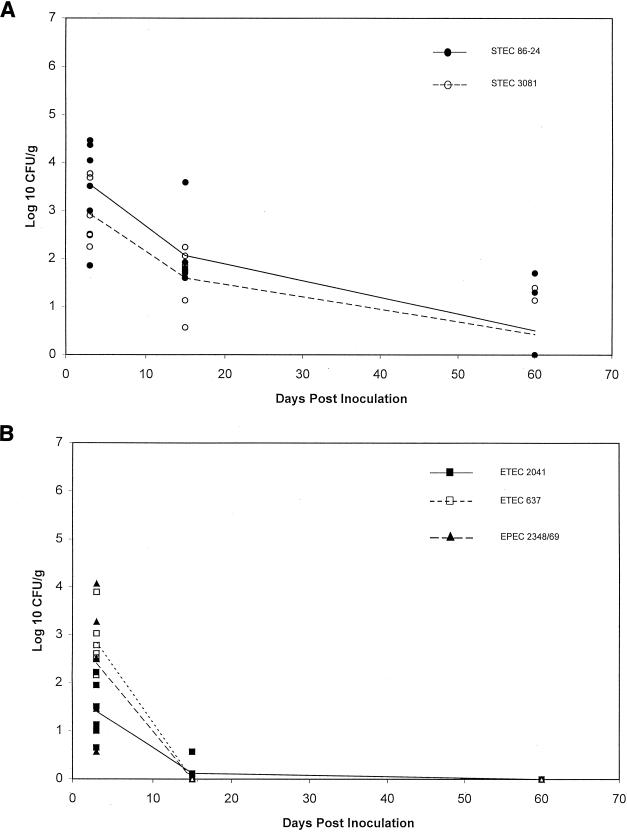
The objective of treatment 2 was to determine if strains that persisted for 2 months in treatment 1 would still persist if they were inoculated at a lower dose. Since the magnitude and duration of colonization by ETEC 2041 were similar to those for the STEC strains, and these three strains persisted longer than the other two strains in sheep given treatment 1, the dose of STEC 86-24, STEC 3081, and ETEC 2041 was lowered to 107 CFU/strain in treatment 2. The dose of ETEC 637 and EPEC E2348/69 was left at 1010 CFU/strain. Six sheep were given this treatment regimen. Again, all of the strains were recovered from all of the sheep during the initial period p.i. (Fig. 2). As was seen with treatment 1, there was a wide variation in the magnitude of shedding for individual sheep.
FIG. 2.
Fecal shedding of various E. coli strains from six sheep inoculated with a cocktail containing five strains. STEC 86-24, STEC 3081, and ETEC 2041 were given at a dose of 107 CFU/strain/animal, and EPEC E2348/69 and ETEC 637 were given at 1010 CFU/strain/animal (treatment 2). Lines represent the means for each strain. (A) Strains STEC 86-24 and STEC 3081; (B) strains ETEC 2041, ETEC 637, and EPEC E2348/69.
At 2 weeks p.i., STEC 86-24 was recovered from 6 of 6 sheep and STEC 3081 was detected in 5 of 6 sheep. In contrast to treatment 1, ETEC 2041 was recovered only from 1 of 6 sheep. At this time the magnitudes of shedding of both STEC strains were significantly greater than those of the other three strains (P ≤ 0.002).
At 2 months p.i., STEC 86-24 and STEC 3081 were each recovered from 2 of 6 sheep and the other three strains were not recovered. At necropsy, only STEC 86-24 and STEC 3081 were recovered from the rectal contents (feces) of two sheep. None of the cocktail strains were recovered from the other necropsy samples.
Treatment 3.
