Abstract
Background:
With the rise in obesity, there has been a concomitant increase in prescription medications associated with weight gain.
Objective:
To quantify the magnitude of association between putative weight-promoting medications and 3-year weight change in a diverse cohort of postmenopausal women in the Women’s Health Initiative (WHI)
Methods:
This is a prospective observational cohort study, considering 40 sites in the WHI and a cohort of 76,252 post-menopausal women aged 50-79 years, with weight measured at both baseline and 3 years, in the WHI-Observational Study. Body mass index (BMI) and waist circumference (WC) were measured at baseline and 3 years. An in-clinic medication inventory identified prescribed medications, including antidepressants, beta-blockers, insulin, and/or glucocorticosteroids. Generalized linear models evaluated if intermittent or persistent use of weight promoting drugs was associated with increased BMI and WC during a 3-year follow up.
Results:
Women with overweight or obesity at baseline were more likely to be taking antidepressants, beta-blockers and/or insulin. Taking at least one putative weight-promoting medication was associated with a greater increase in BMI (0.37 versus 0.27 kg/m2, p=0.0045) and WC (1.10 cm versus 0.89 cm, p=0.0077) over the course of 3 years compared to women not on these medications. Both BMI and WC increased with the number of weight promoting drugs prescribed (p for trend per medication used<0.00001 for both variables). Those who took either antidepressants or insulin, or a combination of antidepressants and beta blockers, were most likely to have a significant increase in BMI compared to non-users.
Conclusion:
Antidepressants, beta blockers, and insulin were associated with weight gain in postmenopausal women. This information may help to inform clinical decision making and efforts to mitigate medication-related weight gain.
Keywords: Obesity, weight gain, weight promoting medications, anti-depressants, psychotropic weight gain, beta blockers, anti-histamines, sleep agents, insulin, steroids
Introduction
Obesity, a serious chronic disease, is a global epidemic that affects both youth and adults, and it is associated with myriad co-morbidities including type 2 diabetes mellitus, cardiovascular disease, and cancer.1 In the United States, obesity has increased steadily over the last three decades. Women have a higher prevalence of obesity at 41.1% compared to 37.9% for men.2 Racial and ethnic minorities, especially non-Hispanic Black women, have disproportionately high levels of obesity.3 Pre-menopausal women tend to have a gynoid fat distribution (i.e. distribution in hip, buttock, and thigh region) whereas post-menopausal women have increased amounts of android fat deposits (i.e. abdominal region).4, 5 Central adiposity is associated with metabolic disorders and an increased risk of type 2 diabetes, cardiovascular disease, and other chronic diseases. 6
As the risk of comorbid conditions increases with obesity, medication use is more prevalent.7 Many of the medications prescribed to treat obesity-related co-morbidities such as hypertension, type 2 diabetes, and depression, have been linked to weight gain. 2, 8, 9 Second-generation antidepressants (SGADs), second-generation antipsychotics (SGAPs), second-generation antihistamines (SGAHs), neuropathic agents, and beta blockers may also be associated with weight gain.7, 10-18 In particular, antidepressant use (the third most frequently prescribed medication class) has increased by roughly 400% from 1988-1994 through 2005-2008. 13 Previous studies indicate that antipsychotics and antidepressants are associated with weight gain and obesity, particularly among women and some racial/ethnic minority groups.19 In large cohorts in the Netherlands, Canada, and the United Kingdom, studies have demonstrated that these medications have been associated with clinically significant weight gain.20-23 Yet large studies investigating weight gain in United States cohorts are largely lacking which is of particular importance due to differences in the prevalence and gender distribution of obesity compared to other Western countries. It is important to note the United States has significantly higher obesity rates than the aforementioned developed countries (United States (39.8%),24 United Kingdom (27.8%),25 Canada (26.8%),26 and the Netherlands (18.8%)27), and it is unclear why this higher degree of excess weight is more commonplace and whether weight promoting medications may play a role in this notable difference. Furthermore, women have a higher likelihood of having obesity in the United States whereas men have higher obesity rates in Canada and the United Kingdom.2, 24-26 Finally, postmenopausal women are of significant interest as those who have obesity and normal weight central obesity are at increased risk for entities such as invasive breast cancer,28 sleep disturbances,29 type 2 diabetes,30 and mortality.31 The association between use of weight promoting medications and the weight status of postmenopausal women within the WHI cohort, the largest international cohort of postmenopausal women, has not been examined previously.
The current analysis evaluates a large sub cohort within the Women’s Health Initiative observational study (WHI-OS). The objective was to analyze the use of drugs suspected to promote weight gain in relation to changes in body mass index (BMI) and waist circumference (WC) in postmenopausal women. Information on the association between putative weight promoting prescription medications and change in body weight may help to inform clinical decision making and spur the development of strategies to avert or minimize these adverse outcomes.
COHORT DESCRIPTION
Methods
The WHI-OS investigates the major causes of morbidity and mortality in postmenopausal women. Details of the study have been reported elsewhere 32. A total of 93,676 eligible women aged 50-79 years at baseline were administered several epidemiologic questionnaires and underwent physical examinations. The current analysis focused on weight change that occurred during the first three years from baseline (1993-1998) to the 3 year follow up visit. Anthropometric measurements were conducted by trained clinic staff, at baseline and year 3. Weight was obtained to the nearest 0.1 kilogram (kg) and height to the nearest 0.1 centimeter (cm) to calculate BMI. Waist circumference (WC) was obtained at the natural waist or narrowest part of the torso to the nearest 0.1 cm. About 18% of the sample did not have anthropometric measurements (BMI and WC) at year 3 and were excluded; 76,252 postmenopausal women were evaluated for BMI change and 76,579 for WC change, respectively. Those who were excluded were of similar age and more likely to be white and less educated.
Medication use was assessed by a medication inventory based on pill bottles brought to the baseline and 3-year clinic visits. Medication exposure was defined as follows: 1) those not taking putative weight-promoting medications either at baseline or at year 3 (reference group); 2) those taking at least one of these putative weight promoting medications either at baseline or year 3 (intermittent users); and 3) those taking the weight promoting medications at both baseline and year 3 (persistent users). Also the total number of weight promoting drugs was taken into account (see below).
The protocol and consent forms were approved by the institutional review board at each of the 40 sites throughout the US. All participants provided written informed consent prior to participation.
