Abstract
Urinary tract infections (UTIs) are one of the most common bacterial infections with uropathogenic Escherichia coli (UPEC) being the most prevalent causative agent in both complicated and uncomplicated UTIs. Antibiotic resistance among UPEC has been already demonstrated against a wide variety of antibiotics and the situation is continuing to deteriorate increasing the rate of recurrence and the difficulty of treatment and prophylaxis. Recently, a big attention has been paid to non-antibiotic approaches as an alternative to conventional antibiotics. Among many strategies, phytotherapy has gained a special attention worldwide. Herbal remedies have been used in traditional medicine since ancient times and they are well known for their effectiveness in treating many health conditions including UTIs. Researches are conducted continuously to validate the use of many medicinal plants against UPEC, investigate their mechanisms of action, and determine their active constituents. Our extensive review of the recent literature revealed that many phytochemicals are shown to target and inhibit a wide variety of bioprocesses in UPEC, such as adhesion, motility, biofilm formation, and quorum sensing. Such natural approaches are very promising in confronting the antibiotic resistance of UPEC and can be further used to develop plant-based strategies and pharmaceutical products to treat and prevent UTIs caused by UPEC.
Keywords: Antibacterial agent, antibiotic resistance, bacterial adhesion, phytochemicals, traditional medicine, urinary tract infections, uropathogenic Escherichia coli
INTRODUCTION
Urinary tract infections (UTIs) are considered one of the most common bacterial infections. Annually, it is estimated that UTIs affect 150 million people worldwide.[1,2] In addition, UTIs cause a serious economic problem because of the huge costs needed to treat or hospitalize people with these infections. For instance, in the United States it has been estimated that $ 6 million is spent annually to treat 11 million people with UTIs.[3] Healthcare-associated UTIs are one of the most frequent healthcare-associated infections (HAI), accounting for 19.0% of HAI in Europe.[4] UTIs represent about 40% of hospital-acquired infections and 50% of bacteremia causes.[5] In general, UTIs frequency in women is higher than in men. Indeed, nearly half of women and 12% of men suffer from UTIs at least once during their lifetime, with recurrent UTIs in 27%–48% of the affected women.[6]
UTI occurs when the pathogen enters the urinary tract system and presents in the urine in quantities of more than 105 colony-forming units per milliliter (CFU/mL).[7] Bacteria are the main causative agent of UTIs with the predominance of Enterobacteriaceae family, such as Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Citrobacter, and Enterobacter. Other common causes include Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, Staphylococcus saprophyticus, Enterococcus faecalis, Streptococcus bovis, and the fungus Candida albicans.[5,8] Among the bacterial causative agents of UTIs, Uropathogenic E. coli (UPEC) is the most prevalent in both complicated and uncomplicated UTIs.[6] UPEC can be disseminated in different ways, including contaminated food, sexual contact, or by fecal–oral route.[9] In addition, E. coli isolates from UTI patients showed a high similarity with those isolated from farm animals (specifically chicken) in terms of the antimicrobial resistance profiles and genetic virulence determinants. This suggests that farm animals could be reservoirs of E. coli strains causing UTI in humans.[10,11]
UPEC is usually treated with antibiotics such as imipenem, ertapenem, amikacin, and nitrofurantoin.[8] However, repeated antibiotic therapies contribute to the emergence of resistant strains.[12] In recent decades, the spread of multidrug-resistant (MDR) UPEC became a serious clinical problem, especially in developing countries as it leads to excessive use of broad-spectrum antibiotics, prolonged hospitalization, and accompanying high cost of treatment.[6] Antibiotic resistance among UPEC isolates has been already demonstrated against a wide variety of antibiotics, including oral first-generation cephalosporins, ampicillin, cefuroxime, cotrimoxazole, TMP-SMZ, nalidixic acid, amoxicillin-clavulanate, aminopenicillins, and cefradine.[8] Moreover, UPEC isolates from pregnant women who have a history of recurrent UTIs have shown a significant increase in resistance to tetracycline, ampicillin, ciprofloxacin, gentamycin, and amikacin.[13] The prevalence of resistance among UPEC in Europe constitutes 11.8% for the third-generation cephalosporins and 22.3% for fluoroquinolones. In the United States, the prevalence of fluoroquinolone-resistant UPEC in hospitals constituted 31.3% for the period 2007–2010.[14]
Due to the continuing and alarming emergence of antibiotic resistance in UPEC and the heavy burden that this resistance carries worldwide, there is a great tendency to develop non-antibiotic antimicrobial strategies for UTIs treatment and prophylaxis. These alternative approaches include vaccines, probiotics, mannosides and galactosides, bacteriophage, pilicides and curlicides, nanoparticles, estrogens, and phytochemicals.[8,12] Among these strategies, phytotherapy has gained a special attention worldwide. Plants represent an essential part of traditional medicine and they have been used throughout the ages as a source of medications. Besides, studies have proven the effectiveness of many medicinal plants in treating and preventing various health conditions. Reviewing the literature, we have noticed that since the beginning of the 21st century there has been a noticeable increase in the number of researches conducted on medicinal plants and more attention have been paid to them as they represent effective, safe, relatively cheap and eco-friendly treatments. In microbiology, this attention towards phytotherapy is also obvious since there have been numerous researches on medicinal plants to confront microorganisms, especially bacteria. Given that targeting the causative agent of infection increases the effectiveness of treatment, and since UPEC is the primary causative agent of UTIs, this review aimed to summarize and discuss the recent studies on antibacterial activity of medicinal plants against UPEC, in addition to their bioactive compounds and possible mechanisms of action. Five databases (PubMed, Scopus, Science Direct, Medline and Cochrane Trials) were searched in August 2020 using the keywords (“medicinal plant” or plant or traditional medicine) AND (“uropathogenic E. coli” or UPEC or UTI). More references were collected by cross-referencing the obtained articles. The majority of studies were after 2010. A textual narrative approach was used to synthesize and obtain the results.
