Abstract
Topoisomerases introduce transient DNA breaks to relax supercoiled DNA, remove catenanes and enable chromosome segregation. Human cells encode six topoisomerases (TOP1, TOP1mt, TOP2α, TOP2β, TOP3α and TOP3β), which act on a broad range of DNA and RNA substrates at the nuclear and mitochondrial genomes. Their catalytic intermediates, the topoisomerase cleavage complexes (TOPcc), are therapeutic targets of various anticancer drugs. TOPcc can also form on damaged DNA during replication and transcription, and engage specific repair pathways, such as those mediated by tyrosyl-DNA phosphodiesterase 1 (TDP1) and TDP2 and by endonucleases (MRE11, XPF–ERCC1 and MUS81). Here, we review the roles of topoisomerases in mediating chromatin dynamics, transcription, replication, DNA damage repair and genomic stability, and discuss how deregulation of topoisomerases can cause neurodegenerative diseases, immune disorders and cancer.
DNA topoisomerases are vital enzymes that solve DNA topological problems that result from strand separation during replication and transcription1,2. Topoisomerases are emerging as important factors in a wide range of fundamental metabolic processes in both the nuclear and mitochondrial genomes. The study of topoisomerases is therapeutically relevant to cancer, immune disorders and neurological diseases, and highly effective antibacterial and anticancer agents selectively target bacterial and eukaryotic topoisomerases, respectively3,4.
The six human topoisomerases, TOP1, TOP1mt, TOP2α, TOP2β, TOP3α and TOP3β, have both shared and specialized roles (FIG. 1). For example, both TOP2α and TOP2β relax negatively supercoiled DNA and carry out DNA decatenation, but whereas TOP2α is absolutely required for chromosome segregation, TOP2β is indispensable for transcription in differentiated, non-dividing cells.
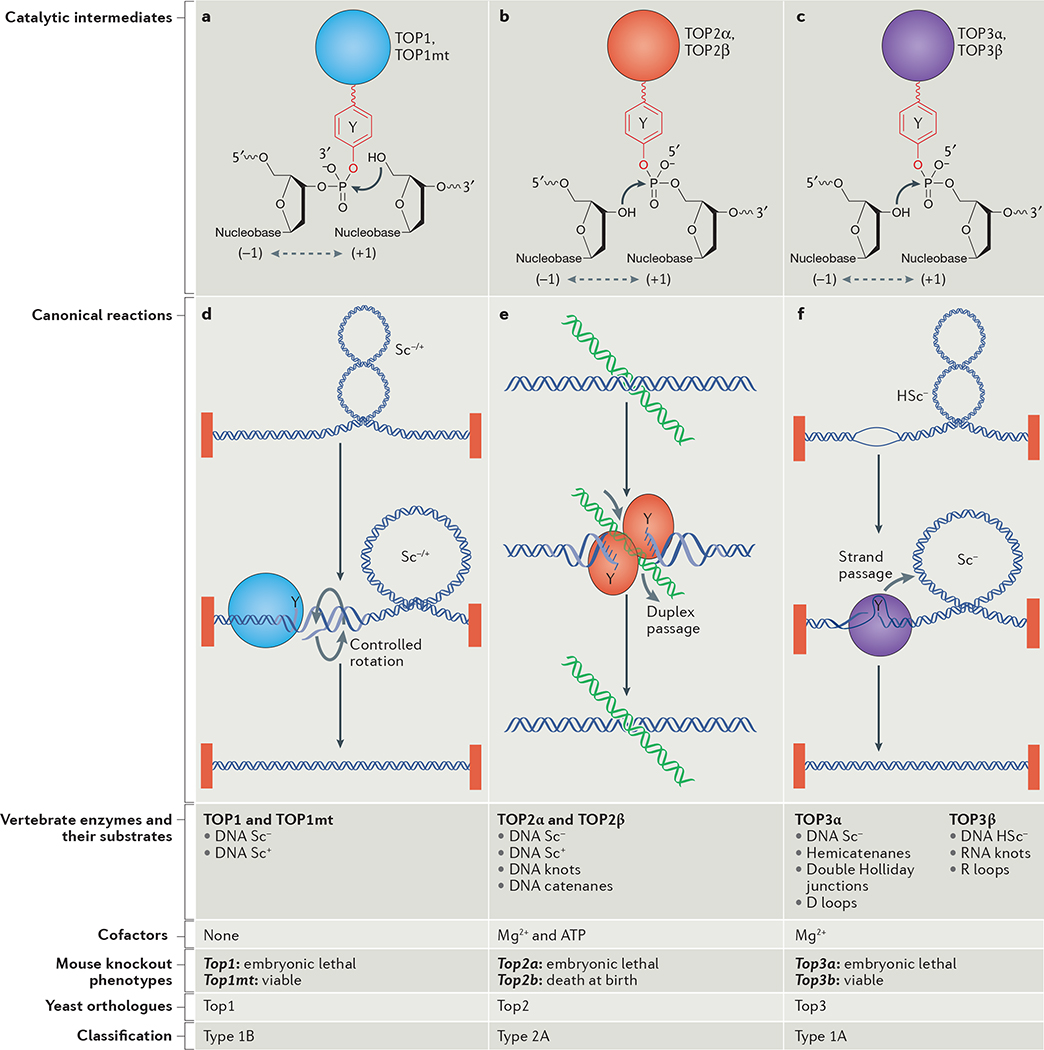
Figure 1 |. Overview of eukaryotic topoisomerases.
a–c | Topoisomerases act by cleaving the DNA phosphodiester backbone and forming transient covalent linkages between a Tyr residue and the DNA 3′ end (TOP1 enzymes) or 5′ end (TOP2 and TOP3 enzymes). Resealing of the breaks is carried out by nucleophilic attack (arrows) of the 5′-hydroxyl end in the case of TOP1 enzymes and the 3′-hydroxyl end in the case of TOP2 and TOP3 enzymes. Base stacking (dashed double-headed arrows) is crucial for the realignment of the DNA ends (like a molecular zipper) and their re-ligation. d | TOP1 enzymes relax both negative and positive supercoils (Sc−/+) by nicking one strand and allowing controlled rotation of the broken strand around the intact strand. TOP1 enzymes can re-ligate non-homologous ends, thereby acting as DNA recombinases. e | TOP2 enzymes function as homodimers to relax both negative and positive supercoils and to resolve catenanes and DNA knots, explaining their essential role in cell division, during which supercoiled circles form catenated daughter molecules. They cleave both DNA strands with a four-base stagger, thereby directing a second duplex to pass through (duplex passage), and re-ligating the DNA following the passage. They require both Mg2+ and ATP hydrolysis for their catalytic cycle. f | TOP3 enzymes only relax hypernegative supercoiling (HSc−) by cleaving one of the two strands of DNA in regions where negative supercoiling promotes their separation and by passing the intact strand through the broken one. Mg2+ is a required metal cofactor. TOP3β can act as an RNA helicase and resolve R loops. See Supplementary information S1 (box), for further information on DNA supercoiling.
Topoisomerases introduce transient DNA breaks using a transesterification mechanism, which is highly reversible and minimizes the risks to genome stability that would otherwise occur owing to strand breakage2,3,5–9 (FIG. 1a–c). Nonetheless, it is now established that topoisomerases can both ensure and endanger genome integrity. Anticancer drugs that target TOP2 enzymes induce genomic translocations that lead to secondary malignancies, and recent work suggests that TOP2β may be especially responsible for these events. Additional studies showed that TOP1 could provoke genome instability by action at sites of endogenous and exogenous DNA damage. The risks associated with strand breakage by topoisomerases suggest that there are aspects of fundamental processes such as transcription that pose unique topological challenges and that cells need a wide repertoire of responses and specific repair pathways to safeguard the dangerous process of introducing transient DNA breaks.
Transesterification.
A chemical reaction in which an organic group of an ester (phosphate ester of a nucleotide) is replaced with the alkoxy group of a nucleophilic alcohol (active Tyr residue of a topoisomerase).
In this Review, we discuss the emerging evidence that topoisomerases have more diverse functions and are more highly specialized than previously appreciated3,5,7,8,10. We discuss recent advances in research on the roles of topoisomerases in mitochondria. Somewhat surprisingly, recent data suggest that topoisomerase-induced DNA damage can have unique cellular roles and that topoisomerases may function in non-canonical ways (that is, by not only changing DNA topology), especially during transcription and in the presence of ribonucleotides that have been incorporated into DNA. Given the breadth of the field, we refer the reader to other reviews covering bacterial topoisomerases2,11, topoisomerase protein structure2,8, post-translational modifications of topoisomerases12 and topoisomerase enzymology2,3,6,8–10,13.
The topoisomerase–chromatin interplay
The biochemistry of topoisomerases and their overlapping activities are well established in the context of naked DNA2,3,5,8 (FIG. 1). By forming topoisomerase cleavage complexes (TOPcc) without strict DNA sequence preference14–17 topoisomerases can function wherever topological problems arise. TOP1 and TOP2 enzymes do not even require DNA to be supercoiled, as they can cleave linear substrates as short as 20 bp18–20. The two type 1B topoisomerases (FIG. 1) have exclusive cellular localization (nuclear for TOP1 and mitochondrial for TOP1mt)21, whereas TOPα, TOP2β and TOP3α are present in both nuclei and mitochondria22,23.
Recent studies have begun to reveal a dynamic picture of the interplay between topoisomerases and chromatin. Not surprisingly, nucleosomes shield DNA from topoisomerases and restrict their reactions to linker DNA24,25. However, even in nucleosome-free regions, it is likely that topoisomerase activities are tightly regulated to preserve the negative supercoiling that is required for initiating transcription26–29 and replication30,31, as well as to minimize the formation of potentially deleterious cleavage complexes (see below). Proteins that bind to negatively supercoiled DNA can shield it from topoisomerases, whereas other proteins directly regulate topoisomerase activities. Hypernegatively supercoiled DNA also tends to form alternative, non-B DNA, such as single-stranded DNA and guanosine quadruplexes, which cannot be processed by TOP1 and TOP2 enzymes32. In addition, recent evidence suggests that topoisomerases are recruited to specific regions by chromatin-remodelling complexes, histone chaperones33,34 and insulator proteins35.
Guanosine quadruplexes.
Nucleic acid structures in which four guanine bases are connected by hydrogen bonds and form a square planar structure. Such guanine tetrads can pile on top of each other to constitute a guanosine quadruplex.
Switch/sucrose non-fermentable (SWI/SNF) complexes (also known as BRG1-associated factor (BAF) complexes36) are ATP-dependent nucleosome remodellers that control nucleosome deposition and histone methylation through the recruitment of the histone methyltransferase Polycomb repressive complex 2 (PRC2). Alterations in the activity of these complexes are increasingly being identified in human cancers. TOP1 and TOP2α are controlled by SMARCA4 (also known as BRG1) the catalytic ATPase subunit of the SWI/SNF complexes. SMARCA4 and other components of the SWI/SNF complexes associate primarily with TOP1, which is recruited to chromatin by SMARCA4 to maintain genomic stability by dissipating negative supercoils and by suppressing the formation of potentially mutagenic DNA structures such as G-quadruplexes and right-handed (Z-form) DNA34. TOPα binding to chromatin is also dependent on the ATPase activity of SMARCA4 and prevents DNA entanglements at mitosis and genomic instability33. SMARCA4 deletion leads to the formation of anaphase bridges, in which sister chromatids remain linked by catenated strands of DNA33 a phenotype also typical of TOP2α deficiency2,7.
Other chromatin proteins are important for the recruitment of TOP1 to specific genomic sites during DNA replication and transcription, such as Tof2 and Fob1 in budding yeast, which form stable cleavage complexes at ribosomal replication fork barriers35. TOP1 is also recruited to transcriptionally active chromatin (marked by histone H3 Lys4 trimethylation (H3K4me3)) by directly binding to the facilitates chromatin transcription (FACT) complex, which is a prominent histone chaperone and transcription elongation factor34. In addition to the recruitment of TOP1 by SMARCA4, the FACT complex has been proposed to recruit TOP1 to non-canonical DNA structures, resulting in DNA breaks at specific recombination sites34. Other proteins that mediate TOP1 binding to chromatin include nucleolin, the large T-antigen replication helicase31, Werner syndrome ATP-dependent helicase (WRN)37, basal transcription factors38–40, the androgen-regulated transcription factor NKX3.1 (REFS 41,42) and the replication checkpoint protein Tof1 (Timeless in humans)43.
TOP2α was proposed to be a key chromosome-scaffolding protein44 and, recently, was shown to be crucial for not only for DNA decatenation but also for chromosome condensation45 and compaction of mitotic chromosomes46. TOP2α sumoylation regulates its decatenating activity to ensure proper segregation of newly replicated DNA during mitosis47–49. Recent studies suggest that TOP2α is recruited to linked chromatids, which are visualized as chromatin threads that connect partially separated chromosomes and are termed anaphase ultrafine bridges (UFBs)50, by binding to the carboxy-terminal domain of TOP2-binding protein 1 (TOPBP1)51, which is a cell cycle checkpoint factor important for rescuing stalled replication forks.
Ultrafine bridges.
(UFBs). A class of mitotic chromatin threads that form bridges linking daughter DNA molecules. UFBs contain DNA and can be stained with antibodies directed against certain helicases but cannot be stained by fluorescent intercalating dyes.
Type 1A topoisomerases also have specific interactions with chromatin that may regulate their function. TOP3β was recently shown to form complexes with the proteins Tudor domain-containing protein 3 (TDRD3) and fragile X mental retardation protein (FMRP)52–54. TDRD3 stabilizes and recruits TOP3β to active promoters (such as MYC and NRAS) that are marked by asymmetrically dimethylated histone H4 Arg3 (H4R3me2a) and H3R17me2a. In such complexes, TOP3β suppresses the formation of R loops at CpG islands (CGIs) in active promoters54. TDRD3 also acts as a bridge connecting TOP3β with the translation regulator FMRP55. Thus, through its selective recruitment to specific chromatin regions, TOP3β is emerging as a novel regulator of both transcription and translation.
R loops.
RNA–DNA hybrids in which a single-stranded RNA hybridizes to a template strand in a DNA duplex and displaces the non-template strand as a loop.
CpG islands.
(CGIs). Genomic regions with a high level of CpG dinucleotides.
Studies of topoisomerase activity at chromatin templates are still at a relatively early stage. Chromatin modifications and chromatin-modifying complexes are likely to be key topoisomerase regulators. The structural elucidation of macromolecular complexes that orchestrate the activities of topoisomerases will be crucial in advancing our understanding of the intricate relationships between topoisomerases and chromatin.
