Abstract
Background
Central venous catheter‐related bloodstream infection is an important cause of mortality and morbidity in newborn infants cared for in neonatal units. Potential strategies to prevent these infections include the use of central venous catheters impregnated with antimicrobial agents.
Objectives
To determine the effect of antimicrobial‐impregnated central venous catheters in preventing catheter‐related bloodstream infection in newborn infants.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 8), MEDLINE (1966 to September 2015), EMBASE (1980 to September 2015), CINAHL (1982 to September 2015), conference proceedings and previous reviews.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing central venous catheters impregnated or coated with any antibiotic or antiseptic versus central venous catheters without antibiotic or antiseptic coating or impregnation in newborn infants.
Data collection and analysis
We extracted data using the standard methods of the Cochrane Neonatal Group, with independent evaluation of risk of bias and data extraction by two review authors.
Main results
We found only one small trial (N = 98). This trial found that silver zeolite‐impregnated umbilical venous catheters reduced the incidence of bloodstream infection in very preterm infants (risk ratio 0.11, 95% confidence interval 0.01 to 0.87; risk difference ‐0.17, 95% CI ‐0.30 to ‐0.04; number needed to treat for benefit 6, 95% CI 3 to 25].
Authors' conclusions
Although the data from one small trial indicates that antimicrobial‐impregnated central venous catheters might prevent catheter‐related bloodstream infection in newborn infants, the available evidence is insufficient to guide clinical practice. A large, simple and pragmatic randomised controlled trial is needed to resolve on‐going uncertainty.
Plain language summary
Antimicrobial‐impregnated central venous catheters to prevent bloodstream infection in newborn infants
Review question: In newborn infants requiring a central venous catheter (CVC), are antimicrobial‐impregnated CVCs compared to standard CVCs effective in preventing acquired bloodstream infection?
Background: Infection in the bloodstream is a frequent and harmful complication for newborn infants who have a central venous catheter (a cannula that extends several centimetres into the infant's blood vessel). While the catheter may provide a secure route for delivering drugs and nutrition, it may also be a locus for infecting organisms to grow and cause long‐term or more severe infection. One potential method of reducing this serious complication is to use central venous catheters that contain antiseptics or antibiotics to stop organisms from sticking to or growing on the catheter.
Study characteristics: We found only one small randomised controlled trial (with 98 very preterm infants participating) that addressed this question.
Key results: This trial showed that central venous catheters containing antiseptics or antibiotics to stop organisms from sticking to or growing on the catheter could reduce the chance of infants developing a bloodstream infection by about 90%. Becasue the trial was small, however, this finding is not certain.
Conclusions: The trial did provide some evidence that antimicrobial‐impregnated central venous catheters can prevent bloodstream infection in newborn infants, but further, large trials are needed to resolve this question fully.
Background
Description of the condition
Bloodstream infection is the most common serious complication associated with the use of central venous catheters (CVCs). The most commonly used CVCs in neonatal practice are umbilical venous catheters or peripherally‐inserted percutaneous CVCs; these are used to deliver drugs, fluids or parenteral nutrition for newborn infants. Micro‐organisms can gain access through the CVC entry site or, less commonly, via the catheter hub into the lumen or the tract of the CVC (Salzman 1995). Microbial pathogens adhere to the material of the CVC and secrete a protective intraluminal or extraluminal biofilm of extracellular polymeric substances (Machado 2009). Bacteria or fungi proliferating within the biofilm are relatively protected from circulating antimicrobial agents, enabling sustained colonisation (Ramirez de Arellano 1994; Stewart 2001). CVC‐associated thrombosis can act as an additional nidus for infection (Thornburg 2008). It is often necessary to remove the CVC in order to clear the infection (Benjamin 2001).
The reported incidence of CVC‐related bloodstream infection ranges from about 5% to 30%, depending on the precise diagnostic criteria used and the demographics of the population (Trotter 1996a; Trotter 1996b; Cartwright 2004; Van der Zwet 2005; Garland 2008; Hoang 2008; Ohki 2008; Olsen 2009; O'Grady 2011). Very preterm (less than 32 weeks gestation) infants are at the highest risk, but inter‐unit variation in the incidence of CVC‐associated bloodstream infection is not fully explained by case mix, and may relate to care or infection control practices (Wong 2012). Other putative risk factors include prolonged use of parenteral nutrition and insertion of the CVC after the first postnatal week (Mahieu 2001c; Mahieu 2001d).
The most common causes of CVC‐related bloodstream infections in newborn infants are coagulase‐negative staphylococci, Gram‐negative bacilli (mainly enteric bacilli), Gram‐positive cocci (Staphylococcus aureus (S. aureus), enterococci), and fungi (predominantly Candida species) (Makhoul 2002; O'Grady 2002; Stoll 2002; Isaacs 2003; Isaacs 2004; Gordon 2006; De Brito 2010). Newborn infants, particularly very preterm infants, with acquired bloodstream infection have a higher risk of mortality and a range of important morbidities that require intensive care and mechanical ventilation: bronchopulmonary dysplasia, necrotising enterocolitis, retinopathy of prematurity, hepatic dysfunction and prolonged hospitalisation (Saint 2000; Mahieu 2001a; Mahieu 2001b; Chapman 2003; Payne 2004; Adams‐Chapman 2006; Hermans 2007; Lahra 2009). Bloodstream infection is also associated with higher rates of several adverse neurodevelopmental outcomes, long‐term disability, vision and hearing impairment, and cerebral palsy (Stoll 2004; Shah 2008; Bassler 2009).
Various strategies to reduce and prevent CVC‐related bloodstream infections have been introduced, often as care bundles. These include strict aseptic precautions when inserting and accessing the CVC, use of needleless intravascular catheter systems and prompt removal when the CVC is no longer needed (O'Grady 2002; Yébenes 2004; Pronovost 2006; Miller 2010; Sannoh 2010; Vanholder 2010; Wirtschafter 2010; Kaplan 2011; O'Grady 2011; Schulman 2011). Care bundles have been shown to reduce bloodstream infection rates in adult, paediatric and neonatal intensive care studies (Pronovost 2006; Miller 2010; Wirtschafter 2010; Kaplan 2011; Schulman 2011). However, despite these strategies, CVC‐related infections remain a major cause of morbidity and mortality in newborn infants and other interventions are required to reduce the infection rates further.
Description of the intervention
CVCs are vascular cannulae terminating in a large (central) vein. In neonatal practice, the most commonly used CVCs are umbilical venous catheters (usually inserted within the first postnatal day), and peripherally‐inserted percutaneous CVCs (inserted via a peripheral vein). Other CVCs that are commonly used in caring for older children or adults, such as those inserted directly via a central vein (typically the femoral or subclavian vein) and subcutaneously "tunnelled" catheters (usually requiring a surgical procedure to insert), are much less commonly used in neonatal care.
