Abstract
SARS-CoV-2 is responsible for coronavirus disease 2019 (COVID-19), progressively extended worldwide countries on an epidemic scale. Along with all the drug treatments suggested to date, currently, there are no approved management protocols and treatment regimens for SARS-CoV-2. The unavailability of optimal medication and effective vaccines against SARS-CoV-2 indicates the requirement for alternative therapies. Probiotics are living organisms that deliberate beneficial effects on the host when used sufficiently and in adequate amounts, and fermented food is their rich source. Probiotics affect viruses by antiviral mechanisms and reduce diarrhea and respiratory tract infection. At this point, we comprehensively evaluated the antiviral effects of probiotics and their mechanism with a particular focus on SARS-CoV-2. In this review, we suggested the conceptual and potential mechanisms of probiotics by which they could exhibit antiviral properties against SARS-CoV-2, according to the previous evidence concerning the mechanism of antiviral effects of probiotics. This study reviewed recent studies that speculate about the role of probiotics in the prevention of the SARS-CoV-2-induced cytokine storm through the mechanisms such as induction of anti-inflammatory cytokines (IL-10), downregulation of pro-inflammatory cytokines (TNF-α, IL-2, IL-6), inhibition of JAK signaling pathway, and act as HDAC inhibitor. Also, the recent clinical trials and their outcome have been reviewed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11756-022-01147-y.
Keywords: SARS-CoV-2, Probiotics, Cytokine storm, Immunological response, COVID-19
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), named novel coronavirus by the international virus classification commission, is responsible for coronavirus disease 2019 (COVID-19). It has been progressively extended to cover all countries on a wide-ranging scale after its first detection in Wuhan, China, in December 2019 (Hashemi et al. 2021). The Severe Acute Respiratory Syndrome (SARS)-CoV and the Middle East Respiratory Syndrome (MERS)-CoV were preceding occurrences of coronaviruses (CoVs), which have been previously considered as significant public health threats (Mousavi et al. 2020a). Coronaviruses are small (ranging from 60 to 140 nm in diameter) positive-sense RNA viruses with spike-like glycoproteins on their envelope, which are based on their appearance (crown-like) under an electron microscope named coronavirus (Singhal 2020). Currently, it seems that SARS-CoV-2 transfers from one person to another by the following routes: person-to-person contact with sneeze or cough or contact with infected people’s secretions. The possibility of SARS-CoV-2 transfer from the fecal-oral route is still unclear; however, it was detected to happen throughout the COVID epidemic (Heymann and Shindo 2020). Although different vaccines have been approved against SARS-CoV-2, the patients are still overgrowing around the world, significantly when control measures are reduced. Until now, four variants of concern (VOC) of SARS-CoV-2 have been classified by World Health Organization (WHO), including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) (Tiecco et al. 2022). In November 2021, Omicron (B.1.1.529), another deadly super variant of SARS-CoV-2, generally makes at least 60 mutations in the virus genome structure compared to the original Wuhan strain, was designated the fifth VOC. According to the WHO, VOC is the most worrying variant of COVID-19.
There are no approved management protocols, and treatment regimens among all the drug treatments suggested to date for SARS-CoV-2 (Cunningham et al. 2020). Numerous clinical trials were conducted to evaluate a variety of medicines such as hydroxychloroquine, lopinavir/ritonavir, corticosteroids, and remdesivir for this disease, and the clinical protocols are being updated continually (Negahdaripour et al. 2022). The effectiveness of most of these medicines is under question for the management of SARS-CoV-2; therefore, the clinical prognosis may be unpredictable according to the lack of clinical evidence. Thus, developing a complementary way to discover new preventive and supportive strategies is one of the serious medical needs these days.
In the last ten years, probiotics (viable form) and postbiotics (non-viable form) have been applied to improve physiological conditions such as progressive development of epithelial barrier function, gut homeostasis, and healthy immune responses (Ashoori et al. 2020). Probiotics are potential biological agents with many valuable features in treating viral infections. In this review, we highlighted the antiviral effects of probiotics and their mechanisms, focusing on their potential ability to manage SARS-CoV-2. These mechanisms are based on the antiviral activity of probiotics and immunological pathways affected by SARS-CoV-2.
Probiotics and their importance in current medicine
Probiotics are determined as living organisms that deliberate beneficial effects on the host when used sufficiently and in adequate amounts (Mohkam et al. 2016; Mousavi et al. 2020b). The most common bacteria used as probiotics are lactic acid bacteria (LAB), especially the genus Lactobacillus. These microorganisms are the essential components of the intestinal microflora and are known as “generally regarded as safe” (GRAS). Members of this genus and their characteristics have been redefined many times. In their latest taxonomic classification in 2020, 23 new members have been added (Zheng et al. 2020). The online searching tool, namely “lactotax” (http://lactotax.embl.de/wuyts/lactotax/), is a handy web-based tool to identify the Lactobacillus family further. The appropriate needed dose for probiotic products is related to their strain. The over-the-counter products have an average of 1–10 billion colony forming units (CFU)/dose, while some products are efficient at the lower range, and some require more CFU for a single dose. This is why human studies define dosage according to the product’s health benefits. Since probiotics are alive, they may die off during storage, so the manufacturer should build in overages so that the potency mentioned on the label doesn’t fall at the end of the product’s shelf life. However, the spore-forming strains resist environmental stress during shelf-life (Sanders et al. 2010, 2016).
Probiotic-containing fermented foods against COVID-19
Fermented foods have been the reach source of probiotics and have been used for thousands of years (Zhao et al. 2019). For instance, kimchi, a traditional Korean fermented food, contains about 200 types of probiotics, or serofluid dishes, existing in Chinese culture, produce probiotic cultures, bacteriocins, and enzymes due to forming complex and distinct bacterial communities (Chen et al. 2016). Leuconostoc mesenteroides and Lactiplantibacillus plantarum (L. plantarum) have been identified as the major species in kimchi. However, numerous studies suggest that LAB contributing to kimchi fermentation include Leuconostoc citreum, Leuconostoc gasicomitatum, Levilactobacillus brevis, Latilactobacillus curvatus, Latilactobacillus sakei subsp. sakei, Lactococcus lactis, Pediococcus pentosaceus, W. confusa, and W. koreensis (Di Cagno et al. 2016). Kimchi LAB exhibit antioxidative, anticancer, immune-stimulatory effects, anti-obesity, and other probiotic activities (Park et al. 2017). It is proposed that fermented cabbage in kimchi, because of their Lactobacillus content, is a proof-of‐concept of dietary management that may augment Nrf2‐associated antioxidant effects helpful in alleviating COVID‐19 severity (Bousquet et al. 2021). The most familiar fermented foods, e.g., probiotic milk, yogurt, and honey, also benefit viral diseases. Human milk contains LAB, which protects against rotavirus (Mirashrafi et al. 2021). Sourdough is the oldest form of slowly fermented leavened bread used as early as 2000 BC by the ancient Egyptians. All sourdough starters were harbored with Leuconostoc citreum, L. plantarum, and Lactococcus lactis, which are almost similar to the microbial profiles of dough prior to fermentation (Rizzello et al. 2015). Sourdough provides gastrointestinal benefits, contains natural prebiotics and probiotics, boosts the number of vitamins and minerals, enhances mood and energy, and has antioxidant properties (Lau et al. 2021).
Traditional kefir, derived from the Caucasus Mountains, is a fermented milk drink with a creamy texture, sour taste, and subtle effervescence (Lopitz-Otsoa et al. 2006). Many microbial species have been identified in kefir grains, commonly including Levilactobacillus brevis, Lacticaseibacillus paracasei subsp. paracasei, L. helveticus, Lactobacillus kefiranofaciens subsp. kefiranofaciens, L. plantarum, Lentilactobacillus kefiri, Lactococcus lactis, Streptococcus thermophiles, Acetobacter lovaniensis, Acetobacter orientalis, Saccharomyces cerevisiae, S. uni sports, Candida kefyr, Kluyveromyces marxianus and Leuconostoc mesenteroides (Prado et al. 2015). Kefir exerts antimicrobial activity and immunostimulatory effects and improves gut dysbiosis (Dimidi et al. 2019). Kefir and kefir derivatives can inhibit viral activity by modulating immune-system responses and/or disrupting viral adhesion. The antiviral mechanisms of kefir involve enhancing macrophage production and boosting the activity of pro-inflammatory cytokines. Kefir has anti-inflammatory activity by inhibiting the activity of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6. Using kefir (and its byproducts) as an inhibitor of the expression of pro-inflammatory cytokines in COVID-19 patients could be a viable policy (Hamida et al. 2021).
Kombucha is a fermented tea beverage in Northeast China around 220 BC and was consumed extensively during the Qin Dynasty. Similar fermented tea beverages became popular in Russia and Eastern Europe (Mousavi et al. 2020b). The bacterial and fungal species presenting in the kombucha typically include acetic acid bacteria (Acetobacter, Gluconobacter), LAB (Lactobacillus, Lactococcus), and yeasts (Saccharomyces, Zygosaccharomyces) (Coton et al. 2017). Kombucha has been displayed to exert effects in animal studies on blood glycemia, oxidative stress, diabetes-induced weight loss, chemically-induced nephrotoxicity, hypercholesterolemia, and indomethacin-induced gastric ulceration (Dimidi et al. 2019). Sauerkraut is one of the most usual form of preserved cabbage originating in the 4th century BC. Sauerkraut is eaten regularly in Germany, other European and Asian countries, and the United States (Raak et al. 2014). Sauerkraut (home-made and shop-bought) has been revealed to comprise Bifidobacterium dentium, Enterococcus faecalis, Lactobacillus casei, Lactobacillus delbrueckii, Staphylococcus epidermidis, Lactobacillus sakei, Lactobacillus curvatus, Lactobacillus plantarum, Lactobacillus brevis, Weissella confusa, Lactococcus lactis, and Enterobacteriaceae (Bati and Boyko 2016). Oral administration of sauerkraut juices in Wistar rats directed to increased activity of glutathione S-transferase (GST) and NAD(P)H: quinone oxidoreductase 1 (NQO1), critical liver and kidney detoxifying enzymes (Krajka-Kuźniak et al. 2011).
The consumption of home-made fermented foods (yogurt, kefir, sauerkraut, kombucha) in commercial products containing probiotics and prebiotics is part of a comprehensive nutritional strategy to enhance the function of the gut microbiota, promote mucosal immunity and potentially upper respiratory tract immunity, be potentially better prepared to face viral or bacterial infections caused by respiratory syndromes (Antunes et al. 2020).