Topoisomerases and transcription
Topoisomerases are required during transcription to manage the DNA supercoiling that accumulates ahead and behind the transcription machinery56 (FIG. 2). Experiments in yeast suggested that either Top1 or Top2 could efficiently provide the topoisomerase activities required for transcription57,58 and that either could enhance the recruitment of RNA polymerase II (Pol II) to promoters59. Recent results in yeast suggest that topoisomerases are required for initiating the transcription of a subset of regulated genes such as GAL and PHO (regulated by galactose and inorganic phosphate, respectively); the requirements for topoisomerases differ depending on the detailed mechanism of gene activation60,61. Importantly, these studies showed that topoisomerase activity is required for transcription initiation but not for elongation or re-initiation. Conversely, another study found that highly expressed genes require both Top1 and Top2, whereas Top1 alone can support genes with low transcription levels26.
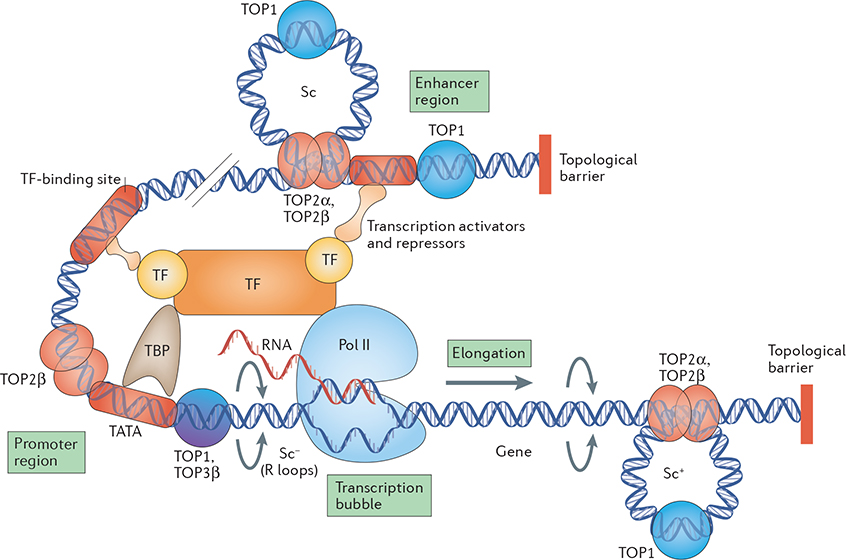
Figure 2 |. Topoisomerases and transcription.
Transcription incurs topological constraints that result from the progression of RNA polymerase II (Pol II). Positive supercoiling (Sc+) of the DNA template takes place ahead of the transcription bubble, which in turn obstructs further Pol II movement, and negative supercoiling (Sc−), which promotes the formation of RNA–DNA hybrids (R loops), accumulates behind it. TOP2 and especially TOP1 enzymes function ahead of Pol II to remove positive supercoils, whereas relaxation of negative supercoils behind the transcription apparatus relies on TOP1 and TOP3β. In addition, TOP1 regulates the activity of the transcription factor TATA-box-binding protein (TBP) at promoter TATA boxes independently of its catalytic activity. The formation of TOP2β-mediated transient DNA double-stranded breaks at promoter regions in certain genes is crucial for transcription activation. TOP1 is also recruited to certain enhancer regions to promote (ligand-dependent) enhancer activation by generating transient DNA single-stranded breaks. Topological barriers are genomic regions where the DNA is not free to rotate around its axis and require TOP1 and TOP2 to relax supercoils (Sc). TF, transcription factor.
Transcription initiation: give me a break?
Early studies showed that TOP1 facilitated the binding of the transcription factor TATA-box-binding protein (TBP) to the TATA box, thereby regulating transcription initiation40 (FIG. 2). However, catalytically dead TOP1-mutant proteins were proficient in both transcription activation and repression, which showed that DNA untwisting activity was not essential38–40. One lesson from these experiments was the necessity to establish the importance of topoisomerase-mediated cleavage, which is typically determined by substitution of the active enzyme with mutants that are incapable of cleaving DNA, such as mutants in which the active site Tyr residue (FIG. 1) was substituted with a Phe residue. A recent study demonstrated that TOP1 tends to be kept inactive at transcription initiation sites to maintain negative supercoiling, which promotes duplex melting at promoters and transcription start sites, and that transcription pause–release requires Pol II-mediated activation of the DNA relaxation activity of TOP1 by bromodomain-containing protein 4 (BRD4)29.
Pause–release.
Regulated pausing and release of RNA polymerase II in promoter-proximal regions, which initiates productive transcription elongation.
The view that topoisomerase activity per se is not necessary at promoters was challenged by the observations62 that transcription at oestrogen-induced promoters required TOP2β and that a relatively long-lived DNA double-stranded break (DSB), induced by TOP2β, persisted during transcription. Notably, in addition to TOP2β recruitment to oestrogen-induced promoters, concomitant recruitment of DNA repair factors such as DNA-dependent protein kinase (DNA-PK) and poly(ADP-ribose) polymerase (PARP) was observed. Although TOP2β activity at certain promoters would not be surprising, the fact that very high levels of breakage were observed was unexpected, as TOP2-mediated relaxation involves very low levels of cleavage in biochemical systems63. The work did not indicate that the TOP2β-mediated cleavage resulted in breaks with genotoxic consequences, and it did not provide evidence that associated repair functions were required to repair TOP2β-induced breaks, although more recent evidence suggests that the formation of oestrogen receptor-induced breaks depends on TOP2β64. A more recent study reported that TOP2β is also recruited to the TMPRSS2 and ERG promoters in an androgen-dependent manner and is required for androgen receptor-dependent gene expression. Furthermore, a consequence of TOP2β-mediated cleavage at these promoters may be the generation of oncogenic TMPRSS2–ERG translocations65. As these translocations are common in prostate cancer, these results suggest that TOP2β activity at promoters may have a substantial role in human tumorigenesis66. Other studies have recently provided further evidence in support of elevated levels of TOP2β-mediated cleavage at promoters64,67,68.
What is responsible for the high levels of breakage induced by TOP2β at gene promoters? We consider three possibilities: first, TOP2β-induced breaks could be relatively rare or accidental. If this is correct, then the observations of TOP2β-mediated breakage fit our current understanding of topoisomerase biochemistry. Second, the breaks could be long-lived. This could happen when the TOP2β re-ligation reaction is temporarily blocked. Both the rare and long-lived TOPcc have the potential to become irreversible, in which case the TOPcc breaks would require repair. Last, the TOP2β-induced breaks could be permanent. In this scenario, the enzyme has been induced by an unknown mechanism to become a nuclease (in the same way that SPO11 functions as a nuclease to initiate meiotic recombination; see BOX 1).
The recording of TOP2β-mediated cleavage at gene promoters could be a technical artefact; for example, it could be a consequence of the assays used to measure TOP2β-induced breaks (primarily chromatin-immunoprecipitation-based assays), which might enrich for cleavage-complex intermediates that could eventually be resealed by the enzyme. In this case, the recorded chromosome aberrations could be explained by the existence of relatively rare DSBs or by the ability of repair enzymes to initiate the removal of a catalytically competent enzyme65. Results from the expression of neuronal genes in the mouse fetal brain have recently provided support for the existence of permanent breaks69. Tyrosyl-DNA phosphodiesterase 2 (TDP2; discussed in detail below), which is a repair enzyme that removes TOP2 that is covalently bound to DNA, is required to maintain the expression of certain neuronal genes as well as genes regulated by the androgen receptor70. Furthermore, a large number of neuronal genes exhibited altered (mainly reduced) expression in developing brains of Tdp2-knockout mice69. Notably, several of these genes are dependent on TOP2β for normal levels of expression71. Taken together, these results suggest that TOP2β-mediated breakage occurs frequently enough during transcription that the damage must be repaired to sustain normal levels of transcription.
A central question is why a long-lasting or permanent break is needed when DNA topoisomerases are intrinsically able to provide a swivelling function. The fact that a site-specific nuclease could substitute for TOP2β at a promoter67 indicates that a DSB is sufficient for transcription activation, but does not explain why a topoisomerase-induced ‘permanent’ DSB would be necessary. One possibility is that it may be needed to mobilize other proteins to promoters, such as other chromatin-remodelling enzymes. A possible advantage of using a topoisomerase to induce chromatin remodelling is that the DNA breaks may still be directly reversed by the topoisomerase before becoming irreversible (that is, permanent).
TOP1 also forms transcription-associated, long-lived DNA breaks at androgen receptor-regulated enhancers42 (FIG. 2). Like the TOP2β-induced breaks, nicking by TOP1 leads to the recruitment of the DNA repair machinery, including the MRE11–RAD50–NBS1 (MRN) complex and components of the base excision repair (BER) pathway, the heterodimer KU70–KU86 and DNA ligase IV of the non-homologous end-joining (NHEJ) pathway, and ataxia telangiectasia and Rad3-related protein (ATR). These observations support the importance of topoisomerase-linked permanent breaks during transcription activation.
The function of topoisomerases in cleaving DNA to generate a DNA damage signal and potentially permanent DNA breaks may not be confined to promoters and enhancers. A recent report showed that Pol II pausing occurs in part owing to the accumulation of superhelical tension and suggested that topoisomerase-mediated breaks may be necessary to release the stalled polymerase72. This brings forward the possibility that topoisomerases persistently cleave the DNA at a much greater frequency than previously anticipated, which could pose threats to genomic stability in the absence of efficient repair (see below). A convincing explanation as to why a persistent break provides a better swivel than a topoisomerase is still lacking.
Transcription elongation
Although there is no global effect of either mutated TOP1 or mutated TOP2 on transcription, the transcription of long genes (in yeast, greater than ~3 kb) is greatly reduced when Top2 is inactivated73. In human cells, to be fully active, TOP1 needs to be phosphorylated74, and a recent study showed that pause–release of Pol II requires the direct activation of TOP1 by Pol II upon the phosphorylation of Pol II by BRD4 (REF. 29). These observations demonstrate that TOP1 and Pol II activities are coupled. Studies in mouse and human cells also point towards a combined role of TOP1 and TOP2β in transcription elongation at long genes. Indeed, trapping TOP1 in a cleavage complex using camptothecin or its clinical derivative topotecan (see below) in cancer cells inhibits transcription elongation75 with increasing efficiency as the genes become longer and contain more introns76. Notably, neuronal cells treated with topotecan also show strong inhibition of expression of long genes (>200 kb) linked to autism77 and synaptic function78. The effect of topotecan is probably not due to TOP1-induced DNA damage because depletion of TOP1 or of TOP2β elicited a similar inhibitory effect on transcription77. These experiments indicate that efficient expression of long genes requires both TOP1 and TOP2 enzymes. Interestingly, an analysis77 found that TOP2β-dependent neuronal genes71 tended to be long genes as well.
Inhibition of transcription elongation by topoisomerase inhibitors has unanticipated therapeutic implications. A screen of small molecules was carried out to identify potential therapeutic agents for Angelman syndrome79. Angelman syndrome arises from point mutations or deletions in the maternal allele of the ubiquitin ligase-encoding gene UBE3A (ubiquitin protein ligase E3A). UBE3A is biallelically expressed in most tissues, but only the maternal allele is expressed in neurons80. Individuals carrying a mutated, maternally derived UBE3A allele exhibit symptoms of Angelman syndrome, with severe neurodevelopmental defects, and the purpose of the screen was to identify molecules that could reactivate the paternal allele. Surprisingly, the TOP1 inhibitor irinotecan was the only US Food and Drug Administration (FDA)-approved drug to come out of the screen79. Further testing extended this result to the other TOP1 inhibitors as well as TOP2 inhibitors, which all led to the indirect reactivation of the silenced allele in primary mouse cortical neurons through inhibition of the transcription of a long, cis-acting antisense transcript derived from the paternal Ube3a locus termed Ube3a-ATS79. A related study has provided further mechanistic insight, showing that topotecan acts not just by trapping TOP1 and forming a physical impediment to elongation ahead of the transcription complex but also by sequestering TOP1, thereby inhibiting its enzymatic activity and inducing the formation of R loops behind the transcription complex in CpG-rich regions, which block the Pol II complex (see below) before it reaches the Ube3a-ATS locus81. Indeed, without TOP1 (or TOP2) to eliminate the negative supercoiling generated by the unwinding of the DNA template behind Pol II, the nascent transcript tends to remain associated with its template, thereby forming R loops (FIG. 2).
Transcription-mediated R-loop formation
At the transcription bubble, nascent transcripts are normally bound to their template only over a short stretch, on average 8 nucleotides or less. Longer RNA–DNA hybrids are termed R loops, and these have the capacity to stop the elongating polymerase82. Certain genomic regions are prone to R-loop formation, such as unmethylated CGIs, the 3′ end of genes, ribosomal DNA loci, highly transcribed genes and recombination sites. R loops are actively removed by RNases H1 and H2, as well as by helicases, including SNX and DEAH box protein 9 (DHX9; also known as RHA)83. As discussed above, TOP3β can suppress R-loop formation in association with TDRD3 (REFS 54,84); purified TOP3β resolves R loops by cleaving the unpaired DNA strand54,85 (FIG. 1f).
Loss of Top1 and Top3 activity in yeast86–88 and reduced activity of TOP1 and TOP3β in human cells54,84,89 lead to an accumulation of R loops. Poisoning of TOP1 by camptothecins also effectively leads to the formation of R loops that give rise to DSBs and activate an ataxia telangiectasia mutated (ATM)-mediated DNA damage response90,91. These R loops could result from a deficiency in TOP1 catalytic activity, but it is plausible that the R loops are directly associated with the trapped TOP1cc and with TOP1cc-induced Pol II arrest91,92. The mechanisms by which R loops give rise to DSBs and activated ATM are not clearly established, although they have been proposed to involve transcription-coupled nucleotide excision repair factors84. In addition, TOP1cc could facilitate the formation of short transcripts, such as antisense transcripts, which could serve as R-loop components93,94.
Topoisomerases in DNA replication
The roles of topoisomerases in replication derive extensively from work carried out in Escherichia coli, which has shown that topological constraints during replication elongation are managed by the concerted actions of DNA gyrase, which relaxes positive supercoils ahead of the replication fork, and the DNA decatenation activity of Topo IV95. Our initial understanding of topoisomerase functions in eukaryotic replication (and chromosome segregation) was based on studies in yeast, in which budding and fission yeast have provided contrasting perspectives. Early experiments in topoisomerase-deficient mutants suggested that neither Top1 nor Top2 was absolutely required for replication initiation or elongation, whereas Top2 was absolutely required for the separation of fully replicated chromosomes. Cells lacking both Top1 and Top2 activities were severely but not entirely deficient in replication elongation58,96,97, leading to Rad53-dependent DNA damage checkpoint activation97. These observations led to a model in which either topoisomerase could relieve positive supercoiling arising from replication fork progression. As we discuss below, the function of individual topoisomerases during ongoing replication and how topoisomerases are recruited to sites of replication initiation and elongation turned out to be subtler than originally described.
