Abstract
Background:
Ghrelin is a gut hormone with numerous physiological effects, including the regulation of energy balance, insulin sensitivity, vascular health, and body composition. Acylated (AG) and des-acylated (DAG) ghrelin constitute approximately 22 % and 78 % of total plasma ghrelin (TG), respectively. Alterations in the TG concentration and the AG/DAG ratio may be implicated in conditions involving energy imbalances and insulin resistant states (e.g., metabolic syndrome or Type 2 diabetes mellitus). Exercise is a therapeutic option that can potentially optimize ghrelin levels. Understanding the precise intensity and dose of exercise to optimize ghrelin levels may lead to targeted interventions to restore metabolic regulation in obesity and other clinical conditions.
Objective:
To perform a systematic review and meta-analysis on the effects of acute exercise on pre-prandial levels of TG, AG, and DAG in healthy adults and to determine if sample demographics or exercise doses moderate such effects.
Methods:
Electronic databases (PubMed, Medline, SPORTDiscus, Web of Science, and Google Scholar) were searched with articles published through August 2020. The following criteria was determined a priori for article inclusion: (i) the study was a randomized controlled trial (RCT),(ii) exercise was an acute bout, (iii) the exercise bout for the intervention group(s)/condition was structured, (iv) the control group/condition received no exercise, (v) participants were adults age 18 or older, (vi) ghrelin was sampled through blood, (vii) there was at least one baseline measure and one post-exercise measure of ghrelin, (viii) there were at least 3 timepoints where ghrelin was measured while participants were fasted to allow for pre-prandial total area-under-the-curve (AUCtotal) calculation, (ix) participants were healthy with no overt disease, (x) interventions were carried out without any environmental manipulations. Standardized mean difference (SMD) with 95 % confidence intervals were calculated using the restricted maximum likelihood estimation Moderator analyses to determine whether the overall pooled effect was influenced by: sex, ghrelin form, method of ghrelin analysis, age, body mass index, body fat percentage, fitness, intensity of exercise bout, duration of exercise bout, energy expenditure, and length of AUCtotal data.
Results:
The analysis included 24 studies that consisted of 52 trials, n = 504 (age 27.0 (8.8) years, BMI 24.7 (2.7) kg/m2) and measured AG (n = 38 trials), DAG (n = 7), and TG (n = 7). The overall model indicated that exercise lowered ghrelin levels compared to control (no exercise); (SMD=−0.44, p < 0.001), and exercise intensity exhibited an inverse relationship with ghrelin levels (regression coefficient (ß)=−0.016, p = 0.04). There was no significant difference by ghrelin form (p = 0.18).
Discussion:
Acute exercise significantly lowers plasma ghrelin levels, with higher intensity exercise associated with greater ghrelin suppression. The majority of studies applied a moderate intensity exercise bout and measured AG, with limited data on DAG. This exercise dose may be clinically significant in individuals with metabolic dysregulation and energy imbalance as a therapy to optimize AG levels. More work is needed to compare moderate and high intensity exercise and the ghrelin response in clinical populations.
Keywords: Gut hormones, Physical activity, Acute exercise
1. Introduction
In 1999, Kojima et al. discovered the hormone ghrelin, via its role as an endogenous ligand to the growth hormone secretagogue receptor 1a (GHSR1a) and the consequent stimulation of growth hormone (GH) release [1]. The biological effects of ghrelin were later found to be much more diverse, which include effects on energy balance, glucose regulation, cardiovascular function, sleep, memory, and the immune system [2,3]. Primarily secreted from the gastric fundus, ghrelin circulates in two major forms: acylated (AG) and desacylated (DAG). The majority of ghrelin exists as DAG (~78 % of total ghrelin (TG)). The less abundant form, AG (about 22 % of TG), exists via a post-translational modification catalyzed by ghrelin o-acyltransferease (GOAT) [4]. AG is the form of ghrelin that binds to GHSR1a.
The identification of AG and DAG and the relative amounts of each has stimulated recent research examining the independent, synergistic and antagonistic roles of these peptides. AG is the most studied and has multiple actions that promote energy storage, including stimulation of appetite, inhibition of insulin release from the pancreas, and increased adiposity via GHSR1a [5–7]. Although the DAG receptor is unknown and earlier reports suggest it was inactive [2], recent work has shown that DAG can antagonize AG in a variety of tissues; specifically by inducing a negative energy balance, reducing fat mass and promoting insulin sensitivity [5,7,8]. Conversely, limited data suggests a potential allied effect of ghrelin on the vasculature, with both displaying potent vasodilatory properties, perhaps via different pathways, suggesting there may be an optimal level of each [9].
Human circulating ghrelin concentrations are altered in numerous clinical conditions [3]. Although TG has been shown to be decreased in obese compared to lean individuals; data on the individual forms is equivocal [10]. Specifically, levels of AG have been shown to be decreased in diabetes mellitus (T2DM) [11], and are either increased or unchanged in obesity [11–13]. DAG may be decreased in obesity, which may contribute to the low TG seen in this population [11]. Importantly, the change in the ghrelin profile may also be implicated in increased insulin resistance and elevated fat mass seen in metabolic disease [11,14]. The physiological effects of ghrelin, coupled with discrepancies in blood concentrations and balance in clinical populations, demonstrates the need for targeted approaches that may effectively optimize these peptides for metabolic and vascular health. Exercise provides a unique therapeutic approach in the treatment of dysregulated ghrelin, obesity, metabolic syndrome, prediabetes, and T2DM [15–17]. Studies examining exercise and ghrelin release are mostly equivocal or only document TG and/or a single (e.g. AG) form of ghrelin [18–35]. The quantification of these peptides in response to exercise is critical to understanding the role of exercise on ghrelin release and ghrelin’s exercise-induced influence on overall glucose regulation and energy balance.
Two previous meta-analyses have investigated the effects of acute exercise on total area-under the curve (AUCtotal) ghrelin data. In both, exercise was found to suppress AG (standardized mean difference (SMD): −0.20 and −0.34) [36,37]. It is important to note that both of these meta-analyses contained only studies that measured AG, and included pre-prandial and post-prandial exercise and ghrelin data. Due to the functional differences in AG and DAG, separate analyses of each form are critical, along with measuring both forms in individual studies. Additionally, as macronutrient content and meal timing impacts both ghrelin levels and fuel utilization during exercise, it is important to pool results where ghrelin is measured in the same feeding state [38–41]. Currently, studies of AG, DAG and TG and their response to exercise is understudied and no clear consensus has emerged from the data; studies have used a variety of exercise prescriptions in their protocols. Therefore, we sought to add to the existing literature by differentiating by form in our analysis, and only including pre-prandial data. Further, including exercise dose and demographical variables in this analysis has allowed us to determine if there is an optimal exercise dose to elicit a response to levels of this hormone. Our results will help provide targets for future studies that will advance the literature surrounding not only how exercise alters ghrelin, but the physiological variables that regulate this response. Understanding how the two forms of ghrelin respond to exercise can help guide future therapies and develop exercise prescriptions tailored to optimize ghrelin levels in distinct clinical populations.
1.1. Objective
Our objective was to perform a systematic review and meta-analysis on the effects of exercise on pre-prandial levels of TG, AG, and DAG in healthy adults. We also sought to determine significant moderators of the ghrelin response such as sample demographics and/or exercise dose.
2. Methods
This meta-analysis and systematic review was performed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [42]. This review was not registered.
2.1. Literature search
Electronic databases (PubMed, Medline, SPORTDiscus, Web of Science, and Google Scholar) were searched by two authors, KA and GZ, with articles published through August 2020 included. The search used the following terms: ((((adult) AND (physical activity OR exercise)) AND ghrelin AND human) NOT (child OR children OR adolescent OR rat OR mouse OR animal))). Reference lists of all relevant studies along with reviews and book chapters were also examined. Articles were limited to randomized controlled trials (RCT) in the English language.
2.2. Article selection
For the purpose of this meta-analysis, the term ‘article’ is used synonymously with ‘study’, and ‘trial’ is the unit included in the meta-analysis. Articles often contained multiple eligible trials that comprised an intervention group and a comparable control.
First, the titles and abstracts of the articles were screened for eligibility. The following criteria were determined a priori for article inclusion: (i) The study was a RCT,(ii) exercise was an acute bout, (iii) the exercise bout for the intervention group/conditions(s) was structured, (iv) the control group/condition received no exercise, (v) participants were adults age 18 or older, (vi) ghrelin was sampled through blood, (vii) there was at least one baseline measure and one post-exercise measure of ghrelin, (viii) there were at least 3 timepoints where ghrelin was measured while participants were fasted to allow for pre-prandial AUCtotal calculation, (ix) participants were healthy with no overt disease, (x) interventions were carried out without any environmental manipulations. Two authors (KA, GZ) independently completed the study selection.
2.3. Data extraction and bias assessment
For studies that met the inclusion criteria, the following data was extracted and tabulated: (i): author, publication year; (ii) continuous variables: sample size, age, ghrelin values, BMI, body fat percentage (BF%), fitness (peak oxygen uptake (VO2peak)), intensity of exercise bout (% VO2peak), duration of exercise bout, exercise energy expenditure (EE), length of AUC time; (iii) categorical variables: sex, ghrelin form (AG, DAG, or TG), and method of ghrelin analysis. Ghrelin values were either entered as pre-prandial AUC data if reported, or each time point was extracted from relevant figures using ImageJ software [43] to allow for manual AUCtotal calculation using the linear trapezoidal method. Extraction was done independently by two authors (KA, GZ) who demonstrated an interclass correlation coefficient of 1.0. If pre-prandial ghrelin time points were not able to be accurately extracted or not reported, the study author was contacted. In the case where authors did not respond to follow up, and standard deviations were not able to be extracted, standard deviations were imputed using the reported baseline values. All values for ghrelin were recorded as pg/mL or converted to such if necessary.
