Abstract
Background:
Childhood adversity is a global health problem affecting 25–50% of children worldwide. Few prior studies have examined the underlying neurochemistry of adversity in adolescents. This cross-sectional study examined spectroscopic markers of trauma in a cohort of adolescents with major depressive disorder (MDD) and healthy controls. We hypothesized that historical adversity would have a negative relationship with spectroscopic measures of glutamate metabolites in anterior cingulate cortex.
Methods:
Adolescent participants (aged 13–21) underwent a semi-structured diagnostic interview and clinical assessment, which included the self-report Childhood Trauma Questionnaire (CTQ), a 28-item assessment of childhood adversity. Proton magnetic resonance spectroscopy (1H-MRS) scans at 3 Tesla of an anterior cingulate cortex (ACC) voxel (8 cm3) encompassing both hemispheres were collected using a 2-dimensional J-averaged sequence to assess N-acetylaspartate (NAA), Glx (glutamate+glutamine) and [NAA]/[Glx] concentrations. Generalized linear models assessed the relationships between CTQ scores and metabolite levels in ACC.
Results:
Thirty-nine participants (17 healthy controls, 22 depressed participants) underwent 1H-MRS and completed the CTQ measures. There were decrements in [NAA]/[Glx] ratio in the ACC of participants with childhood adversity while no significant relationship between CTQ total score and any of the ACC metabolites was found in the combined sample. Exploratory results revealed a positive association between Glx levels and CTQ scores in depressed participants. Conversely the [NAA]/[Glx] ratio had a negative association with total CTQ scores in the depressed participants. Emotional Abuse Scale showed a significant negative relationship with [NAA]/[Glx] ratio in the combined sample when adjusted for depression severity.
Conclusions:
Our findings suggest that childhood adversity may impact brain neurochemical profiles. Further longitudinal studies should examine neurochemical correlates of childhood adversity throughout development and in populations with other psychiatric disorders.
Keywords: Glutamate, NAA, 1H-MRS, Anterior cingulate, ACC, Trauma, Adolescent, Depression
Introduction
Childhood adversity is defined as physical or emotional abuse and/or neglect, or sexual abuse before 18 years old (Nelson et al., 2017). Approximately 85% of adolescents have experienced at least one adverse event during childhood (Soares et al., 2016a, 2016b). Adversity in early life has been linked to a broad range of psychiatric disturbances and ensuing functional impairment in adulthood (Edwards et al., 2003), with increased risk of developing anxiety, affective, and psychotic symptoms across a variety of mental disorders (van Nierop et al., 2015). Other studies have demonstrated that an increased frequency of childhood adversity is associated with more severe psychiatric illness course and outcome (Aas et al., 2016). Childhood adversity is an under-studied contributor to the clinical symptoms and biological underpinnings of depression in adolescents (Sekowski et al., 2020).
The treatment of early onset psychiatric disorders is often challenging due to a poor understanding of the related biologic mechanisms. Proton magnetic resonance spectroscopy (1H-MRS) is a non-invasive imaging method that is utilized to study metabolic changes in the brain. Extant research suggests that adolescents with mood disorders have dysregulated cortical glutamatergic concentrations. The most frequently replicated 1H-MRS finding in major depressive disorder (MDD) is reduced glutamate and Glx (glutamate+glutamine) levels in prefrontal and limbic regions in patients who are currently experiencing a depressive episode (Maddock and Buonocore, 2011). Specifically, there is converging evidence for reduced N-acetylaspartate (NAA) levels and Glx in pregenual ACC (pgACC) (Capizzano et al., 2007; Ende et al., 2006; Yildiz-Yesiloglu and Ankerst, 2006) while y- aminobutyric acid (GABA) levels remain unchanged (Hasler et al., 2007). Glutamatergic dysfunction in MDD is further supported by pharmacological benefits of glutamate modulating substances (Zarate Jr et al., 2005).
Both preclinical and clinical evidence demonstrates that the glutamatergic system is also involved in stress responsivity, adaptability, and related psychopathology (Averill et al., 2017). Prior spectroscopy work demonstrated that increased early stress was associated with reduction in Glx in the hippocampus of depressed patients (Poletti et al., 2016). CTQ trauma index was negatively related to glutamatergic transmission (Glu/Naa) in the medial prefrontal cortex in the adult healthy sample (Duncan et al., 2015). In a study of post-traumatic stress disorder (PTSD), ACC glutamate level was negatively correlated with increased arousal (Meyerhoff et al., 2014). One study showed that children and adolescents with PTSD resulting from maltreatment had lower NAA in the anterior cingulate compared to healthy controls (De Bellis et al, 2000). In adolescents with PTSD, significantly lower Glx levels in the rostral ACC were reported in PTSD relative to healthy controls, as well as in those with remitted symptoms relative to healthy controls (Yang et al., 2015).
Emerging research also suggests that the [NAA]/[Glx] ratio may be a sensitive and dynamic measure of glutamate metabolism (Lewis et al., 2020). NAA is highly abundant in the brain and frequently described as a marker of neuronal viability (Maddock and Buonocore, 2011). NAA and Glx are both involved in the same metabolic pathways. NAA and Glu are linked mainly through the tricarboxylic acid (TCA) and glutamate-glutamine cycles (Moffett et al., 2007). It is proposed that NAA serves as a reservoir for glutamate synthesis (Clark et al., 2006). The correlation between NAA and Glx has been shown in various parts of the brain in healthy individuals (Maddock and Buonocore, 2011; Moreno et al., 2001; Waddell et al., 2011). In individuals with psychiatric disorders the equilibrium of NAA and Glx appears to provide a better correlation with severity of pathology than the individual metabolite levels due to variability in methodology (Coughlin et al., 2015; Kraguljac et al., 2013; Lewis et al., 2020; Martens et al., 2021; Rosso et al., 2017; Walter et al., 2009). The ratio of [Glx]/[NAA] has been suggested as a more sensitive marker of dysregulation in both genetic and clinical studies (Lewis et al., 2020; Martens et al., 2021). Therefore, we also explored the ratio of in the present study.
Exposure to stress appears to change glutamatergic transmission. On one hand this change may invoke plasticity and on the other hand it may exert toxic effects. The neurochemical correlates of trauma and in adolescent mood disorders have been incompletely studied and understood. In this pilot study, we aimed to assess neurometabolic correlates of trauma in a cohort of adolescents using 1H-MRS. Participants had varying degrees of childhood adversity without a PTSD diagnosis except for one adolescent. In this cross-sectional study, our goal was to characterize the association between various types of historical adversity and metabolite levels in an adolescent sample.
