ABSTRACT
Tetracycline-based combinations are increasingly used for serious carbapenem-nonsusceptible Acinetobacter baumannii (CNSAb) infections given their potent in vitro activity, synergism with other agents, and acceptable toxicity profile. Omadacycline is a novel aminomethylcycline with activity against minocycline-resistant pathogens, once daily oral dosing, and favorable pharmacokinetic properties. Given these potential advantages, the in vitro potency and antibacterial activity of omadacycline were evaluated alone and in combination against CNSAb with varying minocycline susceptibility. Broth microdilution testing of 41 CNSAb revealed that omadacycline (MIC50/90: 4/8 mg/L) inhibited 68.3% (28/41) of isolates at ≤4 mg/L and its activity was unaffected by minocycline nonsusceptibility (MIC50/90: 4/8 mg/L; 74.2% [23/31] inhibited at ≤4 mg/L). Ten (5 minocycline susceptible and 5 nonsusceptible) of the 41 CNSAb isolates were then evaluated in time-kill analyses against omadacycline and comparator agents alone and in dual- and triple-drug combinations at the free maximum concentration of drug in serum (fCmax). Amikacin, meropenem, and polymyxin B alone were each bactericidal against 4 of 10 (40%) isolates while omadacycline and sulbactam were bactericidal against 0 (0%) and 1 (10%), respectively. In dual-drug combinations with omadacycline, synergy was observed against 80% of isolates with sulbactam followed by 30% with amikacin or polymyxin B and 0% with meropenem or rifampin. The triple-drug combination of omadacycline, sulbactam, and polymyxin B achieved synergy against just one additional strain over the omadacycline-sulbactam dual combination but significantly reduced the time to 99.9% kill by more than 6 h (4.6 ± 2.8 h vs. 11.3 ± 5.9 h, P < 0.01). These results support the continued investigation into tetracycline-based combinations against CNSAb, particularly those including sulbactam, and suggest that omadacycline may have in vitro advantages over existing tetracycline-derivatives.
IMPORTANCE Treatment of infections due to Acinetobacter baumannii often involves the use of multiple antibiotics simultaneously as combination therapy, but it is unknown which antibiotics are best used together. Tetracycline agents such as minocycline and tigecycline maintain good activity against A. baumannii and are often used with one or more other agents to achieve better killing of the bacteria. Omadacycline is a new tetracycline that may have a role in the treatment of A. baumannii, but no data are available evaluating its interaction with other commonly used drugs such as polymyxin B and sulbactam. Therefore, the purpose of this study was to investigate the antibacterial activity of omadacycline when combined with one or more other agents against carbapenem-resistant strains of A. baumannii. These findings may then be used to design confirmatory studies that could help decide what drugs work best together and what combination of agents should be used for patients.
KEYWORDS: susceptibility, time-kill assay, tetracycline, minocycline, omadacycline, synergy, combination therapy, pharmacodynamics, Acinetobacter baumannii, CRAB
INTRODUCTION
Carbapenem-nonsusceptible Acinetobacter baumannii (CNSAb) remains one of only two Gram-negative pathogens considered both an urgent threat nationally by the Centers for Disease Control and Prevention (CDC) and a critical priority internationally by the World Health Organization (1, 2). The exorbitant morbidity, mortality, and health care costs associated with CNSAb infections are due in large part to the insufficient number of available treatment options with adequate in vitro activity and appreciable clinical efficacy (3). In the United States, 18% of A. baumannii express the difficult-to-treat resistance phenotype and ≥40% are carbapenem nonsusceptible; only cefiderocol, the polymyxins, and the tetracycline-derivatives maintain reliable in vitro potency against this phenotype (4–7). Given the high rates of resistance, importance of time to effective therapy, and the lack of an established standard of care treatment regimen, combination therapy is routinely employed against A. baumannii and is supported by recommendations from the Infectious Diseases Society of America (8). Although clinical studies evaluating combination therapy are conflicting, preclinical data support combinations including a polymyxin with sulbactam, meropenem, rifampin, and/or a tetracycline derivative (9). Despite these data, the optimal combination of agents and dosing regimens to maximize efficacy and minimize toxicity have not been established. As attributable mortality rates for serious CNSAb infections are as high as 70% with current treatment and the prevalence and resistance continue to increase, it is crucial to continue to explore novel potential treatment regimens for this challenging pathogen (10–12).
Omadacycline is a novel aminomethylcycline with structural modifications at the C7 and C9 positions allowing it to circumvent the efflux pumps TetK and TetB and ribosomal protection protein mechanisms TetM and TetO that confer resistance to traditional tetracyclines including minocycline (13). These structural alterations also allow for once daily oral maintenance dosing making it only the second tetracycline-derivative after minocycline with activity against CNSAb available in oral formulation. Additional advantageous pharmacokinetic (PK) properties include significantly lower, concentration-independent protein binding and enhanced epithelial lining fluid penetration (14). Together these factors may make omadacycline a promising alternative to existing tetracycline derivatives for the treatment of A. baumannii, although supporting data are lacking. As such, the objective of this study was to evaluate the in vitro potency of omadacycline and comparator agents against A. baumannii via broth microdilution (BMD) testing and assess the antibacterial activity of each agent alone and in two- and three-drug combinations in time-kill analyses.
RESULTS
Susceptibility testing.