Study quality was assessed using the Cochrane risk-of-bias tool for randomized trials (RoB 2) which includes the following domains: randomization, deviations from interventions, missing outcome data, measurement of outcome data, and results [44]. In each domain are signaling questions, where the risk of bias calculated from each domain is generated from an algorithm. Each study is scored as either “low risk”, “high risk” or “some concern” of bias based on the answers to the signaling questions [22]. Two authors (KA, GZ) independently answered the signaling questions.
2.4. Statistical analysis
The meta-analysis was performed using R Software Version 4.0.2, and the “metafor” and “ggplot2” packages version 2.4 and 3.3, respectively [45,46]. Significance levels for all hypothesis tests were set a priori at p = 0.05. Descriptive data are presented as Mean (Standard Deviation) unless otherwise noted.
A three-level, random effects model with restricted maximum likelihood estimation was chosen to account for any dependence of effect sizes of each trial within the same study, as studies contributed multiple data points [47]. This model accounts for sampling variance of the extracted effect sizes at the level of the subject (level 1), variance of the effect sizes within the same study (level 2), and variance of the extracted effect sizes between studies (level 3). The standardized mean difference (SMD) of the ghrelin AUCtotal between the exercise and control groups of each trial were inputted into the model to determine the pooled effect. Due to the different relative amounts AG and DAG circulate in the body, the use of SMD was considered appropriate. The SMDs of all analyses are expressed as Hedges g, and are interpreted as follows: ≤0.2, 0.2, 0.5, and 0.8 are considered to represent trivial, small, moderate, and large effect sizes, respectively [48].
Subsequent to running overall effect meta-analysis we examined the robustness of the pooled results via publication bias and statistical heterogeneity. Sensitivity analysis was performed utilizing Cook’s distance to determine potential influential studies, along with excluding one study at a time, and rerunning the pooled analysis to determine the robustness of the pooled effect. We conducted a second sensitivity analysis where any trial that measured TG was excluded. Due to evidence showing AG and DAG opposing each other in certain tissues, and AG levels being elevated in obesity and T2DM, we investigated the directionality of the two forms in another three-level, REML model. We inverted the sign of each AG SMD, and signs for DAG SMDs were not changed. Positive values would indicate that exercise had a “favorable effect” and negative values would indicate that exercise had a “unfavorable effect”. Publication bias was adjudicated through visual inspection of Begg’s funnel plot. Last, statistical heterogeneity of the overall model was assessed with Cochrane’s Q. The I2 statistic was used to assess the amount of heterogeneity at each level of the model, with the following interpretation: Values <25 % indicates low risk of heterogeneity, 25–75 % indicates moderate risk of heterogeneity, and >75 % indicates considerable risk of heterogeneity [49].
Separate moderator analyses to determine which nominal variables moderated the overall pooled effect were performed with the following subgroups: sex, ghrelin form, and method of ghrelin analysis. Meta-regressions were also used to determine if the following continuous variables impacted the pooled effect size: age, BMI, BF%, fitness, intensity of exercise bout (only studies that reported %VO2peak were used for this meta-regression), duration of exercise bout, EE, AUCtotal length. The regression coefficient (ß) is reported along with 95 % confidence intervals (CI). We completed moderator analyses with the original model, which included TG, AG, and DAG, and also the model where TG trials were excluded.
3. Results
3.1. Literature search
The PRISMA flow diagram outlining this process is presented in Fig. 1. The initial search identified 1693 articles found via database searches, with an additional 11 articles identified through reference list searching. After title and abstract screening, 1635 articles were excluded, leading to a full text review of 69 eligible articles. After full text review, 24 articles met all inclusion criteria, which contained 52 acceptable trials. The majority of samples contained a young adult population (27.0 (8.8) years), only males (n = 41; only females n = 9; both n = 2) and measured AG (n = 38; DAG: n = 7; TG: n = 7). The full characteristics of included trials are in Table 1.
Fig. 1.
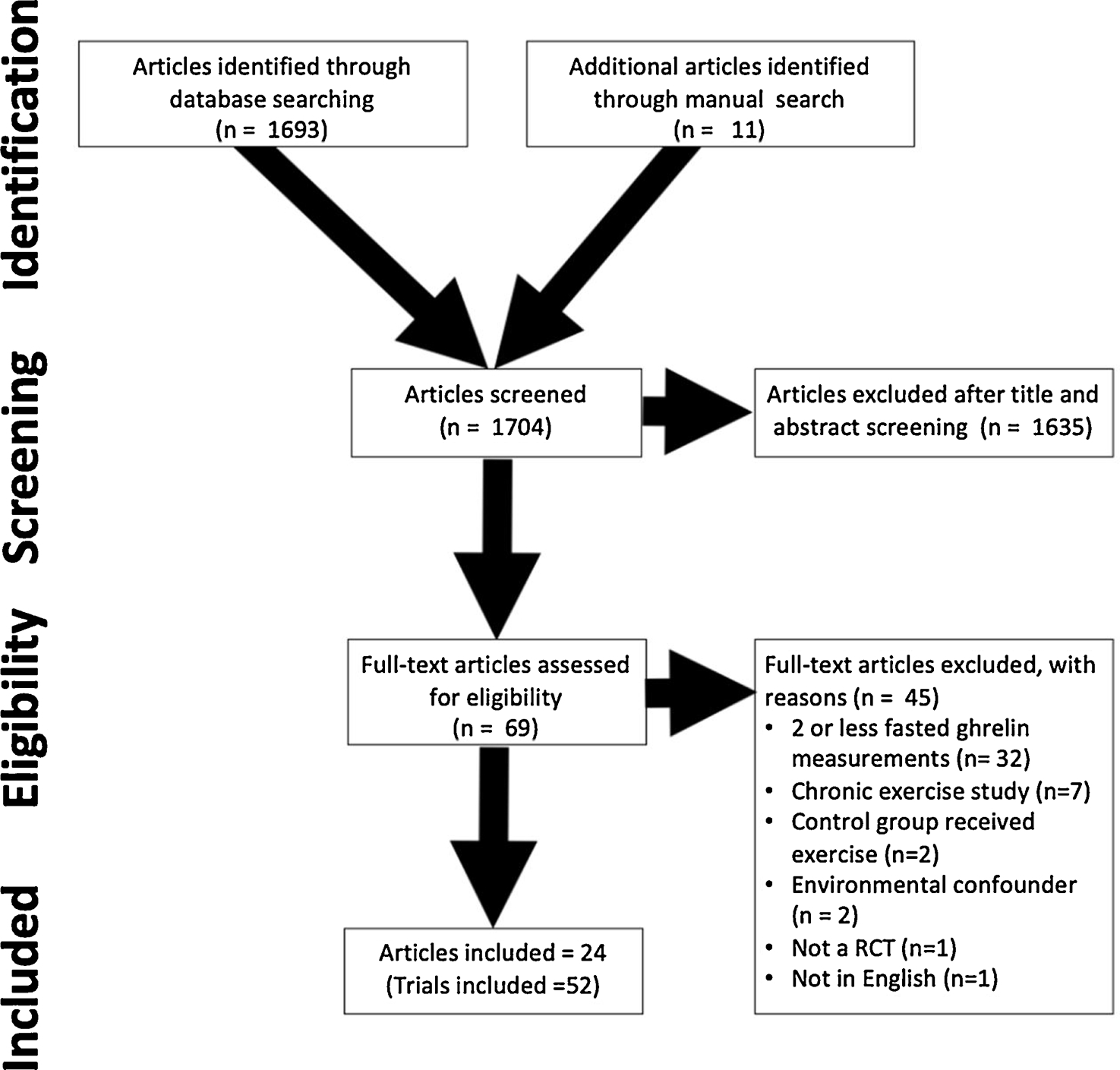
PRISMA Diagram.
Table 1.
Trial Characteristics.
