Abstract
Fibroblasts are the main cell type in the dermis. They are responsible for the synthesis and deposition of structural proteins such as collagen and elastin, which are integrated into the extracellular matrix (ECM). Mouse and human studies using flow cytometry, cell culture, skin reconstitution, and lineage tracing experiments have shown the existence of different subpopulations of fibroblasts, including papillary fibroblasts, reticular fibroblasts, and fibroblasts comprising the dermal papilla at the base of the hair follicle. In recent years, the technological advances in single-cell sequencing have allowed researchers to study the repertoire of cells present in full-thickness skin including the dermis. Multiple groups have confirmed that distinct fibroblast populations can be identified in mouse and human dermis on the basis of differences in the transcriptional profile. Here, we discuss the current state of knowledge regarding dermal fibroblast heterogeneity in healthy mouse and human skin, highlighting the similarities and differences between mouse and human fibroblast subpopulations. We also discuss how fibroblast heterogeneity may provide insights into physiological wound healing and its dysfunction in pathological states such as hypertrophic and keloid scars.
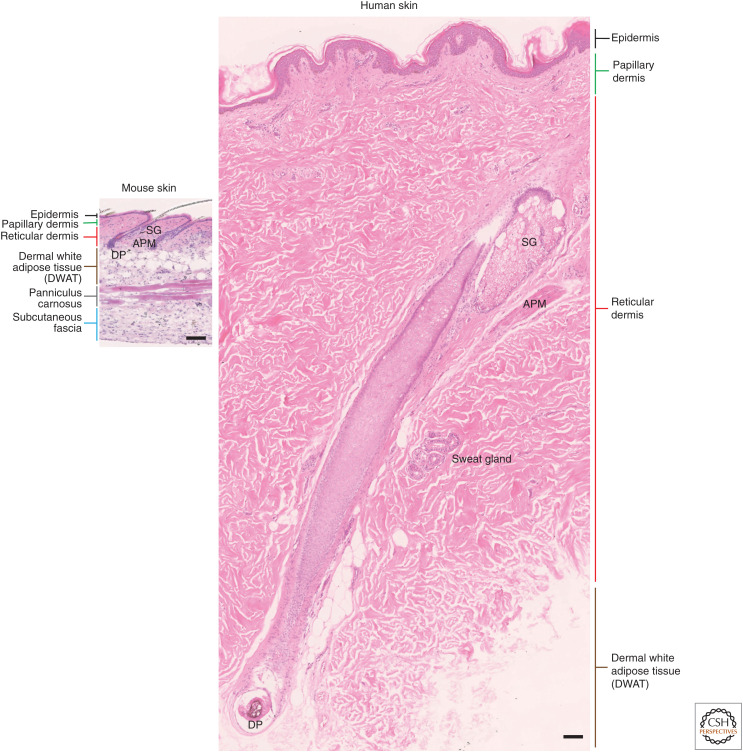
Mammalian skin comprises two layers, a stratified epithelium, the epidermis, and an underlying connective tissue, the dermis, including the subcutaneous fat. The dermis is a structural scaffold that gives strength and elasticity to the skin. It is composed mainly of type I and type III collagens and other collagen subtypes, proteoglycans, and elastin (Watt and Fujiwara 2011). Within the dermis are embedded blood and lymphatic vessels, nerve cells, immune cells, and appendageal structures, which are specialized invaginations of the epithelium: hair follicles, sebaceous, and sweat glands that penetrate the dermis. The dermis microstructure differs between human and mouse skin. In mice, there is a relatively high density of hair follicles and the dermo–epidermal junction is relatively flat. In humans, the density of hair follicles is lower, except in a few locations such as scalp and face, and the epidermis projects into the dermis via rete ridges (Fig. 1).
Figure 1.
Comparison of mouse and human body skin. The epidermis and dermis are thicker in human than in mouse skin. In most body sites, mouse body skin has a higher density of hair follicles compared to human body skin and the sweat glands are segregated to non-hair-bearing regions. In both mouse and human skin, the dermis is composed of a papillary and a reticular layer. Underneath the dermis, in mice, the subcutaneous fat is composed of a dermal with adipose tissue (DWAT), a panniculus carnosus (striated muscle), and the fascia beneath. In human, the dermis is separated from the underlying fascia by a large (many centimeters in the truck area) layer of subcutaneous fat (DWAT). Scale bars, 100 μm. (SG) Sebaceous gland, (APM) arrector pili muscle, (DP) dermal papilla.
In both mouse and human, the dermis is composed of two distinct layers identified by histology (Meigel et al. 1977). The upper, papillary dermis lies immediately beneath the epidermis and reaches a depth of 300–400 μm in humans, depending on age and body site. The papillary dermis is composed of poorly oriented collagen bundles that support the overlying epidermis (Watt and Fujiwara 2011). The lower dermis, called the reticular dermis, is located underneath the papillary dermis (Woodley 2017) and over subcutaneous fat tissue (Driskell et al. 2014). Reticular dermis contains thicker, directionally oriented collagen bundles (Sorrell and Caplan 2004).
Fibroblasts are the main cell type in the dermis and are responsible for the synthesis and deposition of structural proteins such as collagen and elastin, which are integrated into the extracellular matrix (ECM) (Watt and Fujiwara 2011; Shaw and Rognoni 2020; Plikus et al. 2021). In vitro studies in human (Harper and Grove 1979; Schönherr et al. 1993; Sorrell and Caplan 2004; Sorrell et al. 2007; Janson et al. 2012; Woodley 2017) and in vivo studies in mouse skin (Driskell et al. 2013; Kaushal et al. 2015; Rinkevich et al. 2015; Jiang et al. 2018; Abbasi et al. 2020; Leavitt et al. 2020; Goss et al. 2021) have shown the existence of different subpopulations of fibroblasts, including papillary fibroblasts, reticular fibroblasts, arrector pili muscle (APM) fibroblasts, and fibroblasts comprising the dermal papilla and sheath fibroblasts at the base of the hair follicle. Lineage-tracing studies in mice have defined markers of different fibroblast subpopulations and shown how functionally distinct fibroblasts behave during development, wound healing, and fibrosis. Single-cell RNA sequencing (scRNA-seq) studies (Philippeos et al. 2018; Tabib et al. 2018; Joost et al. 2020; Solé-Boldo et al. 2020; Vorstandlechner et al. 2020; Deng et al. 2021; Reynolds et al. 2021) have also revealed heterogeneity in mouse and human skin.
In this review, we discuss the current state of knowledge regarding dermal fibroblast heterogeneity in healthy mouse and human skin, and how different fibroblast subpopulations contribute to wound healing.
FIBROBLAST HETEROGENEITY IN HEALTHY AND WOUNDED ADULT MOUSE SKIN
Heterogeneity of Fibroblasts in Healthy Mouse Skin
Some of the earliest evidence for fibroblast heterogeneity in mouse skin came from studies of the dermal papilla at the base of the hair follicles (Kishimoto et al. 2000; Shimizu and Morgan 2004; Rendl et al. 2008). Mice have several distinct hair follicle types that differ in length, thickness, and in the presence or absence of kinks in the hair shaft (Schlake 2007). Although some genes are expressed in all dermal papillae, the transcription factor Sox2 is specifically expressed in the dermal papillae of guard/awl/auchene follicles (Driskell et al. 2009, 2011). Another example of a specific subset of dermal fibroblasts are the cells of the APM (Fujiwara et al. 2011), which express the α8β1 integrin that mediates adhesion to nephronectin deposited by keratinocytes in the bulge of the hair follicle.