Genotypically, 97.6% (40/41) of CNSAb isolates tested harbored at least 1 aminoglycoside-modifying enzyme and at least 1 Ambler class D blaOXA gene (most commonly blaOXA-23 at 48.8%), while all 41 (100%) carried the class C gene blaADC-25. Nineteen of 41 (46%) also coharbored a class A blaTEM-1B or -1D gene while 32/41 (78%) and 1/41 (2.4%) carried the tet(B) and tet(A) efflux genes, respectively. The phenotypic MIC50, MIC90, MIC range, and percent susceptible for each agent, as applicable, against all 41 CNSAb isolates are summarized in Table 1. Only 12.2% (5/41) of isolates were susceptible to amikacin and none (0%) were susceptible to meropenem (1 intermediate [2.4%] and 40 [97.6%] resistant). Similarly, activity of sulbactam was poor with MIC50/90 values of 32/128 mg/L and just 1 isolate testing susceptible. Polymyxin B displayed the lowest MIC50/90 values of any agent at 0.5/0.5 mg/L although no isolates were considered susceptible as the revised CLSI interpretive criteria only include intermediate and resistant breakpoints for the polymyxins. Minocycline displayed the highest rate of susceptibility overall at 29.3% (12/41) although MIC50/90 values were 16/16 mg/L compared to omadacycline and tigecycline each at 4/8 mg/L. Against the 31 minocycline nonsusceptible isolates, omadacycline and tigecycline MIC50/90 were unchanged at 4/8 mg/L each and 74.2% and 90.3% were inhibited at ≤4 mg/L of omadacycline and tigecycline, respectively. The MIC distributions for each tetracycline derivative against all 41 CNSAb isolates are overlaid in Fig. 1. Overall, isolate AB4 was the most resistant and displayed the highest MICs across all eight agents tested followed by AB6. Isolate AB7 and AB8 were the least resistant of the 10 tested although each were susceptible only to minocycline.
TABLE 1.
Activity of omadacycline and comparator agents against clinical carbapenem-nonsusceptible Acinetobacter baumannii isolates (n = 41)a
| MIC (mg/L) |
Susceptibility (%) |
|||||
|---|---|---|---|---|---|---|
| Agent | 50% | 90% | Range | S | I | R |
| Amikacin | 128 | >256 | 2 to >256 | 12.2 | 24.4 | 63.4 |
| Meropenem | ≥256 | ≥256 | 4 to ≥256 | 0 | 2.4 | 97.6 |
| Minocycline | 16 | 16 | 0.25 to 32 | 29.3 | 19.5 | 51.2 |
| Omadacycline | 4 | 8 | 1 to 16 | NC | NC | NC |
| Polymyxin B | 0.5 | 0.5 | 0.125 to ≥128 | NC | 95.1 | 4.9 |
| Rifampin | 16 | 64 | 4 to >256 | NC | NC | NC |
| Sulbactam | 32 | 128 | 2 to 128 | 2.4 | 0 | 97.6 |
| Tigecycline | 4 | 8 | 1 to 16 | NC | NC | NC |
S, susceptible; I, intermediate; R, resistant; NC, no applicable interpretive criteria available.
FIG 1.
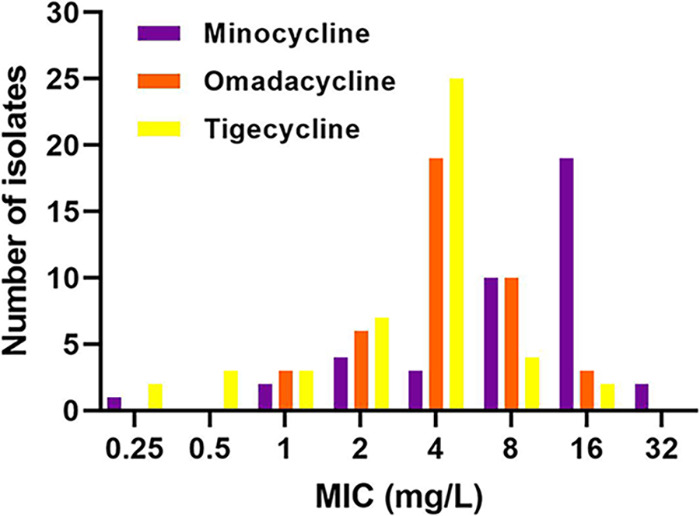
MIC distributions of minocycline, omadacycline, and tigecycline against 41 clinical carbapenem-nonsusceptible Acinetobacter baumannii.
Time-kill experiments.
Table 2 displays the MIC values for each agent against the 10 CNSAb selected for time-kill experiments. Comparing the MIC values in Table 2 to the simulated free maximum concentrations of drug in serum (fCmax) as shown in Table 3 reveals that, although only 3/10 (30%) isolates were considered susceptible to amikacin, 7/10 (70%) had an MIC at or below the fCmax of 55.5 mg/L. All 10 (100%) isolates were considered nonsusceptible to meropenem although 3/10 (30%) had MICs less than or equal to fCmax (40 mg/L) compared to sulbactam for which all (100%) were considered resistant while 7/10 (70%) had an MIC less than or equal to fCmax (60.8 mg/L). The vast majority (80%) of polymyxin MICs were 5 to 10-fold below the fCmax (2.61 mg/L) though none were considered susceptible. Finally, just 1/10 (10%) minocycline isolates had an MIC approximately equal to its fCmax (0.24 mg/L) versus 2/10 (20%) for omadacycline (1.21 mg/L) and 0/10 (0%) for tigecycline (0.08 mg/L).
TABLE 2.
MICs and susceptibility interpretation of omadacycline and comparator agents against 10 carbapenem-nonsusceptible clinical A. baumannii isolates included in time-kill experimentsa
| Isolate | Amikacin | Meropenem | Minocycline | Omadacycline | Polymyxin B | Rifampin | Sulbactam | Tigecycline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AB1 | 32 | I | 16 | R | 0.25 | S | 4 | NC | 0.5 | I | 4 | NC | 128 | R | 8 | NC |
| AB2 | 32 | I | ≥256 | R | 2 | S | 16 | NC | 0.25 | I | 16 | NC | 32 | R | 8 | NC |
| AB3 | 8 | S | 4 | I | 16 | R | 8 | NC | 0.5 | I | 16 | NC | 32 | R | 4 | NC |
| AB4 | >256 | R | 128 | R | 16 | R | 16 | NC | >64 | R | >256 | NC | 16 | R | 16 | NC |
| AB5 | 32 | I | ≥256 | R | 16 | R | 1 | NC | 0.25 | I | 16 | NC | 64 | R | 2 | NC |
| AB6 | >256 | R | ≥256 | R | 8 | I | 4 | NC | >64 | R | 16 | NC | 16 | R | 4 | NC |
| AB7 | 32 | I | ≥256 | R | 1 | S | 2 | NC | 0.25 | I | 8 | NC | 16 | R | 2 | NC |
| AB8 | >256 | R | ≥256 | R | 4 | S | 4 | NC | 0.25 | I | 4 | NC | 32 | R | 2 | NC |
| AB9 | 2 | S | 16 | R | 8 | I | 1 | NC | 0.25 | I | 8 | NC | 16 | R | 1 | NC |
| AB10 | 4 | S | 64 | R | 2 | S | 8 | NC | 0.5 | I | 8 | NC | 64 | R | 4 | NC |
Gray shaded cells represents isolates for which the respective drug’s free maximum concentration of drug in serum (fCmax) was ≥MIC. NC, no applicable interpretive criteria.
