Abstract
Objective:
To examine associations between alcohol use disorder (AUD), its psychiatric comorbidities, and their interactions, with marital outcomes in a diverse high-risk, genetically informative sample.
Method:
Participants included European ancestry (EA, n=4045) and African ancestry (AA, n=1550) individuals from the multigenerational COGA sample (56% female, Mage ~ 41 years). Outcomes were lifetime marriage and divorce. Predictors included lifetime AUD, an alcohol problems polygenic score, and AUD comorbidities, including antisocial personality disorder (ASP), cannabis dependence/abuse (CAN), frequent tobacco use (TOB), and major depressive disorder (MDD). Mixed effect Cox models and generalized linear mixed effects models were fit.
Results:
Among EA participants, those with AUD and CAN were less likely to marry (hazard ratios [HR] 0.70–0.83, ps < 0.01). Among AA participants, those with AUD and TOB were less likely to marry (HRs 0.66–0.82, ps < 0.05) and those with MDD were more likely to marry (HR = 1.34, p < 0.01). Among EA participants, AUD, CAN, TOB, and MDD were associated with higher odds of divorce (ORs 1.59–2.21, ps < 0.01). Among AA participants, no predictors were significantly associated with divorce. Significant random effects indicated genetic and environmental influences on marriage, but only environmental factors on divorce.
Conclusions:
In a high-risk sample, alcohol use disorder was associated with reduced likelihood of marriage in European and African ancestry individuals, and increased risk of divorce in European ancestry individuals. These associations were largely independent of comorbidities. Genetic and environmental background factors contributed to marriage, while only environmental background factors contributed to divorce.
Keywords: alcohol use disorder, marriage, divorce, psychiatric comorbidities, Collaborative Study on the Genetics of Alcoholism (COGA), genetically-informative design
Problematic alcohol use is associated with far-reaching personal and social consequences (Grant et al., 2015; Rehm et al., 2017), including difficulties establishing and maintaining marital relationships (Grant et al., 2015; Kessler et al., 1998). In epidemiological samples, marriage, timing of first marriage, and stability of marriage are associated with alcohol consumption (Leonard & Rothbard, 1999), heavy drinking, and alcohol dependence (Forthofer et al., 1996; Fu & Goldman, 1996; Jang et al., 2017; Waldron et al., 2011). Married individuals consume less alcohol (Fu & Goldman, 1996), in less problematic patterns (Power & Rodgers, 1999), and are less likely to have a lifetime or past-year alcohol use disorder (AUD) diagnosis (Grant et al., 2015) than those that never marry or divorce. Lifetime AUD diagnosis is also associated with increased probability of divorce (Kessler et al., 1998). The associations between AUD and marital outcomes have tangible costs, given substantial literature linking marriage to health and well-being (Manzoli et al., 2007; Schoenborn, 2004) and lower alcohol-related mortality (Herttua et al., 2011).
Despite considerable evidence regarding the associations between AUD and marital outcomes in population-based samples, little is known about these associations in high-risk samples. As others have recently posited (Vanyukov et al., 2016), the factors associated with substance use disorders among individuals low on biological liability may or may not hold among individuals with high biological risk. Much of the prior work linking AUD and marital outcomes comes from population-based samples, and the associations between AUD and marital outcomes observed in the general population may differ from those at high biological risk, such as those from families with a history of alcohol use disorders. For example, children with parental AUD history are less likely to marry (Watt, 2002) and more likely to divorce than those with unaffected parents (Harter, 2000). Moreover, there is evidence that the correlates and consequences of AUD can differ as a function of the biological/genetic risk profile of population under study (Hill et al., 2000; Savage et al., 2018). Taken together, these lines of evidence suggest that the nature of associations between AUD and marital outcomes in higher-risk samples cannot be assumed to be identical to lower-risk population-based samples.
It is equally important to clarify the degree to which the associations between AUD and marital outcomes are independent of or modified by psychiatric conditions that are often comorbid with AUD, such as antisocial personality disorder, other substance use disorders, and major depressive disorder (Grant et al., 2015). In general, these psychiatric comorbidities, like AUD itself, are also associated with atypical age of first marriage (Forthofer et al., 1996) and increased risk of divorce (Kessler et al., 1998). Additionally, there is some evidence that psychiatric comorbidities may magnify the associations between AUD and marital outcomes. Individuals with AUD and other drug use disorders are more likely to be never married or divorced as compared to those with AUD only (Saha et al., 2018). Similarly, individuals with comorbid AUD and major depression are less likely to be married than those with major depression only (Brière et al., 2014).
Genetic epidemiological data indicate that familial factors (genes, rearing environment) contribute to AUD, marital outcomes, and their covariation. There is a substantial degree of genetic influence on AUD, with an estimated heritability of ~50% (Verhulst et al., 2014). Further, marital outcomes aggregate in families (Wolfinger, 2005), and the intergenerational transmission of marriage and divorce reflects both genetic and environmental influences. Twin and family studies also demonstrate that marriage and divorce are genetically influenced (D’Onofrio et al., 2005; Jerskey et al., 2010; Salvatore et al., 2018). Moreover, there is a substantial genetic correlation between AUD and divorce (Salvatore et al., 2017). Evidence also suggests that the associations between AUD and social functioning reflect, in part, shared familial factors (Kendler et al., 2016). These findings underscore the importance of accounting for familial factors to help delineate the associations between AUD and marital outcomes, a limitation of prior studies.
Our goal in this study was to examine the associations between AUD and marital outcomes using the high-risk Collaborative Studies of Genetics of Alcoholism (COGA) sample. This diverse, genetically informative high-risk family sample enriched for AUD vulnerability offers an opportunity to examine the AUD-marital associations while accounting for familial influences. We had three research questions: (1) what are the associations between lifetime AUD and marital outcomes (marriage and divorce); (2) do these associations remain robust when considering polygenic loading for alcohol problems as well as other comorbid externalizing (i.e., conduct or antisocial personality disorder; cannabis dependence/abuse; frequent tobacco use) and internalizing disorders (i.e., major depressive disorder); and (3) do polygenic loading for alcohol problems as well as other comorbid externalizing and internalizing disorders alter the associations between AUD and marital outcomes? We expected that a lifetime AUD diagnosis would be associated with delays in age at first marriage, and a higher likelihood of experiencing divorce. We also hypothesized that among those with a lifetime AUD diagnosis, higher polygenic loading for alcohol problems and comorbid externalizing or internalizing disorders would be associated with lower odds of marriage and a greater likelihood of divorce. We focused on lifetime diagnoses because age of onset was not available for all psychiatric disorders in the COGA sample. This study and hypotheses were preregistered on the Open Science Framework (osf.io/rtyf3). In addition to these pre-registered hypotheses, we leveraged our genetically-informative family-based sample to examine the degree to which genetic and familial rearing environmental factors contributed to the aggregation of marriage and divorce in these high-risk families.
Methods
Sample
Participants came from the Collaborative Study on the Genetics of Alcoholism (COGA), a diverse, family-based study whose objective is to identify genetic variants associated with AUD and related disorders (Begleiter et al., 1995; Bucholz et al., 2017; Reich et al., 1998). Probands were identified through alcohol treatment centers across seven sites in the United States. Probands along with their families were invited to participate if the family was sufficiently large (usually sibships greater than 3, with parents available), and had two or more members in the COGA catchment area. Comparison families were recruited from the same communities. The Institutional Review Board at all data collection sites approved the study, and written consent was obtained from all participants.