Despite all the positive attitudes, experimental studies and trials have not yet confirmed the significant effect of probiotics-containing foods in preventing and treating viral diseases, especially COVID-19. Kinoshita et al. conducted a clinical trial on the dietary intake of yogurt fermented with Lactobacillus delbrueckii ssp.. bulgaricus. This trial revealed that this diary product could not prevent influenza or enhance NK cell activity (Kinoshita et al. 2019). In addition, one should pay serious attention to the interaction between food-food and food-medicine when consuming probiotics containing food. A recent clinical trial indicated that the intake of fermented foods might affect the effects of Pediococcus acidilactici on preventing viral respiratory tract infections (Hishiki et al. 2020). Anti-infectious immune systems are induced just in children who eat less than two fermented foods or yogurt per week along with probiotic strains.
Bioinformatics study and computational analysis of probiotics and COVID-19
Nowadays, bioinformatics tools can be used to analyze the properties of different compounds and even their biological effects with computational mathematical studies with less time and lower cost than laboratory studies and clinical trials (Negahdaripour et al. 2022). Bioinformatics also provides accurate insight for discovering and designing creative therapeutic compounds. Because of the importance and urgency of providing an effective medicine for COVID-19, the notable contribution provided by the computational approach during the ongoing pandemic inspires further efforts toward development and adoption (Eetemadi et al. 2020). We can investigate the potential of probiotics against SARS-CoV-2 through an in-silico analysis and delves into the nature of bacteria-virus interaction by docking approaches.
One of the most exciting new approaches to influence the health and disease system is developing the knowledge of the human microbiome as a biological system. The recently provided opportunity to profoundly understand the complex relationships between the human body and the microbiome environment can be seen as a time to paradigm-shift health. This unique situation is due to the free availability of genomic and proteomic tools, which make unprecedented progress in mining and applying biological and clinical data, including dietary habits, identification of human microbiome species, especially in the gastrointestinal tract, and the expansion of systems biology.
Thus, in the midst of the COVID-19 epidemic, computational technology and bioinformatics as integral parts of probiotic-related research is a potentially quick and practical approach. In this regard, reviewing existing data sources and using a computational approach to analyze available data can be better strategies to understand the effectiveness (or ineffectiveness) of probiotics. For long-term control of this lethal disease, the two main approaches in this field are the microbiome-driven and ensemble-driven docking approaches (Nguyen et al. 2022). Computational methods associated with the microbiome-driven approach can facilitate exploring the human microbiome. This type of in-silico research can answer the mechanism of affecting the human body microbiome on respiratory tract infections and how it may affect the severity of COVID-19. In the two contexts of metagenomics and meta-transcriptomics, several bioinformatics tools for distinguishing GI microbiome offer an understanding of the interaction between microbiome and COVID-19 (Yeoh et al. 2021).
Ensemble-driven docking approaches can lead to discovering accelerated and more accurate therapeutic targets against COVID-19. To that end, COVID-19 data is being shared at an unprecedented rate worldwide. Bioinformatics strategies in drug modeling, molecular binding, molecular dynamics simulation, and ADMET study have been profoundly investigated for screening potential molecules (including probiotics) to combat COVID-19 from multiple databases. In addition, the basic and applied sciences have benefited from the analysis of SARS-CoV-2 data by computational tools. Bioinformatics pushes the experiments about probiotics on SARS-CoV-2 to further research antiviral probiotics and find possible SARS-CoV-2 protein targets.
Immunological aspect and mechanism study
While some strains have their unique features for certain neurological, immunological, and antimicrobial activities, some of the mechanisms of probiotics may be similar between various strains, species, or even genera. For example, many probiotic strains may have the ability to produce short-chain fatty acids or reduce luminal pH in the colon. Probiotics are likely to influence intestinal mucosa by controlling the native microbiota population, preventing the proliferation of harmful bacteria, improving lymphoid tissues in the gut, and enhancing systemic immune responses (Azarang et al. 2020; Gholami et al. 2020). Particular probiotics offset the production of pro-, and anti-inflammatory cytokines, so forming the healthy host-microbe cross-talk is required to preserve inflammatory responses (Karaffova et al. 2017). Probiotics are applied to raise oral vaccine responses and treat enteric infections (Zimmermann and Curtis). Probiotics reduce the incidence of acute infections in the upper respiratory tract and replication of antibiotic use and decrease the duration of every episode (Fonollá et al. 2019b). The single mutant Labile Toxin (LT) (S61 K) reduced the stimulation of tumor necrosis factor (TNF)-α and IL-6 in bone marrow-originated cells, specifying its potential clinical use for allergy and asthma management. Double mutant heat-labile toxin (dmLT) (R192G/L211A) was observed to elevate the production of Th17 cytokine IL-17 A in peripheral blood mononuclear cells. It can elicit dendritic cells (DCs) and improve the rate of IL-17 A+, IFN γ+, and TNFα + secreting CD4 + T cells in the cervical lymph nodes of immunized mice (Jiang et al. 2017b).
Immunologic consequences of probiotic supplementation in human
In the last years, probiotics have been considered a regulator of innate and adaptive (humoral and cellular) immunity. Probiotic’s beneficial effects such as augmented peripheral immunoglobulin production, induction of IgA secretion, and reduced production of pro-inflammatory cytokine has been reported previously (Montazeri-Najafabady et al. 2019). The valuable properties of probiotics could be related to the regulation of immunological pathways. As noted, Bifidobacterium or probiotic bacterial species secreting less endotoxin than Gram-negative bacteria decrease the stimulation of inflammatory intermediates like TNF-α. It was described that the administration of Limosilactobacillus fermentum (L. fermentum) CECT5716 meaningfully declined the infection frequency of influenza virus (IFNV) and augmented natural killer (NK) cell cytolysis activity and TNF-α, anti-influenza specific IgA, and IgM levels (Bajpai et al. 2018). Escherichia coli Nissle (EcN) is one of the Gram-negative probiotics that have bacteriocidal and immunomodulatory effects, for instance, preventing invasion of harmful bacteria to the epithelial cells, inducing secretion of β-defensin to epithelial cells, and regulating T cell propagation (Gholami et al. 2015; Kandasamy et al. 2016). Limosilactobacillus reuteri (L. reuteri) encouraged immune response in Specific Pathogen-Free (SPF) mice. In healthy SPF mice and SPF mice infected with bacterial pathogens, L. reuteri L26 enhanced phagocytic activity and amplified the percentage of T-lymphocytes, CD4 + lymphocytes, NK cells, and regulatory T-cells. Also the upregulated synthesis of pro-inflammatory cytokines (TNF-α, IL-1b, MCP-1) in peripheral blood and mesenteric lymph nodes were noted (Karaffova et al. 2017). Lactobacillus delbrueckii OLL1073R-1 (LDR-1) can release immunomodulatory extracellular polysaccharides (EPSs). EPSs facilitate the collaboration of immunobiotics and host through attachment to pattern recognition receptors (PRRs) expressed in non-immune and immune cells, and it can meaningfully elevate IFN-γ synthesis by murine splenocytes. Elderly individuals that use the immunobiotic LDR-1 yogurt exhibited high NK cell cytolysis activity and decreased the probability of gathering colds (Laiño et al. 2016).
Probiotics are widely recognized to act against viruses by maintaining host immunological responses through induction of immunoeffector cells (interleukins, NK cells, macrophages, immunoglobulins, T-helper cells). The antiviral properties of probiotics, primarily depend on strain specificity, can be through probiotic-virus communication, secretion of antiviral and antibacterial substances, and/or the probiotic-associated regulation of the immunological pathways. LAB can also release various substances exhibiting antiviral and/or antagonistic properties such as hydrogen peroxide (H2O2), lactic acid, bacteriocins, bacteriocin-like substances, short-chain fatty acids, and polysaccharides (Arena et al. 2018).
Immunologic features of SARS-CoV-2
Generally, after virus entry, virus PRRs comprising C-type lectin-like receptors, toll-like receptor (TLR), NOD-like receptor (NLR), and RIG-I-like receptor (RLR) is spotted by the host innate immune system. The SARS-CoV-2 stimulates the synthesis of inflammatory cytokines, the development of DCs, and the production of type I interferons (IFNs) that prevent virus expansion and quicken virus phagocytosis by macrophages. Antigenic peptides of coronavirus are extended by major histocompatibility complex (MHC); or human leukocyte antigen (HLA) in humans) and formerly detected by virus-specific cytotoxic T lymphocytes (CTLs). The antigen presentation of SARS-CoV-2 mainly relies on MHC I molecules, but MHC II also takes part. Presentation of antigens on the cell surfaces then induces the body’s humoral and cellular immunity, modulated by virus-specific B and T cells. The antibody arrangement against the SARS-CoV-2 virus has a standard IgM and IgG production configuration like acute viral infections. The SARS-specific IgM antibodies disappeared 12 weeks after their presentation, although the IgG antibody can remain for an extended period, which shows that the IgG antibody may mostly play a supportive role. The CD4 + and CD8 + T cells count in the peripheral blood of SARS-CoV-2-infected patients are decreased meaningfully. Huang et al. indicated that acute respiratory distress syndrome (ARDS) is the foremost death reason for SARS-CoV-2. Cytokine storm [the lethal unrestrained systemic inflammatory response after the secretion of large quantities of pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGF-β) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10) by active immune cells in SARS-CoV-2 infection] is known as a primary reason for ARDS (Huang et al. 2020b). The immune system attack on the body is stimulated by the cytokine storm, resulting in ARDS and failure of multiple organs, and lastly, death in the acute phase of SARS-CoV-2 infection (Li et al. 2020). Nevertheless, the SARS-CoV-2 N protein conceals the virus from the immune responses.
Effects of probiotics on previous viral infections
The established effects of immunobiotics against viral infection were through modulating innate and adaptive immunity that results in the decline of the period of the disease, the episode counts, and virus cracking (Zelaya et al. 2016).
Ang et al. demonstrated the antiviral properties of L. reuteri against enteroviruses (Coxsackievirus A and Enterovirus 71) (Ang et al. 2016). Other studies displayed the protective effects of Bifidobacterium adolescentis (B. adolescentis) as opposed to Coxsackievirus B3 (CV-B3) (Kim et al. 2014) and Lactiplantibacillus plantarum (L. plantarum) and its culture supernatant against Coxsackievirus B4 (CV-B4) (Arena et al. 2018). In another study, probiotic L. reuteri showed antiviral properties against porcine circovirus type 2 (PCV2) in the intestine by promoting the gut immune response (Karaffova et al. 2017).
Kandasamy et al. 2016 also informed that EcN and Lacticaseibacillus rhamnosus (L. rhamnosus) Strain GG had antiviral activity against rotavirus infection by modulating B cell responses (Kandasamy et al. 2016b). Moreover Bifidobacterium adolescentis SPM0212 and SPM1005 exhibited antiviral properties against the Hepatitis B virus (Lee et al. 2013) and human papillomavirus (Cha et al. 2012), respectively. Also, Bifidobacterium and Lactobacillus spp. exerted inhibitory effects on the herpes simplex virus (An et al. 2012), human influenza virus (Kwak et al. 2013), and human immunodeficiency virus (Fuku et al. 2016).