TABLE 3.
Representative doses and fCmax values simulated for each agent in time-kill experiments
| Agent | Dosea | Cmax (mg/L) | Protein binding (%) | fCmax (mg/L) | Reference |
|---|---|---|---|---|---|
| Amikacin | 15 mg/kg i.v. over 1 h | 60 | 7.5 | 55.5 | (54) |
| Meropenem | 2 g i.v. over 3 h | 40.9 | 2 | 40 | (55) |
| Minocycline | 100 mg i.v. over 1 h | 0.99 | 76 | 0.24 | (56, 57) |
| Omadacycline | 100 mg i.v. over 30 min | 1.51 | 21 | 1.21 | (14) |
| Polymyxin B | 1.5 mg/kg i.v. over 1 h | 6.21 | 58 | 2.61 | (58) |
| Rifampin | 300 mg i.v. over 30 min | 8.90 | 80 | 1.78 | (59) |
| Sulbactam | 1 g i.v. over 30 min | 98 | 38 | 60.8 | (60) |
| Tigecycline | 50 mg i.v. over 30 min | 0.38 | 80 | 0.08 | (61) |
Single doses.
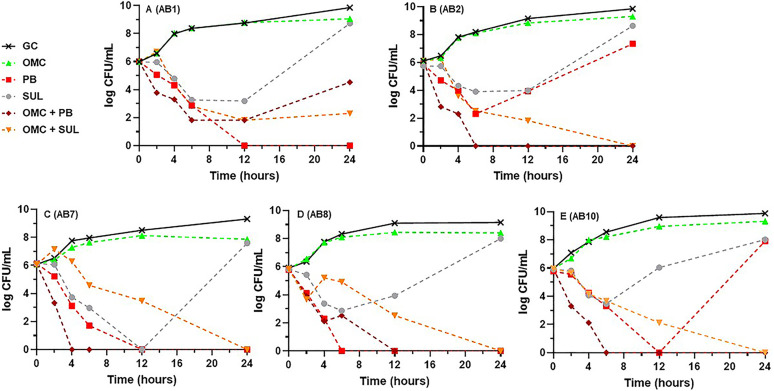
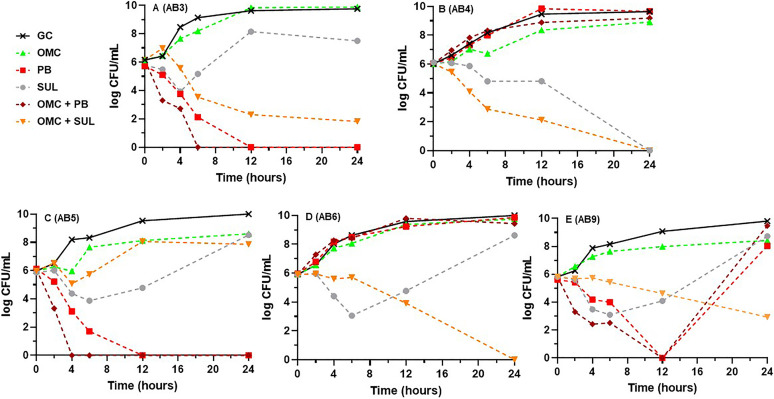
Results of time-kill experiments for omadacycline, polymyxin B, and sulbactam alone and in their respective dual omadacycline-based combinations at fCmax are displayed in Fig. 2 against the five minocycline susceptible isolates and in Fig. 3 against the nonsusceptible isolates. Figures S1 and S2 in the supplemental material display the single- and dual-drug combination results for the other agents tested against the minocycline susceptible and nonsusceptible A. baumannii strains, respectively. Alone, amikacin, meropenem, and polymyxin B were each bactericidal against 4 of 10 (40%) strains. Sulbactam was bactericidal against one strain (10%) while the tetracycline derivatives and rifampin were not bactericidal against any (0%) and 24-h bacterial densities were similar to the drug-free control. Omadacycline with sulbactam was the most active dual combination resulting in synergy against 8/10 (80%) strains (all 5 minocycline susceptible and 3 of 5 nonsusceptible), improvement in bactericidal activity from just 1/10 (10%) with sulbactam alone to 8/10 (80%), and achievement of eradication against 5/10 (50%) tested strains. It was also the only dual combination to have any activity or achieve bactericidal activity against the challenging AB4 and AB6 strains (Fig. 3B and D). The mean (±SD) log10 CFU/mL decrease after exposure to the omadacycline plus sulbactam combination from 0 to 24 h across all 10 isolates was 4.24 ± 2.51. Omadacycline in combination with either amikacin or polymyxin B was synergistic against 3/10 (30%) strains each (2 minocycline susceptible and 1 nonsusceptible), bactericidal against 6/10 (60%) each, and achieved eradication against 5/10 (50%) and 6/10 (60%), respectively. The only instance of antagonism occurred with the combination of omadacycline and polymyxin B compared to polymyxin B alone against AB1 (Fig. 2A; mean 24-h log10 CFU/mL increase from 0 to 4.53). Omadacycline plus rifampin was synergistic and bactericidal against only 2/10 (20%) strains and omadacycline plus meropenem was synergistic against just 1/10 (10%) isolates and did not improve bactericidal activity over meropenem alone (40%) (Fig. S1 and S2). Lastly, the dual combination of meropenem plus polymyxin B was also evaluated against the four strains for which neither meropenem or polymyxin B alone was bactericidal (AB2, 4, 5, and 6). This combination achieved synergy and bactericidal activity against all four strains with a mean (±SD) log10 CFU/mL decrease from 0 to 24 h of 4.77 ± 2.48.
FIG 2.