In the present study, we focused on all COGA participants of European ancestry (EA) and African ancestry (AA) with genome-wide association (GWAS) data, AUD and psychiatric comorbidities phenotypes, and marital outcomes information. We defined two analytic samples within COGA for the marriage and divorce analyses. The first sample, the marriage analytic sample, included 4045 EA participants (from 986 extended families; 2253 (56%) female; Mage = 36.26 years, age range = 19–84 years) and 1550 AA participants (from 503 extended families; 863 (56%) female; Mage = 37.35 years, age range = 21–79 years). This analytic sample excluded those <23 years of age at their assessment (or most recent assessment, for those with multiple assessments) who were without a lifetime AUD diagnosis. We implemented the age minimum to ensure that participants had passed through the period of highest risk for onset of AUD before being classified as unaffected, as guided by epidemiological data regarding age of onset for severe AUD (Grant et al., 2015). Additionally, we excluded those <30 years of age at their assessment (or most recent assessment, for those with multiple assessments) who were unmarried (never married) from the marriage analytic sample. Epidemiological data regarding the age at the majority of first marriages (Goodwin et al., 2009) guided our decision to use age 30 as the cut-off.
The second sample, the divorce analytic sample, was a subset of the marriage analytic sample, and it excluded those married less than 3 years (and not divorced) at their assessment (or most recent assessment, for those with multiple assessments). We implemented this cut-off to ensure that participants had passed through the period of highest risk for divorce before being classified as not divorced, which is typically in the early years of marriage (Cherlin, 1992; Mayol-Garcia et al., 2021). In total, the divorce analytic sample included 3360 EA participants (from 925 extended families; 1938 (58%) female; Mage = 42.30 years, age range = 21–84) and 920 AA participants (from 392 extended families; 532 (58%) female; Mage = 42.94 years, age range = 21–79).
Measures
Marital outcomes.
Marriage and divorce measures came from the Semi-Structured Assessment for the Genetics of Alcoholism interview (SSAGA; for those individuals 18 years of age or older) or adolescent version of the SSAGA (for those individuals between 12–17 years of age; Bucholz et al., 1994; Hesselbrock et al., 1999). Marriage and divorce were limited to legal marriages. We excluded cohabiting marriage-like relationships as there was no information collected regarding the timing of those relationships to calculate age at initiation or dissolution. We examined first marriage and divorce for those with multiple marriages.
Alcohol use disorder.
Lifetime diagnoses (binary) of AUD were made based on the Diagnostic and Statistical Manual of Mental Disorders (5th ed; DSM-5; American Psychiatric Association, 2013) and were assessed from the adult or adolescent version of the SSAGA interviews.
Psychiatric comorbidities.
Lifetime diagnoses (binary) of comorbidities were obtained from the SSAGA. Conduct disorder or antisocial personality disorder (depending on age; ASP) and cannabis dependence or abuse (CAN) diagnoses were measured using DSM-IV criteria (American Psychiatric Association, 1994). We operationally defined lifetime frequent tobacco use (TOB) as someone having smoked a total of at least 100 cigarettes over their lifetime (Bondy et al., 2009) and having a >0 score on the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991), as some people consider themselves non-smokers even when they have consumed >100 cigarettes in a lifetime (Pomerleau et al., 2004). FTND was assessed with a lifetime timeframe, and thus is inclusive of ex-smokers. Absence of frequent tobacco use was defined as non-smokers, or someone with >100 lifetime cigarettes with a zero on FTND. Because FTND was not administered during the early phase of COGA data collection, a separate yes/no question from the SSAGA (“Have you ever smoked cigarettes daily for a month or more?”) was substituted to define frequent tobacco use for those without the FTND data. Lifetime major depressive disorder (MDD) was determined with DSM-IV (American Psychiatric Association, 1994) or DSM-III-R criteria (American Psychiatric Association, 1987).
Genotyping, ancestry, and genetic relatedness matrix.
Participants’ DNA samples were genotyped using the Illumina Human1M array, the Illumina Human OmniExpress 12V1 array, the Illumina 2.5M array, or the Smokescreen genotyping arrays. A full description of data processing, quality control, and imputation is available elsewhere (Lai et al., 2019). EA sample data were imputed to Haplotype Reference Consortium, and the AA sample data to the 1000 Genomes Phase 3. Single nucleotide polymorphisms (SNPs) with a genotyping rate < 0.95, that violated Hardy-Weinberg equilibrium (p < 10−6), or had minor allele frequency < 0.01 were excluded from analysis in both the EA and AA samples.
To avoid population stratification (Cardon & Palmer, 2003), we conducted our analyses separately by ancestry group. Genetic ancestry principal components were computed from GWAS data using Eigenstrat (Price et al., 2006) and the 1000 Genomes, Phase III reference panel. These principal components reflect continuous variation in allele frequencies representing ancestral differences. Individuals were assigned an ancestry classification (European, African, or Other) based on the first two principal components. Our analyses included participants of European and African ancestry. We note that genetic ancestry refers to the population(s) from which an individual’s biological ancestors originated (Peterson et al., 2019), and ancestry and self-report racial/ethnic background are not identical. In the United States, individuals of European ancestry and African ancestry often self-identify as White/European American and Black/African American, respectively.
A genetic relatedness matrix (GRM) was estimated from a pruned set of semi-independent SNPs using the command line software GCTA (Yang et al., 2011) to facilitate estimation of the degree to which genetic factors contributed to marriage and divorce. In view of linkage disequilibrium (LD) differences across ancestral groups (Campbell & Tishkoff, 2008), separate GRMs were estimated for the EA (n SNPs = 423,096) and AA (n SNPs = 562,983) samples.
Alcohol problems polygenic scores (PRS).
Genetic risk for alcohol problems was indexed using genome-wide polygenic scores (PRS). Genome-wide PRS represents the state of the science approach to index an individual’s overall genetic liability for a given trait/behavior using molecular genetic data (Wray et al., 2014). This approach uses the results from a genome-wide association study (GWAS) in a large-scale gene identification discovery sample to calculate a personalized measure of genetic risk for individuals in a target sample. A polygenic score is calculated by summing over the number of alleles for each single nucleotide polymorphism (SNP), weighted by the effect size drawn from a GWAS. Thus, a PRS is a weighted sum of risk-increasing alleles that an individual carries across their genome (for more extensive reviews, see Bogdan et al., 2018). We used PRS-CS “auto” (Ge et al., 2019) to calculate an alcohol problems PRS. This approach employs a Bayesian regression and continuous shrinkage method to correct for the non-independence among nearby SNPs in the genome.
We used ancestry-specific discovery GWAS results to calculate alcohol problems polygenic scores in the COGA EA and AA samples. For the EA sample, the alcohol problems PRS was derived using meta-analyzed GWAS weights (detailed in Barr et al., 2020) from the Psychiatric Genomic Consortium’s GWAS of alcohol dependence analyses (COGA removed; Walters et al., 2018) and UK Biobank’s problem subscale from the Alcohol Use Disorders Identification Test (AUDIT-P) analyses (Sanchez-Roige et al., 2018) for individuals of European ancestry. For the AA sample, the alcohol problems PRS was derived using GWAS weights from the Million Veteran Program GWAS of alcohol use disorder analysis for individuals of African ancestry (Kranzler et al., 2019). Higher polygenic scores indicated higher polygenic loading for alcohol problems.
Covariates.
We included age, sex, birth cohort, and the first ten genetic ancestry principal components (PC1–10) in all analyses. Birth cohort was indexed using four dummy coded variables following the generation status scheme (Bourdon et al., 2020): silent [prior to 1945]; baby boomer [1946 to 1964]; generation X [1965 to 1980]; and millennial [1981 to 1996], with baby boomer set as reference.
Statistical Analysis
We examined time-to-marriage in mixed effects Cox regression models using the coxme package (Therneau, 2020) for R (R Development Core Team, 2019). We examined the likelihood of divorce with generalized linear mixed effects models with a logit link using the lme4qtl package (Ziyatdinov et al., 2018).
In the main effects for the time-to-first-marriage and divorce models, we included AUD, the alcohol problems PRS, and psychiatric comorbidities as predictors. A random intercept for subject with correlation structure defined by a genetic relatedness matrix (GRM) was used to estimate for the additive genetic variance contributing to marriage/divorce. An additional random intercept for family grouping with independence correlation structure was used to account for familial environment variance contributing to marriage/divorce. Age was mean centered. The alcohol problems PRS and 10 PCs were standardized to have a mean of 0 and standard deviation of 1.