Statistical Analysis
Baseline characteristics were summarized in the whole sample, by medication use, by race/ethnicity (Non-Hispanic White, African Americans, Hispanic, Asian/Pacific Islander and Native Americans), by waist circumference (<88 cm or > 88 cm) and, by BMI class (Underweight (BMI<18.5 kg/m2), Normal weight (BMI 18.5-24.9 kg/m2), Overweight (BMI 25-29.9kg/m2), Class 1 obesity- Mild (BMI 30-34.9 kg/m2), Class II obesity – Moderate (BMI 35-39.9 kg/m2) and, Class III obesity - Severe (BMI ≥40 kg/m2).
Candidate weight promoting drugs intake were described as frequencies (proportions) in the whole sample, by weight status and by ethnicity. Generalized linear models evaluated if intermittent or persistent use of weight promoting drugs is associated with increased BMI and WC during a 3-year follow up. For each medication class (Antidepressants, Antipsychotics, Beta Blockers, Insulin and Glucocorticosteroids), and for the drugs that were most likely to be commonly prescribed in combinations and more frequently observed in the data, when more than one medication was taken, simple models assessed both BMI and WC change during the three-year follow-up, stratifying by baseline BMI categories. Since association between weight promoting drugs and increased BMI and WC might be confounded by other factors, multiple models were set up to take into account such confounding. Potential confounders have been selected among those variables that are independently associated with both the exposure and weight gain, supported by evidence obtained through literature.6, 33, 34 Baseline health conditions as diabetes, hypertension and hypercholesterolemia as well as age, education, physical activity, sleep medications, smoking and diet were evaluated as they are well known risk factors for weight gain, but it is largely unknown how these factors interact with weight promoting medications. The research strategy for identifying these factors was based on the knowledge of the field and expertise criteria were involved for evaluating the confounders.
The independent association of the potential mediators, namely the intake of putative weight promoting medications, with weight gain (BMI and WC) was assessed using multivariate linear regression models. Those models included the following covariates: baseline health conditions as diabetes, hypertension and hypercholesterolemia; age (50-59, 60-69, 70-79); education (less than high school or GED, High school or GED, some college, college or higher); physical activity (Total MET hours per week); smoking (Never Smoker, Past Smoker, Current Smoker); sleeping habits (use of sleep medication or not) and sleep duration (less than 4 hours, 4-7 hours, 8-9 hours, more than10 hours); dietary habits (HEI-2005 index score) of the postmenopausal women.
Results
Table 1 shows baseline characteristics of women (N= 76,252) in the whole sample and by medication use, as Supplemental Table 1 shows them stratified by race/ethnicity. While most of the women in the cohort were Non-Hispanic White (n=64,750), most of the major racial/ethnic groups in the United States including American Indian/ Alaskan Native, Asian/ Pacific Islander, African-American, and Hispanic were represented. While a large majority of the cohort (n=67,583, 88.6%) were not taking any putative weight promoting medications, 11.4% (n=8669), were taking such medications. Weight, BMI, and waist circumference was higher in patients who were prescribed weight promoting medications, and there was a continuous rise in these values with the number of weight promoting medications prescribed. As the number of weight promoting medications increased, there were fewer individuals in the highest quartile for physical activity, but the healthy eating index (HEI) score did not seem to differ by number of weight promoting medications. Those on weight promoting medications had a higher likelihood of having insulin dependent diabetes mellitus, treated hypertension, and high cholesterol.
Table 1.
Distribution of Baseline Characteristics in Whole Study Sample and by weight promoting drugs
| All women in sample |
Taking no drugs | Taking 2-3 drugs | Taking > 4 drugs | |
|---|---|---|---|---|
| N=76,252 | N=67,583 | N=7,775 | N=894 | |
| Age | ||||
| Years | 63.5 (7.3) | 63.46 (7.29) | 64.39 (7.18) | 63.22 (6.97) |
| 50-59 | 24,153 31.7% | 21,767 32.2% | 2,107 27.1% | 279 31.2% |
| 60-69 | 34,131 44.8% | 30,124 44.6% | 3,571 45.9% | 436 48.8% |
| 70-79 | 17,968 23.6% | 15,692 23.2% | 2,097 27% | 179 20% |
| Weight | ||||
| Weight, kg | 70.8 (15.4) | 70.27 (15.11) | 74.16 (16.86) | 78.58 (18.92) |
| BMI, kg/m2 | 27 (5.7) | 26.84 (5.58) | 28.3 (6.19) | 29.87 (6.86) |
| Underweight (<18.5) | 910 1.2% | 836 1.2% | 66 0.9% | 8 0.9% |
| Normal (18.5-24.9) | 31,282 41% | 28,555 42.3% | 2,499 32.1% | 228 25.5% |
| Overweight (25.0-29.9) | 26,031 34.1% | 22,992 34% | 2,756 35.5% | 283 31.7% |
| Class 1 Obesity (30.0-34.9) | 11,554 15.2% | 9,873 14.6% | 1,497 19.3% | 184 20.6% |