Most commercially available CVCs are made of a silicone or polyurethane (polytetrafluoroethylene) based material. The intervention under study here is the use of CVCs coated with, or manufactured from, materials impregnated with antibiotic or antiseptic agents. Such catheters have been available in adult practice for several years, and emerging evidence supports their clinical and cost‐effectiveness in preventing bloodstream infection (Alonso‐Echanove 2003; Eggimann 2003; Shorr 2003; Casey 2008; Gilbert 2008; Hockenhull 2008; Halton 2009; Walz 2010; Lambert 2012). A US Centre for Disease Control (CDC) expert consensus statement recommends the "use of a chlorhexidine/silver sulphadiazine or minocycline/rifampicin‐impregnated CVCs in [paediatric and adult] patients whose catheter is expected to remain in place for more than 5 days if, after successful implementation of a comprehensive strategy to reduce rates of [CVC‐related bloodstream infection], the [CVC‐related bloodstream infection] rate is not decreasing" (O'Grady 2011).
How the intervention might work
Use of antimicrobial‐impregnated or coated CVCs might reduce micro‐organism adhesion and survival, thus inhibiting intraluminal or extraluminal biofilm formation. Inhibited or abolished biofilm formation would be expected to reduce the incidence of bloodstream infection, reduce the need for CVC removal, and reduce infection‐associated mortality and morbidity (Cicalini 2004).
In vitro studies suggest that antiseptic‐ and antibiotic‐impregnated CVCs are equally effective at inhibiting microbial adherence and colonisation (Sampath 2001), but a systematic review of trials with mainly adult participants, found that antibiotic‐impregnated (minocycline/rifampicin) CVCs were more effective than antiseptic‐impregnated (chlorhexidine/silver sulphadiazine) CVCs at preventing catheter‐related bloodstream infections (Casey 2008). Although there is the possibility that prolonged use of antibiotic‐impregnated CVCs might select for antibiotic‐resistant micro‐organisms, evidence exists that if bacteria, including methicillin‐resistant Staphylococcus aureus (S. aureus) and vancomycin‐resistant enterococci, are exposed to antibiotic combinations (rather than a single agent) they are less likely to develop antibiotic resistance (Tambe 2001; Munson 2004; Aslam 2007).
Why it is important to do this review
There are important differences between adults and infants that mean that antimicrobial‐impregnated CVCs might work differently. Firstly, infants’ veins are much smaller and so CVCs need to be narrower, with associated higher flow rates per unit of cross‐sectional area. Secondly, CVCs are often kept in place for much longer in infants and the risk of infection may increase over the time a CVC is in place (Schelonka 2006). Thirdly, there is a higher incidence of infection in newborn infants, especially in very preterm and very low birth weight infants, as their immature immune systems and thin, immature skin increase susceptibility (Borghesi 2008). Finally, the consequences of infection in newborn infants are different and more serious than in older children and adults.
Given the potential for the use of antimicrobial‐impregnated CVCs to affect important outcomes for newborn infants, we will undertake a systematic review to identify, appraise and synthesise the available evidence from randomised controlled trials.
Objectives
To determine the effectiveness of antimicrobial‐impregnated CVCs compared to standard CVCs in preventing acquired bloodstream infection in newborn infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials (including cluster randomised trials).
Types of participants
Newborn infants who are to have a CVC placed.
Types of interventions
Intervention: CVCs impregnated or coated with any antibiotic or antiseptic agent.
Control: any other CVC without antibiotic or antiseptic coating or impregnation.
Trials which assess the effect of antibiotic‐impregnated CVCs as part of a package of infection control measures (care bundle) were eligible for inclusion, but we planned to analyse these separately from trials of discreet interventions.
Types of outcome measures
Primary outcomes
Incidence (and rates per 1000 catheter‐days) of laboratory‐confirmed bloodstream infection, where the CVC was in place on the day or the day before the blood sample for microbial culture was obtained. If the CVC was placed on the first postnatal day, then it must have been in place for more than two calendar days on the day that the blood sample for microbial culture was obtained.
Secondary outcomes
Mortality due to all causes prior to hospital discharge and at one year corrected age.
Neurodevelopmental outcomes assessed after 12 months corrected age using validated tools: neurological evaluations; developmental scores; and classifications of disability, including auditory and visual disability. We will define neurodevelopmental impairment as the presence of one or more of the following: non‐ambulant cerebral palsy; developmental quotient more than two standard deviations below the population mean; and blindness (visual acuity less than 6/60) or deafness (any hearing impairment requiring or unimproved by amplification).
Death or neurological impairment assessed after 12 months corrected age.
-
Other morbidity developing after enrolment in trial until discharge from hospital:
bronchopulmonary dysplasia (oxygen supplementation at 36 weeks postmenstrual age);
necrotising enterocolitis (Bell stage 2 or 3);
retinopathy of prematurity, requiring treatment (medical or surgical).
Length of index CVC use (days).
Incidence of CVC removal for suspected or confirmed CVC infection.
Length of stay in the neonatal intensive care and overall hospital stay (days).
Proportion of catheters colonised with antibiotic‐resistant organisms at removal.
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Group.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library (2015, issue 8), MEDLINE (1966 to September 2015), EMBASE (1980 to September 2015), and CINAHL (1982 to September 2015) using a combination of the following text words and MeSH terms:
[infant, newborn OR infant, premature OR infant, low birth weight OR infan* OR neonat*]
AND
[catheters, Indwelling OR catheterization, central venous OR central near3 cathet* OR central near3 cannul* OR central near3 line OR CVC OR CVL OR PCVC OR PICC OR Umbilical, Veins OR UVC OR UAC OR umbilical near3 cathet* OR umbilical near3 cannul* OR umbilical near3 line OR Broviac OR Hickman OR antibiotic impregnat* OR antimicrobial impregnat* OR antibacterial impregnate* OR antiseptic impregnat* OR antibiotic coat* OR antimicrobial coat* OR antibacterial coat* OR antiseptic coat*]
We limited the search outputs with the relevant search filters for clinical trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not apply any language restrictions.
We searched ClinicalTrials.gov and Current Controlled Trials for completed or ongoing trials.
Searching other resources
We examined reference lists in previous reviews and included studies. We searched the proceedings of the annual meetings of the Pediatric Academic Societies (1993 to 2015), the European Society for Pediatric Research (1995 to 2014), the Royal College of Paediatrics and Child Health (2000 to 2015), the Perinatal Society of Australia and New Zealand (2000 to 2015), the European Society for Paediatric Infectious Diseases (2005 to 2014), and the Infectious Diseases Society of America (2003 to 2014). Trials reported only as abstracts were eligible if sufficient information was available from the report, or from contact with the authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Group.
Selection of studies
We screened the title and abstract of all studies identified by the above search strategy; two review authors independently assessed the full articles for all potentially relevant trials. We excluded those studies that did not meet all of the inclusion criteria and stated the reason for exclusion. We discussed any disagreements until consensus was achieved.
Data extraction and management
Two authors independently extracted data using a data collection form to aid extraction of information on design, methodology, participants, interventions, outcomes and treatment effects from each included study. We discussed any disagreements until we reached consensus. If data from the trial reports were insufficient, we contacted the trialists for further information.
Assessment of risk of bias in included studies
We used the criteria and standard methods of the Cochrane Neonatal Group to assess the methodological quality of any included trials. Two authors conducted the assessment of risk of bias. We resolved disagreements in consultation with a third author. We planned to request additional information from the trial authors to clarify methodology and results if necessary.