UROPATHOGENIC ESCHERICHIA COLI: THE MAIN ETIOLOGICAL AGENT OF URINARY TRACT INFECTIONS
UPEC account for about 80% of uncomplicated UTIs, 95% of community-acquired infections, and the half of infections acquired in hospitals, which makes them the most predominant causative agent in both complicated and uncomplicated UTIs.[6]
UPEC is a group of extraintestinal pathogenic E. coli whose origin is believed to be the normal flora in the gut.[5,6] The frequent matches between the UTI-responsible isolates and rectal isolates from the same individual assume that a primary reservoir of UPEC is in the human intestine.[15] The environment of the urinary system is not suitable for bacteria to survive, especially under the constant flow of urine and the innate immune responses that are triggered in response to the infection. However, UPEC developed a number of smart strategies that enable them to survive under these conditions and colonize the urinary tract. These strategies are achieved using a variety of virulence factors such as fimbrial and nonfimbrial adhesins, curli, lipopolysaccharides, surface vesicles, polysaccharide capsule, flagella, secreted toxins, two-component signaling systems and iron acquisition system.[8] These virulence factors can be transferred via genetic elements including transposons, plasmids, bacteriophages, and pathogenicity islands.[5] Using transcriptomics investigations, the global transcription profile of UPEC during UTI has been defined confirming the expression of several virulence genes that play a critical role in UPEC pathogenesis.[8] However, it has been shown that there are a high genomic diversity and considerable differences in the repertoire and expression levels of virulence factors among UPEC strains.[8,16]
The pathogenesis process by which UPEC develop UTI is a complex multi-stage process. This process was well reviewed by Wiles et al.,[16] Terlizzi et al.[8] and Asadi Karam et al.[5] The first stage is the entering of intestinal E. coli into the urinary tract system and colonizing periurethral and vaginal areas and urethra. Some of the colonized bacteria then convert to motile form and ascend into the bladder lumen where they grow as planktonic cells in urine. Using bacterial adhesion factors, UPEC then can adhere to facet cells that line the lumen of the bladder. After that, UPEC are able to enter the cytoplasm of the facet cells and replicate rapidly forming intracellular bacterial communities (IBCs), within which UPEC can reach 105 bacteria per cell. The host immune system responds to the presence of UPEC in the normally sterile urinary tract by inflammatory responses, leading to neutrophil influx, cytokine production, exfoliation of the IBCs-containing bladder facet cells into the urine, and generation of reactive oxygen species, reactive nitrogen species, and other antimicrobial compounds. By the formation of IBCs, the underlying immature urothelial cells become exposed and more susceptible to infection allowing UPEC to invade them and form quiescent intracellular reservoirs, which are less susceptible to many antibiotics. It seems that these inactive reservoirs are able to re-activate and cause recurrent UTI. Also, recurrent UTI may be caused when E. coli strains re-enter the urinary tract system from the gut; however, this requires further studies. Finally, bacteria in the IBCs can convert to the filamentous motile form, flux out of the infected cells, invade naive bladder cells and form new IBCs. Moreover, bacteria escaped from the biofilm structures can ascend and colonize the kidneys causing pyelonephritis. UPEC may also disseminate from the urinary tract to the bloodstream causing bacteremia/septicemia.[5,8,16]
MEDICINAL PLANTS FOR MANAGEMENT AND TREATMENT OF URINARY TRACT INFECTIONS
UTIs are one of the most important health problems at the present time due to their high prevalence in community and hospitals and the economic burden they carry to both patient and government.[3,5] Furthermore, the continuous emergence of uropathogenic strains that are resistant to traditional antibiotics leads to the lack of effective treatments. Bacteria have developed various smart mechanisms to overcome the therapeutic procedures and survive within the urinary system.[12] In addition to the high financial cost (over one billion dollars), the process of developing new antibacterial agent takes a relatively long time (more than 10 years) to reach the market.[17] Therefore, there is a great trend around the world towards medicinal plants as it is estimated that 80% of people and 30% of pharmaceutical formulations are mainly dependent on medicinal plants.[18] Simultaneously, there is an increasing interest within the scientific community towards documenting, validating, and studying these plants. Reviewing the available literature, we have noticed that since the beginning of the 21st century there has been a significant increase in researches on medicinal plants, concerning their effectiveness in treating and preventing UTIs, their mechanisms of action, and the active substances responsible for the antibacterial properties [Figure 1].
Figure 1.
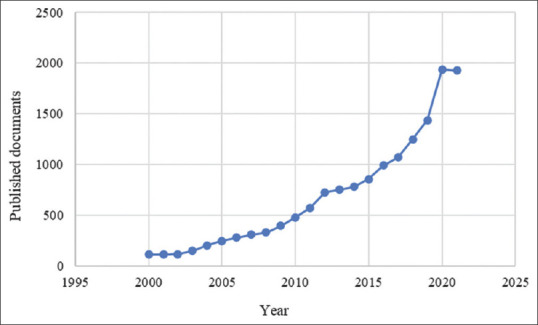
Number of studies published from 2000 to 2021 concerning the use of medicinal plants against UTIs (Scopus database)
The great features of plants are the main reason behind this increasing interest; besides the low cost and the few reported side effects,[18] bacteria potentially are less likely to develop resistance against plant extracts as they contain a wide range of phytochemicals that act against many different molecular targets within bacterial cells.[19,20] The presence of a wide variety of phytochemicals and secondary metabolites in medicinal plants gives them a broad spectrum of properties. These compounds are classified into groups according to their chemical composition; the main groups include flavonoids, quinones, coumarins, tannins, alkaloids, terpenoids, lectins, and polypeptides.[21] Studies have proven the antibacterial activity of many medicinal plants against a wide range of bacterial pathogens, as well as the ability to reverse antibiotic resistance in some cases.[22] The mechanisms by which plants exert therapeutic or protective properties against UTIs are not precisely defined; however, researches are being extensively conducted in this field. There are some mechanisms that have been reported from in vivo and in vitro studies, this includes antiadhesive activity,[23,24,25] inhibition of bacterial growth,[26,27,28] bactericidal activity,[28,29] antibiofilm formation activity,[30,31] inhibition of motility[32,33] and enhancing the antibacterial activity of antibiotics.[34,35] Interestingly, in some studies plants showed activity against UTIs by affecting the urinary cells and not the bacteria themselves.[36,37] The parts of the plant used to treat UTIs vary a lot. In general, certain part of the plant or even the whole plant is used depending on the type of plant and the information from the folk medicine. Medicinal plants are taken orally in their natural form or as extracts, alone or in combination with other foods or drinks or even with other plants, at a dose that is determined according to age, gender, and health status of the person.[18] In their review, Shaheen et al.[18] reviewed 17 plants that are commonly used for the treatment of UTIs, along with their main active phytochemicals and their therapeutic effects.[18]
Given the importance of UPEC as the most common causative agent of complicated and uncomplicated UTIs in hospitals and community, the recent studies (mainly after 2010) conducted to investigate antibacterial effects of medicinal plants against UPEC have been reviewed. The most recently studied medicinal plants against UPEC are the following:
Cranberry
For decades, cranberry has been one of the most popular plants for managing and preventing UTIs. Moreover, it has been extensively studied and its effectiveness against UTIs has been demonstrated in many in vitro and in vivo studies.[38,39]
The American cranberry (Vaccinium macrocarpon) belongs to the evergreen Ericaceae family, whose origin is in the northeastern United States and southern Canada. Native Americans were the first to use cranberry as food, medicine, and dye.[40] Cranberry is rich in a wide range of phytochemicals, including proanthocyanidins (PACs) (419 mg/100 g),[41] anthocyanins (91.5 mg/100 g),[42] flavonols (20–40 mg/100 g),[43] catechins (17 mg/100 g),[44] benzoic and phenolic acids, terpenes, and sterols, among other.[40] PACs are the most bioactive compounds studied in cranberries. Moreover, cranberry content of PACs is the highest among other foods.[40]
Understanding bioavailability and metabolism of cranberry compounds in human body would contribute to identifying their active substances against UPEC; in addition to determining the appropriate dose ensuring that active substances reach the urine in effective quantities. Many studies have been carried out to evaluate the bioavailability of cranberry phytochemicals in human urine showing the presence of anthocyanin glycosides,[45] salicyluric acid, hippuric acid, dihydroxybenzoic acid isomers, and quercetin glucuronide.[46] Some polyphenols are absorbed at the intestinal level and undergo metabolism to produce phase-I and phase-II metabolites, which are then secreted in urine after the first hours of intake.[47] The large part of polyphenols is metabolized by the intestinal flora producing phenolic metabolites such as simple phenols and phenolic acids. These metabolites need a longer time to be secreted in urine.[47,48,49] Comparing to free forms, conjugated forms of phenolic compounds have been shown to be prevalent in human urine after the intake of cranberry.[49]