Mean log10 CFU/mL versus time profiles for omadacycline, polymyxin B, and sulbactam alone versus each respective omadacycline-based dual drug combination at the free maximum concentration of drug in serum (fCmax) against each of the 5 minocycline susceptible A. baumannii strains. Curves represent average concentrations from triplicate experiments. (A) AB1. (B) AB2. (C) AB7. (D) AB8. (E) AB10. GC, growth control; OMC, omadacycline; PB, polymyxin B; SUL, sulbactam.
FIG 3.
Mean log10 CFU/mL versus time profiles for omadacycline, polymyxin B, and sulbactam alone versus each respective omadacycline-based dual drug combination at fCmax against each of the 5 minocycline-nonsusceptible A. baumannii strains. Curves represent average concentrations from triplicate experiments. (A) AB3. (B) AB4. (C) AB5. (D) AB6. (E) AB9.
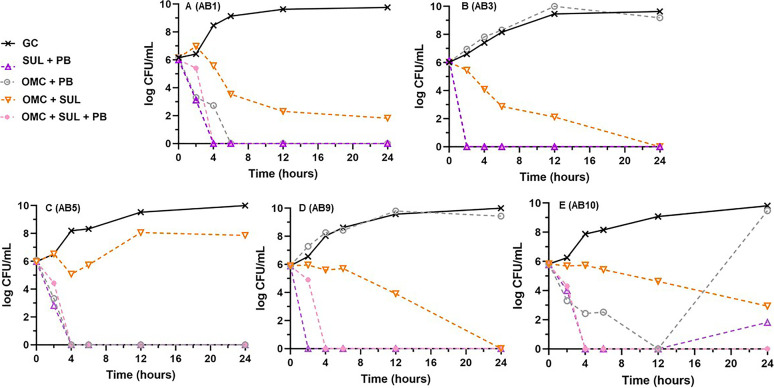
Based on results from the two-drug combination experiments, the triple combination of omadacycline plus sulbactam plus polymyxin B was evaluated to assess whether the antibacterial activity could be improved over the omadacycline plus sulbactam two drug combination. Consequently, the dual combination of sulbactam plus polymyxin B was tested against all 10 strains to allow for comparison to the triple combination and demonstrated 50% synergy (3 minocycline susceptible and 2 nonsusceptible) and 100% bactericidal activity (10/10), which was improved over 4/10 (40%) for polymyxin B alone. The mean (±SD) log10 CFU/mL decrease after exposure to the sulbactam plus polymyxin B combination from 0 to 24 h across all 10 isolates was 5.8 ± 0.64. Figure 4 displays the results of the omadacycline plus sulbactam plus polymyxin B triple combination versus the three comparative dual combinations against a representative subset of five strains (minocycline susceptible strains AB1 and AB10 and nonsusceptible strains AB3, AB5, and AB9). The triple combination of omadacycline plus sulbactam plus polymyxin B achieved synergy against only one (10%) additional strain (AB5; 9/10) over the omadacycline plus sulbactam combination (8/10). The triple combination did achieve a ≥3 log10 CFU/mL reduction almost 7 h sooner on average than the omadacycline and sulbactam dual combination (4.6 ± 2.8 h versus 11.3 ± 5.9 h, P < 0.01). Additionally, eradication was increased from 5/10 (50%) with omadacycline plus sulbactam to 10/10 (100%) and was not affected by susceptibility to minocycline. The mean (±SD) log10 CFU/mL decrease after exposure to the omadacycline plus sulbactam plus polymyxin B triple combination from 0 to 24 h across all 10 isolates was 5.99 ± 0.11. While the activity of the triple combination was improved over that of either the omadacycline plus sulbactam or omadacycline plus polymyxin B dual combinations, it was virtually indistinguishable from the sulbactam plus polymyxin B dual combination (Fig. 4).
FIG 4.
Mean log10 CFU/mL versus time profiles for the triple combination of omadacycline plus sulbactam plus polymyxin B versus each respective two drug combination against a representative subset of 5 A. baumannii strains (2 minocycline susceptible, 3 nonsusceptible). Curves represent average concentrations from triplicate experiments. (A) AB1. (B) AB3. (C) AB5. (D) AB9. (E) AB10. AMK, amikacin; MER, meropenem; RIF, rifampin.
DISCUSSION
The inability to achieve bactericidal activity in vitro, the obstinate link between resistance and delays in time to effective antimicrobial therapy, and the high mortality rates associated with monotherapy against CNSAb strongly support the use of combination therapy (8, 15). Even when faced with favorable MICs, monotherapy is often inadequate as was demonstrated well in the recent cefiderocol CREDIBLE-CR study in which 85% of patients received cefiderocol monotherapy versus 72% combination therapy in the best available therapy (BAT) group (16). Despite 92% of A. baumannii cefiderocol MICs being ≤2 mg/L, 28-day mortality was twice as high for cefiderocol compared to the BAT group. Although combination therapy is standard and endorsed by national and international societies and guidelines, the optimal combination for serious CNSAb infections has remained elusive (3, 8). As the traditional polymyxin-carbapenem combination has fallen out of favor due to refuting controlled trial data and concerns over pharmacokinetic-pharmacodynamic (PK/PD), toxicity, and the development of carbapenem resistance, investigation into alternative combination regimens for CNSAb is sorely needed (17–26).