We evaluated the following issues in the 'Risk of bias' tables:
Random sequence generation ‐ we categorised the method used to generate the allocation sequence as:
low risk ‐ any truly random process, e.g. random number table; computer random number generator;
high risk ‐ any non‐random process, e.g. odd or even date of birth; hospital or clinic record number; and
unclear risk ‐ no or unclear information provided.
Allocation concealment ‐ we categorised the method used to conceal the allocation sequence as:
low risk ‐ e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes;
high risk ‐ open random allocation, e.g. unsealed or non‐opaque envelopes, alternation; date of birth; and
unclear ‐ no or unclear information provided.
Blinding ‐ we assessed blinding of participants, clinicians and caregivers, and outcome assessors separately for different outcomes and categorised the methods as:
low risk;
high risk; and
unclear.
Incomplete outcome data ‐ we described the completeness of data, including attrition and exclusions from the analysis, for each outcome and any reasons for attrition or exclusion, where reported. We assessed whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to reinstate missing data in the analyses. We categorised completeness as:
low risk ‐ up to and including10% missing data;
high risk ‐ more than 10% missing data; and
unclear risk ‐ no or unclear information provided.
Overall risk of bias ‐ we made explicit judgements about whether studies were at high risk of bias, according to the criteria suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the likely magnitude and direction of the bias and whether we considered it likely to impact the findings. We planned to explore the impact of the level of bias in sensitivity analyses.
Measures of treatment effect
We analysed the treatment effects in the trial using Review Manager 5.2 (RevMan 2012) and reported risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). We determined the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. An infant was considered only once in an analysis. We planned to exclude infants with multiple enrolments unless we obtained data from the report or investigators relating to the first episode of randomisation. If we could not separate data from the first randomisation, we planned to exclude the study as we would not be able to address the unit of analysis issues that arise from multiple enrolments of the same infant.
We intended to conduct intention‐to‐treat analyses. However, if the allocated CVC placement was unsuccessful, the primary outcome (bloodstream infection when the CVC is in place) for that infant may not have been possible to evaluate.
The participating neonatal unit or section of a neonatal unit was the unit of analysis in cluster randomised trials. We planned to analyse these using an estimate of the intra‐cluster correlation coefficient derived from the trial (if possible), or from another source as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
If we identified both cluster randomised trials and individually randomised trials, we planned to only combine the results from both if there was little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Dealing with missing data
We requested additional data from the trial investigators if data on important outcomes were missing or reported unclearly. Where data were still missing, we examined the impact on effect size estimates in sensitivity analyses.
Assessment of heterogeneity
As only one study was included in any analysis tests for heterogeneity were not applicable.
Assessment of reporting biases
Where we suspected reporting bias, we contacted trial investigators asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such trials in the overall assessment of results in a sensitivity analysis.
Data synthesis
We planned to use the fixed‐effect model in Review Manager 5.2 (RevMan 2012) for meta‐analyses (as per Cochrane Neonatal Group recommendations). Where substantial heterogeneity existed, we planned to examine the potential causes in subgroup and sensitivity analyses.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
Very preterm (less than 32 weeks) infants (versus infants born at or later than 32 weeks).
Type of CVC (umbilical venous catheters versus peripherally‐inserted CVCs versus surgically‐placed or tunnelled central lines, and antimicrobial versus antiseptic impregnation or coating).
Sensitivity analysis
We planned sensitivity analyses to determine if findings were affected by including only studies of adequate methodology (low risk of bias), defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and up to and including a 10% loss to follow‐up.
Results
Description of studies
See the Characteristics of included studies and Characteristics of excluded studies sections.
Characteristics of ongoing studies: PREVAIL
Results of the search
We identified 31 records, for four of which we assessed full‐text articles for eligibility. Only one trial met all of our inclusion criteria (Bertini 2013).
Included studies
We identified one randomised controlled trial (N = 98) that met the inclusion criteria (Bertini 2013).
Excluded studies
We excluded three reports (see Characteristics of excluded studies).
Risk of bias in included studies
We included only one trial (Bertini 2013).
Allocation
The report states that allocation was contained in sealed opaque envelopes.
Blinding
The trial was unblinded.
Incomplete outcome data
Of the 98 infants who were randomised, 12 died within the first week. These 12 infants were not included in the mortality analysis in the primary report.
Effects of interventions
Antimicrobial‐impregnated bloodstream infection (Comparison 1)
Primary outcomes
Catheter‐related bloodstream infection (Analysis 1.1):Bertini 2013 found that a statistically significant reduced incidence in infants allocated to the intervention (RR 0.11, 95% CI 0.01 to 0.87; RD ‐0.17, 95% CI ‐0.30 to ‐0.04; NNTB 6, 95% CI 3 to 25; Figure 1).
1.1. Analysis.
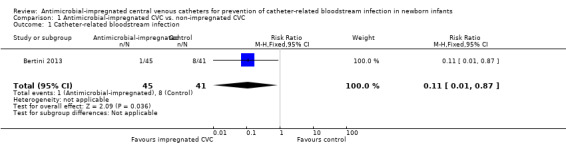
Comparison 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, Outcome 1 Catheter‐related bloodstream infection.
1.
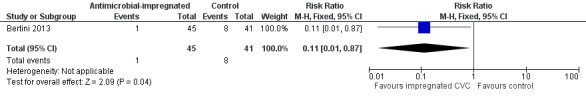
Forest plot of comparison: 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, outcome: 1.1 Catheter‐related bloodstream infection.
Secondary outcomes
Mortality due to all causes prior to hospital discharge (Analysis 1.2):Bertini 2013 did not find a statistically significant difference (RR 0.73, 95% CI 0.21 to 2.53; RD ‐0.03, 95% CI ‐0.16 to 0.10; Figure 2).
Neurodevelopmental outcomes: Not reported.
Death or neurological impairment assessed after 12 months corrected age: Not reported.
-
Other morbidity developing after enrolment in trial until discharge from hospital:
Bronchopulmonary dysplasia (oxygen supplementation at 36 weeks postmenstrual age; Analysis 1.3): Bertini 2013 did not find a statistically significant difference (RR 0.80, 95% CI 0.45 to 1.42; RD ‐0.08, 95% CI ‐0.28 to 0.12; Figure 3).
Necrotising enterocolitis (Bell stage 2 or 3; Analysis 1.4): Bertini 2013 reported the incidence of "necrotising enterocolitis [defined] by Bell's criteria" and did not find a statistically significant difference (RR 0.46, 95% CI 0.04 to 4.84; RD ‐0.03, 95% CI ‐0.11 to 0.05; Figure 4). It is unclear if this analysis included infants with Bell stage 1 disease (as well as Bell stage 2 or 3).
Retinopathy of prematurity, requiring treatment (medical or surgical; Analysis 1.5): Bertini 2013 reported the incidence of retinopathy of prematurity as not statistically significantly different (RR 0.46, 95% CI 0.12 to 1.70; RD ‐0.08, 95% CI ‐0.21 to 0.05; Figure 5). It is unclear if this analysis included infants with retinopathy that did not require treatment (as well as those who did).