| Trial | Sample | Exercise | SMD | Ghrelin Percent Difference (Exercise vs Control AUCtotal) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| (Sex) Size | Age (years) | BMI (kg/m2) | Mode | Intensity | Duration (min) | TG | AG | DAG | ||
|
| ||||||||||
| Alajmi et al. 1 [18] | (F) 8 | 22.3 ± 2.5 | 22.3 ± 2.3 | AEx | 73.3 ± 0.6 % VO2peak | 60.0 ± 0.0 | −0.79 | – | −37.0% | – |
| Alajmi et al. 2 [18] | (M) 8 | 22.6 ± 3.8 | 23.1 ± 2.1 | AEx | 70.9 ± 1.4 % VO2peak | 60.0 ± 0.0 | −0.93 | – | −50.5% | – |
| Balaguera-Cortes et al. 1 [77] | (M) 10 | 21.3 ± 1.4 | 23.7 ± 2.0 | REx | 3 sets of 12 repetitions (or to failure) | 45.0 ± 0.0 | −0.54 | – | −19.4% | – |
| Balaguera-Cortes et al. 2 [77] | (M) 10 | 21.3 ± 1.4 | 23.7 ± 2.0 | AEx | 71.0 ± 7.0 % VO2peak | 45.0 ± 0.0 | 0.078 | – | 3.02 % | – |
| Bishop et al. [19] | (M) 9 | 24 ± 2.0 | 22.8 ± 1.9 | AEx | 73.1 ± 3.7 % VO2peak | 60.0 ± 0.0 | −1.7 | – | −64.4% | – |
| Broom et al.a [22] | (M) 9 | 21.2 ± 0.7 | 22.2 ± 0.7 | AEx | 72.0 ± 2.0 % VO2peak | 60.0 ± 0.0 | −0.24 | – | −46.7% | – |
| Broom et al. b,1 [23] | (M) 11 | 21.1 ± 0.3 | 23.1 ± 0.4 | REx | 80 % of 12 repetitionmax | 90.0 ± 0.0 | −0.19 | – | −19.2% | – |
| Broom et al. b,2 [23] | (M) 11 | 21.1 ± 0.3 | 23.1 ± 0.4 | AEx | 69.0 ± 2.0 % VO2peak | 60.0 ± 0.0 | −0.30 | – | −29.7% | – |
| Broom et al. c,1 [21] | (M) 8 | 21.7 ± 1.7 | 24.5 ± 2.4 | AEx | 52.0 ± 3.0 % VO2peak | 55.0 ± 7.0 | −0.30 | – | −12.2% | – |
| Broom et al. c,2 [21] | (M) 8 | 21.7 ± 1.7 | 24.5 ± 2.4 | AEx | 75.0 ± 4.0 % VO2peak | 36.0 ± 5.0 | −0.16 | – | −15.8% | – |
| Broom et al. c,3 [21] | (M) 9 | 23.2 ± 2.1 | 22.7 ± 1.5 | AEx | 70.0 ± 2.0 % VO2peak | 45.0 ± 0.0 | −0.30 | – | −26.8% | – |
| Broom et al. c,4 [21] | (M) 9 | 23.2 ± 2.1 | 22.7 ± 1.5 | AEx | 70.0 ± 2.0 % VO2peak | 90.0 ± 0.0 | −0.42 | – | −38.5% | – |
| Burns et al. 1 [20] | (M) 9 | 24.5 + 1.3 | 25.1 + 1.2 | AEx | 71.5 ± 2.5 % VO2peak | 60.0 ± 0.0 | −0.32 | −8.3% | – | – |
| Burns et al. 2 [20] | (F) 9 | 23.4 + 1.0 | 22.5 + 0.8 | AEx | 75.5 ± 3.0 % VO2peak | 60.0 ± 0.0 | −0.94 | −12.8% | – | – |
| Crabtree et al. 1 [80] | (M) 15 | 22.5 ± 3.1 | 24.2 ± 2.4 | AEx | 70.0 ± 0.0 % VO2peak | 60.0 ± 0.0 | −0.49 | −17.1% | – | – |
| Crabtree et al. 2 [80] | (M) 15 | 22.5 ± 3.1 | 24.2 ± 2.4 | AEx | 70.0 ± 0.0 % VO2peak | 60.0 ± 0.0 | −0.87 | – | −44.4% | – |
| Dorling et al. 1 [50] | (M) 12 | 20.9 ± 3.5 | 23.5 ± 2.7 | AEx | 71.0 ± 2.0 % VO2peak | 60.0 ± 0.0 | −0.76 | – | – | −18.7% |
| Dorling et al. 2 [50] | (M) 12 | 21.3 ± 3.6 | 23.5 ± 2.3 | AEx | 70.0 ± 2.0 % VO2peak | 60.0 ± 0.0 | −1.2 | – | – | −33.1% |
| Dorling et al. 3 [50] | (M) 12 | 20.9 ± 3.5 | 23.5 ± 2.7 | AEx | 71.0 ± 2.0 % VO2peak | 60.0 ± 0.0 | −2.4 | – | −60.0% | – |
| Dorling et al. 4 [50] | (M) 12 | 21.3 ± 3.6 | 23.5 ± 2.3 | AEx | 70.0 ± 2.0 % VO2peak | 60.0 ± 0.0 | −2.3 | – | −58.6% | – |
| Douglas et al. 1 [65] | (F) 10 | 38.1 ± 16.7 | 21.8 ± 1.6 | AEx | 49.0 ± 26.0 % VO2peak | 60.0 ± 0.0 | 0.17 | – | – | 8.1 % |
| Douglas et al. 2 [65] | (F) 11 | 45.5 ± 13.2 | 28.7 ± 2.8 | AEx | 57.0 ± 4.3 % VO2peak | 60.0 ± 0.0 | 0.20 | – | – | 28.7 % |
| Douglas et al. 3 [65] | (M) 10 | 33.8 ± 13.1 | 22.9 ± 1.4 | AEx | 59.3 ± 2.9 % VO2peak | 60.0 ± 0.0 | −0.055 | – | – | −3.3% |
| Douglas et al. 4 [65] | (M) 12 | 44.9 ± 13.2 | 29.3 ± 3.1 | AEx | 57.9 ± 2.2 % VO2peak | 60.0 ± 0.0 | −0.091 | – | – | −8.9% |
| Douglas et al. 5 [65] | (F) 10 | 38.1 ± 16.7 | 21.8 ± 1.6 | AEx | 49.0 ± 26.0 % VO2peak | 60.0 ± 0.0 | −0.023 | – | −1.1% | – |
| Douglas et al. 6 [65] | (F) 11 | 45.5 ± 13.2 | 28.7 ± 2.8 | AEx | 57.0 ± 4.3 % VO2peak | 60.0 ± 0.0 | 0.15 | – | 14.0 % | – |
| Douglas et al. 7 [65] | (M) 10 | 33.8 ± 13.1 | 22.9 ± 1.4 | AEx | 59.3 ± 2.9 % VO2peak | 60.0 ± 0.0 | −0.24 | – | −10.7% | – |
| Douglas et al. 8 [65] | (M) 12 | 44.9 ± 13.2 | 29.3 ± 3.1 | AEx | 57.9 ± 2.2 % VO2peak | 60.0 ± 0.0 | −0.29 | – | −29.0% | – |
| Erdmann et al. 1 [24] | (M)2, (F) 5 | 24.4 ± 0.6 | 21.4 ± 0.8 | AEx | Cycling at 50W | 30.0 ± 0.0 | 0.64 | 33.2 % | – | – |
| Erdmann et al. 2 [24] | (M)2, (F) 5 | 24.4 ± 0.6 | 21.4 ± 0.8 | AEx | Cycling at 100W | 30.0 ± 0.0 | 0.30 | 18.3 % | – | – |
| Gholipour et al. [69] | (M) 9 | 20.6 ± 1.4 | 32.7 ± 2.5 | AEx | 59.9 ± 0.0 % VO2peak a | 27.0 ± 0.0 | −2.3 | – | −37.8% | – |
| Hagobian et al.1 [25] | (M) 11 | 22.0 ± 2.0 | 26.0 ± 4.0 | AEx | 70.0 % VO2peak | 82.0 ± 13.0 | −0.079 | – | −4.0% | – |
| Hagobian et al. 2 [25] | (F)10 | 21.0 ± 2.0 | 24.0 ± 2.0 | AEx | 70.0 % VO2peak | 84.0 ± 17.0 | −0.14 | – | −8.3% | – |
| Kawano et al. 1 [26] | (M) 15 | 24.4 ± 1.7 | 22.1 ± 2.0 | REx | 64.8 ± 6.9 % VO2peak | 30.0 ± 0.0 | −0.42 | – | −27.5% | – |
| Kawano et al. 2 [26] | (M) 15 | 24.4 ± 1.7 | 22.1 ± 2.0 | AEx | 63.9 ± 7.5 % VO2peak | 30.0 ± 0.0 | −0.46 | – | −30.7% | – |
| Kelly et al. [27] | (M) 10 | 21.4 ± 1.3 | 23.9 ± 2.1 | AEx | 70.0 ± 0.0 % VO2peak | 45.0 ± 0.0 | 0.16 | – | 8.9 % | – |
| King et al. a [28] | (M) 9 | 22.2 ± 0.8 | 23.6 ± 0.4 | AEx | 68.8 ± 0.8 % VO2peak | 90.0 ± 0.0 | −1.1 | – | −49.6% | – |
| King et al. b [29] | (M) 14 | 21.9 ± 0.5 | 23.4 ± 0.6 | AEx | 45.2 ± 2.0 % VO2peak | 60.0 ± 0.0 | 0.0015 | – | −0.09 % | – |
| Larsen et al. 1 [30] | (M) 12 | 48.0 ± 5.0 | 29.9 ± 1.9 | AEx | 75.0 ± 1.0 % VO2peak | 50.0 ± 0.0 | −0.40 | – | −20.6% | – |
| Larsen et al. 2 [30] | (M) 12 | 48.0 ± 5.0 | 29.9 ± 1.9 | REx | 27.0 ± 1.0 % VO2peak | 30.0 ± 0.0 | −0.099 | – | −4.9% | – |
| Larsen et al. 3 [30] | (M) 12 | 48.0 ± 5.0 | 29.9 ± 1.9 | CEx | REx:29.0 ± 1.0, Aex: 74.0 ± 1.0 % VO2peak | 40.0 ± 0.0 | 0.031 | – | −1.6 % | – |
| Mattin et al. 1 [31] | (M) 12 | 26.0 ± 5.0 | 25.5 ± 3.5 | AEx | 40.0 ± 0.0 % VO2peak | 60.0 ± 0.0 | 0.35 | – | −13.4 % | – |
| Mattin et al. 2 [31] | (M) 12 | 26.0 ± 5.0 | 26.5 ± 3.5 | AEx | 70.0 ± 0.0 % VO2peak | 60.0 ± 0.0 | −0.82 | – | −28.3% | – |
| Metcalfe et al. 1 [32] | (M) 8 | 21.0 ± 2.0 | 25.0 ± 4.0 | AEx | Cycling at 60 W plus 2 all out sprints against a resistance of 7.5 % of bodyweight | 10.0 ± 0.0 | −0.88 | – | −51.9% | – |
| Metcalfe et al. 2 [32] | (M) 8 | 21.0 ± 2.0 | 25.0 ± 4.0 | AEx | 53.0 ± 5.0 % VO2peak | 30.0 ± 0.0 | −0.059 | – | −3.5% | – |
| Stokes et al. 1 [33] | (M) 7 | 26.0 ± 3.0 | 24.9 ± 5.0 | AEx | Cycling for 4 min at 60 W, 30 s at 80 W, 30 s at 100 W then a 5-minute rest period. Ending with a 30 sec sprint at 7.0 % of bodyweight | 5.5 ± 0.0 | −0.44 | −12.8% | – | – |
| Stokes et al. 2 [33] | (M) 7 | 26.0 ± 3.0 | 24.9 ± 5.0 | AEx | Cycling for 4 min at 60 W, 30 s at 80 W, 30 s at 100 W, then a 5-minute rest period. Ending with a 30 sec sprint at 9.0 % of bodyweight | 5.5 ± 0.0 | −0.31 | −9.0% | – | – |
| Tiryaki-Sonmez et al. 1 [70] | (F) 9 | 22.8 ± 1.4 | 28.3 ± 1.8 | AEx | 53.1 ± 3.31 % VO2peak | 60.0 ± 0.0 | −0.39 | – | −8.1% | – |
| Tiryaki-Sonmez et al. 2 [70] | (F) 9 | 22.8 ± 1.4 | 28.3 ± 1.8 | AEx | 53.1 ± 3.31 % VO2peak | 60.0 ± 0.0 | 0.027 | – | – | −0.45 % |
| Wasse et al. 1 [34] | (M) 11 | 22.7 ± 2.3 | 23.4 ± 2.4 | AEx | 71.7 ± 2.5 % VO2peak | 60.0 ± 0.0 | −0.44 | – | −30.2% | – |
| Wasse et al. 2 [34] | (M) 11 | 22.7 ± 2.3 | 23.4 ± 2.4 | AEx | 70.3 ± 4.0 % VO2peak | 60.0 ± 0.0 | −0.51 | – | −32.8% | – |
| Vatansever-Ozen et al. [35] | (M) 10 | 20.1 ± 0.17 | 22.0 ± 0.44 | AEx | 54.6 ± 3.5 % VO2peak a | 120.0 ± 0.0 | −0.34 | – | −5.9% | – |
Aerobic Exercise (AEx), Combined Exercise (CEx), Resistance Exercise (REx).