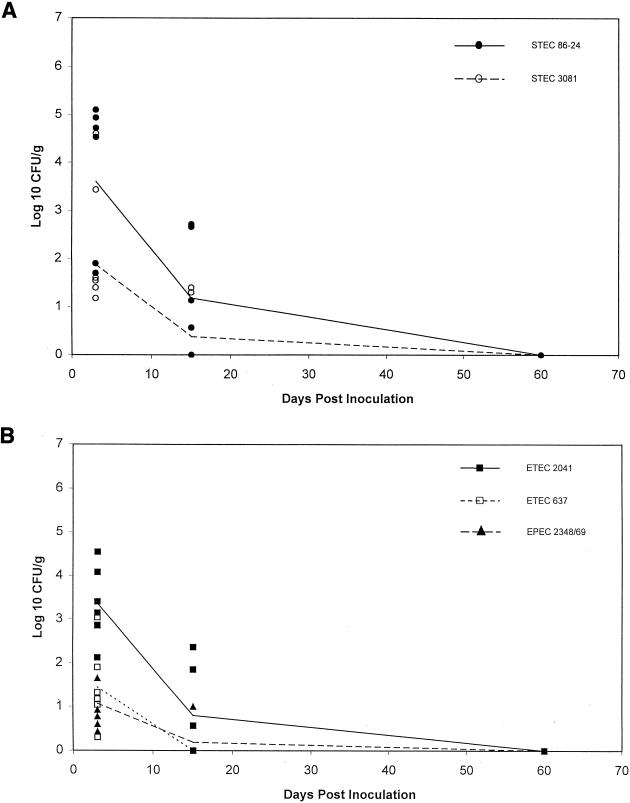
The primary purpose of treatment 3 was to produce donor animals for the transmission experiment. Treatment 2 also demonstrated that both STEC strains, but not ETEC 2041, persisted when the inoculum dose was lowered to 107 CFU. In treatment 3 the dose of ETEC 2041 was raised back to 1010 CFU and the STEC dose remained at 107 CFU/strain. ETEC 637 and EPEC E2348/69 were given at 1010 CFU/strain. Six sheep were given this treatment regimen. As was seen in the previous experiments, all of the strains were recovered from all of the sheep during the initial period p.i. (Fig. 3). The initial magnitudes of shedding of STEC 86-24 and ETEC 2041 tended to be greater than those of the other strains, but the differences were not significant.
FIG. 3.
Fecal shedding of various E. coli strains from six sheep inoculated with a cocktail containing five strains. STEC 86-24 and STEC 3081 were given at a dose of 107 CFU/strain/animal, and ETEC 2041, EPEC E2348/69, and ETEC 637 were given at 1010 CFU/strain/animal (treatment 3). Lines represent the means for each strain. (A) Strains STEC 86-24 and STEC 3081; (B) strains ETEC 2041, ETEC 637, and EPEC E2348/69.
At 2 weeks p.i., STEC 86-24 was recovered from 4 of 6 sheep, STEC 3081 from 2 of 6 sheep, and ETEC 2041 from 3 of 6 sheep. The magnitudes of shedding of STEC 86-24 and ETEC 2041 at this time also tended to be greater than those of the other strains, but the differences were not significant. At 2 months p.i., none of the inoculum strains were recovered from any of the sheep given treatment 3.
Dose effect.
The magnitudes of shedding of both STEC 86-24 and STEC 3081 were significantly different (P ≤ 0.05) between treatment 1 (1010-CFU inoculum) and treatment 2 or 3 (107-CFU inoculum) during the initial period (Fig. 1A, 2A, and 3A). By 2 weeks p.i., the magnitude of STEC shed was not significantly different between sheep given treatment 1 and those given treatment 2. The magnitude of shedding of ETEC 2041 was significantly different (P ≤ 0.05) between all three treatments during the initial period (Fig. 1B, 2B, and 3B). The magnitude of shedding of ETEC 2041 continued to be significantly different (P ≤ 0.05) between treatment 1 and treatment 2 at 2 weeks and 2 months p.i. This is in contrast to both STEC strains.
Infectious dose of in vitro-grown STEC.
In subsequent experiments, the dose of both STEC strains in the cocktail was lowered to 105 CFU/strain and the other strains remained at 1010 CFU/strain. STEC 86-24 was recovered from 4 of 6 sheep during the initial period p.i. (Table 2; range, <50 to 104 CFU/g). It persisted in two sheep at 2 weeks p.i. (104 to 105 CFU/g) and in one of these sheep at 2 months p.i. (<50 CFU/g). STEC 3081 was recovered from 2 of 6 sheep during the initial period (<50 CFU/g) but was not recovered at 2 weeks or 2 months p.i. The dose of both STEC strains was further lowered to 104 CFU/strain, and the other strains remained at 1010 CFU/strain. In this experiment STEC 86-24 was recovered from 2 of 6 sheep during the initial period (<50 CFU/g) but was not recovered at 2 weeks or 2 months p.i. STEC 3081 was not recovered at any time from any of the sheep given the 104-CFU dose. In these experiments the other three strains in the cocktail were recovered at similar magnitudes and times as those described previously (treatments 1 to 3). At necropsy ETEC 2041 was recovered from the rumina, ilea, ceca, and rectums, (range, ≥50 to 105 CFU/g) of 2 of 12 sheep. These two sheep were sharing a room, and one of them shed ETEC 2041 in moderate numbers (range, 102 to 105 CFU/g) throughout the study. ETEC 2041 was recovered from the second animal only at enrichment levels (<50 CFU/g) at 2 weeks and 2 months p.i.