As previously defined, the antiviral activities of probiotics are strain-dependent, and some probiotics have demonstrated antagonist activity more than others. For example, L. fermentum ACA-DC179, E. faecium PCK38, L. plantarum PCA236, Lactiplantibacillus pentosus (L. pentoses) PCA227, B. animalis subsp. lactis BB-12, Lacticaseibacillus casei (L. casei) shirota, and B. longum SP07/3 revealed the most antiviral activities (Al Kassaa et al. 2014). Other published articles already displayed that L. pentosus (Kiso et al. 2013)d rhamnosus have advantageous properties as opposed to severe viral infections triggered by influenza viruses (Song et al. 2016). Dietary supplements of Lactobacillus and Leuconostoc probiotics may encourage health benefits against influenza (Bae et al. 2018b). Regular ingestion of L. casei shirota lowered plasma Cytomegalovirus (CMV) and Epstein–Barr virus (EBV) antibody titers, an impact that can be clarified as an advantage to overall immune status (Gleeson et al. 2016b). The significant decrease in the intestinal and serum Human Rotavirus (HRV)-specific Ab responses in EcN-colonized piglets compared with control piglets were in line with the reduction in fecal HRV shedding titers and diarrhea in the EcN group (Kandasamy et al. 2016b).
In recent times, a clinical study performed on humans proved the anti-rotaviral properties of probiotics by representing the limitation of the duration of diarrhea, which advised that these beneficial microbes might be suitable for the management of acute rotaviral gastroenteritis or as a substitute treatment without adverse effects (Bajpai et al. 2018).
Administration of C. pseudo diphtheriaticum to infant mice raised the production of IFN-β, TNF-α, and IL-6. Simultaneously, it also enhanced the secretion of IL-10, whose most prominent role contributes to restricting inflammation during Respiratory Syncytial Virus (RSV) infection, which subsequently reduces damaging effects in response to RSV (Kanmani et al. 2017).
One study suggests that EPS from L. delbrueckii OLL1073R-1 (LDR-1) could improve intestinal innate antiviral response and prevent intestinal viruses such as rotavirus (Kanmani et al. 2018). Microbial dysbiosis due to viral infection can also be replaced by probiotic supplementation. In a recent experiment, a significant reduction in the counts of Lactobacillus and Bifidobacterium strains has been observed due to COVID-19 (Xu et al. 2020). However, the data from another unpublished study represented that the probiotic supplementation (Lactobacillus acidophilus and Bacillus clausii) of infected animals with coronavirus did not affect the severity of infection and expression of coronavirus receptors (Feng et al. 2020). We provided a list of probiotic strains that had an impact on different viral infections and a concise mechanism of action as well as the cell type affected. We summarized the majority of antiviral probiotics with other information, including probiotic strain, target virus, mechanism of antiviral activity, and examined host cell type in Table S1.
Mechanism of antiviral activity of probiotics
Some mechanisms make probiotic therapy of viral infection successful: (1) direct interaction between probiotic and virus, (2) production of antiviral inhibitory metabolites, and (3) stimulation of the immune system (Al Kassaa et al. 2014; Drider et al. 2016; Ichinohe et al. 2011; Wu et al. 2013). Moreover, it was suggested to consider the effect of probiotics on the epithelial cells of the eukaryotic host and microbiota that can modify the number of calcium ions and electrolyte potential (Hoffmann et al. 2017; Olaya Galán et al. 2016).
Probiotics are well known for maintaining immune responses, which can boost the immune responses versus the virus, inducing antibodies, T-lymphocyte cells (especially Th cells), NK cells, interleukins, and mononuclear phagocytic cells (Maragkoudakis et al. 2010). As another possible mechanism of probiotics for immunomodulation, they can activate IFNs signaling that cause increasing the expression of several IFN-stimulated genes translated to proteins that can neutralize the virus transcription (Sadler and Williams 2008).
Probiotic strains that can beneficially regulate mucosal immunity are called “immunobiotics” (Shigemori and Shimosato 2017). Treatments with immunobiotics can regulate the TLR signaling regulator expression and generate of cytokines/chemokines, modify the rotaviral-induced inflammation (Ishizuka et al. 2016; Villena et al. 2016). Wang et al. (Wang et al. 2010) revealed that probiotics could inhibit the replication rate of the Newcastle disease virus.
Probiotics can produce different substances with antiviral features. The inhibition of viral reproduction in tissue cultures by various bacteriocins, lactic acid (Klebanoff et al. 1999), and hydrogen peroxide (Dembinski et al. 2014) has been presented in some studies (Chikindas et al. 2018). Some studies showed that enterocins such as CRL35 or ST4V (produced by different subtypes of Enterococcus mundtii) and ST5Ha (produced by Enterococcus faecium) have virostatic effects on herpes simplex virus (HSV) subtypes, coxsackie, and poliovirus (Quintana et al. 2014; Todorov et al. 2005, 2010; Wachsman et al. 2003). The effectiveness of bacteriocins of Bifidobacteria and Lactobacilli against rotavirus and adenovirus species (Choi et al. 2009), together with subtilisin, was proved effective against HSV-1 and HSV-2 (Torres et al. 2013). L. delbrueckii bacteriocin revealed anti-influenza virus activity (Serkedjieva et al. 2000). Furthermore, some classic probiotic species may trigger the production of several interleukins (namely 12, 22, 25, and 33), intestinal transforming growth factor via antigen-presenting cells; IL22 by innate immune cells; IL12, IL25, IL10, and TGF via antigen-presenting cells; improving GI wall performance, reducing effector cells and modifying immune cell systems (Vlasova et al. 2016). L. casei shirota supplementation in the HIV-infected children caused significant raises in CD4 + cells significantly on the Th17 subset, in combination with a remarkable fall in the amount of activated CD8 + cells (Ishizaki et al. 2017). Collectively, intervention in DCs differentiation and maturation process helps to amplify TLR/nuclear factor-κB signaling pathway, and regulate the inflammatory mediators were partially causing the protective effects of L. rhamnosus GG (Jiang et al., 2017a). Several possible mechanisms can provide partial protection versus human rotavirus infection induced by EcN: (a) directly affects the viral infection and (b) kills the virus by modifying the immune system of the host (Karaffova et al. 2017).
Probiotic-derived peptides may stop endocytosis via interference in clathrin-coated pit formation, which is necessary for the virus to enter the endosomes and, consequently, inhibit the viral transcriptional complex. It was shown in a study that there is a conjugation between oligopeptides of probiotic strains and the viral capsids that can disrupt the lipid membrane, followed by pore formation at specific concentrations, which lets the viral component diffuse from viral cells (Bajpai et al. 2018).
The genome sequencing of several Lactobacillus and Bifidobacterium strains reported the presence of some components such as surface layer glycoprotein related to the cell envelope. It demonstrated a correlation between the ability to attach to host cells and the ability of strains to counteract the antiviral effects regardless of the impact of their metabolites. These proteins play an essential role in signaling DCs and T cell functioning (Abdelhamid et al. 2019).
Furthermore, it is proven that when the elasticity of the probiotic cell membrane increases, the immune system regulatory mediators such as IL-12 and IFN-γ and anti-inflammatory mediators such as macrophage and nitric oxides increase (Мokrozub VV et al. 2015). An animal study suggested that due to antagonistic properties and the ability to adhere to mice cells, using these probiotic strains may protect against viral pathogens (Servin 2004).
The systemic activity of probiotics in some body compartments reduces viral replication. Taking a specific strain of Lacticaseibacillus paracasei (L. paracasei) decreases the inflammatory mediators, such as IFN-γ, TNF-α, and IL-17 in the respiratory system (Dos Santos Pereira Andrade et al. 2017). S-layer protein of probiotics may also exhibit antiviral effects. For instance, S- the layer protein of Lactobacillus can stimulate the mouse DCs activation, H2N9 virus invasion of DCs inhibition, and IFN signaling pathway stimulation could be a promising antiviral compound against H2N9 infection (Gao et al. 2016).
Besides, proteins produced by probiotic strains (e.g., L. casei and B. adolescentis) directly interact with viral surface glycoproteins (VP4 or VP7) and prohibit the virus entry into the MA104 cells and/or virus adhesion (Fernandez-Duarte et al. 2018).
In an attempt to find a potential treatment for SARS-CoV-2, we assumed using probiotics since their previous effects on viral infections and their antiviral mechanisms could improve the health status of people with many different infectious diseases.
Proposed mechanism of probiotics for protection against SARS-CoV-2
There is no available vaccine and a prominent worthwhile, clinically approved treatment for SARS-CoV-2. The best strategies are preventing SARS-CoV-2 by maintaining high hygiene by washing our hands, refusing connection with infected people, and reinforcing our immune system.
There is a strong evidence of the connections between gut microbiome structure and composition and health or disease. It has been recently observed that a modification of the physiological homeostasis of intestinal microbiota, also known as dysbiosis, is associated with some disorders. Dysbiosis related to loss of strain diversity was correlated with various diseases, from antibiotic-associated diarrhea to type 2 diabetes or common infectious diseases (Le Chatelier et al. 2013). Janda et al. (2021) suggested that changing microbiome composition in the elderly, obese people, and those with underlying chronic disease may expose these people more extensively to the fetal adverse effects of COVID-19 than other individuals. In that study, the opinion was that “A healthy microbiome could be one of the factors responsible for lower case fatality ratio on COVID-19 patients”. So, rebalancing the gut microbiome composition with probiotics could be an excellent strategy to counter COVID-19 and decrease its fetal rate. In addition, patients with COVID-19 developed bacterial imbalances and a decrease in the count of gut microbiota, especially in the strains of Lactobacillus and Bifidobacteria (Xu et al. 2020). Interestingly, some clinical trials have shown that taking a combination of probiotics, including L. rhamnosus GG, live B. subtilis, and E. faecalis to admitted patients in the ICU who have been inhaled with a ventilator has been able to reduce their risk of ventilator-associated pneumonia (Morrow et al. 2010; Zeng et al. 2016). Studies have also shown that probiotics can have anti-coronavirus effects; however, these effects have not yet been studied on the SARS-CoV-2 virus (Baud et al. 2020).