Exercise Intensity was calculated using a time-weighted average if several intensities were used in a single exercise bout.
3.2. Risk of Bias
Overall, studies were deemed to have a low risk of bias (Electronic Supplementary Material (ESM) Appendix S1). Due to the nature of study designs, which compared exercise to a resting control, it is impossible for participants and researchers to be blinded to the treatment assignment. However, due to the analysis methods of the plasma samples (e.g., batch analysis with ELISAs) for ghrelin, the absence of blinding was not deemed high risk to bias results.
3.3. Pooled effect
The overall model indicated that exercise had a significant, small to approaching moderate, suppressive effect on ghrelin levels (SMD=−0.44, 95 % confidence interval (CI): −0.65 to −0.23, p < 0.001, Fig. 2). The overall model had significant heterogeneity (Cochrane’s Q = 78.9, df = 51, p < 0.001) with further analysis revealing no within-study (level 2) heterogeneity (I2 = 0 %, p = 1.0), and moderate between-study (level 3) heterogeneity (I2 = 41.27 %, p < 0.01).
Fig. 2.
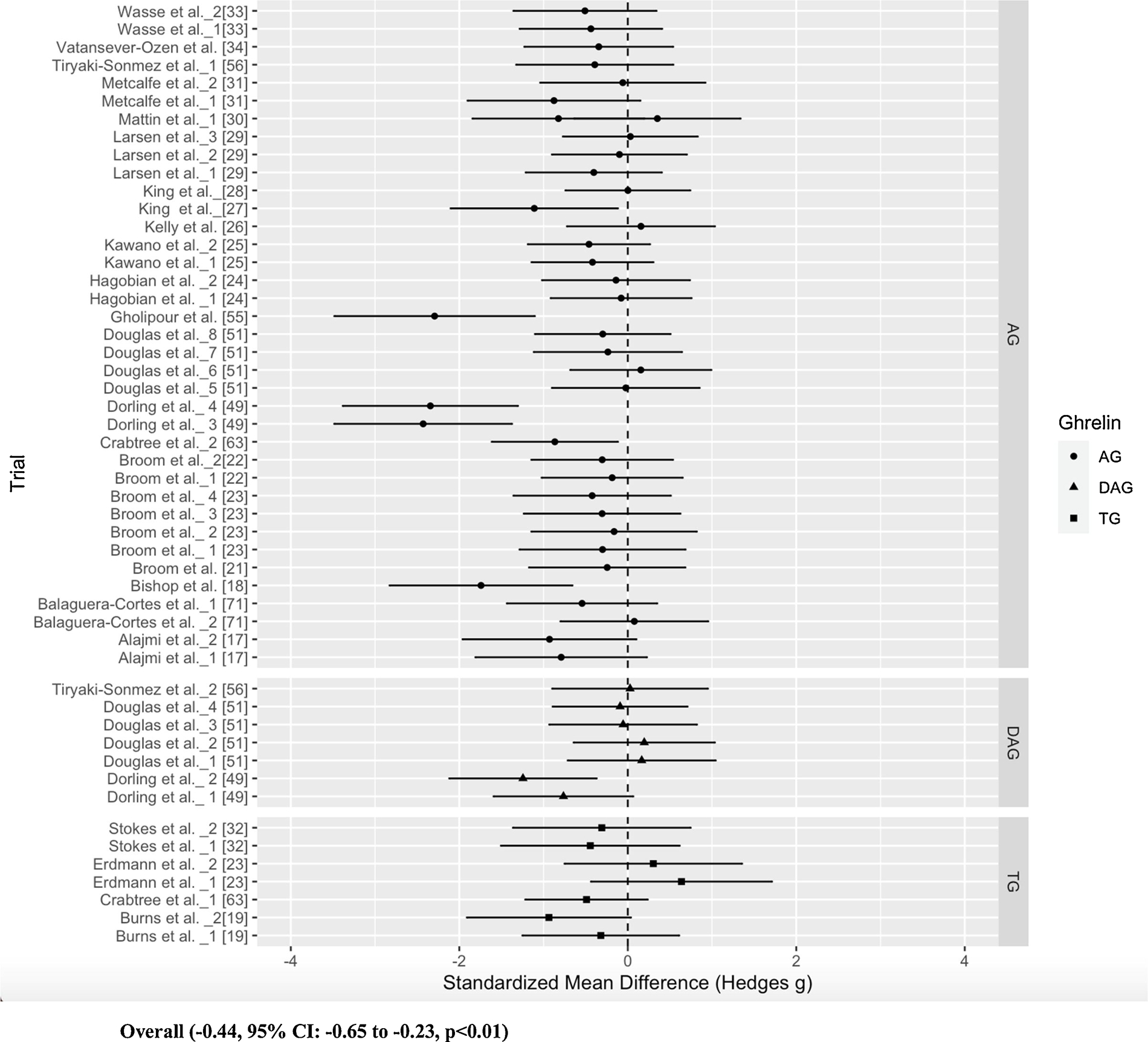
Forest Plot of the overall model sorted by ghrelin form (AG, DAG, TG).
3.4. Cook’s distance and publication bias
Cook’s distance identified one study as an influential study (ESM Appendix S2). When this study was removed from the pooled analysis, the effect of exercise on ghrelin levels remained significant but the SMD became weaker (SMD= −0.33, p < 0.01), and the heterogeneity nonsignificant. It is unclear why this study had such an effect on the model, beyond the trials having the strongest effect sizes and the study being published most recently. All other studies removed during the sensitivity analysis had no substantial effect on the overall model. Visual inspection of the funnel plot revealed asymmetry (ESM Appendix S4). The plot shows that several studies with large effect sizes drive the asymmetry, with one of those studies detected as the influential study by Cook’s Distance [50].
3.5. Subgroup analyses
Moderator data is listed in Table 2. The subgroup analyses revealed nonsignificant moderation by sex (p = 0.37), ghrelin form (p = 0.18), and ghrelin analysis method (p = 0.13). Concerning meta-regressions, exercise intensity was found to be a significant moderator with an inverse relationship with ghrelin levels (ß=−0.016, p = 0.04, Fig. 3). In addition, exercise intensity explained a portion of the heterogeneity in the overall model, decreasing the variance of effect sizes between studies (level 2, I2 = 35.6 %, p < 0.01). When the influential study was removed from this meta-regression, the relationship remained significant (ß=−0.017, p = 0.01) [50]. All other meta-regressions were nonsignificant: age (p = 0.18), BMI (p = 0.69), BF% (p = 0.60), fitness (p = 0.33), EE (p = 0.14), AUCtotal length (p = 0.15), and exercise duration (p = 0.77).
Table 2.
Moderator Analysis Data.
| Moderator Variable | p value | Comparison |
|---|---|---|
|
| ||
| Age | 0.18 | Meta-regression (β = −0.017, 95% CI= −0.008 to 0.043) |
| Sex | 0.37 | Male (ES= −0.50, 95% CI= −0.60 to 0.22) Female (ES= −0.32, 95% CI= −0.72 to 0.09) TG (ES= −0.23, 95% CI= −0.78 to 0.63) |
| Ghrelin Form | 0.18 | AG (ES= −0.52, 95% CI= −0.82 to 0.08) DAG (ES= −0.15, 95% CI= −0.62 to 0.32) |
| BMI | 0.69 | Meta-regression (β = −0.013, 95% CI= −0.076 to 0.051) |
| BF% | 0.60 | Meta-regression (β = −0.014, 95% CI= −0.067 to 0.039) |
| Fitness | 0.33 | Meta-regression (β = −0.008, 95% CI= −0.024 to 0.008) |
| Exercise Intensity (% VO2peak) | 0.04 | Meta-regression (β = −0.016, 95% CI= −0.032 to −0.001) |
| Exercise Duration | 0.77 | Meta-regression (β = −0.001, 95% CI= −0.01 to 0.007) |
| EE | 0.14 | Meta-regression (β = −0.0001, 95% CI= −0.0002 to 0.00003) |
| AUCtotal length | 0.15 | Meta-regression (β = −0.21, 95% CI= −0.50 to 0.08) |
| Ghrelin Analysis Method | 0.13 | ELISA (ES= −0.56, 95% CI= −0.98 to 0.12) RIA (ES = 0.04, 95% CI= −0.73 to 1.08) xMAP (ES= −0.13, 95% CI= −0.62 to 0.37) |
Fig. 3.