TABLE 2.
Infectious dose of in vitro-grown E. coli O157:H7 in sheep
| Strain | Time p.i. | No. of animals positive/no. inoculated at a STEC dosea of:
|
|
|---|---|---|---|
| 105 CFUa | 104 CFU | ||
| 86-24 | Initial | 4/6 | 2/6 |
| 2 wk | 2/6 | 0/6 | |
| 2 mo | 1/6 | 0/6 | |
| 3081 | Initial | 2/6 | 0/6 |
| 2 wk | 0/6 | 0/6 | |
| 2 mo | 0/6 | 0/6 | |
| 2041 | Initial | 6/6 | 6/6 |
| 2 wk | 6/6 | 3/6 | |
| 2 mo | 2/6 | 0/6 | |
| 637 | Initial | 6/6 | 4/6 |
| 2 wk | 2/6 | 1/6 | |
| 2 mo | 0/6 | 0/6 | |
| E2348/69 | Initial | 6/6 | 5/6 |
| 2 wk | 0/6 | 3/6 | |
| 2 mo | 0/6 | 0/6 | |
STEC 86-24 and 3081 were inoculated at 105 or 104 CFU/strain/animal, and ETEC 2041, ETEC 637, and EPEC E2348/69 were inoculated at 1010 CFU/strain/animal.
Transmission between sheep.
The sheep in treatment 3 were used as donors for the transmission experiment. The infected donor sheep transmitted all five of the strains in the cocktail to naïve sheep (Table 3). STEC 86-24 was transmitted to naïve sheep on 4 of 5 occasions when the donor was shedding ≤104 CFU/g at the time it was placed in the room with the naïve animal and to 1 of 1 naïve sheep when the donor was shedding 105 CFU/g. STEC 3081 was transmitted to 2 of 6 naïve sheep when the donor was shedding ≤104 CFU/g. ETEC 2041 was transmitted to 3 of 3 naïve sheep when the donor was shedding ≤104 CFU/g and to 3 of 3 naïve sheep when the donor was shedding ≥105 CFU/g. EPEC strain E2348/69 was transmitted to 1 of 6 naïve sheep when the donor was shedding ≤104 CFU/g. ETEC 637 was transmitted to 0 of 5 naïve sheep when the donor was shedding ≤104 CFU/g and to 1 of 1 naïve sheep when the donor was shedding 105 CFU/g. Although both STEC strains and ETEC 2041 were transmitted more frequently to naïve sheep, EPEC E2348/69 was transmitted to one sheep when the donor was shedding ≤102 CFU/g of that strain.
TABLE 3.
Transmission of E. coli cocktail strains to six naïve sheep
| Strain | Pathotype | Time | No. of naïve animals positive/no. exposed to inoculated donors sheddingaE. coli at the following CFU/g:
|
|||||
|---|---|---|---|---|---|---|---|---|
| <102 | 102 | 103 | 104 | 105 | 106 | |||
| 86-24 | STEC | Initial | 1/2 | 0/1 | 2/2 | 0/1 | ||
| 2 wk | 0/2 | 1/1 | 2/2 | 1/1 | ||||
| 2 mo | 0/2 | 0/1 | 0/2 | 0/1 | ||||
| 3081 | STEC | Initial | 0/2 | 0/1 | 1/1 | 1/2 | ||
| 2 wk | 0/2 | 0/1 | 0/1 | 1/2 | ||||
| 2 mo | 0/2 | 0/1 | 0/1 | 0/2 | ||||
| 2041 | ETEC | Initial | 1/1 | 2/2 | 2/2 | 0/1 | ||
| 2 wk | 1/1 | 1/2 | 0/2 | 1/1 | ||||
| 2 mo | 0/1 | 0/2 | 0/2 | 0/1 | ||||
| E2348/69 | EPEC | Initial | 1/4 | 0/1 | 0/1 | |||
| 2 wk | 0/4 | 0/1 | 0/1 | |||||
| 2 mo | 0/4 | 0/1 | 0/1 | |||||
| 637 | ETEC | Initial | 0/2 | 0/3 | 1/1 | |||
| 2 wk | 0/2 | 0/3 | 0/1 | |||||
| 2 mo | 0/2 | 0/3 | 0/1 | |||||
At the time of exposure to a naïve animal.