Length of index CVC use (days; Analysis 1.6):Bertini 2013 found that a statistically significant increase in duration of UVC (days) in infants allocated to the intervention (MD 2.3, 95% CI 0.38 to 4.22; Figure 6).
Incidence of CVC removal for suspected or confirmed CVC infection: Not reported.
Length of stay in the neonatal intensive care and overall hospital stay (Analysis 1.7):Bertini 2013 did not report data on length of stay in neonatal intensive care. Bertini 2013 found a statistically significant shorter duration of stay in hospital for infants allocated to the intervention (MD ‐15.00 days, 95% CI ‐29.41 to ‐0.59; Figure 7).
Proportion of catheters colonised with antibiotic‐resistant organisms at removal: Stated as "similar" between groups but data not presented.
1.2. Analysis.
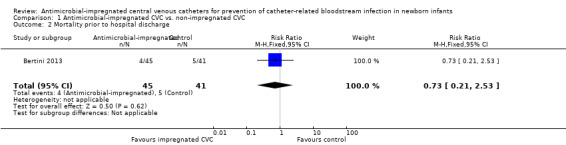
Comparison 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, Outcome 2 Mortality prior to hospital discharge.
2.
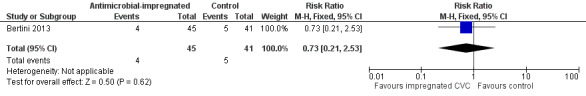
Forest plot of comparison: 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, outcome: 1.2 Mortality prior to hospital discharge.
1.3. Analysis.
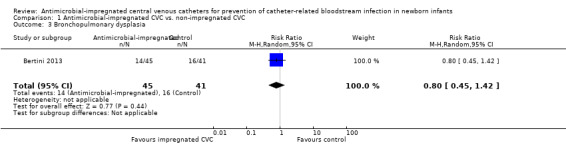
Comparison 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, Outcome 3 Bronchopulmonary dysplasia.
3.
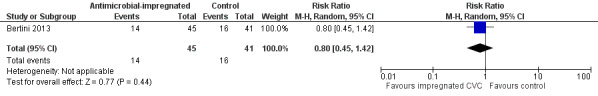
Forest plot of comparison: 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, outcome: 1.3 Bronchopulmonary dysplasia.
1.4. Analysis.
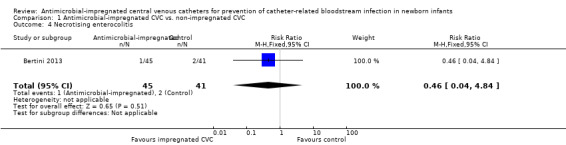
Comparison 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, Outcome 4 Necrotising enterocolitis.
4.
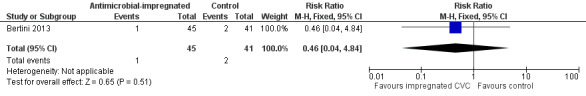
Forest plot of comparison: 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, outcome: 1.4 Necrotising enterocolitis.
1.5. Analysis.
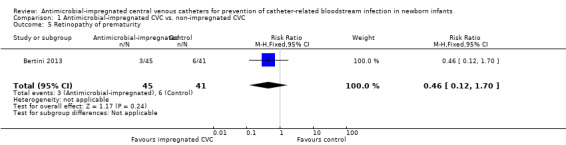
Comparison 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, Outcome 5 Retinopathy of prematurity.
5.
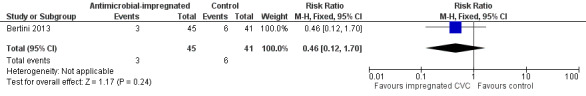
Forest plot of comparison: 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, outcome: 1.5 Retinopathy of prematurity.
1.6. Analysis.
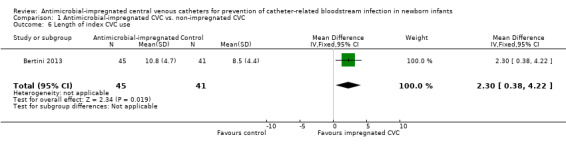
Comparison 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, Outcome 6 Length of index CVC use.
6.
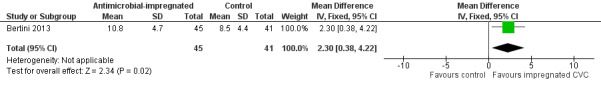
Forest plot of comparison: 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, outcome: 1.6 Length of index CVC use.
1.7. Analysis.
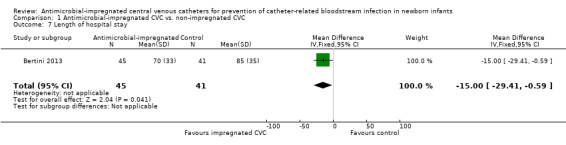
Comparison 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, Outcome 7 Length of hospital stay.
7.
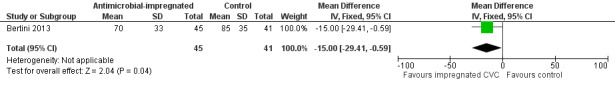
Forest plot of comparison: 1 Antimicrobial‐impregnated CVC vs. non‐impregnated CVC, outcome: 1.7 Length of hospital stay.
Subgroup analyses
Very preterm (less than 32 weeks) infants (versus infants born at or later than 32 weeks): All participants were less than 31 weeks' gestation at birth.
Type of CVC (umbilical venous catheters versus peripherally‐inserted CVCs versus surgically‐placed or tunnelled central lines, and antibiotic‐ versus antiseptic‐impregnation or coating): Bertini 2013 only assessed silver zeolite‐impregnated umbilical venous catheters.
Discussion
Summary of main results
We found only one trial for inclusion in this review. This trial had various methodological limitations, including lack of blinding and incomplete inclusion of all randomised participants. The trial reported a statistically significant reduction in the incidence of catheter‐related bloodstream infection, a statistically significant increase in the length of duration of use of the CVC, and a borderline statistically significant reduction in total length of stay in hospital. There was no evidence of an effect on mortality.
Overall completeness and applicability of evidence
We identified only one small trial (N = 98) for inclusion in this review. This trial provided some evidence that use of antiseptic‐impregnated umbilical venous catheters reduced the risk of bloodstream infection in very preterm infants. The control events rate (20% incidence of catheter‐related bloodstream infection) is comparable to other published studies and the findings are likely to be generally applicable to healthcare settings in high‐income and middle‐income countries with similar populations and care practices (Van der Zwet 2005; Olsen 2009; Wong 2012).
The available data are insufficient to exclude plausible and important effects on important secondary outcomes. For example, for mortality, wide 95% confidence interval bounds included a five‐fold reduced risk and a more than two‐fold increased risk. Long‐term (post discharge) data have not been reported in this trial.