Although there is not yet a clear comprehension of how American cranberry could relief UTI symptoms or act against UPEC, many mechanisms have already been suggested. Based on the available data from in vitro and in vivo studies, the main mechanisms can be summarized as follows: (1) Obstruction of the bacterial adhesion to bladder cells, which is the most reported mechanism, (2) downregulation or interference with several bacterial virulence factors which results in reducing the bacterial ability to survive in urinary tract and establish a successful infection, (3) repressing the inflammatory cascades triggered by the immune system as a response to the bacterial invasion.[50]
For a long time, PACs were believed to represent the active part responsible for the cranberry effectiveness against UTIs. Interestingly, it has been shown that PACs are not significantly bioavailable as they are not absorbed to a relevant extent in the intestine.[51,52] The antiadhesive properties of PACs against UPEC have been mainly attributed to A-type. Several studies have shown that the unusual double A-type linkages in A-type PACs are likely responsible for their antiadhesive properties,[53,54] whereas B-type PACs have been shown to have no antiadhesive properties against UPEC.[55] Moreover, It has been suggested that A-type PACs interfere with P-fimbriae on P-fimbriated UPEC and inhibit their ability to adhesion.[56] Purified PACs from cranberry extracts were able to block mannose-sensitive agglutination of UPEC with yeast cells (Saccharomyces cerevisiae),[57] indicating the inhibition of Type 1 fimbriae that usually mediate the bacterial mannose-sensitive agglutination with yeast cells.[58] Furthermore, an ex vivo experiment has shown that after consuming cranberry, urine from volunteers significantly inhibited UPEC adhesion to human T24 bladder cells.[59] Under in vitro conditions, Rafsanjany et al.[59] investigated the differences in antiadhesive properties between PAC-containing and PAC-depleted cranberry extracts. Results showed that PAC-containing extract inhibited UPEC internalization into bladder cells leading to bacterial agglomeration on the surfaces of bladder cells. Furthermore, this extract also inhibited bacterial adhesion of both mannose-sensitive type 1 fimbriae and P-and F1C-fimbriae dominated UPEC strains. As a feedback, an up-regulation in the expression of these fimbriae was observed in the respective strains. Interestingly, PAC-depleted extract showed an antiadhesive effect only in the case of Type 1 fimbriae dominated UPEC strain.[59]
The high levels of Extended-Spectrum β-lactamases are considered the main cause of resistance in UPEC with the predominance of the CTX-M β-lactamase family in many countries. In particular, CTX-M-15 genotype is the most prevalent in UPEC strains.[60,61] Cysticlean® is a cranberry product which contains 240 mg of PACs per capsule. In an in vitro study, Samarasinghe et al.[62] showed that treating CTX-M-15 UPEC isolate with Cysticlean® lead to a significant reduction in the expression of β-lactamases and many virulence factors genes, including genes encoding for iron acquisition system (ChuA), toxins (SAT, and USP), β-lactamases (CTX-M), protectins (KPSM, SoxS, RecA and TraT) and transporters (IdfB and HcaT). However, the same results may not be obtained in vivo due to the microbial catabolism of most PAC compounds by gut microbiota, as previously mentioned. Interestingly, PACs microbial-derived metabolites (benzoic acid, vanillic acid, catechol, phenylacetic acid, and 3,4-dihydroxyphenylacetic acid) have shown antiadhesive properties against UPEC in vitro and in ex vivo study.[55] Thus, the presence of these compounds in urine may contribute to preventing UTI. Another mechanism by which cranberry may affect UPEC adhesion to host cells is changing bacterial surface hydrophobicity, which is the initial factor in adhesion process.[63] In vitro, cranberry phenolic compounds (procyanidin A dimer, dihydroferulic acid glucuronide, myricetin, quercetin glucoside, and prodelphinidin B) have significantly decreased UPEC surface hydrophobicity.[64] Interestingly, under ex vivo conditions, it was observed that the inhibitory effect of cranberry toward UPEC adhesion to bladder cells differs between males and females.[25] This observation has been corelated with Tamm–Horsfall protein (THP) level which significantly increased after cranberry intake to a more extent in males than in females. THP is a mannose-containing glycoprotein produced only by the renal tubular cells in kidney and considered as an innate immune response.[65] Thus, authors concluded that undefined cranberry metabolites inhibit UPEC adhesion to bladder cells indirectly by stimulating THP production, which binds to type 1 fimbriae in UPEC via their mannose moieties and flush them out with urine. This mechanism could not be observed in vitro studies where bladder cells are used since THP is secreted only by kidney. This could also explain some of the contravention between in vitro and in vivo data.
UPEC motility also has been shown to be affected by cranberry compounds.[32] Indeed, the exposure to either cranberry powder or purified cranberry PACs has resulted in downregulated expression of flagellin gene (fliC), and subsequently, flagella synthesis was inhibited. Because flagella are required for bacterial swimming and swarming motility,[66] these two types of motility were thus hindered by cranberry compounds.
With regard to UPEC biofilms, there are contradictory results in literature. For instance, two cranberry isolated flavonols (myricetin and quercitrin) have been reported to reduce UPEC biofilm formation in vitro,[64] whereas in another study, cranberry did not show any effect on biofilm formation in UPEC.[59] However, clinical UPEC strains produced more biofilm in urine after cranberry juice consumption.[67]
Notwithstanding the above, some studies have reported that cranberry products did not have bacteriostatic activity[32,68,69] or effect on curli formation in UPEC.[59]
Despite numerous in vivo and in vitro studies on cranberries and their products, there is a confusion and inconsistency in the results in terms of their effectiveness in preventing UTIs. Although most in vitro results showed antibacterial activity, many clinical data contradicted them showing insignificant efficacy in preventing UTIs.[70] Thus, there are not clear recommendations to use cranberry products for UTIs prevention. In their meta-analyzes, Jepson et al.[70] concluded that cranberry products were not significantly effective in the prophylaxis of UTIs. Analyzing the available literature, this inconsistency can be explained by many reasons: (1) Most studies are carried out on PACs compounds while other compounds could have an effect no less important than that of PACs, or they could exhibit a synergetic effect that leads to a more effectiveness against UPEC.[59,64] For example, recent studies have shown that PACs-free cranberry extract[59] and other cranberry phytochemicals[64] have also antibacterial properties against UPEC. In addition, the limited bioavailability of PACs suggests that their microbial-metabolites are more likely to be responsible for the antibacterial activity in the urinary tract. (2) There are differences in PACs concentrations in the used forms of cranberry (juice, capsules or tablets). To the best of our knowledge, there is not yet a standardized concentration of PACs proved to prevent UTIs. However, it has been suggested that at least 36 mg of cranberry PAC equivalents per day (given in two doses in the morning and the evening) is required to have anti-adhesion efficacy on UPEC.[53] (3) There is a chemical heterogeneity in the used cranberry products due to manufacturing processes. (4) UPEC strains with different predominant adhesins could be affected differently by cranberry products, as mentioned above.
From the current state of knowledge we can conclude that cranberry could represent a non-pharmaceutical alternative for low-dose antibiotics in recurrent uncomplicated UTIs prophylaxis. Cranberry antibacterial effects against UPEC that have been reported in numerous in vitro and in vivo studies seem to be mediated by several cranberry phytochemicals and metabolites and not only by the PAC compounds, but likely by their microbial-derived metabolites. The effect of cranberry compounds seems not to be limited to direct interaction with UPEC but also to interaction with bladder and kidney cells. All together, these mechanisms can lead finally to anti-UTI activity. However, there is a need to standardize the use of cranberry in managing and preventing UTIs, in terms of form, dosage, and duration. In addition, more studies should be conducted to determine the bioactive compounds in cranberries against UPEC and the appropriate concentrations that give antibacterial effect. The precise identification of these parameters may enable the development of cranberry-based pharmaceutical forms more effective against UPEC.
