Abstract
Background
Angiogenesis-related marker vascular cell adhesion molecule-1 (VCAM-1) has been shown to be elevated in urothelial carcinoma of the bladder (UCB), but its predictive/prognostic role has not been determined. Thus, this study aimed to investigate the predictive/prognostic role of VCAM-1 for patients who have UCB treated with radical cystectomy (RC).
Methods
The study enrolled 1036 patients with clinically non-metastatic advanced UCB who underwent RC, and plasma VCAM-1 was evaluated preoperatively. The correlation of plasma VCAM-1 with pathologic and survival outcomes was assessed using binominal logistic regression and multivariable Cox regression analyses. Discrimination was assessed using the area under the curve and concordance indices. The clinical net benefit was evaluated using decision curve analysis (DCA).
Results
Preoperative VCAM-1 was significantly elevated in patients with adverse pathologic features. Higher VCAM-1 levels were independently associated with increased risk of lymph-node-metastasis (LNM), ≥pT3 disease, and non-organ-confined disease (NOCD (p < 0.001 for each). Preoperative plasma VCAM-1 was independently associated with recurrence-free survival (RFS), cancer-specific survival (CSS), and overall survival (OS) in pre- and postoperative multivariable models. Adding VCAM-1 to these predictive models improved their discriminatory ability to predict all outcomes by a significant margin. In the DCA, VCAM-1 addition to the reference models for prediction of LNM, NOCD, RFS, and CSS resulted in relevant improvement.
Conclusions
Elevated plasma VCAM-1 was associated with biologically and clinically aggressive UCB disease features. After validation, preoperative VCAM-1 may serve as a biomarker to help identify patients likely to benefit from intensified/multimodal therapy. In addition, VCAM-1 improved the discriminatory power of predictive/prognostic models and can be used to refine personalized clinical decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-022-11575-4.
Radical cystectomy (RC) with lymph node dissection is the standard treatment for very-high-risk non-muscle-invasive and muscle-invasive urothelial carcinoma of the bladder (UCB).1,2 Despite definitive therapy with curative intent, the 5-year overall survival (OS) of patients remains below 60 %.3,4 Thus, various clinical and pathologic factors have been explored to improve the risk stratification of patients with UCB and to facilitate clinical decision-making and patient counseling.5–7
Unfortunately, the current outcome prediction models remain suboptimal, likely because of a failure to capture the full potential of host-tumor interactions.8 Additionally, clinical, radiologic, and pre-RC pathologic factors pose significant limitations for outcome prediction, thus limiting accurate personalized clinical decision-making.5,9 Identification of preoperative biomarkers capturing each tumor’s biologic and clinical potential is crucial to improvement of risk stratification for patients with UCB.10
Vascular cell adhesion molecule-1 (VCAM-1), which mediates cellular adhesion, is a 90-kDa transmembrane glycoprotein transiently expressed on the surface of different types of vascular endothelial and stromal cells in response to vascular endothelial growth factors (VEGFs) and cytokines.11,12 A close association exists between VCAM-1 and inflammation, immunologic disorders, tumor angiogenesis, and metastasis.11,13 Moreover, findings have shown that VCAM-1 is associated with oncologic outcomes in several cancers, with several studies reporting elevated serum VCAM-1 levels in patients with UCB.14–16 However, the predictive/prognostic value of blood VCAM-1 levels in patients who have UCB treated with RC remains uninvestigated.
We hypothesized that patients with clinically non-metastatic advanced UCB harboring occult metastases exhibit elevated plasma VCAM-1 levels, which are associated with features of aggressive disease and poor survival despite apparently effective disease control. We enrolled a large consecutive cohort of patients who had clinically non-metastatic advanced UCB treated with RC and pelvic lymphadenectomy to investigate the relationship between preoperative plasma VCAM-1 levels and established features of bladder cancer invasion, metastasis, and survival outcomes. Beyond multivariable modeling, we used predictive accuracy testing and decision curve analysis (DCA) to assess the value of preoperative VCAM-1 as a biomarker in real-world clinical scenarios.
Material and Methods
Patient Selection
All procedures were undertaken with the approval and oversight of the Institutional Review Board for the Protection of Human Subjects (1011011386 and 069826900). This retrospective study included a consecutive cohort of 1036 patients treated with RC for non-metastatic UCB between 2003 and 2015. No patient received neoadjuvant chemotherapy (NAC) or radiotherapy. Adjuvant chemotherapy (AC) was administered to 167 patients (16.1 %) at the clinicians' discretion based on tumor stage and overall health status.
Measurement of Plasma VCAM-1 Levels
Preoperative plasma samples (subsequently used for VCAM-1 testing) were typically collected on the morning of surgery after an overnight fast. Their collection and measurement were performed as described previously.12 Briefly, blood was collected into 8-mL Vacutainer CPT tubes containing 0.1 mL of molar sodium citrate (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA) and centrifuged at 1500 g for 20 min at room temperature. The top layer corresponding to plasma was decanted using sterile transfer pipettes. The plasma was immediately frozen and stored at −80 °C in polypropylene cryopreservation vials (Nalgene, Nalge Nunc, Rochester, NY, USA). Quantitative immunoassays were used to measure VCAM-1 levels (R&D systems, Minneapolis, MN, USA).
