Abstract
Cell polarity regulators are ubiquitous, evolutionary conserved multifunctional proteins. They contain a variety of protein–protein interaction domains endowing them the capacity to interact with cytoskeleton structures, membrane components and multiple regulatory proteins. In this way, they act in complexes and are pivotal for cell growth and differentiation, tissue formation, stability and turnover, cell migration, wound healing, and others. Hence some of these proteins are tumor suppressors.
These cellular processes rely on the establishment of cell polarity characterized by the asymmetric localization of proteins, RNAs, membrane domains, or organelles that together condition cell shape and function. Whether apparently stable, as in epithelia or neurons, or very dynamic, as in immune cells, cell polarity is an active process. It involves cytoskeleton reorganization and targeted intracellular traffic, and results in cellular events such as protein synthesis, secretion and assembly taking place at defined cell poles. Multiple polarity regulators orchestrate these processes.
Immune cells are particularly versatile in rapidly polarizing and assuming different shapes, so to swiftly adopt specialized behaviors and functions. Polarity regulators act in various ways in different immune cell types and at their distinct differentiation states. Here we review how cell polarity regulators control different processes and functions along T lymphocyte physiology, including cell migration through different tissues, immunological synapse formation and effector functions.
Keywords: Cell polarity, T lymphocytes, Signaling, Cytoskeleton, Lymphocyte migration, Immunological synapse
Cell polarity is an evolutionary conserved process found in most organisms, from yeast to mammals. It is the ability of cells to be morphologically, structurally and functionally organized along defined polarity axes. It is characterized by the asymmetric distribution of cellular proteins, membranes, organelles and processes within the cell, endowing it with functionally distinct subcellular domains. It is crucial during embryogenesis, and for maintenance of tissue architecture and homeostasis, wound healing, immune responses, etc. Epithelial cells and neurons are well known examples of polarized cells, but other cells may undergo permanent or transient polarized states, as some secretory cells and immune cells. Cell polarity is an active process triggered and maintained by extracellular cues conveyed by neighboring cells and/or the surrounding microenvironment. Even stably polarized tissues, like the epithelium, need to maintain polarization cues to control the transition between mesenchymal to epithelial states and back. Extracellular cell polarity cues are translated into a tightly regulated cascade of events that, if altered, may lead to pathological states, including developmental abnormalities or tumorigenesis [1,2].
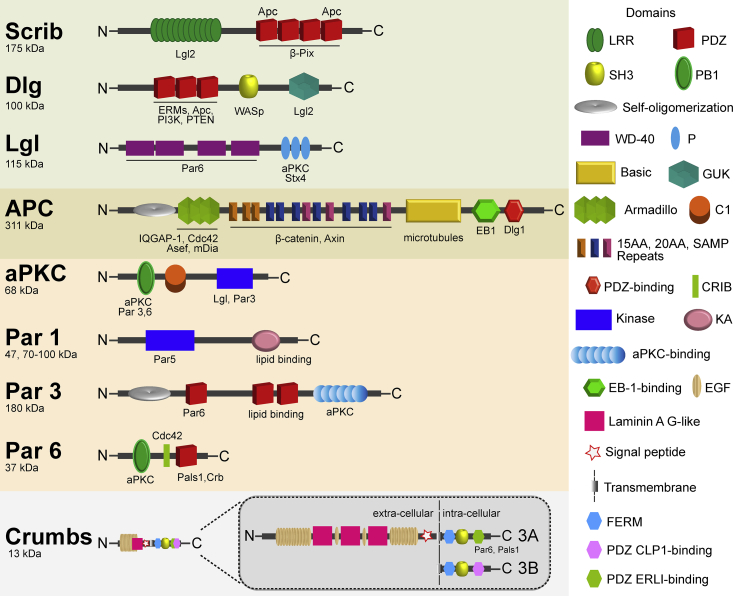
Cell polarity regulators are crucial to orchestrate these intracellular cascades. They are multifunctional proteins displaying a variety of protein–protein interaction domains granting them the capacity to interact with multiple effector regulatory proteins. Some of them have enzymatic activity as protein Ser/Thr kinases (Fig. 1). Protein complexes containing several polarity regulators and the associated effector proteins (polarity complexes) are functional units controlling different forms of cell polarity that are the result of intricate interactomes [3]. Three evolutionary conserved polarity complexes have been extensively studied in various cell types, biological processes and organisms. These include the Scribble, Par and Crumbs complexes that interplay to efficiently control cell polarity. The PDZ-rich scaffold protein Scribble interacts with the MAGUK family member Dlg1 and the cortical cytoskeleton proteins Lethal giant larvae (Lgl1 and 2) [4]. Moreover, Adenomatous Polyposis Coli (APC) associates with the Scribble complex via Dlg1 in a Par6 and aPKC-dependent manner [5]. The Par complex is formed by the PDZ domain-containing adaptors Par3 and Par6, and atypical protein kinase C (aPKC). As aPKC, Par1 is a Ser/Thr kinase, but with different interaction domains. Finally, the Crumbs complex contains the transmembrane protein Crb3, the PDZ-containing scaffold PATJ and the multidomain protein Pals1. In this way, polarity regulators orchestrate complex cellular processes such as the organization of the different cytoskeleton networks, protein stability, intracellular vesicle traffic and targeting, gene transcription, etc. They are pivotal for cell growth and differentiation, tissue formation, stability and turnover, cell migration, and others. Polarity complexes can facilitate either stable polarized states leading to specialized functional areas in tissues and organs, or adaptable polarization occurring, for instance, during wound healing or immune cell responses.
Fig. 1.
Cell polarity regulators involved in immune cell functions. Schematic representation of structures of cell polarity regulators described to be involved in immune cells. Protein interaction domain representations are depicted on the right, and the known interacting protein partners are shown below each domain. Each protein's molecular mass in kDa is depicted below its name. Members of the different polarity complexes, Scribble, Par-APKC and Crumbs are grouped over the same color background. APC is shown as interacting with both Scribble and Par/aPKC polarity complexes.
Among the hallmarks of cell polarity, centrosome positioning and the subsequent microtubule network reorganization are crucial to coordinate cell organization, intracellular transport and cell signaling. They determine cellular features as distinct as the axis of cell division and directionality of migration, and eventually the efficiency of immune cell responses. Polarity complexes are central for centrosome positioning, likely acting as coordinators of extracellular and intracellular cues [6]. Interestingly, even during similar cellular processes, different cells position their centrosome at different locations. For instance, migrating astrocytes have their centromere between the nucleus and the front edge, whereas immune cells, as dendritic cells and lymphocytes, position it between the nucleus and the rear end [[7], [8], [9]]. Migration on different substrates conditions front versus back centrosome position, which thus appears conditioned by extracellular cues [10]. Moreover, T cells encountering an antigen presenting cell switch centrosome position from the back to the front, therefore setting the bases for a new process [11].
