ABSTRACT
Aims/Introduction
Glucagon‐like peptide‐1 receptor agonists (GLP‐1Ras) are widely used to treat type 2 diabetes. They not only reduce glucose, but also have a positive effect on weight loss. However, few studies have reported the effect of GLP‐1Ras on fat distribution.
Materials and Methods
PubMed, Cochrane, Embase and ClinicalTrials.gov were searched for randomized controlled trials on GLP‐1Ras and type 2 diabetes, published from inception to June 2021. Our main outcomes were the reductions of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT). Other anthropometric outcomes were also assessed. We used the Cochrane Collaboration tools to assess the risk of bias in the included studies. The quality of the evidence was assessed using the Grades of Recommendation, Assessment, Development and Evaluation profiler version 3.6. Review Manager 5.4.1 and Stata 16.0 were used for data analysis.
Results
A total of 10 studies involving 541 patients were included. Compared with the control groups, the GLP‐1Ras groups showed reductions in VAT (standard mean difference −0.54, 95% confidence interval [CI] −0.92, −0.17, I 2 = 79%, P = 0.005) and SAT (standard mean difference −0.44, 95% CI −0.60, −0.27, I 2 = 44%, P < 0.00001). In addition, bodyweight (weighted mean difference −3.59, 95% CI −4.30, −2.88, I 2 = 0%, P < 0.00001), waist circumference (weighted mean difference −3.09, 95% CI −4.66, −1.52, I 2 = 70%, P = 0.0001) and body mass index (weighted mean difference −1.11, 95% CI −1.35, −0.86, I 2 = 47%, P < 0.00001) were significantly decreased. According to the Grades of Recommendation, Assessment, Development and Evaluation approach, the level of evidence was low or moderate.
Conclusion
This study highlights that GLP‐1Ras, especially liraglutide and exenatide, might play an active role in fat distribution in patients with type 2 diabetes. After treatment with GLP‐1Ras, both VAT and SAT decreased, and the decrease of VAT was numerically greater than that of SAT.
Keywords: Fat distribution, Glucagon‐like peptide‐1, Type 2 diabetes
![]()
INTRODUCTION
Diabetes is one of the most common diseases worldwide, and 90% of people with diabetes have type 2 diabetes 1 . Type 2 diabetes is caused by a combination of reduced insulin production by pancreatic β‐cells and peripheral insulin resistance 2 . Insulin resistance is a major feature not only of type 2 diabetes, but also of a range of atherogenic diseases 3 and obesity 4 . However, body fat distribution is a key determinant of insulin sensitivity 5 . Body fat distribution might be more important than obesity in determining insulin resistance, the possible risk of type 2 diabetes and cardiovascular disease 3 .
Traditionally, human adipose tissue is mainly distributed in two regions with different metabolic characteristics: subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) 6 . VAT has been linked to the pathology of a variety of diseases, including insulin resistance; dysregulation of glucose and lipid metabolism; and increased susceptibility to prostate, breast and colon cancer 6 . Accumulation of VAT also determines the overall cardiovascular risk profile, and increases the risk of arterial hypertension and ischemic heart disease 6 .
An increasing number of patients with type 2 diabetes are being treated Glucagon‐like peptide‐1 receptor agonists (GLP‐1Ras) 7 . GLP‐1Ras are biological agents that suppress glycemic levels by slowing gastric emptying, reducing food intake and postprandial glucagon, and increasing glucose‐dependent insulin secretion 8 . It not only significantly lowers glucose levels and reduces the incidence of hypoglycemia, but also has important advantages in controlling obesity and cardiovascular risk 9 . Multiple trials have shown that GLP‐1Ras can significantly reduce the weight of patients with type 2 diabetes and obesity 9 ; however, few studies have examined the effect of GLP‐1Ras on fat distribution. Because fat distribution is closely related to the risk of insulin resistance, type 2 diabetes, cardiovascular and cerebrovascular diseases, and so on, we carried out a systematic review and meta‐analysis of randomized trials of GLP‐1Ras in the treatment of type 2 diabetes, which included indicators related to fat distribution.
METHODS
This systematic review and meta‐analysis was carried out in accordance with the Preferred Reporting Items for Systematic reviews and Meta‐analyses (PRISMA) statement 10 . The protocol for this review was registered with PROSPERO (ID: CRD42021242197).
Data sources and search strategy
Embase, Cochrane, PubMed and ClinicalTrials.gov. were searched for studies published from inception to June 2021. In addition, we identified trials that were not published or completed on ClinicalTrials.gov. The search strategy combined free‐text and MeSH terms as follows: (“subcutaneous adipose tissue” OR “visceral adipose tissue” OR “SAT” OR “VAT” OR “abdominal adiposity”) AND (“glucagon‐like peptide‐1 agonists” OR “glucagon like peptide*” OR “GLP‐1” OR “albiglutide” OR “dulaglutide” OR “Exenatide” OR “Liraglutide” OR “lixisenatide” OR “semaglutide” OR “taspoglutide”) AND (randomized controlled trial [Publication Type]). This search strategy was also adapted for other databases.
Study selection
The inclusion criteria were as follows: (i) patients diagnosed with type 2 diabetes; (ii) randomized controlled trials (RCTs); (iii) studies comparing GLP‐1Ras with placebo or active comparator drugs; and (iv) studies reporting results on VAT or SAT, or presenting adequate data to calculate them. The exclusion criteria were: (i) non‐English publications; (ii) reviews, brief reports, conference abstracts, animal experiments and cell experiments; (iii) incomplete basic data or relevant data unobtainable through data transformation; (iv) non‐adult patients; and (v) unpublished or incomplete trials. We used a combination of EndNote X9 and manual exclusion to exclude duplicate documents. Then, according to the aforementioned inclusion and exclusion criteria, we first screened the title and abstract, followed by the full text. Screening was carried out independently by two authors then cross‐checked. If there was a disagreement, a third author was consulted to decide whether to include the study.
