Abstract
Kynurenine pathway is the main route of tryptophan metabolism and produces several metabolites with various biologic properties. It has been uncovered that several cardiovascular diseases are associated with the overactivation of kynurenine pathway and kynurenine and its metabolites have diagnostic and prognostic value in cardiovascular diseases. Furthermore, it was found that several kynurenine metabolites can differently affect cardiovascular health. For instance, preclinical studies have shown that kynurenine, xanthurenic acid and cis-WOOH decrease blood pressure; kynurenine and 3-hydroxyanthranilic acid prevent atherosclerosis; kynurenic acid supplementation and kynurenine 3-monooxygenase (KMO) inhibition improve the outcome of stroke. Indoleamine 2,3-dioxygenase (IDO) overactivity and increased kynurenine levels improve cardiac and vascular transplantation outcomes, whereas exacerbating the outcome of myocardial ischemia, post-ischemic myocardial remodeling, and abdominal aorta aneurysm. IDO inhibition and KMO inhibition are also protective against viral myocarditis. In addition, dysregulation of kynurenine pathway is observed in several conditions such as senescence, depression, diabetes, chronic kidney disease (CKD), cirrhosis, and cancer closely connected to cardiovascular dysfunction. It is worth defining the exact effect of each metabolite of kynurenine pathway on cardiovascular health. This narrative review is the first review that separately discusses the involvement of kynurenine pathway in different cardiovascular diseases and dissects the underlying molecular mechanisms.
Keywords: Kynurenine pathway, cardiovascular disease, hypertension, atherosclerosis, myocardial infraction, transplantation
Introduction
Cardiovascular diseases, particularly ischemic heart disease and stroke, account for a major proportion of total deaths and disability-adjusted life years (DALYs).1,2 In addition, there has been an increase in the prevalence of leading cardiovascular risk factors such as hypertension, dyslipidemia, and hyperglycemia during the last decades. 1 So far, many studies have attempted to discover new markers and therapeutic targets for early diagnosis and better management of cardiovascular diseases.3,4 This progress in the prevention, detection, and treatment of cardiovascular diseases resulted in a remarkable decrease in the incidence and mortality of cardiovascular diseases during recent years. 5
Kynurenine pathway, as a major route of tryptophan degradation, is involved in several biological processes such as immune-regulation, cancer, inflammation, and metabolism.6,7 Different metabolites produced in kynurenine pathway such as kynurenine, kynurenic acid, 3-hydroxykynurenine, 3-hydroxyanthranilic acid were shown to be involved in such effects. For instance, the immunomodulatory effects of kynurenine and kynurenic acid and their function through aryl hydrocarbon receptor (AHR) have been extensively studied in different types of cancer.8-11 Recent studies showed that kynurenine and its metabolites are markedly involved in the pathophysiology of cardiovascular diseases, and pharmacological interventions to modulate kynurenine pathway possess therapeutic value in several cardiovascular diseases.12-16 Furthermore, several cardiovascular risk factors such as obesity, hyperglycemia, dyslipidemia, high blood pressure, smoking, and aging lead to the overactivation of kynurenine pathway.16-20 Moreover, higher levels of kynurenine and its metabolites are found in other diseases such as cancer, CKD, cirrhosis, diabetes, and depression that are known for their cardiovascular complications.10,21-23 Regarding these findings, a deep insight into the involvement of kynurenine pathway in the pathophysiology of cardiovascular diseases can help expand its clinical application and improve the management of cardiovascular diseases. This narrative review aimed to illuminate the diverse effects of kynurenine and its metabolites on different cardiovascular diseases and illustrate the underlying molecular mechanisms. Thereafter, it would be explained how kynurenine may link cardiovascular diseases to senescence, cancer, CDK, cirrhosis, diabetes, and depression which are heavily linked to cardiovascular health.
Kynurenine Pathway
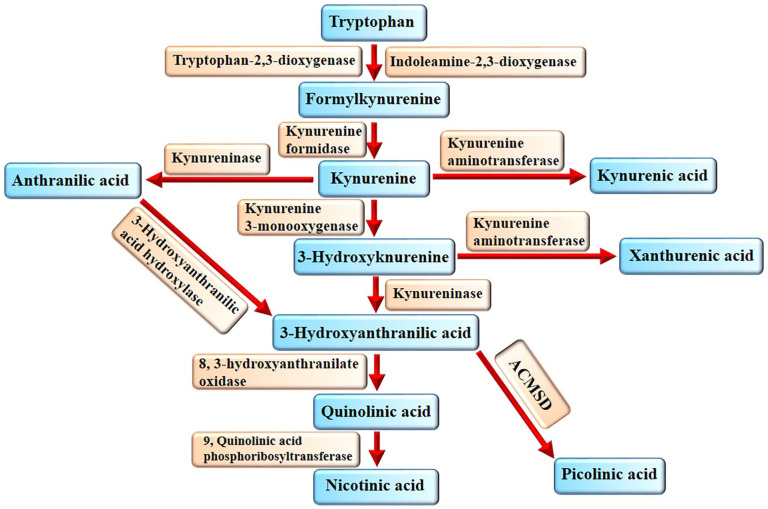
Tryptophan is an essential amino acid that is mainly catabolized through kynurenine pathway in the human body. 24 Besides, tryptophan is the substrate for the synthesis of serotonin, melatonin, and indole metabolites.24-26 IDO and tryptophan 2,3-dioxygenase (TDO) are the first and rate-limiting enzymes of kynurenine pathway. TDO is predominantly expressed in the liver and IDO in other organs. 27 IDO and TDO produce formylkynurenine, which will be converted to kynurenine by kynurenine formidase. Kynurenine undergoes 3 different enzymatic activities. Kynurenine aminotransferase (KAT) converts kynurenine to kynurenic acid. In addition, kynurenine can be converted to anthranilic acid and 3-hydroxykynurenine by kynureninase and KMO, respectively (Figure 1). Thereafter, 3-hydroxykynurenine can be converted to 3-hydroxyanthranilic acid and xanthurenic acid by kynureninase and KAT, respectively. Also, 3-hydroxyanthranilic acid hydroxylase can convert anthranilic acid to 3-hydroxyanthranilic acid.28-30 Eventually, quinolinic acid, nicotinic acid, and picolinic acid are produced from 3-hydroxyanthranilic acid in the last of kynurenine pathway.28-30 Quinolinic acid is produced by 3-hydroxyanthranilate-3,4-dioxygenase form 3-hydroxyanthranilic acid.
Figure 1.
Kynurenine pathway. IDO and TDO convert tryptophan into formylkynurenine, which then will be converted into kynurenine by kynurenine formidase. From this point, the pathway divides into 3 arms. Anthranilic acid, 3-hydroxykynurenine and kynurenic acid are the first products of these arms. Kynureninase and 3-hydroxyanthranilic acid hydroxylase can convert 3-hydroxykynurenine and anthranilic acid into 3-hydroxyanthranilic acid, respectively. Quinolinic acid is produced by 3-hydroxyanthranilate-3,4-dioxygenase form 3-hydroxyanthranilic acid. Phosphoribosyltransferase produces nicotinic acid from quinolinic acid and 7, 2-amino-3-carboxymuconic acid semialdehyde decarboxylase (ACMSD) produces picolinic acid from 3-hydroxyanthranilic acid.
Phosphoribosyltransferase produces nicotinic acid from quinolinic acid and 7, 2-amino-3-carboxymuconic acid semialdehyde decarboxylase (ACMSD) produces picolinic acid from 3-hydroxyanthranilic acid. 31
However, IDO1, IDO2, and TDO catalyze the same reaction, they have different patterns of tissue expression. IDO1 is expressed in different organs throughout the body, TDO is mainly expressed in the liver and IDO2 is mainly expressed in the liver, kidney and antigen-presenting cells. 31 IDO in this article is considered IDO1 unless otherwise mentioned.
During inflammation, several inflammatory mediators such as interleukin (IL) 1β, tumor necrosis factor-α (TNF-α), and particularly interferon-γ (IFN-γ) instigate janus kinase (JAK)/signal transducer and activator of transcription 1 (STAT1)-mediated expression of IDO and activate kynurenine pathway.32-36 Furthermore, the inflammatory response may augment particular branches of kynurenine pathway and result in increased concentration of specific metabolites of kynurenine pathway. 33 IDO is produced by dendritic cells, macrophages and other inflammatory cells, which produces kynurenine, stimulates AHR and enhances T regulatory (Reg) cells differentiation.37,38 AHR is a nuclear receptor that regulates target gene expression. It alters immune cells differentiation in favor of T Reg cells differentiation and provides an immune-suppressive microenvironment in inflammatory diseases and cancer.11,39 Increased T Reg cells abundance downregulates immune response and partly alleviates the initial inflammation. 40 However, this scenario is not always helpful and kynurenine/AHR axis exerts various effects on different organs. Moreover, kynurenine pathway consists of several metabolites with various biologic effects. However, alleviation of immune response can be helpful in inflammatory and autoimmune disease, cancer cells take advantage of this opportunity and silently grow and proliferate in the immune-suppressive microenvironment produced by kynurenine/AHR. 10 Indeed, cancer cells have an increased expression of IDO and TDO that allows them to consume tryptophan and produce a higher amount of kynurenine and its metabolites. 41 Hence, they can increase T Reg cells response and prevent T cells cytotoxic response. In addition, it was observed that some metabolites of kynurenine such as 3-hydroxyanthranilic and quinolinic acid can selectively induce apoptosis in murine Th1 cells in vitro. 42 This warrants the higher plasma kynurenine/tryptophan ratio in patients with cancer.10,41 Herein, several inhibitors of the kynurenine pathway particularly IDO inhibitors such as indoximod, epacadostat, and navoximod are used in cancer clinical trials. 10
Kynurenine Pathway and Blood Pressure
Recently, it has been uncovered that kynurenine pathway is involved in regulating blood pressure and certain metabolites of kynurenine modulated blood pressure in animal studies.16,43,44 Higher plasma concentrations of kynurenine and its metabolites such as kynurenic acid, 3-hydroxykynurenine, and anthranilic acid were detected in the rat model of renovascular hypertension. 43 Renal artery occlusion increased the plasma level of angiotensin (Ang) II, which was positively correlated with the plasma level of kynurenine and negatively correlated with the plasma level of tryptophan. 43 Recently, Wu et al 45 reported that a higher kynurenine/tryptophan ratio (⩾54.7 × 10−3) predicts the effectiveness of Ang receptor blockers (ARBs) on macroalbuminuria (sensitivity 90.0%, specificity 50%) in patients with diabetic kidney disease. In addition, it was shown that ARBs decrease kynurenic acid production and KAT activity in rats’ kidneys in vitro. 46 Likewise, it was found that patients with CKD who receive renin-Ang-aldosterone system (RAAS) inhibitors have a lower kynurenine/tryptophan ratio compared with those not receiving these drugs. 21 These findings show that Ang II positively regulates kynurenine pathway. Also, kynurenine pathway activation in hypertension can be a sign of RAAS overactivity.
Endothelial dysfunction upregulates IDO and increases kynurenine production. Kynurenine activates adenylate and soluble guanylate cyclase pathways and dilates arteries. 16 Endotoxemia enhanced endothelial IDO production in the mice model, thereby increasing kynurenine level and decreasing blood pressure. 16 Consistently, pharmacological inhibition of IDO increased blood pressure in mice with systemic inflammation. 16 In addition, in a dose-dependent manner, kynurenine lowered blood pressure in spontaneously hypertensive rats. 16 Kynurenine, in an endothelial-independent manner, and tryptophan, in an endothelial-dependent manner, led to vasorelaxation in preconstricted coronary arteries in a porcine model. 16 Similarly, it has been observed that IDO is considerably overexpressed in inflamed heart tissue and endothelial cells in the heart and kidney of patients with sepsis. 47 Additionally, kynurenine/tryptophan ratio was positively and vigorously correlated with inotrope requirements in those patients. 47
Kynurenine is strongly produced in patients with idiopathic pulmonary arterial hypertension (PAH) and contributes to lower pulmonary arterial blood pressure by activating nitric oxide (NO)/cGMP and cAMP pathways in the pulmonary arteries. 48 In response to hypoxia, IDO−/− mice showed significantly more increase in mean pulmonary arterial pressure and medial thickness of pulmonary arteries. 49 Endothelial IDO serves as a protective mechanism against PAH and pulmonary arterial remodeling. 49 In addition, IDO overexpression provided more protection against PAH in a mice model. 49 IDO activity led to increased apoptosis of vascular smooth muscle cells (VSMCs) in pulmonary hypertension by depolarizing mitochondrial membrane and releasing cytochrome C. 49 Besides, IDO activity modulated the pathologic differentiation of VSMCs in PAH. 49
IDO is downregulated in endothelial cells of patients with preeclampsia.50-52 In addition, it was uncovered that low IDO expression in the human placenta is correlated with more severe maternal hypertension or proteinuria in patients with preeclampsia. 52 Consistently, IDO knockout has been associated with slightly increased blood pressure and preeclampsia phenotypes such as renal glomerular endotheliosis and proteinuria in mice. 53 Even in the presence of lower IDO expression, increased tryptophan availability for IDO contributed to vasorelaxation. 50 Kynurenine activated large-conductance Ca2+-activated K+ channels (BKCa) channels in VSMCs of women with or without preeclampsia. 54 Kynurenine enhances the amplitude but not the frequency of spontaneous transient outward currents through BKCa. 54 Sakakibara et al 55 also revealed that kynurenine activates voltage-dependent K+ channels (Kv7 channels) in VSMCs of both humans and rats to induce hyperpolarization of these cells and accelerate vasodilation. These effects have been associated with dilatation of omental and myometrial resistance arteries. 54 Consistently, the hypotensive effect of kynurenine in rats was partly attenuated by linopirdine, a selective inhibitor of Kv7 channel. 55
Fazio et al 44 uncovered that xanthurenic acid is the most effective metabolite of kynurenine for vasorelaxation. They found that the serum level of xanthurenic acid is increased by several folds in lipopolysaccharide (LPS)-mediated endotoxemia. Furthermore, systemic administration of xanthurenic acid reduced blood pressure in mice. 44 Xanthurenic acid upregulated endothelial nitric oxide (eNOS) to induce hypotension and its effect was reversed by eNOS inhibitor. In addition, administration of KMO inhibitors increased the serum levels of kynurenine, whereas attenuated LPS-induced hypotension in mice. 44 These findings suggest that xanthurenic acid is a highly effective vasodilator metabolite of kynurenine pathway that can be used for future drug development. Additionally, Kwok et al 56 found that the KAT-1 gene possesses a missense mutation, E61G, in every strain of spontaneously hypertensive rats. This finding may justify the importance of kynurenic acid in hypertension.