Java tea (Orthosiphon stamineus leaves)
O. stamineus is a medicinal plant from Lamiaceae family. Other common names are misai kucing and kumis kucing. This plant is widely grown in tropical regions especially Southeast Asia. Due to the wide range of medicinal benefits, O. stamineus has been widely used in traditional medicine. Java tea is an herbal tea made from the leaves of O. stamineus and is well known for its valuable pharmacological properties including antibacterial, antioxidant, anti-inflammatory, antihypertensive, hepatoprotective, and vasodilatation.[71] Moreover, this plant is listed in many pharmacopoeias as a treatment for urinary tract disorders such as urethritis, cystitis and nephritis.[71]
From the chemical point of view, O. stamineus mainly contains glycosides, bioactive proteins, a high level of potassium and a rich content of phenolic compounds.[72] Particularly, the leaves of O. stamineus contain flavonoids (0.5%–0.7% w/w), especially, methoxylated flavones, prenylated flavones, eupatoretin, di-caffeoyltartrat and rosmarinic acid, diterpens, triterpenes, and volatile oil.[73]
Aqueous extract from O. stamineus leaves (Orthosiphon Water Extract, OWE) has been shown to have significant antiadhesive properties towards UPEC, in both in vitro and in vivo studies. In mice infected with UPEC, OWE led to a significant reduction of bacteria within the urinary tract; moreover, treating mice with OWE before infection reduced UPEC ability to colonize the bladder.[74] O. stamineus leaves also showed a significant inhibition of UPEC adhesion to human T24 bladder cells.[23,75] Interestingly, OWE transformed UPEC lifestyle from sessile to a more motile one which was correlated with an upregulated flagellar gene fliC and a downregulated fimbrial gene fimH.[74,76] The same observations were made by Beydokhti et al.,[73] as they reported a significant downregulation of curli and chaperone–usher associated fimbriae (type 1-, F1C-, and P fimbriae) after treating UPEC with tannin-depleted extract (OWEøtannin). Using OWEøtannin was explained by that the main tannins from O. stamineus leaves (rosmarinic acid and cichoric acid) will not reach the urine as they cannot be absorbed at the intestinal level; therefore, such tannins will give false-positive results in vitro.[73] In a comparison between aqueous and acetonic extracts of O. stamineus leaves with respect to antiadhesive properties towards UPEC, the acetonic extract was shown to have higher efficacy due to its enriched content of polymethoxylated flavones, specifically, the B-ring methoxylation in ladanein, sinensetin, salvigenin, 5,6 74’-tetramethoxyflavone and 5-hydroxy-6,73’,4’-tetramethoxyflavone.[23] The acetonic extract resulted in significant downregulation of type 1 fimbriae and curli genes. Interestingly, the whole extract showed a higher antiadhesive effect compared to isolated polymethoxylated flavones[23] which may indicate the presence of other active compounds or an overall synergistic effect between the extract constituents.
O. stamineus leaves also have been shown to inhibit other bioprocesses in UPEC such as quorum sensing[74] and iron acquisition.[73] It has been found that the downregulation of bacterial iron-acquisition genes by OWE is a feedback mechanism in response to the high iron levels in the extract.[73] Bacterial ABC transporters contribute to bacterial survival within host as they are involved in transporting vital substances (sugars, lipids, metal ions and proteins) across the inner bacterial membrane.[77] Interestingly, it has been reported that OWEøtannin significantly interacted with these transporters pathways in UPEC.[73] However, O. stamineus leaves seem not to have a cytotoxic effect towards neither UPEC nor urinary cells.[23,74,75]
Summarizing, O. stamineus leaves inhibit the adhesion of UPEC by causing a significant downregulation of several adhesins genes, especially those assembled by chaperone-usher pathway.[73,74,76] Moreover, O. stamineus leaves create an unsuitable environment with a high level of iron which may cause the bacteria to transform from sessile to motile lifestyle in an attempt to reach a more appropriate habitat. This transformation is simultaneous with increased expression of flagella genes.[78,79] Another antiadhesive mechanism of O. stamineus leaves is to interact directly with bladder cells hindering the adhesion of UPEC to them.[75]
Through a review of literature and the aforementioned positive results, it is obvious that O. stamineus has effective and promising properties in fighting UTIs caused by UPEC. However, these antibacterial properties need further studies to understand their mechanisms of action and to determine the active substances and their bioavailability within the human body. This in turn may lead to develop an effective UTI therapeutic approach or drug formulas based on this valuable plant.
Corn silk (Stigma Maydis)
Zea mays L. (commonly maize or corn) is one of the most common and widespread crops worldwide. The most producing countries of maize are the United States, China and Brazil. Maize belongs to the grass family Poaceae and is cultivated almost worldwide. All parts of the maize plant are used for the purposes of food, feed, and various industries.[80] Corn silk (Stigma Maydis) is the yellowish thread-like strands of maize plant which have been used for a long time in traditional medicine to treat many health conditions, including UTIs, cystitis, stones, and gravel.[81]
Corn silk contains a wide range of phytochemicals such as flavonoids, tannins, alkaloids, steroids, saponins, chlorogenic acid, and allantoin. In addition, it contains proteins, vitamins, minerals, carbohydrates, and volatiles oils.[81,82] Maysin and its derivatives are known to be a unique C-glucosyl-flavonoids in corn silk.[82,83] Interestingly, it has been shown that C-glucosyl-flavonoids from Crataegus spp. are well bioavailable after oral intake in rat model.[84]
Reviewing the available literature, we concluded that corn silk bioactivity against UTIs is mainly attributed to its antiadhesive effect. In a clinical trial, oral intake of aqueous corn silk extract by UTI patients significantly reduced clinical symptoms and blood cells in urine.[85] Furthermore, in vitro studies have reported an inhibition of corn silk-treated UPEC adhesion to human T24 bladder cells.[75,82] Moreover, it has been shown that this antiadhesive activity is a result of interacting with the bacterial cells without affecting bladder cells viability[75] and with no direct cytotoxic effect towards E. coli.[86]
From the previous data, it can be suggested that C-glucosyl-flavonoids in corn silk are one of the bioactive compounds responsible for the antiadhesive activity against UPEC. However, further studies are needed to validate the traditional use of corn silk for UTI treatment and management.
Celery fruits
Apium graveolens L. (also called Fructus Apii graveolens or celery fruits) is a member of Apiaceae family that is cultivated worldwide and also can grow wild.[87] This plant has been used traditionally in Ayurvedic, Persian, European, Danish, and Philippians folk medicine for treating gut diseases, visceral spasms, kidney stones and also uncomplicated UTIs due to its diuretic and anti-inflammatory activity.[87,88,89]
Celery fruits are rich in a wide variety of phytochemicals. Mainly, they contain flavonoids, flavonoid glycosides, tannin, saponin and volatile oil (2%–3%) (mainly R-(+)-limonene, phthalides, and β-selinene). In addition, they contain fat, protein, Vitamin C, β-carotene, sodium, calcium and phosphorus, among others.[87,89]
Recently, studies have been conducted to validate the use of celery fruits as a therapeutic agent for UTIs showing a strong concentration-dependent antiadhesive activity against UPEC.[24,88] Grube et al.[88] attributed this activity to the presence of phthalides in hydroalcoholic and acetonic extract of celery fruits, particularly, sedanenolide and Z-ligustilide phthalides. Sarshar et al.[24] conducted similar in vitro and in vivo investigations to assess antiadhesive properties of celery fruits against UPEC. The hydroalcoholic extract celery fruits has shown a dose-dependent antiadhesive and anti-quorum sensing activity toward UPEC strains. Additionally, when mice were treated with celery fruits extract before being transurethrally infected with UPEC, a significant reduction in bacterial load in bladder was observed.[24] Since the extract did not affect the viability of UPEC or the bladder cells, it is assumed that the antibacterial activity is more likely to be due to the interference with bacterial adhesion and quorum-sensing system in UPEC.