Fibroblast Populations during Mouse Skin Development
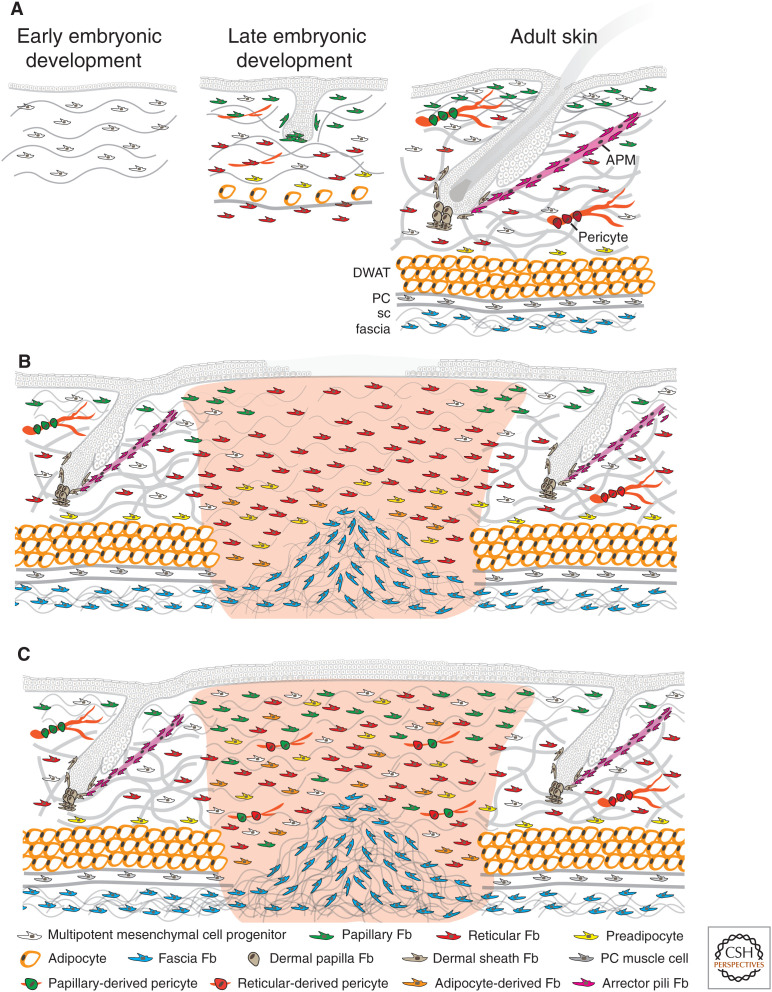
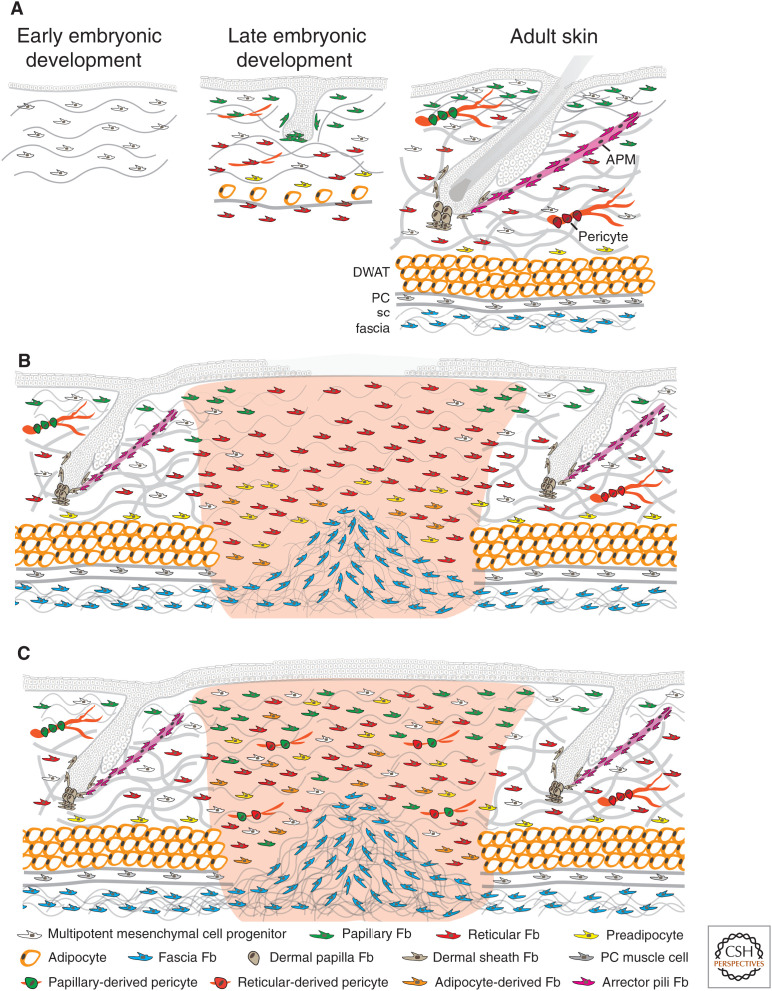
In characterizing APM and dermal papilla fibroblasts, flow cytometry, cell culture, skin reconstitution, and lineage-tracing techniques were applied, leading us to consider whether the same methodology could be used to characterize fibroblast populations within the papillary and reticular dermis of the mouse. For this, we made extensive use of Pdgfrα expression as a pan-fibroblast marker (Kalluri and Zeisberg 2006; Collins et al. 2011). Focusing on mouse back skin, we found that at embryonic day 12.5 (E12.5), there was a multipotent mesenchymal cell progenitor expressing Pdgfrα, Dlk1, and Lrig1. At E14.5, a subset of these cells differentiated into Sox2+ dermal papillae (Pdgfrα+, Cd26−, Sox2+). At approximately E16.5, the mesenchymal progenitors differentiated into papillary (Pdgfrα+, Dlk1−, Blimp1+, Lrig1+) and reticular fibroblasts (Pdgfrα+, Dlk1+, Blimp1−). The papillary fibroblasts formed the dermal papillae of zigzag hairs and the APM, whereas the reticular fibroblasts gave rise to pre- and mature adipocytes (Fig. 2A; Table 1; Driskell et al. 2013).
Figure 2.
Dermal cell organization during mouse skin development (A) and early (B) and advanced adult wound healing (C). In early embryonic development, there are multipotent mesenchymal cell progenitors present in the dermis. In late embryonic development, the mesenchymal progenitors have differentiated into papillary and reticular fibroblasts (Fb). The papillary fibroblasts form the dermal papillae of developing hair follicles, whereas the reticular fibroblasts gave rise to preadipocytes and mature adipocytes. In mouse adult skin, many hair follicles project into the dermis. There are specific fibroblasts in the dermal papilla at the base of each follicle, in the dermal sheath around each follicle, and in the APM that connects to the hair follicle bulge. The papillary dermis is composed of multipotent mesenchymal cell progenitors, papillary fibroblasts, and papillary derived pericytes. The reticular dermis is composed of multipotent mesenchymal cell progenitors, reticular fibroblasts, preadipocytes, and reticular derived pericytes. The subcutaneous fat is mainly composed by mature adipocytes in the dermal white adipose tissue (DWAT), panniculus carnosus (PC), muscle cells in the panniculus muscle layer, and fascia fibroblasts in the subcutaneous (sc) fascia. (A) In wounded adult skin, the initial wave of dermal repair is dominated by reticular fibroblasts; papillary fibroblasts from the upper dermis follow later. Fascia fibroblasts collectively migrate into the wound to contribute to the repair of deep full thickness wounds in the early phase. Also, preadipocytes and adipocytes in the lower dermis start to be recruited. At this stage, fibroblasts are highly proliferative and start to secrete collagen fibers. Reepithelialization is not complete. (B) In the advanced phase of wound healing, reepithelialization is complete and reticular fibroblasts, papillary fibroblasts, preadipocytes, adipocytes, and multipotent mesenchymal cell progenitors have migrated into the wound and are randomly distributed. More fascia fibroblasts from the subcutaneous fascia may have migrated and expanded into the lower area of deep full thickness wounds. At this stage, collagen fibers are secreted and remodeled by fibroblasts.
Table 1.
Experimentally validated markers of dermal cell populations in mouse and human
| Cell type | Mouse markers | Human markers | References |
|---|---|---|---|
| Pan-fibroblast | Pdgfrα*, Pdgfrβ*, Col1a1, Vim, Cd90, Lum, Dcn | PDGFRα*, COL1A1, FAP, VIM, CD90, LUM, DCN | Saalbach et al. 1998; Kalluri and Zeisberg 2006; Collins et al. 2011; Philippeos et al. 2018 |
| Dermal papilla fibroblast | Crabp1, Alkphos, Promolin1, Blimp1, Lef1 | VERSICAN, MZF1, MEF2C, BLIMP1, LEF1 | Higgins et al. 2013; Telerman et al. 2017; Heitman et al. 2020 |
| Dermal sheath fibroblast | A-Sma*, Acan, Myh10, Mlck, Myl9 | A-SMA*, GREM2, SM22, MYH11, MYL9, MEF2C | Higgins et al. 2013; Niiyama et al. 2018; Heitman et al. 2020 |
| Papillary fibroblast | Lrig1, Blimp1, Cd39, Cd26*, Col6a5, Col23a1, Apcdd1, Hspb3, Fap | APCDD1, COL18A1, ENPP2, COL6A5, CD39, CD26*, COL23A1, HSPB3, WIF1 | Driskell et al. 2013; Rinkevich et al. 2015; Jiang et al. 2018; Philippeos et al. 2018; Korosec et al. 2019; Solé-Boldo et al. 2020 |
| Reticular fibroblast | Dlk1, Sca1*, Prrx1 | A-SMA*, MGP, PPARγ, CNN1, COL11A1, TGM2, CD36, CTHRC1, CXCL1, EMGN, MGST1 | Janson et al. 2012; Driskell et al. 2013; Rinkevich et al. 2015; Jiang et al. 2018; Philippeos et al. 2018; Korosec et al. 2019; Solé-Boldo et al. 2020 |
| Arrector pili muscle fibroblast | A-Sma*, Itga8, Sm22α, Npnt | Not described in human | Fujiwara et al. 2011 |
| Preadipocyte fibroblast | Cd34, Sca1, Pdgfrα* | CD34, PDGFRα*, CD24, CD29 | Rodeheffer et al. 2008; Festa et al. 2011; Berry and Rodeheffer 2013; Rivera-Gonzalez et al. 2014 |
| Adipocyte | Adiponectin, Perilipin, Pparγ, Fabp4 | ADIPONECTIN, PERILIPIN | Festa et al. 2011; Rivera-Gonzalez et al. 2014 |
| Pericyte | A-Sma*, Cd146, Rgs5, Tbx18, Pdgfrβ*, Ng2, Pdgfrα* | A-SMA*, CD146, RGS5, TBX18, PDGFRβ* | Guimarães-Camboa et al. 2017; Yamazaki et al. 2017; Goss et al. 2021 |
| Fascia fibroblast | Cd26*, En1, Sca1*, Nov, Gpx3 | A-SMA*, CD26*, CD44, NOV | Correa-Gallegos et al. 2019 |
| Dermo–hypodermal junction fibroblast | Not described in mouse | KLF9, NTN9 | Korosec et al. 2019; Haydont et al. 2020 |
Shared population markers between mouse and human are highlighted in bold, while markers, which are not population-specific, have been highlighted with an asterisk.