Quality of the evidence
Although allocation was concealed, the trial intervention was not blinded to caregivers and investigators, and surveillance bias may have influenced the assessment of some outcomes, including bloodstream infection. Clinicians' subjective assessment of when to investigate for infection may have been affected by the perceived clinical efficacy of antimicrobial impregnated CVCs. The unblinded design may also have influenced care practices. For example, a perception that antimicrobial‐impregnated CVCs prevent infection may influence healthcare staff's adherence to other infection control practices. Lack of blinding may also influence the timing of the decision to remove the CVC, which may be reflected in the longer duration of use of antibiotic‐impregnated CVCs reported in the trial.
Intention‐to‐treat analysis for mortality
Of the 98 infants who were randomised, 12 died within the first week. These infants were not included in the mortality analysis in the primary report. The reason for exclusion from analysis after randomisation is not clear. We have contacted the principal investigator seeking these data (not yet obtained) which can be included in an intention‐to‐treat re‐analysis in an update of this review.
Potential biases in the review process
We found only one trial for inclusion in this review. Although we conducted a comprehensive search, including conference proceedings, we cannot exclude fully the possibility of publication bias since do not know whether other published (but not indexed) or unpublished trials exist.
Definition of catheter‐related bloodstream infection
Our protocol defined catheter‐related bloodstream infection as culture of a micro‐organism from blood obtained when the "CVC was in place on the day or the day before the blood sample for microbial culture was obtained". The included trial's protocol defined catheter‐related bloodstream infection as culture of a micro‐organism from blood "concordant with organism colonising the UVC tip" obtained "when the UVC is in place or within 48 hours of central line removal". We made a post hoc, pragmatic, consensus decision to accept this definition of the primary outcome since (i) it was very similar to our a priori definition (allowing a 48 hours rather than 24 hours window post CVC removal for obtaining blood cultures), and (ii) it was unlikely to be possible to obtain trial data revised to fit our review definition.
Agreements and disagreements with other studies or reviews
The current evidence from studies in which adults participated suggests that antimicrobial‐impregnated CVCs are clinically effective and cost‐effective in preventing bloodstream infection (Alonso‐Echanove 2003; Eggimann 2003; Shorr 2003; Casey 2008; Gilbert 2008; Hockenhull 2008; Halton 2009; Walz 2010; Lambert 2012). In the paediatric population, there are more limited and inconsistent data available (Lenz 2010, Weber 2012).
Other antimicrobial‐impregnated or coated devices for newborn infants, including ventriculoperitoneal shunts, external ventricular drain catheters, and tympanostomy tubes, have been studied and assessed in randomised controlled trials. The limited data currently available do not provide consistent or definite evidence of benefit or harm (Licameli 2008; Muttaiyah 2010; Demetriades 2011; Kandasamy 2011; Thomas 2012).
Authors' conclusions
Implications for practice.
The available data from one, methodologically‐weak trial, although indicating that antimicrobial‐impregnated umbilical venous catheters might reduce the incidence of bloodstream infections in very preterm infants, are insufficient to guide clinical practice.
Implications for research.
Further trials are required to assess the effectiveness of antimicrobial‐impregnated CVCs for preventing bloodstream infections in newborn infants. The trials should be large, simple and pragmatic, and ideally should blind caregivers and investigators to the intervention to avoid introducing bias. Trials might compare either antibiotic‐ or antiseptic‐impregnated CVCs with non‐impregnated standard CVCs. Participants may include any newborn infants cared for in neonatal care centres who require CVC placement, or may be restricted to infants who are at a very high risk of catheter‐related bloodstream infection, such as very, or extremely, preterm infants. As well as assessing the impact on the incidence of bloodstream infection and mortality, trials should be powered to detect plausible effects on important secondary outcomes, including acute neonatal morbidity, duration of hospital admission, and neurodevelopmental outcomes. There are theoretical concerns about potential harm due to emergence of resistant strains of bacteria and drug toxicity, which could be assessed within and between trials. Given the costs associated with this intervention, it is also important to embed a health economics assessment within any trial design.
Acknowledgements
This report is independent research funded by a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Data and analyses
Comparison 1. Antimicrobial‐impregnated CVC vs. non‐impregnated CVC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related bloodstream infection | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.87] |
| 2 Mortality prior to hospital discharge | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.21, 2.53] |
| 3 Bronchopulmonary dysplasia | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.45, 1.42] |
| 4 Necrotising enterocolitis | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 4.84] |
| 5 Retinopathy of prematurity | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.12, 1.70] |
| 6 Length of index CVC use | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [0.38, 4.22] |
| 7 Length of hospital stay | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐13.00 [‐29.41, ‐0.59] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bertini 2013.
| Methods | Randomised controlled trial | |
| Participants | Preterm newborn infants (< 31 weeks' gestation) who needed an umbilical venous catheter (UVC) for parenteral nutrition, therapy, or both during the first week after birth. | |
| Interventions | 1. Silver zeolite‐impregnated UVC (Vygon Lifecath PICC ExpertTM), 4 to 5 French gauge (Fr). 2. Another polyurethane UVC (ArgyleTM), 3.5 to 5 Fr. |
|
| Outcomes | Incidence of "definite" catheter‐related bloodstream infection: micro‐organism grown on peripheral, percutaneously‐obtained blood culture concordant with organism colonising the UVC tip in infants with clinical manifestation of infection, with a UVC in place or within 48 hours of central line removal, and without other apparent sources of bloodstream infection. | |
| Notes | Location: Tertiary Neonatal Unit, Florence, Italy (2007 to 2009). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Allocation contained in sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 98 infants who were randomised, 12 died within the first week. These 12 infants were not included in the mortality analysis in the primary report ‐ we have sought (but not yet obtained) this information from the investigators (email to cdani@unifi.it on 07/05/14) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Seidler 2006 | In vitro study. |
| Stevens 2011 | In vitro study. |
| Venkatesh 2009 | Study of catheter‐lock solutions. |
Characteristics of ongoing studies [ordered by study ID]
PREVAIL.
| Trial name or title | PREVAIL |
| Methods | Randomised controlled trial |
| Participants | Infants in neonatal units for whom placement of a 1 French percutaneously‐inserted CVC is planned |
| Interventions | CVC with rifampicin and miconazole coating or identical catheter without antimicrobial coating |
| Outcomes | Time to first positive blood culture taken 24 after randomisation and up to 48 hours after CVC removal |
| Starting date | June 2015 |
| Contact information | sam.oddie@bthft.nhs.uk |
| Notes |
Differences between protocol and review
Definition of catheter‐related bloodstream infection
Our protocol defined catheter‐related bloodstream infection as culture of a micro‐organism from blood obtained when the "CVC was in place on the day or the day before the blood sample for microbial culture was obtained". The included trial's protocol defined catheter‐related bloodstream infection as culture of a micro‐organism from blood "concordant with organism colonising the UVC tip" obtained "when the UVC is in place or within 48 hours of central line removal". We made a post hoc, pragmatic, consensus decision to accept this definition of the primary outcome since (i) it was very similar to our a priori definition (allowing a 48 hours rather than 24 hours window post CVC removal for obtaining blood cultures), and (ii) it was unlikely to be possible to obtain trial data revised to fit out review definition.
Contributions of authors
All authors contributed to the development of the protocol and production of the review..