Immune cells are mostly versatile and have to rapidly pass from resting to activated states in order to mount immune responses. They migrate between blood, lymphatics, lymphoid organs and other tissues to patrol and execute their functions. Several polarity complexes have been described in immune cells that play key regulatory roles in lymphocyte migration and lymphocyte response to antigen (Fig. 1). We review here how several common polarity regulators orchestrate these two different processes crucial for lymphocyte function. Polarity regulators are also involved in asymmetric cell division during lymphocyte development or differentiation [[12], [13], [14]], but these processes are out of the scope of this review.
Polarization in immune cells
In order to accomplish their specific functions, immune cells undergo cell polarization at different stages of their life cycle. Three main examples of this polarization are migration, immunological synapse formation and asymmetric cell division.
Immune cells are part of and move between different tissues and organs, including blood, lymph, lymphoid organs, dermis, intestinal epithelia and lamina propria. They also infiltrate many other organs under physiological and pathological conditions. Immune cells migrate between different sites in response to environmental signals, including chemokines, inflammatory mediators or microbial components. In order to migrate, immune cells change shape and polarize redistributing various intracellular components, such as the actin-myosin and microtubule cytoskeleton, and membranous compartments as the Golgi, endosomes, lysosomes and mitochondria. Landmarks of immune cell migration are the extension of a leading lamellipodium at the front and the retraction of the cell rear [8,9,15]. Although following some general rules, different immune cells (i.e. dendritic cells, T and B lymphocytes and neutrophils) may utilize different motility regulatory mechanisms and exert different responses to environmental cues.
In lymphoid organs, T and B lymphocytes encounter antigen-presenting cells and polarize towards them, forming a cell–cell communication platform termed the immunological synapse. Although in a different manner than in migrating cells, T and B lymphocytes reorganize intracellular organelles and structural components towards antigen-presenting cells, including the actin and microtubule cytoskeletons together with the centrosome, the Golgi, endo-lysosomal compartments and mitochondria. Lymphocyte polarization within immunological synapses occurs as a reversible transition from the migrating polarized lymphocyte, and it is controlled by a fine balance between antigen receptor signaling, adhesion receptor interactions and chemokine receptor signaling. In this setting, polarization regulates T cell activation, leading to proliferation and cytokines production, and to effector functions, as polarized secretion of cytokines, vesicles and cytotoxic granules [[16], [17], [18], [19]]. Cell polarization in B cell immunological synapses is important for antigen capture, processing and presentation to T cells [20].
Lymphocyte activation may also lead to cell division, which in turn may occur asymmetrically, mediating different cell polarization features and leading to different immune cell fate [13,21].
These three types of polarization involve similar intracellular structures, but the result is different. They are therefore differently regulated. The balance between polarity regulatory molecules and response to extracellular and intracellular cues may account for these differences.
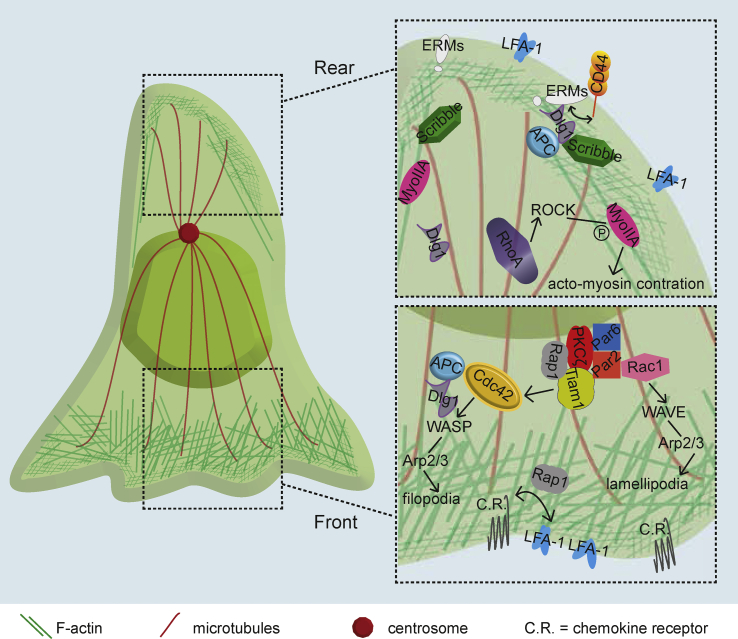
Cell polarization during immune cell migration. Involvement of polarity regulators
Immune cell polarization during migration involves the dynamic reorganization of cytoskeleton networks and the specialized distribution of signaling proteins and adhesion molecules. The specific relocalization of the latter at the plasma membrane is also of key importance for the ability of cells to rapidly adapt their adhesiveness and modulate their migration features to navigate within tissues of different stiffness, scan antigen presenting cell surface or transmigrate blood vessel endothelial barriers [22] (Fig. 2).
Fig. 2.
Cell polarity complexes involved in T cell migration. Simplified scheme of a migrating T cell exhibiting a front-rear polarity. T cell polarization starts with the stimulation of chemokine receptors that triggers in turn the activation of adhesion proteins as LFA-1 integrin and actin and microtubule cytoskeleton remodeling, leading to morphological changes. Polarity regulators localizing at the leading edge scaffold various signaling molecules, including the small GTPases Rac1 and Cdc42, ensuring actin and microtubule cytoskeleton balance and the formation of a leading lamellipodium. At the cell rear, a protrusion called uropod forms and concentrates ERM proteins, linking membrane components with the cortical actin cytoskeleton, and adhesions molecules such as CD44, ICAM-1,2,3 that interact with ERMs and integrins, such as LFA-1. RhoA signaling is key to maintain these subcellular organization, while myosin-IIA facilitates contraction of this region thus supporting T cell migration.
Active actin polymerization generates a lamellipodium at the leading edge, which determines directionality, while myosin concentrated at the back mediates contraction of the cell rear. Front growth and rear contraction ensure together cell progression [23]. Microtubules also reorganize as the centrosome positions between the nucleus and the rear, and ensures localization of organelles (e.g. Golgi, endosomes, lysosomes, mitochondria) and therefore intracellular relocalization of membrane, proteins, lipids and energy. The way organelle positioning influences cell migration is poorly known. The Golgi secretory system may provide lipids and proteins necessary for migration [24], while recycling endosomes and lysosomes may provide membrane, adhesion receptors and signaling molecules to cell areas needing to expand [25,26]. Perturbing microtubules with drugs, does not prevent motility but alters directionality and sustained migration in T cells [27], suggesting a crosstalk between chemokine receptors transducing environmental cues, microtubule orientation and actin-dependent lamellipodium extension. A tight balance of activation of different Rho family GTPases is responsible of transducing signals to actin and microtubule networks that control directionality versus stop in T cells [15,[28], [29], [30]]. Moreover, coordinated activation of actin nucleation machineries at the front and the back of dendritic cells controls their migratory behavior during immature and mature stages [31].
