Abstract
Aim:
To evaluate the efficacy and safety of corticosteroids for treating hospitalized COVID-19 patients.
Materials & methods:
Efficacy outcomes included time to negative SARS-CoV-2 tests, length of stay, duration and incidence of intensive unit care stay, incidence of mortality and duration and incidence of mechanical ventilation. Safety outcomes included the incidence of adverse events and severe adverse events, incidence of hyperglycemia and incidence of nosocomial infections.
Results:
Ninety-five randomized controlled trials (RCTs) and observational studies (n = 42,205) were included. Corticosteroids were associated with increased length of stay (based on RCT only), increased time to negative tests, decreased length of mechanical ventilation and increased odds of hyperglycemia.
Conclusion:
Corticosteroids should be considered in patients requiring mechanical ventilation, and glycemic monitoring may be needed when administering corticosteroids.
Keywords: : corticosteroids, COVID-19, length of stay, mechanical ventilation, mortality, SARS-CoV-2
In March 2020 the WHO designated COVID-19, which is caused by SARS-CoV-2, as a global pandemic [1–4]. The spread of SARS-CoV-2 has been considered to be a severe public health crisis due to its high transmission, hospitalization and mortality rates [5–8]. One of the largest factors attributed to the high mortality rate associated with COVID-19 was the depletion of hospital resources as a result of surges in cases [9–11]. The care of critically ill COVID-19 patients require valuable and scarce supplies such as ICU beds, ventilators, personal protective equipment and hospital staff [12]; thus, physicians have sometimes been forced to triage critically ill COVID-19 patients [13] and divert resources from other specialties such as oncology, leading to the rescheduling of appointments for non-COVID patients [14].
Recent advancements in SARS-CoV-2 vaccination programs, including the US FDA-approved Comirnaty vaccine produced by Pfizer-BioNTech [15], aims to curb the transmission of the virus, induce herd immunity and relieve the pressure of the pandemic on hospital resources. However, new viral variants of interest (VOIs), such as Lambda and Delta [16], threaten to introduce new COVID-19 surges in the coming months with their increased infectivity [17], immune resistance [17,18] and reduced sensitivity to antibody neutralization [19]. These newly discovered variants, in addition to widespread vaccine hesitancy movements [20] and poor vaccine accessibility in low- to middle-income countries (LMICs) [21] are likely to extend the pandemic well into 2022 [22]. Therefore, further investigations are still required to determine the optimal treatment regimen for the management of COVID-19 surges in the coming months.
Currently, most of the proposed COVID-19 treatment regimens consist of repurposed pharmacological compounds that were initially chosen based on their antiviral efficacy against SARS-CoV-2 in vitro or in small observational studies. However, many repurposed regimens such as hydroxychloroquine and lopinavir/ritonavir have been consistently shown in randomized controlled trials (RCTs) and meta-analyses to confer no efficacy against SARS-CoV-2 in clinical settings [23–26]. In early 2020, a series of observational and prognostic studies brought a new category of repurposed therapies, corticosteroids, into the limelight [27]. These early findings are corroborated by encouraging results from the RECOVERY trial, which is the world's largest clinical trial for COVID-19 treatments to date [28]. The RECOVERY trial concluded that dexamethasone may confer survival benefits among patients receiving mechanical ventilation. Unfortunately, no other authoritative RCTs, including the REMAP-CAP [29] and CoDEX trial [30], were able to identify a significant beneficial effect associated with corticosteroids. This was partially due to insufficient sample sizes as a result of premature trial terminations following the publication of results from the RECOVERY trial [31]. Additionally, pre-pandemic studies of corticosteroids in other viral pneumonias, including the original 2003 SARS-CoV [32], the Middle East respiratory syndrome (MERS) [33] and influenza [34], have associated the use of corticosteroids with delayed viral clearance and increased mortality. These conflicting findings call into question the efficacy of corticosteroids, prompting further investigations into this category of treatments.
Biologically, the rationale for the use of corticosteroids in the treatment of COVID-19 is to reduce excessive host inflammatory responses in the lungs through the inflammatory phase of the disease, during which immunopathological factors play a bigger role in disease progression compared with viral replication [35,36]. For some patients, impaired interferon responses [37,38] and the activation of intracellular antiviral pathways triggered by viral RNA may lead to symptoms that mimic macrophage activation syndrome (MAS) during the inflammatory phase [39]. MAS is associated with impaired cytolytic capabilities of CD8+ T cells and natural killer (NK) cells [40,41], which are normally tasked with lysing infected cells to prevent viral replication and the excessive secretion of inflammatory cytokines [42]. This impairment is driven by high levels of pro-inflammatory cytokine IL-6, which forms a vicious cycle of cytokine-driven cytokine secretion [43] leading to cytokine storms [39]. IL-6 is also implicated in the excessive differentiation of Th2 cells and Th17 cells while inhibiting the differentiation of antiviral Th1 cells [40,44–46]. This action prevents the host immune system from mounting an effective antiviral response while inducing a nonproductive, eosinophilic immune response [47]. COVID-19 patients with elevated IL-6 levels and eosinophilic immune responses have been associated with the development of severe lung injuries and acute respiratory distress syndrome (ARDS) [48,49].
As one of the most commonly used therapies for cytokine storms and MAS in patients with autoimmune and inflammatory diseases, corticosteroids appear to be the ideal regimen for treating COVID-related hyperinflammatory disorders [50]. With appropriate administration times and dosages, it is proposed that corticosteroids can reduce the production of proinflammatory cytokines and inhibit overactive immune cells, thus preventing lung damage and ARDS [51–53]. On the other hand, corticosteroids' immunosuppressive properties may impair viral clearance and increase the rate of dangerous nosocomial infections [54]. It is evident that the role of corticosteroids requires further clarification to examine whether its immunomodulatory efficacy outweighs its adverse effects. Thus, the current meta-analysis assessed the efficacy and safety of corticosteroids compared with standard of care, and studied the impact of factors such as dosage and time of administration on patient-important outcomes.
Methods
We conducted this systematic review and meta-analysis in accordance with the PRISMA 2020 guidelines [55]. The completed PRISMA 2020 checklist is provided in Supplementary Table 1. This review was prospectively registered on PROSPERO (CRD42021233108), the international prospective register of systematic reviews [56].
Study identification
To identify relevant studies for inclusion in this review, we created an English search strategy based on database-specific COVID-19 search strings provided by the Rudolph Matas Library of the Health Sciences, Tulane University (LA, USA) [57] in combination with treatment-relevant keywords such as ‘dexamethasone’, ‘prednisone’, ‘methylprednisolone’, ‘corticosteroid’, ‘glucocorticoid’ and ‘steroid’ etc. Using this strategy, we searched the following databases from 1 January 2020 to 12 July 2021: Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (EMBASE), and PubMed. Additionally, we utilized a Chinese search strategy equivalent to our English strategy, and systematically searched the following Chinese literature databases from 1 January 2020 to 12 July 2021: Wanfang Data, Wanfang Med Online, SinoMed, China National Knowledge Infrastructure (CNKI) and Chongqing VIP Information (CQVIP). The search strategies used for the database searches are appended in Supplementary Tables 2–9. Last, we hand searched the reference sections of six previous systematic reviews [34,58–62] to identify relevant studies that were not identified by our database searches.
Eligibility criteria
We included both randomized and non-randomized comparative studies that met the following inclusion criteria: compared any corticosteroid therapy with standard of care, or compared any corticosteroid therapy with an adjuvant therapy to adjuvant therapy alone, and included hospitalized COVID-19 patients. We only included studies using adjuvant therapies if the adjuvant therapy between the control and experimental arms are the same in order to minimize the effect of the adjuvant therapies on treatment outcomes.
Outcome measures
Our efficacy outcomes included: time to negative conversion of SARS-CoV-2 tests, overall length of hospitalization, length of ICU hospitalization, incidence of progression to ICU hospitalization, incidence of mortality, incidence of progression to mechanical ventilation and length of mechanical ventilation. The safety outcomes included: incidence of all-cause adverse events, incidence of investigator-defined severe adverse events, incidence of hyperglycemia or corticosteroid-induced diabetes mellitus and incidence of nosocomial bacterial or fungal infections.
Study selection
All title/abstract entries identified from our literature searches were entered into an independent and in duplicate title/abstract screening process via Rayyan (https://www.rayyan.ai/) [63]. Abstract entries that were deemed eligible were then entered into a subsequent in duplicate full-text screening process. During the selection process, all disagreements were referred to a senior author for resolution. The PRISMA flowchart [64] depicting our study selection process is shown in Figure 1.
Figure 1. . PRISMA flowchart for the identification and selection of studies.
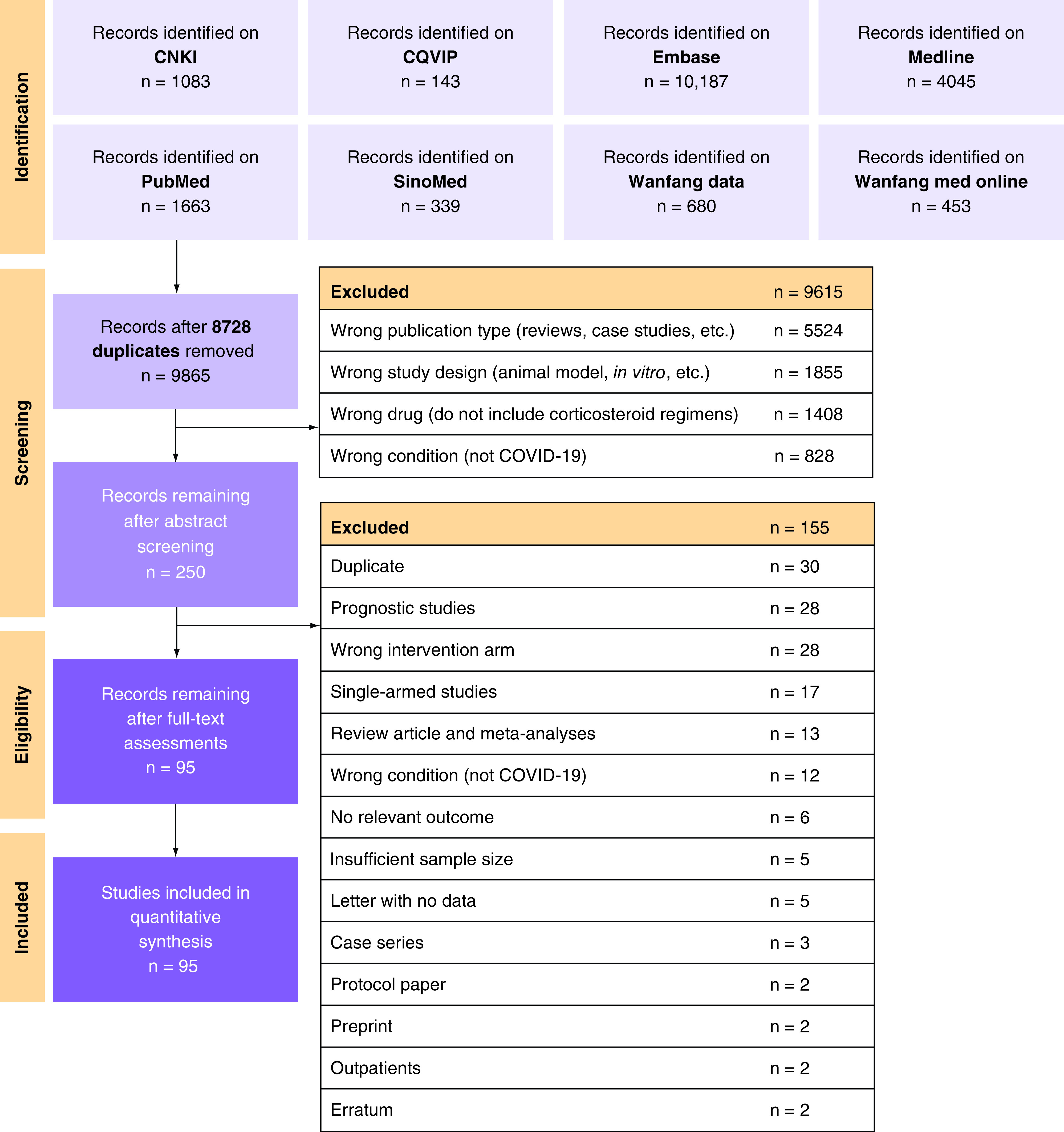
CNKI: China national knowledge infrastructure; CQVIP: Chongqing VIP information; EMBASE: Excerpta medica database; MEDLINE: Medical literature analysis and retrieval system online; PRISMA: Preferred reporting items for systematic reviews and Meta-Analyses.
Data extraction
We performed data extraction independently and in duplicate using an extraction sheet developed a priori. Categories of extracted information included: patient demographics and baseline characteristics, descriptions of study methodology, treatment descriptions and outcome measures. The full list of extracted items are listed on our PROSPERO registration page. For observational studies reporting data for both matched and unmatched cohorts, we only extracted and analyzed the data from matched cohorts. All disagreements were resolved by recruiting a senior author to review the data.
Missing data
For studies with missing data required for meta-analysis, including mean values for continuous outcomes and measures of variance, we made attempts to contact the corresponding authors to obtain unpublished data. For studies that presented median and interquartile range (IQR) for continuous outcomes, we used methods recommended by Luo et al. [65] and Wan et al. [66] to estimate the mean and standard deviation (SD) for meta-analysis, given that the data is normally distributed as examined using methods proposed by Shi et al. [67]. We did not include skewed median and IQR values in the meta-analysis and elected to describe these results narratively.
Risk of bias assessment
The risk of bias in our included RCTs were assessed independently and in duplicate using version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB2) [68]. The risk of bias of non-randomized comparative studies was assessed in duplicate using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool [69]. All disagreements were referred to a senior author for resolution.
Quality of evidence
We assessed the quality of evidence for our outcomes using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [70,71]. A summary of the meta-analysis outcomes and GRADE ratings were presented in a GRADE summary of findings table [72] generated using GRADEpro (https://gradepro.org/) [73].
Statistical analysis
All statistical analyses were conducted using R v4.1.1 (https://www.r-project.org/) [74]. For outcomes with more than two studies presenting eligible data, we performed random-effects meta-analyses using the meta v4.19 library (https://cran.r-project.org/web/packages/meta/) [75]. For dichotomous/incidence outcomes, we expressed the treatment effect as odds ratios (ORs) and 95% CIs for data pooling. The treatment effects of continuous outcomes were expressed as mean differences (MDs) and 95% CIs. Results from individual studies and data synthesis were displayed on forest plots.