The interactive effects models included two-way interaction terms to examine multiplicative relationships between lifetime AUD, the alcohol problems PRS, and the psychiatric comorbidities (ASP, CAN, TOB, MDD), in addition to their main effects.
The initial specification of the models predicting marriage converged, and no adjustments were necessary. The initial model specifications of the models predicting divorce did not converge. Troubleshooting revealed that the subject-level random intercept had very low variance, suggesting that additive genetic variance did not make a substantive contribution to the likelihood of divorce in our sample. Convergence was achieved by removing the subject-level random intercept. Nakagawa’s pseudo-R2 are presented for random effects from the generalized linear mixed effects model predicting divorce (Nakagawa et al., 2017).
In all mixed effects Cox regression models, violations of the proportional hazards assumption for fixed effects were investigated with a non-zero slope of Schoenfeld residuals versus time using the cox.zph function in the survival package (Therneau et al., 2021) in R.
In all models, we explored potential sex differences in patterns of results. Because there was no evidence of sex differences, results are reported with females and males combined, with sex included as a covariate in all models. For all a priori hypotheses, we used a p-value threshold of p < .05 for inference criteria.
Results
Descriptive Statistics
Descriptive statistics for key study variables for the marriage and divorce analytic samples are presented in Table 1. Representativeness analyses of the marriage and divorce analytic samples are summarized in Supplement, section S1. The divorce analytic sample was derived from the marriage analytic sample, as marriage is a prerequisite for divorce. Thus, in the EA and AA samples respectively, 283 and 521 participants were excluded as they were never married. Further, 283 and 110 additional participants in the EA and AA marital analytic samples, respectively, were removed to form the divorce analytic sample because they had not yet been married long enough to pass through the initial risk period of divorce (i.e., less than 3 years without divorcing). Differences between the marriage and divorce samples as a function of AUD and comorbid disorders are summarized in the Supplement, section S1.
Table 1.
Descriptive statistics for key study variables for the marriage and divorce analytic samples among European and African ancestry individuals
| Marriage Analytic Sample | Divorce Analytic Sample | ||||||||
|
|
|
||||||||
| EA | AA | EA | AA | ||||||
|
|
|
||||||||
| N | M (SD) | N | M (SD) | N | M (SD) | N | M (SD) | ||
|
|
|
||||||||
| Age at assessment | 4045 | 42.30 (12.66) | 1550 | 40.21 (9.62) | Age at assessment | 3360 | 44.26 (12.66) | 920 | 42.94 (9.62) |
| Age at First Marriage | 3640 | 23.24 (4.32) | 1029 | 24.64 (5.69) | -- | -- | -- | -- | |
| Age at Last Observation (if censored; marriage analyses only) | 405 | 36.26 (6.49) | 521 | 37.35 (6.16) | -- | -- | -- | -- | |
|
|
|
||||||||
| Percentage | Percentage | ||||||||
|
| |||||||||
| Ever married | 89.99 | 66.39 | Ever divorced | 36.04 | 42.17 | ||||
| Female | 55.70 | 55.68 | Female | 57.68 | 57.83 | ||||
| Silent Generation | 18.57 | 8.06 | Silent Generation | 22.20 | 12.83 | ||||
| Baby Boomer | 48.18 | 54.90 | Baby Boomer | 51.85 | 61.85 | ||||
| Generation X | 19.38 | 24.32 | Generation X | 16.58 | 18.26 | ||||
| Millennial | 13.87 | 12.71 | Millennial | 9.38 | 7.07 | ||||
| AUD | 51.99 | 50.65 | AUD | 49.05 | 45.11 | ||||
| ASP | 14.88 | 20.84 | ASP | 12.95 | 17.72 | ||||
| CAN | 27.44 | 29.03 | CAN | 24.20 | 25.11 | ||||
| TOB | 50.19 | 53.48 | TOB | 50.60 | 49.57 | ||||
| MDD | 22.18 | 16.39 | MDD | 21.28 | 16.85 | ||||
Note. EA = European ancestry; AA = African ancestry. M = mean. SD = standard deviation. The divorce analytic sample is a subset of the final marriage analytic sample. AUD = alcohol use disorder. ASP = conduct disorder or antisocial personality disorder. CAN = cannabis dependence or abuse. TOB = frequent tobacco use. MDD = major depressive disorder. Birth cohort was dummy-coded indexing generation status, defined as: silent [b. prior to 1945]; baby boomer [b. 1946 to 1964]; generation X [b. 1965 to 1980]; and millennial [b. 1981 to 1996].
Time-to-event (first marriage) analyses
Results for the testing of violations of the proportional hazard associated with a predictor remains proportional over time are presented in Supplement, Tables S1–S4 and visually depicted in Supplement, Figures S1–S4.
EA Marriage.
4045 participants were retained for analysis. 3640 participants were married and the remaining 405 were censored. The mixed effects Cox regression model of marriage indicated that the variance component associated with the additive genetic random effect was 0.30 (SD = 0.55). In the context of Cox mixed-effects models, this effect does not have a direct conversion to estimate heritability. Rather, this component is exponentiated and interpreted as the relative risk of marriage attributable to additive genetic factors for individuals who are one standard deviation above or below the baseline level of risk (Pankratz et al., 2005). This corresponds to a relative risk of marriage that is 1.73 (i.e., exp(0.55) = 1.73) higher or lower than the baseline hazard rate, meaning that the individual-specific relative risk of marriage attributable to genetic relatedness for subjects who are one standard deviation above or below the baseline level of risk was 1.73 times larger or smaller than the average likelihood of marriage. Said another way, one’s individual likelihood of marriage increases when they are genetically more similar to others who are married, and decreases when they are genetically more similar to others who are unmarried. The variance component associated with the familial environment random effect was 0.30 (SD = 0.54). This corresponds to a relative risk of marriage that is 1.72 higher or lower than the baseline hazard rate, meaning that the family-specific relative risk of marriage was 1.72 times larger or smaller than the average likelihood of marriage for families who are one standard deviation above or below the baseline level of risk. In other words, likelihood of being married (or not) clusters within families.
The results from the models of marriage as a function of AUD, PRS, and psychiatric comorbidities are summarized in Table 2. AUD and CAN were associated with lower odds of marriage. TOB was associated with higher odds of marriage. There were no statistically significant associations between the alcohol problems PRS, ASP, and MDD and marriage. Relative to baby boomers, odds of marriage were 79% higher for those in the silent generation, and 47% lower among millennials. Odds of marriage were 72% higher among females compared to males. Age at assessment was not associated with odds of marriage.
Table 2.
Hazard ratio of timing of first marriage as a function of alcohol use disorder, alcohol problems polygenic score, and psychiatric comorbidities in the European ancestry sample
| Main Effects Only |
Main & Interaction Effects |
|||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
|
| ||||
| Age (years) | 1.00 | [0.99, 1.01] | 1.00 | [0.99, 1.01] |
| Sex (female) | 1.72 | [1.58, 1.88] | 1.73 | [1.59, 1.89] |
| Silent Generation | 1.79 | [1.47, 2.19] | 1.81 | [1.49, 2.22] |
| Generation X | 0.80 | [0.70, 0.92] | 0.80 | [0.69, 0.92] |
| Millennial | 0.53 | [0.45, 0.62] | 0.52 | [0.45, 0.62] |
| AUD | 0.83 | [0.75, 0.91] | 0.88 | [0.76, 1.00] |
| Alcohol problems PRS | 0.98 | [0.94, 1.02] | 0.97 | [0.91, 1.03] |
| ASP | 1.00 | [0.88, 1.13] | 1.28 | [0.98, 1.67] |
| CAN | 0.70 | [0.62, 0.78] | 0.85 | [0.69, 1.04] |
| TOB | 1.20 | [1.09, 1.32] | 1.21 | [1.06, 1.37] |
| MDD | 1.07 | [0.96, 1.18] | 1.01 | [0.86, 1.18] |
| AUD × PRS | - | 1.02 | [0.94, 1.11] | |
| AUD × ASP | - | 0.74 | [0.55, 1.00] | |
| AUD × CAN | - | 0.78 | [0.62, 0.99] | |
| AUD × TOB | - | 0.98 | [0.82, 1.17] | |
| AUD × MDD | - | 1.10 | [0.90, 1.34] | |
Note. HR = hazard ratio; CI = confidence interval. AUD = alcohol use disorder. PRS = alcohol problems polygenic score. ASP = conduct disorder or antisocial personality disorder. CAN = cannabis dependence or abuse. TOB = frequent tobacco use. MDD = major depressive disorder. All analyses included genetic ancestry principal components (PC1–10) as covariates in the models. Birth cohort was dummy-coded indexing generation status, defined as: silent [b. prior to 1945]; baby boomer [b. 1946 to 1964]; generation X [b. 1965 to 1980]; and millennial [b. 1981 to 1996], with baby boomer set as reference.