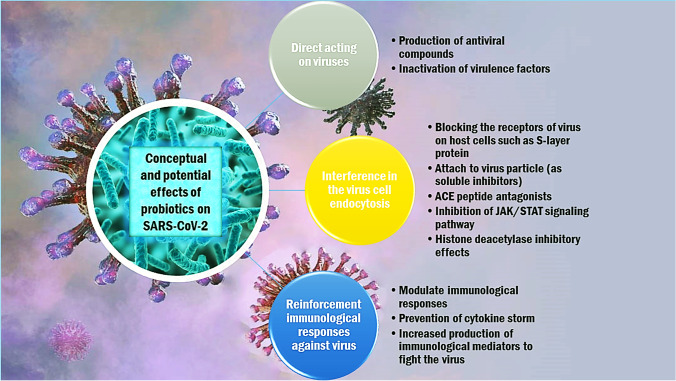
During the last decades, several probiotics prevented and/or decreased the duration of either bacterial or viral infections. There is no scientific basis for applying probiotics to specifically protect, prevent, or treat COVID-19. However, based on an indirect assumption, some clinical researchers have recommended using probiotic supplements for patients with COVID-19 (Mak et al. 2020). In the following section, we discuss the possible mechanisms of probiotics that may affect SARS-CoV-2 (Fig. 1). The conceptual and potential antiviral mechanisms of probiotics against SARS-CoV-2 can be classified into three categories as follow:
Fig. 1.
Proposed mechanisms of antiviral effects attributed to probiotics against SARAS-CoV-2
Direct effect on viruses
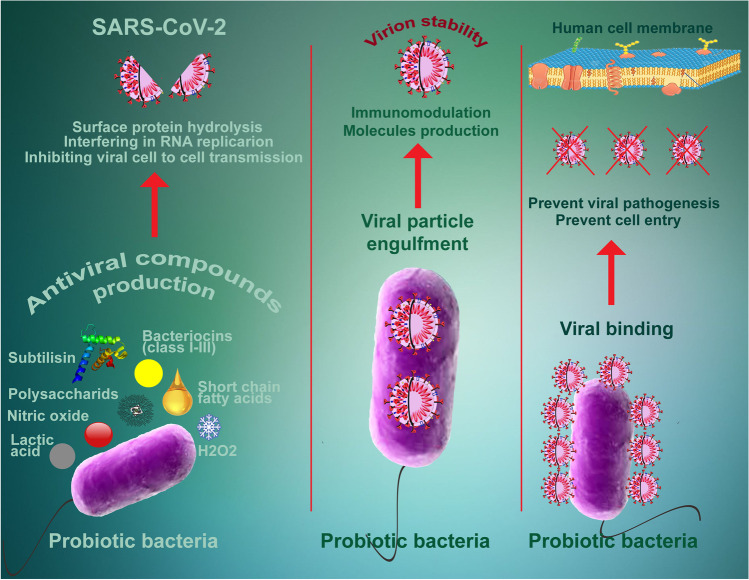
Probiotics can act against viral infections directly by producing antiviral metabolites like lactate, H2O2, bacteriocins, polysaccharides, short-chain fatty acids, subtilisin, nitric oxide, probiotic peptides; and inactivation of virus virulence factor (Fig. 2). Wang et al. reported that a probiotic strain of Bacillus subtilis and its lipoheptapeptide metabolite, surfactin, could be attached to viral particles of transmissible gastroenteritis coronavirus after entering the animal intestinal epithelial cells, inhibit their activity, and finally, inactivate the virus life cycle (Wang et al. 2017). Levilactobacillus brevis (L. brevis) strain CD2 can directly interact with the herpes simplex virus type 2 through its cell wall components and inhibit viral activity (Mastromarino et al. 2011).
Fig. 2.
Schematic presentation of the direct antiviral mechanism of the probiotics against SARS-CoV-2. Probiotics can affect the virus by directly binding to viral particles, producing antiviral compounds, and antiviral effects through hydrogen peroxide, nitric oxide, bacteriocins and subtilisin
Interference in virus endocytosis
SARS-CoV-2 enters target cells through the angiotensin-converting enzyme (ACE) receptor-2 (Sommerstein et al., 2020). It was initially suggested that compounds that could block the renin-angiotensin system, such as ACE inhibitors, may be the potential therapeutic tools for SARS-CoV-2 (Gurwitz 2020). Probiotic strains have affected the activity of ACE, both directly and indirectly. Peptide metabolites produced by the gut microbiota can now block the active site of the ACE during food fermentation and inhibit its function. Furthermore, cell body masses of probiotics are ACE inhibitors. Therefore, since ACE receptors are the main gateway for SARS-CoV-2 to enter GI cells, probiotics can potentially prevent the virus from entering the body by inhibiting ACE receptors (Olaimat et al. 2020).
Moreover, probiotics release ACE-inhibiting peptides may be one of the possible mechanisms by which probiotics could block the virus entry into the cells (Ramchandran and Shah 2008). A recent in-silico computational study on molecular dynamics of probiotic metabolites clearly showed the realism of this idea. Anwar et al. bioinformatically evaluated the antiviral effect of metabolites of L. plantarum through multiple mechanistic approaches and showed their potency in blocking the viral entrance to cells via binding to RdRp, RBD, and ACE2 (Anwar et al. 2020).
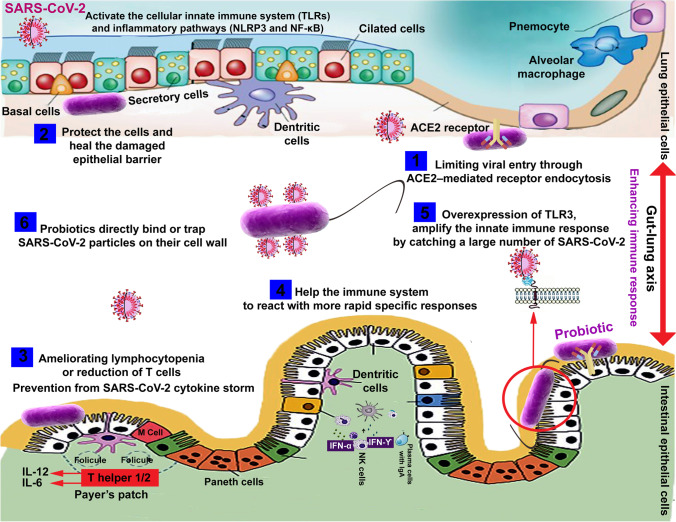
Besides, some protein kinase (NAK family), including adaptor-associated protein kinase 1 (AAK1) and cyclin G-associated kinase (GAK), cause viral endocytosis and are affected by janus kinase (JAK) inhibitors (baricitinib), which block virus entry to pneumocytes (Jamilloux et al. 2019). JAK inhibitors may also be advantageous in the cytokine dysregulation associated with SARS-CoV-2 (Richardson et al. 2020; Stebbing et al. 2020) as they could affect the host inflammatory response and virus entry to the cells. Previously, it was reported that probiotics could inhibit the JAK/STAT signaling pathway. Streptococcus thermophilus prevented the reduction in TER induced by TNF-α- and IFN-γ and increased epithelial permeability by inhibiting the JAK/STAT signaling pathway (Resta-Lenert and Barrett 2006). The expressions of these inflammatory mediators through STAT-1/STAT-3 activation and JAK2 inactivation are relieved significantly by L. plantarum, L. rhamnosus, and L. acidophilus (Lee et al. 2010). Figure 3 schematically depicts the set of mechanisms involved in the probiotic effect on the virus entering the human cells and the inflammatory responses involved in probiotic’s antiviral activity.
Fig. 3.
Inhibitory effects of probiotics and their metabolites on viral cell endocytosis. Probiotics metabolites can block viral attachment by steric hindrance and cover receptor sites in a non-specific manner and induction of mucosal regeneration, thus binding virus particles and inhibiting adherence to epithelial cells, leading to inhibition of virus replication
Reinforcing immunological responses against viruses
When a series of cytokines, including TNF-α, IL-(1β, 2, 6), IFN- (α, β, γ), and MCP, induce the production of free radicals that cause SARS-CoV-2 ARDS and multiple organ failure, release, cytokine storm occurs (Tisoncik et al. 2012).
Huang et al. found a correlation between serum level of some ILs (2, 7, 10), granulocyte colony-stimulating factor, and TNF-α and the severity of COVID-19 (Huang et al. 2020). Diao et al. found that the severity of COVID-19 is associated with TNF-α, IL-6, and IL-10 levels (Diao et al. 2020). Immunosuppressive approaches for cytokine storm include the immune response directed by the regulation of T cells, the inhibition of TNF-α, IFN-γ, IL-1, and JAK (Behrens and Koretzky 2017), inhibition of cytokine signaling (Kedzierski et al. 2014), and histone deacetylase (HDAC) inhibitors (Li et al. 2008).
There are generally two main mechanisms for the immunomodulatory effects of using probiotics. First, they stimulate the production of IL-12 and subsequently activate NK, Th1, and Th2 immune cells, which are considered immunostimulatory effects and are used against infectious diseases or allergies. Second, cooperation in the production of IL-10 and the activation of regulatory T cells that modulate the acquired immune system are considered immunoregulatory effects (Chiba et al. 2010).
Probiotic’s immunomodulatory properties against SARS-CoV-2 may be modulated through many different functions such as the increase of NK cells, T helper cells, immunoglobulins, macrophages, and CD4+; the decrease of CD8+; induction of interleukin 10 and TGF-β; suppression of .TNF-α, IL-2, and IL-6. Also, probiotics show their beneficial properties against SARS-CoV-2 by inhibiting the JAK/STAT signaling pathway and HDAC. Impairment of viral replication in targeted cells can be caused by Type I of INFs that have antiviral activities. Scagnolari et al. suggested that IFN-β could act on SARS-CoV better than INF-α (Scagnolari et al. 2004). It was disclosed that Lactobacillus and Bifidobacterium strains induce different IFN-β profiles in DCs (Weiss et al. 2011). In another study, L. acidophilus NCFM highly induced IFN-β expression in murine DCs (Weiss et al. 2010).
Resta-Lenert and Barrett informed that probiotics prevented from harmful effects of TNF-α and IFN-γ on epithelial function (Resta-Lenert and Barrett 2006). Another study reported that oral administration of L. acidophilus strain SW1-induced suppression of pro-inflammatory cytokine TNF-α through a Treg-dependent manner (Resta-Lenert and Barrett 2006). Borruel et al. detected a substantial reduction in pro-inflammatory TNF-α in inflamed mucosa with L. casei and Lactobacillus delbrueckii subsp. bulgaricus (Borruel et al. 2002). Lately, Karamese et al. assessed probiotics effects on the immune system via the upregulation of anti-inflammatory cytokines (e.g., IL-10) and the downregulation of pro-inflammatory cytokines (e.g., TNF-α and IL-6) by supplementing a Lactobacillus and Bifidobacterium species mixture to rats (Karamese et al. 2016).L. fermentum L930, L. paracasei L350 and B. animalis subsp. animalis IM386 demonstrated significant downregulation of pro-inflammatory cytokines IL-12 and IL-6 (Citar et al. 2015). Also, B. breve reserved inflammation by inducing pro-inflammatory cytokine IL-10 (Jeon et al. 2012).