Pathologic Evaluation and Patient Management
All surgical specimens were processed according to standard pathologic procedures as previously described.17 The 2002 American Joint Committee on Cancer tumor-node-metastasis (TNM) classification was used for pathologic staging, and the 1973 WHO/ISUP consensus classification was used for grading.
All the patients were followed up under the relevant institutional protocols and local guidelines. In general, the routine follow-up assessment included physical examination, radiologic imaging, and urine cytology every 3 months for 2 years. Between the second and fifth years, the follow-up assessment was performed semiannually, with follow-up evaluation subsequently performed annually in most cases. Recurrence was defined as any local recurrence (in the retroperitoneum or renal fossa) or distant metastasis. Recurrences in the bladder or contralateral upper urinary tract were considered as second primaries.
Statistical Analysis
Categorical variables are reported as frequencies and proportions. Continuous coded variables are reported as medians and interquartile ranges (IQRs).
Given the non-normal distribution of the preoperative VCAM-1 levels, a log transformation was performed to reduce skewness and allow valid inference in the multivariable analysis. Patient characteristics and median preoperative plasma VCAM-1 levels were treated as categorical variables. Thus, group comparisons were performed using Mann-Whitney U or Kruskal-Wallis tests with subsequent significance testing as appropriate.
Binominal logistic regression analysis was performed to assess the association between preoperative VCAM-1 plasma levels and lymph node metastasis (LNM), ≥pT3 disease, or any non-organ-confined disease (NOCD, defined as ≥pT3 disease and/or LNM). In receiver operating characteristics (ROCs) curves, the area under the curve (AUC) was calculated to determine the predictive accuracy of the multiple logistic regression models. DeLong’s test was used to test the statistical significance between AUC differences.
Recurrence-free survival (RFS), cancer-specific survival (CSS), and OS were graphically visualized using the Kaplan-Meier method. The difference between groups was assessed using a log-rank test. Uni- and multivariable Cox regression models were used to investigate the associations of VCAM-1 with RFS, CSS, and OS. Clinical and pathologic tumor grades were excluded as variables for all predictive models because most patients had high-grade UCB. Separate Cox regression models that featured either preoperative clinical variables or postoperative histopathologic variables were developed. Their discriminatory ability and the additional information provided by plasma VCAM-1 levels were tested using Harrel’s concordance index (C-index).
The additional clinical net benefit of VCAM-1 also was evaluated using decision curve analysis (DCA) to investigate whether preoperative plasma VCAM-1 levels improved the accuracy of separate predictive and prognostic models and whether these models had a relevant net benefit in pre- or postoperative settings.18 Separate reference models that represented either the pre- or postoperative setting were created, to which VCAM-1 was added to assess the difference in predictive values. In addition, subgroup analyses were conducted among the patients with cT1, cT2, and pT2N0.
All p values were two-sided, and significance was defined as a p value lower than 0.05. Statistical analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and Stata/MP 14.2 statistical software (Stata Corp., College Station, TX, USA).
Results
Association Between Patient Characteristics and VCAM-1
Table 1 outlines the characteristics of the included patients. The median cohort age was 67 years (IQR, 60–73 years). The median plasma VCAM-1 levels were significantly higher for the patients with adverse pathologic features such as LNM and advanced pathologic tumor stage (p < 0.05).
