Abstract
In this study, the modulatory effects of anthracene (ANT) and benz[a]anthracene (BEN) on biochemical markers associated with neurodegeneration were assessed in mouse hippocampal neuronal cells (HT-22). Neuronal cells were cultured and exposed to ANT and BEN (25–125 µM) for 5 days, and the cell viability was determined via MTT assay. Morphological characteristics of the cells were assessed using a compound microscope. Biochemical parameters such as acetylcholinesterase (AChE), monoamine oxidase (MAO) and adenosine deaminase (ADA) activities as well as oxidative stress biomarkers (catalase [CAT], glutathione -S- transferase [GST] activities and Glutathione [GSH] levels) and nitric oxide [NO] levels were assessed after cells were treated with ANT and BEN for two days. The results showed that cell viability reduced with an increase in exposure time. After the fifth day of treatment, BEN and ANT (125 µM) reduced percentage viability to 41 and 38.1%, respectively. Light micrographs showed shrinkage of cells, neuronal injury and cell death in cells treated with higher concentrations of BEN and ANT (50 and 125 µM). Furthermore, AChE and MAO activities reduced significantly after treatment for 48 h with ANT and BEN. A significant decrease in CAT and GST activities and low GSH levels were observed after treatment with BEN and ANT. However, both polycyclic aromatic hydrocarbons caused a significant increase in ADA activity and NO levels. These results suggest that ANT and BEN may induce neurodegeneration in neuronal cells via oxidative stress-induced-neuronal injury, disruption of cholinergic, monoaminergic and purinergic transmission, and increased nitric oxide levels.
Keywords: Anthracene, Benz[a]anthrancene, Polycyclic aromatic hydrocarbons, Neurodegeneration, Oxidative stress, Cholinergic deficit, Antioxidant enzymes
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are persistent organic pollutants with carcinogenic and mutagenic features [1]. These compounds consist of carbon and hydrogen atoms devoid of substitution branch with additional nonpolar and neutral characteristics, making them cumbersome for different organisms to degrade [1, 2]. Many PAHs' persistent nature has caused a global environmental issue as they occur as air, water, and soil pollutants. Some PAHs are anthropogenic in nature and are mostly released into the environment due to the partial combustion of organic matter [3, 4]. Humans are exposed to PAHs via ingestion, inhalation and skin absorption, making these compounds exact their toxic effects on different organs in the body [4]. A previous report has shown that exposure to PAHs may trigger cardiovascular diseases and pulmonary problems [5]. Some PAHs also induced immunotoxicity, reproductive toxicity and endocrine-disrupting effects [6–9]. They have also been reported to induce neurotoxicity in the central nervous system [4, 10].
Moreover, most studies have been on the neurotoxicity of benzo[a]pyrene via an induction of oxidative stress [11], neurobehavioral impairment [12–14], developmental neurotoxicity [15], changes in synaptic dopamine levels [16] and a disruption of cholinergic and serotonergic transmission [17]. Exposure to phenanthrene also induced reactive oxygen species production, changes in acetylcholinesterase activity [18] and neurobehavioral deficit vial alteration in neuronal connectivity and muscle function in Zebrafish [19]. Anthracene (ANT) is a three-ring PAH and a significant component of coal tar [20]. Though some reports have considered it non-carcinogenic; however, it exhibits a toxic response in aquatic systems [21, 22]. However, there are some indications that anthracene and Benz[a]anthracene exhibit potential carcinogenic and genotoxic effects [23, 24]. Benz[a]athracene (BEN) is one of the components of tobacco smoke [25]. A previous report showed that BEN caused developmental and cardiovascular toxicities in a zebrafish model [26]. Exposure to BEN also induced developmental toxicity and DNA damage in larvae of Japanese medaka [27]. However, there are limited studies on the neurotoxic actions of ANT and BEN. In this study, we investigated the neurotoxic effect of ANT and BEN in hippocampal neuronal cells via an assessment of neuronal oxidative stress and neurochemicals associated with neurodegeneration.
Materials and methods
Chemicals
Fetal bovine serum (FBS), phosphate-buffered saline, epinephrine, trichloroacetic acid, Acetylcholine iodide, 5,5′-dithiobisnitrobenzoic acid (DTNB), Griess reagent and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethylsulfoxide (DMSO) were obtained from Sigma Aldrich (St Louis, USA). Anthracene and benz[a]athracene were also sourced from Sigma Aldrich (Germany).
Cell culture
Hippocampal neuronal cell lines (Salk Institute, La Jolla, USA) were cultured in Dulbecco's modified eagle medium, fetal bovine serum (10%) and penicillin with streptomycin (2%) kept in a CO2 incubator set at 37 °C. After the cells had grown to about 60–70% confluence, they were trypsinized and plated in 96 or 24 well plates depending on the experiment. The cells were plated into the following groups: Control [Cont], Vehicle [Veh], 25 µM (0.025% DMSO) Athrancene [ANT25], 50 µM [ANT50] and 125 µM Anthracene [ANT125], 25 µM benz[a]anthracene [BEN25], 50 µM benz[a]athracene [BEN50] and 125 µM benz[a]athracene [BEN125]. The cells were incubated in a growth medium containing 0.025% DMSO (Veh), BEN and ANT [28].
Cell viability assay
Cells were plated in 96-well plates at 37 °C in a humidified atmosphere at 5% CO2 and treated differently with ANT and BEN (25–125 µM) as shown in the groupings above for 24, 72 and 120 h. The medium in each well was changed every 48 h and replaced with a fresh medium. After each experiment (48, 72 and 120 h), a fresh medium was added to each well alongside MTT (20 µL, 1 mg/mL) and was incubated for 4 h. After the incubation period, the solution was aspirated, and 100 µL of DMSO was added to dissolve the formazan crystals formed. A microplate reader was used to measure the absorbance of the solution at 570 nm. Cell viability was calculated and expressed as the percentage absorbance of treated cells relative to untreated cells.
Microscopic analysis
Cells were plated in 24-well plates and were treated according to the treatment groups stated above. The plates were incubated separately for 24, 72 and 120 h. The medium was removed every 24 h and replaced with fresh medium and appropriate treatments. The plates were viewed using the phase-contrast imaging of a Compound Fluorescent Microscope (Olympus Eclipse 80i, Japan) with Nikon Digital Sight DS-Fi1 Camera.
Harvesting of cells and homogenate preparation
Cells were treated with BEN and ANT in 24-well plates for 48 h using the above treatment groupings. The cells were harvested using a lysis buffer, homogenized at 12,000 × g, and the supernatant fraction was taken and kept at − 20 °C for further analysis. The protein content of cell homogenates from each treatment group, control and vehicle, was determined using the Pierce BCA protein assay kit (Thermoscientific).