Antibody titers.
Preexisting titers of antibody to Stx1 (geometric mean, 1:142; range, 1:32 to 1:1,000) but not to Stx2 were found in 8 of 18 sheep tested in this study. Two months p.i., 5 of 18 sheep had ≥4-fold increases in Stx1 antibody titers. None of the sheep had antibody to Stx2 after challenge. Preexisting antibody to the O157 lipopolysaccharide was detected in 1 of 16 sheep. Titers of antibody to O157 increased ≥2-fold at 2 months p.i. in 6 of 16 sheep tested.
None of the sheep were clinically ill during the study, and no free fecal toxin was detected during the initial period p.i. in the 14 sheep tested (magnitude of fecal STEC shedding, nondetectable to 105 CFU/g). Whenever water buckets were culture positive for one of the cocktail strains (14 of 62), at least one of the sheep in the room was shedding that strain in its feces. The cocktail strains were not recovered from the four uninoculated control sheep.
DISCUSSION
Regarding our hypothesis that STEC strains are better adapted than other pathotypes of E. coli for colonization and persistence in sheep, we found no consistent differences between STEC strains and the other strains in the frequency or intensity of colonization during the initial period, nor were we able to demonstrate that STEC strains were transmitted between sheep more readily than strains representing other pathotypes. However, the STEC strains tended to persist longer than did the other pathotypes. This tendency for longer persistence of STEC was evident at 2 weeks but more apparent at 2 months p.i. Furthermore, greater persistence could be demonstrated when STEC strains were inoculated at a dose 1,000-fold lower than the doses of two of the other three strains. The inoculum doses of STEC used in this study were varied from quite high (1010 CFU) down to a dosage that might be expected to occur naturally (104 to 105 CFU). The highest doses were used to compare colonization potentials among strains rather than to reflect precisely what is thought to occur in nature. Previous studies with cattle naturally infected with E. coli O157:H7 indicate that some animals shed as much as 105 CFU/g of feces (53, 59).
At necropsy (2 months p.i.) both STEC 86-24 and STEC 3081 were recovered only from the rectal feces and not from any other tissue or intestinal contents. It is not known where STEC strains colonize the alimentary tracts of mature ruminants, although this study and others (11, 14) suggest that the lower intestinal tract may be a likely site. Brown et al., however, recovered E. coli O157:H7 primarily from the rumina of calves necropsied 2 to 4 weeks p.i. (4). There are differences in the magnitude and duration of colonization by E. coli O157:H7 between calves and mature cattle (11). Perhaps the site(s) colonized also differs with age. We assume that the STEC persisted in the sheep. However, there is no evidence in this study to rule out the possibility that the STEC strains were continuously reintroduced from the environment by ingestion.
The 50% infectious dose of in vitro-grown STEC 86-24 and 3081 inoculated as a cocktail was approximately 105 CFU (Table 2). However, data from the transmission study indicated that an animal may be infectious when it is shedding considerably less than 105 CFU/g (Table 3). Sheep shedding as little as 102 to 103 CFU/g of STEC transmitted these strains to some naïve sheep. ETEC 2041 and EPEC 2348/69 shed at comparable levels also resulted in the transmission of those strains to some naïve sheep. In contrast, ETEC 637 was transmitted only when an animal was shedding at least 105 CFU/g. In any case, we do not know the route of transmission to the naïve sheep or the actual dose of a particular strain that the naïve sheep received. Penmates were in contact with one another throughout the study and shared both water and feed sources. Water troughs have been proposed as an environmental reservoir and a source of E. coli O157:H7 contamination for cattle (16, 26, 53). Both STEC strains and ETEC 2041 were transmitted to a greater percentage of naïve sheep than were EPEC E2348/69 or ETEC 637. Transmission of E. coli O157:H7 from experimentally inoculated donors to naïve sheep has been documented previously (34, 37). In contrast to the findings of the prior study (37), our data indicated that the transmitted strains could be recovered for up to 2 weeks from some sheep. These differences could be due to the STEC strains that were used or to differences between sheep.
