Abstract
Antibiotics do not differentiate between good and bad germs, disrupting normal microflora and causing vitamin deficiency in the human body. They also kill healthy bacteria in the gut and genital tract on a large scale, weakening the host's defense mechanism. Probiotics are a colony of bacteria that live in our intestines and are regarded as a metabolic 'organ' due to their beneficial effects on human health, including metabolism and immunological function. They are used in clinical settings to prevent and treat conditions such as diarrhoea, colon cancer, hypertension, diabetes, acute pancreatitis, Helicobacter pylori infection, ventilator-associated pneumonia, migraine and autism. Probiotics may modify immunological activity by increasing innate and adaptive immune responses, altering microbial habitat in the intestine, improving gut barrier function, competitive adherence to the mucosa and epithelium, and producing antimicrobial compounds. The aim of this study is to index that further in depth researches to be conducted on probiotics pivotal role in the prophylaxis and therapeutic usage for a variety of disease that may or may not have treatment alternatives. Key words such as probiotics, microbiota, prophylactics, and therapeutic applications were searched extensively in research databases such as PubMed, PubMed Central (PMC), Scopus, Web of Science, Research Gate, Google Scholar, and Cochrane Library. This concise narrative review article summarized primarily the history, selection, mechanism/mode of action, recent advances in prophylactic and therapeutic applications, and future directions in the use of probiotics for prophylactic and therapeutic applications.
Keywords: Probiotics, Microbiota, Prophylactic, Therapeutics
Probiotics; Microbiota; Prophylactic; Therapeutics
1. Introduction to probiotics
Antibiotics are used to treat a wide spectrum of potentially fatal bacterial infections, both prophylactically and curatively [1]. Antibiotics have been exposed to a vast portion of the world's population, either directly or indirectly, due to their importance. Antibiotic medication, on the other hand, is linked to: 1) severe side effects; 2) it fails to distinguish between good and harmful microorganisms, disrupting normal microflora and resulting in vitamin shortage in the human body (Vitamin B, Vitamin K, and so on) [2]. Antibiotic usage on a large scale indiscriminately destroys normal gut and genital tract bacteria, impairing the host's defence mechanisms. Researchers from all around the world are looking for an alternative supply as a result of these issues. Many recent studies have shown that altering the commensal microbiota can help prevent and treat a variety of gastrointestinal infections [1, 2, 3]. The human microbiota is a varied collection of microorganisms that live in the human gastrointestinal, respiratory, cutaneous, oral, and genitourinary tracts. It is estimated that the microbiota weighs between 1-2 kg [4, 5]. After the surface area of the respiratory system, the gastro intestinal tract (GIT) has the second highest surface area [6]. The gut microbiota is formed during birth and is acquired naturally from the mother via vertical transmission (from the mother's birth canal) and the environment. The authors concluded that the GIT of a new-born is nearly sterile, in contrast to the microflora of adults. The style of delivery, cleanliness level, and mode of feeding, as well as the mother's flora, genetic factors, and medication use, all influence the microflora's proliferation after birth, which is influenced by the mother's flora, genetic factors, and medication use [6, 7]. The human gut microbiota has around 1000 phylotypes, according to recent research employing molecular methods. Anaerobes make up the majority of bacterial species (97%) [8]. The human gut microbiota has around 1000 phylotypes, according to recent research employing molecular methods. Anaerobes make up the majority of bacterial species (97%) [9, 10, 11, 12]. Bacteroidetes (Porphyromonas, Prevotella, Bacteroides), Firmicutes (Ruminococcus, Clostridium, Lactobacillus, and Eubacteria), and Actinobacteria (Bifidobacterium) make up the gut microbiome, with Bifidobacterium and Bactericides accounting for the majority of intestinal flora [13] .
Microbial antagonism is a phenomena in which normal floras hinder pathogenic germs from colonizing and hence provide health advantages to the host. Competition between floras pushes harmful bacteria out of the intestines, improves levels of vital nutrients like short-chain fatty acids, vitamins, arginine, cysteine, and glutamine amino acids, growth factors, and antioxidants, and strengthens the immune system [14, 15, 16]. As a result, effective epithelial barrier-protective therapies against GIT discomfort and other implicated variables, such as mucosa-associated E. coli, must be created employing competitive probiotic bacteria-based dietary items. The microbe employed to regulate microorganisms should be nontoxic to the host and have no effect on the human gut's natural biome [14, 17]. Probiotics boost some types of micro flora in the intestine, but not the overall bacterial population. Probiotic antibacterial effect is linked to the production of organic acids, ethanol, hydrogen peroxide, or protein-containing components (bacteriocins) [18, 19]. Probiotics are the second most important immune defense system once antibiotics become ineffective owing to antibiotic resistance, according to the WHO. Microbial interference therapy is the name for this type of treatment [20]. In nutraceutical science, ingestible living microorganisms known as "probiotics" are valued for their capacity to provide customers with a variety of health benefits [21].
1.1. Flora imbalance
The defense mechanisms that defend the mammalian GIT from bacterial colonization are extremely complicated. Intestinal inflammation, which is caused by an imbalance in gut flora between pathogenic and commensal bacteria, causes the mucus layer to thin or disappear, allowing bacteria and their metabolites to pass through the mucus layer and invade intestinal epithelial cells, resulting in a reduction in intestinal mechanical barrier function [22]. Most mammals' GI tracts are colonized by two types of bacteria: 1) native microflora and 2) invasive pathogenic germs. Sustaining intestinal immunity and homeostasis requires a healthy gut microbiota. A shift in this equilibrium could have negative pathophysiological consequences [23].
1.2. History and definition
Probiotics are a type of bacteria that live in our intestines and are regarded a metabolic 'organ' because of their beneficial effects on human health, including metabolism and immunological function [24].
Microorganisms initially appeared on Earth 3.8 billion years ago, significantly earlier than the Homo genus, which appeared 2.5 million years ago in Africa. As a result, bacteria had a lot more time outside of humans to modify and adapt by inventing survival strategies that allowed them to survive in even the most hostile situations [10]. The use of live microorganisms in food, particularly bacteria that produce lactic acid, has a long history of maintaining and improving human health. Fermented dairy foods were employed to keep ancient civilizations healthy, such as the Greeks and Romans. In 76 BC, a Roman historian advised using milk fermentation products to cure gastroenteritis. Microorganisms in fermented foods and their impact on human health, on the other hand, have just recently been researched [19, 25]. In fact, probiotics have a modern history that dates back to the early 1900s. Louis Pasteur found the microorganisms that cause fermentation, whereas Metchnikoff initially sought to determine the microbes' potential impact on human health. He linked Bulgarian rural people's longer lives to the fact that they live longer [26].
1.3. Selection criteria and minimal requirement of probiotics
Probiotic strains for food applications should ideally come from humans, be acid and bile tolerant, adhere to GIT linings, compete with pathogenic bacteria, and have a safe dosage for human use [16]. Probiotics selection criteria includes; 1) benefit the host, 2) survive transit through the intestines, 3) cling to the intestinal epithelial cell membrane, 4) create antibiotic substances against infections, and 5) stabilize the intestinal microflora [17].
1.4. Minimal requirement of probiotics
1) The microorganisms must be given alive and in large enough quantities; 2) The strains must be genetically identified, assigned letters, numbers or names, and categorised according to current nomenclature. 3) Appropriately sized and organized experiments must be performed to designate a strain as a probiotics, as well as the strain(s) being used on the host (like human, livestock, companion animal, etc.) for which probiotics are intended; 4) It's possible that strains that have been shown to help with one ailment won't help with another; 5) Throughout the testing procedure, human probiotic strains used in animal studies should be labelled as such [27]. Despite their beneficial use for a variety of diseases, research on probiotics is limited. The goal of this study is to indicate that more in-depth research on probiotics pivotal role in prophylaxis and therapeutic usage for a variety of diseases that may or may not have treatment alternatives. This paper attempts to review the definition of probiotics, the history of probiotics, the mechanism of probiotic action, and the description of prophylactic and therapeutic effects on selected areas of human disease by using a consortium of beneficial bacterial species that can easily adapt to and tolerate the human gut environment and impart health benefits.
2. Mechanism of probiotics action
Apart from their beneficial benefits, probiotics' mode of action has been loosely defined as improving the host's "microbial balance" or the ambient environment's balance, although molecular mechanisms are rarely examined further because there are so many alternative processes described for them. They protect epithelial membranes from harmful microorganisms by passing through the stomach and mucous membranes when consumed orally. Probiotic bacteria like Bifidobacterium and Lactobacillus which create acids like lactic acid, propionic acid, and acetic acid, which lower pH and inhibit harmful bacteria from growing. Their role in immunomodulation has also been hypothesized as a mechanism of action [16, 17, 28]. Lactobacillus contains 261 species (as of March 2020) that are extremely diverse on phenotypic, ecological, and genotypic levels. Because the genus Lactobacillus was reclassified into 25 genera, including the emended genus Lactobacillus, the new names for important probiotics were mentioned in the manuscript [29].
Antimicrobial compounds produced by probiotics target infections, limit adhesion sites, compete for nutrients, destroy toxin receptors, and modulate immunity. The theorized mechanism of probiotic action is summarized here: 1) Preventing harmful germs from adhering to the intestinal epithelium 2) Pathogenic bacteria are inhibited from growing by binding to gram-negative bacteria. 3) Maintaining normal short-chain fatty acid levels (SCFAs). 4) Colonocyte multiplication to repair intestinal permeability. 5) Increased electrolyte absorption in the intestine. 6) Improving the immunological response in the intestine. 7) Lipid metabolism regulation. 8) Intestinal pro-inflammatory cytokines are suppressed [11, 17, 19, 30, 31].
