Abstract
Background:
Sleep dysfunction and sleep disorders are important comorbidities of psoriasis. Not only do these sleep comorbidities contribute to reduced quality of life, but they may also lead to worsening psoriasis and increased susceptibility to cardiometabolic diseases. While psoriasis and sleep dysfunction are thought to be linked by itch, depression, and immune system dysregulation, the relationship between psoriasis and sleep dysfunction is not yet fully understood.
Objective:
We sought to compare previous studies characterizing the gut microbiome in psoriasis and sleep dysfunction and examine the potential relevance of shared findings on cardiometabolic and overall health.
Methods:
We performed literature searches of PubMed and Embase databases to find studies evaluating the gut microbiome in psoriasis, sleep dysfunction, and cardiometabolic diseases.
Results:
Studies characterizing the gut microbiome in psoriasis and sleep dysfunction reveal shared findings, specifically an increased Firmicutes to Bacteroidetes ratio and reduced abundance of short chain fatty acid-producing bacteria. These dysbiotic features have also been shown to promote systemic inflammation and cardiometabolic disease.
Conclusion:
In favoring an increased Firmicutes to Bacteroidetes ratio and reduced abundance of short chain fatty acid-producing bacteria, sleep dysfunction could be contributing to worsening psoriasis and cardiometabolic comorbidities through intestinal dysbiosis. Future studies are needed to determine whether gut- and sleep-targeting interventions could be therapeutic in psoriasis patients with poor sleep.
Keywords: psoriasis, sleep dysfunction, sleep disorder, sleep deprivation, obstructive sleep apnea, insomnia, microbiome, microbiota, dysbiosis, flora
1. Introduction
Psoriasis is a chronic inflammatory skin condition, affecting 2 to 4% of the U.S. population. There are five main variants of psoriasis with the most common being chronic plaque psoriasis, which is characterized by well-defined, erythematous, scaly plaques that for many patients are pruritic, painful, and disfiguring. Psoriasis is associated with a reduced quality of life as well as significant morbidity and mortality, with psoriasis comorbidities including psoriatic arthritis, cardiovascular disease, metabolic syndrome, anxiety, depression, and sleep disorders.1,2 Compared to the general population, psoriasis patients experience an increased prevalence of obstructive sleep apnea (OSA) (36–81.8% vs 2–4%), restless leg syndrome (15.1–18% vs 5–10%), and insomnia (5.9%−44.8% vs. 10% for chronic insomnia) according to a 2016 systematic review.3 Low quality sleep is also very prevalent in this population, with a study by Smith et al. observing that 58.4% of psoriasis patients endorse sleep difficulty and 38.8% report getting less than seven hours of sleep per night,4 the minimum number of hours recommended by the American Academy of Sleep Medicine.5 This high rate of sleep dysfunction is especially concerning in psoriasis patients, as sleep disorders correlate with a lower quality of life and intense daytime fatigue in this population.6 Additionally, sleep dysfunction is independently associated with an increased risk of many psoriasis comorbidities, notably cardiovascular disease, obesity, diabetes, anxiety, and depression.2,7–10 Finally, sleep dysfunction may also play a role in triggering or potentiating psoriatic disease, as night shift workers have been shown to exhibit an increased prevalence of psoriasis as well as more severe and frequent psoriasis flares, implicating circadian rhythm disruption as a potential psoriasis risk factor.11
While the connection between sleep dysfunction and psoriasis is not yet completely understood, pruritus, concomitant depression, and pain with psoriatic arthritis are all likely to be involved.3 Additionally, recent research has uncovered a bidirectional relationship between sleep and the immune system, with sleep regulating immune cell memory formation and cytokine profiles affecting quality and quantity of sleep.12 Through its immune-modulatory effects, sleep dysfunction could be contributing to chronic inflammation in psoriasis patients, potentially influencing disease activity as seen in other chronic inflammatory conditions.13 Conversely, chronic inflammation in psoriasis patients could be one of the factors promoting sleep dysfunction in these patients.
The gut microbiome is an important regulator of immune activity and the circadian rhythm, and thus may play a role in the relationship between sleep and psoriasis.14 The gut microbiome consists of all the microorganisms and their genomes that live within the human gastrointestinal tract. It is influenced by numerous factors and considered an important disease-regulator in many chronic diseases, including psoriasis and cardiometabolic disease, when in a state of dysbiosis or imbalance.15,16 In human and animal studies, shared patterns of intestinal dysbiosis have been related to psoriasis, OSA, and sleep disturbance, including an elevated ratio of bacterial phyla Firmicutes to Bacteroidetes (F/B ratio) and a relative reduction in short chain fatty acid- (SCFA-) producing bacteria. These features of intestinal dysbiosis have been shown to contribute to systemic inflammation and cardiometabolic disease, suggesting that the gut microbiome could be an important factor linking psoriasis and sleep dysfunction and the cardiometabolic comorbidities associated with both of these conditions.
In this review, we examine the findings from previous psoriasis and sleep gut microbiome studies and identify shared characteristics, specifically an increased F/B ratio and reduced abundance of SCFA-producing bacteria. We also review evidence suggesting that both of these microbial alterations promote systemic inflammation and cardiometabolic disease. The health-related implications of these associations for patients with both psoriasis and sleep dysfunction are reviewed, with special regard to psoriasis progression and cardiometabolic health. Finally, we explore future areas of research and the potential therapeutic role of sleep- or gut-targeting interventions in psoriasis patients that have sleep dysfunction or sleep disorders.