The magnitude and duration of colonization of STEC 3081 in sheep given 1010 CFU were similar to those obtained with cattle, where persistence of this strain was documented for 7 weeks in 2 of 9 mature cattle given the same dose (11). When the inoculum dose was lowered to 107 CFU, strain 3081 was recovered from 2 of 5 adult cattle 1 to 2 days p.i. and was not recovered after that. This is in contrast to our data with sheep, wherein 6 of 12 sheep given 107 CFU of STEC 3081 were shedding at 2 weeks p.i. and 2 of 12 remained culture positive for at least 2 months. Sheep were used as a ruminant model because they are less expensive and easier to handle than cattle. In addition, sheep are naturally infected with E. coli O157:H7 and other STEC strains (7, 18, 29, 36).
Some of the differences between strains in the magnitude and duration of colonization may be due to host specificity, since neither EPEC E2348/69, ETEC 637, nor ETEC 2041 was originally isolated from a ruminant. However, pathogenic E. coli strains with virulence traits similar to those of EPEC E2348/69 and ETEC 637 (eae or F5 and STa) infect and cause disease in both lambs and calves (40, 42). The persistent colonization of ETEC 2041 in mature ruminants was unexpected. In contrast to F5+ ETEC strains (such as ETEC 637), F4+ ETEC strains are generally regarded as pig pathogens and are not commonly isolated from other species (42). The magnitude and duration of colonization by ETEC 2041 in sheep were significantly more dose dependent than those for the STEC strains (Fig. 1 and 2). Our data also suggest that there may have been exclusionary or suppressive competition for some niche between the STEC strains and ETEC 2041. When ETEC 2041 and the STEC strains were given at 1010 CFU, all three strains persisted for 2 months in some sheep (Fig. 1). When all three strains were given at 107 CFU, only the STEC strains persisted (Fig. 2). When ETEC 2041 was given at a 1,000-fold-higher dose than the STEC strains, neither the STEC strains nor ETEC 2041 persisted past 2 weeks p.i. (Fig. 3). As part of another study, we inoculated several sheep with 1010 CFU of STEC 86-24 only (8). Fecal shedding during the initial period and 2 weeks p.i. was somewhat greater (1 to 2 log10 CFU/g) than when the strain was given as part of a cocktail. At 2 months p.i., the magnitude of shedding and the percentage of culture-positive sheep that were inoculated with STEC 86-24 only were similar to those found when the strain was inoculated as part of a cocktail. This also suggests that there was competition between the strains in the cocktail and that this competition may have influenced the magnitude of STEC shedding during the initial and 2-week periods of the study reported here. Regardless of the competition between the strains in the cocktail, all of the inoculum strains were subjected to competition with the endogenous flora. Our data suggest that the STEC strains were able to establish a population and perhaps exploit a niche more consistently than the other pathotypes of E. coli.
If the E. coli O157:H7 strains selected for this study are representative of STEC in general, the tendency for STEC to persist in some animals may explain how the ruminant reservoir is maintained. Only a few persistent shedders in a herd would be enough to transmit STEC to naïve animals. Our data suggest that the infectious dose of in vivo-grown STEC may be lower than the 105 CFU reported here for in vitro-grown STEC. Our initial focus was on transmission by sheep shedding low numbers (at or below the 50% infectious dose for in vitro-grown organisms) of STEC bacteria, because such transmission is relevant to natural transmission even though such an experimental design does not allow determination of the infectious dose for the in vivo-grown organisms. However, studies to determine the infectious dose of in vivo-grown STEC are now in progress. Studies with additional strains of E. coli will be required to determine if an enhanced ability to persist is a general attribute of STEC or occurred merely by chance in the two strains selected for this study. It would also be useful to determine if the persistence attribute in the STEC strains studied here would be apparent in nonruminant animals and if any of the known putative virulence factors of STEC strains (intimin, Shiga toxin, and enterohemolysin) contribute to their persistence in ruminants.
ACKNOWLEDGMENTS
We thank Ilze Matise, Pedro Navarro, Dawn Wiarda, Joe Spoo, and Nathan Eslick for assistance, Daniel Nordman for statistical analysis, and Scott Hurd for helpful discussions.
This work was supported in part by the Frank K. Ramsey endowment and NIH grant AI-41328.