3. Clinical application of probiotics
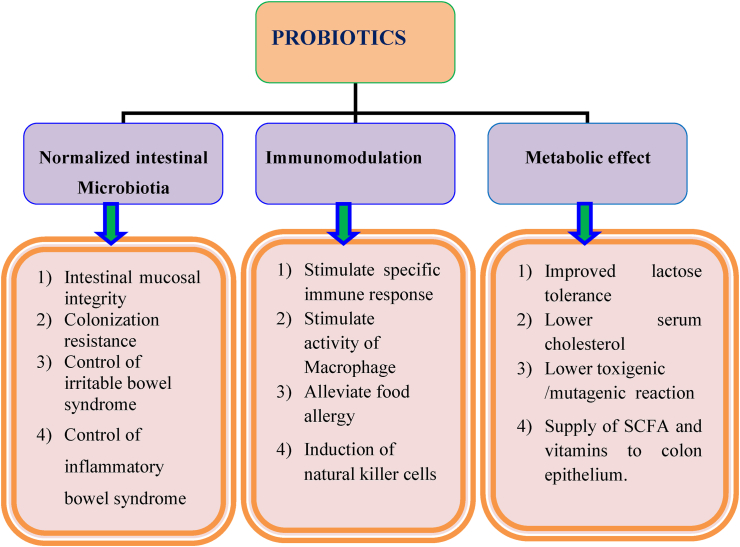
Probiotics can help with a number of acute and chronic infectious disorders, in addition to stomach problems as shown in the Figure 1 [32]. Many factors can weaken the body's resistance, resulting in inflammatory, viral, neoplastic, and degenerative diseases. Other treatments, like as antibiotics, irradiation, and immunosuppressive therapy, may alter the usual flora makeup of the stomach. The goal of functional food products like probiotics is to improve cellular well-being and accelerate the implementation of cells' natural defense mechanisms [18].
Figure 1.
Schematic representation of various roles of probiotics [33].
Several studies have looked into the effects of probiotics on human disease, diseases, mood, behaviours, and performance. Pharmacological therapy, dietary intervention, and the use of alternative substances like probiotics should all be incorporated in the treatment plan, according to the authors. Probiotics are known to have immune-modulatory effects on the host, making them a promising therapeutic and preventive option for a variety of illnesses, including inflammatory disease. Consumption of probiotics in the form of powder, capsules, and drinks helps to restore the beneficial microflora in the gut, which benefits humans by boosting their immune systems [2, 14, 34, 35].
4. Prophylaxis uses of probiotics
4.1. Cclostridium difficile colitis infection in hospitalized patients
Clostridium difficile colitis is an opportunistic infection caused by antibiotic-induced changes in the normal gut flora. The most usually associated antibiotics include clindamycin, fluoroquinolones, broad-spectrum penicillin, and cephalosporin. Clostridium difficile colitis infection is a condition that ranges in severity, with the most severe instances requiring admission to an intensive care unit and being deadly. Probiotics are living non-pathogenic bacteria that colonize the gastrointestinal system and create a lytic peptide that inhibits Clostridium difficile toxin activity. Saccharomyces boulardii produces a protease that inhibits Clostridium difficile toxin activity [36].
4.2. Probiotics prevent postoperative infections
The microbiome is now known to influence a wide range of pathologic and normal processes, and manipulating it may improve patient outcomes while also providing host health advantages. The goal of prophylactic probiotics is to keep "good" bacteria colonized in order to contend with Clostridium difficile overgrowth (CD). Clostridium difficile toxins A and B are able to be neutralized by some probiotics. Probiotics have protective properties, according to data from in vitro and preclinical experiments [37]. Physical injury to the intestinal mucosa, which causes disruption of the gut barrier and increased intestinal permeability, as well as microbial imbalance and decreased immunodeficiency in the postoperative patient, are the most common causes of bacterial translocation. Patients undergoing abdominal surgery for medical conditions such as biliary cancer surgery, pancreaticoduodenectomy, and liver transplantation are at risk for urinary tract infection (UTI), pneumonia, wound infection, intra-abdominal abscess, and cholangitis [38]. Clostridium difficile colitis rates are reduced when probiotics are given alongside antibiotics to adult patients with non-surgical infections. This is a promising infection-prevention strategy that could reduce morbidity, antibiotic therapy duration, hospital stay length, and the risk of antimicrobial resistance emergence [37, 38].
4.3. Probiotics in very low birth weight infants
The leading causes of neonatal morbidity and mortality in new-borns with very low birth weight (VLBW) are nosocomial infection and necrotizing enterocolitis. Endogenous host factors such as gestational age and immune response immaturity have been hypothesized as part of a complicated etiology for both diseases. Furthermore, environmental factors such as enteral nutrition and exposure to an endemic hospital environment are important because they influence abnormal gastrointestinal colonization and entero-pathogenic bacteria translocation via vulnerable intestinal mucosa [39, 40]. The prevention of necrotizing enterocolitis surgery, any abdominal surgery, and all-cause death was associated to the usage of Lactobacillus acidophilus/Bifidobacterium infantis probiotics. Cross-talk between the developing immune system and gut microbes may be the source of probiotics [40].
4.4. Prophylactic intervention of probiotics in cancer (carcinogenesis)
Colorectal cancer patients had greater populations of Bacteroides and Prevotella, whereas people with colorectal adenoma had higher populations of Dorea spp. and faecal bacteria spp. in their colonic microbiota. These species have the potential to create carcinogens and tumor-promoting compounds such heterocyclic amines and bile acids [33, 41, 42]. Not only do probiotic strains prevent infections, but some of them also have anticancer properties in rodents and people [43]. Probiotic bacteria have been proven to have an important function in immunomodulation and have anticancer effects in the future. Bacterial strains may be involved in the detection and degradation of possible carcinogens, as well as the synthesis of short-chain fatty acids, which regulate cell death and proliferation and are known as immune system signalling molecules. Heat-killed probiotic bacteria combined with radiation showed a positive effect on improving cancer cell immunological recognition [44]. Lactobacillus and Bifidobacterium are two of the most common probiotic bacteria found in lactic acid bacteria (LAB), and studies have demonstrated that both strains can mediate anticancer responses. This is due to the fact that lactic acid cultures can alter the activity of faecal enzymes such alpha-glucuronidase, azoreductase, and nitroreductase, all of which have a role in the development of colon cancer [41]. The researchers discovered that giving colorectal cancer patients a symbiotic combination of Lacticaseibacillus rhamnosus GG, Bifidobacterium lactis Bb12, and oligofructose-enriched inulin for 12 weeks resulted in favorable changes in the gut microbiome, with high levels of lactobacilli and bifidobacteria and low levels of Clostridium perfringens. Multiple studies have demonstrated that Lacticasei bacilli casei improves the immune-responsive activities of T cells, macrophages, natural killer cells, and T cells in the fight against cancer. It has also been demonstrated to reduce the side effects of radiotherapy and chemotherapy in the treatment of colorectal cancer [33].
The exact methods through which probiotics may suppress cancer are still being researched. unknown [45, 46]. However, such pathways could include the complex connection between nutrition, gut flora, and host energy metabolism, which causes a number of impacts [46], specifically, 1) carcinogen neutralization; 2) through increasing mucin, defensins, and immunoglobulin A (IgA) synthesis and altering pro-inflammatory cytokine and chemokine responses, contribute in the repair of the intestinal barrier and enhancement of its function; 3) Vitamin and short-chain fatty acid (SCFA) synthesis, as well as nutrition and growth signals for the intestinal epithelium, which may aid in the prevention of colon and other cancers; 4) increased production of cytokines (IL-2 and IL-12), antioxidants, and anti-angiogenic factors; 5) lower intestinal pH; 6) modulation and enhancement of the host's innate and adaptive immune responses by secreting anti-inflammatory molecules and influencing the helper T-cell response and regulation; Antiproliferative effects via apoptosis and cell differentiation regulation; 7) blockade of immunological checkpoint; 8) intestinal microflora modulation; 9) Controlling apoptosis and cell differentiation that result in antiproliferative effects; 10) inhibition tyrosine kinase and [47, 48] other signalling pathways; and 11) Conversion of linoleic acid to conjugated linoleic acid (CLA), an anti-inflammatory polyunsaturated fatty acid that aids in cancer cell viability reduction and apoptosis induction [49, 50, 51].
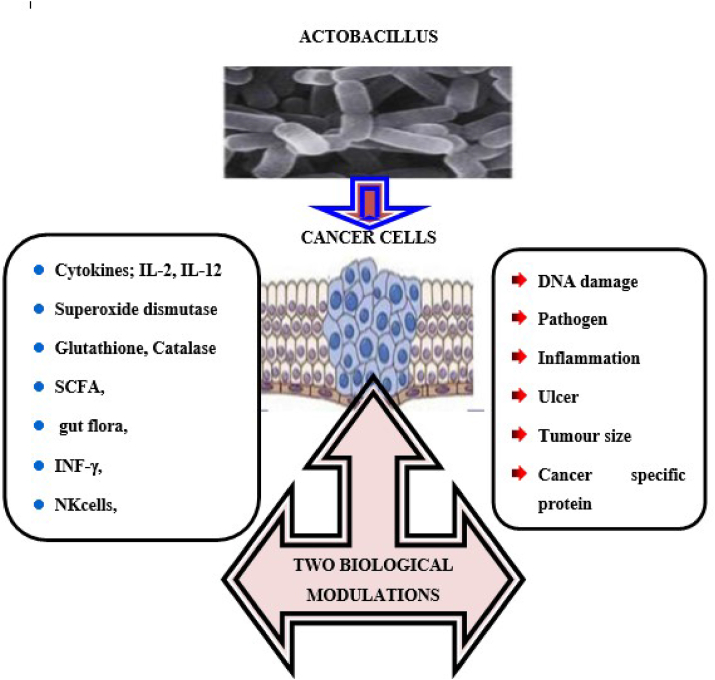
In response to Lactobacillus, some biological factors increased, such as cytokines, interleukins (IL-2, IL-12), antioxidants (SOD, CAT, GSH), indigenous microbial flora, interferons (IFN-), and immune cells (TH cells, NK cells), while others decreased, such as DNA damage, pathogens, inflammation, ulcers, tumor size, cancer specific proteins, polyamine contents, and pro-carcinogenic enzymes Figure 2 [42].
Figure 2.
Lactobacillus triggered biological changes in cancer cells [42].
