Abstract
Ghrelin is a peptide released by the endocrine cells of the stomach and the neurons in the arcuate nucleus of the hypothalamus. It modulates both peripheral and central functions. Although ghrelin has emerged as a potent stimulator of growth hormone release and as an orexigenic neuropeptide, the wealth of literature suggests its involvement in the pathophysiology of affective disorders including depression. Ghrelin exhibits a dual role through the advancement and reduction of depressive behavior with nervousness in the experimental animals. It modulates depression-related signals by forming neuronal networks with various neuropeptides and classical neurotransmitter systems. The present review emphasizes the integration and signaling of ghrelin with other neuromodulatory systems concerning depressive disorders. The role of ghrelin in the regulation of neurosynaptic transmission and depressive illnesses implies that the ghrelin system modulation can yield promising antidepressive therapies.
Keywords: Ghrelin, GHSR, Depression, Psychotic disorders, Neurotransmitter
Graphical abstract
Highlights
-
•
Ghrelin is the orexigenic type of neuropeptide.
-
•
It binds with the growth hormone secretagogue receptor (GHSR).
-
•
GHSR is ubiquitously present in the various brain regions.
-
•
Ghrelin is involved in the regulation of depression-related behavior.
-
•
The review focuses on the neurotransmission and signaling of ghrelin in neuropsychiatric and depressive disorders.
1. Introduction
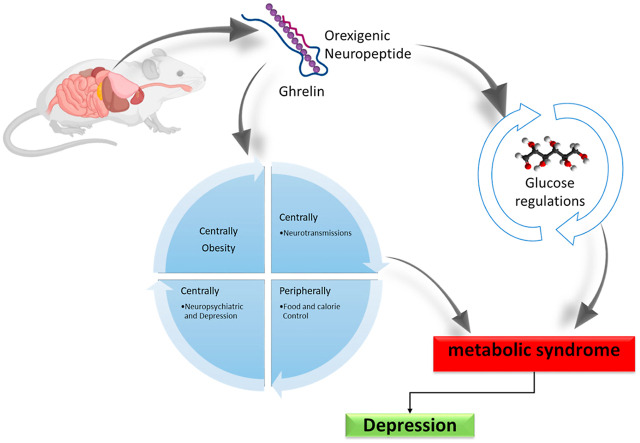
Ghrelin is an orexigenic hormone, isolated from the rat stomach (Kojima et al., 1999). It is synthesized in the body and binds with the growth hormone secretagogue receptor (GHSR). It regulates the somatotropic secretion of hormones from the pituitary gland (Andrews, 2019). GHSR is mainly found in the arcuate nucleus of the hypothalamus (ARC). Ghrelin stimulates orexigenic neuropeptide Y (NPY) in the ARC via GHSR (Delhanty and van der Lely, 2011). In fact, ghrelin enhances food intake, and thus promotes weight gain by the activation of NPY neurons in the ARC, leading to its popular tag “The Hunger Hormone” (Andrews, 2019). Majorly, ghrelin is released by the stomach and many other organs including bowels, kidneys, lungs, thyroid, hypothalamus, and pituitary gland (Kojima et al., 2005). However, it is also found in trace amounts in the various brain regions (Cabral et al., 2017). Ghrelin is prepared and synthesized endogenously from preproghrelin, which also yields obstraitin in minute quantities (Zhang et al., 2008). Obstratin produces physiological variations in the body, like the decline in pancreatic secretions and enhancement of memory and cognition (Gargantini et al., 2013). The discovery of ghrelin and its receptors is a milestone achievement that emphasizes ghrelin-related endocrine mechanisms. Ghrelin governs physiological functions such as the predominant hypothalamus nucleus, which regulates hunger, and the ARC (Marino et al., 2011). Ghrelin and its components are synthesized by many adjacent neurons in the third ventricle. These neurons of the ventricles form ghrelin to act within the brain circuitry in different regions (Cowley et al., 2003). Several studies have established the involvement of ghrelin in the regulation and metabolism of glucose (Qian et al., 2019). Few studies demonstrated that ablation of ghrelin does not affect the change in body weight as well as obesity and adiposity, though normalized glucose metabolism and homeostasis (Castaneda et al., 2010).
The significance of ghrelin in the regulation and metabolism implies that it may be useful for regulating normal blood glucose levels, and might be a therapeutic target for diabetes (Shankar et al., 2020). The role of ghrelin was studied in insulin resistance and regulation of glucose which revealed that ghrelin was effective in regulating glucose levels (Chabot et al., 2014). Similarly, earlier research also stated that exogenous ghrelin administration increases plasma insulin levels in diabetic rats (Elabadlah et al., 2020). Metabolic syndromes and neurological disorders are leading threats to public health and represent a global socioeconomic health burden. Available literature indicates that obesity, food restriction, and calorie control lead to depressive behavior and changes in the thought process. Ghrelin levels can minimize the stress-associated depression-like behaviors but may cause derangement in metabolic profile. Ghrelin also participates in the regulation of circadian rhythm. The intravenous injection of ghrelin for treatment of non-rapid eye movement (NREM) sleep, showed that it fosters sleep, although the effect varies for time and gender (Watson et al., 2012). Several human and animal studies postulated that intravenous administration of ghrelin increases the cortisol and aldosterone concentrations (Leggio et al., 2014). Night-time secretions of cortisol, growth hormone (GH), leutinizing hormone (LH), follicle-stimulating hormone (FSH), and hypothalamic-pituitary-thyroid (HPT) hormones are persuaded by the ghrelin (Stone et al., 2020). Additionally, ghrelin shows a major action in cognition including learning and memory, which implies its involvement in the etiopathogenesis of central nervous system (CNS) disorders, especially manic disorder (Turan et al., 2014). Several study results from longitudinal aging revealed that a three-year follow-up study on old age patients showed positive enforcement of ghrelin in chronically ill depressive male patients (van Andel et al., 2022). Neurobiological mechanisms between depression, obesity, and anxiety have not been derived yet. However, the known increase in the comorbidity of anxiety and obesity may alter the feeding behavior, and subsequent depression (Shintani et al., 2001). An association between psychological disorders and increased visceral fat accumulation has been reported. Ghrelin has been a subject of many studies that focus on the pathophysiological mechanisms of appetite (Cummings et al., 2001; Patterson et al., 2011; Tschöp et al., 2001). Earlier research showed that ghrelin stimulates the hypothalamic-pituitary-adrenal (HPA) axis. Additionally, hormone production and gene expression are both increased by ghrelin in the hypothalamus (Wittekind and Kluge, 2015; Ziko et al., 2018). A ghrelin-stimulated release of adrenocorticotropic hormone (ACTH) and cortisol has also been documented in animals (Kluge et al., 2013; Wittekind and Kluge, 2015; Wren et al., 2000). Additionally, it suppressed the release of hormones responsible for the hypothalamic-pituitary-gonadotrophic (HPG) axis, like LH (Kluge et al., 2007) and FSH (Kluge et al., 2009; Steiger et al., 2011). Ghrelin impacted the HPT axis in humans, producing an increase in thyroxine levels (Kluge et al., 2012).
The role of ghrelin in appetite regulation, cognition, emotions, and other psychological conditions has received attention for therapeutic manipulation using ghrelin in several pathologies, including obesity and depression (Schellekens et al., 2012). Ghrelin is also implicated in sleep and memory (Carlini et al., 2002). The characteristic episodes of depression are repeatedly conveyed by sleep disturbances, hunger, and memory alterations. These can be related to the involvement of ghrelin. Pharmacotherapy of depression has been evolving over the past few decades. The treatment approaches have been changing following our understanding of the disease pathophysiology. These treatment approaches also bring forth numerous limitations. Hence, following the recognition and establishment of ketamine as an antidepressant, there is a renewed interest in exploring monoaminergic pathways for the development of newer antidepressants (Morin et al., 2018). In the present review, we focused on the role of ghrelin in the regulation of neurosynaptic transmission in depressive disorders.
2. Methods
Several electronic search engines were used to conduct the literature review, including PubMed, Scopus, Science Direct, Web of Science, and Google Scholar. In the present review, all the authors separately conducted their literature searches. The eligibility criteria for inclusion of the articles in this review were clinical or pre-clinical findings reported in the English language.
3. Physiological roles of ghrelin
3.1. Ghrelin and depression
Although ghrelin is widely explored as a gut-derived appetite-stimulating hormone. It promotes food intake by stimulating neuronal activity of orexigenic NPY and agouti-related protein in the ARC (Chen et al., 2017). However, it is worth noting that ghrelin receptors are ubiquitously present in the CNS including in regions like the amygdala, hippocampus, nucleus accumbens, and ventral tegmental area (VTA) (Ferrini et al., 2009), which exhibit an essential role-play in the regulation of depression-related behaviors (Bali and Jaggi, 2015; Desai et al., 2014). Paradoxically, central ghrelin injection alleviates depression-like behavior triggered by prolonged unpredictable moderate stress (Huang et al., 2017). Besides the hypothalamus, ghrelin also regulates synaptic plasticity in neuronal circuits in the VTA and hippocampus, which can be correlated with its antidepressant effect (Serrenho et al., 2019). Viewed collectively, the available information suggests that in addition to orexigenic activity, ghrelin also produces an antidepressant effect by acting centrally.
Ghrelin is involved in many neurotransmission and signaling mechanisms in the brain. Several neurotransmitter systems are involved in the regulation of depressive disorders. The dopaminergic function of the brain is important in the control of mood and behavior related to the brain's reward system. According to previously reported evidence, decreased levels of dopamine may elicit certain symptoms linked with depression (Belujon and Grace, 2017). Furthermore, earlier research indicated that ghrelin was prompted neuroprotection via the GHSR and promotes neuronal circuit activity related to learning and memory, as well as reward-seeking behavior. Ghrelin binds to sunstantia nigra pars compacta (SNpc) cells and electrically stimulates SNpc dopamine neurons, increasing tyrosine hydroxylase mRNA and dopamine levels in the dorsal striatum (Andrews et al., 2009). The research study also showed that in lipopolysaccharide-induced inflammation, ghrelin exhibits substantial inhibitory effects on proinflammatory cytokines such as interleukin 1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) (Ereş et al., 2019). Central administration of ghrelin increases dopamine release in the nucleus accumbens shell via activation of ghrelin receptors in the VTA, which may be causally linked with its antidepressant property. However, systemic treatment with ghrelin receptor antagonist JMV2959 prevented the ghrelin triggered dopamine release in the nucleus accumbens shell (Edvardsson et al., 2021). However, the particular mechanism for the involvement of ghrelin in manic disorders is not yet expanded or known, and diverse assumptions have been explored. The sedentary lifestyle is correlated with the problems of synthesis and metabolism of lipoproteins and different types of chylomicrons. In the depressed casualties, these changes were more pronounced and it is supported by the decreased alliance between the lipoprotein expressions and appearance. The disturbances in mood and depression are significantly altered by changes in lifestyle-related factors like smoking, and alcoholism (Marazziti et al., 2014; van Reedt Dortland et al., 2010). Ghrelin shares normal pathophysiologic systems and is also involved in the regulation of the hunger pattern.
3.2. Role of ghrelin
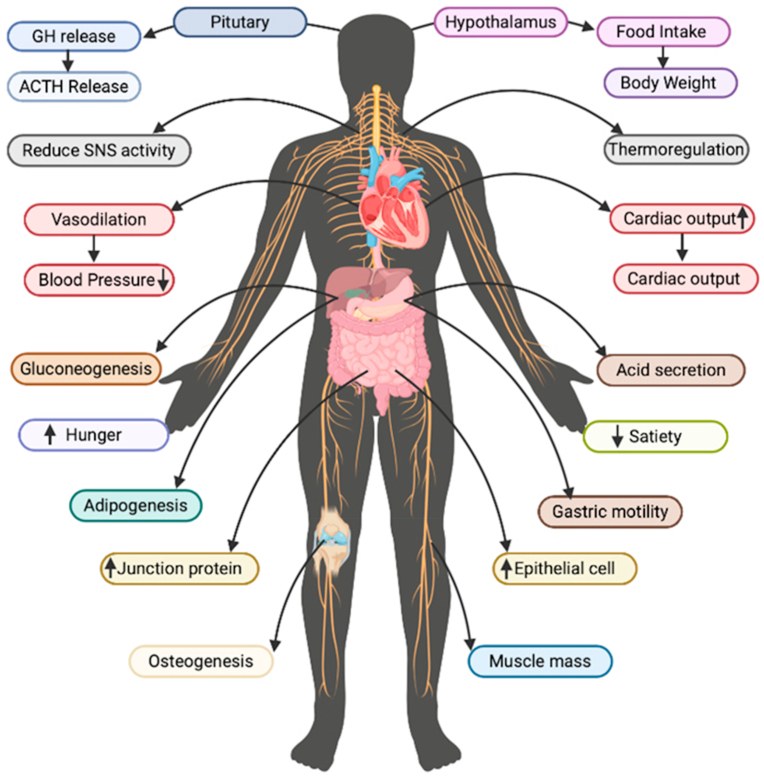
Ghrelin plays many vital roles and regulates different functions. It is a quick-progressing hormone that is assumed to be involved in yearning and hunger. Ghrelin is secreted in early fetal development and promotes lung growth (Steculorum and Bouret, 2011). This discovery is important to a method known as “neurotrophic,” which refers to the memory's ability to learn and adapt to new situations. Ghrelin, according to research, enters the hippocampus via the circulation and controls nerve-cell connections to improve learning and memory. Ghrelin levels are greater when the stomach is empty, making learning easier (Chen et al., 2009). Ghrelin has appeared to assume a role in the prevention of unhappiness and nervousness. Mice inadequate in ghrelin have additionally appeared to display social shirking (Wittekind and Kluge, 2015). The different roles and functions of ghrelin are depicted in Fig. 1.
Fig. 1.
Biological functions of ghrelin on different body systems.