Table 1.
Patient demographicsa
| Overall | Stratified by median log VCAM-1 levels | |||
|---|---|---|---|---|
| Characteristic | (n = 1036) n (%) |
Low VCAM-1 (n = 518) n (%) |
High VCAM-1 (n = 518) n (%) |
p Value |
| Median age: years (IQR) | 67 (60–73) | 66 (59–72) | 67 (60–74) | 0.10 |
| Gender | 0.02 | |||
| Male | 814 (79) | 422 (81) | 392 (76) | |
| Female | 222 (21) | 96 (19) | 126 (24) | |
| Blood transfusion | 268 (26) | 122 (24) | 146 (28) | 0.09 |
| Thrombocytosis | 113 (11) | 56 (11) | 57 (11) | >0.9 |
| Clinical tumor grade | >0.9 | |||
| 2 | 6 (0.6) | 3 (0.6) | 3 (0.6) | |
| 3 | 1,022 (99) | 512 (99) | 510 (99) | |
| Unknown | 8 | 3 | 5 | |
| Clinical tumor stage | 0.06 | |||
| cTa | 23 (2.2) | 12 (2.3) | 11 (2.1) | |
| cTis | 105 (10) | 42 (8.2) | 63 (12) | |
| cT1 | 336 (33) | 156 (30) | 180 (35) | |
| cT2 | 498 (48) | 268 (52) | 230 (45) | |
| cT3 | 38 (3.7) | 19 (3.7) | 19 (3.7) | |
| cT4 | 29 (2.8) | 18 (3.5) | 11 (2.1) | |
| Unknown | 7 | 3 | 4 | |
| Pathologic tumor grade | 0.8 | |||
| 1 | 62 (6.0) | 29 (5.6) | 33 (6.4) | |
| 2 | 11 (1.1) | 5 (1.0) | 6 (1.2) | |
| 3 | 963 (93) | 484 (93) | 479 (92) | |
| Pathologic tumor stage | <0.001 | |||
| pT0 | 62 (6.0) | 29 (5.6) | 33 (6.4) | |
| pTa | 22 (2.1) | 9 (1.7) | 13 (2.5) | |
| pTis | 131 (13) | 67 (13) | 64 (12) | |
| pT1 | 162 (16) | 104 (20) | 58 (11) | |
| pT2 | 248 (24) | 133 (26) | 115 (22) | |
| pT3 | 281 (27) | 122 (24) | 159 (31) | |
| pT4 | 130 (13) | 54 (10) | 76 (15) | |
| Positive surgical margins | 95 (9.2) | 39 (7.5) | 56 (11) | 0.07 |
| Lymphovascular invasion | 295 (28) | 138 (27) | 157 (30) | 0.2 |
| Concomitant carcinoma in situ | 572 (55) | 309 (60) | 263 (51) | 0.004 |
| Lymph node involvement | 263 (25) | 96 (19) | 167 (32) | <0.001 |
| Adjuvant chemotherapy | 167 (16) | 78 (15) | 89 (17) | 0.4 |
VCAM-1 vascular cell adhesion molecule-1; IQR interquartile range
aWilcoxon rank sum test; Pearson’s chi-squared test; Fisher’s exact test
Association Between Advanced Pathologic Features and VCAM-1
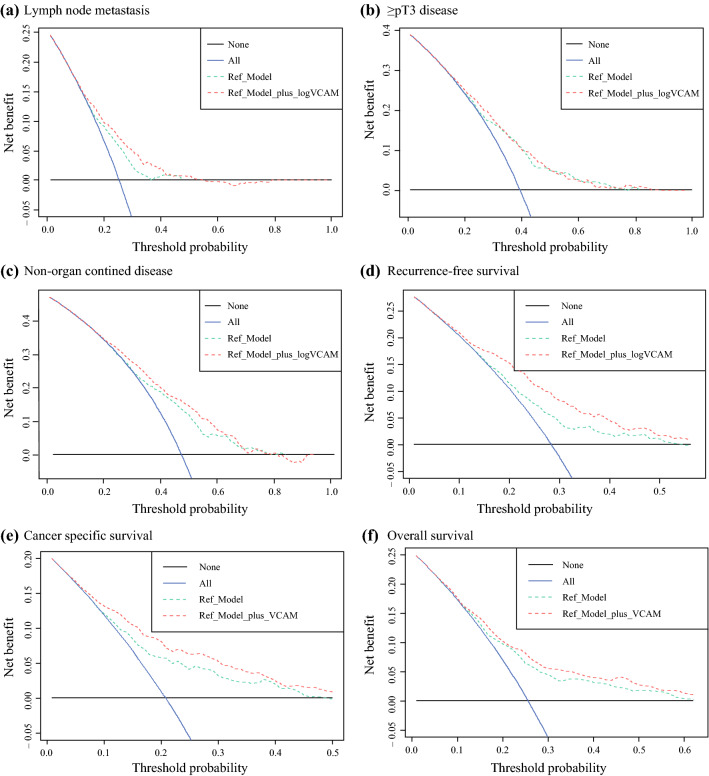
Multivariable logistic regression modeling showed significant association between elevated preoperative plasma VCAM-1 levels and an increased risk of LNM (odds ratio [OR], 2.25; 95 % confidence interval [CI], 1.72–2.97; p < 0.001), ≥pT3 disease (OR, 1.77; 95 % CI, 1.38–2.28; p < 0.001), and NOCD (OR, 1.77; 95 % CI, 1.38–2.28; p < 0.001) (Table 2). In the ROC curve analysis, addition of VCAM-1 to the reference model comprising age, sex, and clinical tumor stage improved its discriminatory ability to predict LNM (+5.8 %; p < 0.001), ≥pT3 disease (+2.0 %; p = 0.008), and NOCD (+4.4 %; p < 0.001). Addition of VCAM-1 to the reference model in DCA improved the net benefit for predicting LNM and NOCD (Fig. 1).
Table 2.
Logistic regression modeling
| Lymph node involvement | pT3/4 disease | Any non-organ-confined disease | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95 % CI | p Value | OR | 95 % CI | p Value | OR | 95 % CI | p Value |
| logVCAM-1 | 2.25 | 1.72–2.97 | <0.001 | 1.77 | 1.38–2.28 | <0.001 | 1.77 | 1.38–2.28 | <0.001 |
| Age | 1.00 | 0.98–1.01 | 0.6 | 1.03 | 1.01–1.04 | <0.001 | 1.03 | 1.01–1.04 | <0.001 |
| Gender | |||||||||
| Male | – | – | – | – | – | – | |||
| Female | 1.38 | 0.97–1.94 | 0.07 | 1.02 | 0.74–1.41 | 0.9 | 1.02 | 0.74–1.41 | 0.9 |
| Clinical tumor stage | |||||||||
| cTa/cTis/cT1 | – | – | – | – | – | – | |||
| cT2 | 2.75 | 2.00–3.80 | <0.001 | 2.91 | 2.20–3.87 | <0.001 | 2.91 | 2.20–3.87 | <0.001 |
| cT3/cT4 | 3.74 | 2.11–6.56 | <0.001 | 9.63 | 5.38–18.0 | <0.001 | 9.63 | 5.38–18.0 | <0.001 |
| AUC with VCAM-1 | 0.687 | 0.695 | 0.720 | ||||||
| AUC without VCAM-1 | 0.629 | 0.675 | 0.676 | ||||||
OR odds ratio, CI confidence interval, VCAM-1 vascular cell adhesion molecule-1, AUC area under the curve
Fig. 1.
