Abstract
This article aimed to describe the current use scenario, alternative feed additives, modes of action and ameliorative effects in broiler production. Alternative feed additives have promising importance in broiler production due to the ban on the use of certain antibiotics. The most used antibiotic alternatives in broiler production are phytogenics, organic acids, prebiotics, probiotics, enzymes, and their derivatives. Antibiotic alternatives have been reported to increase feed intake, stimulate digestion, improve feed efficiency, increase growth performance, and reduce the incidence of diseases by modulating the intestinal microbiota and immune system, inhibiting pathogens, and improving intestinal integrity. Simply, the gut microbiota is the target to raise the health benefits and growth-promoting effects of feed additives on broilers. Therefore, naturally available feed additives are promising antibiotic alternatives for broilers. Then, summarizing the category, mode of action, and ameliorative effects of potential antibiotic alternatives on broiler production may provide more informed decisions for broiler nutritionists, researchers, feed manufacturers, and producers.
Keywords: antibiotic alternatives, broiler, feed additives, phytogenic, prebiotic, probiotic
Introduction
The poultry sector is one of the largest food industries in the globe (1). In the near future, by 2050, it would be projected to be 121% of the year 2005 production (2). It has continual growth and industrialization in many parts of the world (3). Particularly, broiler production has shown exponential growth in global meat consumption and business profit, which will be higher in the next century (4–6). This could be because of its comparative advantages including good quality of nutrition, delicious taste, low-fat content, short production period, low production cost, rapid economic progress, and affordable price even for poor levels of society (7, 8). The production has ascended from 9 to 132 million tons in the year range of 1961 to 2019 (3). Seventeen percent of global output is produced in the United States, which is the world's largest poultry meat producer followed by China and Brazil (3).
Per capita, meat consumption has been an increase in the world in which poultry meat accounts 70% of total meat consumed. Over 66 billion broilers are slaughtered in the world each year (9). From these amounts of slaughtered birds, nearly 110 million tons are produced per annum. Per capita broiler meat consumption is higher in developed countries (10). For example, the average broiler meat consumption per capita in the United States, Brazil, and China is 48, 44.2, and 8.3 kg/head/yr, respectively, in 2017 (11). These exponential broiler meat demands are an alarm to boost production.
Antibiotics have been used for many decades in the poultry industry to enhance production, promote growth performance, and protect birds from pathogenic microbes (12–16). For example, supplementation of broilers' diet with antibiotics could increase body weight gain by 5.8 % (17). This improvement was explained by improved appetite and feed conversion efficiency, stimulation of the immune system, and increased vitality and regulation of the intestinal microflora (18).
Antibiotics are also important for fighting infectious pathologies (16, 19, 20) such as necrotic enteritis and coccidiosis (21). Broadly, antibiotics are used in phytosanitary treatments, feed additives, and prophylactic treatments in animals and humans.
Despite its important role, improper uses of antibiotics in animal farming have been reported to increase antimicrobial resistance bacteria as a public health threat (22–24), residues in animal products, and cause environmental pollution (25, 26). Consequently, the use of antibiotics as growth promoters was banned by the European Union in 2005 (27) and China in 2020 (28). To minimize health risks, consumers have great preferences for conventional broiler meat, resulting in shift to antibiotic-free broiler meat production around the globe (13, 14). The ban on antibiotic use, combined with consumers' preferences, provoked scholars to look for antibiotic alternatives (29). This is important to apply sustainable feeding strategies of potential antibiotic alternatives for increasing antibiotic-free broiler meat production (30, 31). Therefore, this review aimed to explain the current use scenario, mode of action, ameliorative effects, and feeding strategies of different antibiotic alternatives including phytogenic groups (marine algae, herbs, plant extract, and essential oils), prebiotics, probiotics, and enzymes in broiler production.
Current Scenario of Antibiotic Use
The intensity of using antibiotics could vary among nations (32). China is among the world's leading antibiotic producers and consumer, particularly in livestock products (4, 33). This was supported by Ziping (34), who reported that antibiotic use in China is 5 times higher than the international average. Although antimicrobial use in animal production in China increased until 2014, it has fallen in recent years (34). Antimicrobial consumption is projected to be 67% by 2030 and nearly double in Russia, Brazil, China, India, and South Africa (4).
Although antimicrobial consumption in livestock has received little attention, an expert opinion suggests that global consumption of antimicrobials in animals is twice more than in humans (4, 35). In many countries, most commercial broiler producers have reported antibiotic use, i.e., in Ghana (97%) (16), Nepal (90%) (36), Nigeria (89%) (37), Bangladesh (98%) (38), and the United States (40%) (39). Broiler farm intensification could be a driving force for the use of antibiotics as feed additives in developed countries, whereas increasing demand for poultry meat and eggs for food security could be a factor in the developing world and may lead to the risk of developing antibiotic-resistant microbes (40–43).
Globally, the most commonly applied antibiotics to food animal production include tetracyclines, sulfonamides, and penicillins (44). However, this review finds that there are differences in using antibiotics types in different nations that might be due to antibiotic-producing capability, access, price, and banned antibiotics policy platform. Tetracycline, aminoglycosides, penicillins, and fluoroquinolones in Ghana (45), tetracycline, penicillins, and sulfonamides in South Africa (46), bacitracin, tylosin, tetracycline, salinomycin, virginiamycin, and bambermycin in North America (29), and erythromycin, penicillins, tylosin, tetracycline, and vancomycin in China (34) are commonly used antibiotics.
Although the use of antibiotics has ameliorative effects as mentioned above, it has been banned for a decade in different countries because of potential development of antibiotic-resistant human pathogenic bacteria (15, 47, 48). The European Union (EU) has banned non-therapeutic antibiotics used as growth promoters and feed additives in animal production since 2006 (42). Although the ban was applied before a decade and consumers have preferred organic livestock products, antibiotics are still used in livestock as growth promoters. Therefore, feed additives could be familiar as antibiotic alternatives in the poultry production sector, with a great interest in improving growth performance and feed conversion ratio, maintaining healthy intestinal microbial populations, and improving the overall health of birds (20, 49–52).
Feed Additives as Antibiotic Alternatives
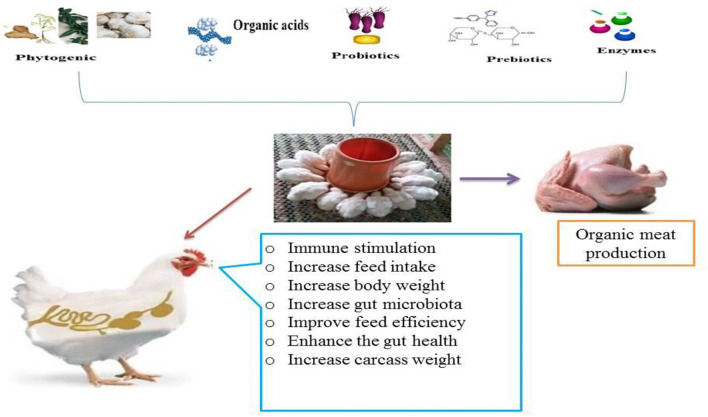
Feed additives are non-nutritive natural products added to basal diet as minor components of the diet to improve feed quality and food from animal origins and improve animal performance and health. They also promote ingestion, absorption, nutrient assimilation, and growth of animals by affecting physiological processes such as immune function and stress resistance (53). It has been reported that feed additives could be used as antibiotic alternatives for broilers to reduce mortality rates and enhance performance without jeopardizing the environment and consumer health (20). The common feed additives tested in poultry are phytogenic feed additive groups including essential oils (20, 51, 52, 54), herbal extracts (55–57), organic acids (58, 59), and others like prebiotics (15, 60), probiotics (15, 61), and enzymes (62–64) (Figure 1).
Figure 1.
Schematic diagram of an alternative feed additive in broiler diet.
Phytogenic Feed Additives
Phytogenic feed additives are plant-origin extracted compounds that include a wide range of substances such as herbs, spices, botanicals, oleoresins, and essential oils that are used in poultry production (65–73) (Table 1). According to Madhupriya et al.' (68) explanation, PFAs are natural, less toxic, residue-free, and ideal feed additives for poultry compared with synthetic antibiotics.
Table 1.
Phytogenic feed additives and ameliorative effects on broiler production.
| Feed additives | Level of supplementation | Findings | Sources |
|---|---|---|---|
| Essential oils (Origanum genus) | 300–600 g/kg | Increase in the average daily gain | (74) |
| Cinnamon | 2 g/kg | Improve growth performance | (75) |
| Lippia Javanica leaf meal | 5 g/kg | Improve daily gain and slaughter weight | (76) |
| The mixture of garlic and black pepper powder | 5 g/kg) and 1 g/kg) | Increase in weight gain | (77) |
| Pennyroyal (Mentha pulegium L.) | 2% | Increase in average daily gain | (78) |
| Neem (Azadirachta indica) |
7 g/kg | Favorable influences on the immune | (79) |
| B. subtilis with enramycin | UBT-MO2/kg | Increase in body weight and relative weight of the thymus | (80) |
| Milk kefir | 2% | Improvement on body mass and chicken consumption index | (81) |
Herbs are flowering plants whose stem does not become woody and persistent, and are valued for their medical properties, flavor, and scent (51), whereas spices are pungent or aromatic substances of vegetable origin that are used as seasonings and preservatives (51). Botanicals or photobiotics are parts of a plant like roots, leaves, and barks, which are used to make drugs for medical use. Essential oils are any class of volatile oils obtained from plants; the possess the odor and other characteristic properties of plants and are used chiefly in the manufacture of perfumes, flavors, and pharmaceuticals (51). The most widely used herbs and spices for Phyto feed additives in poultry production are oregano, thyme, garlic, horseradish, chili, cayenne, pepper, peppermint, cinnamon, anise, clove, rosemary derivatives, citrus, and sage (68, 82).
A growing body of evidence has shown that supplementation of phytogenic feed additives in broilers' diet improve intestinal functions (83, 84), increase nitrogen retention and fiber digestibility, enhance growth performance (85), reduce inflammation (86), and improve anti-oxidative (51, 87) and antimicrobial activities (88) (Table 1). Altogether, the above findings suggest that PFAs have beneficial effects to improve performance and broiler health (54, 73, 75–77, 89, 90).
Phytogenic Mode of Action
Studies have shown that the growth and health-promoting effects of PFAs are associated with their biological activities including antimicrobial, antioxidant, immunomodulatory, and anti-inflammatory (54, 68, 91–93). For instance, Superliv concentrate premix (SCP), AV/HGP/16 premix (AVHGP), and bacteriostatic herbal growth promoter (BHGP) have been increasing the feed efficiency of broilers by modulation of the muscle mTOR pathway and hepatic lipolytic programs; thus, they are promising for muscle protein synthesis and hepatic lipogenesis reduction (94). This is aligned with (95, 96) that have shown that PFAs modulate the expression of feeding-related hypothalamic neuropeptides and result in feed efficiency (FE) improvement. FE is also controlled by peripheral intermediary metabolism like lipid metabolism and protein synthesis-associated signaling pathways, which are modulated by bioactivities of PFAs.
PFAs also improve the palatability, digestibility, absorption of the feed nutrients, control animal intestinal microbiome structure, improve performance and feed quality through positively reflected of biological activities of plant secondary compounds with the action of antioxidative properties and slow microbial growth in poultry (97–99). In addition, they have been shown to enhance gut health by reducing bacterial colony populations, lessening fermentation products including ammonia and biogenic amines, decreasing the activity of the gut-associated lymphatic system, and increasing prececal nutrient digestion. Beneficial phytogenic compounds derived from their bioactive molecules are carvacrol, thymol, cineole, linalool, anethole, eugenol, capsaicin, allicin, allyl isothiocyanate, and piperine (65, 68). Most of these active secondary plant metabolites belong to the classes of isoprene derivatives, flavonoids, and glucosinolates, which act as antibiotics or antioxidants (100, 101).
