Abstract
Background: Thyroid hormones play a significant role in bone development and maintenance, with triiodothyronine (T3) particularly being an important modulator of osteoblast differentiation, proliferation, and maintenance. However, details of the biological processes (BPs) and molecular pathways affected by T3 in osteoblasts remain unclear.
Methods: To address this issue, primary cultures of human adipose-derived mesenchymal stem cells were subjected to our previously established osteoinduction protocol, and the resultant osteoblast-like cells were treated with 1 nm or 10 nm T3 for 72 h. RNA sequencing (RNA-Seq) was performed using the Illumina platform, and differentially expressed genes (DEGs) were identified from the raw data using Kallisto and DESeq2. Enrichment analysis of DEGs was performed against the Gene Ontology Consortium database for BP terms using the R package clusterProfiler and protein network analysis by STRING.
Results: Approximately 16,300 genes were analyzed by RNA-Seq, with 343 DEGs regulated in the 1 nm T3 group and 467 upregulated in the 10 nm T3 group. Several independent BP terms related to bone metabolism were significantly enriched, with a number of genes shared among them (FGFR2, WNT5A, WNT3, ROR2, VEGFA, FBLN1, S1PR1, PRKCZ, TGFB3, and OSR1 for 1nM T3; and FZD1, SMAD6, NOG, NEO1, and ENG for 10 nm T3). An osteoblast-related search in the literature regarding this set of genes suggests that both T3 doses are unfavorable for osteoblast development, mainly hindering BMP and canonical and non-canonical WNT signaling.
Conclusions: Therefore, this study provides new directions toward the elucidation of the mechanisms of T3 action on osteoblast metabolism, with potential future implications for the treatment of endocrine-related bone pathologies.
Keywords: osteobalst, triiodothyronine, BMP—smad signaling pathway, RNA-seq, TGF-beta signaling pathway
Introduction
The skeletal system undergoes intense metabolic activity, maintaining a continuous process of bone remodeling through the action of bone cells. Osteoblasts originate from mesenchymal stem cells and are responsible for the synthesis of the bone extracellular matrix, deposition and mineralization of new bone, thereby promoting bone formation (Hadjidakis and Androulakis, 2006; Cawthray et al., 2017). Additionally, osteoblasts control bone remodeling by modulating osteoclastogenesis and bone resorption by the osteoclasts (Hadjidakis and Androulakis, 2006; Feng and McDonald, 2011; Boyce et al., 2012; Hayden et al., 2014).
Thyroid hormones (THs) act as regulators of the bone remodeling process and influence formation of the skeletal system (Hadjidakis and Androulakis, 2006; Boyce et al., 2012; Cawthray et al., 2017). Osteoblasts are known to express nuclear receptors for THs, namely, thyroid hormone receptor beta (THRB) and alpha (THRA) (Abu et al., 1997; Kim and Mohan, 2013). The THs triiodothyronine (T3) and thyroxine (T4) are especially essential for bone development and maintenance (Bassett et al., 2003; Straub, 2014), as changes in their levels may affect bone metabolism and cause abnormalities, such as changes in the bone mineral density (Waung et al., 2012; Kim and Mohan, 2013; Wojcicka et al., 2013).
Although T3 in particular is known to play an important role in osteoblastogenesis (Klaushofer et al., 1995; Waung et al., 2012; Kim and Mohan, 2013; Wojcicka et al., 2013; Olímpio et al., 2019), its exact biological and molecular mechanisms of action have not been fully elucidated (Harvey et al., 2002; Waung et al., 2012; Kim and Mohan, 2013; Pascual and Aranda, 2013). Therefore, in this study, we aimed to evaluate the effects of different T3 doses on gene expression in osteoblast-like cells, differentiated from human adipose-derived mesenchymal stem cells (hASCs), through a global transcriptome analysis, using RNA sequencing (RNA-Seq) techniques. Overall, our data provide innovative information that adds to existing knowledge about bone development and will help toward clarifying the role that T3 plays in the pathophysiological mechanisms of bone diseases.
Materials and Methods
Cell Culture
This study was approved by the Ethics Committee of the Botucatu Medical School, São Paulo State University (UNESP; Approval No.3216-2009). Primary cultures of the previously characterized model of hASCs (Olimpio et al., 2018) from three donors were provided by the Experimental Research Unit (Unipex) cell bank of UNESP. The methods used to culture the hASCs and to induce their differentiation into osteoblast-like cells were carried out as previously described (for details, see Olimpio et al., 2018). In brief, hASCs were isolated from subcutaneous adipose tissue obtained from three patients undergoing abdominoplasty, up to 50 years of age with normal erythrocyte sedimentation rate (ESR). Subcutaneous adipose tissue samples were then submitted to enzymatic digestion. The isolated hASCs were plated at a density of 2 × 105 in a T25 flask, and grown in a complete medium, defined as Dulbecco’s modified Eagle medium (DMEM), containing 10% fetal bovine serum (FBS) with 1% penicillin-streptomycin and 0.1% gentamicin (10 mg/ml; Invitrogen). Upon reaching 70% confluency, cells were trypsinized and transferred to a T75 flask for cell expansion. All cell cultures were maintained at 37°C in a humidified atmosphere with 5% CO2. For hASC differentiation into osteoblasts, cells were kept in complete DMEM supplemented with 0.1 μM dexamethasone (Sigma-Aldrich), 50 μM ascorbic acid (Sigma-Aldrich), and 10 mm β-glycerophosphate (Sigma-Aldrich) for 16 days. The resulting osteoblast-like cells were then treated with either 1 nm or 10 nm T3 for 72 h. Osteoblast-like cells grown in the absence of T3 were used as controls.
RNA Sequencing and Bioinformatics
Total RNA was extracted from the osteoblast-like cells using the TRIzol reagent method (Invitrogen, Carlsbad, CA, United States). The cDNA library preparation, RNA sequencing, and bioinformatics analysis were carried out using previously described methods (de Oliveira et al., 2020). DEGs were classified as being upregulated or downregulated on the basis of fold-change (FC) values > 1.5, with p < 0.05. The Gene Ontology (GO) enrichment analysis for biological process (BP) terms was performed with the clusterProfiler R package, using a p-value-adjusted false discovery rate and a p-value of < 0.05. Pre-analysis of the GO data was performed, and terms distant from the area of interest were excluded. For the enriched GO terms grouped by similarity (0.7) representation, interactive graphs and TreeMaps were created using REVIGO (http://revigo.irb.hr/) (Supek et al., 2011). DEGs were also analyzed with respect to their protein-protein interactions (PPI) using STRING. Interaction maps were generated considering the following levels of evidence: homology, coexpression, experimentally determined interactions, database-annotated interactions, and text mining. Enriched GO terms were assessed by having an FDR < 0.05.
