Abstract
At the present, association of mitochondrial dysfunction and progression of neurological disorders has gained significant attention. Defects in mitochondrial network dynamics, point mutations, deletions, and interaction of pathogenomic proteins with mitochondria are some of the possible underlying mechanisms involved in these neurological disorders. Mitochondrial genetics, defects in mitochondrial oxidative phosphorylation machinery, and reactive oxygen species production might share common crosstalk in the progression of these neurological disorders. It is of significant interests to explore and develop therapeutic strategies aimed at correcting mitochondrial abnormalities. This review provided insights on mitochondrial dysfunction/mutations involved in the progression of Alzheimer’s disease, Huntington’s disease, and epilepsy with a special focus on Parkinson’s disease pathology. Along with the deleterious effects of mitochondrial mutations in aforesaid neurological disorders, this paper unraveled the available therapeutic strategy, specifically aiming to improve mitochondrial dysfunction, drugs targeting mitochondrial proteins, gene therapies aimed at correcting mutant mtDNA, peptide-based approaches, and lipophilic cations.
Keywords: adenosine-triphosphate deficiency, mitochondrial fission/fusion, mitochondrial mutations, neurodegenerative disorders, oxidative phosphorylation, therapeutic interventions
Introduction
Among all organs, the brain occupies approximately 2% of the total body weight percentage. Owing to its increased weight, approximately 15% of the total cardiac output is received by the brain; furthermore, it also utilizes a major portion approximately 20% of the total oxygen produced by the body. Excessive energy requirements of the brain are crucial for the proper functioning of neurons and maintenance of ionic gradient across the plasma membrane. Impairment in oxygen demand and supply cycle leads to neuronal cell death (Zhu et al., 2018). Apart from being a major source for adenosine-triphosphate (ATP) production, mitochondria are necessary for regulating homeostasis and cell survival. However, defects in mitochondrial function are associated with the increased incidence of cell death and reactive oxygen species (ROS) generation (Bhatti et al., 2017). Nevertheless, mitochondrial ROS are implicated in inducing mutations in mitochondrial DNA (mtDNA) and play a key role in the development and progression of neurological disorders.
Search and Selection Criteria
In this narrative review, PubMed database was searched for articles published from 1988 to 2021 using keywords such as neurological disorders, neurodegenerative disorders, mitochondrial dysfunction, mutations in mitochondria, parkin, α-synuclein, PARK1, therapies targeting mitochondrial dysfunction, and antioxidant therapies. Other than PubMed, official websites such as https://doi.org/10.19723/j.issn.1671-167X.2020.05.009, https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page, https://www.verywellhealth.com/the-4-as-of-alzheimers-disease-98591, were also used for data retrieval and collection.
Genetics of Mitochondria
Defects in mitochondrial oxidative phosphorylation machinery, reactive oxygen species production, and neurological disorders
Mitochondria maintain cellular homeostasis by producing numerous redox enzymes. Under stress conditions, it augments massive ROS production, influences the overall mitochondrial health and quality control function. Even though multiple cellular compartments are associated with ROS production, a majority of the intracellular ROS is delivered back to mitochondria (Rose et al., 2017). This process is well recognized as mitochondrial oxidative phosphorylation (OXPHOS). OXPHOS machinery is located on the inner surface of the mitochondrial membrane and composed of protein complexes having five multi-subunits. Each multi-subunit in respiratory chain complexes are encoded by the nucleus that is commonly rereferred as: (complex I), (complex II), (complex III), (complex IV), and ATP synthase (complex V). When compared with all of the respiratory chain complexes, complex I consists of a maximum number of mitochondrial encoded proteins (seven polypeptide chains) (Table 1). Abnormalities in mitochondria function are the major determinants for various apoptotic pathways, dysregulation of metabolic hemostasis, and fusion/fission process. Inability to produce ATP, defects in mitochondrial function may progress towards deleterious mutations in mitochondria. As per the mitochondrial free-radical theory, mitochondria produce excessive ROS in aging (Nunnari and Suomalainen, 2012). Elevated oxidative load causes an imbalance in proper mitochondrial functioning, ultimately leading to increased cell death. Excessive ROS in turn stimulate the cytosolic signaling molecules crucial for initiating intrinsic mitochondrial apoptotic cascade (Suomalainen and Battersby, 2018).
Table 1.
Encoding of protein subunits in respiratory enzyme complexes: component of oxidative phosphorylation
| Respiratory enzyme complex | Mitochondrion | Nucleus |
|---|---|---|
| Complex I | 7 | > 33 |
| Complex II | 0 | 4 |
| Complex III | 1 | 10 |
| Complex IV | 3 | 10 |
| Complex V | 2 | 10 |
Mitochondrial DNA
mtDNA is a circular-shaped double-stranded molecule of 16.569-kb having 37 genes. Approximately 13 structural genes encode for respiratory chain subunits while the remaining 24 genes are divided into 2 rRNA genes and 22 tRNA genes, though many of them are encoded by the nuclear genome (Kotrys and Szczesny, 2019). mtDNA is highly susceptible to mutational alterations. Compared with nuclear DNA (nDNA), mtDNA is 10 times more susceptible to mutations and associated changes. Studies have confirmed the presence of antioxidant and DNA repair enzymes such as 8-oxoguanine DNA glycosylase (OGG1) and mutY DNA glycosylase in mitochondria, which exhibit preventive potential against DNA mutation. Unlike nDNA, mtDNA is not layered by histones (histones act as a sheath for protection), which makes mtDNA more vulnerable to damage (Kaarniranta et al., 2019). Balanced mitochondrial quality control is required for maintaining effective communication with a nucleus. OXPHOS causes a significant increase in ROS-mediated damage that initiates retrograde signaling in the mitochondrial pathway. Stimulation of retrograde signaling often activates an adaptive nuclear response that leads to impairment in mtDNA and calcineurin-dependent activation of NF-κB. Long mitochondrial disruption has been known to be associated with the expression of more than 40 nuclear genes (Chowdhury et al., 2020). The relevance of mutations in mtDNA could be further understood by knowing the basic differences between nuclear and mitochondrial genetics. Table 2 describes the genetic classification of selected mitochondrial diseases (Kazak et al., 2012).
Table 2.
Classification of the more common forms of mitochondrial diseases on the basis of genetics
| Genome | Gene | mtDNA | Clinical phenotype |
|---|---|---|---|
| mtDNA | Single deletion | KSS, ocular myopathy, PS | |
| mtDNA | tRNALeu(UUR) | Single deletion | MELAS |
| tRNALys | Single deletion | MERRF | |
| Other tRNAs | Single deletion | Multiple phenotypes | |
| ATPase6 | Single deletion | NARP/MILS | |
| ND1, ND4, ND6 | Single deletion | LHON | |
| ND1, ND4 | Single deletion | Myopathy, multisystemic diseases | |
| Cyt b | Single deletion | Myopathy, multisystemic diseases | |
| COX III | Single deletion | Myopathy, multisystemic diseases | |
| nDNA | SCO1 | Single deletion | Hepatoencephalomyopathy |
| SCO2 | Single deletion | Cardioencephalomyopathy | |
| COX10 | Single deletion | Nephroencephalomyopathy | |
| COX 15 | Single deletion | Cardioencephalomyopathy | |
| ATP 12 | Single deletion | Fatal infantile | |
| TP | Multiple deletions | MNGIE | |
| ANT1 | Multiple deletions | adPEO* | |
| Twinkle | Multiple deletions | adPEO* | |
| POLG | Multiple deletions | ad/arPEO* | |
| dGK | Depletion | Hepatocerebral syndrome | |
| TK2 SMA | Depletion | Myopathy, SMA | |
| TAZ | Depletion | Barth syndrome | |
| OPA1 | Depletion | Optic atrophy |
ad/arPEO: Autosomal recessive progressive external ophthalmoplegia; adPEO: autosomal dominant progressive external ophthalmoplegia; ANT1: adenine nucleotide translocator-1; ATP: adenosine triphosphate; ATPase6: adenosine triphosphate synthase 6; COX: cyclooxygenase; Cyt b: cytochrome B; DGK: diacylglycerol kinase; KSS: Kearns- Sayre syndrome; LHON: leber hereditary optic neuropathy; MELAS: mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes; MERRF: myoclonic epilepsy with ragged-red fibers; MILS: maternally inherited leigh syndrome; MNGIE: mitochondrial neurogastrointestinal encephalomyopathy; NARP: neuropathy, ataxia and retinitis pigmentosa; ND1,4 and 6: NADH dehydrogenase subunit 6; OPA1: mitochondrial dynamin like GTPase; POLG: DNA polymerase subunit gamma; PS: Pearson’s syndrome; SCO1: synthesis of cytochrome c oxidase 1; SMA: spinal muscular atrophy; TAZ: tafazzin; TK2: thymidine kinase 2. mtDNA indicates alteration in mtDNA secondary to nDNA mutations. *Indicates proximal weakness, neuropathy, psychiatric disorders, parkinsonism. Information in Table 2 is modified from the studies of DiMauro (2004) and Muravchick (2008).
Crosstalk between reactive oxygen species and mitochondrial DNA dysfunction
Accumulating evidence indicates the implication of ROS in mtDNA dysfunction. Mitochondria undergo the continuous process of mitochondrial network dynamics to meet the constant cellular ATP requirements i.e., for fulfilling cellular metabolic needs and uniform distribution of mitochondria across the cell (Westermann, 2012). Mitofusin 1 (MFN1), Mitofusin 2 (MFN2), and optic atrophy-1 (OPA1) proteins regulate the mitochondrial fusion process. Former 2 proteins are present on the mitochondrial outer membrane while OPA1 is present on the inner side of the mitochondrial membrane. The fission process is regulated by dynamin-related protein 1 (Drp 1) and fission 1 (Chan, 2020). Abnormality in these proteins is related to excessive ROS production, synaptic dysfunction followed by synaptic loss. Increased malonaldehyde production and DNA fragmentation are the subsequent consequences of enhanced ROS. Proper mitochondrial functioning is essential for the regulation and maintenance of central nervous system (CNS) events (Yan et al., 2012).