Ghrelin has endocrine, paracrine, or autocrine characteristics. Previous research also shows that a low level of ghrelin induces sleep with a significant improvement in sleep time (García-García et al., 2014). Ghrelin is also known to stimulate several hormones from the pituitary gland (Takaya et al., 2000). If the parallel nerve center is expelled (as found in rodents considers), taking care of it turns out to be less successive, prompting serious weight reduction and passing. On the off chance that the ventromedial nerve center is evacuated, taking care of expanding prompting weight gain and serious heftiness (Tschöp et al., 2001). Ghrelin and its receptors are likewise found in the heart and the aorta (Mao et al., 2014). It, also, appeared to repress insulin emission in certain examinations (Wierup et al., 2004).
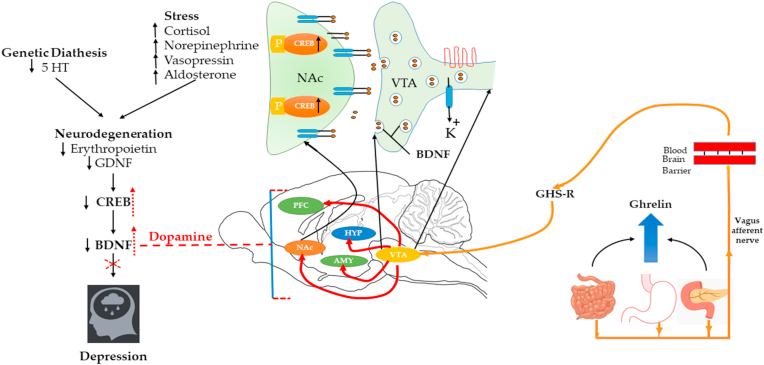
Ghrelin is involved in the molecular mechanisms in the brain and also mediates the various functions in the nervous system. In a depressed state, neurogenesis reduces the level of BDNF while increasing the concentration of cortisol above the normal level. The level of CREB is also decreased, followed by changes in behavior and an increased depressive state (Oh et al., 2006; Wang and Mao, 2019). Ghrelin stimulates GHS-R in vagal afferent nerve fibers in the stomach, resulting in the VTA and projected dopaminergic (mesolimbic) pathways, which in turn to, activation of the dopaminergic region and an increase in the BDNF level, ultimately CREB level is also increased and relieves the depressive symptoms and mood (Abizaid et al., 2006; Jerlhag et al., 2007; Skibicka et al., 2012). Several animal studies also revealed that ghrelin improves cognitive function via activation of the cAMP- CREB signaling pathway (Li et al., 2021). Furthermore, a recent line of investigation suggested that ghrelin is involved in neuronal morphogenesis via modulation of CREB levels in the damaged nerve by neurite outgrowth process formation (Stoyanova and Lutz, 2021) (Fig. 2). Recent studies have indicated that ghrelin administration causes dendritic spine remodeling in the hippocampus, and increases the production of certain BDNF-mRNA species (Perea Vega et al., 2021). Increased BDNF in dopamine-dependent VTA to dopamine-sensitive nucleus accumbens (NAc) leads to the anti-depressant activity with the help of transcription factor CREB by phosphorylation (Pittenger and Duman, 2008; Ruhé et al., 2007). Several preliminary investigations have shown that patients with functional dyspepsia have increased expression of GDNF (Choi et al., 2017). Moreover, a few studies have also postulated that increased expression of GDNF leads to activation of Pax 6, which leads to inhibiting the expression of ghrelin and somatotrophin, and glucagon (Rozanska et al., 2020).
Fig. 2.
Ghrelin signling in depression.
Most of the studies have demonstrated the anti-depressive mechanisms of ghrelin. However, a few studies reported a negative impact as ghrelin itself produces depressive behavior and patterns in animals. Previously, it was reported that an increased level of ghrelin in animals could be because of calorie restriction. Chronic social defeat stress (CSDS), is when social stress induces depressive-like symptoms (Chuang et al., 2010). The GHSR-1 knockout mice demonstrated significantly increased depression-like behavior in response to CSDS (Walker, 2014). Delivery of a neuroprotective agent that increased neurogenesis and had significant antidepressant efficacy (Walker, 2014). A reduction in depression and related behaviors confirm the involvement of ghrelin with GHSR-1 activation (Serrenho et al., 2019).
Acute treatment of ghrelin in mice is shown to have anti-depressant properties (Carlini et al., 2002). The anti-depressant effect of ghrelin was shown to decrease gene expression and arginine vasopressin (AVP) levels in plasma, which suggests the antidepressant effect was partly via expression of AVP (Poretti et al., 2016). Ultimately, ghrelin appeared to increase noradrenergic (Carlini et al., 2002) transmissions similar to serotonergic transmission (Skibicka et al., 2012). Hence, adrenergic and serotonergic activation would be responsible for its anti-depressant effect (Jeon and Kim, 2016). On the other hand, two examinations suggest the depressogenic properties of ghrelin (Skibicka et al., 2012). Long-term administration of ghrelin for more than about a month in rodents was related to a reduction of serotonin activity in the raphe nuclei (Skibicka et al., 2012). Ghrelin anti-sense DNA injection in the lateral ventricle of rodents actuated stimulants like impacts (Kanehisa et al., 2006). It has been found that ghrelin is detected in the patients having major depressive disorder (MDD) (Sorri et al., 2018), elevated (Esel et al., 2005), and equivalent, individually (Kurt et al., 2007; Matsuo et al., 2012). Depressive symptoms in healthy people brought about by catecholamine exhaustion were related to decreased ghrelin plasma levels (Cabral et al., 2012; Homan et al., 2013). It is suggested that inhibiting the body's response to ghrelin signals might be one method to help weight control by decreasing nourishment and increasing vitality. However, the above study explored that interruption of ghrelin signaling, might increase anxiety and depression (Zigman and Elmquist, 2003).
Remarkably, variations in estrogen levels may have a role in depressive-like behavior in postpartum females with oscillations inside the brain's BDNF-related components. Protein regulators of inflammation (BDNF and cytokines) are known to drive several intracellular signaling pathways linked to neuropsychological illness. Research studies suggested that patients with cancer and/or autoimmune diseases might be more likely to experience depressive symptoms due to pro-inflammatory cytokines (Numakawa et al., 2014). The GDNF protein and mRNA expression in the serum and hippocampus of depressive people have been shown to be decreased according to the findings in the former study (Lin and Tseng, 2015), which points to the importance of GDNF in the development of depression. The ghrelin affects mood and restricted the food intake of laboratory mice. The modified mice showed essentially more prominent social shirking than their wild-type partners, demonstrating a fuel of depression-like symptoms. Calorie limitation and losing weight could have an antidepressant effect and could be strengthening this illness, In future examinations, it needs to figure out which territory in the brain ghrelin causes these antidepressant-like effects (Chuang and Zigman, 2010). The previously reported studies have shown a reduced level of ghrelin in the sadness (Gecici et al., 2005; Gueler et al., 2007). Many studies have shown that the extent of ghrelin can not be comparable in normal and depressive patients (Ozsoy et al., 2014). The MDD is a severe mental condition characterized by weight loss, trouble sleeping, and tiredness with diminished physical capacity (Mischoulon et al., 2011). MDD pathophysiology is linked to HPA axis malfunction, abnormalities in the immune system, and alterations in monoamine receptors (De Kloet et al., 2005). Recent research has found that various energy-regulated hormones, such as Nesfatin-1 and ghrelin, exhibit endocrine disruptions in MDD patients (Açıkel et al., 2021; Ari et al., 2011; Bloem et al., 2012; Karadeniz et al., 2020). Ghrelin and Nesfatin-1 are recognized for antagonistically altering hormones, and they are linked to the seriousness of the illness. Patients' expectations and the efficacy of medications may also be affected by nesfatin-1 (Açıkel et al., 2021) and ghrelin levels (Hosoda et al., 2000; Nonogaki et al., 2008; Yosten and Samson, 2009).
4. Ghrelin variants and isoforms
Many isoforms of ghrelin are available like octanoylated ghrelin and diacyl ghrelin (Ferré et al., 2019). The ghrelin gene locus has been complicated by the presence of antisense transcripts, called ghrelin-OS (ghrelin opposite strand), in addition to producing multiple sense transcripts (Seim et al., 2007). The functional importance of these transcripts is still to be determined. Exon 3-erased preproghrelin, when recommended by the label, is the product of entering exon 3 of the ghrelin transcript (Jeffery et al., 2005; Volante et al., 2009). This mutant generates a 91 amino acid pre-propeptide that, unlike the wild type and pre-Gln14-ghrelin, encrypts only ghrelin but does not comprise the obestatin-coding region. This induces a frameshift, but it would still enable the production of the matured ghrelin peptide as well as the development of a new C-terminal peptide (Yeh et al., 2005). Other forms of ghrelin include des-Gln14-ghrelin and ghrelin analogs (Hosoda et al., 2003; Jeffery et al., 2003, 2005). Pre pro-des Gln14-ghrelin emerges from a three-base pair, 5′ truncation of exon 2, delivering a 116 amino corrosive preproghrelin peptide and has comparable capacities to ghrelin (Bedendi et al., 2003; Hosoda et al., 2000; Jeffery et al., 2005). Recently, substitute splicing has led to many new variants of the preproghrelin sequence, including obestatin-only transcripts. These new variants have a better ratio of ghrelin to obestatin.
5. Ghrelin and signaling pathways
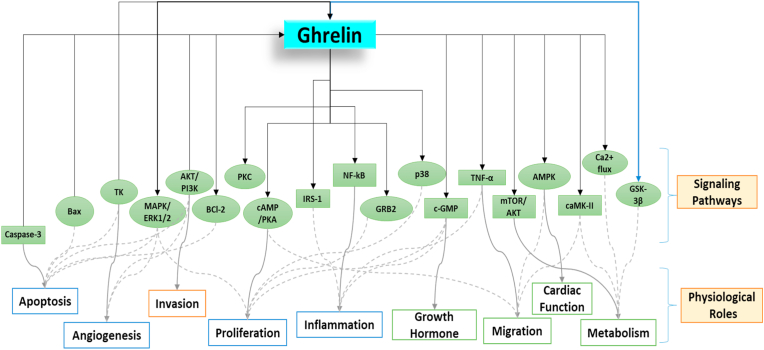
Ghrelin activates and increases calcium influx in Xenopus oocytes by GHSR (Howard et al., 1996). It seems to have a large variety of physiological influences and has been shown to activate motion via the different pathways as shown in Fig. 3. The vital molecular functions of ghrelin are through its ability to modulate the signal-regulated kinase 1/2 (ERK1/2) pathway, and mitogen-initiated protein kinase (MAPK) (Delhanty et al., 2006; Nanzer et al., 2004; Wang et al., 2012). Regulation of angiogenesis (Wang et al., 2012), inflammation (Jacob et al., 2010), inhibition of apoptosis (Favaro et al., 2012), and cell migration (Wang et al., 2012). Ghrelin signals through the Akt/phosphatidylinositol-3-kinase (PI3K) pathway to control expansion (Delhanty et al., 2006; Murata et al., 2002) angiogenesis (Wang et al., 2012), cell migration, and invasion (Duxbury et al., 2003; Wang et al., 2012) to repress apoptosis, and influence metabolism (Barazzoni et al., 2007). Fig. 3 indicates whether ghrelin activates (→) or restrains (←) signaling pathways, or whether both activate and restrain have been described (↔) and these are connected to the observed physiological impacts that are intervened by ghrelin signaling.
Fig. 3.
Involvement of ghrelin in different signaling pathways and its physiological roles in impacting the different situations.
5.1. Ghrelin, agouti-related peptide (AgRP) and NPY
Previous research data demonstrated the molecular action of ghrelin through its ability to modulate the GHSR-1a receptor. It is mostly found in the hypothalamic neurons of the brain that express NPY and AgRP (Narayanaswami, 2012). The implications of ghrelin ligands, on the other hand, demonstrate that ghrelin-induced hunger and gain in body weight do not appear to occur (Schmid et al., 2005). Furthermore, it is being investigated if anorectic actions of NPY-Y1 antagonists or opposing molecular pathways in genetically engineered mice contribute to this evident alteration (Schmid et al., 2005). Subsequent investigations demonstrate that ghrelin triggers stronger AgRP mRNA activity than NPY mRNA, suggesting the highest impact of ghrelins through receptors of the melanocortin system (Cone, 2005). According to a recent study, ghrelin's effects on energy balance are mediated by the CNS and regulated by the PPARG Control (Castañeda et al., 2010). Ghrelin activity is associated with both the CNS and the melanocortin mechanism (Dietrich et al., 2010).
5.2. Ghrelin and blood-brain barrier (BBB)
There is no definitive evidence to support ghrelin's presence in the brain's GHS-1a receptors. Many studies have also suggested that ghrelin could have connections to its receptor through circumventricular organs such as the median eminence. Some investigations also demonstrate that in the presence of a less protected BBB, ghrelin enters the ARC directly (Banks et al., 2008). In ARC, GHSR-1a expressing neurons (NPY/AGRP) distributed through the BBB, including ghrelin, will bind to specific receptors beyond the BBB and trigger hypothalamic activity (Wang et al., 2012). It has been stated that ghrelin can cross the BBB through facilitated diffusion or passive transport. Animal study data explored the ability of ghrelin to penetrate the BBB and found it strongly reliant on its species of origin and acylation state. When mice ghrelin was compared to acylated human ghrelin, the lateral one exhibited promise and well-directed BBB saturation. The difference in amino acid content in the peptide chain between human and mouse ghrelin may account for the higher penetration of human ghrelin (Banks et al., 2008).
When compared to acylated mouse ghrelin, de-acylated mouse ghrelin has a greater ability to reach the BBB. Ghrelin receptor antagonists have been shown to partially inhibit the orexigenic activity of routinely delivered ghrelin by injection directly into the ventral tegmental region of the brainstem, showing that bioactive ghrelin molecules penetrate beyond the BBB at least to some extent (Dickson and Mercer, 2016). When ghrelin is delivered directly to the rat's brainstem, it enhances food intake, suggesting that ghrelin has yet another functionally significant CNS target region. Because NPY is synthesized in the hippocampal region of the solitary tract (NTS), it can mimic the effect of ghrelin mediated through the brainstem. According to some research, vagotomy can remove ghrelin-induced orexigenic effects in mice and rats. Findings that contradict suggestions of a primarily direct hypothalamus or targeted mechanism based on CNS. Additional concern derives from careful investigations that have failed to identify any primary role of the nerve fibers, mainly the vagus nerve (Walker, 2014).