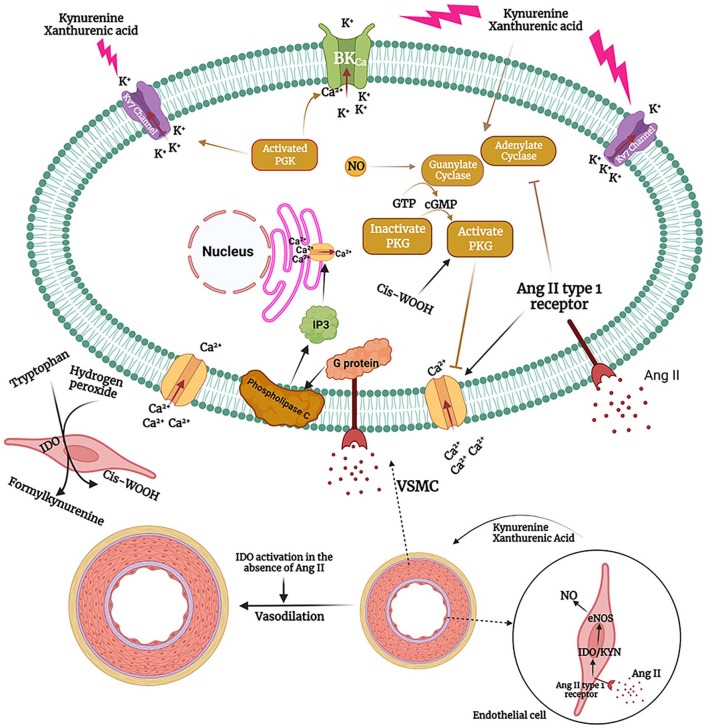
Previously, it has been shown that stimulation of Ang II type 1 receptor, a G protein-coupled receptor, internalizes and degrades BKCa, increases intracellular Ca2+, inhibits NO/guanylate cyclase system, and leads to vasoconstriction in VSMCs, whereas Ang II receptors in endothelial cells may activate compensatory vasodilation mechanisms57-61 (Figure 2). Ang II type 1 receptor stimulation activates G protein-coupled receptor, subsequently activates phospholipase C and increases inositol-1,4,5-triphosphate (IP3) in VSMCs. 61 IP3 stimulates sarcoplasmic reticulum Ca2+ efflux and begins contraction. 61 Ang II type 1 receptor stimulation also leads to extracellular Ca2+ influx. 61 IDO overexpression in endothelial cells, subsequent to Ang II stimulation, activates NO/guanylate cyclase. Guanylate cyclase converts GTP to cGMP, which subsequently activates protein kinase G (PKG), opens K+ channels, hyperpolarizes VSMCs, and blocks the Ca2+ channels. 62 Hence, IDO is mechanistically acting against Ang II to decrease blood pressure (Figure 2).
Figure 2.
The effect of Ang II and kynurenine pathway on vascular tonicity and blood pressure. Ang II stimulates Ang II type 1 receptor on VSMCs, which is a G protein-coupled receptor. G protein activates phospholipase C, leading to IP3 production from membrane phospholipids. IP3 opens the Ca2+ channels on sarcoplasmic reticulum membrane and increases cytoplasmic Ca2+ concentration. In addition, Ang II type 1 receptor stimulation leads to inhibition of guanylate cyclase, downregulation of BKCa and extracellular Ca2 influx. In the endothelial cells, Ang II upregulates IDO and activates kynurenine pathway. Kynurenine metabolites, particularly kynurenine itself and xanthurenic acid, activate guanylate cyclase and adenylate cyclase. Guanylate cyclase converts GTP into cGMP, thereby activating PKG. PKG activates K+ channels and leads to VSMCs hyperpolarization and relaxation. Furthermore, kynurenine pathway can enhance eNOS function in endothelial cells to lower blood pressure. In addition to its hypertensive effects, Ang II activates kynurenine pathway, which acts as a counter-regulatory mechanism. Pharmacological augmentation of kynurenine pathway or attenuation of Ang II signaling pathway helps to lower blood pressure. In addition, overactivation of kynurenine pathway in the absence of increased concentrations of Ang II such as those observed in sepsis leads to hypotension. Interestingly, inflammatory response and increased concentration of hydrogen peroxide can lead to cis-WOOH production as a by-product of kynurenine pathway in the endothelial cells. Cis-WOOH can readily activate PKG1α, induce vasorelaxation and decrease blood pressure.
IDO needs the reduction of Fe3+ to Fe2+ for its enzymatic activity. 63 Interestingly, Stanley et al 63 showed that oxidation of tryptophan by IDO in the presence of hydrogen peroxide can produce a byproduct named cis-WOOH. Cis-WOOH is generated by oxidatively activated dioxygenase activity of IDO. Inflammation results in the increased concentration of hydrogen peroxide and IDO produces singlet molecular oxygen ( 1 O2) from hydrogen peroxide. This reaction is associated with stereoselective oxidation of l-tryptophan to a tricyclic hydroperoxide via the oxidatively activated dioxygenase activity of IDO. Cis-WOOH decreases arterial tonicity and reduces blood pressure through PKG1α. 63 PKG1α function accelerates vasorelaxation and it is also deeply involved in the long-lasting blood pressure-lowering effect of NO. 64 Cis-WOOH can be generated in the endothelial cells. Cis-WOOH effectively and readily reacts with PKG1α, but it reacts slowly with arterial types of glutathione peroxidases and peroxiredoxins which prolongs its function. 65 Developing new drugs based on Cis-WOOH may be helpful for adequate control of blood pressure in hypertensive subjects.
Regarding these findings, IDO, kynurenine and kynurenine metabolites serve as both prognostic markers and therapeutic targets in hypertension. Kynurenine pathway is upregulated by Ang II and helpfully attenuates the deleterious effects of Ang II on hypertension. Future studies can further illuminate the clinical application of kynurenine, its metabolites and inhibitors of kynurenine pathway in the management of systemic hypertension, PAH, preeclampsia, and hypotension.
Kynurenine Pathway and Atherosclerosis
Atherosclerosis is a prevalent vascular condition and a major cause for the initiation and progression of other cardiovascular diseases.66,67 It is a local but chronic inflammatory response of the immune system that crucially endangers cardiovascular health in the long-term. 68 Both progression of atherosclerosis and rupture of an atherosclerotic plaque can result in major cardiovascular events67,68 and pharmacological management of atherosclerosis markedly prevents cardiovascular death.69,70
Both innate and adaptive immune cells, particularly macrophages, are markedly involved in the pathogenesis of atherosclerosis. 71 Oxidized lipoproteins stimulate immune response and lead to the release of numerous inflammatory cytokines such as IL1β, IL6, and TNF-α. 71 Furthermore, decreased abundance of T Reg cells and overactivity of T helper (Th)1, Th17, and B cells in response to ApoB, as a core protein of low-density lipoprotein (LDL), contribute to atherosclerosis progression. 67
It has been uncovered that Kynurenine and its metabolites are heavily implicated in the pathophysiology of atherosclerosis. 72 Overactivation of kynurenine pathway and increased plasma levels of kynurenic acid, 3-hydroxykynurenine, anthranilic acid, and 3-hydroxyanthranilic acid have been associated with a higher risk of acute myocardial infarction in patients with stable angina pectoris, particularly in diabetic and pre-diabetic subgroups. 73
Cason et al 15 measured the correlation between tryptophan, indole metabolites and kynurenine metabolites and the presence of advanced atherosclerosis in 100 patients who underwent carotid endarterectomy or limb revascularization. The study revealed that tryptophan, indole, indole-3-propionic acid, and indole-3-aldehyde levels were significantly associated with decreased risk of advanced atherosclerosis, whereas kynurenine/tryptophan ratio significantly and positively correlated with the presence of advanced atherosclerotic plaque. 15 Furthermore, kynurenine/tryptophan ratio was positively associated with a postoperative cardiac complication and major adverse events during the follow-up period. 15 Interestingly, it was shown that deviation within kynurenine pathway, particularly overproduction of quinolinic acid and decreased production of kynurenic acid, were associated with aggravation of local inflammatory response, carotid artery atherosclerosis, and increased need of patients for surgery because of an unstable carotid plaque. 72 It was observed that kynurenic acid and AHR could suppress the immune response, thereby improving carotid plaque stability. 72 KAT-1 (95% CI, OR 0.058 [0.006-0.541]) and KAT-2 (95% CI, OR 0.395 [0.146-1.069]) independently protected against carotid atherosclerotic plaque being symptomatic, while KMO (95% CI, OR 1.460 [0.896-2.377]) and kynureninase (95% CI, OR 1.540 [0.942-2.519]) were associated with non-significant increase in the risk of atherosclerotic plaque being symptomatic. 72 These enzymes may be useful markers for screening patients who will need therapeutic intervention in the near future.
IDO deletion decreased blood levels of IL10 and accelerated atherosclerosis plaque formation in hypercholesterolemic ApoE−/− mice. 74 Likewise, IDO inhibition by 1-methyl tryptophan (1-MT), an IDO inhibitor, has been associated with larger atherosclerotic lesions in the aortic root, increased levels of TNF-α, monocyte chemoattractant protein 1 (MCP-1), and vascular cell adhesion protein 1 (VCAM1) and increased CD68+ macrophage infiltration into the atherosclerotic lesion. 75 A derivative of anthranilic acid, 3,4-dimethoxycinna-moyl anthranilic acid (3,4-DAA), decreased the production of IL6, TNF-α, C-X-C motif ligand 1 (CXCL1) and granulocyte macrophage colony-stimulating factor (GMCSF) in human atheroma cells and reduced atherosclerotic plaque size and lesional macrophage abundance in ApoE−/− mice. 74
Polyzos et al 76 indicated that systemic attenuation of IDO by 1-MT increases vascular inflammation and augments the expression of MCP-1 and VCAM1 in ApoE−/− mice. IDO inhibition also accelerated M1 subtype macrophages polarization and expedited aortic atherosclerotic plaque formation (Figure 3). IDO inhibition also increased total cholesterol and very low-density lipoprotein (VLDL)/high-density lipoprotein (HDL) ratio in ApoE−/− mice. Supplementation of exogenous 3-hydroxyanthranilic acid alleviated vascular inflammation and reversed the atherogenic effect of 1-MT. 76 Attenuation of inflammatory cells infiltration into the vessel’s wall is a crucial mechanism involved in the protective effects of IDO. 77 Increased activity of inflammatory cells, in particular M1 macrophages, not only contributes to atherosclerotic plaque formation, but also leads to plaques destabilization. 78
Figure 3.
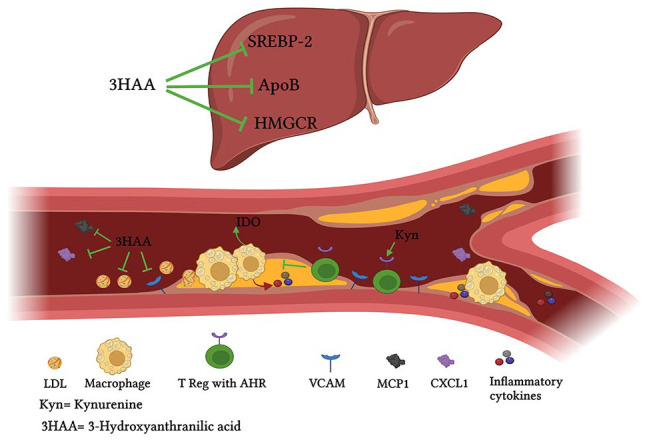
The effect of kynurenine and its metabolites on atherosclerosis. Inflammation and accumulation of oxidized lipoprotein stimulate the expression of IDO in inflammatory cells such as macrophages. Increased production of kynurenine accelerates T Reg cells differentiation and alleviates local inflammatory response. Kynurenine metabolites, particularly 3-hydroxyanthranilic acid, can vigorously decrease inflammatory cytokines release. In addition, 3-hydroxyanthranilic acid prevents the uncontrolled release of chemokines and hinders the infiltration of inflammatory cells into the vessel’s wall. It has been reported that 3-hydroxyanthranilic acid can attenuate hepatic lipogenesis and decrease blood level of LDL by downregulating SREBP-2, HMGCR, and ApoB.
It was found that 3-hydroxyanthranilic acid prevents inflammasome activation and IL1β production in macrophages.14,79 In addition, Berg et al 14 showed that 3-hydroxyanthranilic acid could suppress the expression and nuclear translocation of sterol regulatory element binding protein-2 (SREBP-2) and secretion of apolipoprotein B in hepatocytes. Furthermore, 3-hydroxyanthranilic acid prevented inflammasome activation and IL1β production in macrophages.14,79 Prevention of 3-hydroxyanthranilic acid degradation by inhibiting 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) downregulated hepatic expression of SREBP-2 and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), decreased hepatic lipid accumulation, reduced plasma levels of triglyceride and cholesterol and ameliorated atherosclerosis in LDLR−/− mice. 14 SREBP-2 is a transcription factor that increases the expression of HMGCR to enhance the synthesis of cholesterol 80 (Figure 3). Interestingly, 3-hydroxyanthranilic acid can strongly inhibit the oxidation of LDL and lower oxidized LDL intake in macrophages, thereby ameliorating the initiation and progression of atherosclerosis79,81 (Figure 3).
Previously, it was observed that plasmacytoid dendritic cells are markedly involved in preventing atherosclerosis in both humans and animals. 82 Plasmacytoid dendritic cells produce IDO and stimulate T Reg cells differentiation. 82 IDO expression in dendritic cells is associated with increased activity of T Reg cells in vessels’ walls and reciprocally T Reg cells with their cytotoxic T-lymphocyte-associated protein 4 (CTLA4) surface receptor instigate IDO expression in dendritic cells. 83 The self-augmentation cycle between T Reg cells and IDO mitigates vascular inflammation and protects against atherosclerosis. 83 Consistently, it has been observed that IDO is significantly overexpressed in atherosclerotic plaques, particularly in lesional macrophages and in coordination with T Reg cells activity. 84 Even it was observed that the beneficial effects of eicosapentaenoic acid on the repression of atherosclerosis are mediated by induction of IDO and differentiation of T Reg cells in the lesion. Likewise, IDO inhibitor reversed the protective effects of eicosapentaenoic on atherosclerosis. 85 Similarly, it has been revealed that the protective effects of mesenchymal stem cells on atherosclerosis also rely on IDO. 86
In an NF-kB-dependent manner, IDO increases the expression of tissue factor in macrophages of atherosclerotic plaques which can increase the risk of thrombogenesis and acute cardiovascular events. Consistently, inhibition of IDO or AHR decreases tissue factor expression in macrophages. 87 It was shown that kynurenine but not 3-hydroxyanthranilic and quinolinic acids could induce tissue factor expression in macrophages in the presence of an IDO inhibitor. 88
These findings suggest that the kynurenine pathway is a compensatory mechanism that is activated in atherosclerosis and attempts to protect against atherosclerosis. Metabolites and enzymes of kynurenine pathway can help to find patients who are at high risk of atherosclerotic plaque formation or instability. Moreover, targeted therapy for kynurenine pathways, such as those increasing anthranilic acid and 3-hydroxyanthranilic acid, have therapeutic value in atherosclerosis.