Elevated ROS, impairment in mitochondrial dynamics, mitochondrial mutations, protein aggregation, and numerous environmental conditions may alter ATP production and metabolism in mitochondria. These changes are often linked with mitochondrial dysfunction mediated neurological disorders (Sies and Jones, 2020). Figure 1 shows the possible mechanism of mitochondrial dysfunction and its link with neurological disorders. Table 3 represents a wide array of clinical signs and symptoms for these neurological disorders. However, mutations/deletions in mtDNA may be sporadic or maternally transmitted. Mitochondria have two facets that are necessary for leading a healthy life, whereas, on the other hand, it is related to neuronal cell death. Mitochondrial damage in neurological disorders has led to the tremendous development of mitochondrial-directed therapeutic strategies.
Figure 1.
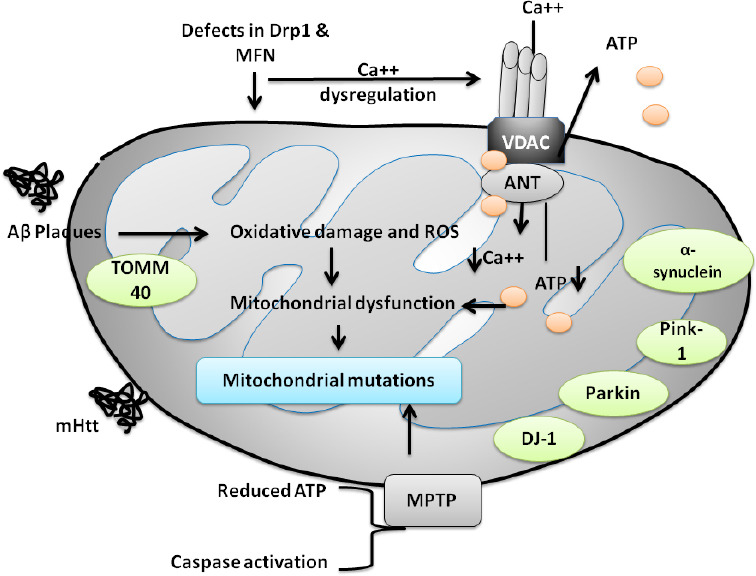
Diagrammatic representation of the pathways involved in mitochondrial dysfunction in neurodegenerative disorders.
MPTP-induced PD is a well-established model for PD. The figure shows the mechanism involved in mitochondrial dysfunction concerning MPTP. MPTP may induce alterations in ATP production, activation of apoptotic pathways, and mutational changes in DJ-1, parkin, PINK-1, α-synuclein. mHtt is associated with mutational changes in HD, mHtt worsens the disease condition by increasing the generation of ROS and oxidative load. In the case of AD, Aβ plaque accumulation is a key contributor in disease progression and development and activates various oxidative stress markers, which may possess the capability to alter mitochondrial fission proteins such as Drp1, MFN. Modifications in Drp1, MFN further causes activation of several other pathways including dysregulation in calcium hemostasis, reduction in ATP production that are considered to be important pathways involved in mitochondrial abnormalities, dysfunction, and mutational changes. Aβ: Amyloid-beta; AD: Alzheimer’s disease; DJ-1: protein deglycase; Drp 1: dynamin-related protein 1; Drp1: dynamin related protein; HD: Huntington’s disease; MFN: mitofusin; mHtt: mutant huntingtin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; OXPHOS: oxidative phosphorylation; PD: Parkinson’s disease.
Table 3.
Signs and symptoms encountered in mitochondrial disorders
| Disease | Mutation | Signs and symptoms |
|---|---|---|
| Kearns-Sayre syndrome | Large-scale mtDNA deletionĀ | Ataxia, peripheral neuropathy, muscle weakness, ophthalmoplegia, apoptosis, pigmentary retinopathy, sideroblastic anemia, diabetes mellitus, short stature, hypoparathyroidism, cardiomyopathy, conduction defects, |
| Myoclonic epilepsy with ragged-red fibers | mtDNA point mutation, tRNA abnormality | Seizures, ataxia, myoclonus, psychomotor regression, peripheral neuropathy, muscle weakness, short stature, sensorineural hearing loss, lactic acidosis, ragged-red fibers on muscle biopsy |
| Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes | mtDNA point mutation, tRNA abnormality | Seizures, ataxia, myoclonus, psychomotor regression, hemiparesis, cortical blindness, migraine, dystonia, peripheral neuropathy, muscle weakness, diabetes mellitus, short stature, cardiomyopathy, conduction defects, intestinal pseudoobstruction, sensorineural hearing loss, Fanconi syndrome, lactic acidosis, ragged-red fibers on muscle biopsy |
| Progressive external ophthalmoplegia | Large-scale mtDNA mutation | Muscle weakness, ophthalmoplegia, apoptosis, lactic acidosis, ragged-red fibers on muscle biopsy |
| Pearson’s syndrome | Large-scale mtDNA mutation | Anemia, pancreatic dysfunction, Fanconi syndrome, lactic acidosis, ragged-red fibers on muscle biopsy |
| Neuropathy, ataxia, and retinitis pigmentosa | mtDNA point mutations, mRNA abnormality | Ataxia, peripheral neuropathy, muscle weakness, pigmentary retinopathy, optic atrophy, sensorineural hearing loss |
| Maternally inherited Leigh’s syndrome | mtDNA point mutations, mRNA abnormality | Seizures, ataxia, psychomotor regression, dystonia, muscle weakness, pigmentary retinopathy, optic atrophy, cardiomyopathy, lactic acidosis |
| Leber’s hereditary optic neuropathy | Multiple mtDNA point mutations, mRNA abnormality | Dystonia, optic atrophy, cardiac conduction defects |
mRNA: Messenger RNA; mtDNA: mitochondrial DNA; tRNA: transfer RNA. Information in Table 3 is modified from the study of Muravchick (2008).
Mitochondrial Mutations and Neurological Disorders
Mitochondrial mutations are primarily linked with the changes in post-mitotic cells, which exhibit increased metabolism in muscles and neurons, signifying their roles in mitochondrial disorders. Mutations can be either heteroplasmic or homoplasmic; however, most of them heteroplasmic whereas homoplasmic mutations are linked with the increased expression of mitochondrial inheritance (Pintoa and Moraesa, 2014).
Alzheimer’s disease and mitochondrial alterations
Alzheimer’s disease (AD) is a complex, multifaceted disease mostly affecting people with advancing age. It is characterized by failure of acquired skills causing agnosia, aphasia, apraxia with interpersonal and social deterioration (https://www.verywellhealth.com/the-4-as-of-alzheimers-disease-98591). Dystrophic neuritis, loss of dendritic branches, and alterations in dendritic spines in AD brains are connected with the changes in mitochondria (Baloyannis, 2009). Overexpression of amyloid precursor protein (APP) in transgenic mice is also coupled with the clogged mitochondrial protein import machinery. Deficient ATP production, increased ROS, cytochrome-c (COX), inhibition of complex I, and apoptosis induction are the key contributors involved in mitochondrial dysfunction (Wilkins and Swerdlow, 2017). Lipid peroxidation caused by excess ROS yields toxic aldehyde causing impairment in activities of mitochondrial enzymes namely: alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase (Sies and Jones, 2020). Mitochondrial damage in AD is represented through the inhibition of complex I and COX, ultimately leading to decreased cell energy and augmented tau phosphorylation causing progression of mitochondrial damage in AD (Wang et al., 2020).
Mutations in mtDNA are often correlated well with neurological impairment. Mitochondrial mutations can account for the degree, pattern, and load segregation across the whole brain as well as among each neuron in neurological disorders. Mutational modifications in the TOMM40 gene are found to be the crucial contributor and important risk factor for AD (Ferencz et al., 2012; Roses et al., 2013). The probability for AD development is facilitated by the varying poly-T 336 homopolymers in TOMM40 gene (rs10542523), which is in linkage disequilibrium 337 with APOE (Lutz et al., 2010; Roses et al., 2013).
Blood and brain tissue samples collected from experimental animal models of AD have revealed that the TOMM40 gene is a potential biomarker for AD. Microtubule-associated protein tau (MAPT) gene encodes for tau protein (Lee et al., 2012; Chong et al., 2013), even a point mutation in the MAPT gene can lead to its hyperphosphorylation, aggregation, and plaque deposition. Intriguingly, it also reduces the binding ability of tau to microtubules ultimately making it more unstable. Unstable microtubules affect axonal mitochondrial transport disrupting synaptic communication (Strang et al., 2019). Moreover, the activity of the respiratory complex, rate of fission, and fusion are decreased in hyperphosphorylated mutant tau in SY5Y cells increasing their susceptibility towards oxidative stress (Schulz et al., 2012). In brief, mutations in the MAPT gene directly affect mitochondrial function and dynamics. Evidence supports that mutations in presenilin-1 are implicated in mitochondrial dysfunction-induced AD (Hansson et al., 2004).
Mutations can be repaired by the available DNA repair pathways such as the base excision repair (BER) pathway; it is based upon the modification of the base through alkylation, deamination, and oxidation processes. Affected as well as non-affected regions of the brain represent BER deficiency, suggesting that BER impairment is common in AD brains. Diminished activities of enzymes such as uracil DNA glycosylase, OGG1, and DNA polymerase beta are responsible for causing an imbalance in the BER mechanism in the clinical condition of AD (Chatterjee and Walker, 2017). The ability to repair mitochondrial nDNA is reduced in AD patients. Mitochondrial dysfunction and oxidative damage are the core contributors involved in AD pathogenesis, although the exact mechanism underlying the disease pathology is still debated (Lin and Beal, 2006; Wang et al., 2020).