Roles of topoisomerases in replication initiation
TOP1 and TOP2 enzymes have been found localized to origins of DNA replication98–100; however, studies in metazoan cells have not demonstrated a direct role for any topoisomerase in replication initiation (reviewed in REF. 101). Xenopus laevis oocyte extracts are proficient in replication initiation when Top2α is depleted (such extracts do not contain Top2β). In this system, Top2α was suggested to suppress the licensing and firing of replication origin clusters by interfering with the loading of the MCM2-7 replicative helicase at the origin102. In mammalian cells, conditional knockdown of both TOP2α and TOP2β simultaneously did not affect replication initiation or elongation103. Taken together, these results show that type 2 topoisomerases are not required for replication initiation. It is possible that topoisomerases need to be kept inactive at replication origins to maintain negative supercoiling and to allow priming from the single-stranded origin template30 (FIG. 3a).
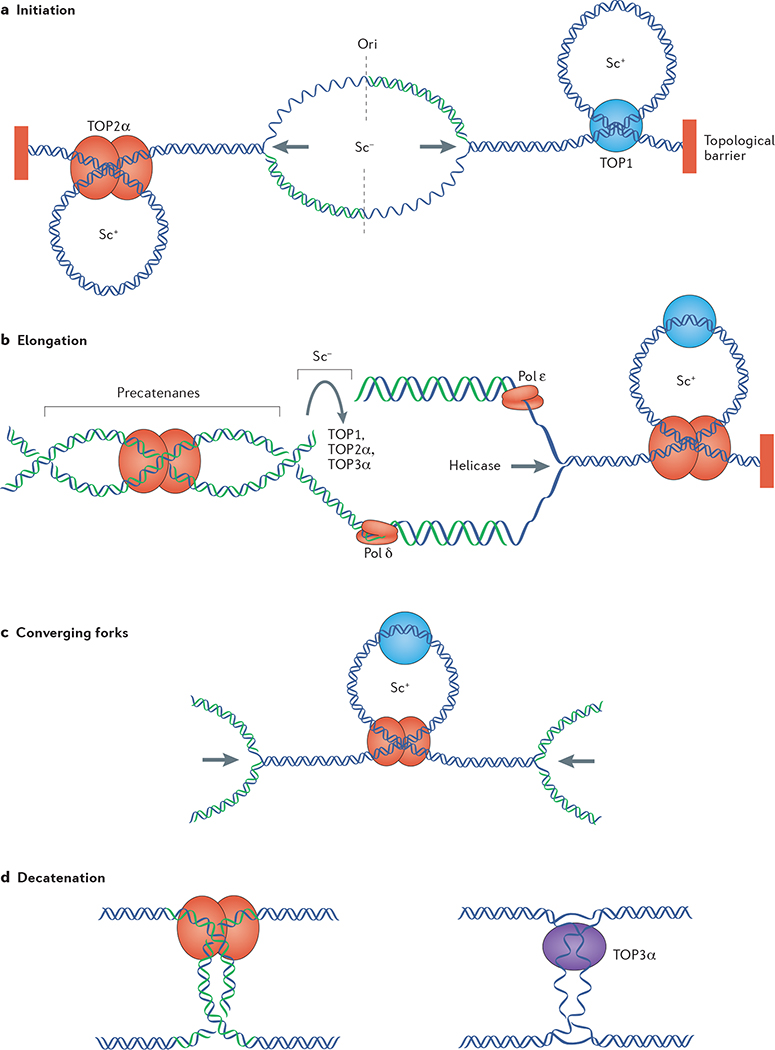
Figure 3 |. Functions of topoisomerases in DNA replication.
a | Initiation of DNA replication requires separation of the two parental strands, which generates negative supercoiling (Sc−) at the origin of replication «and positive supercoiling (Sc+) in the flanking regions owing to topological barriers, such as nuclear matrix attachment sites or insulators, where the DNA is not free to rotate around its axis. Positive supercoiling is dissipated by TOP1 and TOP2α to allow replication fork progression (indicated by the arrows). b | Replication elongation generates positive supercoiling ahead of the replication fork and negative supercoiling behind it. Positive supercoiling is removed by TOP1 and TOP2α, whereas negative supercoiling can be removed by TOP1, TOP2α or TOP3α. TOP2α can also remove precatenanes, which are formed when the fork rotates during elongation. c | Converging forks generate high positive supercoiling between them. d | Upon replication completion, catenanes are removed by TOP2α (left) and hemicatenanes are removed by TOP3α (right). Pol, DNA polymerase.
Replication origin clusters.
Replication origins that tend to fire in coordinated clusters and usually in the same genomic region during S phase.
One potential function for topoisomerases in replication initiation may be blocking of aberrant replication initiation. Reconstituted in vitro bacterial replication systems require a topoisomerase to prevent erroneous initiation at sites of R loops other than replication origins104. Recent results in yeast suggest that topoisomerases may perform similar functions in eukaryotes. Yeast cells lacking RNase H activity, which is necessary for resolving R loops, exhibit aberrant replication initiation in ribosomal genes and absolutely require Top1 for viability105, which suggests that the high levels of transcription in the ribosomal gene cluster leads to elevated levels of supercoiling that enhance R-loop formation. This effect is circumvented by the degradation of the RNA in R loops by RNase H, and accentuated by elevated supercoiling that arises when Top1 is absent. However, it is possible that the synthetic lethality observed in cells lacking Top1 and RNase H may arise from other genotoxic properties of R loops90.
Replication elongation
As the replication fork progresses, positive supercoiling accumulates ahead of the replication fork, which can be relaxed by either TOP1 or TOP2 (FIG. 3b). Most models have postulated that TOP1 acts ahead of the fork and that TOP2 acts behind the fork. Trapping of TOP1 by camptothecin leads to replication stalling and replication fork run-off, a result consistent with TOP1 acting ahead of the fork106. Of note, TOP2α has a distinct preference for relaxation of positively supercoiled DNA63, suggesting that it too may be capable of acting in front of the replication fork.
Replication fork run-off.
A consequence of replication fork collision with a TOP1 cleavage complex (TOP1cc) on the leading strand template, resulting in the formation of an irreversible TOP1cc and a DNA double-stranded end.
Rotation of the replication fork can alleviate the superhelical tension, but it also leads to interweaving of the daughter chromatids behind the replisome107,108 (FIG. 3b). The resulting sister chromatid intertwines are commonly referred to as precatenanes. Formally, precatenanes are not topologically equivalent to catenanes, as they are present in structures that contain DNA strand breaks at the rear of the DNA polymerases and therefore are not unique topological isomers of DNA. However, upon the completion of replication, precatenanes that are not removed will be converted to catenanes109 that will need to be removed by TOP2α109,110.
Catenanes.
Topologically interlocked cyclic DNA molecules. Long, linear DNA molecules can approximate these properties and be referred to as catenated, even though this is not formally correct.
What drives the choice between relaxation of positive supercoils ahead of the replication fork versus fork rotation that generates precatenanes? Relaxation of positive supercoiling is likely to be simpler because fork rotation requires the dislocation of large replication machineries, which probably impedes their efficient movement. However, relaxation of positive supercoiling is sometimes not possible. For example, when replication forks converge (FIG. 3c), physical constraints may prevent access of topoisomerases to DNA ahead of the forks111,112. There are several other contexts in which fork rotation seems to be enhanced, including at heterochromatin and especially at centromeric heterochromatin (reviewed in REF. 113). The regions where fork rotation is favoured would be expected to be enriched for catenanes and hemicatenanes, which if not resolved by TOP2 or TOP3 enzymes114, are potentially at risk of incomplete resolution before mitosis. However, if the catenation of DNA needs to be maintained for proper chromosome structure and sister chromatid cohesion (reviewed in REF. 113), then the mechanics at replication forks might regulate precatenane formation to ensure catenation at appropriate locations.
Hemicatenanes.
A junction of two homologous DNA double helices, in which one strand of a duplex is interlinked with the complementary strand of the other duplex. Unlike catenanes, hemicatenanes can occur in linear DNA molecules.
Recently, replication pause sites in yeast were found to stimulate fork rotation (and therefore the generation of precatenanes)115. Replication pause sites are frequently associated with very stable protein–DNA complexes. An intriguing hypothesis is that persistent protein–DNA complexes can restrict the accessibility to DNA or action of topoisomerases that normally relax positive supercoiling, thereby stimulating fork rotation instead. The Tof1–Csm3 heterodimer is part of the fork protection complex116 and minimizes fork rotation, which is consistent with the observations that Tof1–Csm3 restricts replication rates by binding to pause sites117 and that Tof1 is a Top1-binding partner43. Also, the human orthologue of the Tof1–Csm3 heterodimer, Timeless–Tipin, is required for replication elongation and for avoiding fork breakage118,119, potentially by regulating TOP1 activity and preventing replication fork collapse at TOP1 cleavage sites43,119.
Fork protection complex.
A complex consisting of Timeless, Tipin and other components that stabilizes stalled replication forks and helps to coordinate leading- and lagging-strand synthesis and replication checkpoint signalling.
Topological tension may not be dependent only on local chromosomal features. Studies in budding yeast suggested that long chromosomes accumulate greater superhelical stress than short chromosomes120. Of note, deletion of TOP1 results in defects in the replication of long, but not short, chromosomes121. Similar negative effects on replication were seen in long chromosomes in cells lacking components of the condensin-related complex, Smc5–Smc6 (REF. 121). These results might be explained by enhanced levels of precatenane formation in the absence of functional TOP1 and condensin, which is involved in the resolution of precatenanes. Considering that catenanes are genotoxic115, these results suggest that TOP1 is also acting ahead of the fork to relax positive supercoils, thereby attenuating replication rotation and precatenane formation behind the forks.
Termination and chromosome segregation
Several studies implicate both TOP2 and TOP3 enzymes in replication termination and sister chromatid separation99,114,122–124 (FIG. 3d). TOP2 is required particularly at the point where replication forks converge112. It should be noted that type 1A topoisomerases probably contribute to decatenation following replication termination at least in some contexts, but type 2 topoisomerases are probably the most important decatenases. In yeast, Top2 was present at sites of replication termination122 and catalytically inactive Top2 interfered with the completion of replication. Subsequently, Top2 was found to be recruited to yeast termination region (TER) sites before the arrival of replication forks, to promote fork fusion125. Top2 was thus proposed to decatenate sister chromatids following fork fusion125, as its activity is required to preserve genome integrity, presumably by allowing completion of replication, and to prevent breakage of chromosomes at subsequent steps of cell division. In addition to the recruitment of Top2 to replication fork barriers and TER sites125, Sgs1, the yeast orthologue of the human BLM helicase, promotes Top2-mediated decatenation126. RECQL5, a mammalian RecQ helicase, also facilitates human TOP2a-dependent separation of interlocked sister chromatids by interacting with TOP2α during S phase127. The interaction of BLM with TOP2α seems to be important for BLM function during homologous recombination128, and it has also been suggested to be relevant for chromosomal decatenation129.
What are the cellular consequences of a failure to properly decatenate chromosomes? When Top2 activity is experimentally abolished in yeast, chromosomes fail to separate and mitosis is blocked, possibly leading to endoreduplication4,130 and ultimately to cell senescence or cell death131. In other cases — for example, when replication cannot be completed during S phase — the unreplicated chromosomal regions remain connected by UFBs, which were first identified as structures coated with BLM132. Subsequently, Polo-like kinase-interacting checkpoint helicase (PICH; also known as ERCC6L) was shown to localize to UFBs with BLM133. UFBs have some, but not all, of the characteristics of more conventional chromatin bridges, but they arise frequently even in what appear to be normal mitoses. The DNA in conventional chromatin bridges is readily stained by DNA intercalating dyes and is bound by histones, whereas UFBs cannot be visualized by DNA staining133. UFBs appear to arise at specific loci, especially centromeres. It is noteworthy that at least some of the specific loci are sites that may accumulate precatenanes. What is the evidence that UFBs arise from unresolved catenation? TOP2 inhibition greatly increases the number of UFBs, and the distribution of UFBs to specific loci becomes less well defined133.
Endoreduplication.
Repeated replication of the nuclear genome in the absence of cell division; it can lead to polyploidy.
The finding that UFBs arise in part from uncompleted decatenation is consistent with TOP2α being crucial for avoiding their formation134. Interestingly, there seem to be several factors that participate in recruiting TOP2α to UFBs, including BLM and PICH50 and probably TOPBP1 (REF. 51). TOP3α, which is known to interact with BLM, has single-stranded DNA decatenase activity and resolves double Holliday junctions, and may also assist in the resolution of UFBs. Mutations in the chromatin-remodelling BAF complexes, which are frequently found in human cancers135, resulted in the frequent formation of UFBs, a predicted consequence of incomplete decatenation of chromosomes33. The study also showed that TOP2α associates with the BAF250 subunit and that a deficiency of BAF complexes reduces the recruitment of TOP2α to a large number of chromosomal sites33. These results provide some of the strongest evidence that failure to properly decatenate chromosomes can be oncogenic and that topoisomerases are tightly regulated by chromatin-remodelling complexes.
Given the importance of topoisomerases in cell division, topoisomerase deficiency during cell division was proposed to lead to cell cycle arrest. Treating cells with the TOP2 catalytic inhibitor ICRF-193 or its derivatives slows (but does not block) progression through mitosis136–139. This observation suggested that cells might monitor the separation of catenated DNA molecules before mitosis. Interestingly, cells that go through mitosis in the complete absence of a type 2 topoisomerase, and therefore have highly catenated DNA molecules, do not show mitotic delay, leading to the suggestion that TOP2 is required for a ‘topoisomerase checkpoint’ (REFS 122,140). Several genes have been reported to be involved in what has also been termed a decatenation checkpoint, including the tumour suppressors BRCA1 (REF. 141) and PTEN142 and genes required for the spindle assembly checkpoint143. It is likely that abrogation of checkpoints that ensure complete chromosome decatenation has the potential to generate severe genomic instability.
Topoisomerases and chromosome condensation
After completing replication, chromosomes undergo compaction (condensation) as a prerequisite for entering mitosis. TOP2α has been identified as a main component of the chromosome scaffold44. The roles of TOP2 in chromosome condensation have been reviewed very recently113,144,145 and are not described in detail here. The salient features have recently been elaborated in an elegant and simple in vitro reconstitution45. One key feature that deserves comment here is the utilization of positive supercoiling that is mediated by condensin to ensure complete chromosome decatenation. Positive supercoiling takes place on catenated centromeric plasmids when Saccharomyces cerevisiae cells undergo mitosis. Top2 is directed to the interlinked DNA and decatenates the molecules in response to mitotic positive supercoiling, suggesting that DNA topological constraints impel Top2 to catalyse decatenation to ensure successful cell division146. Also, in budding yeast, longer chromosomes and topological stress induced by perinuclear attachment of telomeres promote the retention of intertwined sister chromatids interlocked at the ribosomal DNA array, leading to late resolution of this locus by Top2 at anaphase120.