As another possible mechanism to suppress cytokine storm induced by SARS-CoV-2, probiotic’s short-chain fatty acids (butyrate) can epigenetically regulate the expression of host genes via HDAC inhibition. Valeric acid produced by M. massiliensis MRx0029 exhibited HDAC inhibition activity. Yuille et al. also observed that R. intestinalis MRx0071, M. massiliensis MRx0029, and B. massiliensis MRx1342 are potent inhibitors for class I HDACs, particularly HDAC2 (Yuille et al. 2018). Elucidation of the immune response has also been reported by producing the L. plantarum metabolites, including γ-aminobutyric acid (GABA), lactic and/or acetic acid, and plantaricin (Albarracin et al. 2017). Despite all the explanations and information provided, to date, the reasons for the use of probiotics against SARS-CoV-2 have been derived only from hypothetical suggestions and, thus, it is not possible to make an accurate and evidence-based conclusion unless proper preclinical and clinical studies are designed, and the results are analyzed blindly and randomly.
Clinical trials related to probiotic’s effects on COVID-19
Oral or parenteral administration of probiotic dietary supplements to relieve the symptoms of COVID-19 in humans by strengthening the host immune response and improving intestinal microbiota has been considered by scientists and physicians since the beginning of the epidemic. Previously, in-vitro and in-vivo studies have shown that different probiotic strains can combat SARS-CoV-2 or its associated symptoms. Studies have shown that the consumption of specific fermented foods increases the microbiota profile of the gut and can strengthen the immune system against viral infections.
The fascinating and vital link between probiotics, the body microbiome, and COVID-19 led to the designing of several clinical trials for treating COVID-19 using those probiotics that may have a high antiviral effect. To date, there are about 35 clinical trials performed in different hospitals and institutes to evaluate the effects of various strains of probiotics on patients with COVID-19, of which 26 have been completed, and the rest are continued (Table 1). These studies are based on strong evidence: (1) probiotics affect viruses through mechanisms described above, (2) probiotics reduce diarrhea and respiratory tract infection, and (3) they are affordable and available with low side effects. All these studies aim to evaluate the effect of probiotics on the duration and severity of COVID-19 and their effect on the evolution of oral and fecal microbiota in symptomatic patients, all in moderate forms of the COVID-19 disease. In one of these double-blind studies, the scientists used two probiotic strains and a placebo containing potato starch and magnesium stearate. In another study, a probiotic mixture was used to improve the symptoms and reduce the hospitalization days.
Table 1.
Clinical trials conducted for probiotics against coronavirus disease 2019 registered at ClinicalTrials.gov posted from May 2020 to December 2021
| Location | Official Title | Dosage form | Probiotic Strain | Number of Participants |
|---|---|---|---|---|
| Austria | Synbiotic Therapy of Gastrointestinal Symptoms During Covid-19 Infection: A Randomized, Double-blind, Placebo-Controlled, Telemedicine Study (SynCov Study) | Oral powder containing ten strains 5*109 CFU/sachet | Bifidobacterium bifidum, Bifidobacterium lactis, Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus paracasei, Lactobacillus plantarum, Lactobacillus rhamnosus and Ligilactobacillus salivarius | 30 |
| Canada | Evaluation of the Efficacy of Probiotics to Reduce the Duration and Symptoms of COVID-19 (PROVID-19 Study): a Randomized, Double-blind, Controlled Trial | Oral capsule containing 2 strains 10*109 CFU/ capsule | Not specified | 17 |
| Canada | Evaluation of the Efficacy of Probiotics Taken During the Acute Phase of COVID-19 to Reduce the Occurrence of Long COVID | Oral capsule containing 2 strains 10*109 CFU/capsule | Not specified | 618 |
| Canada | Randomized Single Blinded Clinical Study of Efficacy of Intranasal Probiotic Treatment to Reduce Severity of Symptoms in COVID19 Infection | Nasal irrigations with probiotic 2.4 *109 CFU/dose | Lactococcus lactis W136 | 23 |
| Hong Kong | A Randomized-controlled Trial of an Oral Microbiome Immunity Formula in Reducing Development of Long-term Co-morbidities in Recovered COVID-19 Patients | Oral powder containing three strains 10*109 CFU/sachet | 3 Bifidobacterial strain | 280 |
| Mexico | Efficacy and Safety of Lactobacillus plantarum and Pediococcus acidilactici as Co-adjuvant Therapy for Reducing the Risk of Severe Disease in Adults With SARS-CoV-2 and Its Modulation of the Fecal Microbiota: A Randomized Clinical Trial | Oral capsule containing two strains 10*109 CFU/capsule | Three Lactobacillus plantarum strains and Pediococcus acidilactici | 300 |
| Pakistan | Study to Investigate the Treatment Effect of Probiotic Streptococcus salivarius K12 in Hospitalised Patients (Non-ICU) With COVID-19 | Oral tablet containing two strains 1*109 CFU/tablet | Streptococcus salivarius K12 | 50 |
| Russia | Efficacy of Probiotics (Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum subsp. infantis, and Bifidobacterium longum) in the Treatment of Hospitalized Patients with Novel | Oral capsule containing 4 strains 4*109 CFU/capsule | Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum subsp. infantis and Bifidobacterium longum | 200 |
| Spain | The Intestinal Microbiota as a Therapeutic Target in Hospitalized Patients With COVID-19 Infection | Oral tablet containing 2 strains 1*109 CFU/tablet | Not specified | 41 |
| Spain | Administration of Ligilactobacillus salivarius MP101 in an Elderly Nursing Home During the COVID Pandemics |
Fermented milk containing 10*109 CFU/dose |
Ligilactobacillus salivarius MP101 | 25 |
| Spain | Changes in Viral Load in Patients With COVID-19 Disease After Dietary Supplementation with Probiotics: A Randomized Clinical Trial | Oral powder containing three strains plus vitamin D, zinc and selenium | Bifidobacterium longum, Bifidobacterium animalis subsp. lactis and Lactobacillus rhamnosus | 96 |
| Spain | Multicenter, Randomized, Double-blind Parallel Group Pilot Study to Evaluate the Effect of the Consumption of a Lactobacillus strain on the Incidence of Covid-19 in the Elderly | Oral capsule containing strain 3*109 CFU/capsule | A Lactobacillus strain | 201 |
| Spain | The Effect of Consumption of Lactobacillus coryniformis K8 on Healthcare Personnel Exposed to COVID-19 | Oral capsule containing a strain 3*109 CFU/capsule | Lactobacillus coryniformis K8 | 314 |
| Sweden | Exploratory Study on the Effects of Probiotic Supplementation on SARS-CoV-2 Antibody Response in Healthy Adults | Oral capsule containing 10*108 probiotics + 10 ug vitamin D3 | Lactobacillus reuteri | 161 |
| Ukraine | Role of Nutritional Support with Probiotics in Adult Outpatients with Symptomatic | Oral capsule containing two strains 5*109 CFU/capsule | A mixture of Bifidobacteria and Lactobacillus | 300 |
| United states | A randomized trial of the effect of Lactobacillus on the microbiome of household contacts | Oral capsule containing a strain 1*109 CFU/capsule | Lactobacillus rhamnosus GG | 182 |
In the study performed in Montreal, Quebec, Canada, the safety and validity of nasal irrigation with probiorinse of Lactococcus lactis W136 and nasal irrigation with saline were compared. Another study in Sweden used a combination of L. reuteri DSM 17,938 + vitamin D as the treatment group and placebo + vitamin D combination as a control group to observe the impact of probiotics on SARS-CoV-2 specific antibody response upon and after infection in healthy adults. A study conducted in Brazil examined the effect of oral gel containing Streptococcus salivarius K12 and L. brevis CD2 and oral gel containing placebo on preventing lung colonization and progression to bacterial pneumonia in patients with severe COVID-19 on the first ICU day. In Mexico City, the researchers used Nutritional Supportive System (NSS) to see if it reduces complications in patients with COVID-19 in stage III with co-morbidities. NSS contains a combination of three B vitamins (B1, B6, B12), Probiotics Saccharomyces boulardii CNCM I-745 “Floratil” (Saylan et al. 2017).
The therapeutic consequence of probiotics in COVID-19 patients
Although several mechanisms have been proposed for the anti-SARS-Co-2 effects of probiotics and its new variant Omicron, the results of clinical trials have not yet supported these perspectives. Clinical trials have been underway for two years, and it needed a longer time to report the outcomes. However, by carefully studying the results of recently published clinical trials, the significant consequences of prebiotics in relieving the symptoms of COVID-19 can be divided into three categories: modulating levels of immune mediators, restoration of healthy gut microbiota, and modifying the Gut-Lung Axis. The effects on viral titers and interferon and antibody production are also proposed; however, there is no sufficient clinical data.
Conclusions
Collectively, probiotics can prevent the SARS-CoV-2-induced cytokine storm through the aforementioned mechanisms, such as induction of anti-inflammatory cytokines (IL-10) and downregulation of pro-inflammatory cytokines (TNF-α, IL-2, IL-6), inhibition of JAK signaling pathway, and act as HDAC inhibitor. Further in vitro, in vivo, and clinical trial studies should be done to confirm our proposed mechanism.