Assay of nitric oxide levels
One hundred microliters of the samples were mixed with 100 µL of Griess reagent and a mixture of vanadium chloride (200 µL, 0.2%) and HCl (5%). The solution was incubated for 1 h at 37 °C, and absorbance was read at 548 nm. NO produced was measured through the reduction of nitrate to nitrite by vanadium chloride. Nitrite produced was measured as micromol. per milligram protein.
Determination of catalase (CAT) activity
Catalase activity in the homogenate samples was determined according to the method of Sinha [29]. In brief, 25 µL of each tissue homogenate sample was reacted with 0.1 mL 2 M H2O2 in the presence of 0.25 mL 0.01 M phosphate buffer (pH 7.0). The reaction was stopped by the addition of 0.4 mL dichromate acetic acid. The absorbance of the reaction mixture was measured at 620 nm. A standard curve was prepared by reacting 0.4 mL of 2 M H2O2 with 2 mL dichromate acetic acid in the presence of 1.0 mL 0.01 M sodium phosphate buffer (pH 7.0). The catalase activity was calculated and expressed as µmol H2O2 consumed/mg protein.
Determination of glutathione-s-transferase activity
According to Habig et al. [30], this assay was carried out with slight modification. It involved the pre-incubation of reaction mixture containing 0.1 mL of 0.25 M phosphate buffer (pH 6.5), 25 mM 1- chloro-2,4-dinitrobenzene (CDNB) and 0.7 mL of distilled water for 5 min at 37 °C. The reaction was started by adding 25 µL of the tissue homogenate and 20 µL of 25 mM glutathione as substrate. The absorbance of the reaction mixture was monitored after 5 min at 340 nm in a spectrophotometer. The reaction mixture without enzyme was used as a blank. The activity of GST was calculated and expressed as µmol/min/mg protein.
Estimation of glutathione content
Trichloroacetic acid (10%) was added to the cell homogenates to achieve deproteinization. The solution obtained was centrifuged for 5 min at a speed of 3500 rpm. The supernatant (100 µL) obtained was removed and placed in 96 well plates followed by the addition of 50 µL of 5,5-dithio-bis(2-nitrobenzoic) acid (DTNB). After 5 min, the absorbance of the yellow solution formed was measured at 415 nm [30]. A standard curve was obtained to measure the concentration of GSH.
Determination of reactive oxygen species (ROS) production
Intracellular ROS levels were measured in treated and untreated cells using 2,7-dichlorohydrofluorescin diacetate (H2DCFDA) as an indicator. Cells were seeded in 96 well plates and were treated differently with 25, 50 and 125 µM of ANT and BEN. Control cells were not treated with the PAHs. After 48 h, 10 µM H2DCFDA was added to the control and treated cells, respectively. The plate was incubated for 30 min in the dark at 37 °C. ROS level was determined by measuring fluorescence intensity in a microplate reader at excitation (488 nm) and emission (525 nm) spectra. ROS produced was measured as percentage control.
Apoptosis assay
Cell death was determined in PAH-treated neuronal cells using ethidium bromide and acridine orange dual stains. Neuronal cells were seeded in 6 well plates, and after 24 h, they were treated with 25, 50 and 125 µM of ANT and BEN. Control cells were not treated with PAHs. After 48 h, the cells were rinsed with PBS and 20 µL of prepared ethidium bromide (100 mg/mL) and acridine orange (100 mg/mL) (1:1) solution was added to each well. Each well was viewed using a Compound Fluorescent Microscope (Olympus Eclipse 80i, Japan) with Nikon Digital Sight DS-Fi1 Camera [31].
Acetylcholinesterase (AChE) activity assay
Acetylcholinesterase activity was assayed according to the method described by Ellman et al. [32]. The reaction mixture was made up of 50 μL of distilled water, 50 μL of 0.1 M potassium phosphate buffer (pH 7.4), 30 μL of DTNB, 25 μL of tissue homogenate, and 30 μL of 8 mM acetylthiocholine. After that, the reaction was monitored for 5 min at 412 nm in a spectrophotometer. The AChE activity was calculated and expressed as mmolAcSch/h/mg protein.
MAO activity assay
MAO activity in the cell homogenates was determined using an MAO activity assay kit (Sigma, St Louis USA) following the manufacturer's instruction. The kit uses a fluorimetric method to determine MAO activity when p-tyramine, a substrate of MAO-A and MAO-B, reacts with the enzyme.
Adenosine deaminase (ADA) activity assay
In this assay, 21 mmol/L of adenosine was added to 50 µL brain homogenate reacted with 21 mmol/L of phosphate buffer (pH 6.5) and incubated for 60 min at 37 °C. The amount of ammonia produced was monitored, and ADA activity was evaluated.
Statistical analysis
Graph Pad Prism (San Diego, CA, USA) was used to analyse all the data obtained from the study. One way analysis of variance (ANOVA) was used to analyze the mean, while appropriate post-hoc treatment was applied. Significant differences were observed at p < 0.05 while the data were expressed as mean ± SD. The bivariate relationship among the biomarker variables was determined by correlation analysis using R-Package.
Results
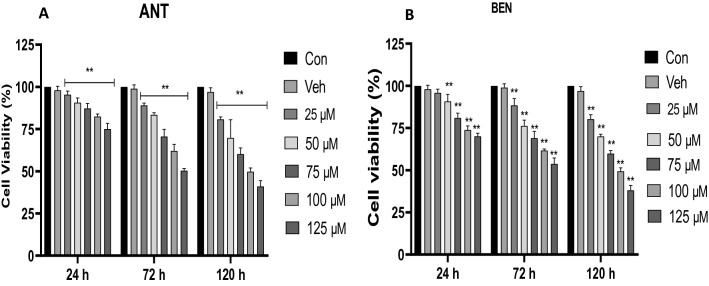
Figures 1a, b reveal the effect of ANT and BEN on cell viability after exposure to HT-22 cells for 24–120 h. Exposure to ANT reduced cell viability and was concentration-dependent. At the highest concentration (125 µM), 75% cell viability was observed after 24 h. However, after exposure for 72 h, the percentage of viable cells reduced to 50.4% at the highest concentration tested (125 µM). A further reduction in percentage cell viability (41.0%) was observed at the highest dose tested after exposure for 120 h (Fig. 1a). Similarly, treatment with BEN (25–125 µM) also reduced cell viability after 24–120 h. After 120 h treatment, a percentage cell viability of 38.1% was observed at the highest concentration tested (125 µM).
Fig. 1.