Decision curve analysis (DCA) for the benefit of preoperative vascular cell adhesion molecule 1 (VCAM-1) after inclusion in a preoperative model for advanced disease and survival prediction. The preoperative reference model consisted of age, sex, and clinical tumor stage, and the additional net benefit of preoperative VCAM-1 was investigated. a Lymph node metastasis. b ≥pT3 disease. c Non-organ-confined disease. d Recurrence-free survival. e Cancer-specific survival. f Overall survival. The x axis shows the threshold probabilities. The y axis measures the net benefit, calculated by adding the true-positives and subtracting the false-positives. The horizontal line representing the x axis assumes that no patients experience the event, whereas the grey line assumes that all patients will experience the event at a specific threshold probability. The dashed black line represents the net benefit of the reference Cox and logistic regression model. The dashed red line represents the net benefit of the same Cox and logistic regression models that also include the preoperative VCAM-1 plasma levels as a variable.
For the patients with cT1 and cT2, elevated preoperative plasma VCAM-1 levels also were significantly associated with an increased risk of LNM, ≥pT3 disease, and NOCD (Table S1). Adding VCAM-1 to the reference model improved the discriminatory ability to predict these outcomes.
Association Between Survival Outcomes and VCAM-1 in the Preoperative Model
The median follow-up period for the alive patients was 37 months. The patients with higher median pretreatment VCAM-1 plasma levels had poorer RFS, CSS, and OS in the respective log-rank tests (all p < 0.001; Fig. S1).
In a multivariable Cox regression analysis that included available preoperative variables (i.e., age, sex, and clinical tumor stage), higher pretreatment VCAM-1 plasma levels were independently associated with poorer RFS (hazard ratio [HR], 2.90; 95 % CI, 2.46–3.41; p < 0.001), CSS (HR, 2.90; 95 % CI, 2.45–3.44; p < 0.001), and OS (HR, 1.77; 95 % CI, 1.52–2.05; p < 0.001) (Table 3). Adding the preoperative VCAM-1 plasma levels improved the C-indices of the same reference models for prognostication of RFS (+10.1 %), CSS (+8.7 %), and OS (+3.0 %). In the DCA, addition of VCAM-1 to the reference models for prognosis of RFS and CSS (Fig. 1) improved the net benefit of threshold probabilities from 10 % to 50 %.
Table 3.
Multivariable Cox regression analysis that included available preoperative variables
| Recurrence-free survival | Cancer-specific survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95 % CI | p Value | HR | 95 % CI | p Value | HR | 95 % CI | p Value |
| logVCAM-1 | 2.90 | 2.46–3.41 | <0.001 | 2.90 | 2.45–3.44 | <0.001 | 1.77 | 1.52–2.05 | <0.001 |
| Age | 1.02 | 1.01–1.03 | 0.004 | 1.02 | 1.01–1.04 | <0.001 | 1.05 | 1.04–1.06 | <0.001 |
| Gender | |||||||||
| Male | – | – | – | – | – | – | |||
| Female | 1.52 | 1.19–1.95 | <0.001 | 1.64 | 1.27–2.12 | <0.001 | 1.36 | 1.12–1.65 | 0.002 |
| Clinical tumor stage | |||||||||
| cTa/cTis/cT1 | – | – | – | – | – | – | |||
| cT2 | 1.96 | 1.55–2.47 | <0.001 | 2.09 | 1.63–2.69 | <0.001 | 1.72 | 1.44–2.06 | <0.001 |
| cT3/cT4 | 2.17 | 1.44–3.28 | <0.001 | 2.38 | 1.56–3.64 | <0.001 | 1.98 | 1.44–2.73 | <0.001 |
| C-index with VCAM-1 | 0.703 | 0.715 | 0.664 | ||||||
| C-index without VCAM-1 | 0.602 | 0.628 | 0.634 | ||||||
HR hazard ratio, CI confidence interval, VCAM-1 vascular cell adhesion molecule-1
For the patients with cT1 and cT2, higher pretreatment plasma VCAM-1 levels also were independently associated with poorer RFS, CSS, and OS (Table S2). Adding the preoperative plasma VCAM-1 levels improved the C-indices of the same reference models for the prediction of all these outcomes.