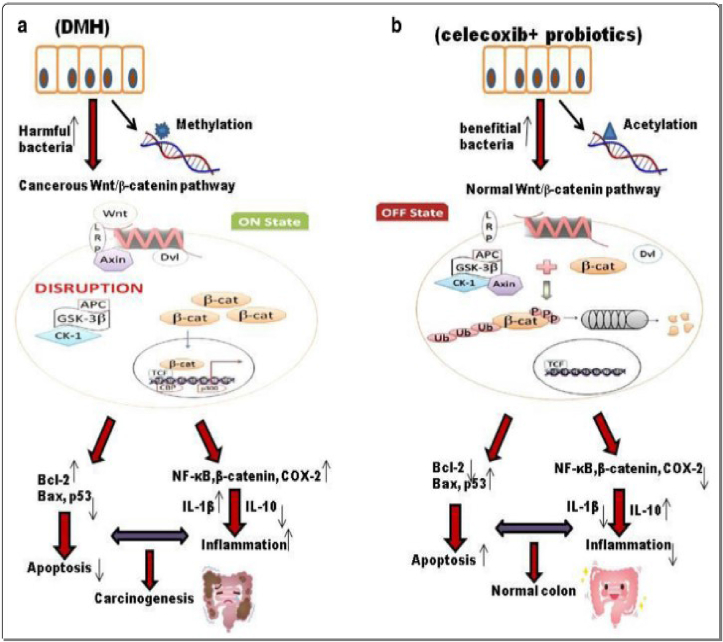
It was discovered that giving rats a combination of probiotics and celecoxib one week before inducing colorectal cancer reduced the expression of the proto-oncogene K-ras while increasing the expression of the tumor suppressor p53, which supports previous findings. This could be owing to the combined impact of probiotics and celecoxib as shown in Figure 3, which could have inhibited cell growth by activating tumor suppressor genes while preserving cell activity and cell cycle. A combination of therapies like this could have helped to maintain gut integrity and increase immune response, resulting in reduced DNA damage [52]. Celecoxib's anti-inflammatory properties resulted in fewer colonic tumours due to down-regulation of K-ras and up-regulation of p53, which can directly induce Bax-mediated apoptosis [52, 53, 54].
-
(a)
DNA methylation induced by differential methylation hybridization (DMH) activates the Wnt pathway, which is accelerated by the overexpression of pro-inflammatory markers. Furthermore, carcinogenesis is caused by the presence of harmful bacteria, the production of toxic metabolites, and a reduction in pro-apoptotic markers.
-
(b)
Probiotics and celecoxib administration modulates the Wnt signaling pathway and improves the gut microbiome, potentially leading to reduced inflammation and increased apoptotic markers, thus preventing carcinogenesis [52].
Figure 3.
A diagrammatic illustration of colorectal cancer incidences [52].
4.5. Probiotics and enteral nutrition in acute pancreatitis
The infection of necrotic pancreatic tissue is thought to be caused by bacterial translocation from the intestines, which is preceded by three pathophysiological processes: 1) Bacterial overgrowth in the small intestine due to reduced bowel motility, 2) Local mucosal and systemic immune system failure, and 3) Increased intestinal permeability, resulting in bacterial translocation to other organs, such as the pancreas. Reduced bacterial translocation may reduce the rate of subsequent infection, as well as mortality and morbidity, in pancreatic necrosis. One of the features of acute pancreatitis pathogenesis is bacterial translocation from the stomach to necrotic pancreatic tissue [55]. Bacterial translocation and pancreatic necrosis late infection are likely to be prevented in humans with prophylactic antibiotics, enteral nutrition, or probiotics. Pre-treatments with probiotics have been shown to maintain intestinal barrier function in a mouse model of acute pancreatitis, even in severe pancreatitis, whereas treatment with probiotics after pancreatitis induction did not. As a result, probiotic delivery timing is crucial to their effectiveness. Although there has only been evidence of a link between the timing of probiotic administration and the onset of pancreatitis in animal models, it is possible that the same is true in humans [55, 56].
4.6. Late-onset sepsis in preterm infants
Gram-negative bacteria are less frequent than Gram-positive bacteria to cause Late-onset sepsis (LOS), but they are associated with a more severe clinical presentation, higher mortality, and a greater risk of neonatal morbidity. Sepsis can be caused by a low birth weight, being born prematurely, or being admitted to an intensive care unit. Premature babies pick up colonizing germs in the intensive care unit. Antibiotic use after delivery and lengthy hospitalization diminish the microbiome's microbial diversity [56, 57]. Colonization with commensals may improve in this population at risk for LOS after therapy with oral probiotics. The effect of probiotics is also influenced by the strain, dosage, and intended use. A randomized control trial employing a probiotic combination of Bifidobacterium infantis, Streptococcus thermophiles, and Bifidobacterium lactis recently published revealed a substantial reduction in LOS in the preterm subgroup of 28–31 weeks gestation [56].
4.7. Necrotizing enterocolitis in preterm infants
Necrotizing enterocolitis s one of the primary causes of neonatal mortality (at least 20–30%) and morbidity among very-low-birth-weight (VLBW) infants (defined as those weighing fewer than 1,500 g at birth) (1.5KG). In premature neonates, gut colonization with probiotic organisms such as bifidobacteria and lactobacilli is low and usually delayed; there may be a shift to potentially hazardous bacteria as a result of cesarean birth, delayed nursing, or postnatal antibiotic treatment [58]. Enteral supplementation of probiotics prevents severe Necrotizing enterocolitis and all-cause mortality in preterm infants [59]. Prophylactic enteral probiotics (live microbial supplements) may help to reduce Necrotizing enterocolitis and its associated morbidity by slowing bacterial migration across the mucosa, competitive exclusion of dangerous bacteria, and improving the host's immune responses. Lactobacillus acidophilus and Bifidobacterium infantis-containing probiotics have been linked to a lower risk of Necrotizing enterocolitis surgery, any abdominal surgery, and all-cause death [39, 60]. Probiotics are hypothesized to help beneficial microbial flora colonize the gut, prevent pathogen colonization, increase the maturity and function of the gut mucosal barrier, and affect the immune system. Probiotics are often administered for two weeks, beginning on the first day of life in new-borns who are not on antibiotics or after antibiotic therapy has been stopped [58, 59, 60].
4.8. Ventilator associated pneumonia
The most prevalent infection in the intensive care unit (ICU) is ventilator-associated pneumonia (VAP), which is linked to a high rate of morbidity and mortality. Probiotics have lately emerged as a new weapon in the fight against VAP. By improving intestinal barrier function, increasing host cell antimicrobial peptides, regulating the composition of the intestinal flora, and reducing pathogenic bacteria overgrowth and bacterial translocation through local and systemic actions, probiotic bacteria are thought to reduce the development of VAP. Probiotics have also been demonstrated to be safe and effective in the prevention and treatment of VAP in ICU patients in various trials [61, 62, 63].
5. Therapeutic use of probiotics
The biggest therapeutic effects of probiotics are defined by their direct or indirect action on the gastrointestinal tract (GIT). This is owing to the fact that probiotic micro flora are effective not only because of their activity and contact with the micro flora of the host cell mucous membranes, but also because they are taken orally. However, because not all probiotic microorganisms are prevalent in the human gut flora, the advantages of one species may not apply to others [24].
5.1. Therapeutic effect of probiotics on helicobacter pylori eradication
The gram-negative, flagellated bacteria Helicobacter pylori is typically found on the luminal surface of the stomach epithelium. Because of severe side effects and changes in natural flora, the efficacy of standard triple therapy (STT) in treating Helicobacter pylori infection has declined [64]. Probiotics improve immune-regulation functions, which operate as an antidote to Helicobacter pylori infection, and hence inhibit Helicobacter pylori infection. Probiotics are beneficial to human health and can be used as a supplement to help enhance Helicobacter pylori eradication rates, reduce treatment-related side effects, and reduce Helicobacter pylori-related gastrointestinal inflammation. However, contradictory test results have made it difficult to make conclusions about probiotics' capacity to treat Helicobacter pylori [4, 24, 65]. Lactobacillus may be more effective at eradicating Helicobacter pylori than other probiotics. The efficacy of probiotics in treating Helicobacter pylori has been attributed to several factors. Probiotic microorganisms, for example, can affect Helicobacter pylori activity both immunologically and non-immunologically ways. They may also have direct bacteriostatic or bactericidal effects, lower cytokine production, boost the gastric defense system, interact with the host's innate immune system, and have direct bacteriostatic or bactericidal effects [64]. By altering L. lactis to produce Helicobacter pylori lipoprotein Lpp20, Zhang et al. developed a vaccine against Helicobacter pylori. This customized probiotic elicited an immune response in animals when tested in vivo. In another experiment, Bacillus subtilis spores were engineered to express the Helicobacteri pylori urease B protein on the surface [4, 24].
5.2. Therapeutic effect of probiotics in autoimmune and inflammatory disorders
Molecular mimicry, self-antigen modification, bystander activation, and immune reactivity modulation are thought to be the four pathways that cause autoimmune disease. Probiotics are used to treat autoimmune diseases such systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) Celiac and Crohn's disease, ulcerative colitis, multiple sclerosis, Sjogren's syndrome, systemic sclerosis, antiphospholipid syndrome, myasthenia gravis, and type 1 diabetes [10].
In mechanistic studies of probiotics, there have been at least 4 metabolic processes implicated, implying that the microbial–immune system interaction has been connected to: 1) Production and signalling of short-chain fatty acids, 2) Tryptophan metabolism and aryl hydrocarbon receptor activation, 3) Nucleoside signalling in the gut, and 4) Activation of the Intestinal Histamine-2 Receptor. In rheumatoid arthritis, ulcerative colitis, and multiple sclerosis, microbial alteration by probiotics has been found to alleviate gastrointestinal symptoms and multi-organ inflammation in several randomized controlled trials [14, 66]. Probiotics may improve a person's health by regulating their immune function [67].
5.3. Therapeutic effect of probiotic use in the management of hypertension
Hypertension (HTN) has emerged as a major risk factor for cardiovascular, cerebrovascular, and renal illness around the world. In the development of hypertension, certain gut microbial strains may have a pathogenic or protective function. Dysbiosis of the gut microbiota has been linked to HTN progression in animal models and humans, according to recent research [68].