Parkinson’s disease
Parkinson’s disease (PD) accounts for the second most prevalent movement and widespread movement disorder. Elderly people aged over 65 years are most commonly affected by PD with a worldwide prevalence of around 2%. Degenerated dopaminergic neurons, depleted dopamine in basal ganglia, and intra-cytoplasmic inclusions containing fibrillar α-synuclein leading to the formation of Lewy bodies are some of the characteristic hallmarks of PD (Kouli et al., 2018). Genetical examination of patients with familial PD has led to the identification of numerous mutated genes such as α-synuclein (PARK1/4), parkin (PARK2), PINK1 (PARK6), DJ-1 (PARK7), LRRK2 (PARK8), and ATP13A2 (PARK9), whereas more rarely associated genes include PARK3, UCHL1 (PARK5), GIGYF2 (PARK11), HTRA2 (PARK13), PLA2G6 (PARK14), and DNA polymerase G gamma (POLG) that are more often related to the prevailing risk factors for PD. However, they were not accounted for the causative mutation. Figure 2 briefly represents the associated abnormalities and mutations in mitochondrial genes and their involvement in neurodegenerative disorders (Schapira, 2011).
Figure 2.
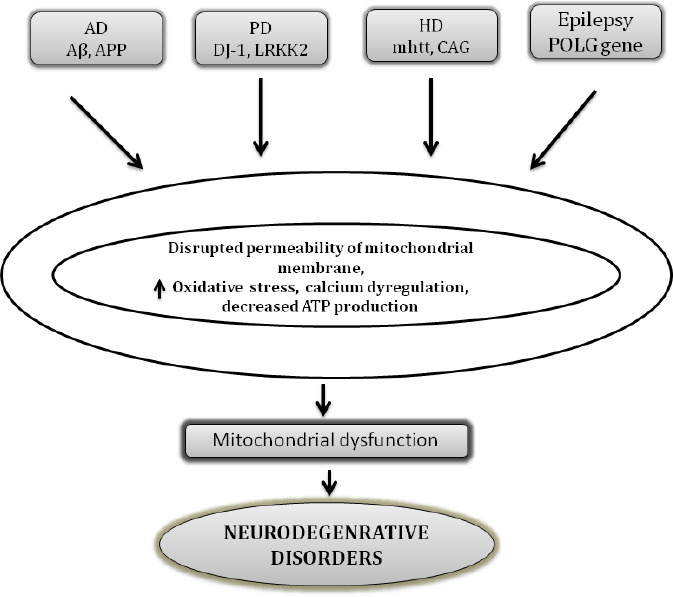
Major proteins involved in mitochondrial abnormalities in various neurodegenerative disorders.
Aβ: Amyloid-beta; AD: Alzheimer’s disease; APP: amyloid precursor protein; CAG: cytosine, adenine, and guanine; DJ-1: protein deglycase; HD: Huntington’s disease; LRRK2: Leucine Rich Repeat Kinase 2; mhtt: mutant huntingtin; PD: Parkinson’s disease; POLG: DNA polymerase gamma.
α- Synuclein
α-Synuclein (α-syn), is a presynaptic neuronal protein composed of 140 amino acid fibrillar protein that is susceptible to aggregation. The presence of water-repelling the amyloid-beta domain is an important factor, which is accountable for its aggregating property. Lewy bodies composed of a fibrillar form of α-syn are one of the major contributors involved in the pathogenesis of PD (Goedert and Spillantini, 2017). Numerous studies indicate that disruption in the activity of mitochondrial respiratory complex-I, excessive mitochondrial ROS production, and reduction in mitochondrial membrane potential are some of the deteriorating effects associated with enhanced mitochondrial deposition of α-syn (Puspita et al., 2017). From the above, it is evident that α-syn accumulation and alterations in mitochondrial processes are interrelated pathological events that play a key role in α-syn-associated mitochondrial abnormalities in the progression and development of PD (Puspita et al., 2017). Genetic alterations of a single base pair (A530T, A30P, and E46K) and genomic duplications in PARK1 and SNCA region of α-syn are correlated well with an autosomal dominant PD (Polymeropoulos et al., 1997; Kruger et al., 1998; Singleton et al., 2003). Overexpression of human A53T α-syn mutation in a transgenic mouse model is linked with DNA double-strand break in neurons, and mitochondrial DNA damage in motor neurons (Martin et al., 2006). Abnormalities in mitochondria and α-syn protein accumulation are implicated in PD pathogenesis. The ability of α-syn to mediate oxidative stress may be one of the possible mechanisms involved in mitochondrial dysfunction and protein accumulation in the case of α-synuclein. An increase in activity of cytotoxic enzymes such as lactate dehydrogenase-II, carbonic anhydrase-II, the enzyme responsible for the maintenance of acid-base balance, and the increase in activity of highly abundant alpha-enolase also relate to the disease worsening and progression. Thus, modification in the activities of enzymes and proteins responsible for energy production and metabolism is highly susceptible to oxidative damage linked with mutant α-syn (Poon et al., 2005).
Parkin
Young-onset autosomal recessive Parkinson’s disease was found to be associated with the mutations in the parkin gene (PARK2) (Fang et al., 2019). Parkin localized on the outer layer of the mitochondrial surface diminishes the release of COX and inhibits mitochondrial swelling playing a key role in guarding cells against cellular apoptosis (Zhang et al., 2016). The protective role of parkin has been demonstrated in parkin knock out mouse and fly models, which illustrates that mice and flies lacking parkin are more prone to develop mitochondrial abnormalities such as mitochondrial blebbing, disintegrated cristae, and the decline in activities of complex I and IV of the mitochondrial respiratory chain (Shim et al., 2011; Steele et al., 2014; Fichi et al., 2019). Contrary to this, mice overexpressing parkin observed reduced dopaminergic cell loss in the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) model of PD. These protective responses confer mitochondrial defense and decrease in α-syn. Parkin is necessary for the maintenance and regulation of mitochondrial dynamics and autophagy (Bian et al., 2012; Sun et al., 2012).
Phosphatase and tensin homolog-induced putative kinase 1
Phosphatase and tensin homolog-induced putative kinase 1 (PINK1), a protein kinase localized to mitochondrial membranes, is predominantly expressed in neurons of the human brain. After parkin, genetic alterations linked with PINK1 are the second most prominent cause contributing to the development and progression of early-onset autosomal recessive PD. Mitochondrial abnormalities noted in the PINK1 knock-out mouse model are quite similar to those noted in parkin such as decreased mitochondrial membrane potential, structural changes in mitochondria, increased activity of caspase, and enhanced oxidative load (Gonçalves and Morais, 2021). Furthermore, mitochondrial proteins in the midbrain, striatum, and cerebral cortex are responsible for regulating metabolic hemostasis and mitochondrial membrane potential also undergo alteration in the disease progression. In terms of specificity, substantia nigra in the midbrain region was severely affected and exhibits a maximum number of defective mitochondria. Furthermore, alterations in these mitochondrial proteins are connected with the mutations in PINK1. Mutations in PINK1 induce structural changes in mitochondria and serve as an important mediator of ROS production (Kausar et al., 2018).
Protein deglycase DJ-1 and leucine-rich repeat kinase 2
Mutations in protein deglycase DJ-1 and leucine-rich repeat kinase 2 (LRRK2) are indicated in the sporadic form of PD. Approximately 1–2% of cases of early-onset PD are associated with DJ-1, (Repici and Giorgini, 2019), whereas LRRK2 is known to induce autosomal-dominant cases of PD. The majority of the cases associated with DJ-1 and LRRK2 are related to familial cases of PD, with less frequent cases for the occurrence of sporadic late-onset PD (Kessler et al, 2018; Repici and Giorgini, 2019). Several lines of experimental evidence indicate that knockdown or overexpression of DJ-1 directly or indirectly corresponds to increased susceptibility for cellular death. Though DJ-1 possesses the capability to escape cellular death owing to its cytoprotective potential; however, its cytoprotective potential is limited to prevent oxidative stress-induced cell death, and it does not seem to confer protection against cell death occurring due to any other causes (Mihoub et al., 2017; Raninga et al., 2017). Cytoprotective property is subjected to the alterations in cysteine residues of DJ-1. Under the conditions of extreme oxidative stress, cysteine residues undergo modification to either cysteine-sulfinic or cysteine-sulfonic acid, or both. Interestingly, DJ-1 has also been reported to exhibit neuroprotective effects possibly by acting as transcriptional co-activator protease or a molecular chaperone (Takahashi-Niki et al., 2017).
Knowledge about the physiological function of LRRK2 protein is limited, but as mentioned above, it is involved in the progression and development of autosomal recessive dominant cases of PD. The precise physiological role of LRRK2 in this protein is unidentified, but the presence of multiple functional domains suggests involvement in a wide array of functions. A possible site of action for the LRRK2 mechanism is mitochondria. A brief update about mitochondrial DNA damage in neurological disorders is shown in Table 4 (West et al., 2005).
Table 4.
Indication for the participation of mitochondrial DNA damage in neurological disorders
| Disease | Pathogenesis | Genetic factors |
|---|---|---|
| Alzheimer’s disease | Aβ plaques, neurofibrilary tangles | APP, Tau, PS1, PS2, APOE4, TOMM40 gene, MAPT |
| Parkinson’s disease | α-Synuclein | Parkin, LRRK2, DJ-1, α-synuclein, PINK1 |
| Huntington’s disease | N-terminal polyglutamine | Htt, Drp1, p53, HTRA2, |
| Myoclonic epilepsy and ragged red fibers | 8344 A->G | MT-TK |
| Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes | A-to-G transition at nucleotide (m.3243A>G). tRNALeu(UUA/UUG) are causative in nature. | MT-TL1 |
APOE: Apolipoprotein E; APP: amyloid precursor protein; Aβ: amyloid beta; DJ1: protein deglycase DJ-1/Parkinson disease protein 7; DRP1: dynamin-related protein 1; HTRA2: HtrA serine peptidase 2; Htt: Huntingtin; LRRK2: leucine rich repeat kinase 2; MAPT: microtubule associated protein tau; MT-TK: mitochondrially encoded TRNA-Lys (AAA/G); MT-TL1: mitochondrially encoded TRNA-Leu (UUA/G) 1); PINK1: PTEN-induced kinase 1; PS1 and 2: presenelin 1 and 2; TOMM40: translocase of outer mitochondrial membrane 40. Information in Table 4 is a summary of a study from Norat et al. (2020).