Besides, not all probiotics involve the exact mechanisms of action in different diseases, and strain specificity is vital to explain the right probiotic for the proper indication. They selected an appropriate combination of various strains with additional features, a variety of probiotics with prebiotics and vitamins, which may be an excellent strategy for effective formulation against SARS-CoV-2. So far, all the logic used to prove probiotics against SARS-CoV-2 has been proposed in an indirect or in-silico study. This study recommends that one avoid excessive and blind consumption of various probiotic strains until a better understanding of the pathogenesis of SARS-CoV-2 and its effect on the intestinal environment. In addition, several preclinical and randomized double-blind clinical trials should be designed to become more aware of the exact impacts of specific probiotic strains on COVID-19 patients.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 35.3 KB)
Acknowledgements
The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ACE
Angiotensin-converting enzyme
- AAK1
Adaptor associated protein kinase 1
- CoVs
Coronaviruses
- COVID-19
Coronavirus disease 2019
- CV-B4
Coxsackievirus B4
- CMV
Cytomegalovirus
- CTLs
Cytotoxic T lymphocytes
- DCs
Dendritic cells
- dmLT
Double mutant heat-labile toxin
- EBV
Epstein–Barr virus
- EcN
Escherichia coli Nissle
- EPSs
Extracellular polysaccharides
- GAK
G-associated kinase
- GRAS
Generally regarded as safe
- HSV
Herpes simplex virus
- HDAC
Histone deacetylase
- HLA
Human leukocyte antigen
- HRV
Human Rotavirus
- H2O2
Hydrogen peroxide
- IFNV
Influenza virus
- IFNs
Interferons
- JAK
Janus kinase
- LT
Labile Toxin
- LAB
Lactic Acid Bacteria
- MERS-CoV
Middle East respiratory syndrome
- NK
Natural killer
- NLR
NOD-like receptor
- PRRs
Pattern recognition receptors
- PCV2
Porcine circovirus type 2
- RSV
Respiratory Syncytial Virus
- RLR
RIG-I-like receptor
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SPF
Specific pathogen-free
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
Declarations
Conflict of interest
The authors report no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelhamid AG, El-Masry SS, El-Dougdoug NK. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019;10:337–350. doi: 10.1007/s13167-019-00184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kassaa I, Hober D, Hamze M, Chihib NE, Drider D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob Proteins. 2014;6:177–185. doi: 10.1007/s12602-014-9162-6. [DOI] [PubMed] [Google Scholar]
- Albarracin L, Kobayashi H, Iida H, Sato N, Nochi T, Aso H, Salva S, Alvarez S, Kitazawa H, Villena J. Transcriptomic analysis of the innate antiviral immune response in porcine intestinal epithelial cells: influence of immunobiotic lactobacilli. Front Immunol. 2017;8:57. doi: 10.3389/fimmu.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An HM, Lee DK, Kim JR, Lee SW, Cha MK, Lee KO, Ha NJ. Antiviral activity of Bifidobacterium adolescentis SPM 0214 against herpes simplex virus type 1. Arch Pharm Res. 2012;35:1665–1671. doi: 10.1007/s12272-012-0918-9. [DOI] [PubMed] [Google Scholar]
- Ang LYE, Too HKI, Tan EL, Chow T-KV, Shek P-CL, Tham E, Alonso S. Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol J. 2016;13:111. doi: 10.1186/s12985-016-0567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes AEC, Vinderola G, Xavier-Santos D, Sivieri K. Potential contribution of beneficial microbes to face the COVID-19 pandemic. Food Res Int. 2020;136:109577–109577. doi: 10.1016/j.foodres.2020.109577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar F, Altayb HN, Al-Abbasi FA, Al-Malki AL, Kamal MA, Kumar V (2020) Antiviral effects of probiotic metabolites on COVID-19. J Biomol Struct Dyn 1–10. 10.1080/07391102.2020.1775123 [DOI] [PMC free article] [PubMed]
- Arena MP, Elmastour F, Sane F, Drider D, Fiocco D, Spano G, Hober D. Inhibition of coxsackievirus B4 by Lactobacillus plantarum. Microbiol Res. 2018;210:59–64. doi: 10.1016/j.micres.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Ashoori Y, Mohkam M, Heidari R, Abootalebi SN, Mousavi SM, Hashem SA, Golkar N, Gholami A (2020) Development and in-vivo characterization of probiotic lysate treated chitosan nanogel as a novel biocompatible formulation for wound healing. Biomed Res Int In-press. 10.1155/2020/8868618. [DOI] [PMC free article] [PubMed]
- Azarang A, Farshad O, Ommati MM, Jamshidzadeh A, Heidari R, Abootalebi SN, Gholami A (2020) Protective role of probiotic supplements in hepatic steatosis: a rat model study. Biomed Res Int 2020:5487659. 10.1155/2020/5487659 [DOI] [PMC free article] [PubMed]
- Bae J-Y, Kim JI, Park S, Yoo K, Joo W, Park MS, Lee I, Park M-S. Effects of lactobacillus plantarum and Leuconostoc mesenteroides probiotics on human seasonal and avian influenza viruses. J Microbiol Biotechnol. 2018;28:893–901. doi: 10.4014/jmb.1804.04001. [DOI] [PubMed] [Google Scholar]
- Bajpai VK, Chandra V, Kim N-H, Rai R, Kumar P, Kim K, Aeron A, Kang SC, Maheshwari DK, Na M, Rather IA, Park Y-H. Ghost probiotics with a combined regimen: a novel therapeutic approach against the Zika virus, an emerging world threat. Crit Rev Biotechnol. 2018;38:438–454. doi: 10.1080/07388551.2017.1368445. [DOI] [PubMed] [Google Scholar]
- Bati VV, Boyko NV. The microbial diversity and its dynamics in the ethnic fermented foods of the Black Sea region. Mikrobiol Z. 2016;78:53–64. doi: 10.15407/microbiolj78.05.053. [DOI] [PubMed] [Google Scholar]
- Baud D, Dimopoulou Agri V, Gibson GR, Reid G, Giannoni E. Using probiotics to flatten the curve of Coronavirus Disease COVID-2019 pandemic. Front Public Health. 2020;8:186. doi: 10.3389/fpubh.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69:1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- Borruel N, Carol M, Casellas F, Antolín M, de Lara F, Espín E, Naval J, Guarner F, Malagelada JR. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659–664. doi: 10.1136/gut.51.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J, Anto JM, Czarlewski W, Haahtela T, Fonseca SC, Iaccarino G, Blain H, Vidal A, Sheikh A, Akdis CA, Zuberbier T, ARIA group Cabbage and fermented vegetables: From death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy. 2021;76:735–750. doi: 10.1111/all.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha MK, Lee DK, An HM, Lee SW, Shin SH, Kwon JH, Kim KJ, Ha NJ. Antiviral activity of Bifidobacterium adolescentis SPM1005-A on human papillomavirus type 16. BMC Med. 2012;10:72. doi: 10.1186/1741-7015-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Zhao Y, Wu Z, Liu R, Xu R, Yan L, Li H. Metagenomic data of fungal internal transcribed spacer from serofluid dish, a traditional Chinese fermented food. Genom Data. 2016;7:134–136. doi: 10.1016/j.gdata.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, Shimizu T, Yamashiro Y, Kiyoshima-Shibata J, Nanno M, Nomoto K. Well-controlled pro-inflammatory cytokine responses of Peyer’s patch cells to probiotic Lactobacillus Casei. Immunology. 2010;130:352–362. doi: 10.1111/j.1365-2567.2009.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikindas ML, Weeks R, Drider D, Chistyakov VA, Dicks LM. Functions and emerging applications of bacteriocins. Curr Opin Biotechnol. 2018;49:23–28. doi: 10.1016/j.copbio.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-J, Song J-H, Ahn Y-J, Baek S-H, Kwon D-H. Antiviral activities of cell-free supernatants of yogurts metabolites against some RNA viruses. Eur Food Res Technol. 2009;228:945–950. doi: 10.1007/s00217-009-1009-0. [DOI] [Google Scholar]
- Citar M, et al. Human intestinal mucosa-associated Lactobacillus and Bifidobacterium strains with probiotic properties modulate IL-10, IL-6 and IL-12 gene expression in THP-1 cells. Benef Microbes. 2015;6:325–336. doi: 10.3920/BM2014.0081. [DOI] [PubMed] [Google Scholar]
- Coton M, Pawtowski A, Taminiau B, Burgaud G, Deniel F, Coulloumme-Labarthe L, Fall A, Daube G, Coton E (2017) Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol Ecol 93. 10.1093/femsec/fix048 [DOI] [PubMed]
- Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24:91–91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembinski JL, Hungnes O, Hauge AG, Kristoffersen AC, Haneberg B, Mjaaland S. Hydrogen peroxide inactivation of influenza virus preserves antigenic structure and immunogenicity. J Virol Methods. 2014;207:232–237. doi: 10.1016/j.jviromet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Di Cagno R, Filannino P, Gobbetti M. Fermented foods: fermented vegetables and other products. In: Caballero B, Finglas PM, Toldrá F, editors. Encyclopedia of food and health. Oxford: Academic; 2016. pp. 668–674. [Google Scholar]
- Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y (2020) Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19). medRxiv, 2020.2002.2018.20024364. 10.3389/fimmu.2020.00827 [DOI] [PMC free article] [PubMed]
- Dimidi E, Cox SR, Rossi M, Whelan K (2019) Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 11:1806. 10.3390/nu11081806 [DOI] [PMC free article] [PubMed]
- Dos Santos Pereira Andrade AC, et al. Daily ingestion of the probiotic Lactobacillus paracasei ST11 decreases Vaccinia virus dissemination and lethality in a mouse model. Benef Microbes. 2017;8:73–80. doi: 10.3920/BM2016.0074. [DOI] [PubMed] [Google Scholar]
- Drider D, Bendali F, Naghmouchi K, Chikindas ML. Bacteriocins: not only antibacterial agents. Probiotics Antimicrob Proteins. 2016;8:177–182. doi: 10.1007/s12602-016-9223-0. [DOI] [PubMed] [Google Scholar]
- Eetemadi A, Rai N, Pereira BMP, Kim M, Schmitz H, Tagkopoulos I (2020) The computational diet: a review of computational methods across diet, microbiome, and health. Front Microbiol 393. 10.3389/fmicb.2020.00393 [DOI] [PMC free article] [PubMed]
- Feng Z, Wang Y, Qi W (2020) The small intestine, an underestimated site of SARS-CoV-2 infection: from red queen effect to probiotics. Preprint. 10.20944/preprints202003.0161.v1