Kynurenine Pathway in Ischemic Heart Disease and Cardiac Remodeling
Hordaland Health Study uncovered that increased kynurenine/tryptophan ratio predicts a higher risk of acute coronary events such as unstable angina, non-fatal or fatal myocardial infarction, and sudden death among patients.89,90 In addition, the adjusted hazard ratio (HR) was 1.57 (1.03-2.39) for the fourth quartile, compared with the first quartile of kynurenine/tryptophan ratio. 89 Similarly, 55 months follow-up of 3224 patients after elective coronary angiography revealed that urine kynurenine/tryptophan ratio is significantly and positively correlated with major coronary events, acute myocardial infarction, and all-cause and cardiovascular mortality. 91 In addition, increased levels of kynurenic acid, 3-hydroxykynurenine, anthranilic acid, and 3-hydroxyanthranilic acid predict a higher risk of acute myocardial infarction in patients with stable angina pectoris, particularly in those with impaired glucose metabolism. 73
It was unveiled that the plasma levels of kynurenine pathway metabolites such as kynurenine, kynurenic acid, and 3-hydroxyanthranilic acid increase after cardiopulmonary resuscitation in rats and pigs.92,93 In addition, it was observed that increased plasma levels of kynurenic acid and 3-hydroxyanthranilic acid correlate with markedly lower ejection fraction and stroke volume. 92 Interestingly, 6 months follow-up of 292 patients after myocardial infarction showed that increased activity of IDO is significantly associated with left ventricular remodeling. 94 Moreover, IDO activity was superior to N-terminal Pro-BNP (NTproBNP), suppression of tumorigenicity 2 (ST2), Galectin 3, or C-reactive protein (CRP) for predicting cardiac remodeling after myocardial infarction. 94
Melhem et al 13 reported that pharmacological inhibition or genetic deletion of IDO could improve myocardial injury, and dysfunction after myocardial infarction. The study exhibited that distinct loss of function of IDO in endothelial cells but not in smooth muscle cells, inflammatory cells, or cardiomyocytes can ameliorate cardiomyocytes contractility, cardiac function and ventricular remodeling after myocardial infarction in mice. 13 Furthermore, kynurenine reversed the protective effects of IDO deletion, stimulated AHR-mediated production of reactive oxygen species (ROS), and activated apoptosis. 13
Liu et al 95 found that IDO is upregulated in cardiac hypertrophy. They uncovered that pharmacological inhibition of IDO with PF-06840003, an IDO inhibitor, partly attenuates Ang II-mediated cardiac hypertrophy in mice. Adenoviral overexpression of IDO enhanced Ang II-mediated hypertrophic cardiomyopathy in vitro. 95 Furthermore, siRNA-mediated knockdown of IDO prevented Ang II-mediated cardiomyocytes growth and decreased the expression of hypertrophy-associated genes such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and β-myosin heavy chain (β-Mhc) in rat cardiomyocytes in vitro. 95 It was illuminated that IDO activates ribosomal protein synthesis through activation of phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin complex 1 (mTORC1) in cardiomyocytes and pharmacological inhibition of PI3K by pictilisib, AKT by perifosine or mTORC1 by rapamycin abrogates the effect of IDO on Ang II-mediated cardiomyopathy. 95 Additionally, 1-MT stimulated apoptosis in human cardiac myofibroblasts that can profoundly prevent cardiac fibrosis. 96
On the contrary, there are studies claiming that kynurenine is responsible for the protective effects of remote ischemic preconditioning in myocardial infarction.97,98 IDO inhibition by 1-MT reverted the protective effects of remote ischemic preconditioning in rats. 98 In addition, administration of kynurenine (300 mg/kg body weight) before myocardial ischemia reduced infarct size in the rat model. 97
However controversial, it was shown that kynurenine pathway is enormously involved in the pathogenesis of myocardial infarction. Furthermore, kynurenine and its metabolites can be used as prognostic markers for surveillance of myocardial infarction or detect patients who are at high risk of coronary artery disease and post-intervention adverse events. 99 Although IDO and kynurenine have been well-studied in ischemic heart disease and cardiac hypertrophy, the effect of other enzymes and metabolites of kynurenine pathway in these conditions remains to be known. Future studies in this regard may help to develop new medications for the treatment of myocardial infarction by targeting specific members of kynurenine pathway.
Kynurenine Pathway and Heart Failure
Recently it was shown that heart failure is associated with increased plasma levels of kynurenine, kynurenic acid, 3-hydroxykynurenine, quinolinic acid, kynurenine/tryptophan ratio, and 3-hydroxykynurenine/xanthurenic acid ratio.100,101 Dschietzig et al 102 evaluated the predictive value of plasma kynurenine in 114 patients with congestive heart failure (CHF). The plasma levels of kynurenine and NT-proBNP increased in patients with greater New York Heart Association (NYHA) grade. Moreover, 6 months follow-up of these patients revealed that elevated plasma levels of kynurenine but not NT-proBNP were associated with increased risk of death among patients with CHF. 102 Konishi et al 103 revealed that kynurenine significantly and negatively correlates with handgrip strength, peak oxygen consumption, and 6-minute walk distance in patients with heart failure. Ten years follow-up of 202 patients with heart failure showed that baseline kynurenine (95% CI, HR 1.4 (1.1-1.9)) quinolinic acid (95% CI, HR 1.8 [1.1-2.9]), 3-hydroxykynurenine (95% CI, HR 1.8 [1.2-2.6]), 3-hydroxykynurenine/xanthurenic acid ratio (95% CI, HR 1.7 [1.3-2.2]), and kynurenine/tryptophan ratio (95% CI, HR 1.55 [1.1-2.2]) are associated with significant increase in mortality among these patients. 100 However, xanthurenic acid showed a negative correlation with heart failure-related mortality. 100
Previously, it was mentioned that the plasma level of kynurenine is elevated in patients with heart failure with either reduced or preserved ejection fraction. 103 Patients with heart failure have elevated levels of inflammatory cytokines suggesting that chronic and systemic inflammation may be a precipitating factor for heart failure progression. 104 Systemic inflammation can be a consequence of heart failure, as well. 104 Higher levels of inflammatory cytokines such as IL1β and TNF-α are common findings in heart failure. 105 Previously, it has been mentioned that inflammatory cytokines such as IL1β, TNF-α, and particularly IFN-γ can induce IDO expression and activate kynurenine pathway.32-36 Garcia et al 106 showed that IFN-γ knockout exacerbates left ventricular hypertrophy and diastolic dysfunction and increases myocardial fibrosis in the mice model of diastolic heart failure. Furthermore, recombinant IFN-γ ameliorated cardiac hypertrophy in both IFN-γ knockout mice and wild-type mice. 106 Moreover, Kimura et al 107 revealed that IFN-γ/STAT5 axis, an inducer of IDO expression, protects against pressure overload-mediated cardiac hypertrophy in mice. Similarly, kynurenine can prevent fibroblast and collagen deposition in damaged tissue and improve the healing process. 108 In contrast, IDO/kynurenine pathway aggravated post-ischemic ventricular remodeling in mice. 13 Besides, kynurenic acid, anthranilic acid, 3-hydroxykynurenine, and 3-hydroxyanthranilic acid impair myocardial mitochondrial respiration, which heavily disrupts cardiac energy and calcium homeostasis, increases ROS production, and contributes to the development of heart failure.109-111 Nevertheless, more studies are necessary to define the exact role of kynurenine pathway in the development and progression of heart failure.
Kynurenine Pathway, Heart Rate, and Arrhythmias
Bądzyńska et al 112 indicated that kynurenic acid 25 mg/kg/day for 3 weeks could selectively decrease heart rate in rats without a significant impact on mean arterial pressure. Shayesteh et al 113 revealed that 1-MT, in a dose-dependent manner, ameliorates cirrhosis-induced chronotropic dysfunction and improves heart response to epinephrine. Metabolomics analysis of 502 patients showed that quinolinic acid was positively correlated with atrial fibrillation (95% CI, OR 1.15 [1.01-1.32]). 101 Quinolinic acid impairs cellular Ca2+ homeostasis and increases intracellular Ca2+ concentration. 114 Quinolinic acid also contributes to lipid peroxidation 115 and ROS production. 116
ROS plays an important role in the pathophysiology of atrial fibrillation. 117 ROS induces arrhythmogenic effects through several mechanisms, including alteration in Na+ currents, increasing intracellular Ca2+ concentration, impairment of gap junctions, fibrosis, and extracellular matrix disruption. 118 These mechanisms contribute to early afterdepolarization, ectopic, and re-entry arrhythmias. 118
On the other hand, inflammation upregulates IDO and increases kynurenine production. Inflammation and cardiac arrhythmia have a highly complex association, and inflammation contributes to arrhythmia. 119 Moreover, inflammation participates in atrial remodeling through several pathways. 120 Since the kynurenine pathway is usually activated in inflammation, uncovering the association between kynurenine metabolites and arrhythmias can be helpful for better management of arrhythmias.
Kynurenine Pathway and Stroke
Stroke is defined as the sudden onset of neurological symptoms, including motor, sensory, and cognitive symptoms due to ischemia or hemorrhage in the central nervous system (CNS). 121 Ischemic stroke constitutes the major proportion of stroke. 121 Stroke is a leading cause of death and disability worldwide. 122 Several mechanisms are involved in the pathophysiology of neuronal damage after stroke, including mitochondrial dysfunction, neuroinflammation, glutamate-induced excitotoxicity, and oxidative stress.123,124
Studies on mice models indicated that ischemic stroke is associated with increased expression of IDO in the neuronal tissue of hippocampus and cerebral arterioles.125,126 IDO activity positively correlated with high sensitivity C-reactive protein (hs-CRP), national institute of health stroke scale (NIHSS), and infarct volume in patients with stroke.127,128 Furthermore, patients with poor outcomes possessed higher kynurenine/tryptophan ratios. 128 Brain resident phagocytes, including monocytes, macrophages, dendritic cells, and microglia express IDO. As previously mentioned, IDO is upregulated in inflammation. 129 In addition, it was shown that IDO expression increases in neuronal progenitor cells after 3 and 8 hours of oxygen and glucose deprivation. 130 IDO overexpression predicted neuronal progenitor cells death and IDO inhibition improved neuronal progenitor cells survival during oxygen and glucose deprivation in vitro. 130 However, Jackman et al 126 revealed that either IDO inhibition or deletion could not change the neurological function, total brain infarct size, and edema following ischemic stroke in mice.
It has been reported that post-ischemic activation of AHR increases astrogliosis and downregulates neurogenesis in adult mice, whereas AHR knockout decreases inflammatory markers such as IL1β, IL6, IFN-γ, CXCL1 and upregulates neurogenesis factors such as neurogenin 1 and neurogenin 2 after stroke. 131 Consistently, AHR antagonist significantly alleviated neurogenic inflammation, decreased oxidative stress and inflammatory cytokines release, and reduced infarct size after ischemic stroke in mice and rats.132,133 Ischemia increased total and nuclear AHR levels in neurons both in vivo and in vitro. Pharmacological inhibition or genetic loss of AHR reduced infarct size and decreased neurological deficit 48 hours after ischemia. 134 AHR inhibits cAMP response element (CRE)-binding protein (CREB) mediated neuronal brain-derived neurotrophic factor (BDNF) gene expression.134,135 CREB signaling prevents apoptosis and prolongs neuronal cells survival. 136 Hence, AHR stimulation accelerates the apoptosis of neurons.134 Kynurenine, as an AHR agonist, is increased after stroke and may induce neurologic damage via AHR activation. 134
Conversely, intraperitoneal administration of kynurenine sulfate (30, 100, and 300 mg/kg) before middle cerebral artery occlusion dose-dependently decreased infarct size and hippocampal CA1 pyramidal cells loss in mice, as well as preventing post-stroke behavioral changes and hypermobility in gerbils. 137 Even post-ischemic chronic administration of kynurenine sulfate (300 mg/kg/day) ameliorated neuronal damage in rats. 138 However, Gellért et al 139 reported that post-ischemic single-dose administration of kynurenine sulfate (300 mg/kg/day) aggravated the outcome of focal cerebral ischemia/reperfusion mediated by middle cerebral artery occlusion. Intraperitoneal injection of KMO inhibitors such as (m-nitrobenzoyl)-alanine (400 mg/kg) or Ro 61-8048 (40 mg/kg) 3 times at 1, 30, and 180 minutes after bilateral carotid artery occlusion significantly decreased infarct size in rats and markedly confined pyramidal neurons lesion in the hippocampal CA1 region of gerbils. 140 In addition, intraperitoneal administration of 1 mmol/kg of kynurenic acid analog 1 hour before and 2, 5, 23, and 29 hours after global forebrain ischemia significantly prevented pyramidal neurons damage in the hippocampus CA1 zone and improved synaptic response and electrophysiological function of neurons. 141 Interestingly, it was found that patients with post-ischemic cognitive decline have a higher quinolinic acid/kynurenic acid ratio. 142 Quinolinic acid and kynurenic acid are the agonist and antagonist of N-Methyl-D-aspartate (NMDA) receptor, respectively. 143 Excessive NMDA receptor stimulation by glutamate leads to intracellular Ca2+ overload, ROS production, mitochondrial damage, and mediates excitotoxic neuronal death in ischemic stroke. 144
KMO leads to the conversion of kynurenine into quinolinic acid, a neurotoxic metabolite of the kynurenine pathway. 145 KMO inhibition not only prevents the production of quinolinic acid but also increases the substrate for KAT and kynurenic acid production. 146 KMO inhibitors prevent neuronal loss and behavioral changes in several neurodegenerative diseases and psychiatric conditions such as Alzheimer’s disease, Huntington’s disease, and depression.146,147 KMO inhibitors, particularly JM6 and Ro 61-8048, do not cross the blood-brain barrier. Accordingly, it seems that peripheral inhibition of KMO is responsible for the protective effects of KMO inhibitors. 146
All in all, previous findings from preclinical studies show that kynurenic acid supplementation, KMO inhibition, AHR inhibition or simultaneous kynurenine supplementation and AHR inhibition may improve the outcome of stroke. In addition, the opposite effects of kynurenine and kynurenic acid supplementation and AHR inhibition on stroke can indicate that kynurenine has major biological functions that are not mediated through AHR.
Kynurenine Pathway and Aneurysm
Ang II is a major instigator of the aneurysm or at least accelerates its progression. 148 Ang II activates several inflammatory signaling pathways such as nuclear factor kappa B (NF-κB) signaling pathway, upregulates matrix metalloproteinases, and damages the vessel’s wall. 148 Ang II is widely used to induce abdominal aorta aneurysm in animal studies.148,149 Ang II, in an IFN-γ-dependent manner, stimulates the expression of IDO in the vessel’s wall. 150 Ang II infusion notably increased the risk of abdominal aorta aneurysm in Apoe−/− mice, but not in Apoe−/− mice with IDO deletion. 150 IDO overactivity has been reported to be involved in the apoptosis of VSMCs.49,151 Consistently, IDO knockout improved the survival of VSMCs and protected against Ang II-mediated abdominal aorta aneurysm in LDLR−/− mice. 151 It was observed that 3-hydroxyanthranilic acid, IDO, and kynureninase are vigorously expressed in the neighboring non-aneurysmal segments of human abdominal aorta aneurysm samples. 150 Similarly, kynureninase and KMO were upregulated in macrophages of aortic atherosclerotic aneurysm in patients who underwent aortic surgery. 152 Furthermore, 3-hydroxyanthranilic acid, in an NF-kB-dependent manner, increases the expression of matrix metalloproteinase 2 in VSMCs and accelerates the formation and exacerbation of Ang II-mediated abdominal aorta aneurysm in both Apoe−/− and Apoe−/−/IDO−/− mice. 150 Moreover, kynureninase knockdown strongly decreased 3-hydroxyanthranilic acid and matrix metalloproteinase 2 and partly prevented Ang II-mediated abdominal aorta aneurysm in mice. 150 Abdominal aorta aneurysm remains without an effective medication and large size and symptomatic aneurysms are repaired by open or endovascular surgery. 153 Maybe pharmacological intervention targeting kynurenine pathway, particularly targeting kynureninase, KMO, and 3-hydroxyanthranilic acid, contribute to the management of abdominal aorta aneurysm.