(ACAN) Aggrecan core protein, (ALKPHOS) alkaline phosphatase, (APCDD1) adenomatosis polyposis coli down-regulated 1, (BLIMP1) B lymphocyte-induced maturation protein-1, (CD146) cluster of differentiation 146 (melanoma cell adhesion molecule), (CD24) cluster of differentiation 24 (small cell lung carcinoma cluster 4 antigen), (CD26) cluster of differentiation 26 (dipeptidyl peptidase 4), (CD29) cluster of differentiation 29 (integrin β1), (CD34) cluster of differentiation 34 (hematopoietic progenitor cell antigen), (CD39) cluster of differentiation 39 (ectonucleoside triphosphate diphosphohydrolase 1), (CD44) cluster of differentiation 44 (homing function and Indian blood group system), (CD90) cluster of differentiation 90 (Thy-1 cell surface antigen), (CNN1) Calponin-1, (COL11A1) collagen, type XI α1 chain, (COL18A1) collagen, type XVIII α1 chain, (COL1A1) collagen, type I α1 chain, (COL23A1) collagen, type XXIII α1 chain, (COL6A1) collagen, type VI α1 chain, (CRABP1) cellular retinoic acid-binding protein 1, (CTHRC1) collagen triple helix repeat-containing 1, (CXCL1) growth-regulated α protein, (DCN) decorin, (DLK1) delta-like noncanonical Notch ligand 1, (EN1) Engrailed homeobox 1, (ENPP2) ectonucleotide pyrophosphatase/phosphodiesterase 2, (FAP) fibroblast activation protein (prolyl endopeptidase), (GPX3) glutathione peroxidase 3, (GREM2) Gremlin 2 (DAN family BMP antagonist), (HSPB3) heat shock protein β3, (ITGA8) integrin subunit α8, (KLF9) Krüppel-like factor 9, (LEF1) lymphoid enhancer-binding factor 1, (LRIG1) leucine-rich repeats and immunoglobulin-like domains protein 1, (LUM) Lumican, (MEF2C) myocyte enhancer factor 2C, (MEF2C) myocyte enhancer factor 2C, (MGST1) microsomal glutathione S-transferase 1, (MLCK) myosin light-chain kinase, (MYH10) myosin heavy chain 10, (MYH11) myosin heavy chain 11, (MYL9) myosin light chain 9, (MZF1) myeloid zinc finger 1, (NG2) neural/glial antigen 2 (melanoma-associated chondroitin sulfate proteoglycan), (NOV) nephroblastoma overexpressed, (NPNT) nephronectin, (NTN9) Neurturin 9, (PDGFR-α) platelet-derived growth factor receptor α, (PDGFR-β) platelet-derived growth factor receptor β, (PRRX1) paired related homeobox 1, (RGS5) regulator of G-protein signaling 5, (SCA1) stem cell antigen 1, (SM22) smooth muscle protein 22, (TBX18) T-box transcription factor 18, (TGM2) protein-glutamine γ-glutamyltransferase 2, (VIM) Vimentin, (WIF1) WNT inhibitory factor 1, (A-SMA) α smooth muscle actin.
Using similar approaches, Longaker's laboratory identified distinct mouse embryonic fibroblast lineages within the dorsal dermis. The Engrailed-1 (En1)-positive (En1+) lineage is the primary contributor to connective tissue deposition and organization during embryonic development, cutaneous wounding, radiation fibrosis, and cancer stroma formation (Rinkevich et al. 2015). Cd26 is highly expressed by the En1+ fibroblast lineage. Cd26-specific fibroblast ablation results in a diminution of connective tissue deposition in wounds in adult murine skin (Rinkevich et al. 2015). In contrast, En1− fibroblasts enhance dermal development and regeneration (Jiang et al. 2018).
Paired-related homeobox 1 (Prrx1)-positive (Prrx1+) and Prrx1-negative (Prrx1−) mouse embryonic fibroblast lineages have been described within the ventral dermis (Leavitt et al. 2020). Similar to En1+ fibroblasts in the dorsal dermis, Prrx1+ delineates at least two distinct embryonic fibroblast populations (Leavitt et al. 2020). Prrx1+ fibroblasts from the ventral lineage have been shown to be scar-forming fibroblasts. Those cells are responsible for most of the collagen production in the dermis following radiation, wounding, and in tumor stroma formation (Hu et al. 2018).
Fibroblast Populations in Adult Mouse Skin
In recent years, scRNA-seq of adult mouse skin has confirmed the existence of a wide variety of fibroblast subtypes. Joost et al. (2020) identified seven subtypes, four of which were significantly expanded in either anagen or telogen, demonstrating that the composition of fibroblasts is influenced by the hair cycle. Markers of the four major fibroblast subpopulations have provided information about their distribution within the skin. F1 (Sparchi/Dcn+) and F2 (Sparclo/Dcn+) are uniformly distributed in the dermis, with F1 being enriched in anagen and F2 in telogen. F3 (Gpx3+/Plac8lo) are spatially segregated to the hypodermis, and F4 (Gpx3+/Plac8hi) are located within the adventitia (Joost et al. 2020). It is hypothesized that F1 and F2 represent a single fibroblast population that shows changes in gene expression in response to different stages of the hair cycle (Joost et al. 2020).
Pericytes Emerge from Fibroblast Populations
Pericytes are perivascular cells embedded within the basement membrane of blood vessels and can be found within arterial, venous, and lymphatic vascular channels in most tissues (Hall 2006; Paquet-Fifield et al. 2009). Pericytes interact with endothelial cells to regulate blood flow via vessel constriction, maintain vessel wall integrity, regulate vascular permeability, and control angiogenesis through directing endothelial cell proliferation and migration (Thomas et al. 2017). Pericytes express a range of markers including Rgs5 (Bondjers et al. 2003), Pdgfrβ (Armulik et al. 2011), Nestin (Birbrair et al. 2013), Tbx18 (Guimarães-Camboa and Evans 2017), Cd146 (Dvoretskiy et al. 2019), and neural/glial antigen 2 (Ng2) (Armulik et al. 2011). However, none of these markers is pericyte specific as they can also be expressed by other perivascular cell populations, including adventitial fibroblasts and endothelial cells.
Although a small proportion of embryonic pericytes in E15.5 mouse skin have a hematopoietic origin (Yamazaki et al. 2017), the lineage relationships of most pericytes were unknown until recently. Goss et al. discovered that Ng2+ pericytes can be categorized into four subpopulations based on expression of Pdgfrα and Pdgfrβ. Lineage tracing shows that Ng2+ cells do not give rise to dermal fibroblasts and that the origins of Ng2+ pericytes depend on their location (Goss et al. 2021). Thus, papillary fibroblasts (Lrig1+ lineage) give rise to pericytes in the upper dermis, whereas pericytes in the lower dermis are primarily derived from reticular Dlk1+ fibroblasts. Notwithstanding the different origins of skin pericytes, most scRNA-seq studies in mouse skin have identified only one pericyte population (Ge et al. 2020). One potential explanation is that because there are no pericyte-specific markers and clustering methods are based on marker expression, pericyte heterogeneity may be underrecognized.
Mouse Fibroblast Heterogeneity in Physiological Wound Healing
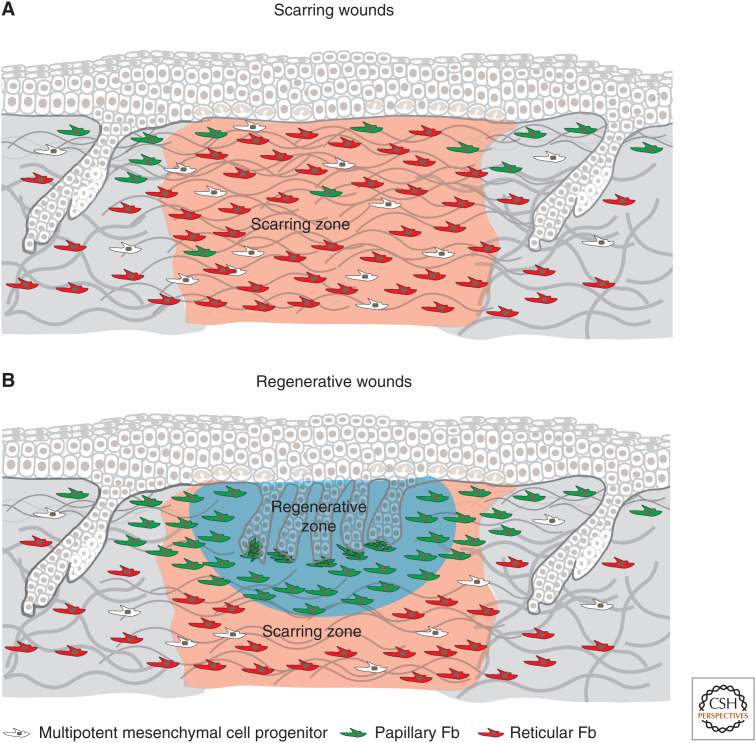
In mouse skin, the wound healing process depends on the size of the wound. Both large (>1 cm2) and small (<1 cm2) wounds heal by a combination of contraction and reepithelialization, which is followed by the formation of new connective tissue leading to scar formation. However, large wounds also contain a regenerative zone, which is rich in fibroblasts in which hair follicle neogenesis occurs (Ito et al. 2007) resulting in regenerative wounds (Fig. 3). Furthermore, the size of the wound that is required for hair follicle neogenesis depends on the model organism; for example, the African spiny mouse is capable of full regeneration of dorsal skin wounds (Seifert et al. 2012).
Figure 3.
Dermal wound healing zones in scarring and regenerative wounds. In mouse skin, the wound healing process differs depending on the size of the wound. Both large (>1 cm2) and small (<1 cm2) wounds heal by a combination of contraction and reepithelialization, which is followed by the formation of new connective tissue leading to scar formation (A). However, large wounds also contain a regenerative zone that is rich in fibroblasts in which hair follicle neogenesis occurs (B). Conversely, a small wound (<1 cm2) repairs without de novo hair follicle formation, resulting in scarring (A).
Loss of Postnatal Fibroblast Quiescent State on Wounding
Dermal maturation is governed by a tight balance of fibroblast proliferation, quiescence, and ECM deposition. There is a switch in fibroblast behavior from highly proliferative in embryonic dermis to quiescent in postnatal skin, associated with ECM deposition (Rognoni et al. 2018). On wounding, this postnatal quiescent state is lost, leading to the activation, proliferation, and migration of different fibroblast lineages at the wound site (Fig. 2B; Shaw and Martin 2016). Intriguingly, in addition to depositing/remodeling ECM in the wound bed, fibroblasts are able to acquire a dermal papilla or adipocyte fate in response to distinct signals promoting hair follicle and DWAT regeneration (Gay et al. 2013; Plikus et al. 2017; Lim et al. 2018).
Adult Fibroblast Populations within the Wound Bed
Lineage-tracing experiments have revealed that, on wounding, mouse fibroblasts in the lower dermis are the first to repopulate the damaged tissue and deposit ECM. Fibroblasts in the papillary dermis move in later and have a role in remodeling and/or appendage regeneration (Driskell et al. 2013; Rognoni et al. 2016). Hair follicle neogenesis in healing wounds occurs predominantly in the central wound bed, suggesting the existence of tissue repair zones with differential regenerative potential (Ito et al. 2007).