2. Methods
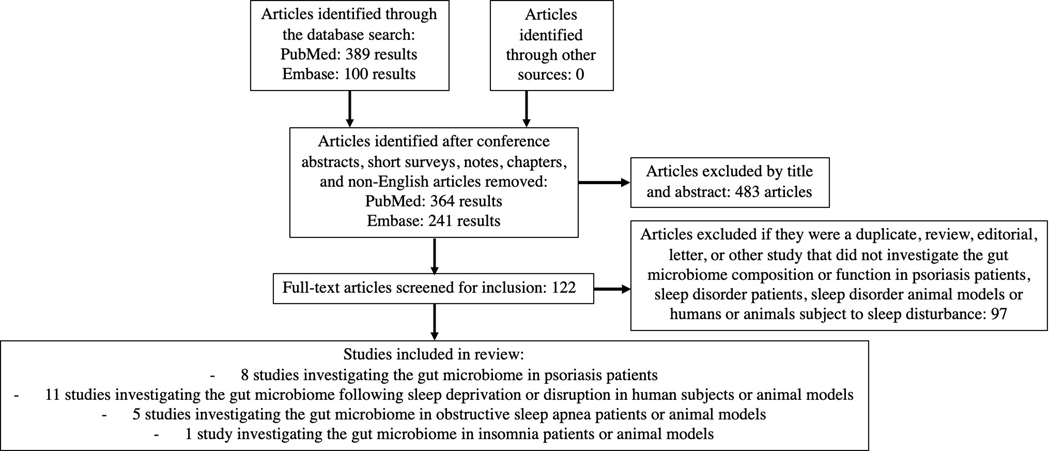
A literature search of PubMed and Embase databases was conducted for the search terms ‘microbiome,’ ‘microbiota,’ OR ‘metagenome’ AND ‘psoriasis,’ ‘psoriatic arthritis,’ ‘sleep dysfunction,’ ‘sleep disorder,’ ‘sleep disruption,’ ‘sleep restriction,’ ‘sleep deprivation,’ ‘obstructive sleep apnea,’ ‘insomnia,’ OR ‘restless leg syndrome’ (Figure 1). Another literature search of PubMed and Embase databases was performed for the search terms ‘microbiome,’ ‘microbiota,’ OR ‘metagenome’ AND ‘cardiovascular disease,’ ‘coronary artery disease,’ ‘atherosclerosis,’ ‘hypertension,’ ‘obesity,’ ‘diabetes,’ OR ‘metabolic disease.’ Our search was limited to English-language articles published prior to April 2nd, 2020. The leading author manually identified observational and interventional human or animal studies investigating the gut microbiome in psoriasis, sleep disorder/disruption, and cardiometabolic disease. Review articles exploring the gut microbiome in cardiometabolic disease were also reviewed.
Figure 1 legend:
PRISMA-based flow-chart of search for studies included in review.
3. Results
We identified eight primary research studies investigating the gut microbiome in psoriasis patients and 16 in sleep disorder patients or animal models or humans or animals subject to sleep disturbance (Table 1). 20 primary research studies or reviews exploring the gut microbiome in cardiovascular or metabolic disease were also included. 31 articles related to the gut microbiome, psoriasis, sleep disorder/disruption, or cardiometabolic disease are referenced to provide additional background, scientific, or clinically relevant information.
Table 1.
Primary studies investigating the gut microbiome in i) psoriasis patients, ii) animals subject to obstructive sleep apnea-mimicking conditions, sleep deprivation, sleep restriction, or circadian rhythm disruption, and iii) individuals with a sleep disorder or volunteers subject to sleep deprivation, sleep restriction, or sleep-wake cycle shift.
| Psoriasis microbiome studies in humans | Subjects | Methods | Reported change in F/B ratio and abundance of SCFA-producing or -relevant bacterial taxa in psoriasis vs. control cohort |
|---|---|---|---|
| Chen et al., 201819 | 32 psoriasis patients vs. 64 age-, gender-, and BMI-matched non-psoriasis controls | 16 sRNA sequencing (hypervariable region V4-V4) | ↑ F/B ratio and ↓ Akkermansia spp. abundance in non-obese, but not obese, psoriasis patients vs. controls |
| Codoñer et al., 201836 | 52 plaque-type psoriasis patients with PASI > 6 vs. 300 healthy subjects who were from the Human Microbiome Project | 16s rRNA sequencing (hypervariable region V3-V4) | ↑Akkermansia and Ruminococcus spp. abundance, ↓ Bacteroides spp. and Faecalibacterium prausnitzii abundance ↓ Bacteroides/Faecalibacterium ratio shown to increase risk of bacterial translocation from the gut to systemic circulation |
| Eppinga et al., 201635 | 29 psoriasis only vs. 31 IBD only vs. 17 HS only vs. 13 concomitant psoriasis and IBD vs. 17 concomitant HS and IBD vs. 33 healthy controls | Quantitative PCR | ↓ Faecalibacterium prausnitzii abundance in psoriasis patients, with even greater reduction in patients with both psoriasis and IBD |
| Hidalgo-Cantabrana et al., 201922 | 19 psoriasis patients vs. 20 geographically matched healthy controls | 16s rRNA sequencing (hypervariable region V2-V3) | ↑ F/B ratio, ↓ Faecalibacterium spp. abundance, and ↑ Ruminococcus spp. and Bifidobacterium spp. abundance |
| Masallat et al., 201621 | 45 psoriasis patients vs. 45 age- and sex-matched healthy controls | Fecal real time PCR | ↑ F/B ratio which positively correlated with PASI score |
| Scher et al., 201537 | 15 skin-only psoriasis patients vs. 16 treatment-naïve psoriatic arthritis patients vs. 17 healthy controls | 16s rRNA sequencing (hypervariable region V1-V2) | ↓ relative abundance of Akkermansia and Ruminococcus spp. in psoriatic arthritis patients vs. skin-only psoriasis patients and psoriatic arthritis patients vs. healthy controls |
| Shapiro et al., 201920 | 24 psoriasis patients vs. 24 age-, BMI- and comorbidity-matched non-psoriasis controls | 16s rRNA sequencing (hypervariable region V4-V4) | ↑ F/B ratio ↓ expression of genes in butyrate synthesis |
| Tan et al., 201734 | 15 psoriasis patients, not on any anti-inflammatory agents, vs. 14 healthy controls | 16s rRNA sequencing (hypervariable region V4-V4) | ↓ Akkermansia muciniphila abundance |
| Sleep microbiome studies in animals | Subjects | Methods | Reported change in F/B ratio and abundance of SCFA-producing or -relevant bacterial taxa in sleep disorder or sleep disrupted vs. control cohort |
| Durgan et al., 201639 | Rats subject to OSA-mimicking conditions and fed either a normal or high-fat diet | 16s rRNA sequencing (hypervariable region V4-V4) | ↑ blood pressure, ↑ F/B ratio, and ↓ SCFA-producing bacteria (belonging to family Ruminococcaceae) in OSA rats that were fed a high-fat vs. normal diet Transplantation of dysbiotic cecal contents of OSA rats on high-fat diet to OSA rats on normal diet resulted in ↑ blood pressure |
| Ma et al., 201940 | Rats subject to 7-day paradoxical sleep deprivation | 16s rRNA sequencing (hypervariable region V4-V4) | ↓ Akkermansia spp. abundance in rats following 7-day paradoxical sleep deprivation ↑ serum levels of IL-6, TNF-alpha, and CRP, ↑ serum levels of HPA-axis related hormones, and ↑ depression-like behavior in rats also observed following intervention |