The novel aminomethylcycline omadacycline is a welcomed addition to the available tetracycline derivatives given its potency against TetB positive, minocycline-resistant CNSAb (~70% of A. baumannii), once daily oral dosing, and favorable PK properties including low protein binding and enhanced intrapulmonary penetration (14, 27, 28). Tetracycline derivatives, including omadacycline, have demonstrated little antibacterial activity against A. baumannii alone in in vitro pharmacodynamic models, as expected given their low free serum concentrations and bacteriostatic nature (29–31). Conversely, tetracycline-based combinations often produce the most potent synergy and bactericidal activity in vitro and in vivo versus nontetracycline-based combinations and available comparative clinical data are encouraging (8, 32–36). In the present study, omadacycline was evaluated alone and in combination for the first time in six different two- and three-drug combinations against CNSAb with varying minocycline susceptibility. Broth microdilution testing of omadacycline demonstrated the second lowest MIC50/90 values at 4/8 mg/L behind polymyxin B despite intentionally enriching our sample with minocycline nonsusceptible isolates (Table 2 and Fig. 1). The in vitro potency observed herein is consistent with previous analyses of omadacycline against larger samples of A. baumannii (37–39). Bactericidal activity was achieved against ≤4 of 10 isolates for any agent in alone in time-kill analyses, in line with the high rates of nonsusceptibility observed among the selected isolates (Table 3). Omadacycline-based combinations were tested in 50 separate time-kill experiments overall (5 per isolate), demonstrating synergy with another agent in 17/50 (34%) and bactericidal activity in 26/50 (52%). Across the 10 isolates included, the highest rate of synergy and degree of bactericidal activity was observed when omadacycline was combined with sulbactam (80%), followed by omadacycline with amikacin or polymyxin B (30% each). The addition of polymyxin to the omadacycline-sulbactam dual combination resulted in more rapid bactericidal activity, although this mimicked the activity of the dual polymyxin-sulbactam combination which was less synergistic (50% versus 80%) than omadacycline-sulbactam. While previous in vitro, in vivo, and clinical data have demonstrated compelling synergy between tetracyclines and sulbactam, this combination is almost never employed clinically despite the added benefit of reduced toxicity compared to polymyxin-based regimens (20, 30, 40–44). Our work adds to the existing literature supporting the further exploration of tetracycline-sulbactam combinations and adds the first set of data evaluating omadacycline in combination against A. baumannii.
Strengths of our study include the use of a broad panel of clinical A. baumannii isolates with varying minocycline susceptibilities and the evaluation of omadacycline alone and in combination with currently preferred agents. Limitations are primarily related to the inherently static, in vitro nature of time-kill experiments including the inability to simulate human PK parameters and other physiologic factors such as protein binding. Nonetheless, time-kill assays are well recognized as suitable and efficient tools for assessing antibacterial activity and PD drug interactions of antibiotic combinations (45). Additionally, as the current study was focused on omadacycline-based combinations, not all possible dual and/or triple combinations were evaluated including some that have demonstrated synergy in previous studies (46). Recent findings suggest rifabutin may be significantly more potent than rifampin against A. baumannii, although this was only evident when testing in nutrient-depleted, mammalian cell culture media, which would not be suitable for the other agents included in this study (47). Finally, testing agents at fCmax could have overestimated the antibacterial activity and limited comparability between agents versus using MIC multiplicatives although MICs were greater than fCmax in the majority of experiments and bactericidal activity was rare in single-drug time-kill experiments.
In conclusion, omadacycline displays potent in vitro activity against CNSAb including strains that harbor TetB and are minocycline resistant. Omadacycline in combination with sulbactam was synergistic and bactericidal against 8/10 (80%) isolates, including strains that were nonsusceptible to every drug tested. In agreement with the growing body of data supporting tetracycline-based combinations against CNSAb, this work adds further impetus to continue to explore tetracycline-sulbactam combinations as a promising regimen toward the goal of maximize antimicrobial efficacy and minimizing toxicity against this challenging pathogen.
MATERIALS AND METHODS
Bacteria and susceptibility testing.
A total of 41 genotypically characterized clinical A. baumannii isolates were selected from the CDC & FDA Antibiotic Resistance Isolate Bank to encompass a range of phenotypes against the agents tested, particularly the tetracycline derivatives (48). Complete genomes were downloaded from the NCBI nucleotide database and resistance genes were identified by BLAST searching against ResFinder 3.1 and CARD-RGI databases (49, 50). Isolates were maintained at −80°C in cation-adjusted Mueller-Hinton broth (CAMHB; Teknova, Hollister, CA, USA) with 20% glycerol and were subcultured twice on tryptic soy agar plates with 5% sheep blood prior to use.
Analytical grade amikacin, meropenem, minocycline, polymyxin B, rifampin, sulbactam, and tigecycline powders were obtained commercially (Sigma-Aldrich, St. Louis, MO, USA). Sulbactam was tested alone as ampicillin has no activity against A. baumannii nor does it impact the activity of sulbactam (51). Analytical grade omadacycline powder was provided by the manufacturer (Paratek Pharmaceuticals, Boston, MA, USA). Stock solutions of each agent were freshly prepared as single-use aliquots at the beginning of each week and kept frozen at −80°C. CAMHB was freshly prepared and used within 12 h of preparation. MICs were determined in triplicate via reference BMD according to Clinical and Laboratory Standards Institute (CLSI) guidelines using the same 0.5 McFarland suspension on the same day (52). Modal MIC values from triplicate BMDs were recorded and are reported as MIC50, MIC90, and MIC range. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality control organisms. Susceptibility interpretations were based on CLSI criteria against A. baumannii where available (52).
Time-kill experiments.
Time-kill experiments were performed in triplicate on the same day against a subset of 10 CNSAb isolates purposefully selected from the original group of 41. These 10 isolates were chosen based on their minocycline susceptibility (5 susceptible and 5 nonsusceptible) and to ensure that each log2 omadacycline BMD MIC across the observed range (1–16 mg/L) was represented at least once. Experiments were performed according to CLSI guidelines modified using a final volume of 2 mL in deep-well, nontissue-treated plates (20). A starting inoculum of ~106 CFU/mL was prepared by suspending three to four isolated colonies selected from a pure overnight culture in 5 mL of sterile saline and adjusting to 0.5 McFarland standard, which was subsequently incubated with agitation to ensure log-phase growth and then diluted 1:100 in CAMHB. Colony counts were performed to ensure final inoculum densities. Time-kill experiments were performed with each agent at its representative plasma fCmax concentration after standard dosing as displayed in Table 3. Single-drug experiments were performed for each agent followed by dual combinations of omadacycline with amikacin, meropenem, polymyxin B, rifampin, and sulbactam. Additionally, triple combinations of omadacycline plus polymyxin B plus either meropenem, rifampin, or sulbactam were tested based on results from the dual combination experiments. A growth control without any antibiotic was included with each experiment. At the prespecified time points of 0, 2, 4, 6, 12, and 24 h, aliquots of 20 μL were removed from the suspensions and serially diluted in log10 dilutions. A 50-μL aliquot was then plated on MH agar plates using an automated spiral plater (Don Whitley WASP Touch, Microbiology International, Frederick, MD) and incubated at 35°C for at least 24 h prior to enumeration. Colony counts were performed using an automated colony counter (ProtoCOL 3 Plus, Synbiosis, Frederick, MD). The theoretical lower limit of quantitation was 100 CFU/mL. Time-kill curves were generated by plotting the average log10 CFU/mL versus time to compare the 24-h killing effects of drugs alone and in dual and triple combinations. Bactericidal activity was defined as ≥3 log10 CFU/mL reduction at 24 h compared to the starting inoculum. Synergy was defined as ≥2 log10 CFU/mL reduction at 24 h compared to the most active drug alone for dual combination experiments and versus the most active dual combination for triple combination experiments. Antagonism was defined as ≥2 log10 CFU/mL increase at 24 h compared to the most active drug alone or dual combination (53).