Zero event studies
For studies reporting zero events in one or both of its treatment arms, we applied treatment arm continuity correction (TACC) [76] to complete the meta-analysis.
Heterogeneity assessment
The presence of heterogeneity was examined using the Cochran's Q test [77] with a significance level of p < 0.10 as recommended by the Cochrane Handbook [78]. Heterogeneity was subsequently quantified using I2 statistics [77,79]. We interpreted 30% <I2 <75% as moderate heterogeneity and I2 ≥75% as serious heterogeneity, as per recommendations from the Cochrane Handbook [78].
Publication bias & small study effects
We created funnel plots for outcomes with 10 or more included studies to identify small study effects within the meta-analysis as an indication for publication bias [78]. Egger's regression test was used to quantitatively evaluate the presence of asymmetry within the funnel plots [80]. We did not draw funnel plots nor conduct Egger's regression test for outcomes with fewer than 10 included studies as Egger's test does not have sufficient power in these circumstances [81].
Meta-regression & subgroup analyses
We performed meta-regression analyses on the proportion of patients with severe disease (defined as per individual investigators' criteria), mean baseline sequential organ failure assessment (SOFA) scores, and follow-up duration for mortality.
Furthermore, we performed the following subgroup analyses: corticosteroid regimens, corticosteroid dose, with methylprednisolone equivalent dose of ≤1.5 mg/kg/day or ≤100 mg/day defined as low dose and methylprednisolone equivalent dose of >1.5 mg/kg/day or >100 mg/day defined as high dose, similar to the threshold set in previous studies [82,83], time from hospitalization to corticosteroid administration, with a time to administration ≤3 days defined as early/immediate administration, and a time to administration >3 days defined as late/delayed administration. Additionally, we performed subgroup analyses by study design (randomized studies vs observational studies) and risk of bias (low/some concerns/moderate risk of bias vs high/serious/critical risk of bias) to examine the impact of different study methodologies.
Results
Included studies
We identified and screened 9865 potentially eligible titles and abstracts (following deduplication using Endnote 20 [https://endnote.com/]). A total of 250 full-text articles were subsequently retrieved and screened. After screening, 10 RCTs [28–30,84–90] and 85 observational studies [83,91–174] were included in this systematic review and meta-analysis (Figure 1), with a total of 42,205 hospitalized COVID-19 patients. In the article selection and data extraction process, we contacted the corresponding authors from seven studies [95,147,149,155,175–177] with unclear or missing data and two authors [149,155] responded to our inquiries. Of the remaining studies, two [95,147] were included with a subset of the reported outcomes without response from authors, and three studies [175–177] were excluded due to a lack of relevant outcomes/insufficient data.
Across all included studies, 37 studies (38.9%) [84,86,89,90,93,94,99,101,104,106,107,112,115,119,121,125,126,130,136,138,142,143,146,147,152–155,161,162,165,167–170,172,174] included treatment arms that assessed methylprednisolone, 10 studies (10.5%) [28,30,88,100,105,114,117,125,141,171] included treatment arms that assessed dexamethasone, 3 studies (3.2%) [29,85,87] administered hydrocortisone, 1 study (1.1%) [92] administered prednisone and 1 study (1.1%) [145] administered prednisolone. The study by Ko et al. [125] had two corticosteroid arms, with one arm receiving dexamethasone and the other arm receiving methylprednisolone. The remaining studies did not specify the exact corticosteroids used and/or only provided equivalent dosages. In terms of adjuvant therapies, 4 studies [93,113,150,160] included tocilizumab as adjuvants, while 1 study [135] used interferon-α2b as an adjuvant.
Forty-six studies (48.4%) [28,83,84,86,90,91,96,98,100,101,104,107–110,112,115–117,120–122,124,130,132,135,137,139,141,143–145,150,151,154,156–158,164,165,168–170,172,173] included all hospitalized COVID-19 patients, 20 studies (21.1%) [87,93,97,99,102,103,126,131,133,134,136,138,142,146,147,152,155,160,163,174] only included hospitalized patients with severe disease, while 6 studies (6.3%) [88,92,106,111,119,128] included hospitalized patients without severe disease. Last, 22 studies (23.2%) [29,30,85,89,94,105,113,114,118,123,125,127,129,140,148,149,153,159,161,162,167,171] only included ICU hospitalized patients, and 1 study (1.1%) [166] only included hospitalized patients receiving mechanical ventilation. Characteristics of the included studies are tabulated in Supplementary Table 12.
Risk of bias
The risk of bias in the included RCTs was evaluated using RoB2 (Figure 2A & Supplementary Table 10). Seven RCTs (70.0%) [29,30,85,86,88–90] were rated as having some concerns in regards to their risk of bias, mainly due to the use of open-label study designs. The remaining 3 RCTs [28,84,87] were rated as having a low risk of bias. No RCT was rated as having a high risk of bias.
Figure 2. . Risk of bias.
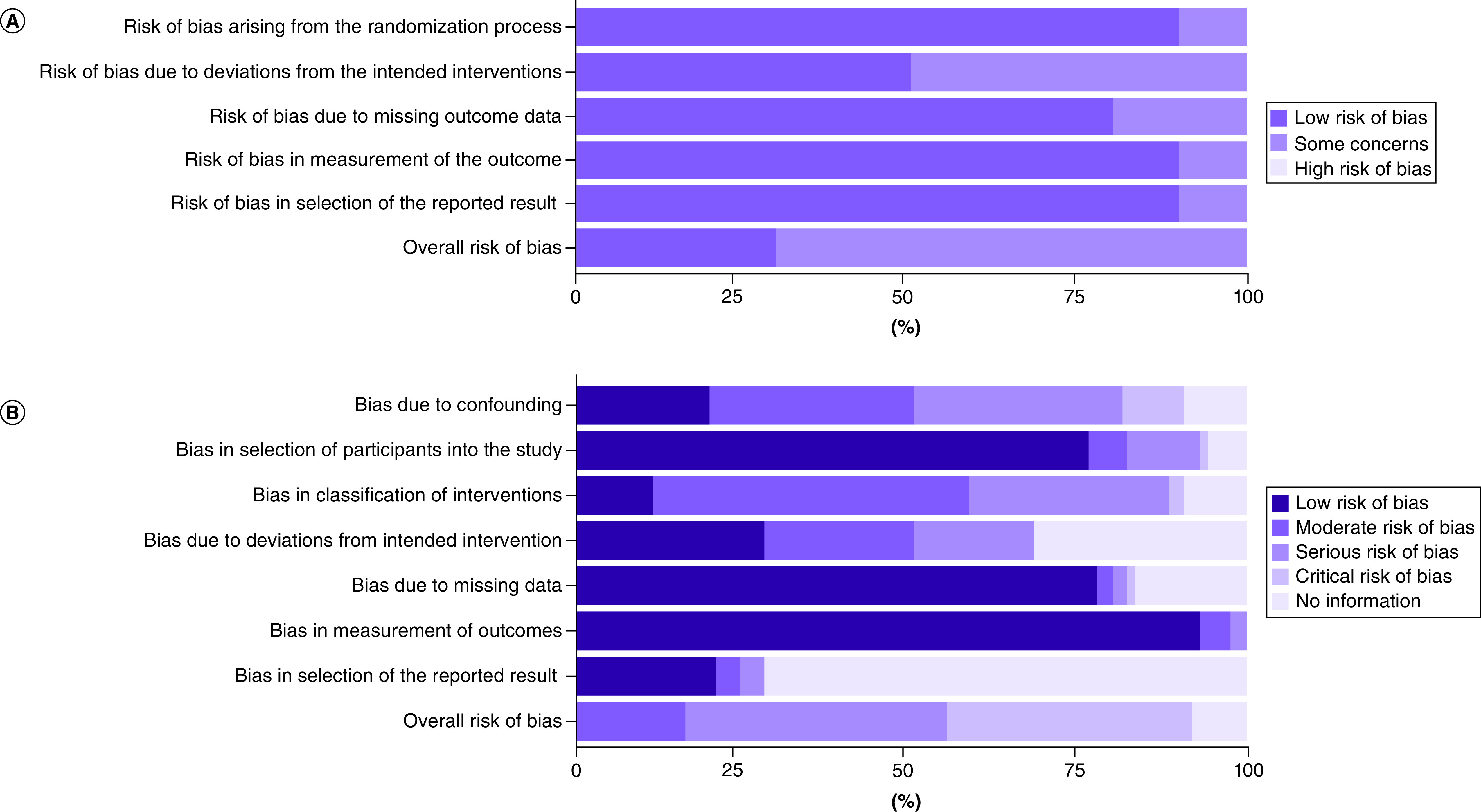
(A) Percentage of studies with risk of bias ratings for randomized controlled trials using RoB2. (B) Percentage of studies with risk of bias ratings for observational studies using ROBINS-I.
ROBINS-I: Risk of bias in non-randomized studies of interventions; RoB2: Revised Cochrane risk of bias tool for randomized trial.
The risk of bias in our included observational studies were assessed using ROBINS-I (Figure 2B & Supplementary Table 11). Thirty-one studies (36.5%) [92,95,96,98,99,103,105–107,109,112,115,118,120,121,126,133,135,139,143,149,150,152,156,160,165,166,170,172–174] were rated as having critical risk of bias, and 33 studies (38.8%) [91,94,97,100,101,108,110,114,117,119,124,125,128–132,134,136,138,140,141,144–147,158,161,163,164,167–169] were rated as having serious risk of bias. Generally, studies were rated as having a critical or serious risk of bias due to confounding factors and poor descriptions of the regimens/interventions used. Fourteen studies (16.5%) [93,102,104,111,113,116,122,137,142,151,154,155,157,162] were rated as having moderate risk of bias, with no studies receiving a rating of low risk of bias. The remaining 7 studies (8.2%) [83,123,127,148,153,159,171] were rated as ‘no information’ due to insufficient reporting of study methodology, treatment descriptions and baseline information.
Treatment outcomes
Time to negative test conversion
Nineteen studies [86,102,106,112,119,121,128,129,131–133,136–138,164,165,169,170,172] with 2785 patients evaluated the effect of corticosteroid administration on time to negative test conversion and were included in the meta-analysis (Figure 3). The overall pooled MD was 1.80 (95% CI: 0.48–3.11) with serious and significant heterogeneity (I2 = 78%; PQ <0.01). There was no noticeable asymmetry on the funnel plot (Supplementary Figure 1), and Egger's regression test showed no significant small-study effects (PEgger = 0.82). There were no significant subgroup differences between the pooled results from observational studies versus RCTs (p = 0.26; Supplementary Figure 2). Similarly, there were no significant subgroup differences between pooled results from high risk of bias studies compared with low risk of bias studies (p = 0.77; Supplementary Figure 3).
Figure 3. . Pooling of mean differences for the outcome of time to negative conversion of SARS-CoV-2 test.
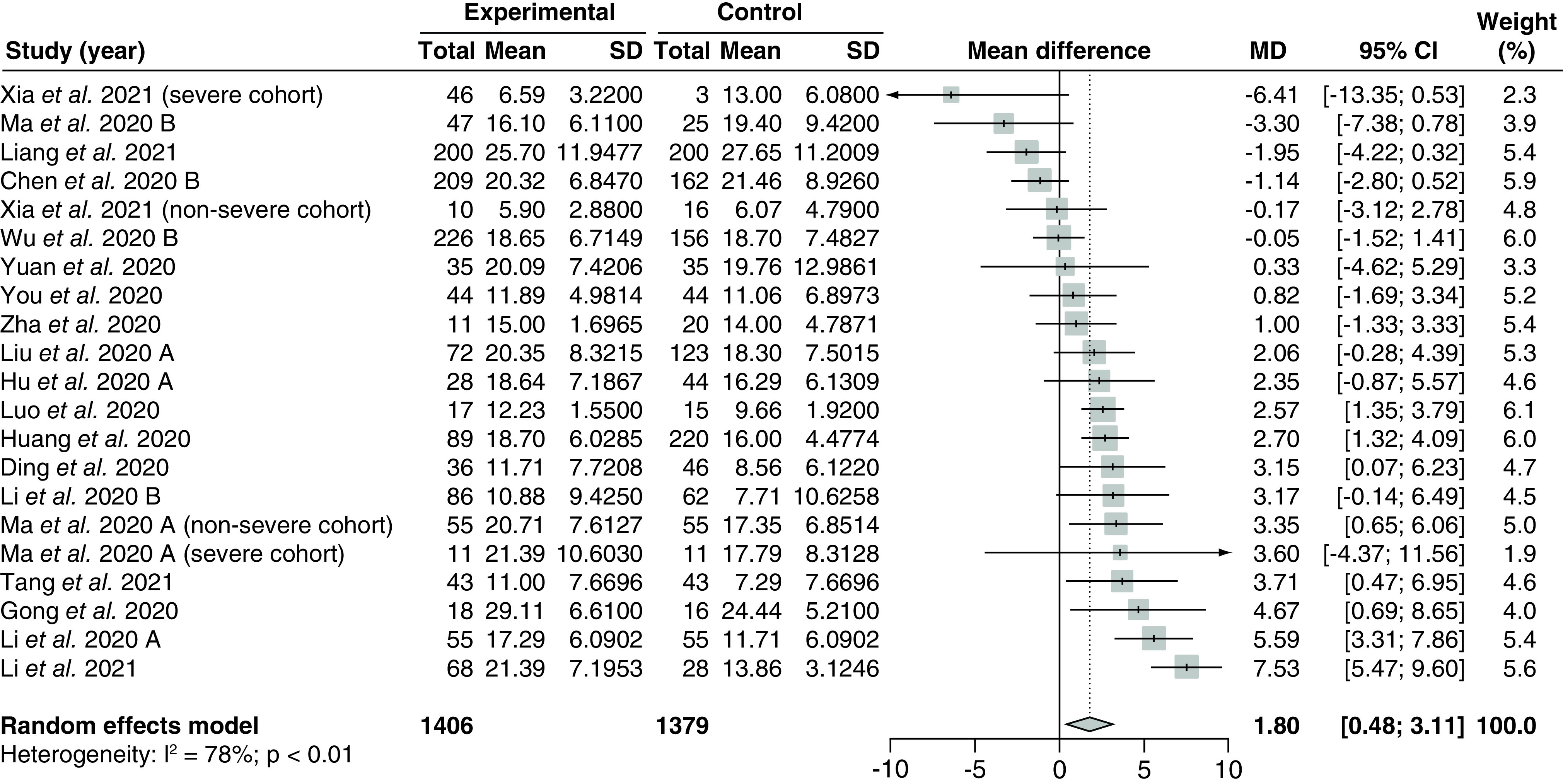
The use of corticosteroids was compared with control groups. Heterogeneity was quantified using I2 statistics. MD <0 indicates beneficial treatment effects of corticosteroids compared with control groups.