Organic Acids as Feed Additives
Organic acids are weak acids that have a carboxylic acid group (R-COOH) and nutritional values and antimicrobial effects in animal feeds (102–104). Organic acids have been used in animal feeds for many years because of the ban on the use of antibiotics (59). In line with these findings (15, 105, 106) reported that organic acids are considered as effective antibiotic alternatives in animal feeds. The most commonly used organic acids in the broilers' diet are acetic, butyric, citric, formic, propionic, malic, tartaric, and lactic acids (15, 28, 107).
The inclusion of organic acids in the broilers' diet has been shown to improve protein and carbohydrate digestibility (108), fight against pathogenic bacteria (105), and (106) enhance the feed conversion rate, nutrient utilization, and growth rate of broilers (109, 110).
Organic Acid Mode of Action
Diets with poor protein quality have more indigestible proteins reaching the GIT, which end up with high protein fermentation (111). This high protein fermentation causes discomfort in the animal body and negatively affects its growth rate because of high volatile fatty acids and ammonia and production of other gases (112). Organic acids are good supplement alternatives in such types of feed to acidify the GIT environment (113) and improve nutrient utilization, which results in activeness of the protease enzyme. For example, Suiryanrayna and Ramana (114) reported stimulation of protein digestion by converting pepsinogen to pepsin by supplementation of organic acids. Moreover, organic acids reduce pH in the GIT, which enhances pepsin activity, and increases the digestibility of nitrogen, phosphorus, and other minerals (15, 115). These acid anions react with calcium, phosphorus, magnesium, and zinc, thus enhancing their digestibility. Peptides produced by pepsin proteolysis stimulate the release of gastrin and cholecystokinin hormones, which regulate protein digestion and absorption (116, 117).
Organic acids have been used as feed preservatives for protecting feed from microbial and fungal deterioration with the mechanism of acidification (118). These are a powerful tool in maintaining the health of the gastrointestinal tract of poultry, resulting in improvement in birds' production performance. For example, sanguinarine suppresses the growth of some harmful acid intolerance bacteria such as E-coli, Salmonella spp., and Clostridium perfringens that cause gastrointestinal distress (119), resulting in enhanced appetite and feed intake and improving growth (120). Reduction of competition for microbial nutrients in the host thereby increases the availability of nutrients (121), consequently increases BWG, and improves FCR (122, 123). Organic acids also affect the histological structure of the gastrointestinal tract; Consequently, improve nutrient absorption, maximized nutrient utilization efficiency, and improved growth performance (54). As a conclusion remark from different studies; organic acids and their salts are used to reduce a load of pathogenic microorganisms in the intestine, activate digestive enzymes, improve digestibility, and increase the absorption of nutrients, gut microflora function, and performance of chickens (Table 2).
Table 2.
Organic acids, their derivatives, and ameliorative effects on broiler production.
| Organic acids | Level of supplementation | Finding | Sources |
|---|---|---|---|
| CA | 2% | Improve epithelial cell proliferation and villi height of gastrointestinal tract | (124) |
| CA, avilamycin | 0.5 and 0.001%, respectively | Significantly increase growth performances at 35 days | (125) |
| BA | 0.2% | Increase CW, breast meat yield, FCR, dressing % and reduce abdominal fat |
(126, 127) |
| SB | 0.6 and 1.2 g/kg | Increase ADG and FCR during 1–21 days period | (123) |
| N-butyric acid and 50% MB | 250–7,000 mg/kg | Reduce Salmonella Typhimurium or Clostridium perfringens | (128) |
| MESB | 800 mg/kg | Higher total body weight, daily gain and FCR at 35 days | (129) |
| PCB | 0.3 g/kg | Increase weight gain | (130) |
| FA | 5 g/kg | Increase BWG, dressing percentage and reduce FCR | (131) |
| KDF | 5 g/kg | Increase BWG, dressing percentage and reduce FCR | (131) |
FCR, feed conversion ratio; CW, carcass weight; SB, sodium butyrate; MESB, microencapsulated sodium butyrate; ADG, average daily gain; PCB, protected calcium butyrate; CA, citric acid; BA, butyric acid; FA, formic acid,; KDF, potassium di-formate; BWG, body weight gain.
Prebiotics Feed Additives
Prebiotics are indigestible carbohydrates by the host animal but can be utilized by useful GIT microorganisms (54, 141–143). Prebiotics are found in different food sources such as oats, barley, dandelion greens, chicory, chia seeds, flax seeds, onion, garlic, almonds, and artichoke (144). Green algae (Chlorophyta) are also considered prebiotic because of the presence of water-soluble sulfated polysaccharides; the perform gut microbiota modulation and immunomodulation, and they have anti-oxidant, antibacterial, anti-hyperlipidemia, and anti-diabetic properties (145).
Potential prebiotics that have been fed to broilers include fructan, oligofructose, inulin, fructooligosaccharides, galactan, galactooligosaccharides, xylooligosaccharides (XOS), pectin, fiber components, and milk oligosaccharides (146–149). Refined functional carbohydrates (RFCs) including mannan-oligosaccharides (MOSs), β-glucan, and D-mannose, which are derived from the cell wall of Saccharomyces cerevisiae, are a readily available source of prebiotics for animal use (150). From these, mannan-oligosaccharides and fructooligosaccharides are the most common commercial feed nutrients in poultry feed production (151). In connection with their economic importance for producers, prebiotics also have no residual effect and do not develop any resistance for broiler product consumers (141).
Supplementation of prebiotics can improve growth performance and antibody titer against infectious bursal disease in broilers (Table 3) (133). Prebiotics are also useful for changing the microbial population of the intestine (31, 149, 152, 153); for example, dietary MOS (1g/kg) increase Lactobacillus and Bifidobacterium contents (154), increase the length of the villain (155), prevention of colon cancer, minimize disease-causing bacteria and increases daily weight gain (156, 157), and medical therapy of broiler (158). Generally, the beneficial effects of prebiotics are alteration of gut microorganisms that enable to increase their numbers, increase digestibility, reduce pathogenic bacteria, increase mineral and vitamin absorbability, maintain optimal intestinal pH, and maximize nutrients utilization (142, 143, 159).
Table 3.
Prebiotics and their ameliorative effects on broiler production.
| Prebiotics | Level of supplementation | Finding | Sources |
|---|---|---|---|
| FOS | 0.25% | Improve productivity of broiler Increase lactobacillus in the ileum |
(132) |
| MOS | 0.05% | Improve productivity of broiler Increase lactobacillus in the ileum |
(132) |
| MOS | 1.5 g/kg | Improve WG and FCR Improve the antibody titer against IBD |
(133) |
| IMO | 5–10 g/ kg | Improve WG Increase feed conversion rate Increase the caecal populations of lactobacilli and bifidobacteria Decrease the caecal Escherichia coli Increase the caecal VFA |
(134) |
| RFC | 50–100 g/t | Improve ADG Decrease cecal Campylobacter counts The high dose also increases FBW |
(135) |
| Autolyzed WY and YCW | 1.5–2 g/kg | Improve BWG, FCR, and Meat yield Positive effect on ileal protein digestibility as well as trypsin and chymotrypsin activities |
(136) |
ADG, average daily gain; FBW, final body weight; FOS, fructo-oligosaccharide; IMO, isomalto-oligosaccharide; IBD, infectious bursal disease; MOS, mannan-oligosaccharide; RFC, refined functional carbohydrate; VFA, volatile fatty acid; WY, whole yeast; YCW, yeast cell wall product.
Prebiotics Mode of Action
Prebiotics can affect host health in different ways, such as production of metabolites like lactic acid, microbial metabolism modification, and increase in epithelium cell integrity (160, 161). Prebiotics are used to modulate the ecosystem of gut elements including alteration of the intestinal microbiota, stimulation of the immune system, improvement of the epithelium, and regulation of interaction between the host and the intestinal microbiota (162).
Prebiotics could be a selective substrate for a limited number of beneficial bacteria to alter the colon microflora in favor of a healthier gastrointestinal environment (149, 152). For example, they serve as a substrate for endogenous beneficial bacteria, thus promoting competitive exclusion of pathogenic microbes and selective colonization by beneficial microbes (60). Mazanko et al. (159) also reported that a prebiotic feed supplement creates an unfavorable condition for pathogenic organisms by altering the pH of the intestine. It establishes a healthy microbial community in the intestine of broilers by enhancing the abundance of Lactobacilli and Bifidobacteria and reducing the titers of Coliform (163, 164). Bifidobacterium and Lactobacillus have manase enzymes; they selectively bind mannan oligosaccharides only for harmful bacteria, which normally do not have this enzyme (157). The effect of mannan oligosaccharides on broilers is increase in the daily weight gain of broilers by 4–8% (156, 157).
The sustainable ability of prebiotics in acidic environments and to remain resistant to distinct digestive enzymes in the small intestine make them an extraordinary tool to boost the growth of beneficial gut microbes that ferment them, leading to production of short-chain fatty acids, vitamins, and other fragmented molecules or some antibacterial substances such as bacteriocin against pathogenic microorganisms (165, 166). These fermented products of beneficial microbes due to prebiotic feed additives also improve the integrity of intestinal epithelial cells, which further increase the absorption of nutrients and enhance the growth performance of animals (115, 162).
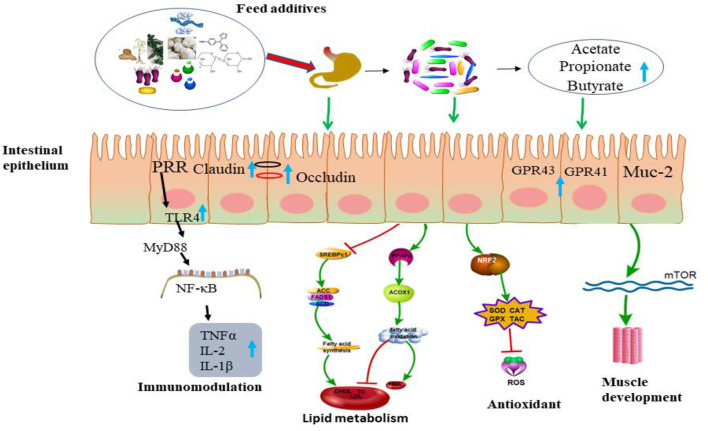
The modulation of the intestinal microbiota with prebiotic feed additives is associated with immune responses (162) (Figure 2). Oligosaccharides have been reported to present immunomodulatory beneficial effects on the gut, such as modifying clearance efficiency of pathogenic bacteria, activating T cell-dependent immune responses, and repression of pro-inflammatory cytokines (167, 168). Inhibiting pathogen colonization with prebiotics can decrease pathogen-associated molecular patterns, which are produced by pathogenic microorganisms (169). The produced molecule can be recognized by pattern recognition receptors (PRRs), including toll-like receptors (TLRs) and NOD-like receptors (NLRs), which are expressed on the surface of sentinel cells (170, 171). Once pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns (PAMPs), sentinel cells such as macrophages, epithelial cells, dendritic cells, and mast cells are activated and produce cytokines for regulation of further innate immune responses (171). They can be recognized by receptors of immune cells, consequently modulating host immunity systems.
Figure 2.