Evidence from subcellular fractionation studies has established a fact that 10% of total LRRK2 protein is coupled with the mitochondrial fraction. Immunohistochemical results further support the localization of LRRK2 to mitochondria. According to previous reports, LRRK2 also shares a cross-talk with TOM20 (Biskup et al., 2006; Gloeckner et al., 2006). This finding was further confirmed by repurposing polyclonal antibodies to amino or carboxyl terminus of LRRK2 to investigate the subcellular localization of LRRK2 in microsomal, synaptic vesicle-enriched, and synaptosomal fractions of rat brain slices and mitochondria.
POLG (mitochondrial DNA polymerase gamma gene)
An earlier report suggests that mitochondrial dysfunction holds significant relevance in pathogenetic forms of PD, specifically sporadic and idiopathic forms (Schapira et al., 2011). Evidence indicates that nuclear-encoded protein (POLG) is implicated in the production, replication, and repairing of mtDNA. Any mutation in the catalytic subunit of PLOG is connected with the depletion or deletions of mtDNA in several types of neurological phenotypes including PD (Stiles et al., 2016; Young and Copeland, 2016). These properties of the catalytic subunit are attributed to its polymerase activity at 59 to 39 and exonuclease activity at 39 to 59. Evidence indicates that clustering of somatic mtDNA deletions occurs in the substantia nigra in PD cases. Moreover, gene products of familial Parkinsonism such as POLG1 have a crosslinking with mitochondrial function. Several lines of evidence explicit that mutations in POLG1 are associated with levodopa-induced Parkinsonism in some families (Hudson et al., 2007; Luoma et al., 2007; Eerola et al., 2010). Mutations in POLG1 lead to the incorporation of defective or incorrect nucleotides in mtDNA causing impairment in the mitochondrial respiratory chain (Orsucci et al., 2011). One report illustrates that homozygous mice having polymerase deficient mutant of POLG1 express a phenotype with enhanced point mutations and mtDNA deletions (Trifunovic et al., 2004). In a few cases, genetic abnormalities occurring in mtDNA due to POLG1 gene mutation can directly or indirectly cause Parkinsonism (Luoma et al., 2007; Eerola et al., 2010). Missense mutations in POLG1 such as a mutation in G737R and R853W are represented in some cases of early-onset Parkinsonism (Davidzon et al., 2006).
Huntington’s disease
Huntington’s disease (HD) is an autosomal dominant neurodegenerative movement disorder. HD is characterized by degeneration of striatal GABAergic medium spiny neurons leading to the development of motor and non-motor symptoms viz involuntary muscle movements, psychiatric disorders, and memory loss (https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page; accessed on October 8, 2021). Mitochondrial defects in HD are attributed to the defects and mutations in the huntingtin (htt) gene and CAG triplet repeat expansion. CAG triplet repeat expansion is responsible for generating a polyglutamine (polyQ) stretch in mutant htt (mhtt) promoting its accumulation in the brain (Neueder et al., 2017). The mhtt produces alterations in ATP production and consumption, mhtt and various other vital cellular processes including endocytosis, intraneuronal trafficking, transcriptional regulation, postsynaptic signaling, and initiation of the apoptotic cascade, probably via causing alteration in mitochondrial network dynamics (Song et al., 2011; Wang et al., 2013; Guo et al., 2016). Another serine protease protein HtrA Serine Peptidase 2 (HTRA2) has been reported to be present on the inter-mitochondrial membrane. HTRA2 known to induce HD-like phenotypical modifications in transgenic mice is located in the inter-mitochondrial membrane. HTRA2 levels were reduced in striatal neurons in conditions where mhtt is expressed. Crosstalk between HTRA2 and mhtt indicates HTRA2 homeostasis is coupled with the specific susceptibility to the striatal neurons (Inagaki et al., 2008). Apart from HTRA2, peroxisome proliferator-activated receptor-g coactivator 1α (PGC-1α) is one of the critical targets, which is involved in the regulation of a few other metabolic processes such as OXPHOS, mitochondrial biogenesis, and adaptive thermogenesis has also been reported (Hollenbeck et al., 2005; Lin et al., 2005; Puigserver et al., 2005; Cui et al., 2006;).
Epilepsy
Epilepsy shares a widespread prevalence of 0.5–0.7% across the globe. It is characterized by episodes of recurrent seizures, synchronized neuronal discharges affecting normal brain functioning. Epileptic seizures present the signs of mitochondrial abnormalities and disparities from normal behavior in the CNS. Mitochondria are the power house of the cells, responsible for ATP production, which is vital for neuronal excitability and survival (Singh et al., 2020). In neurons, balanced mitochondrial functioning is utilized to carry out physiological processes such as neurons Ca2+ hemostasis, redox machinery, developmental and synaptic plasticity, and cell cycle (Singh et al., 2020). High energy demand and lack of regenerative capacity of CNS neurons increase the likelihood of CNS towards mitochondrial dysfunction and increased frequency of occurring epileptic seizure. In response to defects in the mitochondrial respiratory chain, there is a decreased intracellular ATP level, increased neuronal excitability, impairment in Na+/K+ ATPase activity, and reduced mitochondrial membrane potential (Vezzani et al., 2019).
Energy depletion in mitochondria is correlated well with enhanced synaptosomal and astrocytic glutamate release indicating impairment in the glutamate-aspartate transporter in mitochondria (Vezzani et al., 2019; Singh et al., 2020). Figure 3 illustrates the basic mechanism involved in the occurrence of epileptic seizures. Genetic alterations in mtDNA and nuclear genes can also lead to epilepsy. Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS); MERRF, myoclonic epilepsy, and ragged-red fibers (MERRF) are the two disorders that are linked to the mutations in mtDNA. m.8344A>G mutation in the mitochondrial tRNA gene for the lysine gene, MT-TK, was the first-ever reported mutation (Hou et al., 2020). m.8356T>C and m.8361G>A, are other two mutations, which are known to cause the same clinical condition. The first incidence of MELAS came to light in 1984, affecting 1:15,000 individuals worldwide with varying phenotypic expression (Bhatti et al., 2017; Zhu et al., 2018). Maternally inherited neurodegenerative disorder MELAS occurs due to mutation in m.3243A>G. Nearly 80% cases of MELAS are associated with the mutation m.3243A>G of the mitochondrial transfer RNA (tRNA) (Leu (UUR)) gene (MT-TL1) (Montagna et al., 1988; Goto et al., 1990).
Figure 3.
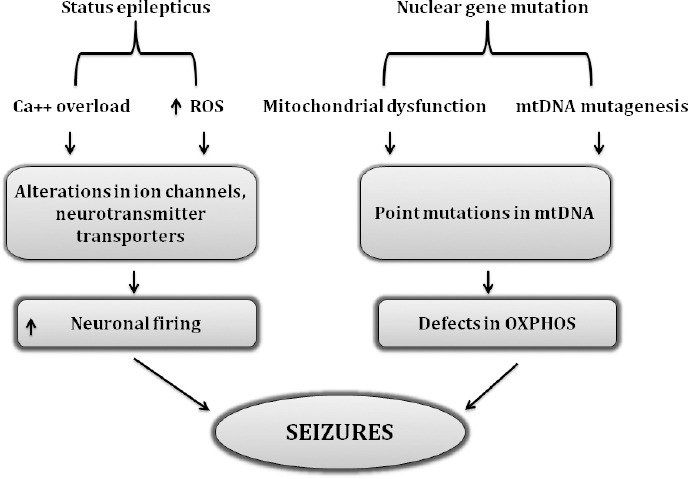
Association of mutational modifications and ROS-related cascade in deterioration of epileptic seizures.
Flow chart indicates the role of nuclear mutations in inducing mitochondrial dysfunction, point mutations in mtDNA ultimately producing defects in OXPHOS machinery which is considered as a prime culprit responsible for causing mitochondrial dysfunction and related changes. On the other hand, the common pathways involved in the initiation of status epilepticus are still the enhanced oxidative load and dysregulation of calcium hemostasis with the result of massive neuronal firing and seizure indicating that these two pathways share common crosstalk in epileptic seizures. Ca++: Calcium overload; mtDNA: mitochondrial DNA; OXPHOS: oxidative phosphorylation; ROS: reactive oxygen species.
Therapeutic Strategies Targeting Mitochondrial Dysfunction
Mitochondrial dysfunction is implicated in the pathophysiology of neurological disorders. Alterations in OXPHOS, enhanced mtDNA deletions, mutations, deregulation in calcium signaling, and impairment in energy metabolism including crosstalk with pathogenic target proteins (e.g., amyloid-β, α-syn, parkin, PINK1, and huntingtin) are the major factors affecting mitochondrial function and biogenesis. It is noteworthy to state that mitochondrial pathology could be potentially involved in the onset of clinical presentation of AD, PD, HD, and epilepsy kind of neurological disorders. Thus, therapeutic interventions targeting to strengthen mitochondrial functions would represent an attractive strategy to improve the pathological alterations observed in these disorders.
Therapeutic approaches targeting mitochondrial dysfunction in neurological disorders are multifaceted. Antioxidants, compounds targeting mitochondria such as alpha-lipoic acid, coenzyme Q10, carnitine, vitamin C, vitamin E, methylene blue, Ginkgo biloba, piracetam, simvastatin, curcumin, and omega-3 polyunsaturated fatty acids are some of the conventional strategies, which are proven to be beneficial (Zhao and Fu, 2021). Current strategies are focusing on proper regulation of (i) mitochondrial fusion/fission process (ii) mitochondrial proteins (iii) gene therapy to decrease mutant mtDNA (iv) peptides and lipophilic cations targeting mitochondria, might represent a novel therapeutic strategy. Recently, newer perspectives in mitochondrial therapeutics are the modulation of mitochondrial network dynamics. Improvement in mitochondrial dynamics is achieved by mitochondrial biogenesis stimulating mitochondrial movement, and nucleotide supplementation (LeitãoRocha et al., 2015).