Percentage cell viability of HT-22 cells after treatment with 25–125 µM of (a) ANT and (b) BEN for 24–72 h. Cells were seeded in 96 well plates, treated with ANT and BEN and incubated for 24–72 h. All data are presented as mean ± SD; n = 3. **p < 0.05 vs Con and Veh
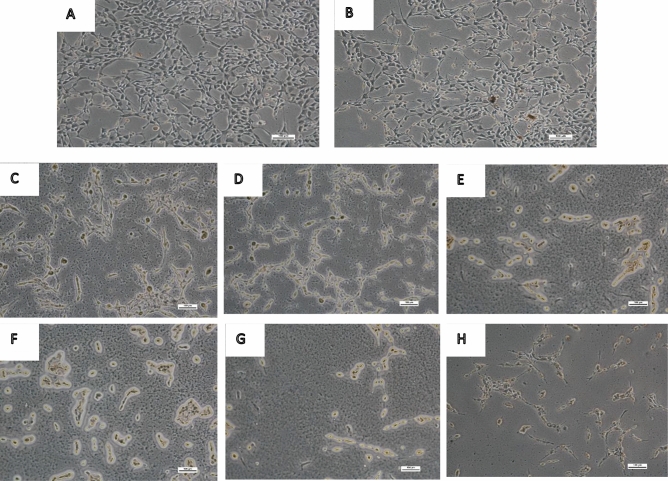
Figure 2 reveals the microscopic examination of HT-22 cells exposed to ANT and BEN (25, 50 and 125 µM) for 48 h. The control group showed viable cells and rapid growth (Fig. 2a). Cells in Fig. 2b were exposed to the vehicle and did not show any shrinkage. However, Fig. 2c, d (cells treated with 25 µM of ANT and BEN) revealed a reduction in cell proliferation and a slight shrinkage of neuronal cells. Figure. 2e, f revealed morphological features of cells treated with 50 µM of BEN and ANT, respectively, which showed a significant inhibition of cell proliferation, shrinkage of cells and neuronal injury. Similarly, treatment with a higher concentration of ANT and BEN (125 µM) also inhibited cell growth, induced neuronal injury and cell death, as shown in Fig. 2 g, h.
Fig. 2.
Microscopic examination of neuronal cells after treatment with ANT and BEN (25, 50 and 125 µM) for 48 h. Cells were seeded in 24 well plates and were treated with ANT and BEN. After 48 h, cells were observed in a microscope in a phase-contrast mode. Magnification – × 100 µm. a control cells; b vehicle; c cells treated with 25 µM ANT; d cells treated with 25 µM BEN; e cells treated with 50 µM ANT; f cells treated with 50 µM BEN; g cells treated with 125 µM ANT; h: cells treated with 125 µM BEN
BEN and ANT also triggered NO production after being exposed to HT-22 cells for 24 h (Fig. 3). NO levels observed in the control were significantly low compared to cells treated with BEN and ANT (25, 50 and 125 µM). In the control cells, 0.035 mmolNOx/mg protein was observed. However, after treatment with ANT and BEN, highest NO levels produced were 0.1023 and 0.110 mmolNOx/mg protein, respectively, at the highest concentration tested (125 µM).
Fig. 3.

Nitric oxide levels of cells after treatment with ANT and BEN for 48 h. All data are presented as mean ± SD; n = 3. **p < 0.05 vs. cont and veh; ***p < 0.05 vs 25 and 50 µM. Cont control, veh vehicle
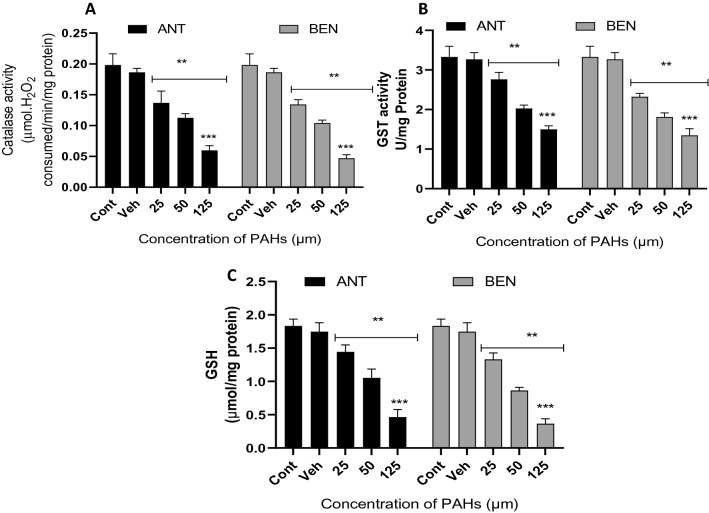
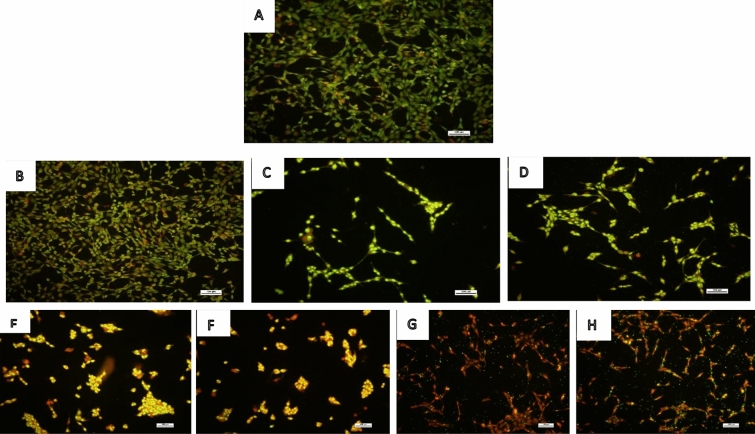
Interestingly, catalase activity was significantly reduced after treatment with different concentrations of BEN and ANT compared to the control (Fig. 4a). Catalase activity of cells in the control group was 0.198 mmol; however, after exposure to ANT and BEN, the enzyme activity reduced to 0.059 and 0.047 mmol, respectively. The result of GST activity, as shown in Fig. 4b, revealed a significant (p < 0.05) reduction in enzyme activity after treatment with ANT and BEN compared to the control. The lowest GST activity was observed in cells treated with 125 µM of ANT or BEN. Similarly, levels of GSH in the cells also reduced significantly (p < 0.05) after treatment with ANT and BEN compared to the control (Fig. 4c). In Fig. 5, ANT and BEN triggered the production of ROS in treated neuronal cells. Higher levels of ROS were produced after treatment with higher concentration (125 µM) of ANT and BEN. The levels of ROS observed in cells treated with ANT and BEN were significantly different from the vehicle control. Figure. 6 shows fluorescence micrographs of control and treated neuronal cells with ANT and BEN (25, 50 and 125 µM). Live cells were observed in the control and vehicle groups, as revealed by the green fluorescence stains in Fig. 6a, b. Bright green and light yellow stains were observed in Fig. 6c, d, which were cells treated with 25 µM of ANT and BEN, respectively. Treatment with 50 and 125 µM of ANT and BEN showed orange/yellow and red fluorescence, respectively, as depicted in Fig. 6e–h.
Fig. 4.