- Fernandez-Duarte KP, Olaya-Galan NN, Salas-Cardenas SP, Lopez-Rozo J, Gutierrez-Fernandez MF. Bifidobacterium adolescentis (DSM 20083) and Lactobacillus casei (Lafti L26-DSL): Probiotics able to block the in vitro adherence of rotavirus in MA104 cells. Probiotics Antimicrob Proteins. 2018;10:56–63. doi: 10.1007/s12602-017-9277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonollá J, Gracián C, Maldonado-Lobón JA, Romero C, Bédmar A, Carrillo JC, Martín-Castro C, Cabrera AL, García-Curiel JM, Rodríguez C, Sanbonmatsu S, Pérez-Ruiz M, Navarro JM, Olivares M. Effects of Lactobacillus coryniformis K8 CECT5711 on the immune response to influenza vaccination and the assessment of common respiratory symptoms in elderly subjects: a randomized controlled trial. Eur J Nutr. 2019;58:83–90. doi: 10.1007/s00394-017-1573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuku N, Alis R, Yvert T, Zempo H, Naito H, Abe Y, Arai Y, Murakami H, Miyachi M, Pareja-Galeano H, Emanuele E, Hirose N, Lucia A. Muscle-related polymorphisms (MSTN rs1805086 and ACTN3 rs1815739) are not associated with exceptional longevity in Japanese centenarians. PLoS ONE. 2016;11:e0166605. doi: 10.1371/journal.pone.0166605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Huang L, Zhu L, Mou C, Hou Q, Yu Q. Inhibition of H9N2 virus invasion into dendritic cells by the S-Layer protein from L. acidophilus ATCC 4356. Front Cell Infect Microbiol. 2016;6:137. doi: 10.3389/fcimb.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami A, Shahin S, Mohkam M, Nezafat N, Ghasemi Y. Cloning, characterization and bioinformatics analysis of novel cytosine deaminase from Escherichia coli AGH09. Int J Pept Res Ther. 2015;21:365–374. doi: 10.1007/s10989-015-9465-9. [DOI] [Google Scholar]
- Gholami A, Dabbaghmanesh MH, Ghasemi Y, Talezadeh P, Koohpeyma F, Montazeri-Najafabady N. Probiotics ameliorate pioglitazone-associated bone loss in diabetic rats. Diabetol Metab Syndr. 2020;12:78. doi: 10.1186/s13098-020-00587-3. [DOI] [Google Scholar]
- Gleeson M, Bishop NC, Struszczak L. Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes: a placebo-controlled, randomized trial. Eur J Appl Physiol. 2016;116:1555–1563. doi: 10.1007/s00421-016-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV‐2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamida RS, Shami A, Ali MA, Almohawes ZN, Mohammed AE, Bin-Meferij MM. Kefir: A protective dietary supplementation against viral infection. Biomed Pharmacother. 2021;133:110974. doi: 10.1016/j.biopha.2020.110974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi SA, et al. Ultra-sensitive viral glycoprotein detection NanoSystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosens Bioelectron. 2021;171:112731. doi: 10.1016/j.bios.2020.112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann DL, Shindo N. COVID-19: what is next for public health? Lancet. 2020;395:542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishiki H, Kawashima T, Tsuji NM, Ikari N, Takemura R, Kido H, Shimojo N (2020) A double-blind, randomized, placebo-controlled trial of heat-killed Pediococcus acidilactici K15 for prevention of respiratory tract infections among preschool children. Nutrients 12:1989. 10.3390/nu12071989 [DOI] [PMC free article] [PubMed]
- Hoffmann HH, Schneider WM, Blomen VA, Scull MA, Hovnanian A, Brummelkamp TR, Rice CM. Diverse viruses require the calcium transporter SPCA1 for maturation and spread. Cell Host Microbe. 2017;22:460–470e465. doi: 10.1016/j.chom.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. PNAS. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki A et al (2017) Effects of short-term probiotic ingestion on immune profiles and microbial translocation among HIV-1-infected vietnamese children. Int J Mol Sci 18. 10.3390/ijms18102185 [DOI] [PMC free article] [PubMed]
- Ishizuka T, Bi X, Nguyen LV, Matsuda K, Pham HV, Phan CTT, Khanh Khu DT, Ichimura H. Immunobiotic Bifidobacteria strains modulate rotavirus immune response in porcine intestinal Epitheliocytes via pattern recognition receptor signaling. PLoS ONE. 2016;11:e0152416. doi: 10.1371/journal.pone.0152416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamilloux Y, Jammal T, Vuitton L, Gerfaud-Valentin M, Kerever S, Sève P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2019;18:102390. doi: 10.1016/j.autrev.2019.102390. [DOI] [PubMed] [Google Scholar]
- Janda L, Mihalčin M, Šťastná M (2021) Is a healthy microbiome responsible for lower mortality in COVID-19? Biologia 76:819–829. 10.2478/s11756-020-00614-8 [DOI] [PMC free article] [PubMed]
- Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, Okumura R, Hara H, Yoshida H, Yamamoto M, Nomoto K, Takeda K. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012;8:e1002714–e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Yang G, Wang Q, Wang Z, Yang W, Gu W, Shi C, Wang J, Huang H, Wang C. Molecular mechanisms underlying protection against H9N2 influenza virus challenge in mice by recombinant Lactobacillus plantarum with surface displayed HA2-LTB. J Biotechnol. 2017;259:6–14. doi: 10.1016/j.jbiotec.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ye L, Cui Y, Yang G, Yang W, Wang J, Hu J, Gu W, Shi C, Huang H, Wang C. Effects of Lactobacillus rhamnosus GG on the maturation and differentiation of dendritic cells in rotavirus-infected mice. Benef Microbes. 2017;8:645–656. doi: 10.3920/BM2016.0157. [DOI] [PubMed] [Google Scholar]
- Kandasamy S, Vlasova AN, Fischer D, Kumar A, Chattha KS, Rauf A, Shao L, Langel SN, Rajashekara G, Saif LJ. Differential effects of Escherichia coli Nissle and Lactobacillus rhamnosus strain GG on human rotavirus binding, infection, and B cell immunity. J Immun. 2016;196:1780–1789. doi: 10.4049/jimmunol.1501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmani P, Clua P, Vizoso-Pinto MG, Rodriguez C, Alvarez S, Melnikov V, Takahashi H, Kitazawa H, Villena J. Respiratory commensal bacteria Corynebacterium pseudodiphtheriticum improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front Microbiol. 2017;8:1613. doi: 10.3389/fmicb.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmani P, Albarracin L, Kobayashi H, Iida H, Komatsu R, Humayun Kober AKM, Ikeda-Ohtsubo W, Suda Y, Aso H, Makino S, Kano H, Saito T, Villena J, Kitazawa H. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol Immunol. 2018;93:253–265. doi: 10.1016/j.molimm.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Karaffova V, Csank T, Mudroňová D, Király J, Revajová V, Gancarčíková S et al (2017) Influence of Lactobacillus reuteri L26 Biocenol on immune response against porcine circovirus type 2 infection in germ-free mice. Benef Microbes 8:367–378 [DOI] [PubMed]
- Karamese M, Aydin H, Sengul E, Gelen V, Sevim C, Ustek D, Karakus E. The Immunostimulatory Effect of Lactic Acid Bacteria in a Rat Model. Iran J Immunol. 2016;13:220–228. [PubMed] [Google Scholar]
- Kedzierski L, Linossi EM, Kolesnik TB, Day EB, Bird NL, Kile BT, Belz GT, Metcalf D, Nicola NA, Kedzierska K, Nicholson SE. Suppressor of Cytokine Signaling 4 (SOCS4) protects against severe cytokine storm and enhances viral clearance during influenza infection. PLOS Pathog. 2014;10:e1004134. doi: 10.1371/journal.ppat.1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Lee DK, Park JE, Park IH, Seo JG, Ha NJ. Antiviral activity of Bifidobacterium adolescentis SPM1605 against Coxsackievirus B3. Biotechnol Biotechnol Equip. 2014;28:681–688. doi: 10.1080/13102818.2014.945237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Maruyama M, Suyama K, Nishijima M, Akamatsu K, Jogamoto A, Katakami K, Saito I. The effects of OLL1073R-1 yogurt intake on influenza incidence and immunological markers among women healthcare workers: a randomized controlled trial. Food Funct. 2019;10:8129–8136. doi: 10.1039/c9fo02128k. [DOI] [PubMed] [Google Scholar]
- Kiso M, Takano R, Sakabe S, Katsura H, Shinya K, Uraki R, Watanabe S, Saito H, Toba M, Kohda N, Kawaoka Y. Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against influenza A virus. Sci Rep. 2013;3:1563. doi: 10.1038/srep01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ, Watts DH, Mehlin C, Headley CM. Lactobacilli and vaginal host defense: activation of the human immunodeficiency virus type 1 long terminal repeat, cytokine production, and NF-kappaB. J Infect Dis. 1999;179:653–660. doi: 10.1086/314644. [DOI] [PubMed] [Google Scholar]
- Krajka-Kuźniak V, Szaefer H, Bartoszek A, Baer-Dubowska W. Modulation of rat hepatic and kidney phase II enzymes by cabbage juices: comparison with the effects of indole-3-carbinol and phenethyl isothiocyanate. Br J Nutr. 2011;105:816–826. doi: 10.1017/S0007114510004526. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Liu R, Kwon JO, Kim MK, Kim AH, Kang SO. Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza A virus. J Microbiol. 2013;51:836–843. doi: 10.1007/s12275-013-3521-y. [DOI] [PubMed] [Google Scholar]
- Laiño J, Villena J, Kanmani P, Kitazawa H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: new insights into molecular interactions with host cells. Microorganisms. 2016;4:27. doi: 10.3390/microorganisms4030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SW, Chong AQ, Chin NL, Talib RA, Basha RK. Sourdough microbiome comparison and benefits. Microorganisms. 2021;9:1355. doi: 10.3390/microorganisms9071355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan B, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork M, Wang P, Ehrlich J, Pedersen SD. O Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- Lee JS, Paek NS, Kwon OS, Hahm KB. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: a novel mechanism. J Gastroenterol Hepatol. 2010;25:194–202. doi: 10.1111/j.1440-1746.2009.06127.x. [DOI] [PubMed] [Google Scholar]
- Lee DK, Kang JY, Shin HS, Park IH, Ha NJ. Antiviral activity of Bifidobacterium adolescentis SPM0212 against Hepatitis B virus. Arch Pharm Res. 2013;36:1525–1532. doi: 10.1007/s12272-013-0141-3. [DOI] [PubMed] [Google Scholar]
- Li N, Zhao D, Kirschbaum M, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. PNAS. 2008;105:4796–4801. doi: 10.1073/pnas.0712051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopitz-Otsoa F, Rementeria A, Elguezabal N, Garaizar J. Kefir: a symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev Iberoam Micol. 2006;23:67–74. doi: 10.1016/s1130-1406(06)70016-x. [DOI] [PubMed] [Google Scholar]
- Mak JWY, Chan FKL, Ng SC. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol Hepatol. 2020;5:644–645. doi: 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragkoudakis PA, Chingwaru W, Gradisnik L, Tsakalidou E, Cencic A. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int J Food Microbiol. 2010;141(Suppl 1):91–97. doi: 10.1016/j.ijfoodmicro.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastromarino P, Cacciotti F, Masci A, Mosca L. Antiviral activity of Lactobacillus brevis towards herpes simplex virus type 2: role of cell wall associated components. Anaerobe. 2011;17:334–336. doi: 10.1016/j.anaerobe.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Mirashrafi S, Moravejolahkami AR, Balouch Zehi Z, Hojjati Kermani MA, Bahreini-Esfahani N, Haratian M, Ganjali Dashti M, Pourhossein M. The efficacy of probiotics on virus titres and antibody production in virus diseases: A systematic review on recent evidence for COVID-19 treatment. Clin Nutr ESPEN. 2021;46:1–8. doi: 10.1016/j.clnesp.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohkam M, Rasoul amini S, Shokri D, Berenjian A, Rahimi F, Sadraeian M, Khalvati B, Gholami A, Ghasemi Y. Characterization and in vitro probiotic assessment of potential indigenous Bacillus strains isolated from soil rhizosphere. Minerva Biotecnol. 2016;28:19–28. [Google Scholar]