The primary hypothesis was that metabolite levels would be significantly different among adolescents with lower CTQ scores and elevated CTQ scores. The secondary hypothesis was that in a sample of healthy and depressed adolescents, measures of adversity (CTQ total score and subscale scores) would demonstrate negative relationships with Glx and NAA in the anterior cingulate cortex (ACC).
Methods
Overview
All study procedures were approved by the Mayo Clinic institutional review board (Rochester, MN, USA) prior to any participant recruitment or research activities. This was an exploratory study of participants who had CTQ scores and reliable spectra data.
Eligibility criteria
Adolescents in the healthy control group had no prior psychiatric diagnosis, no prior psychopharmacologic or psychotherapeutic treatment, and had depression severity raw scores less than 30 on the Children’s Depression Rating Scale, Revised (CDRS-R; [(Poznanski et al., 1984)]). Participants in the depressed group had active diagnoses of unipolar depressive disorders on the (the Schedule for Affective Disorders and Schizophrenia for School Aged Children, K-SADS-PL [(Kaufman et al., 1997)]) diagnostic interview and had CDRS-R raw scores of 30 or greater. Exclusion criteria for all participants consisted of lifetime history of mania or psychosis, the presence of any active substance use disorder except nicotine, orthodontic hardware that would cause artifact in magnetic resonance images, and any contraindication to MRI/MRS as determined by the MRI safety screen and MRI safety codes, such as implanted ferromagnetic material.
Participants and clinical assessments
Adolescents with depressive symptoms between ages 13–21 years were recruited from a psychopharmacology clinic. Control participants (who had no history of psychiatric disorder or treatment) were recruited from pediatric primary care clinics and through community advertising. Written informed consent was obtained from the parents or guardians of participants under 18 years of age, and from participants of 18 years of age and older. Written assent was obtained from participants younger than 18 years. All participants underwent clinical assessment by a board-certified child and adolescent psychiatrist, including semi-structured diagnostic interview K-SADS-PL (Kaufman et al., 1997). Severity of depressive symptoms was rated using the CDRS-R (Poznanski et al., 1984), and the Quick Inventory of Depressive Symptomatology (17-item) Adolescent and Parent Self Report (QIDS-A17-SR; [(Bernstein et al., 2010)]). Healthy control participants (HC) had no prior psychiatric diagnoses based on the K-SADS-PL interview.
Trauma was assessed by Childhood Trauma Questionnaire (CTQ; [(Bernstein et al., 1998)]) total score and subscale scores. CTQ is a 28-item self-reported instrument measuring childhood adversity (Bernstein et al., 1997). Items are rated on a five-point Likert scale ranging from 1 (never true) to 5 (very often true). Scores are generated for five subscales: emotional abuse (EAS), physical abuse (PAS), sexual abuse (SAS), physical neglect (PNS) and emotional neglect (ENS), in addition to a total trauma score. Higher scores indicate greater adversity and trauma. The cut-off scores for each subscale according to CTQ Manual were summarized in Table 1 (Bernstein et al., 1998). Full list of participants, medications, exposure to adversity and comorbities provided in the supplementary materials (Appendix A1). Stimulant medications were held on the day of the scan.
Table 1.
Childhood trauma questionnaire cutoff scores.
| Level of abuse | Emotional abuse | Physical abuse | Sexual abuse | Emotional neglect | Physical neglect |
|---|---|---|---|---|---|
|
| |||||
| None | 8 | 7 | 5 | 9 | 7 |
| Low | 12 | 9 | 7 | 14 | 9 |
| Moderate | 15 | 12 | 12 | 17 | 12 |
| Severe | 16+ | 13+ | 13+ | 18+ | 13+ |
Note: CTQ (Childhood Trauma Questionnaire) Manual cutoff scores and categories for each subscale. Example: Those who scored 8 or less in emotional abuse reported minimal or no trauma. Those who scored 9 or higher in emotional abuse reported some level of emotional abuse.
Proton magnetic resonance spectroscopy
Eligible participants (both healthy and depressed adolescents with no contraindication to magnetic resonance imaging) underwent 1H-MRS scans at 3T to assess glutamatergic metabolite concentrations in anterior cingulate cortex (ACC). Measurements were collected from an 8-cm3 voxel corresponding to the pregenual ACC positioned according to previously published methods (Croarkin et al., 2016; Lewis et al., 2016; Port, Unal, Mrazek, and Marcus, 2008). The voxel was encompassing the pregenual anterior cingulate cortex of both hemispheres (Broadman areas 24a, 24b, and 32) (Fig. 1A). A FAST 3D SPGR sequence was utilized to acquire volumetric data for voxel positioning and tissue segmentation. Spectroscopic data were acquired using a 2-dimensional J-averaged PRESS (2DJ) sequence (TR = 2000 ms, TE = 35–195 ms in 16 steps, TR = 2000 ms, 8 averages, 3-way phase cycling) designed specifically for improved glutamate measurement (Hurd et al., 2004) (Fig. 1B). 2D acquisition methods can examine chemical shift and spin-spin coupling in different dimensions, allowing quantification of J-coupled metabolites with overlapping spectra. J-resolved sequences have the capacity to enhance accuracy of glutamate measurement but this costs diminished repeatability (Maddock and Buonocore, 2011). At the time of the data collection for our study, 2DJ-averaged PRESS for glutamate offered a better focused and improved quantification of glutamate and related metabolites in 3T. The protocol described by Hurd et al., comprises 16 steps with a TE increment of 10 ms per step, starting from a TE of 30–35 ms. This allows adequate coverage of the J-dimension and produces spectra with an effective TE of 105 ms, keeping the loss of signal due to T2 relaxation moderate.
Fig. 1.