| Class II Obesity (35.0-39.9) | 4,104 5.4% | 3,420 5.1% | 571 7.3% | 113 12.6% |
| Class III Obesity (>40) | 2,371 3.1% | 1,907 2.8% | 386 5% | 78 8.7% |
| Waist | ||||
| Circumference (cm) | 84.2 (13.2) | 83.7 (13.03) | 87.8 (14.07) | 92.9 (16.54) |
| <88 | 50,129 65.9% | 45,822 67.5% | 4,282 54.9% | 384 42.9% |
| ≥88 | 25,922 34.1% | 22,068 32.5% | 3,512 45.1% | 511 57.1% |
| Education | ||||
| Less than HS or GED | 3,230 4.3% | 2,779 4.1% | 400 5.2% | 51 5.8% |
| HS or GED | 19,047 25.2% | 16,542 24.7% | 2,235 29% | 270 30.4% |
| Some college | 29,218 38.6% | 25,925 38.7% | 2,961 38.4% | 332 37.4% |
| College or higher | 24,173 32% | 21,826 32.5% | 2,113 27.4% | 234 26.4% |
| Physical Activity | ||||
| Total MET-Hours Per Week | 14.1 (14.4) | 14.4 (14.55) | 11.71 (12.87) | 9.31 (11.09) |
| First quartile | 0.8 (1) | 0.81 (1) | 0.74 (0.98) | 0.7 (0.96) |
| Second quartile | 6.4 (2.1) | 6.41 (2.07) | 6.26 (2.02) | 6.32 (2) |
| Third quartile | 14.5 (2.8) | 14.54 (2.77) | 14.52 (2.74) | 14.45 (2.58) |
| Fourth quartile | 33.4 (13.9) | 33.55 (13.99) | 32.48 (12.88) | 30.89 (11.78) |
| Smoking | ||||
| Never Smoker | 38,887 51.6% | 34,668 52% | 3,788 49.3% | 431 48.9% |
| Past Smoker | 32,209 42.8% | 28,357 42.5% | 3,454 45% | 398 45.1% |
| Current Smoker | 4,201 5.6% | 3,713 5.6% | 435 5.7% | 53 6% |
| HEI-2005 Index score | ||||
| Score | 69.6 (10.4) | 69.63 (10.35) | 69.48 (10.34) | 68.78 (10.56) |
| First quartile | 54.7 (6.4) | 54.68 (6.35) | 54.73 (6.34) | 54.34 (6.69) |
| Second quartile | 67.2 (2.5) | 67.19 (2.45) | 67.07 (2.46) | 67.01 (2.56) |
| Third quartile | 74.2 (1.8) | 74.22 (1.77) | 74.32 (1.76) | 74.36 (1.67) |
| Fourth quartile | 81 (2.8) | 81.03 (2.77) | 80.93 (2.69) | 80.56 (2.61) |
|
Sleep Habits
Do you Take Sleep Medications? |
||||
| No | 54,408 72.5% | 49,285 74% | 4,722 61.7% | 401 45.8% |
| Yes | 20,694 27.6% | 17,285 26% | 2,934 38.3% | 475 54.2% |
|
Number of hours spent sleeping
(baseline) |
||||
| Less than 4 hours | 4,418 5.8% | 3,938 5.9% | 417 5.4% | 63 7.1% |
| 4-7 hours | 22,836 30.1% | 20,457 30.4% | 2,162 28% | 217 24.4% |
| 8-9 hours | 35,283 46.5% | 31,463 46.8% | 3,436 44.4% | 384 43.2% |
| More than 10 hours | 13,287 17.5% | 11,342 16.9% | 1,720 22.2% | 225 25.3% |
| Diabetes | ||||
| No History | 72,505 95.1% | 64,902 96% | 7,010 90.2% | 593 66.3% |
| Insulin Dependent | 798 1.1% | 279 0.4% | 290 3.7% | 229 25.6% |
| Non-Insulin Dependent | 2,949 3.9% | 2,402 3.6% | 475 6.1% | 72 8.1% |
| Hypertension | ||||
| Never hypertensive | 50,847 67.8% | 47,423 71.3% | 3,082 40.3% | 342 39.2% |
| Untreated | 5,815 7.8% | 5,284 8% | 469 6.1% | 62 7.1% |
| Treated hypertensive | 18,337 24.5% | 13,776 20.7% | 4,093 53.6% | 468 53.7% |
|
High cholesterol
requiring pills, ever |
||||
| No | 63,727 85.4% | 57,222 86.5% | 5,844 76.6% | 661 74.9% |
| Yes | 10,932 14.6% | 8,922 13.5% | 1,789 23.4% | 221 25.1% |
| American Indian/Alaska Native | 292 0.4% | 264 0.4% | 22 0.3% | 6 0.7% |
|
Asian/Pacific
Islander |
2,277 3% | 2,114 3.1% | 158 2% | 5 0.6% |
| African American | 5,452 7.2% | 4,872 7.2% | 507 6.5% | 73 8.2% |
| Hispanic | 2,476 3.3% | 2,271 3.4% | 186 2.4% | 19 2.1% |
| Non-Hispanic White | 64,750 85.1% | 57,166 84.8% | 6,802 87.8% | 782 87.8% |
| Unknown | 806 1.1% | 723 1.1% | 77 1% | 6 0.7% |
Data are numbers and column percentages or means (SD) unless otherwise indicated.
BMI Body Mass Index
HEI-2005, Healthy Eating Index-2005 score
Table 1 demonstrates that 57.8% (44,060/76,252) of the WHI cohort had overweight or obesity at baseline. Supplemental Table 2 shows that those with overweight and obesity had lower levels of physical activity (MET-hours/wk) and lower diet quality (lower HEI scores). Women with overweight or obesity also had a higher prevalence of diabetes, hypertension, and high cholesterol requiring medication. By weight status, there was no significant difference in smoking, sleep medication use, or number of hours of sleep.
Women with overweight or obesity were more likely to be prescribed antidepressants, beta-blockers, insulin, and/or glucocorticosteroids (Supplemental Table 3). We assessed frequent combinations of weight-promoting medications in the cohort, and anti-depressants and beta-blockers were found to be most frequently prescribed in combination (Supplemental Table 4). Non-Hispanic white women were most likely to be prescribed antidepressants and beta-blockers, while African-Americans were more likely to be on insulin and glucocorticosteroids (Supplemental Table 5).
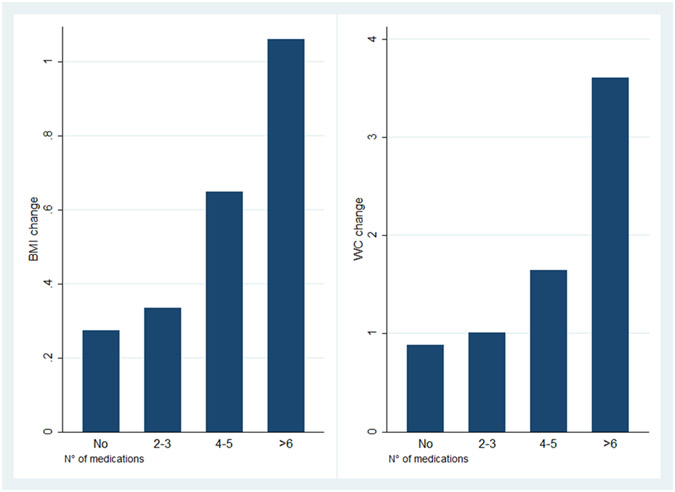
Regardless of medications prescribed, on average all women in the sample gained 0.3 kg over the three years. Women taking at least one weight promoting medication had a greater increase in BMI than those taking none of these medications (0.37 versus 0.27 kg/m2, respectively, p=0.0045) and WC (1.10 cm versus 0.89 cm, respectively, p=0.0077) over the course of 3 years. Both BMI and WC increased with the number of weight- promoting medications (Figure 1).