Activities of CAT and GST and GSH levels in cells treated with ANT and BEN for 48 h. All data are presented as mean ± SD; n = 3. **p < 0.05 vs. cont and veh; ***p < 0.05 vs 25 and 50 µM. Cont control, veh vehicle
Fig. 5.

ROS levels in control and cells treated with ANT and BEN (25, 50 and 125 µM) after 48 h treatment. All data are shown as the mean ± SD; n = 3. **p < 0.05 vs. cont and veh; ***p < 0.05 vs 25 and 50 µM. Cont control, veh vehicle
Fig. 6.
Representation of fluorescence micrographs of cells stained with acridine orange and ethidium bromide mixture after treatment of ANT and BEN. Magnification – × 100 µm. Cells were seeded in 6-well plate and after 24 h, they were treated with ANT and BEN (25, 50 and 125 µM). After, 48 h, cells were stained with a mixture of acridine orange and ethidium bromide (1:1). a control cells; b cells treated with vehicle, c cells treated with 25 µM ANT; d cells treated with 25 µM BEN; e cells treated with 50 µM ANT; f cells treated with 50 µM BEN; g cells treated with 125 µM ANT; h cells treated with 125 µM BEN
Figure 7a reveals the AChE activity of hippocampal neuronal cells exposed to ANT and BEN (25, 50 and 125 µM) for 48 h. Treatment with both PAHs significantly reduced AChE activity in the cells compared to cells in the control group and vehicle. The reduction of AChE activity in the cells, triggered by ANT and BEN, was concentration-dependent. The decrease in AChE activity was observed with an increase in the concentration of ANT and BEN.
Fig. 7.
a AChE, b MAO and c ADA activities of neuronal cells treated with ANT and BEN (25, 50 and 125 µM) for 48 h. All data are presented as mean ± SD; n = 3. **p < 0.05 vs. cont and veh; ***p < 0.05 vs 25 and 50 µM. Cont control, veh vehicle
Similarly, a decrease in MAO activity was also observed after treatment with BEN and ANT (Fig. 7b). Lower MAO activity was observed in cells exposed to BEN and ANT (25, 50 and 125 µM) compared to the vehicle and control group. The lowest AChE activity was observed after treatment with BEN and ANT (125 µM).
Adenosine activity was assessed in hippocampal neuronal cells after treatment with ANT and BEN (25, 50 and 125 µM) for 48 h. A significant (p < 0.05) difference in ADA activity was observed between cells in the control group and cells treated with ANT and BEN (25, 50 and 125 µM) (Fig. 7c). The PAHs triggered a significant increase in ADA activity after 48 h exposure. The increase in ADA activity in cells exposed to ANT and BEN was concentration-dependent, and the highest enzyme activity was observed at the highest dose (125 µM) administered (Fig. 7c).
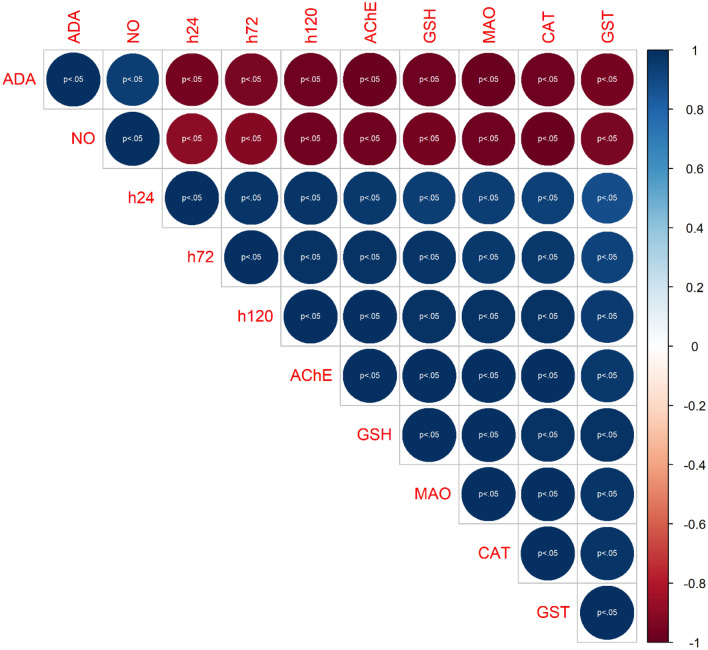
Figure 8 shows the correlation relationship between the biomarker variables investigated in this study, including cell viability. The result showed a strong relationship between the biomarkers except ADA and NO. AChE showed a strong correlation with cell viability for both PAH treatments. Furthermore, MAO and oxidative stress biomarkers also showed a strong correlation with cell viability. However, a significantly weak correlation was observed for NO and ADA compared with cell viability and other biomarkers.
Fig. 8.
Visual representation of bivariate correlation between biomarker variables. ADA Adenosine deaminase, AChE acetylcholinesterase, GSH glutathione, MAO monoamine oxidase, CAT catalase activity, glutathione-S-transferase, h24 cell viability at 24 h, h72 cell viability at 72 h, h120 cell viability at 120 h
Discussion
Some polycyclic aromatic hydrocarbons, such as Benzo [a] pyrene, anthracene, fluoranthene, are potent carcinogens and mutagenic agents [3]. However, the neurotoxic effect of most of these compounds has not been reported. This study investigated the neurotoxic effect of anthracene and benz[a]athrancene in hippocampal neuronal cell lines. It has been established that exposure to some PAHs may induce behavioural changes, learning and memory problems, including cognitive disorders [33]. In this study, BEN and ANT reduced viable cells after exposure for 24–120 h. Though the reduction rate was minimal after 24 h, however, prolonged exposure time alongside a high concentration of BEN and ANT caused a significant decrease in viable cells. The observed reduction in viable cells suggests an inhibition of cell proliferation associated with cell death caused by the PAHs. Our results contradict the report of Tang et al. [34], which revealed that anthracene did not induce cytotoxic effects in neuroblastoma cells. Benzo[a]pyrene induced cytotoxicity in neuroblastoma cells; however, anthracene and chrysene did not induce any cytotoxic effect on the same cells. Higher concentrations of anthracene were used in this study compared to the work of Tang et al. [34].
A previous investigation revealed that exposure to PAHs may alter cell morphology [35]. The light microscopic analysis of cells exposed to BEN and ANT obtained from this study correlated with the reduction of cell viability observed from the MTT assay. Exposure to BEN and ANT triggered shrinkage of cells, cell detachment, neuronal damage, and ultimately cell death in HT-22 cells. Furthermore, inhibition of cell growth was observed, which could be associated with cell death induced by ANT and BEN. Cells exposed to higher concentrations of ANT and BEN with long exposure time exhibited neuronal damage and apoptosis than cells exposed to low concentrations (25 µM).