Results
Characterization of Osteoblast-Like Cells
The RNA-Seq analysis revealed the expression patterns of 16,296 genes in the two groups of T3-treated osteoblast-like cells. Of the 10 most expressed genes from this data set, four encoded bone markers: fibronectin 1 (FN1), osteonectin (SPARC), and collagen type I alpha 1 and 2 chains (COL1A1 and COL1A2). In agreement with other published results, the presence of the nuclear receptors THRA and THRB was also noted, with the former being more abundant in these cells. Additionally, in a previous study conducted by our research group (Olímpio et al., 2019), the presence of genes encoding other bone markers was observed: osteocalcin and alkaline phosphatase proteins, matrix proteins for bone mineralization, and receptor activator of nuclear factor kappa-Β ligand (RANKL).
Transcriptional Regulation by the T3 Treatments
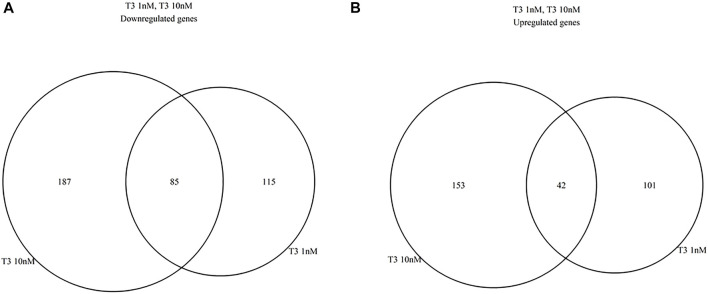
Differential gene expression was analyzed between the T3-treated and control (non-treated) groups (Figures 1, 2, and Supplementary Material). For the 1 nM T3 group, 343 differentially expressed genes (DEGs) were identified, of which 200 were upregulated (58%) and 143 were downregulated (42%). For the 10 nm T3 group, 467 DEGs were identified, of which 272 genes were upregulated and 195 were downregulated (also 58 and 42%, respectively). There was an overlap of roughly 20% among genes regulated by both doses (Figure 3) and, importantly, no gene was altered in opposite directions by one T3 dose compared to the other (not shown).
FIGURE 1.
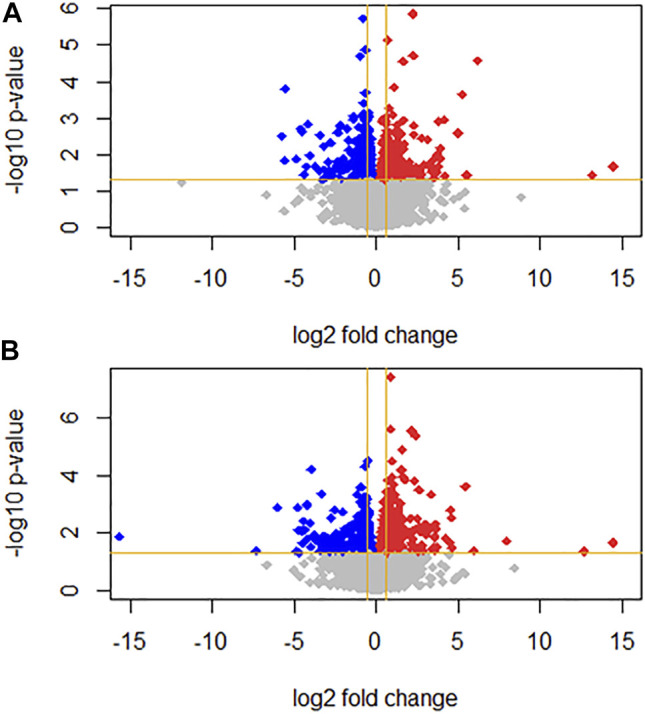
Volcano plots representing gene expression log2 fold change (FC; x axis) and p-value (y axis) for (A) 1 nm T3 and (B) 10 nm T3. Grey dots represent genes with non-significant FC; up- and downregulated genes are represented as red and blue dots, respectively.
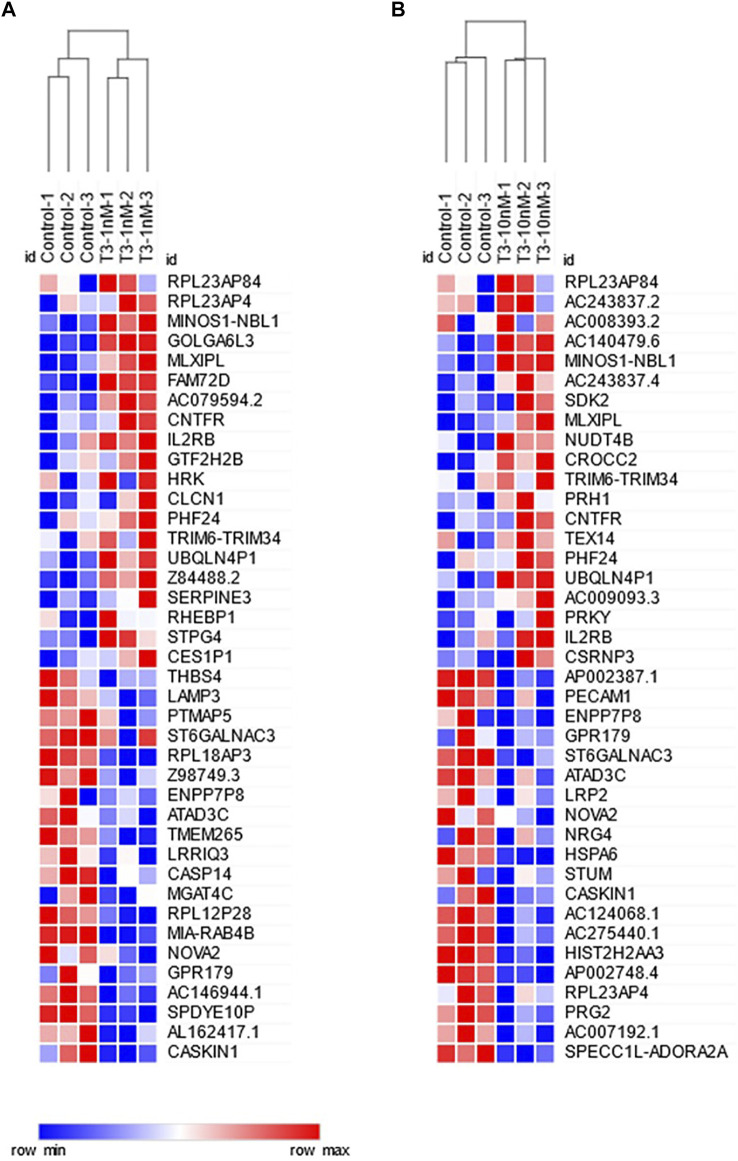
FIGURE 2.
Heatmaps showing the 20 most upregulated (upper half) and 20 most downregulated (lower half) genes for (A) 1 nm T3 and (B) 10 nm T3. Samples (columns) and genes (rows) are hierarchically clustered by mean Euclidean distance.
FIGURE 3.
Venn diagrams summarizing similarities between 1 and 10 nm T3 expression profiles, relative to the Control group; (A), dowregulated genes, (B) upregulated genes.
