Abstract
Most neurodegenerative diseases are characterized by the intracellular or extracellular aggregation of misfolded proteins such as amyloid-β and tau in Alzheimer disease, α-synuclein in Parkinson disease, and TAR DNA-binding protein 43 in amyotrophic lateral sclerosis. Accumulating evidence from both human studies and disease models indicates that intercellular transmission and the subsequent templated amplification of these misfolded proteins are involved in the onset and progression of various neurodegenerative diseases. The misfolded proteins that are transferred between cells are referred to as ‘pathological seeds’. Recent studies have made exciting progress in identifying the characteristics of different pathological seeds, particularly those isolated from diseased brains. Advances have also been made in our understanding of the molecular mechanisms that regulate the transmission process, and the influence of the host cell on the conformation and properties of pathological seeds. The aim of this Review is to summarize our current knowledge of the cell-to-cell transmission of pathological proteins and to identify key questions for future investigation.
The term ‘neurodegenerative disease’ encompasses a large group of conditions that are clinically and pathologically diverse, the majority of which are characterized by the accumulation of misfolded proteins into insoluble aggregates (or inclusions) in the CNS accompanied by a progressive loss of neurons in the affected regions. The protein aggregates involved vary between diseases, for example, amyloid-β (Aβ) and tau aggregates in Alzheimer disease (AD)1,2, misfolded α-synuclein in Parkinson disease (PD)3, TAR DNA-binding protein 43 (TDP43) and superoxide dismutase 1 (SOD1) pathology in amyotrophic lateral sclerosis4, and mutated huntingtin (HTT) in Huntington disease5. Numerous studies have explored the toxic effects of these protein aggregates on the CNS and have investigated the molecular mechanisms underlying the resulting neuronal dysfunction6–9. The findings of these studies have highlighted the crucial role of misfolded proteins in the aetiology and pathogenesis of neurodegenerative diseases.
Interestingly, the spatial distribution of pathological proteins in diseased brains follows stereotypical patterns10,11, which were historically attributed to differences in the vulnerability of the subtypes of neurons in different brain regions12. However, over the past decade, studies using post-mortem brain tissue and various animal and cell models have suggested that many neurodegenerative disease-related pathological proteins undergo cell-to-cell transmission. Following transmission to the recipient cell, pathological proteins act as templates to induce their normal endogenous counterpart protein to misfold, leading to the amplification of the pathological protein conformation13–16, known as ‘templated amplification’. The intercellular transmission and templated amplification of these ‘pathological seeds’ might lead to the spreading of pathological protein aggregates along neuronal networks, which could explain the stereotypical distribution of protein pathology in the brain. Multiple studies have now investigated the nature of these pathological seeds and the mechanisms that modulate the transmission process. For example, studies using post-mortem brain tissue from individuals with neurological disease have shown that different pathological seeds can have unique properties and conformations. The aim of this Review is to summarize the evidence supporting the transmission hypothesis and to discuss the latest progress in this field, particularly regarding our understanding of the cell-to-cell transmission of α-synuclein, Aβ and tau. We also identify key questions for future study.
Evidence of protein transmission
Stereotypical distribution of protein aggregates in diseased brains.
The distribution of pathological protein in the brains of individuals with neurodegenerative disease follows highly predictable spatiotemporal patterns. For example, in individuals with PD, α-synuclein pathology is first found in the olfactory bulb and the dorsal motor nucleus of the glossopharyngeal and vagal nerves. This pathology then spreads in a rostral direction from the brainstem to the midbrain and forebrain, eventually reaching the cerebral cortex11,17. In individuals with AD, pathological tau first appears in the locus coeruleus and transentorhinal cortex, and then spreads to the entorhinal and hippocampal regions, followed by the basal temporal cortex and the insular cortex. In the later stages of AD, tau pathology can be found throughout the neocortex10,18,19. Interestingly, the distribution of Aβ pathology in the brains of individuals with AD follows a different pattern to the distribution of tau. Aβ plaques first develop in the orbitofrontal neocortex and basal temporal cortex, and then spread throughout the neocortex before finally reaching the hippocampus, midbrain, brainstem and cerebellum10,20,21.
Historically, the stereotypical distribution of pathological proteins in the CNS was thought to result from differences in vulnerability between brain regions12 or the progressive spreading of pro-inflammatory cytokines22. For example, neurons that are more vulnerable to α-synuclein pathology in individuals with PD tend to have highly branched axons, slow tonic activity and low levels of Ca2+-buffering proteins12. However, these features do not fully explain the distribution pattern of α-synuclein pathology in diseased brains. During the past decade, multiple studies, which are discussed later in this Review, have demonstrated that templated amplification and dissemination of various pathological proteins can occur in animal and cell models. These observations support the view that this stereotypical involvement of different brain regions is the result of the spreading of pathological proteins between anatomically connected brain areas23.
However, the transmission hypothesis has several limitations. First, the post-mortem studies that identified the stereotypical distribution of pathology are limited by a lack of longitudinal data and therefore do not provide direct evidence of sequential evolvement of different brain regions during disease progression. Nevertheless, PET imaging with ligands for specific pathological proteins has now been used to visualize changes in tau and Aβ pathology over time in the same patients, confirming the major conclusions of post-mortem studies24,25. Second, the stereotypical spreading pattern is not observed in all patients12, indicating that the factors affecting the distribution of pathology can vary between individuals. Finally, some of the brain regions that are anatomically connected to areas containing pathological proteins do not develop pathology and the spreading of pathological proteins is not proportional to the strength of synaptic connections12. Therefore, the selective vulnerability of different neuronal populations could be a crucial modifier of the transmission process12. This selective vulnerability could result from differences in the release or uptake of pathological seeds or in the intracellular environment that modulates the templated amplification process.
The pathological seeds responsible for transmission might not exist in the form of mature aggregates that can be easily detected through histopathological methods. Therefore, to complement traditional histopathological studies, other studies have also been performed to map the seeding activity of pathological proteins isolated from different brain regions. In these studies, potential pathological seeds were isolated from different brain regions and their ability to seed protein aggregation was tested in reporter cell lines26,27. For example, in one study, preparations from brain regions free of tau deposition, including regions that are usually affected further along the Braak staging pathway, could induce tau aggregation in a reporter cell line26. Using similar methods, the origin of tau seeding activity was mapped to the transentorhinal and entorhinal cortices27.
Transplantation.
Strong evidence for the transmission hypothesis comes from studies of patients who have received transplants of human fetal brain-derived cells as a therapy for PD. In these patients, α-synuclein aggregates were observed in the grafted cells, indicating transmission from the host to the graft28,29. More recently, intracerebral deposition of Aβ was found in individuals with iatrogenic Creutzfeldt–Jakob disease (CJD) caused by human-derived growth hormone treatment or dura mater grafts30–34. Intracerebral Aβ deposition was also observed in individuals who had received neuronal grafts but who died from causes other than CJD31,35, indicating that Aβ seeds in transplanted materials could induce Aβ pathology regardless of the existence of CJD pathology. Tau has been detected in several batches of cadaver-derived human growth hormone, but substantial amounts of pathological tau were not identified in individuals with iatrogenic CJD36.
Disease models.
Numerous studies have shown that pathological seeds generated from recombinant proteins, animal models or diseased brains can induce the development of protein pathology in various in vitro and animal models (TABLE 1). Injection of α-synuclein preformed fibrils (misfolded α-synuclein generated from recombinant α-synuclein monomers) or pathological α-synuclein isolated from transgenic mice or human brains, promoted the development of α-synuclein pathology in M83 transgenic mice, which express a familial PD-associated mutant form of human α-synuclein37–39. Importantly, α-synuclein preformed fibrils as well as pathological α-synuclein derived from diseased brains also induced α-synuclein pathology in wild-type mice15,40 and in primary neuronal cultures derived from wild-type mice16,41. Most instances of α-synucleinopathy are sporadic and are not the result of a mutation or amplification of SNCA (the gene encoding α-synuclein). Therefore, induction of α-synuclein aggregation in non-transgenic mice is an important observation that indicates the potential for transmission of α-synuclein in individuals with sporadic PD. Moreover, α-synuclein aggregation in non-human primates was induced by the intracerebral injection of recombinant α-synuclein preformed fibrils or Lewy body-containing extracts from brains of individuals with PD, and the resulting pathology could be found far from the injection site42,43. In both mice and non-human primates, brain regions with α-synuclein pathology also showed neurodegeneration; pathology and neurodegeneration were particularly prominent in the dopaminergic neurons of the substantia nigra15,42,43. In addition to the seeding models described above, the transmission of α-synuclein has also been explored using virus-mediated selective expression of human α-synuclein in the medullary neurons of rats44. In this model, the exogenous α-synuclein protein produced by the medullary neurons was observed to spread in a caudal direction to brain regions such as the pons, midbrain and forebrain.
Table 1 |.
The transmissibility of non-prion protein aggregates
| Protein | Evidence of transmission in humans | Evidence of transmission in experimental models | ||||||
|---|---|---|---|---|---|---|---|---|
| Type of seed | In vitro | In vivo | ||||||
| Non-neuronal cells | WT neurons | Intracerebral injection in transgenic mouse models of disease | Intracerebral injection in WT mice | Transmission to glial cells following intracerebral injection in WT mice | Peripheral injection in transgenic mouse models of disease and WT mice | |||
| α-Synuclein | Fetal mesencephalic cell transplantation28,29 | Synthetic fibrils | Yes246 | Yes16 | Yes37 | Yes15 | No41 | Yes69 |
| Mouse brain lysates | Not tested | Not tested | Yes37 | Not tested | Not tested | Not tested | ||
| Human brain lysates | Yes143 | Yes41 | Yes39 | Yes40 | No41 | Not tested | ||
| Tau | Human-derived growth hormone treatment36 | Synthetic fibrils | Yes13,247 | Yes14 | Yes248 | No14 | No14 | Not tested |
| Mouse brain lysates | Not tested | Not tested | Yes45 | Not tested | Not tested | Yes6 | ||
| Human brain lysates | Yes249 | Yes14 | Yes48 | Yes14,48 | Yes47 | Not tested | ||
| Amyloid-β | Human-derived growth hormone treatment or dura mater grafts30,31,32,33,34,35 | Synthetic fibrils | Yes8 | Not tested | Yes56 | Not tested | Not tested | Not tested |
| Mouse brain lysates | Yes8 | Not tested | Yes55 | Not tested | Not tested | Yes79,250 | ||
| Human brain lysates | Yes8 | Not tested | Yes54,55 | No54 | Not tested | Yes251 | ||
| TDP43 | None | Synthetic fibrils | Yes60 | Not tested | Not tested | Not tested | Not tested | Not tested |
| Mouse brain lysate | Not tested | Not tested | Not tested | Not tested | Not tested | Not tested | ||
| Human brain lysates | Yes61,62 | Not tested | Yes62 | Yes62 | No | Not tested | ||
TDP43, TAR DNA-binding protein 43; WT, wild-type.