Association Between Survival Outcomes and VCAM-1 in the Postoperative Model
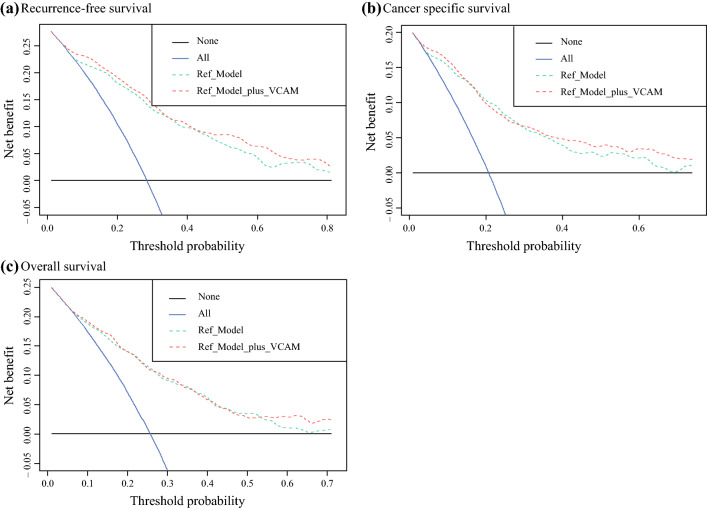
In a multivariable Cox regression model including established postoperative variables, higher pretreatment VCAM-1 plasma levels remained independently associated with poorer RFS (HR, 2.53; 95 % CI, 2.12–3.02; p < 0.001), CSS (HR, 2.49; 95 % CI, 2.06–3.00; p < 0.001), and OS (HR, 1.48; 95 % CI, 1.26–1.73; p < 0.001) (Table 4). Adding the VCAM-1 to the reference models (Table 4) improved the C-indices for prognostication of RFS (+3.2 %), CSS (+2.5 %), and OS (+<1 %). In the DCA, including the preoperative plasma VCAM-1 levels resulted in a net benefit of threshold probabilities from 50 % to 70 % for predicting RFS, from 40 % to 70 % for predicting CSS, and from 60 % to 70 % for predicting OS relative to the reference model (Fig. 2).
Table 4.
Multivariable Cox regression model that included established postoperative variables
| Recurrence-free survival | Cancer-specific survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95 % CI | p Value | HR | 95 % CI | p Value | HR | 95 % CI | p Value |
| logVCAM-1 | 2.53 | 2.12–3.02 | <0.001 | 2.49 | 2.06–3.00 | <0.001 | 1.48 | 1.26–1.73 | <0.001 |
| Age | 1.01 | 1.00–1.02 | 0.13 | 1.02 | 1.00–1.03 | 0.009 | 1.04 | 1.03–1.05 | <0.001 |
| Gender | |||||||||
| Male | – | – | – | – | – | – | |||
| Female | 1.64 | 1.28–2.10 | <0.001 | 1.70 | 1.31–2.19 | <0.001 | 1.40 | 1.15–1.70 | <0.001 |
| Pathologic tumor stage | |||||||||
| pT0/pTa/pTis/pT1 | – | – | – | – | – | – | |||
| pT2 | 1.42 | 0.98–2.07 | 0.06 | 1.39 | 0.93–2.07 | 0.11 | 1.45 | 1.14–1.85 | 0.003 |
| pT3/pT4 | 3.14 | 2.23–4.43 | <0.001 | 2.99 | 2.08–4.31 | <0.001 | 2.50 | 1.96–3.19 | <0.001 |
| Positive surgical margins | 1.47 | 1.08–2.00 | 0.02 | 1.60 | 1.16–2.20 | 0.004 | 1.14 | 0.86–1.52 | 0.3 |
| Lymphovascular invasion | 1.43 | 1.12–1.84 | 0.005 | 1.59 | 1.22–2.06 | <0.001 | 1.23 | 1.01–1.50 | 0.041 |
| Concomitant carcinoma in situ | 1.20 | 0.95–1.50 | 0.12 | 1.04 | 0.82–1.32 | 0.7 | 1.06 | 0.89–1.26 | 0.5 |
| Lymph node involvement | 2.25 | 1.75–2.89 | <0.001 | 2.29 | 1.76–2.97 | <0.001 | 1.96 | 1.59–2.41 | <0.001 |
| Adjuvant chemotherapy | 0.96 | 0.73–1.26 | 0.8 | 1.02 | 0.76–1.35 | >0.9 | 0.87 | 0.69–1.10 | 0.3 |
| C-index with VCAM-1 | 0.785 | 0.801 | 0.740 | ||||||
| C-index without VCAM-1 | 0.753 | 0.776 | 0.735 | ||||||
HR hazard ratio, CI confidence interval, VCAM-1 vascular cell adhesion molecule-1
Fig. 2.
