Abstract
Objectives
To evaluate the potential for long distance airborne transmission of SARS-CoV-2 in indoor community settings and to investigate factors that might influence transmission.
Design
Rapid systematic review and narrative synthesis.
Data sources
Medline, Embase, medRxiv, Arxiv, and WHO COVID-19 Research Database for studies published from 27 July 2020 to 19 January 2022; existing relevant rapid systematic review for studies published from 1 January 2020 to 27 July 2020; and citation analysis in Web of Science and Cocites.
Eligibility criteria for study selection
Observational studies reporting on transmission events in indoor community (non-healthcare) settings in which long distance airborne transmission of SARS-CoV-2 was the most likely route. Studies such as those of household transmission where the main transmission route was likely to be close contact or fomite transmission were excluded.
Data extraction and synthesis
Data extraction was done by one reviewer and independently checked by a second reviewer. Primary outcomes were SARS-CoV-2 infections through long distance airborne transmission (>2 m) and any modifying factors. Methodological quality of included studies was rated using the quality criteria checklist, and certainty of primary outcomes was determined using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework. Narrative synthesis was themed by setting.
Results
22 reports relating to 18 studies were identified (methodological quality was high in three, medium in five, and low in 10); all the studies were outbreak investigations. Long distance airborne transmission was likely to have occurred for some or all transmission events in 16 studies and was unclear in two studies (GRADE: very low certainty). In the 16 studies, one or more factors plausibly increased the likelihood of long distance airborne transmission, particularly insufficient air replacement (very low certainty), directional air flow (very low certainty), and activities associated with increased emission of aerosols, such as singing or speaking loudly (very low certainty). In 13 studies, the primary cases were reported as being asymptomatic, presymptomatic, or around symptom onset at the time of transmission. Although some of the included studies were well conducted outbreak investigations, they remain at risk of bias owing to study design and do not always provide the level of detail needed to fully assess transmission routes.
Conclusion
This rapid systematic review found evidence suggesting that long distance airborne transmission of SARS-CoV-2 might occur in indoor settings such as restaurants, workplaces, and venues for choirs, and identified factors such as insufficient air replacement that probably contributed to transmission. These results strengthen the need for mitigation measures in indoor settings, particularly the use of adequate ventilation.
Systematic review registration
PROSPERO CRD42021236762.
Introduction
Since the early stages of the covid-19 pandemic and the first reports of superspreader events,1 2 the body of evidence suggesting airborne transmission of SARS-CoV-2 in the absence of aerosol generating procedures has grown. However, despite the publication of numerous opinion pieces and narrative reviews in support of airborne transmission of SARS-CoV-2,3 4 5 6 7 8 9 scientific consensus on the relative importance of this route of transmission is lacking. Part of the controversy arises from differences in terminology, definitions, and size thresholds for respiratory particles.10
Traditionally, close contact transmission was assumed to occur through droplets with ballistic trajectory that directly deposit on mucous membranes, whereas airborne transmission was assumed to occur over longer distances via smaller particles (aerosols) that remained suspended in the air and were subsequently inhaled.10 11 Limitations of this dichotomy are well illustrated by the challenge in defining a size range to characterise particles that are droplets or aerosols.6 7 10 12 For example, the World Health Organization threshold is set at 5-10 microns13 whereas in the UK the threshold is based on the work by Milton14 and set to 100 microns.15 This is also complicated by the role of evaporation, as a particle will get smaller as it moves from human sources.
Regardless of terminology and definitions, it is now understood that short range transmission can occur through both droplets and aerosols and that the concentration of respiratory particles is higher at short range than over longer distances.7 11 16 17 Consensus is, however, still lacking on the risk for long distance airborne transmission in indoor settings in the community such as hospitality venues, leisure facilities, workplaces, or apartment blocks. This lack of consensus also reflects the challenging nature of the evidence base, and high quality review level evidence is still needed; some systematic reviews have relied on environmental sampling studies, which only provide indirect evidence of the potential risk of airborne transmission,18 19 20 whereas systematic reviews that have included a wider range of study designs (epidemiological, environmental, and modelling) and settings (healthcare and community) remain inconclusive.21 22 23 24
This gap needs to be addressed from a public health perspective, focusing on long distance transmission (>2 m) in indoor community settings. As evidence on the biological plausibility of long distance airborne transmission is available from environmental and experimental studies,18 21 22 we focused on epidemiological observational studies to assess where and when human-to-human transmission are likely to occur. In this rapid review we systematically identified and examined such studies to evaluate the potential for long distance airborne transmission of SARS-CoV-2 in indoor community settings and to assess the impact of potential modifying factors.
Methods
We used a rapid systematic review approach, following streamlined systematic methodologies to accelerate the review process,25 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines.26 The protocol for this review was registered on PROSPERO before screening took place.27
Data sources and searches
We identified primary studies through two sources. Firstly, we screened studies included in the rapid systematic review by Comber et al for those published from 1 January 2020 to 27 July 2020.21 This systematic review, assessed to be of moderate quality using the AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews, revised) critical appraisal tool,28 contains a comprehensive search strategy and wider inclusion criteria than the current rapid review (studies related to all airborne transmission of SARS-CoV-2) and was the only relevant review available at the time we wrote our protocol.
Secondly, we conducted electronic searches in Ovid Medline, Ovid Embase, medRxiv, Arxiv, and WHO COVID-19 Research Database for studies published from 27 July 2020 to 19 January 2022. The initial search was conducted on 8 February 2021 and last updated on 19 January 2022. The search strategy was drafted by an information scientist and peer reviewed by a second information scientist. Supplementary material 1 (section 1) shows the full search strategy.
Using the studies that met our inclusion criteria, we performed a citation analysis on 1 February 2022 on Web of Science and Cocites (co-citation analysis, forward and backwards snowballing). Although this was not part of the search strategy outlined in the protocol, it was agreed a posteriori by the review team to increase the chance of additional relevant studies being retrieved.
Eligibility criteria for study selection
Our eligibility criteria for study selection were published articles, accepted manuscripts, and preprints reporting on the potential for airborne transmission of SARS-CoV-2 in indoor community (non-healthcare) settings at a distance >2 m (the 2 m threshold is based on UK regulations; we also considered for inclusion non-UK studies that used thresholds based on their respective national recommendations, such as 1.5 m or 6 feet/1.8 m). The aim was to include all observational studies (outbreak investigations and epidemiological case series, cohort, case-control, and cross sectional studies) of any human population in non-healthcare settings. We excluded systematic or narrative reviews, guidelines, opinion pieces, intervention studies, modelling studies, environmental sampling studies without epidemiological investigation, laboratory or virology studies, and animal studies. We also excluded observational studies in which close contact or fomite were the most likely transmission routes (eg, studies reporting on transmission in households).
Screening was performed using Rayyan Systems, a freely available online screening tool.29 Two reviewers independently screened the first 10% of records retrieved from the initial search on title and abstract, with substantial agreement (97.7%; Cohen’s κ=0.61). A single reviewer screened the remainder, and two reviewers independently screened a further 10% (of the total number of records), with almost perfect agreement (99.6%; Cohen’s κ=0.92). All records selected were screened at full text by one reviewer and checked by a second reviewer, with any discrepancies resolved by discussion with a third reviewer.
Outcomes
The primary outcomes were SARS-CoV-2 infections through long distance airborne transmission (at a distance >2 m), and any factors that might have modified the risk of transmission under these conditions. Included measures for SARS-CoV-2 infections were number of covid-19 cases; secondary attack rates; risk, rate, or odds of transmission over the stipulated distances; or any other reported measure related to transmission rate. For the modifying factors, we considered narrative on the type of effect and any potentially relevant information to be acceptable.
Additional outcomes extracted, when available, were time spent in the setting and distance over which airborne transmission was thought to have occurred.
Data extraction and synthesis
We developed a data extraction table to gather information on methods, participants, settings, outcomes, key findings, and any additional relevant information (eg, whether participants wore face coverings). Data extraction was completed for each included study by one reviewer and independently checked by a second reviewer, with discrepancies resolved by discussion. Only evidence directly relevant to the review question was extracted. For example, if studies reported on different outbreaks or on onward transmission that might have happened in different settings, we only extracted the results of outbreaks or settings when distance and transmission routes could be assessed.
A narrative summary of results according to indoor setting was produced.