In conclusion, the traditional use of celery fruits to treat UTIs seems to be validated. However, additional studies are needed to fully understand the mechanism of action and to determine the active compounds as such studies are relatively few. In addition, it is important to study the bioavailability in human in order to standardize the dose and the form of treatment.
Other medicinal plants with antibacterial effects against uropathogenic Escherichia coli
In addition to the previously mentioned medicinal plants, many others are under research to investigate their effectiveness against UPEC; however, researches on them are still relatively few. In Table 1, we summarized other recently studied plants with positive results against UPEC. It is noted that adhesion is the most bacterial bioprocess affected by these plants. The active substances vary according to the type of plant. In general, these plants represent an interesting research area and they should be studied in more depth to determine their antibacterial properties and use them in the fight against UPEC.[98]
Table 1.
Antibacterial effects of some medicinal plants against uropathogenic Escherichia coli
| Plant material | Active phytochemicals | Type of assay | Antibacterial activity |
|---|---|---|---|
| Cinnamon oil | TC | In vitro | TC (560 and 750 µM) decreased the expression of major genes involved in UPEC attachment and invasion to urinary tract epithelial cellss[90] |
| TC (0.25% and 0.50% v/v) inhibited biofilm formation on plates and catheters[91] | |||
| Lock solution containing TC (1%, 1.25% and 1.5% v/v) inactivated UPEC biofilm on polystyrene plates and urinary catheters[91] | |||
| Oregano oil and thyme red oil | Carvacrol and thymol, respectively | In vitro | Carvacrol and thymol (0.01% v/v) inhibited biofilm formation, decreased the hemagglutination ability of UPEC and reduced fimbriae formation and swarming motility of UPEC[30] |
| In the presence of carvacrol and thymol (0.01% v/v), human whole blood was able to more easily kill UPEC[30] | |||
| Essential oils of O. majorana, T. zygis and R. officinalis |
O. majorana: Terpinene-4-ol T. zygis: Linalool R. officinalis: 1,8-cineole, camphor, α-Pinene and β-Pinene |
In vitro | Showed antibacterial and high antibiofilm activity against UPEC[31] |
| A. repens rhizomes | Coumaric acid ester | In vitro | Acetonic extract (>250 µg/mL) showed a significant antiadhesive activity against UPEC attachment to human T24 bladder cells[76] |
| Coumaric acid ester (1000 and 500 µg/mL) reduced the invasion of UPEC into the bladder cells[76] | |||
| Commercial plant secondary metabolites; asiatic acid and ursolic acid | Pentacyclic triterpenes (asiatic acid and ursolic acid) | In vitro | Asiatic and ursolic acid (40 and 50 μg/mL) showed antibacterial effects and significantly reduced the attachment of UPEC to urinary epithelial cells[92] |
| L. pumila var. Alata | N/A | In vivo | Induced apoptosis in bladder epithelial cells and significantly reduced the number of intracellular UPEC[37] |
| Garlic (A. sativum) | N/A | In vitro | Showed antibacterial activity against UPEC (MICs=62.5-100 mg/ml) and inhibited biofilm formation and dispersal[93] |
| O. aristatus leaves | N/A | In vivo (a mouse infection model) and in vitro | Reduced kidney and bladder colonization by UPEC[94] |
| Reduced the bacterial adhesion to T24 bladder cells[94] | |||
| Significantly inhibited bacterial quorum sensing[94] | |||
| Green tea | N/A | In vivo (a rat model) | Intravesical instillation of green tea attenuated the inflammatory response to UPEC-SR71-induced bacterial cystitis[36] |
| Nasturtium (Tropaeoli majoris herba) and horseradish (Armoraciae rusticanae radix) | ITC | In vitro | ITC showed a strong antimicrobial activity against clinical UPEC strains (MIC 90=0.17 mg/ml)[26] |
| ITC reduced bacterial internalization into human T-24 bladder cells[26] | |||
| C. bolivianum | N/A | In vitro | The extract (400 µg/ml) decreased the adhesion, invasion and biofilm formation of UPEC[95] |
| Rosemary (S. rosmarinus) | N/A | In vitro | Rosemary extract (1 and 10 μg/ml) showed a strong bacteriostatic effect against UPEC[57] |
| B. abyssinica | Picolinyl hydrazide | In vitro | The extract showed antibacterial activity against MDR-UPEC (MICs=12.5-50 mg/mL)[96] |
| Neem (A. indica) | N/A | In vitro | Showed inhibitory effect against UPEC[97] |
| A. nilotica | N/A | In vitro | The extract showed antibacterial activity against UPEC (MIC=11.7 mg/mL) and significantly reduced bacterial biofilm formation[98] |
TC: Trans-cinnamaldehyde, ITC: Isothiocyanates, N/A: Not applicable, UPEC: Uropathogenic Escherichia coli, R. officinalis: Rosmarinus officinalis, L. pumila: Labisia pumila, A. sativum: Allium sativum, A. repens: Agropyron repens, O. majorana: Origanum majorana, T. zygis: Thymus zygis, O. aristatus: Orthosiphon aristatus, C. bolivianum: Clinopodium bolivianum, S. rosmarinus: Salvia rosmarinus, B. abyssinica: Bersama abyssinica, A. indica: Azadirachta indica, A. nilotica: Acacia nilotica, MICs: Minimum inhibitory concentrations
CONCLUSION
UTIs represent a global concern and it gets more and more difficult to manage them. The high incidence of recurrence and the rapid emergence of resistance among uropathogens make these infections a serious health problem which requires a big attention from the scientific community. UPEC are the main cause of UTIs accounting for more than 95% of community-acquired infections. UPEC are able to survive within the urinary tract and establish an infection via a wide variety of smart virulence factors. Additionally, the ability of UPEC to resist antibiotics are continuing to increase making the treatment difficult and complicated. Recently, many alternative approaches have been extensively studied to overcome UTIs threat and to develop more effective and economic treatment strategies. Medicinal plants are one the most promising sources of compounds that exert antibacterial activity against UPEC. Since ancient times, plants have been shown to successfully treat and prevent many diseases and today they are highly used by both general public and pharmaceutical companies. A thorough understanding of mechanisms by which plant materials affect UPEC and human body would surely con[98] tribute to the development of reliable and effective plant-based therapeutic approaches. This review presented an overview of the antibacterial activities of medicinal plants against UPEC. In general, it could be said that studies results are very promising in confronting UPEC including MDR strains. However, more investigations are needed to fully comprehend how these plants could treat or prevent UTIs caused by UPEC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–84. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bischoff S, Walter T, Gerigk M, Ebert M, Vogelmann R. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect Dis. 2018;18:56. doi: 10.1186/s12879-018-2960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann R, Mediati DG, Duggin IG, Harry EJ, Bottomley AL. Metabolic adaptations of uropathogenic E.coli in the urinary tract. Front Cell Infect Microbiol. 2017;7:241. doi: 10.3389/fcimb.2017.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ECDC. Sweden: ECDC; 2013. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals 2011–2012. [Google Scholar]