| Gao et al., 201926 | Mice subject to 3-day continuous sleep deprivation | 16s rRNA sequencing (hypervariable V3-V4 region) | ↑ F/B ratio and ↓ Akkermansia, Bacteroides, and Faecalibacterium spp. abundance in mice following 3-day continuous sleep deprivation; corresponded with ↓ plasma melatonin, ↓ anti-inflammatory cytokines, ↑ pro-inflammatory cytokines, and colonic mucosal injury Melatonin supplementation during the experiment reversed the sleep deprivation-induced dysbiosis and colonic mucosal injury |
| Bowers et al., 202024 | Mice subject to a 5-day sleep disruption protocol | 16s rRNA sequencing (hypervariable region V4-V4) | ↑ F/B ratio and ↓ Bifidobacterium spp. and Lactobacillus spp. abundance in sleep disruption vs. control mice |
| Dhaliwal et al., 201842 | Mice subject to chronic sleep deprivation | Quantitative PCR | ↑ intestinal permeability and ↓ cecal SCFA concentration in sleep deprived mice ↑ intestinal permeability prevented in sleep deprived mice that received Lactobacillus plantarum MTCC 9510 supplementation |
| Poroyko et al., 201625 | Mice subject to 4-weeks of sleep fragmentation | 16s rRNA sequencing (hypervariable region V4-V4) | ↑ F/B ratio and change in relative abundance of SCFA-relevant bacteria (↑ Lachnospiraceae, ↑ Ruminococcaceae, and ↓ Lactobacillaceae) following 4-weeks of sleep fragmentation; correlated with ↑ food intake, insulin resistance, and visceral white adipose tissue mass and inflammation Microbial and metabolic alterations reversed following 2-week normal sleep recovery period |
| Aidy et al., 201927 | Mice subject to brief 5-hour sleep deprivation | 16s rRNA sequencing (hypervariable region V3-V5) | ↓ relative Clostridiaceae abundance (family containing butyrate-producing microbes) in sleep deprived vs. control mice |
| Moreno-Indias et al., 201529 | Mice subject to OSA-mimicking conditions | 16s rDNA sequencing (hypervariable region V2-V3) | ↑ F/B ratio and ↓ Bacteroides spp. abundance |
| Tripathi et al., 201873 | Mice subject to OSA-mimicking conditions on a high-fat diet | 16s rRNA sequencing (hypervariable region V4-V4) | ↓ relative Clostridiaceae abundance (family containing butyrate-producing microbes) in experimental v. control mice |
| Lucking et al., 201830 | Guinea pigs subject to OSA-mimicking conditions | 16s rRNA sequencing (hypervariable region V4-V4) | ↓ F/B ratio |
| Voigt et al., 201428 | Mice subject to weekly circadian rhythm disruption fed a normal vs. high-fat, high-sugar diet | 16s rRNA sequencing | Significantly altered microbiome in mice fed a high-fat, high-sugar diet but not mice fed a normal diet after circadian rhythm disruption with ↑ F/B ratio and ↑ Ruminococcus spp. and Akkermansia spp. relative abundance |
| Zhang et al., 201774 | Experimental rats subject to sleep restriction v. control rats | 16s rRNA sequencing (hypervariable region V1-V2) | No significant change in microbiome composition observed |
| Sleep microbiome studies in humans | Subjects | Methods | Reported change in F/B ratio and abundance of SCFA-producing or -relevant bacterial taxa in sleep disorder or sleep disrupted vs. control cohort |
| Ko et al., 201938,75 | 93 OSA patients vs. 20 controls | 16s rRNA sequencing (hypervariable region V3-V4) | No statistically significant difference in F/B ratio between OSA patients and controls ↓ SCFA-producing bacteria abundance correlated with ↑ pro-inflammatory IL-6 levels Lactobacillus spp. abundance positively correlated with homocysteine levels |
| Liu et al., 202043 | 22 volunteers aged 20–35 years old subject to sleep-wake cycle shift | 16s rRNA sequencing (hypervariable region V4-V4) | No significant change in relative abundance of microbial taxa seen after sleep-wake cycle shift ↓ fermentation of acetyl-CoA to butanoate, an important pathway in SCFA metabolism, seen 2 days after sleep-wake cycle shift |
| Benedict et al., 201623 | Within subject crossover study with 9 normal weight volunteers subject partial sleep deprivation vs. normal sleep for 2 nights | 16s rRNA sequencing (hypervariable region V4-V4) | ↑ F/B ratio No difference in fecal SCFA concentrations |
| Liu et al., 201931 | 10 insomnia patients vs. 10 non-insomnia controls | 16s rRNA sequencing (hypervariable region V3-V4) | ↓ F/B ratio |
| Zhang et al., 201774 | 11 healthy volunteers subject to sleep restriction | 16s rRNA sequencing (hypervariable region V1-V2) | No significant change in microbiome composition observed after sleep restriction |
Key: PASI: psoriasis area and severity index, F/B: Firmicutes/Bacteroidetes, SCFA: short-chain fatty acid, IBD: irritable bowel disease, HS: hidradenitis suppurativa, OSA: obstructive sleep apnea, CRP: C-reactive protein, HPA: hypothalamus-pituitary-adrenal
Reviewing the primary investigative studies of the gut microbiome in psoriasis, sleep disorder, and sleep dysfunction, the majority reported significant changes in microbiome composition or function in human patients or animal models of disease relative to controls. We identified two common characteristics that were observed among the majority of these studies, an increased F/B ratio and decreased abundance of short chain fatty acid- (SCFA-) producing bacteria. Both of these findings have also been associated with poor cardiometabolic health outcomes (Table 2).