Quality assessment and certainty of evidence
We used a quality criteria checklist for primary research to assess the methodological quality of each included study.30 This checklist tool is composed of 10 questions, four of which are considered critical (questions on selection bias, group comparability, description of exposure/assessment of transmission routes, and validity of outcome measurements). Strict criteria were used to assess the two critical questions related to exposure and outcome assessment. In particular, a cluster of covid-19 cases in the setting of interest had to be confirmed with viral genomic sequencing to be considered as low risk of bias for validity of outcome measurements. Supplementary material 1 (section 2) lists the 10 questions of the quality criteria checklist.
A study was rated as high methodological quality if the answers were yes to the four critical questions plus at least one of the remaining questions. A study was rated as low methodological quality if answers were no to ≥50% of the critical questions. Otherwise, the study was rated as medium methodological quality. Each study was assessed independently in duplicate, with disagreements resolved by consensus.
Certainty of the evidence was assessed using a variation of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework for systematic reviews without meta-analysis.31 We assessed each of the five GRADE domains (methodological limitations of the studies, indirectness, imprecision, inconsistency, and likelihood of publication bias) and classified them as no limitation or not serious (not important enough to warrant downgrading), serious (downgrading the certainty rating by one level), or very serious (downgrading the certainty rating by two levels). We then classified the body of evidence for a specific outcome as high certainty, moderate certainty, low certainty, or very low certainty.
Patient and public involvement
Patients and members of the public were not involved in this rapid systematic review mainly because of time restrictions. The review question was, however, developed with the input of several public health experts and stakeholders.
Results
Study selection
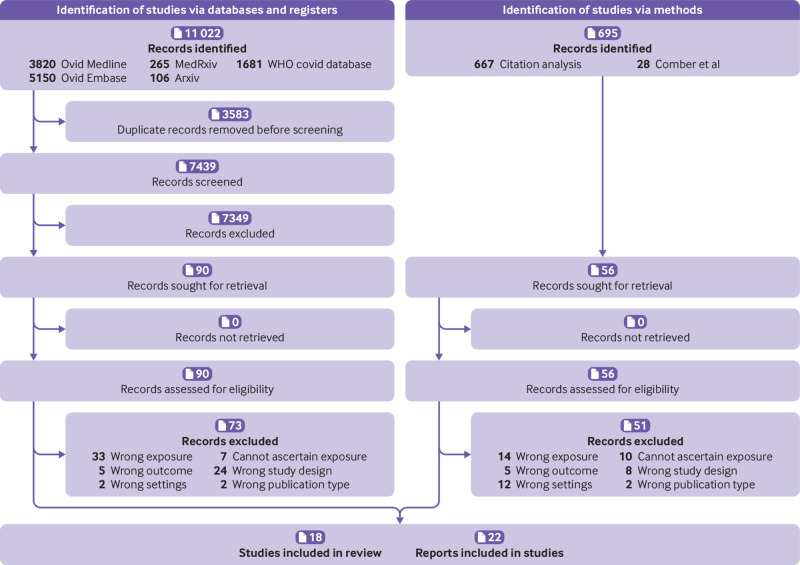
After removal of duplicates, 7439 records were screened for relevance on title and abstract, with 90 reports assessed for eligibility (fig 1). Fifty six additional reports identified from the Comber et al rapid review21 and by citation analysis were also assessed. From these 146 reports, 124 were excluded (see supplementary material 1 (section 3) for list of reasons for exclusion), and 22 reports1 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 relating to 18 studies were included. When two or more reports related to the same study, we considered the most comprehensive report as the main publication.
Fig 1.
Flow of articles through the review
All the studies investigated outbreaks of clusters of SARS-CoV-2 infections, and one study had an analytical component.36 Eight studies were conducted in Asia,34 35 36 37 38 39 40 45 five in Europe,41 43 44 47 48 three in Oceania,32 33 46 and two in the United States.1 42 Three studies reported on transmission between flats in apartment blocks,38 39 40 two in quarantine hotels,32 33 two in restaurants,34 35 two in buses,36 37 one in a food processing factory,41 one in a courtroom,43 one in an office,44 one in a fitness facility,42 one in a department store,45 and four during singing events.1 46 47 48 All the outbreaks occurred in 2020, except for one in January 2021 in South Korea40 (before vaccine rollout started in this country) and one in July 2021 in a quarantine hotel in New Zealand.33
Table 1 and table 2 summarise the studies by setting. Supplementary material 2 provides detailed information on each study.
Table 1.
Summary of included studies, in chronological order by setting: quarantine hotels, restaurants, buses, and apartment blocks
| Reference (quality rating) | Transmission event, setting, date | No of cases | Outcome and exposure assessment | Potential for other transmission routes | Potential for airborne transmission >2 m* | Modifying factors |
|---|---|---|---|---|---|---|
| Li et al,34 Lu et al,49 Zhang et al50 (medium) | Restaurant, China, January 2020 | Ten confirmed cases from three tables | No genomic sequencing. Epidemiological data, video recording, on-site visit, design of air conditioning and ventilation system, experiments to assess airflow and ventilation rates | Close contact or fomite transmission unlikely (except for cases in same household). Transmission from outside event possible for some cases | Possible airborne transmission between primary case and at least two secondary cases; up to 1.4 m (53 min) and 4.6 m (75 min) from primary case | Insufficient air replacement. Directional air flow through air circulation units |
| Shen et al36 (medium) | Buses, China, January 2020 | Twenty four confirmed cases | No genomic sequencing. Questionnaires and interviews, contact tracing data, bus design, and ventilation system | Close contact, fomite transmission, or transmission from outside event possible for some cases | Possible airborne transmission >2 m from primary case (50 min) | Insufficient air replacement. Directional air flow from central heating system |
| Luo et al,37 Ou et al51 (low) | Buses, China, January 2020 | Nine confirmed cases | No genomic sequencing. Epidemiological data, information on loading and unloading stops of all passengers, and seating positions, ventilation systems, tracer gas experiments | Close contact unlikely. Fomite transmission or transmission from outside event possible for some cases | Possible airborne transmission >2 m for some cases (1 hour to 2.5 hours) | Insufficient air replacement. Directional air flow due to exhaust system |
| Lin et al38 (low) | Apartment block, China, January 2020 | Nine confirmed cases from three households | No whole genome sequencing (partial S gene only). Interviews with cases, CCTV of lift, tracer gas and wind speed experiments | Close contact or fomite transmission unlikely (except for cases in same household); transmission from outside event possible for some cases | Possible airborne transmission between cases in one flat to two different flats (up to 10 floors from flat of primary case) | Insufficient air replacement. Directional air flow through drainage and exhaust system |
| Kwon et al35 (high) | Restaurant, South Korea, June 2020 | Three confirmed cases | Genomic sequencing. Contact tracing, interviews, credit card records, video recording, mobile phone location data, on-site visits, air flow measurement, environmental sampling | Close contact, fomite transmission, or transmission from outside event unlikely | Possible airborne transmission between cases seated 4.8 m (21 min) and 6.5 m (5 min) from the primary case | Insufficient air replacement. Directional air flow through air circulation units |
| Hwang et al39 (low) | Apartment block, South Korea, August 2020 | Ten confirmed cases from seven households | No genomic sequencing. Epidemiological data, surface sampling, building assessment | Close contact or fomite transmission unlikely (except for cases in same household). Transmission from outside event possible | Possible airborne transmission through ventilation ducts across floors for some secondary cases | Directional air flow through vertical air duct or floor drain. Insufficient air replacement (unclear) |
| Eichler et al32 (medium) | Quarantine hotel, New Zealand, August-September 2020 | Nine confirmed cases, with one secondary case considered for long distance transmission | Genomic sequencing. Epidemiological data, surveillance video, review of ventilation system in hotel | Close contact or fomite transmission unclear. Transmission from outside event unlikely | Possible airborne transmission from hotel room of the primary case to doorway or corridor for one secondary case | Insufficient air replacement. Directional air flow |
| Han et al40 (low) | Apartment block, South Korea, January 2021 | Five secondary cases (three households) considered for long distance transmission | Genomic sequencing. Epidemiological data, interviews, mobile phone location tracking, surface sampling | Close contact or fomite transmission unlikely (except for cases in same household). Transmission from outside event unlikely | Possible airborne transmission through floor drains across three floors for two secondary cases | Insufficient air replacement. Directional air flow through vertical floor drain |
| Fox-Lewis et al33(high) | Quarantine hotel, New Zealand, July 2021 | Five confirmed cases in two rooms | Genomic sequencing. Epidemiological data, surveillance video, review of ventilation system in hotel | Close contact, fomite transmission, or transmission from outside of event unlikely | Possible airborne transmission from hotel room of primary case to hotel room for at least one secondary case (2.1 m) | Insufficient air replacement. Directional air flow |
This review’s assessment of likelihood of airborne transmission of SARS-CoV-2 over distances >2 m is based on likelihood of it occurring in some, but not necessarily all, transmission events.