Schematic diagram of an alternative feed additive mode of action.
Probiotics Feed Additives
Probiotics are “live strains of strictly selected microorganisms that, when used in adequate amounts, confer a health benefit on the host” (172). Similarly, probiotics are beneficial bacteria that can fight pathogens in the gastrointestinal tract of chickens like subclinical necrotic enteritis (173), stimulate growth (174–176), and improve the immunity of the host (143, 177–179). Probiotics strains have been also providing feeding efficiency improvement, intestinal protection, antioxidant capacity and apoptosis (180), use of nutrients (181), energy digestibility, disappearance of non-starch polysaccharides (182), and microbial profile of cecum and litter (Table 4) (183).
Table 4.
Probiotics and their ameliorative effects on broiler production.
| Probiotics | Level of supplementation | Finding | Sources |
|---|---|---|---|
| Mixture of Bacillus licheniformis and Bacillus subtilis spores | 0.05% | Significantly improve the FCR | (137) |
| Multi-strain probiotic (11 Lactobacillus strains) | 1 g/kg | Increase FCR Improve BWG Increase the caecal populations of lactobacilli and bifidobacteria Increase the caecal VFA Decrease the caecal Escherichia coli |
(134) |
| Protexin | 2 g/kg | Improve the growth performance | (133) |
| Promax | 1 g/L | Improve BW and the hemato-biochemical profile | (138) |
| Normosil | 1 mL/kg | Increase the average daily gain Increase the level of blood erythrocytes Improve carcass quality |
(139) |
| Lact. lactis and L. plantarum | 109 cfu/mL and 1012 cfu/mL, respectively | Lower the serum cholesterol, triglyceride, and total lipid contents Increase contents of blood glucose and total protein | (140) |
BWG, body weight gain; FCR, feed conversion rate; VFA, volatile fatty acid.
Selection and use of microorganisms as feed additives are not an easy task; their risks, handling procedures, and adaptability to the environment should be considered. Some microbes will participate in the spread of antibiotic resistance (enterococcus) and produce toxin substances (Bacillus cereus strains) (184). The recommended dose for most probiotic strains is 10 × 9 colony-forming units of feed (CFU/KG). Care should also be taken when mixing probiotics. The water should be free from any disinfectant or chlorine. Administration or offering of a probiotic feed additive solution should be within 6–12 h after mixing with water. If animals are on antibiotic treatment, it is highly recommended that the treatment be withdrawn 24–48 h before administering probiotics (185).
The most used microorganisms as feed additives in poultry production are bacterial strains, mostly Gram-positive Bifidobacterium, and lactic acid bacteria groups such as Bacillus, Enterococcus, Lactobacillus, Pediococcus, Streptococcus, Aspergillus, Candida, and Saccharomyces. However, fungi and yeast strains are also used, mainly from the species Saccharomyces cerevisiae and Kluyveromyces (184, 186–188).
Probiotics Mode of Action
The main modes of action of probiotics include antagonistic action toward pathogenic bacteria by secreting products that inhibit their development such as bacteriocins, organic acids, and hydrogen peroxide, and competitive exclusion by competing with bacteria for locations in the intestinal mucous membrane to adhere to nutrients (47). Lowering the gut pH through the volatile fatty acids and organic acids produced during probiotic product breakdown is the most common probiotic mode of action (189, 190). The low pH in the intestine suppresses the colonization of pathogens in the digestive tract, thereby competitively inhibiting the effects of pathogens (191). Probiotics are also used to modulate the intestinal microbiota, for immunomodulation, and to improve intestinal integrity (192, 193). Other principal mechanisms of probiotics are competition for binding sites where probiotics adhere to the intestinal epithelium wall, hindering competition and joining of pathogenic microorganisms; this higher concentration of the beneficial microbiota is also the driving force to have an advantage in the competition for nutrients (20). Findings showed that probiotics have nutritional effects, increasing fiber digestion and enzymatic activity in birds to be efficient in feed nutrient utilization (133). The finding of Wang et al. (194) stated that supplementation of broilers with Bacillus subtilis in the diet was more effective in performance in heat stress conditions through the immunity modulated by the microbiota.
Enzymes as Feed Additives
Enzymes are catalysts of biochemical processes that are composed of proteins, amino acids with minerals, and vitamins (195). Enzymes are the most important and useful additives in the animal feed industry (196). They can be obtained from plants, animals, and microorganisms (197). Enzymes, as feed additives in broiler production, are produced by fermentation of fungi and bacteria and are used for maximization of feed conversion efficiency (FCE) (15). Although animals produce endogenous enzymes that are involved in digestion, they do not efficiently degrade feedstuff and take advantage of all their nutritional components; therefore, exogenous enzymes are supplemented to increase animal performance (195, 196, 198). Pectinases, amylases, cellulase, galactosidases, β glucanases, xylanases, associated enzyme phytases, proteases, and lipases are commonly used exogenous enzymes in the animal feed industry (Table 5) (196, 197, 207). These exogenous enzymes are mainly used in monogastric animals like poultry and swine (208).
Table 5.
Enzymes, target substrates, and their benefits in broiler production.
| Broad classes of enzymes | Specific example | Substrate | Target feedstuff | Level of supplementation | Ameliorative Effect | Sources |
|---|---|---|---|---|---|---|
| Carbohydrases | Xylanases | Arabinoxylans | Wheat, rye, triticale, barley, fibrous plant materials | 3,200–24,000 IU/kg | Increase starch and nitrogen digestibility and improve AIDE | (199) |
| α-Galactosidases | Oligosaccharides | Soybean meal, grain, legumes | 50 mg/kg of diet | Improves intestinal histology and morphology | (200) | |
| α-amylase | Starch | Cereal grains, grain legumes | 300–2,250 IU/kg | Improve the apparent ileal digestibility of energy | (201) | |
| β-Glucanases | β-Glucan | Barley, oats, and rye | 20 IU/ | Reduce viscosity, increases dry matter of digesta, and available energy | (202) (203) (204) |
|
| β-Mannanase | Cell wall matrix (fiber components) |
Plant-derived ingredients, fibrous plant materials | 200–400 mg/kg | |||
| Cellulases | 20 IU/kg | |||||
| Hemicellulases | 20 IU/kg | |||||
| Pectinases | 53 IU/ kg | |||||
| Proteases | Proteases | Proteins | All plant protein sources | 30,000 IU/kg | Increase FI and FCR, increase N retention, reduce abdominal fat | (205) |
| Phytases | Phytates | Phytic acid | All plant-derived ingredients | 500 – 1,500 FTU/kg | Increase FI, BW, FCR, CW, and GIT organs length | (206) |
AIDE, apparent ileal digestible energy; BW, body weight; CW, carcass weight; FCR, feed conversion ratio; FI, feed intake.
The supplementation of enzymes for broilers has nutritionally, economically, and environmentally justifiable advantages (209). The use of enzymes in the chicken diet resulted in high feed utilization efficiency, reduction of digesta viscosity, enhanced digestion and absorption of nutrients, and increased feed intake and weight gain (18, 196, 210, 211). Xylanase has increased crude protein digestibility, feed intake, nitrogen and fiber absorption, and weight gain in broilers (211, 212). Phytases increase the utilization of phytate phosphorus in feeds (210). A multi-enzyme complex (Avizyme) composed of xylanases, proteases, and amylases is used to improve nutritional quality, reduce the viscosity of diets, increase body weight, decrease mortality, and increase the amount of net energy (213). It also improves the intestinal health of animals (214). Generally, different studies have reported that the use of exogenous feed enzymes in poultry diets is becoming familiar to overcome the adverse effects of anti-nutritional factors, and improve the digestion of dietary components and bird performance.
Enzymes must be active under physiological conditions prevailing in the animal's digestive tract and must complement the characteristics of dietary ingredients and additives to realize their functions (209, 215).
Enzyme Mode of Action
Each enzyme has a different and interdependent mode of action; its use in combination with feed formulations must be carried out carefully to achieve maximum ameliorative effects (197). Broiler diets containing a large amount of NSP lead to increased digesta viscosity, thus depression in growth performance (216). Carbohydrase enzymes are added to broiler diets to overcome this type of difficulty, consequently improving nutrient utilization and increasing the productivity of birds. For example, hydrolysis of non-starch polysaccharides (NSPs) into smaller oligosaccharides with carbohydrase results in decrease in digesta viscosity and release of encapsulated nutrients (217). Produced small oligosaccharides during NSP hydrolysis could also have a prebiotic advantage (218). The hemicellulose in agro-industrial byproducts, particularly Palm kernel expeller (PKE), is partially hydrolyzed with enzyme treatment, thus obtaining oligosaccharides (DP <6) that have prebiotic-like effects. Based on Chen et al.'s (219) results, the untreated PKE contained 20.93 g/kg oligosaccharides, but after treatment, the oligosaccharide content increased to 28.91 and 59.71 g/kg for PKEENZ and SPKEENZ, respectively. Zhang et al. (220) also reported that smaller oligosaccharides such as xylooligosaccharide (XOS) come from hydrolysis of NSPs, which have been shown to have prebiotic-like effects.
Enzymes act on nutrients having main effects on substrates to which they are directed as well as having side effects. They initiate and control the rate of biological reactions by which substrates are changed into useful products (195). NSP hydrolysis products are fermented by beneficial bacteria such as Bifidobacter and Lactobacilli spp., thus, producing short-chain fatty acids (221). Increased SCFA concentration is often associated with increase in the population of beneficial bacteria and decrease in pathogenic bacteria (19). Some SCFAs are also used as an available energy source to the host for growth (222).
Supplementing glucose oxidase (GOD) in broilers has been reported to increase daily body weight gain, improve meat quality, and enhance digestive ability that is indicated by the nutrients' apparent digestibility and digestive enzymes (223). Different studies also confirm that the increase in body weight gain and FCR of broilers with commercial enzymes is due to the ileal digestibility of crude proteins (224), starch and fat (225), and improvement in ileal non-starch polysaccharide (NSP) digestibility (226). The content of secreted immunoglobulin A and transepithelial electrical resistance are also increased with the GOD supplement, which indicated an enhanced gut barrier. In the general context, dietary GOD supplement could improve the growth performance of broilers in two main mechanisms: 1) by enhancing the digestive function of the gut, which concluded from improved nutrients' apparent digestibility and digestive enzyme, and 2) by increasing the abundance of beneficial bacteria such as F. prausnitzii, Ruminococcaceae, and Firmicutes (223).
Conclusion and Future Research Directions
The ban on certain antibiotics has promoted phytogenics, organic acids, prebiotics, probiotics, and enzymes as alternatives in broiler production. Antibiotic alternatives have comparable advantages to antibiotics to enhance the production performance and well-being of broilers without human health challenges. Moreover, using antibiotic alternatives can increase body weight, average daily gain, carcass weight, feed conversion ratio, and the nutritive value of feed ingredients, and enhance the gut health of broilers. The main provided effects of alternative feed additives includes immune-modulating, enhance digestion, improving nutrient availability, increase absorbability of nutrients, antimicrobial, antioxidant activity, enhancement of gut integrity, intestinal barrier function or improve intestinal health, nutrient for the host, and modulating the host gut microflora. These different modes of action suggest that there could be synbiotic, antagonistic, and synergistic or combative effects between alternatives or other feed nutrients. Therefore, use of alternative feed additives in broiler production should highly promoted and further investigations on interaction effects of combined additives, sub-additive, and with diet nutrient, efficiency of utilization, and level of inclusion could be mandatory.