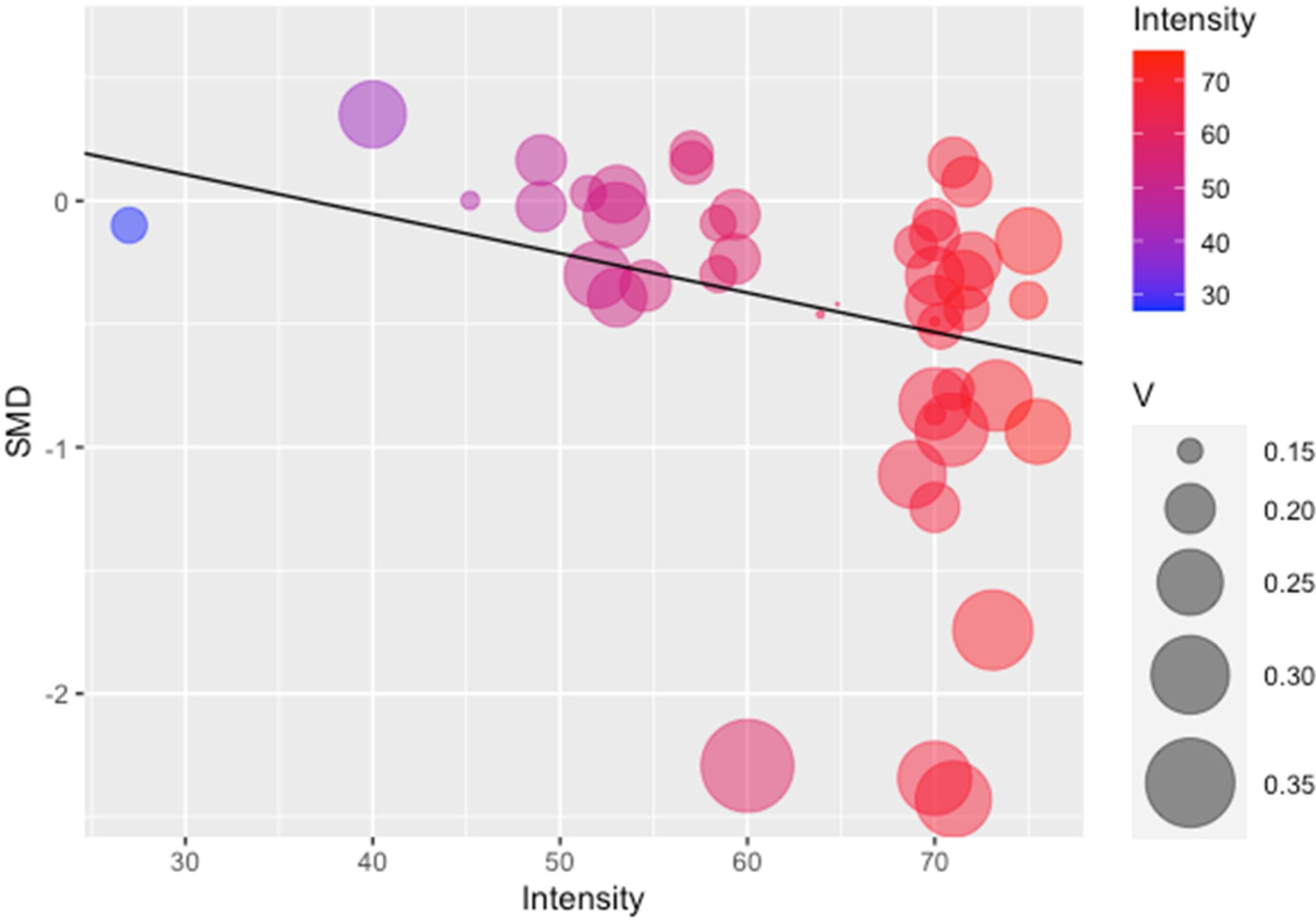
Bubble Plot of the Meta Regression of Exercise Intensity and SMD.
Intensity = %VO2peak; V = Size of trial variance.
3.6. Sensitivity analyses
In the model with only AG and DAG trials, exercise had a significant, moderate effect on ghrelin levels (SMD = 0.36, 95 % CI: 0.17 to 0.54, p < 0.001, ESM Appendix S3). When the influential study was removed from this model, the significance remained (SMD = 0.35, 95 % CI 0.19 to 0.51, p < 0.001). The moderator analysis using this model revealed ghrelin form as the only significant moderator (DAG SMD:−0.25, AG SMD = 0.46, p = 0.003). However, when the influential study was removed from this model, ghrelin form is no longer a significant subgroup (DAG SMD: 0.05, AG SMD = 0.37, p = 0.14) while the meta-regression with exercise intensity became significant (ß = 0.015, p = 0.03).
4. Discussion
4.1. Overall
The purpose of this systematic review and meta-analysis was to examine changes in pre-prandial ghrelin levels in response to an acute bout of exercise in adults, and establish if these effects are moderated by sample demographic characteristics and/or exercise dose. Dysregulated ghrelin occurs in several clinical conditions, including metabolic syndrome and T2DM. An understanding of how exercise may modify ghrelin levels is critical to guide potential future therapies. The results of this analysis suggest that overall exercise suppresses plasma ghrelin levels, and this effect is moderated by exercise intensity; with higher exercise intensities lowering ghrelin to a greater extent. Although there was no statistical difference in the effect of exercise by ghrelin form, it is important to note that the majority of studies measured only AG and as such the data to examine TG, AG, and DAG separately is limited.
Potential mechanisms to explain the suppression of ghrelin in response to exercise are not fully elucidated. However, several hypotheses have been proposed. It is likely that there is considerable overlap and/or redundancy between these potential mechanisms and their contributions may differ between ghrelin forms. One mechanism is that ghrelin levels in humans are known to decrease after intravenous and oral intake of FFA [51,52]. Given that long-chain FFA have been shown to increase post-exercise [53], this may provide a potential explanation for a reduction in ghrelin following exercise. The ghrelin system has other key components that may contribute to the exercise response; however, they remain understudied. Liver-enriched antimicrobial peptide 2 (LEAP2), has been shown to be a GHSR-1a antagonist, blocking the action of AG upon binding [54]. Data further suggests that LEAP2 has the ability to attenuate ghrelin induced food intake and GH secretion in mice, and is sensitive to changes in body weight and feeding status, specifically being suppressed by fasting [55], In addition, during chronic calorie restriction in mice expressing LEAP2, ghrelin levels increased less compared to the control group [56], which may indicate LEAP2 but can also inhibit the secretion of ghrelin. Future work should identify how LEAP2 responds to exercise to further illuminate the ghrelin pathway; specifically, if this hormone is involved in the exercise induced suppression of ghrelin levels we have reported. To our knowledge no study has yet to investigate LEAP2 and its response to exercise. Collectively, current data highlights that there is likely an interaction of multiple hormones that regulate this response.
To address the issue of exercise intensity and ghrelin concentration, it is likely that changes in blood flow distribution and increases in lactate production offer more insight. With increasing exercise intensity, blood flow to the digestive system is progressively reduced, as it is distributed to the working tissues [57]. Since ghrelin is released from the stomach, it is logical that it may be suppressed during and after an exercise bout [58, 59]. This concept is supported by data following gut ischemic injuries and gut hypoxia, in which ghrelin levels are also decreased [60]. Similarly, as skeletal metabolism increases, oxygen demand by working tissues can outstrip supply leading to a greater contribution for anaerobic metabolism and the generation of lactate and hydrogen ions [61]. It is possible that this increase coincides with reduced gut blood flow, but there may be an additional direct effect of lactate. The gastric mucosal cells in the stomach, produce ghrelin contain G-protein receptor 81 (GPR81). Lactate can bind GPR81 and inhibit ghrelin release from the gastric mucosa [62].
4.2. Comparison with other studies
The results of this meta-analysis suggest that exercise suppresses ghrelin levels, in agreement with previously published work [36,37,63]. The pooled effect of exercise on ghrelin levels was small to approaching moderate; the strength of this effect (SMD= −0.44) was stronger than those seen in prior analyses (SMD −0.20 and −0.34) [36,37]. Without the identified influential study [50], our reported effect size (SMD= −0.33) was similar to one of the previous analyses [37]. A possible explanation for the difference in strength of effect sizes between studies is the inclusion of only pre-prandial data. In addition, although we did not report a moderating effect of ghrelin form, as subgroup analyses are observational in nature, it is still possible that our inclusion of TG, AG, and DAG in one model influenced our results. Lastly, the model chosen could have added to the difference in effect sizes between studies. While previous literature utilized random effect models, we chose a three-level nested model. Certain trials in our analysis had separate samples for their control and exercise conditions, while others employed a crossover design. Because the non-independence of data points in our analysis breached the assumptions of typical meta-analysis models, the use of the three-level model was appropriate. Importantly, we extend the literature by reporting exercise intensity as a significant moderator, a result that was not reported by the previous analyses.
4.3. Moderator interpretation
Due to the wide variety of study sample and exercise characteristics, along with heterogeneity being present, exploration through moderator analyses were warranted. Because a majority of these analyses were not significant and likely underpowered, we can only speculate on their interpretation. Hypothesis testing revealed no significant moderation by age, sex, ghrelin form, BMI, BF%, fitness, exercise duration, EE, AUCtotal length, or ghrelin analysis method. It is known that AG levels exhibit a decline in older adults. However, data on how exercise affects the different ghrelin forms across adult age groups is scarce [64]. The trials included in this analysis largely sampled adults younger than 30 years old, where only two studies sampled an average age above that [30,65]. Due to ghrelin’s beneficial role in inflammation, bone mass, and sarcopenia, future studies targeting ghrelin through exercise in middle aged and older adults is needed [66–68].
In addition, the majority of studies in our analysis comprised of males, which suggests that sex as a moderator was underpowered. Limited data has shown that males and females exhibit a sexual dimorphism concerning plasma ghrelin levels, highlighting the importance of exploring sex as a variable in response to exercise interventions [11,65]. We report that the effect of exercise on ghrelin levels were stronger in males (SMD= −0.50) than females (SMD=−0.32), although not reaching statistical significance. These studies varied widely in exercise dose and sample characteristics such as BMI, which adds difficulty in drawing conclusions.