Data extraction
We extracted the following information from the included studies: the last name of the first author, publication year, study design, population, intervention, control, diagnostic method, sample size, duration, baseline body mass index (BMI), key findings (changes in VAT and SAT in each treatment group), quality of trials, mean change and standard deviation of study outcomes from baseline to the end. If the studies we included reported data other than VAT and SAT (baseline mean and standard deviation [SD] and end‐point mean and SD, standard error (SE), 95% confidence interval [CI]), the corresponding formulas were applied to obtain the required data: SDE, change = √(SD2 E,baseline + SD2 E,final – [2 × Corr × SDE,baseline × SDE,final])11, SD = SE × √n 11, SD = √n [upper‐limit − lower‐limit]) / 3.92 11 ; if the studies reported the interquartile range, we used the following formula to obtain the data: ≈ (0.7 + 0.39 / n) / (q3 − q1)2 + (0.3 − 0.39 / n)m 12 , S ≈ (q3 − q1) /( 2Ф−1 [0.75 n − 0.125] / [n + 0.25]) 13 .
Quality assessment
We used Cochrane Collaboration tools 14 to assess the risk of bias in the included studies, it consists of the following domains: selection bias (sequence generation sufficiency, allocation concealment, performance bias (blinding), attrition bias (clarification of failures, incomplete outcome data), reporting bias (selective reporting of the results) and other possible sources of bias. According to these criteria, we divided the trials into three quality levels: low risk, the above domains were all low risk of bias; medium risk, one or two domains were low risk of bias or unclear risk of bias; and high risk, more than two domains were low risk of bias or unclear risk of bias. We evaluated the level of evidence by using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach 15 . In addition, the GRADE profiler version 3.6 was used to create the evidence profile. The evaluation standards included study limitation, inconsistencies of the results, indirectness, imprecision and publishing biases. The GRADE system classifies the quality of evidence in one of four levels: “high,” “moderate,” “low” and “very low.” Data extraction and quality assessment in the present review were completed by two authors, if there were any contradiction, it would be resolved with the third author.
Statistical analysis
Statistical analysis of this review was carried out using Review Manager version 5.4.1 (The Nordic Cochrane Center, The Cochrane Collaboration). For continuous outcomes, we used a fixed effects model to obtain the weighted mean difference (WMD) and 95% CI; if a different measurement method or unit was applied to the study results, then the standard mean difference (SMD) was applied instead. A P‐value <0.05 showed statistical significance. The I 2 index was applied to estimate statistical heterogeneity: I 2 > 50% suggested high heterogeneity, and the statistics were adjusted using a random effects model to reduce the heterogeneity. In addition, we also carried out sensitivity analysis by excluding included studies one by one, and subgroup analysis was carried out for intervention duration, different control groups, baseline BMI, diagnostic methods and types of GLP‐1Ras to find the source of heterogeneity. In each subgroup, I 2 ≤ 50% and combined I 2 > 50% suggested that the classification factor might be the source of heterogeneity. If the source of heterogeneity remained unidentified, we further carried out multivariate regression analysis using Stata 16.0 (StataCorp LP) to determine the source of heterogeneity. A P‐value <0.05 showed that this factor was a source of heterogeneity. Finally, funnel plots were constructed using Review Manager 5.4.1, and Egger's test was carried out using Stata 16.0 to determine whether there was publication bias. Symmetric funnel plots or a P‐value <0.05 on Egger's test showed no publication bias.
RESULTS
Search results
Initially, 272 studies were generated after searching the mentioned databases; 99 articles were deleted for duplication, and the remaining 173 articles were filtered by screening the titles and abstracts. After the initial screening, 27 articles remained, which were screened by reading the full text. In the end, 17 articles were excluded for the following reasons: (i) one article was not in English; (ii) four articles were abstracts of a meeting; (iii) six articles had insufficient data; and (iv) six articles were incomplete or unpublished. Figure 1 shows the detailed literature selection process.
Figure 1.
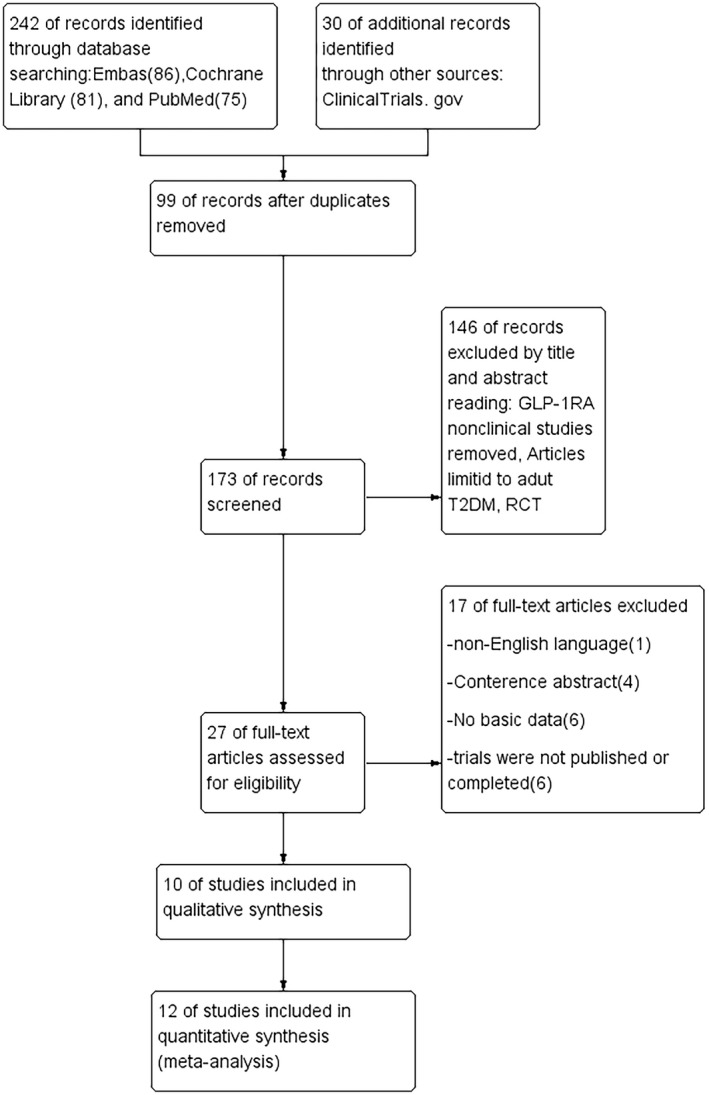
Literature selection process.