New technologies such as scRNA-seq have identified 12 fibroblast populations in the wound bed of mouse skin at 12 d post-wounding, which not only differ in transcription factor and signaling pathway marker/receptor/ligand expression but also in cell cycle state and spatial distribution (Guerrero-Juarez et al. 2019). Furthermore, an scRNA-seq comparison of late-stage (18 d post-wounding) large regenerative and fibrotic wounds identified the presence of three shared myofibroblast populations (Gay et al. 2020). Group 1 and group 2 share an ECM degradation, organization, and preadipocyte signature, although group 2 differs in the inclusion of a proliferation profile. Group 3 displays a transcriptional profile associated with smooth muscle contraction and laminin and collagen interaction (Gay et al. 2020). Within fibrotic wounds, group 3 myofibroblasts are the predominant population (Gay et al. 2020). However, the functional significance of this cellular diversity requires further study, including the role of the wound bed microenvironment in controlling the properties of different fibroblast populations.
Sophisticated multiomics approaches are offering further insights into fibroblast phenotypes (Mascharak et al. 2021). By combining scRNA-seq and ATAC-seq, a recent study revealed that the wound bed microenvironment induces significant chromatin rearrangement in recruited fibroblasts, generating a central, upper dermal regenerative zone and a peripheral, lower dermal scarring zone. Additionally, pharmacological intervention targeting Runx1 and retinoic acid signaling can modulate wound repair outcomes, indicating that the regenerative capacity of fibroblasts can be modulated (Fig. 3; Abbasi et al. 2020). Application of Runx1 inhibitors to healing wounds reduces de novo hair follicle formation and regenerative potential, whereas application of exogenous retinoic acid has the opposite effect, augmenting regeneration and resulting in an increase in new hair follicles (Abbasi et al. 2020).
Dermal–Epidermal Communication during Wound Healing
Spatial fibroblast segregation and interaction with the epidermis are essential for establishing a regenerative zone in the upper dermis, underscoring the importance of the local microenvironment. Key epidermal signals promoting a regenerative niche in the upper dermis include the Wnt/β-catenin and Sonic hedgehog (Shh) pathway (Lichtenberger et al. 2016; Lim et al. 2018). In contrast, sustained activation of Wnt/β-catenin signaling in dermal fibroblasts is insufficient for hair follicle neogenesis, inhibits adipocyte differentiation, and induces fibrotic lesions (Hamburg-Shields et al. 2015; Mastrogiannaki et al. 2016; Rognoni et al. 2016). Conversely, constitutive overexpression of the canonical Wnt/β-catenin transcription factor Lef1 in dermal cells primes the wound bed toward more regenerative skin repair (Phan et al. 2020).
Fibroblast-specific inhibition of β-catenin signaling in mice enhances hair follicle regeneration by preventing the early expansion of lower (profibrotic) dermal cells, whereas Lef1 depletion in dermal cells impairs hair follicle neogenesis, emphasizing its key role in promoting a papillary/dermal papilla fate in wound bed fibroblasts (Rognoni et al. 2016; Phan et al. 2020). Dermal Hedgehog (Hh) activation enables hair follicle regeneration by inducing dermal papilla gene expression and conversion of profibrotic Wnt/β-catenin signaling, promoting active wound fibroblasts to follow a dermal papilla fate in the upper dermis (Lim et al. 2018).
Different Fibroblast Populations Are Influenced by the Local Wound Microenvironment
scRNA-seq has revealed heterogeneous expression of dermal papilla signature genes within active wound bed fibroblasts, suggesting that additional factors such as spatial distribution, cell–cell/ECM interactions, and other signaling pathways are essential for controlling cell plasticity. For example, Wnt/β-catenin signaling is particularly high in scar-forming wounds (Rognoni et al. 2016), and this prolonged Wnt activity is promoted by macrophages that phagocytize and degrade the Wnt inhibitor SFRP4 in the lower wound bed dermis (Gay et al. 2020). A specific macrophage subset promotes proliferation of an activated adipocyte precursor population during wound repair, providing a striking example of how functional heterogeneity is regulated at the cellular level within the skin (Shook et al. 2018).
Increased mechanical tension in the lower wound bed promotes scar formation by reactivating expression of En1 in reticular fibroblasts (Mascharak et al. 2021), consistent with the role of En1 in inducing a profibrotic fibroblast fate during embryonic skin development (Rinkevich et al. 2015). Blocking mechanotransduction by pharmacological or genetic inhibition of Yes-associated protein (YAP) prevents En1 activation and promotes wound regeneration, including recovery of skin appendages and mechanical strength, thereby preventing scarring (Mascharak et al. 2021). The importance of the local microenvironment is also apparent during wound induced angiogenesis, in which the location of the regenerating blood vessel determines which fibroblast subpopulation gives rise to blood vessel–associated Ng2+ pericytes (Goss et al. 2021).
Several fibroblast subpopulations contribute to dermal regeneration, and scRNA-seq and lineage tracing have revealed that interfollicular fibroblasts are the major source of wound bed fibroblasts. The specialized fibroblast subpopulations of the dermal papilla, pericytes, dermal sheath, and APM make little contribution to wound repair (Kaushal et al. 2015; Abbasi et al. 2020; Goss et al. 2021). In contrast, adipocytes of the dermal white adipose tissue (DWAT) can differentiate into wound bed myofibroblasts in response to TGF-β signaling in early wound repair (Zhang et al. 2019). These cells subsequently convert to quiescent adipocytes during the later wound resolution phase when exposed to BMP signals from regenerating wound bed hair follicles (Plikus et al. 2017). It is currently unclear whether this is a response to extrinsic signals or whether adipocytes derived from myofibroblasts maintain an anatomical memory and convert back to their original state.
Another fibroblast population that is relevant to wound repair resides in the fascia beneath the panniculus muscle and contributes to the repair of deep full thickness wounds. Fascia fibroblasts up-regulate the cell–cell adhesion molecule N-cadherin and Cx43 on wounding and enter the wound bed with a swarm-like behavior (Jiang et al. 2020; Wan et al. 2021) in contrast to the individual migration of papillary and dermal fibroblasts (Rognoni et al. 2018). During this coordinated migration, fascia fibroblasts pull their surrounding matrix environment, including immune cells, blood vessels, and nerves, into the wound bed enabling them to quickly seal the wound and provide a provisional wound bed matrix.
A further aspect of wound healing is the effect of aging. Skin aging induces changes in fibroblast composition, lineage identity, and microenvironment, significantly delaying skin repair after wounding (Rognoni et al. 2016; Salzer et al. 2018; Shook et al. 2018). The progressive decrease in the number of fibroblasts during skin aging is balanced by increased formation of membrane protrusions (Marsh et al. 2018). Recent single-cell transcriptomic analyses of wound bed fibroblasts from young and aged mice has revealed distinct subpopulations of fibroblasts with different (proinflammatory) cytokine expression signatures (Mahmoudi et al. 2019). After tissue repair, wound bed fibroblasts reestablish quiescence to maintain skin homeostasis.
FIBROBLAST HETEROGENEITY IN HUMAN HEALTHY AND WOUNDED ADULT SKIN
Human Healthy Fibroblast Heterogeneity
Lineage-tracing studies performed in mice (Driskell et al. 2013; Rinkevich et al. 2015; Jiang et al. 2018) identified distinct fibroblast subpopulations in the dermis, leading to the hypothesis that the same is true in human dermis. Because it is not possible to perform lineage-tracing studies in humans, most of our understanding of human fibroblast subpopulations comes from cell culture experiments and single-cell transcriptional profiling.
Subpopulations and Markers
Human fibroblasts express a range of conserved markers including LUM, DCN, VIM, PDGFRα, and COL1A2 (Philippeos et al. 2018). We performed spatial transcriptional profiling (comparing gene expression in microdissected papillary and reticular dermis) and compared this to single-cell sequencing of a total of 184 cells isolated from human dermis (Philippeos et al. 2018). This revealed that there were at least four populations of fibroblasts present in the dermis. We were able to define cell surface markers that permitted the prospective isolation of populations that showed different functions in both interferon release assays and in supporting the growth of keratinocytes in organotypic 3D cultures (Philippeos et al. 2018).
Technological advances in single cell transcriptomics now permit us to analyze tens of thousands of cells in a single experiment and studies by multiple groups have confirmed that distinct fibroblast populations can be identified in human dermis on the basis of differences in transcriptional profile. Solé-Boldo et al. (2020) analyzed in excess of 15,000 cells derived from sun-protected skin of two young and three older male donors. They identified four subpopulations of fibroblasts. RNA-scope showed one of these to be preferentially localized in the papillary dermis, two in the reticular dermis, and one with a more widespread distribution but a preference for vasculature (Table 2). Additionally, Solé-Boldo et al. compared the transcriptional profile of young and old fibroblast populations and uncovered, via gene ontology analyses, an age-dependent loss of the functional annotations of each cluster, reflecting a reduction in associated genes. Furthermore, the papillary fibroblast subpopulation within aged fibroblasts presented a more reticular gene signature while the reticular fibroblast subpopulation signature was less pronounced. Therefore, aging results in a loss of fibroblast identities (Solé-Boldo et al. 2020). Vorstandlechner et al. (2020) looked at 4764 cells isolated from the abdomen of three healthy donors. Tabib et al. (2018) isolated a total of 8622 cells from six samples of dorsal sun-exposed forearm skin from both male and female subjects. Clustering identified a total of eight cellular populations within the fibroblasts (Table 2), which could be grouped into two major clusters defined by expression of SFRP2/DPP4 and FMO1/LSP1. Tabib et al. (2018) found that staining for SFRP2/DPP4 revealed elongated cells and FMO1/LSP1 was expressed by rounder cells.
Table 2.