The observed shrinkage of cells, neuronal damage and cell death could be linked with nitrosative and oxidative stress. Treatment with ANT and BEN triggered an increase in NO levels in the cell homogenates. A high concentration of NO may trigger nitrosative stress, leading to neuroinflammation and oxidative injury [36]. Nitrosative stress is triggered by the overproduction of nitric oxide and the inability of the cells to neutralize, eliminate, or reduce it [36, 37]. Nitric oxide may react with other reactive oxygen species such as superoxide anions produced during oxidative burst and inflammatory process leading to the formation of more harmful radicals known as peroxynitrites [36]. These harmful radicals are potent oxidizing agents capable of causing DNA fragmentation and inducing lipid peroxidation [38].
Furthermore, the reduction of catalase and GST activities and levels of GSH in cells exposed to ANT and BEN suggest an imbalance in the antioxidant defense system of the neuronal cells, which could be triggered by the overproduction of reactive oxygen species. Previous investigations confirmed that some PAHs triggered oxidative stress caused by the overproduction of reactive oxygen species, leading to the activation of apoptotic signalling processes [39, 40]. The observed reduction in CAT and GST activities and GSH levels suggests the inhibitory effects of ANT and BEN on the antioxidant enzymes and proteins. Catalase plays an important role in the antioxidant defense system by detoxifying harmful radicals to form hydrogen peroxide and water. GST utilizes GSH to transform oxidative stress products to less harmful products. The inhibition of CAT and GST activities and the reduction of GSH levels caused by ANT and BEN may contribute to the observed oxidative injury, neuronal damage, and cell death observed in the microscopic analysis. Furthermore, neuronal cells exhibit low antioxidant defense mechanisms due to high consumption of metabolic oxygen and high levels of polyunsaturated fatty acids. This may further reduce the antioxidant enzymes that were unable to enforce an attack against reactive oxygen species generated by ANT and BEN.
The increase in ROS produced after treatment with ANT and BEN revealed redox imbalance in the neuronal cells compared to the control cells. Higher levels of ROS were observed in cells treated with a higher concentration of ANT and BEN. High levels of ROS have been linked with oxidative stress. ROS have been implicated in the initiation and contribution to cell death [36]. Neuronal cells are prone to ROS production and oxidative stress due to a higher consumption of oxygen and high levels of polyunsaturated fatty acids [41]. The high generation of ROS in the neuronal cells treated with ANT and BEN correlates with early and late neuronal apoptosis and necrosis revealed by the fluorescence micrographs. The ethidium bromide and acridine orange dual staining method is a common technique used to assess cell death. Acridine orange permeates live neuronal cells and emits green fluorescence, while ethidium bromide stains non-viable cells and emits yellow to red fluorescence depending on the stage of neuronal apoptosis [42]. The observed green fluorescence in the control cells revealed the viable cells while the bright green fluorescence observed in neuronal cells treated with 25 µM of ANT and BEN suggest early neuronal apoptosis. After treatment with 50 µM of ANT and BEN, shrinkage of cells was observed and condensation of chromatin followed by yellow fluorescence which revealed that the cells were in late apoptosis. Necrotic cells were observed after treatment with 125 µM of ANT and BEN as shown by the red fluorescence. The fluorescence micrographs revealed that ANT and BEN (50 and 125 µM) may induce late apoptosis and necrosis in brain cells.
Acetylcholinesterase is an important enzyme in the cholinergic system which mediates cholinergic function via the regulation of acetylcholine levels in the synaptic cleft [43]. Acetylcholine is required to transmit nerve impulses from one neuron to another [31, 44]. Hence, an inhibition of acetylcholinesterase may disrupt cholinergic function and neurotransmission and impair memory function and learning activities. In this study, treatment with ANT and BEN rapidly reduced acetylcholinesterase activity in HT-22 cells compared with the control. This result correlates with the reports of Gauthier et al. [18], Vieira et al. [45] and Palanikumar et al. [46], which showed that some PAHs, including anthracene, reduced AChE activity in different models. Currently, the mechanism of action of ANT and BEN for AChE inhibition is unknown. However, previous studies have indicated that some PAHs exhibit mechanisms of action similar to organophosphate pesticides [18, 47]. Cytochrome-P450 enzymes metabolize most organophosphate pesticides to form an oxygenated analogue that phosphorylates the beta-anionic containing ester in the region of the active site of AChE. Nevertheless, most PAHs act on AChE by binding nitro-aromatic compounds at the enzyme's active site [18, 48].
BEN and ANT also reduced monoamine oxidase activity in HT-22 cells compared to the control. Monoamine oxidase is an important regulator of monoaminergic neurotransmission, which controls mood and emotional behaviour, including perception and cognitive functions in the central nervous system [49, 50]. Monoamine oxidase activates the degradation of dietary amines, including serotonin (5-Hydroxytryptamine), tryptamine, dopamine, epinephrine and norepinephrine, tyramine and octopamine. These brain monoamines (dopamine, serotonin, and norepinephrine) act as synaptic neurotransmitters during monoaminergic signalling [51, 52]. Alteration in monoamine oxidase activity may deplete levels of these neurotransmitters, disrupt monoaminergic transmission and induce mitochondrial dysfunction and contribute to the development of neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease [53, 54]. In this study, we examined monoamine oxidase-A activity in neuronal cells treated with BEN and ANT. A significant increase in MAO-A activity was observed after treatment with BEN and ANT compared to the control. This suggests an excessive enzyme activity that may rapidly deplete monoaminergic neurotransmitters, norepinephrine and dopamine. Stephanou et al. [55] reported that treatment with bezo[a]pyrene triggered a decrease in dopamine and norepinephrine in different regions of the brain. This was attributed to an increase in the activity of monoaminergic enzymes. Furthermore, high MAO activity has also been linked with toxic metabolic byproducts such as hydrogen peroxide, ammonia, and amines. These byproducts are capable of activating free radical formation which can trigger oxidative damage to neuronal cells. Hence, a high MAO activity may contribute to radical-induced oxidative damage in the neuronal cells.
Similarly, a high ADA activity was also observed in the neuronal cells after treatment with BEN and ANT. An increase in ADA activity will deplete adenosine levels in the cells. A high ADA activity was also reported in rats' liver after treatment with 7, 12 – dimethylbenz[a]anthracene [56]. ADA is an important enzyme in the purinergic signalling pathway as it activates the enzymatic deamination of adenosine to inosine, hence, regulating adenosine levels [57]. Adenosine is formed from ATP metabolism and has been identified as a regulator of neuronal excitability by reducing excitatory transmission [58, 59]. Furthermore, adenosine also regulates synaptic neurotransmission, and adenosine receptors have been shown to improve memory function [57, 60]. The observed high ADA activity in this study suggests a disruption in purinergic signalling and regulation of adenosine triggered by the treatment with ANT and BEN. Moreover, a high ADA activity will reduce homeostatic levels of adenosine which may impair purinergic signalling and synaptic transmission [61]. Disruption of adenosine levels has also been linked to behavioural problems and memory dysfunction [62]. Hence, ANT and BEN–induced neurotoxicity can be linked to activation of neuronal apoptosis, oxidative damage to neurons and disruption of cholinergic, monoaminergic and purinergic enzymes. There is also an indication that metabolic activation of ANT and BEN may contribute to their neurotoxic effects. Previous studies have established that PAHs requires metabolic activation to exhibit their toxic effects [63–65]. Song et al. [66] reported that metabolites formed from benz[a]anthracene exhibited cytotoxic and genotoxic effects. Hence one of the mechanisms of action of ANT and BEN may be linked to metabolic activation and formation of harmful metabolites.