Exposure distance and time are stated when known; if not stated they are categorised as not clear or not specified.
Table 2.
Summary of included studies, in chronological order by setting: department store, singing events, meat processing plant, fitness facility, courtroom, and office
| Reference (quality rating) | Transmission event, setting, date | No of cases | Outcome and exposure assessment | Potential for other transmission routes | Potential for airborne transmission >2 m* | Modifying factors |
|---|---|---|---|---|---|---|
| Jiang et al45 (low) | Department store, China, January 2020 | Twenty four cases, with 12 secondary cases considered for long distance transmission | No genomic sequencing. Epidemiological data, surveillance video, assessment of ventilation conditions | Close contact, fomite transmission, or transmission from outside event all possible | Unclear airborne transmission across different sections of the store | Not applicable |
| Hamner et al,1 Miller et al52 (low) | Singing event, USA, March 2020 | Fifty two: 32 confirmed cases, 20 probable cases | No genomic sequencing. Telephone interviews | Close contact or transmission from outside event possible for some cases. Fomite transmission unlikely | Possible airborne transmission >2 m for some cases, owing to high secondary attack rate (2.5 hours) | Insufficient air replacement. Increased aerosol emission—singing |
| Charlotte et al48 (low) | Singing event, France, March 2020 | Nineteen: seven confirmed cases, 12 probable cases | No genomic sequencing. Questionnaire and telephone interviews | Close contact possible for some cases. Fomite transmission unlikely. Transmission from outside event possible for at least two cases | Possible airborne transmission >2 m for some cases, owing to high secondary attack rate (2 hours) | Insufficient air replacement. Increased aerosol emission—singing |
| Gunther et al41 (medium) | Meat processing plant, Germany, May-June 2020 | Thirty one confirmed cases | Genomic sequencing. On-site visit (work condition and ventilation system) and information provided by employer on housing, commuting, and workplaces of employees | Close contact and fomite transmission possible for some cases. Transmission from outside event unlikely | Possible airborne transmission for some cases on the production line, up to 12 m from the primary case | Insufficient air replacement. Directional air flow from air circulation system. Increased aerosol emission—physical work (unclear) |
| Groves et al42 (low) | Fitness facility, USA, June 2020 | Twenty one confirmed cases, with10 secondary cases considered for long distance transmission | No genomic sequencing. Questionnaire and on-site assessment | Close contact possible for some cases. Fomite transmission unclear. Transmission from outside event unlikely | Possible airborne transmission >2 m for some cases, owing to high secondary attack rate (1 hour) | Insufficient air replacement. Directional air flow from air fan. Increased aerosol emission—shouting |
| Katelaris et al46 (high) | Singing event, Australia, July 2020 | Thirteen confirmed cases | Genomic sequencing. Interviews with cases, video recording, on-site visit (ventilation system) | Close contact or fomite transmission unlikely (except for five cases in same household). Transmission from outside event unlikely | Possible airborne transmission with secondary cases seated 1-15 m from the primary case (1 hour) | Insufficient air replacement. Increased aerosol emission—singing |
| Vernez et43 (low) | Courtroom, Switzerland, September 2020 | Five confirmed cases | No genomic sequencing. Court records, contact tracing data, and field measurements | Close contact cannot be ruled out, especially for the two secondary cases at 1.5 m from the primary case. Fomite transmission unlikely. Transmission from outside event likely for one secondary case | Possible long distance airborne transmission for three secondary cases (1.5-3 m; 3 hours) | Insufficient air replacement |
| Shah et al47 (medium) | Five singing events, Netherlands, September-October 2020 | Fifty: 48 confirmed cases and two probable cases | Genomic sequencing for seven cases. Phone and email correspondence, questionnaires, epidemiological data, aerosol transmission model | Close contact possible for some cases. Fomite transmission unlikely (except for one event). Transmission from outside event possible for some cases, but unlikely in others | Possible airborne transmission >1.5 m for some cases (1 hour to 2.5 hours) | Increased aerosol emission—singing. Directional air flow (unclear). Insufficient air replacement (unclear) |
| Sarti et al44 (low) | Office, Italy, November 2020 | Five confirmed cases | No genomic sequencing. Telephone interviews | Close contact, fomite transmission, or transmission from outside event possible | Unclear airborne transmission between coworkers | Not applicable |
The review’s assessment of likelihood of airborne transmission of SARS-CoV-2 over distances >2 m is based on likelihood of it occurring in some, but not necessarily all, transmission events.
Exposure distance and time are stated when known; if not stated they are categorised as not clear or not specified.
Quality assessment
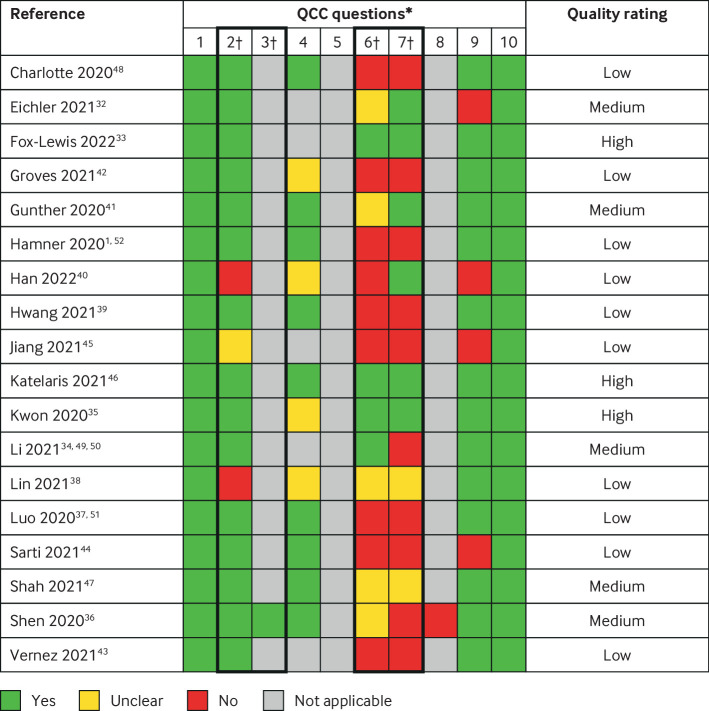
Figure 2 provides details of the methodological quality ratings: three studies were rated as high quality,33 35 46 five as medium quality,32 34 36 41 47 and 10 as low quality.1 37 38 39 40 42 43 44 45 48 These ratings represent the methodological quality of descriptive studies.
Fig 2.
Quality assessment. *Assessments using quality criteria checklist (QCC) for primary research (see supplementary material 1). †Critical questions: 2 on selection bias, 3 on group comparability, 6 on description of exposure/assessment of transmission routes, and 7 on validity of outcome measurements
Transmission settings
Quarantine hotels
Two outbreaks of covid-19 in quarantine hotels were identified, both in New Zealand and involving cases part of the same genomic cluster who had quarantined in separate rooms. The first outbreak, reported by Eichler et al32 (rated as medium methodological quality) occurred in September 2020, and although primary and secondary cases had travelled on the same flight, transmission is believed to have happened in the hotel on day 12 of quarantine, after the primary case had developed symptoms on day 10. No information was provided on the measures in place at this quarantine hotel (eg, use of face coverings). The second outbreak, reported by Fox-Lewis et al33 (rated as high methodological quality) occurred in July 2021. The primary case (asymptomatic) and secondary cases had travelled on different flights and arrived at the hotel on different days. Staff members, all vaccinated, wore full personal protective equipment and were regularly tested. Participants were asked to wear surgical masks when opening doors, but this could not be validated in the investigation. None of the cases (primary or secondary) were vaccinated; the only person who was vaccinated tested negative despite being part of the same travel group as the secondary cases.