MD: Mean difference; SD: Standard deviation.
Ten studies [86,106,112,119,121,136,165,169,170,172] assessing 879 patients compared methylprednisolone versus standard of care, while the remaining studies did not disclose the exact corticosteroid regimens used (Supplementary Figure 4). Including only studies that administered methylprednisolone, the pooled MD was 1.81 (95% CI: 0.33–3.29) with substantially decreased heterogeneity compared with the overall analysis (I2 = 32%; PQ = 0.15). There were no significant subgroup differences between subgroups of studies assessing low fixed-dose regimens versus low weight-adjusted dose regimens (p = 0.53; Supplementary Figure 5), nor were there significant subgroup differences between subgroups of studies providing early administration of corticosteroids versus late administration of corticosteroids (p = 0.95; Supplementary Figure 6). Meta-regression analyses did not demonstrate significant correlations between the treatment effect and the proportion of severe patients (p = 0.70; Supplementary Figure 7) or baseline mean SOFA scores (p = 0.53; Supplementary Figure 8).
Three observational studies [120,122,143] reported median time to negative test conversion with IQR, however, these data could not be imputed due to a lack of normality. Based on the Kruskal–Wallis H test, the Mann-Whitney U test and the Student's t-test, all three studies reported no significant differences in the duration to negative test conversion in patients administered corticosteroids versus control.
Length of stay
Thirty studies [86,88,90,92–94,98,102–104,107,115,116,122,126,128,134,135,138,144,147,151,162,164–167,170,172,173] assessing 10,454 patients compared the effect of corticosteroids versus control on length of stay and were included in the meta-analysis (Figure 4). The overall pooled MD was 1.60 (95% CI: -0.20–3.40) with serious and significant heterogeneity (I2 = 91%; PQ <0.01). There was no funnel plot asymmetry based on visual inspection (Supplementary Figure 9), and Egger's regression test showed no significant small-study effects (PEgger = 0.36).
Figure 4. . Pooling of mean differences for the outcome of length of stay.
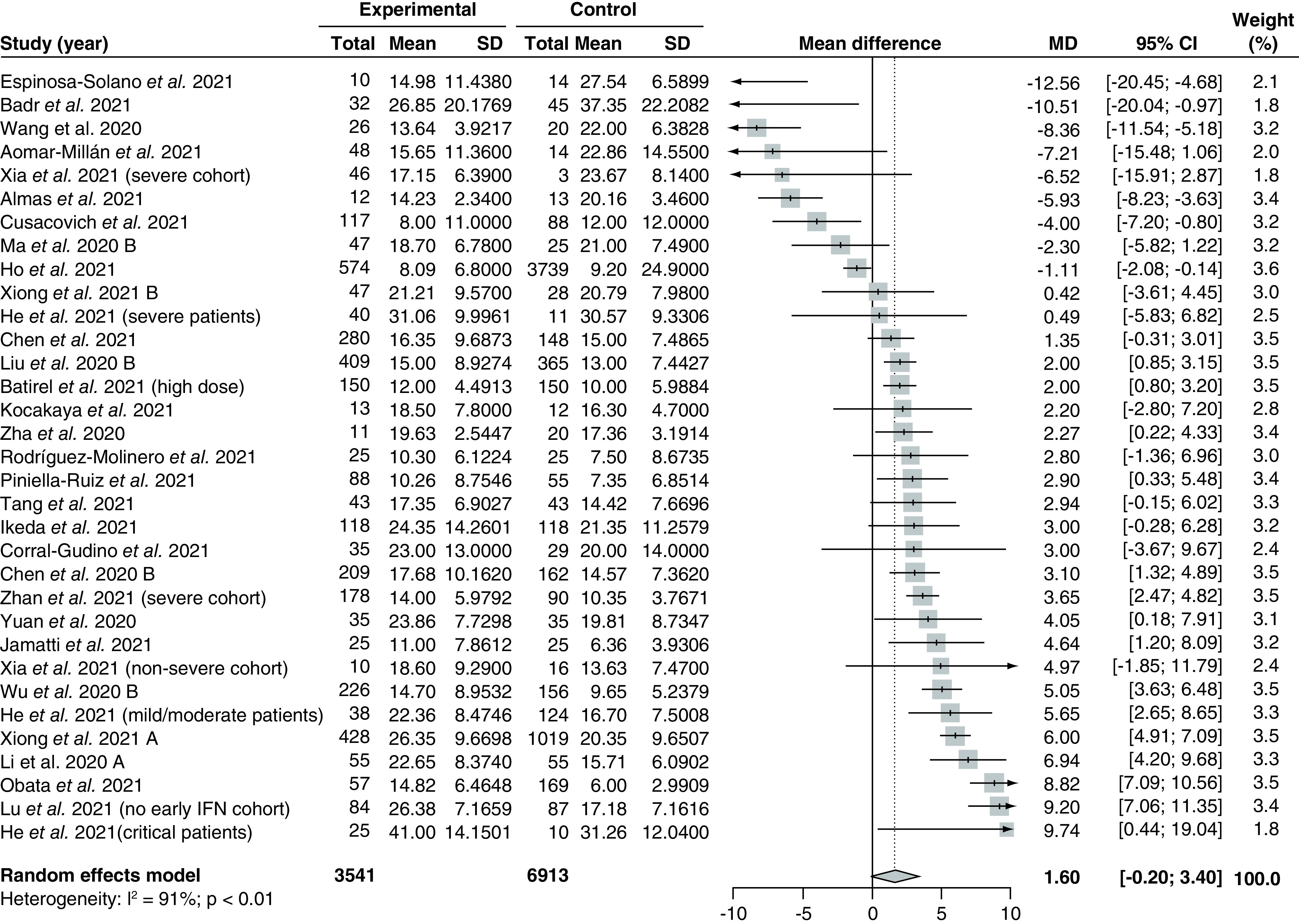
The use of corticosteroids was compared with control groups. Heterogeneity was quantified using I2 statistics. MD <0 indicates beneficial treatment effects of corticosteroids compared with control groups.
IFN: Interferon; MD: Mean difference; SD: Standard deviation.
Subgroup analysis by different study design identified significant between-group differences between the pooled treatment effect from observational studies compared with RCTs (p < 0.05; Supplementary Figure 10). The pooled MD based on 27 observational studies [92–94,98,102–104,107,115,116,122,126,128,134,135,138,144,147,151,162,164–167,170,172,173] was 1.38 (95% CI: -0.62–3.38) with no reduction in heterogeneity (I2 = 92%; PQ <0.01), while the pooled MD based on 3 RCTs [86,88,90] was 3.62 (95% CI: 1.10–6.15) with no heterogeneity (I2 = 0%; PQ = 0.75). There were no significant subgroup differences between pooled results from high risk of bias studies compared with low risk of bias studies (p = 0.13; Supplementary Figure 11).
In addition, there were significant between-group differences between studies using different types of corticosteroids (p < 0.01; Supplementary Figure 12). One study [88] administered dexamethasone and reported the highest MD in length of stay (MD 4.64 [95% CI: 1.20–8.09]). Fourteen studies [86,90,94,104,107,115,126,147,151,162,165,167,170,172] administered methylprednisolone and reported a pooled MD of 0.28 (95% CI: -2.65–3.22). Last, one study [92] administered prednisone and reported the lowest MD of -5.93 (95% CI: -8.23 to -3.63). The remaining studies did not report the exact corticosteroid regimen used in the study.
In terms of subgroup analysis by dosage, there were no significant between-group differences in the pooled MD from studies administering high fixed-dose, high weight-adjusted dose, low fixed-dose, or low weight-adjusted doses of corticosteroids (p = 0.54; Supplementary Figure 13). There were also no significant differences between the pooled MD from studies with early corticosteroids administration versus studies with late corticosteroid administration (p = 0.14; Supplementary Figure 14). Meta-regression analyses did not demonstrate significant correlations between the treatment effect and the proportion of patients with severe disease (p = 0.08; Supplementary Figure 15) or baseline mean SOFA scores (p = 0.28; Supplementary Figure 16).
ICU length of stay
Seven studies [88,98,113,125,140,162,167] assessing 3233 ICU hospitalized patients compared the effect of corticosteroids on ICU length of stay compared with control and were included in the meta-analysis (Figure 5A). The overall pooled MD was -0.01 (95% CI: -3.50–3.47) with serious and significant heterogeneity (I2 = 93%; PQ <0.01). We did not draw the funnel plot and conduct Egger's regression test as there were less than 10 included studies.
Figure 5. . Pooling of mean differences for intensive care unit length of stay and odds ratio for incidence of intensive care unit admission.
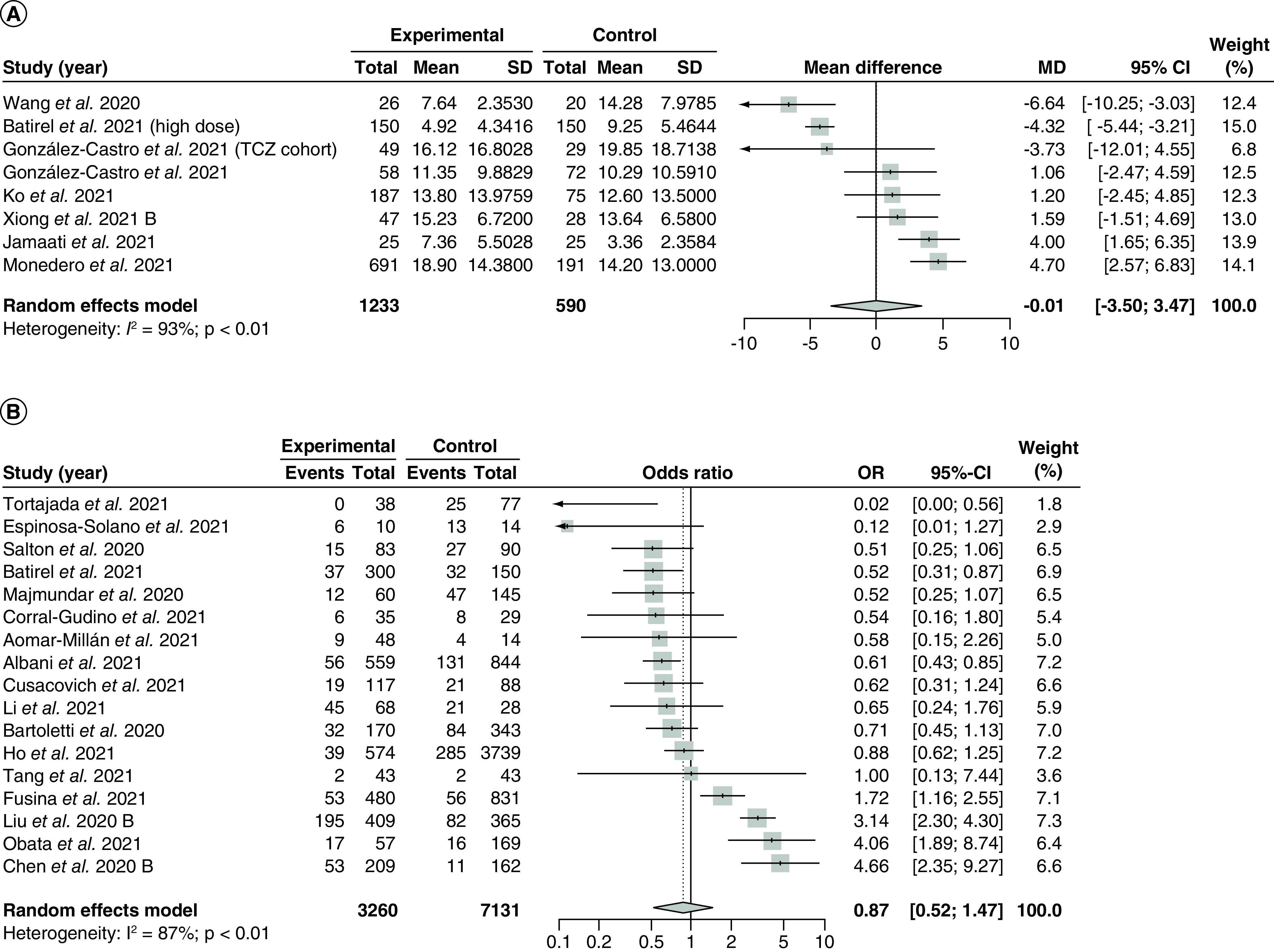
The use of corticosteroids was compared with control groups. Heterogeneity was quantified using I2 statistics. (A) Forest plot for the pooling of MDs for ICU length of stay. MD <0 indicates beneficial treatment effects of corticosteroids compared with control groups. (B) Forest plot for the pooling of ORs for incidence of ICU admission. OR <1 indicates beneficial treatment effects of corticosteroids compared with control groups.
MD: Mean difference; OR: Odds ratio; SD: Standard deviation; TCZ: Tocilizumab.
Subgroup analysis by study design revealed significant differences between the overall MD from observational studies versus RCTs (p < 0.05; Supplementary Figure 17). Across the 6 observational studies [98,113,125,140,162,167], the pooled MD was -0.66 (95% CI: -4.48–3.16) with no reduction in heterogeneity (I2 = 92%; PQ <0.01). Only one study [88] was included in the RCT subgroup, which reported a MD of 4.00 (95% CI: 1.65–6.35). There were no significant subgroup differences between pooled results from high risk of bias studies compared with low risk of bias studies (p = 0.15; Supplementary Figure 18).
There were no significant differences between subgroup results from the subgroup analyses by corticosteroid type (p = 0.14; Supplementary Figure 19) or dose (high fixed-dose vs low weight-adjusted dose, p = 0.75; Supplementary Figure 20). However, there were significant subgroup differences between studies with early administration of corticosteroids versus studies with late administration of corticosteroids (p < 0.01; Supplementary Figure 21). Two studies administering early steroids yielded a pooled MD of 2.00 (-1.63–5.63) with a substantial reduction in heterogeneity compared with the overall meta-analysis (I2 = 0%; PQ = 0.75), while one study administering late corticosteroid yielded an MD of 10.50 (95% CI: 7.57–13.43). Last, meta-regression analyses did not reveal any significant correlations between the treatment effect and the proportion of patients with severe disease (p = 0.82; Supplementary Figure 22) or baseline mean SOFA scores (p = 0.87; Supplementary Figure 23).