- 5.Asadi Karam MR, Habibi M, Bouzari S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol Immunol. 2019;108:56–67. doi: 10.1016/j.molimm.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Kot B. Antibiotic resistance among uropathogenic Escherichia coli. Pol J Microbiol. 2019;68:403–15. doi: 10.33073/pjm-2019-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smelov V, Naber K, Bjerklund Johansen TE. Improved classification of urinary tract infection: Future considerations. Eur Urol Suppl. 2016;15:71–80. [Google Scholar]
- 8.Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol. 2017;8:1566. doi: 10.3389/fmicb.2017.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhakal BK, Kulesus RR, Mulvey MA. Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli. Eur J Clin Invest. 2008;38(Suppl 2):2–11. doi: 10.1111/j.1365-2362.2008.01986.x. [DOI] [PubMed] [Google Scholar]
- 10.Jakobsen L, Spangholm DJ, Pedersen K, Jensen LB, Emborg HD, Agersø Y, et al. Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int J Food Microbiol. 2010;142:264–72. doi: 10.1016/j.ijfoodmicro.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Mellata M, Johnson JR, Curtiss R., 3rd Escherichia coli isolates from commercial chicken meat and eggs cause sepsis, meningitis and urinary tract infection in rodent models of human infections. Zoonoses Public Health. 2018;65:103–13. doi: 10.1111/zph.12376. [DOI] [PubMed] [Google Scholar]
- 12.Zalewska-Piątek BM, Piątek RJ. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains. Acta Biochim Pol. 2019;66:129–38. doi: 10.18388/abp.2018_2787. [DOI] [PubMed] [Google Scholar]
- 13.Habibi A, Khameneie MK. Antibiotic resistance properties of uropathogenic Escherichia coli isolated from pregnant women with history of recurrent urinary tract infections. Trop J Pharm Res. 2016;15:1745. [Google Scholar]
- 14.Edelsberg J, Weycker D, Barron R, Li X, Wu H, Oster G, et al. Prevalence of antibiotic resistance in US hospitals. Diagn Microbiol Infect Dis. 2014;78:255–62. doi: 10.1016/j.diagmicrobio.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis. 1995;172:440–5. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 16.Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85:11–9. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole ST. Who will develop new antibacterial agents? Philos Trans R Soc Lond B Biol Sci. 2014;369:20130430. doi: 10.1098/rstb.2013.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaheen G, Akram M, Jabeen F, Ali Shah SM, Munir N, Daniyal M, et al. Therapeutic potential of medicinal plants for the management of urinary tract infection: A systematic review. Clin Exp Pharmacol Physiol. 2019;46:613–24. doi: 10.1111/1440-1681.13092. [DOI] [PubMed] [Google Scholar]
- 19.Álvarez-Martínez FJ, Barrajón-Catalán E, Encinar JA, Rodríguez-Díaz JC, Micol V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr Med Chem. 2020;27:2576–606. doi: 10.2174/0929867325666181008115650. [DOI] [PubMed] [Google Scholar]
- 20.Álvarez-Martínez FJ, Rodríguez JC, Borrás-Rocher F, Barrajón-Catalán E, Micol V. The antimicrobial capacity of Cistus salviifolius and Punica granatum plant extracts against clinical pathogens is related to their polyphenolic composition. Sci Rep. 2021;11:588. doi: 10.1038/s41598-020-80003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upadhyay A, Upadhyaya I, Kollanoor-Johny A, Venkitanarayanan K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Biomed Res Int. 2014;2014:761741. doi: 10.1155/2014/761741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob Resist Infect Control. 2019;8:118. doi: 10.1186/s13756-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deipenbrock M, Hensel A. Polymethoxylated flavones from Orthosiphon stamineus leaves as antiadhesive compounds against uropathogenic E.coli. Fitoterapia. 2019;139:104387. doi: 10.1016/j.fitote.2019.104387. [DOI] [PubMed] [Google Scholar]
- 24.Sarshar S, Sendker J, Qin X, Goycoolea FM, Asadi Karam MR, Habibi M, et al. Antiadhesive hydroalcoholic extract from Apium graveolens fruits prevents bladder and kidney infection against uropathogenic E.coli. Fitoterapia. 2018;127:237–44. doi: 10.1016/j.fitote.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Scharf B, Sendker J, Dobrindt U, Hensel A. Influence of cranberry extract on tamm-horsfall protein in human urine and its antiadhesive activity against uropathogenic Escherichia coli. Planta Med. 2019;85:126–38. doi: 10.1055/a-0755-7801. [DOI] [PubMed] [Google Scholar]
- 26.Mutters NT, Mampel A, Kropidlowski R, Biehler K, Günther F, Bălu I, et al. Treating urinary tract infections due to MDR E.coli with Isothiocyanates – A phytotherapeutic alternative to antibiotics? Fitoterapia. 2018;129:237–40. doi: 10.1016/j.fitote.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Al-Manhel AJ, Niamah AK. Effect of aqueous and alcoholic plant extracts on inhibition of some types of microbes and causing spoilage of food. Pak J Food Sci. 2015;25:104–9. [Google Scholar]
- 28.Sabadash M, Shulyak A. Canephron® N in the treatment of recurrent cystitis in women of child-bearing age: A randomised controlled study. Clin Phytosci. 2017;3:9. [Google Scholar]
- 29.Dufour V, Stahl M, Baysse C. The antibacterial properties of isothiocyanates. Microbiology (Reading) 2015;161:229–43. doi: 10.1099/mic.0.082362-0. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Kim YG, Lee J. Carvacrol-rich oregano oil and thymol-rich thyme red oil inhibit biofilm formation and the virulence of uropathogenic Escherichia coli. J Appl Microbiol. 2017;123:1420–8. doi: 10.1111/jam.13602. [DOI] [PubMed] [Google Scholar]
- 31.Lagha R, Ben Abdallah F, Al-Sarhan BO, Al-Sodany Y. Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules. 2019;24:1161. doi: 10.3390/molecules24061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hidalgo G, Chan M, Tufenkji N. Inhibition of Escherichia coli CFT073 fliC expression and motility by cranberry materials. Appl Environ Microbiol. 2011;77:6852–7. doi: 10.1128/AEM.05561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranfaing J, Dunyach-Remy C, Lavigne JP, Sotto A. Propolis potentiates the effect of cranberry (Vaccinium macrocarpon) in reducing the motility and the biofilm formation of uropathogenic Escherichia coli. PLoS One. 2018;13:e0202609. doi: 10.1371/journal.pone.0202609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazly Bazzaz BS, Khameneh B, Zahedian Ostad MR, Hosseinzadeh H. In vitro evaluation of antibacterial activity of verbascoside, lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J Phytomed. 2018;8:246–53. [PMC free article] [PubMed] [Google Scholar]
- 35.Bazzaz BS, Sarabandi S, Khameneh B, Hosseinzadeh H. Effect of catechins, green tea extract and methylxanthines in combination with gentamicin agair Staphylococcus aureus and Pseudomonas aeruginosa – Combination therapy against resistant bacteria. J Pharmacopuncture. 2016;19:312–8. doi: 10.3831/KPI.2016.19.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg S, Horowitz R, Coppenhagen-Glazer S, Pizov G, Elia A, Gofrit ON, et al. Intravesical administration of green tea extract attenuates the inflammatory response of bacterial cystitis – A rat model. BJU Int. 2014;114:601–7. doi: 10.1111/bju.12544. [DOI] [PubMed] [Google Scholar]