Close contact and fomite transmission were ruled out by video analysis in both studies, although in the outbreak reported by Eichler et al32 fomite transmission through a communal bin—although unlikely, cannot be ruled out. Video analysis showed that in both outbreaks the doors of the rooms had been opened simultaneously for a short period during which infected respiratory particles could have moved between rooms. Both investigations included a review of the ventilation systems and found that pressure differences between rooms and corridors could support this hypothesis. Long distance airborne transmission between a primary case and at least one secondary case was therefore considered to be the most likely route in both outbreaks.
Restaurants
Two separate outbreaks of covid-19 in restaurants were identified. The first outbreak, in China in January 2020, was mainly reported by Li et al34 (rated as medium methodological quality), with additional evidence provided in two other reports.49 50 This outbreak involved a primary case (with symptom onset later that day) and at least two secondary cases who were seated on tables between 1.4 and 4.6 m away from the primary case. The second outbreak, reported by Kwon et al35 (rated as high methodological quality), occurred in June 2020 in South Korea and involved three people with confirmed covid-19 who belonged to the same genomic cluster. The primary case, which was presymptomatic at the time, sat 6.5 m from one secondary case for five minutes, and 4.8 m from the other secondary cases for 21 minutes, all at different tables.
After extensive epidemiological and environmental investigations, both studies suggested that the most plausible route was long distance airborne transmission, which could have been facilitated by air circulation units generating a directional air flow from the primary to secondary cases combined with lack of air replacement. In both outbreaks close contact and fomite transmission were ruled out based on video surveillance analysis.
Buses and coaches
Two separate outbreaks of covid-19 on buses in China in January 2020 were identified, one on a journey to and from a worship event among lay Buddhists36 and one on a long distance journey using a public coach and minibus.37 51
The outbreak at a worship event was reported by Shen et al36 who conducted a retrospective epidemiological investigation with an analytical component (rated as medium methodological quality). Thirty one of the 300 participants tested positive for SARS-CoV-2 of whom seven were likely to have been infected by close contact transmission during the religious event. The other 23 cases had travelled to the event in the same bus as the primary case and were thought to have been mainly infected during the bus journey, throughout which no one wore face coverings. Those travelling on the bus with the primary case were 11 times more likely to develop covid-19 compared with the other participants (relative risk 11.4, 95% confidence interval 5.1 to 25.4; P<0.01) and 42 times more likely compared with those travelling in the other bus (42.2, 2.6 to 679.3; P<0.01). Close contact transmission, fomite transmission, and transmission from outside the event cannot be ruled out for some of the cases but are unlikely to have accounted for all 23 secondary cases.
The second outbreak, reported by Luo et al37 (rated as low methodological quality) with additional environmental investigations conducted by Ou et al,51 involved one primary case (symptom onset occurred on the day of the event) who had travelled without wearing a face covering on a coach for 2.5 hours with 48 other individuals and then on a minibus for one hour with 12 other individuals. Nine secondary cases were identified, resulting in a secondary attack rate of 15% (95% confidence interval 6% to 24%), with most seated >2 m from the primary case: up to 4.5 m based on one report37 and up to 9.5 m based on the other report.51 Genomic sequencing was not performed and, based on symptom onset dates, it is plausible that more than one primary case was present, reducing our confidence in the distances reported. However, even taking into account all potential primary cases, it is possible that airborne transmission occurred for some secondary cases seated >2 m from a primary case. Some passengers wore face coverings, but none of the secondary cases did.
In both outbreaks, insufficient air replacement and directional airflow from the heating system were hypothesised as promoting long distance airborne transmission, supported by tracer gas experiments in the buses involved in one of the outbreaks.51
Apartment blocks
Three outbreaks of covid-19 in three separate residential apartment blocks were identified. The study by Lin et al38 (rated as low methodological quality) investigated an outbreak involving nine people who tested positive for SARS-CoV-2 in three flats of a 29 storey apartment block in China. The nine cases, identified between 27 January and 13 February 2020, lived in flats that shared drain and sewer pipes connected via an exhaust pipe to the roof. Except for cases in the same household, close contact and fomite transmission were ruled out based on interviews with the cases and partial video analysis (lift only). Some but not all of the cases reported wearing face coverings in the communal areas of the building.
The two other outbreaks were in South Korea. The first, reported by Hwang et al39 (rated as low methodological quality), occurred in August 2020 in an apartment block of 267 flats and involved 10 cases from seven households located around two ventilation ducts (eight cases around one, two around another). The second outbreak, reported by Han et al40 (rated as low methodological quality), occurred in January 2021 in a complex of 260 flats, in which cases located in three flats along the same drainpipe and ventilation duct could not be explained by close contact or fomite transmission. For both outbreaks, transmission routes were mainly investigated through interviews with cases, and therefore recall bias (no video analysis) was possible. All cases reported wearing face coverings in the communal areas of the buildings.
For all three outbreaks, long distance airborne transmission between flats through vertical air ducts or floor drains was deemed possible for at least some of the secondary cases, although environmental investigation (tracer gas experiment) to support this hypothesis was conducted in only one38 of the three studies. In two of the three studies,38 40 the ventilation ducts were found to be malfunctioning, which could have contributed to transmission risk. However, only one of these studies39 tested all residents and only one conducted whole genome sequencing,40 which reduces confidence in the results.
Other indoor settings
The other outbreaks identified in this review occurred in a food processing factory,41 fitness facility,42 courtroom,43 office,44 and department store.45
Gunther et al41 (rated as medium methodological quality) reported on an outbreak in a meat processing plant in Germany in May and June 2020 in which 31 out of the 140 workers on the same shift had tested positive for SARS-CoV-2 and were part of the same genomic cluster. Although close contact or fomite transmission in other areas of the processing plant and outside the factory (some workers shared accommodation and carpools) was possible for some cases, the spatial distribution of the cases suggested that transmission was likely to have occurred on the processing line at distances up to 12 m from the primary case who was asymptomatic. The authors hypothesised that factors such as increased respiratory rates (from physically demanding work), lack of air replacement, and continuous recirculation of cooled unfiltered air might have promoted long distance airborne transmission, but these were not investigated further. Some covid-19 measures were in place, including increased distance between workers and use of single layer face coverings, but adherence was not assessed as part of the study.
Groves et al42 (rated as low methodological quality) reported on an outbreak involving two fitness instructors at classes taught in three different facilities in June and July 2020, although the investigation suggested that close contact and fomite transmission were likely to have occurred in all classes but one. The class in which long distance airborne transmission might have happened was a one hour static cycling class in which bikes were placed at least 1.8 m apart, with doors and windows closed and three large fans directed towards the class participants. The instructor, who had shouted instructions while facing the participants, was identified as being the primary case (with symptom onset the next day) and all 10 class participants had tested positive for SARS-CoV-2 three to six days after the class. Face coverings had not been used during the class.
In an outbreak in a courtroom in Switzerland reported by Vernez et al43 (study rated as low methodological quality), five out of the 10 participants at a three hour hearing held on the 30 September 2020 tested positive for SARS-CoV-2. The use of face coverings was mandatory in the building, but not when seated, and social distancing measures were in place, with a minimum of 1.5 m between each seat. Long distance airborne transmission (1.5-3 m) was likely to have happened between a primary case (with symptom onset on that day) and three secondary cases, although close contact or fomite transmission after the hearing or in the bathroom cannot be ruled out. The hypothesis that a lack of air replacement (doors and windows were closed and there was no mechanical ventilation) might have promoted long distance airborne transmission was supported by field measurements and modelling.
Sarti et al44 (rated as low methodological quality) reported on an outbreak in an office in Italy in which five of six coworkers were identified as cases. One of the five coworkers was identified as the primary case, and transmission happened before symptom onset. The sixth coworker, who was not infected, was not present in the office for the two days before symptom onset of the primary case. This transmission event happened in November and December 2020 when mitigation measures were in place, including social distancing, acrylic panels between desks, hand hygiene, and use of a face covering except when seated at a desk. The office was not well ventilated (no air conditioning and windows were closed), which could have promoted long distance airborne transmission. On the basis of the investigation, however, close contact, fomite transmission, and transmission from outside the event cannot be ruled out, so it is unclear as to whether long distance airborne transmission was the most likely route.