Five studies [94,105,114,129,134] reported median length of ICU stay with IQR. However, as the data were significantly skewed, they could not be imputed and thus were not included in the meta-analysis. Based on the Mann-Whitney U test, two studies [94,129] did not find a significant difference between the median length of ICU stay in patients taking corticosteroids versus control, two studies [105,134] found that the length of ICU stay is significantly longer in patients taking corticosteroids versus control, and one study [114] found that the length of ICU stay is significantly longer in control patients versus patients taking corticosteroids.
ICU admission
Seventeen studies [86,90,91,93,97,98,102,104,107,110,116,131,134,139,144,155,157] with 10,391 patients compared the effect of corticosteroids versus control on incidence of ICU admission and were included in the meta-analysis (Figure 5B). The overall pooled OR was 0.87 (95% CI: 0.52–1.47) with serious and significant heterogeneity (I2 = 87%; PQ <0.01). Visual examination of the funnel plot (Supplementary Figure 24) and Egger's regression test showed no evidence of small-study effects (PEgger = 0.24). There were no significant between-group differences in the subgroup analysis by study design (p = 0.42; Supplementary Figure 25) and by risk of bias (p = 0.61; Supplementary Figure 26).
Five studies [86,90,104,107,155] with 552 patients assessed the efficacy of methylprednisolone versus standard of care. The remaining studies did not disclose the exact type of corticosteroid used. The pooled OR for studies using methylprednisolone was 0.53 (95% CI: 0.29–0.97) with no heterogeneity (I2 = 0%; PQ = 0.71; Supplementary Figure 27).
There were no significant differences between the subgroup results from studies using different dosages of corticosteroids (p = 0.36; Supplementary Figure 28), nor were there any differences between the subgroup results from studies administering early corticosteroids versus late corticosteroids (p = 0.43; Supplementary Figure 29). Last, meta-regression analyses did not reveal significant correlations between the treatment effect and the proportion of patients with severe disease (p = 0.52; Supplementary Figure 30) and baseline mean SOFA score (p = 0.29; Supplementary Figure 31).
Mortality
Eighty-six studies [28–30,83–94,96–99,101–108,110–125,127–129,131–135,137–142,144–164,166–169,171–174] with 40,623 patients compared the effect of corticosteroids versus control in terms of mortality incidence and were included in the meta-analysis (Figure 6). The overall pooled OR was 1.00 (95% CI: 0.84–1.20) with serious and significant heterogeneity (I2 = 76%; PQ <0.01). Visual inspection of the funnel plot (Supplementary Figure 32) and Egger's regression test revealed no evidence of small-study effects (PEgger = 0.44). Subgroup analyses showed that there were no significant differences in the pooled OR between studies of different designs (p = 0.27; Supplementary Figure 33) or between studies rated as high risk of bias versus studies with a low risk of bias (p = 0.17; Supplementary Figure 34).
Figure 6. . Pooling of odds ratios for the outcome of mortality incidence.
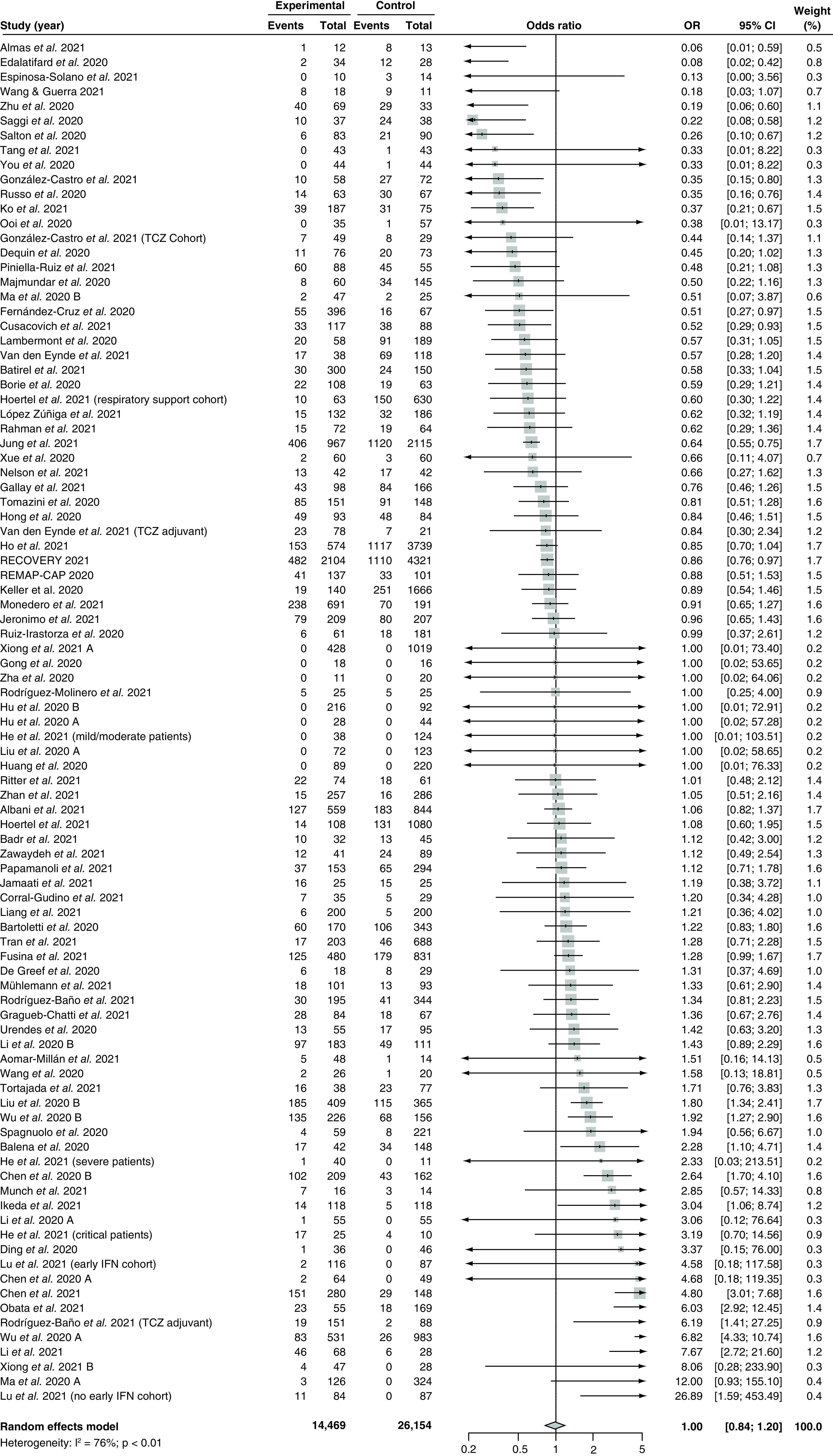
The use of corticosteroids was compared with control groups. Heterogeneity was quantified using I2 statistics.
OR <1 indicates beneficial treatment effects of corticosteroids compared with control groups.
IFN: Interferon; OR: Odds ratio; TCZ: Tocilizumab.
Subgroup analyses by different corticosteroid regimens revealed significant between-group differences (p < 0.05; Supplementary Figure 35). Eight studies [28,30,88,105,114,117,141,171] using dexamethasone yielded a pooled OR of 0.94 (95% CI: 0.78–1.13) with no heterogeneity (I2 = 0%; PQ = 0.72). Three studies [29,85,87] assessed the efficacy of hydrocortisone and yielded a pooled OR of 0.88 (95% CI: 0.11–6.84) with moderate heterogeneity (I2 = 55%; PQ = 0.11). Thirty studies [84,86,89,90,94,99,101,104,106,107,112,115,119,121,138,142,146,147,151–155,161,162,167–169,172,174] assessed the efficacy of methylprednisolone and yielded a pooled OR of 0.62 (95% CI: 0.45–0.85) with moderate heterogeneity (I2 = 36%; PQ <0.05). Last, one study [145] assessed the efficacy of prednisolone with an OR of 0.38 (95% CI: 0.01–13.17), and another [92] assessed the efficacy of prednisone with an OR of 0.06 (95% CI: 0.01–0.59).
There were no significant differences between the subgroup results from studies using different dosages of corticosteroids (p = 0.06; Supplementary Figure 36), nor were there any differences between the subgroup results from studies administering early corticosteroids versus late corticosteroids (p = 0.28; Supplementary Figure 37). Last, meta-regression analyses did not reveal significant correlations between the treatment effect and the proportion of patients with severe disease (p = 0.56; Supplementary Figure 38), baseline mean SOFA score (p = 0.48; Supplementary Figure 39), and follow-up duration (p = 0.14; Supplementary Figure 40).
Incidence of mechanical ventilation
Thirty-seven studies [28,86,88,89,95–98,100–105,107,113,116,122,124,130,131,134,136,138,139,141,144–146,148,155–158,162,164,167] with 20,453 patients compared the effect of corticosteroids versus control in terms of incidence of mechanical ventilation and were included in the meta-analysis (Figure 7A). The overall pooled OR was 1.19 (95% CI: 0.81–1.76) with serious and significant heterogeneity (I2 = 84%; PQ <0.01). Visual inspection of the funnel plot (Supplementary Figure 41) and Egger's regression test showed no evidence of small-study effects (PEgger = 0.90), and there were no significant between-group differences among studies using different study designs (p = 0.73; Supplementary Figure 42) or studies with high risks of bias versus studies with low risks of bias (p = 0.45; Supplementary Figure 43).
Figure 7. . Pooling of odds ratios for incidence of mechanical ventilation and mean differences for length of mechanical ventilation.
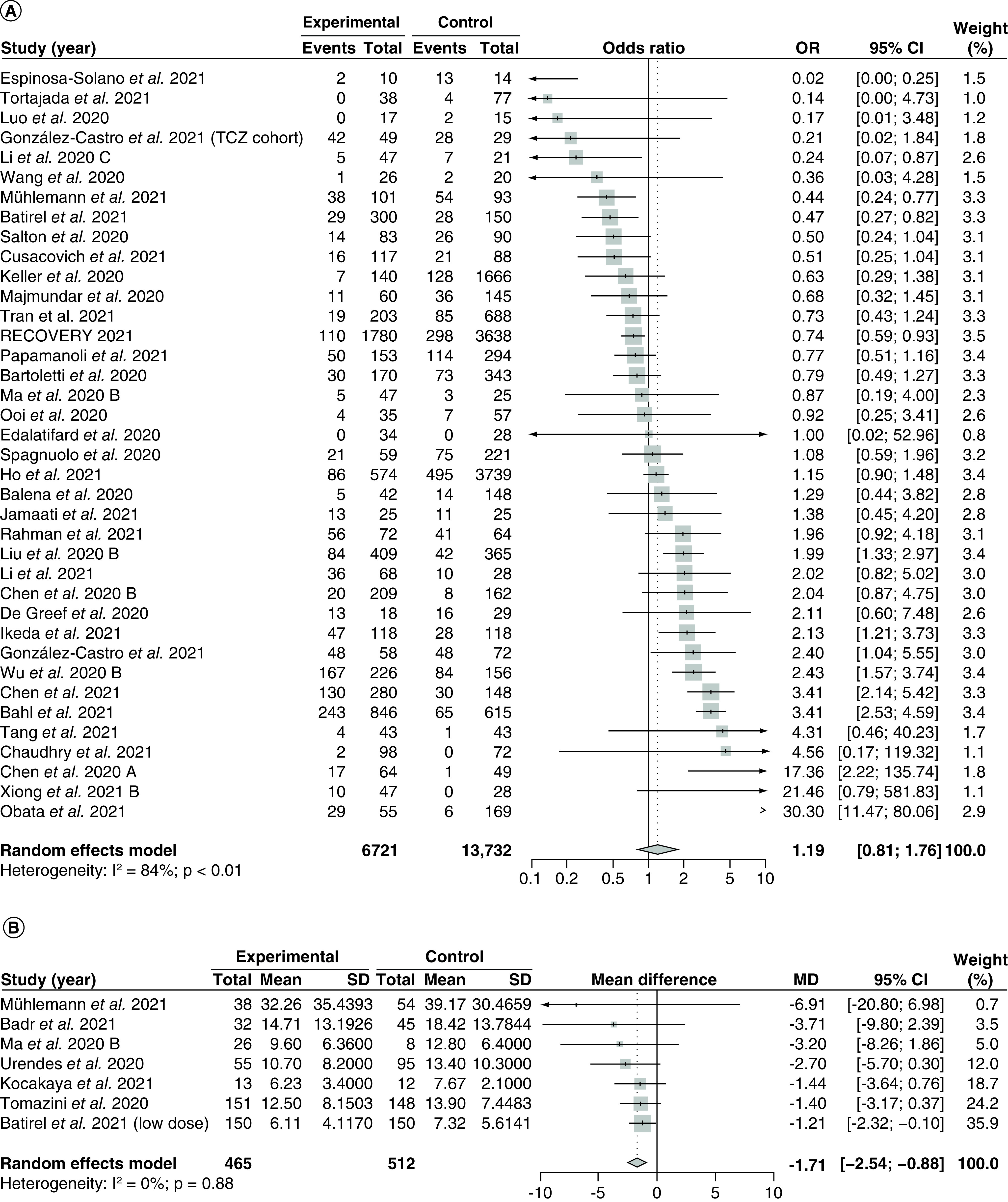
The use of corticosteroids was compared with control groups. Heterogeneity was quantified using I2 statistics. (A) Forest plot for the pooling of ORs for incidence of mechanical ventilation. OR <1 indicates beneficial treatment effects of corticosteroids compared with control groups. (B) Forest plot for the pooling of MDs for length of mechanical ventilation. MD <0 indicates beneficial treatment effects of corticosteroids compared with control groups.
MD: Mean difference; OR: Odds ratio; SD: Standard deviation; TCZ: Tocilizumab.
There were no significant differences between studies using different corticosteroid regimens (p = 0.95; Supplementary Figure 44), studies using different corticosteroid doses (p = 0.56; Supplementary Figure 45), or between studies administering early corticosteroids versus late corticosteroids (p = 0.89; Supplementary Figure 46). Last, meta-regression analyses revealed no significant correlations between the treatment effect and proportion of patients with severe diseases (p = 0.63; Supplementary Figure 47) and baseline mean SOFA score (p = 0.88; Supplementary Figure 48).