Author Contributions
HA carried out the organization and drafting of the manuscript. HZ and TW were involved more in technical editorial support of the drafted manuscript. All authors participated in the evaluation, editing, and approval of the final version of the manuscript.
Funding
This study was funded by the Shandong Key Science and Technology Innovation Program (2019JZZY010704), the China Agriculture Research System-Beijing Team for Poultry Industry, and the Agricultural Science and Technology Innovation Program (ASTIP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Chowdhury EU, Morey A. Intelligent packaging for poultry industry. J App Poul Res. (2019) 28:791–800. 10.3382/japr/pfz098 [DOI] [Google Scholar]
- 2.Alexandratos N, Bruinsma J. World Agriculture Towards 2030/2050: The 2012 Revision Agricultural Development Economics Division, Food and Agriculture Organization of the United Nations. Rome, Italy: (2012). 1–147. Available online at: https://www.fao.org/3/ap106e/ap106e.pdf [Google Scholar]
- 3.FAO (Food and Agriculture Organization) . Gateway to Poultry Production and Products. Paris: 26th World's Poultry Congress; (2022) (accessed December 22, 2022). [Google Scholar]
- 4.Van Boeckel TP, Charles B, Marius G, Bryan TG, Simon AL, Timothy PR, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. (2015) 112:5649–54. 10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mekonnen MM, Neale CM, Ray C, Erickson GE, Hoekstra AY. Water productivity in meat and milk production in the US from 1960 to 2016. Environ Int. (2019) 132:1–12. 10.1016/j.envint.2019.105084 [DOI] [PubMed] [Google Scholar]
- 6.Newswire Globe. Global Poultry (Broiler) Market Analysis & Forecast 2019–2023: Production, Consumption, Import, and Export. France: The World's Largest Market and Research Store; (2019). [Google Scholar]
- 7.Najeeb AP, Mandal PK, Pal UK. Efficacy of fruits (red grapes, gooseberry and tomato) powder as natural preservatives in restructured chicken slices. Int Food Res J. (2014) 21:2431–6. Available online at: http://www.ifrj.upm.edu.my [Google Scholar]
- 8.Petracci M, Mudalal S, Soglia F, Cavani C. Meat quality in fast-growing broiler chickens. World's Poul Sci J. (2015) 71:363–74.30455879 [Google Scholar]
- 9.Faostat, (2019). Food and Agriculture Organization of the United Nations, 2019. Production: Crops. Available online at: http://faostat.fao.org
- 10.FAO . Where is per capita poultry meat consumption highest? Poultry Int. (2018). [Google Scholar]
- 11.AVEC annual report . EU28 Poultry Meat Export Trade, Main Tariff Lines. EU28 Poultry Meat ExportAssociation of Poultry Processors and Poultry Trade in the EU Countries ASBL (2018). p. 1-37. [Google Scholar]
- 12.United states Department of Agriculture . Restrictions on Antibiotic Use for Production Purposes in U.S. Livestock Industries Likely To Have Small Effects on Prices and Quantities. Economic Research Service; (2019). [Google Scholar]
- 13.Dianna VB, Kim MW. Antibiotic-Free Production and Broiler Chicken Meat Safety. Available online at: https://www.food-safety.com/articles/5971-antibiotic-free-production-and-broiler-chicken-meat-safety. (2018) (accessed December 22, 2021).
- 14.Hakimul H, Subir S, Shariful I, Aminul I, Rezaul K, Mohammad EHK, et al. Sustainable antibiotic-free broiler meat production: current trends, challenges, and possibilities in a developing country perspective. Biology. (2020) 9:1–24. 10.3390/biology9110411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letlhogonolo AS, Zahra MH, Tlou GM, Monnye M. The current status of the alternative use to antibiotics in poultry production: an african perspective. Antibiotics. (2020) 9:1–18. 10.3390/antibiotics9090594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paintsil EK, Ofori LA, Akenten CW, Fosu D, Ofori S, Lamshöft M, et al. Antimicrobial usage in commercial and domestic poultry farming in two communities in the Ashanti region of Ghana. Antibiotics. (2021) 10:1–9. 10.3390/antibiotics10070800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman MA, Parvin MS, Sarker RR, Islam MT. Effects of growth promoter and multivitamin-mineral premix supplementation on body weight gain in broiler chickens. J Bangladesh Agril Univ. (2012) 10:245–8. 10.3329/jbau.v10i2.14914 [DOI] [Google Scholar]
- 18.Peric L, Zikic D, Lukic M. Application of alternative growth promoters in broiler production. Biotech Anim Husb. (2009) 25:387–97. 10.2298/BAH0906387P [DOI] [Google Scholar]
- 19.Engberg RM, Hedenann MS, Steenfeldt S, Jensen BB. Influence of Whole Wheat and Xylanase on Broiler Performance and Microbial Composition and Activity in the Digestive Tract. Poultry Science. (2004) 82:925–38. 10.1093/ps/83.6.925 [DOI] [PubMed] [Google Scholar]
- 20.Mehdi Y, Létourneau-Montminy MP, Gaucher M, Chorfi Y, Suresh G, Rouissi T, et al. Use of antibiotics in broiler production: Global impacts and alternatives. Anim Nutr. (2018) 4:170–8. 10.1016/j.aninu.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CSCRA . Canadian Antimicrobial Resistance Surveillance System−2016 Report. Government of Canada, France. (2016). [Google Scholar]
- 22.Nhung NT, Chansiripornchai N, Carrique-Mas JJ. Antimicrobial resistance in bacterial poultry pathogens: a review. Front Vet Sci. (2017) 4:1–17. 10.3389/fvets.2017.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christy M-L, Sampson M, Edson M, Anthony O. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules. (2018) 23:1–48. 10.3390/molecules23040795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oniciuc E, Likotrafiti E, Alvarez-Molina A, Prieto M, Santos J, Alvarez-Ordóñez A. The present and future of whole genome sequencing (WGS) and whole metagenome sequencing (WMS) for surveillance of antimicrobial resistant microorganisms and antimicrobial resistance genes across the food chain. Genes. (2018) 9:1–28. 10.3390/genes9050268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho IT, Santos L. Antibiotics in the aquatic environments: a review of the European scenario. Environ Int. (2018) 94:736–57. 10.1016/j.envint.2016.06.025 [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez RM, Angeles Hernandez JC. Antibiotic and synthetic growth promoters in animal diets: a review of impact and analytical methods. Food Contr. (2017) 72:255–67. 10.1016/j.foodcont.2016.03.001 [DOI] [Google Scholar]
- 27.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. (2005) 84:634–43. 10.1093/ps/84.4.634 [DOI] [PubMed] [Google Scholar]
- 28.Melaku M, Zhong R, Han H, Wan F, Yi B, Zhang H. Butyric and citric acids and their salts in poultry nutrition: effects on gut health and intestinal microbiota. Int J Mol Sci. (2021) 22:1–17. 10.3390/ijms221910392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diarra MS, Malouin F. Antibiotics in canadian poultry productions and anticipated alternatives. Front Microbiol. (2014) 5:282. 10.3389/fmicb.2014.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chand N, Ihsanuddin, Khan RU. Replacement of soybean meal with yeast single cell protein in broiler ration: the effect on performance traits. Pakistan J. Zool. (2014) 46:1753–8. [Google Scholar]
- 31.Khan RU, Naz S, Dhama K. Chromium: pharmacological applications in heat stressed poultry. Int J Pharmacol. (2014) 10:213–317. 10.3923/IJP.2014.213.217 [DOI] [Google Scholar]
- 32.Archawakulathep A, Kim CT, Meunsene D, Handijatno H, Hassim A, Rovira HR, et al. Perspectives on antimicrobial resistance in livestock and livestock products in Asian countries. Wetchasan Sattawaphaet. (2014) 44:5–13.33097262 [Google Scholar]
- 33.Yanhong JH, Benjamin JC. Reducing antibiotic use in livestock, China. Bull World Health Organ. (2020) 98:360–1. 10.2471/blt.19.243501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziping Wu. Antibiotic use and antibiotic resistance in food producing animals in china. 13-15 November 2018. OECD Conference Centre, Paris. (2019). p. 1–32. [Google Scholar]
- 35.Aarestrup F. Sustainable farming: get pigs off antibiotics. Nature. (2012) 486:465–6. 10.1038/486465a [DOI] [PubMed] [Google Scholar]
- 36.Koirala A, Bhandari P, Shewade HD, Tao W, Thapa B, Terry R, et al. Antibiotic use in broiler poultry farms in Kathmandu valley of nepal: which antibiotics and why? Trop Med Infect Dis. (2021) 6:47. 10.3390/tropicalmed6020047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Mustapha AI, Adetunji VO, Heikinheimo A. Risk perceptions of antibiotic usage and resistance: a cross-sectional survey of poultry farmers in Kwara State, Nigeria. Antibiotics. (2020) 9:378. 10.3390/antibiotics9070378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imam T, Gibson JS, Foysal M, Das SB, Gupta SD, Fournié G, et al. Cross-sectional study of antimicrobial usage on commercial broiler and layer chicken farms in Bangladesh. Front Vet Sci. (2020) 7:576113. 10.3389/fvets.2020.576113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moal Aurélie . Consistency Is Vital for Antibiotic-Free Poultry Production. Paris: Adisseo. (2021). [Google Scholar]
- 40.Mousavi S, Ibrahim S, Aroua MK. Sequential nitrification and denitrification in a novel palm shell granular activated carbon twin-chamber upflow bio-electrochemical reactor for treating ammonium-rich wastewater. Bioresour Technol. (2012) 125:256–66. 10.1016/j.biortech.2012.08.075 [DOI] [PubMed] [Google Scholar]
- 41.Shamlo R, Nasr J. Kheiri F. Effects of various levels of pennyroyal (Mentha pulegium L) on carcass characteristics and serum cholesterol in broiler Res Opin Anim Vet Sci. (2014) 4:453–7. [Google Scholar]
- 42.Asad S, Tahseen U, Sarzamin K, Rifat UK. Effect of organic acid supplementation on the performance and ileal microflora of broiler during finishing period, Pakistan J. Zool. (2012) 47:635–9. [Google Scholar]
- 43.Hayden DH, Karla AV, Lixin Z. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals. (2020) 10:1264. 10.3390/ani10081264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Boeckel TP, Glennon EE, Chen D, Gilbert M, Robinson TP, Grenfell BT, et al. Reducing antimicrobial use in food animals. Science. (2017) 357:1350–2. 10.1126/science.aao1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boamah VE, Agyare C, Odoi H, Dalsgaard A. Practices and Factors Influencing the Use of Antibiotics in Selected Poultry Farms in Ghana. J Antimicrob. (2016) 2:1–8. 10.4172/2472-1212.1000120 [DOI] [Google Scholar]
- 46.Smith PW, Agbaje M, LeRoux-Pullen L, Van Dyk D, Debusho LK, Shittu A, et al. Implication of the knowledge and perceptions of veterinary students of antimicrobial resistance for future prescription of antimicrobials in animal health, South Africa. J S Afr Veter Assoc. (2019) 90:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poult Sci. (2003) 82:627–31. 10.1093/ps/82.4.627 [DOI] [PubMed] [Google Scholar]