Mitochondrial topoisomerases
Mitochondrial DNA (mtDNA) is a circular, double-stranded genome (16.5 kb long in humans), which is packaged into DNA–protein assemblies called nucleoids that are attached to the mitochondrial inner membrane in the matrix space. Because cells contain multiple mtDNA copies (thousands in a muscle cell)147, mtDNA contributes up to 30–50% of the overall protein-coding genome. The mtDNA circular structure, its attachment to the inner mitochondrial membrane and its high level of transcription from twin promoters located in a short non-coding region explain the need for mtDNA maintenance by topoisomerases.
Mitochondrial topoisomerases are encoded in the nuclear genome. In vertebrates, two of them are specifically directed to mitochondria: TOP1mt (FIG. 1) and a long TOP3α isoform. Both contain a mitochondrial-targeting sequence (MTS) at their amino terminus. The TOP3A gene encodes both mitochondrial TOP3α and nuclear TOP3α. Mitochondrial TOP3α is produced by an alternative, upstream translation initiation site22. In addition, two other topoisomerases were recently found in human and mouse mitochondria, TOP2α and TOP2β23, which means that no less than four topoisomerases handle mtDNA. The presence of TOP2α and TOP2β in mitochondria23 is probably related to the fact that neither TOP1mt nor TOP3α can decatenate circular duplex DNA following mtDNA replication.
Why do animal mitochondria require so many different topoisomerases, especially considering that in yeast topoisomerases for mitochondrial replication and transcription are unknown? The answer to this question is beginning to emerge from genetic models for TOP1mt and TOP3α. Top1mt-knockout mice have no obvious phenotype but, when challenged with the TOP2 inhibitor doxorubicin, they develop lethal cardiotoxicity with profound alterations of mitochondria ultrastructure and mtDNA copy number148. Furthermore, when Top1mt-knockout mice are challenged with a liver toxin (carbon tetrachloride), they fail to rapidly regenerate their liver and exhibit increased mitophagy149. Both phenotypes suggest that TOP1mt is important for mtDNA replication under conditions in which an organ needs to couple its mtDNA mass with rapid cellular proliferation. In addition, mouse embryonic fibroblasts generated from Top1mt-knockout mice have increased mtDNA negative supercoiling, implying a selective role for TOP1mt in relaxing the negative supercoiling of mtDNA. Thus, TOP1mt is not essential but appears to be crucial for mtDNA replication and structure under certain metabolic conditions.
Genetic studies in Drosophila melanogaster are revealing the specific functions of Top3α in mitochondria. Lack of Top3α is early embryonic lethal. Viability can be rescued by complementation with the short, nuclear Top3α, but these flies, which lack the longer mitochondrial Top3α, are sterile because they lose germline stem cells150. In addition, they have a shortened lifespan and age-related loss of mtDNA and mitochondrial mass151. Thus, these results show that Top3α is crucial for gamete production and mtDNA genomic stability. These functions may relate to the role of TOP3α in mtDNA replication, as the mtDNA regulatory D-loop region contains single-stranded DNA junctions, which are TOP3α substrates.
D-loop.
A DNA structure formed when the two strands are separated and held apart by a third strand that is complementary to one of them. The other strand is displaced and forms a D (DNA) loop.
The role of topoisomerases in mitochondria remains understudied and, to date, the only inhibitors of mitochondrial topoisomerases — lamellarin D for TOP1mt and TOP1 (REF. 152) and doxorubicin for TOP2α and TOP2β148 — poison both the mitochondrial and nuclear topoisomerases. However, based on genetic evidence, it is plausible that TOP1mt and TOP3α mechanistically connect mitochondrial diseases and cardiac and neurological defects, as well as premature ageing.
TOPcc, genomic integrity and disease
A lack of topoisomerase activity may result in failure to complete replication, excess supercoiling, persistent catenation and even knots in DNA. Cells underexpressing TOP1 or TOP1mt exhibit hypernegative supercoiling in their nuclear or mitochondrial genomes, respectively23,34. Insufficient TOP1 activity can induce genomic breaks153 and could generate interference between transcription and replication89. Elevated DNA damage is observed in cancer cells with insufficient SMARCA4 owing to defective recruitment of TOP1 and TOP2α to chromatin33,34. Inactivation of the TOP3β cofactor TDRD3, which serves as a bridge between TOP3β and Arg-methylated histones52,53, leads to TOP3β depletion and increases R-loop formation and chromosome translocations involving the MYC gene54. Moreover, knocking out mitochondrial Top3α results in accelerated ageing, sterility and neurological deficiencies in flies151. The importance of topoisomerases in genomic maintenance may also explain why cancer cells, which are under replicative and transcriptional stress, frequently overexpress both nuclear and mitochondrial topoisomerases (The Cancer Genome Atlas (TCGA) data).
Poisoning topoisomerases by therapeutic agents
Topoisomerase-induced DNA damage is the mechanism of action of a large number of anticancer drugs, which trap TOPcc and thereby are used to induce DNA damage for therapeutic purposes (TABLE 1 and reviewed in REFS 4,9,154,155). The selectivity of topoisomerase poisons for cancer cells remains incompletely understood. The main (not mutually exclusive) hypotheses are that cancer cells overexpress (TCGA data) and rely more on TOP1 and TOP2α for survival, and that DNA damage repair pathways are defective in cancer cells.
Table 1 |.
Drugs, DNA alterations and physiological processes that lead to the formation of persistent TOPcc
| Causes | Consequences for TOP1 enzymes | Consequences for TOP2 enzymes |
|---|---|---|
| Anticancer drugs acting as interfacial inhibitors155 | Trapping of TOP1cc by irinotecan, topotecan, indenoisoquinolines* and tumour-targeting camptothecin derivatives3,154,155 | Trapping of TOP2cc by etoposide, teniposide, doxorubicin, epirubicin, idarubicin and mitoxantrone4 |
| Oxidative DNA lesions (8-oxoguanine, 8-oxoadenosine and 5-hydroxycytosine) | Induction and trapping of TOP1cc218,219 | Induction and trapping of TOP2cc220 |
| Abasic sites and DNA mismatches | Formation of irreversible TOP1cc221 | Formation of irreversible TOP2cc220,222–225 |
| Carcinogenic base adducts (methylated bases, exocyclic adducts, benzo[a]pyrene adducts and crotonaldehyde adducts) | Induction and trapping of TOP1cc226–232 | Induction and trapping of TOP2cc220,233–235 |
| Nicks and DNA strand breaks | Formation of irreversible TOP1cc, double-stranded breaks, genomic deletions and recombination18,167,168,236,237 | Formation of irreversible TOP2cc235 |
| UV lesions (pyrimidine dimers and 6.4-photoproducts) | Induction of TOP1cc238,239 | Enzymatic inhibition240 |
| Ribonucleotide incorporation into DNA | Formation of TOP1cc that generate nicks with 2′,3′-cyclic phosphate ends and short deletions in repeat sequences166–168 | Stabilization of TOP2cc with asymmetrical cleavage20,169,241 |
| Natural and food products | Unknown | Stabilization of TOP2cc by flavones, tea and wine products205 |
| Genetic defects | Unrepaired TOP1cc due to TDP1 defects177,206,210 in cooperation with ATM defects179 | Unrepaired TOP2cc due to TDP2 defects69 |
| Transcription activation | Stabilization of TOP1cc at enhancers42 | Stabilization of TOP2cc at promoters62,65,242,243 |
ATM, ataxia telangiectasia mutated; TDP, tyrosyl-DNA phosphodiesterase; TOPcc, topoisomerase cleavage complex.
Indenoisoquinoline derivatives are in clinical trials.
Because TOP1 is covalently linked to DNA 3′ ends (FIG. 4a), TOP1 inhibitors act as replication poisons by generating replication-induced double-stranded ends when a replication fork collides with a trapped TOP1cc, thereby generating a ‘replication run-off’ lesion106 (FIG. 4b). Replication fork reversal at these blocking sites was also proposed to generate ‘chicken foot’ lesions156 (FIG. 4c), which may be resolved by the MUS81–EME1 endonuclease157,158.
Figure 4 |. Topoisomerases and DNA damage.
a–c | Collision of a replication fork into a TOP1 cleavage complex (TOP1cc; part a) produces replication run-off (part b) in which newly replicated DNA (red) is extended by DNA polymerases up to the 5′ end of the broken DNA, thereby generating a double-stranded end (DSE), which is repaired by homologous recombination. Alternatively, replication fork reversal regenerates a reversible TOP1cc and produces a ‘chicken foot’ structure (part c). d–i | Processing of TOP1cc can produce DNA damage. The −1 base to which TOP1 is covalently linked to cleave DNA (FIG. 1a) is shown as a thick black bar; the red lines represent newly synthesized DNA strands. Efficient re-ligation of TOP1cc relies on stacking and hydrogen bonding with the +1 base (FIG. 1a), which is disrupted by oxidative base damage and mismatches (part d) (TABLE 1). The presence of an abasic site at the +1 position interferes with re-ligation by the 5′-hydroxyl end owing to loss of base stacking and hydrogen bonding (part e). A TOP1cc 5′ end and a pre-existing nick result in loss of the DNA segment between them (gap) and can lead to deletions due to the efficient re-ligation activity of TOP1 (part f). Formation of a TOP1cc at a bulge or a loop can readily generate a stretch of single-stranded DNA at the gap (part g). A TOP1cc opposite to a nick results in a DNA double-stranded break (DSB; part h), which can be resealed by TOP1 (part i) with another broken DNA end (red), thereby generating mutations. j–o | Following ribonucleotide incorporations into the DNA (shown in red) during replication (part j), a TOP1cc forming at a ribonucleotide site (part k) is reversed by nucleophilic attack of the 2′-ribose hydroxyl, which generates a 2′,3′-cyclic phosphate (2′,3′-CP) end (indicated by the arrowhead) with the release of catalytically active TOP1 (part l). Sequential cleavage at a nearby nucleobase by the released TOP1 or by another TOP1 can generate a short deletion (part m) when the TOP1 forms a cleavage complex on the same strand, upstream of the 2′,3′-CP. Alternatively, endonucleolytic cleavage (not shown) or excision of the TOP1cc by tyrosyl-DNA phosphodiesterase 1 (part n) followed by gap filling can repair the DNA while eliminating the ribonucleotide. When the sequential cleavage by a TOP1 is on the opposite strand to the 2′,3′-CP, a DSB is formed (part o).
‘Chicken foot’ lesions.
DNA structures in which newly replicated nascent strands anneal to each other following the retraction of the replication fork. The structure looks like a chicken foot.
Another consequence of persistent TOPcc is the stalling of transcription complexes. As discussed above, TOP1 inhibitors efficiently block transcription elongation, accounting for the preferential impact of TOP1cc on long and on highly transcribed genes76,77,79. Although transcriptional inhibition is believed to contribute only marginally to the anticancer activity of topoisomerase inhibitors, it may offer therapeutic potential for neurological and immune diseases, as demonstrated in animal models of Angelman syndrome79. Similarly, transcription suppression by TOP1 inhibitors was recently shown to protect mice against viral and bacterial infections by suppressing the lethal overexpression of inflammatory immune response genes159.
Poisoning of topoisomerases by DNA alterations
DNA is reactive to endogenous metabolites and exogenous carcinogens160. It is now well established that many DNA alterations interfere with topoisomerase reactions and frequently give rise to elevated levels of TOPcc and DNA damage (FIG. 4; TABLE 1). It is notable that many of these DNA alterations are frequent. Oxidative lesions have been estimated to occur at a rate of approximately 150,000 per cell per day, and base modifications, base losses and single-stranded breaks have each been estimated to occur at a rate of several thousands and up to 105 lesions per cell per day160–163.
Incorporation of ribonucleotides into the DNA by replicative and repair DNA polymerases has emerged to be even more frequent than oxidative lesions, with up to one ribonucleotide incorporated per thousand bases during normal replication in yeast164,165. RNase H2 is the enzyme that efficiently removes these ribonucleotides; however, if TOP1 binds to them before RNase H2, they can be converted into nicks after TOP1 cleavage at the ribose166,167 (FIG. 4j–l). These nicks are generated by nucleophilic attack from the 2′-ribose hydroxyl, which releases TOP1 and generates a 3′ end consisting of a 2′,3′-cyclic phosphate (FIG. 4k). Such nicks cannot be extended by DNA polymerases or joined by ligases166–168. The ribonuclease activity of TOP1 has recently been shown to generate TOP1-mediated short deletions in transcribed sequences that contain short repeats following sequential cleavage by TOP1 upstream from the nick167,168 (FIG. 4m). In addition, it is plausible that TOP1-generated 2′,3′-cyclic phosphate nicks could readily give rise to DSBs when a second TOP1cc forms on the strand opposite to the nick (FIG. 4o). In contrast to TOP1 enzymes, TOP2 and TOP3 enzymes cannot generate nicks at newly incorporated ribonucleotides because of their covalent linkage to the DNA 5′ end, which is too far from the 2′-ribose hydroxyl to release TOP2 or TOP3. However, TOP2cc that form on a ribonucleotide tend to reverse slowly and may also be an important source of genomic alterations20,169.
Repair of TOPcc
Two alternative pathways can remove TOPcc: the TDP excision pathway and the nuclease pathway (FIG. 5). TDP1 and TDP2 have been recently reviewed9,170,171, and we highlight only some points here. The redundancy of the repair pathways is exemplified in yeast, in which knocking out TDP1 results in only weak hypersensitivity to Top1 poisons unless a second pathway, such as the Rad9 cell cycle checkpoint172 or the endonuclease complex Rad1–Rad10 (XPF–ERCC1 in humans; see below), is also inactivated173,174. The substrate specificity of TDP1 extends beyond the repair of nuclear TOP1cc and TOP2cc. TDP1 excises a broad range of 3′-end-blocking lesions, including 3′-phosphoglycolates and chain-terminating nucleoside analogues, both in the nucleus and in mitochondria175,176. The phenotype of TDP1 inactivation depends on the type of mutation (as in the case of the TDP1 mutation that occurs in spinocerebellar ataxia with axonal neuropathy (SCAN1)177), the specific experimental model (gkt (the TDP1 orthologue) deficiency in Drosophila spp. leads to neuronal defects and a shortened life expectancy178) and the presence of other mutations in parallel repair pathways (such as in the ATM pathway179). The removal of TOP1cc by TDP1 leaves a 3′-phosphate, which prevents TDP1 from removing another nucleotide and acting as an exonuclease180,181. However, the 3′-phosphate end cannot be readily processed by DNA polymerases and ligases unless it is first dephosphorylated by polynucleotide kinase phosphatase (PNKP). This explains the importance of PNKP and its scaffolding partner XRCC1 in the repair of TOP1cc182. TDP1 also interacts with and is stabilized by PARP1 at TOP1cc sites183, implicating multiple factors (and possibly pathways) in the repair of TOP1cc by TDP1. Among them, the most dominant pathway for TOP1cc repair in yeast and mammalian cells involves homologous recombination, owing to the formation of DSBs that are produced by the collision of replication forks with TOP1cc (FIG. 4b). Indeed, TOP1cc are toxic in cells with inactivating mutations of homologous recombination components, including Rad50, Rad52 and Mre11 in yeast184 and BRCA1, BRCA2, XRCC2, XRCC3 and RAD52 in chicken DT40 cells185. The interplay between homologous recombination, NHEJ and PARP1 remains to be clarified, as Tdp1 is epistatic with Rad52 in yeast186.