Length of mechanical ventilation
Seven studies [30,94,98,126,138,141,159] with 977 patients receiving mechanical ventilation compared the effect of corticosteroids versus control on the length of mechanical ventilation and were included in the meta-analysis (Figure 7B). Overall, the pooled MD was -1.71 (95% CI: -2.54 to -0.88) with no heterogeneity (I2 = 0%; PQ = 0.88). Because there were less than 10 included studies, we did not draw funnel plots nor did we conduct Egger's regression test. The subgroup analysis by study design (Supplementary Figure 49) and risk of bias (Supplementary Figure 50) showed no significant between-group differences (p = 0.67 for both subgroup analyses).
The subgroup analysis by different corticosteroid regimens (Supplementary Figure 51) and corticosteroid dosage (Supplementary Figure 52) also did not show significant between-group differences (p = 0.91 and p = 0.99 for regimen and dosage subgroups, respectively). Last, meta-regression analyses by the proportion of severe patients (Supplementary Figure 53) and baseline mean SOFA score (Supplementary Figure 54) did not reveal any significant correlations (p = 0.30 and p = 0.39 for the severe proportions and SOFA score regressions, respectively). We did not conduct subgroup analyses by time to administration due to a lack of data.
Five studies [87,114,122,129,155] reported median length of mechanical ventilation with IQR, however, they were not imputed and included in the meta-analysis due to skewed data. One study [87] did not perform any statistical testing, three studies [114,129,155] used the Mann-Whitney U test, and one study [122] used Student's t-test. Out of the four studies with statistical tests, one study [122] (using the t-test) did not find significant differences between the corticosteroid and control groups. Two studies [114,155] found a significantly shorter median length of mechanical ventilation in patients taking corticosteroids, and one study [129] found a significantly shorter median length of mechanical ventilation in the control patients. The study that did not conduct statistical tests [87] reported a median duration of mechanical ventilation of 15 h (IQR: 13–21 h) in the control group, compared with a median duration of 6 h (IQR: 4–20 h) in the corticosteroids group.
Adverse events
Nine studies [30,96,102,111,133,140,155,158,172] with 3409 patients reported incidence of adverse events in the corticosteroid and control groups (Figure 8A). The overall pooled OR was 1.89 (95% CI: 0.98–3.63) with moderate and significant heterogeneity (I2 = 71%; PQ <0.01). There were significant differences between the treatment effect from observational studies versus RCTs (p < 0.01; Supplementary Figure 55), where 8 observational studies [96,102,111,133,140,155,158,172] yielded a pooled OR of 2.17 (95% CI: 1.24–3.81) with similar heterogeneity compared with the overall analysis, and 1 RCT [30] reported an OR of 0.43 (95% CI: 0.14–1.26). In terms of risk of bias, there were no significant between-group differences in the subgroup analysis (p = 0.46; Supplementary Figure 56). We did not draw funnel plots nor conducted Egger's regression tests because less than 10 studies were included in the analysis.
Figure 8. . Pooling of odds ratios for all safety outcomes.
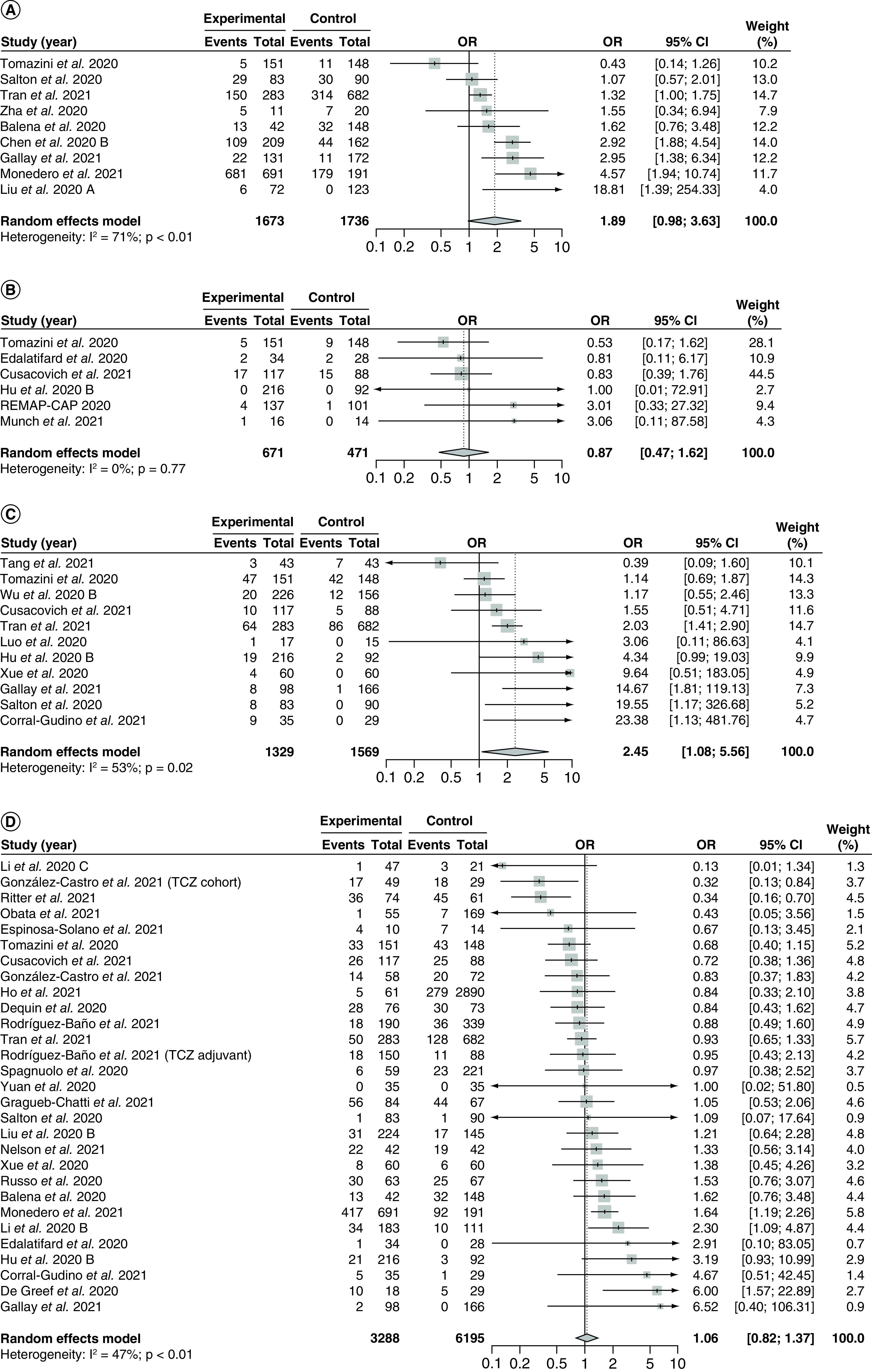
The use of corticosteroids was compared with control groups. Heterogeneity was quantified using I2 statistics. (A) Forest plot for the pooling of ORs for incidence of adverse events. (B) Forest plot for the pooling of ORs for incidence of serious adverse events. (C) Forest plot for the pooling of ORs for incidence of hyperglycemia. (D) Forest plot for the pooling of ORs for incidence of nosocomial infections.
OR <1 indicates beneficial treatment effects of corticosteroids compared with control groups for all safety outcomes.
OR: Odds ratio; TCZ: Tocilizumab.
There were no significant between-group differences by corticosteroid regimen (p = 0.08; Supplementary Figure 57), corticosteroid dosage (p = 0.05; Supplementary Figure 58) and time to administration (p = 0.79; Supplementary Figure 59). Meta-regression analysis showed no significant correlation associated with baseline mean SOFA score (p = 0.74; Supplementary Figure 60). We did not conduct meta-regression analysis by the proportion of patients with severe disease due to a lack of data.
Severe adverse events
Six studies [29,30,87,89,104,120] with 1142 patients reported incidence of severe adverse events (Figure 8B). The pooled OR was 0.87 (95% CI: 0.47–1.62) with no heterogeneity (I2 = 0%; PQ = 0.77). There were no significant between-group differences in the subgroup analysis by study design (p = 0.74; Supplementary Figure 61) or risk of bias (p = 0.96; Supplementary Figure 62). We did not draw funnel plots or conduct Egger's regression test as less than 10 studies were included.
Subgroup analyses by corticosteroid regimen (Supplementary Figure 63) and corticosteroid dosage (Supplementary Figure 64) revealed significant between-group differences (p < 0.01 for both subgroup analyses). Two studies [89,104] used high fixed-dose methylprednisolone and yielded an OR of 0.83 (95% CI: 0.77–0.89) with no heterogeneity (I2 = 0%; PQ = 0.99), while two studies used low fixed-dose hydrocortisone [29,87] and yielded an OR of 3.02 (95% CI: 2.73–3.34) with no heterogeneity (I2 = 0%; PQ = 0.99). One study [120] used a low weight-adjusted dose of unspecified corticosteroid and reported an OR of 1.00 (95% CI: 0.01–72.91), and one study [30] compared dexamethasone versus standard of care and reported an OR of 0.53 (95% CI: 0.17–1.62). There were no between-group differences for subgroup analysis by time to administration (p = 0.73; Supplementary Figure 65), and we did not perform any meta-regression analysis due to a lack of data.
Hyperglycemia
Eleven studies [30,86,90,104,111,120,136,155,158,164,168] with 2898 patients reported incidence of hyperglycemia (Figure 8C) with a pooled OR of 2.45 (95% CI: 1.08–5.56). There was significant and moderate heterogeneity (I2 = 53%; PQ = 0.02). Visual inspection of the funnel plot (Supplementary Figure 66) and Egger's regression test showed no significant small study effects (PEgger = 0.18). No subgroup analysis showed significant between-group differences (study design [p = 0.60; Supplementary Figure 67], risk of bias [p = 0.67; Supplementary Figure 68], corticosteroid regimen [p = 0.16; Supplementary Figure 69], corticosteroid dosage [p = 0.69; Supplementary Figure 70] and time to administration [p = 0.69; Supplementary Figure 71]), and meta-regression analyses showed no correlation between the treatment effect and proportion of severe patients (p = 0.42; Supplementary Figure 72) and baseline mean SOFA score (p = 0.95; Supplementary Figure 73).
Nosocomial infection
Twenty-seven studies [30,85,89,90,96,104,105,107,111,113,114,116,120,129,130,134,140,142,144,149,150,153,155,156,158,168,170] with 9483 patients reported incidence of nosocomial infection (Figure 8D) with a pooled OR of 1.06 (95% CI: 0.82–1.37). There was significant and moderate heterogeneity (I2 = 47%; PQ <0.01). Visual inspection of the funnel plot (Supplementary Figure 74) and Egger's regression test showed no significant small study effects (PEgger = 0.99). No subgroup analysis showed significant between-group differences (study design [p = 0.89; Supplementary Figure 75], risk of bias [p = 0.27; Supplementary Figure 76], corticosteroid regimen [p = 0.70; Supplementary Figure 77], corticosteroid dosage [p = 0.25; Supplementary Figure 78] and time to administration [p = 0.06; Supplementary Figure 79]), and meta-regression analyses showed no correlation between the treatment effect and proportion of severe patients (p = 0.72; Supplementary Figure 80) and baseline mean SOFA score (p = 0.24; Supplementary Figure 81).
Quality of evidence
The summary of findings for primary outcomes is tabulated in Table 1.
Table 1. . Summary of findings, corticosteroid regimens compared with standard of care/adjuvant therapies for the management of hospitalized COVID-19 patients.
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects (95% CI)† | Patients, n (studies, n) | Quality of evidence (GRADE) | ||
|---|---|---|---|---|---|---|
| Risk without corticosteroids | Risk with corticosteroids | Risk difference (95% CI) | ||||
| Time to negative test conversion | – | The mean time in the control group was 15 days | – |
MD 1.80 more days (0.48 more to 3.11 more) |
2,785 (1 RCT, 18 OSs) |
⊕◯◯◯ Very low‡,§,¶ |
| Length of stay | – | The mean time in the control group was 16 days | – |
MD 1.60 more days (0.20 fewer to 3.40 more) |
10,454 (3 RCTs, 27 OSs) |
⊕◯◯◯ Very low‡,§,¶,# |
| ICU length of stay | – | The mean time in the control group was 12 days | – |
MD 0.01 fewer days (3.50 fewer to 3.47 more) |
3,233 (1 RCT, 6 OSs) |
⊕◯◯◯ Very low‡,§,¶,# |
| Incidence of ICU admission |
OR 0.87 (0.52–1.47) |
121 per 1000 |
107 per 1000 (67–168) |
14 fewer per 1000 (54 fewer to 47 more) |
10,391 (2 RCTs, 15 OSs) |
⊕◯◯◯ Very low‡,§,¶,# |
| Mortality |
OR 1.00 (0.84–1.20) |
235 per 1000 |
235 per 1000 (205–269) |
0 fewer per 1000 (30 fewer to 34 more) |
40,623 (10 RCTs, 76 OSs) |
⊕◯◯◯ Very low‡,§,¶,# |
| Incidence of mechanical ventilation |
OR 1.19 (0.81–1.76) |
139 per 1000 |
161 per 1000 (116–221) |
22 more per 1000 (23 fewer to 82 more) |
20,453 (4 RCTs, 33 OSs) |
⊕◯◯◯ Very low‡,§,¶,# |
| Length of mechanical ventilation | – | The mean time in the control group was 6 days | – |
MD 1.71 fewer days (2.54 fewer to 0.88 fewer) |
977 (1 RCT, 6 OSs) |
⊕◯◯◯ Very low‡,§ |
| Incidence of adverse events |
OR 1.89 (0.98–3.63) |
362 per 1000 |
517 per 1000 (512–828) |
155 more per 1000 (5 fewer to 311 more) |
3,409 (1 RCT, 8 OSs) |
⊕◯◯◯ Very low‡,§,# |
| Incidence of severe adverse events |
OR 0.87 (0.47–1.62) |
57 per 1000 |
50 per 1000 (27–89) |
7 fewer per 1000 (30 fewer to 32 more) |
1,142 (4 RCTs, 2 OSs) |
⊕⊕⊕◯ Moderate# |
| Incidence of hyperglycemia |
OR 2.45 (1.08–5.56) |
99 per 1000 |
212 per 1000 (106–379) |
113 more per 1000 (7 more to 280 more) |
2,898 (3 RCTs, 8 OSs) |
⊕⊕◯◯ Low‡,§,†† |
| Incidence of nosocomial infections |
OR 1.06 (0.82–1.37) |
150 per 1000 |
158 per 1000 (126–195) |
8 more per 1000 (24 fewer to 45 more) |
9,483 (4 RCTs, 23 OSs) |
⊕◯◯◯ Very low‡,§,# |
Bold values represent the most important metrics.
GRADE Working Group quality of evidence rating [70].