Decision curve analysis (DCA) for the preoperative vascular cell adhesion molecule 1 (VCAM-1) benefit after inclusion in a postoperative model for survival prediction. The postoperative reference model consisted of established postoperative variables for prediction of recurrence-free survival, cancer-specific survival, and overall survival, and the additional net benefit of preoperative VCAM-1 was investigated. a Recurrence-free survival. b Cancer-specific survival. c Overall survival. The x axis shows the threshold probabilities. The y axis measures the net benefit, calculated by adding the true-positives and subtracting the false-positives. The horizontal line representing the x axis assumes that no patients experience the event, whereas the grey line assumes that all patients will experience the event at a specific threshold probability. The dashed black line represents the net benefit of the reference Cox regression model. The dashed red line represents the net benefit of the same Cox regression models that also include the preoperative VCAM-1 plasma levels as a variable.
For the patients with cT1 and cT2, higher pretreatment plasma VCAM-1 levels were independently associated with poorer RFS, CSS, and OS (Table S3). For the patients with pT2N0, higher pretreatment VCAM-1 plasma levels were independently associated with poorer RFS and CSS. Adding the VCAM-1 to the reference models improved the C-indices for prognostication of these outcomes.
Discussion
Although biomarkers help improve risk stratification by estimating the probability of treatment failure, clinically useful biomarkers for UCB are lacking.19,20 Therefore, we assessed the prognostic significance of preoperative plasma VCAM-1 levels in a large cohort of patients who had UCB treated with RC. We demonstrated that elevated preoperative VCAM-1 levels were associated with worse oncologic outcomes and predicted biologically and clinically aggressive disease. These findings suggest that blood levels of VCAM-1 could help facilitate clinical decision-making regarding perioperative chemotherapy and the extent of lymphadenectomy together with patient counseling.
The mechanism underlying the association of VCAM-1 with survival may be its relationship with tumor angiogenesis and metastasis. Serum VCAM-1 levels were observed to correlate with micro vessel density in breast cancer, suggesting that serum VCAM-1 is a surrogate angiogenesis marker.21 Furthermore, VEGF has been shown to upregulate VCAM-1 expression in endothelial cells.22,23 Additionally, there is an interaction between VCAM-1 and α4β1 integrin during angiogenesis. Findings have shown that VCAM-1 and α4β1 integrin are individually expressed on vascular smooth muscle cells and endothelial cells in the developing vessels of breast cancer.
Administration of an anti-murine VCAM-1 antibody reduced micro vessel formation in Matrigel plug mouse models.24 Furthermore, in vitro exposure to anti-VCAM-1 antibody blocked interleukin (IL)-4 and IL-13-induced tube formation, and angiogenesis induced in vivo by IL-4 and IL-13 was inhibited by an anti-α4 integrin antibody.25
Expression of VCAM-1 also is associated with tumor metastasis.11,13 A comparative gene profile analysis of parental MDA-MB-231 breast cancer cells and in vivo isolates exhibiting lung metastatic activity showed VCAM-1 expression to be upregulated in metastatic breast cancer cells in the lungs.26 Furthermore, mesothelial VCAM-1 expression was negatively associated with RFS and OS in epithelial ovarian cancer.14 Studies also have shown that expression of VCAM-1 is upregulated in lung and colorectal cancers.27, 28 Taken together, cumulative data suggested that increased VCAM-1 enhances tumor angiogenesis, adhesion, and metastasis, leading to advanced disease and increased cancer-related mortality.
To assess whether VCAM-1 can improve the prognostic outcome of established predictors requires more than conventional multivariable analyses.19,20 Analyzing the potential of a novel biomarker to enhance the predictive/prognostic reference model enables us to determine whether the biomarker itself holds unique predictive/prognostic information.19,20 Therefore, we examined whether preoperative VCAM-1 plasma levels improved the accuracy of separate predictive/prognostic models and whether these models had a relevant net benefit in pre- or postoperative settings using DCA. Indeed, we found that addition of VCAM-1 to standard models improved the discriminatory power for predicting LNM, ≥pT3 disease, and NOCD by a statistically and prognostically significant margin. Furthermore, VCAM-1 also considerably improved prognosis of early RFS and CSS. In the DCA, VCAM-1 addition improved the clinical net benefit of several reference models in a wide range of threshold probabilities, suggesting its clinical potential for guiding preoperative risk stratification through improved early outcome prediction. In essence, plasma VCAM-1 seems to improve patient selection for intensified and/or multimodal perioperative systemic therapy with the goal of prognosis.
In addition, VCAM-1 demonstrated the potential to improve outcome prediction if added to a model consisting of established histopathologic variables. In this postoperative setting, our prognostic model exhibited a high discriminatory power (C-Index of 0.79 for RFS prediction and 0.80 for CSS prediction), which could allow for tailored therapy, thereby improving patient care. In the DCA, VCAM-1 addition slightly improved the net benefit of this model for prediction of both RFS and CSS.
Guidelines recommend the use of NAC for cT2-4aN0M0 and the use of AC for pT3/4 and/or pN1 UCB.1 Of clinical interest is whether NAC should be offered for only cT2-4aN0M0 and AC for only pT3/4 and/or pN1, or whether NAC should be offered to all patients with cT2. Therefore, subgroup analyses were performed among cT1, cT2, and pT2N0 patients to assess the role of perioperative chemotherapy. Subsequently, in all these cohorts, adding VCAM-1 to the reference models improved the discriminatory ability to predict LNM, ≥pT3 disease, NOCD, RFS, and CSS by a prognostically significant margin. These results suggested that cT1 patients with elevated VCAM-1 also may benefit from intensified therapy such as NAC because they are more likely to be upstaged at the time of RC. Similarly, pT2N0 patients with elevated VCAM-1 also may benefit from AC because they are more likely to harbor micrometastatic disease. Moreover, in cT2, preoperative VCAM-1 also might serve as a biomarker to identify patients who could avoid NAC because the risk for RFS/CSS is very low. However, caution should be exercised in interpreting these results given that these subgroup analyses had less statistical power.