Gene Ontology Analysis—ClusterProfiler
For 1nM T3, eight and 56 GO biological process (BP) terms were significantly enriched in the up- and down-regulated gene sets, respectively; after manually filtering the terms, we found 11 terms were relevant to the study, all enriched in the down-regulated gene set (Table 1). For the 10 nm T3 group, 49 BP terms were enriched for up-regulated genes, but none reached significance for down-regulated genes. Among the significant terms, the majority was related to embryonic development and none has apparent relation to osteoblast biology; the complete overrepresentation analysis results are presented in the Supplementary Material. The REVIGO tool, which summarizes GO terms on the basis of semantic similarity to reduce redundancy, was used to simplify the results from 1 nm T3 treatment and to clarify the BPs affected (Supek et al., 2011); after analyzing the BP terms with REVIGO, TreeMaps was used to group 11 BP terms into six main terms for the downregulated genes of the 1 nm T3 (Table 1). The main genes involved in the enriched BP terms (Table 2) are examined in the Discussion.
TABLE 1.
GO terms significantly enriched for genes downregulated after 1 nm T3 treatment, manually filtered for relevance to osteoblast biology. The 11 terms were hierarchically grouped under six main terms (bold) using TreeMaps.
| Description | GO ID | Gene count | Gene ratio | Bg ratio | Adj. p-value |
|---|---|---|---|---|---|
| mesenchymal cell proliferation | GO:0010463 | 4 | 4/105 | 44/18670 | 0,027 |
| stem cell proliferation | GO:0072089 | 5 | 5/105 | 120/18670 | 0,036 |
| positive regulation of mesenchymal cell proliferation | GO:0002053 | 3 | 3/105 | 25/18670 | 0,031 |
| regulation of cell morphogenesis involved in differentiation | GO:0010769 | 8 | 8/105 | 301/18670 | 0,031 |
| cell fate commitment | GO:0045165 | 7 | 7/105 | 271/18670 | 0,039 |
| ossification | GO:0001503 | 9 | 9/105 | 398/18670 | 0,031 |
| cellular response to retinoic acid | GO:0071300 | 4 | 4/105 | 69/18670 | 0,036 |
| positive regulation of Wnt signaling pathway | GO:0030177 | 6 | 6/105 | 179/18670 | 0,036 |
| positive regulation of chemotaxis | GO:0050921 | 5 | 5/105 | 135/18670 | 0,044 |
| protein kinase C signaling | GO:0070528 | 4 | 4/105 | 29/18670 | 0,022 |
| peptidyl-tyrosine phosphorylation | GO:0018108 | 9 | 9/105 | 363/18670 | 0,029 |
Gene count, number of differentially expressed (DE) genes associated with the GO term; gene ratio, associated genes/total DE genes; Bg ratio, number of associated genes/total background genes (all genes annotated in the Gene Ontology Consortium database); Adj p-value, false discovery rate-adjusted p-value.
TABLE 2.
Main downregulated genes contributing to enriched GO terms, in the 1 nm T3 group.
| FGFR2 fibroblast growth factor receptor 2 |
| WNT5A Wnt family member 5A |
| WNT3 Wnt family member 3 |
| ROR2 receptor tyrosine kinase like orphan receptor 2 |
| VEGFA vascular endothelial growth factor A |
| FBLN1 fibulin 1 |
| S1PR1 sphingosine-1-phosphate receptor 1 |
| PRKCZ protein kinase C zeta |
| TGFB3 transforming growth factor beta 3 |
| OSR1 oxidative stress responsive kinase 1 |
| AREG amphiregulin |
Gene Ontology Analysis—STRING
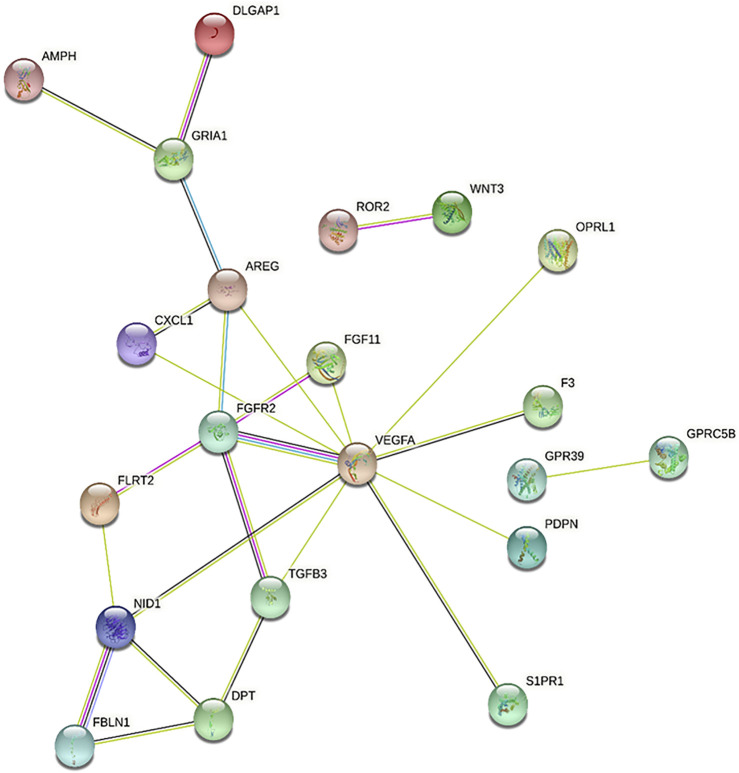
As a second approach for identifying BPs associated with both T3 treatments, we performed PPI analysis using STRING, which is complemented by a GO term enrichment analysis based on predicted interactions (Figure 4 and Supplementary Material). In accordance with ClusterProfiler results, several of the genes downregulated by 1 nm T3 (see Table 2) were also associated with enriched terms and showed interactions with each other at the protein level (Figure 4). These genes enriched terms such as positive regulation of Wnt signaling pathway (GO:0030177), wound healing (GO:0042060) and chemotaxis (GO:0006935), for instance. Interestingly, also for genes upregulated by 10 nm T3, PPI analysis pointed to enriched terms related to osteoblast differentiation, namely, negative regulation of pathway-restricted SMAD protein phosphorylation (GO:0060394) and regulation of BMP signaling pathway (GO:0030510), which will be discussed below.
FIGURE 4.
Protein-protein interaction network among genes downregulated by 1nM T3, as predicted using STRING. Genes that are central to the network, such as VEGFA, FGFR2 and TGFB3, are associated with biological processes enriched by this treatment, as shown in Table 2.
Discussion
Given our current knowledge about the importance of THs for bone development and maintenance (Bochukova et al., 2012; Waung et al., 2012), several in vitro studies have demonstrated the effects of T3 on the expression of osteoblast markers and its modulation of bone cell metabolism (Kim and Mohan, 2013; Williams, 2013; Olímpio et al., 2019). However, the roles played by T3 in osteoblast differentiation, proliferation, development and bone formation remain unclear (Harvey et al., 2002). Considering the importance of cellular models for the study of osteoblasts, we applied an osteoinduction protocol for the differentiation of hASCs, using the cocktail previously established by our research group (Olimpio et al., 2018), and then assessed the effects of 1 and 10 nm T3 doses on the global transcriptome of osteoblast-like cells.