ACKNOWLEDGMENTS
This work was supported by an investigator-initiated research grant awarded to E.W. by Paratek Pharmaceuticals (Boston, MA, USA).
E.W. serves on the speaker’s bureau for Melinta Therapeutics, Astellas Pharma, Abbvie Inc., and Tetraphase Pharmaceuticals and on the advisory board for GenMark Diagnostics and Shionogi & Co., Ltd. All other authors have nothing to disclose.
T.A., A.V., and M.J. contributed to the investigation, data curation, formal analysis, visualization, and writing the original draft manuscript. M.B. and E.W. contributed to conceptualization, methodology, funding acquisition, resources, supervision, administration, formal analysis, and reviewing and editing the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Eric Wenzler, Email: wenzler@uic.edu.
Bonnie Chase Prokesch, Univeristy of Texas Southwestern Medical Center.
REFERENCES
- 1.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Accessed November 14, 2021.
- 2.U.S. Department of Health and Human Services Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States 2019. 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed November 14, 2021.
- 3.Butler DA, Biagi M, Tan X, Qasmieh S, Bulman ZP, Wenzler E. 2019. Multidrug resistant acinetobacter baumannii: resistance by any other name would still be hard to treat. Curr Infect Dis Rep 21:46. doi: 10.1007/s11908-019-0706-5. [DOI] [PubMed] [Google Scholar]
- 4.Flamm RK, Shortridge D, Castanheira M, Sader HS, Pfaller MA. 2019. In Vitro activity of minocycline against U.S. isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus species complex, Stenotrophomonas maltophilia, and Burkholderia cepacia complex: results from the SENTRY Antimicrobial Surveillance Program, 2014 to 2018. Antimicrob Agents Chemother 63:e01154-19. doi: 10.1128/AAC.01154-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI. 2021. Performance standards for antimicrobial susceptibility testing, M100-S31 ed. Clinical and Laboratory Standards Insitute, Wayne, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamano Y. 2019. In vitro activity of cefiderocol against a broad range of clinically important gram-negative bacteria. Clin Infect Dis 69:S544–S551. doi: 10.1093/cid/ciz827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, Palmore TN, Rhee C, Klompas M, Dekker JP, Powers JH, 3rd, Suffredini AF, Hooper DC, Fridkin S, Danner RL, National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI) . 2018. Difficult-to-treat resistance in gram-negative bacteremia at 173 us hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 67:1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2021. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin Infect Dis ciab1013. doi: 10.1093/cid/ciab1013. [DOI] [PubMed] [Google Scholar]
- 9.Mohd Sazlly Lim S, Sime FB, Roberts JA. 2019. Multidrug-resistant Acinetobacter baumannii infections: current evidence on treatment options and the role of pharmacokinetics/pharmacodynamics in dose optimisation. Int J Antimicrob Agents 53:726–745. doi: 10.1016/j.ijantimicag.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Viehman JA, Nguyen MH, Doi Y. 2014. Treatment options for carbapenem-resistant and extensively drug-resistant acinetobacterbaumannii infections. Drugs 74:1315–1333. doi: 10.1007/s40265-014-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. 2019. Antimicrobial susceptibility of Acinetobacter calcoaceticus–Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect Dis 6:S34–S46. doi: 10.1093/ofid/ofy293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai B, Echols R, Magee G, Arjona Ferreira JC, Morgan G, Ariyasu M, Sawada T, Nagata TD. 2017. Prevalence of carbapenem-resistant gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 4:ofx176. doi: 10.1093/ofid/ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draper MP, Weir S, Macone A, Donatelli J, Trieber CA, Tanaka SK, Levy SB. 2014. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother 58:1279–1283. doi: 10.1128/AAC.01066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodvold KA, Burgos RM, Tan X, Pai MP. 2020. Omadacycline: a review of the clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 59:409–425. doi: 10.1007/s40262-019-00843-4. [DOI] [PubMed] [Google Scholar]
- 15.Lodise TP, Kanakamedala H, Hsu WC, Cai B. 2020. Impact of incremental delays in appropriate therapy on the outcomes of hospitalized adult patients with gram-negative bloodstream infections: “Every day matters”. Pharmacotherapy 40:889–901. doi: 10.1002/phar.2446. [DOI] [PubMed] [Google Scholar]
- 16.Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer R, Lodise TP, Naas T, Niki Y, Paterson DL, Portsmouth S, Torre-Cisneros J, Toyoizumi K, Wunderink RG, Nagata TD. 2021. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 21:226–240. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 17.Kaye K, Marchaim D, Thamlikkikul V, Carmeli Y, Chiu CH, Daikos G, Dhar S, Durante-Mangoni E, Gikas A, Kotanidou A, Paul M, Roilides E, Rybak M, Samarkos M, Sims M, Tancheva D, Tsiodros S, Devine G, Ghazyaran V, Pogue J. 2021. Results from the OVERCOME trial: colistin monotherapy versus combination therapy for the treatment of pneumonia or bloodstream infection due to extensively drug-resistant Gram-negative bacilli, abstr 4773. Abstr 31st ECCMID, 9 to 12 July 2021. European Society of Clinical Microbiology and Infectious Diseases. [Google Scholar]