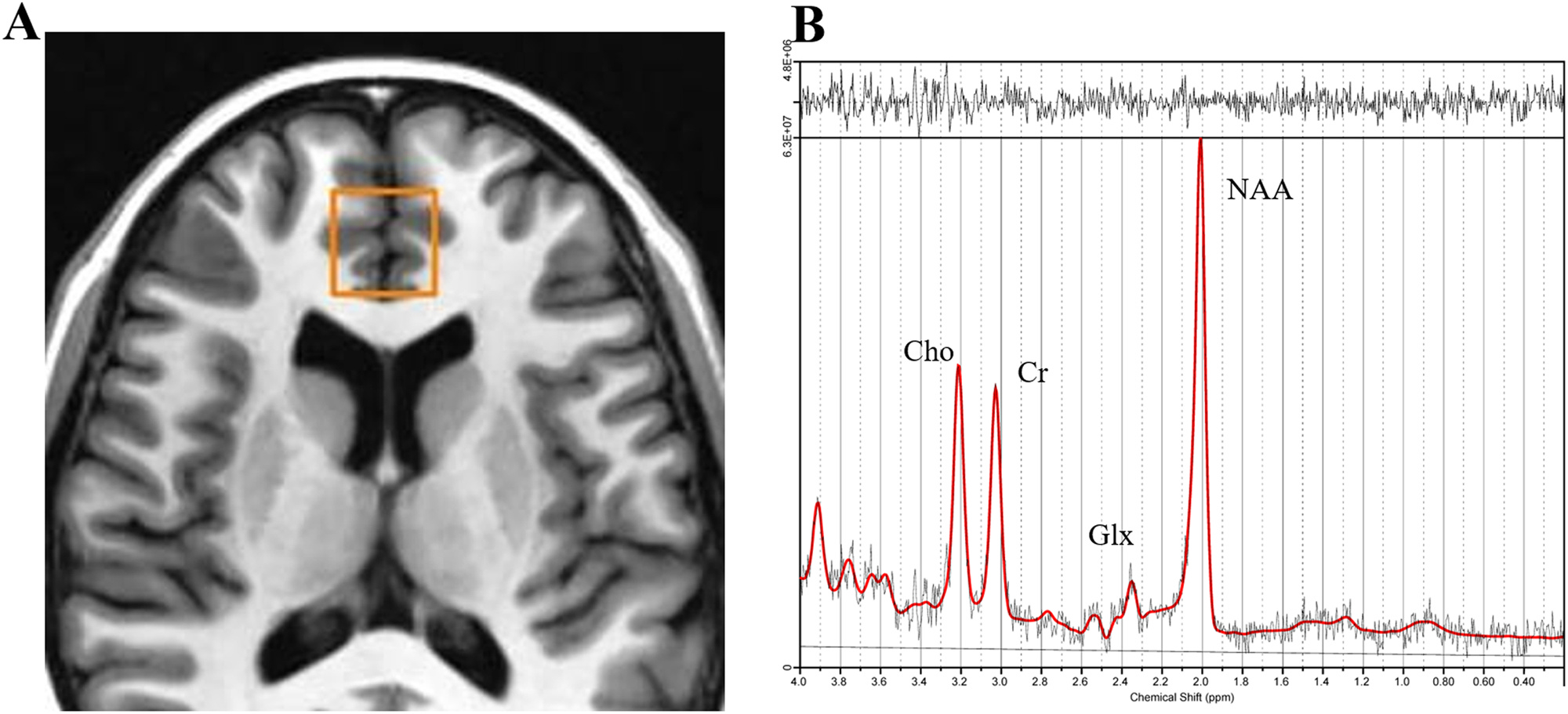
A) Pregenual ACC voxel. Axial view. This voxel encompasses the pregenual ACC of both cerebral hemispheres. B) Spectroscopic data were acquired via a 2D-J TE-averaged PRESS sequence at 3T. Quantitative analysis was performed by LCModel (Provencher, 1993). Also shown are signal peaks of Glx (glutamate + glutamine), choline (Cho), creatine (Cr), and n-acetylaspartate (NAA).
Following the scan, quantitative metabolite concentrations were estimated using LCModel software version 6.3–1 K and a vendor-provided basis set. Scans were reviewed by a neuroradiologist (JDP) to exclude scans with visible artifacts and verify integrity of spectra (Provencher, 2001). Metabolites measured included creatine (Cr), choline (Cho), glutamate (Glu), NAA and Glx. Metabolite concentration measurements were corrected to the cerebrospinal fluid (CSF) fraction according to previously published methods (Lewis et al., 2016). Cramér-Rao lower bounds (Cavassila et al., 2001) is an estimate of the fitting error used as a quality criterion to exclude data sets with unreliable quantification results. Only the measurements with a Cramér-Rao standard deviation less than 20% were included for analysis. Scans with signal-to-noise ratio <10 were excluded. Hence the analyses were restricted to subjects who met strict quality criteria to indicate reliable spectra for each metabolite (Schulte and Boesiger, 2006).
Statistical analyses
Statistical tests were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA) and JMP Pro 14.1.0 (SAS Institute, Inc., Cary, NC, USA) software. The significance level was set at α = 0.05. The Benjamini-Hochberg False Discovery Rate (FDR) method was used to correct for multiple comparisons (Benjamini and Hochberg, 1995). We listed the individual P values in order, from smallest to largest. The smallest P value has a rank of i = 1, then next smallest has i = 2, etc. We compared the each individual P value to its Benjamini-Hochberg critical value, (i/m)Q, where i is the rank, m is the total number of tests, and Q is the false discovery rate we chose. The largest P value that had P < (i/m) Q was significant, and all of the P values smaller than it were also significant, even the ones that were not less than their Benjamini-Hochberg critical value. We chose a relatively high false discovery rate of 0.3, as this is a pilot study for hypothesis generation and planning for future studies.
Demographic and clinical characteristics of the overall sample and each group (healthy controls and depressed participants) were described with the mean and standard deviation for continuous variables and with counts and percentages for categorical variables. Group differences were assessed with independent samples t-tests or Mann-Whitney U tests for continuous variables and Pearson’s chi-squared test for categorical variables. Shapiro-Wilk normality tests revealed that distribution of age and clinical measure were not normal across trauma levels. Therefore, group comparisons across trauma levels were all run with Mann-Whitney U.
The first aim of this study was to compare metabolite levels in two groups of participants based on their report of CTQ: no (or minimal) trauma versus elevated trauma (low, moderate and severe trauma). The “No Trauma” group included participants who did not report any trauma in any of the subscales according to cut-off scores provided in Table 1 (emotional abuse, emotional neglect, physical abuse, physical neglect and sexual abuse). Shapiro-Wilk test revealed that all distributions were normal. Group comparisons were run with student’s t-test.
The secondary aim was relationships between ACC NAA, Glx, and [NAA]/[Glx] and a dimensional measure of trauma (CTQ total scores) and 5 subscales (Emotional Abuse Scale, Emotional Neglect Scale, Physical Abuse Scale, Physical Neglect Scale and Sexual Abuse Scale) were examined using generalized linear models to test the hypothesis in the combined sample as well as subgroups. Dependent variable was the ACC metabolite measure (NAA, Glx, and [NAA]/[Glx] ratio). Separate models were constructed for each metabolite and subscale.
Exploratory analyses included assessment of age, sex, depression severity, and medication status as potential covariates.
Results
Total number of participants were 42. Three participants were excluded because the scans did not meet the quality measures of reliable spectra. All three were in the depressed group with elevated trauma. Two had a comorbid diagnosis of ADHD. 39 patients with both spectroscopy scans and CTQ scores were included in analyses. All participants had valid measurements for both NAA and Glx. Demographic and clinical characteristics are reported in Table 2. Groups did not differ significantly by age or sex. CTQ, CDRS-R and QIDS-A17-SR scores were significantly higher in the depressed group than in healthy controls (p < 0.001). Twelve participants were prescribed psychotropic medication. The most common comorbidities were cannabis use disorder (n = 7, 17.95%) and ADHD (n = 4, 10.26%). There were no differences in metabolite levels in healthy participants versus depressed.