Recombinant proteins.
Proteins that are artificially expressed in, and purified from, bacteria.
Cell-to-cell transmission of tau was first demonstrated with brain extracts obtained from transgenic mice that express the P301S mutant form of human tau and develop tau aggregation with age. Pathological tau-containing extracts from these mice were injected into the brains of mice that express human wild-type tau and do not naturally develop tau pathology. The induction of tau pathology was observed in multiple brain regions in the injected mice45. Synthetic tau preformed fibrils induced tau aggregation in transgenic mice that express the P301L mutant form of human tau but not in wild-type mice46. However, pathological tau derived from the brains of individuals with tauopathies, such as AD, induced tau pathology in wild-type mice14,47, demonstrating that pathological tau proteins in human diseased brains have unique conformations that are not readily recapitulated by their recombinant protein counterparts14,47–50. The transmission of pathological tau has also been demonstrated in transgenic mice that express P301L mutant tau only in layer II of the entorhinal cortex51. In these mice, the tau pathology induced by the mutant form spread to neighbouring cells in the entorhinal cortex and to connected brain regions, including the hippocampus and cingulate cortex. However, the expression pattern of P301L mutant tau in this model has been a matter of debate, as expression has also been detected in cortical regions outside of layer II of the entorhinal cortex, complicating the interpretation of the results52. Nevertheless, restricted expression of mutant tau in layers II and III of the entorhinal cortex of mice has been achieved using an adeno-associated virus-based technique; in these mice, pathological tau was transmitted to the dentate gyrus53.
Injection of synthetic Aβ fibrils or extracts from brain tissue of individuals with AD or from Aβ mouse models into the brains of transgenic mice expressing human Aβ precursor protein (APP) instigated the aggregation of Aβ peptides to form senile plaques54–56. These Aβ plaques were found in brain regions that were far from the injection site but were anatomically connected to it, suggesting that trans-synaptic transmission of Aβ seeds occurs. Interestingly, unlike transmission of α-synuclein and tau, the transmission of Aβ seeds has not been achieved in wild-type mice, which could be a result of the sequence difference between human and mouse Aβ peptides and the low Aβ expression levels in wild-type mice.
Studies have also demonstrated the cell-to-cell transmission of other pathological proteins, including SOD1 (REF.57), mutant HTT58,59 and TDP43 (REFS60–62). Interestingly, TDP43-containing brain extracts from individuals with frontotemporal dementia induced TDP43 aggregation in transgenic mice expressing mutant TPD43 as well as, to a lesser extent, in wild-type mice62.
Transmission from the peripheral nervous system to the CNS.
Some evidence supports the hypothesis that the initial misfolding of α-synuclein occurs in the enteric nervous system and spreads retrogradely to the brainstem. For example, α-synuclein pathology has been detected in the enteric nervous system63–65 and in the submandibular glands66 of individuals with PD. Furthermore, vagotomy or appendix removal has been reported to reduce the risk of PD (evidence suggests the appendix contains a considerable amount of misfolded α-synuclein)65,67. Transmission of pathological seeds from the periphery to the brain has been observed in various animal models. For example, intraperitoneal, intramuscular, intraglossal or intravenous infusion of α-synuclein preformed fibrils into M83 A53T mutant α-synuclein transgenic mice facilitated the development of α-synuclein pathology in the CNS with varying degrees of efficiency68–70. More recently, injection of α-synuclein preformed fibrils into the gastric wall of wild-type mice was shown to induce α-synuclein pathology in the CNS71.
Aβ peptides can be produced in peripheral tissues72–75 and are capable of crossing the blood–brain barrier76,77, suggesting that peripherally derived Aβ contributes to Aβ pathology in the brain. In favour of this hypothesis, Aβ pathology was found in the brains of patients who had received human-derived growth hormone treatment or dura mater grafts, suggesting peripheral-to-CNS transmission of pathological Aβ30–34. Oral, intravenous, intraocular and intranasal administration of Aβ-containing brain extracts to young APP transgenic mice (which do not yet show age-related Aβ pathology) failed to induce cerebral Aβ amyloidosis; however, intraperitoneal administration of the extracts did promote Aβ deposition in the brains of these mice78,79. A study that performed parabiosis between APPPS1 transgenic mice and wild-type mice identified human Aβ in the brains of the wild-type mice, suggesting that Aβ seeds circulating in blood can enter the CNS80. Evidence supporting periphery-to-brain spreading of tau seeds is not as strong as that for Aβ and α-synuclein; however, intraperitoneal injection of brain extracts containing pathological tau did induce tau aggregation in the brains of P301S tau transgenic mice6.
Parabiosis.
The anatomical joining of two individuals.
The transmission process
Intracellular transportation.
The transmission process starts with the formation and amplification of the initial pathological seeds in the donor cell. These seeds are then transported intracellularly to the site of release (FIG. 1). Studies using microfluidic chambers have shown that pathogenic forms of tau, α-synuclein, Aβ and HTT can all be transported along axons in primary neuronal cultures. Both anterograde and retrograde transport of these proteins was observed, although the pathological HTT protein showed a preference for retrograde transmission81–83. Interestingly, Aβ fibrils were transported an estimated ten times more efficiently than pathological forms of α-synuclein and HTT82.
Fig. 1 |. Mechanisms for the transmission of pathological proteins between cells.
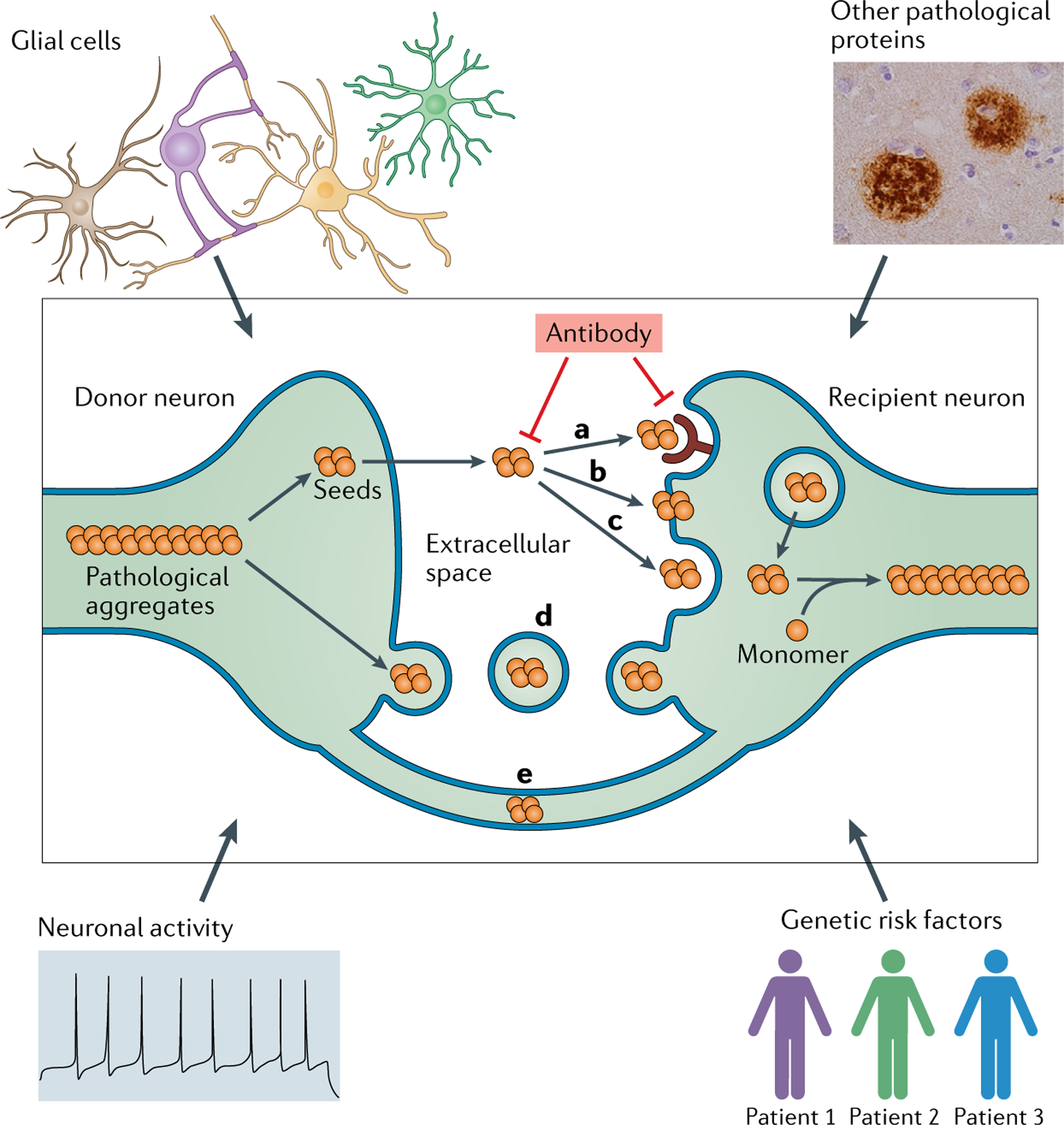
Pathological proteins, or ‘seeds’, are released from donor neurons and enter the extracellular space either as naked protein or in vesicles such as exosomes. Naked protein might be taken up by recipient neurons through receptor-mediated endocytosis (a), direct penetration of the plasma membrane (b) or fluid-phase endocytosis (c). Seeds in vesicles could be internalized through the fusion of vesicles with the plasma membrane (d). Seeds could also be transferred from a donor neuron to a recipient neuron via tunnelling nanotubes that directly connect the two cells (e). The transmission process can be modulated by multiple factors, including the clearance of pathological proteins by glial cells, neuronal activity, genetic risk factors and interaction of the seeds with other pathological proteins. Potential therapeutic interventions include antibodies that target the pathological seeds or the transmission machinery. Adapted from REF.23, Springer Nature Limited.
Microfluidic chambers.
Cell culture chambers that enable the isolation of the axonal or dendritic component from cell bodies.
Release.
The molecular mechanisms responsible for the secretion of pathological proteins by donor cells have been investigated in multiple studies. Even though α-synuclein lacks a secretory signal peptide sequence, the protein can be detected in the plasma and cerebrospinal fluid (CSF) of individuals with PD84,85, supporting the view that α-synuclein is secreted into the extracellular space. Similarly, tau has been detected in the CSF of individuals with AD86 and in the brain interstitial fluid of wild-type mice87. Protein secretion into the extracellular space can occur via multiple routes, including diffusion, classical secretion and unconventional secretion such as pore-mediated translocation, ABC transporter-based secretion, membrane-bound organelle-based secretion and the Golgi bypass pathway.