Data suggest that weight status and obesity can influence ghrelin levels [33], yet how exercise specifically alters responses in lean versus overweight/obese individuals is uncertain. The average BMI in this analysis was 24.7 (2.7) kg/m2, which indicates a need for more studies to sample individuals with BMIs above normal weight; only seven studies in this analysis did so [25,30–32,65,69,70]. The meta-analysis by Douglas et al. found BMI to be a significant moderator; when lean individuals were included in the model, the effect size of the pooled data decreased [37]. We did not observe BMI as a significant moderator, consistent with the meta-analysis by Schubert et al. [36]. We also did not find BF% to significantly moderate ghrelin levels, however many studies did not measure this variable, and those that did most often utilized skinfolds [20,21,28,29,34,35,69,71], an unreliable measure compared to computerized topography, hydrostatic weighing, and dual energy x-ray absorptiometry scans [72,73]. Skin folds do not differentiate between the types of adipose tissue, and prior data indicates TG levels are associated with visceral but not subcutaneous fat [74] the need to further examine adipose tissue type and ghrelin is warranted. Animal models suggesting AG acts to increase fat mass, while DAG has been shown promote the opposite, also necessitates the measurement of both forms individually when looking at body composition [75,76].
Most studies (71 %) in this analysis only measured AG. It is interesting to note the effect sizes of each ghrelin form, which did not reach statistical significance (SMD: TG −0.23, AG −0.52, DAG −0.15, Fig. 2). We did not feel it was appropriate to report weighted effect sizes, due to the biological ratio of AG:DAG and the relative amounts of each. However, we did calculate a percent difference between the exercise and control AUCtotal data, where we report AG decreased by 22.8 % on average, DAG decreased by 0.04 %, and TG decreased by 1.2 % compared to the control condition (Table 1). The difference between effect sizes and percent differences of AG and DAG underpins the importance of measuring the forms individually. As DAG is prevalent in higher amounts compared to AG, it is unsurprising that the effect size of TG may be more closely aligned with this form. As the biological functions of the two forms can oppose each other, a decrease in AG levels can have a different implication than a decrease in DAG. This is further illustrated in obesity, where AG levels have been found to be increased and DAG levels are decreased compared to lean individuals [11,14]. Therefore, a beneficial effect of exercise in this population would be a decrease in AG and an increase in DAG. To address the disparate directionality of AG and DAG, we conducted a sensitivity analysis by running another model with just AG and DAG trials, where the direction of AG SMDs was inverted (ESM Appendix S3). This model strengthened the robustness of our results, indicating that exercise has a favorable effect on ghrelin levels (SMD = 0.36).
Overall, most studies that solely measured AG reported a decrease following exercise. GOAT has been found to be downregulated in certain conditions such as during a positive energy balance and feedback from ghrelin itself [78,79], whether exercise can also induce this effect is an attractive, albeit unstudied, theory that may help explain how exercise induces a suppression of this form. Only three studies measured DAG and reported a decrease [50,65] or no change [70]; whether DAG has a blunted response to exercise compared to AG is unknown. Lastly, TG responses have varied widely, with studies reporting no change [20], a decrease [33,80], or an increase post-exercise [24]. Although we did not find ghrelin analysis method a significant moderator, if blood samples are not treated with a protease inhibitor, AG will degrade and the individual forms cannot be measured accurately [4]. In addition, acidification of the sample before storing aids in stability when frozen [4]. Studies in this analysis reported a variety of blood collection methods, therefore we were unable to add this variable as a moderator and cannot rule out the possibility of collection methods affecting results. Even with the inconsistency in prior data, it is clear that each form of ghrelin may not respond similarly when given an identical exercise dose within studies.
The variety of exercise doses utilized between studies adds to the difficulty in establishing a consensus on ghrelin and exercise data. We report no moderation by exercise duration, a result that is corroborated by the two previous meta analyses [36,37]. Conversely, we report that exercise intensity significantly moderated ghrelin’s relationship with exercise; the higher the intensity, the stronger the suppression of ghrelin. This was a finding that was not significant in the previous meta-analyses [36,37]. Possible explanations include fewer number of studies in their reviews, including post-prandial data, or only focusing on AG in their models. Ghrelin has been shown to stimulate fatty acid oxidation in skeletal muscle [81], and as high-intensity exercise is typically fueled by muscle and liver glycogen over lipids [82], our finding that ghrelin is suppressed more during higher intensities suggests it may aid in energy utilization during exercise. In addition, as previously discussed, lactate and FFA are increasingly produced during high intensity exercise may inhibit ghrelin release [62]. However, more work is needed to understand how intensity alters the ghrelin pathway. The majority of studies utilized a moderate intensity exercise bout and report that ghrelin suppression is achievable at this level. Therefore, more studies that compare high and moderate intensity exercise are needed to demonstrate whether high intensity leads to larger suppression of ghrelin. As intensity was the only significant moderator that reduced but did not eliminate the heterogeneity within our data, there are likely other factors we did not explore and/or that are not reported in the literature that contribute to the relationship.
Both ghrelin and exercise have been shown to be involved in energy balance, with prior work focused on determining if alterations in ghrelin from exercise are due to energy expenditure and intake. We report that energy expenditure is not a significant moderator of exercise-induced ghrelin suppression, in line with the meta-analysis by Schubert et al. [36] Other results indicate the ghrelin is not associated with subsequent relative [83] or ad libitum energy intake post-exercise [25,28]. Although we only included pre-prandial data in our analysis, the majority of studies calculated AUC for the entire visit, which often included both pre- and post-prandial time points. Even with a RCT design, macronutrient content and timing of meals can influence ghrelin levels [38], exercise performance [84], and post-exercise physiological processes [85] which can confound interpretations. Future studies that are designed with exercise and meals should consider calculating separate pre-prandial and post-prandial AUC calculations to help differentiate diet- and/or exercise- induced effects. Overall, data suggests that exercise energy expenditure does not mediate changes to plasma ghrelin, rather this hormone may be more sensitive to meal-induced changes to energy balance [86].
4.4. Implications
Our findings strengthen previous conclusions about the suppressive effect of exercise on ghrelin levels. The rationale for this response is likely multifaceted and complicated by ghrelin form. Decreased AG has been associated with reduced insulin levels, sustained glucose levels, and reduced hunger in lean and obese subjects, suggesting AG may be a mediator of the improvement to insulin sensitivity and suppression of hunger seen in response to exercise [22,23,71,86,87]. Although we did not report a significant moderator effect by ghrelin form, it does highlight the need for more studies examining exercise and its effect on DAG, as the majority of studies measured just AG. Additionally, exercise mode is an understudied facet of the literature that we were underpowered to assess; the majority of studies in our analysis applied an aerobic exercise bout. Due to the opposing effects of AG and DAG on appetite, insulin sensitivity, and energy balance, pinpointing the best exercise prescription to restore or maintain the balance of the two is critical. AG has been found to be elevated in obesity and T2DM [11], and the results of this analysis suggest exercise may be an attractive modality to decrease, and therefore normalize, levels in these populations. Importantly, ghrelin levels have found to be disrupted in conditions beyond those that primarily exhibit energy imbalance and/or insulin resistance; such as heart failure, Parkinson’s disease, multiple sclerosis, and chronic obstructive pulmonary disease [2,3]. Therefore, future work that continues to develop precise exercise doses to target different components of the ghrelin pathway specific to condition is warranted.
The novel finding of this analysis is that exercise intensity was a significant moderator; the higher the exercise intensity, the more ghrelin is suppressed. This has important implications for individualized medicine; optimizing the ghrelin response during exercise can have further downstream effects on insulin sensitivity, appetite, and vascular function [2,10]. However, more work needs to be completed to understand if high intensity exercise is the best prescription for different clinical populations, as we only included studies that sampled healthy adults. Lastly, more exercise training studies that employ a chronic high intensity stimulus is warranted to understand if the suppressive effects of exercise on ghrelin levels can be maintained over time.
4.5. Limitations
This analysis has several limitations. First, we limited our inclusion to studies published in the English language. In addition, the results of this review are restricted to healthy populations, as we excluded major diseases, and are not necessarily comparable to exercise training studies. We recognize that including multiple trials from one study may contribute to analytical issues such as “double counting,” [88] however we feel our choice in applying a nested model helped reduce this effect. It should also be noted that half of the included trials in this analysis occurred from the same laboratory group [18–21,28,29,34,50,65], however we did account for between study variance in our model. Finally, it is possible that many of our moderators were underpowered to detect significant effects, therefore our moderator analyses remain speculative in nature.
4.6. Conclusions
The results of this meta-analysis suggest high intensity exercise may be superior to low and moderate intensities when it pertains to ghrelin suppression, although there is a paucity of data in overweight/obese, female, and older adults. Future work should be concentrated on developing precise exercise prescriptions to best target both AG and DAG during acute exercise, which can later be applied to chronic training interventions. As many facets of ghrelin function remain ambiguous, research should also focus on establishing clear pathways for AG and DAG effects, which can help illuminate how exercise can be applied to lead to clinically meaningful changes to endogenous ghrelin levels.
Supplementary Material
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- VO2peak
peak oxygen uptake
- EE
exercise energy expenditure
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.peptides.2021.170625.
Availability of data and material
The data analyzed in this review are available from the corresponding author upon reasonable request.