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Quality of study was rated as low prior to downgrading or upgrading as a majority of the included studies were observational studies.
Downgraded due to study limitations; a majority of included studies were rated as having serious or critical risk of bias according to ROBINS-I and/or RoB2.
Downgraded due to inconsistency; significant and severe heterogeneity was observed in the analysis.
Downgraded due to imprecision; confidence intervals could not rule out the possibility of no effect (crosses null).
Upgraded due to a large magnitude of effect.
GRADE: Grading of recommendations, assessment, development and evaluation; MD: Mean difference; OR: Odds ratio; OS: Observational study; RCT: Randomized controlled trial.
Discussions
Main findings
The current systematic review and meta-analysis included 10 RCTs and 85 observational studies involving 42,205 hospitalized COVID-19 patients, making it the most comprehensive systematic review available on the use of corticosteroid for treating COVID-19. We found that the use of corticosteroid regimens was not significantly associated with changes in length of ICU hospitalization, nor incidence of mechanical ventilation.
While the overall meta-analysis showed that corticosteroids had no impact on length of stay, the pooled MD from RCT studies showed that corticosteroids may significantly increase the length of stay by 3.62 days, while the 95% CI for the pooled MD from observational studies narrowly crossed the line of no effect. The overall meta-analysis also showed that corticosteroids had no impact on mortality nor incidence of ICU admission, but subgroup analysis by corticosteroid type showed that methylprednisolone may confer a significant survival benefit with a 38% reduction in the odds of death compared with standard of care, while other regimens such as dexamethasone and hydrocortisone conferred no significant survival benefit. Additionally, methylprednisolone may be associated with a significant 47% reduction in the incidence of ICU admission. Across mechanically ventilated patients, we found that corticosteroids significantly reduced length of mechanical ventilation. However, corticosteroids may also significantly increase time to negative conversion of SARS-CoV-2 tests, which could suggest that corticosteroid administration hinders viral clearance.
In terms of safety, the use of corticosteroids was not associated with a significant increase in the odds of adverse events or severe adverse events. However, the pooled OR from observational studies showed that corticosteroids may increase the odds of adverse events by 117%. We also found a significant difference in the odds of serious adverse events between studies using high fixed-dose methylprednisolone versus low fixed-dose hydrocortisone, with methylprednisolone being associated with a significant, 17% reduction in the odds of serious adverse events while hydrocortisone was associated with a significant 202% increase in the odds of serious adverse events. While assessing the odds of hyperglycemia and nosocomial infection – both common adverse events associated with corticosteroids – we found that corticosteroids significantly increased the odds of hyperglycemia by 145%, however, there were no significant increases in nosocomial infection associated with corticosteroids.
Clinical implications for corticosteroid efficacy
In this review, we found that corticosteroids had no protective effect against the incidence and duration of ICU hospitalization nor incidence of progression to mechanical ventilation. These findings are consistent with previous studies assessing the use of corticosteroids in other coronavirus infections such as SARS-CoV and MERS-CoV [178], demonstrating that corticosteroids may not be effective for preventing progression from mild-and-moderate COVID-19 to severe disease, nor is it effective for managing ICU-hospitalized patients. In fact, corticosteroids may significantly increase the length of stay for hospitalized patients (according to pooling of RCT studies) and increase time to viral clearance, potentially placing more strain on limited hospital resources and increasing the risk of viral transmission. As previously speculated, using immunosuppressive therapies indiscriminately for viral infections such as COVID-19 may complicate disease courses and perpetuate illness [179].
Interestingly, while we found that corticosteroids had no significant impact on the incidence of mortality and ICU admission, methylprednisolone appears to have an exceptional efficacy for reducing the odds of death compared with other corticosteroids such as dexamethasone and hydrocortisone. Previous studies, including a triple-blinded RCT [180] and an observational cohort study [125], also found that methylprednisolone may be more efficacious at reducing mortality compared with dexamethasone. In addition, when the meta-analysis is restricted to studies using methylprednisolone only, it was found that methylprednisolone may also significantly reduce incidence of ICU admissions. It was hypothesized that these observations were due to methylprednisolone having greater lung tissue penetration compared with other corticosteroids [181,182], making methylprednisolone a more optimal corticosteroid for treating hyperinflammatory reactions to SARS-CoV-2 and respiratory complications. However, we suspect that over-precision associated with the large quantity of observational studies in the methylprednisolone subgroup may have contributed to our finding as well [183]. Thus we cannot definitively conclude that methylprednisolone is superior compared with other corticosteroids for reducing mortality or ICU admission. Currently, most published RCTs focus on dexamethasone and hydrocortisone [28,29], but less frequently on methylprednisolone. Further investigations using large scale, high-quality RCTs are required to establish the potential impact of methylprednisolone on mortality and ICU admission.
A main finding of our review is that corticosteroids significantly reduced the length of mechanical ventilation in critically ill patients experiencing respiratory failure, while it was not effective in decreasing the odds of mechanical ventilation incidence. Mechanical ventilation is often required in COVID patients experiencing ARDS, defined as a reduction in the area of normoventilated lung due to inflammatory pulmonary edema [184]. While pulmonary edema is one of the main drivers of COVID-related mortality [185,186], prolonged mechanical ventilation is also associated with a higher risk of developing complications such as ventilator-associated pneumonia [187], death, increased hospital and ICU lengths of stay, and increased cost [187,188]. Thus, the use of corticosteroids may substantially improve the outcome of critically ill COVID-19 patients who require mechanical ventilation, as well as conserving valuable ventilator assets during COVID surges.
Compared with previous meta-analyses, the current review did not identify a robust association between the use of corticosteroids and mortality, unlike smaller systematic reviews conducted earlier during the pandemic [58,59,62]. However, our findings regarding prolonged duration of viral clearance and reduced length of mechanical ventilation is strongly supported by the findings of previous meta-analyses [60,61]. In summary, our results suggest that the role of corticosteroids for treating COVID-19 may be limited to severe and critically ill patients receiving mechanical ventilation for reducing length of mechanical ventilation. It may not be effective for reducing mortality or improving other efficacy outcomes for other patient groups. The effect of methylprednisolone on the odds of mortality requires further investigation and clarification. Last, while corticosteroids may be effective in certain patient groups, it is currently unknown whether it is related to SARS-CoV-2, its treatment, or both. Further research may be warranted.
Clinical implications for corticosteroid safety
Similar to previous meta-analyses [58,62], we did not identify any significant associations between the use of corticosteroids and the odds of all-cause adverse events and all-cause severe adverse events. However, excluding the CoDEX trial [30] from the meta-analysis resulted in a significantly increased odds of adverse events based on the treatment effects of the remaining observational studies. Given that the patient characteristics are similar between the CoDEX trial and patients in the observational study, especially as both the CoDEX trial and the observational studies largely excluded mild and moderate patients, a possible explanation of this finding is due to the use of corticosteroids in a subset of control patients in the CoDEX trial, as well as the open-label design which may lead to biased reporting of adverse events in the experimental group. The CoDEX investigators stated that these factors may have shifted the results toward null [30]; consequently, the inclusion of the CoDEX trial would have shifted our pooled results toward null as well.
Additionally, we found that the pooled results from studies using high fixed-dose methylprednisolone yielded a significantly decreased odds of severe adverse events, while studies using low fixed-dose hydrocortisone yielded a significantly increased odds of severe adverse events. As there was no heterogeneity within each of the aforementioned subgroups, and the studies included in the subgroups (including 3 RCTs and 1 propensity-matched cohort study) were unlikely to be substantially biased; we speculate that the differences in corticosteroid doses may explain this observation. It is possible that the high-dose methylprednisolone therapy was more efficacious at controlling COVID symptoms and undesirable effects from adjuvant medications than low-dose hydrocortisone, thus resulting in less reported adverse events. Overall, a majority of the adverse events reported by the subgroup studies were deemed to be unrelated to the intervention [29,87,89,104]. Nevertheless, the sample size supporting this observation is low, and further investigations are needed to confirm this finding.
In this review, we assessed the odds of hyperglycemia and nosocomial infections, two of the most common types of adverse events in patients undergoing systemic corticosteroid treatments [189]. Our review found that the use of corticosteroids is associated with a significant increase in the odds of hyperglycemia compared with control, indicating a need for glycemic monitoring for patients receiving corticosteroid therapy [190]. Interestingly, we did not identify a significant correlation between corticosteroid use and the odds of nosocomial infection, contrary to expectations due to corticosteroids' immunosuppressive properties. This is most likely due to the use of concomitant antimicrobials in COVID-19 patients to prevent superinfections. As the reporting of antibiotic and antifungal use among our included studies were extremely inconsistent, we could not properly evaluate the impact of corticosteroids on the odds of nosocomial infections.
Strengths & limitations
This systematic review and meta-analysis has several strengths. First, we conducted several subgroup analyses to assess sources of heterogeneity and to study the impact of study methodologies on the treatment effect, including comparing the pooled effect from randomized to non-randomized studies and studies with low-moderate risk of bias versus studies with high risk of bias. Secondly, we followed the most up-to-date systematic review guidelines and methods, such as PRISMA 2020, RoB2, and GRADE. Moreover, the large sample size in our analyses improved the power and precision of our study compared with previous systematic reviews.
The major limitation of this study is the inclusion of observational studies. Similar to our previous systematic reviews [25,26], a majority of our included observational studies were rated as having a critical or serious risk of bias according to ROBINS-I, highlighting the poor publication quality associated with pandemic-related literature [191]. The inclusion of observational studies in meta-analyses was also associated with large treatment effects [192,193] and overly precise estimates [194,195], potentially introducing biases into our significant findings. However, it must be noted that our subgroup analysis by study design showed that the findings from observational studies were largely in agreement with findings from RCTs for a majority of the outcomes. In addition, while we conducted numerous subgroup analyses and meta-regressions to explore the sources of heterogeneity and possible factors that may affect the efficacy of corticosteroids, we could not provide insight on the effect of virus variants, the general care provided, vaccination status and underlying conditions on the efficacy of corticosteroids over the course of the pandemic due to a paucity of data. With rapidly changing COVID-19 treatment strategies and the spread of the Omicron variant, future studies may wish to consider the potential impact of these factors.
Conclusion
This comprehensive systematic review and meta-analysis showed that the use of corticosteroids was not significantly associated with changes in length of ICU hospitalization, incidence of ICU admission, incidence of mortality, nor incidence of mechanical ventilation. Additionally, corticosteroids may increase length of stay (based on RCT data only) and increase the duration of viral clearance. However, corticosteroids were significantly associated with reduction in the length of mechanical ventilation; thus, it may confer beneficial effects for critically ill patients requiring mechanical ventilation. There was also evidence suggesting that methylprednisolone may be efficacious in reducing mortality and incidence of ICU admission; however, these findings should be further assessed using RCTs.
In terms of safety, corticosteroids were not associated with increased odds of adverse events or severe adverse events, although these findings need to be interpreted with caution due to potential confounding factors such as corticosteroid dosage. Corticosteroids were also not associated with increased odds of nosocomial infections, albeit the use of adjuvant antimicrobials complicates the interpretation of this finding. Last, corticosteroids use was associated with significantly increased odds of hyperglycemia, indicating a need for glycemic monitoring in COVID-19 patients receiving corticosteroid treatments.
Summary points.
Corticosteroids are a category of drugs repurposed for the management of COVID-19 patients with conflicting evidence regarding its efficacy.
In this systematic review and meta-analysis, data from 10 randomized controlled trials (RCTs) and 85 observational studies (n = 42,205) were analyzed using random-effects meta-analyses to assess its efficacy and safety for treating hospitalized COVID-19 patients.
A majority of included RCTs were rated as having some concerns in terms of risk of bias according to RoB2, while a majority of included observational studies were rated as having serious or critical risk of bias according to ROBINS-I.
The use of corticosteroids was not significantly associated with changes in length of ICU hospitalization, incidence of ICU admission, nor incidence of mechanical ventilation.
The use of corticosteroids was significantly associated with increased length of stay (based on RCT data only) and increased time to negative SARS-CoV-2 test; additionally, the use of corticosteroids was associated with reduced length of mechanical ventilation.
In terms of safety, the use of corticosteroids was not associated with increased odds of adverse events, serious adverse events, or nosocomial infections; however, the use of corticosteroids was associated with increased odds of hyperglycemia.
Some evidence suggests that methylprednisolone may reduce mortality and incidence of ICU admission, and high-dose methylprednisolone pulses may result in reduced incidence of serious adverse events compared with other regimens such as low-dose hydrocortisone; however more research is needed to confirm these findings.
Current evidence supports the use of corticosteroids in the management of critically ill COVID-19 patients requiring corticosteroids, and glycemic monitoring is required for patients undergoing treatments with corticosteroids.