Exosome-based secretion, one of the unconventional secretion pathways, is by far the most extensively studied pathway for the secretion of pathological proteins. Exosomes are membrane-bound extracellular vesicles that are formed as internal vesicles of a multivesicular body and released to the extracellular space by the fusion of the multivesicular body with the plasma membrane. α-Synuclein has been found in exosomes isolated from cell cultures, and human CSF and plasma88–96, suggesting that exosomes could mediate the release of α-synuclein from donor neurons. In support of this hypothesis, levels of α-synuclein were higher in exosomes isolated from the plasma of individuals with PD than in exosomes from healthy controls90. Furthermore, exosomes isolated from individuals with Dementia with Lewy bodies induced α-synuclein aggregation when injected into the brains of wild-type mice97. Similarly, phosphorylated tau (tau aggregates are hyperphosphorylated) was detected in exosomes isolated from the CSF of individuals with AD or from primary neuronal cultures98,99. The transmission of tau-containing exosomes was thought to occur through trans-synaptic connections, as studies using microfluidic devices suggested that synaptic connectivity is required for exosomes to mediate the transfer of tau99.
APP, Aβ and secretases that cleave APP to produce Aβ have been detected in exosomes isolated from cultured cells expressing human APP or the brains of APP transgenic mice100–102. Exosomes isolated from the serum and plasma of individuals with AD contain higher levels of phosphorylated tau and Aβ42 (a major component of Aβ plaques) than exosomes from healthy controls103. Intracerebral injection of Aβ42-containing exosomes facilitated Aβ plaque formation in the brains of 5XFAD transgenic mice, which express AD-associated mutant forms of human APP and presenilin 1 (REF.104). Conversely, some evidence suggests that exosomes deliver proteolytically active enzymes to assist in degrading extracellular Aβ and might therefore also inhibit Aβ pathology105. Despite these findings, the role of exosomes in the secretion of pathological proteins is not yet clear; for example, two studies using two different neuronal cell lines detected only a small fraction of secreted α-synuclein in exosomes106,107. In another study, when tau was expressed at physiological levels in neurons derived from human induced pluripotent stem cells, the protein could not be detected in exosomes108.
Some evidence suggests that transmission of pathological proteins occurs via methods other than exosome-based secretion; for example, an anti-tau antibody blocked the transfer of tau between cultured cells, suggesting that tau was released into the medium as free protein109. If tau had been packaged in exosomes, the protein–antibody interaction would have been prevented by the lipid membrane. In support of this hypothesis, only a very small portion of secreted tau was located in the isolated vesicle fraction108,110. Some evidence suggests that tau could be released through ectosomes111 or by direct translocation110. Another possibility is that pathological seeds are released to the extracellular space after cell death and diffuse into the surrounding area; however, no direct evidence exists that this is the case in diseased brains. Regardless of the route of secretion, diffusion could have a particularly important role in the spreading of Aβ pathology as Aβ aggregates are extracellular. The existence of cerebral amyloid angiopathy in various lines of APP transgenic mice also suggests that diffusion of pathological Aβ from neurons into blood vessels occurs112–115. Further studies are needed to evaluate the relative contributions of different secretion pathways to the release of pathological seeds.
Ectosomes.
Vesicles (0.1–1 μm in diameter) that are budded and released directly from the plasma membrane.
Direct translocation.
Pore-mediated translocation across the plasma membrane.
Uptake.
The internalization of pathological seeds by recipient cells is the next step in the transmission process and multiple mechanisms for this uptake have been proposed. Evidence suggests that misfolded tau and α-synuclein are internalized at the somatodendritic compartment as well as at the axon and presynaptic terminals16,81–83. Heparin sulfate proteoglycan-mediated macropinocytosis was identified as a key mechanism for the uptake of both tau and α-synuclein, which could be blocked by heparin116,117. Wheat germ agglutinin, a drug that facilitates adsorptive endocytosis, promoted the uptake of tau in cultured cells, suggesting that adsorptive endocytosis is involved in the internalization process13. α-Synuclein fibrils are internalized through endocytosis and degraded in lysosomes118; however, α-synuclein monomers can be internalized through direct translocation119. One study found that α-synuclein fibrils bind to the extracellular immunoglobulin-like domains of the transmembrane protein LAG3 (REF.120). Genetic deletion of LAG3 or treatment with anti-LAG3 antibodies reduced the uptake of α-synuclein fibrils and the subsequent induction of α-synuclein pathology in primary neurons in vivo. Irrespective of the specific mechanisms of entry, most studies indicate that endocytosis is the predominant pathway for the internalization of pathological seeds.
Once inside the recipient neuron, pathological seeds need to exit the endosomal vesicle in order to access cytosolic proteins and begin templated amplification. α-Synuclein, tau and mutant HTT fibrils are all able to induce vesicle rupture121,122, which could enable the pathological seeds to access the cytosol123. Finally, the amplification of the transmitted seeds requires the existence of a ‘substrate’, that is, the normal counterparts of pathological proteins in the cytoplasm. The expression level of these substrates could contribute to the selective vulnerability of different neuronal populations7.
In addition to the release–uptake hypothesis described above, tunnelling nanotubes might mediate the direct intercellular transport of pathological proteins. In support of the tunnelling nanotube hypothesis, misfolded tau, α-synuclein and mutant HTT can all be observed in nanotube structures124–126. Interestingly, the addition of aggregated α-synuclein, mutant HTT and tau to cell cultures increased the number of tunnelling nanotubes124–126, which might facilitate the transmission process. Cell-to-cell transmission of α-synuclein via tunnelling nanotubes also occurred in cultured astrocytes and pericytes127,128, indicating that this kind of transmission is not limited to neurons.
Tunnelling nanotubes.
Protrusions that extend from the plasma membrane and enable the communication of cell contents between two cells.
Molecular nature of the seeds
Understanding the molecular nature of pathological seeds in diseased brains will be crucial for the elucidation of transmission mechanisms and the development of targeted therapeutic interventions. However, tools that can track the behaviour of pathological seeds and analyse their properties in human brains are lacking. One way to identify potential seeds is to investigate the seeding ability of different misfolded protein species isolated from diseased brains. The underlying hypo thesis of this kind of investigation is that aggregates with a higher seeding ability are likely to have a greater role in the spreading of pathology. After ultracentrifugation of brain extracts from APP transgenic mice, Langer et al. identified a small fraction of soluble Aβ (<0.05% of total Aβ) that was more proteinase K sensitive, meaning that it forms a more open and less aggregated structure, than insoluble Aβ. However, this soluble Aβ induced greater Aβ pathology than insoluble Aβ when injected into young APP transgenic mice, suggesting that this form of soluble Aβ is a more potent seed than insoluble Aβ for the transmission of Aβ pathology129. Potent soluble Aβ seeds were also identified in brain tissue but not CSF from individuals with AD130. A more detailed analysis found that insoluble Aβ from intracellular membrane fractions had a stronger seeding ability when injected into APP transgenic mice than insoluble Aβ from a general brain homogenate131. This finding suggests that membrane-associated Aβ could be a source of the seeds that contribute to the spreading of pathology.
For intracellular aggregates such as α-synuclein and tau, the pathological seeds must be able to undergo transport between cells while maintaining the ability to induce templated amplification. Very mature aggregates are unlikely to be pathological seeds because of their large size, which would hinder cell-to-cell transmission. Therefore, smaller species that can be readily released and internalized by cells are the more promising candidates. In one study, soluble high-molecular-weight tau was derived from brain interstitial fluid and cortical extracts taken from tau transgenic mice or individuals with AD. This tau species was taken up by cells in primary neuronal cultures, transported intracellularly and passed to connected neurons, suggesting that it could be a pathological seed. Interestingly, the uptake process seems to require phosphorylated tau132.
Mirbaha et al. used size exclusion chromatography to isolate tau repeat domain assemblies ranging from 1 to >100 tau units, and only species containing more than 3 tau units were internalized and induced aggregation in HEK293 cells117. This size threshold was the same for tau assemblies isolated from the brains of individuals with AD, suggesting that trimers are the minimal unit of tau pathological seeds. However, Jackson et al. used a sucrose gradient to isolate tau aggregates from P301S mice and found that only assemblies containing more than 10 tau units could induce tau aggregation in HEK293 cells133. These inconsistent findings are very likely to be the result of the two studies using different systems to evaluate the seeding ability of tau assemblies: Mirbaha et al. used a cell line that expresses the P301S mutant repeat domain of tau, whereas Jackson et al. used a cell line that expresses the full form of P301S mutant tau. A more recent study observed that even the tau monomer, which is traditionally considered to be unstructured, can exist in different conformations134. The monomer derived from sonicated tau fibrils was able to trigger tau aggregation in HEK293 cells, and this observation was replicated using tau monomers isolated from the brains of individuals with AD, suggesting that the pathological tau seed could even be a monomer with a pathological conformation. The difference between this study and the study of tau trimers by Mirbaha et al.117 is that lipofectamine was used in the former to facilitate the transduction of tau into cells.
In an experimental setting, the seeding of α-synuclein pathology by α-synuclein preformed fibrils required the breakdown of fibrils by sonication, suggesting that very long α-synuclein fibrils are not efficient seeds118, likely as a result of inefficient uptake. However, sonication generates a heterogeneous population of short fibrils and potentially also oligomers135, any of which could be responsible for seeding. α-Synuclein oligomers generated from recombinant proteins are soluble and have shown seeding ability in primary neurons; therefore, oligomers are potential pathological seeds136,137. However, the definition of oligomers encompasses a spectrum of α-synuclein aggregates that are all smaller than fibrils but are structurally diverse and have very heterogeneous seeding properties. For example, low-molecular-weight oligomers are less efficient seeds than the larger and more stable oligomers138. One specific α-synuclein oligomer (4-hydroxy-2-nonenal induced) did not show any seeding activity in vivo139, indicating that only oligomers with certain conformations are pathological seeds.
Despite the long list of potential pathological seeds, identifying which candidates are responsible for the spread of protein pathology in individuals with neurological disease will be extremely challenging unless new technologies are developed to track and isolate individual species of misfolded proteins in diseased brains. Another possibility is that the seeds are not pathological proteins and that protein misfolding in neurons is induced by other factors.
Conformational diversity.