- 48.Amaechi N, Amaeze PN. Effect of dietary chloroacetic acid as antibiotic replacer on the gastrointestinal microflora and gut morphology of weanling pigs. Res Opin Anim Vet Sci. (2012) 2:494–8. [Google Scholar]
- 49.Joerger RD. Alternatives to antibiotics: bacteriocins, antimicrobial peptides, and bacteriophages. Poult Sci. (2002) 82:640–7. 10.1093/ps/82.4.640 [DOI] [PubMed] [Google Scholar]
- 50.Waldroup PW, Oviedo-Rondon EO, Fritts CA. Comparison of bio-mos® and antibiotic feeding programs in broiler diets containing copper sulfate. Int J Poult Sci. (2003) 2:28–31. 10.3923/ijps.2003.28.31 [DOI] [Google Scholar]
- 51.Suganya T, Senthilkumar S, Deepa K, Muralidharan J, Gomathi G, Gobiraju S. Herbal feed additives in poultry. Int J Sci Environ Technol. (2016) 5:1137–45. [Google Scholar]
- 52.Qidong Z, Peng S, Bingkun Z. Ling k, Chuanpi X, Zhigang S. Progress on gut health maintenance and antibiotic alternatives in broiler chicken production. Front Nutr. (2021) 8:692839. 10.3389/fnut.2021.692839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jet SM. Florencia NS. Phytogenic feed additives as an alternative to antibiotic growth promoters in poultry nutrition. Advanced studies in the 21st century. Animal Nutr. (2021) 8:1–18. 10.5772/intechopen.99401 [DOI] [Google Scholar]
- 54.Mohamed E, Mohamed T, Heba M, Amira M, Mohamed M, Gehan BA, et al. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poultry Sci. (2022) 101:101696. 10.1016/j.psj.2022.101696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakine Y, Ebru E, Reisli Z, Suzan Y. Effect of garlic powder on the performance, egg traits, and blood parameters of laying hens. J Food Sci. (2006) 86:1336–9. 10.1002/jsfa.2515 [DOI] [Google Scholar]
- 56.Sandra DS, Doris D, Debrabrata B, Irene H. Botanical alternatives to antibiotics for use in organic poultry production. Poult Sci. (2015) 94:1419–30. 10.3382/ps/pev014 [DOI] [PubMed] [Google Scholar]
- 57.Reda FM, El-Saadony MT, El-Rayes TK, Attia AI, El-Sayed SA, Ahmed SY, et al. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilization, blood metabolites and intestinal microbiota. Ital J Anim Sci. (2021) 20:324–35. 10.1080/1828051X.2021.1886001 [DOI] [Google Scholar]
- 58.Gunal M, Yayli G, Kaya O, Karahan N, Sulak O. The effects of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. Poult Sci. (2006) 5:149–55. [Google Scholar]
- 59.Polycarpo GV, Andretta I, Kipper M, Cruz-Polycarpo VC, Dadalt JC, Rodrigues PHM, et al. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult Sci. (2017) 96:3645–53. 10.3382/ps/pex178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biggs P, Parsons CM. The effects of several oligosaccharides on true amino acid digestibility and true metabolizable energy in cecectomized and conventional roosters. Poult Sci. (2007) 86:1161–5. 10.1093/ps/86.6.1161 [DOI] [PubMed] [Google Scholar]
- 61.Awad WA, Bohm J, Razzazi-Fazeli E, Ghareeb K, Zentek J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult Sci. (2006) 85:974–9. 10.1093/ps/85.6.974 [DOI] [PubMed] [Google Scholar]
- 62.Viveros AA, Brenes M, Pizarro G, Castanb M. Effect of enzyme supplementation of a diet based on barly, and actoclave apparent digestibility, growth performance and got morphology of broilers. Anim Feed Sci Technol. (1994) 48:237–51. [Google Scholar]
- 63.Sheiha AM, Abdelnour SA, Abd-El-Hack ME, Khafaga AF, Metwally KA, El-Saadony MT. Effects of dietary biological or chemical-synthesized nanoselenium supplementation on growing rabbits exposed to thermal stress. Animals. (2020) 10:430. 10.3390/ani10030430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reda FM, El-Saadony MT, Elnesr SS, Alagawany M, Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity, and caecal microbiota of Japanese quails. Animals. (2020) 10:754. 10.3390/ani10050754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puvaca NV, Glamocic D, Lević J, Peric L, Milic D. Beneficial effects of phytoadditives in broiler nutrition. World's Poult Sci J. (2013) 69:27–34. 10.1017/S0043933913000032 [DOI] [Google Scholar]
- 66.Bravo D, Pirgozliev V, Rose SPA. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin improves the energy utilization and growth performance of broiler chickens fed a maize-based diet. J Anim Sci. (2014) 92:1531–6. 10.2527/jas.2013-6244 [DOI] [PubMed] [Google Scholar]
- 67.Pirgozliev V, Beccaccia A, Rose SP. Partitioning of dietary energy of chickens fed maize- or wheat-based diets with and without a commercial blend of phytogenic feed additives. J Ani Sci. (2015) 93:1695–702. 10.2527/jas.2014-8175 [DOI] [PubMed] [Google Scholar]
- 68.Madhupriya V, Shamsudeen P, Raj Manohar G, Senthilkumar S, Soundarapandiyan V. Moorthy M. Phyto feed additives in poultry nutrition - A review. Int J Sci Environ Technol. (2018) 7:815–22. [Google Scholar]
- 69.Abdelnour SA, Swelum AA, Salama A, Al-Ghadi MQ, Qattan SYA, Abd ElHack ME, et al. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital J Anim Sci. (2020) 19:1046–56. 10.1080/1828051X.2020.1815598 [DOI] [Google Scholar]
- 70.Ogbuewu IP, Okoro VM, Mbajiorgu CA. Meta-analysis of the influence of phytobiotic (pepper) supplementation in broiler chicken performance. Trop Anim Health Prod. (2020) 52:17–30. 10.1007/s11250-019-02118-3 [DOI] [PubMed] [Google Scholar]
- 71.Abd Elkader AM, Labib S, Taha TF, Althobaiti F, Aldhahrani A, Salem HM, Saad HM, Ibrahim AFM. Phytogenic compounds from avocado (Persea Americana L.) extracts; antioxidant activity, amylase inhibitory activity, the therapeutic potential of type 2 diabetes. Saudi J Biol Sci. (2021) 29:1428–33. 10.1016/j.sjbs.2021.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdel-Moneim AME, El-Saadony MT, Shehata AM, Saad AM, Aldhumri SA, Ouda SM, et al. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J Biol Sci. (2021) 29:1197–209. 10.1016/j.sjbs.2021.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ashour EA, Farsi RM, Alaidaroos BA, Abdel-Moneim AME, El-Saadony MT, Osman AO, et al. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcass traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital J Anim Sci. (2021) 20:1357–72. 10.1080/1828051X.2021.1924087 [DOI] [Google Scholar]
- 74.Peng QY Li JD, Li Z, Duan ZY, Wu YP. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits, and jejunal morphology in broiler chickens. Anim Feed Sci Technol. (2016) 214:148–53. 10.1016/J.ANIFEEDSCI.2016.02.010 [DOI] [Google Scholar]
- 75.Toghyani M, Toghyani M, Gheisari A, Ghalamkari G, Eghbalsaied S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest Sci. (2011) 138:167–73. 10.1016/j.livsci.2010.12.018 [DOI] [Google Scholar]
- 76.Mpofu DA, Marume U, Mlambo V, Hugo A. The effects of Lippia javanica dietary inclusion on growth performance, carcass characteristics, and fatty acid profiles of broiler chickens. Anim Nutri. (2016) 2:160–7. 10.1016/j.aninu.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirubakaran A, Moorthy M, Chitra R, Prabakar G. Influence of combinations of fenugreek, garlic, and black pepper powder on production traits of the broilers. Vet World. (2016) 9:470–4. 10.14202/vetworld.2016.470-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodarzi M, Nanekarani S. Effects of feeding mentha Pulegium L. As an alternative to antibiotics on the performance of broilers. APCBEE Procedia. (2014) 8:53–8. 10.1016/j.apcbee.2014.01.079 [DOI] [Google Scholar]
- 79.Landy N, Ghalamkari G, Toghyani M. Performance, carcass characteristics, and immunity in broiler chickens fed dietary neem (Azadirachta Indica) as an alternative for an antibiotic growth promoter. Livest Sci. (2011) 142:305–9. 10.1016/j.livsci.2011.08.017 [DOI] [Google Scholar]
- 80.Zhang ZF, Cho JH, Kim IH. Effects of Bacillus subtilis Ubt-Mo2 on growth performance, relative immune Organ weight, gas concentration in excreta, and intestinal microbial shedding in broiler chickens. Livest Sci. (2013) 155:343–7. 10.1016/j.livsci.2013.05.021 [DOI] [Google Scholar]
- 81.Toghyani M. Mosavi Sk, Modaresi M, Landy N. Evaluation of kefir as a potential probiotic on growth performance, serum biochemistry and immune responses in broiler chicks. Animal Nutr. (2015) 1:305–9. 10.1016/j.aninu.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mountzouris KC. Phytogenic and probiotic feed additives for broilers: Evidence for growth performance links with gut performance indices. Pages 107–116 in Proceedings of the 2016 World Nutrition Forum. Erber Ag, Austria: (2016). [Google Scholar]
- 83.Platel K, Srinivasan K. Digestive stimulant action of spices: a myth or reality? Indian J Med Res. (2004) 119:167–79. [PubMed] [Google Scholar]
- 84.Wati T, Ghosh TK, Syed B, Haldar S. Comparative efficacy of a phytogenic feed additive and an antibiotic growth promoter on production performance, caecal microbial population and humoral immune response of broiler chickens inoculated with enteric pathogens. Anim Nutr. (2015) 1:213–9. 10.1016/j.aninu.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tazi SM, Mukhtar MA, Mohamed KA, Tabidi MH. Effect of using black pepper as natural feed additive on performance and carcass quality of broiler chicks. Global advanced research. J Agri Sci. (2014) 4:108–13. [Google Scholar]
- 86.Frankic T, Volj CM, Salobir J, Rezar V. Use of herbs and spices and their extracts in animal nutrition. Acta Agric Slov. (2009) 94:95–102. [Google Scholar]
- 87.Cuppett SL, Hall CA. Antioxidant activity of Labiatae. Adv Food Nutri Res. (1998) 42:245–71. 10.1016/S1043-4526%2808%2960097-2 [DOI] [PubMed] [Google Scholar]
- 88.Jarriyawattanachaikul W, Chaveerach P, Chokesajjawatee N. Antimicrobial activity of Thai-Herbal plants against food-borne pathogens E. Coli, S. Aureus, and C. Jejuni. Agri. Agri Sci Procedia. (2016) 11:20–4. 10.1016/J.AASPRO.2016.12.004 [DOI] [Google Scholar]
- 89.Huyghebaert G, Ducatelle R, Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. (2011) 187:182–8. 10.1016/j.tvjl.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 90.Li HL, Zhao PY, Lei Y, Hossain MM, Kim IH. Phytoncide, phytogenic feed additive as an alternative to conventional antibiotics, improved growth performance and decreased excreta gas emission without adverse effect on meat quality in broiler chickens. Livest Sci. (2015) 181:1–6. 10.1016/j.livsci.2015.10.001 [DOI] [Google Scholar]
- 91.Alcicek AH, Bozkurt M, Çabuk M. The effect of a mixture of herbal essential oils, an organic acid or a probiotic on broiler performance. S Afr J Anim Sci. (2004) 34:217–22. [Google Scholar]