The cell rear in migrating lymphocytes and neutrophils appears as a particular protrusive structure, the uropod, which is enriched in adhesion receptors (e.g. ICAM-1-3, CD44, CD43, P-selectin ligand) and proteins of the ezrin, radixin, moesin (ERM) family, which connect adhesion receptors and signaling molecules with the cortical actin network [15,[32], [33], [34], [35], [36], [37]]. The integrin LFA-1 also concentrates at the uropod [38]. Several functions have been assigned to the uropod, including signaling for retraction of the cell rear via myosin-II and Rho GTPases [15], cell–cell interaction for recruitment at points of extravasation [32,34], and passive cell steering under flow [39].
Polarity regulators are good candidates to finely regulate the topographic organization of proteins, cytoskeleton interactions, adhesion and vesicle traffic, because of their ability to interact with multiple effector proteins [3,19]. For instance, in astrocytes or 3T3 fibroblasts undergoing polarized migration during wound healing, Scribble, APC, Dlg1, Par6 and aPKCs, together with the Cdc42 GTPase, ensure the interplay between three cytoskeletal networks (i.e. actin, microtubules and intermediate filaments), cell adhesion and intracellular vesicle traffic [[40], [41], [42]].
The Scribble polarity complex proteins, Scribble, Dlg and Lgl, are central in polarized cell migration via their action on actin cytoskeleton remodeling, vesicle trafficking, and several signaling pathways, as MAPK and PI3K–Akt pathways [43]. In activated, polarized murine and human T cells, Scribble and Dlg were shown to concentrate within and below the uropod, close to other uropod markers, as ezrin or CD44 that display a more cortical distribution [44]. In contrast, Lgl, a third member of the Scribble complex, or members of other polarity complexes as Crumb3, Par3 or aPKCζ displayed a much larger distribution in the cytoplasm. Therefore, cell polarity regulators distribute in a distinct and asymmetrical manner in polarized cells displaying uropods. Interestingly, Scribble silencing inhibited uropod formation, as well as the relocalization of Dlg and the uropod markers ezrin and CD44, supporting the function of these proteins in T cell polarization. Finally, Scribble silencing inhibited spontaneous T cell migration [44]. However, no data were reported on the role of Scribble complex proteins on polarization or migration in response to chemokines.
The polarity complex formed by Par3, Par6 and aPKC protein families was shown to be involved in T cell polarization and migration in response to chemokines [45]. In T lymphocytes, aPKCι and aPKCζ associate with Par6 and are required for a fully polarized T cell phenotype. Indeed, overexpression of aPKCζ, aPKCι and Par6 mutants inhibited T cell polarization, as assessed by the formation of an actin-rich leading edge and a uropod. Overexpression of aPKCζ kinase dead mutants impaired T cell ability to scan dendritic cells, reduced migration speed and persistence. Consistently with other studies, aPKCζ and aPKCι did not get asymmetrically relocalized during T cell polarization and remained localized all over the cytoplasm [44,45]. An activation pathway linking chemokine receptors with Par-aPKCζ-mediated polarization has been described. It involves the activation of the Rap1 GTPase, which in turn activates the Cdc42 GTPase. Cdc42 activation is necessary to phosphorylate and activate aPKCζ, a mechanism that depends on Par components. Tiam1, a guanine nucleotide exchange factor (GEF) that activates the Rac GTPase, interacts with Rap1, Par3 and aPKCζ, connecting them with Rac-induced actin dynamics that drives the formation of the front lamellipodium and T cell polarization. Interestingly, the same study reports the relocalization of aPKCζ to the front edge, suggesting that different extracellular cues may condition aPKCζ localization during T cell migration [27], in contrast with other reports [44,45]. Furthermore, Rap1 mediates chemokine-induced integrin activation [46]. Therefore, Rap1 concomitantly drives inside-out signaling to integrins and T cell polarization.
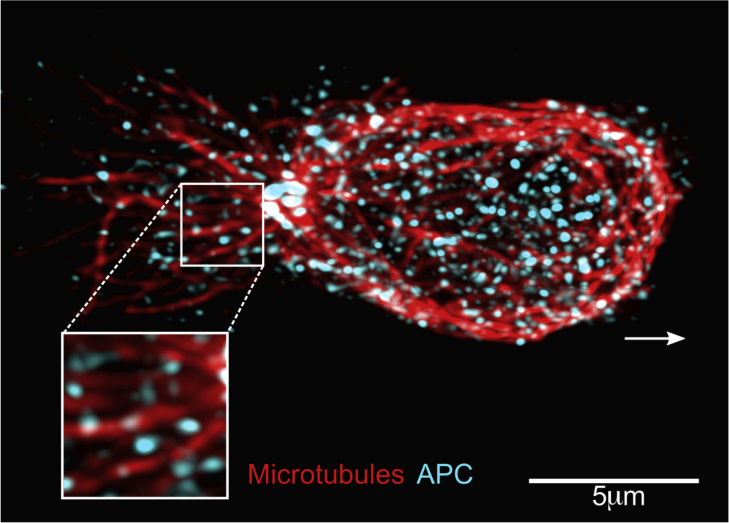
The APC polarity regulator acts at the crossroads of the Scribble-Dlg and Par-aPKC polarity complexes and involves Cdc42 during directed cell migration of astrocytes. Together with Dlg1, APC is implicated in capturing the microtubule 'plus' ends at the leading edge of the migrating cell [5,47,48]. We have recently unveiled that APC is involved in T cell migration by controlling both integrin-mediated cell adhesion and cytoskeleton organization necessary for T cell migration in constraint environments [49]. In migrating T cells, APC displays a broad intracellular distribution mostly associated with microtubules (Fig. 3), suggesting a general rather than a local action during T cell polarization and migration.
Fig. 3.
Localization of the cell polarity regulator APC in migrating T cells. Immunofluorescence of a primary human CD8 T cell migrating on a VCAM-1+chemokine-adhesive substrate, with a distinguishable polarized shape. Contrary to other polarity regulators, APC (puncta in cyan) does not preferentially localizes at a pole of the cells, but rather distributes all along the entire microtubule network (filaments in red), from the centrosome to both the leading edge and the uropod. Inset shows a zoom of the framed region. Arrow shows the direction of migration.