Accumulating evidence indicates that many pathological proteins, including prions, α-synuclein41,140–144, Aβ145–148, tau47,48,149, SOD1 (REF.150) and mutant HTT58, exist in multiple different conformations41,47,48,140–145,148,149,151 (TABLE 2). Different conformations of the same pathological protein have the potential to show dramatically different seeding capacities and spreading behaviours, which in turn could contri bute to the pathological and clinical diversity of neurodegenerative diseases. For example, prion protein exists in many different conformations, known as strains, which contributes to the diversity of prion diseases152.
Table 2 |.
Strains of non-prion protein aggregates
| Protein | Type of seed | Identification of different conformational strains | Strain differences in seeding activity | ||||
|---|---|---|---|---|---|---|---|
| Transmission to glial cells in WT mice | Spreading pattern | Potency | Morphology of aggregates | The ability to seed other proteins | |||
| α-Synuclein | Synthetic fibrils | Yes140,141,142 | No140 | Not tested | Yes140,141,142 | No140,141,142 | Yes140 |
| Mouse brain lysates | Not tested | Not tested | Not tested | Not tested | Not tested | Not tested | |
| Human brain lysates | Yes41,140 | No41 | Yes41 | Yes41 | No41 | Not tested | |
| Tau | Cell lysates | Yes151,252 | No151,252 | Yes151,252 | No151,252 | Yes151,252 | Not tested |
| Mouse brain lysates | Not tested | Not tested | Not tested | Not tested | Not tested | Not tested | |
| Human brain lysates | Yes14,47,160,161,162,253 | Yes47,48,149 | No47,48,149 | Yes47 | Yes47 | Not tested | |
| Amyloid-β | Synthetic fibrils | Yes147 | Not tested | Not tested | Not tested | Not tested | Not tested |
| Mouse brain lysates | Not tested | Not tested | Not tested | Not tested | Yes146 | Not tested | |
| Human brain lysates | Yes145 | Not tested | Not tested | Not tested | Yes148 | Not tested | |
| TDP43 | Synthetic fibrils | Not tested | Not tested | Not tested | Not tested | Not tested | Not tested |
| Mouse brain lysates | Not tested | Not tested | Not tested | Not tested | Not tested | Not tested | |
| Human brain lysates | Not tested | No62 | No62 | Yes9,62 | No62 | Not tested | |
TDP43, TAR DNA-binding protein 43; WT, wild-type.
Although conformational variants of many pathological proteins have now been identified, how these different strains are generated remains unclear. One study demonstrated that the different intracellular environments of oligodendrocytes and neurons could lead to the generation of different α-synuclein strains, which highlights the effect of the local environment on the misfolding process41 (FIG. 2). Studies using artificially generated strains of recombinant proteins have also provided important information about how different strains could develop in diseased brains. For example, repeated seeding of α-synuclein preformed fibrils led to the development of a new strain that could also induce tau pathology in primary neurons140, which suggests that continuous transmission and templated amplification of pathological α-synuclein in diseased brains might also lead to the development of new strains.
Fig. 2 |. Generation of different pathological protein strains.
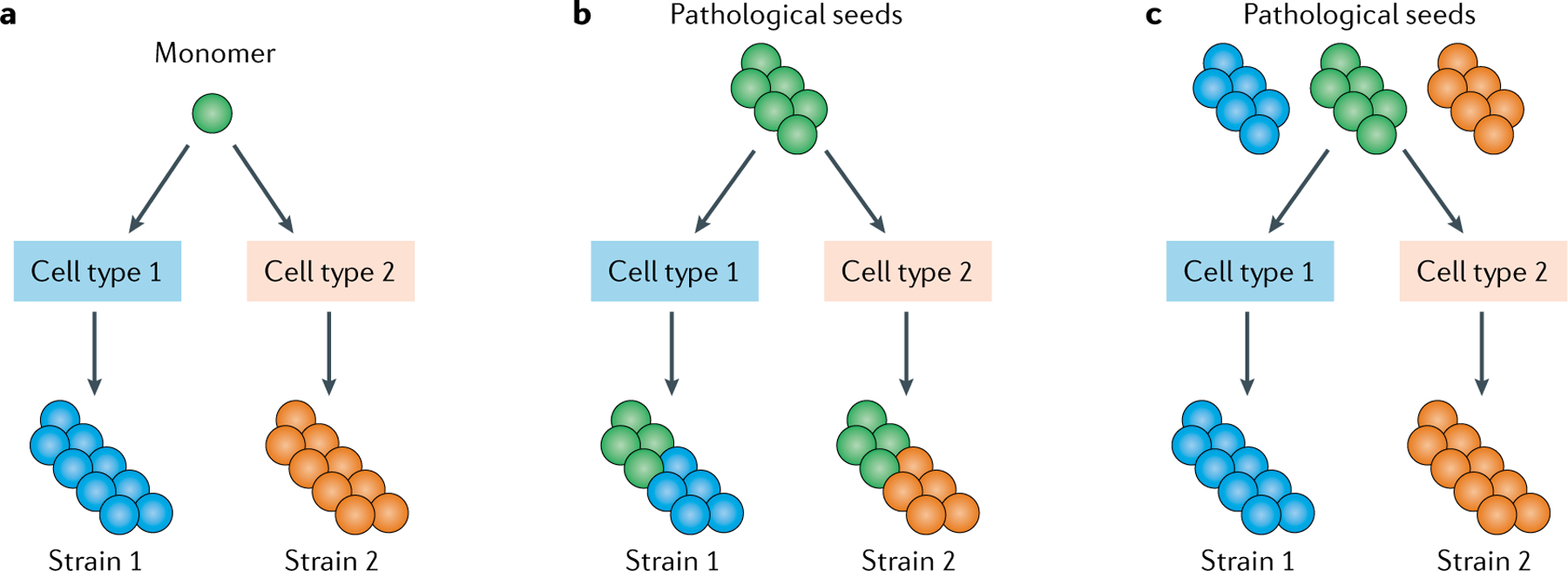
Different intracellular environments can result in different pathological protein strains and several potential mechanisms for this differentiation have been suggested. a | Different intracellular environments could affect the initial protein misfolding process. b | Different intracellular environments could affect the templated amplification process. c | If pathological seeds were a mixture of different conformations or strains, different intracellular environments could lead to the selection and amplification of a specific conformation from the mixture.
The different neurodegenerative disease-related proteins have distinct properties; however, the formation of β-sheet-enriched structures is a shared feature and a crucial step for the formation and amplification of pathological protein conformations. Many studies have tried to illustrate and model the structural basis of the conversion into a pathological conformation153–159. For example, using a seven-amino acid peptide that could form either an α-helix or a β-strand, two studies showed that a pre-formed β-strand can promote the unfolding of an α-helix to form another β-strand155,157. The results of a detailed study of the aggregation process of SOD1 suggested the existence of several distinct steps, including dimer dissociation, metal loss and oligomer formation156. Recently, cryogenic electron microscopy was used to analyse the structure of pathological proteins purified from diseased brains160–164, providing crucial information on the structural basis of the misfolding and aggregation process in diseased brains, including the arrangement and composition of the core of the protein aggregates.
Conformation and potency.
Different conformational variants of pathological seeds can have dramatically different seeding abilities. For example, pathological α-synuclein isolated from oligodendrocytes was conformationally distinct from pathological α-synuclein isolated from neurons, with the oligodendrocyte-derived form having a ~1,000-fold greater seeding ability than the neuron-derived form41. Similarly, pathological tau isolated from brains of individuals with progressive supranuclear palsy (PSP) was conformationally distinct from, and had greater seeding ability than, pathological tau isolated from brains of individuals with AD14. Pathological TDP43 protein isolated from patients with frontotemporal lobar degeneration (FTLD) with a Granulin mutation had a greater seeding ability than those isolated from individuals with sporadic FTLD62. In addition, distinct conformational strains of recombinant α-synuclein have been shown to have different seeding abilties140–142.
One interesting observation is that pathological proteins isolated from diseased brains generally have a greater seeding ability than aggregates generated with recombinant proteins, suggesting that the environment in diseased brains leads to the formation of unique protein conformations that are different from those generated in vitro. For example, pathological tau isolated from tauopathy brain samples induced more tau pathology than synthetic tau fibrils when injected into the brains of wild-type mice14,47. Similarly, the induction of TDP43 aggregation in wild-type mice has only been achieved with pathological TDP43 isolated from brains of individuals with FTLD62. The seeding activity of Aβ aggregates purified from brains of individuals with AD is much higher than the seeding activity of synthetic Aβ aggregates56,130. The seeding ability of α-synuclein derived from diseased brains has not yet been compared with the seeding ability of α-synuclein preformed fibrils. However, in one study, pathological α-synuclein derived from transgenic mouse brains had a greater seeding ability than a pathological form of recombinant α-synuclein37. The extracts from diseased brains that were used in these studies contained other proteins and lipids, in addition to the pathological proteins of interest. Therefore, the high potency of brain-derived seeds could be the result of co-factors in the extracts that promote the seeding process. The development of new technologies to generate highly purified pathological proteins from diseased brains will be needed to exclude the contribution of contaminating proteins to seeding activity.
Conformation and glial cell pathology.
Different conformations of pathological proteins might induce pathology in specific cell types. For example, the injection of pathological tau derived from brains of individuals with AD into tau transgenic or wild-type mice only induced tau pathology in neurons, whereas tau derived from the brains of individuals with cortical basal degeneration (CBD) or PSP induced pathology in neurons, astrocytes and oligodendrocytes47,48,149. Pathology in these cell types is a pathological feature of CBD and PSP. In contrast, the cell type specificity of α-synuclein spreading in the experimental setting does not seem to correlate with clinical features. In individuals with multiple system atrophy, the vast majority of α-synuclein aggregation is in oligodendrocytes; however, the injection of pathological α-synuclein isolated from multiple system atrophy brains into wild-type mice only induced α-synuclein aggregation in neurons41.
Conformation and spreading pattern.
Some evidence suggests that the conformation of pathological seeds modulates the transmission pattern of these pathological proteins along the neuron network. For example, different pathological α-synuclein strains show different spreading patterns after intracerebral injection into wild-type mice41. Similarly, the results of a PET imaging study suggested that the spreading of tau aggregates in individuals with AD is mainly determined by neuronal connectivity, whereas tau aggregates in individuals with PSP spread into brain areas with a high metabolic demand and a lack of trophic support165.
Modifiers of the transmission process
Neuronal activity.