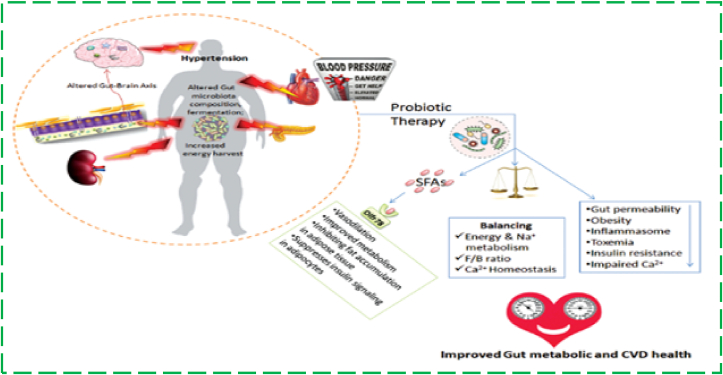
Probiotics work primarily by lowering luminal pH, secreting antimicrobial peptides, inhibiting bacterial invasion, blocking bacterial adhesion to epithelial cells through increased expression of Mucin (MUC2) and Mucin (MUC3) intestinal mucins, enhancing mucous secretion, activating cytokine cascades and immune modulation, and inhibiting (reducing) the concentrations of myeloperoxidase, tumour necrosis factor alpha, nuclear factor kapp Probiotics help improve the integrity of the gastrointestinal (GI) barrier by tightening the mucosal barrier and upregulating growth factors and receptor sites. Through the synthesis of H2O2 and benzoic acid, which block many pathogenic, acid-sensitive bacteria, probiotics promote the proliferation of non-pathogenic commensal bacteria. SCFAs have anti-inflammatory properties in both colony epithelial cells and immune cells [20]. As shown in Figure 4, fermented milk is a functional drink that aids in the reduction of HTN levels. In recent years, the potential of probiotics to lower blood pressure has been connected to the production of bioactive peptides during the fermentation process, such as angiotensin-converting enzyme (ACE) inhibitory peptides, which have a hypotensive effect similar to that of ACE-inhibitor drugs. Aoyagi et al. discovered two tri-peptides that inhibit ACE in sour milk fermented with L. helveticus and Saccharomyces cerevisiae bacteria: 1) isoleucyl–prolyl–proline (Ile-Pro-Pro) and 2) valyl–prolyl–proline (Valyl–Prolyl–Proline) (Val-Pro-Pro). According to research, consuming fermented milk products containing L. casei strain Shirota (LcS) at least three times per week significantly reduces the risk of developing HTN. The antihypertensive effects of L. casei are due to the polysaccharide component (SG1–polysaccharide–glycopeptide complex), which promotes prostaglandin I2 production and decreases peripheral vascular resistance [5].
Figure 4.
Probiotics use in the management of hypertension [20].
5.4. The therapeutic use of probiotics to treat inflammatory bowel disease
Inflammatory bowel disease (IBD), which includes Crohn's disease (CD) and ulcerative colitis (UC), is defined by chronic gut mucosa inflammation, disturbance of the gut barrier function, and a dysbiotic microbiota, all of which often emerge as chronic gastrointestinal mucosa inflammation [31]. Irritable bowel syndrome is a prevalent gastrointestinal disorder that affects the quality of life of millions of people worldwide while also putting a load on healthcare systems. Inflammatory bowel disease patients were found to have a greater permeability defect in their stomach than healthy controls. Lymphocytic proliferation and the production of inflammatory cytokines were shown to be inhibited when lymphocytes consumed customized elimination dies [69, 70].
Researchers recently proposed two possibilities for the role of bacteria in the etiology of Inflammatory Bowel disease (IBD). First, there's a problem with the immune system's response to microorganisms in the natural flora of the intestine. Second, changes in the gut microbiota or a breakdown of the mucosal barrier that results in detrimental immune responses against the mucosa may have a role in the etiology of IBD. Drugs, dietary changes, and the use of alternative substances such as probiotics should all be part of the plan [14, 71].
The generation of pro-inflammatory cytokines is inhibited by fermented milk containing Lactobacilus paracasei L74 CBA. Because it has the ability to prevent the activation of the NF-Kb, Streptococcus salivarius possesses anti-inflammatory properties. Lactobacilus salvarius requires Nucleotide Binding Oligomerization (NOD2) receptors to demonstrate protective benefits, which were also linked to local synthesis of IL-10, which has anti-inflammatory qualities, according to Fernandez et al. In a model of colitis, Duary et al. found that using the probiotic strain Lactiplantibacillus plantarum Lp91 reduced the levels of tumour necrosis factor (TNF) and cyclooxygenase-2 (COX-2) and raised IL-10 expression. For example, probiotic lactic acid bacteria (the most common microorganisms used as probiotics) releasing large amounts of antioxidant enzymes could help minimize oxidative damage, which is useful in the treatment of inflammatory bowel disease. Patients with inflammatory bowel disease who consume probiotic yogurt may enhance intestinal function by increasing the quantity of probiotic bacteria in the gut and colon [14].
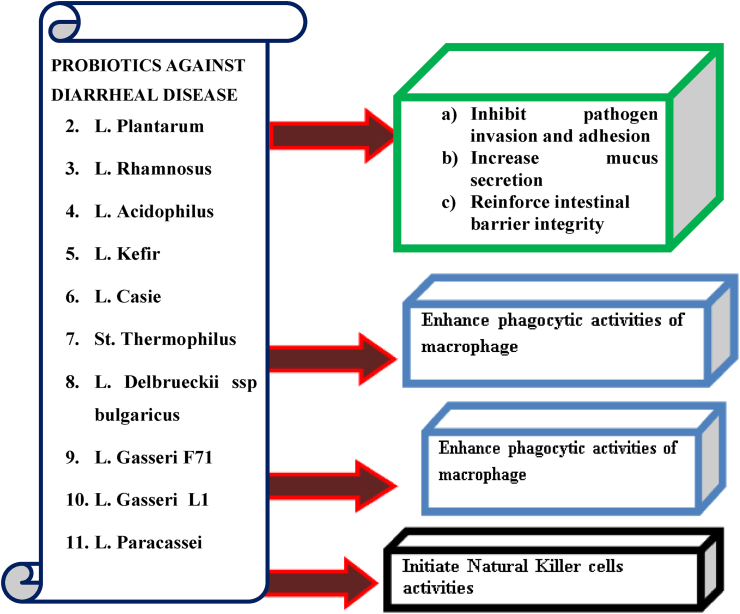
Antibiotic-associated diarrhoea is the most prevalent side effect of antimicrobial therapy. Broad-spectrum antibiotics such amino-penicillin, cephalosporin, and clindamycin are highly related with antibiotic-associated diarrhoea. Antibiotic use is thought to disturb the natural enteric microbiota, resulting in a decrease in native microorganisms in the gastrointestinal system. When the number of these symbiotic bacteria is diminished, the host suffers in a variety of ways [2, 72, 73]. Probiotics have been demonstrated to be effective in the treatment of acute infectious diarrhoea and antibiotic-associated diarrhoea, as well as the prevention of traveller's diarrhoea [74 75]. Repair of the gut microbiota, tissue architectural recovery, and reduction of systemic inflammation were all assisted by probiotic therapy for a long time, but rather than by probiotic therapy for a brief period of time. After probiotic therapy for a long time, all of the major faecal bacteria returned to normal levels, as did IL-10, IFN-, and TNF-α However, the effectiveness of antibiotic-associated diarrhoea probiotics was strain-specific and time-dependent as shown in Figure 5
Figure 5.
Probiotics and their possible mechanism of action in diarrhoea [2].
5.6. The therapeutic use of probiotics oral candidiasis colonization in denture wearers
The usage of dentures increases the risk of developing Candida sp.-related diseases like angular cheilitis and Down syndrome (DS). In all clinical scenarios, local variables such as insufficient occlusal vertical dimension (OVD), trauma caused by an ill-fitting denture, poor denture hygiene, and systemic illnesses and immune system deficiencies are commonly linked to the Candida infection. Lacticaseibacillus rhamnosus, Limosilactobacillus reuteri, and other Lactobacillus strains have been shown to reduce Candida levels in the mouth in investigations [76].
Ishikawa et al indicated that treating Candida-associated stomatitis with a probiotic product containing Bifidobacterium longum, Lactobacillus bulgaris, and Streptococcus thermophilus combined with oral local antifungal medications (nystatin) was more effective than usual therapy. The probiotic product was effective in lowering Candida colonization of the oral cavity in candidiasis-asymptomatic senior denture wearers, suggesting that this multispecies probiotic could be used to prevent oral candidiasis. Candida colonization of the oral surface is thought to increase the risk of invasive fungal infections. Candida infections in senior denture users could be treated with a preparation containing Lacticaseibacillus rhamnosus, Lactobacillus acidophilus, and Bifidum spp. [77]. Because of their potential to boost the immune system, probiotics can reduce opportunistic infections [78].
5.7. The therapeutic use of probiotics in autism spectrum disorder
A probable link between autism spectrum disorder (ASD) and gut bacteria has been discovered in studies [79, 80]. Psychobiotics are a novel type of probiotic that refers to live organisms that have positive impacts on mental health. Probiotics work by adjusting the makeup and/or activity of the gut microbiota through a range of processes, such as bacteriocins and metabolites like lactic, propionic, and acetic acids [79]. Furthermore, recent research has shown that probiotic treatment can help people with ASD by modulating the microbiota–gut–brain axis [81]. By fostering "leaky gut" healing, probiotics may help with ASD rehabilitation by maintaining or improving gut barrier integrity [80]. Probiotics can down regulate gut and CNS inflammatory pathways in species and strain-specific ways by promoting the development of regulatory T cells, lowering LPS levels, providing tolerogenic signals, and raising brain-derived neurotropic factor (BDNF) [82]. Additionally, probiotic immunomodulation may occur by reducing the production of pro-inflammatory cytokines such as IL-12, TNF- α, and INF- α, while raising the expression of anti-inflammatory mediators such as IL-10 and transforming growth factor beta (β-TFGF). By reducing gut inflammation and decreasing dysregulated immunological activity, probiotic supplementation may be effective for treating both gut microbial and behavioral abnormalities in ASD [82, 83].
Finally, several probiotic strains, such as Bifidobacterium sp. (Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, and Bifidobacterium bifidum), Lactobacillus sp. (L. acidophilus, L. helveticus, L. rhamnosus, L. plantarum may impact neurotransmission and emotional states via the vagus nerve-mediated Gut- Brain-Axis (GBA) [82].
5.8. The therapeutic use of probiotics on migraine
Several studies have showed that eliminating particular foods from one's diet can lower the frequency and intensity of migraine headaches. Food hypersensitivity may be indicated by a high level of food-specific immunoglobulin G (IgG). As a result, eating foods that are free of IgG may help to reduce migraine symptoms. Inflammation appears to play a role in migraine genesis, according to growing data. Probiotics may help to reduce migraine headaches by improving the function of the intestinal barrier via the gut-brain axis. Serotonin appears to be a key link in the brain-gut axis, according to several research. However, just 3% of a person's total serotonin is found in the central nervous system. The remainder is contained within the gut. The role of enteric bacteria in serotonin synthesis has been discovered. As a result, modifying the function of the intestine with the right probiotics could be a strategy to help migraine sufferers [70].
5.9. The therapeutic use of for treatment type 1 and type 2 diabetes
Diabetes is a group of metabolic illnesses in which blood sugar levels remain high for an extended period of time. Probiotics have become popular as dietary supplements among the general public and medical community due to their potential importance in promoting health, notably in the prevention and treatment of diabetes [84]. Probiotics are commonly used to alter the gut flora, which aids in the prevention of paediatric obesity and diabetes [85].