Jiang et al45 (rated as low methodological quality) reported on an outbreak linked to a department store that occurred in January 2020 in Tianjin, China, involving 24 cases (six staff and 18 customers). Airborne transmission was considered as the most likely route of transmission between a primary case and 12 secondary cases, which might have been promoted by a lack of air replacement (doors were closed) and high density of people in the store. As genomic sequencing of SARS-CoV-2 was not performed, however, transmission from outside this event cannot be ruled out and, based on symptom onset dates, it is possible that several primary cases were present. On the basis of this investigation, it is unclear whether long distance airborne transmission had occurred in the store.
Singing events
In addition to transmission events associated with specific settings, four epidemiological investigations reporting on outbreaks linked to singing events were identified.
Katelaris et al46 (rated as high methodological quality) reported on an outbreak in Sydney, Australia, linked to a series of four church services held between 15 and 17 July 2020. The probable primary case, a choir member, had sung at each of these one hour services, and 12 secondary cases were identified (2.4% secondary attack rate across the four services), who had sat in the same section of the church, between 1 m and 15 m from the primary case. Viral genomic sequencing of the primary case and 10 secondary cases showed a single genomic cluster, suggesting that transmission had occurred during the church services.
The second epidemiological investigation47 (rated as medium methodological quality; preprint) reported on five singing events held between September and October 2020 in the Netherlands. At the time, national recommendations were in place to reduce covid-19 transmission, and although singing in groups was allowed, physical distancing (>1.5 m) and ventilation were recommended. Each singing event had between nine and 21 attendees, and attack rates of between 53% and 74% were observed. Fomite transmission was deemed unlikely in all but one event, but close contact transmission was considered possible for some of the secondary cases in three of the five events. However, owing to the high secondary attack rates, it is possible that at least some of the secondary cases had been infected via long distance airborne transmission and, even though ventilation through open doors or windows was reported for all events, air exchange rates were likely to have been low in at least three of the five events.
The two other outbreaks occurred in March 2020—that is, during the early stage of the pandemic when no mitigation measures were in place. One of them (70% attack rate, including probable cases) happened in France during a two hour choral rehearsal in a narrow, indoor, non-ventilated space48 (study rated as low methodological quality). The second outbreak (87% secondary attack rate, including probable cases) after a 2.5 hour choral rehearsal on 10 March 2020 in Washington (USA) was initially reported by Hamner et al1 (rated as low methodological quality) and further discussed by Miller et al.52 For both outbreaks, close contact and fomite transmission were only assessed through interviews and cannot be fully ruled out. The high secondary attack rate, however, suggests that long distance airborne transmission might have occurred for at least some of the cases.
The results from the four studies suggest that long distance airborne transmission was likely to have occurred for at least some of the transmission events, and that singing may have increased the amount of aerosol generated by the primary cases, which is consistent with modelling results reported by some of these authors.52 53
Summary and critical analysis of results
Seven of the outbreaks identified1 34 36 37 38 45 48 occurred in the early stage of the pandemic (January-March 2020) when knowledge of covid-19 was limited, especially the incubation period and the extent of asymptomatic or presymptomatic transmission. As a result, most of these studies only conducted symptomatic testing and considered potential secondary cases to be participants with symptom onset soon after the potential exposure event, including the next day. In addition, for the studies conducted in January 2020 in China and in March 2020 in Europe or the US, it is possible that community transmission was higher than perceived at the time.
Therefore, in an outbreak such as the one reported by Luo et al37 where no genomic sequencing was conducted and three of the nine secondary cases developed symptoms or tested positive for SARS-CoV-2 one or two days after exposure, it is plausible that more than one primary case was present and that transmission occurred through means other than long distance airborne transmission. In two of the studies reporting on singing events,1 48 genomic sequencing and asymptomatic testing were not carried out and some of the secondary cases developed symptoms in the days after exposure but because of the high attack rates reported for these outbreaks, it is possible that long distance airborne transmission had happened for at least some of the transmission events. Long distance airborne transmission was also considered possible for two other early studies as a result of detailed epidemiological investigations.34 36 However, the plausibility of long distance airborne transmission for the outbreak in the department store was unclear as other transmission routes could not be ruled out.45
Among the other studies, four33 35 41 46 provided convincing evidence for long distance airborne transmission as a result of detailed epidemiological investigations combined with genomic sequencing. Eichler et al32 also conducted genomic sequencing but their reporting of the epidemiological investigation was not sufficiently exhaustive to exclude other transmission routes (close contact or fomite) for the only secondary cases who could have been infected by long distance airborne transmission. The investigations by Shah et al,47 Hwang et al,39 Groves et al,42 Han et al,40 and Vernez et al43 suggested that long distance airborne transmission was possible for at least some of the transmission events (close contact or fomite could not be fully ruled out), but stronger conclusions could not be drawn owing to methodological limitations (including the absence of genomic sequencing and risk of selection bias). Finally, the likelihood of long distance airborne transmission was unclear in the outbreak in the office reported by Sarti et al44 as, despite the covid-19 measures in place, close contact and fomite transmission could not be completely ruled out on the basis of the investigation.
Eleven of the 18 studies reported on the use of face coverings.33 35 36 37 38 39 40 41 42 43 44 Overall, the information provided was limited, and two of these studies only mentioned that face coverings were compulsory in the settings of interest (quarantine hotel33 and food processing factory41) without reporting on adherence or behaviour (eg, whether workers wore face coverings correctly for the duration of their shift). Based on this limited information, we found no evidence of long distance airborne transmission where participants were known to have worn face coverings for the duration of exposure.
Only one of the outbreaks33 identified occurred at a time when covid-19 vaccines were available, although in this outbreak the primary and secondary cases were not vaccinated.
Grading of the evidence
Table 3 provides the grading of the evidence for each of the primary outcomes: SARS-CoV-2 infection via airborne transmission at a distance >2 m, insufficient air replacement (modifying factor), directional air flow (modifying factor), and increased aerosol emission when singing, speaking loudly, or doing intense physical work (modifying factor). Assessment of modifying factors was considered not applicable for the two outbreaks where the likelihood of long distance airborne transmission had been judged as unclear.
Table 3.
Summary of findings using Grading of Recommendations, Assessment, Development, and Evaluation approach
| Outcome | Effect | No of studies | Certainty in the evidence |
|---|---|---|---|
| SARS-CoV-2 infection through airborne transmission over a distance >2 m | Sixteen studies suggested that long distance airborne transmission was the main transmission route for at least some of the transmission events in the reported outbreaks. Unclear in two studies | 18 | Very low owing to methodological limitations of the studies and serious risk of imprecision and publication bias |
| Modifying factor: insufficient air replacement | Fourteen studies suggested that insufficient air replacement had increased the likelihood of long distance airborne transmission. Unclear in two studies | 16 | Very low owing to methodological limitations of the studies and serious risk of imprecision |
| Modifying factor: directional air flow | Eleven studies suggested that directional air flow might have increased the likelihood of long distance airborne transmission. Unclear in one study | 12 | Very low owing to methodological limitations of the studies and serious risk of imprecision |
| Modifying factor: activities associated with increased emission of aerosols | Five studies (reporting on nine events) suggested that singing and speaking loudly might have increased the likelihood of long distance airborne transmission. Unclear in one study (intense physical work) | 6 | Very low owing to methodological limitations of the studies and serious risk of imprecision and publication bias |
For all four outcomes, the evidence was judged as having methodological limitations owing to study design and to be at serious risk of imprecision owing to small numbers of participants as well as some risk of bias in exposure or outcome assessment, or both. However, the risks of inconsistency and indirectness were judged as not serious as the results were consistent across studies conducted in a range of settings and with different populations and provide evidence of direct relevance to the public health question of interest. The risk of publication bias was judged to be serious for the outcome of SARS-CoV-2 infection through airborne transmission at a distance >2 m and for the modifying factor of activities associated with increased emission of aerosols, but not serious for the modifying factors of insufficient air replacement and directional air flow. As a result, the certainty of evidence was judged as very low for all outcomes.
Because of high heterogeneity between studies, the additional outcomes of time spent in the transmission setting and distance over which airborne transmission was thought to have occurred could not be summarised or graded using the GRADE framework. Exposure timings ranged from five minutes to three hours, and distances were up to 15 m.