5.3. Ghrelin-independent of GHSR-1a
Ghrelin can also exert its effects through a pathway different from GHSR-1a (Zhang et al., 2013). BIM-28163, a clinical analog of ghrelin, inhibits the GHSR-1a receptor in the human midbrain and inhibits ghrelin-induced GH secretion. Although, even in the Dorsomedia hypothalamus, an area that assumes an important role in the regulation of calorie consumption, Either BIM-28163 or ghrelin act as inhibitors and trigger neural signaling, implying the existence of an elusive ghrelin receptor responsible for ghrelin regulation via weight gain. The role of GHSR-1a in regulating an acute feeding stimulus is not fully known. However, it is assumed to modulate ghrelin action on GH secretion. GHSR-knockout mice, on the other hand, do not respond to an intense feeding stimulus, according to research in mice lacking this gene (Fig. 2). The acute ghrelin effect may be controlled by GHSR-1a, but the long-term weight-increasing effect may be mediated by an unidentified ghrelin receptor. Subsequent investigations of continuous Ghrelin therapy in knockout mice will aid in the resolution of this issue (Chabot et al., 2014). Studies also explored that GHSR-1a-knockout mice showed significantly decreased ghrelin activity that included strategies for compensatory mechanisms for the loss of GHSR-1a (Kirchner et al., 2012). Recent research has also revealed that GHSR-1a deficiency protects mice from obesity caused by a high-fat diet for an extended period.
5.4. GHSR-1a intracellular signaling system
The regulation of GHSR-1a is also an important aspect of the control of the body's numerous activities. In the rat ARC, ghrelin increases calcium via the adenylate cyclase – protein kinase A (AC – PKA) pathway. Calcium is one of the most common signaling modes employed by GHS-1a receptors. In GHSR-1a cells, calcium has been found as a key biomarker for detecting ghrelin. Ghrelin tends to initiate signal transduction that leads to the development of food intake control. Ghrelin regulates the inner workings of cells via regulating GH and inflammatory cytokines (Camina, 2006). Recent research suggests that fatty acid production, AMPK activation, and ultimately oxygen radicals with endoplasmic reticular stress in the ARC and ventromedial hypothalamus may further increase ghrelin's orexigenic function. Ghrelin can also inhibit proinflammatory cytokines by activating calmodulin-dependent kinase (CaMKK) and endothelial nitric oxide synthase (eNOS). By boosting ghrelin levels and decreasing fatty acid synthase activity in the hypothalamus, ghrelin has been demonstrated to promote fasting food intake (Xu et al., 2008).
The hypothalamus and pituitary gland have the most GHSR-1a receptors, whereas the hippocampus, midbrain, and hindbrain have the least. GHSR-1b, a shorter version of the receptor arising from alternative gene splicing, exists but does not bind ghrelin. Additionally, GHSR-1a receptors can also be present in vagal and spinal visceral afferents, immunological cells, thyroid, pancreas, spleen, cardiac muscle, ovarian, and adrenal tissue, among other tissues (Yin et al., 2014). Human myocytes were shown to have growth hormone-releasing peptide (GHRP) binding sites. Such a receptor was accidently recognized as CD36, a glycoprotein IV membrane with a far lower affinity for native ghrelin than GHRPs. Several in-vitro investigations examined available facts for the next peripheral ghrelin receptor that affects obesity (Wiedmer et al., 2007). According to the findings, ghrelin receptors may have an important yet undiscovered role in paracrine pancreatic activities. It is also known to trigger 3T3-L1 pre-adipocyte development and to bind directly with a high affinity to non-GHSR-1a cells. Aside from that, mouse pancreatic islets may not express GHSR-1a, but human islets do, implying that acrylate ghrelin may play a less important role than de-acrylate ghrelin. The relationship between acylated and non-acylated kinds of ghrelin has also been shown, mostly in human thyroid and breast cancers. A large quantity of indirect information suggests the presence of functionally relevant non-GHSR-1a ghrelin receptor types (Delhanty et al., 2013). Long-term injection of ghrelin as an anorexigenic peptide has been shown in studies to promote and accelerate weight gain by increasing food consumption and decreasing energy expenses (Misra, 2013). It is known to have GHS-R agonist activity, which is controlled peripherally and directly in the brain, and it generates a feed reaction as well as a decrease in NPY levels (Wren et al., 2000).
The hyperphagic properties of ghrelin are generally linked to brain activity, with a special emphasis on the characterization of key processes related to ghrelin-driven excessive intake. The study was primarily concerned with locating the GHS-R within the feed-related circuitry of the hypothalamus region (Olszewski et al., 2008). This adaptive interchange of ghrelin and other neurotransmitters is essential for brain growth and function. The impact of this product on the consumption of foods with varying caloric density, flavor, essential micronutrients, and appropriateness, as well as the impact of the various aspects of the benefit from the ghrelin profile, represents the interaction of ghrelin as well as other neuroactive variations within the brain (Wren et al., 2000).
6. Central actions of ghrelin
6.1. Ghrelin and neurodegenerative diseases
Previous data postulated the neuroprotective role of ghrelin, soon after its discovery (Frago et al., 2011). However, a huge body of evidence has accumulated, which affirms this perception as well as unequivocally proposes a role of ghrelin in neurogenesis, neural versatility, learning, memory, and neurodegenerative diseases, most notably Alzheimer's disease (AD) (Bayliss and Andrews, 2013; Walker, 2014). Researchers discovered that ghrelin reduces the toxicity of amyloid beta (Aβ)-oligomers by lowering superoxide levels and modulating mitochondrial metabolism in brain cells (Wittekind and Kluge, 2015). Distantly administered Aβ was demonstrated to exacerbates cognitive impairment and neurodegeneration in the hippocampus in the AD animal model (Moon et al., 2009; Nakhate et al., 2018, 2020). Ghrelin receptors are found throughout the CNS, most notably in the hypothalamus, pituitary gland, hippocampus, VTA, and amygdala (Mortazaei et al., 2019; Spencer et al., 2015).
The discoveries of most of the studies are consistent, recommending the memory-improving and neurogenetic impact of ghrelin as determined underneath. Ghrelin has been proven to have cognitive effects, notably in the hippocampus, a region of the brain important for cognitive growth and learning. Ghrelin, for example, would have the choice of penetrating the blood-brain barrier and boosting dendritic spine neuronal assimilation and also long-term stimulatory effects in mice (Diano et al., 2006). Furthermore, recent research has demonstrated that ghrelin can induce hippocampus neurogenesis in both in-vitro and in-vivo tests (Walker, 2014; Zhao et al., 2007). The relevance of these observations is enhanced by the fact that ghrelin has consistently been shown to improve memory performance and spatial learning in animal models (Babri et al., 2013). Except for one study that found a memory-impairing impact of ghrelin (Zhao et al., 2014). Human trials are rare, and the results have been conflicting. Ghrelin serum concentrations have been demonstrated to be both positively connected with verbal learning (Bellar et al., 2013), and adversely correlated with verbal learning and other cognitive functions (Zubieta et al., 2001). Even the placebo-controlled analysis of the effect of ghrelin on the midnight development of motor development did not establish important comparisons between ghrelin and the placebo-controlled condition (Allas et al., 2018). More efficient and reliable investigations are expected to additionally explain the connection between serum ghrelin levels and memory execution in humans. Other later in vitro investigations depicted cell-defensive properties (Wittekind and Kluge, 2015). Ghrelin and ghrelin agonists were also shown to be viable in another animal model of dementia, demonstrating hippocampus neurogenesis (Bayliss et al., 2016; Moon et al., 2009). A team of researchers discovered that peripherally administered ghrelin can reactivate hippocampus neurogenesis (Li et al., 2013). As a result, ghrelin offers neuroprotection without calorie restriction (Bayliss et al., 2016).
The most recent study has shed fresh insight on the several molecular pathways by which ghrelin exerts its neuroprotective and anti-apoptotic actions. Mitochondrial dysfunction has also been involved in dementia pathophysiology (Ma et al., 2014; Morgan et al., 2018). Several investigations have suggested that the phosphatidylinositol-3-kinase enzyme is involved (PI3K) (Chen et al., 2011; Lim et al., 2011). Different systems are likely included in the balance of neurotrophic factors (Stoyanova, 2014) and extracellular signal managed kinase (ERK 1/2) (Chen et al., 2011; Chung et al., 2008). In line with the findings of animal models, ghrelin mRNA production was shown to be lower in three separate areas of the temporal gyrus in human patients with AD compared to unaffected healthy controls in a post-mortem examination (Gahete et al., 2010). Fasting ghrelin blood levels in 14 patients and 14 healthy individuals, categorized by body mass index and age, did not differ significantly in cross-sectional research (Fig. 4, Table 1) (Stylianou et al., 2007).
Fig. 4.
Ghrelin release and actions.
Table 1.
Central effects of ghrelin.
| Central Effects of Ghrelin | |||||
|---|---|---|---|---|---|
| Properties | Alzheimer's disease | Addictive disorders | Stress and Anxiety | Schizophrenia | Parkinson's disease |
| Type of Neuropeptide | Ghrelin mRNA, Acylated ghrelin | Ghrelin mRNA | Ghrelin mRNA, Acylated ghrelin | Ghrelin mRNA | Ghrelin mRNA |
| Receptors | GHS-R1a, GHS-R1b | GHS-R1a | GHS-R1a | GHS-R1a, GHS-R1b | GHS-R1a |
| CNS Distribution | Temporal gyrus, Amygdala, Hypothalamus, Pituitary gland | Amygdala, Orbitofrontal cortex, Anterior insula, Striatum, VTA | Amygdala, Hippocampus, Subgenual anterior cingulate cortex, Paraventricular thalamic nucleus | Hippocampus, NPY cells, ARC | Intra-VTA, Substantia nigra, Nigrostriatal system |
| Biological Actions | Neurogenesis, neural versatility, learning, memory | Mediate the motivation and reinforcing impact of amenable food in both animals and humans | Modulation of in behavior, including such anxiety, mood, and feeding behavior | Decreased elevated level of psychological stress | Attenuate the dopamine in Nigrostriatal system |
| Molecular Pathways | The phosphatidylinositol-3-kinase (PI3K) extracellular sign managed kinase (ERK 1/2) | GABAergic transmission, NMDA antagonism | Improvement in HPA (stress axis) hormone | Adenylate cyclase – protein kinase A (AC – PKA) system, protein kinase C (PLC – PKC) pathway | Co-expression of GHS-R and dopamine receptor-1 (D1R) in substantia nigra, and D1R-induced cAMP aggregation |
| Type of Model | AD and MCI Patient | GHSR-1a-KO mice | GHSR-1a-KO mice | Schizophrenic Patient | PD Patient |
| References | (Bayliss and Andrews, 2013; Walker, 2014) | (Chuang et al., 2011; Cruz et al., 2013; Egecioglu et al., 2011) | (Lodge and Grace, 2011; Walker, 2014) | (Palik et al., 2005; Walker, 2014) | (Jiang et al., 2008; Lee and Koh, 2015) |
6.2. Ghrelin in addictive disorders
It is realized that ghrelin is released at the time of a nutrition deficit and increases the consumption of calories. There has been considerable evidence that ghrelin is needed to mediate the motivation and reinforcing impact of amenable food in both animals and humans (Chuang et al., 2011; Egecioglu et al., 2011). This hypothesis supports the phenomenon of stress feeding as well as the belief that ghrelin interferes with stress-induced particular food in mice (Chuang et al., 2011). The craving for drugs and substance abuse are associated with the hyperactivation of the central ghrelin system (Zallar et al., 2017). A recent study reveals that the endogenous ghrelin system, which may be regulated and targeted to minimize the consequences of cocaine use, as it plays a substantial role in cocaine addiction (You et al., 2021). In addition, a robust correlation between neural pathways governing food and drug incentives has frequently been proposed (Volkow et al., 2011), and the outcomes of human and animal experiments include endocrine in diet and medication rewards. GHSR-1a-knockout mice did not show an improvement in the monoamine neurotransmitter produced inside the NAc, an essential aspect of a specific brain mechanism that was often uncovered with appetizing food (Egecioglu et al., 2011). Moreover, central and general administration of endocrine caused a monoamine neurotransmitter to uncheck within the nucleus accumbens in rodents (Abizaid et al., 2006; Jerlhag and Engel, 2011; Quarta et al., 2009). Comparatively, endocrine injections in humans were correlated with greater stimulation of reward and recognition structures (VTA, amygdala, anterior insula, orbitofrontal cortex) than placebo injections in the correlated grade fMRI research conducted on neural reaction to food video (Malik et al., 2008).
The trigger was shown to be positively associated with self-reported starvation levels. Surprisingly, ghrelin enhanced responsiveness in brain areas involved in attention and memory, as well as food memory (Malik et al., 2008). In addition, GABAergic transmission has consistently been shown to be implicated in alcohol consumption (Cruz et al., 2013), Crucially, the actions of ghrelin on the release of dopamine are regulated by GHSR-1a. At that point, no results have been observed if GHSR-1a has been antagonized (Abizaid et al., 2006; Jerlhag and Engel, 2011). Several neurological and neuropsychiatric disorders have been linked to the NMDA receptor, including AD (Liu et al., 2019), depressive disorder, anxiety, and addiction (Parsons et al., 1998). Moreover, a few studies have suggested that excessive glutamate and consequent overstimulation of NMDA receptors, resulting in excessive Ca2+ influx, are involved in the pathogenesis of several neurodegenerative disorders (Mody and MacDonald, 1995; Sattler and Tymianski, 2000).