Kynurenine Pathway and Myocarditis
Acute viral myocarditis has been associated with decreased serum levels of tryptophan, elevated serum levels of kynurenine and increased IDO activity in the heart and spleen of mice. 154 Furthermore, both IDO−/− and 1-MT-treated mice had significantly better survival, higher IFN-γ and lower viral load than IDO+/+ mice in acute viral myocarditis. 154 Moreover, treatment with kynurenine attenuated the beneficial effects of IDO deletion on mice survival. 154 Previously, it was found that IDO deletion upregulates type I interferon, subsequently hinders viral replication. 155 The expression of IDO also increased in the mice model of chronic viral myocarditis. 156 IDO overexpression in macrophages co-cultured with cardiomyocytes revealed that IDO overactivity increases the release of IL1β, IL6, and TNF-α and ROS. In addition, IDO overactivity was associated with enhanced lactate dehydrogenase (LDH) activity and treatment of macrophages with 1-MT ameliorated these alterations. 156 Kubo et al 157 revealed that KMO−/− mice have better survival and lower inflammatory cells infiltration in the mice model of viral myocarditis, compared with KMO+/+ mice. KMO−/− mice also had markedly higher serum levels of kynurenine, anthranilic acid, and kynurenic acid, and significantly lower levels of chemokines such as chemokine ligand (CCL) 1, CCL2, CCL3, CCL4. 157 Based on these findings, IDO inhibition, KMO inhibition, or a combination of KMO inhibition with supplementation of specific metabolites of kynurenine pathway may help to improve the outcome of myocarditis.
Kynurenine Pathway and Heart Transplantation
During a 1-year follow-up of 98 patients with heart transplantation it was found that compared with those without acute rejection, patients with acute rejection had significantly higher serum IDO activity 1 month after transplantation (3.32 ± 1.56 vs 2.62 ± 1.35). Additionally, it was reported that serum IDO activity was significantly (95% CI, OR 1.4 [1.033-1.876]) associated with cardiac allograft rejection among patients, independent of gender, age or renal function. 158
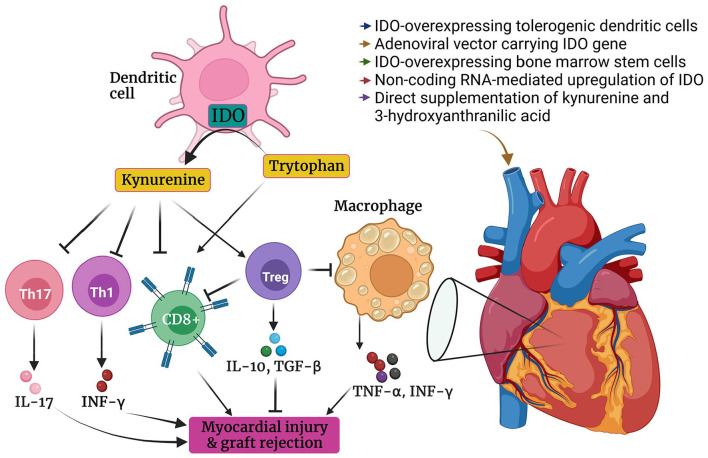
As mentioned previously, activation of kynurenine pathway increases T Reg cells differentiation and induces immune tolerance.10,159,160 Based on this theory, Yang et al 159 investigated the effect of kynurenine pathway on heart transplantation. It was observed that increased expression of IDO and activation of kynurenine pathway is associated with a higher abundance of T Reg cells and predicts decreased risk of graft rejection in rats. Consistently, a higher rejection rate was reported among rats receiving 1-MT. It was shown that adenoviral vector-mediated IDO overexpression by dendritic cells significantly postpones heart allograft rejection in mice and prolongs the survival of animals. 161 Likewise, treatment of heart transplanted rats with IDO-overexpressing bone marrow stem cells markedly improved their ejection fraction and prolonged their survival. 162 In addition, IDO overexpression was associated with a significant increase in IL10, transforming growth factor (TGF)−β1, TGF-β2 and TGF-β3 and T Reg cells abundance. 162 IDO overexpression also decreased IL2, IFN-γ, major histocompatibility complex (MHC) II, CD8+ and CD86+ cell. 162 Intracoronary injection of adenoviral-mediated IDO overexpressing dendritic cells enhanced heart allograft survival in rats and decreased IFN-γ, IL-1, and TNF-α levels 163 (Figure 4). Likewise, several studies indicated that IDO overexpression in the immune cells, induced by gene transfection techniques, protects against heart allograft rejection, reduces CD8+ cells response, prevents inflammatory cytokines release, potentiates T Reg response, and enhances the immunomodulatory effect of other drugs such as cyclosporine to prevent heart allograft rejection in mice and rats164-169 (Table 1). Interestingly, tolerogenic dendritic cells transfer from one heart transplant recipient to another significantly improved graft survival mediated through upregulation of IDO expression, increased T Reg cell differentiation, and suppressed anti-donor T cells response. 170 Interestingly, pretreatment with a single dose of 3-hydroxyanthranilic acid and allogeneic dendritic cells 7 days before transplantation markedly augmented the survival of cardiac allograft in rats and induced tolerance in recipients T cells. 171 Li et al 172 uncovered that intravenous administration of either tryptophan catabolic metabolites or dendritic cells transfected with adenovirus vector carrying IDO gene could downregulate anti-donor T cells function and prolong cardiac allograft survival in mice. Also, their combination was notably superior, compared with each one of them separately. Accordingly, the authors concluded that both tryptophan depletion and accumulation of kynurenine and its metabolites are crucial for cardiac allograft survival 172 (Figure 4). Consistently, it was shown that both tryptophan starvation and tryptophan catabolites lead to general control nonderepressible 2 (GCN2) kinase-dependent downregulation of T cell receptor (TCR) ζ-chain in CD8+ T cells of mice and suppresses T cell-mediated cytotoxic activity. 173 Alloreactive CD8+ T cells are enormously involved in graft rejection and suppression of CD8+ T cells response can markedly prolong heart transplant survival. 174 IDO overexpression ameliorates T helper 17 response, decreases IL17 levels, shifts T cells response toward T Reg response in heart transplantation. 169 IL17 severely instigates inflammatory response in heart transplants and accelerates graft rejection 175 (Figure 4).
Figure 4.
The protective effect of IDO/kynurenine on heart transplantation. Enhancing IDO expression by different methods can induce tolerance in the immune system. Kynurenine accelerates T Reg cells differentiation and suppresses the function of CD8+ cell, T helper 1 and T helper 17 cells in heart transplantation. In addition, IDO overexpression leads to tryptophan depletion. Sufficient amount of tryptophan is necessary for competent function of effector T cells. Subsequently, IDO overexpression increases anti-inflammatory cytokines such as IL10 and TGF-β and decreases pro-inflammatory cytokines such as IL17, TNF-α, and IFN-γ. These alterations prolongs graft survival.
Table 1.
Implication of tryptophan/kynurenine pathway in the survival of heart transplantation.
| Author | Human/animal | Method | Findings |
|---|---|---|---|
| Suarez-Fuentetaja et al 158 | Human | An observational study measuring serum IDO activity in patients with heart transplantation | Patients with acute rejection had higher IDO serum activity after 1 month of transplantation. Also, IDO serum activity independently (95% CI, OR 1.4 [1.033-1.876]) predicted graft rejection among patients with heart transplantation |
| Li et al 164 | Mouse | Treating animals with dendritic cells transfected with adenovirus vector carrying IDO gene | IDO overexpression improved survival, postponed graft rejection, reduced IFN-γ production, and accelerated CD4+ cell death |
| He et al 162 | Rat | Treating animals with bone marrow stem cells transfected with lentiviral vector carrying IDO gene | IDO overexpression prolonged survival, enhanced ejection fraction, decreased CD86+, CD80+ cells, MHC II, IL2 and IFN-γ and increased T Reg cells abundance and the expression of CD274, IL10, TGF-β1, TGF-β2, TGF-β3 in the heart allograft |
| Yang et al 159 | Rat | Treating rats with immutol, cyclosporine and 1-MT | 1-MT significantly blocked kynurenine pathway and decreased graft survival. Activation of kynurenine pathway has been associated with increased abundance of T Reg cells, higher concentrations of IL10, and TGF-β, as well as decreased abundance of CD8+ cells |
| Dai et al 171 | Rat | A single-dose intravenous administration of 3-hydroxyanthranilic acid + allogeneic dendritic cells 7 days before heart transplantation | Pretreatment with the combination of 3-hydroxyanthranilic acid + allogeneic dendritic cells enhanced cardiac allograft survival and suppressed T cells activity |
| Lv et al 161 | Mouse | Treating mice with dendritic cells transfected with adenovirus vector carrying IDO gene | IDO overexpression enhanced both cardiac graft survival and function. Higher IL10/IL6 ratio and lower levels of IFN-γ and IL-2 were found in treated group |
| Li et al 170 | Mouse | Transferring tolerogenic dendritic cells from a tolerant recipient to secondary recipient in mice heart transplantation model | Tolerogenic dendritic cells transfer from a tolerant recipient to another recipient significantly enhanced IDO expression, induced T Reg differentiation, suppressed anti-donor T cells activity and increased graft survival in the secondary recipient |
| Li et al 172 | Mouse | Treating mice with intravenous injection of tryptophan catabolic metabolites, dendritic cells transfected with adenovirus vector carrying IDO gene and their combination | Treating mice with tryptophan catabolic metabolites or adenoviral transfected dendritic cells significantly increased cardiac graft survival, decreased IL2, TNF-α and IFN-γ and increased IL10. Significantly greater improvement was observed in combination therapy |
| Yu et al 169 | Mouse | Cardiac allograft injection with adenovirus vector carrying IDO gene. | Higher IDO expression was detected in treated mice. Increased IDO expression was associated with decreased expression of IL2, IL17, and IFN-γ. IDO enhanced T Reg cells differentiation and prolonged graft survival |
| He et al 166 | Rat | Intravenous injection of DO1-BMSC-exosomes 48 h after heart transplantation | Treated rats showed better cardiac function. Treatment with DO1-BMSC-exosomes augmented T Reg response and suppressed CD8+ response |
| Li et al 176 | Mouse | Measuring microRNAs in mice cardiac transplantation and assessing their effect on cardiac allograft rejection and IDO expression | miR-669b-3p decreased IDO expression, thereby expediting graft rejection |
| Sucher et al 167 | Mouse | Treating heart transplanted mice with cytotoxic T lymphocyte-associated antigen-4 Ig (CTLA4Ig) | CTLA4Ig promoted IDO expression, thereby increasing graft survival. IDO gene deletion accelerated graft rejection. Furthermore, IDO inhibition since transplantation or 50 days after transplantation attenuated the beneficial effects of CTLA4Ig on graft survival. In addition, T Reg cells depletion impairs CTLA4Ig-mediated graft survival |
| Zhang et al 165 | Mouse | Intravenous injection of dendritic cells transfected with adenovirus vector carrying GDF15 gene 7 days before transplantation | GDF15 overexpression upregulated IDO in dendritic cells, thereby inducing tolerance in T cells and delaying graft rejection |
Even, it was demonstrated that IDO and kynurenine pathway are the target of other molecules such as growth/differentiation factor-15 (GDF15) and non-coding RNAs for regulation of cardiac graft survival. They negatively or positively modulate IDO expression, thereby regulating the immune system and graft survival.165,176 Therefore, kynurenine pathway has both prognostic and therapeutic value in heart transplantation. Besides, pharmacological augmentation of kynurenine pathway can ameliorate immune response and prolong the survival of heart transplants.
Kynurenine Pathway and Vascular Transplantation
Similar to heart transplantation, several studies have shown the beneficial effects of kynurenine and its metabolites on vascular transplantation. Herein, Juli et al 177 revealed that post-transplantation mesenchymal stem cells (MSCs) injection into the soft tissue surrounding femoral artery transplant significantly prevented perivascular lymphocyte infiltration, intimal hyperplasia, and graft occlusion in a porcine model of femoral artery transplantation. It was found that MSCs increased IFN-γ, IDO, IL10, and T Reg cells abundance in the vascular graft. 177 IDO expression by VSMCs induces an immune-privileged condition in the medial layer of the artery, which prevented allogeneic T cells infiltration and prolonged graft survival in a mice model. 178 Perivascular infiltration of leukocytes particularly neutrophils and T lymphocytes leads to vascular rejection. 179 The beneficial effect kynurenine on the prevention of perivascular infiltration and vascular graft rejection can generally benefit several organs transplantation. 180
Kynurenine Links Cardiovascular Diseases With Other Diseases
In previous sections, it has been discussed how kynurenine pathway is involved in different cardiovascular diseases. In addition to cardiovascular diseases, kynurenine pathway is involved in a wide variety of other medical conditions which can directly or indirectly affect cardiovascular health. In this section, we briefly discuss the role of kynurenine and its metabolites in some medical conditions and explain their possible interactions with cardiovascular diseases.
Kynurenine Pathway in Aging and Frailty
Aging is related to the impaired function of cells and organs, leading to several diseases such as cardiovascular and metabolic diseases, neurodegenerative diseases, and cancer. 181 Chronic low-grade inflammation or “inflamm-aging” accelerates cardiac and vascular aging, increases the risk of atherosclerosis and atherothrombosis.182,183 Frailty and cardiovascular diseases have mutual interaction, that is, cardiovascular diseases are prevalent among older people with frailty and patients with cardiovascular disease are at higher risk of frailty. 183 Likewise, the removal of senescent cells from tissues was shown to improve several chronic diseases. 181
Frail subjects possess higher serum concentrations of kynurenine, compared to those with robustness and prefrailty. 184 Overactivation and dysregulation of kynurenine pathway are vigorously associated with aging, frailty, weaker grip strength, slower walking speed, and increased inflammatory markers such as TNF-αR1 and IL6. 185 Studies showed that the inhibition of kynurenine pathway prolongs lifespan in Drosophila and worms.186-188 Aging is associated with elevated kynurenine and kynurenine/tryptophan ratio, which correlates with an increased mortality rate among the elderly population.189,190 Increased kynurenine and kynurenine/tryptophan ratio are associated with cardiovascular diseases mortality and all-cause mortality in the Hordaland Health Study. 90 Kynurenine impairs autophagy and induces senescent phenotype in MSCs, which are involved in the regulation of adipogenesis, bone and bone marrow homeostasis. 191 Higher kynurenine levels in older age may be a sign of organs dysfunction such as cardiovascular dysfunction. 90 Also, the increased production of kynurenine and its metabolites can change the clinical course of several cardiovascular diseases, as previously mentioned.