Bold type indicates estimate p < .05.
Results from the models including the interactive effects between AUD, the alcohol problems PRS, and psychiatric comorbidities model are summarized in Table 2. There was a significant AUD by CAN effect, suggesting that CAN modified the association between AUD and marriage. As summarized visually in Figure 1, comorbid AUD and CAN was associated with lower odds of marriage beyond the additive combination of the two effects.
Figure 1.
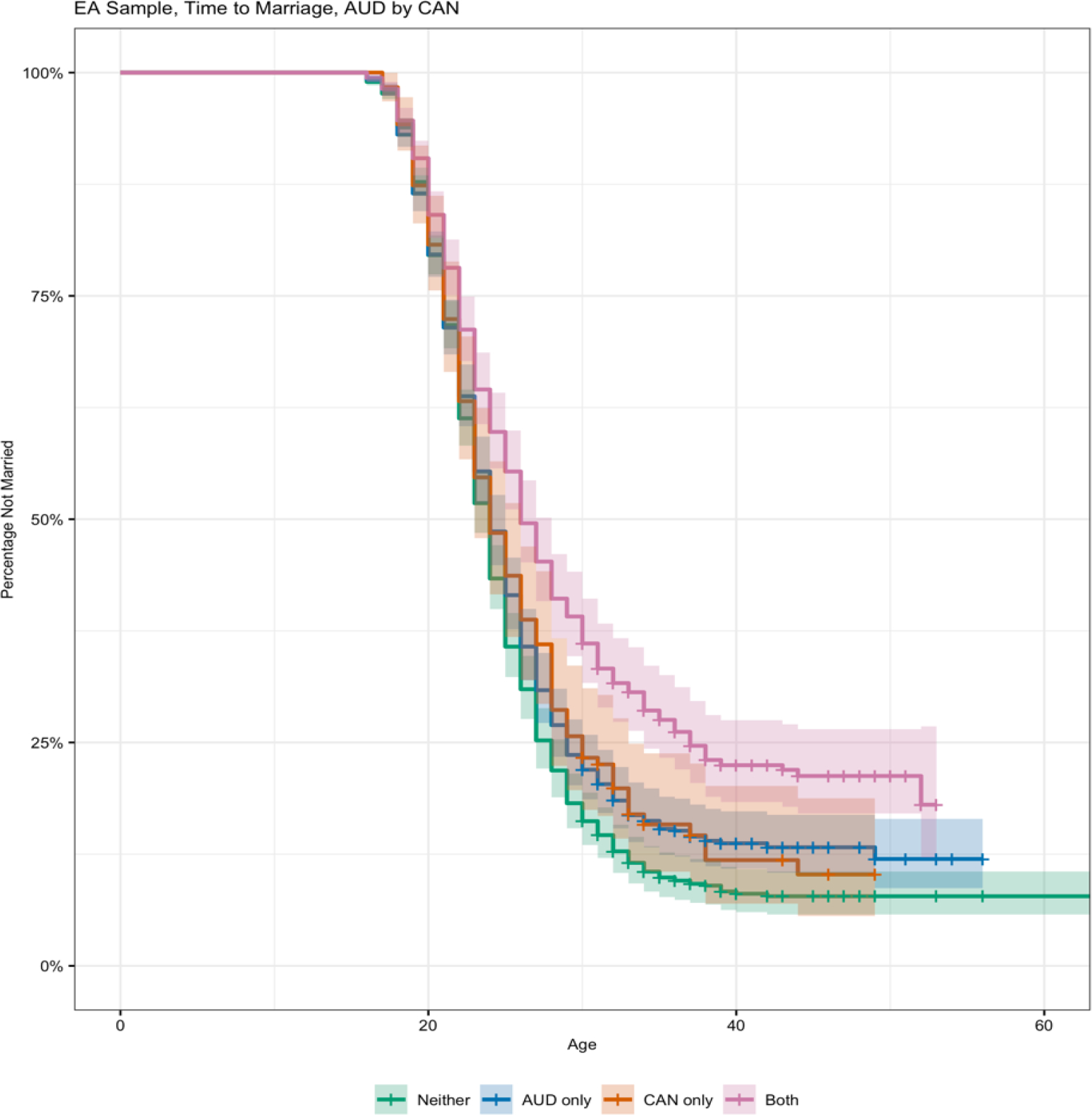
Survival functions for pattern of timing at first marriage by alcohol use disorder (AUD) diagnoses vary as a function of cannabis dependence/abuse (CAN) among individuals of European ancestry
AA Marriage.
1550 participants were retained for analysis. 1029 participants were married and the remaining 521 were censored. The mixed effects cox regression model of marriage indicated that the variance component associated with the additive genetic and familial environment random effects were 0.05 (SD = 0.22) and 0.28 (SD = 0.53). This corresponds to relative risks of marriage that were 1.25 or 1.70 higher or lower than the baseline hazard rate based on genetic makeup and family environment, respectively.
Results from the models of marriage as a function of AUD, the alcohol problems PRS, and psychiatric comorbidities are summarized in Table 3. AUD and TOB were associated with lower odds of marriage. MDD was associated with higher odds of marriage. The alcohol problems PRS, ASP, and CAN were not associated with odds of marriage. Relative to baby boomers, odds of marriage were 136% higher in silent generation participants, and 27% lower among millennials. Age was also related to higher odds of marriage. In the interactive effects model, there was no evidence that the alcohol problems PRS nor psychiatric comorbidities modified the associations between AUD and marriage (Supplement, Table S5).
Table 3.
Hazard ratio of timing of first marriage as a function of alcohol use disorder, alcohol problems polygenic score, and psychiatric comorbidities in the African ancestry group
| HR | 95% CI | |
|---|---|---|
|
| ||
| Age (years) | 1.02 | [1.00, 1.03] |
| Sex (female) | 1.05 | [0.90, 1.22] |
| Silent Generation | 2.36 | [1.60, 3.48] |
| Generation X | 0.92 | [0.73, 1.15] |
| Millennial | 0.73 | [0.54, 0.97] |
| AUD | 0.66 | [0.56, 0.77] |
| Alcohol problems PRS | 1.02 | [0.95, 1.10] |
| ASP | 0.90 | [0.75, 1.09] |
| CAN | 1.01 | [0.84, 1.21] |
| TOB | 0.82 | [0.70, 0.96] |
| MDD | 1.34 | [1.11, 1.62] |
Note. HR = hazard ratio. CI = confidence interval. AUD = alcohol use disorder. PRS = alcohol problems polygenic score. ASP = conduct disorder or antisocial personality disorder. CAN = cannabis dependence or abuse. TOB = frequent tobacco use. MDD = major depressive disorder. Analysis included genetic ancestry principal components (PC1–10) as covariates in the model. Birth cohort was dummy-coded indexing generation status, defined as: silent [b. prior to 1945]; baby boomer [b. 1946 to 1964]; generation X [b. 1965 to 1980]; and millennial [b. 1981 to 1996], with baby boomer set as reference.