Summary of putative markers for fibroblast subpopulations present in normal human skin
| Study | Skin sites | Cluster name | Markers | Inferred function/pathways |
|---|---|---|---|---|
| Solé-Boldo et al. 2020 | Inguinoiliac area | 1 | CTHRC1 | Secretory–reticular |
| 2 | CCL19, APOE | Proinflammatory–reticular | ||
| 3 | APCDD1, COL13A1, COL23A1, COL18A1 | Secretory–papillary | ||
| 9 | ASPN, POSTN, COL11A1, COL24A1 | Mesenchymal, cartilage, bone development | ||
| Vorstandlechner et al. 2020 | Trunk area | FB1 | MFAP5, FAP, COL12A1, LOX, DPP4 | ECM assembly, wound healing, angiogenesis |
| FB2 | APOE | Antimicrobial immune response, leukocyte migration | ||
| FB3 | APCDD1, WIF1 | Cartilage development, leptin signaling | ||
| FB4 | B4GALT1 | Growth factor response | ||
| FB5 | CXCL1, APOE | Antimicrobial immune response, leukocyte migration | ||
| FB6 | ABCDD1, CXCL1, WIF1 | Interferon γ, p38, NF-κB signaling | ||
| Tabib et al. 2018 | Arm area | 0 | SFRP2, DPP4, PCOLCE2, CD55, WIF1, NKD2 | Negative regulation of signaling pathways, regulation, and sequestering of BMP and protein localization to extracellular matrix |
| 1 | FMO1, LSP1, MYOC, IGFBP3, ITM2A, CYGB, C7, AADAC, RAMP2 | Negative regulation of cell movement, lipid clearance, and stress response | ||
| 2 | SFRP2, DPP4, PCOLCE2, CD55 | Negative regulation of signaling pathways, regulation and sequestering of BMP, and protein localization to extracellular matrix | ||
| 3 | SFRP2, DPP4 | |||
| 4 | CRABP1, TNN, DPEP1, COL11A1 | Development of tendon, muscle, circulatory system, and heart | ||
| 5 | FMO1, LSP1, MYOC, IGFBP3 ITM2A, CYGB, and C7 | Negative regulation of signaling pathways, regulation and sequestering of BMP, and protein localization to extracellular matrix | ||
| 6 | SFRP2, DPP4, PCOLCE2, CD55, PRG4, LINC01133, FMO1, LSP1, MYOC, IGFBP3 ITM2A, CYGB, C7 | |||
| 7 | SFRP4, C2orf40, ANGPTL7 | Not defined | ||
| Reynolds et al. 2021 | Breast area | Fb1 | TNFAIP6, IL6, PTGES | Not defined |
| Fb2 | CXCL3, CXCL12 | |||
| Fb3 | TNFAIP6, IL6, PTGES, CD82, CCL19, TNC | |||
| He et al. 2020 | Extremity areas | FB1 | APOD, PTGDS, MFAP4, PCOLCE | Not defined |
| FB2 | MFAP5, SFRP2, WISP2, POSTN, MFAP4, | |||
| FB3 | APCDD1, POSTN, MXRA5, PCOLCE, MFAP5 | |||
| Acensiόn et al. 2021 | Multiple body areas | A | ELN, MMP2, QPCT, SFRP2 | Dermal cell and extracellular matrix (ECM) homeostasis |
| A1 | IGFBP6, PI16, SLPI, WISP2 | |||
| A2 | APCDD1, COL18A1, COMP, NKD2 | |||
| A3 | ELN, RGCC, SGCA, WIF1 | |||
| A4 | FBN1, PCOLCE2, PRG4, SFRP4 | |||
| B | APOE, C7, CYGB, IGFBP7 | Immune surveillance and promoting inflammation | ||
| B1 | CCL2, ITM2A, SPSB1, TNFAIP6 | |||
| B2 | CCDC146, CCL19, CD74, TNFSF13B | |||
| C | DKK3, TNMD, TNN, SFRP1 | Specialized subpopulations, such as dermal papilla cells (type C2) and dermo–hypodermal junction fibroblasts | ||
| C1 | COL11A1, DPEP1, TNMD, WFDC1 | |||
| C2 | COCH, CRABP1, FIBIN, RSPO4 | |||
| C3 | ASPN, F2R, GPM6B, POSTN | |||
| C4 | ANGPTL7, APOD, C2 or f40, TM4SF1 |
More recently, Reynolds et al. analyzed in excess of 500,000 cells derived from embryonic human skin, adult skin, and skin from patients with the inflammatory skin diseases eczema and psoriasis. Healthy adult skin was derived from mammoplasty patients. They identified three fibroblast subpopulations and two pericyte subpopulations (Reynolds et al. 2021). Human pericytes express ACTA2 and RGS5 (Cho et al. 2003; Paquet-Fifield et al. 2009). scRNA-seq of healthy human skin has yet to identify more than one pericyte population; however, analysis of atopic dermatitis and psoriasis samples has revealed the presence of a second pericyte population that is proposed to play a role in leukocyte recruitment and TNF-α-mediated signaling (Reynolds et al. 2021).
He et al. analyzed 39,042 cells from lesional and nonlesional skin of five patients with atopic dermatitis and seven controls (He et al. 2020). They identified three subpopulations of fibroblasts in healthy skin (Table 2). Additionally, they identified a population of fibroblasts that was only present in lesional skin of patients with atopic dermatitis. This was identified by the expression of COL6A5, COL18A1, and CCL2. We have previously observed COL6A5 expression in the papillary dermis of healthy skin (Philippeos et al. 2018) and it is possible that these differences reflect a difference in body site or degree of sun exposure.
All single-cell sequencing approaches use complex statistical transformations to reduce the dimensionality of the input data and permit visualization and analysis. A limitation of the analysis is that the number of clusters identified varies according to the methods of normalization, the dimensionality reduction approach, and the specific parameters chosen for the analysis. It is therefore not surprising that there are differences in the numbers of clusters identified between different studies. To address this, Acensiόn et al. (2021) reanalyzed data published by Tabib et al. (2018), Solé-Boldo et al. (2020), He et al. (2020), and Vorstandlechner et al. (2020). The data were integrated, with the exception of the He et al. data set, which had low cell viability. On this basis, three classes—axes—of fibroblasts were identified, which were designated A–C. Within each class, numerous subpopulations were defined (Table 2). Most of these subpopulations were reproducible across observed datasets, although it remains to be determined to what extent these reflect true subpopulations rather than different cell states or tissue contexts such as different anatomical sites.
Certain fibroblast populations are not well represented within single-cell sequencing data sets and this may reflect difficulties in extraction of cells—for example, hair follicles with associated dermal papillae and dermal sheaths—or degradation of cells during processing—for example, preadipocytes and adipocytes.
Analysis of gene expression patterns within the different fibroblast subpopulations permits the identification of transcriptional markers, expressed in one or more of the clusters. The identity of these markers varies considerably across the different studies (Table 2). It is desirable to identify cell surface markers for the different human fibroblast subpopulations because this allows prospective isolation for therapeutic and experimental approaches, including in vivo targeting of specific subpopulations, for example, using monoclonal antibodies. Based on analysis of 184 cells, we identified CD39 and CD26 (DPP4) as markers of functionally distinct fibroblast subpopulations (Philippeos et al. 2018). In our data, CD26 was coexpressed with MFAP5. Vorstandlechner et al. have confirmed that CD26 (DPP4) is preferentially expressed on the same subpopulation of fibroblasts as MFAP5 (Table 2). Tabib et al. (2018) also found DPP4 expression restricted to a subpopulation of fibroblasts. DPP4 is thus a promising therapeutic target for specific fibroblast cell therapy.
Spatial Localization
Many of the markers identified do not have well-characterized antibodies that can be used for immunolocalization in skin sections. For this reason, many groups have taken advantage of methodological advances in RNA FISH approaches, such as RNAscope (Bio-techne), which permit visualization of transcripts in intact tissue sections. Solé-Boldo et al. looked at the localization of the four different clusters that they identified using RNAscope (Table 2). They found the secretory–papillary population to be preferentially localized in the papillary dermis; the secretory–reticular was preferentially located in the reticular dermis; the mesenchymal population was found in the reticular dermis and in association with hair follicles; and the proinflammatory population was present in both papillary and reticular dermis and also in association with the vasculature.
Fibroblasts can be found both embedded in the dermis itself and in intimate association with the basement membrane of the epidermis, hair follicles, sweat glands, and blood vessels. It seems probable that fibroblasts in these differing contexts will manifest differences in their function and that this will be reflected in gene expression profiles.
Recently, researchers have highlighted another level of complexity within human fibroblast heterogeneity, namely, the fibroblasts from the junction of the dermis and subcutaneous tissue (dermo–hypodermal junction fibroblasts) (Haydont et al. 2020). The existence of a fibroblast population showing adipocyte-like molecular characteristics within the lower reticular dermis has previously been reported in human skin (Korosec et al. 2019). These cells have distinct expression signatures and functional differences from dermal papillary and reticular fibroblasts. They show specific expression of genes involved in ECM synthesis processing and tissue skeleton organization.
What Is the Function of the Different Human Fibroblast Populations?
Gene ontology and pathway analysis provide some insight into the properties of fibroblasts in the identified clusters. Solé-Boldo et al. (2020) found that three out of four of their populations displayed high levels of expression of markers associated with ECM organization, cell adhesion, and collagen fibril organization. In contrast, population 2 possessed a proinflammatory expression profile. In addition, populations 3 and 9 expressed a mesenchymal profile that included markers of muscle and bone development (Table 2).
Reanalysis of existing single-cell data sets has led to the proposal that there are three classes of fibroblasts present in the human dermis that differ in their potential functions (Ascensión et al. 2021). The first, type A, is responsible for ECM maintenance and synthesis; the second, type B, is implicated in immune surveillance and promotion of inflammation; and the third, class C, incorporates numerous different fibroblasts with specialized functions including the dermal papilla.
It is recognized that fibroblasts derived from the upper dermis better support the growth of the epidermis in cell culture (Sorrell et al. 2004; Lee and Cho 2005; Janson et al. 2012). We have shown that fibroblasts with this property can be prospectively isolated using CD90 and CD39 surface markers (Philippeos et al. 2018).
Developmental Programs Regulating Fibroblast Identity
In keeping with classical heterotopic transplantation experiments (Sengel 1990), there is evidence that fibroblasts from different regions of the body have different transcriptional programs (Shaw and Rognoni 2020). Fibroblasts present at different sites in the adult are derived from different embryological origins including neural crest, lateral plate mesoderm, and dermatomyotome (Le Lièvre and Le Douarin 1975; Houzelstein et al. 2000; Sriram et al. 2015). This is reflected in differences in the pattern of expression of Hox genes, which are known master regulators of positional identity during body morphogenesis (Rinn et al. 2006, 2008).