REFERENCES
- 1.Besser T E, Hancock D D, Pritchett L C, McRae E M, Rice D H, Tarr P I. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J Infect Dis. 1997;175:726–729. doi: 10.1093/infdis/175.3.726. [DOI] [PubMed] [Google Scholar]
- 2.Beutin L, Geier D, Steinruck H, Zimmermann S, Scheutz F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol. 1993;31:2483–2488. doi: 10.1128/jcm.31.9.2483-2488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielaszewska M, Janda J, Blahova K, Minarikova H, Jikova E, Karmali M A, Laubova J, Sikulova J, Preston M A, Khakhria R, Karch H, Klazarova H, Nyc O. Human Escherichia coli O157:H7 infection associated with the consumption of unpasteurized goat's milk. Epidemiol Infect. 1997;119:299–305. doi: 10.1017/s0950268897008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown C A, Harmon B G, Zhao T, Doyle M P. Experimental Escherichia coli O157:H7 carriage in calves. Appl Environ Microbiol. 1997;63:27–32. doi: 10.1128/aem.63.1.27-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush E. U.S. swine herd appears free of Escherichia coli O157:H7. Food Safety Digest. 1997;1997:4. [Google Scholar]
- 6.Chalmers R M, Salmon R L, Willshaw G A, Cheasty T, Looker N, Davies I, Wray C. Vero-cytotoxin-producing Escherichia coli O157 in a farmer handling horses. Lancet. 1997;349:1816. doi: 10.1016/s0140-6736(05)61697-2. [DOI] [PubMed] [Google Scholar]
- 7.Chapman P A, Siddons C A, Cerdan Malo A T, Harkin M A. A 1-year study of Escherichia coli O157 in cattle, sheep, pigs and poultry. Epidemiol Infect. 1997;119:245–250. doi: 10.1017/s0950268897007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornick N A, Booher S L, Casey T A, Moon H W. Are ruminants a biological or accidental reservoir for VTEC O157? In: Duffy G, Garvey P, Coia J, Wasteson Y, McDowell D A, editors. Verocytotoxigenic E. coli in Europe: pathogenicity and virulence. Vol. 3. Dublin, Ireland: Teagasc; 2000. pp. 43–50. [Google Scholar]
- 9.Cornick N A, Matise I, Samuel J E, Bosworth B T, Moon H W. Shiga toxin-producing Escherichia coli infection: temporal and quantitative relationships among colonization, toxin production and systemic disease. J Infect Dis. 2000;181:242–251. doi: 10.1086/315172. [DOI] [PubMed] [Google Scholar]
- 10.Cray W C J, Casey T A, Bosworth B T, Rasmussen M A. Effect of dietary stress on fecal shedding of Escherichia coli O157:H7 in calves. Appl Environ Microbiol. 1998;64:1975–1979. doi: 10.1128/aem.64.5.1975-1979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cray W C J, Moon H W. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:1586–1590. doi: 10.1128/aem.61.4.1586-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crichton P B, Old D C. Biotyping of Escherichia coli. J Med Microbiol. 1979;12:473–486. doi: 10.1099/00222615-12-4-473. [DOI] [PubMed] [Google Scholar]
- 13.Dean-Nystrom E A, Bosworth B T, Cray W C J, Moon H W. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect Immun. 1997;65:1842–1848. doi: 10.1128/iai.65.5.1842-1848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean-Nystrom E A, Bosworth B T, Moon H W, O'Brien A D. Bovine infection with Shiga toxin-producing Escherichia coli. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: American Society for Microbiology; 1998. pp. 261–267. [Google Scholar]
- 15.Dean-Nystrom E A, Bosworth B T, Moon H W, O'Brien A D. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect Immun. 1998;66:4560–4563. doi: 10.1128/iai.66.9.4560-4563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faith N G, Shere J A, Brosch R, Arnold K W, Ansay S E, Lee M S, Luchansky J B, Kaspar C W. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl Environ Microbiol. 1996;62:1519–1525. doi: 10.1128/aem.62.5.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farmer J J, Davis B R. H7 antiserum-sorbitol fermentation medium: a single-tube screening medium for detecting Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1985;22:620–625. doi: 10.1128/jcm.22.4.620-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fegan N, Desmarchelier P. Shiga toxin-producing Escherichia coli in sheep and pre-slaughter lambs in eastern Australia. Lett Appl Microbiol. 1999;28:335–339. doi: 10.1046/j.1365-2672.1999.00556.x. [DOI] [PubMed] [Google Scholar]