5.9.1. Type 1 diabetes
Type 1 diabetes is an autoimmune illness defined by the destruction of pancreatic beta (β)-cells by the immune system. Type 1 diabetes is bring about by a combination of environmental influences and genetic predispositions. Increased autoantibodies against beta-cell antigens, as well as insulitis (pancreatic inflammation), precede a decrease in insulin output and beta-cell death in type 1 diabetes [84, 86]. When probiotic strains were ingested, they were found to reduce pro-inflammatory cytokines like IL-6, IL-1, and TNF-α while increasing anti-inflammatory cytokines like TGF-β and IL-10. As a result, probiotics could be able to help prevent type 1 diabetes [86]. Calcinaro et al. looked into the impact of oral probiotic supplementation on the prevention of spontaneous autoimmune diabetes in non-obese diabetic mice. Finally, they discovered that an orally administered probiotic compound containing Bifidobacteria (Bifidobacterium longum, Bifidobacterium infantis, and Bifidobacterium breve) and Lactobacilli (Lactobacillus acidophilus, Lacticaseibacillus casei, Lactobacillus delbrueckii subsp. L. bulgaricus, and Lactiplantibacillus plantarum) had antidiabetic activities. Furthermore, they have demonstrated that exposure to L. plantarum and L. genus can delay or prevent autoimmune diabetes in healthy individuals [84].
5.9.2. Type 2 diabetes
Type 2 Diabetes (T2DM) is an epidemic; consider being a challenge to public health and economy due to its complications that lead to disability [87]. Obesity is hypothesized to be influenced by the gut microbiome, which affects energy extraction, inflammation, appetite and satiety, as well as lipid and glucose metabolism. T2DM has been linked to alterations in the gut microbiome. Short-chain fatty acids, amino acid derivatives, and secondary bile acids, which are produced by the gut microbiota, play a role in metabolic and immunologic processes, suggesting a relationship between the gut microbiota and glucose homeostasis [88, 89]. Overall, probiotics may improve gut integrity, decrease systemic levels of lipopolysaccharides (LPSs), increase Incretins, decrease endoplasmic reticulum (ER) stress, and improve peripheral insulin sensitivity. Probiotics may also have anti-diabetic effects via improving glucose tolerance, regulating lipid metabolism, increasing antioxidant status, and modifying gut flora and SCFA composition. Furthermore, probiotics diminish the inflammatory, autoimmune, and oxidative stress responses [90]. Lactiplantibacillus plantarum and Bifidobacterium lactis are probiotic bacteria that can help reduce the detrimental effects of high-fat meals and even modulate immune responses brought on by inflammatory illnesses. Lacticaseibacillus rhamnosus, when mixed with Lactobacillus gasseri and Bifidobacterium lactis, has been demonstrated to inhibit weight gain in humans, specifically fat tissue mass adiposity, which strengthens the efficacy of probiotics in diabetes [91, 92].
6. Recent advance
Although some recent studies have shown that probiotics can alter the composition of the gut microbiota, the mechanism behind this effect remains uncertain. Oral probiotics improved the disease state by 1) inducing regulatory T cell differentiation and function, 2) reducing inflammatory response, 3) modulating the gut environment, and 4) increasing the proportions of gut microbiota that produce short-chain fatty acids or beneficial metabolites, such as Bifidobacterium, Faecalibacterium, and Akkermansia, among others [93]. Because of the rapid speed of research in human microbiome science, innovative probiotics and prebiotics have become more important. The rules governing the launch of new probiotics and prebiotics differ depending on where you live [94]. So far, modern-day probiotics have been used to help with gastrointestinal issues. Recent advances in culturomics, such as the use of newer methods and gnotobiotic animal models, have created a fertile field for the development of novel host-specific probiotic medicines. While modern probiotics have a promising future, more stringent restrictions are required to develop genuine probiotic products and define innovative probiotics using cutting-edge research and technology [95].
6.1. Recent advance in engineering probiotics for treatment and prophylaxis
Advances in gut bacteria engineering for the development of novel therapeutic modalities aimed at rewiring host microbiome interactions such as host metabolism and immune systems for disease prevention and therapy [96]. According to Advances in Pharmaceutical Approaches to Colon Specific Drug Delivery, an optimal population of probiotic bacteria is critical for the preservation and proper functioning of the digestive system. Inclusion complexes with cyclodextrins efficiently, prolonged release tablets by solid dispersion technology are used to provide probiotics as nutraceutical goods in the form of both conventional pharmaceutical dose forms and traditional non-conventional food products [97].
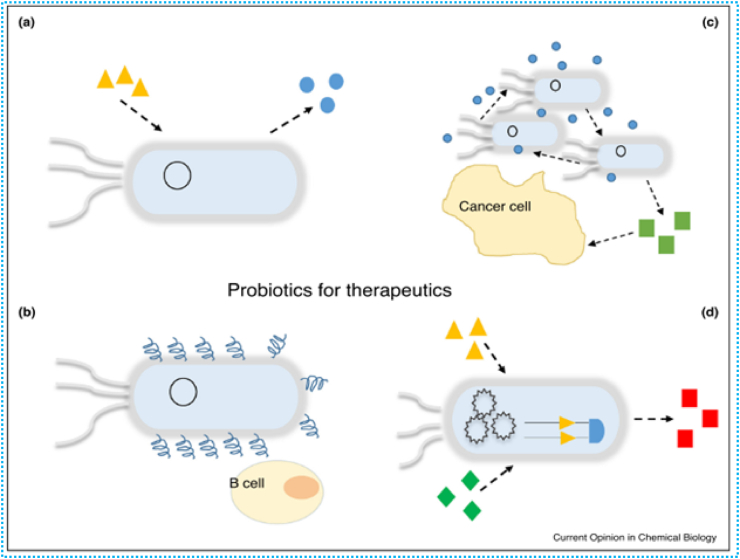
Engineered probiotics have a number of advantages (Figure 6), including enhanced specificity, regulated antimicrobial agent release, and a lower chance of antibiotic resistance. (a) Probiotics are engineered to sense certain molecules released by pathogens (orange triangles) and in response, release the required therapeutic agents for treating infections. (b) Probiotics can be engineered to deliver antigens to treat and prevent allergies in asthmatics. (c) Engineered probiotics can produce anti-tumour factors (green squares) upon detection of quorum sensing signals (blue circles) from the accumulation of probiotics attached to the tumours tissue.(d) Rational design enables probiotics to be equipped with complex circuits to execute more accurate functions. Engineered probiotics can produce the desired therapeutics (red squares) only upon step-wise detection of two molecules (orange triangles and green diamonds) [24].
Figure 6.
A diagram showing how modified probiotics can be used to fight health issues [24].
To tackle multidrug-resistant Enterococcus spp., L. lactis has been genetically engineered. The modified L. lactis was produced to detect the E. faecalis pheromone. Another pathogen that has been addressed by tailored probiotics is C. difficile, a common cause of antibiotic-associated diarrhoea in rich countries. Currently, faecal microbiota transplantation is the most efficacious treatment for C. difficile infection (FMT). Vibrio cholerae, Shigella dysenteriae, and Pseudomonas aeruginosa have all been targeted with probiotics that express specific antigens [24, 97].
Autophagy: a novel anti-inflammatory abilities of probiotics
Autophagy is a metabolic event that occurs within cells and is required for a number of physiological processes. Because of its role in maintaining biological homeostasis in stressful situations, autophagy dysregulation or disruption may be related to human illnesses such as cancer [98]. The autophagy process begins with the development of the phagophore, a double-membrane compartment that engulfs the cargo to be degraded and then shuts to create the autophagosome, which then leads to fusion lysosomes and cargo breakdown by lysosome hydrolases [99, 100].
The discovery of more than 200 genetic risk loci for Inflammatory Bowel disease (IBD) has been made possible by genome-wide association studies (GWAS). Innate immunity, mucosal barrier function, and bacterial identification are all represented by many of these genes [99, 101]. Disrupted epithelial cell function in the gut can lead to intestinal disorders. Additionally, PRR gene defects, including those relating to toll-like receptors, C-type lectin receptors, retinoic-acid-inducible gene-I-like receptors, and nucleotide binding oligomerization domain-2 like receptors, lead to mucosal dysfunction and the subsequent induction of intestinal inflammation [102].
The gene encoding the nucleotide binding oligomerization domain-2 receptor, which detects muramyl dipeptides, a conserved motif seen in both gram-negative and gram-positive bacteria's peptidoglycan, has been extensively established to be the major susceptibility gene for Chronic disease. The strain that was most protective against acute colitis, Bifidobacterium bifidum PI22, was only mildly protective against chronic colitis, whereas Bifidobacterium lactis LA804, which was less protective in the acute model, was the most protective against chronic colitis. In vitro, Lactobacillus helveticus PI5 was not anti-inflammatory, but it was the best at strengthening the epithelial barrier, and as a result, it was able to greatly reduce the severity of acute colitis in mice. Lactobacillus salivarius LA307, interestingly, protected mice from both forms of colitis in a significant way [99, 102].
6.2. Recent advances in managing acute pancreatitis
Acute pancreatitis is the leading cause of gastrointestinal hospitalization in many countries, and its prevalence is increasing. Acute Pancreatitis (AP) pathophysiology involves a complex chain of events involving acinar cell inflammation, immune system involvement, and systemic clinical effects [103]. Probiotic therapy has shown much promise in surgical patients [104]. Recent guidelines advocate against the use of probiotics for severe acute pancreatitis [105]. Probiotics may help with acute pancreatitis by inducing an anti-inflammatory response by stimulating anti-inflammatory cytokines (e.g. interleukin-10), preventing bacterial overgrowth by competing with opportunistic bacteria, decreasing intestinal permeability by strengthening the gastrointestinal barrier, and improving upper gastrointestinal motility. A mixture of Streptococcus, Lactobacillus, and Bifidobacterium was given to an experimental animal model to minimize bacterial translocation and improve the clinical outcome of acute pancreatitis [103, 106].