Fibroblasts derived from the palms and soles have the ability to induce keratin 9 expression in keratinocytes, whereas fibroblasts derived from nonplantar sites do not (Yamaguchi et al. 1999). Androgen receptors have been reported to be expressed more highly in regions of the scalp susceptible to male pattern hair loss (Hibberts et al. 1998). In keeping with this observation, hair follicles transplanted from the posterior scalp to the anterior scalp retain their resistance to androgen-induced miniaturization (Orentreich 1959).
Intrinsic and Extrinsic Regulatory Mechanisms Specifying Fibroblast State
For human tissues including skin, a large fraction of our knowledge of fibroblast identity is derived from single-cell sequencing studies. These represent a snapshot of identity at a specific point in developmental time and space. Fibroblasts can be found both embedded in the dermis itself but also in intimate association with the basement membrane of the epidermis, hair follicles, sweat glands, and APM.
The details of how the transcriptional identity of fibroblasts in these differing tissue contexts is specified remains to be determined. However, cell culture experiments support an interplay between the function and identity of fibroblasts and neighboring cells in the tissue. Coculture experiments have shown reciprocal interactions between keratinocytes and fibroblasts (Maas-Szabowski et al. 1999; Werner et al. 2007). Interactions with ECM elements are also important—fibroblasts cultured in conventional monolayer conditions show differences in gene expression and morphology compared with those cultured in collagen gels (Eckes et al. 1993).
Human Fibroblast Heterogeneity in Physiological Wound Healing
Wound healing in human skin is the result of dynamic and highly regulated processes involving multiple cell types. In the local wound area, a well-defined and coordinated series of events take place: hemostasis, inflammation, new tissue formation, and tissue remodeling.
The Role of Fibroblasts on Wounding
On wounding, fibroblasts migrate into the wounds to restore as much as possible of the skin architecture by producing new ECM and collagen structures to support the other cells associated with effective wound healing, as well as contracting the wound. Additionally, fibroblasts release growth factors and inflammatory cytokines to modulate immune cell functions.
In humans, typical wound healing results in scar formation, which can be caused by fibroblast subpopulation dysfunction and decreased proliferation and/or fibroblast depletion. Although the mechanisms used by mammals to promote rapid wound closure and inflammation are fundamental for the prevention of skin infection and dehydration, the resulting scar formation can also be unfavorable owing to the lack of skin appendages, which leads to defects in the skin's biological and physiological functions. For example, hair follicles and sebaceous glands confer sensory and thermoregulatory functions on the skin (Chen et al. 1997; Li et al. 2011). Therefore, the capability to restore the original state of a healthy skin is highly valued.
Fibroblast Heterogeneity in Pathological States Including Scar and Keloid
Single-cell transcriptional profiling provides an understanding of the cellular populations present in a scar—the end point of cutaneous wound healing. Deng et al. (2021) compared 21,488 cells derived from keloid scars and 19,167 cells derived from normal scars. They found that fibroblasts, endothelial cells, and blood vessel–associated smooth muscle cells accounted for the majority of cells within scars. Fibroblasts made a lesser contribution to keloid scars versus normal scars, which may reflect the expansion of vasculature in keloid scars (Deng et al. 2021). Bioinformatic analysis revealed four clusters and 13 distinct fibroblast populations across the normal scar and keloid scar samples. When the normal scar and keloid scar populations were compared, there was a significant increase in a population of fibroblasts in keloids that corresponded to the subpopulation previously characterized by Solé-Boldo et al. (2020) as mesenchymal and characterized by the expression of POSTN, ASPN, and COL11A1. Other populations identified by Solé-Boldo et al. were relatively decreased. Receptor–ligand analysis found evidence of altered signaling pathways including POSTN and TGFRβ1.
We suggest that dysfunction, decreased proliferation, and/or depletion of fibroblast subpopulations can play different roles in human wound healing, resulting in different repair outcomes, such as the formation of normal scars in physiological tissue repair and fibrosis or ulcers in pathological tissue repair. Understanding dermal fibroblast heterogeneity is challenging, but it is critical to obtaining mechanistic insights into the human wound healing process. These insights could form the basis of developing therapeutics targeting specific subpopulations in wound healing and scarring.
DISCUSSION
Fibroblast Subpopulations in the Human and Mouse
In both human and mouse, scRNA-seq studies have identified multiple distinct populations of fibroblasts. This is in keeping with earlier studies performed via lineage tracing in mice and in cultures of human cells. Despite the anatomical differences between mouse and human skin, many fibroblast population markers are conserved across species (Table 1). This similarity between mouse and human fibroblast subpopulation transcriptomic profiles is reflected in the conservation of signaling pathways (Rognoni et al. 2016; Philippeos et al. 2018; Tabib et al. 2018).
Lessons for Human Wound Healing from the Mouse
Single-cell transcriptomic and epigenetic studies represent a snapshot of cellular identity at a point in developmental time and space. They do not permit us to disentangle the relative contributions of intrinsic transcriptional programs, spatial context within the tissue, and developmental origins. These questions are of particular relevance during development and wound healing, both of which are periods of dynamic change associated with highly coordinated migration and differentiation of multiple cell populations. It is challenging to study dynamic processes in the human; however, powerful genetic and lineage-tracing approaches permit us to do so in the mouse. For this reason, a great deal of our understanding of development and wound healing in the human is inferred from studies in the mouse.
Supporting the similarities in transcriptomic profiles of fibroblasts from mouse and human is the fact that the fundamental principles of dermal maintenance, development, and wound healing are broadly conserved across mammalian species. In the adult steady-state mouse dermis, fibroblasts rarely divide and those that are lost through aging or extrinsic damage are not replaced by division or migration of fibroblasts from neighboring regions (Scharffetter-Kochanek et al. 2000; Wlaschek et al. 2001; Naylor et al. 2011). This is likely to be the case in the human dermis because fibroblast division is rarely observed in histological sections in the steady state and there is a decrease in fibroblast numbers in the dermis with age (Marsh et al. 2018; Solé-Boldo et al. 2020).
During wound healing in the mouse dermis, inflammatory signals lead to an extensive process of cell division and fibroblast migration. Fibroblasts from the reticular dermis migrate into the wound environment first, with fibroblasts from the papillary dermis migrating subsequently. In support of the hypothesis that a similar process is operational in the human dermis, there is an expansion of POSTN+ASPN+ COLL11A1+ fibroblasts in keloid scars (Deng et al. 2021); in healthy skin, this population is preferentially localized to the lower dermis, suggesting that it may correspond to the reticular population of fibroblasts present in the mouse. The appearance of a regenerative zone in the papillary dermis in the mouse (Driskell et al. 2013; Rognoni et al. 2016) does not appear to have a direct correlation in the human because human scars are devoid of hair follicles and other cutaneous appendages. Finally, recent studies have suggested a key role for fascia-derived fibroblasts in healing of mouse wounds (Jiang et al. 2020). This seems less likely to be relevant in the human because at many body sites, particularly on the trunk, the dermis is separated from the underlying fascia by many centimeters of subcutaneous fat.
Open Questions and Ongoing Work
The existence of multiple fibroblast populations has raised a number of important questions that are the subject of ongoing investigation. One unresolved question is to what extent the fibroblast populations present in the human dermis correspond to those within the mouse. This is important because, by necessity, mouse models are used to model dynamic processes such as development and wound healing that are not straightforward to study in humans. Although not all specific markers of fibroblast subpopulations are conserved between human and mouse, it is possible that transcriptional profiles or master transcriptional regulators will be conserved across species.
A second important question is the spatial localization of fibroblast populations within the dermis, in the steady state, in the wound environment, and in scars. Studies in the mouse have shown a distinction between papillary fibroblasts and reticular fibroblasts; however, when the localization of the different fibroblast populations identified by RNA sequencing is assessed through RNA FISH or protein staining in human skin, some populations are not exclusive to either the papillary or the reticular dermis (Philippeos et al. 2018). It is likely that novel spatial transcriptomic approaches will clarify the localization of fibroblasts within the dermis both in terms of depth and with respect to other structures such as the epidermis, hair follicles, glandular structures, and blood vessels, and this is a major focus of ongoing work (Heibel et al. 2020; Ji et al. 2020). The spatial localization of different fibroblast populations within the dermis will likely reflect differences in function. Gene ontology–based approaches have given clues as to these different functions; however, functional experiments through genetically modified mice and organotypic culture of human fibroblasts will be required to understand these processes in greater detail.
It is not clear to what extent the observed differences in transcriptional profiles reflect self-perpetuating intrinsic transcriptional programs versus a consequence of signals arising from ECM or neighboring cells in the tissue context. These questions can be addressed through transplantation, culture, and lineage-tracing experiments. It is possible that the observed transcriptional profiles represent a superposition of different contributory elements. It will also be important to understand whether the identity of fibroblast subpopulations is conserved for an individual clone of cells or whether cells can switch identity from one population to another. Finally, it will be of great importance to understand how fibroblast populations are modified in scarring, malignancy, and aging, because manipulation of fibroblast identity or ablation of specific populations has the potential to offer new therapeutic approaches to treatment.