- 92.Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. (2010) 15:9252–87. 10.3390/molecules15129252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hammer KA, Carson CF. Antibacterial and Antifungal Activities of Essential Oils. In Lipids and Essential Oils as Antimicrobial Agents; First edition, NJ, USA, Hoboken: John Wiley & Sons, Ltd. (2011). p. 255–306. [Google Scholar]
- 94.Joshua JF, Bhaskar G, Sami D. Phytogenic feed additives improve broiler feed efficiency via modulation of intermediary lipid and protein metabolism–related signaling pathways. Poult Sci. (2021) 100:1–11. 10.1016/j.psj.2020.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orlowski S, Flees J, Greene ES, Ashley D, Lee SO, Yang FL, et al. Effects of phytogenic additives on meat quality traits in broiler chickens1. J Anim Sci. (2018) 96:3757–67. 10.1093/jas/sky238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flees J, Greene E, Ganguly B, Dridi S. Phytogenic feedand water-additives improve feed efficiency in broilers via modulation of (an)orexigenic hypothalamic neuropeptide expression. Neuropeptides. (2020) 81:102005. 10.3390/ani11030750 [DOI] [PubMed] [Google Scholar]
- 97.Lambert RJW, Proteus NS, Proteus JC, Nychas G-JE. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol, and carvacrol. J Appl Microbiol. (2001) 91:453–62. [DOI] [PubMed] [Google Scholar]
- 98.Yang C, Chowdhury MAK, Hou Y, Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. (2015) 4:137–56. 10.3390/pathogens4010137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeng Z, Zhang S, Wang H, Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J Anim Sci Biotechnol. (2015) 6:1–10. 10.1186/s40104-015-0004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soliman KM, Badeaa RI. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol. (2002) 40:1669–75. 10.1016/S0278-6915%2802%2900120-5 [DOI] [PubMed] [Google Scholar]
- 101.Burt S. Essential oils: their antibacterial properties and potential applications in foods- a review. Int J Food Microbiol. (2004) 94:223–53. 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 102.Peng M, Salaheen S, Biswas D. Animal health: global antibiotic issues. In Encyclopedia of Agriculture and Food Systems. Van Alfen, NK, Ed Oxford, UK: Academic Press. (2014) 1:346–57. 10.1016/B978-0-444-52512-3.00187-X [DOI] [Google Scholar]
- 103.French D. Chapter five- advances in clinical mass spectrometry. In Advances in Clinical Chemistry. Makowski, GS, Ed. San Francisco, CA, USA; Elsevier. (2017) 79:153–98. 10.1016/bs.acc.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 104.Chahardoli A, Jalilian F, Memariani Z, Farzaei MH, Shokoohinia Y. Analysis of organic acids. Sanches Silva A, Nabavi SF, Saeedi M, Nabavi SM, Eds. In Recent Advances in Natural Products Analysis. Amsterdam, The Netherlands: Elsevier: (2020). p. 767–823. 10.1016/b978-0-12-816455-6.00026-3 [DOI] [Google Scholar]
- 105.Yadav AS, Kolluri G, Gopi M, Karthik K, Malik YS, Dhama K. Exploring alternatives to antibiotics as health promoting agents in poultry- a review. J Exp Boil Agric Sci. (2016) 4:368–83. 10.18006/2016.4%283S%29.368.383 [DOI] [Google Scholar]
- 106.Hermans D, De Laet M. Reaching genetic potential with medium chain fatty acids (MCFAs). Int Poult Prod. (2014) 22:7–9. [Google Scholar]
- 107.Hajati H. Application of organic acids in poultry nutrition. Int J Avian & Wildlife Biol. (2018) 3:324–329. [Google Scholar]
- 108.Adil S, Banday MT, Bhat GA, Mir MS, Rehman M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Veter Med Int. (2010) 8:1–7. 10.4061/2010/479485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hassan HMA, Mohamed MA, Youssef AW, Hassan ER. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal microflora of broilers.. J Anim Sci. (2010) 23:1348–53. 10.5713/AJAS.2010.10085 [DOI] [Google Scholar]
- 110.Qaisrani S, Van Krimpen M, Kwakkel R, Verstegen M, Hendriks W. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poult Sci. (2015) 94:2152–64. 10.3382/ps/pev003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diether NE, Willing BP. Microbial fermentation of dietary protein: an important factor in diet-microbe-host interaction. Microorganisms. (2019) 7:1–14. 10.3390/microorganisms7010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ikker P, Dirkzwager A, Fledderus J, Trevisi P, Le Huërou-Luron I, Lallès J, et al. Dietary protein and fermentable carbohydrates contents influence growth performance and intestinal characteristics in newly weaned pigs. Livest Sci. (2007) 108:194–7. 10.1016/j.livsci.2007.01.057 [DOI] [PubMed] [Google Scholar]
- 113.Partanen KH, Morz Z. Organic acids for performance enhancement in pig diets. Nutr Res Rev. (1999) 12:117–45. 10.1079/095442299108728884 [DOI] [PubMed] [Google Scholar]
- 114.Suiryanrayna MV, Ramana JV. A Review of the effects of dietary organic acids fed to swine. J Anim Sci Biotechnol. (2015) 6:45. 10.1186/s40104-015-0042-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Christian L, Mellor S. The use of organic acids in animal nutrition, with special focus on dietary potassium deformity under European and Austral-Asian conditions. Recent Adv Anim Nutr Aus. (2011) 4:123–30. [Google Scholar]
- 116.Lan Y, Verstegen MWA, Tamminga S, Williams BA. The role of the commensal gut microbial community in broiler chickens. Worlds Poult Sci J. (2005) 61:95–104. 10.1079/WPS200445 [DOI] [Google Scholar]
- 117.Araujo, Robert GAC, Polycarpo GV, Adriano B, Kelry MS, Gabriela V, et al. Performance and economic viability of broiler chickens fed with probiotic and organic acids in an attempt to replace growth-promoting antibiotics. Braz J Poult Sci. (2019) 21:1–7. [Google Scholar]
- 118.Christian L. Effect of dietary sodium deformity on growth performance, nutrient digestibility, gut health, and profitability in broilers. 20th European Symposium on Poultry Nutrition Poster presentation. Available online at: http://en.engormix.com/MA-poultry industry/events/20th-european-symposium-poultry-nutrition-2015-t2254.htm
- 119.Naseri KG, Rahimi S, Khaki P. Comparison of the effects of probiotics, organic acid, and medicinal plant on Campylobacter jejuni challenged broiler chickens. J Agric Sci Technol. (2012) 14:1485–96. [Google Scholar]
- 120.Tschirner K, Susenbeth A, Wolffram S. Influence of Sangrovit supplementation on nitrogen balance and feed intake in growing pigs. Ninth Symposium Vitamins and Additives in the Nutrition of Man and Animal. Jena: Friedrich Schiller University; (2003). p. 45. [Google Scholar]
- 121.Vukic Vramjes M, Wenk C. Influence of Dietary Enzyme Complex on the Performance of Broilers Fed on Bites with and without Antibiotic Supplementation. Br Poult Sci. (1995) 36:265–75. 10.1080/00071669508417774 [DOI] [PubMed] [Google Scholar]
- 122.Bafundo KW, Cox LA, Bywater R. Review Lends Perspective to Recent Sciectific Findings on virginiamycin, antibiotic resistance debate. Feed Stuffs. (2002) 75:26–7. [Google Scholar]
- 123.Lan RX Li SQ, Zhao Z, An L. Sodium butyrate as an effective feed additive to improve growth performance and gastrointestinal development in broilers. Vet Med Sci. (2020) 6:491–9. 10.1002/vms3.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mohammadagheri N, Najafi R, Najafi G. Effects of dietary supplementation of organic acids and phytase on performance and intestinal histomorphology of broilers. Vet Res Forum. (2016) 7:189–95. [PMC free article] [PubMed] [Google Scholar]
- 125.Chowdhury R, Islam K, Khan MJ, Karim MR, Haque MN, Khatun M, et al. Effect of citric acid, avilamycin, and their combination on the performance, tibia ash, and immune status of broilers. Poult Sci. (2009) 88:1616–22. 10.3382/ps.2009-00119 [DOI] [PubMed] [Google Scholar]
- 126.Leeson S, Namkung H, Antongiovanni M, Lee EH. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult Sci. (2005) 84:1418–22. 10.1093/ps/84.9.1418 [DOI] [PubMed] [Google Scholar]
- 127.Panda AK, Rao SVR, Raju MV, Sunder GS. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian Australas J Anim Sci. (2009) 22:1026–31. 10.5713/ajas.2009.80298 [DOI] [Google Scholar]
- 128.Namkung H, Yu H, Gong J, Leeson S. Antimicrobial activity of butyrate glycerides toward salmonella typhimurium and clostridium perfringens. Poult Sci. (2011) 90:2217–22. 10.3382/ps.2011-01498 [DOI] [PubMed] [Google Scholar]
- 129.Song B, Li H, Wu Y, Zhen W, Wang Z, Xia Z, et al. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim Feed Sci Technol. (2017) 232:6–15. 10.1016/j.anifeedsci.2017.07.009 [DOI] [Google Scholar]
- 130.Kaczmarek SA, Barri A, Hejdysz M, Rutkowski A. Effect of different doses of coated butyric acid on growth performance and energy utilization in broilers. Poult Sci. (2016) 95:851–9. 10.3382/ps/pev382 [DOI] [PubMed] [Google Scholar]
- 131.Naela M, Reda M. Korany. Studying the effect of formic acid and potassium deformity on performance, immunity and gut health of broiler chickens;. Animal Nutr. (2016) 2:296–302. 10.1016/j.aninu.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim GB, SeoC MYH, KimI K. Paik Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult Sci. (2011) 90:75–82. 10.3382/ps.2010-00732 [DOI] [PubMed] [Google Scholar]
- 133.Rehman A, Arif M, Sajjad N, Al-Ghadi MQ, Alagawany M, Abd El-Hack ME, et al. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult Sci. (2020) 99:6946–53. 10.1016/j.psj.2020.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saminathan M, Chin CS, Kalavathy R, Norhani A, Yin WH. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J Sci Food Agric. (2014) 94:341–8. 10.1002/jsfa.6365 [DOI] [PubMed] [Google Scholar]
- 135.Froebel LK, Jalukar S, Lavergne TA, Lee JT, Duong T. Administration of dietary prebiotics improves growth performance and reduces pathogen colonization in broiler chickens. Poult Sci. (2019) 98:6668–76. 10.3382/ps/pez537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Emmanuel U, Medani E, Edwin P, Apeh A, Mohammed AQ, Harriet G, et al. Influence of dietary supplementation of autolyzed whole yeast and yeast cell wall products on broiler chickens. Asian-Australas J Anim Sci. (2020) 33:579–87. 10.5713/ajas.19.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Midilli M, Kocabach M, Alp N, Muglah O, Turan N, Yilmaz H, et al. Effects of dietary probiotic and prebiotic supplementation on growth performance and serum IgG concentration of broilers A J. An Sci. (2008) 38:21–7. [Google Scholar]
- 138.Kazi KI. Atiqul Md, Mahedul Md, Khaled MS, Kamrul Md, Mohammad AM. Effects of selected probiotics and synbiotics on growth performance and blood-biochemical changes in broiler chickens. J Bangladesh Agril Univ. (2021) 19:471–6. 10.5455/JBAU.120923 [DOI] [Google Scholar]