Although this was the largest study to investigate the predictive/prognostic value of VCAM-1, it had limitations. First, its retrospective and multicenter design may have resulted in laboratory, pathologic, and surgical workup variations, thus confounding the results. Second, the VCAM-1 value was assessed at a single time point preoperatively and not evaluated for its variability over time. Third, because of the recruitment time for this study, no information was available on the predictive value of VCAM-1 with respect to immunotherapies or NAC. Finally, although next-generation sequencing and immunohistology have shown several other candidate biomarkers, the results often are limited in reproducibility because of cost, intratumor heterogeneity, absence of a standardized approach, and overall complexity,29,30 hindering their implementation into clinical practice. Therefore, given the ease of procurement, low cost, high sample homogeneity, and the potential to improve early outcome prediction and prognosis, VCAM-1 warrants further evaluation as a candidate biomarker for integration into prospective clinical trials.19
Conclusion
To our knowledge, this is the first study to investigate VCAM-1 plasma levels in patients who have UCB treated with RC. Elevated plasma VCAM-1 levels were associated with features of biologically and clinically aggressive disease in patients with UCB. The use of VCAM-1 improved the discriminatory power of predictive/prognostic models by a significant margin. Therefore, VCAM-1 might be a valuable biomarker to guide physicians on the need for perioperative chemotherapy and the extent of lymphadenectomy. Thus, well-designed prospective studies with adequate follow-up evaluation are warranted to validate the use of VCAM-1 as a biomarker to enhance current tools used for risk stratification of patients with UCB and to assess its value in this age of immunotherapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Dr. Laukhtina, Schuettfort, and Pradere are supported by the EUSP Scholarship of the European Association of Urology. Dr. Grossmann is supported by the Zurich Cancer League. Dr. Mori is supported by The Uehara Memorial Foundation.
Funding
Open access funding provided by Medical University of Vienna.
Disclosures
Shahrokh Shariat owns or co-owns the following patents: methods to determine prognosis after therapy for prostate cancer (Grant No. 2002-09-06); methods to determine prognosis after therapy for bladder cancer (Grant No. 2003-06-19); prognostic methods for patients with prostatic disease (Grant No. 2004-08-05); Soluble Fas: urinary marker for the detection of bladder transitional cell carcinoma (Grant No. 2010-07-20). He has a consulting or advisory role for Astellas, Astra Zeneca, Bayer, BMS, Cepheid, Ferring, Ipsen, Jansen, Lilly, MSD, Olympus, Pfizer, Pierre Fabre, Roche, Sanochemia, Sanofi, Takeda, Urogen, and Wolff. Yair Lotan is a consultant for Nanorobotics (consultation starting 5/21), C2I genomics (consultaation starting 10/20), Photocure: Consultant/advisor, scientific study or trial, Astra-Zeneca: Consultant/advisor, Merck: consultant/advisor, Fergene: Consultant, Abbvie: consultant (starting 6/20), Cleveland Diagnostics; consultant, Nucleix consultant, Ambu: consultant, Seattle Genetics: consultant (agreement 12/20), Hitachi: 1/20, Ferring Research: 2/20, verity pharmaceuticals, virtuoso surgical (started 3/21), Nanorobot (started 6/21), Stimit (started 7/21). Research Abbott: Scientific study or trial completed 3/19, Cepheid: Scientific study or trial, Pacific Edge: Scientific study or trial, FKD: Scientific study or trial (completed 2019), MDxHealth: Scientific study or trial, Biocancell: Scientific study or trial (ended 1/20), GenomeDx Biosciences, Inc.: Scientific study or trial, Storz: scientific Study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Burger M, Compérat EM, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)–2019 update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Abdollah F, Gandaglia G, Thuret R, et al. Incidence, survival, and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol. 2013;37:219–225. doi: 10.1016/j.canep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67:241–249. doi: 10.1016/j.eururo.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kluth LA, Black PC, Bochner BH, et al. Prognostic and prediction tools in bladder cancer: a comprehensive review of the literature. Eur Urol. 2015;68:238–253. doi: 10.1016/j.eururo.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Mori K, Mostafaei H, Enikeev DV, et al. Differential effect of sex on outcomes after radical surgery for upper tract and bladder urothelial carcinoma: a systematic review and meta-analysis. J Urol. 2020;204:58–62. doi: 10.1097/JU.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Abufaraj M, Mostafaei H, et al. A systematic review and meta-analysis of variant histology in urothelial carcinoma of the bladder treated with radical cystectomy. J Urol. 2020;204:1129–1140. doi: 10.1097/JU.