Antagonism of the NMDA receptor even lessened the production of ghrelin-induced dopamine in the VTA, a brain rewarding system, indicating the presence of the glutamatergic system (Jerlhag and Engel, 2011). This can be relevant, as this technique is thought to be concerned with the manifestation of habit-forming disorders (Ross and Peselow, 2009), and is hypothesized to possess indications of depressive disorders (Bahi et al., 2013). Surprisingly, endocrine also increased reaction in brain regions involved in attention and memory, resulting in enhanced recollection of food footage. Antagonism of the NMDA receptor additionally dulled ghrelin-induced monoamine neurotransmitter unleash within the VTA, Some main structure inside the brain reward system, which indicates the presence of the glutamatergic system (Jerlhag and Engel, 2011). This can be of significance, as this technique is thought to be related to the looks of habit-forming disorders (Ross and Peselow, 2009), and is calculable to possess signed in spectacular disorders (Pilc et al., 2013). However, endocrine influences the development of fixation, to be specific to its role in tailored learning. Likewise, discoveries from a miscellany assortment of animals ponder to demonstrate that endocrine is related to the pathological process of addiction: endocrine receptor threat attenuated operative organization of alcohol and high alcohol utilization in rodents (Kaur and Ryabinin, 2010).
Previously, endocrine knockout mice have demonstrated slightly less voluntary alcohol intake than wild mice (Bahi et al., 2013). The report postulated that ghrelin antagonism reduced alcohol consumption and locomotor operation in wild mice (Bahi et al., 2013). Furthermore, ghrelin antagonism reduced alcohol intake in rats after 2, 5, and 10 months of active alcohol consumption, as well as the effects of alcohol deprivation and relapse in alcohol consumption (Suchankova et al., 2013). The effects of psychostimulant cocaine have shown similar results. Following the fundamental organization of ghrelin and cocaine and compared with cocaine alone, rats showed improved locomotor movement and conditioned place inclination (Zambrana-Infantes et al., 2018). A focal mixture of ghrelin prompted improved self-organization of heroin and upgraded heroin looking for conduct (Maric et al., 2012), and expanded dopaminergic transmission brought about by nicotine (Palotai et al., 2013). In people, dieting of serum ghrelin in early celibate alcohol subordinate patients was raised contrasted with solid controls. The amount of alcohol consumed before forbearance was shown to be inversely related, but the time of restraint was found to be strongly related to ghrelin serum levels after restraint (Kim et al., 2005). Alcohol was additionally systematically shown to lower the endocrine body fluid levels (Zimmermann et al., 2007). Various studies have also shown that ghrelin levels were low before drinking discontinuation and increased significantly after the start of patience (Leggio et al., 2012). As a result, it has been consistently stated that ghrelin serum concentrations have been positively linked with alcohol. In addition, a new study has shown that inner secretion would elevate the appetite for alcohol.
This effect has not been discovered on the will to drink juice or eat food (Leggio et al., 2014). Two recent studies have also suggested that ghrelin may have a role in human nicotine addiction. It was found that a higher level of ghrelin in the initial 24–48 h after smoking ends was related to expanded wanting and danger of smoking backslide following a month (al’Absi et al., 2014). Another study revealed that intrauterine introduction to tobacco smoke was related to expanded ghrelin levels in adulthood, featuring the solid connection of the ghrelin framework with addictive substances (Paslakis et al., 2014) (Fig. 4).
6.3. Ghrelin and neuropsychiatric conditions
6.3.1. Ghrelin in schizophrenia
Schizophrenia is related to an elevated level of psychological stress. Adjustments in the ghrelin framework appear to be good and beneficial (Lodge and Grace, 2011). Investigations looking at ghrelin levels in schizophrenics and stable controls have found that ghrelin levels in schizophrenics were substantially greater (Palik et al., 2005) with one examination finding decreased serum levels of ghrelin (Togo et al., 2004). Many studies have shown the impact of an antipsychotic drug on ghrelin levels (Togo et al., 2004). While a few examinations propose an expansion of ghrelin because of treatment with atypical antipsychotics (Esen-Danaci et al., 2008; Sentissi et al., 2009) others find no distinction (Himmerich et al., 2005) or abatement in ghrelin levels (Tanaka et al., 2008; Wysokiński et al., 2014). Curiously, antipsychotics found increased ghrelin in patients who experienced a weight gain (Jin et al., 2008), perhaps suggesting a disturbance of the negative criticism instrument of ghrelin emission.
6.3.2. Ghrelin and stress
Numerous findings from human and animal studies point to ghrelin's critical involvement in stress response regulation. The neural circuits that regulate anxiety are essentially connected to those that regulate mood, fear, and anxiety. It has been suggested that the hippocampus, the amygdala, a paraventricular thalamic nucleus, and the hypothalamic structures are involved in the regulation of tension control (Hsu et al., 2014). As a result, a change in the brain circuits that govern pressure is likely to affect temperament and behavior. It was revealed by the selection of neurobiological mechanisms that paved the way for survival by controlling the response to environmental threats by incorporating changes in behavior, such as anxiety, mood, and feeding patterns (Bowers et al., 2014; Fitzsimons et al., 2019). In support of that one, the use of ghrelin usually induces an improvement in HPA (stress axis) hormone. A few studies indicate that the modulation of the HPA axis is linked to a difference in the amount of ghrelin (Azzam et al., 2017; Holubová et al., 2016). Previous research has demonstrated that adrenalectomy results in greater fear learning and increased endogenous ghrelin in the stress response, indicating a stress response that is independent of the HPA axis (Meyer et al., 2014).
In a sequence of elegant studies with rats, it was shown that enhanced GHSR-1a-receptor activation was adequate and essential for increased fear (Buntwal et al., 2019). Systemic injection of the ghrelin receptor agonist improved the memory of anxiety but did not improve the corticotropin-releasing factor encouraging the authors to propose that ghrelin is involved in a novel aspect of the stress response (Meyer et al., 2014). Existing information powerfully showed an ascent of ghrelin serum levels after the presentation of psychological stress in both rodents (Lutter et al., 2008), and people (Ozsoy et al., 2014). Following that, women who revealed a significant number of relational stressors had greater blood ghrelin levels than those who revealed few or no relational stressors (Jaremka et al., 2015). By decreasing blood pressure and enhancing muscle responsiveness to mental stress, ghrelin affects a person's physiological response to stress (Lambert et al., 2011). Even so, the effects of ghrelin are dependent on GH in the amygdala. Treatment with ghrelin and dietary restriction caused anxiolytic-like symptoms in the elevated plus-maze. However, GHSR-1a-knockout mice showed no anxiolytic effects, even when anxiolytic-like effects were seen in all wild mouse models. Anxiolytic symptoms were also observed in another study following an amygdala infusion of ghrelin. Intriguingly, eating between both the infusion of ghrelin and the fear test prevents these anxiolytic effects (Alvarez-Crespo et al., 2012). These discoveries let authors speculate that the elevation of ghrelin because of yearning prompts, a feeding response, however, if no food is accessible, it could be beneficial for endurance if anxiety-like behaviors that would some way or another cut off the rodents from discovering food were put out (Alvarez-Crespo et al., 2012). Several studies in unstressed rodents (Gastón et al., 2017) promote the anxiogenic activity of ghrelin. Ghrelin, on the other hand, has been linked to increased anxiety-like activity and fear memory following chronic stress. This study found that fear memory decreased in response to intense stress, suggesting that anxiolytic effects are associated with ghrelin (Meyer et al., 2014). By doing so, ghrelin may exert anxiolytic effects under high pressure while advancing anxiogenic activity under unstressed and constantly focused conditions.
The contradictory findings regarding the function of ghrelin in anxiety were not readily remedied. The concept that ghrelin has a dual impact, that it may be anxiogenic or anxiolytic depending on the conditions, might explain some but not all the contradictory evidence (Meyer et al., 2014; Spencer et al., 2012). Ghrelin's consequences for rest rely upon the method for an organization with a rest weakening impact after central administration (Szentirmai et al., 2009) and a rest improving impact after peripheral administration (Kluge et al., 2007). Moreover, there is some proof that ghrelin has various impacts depending on the site of its administration in the CNS (Carlini et al., 2004). Furthermore, the prior study did not reveal a link between panic disorder and ghrelin content polymorphism (Hansen et al., 2013; Nakashima et al., 2008).
6.3.3. Ghrelin and insomnia
Ghrelin is also, reported to have an essential role in a sleeping disorder. The nocturnal level of ghrelin in 14 men experiencing a sleeping disorder (Motivala et al., 2009). The sleeping disorder patients indicated fundamentally lower nighttime ghrelin levels. This outcome follows the predictable finding that ghrelin increases males' rest. Deep sleep or non-REM sleep-promoting effects have been observed in teenagers (Schmid et al., 2006), elderly men (Kluge et al., 2009), and depressed men (Steiger et al., 2011). Conversely, ghrelin didn't essentially influence sleep in youth (Kluge et al., 2007) old or depressed (Kluge et al., 2013) females. In men, intense lack of sleep was related to diminished nocturnal ghrelin plasma levels (Dzaja et al., 2004). Similarly, the plasma levels of ghrelin would be higher in the night following sleep deprivation than in the night preceding the sleep deprivation, suggesting a rest-improving effect (Schüssler et al., 2006). Although ghrelin concentrations were shown to decrease throughout insomnia or sleep deprivation. The plasma concentration of ghrelin following a reduction in sleep is best correlated with the finding that chronic insomnia is related to obesity (Schmid et al., 2008; Spiegel et al., 2004; Taheri et al., 2004).
6.3.4. Ghrelin and Parkinson's disease (PD)
PD is a common and disabling age-related mammalian neurodegenerative disorder characterized by the gradual depletion of dopaminergic neurons in the nigra pars compacta (SNc) of the midbrain, the presence of Lewy's body lesions, and the deterioration of transmitting nerve fibers throughout the striatum. Such characteristics of the condition manifest in a variety of motor impairments, like fatigue, sitting tremors, posture dysfunction, and postural instability. In comparison, PD patients exhibit evidence of non-symptoms in the early stage of the disease. These included gastrointestinal (GI) dysfunction, hyposmia, sleep disturbance, and depressive symptoms (Lee and Koh, 2015). Because the ghrelin receptor (GHS-R1a) mRNA is highly distributed across many dopaminergic pathways (Mani et al., 2016).
Ghrelin is essential for the signaling of dopamine. The central administration of ghrelin to the third ventricle causes a rapid rise in locomotive activity and dopamine overload in the nucleus accumbens, which is antagonized by the GHS-R1a antagonist (Jerlhag et al., 2007; Jerlhag and Engel, 2011). Peripheral or intra-VTA ghrelin treatment can also contribute to a rise in the extracellular concentrations of accumbal dopamine and dopamine retention (Abizaid et al., 2006; Quarta et al., 2009). Accordingly, Jiang and colleagues were able to provide the first proof of the neuronal function of ghrelin in nigrostriatal dopamine signaling by co-expressing both GHS-R and dopamine receptor-1 (D1R) in the substantia nigra. Ghrelin activation of the GHS-R has been demonstrated to increase dopamine/D1R-induced cAMP aggregation (Jiang et al., 2008). Because dopamine activity decreases during Parkinson's disease pathology, the augmentation of D1R signaling following ghrelin therapy ensures that ghrelin can counteract the amplification of dopamine activity. As a result, several researchers showed that ghrelin might suppress the dopamine nigrostriatal pathway in several Parkinson's disease models (Andrews et al., 2009; Jiang et al., 2008; Moon et al., 2009). Till now, the majority of ghrelin-induced PD research has examined neuroprotective effects on the nigrostriatal mechanism, neurophysiological regulation of nigral dopaminergic neurons, and nonmotor manifestations of the disorder (Table 1).
6.3.5. Ghrelin and neuroprotection via nigrostriatal system
GHS-R1a is frequently found in dopaminergic nerve signals. Ghrelin has been demonstrated to counteract both dopaminergic neuron deficits in the SNc and dopamine decrease in the striatum. These findings were mediated by GHS-R1a since pretreatment with GHS-R1a antagonist (D-Lys3-GHRP-6) eliminated ghrelin's impact (Jiang et al., 2008). This finding was supported by investigations into numerous MPTP-induced PD in animal models in which animals with PD rescue characteristics were also eliminated through D-Lys3-GHRP-6 administration or GHS-R knockout (Andrews et al., 2009; Moon et al., 2009). Ghrelin offers neuroprotection via mitochondrial activity and microglial activation. This group includes apoptotic pathways related to mitochondria, oxidative stress, and mitochondrial biogenesis. The effect of ghrelin on MPTP-induced apoptosis was previously demonstrated by an increased Bax/Bcl-2 ratio and repression of apoptosis induction by ghrelin treatment (Dong et al., 2009; Jiang et al., 2008; Van Kaer, 2011). This is similar to previous cases of focal cerebral ischemia/reperfusion damage. Ghrelin improved mitochondrial pattern is defined to require the stimulation of the uncoupling protein-2 (UCP2) which removes the effects of ghrelin (Andrews et al., 2009). It is important to remember that ghrelin-induced neuronal activity is not limited to SNc but also occurs in several other brain areas, including the hypothalamus and hippocampus (Abizaid et al., 2006). This indicates that UCP2 is the normal target of ghrelin neuroprotective effects.
It has been hypothesized that AMPK (5′-adenosine monophosphate-activated protein kinase) stimulation and increased mitophagy could also facilitate ghrelin regulation using mitochondrial biogenesis (Bayliss and Andrews, 2013). This hypothesis demands more proof. In PD, ghrelin has neuroprotective effects, including disabling microglia and reducing inflammation. Microglia activated by MPTP and inflammatory mediators such as TNF-5-007 and IL-1β are inhibited by ghrelin in the acute PD mouse model and also in cultures of main mesencephalic neuroglia (Moon et al., 2009).
CRediT authorship contribution statement
Milind V. Masule: Writing – original draft. Sumit Rathod: Writing – original draft. Yogeeta Agrawal: Writing – original draft. Chandragouda R. Patil: Writing – review & editing. Kartik T. Nakhate: Writing – original draft. Shreesh Ojha: Writing – review & editing. Sameer N. Goyal: Writing – review & editing. Umesh B. Mahajan: Conceptualization, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the financial support received under the Early Career Research Award Scheme (File No. ECR/2016/001243) of the Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi, India. The authors also acknowledge for financial support Dr. Shreesh Ojha received from the University Program for Advanced Research (UPAR), United Arab Emirates University, United Arab Emirates.