Study characteristics
Table 1 lists the main features of the included RCTs. Of the 10 articles including 12 randomized controlled trials, all participants (541) were diagnosed as having type 2 diabetes, and three of the articles involved participants with non‐alcoholic fatty liver disease. In seven studies, the intervention was liraglutide; in three studies, the intervention was exenatide. To measure VAT and SAT, one study used computed tomography, and the other nine studies used magnetic resonance imaging. In four of the 12 randomized controlled trials, controls were used as placebos, and controls in the remaining eight RCTs were active comparator drugs. Of the 12 randomized controlled trials, five had an intervention duration of ≤24 weeks, whereas the remaining seven had an intervention duration of >24 weeks.
TABLE 1.
Main characteristics of the included studies
| First authors | Publication year | Study design | Population | Intervention group | Control group | Diagnostic method | Sample size | Duration | Baseline BMI (kg/m2). (Intervention; Control) | Key Findings (Control; Intervention) |
|---|---|---|---|---|---|---|---|---|---|---|
| Yan et al. (a) | 2019 | RCT | Type 2 diabetes mellitus and NAFLD | Liraglutide 1.8 mg/day (0.6 mg/day and then increased by weekly to 1.8 mg/day or the maximum tolerated dose [at least 1.2 mg/day]) +Met |
Insulin glargine 0.2 IU/kg/day +Met |
MRI | 36 | 26 weeks |
30.1 ± 3.3 29.6 ± 3.5 |
VAT↓, SAT↓, |
| Yan et al. (b) | 2019 | RCT | Type 2 diabetes mellitus and NAFLD | Liraglutide 1.8 mg/day (0.6 mg/day and then increased by weekly to 1.8 mg/day or decelerated dose [at least 1.2mg/day]) +Met | Sitagliptin100 mg/day + Met | MRI | 39 | 26 weeks |
30.1 ± 3.3; 29.7 ± 2.8 |
VAT↓, SAT↓ |
| Wang et al. | 2020 | RCT | Type 2 diabetes mellitus and visceral adiposity | Exenatide 10 μg, b.i.d. (exenatide 5 μg, b.i.d., for 4 weeks and then 10 µg b.i.d. for 20 weeks) |
Humalog Mix25 0.4 IU/kg, b.i.d. |
MRI | 95 | 24 weeks |
23.96 ± 1.18; 23.50 ± 1.24 |
VAT↓, SAT↓ |
| Vanderheiden et al. | 2016 | RCT | Type 2 diabetes mellitus (uncontrolled type 2 diabetes requiring high doses of insulin) | Liraglutide 1.8 mg/day (0.6 mg/day and then increased by weekly to 1.8 mg/day) | Placebo | MRI | 71 | 6 months |
40.7 ± 6.7; 41.6 ± 10.4 |
VAT↓, SAT↓ |
| van Eyk et al. | 2019 | RCT | Type 2 diabetes mellitus | Liraglutide 1.8 mg/day (0.6 mg/day and then titrated in 2 weeks to a maximum dose of 1.8 mg/day) | Placebo | MRI | 47 | 26 weeks |
30.4 ± 3.8; 28.6 ± 4.0 |
VAT↓, SAT↓ |
| Liu et al. | 2020 | RCT | Type 2 diabetes mellitus and NAFLD | Exenatide 10 µg bid (exenatide 5 µg bid, for 4 weeks and then 10 µg bid for 20 weeks) |
Insulin glargine 0.1–0.3 IU/Kg, qd |
MRI | 76 | 24 weeks |
28.49 ± 3.02; 27.84 ± 3.10 |
VAT↓, SAT↓ |
| Harreite et al. | 2021 | RCT | Type 2 diabetes mellitus | Exenatide 10 µg, b.i.d. (exenatide 5 µg, b.i.d., for 4 weeks and then 10 µg b.i.d. for 20 weeks) | Placebo 2 mg/week + DAPA 10 mg/day | MRI | 30 | 24 weeks | 31.9 ± 4.6; 30.7 ± 3.5 | VAT↓, SAT↓ |
| Guo et al. (a) | 2020 | RCT | Type 2 diabetes mellitus and NAFLD | Liraglutide 1.8 mg/day (0.6 mg/day and then increased by weekly to 1.8 mg/day) | Insulin glargine 10 IU/day, and titrated by 1 unit each day to achieve a fasting plasma glucose (FPG) < 7 mmol/L | MRI | 48 | 26 weeks | 29.2 ± 4.2; 28.3 ± 3.8 | VAT↓, SAT↓ |
| Guo et al. (b) | 2020 | RCT | Type 2 diabetes mellitus and NAFLD | Liraglutide 1.8 mg/day (0.6 mg/day and then increased by weekly to 1.8 mg/day) | placebo | MRI | 48 | 26 weeks | 29.2 ± 4.2; 28.6 ± 3.7 | VAT↓, SAT↓ |
| Bouchi et al. | 2017 | RCT | Type 2 diabetes mellitus | Liraglutide 0.9 mg/day (0.3 mg/day and then increased by weekly to 0.9 mg/day) + insulin (fixed dose) | Insulin (fixed dose) | CT | 19 | 24 weeks | 27.7 ± 2.5; 28.2 ± 2.5 | VAT↓SAT↓ |
| Bizino et al. | 2020 | RCT | Type 2 diabetes mellitus | Liraglutide 1.8 mg/day (uptitrated to 1.8 mg/day from week 3 onwards) | Placebo | MRI | 50 | 26 weeks | 32.6 ± 4.4; 31.6 ± 3.4 | VAT↓SAT↓ |
| Pastel et al | 2017 | RCT | Type 2 diabetes mellitus | Liraglutide 1.2 mg/day (0.6 mg/day for 2 weeks and then increased to 1.2 mg/day) | Diet | MRI | 30 | 16 weeks | 31.40 ± 3.47; 30.06 ± 4.85 | VAT↓SAT↓ |
Values are expressed as means ± standard deviation. BMI, body mass index; CT, computed tomography; DAPA, dapagliflozin; EXE, exenatide; Met, Metformin; MRI, magnetic resonance imaging; NAFLD, non‐alcoholic fatty liver disease; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Quality assessment evidence
The risk of bias of each included study is summarized in Figure 2. Of the 10 articles (12 randomized controlled trials), four articles were double‐blind, of which Bizino's trials 16 were rated as medium risk because of incomplete outcome data, whereas the remaining three trials 17 , 18 , 19 were rated as low risk. The other eight trials 20 , 21 , 22 , 23 , 24 , 25 were rated as high risk, largely because of open‐label and incomplete outcome data. Using the GRADE profiler version 3.6, overall strength of evidence was evaluated. The evaluation results are as follows: one study result showed “low quality,” and four studies showed “moderate quality,” as shown in Table 2.