- 19.Franck S M, Bosworth B T, Moon H W. Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. J Clin Microbiol. 1998;36:1795–1797. doi: 10.1128/jcm.36.6.1795-1797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garber L P, Wells S J, Hancock D D, Doyle M P, Tuttle J, Shere J A, Zhao T. Risk factors for fecal shedding of Escherichia coli O157:H7 in dairy calves. J Am Vet Med Assoc. 1995;207:46–49. [PubMed] [Google Scholar]
- 21.Gentry M K, Dalrymple J M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980;12:361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon V M, Whipp S C, Moon H W, O'Brien A D, Samuel J E. An enzymatic mutant of Shiga-like toxin II variant is a vaccine candidate for edema disease of swine. Infect Immun. 1992;60:485–490. doi: 10.1128/iai.60.2.485-490.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin P M, Ostroff S M, Tauxe R V, Greene K D, Wells J G, Lewis J H, Blake P A. Illnesses associated with Escherichia coli O157:H7 infections. Ann Intern Med. 1988;109:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 24.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic-uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 25.Hancock D D, Besser T E, Kinsel M L, Tarr P I, Rice D H, Paros M G. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington state. Epidemiol Infect. 1994;113:199–207. doi: 10.1017/s0950268800051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock D D, Besser T E, Rice D H, Ebel E D, Herriot D E, Carpenter L V. Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev Vet Med. 1998;35:11–19. doi: 10.1016/s0167-5877(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 27.Hancock D D, Besser T E, Rice D H, Herriot D E, Tarr P I. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol Infect. 1997;118:193–195. doi: 10.1017/s0950268896007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harmon B G, Brown C A, Tkalcic S, Mueller P O E, Parks A, Jain A V, Zhao T, Doyle M P. Fecal shedding and rumen growth of Escherichia coli O157:H7 in fasted calves. J Food Prot. 1999;62:574–579. doi: 10.4315/0362-028x-62.6.574. [DOI] [PubMed] [Google Scholar]
- 29.Heuvelink A E, van den Biggelaar F L A M, de Boer E, Herbes R G, Melchers W J G, Huis in't Veld J H, Monnens L A H. Isolation and characterization of verocytotoxin-producing Escherichia coli O157 strains from Dutch cattle and sheep. J Clin Microbiol. 1998;36:878–882. doi: 10.1128/jcm.36.4.878-882.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hovde C J, Austin P R, Cloud K A, Williams C J, Hunt C W. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl Environ Microbiol. 1999;65:3233–3235. doi: 10.1128/aem.65.7.3233-3235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R P, Cray W C J, Johnson S T. Serum antibody responses of cattle following experimental infection with Escherichia coli O157:H7. Infect Immun. 1996;64:1879–1883. doi: 10.1128/iai.64.5.1879-1883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keene W E, Sazie E, Kok J, Rice D H, Hancock D D, Balan V K, Zhao T, Doyle M P. An outbreak of Escherichia coli O157:H7 infections traced to jerky made from deer meat. JAMA. 1997;277:1229–1231. doi: 10.1001/jama.1997.03540390059036. [DOI] [PubMed] [Google Scholar]
- 34.Kudva I T, Hatfield P G, Hovde C. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl Environ Microbiol. 1995;61:1363–1370. doi: 10.1128/aem.61.4.1363-1370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudva I T, Hatfield P G, Hovde C J. Characterization of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli serotypes isolated from sheep. J Clin Microbiol. 1997;35:892–899. doi: 10.1128/jcm.35.4.892-899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudva I T, Hatfield P G, Hovde C J. Escherichia coli O157:H7 in microbial flora of sheep. J Clin Microbiol. 1996;34:431–433. doi: 10.1128/jcm.34.2.431-433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudva I T, Hunt C W, Williams C J, Nance U M, Hovde C J. Evaluation of dietary influences on Escherichia coli O157:H7 shedding by sheep. Appl Environ Microbiol. 1997;63:3878–3886. doi: 10.1128/aem.63.10.3878-3886.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laegreid W, Hoffman M, Keen J, Elder R, Kwang J. Development of a blocking enzyme-linked immunosorbent assay for detection of serum antibodies to O157 antigen of Escherichia coli. Clin Diagn Lab Immunol. 1998;5:242–246. doi: 10.1128/cdli.5.2.242-246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O'Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 40.Mainil J G, Jacquemin E R, Kaeckenbeeck A E, Pohl P H. Association between the effacing (eae) gene and the Shiga-like toxin-encoding genes in Escherichia coli isolates from cattle. Am J Vet Res. 1993;54:1064–1068. [PubMed] [Google Scholar]