6.3. Recent advance in antioxidation role of probiotics
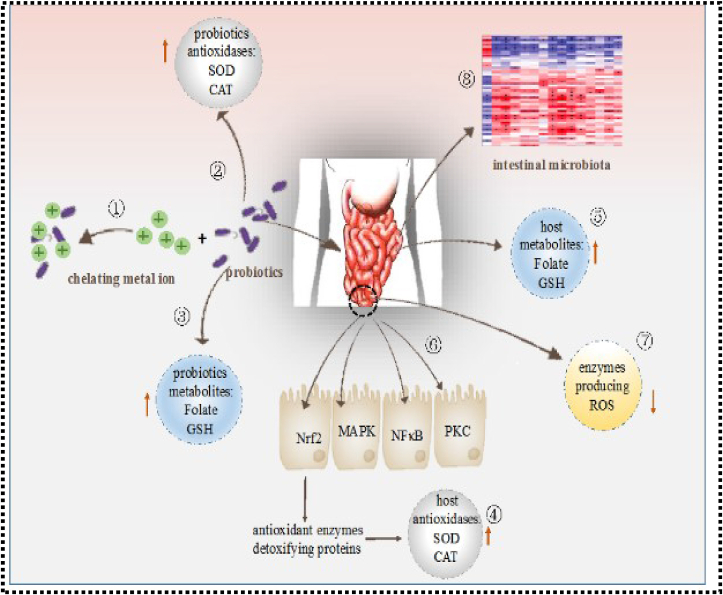
Figure 7 shows the Nobel mechanisms of action of probiotics in autoxidation, which are said to provide health advantages. Probiotic microorganisms have been shown to have considerable antioxidant capacities in vivo and in vitro through a variety of mechanisms: 1) Metal ions are chelated by probiotics. 2) Antioxidases are produced by probiotics. 3) Antioxidant metabolites are produced by probiotics. 4) Probiotics increase the host's antioxidase activity. 5) Probiotics raise the host's antioxidant metabolite levels. 6) Signalling pathways are regulated by probiotics. 7) Probiotics inhibit the activity of enzymes that produce reactive oxygen species (ROS). 8) Probiotics modulate the bacteria in the intestine. Lacticaseibacillus rhamnosus showed substantial antioxidant activity in humans when exposed to high levels of physical stress. Lacticaseibacillus rhamnosuss' ability to raise antioxidant levels and lessen the negative impact of reactive oxygen species may be beneficial to athletes suffering from oxidative stress. Over the last few decades, research has demonstrated that different probiotic bacteria strains can exert antioxidant activity in a variety of ways. However, few reviews on the basis for probiotic antioxidant mechanisms have been published. As a result, the sections that follow provide an overview of what is known about the oxygen resistance mechanisms of various probiotic strains [107].
Figure 7.
Nobel Modes of Action of probiotics in antioxidation [107].
6.4. Recent advance in probiotics on hair toxic element levels
Chelation therapy, which comprises Ethylenediaminetetraacetic acid (EDTA), Meso-2,3-dimercaptosuccinic acid (DMSA), and 2,3-Dimercapto-1-propanesulfonic acid (DMPS), is now the primary method for rapidly removing harmful chemicals from the body. It may, however, cause serious adverse effects such as dehydration, liver failure, renal failure, hypoglycemia, and coagulation insufficiency, as well as reducing the levels of other vital minerals (Ca, Fe, and Zn, for example). In vitro, Lactobacillus gasseri and Limosilactobacillus reuteri can bind dangerous elements like Cd. Limosilactobacillus reuteri Cd70-13 and Pb71-1 eliminated Cd (25%) and Pb (59%) from Rogosa and Sharpe (MRS) culture media, implying that they may adsorb the toxic heavy metals in the intestine. The exopolysaccharide (EPS) of Lacticaseibacillus rhamnosus (L. rhamnosus) may eliminate Al+3 and Cd+2 from water. Bifidobacterium logum can also eliminate copper and lead from water (B. Longum). To summarize the role of probiotics in reducing hair toxicity, 1) after taking probiotics, the number of patients with an abnormal concentration of Hg and Be decreased significantly. 2) taking probiotics for at least six months can help to lower Hg and Be levels significantly [108].
6.5. Recent advance of probiotics for curing and preventing protozoal diseases
Probiotic usage has the potential to disrupt parasite colonization. Faecal microbiota transplantation (FMT), which has gained popularity in recent years, particularly for the treatment of recurrent Clostridium difficile infection, looks relevant in this situation. Inflammatory bowel disease, type 2 diabetes, metabolic disease, and potentially even neuropsychiatric disease could all benefit from faecal microbiota transplantation in the future. FMT is a procedure that involves taking a donor's feces or stool, mixing it with a saline or other solution, straining it, and infusing it into a recipient [109].
7. Conclusion and recomendation
Alternative approaches to combating lethal bacteria are urgently required due to the rise of multidrug-resistant diseases, significant side effects, resistance, and antibiotic depletion. Furthermore, beneficial or antagonistic microbes are required to prevent and treat a variety of noncommunicable diseases as well as those with limited treatment options all over the world, particularly in wealthy countries. Probiotics are known to be one of the most promising biotherapies for overcoming the aforementioned problem by improving specificity, regulating antimicrobial agent release, and lowering the likelihood of antibiotic resistance development. They are also known to have immune-modulatory effects on the host, making them a promising therapeutic and preventive option for a variety of diseases, including inflammatory disease. . Probiotics are chosen for their ability to: 1) benefit the host, 2) survive transit through the intestines, 3) adhere to the intestinal epithelial cell membrane, 4) produce antibiotic compounds to fight infections, and 5) stabilize the intestinal microbiota. Because probiotic effects differ based on dose, situation, and strain, understanding the probiotics’ genus and species is necessary to accomplish the desired effects on the host. Despite the promising health-promoting effects of probiotics, more research into the systemic mechanisms and components that support the beneficial actions is required for the discovery and development of novel microbes derived from our microbial symbionts. It is recommended that in the new future researchers from microbiologist and pharmacologist should design engineered probiotics for those disease that are not able to controlled by the current conventional medicines at large.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to express their gratitude to Mekonnen Addis (DVM, Msc in animal public health, Associate professor at Jimma University in Ethiopia) for suggesting that they write a review for this title.
References
- 1.Kim S., Covington A., Pamer E.G. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017;279(1):90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav A., Chandra H., Maurya V.K. Probiotics: recent advances and future prospects. J. Plant Dev. Sci. 2017;9(11):967–975. [Google Scholar]
- 3.Song H.Y., Zhou L., Liu D.Y., Yao X.J., Li Y. What roles do probiotics play in the eradication of Helicobacter pylori? Current knowledge and ongoing research. Gastroenterol. Res. Pract. 2018;2018 doi: 10.1155/2018/9379480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song H.-Y., Zhou L., Liu D-y, Yao X.-J., Li Y. What roles do probiotics play in the eradication of Helicobacter pylori? Current knowledge and ongoing research. Gastroenterol. Res. Pract. 2018;2018 doi: 10.1155/2018/9379480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noce A., Marrone G., Di Daniele F., Ottaviani E., Wilson Jones G., Bernini R., et al. Impact of gut microbiota composition on onset and progression of chronic non-communicable diseases. Nutrients. 2019;11(5):1073. doi: 10.3390/nu11051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremon C., Barbaro M.R., Ventura M., Barbara G. Pre-and probiotic overview. Curr. Opin. Pharmacol. 2018;43:87–92. doi: 10.1016/j.coph.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Varankovich N.V., Nickerson M.T., Korber D.R. Probiotic-based strategies for therapeutic and prophylactic use against multiple gastrointestinal diseases. Front. Microbiol. 2015;6(685) doi: 10.3389/fmicb.2015.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antza C., Stabouli S., Kotsis V. Gut microbiota in kidney disease and hypertension. Pharmacol. Res. 2018;130:198–203. doi: 10.1016/j.phrs.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 9.O'Toole P.W., Cooney J.C. Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip. Perspect. Infect. Dis. 2008;2008 doi: 10.1155/2008/175285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerner A., Shoenfeld Y., Matthias T. Probiotics: if it does not help it does not do any harm. Really? Microorganisms. 2019;7(4):104. doi: 10.3390/microorganisms7040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen M. Role of probiotics in health and disease–a review. Int. J. Adv. Life Sci. Res. 2019:1–11. [Google Scholar]
- 12.Akshintala V.S., Talukdar R., Singh V.K., Goggins M. The gut microbiome in pancreatic disease. Clin. Gastroenterol. Hepatol. 2019;17(2):290–295. doi: 10.1016/j.cgh.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazloom K., Siddiqi I., Covasa M. Probiotics: how effective are they in the fight against obesity? Nutrients. 2019;11(2):258. doi: 10.3390/nu11020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza MdSS., Barbalho S.M., de Alvares Goulart R., de Carvalho AdCA. The current and future role of drugs and Probiotics in the management of inflammatory bowel disease. J. Biosci. Med. 2015;3(8):76. [Google Scholar]
- 15.Lu H., Zhang C., Qian G., Hu X., Zhang H., Chen C., et al. An analysis of microbiota-targeted therapies in patients with avian influenza virus subtype H7N9 infection. BMC Infect. Dis. 2014;14:359. doi: 10.1186/1471-2334-14-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad A., Khalid S. Elsevier; 2018. Therapeutic aspects of probiotics and prebiotics; pp. 53–91. (Diet, Microbiome and Health). [Google Scholar]
- 17.Kopp-Hoolihan L. Prophylactic and therapeutic uses of probiotics: a review. J. Am. Diet Assoc. 2001;101(2):229–241. doi: 10.1016/S0002-8223(01)00060-8. [DOI] [PubMed] [Google Scholar]
- 18.Zubillaga M., Weill R., Postaire E., Goldman C., Caro R., Boccio J. Effect of probiotics and functional foods and their use in different diseases. Nutr. Res. 2001;21(3):569–579. [Google Scholar]
- 19.Ghasemian A., Eslami M., Shafiei M., Najafipour S., Rajabi A. Probiotics and their increasing importance in human health and infection control. Rev. Med. Microbiol. 2018;29(4):153–158. [Google Scholar]
- 20.Borse S.P., Singh D.P., Upadhyay D., Sharma V., Nivsarkar M.A. Probiotic use in the management of hypertension: a new era of therapeutic management. Indian J. Health Sci. Biomed. Res. (KLEU) 2018;11(3):207. [Google Scholar]
- 21.Patel S., Goyal A. Evolving roles of probiotics in cancer prophylaxis and therapy. Prob. Antimicrob. Proteins. 2013;5 doi: 10.1007/s12602-012-9124-9. [DOI] [PubMed] [Google Scholar]