Cell polarization upon antigen receptor engagement in T and B lymphocytes
When encountering antigen-presenting cells, polarized migrating T lymphocytes slow down their migration and eventually stop in a Rac- and integrin-dependent manner [28,50]. Deceleration of migration depends on TCR signal strength and TCR-antigen affinity [51]. High affinity ligands induce strong morphological changes, including relocalization of the centrosome from the back to the front of the cell, close to the contact with the antigen presenting cells, together with the associated organelles, i.e. Golgi, endosomes and lytic granules [[52], [53], [54]], resorption of the uropod structure and transfer of some of its components to the contact site, including ERMs and ICAMs [55,56]. Finally, the contact site becomes an organized subcellular domain termed the immunological synapse, whereby TCR, co-signaling receptors, adhesion and signaling molecules, and cytoskeletal networks reorganize in a symmetrical manner forming supramolecular activation clusters [[57], [58], [59]]. Synapse structure depends on the interacting antigen presenting cell, appearing multifocal when dendritic cells are engaged [60]. More resolutive microscopy techniques allowed to appreciate smaller molecular structures, as dynamic signaling microclusters containing TCR and signaling molecules [61,62], surrounded by adhesion rings and actin cytoskeleton foci [63,64]. Immunological synapses control of T cell activation and effector functions allowing the efficient communication between the T cell and the antigen presenting cell [17]. Immunological synapse shape and symmetry is ensured by the crosstalk of TCR signal and actin and microtubule cytoskeleton networks [65,66]. Low affinity TCR ligands do not stabilize synapse symmetry and T cells keep an exploratory behavior, termed kinapse, observed in vitro and in vivo, compatible with T cells cumulating low intensity signaling over time [67,68].
TCR signaling triggers and maintains T cell polarity at the immunological synapse, as assessed by centrosome polarization toward the antigen presenting cell. Several molecules were shown to be involved, including TCRζ through its tyrosine-based activation motifs (ITAMs), the tyrosine kinases Lck, which phosphorylates TCRζ ITAMs and ZAP70, which is recruited to TCRζ phosphorylated ITAMs [[69], [70], [71]]. In addition, some membrane lipids, as phosphoinositides locally increased by the balanced activation of PIP kinases and phosphatases, and diacyl glycerol (DAG), regulated by phospholipase C and DAG kinase were shown to be involved [[72], [73], [74], [75]]. Interestingly, the actin nucleating formins diaphanous-1 and Formin-like-1 control TCR-induced centrosome polarization, suggesting a crosstalk between actin dynamics and microtubule polarization [76]. The microtubule-guided motor dynein is involved in centrosome polarization in T cell immunological synapse, suggesting some mechanical wiring pulling microtubules to facilitate centrosome polarization [77]. The scaffold protein ADAP and diacyl glycerol are likely anchoring molecules for dynein at the synapse [74,78].
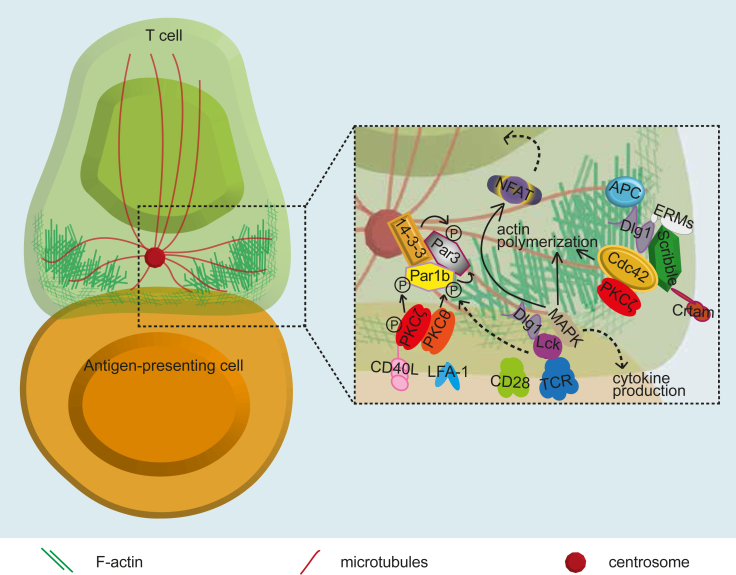
Several polarity regulators are involved in T cell immunological synapses (Fig. 4). Thus, Scribble and Dlg were found to transiently accumulate at the immunological synapse early after its formation, then to be excluded. Scribble knockdown prevents CD3 and PKCθ clustering to anti-CD3+CD28 stimulatory beads [44]. Dlg1 was shown to interact with the TCR signaling machinery via the tyrosine kinase Lck and is involved in actin polymerization, NFAT transcription factor activation and cytokine production, driving T cell activation via p38 MAP kinase [[79], [80], [81], [82]]. Dlg1 is more recruited to the immunological synapse in regulatory T cells (Tregs) and facilitates suppressive function of in vitro-induced Tregs [83,84]. We have shown that Dlg1, through its ERM-mediated interaction with the cortical actin cytoskeleton, ensures the fine microtubule architecture at the immunological synapse, conditioning the dynamics of signaling microclusters and dually regulating early and late T cell activation events [66]. Several mouse Dlg1 KO models were described but in vivo phenotypes were not consistent. Effects on the generation of memory T cells, as well as hyperproliferative response in lymph nodes were reported [[85], [86], [87]].
Fig. 4.
Cell polarity complexes polarity at the T cell immunological synapse. Simplified scheme of an immunological synapse between a T cell (in green) and an antigen-presenting cell (in orange). The formation of an immunological synapse starts with the TCR engagement by its cognate antigen displayed on the surface of an antigen-presenting cell. The TCR and CD28 signal transduction drives cytoskeleton reorganization, characterized by the close polarization of the centrosome to the cell contact and the spreading and retraction of a lamellipodium type membrane extension involving active actin polymerization. The various polarity regulators and partner molecules involved are depicted.
The APC polarity regulator interacts with Dlg1 and preferentially localizes at the microtubule tips at the periphery of the immunological synapse [88,89]. Its silencing in CD4 T cells impaired microtubule network organization, centrosome polarization and microcluster dynamics at the immunological synapse [88], similarly to Dlg1 and ezrin silencing [66], and compromised nuclear translocation and activity of the NFAT transcription factor and IL2 gene expression [88]. Moreover, ApcMin/+ mutant mice displayed impaired differentiation, cytokine production and anti-inflammatory function of intestinal Tregs [88]. In CTLs, Apc regulates actin and microtubule cytoskeleton remodeling at the immunological synapse, controlling synapse morphology and stability and lytic granule dynamics, including targeting and fusion at the synapse. Ultimately, Apc tunes cytotoxic T cell activity, leading to tumor cell killing [89]. Hence, APC, Dlg1 and ERMs may form a molecular complex ensuring the crosstalk between actin and microtubule cytoskelon at the immunological synapse, driving the localization and dynamics of TCR and signaling components as well as the delivery of lytic granules, eventually controlling signaling and effector functions [19].