Kynurenine links depression to cardiovascular diseases
Depression and cardiovascular diseases have a bidirectional relation and the underlying mechanism is complex and multifactorial. 192 Patients with depression are at higher risk of cardiovascular diseases and patients with hypertension, coronary artery diseases and other cardiovascular diseases are more susceptible to depression. 192 In addition, it has been demonstrated that depression increases cardiovascular mortality among patients.193,194 Depression is one of the significant risk factors for coronary artery disease-related mortality. 195 Tryptophan concentrations have been negatively associated with the presence of depression among patients with heart failure. 196 Interestingly, it has been shown that mice with heart failure, particularly those having high kynurenine serum levels and kynurenine/tryptophan ratios, are more susceptible to developing depression. 197 Furthermore, increased expression of KMO and decreased expression of KAT in the heart were strongly associated with depressive behavior in mice with heart failure. 197
Tryptophan depletion and accumulation of toxic metabolites of the kynurenine pathway or shifting from serotonin to kynurenine are involved in the pathophysiology of inflammation-induced depression.198,199 Serotonin regulates the function of the CNS and cardiovascular system. Depletion of tryptophan, as a precursor for serotonin, interferes with the normal function of the CNS and cardiovascular system.200,201 Additionally, toxic metabolites of kynurenine pathway affect the CNS in several ways, including production of ROS, enhancing lipid peroxidation, increasing excitotoxicity, and altering glutamatergic activity.202,203 Intraperitoneal injection of kynurenine in mice resulted in depressive-like behavior. 204 Endotoxin-induced depressive-like symptoms were attenuated in the presence of IDO inhibition, IDO deletion, or IFN-γ receptor deletion.205,206 Depressed patients with co-morbid cardiovascular diseases exhibited greater serum kynurenine/tryptophan ratio, compared to patients with cardiovascular diseases but without depression, which indicates the role of depression in shifting tryptophan metabolism to kynurenine pathway. 207 A meta-analysis by Hunt et al 208 showed that increased risk of depression after treatment with IFN-α in patients with chronic medical conditions could be mediated by the kynurenine pathway activity. Raison et al 209 revealed that the administration of IFN-α elevated the cerebrospinal fluid (CSF) levels of kynurenine and quinolinic acid, which related to the severity of depressive symptoms. Selective serotonin reuptake inhibitors (SSRIs) relieve cytokine-induced depression. 210 Interestingly, Halaris et al 211 revealed that the levels of 3-hydroxykynurenine and quinolinic acid were decreased following treatment with escitalopram, which shows the neuroprotective effect of SSRIs can partly be due to their effect on kynurenine and its metabolites. Kocki et al 212 also uncovered that different antidepressants such as fluoxetine, citalopram, amitriptyline, and imipramine increase the expression of KAT and decrease the expression of KMO in the primary astroglial culture. In addition, these antidepressants increased kynurenic acid/3-hydroxykynurenine ratio. 212
Deregulation of kynurenine pathway seems to be a common finding in depression and cardiovascular diseases which predicts their co-existence. Kynurenine metabolites converge the effect of inflammation and immune system on the CNS and cardiovascular system and can be used as therapeutic and diagnostic targets for both depression and cardiovascular diseases.
Kynurenine links CKD to cardiovascular diseases
The kidney and cardiovascular system have mutual interactions. CKD is a risk factor for heart failure, coronary artery disease, and arrhythmias and increases cardiovascular mortality. 213 In return, cardiovascular dysfunctions such as low-cardiac output, high blood pressure, and endothelial dysfunction impair the kidney’s function and lead to CKD in the long-term. 213
Previously, it has been mentioned how kynurenine pathway is involved in the pathophysiology of hypertension, as a major driver for both cardiovascular and kidney diseases.214,215 Kynurenine levels were negatively correlated with glomerular filtration rate (GFR) among patients with congestive heart failure. 102 The inverse correlation was also demonstrated in both subgroups of heart failure with reduced and preserved ejection fraction. 103 Higher levels of kynurenine pathway metabolites such as kynurenine itself, 3-hydroxykynurenine, kynurenic acid, anthranilic acid, and quinolinic acid in patients with CKD have been associated with increased intima-media thickness and higher expression of inflammatory and coagulatory molecules such as thrombomodulin, von Willbrand factor (vWF), intercellular adhesion molecule 1 (ICAM1), and VCAM1.216,217 Furthermore, increased levels of anthranilic acid and quinolinic acid have been associated with increased plasma levels of prothrombin fragments 1 + 2 and hypercoagulable state among patients with end-stage renal disease (ESRD). 218 Furthermore, metabolomics assessment of 146 patients with ESRD uncovered that 3-hydroxykynurenine is independently associated with the presence of cardiovascular diseases among these patients. 219 Similarly, a higher kynurenine/tryptophan ratio was found among CKD patients with a history of cardiovascular diseases, compared to those without cardiovascular diseases. 21
Higher levels of kynurenic acid, 3-hydroxykynurenine, and kynurenine were found in advanced stages of CKD, compared to primary stages of CKD. 220 Low plasma tryptophan levels were associated with an increased risk of cardiovascular diseases among CKD patients. 220 Likewise, 3-hydroxykynurenine and kynurenine were negatively correlated with estimated glomerular filtration rate (eGFR) among CKD patients. 221 By using the laboratory data of 154 patients with CKD and 42 patients without CKD, Pan et al 222 showed that the logarithm of kynurenine/tryptophan ratio is independently associated with CKD and albuminuria. Also, it was found that IDO activity higher than 0.0466 predicts CKD with a sensitivity of 83.8% and a specificity of 75%. Similarly, the analysis of metabolomics biomarkers of 1741 patients showed that kynurenine (95% CI, OR 1.98 [1.05-3.73]) and kynurenine/tryptophan ratio (95% CI OR 3.20 [1.57-6.51]) significantly predicts the incidence of CKD in the next 8 years after assessment. 223 IDO expression increases during TGF-β-mediated renal fibrosis and partly prevents fibrogenesis. 224 Furthermore, IDO overexpression was shown to be protective against renal inflammation and renal injury.224,225 However, recent studies showed that inhibition of kynurenine pathway ameliorates renal fibrosis.226-228 Pan et al 226 indicated that either IDO deletion or inhibition attenuates glycogen synthase kinase 3 beta (GSK3β) and Wnt/β-catenin pathway, thereby decreasing the expression of pro-fibrotic factors such as smooth muscle alpha-actin (α-SMA), fibronectin, and vimentin and mitigating renal fibrosis in the mice model of acute kidney injury.
Based on previous findings, kynurenine pathway possesses prognostic value in both CKD and cardiovascular diseases. Kynurenine metabolites have therapeutic value in CKD and cardiovascular diseases, which demands future studies. Furthermore, overactivation of kynurenine pathway in CKD or cardiovascular disease may alter the incidence of the other one. Herein, targeted therapy for kynurenine pathway may be a good option to improve the function of both the cardiovascular and urinary systems.
Kynurenine links cirrhosis to cardiovascular diseases
Cirrhosis leads to cardiac dysfunction known as cirrhotic cardiomyopathy. 229 Impaired β-adrenergic function, systemic inflammation, increased production of NO, endocannabinoids, and other endogenous mediators have been implicated in the pathogenesis of cirrhotic cardiomyopathy. 230 In return, cardiac dysfunction due to heart failure, constrictive pericarditis, valvulopathy, myocarditis, and myocardial infarction can lead to liver damage known as cardiac hepatopathy. 231
Patients with acutely decompensated cirrhosis and particularly patients with acute-on-chronic liver failure have higher levels of kynurenine metabolites, compared with healthy individuals and patients with compensated cirrhosis. 22 In addition, higher levels of kynurenine metabolites are associated with nosocomial infection and mortality among patients with acute decompensated cirrhosis. 22 Liver inflammation and fibrosis are associated with an increased plasma kynurenine/tryptophan ratio.232,233 An in-vitro study by Oh et al 234 revealed that inhibition of IDO by 1-MT enhances the apoptosis of hepatic stellate cells, which are the main drivers of liver fibrosis. Furthermore, carbon tetrachloride (CCl4)-induced liver fibrosis was significantly attenuated in IDO−/− mice, compared to wild-type mice. 235 Consistently, treatment with 1-MT alleviates liver inflammation and fibrosis in the rat model of biliary cirrhosis. 113 Treatment with 1-MT also improved cardiac chronotropic response to epinephrine in the rat model of cirrhotic cardiomyopathy. 113
Regarding the involvement of kynurenine metabolites in cardiac remodeling and hepatic fibrosis and the mutual interaction between cirrhosis and cardiac dysfunction, modulation of kynurenine pathway may be a suitable therapeutic approach to preserve the function of the heart and liver.
Kynurenine links cancer to cardiovascular diseases
A higher incidence of cardiovascular events is recorded among cancer patients. For instance, the incidence of cardiovascular events between 108 215 cancer survivors and 523 541 healthy individuals revealed that venous thromboembolism has a significantly higher risk among 18 of 20 site-specific cancers (from HR 1.72 [1.57-1.89] in prostate cancer to HR 9.72 [5.50-17.18] in pancreatic cancer) for more than 5 years after cancer diagnosis. 236 Additionally, higher risks of heart failure, cardiomyopathy, coronary artery diseases, arrhythmia, stroke, pericarditis, and valvular heart diseases were found among cancer survivors. 236 Even, risks of venous thromboembolism, heart failure, and cardiomyopathy were higher among younger patients or those without previous history of cardiovascular diseases. 236 Furthermore, a higher prevalence of cardiovascular risk factors such as hypertension and diabetes were found among cancer survivors. 237 Cardiovascular death constitutes a major proportion of mortality among cancer patients. Of 1 228 328 patients with 28 cancer types, 38% died because of cancer itself, and 11% died of cardiovascular diseases, in particular heart diseases. 238 Herein, cardiovascular adverse effects of anti-cancer drugs remain a great concern in cancer chemotherapy and early detection and management of cardiovascular complications of chemotherapy can markedly improve patients’ lifespan.239,240
Kynurenine serves as a defensive mechanism for cancer cells and contributes to cancer cells’ immune-evasion. 10 Kynurenine/AHR stimulates T Reg cells differentiation and suppresses the cytotoxic function of effector T cells in different types of cancer. 10 Kynurenine pathway is significantly activated in different types of cancer and correlates with the poor prognosis of the disease. 10 Besides, attenuation of kynurenine/AHR signaling may be helpful in cancer chemotherapy. 241 In previous sections, we have comprehensively discussed how impaired kynurenine pathway can alleviate or exacerbate several cardiovascular diseases. As discussed previously, overactivation of kynurenine pathway can aggravate myocardial remodeling, heart failure, and myocardial infarction as the major causes of cardiovascular mortality among cancer survivors. Therefore, kynurenine pathway may be a proper target to improve cancer chemotherapy and relieve the following cardiovascular diseases.
Kynurenine links diabetes to cardiovascular diseases
Cardiovascular diseases such as hypertension, heart failure, coronary artery disease, and peripheral arterial disease are common among patients with diabetes. 242 Diabetes and cardiovascular diseases are affected by similar etiologic factors such as endothelial dysfunction, vascular inflammation, atherosclerosis, RAAS overactivity, and oxidative stress. 242 Interestingly, a 7.6-year follow-up of 2519 patients with coronary artery disease revealed that urine kynurenine/tryptophan ratio is a strong predictor of type 2 diabetes in these patients. Comparing quartile 4 with quartile 1 of kynurenine/tryptophan ratio, showed that kynurenine/tryptophan ratio was independently (95% CI, HR 2.35 [1.39, 3.96]) associated with the incidence of diabetes in the follow-up period. 243 Previously, it was found that overactivity of kynurenine pathway is correlated with insulin resistance. 244 In particular, increased plasma concentrations of xanthurenic acid are associated with the presence of insulin resistance and diabetes (OR 1.15 [1.03-1.27]) among elderly subjects. 245 Furthermore, it was shown that alleviation of insulin resistance by metformin is associated with a decrease in kynurenine/tryptophan ratio. 246 Feeding mice with kynurenine enhanced their weight gain and led to liver steatosis and hyperglycemia. 247 Interestingly, inhibition of kynurenine pathway attenuated olanzapine-mediated weight gain and insulin resistance in mice. 18 Insulin resistance not only leads to diabetes but also contributes to the development of cardiovascular diseases.248,249 Therefore, modulation of kynurenine pathway may improve insulin resistance as a common etiological factor for diabetes and cardiovascular diseases. Furthermore, increased kynurenine metabolites in diabetes or cardiovascular diseases may help to develop the other one.
Conclusion and Future Research Direction
Dysregulation of kynurenine pathway is observed in different cardiovascular diseases and other medical conditions which have mutual interactions with cardiovascular health. Kynurenine metabolites possess diagnostic and prognostic value in cardiovascular diseases. In addition, preclinical studies have shown that augmentation or inhibition of certain branches of kynurenine pathway can alleviate the development or progression of cardiovascular diseases. However, it has been illuminated which metabolites of kynurenine are responsible for the observed effects in some cardiovascular diseases, it remains to be known in most cases. As mentioned, IDO inhibitors such as indoximod, epacadostat, and navoximod are currently used in cancer clinical trials. 10 The same drugs can be used in cardiovascular clinical trials to treat myocardial infarction, heart failure and myocarditis. Furthermore, supplementation of kynurenine and its metabolites or new methods of gene and RNA delivery may be helpful for more efficient management of hypertension, atherosclerosis, heart transplantation and stroke. The kynurenine pathway includes several metabolites and now we just know the cardiovascular effects of some of the upstream metabolites and enzymes of this pathway. Additional studies are needed to provide a better knowledge of cardiovascular effects of downstream metabolites and enzymes of the kynurenine pathway. For instance, it was already shown that KMO inhibition is protective against stroke and myocarditis, 3-hydroxyanthranilic acid prevents atherosclerosis and xanthurenic acid decreases blood pressure. Future studies may show similar effects for other enzymes and metabolites of the kynurenine pathway. Furthermore, future clinical trials are needed to consolidate the results of preclinical studies.
Acknowledgments
We would thank www.biorender.com for helping us in designing parts of the figures.