- 139.Airat K, Fail K, Vyacheslav K, Hamit T, Maksim R, Aleksandra A, et al. Effect of normosil probiotic supplementation on the growth performance and blood parameters of broiler chickens. Indian J Pharmaceut Edu Res. (2020) 54:1046–55. 10.5530/ijper.54.4.199 [DOI] [Google Scholar]
- 140.Deraz SF. Synergetic effects of multispecies probiotic supplementation on certain blood parameters and serum biochemical profile of broiler chickens. J Anim Health Prod. (2018) 6:27–34. 10.17582/JOURNAL.JAHP%2F2018%2F6.1.27.34 [DOI] [Google Scholar]
- 141.Kumprecht I, Zobac P. The effect of probiotic preparations containing Saccharomyces cerevisiae and Enterococcus faecium in diets with different levels of beta-vitamins on chicken broiler performance. Czech J Anim Sci UZPI. (1998) 43:63–70. [Google Scholar]
- 142.Kulshreshtha G, Rathgeber B, Stratton G, Thomas N, Evans F, Critchley A, et al. Feed supplementation with red seaweeds, Chondrus crispus, and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult Sci. (2014) 93:2991–3001. 10.3382/ps.2014-04200 [DOI] [PubMed] [Google Scholar]
- 143.Murate LS, Paião FG, de Almeida AM, Berchieri A, Jr, Shimokomaki M. Efficacy of prebiotics, probiotics, and synbiotics on laying hens and broilers challenged with Salmonella enteritidis. J Poult Sci. (2015) 52:52–6. 10.2141/jpsa.0130211 [DOI] [Google Scholar]
- 144.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition, Types, sources, mechanisms, and clinical applications. Foods. (2019) 8:92. 10.3390/foods8030092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wassie T, Niu K, Xie C, Wang H, Xin W. Extraction techniques, biological activities and health benefits of marine algae Enteromorpha Prolifera polysaccharide. Front Nutr. (2021) 8:747928. 10.3389/fnut.2021.747928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Coppa GV, Zampini L, Galeazzi T, Gabrielli O. Prebiotics in human milk: a review. Dig Liver Dis. (2006) 38:291–4. 10.1016/S1590-8658(07)60013-9 [DOI] [PubMed] [Google Scholar]
- 147.Bird AR, Conlon MA, Christophersen CT, Topping DL. Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef Microbes. (2010) 1:423–31. 10.3920/BM2010.0041 [DOI] [PubMed] [Google Scholar]
- 148.Al-Sultan S. Abdel- Raheem SM, El- Ghareeb WR, Mohamed MHA. Comparative effects of using prebiotic, probiotic, symbiotic and acidifier on growth performance, intestinal microbiology and histomorphology of broiler chicks Japanese. J Veterinary Res. (2016) 64:S187–95. [Google Scholar]
- 149.Amrit PK, Sonali B, Daljeet SD, Eugenie N, Natália C-M, Kamil K, et al. Plant prebiotics and their role in the amelioration of diseases. Biomolecules. (2021) 11:1–25. 10.3390/biom11030440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dallies N, Francois J, Paquet V. A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast. (1998) 14:1297–306. 10.1002/(SICI)1097-0061(1998100)14:14%3C1297::AID-YEA310%3E3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 151.Sun HY, Jiang ZN, Shen X, Xu SX. Report on the use of veterinary antibiotics in China in 2018. China Anim Health. (2019) 21:8–9. [Google Scholar]
- 152.Gibson GR, Fuller R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J Nutr. (2000) 130:391–5. 10.1093/jn/130.2.391s [DOI] [PubMed] [Google Scholar]
- 153.Doyle ME. Alternatives to Antibiotic Use for Growth Promotion in Animal Husbandry; Food Research Institute. Madison, WI: University of Wisconsin-Madison; (2001). [Google Scholar]
- 154.Baurhoo B, Phillip L, Ruiz-Feria CA. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult Sci. (2007) 86:1070–8. 10.1093/ps/86.6.1070 [DOI] [PubMed] [Google Scholar]
- 155.Petersen CB. Comparative effects of ZooLac, Bio-MOS, and Bio-Pro on the performance of broilers to 36 days. Poster In Biotechnology in the Feed Industry Proc Alltechs 14th Annual Symposium; Lyons, TP, Ed. Nicholasville, KY, USA: Archivos de Medicina Veterinaria. (1998). [Google Scholar]
- 156.Shashidhara RG, Devegowda G. Effect of dietary mannan oligosaccharide on broiler breeder production traits and immunity. Poult Sci. (2003) 82:1319–25. 10.1093/ps/82.8.1319 [DOI] [PubMed] [Google Scholar]
- 157.Sinovec Z, Markovic R. Use of pre-biotics in poultry nutrition. Biotehnol Stoc. (2005) 21:235–9. [Google Scholar]
- 158.Spring P. Effects of Mannanoligosaccharide on Different Cecal Parameters and on Cecal Concentrations of enteric Pathogens in Poultry. PhD Thesis, Zurich, Switzerland: Swiss Fed Inst Tech. (1996). [Google Scholar]
- 159.Mazanko MS, Gorlov IF, Prazdnova EV, Makarenko MS, Usatov AV, Bren AB, et al. Bacillus probiotic supplementations improve laying performance, egg quality, hatching of laying hens, and sperm quality of roosters. Probiotics Antimicrob Proteins. (2018) 10:367–73. 10.1007/s12602-017-9369-4 [DOI] [PubMed] [Google Scholar]
- 160.Neupane D, Nepali DB, Devkota N, Sharma MP, Kadaria IP. Effect of Probiotics on production and egg quality of dual-purpose chicken at Kathmundu in Nepal. Bangladesh J Anim Sci. (2019) 48:29–35. 10.3329/bjas.v48i1.44556 [DOI] [Google Scholar]
- 161.Yaqoob M, Abd El-Hack ME, Hassan F, El-Saadony MT, Khafaga A, Batiha G, Yehia N, Elnesr S, Alagawany M, El-Tarabily KA, Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult Sci. (2021) 100 7:101143. 10.1016/j.psj.2021.101143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Po-Yun T, Woo KK. Review: roles of prebiotics in intestinal ecosystem of broilers. Front Vet Sci. (2018) 5:245. 10.3389/fvets.2018.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang Y, Iji PA, Kocher A, Mikkelsen LL, Choct M. Effects of mannanoligosaccharide and fructooligosaccharide on the response of broilers to pathogenic Escherichia coli challenge. Br Poult Sci. (2008) 49:550–9. 10.1080/00071660802290408 [DOI] [PubMed] [Google Scholar]
- 164.Chee SH, Iji PA, Choct M, Mikkelsen LL, Kocher A. Characterization and response of intestinal microflora and mucins to manno-oligosaccharide and antibiotic supplementation in broiler chickens. Br Poult Sci. (2010) 51:368–80. 10.1080/00071668.2010.503477 [DOI] [PubMed] [Google Scholar]
- 165.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. (2011) 5:220–30. 10.1038/ismej.2010.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.BogusŁAwska-Tryk M, Piotrowska A, Burlikowska K. Dietary fructans and their potential beneficial influence on health and performance parameters in broiler chickens. J Cent Eur Agric. (2012) 13:270–88. 10.5513/JCEA01/13.2.104529846691 [DOI] [Google Scholar]
- 167.Troy EB, Kasper DL. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci-Landmark. (2010) 15:25–34. 10.2741/3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bonos E, Christaki E, Abrahim A, Soultos N, Florou-Paneri P. The influence of mannan oligosaccharides, acidifiers and their combination on cecal microflora of Japanese quail (Coturnix japonica). Anaerobe. (2011) 17:436–9. 10.1016/j.anaerobe.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 169.Tizard IR. Veterinary Immunology. 9th ed St Louis, MO: Elsevier. (2013). [Google Scholar]
- 170.Kogut MH. The gut microbiota and host innate immunity: regulators of host metabolism and metabolic diseases in poultry? J Appl Poult Res. (2013) 22:637–46. 10.3382/japr.2013-00741 [DOI] [Google Scholar]
- 171.Gustavo PA-M, Sandy A, Laura MB, Larissa CZ, Ricardo W, Karina RB. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol. (2018) 9:1–19. 10.3389/fimmu.2018.02379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Indikova I, Humphrey TJ, Hilbert F. Survival with a helping hand: campylobacter and microbiota. Front Microbiol. (2015) 6:1–6. 10.3389/fmicb.2015.01266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shini S, Zhang D, Aland RC Li X, Dart PJ, Callaghan MJ, Speight RE, et al. Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency. Poult Sci. (2020) 99:4278–93. 10.1016/j.psj.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Plavnik I, Scott ML. Effects of additional vitamins, minerals, or brewer's yeast upon leg weaknesses in broiler chickens. Poult Sci. (1980) 59:459–64. 10.3382/ps.0590459 [DOI] [PubMed] [Google Scholar]
- 175.Anadón A, Martínez-Larrañaga MR, Martínez M. Probiotics for animal nutrition in the European Union. regulation and safety assessment. Regul Toxicol Pharmacol. (2006) 45:91–5. 10.1016/j.yrtph.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 176.Al-Khalaifa H, Al-Nasser A, Al-Surayee T, Al-Kandari S, Al-Enzi N, Al-Sharrah T, et al. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult Sci. (2019) 98:4465–79. 10.3382/ps/pez282 [DOI] [PubMed] [Google Scholar]
- 177.Guyard-Nicodème M, Keita A, Quesne S, Amelot M, Poëzévara T, Le Berre B, et al. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult Sci. (2016) 95:98–305. 10.3382/ps/pev303 [DOI] [PubMed] [Google Scholar]
- 178.Peralta-Sánchez JM, Martín-Platero AM, Ariza-Romero JJ, Rabelo-Ruiz M, Zurita-González MJ, Baños A, et al. Egg production in poultry farming is improved by probiotic bacteria. Front Microbiol. (2019) 10:1042. 10.3389/fmicb.2019.01042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qamar A, Waheed J, Hamza A, Mohyuddin SG, Lu Z, Namula Z, et al. Chen JJ. The role of intestinal microbiota in chicken health, intestinal physiology, and immunity. J Anim Plant Sci. (2020) 31:342–51. 10.36899/JAPS.2021.2.0221 [DOI] [Google Scholar]
- 180.Wu Y, Wang B, Zeng Z, Liu R, Tang L, Gong L, et al. Effects of probiotics Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 on intestinal barrier function, antioxidative capacity, apoptosis, immune response, and biochemical parameters in broilers. Poult Sci Poultry Science Association Inc. (2019) 98:5028–39. 10.3382/ps/pez226 [DOI] [PubMed] [Google Scholar]
- 181.Singh AK, Tiwari UP, Berrocoso JD, Dersjant-Li Y, Awati A, Jha R. Effects of a combination of xylanase, amylase and protease, and probiotics on major nutrients including amino acids and non-starch polysaccharides utilization in broilers fed different level of fibers. Poult Sci. (2019) 98:5571–81. 10.3382/ps/pez310 [DOI] [PubMed] [Google Scholar]
- 182.Wealleans AL, Walsh MC, Romero LF, Ravindran V. Comparative effects of two multi-enzyme combinations and a Bacillus probiotic on growth performance, digestibility of energy and nutrients, disappearance of non-starch polysaccharides, and gut microflora in broiler chickens. Poult Sci. (2017) 96:4287–97. 10.3382/ps/pex226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.De Cesare A, Caselli E, Lucchi A, Sala C, Parisi A, Manfreda G, et al. Impact of a probiotic-based cleaning product on the microbiological profile of broiler litters and chicken caeca microbiota. Poult Sci. (2019) 98:3602–10. 10.3382/ps/pez148 [DOI] [PubMed] [Google Scholar]