Contributor Information
Milind V. Masule, Email: sum_pharma69@rediffmail.com.
Sumit Rathod, Email: sumit.rathod1@gmail.com.
Yogeeta Agrawal, Email: goyalyogita@rediffmail.com.
Chandragouda R. Patil, Email: pchandragouda@yahoo.com.
Kartik T. Nakhate, Email: kartiknakhate@gmail.com.
Shreesh Ojha, Email: shreeshojha@uaeu.ac.ae.
Sameer N. Goyal, Email: goyal.aiims@gmail.com.
Umesh B. Mahajan, Email: umeshmahajan41@gmail.com.
List of Abbreviations
- 5-HT
5-Hydroxytryptamine
- ACC
Acetyl-CoA Carboxylase
- ACTH
Adrenocorticotropic Hormone
- AD
Alzheimer's disease
- AGRP
Agouti-Related Protein
- Pi3K/Akt
Phosphatidylinositol 3-Kinase/Protein Kinase B Signaling Pathway
- AMPK
AMP-Activated Protein Kinase
- AMY
Amygdala
- ARC
Arcuate Nucleus
- AVP
Arginine Vasopressin
- Aβ
Amyloid beta
- Bax
Bcl-2-associated X protein
- BBB
Blood-Brain Barrier
- Bcl-2
B-cell lymphoma 2
- BDNF
Brain-Derived Neurotrophic Factor
- CaMK-II
Ca2+ and Calmodulin-Dependent Protein Kinase II Pathway
- CaMKK
Calmodulin-Dependent Kinase
- cAMP
Cyclic Adenosine Monophosphate
- cAMP/PKA
cAMP-Dependent Protein Kinase
- CAT
Catalase
- CD-36
Cluster of Differentiation 36
- CNS
Central Nervous System
- CREB
cAMP Response Element-Binding Protein
- CRH
Corticotrophin-Releasing Hormone
- CSDS
Chronic Social Defeat Stress
- D1R
Dopamine Receptor Subtype
- DMH
Dorsomedial Hypothalamic Nucleus
- ECT
Electroconvulsive Therapy
- eNOS
Endothelial Nitric Oxide Synthase
- ERK1/2
Extracellular Sign Controlled Kinase 1/2
- FAC
Fatty Acid Synthase
- FSH
Follicle-Stimulating Hormone
- GABA
Gamma-Aminobutyric Acid
- GDNF
Glial Cell Line-Derived Neurotrophic Factor
- GH
Growth Hormone
- GHSR
Growth Hormone Secretagogue Receptor
- GOAT
Ghrelin O-Acyltransferase
- GRB-2
Growth Factor Receptor-Bound Protein 2
- GSK3-β
Glycogen Synthase Kinase 3 Beta
- HDL
High-Density Lipoprotein
- HPA-axis
Hypothalamic-Pituitary-Adrenal Axis
- HPG axis
Hypothalamic-Pituitary-Gonadal Axis
- HPT axis
Hypothalamus-Pituitary-Thyroid Axis
- HYP
Hypothalamus
- IRS-1
Insulin Receptor Substrate 1
- LDL
Low-Density Lipoproteins
- LH
Luteinizing Hormone
- MAPK
Mitogen Initiated Protein Kinase
- MDD
Major Depressive Disorder
- MPTP
1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine
- mRNA
Messenger RNA
- NAc
Nucleus Accumbens
- NF-kB
Nuclear Factor Kappa-Light-Chain- B Cells
- NMDA
N-Methyl-D-Aspartate
- NPY
Neuropeptide Y
- NREM
Non-Rapid Eye Movement
- NTS
Nucleus of the Tractus Solitarius
- p38
p38 Mitogen-Activated Protein Kinases
- PD
Parkinson's disease
- PFC
Pre-Frontal Cortex
- PI3K
Phosphatidylinositol-3-Kinase
- PKC
Protein Kinase C
- PLC–PKC
Phospholipase C–Protein Kinase C
- PPAR-γ
Peroxisome Proliferator-Activated Receptor Gamma
- ROS
Reactive Oxygen Species
- SNc
Substantia Nigra Pars Compacta
- SNP
Single Nucleotide Polymorphisms
- SOD
Superoxide Dismutase
- TNF-α
Tumor Necrosis Factor Alpha
- TSH
Thyroid-Stimulating Hormone
- UCP-2
Uncoupling Protein-2
- VTA
Ventral Tegmental Area
References
- Abizaid A., Liu Z.-W., Andrews Z.B., Shanabrough M., Borok E., Elsworth J.D., Roth R.H., Sleeman M.W., Picciotto M.R., Tschöp M.H., Gao X.-B., Horvath T.L. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Açıkel B., Hoşoğlu E., Artik A., Yerlikaya F. Increased serum nesfatin-1 levels among adolescents diagnosed with major depressive disorder. Arch. Clin. Psychiatry (São Paulo) 2021;48:16–19. [Google Scholar]
- al'Absi M., Lemieux A., Nakajima M. Peptide YY and ghrelin predict craving and risk for relapse in abstinent smokers. Psychoneuroendocrinology. 2014;49:253–259. doi: 10.1016/j.psyneuen.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allas S., Caixàs A., Poitou C., Coupaye M., Thuilleaux D., Lorenzini F., Diene G., Crinò A., Illouz F., Grugni G. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: a randomized placebo-controlled trial. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Crespo M., Skibicka K.P., Farkas I., Molnár C.S., Egecioglu E., Hrabovszky E., Liposits Z., Dickson S.L. 2012. The Amygdala as a Neurobiological Target for Ghrelin in Rats: Neuroanatomical, Electrophysiological and Behavioral Evidence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews Z.B. Ghrelin: what's the function? J. Neuroendocrinol. 2019;31 doi: 10.1111/jne.12772. e12772–e12772. [DOI] [PubMed] [Google Scholar]
- Andrews Z.B., Erion D., Beiler R., Liu Z.-W., Abizaid A., Zigman J., Elsworth J.D., Savitt J.M., DiMarchi R., Tschoep M., Roth R.H., Gao X.-B., Horvath T.L. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J. Neurosci. 2009;29:14057–14065. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ari M., Ozturk O.H., Bez Y., Oktar S., Erduran D. High plasma nesfatin-1 level in patients with major depressive disorder. Prog. neuro-psychopharmacology Biol. psychiatry. 2011;35:497–500. doi: 10.1016/j.pnpbp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Azzam I., Gilad S., Limor R., Stern N., Greenman Y. Ghrelin stimulation by hypothalamic–pituitary–adrenal axis activation depends on increasing cortisol levels. Endocr. Connect. 2017;6:847–855. doi: 10.1530/EC-17-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babri S., Amani M., Mohaddes G., Mirzaei F., Mahmoudi F. Effects of intrahippocampal injection of ghrelin on spatial memory in PTZ-induced seizures in male rats. Neuropeptides. 2013;47:355–360. doi: 10.1016/j.npep.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Bahi A., Tolle V., Fehrentz J.-A., Brunel L., Martinez J., Tomasetto C.-L., Karam S.M. Ghrelin knockout mice show decreased voluntary alcohol consumption and reduced ethanol-induced conditioned place preference. Peptides. 2013;43:48–55. doi: 10.1016/j.peptides.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Bali A., Jaggi A. An integrative review on role and mechanisms of ghrelin in stress, anxiety and depression. Curr. Drug Targets. 2015;17 doi: 10.2174/1389450116666150518095650. [DOI] [PubMed] [Google Scholar]
- Banks W.A., Burney B.O., Robinson S.M. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood–brain barrier. Peptides. 2008;29:2061–2065. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzoni R., Zanetti M., Cattin M.R., Visintin L., Vinci P., Cattin L., Stebel M., Guarnieri G. Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rats. Obesity. 2007;15:2614–2623. doi: 10.1038/oby.2007.313. [DOI] [PubMed] [Google Scholar]
- Bayliss J.A., Andrews Z.B. Ghrelin is neuroprotective in Parkinson's disease: molecular mechanisms of metabolic neuroprotection. Ther. Adv. Endocrinol. Metab. 2013;4:25–36. doi: 10.1177/2042018813479645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss J.A., Lemus M.B., Stark R., Santos V.V., Thompson A., Rees D.J., Galic S., Elsworth J.D., Kemp B.E., Davies J.S. Ghrelin-AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson's disease. J. Neurosci. 2016;36:3049–3063. doi: 10.1523/JNEUROSCI.4373-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedendi I., Alloatti G., Marcantoni A., Malan D., Catapano F., Ghé C., Deghenghi R., Ghigo E., Muccioli G. Cardiac effects of ghrelin and its endogenous derivatives des-octanoyl ghrelin and des-Gln14-ghrelin. Eur. J. Pharmacol. 2003;476:87–95. doi: 10.1016/s0014-2999(03)02083-1. [DOI] [PubMed] [Google Scholar]
- Bellar D., Glickman E.L., Judge L.W., Gunstad J. Serum ghrelin is associated with verbal learning and adiposity in a sample of healthy, fit older adults. BioMed Res. Int. 2013;2013:202757. doi: 10.1155/2013/202757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P., Grace A.A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 2017;20:1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem B., Xu L., Morava É., Faludi G., Palkovits M., Roubos E.W., Kozicz T. Sex-specific differences in the dynamics of cocaine-and amphetamine-regulated transcript and nesfatin-1 expressions in the midbrain of depressed suicide victims vs. controls. Neuropharmacology. 2012;62:297–303. doi: 10.1016/j.neuropharm.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Bowers C.Y., Coy D.H., Hocart S.J., Tannenbaum G.S. 2014. Methods of Inhibiting the Ghrelin/growth Hormone Secretatogue Receptor Pathway and Uses Thereof. [Google Scholar]
- Buntwal L., Sassi M., Morgan A.H., Andrews Z.B., Davies J.S. Ghrelin-mediated hippocampal neurogenesis: implications for health and disease. Trends Endocrinol. Metabol. 2019;30:844–859. doi: 10.1016/j.tem.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Cabral A., López Soto E.J., Epelbaum J., Perelló M. Is ghrelin synthesized in the central nervous system? Int. J. Mol. Sci. 2017;18:638. doi: 10.3390/ijms18030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A., Suescun O., Zigman J.M., Perello M. Ghrelin indirectly activates hypophysiotropic CRF neurons in rodents. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camina J.P. Cell biology of the ghrelin receptor. J. Neuroendocrinol. 2006;18:65–76. doi: 10.1111/j.1365-2826.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- Carlini V.P., Monzón M.E., Varas M.M., Cragnolini A.B., Schiöth H.B., Scimonelli T.N., de Barioglio S.R. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem. Biophys. Res. Commun. 2002;299:739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- Carlini V.P., Varas M.M., Cragnolini A.B., Schiöth H.B., Scimonelli T.N., de Barioglio S.R. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem. Biophys. Res. Commun. 2004;313:635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- Castaneda T.R., Tong J., Datta R., Culler M., Tschöp M.H. Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Castañeda T.R., Tong J., Datta R., Culler M., Tschöp M.H. Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Chabot F., Caron A., Laplante M., St-Pierre D.H. Interrelationships between ghrelin, insulin and glucose homeostasis: physiological relevance. World J. Diabetes. 2014;5:328. doi: 10.4239/wjd.v5.i3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-Y., Asakawa A., Fujimiya M., Lee S.-D., Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol. Rev. 2009;61:430–481. doi: 10.1124/pr.109.001958. [DOI] [PubMed] [Google Scholar]
- Chen J., Huang S., Chen C., Tsai C., Yeh W., Chou S., Hsieh W., Lu D. Ghrelin induces cell migration through GHS-R, CaMKII, AMPK, and NF-κB signaling pathway in glioma cells. J. Cell. Biochem. 2011;112:2931–2941. doi: 10.1002/jcb.23209. [DOI] [PubMed] [Google Scholar]
- Chen S.-R., Chen H., Zhou J.-J., Pradhan G., Sun Y., Pan H.-L., Li D.-P. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. J. Neurochem. 2017;142:512–520. doi: 10.1111/jnc.14080. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Park Y.S., Kim N., Kim Y.S., Lee S.M., Lee D.H., Jung H.C. Gender differences in ghrelin, nociception genes, psychological factors and quality of life in functional dyspepsia. World J. Gastroenterol. 2017;23:8053. doi: 10.3748/wjg.v23.i45.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J.-C., Cui H., Mason B.L., Mahgoub M., Bookout A.L., Hana G.Y., Perello M., Elmquist J.K., Repa J.J., Zigman J.M. Chronic social defeat stress disrupts regulation of lipid synthesis [S] J. Lipid Res. 2010;51:1344–1353. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J.-C., Perello M., Sakata I., Osborne-Lawrence S., Savitt J.M., Lutter M., Zigman J.M. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J.-C., Zigman J.M. Ghrelin's roles in stress, mood, and anxiety regulation. Int. J. Pept. 2010 doi: 10.1155/2010/460549. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H., Seo S., Moon M., Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3b and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J. Endocrinol. 2008;198:511–522. doi: 10.1677/JOE-08-0160. [DOI] [PubMed] [Google Scholar]
- Cone R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cowley M.A., Smith R.G., Diano S., Tschöp M., Pronchuk N., Grove K.L., Strasburger C.J., Bidlingmaier M., Esterman M., Heiman M.L. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cruz M.T., Herman M.A., Cote D.M., Ryabinin A.E., Roberto M. Ghrelin increases GABAergic transmission and interacts with ethanol actions in the rat central nucleus of the amygdala. Neuropsychopharmacology. 2013;38:364–375. doi: 10.1038/npp.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D.E., Purnell J.Q., Frayo R.S., Schmidova K., Wisse B.E., Weigle D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Delhanty P.J., Neggers S.J., van der Lely A.J. Des-acyl ghrelin: a metabolically active peptide. Ghrelin Syst. 2013;25:112–121. doi: 10.1159/000346059. [DOI] [PubMed] [Google Scholar]
- Delhanty P.J.D., van der Eerden B.C.J., van der Velde M., Gauna C., Pols H.A.P., Jahr H., Chiba H., van der Lely A.-J., van Leeuwen J. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 2006;188:37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- Delhanty P.J.D., van der Lely A.-J. Ghrelin and glucose homeostasis. Peptides. 2011;32:2309–2318. doi: 10.1016/j.peptides.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Desai S.J., Borkar C.D., Nakhate K.T., Subhedar N.K., Kokare D.M. Neuropeptide Y attenuates anxiety- and depression-like effects of cholecystokinin-4 in mice. Neuroscience. 2014;277:818–830. doi: 10.1016/j.neuroscience.2014.07.062. [DOI] [PubMed] [Google Scholar]