Of the factors that could influence the transmission of pathological seeds, neuronal activity is one of the most well studied and has been repeatedly shown to promote the propagation of various pathological proteins99,166–172 (FIG. 1). For example, in a microdialysis study, the concentration of Aβ in the brain interstitial fluid of mice could be modulated by neuronal activity and was correlated with the concentration of lactate, which is a marker of neuronal activity169. Using a similar technique, another study showed that an increase in neuronal activity can rapidly increase the level of extracellular tau in the brain168. In a more recent study, which used a virus-mediated method to overexpress human tau in primary neurons and mice, elevated neuronal activity caused an increase in tau secretion in vitro and in tau transmission in vivo170. Furthermore, a study that used optogenetic and chemogenetic approaches to modulate neuronal activity demonstrated that higher neuronal activity leads to increased tau pathology in mice expressing a mutant form of human tau167. Elevated neuronal activity also caused a rapid increase in extracellular levels of α-synuclein in both primary neuronal cultures and mice, and reduced neuronal activity caused a decrease in extracellular α-synuclein in both experimental models166.
Consistent with a role for neuronal activity in pathological protein transmission, extracellular tau and Aβ levels change according to the sleep–wake cycle, and chronic sleep deprivation, which causes an increase in neuronal activity, can facilitate the secretion and propagation of Aβ and tau172,173. Mechanistically, the increased secretion of tau induced by neuronal activity could be mediated by increased Golgi dynamics174. Given these highly consistent findings, which come from multiple studies using various different models, abnormal neuronal activity, such as the seizure-like activity seen in individuals with AD175 and sleep disorders in individuals with PD176, is likely to facilitate or modulate the spreading of pathological proteins.
Glial cells.
The activation of microglia and astrocytes has been observed in various neurodegenerative diseases; however, the role of these glial cells in the disease process is complicated. Evidence suggests that activated glial cells facilitate the clearance of pathological proteins from the extracellular space and thus inhibit the spreading of pathological seeds from cell to cell. For example, microglial cells can phagocytose both soluble and insoluble forms of tau175,177. Astrocytes can take up tau fibrils in vitro and can also reduce tau pathology in mutant tau transgenic mice178. Microglia and astrocytes are also involved in multiple mechanisms of Aβ clearance. First, glial cells produce Aβ-degrading enzymes, such as matrix metalloproteinases179, tissue plasminogen activator180 and metalloendopeptidases181, which help to clear Aβ peptides. Second, microglia and astrocytes can directly phagocytose fibrillary Aβ176,182.
A specific population of microglial cells associated with neurodegenerative diseases, known as disease-associated microglia, have been identified by single-cell sequencing183. Disease-associated microglia have been detected near Aβ plaques and Aβ particles, suggestiing that this microglial population might be involved in the clearance of Aβ aggregates183. Similarly, evidence suggests that pathological α-synuclein is phagocytosed and cleared by astrocytes and microglia184–186. The overexpression of IL-6 activates microglia and astrocytes and attenuates the spread of pathology induced by α-synuclein preformed fibrils in mice187. Interestingly, in one study, monomeric α-synuclein enhanced microglial phagocytosis, whereas aggregated α-synuclein inhibited this process, suggesting a mechanism by which aggregated α-synuclein can avoid degradation by microglial cells188.
The results of more recent studies suggest that microglia and astrocytes actually facilitate the spreading of pathological proteins and promote disease progression. For example, pharmacological depletion of microglia dramatically suppressed the propagation of tau pathology in an adeno-associated virus-based tau mouse model, and inhibiting exosome secretion by microglia also significantly reduced the spreading of pathological tau both in vitro and in vivo53. These findings suggest that tau can be taken up by microglia and secreted in exosomes. Similarly, tau aggregates develop in astrocytes and oligodendrocytes in the brains of individuals with CBD or PSP189,190, indicating that transmission of tau can occur between neurons and glial cells, or between glial cells191. Microglia might also promote the transmission of Aβ pathology. Apoptosis-associated speck-like protein containing a CARD (ASC) specks secreted by microglia have been shown to bind to and promote the aggregation of Aβ192. In addition, ASC deficiency blocked the seeding of Aβ pathology in APPSwePSEN1dE9 transgenic mice by brain homogenates from aged APPSwePSEN1dE9 mice.
Pathological α-synuclein can be transmitted between neurons and astrocytes193 and, as mentioned earlier, α-synuclein might also be transmitted between astrocytes via tunnelling nanotubes127,128. Using a mouse model that expresses human α-synuclein specifically in oligodendrocytes, we showed that pathological α-synuclein can undergo transmission between different oligodendrocytes, a process that is independent of α-synuclein pathology in neurons41.
Finally, glial cells could also modulate the transmission process by influencing the conformation of the pathological seeds. As discussed earlier, the intracellular environment of oligodendrocytes can modify the conformation of the pathological seeds in a different way to the intracellular environment of neurons, which leads to differences in seeding ability41. Microglia and astrocytes also have a key role in the neurodegenerative process, which has been extensively reviewed elsewhere194–196.
Genetic risk factors.
Evidence suggests that some genetic risk factors for neurodegenerative diseases contribute to disease development and progression by modulating the transmission of pathological proteins. For example, the gene encoding amphiphysin 2 (also known as BIN1) is the second most prevalent risk locus for late-onset AD197. In one study, amphiphysin 2 inhibited tau propagation in vitro by decreasing the endocytosis of pathological tau by primary neurons122. Mutations in the gene encoding leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2), which usually increase the activity of the kinase198–202, are the most common cause of hereditary PD199. LRRK2 activity promoted the propagation of pathological α-synuclein in vitro and in vivo via the phosphorylation of RAB35 (REF.203), which is a small GTPase involved in vesicle trafficking. The effect of LRRK2 activity on the seeding ability of α-synuclein preformed fibrils has also been studied in both cell and animal models204–206. The apolipoprotein E type 4 allele is the strongest genetic risk factor for late-onset AD207. One study used PET imaging to compare the distribution of Aβ and tau in the brains of healthy adults with regional gene expression values from the Allen Human Brain Atlas. The propagation patterns of both Aβ and tau were associated with a lipid metabolism-related genetic profile in which apolipoprotein E has an important role208.
Interaction between pathological proteins.
The co-existence of multiple pathological proteins in diseased brains is common in various neurodegenerative diseases. For example, ~50% of individuals with AD have α-synuclein pathology in the brain in addition to the characteristic Aβ and tau pathology209. Furthermore, ~50% of post-mortem brains from individuals with PDD have sufficient AD co-pathology to warrant a second diagnosis of AD210. The co-existence of different pathologies suggests that one pathological protein could promote the spreading of another.
Histopathological and genetic data suggest that Aβ plaques drive the spreading of tau pathology out of the medial temporal lobe211–215, and this suggestion is supported by studies using various disease models. For example, brain extracts from APP transgenic mice as well as synthetic Aβ fibrils can promote tau aggregation in mutant tau transgenic mice216–219 and synthetic Aβ aggregates can induce tau fibrillization in cells as well as in test tubes217. Furthermore, transgenic mice expressing mutant forms of human APP and tau had greater tau pathology than mice expressing the mutant form of tau only216,220. In a more recent study, pathological tau isolated from the brain tissue of individuals with AD was injected into the brains of mice that had Aβ pathology. In these animals, tau fibrilization was promoted in the dystrophic neurites surrounding Aβ plaque cores221. A similar observation was made in transgenic mice that express the four-repeat domain of human tau as well as a mutant form of human APP185.
The interaction of pathological α-synuclein with Aβ and tau has also been extensively studied. For example, co-incubation of α-synuclein and tau promoted the fibrilization of both proteins222. α-Synuclein preformed fibrils inhibited tau-promoted microtubule assembly and induced tau aggregation in cells overexpressing tau223. Increased phosphorylation of tau has been observed in mice that overexpress a PD-associated mutant form of human α-synuclein224,225. Importantly, one study compared two conformationally distinct α-synuclein strains and found that only one of the strains was able to induce tau aggregation in wild-type primary neurons and in tau transgenic mice140.
The interaction between α-synuclein and Aβ is more complicated than the interaction between α-synuclein and tau. Cross-seeding between α-synuclein and Aβ has been observed using recombinant proteins in vitro226. A transgenic mouse model of tau, Aβ and α-synuclein pathology showed enhancement of all three types of pathology when compared with mouse models of the individual pathologies227. Similarly, expression of the Aβ peptide promoted the formation of α-synuclein pathology in α-synuclein transgenic mice and in mice that had been injected with misfolded α-synuclein228,229. However, in one study, injection of α-synuclein preformed fibrils or brain homogenates from mice expressing mutant human α-synuclein into APP transgenic mice failed to induce Aβ aggregation. In the same mouse line, expression of the A30P form of mutant α-synuclein even reduced the Aβ plaque load230. Therefore, more studies are needed to clarify the interaction between α-synuclein and Aβ pathology.
Therapeutic implications
Studying the transmission and amplification process of the pathological proteins associated with neurodegenerative disease has important therapeutic applications. Cell-based therapies, which aim to replace degenerating cells with healthy ones, have been tested in clinical trials but might not provide long-term benefit because of pathological protein transmission from the patient to the transplanted cells. For example, as discussed earlier, α-synuclein aggregation was found in transplanted fetal ventral mesencephalic neurons in individuals with PD28,29. Therefore, therapies that target the transmission process could slow down disease progression and might improve the outcome of cell therapy.
Currently, passive immunotherapy using antibodies targeting various pathological proteins is being investigated as a potential treatment for neurodegenerative disease. For example, one study systemically administered antibodies against the proximal C-terminal amino acids (91–99) of α-synuclein to mice with lentivirus-mediated overexpression of α-synuclein. In these mice, the antibodies blocked the transportation and aggregation of α-synuclein in axons and reduced axonal degeneration231. In two other studies, antibodies against the C-terminus of α-synuclein were shown to promote α-synuclein clearance and reduce behavioural deficits in α-synuclein transgenic mice232,233. Furthermore, antibodies against misfolded α-synuclein reduced α-synuclein preformed fibril-induced pathology in both primary neurons and wild-type mice234. These anti bodies also ameliorated dopaminergic neuron degeneration and improved motor deficits in mice. Antibodies targeting N-terminal and mid-domain regions of tau prevented the uptake and propagation of pathological tau in cell cultures235. Similarly, antibodies targeting Aβ reduced Aβ levels in animal models and individuals with AD236.
The protective effect of these antibody therapies was thought to be mediated by lysosomal232 or microglial-dependent degradation of pathological proteins237. However, the conformational diversity of the pathological seeds might complicate the development of immunotherapies because antibodies efficient for one pathological strain might not be as effective for another strain. As with any therapy that targets the CNS, developing strategies that enable antibodies to cross the blood–brain barrier will dramatically improve the efficiency of passive immunotherapy for neurodegenerative diseases.