Sources of support
Internal sources
NIHR Cochrane Programme Grant, UK.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
This report is independent research funded by a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
Drs Sam Oddie and William McGuire are co‐investigators in a UK multi‐centre trial of antimicrobial‐impregnated CVC in preterm infants (PREVAIL).
Dr Munisha Balain does not have any conflicts of interest.
New
References
References to studies included in this review
Bertini 2013 {published data only}
- Bertini G, Elia S, Ceciarini F, Dani C. Reduction of catheter‐related bloodstream infections in preterm infants by the use of catheters with the AgION antimicrobial system. Early Human Development 2013;89(1):21‐5. [PUBMED: 22841551] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Seidler 2006 {published data only}
- Seidler M, Salvenmoser S, Muller FM. In vitro effects of micafungin against Candida biofilms on polystyrene and central venous catheter sections. International Journal of Antimicrobial Agents 2006;28(6):568‐73. [PUBMED: 17101265] [DOI] [PubMed] [Google Scholar]
Stevens 2011 {published data only}
- Stevens KN, Croes S, Boersma RS, Stobberingh EE, Marel C, Veen FH, et al. Hydrophilic surface coatings with embedded biocidal silver nanoparticles and sodium heparin for central venous catheters. Biomaterials 2011;32(5):1264‐9. [PUBMED: 21093906] [DOI] [PubMed] [Google Scholar]
Venkatesh 2009 {published data only}
- Venkatesh M, Rong L, Raad I, Versalovic J. Novel synergistic antibiofilm combinations for salvage of infected catheters. Journal of Medical Microbiology 2009;58(Pt 7):936‐44. [PUBMED: 19502361] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
PREVAIL {published data only}
- PREVAIL. Ongoing study June 2015.
Additional references
Adams‐Chapman 2006
- Adams‐Chapman I, Stoll BJ. Neonatal infection and long‐term neurodevelopmental outcome in the preterm infant. Current Opinion in Infectious Diseases 2006;19(3):290‐7. [PUBMED: 16645492] [DOI] [PubMed] [Google Scholar]
Alonso‐Echanove 2003
- Alonso‐Echanove J, Edwards JR, Richards MJ, Brennan P, Venezia RA, Keen J, et al. Effect of nurse staffing and antimicrobial‐impregnated central venous catheters on the risk for bloodstream infections in intensive care units. Infection Control and Hospital Epidemiology 2003;24(12):916‐25. [PUBMED: 14700407] [DOI] [PubMed] [Google Scholar]
Aslam 2007
- Aslam S, Darouiche RO. Prolonged bacterial exposure to minocycline/rifampicin‐impregnated vascular catheters does not affect antimicrobial activity of catheters. The Journal of Antimicrobial Chemotherapy 2007;60(1):148‐51. [PUBMED: 17525051] [DOI] [PubMed] [Google Scholar]
Bassler 2009
- Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 2009;123(1):313‐8. [PUBMED: 19117897] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2001
- Benjamin DK Jr, Miller W, Garges H, Benjamin DK, McKinney RE Jr, Cotton M, et al. Bacteremia, central catheters, and neonates: when to pull the line. Pediatrics 2001;107(6):1272‐6. [PUBMED: 11389242] [DOI] [PubMed] [Google Scholar]
Borghesi 2008
- Borghesi A, Stronati M. Strategies for the prevention of hospital‐acquired infections in the neonatal intensive care unit. The Journal of Hospital Infection 2008;68(4):293‐300. [PUBMED: 18329134] [DOI] [PubMed] [Google Scholar]
Cartwright 2004
- Cartwright DW. Central venous lines in neonates: a study of 2186 catheters. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(6):F504‐8. [PUBMED: 15499142] [DOI] [PMC free article] [PubMed] [Google Scholar]
Casey 2008
- Casey AL, Mermel LA, Nightingale P, Elliott TS. Antimicrobial central venous catheters in adults: a systematic review and meta‐analysis. The Lancet Infectious Diseases 2008;8(12):763‐76. [PUBMED: 19022192] [DOI] [PubMed] [Google Scholar]
Chapman 2003
- Chapman RL, Faix RG. Persistent bacteremia and outcome in late onset infection among infants in a neonatal intensive care unit. The Pediatric Infectious Disease Journal 2003;22(1):17‐21. [PUBMED: 12544403] [DOI] [PubMed] [Google Scholar]
Cicalini 2004
- Cicalini S, Palmieri F, Petrosillo N. Clinical review: new technologies for prevention of intravascular catheter‐related infections. Critical Care 2004;8(3):157‐62. [PUBMED: 15153233] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Brito 2010
- Brito CS, Brito DV, Abdallah VO, Gontijo Filho PP. Occurrence of bloodstream infection with different types of central catheter in critically neonates. The Journal of Infection 2010;60(2):128‐32. [PUBMED: 19944717] [DOI] [PubMed] [Google Scholar]
Demetriades 2011
- Demetriades AK, Bassi S. Antibiotic resistant infections with antibiotic‐impregnated Bactiseal catheters for ventriculoperitoneal shunts. The British Journal of Neurosurgery 2011;25(6):671‐3. [PUBMED: 21707238] [DOI] [PubMed] [Google Scholar]
Eggimann 2003
- Eggimann P, Pittet D. Pathophysiology and prevention of intravascular catheter‐related infection [Physiopathologie et prevention des infections liees aux acces vasculaires]. Medecine et Maladies Infectieuses 2003;33(11):554‐63. [Google Scholar]
Garland 2008
- Garland JS, Alex CP, Sevallius JM, Murphy DM, Good MJ, Volberding AM, et al. Cohort study of the pathogenesis and molecular epidemiology of catheter‐related bloodstream infection in neonates with peripherally inserted central venous catheters. Infection Control and Hospital Epidemiology 2008;29(3):243‐9. [PUBMED: 18220483] [DOI] [PubMed] [Google Scholar]
Gilbert 2008
- Gilbert RE, Harden M. Effectiveness of impregnated central venous catheters for catheter related blood stream infection: a systematic review. Current Opinion in Infectious Diseases 2008;21(3):235‐45. [PUBMED: 18448967] [DOI] [PubMed] [Google Scholar]
Gordon 2006
- Gordon A, Isaacs D. Late onset neonatal Gram‐negative bacillary infection in Australia and New Zealand: 1992‐2002. The Pediatric Infectious Disease Journal 2006;25(1):25‐9. [PUBMED: 16395098] [DOI] [PubMed] [Google Scholar]
Halton 2009
- Halton KA, Cook DA, Whitby M, Paterson DL, Graves N. Cost effectiveness of antimicrobial catheters in the intensive care unit: addressing uncertainty in the decision. Critical Care 2009;13(2):R35. [PUBMED: 19284570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermans 2007