Table 2.
Dysbiotic patterns, an increased Firmicutes/Bacteroidetes ratio and decreased abundance of short chain fatty acid producing bacteria, have been linked to various effects on systemic inflammation and cardiometabolic health.
| Gut microbiome imbalance | Effect on systemic inflammation and cardiometabolic health |
|---|---|
| ↑ F/B ratio | -Associated with an increase in proatherogenic TMAO production -Linked to obesity and insulin resistance in several studies -Observed in heart disease patients and hypertensive mice -Alters carbohydrate metabolism of SCFAs and MCFAs |
| ↓ SCFA-producing bacteria (includes Faecalibacterium, Akkermansia, Ruminococcus, Bifidobacterium, Lactobacillus, Bacteroides spp., and others) | - Observed in obesity, diabetes, insulin resistance, hypertension, atherosclerosis, coronary artery disease, and carotid artery disease patients -Butyrate, a SCFA, has key anti-inflammatory properties and has been shown to suppress Th17 and induce Treg cell development and expansion -Butyrate reduces appetite and supports glucose homeostasis -SCFAs are key energy sources for intestinal epithelial cells -SCFAs help to maintain intestinal epithelial barrier integrity |
Key: F/B: Firmicutes/Bacteroidetes, SCFA: short-chain fatty acid, MCFA: medium-chain fatty acid, TMAO: Trimethylamine-N-oxide
A. Increased Firmicutes to Bacteroidetes ratio in psoriasis and sleep dysfunction
Firmicutes and Bacteroidetes are the most abundant phyla in the gut microbiome,17 and the F/B ratio is considered an important compositional feature of the gut microbiome.18 In four of eight studies that have investigated the gut microbiome in psoriasis patients, a relative imbalance between the two phlya, resulting in an increased F/B ratio, was found.19–22 A significantly increased F/B ratio has also been seen in young normal weight volunteers (n=9) following two nights of partial sleep deprivation versus normal sleep (p<0.05) 23 as well as four studies with mice subject to sleep deprivation.24–27 In a study of circadian rhythm disruption in mice, those fed a high-fat but not a normal diet displayed significantly altered microbiomes, including an increased F/B ratio, suggesting that diet may contribute towards the deleterious effects of circadian rhythm disruption on the gut.28 Poroyko et al. explored the effect of chronic sleep fragmentation (four weeks) on mice intestinal microbiomes, revealing an elevated F/B ratio (p <0.05) that also correlated with an increase in food intake, insulin resistance, and visceral white adipose tissue mass and inflammation. Following a two-week-long recovery period with the mice returning to normal sleep behavior, these microbial and metabolic alterations reversed, suggesting the restorative capacity of proper sleep in repairing sleep-induced microbial and metabolic disturbances.25 In a study by Gao et al., three days of continuous sleep deprivation in mice resulted in elevated F/B ratios as well, with melatonin supplementation during the experiment reversing the sleep-induced dysbiosis.26 Finally, in murine models of OSA, with mice subject to intermittent hypoxia for a six week long period, an increased F/B ratio was reported.29 In contrast to these findings, there have been two studies associating a decreased F/B ratio with poor sleep. In Lucking et al.’s study guinea-pigs exposed to chronic intermittent hypoxia, modeling OSA, developed a lowered F/B ratio30 and in Liu et al.’s study insomnia patients exhibited a reduced F/B ratio relative to healthy controls.31
B. Reduced abundance of short chain fatty acid-producing bacteria in psoriasis and sleep dysfunction
Short chain fatty acids (SCFAs), such as acetate, butyrate, and propionate, are the metabolic end-products of bacterial fermentation.32 SCFAs, especially butyrate and acetate, exhibit important anti-inflammatory effects, reduce oxidative stress, regulate gene expression, and maintain the integrity of the gut epithelial barrier.33 Several studies of psoriasis patients report a relative reduction in intestinal abundance of Akkermansia, Ruminococcus, or Faecalibacterium genera, which are all comprised of mucin-degrading SCFA-producing commensals.19,22,34–37 Analogous findings have been seen in OSA gut microbiome studies, with Ko et al. detecting a reduction in SCFA-producing bacteria in OSA patients (n=93) versus control subjects (n=20).38 In Durgan et al.’s study, researchers subjected mice to OSA-mimicking conditions and saw no effect on blood pressure in those given a normal diet. However, in those fed a high-fat diet, significant increases in blood pressure and decreases in SCFA-producing bacteria were seen, implicating the gut microbiome as instrumental in linking OSA and one of its major comorbidities, hypertension.39 Decreases in SCFA-producing bacteria, specifically belonging to Akkermansia and Faecalibacterium genera, have also been detected in mice subject to seven-day paradoxical sleep deprivation40 and three-day continuous sleep deprivation.26 Decreases in Bifidobacterium and Lactobacillus genera have been seen in mice subject to five days of sleep disruption.24 When used as probiotics in vitro, species of Bifidobacterium and Lactobacillus genera have been shown to increase SCFA production.41 In a study by Dhaliwal et al., sleep deprivation-induced dysbiosis in mice resulted in increased intestinal permeability and reduced abundance of SCFAs, features that were both reversed following subsequent administration of Lactobacillus plantarum MTCC 9510, a probiotic bacterial strain.42 Lastly, in a study of volunteers undergoing an experimental sleep-wake cycle shift, a sleep pattern common in students and shift workers, a decrease in fermentation of acetyl-CoA to butanoate at day two post-intervention was observed. This pathway is related to SCFA metabolism, suggesting that a microbe-mediated decline in SCFA production might be induced by shifting the sleep-wake cycle.43