Figure 5 |. TOPcc repair.
a | Tyrosyl-DNA phosphodiesterase 1 (TDP1) and TDP2 (although much less efficiently and therefore shown in parentheses) cleave the TOP1 tyrosyl–DNA covalent bond (middle), releasing TOP1 and leaving a 3′-phosphate end (right) that needs to be further processed by polynucleotide kinase phosphatase (not shown). b|TOP2 cleavage complexes (TOP2cc) are preferentially repaired by TDP2 and much less efficiently by TDP1 (middle) in vertebrates, releasing TOP2 and leaving a 5′-phosphate (right), which can be readily ligated. Yeast, which do not encode a TDP2 orthologue, use Tdp1 to excise both Top1cc and Top2cc. In the endonuclease pathways (left), topoisomerases are released with the segment of DNA to which they are attached by the action of endonucleases; the polarity is opposite for TOP1cc (part a) and TOP2cc (part b).
Epistatic.
A genetic phenomenon in which an observed phenotype depends on the presence of other genes. Epistatic interactions can arise when different genes encode components of a complex or when two genes function in a linear biochemical pathway.
TDP2 was discovered as a second enzyme that could reverse tyrosyl-linked protein adducts in vertebrate cells70,187. Originally identified as a factor that could repair TOP1–DNA covalent complexes, subsequent studies suggested greater in vitro activity on 5′-tyrosyl–DNA complexes such as TOP2cc and viral replicative intermediates188,189. Following the removal of TOP2 from the DNA 5′ end and regeneration of a 5′-phosphate (FIG. 5), direct re-ligation of the ends can be carried out by end-joining. This directly provides a substrate for NHEJ and would be consistent with the hypersensitivity of cells deficient in the NHEJ factors KU70, KU80, ligase IV or DNA-PK catalytic subunit to TOP2cc185,190, and with the epistatic relationship between TDP2, KU factors and NHEJ191. Yet, as in the case of TOP1cc, homologous recombination also has a role in the processing of TOP2cc190. Taken together, the current literature regarding mammalian cells suggests a simple hypothesis: TDP1 repairs mainly TOP1cc, and TDP2 repairs mainly TOP2cc. Nonetheless, it seems likely that there may be additional specializations (perhaps at the level of recruitment) that dictate which TDP is used.
The alternative pathways for the release of TOPcc rely on endonucleolytic cleavage of the DNA strand to which the topoisomerases are attached171 (FIG. 5). Because these pathways excise the DNA flanking the cleavage complexes, gap-filling polymerases and homologous recombination must restore the integrity of the genome. The role of the 3′-flap endonuclease complex Rad1–Rad10 (XPF–ERCC1) in the repair of TOP1cc was characterized in yeast173,174 and human cells192 and reconstituted in biochemical systems193. Similarly, the 5′-flap endonuclease XPG has been suggested to repair TOP2cc194,195 and the 3′–5′ exonuclease Mre11 could have a role in the excision of both Top1cc and Top2cc196,197. Consistent with the importance of homologous recombination in the repair of Top1cc, the homologous recombination endonuclease CtBP-interacting protein (CtIP; also known as RBBP8) and its yeast orthologue Sae2 (REF. 198) are also involved in the repair of TOP1cc185.
The choice and balance between repair by TDPs or by endonucleases require further elucidation. The ubiquitin–proteasome system appears to be required for efficient TDP activity20,180, which is plausible considering that the tyrosyl–DNA bonds are buried inside the TOPcc and have to be exposed to allow access to TDPs. By contrast, nucleases may be less susceptible to this proteolytic requirement, as they might cleave the DNA some distance away from the TOPcc.
TOPcc in human diseases
A potential aetiological role is suggested for TOPcc in a growing number of human pathological conditions, including cancers, neurodegenerative diseases and autoimmune syndromes. The connection between TOPcc and cancer was first established for secondary leukaemias induced by cancer-targeting TOP2 inhibitors; this has been extensively reviewed199,200 and is not discussed in detail here. Briefly, TOP2β has been proposed as the major cause of etoposide-induced secondary leukaemias201,202, which develop through the formation of oncogenic translocations initiated by trapped TOP2cc at different genomic loci201,202, frequently involving translocations of the mixed lineage leukaemia (MLL) locus with more than 50 partner genes201–203. By contrast, secondary malignancies in patients treated with mitoxantrone involve translocations between the PML and RARA genes and give rise to acute promyelocytic leukaemia201,204. These differences may originate from the cell of origin of the secondary malignancies and may reflect the differential accumulation of therapeutic small molecules in different subsets of cells.
As oncogene-involving translocations could arise from transcription-induced TOP2cc202, it seems plausible that they may share characteristics with transcription-induced damage66. For example, androgens have been proposed to co-recruit the TMPRSS2 and ERG loci to common transcription sites, where TOP2β produces persistent TOPcc9,62,65, resulting in TMPRSS2–ERG fusions, which occur in approximately 50% of prostate cancers (reviewed in REF. 9). Such a mechanism could apply to other TOPcc-related cancer translocations; for example, bioflavonoids, which are present in fruits and vegetables and are TOP2 poisons, were suggested to cause childhood leukaemia205 (TABLE 1). Nevertheless, the detailed biochemical processes that cause these translocations remain poorly understood.
The connection between TOP1cc and neurological disorders was discovered through the presence of a deleterious TDP1 mutation in a large family from Saudi Arabia, with nine members suffering from cerebellar atrophy and ataxia and peripheral neuropathy206 (that is, SCAN1) (TABLE 1). The mutation (H493R) affects the catalytic His residue of TDP1 and causes both reduced catalytic activity and trapping of TDP1 on DNA following the removal of TOP1cc177,207,208. This neurological phenotype was reproduced in flies, in which knocking out gkt resulted in a shortened lifespan and defective climbing ability in females; the phenotype was rescued by neuronal expression of Tdp1 (REF. 178). When Atm is inactivated in mice (which, by itself, confers a mild neuronal phenotype) in addition to Tdp1 inactivation, a profound neuropathological phenotype is observed, with increased TOP1cc, DNA damage and neurodevelopmental defects179. These observations provide evidence for the physiological formation of trapped TOP1cc during normal development and implicate ATM, which is known to directly activate TDP1 (REF. 209), in their removal.
A connection between TOP2cc and neurological diseases was established recently with the discovery of homozygous mutations in TDP2 in consanguineous patients, manifesting with intellectual disability, seizure and ataxia69 (TABLE 1). However, as was seen with Tdp1-knockout mice177,210, Tdp2-knockout mice lack obvious neurological phenotypes in spite of a 25–30% reduction in interneuron density in their cerebellum69. Nevertheless, Tdp2-knockout mice fail to activate the expression of neuronal genes, especially those driven by TOP2β69,71, as well as long neuronal genes such as GABA- and glutamate-associated genes69. These findings are consistent with TDP2 being a transcription regulator that resolves TOP2βcc.
TOP1 autoantibodies (also known as SCL-70 or ATA) are commonly used in the diagnosis of systemic sclerosis (scleroderma) and are associated with poor prognosis and a high mortality rate211. Whether TOP1cc generate these antibodies deserves further investigation, as TOP1cc are a common feature of apoptosis212, and defective processing of apoptotic cells has been implicated in the generation of autoantibodies against nuclear antigens in systemic autoimmune rheumatic diseases, such as scleroderma and systemic lupus erytematosus211.
Conclusions and future perspectives
The past few years have seen a great increase in our understanding of topoisomerase function, especially in vertebrates. As many new topoisomerases were being discovered in the early 1990s, James Wang in a seminal essay asked “why so many?” (REF. 213). We are now beginning to answer that question, and the picture that has emerged is that each of these enzymes has a set of specific functions and that their specialization allows for precise coordination, especially in complicated DNA transactions that are required for replication, transcription and chromosome segregation. Our emerging understanding of chromosome nuclear territories, DNA repair, chromatin-remodelling complexes and super-enhancers, in addition to replication and transcription factories and chromatin looping, underscores the importance of understanding the six vertebrate DNA (and RNA) topoisomerases and integrating that knowledge into our study of genome function.
The biochemistry and biology of TOP1, TOP1mt, TOP2β and TOP3β especially warrant greater investigation. The work discussed above clearly indicates that TOP1 and TOP2β in some contexts have different biochemical activities from what we have come to expect from a topoisomerase: instead of breakage and rapid rejoining, prolonged breakage may have biological roles (BOX 1). Does the resealing of long-lasting TOP1- and TOP2β-induced DNA breaks rely on the topoisomerase, or is the cleavage a type of DNA damage that requires other factors for repair (or perhaps some combination of the two)? In any case, we do not yet understand what the features are at promoters (and perhaps other loci) that apparently lead to elevated levels of topoisomerase-dependent cleavage. As TOP2β is found trapped at some promoters, it is possible that TOP2β is intrinsically different from other topoisomerases. Alternatively, an exciting possibility is that other, as yet unidentified accessory proteins may regulate the cleavage–re-ligation equilibrium of topoisomerases.
Although topoisomerase-induced long-lasting strand breaks may seem like a game of cellular Russian roulette, cells clearly have evolved to use such breakage and even the ensuing genome rearrangements as part of processes such as ribonucleotide excision repair, mismatch repair, meiotic recombination and immunoglobulin gene rearrangements. Although genome rearrangements necessarily involve breakage and subsequent rejoining, cells clearly have the capability of handling strand breakage in their stride. Because topoisomerases are dedicated strand-breaking enzymes, it is not surprising that they are specifically called on when strand breaks are needed, as in the case of cutting of DNA by SPO11 for meiotic recombination214.
We have learned a great deal about how cells process topoisomerase-mediated damage when they are exposed to topoisomerase poisons. We now appreciate that there are two enzymes, TDP1 and TDP2, the major function of which is the release of topoisomerase–DNA adducts. These and other repair proteins probably form stable associations with topoisomerases, perhaps analogous to a DNA first-aid kit. We expected that the study of the repair of topoisomerase-mediated DNA damage would teach us much about cellular responses to anticancer drugs and anticipate that this information will inform the clinical use of anti-topoisomerase agents. What we did not expect was that these studies would also inform us about normal functions of topoisomerases, and about how topoisomerases contribute to the stability and instability of both the nuclear and mitochondrial genomes. The next few chapters in the saga of topoisomerase functions will certainly continue to surprise us.
Supplementary Material
Box 1 |. Non-canonical functions of topoisomerases.
The canonical function of topoisomerases is currently understood to be to change DNA topology by producing transient DNA strand breakage. As discussed in the main text, our current understanding is that topoisomerase function may sometimes break this paradigm; that is, topoisomerases may generate long-lived DNA double-stranded breaks (DSBs) and therefore are not carrying out a full breakage and rejoining reaction. Here, we discuss several other contexts of topoisomerase function.
An exciting, newly discovered non-canonical reaction mediated by type 1A topoisomerases is their ability to function as RNA topoisomerases, as shown initially for Escherichia coli topoisomerase III215, and subsequently for human ΤOΡ3β52,53. More recently, TOP3β was found in association with polyribosomes55, in which the only nucleic acid present is probably RNA. The functional significance of this association is not clear; however, various type 1A topoisomerases, including E. coli TopA and yeast Top3, are also active RNA topoisomerases55. Nevertheless, although the ability to act on RNA appears to be a universal characteristic of type 1A topoisomerases, further studies are warranted to elucidate how RNA is a biologically relevant substrate.
Why would RNA need to be a topoisomerase substrate? One simple consideration may be the need to efficiently handle long RNA transcripts, especially in concert with RNA processing. As TOP3β associates with polyribosomes, topological issues arising during translation should also be considered, although what those issues might be will require further exploration.
A second non-canonical function of topoisomerases was recently suggested following the observation of TOP1 cleavage at ribonucleotides167. Newly incorporated ribonucleotides may be a signal for the DNA mismatch repair pathway owing to the ribonuclease activity of TOP1 on the leading strand165. Although the overall importance of this for mismatch repair has been challenged216, the ability of TOP1 to cleave non-canonical DNA structures represents a unique adaptation of DNA topoisomerases that enables them to participate in other biological processes.
A third non-canonical function of topoisomerases — or, more precisely, topoisomerase-like proteins — is the initiation of meiotic recombination (reviewed in REF. 217). SPO11, a homologue of type 2B topoisomerases, has been shown to make permanent DSBs that are substrates for meiotic recombination. Although this function of SPO11 has been appreciated for some time, it may define a more general paradigm for topoisomerases that moonlight as enzymes that break DNA but leave the resealing for other enzymes.
Acknowledgements
The authors are grateful to numerous colleagues who provided helpful discussion on the diverse topics of this Review, especially D. Levens (US National Cancer Institute (NCI)), J. Berger (Johns Hopkins University, USA), N. Osheroff (Vanderbilt University, USA), P. McKinnon (St. Jude Children’s Research Hospital, USA) and other members of the Pommier and Nitiss laboratories. The authors apologize to colleagues whose work could not be cited owing to space limitations. The authors’ studies are supported by the Center for Cancer Research, the NCI Intramural Program (Z01 BC006161) and NCI grants CA52814 and CA187651 (to J.L.N.).
Footnotes
Competing interests statement
The authors declare no competing interests.
Shortly after this Review was completed, we were saddened to learn that Dr Tao-shih Hsieh, of Duke University, Durham, North Carolina, USA, and Academia Sinica, Taipei, passed away. Hsieh was a true pioneer in the topoisomerase field, with signal accomplishments that included the first isolation of Drosophila topoisomerases, and many elegant biochemical and biological studies on topoisomerase mechanisms. This Review is dedicated to the memory of our friend and colleague.