- Diano S., Farr S.A., Benoit S.C., McNay E.C., da Silva I., Horvath B., Gaskin F.S., Nonaka N., Jaeger L.B., Banks W.A., Morley J.E., Pinto S., Sherwin R.S., Xu L., Yamada K.A., Sleeman M.W., Tschöp M.H., Horvath T.L. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Dickson S.L., Mercer J.G. John Wiley & Sons; 2016. Neuroendocrinology of Appetite. [Google Scholar]
- Dietrich M.O., Antunes C., Geliang G., Liu Z.-W., Borok E., Nie Y., Xu A.W., Souza D.O., Gao Q., Diano S. Agrp neurons mediate Sirt1's action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J. Neurosci. 2010;30:11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Song N., Xie J., Jiang H. Ghrelin antagonized 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. J. Mol. Neurosci. 2009;37:182–189. doi: 10.1007/s12031-008-9162-7. [DOI] [PubMed] [Google Scholar]
- Duxbury M.S., Waseem T., Ito H., Robinson M.K., Zinner M.J., Ashley S.W., Whang E.E. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem. Biophys. Res. Commun. 2003;309:464–468. doi: 10.1016/j.bbrc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Dzaja A., Dalal M.A., Himmerich H., Uhr M., Pollmächer T., Schuld A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. Am. J. Physiol. Endocrinol. Metab. 2004;286:E963–E967. doi: 10.1152/ajpendo.00527.2003. [DOI] [PubMed] [Google Scholar]
- Edvardsson C.E., Vestlund J., Jerlhag E. A ghrelin receptor antagonist reduces the ability of ghrelin, alcohol or amphetamine to induce a dopamine release in the ventral tegmental area and in nucleus accumbens shell in rats. Eur. J. Pharmacol. 2021;899:174039. doi: 10.1016/j.ejphar.2021.174039. [DOI] [PubMed] [Google Scholar]
- Egecioglu E., Skibicka K.P., Hansson C., Alvarez-Crespo M., Friberg P.A., Jerlhag E., Engel J.A., Dickson S.L. Hedonic and incentive signals for body weight control. Rev. Endocr. Metab. Disord. 2011;12:141–151. doi: 10.1007/s11154-011-9166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabadlah H., Hameed R., D'Souza C., Mohsin S., Adeghate E.A. Exogenous ghrelin increases plasma insulin level in diabetic rats. Biomolecules. 2020;10 doi: 10.3390/biom10040633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereş G., Su Akgün Demirtaş C., Toptaş E., Yılmaz A.D., Sengüven B., Kamburoğlu K. Correlations between the peptide hormone ghrelin and proinflammatory cytokines in experimental periodontitis models of female rats at different stages of the life cycle. Arch. Oral Biol. 2019;108 doi: 10.1016/j.archoralbio.2019.104518. [DOI] [PubMed] [Google Scholar]
- Esel E., Ozsoy S., Tutus A., Sofuoglu S., Kartalci S., Bayram F., Kokbudak Z., Kula M. Effects of antidepressant treatment and of gender on serum leptin levels in patients with major depression. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2005;29:565–570. doi: 10.1016/j.pnpbp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Esen-Danaci A., Sarandol A., Taneli F., Ozlen N. 311–Effects of atypical antipsychotics on leptin and ghrelin. Schizophr. Res. 2008;98:161. doi: 10.1016/j.pnpbp.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Favaro E., Granata R., Miceli I., Baragli A., Settanni F., Perin P.C., Ghigo E., Camussi G., Zanone M.M. The ghrelin gene products and exendin-4 promote survival of human pancreatic islet endothelial cells in hyperglycaemic conditions, through phosphoinositide 3-kinase/Akt, extracellular signal-related kinase (ERK) 1/2 and cAMP/protein kinase A (PKA) signall. Diabetologia. 2012;55:1058–1070. doi: 10.1007/s00125-011-2423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré G., Louet M., Saurel O., Delort B., Czaplicki G., M'Kadmi C., Damian M., Renault P., Cantel S., Gavara L., Demange P., Marie J., Fehrentz J.-A., Floquet N., Milon A., Banères J.-L. Structure and dynamics of G protein-coupled receptor-bound ghrelin reveal the critical role of the octanoyl chain. Proc. Natl. Acad. Sci. U. S. A. 2019;116:17525–17530. doi: 10.1073/pnas.1905105116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F., Salio C., Lossi L., Merighi A. Ghrelin in central neurons. Curr. Neuropharmacol. 2009;7:37–49. doi: 10.2174/157015909787602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons S.E.W., Chruścicka B., Druelle C., Stamou P., Nally K., Dinan T.G., Cryan J.F., Schellekens H. A ghrelin receptor and oxytocin receptor heterocomplex impairs oxytocin mediated signalling. Neuropharmacology. 2019;152:90–101. doi: 10.1016/j.neuropharm.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Frago L.M., Baquedano E., Argente J., Chowen J.A. Neuroprotective actions of ghrelin and growth hormone secretagogues. Front. Mol. Neurosci. 2011;4:23. doi: 10.3389/fnmol.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahete M.D., Rubio A., Durán-Prado M., Avila J., Luque R.M., Castano J.P. Expression of Somatostatin, cortistatin, and their receptors, as well as dopamine receptors, but not of neprilysin, are reduced in the temporal lobe of Alzheimer's disease patients. J. Alzheim. Dis. 2010;20:465–475. doi: 10.3233/JAD-2010-1385. [DOI] [PubMed] [Google Scholar]
- García-García F., Juárez-Aguilar E., Santiago-García J., Cardinali D.P. Ghrelin and its interactions with growth hormone, leptin and orexins: implications for the sleep–wake cycle and metabolism. Sleep Med. Rev. 2014;18:89–97. doi: 10.1016/j.smrv.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Gargantini E., Grande C., Trovato L., Ghigo E., Granata R. The role of obestatin in glucose and lipid metabolism. Horm. Metab. Res. 2013;45:1002–1008. doi: 10.1055/s-0033-1351325. [DOI] [PubMed] [Google Scholar]
- Gastón M.S., Cid M.P., Salvatierra N.A. Bicuculline, a GABAA-receptor antagonist, blocked HPA axis activation induced by ghrelin under an acute stress. Behav. Brain Res. 2017;320:464–472. doi: 10.1016/j.bbr.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Gecici O., Serteser M., Emuel M., Demirel R. 2005. Serum Ghrelin and Leptin Levels in Major Depressive Disorders. [Google Scholar]
- Gueler O., Oezbulut O., Kurt E., Alatas G., Serteser M., Gecici O. 2007. Ghrelin and Leptin Levels in Patients with Euthymic Bipolar Disorder and Bipolar Depression. [Google Scholar]
- Hansen C.F., Vrang N., Sangild P.T., Jelsing J. Novel insight into the distribution of L-cells in the rat intestinal tract. Am. J. Transl. Res. 2013;5:347. [PMC free article] [PubMed] [Google Scholar]
- Himmerich H., Fulda S., Künzel H.E., Pfennig A., Dzaja A., Cummings D.E., Pollmächer T. Ghrelin plasma levels during psychopharmacological treatment. Neuropsychobiology. 2005;52:11–16. doi: 10.1159/000086171. [DOI] [PubMed] [Google Scholar]
- Holubová A., Štofková A., Jurčovičová J., Šlamberová R. The effect of neonatal maternal stress on plasma levels of adrenocorticotropic hormone, corticosterone, leptin, and ghrelin in adult male rats exposed to acute heterotypic stressor. Physiol. Res. 2016;65 doi: 10.33549/physiolres.933530. [DOI] [PubMed] [Google Scholar]
- Homan P., Grob S., Milos G., Schnyder U., Hasler G. Reduction in total plasma ghrelin levels following catecholamine depletion: relation to bulimic and depressive symptoms. Psychoneuroendocrinology. 2013;38:1545–1552. doi: 10.1016/j.psyneuen.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Hosoda H., Kojima M., Matsuo H., Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Hosoda H., Kojima M., Mizushima T., Shimizu S., Kangawa K. Structural divergence of human ghrelin: identification of multiple ghrelin-derived molecules produced by post-translational processing. J. Biol. Chem. 2003;278:64–70. doi: 10.1074/jbc.M205366200. [DOI] [PubMed] [Google Scholar]
- Howard A.D., Feighner S.D., Cully D.F., Arena J.P., Liberator P.A., Rosenblum C.I., Hamelin M., Hreniuk D.L., Palyha O.C., Anderson J. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Hsu D.T., Kirouac G.J., Zubieta J.-K., Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front. Behav. Neurosci. 2014;8:73. doi: 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-J., Zhu X.-C., Han Q.-Q., Wang Y.-L., Yue N., Wang J., Yu R., Li B., Wu G.-C., Liu Q., Yu J. Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav. Brain Res. 2017;326:33–43. doi: 10.1016/j.bbr.2017.02.040. [DOI] [PubMed] [Google Scholar]
- Jacob A., Rajan D., Pathickal B., Balouch I., Hartman A., Wu R., Zhou M., Wang P. The inhibitory effect of ghrelin on sepsis-induced inflammation is mediated by the MAPK phosphatase-1. Int. J. Mol. Med. 2010;25:159–164. [PMC free article] [PubMed] [Google Scholar]
- Jaremka L.M., Fagundes C.P., Peng J., Belury M.A., Andridge R.R., Malarkey W.B., Kiecolt-Glaser J.K. Loneliness predicts postprandial ghrelin and hunger in women. Horm. Behav. 2015;70:57–63. doi: 10.1016/j.yhbeh.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery P.L., Herington A.C., Chopin L.K. The potential autocrine/paracrine roles of ghrelin and its receptor in hormone-dependent cancer. Cytokine Growth Factor Rev. 2003;14:113–122. doi: 10.1016/s1359-6101(02)00089-8. [DOI] [PubMed] [Google Scholar]
- Jeffery P.L., Murray R.E., Yeh A.H., McNamara J.F., Duncan R.P., Francis G.D., Herington A.C., Chopin L.K. Expression and function of the ghrelin axis, including a novel preproghrelin isoform, in human breast cancer tissues and cell lines. Endocr. Relat. Cancer. 2005;12:839–850. doi: 10.1677/erc.1.00984. [DOI] [PubMed] [Google Scholar]
- Jeon S.W., Kim Y.-K. Molecular neurobiology and promising new treatment in depression. Int. J. Mol. Sci. 2016;17:381. doi: 10.3390/ijms17030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E., Egecioglu E., Dickson S.L., Douhan A., Svensson L., Engel J.A. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addiction Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E., Engel J.A. Ghrelin receptor antagonism attenuates nicotine-induced locomotor stimulation, accumbal dopamine release and conditioned place preference in mice. Drug Alcohol Depend. 2011;117:126–131. doi: 10.1016/j.drugalcdep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Jiang H., Li L.-J., Wang J., Xie J.-X. Ghrelin antagonizes MPTP-induced neurotoxicity to the dopaminergic neurons in mouse substantia nigra. Exp. Neurol. 2008;212:532–537. doi: 10.1016/j.expneurol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Jin H., Meyer J.M., Mudaliar S., Jeste D.V. Impact of atypical antipsychotic therapy on leptin, ghrelin, and adiponectin. Schizophr. Res. 2008;100:70–85. doi: 10.1016/j.schres.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Akiyoshi J., Kitaichi T., Matsushita H., Tanaka E., Kodama K., Hanada H., Isogawa K. Administration of antisense DNA for ghrelin causes an antidepressant and anxiolytic response in rats. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2006;30:1403–1407. doi: 10.1016/j.pnpbp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Karadeniz S., Yaman H., Bilginer Ç., Hızarcı Bulut S., Yaman S.Ö. Serum nesfatin-1, ghrelin, and lipid levels in adolescents with first episode drug naïve unipolar depression. Nord. J. Psychiatr. 2020;74:613–619. doi: 10.1080/08039488.2020.1772363. [DOI] [PubMed] [Google Scholar]
- Kaur S., Ryabinin A.E. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin. Exp. Res. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Her S.J., Park S.J., Kim D., Park K.S., Lee H.K., Han B.H., Kim M.S., Shin C.S., Kim S.Y. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone. 2005;37:359–369. doi: 10.1016/j.bone.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Heppner K.M., Tschöp M.H. The role of ghrelin in the control of energy balance. Appet. Control. 2012:161–184. doi: 10.1007/978-3-642-24716-3_7. [DOI] [PubMed] [Google Scholar]
- Kluge M., Schmidt D., Uhr M., Steiger A. Ghrelin suppresses nocturnal secretion of luteinizing hormone (LH) and thyroid stimulating hormone (TSH) in patients with major depression. J. Psychiatr. Res. 2013;47:1236–1239. doi: 10.1016/j.jpsychires.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Kluge M., Schüssler P., Schmidt D., Uhr M., Steiger A. Ghrelin suppresses secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in women. J. Clin. Endocrinol. Metab. 2012;97:E448–E451. doi: 10.1210/jc.2011-2607. [DOI] [PubMed] [Google Scholar]
- Kluge M., Schüssler P., Zuber V., Kleyer S., Yassouridis A., Dresler M., Uhr M., Steiger A. Ghrelin enhances the nocturnal secretion of cortisol and growth hormone in young females without influencing sleep. Psychoneuroendocrinology. 2007;32:1079–1085. doi: 10.1016/j.psyneuen.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Kluge M., Uhr M., Bleninger P., Yassouridis A., Steiger A. Ghrelin suppresses secretion of FSH in males. Clin. Endocrinol. 2009;70:920–923. doi: 10.1111/j.1365-2265.2008.03440.x. [DOI] [PubMed] [Google Scholar]
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M., Hosoda H., Nakazato M., Matsuo H. Kangawa K. Ghrelin Struct. Funct. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Kurt E., Guler O., Serteser M., Cansel N., Ozbulut O., Altınbaş K., Alataş G., Savaş H., Gecici O. The effects of electroconvulsive therapy on ghrelin, leptin and cholesterol levels in patients with mood disorders. Neurosci. Lett. 2007;426:49–53. doi: 10.1016/j.neulet.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Lambert E., Lambert G., Ika-Sari C., Dawood T., Lee K., Chopra R., Straznicky N., Eikelis N., Drew S., Tilbrook A. Ghrelin modulates sympathetic nervous system activity and stress response in lean and overweight men. Hypertension. 2011;58:43–50. doi: 10.1161/HYPERTENSIONAHA.111.171025. [DOI] [PubMed] [Google Scholar]