- 22.Pickard J.M., Núñez G. Pathogen colonization resistance in the gut and its manipulation for improved health. Am. J. Pathol. 2019;189(7):1300–1310. doi: 10.1016/j.ajpath.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mushtaq N., Hussain S., Zhang S., Yuan L., Li H., Ullah S., et al. Molecular characterization of alterations in the intestinal microbiota of patients with grade 3 hypertension. Int. J. Mol. Med. 2019;44(2):513–522. doi: 10.3892/ijmm.2019.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua K.J., Kwok W.C., Aggarwal N., Sun T., Chang M.W. Designer probiotics for the prevention and treatment of human diseases. Curr. Opin. Chem. Biol. 2017;40:8–16. doi: 10.1016/j.cbpa.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Espitia P.J., Batista R.A., Azeredo H.M., Otoni C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. Int. 2016;90:42–52. doi: 10.1016/j.foodres.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Gasbarrini G., Bonvicini F., Gramenzi A. Probiotics history. J. Clin. Gastroenterol. 2016;50:S116–S119. doi: 10.1097/MCG.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 27.Reid G., Dhir R. Probiotics: reiterating what they are and what they are not. Front. Microbiol. 2019;10:424. doi: 10.3389/fmicb.2019.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubiger C.B., Orfe L.H., Sudheesh P.S., Cain K.D., Shah D.H., Call D.R. Entericidin is required for a probiotic treatment (Enterobacter sp. strain C6-6) to protect trout from cold-water disease challenge. Appl. Environ. Microbiol. 2015;81(2):658–665. doi: 10.1128/AEM.02965-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng J., Wittouck S., Salvetti E., Franz C., Harris H.M.B., Mattarelli P., et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70(4):2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 30.Khan A.A., Khurshid M., Khan S., Alshamsan A. Gut microbiota and probiotics: current status and their role in cancer therapeutics. Drug Dev. Res. 2013;74(6):365–375. [Google Scholar]
- 31.Chibbar R., Dieleman L.A. The gut microbiota in celiac disease and probiotics. Nutrients. 2019;11(10) doi: 10.3390/nu11102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazir Y., Hussain S.A., Abdul Hamid A., Song Y. Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/3428437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee D., Jain T., Bose S., Bhosale V. Springer; 2018. Importance of probiotics in human health; pp. 539–554. (Functional Food and Human Health). [Google Scholar]
- 34.Yu H.S., Lee N.K., Choi A.J., Choe J.S., Bae C.H., Paik H.D. Anti-inflammatory potential of probiotic strain weissella cibaria JW15 isolated from Kimchi through regulation of NF-kappaB and MAPKs pathways in LPS-induced RAW 264.7 cells. J. Microbiol. Biotechnol. 2019;29(7):1022–1032. doi: 10.4014/jmb.1903.03014. [DOI] [PubMed] [Google Scholar]
- 35.Yamada T., Saito H., Fujieda S. Present state of Japanese cedar pollinosis: the national affliction. J. Allergy Clin. Immunol. 2014;133(3):632–639 e5. doi: 10.1016/j.jaci.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez H., Miller J.E. Do prophylactic probiotics prevent the incidence of Clostridium difficile colitis infection in hospitalized patients? J. Oklahoma State Med. Assoc. 2019;112(1):18–19. [PMC free article] [PubMed] [Google Scholar]
- 37.Franko J., Raman S., Krishnan N., Frankova D., Tee M.C., Brahmbhatt R., et al. Randomized trial of perioperative probiotics among patients undergoing major abdominal operation. J. Am. Coll. Surg. 2019;229(6):533–40. e1. doi: 10.1016/j.jamcollsurg.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Pitsouni E., Alexiou V., Saridakis V., Peppas G., Falagas M.E. Does the use of probiotics/synbiotics prevent postoperative infections in patients undergoing abdominal surgery? A meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2009;65(6):561–570. doi: 10.1007/s00228-009-0642-7. [DOI] [PubMed] [Google Scholar]
- 39.Hartel C., Pagel J., Rupp J., Bendiks M., Guthmann F., Rieger-Fackeldey E., et al. Prophylactic use of Lactobacillus acidophilus/Bifidobacterium infantis probiotics and outcome in very low birth weight infants. J. Pediatr. 2014;165(2):285–289 e1. doi: 10.1016/j.jpeds.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 40.Oncel M.Y., Arayici S., Sari F.N., Simsek G.K., Yurttutan S., Erdeve O., et al. Comparison of Lactobacillus reuteri and nystatin prophylaxis on Candida colonization and infection in very low birth weight infants. J. Matern. Fetal Neonatal Med. 2015;28(15):1790–1794. doi: 10.3109/14767058.2014.968842. [DOI] [PubMed] [Google Scholar]
- 41.Das R., Biswas S., Banerjee E.R. Nutraceutical-prophylactic and therapeutic role of functional food in health. J. Nutr. Food Sci. 2016;6(4):1–17. [Google Scholar]
- 42.Dasari S., Kathera C., Janardhan A., Kumar A.P., Viswanath B. Surfacing role of probiotics in cancer prophylaxis and therapy: a systematic review. Clin. Nutr. 2017;36(6):1465–1472. doi: 10.1016/j.clnu.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Maleki D., Homayouni A., Khalili L., Golkhalkhali B. 2016. Probiotics in Cancer Prevention, Updating the Evidence; pp. 781–791. [Google Scholar]
- 44.Górska A., Przystupski D., Niemczura M.J., Kulbacka J. Probiotic bacteria: a promising tool in cancer prevention and therapy. Curr. Microbiol. 2019;76(8):939–949. doi: 10.1007/s00284-019-01679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranji P., Agah S., Heydari Z., Rahmati-Yamchi M., Alizadeh A.M. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the serum biochemical parameters, and the vitamin D and leptin receptor genes on mice colon cancer. Iran. J. Basic Med. Sci. 2019;22(6):631. doi: 10.22038/ijbms.2019.32624.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plaza-Diaz J., Ruiz-Ojeda F.J., Gil-Campos M., Gil A. Mechanisms of action of probiotics. Adv. Nutr. 2019;10(suppl_1):S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranjbar S., Seyednejad S.A., Azimi H., Rezaeizadeh H., Rahimi R. Emerging roles of probiotics in prevention and treatment of breast cancer: a comprehensive review of their therapeutic potential. Nutr. Cancer. 2019;71(1):1–12. doi: 10.1080/01635581.2018.1557221. [DOI] [PubMed] [Google Scholar]
- 48.Molska M., Reguła J. Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients. 2019;11(10):2453. doi: 10.3390/nu11102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma A. In: Recent Developments in Applied Microbiology and Biochemistry. Buddolla V., editor. Academic Press; 2019. Chapter 4 - importance of probiotics in cancer prevention and treatment; pp. 33–45. [Google Scholar]
- 50.Eslami M., Yousefi B., Kokhaei P., Hemati M., Nejad Z.R., Arabkari V., et al. Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell. Physiol. 2019;234(10):17127–17143. doi: 10.1002/jcp.28473. [DOI] [PubMed] [Google Scholar]
- 51.Libertucci J., Young V.B. The role of the microbiota in infectious diseases. Nat. Microbiol. 2019;4(1):35–45. doi: 10.1038/s41564-018-0278-4. [DOI] [PubMed] [Google Scholar]
- 52.Sharaf L.K., Sharma M., Chandel D., Shukla G. Prophylactic intervention of probiotics (L.acidophilus, L.rhamnosus GG) and celecoxib modulate Bax-mediated apoptosis in 1,2-dimethylhydrazine-induced experimental colon carcinogenesis. BMC Cancer. 2018;18(1):1111. doi: 10.1186/s12885-018-4999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campia I., Doublier S., Aldieri E., Bosia A., Ghigo D., Riganti C. Recent advances in medicinal chemistry 209-245. Recent Adv. Med. Chem. 2019;209:245. [Google Scholar]
- 54.Qorri B., Harless W., Szewczuk M. 2019. Novel molecular mechanism of aspirin targeting neuraminidase-1 impedes epidermal growth factor receptor-signaling platform and induces apoptosis in pancreatic cancer cells. Available at SSRN 3494403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Baal M.C., van Rens M.J., Geven C.B., van de Pol F.M., van den Brink I.W., Hannink G., et al. Association between probiotics and enteral nutrition in an experimental acute pancreatitis model in rats. Pancreatology. 2014;14(6):470–477. doi: 10.1016/j.pan.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Tewari V.V., Dubey S.K., Gupta G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J. Trop. Pediatr. 2015;61(5):377–385. doi: 10.1093/tropej/fmv050. [DOI] [PubMed] [Google Scholar]
- 57.Dong Y., Glaser K., Speer C.P. Late-onset sepsis caused by Gram-negative bacteria in very low birth weight infants: a systematic review. Expert Rev. Anti Infect. Ther. 2019;17(3):177–188. doi: 10.1080/14787210.2019.1568871. [DOI] [PubMed] [Google Scholar]
- 58.Zbinden A., Zbinden R., Berger C., Arlettaz R. Case series of Bifidobacterium longum bacteremia in three preterm infants on probiotic therapy. Neonatology. 2015;107(1):56–59. doi: 10.1159/000367985. [DOI] [PubMed] [Google Scholar]
- 59.AlFaleh K., Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid. Base Child Health. 2014;9(3):584–671. doi: 10.1002/ebch.1976. [DOI] [PubMed] [Google Scholar]
- 60.Goncalves F.L., Soares L.M., Figueira R.L., Simoes A.L., Gallindo R.M., Sbragia L. Evaluation of the expression of I-FABP and L-FABP in a necrotizing enterocolitis model after the use of Lactobacillus acidophilus. J. Pediatr. Surg. 2015;50(4):543–549. doi: 10.1016/j.jpedsurg.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Xie X., Lyu J., Hussain T., Li M. Drug prevention and control of ventilator-associated pneumonia. Front. Pharmacol. 2019;10:298. doi: 10.3389/fphar.2019.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Ruissen M.C., Bos L.D., Dickson R.P., Dondorp A.M., Schultsz C., Schultz M.J. Manipulation of the microbiome in critical illness—probiotics as a preventive measure against ventilator-associated pneumonia. Intens. Care Med. Exp. 2019;7(1):37. doi: 10.1186/s40635-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Branch-Elliman W., Wright S.B., Howell M.D. Determining the ideal strategy for ventilator-associated pneumonia prevention. Cost-benefit analysis. Am. J. Respir. Crit. Care Med. 2015;192(1):57–63. doi: 10.1164/rccm.201412-2316OC. [DOI] [PubMed] [Google Scholar]