Drugs targeting increased mitochondrial fusion/fission process
Emphasis has been laid down towards developing suitable and safer drug candidates aimed at reducing the enhanced mitochondrial fission/fusion processes. These approaches are aimed at maintaining proper balance in mitochondrial network dynamics within the mitochondria. It has been hypothesized that the development of these novel strategies may exert some beneficial actions in upgrading mitochondrial functioning, neuronal activity, and cell survival in neurological disorders associated with oxidative stress and mitochondrial dysfunction. To date, three inhibitors for improving mitochondrial fission are screened through different chemical libraries. Mdivi (Cassidy-Stone, 2008), Dynasore (Macia et al., 2006), and P110 (Qi et al., 2013) are the available inhibitors for correcting impairment in the mitochondrial fission process.
Mdivi
A considerable amount of work has been done on the Mdivi molecule in experimental rodent models for epilepsy and/or seizures for exploring its protective potential (Kim et al., 2016). Qi et al. (2013) have demonstrated that in an in vivo study, Mdivi-1 can inhibit mitochondrial fission in an epileptic rat model. Further, the findings of their study reveal that seizures can cause an increment in the mitochondrial fission and contrary mdivi-1 treatment significantly attenuated oxidative damage with the considerable improvement in neuronal density in the hippocampus. Mdivi also acts as a ROS scavenger against oxidative damage, mitochondrial toxicity ischemia (Qi et al., 2013), oxygen-glucose deprivation (Tang et al., 2013), and rhabdomyolysis (Chlystun et al., 2013). Proven beneficial effects of Mdivi have made it an excellent drug of choice for combating and inhibiting the disproportionate mitochondrial fission and for maintenance of fission-fusion balance normal and the normal functioning of cells.
Dynasore
Dynasore has gained significant relevance as a dynamin GTPase inhibitor for endocytic pathways. It prevents fragmentation and helps in making dynamin-dependent endocytic vesicles. Over 16,000 small molecules were screened by Macìa et al. (2006) to identify their potential to inhibit the mitochondrial fission process. During screening novel compounds, they found a new compound and named it “dynasore”. Due to its remarkable property of inhibiting the fission, dynasore is considered to bring notable changes in the mitochondria-targeted approaches, which claim to balance the mitochondrial fission. Therefore, more stability is provided in mitochondrial network dynamics (Macia et al., 2006).
P110
The development of P110 is another successful strategy under the category of fission inhibitors. Initially, P110 was found to be efficient in blocking the enzyme activity of Drp1, decreasing Fis1 showing improvement in mitochondrial fission in vitro. P110 was further explored for various properties. The evidence further indicates the neuroprotective potential of P110 probably via its inhibitory action on mitochondrial fission and ROS generation. Inhibitory action of P110 on mitochondrial fission is responsible for maintaining sustained mitochondrial integrity and membrane potential. Nevertheless, an additional research is needed to raise the curtain for disclosing its potency as an efficient Drp1 inhibitor (Qi et al., 2013).
Therefore, Mdivi, Dynasore, and P110 represent an interesting strategy to treat neurological disorders that deal with disturbances in mitochondrial fission and dysfunction.
Drugs targeting mitochondrial proteins
Targeting mitochondrial proteins is another fascinating scheme that might represent a unique therapeutic approach directed towards these neurological disorders. Presently, significant attention is being given to developing various mitochondria-directed antioxidants to treat and improve mitochondrial dysfunction. Antioxidant therapy is divided into three main categories: (i) Stoichiometric scavengers: nitroxide spin traps, vitamin E or C (ii) catalytic antioxidants: salen, or metalloporphyrin’s, and lastly (iii) indirect antioxidants: iron chelators. Activation of Nrf2 pathway is the basic mechanism for obtaining the desired protective effects linked with iron chelators, and it also activates various anti-oxidant pathways primarily Nrf2, increasing mitochondrial GSH, and decreasing ROS production (Csire et al., 2020). Vitamin E is a well-known antioxidant that confers neuroprotection against pilocarpine-induced epilepsy. Moreover, these studies have concluded vitamin E can significantly increase the catalase and free fatty acid in the brain. A previous report further indicates the beneficial effects of vitamin E co-medication in improving the cases of refractory epilepsy by substantially reducing the occurrence of seizure frequency in child patients (Mehvari et al., 2016). Although clinical trials with vitamin E are still controversial having numerous unsuccessful attempts for decreasing the occurrence of seizures in pediatric patients and epilepsy animal models (Levy et al., 1992; Xu and Stringer, 2008). Numerous in vitro and in vivo studies indicate the protective potential of taurine against mitochondrial-induced damage and related neurological disorder such as MELAS (Homma et al., 2021). Decreased frequency of stroke-like episodes and alterations in mitochondrial tRNALeu(UUR) is also reported with oral administration of taurine in MELAS patients (Ohsawa et al., 2019). Preventive effects of beta-alanine induced mitochondrial damage, drop in mitochondrial membrane potential, and improvement in apoptosis were also noted in mouse embryonic fibroblasts treated with beta-alanine (Shetewy et al., 2016), which showed that taurine pretreatment protects against mitochondria damage and mitochondria fission in beta-alanine-treated mouse embryonic fibroblasts (Jong et al., 2012). The antioxidant potential of taurine is related to its ability to scavenge hypochlorous acid (HOCl), produced from hydrogen peroxide (H2O2) during ROS generation (Li et al., 2004). This property of taurine is utilized for its ability to prevent mito-oxidative stress, sustenance of intracellular calcium homeostasis against oxidative insult in mitochondria (El Idrissi and Trenkner, 2004; El Idrissi et al., 2008).
Gene therapy to reduce mutant mtDNA
mtDNA is heteroplasmic. To combat this disease condition gene-based therapeutic interventions are considered to be an important approach, which claims its preventive action by maintaining an equilibrium towards wild-type mtDNA by declining the mutant mtDNA below the threshold of these neurological disorders. These strategies are involved in degrading the mutant mtDNA by selective mitochondrial nucleases (Smith and Lightowlers, 2011; Leitão-Rocha et al, 2015). In this context, anti-replicative therapy is one such strategy involving sequence-specific nucleic acids designed to bind mtDNA replication, promoting the propagation and replication of wild-type mtDNA. Peptide nucleic acid has shown beneficial effects in vitro; however, it lacks to modify heteroplasmy under a condition where cells are in intact form due to the presence of an increased amount of mt DNA (Smith and Lightowlers, 2011; Leitão-Rocha et al, 2015).
Current reports suggest that tRNA’s possess the ability to deliver anti-replicative oligo-ribonucleotides to mitochondria through RNA import pathways. tRNAs have been shown to reduce the mtDNA load by 15–35% in Kearns-Sayre syndrome (Comte et al., 2013). Moreover, endonuclease-mediated heteroplasmy is also a newer approach for mutant mtDNA.
Peptides and lipophilic cations targeting mitochondria
The inability of mitochondria-targeted antioxidants to increase the antioxidant levels within mitochondria has posed a serious limitation to its use. At present much enthusiasm has been delivered to innovate and develop mitochondria-specific antioxidant therapies (Armstrong, 2007). Conjugation of lipophilic triphenylphosphonium (TPP+) cation to an antioxidant moiety, like coenzyme Q (MitCoQ) and α-tocopherol (MitoVitE) are the newer approaches in this track (Murphy, 2000). Potential gradient across the mitochondrial inner membrane plays an important role in generating the efficacy of lipophilic cation. Consequently, the proton gradient produces a negative potential of 150 to 180 mV across the inner membrane causing the lipophilic cations to accumulate at 100 to 1000 times in mitochondria. Similarly, MitoVitE is occupied by mitochondria approx. 80-fold more than vitamin E, and considered as far more effective in defending mitochondria against oxidative load as vitamin E itself.
MitoVit E is also found to be 800 times more effective than idebenone, a CoQ10 analog in imparting protection against glutathione depletion in fibroblast cultures obtained from patients with Friedreich ataxia and it is highly approx 350 times more efficacious than trolox, another vitamin E analog (Suárez-Rivero, 2021). Current strategies are now also aimed at developing mitochondria-directed peptides: Szeto-Schiller (SS) peptides (Szeto, 2006). Some analogs of SS peptides are discussed here in the present review. Peptide SS-31 has a significant potency which can be related well by its extensive cellular uptake and distinguished partitioning into mitochondria. Moreover, [3H] SS-31 has high intracellular concentrations, 6-fold higher than extracellular concentrations. Drastic changes in [3H] SS-31 concentration have also been noted in the isolated mitochondrion, which reveals that it was concentrated to about 5000-fold in the mitochondrial fraction. By accumulating in the inner mitochondrial membrane, SS-31 localizes at the site of ROS production thus creating hindrance for ROS mediated damage conferring protection against mito-oxidative damage and further ROS production (Misrani et al., 2021). Reports also provide support to the fact that SS-31 shields neuronal cells against tert-butyl-hydroperoxide caused mitochondrial depolarization and programmed cell death (apoptosis) by declining intracellular ROS levels and caspase activity (Zhao et al., 2005). It reduces the mitochondrial generation of ROS and inhibits PTP and depolarization in isolated mitochondria (Misrani et al., 2004). Recently, Yang et al. (2009) examined the properties of SS-31 and SS-20 to defend against MPTP-induced neurotoxicity in mice.