The aPKCζ polarity regulator was shown to be activated (i.e. phosphorylated at a Thr regulatory site) at the immunological synapse and to control centrosome polarization to the synapse and T cell polarized secretion of interferon (IFN)γ and CD40L towards antigen presenting dendritic cells. This is a crucial event for T helper cell effector function [90]. Interestingly, lytic granule delivery to target cells at the cytotoxic T cell immunological synapse was much less sensitive to aPKCζ inhibition than helper cytokine polarization to the immunological synapse, indicating differential regulation of these two key polarized processes in T cells [91].
Par1b (EMK/MARK2) is one of the four mammalian homologs (Par1a-d) of the Par1 polarity regulator, an evolutionary conserved Ser–Thr kinase implicated in microtubule dynamics via the phosphorylation of microtubule associated proteins (MAPs) [92]. Par1b is phosphorylated on Ser and Thr upon TCR engagement, resulting in 14-3-3 protein binding and the relocalization to the site of contact with the antigen presenting cell, and from the membrane to the cytosol, concentrating Par1b at the cytosol close to the contact site. Par1b phosphorylation needed active Src kinases and was mediated by aPKCζ (on Thr598) and by PKCθ (on Ser400), with no influence of conventional PKCs. A dominant negative form of Par1 inhibits TCR-induced centrosome polarization to the immunological synapse [93]. Therefore, Par1b, PKCζ and PKCθ cooperate to control microtubule polarization at the immunological synapse. Moreover, Par1 phosphorylates Par3 and induces its binding to 14-3-3 proteins, thus inhibiting the assembly of a Par3/Par6/aPKC complex in Drosophila epithelia [94]. Therefore, Par1b phosphorylation of Par3 might control Par3 localization at the immunological synapse area [93]. Further evidence for the importance of Par1b in T cells comes from in vivo mouse KO models, showing a negative regulatory role in immune cells and a key function in lymphocyte homeostasis [95]. It is worth mentioning that other PKCs (e.g. PKCε and PKCη), although not classified as polarity regulators, have been implicated in the recruitment of the centrosome at the immunological synapse [96].
Scribble was reported to be the center of a signaling complex coordinated by the surface protein Crtam (Class I-MHC-restricted T-cell associated molecule), an immunoglobulin family and PDZ-containing protein upregulated on a CD4+ T cell subset. This protein complex is involved in a late phase of T cell polarity and effector functions selectively regulating IFNγ and IL22 cytokine production [97].
Cell polarity is also crucial for B lymphocyte functions in response to antigen. In lymph nodes, B cells recognize antigens tethered to the surface of antigen presenting cells (e.g. follicular dendritic cells or subcapsular sinus macrophages). As part of their B cell antigen receptor complex (BCR), cell surface immunoglobulins interact with antigens and trigger transduction of activating signals to B cells. BCR-induced signaling induces B cell polarization and the formation of an immunological synapse with some similarities with T cell immunological synapses, including actin cytoskeleton-mediated cell spreading and contraction, polarization of the centrosome and clustering of antigen receptors, signaling molecules and integrin adhesion receptors [[98], [99], [100], [101], [102]]. B cell spreading and contraction, and centrosome and lysosome polarization enable antigen capture and internalization, thus facilitating antigen processing and presentation to T cells. Antigen quality may increase this process thus selecting B cells expressing higher affinity immunoglobulins for a more efficient antigen presentation. The GTPase Cdc42 and the aPKCζ are key for these processes [20,101,103].
Conclusion and remaining questions
Several polarity regulators are involved in both lymphocyte migration and antigen-induced responses. The fact that the same molecules act in both processes with different cellular issues is interesting. It is tempting to speculate that polarity regulators may scaffold molecular players from different signaling pathways to build cell polarity in different manners. However, which molecular players and how they interact with polarity regulators remains poorly understood. Rho family GTPases and their activators and inhibitors (GEFs and GAPs, respectively) are likely involved, since their function can be modulated (e.g. through phosphorylation) by antigen, chemokine or integrin-mediated signals. Polarity regulators likely help to keep the balance between Rho, Rac and Cdc42 activities to modulate local actomyosin and microtubule cytoskeleton dynamics capable to induce cell shape changes and directional spreading and retraction leading to polarization features.
However, polarity regulators have numerous protein–protein interaction domains leading to the assembly of large protein interactomes [3], suggesting a much wider and subtle mode of action that remains to be elucidated. Moreover, we do not know whether and how functions of polarity regulators overlap and how they interplay within polarity complexes. For instance, different partners of a polarity complex do not always overlap in time and space [44]. Does it mean that polarity complex partners may differentially interact during certain cellular processes or at different time?
Finally, the importance of polarity regulators in lymphocyte differentiation, activation and effector functions remains to be disentangled, including the involvement of polarity regulators in different T cell subsets. For instance, APC and Dlg1 seem particularly involved in Treg differentiation and regulatory function and more subtly involved in CTL differentiation and cytotoxicity.
Conflicts of interest
Authors declare that they have no competing interests.