- Мokrozub VV, Lazarenko LM, Sichel LM, Babenko LP, Lytvyn PM, Demchenko OM et al (2015) The role of beneficial bacteria wall elasticity in regulating innate immune response. EPMA J 6(1):13 [DOI] [PMC free article] [PubMed]
- Montazeri-Najafabady N, Ghasemi Y, Dabbaghmanesh MH, Talezadeh P, Koohpeyma F, Gholami A. Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics Antimicrob Proteins. 2019;11:1145–1154. doi: 10.1007/s12602-018-9443-6. [DOI] [PubMed] [Google Scholar]
- Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182:1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SM, Hashemi SA, Parvin N, Gholami A, Ramakrishna S, Omidifar N, Moghadami M, Chiang W-H, Mazraedoost S (2020a) Recent biotechnological approaches for treatment of novel COVID-19: from bench to clinical trial. Drug Metab Rev :1–30. 10.1080/03602532.2020.1845201 [DOI] [PubMed]
- Mousavi SM, Hashemi SA, Zarei M, Gholami A, Lai CW, Chiang WH, Omidifar N, Bahrani S, Mazraedoost S. Recent progress in chemical composition, production, and pharmaceutical effects of kombucha beverage: a complementary and alternative medicine. Evid Based Complement Alternat Med. 2020;2020:4397543. doi: 10.1155/2020/4397543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negahdaripour M, Rahbar MR, Mosalanejad Z, Gholami A. Theta-defensins to counter COVID-19 as furin inhibitors: In-silico efficiency prediction and novel compound design. Comput Math Methods Med. 2022;2022:9735626. doi: 10.1155/2022/9735626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QV, Chong LC, Hor Y-Y, Lew L-C, Rather IA, Choi S-B. Role of probiotics in the management of COVID-19: a computational perspective. Nutrients. 2022;14:274. doi: 10.3390/nu14020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaimat AN, Aolymat I, Al-Holy M, Ayyash M, Abu Ghoush M, Al-Nabulsi AA, Osaili T, Apostolopoulos V, Liu SQ, Shah P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci Food. 2020;4:17. doi: 10.1038/s41538-020-00078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya Galán NN, Ulloa Rubiano JC, Velez Reyes FA, Fernandez Duarte KP, Salas Cárdenas SP, Gutierrez Fernandez MF. In vitro antiviral activity of Lactobacillus casei and Bifidobacterium adolescentis against rotavirus infection monitored by NSP4 protein production. J Appl Microbiol. 2016;120:1041–1051. doi: 10.1111/jam.13069. [DOI] [PubMed] [Google Scholar]
- Park KY, Kim HY, Jeong JK. Chap. 20 - Kimchi and its health benefits. In: Frias J, Martinez-Villaluenga C, Peñas E, editors. Fermented foods in health and disease prevention. Boston: Academic; 2017. pp. 477–502. [Google Scholar]
- Prado MR, Blandón LM, Vandenberghe LP, Rodrigues C, Castro GR, Thomaz-Soccol V, Soccol CR. Milk kefir: composition, microbial cultures, biological activities, and related products. Front Microbiol. 2015;6:1177. doi: 10.3389/fmicb.2015.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana VM, Torres NI, Wachsman MB, Sinko PJ, Castilla V, Chikindas M. Antiherpes simplex virus type 2 activity of the antimicrobial peptide subtilosin. J Appl Microbiol. 2014;117:1253–1259. doi: 10.1111/jam.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raak C, Ostermann T, Boehm K, Molsberger F. Regular consumption of sauerkraut and its effect on human health: a bibliometric analysis. Glob Adv Health Med. 2014;3:12–18. doi: 10.7453/gahmj.2014.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandran L, Shah NP. Proteolytic profiles and angiotensin-I converting enzyme and alpha-glucosidase inhibitory activities of selected lactic acid bacteria. J Food Sci. 2008;73:M75–81. doi: 10.1111/j.1750-3841.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterol. 2006;130:731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzello CG, et al. Organic cultivation of Triticum turgidum subsp. durum is reflected in the flour-sourdough fermentation-bread axis. Appl Environ Microbiol. 2015;81:3192–3204. doi: 10.1128/AEM.04161-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach JT, Hörmannsperger G, Huys G. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–185. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, Paraskevakos G, Leyer G. Probiotic use in at-risk populations. JAPhA. 2016;56:680–686. doi: 10.1016/j.japh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Saylan Y, Yilmaz F, Özgür E, Derazshamshir A, Yavuz H, Denizli A. Molecular imprinting of macromolecules for sensor applications. Sensors. 2017;17:898. doi: 10.3390/s17040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagnolari C, Vicenzi E, Bellomi F, Stillitano MG, Pinna D, Poli G, Clementi M, Dianzani F, Antonelli G. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther. 2004;9:1003–1011. doi: 10.1177/135965350400900618. [DOI] [PubMed] [Google Scholar]
- Serkedjieva J, Danova S, Ivanova I. Antiinfluenza virus activity of a bacteriocin produced by Lactobacillus delbrueckii. Appl Biochem Biotechnol. 2000;88:285–298. doi: 10.1385/ABAB:88. [DOI] [Google Scholar]
- Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Shigemori S, Shimosato T. Applications of genetically modified immunobiotics with high immunoregulatory capacity for treatment of inflammatory bowel diseases. Front Immunol. 2017;8:22–22. doi: 10.3389/fimmu.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A review of Coronavirus Disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerstein R, Kochen MM, Messerli FH, Gräni C. Coronavirus Disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? JAHA. 2020;9:e016509. doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JA, Kim HJ, Hong SK, Lee DH, Lee SW, Song CS, Kim KT, Choi IS, Lee JB, Park SY. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J Microbiol Immunol Infect. 2016;49:16–23. doi: 10.1016/j.jmii.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiecco G, Storti S, Degli Antoni M, Focà E, Castelli F, Quiros-Roldan E. Omicron genetic and clinical peculiarities that may overturn SARS-CoV-2 pandemic: a literature review. Int J Mol Sci. 2022;23:1987. doi: 10.3390/ijms23041987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. MMBR. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov S, Wachsman M, Knoetze H, Meincken M, Dicks L. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soya beans. Int J Antimicrob Agents. 2005;25:508–513. doi: 10.1016/j.ijantimicag.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Todorov SD, Wachsman M, Tomé E, Dousset X, Destro MT, TheodoreDicks LM, de Melo Franco BDG, Velho MV, Drider D. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol. 2010;27:869–879. doi: 10.1016/j.fm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Torres NI, Noll KS, Xu S, Li J, Huang Q, Sinko PJ, Wachsman MB, Chikindas ML. Safety, formulation, and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus type 1. Probiotics Antimicrob Proteins. 2013;5:26–35. doi: 10.1007/s12602-012-9123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena J, Vizoso-Pinto MG, Kitazawa H. Intestinal innate antiviral immunity and immunobiotics: beneficial effects against rotavirus infection. Front Immunol. 2016;7:563–563. doi: 10.3389/fimmu.2016.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova AN, Kandasamy S, Chattha KS, Rajashekara G, Saif LJ. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet Immunol Immunopathol. 2016;172:72–84. doi: 10.1016/j.vetimm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman M, Castilla V, Holgado A, Torres R, Sesma F, Coto C. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antiviral Res. 2003;58:17–24. doi: 10.1016/s0166-3542(02)00099-2. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang P, Fu W, Zhang Y, Li T, Pan B, Wei P. Effect of probiotics on Newcastle Disease Virus. Wei Sheng Wu Xue Bao. 2010;50:1664–1669. [PubMed] [Google Scholar]
- Wang X, Hu W, Zhu L, Yang Q (2017) Bacillus subtilis and surfactin inhibit the transmissible gastroenteritis virus from entering the intestinal epithelial cells. Biosci Rep 37. 10.1042/BSR20170082 [DOI] [PMC free article] [PubMed]
- Weiss G, Rasmussen S, Zeuthen LH, Nielsen BN, Jarmer H, Jespersen L, Frøkiaer H. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunol. 2010;131:268–281. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Christensen H, Zeuthen L, Vogensen F, Jakobsen M, Frokiaer H. Lactobacilli and bifidobacteria induce differential interferon-β profiles in dendritic cells. Cytokine. 2011;56:520–530. doi: 10.1016/j.cyto.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Wu S, Jiang ZY, Sun YF, Yu B, Chen J, Dai CQ, Wu XL, Tang XL, Chen XY (2013) Microbiota regulates the TLR7 signaling pathway against respiratory tract influenza a virus infection. Cur Microbiol 67. 10.1007/s00284-013-0380-z [DOI] [PubMed]
- Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh YK, Zuo T, Lui GCY, Zhang F, Liu Q, Li AY, Chung ACK, Cheung CP, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chow KM, Shan Ng SS, Li TCM, Ng RW, Yip TC, Wong GLH, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuille S, Reichardt N, Panda S, Dunbar H, Mulder IE. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE. 2018;13:e0201073. doi: 10.1371/journal.pone.0201073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaya H, Alvarez S, Kitazawa H, Villena J (2016) Respiratory antiviral immunity and immunobiotics: beneficial effects on inflammation-coagulation interaction during influenza virus infection. Front Immunol 7. 10.3389/fimmu.2016.00633 [DOI] [PMC free article] [PubMed]
- Zeng J, Wang CT, Zhang FS, Qi F, Wang SF, Ma S, Wu TJ, Tian H, Tian ZT, Zhang SL, Qu Y, Liu LY, Li YZ, Cui S, Zhao HL, Du QS, Ma Z, Li CH, Li Y, Si M, Chu YF, Meng M, Ren HS, Zhang JC, Jiang JJ, Ding M, Wang YP. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42:1018–1028. doi: 10.1007/s00134-016-4303-x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Liu Y, Latta M, Ma W, Wu Z, Chen P. Probiotics database: a potential source of fermented foods. Int J Food Prop. 2019;22:198–217. doi: 10.1080/10942912.2019.1579737. [DOI] [Google Scholar]
- Zheng J, Stijn W, Elisa S, Charles MAPF, Hugh MBH, Paola M, Paul WO, Bruno P, Peter V, Jens W, Koichi W, Sander W, Giovanna EF, Michael GG, Sarah L. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Curtis N. The influence of probiotics on vaccine responses - A systematic review. Vaccine. 2018;4:207–213. doi: 10.1016/j.vaccine.2017.08.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 35.3 KB)