Figure 2.
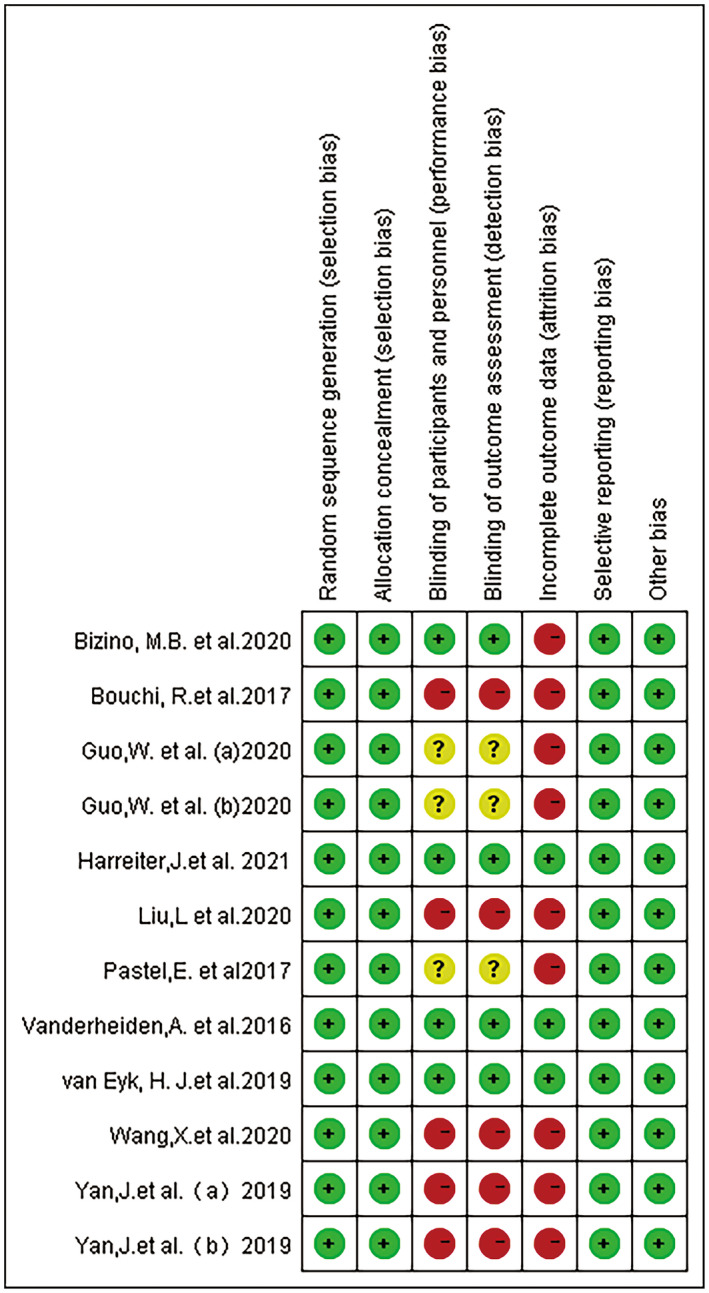
Quality assessment findings using Cochran risk of bias tool.
TABLE 2.
GRADE profile evidence of the included studies
| Quality assessment | No. patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | GLP‐1Ra | Control | Relative (95% CI) | Absolute | ||
| VAT (follow‐up 16–26 weeks; measured with: MRI, CT; Better indicated by lower values) | ||||||||||||
| 12 | Randomized trials | Serious † | Serious ‡ | No serious indirectness | No serious imprecision | None | 296 | 300 | – | MD 0.53 lower (0.7–0.36 lower) § |
⊕⊕OO LOW |
CRITICAL |
| SAT (follow‐up 16–26 weeks; measured with: MRI, CT; Better indicated by lower values) | ||||||||||||
| 12 | Randomized trials | Serious † | No serious inconsistency | No serious indirectness | No serious imprecision | None | 296 | 300 | – | MD 0.44 lower (0.6–0.27 lower) § |
⊕⊕⊕O MODERATE |
CRITICAL |
| Weight (follow‐up 16–26 weeks; Better indicated by lower values) | ||||||||||||
| 11 | Randomized trials | Serious † | No serious inconsistency | No serious indirectness | No serious imprecision | None | 266 | 268 | – | MD 3.59 lower (4.3–2.88 lower) |
⊕⊕⊕O MODERATE |
IMPORTANT |
| Waist (follow‐up 16–26 weeks; Better indicated by lower values) | ||||||||||||
| 9 | Randomized trials | Serious † | No serious inconsistency | No serious indirectness | No serious imprecision | None | 216 | 218 | – | MD 3.43 lower (4.22–2.64 lower) |
⊕⊕⊕O MODERATE |
IMPORTANT |
| BMI (follow‐up 16–26 weeks; Better indicated by lower values) | ||||||||||||
| 10 | Randomized trials | Serious † | No serious inconsistency | No serious indirectness | No serious imprecision | None | 258 | 259 | – | MD 1.11 lower (1.35–0.86 lower) |
⊕⊕⊕O MODERATE |
IMPORTANT |
BMI, body mass index; CT, computed tomography; MRI, magnetic resonance imaging; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Some studies lost follow‐up and failure to adhere to the intention to treat principle when indicated.
I 2 = 79%, P = 0.005, the heterogeneity between studies was large. Although subgroup analysis was carried out, the source of heterogeneity was not identified.
This is a difference in standard deviations. A standard deviation of 0.40–0.70 represents a moderate effect.