Acknowledgements
Research in the authors' laboratory is being supported by grants from La Ligue Nationale contre le Cancer, Equipe Labellisée Ligue 2018, and institutional grants from the Institut Pasteur and INSERM. M.M. is a scholar of the Pasteur Paris University International Doctoral Program, supported by the Institut Pasteur and the European Union Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement 665807 (COFUND-PASTEURDOC). MM is currently funded by “Allocation doctorales 4ème année de thèse-La Ligue Contre Le Cancer”.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Chant J. Cell polarity in yeast. Annu Rev Cell Dev Biol. 1999;15:365–391. doi: 10.1146/annurev.cellbio.15.1.365. [DOI] [PubMed] [Google Scholar]
- 2.Allam A.H., Charnley M., Russell S.M. Context-specific mechanisms of cell polarity regulation. J Mol Biol. 2018;430:3457–3471. doi: 10.1016/j.jmb.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Pires H.R., Boxem M. Mapping the polarity interactome. J Mol Biol. 2018;430:3521–3544. doi: 10.1016/j.jmb.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Ellenbroek S.I., Iden S., Collard J.G. Cell polarity proteins and cancer. Semin Cancer Biol. 2012;22:208–215. doi: 10.1016/j.semcancer.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Etienne-Manneville S., Manneville J.B., Nicholls S., Ferenczi M.A., Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elric J., Etienne-Manneville S. Centrosome positioning in polarized cells: common themes and variations. Exp Cell Res. 2014;328:240–248. doi: 10.1016/j.yexcr.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Etienne-Manneville S. Microtubules in cell migration. Annu Rev Cell Dev Biol. 2013;29:471–499. doi: 10.1146/annurev-cellbio-101011-155711. [DOI] [PubMed] [Google Scholar]
- 8.del Pozo M.A., Sanchez-Mateos P., Sanchez-Madrid F. Cellular polarization induced by chemokines: a mechanism for leukocyte recruitment? Immunol Today. 1996;17:127–131. doi: 10.1016/0167-5699(96)80604-9. [DOI] [PubMed] [Google Scholar]
- 9.Kopf A., Renkawitz J., Hauschild R., Girkontaite I., Tedford K., Merrin J., et al. Microtubules control cellular shape and coherence in amoeboid migrating cells. J Cell Biol. 2020;219 doi: 10.1083/jcb.201907154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Wang Y.L. Centrosome defines the rear of cells during mesenchymal migration. Mol Biol Cell. 2017;28:3240–3251. doi: 10.1091/mbc.E17-06-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Cofreces N.B., Sanchez-Madrid F. Sailing to and docking at the immune synapse: role of tubulin dynamics and molecular motors. Front Immunol. 2018;9:1174. doi: 10.3389/fimmu.2018.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett B.E., Ciocca M.L., Goenka R., Barnett L.G., Wu J., Laufer T.M., et al. Asymmetric B cell division in the germinal center reaction. Science. 2012;335:342–344. doi: 10.1126/science.1213495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham K., Shimoni R., Charnley M., Ludford-Menting M.J., Hawkins E.D., Ramsbottom K., et al. Asymmetric cell division during T cell development controls downstream fate. J Cell Biol. 2015;210:933–950. doi: 10.1083/jcb.201502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thaunat O., Granja A.G., Barral P., Filby A., Montaner B., Collinson L., et al. Asymmetric segregation of polarized antigen on B cell division shapes presentation capacity. Science. 2012;335:475–479. doi: 10.1126/science.1214100. [DOI] [PubMed] [Google Scholar]
- 15.Hind L.E., Vincent W.J., Huttenlocher A. Leading from the back: the role of the uropod in neutrophil polarization and migration. Dev Cell. 2016;38:161–169. doi: 10.1016/j.devcel.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dustin M.L. Hunter to gatherer and back: immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Annu Rev Cell Dev Biol. 2008;24:577–596. doi: 10.1146/annurev.cellbio.24.110707.175226. [DOI] [PubMed] [Google Scholar]
- 17.Dustin M.L., Choudhuri K. Signaling and polarized communication across the T cell immunological synapse. Annu Rev Cell Dev Biol. 2016;32:303–325. doi: 10.1146/annurev-cellbio-100814-125330. [DOI] [PubMed] [Google Scholar]
- 18.Dieckmann N.M., Frazer G.L., Asano Y., Stinchcombe J.C., Griffiths G.M. The cytotoxic T lymphocyte immune synapse at a glance. J Cell Sci. 2016;129:2881–2886. doi: 10.1242/jcs.186205. [DOI] [PubMed] [Google Scholar]
- 19.Mastrogiovanni M., Juzans M., Alcover A., Di Bartolo V. Coordinating cytoskeleton and molecular traffic in T cell migration, activation, and effector functions. Front Cell Dev Biol. 2020;8:591348. doi: 10.3389/fcell.2020.591348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obino D., Lennon-Dumenil A.M. A critical role for cell polarity in antigen extraction, processing, and presentation by B lymphocytes. Adv Immunol. 2014;123:51–67. doi: 10.1016/B978-0-12-800266-7.00001-7. [DOI] [PubMed] [Google Scholar]
- 21.Chang J.T. Polarity and lymphocyte fate determination. Curr Opin Cell Biol. 2012;24:526–533. doi: 10.1016/j.ceb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barreiro O., de la Fuente H., Mittelbrunn M., Sanchez-Madrid F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol Rev. 2007;218:147–164. doi: 10.1111/j.1600-065X.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K.M., Sixt M. Mechanisms of 3D cell migration. Nat Rev Mol Cell Biol. 2019;20:738–752. doi: 10.1038/s41580-019-0172-9. [DOI] [PubMed] [Google Scholar]
- 24.Ravichandran Y., Goud B., Manneville J.B. The Golgi apparatus and cell polarity: roles of the cytoskeleton, the Golgi matrix, and Golgi membranes. Curr Opin Cell Biol. 2020;62:104–113. doi: 10.1016/j.ceb.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 25.De Franceschi N., Hamidi H., Alanko J., Sahgal P., Ivaska J. Integrin traffic - the update. J Cell Sci. 2015;128:839–852. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bretou M., Saez P.J., Sanseau D., Maurin M., Lankar D., Chabaud M., et al. Lysosome signaling controls the migration of dendritic cells. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aak9573. [DOI] [PubMed] [Google Scholar]
- 27.Gerard A., Mertens A.E., van der Kammen R.A., Collard J.G. The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. J Cell Biol. 2007;176:863–875. doi: 10.1083/jcb.200608161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cernuda-Morollon E., Millan J., Shipman M., Marelli-Berg F.M., Ridley A.J. Rac activation by the T-cell receptor inhibits T cell migration. PLoS One. 2010;5:12393. doi: 10.1371/journal.pone.0012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson C.D., Ridley A.J. Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol. 2018;217:447–457. doi: 10.1083/jcb.201612069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takesono A., Heasman S.J., Wojciak-Stothard B., Garg R., Ridley A.J. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS One. 2010;5:8774. doi: 10.1371/journal.pone.0008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas P., Maiuri P., Bretou M., Saez P.J., Pierobon P., Maurin M., et al. Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat Cell Biol. 2016;18:43–53. doi: 10.1038/ncb3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.del Pozo M.A., Cabañas C., Montoya M.C., Ager A., Sánchez-Mateos P., Sánchez-Madrid F. ICAMs redistributed by chemokines to cellular uropods as a mechanism for recruitment of T lymphocytes. J Cell Biol. 1997;137:493–508. doi: 10.1083/jcb.137.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrador J.M., Alonso-Lebrero J.L., del Pozo M.A., Furthmayr H., Schwartz-Albiez R., Calvo J., et al. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J Cell Biol. 1997;138:1409–1423. doi: 10.1083/jcb.138.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrador J.M., Nieto M., Alonso-Lebrero J.L., del Pozo M.A., Calvo J., Furthmayr H., et al. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood. 1998;91:4632–4644. [PubMed] [Google Scholar]