- Hermans D, Talbotec C, Lacaille F, Goulet O, Ricour C, Colomb V. Early central catheter infections may contribute to hepatic fibrosis in children receiving long‐term parenteral nutrition. Journal of Pediatric Gastroenterology and Nutrition 2007;44(4):459‐63. [PUBMED: 17414144] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoang 2008
- Hoang V, Sills J, Chandler M, Busalani E, Clifton‐Koeppel R, Modanlou HD. Percutaneously inserted central catheter for total parenteral nutrition in neonates: complication rates related to upper versus lower extremity insertion. Pediatrics 2008;121(5):e1152‐9. [PUBMED: 18390957] [DOI] [PubMed] [Google Scholar]
Hockenhull 2008
- Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dündar Y, et al. The clinical effectiveness and cost‐effectiveness of central venous catheters treated with anti‐infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technology Assessment 2008;12(12):iii‐iv, xi‐xii, 1‐154. [PUBMED: 18405471] [DOI] [PubMed] [Google Scholar]
Isaacs 2003
- Isaacs D, The Australasian Study Group For Neonatal Infections. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(2):F89–93. [PUBMED: 12598493] [DOI] [PMC free article] [PubMed] [Google Scholar]
Isaacs 2004
- Isaacs D, Fraser S, Hogg G, Li HY. Staphylococcus aureus infections in Australasian neonatal nurseries. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(4):F331‐5. [PUBMED: 15210669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kandasamy 2011
- Kandasamy J, Dwan K, Hartley JC, Jenkinson MD, Hayhurst C, Gatscher S, et al. Antibiotic‐impregnated ventriculoperitoneal shunts‐‐a multi‐centre British paediatric neurosurgery group (BPNG) study using historical controls. Child's Nervous System 2011;27:575‐81. [PUBMED: 20953871] [DOI] [PubMed] [Google Scholar]
Kaplan 2011
- Kaplan HC, Lannon C, Walsh MC, Donovan EF, Ohio Perinatal Quality Collaborative. Ohio statewide quality‐improvement collaborative to reduce late onset sepsis in preterm infants. Pediatrics 2011;127(3):427‐35. [PUBMED: 21339274] [DOI] [PubMed] [Google Scholar]
Lahra 2009
- Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13‐year hospital cohort study. Pediatrics 2009;123(5):1314‐9. [PUBMED: 19403497] [DOI] [PubMed] [Google Scholar]
Lambert 2012
- Lambert A, Lemarignier‐Nueffer C, Iooss P, Roncalez D. Anti‐infective‐treated central venous catheters clinical effectiveness and safety [Evaluation de l'interet clinique et de la tolerance des catheters veineux centraux impregnes d'antimicrobiens]. Journal de Pharmacie Clinique 2012;31(2):73‐87. [Google Scholar]
Lenz 2010
- Lenz AM, Vassallo JC, Moreno GE, Althabe M, Gómez S, Magliola R, et al. Prevention of catheter‐related infection: usefulness and cost‐effectiveness of antiseptic catheters in children. Archivos Argentinos Pediatria 2010;108(3):209‐15. [PUBMED: 20544135] [DOI] [PubMed] [Google Scholar]
Licameli 2008
- Licameli G, Johnston P, Luz J, Daley J, Kenna M. Phosphorylcholine‐coated antibiotic tympanostomy tubes: are post‐tube placement complications reduced?. International Journal of Pediatric Otorhinolaryngology 2008;72(9):1323‐8. [PUBMED: 18635268] [DOI] [PubMed] [Google Scholar]
Machado 2009
- Machado JD, Suen VM, Figueiredo JF, Marchini JS. Biofilms, infection, and parenteral nutrition therapy. Journal of Parenteral and Enteral Nutrition 2009;33(4):397–403. [PUBMED: 19401480] [DOI] [PubMed] [Google Scholar]
Mahieu 2001a
- Mahieu LM, Dooy JJ, Muynck AO, Melckebeke G, Ieven MM, Reempts PJ. Microbiology and risk factors for catheter exit‐site and hub colonization in neonatal intensive care unit patients. Infection Control and Hospital Epidemiology 2001;22(6):357‐62. [PUBMED: 11519913] [DOI] [PubMed] [Google Scholar]
Mahieu 2001b
- Mahieu LM, Buitenweg N, Beutels P, Dooy JJ. Additional hospital stay and charges due to hospital‐acquired infections in a neonatal intensive care unit. The Journal of Hospital Infection 2001;47(3):223‐9. [PUBMED: 11247683] [DOI] [PubMed] [Google Scholar]
Mahieu 2001c
- Mahieu LM, Muynck AO, Ieven MM, Dooy JJ, Goossens HJ, Reempts PJ. Risk factors for central vascular catheter‐associated bloodstream infections among patients in a neonatal intensive care unit. The Journal of Hospital Infection 2001;48(2):108‐16. [PUBMED: 11428877] [DOI] [PubMed] [Google Scholar]
Mahieu 2001d
- Mahieu LM, Dooy JJ, Lenaerts AE, Ieven MM, Muynck AO. Catheter manipulations and the risk of catheter‐associated bloodstream infection in neonatal intensive care unit patients. The Journal of Hospital Infection 2001;48(1):20‐6. [PUBMED: 11358467] [DOI] [PubMed] [Google Scholar]
Makhoul 2002
- Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late‐onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 2002;109(1):34‐9. [PUBMED: 11773539] [DOI] [PubMed] [Google Scholar]
Miller 2010
- Miller MR, Griswold M, Harris JM 2nd, Yenokyan G, Huskins WC, Moss M, et al. Decreasing PICU catheter‐associated bloodstream infections: NACHRI's quality transformation efforts. Pediatrics 2010;125(2):206‐13. [PUBMED: 20064860] [DOI] [PubMed] [Google Scholar]
Munson 2004
- Munson EL, Heard SO, Doern GV. In vitro exposure of bacteria to antimicrobial impregnated‐central venous catheters does not directly lead to the emergence of antimicrobial resistance. Chest 2004;126(5):1628‐35. [PUBMED: 15539737] [DOI] [PubMed] [Google Scholar]
Muttaiyah 2010
- Muttaiyah S, Ritchie S, John S, Mee E, Roberts S. Efficacy of antibiotic‐impregnated external ventricular drain catheters. Journal of Clinical Neuroscience 2010;17(3):286‐8. [PUBMED: 20074964] [DOI] [PubMed] [Google Scholar]
O'Grady 2002
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter‐related infections. Infection Control and Hospital Epidemiology 2002;23(12):759‐69. [PUBMED: 12517020] [DOI] [PubMed] [Google Scholar]
O'Grady 2011
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter‐related infections. American Journal of Infection Control 2011;39(4 Suppl 1):S1‐34. [PUBMED: 21511081] [DOI] [PubMed] [Google Scholar]
Ohki 2008
- Ohki Y, Yoshizawa Y, Watanabe M, Kuwashima M, Morikawa A. Complications of percutaneously inserted central venous catheters in Japanese neonates. Pediatrics International 2008;50(5):636‐9. [PUBMED: 19261110] [DOI] [PubMed] [Google Scholar]
Olsen 2009
- Olsen AL, Reinholdt J, Jensen AM, Andersen LP, Jensen ET. Nosocomial infection in a Danish Neonatal Intensive Care Unit: a prospective study. Acta Paediatrica 2009;98(8):1294‐9. [PUBMED: 19438843] [DOI] [PubMed] [Google Scholar]