This study revealed that exposure of ANT and BEN to HT-22 cells inhibited cell growth, induced shrinkage of cells and cell death. The observed morphological changes of the neuronal cells could be attributed to radical-induced neuronal damage as revealed by reduction of CAT and GST activities, a decrease in GSH levels and a significant increase in NO levels. Furthermore, ANT and BEN also triggered cholinergic dysfunction via an inhibition of acetylcholinesterase activity and induced high MAO and ADA activity. Our findings suggest that an increase in concentrations and long term exposure to ANT and BEN may induce neurodegeneration in neuronal cells. Moreover, Further studies on the metabolic activation of ANT and BEN is important to identify the toxic metabolites responsible for their neurotoxic effects in neuronal cells.
Authors contributions
TAO and AOO conceived the work. TAO designed the experiments and performed them. TAO and AOO wrote, read and approved the manuscript.
Funding
Authors did not receive funding for the study.
Availability of data and materials
The data used in the study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Nam TH, Jeon HJ, Mo HH, Cho K, Ok YS, Lee SE. Determination of biomarkers for polycyclic aromatic hydrocarbons (PAHs) toxicity to earthworm (Eisenia fetida) Environ Geochem Health. 2015;37(6):943–951. doi: 10.1007/s10653-015-9706-z. [DOI] [PubMed] [Google Scholar]
- 2.Mastral AM, Callen MS. A Review on polycyclic aromatic hydrocarbon (PAH) emissions from energy generation. Environ Sci Technol. 2000;34(15):3051–3057. doi: 10.1021/es001028d. [DOI] [Google Scholar]
- 3.Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt J Pet. 2016;25(1):107–123. doi: 10.1016/j.ejpe.2015.03.011. [DOI] [Google Scholar]
- 4.Das SK, Aparna S, Patri M. Chronic waterborne exposure to benzo[a]pyrene induces locomotor dysfunction and development of neurodegenerative phenotypes in zebrafish. Neurosci Lett. 2020 doi: 10.1016/j.neulet.2019.134646. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Yang L, Zhang B, Liu G, Wang C, Zhang Y, Wang T. Neurobehavioral performance of PAH exposure in male coal miners in Shanxi, China: a cross-sectional study. Int Arch Occup Environ Health. 2020;93(6):707–714. doi: 10.1007/s00420-020-01521w. [DOI] [PubMed] [Google Scholar]
- 6.Ramesh A, Harris KJ, Archibong AE (2017) Reproductive and developmental toxicology. Chapter 40—reproductive toxicity of polycyclic aromatic hydrocarbons, 2nd edn. Acadmic Press, Elsevier. Edited Ramesh C Gupta. 10.1016/B978-0-12-804239-7.00040-8
- 7.Jeng HA, Pan C-H, Lin W-Y, Wu M-T, Taylor S, Chang-Chien G-P, Zhou G, Diawara N. Biomonitoring of polycyclic aromatic hydrocarbons from coke oven emissions and reproductive toxicity in nonsmoking workers. J Hazard Mater. 2013;244–245:436–443. doi: 10.1016/j.jhazmat.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Ungvári É, Monori I, Megyeri A, Csiki Z, Prokisch J, Sztrik A, Jávor A, Benkő I. Protective effects of meat from lambs on selenium nanoparticle supplemented diet in a mouse model of polycyclic aromatic hydrocarbon-induced immunotoxicity. Food Chem Toxicol. 2014;64:298–306. doi: 10.1016/j.fct.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Hart LJ, Smith SA, Smith BJ, Robertson J, Besteman EG, Holladay SD. Subacute immunotoxic effects of the polycyclic aromatic hydrocarbon 7,12-dimethylbenzanthracene (DMBA) on spleen and pronephros leukocytic cell counts and phagocytic cell activity in tilapia (Oreochromis niloticus) Aquat Toxicol. 1998;41(1–2):17–29. doi: 10.1016/s0166-445x(97)00075-1. [DOI] [Google Scholar]
- 10.Bollinger CE, McCallister M, Clark R, Rhoades R, Maguire M, Savage RE, Jiao Y, Harris KJ, Ramesh A, Lochotzki H, Hood DB (2020) Polycyclic aromatic hydrocarbons: implications for developmental, molecular, and behavioral neurotoxicity. In: Handbook of toxicology of chemical warfare agents, pp 279–297. Academic Press, Elsevier. Edited by Ramesh C. Gupta. 10.1016/b978-0-12-819090-6.00019-2
- 11.Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol. 2006;26(5):427–438. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- 12.Cheng S-Q, Xia Y-Y, He J-L, Liu X-Q, Chen X-M, Ding Y-B, Wang Y-X, Peng B, Tu B-J. Neurotoxic effect of subacute benzo(a)pyrene exposure on gene and protein expression in Sprague-Dawley rats. Environ Toxicol Pharmacol. 2013;36(2):648–658. doi: 10.1016/j.etap.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Li C-L, Tu B-J, Yang K, Mo T-T, Zhang R-Y, Cheng S-Q, Chen C-Z, Jiang X-J, Han T-L, Peng B, Baker PN, Xia Y-Y. Integrated epigenetics, transcriptomics, and metabolomics to analyze the mechanisms of Benzo[a]pyrene neurotoxicity in the hippocampus. Toxicol Sci. 2018;166(1):65–81. doi: 10.1093/toxsci/kfy192. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Tang Y, Jiang X, Qi Y, Cheng S, Qiu C, Peng B, Tu B. Early postnatal Benzo(a)pyrene exposure in Sprague-Dawley rats causes persistent neurobehavioral impairments that emerge postnatally and continue into adolescence and adulthood. Toxicol Sci. 2012;125(1):248–261. doi: 10.1093/toxsci/kfr265. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y-C, Wu C-Y, Hu C-H, Pai T-W, Chen Y-R, Wang W-D. Integrated hypoxia signaling and oxidative stress in developmental neurotoxicity of Benzo[a]Pyrene in Zebrafish Embryos. Antioxidants. 2020;9(8):731. doi: 10.3390/antiox9080731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K, Jiang X, Cheng S, Bai L, Xia Y, Chen C, Meng P, Wang J, Li C, Tang Q, Cao X, Tu B. Synaptic dopamine release is positively regulated by SNAP-25 that involves in benzo[a]pyrene-induced neurotoxicity. Chemosphere. 2019;237:124378. doi: 10.1016/j.chemosphere.2019.124378. [DOI] [PubMed] [Google Scholar]