- 41.Mechie S C, Chapman P A, Siddons C A. A fifteen month study of Escherichia coli O157:H7 in a dairy herd. Epidemiol Infect. 1997;118:17–25. doi: 10.1017/s0950268896007194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moon H W. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr Top Microbiol Immunol. 1990;151:147–165. doi: 10.1007/978-3-642-74703-8_8. [DOI] [PubMed] [Google Scholar]
- 43.Moon H W, Whipp S C. Development of resistance with age by swine intestine to effects of enteropathogenic Escherichia coli. J Infect Dis. 1970;122:220–223. doi: 10.1093/infdis/122.3.220. [DOI] [PubMed] [Google Scholar]
- 44.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakazawa M, Akiba M. Swine as a potential reservoir of Shiga toxin-producing Escherichia coli O157:H7 in Japan. Emerg Infect Dis. 1999;5:833–834. doi: 10.3201/eid0506.990618. . [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahn K, Renwick S A, Johnson R P, Wilson J B, Clarke R C, Alves D, McEwen S, Lior H, Spika J. Persistence of Escherichia coli O157:H7 in dairy cattle and the dairy farm environment. Epidemiol Infect. 1997;119:251–259. doi: 10.1017/s0950268897007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice D H, Hancock D D, Besser T E. Verotoxigenic E. coli O157:H7 colonization of wild deer and range cattle. Vet Rec. 1995;137:524. doi: 10.1136/vr.137.20.524. [DOI] [PubMed] [Google Scholar]
- 48.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 49.Rios M, Prado V, Trucksis M, Arellano C, Borie C, Alexandre M, Fica A, Levine M M. Clonal diversity of Chilean isolates of enterohemorrhagic Escherichia coli from patients with hemolytic-uremic syndrome, asymptomatic subjects, animal reservoirs, and food products. J Clin Microbiol. 1999;37:778–781. doi: 10.1128/jcm.37.3.778-781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubini S, Cardeti G, Amiti S, Manna G, Onorati R. E. coli O157 in raw sheep milk. Int VTEC/STEC News. 1999;12:2. [PubMed] [Google Scholar]
- 51.Sargeant J M, Hafer D J, Gillespie J R, Oberst R D, Flood S J A. Prevalence of Escherichia coli O157:H7 in white-tailed deer sharing rangeland with cattle. J Am Vet Med Assoc. 1999;215:792–794. [PubMed] [Google Scholar]
- 52.Sarmiento J I, Casey T A, Moon H W. Postweaning diarrhea in swine: experimental model of enterotoxigenic Escherichia coli infection. Am J Vet Res. 1988;49:1154–1159. [PubMed] [Google Scholar]
- 53.Shere J A, Bartlett K J, Kaspar C W. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl Environ Microbiol. 1998;64:1390–1399. doi: 10.1128/aem.64.4.1390-1399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith H W, Linggood M A. Further observations on Escherichia coli enterotoxins with particular regard to those produced by atypical piglet strains and by calf and lamb strains: the transmissible nature of these enterotoxins and of a K antigen possessed by calf and lamb strains. J Med Microbiol. 1972;5:243–250. doi: 10.1099/00222615-5-2-243. [DOI] [PubMed] [Google Scholar]
- 55.Snedecor G W, Cochran W G. Statistical methods. 8th ed. Ames: Iowa State University Press; 1989. [Google Scholar]
- 56.Spika J S, Parsons J E, Nordenberg D, Wells J G, Gunn R A, Blake P A. Hemolytic-uremic syndrome and diarrhea associated with Escherichia coli O157:H7 in a day care center. J Pediatr. 1986;109:287–291. doi: 10.1016/s0022-3476(86)80386-9. [DOI] [PubMed] [Google Scholar]
- 57.Wallace J S, Cheasty T, Jones K. Isolation of Vero cytotoxin-producing Escherichia coli O157 from wild birds. J Appl Microbiol. 1997;82:399–404. doi: 10.1046/j.1365-2672.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- 58.Wells J G, Shipman L D, Greene K D, Sowers E G, Green J H, Cameron D N, Downes F P, Martin M L, Griffin P M, Ostroff S M, Potter M E, Tauxe R V, Wachsmuth I K. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like toxin-producing E. coli from dairy cattle. J Clin Microbiol. 1991;29:985–989. doi: 10.1128/jcm.29.5.985-989.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao T, Doyle M P, Shere J, Garber L. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl Environ Microbiol. 1995;61:1290–1293. doi: 10.1128/aem.61.4.1290-1293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]