Bold type indicates estimate p < .05.
Analyses of likelihood of divorce
EA Divorce.
3360 participants were retained for analysis. The random intercept for family grouping accounted for 16.30% of the variance in divorce. Results from the models of divorce as a function of AUD, PRS, and psychiatric comorbidities are summarized in Table 4. AUD, CAN, TOB, and MDD were associated with higher likelihood of divorce. The alcohol problems PRS was not associated with divorce. Relative to baby boomers, odds of divorce were 54% lower for those in the silent generation, and 70% lower in millennials. Odds of divorce were 42% higher among females. Odds of divorce were 2% higher per year of age (at assessment). In the interactive effects model, no multiplicative interaction terms were associated with the likelihood of divorce (Supplement, Table S6).
Table 4.
Likelihood of divorce as a function of alcohol use disorder, alcohol problems polygenic score, and psychiatric comorbidities in the European and African ancestry samples
| European Ancestry |
African Ancestry |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
|
| ||||
| Intercept | 0.22 | [0.18, 0.28] | 0.68 | [0.46, 1.01] |
| Age (years) | 1.02 | [1.00, 1.03] | 1.06 | [1.03, 1.09] |
| Sex (female) | 1.42 | [1.18, 1.72] | 1.05 | [0.74, 1.48] |
| Silent Generation | 0.46 | [0.31, 0.69] | 0.2 | [0.09, 0.42] |
| Generation X | 0.57 | [0.42, 0.78] | 0.74 | [0.43, 1.29] |
| Millennial | 0.3 | [0.20, 0.45] | 0.85 | [0.40, 1.82] |
| AUD | 1.87 | [1.53, 2.28] | 0.97 | [0.68, 1.38] |
| Alcohol problems PRS | 1.03 | [0.94, 1.13] | 1.15 | [0.99, 1.35] |
| ASP | 1.12 | [0.86, 1.46] | 1.00 | [0.65, 1.54] |
| CAN | 1.59 | [1.27, 1.99] | 1.26 | [0.85, 1.89] |
| TOB | 1.9 | [1.57, 2.30] | 1.37 | [0.98, 1.92] |
| MDD | 2.21 | [1.78, 2.74] | 1.38 | [0.91, 2.08] |
Note. OR = odds ratio. CI = confidence interval. AUD = alcohol use disorder. PRS = alcohol problems polygenic score. ASP = conduct disorder or antisocial personality disorder. CAN = cannabis dependence or abuse. TOB = frequent tobacco use. MDD = major depressive disorder. Analysis included genetic ancestry principal components (PC1-10) as covariates in the model. Birth cohort was dummy-coded indexing generation status, defined as: silent [b. prior to 1945]; baby boomer [b. 1946 to 1964]; generation X [b. 1965 to 1980]; and millennial [b. 1981 to 1996], with baby boomer set as reference.
Bold type indicates estimate p < .05
AA Divorce.
920 participants were retained for analysis. The random intercept for family grouping accounted for 11.59% of the variance in divorce. Results from the models of divorce as a function of AUD, PRS, and psychiatric comorbidities are summarized in Table 4. AUD, the alcohol problems PRS, and psychiatric comorbidities were not associated with divorce. Relative to baby boomers, odds of divorce were 80% lower for those in the silent generation. Odds of divorce were 6% higher per year of age (at assessment). In the interactive effects model, no multiplicative interaction terms were associated with the likelihood of divorce (Supplement, Table S7).
Supplemental analyses
While our pre-registered hypotheses refer specifically to whether the associations between AUD and marital behaviors were robust to or modified by the alcohol problems PRS and other common psychiatric comorbidities, results from models run with each predictor included in a separate model predicting age at marriage and likelihood of divorce are summarized in Supplement Table S8 and Table S9, respectively. In addition, the results of the association between the alcohol problems PRS and AUD are summarized in Supplement Table S10.
Discussion
Although AUD is associated with marriage and divorce in population-based samples, little is known about whether these associations are also observed in high-risk samples. In the present study, we examined the associations between lifetime AUD and marital outcomes in a sample enriched for AUD vulnerability. We tested whether the effects of AUD on marital behaviors are robust to or modified by one’s polygenic loading for alcohol problems and psychiatric comorbidities in a high-risk sample of European and African ancestry participants. Importantly, our analysis allowed for simultaneous estimation of these phenotypic AUD-marital outcome associations while accounting for familial factors that are genetically and environmentally clustered in the multigenerational COGA sample.
We first asked whether AUD was associated with marriage. In the EA sample, we extended the previous findings from population-based samples (Fu & Goldman, 1996; Grant et al., 2015; Waldron et al., 2011) and found that lifetime AUD was also associated with a reduced likelihood of marriage in a high-risk sample, after controlling for alcohol problems PRS and common psychiatric comorbidities. Consistent with previous findings that psychiatric disorders are associated with marital outcomes (Forthofer et al., 1996), we found that cannabis dependence/abuse was associated with lower likelihood of marriage. These associations may partly reflect the incompatibility between problematic substance use and the social roles expectations typically associated with marriage. Frequent tobacco use was associated with higher likelihood of marriage. Although not expected, this finding mirrors prior evidence that there is less role conflict between marriage and tobacco compared to other substances of abuse (Salvatore et al., 2019). Importantly, the effect of AUD on marriage was independent of the effects of common psychiatric comorbidities and polygenic loading for alcohol problems. Expanding on previous literature, we also found that the association between AUD and lower odds of marriage was more pronounced among those with comorbid cannabis dependence/abuse.
In the AA sample, we found that AUD and frequent tobacco use were associated with a lower likelihood of marriage. We note that the direction of association for tobacco is opposite from what was observed in the European ancestry group. Although the exact mechanisms that give rise to this pattern of effects is not clear, it is consistent with prior evidence that there are racial/ethnic differences in the associations between complex behavioral health outcomes and marriage (Sobal et al., 2009), and may reflect differences in how smoking is perceived of on the marriage market. Additionally, since our measure of frequent tobacco use was inclusive of lifetime regular smokers (i.e., not excluding former smokers), it is also possible that there were more ex-smokers in the European ancestry group, given prior evidence that Black smokers experience lower rates of successful smoking cessation than White smokers (King et al., 2004). Contrary to our expectation, major depressive disorder was associated with a higher likelihood of marriage. Although the exact mechanisms underlying this effect are unknown, we note that others have documented associations among marriage and depression in African American samples (Assari, 2017; Bennett et al., 1989).
We next examined the associations between AUD and divorce. In the EA sample, we found that lifetime AUD and psychiatric comorbidities, including cannabis dependence/abuse, frequent tobacco use, and major depressive disorder, were all independently associated with higher likelihood of divorce, while also accounting for the alcohol problems polygenic score. These findings are consistent with prior evidence in population-based samples (Kessler et al., 1998; Waldron et al., 2011). Neither the alcohol problems polygenic score nor psychiatric comorbidities altered the association between AUD and divorce. This highlights the unique predictive power of AUD on divorce above and beyond the potentially confounding effects of genetic predispositions and common comorbid externalizing and internalizing disorders.
In the AA sample, AUD was not associated with divorce. Moreover, none of the psychiatric comorbidities were associated with divorce. These null effects may partly be attributable to reduced statistical power due the smaller AA sample size. Alternatively, because the divorce analytic sample was a subsample of those who got married, we explored whether the null effects might reflect a thresholding effect of SES among those who married. However, supplemental analyses did not support this possibility. Although those in our AA sample who married had higher educational attainment than those who did not, the difference was negligible (12.64 vs. 12.26 years; Supplement, section S2). AA populations are underrepresented in studies of the social correlates and consequences of AUD and psychiatric disorders, and our findings should be considered as initial evidence. It is also possible that ethnic differences in alcohol use behaviors (Zapolski et al., 2014) and marital outcomes (Bryant et al., 2010; Dixon, 2009; Raley & Sweeney, 2020) could be driving differences in the associations of AUD and other psychiatric comorbidities and divorce observed in this study. Clearly, additional research on AUD and marital outcomes/processes in diverse populations is needed.