Our results confirm the responsiveness of hASC-derived osteoblast-like cells to T3. Several osteoblast lineages have been previously shown to respond to T3 (Klaushofer et al., 1995; Harvey et al., 2002; Waung et al., 2012; Kim and Mohan, 2013; Wojcicka et al., 2013; Olímpio et al., 2019), and the present study likewise shows that T3 at both tested doses affected genes related to bone metabolism, in BPs such as mesenchymal cell proliferation and ossification, thus confirming this human primary cell line as a suitable experimental model. Overall, it was noticeable that both T3 doses had similar effects on a subset of the DEGs, but that was not the case for the biological processes affected, which were markedly different.
The osteoblast differentiation and maintenance processes can be regulated by both mechanical and biochemical pathways (Wittkowske et al., 2016), and here we show effects of T3 on the latter. With regard to the 1 nm T3 effects, the downregulated expression of several genes related to cell differentiation and proliferation, chemotaxis, and ossification, found in this study is in agreement with the decrease of mineralized matrix formation found in our previous work (Olímpio et al., 2019). For this dose, the genes involved, summarized in Table 2, are related to osteoblast differentiation through the BMP and WNT pathways, as discussed below.
Transforming growth factor beta (TGF-β) and members of its superfamily, such as BMPs and growth/differentiation factors (GDFs), exert their effects by activating the serine/threonine kinases type I and II receptor complex, which initiates Smad-dependent or -independent intracellular signaling. Smad-dependent signaling involves the phosphorylation of R-Smads (Smads 2/3 for TGF-β and Smads 1/5/8 for BMPs/GDFs), which form complexes with Co-Smads (Smad4) that then translocate to the nucleus to activate transcription factors. Smad-independent signaling involves molecules of the mitogen-activated protein kinase (MAPK) pathways, such as extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNK), and p38. The Smad-dependent BMP pathway is known to be regulated by the inhibitory Smads (I-Smads) Smad 6 and 7, which act to suppress the pathway (Blair et al., 2002; Huang et al., 2007; Yan et al., 2009; Chen et al., 2012). Moreover, although the structure of Smad 9 (also known as Smad 8) matches that of an R-Smad, recent studies have shown that its inhibition of the BMP pathway occurs through mechanisms distinct from those of the I-Smads (Tsukamoto et al., 2015; Salazar et al., 2016).
In this context, the products of the fibroblast growth factor receptor 2 (FGF2R), sphingosine-1-phosphate receptor 1 (SP1R1), fibulin-1 (FBLN1), and oxidative stress responsive kinase 1 (OSR1) genes, which were all downregulated by 1nM T3, act synergistically on the BMP pathway to promote cell differentiation and osteoblastic function. FGF2R increases the expression of BMP receptor type 1B and, consequently, the effects of BMP-2, which phosphorylates the R-Smads and induces the activity of alkaline phosphatase (Singhatanadgit et al., 2006). SP1R1 enhances the BMP-2-promoted phosphorylation of ERK 1/2 and R-Smads (Sato et al., 2012). Fibulin-1, an extracellular matrix glycoprotein encoded by the FBLN1 gene, interacts physically with BMP-2 and is necessary for the transcriptional activation of the osteogenic lineage marker Osterix and alkaline phosphatase (Cooley et al., 2014). Finally, the OSR1 gene encodes a serine/threonine protein kinase which regulates downstream kinases and can increase the expression of BMP-4 and RUNX2 (Karvande et al., 2018).
The downregulation of vascular endothelial growth factor A (VEGFA) gene expression by 1 nm T3 also reinforces the conclusion that this TH dose hampers cell differentiation and proliferation, given that literature describes this gene product as being the most abundant member of the VEGF family, stimulating osteogenesis by participating in the final phases of osteoblast differentiation, in addition to acting on cell migration and proliferation (Yang et al., 2012; Hu and Olsen, 2016). It is also known that BMPs can stimulate VEGF expression in osteoblasts, promoting bone formation and angiogenesis during bone development (Deckers et al., 2002; Zhang et al., 2009).
Aside from being part of the TGF-β family and associated with the BMP pathway and bone formation, TGF-β3 has been demonstrated in a few studies to be involved in bone development as well (Chen et al., 2012; Wu et al., 2016). In this study, it was downregulated by 1 nm T3 and was associated, for instance, with the BP term ossification.
As mentioned above, 1 nm T3 also regulated the WNT canonical and non-canonical signaling pathways. The canonical WNT pathway depends on the activity of β-catenin as a transcription factor and plays an important role in bone metabolism. The binding of WNT proteins to their transmembrane receptor Frizzled and co-receptors lipoprotein receptor-related proteins 5 and 6 (LRP5/6) inhibits the degradation of β-catenin, which then accumulates in the cytoplasm (Baron and Rawadi, 2007) and translocates to the nucleus where it affects RUNX2 gene transcription and promotes osteoblast differentiation and bone formation (Gaur et al., 2005; Li et al., 2005). By contrast, the non-canonical WNT pathway is independent of β-catenin and involves the activation of JNK (cellular polarity pathway) or nuclear factor of activated T-cells (NFAT) (Wnt/Ca2+ pathway) instead, either of which leads to the transcriptional activation of osteoblastic target genes (Enomoto et al., 2009; Gregory et al., 2010; Bretón-Romero et al., 2016).
The WNT5A, WNT3, and tyrosine-protein kinase transmembrane receptor ROR2 (ROR2) genes, which were downregulated by 1nM T3, play important roles in bone metabolism. WNT5A, a member of the WNT family of soluble ligands, interacts with its receptor ROR2 on the cell surface, triggering the non-canonical WNT cell polarity pathway (WNT/JNK). By contrast, WNT3 activates the canonical WNT pathway by binding to Frizzled and LRP5/6 (Gaur et al., 2005; Li et al., 2005). These genes are involved in osteoblast differentiation and proliferation, bone mineralization, and cell migration (Nishita et al., 2006; Enomoto et al., 2009; Sebastian et al., 2017), as previously shown in the pre-osteoblastic cell lines MC3T3 and SaOS-2, differentiated human mesenchymal stem cells, and mouse bone cell cultures (Liu et al., 2007a; Liu et al., 2007b).
The regulation of chemotaxis (via FGFR2, S1PR1, OSR1, and VEGFA), WNT signaling pathways (via WNT5A, WNT3, ROR2), and cellular responses to retinoic acid by 1 nm T3 are corroborated by the literature for FGFR2 (Kim et al., 2007), S1PR1 (Garnero, 2014), VEGFA (Yang et al., 2012; Hu and Olsen, 2016), and the WNT pathway (Gaur et al., 2005; Li et al., 2005) but not for OSR1. Moreover, although previous studies have demonstrated that retinoic acid is involved in osteogenic differentiation, proliferation, and mineralization and is related to the BMP and WNT pathways (Blum and Begemann, 2015; Draut et al., 2019; Roa et al., 2019), there are no published studies on its role in osteoblast migration, which could be a potential target for future study.