- Lee H.M., Koh S.-B. Many faces of Parkinson's disease: non-motor symptoms of Parkinson's disease. J. Mov. Disord. 2015;8:92–97. doi: 10.14802/jmd.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L., Ferrulli A., Cardone S., Nesci A., Miceli A., Malandrino N., Capristo E., Canestrelli B., Monteleone P., Kenna G.A. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addiction Biol. 2012;17:452–464. doi: 10.1111/j.1369-1600.2010.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L., Zywiak W.H., Fricchione S.R., Edwards S.M., Suzanne M., Swift R.M., Kenna G.A. Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol. Psychiatr. 2014;76:734–741. doi: 10.1016/j.biopsych.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Kim Y., Kim S., Park S. Ghrelin-induced hippocampal neurogenesis and enhancement of cognitive function are mediated independently of GH/IGF-1 axis: lessons from the spontaneous dwarf rats. Endocr. J. 2013;60:1065–1075. doi: 10.1507/endocrj.ej13-0045. [DOI] [PubMed] [Google Scholar]
- Li Y.-H., Qing-Xiu L., Wang J.-S., Xiang H., Zhang R.-F., Huang C.-Q. Ghrelin improves cognition via activation of the cAMP- CREB signalling pathway in depressed male C57BL/6J mice. Int. J. Neurosci. 2021:1–13. doi: 10.1080/00207454.2021.1928114. [DOI] [PubMed] [Google Scholar]
- Lim E., Lee S., Li E., Kim Y., Park S. Ghrelin protects spinal cord motoneurons against chronic glutamate-induced excitotoxicity via ERK1/2 and phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3β pathways. Exp. Neurol. 2011;230:114–122. doi: 10.1016/j.expneurol.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Lin P.Y., Tseng P.T. Decreased glial cell line-derived neurotrophic factor levels in patients with depression: a meta-analytic study. J. Psychiatr. Res. 2015;63:20–27. doi: 10.1016/j.jpsychires.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Liu J., Chang L., Song Y., Li H., Wu Y. The role of NMDA receptors in Alzheimer's disease. Front. Neurosci. 2019 doi: 10.3389/fnins.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D.J., Grace A.A. Developmental pathology, dopamine, stress and schizophrenia. Int. J. Dev. Neurosci. 2011;29:207–213. doi: 10.1016/j.ijdevneu.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M., Krishnan V., Russo S.J., Jung S., McClung C.A., Nestler E.J. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J. Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Yuan L., Yu H., Xi Y., Xiao R. Mitochondrial dysfunction and oxidative damage in the brain of diet-induced obese rats but not in diet-resistant rats. Life Sci. 2014;110:53–60. doi: 10.1016/j.lfs.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Malik S., McGlone F., Bedrossian D., Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metabol. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mani B.K., Osborne-Lawrence S., Vijayaraghavan P., Hepler C., Zigman J.M. β1-Adrenergic receptor deficiency in ghrelin-expressing cells causes hypoglycemia in susceptible individuals. J. Clin. Invest. 2016;126:3467–3478. doi: 10.1172/JCI86270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Tokudome T., Kishimoto I. Ghrelin as a treatment for cardiovascular diseases. Hypertension (New York) 2014;64:450–454. doi: 10.1161/HYPERTENSIONAHA.114.03726. [DOI] [PubMed] [Google Scholar]
- Marazziti D., Rutigliano G., Baroni S., Landi P., Dell'Osso L. Metabolic syndrome and major depression. CNS Spectr. 2014;19:293–304. doi: 10.1017/S1092852913000667. [DOI] [PubMed] [Google Scholar]
- Maric T., Sedki F., Ronfard B., Chafetz D., Shalev U. A limited role for ghrelin in heroin self-administration and food deprivation-induced reinstatement of heroin seeking in rats. Addiction Biol. 2012;17:613–622. doi: 10.1111/j.1369-1600.2011.00396.x. [DOI] [PubMed] [Google Scholar]
- Marino J.S., Xu Y., Hill J.W. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol. Metabol. 2011;22:275–285. doi: 10.1016/j.tem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Nakano M., Nakashima M., Watanuki T., Egashira K., Matsubara T., Watanabe Y. Neural correlates of plasma acylated ghrelin level in individuals with major depressive disorder. Brain Res. 2012;1473:185–192. doi: 10.1016/j.brainres.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Meyer R.M., Burgos-Robles A., Liu E., Correia S.S., Goosens K.A. A ghrelin–growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol. Psychiatr. 2014;19:1284–1294. doi: 10.1038/mp.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D., Eddy K.T., Keshaviah A., Dinescu D., Ross S.L., Kass A.E., Franko D.L., Herzog D.B. Depression and eating disorders: treatment and course. J. Affect. Disord. 2011;130:470–477. doi: 10.1016/j.jad.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M. Obesity pharmacotherapy: current perspectives and future directions. Curr. Cardiol. Rev. 2013;9:33–54. doi: 10.2174/157340313805076322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I., MacDonald J.F. NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends Pharmacol. Sci. 1995;16:356–359. doi: 10.1016/s0165-6147(00)89070-7. [DOI] [PubMed] [Google Scholar]
- Moon M., Kim H.G., Hwang L., Seo J.-H., Kim Sehee, Hwang S., Kim Soonyong, Lee D., Chung H., Oh M.S. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson's disease by blocking microglial activation. Neurotox. Res. 2009;15:332–347. doi: 10.1007/s12640-009-9037-x. [DOI] [PubMed] [Google Scholar]
- Morgan A.H., Rees D.J., Andrews Z.B., Davies J.S. Ghrelin mediated neuroprotection-A possible therapy for Parkinson's disease? Neuropharmacology. 2018;136:317–326. doi: 10.1016/j.neuropharm.2017.12.027. [DOI] [PubMed] [Google Scholar]
- Morin V., Hozer F., Costemale-Lacoste J.-F. The effects of ghrelin on sleep, appetite, and memory, and its possible role in depression: a review of the literature. Encephale. 2018;44:256–263. doi: 10.1016/j.encep.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Mortazaei S., Sahraei H., Bahari Z., Meftahi G.H., Pirzad Jahromi G., Hatef B. Ventral tegmental area inactivation alters hormonal, metabolic, and locomotor responses to inescapable stress. Arch. Physiol. Biochem. 2019;125:293–301. doi: 10.1080/13813455.2018.1455711. [DOI] [PubMed] [Google Scholar]
- Motivala S.J., Tomiyama A.J., Ziegler M., Khandrika S., Irwin M.R. Nocturnal levels of ghrelin and leptin and sleep in chronic insomnia. Psychoneuroendocrinology. 2009;34:540–545. doi: 10.1016/j.psyneuen.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M., Okimura Y., Iida K., Matsumoto M., Sowa H., Kaji H., Kojima M., Kangawa K., Chihara K. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J. Biol. Chem. 2002;277:5667–5674. doi: 10.1074/jbc.M103898200. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Akiyoshi J., Hatano K., Hanada H., Tanaka Y., Tsuru J., Matsushita H., Kodama K., Isogawa K. Ghrelin gene polymorphism is associated with depression, but not panic disorder. Psychiatr. Genet. 2008;18:257. doi: 10.1097/YPG.0b013e328306c979. [DOI] [PubMed] [Google Scholar]
- Nakhate K., Borkar C., Bharne A. In: Cognitive Informatics, Computer Modelling, and Cognitive Science. Sinha G.R., Suri J.S., editors. Academic Press; 2020. Chapter 2 - functional neuroanatomy and disorders of cognition; pp. 21–47. [Google Scholar]
- Nakhate K.T., Bharne A.P., Verma V.S., Aru D.N., Kokare D.M. Plumbagin ameliorates memory dysfunction in streptozotocin induced Alzheimer's disease via activation of Nrf 2/ARE pathway and inhibition of β-secretase. Biomed. Pharmacother. 2018;101:379–390. doi: 10.1016/j.biopha.2018.02.052. [DOI] [PubMed] [Google Scholar]
- Nanzer A.M., Khalaf S., Mozid A.M., Fowkes R.C., Patel M.V., Burrin J.M., Grossman A.B., Korbonits M. Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen-activated protein kinase pathway. Eur. J. Endocrinol. 2004;151:233–240. doi: 10.1530/eje.0.1510233. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V. University of Kentucky; 2012. Diet-induced Obesity: Dopaminergic and Behavioral Mechanisms as Outcomes and Predictors. [Google Scholar]
- Nonogaki K., Ohba Y., Sumii M., Oka Y. Serotonin systems upregulate the expression of hypothalamic NUCB2 via 5-HT2C receptors and induce anorexia via a leptin-independent pathway in mice. Biochem. Biophys. Res. Commun. 2008;372:186–190. doi: 10.1016/j.bbrc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Numakawa T., Richards M., Nakajima S., Adachi N., Furuta M., Odaka H., Kunugi H. The role of brain-derived neurotrophic factor (BDNF) in comorbid depression: possible linkage with steroid hormones, cytokines, and nutrition. Front. Psychiatr. 2014;5:136. doi: 10.3389/fpsyt.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh A.K., Greene A.K., Mulliken J.B., Rogers G.F. Prevention of temporal depression that follows fronto-orbital advancement for craniosynostosis. J. Craniofac. Surg. 2006;17:980–985. doi: 10.1097/01.scs.0000230015.16401.1d. [DOI] [PubMed] [Google Scholar]
- Olszewski P.K., Schiöth H.B., Levine A.S. Ghrelin in the CNS: from hunger to a rewarding and memorable meal? Brain Res. Rev. 2008;58:160–170. doi: 10.1016/j.brainresrev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy S., Besirli A., Abdulrezzak U., Basturk M. Serum ghrelin and leptin levels in patients with depression and the effects of treatment. Psychiatry Investig. 2014;11:167. doi: 10.4306/pi.2014.11.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palik E., Faludi G., Cseh K. Possible connection between ghrelin, resistin and TNF-alpha levels and the metabolic syndrome caused by atypical antipsychotics. Neuropsychopharmacol. Hungarica a Magy. Pszichofarmakologiai Egyes. Lapja= Off. J. Hungarian Assoc. Psychopharmacol. 2005;7:132–139. [PubMed] [Google Scholar]
- Palotai M., Bagosi Z., Jászberényi M., Csabafi K., Dochnal R., Manczinger M., Telegdy G., Szabó G. Ghrelin amplifies the nicotine-induced dopamine release in the rat striatum. Neurochem. Int. 2013;63:239–243. doi: 10.1016/j.neuint.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Parsons C.G., Danysz W., Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11:523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- Paslakis G., Buchmann A.F., Westphal S., Banaschewski T., Hohm E., Zimmermann U.S., Laucht M., Deuschle M. Intrauterine exposure to cigarette smoke is associated with increased ghrelin concentrations in adulthood. Neuroendocrinology. 2014;99:123–129. doi: 10.1159/000363325. [DOI] [PubMed] [Google Scholar]
- Patterson M., Bloom S.R., Gardiner J.V. Ghrelin and appetite control in humans—potential application in the treatment of obesity. Peptides. 2011;32:2290–2294. doi: 10.1016/j.peptides.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Perea Vega M.L., Sanchez M.S., Fernández G., Paglini M.G., Martin M., de Barioglio S.R. Ghrelin treatment leads to dendritic spine remodeling in hippocampal neurons and increases the expression of specific BDNF-mRNA species. Neurobiol. Learn. Mem. 2021;179 doi: 10.1016/j.nlm.2021.107409. [DOI] [PubMed] [Google Scholar]
- Pilc A., Wierońska J.M., Skolnick P. Glutamate-based antidepressants: preclinical psychopharmacology. Biol. Psychiatr. 2013;73:1125–1132. doi: 10.1016/j.biopsych.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Pittenger C., Duman R.S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Poretti M.B., Sawant R.S., Rask-Andersen M., de Cuneo M.F., Schiöth H.B., Perez M.F., Carlini V.P. Reduced vasopressin receptors activation mediates the anti-depressant effects of fluoxetine and venlafaxine in bulbectomy model of depression. Psychopharmacology (Berl) 2016;233:1077–1086. doi: 10.1007/s00213-015-4187-4. [DOI] [PubMed] [Google Scholar]
- Qian J., Morris C.J., Caputo R., Garaulet M., Scheer F.A.J.L. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int. J. Obes. 2019;43:1644–1649. doi: 10.1038/s41366-018-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D., Di Francesco C., Melotto S., Mangiarini L., Heidbreder C., Hedou G. Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem. Int. 2009;54:89–94. doi: 10.1016/j.neuint.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Ross S., Peselow E. The neurobiology of addictive disorders. Clin. Neuropharmacol. 2009;32:269–276. doi: 10.1097/wnf.0b013e3181a9163c. [DOI] [PubMed] [Google Scholar]
- Rozanska O., Uruska A., Zozulinska-Ziolkiewicz D. Brain-derived neurotrophic factor and diabetes. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhé H.G., Mason N.S., Schene A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol. Psychiatr. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Sattler R., Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J. Mol. Med. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Schellekens H., Finger B.C., Dinan T.G., Cryan J.F. Ghrelin signalling and obesity: at the interface of stress, mood and food reward. Pharmacol. Ther. 2012;135:316–326. doi: 10.1016/j.pharmthera.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Schmid D.A., Held K., Ising M., Uhr M., Weikel J.C., Steiger A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology. 2005;30:1187–1192. doi: 10.1038/sj.npp.1300670. [DOI] [PubMed] [Google Scholar]