Our analysis allowed us to examine the associations between AUD and marital outcomes while accounting for and estimating the familial factors that contribute to marriage and divorce. Consistent with prior findings from population-based EA samples (D’Onofrio et al., 2005; Jerskey et al., 2010), we found significant familial clustering for marriage, with both latent genetic and familial environmental factors contributing to the aggregation of marriage across ancestries in these high-risk families. We also found that familial environment contributed to the aggregation of divorce in families. It is notable that we did not find evidence of genetic factors contributing to the familial aggregation of divorce in either the EA or AA samples, which is different from evidence in samples of EA population cohorts (Jerskey et al., 2010; Salvatore et al., 2018). These findings underscore the importance of accounting for familial influences to understand the associations between AUD and marital outcomes.
Limitations
Our results should be interpreted within the context of the following limitations. First, COGA is a high-risk sample with most participants from extended families enriched for AUDs. Findings may not be generalizable to other populations or samples ascertained with different risk profiles. Second, we used lifetime diagnoses of AUD and psychiatric comorbidities in view of incomplete age of onset information for these conditions within the COGA sample. As such, our analyses do not provide insights regarding the direction of the effects. Future work incorporating timing of diagnoses would be valuable. Third, our analyses included only participants’ AUD and psychiatric comorbidities information. Partner effects may play a role as well. For example, there is evidence that a spouse’s AUD is associated with their partner’s as well as their own marital adjustment (Cranford et al., 2011). Spousal discordance in their alcohol use patterns is linked with greater marital dissatisfaction (Homish & Leonard, 2007) and greater likelihood of divorce (Ostermann et al., 2005). Thus, incorporating dyadic data is an important next step in future research. Fourth, we focused on legal marriage, and first marriage/divorce for those with multiple marriages. Future research can consider marriage-like cohabiting relationships as well as remarriages.
Fifth, we considered whether the associations between AUD and marital outcomes were robust to or modified by polygenic loading for alcohol problems. Polygenic scores provide a useful global marker of genetic risk, though we recognize that at present polygenic scores generally account for a small amount of variance in alcohol phenotypes (Kranzler et al., 2019; Sanchez-Roige et al., 2018; Walters et al., 2018). Sixth, in the mixed effect Cox regression analyses of predicting marriage, several violations of the proportional hazards assumptions were detected. In the presence of a violation of proportional hazards assumptions, the estimated coefficient is interpretable as an average effect over time, although the coefficient can be biased to the extent that the slope of plotted residuals across time varies in magnitude and direction (Xu & Gamst, 2007). Seventh, we explored and did not find any evidence of sex differences in the pattern of results. However, likelihood of marriage and divorce were higher among females compared to males, which mirrors national data (Aughinbaugh et al., 2013).
Finally, we conducted analyses separately by ancestry group, and did not formally test for ethnic-racial differences. Our study thus is not informative about any factors that might contribute to the differences across ancestry groups, and we urge caution in making comparisons of results across the ancestry groups. Incorporating diverse populations in biomedical research and understanding sources of disparities between different sociocultural groups remains an important topic for future research.
In conclusion, the present study adds to the literature by examining the associations between alcohol use disorder and marital outcomes in a diverse sample enriched for risk. We capitalized on COGA’s multigenerational family-based sample to account for familial components (measured genetic relatedness and familial environment) to better understand the phenotypic associations between AUD and marital outcomes. AUD was associated with marriage in both EA and AA samples, and these effects were largely independent of polygenic predispositions for alcohol problems and common comorbid externalizing and internalizing disorders, and also held when controlling for shared familial factors that contribute to marriage. AUD was associated with divorce in the EA, but not AA, sample. Our results demonstrate the importance of AUD for both marriage and divorce even in a high-risk population. Future studies addressing questions such as what mechanisms contribute to these associations and the degree to which the associations reflect causal and non-causal processes would further help in clarifying the social consequences of AUD.
Supplementary Material
Public health significance:
The social correlates/consequences of alcohol use disorder in high-risk populations are not well established. The results from this study indicate that, in a sample enriched for risk, those with alcohol use disorder and related conditions were less likely to marry, and more likely to divorce, with some evidence for differences in European and African ancestry groups.
Acknowledgements
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, T. Foroud; Scientific Director, A. Agrawal; Translational Director, D. Dick, includes eleven different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, T. Foroud, Y. Liu, M. Plawecki); University of Iowa Carver College of Medicine (S. Kuperman, J. Kramer); SUNY Downstate Health Sciences University (B. Porjesz, J. Meyers, C. Kamarajan, A. Pandey); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, R. Hart, J. Salvatore); The Children’s Hospital of Philadelphia, University of Pennsylvania (L. Almasy); Virginia Commonwealth University (D. Dick); Icahn School of Medicine at Mount Sinai (A. Goate, P. Slesinger); and Howard University (D. Scott). Other COGA collaborators include: L. Bauer (University of Connecticut); J. Nurnberger Jr., L. Wetherill, X., Xuei, D. Lai, S. O’Connor, (Indiana University); G. Chan (University of Iowa; University of Connecticut); D.B. Chorlian, J. Zhang, P. Barr, S. Kinreich, G. Pandey (SUNY Downstate); N. Mullins (Icahn School of Medicine at Mount Sinai); A. Anokhin, S. Hartz, E. Johnson, V. McCutcheon, S. Saccone (Washington University); J. Moore, Z. Pang, S. Kuo (Rutgers University); A. Merikangas (The Children’s Hospital of Philadelphia and University of Pennsylvania); F. Aliev (Virginia Commonwealth University); H. Chin and A. Parsian are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting- Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
Financial Support
This work was supported by the National Institutes of Health (NIH) Grants R01AA028064 (PI: Salvatore) and K01AA024152 (PI: Salvatore) from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The Collaborative Study on the Genetics of Alcoholism (COGA) is supported by NIH Grant U10AA008401 (PI: Porjesz)
Footnotes
Conflicts of Interest: None.
Some results presented in this paper were previously presented at the Annual Research Society on Alcoholism Scientific Meeting in June, 2021.