Footnotes
Author’ Contributions: Moein Ala conceptualized this article. Moein Ala and Seyed Parsa Eftekhar performed the literature search and wrote the draft. Moein Ala edited the article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Moein Ala  https://orcid.org/0000-0001-5951-4864
https://orcid.org/0000-0001-5951-4864
Seyed Parsa Eftekhar  https://orcid.org/0000-0002-1175-4380
https://orcid.org/0000-0002-1175-4380
References
- 1. India State-Level Disease Burden Initiative CVD Collaborators. The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health. 2018;6:e1339-e1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali S, Misganaw A, Worku A, et al. The burden of cardiovascular diseases in Ethiopia from 1990 to 2017: evidence from the Global Burden of Disease Study. Int Health. 2021;13:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bakirhan NK, Ozcelikay G, Ozkan SA. Recent progress on the sensitive detection of cardiovascular disease markers by electrochemical-based biosensors. J Pharm Biomed Anal. 2018;159:406-424. [DOI] [PubMed] [Google Scholar]
- 4. Thomas MR, Lip GY. Novel risk markers and risk assessments for cardiovascular disease. Circ Res. 2017;120:133-149. [DOI] [PubMed] [Google Scholar]
- 5. Amini M, Zayeri F, Salehi M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: results from global burden of disease study 2017. BMC Public Health. 2021;21:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry. 2020;25:131-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dadvar S, Ferreira DMS, Cervenka I, Ruas JL. The weight of nutrients: kynurenine metabolites in obesity and exercise. J Intern Med. 2018;284:519-533. [DOI] [PubMed] [Google Scholar]
- 8. Huang JY, Butler LM, Midttun, et al. A prospective evaluation of serum kynurenine metabolites and risk of pancreatic cancer. PLoS One. 2018;13:e0196465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ala M. The footprint of kynurenine pathway in every cancer: a new target for chemotherapy. Eur J Pharmacol. 2021;896:173921. [DOI] [PubMed] [Google Scholar]
- 11. Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197-203. [DOI] [PubMed] [Google Scholar]
- 12. Song P, Ramprasath T, Wang H, Zou MH. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol Life Sci. 2017;74:2899-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Melhem NJ, Chajadine M, Gomez I, et al. Endothelial cell indoleamine 2, 3-dioxygenase 1 alters cardiac function after myocardial infarction through kynurenine. Circulation. 2021;143:566-580. [DOI] [PubMed] [Google Scholar]
- 14. Berg M, Polyzos KA, Agardh H, et al. 3-Hydroxyanthralinic acid metabolism controls the hepatic SREBP/lipoprotein axis, inhibits inflammasome activation in macrophages, and decreases atherosclerosis in Ldlr−/− mice. Cardiovascular research. 2020;116:1948-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cason CA, Dolan KT, Sharma G, et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg. 2018;68:1552-1562.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Liu H, McKenzie G, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Favennec M, Hennart B, Caiazzo R, et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity. 2015;23:2066-2074. [DOI] [PubMed] [Google Scholar]
- 18. Oxenkrug G, Summergrad P. Benserazide, an inhibitor of peripheral kynurenine metabolism, attenuates olanzapine-induced weight gain, insulin resistance, and dyslipidemia in C57Bl/6j mice. Mol Neurobiol. 2020;57:135-138. [DOI] [PubMed] [Google Scholar]
- 19. de Bie J, Guest J, Guillemin GJ, Grant R. Central kynurenine pathway shift with age in women. J Neurochem. 2016;136:995-1003. [DOI] [PubMed] [Google Scholar]
- 20. Martinsons A, Rudzite V, Jurika E, Silava A. The relationship between kynurenine, catecholamines, and arterial hypertension in mesangioproliferative glomerulonephritis. Adv Exp Med Biol. 1996;398:417-419. [DOI] [PubMed] [Google Scholar]
- 21. Cernaro V, Loddo S, Macaione V, et al. RAS inhibition modulates kynurenine levels in a CKD population with and without type 2 diabetes mellitus. Int Urol Nephrol. 2020;52:1125-1133. [DOI] [PubMed] [Google Scholar]
- 22. Clària J, Moreau R, Fenaille F, et al. Orchestration of tryptophan-kynurenine pathway, acute decompensation, and acute-on-chronic liver failure in cirrhosis. Hepatology. 2019;69:1686-1701. [DOI] [PubMed] [Google Scholar]
- 23. Ogyu K, Kubo K, Noda Y, et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16-25. [DOI] [PubMed] [Google Scholar]
- 24. Guillemin GJ, Cullen KM, Lim CK, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884-12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur J Pharmacol. 1999;375:87-100. [DOI] [PubMed] [Google Scholar]
- 26. Gibson EL. Tryptophan supplementation and serotonin function: genetic variations in behavioural effects. Proc Nutr Soc. 2018;77:174-188. [DOI] [PubMed] [Google Scholar]
- 27. Badawy AAB. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:10:1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwarcz R, Stone TW. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacol. 2017;112:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heyes MP, Saito K, Crowley JS, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115 (Pt 5):1249-1273. [DOI] [PubMed] [Google Scholar]
- 30. Wang Q, Liu D, Song P, Zou M-H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci. 2015;20:1116-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Török N, Tanaka M, Vécsei L. Searching for peripheral biomarkers in neurodegenerative diseases: the tryptophan-kynurenine metabolic pathway. Int J Mol Sci. 2020;21:9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maria NI, van Helden-Meeuwsen CG, Brkic Z, et al. Association of increased Treg cell levels with elevated indoleamine 2,3-dioxygenase activity and an imbalanced kynurenine pathway in interferon-positive primary Sjögren’s syndrome. Arthritis Rheumatol. 2016;68:1688-1699. [DOI] [PubMed] [Google Scholar]
- 33. Alberati-Giani D, Ricciardi-Castagnoli P, Köhler C, Cesura AM. Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells. J Neurochem. 1996;66:996-1004. [DOI] [PubMed] [Google Scholar]
- 34. Robinson CM, Hale PT, Carlin JM. The role of IFN-γ and TNF-α-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res. 2005;25:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Currier AR, Ziegler MH, Riley MM, Babcock TA, Telbis VP, Carlin JM. Tumor necrosis factor-alpha and lipopolysaccharide enhance interferon-induced antichlamydial indoleamine dioxygenase activity independently. J Interferon Cytokine Res. 2000;20:369-376. [DOI] [PubMed] [Google Scholar]
- 36. Cheng C-W, Shieh P-C, Lin Y-C, et al. Indoleamine 2,3-dioxygenase, an immunomodulatory protein, is suppressed by (-)-epigallocatechin-3-gallate via blocking of gamma-interferon-induced JAK-PKC-delta-STAT1 signaling in human oral cancer cells. J Agric Food Chem. 2010;58:887-894. [DOI] [PubMed] [Google Scholar]
- 37. Pemberton LA, Kerr SJ, Smythe G, Brew BJ. Quinolinic acid production by macrophages stimulated with IFN-γ, TNF-α, and IFN-α. J Interferon Cytokine Res. 1997;17:589-595. [DOI] [PubMed] [Google Scholar]
- 38. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neavin DR, Liu D, Ray B, Weinshilboum RM. The role of the aryl hydrocarbon receptor (AHR) in immune and inflammatory diseases. Int J Mol Sci. 2018;19:3851. doi: 10.3390/ijms19123851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harden JL, Egilmez NK. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest. 2012;41:738-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puccetti P, Fallarino F, Italiano A, et al. Accumulation of an endogenous tryptophan-derived metabolite in colorectal and breast cancers. PLoS One. 2015;10:e0122046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003;527:183-190. [DOI] [PubMed] [Google Scholar]
- 43. Bartosiewicz J, Kaminski T, Pawlak K, Karbowska M, Tankiewicz-Kwedlo A, Pawlak D. The activation of the kynurenine pathway in a rat model with renovascular hypertension. Exp Biol Med. 2017;242:750-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fazio F, Carrizzo A, Lionetto L, et al. Vasorelaxing action of the kynurenine metabolite, xanthurenic acid: the missing link in endotoxin-induced hypotension? Front Pharmacol. 2017;8:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu MH, Lin C-N, Chiu DT, Chen S-T. Kynurenine/tryptophan ratio predicts angiotensin receptor blocker responsiveness in patients with diabetic kidney disease. Diagnostics. 2020;10:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zakrocka I, Kocki T, Turski WA. The effect of three angiotensin-converting enzyme inhibitors on kynurenic acid production in rat kidney in vitro. Pharmacol Rep. 2017;69:536-541. [DOI] [PubMed] [Google Scholar]
- 47. Changsirivathanathamrong D, Wang Y, Rajbhandari D, et al. Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit Care Med. 2011;39:2678-2683. [DOI] [PubMed] [Google Scholar]
- 48. Nagy BM, Nagaraj C, Meinitzer A, et al. Importance of kynurenine in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2017;313:L741-L751. [DOI] [PubMed] [Google Scholar]
- 49. Xiao Y, Christou H, Liu L, et al. Endothelial indoleamine 2,3-dioxygenase protects against development of pulmonary hypertension. Am J Respir Crit Care Med. 2013;188:482-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Broekhuizen M, Klein T, Hitzerd E, et al. l-Tryptophan-induced vasodilation is enhanced in preeclampsia: studies on its uptake and metabolism in the human placenta. Hypertension. 2020;76:184-194. [DOI] [PubMed] [Google Scholar]
- 51. Kudo Y, Boyd CA, Sargent IL, Redman CW. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am J Obstet Gynecol. 2003;188:719-726. [DOI] [PubMed] [Google Scholar]
- 52. Iwahashi N, Yamamoto M, Nanjo S, Toujima S, Minami S, Ino K. Downregulation of indoleamine 2, 3-dioxygenase expression in the villous stromal endothelial cells of placentas with preeclampsia. J Reprod Immunol. 2017;119:54-60. [DOI] [PubMed] [Google Scholar]
- 53. Santillan MK, Pelham CJ, Ketsawatsomkron P, et al. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep. 2015;3:e12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Worton SA, Pritchard HAT, Greenwood SL, et al. Kynurenine relaxes arteries of normotensive women and those with preeclampsia. Circ Res. 2021;128:1679-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sakakibara K, Feng G-G, Li J, et al. Kynurenine causes vasodilation and hypotension induced by activation of KCNQ-encoded voltage-dependent K+ channels. J Pharmacol Sci. 2015;129:31-37. [DOI] [PubMed] [Google Scholar]
- 56. Kwok JB, Kapoor R, Gotoda T, et al. A missense mutation in kynurenine aminotransferase-1 in spontaneously hypertensive rats. J Biol Chem. 2002;277:35779-35782. [DOI] [PubMed] [Google Scholar]
- 57. Pueyo ME, Michel JB. Angiotensin II receptors in endothelial cells. Gen Pharmacol Vascul Sys. 1997;29:691-696. [DOI] [PubMed] [Google Scholar]
- 58. Yayama K, Okamoto H. Angiotensin II-induced vasodilation via type 2 receptor: role of bradykinin and nitric oxide. Int Immunopharmacol. 2008;8:312-318. [DOI] [PubMed] [Google Scholar]
- 59. Leo MD, Bulley S, Bannister JP, Kuruvilla KP, Narayanan D, Jaggar JH. Angiotensin II stimulates internalization and degradation of arterial myocyte plasma membrane BK channels to induce vasoconstriction. Am J Physiol Cell Physiol. 2015;309:C392-C402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Forrester SJ, Booz GW, Sigmund CD, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98:1627-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kirabo A, Sayeski PP. Jak2 tyrosine kinase: a potential therapeutic target for AT1 receptor mediated cardiovascular disease. Pharmaceuticals. 2010;3:3478-3493. [Google Scholar]
- 62. Nossaman B, Pankey E, Kadowitz P. Stimulators and activators of soluble guanylate cyclase: review and potential therapeutic indications. Crit Care Res Pract. 2012;2012:290805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stanley CP, Maghzal GJ, Ayer A, et al. Singlet molecular oxygen regulates vascular tone and blood pressure in inflammation. Nature. 2019;566:548-552. [DOI] [PubMed] [Google Scholar]
- 64. Feelisch M, Akaike T, Griffiths K, et al. Long-lasting blood pressure lowering effects of nitrite are NO-independent and mediated by hydrogen peroxide, persulfides, and oxidation of protein kinase G1α redox signalling. Cardiovasc Res. 2020;116:51-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Queiroz RF, Stanley CP, Wolhuter K, et al. Hydrogen peroxide signaling via its transformation to a stereospecific alkyl hydroperoxide that escapes reductive inactivation. Nat Commun. 2021;12:6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203-212. [DOI] [PubMed] [Google Scholar]
- 67. Ley K. Role of the adaptive immune system in atherosclerosis. Biochem Soc Trans. 2020;48:2273-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Abdullah SM, Defina LF, Leonard D, et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease: results from the Cooper Center Longitudinal Study. Circulation. 2018;138:2315-2325. [DOI] [PubMed] [Google Scholar]
- 71. Zhao TX, Mallat Z. Targeting the immune system in atherosclerosis: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1691-1706. [DOI] [PubMed] [Google Scholar]
- 72. Baumgartner R, Berg M, Matic L, et al. Evidence that a deviation in the kynurenine pathway aggravates atherosclerotic disease in humans. J Intern Med. 2021;289:53-68. [DOI] [PubMed] [Google Scholar]
- 73. Pedersen ER, Tuseth N, Eussen SJ, et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2015;35:455-462. [DOI] [PubMed] [Google Scholar]
- 74. Cole JE, Astola N, Cribbs AP, et al. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci. 2015;112:13033-13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Polyzos KA, Ovchinnikova O, Berg M, et al. Abstract 368: inhibition of IDO-mediated tryptophan metabolism aggravates atherosclerosis in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2015;35:A368-A368. [Google Scholar]
- 76. Polyzos KA, Ovchinnikova O, Berg M, et al. Inhibition of indoleamine 2,3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe−/− mice. Cardiovasc Res. 2015;106:295-302. [DOI] [PubMed] [Google Scholar]
- 77. Chajadine M, Laurans L, Royere C, Esposito B, Chassaing B, Taleb S. Role of intestinal indoleamine 2, 3-dioxygenase 1 in atherosclerosis. Arch Cardiovasc Dis Suppl. 2021;13:175. [Google Scholar]
- 78. Ding S, Lin N, Sheng X, et al. Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORα-dependent manner. J Pineal Res. 2019;67:e12581. [DOI] [PubMed] [Google Scholar]
- 79. Zhang L, Ovchinnikova O, Jönsson A, et al. The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur Heart J. 2012;33:2025-2034. [DOI] [PubMed] [Google Scholar]
- 80. Madison BB. Srebp2: a master regulator of sterol and fatty acid synthesis. J Lipid Res. 2016;57:333-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Christen S, Thomas SR, Garner B, Stocker R. Inhibition by interferon-gamma of human mononuclear cell-mediated low density lipoprotein oxidation. Participation of tryptophan metabolism along the kynurenine pathway. J Clin Investig. 1994;93:2149-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yun TJ, Lee JS, Machmach K, et al. Indoleamine 2,3-dioxygenase-expressing aortic plasmacytoid dendritic cells protect against atherosclerosis by induction of regulatory T cells. Cell Metab. 2016;24:886. [DOI] [PubMed] [Google Scholar]
- 83. Forteza MJ, Polyzos KA, Baumgartner R, et al. Activation of the regulatory T-cell/indoleamine 2,3-dioxygenase axis reduces vascular inflammation and atherosclerosis in hyperlipidemic mice. Front Immunol. 2018;9:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Niinisalo P, Oksala N, Levula M, et al. Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere vascular study. Ann Med. 2010;42:55-63. [DOI] [PubMed] [Google Scholar]