The PRKCZ gene, also downregulated by 1nM T3, encodes the atypical protein kinase C-zeta (PKCζ) from the PKC family. These proteins are described in the literature as being associated with various cell types and cellular processes, with recent studies showing their exact functions and associations with several diseases (Gopalakrishna and Jaken, 2000; Kang, 2014). However, there are as yet no studies describing the occurrence of PKCζ in osteoblasts, albeit three studies on global data have indicated its association with osteoporosis and osteosarcoma diseases (Du et al., 2014; Zhang et al., 2019; Zhou et al., 2020). Such data suggest that this molecule could be a potential biomarker for bone tissue and bone-related pathologies and is therefore worthy of further study.
In its turn, as shown by STRING Gene Ontology analysis, 10 nm T3 enriched the regulation of the BMP signaling pathway (GO: 0030510) by upregulating suppressive genes, such as SMAD6, NOG, NEO1, and ENG. This treatment enriched the negative regulation of pathway-restricted SMAD protein phosphorylation (GO:0060394), by increased expression of SMAD6, NOG, and ENG. According to the literature, Smad 6, NOG, and NEO1 inhibit the action of BMP. BMP acts by phosphorylation of the Smad proteins and is known to be regulated by the inhibitory Smads (I-Smads) Smad 6 and Smad 7, which act to suppress the pathway. NOG is an antagonist linker that binds to BMP receptors (Huang et al., 2007; Chen et al., 2012) and is a critical regulator of BMP activity during skeletogenesis and joint formation (Shi and Massagué, 2003). Neogenin 1, the protein encoded by NEO1, is a netrin receptor, considered to be a suppressor of BMP signaling (Abdullah et al., 2021). In addition, studies demonstrate that neogenin acts as a receptor for BMPs, and the signal transduction negatively regulates BMP-induced osteoblastic differentiation (Hagihara et al., 2011).
On the other hand, ENG encodes a transmembrane glycoprotein that operates as a co-receptor to the TGF-β receptor family to activate the BMP pathway by as-yet-unknown mechanisms and is involved in BMP-induced osteogenic differentiation (Ishibashi et al., 2010; Wang et al., 2014). Our results demonstrate that increased expression of the ENG gene enriched the negative regulation of pathway-restricted Smad protein phosphorylation. In this way, ENG could participate in the inhibition of an I-Smad to favor the osteogenic differentiation.
Additionaly, 10 nm T3 enhanced the expression of BMP/Smad target genes such as the ID1 gene, which is usually upregulated following BMP-induced osteogenic stimulation and its transcription is downregulated by TGF-β (Kang et al., 2003). The role of ID1 in osteoblast differentiation has not yet been clarified. Previous studies showed that during osteogenesis, the expression of ID1 is initially elevated to support the proliferation of progenitor cells and then is downregulated during terminal osteoblast differentiation (Peng et al., 2004), and overexpressing ID1 can stimulate osteoclast differentiation (Yuen et al., 2010).
Therefore, our results suggest that 10 nm T3 affects bone metabolism, by increasing the expression of genes that inhibit the BMP pathway and possibly increasing osteoclastogenesis. These results are in accordance with previous studies by our group and others, in which 10 nM T3 induced the expression of RANKL mRNA in (Miura et al., 2002; Olímpio et al., 2019) and the levels of OPG mRNA and protein decreased, which can favor RANKL binding to its receptor, activating osteoclastogenesis, and has a negative effect on net bone matrix formation. (Li et al., 2000; Olímpio et al., 2019).
It should be noted that other studies support a stimulating role for T3 on osteoblast differentiation and bone mineralization. Two studies (Boeloni et al., 2009; Cheng et al., 2016), found T3 to increase markers of osteoblast differentiation and ossification at doses similar to those we used. However, in the study by Boeloni and co-workers, maximum results were obtained with 10 pM T3, while most variables remained unchanged at 1 nm, which we consider to be closer to a physiological dose based on previous studies (Saraiva et al., 2008; Olímpio et al., 2019). Likewise, in two other studies (Chen et al., 2020; Yi et al., 2020), a 100 nm T3 dose was used for most experiments, which is well above our maximum 10 nm dose. Perhaps more important, these two studies were performed with cells from fetal origins, while we used mesenchymal stem cells obtained from adult donors, which could explain the different responses observed, since thyroid hormones may play different roles in early development and adulthood. Besides, all of these works used mouse or rat-derived cells, while the present study employed cells from human origin. These discrepancies underscore the need to address, in future studies, whether context-dependent shifts in osteoblast response to T3 indeed occur.
It has already been established that T3 stimulates the expression of several differentiation markers (Klaushofer et al., 1995; Varga et al., 1997; Harvey et al., 2002; Waung et al., 2012; Kim and Mohan, 2013; Wojcicka et al., 2013). The technique used in this study was not able to detect some of the genes that are recognized to be expressed in fully differentiated osteoblasts, such as SP7 (Osterix), TNFSF11 (RANKL), and BSP (bone sialoprotein). However, in a previous study (Olimpio et al., 2018), we had detected these genes by RT-qPCR, demonstrating that the osteoinduction methodology used ensures osteoblast-like differentiation. We believe that these gene transcripts have remained below the detection limit of the RNA-Seq technique. Nonetheless, to the best of our knowledge, there are no published studies on the activity of T3 in osteoblasts, making the present study an innovative and unique presentation of the biological markers affected by different doses of this TH. Considering that high doses of T3 can modify bone metabolism, causing abnormalities and culminating in pathologies in vivo, and given the problems encountered by patients with thyroid cancer receiving thyroid-stimulating hormone suppression therapy post thyroidectomy (Hannoush and Weiss, 2016).
Our findings on the signaling pathways potentially affected by the two doses of T3 highlight some essential points T3 in osteoblast-like cell metabolism: 1) Both doses of T3 appear to negatively influence terminal cell differentiation by inhibiting signaling pathways that are relevant to osteoblast development; 2) The effects of 10 nm T3 were likely due to BMP signaling pathway inhibition through upregulation of the expression of inhibitory genes; 3) The 1 nm T3 treatment also seems to affect the BMP signaling pathway by inhibiting the synergistic expression of genes in the pathway as well as inhibiting genes essential to the canonical and non-canonical WNT signaling pathways; 4) Both doses of T3 modulated genes related to cell migration and chemotaxis, suggesting a previously unknown role of this TH in these important biological functions; and 5) Several genes and BPs that have been scarcely studied and described in osteoblasts were revealed.
Data Availability Statement
The data presented in the study are deposited in GEO DataSets, accession number GSE205678.