References
- American Psychiatric Association. (1987). Diagnostic and Statistical Manual of Mental Disorders (3rd rev. ed.). American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Publishing. [Google Scholar]
- Assari S (2017). Social Determinants of Depression: The Intersections of Race, Gender, and Socioeconomic Status. Brain Sciences, 7(12), 156. 10.3390/brainsci7120156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aughinbaugh A, Robles O, & Sun H (2013). Marriage and divorce: Patterns by gender, race, and educational attainment. Monthly Labor Review. 10.21916/mlr.2013.32 [DOI] [Google Scholar]
- Barr PB, Ksinan A, Su J, Johnson EC, Meyers JL, Wetherill L, Latvala A, Aliev F, Chan G, Kuperman S, Nurnberger J, Kamarajan C, Anokhin A, Agrawal A, Rose RJ, Edenberg HJ, Schuckit M, Kaprio J, & Dick DM (2020). Using polygenic scores for identifying individuals at increased risk of substance use disorders in clinical and population samples. Translational Psychiatry, 10(1), 196. 10.1038/s41398-020-00865-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Reich T, Hesselbrock V, Porjesz B, Li T-K, Schuckit MA, Edenberg HJ, & Rice JP (1995). The collaborative study on the genetics of alcoholism. Alcohol Health and Research World, 19, 228–228.31798102 [Google Scholar]
- Bennett NG, Bloom DE, & Craig PH (1989). The Divergence of Black and White Marriage Patterns. American Journal of Sociology, 95(3), 692–722. 10.1086/229330 [DOI] [Google Scholar]
- Bogdan R, Baranger DAA, & Agrawal A (2018). Polygenic Risk Scores in Clinical Psychology: Bridging Genomic Risk to Individual Differences. Annual Review of Clinical Psychology, 14(1), 119–157. 10.1146/annurev-clinpsy-050817-084847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy SJ, Victor JC, & Diemert LM (2009). Origin and use of the 100 cigarette criterion in tobacco surveys. Tobacco Control, 18(4), 317–323. 10.1136/tc.2008.027276 [DOI] [PubMed] [Google Scholar]
- Bourdon JL, Tillman R, Francis MW, Dick DM, Stephenson M, Kamarajan C, Edenberg HJ, Kramer J, Kuperman S, Bucholz KK, & McCutcheon VV (2020). Characterization of Service Use for Alcohol Problems Across Generations and Sex in Adults With Alcohol Use Disorder. Alcoholism: Clinical and Experimental Research, 44(3), 746–757. 10.1111/acer.14290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brière FN, Rohde P, Seeley JR, Klein D, & Lewinsohn PM (2014). Comorbidity between major depression and alcohol use disorder from adolescence to adulthood. Comprehensive Psychiatry, 55(3), 526–533. 10.1016/j.comppsych.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CM, Wickrama KAS, Bolland J, Bryant BM, Cutrona CE, & Stanik CE (2010). Race matters, even in marriage: identifying factors linked to marital outcomes for African Americans. Journal of Family Theory & Review, 2(3), 157–174. 10.1111/j.1756-2589.2010.00051.x [DOI] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, & Schuckit MA (1994). A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol, 55(2), 149–158. 10.15288/jsa.1994.55.149 [DOI] [PubMed] [Google Scholar]
- Bucholz KK, McCutcheon VV, Agrawal A, Dick DM, Hesselbrock VM, Kramer JR, Kuperman S, Nurnberger JI Jr, Salvatore JE, Schuckit MA, Bierut LJ, Foroud TM, Chan G, Hesselbrock M, Meyers JL, Edenberg HJ, & Porjesz B (2017). Comparison of Parent, Peer, Psychiatric, and Cannabis Use Influences Across Stages of Offspring Alcohol Involvement: Evidence from the COGA Prospective Study. Alcoholism: Clinical and Experimental Research, 41(2), 359–368. 10.1111/acer.13293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC, & Tishkoff SA (2008). African Genetic Diversity: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping. Annual Review of Genomics and Human Genetics, 9(1), 403–433. 10.1146/annurev.genom.9.081307.164258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, & Palmer LJ (2003). Population stratification and spurious allelic association. The Lancet, 361(9357), 598–604. 10.1016/S0140-6736(03)12520-2 [DOI] [PubMed] [Google Scholar]
- Cherlin AJ (1992). Marriage, divorce, remarriage. Harvard University Press. [Google Scholar]
- Cranford JA, Floyd FJ, Schulenberg JE, & Zucker RA (2011). Husbands’ and wives’ alcohol use disorders and marital interactions as longitudinal predictors of marital adjustment. Journal of Abnormal Psychology, 120(1), 210–222. 10.1037/a0021349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer E, Emery RE, Slutske WS, Heath AC, Madden PA, & Martin NG (2005). A genetically informed study of marital instability and its association with offspring psychopathology. Journal of Abnormal Psychology, 114(4), 570–586. 10.1037/0021-843X.114.4.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon P (2009). Marriage Among African Americans: What Does the Research Reveal? Journal of African American Studies, 13(1), 29–46. 10.1007/s12111-008-9062-5 [DOI] [Google Scholar]
- Forthofer MS, Kessler RC, Story AL, & Gotlib IH (1996). The Effects of Psychiatric Disorders on the Probability and Timing of First Marriage. Journal of Health and Social Behavior, 37(2), 121–132. 10.2307/2137268 [DOI] [PubMed] [Google Scholar]
- Fu H, & Goldman N (1996). Incorporating Health into Models of Marriage Choice: Demographic and Sociological Perspectives. Journal of Marriage and Family, 58(3), 740–758. 10.2307/353733 [DOI] [Google Scholar]
- Ge T, Chen C-Y, Ni Y, Feng Y-CA, & Smoller JW (2019). Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nature Communications, 10(1), 1776. 10.1038/s41467-019-09718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin P, McGill B, & Chandra A (2009). Who marries and when? Age at first marriage in the United States: 2002. NCHS Data Brief(19), 1–8. [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, & Hasin DS (2015). Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72(8), 757–766. 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter SL (2000). Psychosocial adjustment of adult children of alcoholics: A review of the recent empirical literature. Clinical Psychology Review, 20(3), 311–337. 10.1016/S0272-7358(98)00084-1 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom K-O (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Herttua K, Martikainen P, Vahtera J, Kivimäki M, & Brayne C (2011). Living Alone and Alcohol-Related Mortality: A Population-Based Cohort Study from Finland. PLoS Medicine, 8(9), e1001094. 10.1371/journal.pmed.1001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, & Hesselbrock V (1999). A validity study of the SSAGA-a comparison with the SCAN. Addiction, 94(9), 1361–1370. 10.1046/j.1360-0443.1999.94913618.x [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, & Locke J (2000). Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biological Psychiatry, 48(4), 265–275. 10.1016/S0006-3223(00)00841-6 [DOI] [PubMed] [Google Scholar]
- Homish GG, & Leonard KE (2007). The drinking partnership and marital satisfaction: The longitudinal influence of discrepant drinking. Journal of Consulting and Clinical Psychology, 75(1), 43–51. 10.1037/0022-006X.75.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang BJ, Patrick ME, & Schuler MS (2017). Substance Use Behaviors and the Timing of Family Formation During Young Adulthood. Journal of Family Issues, 39(5), 1396–1418. 10.1177/0192513X17710285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerskey BA, Panizzon MS, Jacobson KC, Neale MC, Grant MD, Schultz M, Eisen SA, Tsuang MT, & Lyons MJ (2010). Marriage and divorce: A genetic perspective. Personality and Individual Differences, 49(5), 473–478. 10.1016/j.paid.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Karriker-Jaffe KJ, Sundquist J, & Sundquist K (2016). Social and economic consequences of alcohol use disorder: a longitudinal cohort and co-relative analysis. Psychological Medicine, 47(5), 925–935. 10.1017/S0033291716003032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Walters EE, & Forthofer MS (1998). The Social Consequences of Psychiatric Disorders, III: Probability of Marital Stability. American Journal of Psychiatry, 155(8), 1092–1096. 10.1176/ajp.155.8.1092 [DOI] [PubMed] [Google Scholar]