Figure 1. BMI and WC increase over 3 years with increasing number of weight-promoting medications.
BMI- Body Mass Index
WC- Waist Circumference
In Table 2, we present medications associated with BMI changes at 3 years by medication groups and stratifying by BMI classes. In each model, the specific drug taken, which represents the exposure, is defined as a categorical variable with three levels: non-user, intermittent user, persistent user. We observed that the effect of taking a particular drug led to weight variation which increased or decreased with the intensity of its use and with the severity of BMI class. As such, the regression coefficient is thus interpretable as a trend effect. Those who took either antidepressants or insulin were most likely to have a significant increase in BMI whereas patients on glucocorticosteroids lost weight over the same time course. Overall women on anti-depressants had a 0.14 BMI unit increase over 3 years, while women with class I (mild) obesity showed 0.25 BMI unit increase. In those taking a combination of antidepressants and beta blockers versus non-users, a 0.25 BMI unit increase was observed, while in women with overweight, a 0.39 BMI unit increase was reported. Overall women on OTC insulin had 0.21 BMI unit increase over 3 years of follow up, while those with normal weight status, overweight, class II (moderate) Obesity, and Class III (severe) obesity had an increase in BMI of 0.39, 0.32, 036, 1.87, respectively. Overall beta blockers in combination with glucocorticosteroids, was associated with 0.40 BMI unit decrease while in women with overweight and obesity the decrease was respectively, 0.7 and 1.51 BMI units.
Table 2.
Medications Associated to BMI Changes at 3 years follow-up
| Medications | All Women in Sample |
Underweight | Normal Weight | Overweight | Class I Obesity | Class II Obesity |
Class III Obesity |
|---|---|---|---|---|---|---|---|
| N=74,291 | N=910 | N=31,282 | N=26,031 | N=11,554 | N=4,104 | N=2,371 | |
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Antidepressants | 0.14§ (0.02) | 0.37 (0.2) | 0.14§ (0.03) | 0.21§ (0.03) | 0.25§ (0.05) | 0.19* (0.09) | 0.66 (0.34) |
| Antipsychotics | 0.27 (0.3) | NA | 0.62 (0.43) | 0.09 (0.34) | 0.46 (0.97) | 0.44 (0.95) | 1.55 (3.49) |
| Beta Blockers | 0 (0.02) | 0.19 (0.18) | 0.02 (0.03) | 0.05* (0.03) | 0.02 (0.04) | 0.02 (0.08) | 0.42 (0.33) |
| OTC Insulin | 0.21▯ (0.06) | −1.08 (0.88) | 0.39▯ (0.12) | 0.32▯ (0.09) | 0.18 (0.11) | 0.36* (0.14) | 1.87§ (0.53) |
| Glucocorticosteroids | −0.15† (0.05) | −0.43 (0.33) | 0.01 (0.07) | −0.18* (0.07) | −0.35† (0.11) | −0.15 (0.22) | 0.28 (0.93) |
| Most frequent combinations | All Women in Sample |
Underweight | Normal Weight | Overweight | Class I Obesity | Class II Obesity |
Class III Obesity |
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Antidep & Antipsych | 0.37 (0.48) | NA | 0.12 (0.54) | 0.51 (0.56) | −0.03 (2.56) | 0.22 (1.51) | NA |
| Antidep & BetaBlock | 0.25▯ (0.07) | 0.33 (0.83) | 0.29† (0.1) | 0.39§ (0.09) | 0.17 (0.14) | −0.05 (0.25) | 1.32 (0.95) |
| Antidep & Insulin | 0.05 (0.21) | −2.32 (2.5) | 1.22 (0.72) | 0.86† (0.42) | 0.16 (0.35) | 0.05 (0.39) | 1.37 (1.58) |
| Antidep & Glucocort | 0.15 (0.17) | −0.34 (0.95) | 0.58† (0.24) | −0.14 (0.21) | −0.05 (0.32) | −0.13 (0.84) | 2.33 (2.85) |
| BetaBlock & Insulin | 0.26 (0.21) | NA | −0.53 (0.47) | 0.38 (0.29) | 0.55 (0.33) | 0.3 (0.45) | 1.92 (2.74) |
| BetaBlock & Glucocort | −0.4* (0.2) | 1.49 (1.25) | −0.21 (0.23) | −0.7† (0.32) | −0.38 (0.41) | −1.51† (0.62) | 2.82 (9.87) |
| Insulin & Glucocort | −0.57 (0.63) | NA | 0.74 (1.14) | −0.97 (1.01) | 1.45 (2.56) | −1.09 (1.23) | 1.47 (4.03) |
P<0.05
P<0.01
P<0.001
P<0.0001
borderline 0.05
P values refer to linear regressions run overall and stratifying by BMI class
BMI Body Mass Index
OTC Over The Counter
Differences in BMI stratified by race/ethnicity are shown in Supplemental Table 6. Non-Hispanic White women were more likely to have an increase in BMI than other groups for all medications except antidepressants which were associated to a higher BMI increase in the Hispanic group.
Overall women on anti-depressants had 0.47 cm WC increase at 3 years follow up, while with with class I (mild) obesity showed 0.63 cm WC increase. Overall women on OTC insulin had 0.4 cm WC increase over 3 years of follow up, while those with normal weight and Class III (severe) obesity increased respectively by 0.96 and 1.24 cm. In those taking a combination of antidepressants and beta blockers versus non-users a 0.51 cm WC increase was observed, while in women with overweight and class I (mild) obesity 0.78 and 1.11 cm WC increase, respectively. (Table 3).
Table 3.