Meta‐analysis
A total of 10 studies (12 RCTs), including a total of 541 patients, contributed to the VAT analysis. Compared with the control group, VAT was significantly reduced in the GLP‐1Ras group (SMD −0.54, 95% CI −0.92, −0.17, I 2 = 79%, P = 0.005; Figure 3). A total of 10 studies (12 randomized controlled trials) including a total of 541 patients contributed to the SAT analysis. The GLP‐1Ras group showed a decrease in SAT compared with the control group (SMD −0.44, 95% CI −0.60, −0.27, I 2 = 44%, P < 0.00001; Figure 4).
Figure 3.
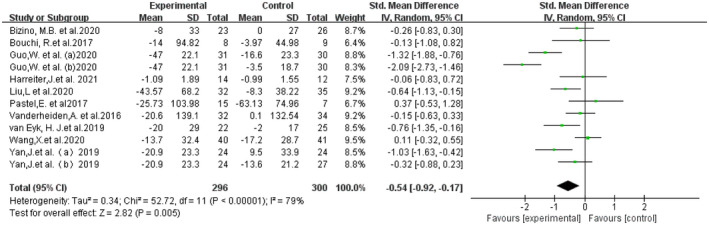
Forest plot comparing the post‐treatment visceral adipose tissue (VAT) of the control and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) groups.
Figure 4.
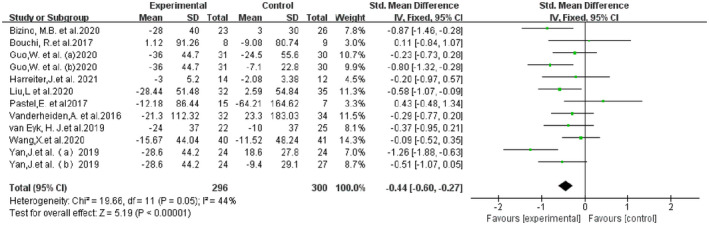
Forest plot comparing the post‐treatment subcutaneous adipose tissue (SAT) of the control and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) groups.
Effect on anthropometric outcomes of interest
Other anthropometric outcomes were also significantly reduced in the GLP‐1Ras group compared with the control group: bodyweight (WMD −3.59, 95% CI −4.30, −2.88, I 2 = 0, P < 0.00001), WC (WMD −3.09, 95% CI −4.66, −1.52, I 2 = 70%, P = 0.0001), BMI (WMD −1.11, 95% CI −1.35, −0.86, I 2 = 47%, P < 0.00001; Figures S1–S3).
Subgroup analysis, meta‐regression and sensitivity analysis
For VAT and WC, I 2 was >50% after the combined effect amounts showed high heterogeneity. We carried out subgroup analyses based on intervention duration, different control groups, baseline BMI, types of GLP‐1Ras and diagnostic methods. The results are shown in Table 3 and Table 4.
TABLE 3.
Subgroup analyses of effect of glucagon‐like peptide‐1 receptor agonist on visceral adipose tissue
| No. studies | SMD | 95% CI | I 2 | P * | I 2 between groups | P ** | |
|---|---|---|---|---|---|---|---|
| Intervention duration | 80% | 0.03 | |||||
| >24 weeks | 7 | −0.83 | −1.34, −0.33 | 82% | <0.00001 | ||
| ≤24 weeks | 5 | −0.12 | −0.49, 0.26 | 39% | 0.16 | ||
| Control group | 0% | 0.46 | |||||
| Placebo | 4 | −0.80 | −1.71, 0.12 | 88% | <0.0001 | ||
| Active comparator drugs | 8 | −0.42 | −0.83, −0.01 | 72% | 0.0009 | ||
| Baseline BMI | 74.1% | 0.02 | |||||
| Obesity (>30 kg/m2) | 7 | −0.36 | −0.67, −0.06 | 42% | 0.11 | ||
| Overweight (25–30 kg/m2) | 4 | −1.08 | −1.84, −0.33 | 83% | 0.0006 | ||
| Normal (<25 kg/m2) | 1 | 0.11 | −0.32, 0.55 | ‐ | ‐ | ||
| Types of GLP‐1RA | 41.5% | 0.19 | |||||
| Liraglutide | 9 | −0.66 | −1.13, −0.20 | 80% | <0.00001 | ||
| Exenatide | 3 | −0.20 | −0.71, 0.31 | 61% | 0.08 | ||
| Diagnostic method | 0% | 0.4 | |||||
| MRI | 11 | −0.57 | −0.97, −0.17 | 81% | <0.00001 | ||
| CT | 1 | −0.13 | −1.08, 0.82 | ‐ | ‐ |
BMI, body mass index; CI, confidence interval; CT, computed tomography; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; MRI, magnetic resonance imaging; SMD, standard mean difference; VAT, visceral adipose tissue.
P‐value for heterogeneity within each subgroup.
P‐value for heterogeneity between subgroup.
TABLE 4.
Subgroup analyses of effect of glucagon‐like peptide‐1 receptor agonist on waist circumference
| No. studies | WMD | 95% CI | I 2 | P * | I 2 between groups | P ** | |
|---|---|---|---|---|---|---|---|
| Intervention duration | 0% | 0.72 | |||||
| >24 weeks | 6 | −3.22 | −5.11, −1.33 | 79% | 0.0002 | ||
| ≤24 weeks | 3 | −2.46 | −6.12,1.20 | 41% | 0.19 | ||
| Control group | 69.6% | 0.07 | |||||
| Placebo | 3 | −4.53 | −5.86, −3.21 | 23% | 0.27 | ||
| Active comparator drugs | 6 | −2.13 | −4.37, −0.12 | 71% | 0.005 | ||
| Baseline BMI | 86.7% | 0.006 | |||||
| Obesity (>30 kg/m2) | 6 | −1.85 | −3.79, 0.10 | 61% | 0.02 | ||
| Over weight (25–30 kg/m2) | 3 | −5.00 | −6.14, −3.86 | 0% | 0.84 | ||
| Types of GLP‐1RA | 0% | 0.95 | |||||
| Liraglutide | 7 | −3.05 | −4.93, −1.17 | 76% | 0.0003 | ||
| Exenatide | 2 | −3.18 | −6.70, 0.33 | 45% | 0.18 |
BMI, body mass index; CI, confidence interval; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; MD, mean difference; WC, waist circumference; WMD, weighted mean difference.