Author Contributions
BR: Conceptualization, Methodology, Data curation, Writing—original draft, and Writing—review and editing. LM, ID, MO, RO, MS, and BG: Visualization, Investigation, Data curation, Writing—original draft, and Writing—review and editing. SC: Methodology. CN: Conceptualization, Methodology, Data curation, Writing—original draft, Writing—review and editing, and Supervision.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (the São Paulo Research Foundation) [grant numbers 2014/16406-9 and 2015/26747-0]. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation processes. This manuscript has been proofread by native English speakers, the edit was performed by professional editors at Editage, a division of Cactus Communications (JOB CODE CENOG_3).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2022.886136/full#supplementary-material
References
- Abdullah A., Herdenberg C., Hedman H. (2021). Netrin-1 Functions as a Suppressor of Bone Morphogenetic Protein (BMP) Signaling. Sci. Rep. 11, 8585. 10.1038/s41598-021-87949-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu E. O., Bord S., Horner A., Chatterjee V. K. K., Compston J. E. (1997). The Expression of Thyroid Hormone Receptors in Human Bone. Bone 21, 137–142. 10.1016/s8756-3282(97)00097-5 [DOI] [PubMed] [Google Scholar]
- Baron R., Rawadi G. (2007). Targeting the Wnt/β-Catenin Pathway to Regulate Bone Formation in the Adult Skeleton. Endocrinology 148, 2635–2643. 10.1210/en.2007-0270 [DOI] [PubMed] [Google Scholar]
- Bassett J. H. D., Harvey C. B., Williams G. R. (2003). Mechanisms of Thyroid Hormone Receptor-specific Nuclear and Extra Nuclear Actions. Mol. Cell. Endocrinol. 213, 1–11. 10.1016/j.mce.2003.10.033 [DOI] [PubMed] [Google Scholar]
- Blair H. C., Zaidi M., Schlesinger P. H. (2002). Mechanisms Balancing Skeletal Matrix Synthesis and Degradation. Biochem. J. 364, 329–341. 10.1042/bj20020165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum N., Begemann G. (2015). Osteoblast De- and Redifferentiation Are Controlled by a Dynamic Response to Retinoic Acid during Zebrafish Fin Regeneration. Development 142, 2894–2903. 10.1242/dev.120204 [DOI] [PubMed] [Google Scholar]
- Bochukova E., Schoenmakers N., Agostini M., Schoenmakers E., Rajanayagam O., Keogh J. M., et al. (2012). A Mutation in the Thyroid Hormone Receptor Alpha Gene. N. Engl. J. Med. 366, 243–249. 10.1056/nejmoa1110296 [DOI] [PubMed] [Google Scholar]
- Boeloni J. N., Ocarino N. M., Melo A. B., Silva J. F., Castanheira P., Goes A. M., et al. (2009). Dose-Dependent Effects of Triiodothyronine on the Osteogenic Differentiation of Rat Bone Marrow Mesenchymal Stem Cells. Horm. Res. 72 (2), 88–97. 10.1159/000232161 [DOI] [PubMed] [Google Scholar]
- Boyce B. F., Rosenberg E., de Papp A. E., Duong L. T. (2012). The Osteoclast, Bone Remodelling and Treatment of Metabolic Bone Disease. Eur. J. Clin. Investigation 42, 1332–1341. 10.1111/j.1365-2362.2012.02717.x [DOI] [PubMed] [Google Scholar]
- Bretón-Romero R., Feng B., Holbrook M., Farb M. G., Fetterman J. L., Linder E. A., et al. (2016). Endothelial Dysfunction in Human Diabetes Is Mediated by Wnt5a–JNK Signaling. Arteriosclerosis, Thrombosis, Vasc. Biol. 36, 561–569. 10.1161/ATVBAHA.115.306578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthray J., Wasan E., Wasan K. (2017). Bone-seeking Agents for the Treatment of Bone Disorders. Drug Deliv. Transl. Res. 7, 466–481. 10.1007/s13346-017-0394-3 [DOI] [PubMed] [Google Scholar]
- Chen G., Deng C., Li Y.-P. (2012). TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Biol. Sci. 8, 272–288. 10.7150/ijbs.2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Hu Y., Jiang T., Xia C., Wang Y., Gao Y. (2020). Triiodothyronine Potentiates BMP9-Induced Osteogenesis in Mesenchymal Stem Cells Through the Activation of AMPK/p38 Signaling. Front. Cell Dev. Biol. 8, 725. 10.3389/fcell.2020.00725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Xing W., Pourteymoor S., Mohan S. (2016). Effects of Thyroxine (T4), 3,5,3′-Triiodo-L-Thyronine (T3) and Their Metabolites on Osteoblast Differentiation. Calcif. Tissue Int. 99, 435–442. 10.1007/s00223-016-0159-x [DOI] [PubMed] [Google Scholar]
- Cooley M. A., Harikrishnan K., Oppel J. A., Miler S. F., Barth J. L., Haycraft C. J., et al. (2014). Fibulin-1 Is Required for Bone Formation and Bmp-2-Mediated Induction of Osterix. Bone 69, 30–38. 10.1016/j.bone.2014.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira M., Mathias L. S., Rodrigues B. M., Mariani B. G., Graceli J. B., De Sibio M. T., et al. (2020). The Roles of Triiodothyronine and Irisin in Improving the Lipid Profile and Directing the Browning of Human Adipose Subcutaneous Cells. Mol. Cell. Endocrinol. 506, 110744. 10.1016/j.mce.2020.110744 [DOI] [PubMed] [Google Scholar]
- Deckers M. M. L., van Bezooijen R. L., van der Horst G., Hoogendam J., van der Bent C., Papapoulos S. E., et al. (2002). Bone Morphogenetic Proteins Stimulate Angiogenesis through Osteoblast-Derived Vascular Endothelial Growth Factor A. Endocrinology 143, 1545–1553. 10.1210/endo.143.4.8719 [DOI] [PubMed] [Google Scholar]
- Draut H., Liebenstein T., Begemann G. (2019). New Insights into the Control of Cell Fate Choices and Differentiation by Retinoic Acid in Cranial, Axial and Caudal Structures. Biomolecules 9, 860. 10.3390/biom9120860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Yang J., Yang D., Tian W., Zhu Z. (2014). The Genetic Basis for Inactivation of Wnt Pathway in Human Osteosarcoma. BMC Cancer 14, 450. 10.1186/1471-2407-14-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M., Hayakawa S., Itsukushima S., Ren D. Y., Matsuo M., Tamada K., et al. (2009). Autonomous Regulation of Osteosarcoma Cell Invasiveness by Wnt5a/Ror2 Signaling. Oncogene 28, 3197–3208. 10.1038/onc.2009.175 [DOI] [PubMed] [Google Scholar]
- Feng X., McDonald J. M. (2011). Disorders of Bone Remodeling. Annu. Rev. Pathol. Mech. Dis. 6, 121–145. 10.1146/annurev-pathol-011110-130203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnero P. (2014). New Developments in Biological Markers of Bone Metabolism in Osteoporosis. Bone 66, 46–55. 10.1016/j.bone.2014.05.016 [DOI] [PubMed] [Google Scholar]