Medications Associated to WC (cm) changes at 3 years follow-up
| Medications | All Women in Sample |
Underweight | Normal Weight | Overweight | Class I Obesity | Class II Obesity |
Class III Obesity |
|---|---|---|---|---|---|---|---|
| N=76,579 | N=906 | N=31,096 | N=25,876 | N=11,492 | N=4,073 | N=2,354 | |
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Antidepressants | 0.47§ (0.05) | 0.57 (0.47) | 0.48§ (0.08) | 0.52§ (0.09) | 0.63§ (0.14) | 0.18 (0.24) | 0.42 (0.35) |
| Antipsychotics | 1.14 (0.69) | NA | 0.44 (1.16) | 0.01 (1.02) | 2.71 (2.86) | 4.21 (2.65) | 5.21 (3.4) |
| Beta Blockers | −0.05 (0.05) | 0.35 (0.42) | 0.01 (0.07) | −0.04 (0.08) | −0.05 (0.12) | −0.04 (0.22) | 0.15 (0.34) |
| OTC Insulin | 0.4† (0.14) | −1.35 (2.04) | 0.96† (0.32) | 0.38 (0.28) | 0.32 (0.33) | 0.39 (0.4) | 1.24* (0.56) |
| Glucocorticosteroids | −0.07 (0.13) | −0.3 (0.79) | 0.26 (0.19) | −0.19 (0.21) | −0.31 (0.34) | −0.11 (0.6) | −0.44 (0.98) |
| Most frequent combinations | All Women in Sample |
Underweight | Normal Weight | Overweight | Class I Obesity | Class II Obesity |
Class III Obesity |
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Antidep & Antipsych | 0.89 (1.11) | NA | −0.05 (1.45) | −0.63 (1.71) | 5.89 (7.56) | 9.63* (4.19) | NA |
| Antidep & BetaBlock | 0.51† (0.17) | 1.12 (1.92) | 0.54 (0.29) | 0.78† (0.28) | 1.11† (0.41) | −0.99 (0.69) | −0.26 (0.98) |
| Antidep & Insulin | 0.29 (0.5) | −1.95 (5.75) | 3.96* (1.86) | 0.94 (1.27) | 0.66 (1.02) | −0.02 (1.09) | −0.07 (1.64) |
| Antidep & Glucocort | 0.24 (0.4) | −0.97 (2.18) | 0.18 (0.63) | 0.08 (0.65) | −0.13 (0.95) | 4.83* (2.32) | 0.34 (2.95) |
| BetaBlock & Insulin | −0.17 (0.48) | NA | −1.13 (1.28) | 0.56 (0.87) | 0.93 (0.97) | −1.05 (1.25) | −1.58 (2.83) |
| BetaBlock & Glucocort | −0.35 (0.47) | 4.13 (2.87) | 1.03 (0.61) | −1.66 (1.01) | −1.3 (1.2) | −3.06 (1.71) | NA |
| Insulin & Glucocort | −0.34 (1.51) | NA | 2.97 (3.08) | −1.47 (3.06) | −1.81 (7.56) | −2.08 (3.42) | 1.71 (4.56) |
P<0.05
P<0.01
P<0.001
P<0.0001
borderline 0.05
P values refer to linear regressions run overall and stratifying by BMI class
WC Waist Circumference
OTC Over The Counter
P values refer to linear regressions run overall and stratifying by BMI class
Differences in waist circumference stratified by race/ethnicity are shown in Supplemental Table 7, women of Hispanic origin were more likely to have an increase in waist circumference than other groups when taking antidepressants. Similarly, Asian/ Pacific Islanders were most likely to have an increase in waist circumference if they were taking insulin. African-American women showed the highest increase in WC if taking antipsychotics. In Non-Hispanic White women, there was a greater increase in WC than other groups when taking Beta blockers. Antidepressant and antipsychotic combinations were most likely to be associated with an increase in waist circumference, especially in African-American women. Hispanic women were most likely to have weight gain when taking a combination of anti-depressants and glucocorticosteroids.
Even after adjusting for baseline health conditions and other covariates (physical activity, smoking, sleep medications, diet), participants who were on anti-depressants had a higher likelihood of an increase in BMI or WC, and also the associations with other drugs remained unchanged (Table 4,5 and Supplemental Tables 8 & 9)). The higher the number of putative weight promoting medications, the greater the increase in BMI, adjusting for other covariates (Figure 1).
Table 4.
Multiple regression models investigating factors associated to BMI Changes
| Model | All Women in Sample n=73,525 |
Underweight | Normal Weight |
Overweight | Class I Obesity | Class II Obesity |
Class III Obesity |
|---|---|---|---|---|---|---|---|
| N=72291 | N=850 | N=29,849 | N=24,640 | N=10,891 | N=3,854 | N=2,207 | |
| Antidepressants | 0.17 - 0.0001 | 0.43 - 0.043 | 0.12 - 0.0001 | 0.23 - 0.0001 | 0.28 - 0.0001 | 0.27 - 0.002 | 0.63 - 0.073 |
| Antipsychotics | 0.3 - 0.32 | NA | 0.57 - 0.199 | 0.23 - 0.501 | 0.44 - 0.645 | 0.39 - 0.685 | 1.93 - 0.57 |
| Betablockers | 0.02 - 0.311 | 0.18 - 0.378 | 0.02 - 0.47 | 0.04 - 0.159 | 0.04 - 0.357 | 0.01 - 0.866 | −0.26 - 0.451 |
| OTC Insulin | 0.44 - 0.0001 | −1.11 - 0.274 | 0.34 - 0.008 | 0.38 - 0.0001 | 0.34 - 0.008 | 0.79 - 0.0001 | 1.29 - 0.04 |
| Glucocorticosteroids | −0.13 - 0.017 | −0.66 - 0.07 | 0 - 0.993 | −0.18 - 0.015 | −0.31 - 0.007 | −0.12 - 0.608 | −0.18 - 0.847 |
Each row represents a model which main regressor is a weight promoting drug class, as each column represent a stratus by BMI class (except for the first column that represents all sample). All models are adjusted for baseline health conditions as diabetes, hypertension and hypercholesterolemia, physical activity, sleep medications, smoking and diet.
Values represent regression coefficients along with p-values (β - p value). Regression coefficients show the average change in BMI observed moving from non-user to intermittent user to persistent user relatively to the specific drug. Regression coefficients of covariates are shown in table S6.
Table 5.