P‐value for heterogeneity within each subgroup.
P‐value for heterogeneity between subgroup.
For VAT, subgroup analyses based on intervention duration showed that for an intervention duration >24 weeks, SMD −0.83, 95% CI −1.34, −0.33 and I 2 = 82%, P < 0.00001; for an intervention duration ≤24 weeks, SMD −0.12, 95% CI −0.49, 0.26 and I 2 = 39%, P = 0.16. Subgroup analyses based on baseline BMI showed that for obesity (baseline BMI >30 kg/m2), SMD −0.36, 95% CI −0.67, −0.06 and I 2 = 42%, P = 0.11; for overweight (baseline BMI 25–30 kg/m2), SMD −1.08, 95% CI −1.84, −0.33 and I 2 = 83%, P = 0.0006. Subgroup analyses based on different control groups, types of GLP‐1Ras and diagnostic methods showed that the I 2 of each subgroup was >50%.
The results of subgroup analysis suggested that intervention duration and baseline BMI might be the potential source of heterogeneity; however, different control groups, types of GLP‐1Ras and diagnostic methods were all non‐heterogeneous sources.
For WC, subgroup analyses based on intervention duration showed that for an intervention duration >24 weeks, WMD −3.22, 95% CI −5.11, −1.33 and I 2 = 79%, P = 0.0002; for an intervention duration ≤24 weeks, WMD −2.46, 95% CI −6.12, 1.20 and I 2 = 41%, P = 0.19. Subgroup analyses based on different control groups showed that for the placebo control group, WMD −4.53, 95% CI 5.86, −3.21 and I 2 = 23%, P = 0.27; for the active comparator drug control group, WMD −2.13, 95% CI −4.37, −0.12 and I 2 = 71%, P = 0.005). Subgroup analyses based on baseline BMI showed that for obesity (baseline BMI >30 kg/m2), WMD −1.85, 95% CI −3.79, 0.10 and I 2 = 61%, P = 0.02; for overweight (baseline BMI 25–30 kg/m2), WMD −5.00, 95% CI −6.14, −3.86 and I 2 = 0%, P = 0.84. Subgroup analyses based on types of GLP‐1Ras showed that for liraglutide, WMD −3.05, 95% CI −4.93, −1.17 and I 2 = 76%, P = 0.0003; for exenatide, WMD −3.18, 95% CI −6.70, 0.33 and I 2 = 45%, P = 0.18, and the results of subgroup analysis suggested that intervention duration, different control groups, baseline BMI and types of GLP‐1Ras were all possible sources of heterogeneity.
Multivariate meta‐regression was carried out according to the aforementioned factors, and the results showed that, for VAT, the regression was P > 0.05 for all the aforementioned factors. For WC, the regression for the control group was P = 0.035. The regression for BMI was P = 0.016, and the results showed that the two variables, different control groups and the baseline BMI were potential sources of heterogenicity. Sensitivity analysis was carried out by excluding the included references individually, and the results suggest that no single study significantly altered the ultimate heterogeneity.
Publication bias
The funnel plots for VAT, SAT, bodyweight, WC and BMI were visually symmetrical (Figures S4‐S8). Egger's test showed P = 0.5573 for VAT, P = 0.2950 for SAT, P = 0.2498 for bodyweight, P = 0.1987 for WC and P = 0.3618 for BMI. The publication bias was modest.
DISCUSSION
To our knowledge, this is the first systematic analysis to examine the effect of GLP‐1Ras on fat distribution. Although GLP‐1Ras in our included studies only included two types (liraglutide and exenatide), the present results showed that compared with other antidiabetic drugs or the placebo, at least these two types of GLP‐1Ras reduced both VAT and SAT, and the decrease of VAT was numerically greater than that of SAT (SMD −0.54, 95% CI −0.92, −0.17, I 2 = 79%, P = 0.005). The GLP‐1Ras group also significantly reduced bodyweight 16 , 17 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , WC 16 , 17 , 19 , 20 , 22 , 23 , 25 and BMI 16 , 17 , 19 , 20 , 21 , 22 , 23 , 25 .
Although no meta‐analysis of the effect of GLP‐1Ras on fat distribution has been found, a study by van Eyk et al. showed that liraglutide reduced VAT compared with placebo, and found a similar, but stronger, association between a reduction in VAT and a reduction in glycated hemoglobin after treatment; although a reduction in SAT was also associated with a reduction in glycated hemoglobin after treatment, reduction in other adipose tissue was not associated with a reduction in glycated hemoglobin levels 19 . Additionally, a study by Bi et al. showed that after 6 months of treatment with exenatide, pioglitazone or insulin, exenatide and pioglitazone significantly reduced VAT, and the decrease was greater than that of pioglitazone; exenatide also reduced SAT, whereas insulin and biaglitazone did not 26 . Furthermore, a study by Wang et al. observed greater decreases in weight, BMI, WC, VAT and SAT in the exenatide group than in the Humalog Mix25 group 21 .
There are several hypotheses regarding the mechanism of GLP‐1Ras influence on fat distribution and weight loss, including: (i) in diabetes and obese patients, the number of glucagon‐like peptide‐1 (GLP‐1) receptors on intra‐abdominal fat cells is significantly higher than that on subcutaneous fat cells, and GLP‐1 subsequently causes cell decomposition by activating GLP‐1 receptors. A more interesting finding was that high concentrations of GLP‐1 (10−10 M) promoted adipocyte decomposition, whereas low concentrations of GLP‐1 (10−12 M) promoted adipocyte synthesis; (ii) GLP‐1 acts on the receptor in the nucleus of the solitary tract, and suppresses food intake and appetite through the brain's limbic reward system; (iii) taste can also suppress appetite, as GLP‐1 receptors are found on cells in both savory and sweet taste buds; and (iv) GLP‐1 inhibits gastric emptying by combining with GLP‐1 receptors in the gastrointestinal tract 27 .