C. Elevated Firmicutes to Bacteroidetes ratio and reduced abundance of short chain fatty acid-producing bacteria in cardiometabolic disease
Metabolic and cardiovascular diseases are responsible for the majority of morbidity and mortality associated with both psoriasis and sleep dysfunction,44–46 and there is compelling evidence to suggest that the gut microbiota can act to promote or prevent these disease states. In numerous human and animal studies, an elevated F/B ratio and/or reduced abundance of SCFA-producing bacteria have been associated with poor cardiometabolic health (Figure 2).47,48
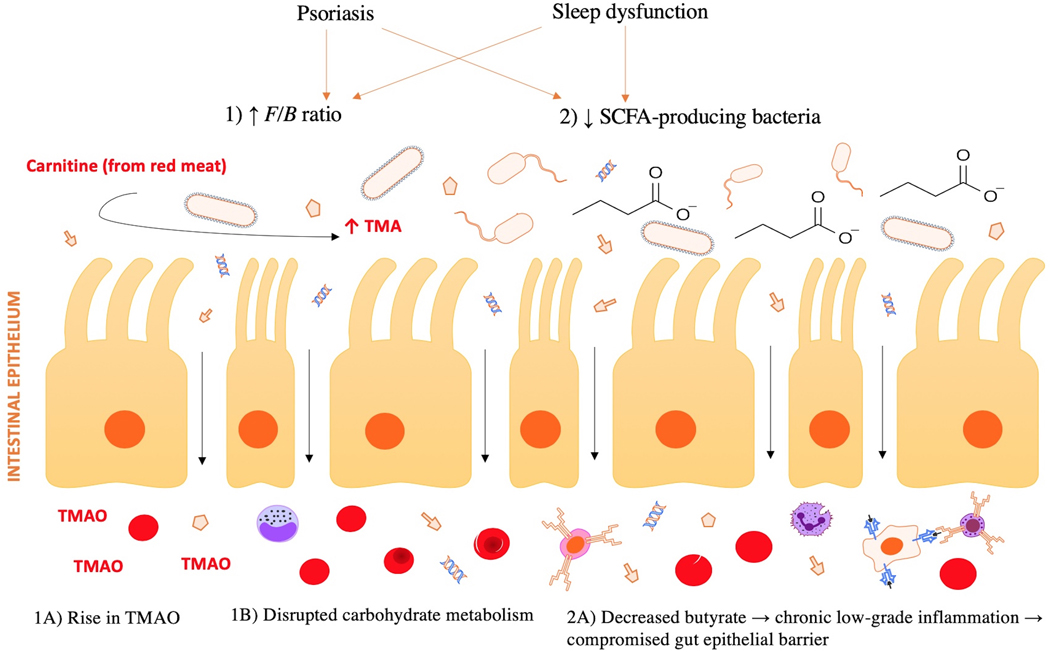
Figure 2 legend:
Effects of an elevated F/B ratio and decreased SCFA-producing bacteria on cardiometabolic health and psoriatic disease. Psoriasis and sleep dysfunction have both been demonstrated to promote intestinal dysbiosis, partially characterized by an 1) increased F/B ratio and 2) reduced SCFA-producing bacteria within the gut. 1A) An increased F/B ratio is associated with high TMAO production, which is linked to greater risk of cardiovascular disease. 1B) An increased F/B ratio promotes obesity and insulin resistance, in part due to its influence on carbohydrate metabolism. 2A) Loss of butyrate, an anti-inflammatory SCFA, permits a chronic, low-grade inflammatory state, impairing the integrity of the gut epithelial barrier. This allows for bacterial DNA and other antigens to translocate from the gut to systemic circulation, where they may act to trigger an immune response at distant sites, such as skin and joints.
An elevated F/B ratio has been associated with obesity and insulin resistance in several human and animal studies.49 For example, Ley et al. found a decrease in intestinal Firmicutes and increase in intestinal Bacteroidetes to correlate with a decrease in weight in obese individuals undergoing dietary changes. When the same individuals returned to their prior dietary habits and gained weight, a rise in Firmicutes and decline in Bacteroidetes, equating to an elevated F/B ratio, ensued.50 The relationship between the F/B ratio and weight may be in part due to the influence of the F/B ratio on carbohydrate metabolism and the relative abundance of SCFAs acetate and butyrate. A high F/B ratio is seen to correlate with high acetate and low butyrate levels. High acetate is linked to an increase in ghrelin, the appetite-stimulating hormone, and low butyrate is permissive to chronic, low-grade inflammation.51–53
An elevated F/B ratio has also been linked to cardiovascular disease, with several animal studies identifying an elevated F/B ratio in hypertensive models.54,55 In research involving human subjects, significant increases in Firmicutes (37.06% v. 32.06%) and decreases in Bacteroidetes (56.12% v. 60.92%) concentrations were seen in chronic heart disease patients (n=29) relative to healthy controls (n=35) (p<0.05).56 Increased F/B ratios have also been identified in fecal samples of high trimethylamine-N-oxide (TMAO) producers.57 TMAO is a proatherogenic metabolite produced via the conversion of dietary carnitine, in eggs and red meat, to trimethyl amine (TMA) by certain bacterial species58 and its concentration positively correlates with risk of atherosclerosis and major cardiovascular events (myocardial infarction, stroke, and death).59
A relative decline in microbe-derived SCFAs is shown to be detrimental to cardiometabolic health as well. SCFAs, especially acetate and butyrate, have many beneficial effects for the host: reducing appetite, supporting glucose homeostasis, modulating the immune system to favor regulatory T cell expansion, a cell type integral in producing anti-inflammatory cytokines and preventing autoimmunity, and maintaining the gut barrier integrity.60,61 Loss of SCFAs allows for the development of a local inflammatory response within the gut and compromises the function of tight junctions between mucosal cells, weakening the gut barrier and its ability to regulate the presentation of pro-inflammatory antigens to systemic circulation. When this occurs, a diffuse inflammatory response is triggered that has been linked to obesity, insulin resistance, and heart disease pathophysiology.61–63
Significantly reduced concentrations of SCFA-producing bacteria have been identified in fecal samples of patients with obesity, diabetes, insulin resistance, hypertension, atherosclerosis, coronary artery disease, and carotid artery disease compared to healthy controls.48,64–67 For example, in chronic heart failure patients (n=53) relative to healthy controls (n=41), researchers Cui et al. identified significant differences in microbiome composition including reduced Faecalibacterium prausnitzii, one of the most important butyrate-producing species in the gut microbiome of humans. Through functional metagenome analysis, they also identified downregulation of microbe-derived butyryl-CoA:acetate CoA transferase, a crucial enzyme in butyrate synthesis, in chronic heart failure patients compared to controls.68
4. Discussion
While studies of the gut microbiome in psoriasis, sleep dysfunction, sleep disorders, and cardiometabolic diseases have been done independently, here we have identified shared characteristics of these studies including an increased F/B ratio and decreased abundance of SCFA-producing bacteria. Not only do such findings suggest a role of dysbiosis in these conditions, but they also suggest that the gut microbiome could be an important element linking psoriasis, sleep dysfunction, and associated cardiometabolic comorbidities. For the psoriasis patient with a comorbid sleep disorder, the dysbiosis induced by these conditions could potentially contribute to poor cardiometabolic outcomes and refractory psoriasis management.