- 37.Fazliana M, Ramos NL, Lüthje P, Sekikubo M, Holm A, Wan Nazaimoon WM, et al. Labisia pumila var.alata reduces bacterial load by inducing uroepithelial cell apoptosis. J Ethnopharmacol. 2011;136:111–6. doi: 10.1016/j.jep.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Guay DR. Cranberry and urinary tract infections. Drugs. 2009;69:775–807. doi: 10.2165/00003495-200969070-00002. [DOI] [PubMed] [Google Scholar]
- 39.Fu Z, Liska D, Talan D, Chung M. Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: A systematic review and meta-analysis. J Nutr. 2017;147:2282–8. doi: 10.3945/jn.117.254961. [DOI] [PubMed] [Google Scholar]
- 40.Neto CC, Vinson JA. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. New Jersey: Humana Press; 2011. Cranberry; pp. 107–30. [Google Scholar]
- 41.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004;134:613–7. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54:4069–75. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 43.Vvedenskaya IO, Rosen RT, Guido JE, Russell DJ, Mills KA, Vorsa N. Characterization of flavonols in cranberry (Vaccinium macrocarpon) powder. J Agric Food Chem. 2004;52:188–95. doi: 10.1021/jf034970s. [DOI] [PubMed] [Google Scholar]
- 44.Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, et al. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006;54:9966–77. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- 45.Ohnishi R, Ito H, Kasajima N, Kaneda M, Kariyama R, Kumon H, et al. Urinary excretion of anthocyanins in humans after cranberry juice ingestion. Biosci Biotechnol Biochem. 2006;70:1681–7. doi: 10.1271/bbb.60023. [DOI] [PubMed] [Google Scholar]
- 46.Valentova K, Stejskal D, Bednar P, Vostalova J, Cíhalík C, Vecerova R, et al. Biosafety, antioxidant status, and metabolites in urine after consumption of dried cranberry juice in healthy women: A pilot double-blind placebo-controlled trial. J Agric Food Chem. 2007;55:3217–24. doi: 10.1021/jf0636014. [DOI] [PubMed] [Google Scholar]
- 47.McKay DL, Chen CY, Zampariello CA, Blumberg JB. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015;168:233–40. doi: 10.1016/j.foodchem.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 48.Monagas M, Urpi-Sarda M, Sánchez-Patán F, Llorach R, Garrido I, Gómez-Cordovés C, et al. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1:233–53. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Zuo Y, Vinson JA, Deng Y. Absorption and excretion of cranberry-derived phenolics in humans. Food Chem. 2012;132:1420–8. doi: 10.1016/j.foodchem.2011.11.131. [DOI] [PubMed] [Google Scholar]
- 50.Vasileiou I, Katsargyris A, Theocharis S, Giaginis C. Current clinical status on the preventive effects of cranberry consumption against urinary tract infections. Nutr Res. 2013;33:595–607. doi: 10.1016/j.nutres.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Rzeppa S, Bittner K, Döll S, Dänicke S, Humpf HU. Urinary excretion and metabolism of procyanidins in pigs. Mol Nutr Food Res. 2012;56:653–65. doi: 10.1002/mnfr.201100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zumdick S, Deters A, Hensel A. in vitro intestinal transport of oligomeric procyanidins (DP 2 to 4) across monolayers of Caco-2 cells. Fitoterapia. 2012;83:1210–7. doi: 10.1016/j.fitote.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Howell AB, Botto H, Combescure C, Blanc-Potard AB, Gausa L, Matsumoto T, et al. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: A multicentric randomized double blind study. BMC Infect Dis. 2010;10:94. doi: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foo LY, Lu Y, Howell AB, Vorsa N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–8. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 55.de Llano DG, Esteban-Fernández A, Sánchez-Patán F, Martínlvarez PJ, Moreno-Arribas MV, Bartolomé B. Anti-adhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic Escherichia coli in bladder epithelial cell cultures. Int J Mol Sci. 2015;16:12119–30. doi: 10.3390/ijms160612119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta A, Dwivedi M, Mahdi AA, Nagana Gowda GA, Khetrapal CL, Bhandari M. Inhibition of adherence of multi-drug resistant E.coli by proanthocyanidin. Urol Res. 2012;40:143–50. doi: 10.1007/s00240-011-0398-2. [DOI] [PubMed] [Google Scholar]
- 57.Marcon J, Schubert S, Stief CG, Magistro G. in vitro efficacy of phytotherapeutics suggested for prevention and therapy of urinary tract infections. Infection. 2019;47:937–44. doi: 10.1007/s15010-019-01315-4. [DOI] [PubMed] [Google Scholar]
- 58.Müller CM, Aberg A, Straseviçiene J, Emody L, Uhlin BE, Balsalobre C. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog. 2009;5:e1000303. doi: 10.1371/journal.ppat.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rafsanjany N, Senker J, Brandt S, Dobrindt U, Hensel A. in vivo consumption of cranberry exerts ex vivo antiadhesive activity against FimH-dominated uropathogenic Escherichia coli: A combined in vivo, ex vivo, and in vitro study of an extract from Vaccinium macrocarpon. J Agric Food Chem. 2015;63:8804–18. doi: 10.1021/acs.jafc.5b03030. [DOI] [PubMed] [Google Scholar]
- 60.Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M β-lactamases: The worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents. 2010;35:316–21. doi: 10.1016/j.ijantimicag.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Cai JC, Zhang R, Hu YY, Zhou HW, Chen GX. Emergence of Escherichia coli sequence type 131 isolates producing KPC-2 carbapenemase in China. Antimicrob Agents Chemother. 2014;58:1146–52. doi: 10.1128/AAC.00912-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samarasinghe S, Reid R, Al-Bayati M. The anti-virulence effect of cranberry active compound proanthocyanins (PACs) on expression of genes in the third-generation cephalosporin-resistant Escherichia coli CTX-M-15 associated with urinary tract infection. Antimicrob Resist Infect Control. 2019;8:181. doi: 10.1186/s13756-019-0637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otto K, Norbeck J, Larsson T, Karlsson KA, Hermansson M. Adhesion of type 1-fimbriated Escherichia coli to abiotic surfaces leads to altered composition of outer membrane proteins. J Bacteriol. 2001;183:2445–53. doi: 10.1128/JB.183.8.2445-2453.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodríguez-Pérez C, Quirantes-Piné R, Uberos J, Jiménez-Sánchez C, Peña A, Segura-Carretero A. Antibacterial activity of isolated phenolic compounds from cranberry (Vaccinium macrocarpon) against Escherichia coli. Food Funct. 2016;7:1564–73. doi: 10.1039/c5fo01441g. [DOI] [PubMed] [Google Scholar]
- 65.Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E.coli from binding to uroplakin Ia and Ib receptors. J Biol Chem. 2001;276:9924–30. doi: 10.1074/jbc.M008610200. [DOI] [PubMed] [Google Scholar]
- 66.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–44. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tapiainen T, Jauhiainen H, Jaakola L, Salo J, Sevander J, Ikäheimo I, et al. Biofilm formation and virulence of uropathogenic Escherichia coli in urine after consumption of cranberry-lingonberry juice. Eur J Clin Microbiol Infect Dis. 2012;31:655–62. doi: 10.1007/s10096-011-1355-2. [DOI] [PubMed] [Google Scholar]