References
- 1.Watson JD & Crick FH Genetical implications of the structure of deoxyribonucleic acid. Nature 171, 964–967 (1953). [DOI] [PubMed] [Google Scholar]
- 2.Wang JC Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3, 430–440 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Pommier Y, Leo E, Zhang H & Marchand C DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 17, 421–433 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitiss JL Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 9, 338–350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SH, Chan NL & Hsieh TS New mechanistic and functional insights into DNA topoisomerases. Annu. Rev. Biochem. 82, 139–170 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Champoux JJ DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 70, 369–413 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Nitiss JL DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 9, 327–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vos SM, Tretter EM, Schmidt BH & Berger JM All tangled up: how cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 12, 827–841 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashour ME, Atteya R & El-Khamisy SF Topoisomerase-mediated chromosomal break repair: an emerging player in many games. Nat. Rev. Cancer 15, 137–151 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Koster DA, Crut A, Shuman S, Bjornsti MA & Dekker NH Cellular strategies for regulating DNA supercoiling: a single-molecule perspective. Cell 142, 519–530 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse-Dinh YC Bacterial and archeal type I topoisomerases. Biochim. Biophys. Acta 1400, 19–27 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Chikamori K et al. DNA topoisomerase II enzymes as molecular targets for cancer chemotherapy. Curr. Cancer Drug Targets 10, 758–771 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Seol Y & Neuman KC Single-molecule measurements of topoisomerase activity with magnetic tweezers. Methods Mol. Biol. 778, 229–241 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Spitzner JR & Muller MT A consensus sequence for cleavage by vertebrate DNA topoisomerase II. Nucleic Acids. Res. 16, 5533–5556 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter SE & Champoux JJ The basis for camptothecin enhancement of DNA breakage by eukaryotic topoisomerase I. Nucleic Acids Res. 17, 8521–8532 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capranico G, Kohn KW & Pommier Y Local sequence requirements for DNA cleavage by mammalian topoisomerase II in the presence of doxorubicin. Nucleic Acids Res. 18, 6611–6619 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaxel C, Capranico G, Kerrigan D, Kohn KW & Pommier Y Effect of local DNA sequence on topoisomerase I cleavage in the presence or absence of camptothecin. J. Biol. Chem. 266, 20418–20423 (1991). [PubMed] [Google Scholar]
- 18.Pourquier P et al. Trapping of mammalian topoisomerase I and recombinations induced by damaged DNA containing nicks or gaps: importance of DNA end phosphorylation and camptothecin effects. J. Biol. Chem. 272, 26441–26447 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Capranico G, Tinelli S, Zunino F, Kohn KW & Pommier Y Effects of base mutations on topoisomerase II DNA cleavage stimulated by mAMSA in short DNA oligomers. Biochemistry 32, 145–152 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Gao R et al. Proteolytic degradation of topoisomerase II (Top2) enables the processing of Top2-DNA and RNA covalent complexes by tyrosyl-DNA-phosphodiesterase 2 (TDP2). J. Biol. Chem. 289, 17960–17969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H et al. Human mitochondrial topoisomerase I. Proc. Natl Acad. Sci. USA 98, 10608–10613 (2001). Demonstrates a specific mammalian type 1B topoisomerase that is localized to mitochondria.
- 22.Wang Y, Lyu YL & Wang JC Dual localization of human DNA topoisomerase IIIa to mitochondria and nucleus. Proc. Natl Acad. Sci. USA 99, 12114–12119 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H et al. Increased negative supercoiling of mtDNA in TOP1mt knockout mice and presence of topoisomerases IIα and IIβ in vertebrate mitochondria. Nucleic Acids Res. 42, 7259–7267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capranico G, Jaxel C, Roberge M, Kohn KW & Pommier Y Nucleosome positioning as a critical determinant for the DNA cleavage sites of mammalian DNA topoisomerase II in reconstituted simian virus 40 chromatin. Nucleic Acids Res. 18, 4553–4559 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salceda J, Fernandez X & Roca J Topoisomerase II, not topoisomerase I, is the proficient relaxase of nucleosomal DNA. EMBO J. 25, 2575–2583 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kouzine F et al. Transcription-dependent dynamic supercoiling is a short-range genomic force. Nat. Struct. Mol. Biol. 20, 396–403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naughton C et al. Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures. Nat. Struct. Mol. Biol. 20, 387–395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J & Wang M Interplay between DNA supercoiling and transcription elongation. Transcription 5, e28636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baranello L et al. RNA polymerase II regulates topoisomerase 1 activity to favor efficient transcription. Cell 165, 357–371 (2016). Demonstrates the importance of DNA supercoiling and TOP1 in transcription pause–release, and novel TOP1 and Pol II interactions.
- 30.Pommier Y, Kohlhagen G, Wu C & Simmons DT Mammalian DNA topoisomerase I activity and poisoning by camptothecin are inhibited by simian virus 40 large T antigen. Biochemistry 37, 3818–3823 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Seinsoth S, Uhlmann-Schiffler H & Stahl H Bidirectional DNA unwinding by a ternary complex of T antigen, nucleolin and topoisomerase I. EMBO Rep. 4, 263–268 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchand C, Pourquier P, Laco GS, Jing N & Pommier Y Interaction of human nuclear topoisomerase I with guanosine quartet-forming and guanosine-rich single-stranded DNA and RNA oligonucleotides. J. Biol. Chem. 277, 8906–8911 (2002). [DOI] [PubMed] [Google Scholar]
- 33. Dykhuizen EC et al. BAF complexes facilitate decatenation of DNA by topoisomerase IIα. Nature 497, 624–627 (2013). Demonstrates the interaction of chromatin-remodelling complexes with topoisomerases as a mechanism of enhancing decatenation by type 2 enzymes.
- 34.Husain A et al. Chromatin remodeller SMARCA4 recruits topoisomerase 1 and suppresses transcription-associated genomic instability. Nat. Commun. 7, 10549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krawczyk C, Dion V, Schar P & Fritsch O Reversible Top1 cleavage complexes are stabilized strand-specifically at the ribosomal replication fork barrier and contribute to ribosomal DNA stability. Nucleic Acids Res. 42, 4985–4995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hohmann AF & Vakoc CR A rationale to target the SWI/SNF complex for cancer therapy. Trends Genet. 30, 356–363 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laine JP et al. Werner protein stimulates topoisomerase I DNA relaxation activity. Cancer Res. 63, 7136–7146 (2003). [PubMed] [Google Scholar]
- 38.Merino A, Madden KR, Lane WS, Champoux JJ & Reinberg D DNA topoisomerase I is involved in both repression and activation of transcription. Nature 365, 227–232 (1993). [DOI] [PubMed] [Google Scholar]
- 39.Kretzschmar M, Meisterernst M & Roeder RG Identification of human DNA topoisomerase I as a cofactor for activator-dependent transcription by RNA polymerase II. Proc. Natl Acad. Sci. USA 90, 11508–11512 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shykind BM, Kim J, Stewart L, Champoux JJ & Sharp PA Topoisomerase I enhances TFIID–TFIIA complex assembly during activation of transcription. Genes Dev. 11, 397–407 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Bowen C et al. NKX3.1 homeodomain protein binds to topoisomerase I and enhances its activity. Cancer Res. 67, 455–464 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Puc J et al. Ligand-dependent enhancer activation regulated by topoisomerase-I activity. Cell 160, 367–380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park H & Sternglanz R Identification and characterization of the genes for two topoisomerase I-interacting proteins from Saccharomyces cerevisiae. Yeast 15, 35–41 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Earnshaw WC & Laemmli UK Silver staining the chromosome scaffold. Chromosoma 89, 186–192 (1984). [DOI] [PubMed] [Google Scholar]
- 45.Shintomi K, Takahashi TS & Hirano T Reconstitution of mitotic chromatids with a minimum set of purified factors. Nat. Cell Biol. 17, 1014–1023 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Farr CJ, Antoniou-Kourounioti M, Mimmack ML, Volkov A & Porter AC The α isoform of topoisomerase II is required for hypercompaction of mitotic chromosomes in human cells. Nucleic Acids Res. 42, 4414–4426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azuma Y, Arnaoutov A & Dasso M SUMO-2/3 regulates topoisomerase II in mitosis. J. Cell Biol. 163, 477–487 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachant J, Alcasabas AA, Blat Y, Kleckner N & Elledge SJ The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9, 1169–1182 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Dawlaty MM et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIα. Cell 133, 103–115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nielsen CF et al. PICH promotes sister chromatid disjunction and co-operates with topoisomerase II in mitosis. Nat. Commun. 6, 8962 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broderick R, Nieminuszczy J, Blackford AN, Winczura A & Niedzwiedz W TOPBP1 recruits TOP2A to ultra-fine anaphase bridges to aid in their resolution. Nat. Commun. 6, 6572 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoll G et al. Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat. Neurosci. 16, 1228–1237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu D et al. Top3β is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat. Neurosci. 16, 1238–1247 (2013). References 52 and 53 suggest a role for TOP3β in RNA metabolism and in the genesis of neurodevelopmental disorders.
- 54.Yang Y et al. Arginine methylation facilitates the recruitment of TOP3B to chromatin to prevent R loop accumulation. Mol. Cell 53, 484–497 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmad M et al. RNA topoisomerase is prevalent in all domains of life and associates with polyribosomes in animals. Nucleic Acids Res. 44, 6335–6349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu LF & Wang JC Supercoiling of the DNA template during transcription. Proc. Natl Acad. Sci. USA 84, 7024–7027 (1987). A seminal work in the topoisomerase field that provides a model for how the process of transcription can result in DNA supercoiling.
- 57.Bermejo R et al. Genome-organizing factors Top2 and Hmo1 prevent chromosome fragility at sites of 5 phase transcription. Cell 138, 870–884 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Brill SJ, DiNardo S, Voelkel-Meiman K 6 Sternglanz, R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. Nature 326, 414–416 (1987). [DOI] [PubMed] [Google Scholar]
- 59.Sperling AS, Jeong KS, Kitada T & Grunstein M Topoisomerase II binds nucleosome-free DNA and acts redundantly with topoisomerase I to enhance recruitment of RNA Pol II in budding yeast. Proc. Natl Acad. Sci. USA 108, 12693–12698 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedersen JM et al. DNA topoisomerases maintain promoters in a state competent for transcriptional activation in Saccharomyces cerevisiae. PLoS Genet. 8, e1003128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roedgaard M, Fredsoe J, Pedersen JM, Bjergbaek L & Andersen AH DNA topoisomerases are required for preinitiation complex assembly during GAL gene activation. PLoS ONE 10, e0132739 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ju BG et al. A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 (2006). A landmark paper that suggests that TOP2β makes long-lasting breaks at certain promoters.
- 63.McClendon AK, Rodriguez AC & Osheroff N Human topoisomerase IIa rapidly relaxes positively supercoiled DNA: implications for enzyme action ahead of replication forks. J. Biol. Chem. 280, 39337–39345 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Williamson LM & Lees-Miller SP Estrogen receptor α-mediated transcription induces cell cycle-dependent DNA double-strand breaks. Carcinogenesis 32, 279–285 (2011). [DOI] [PubMed] [Google Scholar]
- 65. Haffner MC et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat. Genet. 42, 668–675 (2010). A demonstration that TOP2β-induced breaks at promoters can lead to oncogenic translocations.
- 66.Haffner MC, De Marzo AM, Meeker AK, Nelson WG & Yegnasubramanian S Transcription-induced DNA double strand breaks: both oncogenic force and potential therapeutic target? Clin. Cancer Res. 17, 3858–3864 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trotter KW, King HA & Archer TK Glucocorticoid receptor transcriptional activation via the BRG1-dependent recruitment of TOP2β and Ku70/86. Mol. Cell. Biol. 35, 2799–2817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Madabhushi R et al. Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell 161, 1592–1605 (2015). Suggests that TOP2β-induced breaks at promoters may arise from an intrinsic property of TOP2β.
- 69. Gomez-Herreros F et al. TDP2 protects transcription from abortive topoisomerase activity and is required for normal neural function. Nat. Genet. 46, 516–521 (2014). Suggests that long-lived TOP2β-induced breaks at neuronal promoters may arise frequently.
- 70. Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K & Caldecott KW A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 461,674–678 (2009). Describes the identification of TDP2 and suggests that TDP2 has a crucial role in repairing TOP2-mediated DNA damage.
- 71.Lyu YL et al. Role of topoisomerase IIβ in the expression of developmentally regulated genes. Mol. Cell. Biol. 26, 7929–7941 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bunch H et al. Transcriptional elongation requires DNA break-induced signalling. Nat. Commun. 6, 10191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joshi RS, Pina B & Roca J Topoisomerase II is required for the production of long Pol II gene transcripts in yeast. Nucleic Acids Res. 40, 7907–7915 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pommier Y, Kerrigan D, Hartman KD & Glazer RI Phosphorylation of mammalian DNA topoisomerase I and activation by protein kinase C. J. Biol. Chem. 265, 9418–9422 (1990). [PubMed] [Google Scholar]
- 75.Ljungman M & Hanawalt PC The anti-cancer drug camptothecin inhibits elongation but stimulates initiation of RNA polymerase II transcription. Carcinogenesis 17, 31–35 (1996). [DOI] [PubMed] [Google Scholar]
- 76.Solier S et al. Transcription poisoning by topoisomerase I is controlled by gene length, splice sites, and miR-142-3p. Cancer Res. 73, 4830–4839 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. King IF et al. Topoisomerases facilitate transcription of long genes linked to autism. Nature 501, 58–62 (2013). Shows a unique requirement for topoisomerases in the transcription of very long genes.