- 35.Serrador J.M., Urzainqui A., Alonso-Lebrero J.L., Cabrero J.R., Montoya M.C., Vicente-Manzanares M., et al. A juxta-membrane amino acid sequence of P-selectin glycoprotein ligand-1 is involved in moesin binding and ezrin/radixin/moesin-directed targeting at the trailing edge of migrating lymphocytes. Eur J Immunol. 2002;32:1560–1566. doi: 10.1002/1521-4141(200206)32:6<1560::AID-IMMU1560>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Serrador J.M., Vicente-Manzanares M., Calvo J., Barreiro O., Montoya M.C., Schwartz-Albiez R., et al. A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting. J Biol Chem. 2002;277:10400–10409. doi: 10.1074/jbc.M110694200. [DOI] [PubMed] [Google Scholar]
- 37.Yonemura S., Hirao M., Doi Y., Takahashi N., Kondo T., Tsukita S., et al. Ezrin/Radixin/Moesin (ERM) proteins bind to a positively charged aminoacid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43 and ICAM-2. J Cell Biol. 1998;140:885–895. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith A., Carrasco Y.R., Stanley P., Kieffer N., Batista F.D., Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valignat M.P., Negre P., Cadra S., Lellouch A.C., Gallet F., Henon S., et al. Lymphocytes can self-steer passively with wind vane uropods. Nat Commun. 2014;5:5213. doi: 10.1038/ncomms6213. [DOI] [PubMed] [Google Scholar]
- 40.Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27:6970–6980. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 41.Seetharaman S., Etienne-Manneville S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020;30:720–735. doi: 10.1016/j.tcb.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Gomes E.R., Jani S., Gundersen G.G. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Bonello T.T., Peifer M. Scribble: a master scaffold in polarity, adhesion, synaptogenesis, and proliferation. J Cell Biol. 2019;218:742–756. doi: 10.1083/jcb.201810103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludford-Menting M.J., Oliaro J., Sacirbegovic F., Cheah E.T., Pedersen N., Thomas S.J., et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Real E., Faure S., Donnadieu E., Delon J. Cutting edge: atypical PKCs regulate T lymphocyte polarity and scanning behavior. J Immunol. 2007;179:5649–5652. doi: 10.4049/jimmunol.179.9.5649. [DOI] [PubMed] [Google Scholar]
- 46.Katagiri K., Ohnishi N., Kabashima K., Iyoda T., Takeda N., Shinkai Y., et al. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5:1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 47.Etienne-Manneville S. APC in cell migration. Adv Exp Med Biol. 2009;656:30–40. doi: 10.1007/978-1-4419-1145-2_3. [DOI] [PubMed] [Google Scholar]
- 48.Etienne-Manneville S., Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 49.Mastrogiovanni M., Vargas P., Rose T., Cuche C., Juzans M., Esposito E., et al. The tumor suppressor adenomatous polyposis coli regulates T lymphocyte migration. Sci Adv. 2022;8 doi: 10.1126/sciadv.abl5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dustin M.L., Bromley S.K., Kan Z., Peterson D.A., Unanue E.R. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreau H.D., Lemaitre F., Garrod K.R., Garcia Z., Lennon-Dumenil A.M., Bousso P. Signal strength regulates antigen-mediated T-cell deceleration by distinct mechanisms to promote local exploration or arrest. Proc Natl Acad Sci U S A. 2015;112:12151–12156. doi: 10.1073/pnas.1506654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kupfer A., Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984;133:2762–2766. [PubMed] [Google Scholar]
- 53.Das V., Nal B., Dujeancourt A., Thoulouze M.I., Galli T., Roux P., et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 54.Stinchcombe J.C., Majorovits E., Bossi G., Fuller S., Griffiths G.M. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 55.Roumier A., Olivo-Marin J.C., Arpin M., Michel F., Martin M., Mangeat P., et al. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 2001;15:715–728. doi: 10.1016/s1074-7613(01)00225-4. [DOI] [PubMed] [Google Scholar]
- 56.Montoya M.C., Sancho D., Bonello G., Collette Y., Langlet C., He H.T., et al. Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat Immunol. 2002;3:159–168. doi: 10.1038/ni753. [DOI] [PubMed] [Google Scholar]
- 57.Monks C.R., Freiberg B.A., Kupfer H., Sciaky N., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 58.Dustin M.L., Chakraborty A.K., Shaw A.S. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2:a002311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 60.Brossard C., Feuillet V., Schmitt A., Randriamampita C., Romao M., Raposo G., et al. Multifocal structure of the T cell - dendritic cell synapse. Eur J Immunol. 2005;35:1741–1753. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 61.Yokosuka T., Sakata-Sogawa K., Kobayashi W., Hiroshima M., Hashimoto-Tane A., Tokunaga M., et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 62.Campi G., Varma R., Dustin M.L. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto-Tane A., Sakuma M., Ike H., Yokosuka T., Kimura Y., Ohara O., et al. Micro-adhesion rings surrounding TCR microclusters are essential for T cell activation. J Exp Med. 2016;213:1609–1625. doi: 10.1084/jem.20151088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumari S., Depoil D., Martinelli R., Judokusumo E., Carmona G., Gertler F.B., et al. Actin foci facilitate activation of the phospholipase C-gamma in primary T lymphocytes via the WASP pathway. Elife. 2015;4:4953. doi: 10.7554/eLife.04953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sims T.N., Soos T.J., Xenias H.S., Dubin-Thaler B., Hofman J.M., Waite J.C., et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 66.Lasserre R., Charrin S., Cuche C., Danckaert A., Thoulouze M.I., de Chaumont F., et al. Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J. 2010;29:2301–2314. doi: 10.1038/emboj.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dustin M.L. Modular design of immunological synapses and kinapses. Cold Spring Harb Perspect Biol. 2009;1:a002873. doi: 10.1101/cshperspect.a002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moreau H.D., Bousso P. Visualizing how T cells collect activation signals in vivo. Curr Opin Immunol. 2014;26:56–62. doi: 10.1016/j.coi.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Lowin-Kropf B., Shapiro V.S., Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol. 1998;140:861–871. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsun A., Qureshi I., Stinchcombe J.C., Jenkins M.R., de la Roche M., Kleczkowska J., et al. Centrosome docking at the immunological synapse is controlled by Lck signaling. J Cell Biol. 2011;192:663–674. doi: 10.1083/jcb.201008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blanchard N., Di Bartolo V., Hivroz C. In the immune synapse, ZAP-70 controls T cell polarization and recruitment of signaling proteins but not formation of the synaptic pattern. Immunity. 2002;17:389–399. doi: 10.1016/s1074-7613(02)00421-1. [DOI] [PubMed] [Google Scholar]