- 68.O’May C, Tufenkji N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl Environ Microbiol. 2011;77:3061–7. doi: 10.1128/AEM.02677-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hidalgo G, Ponton A, Fatisson J, O’May C, Asadishad B, Schinner T, et al. Induction of a state of iron limitation in uropathogenic Escherichia coli CFT073 by cranberry-derived proanthocyanidins as revealed by microarray analysis. Appl Environ Microbiol. 2011;77:1532–5. doi: 10.1128/AEM.02201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ashraf K, Sultan S, Adam A. Orthosiphon stamineus Benth.is an outstanding food medicine: Review of phytochemical and pharmacological activities. J Pharm Bioallied Sci. 2018;10:109–18. doi: 10.4103/jpbs.JPBS_253_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tezuka Y, Stampoulis P, Banskota AH, Awale S, Tran KQ, Saiki I, et al. Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. Chem Pharm Bull (Tokyo) 2000;48:1711–9. doi: 10.1248/cpb.48.1711. [DOI] [PubMed] [Google Scholar]
- 73.Beydokhti SS, Stork C, Dobrindt U, Hensel A. Orthosipon stamineus extract exerts inhibition of bacterial adhesion and chaperon-usher system of uropathogenic Escherichia coli – A transcriptomic study. Appl Microbiol Biotechnol. 2019;103:8571–84. doi: 10.1007/s00253-019-10120-w. [DOI] [PubMed] [Google Scholar]
- 74.Sarshar S, Brandt S, Asadi Karam MR, Habibi M, Bouzari S, Lechtenberg M, et al. Aqueous extract from Orthosiphon stamineus leaves prevents bladder and kidney infection in mice. Phytomedicine. 2017;28:1–9. doi: 10.1016/j.phymed.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Rafsanjany N, Lechtenberg M, Petereit F, Hensel A. Antiadhesion as a functional concept for protection against uropathogenic Escherichia coli: In vitro studies with traditionally used plants with antiadhesive activity against uropathognic Escherichia coli. J Ethnopharmacol. 2013;145:591–7. doi: 10.1016/j.jep.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 76.Beydokthi SS, Sendker J, Brandt S, Hensel A. Traditionally used medicinal plants against uncomplicated urinary tract infections: Hexadecyl coumaric acid ester from the rhizomes of Agropyron repens (L.) P. Beauv. with antiadhesive activity against uropathogenic E. coli. Fitoterapia. 2017;117:22–7. doi: 10.1016/j.fitote.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Green ER, Mecsas J. Bacterial secretion systems: An overview. Microbiol Spectr. 2016;4(4):1–13. doi: 10.1128/microbiolspec.VMBF-0012-2015. doi: 10.1128/microbiolspec. VMBF-0012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lane MC, Lockatell V, Monterosso G, Lamphier D, Weinert J, Hebel JR, et al. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect Immun. 2005;73:7644–56. doi: 10.1128/IAI.73.11.7644-7656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spurbeck RR, Stapleton AE, Johnson JR, Walk ST, Hooton TM, Mobley HL. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: Contribution of ygi and yad fimbriae. Infect Immun. 2011;79:4753–63. doi: 10.1128/IAI.05621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scott MP, Emery M. Encyclopedia of Food Grains. 2nd ed. Vol. 1. Netherlands: Elsevier Inc.; 2016. Overview; pp. 99–104. [Google Scholar]
- 81.Hasanudin K, Hashim P, Mustafa S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules. 2012;17:9697–715. doi: 10.3390/molecules17089697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rafsanjany N, Sendker J, Lechtenberg M, Petereit F, Scharf B, Hensel A. Traditionally used medicinal plants against uncomplicated urinary tract infections: Are unusual, flavan-4-ol- and derhamnosylmaysin derivatives responsible for the antiadhesive activity of extracts obtained from stigmata of Zea mays L. against uropathogenic E. coli and Benzethonium chloride as frequent contaminant faking potential antibacterial activities? Fitoterapia. 2015;105:246–53. doi: 10.1016/j.fitote.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 83.Waiss AC, Chan BG, Elliger CA, Wiseman BR, McMillian WW, Widstrom NW, et al. Maysin, a flavone glycoside from corn silks with antibiotic activity toward corn earworm13. J Econ Entomol. 1979;72:256–8. [Google Scholar]
- 84.Ma LY, Liu RH, Xu XD, Yu MQ, Zhang Q, Liu HL. The pharmacokinetics of C-glycosyl flavones of Hawthorn leaf flavonoids in rat after single dose oral administration. Phytomedicine. 2010;17:640–5. doi: 10.1016/j.phymed.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 85.Sahib A, Mohammed I, Hamdan S. Use of aqueous extract of corn silk in the treatment of urinary tract infection. J Intercult Ethnopharmacol. 2012;1:93. [Google Scholar]
- 86.Eman AA. Evaluation of antioxidant and antibacterial activities of Egyptian Maydis stigma (Zea mays hairs) rich in some bioactive constituents. J Am Sci. 2011;7:726–9. [Google Scholar]
- 87.Akbar S. Handbook of 200 Medicinal Plants. Cham: Springer International Publishing; 2020. (Apiaceae/Umbelliferae) pp. 295–306. [Google Scholar]
- 88.Grube K, Spiegler V, Hensel A. Antiadhesive phthalides from Apium graveolens fruits against uropathogenic E.coli. J Ethnopharmacol. 2019;237:300–6. doi: 10.1016/j.jep.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 89.European Medicines Agency. Assessment report on Levisticum officinale Koch, radix. 2012;44:1–20. [Google Scholar]
- 90.Amalaradjou MA, Narayanan A, Venkitanarayanan K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J Urol. 2011;185:1526–31. doi: 10.1016/j.juro.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 91.Amalaradjou MA, Narayanan A, Baskaran SA, Venkitanarayanan K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J Urol. 2010;184:358–63. doi: 10.1016/j.juro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 92.Dorota W, Marta K, Dorota TG. Effect of asiatic and ursolic acids on morphology, hydrophobicity, and adhesion of UPECs to uroepithelial cells. Folia Microbiol (Praha) 2013;58:245–52. doi: 10.1007/s12223-012-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gambogou B, Anani K, Karou SD, Ameyapoh YA, Simpore J. Effect of Aqueous garlic extract on biofilm formation and antibiotic susceptibility of multidrug-resistant uropathogenic Escherichia coli clinical isolates in Togo. Int J Adv Multidiscip Res. 2018;5:45–55. [Google Scholar]
- 94.Sarshar S, Asadi Karam M, Habibi M, Bouzari S, Qin X, Goycoolea F, et al. Cytoprotective and antiadhesive effects of aqueous leaf extract from Orthosiphon aristatus against uropathogenic E.coli. Planta Med. 2016;81:S1–381. [Google Scholar]
- 95.Mohanty S, Kamolvit W, Zambrana S, Sandström C, Gonzales E, Östenson CG, et al. Extract of Clinopodium bolivianum protects against E.coli invasion of uroepithelial cells. J Ethnopharmacol. 2017;198:214–20. doi: 10.1016/j.jep.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 96.Ameya G, Manilal A, Idhayadhulla A. Phytochemical analysis and antimicrobial activity of Bersama abyssinica Fresen against multidrug-resistant bacterial uropathogens: Picolinyl hydrazide is a major compound. J Herbs Spices Med Plants. 2019;25:389–400. [Google Scholar]
- 97.Fatima A. Phytochemical screening and antibacterial activity of neem extracts on uropathogens. Pure Appl Biol. 2020;9:148–53. [Google Scholar]
- 98.Elamary RB, Albarakaty FM, Salem WM. Efficacy of Acacia nilotica aqueous extract in treating biofilm-forming and multidrug resistant uropathogens isolated from patients with UTI syndrome. Sci Rep. 2020;10:11125. doi: 10.1038/s41598-020-67732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]