- 17.Slotkin TA, Skavicus S, Ko A, Levin ED, Seidler FJ. The Developmental neurotoxicity of tobacco smoke can be mimicked by a combination of nicotine and Benzo[a]Pyrene: effects on cholinergic and serotonergic systems. Toxicol Sci. 2019;167(1):293–304. doi: 10.1093/toxsci/kfy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauthier PT, Norwood WP, Prepas EE, Pyle GG. Behavioural alterations from exposure to Cu, phenanthrene, and Cu-phenanthrene mixtures: linking behaviour to acute toxic mechanisms in the aquatic amphipod, Hyalella azteca. Aquat Toxicol. 2016;170:377–383. doi: 10.1016/j.aquatox.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Chen Y, Huang C, Dong Q, Roper C, Tanguay RL, Zhu Y, Zhang Y. Neurodevelopmental toxicity assessments of alkyl phenanthrene and Dechlorane Plus co-exposure in zebrafish. Ecotoxicol Environ Saf. 2019;180:762–769. doi: 10.1016/j.ecoenv.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 20.Lawal AT. Polycyclic aromatic hydrocarbons. A review. Cogent Environ Sci. 2017;3(1):1339841. doi: 10.1080/23311843.2017.1339841. [DOI] [Google Scholar]
- 21.Honda M, Suzuki N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int J Environ Res Public Health. 2020;17(4):1636. doi: 10.3390/ijerph17041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badreddine S, Abdelhafidh K, Dellali M, Mahmoudi E, Sheehan D, Hamouda B. The effects of anthracene on biochemical responses of Mediterranean mussels Mytilus galloprovincialis. Chem Ecol. 2017;33(4):309–324. doi: 10.1080/02757540.2017.1309393. [DOI] [Google Scholar]
- 23.Rengarajan T, Rajendran P, Nandakumar N, Lokeshkumar B, Rajendran P, Nishigaki I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac J Trop Biomed. 2015;5(3):182–189. doi: 10.1016/s2221-1691(15)30003-4. [DOI] [Google Scholar]
- 24.Katz IS, Albuquerque LL, Suppa AP, da Silva GB, Jensen JR, Borrego A, Massa S, Starobinas N, Cabrera WH, De Franco M, Borelli P, Ibanez OM, Ribeiro OG. 7,12-Dimethylbenz(a)anthracene-induced genotoxicity on bone marrow cells from mice phenotypically selected for low acute inflammatory response. DNA Repair. 2016;37:43–52. doi: 10.1016/j.dnarep.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Rodgman A, Perfetti T. The composition of cigarette smoke: a catalogue of the polycyclic aromatic hydrocarbons. Beiträge zur Tabakforschung. 2006;22(1):13–69. doi: 10.2478/cttr-2013-0817. [DOI] [Google Scholar]
- 26.Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217(3):308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Le Bihanic F, Sommard V, Perrine DL, Pichon A, Grasset J, Berrada S, Budzinski H, Cousin X, Morin B, Cachot J. Environmental concentrations of benz[a]anthracene induce developmental defects and DNA damage and impair photomotor response in Japanese medaka larvae. Ecotoxicol Environ Saf. 2015;113:321–328. doi: 10.1016/j.ecoenv.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Smith J, Neupane R, McAmis W, Singh U, Chatterjee S, Raychoudhury S. Toxicity of polycyclic aromatic hydrocarbons involves NOX2 activation. Toxicol Rep. 2019;6:1176–1181. doi: 10.1016/j.toxrep.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha A. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 30.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 31.Olasehinde TA, Olaniran AO, Okoh AI. Neuroprotective effects of some seaweeds against Zn - induced neuronal damage in HT-22 cells via modulation of redox imbalance, inhibition of apoptosis and acetylcholinesterase activity. Metab Brain Dis. 2019;34(6):1615–1627. doi: 10.1007/s11011-019-00469-2. [DOI] [PubMed] [Google Scholar]
- 32.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 33.Peiffer J, Cosnier F, Grova N, Nunge H, Salquebre G, Decret MJ, Cossec B, Rychen G, Appenzeller BM, Schroeder H. Neurobehavioral toxicity of a repeated exposure (14 days) to the airborne polycyclic aromatic hydrocarbon fluorene in adult Wistar male rats. PLoS ONE. 2013;8(8):e71413. doi: 10.1371/journal.pone.0071413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y, Donnelly KC, Tiffany-Castiglioni E, Mumtaz MM. Neurotoxicity of polycyclic aromatic hydrocarbons and simple chemical mixtures. J Toxicol Environ Health A. 2003;66(10):919–940. doi: 10.1080/15287390306455. [DOI] [PubMed] [Google Scholar]
- 35.Bai H, Wu M, Zhang H, Tang G. Chronic polycyclic aromatic hydrocarbon exposure causes DNA damage and genomic instability in lung epithelial cells. Oncotarget. 2017;8(45):79034–79045. doi: 10.18632/oncotarget.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Fukuto FM, J, Wink DA, The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem. 2004;385:1–10. doi: 10.1515/BC.2004.001. [DOI] [PubMed] [Google Scholar]
- 38.Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arterioscler Thromb Vasc Biol. 2000;20(7):1716–1723. doi: 10.1161/01.atv.20.7.1716. [DOI] [PubMed] [Google Scholar]
- 39.Sarma SN, Blais JM, Chan HM. Neurotoxicity of alkylated polycyclic aromatic compounds in human neuroblastoma cells. J Toxicol Environ Health A. 2017;80(5):285–300. doi: 10.1080/15287394.2017.1314840. [DOI] [PubMed] [Google Scholar]
- 40.Patri M, Singh A. Protective effects of noradrenaline on benzo[a]pyrene-induced oxidative stress responses in brain tumor cell lines. In Vitro Cell Dev Biol Anim. 2019;55(8):665–675. doi: 10.1007/s11626-019-00378-9. [DOI] [PubMed] [Google Scholar]
- 41.Olasehinde TA, Olaniran AO, Okoh AI. Sulfated polysaccharides of some seaweeds exhibit neuroprotection via mitigation of oxidative stress, cholinergic dysfunction and inhibition of Zn - induced neuronal damage in HT-22 cells. BMC Complement Med Ther. 2020;20(1):251. doi: 10.1186/s12906-020-03047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maney V, Singh M. An in vitro assessment of novel chitosan/bimetallic PtAu nanocomposites as delivery vehicles for doxorubicin. Nanomedicine. 2017;12:2625–2640. doi: 10.2217/nnm-2017-0228. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer's disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14(1):101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanciu GD, Luca A, Rusu RN, Bild V, Beschea Chiriac SI, Solcan C, Bild W, Ababei DC. Alzheimer's disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules. 2019;10(1):40. doi: 10.3390/biom10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vieira LR, Sousa A, Frasco MF, Lima I, Morgado F, Guilhermino L. Acute effects of Benzo[a]pyrene, anthracene and a fuel oil on biomarkers of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Sci Total Environ. 2008;395(2–3):87–100. doi: 10.1016/j.scitotenv.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 46.Palanikumar L, Kumaraguru AK, Ramakritinan CM, Anand M. Biochemical response of anthracene and benzo [a] pyrene in milkfish Chanos chanos. Ecotoxicol Environ Saf. 2012;75(1):187–197. doi: 10.1016/j.ecoenv.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 47.Holth TF, Tollefsen KE. Acetylcholine esterase inhibitors in effluents from oil production platforms in the North Sea. Aquat Toxicol. 2012;112–113:92–98. doi: 10.1016/j.aquatox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Pohanka M. Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155(3):219–229. doi: 10.5507/bp.2011.036. [DOI] [PubMed] [Google Scholar]