Footnotes
Editors: Xing Dai, Sabine Werner, Cheng-Ming Chuong, and Maksim Plikus
Additional Perspectives on Wound Healing: From Bench to Bedside available at www.cshperspectives.org
REFERENCES
- Abbasi S, Sinha S, Labit E, Rosin NL, Yoon G, Rahmani W, Jaffer A, Sharma N, Hagner A, Shah P, et al. 2020. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell 27: 396–412.e6. 10.1016/j.stem.2020.07.008 [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Betsholtz C. 2011. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215. 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Ascensión AM, Fuertes-Álvarez S, Ibañez-Solé O, Izeta A, Araúzo-Bravo MJ. 2021. Human dermal fibroblast subpopulations are conserved across single-cell RNA sequencing studies. J Invest Dermatol 141: 1735–1744.e35. 10.1016/j.jid.2020.11.028 [DOI] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. 2013. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 15: 302–308. 10.1038/ncb2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. 2013. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22: 2298–2314. 10.1089/scd.2012.0647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondjers C, Kalén M, Hellström M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. 2003. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol 162: 721–729. 10.1016/S0002-9440(10)63868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. 1997. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 91: 789–798. 10.1016/S0092-8674(00)80467-5 [DOI] [PubMed] [Google Scholar]
- Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. 2003. Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 17: 440–442. [DOI] [PubMed] [Google Scholar]
- Collins CA, Kretzschmar K, Watt FM. 2011. Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin. Development 138: 5189–5199. 10.1242/dev.064592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Gallegos D, Jiang D, Christ S, Ramesh P, Ye H, Wannemacher J, Gopal SK, Yu Q, Aichler M, Walch A, et al. 2019. Patch repair of deep wounds by mobilized fascia. Nature 576: 287–292. 10.1038/s41586-019-1794-y [DOI] [PubMed] [Google Scholar]
- Deng CC, Hu YF, Zhu DH, Cheng Q, Gu JJ, Feng QL, Zhang LX, Xu YP, Wong D, Rong Z, et al. 2021. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat Commun 12: 3709. 10.1038/s41467-021-24110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. 2009. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 136: 2815–2823. 10.1242/dev.038620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, Watt FM. 2011. Hair follicle dermal papilla cells at a glance. J Cell Sci 124: 1179–1182. 10.1242/jcs.082446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, et al. 2013. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504: 277–281. 10.1038/nature12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Jahoda CAB, Chuong CM, Watt FM, Horsley V. 2014. Defining dermal adipose tissue. Exp Dermatol 23: 629–631. 10.1111/exd.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretskiy S, Garg K, Munroe M, Pincu Y, Mahmassani ZS, Coombs C, Blackwell B, Garcia G, Waterstradt G, Lee I, et al. 2019. The impact of skeletal muscle contraction on CD146 +Lin− pericytes. Am J Physiol Cell Physiol 317: C1011–C1024. 10.1152/ajpcell.00156.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckes B, Mauch C, Hüppe G, Krieg T. 1993. Downregulation of collagen synthesis in fibroblasts within three-dimensional collagen lattices involves transcriptional and posttranscriptional mechanisms. FEBS Lett 318: 129–133. 10.1016/0014-5793(93)80006-G [DOI] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. 2011. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146: 761–771. 10.1016/j.cell.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. 2011. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144: 577–589. 10.1016/j.cell.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, et al. 2013. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med 19: 916–923. 10.1038/nm.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Ghinatti G, Guerrero-Juarez CF, Ferrer RA, Ferri F, Lim CH, Murakami S, Gault N, Barroca V, Rombeau I, et al. 2020. Phagocytosis of Wnt inhibitor SFRP4 by late wound macrophages drives chronic Wnt activity for fibrotic skin healing. Sci Adv 6: eaay3704. 10.1126/sciadv.aay3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Tan SJ, Wang SH, Li L, Sun XF, Shen W, Wang X. 2020. Single-cell transcriptome profiling reveals dermal and epithelial cell fate decisions during embryonic hair follicle development. Theranostics 10: 7581–7598. 10.7150/thno.44306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss G, Rognoni E, Salameti V, Watt FM. 2021. Distinct fibroblast lineages give rise to NG2+ pericyte populations in mouse skin development and repair. Front Cell Dev Biol 9: 675080. 10.3389/fcell.2021.675080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Juarez CF, Dedhia PH, Jin S, Ruiz-Vega R, Ma D, Liu Y, Yamaga K, Shestova O, Gay DL, Yang Z, et al. 2019. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat Commun 10: 650. 10.1038/s41467-018-08247-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães-Camboa N, Evans SM. 2017. Are perivascular adipocyte progenitors mural cells or adventitial fibroblasts? Cell Stem Cell 20: 587–589. 10.1016/j.stem.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Bishop WB, et al. 2017. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20: 345–359.e5. 10.1016/j.stem.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AP. 2006. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol Pathol 34: 763–775. 10.1080/01926230600936290 [DOI] [PubMed] [Google Scholar]
- Hamburg-Shields E, DiNuoscio GJ, Mullin NK, Lafyatis R, Atit RP. 2015. Sustained β-catenin activity in dermal fibroblasts promotes fibrosis by up-regulating expression of extracellular matrix protein-coding genes. J Pathol 235: 686–697. 10.1002/path.4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RA, Grove G. 1979. Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science 204: 526–527. 10.1126/science.432659 [DOI] [PubMed] [Google Scholar]
- Haydont V, Neiveyans V, Perez P, Busson E, Lataillade J, Asselineau D, Fortunel O. 2020. Fibroblasts from the human skin dermo-hypodermal junction are distinct from dermal papillary and reticular fibroblasts and from mesenchymal stem cells and exhibit a specific molecular profile related to extracellular matrix organization and modeling. Cells 9: 368. 10.3390/cells9020368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Suryawanshi H, Morozov P, Gay-Mimbrera J, Del Duca E, Kim EJ, Kameyama N, Estrada Y, Der E, Krueger JG, et al. 2020. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J Allergy Clin Immunol 145: 1615–1628. 10.1016/j.jaci.2020.01.042 [DOI] [PubMed] [Google Scholar]
- Heibel HD, Hooey L, Cockerell CJ. 2020. A review of noninvasive techniques for skin cancer detection in dermatology. Am J Clin Dermatol 21: 513–524. 10.1007/s40257-020-00517-z [DOI] [PubMed] [Google Scholar]
- Heitman N, Sennett R, Mok KW, Saxena N, Srivastava D, Martino P, Grisanti L, Wang Z, Ma'ayan A, Rompolas P, et al. 2020. Dermal sheath contraction powers stem cell niche relocation during hair cycle regression. Science 367: 161–166. 10.1126/science.aax9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberts NA, Howell AE, Randall VA. 1998. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol 156: 59–65. 10.1677/joe.0.1560059 [DOI] [PubMed] [Google Scholar]
- Higgins CA, Chen JC, Cerise JE, Jahoda CAB, Christiano AM. 2013. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci 110: 19679–19688. 10.1073/pnas.1309970110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzelstein D, Chéraud Y, Auda-Boucher G, Fontaine-Pérus J, Robert B. 2000. The expression of the homeobox gene Msx1 reveals two populations of dermal progenitor cells originating from the somites. Development 127: 2155–2164. 10.1242/dev.127.10.2155 [DOI] [PubMed] [Google Scholar]
- Hu MS, Borrelli MR, Hong WX, Malhotra S, Cheung ATM, Ransom RC, Rennert RC, Morrison SD, Lorenz HP, Longaker MT. 2018. Embryonic skin development and repair. Organogenesis 14: 46–63. 10.1080/15476278.2017.1421882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis GE. 2007. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316–320. 10.1038/nature05766 [DOI] [PubMed] [Google Scholar]
- Janson DG, Saintigny G, van Adrichem A, Mahé C, El Ghalbzouri A. 2012. Different gene expression patterns in human papillary and reticular fibroblasts. J Invest Dermatol 132: 2565–2572. 10.1038/jid.2012.192 [DOI] [PubMed] [Google Scholar]
- Ji AL, Rubin AJ, Thrane K, Jiang S, Reynolds DL, Meyers RM, Guo MG, George BM, Mollbrink A, Bergenstråhle J, et al. 2020. Multimodal analysis of composition and spatial architecture in human squamous cell carcinoma. Cell 182: 497–514.e22. 10.1016/j.cell.2020.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Correa-Gallegos D, Christ S, Stefanska A, Liu J, Ramesh P, Rajendran V, De Santis MM, Wagner DE, Rinkevich Y. 2018. Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat Cell Biol 20: 422–431. 10.1038/s41556-018-0073-8 [DOI] [PubMed] [Google Scholar]
- Jiang D, Christ S, Correa-Gallegos D, Ramesh P, Gopal SK, Wannemacher J, Mayr CH, Lupperger V, Yu Q, Ye H, et al. 2020. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat Commun 11: 5653. 10.1038/s41467-020-19425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost S, Annusver K, Jacob T, Sun X, Dalessandri T, Sivan U, Sequeira I, Sandberg R, Kasper M. 2020. The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 26: 441–457.e7. 10.1016/j.stem.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. 2006. Fibroblasts in cancer. Nat Rev Cancer 6: 392–401. 10.1038/nrc1877 [DOI] [PubMed] [Google Scholar]
- Kaushal GS, Rognoni E, Lichtenberger BM, Driskell RR, Kretzschmar K, Hoste E, Watt FM. 2015. Fate of Prominin-1 expressing dermal papilla cells during homeostasis, wound healing and Wnt activation. J Invest Dermatol 135: 2926–2934. 10.1038/jid.2015.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. 2000. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 14: 1181–1185. 10.1101/gad.14.10.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosec A, Frech S, Gesslbauer B, Vierhapper M, Radtke C, Petzelbauer P, Lichtenberger BM. 2019. Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin. J Invest Dermatol 139: 342–351. 10.1016/j.jid.2018.07.033 [DOI] [PubMed] [Google Scholar]
- Leavitt T, Hu MS, Borrelli MR, Januszyk M, Garcia JT, Ransom RC, Mascharak S, desJardins-Park E, Litzenburger UM, Walmsley GG, et al. 2020. Prrx1 fibroblasts represent a pro-fibrotic lineage in the mouse ventral dermis. Cell Rep 33: 108356. 10.1016/j.celrep.2020.108356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Cho KH. 2005. The effects of epidermal keratinocytes and dermal fibroblasts on the formation of cutaneous basement membrane in three-dimensional culture systems. Arch Dermatol Res 296: 296–302. 10.1007/s00403-004-0529-5 [DOI] [PubMed] [Google Scholar]