Table 2.
Demographics and clinical characteristics.
| Mean (SD) | Combined sample | Healthy controls | Depressed | p | No trauma | Elevated trauma | p |
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | 39 | 17 | 22 | 18 | 21 | ||
| Age | 16.36 (2.08) | 16.65 (2.21) | 16.14 (2.01) | 0.46 | 16.39 (1.94) | 16.33 (2.24) | 0.4 |
| Female, % | 24 (62) | 12 (70.6) | 12 (54.5) | 0.3 | 11 (61.1) | 13 (61.9) | 0.9 |
| CDRS-R | 35.03 (17.9) | 19.12 (2.89) | 47.32 (14.49) | <0.001 | 24.33 (11.89) | 44.19 (17.24) | <0.001 |
| QIDS-SR-A17 | 7.9 (6.28) | 2.71 (2.14) | 11.9 (5.4) | <0.001 | 3.72 (3.78) | 11.48 (6.28) | <0.001 |
| CTQ Total Score | 36.54 (11.01) | 28.35 (3.28) | 42.86 (10.72) | <0.001 | 27.67 (3.14) | 44.14 (9.45) | <0.001 |
| CTQ EAS | 8.82 (4.08) | 6.06 (1.39) | 10.95 (4.2) | <0.001 | 5.83 (0.99) | 11.38 (3.98) | <0.001 |
| CTQ ENS | 8.54 (3.95) | 6.41 (1.91) | 10.18 (4.36) | 0.002 | 6.06 (1.66) | 10.67 (4.13) | <0.001 |
| CTQ PAS | 6.21 (1.96) | 5.18 (0.53) | 7 (2.29) | 0.006 | 5.28 (0.67) | 7 (2.35) | 0.006 |
| CTQ PNS | 6.41 (2.21) | 5.71 (1.36) | 6.95 (2.59) | 0.124 | 5.33 (0.6) | 7.33 (2.65) | 0.012 |
| CTQ SAS | 6.49 (4.12) | 5 (0) | 7.64 (5.24) | 0.015 | 5 (0) | 7.76 (5.34) | 0.001 |
Note: Trauma level is based on Childhood Trauma Questionnaire (CTQ) scoring guide. No trauma group reported none or minimal exposure in any of the subscales. Sex differences across groups were assessed with Chi-Square. Age across healthy vs depressed student’s t-test. Shapiro-Wilk normality test revealed that distribution of age and clinical measure were not normal. Therefore group comparisons across trauma levels were all run with Mann-Whitney U. (EAS, Emotional Abuse Subscale; ENS, Emotional Neglect Subscale; PAS, Physical Abuse Subscale; PNS, Physical Neglect Subscale; SAS, Sexual Abuse Subscale).
Primary outcome: 1H-MRS measured ACC metabolite comparisons between trauma groups
Metabolite levels were compared in two groups of participants based on trauma level. Means and standard errors of [Cr], [Cho], [NAA], [Glx], and [NAA]/[Glx] are reported in Table 3. There was a significant difference in mean [NAA]/[Glx] between “No Trauma” and “Elevated Trauma” (p = 0.047, pFDR = 0.282). The ratio was greater in the “No Trauma” group. We also calculated a Hedges g (due to difference in sample sizes) to have a standardized effect size for mean differences across trauma levels. The effect sizes for NAA, Glx, and [NAA]/[Glx] were respectively g = 2.04, g = 1.25, and g = 0.66.
Table 3.
1H-MRS anterior cingulate cortex metabolites across trauma levels.
| ACC (2DJ) metabolites mean (SE) | Combined sample n = 39 | No trauma n = 18 | Elevated trauma n= 21 | p | p FDR |
|---|---|---|---|---|---|
|
| |||||
| Glutamate | 87.93 (1.96) | 87.32 (2.87) | 88.45 (2.75) | 0.779 | 0.779 |
| [Glx] | 113.22 (3.27) | 110.05 (5.58) | 115.94 (3.78) | 0.377 | 0.566 |
| N-acetyl aspartate | 84.72 (1.17) | 86.55 (2.15) | 83.15 (1.11) | 0.15 | 0.414 |
| [NAA]/[Glx] | 0.77 (0.02) | 0.81 (0.03) | 0.73 (0.02) | 0.047* | 0.282* |
| Creatine | 59.7 (0.87) | 60.9 (1.5) | 58.67 (0.97) | 0.207 | 0.414 |
| Choline | 21.06 (0.43) | 21.21 (0.63) | 20.94 (0.61) | 0.762 | 0.779 |
Cut-off score was based on CTQ scoring guidelines. See Table 1. Glutamine was not measured. Glx: glutamate+glutamine. Shapiro-Wilk test revealed that all distributions were normal. Group comparisons run with student’s t-test. False discovery rate was chosen as 0.3.
statistically significant.
Secondary outcome: relationship between CTQ Total score and subscales and 1H-MRS measured ACC metabolites
Table 4 summarized the results of the models with independent variable CTQ total trauma score in the combined sample. There were no significant associations of the total score in the combined sample. In Table 4, unstandardized parameter estimates and p-values of models assessing the relationship between ACC metabolites and all 5 CTQ subscales were reported in healthy and depressed subgroups.
Table 4.
Relationships between CTQ scores and 1H-MRS measured ACC metabolites.