Multiple regression models investigating factors associated to WC Changes
| Model | All Women in Sample n=73,857 |
Underweight | Normal Weight |
Overweight | Class I Obesity | Class II Obesity |
Class III Obesity |
|---|---|---|---|---|---|---|---|
| Antidepressants | 0.48 - 0.0001 | 0.5 - 0.328 | 0.44 - 0.0001 | 0.52 - 0.0001 | 0.72 - 0.0001 | 0.26 - 0.299 | 0.34 - 0.358 |
| Antipsychotics | 1.16 - 0.1 | NA | 0.02 - 0.985 | 0.22 - 0.828 | 2.77 - 0.332 | 4.17 - 0.118 | 4.84 - 0.161 |
| Betablockers | 0 - 0.97 | 0.25 - 0.608 | 0.04 - 0.629 | −0.02 - 0.826 | −0.02 - 0.852 | −0.1 - 0.683 | 0.17 - 0.642 |
| OTC Insulin | 0.75 - 0.0001 | −0.5 - 0.837 | 0.81 - 0.023 | 0.23 - 0.482 | 0.97 - 0.011 | 1.31 - 0.007 | 1.17 - 0.09 |
| Glucocorticosteroids | −0.01 - 0.962 | −0.61 - 0.48 | 0.32 - 0.104 | −0.12 - 0.575 | −0.25 - 0.465 | −0.22 - 0.727 | −0.67 - 0.522 |
Each row represents a model which main regressor is a weight promoting drug class, as each column represent a stratus by BMI class (except for the first column that represents all sample). All models are adjusted for baseline health conditions as diabetes, hypertension and hypercholesterolemia, physical activity, sleep medications, smoking and diet.
Values represent regression coefficients along with p-values (β - p value). Regression coefficients show the average change in WC observed moving from non-user to intermittent user to persistent user relatively to the specific drug. Regression coefficients of covariates are shown in table S5.
Discussion
Use of several classes of putative weight promoting medications was associated with an increase in BMI and waist circumference over 3 years among post-menopausal women in the WHI cohort. The primary classes of medications associated with weight gain were antidepressants, anti-hypertensives, and insulin.35 Study participants who took either antidepressants or those with diabetes who took insulin were most likely to have greater increases in BMI. It is important to note that these statistically significant BMI and WC changes likely demonstrate clinical significance. There has been a steady increase in BMI and WC over time in developed and developing countries throughout the world, but the role of putative weight promoting medications in this epidemic is largely overlooked. In the clinical setting, health care providers may address diet quality and/or physical activity, but there is rarely a focus on whether a patient’s previous or current medications have led to weight gain. As such, strategies to mitigate medication induced weight gain are not incorporated into clinical care.
Despite adjusting for factors usually known to be associated with BMI and WC, such as physical activity, diet quality, sleep duration, smoking status, several putative weight-promoting medications were associated with weight gain over 3 years. The data highlight that, when weight promoting medications are prescribed, it may be helpful to encourage regular physical activity, adequate sleep, and attention to diet quality.36-38 Yet, it is important to note that even with positive lifestyle interventions, medications may still lead to weight change. Antidepressants have a well-documented history of numerous side effects, particularly weight gain. 39, 40 Older generation antidepressants like tricyclics (TCA) have been shown to cause an increase in BMI and WC. In a study by Berken and colleagues, low doses of the TCAs amitriptyline, nortriptyline and imipramine were correlated with weight gain of up to 2 lbs. per month.41 Newer antidepressants, namely citalopram, escitalopram, sertraline, paroxetine, venlafaxine, duloxetine, and mirtazapine have also been associated with significant increases in body weight.18 There has been a 400% rise in prescriptions for antidepressants since 1988 with a concomitant rise in adult and pediatric obesity since 1980, and it has been postulated that the increase in antidepressants is a contributor to the obesity epidemic. The hypothalamic-pituitary-adrenal (HPA) axis is activated in stress, dysregulated in obesity and metabolic syndrome, and it shares a common pathophysiologic pathway with major depressive disorder (MDD)9; how antidepressants modify these pathways and exacerbate weight gain is largely unknown. When a patient has a predisposition to weight gain and would benefit from an anti-depressant, bupropion is an agent which has been more likely to demonstrate weight loss than weight gain,42 and it also currently prescribed in combination with naltrexone as a FDA approved weight loss medication in the US.43
Medication-related weight gain may affect patient medication compliance with these medications.44 Patients taking antihypertensive drugs may experience weight gain through multiple pathways, including direct obesogenic effects of medications or other mechanisms. For example, beta-blockers may reduce total energy expenditure by 5% to 10% which corresponds to a reduction of 100 to 200 kcal/d in utilized energy 45, 46, and fatigue and reduced exercise intolerance may exacerbate the weight gain. Randomized, controlled studies in patients with or at increased risk for cardiovascular disease have also found a correlation between beta blocker use and risk of diabetes mellitus. In patients with abdominal obesity, the use of beta blockers impaired fasting glucose and increased triglycerides, which may contribute to the risk of cardiovascular disease.6, 47 If beta blockers are an essential medication for a patient, the patient would likely have less weight gain on carvedilol when compared to other medications in this class.48
Type of medication, dosage, and race/ethnicity may have important interrelationships. In our study, non-Hispanic White women were most likely to be prescribed anti-depressants and beta-blockers. African-Americans were more likely to have diabetes and be on insulin and/or glucocorticosteroids. Previous studies have shown that African Americans, in addition to Native American, Pacific Islander, and Hispanic patients, are more likely to have weight gain when taking weight promoting medications. 49 Excess adiposity, particularly in type 2 diabetes is associated with insulin resistance, which promotes dyslipidemia and beta-cell dysfunction, further exacerbating insulin resistance and weight gain. In order to mitigate the likelihood of weight gain, other medications within the medication classes studied which are less likely to promote weight gain should be considered. In the United Kingdom Prospective Diabetes Study (UKPDS), patients in the insulin-treated intensive intervention cohort gained an average of 6.5kg. Within that group, weight gain was even higher in patients who were more than 120% of their ideal body weight at baseline.14 In addition to the aforementioned health issues, medications might influence food preferences (towards high energy dense foods),and lead to increased sugary beverage consumption due to dry mouth, increased hunger and/or delayed satiety with concomitant increased food intake, down regulation of body fat stores, fatigue (resulting in decreased exercise tolerance and lower physical activity levels), and reductions in resting metabolic rate and diet-induced thermogenesis. Weight gain associated with insulin treatment is almost exclusively due to an increase in fat mass consequent to decrease 24-hour energy expenditure 50 and the same has been observed during glucocorticosteroid therapy 40 with a typical increase in truncal fat stores. Many of the medications associated with weight gain are prescribed for chronic conditions and are often taken in combination.