Active immunization has also been explored as a potential therapy for neurodegenerative diseases. For example, immunization with α-synuclein proteins or peptides reduced α-synuclein accumulation and behavioural deficits in various α-synuclein transgenic models238–240. As internalization of pathological seeds is a key process for transmission, researchers are also investigating the potential of therapies that target the molecules involved in the uptake of pathological protein. For example, synthetic heparin mimics blocked the heparin sulfate proteoglycan-mediated uptake of pathological tau and α-synuclein in vivo and in vitro116, and antibodies targeting LAG3 blocked the transmission of pathological α-synuclein120. In addition to targeting the pathological seeds, reducing the concentration of the substrate, that is, the normal protein counterparts, using approaches such as antisense oligonucleotides is another strategy to inhibit the transmission process241. Finally, analysing pathological proteins in the CSF or even the blood of patients could facilitate the early and accurate diagnosis of various neurodegenerative diseases. For example, protein misfolding cyclic amplification has been used to detect pathological α-synuclein in the CSF as a diagnostic strategy for PD242. A similar technique was also used to detect aggregated tau in brains of individuals with AD243.
Protein misfolding cyclic amplification.
The amplification of misfolded protein by repeated incubation with corresponding monomers.
Conclusions and future directions
During the past 5 years, the focus of neurodegenerative research has shifted from testing the transmission hypothesis to exploring the underlying molecular mechanisms of the transmission process. Given the complexity and therapeutic significance of the mechanisms underlying the transmission of pathological proteins in neurological diseases, this topic is likely to continue being the focus of the field for some time.
One challenge for the field is that the vast majority of studies were performed using preformed fibrils generated from recombinant proteins. However, accumulating evidence demonstrates crucial conformational and biological differences between preformed fibrils and pathological aggregates isolated from diseased brains14,62. In the future, analysing the cell-to-cell transmission of pathological proteins from diseased brains will be essential. However, these kinds of studies are currently constrained by the limited availability of brain tissue and the small amounts of pathological proteins that can be isolated from diseased brains. Therefore, developing improved methods to purify and amplify these pathological seeds from diseased brains will be extremely beneficial for the field.
Another major challenge to understanding the molecular mechanisms underlying transmission is the conformational diversity of the pathological proteins. As different strains of an increasing number of pathological proteins have been identified, conformational diversity now seems to be a phenomenon that is common to the majority of neurodegenerative disease-associated proteins, and many more strains are likely to have not yet been discovered. Analysing the distribution and interaction of different protein strains in diseased brains is extremely challenging. However, the recent development of conformation-specific antibodies244,245 could facilitate the discovery and analysis of different strains in brains affected by neurodegenerative disease.
Despite the progress made, several key gaps in our knowledge remain. First, we do not yet know how pathological seeds form in diseased brains nor do we understand the molecular nature of these seeds. Second, whether pathological seeds cause neuronal and/or glial cell toxicity is unclear. Finally, although different strains of various pathological proteins have now been identified, how these different strains are generated remains unknown. The development of new technologies to track, isolate and analyse pathological proteins in diseased brains will be essential in addressing these important questions.
Key points.
Cell-to-cell transmission and the subsequent amplification of pathological proteins is emerging as a common mechanism for the progression of various neurodegenerative diseases.
Transmission within the CNS as well as from the peripheral nervous system to the CNS has been reported for multiple pathological proteins.
Multiple molecular mechanisms involved in the secretion, uptake and transport of pathological seeds have been identified.
Neurodegenerative disease-related pathological proteins are conformationally diverse.
Various factors can modulate the transmission process, including neuronal activity, glial cells, genetic risk factors and interactions with other pathological proteins.
Antibodies against pathological seeds, which are designed to block the transmission process, are currently in clinical trials.
Acknowledgements
The authors thank the Michael J. Fox Foundation.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Glenner GG & Wong CW Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun 120, 885–890 (1984). [DOI] [PubMed] [Google Scholar]
- 2.Kosik KS, Joachim CL & Selkoe DJ Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl Acad. Sci. USA 83, 4044–4048 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spillantini MG, Crowther RA, Jakes R, Hasegawa M & Goedert M -Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl Acad. Sci. USA 95, 6469–6473 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann M et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006). [DOI] [PubMed] [Google Scholar]
- 5.DiFiglia M et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Clavaguera F et al. Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol. 127, 299–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luna E et al. Differential α-synuclein expression contributes to selective vulnerability of hippocampal neuron subpopulations to fibril-induced toxicity. Acta Neuropathol. 135, 855–875 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyagi A et al. Aβ and tau prion-like activities decline with longevity in the Alzheimer’s disease human brain. Sci. Transl. Med 11, eaat8462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laferriere F et al. TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates. Nat. Neurosci 22, 65–77 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Braak H & Braak E Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Braak H et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Surmeier DJ, Obeso JA & Halliday GM Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci 18, 101–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo JL & Lee VM Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem 286, 15317–15331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo JL et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med 213, 2635–2654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luk KC et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpicelli-Daley LA et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braak H & Del Tredici K Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv. Anat. Embryol. Cell Biol 201, 1–119 (2009). [PubMed] [Google Scholar]
- 18.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H & Del Tredici K Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braak H & Del Tredici K The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 121, 171–181 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Thal DR, Rub U, Orantes M & Braak H Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Thal DR et al. Sequence of Aβ-protein deposition in the human medial temporal lobe. J. Neuropathol. Exp. Neurol 59, 733–748 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Glass CK, Saijo K, Winner B, Marchetto MC & Gage FH Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo JL & Lee VM Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med 20, 130–138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholl M et al. PET imaging of tau deposition in the aging human brain. Neuron 89, 971–982 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz AJ et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain 139, 1539–1550 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Furman JL et al. Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 133, 91–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman SK, Del Tredici K, Thomas TL, Braak H & Diamond MI Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol. 136, 57–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kordower JH, Chu Y, Hauser RA, Freeman TB & Olanow CW Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med 14, 504–506 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Li JY et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med 14, 501–503 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Jaunmuktane Z et al. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 525, 247–250 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Cali I et al. Iatrogenic Creutzfeldt-Jakob disease with amyloid-β pathology: an international study. Acta Neuropathol. Commun 6, 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frontzek K, Lutz MI, Aguzzi A, Kovacs GG & Budka H Amyloid-β pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt-Jakob disease after dural grafting. Swiss Med. Wkly 146, w14287 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Hamaguchi T et al. Significant association of cadaveric dura mater grafting with subpial Aβ deposition and meningeal amyloid angiopathy. Acta Neuropathol. 132, 313–315 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Herve D et al. Fatal Aβ cerebral amyloid angiopathy 4 decades after a dural graft at the age of 2 years. Acta Neuropathol. 135, 801–803 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Ritchie DL et al. Amyloid-β accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol. 134, 221–240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duyckaerts C et al. Neuropathology of iatrogenic Creutzfeldt-Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Aβ pathology. Acta Neuropathol. 135, 201–212 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Luk KC et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med 209, 975–986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mougenot AL et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging 33, 2225–2228 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Watts JC et al. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl Acad. Sci. USA 110, 19555–19560 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masuda-Suzukake M et al. Prion-like spreading of pathological α-synuclein in brain. Brain 136, 1128–1138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng C et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557, 558–563 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Recasens A et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol 75, 351–362 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Shimozawa A et al. Propagation of pathological α-synuclein in marmoset brain. Acta Neuropathol. Commun 5, 12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulusoy A et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol. Med 5, 1119–1127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clavaguera F et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol 11, 909–913 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peeraer E et al. Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiol. Dis 73, 83–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narasimhan S et al. Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J. Neurosci 37, 11406–11423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clavaguera F et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl Acad. Sci. USA 110, 9535–9540 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lasagna-Reeves CA et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep 2, 700 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W et al. Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer’s and Pick’s diseases. Elife 8, e43584 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Calignon A et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yetman MJ, Lillehaug S, Bjaalie JG, Leergaard TB & Jankowsky JL Transgene expression in the Nop-tTA driver line is not inherently restricted to the entorhinal cortex. Brain Struct. Funct 221, 2231–2249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asai H et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci 18, 1584–1593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kane MD et al. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J. Neurosci 20, 3606–3611 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer-Luehmann M et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Stohr J et al. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc. Natl Acad. Sci. USA 109, 11025–11030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munch C, O’Brien J & Bertolotti A Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl Acad. Sci. USA 108, 3548–3553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nekooki-Machida Y et al. Distinct conformations of in vitro and in vivo amyloids of huntingtin-exon1 show different cytotoxicity. Proc. Natl Acad. Sci. USA 106, 9679–9684 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren PH et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell Biol 11, 219–225 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen AK et al. Induction of amyloid fibrils by the C-terminal fragments of TDP-43 in amyotrophic lateral sclerosis. J. Am. Chem. Soc 132, 1186–1187 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Nonaka T et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 4, 124–134 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Porta S et al. Patient-derived frontotemporal lobar degeneration brain extracts induce formation and spreading of TDP-43 pathology in vivo. Nat. Commun 9, 4220 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wakabayashi K, Takahashi H, Ohama E & Ikuta F Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 79, 581–583 (1990). [DOI] [PubMed] [Google Scholar]
- 64.Wakabayashi K, Takahashi H, Takeda S, Ohama E & Ikuta F Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 76, 217–221 (1988). [DOI] [PubMed] [Google Scholar]
- 65.Killinger BA et al. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci. Transl. Med 10, eaar5280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Tredici K, Hawkes CH, Ghebremedhin E & Braak H Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 119, 703–713 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Svensson E et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol 78, 522–529 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Breid S et al. Neuroinvasion of α-synuclein prionoids after intraperitoneal and intraglossal inoculation. J. Virol 90, 9182–9193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sacino AN et al. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl Acad. Sci. USA 111, 10732–10737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ayers JI et al. Robust central nervous system pathology in transgenic mice following peripheral injection of α-synuclein fibrils. J. Virol 91, e02095–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uemura N et al. Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener 13, 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li QX et al. Proteolytic processing of Alzheimer’s disease beta A4 amyloid precursor protein in human platelets. J. Biol. Chem 270, 14140–14147 (1995). [DOI] [PubMed] [Google Scholar]