Emerging Perspectives in Therapeutics
Mitochondrial biogenesis
Mitochondrial diseases are characterized by defective mitochondrial network dynamics i.e., bioenergetics defects. Therefore, therapeutic approaches for improving cellular ATP production would represent an exciting strategy. Peroxisome proliferator-activate receptor (PPAR) and PGC-1α, one of its co-activators are major contributors in initiating biogenesis, demonstrating PPAR/PGCtrα as an important drug target. It has been reported that transgenic mice expressing PGC-1α, or treatment with bezafibrate (PPAR agonist) induces mitochondrial biogenesis, prevents mitochondrial myopathy in mice having deficient COX activity in skeletal muscle. However, treatment with bezafibrate failed to increase ATP production via mitochondrial biogenesis in COX efficient animals though it shows the improvement in PGCgh expression and preventing disease conditions. In a mouse model of encephalopathy, bezafibrate exhibits the neuroprotective potential in mitochondrial encephalopathy (Dumont et al., 2012; Noe et al., 2013). Lysine deacetylases (KDACs) regulate mitochondrial biogenesis through altering the activity of PGCrou gene expression implying that KDAC modulatory drugs represent a successful strategy in improving mitochondrial biogenesis (Guedesesisr and Oliveira, 2013).
Movement and mitophagy
Activation of mitochondrial movement is a promising scheme to rule over the abnormalities in mitochondrial distribution as some neurons demand extensive energy and so mitochondria must travel those distant synapses. In AD and HD brains, tubulin acetylation is significantly reduced; disturbances in microtubule-dependent mitochondrial transport are evident by their cellular models (Dan et al., 2018). Microtubule deacetylase inhibitors such as HDAC6 in conjunction with tubacin have been reported to augment motor recruitment, promoting two-directional mitochondrial transport in cellular models of HD. One more HDAC6 inhibitor tuba statin A has shown beneficial effects in mitochondrial transport in hippocampal neurons of amyloid-β induced AD rats (Dompierre et al., 2007; Govindarajan et al., 2013).
Mitophagy dysregulation is associated with neurodegeneration and can be either defective or excessive (Wanderoy et al., 2021). In the case of PD, the PINK1/parkin pathway has emerged as a major contributor to mammalian mitophagy. Disrupted mitochondria with distorted Δψm cannot import and process PINK1, causing its accumulation outside the mitochondria recruiting the ubiquitin-ligase Parkin. Ubiquitinated mitochondria are identified by ubiquitin-binding adaptors like p62 and HDAC6. This recognition process facilitates its interaction with the autophagosomal protein. Microtubule-associated protein 1A/1B-light chain 3 is a soluble protein that establishes a cascade that defines the mitochondrial digestion and functioning of autophagosome-lysosome fusion (Quinn et al., 2020). Presently, the (patho) physiological factors that determine neuronal mitophagy and the mechanisms for treating injured mitochondria in isolated synapses are poorly understood presenting a terrific challenge for the discovery and development of mitophagy-associated therapeutic strategies.
Nucleotide supplementation
Advancements have been made for treating imbalance in mitochondrial functioning starting from its dysfunction to mtDNA depletion. Deoxyribonucleotides supplements are an emerging perspective designed to improve mtDNA depletion in fibroblasts of patients having mutations in enzymes associated with the regulation and control of mitochondrial nucleotide and deoxynucleotide pools. The most common enzymes linked with this function are deoxy-guanosine kinase and thymidine phosphorylase (TYMP). Deoxyguanosine kinase DGUOK and TYMP genes encode TP. In a TP knockout mouse, mtDNA depletion has been modified by dCtd or tetrahydro uridine treatment, a nucleotide catabolism inhibitor (Camara et al., 2014). TYMP1 mutations encoding TP are related to the onset of mitochondrial neuro-gastro-intestinal encephalomyopathy (Nishino et al., 1999).
Conclusion
Oxidative stress, mitochondrial dysfunction, and mitochondrial mutations have interrelated to the pathogenesis of neurological disorders. Increased oxidative load augments ROS production leading to dysfunction and mutations in mitochondria. Mitochondria are the power house of the cell that regulates important aspects of cellular functions in a wide variety of cell organelles such as the nucleus and endoplasmic reticulum mutations. Mutations in mtDNA genome may hamper the proper functioning of mitochondria and cellular functions causing perturbations in mitochondrial stability eliciting a series of changes in nuclear gene expression. Therefore, it is of significant interest to explore and examine the mitochondrial mutations involved in the progression, development, and worsening of neurological disorders. Further, identification of these mutations and associated changes may also open new horizons for the development of therapeutic strategies aimed at improving these conditions. Nevertheless, the precise mechanisms underlying these abnormalities and mutations in mitochondria need to be explored. Some of the achievements done so far concerning identification of treatment strategies include molecular approaches such as (i) targeted re-expression of the mutated gene (ii) manipulating mtDNA heteroplasmy (iii) stabilizing mutant mt-tRNA (iv) bypassing the block of the respiratory chain are in pipeline for establishing their role in improving these abnormalities. Identification of mutations in mitochondria could be an attractive approach for the development of novel drugs aimed to combat the abnormalities in mitochondria and associated alterations which might have resulted due to these mutations.
Acknowledgments:
I express my sincere gratitude towards my mentors Dr. Atish Prakash (Scientist, Eurofins PSS Insourcing solutions, Malvern, PA, USA), Dr. Puneet Kumar Bansal (Associate Professor of Pharmacology & Head, Department of Pharmacology, Central University of Punjab, Bathinda, Punjab, India), and Dr. Avril V. Somlyo (Professor, Department of Molecular Physiology and Biological Physics, School of Medicine, University of Virginia, Charlottesville, VA, USA) for their guidance and valuable inputs. I am thankful to Mr. Praveen Garg, Chairman, ISF College of Pharmacy, Moga, Punjab, India for his continuous support and encouragement.
Footnotes
Conflicts of interest: The author declares no conflicts of interest.
P-Reviewer: Naef V; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Armstrong JS. Mitochondrial medicine:pharmacological targeting of mitochondria in disease. Br J Pharmacol. 2007;151:1154–1165. doi: 10.1038/sj.bjp.0707288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloyannis SJ. Dendritic pathology in Alzheimer's disease. J Neurol Sci. 2009;283:153–157. doi: 10.1016/j.jns.2009.02.370. [DOI] [PubMed] [Google Scholar]
- 3.Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders-A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian M, Liu J, Hong X, Yu M, Huang Y, Sheng Z, Fei J, Huang F. Overexpression of parkin ameliorates dopaminergic neurodegeneration induced by 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. PLoS One. 2012;7:e39953. doi: 10.1371/journal.pone.0039953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 6.Bulst S, Holinski-Feder E, Payne B, Abicht A, Krause S, Lochmüller H, Chinnery PF, Walter MC, Horvath R. In vitro supplementation with deoxynucleoside monophosphates rescues mitochondrial DNA depletion. Mol Genet Metab. 2012;107:95–103. doi: 10.1016/j.ymgme.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cámara Y, González-Vioque E, Scarpelli M, Torres-Torronteras J, Caballero A, Hirano M, Martí R. Administration of deoxyribonucleosides or inhibition of their catabolism as a pharmacological approach for mitochondrial DNA depletion syndrome. Hum Mol Genet. 2014;23:2459–2467. doi: 10.1093/hmg/ddt641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DC. Mitochondrial dynamics and its involvement in disease. Ann Rev Pathol. 2020;15:235–259. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. 2017;58:235–263. doi: 10.1002/em.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chlystun M, Campanella M, Law AL, Duchen MR, Fatimathas L, Levine TP, Gerke V, Moss SE. Regulation of mitochondrial morphogenesis by annexin A6. PLoS One. 2013;8:e53774. doi: 10.1371/journal.pone.0053774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong MS, Goh LK, Lim WS, Chan M, Tay L, Chen G, Feng L, Ng TP, Tan CH, Lee TS. Gene expression profiling of peripheral blood leukocytes shows consistent longitudinal downregulation of TOMM40 and upregulation of KIR2DL5A, PLOD1, and SLC2A8 among fast progressors in early Alzheimer's disease. J Alzheimers Dis. 2013;34:399–405. doi: 10.3233/JAD-121621. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury AR, Zielonka J, Kalyanaraman B, Hartley RC, Murphy MP, Avadhani NG. Mitochondria-targeted paraquat and metformin mediate ROS production to induce multiple pathways of retrograde signaling:A dose-dependent phenomenon. Redox Biol. 2020;36:101606. doi: 10.1016/j.redox.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comte C, Tonin Y, Heckel-Mager AM, Boucheham A, Smirnov A, Auré K, Lombès A, Martin RP, Entelis N, Tarassov I. Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with Kearns Sayre Syndrome. Nucleic Acids Res. 2013;41:418–433. doi: 10.1093/nar/gks965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csire G, Canabady-Rochelle L, Averlant-Petit MC, Selmeczi K, Stefan L. Both metal-chelating and free radical-scavenging synthetic pentapeptides as efficient inhibitors of reactive oxygen species generation. Metallomics. 2020;12:1220–1229. doi: 10.1039/d0mt00103a. [DOI] [PubMed] [Google Scholar]
- 16.Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Dan Wei, Gao N, Li L, Zhu JX, Diao L, Huang J, Han QJ, Wang S, Xue H, Wang Q, Wu QF, Zhang X, Bao L. α-Tubulin acetylation restricts axon over branching by dampening microtubule plus-end dynamics in neurons. Cereb Cortex. 2018;28:3332–3346. doi: 10.1093/cercor/bhx225. [DOI] [PubMed] [Google Scholar]