We found some racial differences in these relationships. With anti-depressants and antipsychotics in combination, African-American women were most likely to have an increase in waist circumference. Hispanic women were most likely to have weight gain on a combination of anti-depressants and glucocorticosteroids. However, these findings should be interpreted cautiously due to multiple comparisons and they may be due to chance. Individual susceptibility and genetic predisposition to weight gain may be contributory 51. Options to mitigate the weight gain may include proactive lifestyle modifications, reduction in dose, change to another agent, or discontinuation of the medication altogether. If alternative medications are not an option, lifestyle factors such as diet quality, physical activity level, and sleep quality and duration warrant emphasis.
This study has important strengths and limitations. The strengths include prospective follow up of a large cohort, inclusion of a racially and ethnically diverse sample, use of medication inventories, and measured weights and waist circumference at baseline and 3 years. However, limitations also warrant consideration. We have limited information regarding the indications for the prescription of some of the medications studied, and the underlying health condition may confound the results. Some disorders, such as major depressive disorder, are associated with higher rates of obesity, and depression severity might increase the risk of overweight and obesity. While antidepressant use may contribute to overweight/obesity, it cannot be ruled out that participants using antidepressants have a more severe form of depression than those not using antidepressants. However, information about depression was not collected routinely in the cohort. Similarly, concomitant treatment with other psychotropics such as antipsychotics could exacerbate weight gain but may also signal a more severe form of illness or different diagnoses altogether, such as bipolar disorder or a psychotic disorder. Without controlling for the specific diagnosis and its severity, it is not possible to directly attribute risk to the medication alone. Finally, there does appear to be an increased risk of obesity with psychiatric disorders themselves, and this is complicated/compounded by many of the medications.
Conclusions
Many medications prescribed for hypertension, diabetes, depression, and/or mental conditions are associated with unintentional weight gain. Post-menopausal women, a group with a high prevalence of overweight and/or obesity, are already at risk for an increase in BMI and WC over time. Racial and ethnic minority women, groups with a higher weight status at baseline, also may be particularly susceptible to weight gain associated with the use of prescription medications. Before patients are prescribed weight promoting medications, clinicians should be vigilant about whether the medication is necessary, whether alternative agents are available, and utilize only the lowest dose necessary to achieve the desired effect. Information about weight-promoting medications may help to inform clinical decision making and support increased attention to lifestyle modifications and other strategies to mitigate these side effects.
Supplementary Material
Supplemental Table 6. BMI changes associated with medications and medication combinations in various subgroups at 3 years of follow-up
Supplemental Table 7. Waist circumference changes (cm) associated with medications and medication combinations in various subgroups
Supplemental Table 8. Covariates of multiple regression models investigating factors associated to BMI Changes
Supplemental Table 9. Covariates of multiple regression models investigating factors associated to WC Changes
Supplemental Table 2. Distribution of Baseline Characteristics in Study Sample by Weight Class
Supplemental Table 3: Baseline Weight Promoting Medication Utilization by Weight Class
Supplemental Table 1. Distribution of Baseline Characteristics in Study Sample by Race/Ethnicity
Supplemental Table 5: Baseline Weight Promoting Medication Utilization by Race/Ethnicity
Supplemental Table 4: Baseline Weight Promoting Medications Combinations by Weight Class
Primary Funding Sources:
National Institutes of Health and Massachusetts General Hospital Executive Committee on Research (ECOR)(FCS), National Institutes of Health NIDDK P30 DK040561 (FCS) and L30 DK118710 (FCS)
Footnotes
Conflicts of Interest: No conflicts of interest.
References
- 1.Fruh SM. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract 2017; 29(S1): S3–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA 2018; 319(16): 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level Among Adults in the United States, 2013-2016. JAMA 2018; 319(23): 2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 2015; 402: 113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapoor E, Collazo-Clavell ML, Faubion SS. Weight Gain in Women at Midlife: A Concise Review of the Pathophysiology and Strategies for Management. Mayo Clin Proc 2017; 92(10): 1552–1558. [DOI] [PubMed] [Google Scholar]
- 6.Cooper-DeHoff RM, Wen S, Beitelshees AL, Zineh I, Gums JG, Turner ST et al. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension 2010; 55(1): 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randhawa AK, Parikh JS, Kuk JL. Trends in medication use by body mass index and age between 1988 and 2012 in the United States. PLoS One 2017; 12(9): e0184089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kivimaki M, Hamer M, Batty GD, Geddes JR, Tabak AG, Pentti J et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care 2010; 33(12): 2611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SH, Paz-Filho G, Mastronardi C, Licinio J, Wong ML. Is increased antidepressant exposure a contributory factor to the obesity pandemic? Transl Psychiatry 2016; 6: e759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastronardi C, Paz-Filho GJ, Valdez E, Maestre-Mesa J, Licinio J, Wong ML. Long-term body weight outcomes of antidepressant-environment interactions. Mol Psychiatry 2011; 16(3): 265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patten SB, Williams JV, Lavorato DH, Brown L, McLaren L, Eliasziw M. Major depression, antidepressant medication and the risk of obesity. Psychother Psychosom 2009; 78(3): 182–6. [DOI] [PubMed] [Google Scholar]
- 12.Ratliff JC, Barber JA, Palmese LB, Reutenauer EL, Tek C. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring) 2010; 18(12): 2398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravindran PP, Zang W, Renukunta S, Mansour R, Denduluri S. Effect of comedication of bupropion and other antidepressants on body mass index. Ther Adv Psychopharmacol 2015; 5(3): 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes Metab 2007; 9(6): 799–812. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int Clin Psychopharmacol 2014; 29(4): 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stimpel M, Koch B, Weber MA. Comparison between moexipril and atenolol in obese postmenopausal women with hypertension. Maturitas 1998; 30(1): 69–77. [DOI] [PubMed] [Google Scholar]