| Scores | CTQ TOTAL |
CTQ EAS |
CTQ ENS |
CTQ SAS |
CTQ PAS |
CTQ PNS |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | β | p | β | p | β | p | β | p | β | p | β | p | |
|
| |||||||||||||
| NAA | Healthy | −1.015 | 0.067 | 0.058 | 0.968 | −2.016 | 0.029 * | 0 | – | −1.23 | 0.743 | −1.813 | 0.194 |
| Depressed | −0.012 | 0.918 | −0.152 | 0.618 | 0.104 | 0.724 | 0.086 | 0.728 | 0.095 | 0.866 | −0.672 | 0.16 | |
| Combined | −0.181 | 0.077 | −0.424 | 0.13 | −0.103 | 0.164 | −0.101 | 0.722 | −0.508 | 0.392 | −1.092d | 0.03 * | |
| Glx | Healthy | −0.7 | 0.636 | −0.189 | 0.957 | −1.7 | 0.501 | 0 | – | 4.317 | 0.638 | −1.184 | 0.741 |
| Depressed | 0.738 | 0.05 | 2.014a | 0.034 * | 1.013 | 0.302 | 0.826 | 0.312 | 1.949 | 0.297 | 0.184 | 0.913 | |
| Combined | 0.134 | 0.652 | 0.616 | 0.44 | 0.058 | 0.944 | 0.446 | 0.574 | 0.701 | 0.674 | −0.533 | 0.719 | |
| NAA/Glx | Healthy | −0.002 | 0.797 | 0.000008 | 1 | −0.003 | 0.858 | 0 | – | −0.035 | 0.482 | −0.002 | 0.921 |
| Depressed | −0.006c | 0.012 * | −0.017b | 0.004 * | −0.007 | 0.316 | −0.006 | 0.235 | −0.015 | 0.233 | −0.009 | 0.393 | |
| Combined | −0.003 | 0.12 | −0.009 | 0.063 | −0.004 | 0.468 | −0.005 | 0.288 | −0.01 | 0.313 | −0.006 | 0.475 | |
Note: Each β and p-value pair was generated via separate generalized linear models. Scores were the only independent variables in these models. The results of models with added covariates such as age, sex, depression severity, current psychotropic medication use are available in Appendix A2. Number of healthy participants n = 17, number of depressed participants n = 22, combined sample n = 39.
statistically significant.
standardized estimate = 0.412.
standardized estimate = −0.523.
standardized estimate = −0.471.
standardized estimate = −0.328.
In the combined sample physical neglect showed significant negative association with NAA levels. In the depressed sample, emotional abuse score was significant for both Glx (β = 2.014, p = 0.034) and [NAA]/ [Glx] ratio (β = −0.017, p = 0.004). In this sample, CTQ total score showed a significant negative association with [NAA]/[Glx] ratio (β = −0.006, p = 0.012). In the healthy group, emotional neglect showed a significant negative relationship to NAA levels (β = −2.016, p = 0.029). We calculated the standardized the regression coefficients by multiplying them by the standard deviation of the predictor (independent variable, X, CTQ scores) and dividing them by the standard deviation of the response (dependent variable, Y, metabolites levels). These are reported in the footnote of Table 4. All significant estimates were in the small to medium range.
Exploratory analyses: exploring age, sex, depression severity and medication use
We explored age, sex, depression severity and medication use as potential covariates in the significant models. Psychotropic medications use was not a significant covariate in any of the analyses. Results are summarized in the supplementary materials (Appendix A.2).
In the healthy control group for emotional neglect was not significantly associated with NAA after adjusting for age. Sex and depression severity were not confounding.
In the depressed group, CTQ total score showed a significant positive relationship with [Glx] when adjusted for for depression severity (β = 0.828, p = 0.046). The negative significant relationship with total CTQ score and [NAA]/[Glx] ratio remained significant after adjusting for age, sex and depression severity. Emotional abuse showed significant negative associations with [Glx] and [NAA]/[Glx] ratio. These results remained significant after adjusting for age, sex and depression severity.
In the combined sample, the association of physical neglect and NAA was rendered nonsignificant after adjusting for depression severity. Emotional abuse showed significant association with [NAA]/[Glx] in the combined sample only after adjusting for depression severity.
Discussion
This preliminary analysis explored potential correlations between CTQ measures of childhood adversity and ACC metabolite levels in an adolescent cohort of depressed and healthy individuals. In terms of metabolite levels across trauma levels, [NAA]/ [Glx] ratio was the only measure that was significantly smaller in the elevated trauma group. This is in line with prior evidence suggesting a relationship in the same direction with worse mental health outcomes (Averill et al., 2017; Lewis et al., 2020; Martens et al., 2021). Medium to large effect sizes in metabolite levels across trauma levels are reassuring and emphasizing that sample size may be a limiting factor in our results.
In the secondary and exploratory analyses, the most robust findings were the associations between Glx or [NAA]/ [Glx] ratio and overall trauma severity or emotional abuse in the depressed group. Depression in this sample was notable confounding factor. This is supported by the significant positive association between overall childhood adversity and ACC [Glx] in the depressed group, although only when adjusted for depression severity. Among different subtypes of trauma, emotional abuse was the adversity driving the association to metabolites which were significant after adjusting for age, sex or CDRS-R independently. Small to medium standardized parameter estimates may suggest that there is a role of glutamate in pathophysiology of adversity and it warrants further exploration from different aspects. Our findings were comparable with another recent study (Averill et al., 2020) investigating the relationship of trauma and neurometabolites in adults with MDD. Exploratory findings in this study suggested a significant positive correlation between early life stress and occipital glutamine but not glutamate. Post-hoc analyses showed that the association with glutamine was driven by the emotional abuse subscale. Authors found that in a smaller subset (n = 11), those with childhood emotional abuse appeared to have increased occipital glutamate neurotransmission as reflected by increased glutamate/glutamine cycling and glutamine level. This is also supportive of the positive directions of associations with Glx and emotional abuse in our sample despite the differences in age (13–21 age range versus 18–65 age range) and medication status of samples. Averill et al. included 36 non-medicated adult MDD patients, whereas our sample included 22 depressed adolescents, more than half were on psychotropic medication. However, our exploratory analyses did not support the idea of consequences of psychotropic use in neurochemical levels. Another difference is that the spectroscopy voxel was selected from occipital cortex versus ACC scans in our study. However, it is noteworthy that emotional abuse was related to Glx expression in both studies as a prominent part of overall trauma.
We also acknowledge that comorbidities in our sample introduced heterogeneity which may contributed to some of the nonsignificant results. Cannabis use disorder was comorbid in seven of the depressed participants and one healthy control. Cannabis use was suggested to lower NAA in DLPFC in adolescents (Sneider et al., 2013). ADHD was comorbid in four of the depressed participants. Frontal/striatal glutamatergic resonances (Glx) were elevated in the children with ADHD compared with healthy control subjects, but no differences were noted in NAA, Cho, or Cr metabolite ratios (MacMaster et al., 2003). Although not specifically in a childhood trauma cohort, another study in patients with PTSD reported that within dorsal ACC, there was a positive linear relationship between Glx concentrations and current stress disorder symptoms (Harnett et al., 2017). In our study, the direction between Glx levels and trauma severity was also consistently positive.