References
- [1].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K, Ghrelin is a growth-hormone-releasing acylated peptide from stomach, Nature 402 (1999) 656–660. [DOI] [PubMed] [Google Scholar]
- [2].Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. , Ghrelin. Mol Metab. 4 (2015) 437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Colldén G, Tschöop MH, Müller TD, Therapeutic potential of targeting the ghrelin pathway, Int. J. Mol. Sci 18 (2017) 798. Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, et al. , Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men, J. Clin. Endocrinol. Metab 93 (2008) 1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gauna C, Meyler FM, Janssen J, Delhanty PJD, Abribat T, van Koetsveld P, et al. , Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity, J. Clin. Endocrinol. Metab 89 (2004) 5035–5042. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- [6].Neary NM, Druce MR, Small CJ, Bloom SR, Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect, Gut 55 (2006) 135. BMJ Publishing Group. [PMC free article] [PubMed] [Google Scholar]
- [7].Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, et al. , Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin, Gut 54 (2005) 18–24. BMJ Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gauna C, Delhanty PJD, Hofland LJ, Janssen JAMJL, Broglio F, Ross RJM, et al. , Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes, J. Clin. Endocrinol. Metab 90 (2005) 1055–1060. [DOI] [PubMed] [Google Scholar]
- [9].Kleinz MJ, Maguire JJ, Skepper JN, Davenport AP, Functional and immunocytochemical evidence for a role of ghrelin and des-octanoyl ghrelin in the regulation of vascular tone in man, Cardiovasc. Res 69 (2006) 227–235. Elsevier Science. [DOI] [PubMed] [Google Scholar]
- [10].Gortan Cappellari G, Barazzoni R, Ghrelin forms in the modulation of energy balance and metabolism, Eat Weight Disord. – Stud. Anorex Bulim Obes 24 (2019) 997–1013. [DOI] [PubMed] [Google Scholar]
- [11].Rodríguez A, Gómez-Ambrosi J, Catalán V, Gil MJ, Becerril S, Sáinz N, et al. , Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes, Int. J. Obes 33 (2009) 541–552, 2005. [DOI] [PubMed] [Google Scholar]
- [12].Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, et al. , Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome, J. Clin. Endocrinol. Metab 92 (2007) 3935–3940. [DOI] [PubMed] [Google Scholar]
- [13].Malin SK, Samat A, Wolski K, Abood B, Pothier CE, Bhatt DL, et al. , Improved acylated ghrelin suppression at 2 years in obese patients with type 2 diabetes: effects of bariatric surgery vs standard medical therapy, Int. J. Obes 38 (2014) 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Razzaghy-Azar M, Nourbakhsh M, Pourmoteabed A, Nourbakhsh M, Ilbeigi D, Khosravi M, An evaluation of acylated ghrelin and obestatin levels in childhood obesity and their association with insulin resistance, metabolic syndrome, and oxidative stress, J. Clin. Med 5 (2016) 61. Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Henriksen EJ, Invited review: effects of acute exercise and exercise training on insulin resistance, J Appl. Physiol. Bethesda Md. 1985 93 (2002) 788–796. [DOI] [PubMed] [Google Scholar]
- [16].Thompson JK, Jarvie GJ, Lahey BB, Cureton KJ, Exercise and obesity: etiology, physiology, and intervention, Psychol. Bull 91 (1982) 55–79. [PubMed] [Google Scholar]
- [17].Shephard RJ, Balady GJ, Exercise as cardiovascular therapy, Circulation 99 (1999) 963–972. [DOI] [PubMed] [Google Scholar]
- [18].Alajmi N, Deighton K, King JA, Reischak-Oliveira A, Wasse LK, Jones J, et al. , Appetite and energy intake responses to acute energy deficits in females versus males, Med. Sci. Sports Exerc 48 (2016) 412. Wolters Kluwer Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bishop NC, Hayashida H, Clark M, Coombs C, Miller S, Stensel DJ, Effect of acute and regular exercise on growth hormone secretagogue receptor-1a expression in human lymphocytes, T cell subpopulation and monocytes, Brain Behav. Immun 39 (2014) 172–179. Elsevier. [DOI] [PubMed] [Google Scholar]
- [20].Burns SF, Broom DR, Miyashita M, Mundy C, Stensel DJ, A single session of treadmill running has no effect on plasma total ghrelin concentrations, J. Sports Sci 25 (2007) 635–642. Routledge. [DOI] [PubMed] [Google Scholar]
- [21].Broom DR, Miyashita M, Wasse LK, Pulsford R, King JA, Thackray AE, et al. , Acute effect of exercise intensity and duration on acylated ghrelin and hunger in men, J. Endocrinol 232 (2017) 411–422. [DOI] [PubMed] [Google Scholar]
- [22].Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M, Exercise-induced suppression of acylated ghrelin in humans, J. Appl. Physiol. Bethesda Md. 1985 102 (2007) 2165–2171. [DOI] [PubMed] [Google Scholar]
- [23].Broom DR, Batterham RL, King JA, Stensel DJ, Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males, Am. J. Physiol. Regul. Integr. Comp. Physiol 296 (2009) R29–35. [DOI] [PubMed] [Google Scholar]
- [24].Erdmann J, Tahbaz R, Lippl F, Wagenpfeil S, Schusdziarra V, Plasma ghrelin levels during exercise - effects of intensity and duration, Regul. Pept 143 (2007) 127–135. [DOI] [PubMed] [Google Scholar]
- [25].Hagobian TA, Yamashiro M, Hinkel-Lipsker J, Streder K, Evero N, Hackney T, Effects of acute exercise on appetite hormones and ad libitum energy intake in men and women, Appl. Physiol. Nutr. Metab 38 (2013) 66–72. NRC Research Press. [DOI] [PubMed] [Google Scholar]
- [26].Kawano H, Mineta M, Asaka M, Miyashita M, Numao S, Gando Y, et al. , Effects of different modes of exercise on appetite and appetite-regulating hormones, Appetite 66 (2013) 26–33. Elsevier. [DOI] [PubMed] [Google Scholar]
- [27].Kelly P, Guelfi K, Wallman K, Fairchild T, Mild dehydration does not reduce postexercise appetite or energy intake, Med. Sci. Sports Exerc 44 (2012) 516–524. [DOI] [PubMed] [Google Scholar]
- [28].King JA, Miyashita M, Wasse LK, Stensel DJ, Influence of prolonged treadmill running on appetite, energy intake and circulating concentrations of acylated ghrelin, Appetite 54 (2010) 492–498. [DOI] [PubMed] [Google Scholar]
- [29].King JA, Wasse LK, Broom DR, Stensel DJ, Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin, Med. Sci. Sports Exerc 42 (2010) 485–492. [DOI] [PubMed] [Google Scholar]
- [30].Larsen PS, Donges CE, Guelfi KJ, Smith GC, Adams DR, Duffield R, Effects of aerobic, strength or combined exercise on perceived appetite and appetite-related hormones in inactive middle-aged men, Int. J. Sport Nutr. Exerc. Metab 27 (2017) 389–398. [DOI] [PubMed] [Google Scholar]
- [31].Mattin LR, Yau AM, McIver V, James LJ, Evans GH, The effect of exercise intensity on gastric emptying rate, appetite and gut derived hormone responses after consuming a standardised semi-solid meal in healthy males, Nutrients 10 (2018) 787. Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Metcalfe RS, Koumanov F, Ruffino JS, Stokes KA, Holman GD, Thompson D, et al. , Physiological and molecular responses to an acute bout of reduced-exertion high-intensity interval training (REHIT), Eur. J. Appl. Physiol 115 (2015) 2321–2334. Springer. [DOI] [PubMed] [Google Scholar]
- [33].Stokes KA, Sykes D, Gilbert KL, Chen J-W, Frystyk J, Brief, high intensity exercise alters serum ghrelin and growth hormone concentrations but not IGF-I, IGF-II or IGF-I bioactivity, Growth Horm. IGF Res 20 (2010) 289–294. [DOI] [PubMed] [Google Scholar]
- [34].Wasse LK, Sunderland C, King JA, Miyashita M, Stensel DJ, The influence of vigorous running and cycling exercise on hunger perceptions and plasma acylated ghrelin concentrations in lean young men, Appl. Physiol. Nutr. Metab 38 (2013) 1–6. NRC Research Press. [DOI] [PubMed] [Google Scholar]
- [35].Vatansever-Ozen S, Tiryaki-Sonmez G, Bugdayci G, Ozen G, The effects of exercise on food intake and hunger: relationship with acylated ghrelin and leptin, J. Sports Sci. Med 10 (2011) 283. Dept. of Sports Medicine, Medical Faculty of Uludag University. [PMC free article] [PubMed] [Google Scholar]
- [36].Schubert MM, Sabapathy S, Leveritt M, Desbrow B, Acute exercise and hormones related to appetite regulation: a meta-analysis, Sports Med. 44 (2014) 387–403. Springer. [DOI] [PubMed] [Google Scholar]
- [37].Douglas JA, Deighton K, Atkinson JM, Sari-Sarraf V, Stensel DJ, Atkinson G, Acute exercise and appetite-regulating hormones in overweight and obese individuals: a meta-analysis, J. Obes 2016 (2016). Hindawi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, et al. , Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates, J. Clin. Endocrinol. Metab 93 (2008) 1971–1979. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jakubowicz D, Froy O, Wainstein J, Boaz M, Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults, Steroids 77 (2012) 323–331. Elsevier. [DOI] [PubMed] [Google Scholar]
- [40].Hawley JA, Burke LM, Effect of meal frequency and timing on physical performance, Br. J. Nutr 77 (1997) S91–103. Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- [41].Bennard P, Doucet E, Acute effects of exercise timing and breakfast meal glycemic index on exercise-induced fat oxidation, Appl. Physiol. Nutr. Metab 31 (2006) 502–511. NRC Research Press. [DOI] [PubMed] [Google Scholar]
- [42].Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS Med. 6 (2009), e1000097. Public Library of Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schneider CA, Rasband WS, Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis, Nat. Methods 9 (2012) 671–675. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. , RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ (2019) 366. British Medical Journal Publishing Group. [DOI] [PubMed] [Google Scholar]