- 72.Chauveau A., Le Floc'h A., Bantilan N.S., Koretzky G.A., Huse M. Diacylglycerol kinase alpha establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci Signal. 2014;7:ra82. doi: 10.1126/scisignal.2005287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Floc'h A., Tanaka Y., Bantilan N.S., Voisinne G., Altan-Bonnet G., Fukui Y., et al. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J Exp Med. 2013;210:2721–2737. doi: 10.1084/jem.20131324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X., Kapoor T.M., Chen J.K., Huse M. Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II. Proc Natl Acad Sci U S A. 2013;110:11976–11981. doi: 10.1073/pnas.1306180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quann E.J., Merino E., Furuta T., Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 76.Gomez T.S., Kumar K., Medeiros R.B., Shimizu Y., Leibson P.J., Billadeau D.D. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin-Cofreces N.B., Robles-Valero J., Cabrero J.R., Mittelbrunn M., Gordon-Alonso M., Sung C.H., et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol. 2008;182:951–962. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Combs J., Kim S.J., Tan S., Ligon L.A., Holzbaur E.L., Kuhn J., et al. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci U S A. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xavier R., Rabizadeh S., Ishiguro K., Andre N., Ortiz J.B., Wachtel H., et al. Discs large (Dlg1) complexes in lymphocyte activation. J Cell Biol. 2004;166:173–178. doi: 10.1083/jcb.200309044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Round J.L., Humphries L.A., Tomassian T., Mittelstadt P., Zhang M., Miceli M.C. Scaffold protein Dlgh1 coordinates alternative p38 kinase activation, directing T cell receptor signals toward NFAT but not NF-kappaB transcription factors. Nat Immunol. 2007;8:154–161. doi: 10.1038/ni1422. [DOI] [PubMed] [Google Scholar]
- 81.Round J.L., Tomassian T., Zhang M., Patel V., Schoenberger S.P., Miceli M.C. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J Exp Med. 2005;201:419–430. doi: 10.1084/jem.20041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crocetti J., Silva O., Humphries L.A., Tibbs M.D., Miceli M.C. Selective phosphorylation of the Dlg1AB variant is critical for TCR-induced p38 activation and induction of proinflammatory cytokines in CD8+ T cells. J Immunol. 2014;193:2651–2660. doi: 10.4049/jimmunol.1401196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zanin-Zhorov A., Kumari S., Hippen K.L., Merkel S.C., MacMillan M.L., Blazar B.R., et al. Human in vitro-induced regulatory T cells display Dlgh1dependent and PKC-theta restrained suppressive activity. Sci Rep. 2017;7:4258. doi: 10.1038/s41598-017-04053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zanin-Zhorov A., Lin J., Scher J., Kumari S., Blair D., Hippen K.L., et al. Scaffold protein Disc large homolog 1 is required for T-cell receptor-induced activation of regulatory T-cell function. Proc Natl Acad Sci U S A. 2012;109:1625–1630. doi: 10.1073/pnas.1110120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gmyrek G.B., Graham D.B., Sandoval G.J., Blaufuss G.S., Akilesh H.M., Fujikawa K., et al. Polarity gene discs large homolog 1 regulates the generation of memory T cells. Eur J Immunol. 2013;43:1185–1194. doi: 10.1002/eji.201142362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Humphries L.A., Shaffer M.H., Sacirbegovic F., Tomassian T., McMahon K.A., Humbert P.O., et al. Characterization of in vivo Dlg1 deletion on T cell development and function. PLoS One. 2012;7:45276. doi: 10.1371/journal.pone.0045276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stephenson L.M., Sammut B., Graham D.B., Chan-Wang J., Brim K.L., Huett A.S., et al. DLGH1 is a negative regulator of T-lymphocyte proliferation. Mol Cell Biol. 2007;27:7574–7581. doi: 10.1128/MCB.00439-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aguera-Gonzalez S., Burton O.T., Vazquez-Chavez E., Cuche C., Herit F., Bouchet J., et al. Adenomatous polyposis coli defines Treg differentiation and anti-inflammatory function through microtubule-mediated NFAT localization. Cell Rep. 2017;21:181–194. doi: 10.1016/j.celrep.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 89.Juzans M., Cuche C., Rose T., Mastrogiovanni M., Bochet P., Di Bartolo V., et al. Adenomatous polyposis coli modulates actin and microtubule cytoskeleton at the immunological synapse to tune CTL functions. Immunohorizons. 2020;4:363–381. doi: 10.4049/immunohorizons.2000044. [DOI] [PubMed] [Google Scholar]
- 90.Bertrand F., Esquerre M., Petit A.E., Rodrigues M., Duchez S., Delon J., et al. Activation of the ancestral polarity regulator protein kinase C zeta at the immunological synapse drives polarization of Th cell secretory machinery toward APCs. J Immunol. 2010;185:2887–2894. doi: 10.4049/jimmunol.1000739. [DOI] [PubMed] [Google Scholar]
- 91.Bertrand F., Muller S., Roh K.H., Laurent C., Dupre L., Valitutti S. An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proc Natl Acad Sci U S A. 2013;110:6073–6078. doi: 10.1073/pnas.1218640110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drewes G., Ebneth A., Mandelkow E.M. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- 93.Lin J., Hou K.K., Piwnica-Worms H., Shaw A.S. The polarity protein Par1b/EMK/MARK2 regulates T cell receptor-induced microtubule-organizing center polarization. J Immunol. 2009;183:1215–1221. doi: 10.4049/jimmunol.0803887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benton R., St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- 95.Hurov J.B., Stappenbeck T.S., Zmasek C.M., White L.S., Ranganath S.H., Russell J.H., et al. Immune system dysfunction and autoimmune disease in mice lacking Emk (Par-1) protein kinase. Mol Cell Biol. 2001;21:3206–3219. doi: 10.1128/MCB.21.9.3206-3219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quann E.J., Liu X., Altan-Bonnet G., Huse M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat Immunol. 2011;12:647–654. doi: 10.1038/ni.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yeh J.H., Sidhu S.S., Chan A.C. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell. 2008;132:846–859. doi: 10.1016/j.cell.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 98.Batista F.D., Iber D., Neuberger M.S. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 99.Carrasco Y.R., Fleire S.J., Cameron T., Dustin M.L., Batista F.D. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20:589–599. doi: 10.1016/s1074-7613(04)00105-0. [DOI] [PubMed] [Google Scholar]
- 100.Depoil D., Weber M., Treanor B., Fleire S.J., Carrasco Y.R., Harwood N.E., et al. Early events of B cell activation by antigen. Sci Signal. 2009;2:pt1. doi: 10.1126/scisignal.263pt1. [DOI] [PubMed] [Google Scholar]
- 101.Fleire S.J., Goldman J.P., Carrasco Y.R., Weber M., Bray D., Batista F.D. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–741. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 102.Mattila P.K., Feest C., Depoil D., Treanor B., Montaner B., Otipoby K.L., et al. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity. 2013;38:461–474. doi: 10.1016/j.immuni.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 103.Yuseff M.I., Reversat A., Lankar D., Diaz J., Fanget I., Pierobon P., et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity. 2011;35:361–374. doi: 10.1016/j.immuni.2011.07.008. [DOI] [PubMed] [Google Scholar]