Limitations
First, the present data for this exploratory study comes from a cross-sectional study. Cross-sectional analyses cannot causally link trauma and depressive symptoms. Additionally, we suspect our small sample size is limiting power in analyses of sub-sets with respect to specific comorbidities, sex, age, or race. Longitudinal studies are needed to explore the development of depression in youth with history of trauma and to portray brain-based differences between early manifestations of psychiatric disorders. Replication with a larger sample is needed. Validity of self-report in adversity may be questioned as it is prone to recall bias and may depend on participant’s wellbeing at the time of measurement. When comparing 1H-MRS results across studies, participant related factors (i.e. age, sex, medication status) and methodological factors such as 1H-MRS sequences and acquisition parameters, anatomic region sampled, processing techniques and quantification strategies must be considered. We acknowledge that 2D-J averaged PRESS is not a widely adopted method. To this date, even though there are multiple 1H-MRS acquisition sequences that are capable of measuring glutamate and related metabolites, newer techniques are still being developed (Al-Ie-dani et al., 2018; Jensen et al., 2017; Lally et al., 2016; Liu et al., 2017). These attempts are driven by the fact that Glutamate and Glutamine both give rise to a complex proton MR spectrum, characterized by the coupled spins of the C2–C4 hydrogen nuclei and J-modulated peak phases. The reality is that regardless of technique, precision for glutamatergic metabolites is less than NAA or Cho (Mullins et al., 2008). Comparisons of these methods in large clinical human populations are lacking. It is not yet possible to definitively say that one method is superior.
Funding
This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and National Institute of Mental Health Grants R01 MH124655 and R01 MH113700. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by a grant from the Brain & Behavior Research Foundation (C.P.L., 2018 NARSAD Young Investigator Grant 27488, Alan G. Ross Memorial Investigator).
Appendix A1. Participant’s psychotropic medications and comorbidities at the time of the 1H-MRS scan
| Participant | Group | Trauma Exposure | Medication (s) and Total Daily Dose | Comorbidities |
|---|---|---|---|---|
|
| ||||
| 1 | Dep/Med − | EAS, PAS, ENS | none | cannabis use |
| 2 | Dep/Med + | No Trauma | Fluoxetine, methylphenidate hydrochloride ER | ADHD |
| 3 | HC | No Trauma | none | none |
| 4 | HC | No Trauma | none | none |
| 5 | HC | EAS | none | none |
| 6 | HC | No Trauma | none | none |
| 7 | Dep/Med − | EAS, SAS,PNS | none | panic disorder |
| 8 | HC | No Trauma | none | none |
| 9 | Dep/Med + | EAS, SAS, ENS | sertraline, aripiprazole | none |
| 10 | Dep/Med + | SAS | fluoxetine | alcohol use disorder in remission 8 months, anxiety disorder NOS |
| 11 | HC | No Trauma | none | none |
| 12 | HC | No Trauma | none | none |
| 13 | HC | ENS | none | subthreshold depressive symptoms |
| 14 | Dep/Med − | EAS, ENS | none | none |
| 15 | Dep/Med − | EAS, PAS, ENS, PNS | none | cannabis use disorder |
| 16 | Dep/Med + | EAS, SAS | sertraline | none |
| 17 | HC | No Trauma | none | none |
| 18 | Dep/Med − | EAS, SAS, ENS, PNS | none | alcohol |
| cannabis | ||||
| nicotine (all since 2014) | ||||
| 19 | Dep/Med − | EAS, ENS, PNS | none | none |
| 20 | Dep/Med − | EAS, SAS | none | ADHD, Osgood Schlatter |
| 21 | Dep/Med + | EAS, SAS, ENS | Fluoxetine, dextroamphetamine saccharate | ADHD, GAD, Motor Tic Disorder |
| 22 | Dep/Med + | EAS, ENS, PNS | fluoxetine | cannabis use disorder |
| 23 | Dep/Med − | EAS, ENS | none | persistent dysthymic disorder (8 years) |
| 24 | Dep/Med + | No Trauma | fluoxetine | restless leg syndrome, migraines |
| 25 | Dep/Med − | EAS, PAS, ENS, PNS | none | persistent dysthymic disorder, cannabis use disorder |
| 26 | HC | No Trauma | none | none |
| 27 | HC | PNS | none | none |
| 28 | Dep/Med + | No Trauma | duloxetine | ADHD, minor depressive disorder |
| 29 | Dep/Med + | No Trauma | bupropion hydrochloride xl | none |
| 30 | HC | PNS | none | cannabis use disorder |
| 31 | HC | No Trauma | none | none |
| 32 | Dep/Med − | EAS, PAS, SAS, ENS | none | PTSD |
| 33 | HC | No Trauma | none | mild concussion history |
| 34 | Dep/Med + | EAS, PAS, SAS | fluoxetine | cannabis use disorder |
| 35 | Dep/Med + | No Trauma | amitriptyline | learning disability, chronic headaches |
| 36 | Dep/Med + | EAS, PAS, SAS, ENS | fluoxetine | none |
| 37 | HC | No Trauma | none | none |
| 38 | HC | No Trauma | none | none |
| 39 | HC | No Trauma | none | none |
All stimulants were held on the day of MRS scan for the parent study. “No Trauma” group reported none or minimal trauma based on cut-off scores provided in CTQ Manual. See Table 1 in the manuscript. Emotional abuse (EAS), physical abuse (PAS), sexual abuse (SAS), physical neglect (PNS) and emotional neglect (ENS). PTSD: post-traumatic stress disorder. ADHD: attention deficit hyperactivity disorder. GAD: generalized anxiety disorder. NOS: not otherwise specified. Healthy Control (HC), Dep/Med+(Depressed Subject treated with an antidepressant), Dep/Med− (Depressed Subject unmedicated)
Appendix A.2. Exploratory analyses and multivariate models
| ACC 2DJ | Independent Variable | CTQ Total Score | CTQ EAS | ||||
|
| |||||||
| NAA/Glx | Depressed Group, n = 22 | Depressed Group, n = 22 | Combined Sample, n = 39 | ||||
| Co-vary | β | p-value | β | p-value | β | p-value | |
| None | −0.006 | 0.012 | −0.017 | 0.004 | −0.009 | 0.063 | |
| Age | −0.006 | 0.012 | −0.017 | 0.004 | −0.009 | 0.072 | |
| Sex | −0.006 | 0.012 | −0.017 | 0.003 | −0.009 | 0.058 | |
| CDRS-R | −0.006 | 0.021 | −0.018 | 0.007 | −0.014 | 0.027 | |
|
| |||||||
|
| |||||||
| ACC 2DJ | Independent Variable | CTQ Total Score | CTQ EAS | ||||
|
| |||||||
| Glx | Depressed Group, n = 22 | Depressed Group, n = 22 | |||||
| Co-vary | β | p-value | β | p-value | |||
| None | 0.738 | 0.05 | 2.014 | 0.034 | |||
| Age | 0.732 | 0.053 | 2.061 | 0.036 | |||
| Sex | 0.712 | 0.051 | 2.222 | 0.017 | |||
| CDRS-R | 0.828 | 0.046 | 2.352 | 0.026 | |||
|
| |||||||
|
| |||||||
| ACC 2DJ | Independent Variable | CTQ ENS | CTQ PNS | ||||
|
| |||||||
| NAA | Healthy Group, n = 17 | Combined Sample, n = 39 | |||||
| Co-vary | β | p-value | β | p-value | |||
| None | −2.016 | 0.029 | −1.092 | 0.03 | |||
| Age | −1.814 | 0.064 | −1.17 | 0.019 | |||
| Sex | −2.016 | 0.028 | −0.973 | 0.05 | |||
| CDRS-R | −2.544 | 0.014 | −0.683 | 0.196 | |||
Note: Each β and p-value pair represents the coefficient and p-value of CTQ score from a separate generalized linear model. EAS, Emotional Abuse Subscale; ENS, Emotional Neglect Subscale; PAS, Physical Abuse Subscale; PNS, Physical Neglect Subscale; SAS, Sexual Abuse Subscale.