- 73.Evin G, Zhu A, Holsinger RM, Masters CL & Li QX Proteolytic processing of the Alzheimer’s disease amyloid precursor protein in brain and platelets. J. Neurosci. Res 74, 386–392 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Citron M et al. Excessive production of amyloid beta-protein by peripheral cells of symptomatic and presymptomatic patients carrying the Swedish familial Alzheimer disease mutation. Proc. Natl Acad. Sci. USA 91, 11993–11997 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuo YM et al. Elevated Aβ42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AβPP metabolism. Am. J. Pathol 156, 797–805 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zlokovic BV et al. Brain uptake of circulating apolipoproteins J and E complexed to Alzheimer’s amyloid beta. Biochem. Biophys. Res. Commun 205, 1431–1437 (1994). [DOI] [PubMed] [Google Scholar]
- 77.Deane R & Zlokovic BV Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res 4, 191–197 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Eisele YS et al. Induction of cerebral β-amyloidosis: intracerebral versus systemic Aβ inoculation. Proc. Natl Acad. Sci. USA 106, 12926–12931 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eisele YS et al. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science 330, 980–982 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bu XL et al. Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol. Psychiatry 23, 1948–1956 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Wu JW et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem 288, 1856–1870 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brahic M, Bousset L, Bieri G, Melki R & Gitler AD Axonal transport and secretion of fibrillar forms of α-synuclein, Aβ42 peptide and HTTExon 1. Acta Neuropathol. 131, 539–548 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freundt EC et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann. Neurol 72, 517–524 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El-Agnaf OM et al. Detection of oligomeric forms of α-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 20, 419–425 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Mollenhauer B et al. Direct quantification of CSF α-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol 213, 315–325 (2008). [DOI] [PubMed] [Google Scholar]
- 86.Buch K et al. Tau protein. A potential biological indicator for early detection of Alzheimer disease [German]. Nervenarzt 69, 379–385 (1998). [DOI] [PubMed] [Google Scholar]
- 87.Yamada K et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J. Neurosci 31, 13110–13117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emmanouilidou E et al. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci 30, 6838–6851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alvarez-Erviti L et al. Lysosomal dysfunction increases exosome-mediated α-synuclein release and transmission. Neurobiol. Dis 42, 360–367 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi M et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 128, 639–650 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kunadt M et al. Extracellular vesicle sorting of α-synuclein is regulated by sumoylation. Acta Neuropathol. 129, 695–713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danzer KM et al. Exosomal cell-to-cell transmission of α synuclein oligomers. Mol. Neurodegener 7, 42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kong SM et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-synuclein externalization via exosomes. Hum. Mol. Genet 23, 2816–2833 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Tsunemi T, Hamada K & Krainc D ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. J. Neurosci 34, 15281–15287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pan-Montojo F et al. Environmental toxins trigger PD-like progression via increased α-synuclein release from enteric neurons in mice. Sci. Rep 2, 898 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poehler AM et al. Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment. Autophagy 10, 2171–2192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ngolab J et al. Brain-derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology. Acta Neuropathol. Commun 5, 46 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saman S et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem 287, 3842–3849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener 12, 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vingtdeux V et al. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J. Biol. Chem 282, 18197–18205 (2007). [DOI] [PubMed] [Google Scholar]
- 101.Sharples RA et al. Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 22, 1469–1478 (2008). [DOI] [PubMed] [Google Scholar]
- 102.Perez-Gonzalez R, Gauthier SA, Kumar A & Levy E The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J. Biol. Chem 287, 43108–43115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fiandaca MS et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 11, 600–607.e1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dinkins MB, Dasgupta S, Wang G, Zhu G & Bieberich E Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 35, 1792–1800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.An K et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Mol. Brain 6, 47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Melachroinou K et al. Deregulation of calcium homeostasis mediates secreted α-synuclein-induced neurotoxicity. Neurobiol. Aging 34, 2853–2865 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Ejlerskov P et al. Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome-lysosome fusion. J. Biol. Chem 288, 17313–17335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chai X, Dage JL & Citron M Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol. Dis 48, 356–366 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Kfoury N, Holmes BB, Jiang H, Holtzman DM & Diamond MI Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem 287, 19440–19451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Katsinelos T et al. Unconventional secretion mediates the trans-cellular spreading of Tau. Cell Rep. 23, 2039–2055 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Dujardin S et al. Ectosomes: a new mechanism for non-exosomal secretion of tau protein. PLoS One 9, e100760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davis J et al. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J. Biol. Chem 279, 20296–20306 (2004). [DOI] [PubMed] [Google Scholar]
- 113.Herzig MC et al. Aβ is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat. Neurosci 7, 954–960 (2004). [DOI] [PubMed] [Google Scholar]
- 114.Calhoun ME et al. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc. Natl Acad. Sci. USA 96, 14088–14093 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weller RO et al. Cerebral amyloid angiopathy: amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am. J. Pathol 153, 725–733 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holmes BB et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl Acad. Sci. USA 110, E3138–E3147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mirbaha H, Holmes BB, Sanders DW, Bieschke J & Diamond MI Tau trimers are the minimal propagation unit spontaneously internalized to seed intracellular aggregation. J. Biol. Chem 290, 14893–14903 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karpowicz RJ Jr. et al. Selective imaging of internalized proteopathic α-synuclein seeds in primary neurons reveals mechanistic insight into transmission of synucleinopathies. J. Biol. Chem 292, 13482–13497 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee HJ et al. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int. J. Biochem. Cell Biol 40, 1835–1849 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Mao X et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, aah3374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Flavin WP et al. Endocytic vesicle rupture is a conserved mechanism of cellular invasion by amyloid proteins. Acta Neuropathol. 134, 629–653 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Calafate S, Flavin W, Verstreken P & Moechars D Loss of Bin1 promotes the propagation of tau pathology. Cell Rep. 17, 931–940 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Falcon B, Noad J, McMahon H, Randow F & Goedert M Galectin-8-mediated selective autophagy protects against seeded tau aggregation. J. Biol. Chem 293, 2438–2451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Costanzo M et al. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J. Cell Sci 126, 3678–3685 (2013). [DOI] [PubMed] [Google Scholar]
- 125.Abounit S, Wu JW, Duff K, Victoria GS & Zurzolo C Tunneling nanotubes: a possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 10, 344–351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abounit S et al. Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 35, 2120–2138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rostami J et al. Human astrocytes transfer aggregated alpha-synuclein via tunneling nanotubes. J. Neurosci 37, 11835–11853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dieriks BV et al. α-synuclein transfer through tunneling nanotubes occurs in SH-SY5Y cells and primary brain pericytes from Parkinson’s disease patients. Sci. Rep 7, 42984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Langer F et al. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J. Neurosci 31, 14488–14495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fritschi SK et al. Highly potent soluble amyloid-β seeds in human Alzheimer brain but not cerebrospinal fluid. Brain 137, 2909–2915 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marzesco AM et al. Highly potent intracellular membrane-associated Aβ seeds. Sci. Rep 6, 28125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takeda S et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat. Commun 6, 8490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jackson SJ et al. Short fibrils constitute the major species of seed-competent tau in the brains of mice transgenic for human P301S Tau. J. Neurosci 36, 762–772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mirbaha H et al. Inert and seed-competent tau monomers suggest structural origins of aggregation. Elife 7, e36584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polinski NK et al. Best practices for generating and using alpha-synuclein pre-formed fibrils to model Parkinson’s disease in rodents. J. Parkinsons Dis 8, 303–322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Danzer KM, Krebs SK, Wolff M, Birk G & Hengerer B Seeding induced by alpha-synuclein oligomers provides evidence for spreading of α-synuclein pathology. J. Neurochem 111, 192–203 (2009). [DOI] [PubMed] [Google Scholar]
- 137.Danzer KM et al. Different species of α-synuclein oligomers induce calcium influx and seeding. J. Neurosci 27, 9220–9232 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pieri L, Madiona K & Melki R Structural and functional properties of prefibrillar α-synuclein oligomers. Sci. Rep 6, 24526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fagerqvist T et al. Off-pathway alpha-synuclein oligomers seem to alter alpha-synuclein turnover in a cell model but lack seeding capability in vivo. Amyloid 20, 233–244 (2013). [DOI] [PubMed] [Google Scholar]
- 140.Guo JL et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154, 103–117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bousset L et al. Structural and functional characterization of two α-synuclein strains. Nat. Commun 4, 2575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peelaerts W et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015). [DOI] [PubMed] [Google Scholar]
- 143.Prusiner SB et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl Acad. Sci. USA 112, E5308–E5317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Woerman AL et al. Propagation of prions causing synucleinopathies in cultured cells. Proc. Natl Acad. Sci. USA 112, E4949–E4958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu JX et al. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154, 1257–1268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heilbronner G et al. Seeded strain-like transmission of β-amyloid morphotypes in APP transgenic mice. EMBO Rep. 14, 1017–1022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Petkova AT et al. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science 307, 262–265 (2005). [DOI] [PubMed] [Google Scholar]
- 148.Watts JC et al. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc. Natl Acad. Sci. USA 111, 10323–10328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boluda S et al. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 129, 221–237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Furukawa Y, Kaneko K, Yamanaka K & Nukina N Mutation-dependent polymorphism of Cu,Zn-superoxide dismutase aggregates in the familial form of amyotrophic lateral sclerosis. J. Biol. Chem 285, 22221–22231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sanders DW et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aguzzi A, Heikenwalder M & Polymenidou M Insights into prion strains and neurotoxicity. Nat. Rev. Mol. Cell Biol 8, 552–561 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Kelly JW Alternative conformations of amyloidogenic proteins govern their behavior. Curr. Opin. Struct. Biol 6, 11–17 (1996). [DOI] [PubMed] [Google Scholar]
- 154.Dobson CM Protein misfolding, evolution and disease. Trends Biochem. Sci 24, 329–332 (1999). [DOI] [PubMed] [Google Scholar]
- 155.Ding F, LaRocque JJ & Dokholyan NV Direct observation of protein folding, aggregation, and a prion-like conformational conversion. J. Biol. Chem 280, 40235–40240 (2005). [DOI] [PubMed] [Google Scholar]
- 156.Khare SD, Caplow M & Dokholyan NV The rate and equilibrium constants for a multistep reaction sequence for the aggregation of superoxide dismutase in amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 101, 15094–15099 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peng Y & Hansmann UH Helix versus sheet formation in a small peptide. Phys. Rev. E Stat. Nonlin. Soft Matter Phys 68, 041911 (2003). [DOI] [PubMed] [Google Scholar]