- 64.Jung J.H., Cho I.K., Lee C.H., Song G.G., Lim J.H. Clinical outcomes of standard triple therapy plus probiotics or concomitant therapy for Helicobacter pylori infection. Gut Liver. 2018;12(2):165. doi: 10.5009/gnl17177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gulzar N., Saleem I.M., Rafiq S., Nadeem M. IntechOpen; 2019. Therapeutic potential of probiotics and prebiotics. (Oral Health by Using Probiotic Products). [Google Scholar]
- 66.Liu Y., Alookaran J.J., Rhoads J.M. Probiotics in autoimmune and inflammatory disorders. Nutrients. 2018;10(10):1537. doi: 10.3390/nu10101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao Q., Dong B.R., Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015;(2) doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- 68.Kang Y., Cai Y. Gut microbiota and hypertension: from pathogenesis to new therapeutic strategies. Clin. Res. Hepatol. Gastroenterol. 2018;42(2):110–117. doi: 10.1016/j.clinre.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Harper A., Naghibi M.M., Garcha D. The role of bacteria, probiotics and diet in irritable bowel syndrome. Foods. 2018;7(2):13. doi: 10.3390/foods7020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie Y., Zhou G., Xu Y., He B., Wang Y., Ma R., et al. Effects of diet based on IgG elimination combined with probiotics on migraine plus irritable bowel syndrome. Pain Res. Manag. 2019;2019 doi: 10.1155/2019/7890461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaylaa M., Alard J., Kassaa I.A., Peucelle V., Boutillier D., Desramaut J., et al. Autophagy: a novel mechanism involved in the anti-inflammatory abilities of probiotics. Cell. Physiol. Biochem. 2019;53(5):774–793. doi: 10.33594/000000172. [DOI] [PubMed] [Google Scholar]
- 72.Maziade P.J., Andriessen J.A., Pereira P., Currie B., Goldstein E.J. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr. Med. Res. Opin. 2013;29(10):1341–1347. doi: 10.1185/03007995.2013.833501. [DOI] [PubMed] [Google Scholar]
- 73.Issa I., Moucari R. Probiotics for antibiotic-associated diarrhea: do we have a verdict? World J. Gastroenterol. 2014;20(47):17788–17795. doi: 10.3748/wjg.v20.i47.17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bae J.M. Prophylactic efficacy of probiotics on travelers' diarrhea: an adaptive meta-analysis of randomized controlled trials. Epidemiol. Health. 2018;40 doi: 10.4178/epih.e2018043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ling Z., Liu X., Cheng Y., Luo Y., Yuan L., Li L., et al. Clostridium butyricum combined with Bifidobacterium infantis probiotic mixture restores fecal microbiota and attenuates systemic inflammation in mice with antibiotic-associated diarrhea. BioMed Res. Int. 2015;2015:9. doi: 10.1155/2015/582048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu L., Zhou M., Young A., Zhao W., Yan Z. In vivo effectiveness and safety of probiotics on prophylaxis and treatment of oral candidiasis: a systematic review and meta-analysis. BMC Oral Health. 2019;19(1):140. doi: 10.1186/s12903-019-0841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishikawa K.H., Mayer M.P., Miyazima T.Y., Matsubara V.H., Silva E.G., Paula C.R., et al. A multispecies probiotic reduces oral Candida colonization in denture wearers. J. Prosthodont. 2015;24(3):194–199. doi: 10.1111/jopr.12198. [DOI] [PubMed] [Google Scholar]
- 78.Junqueira J.C., Fuchs H.B., Mylonakis E. 2019. Probiotic Bacteria-Directed Prevention or Treatment of Fungal Infection. (Google Patents). [Google Scholar]
- 79.Rosenfeld C.S. Microbiome disturbances and autism spectrum disorders. Drug Metabol. Dispos. 2015;43(10):1557–1571. doi: 10.1124/dmd.115.063826. [DOI] [PubMed] [Google Scholar]
- 80.Shaaban S.Y., El Gendy Y.G., Mehanna N.S., El-Senousy W.M., El-Feki H.S., Saad K., et al. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr. Neurosci. 2018;21(9):676–681. doi: 10.1080/1028415X.2017.1347746. [DOI] [PubMed] [Google Scholar]
- 81.Li Q., Zhou J.M. The microbiota–gut–brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience. 2016;324:131–139. doi: 10.1016/j.neuroscience.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 82.El Khatib R., Karam-Sarkis D., Waligora-Dupriet A.-J., Butel M.-J. IntechOpen; 2019. Could Gut Modulation through Probiotic Supplementation Be Beneficial in Autism Spectrum Disorder? Prebiotics and Probiotics-Potential Benefits in Human Nutrition and Health. [Google Scholar]
- 83.Morshedi M., Hashemi R., Moazzen S., Sahebkar A., Hosseinifard E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J. Neuroinflammation. 2019;16(1):231. doi: 10.1186/s12974-019-1611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rabiee M.R., Babajafari S. Probiotics and diabetes: a review. Int. J. Nutr. Sci. June. 2018;3(2):73–81. [Google Scholar]
- 85.Vyas N., Nair S., Rao M., Miraj S.S. Elsevier; 2019. Childhood obesity and diabetes: role of probiotics and prebiotics; pp. 363–376. (Global Perspectives on Childhood Obesity). [Google Scholar]
- 86.Mishra S., Wang S., Nagpal R., Miller B., Singh R., Taraphder S., et al. Probiotics and prebiotics for the amelioration of type 1 diabetes: present and future perspectives. Microorganisms. 2019;7(3):67. doi: 10.3390/microorganisms7030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobyliak N., Falalyeyeva T., Mykhalchyshyn G., Kyriienko D., Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes & Metabolic Syndrome. Clin. Res. Rev. 2018;12(5):617–624. doi: 10.1016/j.dsx.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 88.Caesar R. Pharmacologic and non-pharmacologic therapies for type 2 diabetes on the gut microbiota. Can. J. Diabetes. 2019 doi: 10.1016/j.jcjd.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 89.Okesene-Gafa K.A.M., Brown J., McCowan L., Crowther C.A. Probiotics for treating women with gestational diabetes for improving maternal and fetal health and well-being. Cochrane Database Syst. Rev. 2018;2018(2):CD012970. doi: 10.1002/14651858.CD012970.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun Z., Sun X., Li J., Li Z., Hu Q., Li L., et al. Using probiotics for type 2 diabetes mellitus intervention: advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 2019:1–14. doi: 10.1080/10408398.2018.1547268. [DOI] [PubMed] [Google Scholar]
- 91.Belizario J.E., Faintuch J., Garay-Malpartida M. Gut microbiome dysbiosis and immunometabolism: new Frontiers for treatment of metabolic diseases. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/2037838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kang Y., Cai Y. The development of probiotics therapy to obesity: a therapy that has gained considerable momentum. Hormones (Basel) 2018;17(2):141–151. doi: 10.1007/s42000-018-0003-y. [DOI] [PubMed] [Google Scholar]
- 93.Choi H.-J. Recent advances on next-generation probiotics linked to the gut microbiome. Food Sci. Ind. 2019;52(3):261–271. [Google Scholar]
- 94.Kumar H., Salminen S., Verhagen H., Rowland I., Heimbach J., Banares S., et al. Novel probiotics and prebiotics: road to the market. Curr. Opin. Biotechnol. 2015;32:99–103. doi: 10.1016/j.copbio.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 95.Kumar R., Sood U., Gupta V., Singh M., Scaria J., Lal R. Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. Indian J. Microbiol. 2019:1–14. doi: 10.1007/s12088-019-00808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hwang I.Y., Chang M.W. Engineering commensal bacteria to rewire host–microbiome interactions. Curr. Opin. Biotechnol. 2020;62:116–122. doi: 10.1016/j.copbio.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Sanap D.S., Garje M.A., Godge G.R. Probiotics, their health benefits and applications for development of human health: a review. J. Drug Deliv. Therapeut. 2019;9(4-s):631–640. [Google Scholar]
- 98.Kim S., Eun H.S., Jo E.-K. Roles of autophagy-related genes in the pathogenesis of inflammatory bowel disease. Cells. 2019;8(1):77. doi: 10.3390/cells8010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alard J., Peucelle V., Boutillier D., Breton J., Kuylle S., Pot B., et al. New probiotic strains for inflammatory bowel disease management identified by combining in vitro and in vivo approaches. Benef. Microbes. 2018;9(2):317–331. doi: 10.3920/BM2017.0097. [DOI] [PubMed] [Google Scholar]
- 100.Zaylaaa M., Alarda J., Al Kassaab I., Peucellea V., Boutilliera D., Desramauta J., et al. Autophagy: a novel mechanism involved in the anti-inflammatory abilities of probiotics. Cell. Physiol. Biochem. 2019;53(5):774–793. doi: 10.33594/000000172. [DOI] [PubMed] [Google Scholar]
- 101.Escudero-Hernández C., Koch S. Springer; 2019. The epithelial barrier; pp. 329–345. (Molecular Genetics of Inflammatory Bowel Disease). [Google Scholar]
- 102.Fujimoto K., Uematsu S. In: Mucosal Vaccines. second ed. Kiyono H., Pascual D.W., editors. Academic Press; 2020. Chapter 6 - innate immunity at mucosal surfaces; pp. 101–116. [Google Scholar]
- 103.Pan L.-L., Li J., Shamoon M., Bhatia M., Sun J. Recent advances on nutrition in treatment of acute pancreatitis. Front. Immunol. 2017;8:762. doi: 10.3389/fimmu.2017.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomson J.-E., Van Dijk S.M., Brand M., Van Santvoort H.C., Besselink M.G. 2018. Managing Infected Pancreatic Necrosis. [DOI] [PubMed] [Google Scholar]
- 105.Mandalia A., Wamsteker E.J., DiMagno M.J. Vol. 7. 2018. Recent Advances in Understanding and Managing Acute Pancreatitis. (F1000Res). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Den Nieuwboer M., Claassen E. Dealing with the remaining controversies of probiotic safety. Benef. Microbes. 2019;10(6):605–616. doi: 10.3920/BM2018.0159. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y., Wu Y., Wang Y., Xu H., Mei X., Yu D., et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9(5):521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fang S., Cheng D., Huang Y., Hsu T., Lu H. Pilot study of the influence of probiotics on hair toxic element levels after long-term supplement with different Lactic Acid bacteria strains. J. Prob. Health. 2018;6:1–8. [Google Scholar]
- 109.Stensvold C.R., Van Der Giezen M. Associations between gut microbiota and common luminal intestinal parasites. Trends Parasitol. 2018;34(5):369–377. doi: 10.1016/j.pt.2018.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.