- 18.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L. 2018. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 19.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papst L, Beović B, Pulcini C, Durante-Mangoni E, Rodríguez-Baño J, Kaye KS, Daikos GL, Raka L, Paul M, ESGAP, ESGBIS, ESGIE and the CRGNB treatment survey study group . 2018. Antibiotic treatment of infections caused by carbapenem-resistant Gram-negative bacilli: an international ESCMID cross-sectional survey among infectious diseases specialists practicing in large hospitals. Clin Microbiol Infect 24:1070–1076. doi: 10.1016/j.cmi.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) . 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 23.Muggeo A, Guillard T, Barbe C, Thierry A, Bajolet O, Vernet-Garnier V, Limelette A, Brasme L, De Champs C, CARBAFREST Group . 2017. Factors associated with carriage of carbapenem-non-susceptible Enterobacteriaceaein North-Eastern France and outcomes of infected patients. J Antimicrob Chemother 72:1496–1501. doi: 10.1093/jac/dkw590. [DOI] [PubMed] [Google Scholar]
- 24.Aitken SL, Sahasrabhojane PV, Kontoyiannis DP, Savidge TC, Arias CA, Ajami NJ, Shelburne SA, Galloway-Peña JR. 2021. Alterations of the oral microbiome and cumulative carbapenem exposure are associated with stenotrophomonas maltophilia infection in patients with acute myeloid leukemia receiving chemotherapy. Clin Infect Dis 72:1507–1513. doi: 10.1093/cid/ciaa778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armand-Lefèvre L, Angebault C, Barbier F, Hamelet E, Defrance G, Ruppé E, Bronchard R, Lepeule R, Lucet JC, El Mniai A, Wolff M, Montravers P, Plésiat P, Andremont A. 2013. Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 57:1488–1495. doi: 10.1128/AAC.01823-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagenlehner F, Lucenteforte E, Pea F, Soriano A, Tavoschi L, Steele VR, Henriksen AS, Longshaw C, Manissero D, Pecini R, Pogue JM. 2021. Systematic review on estimated rates of nephrotoxicity and neurotoxicity in patients treated with polymyxins. Clin Microbiol Infect 5:P671–686. doi: 10.1016/j.cmi.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Lomovskaya O, Sun D, Rubio-Aparicio D, Nelson KJ, Thamlikitkul V, Dudley MN, Redell MA. 2018. Absence of TetB identifies minocycline-susceptible isolates of Acinetobacter baumannii. Int J Antimicrob Agents 52:404–406. doi: 10.1016/j.ijantimicag.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, McElheny CL, Mettus RT, Shanks RMQ, Doi Y. 2017. Contribution of the TetB efflux pump to minocycline susceptibility among carbapenem-resistant Acinetobacter baumannii strains. Antimicrob Agents Chemother 61:e01176-17. doi: 10.1128/AAC.01176-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noel AR, Attwood M, Bowker KE, MacGowan AP. 2021. In vitro pharmacodynamics of omadacycline against Escherichia coli and Acinetobacter baumannii. J Antimicrob Chemother 76:667–670. doi: 10.1093/jac/dkaa508. [DOI] [PubMed] [Google Scholar]
- 30.Beganovic M, Daffinee KE, Luther MK, LaPlante KL. 2021. Minocycline alone and in combination with polymyxin B, meropenem, and sulbactam against carbapenem-susceptible and -resistant Acinetobacter baumannii in an in vitro Pharmacodynamic model. Antimicrob Agents Chemother 65:e01680-20. doi: 10.1128/AAC.01680-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagihara M, Housman ST, Nicolau DP, Kuti JL. 2014. In vitro pharmacodynamics of polymyxin B and tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 58:874–879. doi: 10.1128/AAC.01624-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aranzana-Climent V, Buyck JM, Smani Y, Pachón-Diaz J, Marchand S, Couet W, Grégoire N. 2020. Semi-mechanistic PK/PD modelling of combined polymyxin B and minocycline against a polymyxin-resistant strain of Acinetobacter baumannii. Clin Microbiol Infect 26:1254.e9-1254-e15. doi: 10.1016/j.cmi.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Rao GG, Ly NS, Diep J, Forrest A, Bulitta JB, Holden PN, Nation RL, Li J, Tsuji BT. 2016. Combinatorial pharmacodynamics of polymyxin B and tigecycline against heteroresistant Acinetobacter baumannii. Int J Antimicrob Agents 48:331–336. doi: 10.1016/j.ijantimicag.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowers DR, Cao H, Zhou J, Ledesma KR, Sun D, Lomovskaya O, Tam VH. 2015. Assessment of minocycline and polymyxin B combination against Acinetobacter baumannii. Antimicrob Agents Chemother 59:2720–2725. doi: 10.1128/AAC.04110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fragkou PC, Poulakou G, Blizou A, Blizou M, Rapti V, Karageorgopoulos DE, Koulenti D, Papadopoulos A, Matthaiou DK, Tsiodras S. 2019. The role of minocycline in the treatment of nosocomial infections caused by multidrug, extensively drug and pandrug resistant Acinetobacter baumannii: a systematic review of clinical evidence. Microorganisms 7:159. doi: 10.3390/microorganisms7060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lashinsky JN, Henig O, Pogue JM, Kaye KS. 2017. Minocycline for the treatment of multidrug and extensively drug-resistant A. baumannii: a review. Infect Dis Ther 6:199–211. doi: 10.1007/s40121-017-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P-Y, Ko W-C, Lee W-S, Lu P-L, Chen Y-H, Cheng S-H, Lu M-C, Lin C-Y, Wu T-S, Yen M-Y, Wang L-S, Liu C-P, Shao P-L, Lee Y-L, Shi Z-Y, Chen Y-S, Wang F-D, Tseng S-H, Lin C-N, Chen Y-H, Sheng W-H, Lee C-M, Tang H-J, Hsueh P-R. 2021. In vitro activity of cefiderocol, cefepime/enmetazobactam, cefepime/zidebactam, eravacycline, omadacycline, and other comparative agents against carbapenem-non-susceptible Pseudomonas aeruginosa and Acinetobacter baumannii isolates associated from bloodstream infection in Taiwan between 2018–2020. J Microbiol Immunol Infect S1684-1182(21)00186-9. doi: 10.1016/j.jmii.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Huband MD, Pfaller MA, Shortridge D, Flamm RK. 2019. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: results from the SENTRY Antimicrobial Surveillance Programme, 2017. J Glob Antimicrob Resist 19:56–63. doi: 10.1016/j.jgar.