- 158.Redler RL & Dokholyan NV The complex molecular biology of amyotrophic lateral sclerosis (ALS). Prog. Mol. Biol. Transl. Sci 107, 215–262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guenther EL et al. Atomic structures of TDP-43 LCD segments and insights into reversible or pathogenic aggregation. Nat. Struct. Mol. Biol 25, 463–471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Falcon B et al. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561, 137–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Falcon B et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568, 420–423 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fitzpatrick AWP et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Arakhamia T et al. Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell 180, 633–644.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang W et al. Novel tau filament fold in corticobasal degeneration. Nature 10.1038/s41586-020-2043-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cope TE et al. Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain 141, 550–567 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yamada K & Iwatsubo T Extracellular alpha-synuclein levels are regulated by neuronal activity. Mol. Neurodegener 13, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu JW et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci 19, 1085–1092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yamada K et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med 211, 387–393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bero AW et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci 14, 750–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schultz MK Jr. et al. Pharmacogenetic neuronal stimulation increases human tau pathology and trans-synaptic spread of tau to distal brain regions in mice. Neurobiol. Dis 118, 161–176 (2018). [DOI] [PubMed] [Google Scholar]
- 171.Pooler AM, Phillips EC, Lau DH, Noble W & Hanger DP Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 14, 389–394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Holth JK et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kang JE et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mohamed NV, Desjardins A & Leclerc N Tau secretion is correlated to an increase of Golgi dynamics. PLoS One 12, e0178288 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Luo W et al. Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci. Rep 5, 11161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee SJ, Seo BR & Koh JY Metallothionein-3 modulates the amyloid beta endocytosis of astrocytes through its effects on actin polymerization. Mol. Brain 8, 84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bolos M et al. Direct evidence of internalization of tau by microglia in vitro and in vivo. J. Alzheimers Dis 50, 77–87 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Martini-Stoica H et al. TFEB enhances astroglial uptake of extracellular tau species and reduces tau spreading. J. Exp. Med 215, 2355–2377 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yan P et al. Matrix metalloproteinase-9 degrades amyloid-β fibrils in vitro and compact plaques in situ. J. Biol. Chem 281, 24566–24574 (2006). [DOI] [PubMed] [Google Scholar]
- 180.Melchor JP & Strickland S Tissue plasminogen activator in central nervous system physiology and pathology. Thromb. Haemost 93, 655–660 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamamoto N et al. Leptin inhibits amyloid beta-protein degradation through decrease of neprilysin expression in primary cultured astrocytes. Biochem. Biophys. Res. Commun 445, 214–217 (2014). [DOI] [PubMed] [Google Scholar]
- 182.Lee CY & Landreth GE The role of microglia in amyloid clearance from the AD brain. J. Neural Transm 117, 949–960 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Keren-Shaul H et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017). [DOI] [PubMed] [Google Scholar]
- 184.Loria F et al. α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. 134, 789–808 (2017). [DOI] [PubMed] [Google Scholar]
- 185.Stefanova N et al. Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol 179, 954–963 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fellner L et al. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia 61, 349–360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Koller EJ, Brooks MM, Golde TE, Giasson BI & Chakrabarty P Inflammatory pre-conditioning restricts the seeded induction of α-synuclein pathology in wild type mice. Mol. Neurodegener 12, 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Park JY, Paik SR, Jou I & Park SM Microglial phagocytosis is enhanced by monomeric α-synuclein, not aggregated α-synuclein: implications for Parkinson’s disease. Glia 56, 1215–1223 (2008). [DOI] [PubMed] [Google Scholar]
- 189.Lee VM, Goedert M & Trojanowski JQ Neurodegenerative tauopathies. Annu. Rev. Neurosci 24, 1121–1159 (2001). [DOI] [PubMed] [Google Scholar]
- 190.Williams DR & Lees AJ Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 8, 270–279 (2009). [DOI] [PubMed] [Google Scholar]
- 191.Narasimhan S et al. Human tau pathology transmits glial tau aggregates in the absence of neuronal tau. J. Exp. Med 217, e20190783 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Venegas C et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 552, 355–361 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Cavaliere F et al. In vitro α-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains. Neurobiol. Dis 103, 101–112 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Golde TE Harnessing immunoproteostasis to treat neurodegenerative disorders. Neuron 101, 1003–1015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hickman S, Izzy S, Sen P, Morsett L & El Khoury J Microglia in neurodegeneration. Nat. Neurosci 21, 1359–1369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Song WM & Colonna M The identity and function of microglia in neurodegeneration. Nat. Immunol 19, 1048–1058 (2018). [DOI] [PubMed] [Google Scholar]
- 197.Seshadri S et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303, 1832–1840 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Greggio E et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis 23, 329–341 (2006). [DOI] [PubMed] [Google Scholar]
- 199.Healy DG et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 7, 583–590 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sheng Z et al. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci. Transl. Med 4, 164ra161 (2012). [DOI] [PubMed] [Google Scholar]
- 201.Steger M et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5, e12813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.West AB et al. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl Acad. Sci. USA 102, 16842–16847 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bae EJ et al. LRRK2 kinase regulates alpha-synuclein propagation via RAB35 phosphorylation. Nat. Commun 9, 3465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao HT et al. LRRK2 antisense oligonucleotides ameliorate alpha-synuclein inclusion formation in a Parkinson’s disease mouse model. Mol. Ther. Nucleic Acids 8, 508–519 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Henderson MX, Peng C, Trojanowski JQ & Lee VMY LRRK2 activity does not dramatically alter α-synuclein pathology in primary neurons. Acta Neuropathol. Commun 6, 45 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Volpicelli-Daley LA et al. G2019S-LRRK2 expression augments α-synuclein sequestration into inclusions in neurons. J. Neurosci 36, 7415–7427 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Corder EH et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923 (1993). [DOI] [PubMed] [Google Scholar]
- 208.Sepulcre J et al. Neurogenetic contributions to amyloid beta and tau spreading in the human cortex. Nat. Med 24, 1910–1918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hamilton RL Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol. 10, 378–384 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Irwin DJ et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol 72, 587–598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sepulcre J et al. In vivo tau, amyloid, and gray matter profiles in the aging brain. J. Neurosci 36, 7364–7374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hardy JA & Higgins GA Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992). [DOI] [PubMed] [Google Scholar]
- 213.Hardy J & Selkoe DJ The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002). [DOI] [PubMed] [Google Scholar]
- 214.Jack CR Jr. et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jack CR Jr. et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bolmont T et al. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP × Tau transgenic mice. Am. J. Pathol 171, 2012–2020 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vasconcelos B et al. Heterotypic seeding of Tau fibrillization by pre-aggregated Aβ provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol. 131, 549–569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gotz J, Chen F, van Dorpe J & Nitsch RM Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Aβ42 fibrils. Science 293, 1491–1495 (2001). [DOI] [PubMed] [Google Scholar]
- 219.Gomes LA et al. Aβ-induced acceleration of Alzheimer-related tau-pathology spreading and its association with prion protein. Acta Neuropathol. 138, 913–941 (2019). [DOI] [PubMed] [Google Scholar]
- 220.Lewis J et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491 (2001). [DOI] [PubMed] [Google Scholar]
- 221.He Z et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med 24, 29–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Giasson BI et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 300, 636–640 (2003). [DOI] [PubMed] [Google Scholar]
- 223.Oikawa T et al. α-synuclein fibrils exhibit gain of toxic function, promoting tau aggregation and inhibiting microtubule assembly. J. Biol. Chem 291, 15046–15056 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Haggerty T et al. Hyperphosphorylated Tau in an α-synuclein-overexpressing transgenic model of Parkinson’s disease. Eur. J. Neurosci 33, 1598–1610 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wills J et al. Tauopathic changes in the striatum of A53T α-synuclein mutant mouse model of Parkinson’s disease. PLoS One 6, e17953 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ono K, Takahashi R, Ikeda T & Yamada M Cross-seeding effects of amyloid β-protein and α-synuclein. J. Neurochem 122, 883–890 (2012). [DOI] [PubMed] [Google Scholar]
- 227.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ & LaFerla FM Synergistic interactions between Aβ, tau, and α-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci 30, 7281–7289 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Masliah E et al. β-amyloid peptides enhance α-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc. Natl Acad. Sci. USA 98, 12245–12250 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bassil F et al. Amyloid-beta (Aβ) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of Lewy body disorders with Aβ pathology. Neuron 105, 260–275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bachhuber T et al. Inhibition of amyloid-β plaque formation by α-synuclein. Nat. Med 21, 802–807 (2015). [DOI] [PubMed] [Google Scholar]
- 231.Spencer B et al. Anti-α-synuclein immunotherapy reduces α-synuclein propagation in the axon and degeneration in a combined viral vector and transgenic model of synucleinopathy. Acta Neuropathol. Commun 5, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Masliah E et al. Passive immunization reduces behavioral and neuropathological deficits in an α-synuclein transgenic model of Lewy body disease. PLoS One 6, e19338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 233.Games D et al. Reducing C-terminal-truncated α-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J. Neurosci 34, 9441–9454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tran HT et al. α-synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 7, 2054–2065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nobuhara CK et al. Tau antibody targeting pathological species blocks neuronal uptake and interneuron propagation of tau in vitro. Am. J. Pathol 187, 1399–1412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sevigny J et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016). [DOI] [PubMed] [Google Scholar]
- 237.Bae EJ et al. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J. Neurosci 32, 13454–13469 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mandler M et al. Active immunization against α-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol. Neurodegener 10, 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Masliah E et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 46, 857–868 (2005). [DOI] [PubMed] [Google Scholar]
- 240.Mandler M et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson’s disease clinical trials. Acta Neuropathol. 127, 861–879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Scoles DR & Pulst SM Oligonucleotide therapeutics in neurodegenerative diseases. RNA Biol. 15, 707–714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shahnawaz M et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 74, 163–172 (2017). [DOI] [PubMed] [Google Scholar]
- 243.Kraus A et al. Seeding selectivity and ultrasensitive detection of tau aggregate conformers of Alzheimer disease. Acta Neuropathol. 137, 585–598 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Covell DJ et al. Novel conformation-selective alpha-synuclein antibodies raised against different in vitro fibril forms show distinct patterns of Lewy pathology in Parkinson’s disease. Neuropathol. Appl. Neurobiol 43, 604–620 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gibbons GS et al. Detection of Alzheimer disease (AD)-specific tau pathology in AD and nonAD tauopathies by immunohistochemistry with novel conformation-selective tau antibodies. J. Neuropathol. Exp. Neurol 77, 216–228 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Luk KC et al. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl Acad. Sci. USA 106, 20051–20056 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Frost B, Jacks RL & Diamond MI Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem 284, 12845–12852 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Iba M et al. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci 33, 1024–1037 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Holmes BB et al. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl Acad. Sci. USA 111, E4376–E4385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Eisele YS et al. Multiple factors contribute to the peripheral induction of cerebral β-amyloidosis. J. Neurosci 34, 10264–10273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Burwinkel M, Lutzenberger M, Heppner FL, Schulz-Schaeffer W & Baier M Intravenous injection of β-amyloid seeds promotes cerebral amyloid angiopathy (CAA). Acta Neuropathol. Commun 6, 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kaufman SK et al. Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron 92, 796–812 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Taniguchi-Watanabe S et al. Biochemical classification of tauopathies by immunoblot, protein sequence and mass spectrometric analyses of sarkosyl-insoluble and trypsin-resistant tau. Acta Neuropathol. 131, 267–280 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