A. Sleep-induced dysbiosis contributing to cardiometabolic disease risk in psoriasis patients
With cardiometabolic disease being a well-established comorbidity of both psoriasis and sleep dysfunction, the association of psoriasis, sleep dysfunction, and cardiometabolic disease with the two detrimental patterns of dysbiosis reviewed (an increased F/B ratio and reduced abundance of SCFA-producing bacteria) suggest that the gut dysbiosis seen in psoriasis and sleep dysfunction could be contributing to compromised cardiometabolic health in these patients. For the patient with both psoriasis and sleep dysfunction, this may have special relevance as poor sleep could be exacerbating pre-existing psoriasis-related dysbiosis to further increase the patient’s risk of cardiometabolic disease development. A Taiwanese study by Chiu et al. demonstrated that psoriasis patients with a comorbid sleep disorder (n=2,223) had a higher risk of ischemic heart disease (HR, 1.25, 95% CI 1.22–1.28) and stroke (HR 1.24, 95% CI 1.16–1.33) compared to psoriasis patients without a comorbid sleep disorder (n=97,405).69 While they did not investigate whether the gut microbiome played a role in this association, studies comparing the gut microbiome composition and function in psoriasis patients with versus without sleep dysfunction could lead to novel insights regarding the relationship between psoriasis and cardiometabolic disease and the possible augmenting role of sleep dysfunction.
B. Sleep-induced dysbiosis contributing to psoriasis severity and progression
Sleep dysfunction might also be contributing to incomplete disease control in psoriasis patients by potentiating presumably pathogenic patterns of intestinal dysbiosis. In a recent study of over 3,000 psoriasis patients, reports of sleep difficulty and low sleep quantity were both higher in those who had moderate (OR 1.59, 95% CI [1.30–1.94, 1.41 [1.16–1.72]) and severe (2.40 [1.87–3.08], 1.40 [1.11–1.76]) psoriasis relative to mild psoriasis.4 While it is likely that the high rate of sleep dysfunction in more severe patients is in part because of an increased intensity of symptoms such as itch, pain, depression, or anxiety, it is also possible that poor sleep could be contributing to psoriasis severity via its detrimental effects on the gut. It is suggested that the gut epithelial permeability implicated in cardiometabolic disease pathophysiology may also play a role in psoriasis disease and progression, by allowing intestinal bacterial antigens to reach extraintestinal sites such as skin and joints where they then trigger an inflammatory reaction to cause psoriatic plaque formation or arthritis symptoms, respectively.36,70 In fact, studies by Sharma et al. and Shapiro et al. implicate advanced dysbiosis and intestinal inflammation in the progression from cutaneous psoriasis to psoriatic arthritis.20,71 Future investigations of sleep dysfunction induced dysbiosis on psoriasis severity and progression could examine the therapeutic utility of sleep- and gut-targeting interventions in psoriasis management.
5. Limitations and future research
Limitation:
Inherent limitations of microbiome research are the high interpersonal variabilities and controlling for environmental factors, geographic features, stage of disease, and experimental conditions that are all capable of influencing the gut microbiome, adding noise that may make identifying disease-causing bacteria even more challenging.
Solution:
Longitudinal studies with more standardized protocols as well as careful matching technique could assist in distinguishing microbiome fluctuations in response to environmental factors versus predominant, disease-relevant changes.
Limitation:
Due to the low number of human studies that have been done analyzing the gut microbiome in sleep disorders and sleep dysfunction, comparisons between animal and human studies made here are limited due to the potential differences in host-microbe interactions between species.
Solution:
An increase in human studies analyzing the gut microbiome as it relates to sleep disorders and sleep behavior would lead to improved reliability in comparing studies.
Limitation:
While psoriasis and sleep dysfunction microbiome studies depict similar findings, the microbiome changes in patients with both psoriasis and sleep dysfunction have not been explored.
Solution:
Studies analyzing the gut microbiome in patients that have psoriasis-only, sleep disorder-only, and concomitant psoriasis and sleep disorder are necessary to truly understand how the presence of sleep dysfunction alters the gut microbiome of psoriasis patients, and how that affects disease severity and comorbidity development.
6. Conclusion
In this review, we highlight similar patterns of dysbiosis identified in both psoriasis and sleep dysfunction human and animal studies: an increase in F/B ratio and decrease in SCFA-producing bacteria. Given that both features have been implicated in promoting systemic inflammation and cardiometabolic disease, we proposed that poor sleep in psoriasis patients could be contributing to increased disease activity as well as less favorable cardiometabolic outcomes due to its dysbiosis-inducing effects. Understanding the connection between sleep dysfunction and psoriasis is likely to have clinical relevance for the practicing dermatologist, as future research in this field may show that screening for and treating sleep disorders in psoriasis patients leads to long- and short-term benefits. Sleep-focused interventions to be explored include melatonin supplementation, continuous positive airway pressure machines for OSA patients, relaxation techniques, and light therapy.72 Future research could examine whether gut-altering therapies, such as probiotics, antibiotics, or dietary changes designed to ameliorate dysbiosis could be beneficial in psoriasis patients suffering from a sleep disorder or chronic sleep dysfunction as they may help to mitigate the detrimental effects of sleep-induced dysbiosis on psoriasis progression and comorbidity development.
Funding:
This review received no specific grant from any funding agency in the public, commercial, or non-for-profit sectors. T.B. has served as a consultant for Abbvie, Pfizer, Novartis, and Meiji Pharmaceuticals. She is currently an investigator for Amgen, Janssen, Pfizer, Regeneron, and Sun Pharma. W.L. is funded in part by grants from the National Institutes of Health (U01AI119125) and has served as a research investigator for Abbvie, Amgen, Janssen, Novartis, Pfizer, Regeneron, Sanofi, and TRex Bio.