Discussion
Evidence from the outbreak investigations discussed in this review suggests that airborne transmission of SARS-CoV-2 from an infectious individual to others located >2 m away can occur in different indoor non-healthcare settings. The results of this review show that when long distance transmission occurred, one or more factors were thought to have contributed. Modifying factors such as insufficient air replacement and singing are likely to result in an increased concentration of infectious respiratory particles within the indoor space, whereas factors such as directional air flow are likely to allow viable virus to travel further in a certain direction, which could potentially infect someone downstream of a primary case. The results of this review therefore confirm the importance of the role of ventilation to mitigate the risk of long distance aerosol transmission.54 55 56 57
A total of eight events (from four studies) in which singing may have contributed to long distance airborne transmission were identified.1 46 47 48 These results are in line with experimental and modelling studies that have reported on singing and aerosol generation, suggesting that more virus-containing respiratory particles tend to be emitted when singing compared with speaking or breathing.53 58 More generally, the quantity of respiratory particles emitted increases with loudness of vocalisation,59 60 which was thought to have contributed to long distance aerosol transmission in a fitness facility.42
In 13 out of 18 studies identified,33 34 35 36 37 41 42 43 44 45 46 47 48 suspected primary cases were asymptomatic, presymptomatic, or near the time of symptom onset when transmission occurred. This finding is consistent with wider evidence that people with asymptomatic or presymptomatic SARS-CoV-2 infection can contribute to the community spread of covid-19,61 62 63 including from long distance airborne transmission.
Although the evidence on face coverings was limited, no outbreaks in which participants had been wearing face coverings for the duration of the exposure were identified. Evidence suggests that face coverings can reduce the number of respiratory particles emitted from the nose and mouth.64 However, it is not possible to deduce from the evidence assessed in this review if wearing a face covering can prevent or reduce the risk of long distance transmission of SARS-CoV-2.
Most of the outbreaks we identified occurred at a time when population immunity was limited, either naturally acquired or vaccine mediated. This limits the applicability of our findings to the current context, although there is evidence that transmission of SARS-CoV-2 after vaccination does occur.65 While the lack of evidence identified in vaccinated populations may to some extent reflect the successes of vaccine rollout, there may also be a time lag in publication of outbreak reports since vaccine programmes were initiated. There may also be less interest in publishing reports on SARS-CoV-2 associated outbreaks over time.
The evidence from our rapid systematic review was deemed to be of very low certainty based on 18 studies. The relatively small number of studies identified could suggest that outbreaks related to long distance airborne transmission are rare, although also likely to result from difficulties in identifying such events or to under-reporting—for example, in countries without sufficient contact tracing. It can also be partly explained by the level of detail needed to assess transmission routes. Indeed, even outbreak investigations that follow reporting guidelines such as the Outbreak Reports and Intervention studies Of Nosocomial infection (ORION) statement published by the Canada Communicable Disease Report66 are not necessarily thorough enough to be able to fully rule out other transmission routes. As a result, several outbreaks in which long distance airborne transmission may have happened were excluded on full text, including a few reports on clusters in aeroplanes that did not properly consider transmission routes during boarding and disembarking.67 68 69 70 Finally, the wider challenges of the pandemic should be acknowledged, including the limited resources in public health teams to conduct detailed epidemiological investigations.
The outcomes were rated as being of very low certainty using the GRADE framework, although this reflects the principles of GRADE rather than a lack of quality of the included studies because in traditional evidence hierarchies, outbreak investigations are classed as a low level of evidence. However, some of the included studies were well conducted investigations of covid-19 outbreaks and their contribution to this particular research question should not be underestimated—they provide critical insight where other types of study are just not feasible.71 The GRADE framework was developed to inform clinical practice where randomised controlled trials are feasible, and linear causal pathways are more often the norm. Public health research does not always fit easily within this framework and the question of what level of public health evidence is sufficient to support decision making for a novel infection warrants further consideration.
Comparison with other studies
These findings are an important addition to the wider body of evidence that supports the biological plausibility of airborne transmission as a potentially important route of transmission in certain scenarios. The wider evidence includes experimental evidence from animal studies72 as well as experimental studies that have shown that SARS-CoV-2 can remain viable in artificially generated aerosols for up to 16 hours, and that the stability and viability depends on environmental factors such as temperature, humidity, and exposure to sunlight.22 Similarly, biological monitoring studies have shown that SARS-CoV-2 RNA can be detected in exhaled breath and environmental air samples, but the evidence on viable virus remains limited to a few studies that mostly detected infectious virus in air samples collected at <2 m from the infectious individual.22 23 24 These experimental and biological studies provide evidence that SARS-CoV-2 can be viable in aerosols and therefore support the epidemiological evidence from this rapid review, and from others22 23 that suggest that airborne transmission can happen in some settings.
Strengths and limitations of this review
This rapid systematic review critically assessed the likelihood of long distance airborne transmission of SARS-CoV-2 using only direct real world evidence from observational studies from indoor non-healthcare settings. The application of inclusion criteria that focused the critical appraisal on those studies, which involved comprehensive investigations, is a key strength of our approach: some of these studies not only included epidemiological data, but also genomic analysis, video surveillance, analysis of seating arrangements, and environmental hypothesis testing.
The main limitation of selecting studies of only real world human-to-human transmission events is that scenarios where transmission has not occurred were not included, and likewise where transmission events have not been detected by contact tracing systems, which could be seen as a form of publication bias. All the evidence is from retrospective epidemiological investigations of outbreaks and therefore this review cannot make inferences on the extent to which long distance airborne transmission occurs or the contribution it may have on community rates of transmission: these remain critical questions for policy and practice. In addition, most of the outbreaks occurred before vaccine rollouts and it is unclear how these results apply to populations with a high level of immunity to infection. Finally, and as with all reviews assessing evidence related to covid-19, this rapid review is limited by the fact that the evidence assessed is from an emerging specialty.
Future work and policy implications
Well conducted outbreak investigations continue to be needed to assess the potential for long distance airborne transmission in vaccinated populations, and with more transmissible SARS-CoV-2 variants such as omicron. To assess transmission routes, such outbreak investigations should deploy robust and mixed methods, ranging from genomic analysis to environmental assessment, and they should be conducted as early as possible to reduce recall bias.
The results from this rapid systematic review highlight the need to ensure measures to mitigate SARS-CoV-2 long distance transmission in indoor settings, especially in poorly ventilated spaces. Identification of poorly ventilated public spaces should be undertaken and improvements made. Other factors such as directional air flow or singing that could increase the risk for long distance airborne transmission should also be considered in risk mitigation.
A need also exists to develop a new framework, or to adapt the existing GRADE framework, to support a pragmatic and consistent approach to the collation, interpretation, and synthesis of epidemiological investigations, especially when other types of studies are not feasible. The question of what level of public health evidence is sufficient to support decision making for a novel infection warrants further consideration.
Conclusion
This rapid review found evidence suggesting that long distance (>2 m) airborne transmission of SARS-CoV-2 might happen in indoor non-healthcare settings, and that it can occur from people who are asymptomatic or presymptomatic. All transmission events identified occurred alongside factors believed to have contributed to this type of transmission, including lack of air replacement (absence or little ventilation with fresh air), directional air flow (mainly through air circulation systems), and activities such as singing that increased aerosol emission. In the review, we found no evidence of long distance airborne transmission occurring without one or more of these factors present.
Based on the results from this review, indoor non-healthcare settings that might be at risk of long distance airborne transmission include hospitality settings such as restaurants, public transport, and workplaces with inadequate ventilation, as well as settings where activities resulting in increased aerosol emission, such as singing or speaking loudly are carried out.
These results highlight the importance of assessing ventilation, especially in indoor spaces where people meet others from outside their household. Particular attention should be given to ventilation in settings with activities that might increase the number of respiratory particles, for example, singing. Where ventilation is assessed to be inadequate, improvements should be made.
What is already known on this topic
The risk of SARS-CoV-2 transmission is likely to be greatest when in close proximity (<2 m) to someone who is infected
The potential for long distance airborne transmission (>2 m) is unclear, although widespread reporting of superspreader events suggests it may occur
Emission rates of respiratory particles released vary considerably between individuals but are generally higher for singing and speaking compared with breathing and tend to increase with loudness of vocalisation
What this study adds
The findings from this rapid systematic review suggest that long distance airborne transmission of SARS-CoV-2 might happen in indoor settings such as restaurants, public transport, workplaces, or choir venues
These results show that factors such as insufficient air replacement, directional air flow, and activities associated with increased emissions of respiratory particles (eg, singing or speaking loudly) might contribute to long distance airborne transmission
Well conducted epidemiological investigations can provide critical insight into transmission routes, especially when other types of studies are not feasible; the question of what level of public health evidence is sufficient to support decision making for a novel infection warrants further consideration
Acknowledgments
We thank colleagues within the UK Health Security Agency for their support into specific aspects of this review, especially Jason Kwasi Sarfo-Annin, Bethany Walters, and Marialena Trivella. JCP is supported by a British Heart Foundation accelerator award (AA/18/7/34219) and works in a unit that is supported by the University of Bristol and UK Medical Research Council (MC_UU_00011/6) and was also supported by a secondment to the COVID-19 Rapid Evidence Service.