- Gaur T., Lengner C. J., Hovhannisyan H., Bhat R. A., Bodine P. V. N., Komm B. S., et al. (2005). Canonical WNT Signaling Promotes Osteogenesis by Directly Stimulating Runx2 Gene Expression. J. Biol. Chem. 280, 33132–33140. 10.1074/jbc.m500608200 [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Jaken S. (2000). Protein Kinase C Signaling and Oxidative Stress. Free Radic. Biol. Med. 28, 1349–1361. 10.1016/s0891-5849(00)00221-5 [DOI] [PubMed] [Google Scholar]
- Gregory M. A., Phang T. L., Neviani P., Alvarez-Calderon F., Eide C. A., O'Hare T., et al. (2010). Wnt/Ca2+/NFAT Signaling Maintains Survival of Ph+ Leukemia Cells upon Inhibition of Bcr-Abl. Cancer Cell 18, 74–87. 10.1016/j.ccr.2010.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjidakis D. J., Androulakis I. I. (2006). Bone Remodeling. Ann. N. Y. Acad. Sci. 1092, 385–396. 10.1196/annals.1365.035 [DOI] [PubMed] [Google Scholar]
- Hagihara M., Endo M., Hata K., Higuchi C., Takaoka K., Yoshikawa H., et al. (2011). Neogenin, a Receptor for Bone Morphogenetic Proteins. J. Biol. Chem. 286 (7), 5157–5165. 10.1074/jbc.m110.180919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannoush Z. C., Weiss R. E. (2016). Thyroid Hormone Replacement in Patients Following Thyroidectomy for Thyroid Cancer. Rambam Maimonides Med. J. 7, e0002. 10.5041/rmmj.10229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey C. B., O'Shea P. J., Scott A. J., Robson H., Siebler T., Shalet S. M., et al. (2002). Molecular Mechanisms of Thyroid Hormone Effects on Bone Growth and Function. Mol. Genet. Metabolism 75, 17–30. 10.1006/mgme.2001.3268 [DOI] [PubMed] [Google Scholar]
- Hayden R. S., Fortin J.-P., Harwood B., Subramanian B., Quinn K. P., Georgakoudi I., et al. (2014). Cell-Tethered Ligands Modulate Bone Remodeling by Osteoblasts and Osteoclasts. Adv. Funct. Mat. 24, 472–479. 10.1002/adfm.201302210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Olsen B. R. (2016). Osteoblast-derived VEGF Regulates Osteoblast Differentiation and Bone Formation during Bone Repair. J. Clin. Investigation 126, 509–526. 10.1172/jci82585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Yang S., Shao J., Li Y. P. (2007). Signaling and Transcriptional Regulation in Osteoblast Commitment and Differentiation. Front. Biosci. 12, 3068–3092. 10.2741/2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi O., Ikegame M., Takizawa F., Yoshizawa T., Moksed M. A., Iizawa F., et al. (2010). Endoglin Is Involved in BMP-2-Induced Osteogenic Differentiation of Periodontal Ligament Cells through a Pathway Independent of Smad-1/5/8 Phosphorylation. J. Cell. Physiol. 222, 465–473. 10.1002/jcp.21968 [DOI] [PubMed] [Google Scholar]
- Kang J.-H. (2014). Protein Kinase C (PKC) Isozymes and Cancer. New J. Sci. 2014, 1–36. 10.1155/2014/231418 [DOI] [Google Scholar]
- Kang Y., Chen C.-R., Massagué J. (2003). A Self-Enabling TGFβ Response Coupled to Stress Signaling: Smad Engages Stress Response Factor ATF3 for Id1 Repression in Epithelial Cells. Mol. Cell 11 (4), 915–926. 10.1016/s1097-2765(03)00109-6 [DOI] [PubMed] [Google Scholar]
- Karvande A., Kushwaha P., Ahmad N., Adhikary S., Kothari P., Tripathi A. K., et al. (2018). Glucose Dependent miR-451a Expression Contributes to Parathyroid Hormone Mediated Osteoblast Differentiation. Bone 117, 98–115. 10.1016/j.bone.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Kim H.-Y., Mohan S. (2013). Role and Mechanisms of Actions of Thyroid Hormone on the Skeletal Development. Bone Res. 1, 146–161. 10.4248/br201302004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Kim S. Y., Kwon C. H., Kim Y. K. (2007). Differential Effect of FGF and PDGF on Cell Proliferation and Migration in Osteoblastic Cells. Growth factors. 25, 77–86. 10.1080/08977190701398977 [DOI] [PubMed] [Google Scholar]
- Klaushofer K., Varga F., Glantschnig H., Fratzl-Zelman N., Czerwenka E., Leis H. J., et al. (1995). The Regulatory Role of Thyroid Hormones in Bone Cell Growth and Differentiation. J. Nutr. 125, 1996S–2003S. 10.1093/jn/125.suppl_7.1996S [DOI] [PubMed] [Google Scholar]
- Li J., Sarosi I., Yan X.-Q., Morony S., Capparelli C., Tan H.-L., et al. (2000). RANK Is the Intrinsic Hematopoietic Cell Surface Receptor that Controls Osteoclastogenesis and Regulation of Bone Mass and Calcium Metabolism. Proc. Natl. Acad. Sci. U.S.A. 97 (4), 1566–1571. 10.1073/pnas.97.4.1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Liu P., Liu W., Maye P., Zhang J., Zhang Y., et al. (2005). Dkk2 Has a Role in Terminal Osteoblast Differentiation and Mineralized Matrix Formation. Nat. Genet. 37, 945–952. 10.1038/ng1614 [DOI] [PubMed] [Google Scholar]
- Liu Y., Bhat R. A., Seestaller-Wehr L. M., Fukayama S., Mangine A., Moran R. A., et al. (2007). The Orphan Receptor Tyrosine Kinase Ror2 Promotes Osteoblast Differentiation and Enhances Ex Vivo Bone Formation. Mol. Endocrinol. 21, 376–387. 10.1210/me.2006-0342 [DOI] [PubMed] [Google Scholar]
- Liu Y., Ross J. F., Bodine P. V. N., Billiard J. (2007). Homodimerization of Ror2 Tyrosine Kinase Receptor Induces 14-3-3β Phosphorylation and Promotes Osteoblast Differentiation and Bone Formation. Mol. Endocrinol. 21, 3050–3061. 10.1210/me.2007-0323 [DOI] [PubMed] [Google Scholar]
- Miura M., Tanaka K., Komatsu Y., Suda M., Yasoda A., Sakuma Y., et al. (2002). A Novel Interaction between Thyroid Hormones and 1,25(OH)2D3 in Osteoclast Formation. Biochem. Biophysical Res. Commun. 291, 987–994. 10.1006/bbrc.2002.6561 [DOI] [PubMed] [Google Scholar]
- Nishita M., Yoo S. K., Nomachi A., Kani S., Sougawa N., Ohta Y., et al. (2006). Filopodia Formation Mediated by Receptor Tyrosine Kinase Ror2 Is Required for Wnt5a-Induced Cell Migration. J. Cell Biol. 175, 555–562. 10.1083/jcb.200607127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olimpio R. M. C., de Oliveira M., De Sibio M. T., Moretto F. C. F., Deprá I. C., Mathias L. S., et al. (2018). Cell Viability Assessed in a Reproducible Model of Human Osteoblasts Derived from Human Adipose-Derived Stem Cells. PLOS ONE 13, e0194847. 10.1371/journal.pone.0194847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olímpio R. M. C., Moretto F. C. F., De Sibio M. T., de Oliveira M., Mathias L. S., Gonçalves B. M., et al. (2019). The Importance of Estrogen for Bone Protection in Experimental Hyperthyroidism in Human Osteoblasts. Life Sci. 231, 116556. 10.1016/j.lfs.2019.116556 [DOI] [PubMed] [Google Scholar]