- 18.Davidzon G, Greene P, Mancuso M, Klos KJ, Ahlskog JE, Hirano M, DiMauro S. Early-onset familial parkinsonism due to POLG mutations. Ann Neurol. 2006;59:859–862. doi: 10.1002/ana.20831. [DOI] [PubMed] [Google Scholar]
- 19.Dompierre JP, Godin JD, Charrin BC, Cordelières FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumont M, Stack C, Elipenahli C, Jainuddin S, Gerges M, Starkova N, Calingasan NY, Yang L, Tampellini D, Starkov AA, Chan RB, Di Paolo G, Pujol A, Beal MF. Bezafibrate administration improves behavioral deficits and tau pathology in P301S mice. Hum Mol Genetics. 2012;21:5091–5105. doi: 10.1093/hmg/dds355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eerola J, Luoma PT, Peuralinna T, Scholz S, Paisan-Ruiz C, Suomalainen A, Singleton AB, Tienari PJ. POLG1 polyglutamine tract variants associated with Parkinson's disease. Neurosci Lett. 2010;477:1–5. doi: 10.1016/j.neulet.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Idrissi A, Trenkner E. Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem Res. 2004;29:189–197. doi: 10.1023/b:nere.0000010448.17740.6e. [DOI] [PubMed] [Google Scholar]
- 23.El Idrissi A. Taurine increases mitochondrial buffering of calcium:role in neuroprotection. Amino Acids. 2008;34:321–328. doi: 10.1007/s00726-006-0396-9. [DOI] [PubMed] [Google Scholar]
- 24.Fang YQ, Mao F, Zhu MJ, Li XH. Compound heterozygous mutations in PARK2 causing early-onset Parkinson disease:a case report. Medicine. 2019;98:e14228. doi: 10.1097/MD.0000000000014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferencz B, Karlsson S, Kalpouzos G. Promising genetic biomarkers of preclinical Alzheimer's disease:the influence of APOE and TOMM40 on brain integrity. Int J Alzheimers Dis. 2012;2012:421–452. doi: 10.1155/2012/421452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fichi G, Naef V, Barca A, Longo G, Fronte B, Verri T, Santorelli FM, Marchese M, Petruzzella V. Fishing in the cell powerhouse:zebrafish as a tool for exploration of mitochondrial defects affecting the nervous system. Int J Mol Sci. 2019;20:2409. doi: 10.3390/ijms20102409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, Meitinger T, Kolch W, Prokisch H, Ueffing M. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 28.Goedert M, Jakes R, Spillantini MG. The synucleinopathies:twenty years on. J Parkinsons Dis. 2017;7(s1):51–69. doi: 10.3233/JPD-179005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonçalves FB, Morais VA. PINK1:a bridge between mitochondria and Parkinson's disease. Life. 2021:11–371. doi: 10.3390/life11050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto Y, Nonaka I, Horai S. A mutation in the tRNA (Leu) (UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 31.Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schlüter OM, Bradke F, Lu J, Fischer A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer's disease. EMBO Mol Med. 2013;5:52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guedes-Dias P, Oliveira JM. Lysine deacetylases and mitochondrial dynamics in neurodegeneration. Biochim Biophys Acta. 2013;832:1345–1359. doi: 10.1016/j.bbadis.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Guo X, Sun X, Hu D, Wang YJ, Fujioka H, Vyas R, Chakrapani S, Joshi AU, Luo Y, Mochly-Rosen D, Qi X. VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington's disease. Nat Commun. 2016;7:12646. doi: 10.1038/ncomms12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. J Biol Chem. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- 35.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homma K, Toda E, Osada H, Nagai N, Era T, Tsubota K, Okano H, Ozawa Y. Taurine rescues mitochondria-related metabolic impairments in the patient-derived induced pluripotent stem cells and epithelial-mesenchymal transition in the retinal pigment epithelium. Redox Biol. 2021;41:101921. doi: 10.1016/j.redox.2021.101921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou Y, Zhao XT, Xie ZY, Yuan Y, Wang ZX. J Peking Univ Health Sci. 2020;52:851–855. doi: 10.19723/j.issn.1671-167X.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, Venables G, Griffiths P, Burn DJ, Turnbull DM, Chinnery PF. Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol. 2007;64:553–557. doi: 10.1001/archneur.64.4.553. [DOI] [PubMed] [Google Scholar]
- 39.Inagaki R, Tagawa K, Qi ML, Enokido Y, Ito H, Tamura T, Shimizu S, Oyanagi K, Arai N, Kanazawa I, Wanker EE, Okazawa H. Omi/HtrA2 is relevant to the selective vulnerability of striatal neurons in Huntington's disease. Eur J Neurosci. 2008;28:30–40. doi: 10.1111/j.1460-9568.2008.06323.x. [DOI] [PubMed] [Google Scholar]
- 40.Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine:Prevention of mitochondrial oxidant production. Amino Acids. 2012;42:2223–2232. doi: 10.1007/s00726-011-0962-7. [DOI] [PubMed] [Google Scholar]
- 41.Kaarniranta K, Pawlowska E, Szczepanska J, Jablkowska A, Blasiak J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD) Int J Mol Sci. 2019;20:2374. doi: 10.3390/ijms20102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kausar S, Wang F, Cui H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells. 2018;7:274. doi: 10.3390/cells7120274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kazak L, Reyes A, Holt IJ. Minimizing the damage:repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012;13:659–671. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- 44.Kessler C, Atasu B, Hanagasi H, Simón-Sánchez J, Hauser A, K Pak, M Bilgic, B Erginel-Unaltuna N, Gurvit H, Gasser T, Lohmann E. Role of LRRK2 and SNCA in autosomal dominant Parkinson's disease in Turkey. Parkinsonism Relat Disord. 2018;48:34–39. doi: 10.1016/j.parkreldis.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Lee JY, Park K J, Kim WH, Roh GS. A mitochondrial division inhibitor, Mdivi-1, inhibits mitochondrial fragmentation and attenuates kainic acid-induced hippocampal cell death. BMC Neurosci. 2016:17–33. doi: 10.1186/s12868-016-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotrys AV, Szczesny RJ. Mitochondrial gene expression and beyond-novel aspects of cellular physiology. Cells. 2019:9–17. doi: 10.3390/cells9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kouli A, Torsney KM, Kuan WL. Parkinson's disease:etiology, neuropathology, and pathogenesis In:Parkinson's disease:pathogenesis and clinical aspects. In: Stoker TB, Greenland JC, editors. [Internet] Brisbane: Codon Publications; 2018. [PubMed] [Google Scholar]
- 48.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 49.Lee TS, Goh L, Chong MS, Chua SM, Chen GB, Feng L, Lim WS, Chan M, Ng TP, Krishnan KR. Downregulation of TOMM40 expression in the blood of Alzheimer disease subjects compared with matched controls. J Psychiatr Res. 2012;46:828–830. doi: 10.1016/j.jpsychires.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Leitão-Rocha A, Guedes-Dias P, Pinho BR, Oliveira JM. Trends in mitochondrial therapeutics for neurological disease. Cur Med Chemistry. 2015;22:2458–2467. doi: 10.2174/0929867322666150209160317. [DOI] [PubMed] [Google Scholar]
- 51.Levy SL, Burnham WM, Bishai A, Hwang PA. The anticonvulsant effects of vitamin E:a further evaluation. Can J Neurol Sci. 1992;19:201–203. [PubMed] [Google Scholar]
- 52.Li JX, Pang YZ, Tang CS, Li ZQ. Protective effect of taurine on hypochlorous acid toxicity to nuclear nucleoside triphosphatase in isolated nuclei from rat liver. World J Gastroenterol. 2004;10:694–698. doi: 10.3748/wjg.v10.i5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 2005. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 55.Linnertz C, Saunders AM, Lutz MW, Crenshaw DM, Grossman I, Burns DK, Whitfield KE, Hauser MA, McCarthy JJ, Ulmer M, Allingham R, Welsh-Bohmer KA, Roses AD, Chiba-Falek O. Characterization of the poly-T variant in the TOMM40 gene in diverse populations. PLoS One. 2012;7:e30994. doi: 10.1371/journal.pone.0030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luoma PT, Eerola, J, Ahola S, Hakonen AH, Hellström O, Kivistö KT, Tienari PJ, Suomalainen A. Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology. 2007;69:1152–1159. doi: 10.1212/01.wnl.0000276955.23735.eb. [DOI] [PubMed] [Google Scholar]
- 57.Lutz MW, Crenshaw DG, Saunders AM, Roses AD. Genetic variation at a single locus and age of onset for Alzheimer's disease. Alzheimers Dement. 2010;6:125–131. doi: 10.1016/j.jalz.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehvari J, Motlagh FG, Najafi M, Ghazvini MR, Naeini AA, Zare M. Effects of vitamin E on seizure frequency, electroencephalogram findings, and oxidative stress status of refractory epileptic patients. Adv Biomed Res. 2016:5–36. doi: 10.4103/2277-9175.178780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mihoub M, Abdallah J, Richarme G. Protein repair from glycation by glyoxals by the DJ-1 family maillard deglycases. Adv Exp Med Biol. 2017;1037:133–147. doi: 10.1007/978-981-10-6583-5_9. [DOI] [PubMed] [Google Scholar]
- 62.Misrani A, Tabassum S, Yang L. Mitochondrial dysfunction and oxidative stress in Alzheimer's disease. Front Aging Neurosci. 2021;13:57. doi: 10.3389/fnagi.2021.617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montagna P, Gallassi R, Medori R, Govoni E, Zeviani M, Di Mauro S, Lugaresi E, Andermann F. MELAS syndrome:characteristic migrainous and epileptic features and maternal transmission. Neurology. 1988;38:751–754. doi: 10.1212/wnl.38.5.751. [DOI] [PubMed] [Google Scholar]