Studies have found that VAT plays a more important role in various metabolic abnormalities related to obesity than SAT. The increase in VAT not only reduces insulin sensitivity, but also increases the concentration of free fatty acids in hepatic portal blood 28 . Similarly, some studies found that after matching VAT, individuals with high or low SAT showed no difference in insulin sensitivity, whereas individuals with matched abdominal SAT, but high or low accumulation of VAT, showed significant differences in insulin resistance and glucose tolerance 29 . Thus, VAT is a better predictor of insulin resistance, even in patients with normal weight 30 .
A growing body of evidence has shown that VAT is associated with the occurrence of a variety of diseases, and excess visceral fat disrupts the secretion of adipocytokines, leading to the pathological features of metabolic syndrome and non‐alcoholic steatohepatitis 30 . In addition, active metabolism of VAT is the source of cellular and humoral inflammation in patients with obesity and coronary heart disease 31 . A prospective long‐term follow‐up study, the Framingham Heart Study, reported that VAT was an independent predictor of cardiovascular events 31 . Even more surprising is that VAT was not only significantly positively associated with the risk of colorectal adenomas 32 , but was also an important prognostic indicator of acute pancreatitis severity 33 . Therefore, in the treatment of patients with type 2 diabetes, it would be prudent to choose a drug that can not only regulate glucose metabolism, but also reduce VAT. The present study provides part of evidence that GLP‐1Ras (liraglutide and exenatide) change fat distribution and especially reduce VAT.
In the present meta‐analysis, after the study combination, the heterogeneity of VAT and WC was greater. Previous studies have shown that, for non‐elderly people, WC can be used as a reliable alternative indicator to estimate VAT, whereas BMI can be used as a reliable indicator to estimate SAT 34 . On the basis of the consistency of changes between WC and VAT, we carried out subgroup analysis for these two outcome indicators, as the heterogeneity in BMI, bodyweight and SAT was relatively small, we did not explore the sources of heterogeneity. Subgroup analysis based on the five‐factor intervention duration, different control groups, baseline BMI, diagnostic methods and types of GLP‐1Ras failed to find the definitive source of heterogeneity. In addition, considering that other drugs used in combination with GLP‐1Ras in the intervention group might also have had an impact on the outcome indicators, the same combination of drugs was also used in the control group after careful comparison, resulting in the elimination of the influence of this factor on outcomes. As a result, we did not carry out any further analysis.
To find the source of heterogeneity, we further carried out a meta‐regression. For VAT, subgroup analyses based on the intervention duration showed a significant difference for an intervention duration >24 weeks (I 2 = 82%, P < 0.00001), as compared with an intervention duration of ≤24 weeks (I 2 = 39%, P = 0.16). Subgroup analyses based on baseline BMI showed a significant difference for obesity (I 2 = 42%, P = 0.11), as compared with overweight (I 2 = 83%, P = 0.0006). The results of the subgroup analysis suggested that the intervention duration and baseline BMI might be the potential source of heterogeneity. However, the regression was P > 0.05 for all the aforementioned factors by multivariate meta‐regression. The source of heterogeneity might exist in other aspects, which requires further investigation.
For WC, different control groups and baseline BMIs were potential sources of heterogeneity. Subgroup analyses based on baseline BMI showed a significant result for obesity (baseline BMI >30 kg/m2), WMD −1.85, 95% CI −3.79, 0.10, I 2 = 61%, P = 0.02, as compared with overweight (baseline BMI 25–30 kg/m2), WMD −5.00, 95% CI −6.14, −3.86, I 2 = 0%, P = 0.84. This suggests that GLP‐1Ras might be more significantly associated with WC reduction in cases with an initial BMI of 25–30 kg/m2. These results were more robust; therefore, GLP‐1Ras might be more appropriate for patients who are overweight. However, as the initial BMI in all studies in which WC was considered as the outcome index in the selected articles was > 5 kg/m2, the influence of GLP‐1Ras on WC in patients with normal initial BMI requires further investigation.
We also assessed the level of evidence using the GRADE approach. According to the GRADE approach, the quality of the evidence was only low (VAT) and intermediate (the remaining four indicators) due to the following reasons: (i) some studies lost follow up, and failed to adhere to the intention‐to‐treat principle when indicated; and (ii) unexplained heterogeneity.
The present study had some limitations. First, all the included studies were in English, which might have led to publication bias or selection bias. Second, not all the experimental controls we selected were placebos, which might have increased the heterogeneity; however, in the present study, we carried out a subgroup analysis for the type of control group to determine the source of heterogeneity. Third, some of the results of the present study showed high heterogeneity; these need to be clarified by further research. Although we carried out subgroup analysis and meta‐regression, no clear source of heterogeneity was found for VAT. Fourth, the analyses were only from the studies of liraglutide and exenatide, whether other GLP‐1Ras have the same effect requires further study.
The present study is the first meta‐analysis of the effect of GLP‐1Ras on fat distribution. At least liraglutide and exenatide in GLP‐1Ras are associated with decreased VAT, as well as SAT. Because of the limitations of related literature, we were unable to study the effect of all types of GLP on fat distribution, and we also did not specifically study the effect of GLP‐1Ras on specific visceral fat distribution, such as epicardial adipose tissue and parapericardial adipose tissue. Future large‐scale RCTs are required to confirm these findings.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: N/A.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Figure S1 | Forest plot comparing the post‐treatment weight of the control and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) groups.
Figure S2 | Forest plot comparing the post‐treatment waist circumference (WC) of the control and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) groups.
Figure S3 | Forest plot comparing the post‐treatment body mass index (BMI) of the control and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) groups.
Figure S4 | Funnel plots for visceral adipose tissue (VAT).
Figure S5 | Funnel plots for subcutaneous adipose tissue (SAT).
Figure S6 | Funnel plots for weight.
Figure S7 | Funnel plots for waist circumference (WC).
Figure S8 | Funnel plots for body mass index (BMI).
ACKNOWLEDGMENTS
We thank Erika 10, PhD, from Wiley Editing Services (https://wileyeditingservices.com/cn/), for editing the English text of a draft of this manuscript.