Web extra.
Extra material supplied by authors
Supplementary information: supplementary material 1: search strategy, quality criteria checklist, and list of excluded studies
Supplementary information: supplementary material 2: data extraction of included studies
Contributors: DD and JCP contributed equally. JCP, RC, NPS, and DD designed the review, with input from EOC and AB. NPS conducted the literature searches and citation analysis. JCP, DD, and IT conducted the screening and data extraction. DD and JCP conducted the critical appraisal, interpreted the findings, and drafted the manuscript, with further input from RC, EOC, and AB. RC supervised the study and is the study guarantor. All authors had direct access to the data, or access was provided as requested. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: No specific funding.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; AB reports grant funding from UK Research and Innovation (UKRI) and from PROTECT (National Core Study on Transmission and the Environment); no other relationships or activities that could appear to have influenced the submitted work.
The manuscript’s guarantor (RC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: This paper will be shared widely within the UK Health Security Agency and used to inform relevant guidance. It will also be shared with relevant stakeholders, policy makers, and other public health agencies within the United Kingdom and internationally. Further dissemination will include our website https://ukhsalibrary.koha-ptfs.co.uk/covid19rapidreviews/ and through colleagues working in covid-19 evidence synthesis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
Data are from published research and therefore are in the public domain. Extracted data are available in supplementary material 2.
References
- 1. Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at a choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:606-10. 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 2. Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis 2020;93:339-44. 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morawska L, Milton DK. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin Infect Dis 2020;71:2311-3. 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021;397:1603-5. 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang JW, Marr LC, Li Y, Dancer SJ. Covid-19 has redefined airborne transmission. BMJ 2021;373:n913. 10.1136/bmj.n913. [DOI] [PubMed] [Google Scholar]
- 6. Tang JW, Bahnfleth WP, Bluyssen PM, et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Hosp Infect 2021;110:89-96. 10.1016/j.jhin.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samet JM, Prather K, Benjamin G, et al. Airborne Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): What We Know. Clin Infect Dis 2021;73:1924-6. 10.1093/cid/ciab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang CC, Prather KA, Sznitman J, et al. Airborne transmission of respiratory viruses. Science 2021;373:eabd9149. 10.1126/science.abd9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen J, Kong M, Dong B, Birnkrant MJ, Zhang J. Airborne transmission of SARS-CoV-2 in indoor environments: A comprehensive review. Sci Technol Built Environ 2021;27:1331-67. 10.1080/23744731.2021.1977693. [DOI] [Google Scholar]
- 10. Randall K, Ewing ET, Marr LC, Jimenez JL, Bourouiba L. How did we get here: what are droplets and aerosols and how far do they go? A historical perspective on the transmission of respiratory infectious diseases. Interface Focus 2021;11:20210049. 10.1098/rsfs.2021.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marr LC, Tang JW. A Paradigm Shift to Align Transmission Routes With Mechanisms. Clin Infect Dis 2021;73:1747-9. 10.1093/cid/ciab722. [DOI] [PubMed] [Google Scholar]
- 12. Prather KA, Marr LC, Schooley RT, McDiarmid MA, Wilson ME, Milton DK. Airborne transmission of SARS-CoV-2. Science 2020;370:303-4. 10.1126/science.abf0521. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. 2020. [Cited 14 March 2022] www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions.
- 14. Milton DK. A Rosetta Stone for Understanding Infectious Drops and Aerosols. J Pediatric Infect Dis Soc 2020;9:413-5. 10.1093/jpids/piaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SAGE-EMG. Application of physical distancing and fabric face coverings in mitigating the B117 variant SARS-CoV-2 virus in public, workplace and community setting. 2021. [Cited 14 February 2022] https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1008199/S1029-EMG-face-coverings-distancing-13-jan.pdf.
- 16. Tang JW, Marr LC, Milton DK. Aerosols should not be defined by distance travelled. J Hosp Infect 2021;115:131-2. 10.1016/j.jhin.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen W, Zhang N, Wei J, Yen H-L, Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build Environ 2020;176:106859. 10.1016/j.buildenv.2020.106859. [DOI] [Google Scholar]
- 18. Noorimotlagh Z, Jaafarzadeh N, Martínez SS, Mirzaee SA. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ Res 2021;193:110612. 10.1016/j.envres.2020.110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ribaric NL, Vincent C, Jonitz G, Hellinger A, Ribaric G. Hidden hazards of SARS-CoV-2 transmission in hospitals: A systematic review. Indoor Air 2022;32:e12968. 10.1111/ina.12968. [DOI] [PubMed] [Google Scholar]
- 20. Aghalari Z, Dahms H-U, Sosa-Hernandez JE, Oyervides-Muñoz MA, Parra-Saldívar R. Evaluation of SARS-COV-2 transmission through indoor air in hospitals and prevention methods: A systematic review. Environ Res 2021;195:110841. 10.1016/j.envres.2021.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Comber L, O Murchu E, Drummond L, et al. Airborne transmission of SARS-CoV-2 via aerosols. Rev Med Virol 2021;31:e2184. 10.1002/rmv.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Public Health Agency of Canada . Rapid Review on SARS-CoV-2 Aerosol Transmission, Update 2. 2021. www.nccmt.ca/covid-19/covid-19-evidence-reviews/418.
- 23.Antimicrobial resistance and healthcare associated infection (ARHAI) Scotland. Rapid review of the literature: Assessing the infection prevention and control measures for the prevention and management of COVID-19 in health and care settings (version 25). 2022. [Cited 8 April 2022] www.nipcm.hps.scot.nhs.uk/web-resources-container/rapid-review-of-the-literature-assessing-the-infection-prevention-and-control-measures-for-the-prevention-and-management-of-covid-19-in-healthcare-settings.
- 24. Bak A, Mugglestone MA, Ratnaraja NV, et al. SARS-CoV-2 routes of transmission and recommendations for preventing acquisition: joint British Infection Association (BIA), Healthcare Infection Society (HIS), Infection Prevention Society (IPS) and Royal College of Pathologists (RCPath) guidance. J Hosp Infect 2021;114:79-103. 10.1016/j.jhin.2021.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tricco AC, Langlois E, Straus SE. Rapid reviews to strengthen health policy and systems: a practical guide: World Health Organization; 2017. https://apps.who.int/iris/bitstream/handle/10665/258698/9789241512763-eng.pdf.
- 26. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med 2021;18:e1003583. 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer J, Pearce-Smith N, Duval D, et al. Airborne transmission of SARS-CoV-2 over distances greater than two metres: a rapid review protocol. PROSPERO. 2021;CRD42021236762.
- 28. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Academy of Nutrition and Dietetics. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. 2016. [Cited 21 February 2022] www.andeal.org/evidence-analysis-manual.