- 78.Mabb AM et al. Topoisomerase 1 inhibition reversibly impairs synaptic function. Proc. Natl Acad. Sci. USA 111, 17290–17295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang HS et al. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature 481, 185–189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mabb AM, Judson MC, Zylka MJ & Philpot BD Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci. 34, 293–303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Powell WT et al. R-loop formation at Snord116 mediates topotecan inhibition of Ube3a-antisense and allele-specific chromatin decondensation. Proc. Natl Acad. Sci. USA 110, 13938–13943 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drolet M Growth inhibition mediated by excess negative supercoiling: the interplay between transcription elongation, R-loop formation and DNA topology. Mol. Microbiol. 59, 723–730 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Santos-Pereira JM & Aguilera A R loops: new modulators of genome dynamics and function. Nat. Rev. Genet. 16, 583–597 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Sollier J et al. Transcription-coupled nucleotide excision repair factors promote R-loop-induced genome instability. Mol. Cell 56, 777–785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson-Sali T & Hsieh TS Preferential cleavage of plasmid-based R-loops and D-loops by Drosophilarase IIIβ. Proc. Natl Acad. Sci. USA 99, 7974–7979 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Hage A, French SL, Beyer AL & Tollervey D Loss of topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes Dev. 24, 1546–1558 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim N & Jinks-Robertson S Guanine repeat-containing sequences confer transcription-dependent instability in an orientation-specific manner in yeast. DNA Repair 10, 953–960 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yadav P, Owiti N & Kim N The role of topoisomerase I in suppressing genome instability associated with a highly transcribed guanine-rich sequence is not restricted to preventing RNA:DNA hybrid accumulation. Nucleic Acids Res. 44, 718–729 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tuduri S et al. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nat. Cell Biol. 11, 1315–1324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sordet O, Nakamura AJ, Redon CE & Pommier Y DNA double-strand breaks and ATM activation by transcription-blocking DNA lesions. Cell Cycle 9, 274–278 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sordet O et al. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO Rep. 10, 887–893 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sordet O et al. Hyperphosphorylation of RNA polymerase II in response to topoisomerase I cleavage complexes and its association with transcription- and BRCA1-dependent degradation of topoisomerase I. J. Mol. Biol. 381,540–549 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marinello J et al. Dynamic effects of topoisomerase I inhibition on R-loops and short transcripts at active promoters. PLoS ONE 11, e0147053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marinello J, Chillemi G, Bueno S, Manzo SG & Capranico G Antisense transcripts enhanced by camptothecin at divergent CpG-island promoters associated with bursts of topoisomerase I–DNA cleavage complex and R-loop formation. Nucleic Acids Res. 41, 10110–10123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sissi C & Palumbo M In front of and behind the replication fork: bacterial type IIA topoisomerases. Cell. Mol. Life Sci. 67, 2001–2024 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim RA & Wang JC Function of DNA topoisomerases as replication swivels in Saccharomyces cerevisiae. J. Mol. Biol. 208, 257–267 (1989). [DOI] [PubMed] [Google Scholar]
- 97.Bermejo R et al. Top1- and Top2-mediated topological transitions at replication forks ensure fork progression and stability and prevent DNA damage checkpoint activation. Genes Dev. 21, 1921–1936 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdurashidova G et al. Functional interactions of DNA topoisomerases with a human replication origin. EMBO J. 26, 998–1009 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Falaschi A Binding of DNA topoisomerases I and II to replication origins. Methods Mol. Biol 582, 131–143 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Hu HG, Baack M & Knippers R Proteins of the origin recognition complex (ORC) and DNA topoisomerases on mammalian chromatin. BMC Mol. Biol. 10, 36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rampakakis E, Gkogkas C, Di Paola D & Zannis-Hadjopoulos M Replication initiation and DNA topology: the twisted life of the origin. J. Cell Biochem. 110, 35–43 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Gaggioli V, Le Viet B, Germe T & Hyrien O DNA topoisomerase IIa controls replication origin cluster licensing and firing time in Xenopus egg extracts. Nucleic Acids Res. 41,7313–7331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonzalez RE et al. Effects of conditional depletion of topoisomerase II on cell cycle progression in mammalian cells. Cell Cycle 10, 3505–3514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaguni JM & Kornberg A Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell 38, 183–190 (1984). [DOI] [PubMed] [Google Scholar]
- 105.Stuckey R, Garcia-Rodriguez N, Aguilera A & Wellinger RE Role for RNA:DNA hybrids in origin-independent replication priming in a eukaryotic system. Proc. Natl Acad. Sci. USA 112, 5779–5784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strumberg D et al. Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5′-phosphorylated DNA double-strand breaks by replication runoff. Mol. Cell. Biol. 20, 3977–3987 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Postow L, Peter BJ & Cozzarelli NR Knot what we thought before: the twisted story of replication. Bioessays 21,805–808 (1999). [DOI] [PubMed] [Google Scholar]
- 108.Postow L, Crisona NJ, Peter BJ, Hardy CD & Cozzarelli NR Topological challenges to DNA replication: conformations at the fork. Proc. Natl Acad. Sci. USA 98, 8219–8226 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lucas I, Germe T, Chevrier-Miller M & Hyrien O Topoisomerase II can unlink replicating DNA by precatenane removal. EMBO J. 20, 6509–6519 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cebrian J et al. Direct evidence for the formation of precatenanes during DNA replication. J. Biol. Chem. 290, 13725–13735 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sundin O & Varshavsky A Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell 21, 103–114 (1980). [DOI] [PubMed] [Google Scholar]
- 112.Sundin O & Varshavsky A Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell 25, 659–669 (1981). [DOI] [PubMed] [Google Scholar]
- 113.Baxter J ‘Breaking up is hard to do’: the formation and resolution of sister chromatid intertwines. J. Mol. Biol. 427, 590–607 (2015). [DOI] [PubMed] [Google Scholar]
- 114.Lee SH, Siaw GE, Willcox S, Griffith JD & Hsieh TS Synthesis and dissolution of hemicatenanes by type IA DNA topoisomerases. Proc. Natl Acad. Sci. USA 110, E3587–E3594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schalbetter SA, Mansoubi S, Chambers AL, Downs JA & Baxter J Fork rotation and DNA precatenation are restricted during DNA replication to prevent chromosomal instability. Proc. Natl Acad. Sci. USA 112, E4565–E4570 (2015). An elegant demonstration of the genetic control of precatenane formation.
- 116.Leman AR & Noguchi E Local and global functions of Timeless and Tipin in replication fork protection. Cell Cycle 11, 3945–3955 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McFarlane RJ, Mian S & Dalgaard JZ The many facets of the Tim–Tipin protein families′ roles in chromosome biology. Cell Cycle 9, 700–705 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Chou DM & Elledge SJ Tipin and Timeless form a mutually protective complex required for genotoxic stress resistance and checkpoint function. Proc. Natl Acad. Sci. USA 103, 18143–18147 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hosono Y et al. Tipin functions in the protection against topoisomerase I inhibitor. J. Biol. Chem. 289, 11374–11384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Titos I, Ivanova T & Mendoza M Chromosome length and perinuclear attachment constrain resolution of DNA intertwines. J. Cell Biol. 206, 719–733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kegel A et al. Chromosome length influences replication-induced topological stress. Nature 471, 392–396 (2011). A novel observation concerning the effects of chromosome size in yeast on topological aspects of DNA replication.
- 122.Baxter J & Diffley JF Topoisomerase II inactivation prevents the completion of DNA replication in budding yeast. Mol. Cell 30, 790–802 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Cuvier O, Stanojcic S, Lemaitre JM & Mechali M A topoisomerase II-dependent mechanism for resetting replicons at the S–M-phase transition. Genes Dev. 22, 860–865 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.DiNardo S, Voelkel K & Sternglanz R DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc. Natl Acad. Sci. USA 81, 2616–2620 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fachinetti D et al. Replication termination at eukaryotic chromosomes is mediated by Top2 and occurs at genomic loci containing pausing elements. Mol. Cell 39, 595–605 (2010). Details how replication is terminated at pausing elements and how type 2 topoisomerases are recruited.
- 126.Watt PM, Louis EJ, Borts RH & Hickson ID Sgs1: a eukaryotic homolog of E. coli RecQ that interacts with topoisomerase II in vivo and is required for faithful chromosome segregation. Cell 81, 253–260 (1995). [DOI] [PubMed] [Google Scholar]
- 127.Ramamoorthy M et al. RECQL5 cooperates with topoisomerase II α in DNA decatenation and cell cycle progression. Nucleic Acids Res. 40, 1621–1635 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Russell B et al. Chromosome breakage is regulated by the interaction of the BLM helicase and topoisomerase IIα. Cancer Res. 71,561–571 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rouzeau S et al. Bloom’s syndrome and PICH helicases cooperate with topoisomerase IIα in centromere disjunction before anaphase. PLoS ONE 7, e33905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holm C, Stearns T & Botstein D DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol. Cell. Biol. 9, 159–168 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tovar C et al. Small-molecule inducer of cancer cell polyploidy promotes apoptosis or senescence: implications for therapy. Cell Cycle 9, 3364–3375 (2010). [DOI] [PubMed] [Google Scholar]
- 132.Chan KL, North PS & Hickson ID BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J. 26, 3397–3409 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chan KL & Hickson ID New insights into the formation and resolution of ultra-fine anaphase bridges. Semin. Cell Dev. Biol. 22, 906–912 (2011). [DOI] [PubMed] [Google Scholar]
- 134.Liu Y, Nielsen CF, Yao Q & Hickson ID The origins and processing of ultra fine anaphase DNA bridges. Curr. Opin. Genet. Dev. 26, 1–5 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Kadoch C et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 45, 592–601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Skoufias DA, Lacroix FB, Andreassen PR, Wilson L & Margolis RL Inhibition of DNA decatenation, but not DNA damage, arrests cells at metaphase. Mol. Cell 15, 977–990 (2004). [DOI] [PubMed] [Google Scholar]
- 137.Haggarty SJ et al. Small molecule modulation of the human chromatid decatenation checkpoint. Chem. Biol. 10, 1267–1279 (2003). [DOI] [PubMed] [Google Scholar]
- 138.Deming P B. et al. The human decatenation checkpoint. Proc. Natl Acad. Sci. USA 98, 12044–12049 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Downes CS et al. A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature 372, 467–470 (1994). [DOI] [PubMed] [Google Scholar]
- 140.Furniss KL et al. Direct monitoring of the strand passage reaction of DNA topoisomerase II triggers checkpoint activation. PLoS Genet. 9, e1003832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lou Z, Minter-Dykhouse K & Chen J BRCA1 participates in DNA decatenation. Nat. Struct. Mol. Biol. 12, 589–593 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Kang X et al. PTEN stabilizes TOP2A and regulates the DNA decatenation. Sci. Rep. 5, 17873 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diaz-Martinez LA et al. PIASγ is required for faithful chromosome segregation in human cells. PLoS ONE 1, e53 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kschonsak M & Haering CH Shaping mitotic chromosomes: from classical concepts to molecular mechanisms. Bioessays 37, 755–766 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bell JC & Straight AF Condensing chromosome condensation. Nat. Cell Biol. 17, 964–965 (2015). [DOI] [PubMed] [Google Scholar]
- 146.Baxter J et al. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science 331, 1328–1332 (2011). [DOI] [PubMed] [Google Scholar]
- 147.D’Erchia AM et al. Tissue-specific mtDNA abundance from exome data and its correlation with mitochondrial transcription, mass and respiratory activity. Mitochondrion 20, 13–21 (2015). [DOI] [PubMed] [Google Scholar]
- 148.Khiati S et al. Mitochondrial topoisomerase I (Top1mt) is a novel limiting factor of doxorubicin cardiotoxicity. Clin. Cancer Res. 20, 4873–4881 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khiati S et al. Lack of mitochondrial topoisomerase I (TOP1mt) impairs liver regeneration. Proc. Natl Acad. Sci. USA 112, 11282–11287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wu J, Feng L & Hsieh TS Drosophila topo IIIa is required for the maintenance of mitochondrial genome and male germ-line stem cells. Proc. Natl Acad. Sci. USA 107, 6228–6233 (2010). A demonstration that human type 1A topoisomerases can function on RNA substrates.
- 151. Tsai HZ, Lin RK & Hsieh TS Drosophila mitochondrial topoisomerase III alpha affects the aging process via maintenance of mitochondrial function and genome integrity. J. Biomed. Sci. 23, 38 (2016). A clear demonstration of the role of Top3α in mitochondria.
- 152.Khiati S et al. Poisoning of mitochondrial topoisomerase I by lamellarin D. Mol. Pharmacol. 86, 193–199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miao ZH et al. Nonclassic functions of human topoisomerase I: genome-wide and pharmacologic analyses. Cancer Res. 67, 8752–8761 (2007). [DOI] [PubMed] [Google Scholar]
- 154.Pommier Y & Marchand C Interfacial inhibitors: targeting macromolecular complexes. Nat. Rev. Drug Discov. 11, 25–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pommier Y Drugging topoisomerases: lessons and challenges. ACS Chem. Biol. 8, 82–95 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ray Chaudhuri A et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat. Struct. Mol. Biol. 19, 417–423 (2012). [DOI] [PubMed] [Google Scholar]
- 157.Regairaz M et al. Mus81-mediated DnA cleavage resolves replication forks stalled by topoisomerase I–DNA complexes. J. Cell Biol. 195, 739–749 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fu H et al. The DNA repair endonuclease Mus81 facilitates fast DNA replication in the absence of exogenous damage. Nat. Commun. 6, 6746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rialdi A et al. Topoisomerase 1 inhibition suppresses inflammatory genes and protects from death by inflammation. Science 352, aad7993 (2016). A very recent paper that highlights a therapeutic potential for inhibiting TOP1.
- 160.Barnes DE & Lindahl T Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu. Rev. Genet. 38, 445–476 (2004). [DOI] [PubMed] [Google Scholar]
- 161.Beckman KB & Ames BN Oxidative decay of DNA. J. Biol. Chem. 272, 19633–19636 (1997). [DOI] [PubMed] [Google Scholar]
- 162.Vilenchik MM & Knudson AG Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc. Natl Acad. Sci. USA 100, 12871–12876 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hoeijmakers JH DNA damage, aging, and cancer. N. Engl. J. Med. 361, 1475–1485 (2009). [DOI] [PubMed] [Google Scholar]
- 164.Nick McElhinny SA et al. Genome instability due to ribonucleotide incorporation into DNA. Nat. Chem. Biol. 6, 774–781 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Williams JS, Lujan SA & Kunkel TA Processing ribonucleotides incorporated during eukaryotic DNA replication. Nat. Rev. Mol. Cell Biol. 17, 350–363 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sekiguchi J & Shuman S Site-specific ribonuclease activity of eukaryotic DNA topoisomerase I. Mol. Cell 1, 89–97 (1997). [DOI] [PubMed] [Google Scholar]
- 167. Kim N et al. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332, 1561–1564 (2011). A demonstration that incorporation of ribonucleotides leads to trapping of TOP1, with mutagenic consequences.