- 49.Naoi M, Riederer P, Maruyama W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: genetic and environmental factors involved in type A MAO expression. J Neural Transm. 2016;123(2):91–106. doi: 10.1007/s00702-014-1362-4. [DOI] [PubMed] [Google Scholar]
- 50.Adefegha SA, Oboh G, Olasehinde TA. Alkaloid extracts from shea butter and breadfruit as potential inhibitors of monoamine oxidase, cholinesterases, and lipid peroxidation in rats' brain homogenates: a comparative study. Comp Clin Pathol. 2016;25:1213–1219. doi: 10.1007/s00580-016-2331-0. [DOI] [Google Scholar]
- 51.Di Giovanni G, Svob Strac D, Sole M, Unzeta M, Tipton KF, Mück-Šeler D, Bolea I, Della Corte L, Nikolac Perkovic M, Pivac N, Smolders IJ, Stasiak A, Fogel WA, De Deurwaerdère P. Monoaminergic and histaminergic strategies and treatments in brain diseases. Front Neurosci. 2016 doi: 10.3389/fnins.2016.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics☆. Adv Drug Deliv Rev. 2008;60(13–14):1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quartey MO, Nyarko JNK, Pennington PR, Heistad RM, Klassen PC, Baker GB, Mousseau DD. Alzheimer disease and selected risk factors disrupt a co-regulation of monoamine Oxidase-A/B in the hippocampus, but not in the cortex. Front Neurosci. 2018 doi: 10.3389/fnins.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Šimić G, Babić Leko M, Wray S, Harrington CR, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L, De Silva R, Di Giovanni G, Wischik CM, Hof PR. Monoaminergic neuropathology in Alzheimer's disease. Prog Neurobiol. 2017;151:101–138. doi: 10.1016/j.pneurobio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephanou P, Konstandi M, Pappas P, Marselos M. Alterations in central monoaminergic neurotrasmission induced by polycyclic aromatic hydrocarbons in rats. Eur J Drug Metab Pharmacokinet. 1998;23(4):475–481. doi: 10.1007/BF03189998. [DOI] [PubMed] [Google Scholar]
- 56.Ozturk IC, Batcioglu K. Investigation of the relationship between nitric oxide metabolites' levels and adenosine deaminase activity in 7,12-dimethylbenz[a]anthracene induced mouse liver. J Biochem Mol Toxicol. 2002;16(5):260–262. doi: 10.1002/jbt.10041. [DOI] [PubMed] [Google Scholar]
- 57.Marisco PC, Carvalho FB, Rosa MM, Girardi BA, Gutierres JM, Jaques JAS, Salla APS, Pimentel VC, Schetinger MRC, Leal DBR, Mello CF, Rubin MA. Piracetam prevents scopolamine-induced memory impairment and decrease of NTPDase, 5′-nucleotidase and adenosine deaminase activities. Neurochem Res. 2013;38(8):1704–1714. doi: 10.1007/s11064-013-1072-6. [DOI] [PubMed] [Google Scholar]
- 58.Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48(6):1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sperlagh B, Sylvester Vizi E. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and basal ganglia: pharmacological and clinical aspects. Curr Top Med Chem. 2011;11(8):1034–1046. doi: 10.2174/156802611795347564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunha RA. How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem. 2016;139(6):1019–1055. doi: 10.1111/jnc.13724. [DOI] [PubMed] [Google Scholar]
- 61.Polachini CRN, Spanevello RM, Casali EA, Zanini D, Pereira LB, Martins CC, Baldissareli J, Cardoso AM, Duarte MF, Da Costa P, Prado ALC, Schetinger MRC, Morsch VM. Alterations in the cholinesterase and adenosine deaminase activities and inflammation biomarker levels in patients with multiple sclerosis. Neuroscience. 2014;266:266–274. doi: 10.1016/j.neuroscience.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 62.Sauer AV, Hernandez RJ, Fumagalli F, Bianchi V, Poliani PL, Dallatomasina C, Riboni E, Politi LS, Tabucchi A, Carlucci F, Casiraghi M, Carriglio N, Cominelli M, Forcellini CA, Barzaghi F, Ferrua F, Minicucci F, Medaglini S, Leocani L, La Marca G, Notarangelo LD, Azzari C, Comi G, Baldoli C, Canale S, Sessa M, D’Adamo P, Aiuti A. Alterations in the brain adenosine metabolism cause behavioral and neurological impairment in ADA-deficient mice and patients. Sci Rep. 2017;7(1):40136. doi: 10.1038/srep40136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kleiner HE, Vulimiri SV, Reed MJ, Uberecken A, Digiovanni J. Role of cytochrome P450 1a1 and 1b1 in the metabolic activation of 7,12-Dimethylbenz[a]anthracene and the effects of naturally occurring furanocoumarins on skin tumor initiation. Chem Res Toxicol. 2002;15(2):226–235. doi: 10.1021/tx010151v. [DOI] [PubMed] [Google Scholar]
- 64.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and1B1. Cancer Sci. 2004;95(1):1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyata M, Furukawa M, Takahashi K, Gonzalez FJ, Yamazoe Y. Mechanism of 7,12-Dimethylbenz[a]anthracene-induced immunotoxicity: role of metabolic activation at the target organ. Jpn J Pharmacol. 2001;86(3):302–309. doi: 10.1254/jjp.86.302. [DOI] [PubMed] [Google Scholar]
- 66.Song M-K, Kim Y-J, Song M, Choi H-S, Park Y-K, Ryu J-C. Formation of a 3,4-diol-1,2-epoxide metabolite of benz[a]anthracene with cytotoxicity and genotoxicity in a human in vitro hepatocyte culture system. Environ Toxicol Pharmacol. 2012;33(2):212–225. doi: 10.1016/j.etap.2011.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the study are available from the corresponding author on reasonable request.