Abbreviations:
- OSA
(obstructive sleep apnea)
- SCFA
(short chain fatty acid)
- F/B
(Firmicutes to Bacteroidetes ratio)
- TMAO
(trimethylamine-N-oxide)
- NREM
(non-rapid eye movement)
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
References
- 1.Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4:35. doi: 10.1186/1477-7525-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveira M de FSP, Rocha B de O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90(1):9–20. doi: 10.1590/abd1806-4841.20153038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: A systematic review. Sleep Med Rev. 2016;29:63–75. doi: 10.1016/j.smrv.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 4.Smith MP, Ly K, Thibodeaux Q, et al. Factors Influencing Sleep Difficulty and Sleep Quantity in the Citizen Pscientist Psoriatic Cohort. Dermatol Ther (Heidelb). 2019;9(3):511–523. doi: 10.1007/s13555-019-0306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society | Journal of Clinical Sleep Medicine. Accessed March 31, 2020. 10.5664/jcsm.4758 [DOI] [Google Scholar]
- 6.Krajewska-Włodarczyk M, Owczarczyk-Saczonek A, Placek W. Sleep disorders in patients with psoriatic arthritis and psoriasis. Reumatologia. 2018;56(5):301–306. doi: 10.5114/reum.2018.79501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo CM, Lee PH. Prevalence and impacts of poor sleep on quality of life and associated factors of good sleepers in a sample of older Chinese adults. Health Qual Life Outcomes. 2012;10:72. doi: 10.1186/1477-7525-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalmbach DA, Arnedt JT, Song PX, Guille C, Sen S. Sleep Disturbance and Short Sleep as Risk Factors for Depression and Perceived Medical Errors in First-Year Residents. Sleep. 2017;40(3). doi: 10.1093/sleep/zsw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41(6). doi: 10.1093/sleep/zsy047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatima Y, Doi S a R, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17(11):1154–1166. doi: 10.1111/obr.12444 [DOI] [PubMed] [Google Scholar]
- 11.Li W, Qureshi AA, Schernhammer ES, Han J. Rotating night shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133(2):565–567. doi: 10.1038/jid.2012.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besedovsky L, Lange T, Haack M. The Sleep-Immune Crosstalk in Health and Disease. Physiological Reviews. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm res. 2007;56(2):51–57. doi: 10.1007/s00011-006-6067-1 [DOI] [PubMed] [Google Scholar]
- 14.al YL et. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression | Kopernio. Accessed February 23, 2020. [DOI] [Google Scholar]
- 15.Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the Human Microbiome. Nutr Rev. 2012;70(Suppl 1):S38–S44. doi: 10.1111/j.1753-4887.2012.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements SJ R Carding S. Diet, the intestinal microbiota, and immune health in aging. Crit Rev Food Sci Nutr. 2018;58(4):651–661. doi: 10.1080/10408398.2016.1211086 [DOI] [PubMed] [Google Scholar]
- 17.Rinninella E, Raoul P, Cintoni M, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1). doi: 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 19.Chen Y-J, Ho HJ, Tseng C-H, Lai Z-L, Shieh J-J, Wu C-Y. Intestinal microbiota profiling and predicted metabolic dysregulation in psoriasis patients. Exp Dermatol. 2018;27(12):1336–1343. doi: 10.1111/exd.13786 [DOI] [PubMed] [Google Scholar]
- 20.Shapiro J, Cohen NA, Shalev V, Uzan A, Koren O, Maharshak N. Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J Dermatol. 2019;46(7):595–603. doi: 10.1111/1346-8138.14933 [DOI] [PubMed] [Google Scholar]
- 21.Masallat D, Moemen D, State A. Gut bacterial microbiota in psoriasis: A case control study. African Journal of Microbiology Research. 2016;10:1337–1343. doi: 10.5897/AJMR2016.8046 [DOI] [Google Scholar]
- 22.Hidalgo-Cantabrana C, Gómez J, Delgado S, et al. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br J Dermatol. Published online March 28, 2019. doi: 10.1111/bjd.17931 [DOI] [PubMed] [Google Scholar]
- 23.Benedict C, Vogel H, Jonas W, et al. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Molecular Metabolism. 2016;5(12):1175–1186. doi: 10.1016/j.molmet.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowers SJ, Vargas F, González A, et al. Repeated sleep disruption in mice leads to persistent shifts in the fecal microbiome and metabolome. PLoS ONE. 2020;15(2):e0229001. doi: 10.1371/journal.pone.0229001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poroyko VA, Carreras A, Khalyfa A, et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Scientific Reports. 2016;6(1):1–11. doi: 10.1038/srep35405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao T, Wang Z, Dong Y, et al. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res. 2019;67(1):e12574. doi: 10.1111/jpi.12574 [DOI] [PubMed] [Google Scholar]
- 27.Aidy SE, Bolsius YG, Raven F, Havekes R. A brief period of sleep deprivation leads to subtle changes in mouse gut microbiota. Journal of Sleep Research. n/a(n/a):e12920. doi: 10.1111/jsr.12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voigt RM, Forsyth CB, Green SJ, et al. Circadian Disorganization Alters Intestinal Microbiota. PLOS ONE. 2014;9(5):e97500. doi: 10.1371/journal.pone.0097500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno-Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45(4):1055–1065. doi: 10.1183/09031936.00184314 [DOI] [PubMed] [Google Scholar]
- 30.Lucking EF, O’Connor KM, Strain CR, et al. Chronic intermittent hypoxia disrupts cardiorespiratory homeostasis and gut microbiota composition in adult male guinea-pigs. EBioMedicine. 2018;38:191–205. doi: 10.1016/j.ebiom.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Lin W, Chen S, et al. Gut Microbiota as an Objective Measurement for Auxiliary Diagnosis of Insomnia Disorder. Front Microbiol. 2019;10. doi: 10.3389/fmicb.2019.01770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10. doi: 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canani RB. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases | Kopernio. Accessed April 9, 2020. https://kopernio.com/viewer?doi=10.3748%2Fwjg.v17.i12.1519&token=WzEzNjc0MTcsIjEwLjM3NDgvd2pnLnYxNy5pMTIuMTUxOSJd.Q8B2vhifrmJEevTGaE9MhnlqIWs [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan L, Zhao S, Zhu W, et al. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp Dermatol. 2018;27(2):144–149. doi: 10.1111/exd.13463 [DOI] [PubMed] [Google Scholar]
- 35.Eppinga H, Sperna Weiland CJ, Thio HB, et al. Similar Depletion of Protective Faecalibacterium prausnitzii in Psoriasis and Inflammatory Bowel Disease, but not in Hidradenitis Suppurativa. J Crohns Colitis. 2016;10(9):1067–1075. doi: 10.1093/ecco-jcc/jjw070 [DOI] [PubMed] [Google Scholar]