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 8.Karakiewicz PI, Shariat SF, Palapattu GS, et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol. 2006;176(4 Pt 1):1354–1361. doi: 10.1016/j.juro.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51:137–149. doi: 10.1016/j.eururo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Miura N, Mostafaei H, et al. Prognostic value of preoperative hematologic biomarkers in urothelial carcinoma of the bladder treated with radical cystectomy: a systematic review and meta-analysis. Int J Clin Oncol. 2020;25:1459–1474. doi: 10.1007/s10147-020-01690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong DH, Kim YK, Kim MR, Jang JH, Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int J Mol Sci. 2018;19(4):1057. doi: 10.3390/ijms19041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shariat SF, Anwuri VA, Lamb DJ, Shah NV, Wheeler TM, Slawin KM. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy. J Clin Oncol. 2004;22:1655–1663. doi: 10.1200/JCO.2004.09.142. [DOI] [PubMed] [Google Scholar]
- 13.Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1): an increasing insight into its role in tumorigenicity and metastasis. Int J Cancer. 2015;136:2504–2514. doi: 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- 14.Scalici JM, Arapovic S, Saks EJ, et al. Mesothelium expression of vascular cell adhesion molecule-1 (VCAM-1) is associated with an unfavorable prognosis in epithelial ovarian cancer (EOC) Cancer. 2017;123:977–984. doi: 10.1002/cncr.30415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Lyu J, Bell Burdett K, et al. Prognostic and predictive biomarkers in patients with metastatic colorectal cancer receiving regorafenib. Mol Cancer Therapeut. 2020;19:2146–2154. doi: 10.1158/1535-7163.MCT-20-0249. [DOI] [PubMed] [Google Scholar]
- 16.Tas F, Karabulut S, Bilgin E, Duranyildiz D. Serum levels of vascular cell adhesion molecule-1 (VCAM-1) may have diagnostic, predictive, and prognostic roles in patients with lung cancer treated with platinum-based chemotherapy. Tumour Biol. 2014;35:7871–7875. doi: 10.1007/s13277-014-2050-6. [DOI] [PubMed] [Google Scholar]
- 17.Svatek RS, Shariat SF, Novara G, et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011;107:898–904. doi: 10.1111/j.1464-410X.2010.09628.x. [DOI] [PubMed] [Google Scholar]
- 18.D’Andrea D, Soria F, Zehetmayer S, et al. Diagnostic accuracy, clinical utility, and influence on decision-making of a methylation urine biomarker test in the surveillance of non-muscle-invasive bladder cancer. BJU Int. 2019;123:959–967. doi: 10.1111/bju.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotan Y, Shariat SF, Schmitz-Dräger BJ, et al. Considerations on implementing diagnostic markers into clinical decision-making in bladder cancer. Urol Oncol. 2010;28:441–448. doi: 10.1016/j.urolonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Shariat SF, Lotan Y, Vickers A, et al. Statistical consideration for clinical biomarker research in bladder cancer. Urol Oncol. 2010;28:389–400. doi: 10.1016/j.urolonc.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrne GJ, Ghellal A, Iddon J, et al. Serum soluble vascular cell adhesion molecule-1: role as a surrogate marker of angiogenesis. J Natl Cancer Inst. 2000;92:1329–1336. doi: 10.1093/jnci/92.16.1329. [DOI] [PubMed] [Google Scholar]
- 22.Fearnley GW, Odell AF, Latham AM, et al. VEGF-A isoforms differentially regulate ATF-2-dependent VCAM-1 gene expression and endothelial-leukocyte interactions. Mol Biol Cell. 2014;25:2509–2521. doi: 10.1091/mbc.e14-05-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 24.Garmy-Susini B, Jin H, Zhu Y, Sung RJ, Hwang R, Varner J. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–1551. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukushi J, Ono M, Morikawa W, Iwamoto Y, Kuwano M. The activity of soluble VCAM-1 in angiogenesis stimulated by IL-4 and IL-13. J Immunol Baltim Md. 1950;2000(165):2818–2823. doi: 10.4049/jimmunol.165.5.2818. [DOI] [PubMed] [Google Scholar]
- 26.Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MR, Jang JH, Park CS, et al. A human antibody that binds to the sixth Ig-like domain of VCAM-1 blocks lung cancer cell migration in vitro. Int J Mol Sci. 2017;18(3):566. doi: 10.3390/ijms18030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahdiyeh S, Frouzandeh M, Bahar M, Samira S. Study of VCAM-1 gene expression in normal and tumoral tissues in patients with colorectal cancer. J Biotechnol Biomed Sci. 2017;1:19–26. [Google Scholar]
- 29.Tripathi A, Grivas P. The utility of next-generation sequencing in advanced urothelial carcinoma. Eur Urol Focus. 2020;6:41–44. doi: 10.1016/j.euf.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Ilijazi D, Abufaraj M, Hassler MR, Ertl IE, D’Andrea D, Shariat SF. Waiting in the wings: the emerging role of molecular biomarkers in bladder cancer. Expert Rev Mol Diagn. 2018;18:347–356. doi: 10.1080/14737159.2018.1453808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.