- King G, Polednak A, Bendel RB, Vilsaint MC, & Nahata SB (2004). Disparities in smoking cessation between African Americans and Whites: 1990–2000. American Journal of Public Health, 94(11), 1965–1971. 10.2105/ajph.94.11.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Zhou H, Kember RL, Vickers Smith R, Justice AC, Damrauer S, Tsao PS, Klarin D, Baras A, Reid J, Overton J, Rader DJ, Cheng Z, Tate JP, Becker WC, Concato J, Xu K, Polimanti R, Zhao H, & Gelernter J (2019). Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nature Communications, 10(1), 1499. 10.1038/s41467-019-09480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D, Wetherill L, Bertelsen S, Carey CE, Kamarajan C, Kapoor M, Meyers JL, Anokhin AP, Bennett DA, Bucholz KK, Chang KK, De Jager PL, Dick DM, Hesselbrock V, Kramer J, Kuperman S, Nurnberger JI, Raj T, Schuckit M, …, & Foroud T (2019). Genome-wide association studies of alcohol dependence, DSM-IV criterion count and individual criteria. Genes, Brain & Behavior, 18(6), 10.1111/gbb.12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KE, & Rothbard JC (1999). Alcohol and the marriage effect. Journal of Studies on Alcohol, Supplement(s13), 139–146. 10.15288/jsas.1999.s13.139 [DOI] [PubMed] [Google Scholar]
- Manzoli L, Villari P, Pirone GM, & Boccia A (2007). Marital status and mortality in the elderly: A systematic review and meta-analysis. Social Science & Medicine, 64(1), 77–94. 10.1016/j.socscimed.2006.08.031 [DOI] [PubMed] [Google Scholar]
- Mayol-Garcia Y, Gurrentz B, & Kreider RM (2021). Number, timing, and duration of marriages and divorces: 2016. [Google Scholar]
- Nakagawa S, Johnson PCD, & Schielzeth H (2017). The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. Journal of The Royal Society Interface, 14(134), 20170213. 10.1098/rsif.2017.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Sloan FA, & Taylor DH (2005). Heavy alcohol use and marital dissolution in the USA. Social Science & Medicine, 61(11), 2304–2316. 10.1016/j.socscimed.2005.07.021 [DOI] [PubMed] [Google Scholar]
- Pankratz VS, de Andrade M, & Therneau TM (2005). Random-effects Cox proportional hazards model: General variance components methods for time-to-event data. Genetic Epidemiology, 28(2), 97–109. 10.1002/gepi.20043 [DOI] [PubMed] [Google Scholar]
- Peterson RE, Kuchenbaecker K, Walters RK, Chen C-Y, Popejoy AB, Periyasamy S, Lam M, Iyegbe C, Strawbridge RJ, Brick L, Carey CE, Martin AR, Meyers JL, Su J, Chen J, Edwards AC, Kalungi A, Koen N, Majara L, … & Duncan LE (2019). Genome-wide Association Studies in Ancestrally Diverse Populations: Opportunities, Methods, Pitfalls, and Recommendations. Cell, 179(3), 589–603. 10.1016/j.cell.2019.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Snedecor SM, & Mehringer AM (2004). Defining a never-smoker: Results from the nonsmokers survey. Addictive Behaviors, 29(6), 1149–1154. 10.1016/j.addbeh.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Power C, & Rodgers B (1999). Heavy alcohol consumption and marital status: disentangling the relationship in. Addiction, 94(10), 1477–1487. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, & Reich D (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics, 38(8), 904–909. 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2019). R: A language and environment for statistical computing. In R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Raley RK, & Sweeney MM (2020). Divorce, Repartnering, and Stepfamilies: A Decade in Review. Journal of Marriage & Family, 82(1), 81–99. 10.1111/jomf.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Gmel GE, Gmel G, Hasan OSM, Imtiaz S, Popova S, Probst C, Roerecke M, Room R, Samokhvalov AV, Shield KD, & Shuper PA (2017). The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction, 112(6), 968–1001. 10.1111/add.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li T-K, Conneally PM, Nurnberger JI Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, … & Begleiter, H. (1998). Genome-wide search for genes affecting the risk for alcohol dependence. American Journal of Medical Genetics, 81(3), 207–215. [PubMed] [Google Scholar]
- Saha TD, Grant BF, Chou SP, Kerridge BT, Pickering RP, & Ruan WJ (2018). Concurrent use of alcohol with other drugs and DSM-5 alcohol use disorder comorbid with other drug use disorders: Sociodemographic characteristics, severity, and psychopathology. Drug and Alcohol Dependence, 187, 261–269. 10.1016/j.drugalcdep.2018.03.006 [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Gardner CO, & Kendler KS (2019). Marriage and reductions in men’s alcohol, tobacco, and cannabis use. Psychological Medicine, 50(15), 2634–2640. 10.1017/S0033291719002964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Larsson Lönn S, Sundquist J, Lichtenstein P, Sundquist K, & Kendler KS (2017). Alcohol use disorder and divorce: evidence for a genetic correlation in a population-based Swedish sample. Addiction, 112(4), 586–593. 10.1111/add.13719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Larsson Lönn S, Sundquist J, Sundquist K, & Kendler KS (2018). Genetics, the Rearing Environment, and the Intergenerational Transmission of Divorce: A Swedish National Adoption Study. Psychological Science, 29(3), 370–378. 10.1177/0956797617734864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Palmer AA, Fontanillas P, Elson SL, Adams MJ, Howard DM, Edenberg HJ, Davies G, Crist RC, Deary IJ, McIntosh AM, & Clarke T-K (2018). Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in Two Population-Based Cohorts. American Journal of Psychiatry, 176(2), 107–118. 10.1176/appi.ajp.2018.18040369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JE, Salvatore JE, Aliev F, Edwards AC, Hickman M, Kendler KS, Macleod J, Latvala A, Loukola A, Kaprio J, Rose RJ, Chan G, Hesselbrock V, Webb BT, Adkins A, Bigdeli TB, Riley BP, & Dick DM (2018). Polygenic Risk Score Prediction of Alcohol Dependence Symptoms Across Population-Based and Clinically Ascertained Samples. Alcoholism, clinical and experimental research, 42(3), 520–530. 10.1111/acer.13589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn CA (2004). Marital status and health: United States, 1999–2002. Adv Data(351), 1–32. [PubMed] [Google Scholar]
- Sobal J, Hanson KL, & Frongillo EA (2009). Gender, Ethnicity, Marital Status, and Body Weight in the United States. Obesity, 17(12), 2223–2231. 10.1038/oby.2009.64 [DOI] [PubMed] [Google Scholar]
- Therneau TM (2020). coxme: Mixed Effects Cox Models. https://CRAN.R-project.org/package=coxme [Google Scholar]
- Therneau TM, Lumley T, Atkinson E, & Crowson C (2021). survival: Survival Analysis. https://cran.r-project.org/web/packages/survival/
- Vanyukov MM, Tarter RE, Conway KP, Kirillova GP, Chandler RK, & Daley DC (2016). Risk and resistance perspectives in translation-oriented etiology research. Translational Behavioral Medicine, 6(1), 44–54. 10.1007/s13142-015-0355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, & Kendler KS (2014). The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychological Medicine, 45(5), 1061–1072. 10.1017/S0033291714002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron M, Heath AC, Lynskey MT, Bucholz KK, Madden PAF, & Martin NG (2011). Alcoholic Marriage: Later Start, Sooner End. Alcoholism:Clinical and Experimental Research, 35(4), 632–642. 10.1111/j.1530-0277.2010.01381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, Aliev F, Bacanu S-A, Batzler A, Bertelsen S, Biernacka JM, Bigdeli TB, Chen L-S, Clarke T-K, Chou Y-L, Degenhardt F, Docherty AR, Edwards AC, Fontanillas P,… & Agrawal A(2018). Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nature Neuroscience, 21(12), 1656–1669. 10.1038/s41593-018-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt TT (2002). Marital and Cohabiting Relationships of Adult Children of Alcoholics: Evidence from the National Survey of Families and Households. Journal of Family Issues, 23(2), 246–265. 10.1177/0192513X02023002004 [DOI] [Google Scholar]
- Wolfinger NH (2005). Understanding the divorce cycle : The children of divorce in their own marriages. Cambridge University Press. [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, & Middeldorp CM (2014). Research Review: Polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry, 55(10), 1068–1087. 10.1111/jcpp.12295 [DOI] [PubMed] [Google Scholar]
- Xu R, & Gamst A (2007). On proportional hazards assumption under the random effects models. Lifetime data analysis., 13(3), 317–332. 10.1007/s10985-007-9041-5 [DOI] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, & Visscher PM (2011). GCTA: A Tool for Genome-wide Complex Trait Analysis. American journal of human genetics, 88(1), 76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski TCB, Pedersen SL, McCarthy DM, & Smith GT (2014). Less drinking, yet more problems: Understanding African American drinking and related problems. Psychological Bulletin, 140(1), 188–223. 10.1037/a0032113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyatdinov A, Vázquez-Santiago M, Brunel H, Martinez-Perez A, Aschard H, & Soria JM (2018). lme4qtl: linear mixed models with flexible covariance structure for genetic studies of related individuals. BMC Bioinformatics, 19(1), 68. 10.1186/s12859-018-2057-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