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Carvalhaes CG, Huband MD, Reinhart HH, Flamm RK, Sader HS. 2019. Antimicrobial activity of omadacycline tested against clinical bacterial isolates from hospitals in mainland China, Hong Kong, and Taiwan: Results from the SENTRY Antimicrobial Surveillance Program (2013 to 2016). Antimicrob Agents Chemother 63:e02262-18. doi: 10.1128/AAC.02262-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Housman ST, Hagihara M, Nicolau DP, Kuti JL. 2013. In vitro pharmacodynamics of human-simulated exposures of ampicillin/sulbactam, doripenem and tigecycline alone and in combination against multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother 68:2296–2304. doi: 10.1093/jac/dkt197. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Shu Y, Zhu F, Feng B, Zhang Z, Liu L, Wang G. 2021. Comparative efficacy and safety of combination therapy with high-dose sulbactam or colistin with additional antibacterial agents for multiple drug-resistant and extensively drug-resistant Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Glob Antimicrob Resist 24:136–147. doi: 10.1016/j.jgar.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Ku NS, Lee S-H, Lim Y-S, Choi H, Ahn JY, Jeong SJ, Shin SJ, Choi JY, Choi YH, Yeom J-S, Yong D, Song YG, Kim JM. 2019. In vivo efficacy of combination of colistin with fosfomycin or minocycline in a mouse model of multidrug-resistant Acinetobacter baumannii pneumonia. Sci Rep 9:17127–17127. doi: 10.1038/s41598-019-53714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinc G, Demiraslan H, Elmali F, Ahmed SS, Metan G, Alp E, Doganay M. 2013. Efficacy of sulbactam and its combination with imipenem, colistin and tigecycline in an experimental model of carbapenem-resistant Acinetobacter baumannii sepsis. Chemotherapy 59:325–329. doi: 10.1159/000356755. [DOI] [PubMed] [Google Scholar]
- 44.Wenzler E, Bunnell KL, Danziger LH. 2018. Clinical use of the polymyxins: the tale of the fox and the cat. Int J Antimicrob Agents 51:700–706. doi: 10.1016/j.ijantimicag.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 45.Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, Drusano GL, Hoover JL. 2019. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 63:e02307-18. doi: 10.1128/AAC.02307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim T-P, Tan T-Y, Lee W, Sasikala S, Tan T-T, Hsu L-Y, Kwa AL. 2009. In vitro activity of various combinations of antimicrobials against carbapenem-resistant Acinetobacter species in Singapore. J Antibiot (Tokyo) 62:675–679. doi: 10.1038/ja.2009.99. [DOI] [PubMed] [Google Scholar]
- 47.Luna B, Trebosc V, Lee B, Bakowski M, Ulhaq A, Yan J, Lu P, Cheng J, Nielsen T, Lim J, Ketphan W, Eoh H, McNamara C, Skandalis N, She R, Kemmer C, Lociuro S, Dale GE, Spellberg B. 2020. A nutrient-limited screen unmasks rifabutin hyperactivity for extensively drug-resistant Acinetobacter baumannii. Nat Microbiol 5:1134–1143. doi: 10.1038/s41564-020-0737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutgring JD, Machado M-J, Benahmed FH, Conville P, Shawar RM, Patel J, Brown AC. 2018. FDA-CDC antimicrobial resistance isolate bank: a publicly available resource to support research, development, and regulatory requirements. J Clin Microbiol 56:e01415-17. doi: 10.1128/JCM.01415-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FSL, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corbella X, Ariza J, Ardanuy C, Vuelta M, Tubau F, Sora M, Pujol M, Gudiol F. 1998. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J Antimicrob Chemother 42:793–802. doi: 10.1093/jac/42.6.793. [DOI] [PubMed] [Google Scholar]
- 52.CLSI. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. Document M100-S30. Clinical and Laboratory Standards Insitute, Wayne, PA. [Google Scholar]
- 53.CLSI. 1999. Methods for determining bactericidal activity of antimicrobial agents, M26-A ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 54.White BP, Lomaestro B, Pai MP. 2015. Optimizing the initial amikacin dosage in adults. Antimicrob Agents Chemother 59:7094–7096. doi: 10.1128/AAC.01032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubino CM, Bhavnani SM, Loutit JS, Morgan EE, White D, Dudley MN, Griffith DC. 2018. Phase 1 study of the safety, tolerability, and pharmacokinetics of vaborbactam and meropenem alone and in combination following single and multiple doses in healthy adult subjects. Antimicrob Agents Chemother 62:e02228-17. doi: 10.1128/AAC.02228-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cornely OA, Arenz D, Barraud O, Bayliss M, Dimitriou V, Lovering AM, Alasdair Macgowan A, Cammarata SK, Fusaro K, Griffith DC, Morgan EE, Loutit JS. 2018. Phase I study to evaluate the safety and pharmacokinetics of single and multiple ascending doses of intravenous minocycline in healthy adult subjects, poster #P1387. The IDWeek Annual Meeting 2018, San Fransico, CA. [Google Scholar]
- 57.Minocin (minocycline) [package insert]. 2015. The Medicines Company, Parsippany, NJ. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050444s049lbl.pdf. Revised April 2015. Accessed November 14, 2021. [Google Scholar]
- 58.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 59.Rifadin (rifampin) [package insert]. 2019. Sanofi-Aventis, Bridgewater, NJ. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/050420s080,050627s023lbl.pdf. Revised February 2019. Accessed November 14, 2021. [Google Scholar]
- 60.Unasyn (ampicillin/sulbactam) [package insert]. 2008. Pfizer, Inc., New York, NY. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050608s029lbl.pde. Revised April 2007. Accessed November 14, 2021. [Google Scholar]
- 61.Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. 2005. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis 41(Suppl 5):S333–4340. doi: 10.1086/431674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.00542-22-s0001.pdf, PDF file, 0.8 MB (796.4KB, pdf)