Supplementary Material
Acknowledgments
The authors would like to acknowledge E Lapshina and M Mellett of the Faculty of Science at McGill University for their contributions in performing preliminary database searches before the commencement of the review process to determine the feasibility of their research methods. In addition, the authors would like to express their most sincere gratitude towards Marco Confalonieri of Azienda Sanitaria Universitaria Giuliano Isontina (ASUGI) for providing them with unpublished length of stay data for analysis. They would also like to express their appreciation for Andrea R Levine of the University of Maryland School of Medicine for her assistance with interpreting the mortality data in her study.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fvl-2021-0244
Author contributions
F Zhou was responsible for conception and design of the work, as well as for supervising and contributing to all parts of the data acquisition process (including article screening, full-text retrieval, data extraction and risk of bias analyses), drafting of the final manuscript and revised the final manuscript critically for important intellectual content. J Deng was responsible for conception and design of the work, as well as for search strategy development, conducting database searches, performing data analyses (including meta-analyses, meta-regressions, subgroup analyses and GRADE ratings) and drafting of the final manuscript. K Heybati, QK Zuo, S Ali, W Hou, CY Wong, HB Ramaraju, O Chang, T Dhivagaran and Z Silver contributed to all parts of the data acquisition process (including article screening, full-text retrieval, data extraction and risk of bias analyses) and revised the final manuscript critically for important intellectual content. All authors have given final approval for the final version of this manuscript to be submitted for publication and all authors agree to be held accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
There are no relevant ethical disclosures as only published, aggregate patient data was used for this study. Aggregate patient data extracted by study authors are available upon reasonable request.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 91(1), 157–160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahase E. COVID-19: WHO declares pandemic because of ‘alarming levels’ of spread, severity, and inaction. BMJ 368, m1036 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili SM, Bahreini E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online 22, 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 215, 108427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Arienzo M, Coniglio A. Assessment of the SARS-CoV-2 basic reproduction number, based on the early phase of COVID-19 outbreak in Italy. Biosaf. Health 2(2), 57–59 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahajan S, Caraballo C, Li SX et al. SARS-CoV-2 infection hospitalization rate and infection fatality rate among the non-congregate population in Connecticut. Am. J. Med. 134(6), 812–816.e2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer CK, Retnakaran R. Rates of COVID-19-associated hospitalization in British Columbia and Ontario: time course of flattening the relevant curve. Can. J. Public Health 111(5), 636–640 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macedo A, Gonçalves N, Febra C. COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis. Ann. Epidemiol. 57, 14–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoukat A, Wells CR, Langley JM, Singer BH, Galvani AP, Moghadas SM. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. CMAJ 192(19), E489–E496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R, Rivers C, Tan Q, Murray MB, Toner E, Lipsitch M. Estimated demand for US hospital inpatient and intensive care unit beds for patients with COVID-19 based on comparisons with Wuhan and Guangzhou, China. JAMA Netw. Open 3(5), e208297 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett K, Khan YA, Mac S, Ximenes R, Naimark DMJ, Sander B. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. CMAJ 192(24), E640–E646 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen-Crowe B, Sutherland M, McKenney M, Elkbuli A. A closer look into global hospital beds capacity and resource shortages during the COVID-19 pandemic. J. Surg. Res. 260, 56–63 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maves RC, Downar J, Dichter JR et al. Triage of scarce critical care resources in COVID-19 an implementation guide for regional allocation: an expert panel report of the task force for mass critical care and the American College of Chest Physicians. Chest 158(1), 212–225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jazieh AR, Akbulut H, Curigliano G et al. Impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob. Oncol. 6, 1428–1438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FDA Office of the Commissioner. ‘FDA approves first COVID-19 vaccine’ (2021). https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
- 16.Chitsike L, Duerksen-Hughes P. Keep out! SARS-CoV-2 entry inhibitors: their role and utility as COVID-19 therapeutics. Virol. J. 18(1), 154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura I, Kosugi Y, Wu J et al. SARS-CoV-2 Lambda variant exhibits higher infectivity and immune resistance. bioRxiv (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farinholt T, Doddapaneni H, Qin X et al. Transmission event of SARS-CoV-2 Delta variant reveals multiple vaccine breakthrough infections. medRxiv (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planas D, Veyer D, Baidaliuk A et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596(7871), 276–280 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines (Basel) 9(2), 160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancet Commission on COVID-19 Vaccines and Therapeutics Task Force Members. Urgent needs of low-income and middle-income countries for COVID-19 vaccines and therapeutics. Lancet 397(10274), 562–564 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 368(6493), 860–868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RECOVERY Collaborative Group, Horby P, Mafham M et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N. Engl. J. Med. 383(21), 2030–2040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 396(10259), 1345–1352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng J, Zhou F, Hou W et al. Efficacy of lopinavir-ritonavir combination therapy for the treatment of hospitalized COVID-19 patients: a meta-analysis. Future Virol. 17(3), 169–189 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng J, Zhou F, Heybati K et al. Efficacy of chloroquine and hydroxychloroquine for the treatment of hospitalized COVID-19 patients: a meta-analysis. Future Virol. 17(2), 95–118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C, Chen X, Cai Y et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180(7), 934–943 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RECOVERY Collaborative Group, Horby P, Lim WS et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 384(8), 693–704 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angus DC, Derde L, Al-Beidh F et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 324(13), 1317–1329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomazini BM, Maia IS, Cavalcanti AB et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 324(13), 1307–1316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA 324(13), 1292–1295 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Lee N, Allen Chan KC, Hui DS et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J. Clin. Virol. 31(4), 304–309 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arabi YM, Mandourah Y, Al-Hameed F et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 197(6), 757–767 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Ye Z, Wang Y, Colunga-Lozano LE et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ 192(27), E756–E767 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taboada M, Caruezo V, Naveira A, Atanassoff PG. Corticosteroids and the hyper-inflammatory phase of the COVID-19 disease. J. Clin. Anesth. 66, 109926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 6(1), 255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco-Melo D, Nilsson-Payant BE, Liu WC et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181(5), 1036–1045.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Ren L, Zhang L et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 27(6), 883–890.e2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelaia C, Tinello C, Vatrella A, De Sarro G, Pelaia G. Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Ther. Adv. Respir. Dis. 14, 1753466620933508 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmadpoor P, Rostaing L. Why the immune system fails to mount an adaptive immune response to a COVID-19 infection. Transpl. Int. 33(7), 824–825 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front. Microbiol. 10, 1057 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front. Immunol. 10, 119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres Acosta MA, Singer BD. Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. Eur. Respir. J. 56(3), 2002049 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotez PJ, Bottazzi ME, Corry DB. The potential role of Th17 immune responses in coronavirus immunopathology and vaccine-induced immune enhancement. Microbes Infect. 22(4–5), 165–167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 53(3), 368–370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roncati L, Nasillo V, Lusenti B, Riva G. Signals of T2 immune response from COVID-19 patients requiring intensive care. Ann. Hematol. 99(6), 1419–1420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gil-Etayo FJ, Suàrez-Fernández P, Cabrera-Marante O et al. T-helper cell subset response is a determining factor in COVID-19 progression. Front. Cell. Infect. Microbiol. 11, 624483 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 323(18), 1824–1836 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Li J, Zhan Y et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect. Immun. 72(8), 4410–4415 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis SR, Pritchard MW, Thomas CM, Smith AF. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst. Rev. 7, CD004477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223), 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhodes A, Evans LE, Alhazzani W et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 43(3), 304–377 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 46(5), 846–848 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iannaccone G, Scacciavillani R, Del Buono MG et al. Weathering the cytokine storm in COVID-19: therapeutic implications. Cardiorenal Med. 10(5), 277–287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schiavo JH. PROSPERO: an international register of systematic review protocols. Med. Ref. Serv. Q. 38(2), 171–180 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Hicks E. ‘Library guides: TU GOARN: COVID-19 search strategies’ (2020). https://libguides.tulane.edu/TESTCovid-19/COVID-19SearchStrings
- 58.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324(13), 1330–1341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit. Care 24(1), 696 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tlayjeh H, Mhish OH, Enani MA et al. Association of corticosteroids use and outcomes in COVID-19 patients: a systematic review and meta-analysis. J. Infect. Public Health 13(11), 1652–1663 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Yang W, Chen P et al. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: a systematic review and meta-analysis. PLoS ONE 16(4), e0249481 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pulakurthi YS, Pederson JM, Saravu K et al. Corticosteroid therapy for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Medicine 100(20), e25719 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5(1), 210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27(6), 1785–1805 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi J, Luo D, Wan X et al. Detecting the skewness of data from the sample size and the five-number summary. arXiv (2020) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 68.Sterne JAC, Savović J, Page MJ et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Sterne JA, Hernán MA, Reeves BC et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650), 924–926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murad MH. Clinical Practice Guidelines: a primer on development and dissemination. Mayo Clin. Proc. 92(3), 423–433 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64(4), 383–394 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Akl EA, Schünemann HJ. Using systematic reviews in guideline development: the GRADE approach. Res. Synth. Methods 10(3), 312–329 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Chan KCB. Data analysis using R programming. Adv. Exp. Med. Biol. 1082, 47–122 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Fleiss JL. The statistical basis of meta-analysis. Stat. Methods Med. Res. 2(2), 121–145 (1993). [DOI] [PubMed] [Google Scholar]
- 76.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 23(9), 1351–1375 (2004). [DOI] [PubMed] [Google Scholar]
- 77.West SL, Gartlehner G, Mansfield AJ et al. Comparative Effectiveness Review Methods: Clinical Heterogeneity. Agency for Healthcare Research and Quality, MD, USA: (2010). [PubMed] [Google Scholar]
- 78.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley, NJ, USA: (2008). [Google Scholar]
- 79.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 327(7414), 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fagerland MW. Evidence-based medicine and systematic reviews. In: Research in Medical and Biological Sciences. Elsevier, Amsterdam, Netherlands : 431–461 (2015). [Google Scholar]
- 82.Monreal E, Sainz de la Maza S, Natera-Villalba E et al. High versus standard doses of corticosteroids in severe COVID-19: a retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 40(4), 761–769 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.López Zúñiga MÁ, Moreno-Moral A, Ocaña-Granados A et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS ONE 16(1), e0243964 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeronimo CMP, Farias MEL, Val FFA et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin. Infect. Dis. 72(9), e373–e381 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dequin PF, Heming N, Meziani F et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 324(13), 1298–1306 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang X, Feng YM, Ni JX et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration 100(2), 116–126 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munch MW, Meyhoff TS, Helleberg M et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol. Scand. 65(10), 1421–1430 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jamaati H, Hashemian SM, Farzanegan B et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur. J. Pharmacol. 897, 173947 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edalatifard M, Akhtari M, Salehi M et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur. Respir. J. 56(6), 2002808 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: an open-label randomized trial (GLUCOCOVID). Wien. Klin. Wochenschr. 133(7–8), 303–311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albani F, Fusina F, Granato E et al. Corticosteroid treatment has no effect on hospital mortality in COVID-19 patients. Sci. Rep. 11(1), 1015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Almas T, Ehtesham M, Khan AW et al. Safety and efficacy of low-dose corticosteroids in patients with non-severe coronavirus disease 2019: a retrospective cohort study. Cureus 13(1), e12544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aomar-Millán IF, Salvatierra J, Torres-Parejo Ú, Nuñez-Nuñez M, Hernández-Quero J, Anguita-Santos F. Glucocorticoids alone versus tocilizumab alone or glucocorticoids plus tocilizumab in patients with severe SARS-CoV-2 pneumonia and mild inflammation. Med. Clin. 156(12), 602–605 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Badr M, De Oliveira B, Abdallah K et al. Effects of methylprednisolone on ventilator-free days in mechanically ventilated patients with acute respiratory distress syndrome and COVID-19: a retrospective study. J. Clin. Med. Res. 10(4), 760 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bahl A, Johnson S, Chen NW. Timing of corticosteroids impacts mortality in hospitalized COVID-19 patients. Intern. Emerg. Med. 16(6), 1593–1603 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balena F, Bavaro DF, Fabrizio C et al. Tocilizumab and corticosteroids for COVID-19 treatment in elderly patients. J.Gerontol. Geriatrics 68, 197–203 (2020). [Google Scholar]
- 97.Bartoletti M, Marconi L, Scudeller L et al. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin. Microbiol. Infect. 27(1), 105–111 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Batirel A, Demirhan R, Eser N, Körlü E, Tezcan ME. Pulse steroid treatment for hospitalized adults with COVID-19. Turk. J. Med. Sci. 51(5), 2248–2255 (2021). [DOI] [PubMed] [Google Scholar]
- 99.Borie R, Savale L, Dossier A et al. Glucocorticoids with low-dose anti-IL1 anakinra rescue in severe non-ICU COVID-19 infection: a cohort study. PLoS ONE 15(12), e0243961 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaudhry Z, Shawe-Taylor M, Rampling T et al. Short durations of corticosteroids for hospitalised COVID-19 patients are associated with a high readmission rate. J. Infect. 82(6), 276–316 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen Y, Li L. Influence of corticosteroid dose on viral shedding duration in patients with COVID-19. Clin. Infect. Dis. 72(7), 1298–1300 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Q, Song Y, Wang L et al. Corticosteroids treatment in severe patients with COVID-19: a propensity score matching study. Expert Rev. Respir. Med. 15(4), 543–552 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen H, Xie J, Su N et al. Corticosteroid therapy is associated with improved outcome in critically ill patients with COVID-19 with hyperinflammatory phenotype. Chest 159(5), 1793–1802 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cusacovich I, Aparisi Á, Marcos M et al. Corticosteroid pulses for hospitalized patients with COVID-19: effects on mortality. Mediators Inflamm. 2021, 6637227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Greef E, Vandebroek A, Vanfleteren L, De Corte W, Lamote S. The impact of dexamethasone on clinical outcome of patients with COVID-19 admitted to ICU. A retrospective, single-center cohort study. Intensive Care Medicine Experimental 8(2), 000819 (2020). [Google Scholar]