- 31. Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med 2017;22:85-7. 10.1136/ebmed-2017-110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eichler N, Thornley C, Swadi T, et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 during Border Quarantine and Air Travel, New Zealand (Aotearoa). Emerg Infect Dis 2021;27:1274-8. 10.3201/eid2705.210514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fox-Lewis A, Williamson F, Harrower J, et al. Airborne Transmission of SARS-CoV-2 Delta Variant within Tightly Monitored Isolation Facility, New Zealand (Aotearoa). Emerg Infect Dis 2022;28:501-9. 10.3201/eid2803.212318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Qian H, Hang J, et al. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build Environ 2021;196:107788. 10.1016/j.buildenv.2021.107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwon KS, Park JI, Park YJ, Jung DM, Ryu KW, Lee JH. Evidence of Long-Distance Droplet Transmission of SARS-CoV-2 by Direct Air Flow in a Restaurant in Korea. J Korean Med Sci 2020;35:e415. 10.3346/jkms.2020.35.e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen Y, Li C, Dong H, et al. Community Outbreak Investigation of SARS-CoV-2 Transmission Among Bus Riders in Eastern China. JAMA Intern Med 2020;180:1665-71. 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo K, Lei Z, Hai Z, et al. Transmission of SARS-CoV-2 in Public Transportation Vehicles: A Case Study in Hunan Province, China. Open Forum Infect Dis. 2020;7:ofaa430. 10.1093/ofid/ofaa430 [DOI] [PMC free article] [PubMed]
- 38. Lin G, Zhang S, Zhong Y, et al. Community evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission through air. Atmos Environ (1994) 2021;246:118083. 10.1016/j.atmosenv.2020.118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hwang SE, Chang JH, Oh B, Heo J. Possible aerosol transmission of COVID-19 associated with an outbreak in an apartment in Seoul, South Korea, 2020. Int J Infect Dis 2021;104:73-6. 10.1016/j.ijid.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han T, Park H, Jeong Y, et al. COVID-19 Cluster Linked to Aerosol Transmission of SARS-CoV-2 via Floor Drains. J Infect Dis 2022;225:1554-60. 10.1093/infdis/jiab598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Günther T, Czech-Sioli M, Indenbirken D, et al. SARS-CoV-2 outbreak investigation in a German meat processing plant. EMBO Mol Med 2020;12:e13296. 10.15252/emmm.202013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Groves LM, Usagawa L, Elm J, et al. Community Transmission of SARS-CoV-2 at Three Fitness Facilities - Hawaii, June-July 2020. MMWR Morb Mortal Wkly Rep 2021;70:316-20. 10.15585/mmwr.mm7009e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vernez D, Schwarz S, Sauvain JJ, Petignat C, Suarez G. Probable aerosol transmission of SARS-CoV-2 in a poorly ventilated courtroom. Indoor Air 2021;31:1776-85. 10.1111/ina.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarti D, Campanelli T, Rondina T, Gasperini B. COVID-19 in Workplaces: Secondary Transmission. Ann Work Expo Health 2021;65:1145-51. 10.1093/annweh/wxab023. [DOI] [PubMed] [Google Scholar]
- 45. Jiang G, Wang C, Song L, et al. Aerosol transmission, an indispensable route of COVID-19 spread: case study of a department-store cluster. Front Environ Sci Eng 2021;15:46. 10.1007/s11783-021-1386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Katelaris AL, Wells J, Clark P, et al. Epidemiologic Evidence for Airborne Transmission of SARS-CoV-2 during Church Singing, Australia, 2020. Emerg Infect Dis 2021;27:1677-80. 10.3201/eid2706.210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shah AA, Dusseldorp F, Veldhuijzen IK, et al. High SARS-CoV-2 attack rates following exposure during five singing events in the Netherlands, September-October 2020 (v2, July 2021). medRxiv. 2021:2021.03.30.21253126. 10.1101/2021.03.30.21253126. [DOI]
- 48. Charlotte N. High Rate of SARS-CoV-2 Transmission Due to Choir Practice in France at the Beginning of the COVID-19 Pandemic. J Voice 2020:S0892-1997(20)30452-5. 10.1016/j.jvoice.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lu J, Gu J, Li K, et al. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerg Infect Dis 2020;26:1628-31. 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang N, Chen X, Jia W, et al. Evidence for lack of transmission by close contact and surface touch in a restaurant outbreak of COVID-19. J Infect 2021;83:207-16. 10.1016/j.jinf.2021.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ou C, Hu S, Luo K, et al. Insufficient ventilation led to a probable long-range airborne transmission of SARS-CoV-2 on two buses. Build Environ 2022;207:108414. 10.1016/j.buildenv.2021.108414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller SL, Nazaroff WW, Jimenez JL, et al. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2021;31:314-23. 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schijven J, Vermeulen LC, Swart A, Meijer A, Duizer E, de Roda Husman AM. Quantitative microbial risk assessment for airborne transmission of SARS-CoV-2 via breathing, speaking, singing, coughing, and sneezing. Environ Health Perspect 2021;129:47002. 10.1289/EHP7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morawska L, Tang JW, Bahnfleth W, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int 2020;142:105832. 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morawska L, Allen J, Bahnfleth W, et al. A paradigm shift to combat indoor respiratory infection. Science 2021;372:689-91. 10.1126/science.abg2025. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization (WHO). Coronavirus disease (COVID-19): How is it transmitted? (last updated 23 December 2021). 2021. [Cited 14 March 2022] www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted.
- 57.UK Health Security Agency (UKHSA). COVID-19: ventilation of indoor spaces to stop the spread of coronavirus (published 4 March 2021, updated 15 September 2021). 2021. [Cited 14 March 2022] www.gov.uk/government/publications/covid-19-ventilation-of-indoor-spaces-to-stop-the-spread-of-coronavirus.
- 58. Alsved M, Nygren D, Thuresson S, Medstrand P, Fraenkel C-J, Löndahl J. SARS-CoV-2 in Exhaled Aerosol Particles from COVID-19 Cases and Its Association to Household Transmission. Clin Infect Dis 2022;ciac202. Published online Mar 10. doi/10.1093/cid/ciac202/6546685#354016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gregson FKA, Watson NA, Orton CM, et al. Comparing aerosol concentrations and particle size distributions generated by singing, speaking and breathing. Aerosol Sci Technol 2021;55:681-91. 10.1080/02786826.2021.1883544. [DOI] [Google Scholar]
- 60. Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep 2019;9:2348. 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alene M, Yismaw L, Assemie MA, et al. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis. PLoS One 2021;16:e0249090. 10.1371/journal.pone.0249090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. JAMMI 2020;5:223-34. 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buitrago-Garcia D, Ipekci AM, Heron L, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: update of a living systematic review and meta-analysis (v2, March 2022). medRxiv. 2022:2022.01.20.22269581. 10.1101/2022.01.20.22269581. [DOI] [PMC free article] [PubMed]
- 64.Harrison S, Duval D, Walters B, Sadler L, Pearce-Smith N, Clark R. The effectiveness of face coverings to reduce transmission of COVID-19 in community settings - A rapid review (update 2). UKHSA COVID-19 Rapid Evidence Service, 2021. [Cited 28 March 2022] https://ukhsa.koha-ptfs.co.uk/cgi-bin/koha/opac-retrieve-file.pl?id=cfd006713bdc311c9bc9e4e029fb4f47.
- 65.Harrison S, Simmons Z, Ghataure A, Pearce-Smith N, Clark R. The effect of vaccination on transmission of COVID-19 - A rapid evidence briefing (update 1). UKHSA COVID-19 Rapid Evidence Service, 2022. [Cited 28 April 2022] https://ukhsa.koha-ptfs.co.uk/cgi-bin/koha/opac-retrieve-file.pl?id=17de0b088ac2fa00f643b7037ca51665.
- 66. Stone SP, Cooper BS, Kibbler CC, et al. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. J Antimicrob Chemother 2007;59:833-40. 10.1093/jac/dkm055. [DOI] [PubMed] [Google Scholar]
- 67. Khanh NC, Thai PQ, Quach H-L, et al. Transmission of SARS-CoV 2 during long-haul flight. Emerg Infect Dis 2020;26:2617-24. 10.3201/eid2611.203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Toyokawa T, Shimada T, Hayamizu T, et al. Transmission of SARS-CoV-2 during a 2-h domestic flight to Okinawa, Japan, March 2020. Influenza Other Respir Viruses 2022;16:63-71. 10.1111/irv.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Speake H, Phillips A, Chong T, et al. Flight-associated transmission of severe acute respiratory syndrome coronavirus 2 corroborated by whole-genome sequencing. Emerg Infect Dis 2020;26:2872-80. 10.3201/eid2612.203910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Swadi T, Geoghegan JL, Devine T, et al. Genomic evidence of in-flight transmission of SARS-CoV-2 despite predeparture testing. Emerg Infect Dis 2021;27:687-93. 10.3201/eid2703.204714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fox MP, Murray EJ, Lesko CR, Sealy-Jefferson S. On the Need to Revitalize Descriptive Epidemiology. Am J Epidemiol 2022;kwac056. 10.1093/aje/kwac056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kutter JS, de Meulder D, Bestebroer TM, et al. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nat Commun 2021;12:1653. 10.1038/s41467-021-21918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: supplementary material 1: search strategy, quality criteria checklist, and list of excluded studies
Supplementary information: supplementary material 2: data extraction of included studies
Data Availability Statement
Data are from published research and therefore are in the public domain. Extracted data are available in supplementary material 2.