- 64.Murphy MP, Smith RA. Drug delivery to mitochondria:the key to mitochondrial medicine. Adv Drug Deliv Rev. 2000;41:235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 65.Neueder A, Landles C, Ghosh R, Howland D, Myers RH, Faull R, Tabrizi SJ, Bates GP. The pathogenic exon 1 HTT protein is produced by incomplete splicing in Huntington's disease patients. Sci Rep. 2017;7:1307. doi: 10.1038/s41598-017-01510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–692. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- 67.Noe N, Dillon L, Lellek V, Diaz F, Hida A, Moraes CT, Wenz T. Bezafibrate improves mitochondrial function in the CNS of a mouse model of mitochondrial encephalopathy. Mitochondrion. 2013;13:417–426. doi: 10.1016/j.mito.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norat P, Soldozy S, Sokolowski JD, Gorick CM, Kumar JS, Chae Y, Yağmurlu K, Prada F, Walker M, Levitt MR, Price RJ, Tvrdik P, Kalani M. Mitochondrial dysfunction in neurological disorders:Exploring mitochondrial transplantation. NPJ Regen Med. 2020:5–22. doi: 10.1038/s41536-020-00107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nunnari J, Suomalainen A. Mitochondria:in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohsawa Y, Hagiwara H, Nishimatsu S I, Hirakawa A, Kamimura N, Ohtsubo H, Fukai Y, Murakami T, Koga Y, Goto YI, Ohta S, Sunada Y, KN01 Study Group. Taurine supplementation for prevention of stroke-like episodes in MELAS:a multicentre, open-label, 52-week phase III trial. J Neurol Neurosurg Psychiatry. 2019;90:529–536. doi: 10.1136/jnnp-2018-317964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orsucci D, Caldarazzo Ienco E, Mancuso M, Siciliano G. POLG1-related and other “mitochondrial Parkinsonisms”:an overview. J Mol Neurosci. 2011;44:17–24. doi: 10.1007/s12031-010-9488-9. [DOI] [PubMed] [Google Scholar]
- 72.Pintoa M, Moraesa CT. Mitochondrial genome changes and neurodegenerative diseases. Biochim Biophys Acta. 2014;1842:1198–1207. doi: 10.1016/j.bbadis.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 74.Poon HF, Frasier M, Shreve N, Calabrese V, Wolozin B, Butterfield DA. Mitochondrial associated metabolic proteins are selectively oxidized in A30P alpha-synuclein transgenic mice--a model of familial Parkinson's disease. Neurobiol Dis. 2005;18:492–498. doi: 10.1016/j.nbd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 2005;29:S5–9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- 76.Puspita L, Chung SY, Shim JW. Oxidative stress and cellular pathologies in Parkinson's disease. Mol Brain. 2017:10–53. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quinn P, Moreira PI, Ambrósio AF, Alves CH. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol Commun. 2020:8–189. doi: 10.1186/s40478-020-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raninga PV, Di Trapani G, Tonissen KF. The multifaceted roles of DJ-1 as an antioxidant. Adv Exp Med Biol. 2017;1037:67–87. doi: 10.1007/978-981-10-6583-5_6. [DOI] [PubMed] [Google Scholar]
- 80.Repici M, Giorgini F. DJ-1 in Parkinson's disease:clinical insights and therapeutic perspectives. J Clin Med. 2019;8:1377. doi: 10.3390/jcm8091377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rose J, Brian C, Woods J, Pappa A, Panayiotidis MI, Powers R, Franco R. Mitochondrial dysfunction in glial cells:implications for neuronal homeostasis and survival. Toxicology. 2017;391:109–115. doi: 10.1016/j.tox.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, Huentelman MJ, Welsh-Bohmer KA, Reiman EM. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer's disease. Pharmacogenomics J. 2010;10:375–384. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roses AD, Lutz MW, Crenshaw DG, Grossman I, Saunders AM, Gottschalk WK. TOMM40 and APOE:requirements for replication studies of association with age of disease onset and enrichment of a clinical trial. Alzheimers Dement. 2013;9:132–136. doi: 10.1016/j.jalz.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 84.Schapira AH. Mitochondrial pathology in Parkinson's disease. Mt Sinai J Med New York. 2011;78:872–881. doi: 10.1002/msj.20303. [DOI] [PubMed] [Google Scholar]
- 85.Schulz KL, Eckert A, Rhein V, Mai S, Haase W, Reichert AS, Jendrach M, Müller WE, Leuner K. A new link to mitochondrial impairment in tauopathies. Mol Neurobiol. 2012;46:205–216. doi: 10.1007/s12035-012-8308-3. [DOI] [PubMed] [Google Scholar]
- 86.Shetewy A, Shimada-Takaura K, Warner D, Jong CJ, Mehdi AB, Alexeyev M, Takahashi K, Schaffer SW. Mitochondrial defects associated with beta-alanine toxicity:relevance to hyper-beta-alaninemia. Mol Cell Biochem. 2016;416:11–22. doi: 10.1007/s11010-016-2688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shim JH, Yoon SH, Kim KH, Han JY, Ha JY, Hyun DH, Paek SH, Kang UJ, Zhuang X, Son JH. The antioxidant Trolox helps recovery from the familial Parkinson's disease-specific mitochondrial deficits caused by PINK1- and DJ-1-deficiency in dopaminergic neuronal cells. Mitochondrion. 2011;11:707–715. doi: 10.1016/j.mito.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 88.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 89.Singh S, Singh TG, Rehni AK. An insight into molecular mechanisms and novel therapeutic approaches in epileptogenesis. CNS Neurol Disord Drug Targets. 2020;19:750–779. doi: 10.2174/1871527319666200910153827. [DOI] [PubMed] [Google Scholar]
- 90.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 91.Smith PM, Lightowlers RN. Altering the balance between healthy and mutated mitochondrial DNA. J Inherit Metab Dis. 2011;34:309–313. doi: 10.1007/s10545-010-9122-6. [DOI] [PubMed] [Google Scholar]
- 92.Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, Masliah E, Ellisman, Rouiller I, Schwarzenbacher R, Bossy B, Perkins G., Bossy-Wetzel E. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steele SL, Prykhozhij SV, Berman JN. Zebrafish as a model system for mitochondrial biology and diseases. Transl Res. 2014;163:79–98. doi: 10.1016/j.trsl.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 94.Stiles AR, Simon MT, Stover A, Eftekharian S, Khanlou N, Wang HL, Magaki S, Lee H, Partynski K, Dorrani N, Chang R, Martinez-Agosto JA, Abdenur JE. Mutations in TFAM, encoding mitochondrial transcription factor A, cause neonatal liver failure associated with mtDNA depletion. Mol Genet Metab. 2016;119:91–99. doi: 10.1016/j.ymgme.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Strang KH, Golde TE, Giasson BI. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab Invest. 2019;99:912–928. doi: 10.1038/s41374-019-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suárez-Rivero JM, Pastor-Maldonado CJ, Povea-Cabello S, Álvarez-Córdoba M, Villalón-García I, Munuera-Cabeza M, Suárez-Carrillo A, Talaverón-Rey M, Sánchez-Alcázar JA. Coenzyme Q10Analogues:benefits and challenges for therapeutics. Antioxidants. 2021:10–236. doi: 10.3390/antiox10020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun Y, Vashisht AA, Tchieu J, Wohlschlegel JA, Dreier L. Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy. J Biol Chem. 2012;287:40652–40660. doi: 10.1074/jbc.M112.419721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suomalainen A, Battersby BJ. Mitochondrial diseases:the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol. 2018;19:77–92. doi: 10.1038/nrm.2017.66. [DOI] [PubMed] [Google Scholar]
- 99.Szeto HH. Mitochondria-targeted peptide antioxidants:novel neuroprotective agents. AAPS J. 2006;8:E521–531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahashi-Niki K, Niki T, Iguchi-Ariga S, Ariga H. Transcriptional regulation of DJ-1. Adv Exp Med Biol. 2017;1037:89–95. doi: 10.1007/978-981-10-6583-5_7. [DOI] [PubMed] [Google Scholar]
- 101.Tang WX, Wu WH, Qiu HY, Bo H, Huang SM. Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. J Nephrol. 2013;26:1073–1082. doi: 10.5301/jn.5000268. [DOI] [PubMed] [Google Scholar]
- 102.Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459–472. doi: 10.1038/s41582-019-0217-x. [DOI] [PubMed] [Google Scholar]
- 103.Wanderoy S, Hees JT, Klesse R, Edlich F, Harbauer AB. Kill one or kill the many:interplay between mitophagy and apoptosis. Biol Chem. 2020;402:73–88. doi: 10.1515/hsz-2020-0231. [DOI] [PubMed] [Google Scholar]
- 104.Wang JQ, Chen Q, Wang X, Wang QC, Wang Y, Cheng HP, Guo C, Sun Q, Chen Q, Tang TS. Dysregulation of mitochondrial calcium signaling and superoxide flashes cause mitochondrial genomic DNA damage in Huntington disease. J Biol Chem. 2013;288:3070–3084. doi: 10.1074/jbc.M112.407726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang W, Zhao F, Ma X, Perry G, Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer's disease:recent advances. Mol Neurodegener. 2020:15–30. doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta. 2012;1817:1833–1838. doi: 10.1016/j.bbabio.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 108.Wilkins HM, Swerdlow RH. Amyloid precursor protein processing and bioenergetics. Brain Res Bull. 2017;133:71–79. doi: 10.1016/j.brainresbull.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu K, Stringer JL. Antioxidants and free radical scavengers do not consistently delay seizure onset in animal models of acute seizures. Epilepsy Behav. 2008;13:77–82. doi: 10.1016/j.yebeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Rad Biol Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. 2009;11:2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Young MJ, Copeland WC. Human mitochondrial DNA replication machinery and disease. Curr Opin Genet Dev. 2016;38:52–62. doi: 10.1016/j.gde.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang HT, Mi L, Wang T, Yuan L, Li XH, Dong LS, Zhao P, Fu JL, Yao BY, Zhou ZC. PINK1/Parkin-mediated mitophagy play a protective role in manganese induced apoptosis in SH-SY5Y cells. Toxicol In Vitro. 2016;34:212–219. doi: 10.1016/j.tiv.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 114.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochemical Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 115.Zhao Z, Fu A. Mitochondrial therapy:a new strategy for treating mitochondrion-associated diseases. Chin J Biotechnol. 2021;37:1168–1177. doi: 10.13345/j.cjb.200383. [DOI] [PubMed] [Google Scholar]
- 116.Zhu XH, Lu M, Chen W. Quantitative imaging of brain energy metabolisms and neuroenergetics using in vivo X-nuclear 2H, 17O and 31P MRS at ultra-high field. J Magn Reson. 2018;292:155–170. doi: 10.1016/j.jmr.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