- Le Lièvre CS, Le Douarin NM. 1975. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 34: 125–154. [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. 2011. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147: 1615–1627. 10.1016/j.cell.2011.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger BM, Mastrogiannaki M, Watt FM. 2016. Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat Commun 7: 10537. 10.1038/ncomms10537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CH, Sun Q, Ratti K, Lee SH, Zheng Y, Takeo M, Lee W, Rabbani P, Plikus MV, Cain JE, et al. 2018. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat Commun 9: 4903. 10.1038/s41467-018-07142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas-Szabowski N, Shimotoyodome A, Fusenig NE. 1999. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci 112: 1843–1853. 10.1242/jcs.112.12.1843 [DOI] [PubMed] [Google Scholar]
- Mahmoudi S, Mancini E, Xu L, Moore A, Jahanbani F, Hebestreit K, Srinivasan R, Li X, Devarajan K, Prélot L, et al. 2019. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 574: 553–558. 10.1038/s41586-019-1658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh E, Gonzalez DG, Lathrop EA, Boucher J, Greco V. 2018. Positional stability and membrane occupancy define skin fibroblast homeostasis in vivo. Cell 175: 1620–1633.e13. 10.1016/j.cell.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascharak S, desJardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, Chen K, Duoto B, Chinta M, Foster DS, et al. 2021. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372: eaba2374. 10.1126/science.aba2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki M, Lichtenberger BM, Reimer A, Collins CA, Driskell RR, Watt FM. 2016. β-Catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J Invest Dermatol 136: 1130–1142. 10.1016/j.jid.2016.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigel WN, Gay S, Weber L. 1977. Dermal architecture and collagen type distribution. Arch Dermatol Res 259: 1–10. 10.1007/BF00562732 [DOI] [PubMed] [Google Scholar]
- Naylor EC, Watson REB, Sherratt MJ. 2011. Molecular aspects of skin ageing. Maturitas 69: 249–256. 10.1016/j.maturitas.2011.04.011 [DOI] [PubMed] [Google Scholar]
- Niiyama S, Ishimatsu-Tsuji Y, Nakazawa Y, Yoshida Y, Soma T, Ideta R, Mukai H, Kishimoto J. 2018. Gene expression profiling of the intact dermal sheath cup of human hair follicles. Acta Derm Venereol 98: 694–698. 10.2340/00015555-2949 [DOI] [PubMed] [Google Scholar]
- Orentreich N. 1959. Autografts in alopecias and other selected dermatological conditions. Ann NY Acad Sci 83: 463–479. 10.1111/j.1749-6632.1960.tb40920.x [DOI] [PubMed] [Google Scholar]
- Paquet-Fifield S, Schlüter H, Li A, Aitken T, Gangatirkar P, Blashki D, Koelmeyer R, Pouliot N, Palatsides M, Ellis S, et al. 2009. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest 119: 2795–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan QM, Fine GM, Salz L, Herrera GG, Wildman B, Driskell IM, Driskell RR. 2020. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. eLife 9: e60066. 10.7554/eLife.60066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, et al. 2018. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol 138: 811–825. 10.1016/j.jid.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC, et al. 2017. Regeneration of fat cells from myofibroblasts during wound healing. Science 355: 748–752. 10.1126/science.aai8792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, Driskell RR, Rosenthal N, Biernaskie J, Horsley V. 2021. Fibroblasts: origins, definitions, and functions in health and disease. Cell 184: 3852–3872. 10.1016/j.cell.2021.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. 2008. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev 22: 543–557. 10.1101/gad.1614408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, Goh I, Botting RA, Huang N, Olabi B, Dubois A, et al. 2021. Developmental cell programs are co-opted in inflammatory skin disease. Science 371: eaba6500. 10.1126/science.aba6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, et al. 2015. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151. 10.1126/science.aaa2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Bondre C, Gladstone HB, Brown POB, Chang HY. 2006. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet 2: e119. 10.1371/journal.pgen.0020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Wang JK, Allen N, Brugmann SA, Mikels AJ, Liu H, Ridky TW, Stadler HS, Nusse R, Helms JA, et al. 2008. A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev 22: 303–307. 10.1101/gad.1610508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gonzalez G, Shook B, Horsley V. 2014. Adipocytes in skin health and disease. Cold Spring Harb Perspect Med 4: a015271. 10.1101/cshperspect.a015271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. 2008. Identification of white adipocyte progenitor cells in vivo. Cell 135: 240–249. 10.1016/j.cell.2008.09.036 [DOI] [PubMed] [Google Scholar]
- Rognoni E, Watt FM. 2018. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol 28: 709–722. 10.1016/j.tcb.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Gomez C, Pisco OA, Rawlins EL, Simons BD, Watt FM, Driskell RR. 2016. Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 143: 2522–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Pisco AO, Hiratsuka T, Sipilä KH, Belmonte JM, Mobasseri SA, Philippeos C, Dilão R, Watt FM. 2018. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol Systems Biol 14: e8174. 10.15252/msb.20178174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach A, Kraft R, Herrmann K, Haustein UF, Anderegg U. 1998. The monoclonal antibody AS02 recognizes a protein on human fibroblasts being highly homologous to Thy-1. Arch Dermatol Res 290: 360–366. 10.1007/s004030050318 [DOI] [PubMed] [Google Scholar]
- Salzer MC, Lafzi A, Berenguer-Llergo A, Youssif C, Castellanos A, Solanas G, Peixoto FO, Attolini CSO, Prats N, Aguilera M, et al. 2018. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 175: 1575–1590.e22. 10.1016/j.cell.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Scharffetter-Kochanek K, Brenneisen P, Wenk J, Herrmann G, Ma W, Kuhr L, Meewes C, Wlaschek M. 2000. Photoaging of the skin from phenotype to mechanisms. Exp Gerontol 35: 307–316. 10.1016/S0531-5565(00)00098-X [DOI] [PubMed] [Google Scholar]
- Schlake T. 2007. Determination of hair structure and shape. Semin Cell Dev Biol 18: 267–273. 10.1016/j.semcdb.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Schönherr E, Beavan LA, Hausser H, Kresse H, Culp LA. 1993. Differences in decorin expression by papillary and reticular fibroblasts in vivo and in vitro. Biochem J 290: 893–899. 10.1042/bj2900893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. 2012. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489: 561–565. 10.1038/nature11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengel P. 1990. Pattern formation in skin development. Int J Dev Biol 34: 33–50. [PubMed] [Google Scholar]
- Shaw TJ, Martin P. 2016. Wound repair: a showcase for cell plasticity and migration. Curr Opin Cell Biol 42: 29–37. 10.1016/j.ceb.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Shaw TJ, Rognoni E. 2020. Dissecting fibroblast heterogeneity in health and fibrotic disease. Curr Rheumatol Rep 22: 33. 10.1007/s11926-020-00903-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Morgan BA. 2004. Wnt signaling through the β-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J Invest Dermatol 122: 239–245. 10.1046/j.0022-202X.2004.22224.x [DOI] [PubMed] [Google Scholar]
- Shook BA, Wasko BR, Rivera-Gonzalez GC, Salazar-Gatzimas E, López-Giráldez F, Dash BC, Muñoz-Rojas AR, Aultman KD, Zwick RK, Lei V, et al. 2018. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362: eaar2971. 10.1126/science.aar2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Boldo L, Raddatz G, Schütz S, Mallm JP, Rippe K, Lonsdorf AS, Rodríguez-Paredes M, Lyko F. 2020. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun Biol 3: 188. 10.1038/s42003-020-0922-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI. 2004. Fibroblast heterogeneity: more than skin deep. J Cell Sci 117: 667–675. 10.1242/jcs.01005 [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Baber MA, Caplan AI. 2004. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol 200: 134–145. 10.1002/jcp.10474 [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Baber MA, Caplan AI. 2007. Clonal characterization of fibroblasts in the superficial layer of the adult human dermis. Cell Tissue Res 327: 499–510. 10.1007/s00441-006-0317-y [DOI] [PubMed] [Google Scholar]
- Sriram G, Bigliardi PL, Bigliardi-Qi M. 2015. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur J Cell Biol 94: 483–512. 10.1016/j.ejcb.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Tabib T, Morse C, Wang T, Chen W, Lafyatis R. 2018. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J Invest Dermatol 138: 802–810. 10.1016/j.jid.2017.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telerman SB, Rognoni E, Sequeira I, Pisco AO, Lichtenberger BM, Culley OJ, Viswanathan P, Driskell RR, Watt FM. 2017. Dermal Blimp1 acts downstream of epidermal TGFβ and Wnt/β-catenin to regulate hair follicle formation and growth. J Invest Dermatol 137: 2270–2281. 10.1016/j.jid.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Cowin AJ, Mills SJ. 2017. The importance of pericytes in healing: wounds and other pathologies. Int J Mol Sci 18: 1129. 10.3390/ijms18061129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstandlechner V, Laggner M, Kalinina P, Haslik W, Radtke C, Shaw L, Lichtenberger BM, Tschachler E, Ankersmit HJ, Mildner M. 2020. Deciphering the functional heterogeneity of skin fibroblasts using single-cell RNA sequencing. FASEB J 34: 3677–3692. 10.1096/fj.201902001RR [DOI] [PubMed] [Google Scholar]
- Wan L, Jiang D, Correa-Gallegos D, Ramesh P, Zhao J, Ye H, Zhu S, Wannemacher J, Volz T, Rinkevich Y. 2021. Connexin43 gap junction drives fascia mobilization and repair of deep skin wounds. Matrix Biol 97: 58–71. 10.1016/j.matbio.2021.01.005 [DOI] [PubMed] [Google Scholar]
- Watt FM, Fujiwara H. 2011. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol 3: a005124. 10.1101/cshperspect.a005124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H. 2007. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 127: 998–1008. 10.1038/sj.jid.5700786 [DOI] [PubMed] [Google Scholar]
- Wlaschek M, Tantcheva-Poór I, Naderi L, Ma W, Schneider LA, Razi-Wolf Z, Schüller J, Scharffetter-Kochanek K. 2001. Solar UV irradiation and dermal photoaging. J Photochem Photobiol B 63: 41–51. 10.1016/S1011-1344(01)00201-9 [DOI] [PubMed] [Google Scholar]
- Woodley DT. 2017. Distinct fibroblasts in the papillary and reticular dermis: implications for wound healing. Dermatol Clin 35: 95–100. 10.1016/j.det.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Itami S, Tarutani M, Hosokawa K, Miura H, Yoshikawa K. 1999. Regulation of keratin 9 in nonpalmoplantar keratinocytes by palmoplantar fibroblasts through epithelial-mesenchymal interactions. J Invest Dermatol 112: 483–488. 10.1046/j.1523-1747.1999.00544.x [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, Yamamoto S, Ema M, Mukouyama YS. 2017. Tissue myeloid progenitors differentiate into pericytes through TGF-β signaling in developing skin vasculature. Cell Rep 18: 2991–3004. 10.1016/j.celrep.2017.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, Zhu Y, Ghaben AL, Wang MY, Li N, et al. 2019. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest 129: 5327–5342. 10.1172/JCI130239 [DOI] [PMC free article] [PubMed] [Google Scholar]