- Pascual A., Aranda A. (2013). Thyroid Hormone Receptors, Cell Growth and Differentiation. Biochim. Biophys. Acta 1830, 3908–3916. 10.1016/j.bbagen.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Peng Y., Kang Q., Luo Q., Jiang W., Si W., Liu B. A., et al. (2004). Inhibitor of DNA Binding/Differentiation Helix-Loop-Helix Proteins Mediate Bone Morphogenetic Protein-Induced Osteoblast Differentiation of Mesenchymal Stem Cells. J. Biol. Chem. 279, 32941–32949. 10.1074/jbc.m403344200 [DOI] [PubMed] [Google Scholar]
- Roa L. A., Bloemen M., Carels C. E. L., Wagener F. A. D. T. G., Von den Hoff J. W. (2019). Retinoic Acid Disrupts Osteogenesis in Pre-osteoblasts by Down-Regulating WNT Signaling. Int. J. Biochem. Cell Biol. 116, 105597. 10.1016/j.biocel.2019.105597 [DOI] [PubMed] [Google Scholar]
- Salazar V. S., Gamer L. W., Rosen V. (2016). BMP Signalling in Skeletal Development, Disease and Repair. Nat. Rev. Endocrinol. 12, 203–221. 10.1038/nrendo.2016.12 [DOI] [PubMed] [Google Scholar]
- Saraiva P. P., Teixeira S. S., Padovani C. R., Nogueira C. R. (2008). Triiodothyronine (T3) Does Not Induce Rankl Expression in Rat Ros 17/2. Arq. Bras. Endocrinol. Metabol. 52 (1), 109–113. 10.1590/s0004-27302008000100015 [DOI] [PubMed] [Google Scholar]
- Sato C., Iwasaki T., Kitano S., Tsunemi S., Sano H. (2012). Sphingosine 1-phosphate Receptor Activation Enhances BMP-2-Induced Osteoblast Differentiation. Biochem. Biophysical Res. Commun. 423, 200–205. 10.1016/j.bbrc.2012.05.130 [DOI] [PubMed] [Google Scholar]
- Sebastian A., Hum N. R., Murugesh D. K., Hatsell S., Economides A. N., Loots G. G. (2017). Wnt Co-receptors Lrp5 and Lrp6 Differentially Mediate Wnt3a Signaling in Osteoblasts. PLOS ONE 12, e0188264. 10.1371/journal.pone.0188264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massagué J. (2003). Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell 113 (6), 685–700. 10.1016/s0092-8674(03)00432-x [DOI] [PubMed] [Google Scholar]
- Singhatanadgit W., Salih V., Olsen I. (2006). Up-regulation of Bone Morphogenetic Protein Receptor IB by Growth Factors Enhances BMP-2-Induced Human Bone Cell Functions. J. Cell. Physiol. 209, 912–922. 10.1002/jcp.20799 [DOI] [PubMed] [Google Scholar]
- Straub R. H. (2014). Interaction of the Endocrine System with Inflammation: a Function of Energy and Volume Regulation. Arthritis Res. Ther. 16, 203. 10.1186/ar4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F., Bošnjak M., Škunca N., Šmuc T. (2011). REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 6, e21800. 10.1371/journal.pone.0021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto S., Mizuta T., Fujimoto M., Ohte S., Osawa K., Miyamoto A., et al. (2015). Smad9 Is a New Type of Transcriptional Regulator in Bone Morphogenetic Protein Signaling. Sci. Rep. 4, 7596. 10.1038/srep07596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga F., Rumpler M., Luegmayr E., Fratzl-Zelman N., Glantschnig H., Klaushofer K. (1997). Triiodothyronine, a Regulator of Osteoblastic Differentiation: Depression of Histone H4, Attenuation of C-Fos/c-Jun, and Induction of Osteocalcin Expression. Calcif. Tissue Int. 61, 404–411. 10.1007/s002239900356 [DOI] [PubMed] [Google Scholar]
- Wang R. N., Green J., Wang Z., Deng Y., Qiao M., Peabody M., et al. (2014). Bone Morphogenetic Protein (BMP) Signaling in Development and Human Diseases. Genes & Dis. 1, 87–105. 10.1016/j.gendis.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung J. A., Bassett J. H. D., Williams G. R. (2012). Thyroid Hormone Metabolism in Skeletal Development and Adult Bone Maintenance. Trends Endocrinol. Metabolism 23, 155–162. 10.1016/j.tem.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Williams G. R. (2013). Thyroid Hormone Actions in Cartilage and Bone. Eur. Thyroid. J. 2, 3–13. 10.1159/000345548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkowske C., Reilly G. C., Lacroix D., Perrault C. M. (2016). In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 4, 87. 10.3389/fbioe.2016.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcicka A., Bassett J. H. D., Williams G. R. (2013). Mechanisms of Action of Thyroid Hormones in the Skeleton. Biochimica Biophysica Acta (BBA) - General Subj. 1830, 3979–3986. 10.1016/j.bbagen.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Wu M., Chen G., Li Y.-P. (2016). TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 4, 16009. 10.1038/boneres.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Liu Z., Chen Y. (2009). Regulation of TGF- Signaling by Smad7. Acta biochimica biophysica Sinica 41, 263–272. 10.1093/abbs/gmp018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-Q., Tan Y.-Y., Wong R., Wenden A., Zhang L.-K., Rabie A. B. M. (2012). The Role of Vascular Endothelial Growth Factor in Ossification. Int. J. Oral Sci. 4, 64–68. 10.1038/ijos.2012.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Zhong T., Huang Y., Huang S. (2020). Triiodothyronine Promotes the Osteoblast Formation by Activating Autophagy. Biophys. Chem. 267, 106483. 10.1016/j.bpc.2020.106483 [DOI] [PubMed] [Google Scholar]
- Yuen H.-F., Chiu Y.-T., Chan K.-K., Chan Y.-P., Chua C.-W., McCrudden C. M., et al. (2010). Prostate Cancer Cells Modulate Osteoblast Mineralisation and Osteoclast Differentiation through Id-1. Br. J. Cancer 102, 332–341. 10.1038/sj.bjc.6605480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Qiu T., Wu X., Wan C., Shi W., Wang Y., et al. (2009). Sustained BMP Signaling in Osteoblasts Stimulates Bone Formation by Promoting Angiogenesis and Osteoblast Differentiation. J. Bone Mineral Res. 24, 1224–1233. 10.1359/jbmr.090204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Ding L., Li X., Fan H. (2019). Identification of Biomarkers Associated with the Recurrence of Osteosarcoma Using ceRNA Regulatory Network Analysis. Int. J. Mol. Med. 43, 1723–1733. 10.3892/ijmm.2019.4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yang L., Wang H., Chen X., Jiang W., Wang Z., et al. (2020). Alterations in DNA Methylation Profiles in Cancellous Bone of Postmenopausal Women with Osteoporosis. FEBS Open Bio 10, 1516–1531. 10.1002/2211-5463.12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in the study are deposited in GEO DataSets, accession number GSE205678.