- 85. Nakajima K, Yamashita T, Kita T, et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1963-1972. [DOI] [PubMed] [Google Scholar]
- 86. Zhang X, Huang F, Li W, et al. Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate atherosclerosis. Front Immunol. 2018;9:878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Watanabe Y, Koyama S, Matsuura Y, et al. Abstract 158: indoleamine 2,3-dioxygenase 1 is expressed in macrophages in human coronary atherosclerotic plaque and is associated with tissue factor expression in activated THP-1 macrophages. Arterioscler Thromb Vasc Biol. 2018;38:A158-A158. [Google Scholar]
- 88. Watanabe Y, Koyama S, Yamashita A, et al. Indoleamine 2,3-dioxygenase 1 in coronary atherosclerotic plaque enhances tissue factor expression in activated macrophages. Res Pract Thromb Haemost. 2018;2:726-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sulo G, Vollset SE, Nygård O, et al. Neopterin and kynurenine–tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int J Cardiol. 2013;168:1435-1440. [DOI] [PubMed] [Google Scholar]
- 90. Zuo H, Ueland PM, Ulvik A, et al. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: the Hordaland Health Study. Am J Epidemiol. 2016;183:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pedersen ER, Svingen GF, Schartum-Hansen H, et al. Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J. 2013;34:2689-2696. [DOI] [PubMed] [Google Scholar]
- 92. Brunelli L, Ristagno G, Bagnati R, et al. A combination of untargeted and targeted metabolomics approaches unveils changes in the kynurenine pathway following cardiopulmonary resuscitation. Metabolomics. 2013;9:839-852. [Google Scholar]
- 93. Ristagno G, Fries M, Brunelli L, et al. Early kynurenine pathway activation following cardiac arrest in rats, pigs, and humans. Resuscitation. 2013;84:1604-1610. [DOI] [PubMed] [Google Scholar]
- 94. Vodovar N, Saadi M, Paven E, et al. P5444Plasma Indoleamine 2,3-dioxygenase is highly predictive of cardiac remodeling after myocardial infarction. Eur Heart J. 2019;40:ehz746.0400. [Google Scholar]
- 95. Liu Y, Li S, Gao Z, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) promotes cardiac hypertrophy via a PI3K-AKT-mTOR-dependent mechanism. Cardiovasc Toxicol. 2021;21:655-668. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96. Lee J-W, Oh JE, Rhee K-J, et al. Co-treatment with interferon-γ and 1-methyl tryptophan ameliorates cardiac fibrosis through cardiac myofibroblasts apoptosis. Mol Cell Biochem. 2019;458:197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chao de la Barca JM, Bakhta O, Kalakech H, et al. Metabolic signature of remote ischemic preconditioning involving a cocktail of amino acids and biogenic amines. J Am Heart Assoc. 2016;5:e003891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bakhta O, Pascaud A, Dieu X, et al. Tryptophane-kynurenine pathway in the remote ischemic conditioning mechanism. Basic Res Cardiol. 2020;115:13. [DOI] [PubMed] [Google Scholar]
- 99. Tong Q, Song J, Yang G, Fan L, Xiong W, Fang J. Simultaneous determination of tryptophan, kynurenine, kynurenic acid, xanthurenic acid and 5-hydroxytryptamine in human plasma by LC-MS/MS and its application to acute myocardial infarction monitoring. Biomed Chromatogr. 2018;32:e4156. [DOI] [PubMed] [Google Scholar]
- 100. Lund A, Nordrehaug JE, Slettom G, et al. Correction: plasma kynurenines and prognosis in patients with heart failure. PLoS One. 2020;15:e0230056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Razquin C, Ruiz-Canela M, Toledo E, et al. Metabolomics of the tryptophan-kynurenine degradation pathway and risk of atrial fibrillation and heart failure: potential modification effect of Mediterranean diet. Am J Clin Nutr. 2021;114:1646-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dschietzig T, Kellner KH, Sasse K, et al. Plasma kynurenine predicts severity and complications of heart failure and associates with established biochemical and clinical markers of disease. Kidney Blood Press Res. 2019;44:765-776. [DOI] [PubMed] [Google Scholar]
- 103. Konishi M, Ebner N, Springer J, et al. Impact of plasma kynurenine level on functional capacity and outcome in heart failure - results from studies investigating co-morbidities aggravating heart failure (SICA-HF). Circ J. 2016;81:52-61. [DOI] [PubMed] [Google Scholar]
- 104. Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269-285. [DOI] [PubMed] [Google Scholar]
- 105. Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23-35. [DOI] [PubMed] [Google Scholar]
- 106. Garcia AG, Wilson RM, Heo J, et al. Interferon-γ ablation exacerbates myocardial hypertrophy in diastolic heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H587-H596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kimura A, Ishida Y, Furuta M, et al. Protective roles of interferon-γ in cardiac hypertrophy induced by sustained pressure overload. J Am Heart Assoc. 2018;7:e008145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dolivo DM, Larson SA, Dominko T. Tryptophan metabolites kynurenine and serotonin regulate fibroblast activation and fibrosis. Cell Mol Life Sci. 2018;75:3663-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Baran H, Staniek K, Kepplinger B, Gille L, Stolze K, Nohl H. Kynurenic acid influences the respiratory parameters of rat heart mitochondria. Pharmacology. 2001;62:119-123. [DOI] [PubMed] [Google Scholar]
- 110. Baran H, Staniek K, Bertignol-Spörr M, Attam M, Kronsteiner C, Kepplinger B. Effects of various kynurenine metabolites on respiratory parameters of rat brain, liver and heart mitochondria. Int J Tryptophan Res. 2016;9:17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bisaccia G, Ricci F, Gallina S, Di Baldassarre A, Ghinassi B. Mitochondrial dysfunction and heart disease: critical appraisal of an overlooked association. Int J Mol Sci. 2021;22:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bądzyńska B, Zakrocka I, Turski WA, Olszyński KH, Sadowski J, Kompanowska-Jezierska E. Kynurenic acid selectively reduces heart rate in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:673-679. [DOI] [PubMed] [Google Scholar]
- 113. Shayesteh S, Guillemin GJ, Rashidian A, et al. 1-Methyl tryptophan, an indoleamine 2,3-dioxygenase inhibitor, attenuates cardiac and hepatic dysfunction in rats with biliary cirrhosis. Eur J Pharmacol. 2021;908:174309. [DOI] [PubMed] [Google Scholar]
- 114. Vandresen-Filho S, Severino PC, Constantino LC, et al. N-methyl-D-aspartate preconditioning prevents quinolinic acid-Induced deregulation of glutamate and calcium homeostasis in mice hippocampus. Neurotox Res. 2015;27:118-128. [DOI] [PubMed] [Google Scholar]
- 115. Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem Res. 1991;16:1139-1143. [DOI] [PubMed] [Google Scholar]
- 116. Santamaría A, Galván-Arzate S, Lisý V, et al. Quinolinic acid induces oxidative stress in rat brain synaptosomes. Neuroreport. 2001;12:871-874. [DOI] [PubMed] [Google Scholar]
- 117. Samman Tahhan A, Sandesara PB, Hayek SS, et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm. 2017;14:1849-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sovari AA. Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol Res Pract. 2016;2016:9656078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vonderlin N, Siebermair J, Kaya E, Köhler M, Rassaf T, Wakili R. Critical inflammatory mechanisms underlying arrhythmias. Herz. 2019;44:121-129. [DOI] [PubMed] [Google Scholar]
- 120. Korantzopoulos P, Letsas KP, Tse G, Fragakis N, Goudis CA, Liu T. Inflammation and atrial fibrillation: A comprehensive review. J Arrhythm. 2018;34:394-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Morotti A, Poli L, Costa P. Acute stroke. Thieme Medical Publishers; 2019:061-072. [Google Scholar]
- 123. Yang Q, Huang Q, Hu Z, Tang X. Potential neuroprotective treatment of stroke: targeting excitotoxicity, oxidative stress, and inflammation. Front Neurosci. 2019;13:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. He Z, Ning N, Zhou Q, Khoshnam SE, Farzaneh M. Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med. 2020;146:45-58. [DOI] [PubMed] [Google Scholar]
- 125. Hoshi M, Saito K, Murakami Y, et al. Marked increases in hippocampal neuron indoleamine 2, 3-dioxygenase via IFN-gamma-independent pathway following transient global ischemia in mouse. Neurosci Res. 2009;63:194-198. [DOI] [PubMed] [Google Scholar]
- 126. Jackman KA, Brait VH, Wang Y, et al. Vascular expression, activity and function of indoleamine 2,3-dioxygenase-1 following cerebral ischaemia–reperfusion in mice. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:471-481. [DOI] [PubMed] [Google Scholar]
- 127. Mo X, Pi L, Yang J, Xiang Z, Tang A. Serum indoleamine 2,3-dioxygenase and kynurenine aminotransferase enzyme activity in patients with ischemic stroke. J Clin Neurosci. 2014;21:482-486. [DOI] [PubMed] [Google Scholar]
- 128. Brouns R, Verkerk R, Aerts T, et al. The role of tryptophan catabolism along the kynurenine pathway in acute ischemic stroke. Neurochem Res. 2010;35:1315-1322. [DOI] [PubMed] [Google Scholar]
- 129. Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119:197-209. [DOI] [PubMed] [Google Scholar]
- 130. Wang J, Wang B, Jiang L, Zhou K, Yang G-Y, Jin K. The effect of IDO on neural progenitor cell survival under oxygen glucose deprivation. Front Cell Neurosci. 2020;14:581861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chen W-C, Chang L-H, Huang S-S, et al. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflammation. 2019;16:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tanaka M, Fujikawa M, Oguro A, Itoh K, Vogel CFA, Ishihara Y. Involvement of the microglial aryl hydrocarbon receptor in neuroinflammation and vasogenic edema after ischemic stroke. Cells. 2021;10:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kwon J-I, Heo H, Ham SJ, et al. Aryl hydrocarbon receptor antagonism before reperfusion attenuates cerebral ischaemia/reperfusion injury in rats. Sci Rep. 2020;10:14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cuartero MI, Ballesteros I, de la Parra J, et al. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation. 2014;130:2040-2051. [DOI] [PubMed] [Google Scholar]
- 135. Lin CH, Chen CC, Chou CM, et al. Knockdown of the aryl hydrocarbon receptor attenuates excitotoxicity and enhances NMDA-induced BDNF expression in cortical neurons. J Neurochem. 2009;111:777-789. [DOI] [PubMed] [Google Scholar]
- 136. Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11-14. [DOI] [PubMed] [Google Scholar]
- 137. Gigler G, Szénási G, Simó A, et al. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur J Pharmacol. 2007;564:116-122. [DOI] [PubMed] [Google Scholar]
- 138. Robotka H, Sas K, Agoston M, et al. Neuroprotection achieved in the ischaemic rat cortex with L-kynurenine sulphate. Life Sci. 2008;82:915-919. [DOI] [PubMed] [Google Scholar]
- 139. Gellért L, Knapp L, Németh K, et al. Post-ischemic treatment with L-kynurenine sulfate exacerbates neuronal damage after transient middle cerebral artery occlusion. Neuroscience. 2013;247:95-101. [DOI] [PubMed] [Google Scholar]
- 140. Cozzi A, Carpenedo R, Moroni F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61-8048) in models of focal or global brain ischemia. J Cereb Blood Flow Metab. 1999;19:771-777. [DOI] [PubMed] [Google Scholar]
- 141. Gellért L, Fuzik J, Göblös A, et al. Neuroprotection with a new kynurenic acid analog in the four-vessel occlusion model of ischemia. Eur J Pharmacol. 2011;667:182-187. [DOI] [PubMed] [Google Scholar]
- 142. Cogo A, Mangin G, Maïer B, et al. Increased serum QUIN/KYNA is a reliable biomarker of post-stroke cognitive decline. Mol Neurodegener. 2021;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Stone TW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol Sci. 2000;21:149-154. [DOI] [PubMed] [Google Scholar]
- 144. Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain. 2018;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schwarcz R, Whetsell WO, Jr., Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316-318. [DOI] [PubMed] [Google Scholar]
- 146. Zwilling D, Huang S-Y, Sathyasaikumar KV, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Parrott JM, O’Connor JC. Kynurenine 3-monooxygenase: an influential mediator of neuropathology. Front Psychiatry. 2015;6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wu QY, Cheng Z, Zhou Y-Z, et al. A novel STAT3 inhibitor attenuates angiotensin II-induced abdominal aortic aneurysm progression in mice through modulating vascular inflammation and autophagy. Cell Death Dis. 2020;11:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Xue F, Yang J, Cheng J, et al. Angiotensin-(1-7) mitigated angiotensin II-induced abdominal aortic aneurysms in apolipoprotein E-knockout mice. Br J Pharmacol. 2020;177:1719-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang Q, Ding Y, Song P, et al. Tryptophan-derived 3-hydroxyanthranilic acid contributes to angiotensin II-induced abdominal aortic aneurysm formation in mice in vivo. Circulation. 2017;136:2271-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Metghalchi S, Vandestienne M, Haddad Y, et al. Indoleamine 2 3-dioxygenase knockout limits angiotensin II-induced aneurysm in low density lipoprotein receptor-deficient mice fed with high fat diet. PLoS One. 2018;13:e0193737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nishimura M, Yamashita A, Matsuura Y, et al. Upregulated kynurenine pathway enzymes in aortic atherosclerotic aneurysm: macrophage kynureninase downregulates inflammation. J Atheroscler Thromb. 2021;28:1214-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Golledge J, Moxon J, Singh T, Bown M, Mani K, Wanhainen A. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med. 2020;288:6-22. [DOI] [PubMed] [Google Scholar]
- 154. Hoshi M, Matsumoto K, Ito H, et al. l-tryptophan-kynurenine pathway metabolites regulate type I IFNs of acute viral myocarditis in mice. J Immunol. 2012;188:3980-3987. [DOI] [PubMed] [Google Scholar]
- 155. Hoshi M, Saito K, Hara A, et al. The absence of IDO upregulates type I IFN production, resulting in suppression of viral replication in the retrovirus-infected mouse. J Immunol. 2010;185:3305-3312. [DOI] [PubMed] [Google Scholar]
- 156. Guo G, Sun L, Yang L, Xu H. IDO1 depletion induces an anti-inflammatory response in macrophages in mice with chronic viral myocarditis. Cell Cycle. 2019;18:2598-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kubo H, Hoshi M, Mouri A, et al. Absence of kynurenine 3-monooxygenase reduces mortality of acute viral myocarditis in mice. Immunol Lett. 2017;181:94-100. [DOI] [PubMed] [Google Scholar]
- 158. Suarez-Fuentetaja N, Domenech-Garcia N, Paniagua-Martin MJ, et al. Indoleamine, 2-3 dioxygenase activity could be an early marker of graft rejection in heart transplantation. Transplant Proc. 2012;44:2645-2648. [DOI] [PubMed] [Google Scholar]
- 159. Yang L, Ma J, He Q, Li X. Immutol regulates CD4+Tregs, CD8+Tregs and pDCs via IDO signaling pathway to induce immune tolerance in rat heart allograft transplant. Transpl Immunol. 2021;68:101393. [DOI] [PubMed] [Google Scholar]
- 160. Ala M. Tryptophan metabolites modulate inflammatory bowel disease and colorectal cancer by affecting immune system. Int Rev Immunol. 2021;2021:1-20. [DOI] [PubMed] [Google Scholar]
- 161. Lv Y, Pang X, Jia P, Jia D. Combined therapy of infusion of DC from rats with higher expression of IDO and CD40L on rejection post heart transplantation. Eur Rev Med Pharmacol Sci. 2018;22:7977-7984. [DOI] [PubMed] [Google Scholar]
- 162. He JG, Li BB, Zhou L, Yan D, Xie Q-L, Zhao W. Indoleamine 2,3-dioxgenase-transfected mesenchymal stem cells suppress heart allograft rejection by increasing the production and activity of dendritic cells and regulatory T cells. J Investig Med. 2020;68:728-737. [DOI] [PubMed] [Google Scholar]