- 17.Toups MS, Myers AK, Wisniewski SR, Kurian B, Morris DW, Rush AJ et al. Relationship between obesity and depression: characteristics and treatment outcomes with antidepressant medication. Psychosom Med 2013; 75(9): 863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uguz F, Sahingoz M, Gungor B, Aksoy F, Askin R. Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatry 2015; 37(1): 46–8. [DOI] [PubMed] [Google Scholar]
- 19.Gates ML, Wilkins T, Ferguson E, Walker V, Bradford RK, Yoo W. Gender and race disparities in weight gain among offenders prescribed antidepressant and antipsychotic medications. Health Justice 2016; 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bet PM, Hugtenburg JG, Penninx BW, Hoogendijk WJ. Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol 2013; 23(11): 1443–51. [DOI] [PubMed] [Google Scholar]
- 21.Grundy A, Cotterchio M, Kirsh VA, Kreiger N. Associations between anxiety, depression, antidepressant medication, obesity and weight gain among Canadian women. PLoS One 2014; 9(6): e99780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keers R, Aitchison KJ. Gender differences in antidepressant drug response. Int Rev Psychiatry 2010; 22(5): 485–500. [DOI] [PubMed] [Google Scholar]
- 23.Noordam R, Aarts N, Tiemeier H, Hofman A, Stricker BH, Visser LE. Sex-specific association between antidepressant use and body weight in a population-based study in older adults. J Clin Psychiatry 2015; 76(6): e745–51. [DOI] [PubMed] [Google Scholar]
- 24.(CDC) CfDCaP. Adult Obesity Facts. In, 2019. [Google Scholar]
- 25.Library UKPHoC. Obesity Statistics. In, 2019. [Google Scholar]
- 26.Canada S. Overweight and Obese Adults, 2018. In, 2018. [Google Scholar]
- 27.(WHO) WHO. Nutrition, Physical Activity, and Obesity- The Netherlands. In, 2013. [Google Scholar]
- 28.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women's Health Initiative Randomized Clinical Trials. JAMA Oncol 2015; 1(5): 611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naufel MF, Frange C, Andersen ML, Girao M, Tufik S, Beraldi Ribeiro E et al. Association between obesity and sleep disorders in postmenopausal women. Menopause 2018; 25(2): 139–144. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, Cui J, Wang A, Mu Y, Yan Y, Liu F et al. Association Between Age at Natural Menopause and Risk of Type 2 Diabetes in Postmenopausal Women With and Without Obesity. J Clin Endocrinol Metab 2019; 104(7): 3039–3048. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Liu B, Snetselaar LG, Wallace RB, Caan BJ, Rohan TE et al. Association of Normal-Weight Central Obesity With All-Cause and Cause-Specific Mortality Among Postmenopausal Women. JAMA Netw Open 2019; 2(7): e197337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials 1998; 19(1): 61–109. [DOI] [PubMed] [Google Scholar]
- 33.Blumenthal SR, Castro VM, Clements CC, Rosenfield HR, Murphy SN, Fava M et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry 2014; 71(8): 889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domecq JP, Prutsky G, Wang Z, Elraiyah T, Brito JP, Mauck K et al. Drugs commonly associated with weight change: umbrella systematic review and meta-analysis (Protocol). Syst Rev 2012; 1: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ness-Abramof R, Apovian CM. Drug-induced weight gain. Drugs Today (Barc) 2005; 41(8): 547–55. [DOI] [PubMed] [Google Scholar]
- 36.Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet 2016; 387(10031): 1947–56. [DOI] [PubMed] [Google Scholar]
- 37.Davison KM. The relationships among psychiatric medications, eating behaviors, and weight. Eat Behav 2013; 14(2): 187–91. [DOI] [PubMed] [Google Scholar]
- 38.Henderson DC. Weight gain with atypical antipsychotics: evidence and insights. J Clin Psychiatry 2007; 68 Suppl 12: 18–26. [PubMed] [Google Scholar]
- 39.Fernstrom MH. Drugs that cause weight gain. Obes Res 1995; 3 Suppl 4: 435S–439S. [DOI] [PubMed] [Google Scholar]
- 40.Pijl H, Meinders AE. Bodyweight change as an adverse effect of drug treatment. Mechanisms and management. Drug Saf 1996; 14(5): 329–42. [DOI] [PubMed] [Google Scholar]
- 41.Berken GH, Weinstein DO, Stern WC. Weight gain. A side-effect of tricyclic antidepressants. J Affect Disord 1984; 7(2): 133–8. [DOI] [PubMed] [Google Scholar]
- 42.Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol 2016; 6(2): 99–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010; 376(9741): 595–605. [DOI] [PubMed] [Google Scholar]
- 44.Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat 2017; 13: 2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ripley TL, Saseen JJ. beta-blockers: a review of their pharmacological and physiological diversity in hypertension. Ann Pharmacother 2014; 48(6): 723–33. [DOI] [PubMed] [Google Scholar]
- 46.Sharma AM, Pischon T, Hardt S, Kunz I, Luft FC. Hypothesis: Beta-adrenergic receptor blockers and weight gain: A systematic analysis. Hypertension 2001; 37(2): 250–4. [DOI] [PubMed] [Google Scholar]
- 47.Cooper-Dehoff RM, Hou W, Weng L, Baillie RA, Beitelshees AL, Gong Y et al. Is diabetes mellitus-linked amino acid signature associated with beta-blocker-induced impaired fasting glucose? Circ Cardiovasc Genet 2014; 7(2): 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pischon T, Sharma AM. Use of beta-blockers in obesity hypertension: potential role of weight gain. Obes Rev 2001; 2(4): 275–80. [DOI] [PubMed] [Google Scholar]
- 49.Feldstein AC, Nichols GA, Smith DH, Rosales AG, Perrin N. Weight change and glycemic control after diagnosis of type 2 diabetes. J Gen Intern Med 2008; 23(9): 1339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlson MG, Campbell PJ. Intensive insulin therapy and weight gain in IDDM. Diabetes 1993; 42(12): 1700–7. [DOI] [PubMed] [Google Scholar]
- 51.Alomar MJ. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm J 2014; 22(2): 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 6. BMI changes associated with medications and medication combinations in various subgroups at 3 years of follow-up
Supplemental Table 7. Waist circumference changes (cm) associated with medications and medication combinations in various subgroups
Supplemental Table 8. Covariates of multiple regression models investigating factors associated to BMI Changes
Supplemental Table 9. Covariates of multiple regression models investigating factors associated to WC Changes
Supplemental Table 2. Distribution of Baseline Characteristics in Study Sample by Weight Class
Supplemental Table 3: Baseline Weight Promoting Medication Utilization by Weight Class
Supplemental Table 1. Distribution of Baseline Characteristics in Study Sample by Race/Ethnicity
Supplemental Table 5: Baseline Weight Promoting Medication Utilization by Race/Ethnicity
Supplemental Table 4: Baseline Weight Promoting Medications Combinations by Weight Class