Footnotes
Statement of Interest
Dr. Lewis. received grant support from the Brain & Behavior Research Foundation as the Alan G. Ross Memorial Investigator. He has been a site investigator for multicenter trials funded by Neuronetics, Inc. and NeoSync, Inc. Dr. Port has served as an imaging consultant for Takeda Pharmaceutical Company, Ltd., Biomedical Systems Corp., and Neuronetics, Inc.
Dr. Choi is a scientific advisory board member for Peptron Inc. Dr Croarkin has received research grant support from Pfizer, Inc; equipment support from Neuronetics, Inc; equipment support from MagVenture, Inc; and supplies and genotyping services from Assurex Health, Inc for investigator-initiated studies. He was the primary investigator for a multicenter study funded by Neuronetics, Inc and a site primary investigator for a study funded by NeoSync, Inc. Dr Croarkin has served as a consultant for Engrail Therapeutics, Myriad Neuroscience, Procter & Gamble Companyand Sunovion. The other authors report no financial relationships with commercial interests.
References
- Aas M, Andreassen OA, Aminoff SR, Færden A, Romm KL, Nesvåg R, Melle I, 2016. A history of childhood trauma is associated with slower improvement rates: Findings from a one-year follow-up study of patients with a first-episode psychosis. BMC Psychiatry 16 (1), 1–8. 10.1186/s12888-016-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Iedani O, Arm J, Ribbons K, Lea R, Lechner-Scott J, Ramadan S, 2018. Diurnal stability and long-term repeatability of neurometabolites using single voxel 1H magnetic resonance spectroscopy. Eur. J. Radiol 108, 107–113. 10.1016/j.ejrad.2018.09.020. [DOI] [PubMed] [Google Scholar]
- Averill LA, Abdallah CG, Fenton LR, Fasula MK, Jiang L, Rothman DL, Sanacora G, 2020. Early life stress and glutamate neurotransmission in major depressive disorder. Eur. Neuropsychopharmacol 35, 71–80. 10.1016/j.euroneuro.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG, 2017. Glutamate dysregulation and glutamatergic therapeutics for PTSD: evidence from human studies. Neurosci. Lett 649, 147–155. 10.1016/j.neulet.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. So. Se. B (Stat. Methodol.) 57 (1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L, 1997. Validity of the childhood trauma questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry 36 (3), 340–348. 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J 1998. Childhood trauma questionnaire. Assessment of Family Violence: a Handbook for Researchers and Practitioners < 10.1037/t02080-000>. [DOI] [Google Scholar]
- Bernstein IH, Rush AJ, Trivedi MH, Hughes CW, Macleod L, Witte BP, Emslie GJ, 2010. Psychometric properties of the quick inventory of depressive symptomatology in adolescents. Int. J. Methods Psychiatr. Res 19 (4), 185–194. 10.1002/mpr.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capizzano AA, Jorge RE, Acion LC, Robinson RG, 2007. In vivo proton magnetic resonance spectroscopy in patients with mood disorders: a technically oriented review. J. Magn. Reson. Imaging 26 (6), 1378–1389. 10.1002/jmri.21144. [DOI] [PubMed] [Google Scholar]
- Cavassila S, Deval S, Huegen C, Van Ormondt D, Graveron-Demilly D, 2001. Cramér-Rao bounds: an evaluation tool for quantitation. NMR Biomed 14 (4), 278–283. 10.1002/nbm.701. [DOI] [PubMed] [Google Scholar]
- Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, Pyne-Geithman GJ, 2006. N-acetylaspartate as a reservoir for glutamate. Med. Hypotheses 67 (3), 506–512. 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Tanaka T, Marsman A, Wang H, Bonekamp S, Kim PK, Pomper M, 2015. Decoupling of N-acetyl-aspartate and glutamate within the dorsolateral prefrontal cortex in schizophrenia. Curr. Mol. Med 15 (2), 176–183. 10.2174/1566524015666150303104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croarkin P, Lewis C, Vande Voort J, Kohli J, Kozel F, Frye M, Port J 2016. Spectroscopic glutamate correlates of trauma and anhedonia in adolescents. In: Proceedings of the Paper Presented at the Neuropsychopharmacology [Google Scholar]
- De Bellis MD, Keshavan MS, Spencer S, Hall J, 2000. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am. J. Psychiatry 157 (7), 1175–1177. 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Hayes DJ, Wiebking C, Tiret B, Pietruska K, Chen DQ, Doyon J, 2015. Negative childhood experiences alter a prefrontal-insular-motor cortical network in healthy adults: a preliminary multimodal rsfMRI-fMRI-MRS-dMRI study. Human Brain Map 36 (11), 4622–4637. 10.1002/hbm.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF, 2003. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am. J. Psychiatry 160 (8), 1453–1460. 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- Ende G, Demirakca T, Tost H, 2006. The biochemistry of dysfunctional emotions: proton MR spectroscopic findings in major depressive disorder. Prog. Brain Res 156, 481–501. 10.1016/S0079-6123(06)56027-3. [DOI] [PubMed] [Google Scholar]
- Harnett NG, Wood KH, Ference III EW, Reid MA, Lahti AC, Knight AJ, Knight DC, 2017. Glutamate/glutamine concentrations in the dorsal anterior cingulate vary with post-traumatic stress disorder symptoms. J. Psychiatr. Res 91, 169–176. 10.1016/j.jpsychires.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC, 2007. Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 64 (2), 193–200. 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ, 2004. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn. Reson. Med 51, 435–440. 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- Jensen JE, Auerbach RP, Pisoni A, Pizzagalli DA, 2017. Localized MRS reliability of in vivo glutamate at 3 T in shortened scan times: a feasibility study. NMR Biomed 30 (11), e3771 10.1002/nbm.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N, 1997. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36 (7), 980–988. 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Reid MA, Lahti AC, 2013. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry 70 (12), 1294–1302. 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, An L, Banerjee D, Niciu MJ, Luckenbaugh DA, Richards EM, Nugent AC, 2016. Reliability of 7T 1H-MRS measured human prefrontal cortex glutamate, glutamine, and glutathione signals using an adapted echo time optimized PRESS sequence: a between-and within-sessions investigation. J. Magn. Reson. Imag 43 (1), 88–98. 10.1002/jmri.24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CP, Port JD, Blacker CJ, Sonmez AI, Seewoo BJ, Leffler JM, Croarkin PE, 2020. Altered anterior cingulate glutamatergic metabolism in depressed adolescents with current suicidal ideation. Transl. Psychiatry 10 (1), 1–12. 