- 163. Li J, Meinhardt A, Roehrich M-E, et al. Indoleamine 2,3-dioxygenase gene transfer prolongs cardiac allograft survival. Am J Physiol Heart Circ Physiol. 2007;293:H3415-H3423. [DOI] [PubMed] [Google Scholar]
- 164. Li C, Liu T, Zhao N, Zhu L, Wang P, Dai X. Dendritic cells transfected with indoleamine 2,3-dioxygenase gene suppressed acute rejection of cardiac allograft. Int Immunopharmacol. 2016;36:31-38. [DOI] [PubMed] [Google Scholar]
- 165. Zhang Y, Zhang G, Liu Y, et al. GDF15 regulates Malat-1 circular RNA and inactivates NFκB signaling leading to immune tolerogenic DCs for preventing alloimmune rejection in heart transplantation. Front Immunol. 2018;9:2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. He JG, Xie QL, Li BB, Zhou L, Yan D. Exosomes derived from IDO1-overexpressing rat bone marrow mesenchymal stem cells promote immunotolerance of cardiac allografts. Cell Transplant. 2018;27:1657-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sucher R, Fischler K, Oberhuber R, et al. IDO and regulatory T cell support are critical for cytotoxic T lymphocyte-associated Ag-4 Ig-mediated long-term solid organ allograft survival. J Immunol. 2012;188:37-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Popp FC, Eggenhofer E, Renner P, et al. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55-60. [DOI] [PubMed] [Google Scholar]
- 169. Yu G, Dai H, Chen J, et al. Gene delivery of indoleamine 2,3-dioxygenase prolongs cardiac allograft survival by shaping the types of T-cell responses. J Gene Med. 2008;10:754-761. [DOI] [PubMed] [Google Scholar]
- 170. Li M, Zhang X, Zheng X, et al. Tolerogenic dendritic cells transferring hyporesponsiveness and synergizing T regulatory cells in transplant tolerance. Int Immunol. 2008;20:285-293. [DOI] [PubMed] [Google Scholar]
- 171. Dai X, Zhu BT. Suppression of T-cell response and prolongation of allograft survival in a rat model by tryptophan catabolites. Eur J Pharmacol. 2009;606:225-232. [DOI] [PubMed] [Google Scholar]
- 172. Li C, Sun Z, Yuan F, et al. Mechanism of indoleamine 2, 3-dioxygenase inhibiting cardiac allograft rejection in mice. J Cell Mol Med. 2020;24:3438-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752-6761. [DOI] [PubMed] [Google Scholar]
- 174. Mathern DR, Horwitz K, Heeger J PS, Absence of recipient C3aR1 signaling limits expansion and differentiation of alloreactive CD8+ T cell immunity and prolongs murine cardiac allograft survival. Am J Transplant. 2019;19:1628-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Itoh S, Nakae S, Axtell RC, et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30:235-240. [DOI] [PubMed] [Google Scholar]
- 176. Li C, Wang X, Yuan F, et al. MiR-669b-3p regulates CD4(+) T cell function by down-regulating indoleamine-2, 3-dioxygenase. Transpl Immunol. 2020;62:101320. [DOI] [PubMed] [Google Scholar]
- 177. Jui HY, Lin CH, Hsu WT, et al. Autologous mesenchymal stem cells prevent transplant arteriosclerosis by enhancing local expression of interleukin-10, interferon-γ, and indoleamine 2,3-dioxygenase. Cell Transplant. 2012;21:971-984. [DOI] [PubMed] [Google Scholar]
- 178. Cuffy MC, Silverio AM, Qin L, et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-γ contributes to medial immunoprivilege. J Immunol. 2007;179:5246-5254. [DOI] [PubMed] [Google Scholar]
- 179. Sadahiro M, McDonald TO, Allen MD. Reduction in cellular and vascular rejection by blocking leukocyte adhesion molecule receptors. Am J Pathol. 1993;142:675-683. [PMC free article] [PubMed] [Google Scholar]
- 180. Zulpaite R, Miknevicius P, Leber B, Strupas K, Stiegler P, Schemmer P. Tryptophan metabolism via kynurenine pathway: role in solid organ transplantation. Int J Mol Sci. 2021;22:1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Song S, Lam EW, Tchkonia T, Kirkland JL, Sun Y. Senescent cells: emerging targets for human aging and age-related diseases. Trends Biochem Sci. 2020;45:578-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Liberale L, Montecucco F, Tardif J-C, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J. 2020;41:2974-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. 2020;1216:55-64. [DOI] [PubMed] [Google Scholar]
- 184. Jang IY, Park JH, Kim JH, et al. The association of circulating kynurenine, a tryptophan metabolite, with frailty in older adults. Aging. 2020;12:22253-22265. [DOI] [PubMed] [Google Scholar]
- 185. Westbrook R, Chung T, Lovett J, et al. Kynurenines link chronic inflammation to functional decline and physical frailty. JCI Insight. 2020;5:e136091. doi: 10.1172/jci.insight.136091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Oxenkrug GF, Navrotskaya V, Voroboyva L, Summergrad P. Extension of life span of Drosophila melanogaster by the inhibitors of tryptophan-kynurenine metabolism. Fly. 2011;5:307-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. van der Goot AT, Zhu W, Vázquez-Manrique RP, et al. Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc Natl Acad Sci USA. 2012;109:14912-14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Segall PE, Timiras PS. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech Ageing Dev. 1976;5:109-124. [DOI] [PubMed] [Google Scholar]
- 189. Pertovaara M, Raitala A, Lehtimäki T, et al. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497-499. [DOI] [PubMed] [Google Scholar]
- 190. Kim BJ, Hamrick MW, Yoo HJ, et al. The detrimental effects of kynurenine, a tryptophan metabolite, on human bone metabolism. J Clin Endocrinol Metab. 2019;104:2334-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kondrikov D, Elmansi A, Bragg RT, et al. Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway. Exp Gerontol. 2020;130:110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zhang Y, Chen Y, Ma L. Depression and cardiovascular disease in elderly: current understanding. J Clin Neurosci. 2018;47:1-5. [DOI] [PubMed] [Google Scholar]
- 193. Wei J, Hou R, Zhang X, et al. The association of late-life depression with all-cause and cardiovascular mortality among community-dwelling older adults: systematic review and meta-analysis. Br J Psychiatry. 2019;215:449-455. [DOI] [PubMed] [Google Scholar]
- 194. Meng R, Yu C, Liu N, et al. Association of depression with all-cause and cardiovascular disease mortality among adults in China. JAMA Netw Open. 2020;3:e1921043-e1921043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802-813. [DOI] [PubMed] [Google Scholar]
- 196. Bekfani T, Neugebauer S, Bekhite ELsaied M, et al. Kynurenine and spermidine are associated with reduced skeletal muscle function, expression of PPARa and elevated depression score in patients with heart failure. Circulation. 2019;140:A16513-A16513. [Google Scholar]
- 197. Su J, Wang J, Ma Y, et al. Inflammation associated with chronic heart failure leads to enhanced susceptibility to depression. FEBS J. 2019;286:2769-2786. [DOI] [PubMed] [Google Scholar]
- 198. Dantzer R. Role of the kynurenine metabolism pathway in inflammation-induced depression: preclinical approaches. In: Dantzer R, Capuron L, eds. Inflammation-Associated Depression: Evidence, Mechanisms and Implications. Springer International Publishing; 2017;117-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198-209. [DOI] [PubMed] [Google Scholar]
- 200. Watts SW. Serotonin and sensory nerves: Meeting in the cardiovascular system. Vasc Pharmacol. 2014;63:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Shively CA, Silverstein-Metzler M, Justice J, Willard SL. The impact of treatment with selective serotonin reuptake inhibitors on primate cardiovascular disease, behavior, and neuroanatomy. Neurosci Biobehav Rev. 2016;74:433-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356-1365. [DOI] [PubMed] [Google Scholar]
- 203. Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;6:e918-e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. O’Connor JC, Lawson MA, André C, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. O’Connor JC, Andre C, Wang Y, et al. Interferon-γ and tumor necrosis factor-α mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Murakami Y, Ishibashi T, Tomita E, et al. Depressive symptoms as a side effect of Interferon-α therapy induced by induction of indoleamine 2,3-dioxygenase 1. Sci Rep. 2016;6:29920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Nikkheslat N, Zunszain PA, Horowitz MA, et al. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun. 2015;48:8-18. [DOI] [PubMed] [Google Scholar]
- 208. Hunt C, Macedo e, Cordeiro T, Suchting R, et al. Effect of immune activation on the kynurenine pathway and depression symptoms – a systematic review and meta-analysis. Neurosci Biobehav Rev. 2020;118:514-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961-966. [DOI] [PubMed] [Google Scholar]
- 211. Halaris A, Myint AM, Savant V, et al. Does escitalopram reduce neurotoxicity in major depression? J Psychiatr Res. 2015;66-67:118-126. [DOI] [PubMed] [Google Scholar]
- 212. Kocki T, Wnuk S, Kloc R, Kocki J, Owe-Larsson B, Urbanska EM. New insight into the antidepressants action: modulation of kynurenine pathway by increasing the kynurenic acid/3-hydroxykynurenine ratio. J Neural Transm. 2012;119:235-243. [DOI] [PubMed] [Google Scholar]
- 213. Di Lullo L, House A, Gorini A, Santoboni A, Russo D, Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. 2015;20:259-272. [DOI] [PubMed] [Google Scholar]
- 214. Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74:120-131. [DOI] [PubMed] [Google Scholar]
- 215. Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5:543-551. [DOI] [PubMed] [Google Scholar]
- 216. Pawlak K, Myśliwiec M, Pawlak D. Kynurenine pathway - a new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv Med Sci. 2010;55:196-203. [DOI] [PubMed] [Google Scholar]
- 217. Pawlak K, Kowalewska A, Mysliwiec M, Pawlak D. Kynurenine and its metabolites–kynurenic acid and anthranilic acid are associated with soluble endothelial adhesion molecules and oxidative status in patients with chronic kidney disease. Am J Med Sci. 2009;338:293-300. [DOI] [PubMed] [Google Scholar]
- 218. Pawlak K, Mysliwiec M, Pawlak D. Hypercoagulability is independently associated with kynurenine pathway activation in dialysed uraemic patients. Thromb Haemost. 2009;102:49-55. [DOI] [PubMed] [Google Scholar]
- 219. Pawlak K, Domaniewski T, Mysliwiec M, Pawlak D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis. 2009;204:309-314. [DOI] [PubMed] [Google Scholar]
- 220. Konje VC, Rajendiran TM, Bellovich K, et al. Tryptophan levels associate with incident cardiovascular disease in chronic kidney disease. Clin Kidney J. 2021;14:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Pawlak K, Tankiewicz J, Mysliwiec M, Pawlak D. Tissue factor/its pathway inhibitor system and kynurenines in chronic kidney disease patients on conservative treatment. Blood Coagul Fibrinolysis. 2009;20:590-594. [DOI] [PubMed] [Google Scholar]
- 222. Pan B, Zhang F, Sun J, et al. Correlation of indoleamine-2,3-dioxygenase and chronic kidney disease: a pilot study. J Immunol Res. 2021;2021:8132569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Lee H, Jang HB, Yoo MG, Park SI, Lee HJ. Amino acid metabolites associated with chronic kidney disease: an eight-year follow-up Korean epidemiology study. Biomedicines. 2020;8:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Matheus LHG, Simão GM, Amaral TA, et al. Indoleamine 2, 3-dioxygenase (IDO) increases during renal fibrogenesis and its inhibition potentiates TGF-β 1-induced epithelial to mesenchymal transition. BMC Nephrol. 2017;18:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Liu K, Yang Y, Chen Y, Li S, Gong Y, Liang Y. The therapeutic effect of dendritic cells expressing indoleamine 2,3-dioxygenase (IDO) on an IgA nephropathy mouse model. Int Urol Nephrol. 2020;52:399-407. [DOI] [PubMed] [Google Scholar]
- 226. Pan B, Zhang H, Hong Y, Ma M, Wan X, Cao C. Indoleamine-2,3-dioxygenase activates Wnt/β-catenin inducing kidney fibrosis after acute kidney injury. Gerontology. 2021;67:611-619. [DOI] [PubMed] [Google Scholar]
- 227. Chen L, Chen DQ, Liu JR, et al. Unilateral ureteral obstruction causes gut microbial dysbiosis and metabolome disorders contributing to tubulointerstitial fibrosis. Exp Mol Med. 2019;51:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Irsik DL, Xu J, Bollag WB, Isales CM. Kynurenine induces renal fibrosis which may contribute to bone loss in aging. FASEB J. 2019;33:570.2-570.2. [Google Scholar]
- 229. Izzy M, VanWagner LB, Lin G, et al. Redefining cirrhotic cardiomyopathy for the modern era. Hepatology. 2020;71:334-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Drăghici T, Negreanu L, Bratu OG, et al. Liver abnormalities in patients with heart failure. Arch Balk Med Union. 2018;53:76-81. [Google Scholar]
- 232. Kardashian A, Ma Y, Yin MT, et al. High Kynurenine: Tryptophan Ratio Is Associated With Liver Fibrosis in HIV-Monoinfected and HIV/Hepatitis C Virus–Coinfected Women. Oxford University Press US; 2019:ofz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Yang R, Gao N, Chang Q, Meng X, Wang W. The role of IDO, IL-10, and TGF-β in the HCV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Med Virol. 2019;91:265-271. [DOI] [PubMed] [Google Scholar]
- 234. Oh JE, Shim KY, Lee JI, Choi SI, Baik SK, Eom YW. 1-Methyl-L-tryptophan promotes the apoptosis of hepatic stellate cells arrested by interferon-γ by increasing the expression of IFN-γRβ, IRF-1 and FAS. Int J Mol Med. 2017;40:576-582. [DOI] [PubMed] [Google Scholar]
- 235. Zhong W, Gao L, Zhou Z, et al. Indoleamine 2,3-dioxygenase 1 deficiency attenuates CCl4-induced fibrosis through Th17 cells down-regulation and tryptophan 2,3-dioxygenase compensation. Oncotarget. 2017;8:40486-40500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Rubens M, Appunni S, Ramamoorthy V, et al. Prevalence of cardiovascular risk factors among cancer patients in the United States. Metab Syndr Relat Disord. 2019;17:397-405. [DOI] [PubMed] [Google Scholar]
- 238. Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007-1017. [DOI] [PubMed] [Google Scholar]
- 240. Monsuez J-J. Detection and prevention of cardiac complications of cancer chemotherapy. Arch Cardiovasc Dis. 2012;105:593-604. [DOI] [PubMed] [Google Scholar]
- 241. Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin Cancer Res. 2019;25:1462-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rebnord EW, Strand E, Midttun, et al. The kynurenine:tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia. 2017;60:1712-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Oxenkrug GF, Turski WA, Zgrajka W, Weinstock JV, Summergrad P. Tryptophan-kynurenine metabolism and insulin resistance in hepatitis C patients. Hepat Res Treat. 2013;2013:149247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Reginaldo C, Jacques P, Scott T, Oxenkrug G, Selhub J, Paul L. Xanthurenic acid is associated with higher insulin resistance and higher odds of diabetes. FASEB J. 2015;29:919.20. [Google Scholar]
- 246. Muzik O, Burghardt P, Yi Z, Kumar A, Seyoum B. Successful metformin treatment of insulin resistance is associated with down-regulation of the kynurenine pathway. Biochem Biophys Res Commun. 2017;488:29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Rojas IY, Moyer BJ, Ringelberg CS, et al. Kynurenine-induced aryl hydrocarbon receptor signaling in mice causes body mass gain, liver steatosis, and hyperglycemia. Obesity. 2021;29:337-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Lu MC, Fang W-C, Li WC, Yeh W-C, Shieh Y-H, Chen J-Y. The association between insulin resistance and cardiovascular disease risk: a community-based cross-sectional study among Taiwanese people aged over 50 years. Int J Environ Res Public Health. 2020;17:7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018;14:491-509. [DOI] [PubMed] [Google Scholar]