J Diabetes Investig. 2022; 13: 1149–1160
References
- 1. Sharma D, Verma S, Vaidya S, et al. Recent updates on GLP‐1 agonists: current advancements & challenges. Biomed Pharmacother 2018; 108: 952–962. [DOI] [PubMed] [Google Scholar]
- 2. Galicia‐Garcia U, Benito‐Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci 2020; 21: 6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carey DG, Jenkins AB, Campbell LV, et al. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 1996; 45: 633–638. [DOI] [PubMed] [Google Scholar]
- 4. Al‐Goblan AS, Al‐Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 2014; 7: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846. [DOI] [PubMed] [Google Scholar]
- 6. Shuster A, Patlas M, Pinthus JH, et al. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol 2012; 85: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun F, Wu S, Wang J, et al. Effect of glucagon‐like peptide‐1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta‐analysis. Clin Ther 2015; 37: 225–241.e8. [DOI] [PubMed] [Google Scholar]
- 8. Jiang Y, Liu J, Chen X, et al. Efficacy and safety of glucagon‐like peptide 1 receptor agonists for the treatment of type 2 diabetes mellitus: a network meta‐analysis. Adv Ther 2021; 38: 1470–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dogruel H, Balci MK. Development of therapeutic options on type 2 diabetes in years: glucagon‐like peptide‐1 receptor agonist's role intreatment; from the past to future. World J Diabetes 2019; 10: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 11. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019; 10: ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat Methods Med Res 2018; 27: 1785–1805. [DOI] [PubMed] [Google Scholar]
- 13. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bizino MB, Jazet IM, de Heer P, et al. Placebo‐controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre‐specified secondary study on ectopic fat accumulation. Diabetologia 2020; 63: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harreiter J, Just I, Leutner M, et al. Combined exenatide and dapagliflozin has no additive effects on reduction of hepatocellular lipids despite better glycaemic control in patients with type 2 diabetes mellitus treated with metformin: EXENDA, a 24‐week, prospective, randomized, placebo‐controlled pilot trial. Diabetes Obes Metab 2021; 23: 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vanderheiden A, Harrison LB, Warshauer JT, et al. Mechanisms of action of liraglutide in patients with type 2 diabetes treated with high‐dose insulin. J Clin Endocrinol Metab 2016; 101: 1798–1806. [DOI] [PubMed] [Google Scholar]
- 19. van Eyk HJ, Paiman EHM, Bizino MB, et al. A double‐blind, placebo‐controlled, randomised trial to assess the effect of liraglutide on ectopic fat accumulation in South Asian type 2 diabetes patients. Cardiovasc Diabetol 2019; 18: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan J, Yao B, Kuang H, et al. Liraglutide, Sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology 2019; 69: 2414–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang X, Zhao X, Gu Y, et al. Effects of exenatide and humalog Mix25 on fat distribution, insulin sensitivity, and β‐cell function in normal bmi patients with type 2 diabetes and visceral adiposity. J Diabetes Res 2020; 2020: 9783859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Yan H, Xia MingFeng, et al. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev 2020; 36: e3292. [DOI] [PubMed] [Google Scholar]
- 23. Guo W, Tian W, Lin LU, et al. Liraglutide or insulin glargine treatments improves hepatic fat in obese patients with type 2 diabetes and nonalcoholic fatty liver disease in twenty‐six weeks: a randomized placebo‐controlled trial. Diabetes Res Clin Pract 2020; 170: 108487. [DOI] [PubMed] [Google Scholar]
- 24. Bouchi R, Nakano Y, Fukuda T, et al. associated with ameliorations of hepatic steatosis, albuminuria, and micro‐inflammation in type 2 diabetic patReduction of visceral fat by liraglutide isients with insulin treatment: a randomized control trial. Endocr J 2017; 64: 269–281. [DOI] [PubMed] [Google Scholar]
- 25. Pastel E, McCulloch L, Ward R, et al. GLP‐1 analogue‐induced weight loss does not improve obesity‐induced AT dysfunction. Clin Sci 2017; 131: 343–353. [DOI] [PubMed] [Google Scholar]
- 26. Bi Y, Zhang B, Xu W, et al. Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug‐naive subjects with type 2 diabetes. Acta Diabetol 2014; 51: 865–873. [DOI] [PubMed] [Google Scholar]
- 27. Shi LI, Zhu J, Yang P, et al. Comparison of exenatide and acarbose on intra‐abdominal fat content in patients with obesity and type‐2 diabetes: a randomized controlled trial. Obes Res Clin Pract 2017; 11: 607–615. [DOI] [PubMed] [Google Scholar]
- 28. Kim C, Park J, Park J, et al. Comparison of body fat composition and serum adiponectin levels in diabetic obesity and non‐diabetic obesity. Obesity 2006; 14: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 29. Després J‐P, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 30. Schäffler A, Schölmerich J, Büchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue–emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 2005; 2: 273–280. [DOI] [PubMed] [Google Scholar]
- 31. Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014; 233: 104–112. [DOI] [PubMed] [Google Scholar]
- 32. Hu H, Cai Y, Huang J, et al. Visceral adipose tissue and the risk of colorectal adenomas: a meta‐analysis of observational studies. Eur J Cancer Prev 2015; 24: 462–469. [DOI] [PubMed] [Google Scholar]
- 33. Kuan LL, Dennison AR, Garcea G. Association of visceral adipose tissue on the incidence and severity of acute pancreatitis: a systematic review. Pancreatology 2020; 20: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 34. Ping Z, Pei X, Xia P, et al. Anthropometric indices as surrogates for estimating abdominal visceral and subcutaneous adipose tissue: a meta‐analysis with 16,129 participants. Diabetes Res Clin Pract 2018; 143: 310–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Forest plot comparing the post‐treatment weight of the control and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) groups.
Figure S2 | Forest plot comparing the post‐treatment waist circumference (WC) of the control and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) groups.
Figure S3 | Forest plot comparing the post‐treatment body mass index (BMI) of the control and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) groups.
Figure S4 | Funnel plots for visceral adipose tissue (VAT).
Figure S5 | Funnel plots for subcutaneous adipose tissue (SAT).
Figure S6 | Funnel plots for weight.
Figure S7 | Funnel plots for waist circumference (WC).
Figure S8 | Funnel plots for body mass index (BMI).