- Schmid D.A., Wichniak A., Uhr M., Ising M., Brunner H., Held K., Weikel J.C., Sonntag A., Steiger A. Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacology. 2006;31:832–844. doi: 10.1038/sj.npp.1300923. [DOI] [PubMed] [Google Scholar]
- Schmid S.M., Hallschmid M., Jauch-Chara K., Born J., Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J. Sleep Res. 2008;17:331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Schüssler P., Uhr M., Ising M., Weikel J.C., Schmid D.A., Held K., Mathias S., Steiger A. Nocturnal ghrelin, ACTH, GH and cortisol secretion after sleep deprivation in humans. Psychoneuroendocrinology. 2006;31:915–923. doi: 10.1016/j.psyneuen.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Seim I., Collet C., Herington A.C., Chopin L.K. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genom. 2007;8:1–16. doi: 10.1186/1471-2164-8-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentissi O., Grouselle D., Viala A., Bourdel M.C., Olié J.P., Epelbaum J., Poirier M.F. Ghrelin and leptin levels in schizophrenic patients treated with antipsychotic monotherapy. J. Clin. Psychopharmacol. 2009;29:304–306. doi: 10.1097/JCP.0b013e3181a390ed. [DOI] [PubMed] [Google Scholar]
- Serrenho D., Santos S.D., Carvalho A.L. The role of ghrelin in regulating synaptic function and plasticity of feeding-associated circuits. Front. Cell. Neurosci. 2019;13:205. doi: 10.3389/fncel.2019.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K., Gupta D., Mani B.K., Findley B.G., Lord C.C., Osborne-Lawrence S., Metzger N.P., Pietra C., Liu C., Berglund E.D., Zigman J.M. Acyl-ghrelin is permissive for the normal counterregulatory response to insulin-induced hypoglycemia. Diabetes. 2020;69:228–237. doi: 10.2337/db19-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani M., Ogawa Y., Ebihara K., Aizawa-Abe M., Miyanaga F., Takaya K., Hayashi T., Inoue G., Hosoda K., Kojima M. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- Skibicka K.P., Hansson C., Egecioglu E., Dickson S.L. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addiction Biol. 2012;17:95–107. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorri A., Järventausta K., Kampman O., Lehtimäki K., Björkqvist M., Tuohimaa K., Hämäläinen M., Moilanen E., Leinonen E. Effect of electroconvulsive therapy on brain-derived neurotrophic factor levels in patients with major depressive disorder. Brain Behav. 2018;8 doi: 10.1002/brb3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S.J., Emmerzaal T.L., Kozicz T., Andrews Z.B. Ghrelin's role in the hypothalamic-pituitary-adrenal axis stress response: implications for mood disorders. Biol. Psychiatr. 2015;78:19–27. doi: 10.1016/j.biopsych.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Spencer S.J., Xu L., Clarke M.A., Lemus M., Reichenbach A., Geenen B., Kozicz T., Andrews Z.B. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol. Psychiatr. 2012;72:457–465. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Spiegel K., Tasali E., Penev P., Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Steculorum S.M., Bouret S.G. Developmental effects of ghrelin. Peptides. 2011;32:2362–2366. doi: 10.1016/j.peptides.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A., Dresler M., Schüssler P., Kluge M. Ghrelin in mental health, sleep, memory. Mol. Cell. Endocrinol. 2011;340:88–96. doi: 10.1016/j.mce.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Stone L.A., Harmatz E.S., Goosens K.A. Ghrelin as a stress hormone: implications for psychiatric illness. Biol. Psychiatr. 2020;88:531–540. doi: 10.1016/j.biopsych.2020.05.013. [DOI] [PubMed] [Google Scholar]
- Stoyanova I., Lutz D. Ghrelin-mediated regeneration and plasticity after nervous system injury. Front. Cell Dev. Biol. 2021;9:583. doi: 10.3389/fcell.2021.595914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanova I.I. Ghrelin: a link between ageing, metabolism and neurodegenerative disorders. Neurobiol. Dis. 2014;72:72–83. doi: 10.1016/j.nbd.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Stylianou C., Galli-Tsinopoulou A., Koliakos G., Fotoulaki M., Nousia-Arvanitakis S. Ghrelin and leptin levels in young adults with cystic fibrosis: relationship with body fat. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2007;6:293–296. doi: 10.1016/j.jcf.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Suchankova P., Steensland P., Fredriksson I., Engel J.A., Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai É., Kapás L., Sun Y., Smith R.G., Krueger J.M. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proc. Natl. Acad. Sci. USA. 2009;106:14069–14074. doi: 10.1073/pnas.0903090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S., Lin L., Austin D., Young T., Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya K., Ariyasu H., Kanamoto N., Iwakura H., Yoshimoto A., Harada M., Mori K., Komatsu Y., Usui T., Shimatsu A. Ghrelin strongly stimulates growth hormone release in humans. J. Clin. Endocrinol. Metab. 2000;85:4908–4911. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Morinobu S., Ichimura M., Asakawa A., Inui A., Hosoda H., Kangawa K., Yamawaki S. Decreased levels of ghrelin, cortisol, and fasting blood sugar, but not n-octanoylated ghrelin, in Japanese schizophrenic inpatients treated with olanzapine. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2008;32:1527–1532. doi: 10.1016/j.pnpbp.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Togo T., Hasegawa K., Miura S., Hosojima H., Kojima K., Shoji M., Kase A., Uchikado H., Iseki E., Kosaka K. Serum ghrelin concentrations in patients receiving olanzapine or risperidone. Psychopharmacology (Berl) 2004;172:230–232. doi: 10.1007/s00213-003-1642-4. [DOI] [PubMed] [Google Scholar]
- Tschöp M., Weyer C., Tataranni P.A., Devanarayan V., Ravussin E., Heiman M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- Turan B., Siva Z.O., Uluduz D., Konukoglu D., Erenler F., Saip S., Goksan B., Siva A. The impact of depression and ghrelin on body weight in migraineurs. J. Headache Pain. 2014;15:1–7. doi: 10.1186/1129-2377-15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Andel M., van Schoor N.M., Korten N.C., Heijboer A.C., Drent M.L. Ghrelin, leptin and high-molecular-weight adiponectin in relation to depressive symptoms in older adults: results from the Longitudinal Aging Study Amsterdam. J. Affect. Disord. 2022;296:103–110. doi: 10.1016/j.jad.2021.09.069. [DOI] [PubMed] [Google Scholar]
- Van Kaer L. Glatiramer acetate for treatment of MS: regulatory B cells join the cast of players. Exp. Neurol. 2011;227:19–23. doi: 10.1016/j.expneurol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reedt Dortland A.K.B., Giltay E.J., Van Veen T., Zitman F.G., Penninx B.W.J.H. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr. Scand. 2010;122:30–39. doi: 10.1111/j.1600-0447.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- Volante M., Rosas R., Ceppi P., Rapa I., Cassoni P., Wiedenmann B., Settanni F., Granata R., Papotti M. Obestatin in human neuroendocrine tissues and tumours: expression and effect on tumour growth. J. Pathol. A J. Pathol. Soc. Gt. Britain Irel. 2009;218:458–466. doi: 10.1002/path.2551. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Fowler J.S., Tomasi D., Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Brain imaging Behav. Neurosci. 2011;1–24 doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Walker A.K. 2014. Investigating the Enteroendocrine-Brain Axis: Ghrelin Cell and ECL Cell Physiology and Ghrelin Action on Mood and Complex Eating. [Google Scholar]
- Wang L., Chen Q., Li G., Ke D. Ghrelin stimulates angiogenesis via GHSR1a-dependent MEK/ERK and PI3K/Akt signal pathways in rat cardiac microvascular endothelial cells. Peptides. 2012;33:92–100. doi: 10.1016/j.peptides.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Wang J.Q, Mao L. The ERK pathway: molecular mechanisms and treatment of depression. Mol. Neurobiol. 2019;56:6197–6205. doi: 10.1007/s12035-019-1524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C.J., Baghdoyan H.A., Lydic R. Neuropharmacology of sleep and wakefulness: 2012 update. Sleep Med. Clin. 2012;7:469–486. doi: 10.1016/j.jsmc.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmer P., Nogueiras R., Broglio F., D’alessio D., Tschöp M.H. Ghrelin, obesity and diabetes. Nat. Clin. Pract. Endocrinol. Metabol. 2007;3:705–712. doi: 10.1038/ncpendmet0625. [DOI] [PubMed] [Google Scholar]
- Wierup N., Yang S., McEvilly R.J., Mulder H., Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J. Histochem. Cytochem. 2004;52:301–310. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- Wittekind D.A., Kluge M. Ghrelin in psychiatric disorders–a review. Psychoneuroendocrinology. 2015;52:176–194. doi: 10.1016/j.psyneuen.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Wren A.M., Small C.J., Ward H.L., Murphy K.G., Dakin C.L., Taheri S., Kennedy A.R., Roberts G.H., Morgan D.G.A., Ghatei M.A. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Wysokiński A., Kowalski M.L., Kłoszewska I. Serum levels of desacyl ghrelin in patients with schizophrenia on clozapine monotherapy. Psychiatr. Clin. Neurosci. 2014;68:833–840. doi: 10.1111/pcn.12199. [DOI] [PubMed] [Google Scholar]
- Xu X., Sook Jhun B., Hoon Ha C., Jin Z.-G. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology. 2008;149:4183–4192. doi: 10.1210/en.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh A.H., Jeffery P.L., Duncan R.P., Herington A.C., Chopin L.K. Ghrelin and a novel preproghrelin isoform are highly expressed in prostate cancer and ghrelin activates mitogen-activated protein kinase in prostate cancer. Clin. Cancer Res. 2005;11:8295–8303. doi: 10.1158/1078-0432.CCR-05-0443. [DOI] [PubMed] [Google Scholar]
- Yin Y., Li Y., Zhang W. The growth hormone secretagogue receptor: its intracellular signaling and regulation. Int. J. Mol. Sci. 2014;15:4837–4855. doi: 10.3390/ijms15034837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten G.L.C., Samson W.K. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am. J. Physiol. Integr. Comp. Physiol. 2009;297:R330–R336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z.-B., Galaj E., Alén F., Wang B., Bi G.-H., Moore A.R., Buck T., Crissman M., Pari S., Xi Z.-X., Leggio L., Wise R.A., Gardner E.L. Involvement of the ghrelin system in the maintenance and reinstatement of cocaine-motivated behaviors: a role of adrenergic action at peripheral β1 receptors. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2021 doi: 10.1038/s41386-021-01249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallar L.J., Farokhnia M., Tunstall B.J., Vendruscolo L.F., Leggio L. The role of the ghrelin system in drug addiction. Int. Rev. Neurobiol. 2017;136:89–119. doi: 10.1016/bs.irn.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Zambrana-Infantes E., Rosell Del Valle C., Ladrón de Guevara-Miranda D., Galeano P., Castilla-Ortega E., Rodríguez De Fonseca F., Blanco E., Santín L.J. Palmitoylethanolamide attenuates cocaine-induced behavioral sensitization and conditioned place preference in mice. Pharmacol. Biochem. Behav. 2018;166:1–12. doi: 10.1016/j.pbb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Zhang J.V., Jahr H., Luo C.-W., Klein C., Van Kolen K., Ver Donck L., De A., Baart E., Li J., Moechars D. Obestatin induction of early-response gene expression in gastrointestinal and adipose tissues and the mediatory role of G protein-coupled receptor, GPR39. Mol. Endocrinol. 2008;22:1464–1475. doi: 10.1210/me.2007-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Yu G., Huang Y., Xu D., Ren J., Jiang L., Wu C., Tong D. Expression of ghrelin and GHSR-1a in mammary glands of dairy goat during the lactation and the effects of gherlin on regulation of mammary function in vitro. Mol. Cell. Endocrinol. 2013;370:20–31. doi: 10.1016/j.mce.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Zhao H., Liu G., Wang Q., Ding L., Cai H., Jiang H., Xin Z. Effect of ghrelin on human endothelial cells apoptosis induced by high glucose. Biochem. Biophys. Res. Commun. 2007;362:677–681. doi: 10.1016/j.bbrc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Liu H., Xiao K., Yu M., Cui L., Zhu Q., Zhao R., Li G.-D., Zhou Y. Ghrelin administration enhances neurogenesis but impairs spatial learning and memory in adult mice. Neuroscience. 2014;257:175–185. doi: 10.1016/j.neuroscience.2013.10.063. [DOI] [PubMed] [Google Scholar]
- Zigman J.M., Elmquist J.K. Minireview: from anorexia to obesity—the yin and yang of body weight control. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- Ziko I., Sominsky L., De Luca S.N., Lelngei F., Spencer S.J. Acylated ghrelin suppresses the cytokine response to lipopolysaccharide and does so independently of the hypothalamic-pituitary-adrenal axis. Brain Behav. Immun. 2018;74:86–95. doi: 10.1016/j.bbi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Zimmermann U.S., Buchmann A., Steffin B., Dieterle C., Uhr M. Clinical study: alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychosocial stress. Addiction Biol. 2007;12:17–21. doi: 10.1111/j.1369-1600.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Zubieta J.-K., Huguelet P., O'Neil R.L., Giordani B.J. Cognitive function in euthymic bipolar I disorder. Psychiatr. Res. 2001;102:9–20. doi: 10.1016/s0165-1781(01)00242-6. [DOI] [PubMed] [Google Scholar]