- 36.Codoñer FM, Ramírez-Bosca A, Climent E, et al. Gut microbial composition in patients with psoriasis. Scientific Reports. 2018;8(1):1–7. doi: 10.1038/s41598-018-22125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis & Rheumatology (Hoboken, NJ). 2015;67(1):128–139. doi: 10.1002/art.38892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko C-Y, Liu Q-Q, Su H-Z, et al. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci. 2019;133(7):905–917. doi: 10.1042/CS20180891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durgan DJ, Ganesh BP, Cope JL, et al. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension. 2016;67(2):469–474. doi: 10.1161/HYPERTENSIONAHA.115.06672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma W, Song J, Wang H, et al. Chronic paradoxical sleep deprivation-induced depression-like behavior, energy metabolism and microbial changes in rats. Life Sci. 2019;225:88–97. doi: 10.1016/j.lfs.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 41.LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16. doi: 10.1186/s12934-017-0691-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhaliwal J, Singh DP, Singh S, et al. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J Appl Microbiol. 2018;125(1):257–269. doi: 10.1111/jam.13765 [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Wei Z-Y, Chen J, et al. Acute Sleep-Wake Cycle Shift Results in Community Alteration of Human Gut Microbiome. mSphere. 2020;5(1). doi: 10.1128/mSphere.00914-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: A population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163(3):586–592. doi: 10.1111/j.1365-2133.2010.09941.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malhotra A, Loscalzo J. Sleep and Cardiovascular Disease: An Overview. Prog Cardiovasc Dis. 2009;51(4):279–284. doi: 10.1016/j.pcad.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Depner CM, Stothard ER, Wright KP. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14(7):507. doi: 10.1007/s11892-014-0507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sciences. 2018;214:153–157. doi: 10.1016/j.lfs.2018.10.063 [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Devaraj S. Gut Microbiome in Obesity, Metabolic Syndrome, and Diabetes. Curr Diab Rep. 2018;18(12):129. doi: 10.1007/s11892-018-1104-3 [DOI] [PubMed] [Google Scholar]
- 49.Castaner O, Goday A, Park Y-M, et al. The Gut Microbiome Profile in Obesity: A Systematic Review. Int J Endocrinol. 2018;2018:4095789. doi: 10.1155/2018/4095789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. doi: 10.1038/nature18309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komaroff AL. The Microbiome and Risk for Obesity and Diabetes. JAMA. 2017;317(4):355–356. doi: 10.1001/jama.2016.20099 [DOI] [PubMed] [Google Scholar]
- 53.Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients. 2011;3(10):858–876. doi: 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adnan S, Nelson JW, Ajami NJ, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96–104. doi: 10.1152/physiolgenomics.00081.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui L, Zhao T, Hu H, Zhang W, Hua X. Association Study of Gut Flora in Coronary Heart Disease through High-Throughput Sequencing. Biomed Res Int. 2017;2017:3796359. doi: 10.1155/2017/3796359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho CE, Taesuwan S, Malysheva OV, et al. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Molecular Nutrition & Food Research. 2017;61(1):1600324. doi: 10.1002/mnfr.201600324 [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M, Zhou Q, Dorfman RG, et al. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016;16. doi: 10.1186/s12876-016-0500-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chambers ES, Preston T, Frost G, Morrison DJ. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr Nutr Rep. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasini E, Aquilani R, Testa C, et al. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016;4(3):220–227. doi: 10.1016/j.jchf.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 63.Zhou X, Li J, Guo J, et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6(1):66. doi: 10.1186/s40168-018-0441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan Q, Gu Y, Li X, et al. Alterations of the Gut Microbiome in Hypertension. Front Cell Infect Microbiol. 2017;7. doi: 10.3389/fcimb.2017.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jie Z, Xia H, Zhong S-L, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi: 10.1038/s41467-017-00900-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Q, Gao R, Zhang Y, et al. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol Genomics. 2018;50(10):893–903. doi: 10.1152/physiolgenomics.00070.2018 [DOI] [PubMed] [Google Scholar]
- 67.Karlsson FH, Fåk F, Nookaew I, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. doi: 10.1038/ncomms2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui X, Ye L, Li J, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Scientific Reports. 2018;8(1):635. doi: 10.1038/s41598-017-18756-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiu H-Y, Hsieh C-F, Chiang Y-T, et al. Concomitant Sleep Disorders Significantly Increase the Risk of Cardiovascular Disease in Patients with Psoriasis. PLoS One. 2016;11(1). doi: 10.1371/journal.pone.0146462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14. doi: 10.1186/s12876-014-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adarsh MB, Dogra S, Vaiphei K, Vaishnavi C, Sinha SK, Sharma A. Evaluation of subclinical gut inflammation using faecal calprotectin levels and colonic mucosal biopsy in patients with psoriasis and psoriatic arthritis. Br J Dermatol. 2019;181(2):401–402. doi: 10.1111/bjd.17745 [DOI] [PubMed] [Google Scholar]
- 72.Abad VC, Guilleminault C. Diagnosis and treatment of sleep disorders: a brief review for clinicians. Dialogues Clin Neurosci. 2003;5(4):371–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tripathi A, Melnik AV, Xue J, et al. Intermittent Hypoxia and Hypercapnia, a Hallmark of Obstructive Sleep Apnea, Alters the Gut Microbiome and Metabolome. mSystems. 2018;3(3). doi: 10.1128/mSystems.00020-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.al SLZ et. Human and rat gut microbiome composition is maintained following sleep restriction | Kopernio. Accessed February 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ko C-Y, Fan J-M, Hu A-K, et al. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 2019;9(5):e01287. doi: 10.1002/brb3.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]