Payne 2004
- Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics 2004;114(2):348‐55. [PUBMED: 15286215] [DOI] [PubMed] [Google Scholar]
Pronovost 2006
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. New England Journal of Medicine 2006;355(26):2725‐32. [PUBMED: 17192537] [DOI] [PubMed] [Google Scholar]
Ramirez de Arellano 1994
- Ramirez de Arellano E, Pascual A, Martinez‐Martinez L, Perea EJ. Activity of eight antibacterial agents on staphylococcus epidermidis attached to Teflon catheters. Journal of Medical Microbiology 1994;40(1):43‐7. [PUBMED: 8289214] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Saint 2000
- Saint S, Veenstra DL, Lipsky BA. The clinical and economic consequences of nosocomial central venous catheter‐related infection: are antimicrobial catheters useful?. Infection Control and Hospital Epidemiology 2000;21(6):375‐80. [PUBMED: 10879567] [DOI] [PubMed] [Google Scholar]
Salzman 1995
- Salzman MB, Rubin LG. Intravenous catheter‐related infections. Advances in Pediatric Infectious Diseases 1995;10:337‐68. [PUBMED: 7718211] [PubMed] [Google Scholar]
Sampath 2001
- Sampath LA, Tambe SM, Modak SM. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infection Control and Hospital Epidemiology 2001;22(10):640‐6. [PUBMED: 11776351] [DOI] [PubMed] [Google Scholar]
Sannoh 2010
- Sannoh S, Clones B, Munoz J, Montecalvo M, Parvez B. A multimodal approach to central venous catheter hub care can decrease catheter‐related bloodstream infection. American Journal of Infection Control 2010;38(6):424‐9. [PUBMED: 20137829] [DOI] [PubMed] [Google Scholar]
Schelonka 2006
- Schelonka RL, Scruggs S, Nichols K, Dimmitt RA, Carlo WA. Sustained reductions in neonatal nosocomial infection rates following a comprehensive infection control intervention. Journal of Perinatology 2006;26(3):176‐9. [PUBMED: 16341027] [DOI] [PubMed] [Google Scholar]
Schulman 2011
- Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, et al. Statewide NICU central‐line‐associated bloodstream infection rates decline after bundles and checklists. Pediatrics 2011;127(3):436‐44. [PUBMED: 21339265] [DOI] [PubMed] [Google Scholar]
Shah 2008
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. The Journal of Pediatrics 2008;153(2):170‐5. [PUBMED: 18534228] [DOI] [PubMed] [Google Scholar]
Shorr 2003
- Shorr AF, Humphreys CW, Helman DL. New choices for central venous catheters: potential financial implications. Chest 2003;124(1):275‐84. [PUBMED: 12853534] [PubMed] [Google Scholar]
Stewart 2001
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. The Lancet 2001;358(9276):135‐8. [PUBMED: 11463434] [DOI] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late‐onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285‐91. [PUBMED: 12165580] [DOI] [PubMed] [Google Scholar]
Stoll 2004
- Stoll BJ, Hansen NI, Adams‐Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low‐birth‐weight infants with neonatal infection. JAMA 2004;292(19):2357‐65. [PUBMED: 15547163] [DOI] [PubMed] [Google Scholar]
Tambe 2001
- Tambe SM, Sampath L, Modak SM. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. The Journal of Antimicrobial Chemotherapy 2001;47(5):589‐98. [PUBMED: 11328769] [DOI] [PubMed] [Google Scholar]
Thomas 2012
- Thomas R, Lee S, Patole S, Rao S. Antibiotic‐impregnated catheters for the prevention of CSF shunt infections: a systematic review and meta‐analysis. British Journal of Neurosurgery 2012;26(2):175‐84. [PUBMED: 21973061] [DOI] [PubMed] [Google Scholar]
Thornburg 2008
- Thornburg CD, Smith PB, Smithwick ML, Cotten CM, Benjamin DK Jr. Association between thrombosis and bloodstream infection in neonates with peripherally inserted catheters. Thrombosis Research 2008;122(6):782‐5. [PUBMED: 17997477] [DOI] [PubMed] [Google Scholar]
Trotter 1996a
- Trotter CW. Percutaneous central venous catheter‐related sepsis in the neonate: an analysis of the literature from 1990 to 1994. Neonatal Network 1996;15(3):15‐28. [PUBMED: 8715646] [PubMed] [Google Scholar]
Trotter 1996b
- Trotter CW. Percutaneous central venous catheters in neonates: a descriptive analysis and evaluation of predictors for sepsis. The Journal of Perinatal & Neonatal Nursing 1996;10(2):56‐71. [PUBMED: 8868627] [DOI] [PubMed] [Google Scholar]
Van der Zwet 2005
- Zwet WC, Kaiser AM, Elburg RM, Berkhof J, Fetter WP, Parlevliet GA, et al. Nosocomial infections in a Dutch neonatal intensive care unit: surveillance study with definitions for infection specifically adapted for neonates. The Journal of Hospital Infection 2005;61(4):300‐11. [PUBMED: 16221510] [DOI] [PubMed] [Google Scholar]
Vanholder 2010
- Vanholder R, Canaud B, Fluck R, Jadoul M, Labriola L, Marti‐Monros, A, et al. Catheter‐related blood stream infections (CRBSI): a European view. Nephrology, Dialysis, Transplantation 2010;25(6):1753–6. [PUBMED: 20466662] [DOI] [PubMed] [Google Scholar]
Walz 2010
- Walz JM, Memtsoudis SG, Heard SO. Prevention of central venous catheter bloodstream infections. Journal of Intensive Care Medicine 2010;25(3):131‐8. [PUBMED: 20089527] [DOI] [PubMed] [Google Scholar]
Weber 2012
- Weber JM, Sheridan RL, Fagan S, Ryan CM, Pasternack MS, Tompkins RG. Incidence of catheter‐associated bloodstream infection after introduction of minocycline and rifampin antimicrobial‐coated catheters in a pediatric burn population. Journal of Burn Care & Research 2012;33(4):539‐43. [PUBMED: 22210071] [DOI] [PubMed] [Google Scholar]
Wirtschafter 2010
- Wirtschafter DD, Pettit J, Kurtin P, Dalsey M, Chance K, Morrow HW, et al. A state wide quality improvement collaborative to reduce neonatal central line‐associated blood stream infections. Journal of Perinatology 2010;30(3):170–81. [PUBMED: 19940855] [DOI] [PubMed] [Google Scholar]
Wong 2012
- Wong J, Dow K, Shah PS, Andrews W, Lee S. Percutaneously placed central venous catheter‐related sepsis in Canadian neonatal intensive care units. American Journal of Perinatology 2012;29(8):629‐34. [PUBMED: 22566117] [DOI] [PubMed] [Google Scholar]
Yébenes 2004
- Yébenes JC, Vidaur L, Serra‐Prat M, Sirvent JM, Batlle J, Motje M, et al. Prevention of catheter related blood stream infection in critically ill patients using a disinfectable, needle free connector: a randomised controlled trial. American Journal of Infection Control 2004;32(5):291‐5. [PUBMED: 15292895] [DOI] [PubMed] [Google Scholar]