- 184.Patel SG, Raval AP, Bhagwat SR, Sadrasaniya DA, Patel AP, Joshi SS. Effects of probiotics supplementation on growth performance, feed conversion ratio, and economics of broilers. J Ani Res. (2015) 5:155–60. [Google Scholar]
- 185.Mizak L, Gryko R, Kwiatek M, Parasion S. Probiotics in animal nutrition. Zycie Weter. (2012) 87:736–42. [Google Scholar]
- 186.Bajagai YS, Klieve AV, Peter JD BW L. Probiotics in animal nutrition- production, impact, and regulation. Makkar HPS, editor FAO Anim Prod Heal FAO Animal Production and Health. (2016) 179:1–89. [Google Scholar]
- 187.Markowiak P, Slizewska K. The role of probiotics, prebiotics, and synbiotics in animal nutrition. Gut Pathog BioMed Central. (2018) 10:1–20. 10.1186/s13099-018-0250-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zommiti M, Chikindas ML, Ferchichi M. Probiotics-live biotherapeutics: a story of success, limitations, and future prospects-not only for humans. Probiotics antimicrob proteins. Probio Antimicro Prot. (2020) 12:1266–89. 10.1007/s12602-019-09570-5 [DOI] [PubMed] [Google Scholar]
- 189.Khan RU, Naz S. The applications of probiotics in poultry production. Worlds Poult Sci J. (2013) 69:621–32. 10.1017/S0043933913000627 [DOI] [Google Scholar]
- 190.AlFatah MA. Probiotic modes of action and its effect on biochemical parameters and growth performance in poultry. Iran J Appl Anim Sci. (2020) 10:9–15. [Google Scholar]
- 191.Sandvang D, Skjoet-Rasmussen L, Cantor MD, Mathis GF, Lumpkins BS, Blanch A. Effects of feed supplementation with 3 different probiotic Bacillus strains and their combination on the performance of broiler chickens challenged with Clostridium perfringens. Poult Sci. (2021) 100:1–10. 10.1016/j.psj.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Abd El-Hack ME, Alaidaroos BA, Farsi RM, Abou-Kassem DE, El-Saadony MT, Saad AM, et al. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles, and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality, and cecal microbial load. Animals. (2021) 11:1–24. 10.3390/ani11071878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Celina EB, Tamiris NSS. Probiotics as a Promising Additive in Broiler Feed: Advances and Limitations, Advances in Poultry Nutrition Research. (2021). [Google Scholar]
- 194.Wang C, Shi C, Su W, Jin M, Xu B, Hao L, et al. Dynamics of the physicochemical characteristics, microbiota, and metabolic functions of soybean meal and corn mixed substrates during two-stage solid-state. App Environmen Sci. (2020) 5:1–17. 10.1128/mSystems.00501-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Alagawany Elnesr Farag . The role of exogenous enzymes in promoting growth and improving nutrient digestibility in poultry. IJVR. (2018) 19:157–64. [PMC free article] [PubMed] [Google Scholar]
- 196.Ojha BK, Singh PK, Shrivastava N. Enzymes in the Animal Feed Industry. in Enzymes in Food Biotechnology. Mohammed, K., Ed.; Cambridge, MA, USA: Academic Press; (2019). p. 93–109. 10.1016/B978-0-12-813280-7.00007-4 [DOI] [Google Scholar]
- 197.Velázquez-De Lucio BS, Hernández-Domínguez EM, Villa-García M, Díaz-Godínez G, Mandujano-Gonzalez V, Mendoza-Mendoza B, et al. Exogenous enzymes as zootechnical additives in animal feed: a review. Catalysts. (2021) 11:851. 10.3390/catal11070851 [DOI] [Google Scholar]
- 198.Ravindran V. Feed enzymes: the science, practice, and metabolic realities. J Appl Poult Res. (2013) 22:628–36. 10.3382/japr.2013-00739 [DOI] [Google Scholar]
- 199.Amerah A, Romero L, Awati A, Ravindran V. Effect of exogenous xylanase, amylase, and protease as single of combined activities on nutrient digestibility and growth performance of broilers fed corn/soy diets. Poult Sci. (2017) 96:807–16. 10.3382/ps/pew297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shimaa A, Mohamed A, Ahmed A, Ahmed A, Saleh, Shafika A, Doaa M, et al. Effect of dietary supplementation of alpha-galactosidase on the growth performance, ileal digestibility, intestinal morphology, and biochemical parameters in broiler chickens. BMC Vet Res. (2020) 16:144. 10.1186/s12917-020-02359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rao S, Raju M, Nagalakshmi D, Prakash B, Paul S. Effect of supplementation of graded concentrations of xylanase and α-amylase on performance, slaughter variables and energy digestibility in broiler chicken fed corn-soybean meal based diet. J App Poul Res. (2021) 30:100139. 10.1016/j.japr.2021.100139 [DOI] [Google Scholar]
- 202.Cowieson A, Bedford M, Ravindran V. Interactions between xylanase and glucanase in maize-soy-based diets for broilers. Br Poult Sci. (2010) 51:246–57. 10.1080/00071661003789347 [DOI] [PubMed] [Google Scholar]
- 203.Rambabu D, Reddy Ravinder V, Qudratullah S, Reddy M, Reddy K. Effect of exogenous enzyme supplementation on certain growth and carcass characteristics of broiler chicks. Indian J Poul Sci. (2011) 46:189–94. [Google Scholar]
- 204.Saeed M, Ayaşan T, Alagawany M. MEA El-HackMA, Abdel-Latif, AK Patra. The role of ß-mannanase (Hemicell) in improving poultry productivity, health and environment. Braz J Poult Sci. (2019) 21:1–8. 10.1590/1806-9061-2019-1001 [DOI] [Google Scholar]
- 205.Alhidary IA, Abdelrahman MA, Albadani H, Khan RU, Selvaggi M, Laudadio V, et al. Impact of microbial protease enzyme and dietary crude protein levels on growth and nutrients digestibility in broilers over 15–28 days. Animals. (2021) 11:2499. 10.3390/ani11092499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alshamiri MMA, Ali SAM, Abdalla HO, Ahmed HB. The effect of supplementing different levels of phytase enzyme on performance, some carcass properties and economics of broiler chickens. Agrobiological Records. (2021) 4:14–22. 10.47278/journal.abr/2020.025 [DOI] [Google Scholar]
- 207.Adeola O, Cowieson AJ. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve non-ruminant animal production. J Anim Sci. (2011) 89:3189–218. 10.2527/jas.2010-3715 [DOI] [PubMed] [Google Scholar]
- 208.Ugwuanyi JO. Enzymes for nutritional enrichment of agro-residues as livestock feed. In: Gurpreet SD, Surinder K, editors. Agro-Industrial Wastes as Feedstock for Enzyme, Production. Cambridge, MA: Academic Press. (2016). pp. 233–60. 10.1016/B978-0-12-802392-1.00010-1 [DOI] [Google Scholar]
- 209.Doskovic V, Bogosav, Jevic-Boskovic S, Pavlovski Z, Milosevic B, Skrbic Z, et al. Enzymes in broiler diets with special reference to protease. World's Poul Sci J. (2013) 69:343–60. 10.1017/S0043933913000342 [DOI] [Google Scholar]
- 210.Khattak FM, Pasha TN, Hayat Z, Mahmud A. Enzymes in poultry nutrition. J Anim Plant Sci. (2006) 16:1–2. [Google Scholar]
- 211.Mabelebele M, Gous RM, Siwela M, O'Neil H, Iji P. Performance of broiler chickens fed South African sorghum-based diets with xylanase. S Afr J Anim Sci. (2017) 47:679–87. [Google Scholar]
- 212.Babalola TO, Apata DF, Atteh JO. Effect of β-xylanase supplementation of boiled castor seed meal-based diets on the performance, nutrient absorbability and some blood constituents of pullet chicks. Trop Sci. (2006) 46:216–23. 10.1002/ts.181 [DOI] [Google Scholar]
- 213.Café MB, Borges CA, Fritts CA, Waldroup PW. Avizyme improves performance of broilers fed corn-soybean meal-based diets. J Appl Poult Res. (2002) 11:29–33. 10.1093/japr/11.1.29 [DOI] [Google Scholar]
- 214.Ohimain EI, Ofongo R. Enzyme supplemented poultry diets: benefits so far-A review. Int J Adv Biotechnol Res. (2014) 3:31–9. [Google Scholar]
- 215.Tuoying AO. Exogenous Enzymes and Organic Acids in the Nutrition of Broiler Chicks: Effects on Growth Performance and in vitro and in vivo Digestion. Ph D Thesis, The Graduate School University of Kentucky (2005). [Google Scholar]
- 216.Jia W, Slominski BA, Bruce HL, Blank G, Crow G, Jones O. Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during sub clinical clostridium perfingens challenge. Poult Sci. (2009) 88:132–40. 10.3382/ps.2008-00204 [DOI] [PubMed] [Google Scholar]
- 217.Knudsen KEB. Fibre and non-starch polysaccharide content and variation in common crops used in broiler diets. Poult Sci. (2014) 93:2380–93. 10.3382/ps.2014-03902 [DOI] [PubMed] [Google Scholar]
- 218.Courtin C, Broekaert W, Swenen K, Lescroart O, Onagbesan O, Buyse J, et al. Dietary inclusion of wheat bran ararbinoxylooligosacchairdes induces beneficial nutritional effects in chickens. Cereal Chem. (2008) 85:607–13. 10.1094/CCHEM-85-5-0607 [DOI] [Google Scholar]
- 219.Chen H, Liu S, Xu XR, Zhou GJ, Liu SS, Yue WZ. Antibiotics in the coastal environment of the hailing bay region, South China Sea: spatial distribution, source analysis and ecological risks. Mar Pollut Bull. (2015) 95:365–673. 10.1016/j.marpolbul.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 220.Zhang L, Xu J, Lei L, Jiang Y, Gao F, Zhou G. Effects of Xylanase supplementation on growth performance, nutrient digestibility and non-starch polysaccharide degradation in difference sections of the gastro-intestinal tract of broilers. Asian Austr J Ani Sci. (2014) 27:855–86. 10.5713/ajas.2014.14006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee S, Apajalahti K, Vienola G, Gonzalez-Ortiz C. Bedford M. Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Ani Feed Sci Technol. (2017) 234:29–42. 10.1016/j.anifeedsci.2017.07.017 [DOI] [Google Scholar]
- 222.Ravangard A, Houshmand M, Khajvi K, Naghihi R. Performance and cecal bacteria counts of broilers fed low protein diets with and without a combination of probiotic and prebiotic. Br J Poul Sci. Nutrition. (2017) 3:75–82. 10.1590/1806-9061-2016-0319 [DOI] [Google Scholar]
- 223.Shengru W, Li T, Niu H, Zhu Y, Liu Y, Duan Y, et al. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult Sci. (2019) 98:828–41. 10.3382/ps/pey393 [DOI] [PubMed] [Google Scholar]
- 224.Simbaya J, Slominski B, Guenter W, Morgan A, Campbell L. The effects of protease and carbohydrase supplementation on the nutritive value of canola meal for poultry: in vitro and in vivo studies. Anim Feed Sci Technol. (1996) 61:219–34. 10.1016/0377-8401(95)00939-6 [DOI] [Google Scholar]
- 225.Zanella I, Sakomura N, Silversides F, Fiqueirdo A, Pack M. Effect of enzyme supplementation of broiler diets based on maize and soybeans. Poult Sci. (1999) 78:561–8. 10.1093/ps/78.4.561 [DOI] [PubMed] [Google Scholar]
- 226.Cowieson J, Adeola O. Carbohydrases, protease, and phytase have an additive beneficial effect in nutritionally marginal diets for broiler chicks. Poult Sci. (2005) 84:1860–7. 10.1093/ps/84.12.1860 [DOI] [PubMed] [Google Scholar]