- 168.Huang SY, Ghosh S & Pommier Y Topoisomerase I alone is sufficient to produce short DNA deletions and can also reverse nicks at ribonucleotide sites. J. Biol. Chem. 290, 14068–14076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Y, Thyssen A, Westergaard O & Andersen AH Position-specific effect of ribonucleotides on the cleavage activity of human topoisomerase II. Nucleic Acids Res. 28, 4815–4821 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pommier Y et al. Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2). DNA Repair 19, 114–129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Menon V & Povirk LF End-processing nucleases and phosphodiesterases: an elite supporting cast for the non-homologous end joining pathway of DNA double-strand break repair. DNA Repair 43, 57–68 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Pouliot JJ, Yao KC, Robertson CA & Nash HA Yeast gene for a Tyr-DNA phosphodiesterase that repairs topo I covalent complexes. Science 286, 552–555 (1999). [DOI] [PubMed] [Google Scholar]
- 173.Liu C, Pouliot JJ & Nash HA Repair of topoisomerase I covalent complexes in the absence of the tyrosyl-DNA phosphodiesterase Tdp1. Proc. Natl Acad. Sci. USA 99, 14970–14975 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vance JR & Wilson TE Yeast Tdp1 and Rad1–Rad10 function as redundant pathways for repairing Top1 replicative damage. Proc. Natl Acad. Sci. USA 99, 13669–13674 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang SY et al. TDP1 repairs nuclear and mitochondrial DNA damage induced by chain-terminating anticancer and antiviral nucleoside analogs. Nucleic Acids Res. 41, 7793–7803 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Das BB, Dexheimer TS, Maddali K & Pommier Y Role of tyrosyl-DNA phosphodiesterase (TDP1) in mitochondria. Proc. Natl Acad. Sci. USA 107, 19790–19795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hirano R et al. Spinocerebellar ataxia with axonal neuropathy: consequence of a Tdp1 recessive neomorphic mutation? EMBO J. 26, 4732–4743 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guo D, Dexheimer TS, Pommier Y & Nash HA Neuroprotection and repair of 3′-blocking DNA ends by glaikit (gkt) encoding Drosophila tyrosyl-DNA phosphodiesterase 1 (TDP1). Proc. Natl Acad. Sci. USA 111, 15816–15820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Katyal S et al. Aberrant topoisomerase-1 DNA lesions are pathogenic in neurodegenerative genome instability syndromes. Nat. Neurosci. 17, 813–821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Debethune L, Kohlhagen G, Grandas A & Pommier Y Processing of nucleopeptides mimicking the topoisomerase I-DNA covalent complex by tyrosyl-DNA phosphodiesterase. Nucleic Acids Res. 30, 1198–1204 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Interthal H, Chen HJ & Champoux JJ Human Tdp1 cleaves a broad spectrum of substrates including phosphoamide linkages. J. Biol. Chem. 280, 36518–36528 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Plo I et al. Association of XRCC1 and tyrosyl DNA phosphodiesterase (Tdp1) for the repair of topoisomerase I-mediated DNA lesions. DNA Repair 2, 1087–1100 (2003). [DOI] [PubMed] [Google Scholar]
- 183.Das BB et al. PARP1–TDP1 coupling for the repair of topoisomerase I-induced DNA damage. Nucleic Acids Res. 42, 4435–4449 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nitiss J & Wang JC DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc. Natl Acad. Sci. USA 85, 7501–7505 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maede Y et al. Differential and common DNA repair pathways for topoisomerase I- and II-targeted drugs in a genetic DT40 repair cell screen panel. Mol. Cancer Ther. 13, 214–220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pouliot JJ, Robertson CA & Nash HA Pathways for repair of topoisomerase I covalent complexes in Saccharomyces cerevisiae. Genes Cells 6, 677–687 (2001). [DOI] [PubMed] [Google Scholar]
- 187.Zeng Z et al. TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1. Nucleic Acids Res. 40, 8371–8380 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Virgen-Slane R et al. An RNA virus hijacks an incognito function of a DNA repair enzyme. Proc. Natl Acad. Sci. USA 109, 14634–14639 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Koniger C et al. Involvement of the host DNA-repair enzyme TDP2 in formation of the covalently closed circular DNA persistence reservoir of hepatitis B viruses. Proc. Natl Acad. Sci. USA 111, E4244–E4253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Malik M, Nitiss KC, Enriquez-Rios V & Nitiss JL Roles of nonhomologous end-joining pathways in surviving topoisomerase II-mediated DNA damage. Mol. Cancer Ther. 5, 1405–1414 (2006). [DOI] [PubMed] [Google Scholar]
- 191.Gomez-Herreros F et al. TDP2-dependent nonhomologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 9, e1003226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang YW et al. Poly(ADP-ribose) polymerase and XPF–ERCC1 participate in distinct pathways for the repair of topoisomerase I-induced DNA damage in mammalian cells. Nucleic Acids Res. 39, 3607–3620 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Takahata C et al. Repair synthesis step involving ERCC1–XPF participates in DNA repair of the Top1–DNA damage complex. Carcinogenesis 36, 841–851 (2015). [DOI] [PubMed] [Google Scholar]
- 194.Malik M & Nitiss JL DNA repair functions that control sensitivity to topoisomerase-targeting drugs. Eukaryot. Cell 3, 82–90 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kametani Y et al. FEN1 participates in repair of the 5′-phosphotyrosyl terminus of DNA single-strand breaks. Carcinogenesis 37, 56–62 (2015). [DOI] [PubMed] [Google Scholar]
- 196.Hamilton NK & Maizels N MRE11 function in response to topoisomerase poisons is independent of its function in double-strand break repair in Saccharomyces cerevisiae. PLoS ONE 5, e15387 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sacho EJ & Maizels N DNA repair factor MRE11/RAD50 cleaves 3′-phosphotyrosyl bonds and resects DNA to repair damage caused by topoisomerase 1 poisons. J. Biol. Chem. 286, 44945–44951 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sartori AA et al. Human CtIP promotes DNA end resection. Nature 450, 509–514 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pendleton M et al. Topoisomerase II and leukemia. Ann. NY Acad. Sci. 1310, 98–110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cowell IG & Austin CA Do transcription factories and TOP2B provide a recipe for chromosome translocations in therapy-related leukemia? Cell Cycle 11, 3143–3144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cowell IG et al. Model for MLL translocations in therapy-related leukemia involving topoisomerase IIβ-mediated DNA strand breaks and gene proximity. Proc. Natl Acad. Sci. USA 109, 8989–8994 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Azarova AM et al. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc. Natl Acad. Sci. USA 104, 11014–11019 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lovett BD et al. Near-precise interchromosomal recombination and functional DNA topoisomerase II cleavage sites at MLL and AF-4 genomic breakpoints in treatment-related acute lymphoblastic leukemia with t(4;11) translocation. Proc. Natl Acad. Sci. USA 98, 9802–9807 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Joannides M et al. Molecular pathogenesis of secondary acute promyelocytic leukemia. Mediterr. J. Hematol. Infect. Dis. 3, e2011045 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ketron AC & Osheroff N Phytochemicals as anticancer and chemopreventive topoisomerase II poisons. Phytochem. Rev 13, 19–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Takashima H et al. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 32, 267–272 (2002). [DOI] [PubMed] [Google Scholar]
- 207.Interthal H et al. SCAN1 mutant Tdp1 accumulates the enzyme–DNA intermediate and causes camptothecin hypersensitivity. EMBO J. 24, 2224–2233 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.He X et al. Mutation of a conserved active site residue converts tyrosyl–DNA phosphodiesterase I into a DNA topoisomerase I-dependent poison. J. Mol. Biol. 372, 1070–1081 (2007). [DOI] [PubMed] [Google Scholar]
- 209.Das BB et al. Optimal function of the DNA repair enzyme TDP1 requires its phosphorylation by ATM and/or DNA-PK. EMBO J. 28, 3667–3680 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Katyal S et al. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 26, 4720–4731 (2007). Demonstrates the synergistic roles of TDP1 and ATM in mouse models of neurodegenerative disorders.
- 211.Mahler M, Silverman ED, Schulte-Pelkum J & Fritzler MJ Anti-Scl-70 (topo-I) antibodies in SLE: myth or reality? Autoimmun. Rev. 9, 756–760 (2010). [DOI] [PubMed] [Google Scholar]
- 212.Sordet O, Khan QA & Pommier Y Apoptotic topoisomerase I-DNA complexes induced by oxygen radicals and mitochondrial dysfunction. Cell Cycle 3, 1095–1097 (2004). [PubMed] [Google Scholar]
- 213.Wang JC DNA topoisomerases: why so many? J. Biol. Chem. 266, 6659–6662 (1991). [PubMed] [Google Scholar]
- 214.Keeney S, Lange J & Mohibullah N Self-organization of meiotic recombination initiation: general principles and molecular pathways. Annu. Rev. Genet. 48, 187–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wang H, Di Gate RJ & Seeman NC An RNA topoisomerase. Proc. Natl Acad. Sci. USA 93, 9477–9482 (1996). The first demonstration that type 1A topoisomerases can work on RNA.
- 216.Yao NY, Schroeder JW, Yurieva O, Simmons LA & O’Donnell ME Cost of rNTP/dNTP pool imbalance at the replication fork. Proc. Natl Acad. Sci. USA 110, 12942–12947 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lam I & Keeney S Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb. Perspect. Biol. 7, a016634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pourquier P et al. Induction of reversible complexes between eukaryotic DNA topoisomerase I and DNA-containing oxidative base damages. J. Biol. Chem. 274, 8516–8523 (1999). [DOI] [PubMed] [Google Scholar]
- 219.Lesher DT, Pommier Y, Stewart L & Redinbo MR 8-Oxoguanine rearranges the active site of human topoisomerase I. Proc. Natl Acad. Sci. USA 99, 12102–12107 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sabourin M & Osheroff N Sensitivity of human type II topoisomerases to DNA damage: stimulation of enzyme-mediated DNA cleavage by abasic, oxidized and alkylated lesions. Nucleic Acids Res. 28, 1947–1954 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pourquier P et al. Effects of uracil incorporation, DNA mismatches, and abasic sites on cleavage and religation activities of mammalian topoisomerase I. J. Biol. Chem. 272, 7792–7796 (1997). [DOI] [PubMed] [Google Scholar]
- 222.Kingma PS, Corbett AH, Burcham PC, Marnett LJ & Osheroff N Abasic sites stimulate double-stranded DNA cleavage mediated by topoisomerase II. J. Biol. Chem. 270, 21441–21444 (1995). [DOI] [PubMed] [Google Scholar]
- 223.Kingma PS & Osheroff N Topoisomerase II-mediated DNA cleavage and religation in the absence of base pairing. Abasic lesions as a tool to dissect enzyme mechanism. J. Biol. Chem. 273, 17999–18002 (1998). [DOI] [PubMed] [Google Scholar]
- 224.Kingma PS & Osheroff N Apurinic sites are position-specific topoisomerase II poisons. J. Biol. Chem. 272, 1148–1155 (1997). [DOI] [PubMed] [Google Scholar]
- 225.Deweese JE, Burgin AB & Osheroff N Using 3′-bridging phosphorothiolates to isolate the forward DNA cleavage reaction of human topoisomerase IIa. Biochemistry 47, 4129–4140 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pourquier P, Bjornsti MA & Pommier Y Induction of topoisomerase I cleavage complexes by the vinyl chloride adduct 1,N6-ethenoadenine. J. Biol. Chem. 273, 27245–27249 (1998). [DOI] [PubMed] [Google Scholar]
- 227.Pommier Y et al. Benzo[a]pyrene epoxide adducts in DNA are potent inhibitors of a normal topoisomerase I cleavage site and powerful inducers of other topoisomerase I cleavages. Proc. Natl Acad. Sci. USA 97, 2040–2045 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pommier Y et al. Position-specific trapping of topoisomerase I–DNA cleavage complexes by intercalated benzo[a]- pyrene diol epoxide adducts at the 6-amino group of adenine. Proc. Natl Acad. Sci. USA 97, 10739–10744 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pommier Y et al. Different effects on human topoisomerase I by minor groove and intercalated deoxyguanosine adducts derived from two polycyclic aromatic hydrocarbon diol epoxides at or near a normal cleavage site. J. Biol. Chem. 277, 13666–13672 (2002). [DOI] [PubMed] [Google Scholar]
- 230.Pourquier P et al. Topoisomerase I-mediated cytotoxicity of N-methyl-N′-nitro-N-nitrosoguanidine: trapping of topoisomerase I by the O6-methylguanine. Cancer Res. 61,53–58 (2001). [PubMed] [Google Scholar]
- 231.Antony S et al. Enhancement of camptothecin-induced topoisomerase I cleavage complexes by the acetaldehyde adduct N2-ethyl-2′-deoxyguanosine. Nucleic Acids Res. 32, 5685–5692 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dexheimer TS, Kozekova A, Rizzo CJ, Stone MP & Pommier Y The modulation of topoisomerase I-mediated DNA cleavage and the induction of DNA–topoisomerase I crosslinks by crotonaldehyde-derived DNA adducts. Nucleic Acids Res. 36, 4128–4136 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Khan QA et al. Position-specific trapping of topoisomerase II by benzo[a]pyrene diol epoxide adducts: implications for interactions with intercalating anticancer agents. Proc. Natl Acad. Sci. USA 100, 12498–12503 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Velez-Cruz R et al. Exocyclic DNA lesions stimulate DNA cleavage mediated by human topoisomerase IIα in vitro and in cultured cells. Biochemistry 44, 3972–3981 (2005). [DOI] [PubMed] [Google Scholar]
- 235.Deweese JE & Osheroff N The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 37, 738–748 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pommier Y, Jenkins J, Kohlhagen G & Leteurtre F DNA recombinase activity of eukaryotic DNA topoisomerase I: effects of camptothecin and other inhibitors. Mutat. Res. 337, 135–145 (1995). [DOI] [PubMed] [Google Scholar]
- 237.Wang X, Henningfeld KA & Hecht SM DNA topoisomerase I-mediated formation of structurally modified DNA duplexes. Effects of metal ions and topoisomerase I inhibitors. Biochemistry 37, 2691–2700 (1998). [DOI] [PubMed] [Google Scholar]
- 238.Lanza A, Tornatelli S, Rodolfo C, Scanavini MC & Pedrini AM Human DNA topoisomerase I-mediated cleavages stimulated by ultraviolet light-induced DNA damage. J. Biol. Chem. 271,6978–6986 (1996). [DOI] [PubMed] [Google Scholar]
- 239.Subramanian D, Rosenstein BS & Muller MT Ultraviolet-induced DNA damage stimulates topoisomerase I–DNA complex formation in vivo: possible relationship with DNA repair. Cancer Res. 58, 976–984 (1998). [PubMed] [Google Scholar]
- 240.Corbett AH, Zechiedrich EL, Lloyd RS & Osheroff N Inhibition of eukaryotic topoisomerase II by ultraviolet-induced cyclobutane pyrimidine dimers. J. Biol. Chem. 266, 19666–19671 (1991). [PubMed] [Google Scholar]
- 241.Wang Y, Knudsen BR, Bjergbaek L, Westergaard O & Andersen AH Stimulated activity of human topoisomerases IIα and Πβ on RNA-containing substrates. J. Biol. Chem. 274, 22839–22846 (1999). [DOI] [PubMed] [Google Scholar]
- 242.Ju BG & Rosenfeld MG A breaking strategy for topoisomerase Πβ/PARP-1-dependent regulated transcription. Cell Cycle 5, 2557–2560 (2006). [DOI] [PubMed] [Google Scholar]
- 243.Lis JT & Kraus WL Promoter cleavage: a TopoIIβ and PARP-1 collaboration. Cell 125, 1225–1227 (2006) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.