- [45].Viechtbauer W, Conducting meta-analyses in R with the metafor package, J. Stat. Softw 36 (2010) 1–48. UCLA Statistics. [Google Scholar]
- [46].Wickham H, ggplot2: Elegant Graphics for Data Analysis, springer, 2016. [Google Scholar]
- [47].Assink M, Wibbelink CJM, Fitting three-level meta-analytic models in R: a step-by-step tutorial, Quant. Methods Psychol 12 (2016) 154–174. [Google Scholar]
- [48].Cohen J, Statistical Power Analysis for the Behavioural Sciences, 2nd edn, L. Erlbaum Associates, Hillsdale, NJ, 1988. [Google Scholar]
- [49].Higgins JPT, Thompson SG, Deeks JJ, Altman DG, Measuring inconsistency in meta-analyses, BMJ 327 (2003) 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dorling JL, Clayton DJ, Jones J, Carter WG, Thackray AE, King JA, et al. , A randomized crossover trial assessing the effects of acute exercise on appetite, circulating ghrelin concentrations, and butyrylcholinesterase activity in normal-weight males with variants of the obesity-linked FTO rs9939609 polymorphism, Am. J. Clin. Nutr 110 (2019) 1055–1066. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- [51].Feltrin KL, Patterson M, Ghatei MA, Bloom SR, Meyer JH, Horowitz M, et al. , Effect of fatty acid chain length on suppression of ghrelin and stimulation of PYY, GLP-2 and PP secretion in healthy men, Peptides 27 (2006) 1638–1643. [DOI] [PubMed] [Google Scholar]
- [52].Greenman Y, Golani N, Gilad S, Yaron M, Limor R, Stern N, Ghrelin secretion is modulated in a nutrient- and gender-specific manner, Clin. Endocrinol. (Oxf.) 60 (2004) 382–388. [DOI] [PubMed] [Google Scholar]
- [53].Peake JM, Tan SJ, Markworth JF, Broadbent JA, Skinner TL, Cameron-Smith D, Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise, Am. J. Physiol. Endocrinol. Metab 307 (2014) E539–552. [DOI] [PubMed] [Google Scholar]
- [54].Gupta D, Ogden SB, Shankar K, Varshney S, Zigman JM, A LEAP 2 conclusions? Targeting the ghrelin system to treat obesity and diabetes, Mol. Metab 46 (2021), 101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mani BK, Puzziferri N, He Z, Rodriguez JA, Osborne-Lawrence S, Metzger NP, et al. , LEAP2 changes with body mass and food intake in humans and mice, J. Clin. Invest 129 (2019) 3909–3923. American Society for Clinical Investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ge X, Yang H, Bednarek MA, Galon-Tilleman H, Chen P, Chen M, et al. , LEAP2 is an endogenous antagonist of the ghrelin receptor, Cell Metab. 27 (2018) 461–469, e6. [DOI] [PubMed] [Google Scholar]
- [57].Clausen JP, Effect of physical training on cardiovascular adjustments to exercise in man, Physiol. Rev 57 (1977) 779–815. American Physiological Society. [DOI] [PubMed] [Google Scholar]
- [58].Hazell TJ, Islam H, Townsend LK, Schmale MS, Copeland JL, Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: potential mechanisms, Appetite 98 (2016) 80–88. [DOI] [PubMed] [Google Scholar]
- [59].Wasse LK, Sunderland C, King JA, Batterham RL, Stensel DJ, Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY, J. Appl. Physiol. Bethesda Md. 1985 112 (2012) 552–559. [DOI] [PubMed] [Google Scholar]
- [60].Wu R, Dong W, Ji Y, Zhou M, Marini CP, Ravikumar TS, et al. , Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion, PLoS One 3 (2008) e2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cairns SP, Lactic acid and exercise performance : culprit or friend? Sports Med. Auckl. NZ 36 (2006) 279–291. [DOI] [PubMed] [Google Scholar]
- [62].Engelstoft MS, Park W, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, et al. , Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells, Mol. Metab 2 (2013) 376–392. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zouhal H, Sellami M, Saeidi A, Slimani M, Abbassi-Daloii A, Khodamoradi A, et al. , Effect of physical exercise and training on gastrointestinal hormones in populations with different weight statuses, Nutr. Rev 77 (2019) 455–477. [DOI] [PubMed] [Google Scholar]
- [64].Nass R, Farhy LS, Liu J, Pezzoli SS, Johnson ML, Gaylinn BD, et al. , Age-dependent decline in acyl-ghrelin concentrations and reduced association of acyl-ghrelin and growth hormone in healthy older adults, J. Clin. Endocrinol. Metab 99 (2014) 602–608. Oxford Academic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Douglas JA, King JA, Clayton DJ, Jackson A, Sargeant JA, Thackray AE, et al. , Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women, Int. J. Obes 41 (2017) 1737–1744. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Delhanty PJD, van der Eerden B.C.J., van Leeuwen J.P.T.M., Ghrelin and bone, BioFactors 40 (2014) 41–48. [DOI] [PubMed] [Google Scholar]
- [67].Thompson D, Aging and sarcopenia, J. Musculoskelet. Neuronal Interact 7 (2007) 344. Hylonome. [PubMed] [Google Scholar]
- [68].da S Pereira JA, da Silva FC, de Moraes-Vieira PMM, The impact of ghrelin in metabolic diseases: an immune perspective, J. Diabetes Res 2017 (2017). Hindawi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gholipour M, Kordi MR, Taghikhani M, Ravasi AA, Gaeini AA, Tabrizi A, Possible role for growth hormone in suppressing acylated ghrelin and hunger ratings during and after intermittent exercise of different intensities in obese individuals, Acta Med. Iran (2014) 29–37. [PubMed] [Google Scholar]
- [70].Tiryaki-Sonmez G, Ozen S, Bugdayci G, Karli U, Ozen G, Cogalgil S, et al. , Effect of exercise on appetite-regulating hormones in overweight women, Biol. Sport 30 (2013) 75. Institute of Sport. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].King JA, Wasse LK, Stensel DJ, Nimmo MA, Exercise and ghrelin. A narrative overview of research, Appetite 68 (2013) 83–91. [DOI] [PubMed] [Google Scholar]
- [72].Duren DL, Sherwood RJ, Czerwinski SA, Lee M, Choh AC, Siervogel RM, et al. , Body composition methods: comparisons and interpretation, J. Diabetes Sci. Technol 2 (2008) 1139–1146. SAGE Publications Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rodríguez G, Moreno LA, Blay MG, Blay VA, Fleta J, Sarría A, et al. , Body fat measurement in adolescents: comparison of skinfold thickness equations with dual-energy X-ray absorptiometry, Eur. J. Clin. Nutr 59 (2005) 1158–1166. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- [74].Sondergaard E, Gormsen LC, Nellemann B, Vestergaard ET, Christiansen JS, Nielsen S, Visceral fat mass is a strong predictor of circulating ghrelin levels in premenopausal women, Eur. J. Endocrinol 160 (2009) 375–379. [DOI] [PubMed] [Google Scholar]
- [75].Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, et al. , Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin, Gut 54 (2005) 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. , GOAT links dietary lipids with the endocrine control of energy balance, Nat. Med 15 (2009) 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Balaguera-Cortes L, Wallman KE, Fairchild TJ, Guelfi KJ, Energy intake and appetite-related hormones following acute aerobic and resistance exercise, Appl. Physiol. Nutr. Metab 36 (2011) 958–966. NRC Research Press. [DOI] [PubMed] [Google Scholar]
- [78].Khatib MN, Gaidhane S, Gaidhane AM, Simkhada P, Zahiruddin QS, Ghrelin O acyl transferase (GOAT) as a novel metabolic regulatory enzyme, J. Clin. Diagn. Res 9 (2015) LE01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kitazawa T, Hiraga T, Teraoka H, Yaosaka N, Kaiya H, Correlation of ghrelin concentration and ghrelin, ghrelin-O-acetyltransferase (GOAT) and growth hormone secretagogue receptor 1a mRNAs expression in the proventriculus and brain of the growing chicken, Peptides 63 (2015) 134–142. [DOI] [PubMed] [Google Scholar]
- [80].Crabtree DR, Chambers ES, Hardwick RM, Blannin AK, The effects of high-intensity exercise on neural responses to images of food, Am. J. Clin. Nutr 99 (2014) 258–267. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- [81].Kraft EN, Cervone DT, Dyck DJ, Ghrelin stimulates fatty acid oxidation and inhibits lipolysis in isolated muscle from male rats, Physiol. Rep (2019) [Internet]. [cited 2020 Oct 7];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6453820/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Balsom PD, Gaitanos G, Söderlund K, Ekblom B, High-intensity exercise and muscle glycogen availability in humans, Acta Physiol. Scand 165 (1999) 337–346. BLACKWELL SCIENTIFIC PUBLICATIONS. [DOI] [PubMed] [Google Scholar]
- [83].Deighton K, Barry R, Connon CE, Stensel DJ, Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise, Eur. J. Appl. Physiol 113 (2013) 1147–1156. Springer. [DOI] [PubMed] [Google Scholar]
- [84].Ormsbee MJ, Bach CW, Baur DA, Pre-exercise nutrition: the role of macronutrients, modified starches and supplements on metabolism and endurance performance, Nutrients 6 (2014) 1782–1808. Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Roy BD, Tarnopolsky MA, Influence of differing macronutrient intakes on muscle glycogen resynthesis after resistance exercise, J. Appl. Physiol 84 (1998) 890–896. American Physiological Society. [DOI] [PubMed] [Google Scholar]
- [86].King JA, Wasse LK, Ewens J, Crystallis K, Emmanuel J, Batterham RL, et al. , Differential acylated ghrelin, peptide YY3–36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction, J. Clin. Endocrinol. Metab 96 (2011) 1114–1121. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- [87].Ueno H, Shiiya T, Mizuta M, Mondal SM, Nakazato M, Plasma ghrelin concentrations in different clinical stages of diabetic complications and glycemic control in Japanese diabetics, Endocr. J 54 (2007) 895–902. [DOI] [PubMed] [Google Scholar]
- [88].Senn SJ, Overstating the evidence–double counting in meta-analysis and related problems, BMC Med. Res. Methodol 9 (10) (2009). Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this review are available from the corresponding author upon reasonable request.