- 106.Ding C, Feng X, Chen Y et al. Effect of corticosteroid therapy on the duration of SARS-CoV-2 clearance in patients with mild COVID-19: a retrospective cohort study. Infect. Dis. Ther. 9(4), 943–952 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Espinosa-Solano M, Gonzalez-Vergara D, Ferrer-Galvan M et al. Repeated pulses of methyl-prednisolone in adults hospitalized with COVID-19 pneumonia and acute respiratory distress syndrome: a preliminary before–after study (CortiCOVID Study). Open Respiratory Archives 3(2), 100086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A et al. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob. Agents Chemother. 64(9), e01168–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu E, Ma R, Wang L, Fu H, Li W. Study on clinical characteristics of 173 cases of COVID-19 and effect of glucocorticoid on nucleic acid negative conversion. Am. J. Transl. Res. 13(5), 4251–4265 (2021). [PMC free article] [PubMed] [Google Scholar]
- 110.Fusina F, Albani F, Granato E et al. Effect of corticosteroids on mortality in hospitalized COVID-19 patients not receiving invasive mechanical ventilation. Clin. Pharmacol. Ther. 109(6), 1660–1667 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gallay L, Tran VT, Perrodeau E et al. Fourteen-day survival among older adults with severe infection with severe acute respiratory syndrome coronavirus 2 treated with corticosteroid: a cohort study. Clin. Microbiol. Infect. 27(8), 1145–1150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gong Y, Guan L, Jin Z, Chen S, Xiang G, Gao B. Effects of methylprednisolone use on viral genomic nucleic acid negative conversion and CT imaging lesion absorption in COVID-19 patients under 50 years old. J. Med. Virol. 92(11), 2551–2555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.González-Castro A, Cuenca Fito E, Fernández A, Escudero Acha P, Rodríguez Borregán JC, Peñasco Y. Impact of corticosteroid therapy on the survival of critical COVID-19 patients admitted into an intensive care unit. Rev. Esp. Anestesiol. Reanim. 69(2), 120–121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gragueb-Chatti I, Lopez A, Hamidi D et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: a multicenter retrospective study. Ann. Intensive Care 11(1), 87 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.He R, Liu L, Xu HX et al. Observation on the efficacy of glucocorticoid in the treatment of novel coronavirus pneumonia. Acta Universitatis Medicinalis Anhui 56(6), 991–994 (2021). [Google Scholar]
- 116.Ho KS, Narasimhan B, Difabrizio L et al. Impact of corticosteroids in hospitalised COVID-19 patients. BMJ Open Respir. Res. 8(1), e000766 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoertel N, Sánchez-Rico M, Vernet R et al. Dexamethasone use and mortality in hospitalized patients with coronavirus disease 2019: a multicentre retrospective observational study. Br. J. Clin. Pharmacol. 87(10), 3766–3775 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hong G, Patel M, Tusha J et al. Corticosteroid treatment in patients with severe COVID-19 pneumonia. Chest 158(4), A599 (2020). [Google Scholar]
- 119.Hu Z, Lv Y, Xu C et al. Clinical use of short-course and low-dose corticosteroids in patients with non-severe COVID-19 during pneumonia progression. Front Public Health 8, 355 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu Y, Wang T, Hu Z et al. Clinical efficacy of glucocorticoid on the treatment of patients with COVID-19 pneumonia: a single-center experience. Biomed. Pharmacother. 130, 110529 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang R, Zhu C, Wang Jian et al. Corticosteroid therapy is associated with the delay of SARS-CoV-2 clearance in COVID-19 patients. Eur. J. Pharmacol. 889, 173556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ikeda S, Misumi T, Izumi S et al. Corticosteroids for hospitalized patients with mild to critically-ill COVID-19: a multicenter, retrospective, propensity score-matched study. Sci. Rep. 11(1), 10727 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jung C, Wernly B, Fjølner J et al. Steroid use in elderly critically ill COVID-19 patients. Eur. Respir. J. 58(4), 2100979 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Keller MJ, Kitsis EA, Arora S et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J. Hosp. Med. 15(8), 489–493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ko JJ, Wu C, Mehta N, Wald-Dickler N, Yang W, Qiao R. A Comparison of methylprednisolone and dexamethasone in intensive care patients with COVID-19. J. Intensive Care Med. 36(6), 673–680 (2021). [DOI] [PubMed] [Google Scholar]
- 126.Kocakaya D, Olgun Yildizeli Ş, Balcan B, Eryuksel E, Karakurt S. Does methylprednisolone affect time to recovery in COVID-19 pneumonia? Marmara Medical J. 34(2), 120–126 (2021). [Google Scholar]
- 127.Lambermont B, Ernst M, Demaret P et al. Corticosteroid therapy is associated with a decrease in mortality in a multicenter cohort of mechanically ventilated COVID-19 patients. Intensive Care Medicine Experimental 8, 73 (2020).33313986 [Google Scholar]
- 128.Li Q, Li W, Jin Y et al. Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 pneumonia: a retrospective cohort study. Infect. Dis. Ther. 9(4), 823–836 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y, Meng Q, Rao X et al. Corticosteroid therapy in critically ill patients with COVID-19: a multicenter, retrospective study. Crit. Care 24(1), 698 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Y, Zhou X, Li T et al. Corticosteroid prevents COVID-19 progression within its therapeutic window: a multicentre, proof-of-concept, observational study. Emerg. Microbes Infect. 9(1), 1869–1877 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y, Li J, Ke J et al. Adverse outcomes associated with corticosteroid use in critical COVID-19: a retrospective multicenter cohort study. Front. Med. 8, 604263 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang MY, Chen P, He M et al. Corticosteroids treatment of patients with coronavirus disease 2019: a propensity score matching study. Curr. Med. Sci. 41(1), 24–30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu X, Zhu T, Wang M et al. A retrospective analysis of adjuvant glucocorticoid therapy for severe COVID-19. Chinese J. Lung Dis. 13(5), 586–591 (2020). [Google Scholar]
- 134.Liu J, Zhang S, Dong X et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J. Clin. Invest. 130(12), 6417–6428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu Y, Liu F, Tong G et al. Clinical evidence of an interferon-glucocorticoid therapeutic synergy in COVID-19. Signal Transduct. Target. Ther. 6(1), 107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luo B, Peng H, Li Y, Li Y, Li R, Tan X. Clinical efficacy of glucocorticoids in the treatment of severe COVID-19. Med. Sci. J. Central South China 48(6), 628–632 (2020). [Google Scholar]
- 137.Ma Y, Zeng H, Zhan Z et al. Corticosteroid use in the treatment of COVID-19: a multicenter retrospective study in Hunan, China. Front. Pharmacol. 11, 1198 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ma Q, Qi D, Deng XY et al. Corticosteroid therapy for patients with severe novel coronavirus disease 2019. Eur. Rev. Med. Pharmacol. Sci. 24, 8194–8201 (2020). [DOI] [PubMed] [Google Scholar]
- 139.Majmundar M, Kansara T, Lenik JM et al. Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York metropolitan region. PLoS ONE 15(9), e0238827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Monedero P, Gea A, Castro P et al. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit. Care 25(1), 2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mühlemann B, Thibeault C, Hillus D et al. Impact of dexamethasone on SARS-CoV-2 concentration kinetics and antibody response in hospitalized COVID-19 patients: results from a prospective observational study. Clin. Microbiol. Infect. 27(10), 1520.e7–1520.e10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nelson BC, Laracy J, Shoucri S et al. Clinical outcomes associated with methylprednisolone in mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 72(9), e367–e372 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ni Q, Ding C, Li Y et al. Effect of low-to-moderate dose glucocorticoids on viral clearance in COVID-19: a retrospective study. Chinese Journal of Clinical Infectious Diseases 13(1), 21–24 (2020). [Google Scholar]
- 144.Obata R, Maeda T, Rizk D, Kuno T. Increased secondary infection in COVID-19 patients treated with steroids in New York City. Jpn J. Infect. Dis. 74(4), 307–315 (2021). [DOI] [PubMed] [Google Scholar]
- 145.Ooi ST, Parthasarathy P, Lin Y et al. Antivirals with adjunctive corticosteroids prevent clinical progression of early coronavirus 2019 pneumonia: a retrospective cohort study. Open Forum Infect. Dis. 7(11), ofaa486 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Papamanoli A, Yoo J, Grewal P et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur. J. Clin. Invest. 51(2), e13458 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Piniella-Ruiz E, Bellver-Álvarez MT, Mestre-Gómez B et al. Impact of systemic corticosteroids on mortality in older adults with critical COVID-19 pneumonia. J. Gerontol. A Biol. Sci. Med. Sci. 76(8), e127–e132 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rahman O, Trigonis R, Craft M et al. Corticosteroid use in severely hypoxemic COVID-19 patients. Crit. Care Med. 49(S1), 124 (2021). [Google Scholar]
- 149.Ritter LA, Britton N, Heil EL et al. The impact of corticosteroids on secondary infection and mortality in critically ill COVID-19 patients. J. Intensive Care Med. 36(10), 1201–1208 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodríguez-Baño J, Pachón J, Carratalà J et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19). Clin. Microbiol. Infect. 27(2), 244–252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodríguez-Molinero A, Pérez-López C, Gálvez-Barrón C et al. Association between high-dose steroid therapy, respiratory function, and time to discharge in patients with COVID-19: cohort study. Med. Clin. 156(1), 7–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ruiz-Irastorza G, Pijoan JI, Bereciartua E et al. Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: an observational comparative study using routine care data. PLoS One 15(9), e0239401 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Russo FM, Zacchetti L, Manesso L et al. Steroid immunomodulation in SARS-CoV-2 patients. Intensive Care Medicine Experimental 8(2), 000801 (2020). [Google Scholar]
- 154.Saggi SJ, Nath S, Culas R et al. Early experience with methylprednisolone on SARS-CoV-2 infection in the African–American population, a retrospective analysis. Clin. Med. Insights Circ. Respir. Pulm. Med. 14, 1179548420980699 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Salton F, Confalonieri P, Meduri GU et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect. Dis. 7(10), ofaa421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Spagnuolo V, Guffanti M, Galli L et al. Viral clearance after early corticosteroid treatment in patients with moderate or severe COVID-19. Sci. Rep. 10(1), 21291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tortajada C, Colomer E, Andreu-Ballester JC, Esparcia A, Oltra C, Flores J. Corticosteroids for COVID-19 patients requiring oxygen support? Yes, but not for everyone: effect of corticosteroids on mortality and intensive care unit admission in patients with COVID-19 according to patients' oxygen requirements. J. Med. Virol. 93(3), 1817–1823 (2021). [DOI] [PubMed] [Google Scholar]
- 158.Tran VT, Mahévas M, Bani-Sadr F et al. Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. Clin. Microbiol. Infect. 27(4), 603–610 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Urendes ML, Meza Samaniego F, Palencia C et al. Corticosteroid use related factors in the treatment of admitted patients in ICU with COVID-19 pneumonia. Intensive Care Medicine Experimental 8(2), 001130 (2020). [Google Scholar]
- 160.Van den Eynde E, Gasch O, Oliva JC et al. Corticosteroids and tocilizumab reduce in-hospital mortality in severe COVID-19 pneumonia: a retrospective study in a Spanish hospital. Infect. Dis. 53(4), 291–302 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang T, Guerra LA. Glucocorticoids improve 30-day survival probability in critical coronavirus disease 2019 patients. Infect. Dis. Clin. Pract. 29(1), e20–e22 (2021). [Google Scholar]
- 162.Wang Y, Jiang W, He Q et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct. Target. Ther. 5(1), 57 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu J, Huang J, Zhu G et al. Systemic corticosteroids and mortality in severe and critical COVID-19 patients in Wuhan, China. J. Clin. Endocrinol. Metab. 105(12), e4230–e4239 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu C, Hou D, Du C et al. Corticosteroid therapy for coronavirus disease 2019-related acute respiratory distress syndrome: a cohort study with propensity score analysis. Crit. Care 24(1), 643 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xia Q, Dai W, Xu K et al. Clinical efficacy of methylprednisolone and the combined use of lopinavir/ritonavir with arbidol in treatment of coronavirus disease 2019. J. Med. Virol. 93(7), 4446–4453 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xiong X, Jiao X, Li H, Zeng S, Wang Y, Gao Q. Glucocorticoid benefits the ventilatory function of severe/critical COVID-19 patients. J. Infect. 82(1), e33–e35 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xiong B, He LM, Qin YY et al. Effectiveness of adjunctive corticosteroid therapy in patients with severe COVID-19: a retrospective cohort study. World J. Clin. Cases. 9(15), 3546–3558 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xue P, Liu X, Lei C, Wang J, Tu Y, Tan Q. Clinical observation of short-term and low-dose glucocorticosteroid in treatment of moderate COVID-19 in elderly patients. Shandong Med. J. 15(60), 1–5 (2020). [Google Scholar]
- 169.You X, Wu CH, Fu YN et al. The use of methylprednisolone in COVID-19 patients: a propensity score matched retrospective cohort study. PLoS ONE 15(12), e0244128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yuan M, Xu X, Xia D et al. Effects of corticosteroid treatment for non-severe COVID-19 pneumonia: a propensity score-based analysis. Shock 54(5), 638–643 (2020). [DOI] [PubMed] [Google Scholar]
- 171.Zawaydeh Q, Husain A, Cotton S, Malhotra A. The effect of steroid dosing on risk of secondary infection in COVID-19 critically ill patients. Am. J. Respir. Crit. Care Med. 203, A3806 (2021). [Google Scholar]
- 172.Zha L, Li S, Pan L et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med. J. Aust. 212(9), 416–420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhan Y, Shang J, Gu Y, Huang Q, Xie J. Efficacy of corticosteroid in patients with COVID-19: a multi-center retrospective study and meta-analysis. J. Med. Virol. 93(7), 4292–4302 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu HM, Li Y, Li BY et al. Effect of methylprednisolone in severe and critical COVID-19: analysis of 102 cases. World J. Clin. Cases 8(23), 5952–5961 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Heaton J, Malhotra P, Stockton P, Rossiter L. Late breaking abstract – corticosteroids reduce mortality and prevent ICU admission in COVID-19 pneumonia – results from a rapid clinical review of 191 patients. Eur. Respir. J. 56, 3586 (2020). [Google Scholar]
- 176.Guaraldi G, Banchelli F, Milic J et al. Methylprednisolone as rescue therapy after tocilizumab failure in patients with severe COVID-19 pneumonia. Clin. Exp. Rheumatol. 39(5), 1141 (2021). [DOI] [PubMed] [Google Scholar]
- 177.Ji J, Wu M, Zhong L et al. Early, low-dose, short-term methylprednisolone decreased the mortality in critical COVID-19 patients: a multicenter retrospective cohort study. J. Infect. 82(4), 84–123 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li H, Chen C, Hu F et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia 34(6), 1503–1511 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet 395(10230), 1111 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ranjbar K, Moghadami M, Mirahmadizadeh A et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect. Dis. 21(1), 337 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meduri GU, Siemieniuk RAC, Ness RA, Seyler SJ. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J. Intensive Care Med. 6, 53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Burton GH, Cooke NT, Tetley TD. Prednisone and methylprednisolone disposition in the lung. Lancet 2(8363), 1371 (1983). [DOI] [PubMed] [Google Scholar]
- 183.Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 16(2), e1002742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hajjar LA, Costa IBS da S, Rizk SI et al. Intensive care management of patients with COVID-19: a practical approach. Ann. Intensive Care 11(1), 36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dagens A, Sigfrid L, Cai E et al. Scope, quality, and inclusivity of clinical guidelines produced early in the COVID-19 pandemic: rapid review. BMJ 369, m1936 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cui X, Chen W, Zhou H et al. Pulmonary edema in COVID-19 patients: mechanisms and treatment potential. Front. Pharmacol. 12, 664349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Loss SH, de Oliveira RP, Maccari JG et al. The reality of patients requiring prolonged mechanical ventilation: a multicenter study. Rev. Bra. Ter. Intensiva 27(1), 26–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Esteban A, Anzueto A, Frutos F et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 287(3), 345–355 (2002). [DOI] [PubMed] [Google Scholar]
- 189.Liu D, Ahmet A, Ward L et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 9(1), 30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J. Diabetes 6(8), 1073–1081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jung RG, Di Santo P, Clifford C et al. Methodological quality of COVID-19 clinical research. Nat. Commun. 12(1), 943 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ioannidis JP, Haidich AB, Pappa M et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 286(7), 821–830 (2001). [DOI] [PubMed] [Google Scholar]
- 193.Haidich AB. Meta-analysis in medical research. Hippokratia 14(Suppl. 1), 29–37 (2010). [PMC free article] [PubMed] [Google Scholar]
- 194.Mueller M, D'Addario M, Egger M et al. Methods to systematically review and meta-analyse observational studies: a systematic scoping review of recommendations. BMC Med. Res. Methodol. 18(1), 44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ 316(7125), 140–144 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