10.1038/s41398-020-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CP, Port JD, Frye MA, Vande Voort JL, Ameis SH, Husain MM, Croarkin PE, 2016. An exploratory study of spectroscopic glutamatergic correlates of cortical excitability in depressed adolescents. Front. Neural Circ 10, 98. 10.3389/fncir.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-L, Li L, Li J-N, Rong J-H, Liu B, Hu Z-X, 2017. Reliability of glutamate quantification in human nucleus accumbens using proton magnetic resonance spectroscopy at a 70-cm wide-bore clinical 3T MRI system. Front. Neurosci 11, 686. 10.3389/fnins.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Carrey N, Sparkes S, Kusumakar V, 2003. Proton spectroscopy in medication-free pediatric attention-deficit/hyperactivity disorder. Biol. Psychiatry 53 (2), 184–187. 10.1016/S0006-3223(02)01401-4. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH, 2011. MR spectroscopic studies of the brain in psychiatric disorders. In: Carter C, Dalley JF (Eds.), Brain Imaging in Behavioral Neuroscience. Springer, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- Martens L, Herrmann L, Colic L, Li M, Richter A, Behnisch G, Walter M, 2021. Met carriers of the BDNF Val66Met polymorphism show reduced Glx/NAA in the pregenual ACC in two independent cohorts. Sci. Rep 11 (1), 1–12. 10.1038/s41598-021-86220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Mon A, Metzler T, Neylan TC, 2014. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep 37 (5), 893–900. 10.5665/sleep.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM, 2007. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol 81 (2), 89–131. 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Ross BD, Blüml S, 2001. Direct determination of the N-acetyl-l-aspartate synthesis rate in the human brain by 13C MRS and [1–13C] glucose infusion. J. Neurochem 77 (1), 347–350. 10.1046/j.1471-4159.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C, 2008. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn. Reson. Med 60 (4), 964–969. 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- Nelson J, Klumparendt A, Doebler P, Ehring T, 2017. Childhood maltreatment and characteristics of adult depression: meta-analysis. Br. J. Psychiatry 210 (2), 96–104. 10.1192/bjp.bp.115.180752. [DOI] [PubMed] [Google Scholar]
- Poletti S, Locatelli C, Falini A, Colombo C, Benedetti F, 2016. Adverse childhood experiences associate to reduced glutamate levels in the hippocampus of patients affected by mood disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatry 71, 117–122. 10.1016/j.pnpbp.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Port JD, Unal SS, Mrazek DA, Marcus SM, 2008. Metabolic alterations in medication-free patients with bipolar disorder: a 3T CSF-corrected magnetic resonance spectroscopic imaging study. Psychiatry Res. Neuroimag 162 (2), 113–121. 10.1016/j.pscychresns.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R, 1984. Preliminary studies of the reliability and validity of the children’s depression rating scale. J. Am. Acad. Child Psychiatry 23 (2), 191–197. 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine 30 (6), 672–679. 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 2001. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14 (4), 260–264. 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Crowley DJ, Silveri MM, Rauch SL, Jensen JE, 2017. Hippocampus glutamate and N-acetyl aspartate markers of excitotoxic neuronal compromise in posttraumatic stress disorder. Neuropsychopharmacology 42 (8), 1698–1705. 10.1038/npp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte RF, Boesiger P, 2006. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed 19 (2), 255–263. 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- Sekowski M, Gambin M, Cudo A, Wozniak-Prus M, Penner F, Fonagy P, Sharp C, 2020. The relations between childhood maltreatment, shame, guilt, depression and suicidal ideation in inpatient adolescents. J. Affect. Disord 276, 667–677. 10.1016/j.jad.2020.07.056. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Mashhoon Y, Silveri MM, 2013. A review of magnetic resonance spectroscopy studies in marijuana using adolescents and adults. J. Addict. Res. Ther 10.4172/2155-6105.S4-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares ALG, Howe LD, Matijasevich A, Wehrmeister FC, Menezes AM, Gonçalves H, 2016a. Adverse childhood experiences: prevalence and related factors in adolescents of a Brazilian birth cohort. Child Abuse Negl 51, 21–30. 10.1016/j.chiabu.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares ALG, Howe LD, Matijasevich A, Wehrmeister FC, Menezes AMB, Gonçalves H, 2016b. Adverse childhood experiences: prevalence and related factors in adolescents of a Brazilian birth cohort. Child Abuse Negl 51, 21–30. 10.1016/j.chiabu.2015.11.017. <http://www.sciencedirect.com/science/article/pii/S014521341500441X>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nierop M, Viechtbauer W, Gunther N, Van Zelst C, De Graaf R, Ten Have M, Van Winkel R, 2015. Childhood trauma is associated with a specific admixture of affective, anxiety, and psychosis symptoms cutting across traditional diagnostic boundaries. Psychol. Med 45 (6), 1277–1288. 10.1017/S0033291714002372. [DOI] [PubMed] [Google Scholar]
- Waddell KW, Zanjanipour P, Pradhan S, Xu L, Welch EB, Joers JM, Gore JC, 2011. Anterior cingulate and cerebellar GABA and Glu correlations measured by 1H J-difference spectroscopy. Magn. Resonan. Imag 29 (1), 19–24. 10.1016/j.mri.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Northoff G, 2009. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch. Gen. Psychiatry 66 (5), 478–486. 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- Yang ZY, Quan H, Peng ZL, Zhong Y, Tan ZJ, Gong QY, 2015. Proton magnetic resonance spectroscopy revealed differences in the glutamate+ glutamine/creatine ratio of the anterior cingulate cortex between healthy and pediatric post-traumatic stress disorder patients diagnosed after 2008 W enchuan earthquake. Psychiatry Clin. Neurosci 69 (12), 782–790. 10.1111/pcn.12332. [DOI] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP, 2006. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. Neuroimag 147 (1), 1–25. 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Manji HK, 2005. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol. Psychiatry 57 (4), 430–432. 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]


